Abstract
Background
Several beta‐lactams are recommended as single agents for the treatment of febrile neutropenia.
Objectives
To compare the effectiveness of different anti‐pseudomonal beta‐lactams as single agents in the treatment of febrile neutropenia. To compare the development of bacterial resistance, bacterial and fungal superinfections during or following treatment with the different beta‐lactams.
Search methods
We searched the Cochane Register of Controlled Trials (CENTRAL), Issue 3, 2010. MEDLINE, EMBASE, LILACS, FDA drug applications, conference proceedings and ongoing clinical trial databases up to August 2010. References of included studies were scanned.
Selection criteria
Randomised controlled trials (RCTs) comparing an antipseudomonal beta‐lactam to another antipseudomonal beta‐lactam antibiotic, both given alone or with the addition of the same glycopeptide to both study arms, for the initial treatment of fever and neutropenia among cancer patients.
Data collection and analysis
Two review authors applied inclusion criteria and extracted the data independently. Missing data were sought. Risk ratios (RR) were calculated with 95% confidence intervals (CI), and pooled using the fixed effect model. The primary outcome was all‐cause mortality. Risk of bias was assessed using a domain‐based evaluation and its effect of results was assessed through sensitivity analyses.
Main results
Forty‐four trials were included. The antibiotics assessed were cefepime, ceftazidime, piperacillin‐tazobactam, imipenem and meropenem. Adequate allocation concealment and generation were reported in about half of the trials and only two trials were double‐blinded. The risk for all‐cause mortality was significantly higher with cefepime compared to other beta‐lactams (RR 1.39, 95% CI 1.04 to 1.86, 21 trials, 3471 participants), without heterogeneity and with higher RRs in trials at low risk for bias. There were no differences in secondary outcomes but for a non‐significantly higher rate of bacterial superinfections with cefepime. Mortality was significantly lower with piperacillin‐tazobactam compared to other antibiotics (RR 0.56, 95% CI 0.34 to 0.92, 8 trials, 1314 participants), without heterogeneity. Carbapenems resulted in similar all‐cause mortality and a lower rate of clinical failure and antibiotic modifications as compared to other antibiotics, but a higher rate of diarrhea caused by Clostridium difficile.
Authors' conclusions
Current evidence supports the use of piperacillin‐tazobactam in locations where antibiotic resistance profiles do not mandate empirical use of carbapenems. Carbapenems result in a higher rate of antibiotic‐associated and Clostridium difficile‐associated diarrhea. There is a high level of evidence that all‐cause mortality is higher with cefepime compared to other beta‐lactams and it should not be used as monotherapy for patients with febrile neutropenia.
Plain language summary
Single‐agent antibiotic treatment for cancer patients with fever and low white blood cell counts
Cancer patients develop neutropenia, a decrease in the neutrophil subset of the white blood cells, as a result of chemotherapy. Neutropenia exposes patients to infections, mainly bacterial. Without antibiotic treatment these infections may be fatal, therefore antibiotic treatment is administered when a patient with neutropenia develops fever. The objective of this review was to compare antibiotic treatments currently recommended in consensus guidelines for the initial treatment of cancer patients with fever and neutropenia.
We identified 44 studies comparing different antibiotics. Cefepime resulted in significantly higher mortality compared to all other antibiotics combined, at the end of patients' hospital stay or 30 days after entry into the study. The risk was 39% higher with cefepime, ranging from 4 to 86% increased risk. We did not find an explanation for this when looking into other outcomes reported in the primary studies. Piperacillin‐tazobactam resulted in lower mortality than other antibiotics. The other antibiotics (ceftazidime, imipenem and meropenem) showed comparable efficacy, with a lower rate of antibiotic changes for imipenem or meropenem and a higher rate of severe diarrhea with these two antibiotics.
We conclude that piperacillin‐tazobactam might be the preferred antibiotic for the treatment of cancer patients with fever and neutropenia and that cefepime should not be used. Antibiotic selection (other than cefepime) depends on the individual patient and the type of bacteria prevalent in the specific hospital.
Background
Description of the condition
Neutropenia is defined as a reduction of the neutrophil count below 500/mm3. Chemotherapy is the major cause among cancer patients. Other causes include radiation therapy and bone marrow involvement by the primary tumour (De Pauw 2000). Susceptibility to infections increases as the neutrophil count decreases below 1000 cells/mm3. Very low counts (below 100 cells/mm3) and longer duration of neutropenia increase the risk (Bodey 1944). Other deficits in the immune response, breaks in skin and mucosa, indwelling catheters, and invasive procedures further increase patients' susceptibility to infection (De Pauw 2000).
Fever develops in 57% to 94% of patients in different studies during an episode of neutropenia (Engels 1998) with a rate of 20 to 40 fever‐days per 100 days with neutrophil count below 500/mm3 (Storring 1977). Infection is documented in 56% (24 to 94%) of patients with fever and neutropenia, while bacteraemia is documented in 24% (4% to 57%, Paul 2004). Short‐term mortality for bacteraemia with neutropenia ranges between 8% in randomised trials to 23% in observational series (Paul 2004; Velasco 2003). Appropriate empirical antibiotic treatment may halve overall mortality (Ibrahim 2000; Leibovici 1998). The ability of clinicians to predict infection in febrile neutropenic patients is low (sensitivity 70%, specificity 62% in one study, Lawson 1984), and thus all febrile neutropenic patients are treated with broad‐spectrum antibiotics.
The microbiological etiology of an infection in the neutropenic patient depends on underlying disease and type of chemotherapy, extent of the patient's exposure to the healthcare environment, use of prophylactic or therapeutic antibiotics, duration of neutropenia, and other factors. In general, the rate of Gram‐positive infections has been increasing throughout the last three decades, probably in relation to the extensive use of long‐term intravascular catheter (De‐bock 2001). Centers in the US, Italy and in the MASCC cohorts (Multinational Association for Supportive Care in Cancer) report a predominance of Gram‐positive bacteremia (Klastersky 2007; Raad 2007;Tumbarello 2009 ). However, epidemiology is highly local with some centers, more in the middle east and developing countries, reporting a predominance of Gram‐negative bacteria (Baskaran 2007; Chen 2009; Kanafani 2007; Paul 2007).
Description of the intervention
Beta‐lactam monotherapy has been shown to be as effective and potentially safer than combination therapy consisting of a beta‐lactam plus an aminoglycoside (Paul 2004). Management guidelines from various societies endorse monotherapy for patients in need for intravenous antibiotic treatment, with or without the addition of a glycopeptide (ECIL 2009; Hughes 2002; Link 2003; Tamura 2005). The guidelines recommend that "a third or fourth‐generation cephalosporin (ceftazidime or cefepime) or a carbapenem (imipenem‐cilastatin or meropenem) may be used successfully as monotherapy" and that "piperacillin‐tazobactam has also been found to be effective as monotherapy, but its use has not been studied as extensively as that of the other agents".
How the intervention might work
Beta‐lactams differ somewhat in their antibacterial spectrum of activity. Cefepime and ceftazidime are advanced oxymino‐cephalosporins. Cefepime offers, in‐vitro, a broader spectrum than ceftazidime, with enhanced activity against Gram‐positive (methicillin‐sensitive Staphylococcus aureus and Streptococcus pneumoniae) and Gram‐negative bacteria (Fritsche 2003; Pfaller 1997; Sanders 1993). The major defence mechanism of Gram‐negative bacteria are beta‐lactamases, enzymes capable of cleaving the beta‐lactam ring thereby inactivating beta‐lactams; cefepime is resistant to some of the extended‐spectrum beta‐lactamases (ESBLs) which inactivate ceftazidime (Jacoby 2005; Sanders 1993). Piperacillin‐tazobactam, a penicillin beta‐lactam combined with a beta‐lactamase inhibitor, shows variable in‐vitro activity against ESBL‐producing bacteria (Burgess 2004; Li 2004; Pfaller 1997). However, the activity of both cefepime and piperacillin‐tazobactam against ESBL‐positive Gram‐negative bacteria is limited by the inoculum effect, with diminished activity as the size of the bacterial inoculum increases (Burgess 2004; Jacoby 2005). Carbapenems offer a broad spectrum of activity against ESBL‐producing Gram‐negative and Gram‐positive bacteria with the exception of methicillin‐resistant Staphylococcus aureus. Clinical outcomes with carbepenems are probably superior to those obtained with other beta‐lactams for ESBL‐producing Gram‐negative bacteria (Paterson 2004).
Beta‐lactams differ also in their propensity to induce resistance. Resistance induction may affect the outcome of the treated infection and/or subsequent infections. The beta‐lactams recommended for use in febrile neutropenia are poor inducers of AmpC chromosomal beta‐lactamases and are thus active against Gram‐negative bacteria with inducible beta‐lactamases (Goldstein 2002). Carbapenems, however, and to a lesser extent cefepime, have a lower propensity for selection of resistant (derepressed) mutants, highly resistant to broad‐spectrum cephalosporins (Goldstein 2002). Clinical studies have shown correlations between the use of ceftazidime or piperacillin‐tazobactam, and subsequent isolation of broad‐spectrum cephalosporin‐resistant Enterobacter spp. (Johnson 1990; Schwaber 2003). Development of resistance during treatment of Enterobacter infections has also been shown with ceftazidime (Chow 1991; Kaye 2001). Cefepime was advantageous in animal models (Pechere 1992) and small non‐comparative series have suggested a clinical advantage (Sanders 1996). However, broad‐spectrum resistance may be selected in‐vitro also with cefepime and involves selection of mutants with altered permeability conferring resistance to other antibiotic classes (Fung‐Tomc 1996) Clinical studies comparing the risk of resistance induction with cefepime versus other broad‐spectrum cephalosporins are lacking.
Why it is important to do this review
Recommended beta‐lactams have been compared in several trials, most commonly comparing a newly introduced antibiotic to an established beta‐lactam. No single beta‐lactam is currently preferred. Previous meta‐analyses targeted specific beta‐lactams: ceftazidime (Sanders 1991), imipenem‐cilastatin (Deaney 1996), and ceftriaxone (Furno 2000), and included studies comparing monotherapy to combination therapy. In a previous systematic review we demonstrated increased mortality with cefepime (Other published versions of this review; Yahav 2007), a finding that was refuted by a subsequent analysis conducted by the FDA (FDA 2009; FDA 2010).
We assembled comparative trials assessing anti‐pseudomonal beta‐lactams administered as single agents (defined as a beta‐lactam without an aminoglycoside) for the treatment of febrile neutropenia in cancer patients to assess whether there is an advantage to one of the recommended beta‐lactams and to clarify the uncertainty regarding cefepime for this indication.
Objectives
To compare the effectiveness of different anti‐pseudomonal beta‐lactams given as single agent, without an aminoglycoside, in the treatment of febrile neutropenia.
To compare the development of bacterial resistance, bacterial and fungal superinfections during or following treatment with the different beta‐lactams.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Cancer patients of any age with febrile neutropenia . Since the definitions of neutropenia will vary to some extent from study to study, we accepted and documented the definitions for fever and for neutropenia used in the studies.
Types of interventions
Any beta‐lactam antibiotic with a spectrum of coverage comprising Pseudomonas aeruginosa ("antipseudomonal") versus a different antipseudomonal beta‐lactam antibiotic. We included only antibiotics given intravenously, as single agents (without an aminoglycoside) or in combination with a glycopeptide, for the initial empirical treatment for febrile neutropenia. The glycopeptide antibiotic (vancomycin, or teicoplanin) must have been added to both trial arms using the same dose, schedule and timing of administration for inclusion. We excluded interventions where an aminoglycoside was added to the beta‐lactam. Thus, we assessed the beta‐lactam monotherapies currently recommended in consensus guidelines for the treatment of febrile neutropenia, for those in need of intravenous treatment, with or without a glycopeptide (Hughes 2002; Link 2003; Tamura 2002; ECIL 2009). We permitted the inclusion of a glycopeptide because it should not affect the efficacy of the beta‐lactam (when given in both study arms) and since selection of the beta‐lactam is separate from the decision of whether to add a glycopeptide (Hughes 2002; Paul 2005; Paul 2005a).
The specific beta‐lactam antibiotics addressed by the current review included:
Penicillins: piperaciilin, ticarcillin, azlocillin ‐ all with or without a beta‐lactamase inhibitor
Cephalosporins: ceftazidime, cefepime, cefoperazone, cefpirome, cefpiramide, ceftobiprole ‐ all with or without a beta‐lactamase inhibitor
Monobactam: aztreonam
Carbapenems: imipenem, meropenem
Types of outcome measures
We included trials assessing one or more of the review‐defined outcomes. We excluded pharmacokinetic/ pharmacodynamic trials assessing only drug levels. Studies with a dropout rate above 30% were excluded from the review, unless data were available by intention‐to‐treat for at least one of the review‐defined outcomes.
Primary outcomes
All‐cause 30‐day mortality. When 30‐day mortality was not reported, we collected all‐cause mortality at end of study follow‐up and documented the follow‐up definitions.
Secondary outcomes
Clinical failure: Failure of antibiotic treatment defined as continued signs or symptoms of infection or the need for antibiotic modifications (change or addition). This outcome corresponds to the outcome of treatment response (success of initial empirical antibiotic therapy without any modification), as recommended by current guidelines regarding the methodology of clinical trials of patients with cancer and febrile neutropenia (Feld 2002).
Microbiological failure: defined as persistence of the infecting pathogen among patients with microbiologically documented infections.
Infection‐related mortality.
Antibiotic modifications: addition of glycopeptides, antifungal and any need for modification.
Duration of hospital stay (accounting for inclusion or exclusion of patients who died).
Development of resistance: change in susceptibility of pathogens isolated at initiation of antibiotic therapy.
Superinfection: new, persistent, or worsening symptoms and/or signs of infection associated with the isolation of a new pathogen (different pathogen, or same pathogen with different susceptibilities) or the development of a new site of infection
Colonisation by resistant bacteria: the isolation of bacteria during or following antibiotic therapy, without signs or symptoms of infection.
Adverse events: incidence of any adverse event, discontinuation of treatment due to adverse events and specific adverse events (diarrhea, pseudomembranous colitis, dermatological, neurological, nephrotoxicity, hepatotoxicity and anaphylaxis).
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search in an attempt to identify all relevant studies regardless of year of publication, language or publication status. We combined each of the specific antibiotics with the terms (neutropen* OR neutropaen* OR granulocytopen* OR granulocytopaen*). The following antibiotic names were used in the search: 'penicillin', 'ceftazidime', 'cefepime', 'cefoperazone', 'cefpiramide', 'ceftobiprole', cephalosporin*', 'piperaciilin', 'piperacillin‐tazobactam', 'ticarcillin', 'azlocillin', 'imipenem*', 'imipenem‐cilastatin', 'meropenem', 'carbapenem', 'monobactam', 'aztreonam' and 'beta‐lactam*'.
The following databases were searched:
Cochrane Central Register of Controlled Trials (CENTRAL), (Cochrane Library Issue 3, 2010) PubMed ‐ 1966 to 8.2010 LILACS ‐ 1982 to 8.2010
We limited the search strategy to randomised controlled trials in all databases except CENTRAL using the search strategy suggested in the Cochrane Handbook (Cochrane 2008). See Appendix 1.
Searching other resources
We searched the following conference proceedings for unpublished trials: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 1995 to 2009; European Congress of Clinical Microbiology and Infectious Diseases 2001 to 2009; Annual Meeting of the Infectious Diseases Society of America (IDSA) 2001 to 2009; and The American Society of Hematology 2001 to 2008. In addition, we searched Current Controlled Trials in the metaRegister of controlled clinical trials for ongoing and unpublished trials (http://www.controlled‐trials.com/mrct/). This sources covers several trial registries, including the NIH ClinicalTrials.gov Register. We searched the web for new drug application (NDA) documents of the US Food and Drug Administration (FDA) that included unpublished studies.
The bibliographies of all included studies and pertinent reviews were scanned for additional references. We requested from pharmaceutical companies data regarding unpublished studies.
Since, the publication of the first version of the current review (Paul 2006) and a second analysis of the specific efficacy and safety of cefepime (Yahav 2007), the FDA has conducted an investigation into the safety of cefepime for different indications, including febrile neutropenia (FDA 2009; FDA 2010). The review assessed mortality in trials that included cefepime in one of the comparator arms and included unpublished data from the following sources:
Unpublished RCTs available to the sponsoring pharmaceutical company (supplied by request from the pharmaceutical company)
Primary data from the pharmaceutical company on 30‐day all‐cause mortality for all randomized patients
We searched the accompanying documents of the FDA review describing the analysis for additional trials and mortality data that were not available through the published trial reports. However, we planned to include new trials only if we could confirm that the design and inclusion criteria were compatible with our review inclusion criteria.
Data collection and analysis
Selection of studies
One review author performed the search and inspected the abstract of each reference identified (MP). When relevant articles were identified, the full article was obtained and inspected independently by two review authors who applied inclusion criteria (MP, DY or AB).
Data extraction and management
Data from included trials were independently extracted by two review authors into a data extraction sheet (MP, AB, DY). Differences in the data extracted were resolved by discussion with a third review author (AF or LL). Justification for excluding studies from the review was documented.
The following data were extracted, checked, and recorded:
Trial characteristics
Years (start and end of recruitment) and countries of study.
Trial sponsor.
Publication status: published in journal; abstract/ proceeding; unpublished.
Follow up duration and the timing for mortality outcome assessment.
Ethical standards: ethics committee, patient consent.
Performance of surveillance cultures.
Patient characteristics
Definition of neutropenia in inclusion criteria.
Age: mean or median, and percentage of children under 18 years.
Number of patients receiving anti‐bacterial prophylaxis: quinolone/s, others.
Number of patients receiving anti‐fungal prophylaxis.
Number of patients with central venous catheter.
Infection characteristics
Number of patients with clinically documented infections, microbiologically documented infections, and fever of unknown origin .
Number of patients with and type of bacteraemia: single Gram negative, single Gram positive, polymicrobial.
Specific bloodstream isolates: Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, Enterobacter spp., Acinetobacter spp., other Gram‐negatives, Coagulase‐negative Staphylococci, Staphylococcus aureus, Enterococcus spp., Streptococcus pneumoniae, other Streptococci, other Gram‐positives.
Empirical antibiotic/s coverage of bloodstream pathogens isolated at onset of infectious episode.
Intervention characteristics
Antibiotics type and dose.
Outcome measures
As specified above with the number of patients evaluated per outcome.
Assessment of risk of bias in included studies
Two reviewers assessed risk of bias of each study, independently (MP, AB or DY). We used domain‐based evaluation, examining the following domains: allocation sequence generation, allocation concealment, blinding, incomplete outcome data for the outcomes of mortality and clinical failure with the number of patients excluded from outcome assessment and inclusion of patients more than once in the trial (listed under "other bias"). Each item was scored as yes or no, for low or high risk for bias, respectively. Where data/ outcome were not reported or details were not clear the item was scored as unclear.
Mortality and clinical failure were extracted preferentially by intention to treat, including all individuals randomised in the outcome assessment. Where not possible, data as treated (per protocol) were extracted. We scored trials in which the number of patients evaluated was lower than the number of patients randomized as high risk for bias for incomplete outcome data assessment, regarding attrition and exclusions similarly. This domain was scored as unclear if the outcome was not reported or if the number of randomized patients was not provided.
For blinding, only double‐blinded trials (blinding of patient and carer) were scored as low risk. Trials recruiting patients more than once into the trial (randomizing and evaluating episodes rather than patients) were scored as high risk for bias.
We could not assess selective outcome reporting, since most trials were conducted prior to regulations on trial registry and the trials' protocols were not available to us.
Measures of treatment effect
Relative risks (RR) for dichotomous data and weighted mean differences for continuous outcomes were calculated, with 95% confidence intervals (CI). In sensitivity analyses we calculated the risk difference per 1000 participants.
Unit of analysis issues
Trials of febrile neutropenia frequently randomise febrile episodes instead of patients, without adjusting for intra‐patient correlations. This is methodologically incorrect as the statistical tests used assume independence between the cases analysed. When extracting data this can be corrected only if the number of patients is known per group and the outcome is known for each patient's first entry. When available, we extracted outcomes for patients' first entry. Otherwise, we extracted outcomes per episode and recorded this (see under risk of bias assessment, "other bias").
Dealing with missing data
We contacted the first or corresponding author of each included study, for clarifications and further information that was not available in the publication. See also mortality data taken from FDA analyses under "Searching other resources ".
Assessment of heterogeneity
Heterogeneity in the results of the trials was assessed using a Chi‐square test of heterogeneity (p < 0.1) and the I2 measure of inconsistency (I2 > 50%) (Higgins 2003).
Assessment of reporting biases
Funnel plots for mortality and failure (1/standard error plotted against odds ratios) were visually examined in order to estimate potential selection bias (publication or other) in comparisons including 10 or more trials.
Data synthesis
We use the fixed effect model throughout the review. If the analysis was heterogenous (I2 > 50%), we conducted sensitivity/ subgroup analyses to investigate heterogeneity (see below) and did not try to pool all studies in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
In case of heterogeneity or to investigate differences detected between antibiotics, we conducted subgroup analyses of patients with clinically documented infections, where data were available. The outcome of microbiological failure was based on the subgroup of patients with microbiologically documented infections per definition.
Sensitivity analysis
To assess the effect of study risk of bias (see items listed above) on outcomes we performed sensitivity analyses, mainly for allocation concealment, basing on previous evidence showing over‐estimation of effects with inadequate or unclear allocation concealment (Moher 1998; Schulz 1995). In comparisons where a significant difference was observed, we conducted a sensitivity analysis using risk difference instead of risk ratios to permit the inclusion of trials with no events in both study groups (and to compare with the FDA analyses of cefepime). For the analysis of all‐cause mortality for cefepime, we conducted post‐hoc analyses of antibiotic dose and publication status.
Results
Description of studies
Results of the search
The search resulted in a large number of studies, of which 70 were deemed potentially eligible. Twenty‐six studies were excluded, mostly due to incompatible comparisons (addition of an aminoglycoside to one or both study arms), non‐random design or the assessment of non‐neutropenic patients. The reasons for exclusion are detailed in the characteristics of excluded studies table.
Included studies
Forty‐four trials were included comparing one antipseudomonal beta‐lactam to another, without or without a glycopeptide, among neutropenic cancer patients. These are described in the characteristics of included studies tables. The studies were conducted between 1985 to 2009 (year study ended), published between 1988 to 2010 and conducted worldwide. Notably, 14 were conducted in the US (year end 1985 to 2001) and 7 in Turkey (year end 1998 to 2009). Forteen were multicenter studies, while all others were conducted in a single hospital. Thirty‐seven trials were published in full, four as conference proceedings (Bickers 1990; Cornely 2001; Oppenheim 2000; Shichmanter 2004) and three were identified in the new drug applications of cefepime to the FDA (Aoun 1997; Glauser 1997; Ramphal 1996). No new trials were identified in the FDA analysis of cefepime (FDA 2009).
The comparisons performed are shown in Figure 1. The most frequent comparison was between cefepime and ceftazidime (nine trials). Only one trial compared piperacillin‐tazobactam versus ceftazidime. Missing from the figure are six comparisons, each performed in a single trial: cefepime versus an unspecific carbapenem (Tamura 2002) or panipenem (Kwon 2008), ceftazidime versus ticarcillin‐clavulanate (Bodey 1990) or piperacillin (Anaissie 1988) and imipenem versus aztreonam (Raad 1996) or meropenem (Shah 1996). Vancomycin was added to both study arms in five studies.
1.
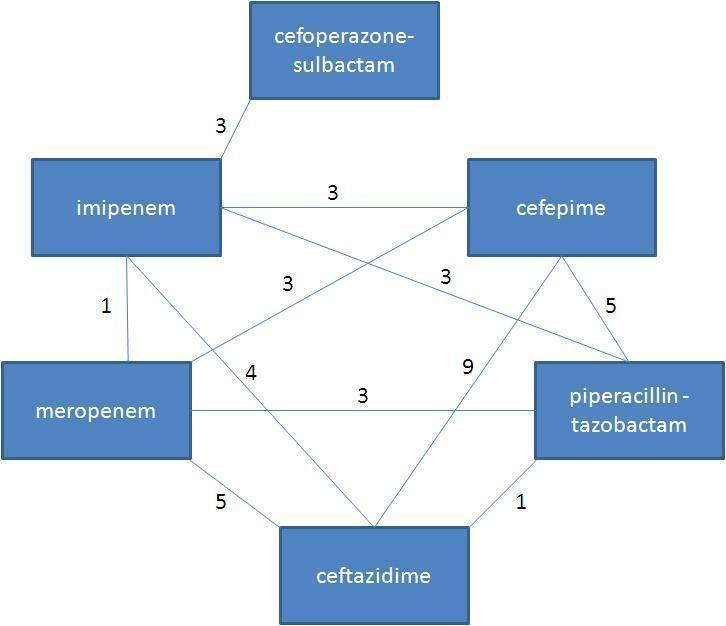
Comparisons identified for this review
The following comparisons were performed in our review, each stratified by the specific antibiotic comparison:
Cefepime versus other
Ceftazidime versus other
Piperacillin‐tazobactam versus other
Carbapenem versus other
The median number of included patients was 142 (range 40 to 528), with study size inversely correlated with study years (Spearman's rho p = 0.002, correlation coefficient = ‐0.49). Eight trials included children alone, two included adults and children and the remaining assessed adults alone. Neutropenia was defined as less than 500/ml3 in 12 trials, while 28 trials allowed also the inclusion of patients with neutrophil counts <1000/ml3 that were expected to drop to < 500/ml3 (three trials did not define neutropenia). The median prevalences of central venous catheter, antibiotic and antifungal prophylaxis on study entry were 68% (reported in 27 studies), 28% (reported in 28 studies) and 45% (reported in 12 studies), respectively, all ranging from 0 to 100%. The median percentage of bacteremias caused by Gram‐negative bacteria was 39.7% (7.7 to 100%, 26 studies) and the median percentage of bacteria resistant to one of the study drugs was 25% (0 to 59%, 14 studies). None of these clinical data were correlated with the study year.
All‐cause mortality was reported in 37/44 trials, the rate of superinfections in 19 and duration of hospital stay in six. All trials reported on clinical failure (the primary outcome in all trials), defined as no improvement, deterioration, or need for antibiotic modifications, where need for modifications was the predominant reason for failure assignment. The timing for reporting of mortality was fixed (e.g. 30 days post inclusion) in six trials; in 25 trials the time point was stated but not fixed (e.g. end of treatment, 7 days after end of treatment, in‐hospital, etc) and in 13 it was not stated. Data for duration of hospital stay were infrequently and variably reported as means or medians, thus we did not try to pool this outcome. Resistance development was reported in a single trial (Anaissie 1988); none of the trials conducted surveillance cultures. Risk ratios for clinical failure were correlated with those for any antibiotic modification; risk ratios for mortality were not correlated with those for clinical failure, microbiological failure or other secondary outcomes (data not shown).
Approval of an ethics committee was reported in 24 studies and patient consent in 24 of the 44 included trials.
Risk of bias in included studies
Adequate allocation concealment and generation were assigned to about half of the trials each (risk of bias tables Figure 2 and Figure 3), based on the publication report, contact with the authors or presumed by the trial authors' previous publications (the latter in four trials: (Anaissie 1988; Bodey 1990; Raad 2003; Rolston 1992). Thirty‐one trials were open‐labelled, only outcome assessors were blinded in 10, the patient in one, while only two trials were double (Feld 2000) or triple (Chandrasekar 2000) blinded (only double‐blinded trials assigned low risk for bias).
2.
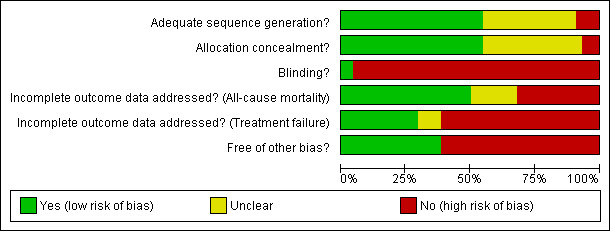
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
3.
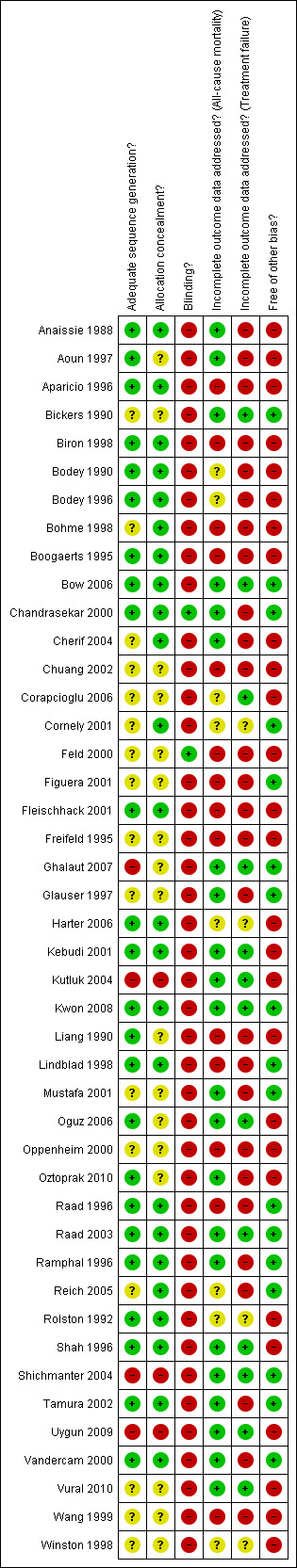
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Half of the studies were assigned low risk for bias related to incomplete outcome data reporting for mortality, since the outcome was reported for all randomized patients; about 30% were assigned high‐risk when not all patients were evaluated for mortality; and the remaining were assigned unclear status since mortality was not reported or the number of randomized patients was unclear. The percentage of studies at high risk for bias due to incomplete outcome reporting was higher for clinical failure than for mortality (Figure 2).
Twenty‐seven trials permitted the inclusion of patients more than once for different febrile neutropenic episodes. In these trials outcomes were reported per episode and most commonly were not reported separately for patient's first randomization (see risk of bias tables). Episodes were used as the denominator in the meta‐analysis of these trials.
Twenty‐three trials were sponsored by a pharmaceutical company producing one of the study drugs, five trials stated specifically that the trial was not funded or received academic funding only, while this information was not provided in 16 studies.
Effects of interventions
Cefepime versus other (RR<1 in favour of cefepime)
All‐cause mortality was significantly higher with cefepime as compared with other antibiotics, RR 1.39 [1.04, 1.86], without heterogeneity (I2=0%), 21 trials, 3471 participants Analysis 1.1. The RRs were >1 in the comparisons versus ceftazidime, carbapenems or piperacillin‐tazobactam and statistically significant only overall. To investigate increased mortality we conducted several sensitivity analyses. The differences between cefepime and comparators were higher with adequate allocation concealment (RR 1.74 [1.18, 2.56], Analysis 1.3) and generation (RR 1.79 [1.22, 2.64], Analysis 1.4), with blinding of outcome assessors or double‐blinding (Analysis 1.5) and with intention to treat analysis (Analysis 1.6), as compared to unclear or higher risk for bias for these categories. RRs were higher in published data and published studies as compared with unpublished data (unpublished studies or data obtained through correspondence with authors) or unpublished studies (Analysis 1.7 and Analysis 1.8). Mortality was significantly higher with cefepime in adequately concealed trials when used in the recommended daily dose (4gr/day for adults and 150 mg/kg/day for children) and also in trials where lower doses were used regardless of trial's risk of bias (trials were too few for separate analysis), Analysis 1.9. When re‐analysing Analysis 1.1 using risk differences, to include studies with 0 events in both study arms, the result was still statistically significant, risk difference 16.2/1000 episodes [1.7, 30.6], p = 0.028.
1.1. Analysis.
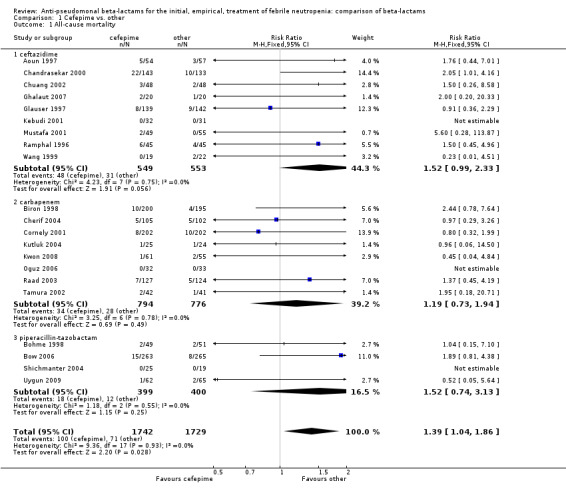
Comparison 1 Cefepime vs. other, Outcome 1 All‐cause mortality.
1.3. Analysis.
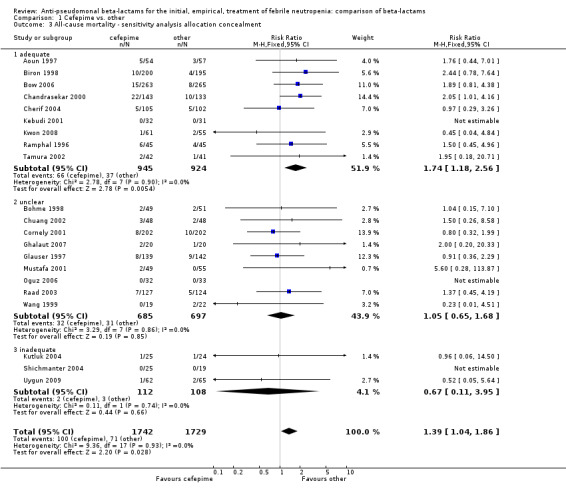
Comparison 1 Cefepime vs. other, Outcome 3 All‐cause mortality ‐ sensitivity analysis allocation concealment.
1.4. Analysis.
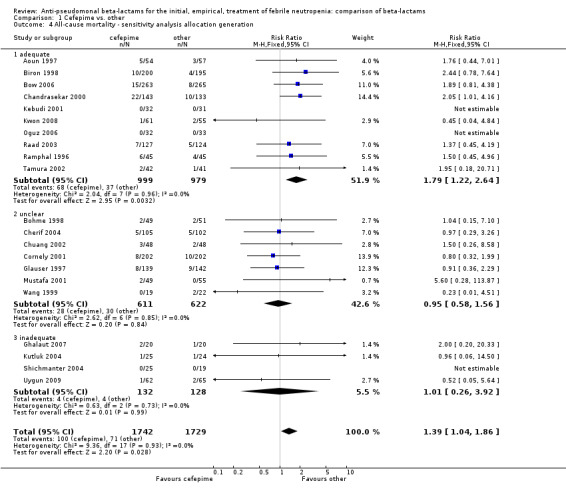
Comparison 1 Cefepime vs. other, Outcome 4 All‐cause mortality ‐ sensitivity analysis allocation generation.
1.5. Analysis.
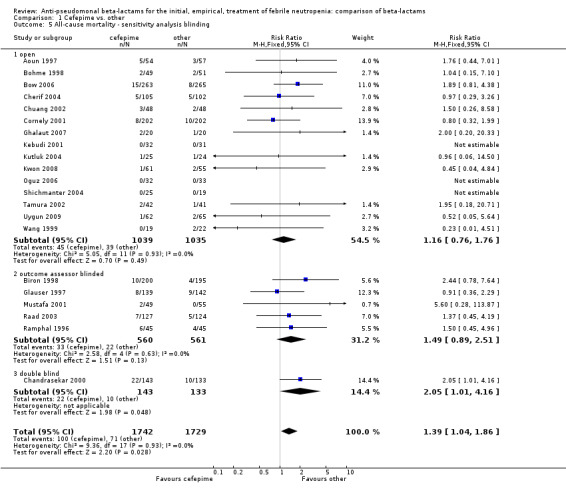
Comparison 1 Cefepime vs. other, Outcome 5 All‐cause mortality ‐ sensitivity analysis blinding.
1.6. Analysis.
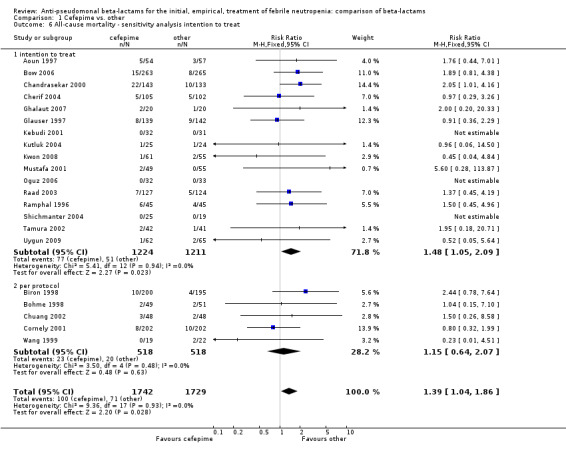
Comparison 1 Cefepime vs. other, Outcome 6 All‐cause mortality ‐ sensitivity analysis intention to treat.
1.7. Analysis.
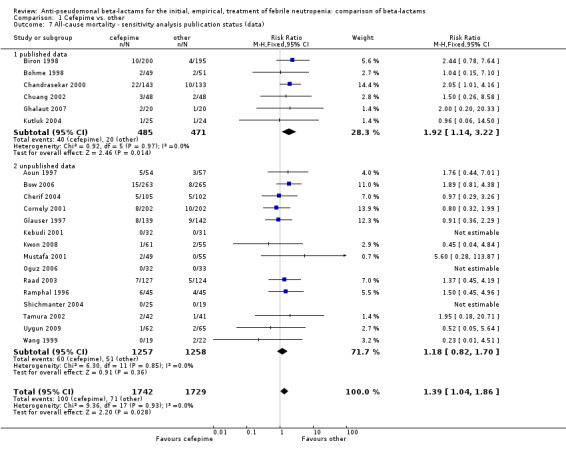
Comparison 1 Cefepime vs. other, Outcome 7 All‐cause mortality ‐ sensitivity analysis publication status (data).
1.8. Analysis.
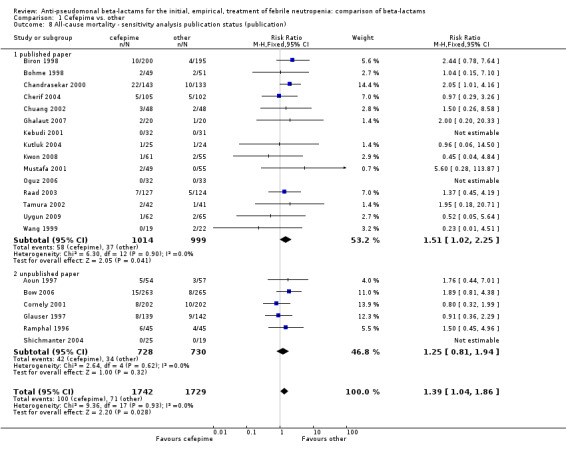
Comparison 1 Cefepime vs. other, Outcome 8 All‐cause mortality ‐ sensitivity analysis publication status (publication).
1.9. Analysis.
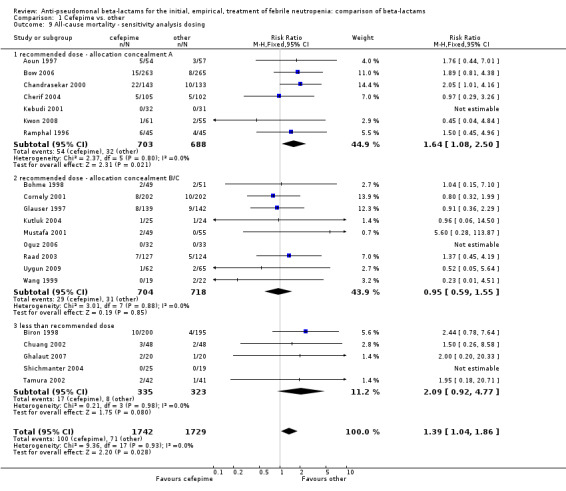
Comparison 1 Cefepime vs. other, Outcome 9 All‐cause mortality ‐ sensitivity analysis dosing.
In the recent FDA investigation into the safety of cefepime (FDA 2009; FDA 2010), data on 30‐day (rather than end of follow‐up) mortality per patient were obtained for some the trials included in our analysis. When re‐analysing the comparison using FDA's data on 30‐day mortality for trials included in our analysis (15 trials) and our data for trials not included in the FDA analysis ‐ 6 trials (Ghalaut 2007; Kutluk 2004; Kwon 2008; Oguz 2006; Shichmanter 2004; Uygun 2009), results were similar to our main analysis (RR 1.37 [1.03, 1.83]), Analysis 1.2. The risk difference was 15.4/1000 episodes [1.2, 29.7], p = 0.034 This analysis includes more patients because the FDA obtained mortality data for all randomized patients (intention to treat analysis).
1.2. Analysis.
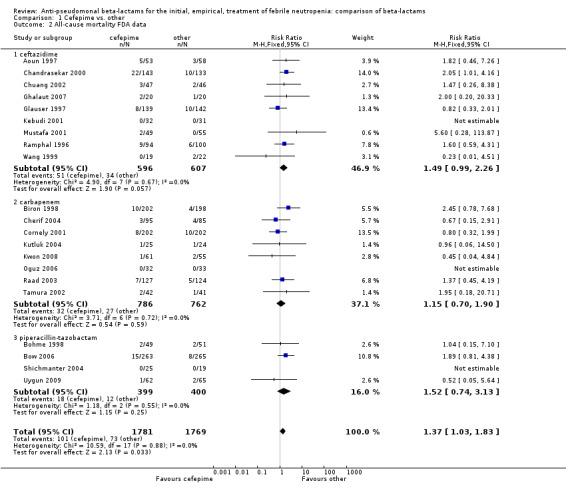
Comparison 1 Cefepime vs. other, Outcome 2 All‐cause mortality FDA data.
No differences were detected overall between cefepime and comparators with regard to all secondary outcomes. Clinical failure was not different overall (RR 1.02 [0.95, 1.09], 21 trials, 3028 participants, Analysis 1.11). Sensitivity analysis by allocation concealment (Analysis 1.12) and subgroup analysis for patients with clinically‐documented infections did not reveal new differences. There were no significant differences as regards microbiological failure (Analysis 1.14), any modifications to the antibiotic assigned antibiotic (Analysis 1.15) or any superinfection. There were fewer additions of glycopeptides and antifungal drugs with cefepime as compared to carbapenems, but the differences overall were not statistically significant (Analysis 1.16; Analysis 1.17). The RR for bacterial superinfections was 1.70 [0.94, 3.09] (Analysis 1.19). Duration of hospital stay was reported in three trials (Kebudi 2001; Oguz 2006; Uygun 2009 with no significant differences between trial arms considering all randomized patients.
1.11. Analysis.
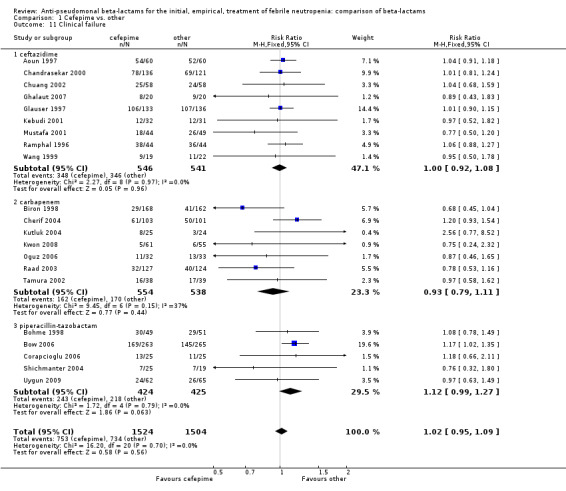
Comparison 1 Cefepime vs. other, Outcome 11 Clinical failure.
1.12. Analysis.
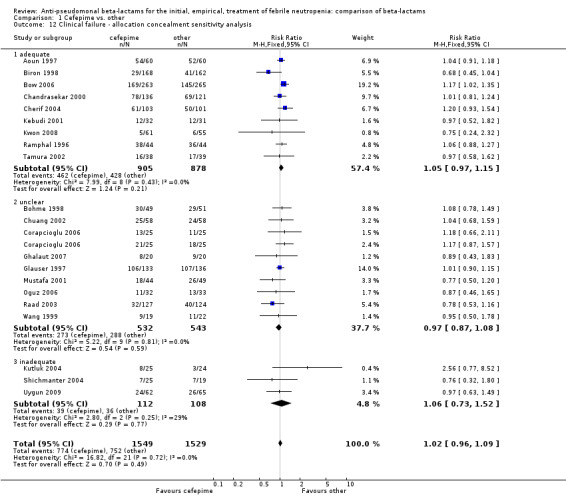
Comparison 1 Cefepime vs. other, Outcome 12 Clinical failure ‐ allocation concealment sensitivity analysis.
1.14. Analysis.
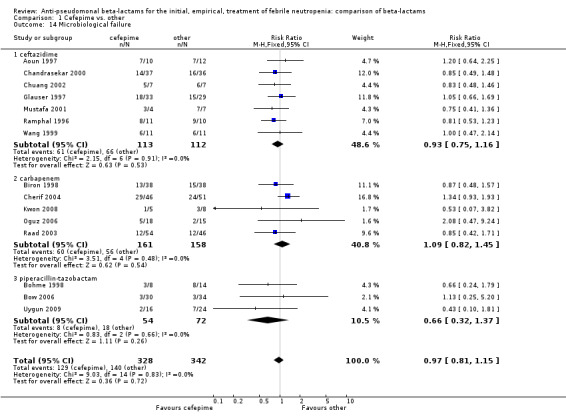
Comparison 1 Cefepime vs. other, Outcome 14 Microbiological failure.
1.15. Analysis.
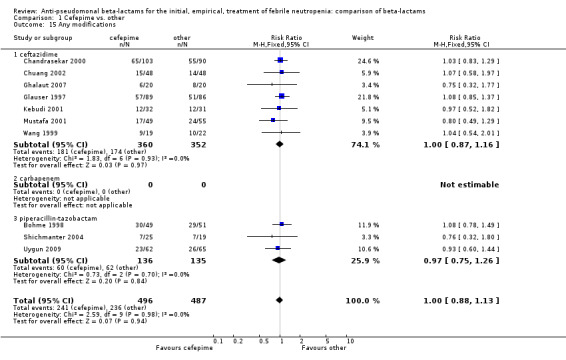
Comparison 1 Cefepime vs. other, Outcome 15 Any modifications.
1.16. Analysis.
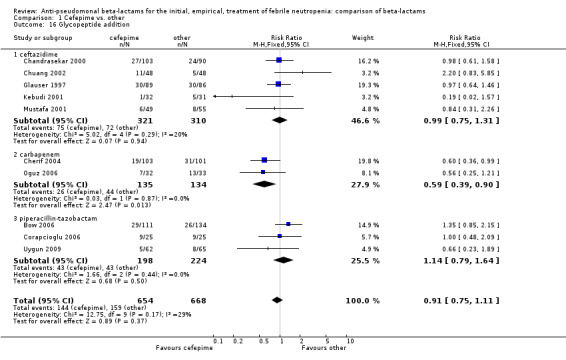
Comparison 1 Cefepime vs. other, Outcome 16 Glycopeptide addition.
1.17. Analysis.
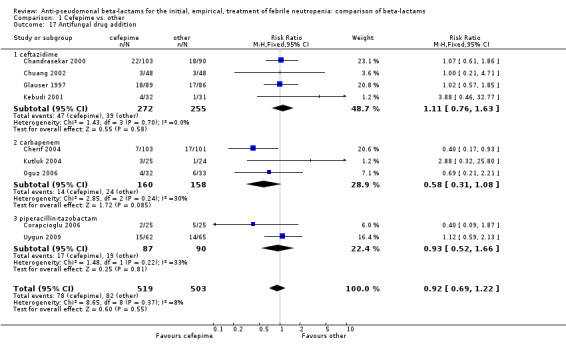
Comparison 1 Cefepime vs. other, Outcome 17 Antifungal drug addition.
1.19. Analysis.
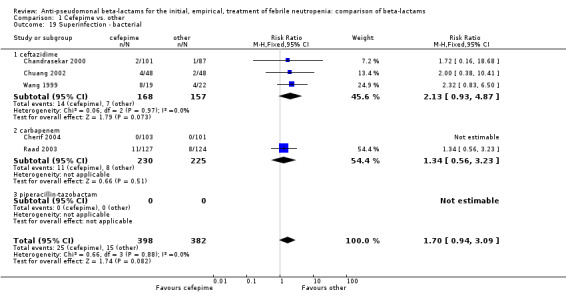
Comparison 1 Cefepime vs. other, Outcome 19 Superinfection ‐ bacterial.
There were significantly fewer adverse events with cefepime overall, a difference derived mainly from the comparison versus carbapenems (Analysis 1.20), but there was no statistically significant difference in adverse events requiring discontinuation of treatment (Analysis 1.21). There was no significant heterogeneity in all analyses.
1.20. Analysis.
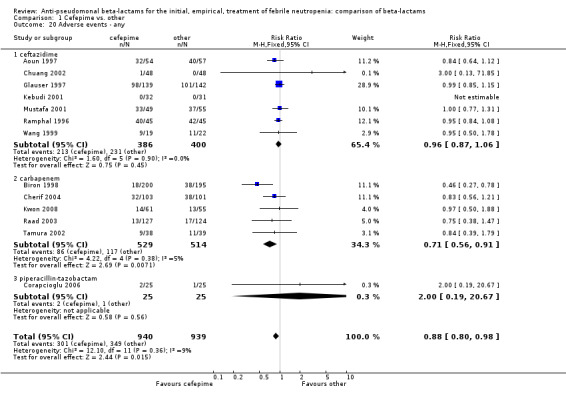
Comparison 1 Cefepime vs. other, Outcome 20 Adverse events ‐ any.
1.21. Analysis.
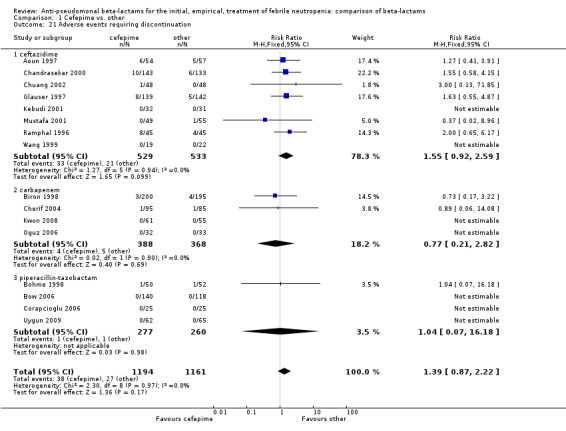
Comparison 1 Cefepime vs. other, Outcome 21 Adverse events requiring discontinuation.
In summary, cefepime resulted in significantly higher all‐cause mortality than comparator antibiotics and no other differences in secondary outcomes. The level of evidence for this finding is high, given the lack of heterogeneity, consistent findings with adequate randomization methods and the FDA's re‐analysis using 30‐day mortality data for all randomized patients. A higher rate of bacterial superinfections (without statistical significance) was the only possible explanatory finding.
Ceftazidime versus other (RR<1 in favour of ceftazidime)
All‐cause mortality was not significantly different for ceftazidime versus other antibiotics overall, RR 0.81 [0.59, 1.13], 19 trials, 3335 participants (Analysis 2.1). When excluding the comparison with cefepime from the analysis, the RR was 1.10 [0.66, 1.84].
2.1. Analysis.
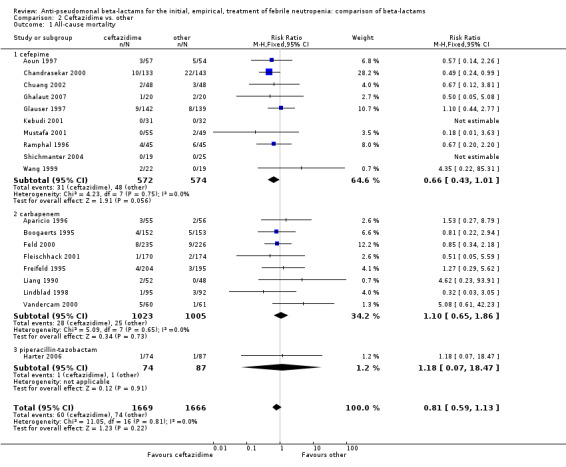
Comparison 2 Ceftazidime vs. other, Outcome 1 All‐cause mortality.
Clinical failure was significantly more common with ceftazidime as compared to carbapanems, RR 1.16 [1.07, 1.26] (21 trials, 4204 participants Analysis 2.3), with moderate heterogeneity (I2= 38%). Consistently, antibiotic modifications of any kind and the addition of glycopeptides were more frequent with ceftazidime as compared to carbapanems (Analysis 2.7; Analysis 2.8). The addition of antifungals was not more frequent with ceftazidime (Analysis 2.9) There were no differences between ceftazidime and other antibiotics overall with these outcomes and no difference compared to all antibiotics with regard to infection‐related mortality (Analysis 2.2) and microbiological failure (Analysis 2.6). There was no significant difference in superinfections overall, with RRs favouring comparator antibiotics (RR 1.20 [0.95, 1.52], Analysis 2.6), and only few studies reporting bacterial superinfections specifically (Analysis 2.11). The analysis of clinical failure was sensitive to randomization methods, with trials of unclear allocation concealment or generation methods showing disadvantage to ceftazidime with significant heterogeneity (I2=65%) and trials describing adequate methods showing a RR near 1 without heterogeneity (Analysis 2.4; Analysis 2.5). Single trials comparing ceftazidime to piperacillin (Anaissie 1988) or ticarcillin‐clavulanate (Bodey 1990) were outliers in the analyses of clinical and microbiological failure, showing advantage to ceftazidime, and contributed to heterogeneity in these comparisons. Their exclusion abolished heterogeneity with no change in the results described above. There was no significant heterogeneity in other analyses.
2.3. Analysis.
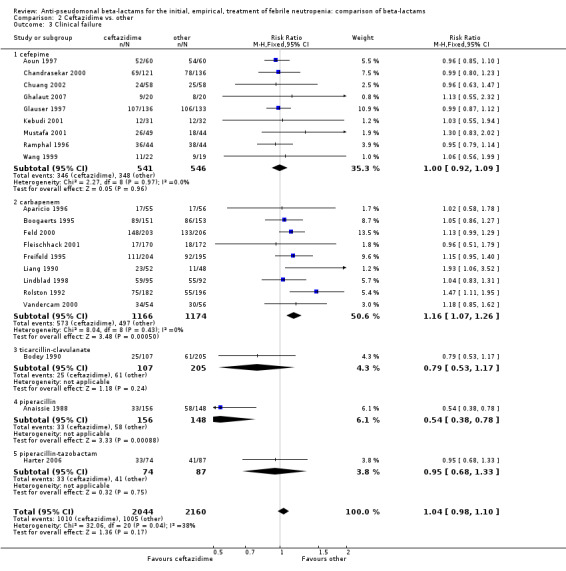
Comparison 2 Ceftazidime vs. other, Outcome 3 Clinical failure.
2.7. Analysis.
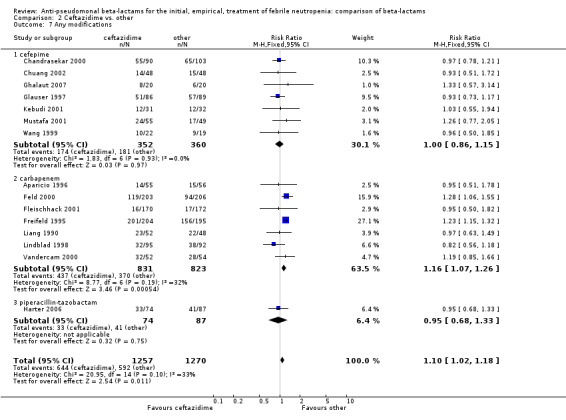
Comparison 2 Ceftazidime vs. other, Outcome 7 Any modifications.
2.8. Analysis.
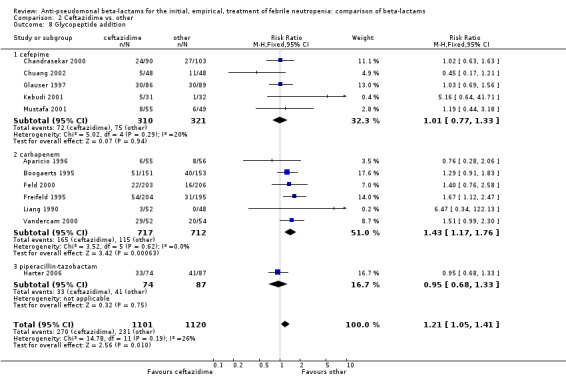
Comparison 2 Ceftazidime vs. other, Outcome 8 Glycopeptide addition.
2.9. Analysis.
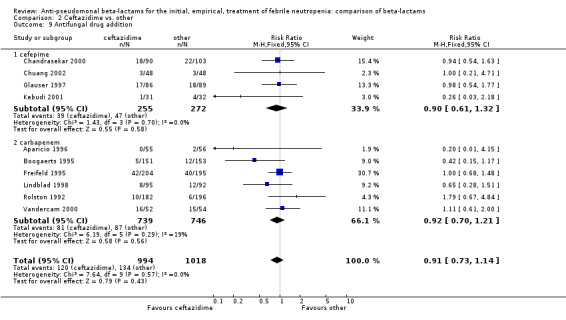
Comparison 2 Ceftazidime vs. other, Outcome 9 Antifungal drug addition.
2.2. Analysis.
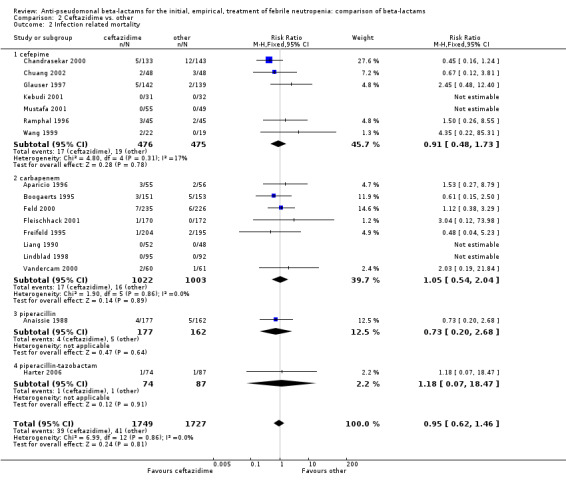
Comparison 2 Ceftazidime vs. other, Outcome 2 Infection related mortality.
2.6. Analysis.
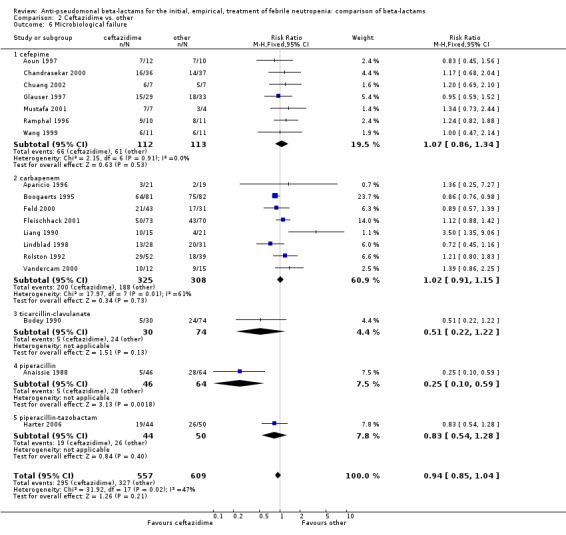
Comparison 2 Ceftazidime vs. other, Outcome 6 Microbiological failure.
2.11. Analysis.
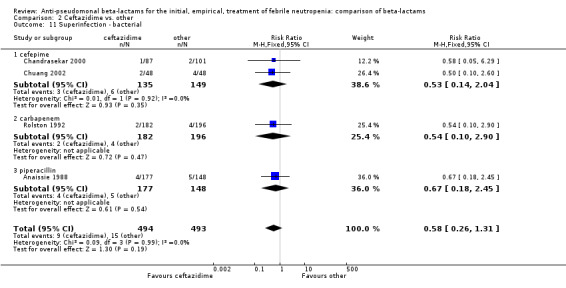
Comparison 2 Ceftazidime vs. other, Outcome 11 Superinfection ‐ bacterial.
2.4. Analysis.
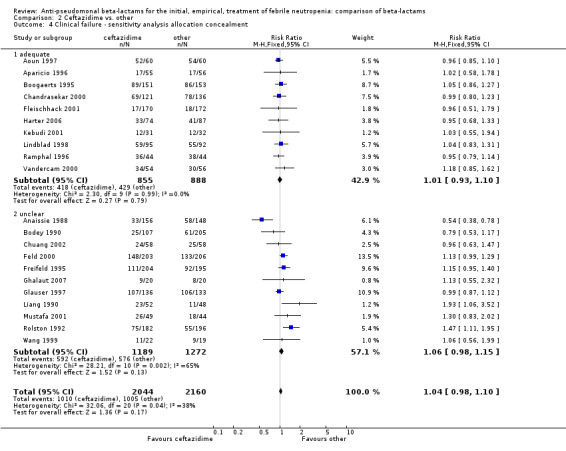
Comparison 2 Ceftazidime vs. other, Outcome 4 Clinical failure ‐ sensitivity analysis allocation concealment.
2.5. Analysis.
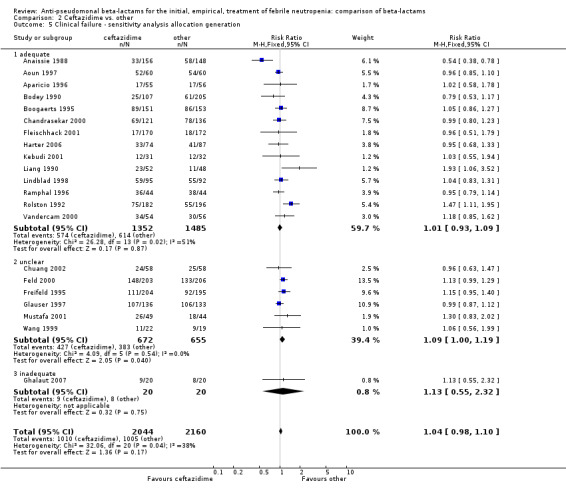
Comparison 2 Ceftazidime vs. other, Outcome 5 Clinical failure ‐ sensitivity analysis allocation generation.
The analysis of any adverse event was highly heterogenous, thus we did not try to compile it (Analysis 2.12). Ceftazidime caused much less adverse events than carbapenems in two trials (Freifeld 1995; Rolston 1992); in both on account of more gastrointestinal events and pseudomembranous colitis with imipenem. Similar results were seen in one trial comparing ceftazidime to piperacillin (Anaissie 1988), where allergic skin reactions also contributed to the difference. Tha analysis of adverse events requiring discontinuation was not heterogenous and showed a non‐significant advantage to ceftazidime, RR 0.69 [0.47, 1.03] (Analysis 2.13).
2.12. Analysis.
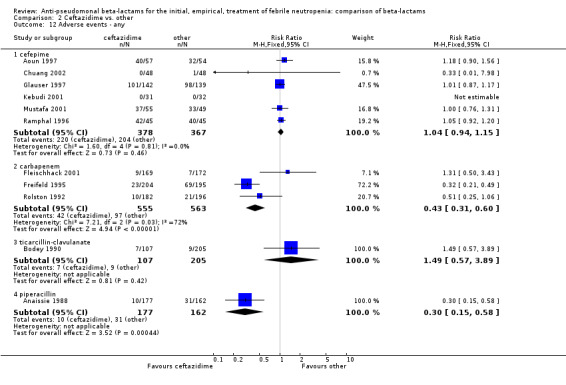
Comparison 2 Ceftazidime vs. other, Outcome 12 Adverse events ‐ any.
2.13. Analysis.
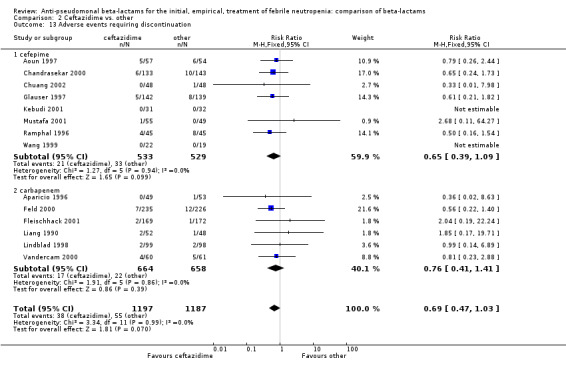
Comparison 2 Ceftazidime vs. other, Outcome 13 Adverse events requiring discontinuation.
In summary, there was no difference between ceftazidime and comparator drugs in all‐cause mortality, except for cefepime. More clinical failures occurred with ceftazidime compared to carbapenems, on account of antibiotic modifications, but this analysis was sensitive to randomization methods and with adequate randomization methods no difference was observed. There were fewer adverse events with ceftazidime, mainly diarrhea and pseudomembranous colitis when compared to imipenem.
Piperacillin‐tazobactam versus other (RR<1 favours piperacillin‐tazobactam)
All‐cause mortality was lower with piperacillin‐tazobactam versus all other antibiotics , RR 0.56 [0.34, 0.92], 8 trials, 1314 participants (Analysis 3.1). The difference was statistically significant also in the comparison restricted to carbapenems, RR 0.46 [0.22, 0.95]. RRs were similar in adequately concealed trials and in those where allocation concealment methods were unclear (Analysis 3.2). The risk difference, including one trial with no deaths was, ‐26.9/1000 episodes [‐ 4.4, ‐ 49.5], p = 0.02. There was no heterogeneity in these analyses (I2=0%).
3.1. Analysis.
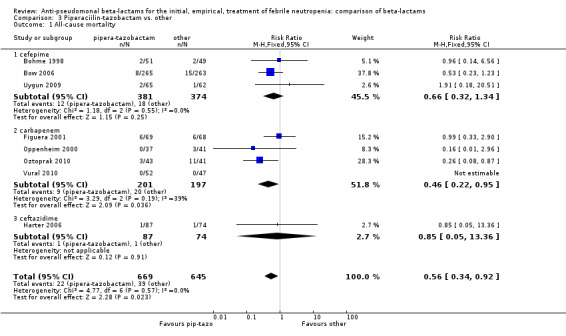
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 1 All‐cause mortality.
3.2. Analysis.
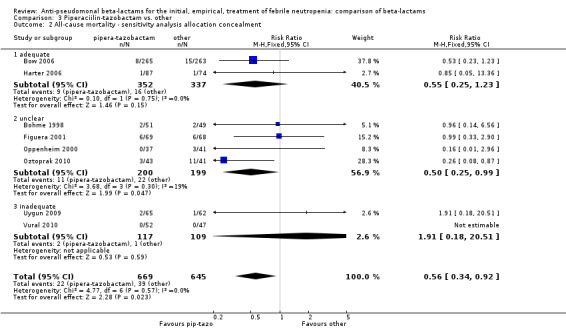
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 2 All‐cause mortality ‐ sensitivity analysis allocation concealment.
There was no statistically significant difference with regard to infection‐related mortality, RR 0.62 [0.29, 1.34] (Analysis 3.3). There was no significant difference in clinical failure overall, with a statistically non‐significance advantage to piperacillin ‐tazobactam when compared to cefepime, 0.89 [0.79, 1.01], Analysis 3.4, and this analysis was not sensitive to the methods of allocation concealment (Analysis 3.5). There were no significant differences between piperacillin‐tazobactam and comparators with regard to microbiological failure (Analysis 3.6), any antibiotic modifications (Analysis 3.7), the addition of glycopeptides (Analysis 3.8) or the addition of antifungals (Analysis 3.9). No trials reported on superinfections.
3.3. Analysis.
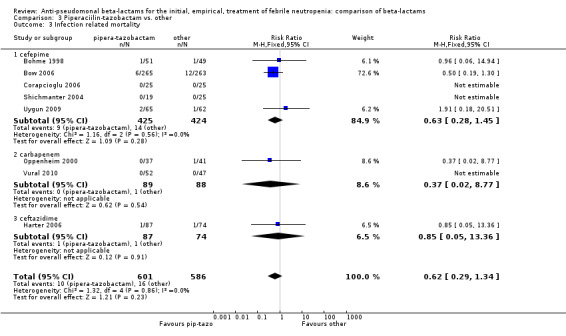
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 3 Infection related mortality.
3.4. Analysis.
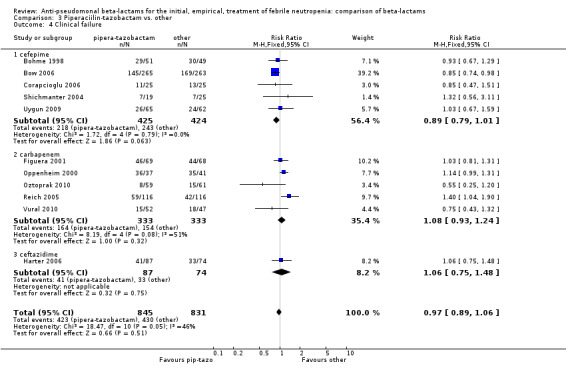
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 4 Clinical failure.
3.5. Analysis.
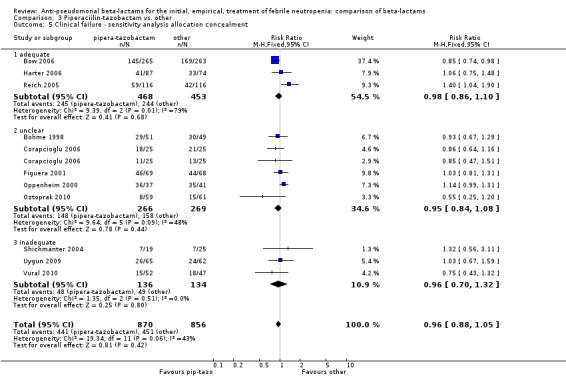
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 5 Clinical failure ‐ sensitivity analysis allocation concealment.
3.6. Analysis.
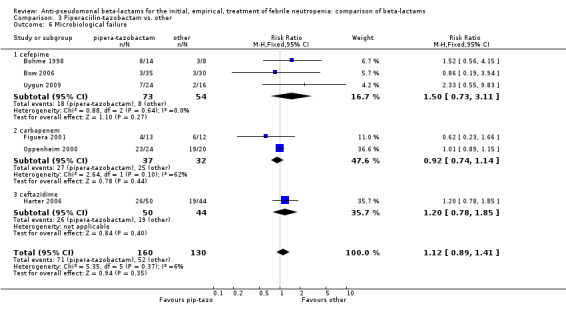
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 6 Microbiological failure.
3.7. Analysis.
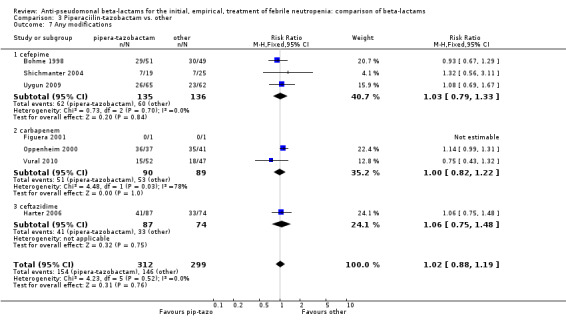
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 7 Any modifications.
3.8. Analysis.
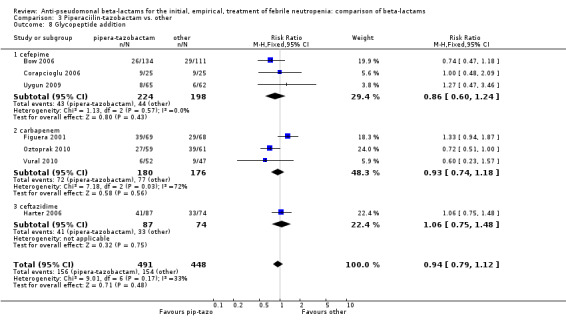
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 8 Glycopeptide addition.
3.9. Analysis.
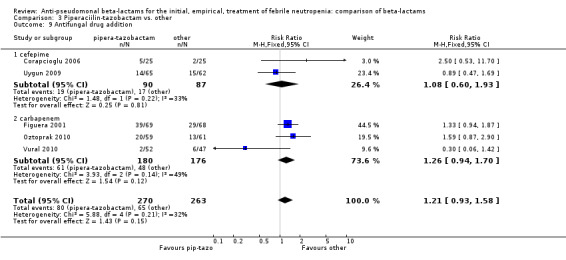
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 9 Antifungal drug addition.
There were significantly fewer adverse events with piperacillin‐tazobactam in an analysis including three trials comparing it to imipenem and one trial comparing it to cefepime, with some heterogeneity, RR 0.39 [0.24, 0.65], I2=12% (Analysis 3.10). Adverse events requiring discontinuation occurred in one trial only; the compiled risk difference for discontinuations including no events trials was ‐0.1/1000 participants [‐15.9, +14.9], 5 trials, 636 participants (Analysis 3.11).
3.10. Analysis.
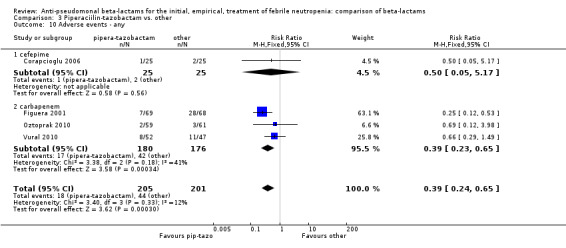
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 10 Adverse events ‐ any.
3.11. Analysis.
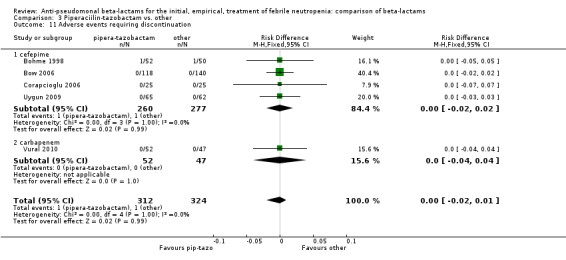
Comparison 3 Piperaciilin‐tazobactam vs. other, Outcome 11 Adverse events requiring discontinuation.
In summary, piperacillin‐tazobactam resulted in lower all‐cause mortality, compared mainly to cefepime and carbapanems. A benefit was observed also with regard to adverse events. The level of evidence for this finding is moderate because most trials had unclear allocation concealment.
Carbapenems versus other (RR<1 favours carbapenems)
There was no difference between carbapenems and comparators in all‐cause mortality overall, RR 1.16 [0.87, 1.55], 22 trials, 2861 participants, without heterogeneity (Analysis 4.1). No difference was seen both with adequately concealed randomization or unclear methods (Analysis 4.2). When excluding the comparison versus cefepime, the RR was 1.39 [0.96, 2.00].
4.1. Analysis.
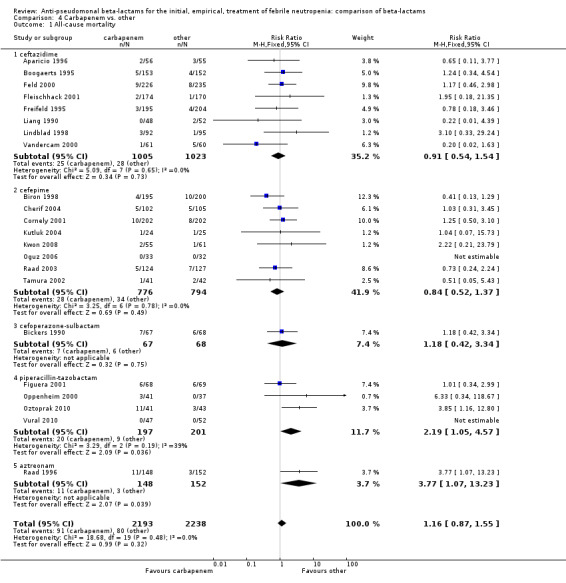
Comparison 4 Carbapenem vs. other, Outcome 1 All‐cause mortality.
4.2. Analysis.
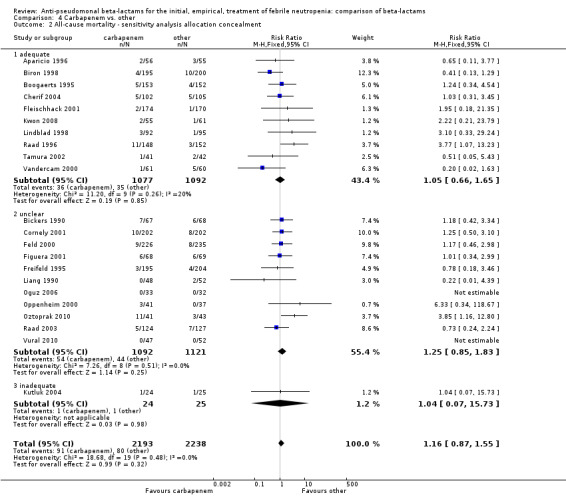
Comparison 4 Carbapenem vs. other, Outcome 2 All‐cause mortality ‐ sensitivity analysis allocation concealment.
As previously shown, carbapenems resulted in a higher rate of clinical success and lower rate of any and glycopeptide antibiotic modifications when compared to ceftazidime (Analysis 4.4, Analysis 4.7; Analysis 4.8). There were significantly less clinical failures with carbapenems versus all other antibiotics combined, RR 0.93 [0.88, 0.99], 25 trials, 5159 participants (Analysis 4.4) and less need for antibiotic modifications, RR 0.89 [0.82, 0.96] (Analysis 4.7), with moderate heterogeneity in both analyses (I2 35 to 39%). The difference in clinical failure was more pronounced and heterogenous in trials with unclear allocation concealment methods compared to those with adequate methods (Analysis 4.5). There was no difference in the rate of infection‐related mortality (Analysis 4.3) and microbiological failure (Analysis 4.6). The analyses of glycopeptide and antifungal drug additions were heterogenous and thus not compiled. Antifungals drugs were added more to the carbapenem arm in all comparisons except for that with piperacillin‐tazobactam; when excluding the comparison with piperacillin‐tazobactam there was a significant advantage to comparator antibiotics, RR 1.29 [1.03, 1.61] (Analysis 4.9). However, there were no differences between carbapenems and comparators with regard to superinfections overall (Analysis 4.10), fungal (Analysis 4.11) or bacterial Analysis 4.12, with only few trials contributing to the latter analyses. Duration of hospital stay was shorter with meropenem as compared to ceftazidime in one trial (mean 17 versus 22 days, Vandercam 2000), similar in two other trials comparing meropenem to ceftazidime (Fleischhack 2001) or cefepime (Oguz 2006) and longer in one trial comparing carbapenems to piperacillin‐tazobactam (Oztoprak 2010).
4.4. Analysis.
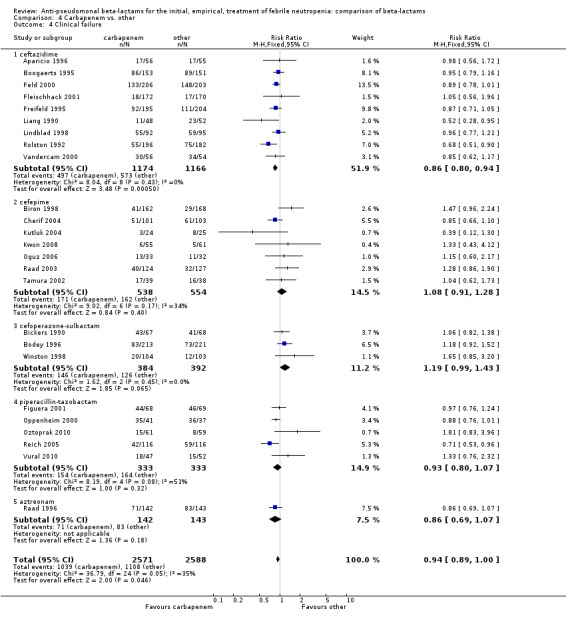
Comparison 4 Carbapenem vs. other, Outcome 4 Clinical failure.
4.7. Analysis.
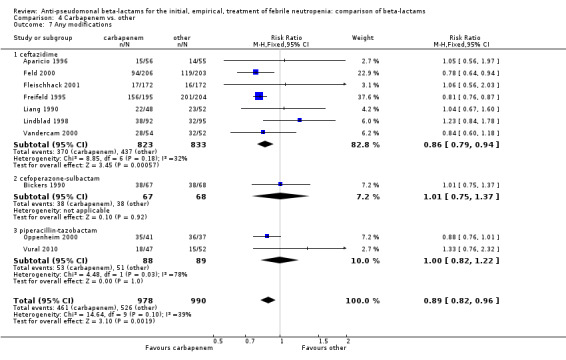
Comparison 4 Carbapenem vs. other, Outcome 7 Any modifications.
4.8. Analysis.
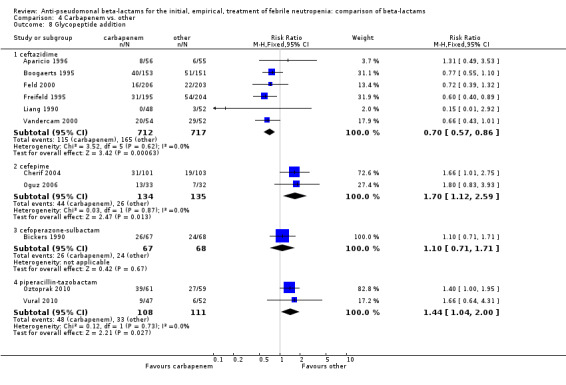
Comparison 4 Carbapenem vs. other, Outcome 8 Glycopeptide addition.
4.5. Analysis.
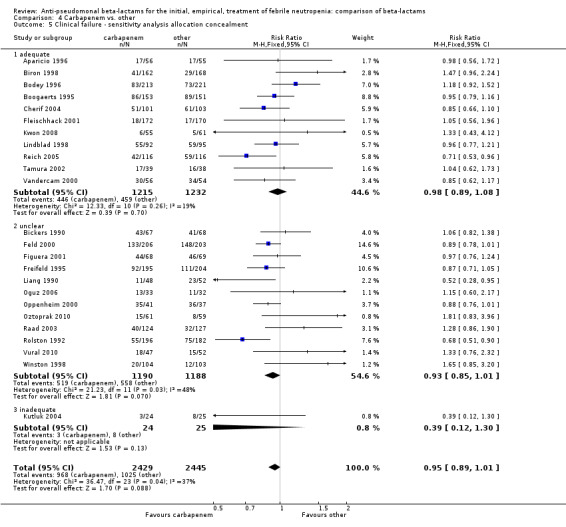
Comparison 4 Carbapenem vs. other, Outcome 5 Clinical failure ‐ sensitivity analysis allocation concealment.
4.3. Analysis.
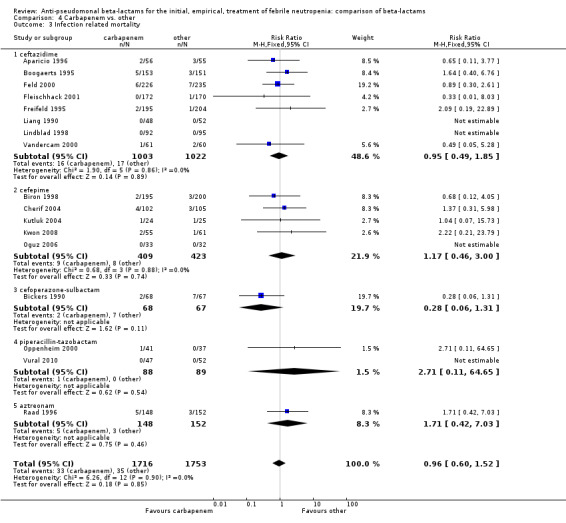
Comparison 4 Carbapenem vs. other, Outcome 3 Infection related mortality.
4.6. Analysis.
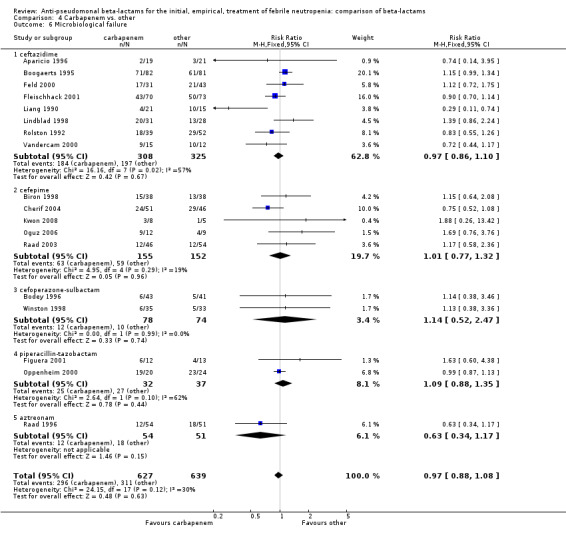
Comparison 4 Carbapenem vs. other, Outcome 6 Microbiological failure.
4.9. Analysis.
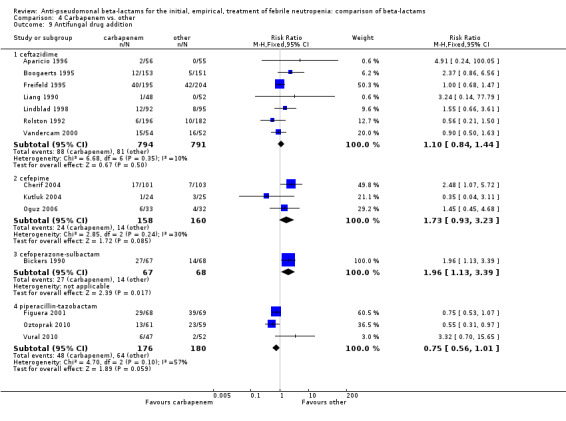
Comparison 4 Carbapenem vs. other, Outcome 9 Antifungal drug addition.
4.10. Analysis.
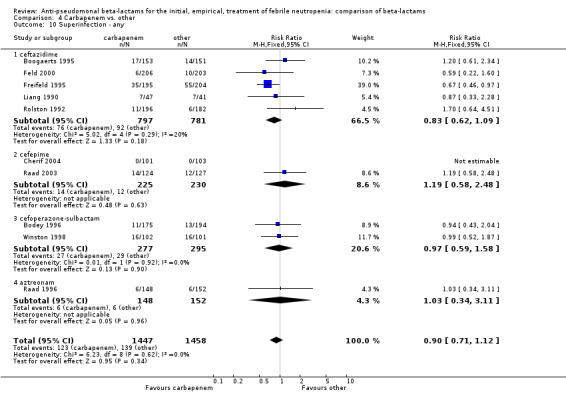
Comparison 4 Carbapenem vs. other, Outcome 10 Superinfection ‐ any.
4.11. Analysis.
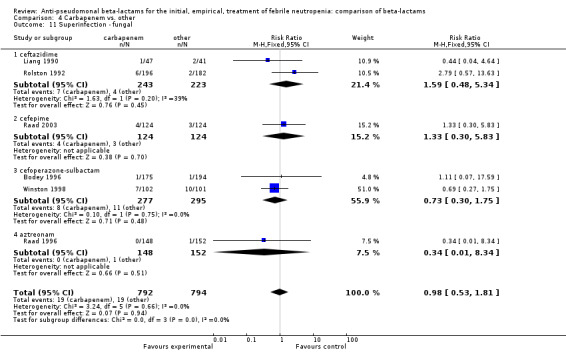
Comparison 4 Carbapenem vs. other, Outcome 11 Superinfection ‐ fungal.
4.12. Analysis.
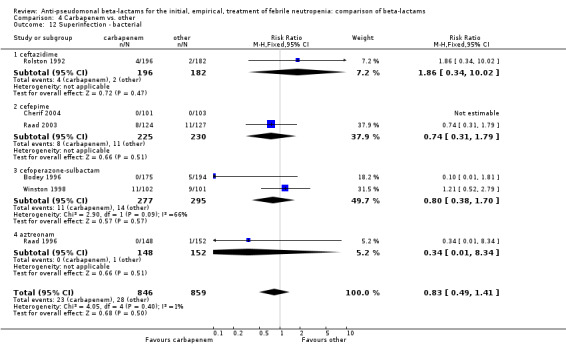
Comparison 4 Carbapenem vs. other, Outcome 12 Superinfection ‐ bacterial.
Adverse events were significantly more frequent with imipenem when compared to other beta‐lactams, RR 1.68 [1.44, 1.96] and with significant heterogeneity (Analysis 4.13). The overall analysis was significantly heterogenous and thus not compiled. There was a higher rate of seizures with imipenem compared to other antibiotics, RR 3.18 [1.10, 9.18] (Analysis 4.15), a higher rate of pseudomembranous colitis for carbapenems versus cephalosporins, 1.94 [1.24, 3.04] (Analysis 4.16) and a higher rate of other gastrointestinal adverse events including diarrhea and vomiting overall, 2.00 [1.63, 2.46] (Analysis 4.17), with significant heterogeneity. The latter comparison was heterogenous (I2=68%); the definitions for other gastrointestinal events were heterogenous, but most trials showed varying degrees of advantage to comparator drugs. Discontinuations due to adverse events were reported in fewer trials and there was no significant difference between carbapenems and other antibiotics (Analysis 4.14).
4.13. Analysis.
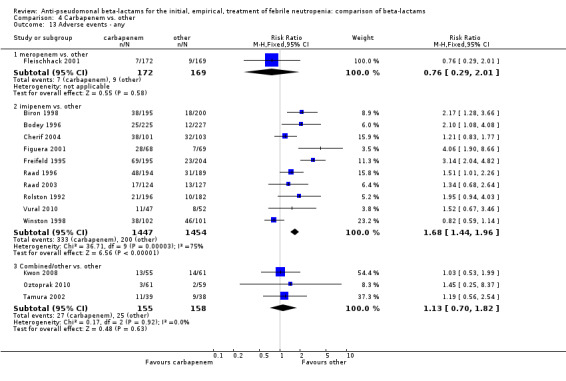
Comparison 4 Carbapenem vs. other, Outcome 13 Adverse events ‐ any.
4.15. Analysis.
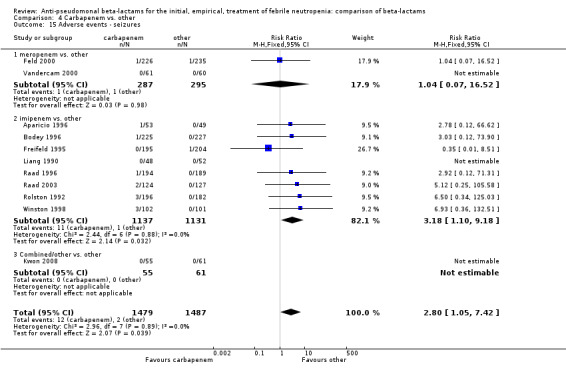
Comparison 4 Carbapenem vs. other, Outcome 15 Adverse events ‐ seizures.
4.16. Analysis.
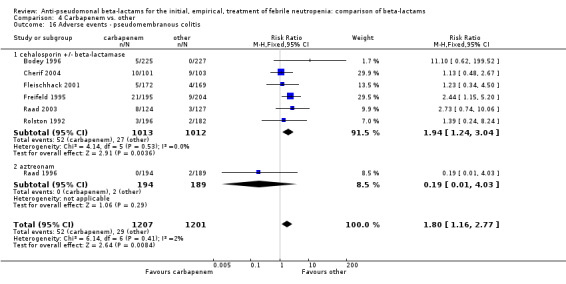
Comparison 4 Carbapenem vs. other, Outcome 16 Adverse events ‐ pseudomembranous colitis.
4.17. Analysis.
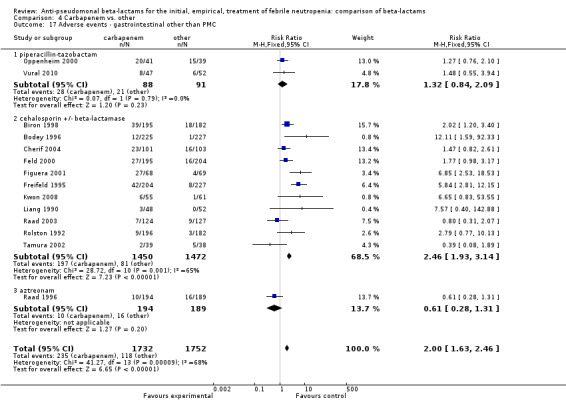
Comparison 4 Carbapenem vs. other, Outcome 17 Adverse events ‐ gastrointestinal other than PMC.
4.14. Analysis.
Comparison 4 Carbapenem vs. other, Outcome 14 Adverse events requiring discontinuation.
In summary, carbapenems showed similar all‐cause mortality and a lower rate of clinical failure and antibiotic modifications as compared to other antibiotics. Carbapenems resulted in a higher rate of adverse events, mainly gastrointestinal including pseudomembranous colitis. The level of evidence for this finding is low, mainly due to significant heterogeneity. Imipenem caused a higher rate of seizures.
Other
One trial comparing imipenem versus meropenem was not included in previous analyses (Shah 1996). It showed no differences in primary or secondary outcomes, within the limitations of a small trial (61 patients).
Discussion
We compiled trials comparing different antipseudomonal beta‐lactams administered as single agents, with or without the addition of a glycopeptide, in the treatment of febrile neutropenia. The most significant finding of this review is that cefepime resulted in higher all‐cause mortality at 30 days and end of follow‐up when compared to carbapenems, ceftazidime or piperacillin‐tazobactam. This finding was robust to several sensitivity analyses, consistently more pronounced in trials with lower risk of bias, resulting in a high level of evidence. The reasons for increased mortality were not apparent in the analysis of secondary outcomes; we did not find a disadvantage to cefepime as regards clinical failure, microbiological eradication of infection or adverse events. There were non‐significantly more bacterial superinfections with cefepime. The other finding was lower all‐cause mortality with piperacillin‐tazobactam compared to other beta‐lactams, with a moderate level of evidence. Carbapenems were associated with a lower rate of clinical failure and antibiotic modifications than the other antibiotics, but caused significantly more adverse events, mainly diarrhea and pseudomembranous colitis, which may be significant problems in cancer patients (low level of evidence). Imipenem caused a significantly higher rate of seizures.
In all but two trials carers were not blinded to the assigned antibiotic regimen. Thus, the outcome of clinical failure should be viewed with caution. Defined by current guidelines as no response or need for antibiotic modification (Feld 2002), it consists mainly of antibiotic modifications (addition of a glycopeptide, aminoglycoside, antifungal drug or beta‐lactam switch). Antibiotic modifications are naturally prone to bias in trials comparing a novel antibiotic to a commonly used antibiotic. In addition, this composite outcome comprises many possible complications, including the mere continuation of fever that is very common among neutropenic cancer patients as long as neutropenia persists, true failure of the treatment, a new bacterial superinfection or a new fungal infections that carries ominous consequences. On the other hand, it is difficult to define clinical failure differently, disregarding antibiotic modifications, since ultimately a patient either resolves the infection without or without antibiotic modifications or dies. In all analyses, results for all‐cause mortality were not correlated with clinical failure, reported as primary outcome in all trials. Our interpretation is that the outcomes relevant in the assessment of treatment for febrile neutropenia are all‐cause mortality, hospital stay, bacterial and fungal superinfections defined with commonly accepted criteria for healthcare associated infections (CDC/NHSN 2008 or fungal infections (De Pauw 2008), respectively, and adverse events, mainly Clostridium‐difficile‐associated colitis. Basing the conclusions of our review on these outcomes, cefepime should not be used for the treatment of neutropenic cancer patients due to higher mortality, piperacillin‐tazobactam or ceftazidime (in this order) are the preferred beta‐lactams and carbapenems should be reserved for cases where baseline resistance rates prohibit the use of piperacillin‐tazobactam, due to a higher rate of adverse events.
Since the first version of this review (Paul 2006; Yahav 2007) and because of its results, the FDA has conducted a review on the safety of cefepime (FDA 2009; FDA 2010). This analysis included both neutropenic and non‐neutropenic patients. They concluded that "no statistically significant increase in mortality was seen in cefepime‐treated patients compared to comparator‐treated patients... and,... Based on the results of FDA's meta‐analyses, the FDA has determined that cefepime remains an appropriate therapy for its approved indications". Notably, cefepime is the only FDA‐approved monotherapy for febrile neutropenia. TheFDA's analysis was highly transparent, its results are openly available to the public on the web (FDA 2009) and all our questions regarding this investigation were duly answered by the medical officer. However, we have several concerns with the results of this analysis. The analysis was dependent on data provided by Bristol Myers Squibb (BMS), the producer of cefepime. BMS supplied previously unpublished trials and complemented mortality data that were previous unavailable in published trials. Unfortunately, the FDA did not attempt to corroborate the data supplied by BMS through correspondence with the academic researchers involved in the unpublished trials and could not obtain data on the trial methods substantiating a randomized design for the new trials that BMS supplied. The FDA's analysis includes 30 unpublished trials, where BMS declared that "All the non proprietary data available from Bristol‐Myers Squibb Company (BMS) on cefepime is currently available in the published literature" in correspondence with us at the time we conducted the original review and asked the company for additional data (personal correspondence, available upon request from the authors). In published trials (including complementary data on mortality supplied by authors or BMS) mortality was significantly higher with cefepime, RR 1.26 [1.08, 1.46]. In new trials, previously unavailable to the FDA nor published in conference proceedings or elsewhere, mortality was significantly lower with cefepime, RR 0.77 [0.61, 0.98] (Leibovici 2010). The combined results showing that cefepime is not associated with higher mortality is suspect.
The FDA's analysis of cefepime for febrile neutropenia, was based on a meta‐analysis of trials including cefepime versus any other antibiotic, with or without an aminoglycoside in one or both study arms (FDA 2009) and showed no statistically significant difference for cefepime versus comparators (risk difference 9.67/1000 episodes [‐2.87, 22.21]) (FDA 2010). Thus, cefepime was approved for its indication of monotherapy for febrile neutropenia based on an analysis that included trials assessing it in combination with aminoglycosides. The current analysis of monotherapy trials only, including FDA's data on 30‐day mortality by intention to treat, shows higher mortality with cefepime (Analysis 1.2). The FDA entitled their study "meta‐analysis of a possible signal of increased mortality associated with cefepime use". It should be noted that association is inferred from observational studies; randomized controlled trials can establish causality. The level of evidence for this finding is high, because it is based on the results of randomized controlled trials; there is no inconsistency in results; the evidence directly answers the healthcare question we posed; publication bias is unlikely given our systematic search and the FDA's analysis of the sponsor's trials; and a dose‐response relationship was observed (Guyatt 2008).
The reasons for increased mortality with cefepime remain unexplained. The FDA conducted a patient‐level analysis of a set of trials selected by the sponsor. There were no significant differences in baseline patient characteristics explaining the increased mortality among patients assigned to cefepime, but comparative clinical data relevant to cancer patients was missing (malignancy status unknown in 87% of the febrile neutropenia trials). A detailed assessment of the circumstances leading to death in seven febrile neutropenia trials failed to identify a common cause. However, It is very difficult to identify the cause for death in cancer patients with fever and neutropenia retrospectively (Bodey 1992). Recent reports have described non‐convulsive status epilepticus among patients treated with cefepime, mainly among patients with impaired renal function (Lichaa 2010; Martin Herrera 2009; Shaheen 2009; Thabet 2009). A recent study showed that free drug concentrations above MIC of cefepime are required >60% of time for microbiological cure of Pseudomonas aeruginosa infections that are prevalent among neutropenic patients (Crandon 2010). The authors stated that cefepime doses of at least 2 g every 8 h are required to achieve this target against susceptible P. aeruginosa organisms in patients with normal renal function. In another study, conventional dose and intermittent administration of cefepime at 2gr twice daily achieved only 54% and 28% of the target drug concentrations against P. aeruginosa and Acinetobacter baumannii, respectively (Roos 2006). Thus, two explanations for the increased mortality may exist; inadequate dosing/ administration schedule or an adverse event that was not documented in randomized controlled trials. However, the most likely cause for increased mortality among patients with febrile neutropenia is lack of antibacterial efficacy and our ability to assess this outcome is limited.
Authors' conclusions
Implications for practice.
The decision of the beta‐lactam used for the treatment of febrile neutropenia is dependent on local epidemiology and susceptibility patterns in the ward or hospital (Rolston 2006). Our review provides evidence on the comparative efficacy and toxicity of the available beta‐lactams when administered in a given epidemiological setting. Based on this review, cefepime should not be used for patients with febrile neutropenia. Piperacillin‐tazobactam should be the preferred beta‐lactam for use as single agent, with or without a glycopeptide, in settings where resistance of Gram‐negative bacteria to piperacillin‐tazobactam is not prevalent (lower than 25%). Ceftazidime, imipenem and meropenem can be used, considering that the carbapenems are associated with a higher rate of antibiotic‐associated and Clostridium difficile‐associated diarrhea. The decision whether to used a lower‐spectrum beta‐lactam (with or without an antipseudomonal spectrum of coverage) combined with an aminoglycoside to increase the spectrum of coverage or to select a broader‐spectrum beta‐lactam alone is difficult. Evidence shows that mortality is lower with the broader‐spectrum beta‐lactam and nephrotoxicity is significantly higher with combination therapy (Paul 2003; Paul 2004).
Implications for research.
Future trials assessing antibiotic treatment for febrile neutropenia should report outcomes that are important to the individual patient and for decision making. All cause mortality should be reported by intention to treat in all trials and also as‐treated in non‐inferiority trials (Piaggio 2006). Clinical failure, as currently defined, is not an appropriate surrogate outcome since it does not portend different in all‐cause mortality. Patients should be included only once in the trial or results reported for patients' first randomization, since analyses relying on episodes are incorrect. The outcomes of bacterial and fungal superinfections should be defined using established and replicable definitions. While definitions for fungal infections have been specifically devised (De Pauw 2008), definitions for other infections, including catheter‐related infections, have not been defined specifically for neutropenic patients. We suggest that the CDC/NHSN definitions for healthcare associated infections be used (CDC/NHSN 2008). Length of hospital stay is a highly relevant outcome to the individual patient and should be reported.
All randomized trials conducted should be registered in accessible trial registries and their results should be made publicly available if not published, to avoid situations such the one described herein for cefepime. As for cefepime, full data on trial methods, trial locations, inclusion criteria, patient characteristics and outcomes for the unpublished trials included in the FDA's analysis should be reported. Given the alternative antibiotics currently available, we see no further need or justification for trials assessing its efficacy/ safety among neutropenic cancer patient.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 11, 2010
| Date | Event | Description |
|---|---|---|
| 26 February 2014 | Amended | Contact details updated. |
| 18 November 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the authors who provided supplemental data for their trials (Table 1).
1. Additional data from authors or unpublished sources.
| Study ID | Contact with authors | Unpublished data | Data obtained | |
| Anaissie 1988 | No | No | No data requested, old study | u |
| Aoun 1997 +V | No | Yes | All data obtained from NDA document: study AI411‐198. Additional data requested from Bristol‐Myers Squibb without response. | |
| Aparicio 1996 | Yes | Yes | Randomisation procedures and blinding, infection‐related mortality. | |
| Bauduer 2001 | No | No | Data requested, no contact with author | |
| Bickers 1990 | Yes | No | Author sent a book chapter which served as primary publication. | |
| Biron 1998 | No | No | All necessary data obtained from primary publication | |
| Bodey 1990 | No | No | No data requested, old study | |
| Bodey 1996 | Yes | No | Requested mortality data, which were no longer available | |
| Bohme 1998 | No | No | Data requested, no contact with author | |
| Boogaerts 1995 | Yes | Yes | Author supplied randomisation methods and timing of outcome collection | |
| Bow 2003 | Yes | Yes | All data from conference proceedings. Authos supplied all‐cause mortality data and randomisation procedures. | |
| Chandrasekar 2000 | Yes | Yes | Author supplied randomisation methods. Full data obtained from NDA document: study AI411‐204. | |
| Cherif 2004 | Yes | Yes | Author supplied randomisation methods and overall mortality data | |
| Chuang 2002 | No | No | Data requested, no contact with author | |
| Corapcioglu 2006 | No | No | Data requested, no contact with author | |
| Cornely 2001 | Yes | Yes | Author supplied overall mortality data | |
| Feld 2000 | Yes | No | Asked about randomisation procedures, author replied that data with AstraZeneca | |
| Figuera 2001 | No | No | Data requested, no contact with author | |
| Fleishhack 2001 | Yes | Yes | Randomisation procedures and blinding. | |
| Freifeld 1995 | No | No | Requested mortality by intention to treat and randomisation procedures, author replied that data are no longer available | |
| Ghalaut 2007 | No | No | Data requested, no contact with author | |
| Glauser 1997 | No | Yes | All data obtained from NDA document: study AI411‐189. Additional data requested from Bristol‐Myers Squibb without response. | |
| Harter 2006 | No | No | Data requested, no contact with author | |
| Kebudi 2001 | Yes | Yes | Mortality data, all randomisation procedures, microbiological data, and drug modifications. | |
| Kutuk 2004 | Yes | Yes | Randomisation procedures and blinding, number of patients randomised and infection‐related mortality. | |
| Kwon 2008 | Yes | Yes | Author supplied data on randomization generation and mortality | |
| Liang 1990 | Yes | Yes | Randomisation procedures and blinding. No further data available. | |
| Lindblad 1998 | No | No | Data requested, no contact with author | |
| Mustafa 2001 | No | Yes | Data in publication complemented with unpublished data found in new drug approval (NDA) document: study AI411‐131. | |
| Oguz 2006 | Yes | Yes | Author supplied data on randomization generation and mortality | |
| Oppenheim 2000 | Yes | Yes | Author sent results presented in a conference. Consequently, the trial was terminated early due to problems with drug supply and never published. | |
| Raad 1996 | No | No | No data requested | |
| Raad 2003 | Yes | Yes | Randomisation generation and all‐cause 30‐day mortality | |
| Ramphal 1997 | Yes | Yes | Author sent a book chapter describing part of the study. Full data obtained from NDA document: study AI411‐131. Additional data requested from Bristol‐Myers Squibb without response. | |
| Reich 2005 | No | No | Data requested, no contact with author | |
| Rolston 1992 | Yes | No | Requested mortality data, which were no longer available | |
| Shah 2006 | Yes | Yes | Author supplied randomisation methods | |
| Shichmanter 2004 | Yes | Yes | Randomisation procedures and mortality. | |
| Tamura 2002 | Yes | Yes | Mortality by intention to treat, all randomisation procedures. | |
| Uygun 2009 | Yes | Yes | Author supplied data on randomization generation and mortality | |
| Vandercam 2000 | Yes | Yes | Denominator for mortality, all randomisation procedures, re‐entries. | |
| Vural 2010 | No | No | Data requested, author will try get the data | |
| Wang 1999 | Yes | Yes | Author supplied overall mortality data | |
| Winston 1998 | No | No | Data requested, no contact with author |
All pharmaceutical companies sponsoring included studies were contacted with a request for complementary data on studies we identified, and data regarding further un‐identified/ unpublished studies. Pfizer (cefoperazone‐sulbactam) responded that an exhaustive search of all records and data was performed. No further studies were identified. Data for identified studies were not in possession of the company. GlaxoSmithKline (ceftazidime) conducted and sent us the results of an exhaustive search on all trials assessing ceftazidime for febrile neutropenia. No new studies were identified. Bristol‐Myers Squibb responded no data were available to the company (see discussion).
Appendices
Appendix 1. Search Srategy
| #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 |
randomized controlled trial [pt] controlled clinical trial [pt] randomized [tiab] placebo [tiab] drug therapy [sh] randomly [tiab] trial [tiab] groups [tiab] #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 animals [mh] not (humans [mh] and animals [mh]) #9 not #10 |
Data and analyses
Comparison 1. Cefepime vs. other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 1.1 ceftazidime | 9 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.99, 2.33] |
| 1.2 carbapenem | 8 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.94] |
| 1.3 piperacillin‐tazobactam | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.74, 3.13] |
| 2 All‐cause mortality FDA data | 21 | 3550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.03, 1.83] |
| 2.1 ceftazidime | 9 | 1203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.99, 2.26] |
| 2.2 carbapenem | 8 | 1548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.70, 1.90] |
| 2.3 piperacillin‐tazobactam | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.74, 3.13] |
| 3 All‐cause mortality ‐ sensitivity analysis allocation concealment | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 3.1 adequate | 9 | 1869 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.18, 2.56] |
| 3.2 unclear | 9 | 1382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.68] |
| 3.3 inadequate | 3 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.95] |
| 4 All‐cause mortality ‐ sensitivity analysis allocation generation | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 4.1 adequate | 10 | 1978 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.22, 2.64] |
| 4.2 unclear | 7 | 1233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.56] |
| 4.3 inadequate | 4 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.92] |
| 5 All‐cause mortality ‐ sensitivity analysis blinding | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 5.1 open | 15 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.76, 1.76] |
| 5.2 outcome assessor blinded | 5 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.89, 2.51] |
| 5.3 double blind | 1 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.01, 4.16] |
| 6 All‐cause mortality ‐ sensitivity analysis intention to treat | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 6.1 intention to treat | 16 | 2435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.05, 2.09] |
| 6.2 per protocol | 5 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.64, 2.07] |
| 7 All‐cause mortality ‐ sensitivity analysis publication status (data) | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 7.1 published data | 6 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.14, 3.22] |
| 7.2 unpublished data | 15 | 2515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.82, 1.70] |
| 8 All‐cause mortality ‐ sensitivity analysis publication status (publication) | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 8.1 published paper | 15 | 2013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.02, 2.25] |
| 8.2 unpublished paper | 6 | 1458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.81, 1.94] |
| 9 All‐cause mortality ‐ sensitivity analysis dosing | 21 | 3471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.04, 1.86] |
| 9.1 recommended dose ‐ allocation concealment A | 7 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.08, 2.50] |
| 9.2 recommended dose ‐ allocation concealment B/C | 9 | 1422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.55] |
| 9.3 less than recommended dose | 5 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.92, 4.77] |
| 10 Infection related mortality | 17 | 2632 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.74, 1.80] |
| 10.1 ceftazidime | 7 | 951 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.07] |
| 10.2 carbapenem | 5 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.33, 2.18] |
| 10.3 piperacillin‐tazobactam | 5 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.69, 3.62] |
| 11 Clinical failure | 21 | 3028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
| 11.1 ceftazidime | 9 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 11.2 carbapenem | 7 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.11] |
| 11.3 piperacillin‐tazobactam | 5 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.27] |
| 12 Clinical failure ‐ allocation concealment sensitivity analysis | 21 | 3078 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 12.1 adequate | 9 | 1783 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.15] |
| 12.2 unclear | 9 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 12.3 inadequate | 3 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.73, 1.52] |
| 13 Failure for clinically documented infections | 11 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.35] |
| 13.1 ceftazidime | 5 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 13.2 carbapenem | 5 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.85, 1.62] |
| 13.3 piperacillin‐tazobactam | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 14 Microbiological failure | 15 | 670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.15] |
| 14.1 ceftazidime | 7 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
| 14.2 carbapenem | 5 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.45] |
| 14.3 piperacillin‐tazobactam | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.37] |
| 15 Any modifications | 10 | 983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| 15.1 ceftazidime | 7 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.16] |
| 15.2 carbapenem | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 piperacillin‐tazobactam | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.75, 1.26] |
| 16 Glycopeptide addition | 10 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.11] |
| 16.1 ceftazidime | 5 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.75, 1.31] |
| 16.2 carbapenem | 2 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.90] |
| 16.3 piperacillin‐tazobactam | 3 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.79, 1.64] |
| 17 Antifungal drug addition | 9 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.22] |
| 17.1 ceftazidime | 4 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.63] |
| 17.2 carbapenem | 3 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.31, 1.08] |
| 17.3 piperacillin‐tazobactam | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.66] |
| 18 Superinfection ‐ any | 7 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| 18.1 ceftazidime | 5 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.52, 1.49] |
| 18.2 carbapenem | 2 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.40, 1.74] |
| 18.3 piperacillin‐tazobactam | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Superinfection ‐ bacterial | 5 | 780 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.94, 3.09] |
| 19.1 ceftazidime | 3 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.93, 4.87] |
| 19.2 carbapenem | 2 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.56, 3.23] |
| 19.3 piperacillin‐tazobactam | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Adverse events ‐ any | 13 | 1879 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.98] |
| 20.1 ceftazidime | 7 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 20.2 carbapenem | 5 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.56, 0.91] |
| 20.3 piperacillin‐tazobactam | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.67] |
| 21 Adverse events requiring discontinuation | 16 | 2355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.87, 2.22] |
| 21.1 ceftazidime | 8 | 1062 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.92, 2.59] |
| 21.2 carbapenem | 4 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.21, 2.82] |
| 21.3 piperacillin‐tazobactam | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.18] |
1.10. Analysis.
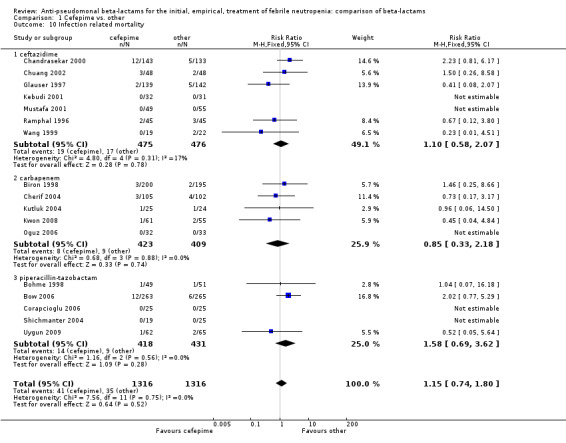
Comparison 1 Cefepime vs. other, Outcome 10 Infection related mortality.
1.13. Analysis.
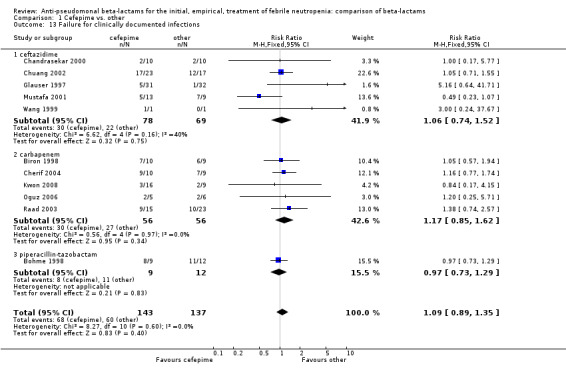
Comparison 1 Cefepime vs. other, Outcome 13 Failure for clinically documented infections.
1.18. Analysis.
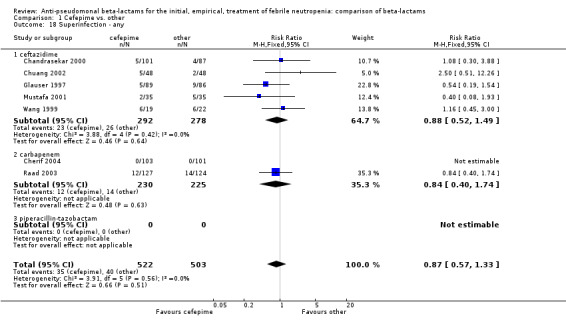
Comparison 1 Cefepime vs. other, Outcome 18 Superinfection ‐ any.
Comparison 2. Ceftazidime vs. other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 19 | 3335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.13] |
| 1.1 cefepime | 10 | 1146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.01] |
| 1.2 carbapenem | 8 | 2028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.65, 1.86] |
| 1.3 piperacillin‐tazobactam | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.07, 18.47] |
| 2 Infection related mortality | 17 | 3476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.46] |
| 2.1 cefepime | 7 | 951 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.48, 1.73] |
| 2.2 carbapenem | 8 | 2025 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.04] |
| 2.3 piperacillin | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.20, 2.68] |
| 2.4 piperacillin‐tazobactam | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.07, 18.47] |
| 3 Clinical failure | 21 | 4204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 3.1 cefepime | 9 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 3.2 carbapenem | 9 | 2340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.07, 1.26] |
| 3.3 ticarcillin‐clavulanate | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.17] |
| 3.4 piperacillin | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.38, 0.78] |
| 3.5 piperacillin‐tazobactam | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Clinical failure ‐ sensitivity analysis allocation concealment | 21 | 4204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 4.1 adequate | 10 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 4.2 unclear | 11 | 2461 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.15] |
| 5 Clinical failure ‐ sensitivity analysis allocation generation | 21 | 4204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 5.1 adequate | 14 | 2837 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 5.2 unclear | 6 | 1327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.00, 1.19] |
| 5.3 inadequate | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.55, 2.32] |
| 6 Microbiological failure | 18 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 6.1 cefepime | 7 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.34] |
| 6.2 carbapenem | 8 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 6.3 ticarcillin‐clavulanate | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.22] |
| 6.4 piperacillin | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.59] |
| 6.5 piperacillin‐tazobactam | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.28] |
| 7 Any modifications | 15 | 2527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.02, 1.18] |
| 7.1 cefepime | 7 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.15] |
| 7.2 carbapenem | 7 | 1654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.07, 1.26] |
| 7.3 piperacillin‐tazobactam | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 8 Glycopeptide addition | 12 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.05, 1.41] |
| 8.1 cefepime | 5 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.77, 1.33] |
| 8.2 carbapenem | 6 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.17, 1.76] |
| 8.3 piperacillin‐tazobactam | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 9 Antifungal drug addition | 10 | 2012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| 9.1 cefepime | 4 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.32] |
| 9.2 carbapenem | 6 | 1485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 10 Superinfection ‐ any | 12 | 2783 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.95, 1.52] |
| 10.1 cefepime | 5 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.68, 1.93] |
| 10.2 carbapenem | 5 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.91, 1.60] |
| 10.3 ticarcillin‐clavulanate | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.70] |
| 10.4 piperacillin | 1 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.64, 4.35] |
| 11 Superinfection ‐ bacterial | 4 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.31] |
| 11.1 cefepime | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| 11.2 carbapenem | 1 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.10, 2.90] |
| 11.3 piperacillin | 1 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.45] |
| 12 Adverse events ‐ any | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 cefepime | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.15] |
| 12.2 carbapenem | 3 | 1118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.60] |
| 12.3 ticarcillin‐clavulanate | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.57, 3.89] |
| 12.4 piperacillin | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.15, 0.58] |
| 13 Adverse events requiring discontinuation | 14 | 2384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.47, 1.03] |
| 13.1 cefepime | 8 | 1062 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.39, 1.09] |
| 13.2 carbapenem | 6 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.41, 1.41] |
2.10. Analysis.
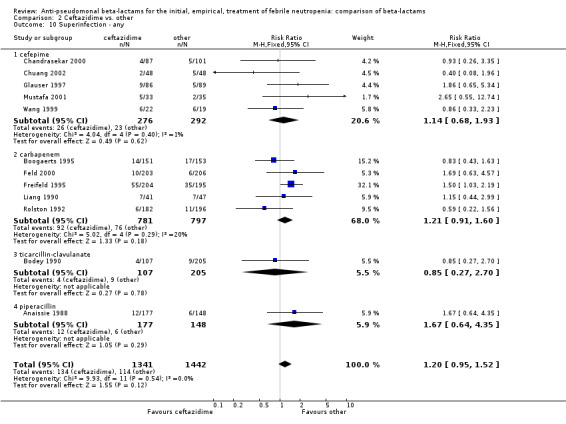
Comparison 2 Ceftazidime vs. other, Outcome 10 Superinfection ‐ any.
Comparison 3. Piperaciilin‐tazobactam vs. other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 8 | 1314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.34, 0.92] |
| 1.1 cefepime | 3 | 755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.34] |
| 1.2 carbapenem | 4 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.22, 0.95] |
| 1.3 ceftazidime | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.05, 13.36] |
| 2 All‐cause mortality ‐ sensitivity analysis allocation concealment | 8 | 1314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.34, 0.92] |
| 2.1 adequate | 2 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| 2.2 unclear | 4 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 0.99] |
| 2.3 inadequate | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.18, 20.51] |
| 3 Infection related mortality | 8 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.29, 1.34] |
| 3.1 cefepime | 5 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.45] |
| 3.2 carbapenem | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.77] |
| 3.3 ceftazidime | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.05, 13.36] |
| 4 Clinical failure | 11 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.06] |
| 4.1 cefepime | 5 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.01] |
| 4.2 carbapenem | 5 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.24] |
| 4.3 ceftazidime | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.48] |
| 5 Clinical failure ‐ sensitivity analysis allocation concealment | 11 | 1726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 5.1 adequate | 3 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| 5.2 unclear | 5 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 5.3 inadequate | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.32] |
| 6 Microbiological failure | 6 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.89, 1.41] |
| 6.1 cefepime | 3 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.73, 3.11] |
| 6.2 carbapenem | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 6.3 ceftazidime | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.78, 1.85] |
| 7 Any modifications | 7 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.19] |
| 7.1 cefepime | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.33] |
| 7.2 carbapenem | 3 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.22] |
| 7.3 ceftazidime | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.48] |
| 8 Glycopeptide addition | 7 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 8.1 cefepime | 3 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 8.2 carbapenem | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.74, 1.18] |
| 8.3 ceftazidime | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.48] |
| 9 Antifungal drug addition | 5 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.93, 1.58] |
| 9.1 cefepime | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.93] |
| 9.2 carbapenem | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.94, 1.70] |
| 10 Adverse events ‐ any | 4 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.65] |
| 10.1 cefepime | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 10.2 carbapenem | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.23, 0.65] |
| 11 Adverse events requiring discontinuation | 5 | 636 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 11.1 cefepime | 4 | 537 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 11.2 carbapenem | 1 | 99 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
Comparison 4. Carbapenem vs. other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 22 | 4431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.55] |
| 1.1 ceftazidime | 8 | 2028 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.54] |
| 1.2 cefepime | 8 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.37] |
| 1.3 cefoperazone‐sulbactam | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.42, 3.34] |
| 1.4 piperacillin‐tazobactam | 4 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.05, 4.57] |
| 1.5 aztreonam | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.77 [1.07, 13.23] |
| 2 All‐cause mortality ‐ sensitivity analysis allocation concealment | 22 | 4431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.55] |
| 2.1 adequate | 10 | 2169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.65] |
| 2.2 unclear | 11 | 2213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.83] |
| 2.3 inadequate | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 3 Infection related mortality | 17 | 3469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.60, 1.52] |
| 3.1 ceftazidime | 8 | 2025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.85] |
| 3.2 cefepime | 5 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.46, 3.00] |
| 3.3 cefoperazone‐sulbactam | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.06, 1.31] |
| 3.4 piperacillin‐tazobactam | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.65] |
| 3.5 aztreonam | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.42, 7.03] |
| 4 Clinical failure | 25 | 5159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.89, 1.00] |
| 4.1 ceftazidime | 9 | 2340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.80, 0.94] |
| 4.2 cefepime | 7 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 4.3 cefoperazone‐sulbactam | 3 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.99, 1.43] |
| 4.4 piperacillin‐tazobactam | 5 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.07] |
| 4.5 aztreonam | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.07] |
| 5 Clinical failure ‐ sensitivity analysis allocation concealment | 24 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.01] |
| 5.1 adequate | 11 | 2447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.08] |
| 5.2 unclear | 12 | 2378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.01] |
| 5.3 inadequate | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.30] |
| 6 Microbiological failure | 18 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.08] |
| 6.1 ceftazidime | 8 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 6.2 cefepime | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.77, 1.32] |
| 6.3 cefoperazone‐sulbactam | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.52, 2.47] |
| 6.4 piperacillin‐tazobactam | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.35] |
| 6.5 aztreonam | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.34, 1.17] |
| 7 Any modifications | 10 | 1968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.96] |
| 7.1 ceftazidime | 7 | 1656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 7.2 cefoperazone‐sulbactam | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.37] |
| 7.3 piperacillin‐tazobactam | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.22] |
| 8 Glycopeptide addition | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 ceftazidime | 6 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.86] |
| 8.2 cefepime | 2 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.12, 2.59] |
| 8.3 cefoperazone‐sulbactam | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.71, 1.71] |
| 8.4 piperacillin‐tazobactam | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.04, 2.00] |
| 9 Antifungal drug addition | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 ceftazidime | 7 | 1585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 9.2 cefepime | 3 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.93, 3.23] |
| 9.3 cefoperazone‐sulbactam | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.13, 3.39] |
| 9.4 piperacillin‐tazobactam | 3 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.01] |
| 10 Superinfection ‐ any | 10 | 2905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.12] |
| 10.1 ceftazidime | 5 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.09] |
| 10.2 cefepime | 2 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.48] |
| 10.3 cefoperazone‐sulbactam | 2 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.59, 1.58] |
| 10.4 aztreonam | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.34, 3.11] |
| 11 Superinfection ‐ fungal | 6 | 1586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.81] |
| 11.1 ceftazidime | 2 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.48, 5.34] |
| 11.2 cefepime | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.30, 5.83] |
| 11.3 cefoperazone‐sulbactam | 2 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.30, 1.75] |
| 11.4 aztreonam | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.34] |
| 12 Superinfection ‐ bacterial | 6 | 1705 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.49, 1.41] |
| 12.1 ceftazidime | 1 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.34, 10.02] |
| 12.2 cefepime | 2 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.31, 1.79] |
| 12.3 cefoperazone‐sulbactam | 2 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.70] |
| 12.4 aztreonam | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.34] |
| 13 Adverse events ‐ any | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 meropenem vs. other | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.29, 2.01] |
| 13.2 imipenem vs. other | 10 | 2901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.44, 1.96] |
| 13.3 Combined/other vs. other | 3 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.70, 1.82] |
| 14 Adverse events requiring discontinuation | 11 | 2213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.68, 1.90] |
| 14.1 meropenem vs. other | 5 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.70, 2.61] |
| 14.2 imipenem vs. other | 5 | 912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 1.99] |
| 14.3 Combined/other vs. other | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adverse events ‐ seizures | 11 | 2966 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.05, 7.42] |
| 15.1 meropenem vs. other | 2 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.52] |
| 15.2 imipenem vs. other | 8 | 2268 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [1.10, 9.18] |
| 15.3 Combined/other vs. other | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Adverse events ‐ pseudomembranous colitis | 7 | 2408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.16, 2.77] |
| 16.1 cehalosporin +/‐ beta‐lactamase | 6 | 2025 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.24, 3.04] |
| 16.2 aztreonam | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.03] |
| 17 Adverse events ‐ gastrointestinal other than PMC | 14 | 3484 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.63, 2.46] |
| 17.1 piperacillin‐tazobactam | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.84, 2.09] |
| 17.2 cehalosporin +/‐ beta‐lactamase | 11 | 2922 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.93, 3.14] |
| 17.3 aztreonam | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.28, 1.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anaissie 1988.
| Methods | RCT Assessor blinded 1985‐1985 | |
| Participants | Number: 339 episodes Age: med 42, range 16‐85 | |
| Interventions | piperacillin 4grX6 + vancomycin 1grX2 versus ceftazidime 1grX6 + vancomycin 1grX2 A third arm not included in this review assessed all 3 antibiotics combined. | |
| Outcomes | Infection‐related mortality ‐ at 7 days after end of treatment Clinical and microbiological failure Superinfection Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated sequence |
| Allocation concealment? | Low risk | No description, but previous trials in the center were conducted using the pharmacy as the center of randomization and allocation was disclosed only after patients' entered the trial |
| Blinding? All outcomes | High risk | Response to therapy was evaluated by an investigator who was not involved in the patient's care and who was unaware of the regimen used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (10%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; Patients to episodes ratio 0.63 |
Aoun 1997.
| Methods | RCT Open 1993‐1994 | |
| Participants | Number: 111 patients (128 episodes) Age: mean 48.8 SD 14.9 | |
| Interventions | cefepime 2grx3 + vancomycin 30mg/kg/d versus ceftazidime 2grx3 + vancomycin 30mg/kg/d | |
| Outcomes | Overall mortality ‐ at 30 days after end of treatment Clinical and microbiological failure Adverse events | |
| Notes | MC Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomly assigned by a computer‐generated sequence of numbers to receive one of the three regimens |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Response to therapy was evaluated by an investigator who was not involved in the patient's care and who was unaware of the regimen used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (6%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; Patients to episodes ratio 0.87 |
Aparicio 1996.
| Methods | RCT Open 1991‐1994 | |
| Participants | Number: 102 patients (118 episodes) Age: med58, range 15‐75 | |
| Interventions | ceftazidime 2grx3 versus imipenem 500mgx4 | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomization code was generated by computer |
| Allocation concealment? | Low risk | The allocation was performed centrally by telephone call to our secretariat |
| Blinding? All outcomes | High risk | No blinding were used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 13% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (13%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.86 |
Bickers 1990.
| Methods | RCT Open | |
| Participants | Number: 135 patients Age: mean 41 | |
| Interventions | cefoperazone‐sulbactam 2gr/1grx2 versus imipenem 500mgx4 | |
| Outcomes | Overall and infection‐related mortality ‐ at 7 days after end of treatment Clinical failure Drug modifications Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | Low risk | Unit of randomization = patients |
Biron 1998.
| Methods | RCT Assessor blinded 1995‐1996 | |
| Participants | Number: 380 patients (400 episodes) Age: No data | |
| Interventions | cefepime 2grx2 versus imipenem 1grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 7 days after end of treatment Clinical and microbiological failure Adverse events | |
| Notes | MC France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomized using a centralized computer network system, stratified by centre and by first or second inclusion |
| Allocation concealment? | Low risk | Centralized computer network system |
| Blinding? All outcomes | High risk | All 400 cases were reviewed by the study steering committee in a blinded fashion |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 1% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (17%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.95 |
Bodey 1990.
| Methods | RCT Assessor blinded 1985‐1986 | |
| Participants | Number: 352 episodes Age: mean 46, range 15‐82 | |
| Interventions | ticalcillin/clavulanate 4gr/0.1grX6 + vancomycin 1grX2 versus ceftazidime 1grX6 + vancomycin 1grX2 A third arm not included in this review assessed all 3 antibiotics combined. | |
| Outcomes | Clinical and microbiological failure Superinfection Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated sequence |
| Allocation concealment? | Low risk | No description, but previous trials in the center were conducted using the pharmacy as the center of randomization and allocation was disclosed only after patients' entered the trial |
| Blinding? All outcomes | High risk | Response to therapy was evaluated by an investigator who was not involved in the patient's care and who was unaware of the regimen used |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Mortality not reported in study |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (11%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.86 |
Bodey 1996.
| Methods | RCT Assessor blinded 1990‐1993 | |
| Participants | Number: 407 patients (457 episodes) Age: med 51, range 17‐84 | |
| Interventions | imipenem 500mg/m2x versus cefoperazone 2gr sulbactam 1grx3 | |
| Outcomes | Clinical and microbiological failure Superinfection Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated sequence |
| Allocation concealment? | Low risk | Patients were randomly assigned by the pharmacy and randomization was performed by the pharmacy after the patient was entered |
| Blinding? All outcomes | High risk | The physicians caring for the patients were aware of the antibiotic regimen assigned. Evaluation was conducted by investigators not involved in the patient's care and who were blinded to the therapeutic regimen and identity of the patient. |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Mortality not reported |
| Incomplete outcome data addressed? Treatment failure | High risk | 5% dropouts |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.89 |
Bohme 1998.
| Methods | RCT Open 1996‐1996 | |
| Participants | Number: 88 patients (102 episodes) Age: med 45, range 18‐68 | |
| Interventions | piperacillin‐tazobactam 4.5grx3 versus cefepime 2grx3 | |
| Outcomes | Overall and infection‐related mortality Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were centrally randomized by the physician on duty in the emergency room not participating in the study |
| Allocation concealment? | Low risk | Patients were centrally randomized by the physician on duty in the emergency room not participating in the study |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 2% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (2%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.86 |
Boogaerts 1995.
| Methods | RCT Open 1991‐1994 | |
| Participants | Number: 248 patients Age: mean 42, range 14‐78 | |
| Interventions | meropenem 1grx3 versus ceftazidime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical and microbiological failure Superinfection Drug modifications | |
| Notes | MC Europe | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Allocation to one or the other regimen was achieved by an investigator opening the next in a sequence of sealed envelopes containing numbers generated by a computer in each center. sealed envelopes, opaque not mentioned. |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 10% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (10%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.73 |
Bow 2006.
| Methods | RCT Open | |
| Participants | Number: 528 patients Age not given | |
| Interventions | piperacillin‐tazobactam 4.5grx4 versus cefepime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 7 days following end of treatment Clinical and microbiological failure Drug modifications | |
| Notes | MC USA,Canada,Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | Low risk | Unit of randomization = patients |
Chandrasekar 2000.
| Methods | RCT Triple‐blind 1993‐1994 | |
| Participants | Number: 276 patients (315 episodes) Age: med 56, range 18‐82 | |
| Interventions | cefepime 2grx3 versus ceftazidime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 30 days following end of treatment Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | MC USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence. Patients were stratified and randomized based on whether they had a solid tumor or hematologic malignancy. |
| Allocation concealment? | Low risk | Central |
| Blinding? All outcomes | Low risk | Triple blind: patient, carer and an independent reviewer, blinded with respect to treatment assignment assigned clinical responses |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (7%) known per study group |
| Free of other bias? | Low risk | |
Cherif 2004.
| Methods | RCT Open 1996‐1998 | |
| Participants | Number: 180 patients (207 episodes) Age: med 55, range 16‐83 | |
| Interventions | cefepime 2grx3 versus imipenem 500mgx4 | |
| Outcomes | Overall and infection‐related mortality ‐ at 30 days after recruitment Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | MC Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomized study with stratification by center |
| Allocation concealment? | Low risk | Sealed envelopes, opaque not mentioned. |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (1%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.87 |
Chuang 2002.
| Methods | RCT Open 2000‐2001 | |
| Participants | Number: 95 patients (120 episodes) Age: mean 5.5, range 0.3‐15.5 | |
| Interventions | cefepime 25mg/kgx2 versus ceftazidime 25mg/kgx2 | |
| Outcomes | Overall and infection‐related mortality ‐ at 30 days after end of treatment Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | Taiwan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomized in a 1:1 ratio and stratified by type of underlying malignancy |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 20% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (3%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.79 |
Corapcioglu 2006.
| Methods | RCT Open 2004‐2005 |
|
| Participants | Number: 28 patients (50 episodes) Age: med 8.4 ± SD 5.2 | |
| Interventions | piperacillin‐tazobactam 80+10mg/kgX4 versus cefepime 50mg/kgX3 |
|
| Outcomes | Infection‐related mortality (at least 7 days after discontinuation of treatment) Clinical failure, fever duration Drug modifications Adverse events |
|
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Consecutive randomization |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | All‐cause mortality not assessed |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.56 |
Cornely 2001.
| Methods | RCT | |
| Participants | Number: 602 patients (? number randomized unknown) Age: adults | |
| Interventions | meropenem 1grX3 versus cefepime 2grX3 An addition arm not included in this review assessed piperacillin‐tazobactam + aminoglycoside | |
| Outcomes | Overall mortality. Other outcomes given only as percentages in a conference presentation without denominators, | |
| Notes | MC Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomized multicenter in blocks, block size six |
| Allocation concealment? | Low risk | Sealed envelopes, opaque not mentioned. |
| Blinding? All outcomes | High risk | No description |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Number of randomised patients unknown |
| Incomplete outcome data addressed? Treatment failure | Unclear risk | Outcome not reported |
| Free of other bias? | Low risk | Unit of randomization = patients |
Feld 2000.
| Methods | RCT Double‐blind 1991‐1993 | |
| Participants | Number: 411 patients (471 episodes) Age: mean 48.5; range 17‐85 | |
| Interventions | meropenem 1grx3 versus ceftazidime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ 7 days following end of treatment Clinical failure Treatment modifications Superinfections Adverse events | |
| Notes | MC (US, Canada, Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were allocated at random for each neutropenic episode. Blinded drug was distributed based on a schedule that provided a stratified, balanced, block random assignment within each center, stratification was by the presence or absence of prophylactic treatment with antiviral medication. As patients were enrolled for specific neutropenic episodes, they were assigned the next available number and associated randomized treatment |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | Low risk | Double‐blind |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 2% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (13%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.87 |
Figuera 2001.
| Methods | RCT Open 1994‐1996 | |
| Participants | Number: 150 patients Age: mean 38.3; range 16‐73 | |
| Interventions | imipenem 500mgX4 versus piperacillin‐tazobactam 4.5grX4 | |
| Outcomes | Overall mortality ‐ at end of treatment Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 9% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | 9% dropouts |
| Free of other bias? | Low risk | |
Fleischhack 2001.
| Methods | RCT Open 1998‐2000 | |
| Participants | Number: 169 patients (375 episodes) Age: med 7.4, range 0.3‐32.6 | |
| Interventions | meropenem 60mg/kgx3 versus ceftazidime 100mg/kgx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 7 days after end of treatment Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | MC Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation was based on the sequential drawing of sealed envelopes, which had been centrally prepared beforehand in balanced blocks of four groups for each center. Three stratification variables were used: the center, the intensity of chemotherapy and age. |
| Allocation concealment? | Low risk | Sealed envelopes, opaque not mentioned. |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 9% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | 9% dropouts |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.45 |
Freifeld 1995.
| Methods | RCT Open 1986‐1990 | |
| Participants | Number: 501 episodes Age: mean 32, range 4‐80 | |
| Interventions | ceftazidime 30mg/kgx3 versus imipenem 15mg/kgx4 or 12.5mg/kgx4 < 12y | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical failure Superinfection Drus modifications Adverse events | |
| Notes | MC USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 20% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (20%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; number of patients unknown |
Ghalaut 2007.
| Methods | RCT Open |
|
| Participants | Number: 40 patients Age: cefepime mean33.25± SD 15.95, ceftazidime 41.45±18.33 |
|
| Interventions | cefepime 1‐2mgX3 versus ceftazidime 0.5‐2grX3 |
|
| Outcomes | All‐cause mortality Clinical failure Drug modifications |
|
| Notes | India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Forty patients "were divided into 2 groups of 20 patients each |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | Low risk | Unit of randomization = patients |
Glauser 1997.
| Methods | RCT Assessor blinded 1993‐1995 | |
| Participants | Number: 281 patients (324 episodes) Age: med52, mean 50.1, SD 16.6 | |
| Interventions | cefepime 2grx3 versus ceftazidime 2grx3 Additional glycopeptides administered to <1% of patients (in both arms). | |
| Outcomes | Overall and infection‐related mortality ‐ at 30 days after end of treatment Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | MC Europe | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Only outcome assessor blinded |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (4%) known per study group |
| Free of other bias? | Low risk | |
Harter 2006.
| Methods | RCT Open 2000‐2003 |
|
| Participants | Number: 219 patients (243 episodes) Age: ceftazidime median 57, range (21–70); piperacillin‐tazobactam 53 (19–71) |
|
| Interventions | ceftazidime 2grX3 versus piperacillin‐tazobactam 4.5grX3 |
|
| Outcomes | All‐cause and infection‐related mortality Clinical and microbiological failure Drug modifications Adverse events |
|
| Notes | Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randmization list |
| Allocation concealment? | Low risk | Central in the pharmacy |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Number randomized unclear |
| Incomplete outcome data addressed? Treatment failure | Unclear risk | Number randomized unclear |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.90 |
Kebudi 2001.
| Methods | RCT Open 1998‐1998 | |
| Participants | Number: 33 patients (63 episodes) Age: med 7, range 1m‐14y | |
| Interventions | ceftazidime 33mg/kgX3 versus cefepime 50mg/kgX3 | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical failure Drug modifications Adverse events | |
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central in statistics department |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.52 |
Kutluk 2004.
| Methods | RCT single blind ‐ patients | |
| Participants | Number: 30 patients (63 episodes) Age: mean 7.4 SD 3.74 | |
| Interventions | meropenem 20mg/kg x 3 versus cefepime 50mg/kg x 3 | |
| Outcomes | Overall and infection‐related mortality Clinical failure Drug modifications | |
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Patients were randomised according to the order of their admission ‐ alternation upon arrival to department. |
| Allocation concealment? | High risk | Alternation |
| Blinding? All outcomes | High risk | Only the patient was blinded |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.61 |
Kwon 2008.
| Methods | RCT Open 2004‐2005 |
|
| Participants | Number: 116 patients Age: panipenem mean 51.4 +/‐ SD 13.2, cefepime 52.8+/‐13 |
|
| Interventions | Panipenem/betamipron 0.5/0.5 gX3 versus Cefepime 2grX2 |
|
| Outcomes | Overall and infection‐related mortality Clinical and microbiological failure, fever duration Adverse events |
|
| Notes | Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | On the hospital's web‐site (after informed consent was obtained) |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | Low risk | |
Liang 1990.
| Methods | RCT Open 1988‐1989 | |
| Participants | Number: 92 patients (103 episodes) Age: mean 38, range 16‐76 | |
| Interventions | ceftazidime 1grx3 versus imipenem 500mg x 4 | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | Hong‐Kong | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 3% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | 3% dropouts |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.89 |
Lindblad 1998.
| Methods | RCT Open | |
| Participants | Number: 192 patients Age: mean 60, range 17‐85 | |
| Interventions | meropenem 1grX3 versus ceftazidime 2grX3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 72 hours Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | MC Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomly assigned to receive one of the study drugs by sequentially opening sealed envelopes, which had been prepared centrally beforehand in balanced blocks for each center. |
| Allocation concealment? | Low risk | Sealed envelopes (opaque not mentioned) |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 3% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (3%) known per study group |
| Free of other bias? | Low risk | |
Mustafa 2001.
| Methods | RCT Assessor blinded | |
| Participants | Number: 104 patients (149 episodes) Age: med 6, range 5m‐19y | |
| Interventions | cefepime 50mg/kg x 3 versus ceftazidime 50mg/kg x 3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 30 days after end of treatment Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Response was determined by an independent blinded reviewer |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (11%) known per study group |
| Free of other bias? | Low risk | |
Oguz 2006.
| Methods | RCT Open 2003‐2004 |
|
| Participants | Number: 37 patients (65 episodes) Age: cefepime mean 11.16 ± SD 3.64; meropenem 11.15 ± 2.57 |
|
| Interventions | cefepime 50 mg/kgX3 versus meropenem 20 mg/kgX3 |
|
| Outcomes | All cause and infection‐related mortality (at least 7 days after discontinuation of treatment) | |
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated list |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.57 |
Oppenheim 2000.
| Methods | RCT Open | |
| Participants | Number: 80 episodes Age: mean 45.6 | |
| Interventions | piperacillin‐tazobactam 4.5grX3 versus meropenem 1grX3 | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical and microbiological failure | |
| Notes | UK Study terminated early because of problems with supply of one of the study drugs and therefore unpublished. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 2% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (2%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; number of patients unknown |
Oztoprak 2010.
| Methods | RCT Open |
|
| Participants | Number: 127 episodes, 84 patients (120 episodes assessed) Age: mean 45.6 | |
| Interventions | piperacillin‐tazobactam 4.5grX3 versus meropenem 1grX3 or imipenem 500mgX4 | |
| Outcomes | Overall mortality at 30 days
Clinical failure Adverse events |
|
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated random‐number program |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | Mortality data for all patients, but reported per episode and not per patient's first episode |
| Incomplete outcome data addressed? Treatment failure | High risk | 5% dropouts |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.66. Mortality reported per patient. |
Raad 1996.
| Methods | RCT Outcome assessor blinded 1993‐1994 |
|
| Participants | Number: 390 patients Age: imipenem med 52 range (17‐82); aztreonam 48 (18‐81) |
|
| Interventions | Imipenem 500mgX4 + vancomycin 1grX2 versus Aztreonam 2grX4 + vancomycin 1grX2 |
|
| Outcomes | All‐cause and infection‐related mortality Clinical and microbiological failure Bacterial and fungal superinfections Adverse events |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central in hospital's pharmacy, treatment assignment unknown to investigator in charge of determining eligibility |
| Blinding? All outcomes | High risk | Only outcome assessor blinded |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 23% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | 23% dropouts |
| Free of other bias? | Low risk | |
Raad 2003.
| Methods | RCT Assessor blinded 1996‐2001 | |
| Participants | Number: 251 patients Age: med 55, range: 15‐84 | |
| Interventions | cefepime 2grX3 versus imipenem 500mgX4 In addition, glycopeptides added to 25% and aminoglycosides to 6% of patients (in both study arms). | |
| Outcomes | Overall mortality ‐ at 30 days following end of treatment Clinical and microbiological failure Superinfection Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation performed by a computer generated number scheme |
| Allocation concealment? | Low risk | No description, but previous trials in the center were conducted using the pharmacy as the center of randomization and allocation was disclosed only after patients' entered the trial |
| Blinding? All outcomes | High risk | Randomization was done in an evaluator‐blind fashion. The treatment assignments remained unknown to the investigators in charge of determining eligibility, establishing infectious diagnosis, and evaluating outcome and adverse events throughout the study |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | Low risk | |
Ramphal 1996.
| Methods | RCT Open 1989‐1991 | |
| Participants | Number: 90 patients (104 episodes) Age: med41.5, mean 43.5, SD 14.6 | |
| Interventions | cefepime 2grx3 versus ceftazidime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 30 days after end of treatment Clinical and microbiological failure Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | For each study site, the Biostatistics and Data Management group at Bristol‐Myers Squibb provided to the investigator computer‐generated randomization schedules and sequentially‐numbered, sealed envelopes containing a card with the treatment assignment. The sealed envelopes were maintained in the pharmacy at the study site. At the time the subject provided informed consent, the next available sealed envelope was opened by the pharmacist and the treatment assignment was provided to the physician responsible for the subject. |
| Allocation concealment? | Low risk | Sequentially‐numbered, sealed envelopes kept centrally and opened only after informed consent obtained |
| Blinding? All outcomes | High risk | A blinded review of the efficacy was performed by a consultant for the sponsor |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (2%) known per study group |
| Free of other bias? | Low risk | |
Reich 2005.
| Methods | RCT Open 1997‐2001 |
|
| Participants | Number: 236 patients Age: meropenem med 44 range (18–70); piperacillin‐tazobactam 44 (20–63) |
|
| Interventions | meropenem 1grX3 versus piperacillin‐tazobactam 4.5grX3 |
|
| Outcomes | Clinical failure, fever duration | |
| Notes | MC Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Low risk | Sealed envelopes attached to sequentially numbered patient documentation folders (opaque not mentioned) |
| Blinding? All outcomes | High risk | No blinding used |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Outcome not reported |
| Incomplete outcome data addressed? Treatment failure | High risk | 2% dropouts |
| Free of other bias? | Low risk | |
Rolston 1992.
| Methods | RCT Assessor blinded 1986‐1988 | |
| Participants | Number: number randomised unknown; 378 episodes assessed Age: med 46, range: 16‐84 | |
| Interventions | imipenem 12.5mg/kg x 4 versus ceftazidime 1grx6 | |
| Outcomes | Clinical and microbiological failure Superinfection Drug modifications Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomly assigned using a computer‐generated sequence of numbers |
| Allocation concealment? | Low risk | No description, but previous trials in the center were conducted using the pharmacy as the center of randomization and allocation was disclosed only after patients' entered the trial |
| Blinding? All outcomes | High risk | Response to therapy was evaluated by an investigator who was not involved in the patient's care and who was unaware of the regimen used |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Mortaliy not reported in study |
| Incomplete outcome data addressed? Treatment failure | Unclear risk | Number randomized not specified |
| Free of other bias? | High risk | Unit of randomization = episodes; number of patients unknown |
Shah 1996.
| Methods | RCT Open 1992‐1994 |
|
| Participants | Number: 61 patients (66 episodes) Age: imipenem mean 8.6 range (1‐28); meropenem 9.8 (1‐22) |
|
| Interventions | imipenem/cilastatin 1grX3 | |
| Outcomes | meropenem 1grX3 | |
| Notes | Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Sealed opaque envelopes used |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.92 |
Shichmanter 2004.
| Methods | Quasi‐randomised trial (4‐armed trial, only 2 arms used for the current review) Open 2000‐2002 | |
| Participants | Number: 44 patients Age: mean 52, SD 13.6 | |
| Interventions | piperacillin‐tazobactam 4.5grX3 versus cefepime 2grX2 | |
| Outcomes | Infection‐related mortality Clinical failure Drug modifications | |
| Notes | Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | According to patient's room |
| Allocation concealment? | High risk | According to patient's room |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | Low risk | |
Tamura 2002.
| Methods | RCT Open 1999‐2000 |
|
| Participants | Number: 83 patients (the trial included a third arm with 82 patients that received combination therapy and was excluded from this review) Age: mean 52.4 | |
| Interventions | cefepime 1‐2grx2 versus carbapenem (imipenem, panipenem, or meropenem) 0.5‐1grX2 | |
| Outcomes | Overall mortality ‐ at 30 days after end of treatment Clinical failure Adverse events | |
| Notes | MC Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by table of random numbers |
| Allocation concealment? | Low risk | Sealed envelopes, opaque not mentioned |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | number of dropouts (7%) known per study group |
| Free of other bias? | Low risk | |
Uygun 2009.
| Methods | Quasi‐randomized trial Open 2005‐2006 |
|
| Participants | Number: 69 patients (127 episodes) Age: median 4.2 range (0.3‐18) |
|
| Interventions | piperacillin‐tazobactam 80+10mg/kgX4 vs cefepime 50mg/kgX3 |
|
| Outcomes | All‐cause and infection‐related mortality (at end of hospital stay) Clinical and microbiological failure, fever duration and hospital stay Drug modifications Adverse events |
|
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternation |
| Allocation concealment? | High risk | |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.54 |
Vandercam 2000.
| Methods | RCT 1992‐1994 | |
| Participants | Number: 101patients (121 episodes) Age: mean 53, range 17‐86 | |
| Interventions | meropenem 1gx3 versus ceftazidime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at end of treatment Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Sealed opaque envelopes used |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (9%) known per study group |
| Free of other bias? | Low risk | Unit of randomization = episodes (patient re‐entry discontinued during the trial) ; patients to episodes ratio 0.83 |
Vural 2010.
| Methods | RCT Open 2005‐2006 |
|
| Participants | Number: 63 patients (99 episodes) Age: med 5 range (2‐14) |
|
| Interventions | piperacillin‐tazobactam 80+10mg/kgX4 versus imipenem‐cilastatin 15mg/mgX4 |
|
| Outcomes | All‐cause and infection‐related mortality (during the neutropenic episode) Clinical failure Drug modifications Adverse events |
|
| Notes | Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Low risk | |
| Incomplete outcome data addressed? Treatment failure | Low risk | |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.64 |
Wang 1999.
| Methods | RCT Open 1996‐1997 | |
| Participants | Number: 38 patients (45 episodes) Age: mean 44, SD 16 | |
| Interventions | cefepime 2grx 3 versus ceftazidime 2grx3 | |
| Outcomes | Overall and infection‐related mortality ‐ at 14 days after end of treatment Clinical and microbiological failure Drug modifications Adverse events | |
| Notes | Taiwan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | High risk | 9% dropouts |
| Incomplete outcome data addressed? Treatment failure | High risk | Number of dropouts (9%) known per study group |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.84 |
Winston 1998.
| Methods | RCT Open | |
| Participants | Number: 209 patients (213 episodes) Age: med 43, range 16‐81 | |
| Interventions | sulbactam/cefoperazone 2gr/4grx2 versus imipenem 500mgx4 | |
| Outcomes | Clinical and microbiological failure Superinfection Adverse events | |
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description |
| Allocation concealment? | Unclear risk | No description |
| Blinding? All outcomes | High risk | Blinding not used |
| Incomplete outcome data addressed? All‐cause mortality | Unclear risk | Mortaliy not reported in study |
| Incomplete outcome data addressed? Treatment failure | Unclear risk | Denominator refers to sites of infection rather than patient or episode |
| Free of other bias? | High risk | Unit of randomization = episodes; patients to episodes ratio 0.98. Unit used for analysis sites of infection |
RCT ‐ randomised controlled trial (see table of study quality assessment for further details)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauduer 2001 | Aminoglycoside |
| Bodey 1989 | A review |
| Borja 1999 | Correspondence ‐ in response to Winston 1998. Does not add any information |
| Cometta 1995 | Aminoglycoside in both arms |
| Cometta 1996 | A review |
| Cordonnier 1997 | Aminoglycoside in both arms |
| Cornely 2002 | Aminoglycoside in both arms |
| Deaney 1996b | Meta‐analysis |
| Erman 2001 | Aminoglycoside in both arms |
| Fanci 1992 | Aminoglycosides allowed only in one study arm. This is a preliminary report from a larger multicenter study comparing imipenem/ cilastatin (Tienam) versus ceftazidime. Addition of amikacin empirically was allowed by protocol only in the ceftazidime arm. |
| Gomez 2001 | Aminoglycoside in both arms |
| Hoepelman 1993 | Neutropenic patients were excluded from the study |
| Kieft 1994 | Neutropenic patients were excluded from the study |
| Kuye 1993 | A review summarising phase I and III clinical trials of piperacillin‐tazobactam |
| Longree 2001 | Aminoglycoside in both arms |
| Malik 2002 | Comparison with a historic group (49 pts prospectively received meropenem and compared with a 50 pts who received ceftazidime in the immediate past), |
| Marra 1998 | Double‐blind randomised controlled trial conducted as a formulary feasibility study in Canada. 150 patients prescribed imipenem, with or without other medications, meeting inclusion criteria were randomised to imipenem versus piperacillin‐tazobactam. The prescription was for febrile neutropenia in 30 patients, other patients were not neutropenic. We tried to contact the corresponding author unsuccessfully, to obtain outcome data for the subgroup of febrile neutropenic patients. Lacking these data, we excluded this study. |
| Moroni 1987 | Compares ceftazidime‐amikacin vs ceftazidime‐vanco |
| Papachristodoulou 96 | Compares ceftazidime 6g/d versus ceftazidime 6g/d+amikacin 1g/d |
| Rolston 1991 | Non neutropenic patients |
| Sanz 2002 | Aminoglycoside in both arms |
| Smyth 2002 | Pharmacoeconomic study comparing between piperacillin‐tazobactam and meropenem on the randomized population included in Oppenheim 2000. |
| Sumitani 2007 | pharmacokinetics/pharmacodynamics theory model, without a clinical study |
| Tanindi 1994 | Reference could not be obtain. Unknown whether this comparative study is randomized |
| Varthalitis 1996 | RCT comparing cefepime 2gx2 versus cefepime 2gx3 as empiric monotherapy for neutropenic fever. Doesn't compare cefepime to another drug |
| Zarostsky 2001 | Meta‐analysis of trials assessing cefepime infebrile neutropenia |
Characteristics of studies awaiting assessment [ordered by study ID]
Ma 2003.
| Methods | Randomized trial |
| Participants | Neutropenic fever during early phase post stem cell transplantation |
| Interventions | Meropenem versus imipenem/cilastatin |
| Outcomes | Success rate, deaths, adverse events |
| Notes | Report of an abstract from conference. Number of patients randomized/ evaluated not given. Outcomes are shown as percentages only. Authors contacted for full data. |
Differences between protocol and review
In an original version of the protocol we intended to include all beta‐lactams, including antibiotics without an anti‐pseudomonal spectrum of coverage. In a subsequent revised version we limited the review to the comparison of antipseudomonal beta‐lactams.
Contributions of authors
Wrote the protocol: Mical Paul, Dafna Yahav, Abigail Fraser, Leonard Leibovici
Searched for studies and data extraction: Mical Paul, Assaf Bivas, Dafna Yahav
Write the final review: Mical Paul
Revision of the final review: All authors
Sources of support
Internal sources
COCHRANE GYNAECOLOGICAL CANCER REVIEW GROUP, UK.
External sources
No sources of support supplied
Declarations of interest
None declared
Edited (no change to conclusions)
References
References to studies included in this review
Anaissie 1988 {published data only}
- Anaissie EJ, Fainstein V, Bodey GP, Rolston K, Elting L, Kantarjian H, et al. Randomized trial of beta‐lactam regimens in febrile neutropenic cancer patients. American Journal of Medicine 1988;84(3 Pt 2):581‐9. [DOI] [PubMed] [Google Scholar]
Aoun 1997 {unpublished data only}
- Aoun M, Boogaerts MA, Bosly AJ, Schots H. A multicenter, randomized, prospective, comparative study of cefepime plus vancomycin versus ceftazidime plus vancomycin as empiric therapy in the treatment of febrile episodes in granulocytopenic patients with hematological malignancies. Joint clinical/statistical review of NDA 50,679/SE1‐002: study AI411‐198 1997:122‐32.
- Breen J, Ramphal R, Cometta A, Conetta B, Nicaise C. Cefepime versus Ceftazidime as empiric therapy of febrile episodes in neutropenic patients. In: Klastersky J editor(s). Febrile neutropenia. Berlin: Springer‐Verlag, 1997:63‐74. [Google Scholar]
Aparicio 1996 {published and unpublished data}
- Aparicio J, Gomez‐Aldaravi L, Pastor M, Munarriz B, Gomez J, Maestu, et al. Randomized comparison of Ceftazidime and Imipenem in oncologic patients with neutropenic fever [Comparacion aleatorizada de Ceftazidima e Imipenem en pacientes oncologicos con fiebre neutropenica]. Oncologia. IV Congreso Nacional de la SEOM. 1993; Vol. 16, issue 6 ‐ congreso:248.
- Aparicio J, Montalar J, Gomez‐Aldaravi L, Pastor M, Gomez‐Codina J, Herranz C. Ceftazidime versus imipenem as initial monotherapy in oncologic patients with fever and neutropenia: preliminary results of a randomized trial. Neoplasia 1993;10(4):122‐25. [Google Scholar]
- Aparicio J, Oltra A, Llorca C, Montalar J, Herranz C, Gomez‐Codina J, et al. Randomised comparison of ceftazidime and imipenem as initial monotherapy for febrile episodes in neutropenic cancer patients. European Journal of Cancer 1996;32A(10):1739‐43. [DOI] [PubMed] [Google Scholar]
Bickers 1990 {published data only (unpublished sought but not used)}
- Bickers J, Gumbart C, Cavalier J, Vial R, Ostroske DR, Veith R. A prospective randomized trial of cefoperazone plus sulbactam versus imipenem‐cilastatin in febrile neutropenic patients. Clinical Research. 1990; Vol. 38:991A.
- Bickers J, Gumbart C, Vial R. A prospective randomized trial of cefoperazone plus sulbactam versus imipenem‐cilastatin in febrile, neutropenic patients. Program and abstracts of the 18th international congress of chemotherapy. Stockholm, 1993; Vol. abs 569:217.
Biron 1998 {published data only}
- Biron P, Fuhrmann C, Cure H, Viens P, Lefebvre D, Thyss A, et al. Cefepime versus imipenem‐cilastatin as empirical monotherapy in 400 febrile patients with short duration neutropenia. CEMIC (Study Group of Infectious Diseases in Cancer). Journal of Antimicrobial Chemotherapy 1998;42(4):511‐18. [DOI] [PubMed] [Google Scholar]
- Biron P, Fuhrmann C, Kheder K, Viens P, Lefebvre D, Beal J, et al. Cefepime (CEP) vs Imipenem‐cilastatin (IMP) as empiric monotherapy in 400 febrile patients with short duration neutropenia. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Ontario, Canada, 1997.
- Fuhrmann C, Beal J, Escande MC, Lebrun‐Jezekova D, Lefebre D, Lesimple T, et al. Cepemine (CEP) vs Imipenem‐cilastatin (IMP) as empiric monotherapy in 400 febrile patients with short duration neutropenia. Supportive Care in Cancer 1998;6 Suppi(Abs No 74):323. [Google Scholar]
Bodey 1990 {published data only}
- Bodey GP, Fainstein V, Elting LS, Anaissie E, Rolston K, Khardori N, et al. Beta‐lactam regimens for the febrile neutropenic patient. Cancer 1990;65(1):9‐16. [DOI] [PubMed] [Google Scholar]
Bodey 1996 {published data only (unpublished sought but not used)}
- Bodey G, Abi‐Said D, Rolston K, Raad I, Whimbey E. Imipenem or cefoperazone‐sulbactam combined with vancomycin for therapy of presumed or proven infection in neutropenic cancer patients. European Journal of Clinical Microbiology and Infectious Diseases 1996;15(8):625‐34. [DOI] [PubMed] [Google Scholar]
Bohme 1998 {published data only}
- Bohme A, Shah PM, Stille W, Hoelzer D. Cefepime (CEFP) versus piperacillin/tazobactam (PIP/TAZ) as initial antimicrobial therapy in febrile granulocytopenic patients. Annals of Hematology 1996;73(Suppl 2):A44. [Google Scholar]
- Bohme A, Shah PM, Stille W, Hoelzer D. Piperacillin/tazobactam versus cefepime as initial empirical antimicrobial therapy in febrile neutropenic patients: a prospective randomized pilot study. European Journal of Medical Research 1998;3(7):324‐30. [PubMed] [Google Scholar]
Boogaerts 1995 {published and unpublished data}
- Boogaerts MA, Demuynck H, Mestdagh N, Verbist L, Goldstone AH, Kelsey HC, et al. Equivalent efficacies of meropenem and ceftazidime as empirical monotherapy of febrile neutropenic patients. Journal of Antimicrobial Chemotherapy 1995;36(1):185‐200. [DOI] [PubMed] [Google Scholar]
- De‐Pauw B, Donnely P, Boogaerts MA, Mesidagh N, Machin SJ, Goldstone AH, et al. Meropenem or ceftazidime empiric therapy for febrile neutropenic adults. 32nd Intersciense Confernce on Antimicrobial Agents and Chemotherapy. Anaheim, California, USA, 1992; Vol. Abs No 1693.
- Favero A, Bucaneve G, Menichetti F. Empiric monotherapy in neutropenia: a realistic goal?. Scandinavian journal of infectious diseases. Supplementum 1995;96:34‐37. [PubMed] [Google Scholar]
Bow 2006 {published and unpublished data}
- Bow EJ, Noskin GA, Schwarer AP, Laverdiere M, Vesole DH. Efficacy of Piperacillin/Tazobactam as Initial Empiric Therapy of Febrile Neutropenia in Patients with Hematologic Malignancy. 45th Annual Meeting of the Americal Society of Hematology. San Diego, 2003:Abstract no. 1000.
- Bow EJ, Rotstein C, Noskin GA, Laverdiere M, Schwarer AP, Segal BH, et al. A randomized, open‐label, multicenter comparative study of the efficacy and safety of piperacillin‐tazobactam and cefepime for the empirical treatment of febrile neutropenic episodes in patients with hematologic malignancies. Clinical Infectious Diseases 2006;43(4):447‐59. [DOI] [PubMed] [Google Scholar]
- Bow EJ, Schwarer AP, Laverdiere M, Segal BH, Anaissie E. Efficacy of Piperacillin/Tazobactam as Initial Empiric Therapy of Febrile Neutropenia in Patients with Hematologic Malignancy. 43th Interscience conferemce on antimicrobials agents and chemotherapy. Chicago: American Society for Microbiology, 2003:Abstract no. L‐114.
- Noskin GA, Vesole D, Szer J, Sanche S, McCarthy AE. Annual Meeting of the Infectious Diseases Society of America. San Diego, 2003:Abstract no. 373.
Chandrasekar 2000 {published and unpublished data}
- Breen J, Ramphal R, Cometta A, Conetta B, Nicaise C. Cefepime versus Ceftazidime as empiric therapy of febrile episodes in neutropenic patients. In: Klastersky J editor(s). Febrile neutropenia. Berlin: Springer‐Verlag, 1997:63‐74. [Google Scholar]
- Chandrasekar PH, Arnow PM. Cefepime versus ceftazidime as empiric therapy for fever in neutropenic patients with cancer. The Annals of Pharmacotherapy 2000;34(9):989‐95. [DOI] [PubMed] [Google Scholar]
- Hathorn J, Chandrasakar P, Baird I, Arnow P, Waskin A. Double‐blind Comparison of Cefepime vs Ceftazidime to Treat Febrile Neutropenia. 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. 1996.
- Hathorn JW, Chandrasekar PH, Baird I, Arnow P, Velasquez W, Heimenz J. A double‐blind randomized trial of cefepime versus ceftazidime fort the empirical treatment of febrile episodes in neutropenic cancer patients. Joint clinical/statistical review of NDA 50,679/SE1‐002: study AI411‐204 1997:26‐50.
Cherif 2004 {published and unpublished data}
- Cherif H, Bjorkholm M, Engervall P, Johansson P, Ljungman P, Hast R, et al. A prospective, randomized study comparing cefepime and imipenem‐cilastatin in the empirical treatment of febrile neutropenia in patients treated for haematological malignancies. Scandinavian Journal of Infectious Diseases 2004;36(8):593‐600. [DOI] [PubMed] [Google Scholar]
- Cherif H, Engervall P, Bjorkholm M, Kalin M. Comparison of cefepime and imipenem‐cilastatin in the empirical treatment of febrile neutropenia in patients treated for haematological malignancies: a prospective, randomised, multicentre study [abstract]. 8th Congress of the European Hematology Association 2003. Lyon, France, 2003; Vol. abs no. 854.
Chuang 2002 {published data only (unpublished sought but not used)}
- Chuang YY, Hung IJ, Yang CP, Jaing TH, Lin TY, Huang YC. Cefepime versus ceftazidime as empiric monotherapy for fever and neutropenia in children with cancer. Pediatric Infectious Diseases Journal 2002;21(3):203‐209. [DOI] [PubMed] [Google Scholar]
Corapcioglu 2006 {published data only (unpublished sought but not used)}
- Corapcioglu F, Sarper N, Zengin E. Monotherapy with piperacillin/tazobactam versus cefepime as empirical therapy for febrile neutropenia in pediatric cancer patients: a randomized comparison. Pediatric Hematology and Oncology 2006;23(3):177‐186. [DOI] [PubMed] [Google Scholar]
Cornely 2001 {unpublished data only}
- Cornely OA, Reichert D, Buchheidt D, Maschmeyer G, Wilhelm M, Schiel X, et al. Three‐Armed Multicenter Randomized Study on the Empiric Treatment of neutropenic Fever in a High Risk Patient Population (PEG Study III). In ICAAC. Chicago; 2001. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, 2001:P446.
- Maschmeyer G. Cefepime in the initial treatment of high‐risk febrile neutropenic patients [Cefepim in der empirischen Initial therapie bei febrilen neutropenischen Patienten mit mignen erkankungen]. Chemotherapie Journal 2004;13:174‐80. [Google Scholar]
Feld 2000 {published data only (unpublished sought but not used)}
- Pauw B. Initial empiric monotherapy with metropenem or ceftazidime in high risk cancer patients with febrile neutropenia. Supportive Care in Cancer 2000;8 Suppl(Abstr. 49):157. [Google Scholar]
- Feld R, Pauw B, Berman S, Keating A, Ho W. Meropenem (MEM) Versus Ceftazidime (CAZ) for Initial Empiric Therapy in Cancer Patients with Febrile Neutropenia: Results of a Double‐Blind, Randomized, Multicenter Trial. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, California, 1999; Vol. Abs No. 1091:722.
- Feld R, Pauw B, Berman S, Keating A, Ho W. Meropenem versus ceftazidime in the treatment of cancer patients with febrile neutropenia: a randomized, double‐blind trial. Journal of Clinical Oncology 2000;18(21):3690‐98. [DOI] [PubMed] [Google Scholar]
Figuera 2001 {published data only (unpublished sought but not used)}
- Figuera A, Rivero N, Pajuelo F, Font P, Leyra F, De‐la‐Camara R, Arranz R, et al. Initial empiric therapy of febrile neutropenia with antibiotic monotherapy. Piperacillin/tazobactam versus imipenem/cilastatin (1994‐1996). Medicina Clinica 2001;116(16):610‐11. [DOI] [PubMed] [Google Scholar]
Fleischhack 2001 {published and unpublished data}
- Fleischhack G, Hartmann C, Simon A, Wulff B, Havers W, Marklein G, et al. Meropenem versus ceftazidime as empirical monotherapy in febrile neutropenia of paediatric patients with cancer. Journal of Antimicrobial Chemotherapy 2001;47(6):841‐53. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Fleischhack G, Wulff B, Havers W, Hasan C, Bode U. A randomized comparison of ceftazidime versus meropenem in febrile neutropenia in pediatric cancer patients. Supportive Care in Cancer 2000;8 suppl(Abs No 48):156. [Google Scholar]
Freifeld 1995 {published data only (unpublished sought but not used)}
- Fallon J, Rubin M, Hathorn J, Callender D, Albano E, Walsh TJ, et al. Is a carbapenem as effective as a 3rd generation cephalosporin when used as monotherapy in the empiric treatment of the febrile neutropenic patient?. 27th Interscience conference on Antimicrobial Agents Chemotherapy. Washington DC, 1987; Vol. Abs No. 1254.
- Freifeld AG, Walsh T, Marshall D, Gress J, Steinberg SM, Hathorn J, et al. Monotherapy for fever and neutropenia in cancer patients: a randomized comparison of ceftazidime versus imipenem. Journal of Clinical Oncology 1995;13(1):165‐76. [DOI] [PubMed] [Google Scholar]
- Rubin M, Pizzo PA. Monotherapy for empirical management of febrile neutropenic patients. National Cancer Institute Monographies 1990;9:111‐6. [PubMed] [Google Scholar]
Ghalaut 2007 {published data only (unpublished sought but not used)}
- Ghalaut PS, Chaudhry U, Ghalaut VS, Aggarwal S, Sood V, Dixit G. Cefepime versus ceftazidime as empirical therapy for fever in neutropenic patients with haematological malignancies. Indian Journal of Hematology and Blood Transfusion 2007;23(3‐4):104‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glauser 1997 {unpublished data only}
- Breen J, Ramphal R, Cometta A, Conetta B, Nicaise C. Cefepime versus Ceftazidime as empiric therapy of febrile episodes in neutropenic patients. In: Klastersky J editor(s). Febrile neutropenia. Berlin: Springer‐Verlag, 1997:63‐74. [Google Scholar]
- Glauser M, Dekker AW, Marwijkkooy M, Palmblad J, Wood M, Harper P. A multicenter comperative study of cefepime vs. ceftazidime as empiric therapy in the treatment of febrile episodes in neutropenic patients. Joint clinical/statistical review of NDA 50,679/SE1‐002: study AI411‐189 1997:51‐74.
Harter 2006 {published data only (unpublished sought but not used)}
- Harter C, Schulze B, Goldschmidt H, Benner A, Geiss HK, Hoppe‐Tichy T, et al. Piperacillin/tazobactam vs ceftazidime in the treatment of neutropenic fever in patients with acute leukemia or following autologous peripheral blood stem cell transplantation: a prospective randomized trial. Bone Marrow Transplantation 2006;37(4):373‐79. [DOI] [PubMed] [Google Scholar]
Kebudi 2001 {published and unpublished data}
- Kebudi R, Gorgun O, Ayan I, Gurler N, Akici F, Toreci K. Randomized comparison of cefepime versus ceftazidime monotherapy for fever and neutropenia in children with solid tumors. Medical and Pediatric Oncology 2001;36(4):434‐341. [DOI] [PubMed] [Google Scholar]
Kutluk 2004 {published and unpublished data}
- Kutluk T, Kurne O, Akyuz C, Ceyhan M, Kanra G, Buyukpamukcu M, et al. Cefepime vs. Meropenem as empirical therapy for neutropenic fever in children with lymphoma and solid tumours. Pediatric Blood & Cancer 2004;42(3):284‐86. [DOI] [PubMed] [Google Scholar]
Kwon 2008 {published and unpublished data}
- Kwon KT, Cheong HS, Rhee JY, Wi YM, Ryu SY, Heo ST, et al. Panipenem versus cefepime as empirical monotherapy in adult cancer patients with febrile neutropenia: a prospective randomized trial. Japanese Journal of Clinical Oncology 2008;38(1):49‐55. [DOI] [PubMed] [Google Scholar]
Liang 1990 {published and unpublished data}
- Liang R, Yung R, Chiu E, Chau PY, Chan TK, Lam WK, et al. Ceftazidime versus imipenem‐cilastatin as initial monotherapy for febrile neutropenic patients. Antimicrobial Agents and Chemotherapy 1990;34(7):1336‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindblad 1998 {published data only (unpublished sought but not used)}
- Lindblad R, Rodjer S, Adriansson M, Andreasson B, Backstrom B, Johansson P, et al. Empiric monotherapy for febrile neutropenia‐‐a randomized study comparing meropenem with ceftazidime. Scandinavian Journal of Infectious Diseases 1998;30(3):237‐43. [DOI] [PubMed] [Google Scholar]
Mustafa 2001 {published and unpublished data}
- Mustafa MM, Carlson L, Tkaczewski I, McCracken GH Jr, Buchanan GR. Comparative study of cefepime versus ceftazidime in the empiric treatment of pediatric cancer patients with fever and neutropenia. Pediatric Infectious Diseases Journal 2001;20(3):362‐69. [DOI] [PubMed] [Google Scholar]
Oguz 2006 {published and unpublished data}
- Oguz A, Karadeniz C, Citak EC, Cil V, Eldes N. Experience with cefepime versus meropenem as empiric monotherapy for neutropenia and fever in pediatric patients with solid tumors. Pediatric Hematoly Oncology 2006;23(3):245‐53. [DOI] [PubMed] [Google Scholar]
Oppenheim 2000 {unpublished data only}
- Oppenheim BA, Morgenstern GR, Chang J. Safety and efficacy of piperacillin/tazobactam versus meropenem in the treatment of febrile neutropenia. Proceeding of the 7th Conference of the Federation of Infection Societies. 2000:page 67, 52.
Oztoprak 2010 {published data only}
- Oztoprak N, Piskin N, Aydemir H, Celebi G, Akduman D, Keskin AS, et al. Piperacillin–tazobactam Versus Carbapenem Therapy With andWithout Amikacin as Empirical Treatment of Febrile Neutropeniain Cancer Patients: Results of an Open Randomized Trialat a University Hospital. Japanese Journal of Clinical Oncology 2010;40(8):761‐67. [DOI] [PubMed] [Google Scholar]
Raad 1996 {published data only}
- Raad II, Whimbey EE, Rolston KV, Abi‐Said D, Hachem RY, Pandya RG, et al. A comparison of aztreonam plus vancomycin and imipenem plus vancomycin as initial therapy for febrile neutropenic cancer patients. Cancer 1996;77(7):1386‐94. [DOI] [PubMed] [Google Scholar]
Raad 2003 {published and unpublished data}
- Raad II, Escalante C, Hachem RY, Hanna HA, Husni R, Afif C, Boktour MR, et al. Treatment of febrile neutropenic patients with cancer who require hospitalization: a prospective randomized study comparing imipenem and cefepime. Cancer 2003;98(5):1039‐47. [DOI] [PubMed] [Google Scholar]
Ramphal 1996 {unpublished data only}
- Breen J, Ramphal R, Cometta A, Conetta B, Nicaise C. Cefepime versus Ceftazidime as empiric therapy of febrile episodes in neutropenic patients. In: Klastersky J editor(s). Febrile Neutropenia. Berlin: Springer‐Verlag, 1997:63‐74. [Google Scholar]
- Ramphal R, Gucalp R, Rotstein C, Cimino M, Oblon D. Clinical experience with single agent and combination regimens in the management of infection in the febrile neutropenic patient. The American Journal of Medicine 1996;100(suppl 6A):83S‐89S. [DOI] [PubMed] [Google Scholar]
- Ramphal R, Haddow A, McCracken GH. A comparative study of cefepime vs. ceftazidime in the treatment of adult cancer patients with fever and neutropenia. Joint clinical/statistical review of NDA 50,679/SE1‐002: study AI411‐131 1997:75‐89.
Reich 2005 {published data only (unpublished sought but not used)}
- Reich G, Cornely OA, Sandherr M, Kubin T, Krause S, Einsele H, et al. Empirical antimicrobial monotherapy in patients after high‐dose chemotherapy and autologous stem cell transplantation: a randomised, multicentre trial. British Journal of Haematology 2005;130(2):265‐70. [DOI] [PubMed] [Google Scholar]
Rolston 1992 {published data only (unpublished sought but not used)}
- Rolston KV, Berkey P, Bodey GP, Anaissie EJ, Khardori NM, Joshi JH, et al. A comparison of imipenem to ceftazidime with or without amikacin as empiric therapy in febrile neutropenic patients. Archives of Internal Medicine 1992;152(2):283‐91. [PubMed] [Google Scholar]
Shah 1996 {published and unpublished data}
- Shah PM, Heller A, Fuhr HG, Walther F, Halir S, Schaumann R, et al. Empirical monotherapy with meropenem versus imipenem/cilastatin for febrile episodes in neutropenic patients. Infection 1996;24(6):480‐84. [DOI] [PubMed] [Google Scholar]
Shichmanter 2004 {unpublished data only}
- Shichmanter R, Miller E, Shtalrid M, Landau Z. Empiric antibiotic treatment of adults with neutropenic fever in a general internal medicine ward. In 14th European Congress of Clinical Microbiology and Infectious Diseases. 14th European Congress of Clinical Microbiology and Infectious Diseases. 2004; Vol. European Society of Clinical Microbiology and Infectious Diseases.
Tamura 2002 {published and unpublished data}
- Tamura K, Matsuoka H, Tsukada J, Masuda M, Ikeda S, Matsuishi E. Cefepime or carbapenem treatment for febrile neutropenia as a single agent is as effective as a combination of 4th‐generation cephalosporin + aminoglycosides: comparative study. American Journal of Hematology 2002;71(4):248‐55. [DOI] [PubMed] [Google Scholar]
Uygun 2009 {published and unpublished data}
- Uygun V, Karasu GT, Ogunc D, Yesilipek A, Hazar V. Piperacillin/tazobactam versus cefepime for the empirical treatment of pediatric cancer patients with neutropenia and fever: a randomized and open‐label study. Pediatric Blood Cancer 2009;53(4):610‐4. [DOI] [PubMed] [Google Scholar]
Vandercam 2000 {published and unpublished data}
- Vandercam B, Gerain J, Humblet Y, Ferrant A, Wauters G, Moreau M, et al. Meropenem versus ceftazidime as empirical monotherapy for febrile neutropenic cancer patients. Annals of Hematology 2000;79(3):152‐57. [DOI] [PubMed] [Google Scholar]
Vural 2010 {published data only (unpublished sought but not used)}
- Vural S, Erdem E, Gulec SG, Yildirmak Y, Kebudi R. Imipenem‐cilastatin versus piperacillin‐tazobactam as monotherapy in febrile neutropenia. Pediatrics International EPUB;EPUB:EPUB. [DOI] [PubMed] [Google Scholar]
Wang 1999 {published and unpublished data}
- Wang FD, Liu CY, Hsu HC, Gau JP, Chau WK, Haung ML, et al. A comparative study of cefepime versus ceftazidime as empiric therapy of febrile episodes in neutropenic patients. Chemotherapy 1999;45(5):370‐79. [DOI] [PubMed] [Google Scholar]
Winston 1998 {published data only (unpublished sought but not used)}
- Winston DJ, Bartoni K, Bruckner DA, Schiller GJ, Territo MC. Randomized comparison of sulbactam/cefoperazone with imipenem as empirical monotherapy for febrile granulocytopenic patients. Clinical Infectious Diseses 1998;26(3):576‐83. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bauduer 2001 {published data only}
- Bauduer F, Cousin T, Boulat O, Attal M, Molina L, Rossi JF. A Randomized Prospective Multicenter Trial of Cefpirome vs. Piperacillin‐Tazobactam in Febrile Neutropenia. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy.. San Francisco, California, 1999; Vol. Abs No 1089:720.
- Bauduer F, Cousin T, Boulat O, Rigal‐Huguet F, Molina L, Fegueux N, et al. A randomized prospective multicentre trial of cefpirome versus piperacillin‐tazobactam in febrile neutropenia. Leukemia and Lymphoma 2001;42(3):379‐86. [DOI] [PubMed] [Google Scholar]
Bodey 1989 {published data only}
- Bodey GP. Evolution of antibiotic therapy for infection in neutropenic patients: studies at MD Anderson Hospital. Reviews of Infectious Diseases 1989;11 Suppl 7:S1582‐S1590. [DOI] [PubMed] [Google Scholar]
Borja 1999 {published data only}
Cometta 1995 {published data only}
- Cometta A, Zinner S, Bock R, Calandra T, Gaya H, Klastersky J, et al. Piperacillin‐tazobactam plus amikacin versus ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Antimicrobial Agents and Chemotherapy 1995;39(2):445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cometta 1996 {published data only}
- Cometta A, Glauser MP. Empiric antibiotic monotherapy with carbapenems in febrile neutropenia: a review. Journal of Chemotherapy 1996;8(5):375‐81. [DOI] [PubMed] [Google Scholar]
Cordonnier 1997 {published data only}
- Cordonnier C, Delmer A, LeBlond V. A multi‐investigator comparative study of cefepime and ceftazidime, in combination with amikacin for the treatment of patients with fever and neutropenia. NDA AI411‐186.
- Cordonnier C, Herbrecht R, Pico JL, Gardembas M, Delmer A, Delain M, et al. Cefepime/amikacin versus ceftazidime/amikacin as empirical therapy for febrile episodes in neutropenic patients: a comparative study. The French Cefepime Study Group. Clinical Infectious Diseases 1997;24(1):41‐51. [DOI] [PubMed] [Google Scholar]
Cornely 2002 {published data only}
- Cornely OA, Bethe U, Seifert H, Breuer K, Schütt‐Gerowitt H, Salzberger B, et al. A randomized monocentric trial in febrile neutropenic patients: ceftriaxone and gentamicin vs. cefepime and gentamicin. Annals of Hematology 2002;81(1):37‐43. [DOI] [PubMed] [Google Scholar]
Deaney 1996b {published data only}
- Deaney NB, Tate H. A meta‐analysis of clinical studies of imipenem‐cilastatin for empirically treating febrile neutropenic patients. Journal of Antimicrobial Chemotherapy 1996;37(5):975‐86. [DOI] [PubMed] [Google Scholar]
Erman 2001 {published data only}
- Erman M, Akova M, Akan H, Korten V, Ferhanoglu B, Koksal I, et al. Comparison of cefepime and ceftazidime in combination with amikacin in the empirical treatment of high‐risk patients with febrile neutropenia: a prospective, randomized, multicenter study. Scandinavian Journal of Infectious Diseases 2001;33(11):827‐31. [PUBMED: 11760163] [DOI] [PubMed] [Google Scholar]
Fanci 1992 {published data only}
- Fanci R, Leoni F, Ciolli S, Caporale R, Longo G, Rossi FP. Randomized assessment of tienam vs. ceftazidime for the empirical therapy of suspected bacterial infections in neutropenic patients: preliminary results. Haematologica 1992;77 Suppl 1:157‐59. [Google Scholar]
Gomez 2001 {published data only}
- Gomez L, Estrada C, Gomez I, Marquez M, Dalmau D, Estany C, et al. Cefepime Plus Amikacin versus Piperacillin‐Tazobactam Plus Amikacin in the Treatment of Patients with Fever and Granulocytopenia. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, DC, USA, 2001:Abstract L‐771, p. 445.
Hoepelman 1993 {published data only}
- Hoepelman AI, Kieft H, Aoun M, Kosmidis J, Strand T, Verhoef J, et al. International comparative study of cefepime and ceftazidime in the treatment of serious bacterial infections. Journal of Antimicrobial Chemotherapy 1993;32 Suppl B:175‐86. [DOI] [PubMed] [Google Scholar]
Kieft 1994 {published data only}
- Kieft H, Hoepelman AI, Rozenberg‐Arska M, Branger JM, Voskuil JH, Geers AB, et al. Cefepime compared with ceftazidime as initial therapy for serious bacterial infections and sepsis syndrome. Antimicrobial Agents and Chemotherapy 1994;38(3):415‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuye 1993 {published data only}
- Kuye O, Teal J, DeVries VG, Morrow CA, Tally FP. Safety profile of piperacillin/tazobactam in phase I and III clinical studies. Journal of Antimicrobial Chemotherapy 1993;31 Suppl A:113‐24. [DOI] [PubMed] [Google Scholar]
Longree 2001 {published data only}
- Longree L, Bock R, Meyer S, et al. A multicenter comparative study of cefepime plus amikacin vs. piperacillin‐tazobactam plus amikacin in the empiric treatment of febrile episodes in neurtopenic cancer patients. CAPTA study group, Belgium. 5th International Meeting on Febrile Neutropenia, Brussels. December 12‐14, 2001.
Malik 2002 {published data only}
- Malik I. Comparison of meropenem with ceftazidime as monotherapy of cancer patients with chemotherapy induced febrile neutropenia. Journal of the Pakistan Medical Association 2002;52(1):15‐18. [PubMed] [Google Scholar]
Marra 1998 {published data only}
- Marra F, Reynolds R, Stiver G, Bryce E, Sleigh K, Frighetto L, MacDougall C, Jewesson P. Piperacillin/tazobactam versus imipenem: a double‐blind, randomized formulary feasibility study at a major teaching hospital. Diagnostic microbiology and infectious disease 1998;31(2):355‐368. [DOI] [PubMed] [Google Scholar]
Moroni 1987 {published data only}
- Moroni C, Viscoli C, Boni L, Garaventa A, Dini G, Haupt R, Bruzzi P, Massimo L, Terragna A. A randomized study of the empirical antibiotic therapy of the infections in neutropenic patients, affected by neoplastic diseases in paediatric age and under a fever attack. Giornale di malattie infettive e parassitarie 1987;39(11):1194‐2008. [Google Scholar]
Papachristodoulou 96 {published data only}
- Papachristodoulou A, Vaslamatzis M, Xynogalos S, Papacharalambous A, Alexopoulos CG. Ceftazidime (CFZ) monotherapy as empirical initial treatment of febrile neutropenia cancer patients (Pts). Annals of Oncology 1996;7(suppl 5):140. [Google Scholar]
Rolston 1991 {published data only}
- Rolston KV, Jones PG, Fainstein V, Khardori NM, Anaissie EJ, Steelhammer L, et al. Single agent therapy for infections in cancer patients: a prospective randomized trial comparing three extended‐spectrum cephalosporins. European Journal of Clinical Microbiology and Infectious Diseases 1991;10(3):139‐45. [DOI] [PubMed] [Google Scholar]
Sanz 2002 {published data only}
- Sanz MA, Lopez J, Lahuerta JJ, Rovira M, Batlle M, Perez C, et al. Cefepime plus amikacin versus piperacillin‐tazobactam plus amikacin for initial antibiotic therapy in haematology patients with febrile neutropenia: results of an open, randomized, multicentre trial. Journal of Antimicrobial Chemotherapy 2002;50(1):79‐88. [DOI] [PubMed] [Google Scholar]
- Sanz MA, Villal L, Lahuerta JJ, Rovira M, Ribera JM, Perez C, et al. Cefepime Plus Amikacin Versus Piperacillin/Tazobactam Plus Amikacin for Initial Antibiotic Therapy in Hematological Patients with Febrile Neutropenia: Results of an Open, Randomized, Multicenter Trial. Abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy. 2000:474.
Smyth 2002 {published data only}
- Smyth ETM, Barr JG, Hogg GM, Oppenheim BA. A pharmacoeconomic evaluation of piperacillin/tazobactam versus meropenem in the treatment of adult febrile neutropenia. Journal of Medical Economics 2002;5:135‐45. [Google Scholar]
Sumitani 2007 {published data only}
- Sumitani Y, Kobayashi Y. Ceftazidime vs cefepime: comparison of two drugs as empirical therapeutic drugs for febrile neutropenia by Monte Carlo simulation method. ECCMID. 2007:Abstract 1722_873.
Tanindi 1994 {published data only}
- Tanindi S, Alpay F, Koseoglu V, Kurekci E, Ozcan O. Comparison of ceftazidime versus sulbactam/cefoperazone monotherapy as empiric therapy for febrile episodes in granulocytopenic children with cancer. Bulletin of Gulhane Military Medical Academy 1994;36(1):1‐6. [Google Scholar]
Varthalitis 1996 {published data only}
- Varthalitis I, Papadopoulou M, Vlachopoulos V, Pectasides D, Minitriadis M, Athanassiou AE. Cefepime (C) monotherapy in febrile patients (PTS) with chemotherapy induced neutropenia (Preliminary results). Annals of Oncology 1996;7(suppl 5):140. [Google Scholar]
Zarostsky 2001 {published data only}
- Zarostsky V, Jaresko GS, Holtom PD. Piperacillin/tazobactam use in febrile neutropenic patients: a meta‐analysis. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, 2001:446.
References to studies awaiting assessment
Ma 2003 {published data only}
- Ma SY, Au WY, Lie AKW, Tse E, Leung AYH, Kwong YL. A prospective randomized trial of meropenem versus imipenem/cilastatin as monotherapy for neutropenic fever during early phase post stem cell transplantation. Blood 2003;102(11):439b (abstract 5484). [Google Scholar]
Additional references
Baskaran 2007
- Baskaran ND, Gan GG, Adeeba K, Sam IC. Bacteremia in patients with febrile neutropenia after chemotherapy at a university medical center in Malaysia. International Journal of Infectious Diseases 2007;11(6):513‐17. [DOI] [PubMed] [Google Scholar]
Bodey 1944
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 1966;64(2):328‐40. [DOI] [PubMed] [Google Scholar]
Bodey 1992
- Bodey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, et al. Fungal infections in cancer patients: an international autopsy survey. European Journal of Clinical Microbiology and Infectious Diseases 1992;11(2):99‐109. [DOI] [PubMed] [Google Scholar]
Burgess 2004
- Burgess DS, Hall RG, 2nd. In vitro killing of parenteral beta‐lactams against standard and high inocula of extended‐spectrum beta‐lactamase and non‐ESBL producing Klebsiella pneumoniae. Diagnostic Microbiology and Infectious Diseases 2004;49(1):41‐6. [DOI] [PubMed] [Google Scholar]
CDC/NHSN 2008
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infectious Control 2008;36(5):309‐32. [DOI] [PubMed] [Google Scholar]
Chen 2009
- Chen CY, Tsay W, Tang JL, Tien HF, Chen YC, Chang SC, et al. Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiology and Infection 2009;EPUB:1‐8. [DOI] [PubMed] [Google Scholar]
Chow 1991
- Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Annals of Internal Medicine 1991;115(8):585‐90. [DOI] [PubMed] [Google Scholar]
Cochrane 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Crandon 2010
- Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy 2010;54(3):1111‐6. [PUBMED: 20038614] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Pauw 2000
- Pauw BE, Donnelly JP. Infections in the immunocompromised Host: General Principles. In:. In: Mandell GL, Bennet JE, Dolin R editor(s). Mandell, Douglas and Bennet's Priciples and Practice of Infectious Diseases. Fifth edition. Philadelphia: Churchil Livingstone, 2000. [Google Scholar]
De Pauw 2008
- Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Mu?oz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 2008;46(12):1813‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐bock 2001
- ‐bock R, Cometta A, Kern WV, Aoun M, Caballero D, Engelhard D, et al. Incidence of Single Agent Gram‐negative Bacteremias (SAGNB)in Neutropenic Cancer Patients (NCP) in EORTC‐IATG Trials of Empirical Therapy for Febrile Neutropenia. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy. 2001:p. 445, abstract n° L‐773.
Deaney 1996
- Deaney NB, Tate H. A meta‐analysis of clinical studies of imipenem‐cilastatin for empirically treating febrile neutropenic patients. Journal of Antimicrobial Chemotherapy 1996;37(5):975‐86. [DOI] [PubMed] [Google Scholar]
ECIL 2009
- International Immunocompromised Host Society, European Conference on Infections in Leukaemic patients. Guidelines for the Management of Bacterial, Fungal and Viral Infections. http://www.ichs.org/ecilslides.htm.
Engels 1998
- Engels EA, Lau J, Barza M. Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta‐analysis. Journal of Clinical Oncology 1998;16(3):1179‐87. [DOI] [PubMed] [Google Scholar]
FDA 2009
- US Food, Drug Administration. Information for Healthcare Professionals: Cefepime. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm167254.htm.
FDA 2010
- Kim PW, Wu YT, Cooper C, Rochester G, Valappil T, Wang Y, et al. Meta‐analysis of a possible signal of increased mortality associated with cefepime use. Clinical Infectious Diseases 2010;51(4):381‐9. [DOI] [PubMed] [Google Scholar]
Feld 2002
- Feld R, Paesmans M, Freifeld AG, Klastersky J, Pizzo PA, Rolston KV, et al. Methodology for clinical trials involving patients with cancer who have febrile neutropenia: updated guidelines of the Immunocompromised Host Society/Multinational Association for Supportive Care in Cancer, with emphasis on outpatient studies. Clinicsl Infectious Diseases 2002;35(12):1463‐8. [DOI] [PubMed] [Google Scholar]
Fritsche 2003
- Fritsche TR, Sader HS, Jones RN. Comparative activity and spectrum of broad‐spectrum beta‐lactams (cefepime, ceftazidime, ceftriaxone, piperacillin/tazobactam) tested against 12,295 staphylococci and streptococci: report from the SENTRY antimicrobial surveillance program (North America: 2001‐2002). Diagnostic Microbiology and Infectious Diseases 2003;47(2):435‐40. [DOI] [PubMed] [Google Scholar]
Fung‐Tomc 1996
- Fung‐Tomc JC, Gradelski E, Huczko E, Dougherty TJ, Kessler RE, Bonner DP. Differences in the resistant variants of Enterobacter cloacae selected by extended‐spectrum cephalosporins. Antimicrobial Agents and Chemotherapy 1996;40(5):1289‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furno 2000
- Furno P, Dionisi MS, Bucaneve G, Menichetti F, Favero A. Ceftriaxone versus beta‐lactams with antipseudomonal activity for empirical, combined antibiotic therapy in febrile neutropenia: a meta‐analysis. Supportive Care in Cancer 2000;8(4):293‐301. [DOI] [PubMed] [Google Scholar]
Goldstein 2002
- Goldstein FW. Cephalosporinase induction and cephalosporin resistance: a longstanding misinterpretation. Clinical Microbiology and Infection 2002;8(12):823‐5. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Sch?nemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2002
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases 2002;34(6):730‐51. [DOI] [PubMed] [Google Scholar]
Ibrahim 2000
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118(1):146‐55. [DOI] [PubMed] [Google Scholar]
Jacoby 2005
- Jacoby GA, Munoz‐Price LS. The new beta‐lactamases. New England Journal of Medicine 2005;352(4):380‐91. [DOI] [PubMed] [Google Scholar]
Johnson 1990
- Johnson MP, Ramphal R. Beta‐lactam‐resistant Enterobacter bacteremia in febrile neutropenic patients receiving monotherapy. Journal of Infectious Diseases 1990;162(4):981‐3. [DOI] [PubMed] [Google Scholar]
Kanafani 2007
- Kanafani ZA, Dakdouki GK, El‐Chammas KI, Eid S, Araj GF, Kanj SS. Bloodstream infections in febrile neutropenic patients at a tertiary care center in Lebanon: a view of the past decade. International Journal of Infectious Diseases 2007;11(5):450‐453. [DOI] [PubMed] [Google Scholar]
Kaye 2001
- Kaye KS, Cosgrove S, Harris A, Eliopoulos GM, Carmeli Y. Risk factors for emergence of resistance to broad‐spectrum cephalosporins among Enterobacter spp. Antimicrobial Agents and Chemotherapy 2001;45(9):2628‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klastersky 2007
- Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, et al. Bacteraemia in febrile neutropenic cancer patients. International Journal of Antimicrobial Agents 2007;30 Suppl 1:S51‐9. [DOI] [PubMed] [Google Scholar]
Lawson 1984
- Lawson RD, Gentry LO, Bodey GP, Keating MJ, Smith TL. A randomized study of tobramycin plus ticarcillin, tobramycin plus cephalothin and ticarcillin, or tobramycin plus mezlocillin in the treatment of infection in neutropenic patients with malignancies. American Journal of Medical Sciences 1984;287(1):16‐23. [DOI] [PubMed] [Google Scholar]
Leibovici 1998
- Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. Journal of Internal Medicine 1998;244(5):379‐86. [DOI] [PubMed] [Google Scholar]
Leibovici 2010
- Leibovici L, Yahav D, Paul M. Excess mortality related to Cefepime: Comment on the FDA review process and conclusions. Lancet Infectious Diseases 2010;Accepted for publication:EPUB. [DOI] [PubMed] [Google Scholar]
Li 2004
- Li C, Nicolau DP, Lister PD, Quintiliani R, Nightingale CH. Pharmacodynamic study of beta‐lactams alone and in combination with beta‐lactamase inhibitors against Pseudomonas aeruginosa possessing an inducible beta‐lactamase. Journal of Antimicrobial Chemotherapy 2004;53(2):297‐304. [DOI] [PubMed] [Google Scholar]
Lichaa 2010
- Lichaa H, Rachoin JS, Cerceo E, Rajput V, Surkis W. Cefepime: An underrecognized cause of nonconvulsive status epilepticus. Journal of Hospital Medicine 2010;5(3):E18‐E19. [DOI] [PubMed] [Google Scholar]
Link 2003
- Link H, B?hme A, Cornely OA, H?ffken K, Kellner O, Kern WV, Mahlberg R, Maschmeyer G, Nowrousian MR, Ostermann H, Ruhnke M, Sezer O, Schiel X, Wilhelm M, Auner HW, Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG‐German Cancer Society). Antimicrobial therapy of unexplained fever in neutropenic patients‐‐guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG‐German Cancer Society). Annals of Hematology 2003;82 Suppl 2:S105‐17. [DOI] [PubMed] [Google Scholar]
Martin Herrera 2009
- Martin Herrera C, Navarro M. [Cefepime‐induced encephalopathy in patients with renal failure]. Nefrologia 2009;29(2):181. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Paterson 2004
- Paterson DL, Ko WC, Gottberg A, Mohapatra S, Casellas JM, Goossens H, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended‐spectrum beta‐lactamases. Clinical infectious Diseases 2004;39(1):31‐7. [DOI] [PubMed] [Google Scholar]
Paul 2003
- Paul M, Soares‐Weiser K, Leibovici. Beta lactam monotherapy versus beta lactam‐aminoglycoside combination therapy for fever with neutropenia: systematic review and meta‐analysis. BMJ 2003;326(7399):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2004
- Paul M, Soares‐Weiser K, Grozinsky S, Leibovici L. Beta‐lactam versus beta‐lactam‐aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database of Systematic Reviews 2004, Issue Issue 3. [Google Scholar]
Paul 2005
- Paul M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics against Gram‐positive infections for febrile neutropenia: systematic review and meta‐analysis of randomized controlled trials. Journal of Antimicrobical Chemotherapy 2005;55(4):436‐444. [DOI] [PubMed] [Google Scholar]
Paul 2005a
- Paul M, Borok S, Fraser A, Vidal L, Cohen M, Leibovici L. Additional anti‐Gram‐positive antibiotic treatment for febrile neutropenic cancer patients. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Paul 2007
- Paul M, Gafter‐Gvili A, Leibovici L, Bishara J, Levy I, Yaniv I, et al. The epidemiology of bacteremia with febrile neutropenia: experience from a single center, 1988‐2004. Israeli Medical Association Journal 2007;9(6):424‐29. [PubMed] [Google Scholar]
Pechere 1992
- Pechere JC, Vladoianu IR. Development of resistance during ceftazidime and cefepime therapy in a murine peritonitis model. Journal of Antimicrobial Chemotherapy 1992;29(5):563‐73. [DOI] [PubMed] [Google Scholar]
Pfaller 1997
- Pfaller MA, Jones RN, Marshall SA, Coffman SL, Hollis RJ, Edmond MB, et al. Inducible amp C beta‐lactamase producing gram‐negative bacilli from blood stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE). Diagnostic Microbiology and Infectious Diseases 1997;28(4):211‐9. [DOI] [PubMed] [Google Scholar]
Piaggio 2006
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ, CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. Journal of the Americal Medical Association 2006;295(10):1152‐1160. [DOI] [PubMed] [Google Scholar]
Raad 2007
- Raad I, Hachem R, Hanna H, Bahna P, Chatzinikolaou I, Fang X, et al. Sources and outcome of bloodstream infections in cancer patients: the role of central venous catheters. European Journal of Clinical Microbiology and Infectious Diseases 2007;26(8):549‐56. [DOI] [PubMed] [Google Scholar]
Rolston 2006
- Rolston KV, Bodey GP. Comment on: empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta‐analysis of randomized controlled trials. Journal of Antimicrobical Chemotherapy 2006;58(2):478. [DOI] [PubMed] [Google Scholar]
Roos 2006
- Roos JF, Bulitta J, Lipman J, Kirkpatrick CM. Pharmacokinetic‐pharmacodynamic rationale for cefepime dosing regimens in intensive care units. The Journal of Antimicrobial cChemotherapy 2006;58(5):987‐93. [PUBMED: 16943209] [DOI] [PubMed] [Google Scholar]
Sanders 1991
- Sanders JW, Powe NR, Moore RD. Ceftazidime monotherapy for empiric treatment of febrile neutropenic patients: a meta‐analysis. Journal of Infectious Diseases 1991;164(5):907‐16. [DOI] [PubMed] [Google Scholar]
Sanders 1993
- Sanders CC. Cefepime: the next generation?. Clinical Infectious Diseases 1993;17(3):369‐79. [PubMed] [Google Scholar]
Sanders 1996
- Sanders WE Jr, Tenney JH, Kessler RE. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clinical Infectious Diseases 1996;23(3):454‐61. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Jama 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schwaber 2003
- Schwaber MJ, Graham CS, Sands BE, Gold HS, Carmeli Y. Treatment with a broad‐spectrum cephalosporin versus piperacillin‐tazobactam and the risk for isolation of broad‐spectrum cephalosporin‐resistant Enterobacter species. Antimicrobial Agents and Chemotherapy 2003;47(6):1882‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaheen 2009
- Shaheen T, Volles D, Calland F, Sifri CD, Mytinger J, Hagspiel K, et al. Cefepime‐associated status epilepticus in an ICU patient with renal failure. Journal of Chemotherapy 2009;21(4):452‐54. [DOI] [PubMed] [Google Scholar]
Storring 1977
- Storring RA, Jameson B, McElwain TJ, Wiltshaw E. Oral non‐absorbed antibiotics prevent infection in acute non‐lymphoblastic leukaemia. Lancet 1977;2(8043):837‐40. [DOI] [PubMed] [Google Scholar]
Tamura 2005
- Tamura K. Clinical guidelines for the management of neutropenic patients with unexplained fever in Japan: validation by the Japan Febrile Neutropenia Study Group. International Journal of Antimicrobial Agents 2005;Suppl 2:S123‐7. [DOI] [PubMed] [Google Scholar]
Thabet 2009
- Thabet F, Al Maghrabi M, Al Barraq A, Tabarki B. Cefepime‐induced nonconvulsive status epilepticus: case report and review. Neurocritical care 2009;10(3):347‐51. [DOI] [PubMed] [Google Scholar]
Tumbarello 2009
- Tumbarello M, Spanu T, Caira M, Trecarichi EM, Laurenti L, Montuori E, et al. Factors associated with mortality in bacteremic patients with hematologic malignancies. Diagnostic Microbiology and Infectious Diseases 2009;64(3):320‐6. [DOI] [PubMed] [Google Scholar]
Velasco 2003
- Velasco E, Byington R, Martins CA, Schirmer M, Dias LM, Goncalves VM. Prospective evaluation of the epidemiology, microbiology, and outcome of bloodstream infections in hematologic patients in a single cancer center. European Journal of Clinical Microbiology & Infectious Diseases 2003;22(3):137‐43. [DOI] [PubMed] [Google Scholar]
Yahav 2007
- Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta‐analysis. Lancet Infect Diseases 2007;7(5):338‐48. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Paul 2006
- Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta‐analysis of randomized controlled trials. Journal of Antimicrobial Chemothereapy 2006;57(2)(2):176‐189. [DOI] [PubMed] [Google Scholar]


