Abstract
Introduction
The prevalence of obesity is increasing globally. The principal aim was to evaluate whether gastric bypass surgery modifies the bioavailability and pharmacokinetic (PK) parameters of omeprazole.
Methods
Controlled, open-label, bioavailability clinical trial in patients undergoing Roux-en-Y gastric bypass (RYGB). Healthy patients with obesity (body mass index >35) were included and assessed for omeprazole PKs before and after RYGB (1 and 6 months). PK sampling was done at baseline and several times up to 12 h after drug dosing. Pre- and post-surgery parameters were compared using paired ANOVA or Wilcoxon tests, and control versus cases using ANOVA or Mann-Whitney tests. Given the post-surgery change in body weight, parameters were corrected by dose/body weight.
Results
Fourteen case and 24 control subjects were recruited; 92% were women (N = 35/38). In patients who underwent RYGB, maximum plasma concentration (C<sub>max</sub>) was significantly reduced at 1 and 6 months after surgery compared with presurgery values (p = 0.001). Regarding the AUC, the values are lower at 1 and 6 months after surgery than at baseline (p < 0.001). The drug clearance was also increased in the first month after surgery. No differences were found between patients 6 months after surgery and controls. C<sub>max</sub> and AUC corrected by dose/body weight were significantly different between the baseline surgery subjects and controls.
Discusion/Conclusions
Omeprazole bioavailability is reduced in patients with obesity at 1 and 6 months after RYGB. However, omeprazole PK parameters 6 months after RYGB are similar to control subjects, and thus no dose correction is required after RYGB for a given indication.
Keywords: Bioavailability, Pharmacokinetics-pharmacodynamics, Bariatric surgery, Omeprazole, Obesity
Introduction
The prevalence of individuals being overweight and obese has increased over 3 decades, reaching over 2 billion individuals worldwide, with a higher percentage of women [1]. This increase and the related comorbidities [2, 3] have become a considerable challenge and burden for the health care systems [4]. Although dietary restriction and physical exercise are the main recommendations to maintain a healthy weight, many patients require bariatric surgery [5]. According to several head-to-head trials [6, 7, 8, 9, 10, 11, 12, 13, 14] and meta-analyses [15, 16], bariatric surgery results in greater weight loss than conventional treatment in the short term (up to 2 years). Regarding efficacy of different bariatric procedures, Roux-en-Y gastric bypass (RYGB) had excellent long-term outcomes for both weight loss and type 2 diabetes remission rates [17, 18, 19].
The digestive consequences of obesity include alterations in the esophagus, small and large bowel, stomach, pancreas, bile ducts, and liver; patients undergoing bariatric surgery, in addition, can be affected by structural alteration after surgery [20]. All of these changes are modifying factors for the oral bioavailability of drugs, with the volume of the stomach and the gastric emptying speed being the most modified parameters.
While changes to the oral bioavailability following bariatric surgery are expected, there is not enough information about the pharmacological implications for patients [21, 22]. This information is important for drugs that are frequently used, such as omeprazole. After RYGB, there is a risk of occurrence of peptic ulcer of the gastrojejunal anastomosis because the acid secretion of the gastric pouch enters into direct contact with the jejunal loop, which has no biliary or pancreatic secretion capable of neutralizing such acidity [21]. Proton pump inhibitors as omeprazole reduce the occurrence of ulceration [23, 24] and can help eradicating Helicobacter pylori [25]; thus, treatment with omeprazole is recommended during the first postoperative year [21].
The omeprazole effective dose for patients submitted to RYGB has not been, yet, established. Omeprazole is formulated as an enteric-coated tablet and, in normal physiology, after passing through the acidic stomach, the coating dissolves and omeprazole is absorbed. RYGB can modify gastric pouch pH as well as gastric transit time, affecting omeprazole bioavailability and effectiveness in patients where the presence of gastric acid injuries is common [21]. The aim of the present study was to evaluate whether RYGB modifies the bioavailability and pharmacokinetic (PK) parameters of omeprazole at 1 and 6 months post-surgery.
Materials and Methods
Design
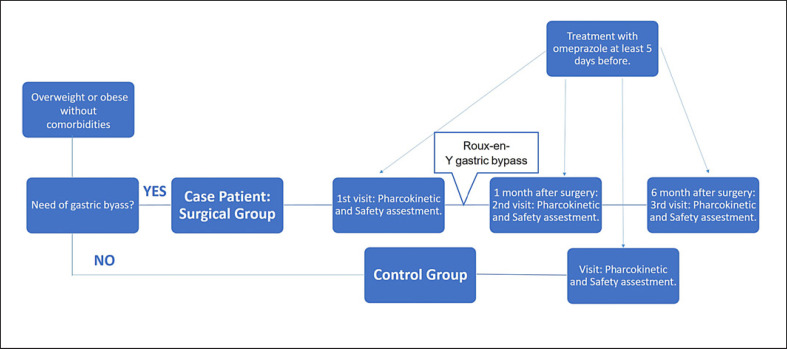
This study was a prospective, open-label PK trial to compare the omeprazole bioavailability parameters in patients who undergo RYGB at three specific stages: prior to RYGB, 1 month post-surgery, and 6 months post-surgery; as well as to compare parameters between those patients with control subjects who had a similar body mass index (BMI) at 6 months post-surgery. The study design is summarized in Figure 1. The patients were recruited in the Department of Endocrinology and all the procedures were performed in the phase I unit (Department of Clinical Pharmacology) in the same hospital.
Fig. 1.
Flow chart of subject assignment and treatment throughout the study.
Subjects
Case patients were obese class II (BMI >35.0 kg/m2) [26] with indication of bariatric surgery, and control patients were subjects who had similar characteristics to case patients at 6 months post-surgery. Both groups comprised patients between 18 and 60 years without comorbidities (except H. pylori) and in good status as determined by medical history, physical examination, and standard biology (they had no clinically significant abnormality based on clinical examination, medical history, clinical chemistry, and hematology or urinalysis results). A negative screening for pregnancy was also required for women of childbearing age. Pregnant or breastfeeding women were excluded from the study, as well as consumers of stimulant drinks (more than 400 mg of caffeine per day), those having contraindications for omeprazole oral intake, and those who had consumed medications 2 weeks prior to the study that could interfere with the study objectives. In all patients who underwent gastric bypass, H. pylori detection (endoscopy and anti-H. pylori antibodies) was performed; it was only considered necessary in control patients if they were symptomatic. Patients were withdrawn if they developed any serious adverse event during the study.
The study was conducted according to the ethical principles set forth in the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice [27]. The study protocol and informed consent documents were approved by the Hospital Clinico San Carlos Ethics Committee (11/094-e) prior to subject enrolment (EudraCT code: 2011-005589-39 and NCT number: 03378960). This study was monitored by UICEC-HCSC. After receiving the information about the study risks and benefits, all the subjects signed the informed consent document.
Sample Size
Considering data in scientific literature regarding the omeprazole intrasubject variance (25%) as a reference, we estimated a sample size of 16 cases and 25 controls to detect significant differences (≥25%) between groups, with a statistical power of 80% and a confidence level of 95%. We estimated about 20% of withdrawal rate in the follow-up.
Study Procedures
Surgical Technique
For laparoscopic RYGB, a 25–35-mL gastric pouch was created along with a 75–100-cm biliopancreatic limb and an alimentary limb of 150 cm. All the surgical procedures were conducted by the same team of surgeons.
Pharmacokinetic Study
Following the confirmation of eligibility and signed informed consent, the subjects were admitted to the phase I unit for the PK assessment. This procedure was performed three times (visits) in case patients (baseline, +1 month post-surgery, and +6 months post-surgery) and only once in control subjects. The same PK evaluation was performed in both case and control patients.
To ensure steady conditions for omeprazole PKs, the subjects were instructed to take at least an oral dose of omeprazole 20 mg per day for the previous 5 days. After overnight fasting, they were admitted to the phase I unit, where they received an oral dose of 20 mg of omeprazole with 200 mL of water and stayed in the unit for 12 h; food intake was not allowed up to 2 h after drug intake. During this time, safety assessment and PK sampling were performed. Venous blood samples for quantitation of omeprazole were obtained before drug dosing and at 1, 2, 2.5, 3, 3.5, 4, 5, 7, 9, and 12 h after omeprazole intake. Each plasma sample was divided into three aliquots and stored at −80°C, before being delivered to the Department of Toxicology and Health Legislation (UCM, Madrid, Spain) for analysis. Study drugs were labeled and managed in accordance with good clinical practice and local regulatory requirements.
Samples Analysis
The blood samples were analyzed in the Department of Toxicology and Health Legislation (UCM, Madrid, Spain) using the method described by Macek et al. [28] as a reference. Plasma concentrations of omeprazole were estimated using Mass Hunter® (B.04.01 version) software.
Safety Assessments
Physical evaluation, hematology, biochemistry, and vital signs were assessed at the beginning and end of each visit to address safety concerns. These assessments were recorded throughout the study, from the inclusion time to 1 day after the last visit in the phase I unit. All adverse events detected were described and graded by severity (mild, moderate, and severe) and potential attributional relationship to omeprazole (Karch and Lasagna algorithm [29]).
Study Variables
The principal variables were the bioavailability parameters of omeprazole as area under the concentration-time curve (AUClast, AUCinf), maximum plasma concentration (Cmax), and time until Cmax is reached (Tmax). For a clearer assessment of the effects of surgery on PK processes, Cmax and AUC were corrected by dose/body weight, given that weight was significantly modified throughout the study in the surgery group, affecting the volume of drug distribution.
Definition of study variables:
AUClast or AUC (0-t): area under the plasma concentration curve, calculated using the linear trapezoidal method until the last measurable concentration.
AUCinf: AUClast + extrapolation to infinity.
Cmax/(dose/body weight): maximum plasma concentration adjusted by dose of omeprazole per kilogram of patient's body weight.
AUClast/(dose/body weight): area under the plasma concentration curve adjusted by dose of omeprazole administered per kilogram of patient's weight.
Clearance (Cl): expressed as the plasma volume totally free of the drug per unit of time and measured in units of volume per units of time.
Omeprazole PK parameters were estimated upon plasma concentration versus time data, by standard noncompartmental methods using Phoenix WinNonlin v.6.1 (Pharsight Corp., Mountain View, CA, USA). Safety was monitored through tolerability, vital signs, clinical laboratory tests, physical examinations, and adverse event assessments.
Statistical Analysis
Continuous variables were described using means and standard deviation, or median and interquartile range when the data were not normally distributed. Qualitative variables were described using frequencies and percentages. Descriptive analyses of PK parameters, including the mean, median, geometric mean, standard deviation, maximum, and minimum were also computed for both groups. Pre- and post-surgery omeprazole PK parameters were compared using paired T-test or Wilcoxon signed-ranks test when normality assumption was violated. To compare the values of both control and case groups after 6 months, the independent samples T-test was used, or the Mann-Whitney U test when the assumptions of the t-test were not met.
Statistical significance was set at <0.05. Statistical analysis was performed using IBM SPPS Statistics version 22 (International Business Machines Corp., Armonk, NY, USA).
Results
Forty-two patients were identified and four refused to participate, resulting in aaaathirty-eight subjects who were enrolled in the study (14 patients with obesity and planned bariatric surgery and 24 control subjects). They were enrolled in the study between April 2012 and June 2014. All subjects received their planned doses of study medication; one of the surgical patients was lost to follow-up at 6 months post-surgery. All subjects were Caucasian (Table 1).
Table 1.
Demographic characteristics of case and control groups
| Control group (n = 24) | Surgical group (n = 14) |
Comparison 1st month after surgery versus baseline p (95% CI) | Comparison 6th month after surgery versus baseline p (95% CI) | Comparison 6th month after surgery versus control p (95% CI) | |||
|---|---|---|---|---|---|---|---|
| baseline (n = 14) | 1st month after surgery (n = 14) | 6th month after surgery (n = 13) | |||||
| Sex, n (%) | |||||||
| Male | 2 (8.3) | 1 (7.1) | 0.896** | ||||
| Female | 22 (91.7) | 13 (92.9) | 0.455** | ||||
| Age, years | |||||||
| Mean ± SD* | 46.4±11.4 | 49.0±8.3 | |||||
| Range | 41 | 29 | |||||
| Weight, kg | |||||||
| Mean ± SD* | 81.8±16.3 | 112.5±13.9 | 96.6±14.7 | 82.1 ±13.1 | <0.001 | <0.001 | 0.952*** |
| Range | 58.4 | 49.6 | 53 | 50 | |||
| Height, cm | |||||||
| Mean ± SD* | 159±7 | 157±4 | |||||
| Range | 31 | 16 | |||||
| BMI, kg m−2 | |||||||
| Mean ± SD* | 32.5±6.7 | 45.6±5.3 | 39.2±5.8 | 33.3±5.2 | <0.001 | <0.001 | 0.718*** |
| Range | 25.3 | 16.5 | 18.10 | 17.9 | |||
SD, standard deviation.
Averages are presented as mean ± SD.
χ2 test.
Student t test.
In all patients who underwent RYGB, H. pylori detection was performed, and 54.1% of patients required H. pylori eradication, as they were positive before surgery. It was not considered necessary to detect or treat H. pylori in the control subjects as they were asymptomatic.
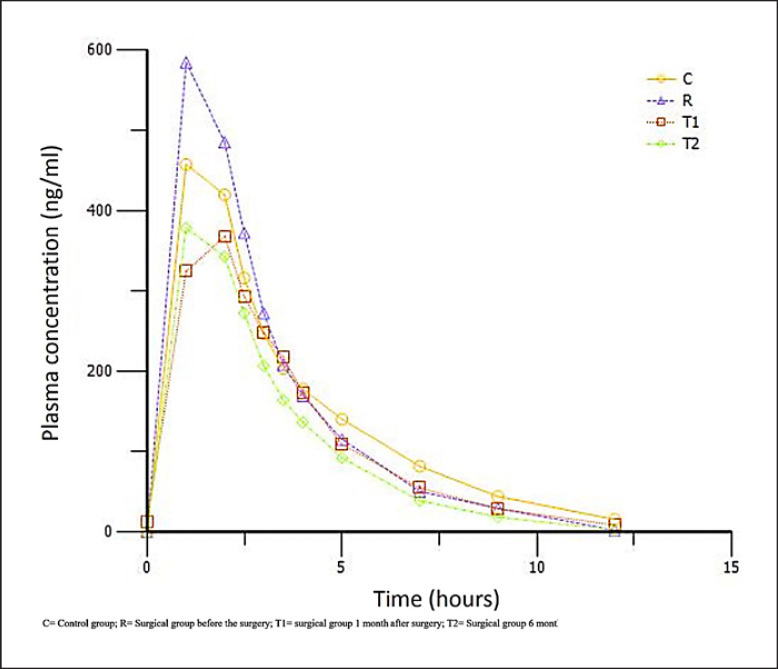
Observation of plasma concentration versus time profiles (Fig. 2) showed obvious changes during the absorption process (Tables 2, 3, and Fig. 3). Case patients presurgery (baseline) had the highest Cmax (749.3 ± 377 ng/mL) and AUC (2,053.8 ± 1,892.7 h ng/mL) values, and the difference was even greater when these parameters are adjusted by omeprazole dose per patient weight. Case patients 1 and 6 months post-surgery showed higher clearance figures (0.03 ± 0.03 mL/h both times). The omeprazole Cmax was reached sooner in case patients at baseline and 6 months post-surgery (median Tmax 1.1 [1.0–2.0] and 1.0 [1–2.5] h) versus in control patients (median Tmax 2.0 [1.0–4.0] h).
Fig. 2.
Observed mean plasma concentration-time profiles of omeprazole over 12 h after oral administration.
Table 2.
Omeprazole PK parameters
| Control group (n = 24) |
Surgical group |
Comparison 1st month after surgery vs. baseline p (95% CI) | Comparison 6th month after surgery vs. baseline p (95% CI) | Comparison 6th month after surgery vs. control p (95% CI) | Comparison surgical group baseline vs. control group p (95% CI) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline (n = 14) |
1 month (n = 14 |
6 months (n = 13) |
||||||||||||||||||||||
| mean ± SD | median | min | max | geometric mean | mean ± SD | median | min | max | geometric mean | mean ± SD | median | min | max | geometric mean | mean ± SD | median | min | max | geometric mean | |||||
| 1.8± 0.7 | 2.0 | 1.0 | 4.0 | 1.4± 0.5 | 1.1 | 1.0 | 2.0 | 1.8± 0.7 | 1.9 | 1.0 | 3.0 | 1.4±0.6 | 1.0 | 1 | 2.5 | 0.103 (−0.05 to −0.01) | 0.593 (−0.03 to 0.01) | 0.159 (−0.03 to −0.002) | 0.125 (−0.10 to 0.76) | |||||
|
| ||||||||||||||||||||||||
| Cmax, ng/mL | 515.9±386.2 | 443.5 | 118.1 | 1,892.6 | 411.6 | 749.3± 377.0 | 618.9 | 342.0 | 1,643.1 | 674.5 | 461.9± 365.7 | 309.1 | 26.2 | 1,229.0 | 318.3 | 486.1±348.5 | 359.2 | 20.4 | 1,154.3 | 343.5 | 0.001 (−429.6 to −145.2) | 0.003 (−466.0 to −123.0) | 0.819 (−231.6 to 291.0) | 0.078 (−4944 to 27.7) |
|
| ||||||||||||||||||||||||
| AUClast, h ng/mL | 1,458.9±1,518.7 | 876.6 | 228.0 | 7,060.8 | 977.6 | 2,053.8± 1,892.7 | 1,214.7 | 502.4 | 7,157.6 | 1,511.2 | 1,408.2± 1,820.1 | 636.3 | 50.3 | 6,730.9 | 688.3 | 1,327.0± 1,373.2 | 888.9 | 16.1 | 4,537.8 | 667.0 | <0.001 (−880.0 to −411.5) | 0.002 (−1,330.2 to −362.1) | 0.796 (−896.1 to 1,159.9) | 0.295 (−1,729.5 to 539.7) |
|
| ||||||||||||||||||||||||
| AUCinf, h ng/mL | 1,659.1±1,642.1 | 954.9 | 246.0 | 7,564.4 | 1,167.0 | 2,071.2± 1,902.7 | 1,226.3 | 503.5 | 7,181.5 | 1,523.7 | 1,680.4± 2,004.8 | 894.8 | 165.1 | 7,194.7 | 973.4 | 1,569.7± 1,393.7 | 1,072.9 | 268.1 | 4,599.2 | 1,073.3 | 0.001 (−900.0 to −300.0) | 0.008 (−1,248.9 to −236.2) | 0.878 (−1,090.3 to 1,269.0) | 0.495 (−1,625.4 to 801.2) |
|
| ||||||||||||||||||||||||
| t1/2, h | 1.3± 1.0 | 1.0 | 0.3 | 4.3 | 0.9± 0.6 | 0.8 | 0.3 | 2.3 | 0.9± 0.8 | 0.6 | 0.3 | 2.8 | 1.4± 1.2 | 1.0 | 0.3 | 4.7 | 0.495 (−0.4 to 0.2) | 0.384 [–0.5 to 1.3) | 0.871 (−0.9 to 0.8) | 0.178 [–0.2 to 1.0) | ||||
|
| ||||||||||||||||||||||||
| CI, mL/h | 0.0234± 0.0192 | 0.0209 | 0.00 | 0.08 | 0.02± 0.01 | 0.01 | 0.0 | 0.04 | 0.03± 0.03 | 0.03 | 0.0 | 0.1 | 0.03± 0.03 | 0.02 | 0 | 0.07 | 0.023 (0.0030-0.0033) | 0.056 (−0.0004 to 0.0264) | 0.616 (−0.198 to 0.0119) | 0.237 (−0.0046 to 0.0182) | ||||
Averages are presented as mean ± SD. SD, standard deviation.
Table 3.
Dose/weight-adjusted omeprazole pharmacokinetic parameters
| Control group (n = 24) | Surgical group (n = 14) |
Comparison 1st month after surgery vs. baseline p (95% CI) | Comparison 6th month after surgery vs. baseline p (95% CI) | Comparison 6th month after surgery vs. control p (95% CI) | Comparison surgical group baseline vs. control group p (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| mean ±SD | baseline mean ± SD | 1 month mean ± SD | 6 months mean ± SD | |||||
| Cmax, ng/mL | 515.9±386.2 | 749.3±377.0 | 461.9±365.7 | 486.1 ±348.5 | 0.001 (−429.6 to −145.2) |
0.003 (−466.0 to −123.0) |
0.819 (−231.6 to 291.0) |
0.078 (−494.4 to 27.7) |
|
| ||||||||
| AUClast, h ng/mL | 1,458.9±1,518.7 | 2,053.8±1,892.7 | 1,408.2±1,820.1 | 1,327.0±1,373.2 | <0.001 (−880.0 to −411.5) |
0.002 (−1,330.2 to −362.1) |
0.796 (−896.1 to 1,159.9) |
0.295 (−1,729.5 to 539.7) |
|
| ||||||||
| Cmax/(dose/weight) | 2,059.34±1,479.78 | 4,340.81±2,554.31 | 2,279.60±1,852.20 | 2,042.34±1,514.49 | <0.001 (−2,894.87 to −1,227.53) |
<0.001 (−3,583.98 to −1,404.53) |
0.974 (−1,025.91 to 1,059.9) |
0.001 (−3,603.13 to −959.80) |
|
| ||||||||
| AUClast/(dose/weight) | 5,844.76±5,684.29 | 12,159.30±12,118.95 | 7,184.81 ±9,704.37 | 5,666.94±6,040.13 | <0.001 (−6,819.17 to −3,129.8) |
0.003 (−11,464.84 to −2,984.72) |
0.930 (−3,883.11 to 4,238.74) |
0.035 (−12168.95 to −460.14) |
|
| ||||||||
| Dose/weight, mg/kg | 0.18±0.05 | 0.15±0.02 | 0.21±0.03 | 0.25±0.04 | <0.001 (0.02–0.04) | <0.001 (0.06–0.08) | 0.788 (−0.03 to 0.04) |
<0.001 (0.004–0.01) |
Averages are presented as mean ± SD. SD, standard deviation.
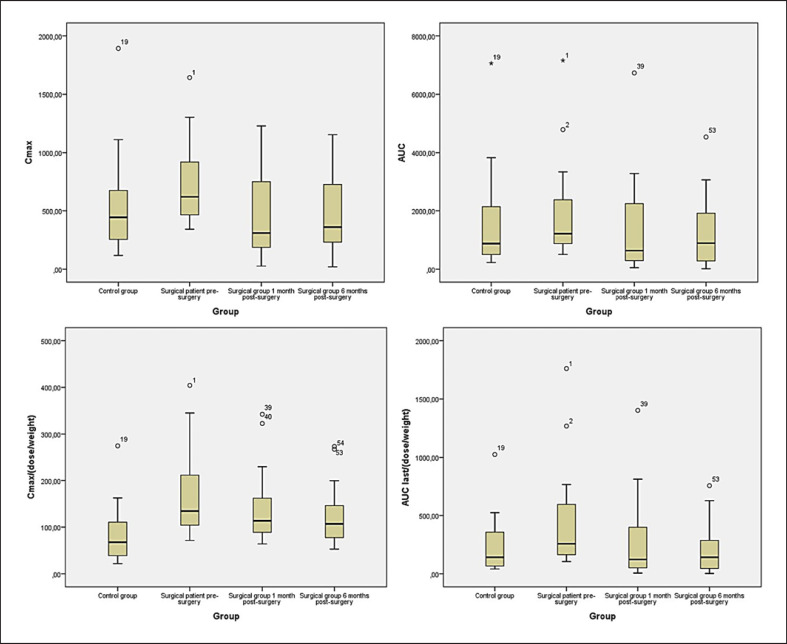
Fig. 3.
Boxplot of PK parameters and dose per weight correction.
In patients with obesity, Cmax decreased 1 month (from 749.3 ± 377 ng/mL to 461.9 ± 365.7 ng/mL; p = 0.001) and 6 months (749.3 ± 377 ng/mL to 486.1 ± 348.5 ng/mL; p = 0.003) post-surgery. Regarding AUC, the values were lower at 1 and 6 months post-surgery (1,408.2 ± 1,820.1 and 1,327.0 ± 1,373.2 h*ng/mL, respectively) than the values for the same patients at baseline (2,053.8 ± 1,892.7 h*ng/mL; p < 0.001 and p = 0.002, respectively). Clearance increased in the first month post-surgery (0.03 ± 0.03 mL/h, p = 0.023).
No differences were found between patients 6 months post-surgery and control subjects. Cmax and AUC showed no significant differences between baseline case subjects and controls (p = 0.078 and p = 0.295), but significant differences were found for both Cmax and AUC when corrected by dose/body weight (Cmaxp = 0.001, IC95–3,603.13 to −959.80; and AUC p = 0.035, IC95–12168.95 to −460.14). Safety was monitored, and no concerns were observed throughout the study period.
Discussion
The PKs of oral drugs are modified by the digestive consequences of obesity and bariatric surgery. This study evaluated whether the digestive changes entailed by RYGB could modify the PK parameters of omeprazole. It was anticipated that patients who undergo bariatric surgery will have an impairment of omeprazole absorption, depending on the anatomical and physiological changes that the surgery produces. Among the changes introduced by RYGB, those that most affect the oral absorption of omeprazole are gastric emptying, isolation of the duodenum, and gastric pH. The data obtained in the present study cannot be extrapolated to patients who have undergone other bariatric surgery techniques.
Omeprazole has some clinical and pharmacodynamic properties that could be affected by anatomical changes to the digestive system and intestinal transit speed [30]. The digestive changes are different throughout the process, from presurgery to stabilization and, ultimately, attaining normal weight; it is important to analyze these stage-specific modifications. When gastric emptying is increased, as in people with obesity, omeprazole is less degraded in the stomach and a greater quantity reaches the duodenum to be absorbed. This could explain why the omeprazole Cmax and AUC in patients with obesity before RYGB are higher than in control patients, despite the larger volume of distribution of the former. The reduction shown in omeprazole Cmax and AUC after RYGB compared to baseline could be explained by an impairment of drug absorption. Omeprazole's absorption surface is reduced after RYGB because the duodenum is isolated, and nothing ingested by these patients goes through it.
RYGB induced a sustained (1 and 6 month) decrease in omeprazole Cmax and AUC versus baseline conditions and a significant increase in clearance values. These results suggest a reduction in drug absorption, due to the changes in the absorption surface, and an increase in drug clearance, due to an effect on enterohepatic circulation, if the bowel transit speed has been increased. It is important to mention that about 20% of omeprazole metabolites are excreted in the bile [30], and thus, they could be available to be absorbed again. Our results are compatible with those (graphical tendency to increase in clearance after surgery, and higher bioavailability vs. healthy subjects) found by Puri et al. [22], in spite of the differences in methods (association of drugs, drug administration technique, and sampling times). Although some PK studies genotype subjects trying to explain possible differences or variability (i.e., CYP2C19/3A4 in case of omeprazole), our main objective regards to comparison of omeprazole bioavailability parameters after surgery versus baseline values.
Omeprazole Cmax and AUC 6 months post-RYGB are similar to those in controls with a similar weight and BMI, suggesting that the case subjects exhibit a higher omeprazole bioavailability, which is apparent when the PK parameters are corrected by dose/body weight.
Our results differ from other studies in the field [21, 31] that detected different values for Tmax, Cmax, and AUC; however, although both are bioavailability clinical trials, there are significant differences in the methods, including the doses of omeprazole administered, the times used for blood sampling, and the weight of the included patients. In addition, Mitrov-Winkelmolen et al. [31] administered omeprazole together with acetyl salicylate acid, which changes the pH of the stomach and therefore the dissolution and absorption conditions of omeprazole. Cmax and Tmax are easily influenced by differences in patient conditions and study procedures; therefore, the most comparable parameter between both studies is the AUC. In both studies, the AUC before bariatric surgery is higher than that obtained afterward. Concerning the differences in the graphic representations of the presurgical AUC in both studies, Mitrov-Winkelmolen et al. [31] performed the last blood extraction only 4 h after dosing (Tlast = 4 h); therefore, the curve represented from there until 12 h is an estimation, which in this case represents a significant percentage of the total. In our patients, the last extraction was 12 h after drug intake; the graph represents the data obtained directly from the patients. Similarly, the study from Collares-Pelizaro et al. [21] showed a reduction in omeprazole absorption during the 90 min after omeprazole intake; this time is not enough to assess the whole process of absorption and therefore noncomparable with our results.
Despite the exploratory character of our study, the results are quite clear, although additional information on the mechanism and bowel dynamics would be beneficial, given that not only the structure but also the function of the bowel is affected by RYGB. Although a decrease in omeprazole bioavailability is clearly shown after RYGB, the similarity of the resulting parameters to those in control subjects allows us to conclude that no dose correction in omeprazole posology is required after RYGB for a given clinical situation. This does not modify the fact that if the needs of the patient increase (e.g., ulcer treatment), dosing should be temporarily increased.
As a study limitation, the originally estimated sample size was difficult to reach due to complexity of patient recruitment, yet the statistically significant results were not affected by the sample reduction. Moreover, as a bioavailability study, the possibility of drawing mechanisms of action and clinical conclusions is limited, and additional studies would add more insight. Among the strengths, compared with previous articles describing drug absorption before and after RYGB, the subjects act as their own controls to minimize variability between the pre- and post-surgery situation. Additionally, the presence of a nonsurgery control group provides an external reference, and due to the applied exclusion criteria, the PKs were not influenced by interacting medication.
Conclusion
Omeprazole absorption is reduced in patients with obesity at 1 and 6 months post-RYGB compared with baseline status. As omeprazole PK parameters 6 months after RYGB are similar to those in the controls, no dose correction in omeprazole posology is required after RYGB for a given indication, but individual needs must be evaluated upon the patient situation.
Considering that omeprazole is a part of the usual drug treatment in these patients, it is important for physicians to know that there is no need to modify the dose post-RYGB. The higher bioavailability of omeprazole observed in patients who were morbidly obese before RYGB versus control subjects indicates that there is no need to increase doses of omeprazole in relation to their higher weight and BMI.
Statement of Ethics
All procedures performed in studies were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. After receiving the information about the study risks and benefits, all the subjects signed the informed consent document. The study protocol and informed consent documents were approved by the Hospital Clinico San Carlos Ethics Committee (11/094-e) prior to subject enrolment (EudraCT code: 2011-005589-39 and NCT number: 03378960). This study was monitored by UICEC-HCSC.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Funding Sources
This study has been funded by the Fundación Mutua Madrileña, VIII call for Health Research, 2011.
Author Contributions
E.V.C. was the clinical trial principal investigator and responsible for the design and development of the study. A.P.-P. implemented the pharmacokinetic modeling. M.A.R. identified and recruited the patients. A.B.R.P., A.S.P., A.J.T.G., and C.M.L. participated in the collection and the data analysis. L.M.C. and F.B. analyzed the blood samples. A.P.P., A.L.-P, and A.B.R.P. participated in the data analysis. The manuscript was written and edited by A.R.P. and A.P.P. All authors read and approved the final version of the manuscript.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.
Acknowledgments
We thank Dr. Ruiz de Leon (Digestive pathology Department, HCSC) for facilitating personal research results for the discussion.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384((9945)):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373((9669)):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376((3)):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 5.Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol. 2019;15:339–355. doi: 10.1038/s41574-019-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sovik TT, Taha O, Aasheim ET, Engström M, Kristinsson J, Björkman S, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. Br J Surg. 2010;97((2)):160–6. doi: 10.1002/bjs.6802. [DOI] [PubMed] [Google Scholar]
- 7.Risstad H, Kristinsson JA, Fagerland MW, le Roux CW, Birkeland KI, Gulseth HL, et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis. 2017;13((9)):1544–1553. doi: 10.1016/j.soard.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017;376((7)):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI. Obes Surg. 2011;21((11)):1650–1656. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 10.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366((17)):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biter LU, van Buuren MMA, Mannaerts GHH, Apers JA, Dunkelgrün M, Vijgen GHEJ. Quality of life 1 year after laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass: a randomized controlled trial focusing on gastroesophageal reflux disease. Obes Surg. 2017;27((10)):2557–2565. doi: 10.1007/s11695-017-2688-4. [DOI] [PubMed] [Google Scholar]
- 12.Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319((3)):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149((7)):707–715. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15((7)):1024–1029. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 15.Park CH, Nam SJ, Choi HS, Kim KO, Kim DH, Kim JW, et al. Comparative efficacy of bariatric surgery in the treatment of morbid obesity and diabetes mellitus: a systematic review and network meta-analysis. Obes Surg. 2019;29:2180–2190. doi: 10.1007/s11695-019-03831-6. [DOI] [PubMed] [Google Scholar]
- 16.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312((9)):934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakobsen GS, Småstuen MC, Sandbu R, Nordstrand N, Hofs⊘ D, Lindberg M, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319((3)):291–301. doi: 10.1001/jama.2017.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24((8)):1126–1135. doi: 10.1007/s11695-014-1354-3. [DOI] [PubMed] [Google Scholar]
- 20.Encinas Sotillos A, Fernández Azuela M, Ortega Anta RM, Cano López JM. Obesidad y aparato digestivo. Med Integral. 2002;36((5)):157–190. [Google Scholar]
- 21.Collares-Pelizaro RVA, Santos JS, Nonino CB, dos Reis Dias LA, Gaitani CM, Salgado W., Jr omeprazole absorption and fasting gastrinemia after Roux-en-Y gastric bypass. Obes Surg. 2017;27((9)):2303–2307. doi: 10.1007/s11695-017-2672-z. [DOI] [PubMed] [Google Scholar]
- 22.Puris E, Pasanen M, Ranta VP, Gynther M, Petsalo A, Käkelä P, et al. Laparoscopic Roux-en-Y gastric bypass surgery influenced pharmacokinetics of several drugs given as a cocktail with the highest impact observed for CYP1A2, CYP2C8 and CYP2E1 substrates. Basic Clin Pharmacol Toxicol. 2019 Aug;125((2)):123–132. doi: 10.1111/bcpt.13234. [DOI] [PubMed] [Google Scholar]
- 23.Coblijn UK, Lagarde SM, de Castro SM, Kuiken SD, van Tets WF, van Wagensveld BA. The influence of prophylactic proton pump inhibitor treatment on the development of symptomatic marginal ulceration in Roux-en-Y gastric bypass patients: a historic cohort study. Surg Obes Relat Dis. 2016 Feb;12((2)):246–252. doi: 10.1016/j.soard.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Wu CYV, Kim SH, Kam KJ, et al. Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: a systematic review and meta-analysis of cohort studies. Surg Endosc. 2014;29((5)):1018–1023. doi: 10.1007/s00464-014-3794-1. [DOI] [PubMed] [Google Scholar]
- 25.Cuesta Hernández M, Pérez Peña C, Matía Martín P, Cabrerizo García L, Pérez-Ferre N, Sánchez-Pernaute A, et al. Helicobacter pylori (HP) infection in obese patients undergoing Roux-en-Y gastric bypass: efficacy of two different treatment regimens in HP eradication. Nutr Hosp. 2015;32((2)):600–5. doi: 10.3305/nh.2015.32.2.9177. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization Body mass index − BMI [cited 2021 Mar 30] Available from: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 27.The World Medical Association Declaration of Helsinki 2008 [cited 2021 Jan 15] Available from: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/doh-oct2008/.
- 28.Macek J, Klíma J, Ptácek P. Rapid determination of omeprazole in human plasma by protein precipitation and liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:282–7. doi: 10.1016/j.jchromb.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977;21((3)):247–254. doi: 10.1002/cpt1977213247. [DOI] [PubMed] [Google Scholar]
- 30.Losec® Summary product characteristics [cited 2020 Jul 15] Available from: https://cima.aemps.es/cima/dochtml/ft/58377/FT_58377.html.
- 31.Mitrov-Winkelmolen L, van Buul-Gast MW, Swank DJ, Overdiek HWPM, van Schaik RHN, Touw DJ. The effect of Roux-en-Y gastric bypass surgery in morbidly obese patients on pharmacokinetics of (acetyl)salicylic acid and omeprazole: the ERY-PAO Study. Obes Surg. 2016 Sep;26((9)):2051–2058. doi: 10.1007/s11695-016-2065-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.