Abstract
Given the general beneficial effects of antioxidants-rich foods on human health and disease prevention, there is a continuous interest in plant secondary metabolites conferring attractive colors to fruits and grains and responsible, together with others, for nutraceutical properties. Cereals and Solanaceae are important components of the human diet, thus, they are the main targets for functional food development by exploitation of genetic resources and metabolic engineering. In this review, we focus on the impact of antioxidants-rich cereal and Solanaceae derived foods on human health by analyzing natural biodiversity and biotechnological strategies aiming at increasing the antioxidant level of grains and fruits, the impact of agronomic practices and food processing on antioxidant properties combined with a focus on the current state of pre-clinical and clinical studies. Despite the strong evidence in in vitro and animal studies supporting the beneficial effects of antioxidants-rich diets in preventing diseases, clinical studies are still not sufficient to prove the impact of antioxidant rich cereal and Solanaceae derived foods on human
Keywords: antioxidants, polyphenols, carotenoids, cereals, Solanaceae, pre-clinical studies, food diet
1. Introduction
The tremendous variety of fruits and grains that provide colour to cereals and Solanaceae, respectively, is mainly due to carotenoids and polyphenols (e.g., flavonoids), two classes of phytochemicals characterized by antioxidant properties. These bioactive compounds, together with ascorbic acid, vitamin E, and others, are considered health-promoting components of the human diet, given their capacity of exerting scavenging activity on reactive oxygen species (ROS) and free radicals, protecting cells and tissues from oxidative stress, and the onset of chronic diseases. Polyphenols exert a well-known beneficial effect on gut microbiota and, at cellular level, trigger the signaling cascade of endogenous antioxidant defences [1,2]. In many crops, the great diversity in fruit and grain colour allows the selection of cultivars with different amounts and types of pigments. Plant breeding has invested significant resources to select pigmented varieties with a higher content in carotenoids and flavonoids and, consequently, higher antioxidant potential [3]. Cereals and Solanaceae are relevant dietary sources of nutrients in the human diet [4] with a vast and well characterized genetic diversity. The available genetic knowledge could allow replacing the current varieties with new ones enriched in pigments and the antioxidant content of food crops could even be reinforced by using specific crop managements. These efforts could translate into vegetables and grains with an increased nutritional value that may result in antioxidant-rich foods.
This review aims at exploring genetics and new cereals and Solanaceae breeding programs for antioxidant-rich foods, particularly carotenoids and polyphenols, together with the effect of agronomic practices and food processing on levels of these compounds, and their potential impact on human health.
2. The Pigment Biosynthetic Pathways
2.1. The Conserved Flavonoid Pathway
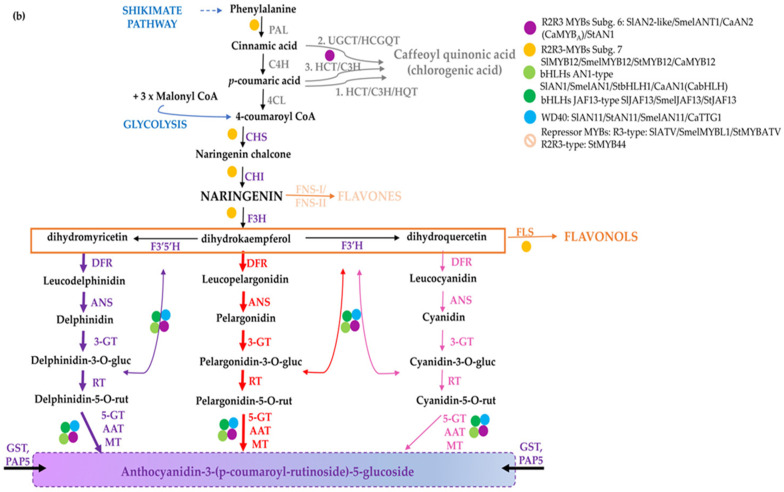
Flavonoids share a general C3–C6–C3 structure with two aromatic rings linked by a three-carbon bridge, where the degree of oxidation of the C-ring defines the various flavonoid sub-classes. They are synthesized through the phenylpropanoid pathway [5,6] (Figure 1), consisting of several branches that, starting from the aromatic amino acid phenylalanine, lead to the synthesis of distinct classes of compounds: anthocyanins, proanthocyanidins (PA or condensed tannins), phlobaphene pigments and the non-pigmented (i.e., not visible to human eye) flavonols, flavones, and isoflavones [7]. Both flavones and flavonols generally have little colour, but they may influence anthocyanin colour through co-pigmentation. The number of hydroxyl groups on the B-ring (side chain decorations) of the flavonoids influences their ability to scavenge different free radicals [8], whereas the stability of anthocyanins is highly influenced by glycosylation and acylation. As an example, anthocyanin 3-disaccharides are generally more stable than 3-monosaccharides and 3,5-disaccharides, and acylation of sugars with aromatic or aliphatic acids further increases their stability, allowing co-pigmentation and a shift towards blue [9]. In general, ferulic acid is predominantly present in cereal grains, whereas chlorogenates are the major phenolic compounds found in solanaceous fruits. In cereals, pigmented tissues mainly accumulate cyanidin and to a lesser extent pelargonidin-derived anthocyanins or phlobaphenes in corn pericarp (Figure 1a). Delphinidin and pelargonidin in the form of anthocyanidin-3-(p-coumaroyl-rutinoside)-5-glucoside are the predominant type in Solanaceae, whereas the latter is restricted to red potato tubers [10] (Figure 1b). The flavonoid pathway is largely regulated at transcriptional level by transcription factors which modulate the spatio-temporal expression of biosynthetic genes [11]. R2R3-MYBs are considered the master regulators of the flavonoid pathway, since they can act as independent regulatory proteins, thereby activating biosynthetic genes and other regulators, e.g., bHLHs. Furthermore, they can also act as members of multimeric complexes known as MBW, consisting of R2R3-MYBs, bHLHs, and WD40s [12].
Figure 1.
The flavonoid biosynthesis and its regulation in cereal and Solanaceae crops. Scheme of the pathway leading to the production of flavonoids and phenolic acids in monocots (a) and dicots (b). Phenylalanine is first deaminated by PAL to produce cinnamic acid, then converted by C4H into p-coumaric acid, which can enter the synthesis of hydroxycinnamic acids (i.e., chlorogenic acid and other phenolics) or it can be conjugated with coenzyme A to produce 4-coumaroyl-CoA by 4CL. CHS catalyses the condensation of p-coumaroyl-CoA with three molecules of malonyl-CoA to naringenin chalcone, then converted to the flavanone naringenin by CHI. Indeed, naringenin may be converted to flavones by FNSI/FNSII (e.g., maysin in maize), to the red phlobaphenes, derived from condensation of the 3-deoxy flavonoids apiferol and luteoforol (a) and to dihydroflavonols, such as dihydrokaempferol (DHK), which can then be used by F3′H to produce dihydroquercetin (DHQ) or by F3′5′H to form dihydromyricetin (DHM) (b). Dihydroflavonols are then converted to flavonols (e.g., kaempferol, quercetin, and myricetin) by FLS. Downstream, DFR reduces the dihydroflavonols to their respective colourless leucoanthocyanidins, which are then converted into the coloured anthocyanidins (e.g., cyanidin, pelargonidin, and delphinidin). The main enzymes catalyzing the reactions in the pathway are reported in violet. Regulatory proteins belonging to diverse classes of transcription factors are marked with coloured dots. Branches leading to different classes of flavonoids and anthocyanins are indicated with diverse colours; in B, thicker purple and red arrows highlight the branch leading to the most abundant derived anthocyanins. The name of the enzymes is detailed in the abbreviation list.
A different regulation of flavonoid biosynthesis occurs in monocots and dicots. In monocots, all anthocyanin biosynthetic genes are coordinately activated by MYB and bHLH proteins In Figure 1a, the biosynthetic pathway leading to the diverse classes of polyphenols in monocots and the major regulatory determinants in maize kernels are shown [13,14,15], for a review refer to [14]. In dicots biosynthetic genes are commonly divided in Early Biosynthetic Genes (EBGs), which catalyse the early steps leading to the production of flavonoids from CHS to naringenin chalcone and related branches, and Late Biosynthetic Genes (LBGs) specific to anthocyanins and proanthocyanidins [16]. These structural genes are differently regulated. Indeed, the expression of the EBGs is modulated by a set of functionally redundant R2R3-MYBs, while the activation of LBGs is regulated by a ternary MBW complex [11]. In Figure 1b, the biosynthetic route and the major regulatory determinants of anthocyanin biosynthesis in tomato fruits are shown [17,18,19].
2.2. Carotenoids Colouring Fruits and Grains
Carotenoids are tetraterpenoids sharing a carbon chain structure with 9–11 conjugated double bonds. The ability of these pigments to absorb light of specific wavelengths is related to the length of the carotenoid chain, which in turn affects colouration in the range from yellow to red [20,21]. Unlike anthocyanins, carotenoids are synthesised in bacteria, algae, and plants [22]. The presence of carotenoids is particularly noticeable in fleshy fruits, such as tomato that accumulate a large amount of the red lycopene [23]. Accumulation of lycopene increases with ripening in tomato fruit and is considered as part of the ripening process [24]. In cereals, the carotenoid β-hydroxylases (HYD) reached the highest expression level at the last stage of development, suggesting that carotenoids (at least xanthophylls) are still actively synthesized in mature grains, raising the nutritional value of kernels [25]. Carotenoids are divided into carotenes (lycopene, α-, and β-carotene) and xanthophylls (lutein, and zeaxanthin) based on the absence or presence of oxygen, respectively, and their biosynthetic route is shown in Figure 2 [26,27,28,29,30,31,32,33,34].
Figure 2.
A simplified overview of carotenoid pathway in cereal and Solanaceae crops. The first step in carotenoid biosynthesis is the condensation of two GGPP molecules to form phytoene catalysed by PSY, which is the main rate-limiting step in solanaceous fruits and cereal grains. Further, the conversion of phytoene to lycopene via sequential desaturation and isomerization reactions is catalysed by a set of four enzymes (PDS, ZISO, ZDS, and CRTISO). Lycopene is at the branch point of carotenoid synthesis since it can be cyclized to ß-carotene or α-carotene by LCYB and LCYE. Downstream, the sequential hydroxylation and epoxidation of these carotenes leads to the production of diverse xanthophylls (e.g., lutein and zeaxanthin). Regulatory proteins belonging to diverse classes of transcription factors are marked with coloured dots. The name of the enzymes is detailed in the abbreviation list.
3. Antioxidants in Cereals
Wheat, rice, and maize are the most important food crops and contribute more than 50% of calories of the human diet, while other cereals, e.g., sorghum and barley, are relevant only in some regions [35]. Due to their relevance in the diet, even a small increase in antioxidant content could have a large impact on health, but only if whole grain flour is used, since bran (pericarp) and germ are the major sources of dietary fibre, phenolics, vitamins, and minerals.
3.1. Wheat
In bread and durum wheat, the most important compounds with antioxidant properties, are phenolic acids (mainly ferulic and p-coumaric acids), and, to a lesser extent, carotenoids (mainly lutein), tocopherols, and tocotrienols (Table 1). Flavonoids also accumulate in wheat grains, mainly as apigenin derivatives [36]. Nevertheless, current evidence ruled out a significant antioxidant effect for this class of compounds [37]. Carotenoids in the form of lutein are the main determinants of the yellow colour of semolina, which has important implications for the marketing of durum wheat end-products [38], while apigenins contribute to the yellow colour of Asian alkaline noodles made from bread wheat [36]. Lastly, although rare in the currently cultivated wheat germplasm, anthocyanins provide the blue, purple, or red colour to the grains of pigmented wheats. Antioxidants have a specific tissue distribution into the grains. Most of the phenolic acids are contributed by outer layers of kernels (pericarp, and aleurone) or by germ [36,39], whereas the higher biomass of endosperm, compared to bran and germ, determine an overall low concentration of phenolic acids in kernels [39]. On the contrary, carotenoids are equally distributed across the kernel, with endosperm having the highest content [40]. The pattern of antioxidant content across kernel layers plays, therefore, a pivotal role for interpreting the variability of this trait in wheat germplasm. As antioxidants are mainly stored in the outer layers of mature grains, surface area to volume ratio of kernels is an important parameter that should be considered for exploiting the natural variation of this trait. Consequently, reducing grain size can improve the total antioxidant content in wholemeal flours at expenses of grain yield. Notably, not all phenolics present in wheat are equally bioavailable, they can be soluble free, soluble conjugated, or insoluble bound linked to polymers of the plant cell wall [41], with a per cent amount of 1, 22, and 77, respectively [42]. Soluble compounds have the highest bioavailability [42], with germination increasing soluble phenolics content and antioxidant activity [43], although some conjugated forms may also exert their biological activity upon cleavage by intestinal microbes [39]. To date, many studies have assessed natural variation in the total antioxidant content and its components in bread [44,45,46] and durum wheat (for phenolic acids [47,48]; for carotenoids and tocols [49,50]) considering varieties, landraces, and wild ancestors [51]. Some evidence might suggest higher amounts of phenolic acids in wild and some primitive subspecies, compared to durum wheat cultivars, and no significant differences between old and modern varieties [52,53], nevertheless these studies do not provide indication on the seed size and, consequently, the results cannot be considered conclusive. On the other hand, some publications, comparing historical and modern wheat varieties (e.g., [37,54]) suggested that breeding has qualitatively affected the profiles of phenolic compounds and isomer forms. Environmental factors (E, including year, location, as well as agronomic practices), genetic (G) effects, and GxE all contribute to determining phenotypic variation for total antioxidant contents [48,49,50,52,55], with environmental effects larger than genotypic differences [56]. Since plants produce secondary metabolites to cope with stress, both E and GxE effects are expected, the latter because of different levels of genetic tolerance to stress among genotypes. On the contrary, carotenoid content is largely controlled by genetic factors with a heritability value as high as 0.7 [57]. Consequently, breeding for yellow semolina colour has succeeded in a substantial quantitative effect on carotenoid content which is indeed significantly increased in modern varieties compared to landraces and old cultivars [49,58]. Two loci on durum wheat chromosomes 7A and 7B which co-localized with PSY genes [59], the rate limiting step of carotenoid biosynthesis [38], have a major effect on carotenoid content [60] and have been targets of successful phenotypic selection for yellow pigment content by recent breeding. As nutraceutical alternative, recent studies on durum wheat mutants in enzymes of the carotenoid biosynthesis provide support to successful engineering of the synthesis of β-carotene for the final goal of provitamin A biofortification of kernel durum wheat [61]. Grain pigmentation due to anthocyanin accumulation in pericarp or in aleurone can also represent a strategy to increased antioxidant activity in wheat. Two loci, Pp3 and Pp-1, originally identified in a tetraploid Abyssinian wheat accession, are responsible for the activation of the anthocyanin biosynthesis pathway in wheat pericarp [62]. Purple pigmentation was initially used to clearly mark feed-type common wheat, but presently purple-grained commercial wheat cultivars for human nutrition have been released in several countries, e.g., the Canadian bread purple wheat AnthoGrain [63]. The genetic trait conferring blue aleurone has been introgressed in cultivated wheat from wheat relatives (Agropyron species and diploid wheat) [64] and then pyramidized with purple pericarp to obtain wheat line with a high anthocyanin content [65].
Table 1.
Typical range of concentrations of phytochemicals in cereal crops. * no antioxidant effect since they are bound.
| Antioxidant Class | Prevalent Compounds | Main Modifications | Distribution into the Grain | Heritability Range for Total Content | Rice (O. sativa) | Wheat (T. aestivum; T. urum) | Maize (Z. mays) | Sorghum (S. bicolor L.) |
|---|---|---|---|---|---|---|---|---|
| Carotenoids | Lutein, Zeaxanthin, β-carotene, β-cryptoxanthin | endosperm, aleurone and germ (cereals) [66] | 0.7 in pigmented wheat and durum wheats [38] and in RILs of durum wheats [57] | negligible amount [67] | 4–12 μg/g [49] | blue: 0.18 μg/g; yellow: 0.13–60 μg/g [68,69] | 0.02 to 0.85 μg/g [70] |
|
| Vitamin E | α and β-tocopherol, α and β-tocotrienols | aleurone, pericarp and germ (maize) | 19.36–63.29 μg/g [71] | 2.81–29.62 μg/g [70] | ||||
| Phenolic acids | ferulic acid, coumaric acid, syringic acid, vanillic acid, caffeic | mainly ester or ether linked to cell wall polimers | aleurone and germ (maize), bran, embryo and endosperm (rice) | 0.63 in tetraploid wheat collection [39] | 78.83–317.4 μg/g [72] | 550–1700 μg/g; 27–45 μg/g [37,39] |
blue: 2.60 mg/g; yellow: 3.2 mg/g; white: 2.6 mg/g [73]; purple: 2.66–17.5 mg/g [74] |
1.0–29.6 mg/g [75] |
| Flavonoids | apigenin derivatives | mainly conjugated (glycosides) | pericarp (maize) | 886–2863 (μg rutin equivalent/g) [76] | 70–110 μg/g [36] | red: 27.53 mg/g [77] | * 0–23 mg/g [78] | |
| Anthocyanins | cyanidin 3-O-glucoside | only conjugated (glycosides) | aleurone, pericarp, cob; pericarp (maize) | 0.93 in pigmented wheat and durum wheats [38] | 87.54 mg (Cyanidin-3-glucoside equivalent/100 g rice grain) [72] | purple: 8–50 μg/g; red: 1–25 μg/g; blue: 80–170 μg/g [38] | blue: 0.66 mg/g; purple: 1.64 mg/g [79]; purple cob: 3.1–12.6 mg/g [79,80] | 1–3 mg/g [35] |
3.2. Rice
The most popular rice cultivars have a white pericarp, but variability for pericarp colour is well present in rice with accessions characterized by red, black, and purple pericarp mainly due to accumulation of proanthocyanidins that show a higher antioxidant content than white rice [81,82]. Two loci, namely Rc and Rd, encoding a bHLH transcription factor and DFR, respectively, control proanthocyanidin biosynthesis [83] and the accumulation of both anthocyanins and proanthocyanidins. Allelic variants in these loci account for a large fraction of the phenotypic variability for flavonoids and phenolic content [84,85], while additional loci and corresponding candidate genes controlling the content of ferulic acid have been identified [85]. In addition, OsB2 and OsC1 (Kala4 and Kala3, respectively) have been proposed as responsible for anthocyanin accumulation in pericarp of black-grained rice [22,86]. A biotechnological approach has significantly contributed to increase the content of pigment with nutritional value in rice. The best-known example is Golden Rice. In rice endosperm of all existing wild and cultivated varieties, ß-carotene and other carotenoids have never been identified as the carotenoid biosynthetic pathway is blocked owing to lack of expression of PSY, PDS, and ZDS in rice endosperm [87,88]. Golden Rice is a genetically modified rice in which the biosynthetic pathway of carotenoids in the endosperm was restored by transforming plants with daffodil PSY and crtI from Erwinia uredovora under the control of an endosperm-specific or constitutive promoter, respectively [87]. Golden Rice plants carrying both transformation events showed 1.6 µg/g DW mean concentration of carotenoids in kernels, still insufficient to prevent diseases correlated with low carotenoid intake. As the limiting step of carotenoid biosynthesis in Golden Rice was recognized to be the daffodil PSY gene, a panel of different heterologous PSY genes was tested and the combination between maize PSY and E. uredovora crtI genes resulted in a carotenoid content up to 37 µg/g DW (Golden Rice 2, [89]), making this genetically modified rice useful to fight carotenoid malnutrition. Bioavailability studies demonstrated that 100–150 g of cooked Golden Rice 2 (50 g uncooked) provides about 435 µg retinol and is as effective as β-carotene supplementation in oil capsules in providing vitamin A to 6–8 years-old Chinese children, reaching 60% of Chinese Recommended Daily Allowance of vitamin A [90].
3.3. Maize
Maize is naturally rich in antioxidants and pigmented varieties of corn could be used as healthy ingredients for the formulation of novel gluten-free products [91]. The antioxidant activity of maize flour is dependent on phenolics, mainly ferulic acid, carotenoids, phlobaphenes, and anthocyanins, which confer different colours to five types of corn (white, yellow, blue, purple, and red). The composition in antioxidants and bio-active compounds (phenolics, proanthocyanidins, carotenoids, and tocols) was recently found to vary in differently pigmented corns [92]. The total phenolic content ranges from 2.60 to 17.56 mg of gallic acid equivalents (GAEs)/g with the lowest content in white corn and the highest in Andean purple corn, whereas yellow corn contains 3.20 mg of (GAEs)/g [73,74]. Phenolic acids are mainly represented by ferulic acid and p-coumaric acid, particularly in pigmented varieties [73]. In maize seeds, anthocyanin accumulation is genetically determined by MYB and bHLH regulatory genes and can occur in the aleurone (blue corn, C1 R1), in the pericarp (purple corn, B1 Pl1) or, eventually, in both, with an anthocyanin content ranging between 0.66 and 1.64 mg of cyanidin 3-glucoside equivalents/g [77]. An alternative source of anthocyanins suitable for the development of dietary supplements is the purple corn cob with a concentration range of 3.1 to 12.6 mg of cyanidin-3-glucoside equivalents/g [79,80]. Finally, red pigmentation depends on the MYB P1 gene, which activates phlobaphene accumulation (27.53 mg/g) in pericarp of maize seeds [91]. Yellow corn varieties contain lutein, zeaxanthin, β-cryptoxanthin, and β-carotenes mainly in the germ, followed by aleurone and endosperm [68,93,94]. Carotenoid concentration can also vary depending on genotypes, ranging from 0.18 μg of β-carotene/g in the blue corn [69] up to 60 μg of xanthophylls/g in high carotenoid varieties [95]. Several synthetic anthocyanin-rich varieties suitable for human consumption (i.e., polenta, sweet corn, and popcorn) have been selected aiming to increase the daily intake of these bioactive compounds [96,97] and when tested the higher scavenging ability of anthocyanin-rich varieties compared to control yellow varieties was confirmed [13].
3.4. Barley
Barley has only a limited use for direct human consumption, especially in the form of hull-less (naked) grains. Whole grain barley was shown to reduce the risk of developing chronic diseases thanks to health-promoting properties of different bioactive compounds, such as β-glucan fibers and antioxidants [98]. Barley is richer in vitamin E (α-tocopherol and α-tocotrienol) than most cereals and contains high levels of free or conjugated phenolic compounds with antioxidant properties ([99], Table 1). In addition, barley genotypes can accumulate anthocyanins in different seed external layers resulting in yellow, blue, purple, or black barley grains. The Ant28 locus on chromosome 3H controls the biosynthesis of proanthocyanidins, that accumulate in the seed coat giving a yellow colour [95]. Two MYB transcription factors on chromosome 4H are the best candidate genes for the accumulation of anthocyanins in the aleurone layer, that results in blue grains [100]. On the other side, the accumulation of anthocyanins in the pericarp, controlled by Ant1 and Ant2 loci that determine the red pigmentation of awns and auricles, results in purple grains [101]. The heterogeneous distribution of these compounds can be exploited through pearling, to separate fractions enriched in specific compounds. For example, Martínez-Subirà et al. [102] have recently observed that the outermost 30% pearling fractions best exploit barley antioxidant capacity and could be used as a valuable source of functional ingredients. Biscuits containing different proportions of whole flour and pearling fractions from a purple hull-less barley genotype showed high in vitro antioxidant capacity [103]. Interestingly, genotypic differences influence the content of bioactive compounds more than the environment, and heat stress at the end of the growing season increased the phenolic compounds and their antioxidant capacity [104].
3.5. Sorghum
Sorghum grains, particularly red varieties, exhibit the highest values of total antioxidant capacity (400–500 μmol of Trolox equiv/g) among several crops (e.g., wheat, rice, oats, barley, maize, and potato) [105,106] and sources of natural antioxidants from plant foods [107,108]. Polyphenols are the major component of antioxidant capacity in sorghum grain and several studies [75,78,109] showed broad genetic diversity for their content, implying that breeding activity can improve antioxidant capacity in sorghum crop. For instance, Habyarimana et al. [109] and Salazar-López et al. [75] came across similar ranges of phenolic contents of, respectively, 0.6–20.73 and 1.0–29.6 mg equivalent of gallic acid/g (mg GAE/g) in diverse sorghum populations, with wild relatives outperforming the cultivated species. Polyphenols, particularly tannins, were long selected against in sorghum [108], because of their astringency and negative effect on animal feeding. As human interest shifted in favour of these compounds, it can be expected that such a trend is going to be reversed with wild relatives representing useful traits donors [110]. Quantitative traits loci for antioxidant content, grain colour, and antioxidant capacity were reported in sorghum [111] and two major genes were proved to be involved in the biosynthesis of polyphenols in grain [108,112]. They encode a MYB (Yellow seed1) and a WD40 (Tannin1) transcription factor, the latter having homology to the A. thaliana TTG1. Several other genes have been associated to variations in grain tannin and antioxidant activity, including two homologs of AtTT10 and AtTT4 [113,114].
4. Antioxidants in Solanaceae
The Solanaceae family comprises economically important staple crops, like tomato, potato, eggplant, and pepper. Millennia of domestication and breeding have selected many varieties, in which the levels and distribution of carotenoid and anthocyanin pigments vary widely in response to developmental and environmental stimuli (Table 2). Their accumulation in fruits is an important determinant of ripeness and quality and is frequently used to easily differentiate the products.
Table 2.
Typical range of concentration of phytochemicals in the edible fruit of Solanaceae varieties. * In eggplant fruits commercial maturity (stage B) precedes the physiological ripening (stage C). n.d. = not detectable.
| Typical Range of Concentration (mg/g) in the Edible Fruit of Cultivated Solanaceae Varieties | |||||||
|---|---|---|---|---|---|---|---|
| Antioxidant Class | Prevalent Compounds | Main Modifications | Distribution into the Fruit | Tomato (S. lycopersicum) | Potato (S. tuberosum) | * Eggplant (S. melongena) | Pepper (C. annum) |
| Carotenoids | lycopene and phytoene (tomato); antheraxanthin, violaxanthin, lutein; zeaxanthin (potato); luteolin (pepper) | n.d. | peel and flesh | Cultivated varieties (mg 100 g−1 FW) 7.8–18.1 (lycopene); 1.0–2.9 (phytoene) [133] | Cultivated varieties (mg/100 g DW): 1.1 (antheraxanthin); 0.8 (violaxanthin); 0.5 (lutein); 0.5 (zeaxanthin in S. tuberosum 4n); 2.2 (zeaxanthin in S. phureja 2n) [134], 0.3–3.6 (mg/100 g DW) in Andean landraces [135] | Local eggplant landraces 0.00146–0.00406 mg g−1 FW [136] | Red cultivars total carotenoids 13.51–43.32 mg/100 g DW; orange cultivars total carotenoids 109.69–190.43 mg/100 g DW; yellow cultivars total carotenoids 15.31–29.70 mg/100 g DW [137,138] |
| Phenolic acids | hydroxycinnamic acids (mainly CGA, caffeic acid) | mainly conjugated with organic acids | mainly in the flesh where they account for 70–90% of total phenolics in eggplant fruits; mainly in tuber skin (potato) | Cultivated varieties (mg 100 g−1 FW) 1.4–3.3 (CGA); 0.1–1.3 (caffeic acid) [133] | Cultivated varieties (tuber skin) 1020–2920 mg/100 g DW, CGA: 2.11 mg g−1 DW (cv. Bionica) [139,140]; total phenolics: 10.1–105.0 mg/100 g DW (Vitellotte), 6.7–108.0 mg/100 g DW (Blue Star) [141] | Cultivated eggplant CGA at stage A of fruit ripening (2319 mg/100 g DW) [142], 4 mg/100 g DW cv. Lunga Napoletana [143] | C. annuum cultivars 119.97 ± 3.44–2060.12 ± 20.56 mg GAE/100 g FW [127] |
| Flavonoids (considered as total content) | flavanones (naringenin chalcone), flavonols (mainly rutin, quercetin) | mainly conjugated (glycosides) | mainly in the fruit peel | Cultivated varieties (mg 100 g−1 FW) 0.9–18.2 (naringenin chalcone); 0.5–4.5 (rutin); 0.7–4.4 (quercetin) [133] | Cultivated varieties (tuber skin): total flavonoid content 510–960 mg/100 g DW [139], total flavonols 0–0,22 (Andean potatoes) and up to 3 (Phureja tubers) mg g−1 DW [144] | Cultivated varieties at commercial maturity (stage B) total flavonoid content 267.7 mg/100 g DW [145] | Cultivated varieties (mg 100 g−1 FW): 2.21 (quercetin); 4.71 (luteolin) (USDA) |
| Flavonoids (Anthocyanins) | mainly delphinidin, petunidin (potato), and pelargonidin-3-(p-coumaroyl-rutinoside9-5-glucoside | mainly conjugated (glycosides) | mainly in the fruit peel | n.d. | Anthocyanins: 16 (Andigenum group) and 41 (Phureja genotype) mg g−1 DW [144], 16.32 mg/100 g DW (Vitellotte), 18.12 mg/100 g DW (Magenta Love) [141] | cv. Lunga Napoletana D3R ∼1.2 mg/100 mg DW [143] | n.d. |
4.1. Tomato
Tomato displays a plethora of pigmented phenotypes; nevertheless, conventional commercial varieties exhibit red pigmented fruits due to the presence of lycopene [115]. Cultivated tomato fruits are generally devoid of anthocyanins [116] and the current black varieties are due to chlorophylls/carotenoids co-pigmentation [117]. The loss of anthocyanins occurred during the first wave of domestication [118], although under favourable environmental conditions (e.g., high light and/or cold temperatures), anthocyanin pigmentation can eventually be observed [119]. Indeed, the anthocyanin biosynthetic machinery is fully present in the fruits of cultivated tomato, but inactive due to mutations that occurred in regulatory players thus leading to the red fruits phenotype [120] for a recent review see [121]. The anthocyanin trait was reintroduced in modern tomato cultivars through the introgression of the Aft [18,122] and the atv genes [123,124] derived from S. chilense and S. cheesmaniae (L. Riley) Fosberg wild relative tomatoes, respectively. The resulting Aft/Aft atv/atv tomato line, commercially known as Sun Black™, shows the anthocyanin pigmentation limited to fruit skin [125]. Interestingly, total carotenoids content is not affected [126]. Using the same parental lines and breeding program of Sun Black™, the University of Oregon developed an independent variety, named “Indigo Rose”, with the same phenotype (https://extension.oregonstate.edu/news/purple-tomato-debuts-indigo-rose). Many biotechnological strategies have been exploited leading to a significant enrichment in lycopene, anthocyanins, and other flavonoids. Silencing the SGR1 gene via genome editing promoted the activity of PSY1 during fruit maturation, which increased the accumulation of lycopene (up to 5-fold) and β-carotene [127]. The pyramiding of mutations in four genes controlling the carotenoid biosynthesis allowed the accumulation of zeaxanthin, a carotenoid with high nutritional value absent in tomato fruits. The BSh allele of the CycB (B) gene (identified in wild accessions) promotes the accumulation of β-carotene up to 80% of total carotenoids. When BSh was introgressed in genotypes carrying high-pigment 3 (hp3, a mutation impairing in zeaxanthin epoxidase) [128], GREEN-STRIPE (gs, a mutation conferring green fruits) and high-pigment mutant hp2dg [129], the quadruple homozygous mutant BSh hp3 gs hp2dg accumulated >500 µg/g DW of zeaxanthin in fruits [130]. Metabolic engineering allows to accumulate anthocyanins up to 2 mg/g FW in Del/Ros1 purple tomatoes, a level comparable to small berries [120] as well as to drive the selective accumulation of other flavonoids. Indeed, the Cathie Martin’s research group developed several transgenic tomato lines enriched in polyphenols (Table 3); among them, the “Bronze” fruits showed the highest antioxidant capacity compared to both red and other polyphenol-enriched lines due to the simultaneous presence of different classes of polyphenols (flavonols, anthocyanins, and stilbenoids) [131,132].
Table 3.
Typical range of concentration of phytochemicals in naturally biofortified and metabolic engineered cereal and Solanaceae Crops.
| Concentration of Phytochemicals (mg/g) Cereal and Solanaceae Crops Obtained by Breeding or Metabolic Engineering | ||||
|---|---|---|---|---|
| Antioxidant Class | Rice (O. sativa) | Tomato (S. lycopersicum) | Potato (S. tuberosum) | Eggplant (S. melongena) |
| Carotenoids | Golden rice: 5.06 μg/g, Golden Rice 2: up to 37 µg/g DW [89] |
SRG1 mutants 5.1× lycopene [126]; “Sun Black” (Aft/Aft atv/atv, peel) 0.2 mg/g DW total carotenoid content [127]; “Bronze” (E8:MYB12, E8:Del/Ros, 35S:StSy) ∼0.55 mg/g DW total carotenoid content [132] | Golden potato (cv. Desiree) 3600-fold increase in beta carotene to 4.7 mg/100 g DW [146]; 4 mg/100 g DW zeaxanthin in S. tuberosum 4n [147]. From traces to 0.33 mg/100 g beta-carotene [148] | eggplant transgenic line EEF48:crtB 0.15 mg g−1 FW of β-carotene [149] |
| Phenolic acids | “Sun Black” (Aft/Aft atv/atv, peel) 0.6 mg/g DW of CGA, 8.6 mg/g DW total phenolic content [127]; “Yellow” E8:MYB12 15 mg g−1 DW CQAs equivalent to 22-fold higher levels, respectively [150] | 3.35-fold increases on average) [151] | ||
| Flavonoids (considered as total content) | “Sun Black” (Aft/Aft atv/atv, peel) 0.8 mg/g DW rutin [132], “Yellow” (E8:MYB12) 72 mg g−1 DW total flavonols equivalent to 65-fold higher levels [150]; “Bronze” (E8:MYB12, E8:Del/Ros1, 35S:StSy), “Indigo” (E8:MYB12, E8:Del/Ros1) ∼15 and 20 mg/g DW total flavonols, respectively [132] | Flavonols (4.50-fold increase on average) [151] | ||
| Flavonoids (Anthocyanins) | “Sun Black” (Aft/Aft atv/atv) 1.2 mg/g DW in fruit peel [127]; Del/Ros1 (E8:Del/Ros1) 14.7 mg g−1; “Indigo” (E8:MYB12, E8:Del/Ros1) ∼5–24 mg g−1 DW [131,132]; “Crimson” (E8:Del/Ros1, E8:AmDFR, f3′5′h) 5.3 ± 1.3 mg/g DW, “Magenta” (E8:Del/Ros1, E8:MYB12, E8:AmDFR, f3′5′h) 7.9 ± 2.3 mg/g DW total anthoyanins [131] | From 0.4 in wt to 3 ug/100 mg Petunidin (7×); from 0.04 in wt to up to 0.3 ug/100 g Pelargonidin (7×) [152] |
||
4.2. Potato
Cultivated potato varieties show a great variability in terms of accumulation of diverse xanthophylls in the tubers flesh and skin [153], ranging from 2 (white-fleshed) to 45 (yellow fleshed) mg/kg of FW [154]. Allelic variation in several genes involved in the biosynthesis of the carotenoids is responsible for the genetic diversity in tuber colour. Two alleles at the BCH2 gene [155] (Figure 2) explaining a large variation in carotenoid content are currently used in breeding [156] with carotenoid accumulation in tubers correlated with high CHY2 expression level [157,158]. The carotenoid profile of most diploid Andean native accessions of the Phureja group (collectively called papa amarilla, due to their yellow-orange tuber flesh colour) is characterized by a marked zeaxanthin accumulation, that can reach up to 85% of the total carotenoids in the tuber, while in Tuberosum group it seldom exceeds 24% [134]. Nevertheless, due to ploidy incompatibility, this trait cannot be easily transferred can be hardly transferred by conventional breeding in tetraploid cultivated varieties. The phenotype “high-zeaxanthin” is sustained by the presence of a defective allele at the ZEP locus (Figure 2) that impairs the synthesis of antheraxanthin and downstream carotenoids resulting in zeaxanthin accumulation, conferring the typical orange colour of the tuber flesh [157,158]. Cultivated potato varieties can also accumulate anthocyanins, mainly delphinidin- and pelargonidin-based molecules [10], conferring red or purple colour, while the Andean potato tubers show a wide variability in flesh and skin colour mainly due to a combination of anthocyanins and carotenoids [158]. Targeted genetic manipulation to improve nutritional quality of potato tubers has been accomplished by biotechnological approaches aiming at enhancing zeaxanthin content as well as other carotenoids. Of relevance, zeaxanthin concentration in tubers was tripled by silencing the LCY-E gene [149] and was increased up to >5 fold by ZEP gene silencing [150], with a single average-sized potato contributing about 0.5–0.6 mg of zeaxanthin in diet.
4.3. Eggplant
The pigmentation of eggplant fruits is associated to the presence of two anthocyanins: delphinidin-3-rutinoside (D3R) and nasunin (NAS), which accumulate at high amounts in the peel of black-purple and lilac genotypes, respectively, due to the activity of an acyltransferase [159]. β-carotene is only marginally present in eggplant fruits, but the transgenic expression of the crtB gene under the control of the fruit specific EEF48 promoter allowed to increase β-carotene content up to 30-fold compared to WT [158]. Cholorogenic acid (CGA) is the major soluble phenolic compound in eggplant predominantly accumulated in fruit flesh [141]. In cultivated eggplant varieties and in their wild relatives (i.e., S. incanum) a large genetic diversity for CGA content has been described, thus allowing breeders to introgress the high-CGA trait into commercial cultivars and to counter-select for low polyphenol oxidases (PPO) activity to limit the browning pleiotropic phenotype [160]. Recently, a gene editing strategy targeting the genes coding for PPO allowed to select genotypes enriched in beneficial phenolic compounds without undesired browning phenotypes [161].
4.4. Pepper
Peppers are characterized by a wide range of colours (varying from red to white), phenolics and carotenoid profiles [138], and pungency. Nevertheless, the molecular identity sustaining the different pigmentations is known only for the orange colour attributed to a splicing mutation in the ZEP gene leading to the accumulation of zeaxanthin [162]. Fruits consumed at the at the green stage are deficient in antioxidant content, due to the progressive accumulation of carotenoids, phenolics, flavonoids, and ascorbic acid in later stages of ripening [163]. Indeed, green pepper fruits have a total carotenoid content of the order of magnitude of 10–50 mg/100 g DW, while at full maturation carotenoid content can peak as high as 3000 mg/kg DW (e.g., in mature red paprika; [164]). The peculiar carotenoids of red peppers are capsanthin, capsorubin, and capsolutein [165]. Red fruits mainly contain xanthophylls including ketoxanthophylls and epoxydated forms of these carotenoids. Pepper carotenoids occur in esterified form, with fatty acids that make them insoluble in mature fruits [164]. Anthocyanin pigmentation is not appreciated by breeders, because restricted to unripe pepper fruits, thus determining a bitter taste and lack of sweetness [10].
5. Impact of Crop Management and Food Processing on Antioxidant Content
Besides genetic factors, different agronomic practices (e.g., fertilization), management (conventional vs. organic) and symbiotic association with arbuscular mycorrhizal fungi can affect polyphenols biosynthetic pathway [166]. In general, higher levels of flavonoids and antioxidants are observed at low nitrogen input [167], for instance, tomato fruits harvested from plants grown with low nitrogen supply tend to have a higher phenolic content [168]. Nevertheless, some exceptions are also reported, and anthocyanin accumulation was found higher in purple-blue potatoes fertilized with 120 kg ha−1 of nitrogen in the absence of phosphorus and potassium fertilization [169]. The effect of crop management (organic vs. conventional practices) cannot be univocally explained. A long-term study of tomatoes showed that total phenolics and flavonoids, in particular quercetin, naringenin, and kaempferol content, were higher in fruits from organic plots, a result associated to the reduced amount of available nitrogen in the organic management [170]. Potatoes from organic cultivation were significantly richer in polyphenols, including phenolic acids, and flavonoids, in comparison to the conventional ones, while higher concentrations of lutein were noted in conventional compared to the organic potatoes [171,172]. However, results are often contradictory due to the strong influence of both environment and genotype, suggesting that the beneficial effects of organic farming can be maximized by using new varieties developed for a low-input agriculture [173,174]. Oat [174] and rice [175] grains as well as eggplant fruits [176] exhibited no differences in the polyphenols content between organic and conventional farming. In some studies, higher soluble phenols and total flavonoids were found in organic pepper as compared to conventional one, in response both to pathogenic pressure and to a lower nitrogen availability [177], while in other works no differences were pointed out [178,179]. In response to higher incidence/severity of pest and disease damage, due to the ban of synthetic pesticides in organic farming and/or fertilization intensity, organic cultivation may lead to an intensive production of secondary metabolites, including those with antioxidant properties, as a defence mechanism [174]. Research on microbial communities that colonize plant surfaces (phyllosphere, and rhizosphere) and internal plant tissues (endosphere) has shown that these microorganisms can also modulate the plant secondary metabolism [180], leading to increased biosynthesis of antioxidant phytochemicals in response to stress [181,182]. Studies on crops treated with arbuscular mycorrhizal fungi have evidenced that secondary metabolites and antioxidant activities varied depending on the variety and on the composition of the mycorrhize inoculum, ranging from no effect to significantly increased antioxidant concentrations [183]. Mixes of Gomus species resulted efficient in increasing antioxidants in tomato (up to 18.5% lycopene) in controlled conditions (greenhouse) [184].
Cereals and Solanaceae often undergo transformation and/or cooking before being eaten and these processing heavily impact on the antioxidant content of foods [10,183]. In cereals, a first significant decay of bioactive compounds is observed after traditional milling with a significant amount of antioxidant lost with the bran [185,186]. Strategies to enhance the antioxidant potential of flours are the emerging techniques based on alternative milling methods (micronization, and microfluidization) [187,188] or dedicated fractionation processes (debranning/pearling and air-classification) to select fractions rich in phytochemicals of the external grain layers [182,189,190]. The level of bioactive compounds is further affected by processing and cooking [191]. In bread-making, major decreases in carotenoids and anthocyanins were shown during kneading and baking phases, respectively [192,193]. In pasta-making, the critical step was represented by the drying process, with a up to half decay in anthocyanins of its original content (in pasta made with purple durum wheat) [183]. Significant is also the partial leaching of bioactive compounds during pasta cooking [183,194]. Quite different is the impact of cooking on rice prepared following the Italian traditional “risotto”-like cooking style, in which during boiling, broth or water is completely reabsorbed by rice grains, causing a limited loss of anthocyanins with respect to carotenoids because of their different solubility [195].
In Solanaceae, after the preliminary preparation procedures (cutting and/or crushing, homogenization, and peeling) which are responsible for the enzymatic oxidation processes with a partial decay of antioxidants, the critical step is represented by thermal processing [191,196,197,198]. In tomato, during processing to produce tomato paste from fresh tomatoes, thermal treatment causes the breaking of protein aggregates where the pigment is associated inside the vegetable matrix, enhancing the carotenoid bioaccessibility [191]. A 3- to 4-fold increase of anthocyanins was found in potato after heating due to the enhanced chemical extractability of anthocyanins and depending on the variety used and the pH used in the extraction process [199]. Besides thermal treatments, other compounds of the food matrix affect the anthocyanin stability, such as metal ions, protein, fibers, and interaction with other phytochemicals [200,201]. In eggplants, thermal treatments lead to a decrease in bioactive compounds, with effects directly correlated with the increasing temperature [202,203]. On the contrary, frying or boiling potatoes may lead to important losses of carotenoids [201]. In purple eggplant, boiling caused more anthocyanin decrease than steaming [201]. A similar phenomenon has been reported for total anthocyanin of purple-fleshed potatoes boiled in 100 ◦C water with respect to steamed potatoes [203]. Innovative non-thermal treatments can be applied to protect bioactive compounds during food processing. High hydrostatic pressure processing was positively applied in tomato purees for preserving the bioactive compounds [204,205], while in chili pepper high hydrostatic pressure combined with a mild thermal treatment increased preservation and bio-accessibility of pigments [181,206].
6. Impact of the Cereal and Solanaceae-Based Foods Enriched in Antioxidants on Human Health: Preclinical and Clinical Studies
Although the in vitro antioxidant activity of plant polyphenols is higher than vitamins E and C [207], their low bioavailability and their conversion into metabolites by the gut microbiota and endogenous metabolism [208,209,210] suggest that polyphenols mainly exert their direct role of scavengers in the gut, whereas at cellular level they can function as molecular signals, able to activate the endogenous antioxidant defences [211,212]. Several studies have shown that polyphenols can activate the NRF2 factor and genes encoding for antioxidant response enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase, but they can also directly modulate their enzymatic activity [212,213]. Polyphenols are also well-known for their anti-inflammatory properties by modulating both cellular and molecular mediators of inflammation [214,215]. Regarding the anti-inflammatory effect, several studies have established that polyphenols can prevent excessive and/or chronic inflammation by reducing the activation of NF-κB, a transcription factor that regulates many genes of the inflammatory response, such as iNOS, COX-2, and pro-inflammatory cytokines, such as IL-6 and TNF-α [216]. They also inhibit the signaling cascade of MAPKs (i.e., p38, JNK, and ERK), again reducing the activation of pro-inflammatory cytokines, iNOS and COX-2 [217] and directly inhibit COX-1 and COX-2 enzymes thus reducing the production of prostaglandins E2 (PGE2) [218].
Taken together, the in vitro data indicate an overall bioactive potential of plant antioxidants for the prevention of oxidative stress and chronic inflammation, nevertheless the effectiveness of antioxidant-rich foods has also to be tested in preclinical studies and validated with human clinical trials.
6.1. Pre-Clinical Studies
Several in vivo studies indicate that supplementation with pigmented rice and wheat may contribute preventing metabolic syndrome by ameliorating the negative effects of high-fat or high-sugar diets. Table 4 summarizes the pre-clinical studies which highlight the health effects of antioxidant-rich cereals and Solanaceae foods. In mice models of diet-induced obesity, black rice was more effective than simvastatin in decreasing cholesterol metabolism and prevented hypertriglyceridemia, weight gain, insulin resistance with an improvement on gut microbiota dysbiosis [219,220,221,222]. Both red and black rice decrease atherosclerotic plaques in rabbits and increase the endogenous antioxidant status in apoliprotein E (apoE)-deficient mice [223,224,225]. Similarly, both black and purple wheat reduced total cholesterol, triglyceride and free fatty acid levels in serum, with restoration of insulin resistance and elevation of antioxidant enzymes [226]. Black rice also exerted a protective action against alcohol-induced liver toxicity and hepatic fibrosis induced by carbon tetrachloride as demonstrated by the decreased plasma levels of aspartate transaminase, alanine transaminase, and gamma glutamyl transferase [227,228]. Finally, an anti-aging effect of black rice was shown in a mouse model of D-galactose-induced senescence, by increasing the endogenous antioxidant response [229] and a neuroprotective effect of purple rice was suggested in rat models of Alzheimer’s disease, by preventing memory impairment and hippocampal neurodegeneration [230]. A wheat-based anthocyanin-rich diet also prevented deficits in working memory in a mouse model of Alzheimer’s disease induced by amyloid-beta and partially reversed episodic memory alterations compared to a diet based on non-pigmented wheat. Furthermore, pigmented wheat reduced alpha-synuclein accumulation in a mouse model of Parkinson’s disease and enhanced the expression of Arginase1 that marks M2 anti-inflammatory microglia in the brain [231]. Evidence from animal studies also support the beneficial effect of polyphenol-rich corn on a variety of chronic diseases, such as cardiovascular disease, obesity, diabetes, cancer, and some genetic diseases [215,232]. Dietary treatment with anthocyanin-rich corn was able to induce a long-lasting cardioprotection against ischemia-reperfusion injury by modulating the microbiota [233,234] and to prevent the cardiotoxic effects induced by doxorubicin, a chemotherapeutic drug widely used against solid and haematological tumors [235]. Several in vivo studies have shown the ability of purple corn to ameliorate hyperglycemia, insulin sensitivity and to retard diabetic nephropathy in diabetic db mice [236,237,238], to prevent high fat diet-induced obesity as well as to reduce obesity-associated inflammation [77,239,240,241]. Purple corn was also effective in reducing the development of trigeminal inflammatory pain in rats [242] and in preserving muscle function in a dystrophic mouse model, in which oxidative stress and inflammation trigger progressive muscle tissue loss [243].
Table 4.
Health effects of antioxidant-rich foods in preclinical studies. HFD, high-fat diet; HCD, high cholesterol diet; ATM, adipose tissue macrophages; Alzheimer’s disease (AD); Parkinson’s disease (PD); CCl4, carbon tetrachloride; MNU1, methyl-1-nitrosourea; GalN, D-galactosamine; and STZ, streptozotocine.
| Antioxidant-Rich Food | Health Effects in Animal Models | Refs. | |
|---|---|---|---|
| Cereals | Black rice | Improved hyperlipidemia and insulin resistance in rats on high-fructose diet | [219] |
| Reduced hyperlipidemia in rats fed HCD | [220] | ||
| Reduced dyslipidemia, induced optimal platelet function in rats fed HFD | [221] | ||
| Regulated cholesterol metabolism and improved dysbiosis of gut microbiota in mice fed HCD | [222] | ||
| Reduced ethanol-induced liver damage in rats | [227] | ||
| Attenuated liver injury and prevented fibrosis in CCl4-treated mice | [228] | ||
| Ameliorative effect in senescent mice induced by D-galactose | [229] | ||
| Black/red rice | Decreased atherosclerotic plaques, increased antioxidant status in rabbit fed HCD and in apoE-deficient mice | [223,224,225] | |
| Purple rice | Prevented neurodegeneration in a rat model of AD | [230] | |
| Black/purple wheat | Prevented obesity, hyperlipidemia, and insulin resistance in mice fed HFD | [226] | |
| Purple wheat | Preventive effect on cognitive functions in mouse models of AD and PD | [231] | |
| Blue corn | Reduced cardiac infarct size following ischemia-reperfusion in rats | [233] | |
| Purple corn | Long lasting cardioprotection against ischemia-reperfusion mediated by microbiota in mice | [234] | |
| Increased survival and reduced cardiac damages against Doxorubicin-induced cardiotoxic effects in mice | [235] | ||
| Reduced diabetes-associated renal fibrosis, angiogenesis, and mesangial and glomerulal inflammation in db/db mice | [236,237] | ||
| Prevented obesity and ameliorated hyperglycemia in mice fed HFD | [217,218,219] | ||
| Reduced obesity-associated inflammation by reprogramming of ATM in mice fed HFD | [77] | ||
| Reduced trigeminal inflammatory pain in rats | [239] | ||
| Delayed progression of muscular dystrophy reducing inflammation and oxidative stress in Sgca null mice | [240] | ||
| Solanaceae | Purple tomato | Delayed cancer development and increased life span in p53-/- mice | [120] |
| Reduced inflammation and induced antioxidant response in rat model of carrageenan-induced paw oedema | [244] | ||
| Bronze tomato | Reduced inflammation markers, modulated gut microbiota in Winnie mice | [132,246] | |
| Purple potato | Prevented gastrointestinal inflammation/cancers in pig fed HFD | [247] | |
| Reduced chronic intestinal inflammation in DSS-induced colitis in mice | [248,249] | ||
| Prevented obesity, hyperlipidemia, and insulin resistance in rats fed HFD | [252,253] | ||
| Attenuated hyperglycemia in STZ-induced diabetic rats. | [254] | ||
| Reduced obesity-associated oxidative damage in rats fed HCD | [256] | ||
| Suppressed GalN-induced hepatotoxicity via inhibition of lipid peroxidation and/or inflammation in rats | [255] | ||
| Purple/red potato | Reduced proliferation of the benzopyrene-induced stomach cancer in mice | [250] | |
| Red potato | Reduced MNU1-induced breast carcinogenesis in rats | [251] | |
| Inhibited hepatic lipid peroxidation in rats | [257] |
Pigmented-rich tomato or potato have a positive impact on health in animal studies. In mice, in vivo studies with polyphenol-rich tomato have suggested potential antitumoral and anti-inflammatory activities. Mice fed with an anthocyanin-rich diet from Del/Ros1 tomato had significantly prolonged lifespan in cancer-prone p53−/− mice [120] compared to red tomato. An extract from a V118 purple tomato significantly reduced carrageenan-induced paw oedema in rats in a dose-dependent manner compared to red conventional tomato, supporting a strong anti-inflammatory activity of purple tomato, associated to a reduction of oxidative stress, as evidenced by reduced lipid peroxidation, nitric oxide production, and increase of detoxifying enzymes [244]. Tomlinson et al. [245] reported strong and specific inhibitory effects on gut barrier pro-inflammatory cytokines and chemokines via SAPK/JNK and p38 MAPK pathways of engineered tomato extracts (enriched in anthocyanins from Del/Ros1 tomato or enriched in flavonols from AtMYB12-overexpressing tomato) by primary murine colonic epithelial cell-based inflammation assays. Based on this study, tomato lines with different combinations of polyphenols (Table 3) have been tested in a mouse model of inflammatory bowel disease (IBD), demonstrating that tomato enriched in flavonols, anthocyanins, and stilbenoids (named Bronze) were able to reduce/delay the symptoms as well as the dysbiotic intestinal microbiota associated to dextran sodium sulfate (DSS)-induced colitis, and showed a significantly diminished IL-6 and TNF-α levels. Interestingly, the combination of different polyphenols was more effective than single flavonoid classes [132,246]. A similar preventive effect on inflammatory cytokines and gut barrier function was observed using purple-fleshed potato on high-fat diet in pigs and on DSS-induced colitis in mice [247,248,249]. Purple and red potatoes reduced benzopyrene-induced stomach cancer and chemically induced breast cancer in rats [250,251]. Purple potato showed an anti-obesity effect in rats or mice and high-fat diet through p38 signaling pathway and UCP3 [252,253]. In addition, purple potatoes were reported to reduce hyperglycemia, oxidative stress, and overall food intake in diabetic rats [254] and to increase the antioxidant response pathway in hypercolesterolemic rats [255,256,257].
6.2. Clinical Studies
The in vitro and animal studies provide some evidence that antioxidants are beneficial for health and, consequently, the increase in antioxidants content has been pursued particularly in cereals and Solanaceae, since they represent relevant dietary sources of nutrients in the human diet. A plethora of scientific evidence, summarized in Table 5, suggests that increased consumption of fruits, vegetables and cereals represents an easy and practical strategy to significantly reduce the incidence of chronic diseases, such as cancer, cardiovascular diseases, type-2 diabetes, obesity, and other aging-related pathologies [250,251,252,253,254,255,256,257,258,259,260]. A large population-based study (i.e., 24,325 men and women aged ≥35 years) has evidenced that a high polyphenol antioxidant content (PAC) score in the diet [261] is associated in both genders with low-grade inflammation, evaluated as C-Reactive Protein levels, white blood cells, platelet count, and granulocyte to lymphocyte ratio [262], with a reduced mortality risk, including cerebrovascular and cancer mortality [263], and with a decelerated biological aging [264]. In addition, a meta-analysis including many randomized controlled trials reported that the consumption of polyphenol-rich berries significantly ameliorated lipid profiles, blood pressure, or vascular functions [265]. Nevertheless, the potential impact of antioxidant-rich cultivars in comparison with conventional cultivars in the context of a generally healthy diet have been studied so far in very few clinical studies and deserves further investigation. Much evidence supports the potential beneficial properties of whole grain cereals, a result associated to their unique composition in vitamins and minerals, unsaturated fatty acids, tocotrienols, tocopherols, soluble and insoluble fiber, phytosterols, stanols, sphingolipids, phytates, lignans, and lipophilic and hydrophilic antioxidants, such as phenolic acids [46,259,266,267,268]. The synergistic effect of these compounds, along with other nutrients, is responsible for health benefits associated to whole grain cereals [269]. Nutritional guidelines suggest increasing whole-grain intake by replacing refined grains with whole grains [270,271]. Many works have been concerned with the antioxidant properties, chemistry, and functions of coloured rice, wheat bran [272], and pigmented maize, nevertheless only very few studies in humans are available in which antioxidant-enriched grains are compared vs. control varieties. A cross-over acute study conducted to evaluate the effect of bread made from the anthocyanin-rich rice cultivar “Riceberry” on the postprandial glycemic response, glucagon-like peptide-1, antioxidant status, and subjective ratings of appetite on 16 volunteers, suggest a reduced glycemic response together with improvement of antioxidant status in healthy subjects [273]. A cross-over design, randomized, dietary intervention on 24 healthy subjects was carried out by Callcott [274] to assess the impact of pigmented rice (purple, red, and brown) consumption on antioxidant status and reduction of biomarkers of oxidative stress and inflammation. This study demonstrates that acute consumption of red and purple rice in a healthy population significantly increases antioxidant activity and decreases plasma MDA and proinflammatory cytokines. The brown rice variety did not affect any parameter tested. A clinical pilot study showed that consumption of anthocyanins from purple corn reduced plasma levels of inflammatory markers and improved the response to Infliximab, a chimeric monoclonal antibody against TNF-α, in Crohn’s Disease patients [275]. Liu and co-workers [276] in a clinical trial on 120 individuals affected with type-2 diabetes found that black wheat intake (69 g/d) for 5 w decreased serum levels of glycated albumin and prevented the increase in TNF-α and IL-6 levels compared with the control group, in which the subjects only received nutritional education and diet control. Although studies with larger sample sizes or longer durations are needed, the authors concluded the partial substitution of black wheat can alleviate or prevent hyperglycemia and inflammation in type-2 diabetes patients or in individuals at high risk of developing type-2 diabetes. In a randomized, single-blind parallel-arm study, Gamel et al. [277] found a modest improvement in plasma markers of inflammation and oxidative stress in overweight and obese adults with evidence of chronic inflammation (high-sensitivity C-reactive protein > 10 mg/L) upon consumption of both purple and regular wheat.
Table 5.
Health effects of antioxidant-rich foods in clinical studies. MDA, malondialdehyde; HCD, high-caloric diet; HCD, TNF-α,Tumor Necrosis Factor-α; and DNA, deoxyribonucleic acid.
| Antioxidant-Rich Food | Health Effects in Clinical Studies | Refs. | |
|---|---|---|---|
| Cereals | Antocyanin-rich rice | A reduced glycaemic response together with improvement of antioxidant status in healthy subjects | [273] |
| Purple/red rice | Significantly increases antioxidant activity and decreases plasma MDA and proinflammatory cytokines in healthy population | [274] | |
| Purple corn | Reduced plasma levels of inflammatory markers and improved the response to Infliximab, a chimeric monoclonal antibody against TNF-α, in Crohn’s Disease patients | [275] | |
| Pigmented wheat | Various medical advantages like obesity, type-2diabetes, cardiovascular disease, and cancer | [272] | |
| Black wheat | Decreased serum levels of glycated albumin and prevented the increase in TNF-α and IL-6 levels in patients with type 2 diabetes | [276] | |
| Purple wheat | Modest improvement in plasma markers of inflammation and oxidative stress in overweight and obese adults with evidence of chronic inflammation | [277] | |
| Solanaceae | Pigmented potatoes | Significant drop in blood pressure without weight gain in healthy adults | [279] |
| Reduced postprandial glycemia and insulinemia in healthy adults | [280,281] | ||
| Purple potatoes | Improved arterial stiffness in healthy adult | [282] |
While in cereal-based foods a relevant increase in the content of beneficial compounds can be better achieved moving from refined to whole grain products, tomato fruits and tomato-based products are both good sources of lycopene as well as other antioxidant components, including ascorbic acid and phenolic compounds. Recent genetic achievements have led to the selection of anthocyanin-rich tomato varieties that could significantly increase the beneficial effect of tomato on human health. Considering the promising results obtained in some in vitro and in vivo preclinical studies investigating the health potential of purple tomato, human studies aimed to validate these findings deserve to be undertaken [121].
Clinical studies showed beneficial effects of pigmented potato consumption in healthy adults through reduced inflammation and DNA damage [278], significant drop in blood pressure without weight gain [279] and reduced postprandial glycemia and insulinemia [280,281]. A small-scale intervention study has shown that purple potatoes improved arterial stiffness in healthy adult [282]. Eggplant rich of bioactive compounds, such as anthocyanins and phenolic acids, has been extensively investigated for its antioxidant properties, for a strong radical scavenging activity [283,284]. Nasunin, the major component of anthocyanin pigment of eggplant, has a potential bioactive role in the control of osteoblastic for bone health since in vitro studies have shown that nasunin prevents MC3T3-E1 osteoblast cells from oxidative damage [285]. It remains to be clarified whether the use of nasunin as a functional food ingredient in the diet may be relevant for human bone health depending on their bioavailability after ingestion [286]. Although the results of previous in vivo studies are promising, as they show that nasunin given to rats as a food supplement is well absorbed from the gastrointestinal tract [287], clinical trials to evaluate the effects of dietary nasunin on bone mass are lacking [283].
7. New Perspectives to Promote Antioxidant Response in Human Cells: Plant miRNAs with Cross-Kingdom Activity
Plant-derived foods also contain a class of small RNA that to date, only a few investigations have been performed for their antioxidant and beneficial effect on human health. Very recently, it has been reported that microRNAs (miRNAs) can transfer and regulate gene expression in a cross-kingdom manner [288]. Stable miRNAs derived from food plants may enter the mammals’ circulatory system and inhibit the production of target mammalian proteins These observations have been received with a mix of enthusiasm and scepticism [289]. This new type of regulatory mechanism, although not well understood, provides a fresh look at the relationship between food consumption and physiology [290]. For example, miRNA designed based on Fragaria vesca miR168, can reduce inflammation and prevent symptoms of multiple sclerosis at physiological concentrations [291]. The mechanism causing immune-modulating activity of plant miRNAs is poorly understood and may be due to the 2′-OH-methylation at the 3′ end and to the capacity of miRNAs to bind to toll-like receptor 3 (TLR3) and impairing TIR-domain-containing adapter-inducing IFN-β signalling. The orthologous from rice, osa-miRNA168, reduced T cell proliferation to a similar extent, thus suggesting the existence of analogous mechanisms in mono and dicotyledonous species [291]. This is also supported by the miRNAs found in human blood, which are mapping to corn, rice, and wheat [292]. There is scope for the breeding of crops with increased amount of miRNAs, as demonstrated by the similarities and differences in expression profiles between tissues of the same genotype as well as between genotypes, for example in alfalfa [293] and maize [294]. Also, it is possible to envision agronomic systems designed to optimize miRNAs biosynthesis [295]. Plant exosome-like nanoparticles can be taken up by immune system cells and exert antioxidant and anti-inflammatory effects [296]. As the exosomes which are absorbed by the intestinal macrophages are known to be rich in miRNAs [297,298], it may be of great interest to establish if they have anti-inflammatory and/or antioxidant functions [299] and develop innovative miRNA-based strategies to boost the impact of Solanaceae and cereals on human health.
8. Does a Diet including Antioxidant-Rich Cereals and Solanaceae Have an Impact on Human Health?
There is a growing interest in natural pigments as phytochemicals in food science and human nutrition, supported by preclinical, clinical, and large-scale population-based studies indicating their effect in preventing chronic diseases and suggesting a generalized concept according to which “the more coloured foods we eat the better the health benefits”. Cereals and Solanaceae have a dominant role in the human diet and improving their content of antioxidant compounds can have a strong impact on the total amount of antioxidant in human diet. A plethora of genetic studies have characterized the genetic diversity of these crops for content and composition in carotenoids and polyphenols and either traditional breeding or metabolic engineering works have been carried out in the last 20 years to select varieties with an increased content of beneficial compounds. Dedicated agronomic management practices and transformation processes can, to some extent, increase and preserve the antioxidant value of grains and fruits, but genetics (particularly genetic engineering) is the most robust strategy to enhance these compounds in edible parts of crops.
The data summarized in Table 1, Table 2 and Table 3 demonstrate the availability of cereals and Solanaceae varieties with an antioxidant content significantly higher than those of the most used varieties and preclinical studies support the potential benefits associated to the utilization of pigment-enriched varieties. Despite the strong evidence of pre-clinical and pilot studies (Table 4 and Table 5) suggesting that high polyphenol-enriched foods regimes lead to preventive effects against chronic diseases, an overall understanding of bioactivity, absorption/bioavailability and interactions among dietary bioactive compounds is still limited in terms of clinical studies. These considerations should influence that clinical studies are conducted to produce clear evidence on the potential impact of antioxidant-rich cereals- and Solanaceae-derived foods on human health.
Bioaccessibility and bioavailability are modified by many different factors, including food processing and mode of food consumption (cooked or fresh food, with or without fruit skin, etc.), chemical properties of the bioactive compounds, the ratios of the different phytochemicals in the ingested food matrix, other compounds present in the food matrix, and modifications which might occur during food digestion. To date, clinical studies (Table 5) are generally focused on specific single compounds and effects are studied on a limited number of markers; the study designs are different and are generally conducted on a small number of subjects with different characteristics (e.g., age, sex, nutritional status, ethnicity, etc.). Also, the influence of genetic factors on the response of individuals to specific phytochemicals must be considered. Further research is required to ascertain the impact of the consumption of antioxidant-rich cereals- and Solanaceae-derived foods on changes in microbiota and associated health benefits. Future studies should focus on understanding the complex interactions on bioaccessibility and bioactivity between multiple phytochemicals and other food components in different food matrices and further human intervention studies should be carried out with antioxidant-rich cereals- and Solanaceae-derived foods to strengthen the available evidence on their efficacy on preventing chronic diseases. Finally, to allow the comparison across the different studies, it is necessary to standardize the design of the experiments with specific reference to: (i) the adoption of common units for the definition of the phytochemicals amount; (ii) the type of extract and the diet administration in clinical studies; (iii) the clinical controls, both diet and model; and (iv) the amount scalability of antioxidant-rich food matrix from preclinical to clinical studies.
In conclusion, the application of pigmented cereals- and Solanaceae-derived foods in the reference healthy diet may lead to a new paradigm in public health nutrition and recommendation that is based on the use of long-term disease preventative measures. Nevertheless, an implementation of robust and comparable clinical trials on the effects of diets including antioxidant-rich cereals- and Solanaceae-derived foods is required.
The scientific gaps highlighted in this paper constitute the rationale of the FACCE JPI project: “SYSTEMIC—Knowledge hub on Nutrition and Food Security (systemic-hub.eu)”.
Gene and Enzyme Abbreviation List
| POD | peroxidase |
| APX | ascorbate peroxidase |
| MDA | malondialdehyde |
| OS | oxidative stress |
| ROS | reactive oxygen species |
| PAL | phenylalanine ammonia lyase |
| C4H | cinnamate 4-hydroxylase |
| C3H | p-coumarate 3′-hydroxlase |
| HQT | hydroxycinnamoyl CoA quinate hydroxycinnamoyl transferase |
| HCT | hydroxycinnamoyl CoA shikimate/quinate hydroxycinnamoyltransferase |
| UGCT | UDP glucose:cinnamate glucosyl transferase |
| HCGQT | hydroxycinnamoyl D-glucose:quinate hydroxycinnamoyl transferase |
| 4CL | 4-coumarate-CoA ligase |
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| F3H | flavanone 3-hydroxylase |
| F3′H | flavonoid 3′-hydroxylase |
| F3′5′H | flavonoid 3′,5′-hydroxylase |
| FNS | flavone synthase |
| FLS | flavonol synthase |
| DFR | dihydroflavonol 4-reductase |
| ANS | anthocyanidin synthase |
| LAR | leucoanthocyanidin reductase |
| ANR | anthocyanidin reductase |
| UFGT | UDP-glucose: flavonoid-3-O-glycosyltransferase |
| 3-GT | flavonoid 3-O-glucosyltransferase |
| RT | rhamnosyl transferase |
| 5-GT | flavonoid 5-O-glucosyl transferase |
| AAT | anthocyanin acyl transferase |
| MT | methyltransferase |
| GST | glutathione S-transferase |
| MATE | multi-antimicrobial extrusion protein |
| PAP5 | Putative Anthocyanin Permease 5 |
| DXS | 1-deoxy-D-xylulose 5-phosphate synthase |
| DXR | 1-deoxy-D-xylulose-5-phosphate reductor isomerase |
| MCT | 2-C-methyl-D-erythritol 4-phosphate cytidylyl transferase |
| CMK | 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase |
| MDS | 2-C-methyl-D-erythritol 2 4-cyclodiphosphate synthase |
| HDS | 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase |
| HDR | 4-hydroxy-3-methylbut-2-enyl diphosphate reductase |
| IPI | Isopentenyl-diphosphate delta-isomerase 1 |
| GGPP | Geranylgeranyl pyrophosphate synthase |
| PSY | Phytoene synthase |
| PDS | Phytoene desaturase |
| ZISO | 15-cis-zeta-carotene isomerase |
| ZDS | Zeta-carotene desaturase |
| CRTISO | Carotenoid isomerase |
| PTOX | Plastid terminal oxydase |
| LCYB1 | Lycopene beta-cyclase |
| CYCB | Chromoplast-specific lycopene beta-cyclase |
| LCYE | Lycopene epsilon cyclase |
| CYP97 | Cytochrome P450 |
| ZEP | Zeaxanthin epoxidase |
| VDE | Violaxanthin de-epoxidase |
| CHY | Beta-carotene hydroxylase |
| NXD1 | Neoxanthin-deficient 1 |
| ABA4 | Abscisic acid deficient 4 |
| CCD | Carotenoid cleavage dioxygenase |
| NCED | 9-cis-epoxycarotenoid dioxygenase |
| BCH2/CH2 | beta-carotene hydroxylase |
| CGA | Chlorogenic Acid |
| bHLH | basic helix loop helix |
| MYB | myeloblastosis |
| WD40 | tryptophan-aspartic acid repeat domains |
| SlAN2-like | S. lycopersicum Anthocyanin 2 like |
| SlAN1 | S. lycopersicum Anthocyanin 1 |
| SlAN11 | S. lycopersicum Anthocyanin 11 |
| SlMYB-ATV | S. lycopersicum MYB-atroviolacea |
| IN1 | Intensifier 1 |
| PAC1 | Pale Aleurone Color 1 |
| SGR1 | Stay Green 1 |
| TTG1 | Transparent Testa Glabra 1 |
| Aft | Anthocyanin fruit tomato |
| Atv | atroviolacea |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| iNOS | inducible nitric oxide synthase |
| COX-2 | cyclooxygenase-2 |
| MAPKs | mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinases |
| ERK | extracellular signal-regulated kinases |
| COX-1 | cyclooxygenase-1 |
| PGE2 | prostaglandins E2 |
| UCP3 | Uncoupling Protein 3 |
| apoE | apoliprotein E |
| SAPK/JNK | |
| IBD | inflammatory bowel disease |
| DSS | dextran sodium sulfate |
| T2DM | Type 2 diabetes |
| CVD | Cardiovascular disease |
| TNF-α | Tumor Necrosis Factor-α |
| IL-6 | Interleukin-6 |
Author Contributions
Conceptualization, L.C.; writing original draft preparation and review and editing, L.B., K.P., A.P., A.M., E.A., M.F., D.B.M.F., E.M., A.T., A.F., R.P., I.G.-R., C.R., M.D.R., C.M.P., G.M., E.H. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SYSTEMIC “An integrated approach to the challenge of sustainable food systems: adaptive and mitigatory strategies to address climate change and malnutrition”, Knowledge hub on Nutrition and Food Security, has received funding from national research funding parties in Belgium (FWO), France (INRA), Germany (BLE), Italy (MIPAAF), Latvia (IZM), Norway (RCN), Portugal (FCT), and Spain (AEI) in a joint action of JPI HDHL, JPI-OCEANS, and FACCE-JPI launched in 2019 under the ERA-NET ERA-HDHL (No. 696295). The research was also partially funded by the project MIND FoodS HUB (Milano Innovation District Food System Hub): Innovative concept for the eco-intensification of agricultural production and for the promotion of dietary patterns for human health and longevity through the creation in MIND of a digital Food System Hub, cofunded by POR FESR 2014-2020_BANDO Call HUB Ricerca e Innovazione, Regione Lombardia to K.P.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez V., Mitjans M., Vinardell M.P. Cytoprotective effects of polyphenols against oxidative damage. In: Watson R.R., Preedy V.R., Sherma Z., editors. Polyphenols in Human Health and Disease. Academic Press; Cambridge, MA, USA: 2014. pp. 275–288. [Google Scholar]
- 3.Marone D., Mastrangelo A.M., Borrelli G.M., Mores A., Laidò G., Russo M.A., Ficco D. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022;172:48–55. doi: 10.1016/j.plaphy.2021.12.037. [DOI] [PubMed] [Google Scholar]
- 4.FAOSTAT. [(accessed on 3 November 2021)]. Available online: https://www.fao.org/faostat/en/#data.
- 5.Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant. Phys. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schijlen E.G., Ric de Vos C.H., van Tunen A.J., Bovy A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry. 2004;65:2631–2648. doi: 10.1016/j.phytochem.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y., Sasaki N., Ohmiya A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008;54:733–749. doi: 10.1111/j.1365-313X.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- 8.Markovic J.M.D., Milenkovic D., Amie D., Popovic-Bijelic A., Mojovic M., Pasti I.A., Markovic Z.S. Energy requirements of the reactions of kaempferol and selected radical species in different media: Towards the prediction of the possible radical scavenging mechanisms. Struct. Chem. 2004;25:1795–1804. doi: 10.1007/s11224-014-0453-z. [DOI] [Google Scholar]
- 9.Zhang Y., Butelli E., Martin C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014;19:81–90. doi: 10.1016/j.pbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Tikunov Y., Schouten R.E., Marcelis L., Visser R., Bovy A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018;6:52. doi: 10.3389/fchem.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert N.W., Davies K.M., Schwinn K.E. Gene regulation networks generate diverse pigmentation patterns in plants. Plant Signal Behav. 2014;9:e29526. doi: 10.4161/psb.29526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan A.C., Espley R.V. MYBs Drive Novel Consumer Traits in Fruits and Vegetables. Trends Plant Sci. 2018;23:693–705. doi: 10.1016/j.tplants.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Petroni K., Pilu R., Tonelli C. Anthocyanins in corn: A wealth of genes for human health. Planta. 2014;240:901–911. doi: 10.1007/s00425-014-2131-1. [DOI] [PubMed] [Google Scholar]
- 14.Petroni K., Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011;181:219–229. doi: 10.1016/j.plantsci.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Ma D., Constabel C.P. MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019;24:275–289. doi: 10.1016/j.tplants.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Chaves-Silva S., Santos A., Chalfun-Júnior A., Zhao J., Peres L., Benedito V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants—Tools for breeding purple varieties of fruits and vegetables. Phytochemistry. 2018;153:11–27. doi: 10.1016/j.phytochem.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Colanero S., Perata P., Gonzali S. What’s behind purple tomatoes? Insight into the mechanisms of anthocyanin synthesis in tomato Fruits. Plant Physiol. 2020;182:1841–1853. doi: 10.1104/pp.19.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Zhang Y. A transcriptional network makes normal tomato fruit not purple. Mol. Plant. 2020;13:11–13. doi: 10.1016/j.molp.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Vershinin A. Biological functions of carotenoids—Diversity and evolution. BioFactors. 1999;10:99–104. doi: 10.1002/biof.5520100203. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano G., Tavazza R., Diretto G., Beyer P., Taylor M.A. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol. 2008;26:139–145. doi: 10.1016/j.tibtech.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Sun T., Yuan H., Cao H., Yazdani M., Tadmor Y., Li L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant. 2018;11:58–74. doi: 10.1016/j.molp.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Fraser P.D., Truesdale M.R., Bird C.R., Schuch W., Bramley P.M. Carotenoid Biosynthesis during Tomato Fruit Development (Evidence for Tissue-Specific Gene Expression) Plant Physiol. 1994;105:405–413. doi: 10.1104/pp.105.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobson G.E., Davies J.N. The tomato. In: Hulme A.C., editor. The Biochemistry of Fruits and Their Products. Academic Press; London, UK: 1971. pp. 437–482. [Google Scholar]
- 25.Qin X., Zhang W., Dubcovsky J., Tian L. Cloning and comparative analysis of carotenoid beta-hydroxylase genes provides new insights into carotenoid metabolism in tetraploid (Triticum turgidum ssp. durum) and hexaploid (Triticum aestivum) wheat grains. Plant Mol. Biol. 2012;80:631–646. doi: 10.1007/s11103-012-9972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Concepción M., Boronat A. Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr. Opin. Plant Biol. 2015;25:17–22. doi: 10.1016/j.pbi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Stanley L., Yuan Y.W. Transcriptional regulation of carotenoid biosynthesis in plants: So many regulators, so little consensus. Front. Plant Sci. 2019;10:1017. doi: 10.3389/fpls.2019.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallabhaneni R., Bradbury L.M., Wurtzel E.T. The carotenoid dioxygenase gene family in maize, sorghum, and rice. Arch. Biochem. Biophys. 2010;504:104–111. doi: 10.1016/j.abb.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barchi L., Pietrella M., Venturini L., Minio A., Toppino L., Acquadro A., Andolfo G., Aprea G., Avanzato C., Bassolino L., et al. A chromosome-anchored eggplant genome sequence reveals key events in Solanaceae evolution. Sci. Rep. 2019;9:11769. doi: 10.1038/s41598-019-47985-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Qudah J.M. Identification and quantification of major carotenoids in some vegetables. Amer. J. Appl. Sci. 2009;6:492. doi: 10.3844/ajassp.2009.492.497. [DOI] [Google Scholar]
- 31.Yuan H., Zhang J., Nageswaran D., Li L. Carotenoid metabolism and regulation in horticultural crops. Hort. Res. 2015;2:15036. doi: 10.1038/hortres.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martel C., Vrebalov J., Tafelmeyer P., Giovannoni J.J. The tomato mads-box transcription factor ripening inhibitor interacts with promoters involved in numerous ripening processes in a colorless nonripening-dependent manner. Plant Physiol. 2011;157:1568–1579. doi: 10.1104/pp.111.181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujisawa M., Nakano T., Shima Y., Ito Y. A large-scale identification of direct targets of the tomato MADS box transcription factor ripening inhibitor reveals the regulation of fruit ripening. Plant Cell. 2013;25:371–386. doi: 10.1105/tpc.112.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Z., Zhang J., Li J., Yang C., Wang T., Ouyang B., Li H., Giovannoni J., Ye Z. A stay-green protein SlSGR1 regulates lycopene and β-carotene accumulation by interacting directly with SlPSY1 during ripening processes in tomato. New Phytol. 2013;198:442–452. doi: 10.1111/nph.12175. [DOI] [PubMed] [Google Scholar]
- 35.Awika J.M. Advances in Cereal Science: Implications to Food Processing and Health Promotion ACS Symposium Series. American Chemical Society; Washington, DC, USA: 2011. Major Cereal Grains Production and Use around the World; pp. 1–13. Chapter 1. [Google Scholar]
- 36.Asenstorfer R., Wang Y., Mares D. Chemical structure of flavonoid compounds in wheat (Triticum aestivum L.) flour that contribute to the yellow colour of Asian Alkaline Noodles. J. Cereal Sci. 2006;43:108–119. doi: 10.1016/j.jcs.2005.09.001. [DOI] [Google Scholar]
- 37.de Camargo A.C., de Souza Silva A.P., Soares J.C., de Alencar S.M., Handa C.L., Cordeiro K.S., Figueira M.S., Sampaio G.R., Torres E.A.F.S., Shahidi F., et al. Do flavonoids from durum wheat contribute to its bioactive properties? A prospective study. Molecules. 2021;26:463. doi: 10.3390/molecules26020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ficco D.B.M., De Simone V., Colecchia S.A., Pecorella I., Platani C., Nigro F., Finocchiaro F., Papa R., De Vita P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aesti-vum L.) and durum (Triticum turgidum L. spp. turgidum var. durum) wheats. J. Agric. Food Chem. 2014;62:8686–8695. doi: 10.1021/jf5003683. [DOI] [PubMed] [Google Scholar]
- 39.Laddomada B., Caretto S., Mita G. Wheat bran phenolic acids: Bioavailability and stability in whole wheat-based foods. Molecules. 2015;20:15666–15685. doi: 10.3390/molecules200915666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borrelli G.M., De Leonardis A.M., Platani C., Troccoli A. Distribution along durum wheat kernel of the components involved in semolina colour. J. Cereal Sci. 2008;48:494–502. doi: 10.1016/j.jcs.2007.11.007. [DOI] [Google Scholar]
- 41.Li L., Shewry P.R., Ward J.L. Phenolic acids in wheat varieties in the healthgrain diversity Screen. J. Agric. Food Chem. 2008;56:9732–9739. doi: 10.1021/jf801069s. [DOI] [PubMed] [Google Scholar]
- 42.Mateo Anson N.M., van den Berg R., Havenaar R., Bast A., Haenen G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009;49:296–300. doi: 10.1016/j.jcs.2008.12.001. [DOI] [Google Scholar]
- 43.Benincasa P., Falcinelli B., Lutts S., Stagnari F., Galieni A. Sprouted Grains: A Comprehensive Review. Nutrients. 2019;11:421. doi: 10.3390/nu11020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Ma D., Sun D., Wang C., Zhang J., Xie Y., Tiancai G. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015;3:328–334. doi: 10.1016/j.cj.2015.04.004. [DOI] [Google Scholar]
- 45.Okarter N., Liu C.S., Sorrells M.E., Liu R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010;119:249–257. doi: 10.1016/j.foodchem.2009.06.021. [DOI] [Google Scholar]
- 46.Rakszegi M., Boros D., Kuti C., Láng L., Bedo Z., Shewry P.R. Composition and end-use quality of 150 wheat lines selected for the healthgrain diversity Screen. J. Agric. Food Chem. 2008;56:9750–9757. doi: 10.1021/jf8009359. [DOI] [PubMed] [Google Scholar]
- 47.Nigro D., Laddomada B., Mita G., Blanco E., Colasuonno P., Simeone R., Gadaleta A., Pasqualone A., Blanco A. Genome wide association mapping of phenolic acids in tetraploid wheats. J. Cereal Sci. 2017;75:25–34. doi: 10.1016/j.jcs.2017.01.022. [DOI] [Google Scholar]
- 48.Laddomada B., Durante M., Mangini G., D’Amico L., Lenucci M.S., Simeone R., Piarulli L., Mita G., Blanco A. Genetic variation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet. Resour. Crop Evol. 2017;64:587–597. doi: 10.1007/s10722-016-0386-z. [DOI] [Google Scholar]
- 49.Digesù A.M., Platani C., Cattivelli L., Mangini G., Blanco A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J. Cereal Sci. 2009;50:210–218. doi: 10.1016/j.jcs.2009.05.002. [DOI] [Google Scholar]
- 50.Groth S., Wittmann R., Longin C.F., Böhm V. Influence of variety and growing location on carotenoid and vitamin E contents of 184 different durum wheat varieties (Triticum turgidum ssp. durum) in Germany. Eur. Food Res. Technol. 2020;246:2079–2092. doi: 10.1007/s00217-020-03557-1. [DOI] [Google Scholar]
- 51.Yilmaz V.A., Brandolini A., Hidalgo A. Phenolic acids and antioxidant activity of wild, feral and domesticated diploid wheats. J. Cereal Sci. 2015;64:168–175. doi: 10.1016/j.jcs.2015.05.005. [DOI] [Google Scholar]
- 52.Pasqualone A., Delvecchio L.N., Mangini G., Taranto F., Blanco A. Variability of total soluble phenolic compounds and antioxidant activity in a collection of tetraploid wheat. Agric. Food Sci. 2014;23:307–316. doi: 10.23986/afsci.47985. [DOI] [Google Scholar]
- 53.Boukid F., Dall’Asta M., Bresciani L., Mena P., Del Rio D., Calani L., Sayar R., Seo Y.W., Yacoubi I., Mejri M. Phenolic profile and antioxidant capacity of landraces, old and modern Tunisian durum wheat. Eur. Food Res. Technol. 2019;245:73–82. doi: 10.1007/s00217-018-3141-1. [DOI] [Google Scholar]
- 54.Di Loreto A., Bosi S., Montero L., Bregola V., Marotti I., Sferrazza R.E., Dinelli G., Herrero M., Cifuentes A. Determination of phenolic compounds in ancient and modern durum wheat genotypes. Electrophoresis. 2018;39:2001–2010. doi: 10.1002/elps.201700439. [DOI] [PubMed] [Google Scholar]
- 55.Zrckova M., Capouchova I., Eliášová M., Paznocht L., Pazderů K., Dvořák P., Konvalina P., Orsák M., Štěrba Z. The effect of genotype, weather conditions and cropping system on antioxidant activity and content of selected antioxidant compounds in wheat with coloured grain. Plant Soil Environ. 2018;64:530–538. doi: 10.17221/430/2018-PSE. [DOI] [Google Scholar]
- 56.Fernandez-Orozco R., Li L., Harflett C., Shewry P.R., Ward J.L. Effects of environment and genotype on phenolic acids in wheat in the healthgrain diversity screen. J. Agric. Food Chem. 2010;58:9341–9352. doi: 10.1021/jf102017s. [DOI] [PubMed] [Google Scholar]
- 57.Blanco A., Colasuonno P., Gadaleta A., Mangini G., Schiavulli A., Simeone R., Digesù A.M., De Vita P., Mastrangelo A.M., Cattivelli L. Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat. J. Cereal Sci. 2011;54:255–264. doi: 10.1016/j.jcs.2011.07.002. [DOI] [Google Scholar]
- 58.Ziegler J.U., Wahl S., Würschum T., Longin C.F.H., Carle R., Schweiggert R.M. Lutein and lutein esters in whole grain flours made from 75 genotypes of 5 triticum species grown at multiple sites. J. Agric. Food Chem. 2015;63:5061–5071. doi: 10.1021/acs.jafc.5b01477. [DOI] [PubMed] [Google Scholar]
- 59.Carrera A., Echenique V., Zhang W., Helguera M., Manthey F., Schrager A., Picca A., Cervigni G., Dubcovsky J. A deletion at the Lpx-B1 locus is associated with low lipoxygenase activity and improved pasta colour in durum wheat, Triticum turgidum ssp. durum. J. Agric. Food Chem. 2007;45:67–77. [Google Scholar]
- 60.Campos K.M., Royo C., Schulthess A., Villegas D., Matus I., Ammar K., Schwember A.R. Association of Phytoene synthase Psy1-A1 and Psy1-B1 allelic variants with semolina yellowness in durum wheat (Triticum turgidum L. var. durum) Euphytica. 2016;207:109–117. doi: 10.1007/s10681-015-1541-x. [DOI] [Google Scholar]
- 61.Yu S., Li M., Dubcovsky J., Tian L. Mutant combinations of lycopene ɛ-cyclase and β-carotene hydroxylase 2 homoeologs increased β-carotene accumulation in endosperm of tetraploid wheat (Triticum turgidum L.) grains. Plant Biotechnol. J. 2021;20:564. doi: 10.1111/pbi.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khlestkina E.K., Röder M.S., Börner A. Mapping genes controlling anthocyanin pigmentation on the glume and pericarp in tetraploid wheat (Triticum durum L.) Euphytica. 2010;171:65–69. doi: 10.1007/s10681-009-9994-4. [DOI] [Google Scholar]
- 63.Top Crop Manager. [(accessed on 15 October 2021)]. Available online: https://www.topcropmanager.com/
- 64.Burešová V., Kopecký D., Bartoš J., Martinek P., Watanabe N., Vyhnánek T., Doležel J. Variation in genome composition of blue-aleurone wheat. Theor. Appl. Genet. 2015;128:273–282. doi: 10.1007/s00122-014-2427-3. [DOI] [PubMed] [Google Scholar]
- 65.Jaafar S.N.S., Baron J., Siebenhandl-Ehn S., Rosenau T., Böhmdorfer S., Grausgruber H. Increased anthocyanin content in purple pericarp × blue aleurone wheat crosses. Plant Breed. 2013;132:546–552. doi: 10.1111/pbr.12090. [DOI] [Google Scholar]
- 66.Ndolo V.U., Beta T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013;139:663–671. doi: 10.1016/j.foodchem.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Gayen D., Ghosh S., Paul S., Sarkar S.N., Datta S.K., Datta K. Metabolic Regulation of Carotenoid-Enriched Golden Rice Line. Front. Plant Sci. 2016;7:1622. doi: 10.3389/fpls.2016.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masisi K., Diehl-Jones W.L., Gordon J., Chapman D., Moghadasian M.H., Beta T. Carotenoids of aleurone, germ, and endosperm fractions of barley, corn and wheat differentially inhibit oxidative stress. J. Agric. Food Chem. 2015;63:2715–2724. doi: 10.1021/jf5058606. [DOI] [PubMed] [Google Scholar]
- 69.Mendoza-Díaz S., Ortiz-Valerio M., Castaño-Tostado E., Figueroa-Cárdenas J., Reynoso-Camacho R., Ramos-Gómez M., Campos-Vega R., Loarca-Piña G. Antioxidant capacity and antimutagenic activity of anthocyanin and carotenoid extracts from nixtamalized pigmented creole maize races (Zea Mays L.) Plant Foods Hum. Nutr. 2012;67:442–449. doi: 10.1007/s11130-012-0326-9. [DOI] [PubMed] [Google Scholar]
- 70.Cardoso L., Pinheiro S.S., da Silva L.L., de Menezes C.B., de Carvalho C.W., Tardin F.D., Queiroz V.A., Martino H.S., Pinheiro-Sant’Ana H.M. Tocochromanols and carotenoids in sorghum (Sorghum bicolor L.): Diversity and stability to the heat treatment. Food Chem. 2015;172:900–908. doi: 10.1016/j.foodchem.2014.09.117. [DOI] [PubMed] [Google Scholar]
- 71.Shammugasamy B., Ramakrishnan Y., Ghazali H.M., Muhammad K. Tocopherol and tocotrienol contents of different varieties of rice in Malaysia. J. Sci. Food Agric. 2015;95:672–678. doi: 10.1002/jsfa.6742. [DOI] [PubMed] [Google Scholar]
- 72.Shao Y., Xu F., Sun X., Bao J., Beta T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014;143:90–96. doi: 10.1016/j.foodchem.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 73.De La Parra C., Serna Saldivar S.O., Liu R.H. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J. Agric. Food Chem. 2007;55:4177–4183. doi: 10.1021/jf063487p. [DOI] [PubMed] [Google Scholar]
- 74.Cevallos-Casals B.A., Cisneros-Zevallos L. Stoichiometric and kinetic studies of phenolic antioxidants from andean purple corn and red-fleshed sweetpotato. J. Agric. Food Chem. 2003;51:3313–3319. doi: 10.1021/jf034109c. [DOI] [PubMed] [Google Scholar]
- 75.Salazar-López N.J., González-Aguilar G.A., Rouzaud-Sández O., Robles-Sánchez M. Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. J. Food Sci. Technol. 2018;55:2021–2030. doi: 10.1007/s13197-018-3116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Y., Jin L., Xiao P., Lu Y., Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009;49:106–111. doi: 10.1016/j.jcs.2008.07.010. [DOI] [Google Scholar]
- 77.Pilu R., Cassani E., Sirizzotti A., Petroni K., Tonelli C. Effect of Flavonoid Pigments on the Accumulation of Fumonisin B1 in the Maize Kernel. J. Appl. Genet. 2011;52:145–152. doi: 10.1007/s13353-010-0014-0. [DOI] [PubMed] [Google Scholar]
- 78.Habyarimana E., Lopez-Cruz M. Genomic Selection for Antioxidant Production in a Panel of Sorghum bicolor and S. bicolor × S. halepense Lines. Genes. 2019;10:841. doi: 10.3390/genes10110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lao F., Giusti M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and hplc approaches: Method comparison and correlation. Food Anal. Methods. 2016;9:1367–1380. doi: 10.1007/s12161-015-0318-0. [DOI] [Google Scholar]
- 80.Tomay F., Marinelli A., Leoni V., Caccia C., Matros A., Mock H.P., Tonelli C., Petroni K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019;17:237. doi: 10.1186/s12967-019-1972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finocchiaro F., Ferrari B., Gianinetti A., Dall’asta C., Galaverna G., Scazzina F., Pellegrini N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nut. Food Res. 2007;51:1006–1019. doi: 10.1002/mnfr.200700011. [DOI] [PubMed] [Google Scholar]
- 82.Min B., McClung A.M., Chen M.H. Phytochemicals and antioxidant capacities in rice brans of different color. J. Food Sci. 2011;76:C117–C126. doi: 10.1111/j.1750-3841.2010.01929.x. [DOI] [PubMed] [Google Scholar]
- 83.Sweeney M.T., Thomson M.J., Pfeil B.E., McCouch S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell. 2006;18:283–294. doi: 10.1105/tpc.105.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shao Y., Zhang G., Bao J. Total phenolic content and antioxidant capacity of rice grains with extremely small size. Afr. J. Agric. Res. 2011;6:2289–2293. [Google Scholar]
- 85.Xu R., Yang Y., Qin R., Li H., Qiu C., Li L., Wei P., Yang J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016;43:529–532. doi: 10.1016/j.jgg.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Oikawa T., Maeda H., Oguchi T., Yamaguchi T., Tanabe N., Ebana K., Yano M., Ebitani T., Izawa T. The Birth of a Black Rice Gene and Its Local Spread by Introgression. Plant Cell. 2015;27:2401–2414. doi: 10.1105/tpc.15.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye X., Al-Babili S., Klöti A., Zhang J., Lucca P., Beyer P., Potrykus I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 88.Schaub P., Al-Babili S., Drake R., Beyer P. Why is golden rice golden (yellow) instead of red? Plant Physiol. 2005;138:441–450. doi: 10.1104/pp.104.057927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paine J.A., Shipton C.A., Chaggar S., Howells R.M., Kennedy M.J., Vernon G., Wright S.Y., Hinchliffe E., Adams J.L., Silverstone A.L., et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005;23:482–487. doi: 10.1038/nbt1082. [DOI] [PubMed] [Google Scholar]
- 90.Tang G. Techniques for measuring vitamin A activity from β-carotene. Am. J. Clin. Nutr. 2012;96:1185S–1188S. doi: 10.3945/ajcn.112.034603. [DOI] [PubMed] [Google Scholar]
- 91.Colombo F., Di Lorenzo C., Petroni K., Silano M., Pilu R., Falletta E., Biella S., Restani P. Pigmented corn varieties as functional ingredients for gluten-free products. Foods. 2021;10:1770. doi: 10.3390/foods10081770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suriano S., Balconi C., Valoti P., Redaelli R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT. 2021;144:111257. doi: 10.1016/j.lwt.2021.111257. [DOI] [Google Scholar]
- 93.Kristina K., Grbeša D. Carotenoid Content and Antioxidant Activity of Hexane Extracts from Selected Croatian Corn Hybrids. Food Chem. 2015;167:402–408. doi: 10.1016/j.foodchem.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Hulshof P.J., Kosmeijer-Schuil T., West C.E., Hollman P.C. Quick screening of maize kernels for provitamin a content. J. Food Compos. Anal. 2007;20:655–661. doi: 10.1016/j.jfca.2006.04.014. [DOI] [Google Scholar]
- 95.Himi E., Yamashita Y., Haruyama N., Yanagisawa T., Maekawa M., Taketa S. Ant28 gene for proanthocyanidin synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica. 2011;188:141–151. doi: 10.1007/s10681-011-0552-5. [DOI] [Google Scholar]
- 96.Lago C., Cassani E., Zanzi C., Landoni M., Trovato R., Pilu R. Development and study of a maize cultivar rich in anthocyanins: Coloured polenta, a new functional food. Plant Breed. 2014;133:210–217. doi: 10.1111/pbr.12153. [DOI] [Google Scholar]
- 97.Lago C., Landoni M., Cassani E., Doria E., Nielsen E., Pilu R. Study and characterization of a novel functional food: Purple popcorn. Mol. Breed. 2013;31:575–585. doi: 10.1007/s11032-012-9816-6. [DOI] [Google Scholar]
- 98.Baik B.-K., Ullrich S.-E. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008;48:233–242. doi: 10.1016/j.jcs.2008.02.002. [DOI] [Google Scholar]
- 99.Idehen E., Tang Y., Sang S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017;25:148–161. doi: 10.1016/j.jfda.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strygina K.V., Börner A., Khlestkina E.K. Identification and characterization of regulatory network components for anthocyanin synthesis in barley aleurone. BMC Plant Biol. 2017;17((Suppl. 1)):184. doi: 10.1186/s12870-017-1122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X.W., Jiang Q.T., Wei Y.M., Liu C. Inheritance analysis and mapping of quantitative trait loci (QTL) controlling individual anthocyanin compounds in purple barley (Hordeum vulgare L.) grains. PLoS ONE. 2017;12:e0183704. doi: 10.1371/journal.pone.0183704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martínez-Subirà M., Romero M.-P., Macià A., Puig E., Romagosa I., Moralejo M. Bioactive compounds and antioxidant capacity in pearling fractions of hulled, partially hull-less and hull-less food barley genotypes. Foods. 2021;10:565. doi: 10.3390/foods10030565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martínez-Subirà M., Romero M.P., Macià A., Puig E., Macià A., Romagosa I., Moralejo M. Purple, high β-glucan, hulless barley as valuable ingredient for functional food. Food Sci. Technol. 2020;131:109582. doi: 10.1016/j.lwt.2020.109582. [DOI] [Google Scholar]
- 104.Martínez-Subirà M., Moralejo M., Puig E., Romero M.-P., Savin R., Romagosa I. Impact of rising temperature in the deposition patterns of bioactive compounds in field grown food barley grains. Plants. 2021;10:598. doi: 10.3390/plants10030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dykes L., Rooney L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World. 2007;52:105–111. doi: 10.1094/CFW-52-3-0105. [DOI] [Google Scholar]
- 106.Alfieri M., Balconi C., Cabassi G., Habyarimana E., Redaelli R. Antioxidant activity in a set of sorghum landraces and breeding lines. Maydica. 2017;62:1–7. [Google Scholar]
- 107.Dicko M.H., Gruppen H., Barro C., Traore A.S., van Berkel W.J., Voragen A.G. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005;31:2671–2688. doi: 10.1007/s10886-005-7619-5. [DOI] [PubMed] [Google Scholar]
- 108.Wu Y., Li X., Xiang W., Zhu C., Lin Z., Wu Y., Li J., Pandravada S., Ridder D.D., Bai G., et al. Presence of tannins in sorghum grains is conditioned by different natural alleles of Tannin1. Proc. Natl. Acad. Sci. USA. 2012;109:10281–10286. doi: 10.1073/pnas.1201700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Habyarimana E., Dall’Agata M., De Franceschi P., Baloch F.S. Genome-wide association mapping of total antioxidant capacity, phenols, tannins, and flavonoids in a panel of Sorghum bicolor and S. bicolor × S. halepense populations using multi-locus models. PLoS ONE. 2019;14:e0225979. doi: 10.1371/journal.pone.0225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Habyarimana E., Lopez-Cruz M., Baloch F.S. Genomic selection for optimum index with dry biomass yield, dry mass fraction of fresh material, and plant height in biomass sorghum. Genes. 2020;11:61. doi: 10.3390/genes11010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mace E., Innes D., Hunt C., Wang X., Tao Y., Baxter J., Hassall M., Hathorn A., Jordan D. The Sorghum QTL stlas: A powerful tool for trait dissection, comparative genomics and crop improvement. Theor. Appl. Genet. 2019;132:751–766. doi: 10.1007/s00122-018-3212-5. [DOI] [PubMed] [Google Scholar]
- 112.Boddu J., Svabek C., Ibraheem F., Jones A.D., Chopra S. Characterization of a deletion allele of a sorghum Myb gene yellow seed1 showing loss of 3-deoxyflavonoids. Plant Sci. 2005;169:542–552. doi: 10.1016/j.plantsci.2005.05.007. [DOI] [Google Scholar]
- 113.Rhodes D.H., Hoffmann L., Jr., Rooney W.L., Ramu P., Morris G.P., Kresovich S. Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L.) Moench] germplasm. J. Agric. Food Chem. 2014;62:10916–10927. doi: 10.1021/jf503651t. [DOI] [PubMed] [Google Scholar]
- 114.Rhodes D., Gadgil P., Perumal R., Tesso T., Herald T.J. Natural Variation and Genome-Wide Association Study of Antioxidants in a Diverse Sorghum Collection. Cereal Chem. 2017;94:190–198. doi: 10.1094/CCHEM-03-16-0075-R. [DOI] [Google Scholar]
- 115.Klee H.J., Giovannoni J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 116.Gonzali S., Mazzucato A., Perata P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009;14:237–241. doi: 10.1016/j.tplants.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Jones C.M., Mes P., Myers J.R. Characterization and inheritance of the Anthocyanin fruit (Aft) tomato. J. Hered. 2003;94:449–456. doi: 10.1093/jhered/esg093. [DOI] [PubMed] [Google Scholar]
- 118.Paauw M., Koes R., Quattrocchio F.M. Alteration of flavonoid pigmentation patterns during domestication of food crops. J. Exp. Bot. 2019;70:3719–3735. doi: 10.1093/jxb/erz141. [DOI] [PubMed] [Google Scholar]
- 119.Bedinger P.A., Chetelat R.T., McClure B., Moyle L.C., Rose J.K., Stack S.M., van der Knaap E., Baek Y.S., Lopez-Casado G., Covey P.A., et al. Interspecific reproductive barriers in the tomato clade: Opportunities to decipher mechanisms of reproductive isolation. Sex. Plant Reprod. 2011;24:171–187. doi: 10.1007/s00497-010-0155-7. [DOI] [PubMed] [Google Scholar]
- 120.Butelli E., Titta L., Giorgio M., Mock H.P., Matros A., Peterek S., Schijlen E.G., Hall R.D., Bovy A.G., Luo J., et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008;26:1301–1308. doi: 10.1038/nbt.1506. [DOI] [PubMed] [Google Scholar]
- 121.Gonzali S., Perata P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants. 2020;9:1017. doi: 10.3390/antiox9101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun C., Deng L., Du M., Zhao J., Chen Q., Huang T., Jiang H., Li C.B., Li C. A transcriptional network promotes anthocyanin biosynthesis in tomato flesh. Mol. Plant. 2020;13:42–58. doi: 10.1016/j.molp.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 123.Cao X., Qiu Z., Wang X., Van Giang T., Liu X., Wang J., Wang X., Gao J., Guo Y., Du Y., et al. A putative R3 MYB repressor is the candidate gene underlying atroviolacium, a locus for anthocyanin pigmentation in tomato fruit. J. Exp. Bot. 2017;68:5745–5758. doi: 10.1093/jxb/erx382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Colanero S., Perata P., Gonzali S. The atroviolacea gene encodes an R3- MYB protein repressing anthocyanin synthesis in tomato plants. Front. Plant Sci. 2018;9:830. doi: 10.3389/fpls.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Povero G., Gonzali S., Bassolino L., Mazzucato A., Perata P. Transcriptional analysis in high-anthocyanin tomatoes reveals synergistic effect of Aft and atv genes. J. Plant Physiol. 2011;168:270–279. doi: 10.1016/j.jplph.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 126.Blando F., Berland H., Maiorano G., Durante M., Mazzucato A., Picarella M.E., Nicoletti I., Gerardi C., Mita G., Andersen Ø.M. Nutraceutical characterization of anthocyanin-rich fruits produced by “Sun Black” tomato line. Front. Nutr. 2019;6:133. doi: 10.3389/fnut.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li X., Wang Y., Chen S., Tian H., Fu D., Zhu B., Luo Y., Zhu H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018;9:559. doi: 10.3389/fpls.2018.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galpaz N., Wang Q., Menda N., Zamir D., Hirschberg J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008;53:717–730. doi: 10.1111/j.1365-313X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 129.Levin I., de Vos C.H., Tadmor Y., Bovy A.G., Lieberman M., Oren-Shamir M., Segev O., Kolotilin I., Keller M., Ovadia R., et al. High pigment tomato mutants—More than just lycopene (a review) Isr. J. Plant Sci. 2006;54:179–190. doi: 10.1560/IJPS_54_3_179. [DOI] [Google Scholar]
- 130.Karniel U., Koch A., Zamir D., Hirschberg J. Development of zeaxanthin-rich tomato fruit through genetic manipulations of carotenoid biosynthesis. Plant Biotechnol. J. 2020;18:2292–2303. doi: 10.1111/pbi.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Butelli E., Bulling K., Hill L., Martin C. Beyond Purple Tomatoes: Combined Strategies Targeting Anthocyanins to Generate Crimson, Magenta, and Indigo Fruit. Horticulturae. 2021;7:327. doi: 10.3390/horticulturae7090327. [DOI] [Google Scholar]
- 132.Scarano A., Butelli E., De Santis S., Cavalcanti E., Hill L., De Angelis M., Giovinazzo G., Chieppa M., Martin C., Santino A. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018;4:75. doi: 10.3389/fnut.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martí R., Roselló S., Cebolla-Cornejo J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers. 2016;8:58. doi: 10.3390/cancers8060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sulli M., Mandolino G., Sturaro M., Onofri C., Diretto G., Parisi B., Giuliano G. Molecular and biochemical characterization of a potato collection with contrasting tuber carotenoid content. PLoS ONE. 2017;12:e0184143. doi: 10.1371/journal.pone.0184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andre C.M., Ghislain M., Bertin P., Oufir M., Herrera M., Hoffmann L., Hausman J.F., Larondelle Y., Evers D. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007;55:366–378. doi: 10.1021/jf062740i. [DOI] [PubMed] [Google Scholar]
- 136.Martínez-Ispizua E., Calatayud Á., Marsal J.I., Mateos-Fernández R., Díez M.J., Soler S., Valcárcel J.V., Martínez-Cuenca M.R. Phenotyping local eggplant varieties: Commitment to biodiversity and nutritional quality preservation. Front. Plant Sci. 2021;12:696272. doi: 10.3389/fpls.2021.696272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mohd Hassan N., Yusof N.A., Yahaya A.F., Mohd Rozali N.N., Othman R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants. 2019;8:469. doi: 10.3390/antiox8100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sora G.T., Haminiuk C.W., da Silva M.V., Zielinski A.A., Gonçalves G.A., Bracht A., Peralta R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015;52:8086–8094. doi: 10.1007/s13197-015-1935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calcio Gaudino E., Colletti A., Grillo G., Tabasso S., Cravotto G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods. 2020;9:1598. doi: 10.3390/foods9111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pacifico D., Musmeci S., del Pulgar J.S., Onofri C., Parisi B., Sasso R., Mandolino G., Lombardi-Boccia G. Caffeic Acid and α-Chaconine Influence the Resistance of Potato Tuber to Phthorimaea operculella (Lepidoptera: Gelechiidae) Am. J. Potato Res. 2019;96:403–413. doi: 10.1007/s12230-019-09726-7. [DOI] [Google Scholar]
- 141.De Masi L., Bontempo P., Rigano D., Stiuso P., Carafa V., Nebbioso A., Piacente S., Montoro P., Aversano R., D’Amelia V., et al. Comparative phytochemical characterization, genetic profile, and antiproliferative activity of polyphenol-rich extracts from pigmented tubers of different solanum tuberosum varieties. Molecules. 2020;25:233. doi: 10.3390/molecules25010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mennella G., Lo Scalzo R., Fibiani M., D’Alessandro A., Francese G., Toppino L., Acciarri N., de Almeida A.E., Rotino G.L. Chemical and bioactive quality traits during fruit ripening in eggplant (S. melongena L.) and allied species. J. Agric. Food Chem. 2012;60:11821–11831. doi: 10.1021/jf3037424. [DOI] [PubMed] [Google Scholar]
- 143.Docimo T., Francese G., Ruggiero A., Batelli G., De Palma M., Bassolino L., Toppino L., Rotino G.L., Mennella G., Tucci M. Phenylpropanoids Accumulation in Eggplant Fruit: Characterization of Biosynthetic Genes and Regulation by a MYB Transcription Factor. Front. Plant Sci. 2016;6:1233. doi: 10.3389/fpls.2015.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hellmann H., Goyer A., Navarre D.A. Antioxidants in potatoes: A functional view on one of the major food crops worldwide. Molecules. 2021;26:2446. doi: 10.3390/molecules26092446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lo Scalzo R., Florio F.E., Fibiani M., Speranza G., Rabuffetti M., Gattolin S., Toppino L., Rotino G.L. Scrapped but not neglected: Insights into the composition, molecular modulation and antioxidant capacity of phenols in peel of eggplant (Solanum melongena L.) fruits at different developmental stages. Plant Physiol. Biochem. 2021;167:678–690. doi: 10.1016/j.plaphy.2021.08.037. [DOI] [PubMed] [Google Scholar]
- 146.Diretto G., Al-Babili S., Tavazza R., Papacchioli V., Beyer P., Giuliano G. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE. 2007;2:e350. doi: 10.1371/journal.pone.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Römer S., Lübeck J., Kauder F., Steiger S., Adomat C., Sandmann G. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab. Eng. 2002;4:263–272. doi: 10.1006/mben.2002.0234. [DOI] [PubMed] [Google Scholar]
- 148.van Eck J., Conlin B., Garvin D.F., Mason H., Navarre D.A., Brown C.R. Enhancing beta-carotene content in potato by RNAi-mediated silencing of the beta-carotene hydroxylase gene. Am. J. Potato Res. 2007;84:331–342. doi: 10.1007/BF02986245. [DOI] [Google Scholar]
- 149.Mishiba K.I., Nishida K., Inoue N., Fujiwara T., Teranishi S., Iwata Y., Takeda S., Koizumi N. Genetic engineering of eggplant accumulating β-carotene in fruit. Plant Cell Rep. 2020;39:1029–1039. doi: 10.1007/s00299-020-02546-8. [DOI] [PubMed] [Google Scholar]
- 150.Luo J., Butelli E., Hill L., Parr A., Niggeweg R., Bailey P., Weisshaar B., Martin C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008;56:316–326. doi: 10.1111/j.1365-313X.2008.03597.x. [DOI] [PubMed] [Google Scholar]
- 151.Li Y., Tang W., Chen J., Jia R., Ma L., Wang S., Wang J., Shen X., Chu Z., Zhu C., et al. Development of Marker-Free Transgenic Potato Tubers Enriched in Caffeoylquinic Acids and Flavonols. J. Agric. Food Chem. 2016;64:2932–2940. doi: 10.1021/acs.jafc.6b00270. [DOI] [PubMed] [Google Scholar]
- 152.Kostyn K., Szatkowski M., Kulma A., Kosieradzka I., Szopa J. Transgenic potato plants with overexpression of dihydroflavonol reductase can serve as efficient nutrition sources. J. Agric. Food Chem. 2013;61:6743–6753. doi: 10.1021/jf400645s. [DOI] [PubMed] [Google Scholar]
- 153.Oertel A., Matros A., Hartmann A., Arapitsas P., Dehmer K.J., Martens S., Mock H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta. 2017;246:281–297. doi: 10.1007/s00425-017-2718-4. [DOI] [PubMed] [Google Scholar]
- 154.Tatarowska B., Milczarek D., Wszelaczyńska E., Pobereżny J., Keutgen N., Keutgen A.J., Flis B. Carotenoids variability of potato tubers in relation to genotype, growing location and year. Am. J. Potato Res. 2019;96:493–504. doi: 10.1007/s12230-019-09732-9. [DOI] [Google Scholar]
- 155.Thorup T.A., Tanyolac B., Livingstone K.D., Popovsky S., Paran I., Jahn M. Candidate gene analysis of organ pigmentation loci in the Solanaceae. Proc. Natl. Acad. Sci. USA. 2020;97:11192–11197. doi: 10.1073/pnas.97.21.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brown C.R., Edwards C.G., Yang C.P., Dean B.B. Segregation of Total carotenoid in high level potato germplasm and its relationship to beta-carotene hydroxylase polymorphism. Am. J. Potato Res. 2006;83:365–372. doi: 10.1007/BF02872013. [DOI] [Google Scholar]
- 157.Wolters A.M., Uitdewilligen J.G., Kloosterman B.A., Hutten R.C., Visser R.G., van Eck H.J. Identification of alleles of carotenoid pathway genes important for zeaxanthin accumulation in potato tubers. Plant Mol. Biol. 2020;73:659–671. doi: 10.1007/s11103-010-9647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bellumori M., Chasquibol Silva N.A., Vilca L., Andrenelli L., Cecchi L., Innocenti M., Balli D., Mulinacci N. A Study on the biodiversity of pigmented andean potatoes: Nutritional profile and phenolic composition. Molecules. 2020;25:3169. doi: 10.3390/molecules25143169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Florio F.E., Gattolin S., Toppino L., Bassolino L., Fibiani M., Lo Scalzo R., Rotino G.L.A. SmelAAT acyltransferase variant causes a major difference in eggplant (solanum melongena L.) peel anthocyanin composition. Int. J. Mol. Sci. 2021;22:9174. doi: 10.3390/ijms22179174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Plazas M., Andújar I., Vilanova S., Hurtado M., Gramazio P., Herraiz F.J., Prohens J. Breeding for chlorogenic acid content in eggplant: Interest and prospects. Not. Bot. Horti. Agrobot. Cluj-Napoca. 2013;41:26. doi: 10.15835/nbha4119036. [DOI] [Google Scholar]
- 161.Maioli A., Gianoglio S., Moglia A., Acquadro A., Valentino D., Milani A.M., Prohens J., Orzaez D., Granell A., Lanteri S., et al. Simultaneous CRISPR/Cas9 editing of three ppo genes reduces fruit flesh browning in Solanum melongena L. Front. Plant Sci. 2020;11:607161. doi: 10.3389/fpls.2020.607161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee S.Y., Jang S.J., Jeong H.B., Lee S.Y., Venkatesh J., Lee J.H., Kwon J.K., Kang B.C. A mutation in Zeaxanthin epoxidase contributes to orange coloration and alters carotenoid contents in pepper fruit (Capsicum annuum) Plant J. 2021;106:1692–1707. doi: 10.1111/tpj.15264. [DOI] [PubMed] [Google Scholar]
- 163.Deepa N., Kaur C., Binoy G., Singh B., Kapoor H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT-Food Sci. Technol. 2007;40:121–129. doi: 10.1016/j.lwt.2005.09.016. [DOI] [Google Scholar]
- 164.Gómez-García M., Ochoa-Alejo N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.) Int. J. Mol. Sci. 2013;14:19025–19053. doi: 10.3390/ijms140919025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matus Z., Deli J., Szabolcs J. Carotenoid composition of yellow pepper during ripening: Isolation of β-cryptoxanthin 5,6-epoxide. J. Agric. Food Chem. 1991;39:1907–1914. doi: 10.1021/jf00011a003. [DOI] [Google Scholar]
- 166.Heimler D., Romani A., Ieri F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017;243:1107–1115. doi: 10.1007/s00217-016-2826-6. [DOI] [Google Scholar]
- 167.Barański M., Srednicka-Tober D., Volakakis N., Seal C., Sanderson R., Stewart G.B., Benbrook C., Biavati B., Markellou E., Giotis C., et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014;112:794–811. doi: 10.1017/S0007114514001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bénard C., Gautier H., Bourgaud F., Grasselly D., Navez B., Caris-Veyrat C., Weiss M., Génard M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009;57:4112–4123. doi: 10.1021/jf8036374. [DOI] [PubMed] [Google Scholar]
- 169.Michalskaa A., Wojdyłob A., Bogucka B. The influence of nitrogen and potassium fertilisation on the content of polyphenolic compounds and antioxidant capacity of colored potato. J. Food Comp. Anal. 2016;47:69–75. doi: 10.1016/j.jfca.2016.01.004. [DOI] [Google Scholar]
- 170.Mitchell A.E., Hong Y.J., Koh E., Barrett D.M., Bryant D.E., Denison R.F., Kaffka S. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J. Agric. Food Chem. 2007;55:6154–6159. doi: 10.1021/jf070344+. [DOI] [PubMed] [Google Scholar]
- 171.Pacifico D., Casciani R., Ritota M., Mandolino G., Onofri C., Moschella A., Parisi B., Cafiero C., Valentini M. NMR-Based metabolomics for organic farming traceability of early potatoes. J. Agric. Food Chem. 2013;61:11201–11211. doi: 10.1021/jf402961m. [DOI] [PubMed] [Google Scholar]
- 172.Kazimierczak R., Średnicka-Tober D., Hallmann E., Kopczyńska K., Zarzyńska K. The impact of organic vs. conventional agricultural practices on selected quality features of eight potato cultivars. Agronomy. 2019;9:799. doi: 10.3390/agronomy9120799. [DOI] [Google Scholar]
- 173.Pacifico D., Paris R. Effect of organic potato farming on human and environmental health and benefits from new plant breeding techniques. is it only a matter of public acceptance? Sustainability. 2016;8:1054. doi: 10.3390/su8101054. [DOI] [Google Scholar]
- 174.Dimberg L.H., Gissén C., Nilsson J. Phenolic compounds in oat grains (Avena sativa L.) grown in conventional and organic systems. Ambio. 2005;34:331–337. doi: 10.1579/0044-7447-34.4.331. [DOI] [PubMed] [Google Scholar]
- 175.Kesarwani A., Chiang P.Y., Chen S.S. Distribution of phenolic compounds and antioxidative activities of rice kernel and their relationships with agronomic practice. Science. 2014;2014:620171. doi: 10.1155/2014/620171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luthria D., Singh A.P., Wilson T., Vorsa N., Banuelos G.S., Vinyard B.T. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: Plant-to-plant variation. Food Chem. 2010;121:406–411. doi: 10.1016/j.foodchem.2009.12.055. [DOI] [Google Scholar]
- 177.Szafirowska A., Elkner K. The comparison of yielding and nutritive value of organic and conventional pepper fruits. Veg. Crops Res. Bull. 2019;71:111–121. doi: 10.2478/v10032-009-0032-9. [DOI] [Google Scholar]
- 178.Chassy A.W., Bui L., Renaud E.N.C., Van Horn M., Mitchell A.E. Three-year comparison of the content of antioxidant microconstituents and several quality characteristics in organic and conventionally managed tomatoes and bell peppers. J. Agric. Food Chem. 2006;54:8244–8252. doi: 10.1021/jf060950p. [DOI] [PubMed] [Google Scholar]
- 179.Marín A., Gil M.I., Flores P., Hellín P., Selma M.V. Microbial quality and bioactive constituents of sweet peppers from sustainable production systems. J. Agric. Food Chem. 2008;56:11334–11341. doi: 10.1021/jf8025106. [DOI] [PubMed] [Google Scholar]
- 180.Kaur S., Suseela V. Unraveling Arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites. 2020;10:335. doi: 10.3390/metabo10080335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hamilton C.E., Gundel P.E., Helander M., Saikkoonen K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012;54:1–10. doi: 10.1007/s13225-012-0158-9. [DOI] [Google Scholar]
- 182.Avio L., Turrini A., Giovannetti M., Sbrana C. Designing the ideotype mycorrhizal symbionts for the production of healthy food. Front. Plant Sci. 2018;9:1089. doi: 10.3389/fpls.2018.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Skendi A. Trends in Wheat and Bread Making. Elsevier; Amsterdam, The Netherlands: 2021. Alternatives to increase the antioxidant capacity of bread with phenolics. Chapter 11. [Google Scholar]
- 184.Giovannetti M., Avio L., Barale R., Ceccarelli N., Cristofani R., Iezzi A., Mignolli F., Picciarelli P., Pinto B., Reali D., et al. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 2012;107:242–251. doi: 10.1017/S000711451100290X. [DOI] [PubMed] [Google Scholar]
- 185.Abdel-Aal E.S., Young J.C., Rabalski I. Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. J. Agric. Food Chem. 2006;54:4696–4704. doi: 10.1021/jf0606609. [DOI] [PubMed] [Google Scholar]
- 186.Ficco D.B.M., De Simone V., De Leonardis A.M., Giovanniello V., Del Nobile M.A., Padalino L., Lecce L., Borrelli G.M., De Vita P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016;205:187–195. doi: 10.1016/j.foodchem.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 187.Kasote D., Tiozon R.N., Sartagoda K.J.D., Itagi H., Roy P., Kohli A., Regina A., Sreenivasulu N. Food processing technologies to develop functional foods with enriched bioactive phenolic compounds in cereals. Front. Plant Sci. 2021;12:2660. doi: 10.3389/fpls.2021.771276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mert I.D. The applications of microfluidization in cereals and cereal-based products: An overview. Crit. Rev. Food Sci. Nutr. 2020;60:1007–1024. doi: 10.1080/10408398.2018.1555134. [DOI] [PubMed] [Google Scholar]
- 189.Ficco D.B.M., Borrelli G.M., Miedico O., Giovanniello V., Tarallo M., Pompa C., De Vita P., Chiaravalle A.E. Effects of grain debranning on bioactive compounds, antioxidant capacity and essential and toxinc trace elements in purple durum wheats. LWT Food Sci. Technol. 2020;118:108734. doi: 10.1016/j.lwt.2019.108734. [DOI] [Google Scholar]
- 190.Ficco D.B.M., Borrelli G.M., Giovanniello V., Platani C., De Vita P. Production of anthocyanin-enriched flours of durum and soft pigmented wheats by air-classification, as a potential ingredient for functional bread. J. Cereal Sci. 2018;79:118–126. doi: 10.1016/j.jcs.2017.09.007. [DOI] [Google Scholar]
- 191.Cilla A., Bosch L., Barberá R., Alegría A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018;68:3–15. doi: 10.1016/j.jfca.2017.01.009. [DOI] [Google Scholar]
- 192.Paznocht L., Kotikova Z., Orsak M., Lachman J., Martinek P. Carotenoid changes of colored-grain wheat flours during bun-making. Food Chem. 2017;277:725–734. doi: 10.1016/j.foodchem.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 193.Bartl P., Albreht A., Skrt M., Tremlova B., Ostadlova M., Smejkal K., Vovk I., Ulrih N.P. Anthocyanins in purple and blue wheat grains and in resulting bread: Quantity, composition, and thermal stability. Int. J. Food Sci. Nutr. 2015;66:514–519. doi: 10.3109/09637486.2015.1056108. [DOI] [PubMed] [Google Scholar]
- 194.Carcea M., Narducci V., Turfani V., Giannini V. Polyphenols in Raw and Cooked Cereals/Pseudocereals/Legume Pasta and Couscous. Foods. 2017;6:80. doi: 10.3390/foods6090080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Melini V., Panfili G., Fratianni A., Acquistucci R. Bioactive compounds in rice on Italian market: Pigmented varieties as a source of carotenoids, total phenolic compounds and anthocyanins, before and after cooking. Food Chem. 2019;277:119–127. doi: 10.1016/j.foodchem.2018.10.053. [DOI] [PubMed] [Google Scholar]
- 196.Rytel E., Tajner-Czopek A., Kita A., Tkaczyńska A., Kucharska A.Z., Sokół-Łętowska A. The Influence of the Production Process on the Anthocyanin Content and Composition in Dried Potato Cubes, Chips, and French Fries Made from Red-Fleshed Potatoes. Appl. Sci. 2021;11:1104. doi: 10.3390/app11031104. [DOI] [Google Scholar]
- 197.Arkoub-Djermoune L., Boulekbache-Makhlouf L., Zeghichi-Hamri S., Bellili S., Boukhalfa F., Madani K. Influence of the thermal processing on the physicochemical properties and the antioxidant activity of a Solanaceae vegetable: Eggplant. J. Food Qual. 2016;39:181–191. doi: 10.1111/jfq.12192. [DOI] [Google Scholar]
- 198.Villa-Rivera M.G., Ochoa-Alejo N. Chili Pepper Carotenoids: Nutraceutical Properties and Mechanisms of Action. Molecules. 2020;25:5573. doi: 10.3390/molecules25235573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lachman J., Hamouz K., Orsák M., Pivec V., Hejtmánková K., Pazderů K., Dvořák P., Čepl J. Impact of selected factors—Cultivar, storage, cooking and baking on the content of anthocyanins in coloured-flesh potatoes. Food Chem. 2012;133:1107–1116. doi: 10.1016/j.foodchem.2011.07.077. [DOI] [Google Scholar]
- 200.Girard A.L., Awika J.M. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol–gluten interactions. Compr. Rev. Food Sci. Food Saf. 2020;19:2164–2199. doi: 10.1111/1541-4337.12572. [DOI] [PubMed] [Google Scholar]
- 201.Van den Berg H., Faulks R., Fernando Granado H., Hirschberg J., Olmedilla B., Sandmann G., Southon S., Stahl W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 2000;80:880–912. doi: 10.1002/(SICI)1097-0010(20000515)80:7<880::AID-JSFA646>3.0.CO;2-1. [DOI] [Google Scholar]
- 202.Zhang Y., Sun Y., Zhang H., Mai Q., Zhang B., Li H., Deng Z. The degradation rules of anthocyanins from eggplant peel and antioxidant capacity in fortified model food system during the thermal treatments. Food Biosci. 2020;38:100701. doi: 10.1016/j.fbio.2020.100701. [DOI] [Google Scholar]
- 203.Chitchumroonchokchai C., Diretto G., Parisi B., Giuliano G., Failla M.L. Potential of golden potatoes to improve vitamin A and vitamin E status in developing countries. PLoS ONE. 2017;12:e0187102. doi: 10.1371/journal.pone.0187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tian J., Chen J., Lv F., Chen S., Chen J., Liu D., Ye X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016;197:1264–1270. doi: 10.1016/j.foodchem.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 205.Patras A., Brunton N., Da Pieve S., Butler F., Downey G. Effect of high-pressure processing on antioxidant activity and instrumental colour of tomato and carrot purees. Innov. Food Sci. Emerg. Technol. 2008;10:16–22. doi: 10.1016/j.ifset.2008.09.008. [DOI] [Google Scholar]
- 206.Andres V., Villanueva M.J., Tenorio M.D. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem. 2016;192:328–335. doi: 10.1016/j.foodchem.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 207.Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- 208.Kay C.D., Kroon P.A., Cassidy A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009;53((Suppl. 1)):S92–S101. doi: 10.1002/mnfr.200800461. [DOI] [PubMed] [Google Scholar]
- 209.Aura A.M., Martin-Lopez P., O’Leary K.A., Williamson G., Oksman-Caldentey K.M., Poutanen K., Santos-Buelga C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005;44:133–142. doi: 10.1007/s00394-004-0502-2. [DOI] [PubMed] [Google Scholar]
- 210.Ozdal T., Sela D.A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Virgili F., Marino M. Regulation of cellular signals from nutritional molecules, a specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008;45:1205–1216. doi: 10.1016/j.freeradbiomed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 212.Speciale A., Anwar S., Canali R., Chirafisi J., Saija A., Virgili F., Cimino F. Cyanidin-3-O-glucoside counters the response to tnf-alpha of endothelial cells by activating nrf2 pathway. Mol. Nutr. Food Res. 2013;57:1979–1987. doi: 10.1002/mnfr.201300102. [DOI] [PubMed] [Google Scholar]
- 213.Speciale A., Chirafisi J., Saija A., Cimino F. Nutritional antioxidants and adaptive cell responses, an update. Curr. Mol. Med. 2011;11:770–789. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- 214.Kim S.H., Son G.H., Bhattacharjee S., Kim H.J., Nam J.C., Nguyen P.D., Hong J.C., Gassmann W. The Arabidopsis immune adaptor SRFR1 interacts with TCP transcription factors that redundantly contribute to effector-triggered immunity. Plant J. 2014;78:978–989. doi: 10.1111/tpj.12527. [DOI] [PubMed] [Google Scholar]
- 215.Pojer E., Mattivi F., Johnson D., Stockley C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013;12:483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- 216.Tunon M.J., Garcia-Mediavilla M.V., Sanchez-Campos S., Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents, modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 2009;10:256–271. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- 217.Vendrame S., Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-kB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015;73:348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 218.Hassimotto N.M., Moreira V., do Nascimento N.G., Souto P.C., Teixeira C., Lajolo F.M. Inhibition of carrageenan-induced acute inflammation in mice by oral administration of anthocyanin mixture from wild mulberry and cyanidin-3-glucoside. Biomed. Res. Int. 2013;2013:146716. doi: 10.1155/2013/146716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guo H., Ling W., Wang Q., Liu C., Hu Y., Xia M., Feng X., Xia X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 2007;62:1–6. doi: 10.1007/s11130-006-0031-7. [DOI] [PubMed] [Google Scholar]
- 220.Salgado J.M., Oliveira A.G., Mansi D.N., Donado-Pestana C.M., Bastos C.R., Marcondes F.K. The role of black rice (Oryza sativa L.) in the control of hypercholesterolemia in rats. J. Med. Food. 2010;13:1355–1362. doi: 10.1089/jmf.2009.0246. [DOI] [PubMed] [Google Scholar]
- 221.Yang Y., Andrews M.C., Hu Y., Wang D., Qin Y., Zhu Y., Ni H., Ling W. Anthocyanin extract from black rice significantly ameliorates platelet hyperactivity and hypertriglyceridemia in dyslipidemic rats induced by high fat diets. J. Agric. Food Chem. 2011;59:6759–6764. doi: 10.1021/jf201079h. [DOI] [PubMed] [Google Scholar]
- 222.Wang H., Liu D., Ji Y., Liu Y., Xu L., Guo Y. Dietary supplementation of black rice anthocyanin extract regulates cholesterol metabolism and improves gut microbiota dysbiosis in C57BL/6J mice fed a high-fat and cholesterol diet. Mol. Nutr. Food Res. 2020;64:1900876. doi: 10.1002/mnfr.201900876. [DOI] [PubMed] [Google Scholar]
- 223.Ling W.H., Cheng Q.X., Ma J., Wang T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J. Nutr. 2001;131:1421–1426. doi: 10.1093/jn/131.5.1421. [DOI] [PubMed] [Google Scholar]
- 224.Ling W.H., Wang L.L., Ma J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J. Nutr. 2002;132:20–26. doi: 10.1093/jn/132.1.20. [DOI] [PubMed] [Google Scholar]
- 225.Xia X., Ling W., Ma J., Xia M., Hou M., Wang Q., Zhu H., Tang Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein e-deficient mice. J. Nutr. 2006;136:2220–2225. doi: 10.1093/jn/136.8.2220. [DOI] [PubMed] [Google Scholar]
- 226.Sharma S., Khare P., Kumar A., Chunduri V., Kumar A., Kapoor P., Mangal P., Kondepudi K.K., Bishnoi M., Garg M. Anthocyanin-biofortified colored wheat prevents high fat diet–induced alterations in mice, nutrigenomics studies. Mol. Nutr. Food Res. 2020;64:1900999. doi: 10.1002/mnfr.201900999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hou Z., Qin P., Ren G. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. Japonica) on chronically alcohol-induced liver damage in rats. J. Agric. Food Chem. 2010;58:3191–3196. doi: 10.1021/jf904407x. [DOI] [PubMed] [Google Scholar]
- 228.Jiang X., Guo H., Shen T., Tang X., Yang Y., Ling W. Cyanidin-3-O-β-glucoside purified from black rice protects mice against hepatic fibrosis induced by carbon tetrachloride via inhibiting hepatic stellate cell activation. J. Agric. Food Chem. 2015;63:6221–6230. doi: 10.1021/acs.jafc.5b02181. [DOI] [PubMed] [Google Scholar]
- 229.Lu X., Zhou Y., Wu T., Hao L. Ameliorative effect of black rice anthocyanin on senescent mice induced by D-galactose. Food Funct. 2014;5:2892–2897. doi: 10.1039/C4FO00391H. [DOI] [PubMed] [Google Scholar]
- 230.Pannangrong W., Wattanathorn J., Muchimapura S., Tiamkao S., Tong-Un T. Purple rice berry is neuroprotective and enhances cognition in a rat model of Alzheimer’s disease. J. Med. Food. 2011;14:688–694. doi: 10.1089/jmf.2010.1312. [DOI] [PubMed] [Google Scholar]
- 231.Tikhonova M.A., Shoeva O.Y., Tenditnik M.V., Ovsyukova M.V., Akopyan A.A., Dubrovina N.I., Amstislavskaya T.G., Khlestkina E.K. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients. 2020;12:3877. doi: 10.3390/nu12123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Salehi B., Sharifi-Rad J., Cappellini F., Reiner Z., Zorzan D., Imran M., Sener B., Kilic M., El-Shazly M., Fahmy N.M., et al. The therapeutic potential of anthocyanins, current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020;11:1300. doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Toufektsian M.C., de Lorgeril M., Nagy N., Salen P., Donati M.B., Giordano L., Mock H.P., Peterek S., Matros A., Petroni K., et al. dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J. Nutr. 2008;138:747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- 234.Trinei M., Carpi A., Menabo’ R., Storto M., Fornari M., Marinelli A., Minardi S., Riboni M., Casciaro F., DiLisa F., et al. Dietary intake of cyanidin-3-glucoside induces a long-lasting cardioprotection from ischemia/reperfusion injury by altering the microbiota. J. Nutr. Biochem. 2022;101:108921. doi: 10.1016/j.jnutbio.2021.108921. [DOI] [PubMed] [Google Scholar]
- 235.Petroni K., Trinei M., Fornari M., Calvenzani V., Marinelli A., Micheli L.A., Pilu R., Matros A., Mock H.P., Tonelli C., et al. Dietary cyanidin 3-glucoside from purple corn ameliorates doxorubicin-induced cardiotoxicity in mice. Nutr. Metab. Cardiovasc. Dis. 2017;27:462–469. doi: 10.1016/j.numecd.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 236.Li J., Kang M.K., Kim J.K., Kim J.L., Kang S.W., Lim S.S., Kang Y.H. Purple corn anthocyanins retard diabetes-associated glomerulosclerosis in mesangial cells and db/db mice. Eur. J. Nutr. 2012;51:961–973. doi: 10.1007/s00394-011-0274-4. [DOI] [PubMed] [Google Scholar]
- 237.Kang M.K., Li J., Kim J.L., Gong J.H., Kwak S.N., Park J.H., Lee J.Y., Lim S.S., Kang Y.H. Purple corn anthocyanins inhibit diabetesassociated glomerular monocyte activation and macrophage infiltration. Am. J. Physiol. Renal. Physiol. 2012;303:F1060–F1069. doi: 10.1152/ajprenal.00106.2012. [DOI] [PubMed] [Google Scholar]
- 238.Kang M.K., Lim S.S., Lee J.Y., Yeo K.M., Kang Y.H. Anthocyanin-rich purple corn extract inhibit diabetes-associated glomerular angiogenesis. PLoS ONE. 2013;8:e79823. doi: 10.1371/journal.pone.0079823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Dietary cyanidin 3-O-β-D-glucoside rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 240.Luna-Vital D.A., de Mejia E.G. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLoS ONE. 2018;13:e0200449. doi: 10.1371/journal.pone.0200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Luna-Vital D., Luzardo-Ocampo I., Cuellar-Nuñez L., Loarca-Pina G., de Mejia E.G. Maize extract rich in ferulic acid and anthocyanins prevents high-fat induced obesity in mice by modulating SIRT1, AMPK, and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020;79:108343. doi: 10.1016/j.jnutbio.2020.108343. [DOI] [PubMed] [Google Scholar]
- 242.Magni G., Marinelli A., Riccio D., Lecca D., Tonelli C., Abbracchio M.P., Petroni K., Ceruti S. Purple Corn Extract as Anti-allodynic Treatment for Trigeminal Pain, Role of Microglia. Front. Cell Neurosci. 2018;12:378. doi: 10.3389/fncel.2018.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Saclier M., Bonfanti C., Antonini S., Angelini G., Mura G., Zanaglio F., Taglietti V., Romanello V., Sandri M., Tonelli C., et al. Nutritional intervention with cyanidin hinders the progression of muscular dystrophy. Cell Death Dis. 2020;11:127. doi: 10.1038/s41419-020-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li H., Deng Z., Liu R., Loewen S., Tsao R. Bioaccessibility, in vitro antioxidant activities and in vivo anti-inflammatory activities of a purple tomato (Solanum lycopersicum L.) Food Chem. 2014;159:353–360. doi: 10.1016/j.foodchem.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 245.Tomlinson M.L., Butelli E., Martin C., Carding S.R. Flavonoids from Engineered Tomatoes Inhibit Gut Barrier Pro-inflammatory Cytokines and Chemokines, via SAPK/JNK and p38 MAPK Pathways. Front. Nutr. 2017;4:61. doi: 10.3389/fnut.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liso M., De Santis S., Scarano A., Verna G., Dicarlo M., Galleggiante V., Campiglia P., Mastronardi M., Lippolis A., Vacca M., et al. Bronze-tomato enriched diet affects the intestinal microbiome under homeostatic and inflammatory conditions. Nutrients. 2018;10:1862. doi: 10.3390/nu10121862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sido A., Radhakrishnan S., Kim S.W., Eriksson E., Shen F., Li Q., Bhat V., Reddivari L., Vanamala J. A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis. J. Nutr. Biochem. 2017;43:11–17. doi: 10.1016/j.jnutbio.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 248.Reddivari L., Wang T., Wu B., Li S. Potato, an Anti-Inflammatory Food. Am. J. Potato Res. 2019;96:164–169. doi: 10.1007/s12230-018-09699-z. [DOI] [Google Scholar]
- 249.Lee S.-J., Shin J.-S., Choi H.-E., Lee K.-G., Cho Y.-W., An H.-J., Jang D.S., Jeong J.-C., Kwon O.-K., Nam J.-H., et al. Chloroform fraction of Solanum tuberosum L. cv Jayoung epidermis suppresses LPS-induced inflammatory responses in macrophages and DSS induced colitis in mice. Food Chem. Toxicol. 2014;63:53–61. doi: 10.1016/j.fct.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 250.Hayashi K., Hibasami H., Murakami T., Terahara N., Mori M., Tsukui A. Induction of apoptosis in cultured human stomach cancer cells by potato anthocyanins and its inhibitory effects on growth of stomach cancer in mice. Food Sci. Technol. Res. 2006;12:22–26. doi: 10.3136/fstr.12.22. [DOI] [Google Scholar]
- 251.Thompson M.D., Thompson H.J., McGinley J.N., Neil E.S., Rush D.K., Holm D.G., Stushnoff C. Functional food characteristics of potato cultivars (Solanum tuberosum L.), phytochemical composition and inhibition. J. Food Compos. Anal. 2009;22:571–576. doi: 10.1016/j.jfca.2008.09.002. [DOI] [Google Scholar]
- 252.Yoon S.S., Rhee Y.H., Lee H.J., Lee E.O., Lee M.H., Ahn K.S., Lim H.T., Kim S.H. Uncoupled protein 3 and p38 signal pathways are involved in antiobesity activity of Solanum tuberosum L. cv. Bora Valley. J. Ethnopharmacol. 2008;118:396–404. doi: 10.1016/j.jep.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 253.Kubow S., Hobson L., Iskandar M.M., Sabally K., Donnelly D.J., Agellonm L.B. Extract of Irish potatoes (Solanum tuberosum L.) decreases body weight gain and adiposity and improves glucose control in the mouse model of diet-induced obesity. Mol. Nutr. Food Res. 2014;58:2235–2238. doi: 10.1002/mnfr.201400013. [DOI] [PubMed] [Google Scholar]
- 254.Singh N., Kamath V., Rajini P.S. Attenuation of hyperglycemia and associated biochemical parameters in STZ-induced diabetic rats by dietary supplementation of potato peel powder. Clin. Chim. Acta. 2005;353:165–175. doi: 10.1016/j.cccn.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 255.Han K.H., Hashimoto N., Shimada K., Sekikawa M., Noda T., Yamauchi H., Hashimoto M., Chiji H., Topping D.L., Fukushima M. Hepatoprotective effects of purple potato extract against D-galactosamine-induced liver injury in rats. Biosci. Biotechnol. Biochem. 2006;70:1432–1437. doi: 10.1271/bbb.50670. [DOI] [PubMed] [Google Scholar]
- 256.Han K.H., Matsumoto A., Shimada K., Sekikawa M., Fukushima M. Effects of anthocyanin-rich purple potato flakes on antioxidant status in F344 rats fed a cholesterol-rich diet. Br. J. Nutr. 2007;98:914–921. doi: 10.1017/S0007114507761792. [DOI] [PubMed] [Google Scholar]
- 257.Han K.H., Shimada K., Sekikawa M., Fukushima M. Anthocyanin-rich red potato flakes affect serum lipid peroxidation and hepatic SOD mRNA level in rats. Biosci. Biotechnol. Biochem. 2007;71:1356–1359. doi: 10.1271/bbb.70060. [DOI] [PubMed] [Google Scholar]
- 258.Boeing H., Bechthold A., Bub A., Ellinger S., Haller D., Kroke A., Leschik-Bonnet E., Muller M.J., Oberritter H., Schulze M., et al. Critical review, vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McRae M.P. Health Benefits of Dietary Whole Grains: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017;16:10–18. doi: 10.1016/j.jcm.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Afshin A., Sur P.J., Fay K.A., Cornaby L., Ferrara G., Salama J.S., Mullany E.C., Abate K.H., Abbafati C., Abebe Z., et al. Health effects of dietary risks in 195 countries, 1990–2017, A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pounis G., Di Castelnuovo A., Bonaccio M., Costanzo S., Persichillo M., Krogh V., Donati M.B., De Gaetano G., Iacoviello L. Flavonoid and lignan intake in a Mediterranean population, proposal for a holistic approach in polyphenol dietary analysis, the Moli-sani Study. Eur. J. Clin. Nutr. 2016;70:338–345. doi: 10.1038/ejcn.2015.178. [DOI] [PubMed] [Google Scholar]
- 262.Pounis G., Bonaccio M., Di Castelnuovo A., Costanzo S., De Curtis A., Persichillo M., Sieri S., Donati M.B., Cerletti C., De Gaetano G., et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. J. Thromb. Haemost. 2016;115:344–352. doi: 10.1160/th15-06-0487. [DOI] [PubMed] [Google Scholar]
- 263.Pounis G., Costanzo S., Bonaccio M., Di Castelnuovo A., de Curtis A., Ruggiero E., Persichillo M., Cerletti C., Donati M.B., de Gaetano G., et al. Reduced mortality risk by a polyphenol-rich diet, an analysis from the Moli-sani study. Nutrition. 2018;48:87–95. doi: 10.1016/j.nut.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 264.Esposito S., Gialluisi A., Costanzo S., Di Castelnuovo A., Ruggiero E., De Curtis A., Persichillo M., Cerletti C., Donati M.B., de Gaetano G., et al. Dietary polyphenol intake is associated with biological aging, a novel predictor of cardiovascular disease, cross-sectional findings from the moli-sani study. Nutrients. 2021;13:1701. doi: 10.3390/nu13051701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Luís Â., Domingues F., Pereira L. Association between berries intake and cardiovascular diseases risk factors, a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018;9:740–757. doi: 10.1039/C7FO01551H. [DOI] [PubMed] [Google Scholar]
- 266.Liu R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- 267.Willett W.C., Rockström J., Loken B., Springmann M., Lang T., Vermeulen S., Garnett T., Tilman D., DeClerck F., Wood A., et al. Food in the Anthropocene. Lancet. 2019;393:447–492. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 268.Seal C.J., Courtin C.M., Venema K., de Vries J. Health benefits of whole grain, Effects on dietary carbohydrate quality, the gut microbiome and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021;20:2742–2768. doi: 10.1111/1541-4337.12728. [DOI] [PubMed] [Google Scholar]
- 269.Lillioja S., Neal A.L., Tapsell L., Jacobs D.R., Jr. Whole grains, type 2 diabetes, coronary heart disease, and hypertension, links to the aleurone preferred over indigestible fiber. Biofactors. 2013;39:242–258. doi: 10.1002/biof.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.U.S. Department of Health and Human Services. U.S. Department of Agriculture [(accessed on 3 November 2021)];2015–2020 Dietary Guidelines for Americans. (8th ed.). 2015 December; Available online: http://health.gov/dietaryguidelines/2015/guidelines/
- 271.EFSA panel on dietetic products, nutrition, and allergies (nda); scientific opinion on dietary reference values for carbohydrates and dietary fibre. [(accessed on 3 November 2021)];EFSA J. 2010 8:1462. Available online: www.efsa.europa.eu. [Google Scholar]
- 272.Gupta R., Meghwal M., Prabhakar P.K. Bioactive compounds of pigmented wheat (Triticum aestivum), potential benefits in human health. Trends Food Sci. Technol. 2021;110:240–252. doi: 10.1016/j.tifs.2021.02.003. [DOI] [Google Scholar]
- 273.Chusak C., Pasukamonset P., Chantarasinlapin P., Adisakwattana S. Postprandial glycemia, insulinemia, and antioxidant status in healthy subjects after ingestion of bread made from anthocyanin-rich riceberry rice. Nutrients. 2020;12:782. doi: 10.3390/nu12030782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Callcott E.T., Blanchard C.L., Snell P., Santhakumar A.B. The anti-inflammatory and antioxidant effects of acute consumption of pigmented rice in humans. Food Funct. 2019;10:8230. doi: 10.1039/C9FO02455G. [DOI] [PubMed] [Google Scholar]
- 275.Liso M., Sila A., Verna G., Scarano A., Donghia R., Castellana F., Cavalcanti E., Pesole P.L., Sommella E.M., Lippolis A., et al. Nutritional Regimes Enriched with Antioxidants as an Efficient Adjuvant for IBD Patients under Infliximab Administration, a Pilot Study. Antioxidants. 2022;11:138. doi: 10.3390/antiox11010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Liu Y., Qiu J., Yue Y., Li K., Ren G. Dietary black-grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes, a randomized controlled trial. Ther. Clin. Risk Manag. 2018;14:247–256. doi: 10.2147/TCRM.S151424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gamel T.H., Abdel-Aal E.M., Tucker A.J., Pare S.M., Faughnan K., O’Brien C.D., Dykun A., Rabalski I., Pickard M., Wright A.J. Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity C-reactive protein, a randomised, single-blind parallel-arm study. Br. J. Nutr. 2020;24:1179–1189. doi: 10.1017/S0007114520002275. [DOI] [PubMed] [Google Scholar]
- 278.Kaspar K.L., Park J.S., Brown C.R., Mathison B.D., Navarre D.A., Chew B.P. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J. Nutr. 2011;141:108–111. doi: 10.3945/jn.110.128074. [DOI] [PubMed] [Google Scholar]
- 279.Vinson J.A., Demkosky C.A., Navarre D.A., Smyda M.A. High-antioxidant potatoes, Acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects. J. Agric. Food Chem. 2012;60:6749–6754. doi: 10.1021/jf2045262. [DOI] [PubMed] [Google Scholar]
- 280.Jokioja J., Linderborg K.M., Kortesniemi M., Nuora A., Heinonen J., Sainio T., Viitanen M., Kallio H., Yang B. Anthocyaninrich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020;310:125797. doi: 10.1016/j.foodchem.2019.125797. [DOI] [PubMed] [Google Scholar]
- 281.Linderborg K.M., Salo J.E., Kalpio M., Vuorinen A.L., Kortesniemi M., Griinari M., Viitanen M., Yang B., Kallio H. Comparison of the postprandial effects of purple-fleshed and yellow-fleshed potatoes in healthy males with chemical characterization of the potato meals. Int. J. Food Sci. Nutr. 2016;67:581–591. doi: 10.1080/09637486.2016.1181157. [DOI] [PubMed] [Google Scholar]
- 282.Tsang C., Smail N.F., Almoosawi S., McDougall G.J.M., Al-Dujaili E.A.S. Antioxidant Rich Potato Improves Arterial Stiffness in Healthy Adults. Plant Foods Hum. Nutr. 2018;73:203–208. doi: 10.1007/s11130-018-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gurbuz N., Uluisik S., Frary A., Frary A., Doganlar S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018;268:602–610. doi: 10.1016/j.foodchem.2018.06.093. [DOI] [PubMed] [Google Scholar]
- 284.Jung E.J., Bae M.S., Jo E.K., Jo Y.H., Lee S.C. Antioxidant activity of different parts of eggplant. J. Med. Plants Res. 2011;5:4610–4615. [Google Scholar]
- 285.Casati L., Pagani F., Braga P.C., Scalzo R.L., Sibilia V. Nasunin, a new player in the field of osteoblast protection against oxidative stress. J. Funct. Foods. 2016;23:474–484. doi: 10.1016/j.jff.2016.03.007. [DOI] [Google Scholar]
- 286.Sensoy I. A review on the relationship between food structure, processing, and bioavailability. Crit. Rev. Food Sci. Nutr. 2014;54:902–909. doi: 10.1080/10408398.2011.619016. [DOI] [PubMed] [Google Scholar]
- 287.Ichiyanagi T., Terahara N., Rahman M.M., Konishi T. Gastrointestinal uptake of nasunin, acylated anthocyanin in eggplant. J. Agric. Food Chem. 2006;54:5306–5312. doi: 10.1021/jf060238s. [DOI] [PubMed] [Google Scholar]
- 288.Zhang L., Chen T., Yin Y., Zhang C.Y., Zhang Y.L. Dietary microRNA-A Novel Functional Component of Food. Adv. Nutr. 2019;10:711–721. doi: 10.1093/advances/nmy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Liu Y.C., Chen W.L., Kung W.H., Huang H.D. Plant miRNAs found in human circulating system provide evidences of cross kingdom RNAi. BMC Genom. 2017;18:112. doi: 10.1186/s12864-017-3502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang H., Li Y., Liu Y., Liu H., Wang H., Jin W., Zhang Y., Zhang C., Xu D. Role of plant MicroRNA in cross-species regulatory networks of humans. BMC Syst. Biol. 2016;10:60. doi: 10.1186/s12918-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Cavalieri D., Rizzetto L., Tocci N., Rivero D., Asquini E., Si-Ammour A., Bonechi E., Ballerini C., Viola R. Plant microRNAs as novel immunomodulatory agents. Sci. Rep. 2016;6:25761. doi: 10.1038/srep25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang K., Li H., Yuan Y., Etheridge A., Zhou Y., Huang D., Wilmes P., Galas D. The complex exogenous RNA spectra in human plasma, An interface with human gut biota? PLoS ONE. 2012;7:e51009. doi: 10.1371/journal.pone.0051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pokoo R., Ren S., Wang Q., Motes C.M., Hernandez T.D., Ahmadi S., Monteros M.J., Zheng Y., Sunkar M. Genotype- and tissue-specific miRNA profiles and their targets in three alfalfa (Medicago sativa L) genotypes. BMC Genom. 2018;19:913. doi: 10.1186/s12864-018-5280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Crisp P.A., Hammond R., Zhou P., Vaillancourt B., Lipzen A., Daum C., Barry K., de Leon N., Buell C.R., Kaeppler S.M., et al. Variation and inheritance of small RNAs in maize inbreds and F1 hybrids. Plant Physiol. 2020;182:318–331. doi: 10.1104/pp.19.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ali O., Ramsubhag A., Jayaraman J. Biostimulant properties of seaweed extracts in plants, implications towards sustainable crop production. Plants. 2021;10:531. doi: 10.3390/plants10030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Dad H.A., Gu T.W., Zhu A.Q., Huang L.Q., Peng L.H. Plant exosome-like nanovesicles, emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021;29:13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bhome R., Del Vecchio F., Lee G.H., Bullock M.D., Primrose J.N., Sayan A.E., Mirnezami A.H. Exosomal microRNAs (exomiRs), Small molecules with a big role in cancer. Cancer Lett. 2018;420:228–235. doi: 10.1016/j.canlet.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., Samykutty A., Zhang L., Yan J., Miller D., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Díez-Sainz E., Lorente-Cebrián S., Aranaz P., Riezu-Boj J.I., Martínez J.A., Milagro F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021;8:586564. doi: 10.3389/fnut.2021.586564. [DOI] [PMC free article] [PubMed] [Google Scholar]