Abstract
Non-alcoholic fatty liver disease (NAFLD) is associated with cardiovascular disease morbimortality. However, it is not clear if NAFLD staging may help identify early or subclinical markers of cardiovascular disease. We aimed to evaluate the association of liver stiffness and serum markers of liver fibrosis with epicardial adipose tissue (EAT) and coronary artery calcium (CAC) in an observational cross-sectional study of 49 NAFLD patients that were seen at Clínica Universidad de Navarra (Spain) between 2009 and 2019. Liver elastography and non-invasive fibrosis markers were used to non-invasively measure fibrosis. EAT and CAC, measured through visual assessment, were determined by computed tomography. Liver stiffness showed a direct association with EAT (r = 0.283, p-value = 0.049) and CAC (r = 0.337, p-value = 0.018). NAFLD fibrosis score was associated with EAT (r = 0.329, p-value = 0.021) and CAC (r = 0.387, p-value = 0.006). The association of liver stiffness with CAC remained significant after adjusting for metabolic syndrome features (including carbohydrate intolerance/diabetes, hypertension, dyslipidaemia, visceral adipose tissue, and obesity). The evaluation of NAFLD severity through liver elastography or non-invasive liver fibrosis biomarkers may contribute to guide risk factor modification to reduce cardiovascular risk in asymptomatic patients. Inversely, subclinical cardiovascular disease assessment, through Visual Scale for CAC scoring, may be a simple and effective measure for patients with potential liver fibrosis, independently of the existence of other cardiovascular risk factors.
Subject terms: Cardiology, Endocrinology, Gastroenterology, Risk factors
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent disease worldwide that has become a challenge for health services due to its known relation to cardiovascular and liver morbimortality1. The presence and severity of fibrosis is the determining factor associated with higher mortality from any cause, although mainly due to cardiovascular disease (CVD)2. NAFLD is now considered an additional independent risk factor for CVD3,3, and its relation with subclinical cardiac damage has already been evidenced5. The association of NAFLD and CVD is complex; insulin resistance, proinflammatory cytokines, lipotoxicity, oxidative stress and endothelial dysfunction may all contribute to the development and progression of CVD6,6.
Coronary artery calcium (CAC)8,8 and epicardial adipose tissue (EAT)10,10 are considered surrogate markers of coronary artery disease and have significantly improved the cardiovascular risk classification in asymptomatic individuals8–11. EAT is a novel clinical biomarker not only associated with accelerated progression of subclinical coronary atherosclerosis10,10, but also mediator of cardiac arrhythmias12, left ventricular diastolic dysfunction13, and stroke14. EAT has important physiological functions15, nonetheless, excessive EAT leads to a proinflammatory state with adverse effects on the myocardium.
Liver biopsy is the reference method for assessing the severity of NAFLD, however, it is an invasive non-cost-effective procedure for a highly prevalent disease. Liver elastography (LE)16 and non-invasive fibrosis markers (e.g. NAFLD Fibrosis Score [NFS]17 and Fibrosis 4 Score [FIB-4]18) have shown high diagnostic precision for advance stages of liver fibrosis (F3–F4). Due to the high prevalence of NAFLD, it is of interest to investigate which patient is at a higher risk for cardiovascular abnormalities. The aim of this study was to assess the association of NAFLD severity, specifically through LE and non-invasive markers of liver fibrosis, with EAT and CAC.
Materials and methods
Study patients
This was a retrospective study of adult patients with NAFLD that were seen at Clínica Universidad de Navarra (Spain) between 2009 and 2019. The research ethics committee of the University of Navarra approved the study protocol (2019.080). Informed consent was obtained from all subjects involved in the study. Eligible patients were those that had LE, whole body scan computed tomography (CT-WBS) or computed tomography thoracic scan (CT-TS), and blood test at the same visit. In our Centre, CT-WBS/CT-TS and laboratory tests are routinely performed on the same day (or within a few days) of the initial visit. Epidemiological, clinical, laboratory and radiological information were obtained from patient records.
All subjects were non-symptomatic with no history of malignancy. Alcohol consumption was specifically investigated by interviewing the patients. Exclusion criteria included previous liver disease of other etiologies, such as alcoholic fatty liver disease, autoimmune or viral hepatitis, drug-induced fatty liver disease (e.g. amiodarone, systemic corticosteroids, valproic acid, carbamazepine, antiretroviral drugs, methotrexate or tamoxifen), autoimmune hepatitis, cholestatic liver disease, genetic liver disease (e.g. haemochromatosis), endocrinological disorders (e.g. hypopituitarism, hypothyroidism), inborn errors of metabolism or nutritional disorders (e.g. starvation, parenteral nutrition), systematic inflammatory disease and malignant disease. Additionally, patients with a personal history of cerebral vascular diseases (including transient ischemic attack), ischemic heart diseases, heart failure, atrial fibrillation, pericarditis, valvular disease or any other heart disease prior to index date were excluded (Fig. 1). All patients had a negative history of alcohol abuse as indicated by a daily ethanol consumption of less than 20 g in women, and less than 30 g in men.
Figure 1.
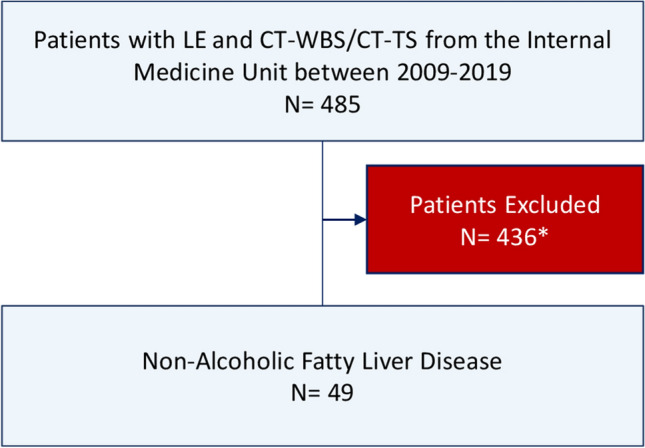
LE liver elastography, CT-WBS computed tomography whole body scan, CT-TS computed tomography thoracic scan. *Of the initial cohort of 485 patients, 436 were excluded for one or more of the following criteria that may have affected cardiovascular outcomes or had a different liver disease: personal history of cardiovascular disease (n = 12); active malignancy (n = 52); endocrine diseases (n = 1); excessive alcohol consumption (n = 125); viral hepatitis (n = 123); autoimmune liver disease (n = 28); toxic hepatitis (n = 10); steatogenic drugs (n = 2); iron overload (n = 8); alfa1-antitripsin deficit (n = 6); cirrhosis (n = 7); inflammatory diseases (n = 10); portal hypertension (n = 12) and other liver disease (cholestasis, cystic fibrosis, amyloidosis, Gilbert’s syndrome, paludism, thalassemia) (n = 40).
Non-invasive evaluation suggestive of fibrosis
We measured the liver stiffness using transient LE (Fibroscan®; Echosens, Paris, France)16. An experienced technician performed LE measurements. Only valid measurements were included. We used NFS17 and FIB-4 Score18 as non-invasive fibrosis serum markers. In NAFLD, a LE < 8.2 kPa is considered as low likelihood of fibrosis and ≥ 8.2 kPa is considered as clinically significant fibrosis (liver biopsy: ≥ F2); a NFS < − 1.455 is an approximate F0–F2 histological fibrosis stage, between ≥ − 1.455 and ≤ 0.676 is an indeterminant score, > 0.676 is an approximate F3–F4 histological fibrosis stage. On the other hand, a FIB-4 Score < 1.30 is an approximate F0–F1 histological fibrosis stage, between ≥ 1.30 and ≤ 2.67 is an indeterminant score, > 2.67 is an approximate F3–F4 histological fibrosis stage.
Whole‑body scan computed tomography protocol
All examinations were performed using a sixty-four-row multidetector CT (64-MDCT) (SOMATOM Definition and SOMATOM Sensation-64, Siemens Healthcare, Forchheim, Germany), with tube voltage and reference tube current time product variable depending on region included. All images were stored in picture archiving and communication system (PACS). The protocol of CT-WBS included: low-dose chest CT (120 kV and 40 mA/s) without contrast material, CAC measurement (120 kV and 138 mA/s) in cases where coronary calcification was identified in low-dose chest CT, abdominopelvic CT (120 kV and 180 mA/s) performed after intravenous injection of 120-mL iodinated contrast medium (Omnipaque TM 300 (iohexol), 300 mg I/mL, GE Healthcare Bio-Sciences, Madrid, Spain), at 2 mL/s. Portal phase was acquired at 65 s.
From January 2020 to May 2020, CT-WBS images were reobtained from PACS to measure EAT, visual scoring of CAC, subcutaneous adipose tissue (SCAT) and visceral adipose tissue (VAT) by a specialized reader. EAT was defined as all adipose tissue enclosed by the visceral pericardium, including all fat directly surrounding the coronary arteries. The radiologist examined the CT-WBS and semiautomatically quantified EAT including voxels with attenuation values between − 45 to − 190 Hounsfield units. Visual evaluation of CAC is feasible and correlates well with the Agatston score showing a good inter-reader evaluation19. We used the ordinal CAC scoring for visual assessment of CAC20. The images were reviewed to evaluate the linear extent of calcium along the four main coronary arteries (right, circumflex, left main and left anterior descending). Calcification was scored as follows: 0 (no visible calcification); 1 (mild calcification; less than one-third of the length of the artery showed calcification); 2 (moderate calcification; one-third to two-thirds of the artery showed calcification); 3 (severe calcification; more than two-thirds of the artery showed calcification). Each patient received a CAC score ranging from 0 to 12 (0: very low risk; 1–3: mild to moderately increased risk; 4–12: moderately to severely increased risk). VAT and SCAT measurement were quantified semi-automatically including voxels with attenuation values between − 45 to − 190 Hounsfield units. The abdominal muscular wall was traced manually to separate VAT from the SCAT. The VAT/SCAT ratio was also measured due to its known correlation to cardiovascular risk, beyond body mass index (BMI) and VAT21.
Statistical analysis
Demographic and clinical characteristics of patients were summarized using mean and standard deviation (SD), median, percentiles 25 (p25) and 75 (p75), and percentages. Correlations were evaluated with the estimation of the product-moment correlation coefficient (r). Multivariable linear regression models were used to assess the relationship between EAT/CAC and the severity of NAFLD, adjusting for potential confounders, including sex, obstructive sleep apnoea syndrome, hyperuricemia, statin therapy, aspirin therapy, smoking, and metabolic syndrome features, such as carbohydrate intolerance, diabetes, hypertension, dyslipidaemia, obesity, VAT, and VAT/SCAT ratio. All analyses were performed with Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). p < 0.05 was considered statistically significant.
Institutional Review Board statement
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of Universidad de Navarra (protocol code 2019.080; 7th of June 2019).
Results
A total of 49 NAFLD patients were included in the analyses. Table 1 displays main demographic and clinical characteristics of patients. Mean age was 58 years (SD: 11.28; range: 18–83). Most patients were men (81.6%) and overweight or obese (85.7%). Impaired fasting glucose or diabetes was diagnosed in 59.2% of patients. Mean EAT was 177.7 cm3 (SD: 95.03, p25: 116.87, p75: 222.30). Median CAC score was 1 (p25: 0, p75: 3, range: 0–9). EAT and CAC were directly correlated (r = 0.429; p = 0.002).
Table 1.
Characteristics of non-alcoholic fatty liver disease patients (n = 49).
| Characteristics | Total |
|---|---|
| Age, y | 58 (11.28) |
| Men, n (%) | 40 (81.63) |
| Waist circumference, cm | 105 (14.01) |
| Body mass index, kg/m2 | 30.1 (5.5) |
| Overweight, n (%) | 21 (42.86) |
| Obesity, n (%) | |
| Class 1 | 14 (28.57) |
| Class 2 | 4 (8.16) |
| Class 3 | 3 (6.12) |
| Total body fat (CUNBAE), % | 33.79 (8.15) |
| VAT, mL | 4558.57 (2113.57) |
| SCAT, mL | 6905.98 (3873.47) |
| VAT/SCAT ratio | 0.74 (0.41) |
| Hypertension, n (%) | 19 (38.78) |
| Impaired fasting glucose, n (%) | 18 (36.73) |
| Diabetes, n (%) | 11 (22.45) |
| Dyslipidaemia, n (%) | 24 (48.98) |
| OSA, n (%) | 7 (14.30) |
| Smoking, n (%) | |
| Current | 10 (20.41) |
| Former | 22 (44.90) |
| Never | 17 (34.69) |
| CVD family history, n (%) | 10 (20.41) |
| Antihypertensive therapy, n (%) | 16 (32.70) |
| Glucose lowering therapy, n (%) | 8 (16.30) |
| Metformin | 5 (10.20) |
| Glinides | 1 (2.00) |
| SGLT2 inhibitors | 3 (6.10) |
| DPP4 inhibitors | 3 (6.10) |
| Statin therapy, n (%) | 13 (26.50) |
| Aspirin therapy, n (%) | 3 (6.10) |
| Hypouricemic therapy, n (%) | 4 (8.20) |
| Glucose, mg/dL | 111 (30) |
| HbA1c, % | 6.0 (1.0) |
| Insulin, U/mL | 16.24 (8.86) |
| HOMA-IR | 1.36 (2.50) |
| Triacylglycerol, mg/dL | 127 (55) |
| Total cholesterol, mg/dL | 188 (44) |
| LDL cholesterol, mg/dL | 120 (57) |
| HDL cholesterol, mg/dL | 50 (15) |
| ALT, IU/L | 36 (23.40) |
| AST, IU/L | 23 (11.57) |
| AST/ALT | 0.7 (0.25) |
| ALP, IU/L, median (p25, p75) | 47 (28, 74) |
| GGT, IU/L | 64 (33.66) |
| Platelet count, × 103/µL | 228 (62.97) |
| Albumin, g/dL | 4.78 (0.32) |
| Urate, mg/dL | 11.2 (15.3) |
| Creatinine, mg/dL | 3.9 (13.8) |
| Urine albumin to creatinine ratio, mg/g | 4.9 (2.2) |
Values are expressed as mean (SD), unless otherwise stated.
ALT Alanine transaminase, ALP alkaline phosphatase, AST aspartate transaminase, BMI body mass index, CUNBAE Clínica Universidad de Navarra-Body Adiposity Estimator, CVD cardiovascular disease. DPP4 dipeptidyl peptidase 4, GGT gamma-glutamyl transpeptidase, HbA1c hemoglobin A1c, HOMA-IR homeostatic model assessment of insulin resistance, OSA obstructive sleep apnea, SCAT subcutaneous adipose tissue, SGLT2 sodium-glucose co-transporter-2, VAT visceral adipose tissue.
Mean liver stiffness was 6.28 kPa (SD: 3.06, p25: 4.30, p75: 7.30, liver stiffness ≥ 8.2 kPa: 16.3% of patients). Mean FIB-4 Score was 1.08 (SD: 0.48, p25: 0.72, p75: 1.40). Mean NFS was − 1.45 (SD: 1.21, p25: − 2.44; p75: − 0.68). According to FIB-4 Score, 73.5% of the patients were classified as with mild fibrosis or absence of significant fibrosis (FIB-4 Score < 1.30) and 26.53% of the patients were classified as with an indeterminant range fibrosis status (FIB-4 Score ≥ 1.30 and ≤ 2.67). No patient had a FIB-4 Score above 2.67. According to NFS, 46.9% of patients had absence of significant liver fibrosis (NFS ≤ 1.455), 49.0% were classified as with an indeterminant range fibrosis status (NFS: ≥ − 1.455 and ≤ 0.676) and 4.1% had presence of significant liver fibrosis (NFS: > 0.676).
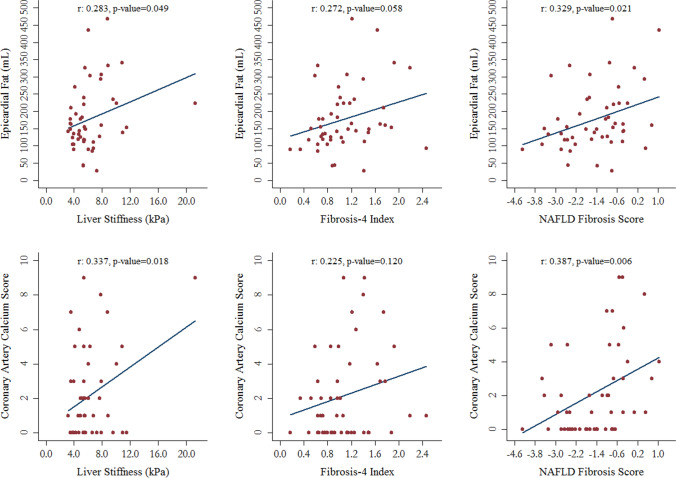
Liver stiffness showed a direct association with both EAT (r = 0.283, p-value = 0.049) and CAC levels (r = 0.337, p-value = 0.018) (Fig. 2). The level of liver fibrosis measured by the NFS was moderately associated with EAT (r = 0.329, p-value = 0.021) and CAC (r = 0.387, p-value = 0.006) (Fig. 2). The correlations between FIB-4 Score and EAT or CAC were direct but relatively weak and not statistically significant (Fig. 2).
Figure 2.
Correlations of liver stiffness and fibrosis serum markers with epicardial fat and coronary artery calcium levels.
The identified associations between liver stiffness and EAT remained largely unchanged after adjusting for sex, carbohydrate intolerance/diabetes, obstructive sleep apnea syndrome, hyperuricemia, and obesity in the multivariable regression models (Table 2). Likewise, NFS relationship with EAT remained after adjusting for sex, carbohydrate intolerance, hypertension, hyperuricemia, statin therapy, aspirin therapy, obesity, smoking and VAT/SCAT ratio as independent predictor variables (Table 2).
Table 2.
Multiple regression analysis with epicardial adipose tissue as the outcome variable.
| Model | Liver stiffness (kPa) | Fibrosis-4 Index | NAFLD Fibrosis Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient | SE | p value | Regression coefficient | SE | p value | Regression coefficient | SE | p value | |
| Unadjusted | 8.767 | 4.342 | 0.049 | 53.805 | 27.719 | 0.058 | 25.819 | 10.815 | 0.021 |
| 1 | 8.719 | 4.258 | 0.046 | 47.390 | 27.845 | 0.096 | 25.188 | 10.629 | 0.022 |
| 2 | 9.222 | 4.435 | 0.043 | 59.920 | 28.850 | 0.043 | 25.763 | 10.974 | 0.023 |
| 3 | 8.730 | 4.397 | 0.053 | 54.709 | 28.652 | 0.062 | 27.294 | 11.401 | 0.021 |
| 4 | 7.976 | 4.441 | 0.079 | 53.526 | 27.542 | 0.058 | 23.771 | 11.268 | 0.040 |
| 5 | 6.845 | 4.147 | 0.106 | 41.906 | 26.421 | 0.120 | 18.752 | 10.702 | 0.086 |
| 6 | 8.642 | 4.151 | 0.043 | 49.594 | 26.720 | 0.070 | 21.557 | 10.777 | 0.051 |
| 7 | 8.305 | 4.109 | 0.049 | 45.050 | 26.717 | 0.099 | 23.946 | 10.277 | 0.024 |
| 8 | 8.704 | 4.325 | 0.050 | 53.989 | 27.588 | 0.056 | 24.328 | 11.001 | 0.032 |
| 9 | 8.735 | 4.358 | 0.051 | 51.416 | 28.340 | 0.076 | 25.375 | 11.458 | 0.032 |
| 10 | 8.816 | 4.346 | 0.048 | 77.369 | 27.545 | 0.007 | 27.409 | 10.833 | 0.015 |
| 11 | 9.045 | 4.573 | 0.054 | 53.191 | 28.681 | 0.070 | 25.942 | 11.233 | 0.026 |
| 12 | − 3.754 | 3.820 | 0.334 | 45.719 | 26.709 | 0.098 | 11.258 | 10.786 | 0.305 |
| 13 | 6.068 | 4.642 | 0.201 | 67.333 | 37.009 | 0.079 | 30.679 | 12.711 | 0.022 |
| 14 | 7.361 | 4.247 | 0.090 | 57.001 | 26.269 | 0.035 | 19.652 | 11.203 | 0.086 |
| 15 | 8.575 | 4.249 | 0.050 | 52.207 | 30.636 | 0.096 | 16.009 | 13.117 | 0.229 |
SE standard error. Model 1: adjusted for sex. Model 2: adjusted for carbohydrate intolerance. Model 3: adjusted for diabetes. Model 4: adjusted for hypertension. Model 5: adjusted for dyslipidaemia. Model 6: adjusted for obstructive sleep apnoea syndrome. Model 7: adjusted for hyperuricemia. Model 8: adjusted for statin therapy. Model 9: adjusted for aspirin therapy. Model 10: adjusted for obesity. Model 11: adjusted for smoking. Model 12: adjusted for visceral adipose tissue. Model 13: adjusted for visceral adipose tissue/subcutaneous adipose tissue ratio. Model 14: adjusted for presence of carbohydrate intolerance, hypertension, dyslipidaemia, and obesity (number of factors). Model 15: adjusted for presence of carbohydrate intolerance/diabetes, hypertension, dyslipidaemia, and obesity (number of factors).
The relationship between liver stiffness and CAC was preserved even after adjustment for sex, obstructive sleep apnea syndrome, hyperuricemia, statin therapy, aspirin therapy, smoking, and metabolic syndrome features, such as VAT, VAT/SCAT ratio, carbohydrate intolerance/diabetes, hypertension, dyslipidemia, and obesity (Table 3). Similarly, the association between NFS and CAC remained statistically significant after adjustment for sex, obstructive sleep apnea syndrome, hyperuricemia, statin therapy, aspirin therapy, smoking, carbohydrate intolerance/diabetes, hypertension, dyslipidemia, obesity, excepting for VAT (p = 0.263) and VAT/SCAT ratio (p = 0.054). In the number of factors multiple regression analysis, the p-value for the LE association with CAC was 0.017 and for the NFS association with CAC was 0.094.
Table 3.
Multiple regression analysis with coronary artery score as the outcome variable.
| Model | Liver stiffness (kPa) | Fibrosis-4 Index | NAFLD Fibrosis Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient | SE | p value | Regression coefficient | SE | p value | Regression coefficient | SE | p value | |
| Unadjusted | 0.289 | 0.118 | 0.018 | 1.227 | 0.775 | 0.120 | 0.840 | 0.292 | 0.006 |
| 1 | 0.288 | 0.116 | 0.017 | 1.063 | 0.782 | 0.180 | 0.824 | 0.288 | 0.006 |
| 2 | 0.286 | 0.121 | 0.022 | 1.194 | 0.812 | 0.148 | 0.862 | 0.294 | 0.005 |
| 3 | 0.276 | 0.115 | 0.021 | 0.963 | 0.779 | 0.223 | 0.733 | 0.303 | 0.020 |
| 4 | 0.230 | 0.113 | 0.048 | 1.209 | 0.715 | 0.097 | 0.657 | 0.288 | 0.027 |
| 5 | 0.202 | 0.095 | 0.040 | 0.670 | 0.626 | 0.290 | 0.505 | 0.247 | 0.047 |
| 6 | 0.285 | 0.108 | 0.011 | 1.076 | 0.721 | 0.143 | 0.687 | 0.282 | 0.019 |
| 7 | 0.287 | 0.119 | 0.020 | 1.196 | 0.791 | 0.137 | 0.833 | 0.295 | 0.007 |
| 8 | 0.284 | 0.103 | 0.008 | 1.241 | 0.682 | 0.075 | 0.688 | 0.266 | 0.013 |
| 9 | 0.286 | 0.108 | 0.011 | 0.886 | 0.736 | 0.235 | 0.636 | 0.292 | 0.035 |
| 10 | 0.308 | 0.122 | 0.015 | 1.603 | 0.822 | 0.057 | 0.921 | 0.302 | 0.004 |
| 11 | 0.255 | 0.122 | 0.043 | 1.353 | 0.774 | 0.087 | 0.842 | 0.293 | 0.006 |
| 12 | 0.282 | 0.118 | 0.024 | − 0.076 | 0.934 | 0.936 | 0.409 | 0.358 | 0.263 |
| 13 | 0.372 | 0.107 | 0.002 | 0.281 | 1.040 | 0.789 | 0.698 | 0.348 | 0.054 |
| 14 | 0.241 | 0.112 | 0.036 | 1.334 | 0.712 | 0.067 | 0.626 | 0.296 | 0.040 |
| 15 | 0.264 | 0.106 | 0.017 | 1.574 | 0.774 | 0.048 | 0.566 | 0.330 | 0.094 |
SE standard error. Model 1: adjusted for sex. Model 2: adjusted for carbohydrate intolerance. Model 3: adjusted for diabetes. Model 4: adjusted for hypertension. Model 5: adjusted for dyslipidaemia. Model 6: adjusted for obstructive sleep apnea syndrome. Model 7: adjusted for hyperuricemia. Model 8: adjusted for statin therapy. Model 9: adjusted for aspirin therapy. Model 10: adjusted for obesity. Model 11: adjusted for smoking. Model 12: adjusted for visceral adipose tissue. Model 13: adjusted for visceral adipose tissue/subcutaneous adipose tissue ratio. Model 14: adjusted for presence of carbohydrate intolerance, hypertension, dyslipidaemia, and obesity (number of factors). Model 15: adjusted for presence of carbohydrate intolerance/diabetes, hypertension, dyslipidaemia, and obesity (number of factors).
Discussion
The existence of liver fibrosis in NAFLD is strongly associated with an increased risk of major adverse cardiovascular events (MACE)22,22, however, it is a challenge to identify subclinical CVD in the highly prevalent NAFLD population. We observed an association of liver stiffness and fibrosis serum markers with subclinical CVD (evaluated through EAT and visual assessment of CAC) in a population of NAFLD patients without personal history of CVD. The novelty of our findings is that fibrosis markers, even at indeterminant range, associate with EAT and CAC. Therefore, our findings support fibrosis screening in NAFLD patients through LE or non-invasive fibrosis markers in clinicians’ daily practice, though they may be useful tools to identify patients with early or subclinical cardiovascular abnormalities.
Cross-sectional and longitudinal studies have found an association between CAC and NAFLD. In 2016, a retrospective study (n = 4731 patients) was conducted to assess the association between NAFLD (diagnosed by ultrasound) and the progression of coronary atherosclerosis (assessed by CT-TS)8. The mean duration of follow-up was 3.9 years. The annual rate of progression of coronary calcium was higher in patients with NAFLD (22%) vs. without NAFLD (17%) regardless of cardiovascular and metabolic risk factors (p = < 0.001). Subsequently, a similar cross-sectional study (n = 2345 patients) confirmed the association between NAFLD and CAC (crude OR: 1.631, 95% CI: 1.295–2.053, adjusted OR: 1.348, 95% CI: 1.030–1.765)9. Moreover, Lee et al.24 found that NAFLD was more closely associated with CAC than abdominal obesity (assessed by waist-hip ratio) after analyzing data from 21,335 male participants. Regarding the presence of fibrosis, our findings are consistent with Song et al. who recently found that non-invasive fibrosis markers (NFS and FIB-4 score) were associated with coronary atherosclerosis measured by Agatston Score (n = 665 NAFLD patients)25. We assessed CAC score through visual assessment, which can be easily measured in non-gated non-contrast CT scans, shows good inter-reader correlation, and has an excellent acceptability for non-cardiac radiologists26.
Moreover, we simultaneously evaluated EAT which is considered an earlier subrogate marker of CVD, mediator of cardiac arrhythmias12 and left ventricular diastolic dysfunction13. In 2013, Mahabadi et al.27 determine that EAT predicted MACE in the general population in participants from the prospective population-based Heinz Nixdorf Recall cohort (n = 4093 participants, age 59.4 years, 47% male) during a follow-up period of 8.0 ± 1.5 years. Doubling of EAT was associated with a 1.5-fold risk of coronary events when adjusting for cardiovascular risk factors (CVRF) (HR: 1.54; 95% CI: 1.09–2.19), which remained unaltered after further adjustment for CAC (HR: 1.50; 95% CI: 1.07–2.11). Ito et al.28, described an association of EAT with an obstructive plaque in patients with zero CAC (n = 1308). EAT may represent an earlier and improved marker of subclinical cardiovascular risk in asymptomatic patients who, therefore, require intensive CVRF treatment. Concerning NAFLD and EAT, in 2014, Petta et al.10 found a higher EAT (assessed by echocardiography) in 147 NAFLD patients with histological severe fibrosis vs. mild fibrosis (8.5 ± 3.0 vs. 7.2 ± 2.3 mm; p = 0.006) even after adjusting for gender, age, prediabetes/diabetes, visceral obesity, NASH, and severe steatosis (OR: 1.22; 95% CI: 1.01–1.47; p = 0.04). Authors concluded that EAT is an independent indicator of the severity of liver fibrosis. Similarly, in 2018, EAT (measured by CT-TS) was significantly associated with NAFLD even after adjusting for main CVRF (OR: 1.407; 95% CI: 1.117–1.773) in a 2238 Chinese cohort29. In the CAESAR (CArdiometabolic risk, Epicardial fat, and Subclinical Atherosclerosis Registry) (n = 2277 patients) registry, both, EAT (assessed by echocardiography) and the presence of NAFLD were associated with CAC, finding a stronger association to CAC with the presence of increased EAT in NAFLD patients11. Interestingly, in a recent prospective, cross-sectional study (n = 100 patients with diabetes)30, EAT (measured by magnetic resonance [MR]) was higher in patients with NAFLD. Liver fat content and liver fibrosis (measured by MR elastography) were positively and independently associated with EAT. Authors concluded that liver fat content and fibrosis may increase cardiovascular risk, and therefore, identification of even mild forms of NAFLD in individuals with diabetes may warrant treatment and aggressive risk factor modification to reduce cardiovascular risk. A recent meta-analysis31 including thirteen studies (n = 2260 patients) confirmed that EAT was significantly increased in NAFLD patients, it correlated with the severity of hepatic steatosis and with fibrosis.
The underlying mechanisms linking NAFLD and CVD are complex. Both entities share common risk factors, nevertheless, NAFLD seems to be an independent risk factor for CVD1,1,1,1. In NAFLD, hepatic fat accumulation results from lipolysis of adipose tissue (AT), dietary fats and de novo lipogenesis with insufficient compensatory fatty acid β-oxidation6. This disbalance leads to plasma lipoprotein abnormalities characterized by atherogenic dyslipidemia, which results in fatty acids accumulation and oxidation within the subendothelial vascular wall contributing to endothelial dysfunction7 and, subsequent atherosclerosis33. Apolipoprotein-B lipoproteins may activate toll-like receptors, and, consequently, innate immune system contributing to systemic inflammation34. Additionally, in obesity, adipocytes suffer hypertrophy and hyperplasia with subsequent hypoxia, along with an increase in the number of inflammatory cells that leads to a downregulation of adiponectin expression and secretion of pro-inflammatory factors through activation of metabolic signaling pathways. The concentrations of inflammatory cytokines in the different phenotypes of obesity35, supports the continuum of AT dysfunction involved in the onset of systemic low-grade inflammation leading to oxidative stress34. The underlying systemic inflammation, in addition, to ectopic dysfunctional fatty tissue accumulation enhances insulin resistance (IR) and may lead to β cell dysfunction in the pancreas34,34. In the liver, IR results in compensatory hyperinsulinemia which increases the fatty acid uptake, alters triglycerides transportation and reduces β-oxidation37. Progressive liver fat accumulation leads to an activation of hepatic macrophages with a broaden intensification of the pro-inflammatory cytokine activity that is associated with oxidative stress-mediated lipotoxicity and gradual accumulation of excess extracellular matrix through hepatic stellate cells activation7. Systemic inflammation and oxidative stress support subclinical organ dysfunction and gradually contributes to the development and progression of atherosclerosis32,32. Furthermore, the secretion of proinflammatory cytokines from EAT reinforces the prevailing systemic proinflammatory activity, which in turn results in pathological changes of the coronary arteries10,10 and/or structural changes of the contiguous myocardium which can eventually lead to the development of cardiac arrhythmias12 and left ventricular diastolic dysfunction13. In summary, AT dysfunction39 and IR34 are important mechanisms linking both entities, although, additional mechanisms, like dysbiosis and genetic susceptibilities, may interplay a role in the pathophysiology of NAFLD and CVD32,32.
Current guidelines recommend detailed CVRF assessment in patients with NAFLD41. Nonetheless, NAFLD is highly prevalent, and clinicians need an accurate and effective CVD assessment tool, regardless of the presence of traditional risk factors. CAC provides predictive evidence beyond that provided by standard CVRF or risk scores in different ethnic groups42. Studies have evidenced that simple visual assessment of the CAC on non-gated thorax CT scan accurately predict incident cardiac events in hospital inpatients43 and in an outpatient setting44. Several studies have evidenced similar prognostic values and a high degree of agreement between simple visual assessment of CAC and Agatston Score CAC assessment45–47. In some populations (i.e. COPD), the visual scaling score of CAC was equally accurate as the Agatston score for prevalent CAD and performed better than the Agatston score in predicting incident cardiac events48. With current evidence, in 2016, the Society of Cardiovascular Computed Tomography (SCCT) and the Society of Thoracic Radiology made a class I recommendation in their guidelines for routine CAC visual assessment on non-gated chest CT scans regardless of the indication for the scan49. In 2018, the SCCT published the CAC Data and Reporting System (CAC-DRS) to create a standardized method to communicate findings of CAC using traditional Agatston score categories and novel visually estimated CAC50. In 2020, the British Society of Cardiovascular Imaging, British Society of Cardiac Computed Tomography, and British Society of Thoracic Imaging recommended the visual quantification of CAC in their consensus statement51.
With strong evidence of CVD as a major comorbidity in NAFLD, the use of a simple visual assessment of CAC in readily available images may help to identify patients who may benefit from aggressive CVRF modification in a primary prevention setting. We consider that CAC assessment through visual assessment may be implemented in daily practice for CVD detailed assessment in patients with NAFLD and potential fibrosis assessed through LE and fibrosis markers. Likewise, as current guidelines recommend, our study highlight the importance of NAFLD screening in persons at high CVD risk not only to identify advanced stages of liver fibrosis but also to open therapeutic possibilities that may control CVRF and prevent CVD.
Finally, it is of interest to investigate if the improvement of NAFLD severity may possibly reduce CVRF. A reduction of carotid intima media thickness has been evidenced as NAFLD severity improves52. Physicians need to be aware of surveillance and early intervention in NAFLD (weight loss, cardiovascular protection, insulin sensitization and lipid reduction) as the strategy to improve cardiovascular and liver outcomes.
Our findings are relevant in clinical practice as it throws light in the mechanism underlying the association of NAFLD severity with subclinical CVD and suggest potential approaches for CVD prevention. Nevertheless, our study has various limitations: first, it is limited by the retrospective design and a relatively small sample size, however, this exploratory study has a clinically well-characterized cohort with suitable evaluation of subclinical CVD and may be useful to warrant further research. Second, our results derived from middle-aged Spanish adults, though it should be interpreted with caution when applied to different populations. Larger studies are needed to clarify the relationship between liver stiffness and non-invasive fibrosis markers with subclinical CVD. Further research should investigate whether reversal of hepatic steatosis/fibrosis is accompanied by a comparable decrease in overall cardiovascular risk and whether subclinical cardiovascular disease evaluation (through EAT and CAC visual scale) is a cost-effective measure, at least for patients with potential liver fibrosis, independently of the existence of other cardiovascular risk factors.
Conclusions
LE and noninvasive serum fibrosis markers may be useful tools for identifying NAFLD patients at risk for cardiac abnormalities. The findings of this study suggest direct associations between LE or noninvasive serum liver fibrosis marker, specifically NFS, with EAT and CAC in patients with NAFLD. The association between LE and CAC remained even after adjusting for metabolic syndrome features (carbohydrate intolerance, diabetes, hypertension, dyslipidaemia, visceral fat, and obesity). Our results highlight the importance of assessing the severity of NAFLD not just to determine potential fibrosis but also to aid clinicians in the task of reducing CVRF. In NAFLD, subclinical CVD assessment, through CAC visual scoring, may be a useful tool in patients with potential liver fibrosis, independently of the existence of other CVRF.
Author contributions
All authors review the manuscript. C.M.P., G.B. and J.E. designed the study. C.M.P. and A.E. performed data collection. C.M.P. wrote the first draft of the manuscript. J.M.N. and J.E. review and edit the manuscript. All authors contributed to the critical discussion of the results and approved previous versions and the final version of the article, including the authorship list.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to containing information that could compromise the privacy of research participants but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action. Diabetes Care. 2017;40(3):419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 4.Budd J, Cusi K. Nonalcoholic fatty liver disease: What does the primary care physician need to know? Am. J. Med. 2020;133(5):536–543. doi: 10.1016/j.amjmed.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Oikonomidou AC, et al. Evaluation of subclinical cardiac damage in biopsy-proven nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2021;34(3):424–430. doi: 10.20524/aog.2021.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology. 2016;63(6):2032–2043. doi: 10.1002/hep.28392. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinn DH, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: A retrospective cohort study. Gut. 2017;66:323–329. doi: 10.1136/gutjnl-2016-311854. [DOI] [PubMed] [Google Scholar]
- 9.Wu R, et al. Nonalcoholic fatty liver disease and coronary artery calcification in a northern Chinese population: A cross sectional study. Sci. Rep. 2017;7(1):9933. doi: 10.1038/s41598-017-09851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petta S, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: Association with the severity of liver disease. J. Hepatol. 2015;62(4):928–933. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Kim BJ, Cheong ES, Kang JG, Kim BS, Kang JH. Relationship of epicardial fat thickness and nonalcoholic fatty liver disease to coronary artery calcification: From the CAESAR study. J. Clin. Lipidol. 2016;10(3):619–626.e1. doi: 10.1016/j.jacl.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Patel K, Hwang T, Se Liebers C, Ng FS. Epicardial adipose tissue as a mediator of cardiac arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2022;322(2):H129–H144. doi: 10.1152/ajpheart.00565.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma W, et al. Association of epicardial fat thickness with left ventricular diastolic function parameters in a community population. BMC Cardiovasc. Disord. 2021;21(1):262. doi: 10.1186/s12872-021-02071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosa MI, et al. Association between epicardial adipose tissue and stroke. Front. Cardiovasc. Med. 2021;8:658445. doi: 10.3389/fcvm.2021.658445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabkin SW. Epicardial fat: Properties, function and relationship to obesity. Obes. Rev. 2007;8(3):253–261. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 16.Foucher J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut. 2006;55(3):403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleiner DE, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Sterling RK, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: Systematic review and meta-analysis. Circ. Cardiovasc. Imaging. 2013;6(4):514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 20.Shemesh J, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541–548. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 21.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Vilar-Gomez E, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology. 2018;155(2):443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 24.Lee MK, et al. Higher association of coronary artery calcification with non-alcoholic fatty liver disease than with abdominal obesity in middle-aged Korean men: The Kangbuk Samsung Health Study. Cardiovasc. Diabetol. 2015;14:88. doi: 10.1186/s12933-015-0253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song DS, Chang UI, Kang SG, Song SW, Yang JM. Noninvasive serum fibrosis markers are associated with coronary artery calcification in patients with nonalcoholic fatty liver disease. Gut Liver. 2019;13(6):658–668. doi: 10.5009/gnl18439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einstein AJ, et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J. Am. Coll. Cardiol. 2010;56(23):1914–1921. doi: 10.1016/j.jacc.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahabadi AA, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2013;61(13):1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, et al. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int. J. Cardiol. 2013;167(6):2852–2858. doi: 10.1016/j.ijcard.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Meng X, et al. Epicardial adipose tissue volume is associated with non-alcoholic fatty liver disease and cardiovascular risk factors in the general population. Ther. Clin. Risk Manag. 2018;14:1499–1506. doi: 10.2147/TCRM.S168345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouha SS, Nguyen P, Bettencourt R, Sirlin CB, Loomba R. Increased severity of liver fat content and liver fibrosis in non-alcoholic fatty liver disease correlate with epicardial fat volume in type 2 diabetes: A prospective study. Eur. Radiol. 2018;28(4):1345–1355. doi: 10.1007/s00330-017-5075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, et al. Association of epicardial adipose tissue with non-alcoholic fatty liver disease: A meta-analysis. Hepatol. Int. 2019;13:757–765. doi: 10.1007/s12072-019-09972-1. [DOI] [PubMed] [Google Scholar]
- 32.Francque SM, van der Graaff D, Kwanten WJ, et al. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65(2):425–43. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. CMLS. 2018;75(18):3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasper P, et al. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021;110(7):921–937. doi: 10.1007/s00392-020-01709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira FG, et al. Metabolically unhealthy and overweight phenotypes are associated with increased levels of inflammatory cytokines: A population-based study. Nutrition. 2022;96:111590. doi: 10.1016/j.nut.2022.111590. [DOI] [PubMed] [Google Scholar]
- 36.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11(3):677. doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou YY, et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018;30(6):631–636. doi: 10.1097/MEG.0000000000001075. [DOI] [PubMed] [Google Scholar]
- 39.Hamjane N, Benyahya F, Nourouti NG, Mechita MB, Barakat A. Cardiovascular diseases and metabolic abnormalities associated with obesity: What is the role of inflammatory responses? A systematic review. Microvasc. Res. 2020;131:104023. doi: 10.1016/j.mvr.2020.104023. [DOI] [PubMed] [Google Scholar]
- 40.Perdomo CM, Garcia-Fernandez N, Escalada J. Diabetic kidney disease, cardiovascular disease and non-alcoholic fatty liver disease: A new triumvirate? J. Clin. Med. 2021;10(9):2040. doi: 10.3390/jcm10092040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol.64(6), 1388–402 (2016). [DOI] [PubMed]
- 42.Detrano R, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 43.Yu C, et al. Incidentally identified coronary artery calcium on non-contrast CT scan of the chest predicts major adverse cardiac events among hospital inpatients. Open Heart. 2021;8:1–6. doi: 10.1136/openhrt-2021-001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J. Cardiovasc. Comput. Tomogr. 2017;11:8–15. doi: 10.1016/j.jcct.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Shao L, et al. Prognostic value of visually detected coronary artery calcification on unenhanced non-gated thoracic computed tomography for prediction of non-fatal myocardial infarction and all-cause mortality. J. Cardiovasc. Comput. Tomogr. 2017;11(3):196–202. doi: 10.1016/j.jcct.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Wu M-T, et al. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: Concordance study with dedicated cardiac CT. Am. J. Roentgenol. 2008;190(4):923–928. doi: 10.2214/AJR.07.2974. [DOI] [PubMed] [Google Scholar]
- 47.Htwe Y, et al. Coronary artery calcification on low-dose computed tomography: Comparison of Agatston and Ordinal Scores. Clin. Imaging. 2015;39(5):799–802. doi: 10.1016/j.clinimag.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt SP, et al. Visual estimate of coronary artery calcium predicts cardiovascular disease in COPD. Chest. 2018;154:579–587. doi: 10.1016/j.chest.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecht HS, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J. Cardiovasc. Comput. Tomogr. 2017;11(1):74–84. doi: 10.1016/j.jcct.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Hecht HS, et al. CAC-DRS: Coronary Artery Calcium Data and Reporting System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT) J. Cardiovasc. Comput. Tomogr. 2018;12(3):185–191. doi: 10.1016/j.jcct.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Williams MC, et al. Reporting incidental coronary, aortic valve and cardiac calcification on non-gated thoracic computed tomography, a consensus statement from the BSCI/BSCCT and BSTI. Br. J. Radiol. 2021;94(1117):20200894. doi: 10.1259/bjr.20200894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatia L, et al. Improvement in non-alcoholic fatty liver disease severity is associated with a reduction in carotid intima-media thickness progression. Atherosclerosis. 2016;246:13–20. doi: 10.1016/j.atherosclerosis.2015.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to containing information that could compromise the privacy of research participants but are available from the corresponding author on reasonable request.