Abstract
Introduction
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease with rising prevalence. Topical corticosteroids (TCS) are recommended as first-line therapy for patients with AD in China; however, corticophobia is a widespread concern, which can manifest as noncompliance: in a previous Chinese study, almost all parents whose children had AD were very concerned about the side effects of TCS and, as a result, nearly half did not use it in the event of recurrence. We propose a TCS-sparing treatment algorithm for the management of infants, children, adolescents, and adults with mild-to-moderate AD, to guide clinical practice in China.
Methods
A panel of eight experts in AD from China and one expert from Germany formed to develop a practical algorithm for the management of mild-to-moderate AD, focusing on pimecrolimus.
Results
Irrespective of body location, all patients with mild AD (including acute flares) and infants with moderate AD should apply the topical calcineurin inhibitor (TCI) pimecrolimus twice daily to the affected area until symptoms disappear. For children, adolescents, and adults with moderate AD, pimecrolimus should be applied twice daily to sensitive skin areas, and a TCI (either pimecrolimus or tacrolimus) should be applied twice daily to other body locations. Short-term administration of TCS, followed by TCI twice daily, is recommended for most patients with moderate AD experiencing acute flares, regardless of lesion site. Emollients should be used regularly.
Conclusions
The algorithm presented intends to simplify treatment of AD in China and guide clinical decision-making.
Keywords: China, Consensus, Pimecrolimus
Key Summary Points
| Why carry out this study? |
| Topical corticosteroids (TCS) are recommended as first-line therapy for patients with atopic dermatitis (AD) in China; however, corticophobia is a concern. |
| The aim of this article was to propose a practical TCS-sparing treatment algorithm for the management of infants, children, adolescents, and adults with mild-to-moderate AD, to guide daily clinical practice in China. |
| What was learned from the study? |
| All authors agreed on a TCS-sparing treatment algorithm for patients with mild-to-moderate AD, with a focus on pimecrolimus (and, when appropriate, tacrolimus) and emollient maintenance therapy |
| The algorithm presented here is intended to simplify the treatment of AD in daily practice in China. |
Introduction
Atopic dermatitis (AD) is a chronic or chronically relapsing, pruritic, inflammatory skin disease [1, 2]. AD is one of the most common noncommunicable skin diseases, and is a global issue, with worldwide prevalence estimated at 15–20% in children (aged 6–14 years) and 1–3% in adults [3]. Prevalence is on the rise, notably in children [4]. Environmental aspects (e.g., air pollution) may influence the epidemiology of the disease, with the prevalence of AD in preschool children aged 3–6 years reportedly differing between urban and rural areas in China [5–7]. In addition, AD has a major impact on the quality of life (QoL) of patients and caregivers [8], who frequently experience depression, anxiety, suicidal ideation, and fatigue/insomnia [9–12].
The pathophysiology of AD is complex and is influenced by genetics [13], impairment of the epidermal barrier [13], the innate and acquired immune system [13], and the exposome (i.e., the sum of external factors an individual is exposed to), including the microbiome and pollution [14]. Due to the heterogeneous nature of the disease, it is characterized by various phenotypes and endotypes, based on and/or impacted by: age [15], disease severity [15, 16], chronicity (acute versus chronic) [15], epidermal barrier impairment (e.g., filaggrin [FLG] status: FLG+ versus FLG–) [15], immune dysregulation (e.g., immunoglobulin E status) [15], microbiome diversity [17], and environmental factors (e.g., air pollution) [5, 7, 18]. Etiological differences between European American, African American, and Asian patients (e.g., intrinsic versus extrinsic AD, immune polarization, epidermal thickness, genetic factors) also exist and influence the characterization of AD [15]. Stratification of AD by phenotypes and endotypes is therefore important for developing a patient-centric treatment strategy, distancing from the “one-size-fits-all” treatment model [15].
The treatment of mild-to-moderate AD generally comprises emollients, topical corticosteroids (TCS), and topical calcineurin inhibitors (TCI) [19], with other therapies (e.g., systemic immunosuppressive agents, phototherapy, biologics) recommended for the management of severe or refractory disease [20, 21]. Currently, there are geographical differences in the management of AD across Asia, owing in part to significant diversity within the region regarding treatment access, socioeconomic circumstances, and cultural beliefs [22]. A survey of 255 dermatologists across Southeast Asia (based in Indonesia, Malaysia, the Philippines, Singapore, Thailand, and Vietnam) found considerable variation in how familiar the respondents were with diagnostic criteria, as well as differences in how and when TCS and TCI were used [22]; this highlights the need for consensus on the optimal treatment regimen [22]. In addition, complementary and alternative medicines (e.g., herbal preparations) are widely used [23]. However, the availability of data from randomized trials in patients with AD is limited [23–25], and the level of use in the management of AD in Asia remains unclear.
TCS are recommended as first-line therapy for short-term treatment of acute flares when lesions are unresponsive to basic therapy (and as long-term therapy for the prevention of relapses) [19]. There are many considerations when selecting a TCS, including galenic formulation, potency, patient age, and area of the body to which medication will be applied [19]. Although TCS have an important role in the management of AD [26], they are associated with several limitations. Corticophobia (i.e., worries associated with use of TCS) is a major consideration due to its potential impact on treatment adherence, and is therefore a widespread concern [27, 28]. In a survey of 300 parents of children with AD conducted in China, 96% were very concerned about the side effects of TCS; as a result, 42% did not use TCS in the event of AD recurrence [27]. Elsewhere, a study of 200 patients with AD in the United Kingdom found that one-third of patients with concerns about TCS admitted to noncompliance with their TCS regimen [29]; similarly, a study of 208 patients with AD in France found that approximately 81% of respondents had fears about TCS and 36% reported nonadherence to their treatment [30].
In addition, use of TCS is associated with skin barrier impairment, skin atrophy, increased risk of skin infections, tachyphylaxis, and misuse/addiction [31, 32]. As such, TCS are not recommended for long-term management or the treatment of sensitive skin areas, which is notably an issue given the chronic nature of AD and the fact that the disease often affects sensitive skin areas (e.g., face, neck, and flexures) [1, 19]. Sensitive skin areas therefore require further consideration when it comes to therapeutic decision-making, and there is a need for TCS-sparing treatment strategies, based on different clinical manifestations (e.g., age, severity of disease).
TCI offer a valid alternative, as they have similar efficacy to low-to-mid potency TCS, and are not associated with the same limitations, such as skin barrier impairment and skin atrophy [33–35].
The aim of this article is to propose a practical TCS-sparing treatment algorithm for the management of infants, children, adolescents, and adults with mild-to-moderate AD, to guide daily clinical practice in China. The algorithm focuses on the role of TCI in the treatment of mild-to-moderate AD, incorporating a TCS-sparing approach, and identifying the role of pimecrolimus for sensitive skin areas. The algorithm has been structured so that primary care physicians (who regularly see patients with AD), as well as pediatricians and dermatologists, can use it. It is intended to support evidence-based treatment guidelines available at both the international and national level.
Methods
A panel of eight experts in AD from China and one expert from Germany (including dermatologists and pediatricians) was established to discuss and create a practical algorithm for the management of AD in patients from China. Professor Zhao and Professor Luger developed the initial draft of the algorithm. This was reviewed and modified with the other authors according to relevant expertise, local knowledge, guidelines, and literature. Ethical approval was not required since no interventional studies were carried out.
Results
Clinical Evidence for the Treatment of Mild-to-Moderate AD
Assessment of Severity of AD
Initial assessment of patients presenting with AD should account for patient age, and the site and severity of lesions, as these factors help to inform optimal management. Several scales are available to measure the severity of AD: Severity Scoring of Atopic Dermatitis (SCORAD), Eczema Activity Severity Index (EASI), Investigator Global Assessment (IGA), Patient-Oriented Eczema Measure (POEM), Dermatology Life Quality Index (DLQI), visual analog scale (VAS), and numeric rating scale (NRS).
These measures can also be combined to further define the severity of AD (mild: SCORAD < 15, EASI < 6; moderate: SCORAD 15–50, EASI 6–23; severe: SCORAD > 50–103; EASI > 23–72), ahead of treatment selection [36].
Maintenance Treatment with Emollients
Emollients are the mainstay, basic, and maintenance therapy for AD [19, 37]. Traditionally, they have been defined as topical formulations with vehicle-type substances lacking active ingredients [19]. However, in recent years, emollient “plus” formulations have been developed: topical formulations with vehicle-type substances and additional active, nonmedicated substances [19]. Emollients comprise a combination of several components including humectants (e.g., lactate, urea,and glycerin) that have water-attracting properties to promote water retention in the stratum corneum (SC), occludents (e.g., petrolatum) to reduce evaporation, and lipids that may supplement the diminished lipid component of the SC [19, 38, 39].
Emollients are recommended in various national and international treatment guidelines to assist physicians in the management of children, adolescents, and adult patients with mild-to-moderate AD [15, 39–42]. Emollients improve symptoms of AD through several mechanisms: reduced pruritus [43], preserved barrier lipid content [44, 45], decreased susceptibility to irritants [46], reduced transepidermal water loss (TEWL) [43], and moisturization and hydration of the skin [43, 46]. Emollient enhancement of the skin barrier from birth may therefore offer an effective AD prevention strategy [47]. Finally, emollients decrease the need for TCS, offering a TCS-sparing treatment approach [48–50].
Antiinflammatory Treatment
A number of pathophysiological mechanisms are implicated in AD and interplay between these leads to inflammatory responses involving T cells, chemokines, and cytokines, driving the development of AD [51].
Topical antiinflammatories, applied directly to the site of inflammation, are central to effective management of AD. The two predominant classes are TCS (numerous different agents with differing potencies and formulations) and TCI (pimecrolimus and tacrolimus) [19]. TCI available for the treatment of AD in Asia are summarized in Table 1 [19, 26, 52–54].
Table 1.
| Recommendation(s) for clinical use | Strength and formulation | ||
|---|---|---|---|
| Pimecrolimus | Management of mild-to-moderate acute flares, in particular those on sensitive skin, but also on other nonsensitive body locations | Infants aged ≥ 3 months to 2 yearsa [55–66]; children aged ≥ 2 years, adolescents, and adults [53]: 1% cream | |
| Tacrolimus | Management of moderate-to-severe acute flares | Proactive use |
Children aged ≥ 2 to 15 years [54]: 0.03% ointment Children aged ≥ 16 years and adults [54]: 0.1% ointment |
AD atopic dermatitis, TCI topical calcineurin inhibitors
TCI may not be available across all countries in Asia. Pimecrolimus is not available in Japan
aAustralia, Brazil, Canada, European Union, India, Indonesia, Israel, New Zealand, Philippines, Russia, Taiwan, and Thailand only
Proactive (i.e., preventative) and intermittent therapy have been recommended to prevent acute flares [67, 68]. Proactive therapy is a combination of long-term, low-dose antiinflammatory treatment with TCI applied two to three times weekly to areas of skin previously affected by AD. Alternatively, intermittent therapy with TCI involves the resumption of treatment at the first signs of a new flare, i.e., pruritus. However, there are no data from randomized controlled clinical trials conducted to date to indicate that proactive therapy provides greater benefit versus intermittent therapy. Additionally, clinical studies investigating adherence to proactive therapy in patients with myocardial infarction showed suboptimal long-term adherence [69].
TCI should be considered an alternative treatment to reduce the use of TCS, as they are not associated with the same side effects. Pimecrolimus 1% cream is approved for mild-to-moderate AD in adults and children aged ≥ 2 years in several countries [53]; pimecrolimus is also approved in infants aged ≥ 3 months (Australia, Brazil, Canada, European Union, India, Indonesia, Israel, New Zealand, Philippines, Russia, Taiwan, and Thailand) [55–64].
Tacrolimus ointment is available in a 0.03% formulation, approved for the treatment of moderate-to-severe AD in patients aged 2–15 years; a 0.1% formulation is licensed for use in patients aged ≥ 16 years [54].
TCI in AD
Ethnicity (Caucasian versus non-Caucasian) had no effect on treatment outcomes with pimecrolimus in pediatric patients with AD [70]. Similarly, in an analysis of pooled data from studies conducted on adult and pediatric patients with AD in Asia, the efficacy and safety of tacrolimus was similar to that observed in studies in the USA, Europe, and Japan [71]. Hence, where data from studies not conducted specifically in Chinese patients are reported in this paper, results can be inferred to guide treatment practice in Chinese patients.
Steroid-Sparing Effects
The steroid-sparing effects of pimecrolimus have been reported in numerous studies, irrespective of patient age and severity of disease [72–76]. In a 1-year, double-blind study in 251 infants (aged 3–23 months) with AD, overall TCS use was substantially lower in patients receiving pimecrolimus versus conventional treatment (64% versus 35%, respectively) [72]. Pimecrolimus also reduced the need for rescue therapy with TCS in a study of 192 adults with moderate-to-severe AD; over a 24-week treatment period, TCS were used on 14% (95% confidence intervals [CI] 8.3, 21.1) and 37% (95% CI 30.4, 44.0) of days in the pimecrolimus versus control group, respectively (p < 0.001) [76]. Similarly, in a 26-week, randomized study in 543 patients (aged ≥ 18 years) with a history of mild-to-moderate AD, the mean number of TCS-free days was significantly higher in patients treated with pimecrolimus versus control (152 days versus 139 days; p < 0.001) at the first signs and/or symptoms of relapse/recurrence [74].
Rapid Relief from Pruritus
Pruritus is a hallmark feature of AD that severely impacts QoL [77]; rapid relief is essential in the management of patients [78]. In a double-blind, vehicle-controlled, randomized study, 174 children and adolescents (aged 2–17 years) with mild-to-moderate AD and moderate-to-severe pruritus received twice-daily application of pimecrolimus or vehicle [78]. Median time to a ≥ 1 point improvement in pruritus score from baseline was significantly reduced in patients treated with pimecrolimus versus vehicle (48 versus 72 h, respectively; p = 0.038) [78]. In addition, significantly more patients achieved complete resolution of pruritus by day 7 with pimecrolimus versus vehicle (37% versus 18%, respectively; p = 0.008) [78]. In infants with mild to very severe AD, rapid onset of action and no disease rebound after discontinuation was seen with pimecrolimus versus vehicle [79].
Reduction in pruritus has also been reported with tacrolimus. In a 6-week, multicenter, double-blind study in 317 patients aged 2–15 years with mild-to-moderate AD, pruritus scores were significantly lower in tacrolimus-treated patients versus vehicle-treated patients (2.1 versus 3.7, respectively; p < 0.0001) [80].
No Impairment of the Epidermal Barrier or Skin Atrophy
A number of studies have reported the beneficial effects of pimecrolimus on the epidermal barrier. When applied to normal skin for 4 weeks, pimecrolimus did not cause skin atrophy, whereas significant epidermal thinning was reported with TCS [34]. Similar results were seen in an 8-week, investigator-blinded study in patients with mild-to-moderate AD comparing the effects of pimecrolimus and TCS on epidermal and dermal thickness [81]. Importantly, pimecrolimus was effective in patients with head and neck AD intolerant of, or dependent on, TCS [82]. Reversion of skin atrophy was also reported during TCS-free intervals [82]. In addition, after 3 weeks of twice-daily treatment with pimecrolimus or vehicle (one on each forearm) in patients with mild-to-moderate AD, there were significant improvements in skin hydration and TEWL with pimecrolimus versus vehicle [35]. Similarly, findings from a double-blind, randomized, placebo-controlled study in a combined group of patients with AD and healthy volunteers demonstrated that tacrolimus ointment, unlike TCS, did not cause skin atrophy [83].
Long-Term Efficacy and Safety
In the 5-year, open-label, randomized, PETITE study, long-term safety and efficacy of pimecrolimus and TCS were assessed in 2418 infants (aged ≥ 3 months to ≤ 12 months) with mild-to-moderate AD [84]. After 5 years, in both groups, > 85% of patients were cleared/almost cleared of overall AD and > 95% of patients cleared/almost cleared of facial AD. The profile and frequency of adverse events were similar in the two groups: there was no evidence for impairment of humoral or cell-mediated immunity in either group [84]. In a separate study in infants and young children with mild-to-severe AD, pimecrolimus treatment for up to 2 years was well tolerated and led to sustained improvements in disease [85].
Various noncomparative trials support the long-term efficacy and safety (up to 1–4 years of treatment) of tacrolimus 0.1% or 0.03% ointment in children, adolescents, and adults with moderate-to-severe AD [86–89].
Improvement of QoL
Pimecrolimus improved the QoL of both patients’ and caregivers’ in various trials [76, 90–92]. In two trials reporting the effects of pimecrolimus in infants (aged 3 months to 2 years), children (aged 2–17 years), and parents, pimecrolimus significantly improved QoL versus control in all groups assessed [90].
Tacrolimus led to significant improvements in health-related QoL versus vehicle in children aged ≥ 2 years and adults with AD [93]. In addition, in Japanese patients with corticophobia (n = 35), following 12 weeks of treatment with tacrolimus ointment, overall QoL score significantly improved from baseline at the end of the study (p < 0.001) [94].
Reduction in AD Flares
In previously mentioned studies, pimecrolimus cream was associated with significantly fewer AD flares versus vehicle in infants, children, adolescents, and adults with AD [72–76, 95].
Sensitive Skin Areas
TCI have greater selectivity versus TCS in targeting cells involved in the inflammatory response at sites affected by AD [96]. As such, TCI (in particular, pimecrolimus) are preferred over TCS in sensitive skin areas [39], and are recommended by European guidelines for the treatment of facial lesions [19]. In terms of TCS, we recommend use for a short period, followed by TCI, in the treatment of acute flares of moderate AD on sensitive skin.
Tolerability and Acceptability
The most common treatment-related adverse events with pimecrolimus are application site reactions, including feelings of warmth and/or burning, pruritus, and erythema/irritation [97]. In various trials, application site reactions following treatment with pimecrolimus were transient and/or mostly mild-to-moderate in severity [73–75, 98].
In two randomized studies of tacrolimus ointment (0.03% or 0.1%) versus vehicle, the most common adverse events with significantly greater incidence than in the vehicle group were sensation of skin burning, flu-like symptoms, and headache; symptoms generally resolved within the first few days of treatment [99]. However, in the authors’ clinical experience, flu-like symptoms and headache have not commonly been reported with real-world application of tacrolimus.
In a 6-week, investigator-blinded study comparing pimecrolimus (n = 71) with tacrolimus ointment 0.03% (n = 70) in patients (aged 2–17 years) with moderate AD, incidence of erythema/irritation was less common (8% versus 19%; p = 0.039) and shorter duration (erythema/irritation lasting > 30 min: 0% versus 85%; p < 0.001) in pimecrolimus-treated versus tacrolimus-treated patients, respectively [100]. Warmth, stinging, and burning were similar between groups; however, adverse events lasting > 30 min were less common in the pimecrolimus group versus tacrolimus (0% versus 67%; p < 0.001) [100]. In addition, pimecrolimus was preferred to tacrolimus ointment across many product features (i.e., ease of application, suitability for face, nonsticky feel, ease of rub-in, and spreadability), with more patients rating ease of application as “excellent” or “very good” (76% versus 59%, respectively; p < 0.02) [100].
Langerhans cells (LCs) are specialized antigen-presenting cells in the epidermis with a pivotal role in cutaneous immune surveillance [101, 102]. In contrast to TCS, which led to depletion of LCs, pimecrolimus did not affect LCs in studies of murine epidermis, and healthy and atopic human skin [101–103]. Tacrolimus was shown to influence the maturation of LCs in vitro; however, in patients with AD, tacrolimus depleted inflammatory dendritic epidermal cells, with no apoptosis of LCs reported [104].
In terms of systemic effects, permeability of pimecrolimus through the skin is lower compared with tacrolimus (9–10 times slower), and much less than TCS (70–110 times slower) [104], decreasing the likelihood of transcutaneous resorption following topical administration, and leading to a reduced risk of systemic effects (e.g., hypothalamic–pituitary–adrenal axis suppression, Cushing’s syndrome, femoral head osteonecrosis, and cataracts) [104]. This may be due, in part, to the higher lipophilicity of pimecrolimus (versus tacrolimus and TCS) [105].
Finally, long-term safety data illustrate no evidence that TCI cause skin malignancies and/or lymphomas [53, 106].
Other Treatments for Severe AD
The majority of cases of AD are mild to moderate in severity, although a subpopulation of patients suffer from severe eczematous skin lesions [36]. Tacrolimus is indicated for the treatment of moderate-to-severe AD [54]; there is some evidence supporting the use of pimecrolimus in this patient population [107]. However, several systemic immunosuppressive therapies are also recommended or under investigation in patients with moderate-to-severe or severe AD [20]. Dupilumab (anti-IL4Rα) [108–110] and cyclosporine A [111–113] are both approved treatments (although cyclosporine A is not approved by the Food and Drug Administration [FDA]), and methotrexate [114, 115], azathioprine [116], mycophenolate mofetil [117, 118], nemolizumab (anti-IL31Rα) [119], tralokinumab (anti-IL13) [120], and Janus kinase (JAK) 1/2 and JAK1/3 inhibitors (e.g., baricitinib [121], abrocitinib [122], tofacitinib [123]) have been investigated and have shown efficacy in this population of patients. Other biologics, such as tezepelumab (anti-thymic stromal lymphopoietin), are also under investigation in early-phase trials [124]. In addition, short-term use of systemic corticosteroids is noted in Asia-Pacific guidelines [125], and occasionally used in China. Finally, phototherapy (e.g., narrow-band ultraviolet B) is recommended in European guidelines [19], and has resolved clinical disease in patients with moderate-to-severe AD [126]. However, patient age and affected body regions must be carefully considered before administering these therapies, and systemic treatment should be reserved for persistent, widespread AD that is unresponsive to other treatment [19, 125], or for patients with prolonged use of high-potency TCS [127]. Regardless of chosen therapy, this should be supplemented with local treatment with TCS and/or TCI [19, 20].
Discussion
TCS-Sparing Treatment Algorithm for Mild-to-Moderate AD in China
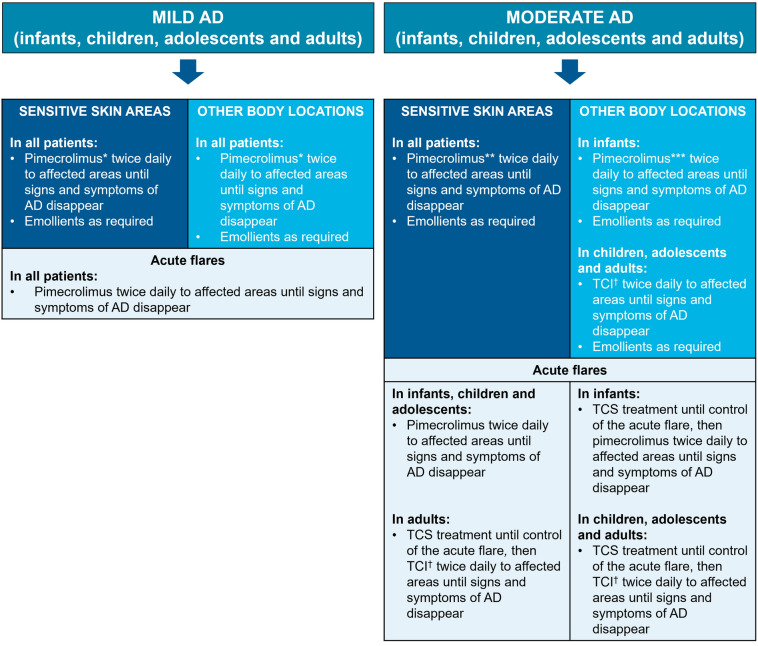
Following discussions, all authors agreed on a TCS-sparing treatment algorithm for patients with mild-to-moderate AD, with a focus on pimecrolimus (and, when appropriate, tacrolimus) and emollient maintenance therapy in infants, children, adolescents, and adults (Fig. 1).
Fig. 1.
Algorithm for the treatment of infants, children, adolescents, and adults with mild-to-moderate AD. AD atopic dermatitis, EU European Union, TCI topical calcineurin inhibitors, TCS topical corticosteroids. *Pimecrolimus 1% cream is indicated for mild-to-moderate AD (children aged ≥ 2 years, adolescents, and adults) [53] and for use in infants aged ≥ 3 months (Australia, Brazil, Canada, European Union, India, Indonesia, Israel, New Zealand, Philippines, Russia, Taiwan, and Thailand only) [55–66]. **Pimecrolimus is recommended in EU guidelines [19] in sensitive skin areas; evidence suggests patient preference for pimecrolimus versus tacrolimus [100]. ***Pimecrolimus is recommended in other body locations versus tacrolimus, as there is a body of evidence to support its efficacy and tolerability profile. †TCI: pimecrolimus 1% cream, or tacrolimus 0.1% (aged ≥ 16 years) or 0.03% (aged 2–15 years) ointment; pimecrolimus is indicated for mild-to-moderate AD, and tacrolimus is indicated for moderate-to-severe AD [53, 54]
Conclusions
AD is one of the most common noncommunicable skin diseases, and a major issue globally and in China. Currently, there is a need for treatment approaches that reduce the use of TCS, owing to their association with corticophobia, adverse events and misuse, and lack of suitability for long-term treatment of AD. We recommend the use of emollients to prevent disease flares. We also recommend a TCS-sparing treatment strategy, focusing on the role of TCI (notably pimecrolimus 1% cream) in the management of infants, children, adolescents, and adults with mild-to-moderate AD. The algorithm presented here is intended to simplify the treatment of AD in daily practice in China.
Acknowledgements
Funding
The writing/editorial support/journal Rapid Service fee were funded by Meda Pharma S.p.A., a Viatris company. All authors were invited to a workshop funded by Viatris, in which travel and accommodation were paid.
Medical Writing, Editorial, and Other Assistance
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Danny Hawker, MSc, and Molly Macpherson, BSc, of Ashfield MedComms, an Ashfield Health company, and funded by Meda Pharma S.p.A., a Viatris company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
ZZ contributed to conceptualization, project administration, supervision and writing (reviewing and editing). X-HG contributed to conceptualization and writing (reviewing and editing). WL contributed to conceptualization and writing (reviewing and editing). HW contributed to conceptualization and writing (reviewing and editing). YL contributed to conceptualization and writing (reviewing and editing). JT contributed to conceptualization and writing (reviewing and editing). XY contributed to conceptualization and writing (reviewing and editing). HZ contributed to conceptualization and writing (reviewing and editing). TL contributed to conceptualization, project administration, supervision and writing (reviewing and editing).
Compliance with Ethics Guidelines
Ethical approval was not required since no interventional studies were carried out: this article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Disclosures
Zuotao Zhao reports personal fees from Novartis, Pfizer, Astellas, Galderma, Meda Pharma S.p.A., a Viatris company, Bayer, LEO, GSK, Janssen, and ALK Pharma, outside the submitted work. Xing-Hua Gao reports personal fees from Novartis, Pfizer, Astellas, Meda Pharma S.p.A., a Viatris company, Sanofi, Lilly, Bayer, LEO, GSK, Pierre Fabre, and Janssen, outside the submitted work. Wei Li reports personal fees from Sanofi, Pfizer, Lilly, Novartis, LEO, Pierre Fabre, Meda Pharma S.p.A., a Viatris company, Astellas, and Galderma, outside the submitted work. Hua Wang has nothing to disclose. Yunsheng Liang reports personal fees and other from Sanofi and Pfizer, and personal fees from Novartis, Astellas, Janssen, LEO, and Meda Pharma S.p.A., a Viatris company, during the conduct of the study. Jianping Tang reports personal fees from Meda Pharma S.p.A., a Viatris company, during the conduct of the study. Xu Yao has nothing to disclose. Hua Zhao reports personal fees from Meda Pharma S.p.A., a Viatris company, during the conduct of the study. Thomas Luger reports grants and personal fees from Meda Pharma S.p.A., a Viatris company, during the conduct of the study.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Watson W, Kapur S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2011;7:S4. doi: 10.1186/1710-1492-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidinger S, Novak N. Atopic dermatitis. Lancet (Lond, Engl) 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 3.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66:8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 4.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 5.Kabashima K, Otsuka A, Nomura T. Linking air pollution to atopic dermatitis. Nat Immunol. 2016;18:5–6. doi: 10.1038/ni.3615. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Yan S, Li F, et al. Prevalence of childhood atopic dermatitis: an urban and rural community-based study in Shanghai, China. PLoS ONE. 2012;7:e36174. doi: 10.1371/journal.pone.0036174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidaka T, Ogawa E, Kobayashi EH, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18:64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 8.Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984–992. doi: 10.1111/j.1742-1241.2006.01047.x. [DOI] [PubMed] [Google Scholar]
- 9.Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Investig Dermatol. 2015;135:984–991. doi: 10.1038/jid.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Investig Dermatol. 2015;135:56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 11.Sandhu JK, Wu KK, Bui TL, Armstrong AW. Association between atopic dermatitis and suicidality: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:178–187. doi: 10.1001/jamadermatol.2018.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng LJ, Chen AW, Luo XY, Wang H. Increased attention deficit/hyperactivity and oppositional defiance symptoms of 6–12 years old Chinese children with atopic dermatitis. Medicine. 2020;99:e20801. doi: 10.1097/MD.0000000000020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40:84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75:63–74. doi: 10.1111/all.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1–11. doi: 10.1016/j.jaci.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh D, Bernstein JA, Khurana Hershey GK, Rothenberg ME, Mersha TB. Leveraging multilayered “Omics” data for atopic dermatitis: a road map to precision medicine. Front Immunol. 2018;9:2727. doi: 10.3389/fimmu.2018.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paller AS, Kong HH, Seed P, et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:26–35. doi: 10.1016/j.jaci.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnass W, Huls A, Vierkotter A, Kramer U, Krutmann J, Schikowski T. Traffic-related air pollution and eczema in the elderly: findings from the SALIA cohort. Int J Hyg Environ Health. 2018;221:861–867. doi: 10.1016/j.ijheh.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol JEADV. 2018;32:657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 20.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 21.Patrizi A, Raone B, Ravaioli GM. Management of atopic dermatitis: safety and efficacy of phototherapy. Clin Cosmet Investig Dermatol. 2015;8:511–520. doi: 10.2147/CCID.S87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan YC, Tay YK, Sugito TL, et al. A study on the knowledge, attitudes and practices of Southeast Asian dermatologists in the management of atopic dermatitis. Ann Acad Med Singap. 2006;35:794–803. [PubMed] [Google Scholar]
- 23.Leung TN, Hon KL. Correction: Eczema therapeutics in children: what do the clinical trials say? Hong Kong Med J. 2015;21:374. [PubMed] [Google Scholar]
- 24.Hon KL, Leung AKC, Leung TNH, Lee VWY. Complementary, alternative and integrative medicine for childhood atopic dermatitis. Recent Pat Inflamm Allergy Drug Discov. 2017;11:114–124. doi: 10.2174/1872213X11666171128142333. [DOI] [PubMed] [Google Scholar]
- 25.Gu S, Yang AW, Xue CC, et al. Chinese herbal medicine for atopic eczema. Cochrane Database Syst Rev. 2013;10:Cd008642. doi: 10.1002/14651858.CD008642.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay YK, Chan YC, Chandran NS, et al. Guidelines for the management of atopic dermatitis in Singapore. Ann Acad Med Singap. 2016;45:439–450. [PubMed] [Google Scholar]
- 27.Li Y, Han T, Li W, Li Y, Guo X, Zheng L. Awareness of and phobias about topical corticosteroids in parents of infants with eczema in Hangzhou, China. Pediatr Dermatol. 2018;35:463–467. doi: 10.1111/pde.13527. [DOI] [PubMed] [Google Scholar]
- 28.Mueller SM, Itin P, Vogt DR, et al. Assessment of “corticophobia” as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatol Treat. 2017;28:104–111. doi: 10.1080/09546634.2016.1201189. [DOI] [PubMed] [Google Scholar]
- 29.Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931–936. doi: 10.1046/j.1365-2133.2000.03473.x. [DOI] [PubMed] [Google Scholar]
- 30.Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808–814. doi: 10.1111/j.1365-2133.2011.10449.x. [DOI] [PubMed] [Google Scholar]
- 31.Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5:416–425. doi: 10.4103/2229-5178.142483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A, Sengupta S, Coondoo A, Jana AK. Topical corticosteroid addiction and phobia. Indian J Dermatol. 2014;59:465–468. doi: 10.4103/0019-5154.139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broeders JA, Ahmed Ali U, Fischer G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: a 15-year experience. J Am Acad Dermatol. 2016;75:410–9.e3. doi: 10.1016/j.jaad.2016.02.1228. [DOI] [PubMed] [Google Scholar]
- 34.Queille-Roussel C, Paul C, Duteil L, et al. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for 4 weeks: a randomized, double-blind controlled study. Br J Dermatol. 2001;144:507–513. doi: 10.1046/j.1365-2133.2001.04076.x. [DOI] [PubMed] [Google Scholar]
- 35.Aschoff R, Schwanebeck U, Brautigam M, Meurer M. Skin physiological parameters confirm the therapeutic efficacy of pimecrolimus cream 1% in patients with mild-to-moderate atopic dermatitis. Exp Dermatol. 2009;18:24–29. doi: 10.1111/j.1600-0625.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 36.Spergel J, Lio P. Management of severe atopic dermatitis (eczema) in children. 2019. https://www.uptodate.com/contents/management-of-severe-atopic-dermatitis-eczema-in-children. Accessed 5 May 2020.
- 37.van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2:CD012119. doi: 10.1002/14651858.CD012119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correa CMM, Nebus J. Management of patients with atopic dermatitis: the role of emollient therapy. Dermatol Res Pract. 2012;2012:836931. doi: 10.1155/2012/836931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luger T, De Raeve L, Gelmetti C, et al. Recommendations for pimecrolimus 1% cream in the treatment of mild-to-moderate atopic dermatitis: from medical needs to a new treatment algorithm. Eur J Dermatol EJD. 2013;23:758–766. doi: 10.1684/ejd.2013.2169. [DOI] [PubMed] [Google Scholar]
- 40.Reda AM, Elgendi A, Ebraheem AI, et al. A practical algorithm for topical treatment of atopic dermatitis in the Middle East emphasizing the importance of sensitive skin areas. J Dermatol Treat. 2019;30:366–373. doi: 10.1080/09546634.2018.1524823. [DOI] [PubMed] [Google Scholar]
- 41.Group of Pediatric Dermatology, Chinese Society of Dermatology, Chinese Medical Association Treatment of atopic eczema (atopic dermatitis) in children: a Chinese expert consensus statement. Chin J Dermatol. 2017;50:784–789. [Google Scholar]
- 42.Group of Immunology, Society of Dermatology, Chinese Medical Association Atopic Dermatitis Expert collaborative research center. Guideline for diagnosis and treatment of atopic dermatitis in China. Clin Educ Gen Pract. 2014;12:606–615. [Google Scholar]
- 43.Sparavigna A, Tenconi B, La Penna L. Efficacy of a novel emollient plus cream in atopic dermatitis: a randomised, vehicle-controlled, double-blind study. J Plast Pathol Dermatol. 2019;15:85–93. [Google Scholar]
- 44.Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045–1060. doi: 10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
- 45.Telofski LS, Morello AP, 3rd, Mack Correa MC, Stamatas GN. The infant skin barrier: can we preserve, protect, and enhance the barrier? Dermatol Res Pract. 2012;2012:198789. doi: 10.1155/2012/198789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kownacki S. The importance of emollients in treating the increasing incidence of atopic eczema. Nurs Times. 2009;105:18–22. [PubMed] [Google Scholar]
- 47.Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–823. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30:71–86. doi: 10.1016/j.sder.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mengeaud V, Phulpin C, Bacquey A, Boralevi F, Schmitt AM, Taieb A. An innovative oat-based sterile emollient cream in the maintenance therapy of childhood atopic dermatitis. Pediatr Dermatol. 2015;32:208–215. doi: 10.1111/pde.12464. [DOI] [PubMed] [Google Scholar]
- 50.Eberlein B, Eicke C, Reinhardt HW, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study) J Eur Acad Dermatol Venereol JEADV. 2008;22:73–82. doi: 10.1111/j.1468-3083.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 51.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 52.Leung TNH, Chow CM, Chow MPY, et al. Clinical guidelines on management of atopic dermatitis in children. Hong Kong J Paediatr. 2013;18:96–104. [Google Scholar]
- 53.Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol. 2015;26:306–315. doi: 10.1111/pai.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LEO Pharma. Protopic (tacrolimus) prescribing information. LEO Pharma revised: 02/2019. 2019. http://www.protopic.com/. Accessed 4 Mar 2020.
- 55.Mylan. Elidel 1% Summary of Product Characteristics (Philippines). 2017.
- 56.Mylan. Elidel 1% Summary of Product Characteristics (Thailand). 2008.
- 57.Mylan. Elidel 1% Summary of Product Characteristics (Indonesia). 2008.
- 58.Mylan. Elidel 1% Summary of Product Characteristics (New Zealand). 2018.
- 59.Mylan. Elidel 1% Summary of Product Characteristics (Australia). 2018.
- 60.Valeant. Elidel 1% Product Monograph (Canada). 2019.
- 61.Mylan. Elidel 1% Prescribing Information (Russia). 2021.
- 62.Mylan. Elidel 1% Prescribing Information (Israel). 2009.
- 63.Mylan. Elidel 1% Summary of Product Characteristics (India). 2018.
- 64.Mylan. Elidel 1% Summary of Product Characteristics (Brazil). 2018.
- 65.Mylan. Elidel 1% Summary of Product Characteristics (Taiwan).
- 66.Viatris. Elidel 1% Summary of Product Characteristics (Denmark).
- 67.Katayama I, Aihara M, Ohya Y, et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66:230–247. doi: 10.1016/j.alit.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Damiani G, Calzavara-Pinton P, Stingeni L, et al. Italian guidelines for therapy of atopic dermatitis—adapted from consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) Dermatol Ther. 2019;32:e13121. doi: 10.1111/dth.13121. [DOI] [PubMed] [Google Scholar]
- 69.Dibao-Dina C, Angoulvant D, Lebeau JP, Peurois JE, Abdallah El Hirtsi K, Lehr-Drylewicz AM. Patients' adherence to optimal therapeutic, lifestyle and risk factors recommendations after myocardial infarction: Six years follow-up in primary care. PLoS ONE. 2018;13:e0202986. doi: 10.1371/journal.pone.0202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eichenfield LF, Lucky AW, Langley RG, et al. Use of pimecrolimus cream 1% (Elidel) in the treatment of atopic dermatitis in infants and children: the effects of ethnic origin and baseline disease severity on treatment outcome. Int J Dermatol. 2005;44:70–75. doi: 10.1111/j.1365-4632.2004.02234.x. [DOI] [PubMed] [Google Scholar]
- 71.Kim KH, Kono T. Overview of efficacy and safety of tacrolimus ointment in patients with atopic dermatitis in Asia and other areas. Int J Dermatol. 2011;50:1153–1161. doi: 10.1111/j.1365-4632.2011.04881.x. [DOI] [PubMed] [Google Scholar]
- 72.Kapp A, Papp K, Bingham A, et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. J Allergy Clin Immunol. 2002;110:277–284. doi: 10.1067/mai.2002.126500. [DOI] [PubMed] [Google Scholar]
- 73.Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110:e2. doi: 10.1542/peds.110.1.e2. [DOI] [PubMed] [Google Scholar]
- 74.Gollnick H, Kaufmann R, Stough D, et al. Pimecrolimus cream 1% in the long-term management of adult atopic dermatitis: prevention of flare progression. A randomized controlled trial. Br J Dermatol. 2008;158:1083–1093. doi: 10.1111/j.1365-2133.2008.08484.x. [DOI] [PubMed] [Google Scholar]
- 75.Sigurgeirsson B, Ho V, Ferrandiz C, Andriano K, Grinienko A, Jimenez P. Effectiveness and safety of a prevention-of-flare-progression strategy with pimecrolimus cream 1% in the management of paediatric atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22:1290–1301. doi: 10.1111/j.1468-3083.2008.02785.x. [DOI] [PubMed] [Google Scholar]
- 76.Meurer M, Folster-Holst R, Wozel G, Weidinger G, Junger M, Brautigam M. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology (Basel, Switzerland) 2002;205:271–277. doi: 10.1159/000065863. [DOI] [PubMed] [Google Scholar]
- 77.Yang EJ, Beck KM, Sekhon S, Bhutani T, Koo J. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66–71. doi: 10.1111/pde.13727. [DOI] [PubMed] [Google Scholar]
- 78.Fowler J, Johnson A, Chen M, Abrams K. Improvement in pruritus in children with atopic dermatitis using pimecrolimus cream 1% Cutis. 2007;79:65–72. [PubMed] [Google Scholar]
- 79.Kaufmann R, Folster-Holst R, Hoger P, et al. Onset of action of pimecrolimus cream 1% in the treatment of atopic eczema in infants. J Allergy Clin Immunol. 2004;114:1183–1188. doi: 10.1016/j.jaci.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 80.Schachner LA, Lamerson C, Sheehan MP, et al. Tacrolimus ointment 0.03% is safe and effective for the treatment of mild to moderate atopic dermatitis in pediatric patients: results from a randomized, double-blind, vehicle-controlled study. Pediatrics. 2005;116:e334–e342. doi: 10.1542/peds.2004-2638. [DOI] [PubMed] [Google Scholar]
- 81.Aschoff R, Schmitt J, Knuschke P, Koch E, Brautigam M, Meurer M. Evaluation of the atrophogenic potential of hydrocortisone 1% cream and pimecrolimus 1% cream in uninvolved forehead skin of patients with atopic dermatitis using optical coherence tomography. Exp Dermatol. 2011;20:832–836. doi: 10.1111/j.1600-0625.2011.01335.x. [DOI] [PubMed] [Google Scholar]
- 82.Murrell DF, Calvieri S, Ortonne JP, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. Br J Dermatol. 2007;157:954–959. doi: 10.1111/j.1365-2133.2007.08192.x. [DOI] [PubMed] [Google Scholar]
- 83.Reitamo S, Rissanen J, Remitz A, et al. Tacrolimus ointment does not affect collagen synthesis: results of a single-center randomized trial. J Investig Dermatol. 1998;111:396–398. doi: 10.1046/j.1523-1747.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- 84.Sigurgeirsson B, Boznanski A, Todd G, et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics. 2015;135:597–606. doi: 10.1542/peds.2014-1990. [DOI] [PubMed] [Google Scholar]
- 85.Papp KA, Werfel T, Folster-Holst R, et al. Long-term control of atopic dermatitis with pimecrolimus cream 1% in infants and young children: a two-year study. J Am Acad Dermatol. 2005;52:240–246. doi: 10.1016/j.jaad.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Reitamo S, Wollenberg A, Schopf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. The European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999–1006. doi: 10.1001/archderm.136.8.999. [DOI] [PubMed] [Google Scholar]
- 87.Kang S, Lucky AW, Pariser D, Lawrence I, Hanifin JM. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44:S58–S64. doi: 10.1067/mjd.2001.109812. [DOI] [PubMed] [Google Scholar]
- 88.Hanifin JM, Paller AS, Eichenfield L, et al. Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis. J Am Acad Dermatol. 2005;53:S186–S194. doi: 10.1016/j.jaad.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 89.Remitz A, Harper J, Rustin M, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. Acta Derm Venereol. 2007;87:54–61. doi: 10.2340/00015555-0167. [DOI] [PubMed] [Google Scholar]
- 90.McKenna SP, Whalley D, de Prost Y, et al. Treatment of paediatric atopic dermatitis with pimecrolimus (Elidel, SDZ ASM 981): impact on quality of life and health-related quality of life. J Eur Acad Dermatol Venereol. 2006;20:248–254. doi: 10.1111/j.1468-3083.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 91.Staab D, Kaufmann R, Brautigam M, Wahn U. Treatment of infants with atopic eczema with pimecrolimus cream 1% improves parents’ quality of life: a multicenter, randomized trial. Pediatr Allergy Immunol. 2005;16:527–533. doi: 10.1111/j.1399-3038.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 92.Whalley D, Huels J, McKenna SP, Van Assche D. The benefit of pimecrolimus (Elidel, SDZ ASM 981) on parents’ quality of life in the treatment of pediatric atopic dermatitis. Pediatrics. 2002;110:1133–1136. doi: 10.1542/peds.110.6.1133. [DOI] [PubMed] [Google Scholar]
- 93.Drake L, Prendergast M, Maher R, et al. The impact of tacrolimus ointment on health-related quality of life of adult and pediatric patients with atopic dermatitis. J Am Acad Dermatol. 2001;44:S65–S72. doi: 10.1067/mjd.2001.109814. [DOI] [PubMed] [Google Scholar]
- 94.Kawashima M. Quality of life in patients with atopic dermatitis: impact of tacrolimus ointment. Int J Dermatol. 2006;45:731–736. doi: 10.1111/j.1365-4632.2006.02705.x. [DOI] [PubMed] [Google Scholar]
- 95.Siegfried E, Korman N, Molina C, Kianifard F, Abrams K. Safety and efficacy of early intervention with pimecrolimus cream 1% combined with corticosteroids for major flares in infants and children with atopic dermatitis. J Dermatolog Treat. 2006;17:143–150. doi: 10.1080/09546630600647297. [DOI] [PubMed] [Google Scholar]
- 96.Stuetz A, Baumann K, Grassberger M, Wolff K, Meingassner JG. Discovery of topical calcineurin inhibitors and pharmacological profile of pimecrolimus. Int Arch Allergy Immunol. 2006;141:199–212. doi: 10.1159/000095289. [DOI] [PubMed] [Google Scholar]
- 97.electronic Medicines Compendium (eMC). Elidel 10mg/g cream summary of product characteristics (SmPC). 2018. https://www.medicines.org.uk/emc/product/4966/smpc. Accessed 7 May 2020.
- 98.Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495–504. doi: 10.1067/mjd.2002.122187. [DOI] [PubMed] [Google Scholar]
- 99.Soter NA, Fleischer AB, Jr, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44:S39–S46. doi: 10.1067/mjd.2001.109817. [DOI] [PubMed] [Google Scholar]
- 100.Kempers S, Boguniewicz M, Carter E, et al. A randomized investigator-blinded study comparing pimecrolimus cream 1% with tacrolimus ointment 0.03% in the treatment of pediatric patients with moderate atopic dermatitis. J Am Acad Dermatol. 2004;51:515–525. doi: 10.1016/j.jaad.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 101.Meingassner JG, Kowalsky E, Schwendinger H, Elbe-Burger A, Stutz A. Pimecrolimus does not affect Langerhans cells in murine epidermis. Br J Dermatol. 2003;149:853–857. doi: 10.1046/j.1365-2133.2003.05559.x. [DOI] [PubMed] [Google Scholar]
- 102.Hoetzenecker W, Ecker R, Kopp T, Stuetz A, Stingl G, Elbe-Burger A. Pimecrolimus leads to an apoptosis-induced depletion of T cells but not Langerhans cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2005;115:1276–1283. doi: 10.1016/j.jaci.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Hoetzenecker W, Meingassner JG, Ecker R, Stingl G, Stuetz A, Elbe-Burger A. Corticosteroids but not pimecrolimus affect viability, maturation and immune function of murine epidermal Langerhans cells. J Investig Dermatol. 2004;122:673–684. doi: 10.1111/j.0022-202X.2004.22324.x. [DOI] [PubMed] [Google Scholar]
- 104.Grassberger M, Steinhoff M, Schneider D, Luger TA. Pimecrolimus—an anti-inflammatory drug targeting the skin. Exp Dermatol. 2004;13:721–730. doi: 10.1111/j.0906-6705.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 105.Billich A, Aschauer H, Aszódi A, Stuetz A. Percutaneous absorption of drugs used in atopic eczema: pimecrolimus permeates less through skin than corticosteroids and tacrolimus. Int J Pharm. 2004;269:29–35. doi: 10.1016/j.ijpharm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 106.Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163–178. doi: 10.1007/s40257-013-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luger TA, Lahfa M, Folster-Holst R, et al. Long-term safety and tolerability of pimecrolimus cream 1% and topical corticosteroids in adults with moderate to severe atopic dermatitis. J Dermatol Treat. 2004;15:169–178. doi: 10.1080/09546630410033781. [DOI] [PubMed] [Google Scholar]
- 108.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 109.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 110.Sanofi Genzyme. Dupixent Summary of Product Characteristics. Sanofi Genzyme revised: 25/06/20. https://www.medicines.org.uk/emc/product/8553/smpc. Accessed 13 Aug 2020.
- 111.Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema—a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2007;21:606–619. doi: 10.1111/j.1468-3083.2006.02023.x. [DOI] [PubMed] [Google Scholar]
- 112.Lee SS, Tan AW, Giam YC. Cyclosporin in the treatment of severe atopic dermatitis: a retrospective study. Ann Acad Med Singap. 2004;33:311–313. [PubMed] [Google Scholar]
- 113.Novartis Pharmaceuticals UK Ltd. Neoral soft gelatin capsules Summary of Product Characteristics. Novartis revised: 27/02/20. https://www.medicines.org.uk/emc/medicine/1307. Accessed 13 Aug 2020.
- 114.El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr. 2013;172:351–356. doi: 10.1007/s00431-012-1893-3. [DOI] [PubMed] [Google Scholar]
- 115.Lyakhovitsky A, Barzilai A, Heyman R, et al. Low-dose methotrexate treatment for moderate-to-severe atopic dermatitis in adults. J Eur Acad Dermatol Venereol. 2010;24:43–49. doi: 10.1111/j.1468-3083.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- 116.Berth-Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147:324–330. doi: 10.1046/j.1365-2133.2002.04989.x. [DOI] [PubMed] [Google Scholar]
- 117.Murray ML, Cohen JB. Mycophenolate mofetil therapy for moderate to severe atopic dermatitis. Clin Exp Dermatol. 2007;32:23–27. doi: 10.1111/j.1365-2230.2006.02290.x. [DOI] [PubMed] [Google Scholar]
- 118.Ballester I, Silvestre JF, Pérez-Crespo M, Lucas A. Severe adult atopic dermatitis: treatment with mycophenolate mofetil in 8 patients. Actas Dermosifiliogr. 2009;100:883–887. doi: 10.1016/S0001-7310(09)72917-5. [DOI] [PubMed] [Google Scholar]
- 119.Silverberg JI, Pinter A, Pulka G, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145:173–182. doi: 10.1016/j.jaci.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 120.Simpson E. Efficacy and safety of tralokinumab monotherapy in adult patients with moderate-to-severe atopic dermatitis: results from two 52-week, phase 3 trials (ECZTRA 1 and ECZTRA 2) SKIN J Cutan Med. 2020;4:s96. doi: 10.25251/skin.4.supp.96. [DOI] [Google Scholar]
- 121.Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80:913 e9–921 e9. doi: 10.1016/j.jaad.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 122.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 123.Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol. 2018;178:424–432. doi: 10.1111/bjd.16014. [DOI] [PubMed] [Google Scholar]
- 124.Puar N, Chovatiya R, Paller AS. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126:21–31. doi: 10.1016/j.anai.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 125.Rubel D, Thirumoorthy T, Soebaryo RW, et al. Consensus guidelines for the management of atopic dermatitis: an Asia-Pacific perspective. J Dermatol. 2013;40:160–171. doi: 10.1111/1346-8138.12065. [DOI] [PubMed] [Google Scholar]
- 126.Tintle S, Shemer A, Suarez-Farinas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128(583–93):e1–4. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623–633. doi: 10.1016/j.jaad.2017.06.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.