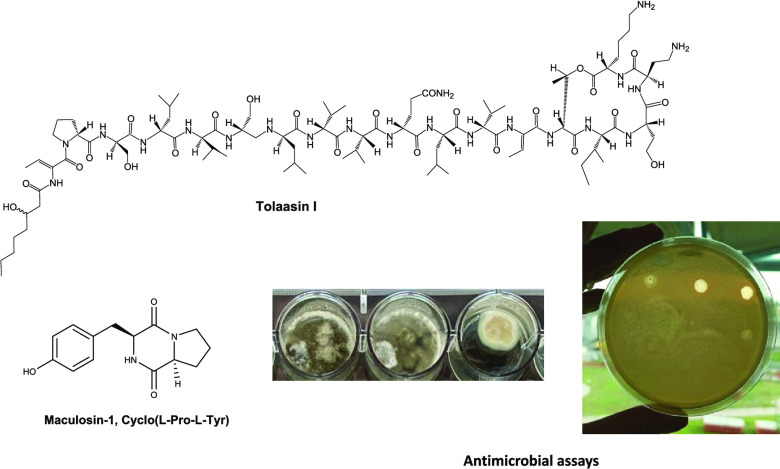
Abstract
Biotic stresses (fungi, bacteria, insects, weeds, etc.) are some of the most important causes of the decrease in the quality and quantity of crops that could become an emergency due to a noteworthy increase in the world population. Thus, to overcome these problems, massive use of chemical pesticides has been carried out with heavy consequences for environmental pollution and food safety. An eco-friendly alternative can be using natural compound-based biopesticides with high efficacy and selectivity. Some bacterial lipodepsipeptides (tolaasins I, II, A, D, and E and WLIP together with hexacetyl- and tetrahydro-tolaasin I and WLIP methyl ester) and cyclic dipeptides (cyclo(l-Pro-l-Tyr), cyclo(d-Pro-l-Tyr), cyclo(l-Pro-l-Val), and cyclo(l-Pro-l-Leu)) were assayed against several pathogenic bacteria and fungi of important agrarian plants. Lipodepsipeptides showed strong growth inhibition of all microorganisms tested in the range of 0.1–0.8 μg/mL, while cyclodipeptides, despite preserving this ability, showed a noteworthily reduced antimicrobial activity being active only in the range of 15–900 μg/mL. Among the lipodepsipeptides and cyclic dipeptides assayed, tolaasin d and cyclo(l-Pro-l-Tyr) (also named maculosin-1) appeared to be the most toxic compounds. Some structure–activity relationships of lipodepsipeptides were also discussed along with their practical application as biopesticides in agriculture.
Keywords: agrarian plant diseases, bacterial and fungal pathogens, biopesticides, lipodepsipeptides, cyclic dipeptides, antimicrobial activity
Introduction
People, since ancient times, had worldwide developed agriculture as the first human activity to produce food in high quantity and quality. This necessity increased over time for a constant increase in the world population, which will be almost 10 billion by 2050.1,2 These aspects, despite the noteworthy technological progress done in agriculture, are becoming an emergency due to the strong reduction of natural sources, the environmental pollution, and climate changes.3,4 Biotic stresses, including microbial pathogens, weeds, insects, etc., represent the main causes of severe losses in agrarian production and food safety. Up to this day, control of these damaging agents has been done with the massive use of synthetic pesticides. The latter can cause environmental pollution, induce resistance in the host plants, and are responsible for the presence of toxic residues in agricultural products.5,6 These problems prompted efforts to develop integrated pest management5 to reduce or eliminate synthetic pesticides significantly. A valid and efficacy alternative is represented by biopesticides, which are easy degradable and represent no risk for human and animal health as strongly required by consumers and by public administrators.3,7
Natural products are the most important source for finding substances with different biological activities, new carbon skeletons to overcome resistance phenomena, and potential applications as new eco-friendly solutions in various fields.6,8
Among these classes of natural bioactive metabolites, there are lipodepsipeptides and cyclic dipeptides. Lipodepsipeptides are biologically active metabolites produced by different bacteria and are constituted by three moieties: (i) a macrocyclic peptide lactone; (ii) a linear peptide; and (iii) fatty acid. These lipodepsipeptides, containing unusual amino acids also with an opposite stereochemistry, are classified according to their primary structures into two groups. Syringotoxins, syringomycins, pseudomycins, and syringostatins belong to the first group. Those containing from 18 to 25 amino acid residues, most of which have a d-stereochemistry, such as syringopeptins, fuscopeptins, tolaasins, and corpeptins, are reported in the second group.9 In the latter one, the C-terminal group forms a lactone ring constituting from 5 (corpeptins, tolaasins, and fuscopeptins) to 8 (syringopeptins) amino acids. The first reported nonapeptides were syringomycins, a subgroup synthesized by the plant pathogenic bacterium Pseudomonas syringae pv. syringae showing antifungal activities. They targeted the fungal plasma membrane, and some studies on their mode of action were also performed.10 Successively, the other nonapeptides syringostatins, syringotoxins, and pseudomicines were produced by P. syringae pv. syringae but isolated from different infected host plants.11 Other lipodepsipeptides such as syringopeptines,12 fuscopetines,13 and corpeptines14,15 were produced by P. syringae pv. syringae, Pseudomonas fuscovaginae, Pseudomonas corrugata, and Pseudomonas cichorii. Lipodepsipeptides in addition to phytotoxic and antifungal activities also showed potential antibiotic activity and thus potential against the bacterial species that have developed resistance to common antibiotics.16
Also, pathogenic bacteria of cultivated mushroom produce lipodepsipeptides with different biological activities such as Burkholderia gladioli pv. agaricicola, Pseudomonas tolaasii, and Pseudomonas reactans. The main bioactive lipodepsipeptides produced by both Pseudomonas strains are tolaasins I and II (1 and 2, Figure 1), which themselves differed in the substitution of the homoserine residue (Hse16) of macrocyclic lactone with a glycine residue,17 and the so-called white line inducing principle (WLIP, 3, Figure 2).18 The role that these metabolites play in the diseases and their biological activities were extensively studied.19P. tolaasii, pathogen of Agaricus bisporus and Pleurotus ostreatus, also showed to produce, despite being in lesser amounts, other tolaasins named tolaasins A, B, C, D, and E (4–8, Figure 1). They differed from tolaasins I and II in the peptide chain, as observed in other lipodepsipeptides of bacterial origin, and preserved the β-hydroxyoctanoyl ϕ group at the N-terminus, except for tolaasin A, in which the acyl moiety was a γ-carboxybutanoyl ϕ chain. When tested on fungi, yeast, and bacteria, they showed antimicrobial activity against Gram-positive bacteria, which appeared to be the most sensitive, and this activity seemed to be related to the structural differences of the analogues.20
Figure 1.
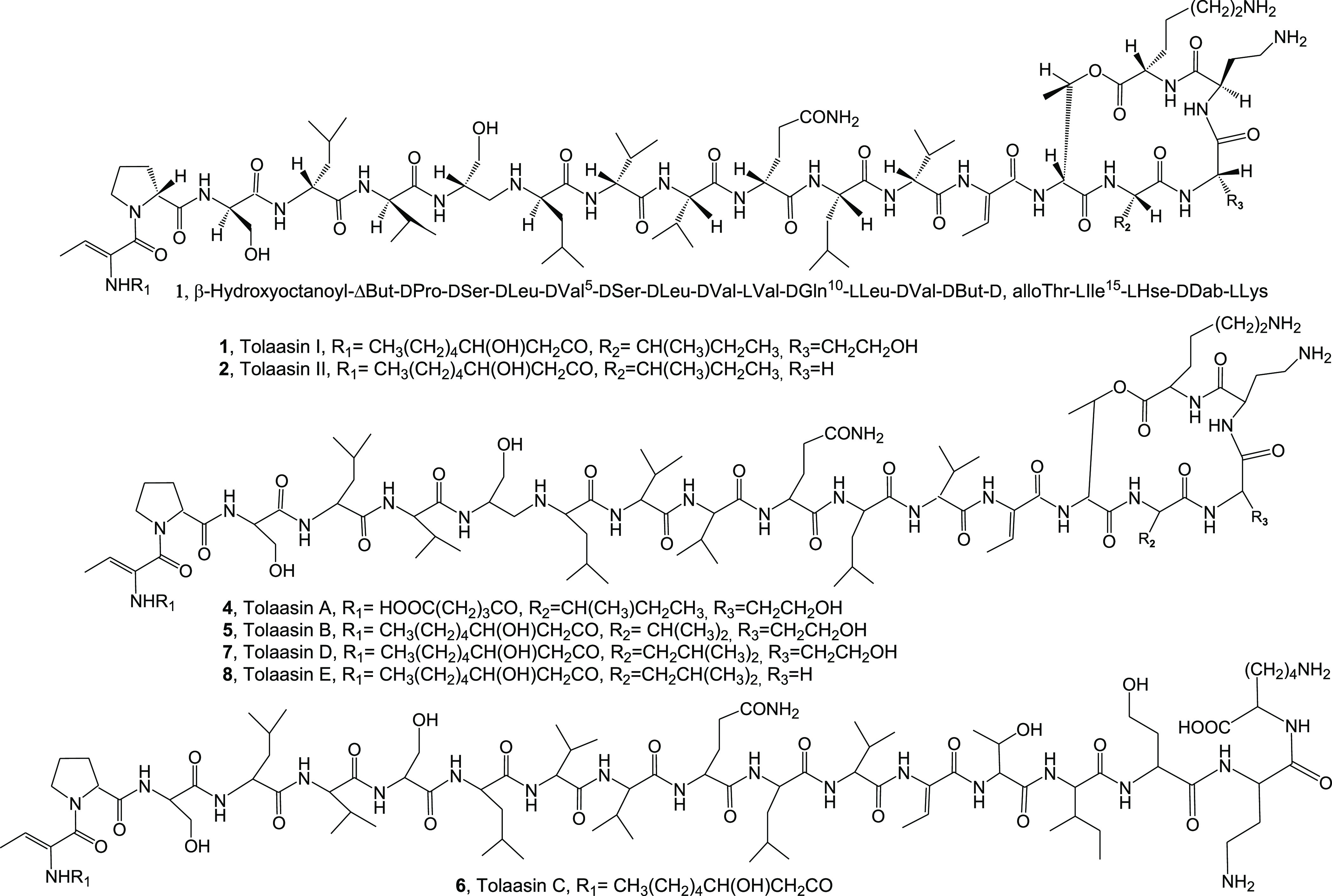
Structures of tolaasins I, II, A-E (1, 2, 4–8).
Figure 2.
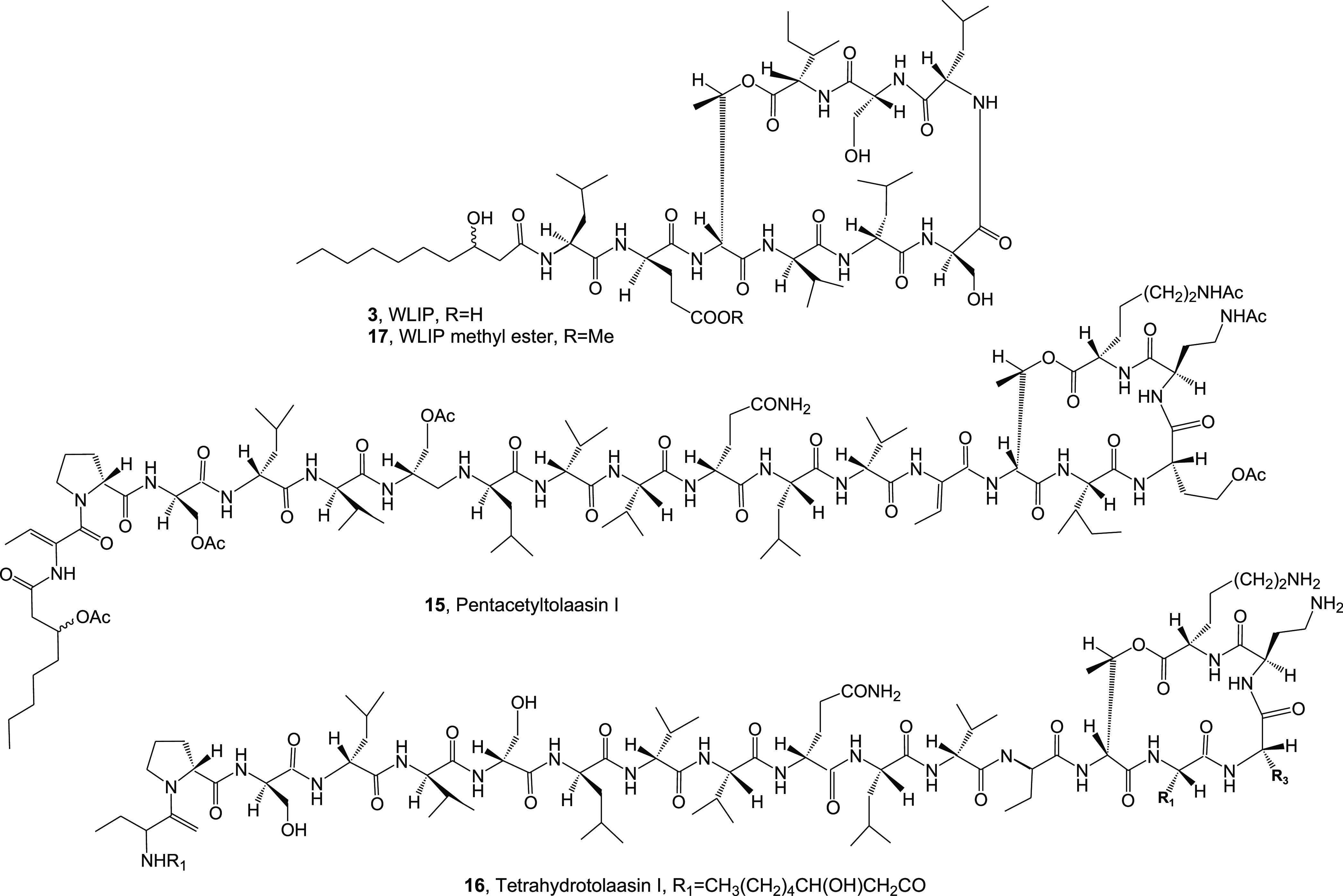
Structures of WLIP, hexacetyl- and tatrahydro-tolaasin I, and methyl ester of WLIP (3, 15–17).
The close naturally occurring cyclopeptides exhibit potent biological activities, including insecticidal, antimicrobial, antifungal, and antiproliferative. They are produced by marine organisms21 and plants.22 A subgroup of this class of natural compounds is the cyclodipeptides, also known as 2,5-diketopiperazines, which showed various biological activities and displayed strong resistance against enzymatic hydrolysis, thus attracting great interest in a variety of fields spanning from functional materials to drug discovery.23
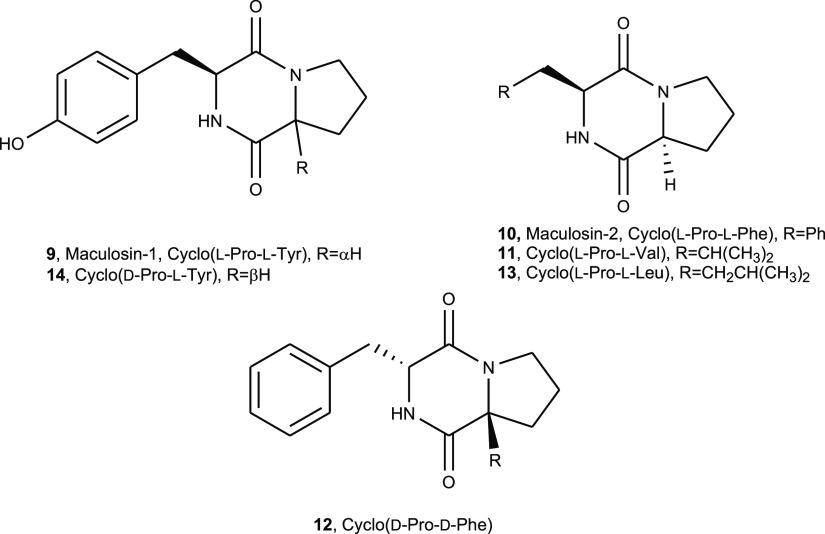
Among 2,5-diketopiperazines, the most known is maculosin-1 (cyclo(l-Pro-l-Tyr)) (9, Figure 3). Compound 9 is a host-specific phytotoxin produced by Alternaria alternata, a pathogen of knapweed.24 The same fungus also synthesizes cyclo(l-Pro-l-Phe) (maculosin-2) and cyclo(Pro-Ala), cyclo(Pro-Val), cyclo(Pro-Hle), cyclo(Pro-Leu), and cyclo(l-Pro-d-Phe), as potential biocontrol agents of knapweed.24
Figure 3.
Structures of 2,5-diketopiperazine maculosin-1 (cyclo(l-Pro-l-Tyr), maculosin-2 (cyclo(l-Pro-l-Phe)), cyclo(l-Pro-l-Val), cyclo(d-Pro-d-Phe), cyclo(l-Pro-l-Leu), and cyclo(d-Pro-l-Tyr) (9–14)).
Compound 9 was also recently isolated from Lysobacter capsici AZ78 and showed antifungal activity against Phytophthora infestans and Plasmopara viticola, two pathogens of important crops.25 Some derivatives of maculosin-1 were also prepared and their antifungal activity, compared to those of the parent compound (9) and maculosin-2 cyclo(l-Pro-l-Phe) (10, Figure 3), was tested against P. infestans. Among them, the azido derivative of 9 showed strong antifungal activity, suggesting its potential use as a biofungicide.26 To corroborate these results, 9 was applied on tomato leaves to prevent the occurrence of late blight lesions.25 These results prompted an in-depth investigation of 2,5-diketopiperazine production by L. capsici. In fact, cyclo(l-Pro-l-Val), cyclo(d-Pro-d-Phe), cyclo(l-Pro-l-Leu), and cyclo(d-Pro-l-Tyr) (11–14, Figure 3) were successively isolated from the same bacterial cultures and were tested together with maculosins-1 and 2 (9 and 10) against the phytopathogenic Gram-positive bacterium Rhodococcus fascians LMG.27 Among all the 2,5-diketopiperazines assayed, compound 11 showed toxicity similar to that of chloramphenicol, a positive control, when used at the same concentration. These results and reported data suggest that 2,5-diketopiperazines could be proposed as potential biopesticides due to their broad activity spectrum against phytopathogenic microorganisms.
Thus, this article reports the antimicrobial activity of five lipodepsipeptides (1, 2, 7, and 8), WLIP (3), two tolaasin I derivatives (15 and 16), one WLIP derivative (17), and four diketopiperazines (9, 11, 13, and 14) against several pathogenic bacteria and fungi of agrarian plants. Results of structure–activity relationships were also discussed.
Materials and Methods
General Experimental Procedures
Optical rotation, 1H NMR spectra, electrospray ionization mass spectrometry (ESI MS) analysis, analytical and preparative thin-layer chromatography (TLC), and column chromatography were performed as previously reported.20,25 Reverse-phase high-performance liquid chromatography (HPLC) of the tolaasin crude mixture was performed as previously reported.20
Production and Purification of Tolaasins from P. tolaasii
Tolaasins I, II, D, and E were produced growing P. tolaasii (strain type NCPPB2192) in liquid King’s B medium stirred culture at 25 °C as previously reported.20 The culture was centrifuged and lyophilized, and tolaasins were purified from the culture filtrates (1.350 L) according to a previously reported method.28 Briefly, after acidification of the culture filtrates, the precipitate was discarded, and tolaasins were precipitated by adding CaCl2. After more steps of washing with small volumes of MeOH followed by washing with small volume of Milli-Q water, the crude residue was desalted by G-10 column chromatography, and the tolaasin-containing fractions were combined and lyophilized to give a white solid residue. The tolaasin mixture (67.6 mg) was purified by HPLC using a reverse-phase semipreparative column eluted with a gradient MeCN-0.1% TFA and afforded tolaasins I, II, D, and E (1, 2, 7, and 8). This procedure was repeated more times to accumulate the tolaasins in discrete amounts. Tolaasins I, II, D, and E were identified by 1H NMR and ESIMS spectra in comparison with those of standard samples. Their purity was >98% as ascertained by HPLC analysis.
Acetylation of Tolaasin I
Tolaasin I (1, 2.0 mg) was dissolved in dry pyridine (100 μL) and acetylated with AC2O (100 μL). The reaction was carried out at room temperature overnight and stopped by adding MeOH. Pyridine was eliminated under a N2 stream of the azeotrope formed by addition of C6H6. The organic residue was purified with TLC using i-PrOH/H2O (8/2, v/v) as an eluent, affording the hexacetyl derivative of tolaasin I (15, 1.9 mg, 85%) as an amorphous solid. Compound 15 had a 1H NMR spectrum (Figure S2, SI) that essentially differs from that of tolassin I (Figure S1, SI), recorded under the same conditions for the presence of the singlets of five acetyl groups in the range of δ 2.2–1.99; ESI MS (+), m/z: 2260 [M + Na]+ (Figure S3, SI).
Hydrogenation of Tolaasin I
Tolaasin I (1, 2.7 mg) was dissolved in MeOH (1 mL) and added to a suspension of 95% PtO2/C in MeOH (1 mL) presaturated with H2 gas for 30 min under stirring. The reaction was performed with H2 at atmospheric pressure at room temperature under stirring in the dark. The reaction was completed after 24 h and stopped by filtration of the catalyst. The solution was evaporated under reduced pressure to give the tetrahydroderivative of tolaasin I (16, 2.6 mg, 96%) as an amorphous solid. The 1H NMR spectrum recorded in CD3OD (Figure S4, SI) essentially differed from that of tolaasin recorded under the same conditions (Figure S1, SI) for the absence of olefinic protons; ESIMS (+) m/z: 1990 [M + H]+ (Figure S5, SI).
Production and Purification of WLIP
P. reactans NCPPB1311 was grown on liquid KB medium at 25 °C under shaking, as previously reported.29 Briefly, the lyophilized culture filtrate (1.4 L) was dissolved in MIlli-Q water (1.3 L) and centrifuged at 10 000 rpm at 15 °C for 30 min. The supernantant was filtered on a Wathman n. 42 paper disk, acidified up to pH 5 with 1 N HCl, and left at room temperature overnight. The precipitate dissolved in Millli-Q water was alkalinized up to pH 7.5 with 1 N NaOH, and the solution was filtered on Whatman n. 40 paper disks. It was acidified up to pH 5 with 1N HCl. The precipitate was collected by centrifugation at 10 000 rpm at 15 °C for 30 min, oven-dried at 50 °C, and then dissolved in MeOH (100 mL). The suspension was then filtered on Whatman n. 42 paper disks, and the filtrate was evaporated under vacuum. The solid residue was washed with MeOH (10 mL), centrifuged at 8000 rpm at 15 °C for 30 min, then dissolved in MeOH (100 mL), and dried under vacuum to give crude WLIP (250 mg). The latter crystallized as white needles (216 mg) with blowing in water vapor, according to the procedure reported by Mortishire-Smith (1991).18 WLIP was identified by 1H NMR and ESIMS spectra in comparison with those of standard samples. Its purity was >98% as ascertained by HPLC analysis.
WLIP Methyl Ester
A solution of WLIP (3, 15.1 mg) was dissolved MeOH (1 mL), and an ethereal solution of diazomethane was added up to a yellow persistent color. The reaction was performed at 0 °C for 48 h and stopped by evaporation of the solution using reduced pressure. The residue was purified by TLC eluted with EtOAc/MeOH/H2O (85/20/10), yielding the methyl ester of WLIP (17, 14.1 mg, 93%) as an amorphous solid. Compound 17 had a 1H NMR spectrum (Figure S6, SI) that essentially differed from that of WLIP (Figure S7, SI) for the presence of the singlet at δ 3.70 due to the ester methyl group; ESIMS (+) m/z: 1289 [M + Na]+, 1275 [M + H]+ (Figure S8, SI).
Production and Purification of 2,5-Diketopiperazines
L. capsici AZ78 cultures were obtained as previously described.30 The lyophilized culture filtrates (20 L) were dissolved in Milli-Q water (2 L) and extracted with EtOAc (3 × 2 L). The corresponding extract was fractionated according the procedure previously reported.27 In particular, the organic extracts were combined and dried under vacuum to give a solid residue (1.56 g). The latter was chromatographed on a silica gel eluted with CHCl3/i-PrOH (9/1) and then with CHCl3/i-PrOH (7/3), yielding 10 groups of homogeneous fractions (F1–F10). The F2 residue (302 mg) was subjected to another fractionation by column chromatography, using CHCl3/i-PrOH (9/1) as an eluent. A total of 10 groups of homogeneous fractions were collected (F2.1–F2.10). The F2.3 residue (13.9 mg) appeared to be a pure metabolite, identified as cyclo(l-Pro-l-Val) (11). The F2.4 residue (51.3 mg) was further purified by TLC, eluted with CHCl3/i-PrOH (9/1), yielding four groups of homogeneous fractions (F2.4.1–F2.4.4). The F2.4.2 residue (36.2 mg) was further purified by more steps of TLC, giving further amounts of 11 (4 mg) and cyclo(l-Pro-l-Leu) (13, 3.7 mg). The residue (62.5 mg) of F3 was further purified by several steps of TLC, yielding further amounts of 11 (5.5 mg), maculosin-1 (cyclo(l-Pro-l-Tyr) (9, 11.9 mg), and cyclo(d-Pro-l-Tyr) (14, 18.4 mg)). Their identity was ascertained by 1H NMR and ESI MS spectra in comparison with those of standards. Their purity was >98% as ascertained by HPLC analysis.
Minimum Inhibitory Concentrations (MIC)
Antimicrobial Assay
The antimicrobial assay was carried out as described in Bassarello et al. (2004)20 with some modifications. Bacteria were grown in LB broth at 25 or 37 °C overnight at 150 rpm. A total of 500 μL of a suspension containing about 108 cfu mL–1 were added to 3 mL of LB soft agar (0.7%) and poured onto plates containing 7 mL of LB broth with agar 1.8%. After agar gelification, 10 μL drops of serial dilutions of different lipodepsipeptides and their derivatives (from 0.1 to 1 μg/mL) and cyclic dipeptides (from 10 to 1000 μg/mL) were tested. After 24 ± 48 h of incubation at 25 or 37 °C, the end serial dilution inhibiting the growth of the bacteria in the area of application of 10 μL solutions was recorded. The plates containing the bacteria alone were used as a control. The experiment was performed in triplicate.
Antifungal Assay
The antifungal activity was performed in 24-well culture plates according to the method previously described31 with some modification. Serial dilutions of different lipodepsipeptides (from 0.1 to 1 μg/mL) and cyclic dipeptides (from 10 to 1000 μg/mL) were dissolved in a volume of 500 μL of ultrapure Milli-Q and finally inoculated with 500 μL of 2× potato dextrose broth (Difco) containing the Colletotrichum truncatum plug of 4 mm × 4 mm diameter. As a control, C. truncatum plugs (4 mm × 4 mm) were grown in 2× PD broth diluted with 500 μL of ultrapure Milli-Q water, and the plates were incubated at 28 °C for 7 days. The MIC was measured as the lowest concentration of antifungal agent at which there was no visible growth of the fungus after incubation. The experiment was performed in triplicate.
Results and Discussion
Bacteria belonging to the Pseudomonas genus were used in this study; all are causal agents of severe diseases of important agrarian plants. Among them, there is Burkholderia caryophylli (syn. Pseudomonas caryophylli) responsible for bacterial wilt of carnation resulting in serious losses in carnation production.32 From its culture filtrates were isolated three polyunsaturated C:17 fatty acids and other three metabolites; the latter were obtained as an interconvertible mixture and named caryoynencins A-C. The latter showed strong antibacterial activity against Gram-positive and Gram-negative bacteria such as Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Klabsiella pneumoniae but had no phytotoxicity.33 Although the culture filtrates exhibited phytotoxicity toward the host and nonhost plants, up to a day, phytotoxins were not isolated from B. caryophylli, but from some preliminary experiments carried out by some of the authors, they should be lipodepsipeptides (private communication). Extensive work was done by some of the authors on the lipopolysaccharides (LPS) present in the outer membrane of this bacterium as in general, LPS plays an important role in the first process of pathogenesis and in particular in the interaction of the plant and pathogen.34 The LPSs of P. caryophylli appeared to be constituted by two homopolysaccharide chains, with the major one built up of (1 → 7)-linked α-cryophyllose [3,6,10-trideoxy-4-C-(d-glycero-1-hydroxyethyl)-d-erythro-d-gulo-decose] residues and the minor one made up of (1 → 7)-linked β-caryose (4,8-cyclo-3,9-dideoxy-l-erythro-d-ido-nonose) residues. A third polysaccharide fraction mainly constituted by heptose and glucose was also isolated.35 The main polysaccharide, named caryophyllane, was constituted by a repeating unit of a novel 4-branched monosaccharide, named caryophyllose, characterized as trideoxy-C-[(R)-1-hydroxyethyl]-d-erythro-d-gulo-decose.36,37 The minor polysaccharide, named carian, was constituted by the repeating unit of new cyclic monosccharide, named caryose, characterized as carbocyclic (4,8-cyclo-3,9-dideoxy-l-erythro-d-ido-nonose).38 Another bacterium used is P. syringae pv. panici, a worldwide diffused pathogen, which induces diseases in different plants including crops such as rice, lilac, millet, and pearl millet.39 In rice, P. syringae pv. panici induces brown stripe disease.40Pseudomonassyringae pv. tabaci was also included among the bacteria used in this study as it induces brown spots on tobacco, a disease named wildfire, with severe economic consequences.41 The same is for P. syringae pv. siringae (Pss), which is the most polyphagous bacterium in the P. syringae complex due to its wide host range, first affecting woody and herbaceous host plants. In early 1990s, Pss caused apical necrosis of mango trees, a severe disease in Southern Spain. A lot of studies had been carried out on this pathogen, whose results are reported in some reviews as that published by Gutiérrez-Barranquero et al.42Pseudomonassyringae pv. japonica, also included in the bacteria tested, induced the black node disease of barley (Hordeum vulgare L.) and wheat (Triticum aestiuum L.) and was initially classified as Pseudomonas striafaciens var. japonica.43 The other three bacteria tested were B. subtilis, Bacillus megaterium, and E. coli, which are laboratory strains. Colletotrichum truncatum was selected, among the phytopathogenic fungi available, as the only strain to test because very low amounts of both lipodespsipeptides and cylodipeptides were available for the antimicrobial assay. The strain of C. truncatum was isolated in Argentina as one of the most dangerous pathogens of soybean inducing anthracnose symptoms with severe epidemics and expressive yield losses.44
All the lipodepsipeptides (tolaasins and WLIP) were produced, purified, and identified as reported in detail in the Materials and Methods section. The two derivatives of tolaasin I and the methyl ester of WLIP were prepared and characterized as reported in detail in the same section and in the Supporting Information. In particular, the 1HNMR spectrum (Figure S2, SI) of the hexacetyl derivative of tolaasin I (15) essentially differed from that of tolassin I (Figure S1, SI), recorded under the same conditions for the singlets of five acetyl groups in the range of δ 2.20–1.99. Its ESIMS (+), spectrum showed the sodiated adduct ion [M + Na]+ at m/z 2260. The 1H NMR spectrum (Figure S4, SI) of the tetrahydro derivative of tolaasin I (16) essentially differed from that of tolaasin I, recorded under the same conditions, for the absence of olefinic protons. Its ESIMS (+) spectrum showed the protonated adduct ion [M + H]+ at m/z 1990. Finally, the 1H NMR spectrum of WLIP methyl ester (17) (Figure S6, SI) essentially differed from that of WLIP (Figure S7, SI), recorded under the same conditions, for the presence as a singlet at δ 3.70 due to the ester methyl group. Its ESIMS (+) spectrum exhibited the sodiated [M + Na]+ and the protonated [M + H]+ adduct ions at m/z 1275 and 1289, respectively.
In the first experiment, the lipodepsipeptide tolaasins I, II, D, and E (1, 2, 7, and 8, Figure 1) and WLIP (3, Figure 2) and their derivatives hexacetyl- and tetrahydro-tolaasin I and WLIP methyl ester (15, 16, and 17, Figure 2) were assayed against all the plant pathogenic and nonpathogenic bacteria and the fungus C. truncatum reported above using antimicrobial and antifungal tests (Figures 4 and 5). The results obtained, summarized in Table 1, showed that among the tolaasins and their two derivatives, the compounds 1, 2, and 7 and the tetrahydro tolaasin I (16) inhibited all the bacteria and the fungus tested with a MIC in the range of 0.1–0.9 μg/mL. Just for the bacteria E. coli, the growth was not inhibited. Tolaasin E and the hexacetyl tolaasin I (8 and 15) did not show activity against the three laboratory bacterial strains of B. subtilis, B. megaterium, and E. coli. However, compounds 8 and 15 showed a MIC in the range of 3–6 and 0.7–1 μg/mL, respectively, against the pathogenic bacteria and C. truncatum. Furthermore, the sensitivity among the bacteria seems similar, while the fungus appeared to be always less sensitive. The highest antimicrobial activity was shown by tolaasin D (7) with a MIC range of 0.1–0.2 μg/mL, and the lesser toxicity was shown by tolaasin E (8) and by the two derivatives of tolaasin I (15 and 16) with a MIC range of 0.7–1 and 0.2–3 μg/mL, respectively. Comparing the very similar activity of tolaasins I and II (1 and 2) seemed that the amino acid residue at the 16 position of the macrolactone ring is not important for the activity as it is l-homoserine (l-Hse) in 1 and l-serine (l-Ser) in 2. l-Hse is also present at the same position in tolaasin D (7); thus, the increased activity showed by the latter, with respect to 1 and 2, could be due to the presence of a different amino acid residue at the 15 position, which is l-leucine (l-Leu) in 7 and l-iso-leucine (l-Ile) in the other two. However, the presence in the same lipodepsipeptide of l-Leu and l-Ser at 15 and 16 positions, respectively, probably induces a noteworthy decrease in antimicrobial activity as observed in tolaasin E (8). The acetylation of the hydroxyl group of the fatty acid, l-Ser, l-Hse, and the primary amino groups of d-2,4-diamino butyric acid (d-Dab) and l-lysine (l-Lys) at positions 17 and 18 of macrocyclic lactone and the hydrogenation of two residue 2-butenylbutiric acid (ΔBut) located at 1 and 13 positions of the linear peptide chain, compared to 1, significantly induced a decrease in activity.
Figure 4.
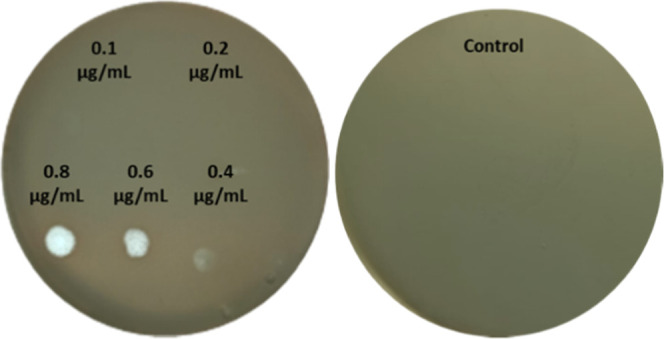
Representative photographs of minimum inhibitory concentration of tolaasin II against the Pseudomonassyringae pv. syringae strain B475.
Figure 5.
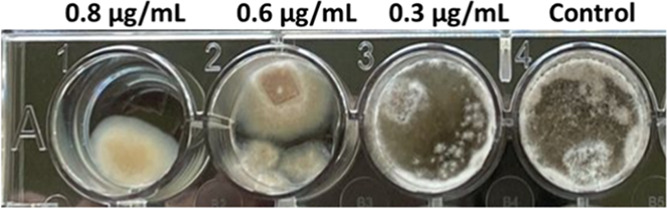
Representative photographs of minimum inhibitory concentration of tolaasin I against the C. truncatum strain 17-5-5.
Table 1. Minimal Inhibitory Concentration of the Lipodepsipeptides Testeda.
| MIC (μg/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | strain | WLIP | WILP methyl ester | hexacetyl tolaasin I (15) | tolaasin D (7) | tolaasin E (8) | tetrahydro tolaasin I (16) | tolaasin I (1) | tolaasin II (2) |
| NCPPB 349 | Pseudomonas caryophylli | - | - | 0.9 | 0.1 | 3 | 0.9 | 0.2 | 0.4 |
| ICMP3955 | Pseudomonas syringae pv. panici | - | - | 0.7 | 0.1 | 3 | 0.9 | 0.2 | 0.4 |
| ICMP2706 | Pseudomonassyringae pv. tabaci | - | - | 0.8 | 0.1 | 4 | 0.8 | 0.3 | 0.4 |
| B475 | Pseudomonassyringae pv. syringae | - | - | 0.8 | 0.1 | 3 | 0.8 | 0.3 | 0.4 |
| ICMP6305 | Pseudomonassyringae pv. japonica | - | - | 0.7 | 0.1 | 4 | 0.9 | 0.3 | 0.4 |
| PY79 | Bacillus subtilis | 0.3 | 0.5 | - | 0.2 | - | 0.2 | 0.3 | 0.4 |
| QMB | Bacillus megaterium | 0.3 | 0.5 | - | 0.2 | - | 0.2 | 0.3 | 0.4 |
| DH5α | Escherichia coli | - | - | - | - | - | - | - | - |
| 17-5-5 | Colletotrichum truncatum | 3 | 5 | 1 | 0.2 | 6 | 3 | 0.6 | 0.8 |
-: no activity.
The nonapeptide WLIP (3) that differed from tolaasins for all the three moieties such as the fatty acid, the linear side peptide chain, and the macrocyclic lactone practically did not inhibit the growth of all pathogenic bacteria, while it exhibited activity against the two laboratory Gram-positive strains B. subtilis and B. megaterium. Despite the lesser activity of tolassins I, II, and D (1, 2, and 7), WLIP showed antifungal activity against C. truncatum with a MIC of 3 μg/mL. Its methyl ester gave very similar activity, suggesting that a lethal methabolism45 could work by hydrolyzing the methyl ester group under physiological conditions.
In a second experiment, using the same bioassay method and the same microorganisms, the antimicrobial activity of the 2,5-diketopiperazines, namely cyclo(l-Pro-l-Tyr), cyclo(l-Pro-l-Val), cyclo(L-Pro-L-Leu), and cyclo(D-Pro-L-Tyr) (9, 11, 13, and 14), was tested. The results of the bioassay, listed in Table 2, showed that all the dicyclopeptides showed activity against all the bacteria except compound 9 on E. coli. The 2,5-diketopiperazine 11 was not toxic. Among the active compounds 9, 13, and 14, the highest antimicrobial activity was showed by maculosin-1 (cyclo(l-Pro-l-Tyr, 9)) with a MIC range of 15–20 μg/mL. The other two compounds (13 and 14) were less active, showing for the pathogenic bacteria and the fungus a MIC range of 500–800 μg/mL, but were more active against the laboratory bacterial strains. The antimicrobial activity of compound 9 is in agreement with its antifungal activity previously reported.25,26 The lack of activity of compound 14 also demonstrated that the configuration d or l of the amino acids that constitute the dicyclopeptide is a very important feature to impart activity. In fact, dicyclopeptides 9 and 14 differed only for the opposite d stereochemistry of proline residue in the second one, and its activity is reduced with respect to that of 9 by 50–60 times. The amino acids which constitute the dicyclopeptides also affect the activity as compound 13, which differs from compound 9 for the substitution of l-Tyr with l-Leu, showing a noteworthy reduction of activity by 40–50 times. Very surprising is the inactivity of dicyclopeptide 11 as recently it showed, among the 2,5-diketopiperazines reported above, the highest activity against R. fascians.27
Table 2. Minimal Inhibitory Concentration of the Cyclodipeptides Testeda.
| MIC (μg/mL) |
|||||
|---|---|---|---|---|---|
| ID | strain | L-Pro-L-Tyr (9) | D-Pro-L-Tyr (14) | L-Pro-L-Leu (13) | L-Pro-L-Val (11) |
| NCPPB 349 | Pseudomonas caryophylli | 15 | 800 | 500 | - |
| ICMP3955 | Pseudomonas syringae pv. panici | 15 | 900 | 700 | - |
| ICMP2706 | Pseudomonassyringae pv. tabaci | 15 | 800 | 500 | - |
| B475 | Pseudomonassyringae pv. syringae | 15 | 800 | 600 | - |
| ICMP6305 | Pseudomonassyringae pv. japonica | 15 | 800 | 600 | - |
| PY79 | Bacillus subtilis | 20 | 35 | 15 | - |
| QMB | Bacillus megaterium | 20 | 30 | 30 | - |
| DH5α | Escherichia coli | - | 20 | 300 | - |
| 17-5-5 | Colletotrichum truncatum | 20 | 800 | 500 | - |
-: no activity.
In conclusion, in testing the antibacterial and antifungal activity, lipodepsipeptides showed growth inhibitory activity 56–60 times higher than that of dicylopeptides. Among the lipodepsipeptides, the nonapeptides such as WLIP, tested on phytopathogenic bacteria and fungus, showed only weaker fungicide activity against C. truncatum. In lipodepsipeptides having a longer peptide side chain, the presence of some amino acid residues of the lactone ring is important to increase the activity as was the effect on the activity of tolaasin D for the presence of l-Ile instead of l-Leu residue. The derivatization of their amino acid residues of both the macrocyclic lactone ring and linear peptide side chain weakly affects inhibitory activity. Finally, for tolaasin D, considering the possibility of its large-scale production using a fermenter, a suitable bioformulation could have a potential for practical application as a bacteriocide and fungicide in agriculture and in particular against the pathogens of important agrarian plants that have developed resistance to the common chemical pesticides.
Acknowledgments
The authors thank Dr. Paola Lavermicocca, Institute of Sciences of Food Production, National Research Council, Bari, Italy, for supplying strains of Pseudomonas caryophilli, Pseudomonas syringae pv. panici, Pseudomonassyringae pv. tabaci, Pseudomonas syringae pv. syringae, and Pseudomonas syringae pv. japonica and Prof. Marcelo A. Carmona, Agrarian Faculty, University of Buenos Aires, for supplying the strain of Colletotrichum truncatum. The authors also thank Prof. Nicola Sante Iacobellis (University of Basilicata, Potenza, Italy) and Prof. Gerardo Puopolo (University of Trento, Trento, Italy) for supplying the culture filtrates, respectively, of P. tolaasii and P. reactans and L. capsici AZ78, and Dr. Silvia Lazzaroni for her technical assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.1c08139.
1H NMR spectrum of tolaasin I in CD3OD at 500 MHz (Figure S1); 1H NMR spectrum of hexacetyltolaasin I in CD3OD at 500 MHz (Figure S2); ESIMS spectrum of hexacetyltolaasin I (Figure S3); 1H NMR spectrum of tetrahydrotolaasin I in CD3OD at 500 MHz (Figure S4); ESIMS spectrum of tetrahydrotolaasin I (Figure S5); 1H NMR spectrum of WLIP in CD3OD at 500 MHz (Figure S6); 1H NMR spectrum of WLIP methyl ester in CD3OD at 500 MHz (Figure S7); and ESIMS spectrum of WLIP methyl ester (Figure S8) (PDF)
Author Contributions
§ S.C. and A.C contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Tilman D.; Balzer C.; Hill J.; Befort B. L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 20260–20264. 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture-Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Rosegrant M. W.; Cline S. A. Global food security: challenges and policies. Science 2003, 302, 1917–1919. 10.1126/science.1092958. [DOI] [PubMed] [Google Scholar]
- Foley J. A.; Ramankutty N.; Brauman K. A.; Cassidy E. S.; Gerber J. S.; Johnston M.; Mueller N. D.; O’Connell C.; Ray D. K.; West P. C.; Balzer C.; Bennett E. M.; Carpenter S. R.; Hill J.; Monfreda C.; Polasky S.; Rockström J.; Sheehan J.; Siebert S.; Tilman D.; Zaks D. P. M. Solutions for a cultivated planet. Nature 2011, 478, 337–342. 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- Oerke E. C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. 10.1017/S0021859605005708. [DOI] [Google Scholar]
- Cimmino A.; Masi M.; Evidente M.; Superchi S.; Evidente A. Fungal phytotoxins with potential herbicidal activity: chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. 10.1039/C5NP00081E. [DOI] [PubMed] [Google Scholar]
- Godfray H. C. J.; Beddington J. R.; Crute I. R.; Lawrence H.; Lawrence D.; Muir J. F.; Sherman P.; Roinson S.; Thomas S. M.; Toulmin C. Food security: the challenge of feeding 9 billion people. Science 2010, 327, 812–818. 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Bender C. L.; Alarcon Chaidez F.; Gross D. C. Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. 10.1128/MMBR.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J. Y.; Brand J. G.; Kaulin Y. A.; Malev V. V.; Schagina L. V.; Blasko K.. The syringomycins: lipodepsipeptide pore formers from plant bacterium Pseudomonas syringae. In Pore-forming Peptides and Proteins; Menestrina G.; Della Serra M.; Lazarovici P., Eds.; Francis and Tasylor: London, U.K., 2003; Vol. 5, pp 260–271. [Google Scholar]
- Ballio A.; Grgurina I.. Bioactive Lipopeptides of Pseudomonas syringae. In Bacterial, Plant & Animal Toxins; Ascenzi P.; Polticelli F.; Visca P., Eds.; Research Signpost: Trivandum, India, 2003; Vol. 76, pp 45–58. [Google Scholar]
- Scaloni A.; Camoni L.; Di Giorgio D.; Scortichini M.; Cozzolino R.; Ballio A. A new syringopeptin produced by a Pseudomonas syringae pv. syringae strain isolated from diseased twigs of laurel. Physiol. Mol. Plant Pathol. 1997, 51, 259–264. 10.1006/pmpp.1997.0124. [DOI] [Google Scholar]
- Ballio A.; Bossa F.; Camoni L.; Di Giorgio D.; Flamand M. C.; Maraite H.; Nitti G.; Pucci P.; Scaloni A. Structure of fuscopeptins, phytotoxic metabolites of Pseudomonas fuscovaginae. FEBS Lett. 1996, 381, 213–216. 10.1016/0014-5793(96)00043-9. [DOI] [PubMed] [Google Scholar]
- Emanuele M. C.; Scaloni A.; Lavermicocca P.; Jacobellis N. S.; Camoni L.; Di Giorgio D.; Pucci P.; Paci M.; Segre A.; Ballio A. Corceptins, new bioactive lipodepsipeptides from cultures of Pseudomonas corrugata. FEBS Lett. 1998, 433, 317–320. 10.1016/S0014-5793(98)00933-8. [DOI] [PubMed] [Google Scholar]
- Huang C. J.; Pauwelyn E.; Ongena M.; Debois D.; Leclère V.; Jacques P.; Bleyaert P.; Höfte M. Characterization of cichopeptins, new phytotoxic cyclic lipodepsipeptides produced by Pseudomonas cichorii SF1-54 and their role in bacterial midrib rot disease of lettuce. Mol. Plant-Microbe Interact. 2015, 28, 1009–1022. 10.1094/MPMI-03-15-0061-R. [DOI] [PubMed] [Google Scholar]
- Bionda N.; Pitteloud J. P.; Cudic P. Cyclic lipodepsipeptides: a new class of antibacterial agents in the battle against resistant bacteria. Future Med. Chem. 2013, 5, 1311–1330. 10.4155/fmc.13.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutkins J. C.; Mortishire-Smith R. J.; Packman L. C.; Brodey C. L.; Rainey P. B.; Johnstone K.; Williams D. H. Structure determination of tolaasin, an extracellular lipodepsipeptide produced by the mushroom pathogen, Pseudomonas tolaasii Paine. J. Am. Chem. Soc. 1991, 113, 2621–2627. 10.1021/ja00007a040. [DOI] [Google Scholar]
- Mortishire-Smith R. J.; Nutkins J. C.; Packman L. C.; Brodey C. L.; Rainey P. B.; Johnstone K.; Williams D. H. Determination of the structure of an extracellular peptide produced by the mushroom saprotroph Pseudomonas reactans. Tetrahedron 1991, 47, 3645–3654. 10.1016/S0040-4020(01)80877-2. [DOI] [Google Scholar]
- Andolfi A.; Cimmino A.; Cantore P. L.; Iacobellis N. S.; Evidente A. Bioactive and structural metabolites of Pseudomonas and Burkholderia species causal agents of cultivated mushrooms diseases. Perspect. Med. Chem. 2008, 2, 81–112. 10.1177/1177391X0800200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassarello C.; Lazzaroni S.; Bifulco G.; Lo Cantore P.; Iacobellis N. S.; Riccio R.; Gomez-Paloma L.; Evidente A. Tolaasins A-E, five new lipodepsipeptides produced by Pseudomonas tolaasii. J. Nat. Prod. 2004, 67, 811–816. 10.1021/np0303557. [DOI] [PubMed] [Google Scholar]
- Albericio F.; Alvarez M.; Bruno P.; Canedo L.; Cuevas C.; Francesch A.; Garcia-Ramos Y.; Just X.; Martin J.; Munt S.. et al. State of the Art in the Synthesis of Complex Natural Marine Peptides. Proceedings of the 4th Asia-Pacific International Peptide Symposium/50th Japanese Peptide Symposium; Osaka, Japan, Nov 7, 2013; pp 13––16.. [Google Scholar]
- Dahiya R.; Dahiya S. Natural bioeffective cyclooligopeptides from plant seeds of Annona genus. Eur. J. Med. Chem. 2021, 214, 113221 10.1016/j.ejmech.2021.113221. [DOI] [PubMed] [Google Scholar]
- Scarel M.; Marchesan S. Diketopiperazine gels: New horizons from the self-assembly of cyclic dipeptides. Molecules 2021, 26, 3376 10.3390/molecules26113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierle A. C.; Cardellina J. H.; Strobel G. A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. U.S.A. 1988, 85, 8008–8011. 10.1073/pnas.85.21.8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo G.; Cimmino A.; Palmieri M. C.; Giovannini O.; Evidente A.; Pertot I. Lysobacter capsici AZ78 produces cyclo (L-Pro-L-Tyr), a 2,5-diketopiperazine with toxic activity against sporangia of Phytophthora infestans and Plasmopara viticola. J. Appl. Microbiol. 2014, 117, 1168–1180. 10.1111/jam.12611. [DOI] [PubMed] [Google Scholar]
- Cimmino A.; Puopolo G.; Perazzolli M.; Andolfi A.; Melck D.; Pertot I.; Evidente A. Cyclo (L-pro-L-tyr), the fungicide isolated from Lysobacter capsici AZ78: a structureactivity relationship study. Chem. Heterocycl. Compd. 2014, 50, 290–295. 10.1007/s10593-014-1475-6. [DOI] [Google Scholar]
- Cimmino A.; Bejarano A.; Masi M.; Puopolo G.; Evidente A. Isolation of 2,5-diketopiperazines from Lysobacter capsici AZ78 with activity against Rhodococcus fascians. Nat. Prod. Res. 2021, 35, 4969–4977. 10.1080/14786419.2020.1756803. [DOI] [PubMed] [Google Scholar]
- Peng J. T.Resistance to Disease in Agaricus bisporus (Lange) Imbach. Ph.D. Thesis, University of Leeds, 1986. [Google Scholar]
- Lo Cantore P.; Lazzaroni S.; Coraiola M.; Serra M. D.; Cafarchia C.; Evidente A.; Iacobellis N. S. Biological characterization of white line-inducing principle (WLIP) produced by Pseudomonas reactans NCPPB1311. Mol. Plant- Microbe Interact. 2006, 19, 1113–1120. 10.1094/MPMI-19-1113. [DOI] [PubMed] [Google Scholar]
- Segarra G.; Puopolo G.; Giovannini O.; Pertot I. Stepwise flow diagram for the development of formulations of non spore-forming bacteria against foliar pathogens: the case of Lysobacter capsici AZ78. J. Biotechnol. 2015, 216, 56–64. 10.1016/j.jbiotec.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Agrillo B.; Mirino S.; Tatè R.; Gratino L.; Gogliettino M.; Cocca E.; Tabli N.; Nabti E.; Palmieri G. An alternative biocontrol agent of soil-borne phytopathogens: A new antifungal compound produced by a plant growth promoting bacterium isolated from North Algeria. Microbiol. Res. 2019, 221, 60–69. 10.1016/j.micres.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.Horticulture in Japan; Asakura Publishing Co. Ltd (in Japanese): Tokyo, Japan, 1994. [Google Scholar]
- Kusumi T.; Ohtani I.; Nishiyama K.; Kakisawa H. Caryoynencins, potent antibiotics from a plant pathogen Pseudomonas caryophylli. Tetrahedron Lett. 1987, 28, 3981–3984. 10.1016/S0040-4039(00)96437-2. [DOI] [Google Scholar]
- Molinaro A.; Newman M. A.; Lanzetta R.; Parrilli M. The structures of lipopolysaccharides from plant-associated Gram-negative bacteria. Eur. J. Org. Chem. 2009, 2009, 5887–5896. 10.1002/ejoc.200900682. [DOI] [Google Scholar]
- Adinolfi M.; Corsaro M. M.; De Castro C.; Evidente A.; Lanzetta R.; Lavermicocca P.; Parrilli M. Analysis of the polysaccharide components of the lipopolysaccharide fraction of Pseudomonas caryophylli. Carbohydr. Res. 1996, 284, 119–133. 10.1016/0008-6215(96)00019-5. [DOI] [Google Scholar]
- Adinolfi M.; Corsaro M. M.; De Castro C.; Lanzetta R.; Parrilli M.; Evidente A.; Lavermicocca P. A novel 4-C-branched sugar from the lipopolysaccharide of the bacterium Pseudomonas caryophylli. Carbohydr. Res. 1995, 267, 307–311. 10.1016/0008-6215(94)00302-V. [DOI] [Google Scholar]
- Adinolfi M.; Corsaro M. M.; De Castro C.; Evidente A.; Lanzetta R.; Mangoni L.; Parrilli M. The relative and absolute configurations of stereocenters in caryophyllose. Carbohydr. Res. 1995, 274, 223–232. 10.1016/0008-6215(95)00127-F. [DOI] [Google Scholar]
- Adinolfi M.; Corsaro M. M.; De Castro C.; Evidente A.; Lanzetta R.; Molinaro A.; Parrilli M. Caryose: a carbocyclic monosaccharide from Pseudomonas caryophylli. Carbohydr. Res. 1996, 284, 111–118. 10.1016/0008-6215(96)00018-3. [DOI] [Google Scholar]
- Young J. M.; Fletcher M. J. Pseudomonas syringae pv. panici (Elliott 1923) Young, Dye & Wilkie 1978 is a doubtful name. Australas. Plant Pathol. 1994, 23, 66–68. 10.1071/APP9940066. [DOI] [Google Scholar]
- Liu H.; Qiu H.; Zhao W.; Cui Z.; Ibrahim M.; Jin G.; Li B.; Zhu B.; Xie G. L. Genome sequence of the plant pathogen Pseudomonas syringae pv. panici LMG 2367. J. Bacteriol. 2012, 194, 5693–5694. 10.1128/JB.01267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapuranga N. A new race of Pseudomonas syringae pv. tabaci on tobacco in Zimbabwe. Plant Dis. 1998, 82, 1404. 10.1094/PDIS.1998.82.12.1404A. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Barranquero J. A.; Cazorla F. M.; de Vicente A. Pseudomonas syringae pv. syringae associated with mango trees, a particular pathogen within the “hodgepodge” of the Pseudomonas syringae complex. Front. Plant Sci. 2019, 10, 570 10.3389/fpls.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M. Pseudomonas syringae pv. japonica (Mukoo 1955) Dye et al. 1980 is a junior synonym of Ps. syringae pv. syringae van Hall 1902. Lett. Appl. Microbiol. 1992, 15, 129–130. 10.1111/j.1472-765X.1992.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Dias M. D.; Dias-Neto J. J.; Santos M. D.; Formento A. N.; Bizerra L. V.; Fonseca M. E. N.; Boiteux L. S.; Café-Filho A. C. Current status of soybean anthracnose associated with Colletotrichum truncatum in Brazil and Argentina. Plants 2019, 8, 459. 10.3390/plants8110459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassal K. A.Biochemistry and Use of Pesticides; Verlag Chemie:Weinheim, Germany. 1990, Vol. 58, pp 429–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.