Abstract
Objective
SARS-CoV-2 infection induces significant inflammatory cytokine production in adults, but infant cytokine signatures in pregnancies affected by maternal SARS-CoV-2 are less well characterized. We aimed to evaluate cytokine profiles of mothers and their infants following COVID-19 in pregnancy.
Study design
Serum samples at delivery from 31 mother-infant dyads with maternal SARS-CoV-2 infection in pregnancy (COVID) were examined in comparison to 29 control dyads (Control). Samples were evaluated using a 13-plex cytokine assay.
Results
In comparison with controls, interleukin (IL)-6 and interferon gamma-induced protein 10 (IP-10) were higher in COVID maternal and infant samples (p < 0.05) and IL-8 uniquely elevated in COVID infant samples (p < 0.05). Significant elevations in IL-6, IP-10, and IL-8 were found among both early (1st/2nd Trimester) and late (3rd Trimester) maternal SARS-CoV-2 infections.
Conclusions
Maternal SARS-CoV-2 infections throughout gestation are associated with increased maternal and infant inflammatory cytokines at birth with potential to impact long-term infant health.
Subject terms: Lymphokines, Interleukins
Introduction
COVID-19 has impacted a growing number of pregnant patients throughout the pandemic, affecting an estimated 14 per 1000 births in the U.S. in 2020, with yet undefined risks for long-term infant health [1]. Throughout the pandemic, clinical studies have consistently shown that maternal SARS-CoV-2 infection during pregnancy generally does not result in vertical transmission from mother to infant [2–4]. While the rarity of vertical SARS-CoV-2 transmission has been well-characterized, the effects of maternal SARS-CoV-2 infection in pregnancy on the developing fetus are less well defined. Of particular importance is the maternal immune activation and inflammation in response to SARS-CoV-2 which has significant potential to cause harm to the developing fetus. Indeed, inflammatory exposures during the perinatal period have been shown to impair infant growth and development in other disease processes [5–7] with a particular risk for alterations in brain development and cognitive function later in life [8].
In acute COVID-19 infections, intense immune activation leads to an overproduction of serum cytokines known as a “cytokine storm”, with significant increases in multiple inflammatory mediators including IL-1, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IP-10 [9, 10]. While pregnant COVID-19 patients have a variety of clinical presentations, SARS-CoV-2 infection in pregnancy has been associated with increased COVID-19 disease severity and higher rates of intensive care unit (ICU) admissions when compared with COVID-19 in non-pregnant patients. Further, in comparison to pregnant patients without SARS-CoV-2 infections, pregnant patients with COVID-19 have an increased risk for preeclampsia and increased maternal mortality [11, 12]. Additionally, there is substantial evidence supporting intrauterine inflammation in pregnancies affected by maternal COVID-19 disease. Multiple studies have identified histological evidence of placental inflammatory lesions [13, 14] and male fetus-specific upregulation of placental interferon response genes and pro-inflammatory cytokines/chemokines [15].
More recent publications have also identified altered expression of maternal inflammatory cytokine profiles and evidence of fetal leukocyte activation through cord blood analysis in limited sample cohorts from pregnancies with COVID-19 in the third trimester [16, 17]. However, no study to date has characterized corresponding maternal and infant cytokine repertoires in larger cohorts, particularly from pregnancies with maternal SARS-CoV-2 infections in early pregnancy. Here we hypothesized that when compared to control pregnancies, SARS-CoV-2 positive mothers and their infants have significant alterations in inflammatory cytokines and that these alterations vary by the gestational timing of maternal COVID-19. In the current study, serum cytokines were evaluated in mother-infant dyads by examining maternal blood and cord blood/infant blood at the time of delivery between SARS-CoV-2 positive and control pregnancies (i.e., contemporary patients negative for SARS-CoV-2 at delivery). In particular, we evaluated changes relative to the gestational timing of maternal SARS-CoV-2 infection.
Subjects and methods
Study design and patient population
Boston Medical Center (BMC) is the largest urban safety-net hospital in New England serving most of the underrepresented minority populations living in the Greater Boston Area. This hospital delivers a significant proportion of pregnant patients from minority groups with comorbidities such as hypertension and obesity and having public insurance, which are characteristics associated with severe SARS-CoV-2 disease [18, 19]. BMC performed universal nasopharyngeal swab PCR screening of all pregnant patients who presented in labor starting in April 2020.
We enrolled mother-infant dyads from the obstetric prenatal clinics, Labor and Delivery, and the Postpartum Unit in a prospective cohort study from July 2020 through June 2021. Sample size was chosen based on previous publications evaluating the impact of inflammatory exposures in the perinatal period [5–7] and COVID-19 specific studies on inflammatory cytokines [9].
To be eligible for the COVID-19 group, patients had to be at least 18 years of age, have a positive SARS-CoV-2 infection at any point during pregnancy as documented by nasopharyngeal swab PCR testing, have a viable singleton gestation pregnancy, and be English or Spanish speaking. Patients were excluded from this cohort if they had received COVID-19 vaccination by the time of delivery, or if they were deemed unable to provide informed consent and agree to study procedures for any medical or social reason.
The control group was enrolled between January and April 2021. Eligibility criteria for the control group were age at least 18 years of age, documented negative SARS-CoV-2 PCR testing throughout their pregnancy without suspected COVID-19, a viable singleton gestation pregnancy, and English or Spanish speaking. Of note, this group was also unvaccinated for SARS-CoV-2.
This study was approved by the Boston University Medical Campus Institutional Review Board. Written informed consent or REDCap e-consent was obtained from all participants. Institutional COVID-19 research protocols were followed with use of a remote consent process and coordination with clinical care blood draws for patients on COVID-19 precautions at the time of delivery.
Sample collection
Maternal blood samples (3 mL in an EDTA tube) were collected by L&D staff or phlebotomy trained research staff. Cord blood samples (3–5 mL in an EDTA tube) were collected by L&D staff in the delivery room. If cord blood could not be obtained, an infant blood sample (0.5–1 mL in a EDTA microtainer) was obtained via heel stick by trained neonatal study staff or bedside nursing staff. Blood was centrifuged within 6 h of collection in our BSL-2+ research laboratory space using COVID-19 specific safety protocols. Plasma was then extracted, aliquoted into 0.5 mL aliquots, and frozen at −80 °C until analysis.
Cytokine analysis
Serum cytokine levels were evaluated using a flow cytometry bead-based LEGENDplex assay (BioLegend). Frozen plasma samples were quick-thawed and analyzed using a13-plex human anti-viral response panel to analyze the following cytokines: IP-10, IL-1β, IL-6, TNF-α, IFN-λ1, IL-8, IL-12p70, IFN- α2, IFN-λ 2/3, GM-CSF, IFN- β, IL-10, and IFN-γ. This panel was selected because it included cytokines previously identified to be altered among adult COVID-19 patients [9, 10] as well as cytokines associated with adverse long-term fetal outcomes in perinatal inflammatory exposures [5–8]. Samples were pre-diluted 1:2 and the kit was run according to manufacturer’s instructions. Sample data were collected using the Cytek® Aurora flow cytometer (Cytek Biosciences) in the Boston University School of Medicine Flow Cytometry Core facility. Cytokine concentrations for each sample were determined from extrapolation from standard curves using the LEGENDplex Data Analysis Software. Pilot testing of 20 cord blood and 20 infant blood samples indicated a very high correlation for this cytokine panel, thus these sample types were grouped together for the purposes of analysis as an “infant” sample for analysis of the full cohort.
Data abstraction
The electronic medical records of the mother-infant dyads enrolled were reviewed. Demographic characteristics such as race, ethnicity, age at delivery, and primary language were recorded in a secure electronic study database. Other maternal data points such as history of chronic illnesses, pregnancy related diagnosis and SARS-CoV-2 infection-related symptoms were abstracted. Trimester and gestational age at the time of infection, severity of infection (hospitalization/ICU), and maternal COVID-19 status at the time of delivery were also collected. For infants, demographics and birth parameters as well as COVID-19 testing status and neonatal symptoms in the first 30 days of life were reviewed. Infants were also tracked for any emergency room visits or readmissions in the first 30 days of life.
Statistical methods
Demographics between the COVID-19 and control groups were compared using t-tests and chi-square as indicated. For cytokine statistical analysis, due to a large proportion of non-detects in several of the cytokines examined, a Kaplan Meier method for left-censored data was used for cytokine statistical analysis. This is a non-parametric technique that estimates a probability distribution based on the number of samples below detected concentrations [20]. This method is preferred over simple substitution methods particularly when there is a large proportion of censored data. This Kaplan Meier method was used to calculate means and standard deviations separately within COVID and control groups and by sample type (maternal blood and cord/infant blood). Mean differences and 95% confidence intervals between the COVID and reference groups were then calculated for each cytokine within both maternal and cord/infant samples. We also examined differences between mean cytokine levels in maternal blood and cord blood/infant blood by timing of SARS-CoV-2 infection during pregnancy. Mean cytokine levels for early COVID (1st/2nd trimester) and late COVID (3rd trimester) were compared to each other and then to the reference group using t-tests for independent samples with unequal variances. Lastly, among the COVID-19 dyads, we calculated the correlation coefficient for individual cytokines between maternal and cord/infant samples. All statistical analysis was performed using SAS software V9.4 (SAS Analytics). Graphical data for cytokine levels was created using Prism Software (Graphpad).
Results
Cohort clinical data
For our prospective study design (Fig. 1) we enrolled pregnant patients with SARS-CoV-2 in early (1st/2nd trimester) and late (3rd trimester) gestational stages of pregnancy along with contemporary controls who had no documented history of COVID-19 and also negative SARS-CoV-2 testing at time of delivery. We identified 146 pregnant individuals with COVID-19 who delivered infants at BMC between July 2020 and April 2021. Of those, 20 were not eligible for approach for the study due to twin gestation (n = 1), non-English or Spanish speaking (n = 11), severe social concerns screened out by providers (n = 3), maternal age <18 years (n = 3), or ICU admission during screening (n = 2). Of the remaining 126 patients, 85 were approached for consent. The remainder were not approached due to availability of research staff and timing during labor (no patient in advanced stages of labor or immediately after delivery were approached). Of those 85 patients, 60 mothers (71%) provided informed consent for the study. From those 60 consented, 31 dyads with complete maternal blood and cord blood matched samples from delivery were selected at random for the cytokine analysis.
Fig. 1. Study design.
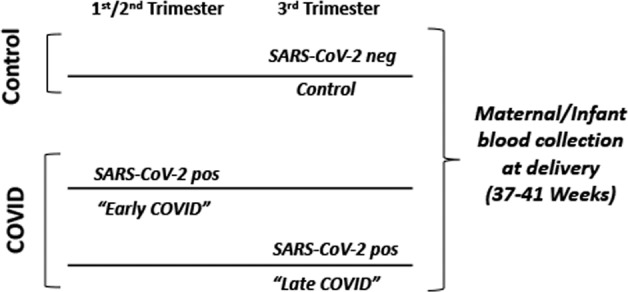
Study design of patient cohorts for serum cytokine analysis. Control: mothers with no report of COVID-19 disease at any time during their pregnancy and negative for SARS-CoV-2 at time of delivery screening. Early COVID: mothers with SARS-CoV-2 positive testing within the 1st or 2nd trimester of pregnancy (1–27 weeks gestation). Late COVID: mothers with SARS-CoV-2 positive testing in the 3rd trimester of pregnancy (28–41 weeks gestation).
For our contemporary control cohort recruitment during the same time period, a total of 256 patients were admitted in labor. Of those, 20 were identified as COVID-19 positive thus ineligible, and others screened out due to non-English or Spanish speaking (n = 23), being screened out by providers due to social concerns (n = 5), or other medical reasons for ineligibility such as twin gestation (n = 6). Of the remaining 202 patients, 41 (20%) were approached with our goal of enrolling 2 control dyads per week due to staff availability and timing of labor. Of the 41 patients approached, 31 (75%) consented to the study. Of those 31 dyads, 29 had complete maternal blood and cord blood matched samples and thus were included in the analysis.
Demographics of the COVID and control cohorts are shown in Table 1. There were no significant differences in maternal age, race, ethnicity, language, delivery mode, gestational age, birth weight, infant sex, or infant outcomes between the two cohorts. The COVID-19 cohort contained 3 patients (9.7%) with infections in the first trimester, 18 patients (58.1%) with infections in the 2nd trimester, and 10 patients (32.3%) with infections in the third trimester. Twenty-eight patients (90.3%) in our COVID cohort had documentation of COVID-19 symptoms at any stage during their pregnancy with 6.6% requiring hospitalization for COVID-19 during pregnancy. Some differences were noted in the presence of various pregnancy co-morbidities and chronic health conditions, with the most significant being intrauterine growth restriction in the COVID-19 group. None of the infants were diagnosed with SARS-CoV-2 infection within 30 days of delivery.
Table 1.
Demographics of COVID-19 versus control mother-infant dyads.
| Variable | Mean (SD) or N (%) Control N = 28 |
Mean (SD) or N(%) COVID-19 N = 31 |
P value |
|---|---|---|---|
| Maternal age at delivery (years) | 29.8 (5.7) | 29.7 (5.6) | 0.96 |
| Maternal Race | 0.17 | ||
| Black | 5 (17.9%) | 2 (6.5%) | |
| White | 5 (17.9%) | 8 (25.8%) | |
| Asian | 1 (3.6%) | 2 (6.5%) | |
| Other | 14 (50.0%) | 19 (61.3%) | |
| Missing | 3 (10.7%) | 0 (0%) | |
| Maternal Ethnicity = Hispanic | 18 (64.3%) | 23 (74.2%) | 0.41 |
| Maternal Primary Language = English | 18 (64.3%) | 14 (45.2%) | 0.14 |
| Health Insurance = Public | 20 (71.4%) | 24 (77.4%) | 0.60 |
| Chronic health conditions | |||
| Any condition | 17 (60.7%) | 21 (67.7%) | 0.57 |
| Diabetes | 0 (0%) | 0 (0%) | N/A |
| Hepatitis C | 2 (7.1%) | 0 (0%) | 0.13 |
| Hypertension | 2 (7.1%) | 1 (3.2%) | 0.49 |
| Obesity | 4 (14.3%) | 3 (9.7%) | 0.58 |
| Thyroid condition | 4 (14.3%) | 1 (3.2%) | 0.13 |
| Substance use disorder | 2 (7.1%) | 1 (3.2%) | 0.49 |
| Other | 15 (53.6%) | 13 (41.9%) | 0.37 |
| Pregnancy co-morbidities | |||
| Any co-morbidity | 23 (82.1%) | 29 (93.6%) | 0.17 |
| Chorioamnionitis | 0 (0%) | 4 (12.9%) | 0.05 |
| Gestational diabetes | 6 (21.4%) | 4 (12.9%) | 0.38 |
| Preeclampsia/Gestational hypertension | 10 (35.7%) | 9 (29.0%) | 0.58 |
| Intrauterine growth restriction | 1 (3.6%) | 7 (22.6%) | 0.03 |
| Preterm labor | 0 (0%) | 2 (6.5%) | 0.17 |
| Other | 16 (57.1%) | 21 (67.7%) | 0.40 |
| Nicotine smoking | 2 (7.1%) | 2 (6.5%) | 0.31 |
| Delivery mode = C-section | 6 (21.4%) | 9 (29.0%) | 0.50 |
| Gestational age at delivery (weeks) | 39.1 (1.3) | 39.3 (1.6) | 0.50 |
| Infant birth weight (g) | 3349 (513.9) | 3306 (547.4) | 0.76 |
| Infant sex = male | 15 (53.6%) | 16 (51.6%) | 0.69 |
| Breastfed infant | 26 (92.9%) | 30 (96.8%) | 0.49 |
| 5 min APGAR score | 8.8 (0.6) | 8.9 (0.5) | 0.32 |
| Infant length of hospitalization (days) | 3.5 (3.0) | 3.1 (2.1) | 0.59 |
| Infant ER visit within 30 days | 3 (11.1%) | 1 (3.2%) | 0.24 |
| Infant re-hospitalization with 30 days | 3 (11.1%) | 1 (3.2%) | 0.24 |
| Infant diagnosed with SARS-CoV-2 within 30 days of delivery | 0 (0%) | 0 (0%) | N/A |
| NICU admissiona | 4 (14.3%) | 4 (12.9%) | 0.88 |
| Mother diagnosed with SARS-CoV-2 within 30 days of delivery | 0 (0%) | N/A | N/A |
| Trimester of COVID-19 infection | N/A | N/A | |
| First | 3 (9.7%) | ||
| Second | 18 (58.1%) | ||
| Third | 10 (32.3%) | ||
| Gestational age at infection (weeks) | N/A | 24.0 (9.0) | N/A |
| Maternal symptoms of COVID-19 at time of SARS-CoV-2 positive testing | N/A | 28 (90.3%) | N/A |
| Maternal COVID-19 symptoms at delivery | N/A | N/A | |
| Confirmed SARS-CoV-2 with active symptoms at delivery | 1 (3.2%) | ||
| Confirmed SARS-CoV-2 and asymptomatic at delivery | 4 (12.9%) | ||
|
Recovered from SARS-CoV-2 infection earlier in pregnancy (1st, 2nd, and 3rd Trimesters) |
26 (83.9%) | ||
| Hospitalized for COVID-19 | N/A | 2 (6.6%) | N/A |
ER emergency room, SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2.
aReasons for NICU admission were myelocystocele, apnea related to late prematurity, subgaleal hemorrhage, bradycardia, NAS, and TTN.
Serum cytokine analysis
Maternal and infant cytokine levels were compared between control and COVID-19 cohorts (Figs. 2–4, Supplementary Table 1) with additional sub-analysis of Control (SARS-CoV-2 negative) vs Early COVID (1st/2nd Trimester) and Late COVID (3rd Trimester) maternal SARS-CoV-2 infections in pregnancy (Figs. 2–4, Supplementary Table 2). Finally, we conducted a correlation analysis of maternal-infant cytokine levels in maternal-infant dyads within the COVID-19 cohort (Figs. 2–4, Supplementary Table 3).
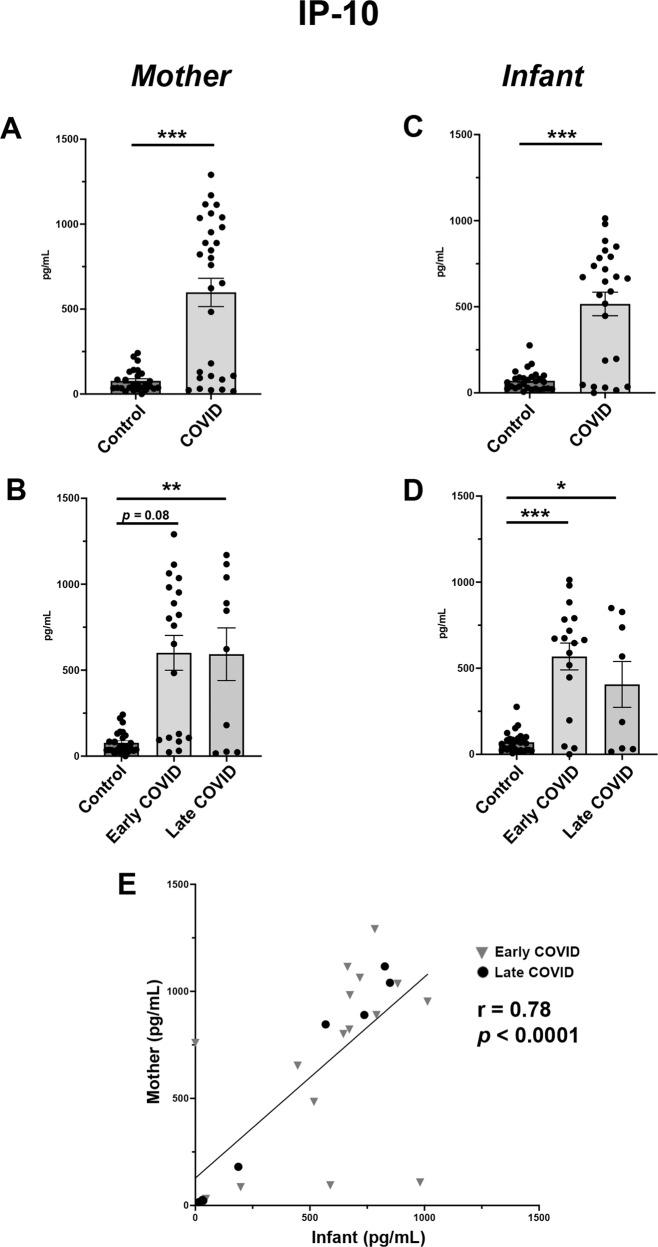
Fig. 2. IP-10 is significantly elevated in maternal and infant serum following COVID-19 infections in pregnancy.
A Maternal serum IP-10 expression in Control vs COVID cohorts. B Maternal serum IP-10 sub-analysis among Control, Early COVID and Late COVID cohorts. C Infant serum IP-10 expression in Control vs COVID cohorts. D Infant serum IP-10 sub-analysis among Control, Early COVID and Late COVID cohorts. E Correlation analysis of IP-10 expression in maternal-infant dyads. Control: as described in Fig. 1. COVID: Mothers positive for SARS-CoV-2 in pregnancy. Early COVID: as described in Fig. 1. Late COVID: as described in Fig. 1. *p < 0.05, **p < 0.01, ***p < 0.001.
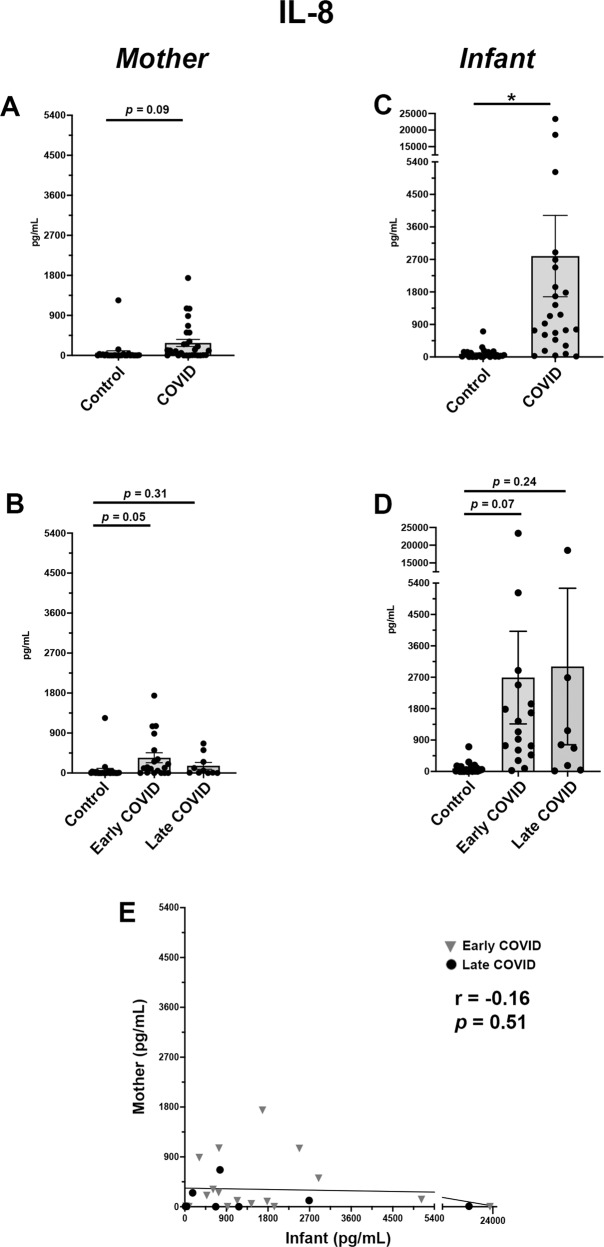
Fig. 4. Uniquely elevated levels of IL-8 in serum of infants from pregnancies affected by maternal SARS-CoV-2 in early pregnancy.
A Maternal serum IL-8 expression in Control vs COVID cohorts. B Maternal serum IL-8 sub-analysis among Control, Early COVID and Late COVID cohorts. C Infant serum IL-8 expression in Control vs COVID cohorts. D Infant serum IL-8 sub-analysis among Control, Early COVID and Late COVID cohorts. E Correlation analysis of IL-8 expression in maternal-infant dyads. Control, COVID, Early COVID, and Late COVID: as described in Figs. 1 and 2. *p < 0.05.
Among all analytes, those with the most significant changes were pro-inflammatory cytokines IP-10, IL-6 and IL-8. For IP-10, both maternal and infant blood showed significantly higher levels in the COVID cohort as compared with Controls (Fig. 2A, C). Interestingly, these elevations were noted in both Early COVID and Late COVID groups. While the most significant elevations in IP-10 maternal levels were found to be in the Late COVID group (Fig. 2B), the highest infant blood levels were noted in Early COVID pregnancies (Fig. 2D). Regression analysis further demonstrated a positive correlation in maternal and infant IP-10 levels among dyads analyzed (Fig. 2E).
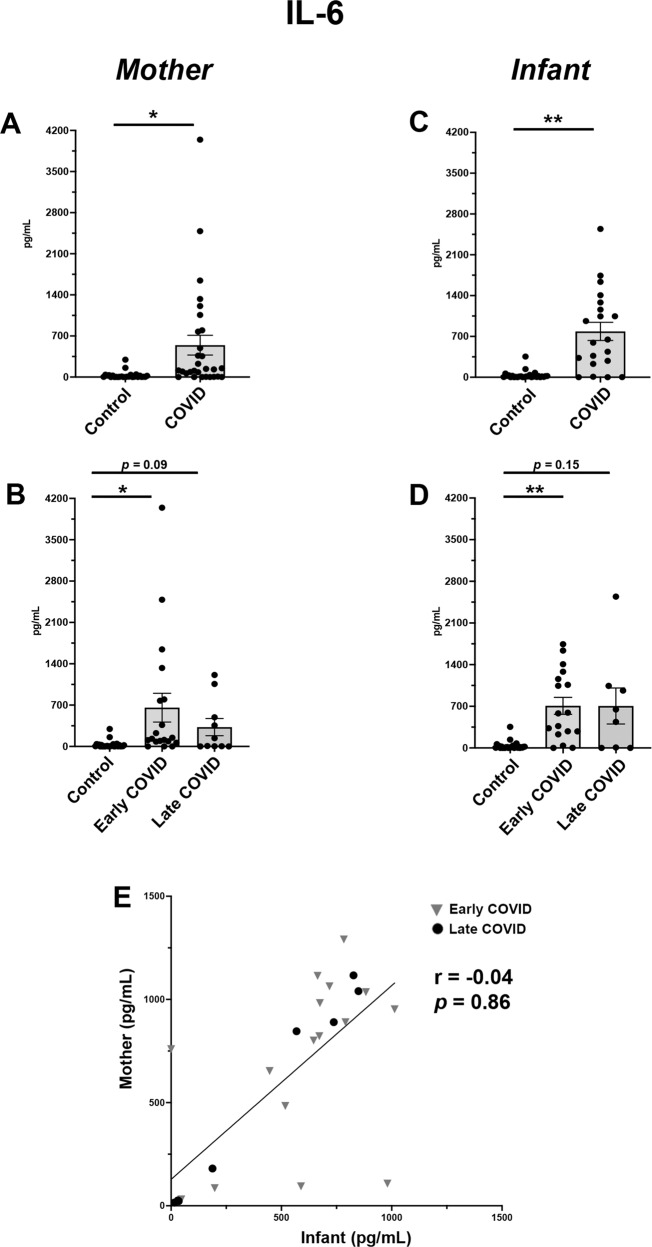
IL-6 analysis also showed significant upregulation in both maternal and infant blood from COVID pregnancies compared with Controls (Fig. 3A, C). When analyzing by gestational stage of SARS-CoV-2 infection, we identified that the Early COVID cohort had the highest IL-6 elevations for both maternal and infant samples (Fig. 3B, D). However, these elevations in IL-6 were not significantly correlated within individual mother-infant dyads (Fig. 3E).
Fig. 3. Maternal and infant serum IL-6 levels are distinctly elevated in SARS-CoV-2 infections early in pregnancy.
A Maternal serum IL-6 expression in Control vs COVID cohorts. B Maternal serum IL-6 sub-analysis among Control, Early COVID and Late COVID cohorts. C Infant serum IL-6 expression in Control vs COVID cohorts. D Infant serum IL-6 sub-analysis among Control, Early COVID and Late COVID cohorts. E Correlation analysis of IL-6 expression in maternal-infant dyads. Control, COVID, Early COVID, and Late COVID: as described in Figs. 1 and 2. *p < 0.05, **p < 0.01.
When evaluating IL-8, only infant samples showed a significant elevation in the COVID cohort as compared with controls (Fig. 4C). While this was significant for total cohort analysis, these values did not reach statistical significance in sub-analysis by gestational stage of SARS-CoV-2 infection (Fig. 4D), likely due to the significant variability in cytokine values among Early and Late COVID sub-groups. Further, the abundance of IL-8 was also notably higher in the infant COVID cohort (Fig. 4C) as compared with the maternal COVID cohort (Fig. 4A). As expected, there was no correlation between maternal and infant cytokine levels among dyads (Fig. 4E).
Among the remaining cytokines, IFN-λ1 showed a trend in elevation among COVID maternal samples, particularly among the Early COVID cohort (Supplementary Fig. 1A, B). While correlation analysis did show significant correlation among dyads (Supplementary Fig. 1E), the correlation significance appeared to originate in highly elevated levels from two dyads with the remainder of the dyads having minimal cytokine values among the infant samples. All other cytokines assayed showed no significant differences in COVID vs Control group comparisons among maternal and infant samples (Supplementary Tables 1, 2, and 3).
Discussion
This study identified a unique repertoire of inflammatory cytokines with significant alterations in maternal-infant dyads from pregnancies affected by maternal SARS-CoV-2 infection, namely IP-10, IL-6, and IL-8. Importantly, these alterations were identified even among the Early COVID cohort, suggesting persistent elevations of inflammatory cytokines in maternal and neonatal circulation months after initial maternal SARS-CoV-2 infection.
The COVID-associated maternal serum elevations of IP-10 and IL-6 were expected findings as both are central components of the COVID-19 cytokine storm [9, 10, 21] and 90% of our maternal COVID-19 cohort was symptomatic at the time of the positive SARS-CoV-2 tests, indicating the majority of our cohort had active disease rather than asymptomatic carrier status. These data also suggest a prolonged upregulation of these cytokines lasting weeks to months after disease onset as as only a small percentage (1%) had active symptoms at the time of delivery sample collection. The differing maternal-infant correlation profiles for IP-10, IL-6 and IL-8 between mothers and infants in the COVID cohort suggest that the infant cytokine elevations are not merely a passive transfer of maternal cytokines passing into fetal circulation. Rather, these changes may be the result of an independent fetal immune response to maternal SARS-CoV-2 in pregnancy, as also suggested by a recent study showing evidence of fetal leukocyte activation in a cohort (n = 3) of cord blood samples from pregnancies affected by maternal COVID-19 in the third trimester [17].
IP-10 (also known as CXCL10) is an established inflammatory chemokine secreted by both immune and parenchymal cell types with varied functions to induce leukocyte chemotaxis, trigger cellular apoptosis, and inhibit vascular growth [22]. In adult COVID-19 patients, IP-10 has a long-lasting serum elevation profile that is unique from its secretion pattern in other viral infections [21]. Our data were congruent with these findings showing persistent IP-10 elevations in maternal and most significantly infant serum from early gestational COVID-19 infections. In pregnancy, elevated maternal IP-10 has been implicated in miscarriage and preeclampsia [23, 24], but the long-term infant effects of IP-10 exposure in the perinatal period are currently undefined.
IL-6 and IL-8 are also both well-characterized inflammatory mediators central in the COVID-19 cytokine response [9, 10]. In pregnancy, elevated serum levels of IL-6 and IL-8 have been associated with gestational pathologies including miscarriage, preeclampsia and preterm delivery [25, 26]. Perinatal exposure to these cytokines has been associated with altered fetal development. In preclinical studies, IL-6 is a central mediator of enhanced postnatal offspring intestinal inflammatory T cell activation following maternal perinatal infection [27]. Additionally, IL-6 and IL-8 elevations in pregnancy have independently been associated with altered infant neurodevelopmental outcomes [28, 29], with prenatal exposures to IL-8 particularly implicated in the risk of schizophrenia later in life [30, 31].
The current study has several limitations. First, our cohorts were of moderate sample size and we did not evaluate a complete repertoire of COVID-19 related clinical variables (i.e., c-reactive protein levels, white blood cell count). As only a small subset of our cohort had these labs available, they were not included as part of this analysis. Our cohort also had a larger proportion of patients with maternal SARS-CoV-2 infections in early gestation as compared with late gestation. Ongoing analysis with larger cohorts will be required to characterize the cytokine profiles of early vs late pregnancy SARS-CoV-2 infections more completely. As the majority of studies on COVID-19 in pregnancy contain samples exclusively from late pregnancy (3rd trimester) infections, our study highlights the importance of incorporating patients with SARS-CoV-2 infection at multiple gestational stages of pregnancy for ongoing studies in this field. In particular, multivariate analysis to correlate anti-viral antibodies with cytokine profiles will be informative to identify how these inflammatory signatures impact maternal and infant SARS- CoV-2 responses, particularly in relation to gestational timing of maternal infection.
This study supports a growing body of evidence that perinatal alterations resulting from maternal COVID-19 in pregnancy have a risk of impacting the health of infants even from in the absence of fetal SARS-CoV-2 transmission. Indeed, of all our clinical parameters evaluated between control and COVID cohorts, the only value which reached statistical significance was an increase in fetal intrauterine growth restriction in the COVID cohort. As there was no accompanying difference in the incidence of preeclampsia, the growth restriction could be an early indicator of primary fetal pathology resulting from altered intrauterine physiology in response to maternal SARS-CoV-2 infection. Future studies involving more extensive patient cohorts and pre-clinical models will be required to characterize the driving mechanisms and developmental impact of the cytokine alterations identified in this study. Finally, our work highlights the importance of long-term follow-up for infants from pregnancies affected by maternal SARS-CoV-2 as an at-risk population of the COVID-19 era.
Supplementary information
Acknowledgements
We would like to acknowledge all of the bedside nurses, midwives, and physicians from the Boston Medical Center Labor and Delivery Unit, Postpartum Unit, and Newborn Intensive Care Unit who assisted us with subject recruitment and facilitation of sample collection, especially Kate Thibault, RN; Lauren Laliberte, RN; Brianna Medeiros, RN, NP; Elizabeth Regan, RN, and Joanna Bushfield, RN; Sigride Jean-Sicard, RN, NP; Elizabeth Woodard, RN, NP; Alice Cruikshank, RN, NP; Bharati Sinha, MD; Ruby Bartolome, DO; and Margaret G. Parker, MD, MPH. We would also like to acknowledge the Maxwell Finland Laboratory of Pediatric Infectious Disease at Boston Medical Center, and the Boston University laboratory of Dr. Jennifer Snyder-Cappione. This study was funded by Boston University Clinical and Translational Science Institute COVID-19 pilot grant program to support this project (1UL1TR001430)
Author contributions
EW, EB, CY, VS, and ET were involved in development of the study protocol and procedures. JB, LJ, ET, and EW enrolled subjects in the study. YD, JB, LJ, ET, and EW collected and processed samples for the study. KC and JC performed the cytokine assays for the study. YD and JD performed the chart abstraction for the study. JH and SP performed the statistical data analysis for the study. ET, YD and EW were involved in data interpretation, figure composition and final manuscript composition. EW provided overall oversight and obtained grant funding for the study. All authors reviewed and edited the manuscript and provided final approval of the submitted version.
Competing interests
This study was funded by Boston University Clinical and Translational Science Institute COVID-19 pilot grant program to support this project (1UL1TR001430).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41372-022-01391-9.
References
- 1.Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–31. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Toro F, Gjoka M, Di Lorenzo G, De Santo D, De Seta F, Maso G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boateng JO, Wachman EM, Turcinovic J, Devera J, Jain M, Jean-Sicard S, et al. SARS-CoV-2 in infant urine and fecal samples after in utero COVID-19 exposure. Pediatr Res. 2021;30:1–5. [DOI] [PMC free article] [PubMed]
- 5.Nazzari S, Fearon P, Rice F, Ciceri F, Molteni M, Frigerio A. Neuroendocrine and immune markers of maternal stress during pregnancy and infant cognitive development. Dev Psychobiol. 2020;62:1100–10. doi: 10.1002/dev.21967. [DOI] [PubMed] [Google Scholar]
- 6.Nist MD, Shoben AB, Pickler RH. Early inflammatory measures and neurodevelopmental outcomes in preterm infants. Nurs Res. 2020;69:S11–s20. doi: 10.1097/NNR.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevenoaks T, Wedderburn CJ, Donald KA, Barnett W, Zar HJ, Stein DJ, et al. Association of maternal and infant inflammation with neurodevelopment in HIV-exposed uninfected children in a South African birth cohort. Brain Behav Immun. 2021;91:65–73. doi: 10.1016/j.bbi.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schepanski S, Buss C, Hanganu-Opatz IL, Arck PC. Prenatal immune and endocrine modulators of offspring’s brain development and cognitive functions later in life. Front Immunol. 2018;9:2186. doi: 10.3389/fimmu.2018.02186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–80. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID. JAMA Pediatr. 2021;175:817–26. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu-Culligan A, Chavan AR, Vijayakumar P, Irshaid L, Courchaine EM, Milano KM, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Medicines. 2021;2:591–610. doi: 10.1016/j.medj.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharps MC, Hayes DJL, Lee S, Zou Z, Brady CA, Almoghrabi Y, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bordt EA, Shook LL, Atyeo C, Pullen KM, De Guzman RM, Meinsohn MC, et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci Transl Med. 2021;13:eabi7428. doi: 10.1126/scitranslmed.abi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Liao Q, Ai J, Yang B, Bai H, Chen J, et al. Immune Response to COVID-19 During Pregnancy. Front Immunol. 2021;12:675476. doi: 10.3389/fimmu.2021.675476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matute JD, Finander B, Pepin D, Ai X, Smith NP, Li JZ, et al. Single-cell immunophenotyping of the fetal immune response to maternal SARS-CoV-2 infection in late gestation. Pediatr Res. 2021;8:1–9. [DOI] [PMC free article] [PubMed]
- 18.Gelaye B, Foster S, Bhasin M, Tawakol A, Fricchione G. SARS-CoV-2 morbidity and mortality in racial/ethnic minority populations: a window into the stress related inflammatory basis of health disparities? Brain Behav Immun Health. 2020;9:100158. doi: 10.1016/j.bbih.2020.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra S, Ma H, Moloney G, Yiu KCY, Darvin D, Landsman D, et al. Increasing concentration of COVID-19 by socioeconomic determinants and geography in Toronto, Canada: an observational study. Ann Epidemiol. 2021;65:84–92. [DOI] [PMC free article] [PubMed]
- 20.Beal D. a macro for calculating summary statistics on left censored environmental data using the Kaplan-Meier method. SDA-09. 2009;9:1–6.
- 21.Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623–35. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22:121–30. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Huang F, Chai X, Yuan W, Ding H, Wu X. The role of IP-10 and its receptor CXCR3 in early pregnancy. Mol Immunol. 2021;140:59–69. doi: 10.1016/j.molimm.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, et al. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern Fetal Neonatal Med. 2007;20:777–92. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95:1–14. doi: 10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Shahshahan Z, Hashemi L. Maternal serum cytokines in the prediction of preterm labor and response to tocolytic therapy in preterm labor women. Adv Biomed Res. 2014;3:126. doi: 10.4103/2277-9175.137864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim AI, McFadden T, Link VM, Han SJ, Karlsson RM, Stacy A, et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science. 2021;373:eabf3002. [DOI] [PubMed]
- 28.Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 2018;21:765–72. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan G, Galdi P, Cabez MB, Borbye-Lorenzen N, Stoye DQ, Lamb GJ, et al. Interleukin-8 dysregulation is implicated in brain dysmaturation following preterm birth. Brain Behav Immun. 2020;90:311–8. doi: 10.1016/j.bbi.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–95. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 31.Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121:46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.