Abstract
There are uncertainties with respect to the transmission of methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA) and Staphylococcus pseudintermedius between dogs and humans. In this study, we investigated concomitant nasal colonization of dogs and humans in three cohorts. Cohort I, households owning dogs: In 42 of 84 households, 66 humans (36.9%) and 10 dogs (8.9%) carried S. aureus. MRSA, attributed to sequence type (ST) 22 and ST130, were detected in two (1.1%) of the humans but in none of the dogs. Typing by means of spa-typing and whole-genome sequencing (WGS) indicated eight transmissions of S. aureus between humans and dogs in 8 of 42 (19.0%) households with human S. aureus carriers, whereas in 11 of 38 (29.0%) households with ≥two persons and S. aureus colonization of humans, 15 human-to-human transmissions were observed (p = 0.43). S. pseudintermedius was isolated from 42 dogs (37.5%), but from only one human (0.6%). In this case, WGS-based typing indicated strong relatedness of this isolate with a canine isolate from the same household. Cohort II, dogs and their owners visiting a veterinary practice: Among 17 humans and 17 dogs attending a veterinary practice, MSSA was detected in three humans and two dogs, and S. pseudintermedius in only six dogs. Cohort III, dogs used for animal-assisted interventions in human healthcare facilities and their owners: MSSA was obtained in 1 of 59 dogs (1.7%) and in 17 of 60 (28.3%) of the dog owners, while S. pseudintermedius was isolated from seven (12%) dogs and one (1.7%) human owner. We conclude that the risk of exchanging S. aureus/MRSA between humans and dogs is higher than that for S. pseudintermedius.
Keywords: zoonosis, transmission of Staphylococcus aureus, household contacts, dogs, humans
1. Introduction
Staphylococcus aureus (S. aureus) is widely disseminated as a colonizer and as an opportunistic pathogen among humans and other animal species. In the human community, it colonizes the anterior nares of about 25–35% of healthy persons [1,2]. The population structure of S. aureus consists of several clonal complexes, some of which are associated with defined animal species, while others are less host-specific [3]. Methicillin-resistant S. aureus (MRSA) is globally prevalent in nosocomial settings as healthcare-associated MRSA (HA-MRSA), which is mainly due to the spread of epidemic clonal lineages [4]. In addition, MRSA emerges in the community without any relation to the healthcare facilities (CA-MRSA, [5]. HA-MRSAs, especially those attributed to lineage ST22, were also observed as nosocomial pathogens in veterinary hospitals [6]. Livestock-associated MRSA (LA-MRSA), attributed to clonal complex (CC) 398 as defined by multilocus sequence typing (MLST), originally emerged in livestock before a particular subpopulation became increasingly prevalent in animal hospitals during the past decade [7,8,9].
Staphylococcus pseudintermedius is a member of the Staphylococcus intermedius group (SIG), besides Staphylococcus intermedius and Staphylococcus delphini [10]. These three coagulase-positive staphylococcal species are commensals of the skin and mucous membranes. Staphylococcus pseudintermedius is the major coagulase-positive staphylococcal species that colonizes dogs and cats, and also the most prevalent causative agent of canine bacterial infections [11]. Methicillin-resistant S. pseudintermedius (MRSP) exhibiting multiple resistance phenotypes has globally emerged as nosocomial pathogens in hospitals for small animals [12]. Although still infrequent, S. pseudintermedius was also isolated from infections in humans after dog bites [13,14], as well as from other kinds of infections, including septicemia [15]. So far, studies on human nasal colonization with S. pseudintermedius in dog-owning households were reported in Korea [16] and Portugal [17], where a prevalence of 3–4.5% was found in humans and 25–65.9% in dogs, respectively.
In 2020, 10.7 million dogs were living in 21% of German households [18]. Dogs and cats are increasingly regarded as family members. Therefore, the question about mutual transmission of pathogens is of particular interest. In households, staphylococci can be transmitted either through direct contact during owner-pet interactions or through secondary contact with contaminated surfaces [19]. Previous studies suggested that S. aureus can be transmitted between dogs and their human owners, as isolates exhibited identical phage patterns [20]. Although considerably less frequent than in humans, S. aureus was described as a colonizer of dogs [21]. The question of whether this colonization is associated with S. aureus carriage by human contact persons has been addressed by a few previous studies in Korea [16], Hong Kong [22], and the United States [23], which resulted in different findings with respect to the frequencies of concomitant colonization of dogs and owners. This could be due to the study design with respect to concomitant sampling of humans and dogs, and to not sampling all household members. Intrahousehold transmission of MRSA from colonized or infected humans to dogs was observed in North America [24,25]. The introduction of human HA-MRSA strains into veterinary hospitals by dogs having acquired them from their owners was reported for MRSA ST22 in Europe [26] and ST5 (“USA100”) in North America [27,28].
Animal-assisted therapeutic interventions (AAI) are, meanwhile, established in human healthcare facilities, such as nursing homes, rehabilitation care centers, and hospitals, in many countries. The aim of AAI is to improve the patients’ emotional, cognitive, or neurological functioning [29]. Although the results on the effectiveness of AAI are altogether heterogeneous [29], it seems to be particularly effective in children and adults suffering from post-traumatic stress disorder symptoms [30]. In this context, the possibility of transmitting of HA-MRSA by dogs used for AAI is of particular interest. So far, this was reported from Canada [31] and the UK [32].
This study aims to answer questions about the emergence of both S. aureus and S. pseudintermedius in humans and dogs, and to analyze the transmission. We sampled humans and dogs differentiated into three cohorts: (I) as household contacts, (II) as visitors at veterinary practices, and (III) in human healthcare settings.
2. Materials and Methods
Three different cohorts of participants were enrolled: households owning dogs (cohort I), dogs and their owners visiting a veterinary practice (cohort II), and dogs used for AAI in human healthcare facilities and their owners (cohort III). The study was performed from 2019–2020.
2.1. Study Populations and Recruitment
Cohort I: The study was performed in the Landkreis Harz region in Central Germany (214,446 inhabitants, 2104.54 km2). There are three primary care hospitals in this area. A total of 83 households were enrolled. Recruitment was based on directly contacting several families owning dogs in five villages and three towns. In a second step, households already participating in the study referred to other dog-owning households. A prerequisite for participation was the agreement of the contacted households, documented by the return of a declaration of consent. The participants were provided with swabs, as well as an instruction for swab-self-collection for humans and dogs, and a short questionnaire asking for information on basic demographic characteristics, such as age, sex, and potential risk factors for colonization with S. aureus (e.g., antibiotic treatment, hospital stay during the six months prior to sampling). The feasibility of self-collection of nasal swabs was previously reported [33]. Households in which dogs with S. aureus carriage were detected were asked to provide a second nasal sample from the dogs and owners 12 months after the initial sampling.
Cohort II: 17 dogs and their owners were randomly selected by a veterinarian and instructed with respect to self-sampling and data provision as in cohort I.
Cohort III: Supported by the organization “Animals as Therapy” (www.tierealstherapie.at/tat-waz/, accessed on 21 February 2022) the owners of dogs used for AAI in human regional healthcare facilities were directly contacted. The further procedure was as described for cohort I.
The study was approved by the ethical committee of the medical faculty of the University of Magdeburg (#33/14).
2.2. Microbiological Analysis
The eSwabTM system (MAST Diagnostics, Reinfeld, Germany) was used for taking swabs from both nostrils of humans and dogs. Aliquots were streaked on CHROMagarTM MRSA (MAST) and, in parallel, on Mueller–Hinton blood agar plates (Oxoid, Wesel, Germany). After incubation at 37 °C for 18–24 h, one suspicious colony was subcultured on sheep blood agar (except in the case of differences in colonial morphology and hemolysis). Confirmation of S. aureus was performed by demonstration of the clumping factor and, additionally, by the tube coagulase test. For proof of the clumping factor, we used a solution of fibrinogen from human plasma (Sigma-Aldrich, Taufkirchen, Germany) of 2 mg/mL 0.85% NaCl. For the tube coagulase test, fresh ready-to-use human plasma (DRK blood donation service, Springe, Germany) was used. In the case of negative results, we performed a PCR for the region of tuf that is specific for S. aureus using the primers and PCR conditions as described [34]. For the identification of S. pseudintermedius, presumptive colonies were subcultured on blood agar plates and subjected to PCR analysis according to [35]. For PCR analysis, genomic DNA was extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany), and lysostaphin (100 mg/L; Sigma, Taufkirchen, Germany) for bacterial lysis.
Antimicrobial susceptibility testing: The following antibiotics were tested: penicillin (PEN), oxacillin (OXA), fosfomycin (FOS), gentamicin (GEN), linezolid (LIN), erythromycin (ERY), clindamycin (CLI), tetracycline (TET), tigecycline (TIG), vancomycin (VAN), teicoplanin (TEI), ciprofloxacin (CIP), trimethoprim/sulfamethoxazole (TRS), fusidic acid–sodium (FUS), rifampicin (RIF), mupirocin (MUP), cefoxitin (CEF), moxifloxacin (MOX), and daptomycin (DAP). The testing was performed using a broth microdilution and applying EUCAST clinical breakpoints for humans (version 9.0, valid from 1 January 2019).
2.3. Molecular Characterization
DNA extraction: Strains were grown overnight in tryptic soy broth at 37 °C. For PCR analysis, genomic DNA was extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany), and lysostaphin (100 mg/L; Sigma, Taufkirchen, Germany) for bacterial lysis.
PCR for resistance genes: PCR analysis was performed for mecA and mecC in order to confirm MRSA as previously described [36].
Spa-typing, BURP and MLST: All S. aureus isolates were subjected to spa-typing as previously described [36]. Related spa-types (costs ≤ 4) were grouped into spa-clonal complexes (spa-CC) by use of the BURP algorithm (Ridom StaphType software version 2.2.1, Ridom, Münster, Germany). The spa-CCs were allocated to MLST CCs (Ridom SpaServer—Spa-types; database of the German National Reference Center for Staphylococci and Enterococci). Subsets of the isolates were subjected to MLST as described elsewhere [36].
2.4. Whole-Genome Sequencing and Phylogenetic Analyses
Whole-genome sequencing: DNA quantification was carried out using the Qubit dsDNA HS Assay Kit (Invitrogen/Thermo Fisher Scientific, Karlsruhe, Germany). A total of 1 ng of extracted DNA was employed for library preparation using the Nextera XT DNA Library Prep Kit according to the manufacturer’s instructions (Illumina, San Diego, CA, USA). Sequencing was performed on an Illumina MiSeq platform in paired-end mode with a final readout of 2 × 250 bp. The quality of the raw read data was assessed by an in-house-developed pipeline (Qcumber-2). Genome assembly and core-genome multilocus sequence typing (cgMLST): The raw read files were imported into Ridom SeqSphere+ version 8.1.0 (Ridom GmbH, Münster, Germany (https://www.ridom.de/seqshpere/ug/v81/Version_History.html, accessed on 21 February 2022) and assembled de novo using SPAdes (version 3.13.1 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3342519/; accessed on 21 February 2022), default settings, integrated in the Linux version of SeqSphere). For the S. aureus isolates, the S. aureus cgMLST scheme version 1.3 (http://pubmed.ncbi.nlm.nih.gov/24759713/; accessed on 21 February 2022), implemented into SeqSphere+, was applied. For S. pseudintermedius, an ad hoc cgMLST was established according to Ridom SeqSphere guidelines, with S. pseudintermedius ED99 as a seed genome (GenBank accession number CP002478). Minimum spanning trees were calculated on the basis of cgMLST by ignoring pairwise missing values. To detect closely related S. aureus and S. pseudintermedius isolates, the threshold for cluster distance determination was set to 10 and 15 cgMLST alleles, respectively.
3. Results
This study included 179, 17, and 60 humans in cohorts I, II, and III, respectively. Moreover, 112, 17, and 59 dogs were included in the three cohorts.
3.1. Cohort I: Humans and Dogs in Household Contacts
Overall, 179 persons participated in the study, representing all members of 84 households. Among these households, 18 were single households (21.4%), 48 comprised two persons (45.2%), and 18 comprised ≥ three persons. In the 84 households, there were 112 dogs, which were included in the study (one dog in 61 households, two dogs in 19 households, ≥three dogs in 4 households).
3.1.1. S. aureus Nasal Carriage
S. aureus was detected in 66/179 human participants (36.9%); 64 (35.8%) carried methicillin-susceptible S. aureus (MSSA), and 2 carried MRSA (1.1%). S. aureus nasal carriage was significantly more frequent among persons suffering from atopic eczema (Table 1). For the other variables, an association with S. aureus carriage was not apparent. For all 112 dogs living in these households, the overall distribution of risk factors for S. aureus carriage was as follows: a previous stay in an animal hospital was reported for 8 (6.6%), a previous antibiotic prescription for 25 (21.5%), skin lesions for 6 (5.4%), and bite injuries for 1 (0.9%). Among these 112 dogs, 10 (7.8%) carried S. aureus (all of which were MSSA). Of these, two were previously treated with an antibiotic, one was treated in a veterinary hospital, and one had a skin lesion.
Table 1.
Characteristics of humans and S. aureus (SA) carriage in 84 households.
| Characteristics | All 179 Persons in 84 Households 1 | 14 Persons in 8 Households with S. aureus in Humans and Dogs |
97 Persons in 34 Households with S. aureus only in Humans |
68 Persons in 42 Households without S. aureus | |||
|---|---|---|---|---|---|---|---|
| carriage | SA positive |
SA negative |
SA positive |
SA negative |
SA positive |
SA negative |
SA negative |
| No. and % among carriers and non-carriers, respectively | |||||||
| all | 66 (36.9) | 113 (63.1) | 12 | 2 | 54 (55.6) | 43 (44.3) | 68 (100.0) |
| female | 34 (51.5) | 61 (54.0) | 8 | 1 | 26 (48.1) | 25 (58.1) | 35 (51.4) |
| male | 32 (48.5) | 52 (46.0) | 4 | 1 | 28 (51.9) | 18 (41.9) | 33 (48.5) |
| previous hospital stay 2 | 11 (16.7) | 15 (13.3) | 3 | 2 | 8 (14.8) | 4 (9.3) | 9 (13.2) |
| antibiotic consumption 2 | 12 (18.2) | 12 (10.6) | 3 | 0 | 9 (16.7) | 4 (9.3) | 8 (11.8) |
| diabetes mellitus | 4 (6.1) | 3 (2.7) | 0 | 0 | 4 (7.4) | 0 | 3 (4.4) |
| skin lesions | 2 (3.0) | 2 (1.8) | 0 | 0 | 2 3.7) | 2 (4.7) | 0 |
| atopic eczema | 10 (15.2) | 4 (3.5%) | 1 | 0 | 0 | 0 | 4 (5.9) |
| ≥2 dogs in the household | 25 (37.8%) | 36 (31.8%) | 5 | 3 | 20 (37) | 16 (37.2%) | 17 (25%) |
1p-values (Fishers exact, two-tailed) for all S. aureus carriers vs. non-carriers and characteristics predisposing to S. aureus nasal carriage: female gender: 0.759; previous hospital stay: 0.149; previous antibiotic consumption: 0.368; skin lesions: 1.0; diabetes mellitus: 0.426; atopic eczema: 0.02; living with ≥2 dogs in the household: 0.65. 2 6 months prior to sampling.
Typing attributed the S. aureus isolates of humans to MLST clonal complexes CC1 (3), CC5 (1), CC7 (4), CC8 (9), CC12 (2), CC15 (12), CC22 (5), CC30 (10), CC45 (15), CC97 (1), CC398 (1), CC130 (1), and ST133 (3). The two MRSA isolates were associated with CC22 (harboring mecA; phenotypically resistant to penicillin, cefoxitin, oxacillin, erythromycin, clindamycin, ciprofloxacin, and moxifloxacin) and CC130 (harboring mecC; phenotypically resistant to penicillin, cefoxitin, and oxacillin only), respectively.
3.1.2. S. aureus Intrahousehold Transmission
Human S. aureus carriage was found in 42 (50.0%) of the 84 households. There were 18 single households with four persons carrying S. aureus. More than one person lived in 66 households (Table 2). Overall, human S.aureus carriage affected 4/18 (22.2%) single households vs. 38/66 (57.6%) non-single households (p = 0.015); or related to the number of persons, 4/18 (22.2%) persons in single households vs. 62/161 (38.5%) persons in non-single households (p = 0.20643). In 17 of the 66 non-single households, more than one person was concomitantly colonized. The observation of identical spa-types and resistance phenotypes in isolates from different individuals from the same household would initially indicate intrahousehold transmission. This was the case for 11 of these 17 households (Supplemental Table S1). Here, we observed 15 transmissions (calculated as the number of humans carrying S. aureus with matching typing characteristics minus one). Based on the 38 non-single households with at least one S. aureus carrier, where transmission could actually happen, the intrahousehold, human-to-human transmission rate was 11/38 (29.0%).
Table 2.
Households, number of persons, and S. aureus colonization of humans.
| Persons/ Household |
No. of Households (n = 84) |
Households with Carriers of S. aureus
(n =42) |
Households with 1 Person Colonized |
Households with ≥ 2 Persons Colonized |
||||
|---|---|---|---|---|---|---|---|---|
| Households | Carrier (%) | All persons | Households | Carrier (%) | All persons | |||
| 1 | 18 | 4 | 4 | 4 (22) | 18 | |||
| ≥2 | 66 | 38 1 | 21 | 21 (50) | 42 | 17 | 41 (82) 2 | 51 |
p-values (Fishers exact, two-tailed): 1S. aureus carriage in single vs. non-single households, 0.0151 2 S. aureus carriage in households with one colonized person vs. households ≥ two colonized persons, 0.0037.
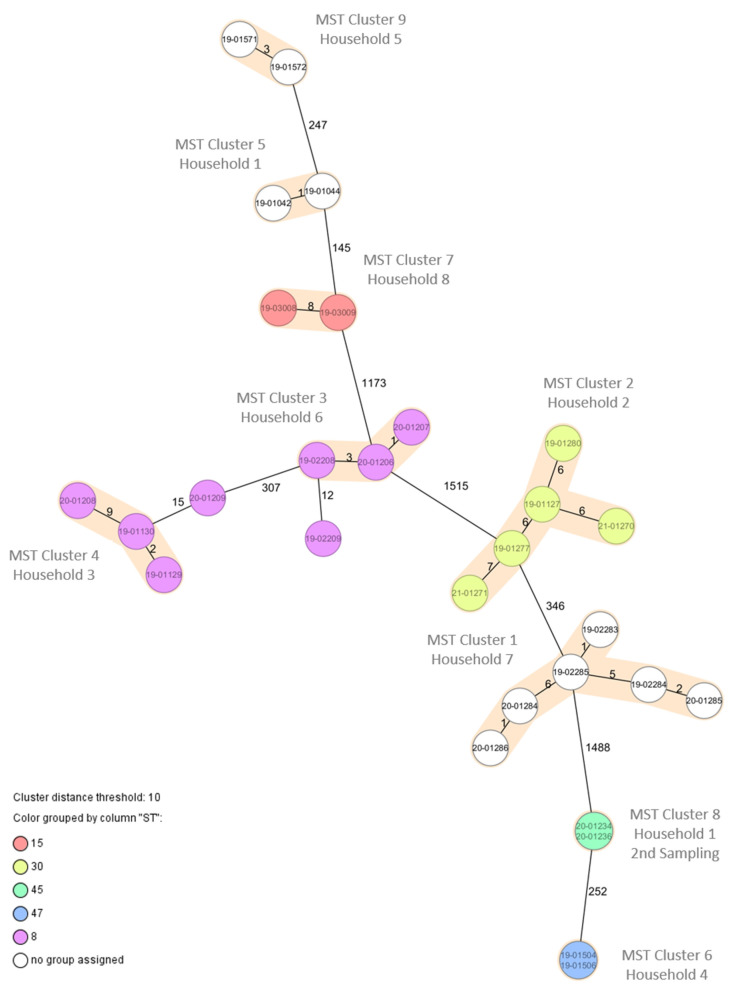
The overall rate of S. aureus carriage among dogs was 10/112 (8.9%), i.e., dogs carrying S. aureus were found in 10/84 households (11.9%). However, of the ten dogs, eight lived in families where humans were also colonized with S. aureus. Among these eight households, there were three with two colonized persons. For all of these households, the isolates of the dogs exhibited the same spa-types and, with one exception, the same resistance phenotypes as those isolates derived from the humans living in the same household (Table 3), suggesting intrahousehold transmission between humans and dogs. This was confirmed by the results from the cgMLST allelic profiles derived from whole-genome sequencing of the 27 isolates (Figure 1). Although we observed 1–15 allelic differences between the isolates, this still indicates very close relatedness and transmission [37]. As one transmission was likely for each of the eight households, the transmission rate was 8/42 (19%) and, thus, was lower than that observed for human-to-human transmission (11/38; p = 0.4306). A second sampling was performed for these eight households after one year, in which six households participated again. Besides a lack of detection of S. aureus in dogs from household No. 1 and, further, No. 2 and No. 4, the results from first sampling were confirmed (Table 3). Interestingly, in household No. 1, a second new dog (puppy) was acquired in that time period, and the human contact person living in that household lost the previous carriage of S. aureus CC15 and changed the nasal colonization to S. aureus CC45, closely related to that carried by the new dog (Table 3).
Table 3.
Demonstration of S. aureus in humans und dogs living in the same households.
| First Sampling | Second Sampling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Household | Individual 1 | Species 2 | Isolate 3 | Spa-Type | CC 4 | Resistance Phenotype 5 | Individual 1 | Species 2 | Isolate 3 | Spa-Type | CC 4 | Resistance Phenotype 5 |
| 1 | H-28 | SA | 19-01042 | t2696 | 15 | PEN | H-28.1 | SA | 20-01234 | t779 | 45 | susceptible |
| H-29 | SA | t091 | 7 | susceptible | H-29.1 | SA | t091 | 7 | susceptible | |||
| D-30 | SA | 19-01044 | t2696 | 15 | susceptible | D-30.1 | negative | |||||
| D-400 | SA | 20-01236 | t779 | 45 | susceptible | |||||||
| 2 | H-62 | SA | 19-01277 | t1577 | 30 | PEN, ERY | H-62.1 | SA | 21-01270 | t1577 | 30 | PEN, ERY |
| H-63 | SA | 19-01127 | t1577 | 30 | PEN, ERY | H-63.1 | SA | 21-01271 | t1577 | 30 | PEN, ERY | |
| D-64 | SP | PEN, OXA, TET, CIP, MOX | D-64.1 | negative | ||||||||
| D-65 | SA | 19-01280 | t1577 | 30 | PEN, ERY | D-65.1 | negative | |||||
| 3 | H-66 | SA | 19-01129 | t9325 | 8 | PEN | H-66.1 | SA | 20-01208 | t9325 | 8 | PEN |
| D-67 | SA | 19-01130 | t9325 | 8 | PEN | D-67.1 | SA, SP | 20-01209 | t9325 | 8 | PEN | |
| 4 | H-176 | SA | 19-01504 | t026 | 45 | susceptible | H-176.1 | negative | ||||
| D-178 | SP | D-178.1 | negative | |||||||||
| D-179 | SA | 19-01506 | t026 | 45 | susceptible | D-179.1 | negative | |||||
| 5 | H-206 | SA | 19-01571 | t084 | 15 | PEN | H-206.1 | No feedback | ||||
| H-207 | negative | H-207.1 | ||||||||||
| D-208 | SA | 19-01572 | t084 | 15 | PEN | |||||||
| 6 | H-312 | SA | 19-02208 | t008 | 8 | PEN, FUS | D-312.1 | SA | 20-01206 | t008 | 8 | PEN, FUS |
| D-313 | SA | 19-02209 | t008 | 8 | PEN, FUS | D-313.1 | SA | 20-01207 | t008 | 8 | PEN, FUS | |
| D-314 | SP | susceptible | deceased | |||||||||
| 7 | H-318 | SA | 19-02283 | t6997 | 30 | PEN | H-318.1 | SA | 20-01284 | t6997 | 30 | PEN |
| H-319 | SA | 19-02284 | t6997 | 30 | PEN | H-319.1 | SA | 20-01286 | t6997 | 30 | PEN | |
| D-320 | SA | 19-02285 | t6997 | 30 | PEN | D-320.1 | SA | t6997 | 30 | PEN | ||
| 8 | H-350 | negative | No feedback | |||||||||
| H-351 | SA | 19-03008 | t935 | 15 | susceptible | H-351.1 | ||||||
| D-352 | SP | TET | D-352.1 | |||||||||
| D-353 | SA | 19-03009 | t935 | 15 | susceptible | D-353.1 | ||||||
1 Individual: H = human, D = dog; 2 Species: SA = S. aureus, SP = S. pseudintermedius; 3 laboratory number; 4 CC = clonal complex; 5 No resistance was detected against: FOS, GEN, LIN, CLI, TIG, VAN, TEI, TRS, RIF, MUP, DAP; susceptible = susceptible to all antibiotics tested.
Figure 1.
Minimum spanning tree based on core-genome multilocus sequence typing allelic profiles of 27 S. aureus isolates from humans and dogs in eight households. Colors indicate the MLST CC (as deduced from WGS data). Brown shades indicate clusters with <10 allelic differences between the isolates.
3.1.3. S. pseudintermedius Colonization of Dogs and Humans
This species was detected in nasal swabs from 42 (37.5%) of the 112 dogs who lived in 36 (42.8%) of the 84 households enrolled in the study. It was obtained from only 1 of the 179 humans (0.6%). Three of these dogs were concomitantly colonized with S. aureus. There was no association of S. pseudintermedius carriage and the dogs’ hospital stays prior to sampling. Canine S. pseudintermedius carriage was not significantly associated with the number of dogs living in the same households (p 0.557, Table 4).
Table 4.
Number of dogs in households carrying S. pseudintermedius.
| Total No. of Households | No. of Households with SP 1 Positive Dogs | Total No. of Dogs | No. of SP 1 Positive Dogs | ||
|---|---|---|---|---|---|
| All | 84 | 36 | 112 | 42 (37.5%) | |
| Households with 1 dog | 61 | 21 (34.4%) | 61 | 21 (34.4%) | |
| Households with ≥2 dogs | 23 | 15 (65.2%) p (0.078) | 51 | 21 (41.2%) p (0.557) | |
| 2 dogs | 19 | 11 | 38 | 14 (36.8%) | |
| 3 dogs | 3 | 3 | 9 | 4 | |
| 4 dogs | 1 | 1 | 4 | 3 | |
1 SP = S. pseudintemedius.
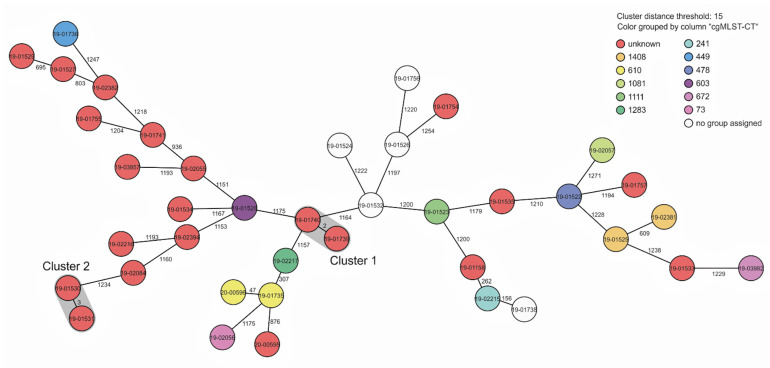
In only 1 of 22 households owning two dogs, S. pseudintermedius was detected in both dogs. These isolates were closely related when subjected to cgMLST, as shown in Figure 2 (cluster 1: two alleles different). In one of the three households owning three dogs, S. pseudintermedius was isolated from all three animals, and in one household owning four dogs, three carried S. pseudintermedius, respectively. In these cases, cgMLST typing revealed no relatedness. In only one household owning three dogs, S. pseudintermedius was detected in two of the three dogs and one of the three humans belonging to the household. The isolates from the human and from dog 1 were closely related (cluster 2; three alleles different), whereas the isolate from dog 2 was clearly not related. Altogether, the population structure of the sample of isolates investigated was highly diverse, as also indicated by attribution of the isolates to at least 12 complex types (Figure 2). The antibiotic resistance phenotypes of the 42 S. pseudintermedius isolates are shown in Table 5. Of these S. pseudintermedius isolates, 28 (68%) were susceptible to all tested antibiotics, 8 exhibited resistance to only one antibiotic, and 7 isolates were resistant to two or more antibiotics. One isolate (MRSP) was resistant to oxacillin (mecA positive), penicillin, erythromycin, clindamycin, tetracycline, and gentamicin. This isolate exhibited MLST ST672, as deduced from WGS data.
Figure 2.
Minimum spanning tree based on core-genome multilocus sequence typing allelic profiles of 39 S. pseudintermedius isolates from humans and dogs in households (cohort I). Colors indicate the cgMLST-CT (as deduced from WGS data). Grey shades indicate clusters with <15 allelic differences between in the isolates.
Table 5.
Antibiotic resistance phenotypes of S. pseudintermedius from dogs in the cohorts.
| Antibiotic Resistance Phenotypes 1 | All | Cohort I | Cohort II | Cohort III |
|---|---|---|---|---|
| PEN | 8 | 7 | 1 | |
| TET | 4 | 4 | ||
| PEN, TET | 5 | 5 | ||
| ERY, CLI | 3 | 1 | 1 | 1 |
| ERY, CLI, TET | 3 | 2 | 1 | |
| PEN, ERY, CLI, TET | 3 | 3 | ||
| PEN, GEN, ERY, CLI, TET, CIP | 1 | 1 | ||
| PEN, OXA, TET, CIP, MOX | 1 | 1 | ||
| PEN, OXA, ERY, CLI, TET, CIP, MOX, TRS | 3 | 3 | ||
| PEN, OXA, GEN, ERY, CLI, CIP, MOX, TRS, FUS | 1 | 1 | ||
| Susceptible to all antibiotics tested | 23 | 18 | 1 | 4 |
| Total | 55 | 42 | 6 | 7 |
1 No resistance was found against LIN, TIG, VAN, TEI, RIF, MUP, DAP, CEF.
3.2. Cohort II: Dogs and Humans after Visiting a Veterinarian Practice
This group was composed of 17 pairs of dogs and their dedicated owners. Nasal S. aureus colonization was detected in 3 of the 17 humans and in 2 of the 17 dogs, which were not dedicated to the human carriers. S. pseudintermedius was obtained from six of the dogs, but none of the humans. Of these isolates, three were revealed as MRSP (Table 5).
3.3. Cohort III: Dogs Active in AAI and Their Owners
S. aureus colonization was detected in 17 (28.3%) of the 60 dog handlers, but in only 1 of the 59 dogs (1.7%) attended by them. The S. aureus isolates were attributed to MLST clonal complexes CC5 (2), CC7 (1), CC15 (3), CC22 (3), CC30 (1), CC34 (1), CC45 (4), and CC398 (2). Among these isolates, four were susceptible to all antibiotics tested; four were resistant to penicillin; one was resistant to erythromycin; and one was resistant to penicillin and to fusidic acid.
S. pseudintermedius was detected in seven of the dogs (11.9%) and in one of the human owners (1.7%), whose dog did not carry S. pseudintermedius. The isolate from the human was multi-resistant (PEN, OXA, FOS, GEN, ERY, CLI, CIP, MOX, TRS, FUS) and mecA- positive (MRSP).
3.4. Results from Spa-Typing and Phenotypic Susceptibility Testing of S. aureus (Cohorts I–III)
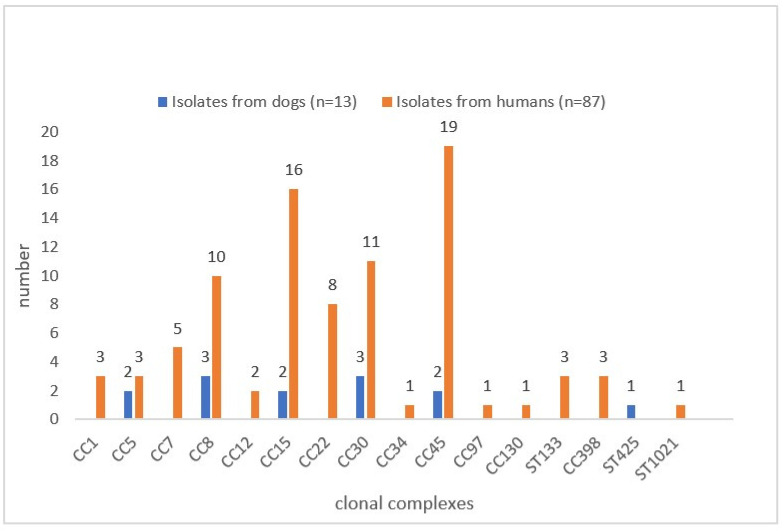
The results from spa-typing of the 87 isolates from humans are presented in supplemental Table S2. The attribution to clonal complexes of these isolates and of the 13 isolates from dogs is shown in Figure 3. Four clonal complexes predominate in this order: CC45 (n = 22), CC15 (n = 18), CC30 (n = 15), and CC8 (n = 13), demonstrated in Figure 3. The results from phenotypical antibiotic susceptibility testing of all detected isolates from the humans and dogs are compiled in supplemental Table S3.
Figure 3.
Distribution of MLST clonal complexes (CC) and sequence types (ST) of S. aureus isolates from humans and dogs from all cohorts.
4. Discussion
S. aureus nasal carriage was observed in 36.9% of the humans living in 42 of 84 households owning dogs. This is more frequent than what has been observed in three previous studies in Central and Northern Germany (26.9% [38], 22% [39]), but in the range of data from other studies, indicating a prevalence of (intermittent) S. aureus carriage of 20–50% [2]. Among the factors that predispose a person for S. aureus carriage, such as diabetes mellitus or skin disorders/allergies, only atopic eczema was associated with S. aureus carriage in this study, which might have been due to the small sample size. The possible influence of living together with dogs on the nasal microbiome [40] and the consequences on S. aureus colonization resulting therefrom remain to be explored. Among the 42 households with S. aureus carriage in humans, there were 17 in which two and more household members were colonized. Eleven of these isolates from different persons exhibited identical typing patterns, suggesting intrafamilial transmission between humans. This is well known and most probably mediated by direct contact and surface contamination in the household [41,42,43]. On the other hand, this means that, in 55.2% (21/38) of the non-single households with human carriers of S. aureus where one member was carrying S. aureus, the other human household members were not affected. We observed concomitant carriage of humans and dogs with closely related S. aureus isolates in eight households, which suggests transmission between humans and dogs (or vice versa). As it was observed in only 8 among the 42 households (19%) with human S. aureus carriers, it seems that transmission between humans and dogs occurs less frequently when compared to intrahousehold human-to-human transmission (39.529.0%, p=0.4306), although this effect was not statistically significant. Similar observations were reported from studies in Spain [44] and the USA [23,45]. Compared with our results, where the typing patterns of S. aureus from dogs and humans were identical and clearly indicated transmission, these studies partly found a higher proportion of different isolates. This might have been due to incomplete screening of all the humans in these households. The frequency distribution of clonal complexes to which the MSSA isolates from humans and dogs in our study were attributed corresponded to that observed in a previous study on nasal S. aureus colonization in Central Germany [36,39]. So far, there are no indications of dog-adapted clonal lineages of S. aureus. The results from a study on the dynamics of S. aureus colonization in humans and dogs in seven Spanish households [44] suggested humans as the original reservoir of S. aureus for dogs. Our results may support this hypothesis, as the overall carriage rates were much higher among humans than among dogs. However, transmission from dogs to humans also seems to be possible, as suggested by our observation that in one participant, a previously colonizing strain was displaced by another strain, which was also found in a dog that was bought shortly before the sampling. We detected MRSA in 2 of the 179 humans (1.1%), but not in the dogs. This prevalence in humans in the community corresponds to results from the previous studies mentioned above. One of these MRSA belonged to MLST clonal complex CC22, representing the majority of HA-MRSA isolates in Germany [46]. The other MRSA was associated with mecC and attributed to clonal lineage ST130, which has a wide range of animal hosts and is rare in humans so far [47]. Dogs acquire MRSA colonization by either animal hospital stay or by contact with humans with MRSA infection or colonization [26,28]; the canine carriage rates in German veterinary practices have previously been assessed and were 2.6%. This is higher than observed in this study. However, the sample size in the comparable cohort (cohort II) of this study was very small. We observed a lower prevalence of nasal S. aureus carriage in the humans handling therapy dogs (cohort III) vs. the humans living with dogs in their households (cohort I). An explanation could be that handlers of therapy dogs practice more careful hygiene, including hand washing, while at home or on duty with their dogs. Hands seem to play an important role in human-to-human transmission via the contamination of surfaces [43]. Another explanation may be that the contact is less intense. The prevalence of S. pseudintermedius carriage among dogs living in households was higher in our study (37.5%) than that reported for dogs from Spain (23%, [44]), but lower than observed in Canada (46%, [27]) and in Korea (66%, [16]). For the latter study, this could be due to the inclusion of rectal swabs, which might have increased the sensitivity of the analysis. In the study reported from Korea [16], the dogs were sampled at admission to veterinary hospitals. Interestingly, we observed no statistically significant association of the number of dogs living in the 36 households owning colonized dogs with the prevalence of nasal S. pseudintermedius colonization. This finding suggests that the acquisition of nasal colonization by transfer between healthy dogs probably does not frequently occur. This is in line with the observed highly diverse population structure, which was also reported for methicillin-susceptible S. pseudintermedius from France [48]. We observed nasal carriage of humans living with dogs in only 0.6% of the persons investigated. This is lower than reported from Korea [16], Spain [44], and Canada [27]. It seems that humans are not regular hosts for S. pseudintermedius. However, adaptation to humans may occur. A recent pilot study has shown diversity between the isolates attributed to the same clonal lineage from infections in humans and in dogs with respect to pathogenicity islands and virulence gene- containing prophages [49]. In this study, methicillin-resistant S. pseudintermedius (MRSP) was only detected in 0.9% of the dogs from households. This might have been due to the low number of dogs with a hospital stay prior to the study. The MRSP isolate exhibited MLST type ST610, which has not been reported so far for MRSP from dogs [12]. The prevalence of MRSP carriage by dogs in the community reported so far is 0.0% (Sweden, [14]), 2.6% (Norway, [50]), and 4.5% (Canada [27]). This is contrasted to the high prevalence observed in dogs undergoing clinical treatment [12,51]. Multi-resistant S. pseudintermedius seems to be associated with particular, animal-hospital-associated S. pseudintermedius clonal lineages [52], whereas isolates, which are susceptible or resistant to only a few antibiotics, represent natural colonizers of dog. Nevertheless, clinical microbiological laboratories should pay attention to the correct identification of S. pseudintermedius, which may be a more common human pathogen than has been recognized so far [53]. Although there is consensus that the benefits of AAI outweigh the risks with respect to the transmission of pathogens [29,54], the question of whether dogs used in AAI may be vectors of MSSA, MRSA, or MRSP remains of interest. Besides older observations [29,30], a current study in a pediatric hospital in the USA has shown mutual transmission of MRSA between dogs and children and the effectiveness of a decolonization procedure on dogs [55]. Guidelines for therapy animal organizations, facilities, and therapy animal handlers providing AAI in healthcare facilities were developed in several countries. SHEA (Society for Healthcare Epidemiology of America) has provided rather detailed guidance [56]. It recommends annual veterinary inspection of the dogs, but not routine screening for MRSA colonization. In contrast, the latter is recommended by the guideline from the German Society for Hospital Hygiene [57]. If the screening of dogs is required (e.g., for outbreak investigations), their handlers should also be screened, as mutual transmission cannot be excluded. So far, there is limited experience with the effectiveness of MRSA (or MSSA or MRSP) decolonization therapies for dogs, which may include the application of a mupirocin nasal ointment. In this context, the finding of high-level mupirocin resistance in MRSA from dogs must be taken into account [58,59]. With respect to alternatives, there is also a single report on the successful application of chlorhexidine [55].
5. Conclusions
We estimate the risk of acquisition of nasal colonization of humans with S. aureus from colonized dogs in households to be smaller than human-to-human transmission. For S. aureus, it seems to be higher than for S. pseudintermedius. Whether, in both species, clonal lineages with a more pronounced capacity for spreading among humans and dogs will emerge should be followed up through further surveillance.
Acknowledgments
We acknowledge the valuable technical assistance of Birgit Pasemann, Petra Vilbrandt, and Mike Henkel, and are indebted to Erika Kleindienst for processing of the manuscript. Moreover, we would also like to thank all participants with their dogs and their provided written consent.
Supplementary Materials
The following supplementary material is available online at https://www.mdpi.com/article/10.3390/microorganisms10040677/s1. Table S1: Results from spa-typing of S. aureus isolates from humans and dogs; Table S2: Antibiotic resistance phenotypes of S. aureus from humans; Table S3: Antibiotic resistance phenotypes of S. aureus from humans.
Author Contributions
Conceptualization, C.C., W.W. and R.K.; recruitment of participants, C.C. and W.W.; primary diagnostics, C.C.; typing and input on analysis, F.L.-N. and R.W. data analysis, C.C.; writing—manuscript draft and feedback collection, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly performed within the framework of the German Research Network on Zoonotic Infectious Diseases (project #1 Health-PREVENT, grant no. 01KI2009F).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest to disclose for this manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakr A., Brégeon F., Mège J.L., Rolain J.M., Blin O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018;9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehraj J., Witte W., Akmatov M.K., Layer F., Werner G., Krause G. Epidemiology of Staphylococcus aureus nasal carriage patterns in the community. Curr. Top. Microbiol. Immunol. 2016;398:55–87. doi: 10.1007/82_2016_497. [DOI] [PubMed] [Google Scholar]
- 3.Cuny C., Friedrich A., Kozytska S., Layer F., Nübel U., Ohlsen K., Strommenger B., Walther B., Wieler L., Witte W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med. Microbiol. 2010;300:109–117. doi: 10.1016/j.ijmm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Witte W., Cuny C., Klare I., Nübel U., Strommenger B., Werner G. Emergence and spread of antibiotic-resistant Gram-positive bacterial pathogens. Int. J. Med. Microbiol. 2008;298:365–377. doi: 10.1016/j.ijmm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Mediavilla J.R., Chen L., Mathema B., Kreiswirth B.N. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr. Opin. Microbiol. 2012;15:588–595. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Walther B., Wieler L.H., Friedrich A.W., Kohn B., Brunnberg L., Lübke-Becker A. Staphylococcus aureus and MRSA colonization rates among personnel and dogs in a small animal hospital: Association with nosocomial infections. Berl. Munch. Tierarztl. Wochenschr. 2009;122:178–185. [PubMed] [Google Scholar]
- 7.Vincze S., Stamm I., Kopp P.A., Hermes J., Adlhoch C., Semmler T., Wieler L.H., Lübke-Becker A., Walther B. Alarming proportions of methicillin-resistant Staphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010–2012. PLoS ONE. 2014;9:e85656. doi: 10.1371/journal.pone.0085656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelbary M.M., Wittenberg A., Cuny C., Layer F., Kurt K., Wieler L.H., Walther B., Skov R., Larsen J., Hasman H., et al. Phylogenetic analysis of Staphylococcus aureus CC398 reveals a sub-lineage epidemiologically associated with infections in horses. PLoS ONE. 2014;9:e88083. doi: 10.1371/journal.pone.0088083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuny C., Witte W. MRSA in equine hospitals and its significance for infections in humans. Vet. Microbiol. 2017;200:59–64. doi: 10.1016/j.vetmic.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald J.R. The Staphylococcus intermedius group of bacterial pathogens: Species re-classification, pathogenesis and the emergence of methicillin resistance. Vet. Dermatol. 2009;20:490–495. doi: 10.1111/j.1365-3164.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 11.Bannoehr J., Guardabassi L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012;23:253–266. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 12.Pires dos Santos T., Damborg P., Moodley A., Guardabassi L. Systematic review on global epidemiology of methicillin-resistant Staphylococcus pseudintermedius: Inference of population structure from multilocus sequence typing data. Front. Microbiol. 2016;7:1599. doi: 10.3389/fmicb.2016.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talan D.A., Goldstein E., Staatz D., Overturf G.D. Staphylococcus intermedius: Clinical presentation of a new human dog bite pathogen. Ann. Emerg. Med. 1989;18:410–413. doi: 10.1016/S0196-0644(89)80582-7. [DOI] [PubMed] [Google Scholar]
- 14.Börjesson S., Gomez-Sanz E., Ekstrom K., Torres C., Gronlund U. Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:839–844. doi: 10.1007/s10096-014-2300-y. [DOI] [PubMed] [Google Scholar]
- 15.Somayaji R., Priyantha M.A., Rubin J.E., Church D. Human infections due to Staphylococcus pseudintermedius, anemerging zoonosis of canine origin: Report of 24 cases. Diagn. Microbiol. Infect. Dis. 2016;85:471–476. doi: 10.1016/j.diagmicrobio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Han J.I., Yang C.H., Park H.M. Prevalence and risk factors of Staphylococcus spp. carriage among dogs and their owners: A cross-sectional study. Vet. J. 2016;212:15–21. doi: 10.1016/j.tvjl.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues A.C., Belas A., Marques C., Cruz L., Gama L.T., Pomba C. Similar articles risk factors for nasal colonization by methicillin-resistant staphylococci in healthy humans in professional daily contact with companion animals in Portugal. Microb. Drug. Resist. 2018;24:434–446. doi: 10.1089/mdr.2017.0063. [DOI] [PubMed] [Google Scholar]
- 18.German federal Ministry of Food and Agriculture. [(accessed on 21 February 2022)]. Available online: https://www.tierwohl-staerken.de/heimtiere/heimtiere-in-deutschland/#.
- 19.Guardabassi L., Schwarz S., Lloyd D. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004;54:321–332. doi: 10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
- 20.Mann P.H. Bacteriophage typing of staphylococci isolated from animals. Canad. J. Pub. Health. 1960;51:153–156. [PubMed] [Google Scholar]
- 21.Kaspar U., Von Lützau A., Schlattmann A., Roesler U., Köck R., Becker K. Zoonotic multidrug-resistant microorganisms among small companion animals in Germany. PLoS ONE. 2018;13:e0208364. doi: 10.1371/journal.pone.0208364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boost M., O`Donoghue M., James A. Investigation of the role of dogs as reservoirs of Staphylococcus aureus and the transmission of strains between pet owners and their dogs. Hong Kong Med. J. 2008;14:15–18. [PubMed] [Google Scholar]
- 23.Van Balen J.C., Landers T., Nutt E., Dent A., Hoet A.E. Molecular epidemiological analysis to assess the influence of pet-ownership in the biodiversity of Staphylococcus aureus and MRSA in dog- and non-dog-owning healthy household. Epidemiol. Infect. 2017;145:1135–1147. doi: 10.1017/S0950268816003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris D.O., Lautenbach E., Zaoutis T., Leckerman K., Edelstein P.H., Rankin S.C. Potential for pet animals to harbour methicillin-resistant Staphylococcus aureus (MRSA) when residing with human MRSA patients. Zoonoses Public Health. 2012;59:286–293. doi: 10.1111/j.1863-2378.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender J.B., Waters K.C., Nerby J., Olsen K.E., Jawahi S. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from pets living in households with MRSA-infected children. Clin. Infect. Dis. 2012;54:49–50. doi: 10.1093/cid/cir714. [DOI] [PubMed] [Google Scholar]
- 26.Loeffler A., McCarthy A., Lloyd D.H., Musilová E., Pfeiffer D.U., Lindsay J.A. Whole-genome com-parison of methicillin-resistant Staphylococcus aureus CC22 SCCmecIV from people and their in-contact pets. Vet. Dermatol. 2013;24:538-e128. doi: 10.1111/vde.12062. [DOI] [PubMed] [Google Scholar]
- 27.Hanselman B.A., Kruth S.A., Rousseau J., Weese J.S. Coagulase positive staphylococcal colonization of humans and their household pet. Can. Vet. J. 2009;50:954–958. [PMC free article] [PubMed] [Google Scholar]
- 28.Faires M.C., Tater K., Weese J.S. An investigation of methicillin-resistant Staphylococcus aureus colonization in people and pets in the same household with an infected person or infected pet. J. Am. Vet. Med. Ass. 2009;235:540–543. doi: 10.2460/javma.235.5.540. [DOI] [PubMed] [Google Scholar]
- 29.Lundqvist M., Carlsson P., Sjödahl R., Theodorsson E., Levin L.Å. Patient benefit of dog-assisted interventions in health care: A systematic review. BMC Complementary Altern. Med. 2017;17:358. doi: 10.1186/s12906-017-1844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hediger K., Wagner J., Künzi K., Haefeli A., Theis F., Grob C., Pauli J., Gerger H. Effectiveness of animal-assisted interventions for children and adults with post-traumatic stress disorder symptoms: A systematic review and meta-analysis. Eur. J. Psychotraumatol. 2021;12:1879713. doi: 10.1080/20008198.2021.1879713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefebvre S.L., Waltner-Toews D., Peregrine A.S., Reid-Smith R., Hodge L., Arroyo L., Weese J.S. Prevalence of zoonotic agents in dogs visiting hospitalized people in Ontario: Implications for infection control. J. Hosp. Infect. 2006;62:458–466. doi: 10.1016/j.jhin.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Enoch D.A., Karas J.A., Slater J.D., Emery M.M., Kearns A.M., Farrington M. MRSA carriage in a pet therapy dog. J. Hosp. Infect. 2005;60:186–188. doi: 10.1016/j.jhin.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Akmatov M.K., Mehraj J., Gatzemeier A., Strömp J., Witte W., Krause G., Pessler F. Serial home-based self-collection of anterior nasal swabs to detect Staphylococcus aureus carriage in a randomized population-based study in Germany. Int. J. Infect. Dis. 2014;25:4–10. doi: 10.1016/j.ijid.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Martineau F., Picard F.J., Ke D., Paradis S., Roy P.H., Ouellette M., Bergeron M.G. Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol. 2001;39:2541–2547. doi: 10.1128/JCM.39.7.2541-2547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki T., Tsubakishita S., Tanaka Y., Sakusabe A., Ohtsuka M., Hirotaki S., Kawakami T., Fukata T., Hiramatsu K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010;48:765–769. doi: 10.1128/JCM.01232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuny C., Layer F., Hansen S., Werner G., Witte W. Nasal Colonization of humans with occupational exposure to raw meat and to raw meat products with methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Toxins. 2019;11:190. doi: 10.3390/toxins11040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal M., Javerliat F., Palmieri M., Mirande C., Van Wamel W., Tavakol M., Verkaik N., Van Belkum A. Genomic evolution of Staphylococcus aureus during artificial and natural colonization of the human nose. Front. Microbiol. 2019;10:1525. doi: 10.3389/fmicb.2019.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehraj J., Akmatov M.K., Strömpl J., Gatzemeier A., Layer F., Werner G., Pieper D.H., Medina E., Witte W., Pessler F., et al. Methicillin-sensitive and methicillin-resistant Staphylococcus aureus nasal carriage in a random sample of non-hospitalized adult population in northern Germany. PLoS ONE. 2014;9:e107937. doi: 10.1371/journal.pone.0107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtfreter S., Grumann D., Balau V., Barwich A., Kolata J., Goehler A., Weiss S., Holtfreter B., Bauerfeind S.S., Döring P., et al. Molecular epidemiology of Staphylococcus aureus in the general population in Northeast Germany: Results of the study of health in Pomerania (SHIP-TREND-0) J. Clin. Microbiol. 2016;54:2774–2785. doi: 10.1128/JCM.00312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misic A.M., Davis M.F., Tyldsley A.S., Hodkinson B.P., Tolomeo P., Hu B., Nachamkin I., Lautenbach E., Morris D.O., Grice E.A. The shared microbiota of humans and companion animals as evaluated from Staphylococcus carriage sites. Microbiome. 2015;3:1–19. doi: 10.1186/s40168-014-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson B.M., Kates A.E., O’Malley S.M., Mills E., Herwaldt L.A., Torner J.C., Dawson J.D., Farina S.A., Klostermann C., Wu J.Y., et al. Staphylococcus aureus in the nose and throat of Iowan families. Epidemiol. Infect. 2018;14:1777–1784. doi: 10.1017/S0950268818001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knox J., Uhlemann A.C., Lowy F.D. Staphylococcus aureus infections: Transmission within households and the community. Trends Microbiol. 2015;7:437–444. doi: 10.1016/j.tim.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wertheim H.F., Melles D.C., Vos M.C., van Leeuwen W., van Belkum A., Verbrugh H.A., Nouwen J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Sanz E., Torres C., Lozano C., Zarazaga M. High diversity of Staphylococcus aureus and Staphylococcus pseudintermedius lineages and toxigenic traits in healthy pet-owning household members. Underestimating normal household contact? Comp. Immunol. Microbiol. Infect. Dis. 2013;36:83–94. doi: 10.1016/j.cimid.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Kottler S., Middleton J.R., Perry J., Weese J.S., Cohn L.A. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus carriage in three populations. J. Vet. Intern. Med. 2010;24:132–139. doi: 10.1111/j.1939-1676.2009.0424.x. [DOI] [PubMed] [Google Scholar]
- 46.Layer F., Stromenger B., Cuny C., Werner G. Eigenschaften, Häufigkeit und Verbreitung von MRSA in Deutschland—Update 2013/2014. [(accessed on 21 February 2022)];Epidemiol. Bull. 2015 Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2015/31/Art_01.html. [Google Scholar]
- 47.Paterson G.K., Harrison E.M., Holmes M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014;22:42–47. doi: 10.1016/j.tim.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haenni M., El Garch F., Miossec C., Madec J.Y., Hocquet D., Valot B. High genetic diversity among methicillin-susceptible Staphylococcus pseudintermedius in dogs in Europe. J. Glob. Antimicrob. Resist. 2020;21:57–59. doi: 10.1016/j.jgar.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Phumthanakorn N., Schwendener S., Donà V., Chanchaithong P., Perreten V., Prapasarakul N. Genomic insights into methicillin-resistant Staphylococcus pseudintermedius isolates from dogs and humans of the same sequence types reveals diversity in prophages and pathogenicity islands. PLoS ONE. 2021;16:e0254382. doi: 10.1371/journal.pone.0254382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kjellman E.E., Slettemeås J.S., Small H., Sunde M. Methicillin-resistant Staphylococcus pseudintermedius (MRSP) from healthy dogs in Norway-occurrence, genotypes and comparison to clinical MRSP. Microbiologyopen. 2015;4:857–866. doi: 10.1002/mbo3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haenni M., de Moraes N.A., Châtre P., Médaille C., Moodley A., Madec J.Y. Characterisation of clinical canine methicillin-resistant and methicillin-susceptible Staphylococcus pseudintermedius in France. J. Glob. Antimicrob. Resist. 2014;2:119–123. doi: 10.1016/j.jgar.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Duim B., Verstappen K.M., Broens E.M., Laarhoven L.M., van Duijkeren E., Hordijk J., de Heus P., Spaninks M., Timmerman A.J., Wagenaar J.A. Changes in the population of methicillin-resistant Staphylococcus pseudintermedius and dissemination of antimicrobial-resistant phenotypes in the Netherlands. J. Clin. Microbiol. 2016;54:283–288. doi: 10.1128/JCM.01288-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viau R., Hujer A.M., Hujer K.M., Bonomo R.A., Jump R.L. Are Staphylococcus intermedius Infections in Humans Cases of Mistaken Identity? A Case Series and Literature Review. Open Forum Infect. Dis. 2015;2:ofv110. doi: 10.1093/ofid/ofv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bert F., Gualano M.R., Camussi E., Pieve G., Voglino G., Siliquini R. Animal assisted intervention: A systematic review of benefits and risks. Eur. J. Integr. Med. 2016;8:695–706. doi: 10.1016/j.eujim.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalton K.R., Ruble K., Redding L.E., Morris O.D., Mueller-Roland N.T., Thorpe J., Agnew J., Carroll K.C., Planet P.J., Rubenstein R.C., et al. Microbial sharing between pediatric patients and therapy dogs during hospital animal-assisted intervention programs. Microorganisms. 2021;9:1054. doi: 10.3390/microorganisms9051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murthy R., Bearman G., Brown S., Bryant K., Chinn R., Hewlett A., George B.G., Goldstein E.J.C., Holzmann-Pazgal G., Rupp M.E., et al. Animals in healthcare facilities: Recommendations to minimize potential risks. Infect. Control. Hosp. Epidemiol. 2015;36:495–516. doi: 10.1017/ice.2015.15. [DOI] [PubMed] [Google Scholar]
- 57.Deutsche Gesellschaft für Krankenhaushygiene Empfehlung zum hygienegerechten Umgang mit Therapiehunden in Krankenhäusern und vergleichbaren Einrichtungen. [(accessed on 21 February 2022)];Hyg. Med. 2017 :42-10. Available online: https://www.krankenhaushygiene.de/pdfdata/hm/2017_10_DGKH_Therapiehunde.pdf. [Google Scholar]
- 58.Caffrey A.R., Quilliam B.J., LaPlante K. Risk factors associated with mupirocin resistance in methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2010;76:206–210. doi: 10.1016/j.jhin.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 59.Kizerwetter-Świda M., Chrobak-Chmiel D., Rzewuska M. High-level mupirocin resistance in methicillin-resistant staphylococci isolated from dogs and cats. BMC Vet. Res. 2019;15:238. doi: 10.1186/s12917-019-1973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.