Abstract
Prostate-specific membrane antigen (PSMA) is expressed in a variety of cancer cells, while the fibroblast activation protein (FAP) is expressed in the microenvironment of tumors. Previously, we reported the ability of iPSMA and iFAP ligands to specifically target PSMA and FAP proteins, as well as the preparation of stable 177Lu2O3 nanoparticles (<100 nm) functionalized with target-specific peptides. This research aimed to evaluate the dosimetry and therapeutic response of Lu2O3-iPSMA and Lu2O3-iFAP nanoparticles activated by neutron irradiation to demonstrate their potential for theranostic applications in nuclear medicine. The biokinetic behavior, radiation absorbed dose, and metabolic activity ([18F]FDG/micro-PET, SUV) in preclinical tumor tissues (athymic mice), following treatment with 177Lu2O3-iPSMA, 177Lu2O3-iFAP or 177Lu2O3 nanoparticles, were assessed. One patient with multiple colorectal liver metastases (PSMA-positive) received 177Lu2O3-iPSMA under a “compassionate use” protocol. Results indicated no significant difference (p < 0.05) between 177Lu2O3-iPSMA and 177Lu2O3-iFAP, regarding tumor radiation absorbed doses (105 ± 14 Gy, 99 ± 12 Gy and 58 ± 7 Gy for 177Lu2O3-iPSMA, 177Lu2O3-iFAP, and 177Lu2O3, respectively) and tumor metabolic activity (SUV of 0.421 ± 0.092, 0.375 ± 0.104 and 1.821 ± 0.891 for 177Lu2O3-iPSMA, 177Lu2O3-iFAP, and 177Lu2O3, respectively) in mice after treatment, which correlated with the observed therapeutic response. 177Lu2O3-iPSMA and 177Lu2O3-iFAP significantly inhibited tumor progression, due to the prolonged tumor retention and a combination of 177Lu radiotherapy and iPSMA or iFAP molecular recognition. There were negligible uptake values in non-target tissues and no evidence of liver and renal toxicity. The doses received by the patient’s liver metastases (42–210 Gy) demonstrated the potential of 177Lu2O3-iPSMA for treating colorectal liver metastases.
Keywords: lutetium oxide nanoparticles, prostate-specific membrane antigen inhibitor, fibroblast activation protein inhibitor, lutetium-177
1. Introduction
Research on nanoparticle-based radiopharmaceuticals for the treatment of various types of cancer is of interest in the field of human health, due to the high tumor retention and high internalization in cancer cells of previously-reported targeted radionanosystems [1,2,3,4].
Lutetium-177 has been widely used as the radionuclide of choice for peptide-targeted endoradiotherapy [5,6]. During the last decade many studies have reported the preclinical evaluation of polymeric and metallic nanoparticles labeled with 177Lu. However, the literature related to the preparation of lutetium nanoparticles is scarce and focused on preparing luminescent doped systems (Lu2O3: Ln3+, Ln = Eu, Er, Yb) or contrast agents [7,8,9,10].
Fibroblast activation protein (FAP) is expressed mainly in activated stromal fibroblasts, present in the tumor microenvironment of most human epithelial tumors, but not on the cell membrane of normal fibroblasts [11].
Prostate-specific membrane antigen (PSMA) is a protein that is overexpressed in 90% of advanced prostate tumors and the neovasculature of the tumor microenvironment of many types of advanced cancer, such as metastatic colon cancer, triple-negative breast cancer, and hepatocarcinoma, among others [12,13,14,15,16].
Previously, our research group reported the synthesis of lutetium sesquioxide nanoparticles functionalized with peptides and activated by neutron irradiation [17,18]. The chemical characterization of the nanosystems showed a well-defined quasi-spherical morphology with a monodisperse and monomodal distribution size (30–45 nm), and displayed characteristic radioluminescent properties. The radioluminescent property is due to the interaction between lutetium atoms and their emitted ionizing radiation. In addition, the Lu electronic configuration (4f14 5d1 6s2) allows the formation of sesquioxides with trivalent ions and the presence of f-f transitions between electrons of 4f orbitals shielded by 5s and 5p electrons, which results in photonic emissions (radioluminescence) with long half-lives and resistance to photobleaching.
The 177Lu2O3-iPSMA nanosystem demonstrated in vivo stability and affinity (Kd = 5.7 nM) for the PSMA protein, while 177Lu2O3-iFAP showed significant membrane binding in stromal cells expressing FAP receptors [17,19].
This research aimed to evaluate the biokinetics, dosimetry and preclinical therapeutic response of Lu2O3-iPSMA and Lu2O3-iFAP nanoparticles activated by neutron irradiation to demonstrate their potential for theranostic applications in nuclear medicine. As a proof of concept, the biokinetics and dosimetry of 177Lu2O3-iPSMA in liver metastases (PSMA-positive) of a patient with advanced colorectal cancer, was also assessed.
2. Materials and Methods
2.1. Synthesis of Lutetium Nanoparticles
Lutetium sesquioxide nanoparticles (Lu2O3 NPs) were synthesized by a precipitation-calcination method, as previously reported [17]. Briefly, a 10-mM solution of LuCl3 (anhydrous; powder; 99.99% trace metals basis; Sigma-Aldrich; Saint Louis, MO, USA) was prepared, onto which a 2-M (NH4)2CO3/NH4OH 1:1 (v/v) mixture was added dropwise until the solution reached pH 9, appearing as a fluffy white precipitate. Stirring was continued for an additional hour. The precipitate was washed with type-I water, employing several centrifugations at 2500× g/30 min, until the washing solution reached pH 7. Subsequently, the product was dried and calcined at 1000 °C for 24 h. The obtained powder was analyzed by transmission electron microscopy (TEM) (JEOL JEM 2010 HT, JEOL Inc, Peabody, MA, USA) and infrared spectroscopy (FT-IR, ATR platform) (PerkinElmer 2000, PerkinElmer, Waltham, MA, USA). Dynamic light scattering (DLS: Nanotrac wave, Model MN401) was also applied to verify the hydrodynamic nanoparticle size.
2.2. Preparation of 177Lu2O3-iPSMA and 177Lu2O3-iFAP Nanoparticles
The 177Lu2O3 nanoparticles were produced by neutron capture. For neutron activation, 12 mg of Lu2O3 nanoparticles were irradiated in the Triga MARK III nuclear reactor of the National Institute for Nuclear Research, Mexico (ININ, Ocoyoacac, Mexico), in two cycles of 20 h each, at a neutron flux of 3 × 1013 n/s.cm2, with an interval of 5 d between the two cycles.
Twenty-four hours after irradiation, the nanoparticles were dispersed in 10 mL of a 19-mM sodium citrate solution. Subsequently, 50 µL (0.1 mM) of the iPSMA peptide (ligand targeting PSMA) [20] or iFAP (ligand targeting FAP) [19] were added. The solution was divided into 1.5-mL volumes, which were added to sterile and apyrogenic 13-mL vials containing 3.5 mL of injectable-grade water. The vials were sealed with aluminum caps. The mixture was vigorously shaken, and the activity was verified in an activimeter (Capintec CRC-55tR). The radionanoparticles were sterilized in an autoclave at 121 °C and 21 psi for 20 min. The final colloidal solutions were subjected to bacterial endotoxin and sterility tests (pharmacopeial methods). Radiochemical purity was verified by ultracentrifugation (2500× g for 0.5 h; 30,000 MW cut-off; Amicon Ultra; Millipore: Burlington, MA, USA), in which functionalized [177Lu]Lu2O3 (177Lu2O3) nanoparticles remained in the filter, while [177Lu]LuCl3 and 177Lu2O3 (not nanoparticles) passed through the filter. The processes were carried out in areas with GMP certification. After completing radioactive decay, one sample was used to verify nanoparticle size distribution by TEM and DLS, as well as nanoparticle functionalization, via IR spectroscopy.
2.3. Cell Culture
The human colorectal cancer cell line HCT116 (ATCC® CCL-247™) was cultured in RPMI-1640 (ATCC® 30-2001™) medium (ATCC, Manassas, VA, USA). The medium was supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution. Cells were grown under a 5% carbon dioxide atmosphere at 37 °C.
2.4. Bidistribution of 177Lu2O3-iPSMA and 177Lu2O3-iFAP Nanoparticles
In vivo studies in healthy mice (CD1 male mice; 8 weeks old; weight from 40 to 42 g) were performed in accordance with ethical regulations and standards for the handling of laboratory animals (Official Mexican Norm NOM-062-ZOO-1999). CD1 mice were injected, via the tail vein, with 177Lu2O3-iPSMA or 177Lu2O3-iFAP nanoparticles (50 μL; 13 MBq). Subsequently, whole-body radioluminescent images were obtained from three mice via a system equipped with a CCD camera (XTREME Imaging System; Bruker, Billerica, MA, USA), at the following post-injection acquisition times: 0.5, 1, 2, 3, 24, 48, 72 and 96 h. The other CD1 mice were sacrificed at the time intervals of 3, 48, 72 and 96 h (n = 3 for each time point) for each radionanosystem. The following organs were dissected: liver, spleen, lungs, pancreas, heart, and kidneys, whose activity was measured in a thallium-doped NaI detector, in order to determine the percentage of the dose injected per organ (%ID) with regard to the total activity initially injected. Two groups of healthy mice (n = 3) (CD1 mice; average weight of 40 g) were injected intravenously with a single dose (12 µg/40 g, 2 MBq) of 177Lu2O3-iPSMA or 177Lu2O3-iFAP nanoparticles and after 2 weeks blood samples were obtained for creatinine and liver enzyme quantitation as described below.
2.5. Bidistribution of Nanoparticles in Mice Bearing HCT116 Tumors
Athymic mice (nude male mice; 7 weeks old; weight from 20 to 22 g) were used for tumor induction by subcutaneous injection of HCT116 cells (1 million cells suspended in 0.2 mL PBS) into the leg muscle.
2.6. Therapeutic Protocol
Nude mice with HCT116-induced tumors (tumor size of 0.06 ± 0.01 g) were divided into 4 groups (n = 5), to receive one of the following radionanoparticle treatments (1–2 MBq/0.05 mL), via intratumoral injection, on days 1, 7 and 14: (a) 177Lu2O3-iPSMA, (b) 177Lu2O3-iFAP, and (c) 177Lu2O3. The fourth untreated group was used as a control. Each week, the width (a) and length (L) of tumors were measured with calipers, in order to determine the volume, using the Equation (1):
| (1) |
After 21 days, the tumor’s metabolic activity was assessed by [18F]FDG (2-deoxy-2-[18F]fluoro-D-glucose) PET/CT imaging. Finally, mice were sacrificed to draw blood samples for quantitation of creatinine and liver enzymes. The radiation absorbed dose assessment was performed by calculating the total number of nuclear transformations (N) that occurred in the tumors through the mathematical integration of the activity functions, Ah(t), [], considering the three injected activities (Equation (2)):
| (2) |
The N values were used with the sphere model included in the OLINDA/EXM code [21], to calculate the radiation doses to the tumors throughout the treatment.
2.7. Metabolic Activity Evaluation
[18F]FDG PET/CT images were obtained using a micro-SPECT/PET/CT equipment (Albira; ONCOVISION; Valencia, Spain). Mice included in the treatment protocol (at day 21) were anesthetized with 2% isoflurane and injected in the caudal vein with 5.55 MBq (0.05 mL) of [18F]FDG for whole-body imaging at 1 h post-injection. The PET field-of-view was 60 mm and the acquisition time was 7.5 min. The CT images were acquired with a current of 700 µA, a 35-kV sure voltage, and 600 projections. The mean standardized uptake value [mean SUV] was calculated using PMOD Data Analysis software.
2.8. Creatinine and Liver Enzyme Quantitation
The blood samples of mice, obtained at the end of each treatment, were used for creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) quantitation. Creatinine was evaluated titrimetrically using the picrate method. AST, ALT, and LDH were quantitated using a Roche Diagnostics kit for UV assays.
2.9. Clinical Study
A 67-year-old woman (weight of 57 kg; height of 162 cm), diagnosed with unresectable liver metastases from colorectal cancer, was enrolled in this study. Three months before enrollment, the patient underwent ultrasound and contrast-enhanced CT of the abdomen, which showed data of advanced metastatic colorectal cancer. [18F]FGD PET/CT, and colonoscopy with biopsy and molecular markers were employed, confirming moderately-differentiated intestinal-type invasive adenocarcinoma (stage IV), mutated KRAS (negative to BRAF, PIK3CA, and AKT1, NRAS mutation), negative for microsatellite instability, and with expression of the four proteins of the DNA repair genes. The primary tumor was located in the right iliac fossa, at the level of the ileocecal valve. The patient presented multiple liver metastases, with the presence of two large necrotic lesions; the largest was in segment IV, with a diameter of approximately 6 cm on the largest axis, which compressed the confluence of the right and left biliary branches and the common hepatic artery, causing severe dilatation of the intrahepatic bile duct. Through an endoscopic retrograde cholangiopancreatography procedure, it was possible to place a stent to drain the left side of the liver, but it was not possible to place a second stent for the right side, due to the obstruction caused by the large necrotic lesion. After resection of the primary colorectal tumor, a process of cholangitis was developed and treated with beta-lactam antibiotics. Subsequently, two external catheters were placed to drain the right side of the liver. The patient was intolerant to Folfox chemotherapy (oxyplatin 70 mg + folinic acid 400 mg + 5-FU 1800 mg), which generated severe hepatomegaly, generalized edema, and a state of prostration. A month and a half after the application of chemotherapy, a compassionate 177Lu2O3-iPSMA protocol was approved (Ethics Committee: Protocol No. 2021-MN01) as a possible alternative for the treatment of liver metastases. The patient signed a consent form after receiving detailed information regarding the aims of the study and agreed to the collection of data.
At the time of 177Lu2O3-iPSMA application, the patient had a leukocyte count of 8.1 × 109/L, platelet count of 456 × 109/L, hemoglobin level of 13.6 g/dL, serum creatinine level of 0.6 g/L, serum bilirubin level of 2.2 g/L (direct bilirubin of 1.7 g/L), serum albumin level of 30 g/L, AST level of 189.0 U/L, ALT level of 135.0 U/L, LDH level of 318.0 U/L, serum gamma glutamyltransferase level of 2666.0 U/L, and serum alkaline phosphatase level of 3413.0 U/L.
99mTc-iPSMA (740 MBq) SPECT/CT imaging of the patient was obtained to evaluate PSMA expression. Afterwards, a dose of 185 MBq of 177Lu2O3-iPSMA nanoparticles was intravenously administered. A hybrid planar/SPECT (2D/3D) quantitation method was applied to obtain biokinetic data, as previously reported [3]. In brief, planar and SPECT/CT images were obtained at 1 h after 177Lu2O3-iPSMA administration, with a dual-head gamma camera (SPECT/CT; Siemens, Munich, Germany). Correction factors (CFHybrid) between imaging modalities were calculated by dividing the activity in the organ of interest quantified by SPECT (reconstructions in kBq/mL) and the activity measured in the organ of interest on planar images. Subsequently, planar images were obtained at 24, 90 and 134 h. CFHybrid was applied in the quantitation of the planar method (A(t)P), to determine the volumetric activity (A(t)VOI), as follows: A(t)VOI = CFHybrid × A(t)P. The fractions of the injected activity in each source organ, including tumors (modeled as geometries of isolated spheres), were fitted to exponential models and radiation absorbed doses were calculated by using the OLINDA/EXM code [21].
2.10. Statistics
Differences among the preclinical treatment groups were analyzed by three-way ANOVA multiple comparisons (Tukey’s Multiple Comparisons Test; alpha of 0.05).
3. Results
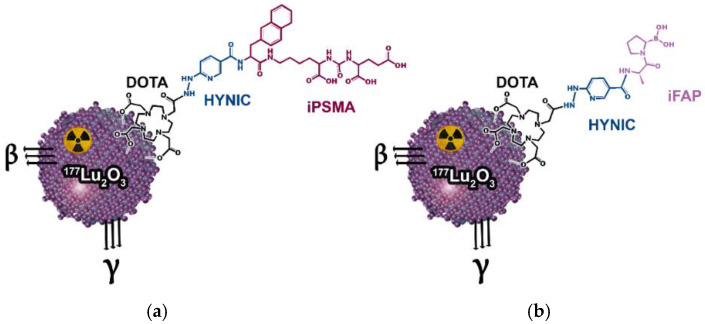
TEM, DLS, and IR analyses of the nanoparticles corroborated the nanometric size (36 nm ± 7 nm by TEM and 105 nm ± 20 nm of hydrodynamic diameter determined by DLS), and the presence of a band at 575 cm−1, corresponding to the Lu–O stretching vibration, which is also proof of the correct crystallization and nanometric scale, as previously reported [17,18]. After neutron irradiation cycles and 24 h of decay, the specific activity of [177Lu]Lu2O3 nanoparticles was 843 MBq/mg. Sterile and apyrogenic colloidal solutions of [177Lu]Lu2O3-iPSMA (177Lu2O3-iPSMA) and [177Lu]Lu2O3-iFAP (177Lu2O3-iFAP) were obtained as pharmaceutical preparations, containing 1350 MBq in 5 mL of 6-mM sodium citrate solution (Figure 1). After complete decay, Lu2O3-iPSMA and Lu2O3-iFAP kept their monodispersed and monomodal distribution behavior and morphological characteristics, determined by DLS and TEM. IR spectra of the functionalized nanoparticles also showed the band at 575 cm−1 and a blue shift of most iFAP and iPSMA characteristic bands (20–100 cm−1), due to the formation of covalent bonds (Lu-DOTA) and changes in the way dipoles vibrate when interacting on the nanoparticle surface, regarding the free ligand.
Figure 1.
Schemes of the (a) 177Lu2O3-iPSMA and (b) 177Lu2O3- iFAP nanosystems.
As expected, the biodistribution profile of 177Lu2O3-iPSMA and 177Lu2O3-iFAP, after intravenous injection into healthy CD1 mice, indicated a considerable hepatic uptake for both nanosystems, without significant differences between them (p < 0.05) (Table 1 and Table 2). The second and third target organ was the spleen and lung, with approximately 15 and 70 times less uptake of nanoparticles than the liver at 3 h, respectively. Radioluminescent images of 177Lu2O3-iPSMA in healthy mice, at different times, also corroborated the liver as the primary target and source organ (Figure 2). Therefore, the intravenous administration of the radionanosystems for targeted treatment of liver metastases or hepatocarcinoma could be possible, since radiotherapy is usually indicated when a significant and inoperable tumor burden affects the hepatic tissue, as long as the nanoparticles are captured and retained in the tumor lesions.
Table 1.
Biodistribution (% injected dose) of 177Lu2O3-iPSMA in CD1 mice at various time points (mean ± SD) (n = 3) after intravenous injection.
| Organ | Time (h) | |||
|---|---|---|---|---|
| 3 | 48 | 72 | 96 | |
| Heart | 0.11 ± 0.03 | 0.08 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| Liver | 23.56 ± 2.13 | 18.08 ± 0.94 | 16.87 ± 1.37 | 14.59 ± 1.72 |
| Lung | 0.34 ± 0.32 | 0.21 ± 0.15 | 0.16 ± 0.09 | 0.12 ± 0.05 |
| Pancreas | 0.12 ± 0.04 | 0.04 ± 0.03 | 0.01 ± 0.01 | 0.00 ± 0.00 |
| Spleen | 1.61 ± 0.59 | 1.18 ± 0.91 | 0.99 ± 0.63 | 0.81 ± 0.35 |
| Kidney | 0.85 ± 0.11 | 0.41 ± 0.18 | 0.22 ± 0.07 | 0.19 ± 0.10 |
| Brain | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Table 2.
Biodistribution (% injected dose) of 177Lu2O3-iFAP in CD1 mice at various time points (mean ± SD) (n = 3) after intravenous injection.
| Organ | Time (h) | |||
|---|---|---|---|---|
| 3 | 48 | 72 | 96 | |
| Heart | 0.13 ± 0.03 | 0.05 ± 0.03 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Liver | 24.98 ± 1.84 | 18.93 ± 2.04 | 17.23 ± 1.41 | 15.01 ± 1.17 |
| Lung | 0.35 ± 0.09 | 0.20 ± 0.11 | 0.14 ± 0.04 | 0.08 ± 0.03 |
| Pancreas | 0.10 ± 0.07 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Spleen | 1.52 ± 0.39 | 1.14 ± 0.49 | 0.79 ± 0.39 | 0.75 ± 0.28 |
| Kidney | 0.98 ± 0.10 | 0.52 ± 0.07 | 0.21 ± 0.07 | 0.18 ± 0.08 |
| Brain | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
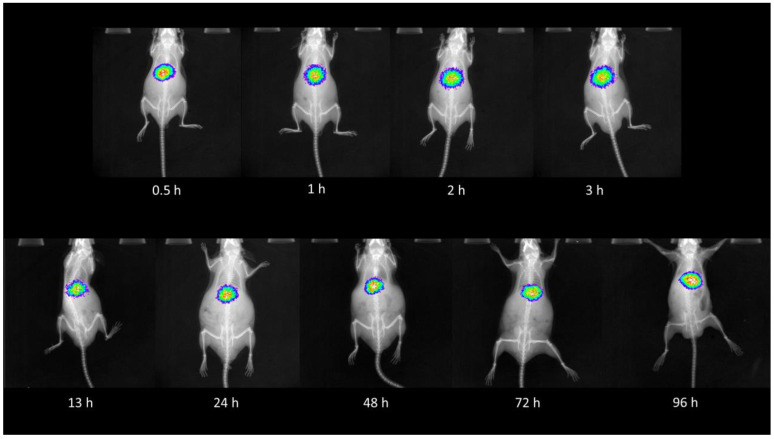
Figure 2.
Radioluminescent images at various time points after the intravenous injection of 177Lu2O3-iPSMA (13 MBq, 0.1 mg) in healthy CD1 mice.
The biokinetic evaluation of 177Lu2O3-iPSMA (Table 3) and 177Lu2O3-iFAP (Table 4) in nude mice bearing HCT116 tumors showed a statistically significant (p < 0.05) higher tumor retention of both nanosystems after intratumoral injection with regard to 177Lu2O3 nanoparticles (Table 5). The mathematical integration of the fit data as exponential models (biokinetic models) until 177Lu complete decay, showed total nuclear transformation values (N) of 597,600 ± 55,576 Bq.s for 177Lu2O3-iPSMA and 586,800 ± 49,878 Bq.s for 177Lu2O3-iFAP, which were significantly (p < 0.05) greater compared to those produced by 177Lu2O3 (339,840 ± 15,632 Bq.s). The differences in the percentage of injected dose retained in the tumors and N values are attributed to the target-specific recognition of iPSMA and iFAP ligands present in the functionalized nanosystems. Some uptake also occurred mainly in the liver and, to a lesser degree, in the spleen and kidney, with practically-negligible retention in other organs.
Table 3.
Biodistribution (% injected dose) at various time points of 177Lu2O3-iPSMA in nude mice bearing HCT116 tumors (mean ± SD) (n = 3), after intratumoral injection.
| Tissue | Time (h) | |||
|---|---|---|---|---|
| 3 | 48 | 72 | 96 | |
| Heart | 0.28 ± 0.11 | 0.19 ± 0.07 | 0.10 ± 0.08 | 0.07 ± 0.02 |
| Lung | 0.30 ± 0.10 | 0.21 ± 0.03 | 0.15 ± 0.04 | 0.11 ± 0.07 |
| Liver | 1.05 ± 0.38 | 0.94 ± 0.21 | 0.88 ± 0.12 | 0.84 ± 0.09 |
| Spleen | 0.39 ± 0.16 | 0.34 ± 0.10 | 0.31 ± 0.09 | 0.28 ± 0.07 |
| Kidney | 0.84 ± 0.19 | 0.63 ± 0.23 | 0.58 ± 0.17 | 0.39 ± 0.21 |
| Tumor (%ID/g) | 80.42 ± 5.87 | 79.32 ± 5.27 | 77.23 ± 6.01 | 75.98 ± 5.41 |
Table 4.
Biodistribution (% injected dose) at various time points of 177Lu2O3-iFAP in nude mice bearing HCT116 tumors (mean ± SD) (n = 3), after intratumoral injection.
| Tissue | Time (h) | |||
|---|---|---|---|---|
| 3 | 48 | 72 | 96 | |
| Heart | 0.22 ± 0.09 | 0.18 ± 0.09 | 0.14 ± 0.07 | 0.05 ± 0.02 |
| Lung | 0.26 ± 0.11 | 0.19 ± 08 | 0.16 ± 0.05 | 0.10 ± 0.04 |
| Liver | 1.18 ± 0.70 | 1.10 ± 0.42 | 0.83 ± 0.22 | 0.81 ± 0.13 |
| Spleen | 0.40 ± 0.14 | 0.35 ± 0.12 | 0.32 ± 0.11 | 0.26 ± 0.09 |
| Kidney | 0.91 ± 0.17 | 0.78 ± 0.15 | 0.62 ± 0.13 | 0.50 ± 0.18 |
| Tumor (%ID/g) | 83.87 ± 5.33 | 81.21 ± 5.64 | 78.58 ± 3.81 | 77.32 ± 4.25 |
Table 5.
Biodistribution (% injected dose) at various time points of 177Lu2O3 in nude mice bearing HCT116 tumors (mean ± SD) (n = 3), after intratumoral injection.
| Tissue | Time (h) | |||
|---|---|---|---|---|
| 3 | 48 | 72 | 96 | |
| Heart | 0.27 ± 0.06 | 0.21 ± 0.08 | 0.16 ± 0.07 | 0.11 ± 0.04 |
| Lung | 0.29 ± 0.13 | 0.25 ± 0.11 | 0.20 ± 0.14 | 0.18 ± 0.05 |
| Liver | 1.69 ± 0.97 | 1.40 ± 0.52 | 1.36 ± 0.45 | 1.29 ± 0.12 |
| Spleen | 0.55 ± 0.28 | 0.49 ± 0.25 | 0.40 ± 0.21 | 0.38 ± 0.14 |
| Kidney | 0.98 ± 0.31 | 0.95 ± 0.29 | 0.88 ± 0.43 | 0.83 ± 0.34 |
| Tumor (%ID/g) | 64.12 ± 4.98 | 57.25 ± 4.56 | 53.58 ± 3.25 | 49.52 ± 3.31 |
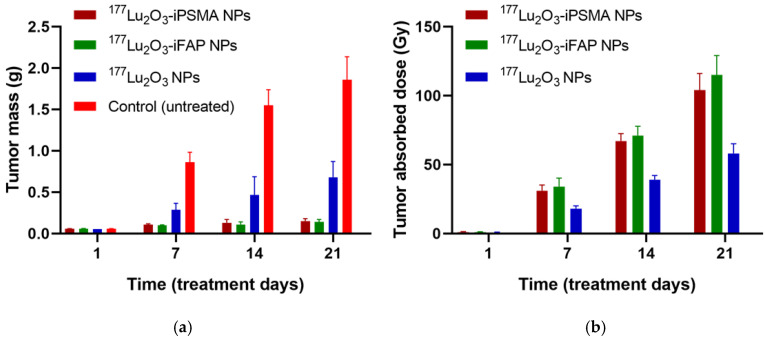
The tumor progression in all mice treated with 177Lu2O3 nanoparticles was significantly inhibited (p < 0.05) with regard to the control group (Figure 3). At 21 d, tumor size in the 177Lu2O3-iPSMA and 177Lu2O3-iFAP groups was 7.5 times smaller than that of the controls and three-fold smaller than in the 177Lu2O3 group (Figure 3). The tumor size progression correlated with the doses delivered to the HCT116 tumors in the order: 177Lu2O3-iFAP (105 ± 14 Gy), 177Lu2O3-iPSMA (99 ± 12 Gy), and 177Lu2O3 (58 ± 7 Gy) nanoparticles, although without significant (p < 0.05) difference between the functionalized nanoparticles (Figure 3).
Figure 3.
(a) Tumor size progression for 177Lu2O3-iPSMA, 177Lu2O3-iFAP, and 177Lu2O3 groups at different days of the treatment, without statistically significant difference (p > 0.05) between 177Lu2O3-iPSMA and 177Lu2O3-iFAP and with statistically significant difference (p < 0.05) of 177Lu2O3-iPSMA and 177Lu2O3-iFAP vs. 177Lu2O3 nanoparticles and the control group; (b) The mean radiation absorbed doses of 177Lu2O3-iPSMA, 177Lu2O3-iFAP, and 177Lu2O3 delivered to HCT116 tumors.
Significant uptake of [18F]FDG in tumors produces high SUV values, indicating high metabolic activity in the viable cells of tumors. As shown in Table 6, the SUV values of the groups of mice treated with nanoparticles were significantly lower with regard to the control group (p < 0.05), while the SUV of the 177Lu2O3 group was significantly greater than that of the 177Lu2O3-iPSMA and 177Lu2O3-iFAP groups (p < 0.05).
Table 6.
Tumor metabolic activity ([18F]FDG/micro-PET; SUV(standard uptake value)), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) serum concentrations in mice bearing HCT116 tumors after 21 days of treatment with 177Lu2O3-iPSMA, 177Lu2O3-iFAP, or 177Lu2O3 nanoparticles (mean ± standard deviation).
| Treatment Group | SUV | Creatinine (mg/dL) |
Aspartate Aminotransferase (AST) (IU/L) | Alanine Aminotransferase (ALT) (IU/L) | Lactate Dehydrogenase (LDH) (IU/L) |
|---|---|---|---|---|---|
| 177Lu2O3-iPSMA | 0.421 ± 0.092 | 0.208 ± 0.032 | 152 ± 13 | 71 ± 6 | 292 ± 37 |
| 177Lu2O3-iFAP | 0.375 ± 0.104 | 0.197 ± 0.042 | 137 ± 16 | 65 ± 7 | 285 ± 44 |
| 177Lu2O3 | 1.821 ± 0.891 | 0.186 ± 0.051 | 142 ± 13 | 69 ± 5 | 294 ± 39 |
| Control | 2.654 ± 0.742 | 0.193 ± 0.061 | 148 ± 15 | 74 ± 8 | 302 ± 42 |
As is known, AST and ALT significantly increase their enzymatic activity after a toxic exposure that affects the integrity of liver cells, while LDH is a stable cytosolic enzyme that is released after cytotoxic damage. Also, elevated creatinine levels indicate impaired kidney function. As shown in Table 6, no significant differences (p < 0.05) in the creatinine, AST, ALT, and LDH values between mice treated with radionanoparticles and those of the control group were found. The same behavior was observed in healthy mice (CD1 mice; average weight of 40 g) at 2 weeks after intravenous injection of the functionalized radionanoparticles, with a single dose of 12 µg/40 g (2 MBq), which indicated that the 177Lu2O3-iPSMA and 177Lu2O3-iFAP radionanosystems do not affect the integrity of liver cells and kidney function.
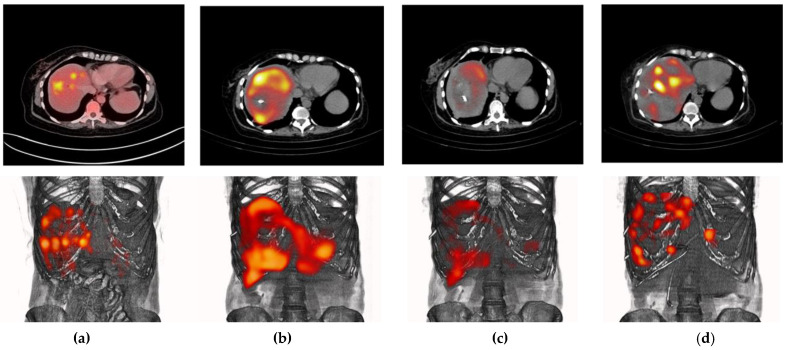
Figure 4 shows the [18F]FDG-micro-PET/CT images of mice after treatment (at 21 d) with 177Lu2O3 (SUVTUMOR = 1.950), 177Lu2O3-iPSMA (SUVTUMOR = 0.365), and 177Lu2O3-iFAP (SUVTUMOR = 0.334), and an untreated mouse (control) (SUVTUMOR = 2.847), in which the low metabolic activity in tumors treated with functionalized radionanoparticles can be observed. The significant difference between nonfunctionalized and functionalized radionanoparticles can be associated with the mechanisms mediated by receptors present in the tumor microenvironment that recognize iPSMA or iFAP ligands bound to the surface of the nanoparticles, leading to large tumor retention, high doses of radiation delivered to the tumor, and a further decrease in tumor metabolic activity.
Figure 4.
[18F]FDG-microPET/CT imaging of the (a) control (untreated mouse), (b) 177Lu2O3, (c) 177Lu2O3-iPSMA, (d) 177Lu2O3-iFAP groups at 21 days of treatment of mice bearing HCT116 tumors.
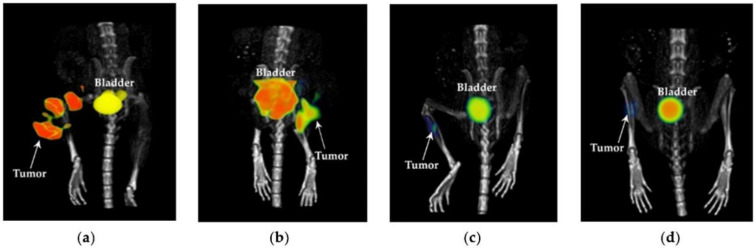
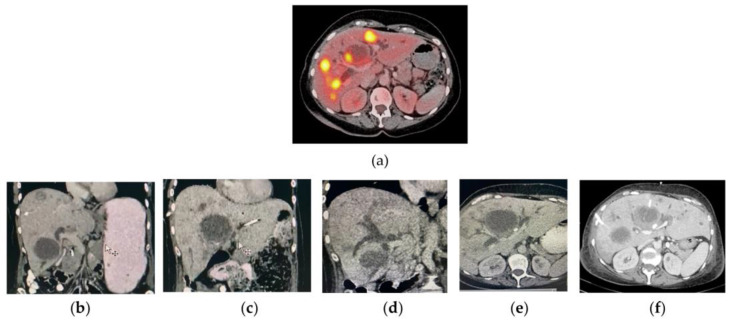
The patient with multiple colorectal liver metastases (PSMA-positive; as determined by [99mTc]Tc-iPSMA SPECT/CT imaging, received 177Lu2O3-iPSMA under a “compassionate use” protocol. The activity, administered intravenously, for the evaluation of the biokinetics and dosimetry in liver metastases, was 185 MBq (0.2 mg/0.8 mL). Figure 5 shows different molecular images of the patient: the [18F]FDG PET/CT images acquired on 27 August 2021, the [99mTc]Tc-iPSMA SPECT/CT images at 3 h and 24 h post-injection (administration on 9 November 2021), and the 177Lu2O3-iPSMA SPECT/CT images acquired on 18 November 2021. A SPECT/CT axial section of the hepatic region is shown at the top of each image. As can be seen in Figure 5, the patient had multiple liver lesions with moderate PSMA expression, which successfully captured the 177Lu2O3-iPSMA nanosystem. It can also be observed that over almost three months, the metastases moved. This occurred due to the formation of several bilomas despite the fact that the patient had a prosthesis (stent) and two biliary catheters, because a necrotic tumor (approximately of 6 cm in diameter) obstructed the bile ducts at an earlier stage of the disease. The described condition of the patient’s liver can be appreciated in CT figures shown in Appendix A (Figure A1).
Figure 5.
Different molecular images (top: axial sections; bottom: PET/CT or SPECT/CT images) of a patient with multiple colorectal liver metastases (PSMA-positive): (a) [18F]FDG PET/CT images acquired on 27 August 2021; [99mTc]Tc-iPSMA SPECT/CT images at (b) 3 h and (c) 24 h post-injection (administration on 9 November 2021); and (d) 177Lu2O3-iPSMA SPECT/CT images acquired on 18 November 2021. It can also be observed that over almost three months, the metastases moved due to the appearance of bilomas and the presence of necrotic tumors. The 177Lu2O3-iPSMA SPECT/CT image was contrast-enhanced to highlight the uptake of the radionanoparticles in tumor lesions as proof of concept. The detailed biokinetic behavior of the source organs is shown in Figure 6.
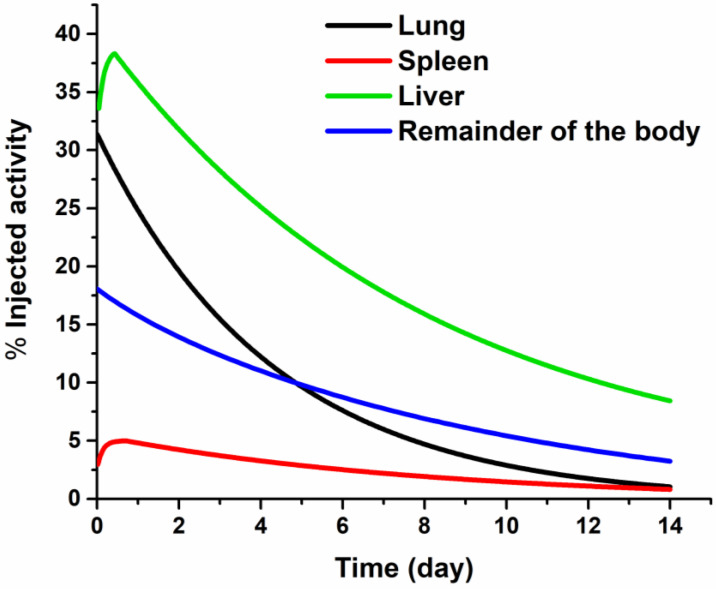
In Figure 5, the 177Lu2O3-iPSMA SPECT/CT image was contrast-enhanced to highlight the uptake of the radionanoparticles in most of the tumor lesions observed in the [18F]FDG PET/CT study as a proof of concept; however, the radionanosystem was also captured in the reticuloendothelial system of the lungs and, to a lesser extent, in the spleen, as observed in the detailed biokinetic behavior of the radiation source organs shown in Figure 6. The biodistribution in the patient does not correlate with that obtained in preclinical studies after intravenous administration of the radionanosystem (Figure 2). Differences in biodistribution patterns are attributed to the severe liver damage of the patient; the reason why there was a redistribution of radionanoparticle uptake by the reticuloendothelial system. The whole-body and abdominal SPECT images can be observed in Appendix A (Figure A2). It is important to emphasize that the biodistribution obtained in the patient in this research cannot be considered as the normal pattern for 177Lu2O3-iPSMA nanoparticles. The relevant point to be considered is that the radionanoparticle’s biodistribution will depend on the degree of disease progression.
Figure 6.
Biokinetic behavior of the source organs after the intravenous administration of 177Lu2O3-iPSMA nanoparticles in a patient with multiple colorectal liver metastases.
Nevertheless, the biokinetic models obtained from the patient for each radiation source organ (Table 7) and metastatic lesions indicated that an administered activity of 1350 MBq would be safe and adequate to generate a total number of nuclear transformations sufficient to produce therapeutic radiation absorbed doses (from 42 to 202 Gy) in the liver metastases (Table 8), without exceeding maximum tolerated doses to any organ (Table 9). For example, it is well-known that with a maximum dose of 7 Gy to the lung parenchyma, after external irradiation (conventional radiotherapy), the probability of symptomatic pneumonia is 5% [22]. In the case presented in this research, the patient would receive 3.42 Gy per 1350 MBq (Table 8), but not homogeneously to the lung parenchyma, since the nanoparticles are trapped by the macrophages of the reticuloendothelial system; a behavior that also occurs in the spleen and liver. In other words, healthy lung parenchyma cells do not trap 177Lu2O3-iPSMA and are not a radiation source tissue; they only receive radiation from the nanoparticles trapped in the alveolar macrophages, which means that the actual radiation dose to healthy lung cells would be much lower than 3.42 Gy per 1350 MBq administered intravenously.
Table 7.
Biokinetic models of 177Lu2O3-iPSMA nanoparticles, calculated from a patient with multiple colorectal liver metastases.
| Organ | Biokinetic Model |
(MBq h/MBq) |
|---|---|---|
| Liver | 86.59 | |
| Lung | 43.33 | |
| Spleen | 9.56 | |
| Remainder of the body | 32.60 |
Table 8.
Total number of nuclear transformations (N) and radiation absorbed doses (Gy/1350 MBq) of 177Lu2O3-iPSMA nanoparticles in liver metastases, from a patient with metastatic colorectal cancer.
| Liver Metastasis (Lesion Number) |
Volume (cm3) |
(MBq h/MBq) |
Dose (Gy) (per 1350 MBq) |
|---|---|---|---|
| L1 | 8.44 | 6.73 | 84.52 |
| L2 | 6.12 | 3.96 | 68.32 |
| L3 | 7.70 | 3.09 | 42.48 |
| L4 | 9.26 | 4.23 | 48.38 |
| L5 | 10.27 | 5.91 | 60.95 |
| L6 | 3.01 | 2.76 | 95.87 |
| L7 | 9.70 | 5.69 | 62.02 |
| L8 | 3.47 | 2.59 | 78.10 |
| L9 | 17.53 | 9.46 | 57.21 |
| L10 | 2.58 | 2.70 | 109.10 |
| L11 | 1.42 | 2.28 | 201.61 |
| L12 | 7.76 | 5.22 | 70.66 |
| L13 | 12.47 | 6.03 | 50.94 |
| L14 | 2.15 | 1.71 | 82.29 |
Table 9.
Radiation absorbed doses (Gy per 1350 MBq) of 177Lu2O3-iPSMA nanoparticles, calculated from a patient with multiple colorectal liver metastases.
| Target Organ | Absorbed Doses |
|---|---|
| (Gy) | |
| Adrenals | 1.18 × 10− × 10−01 |
| Brain | 4.29 × 10−02 |
| Breasts | 7.05 × 10−02 |
| Gallbladder wall | 1.49 × 10−01 |
| Lower large intestine wall | 4.81 × 10−02 |
| Small intestine | 6.37 × 10−02 |
| Stomach wall | 9.79 × 10−02 |
| Upper laege intestine wall | 8.29 × 10−02 |
| Heart | 1.29 × 10−01 |
| Kidneys | 1.08 × 10−01 |
| Liver | 3.21 × 10+00 |
| Lungs | 3.42 × 10+00 |
| Muscle | 7.22 × 10−02 |
| Ovaries | 5.97 × 10−02 |
| Pancreas | 1.43 × 10−01 |
| Red marrow | 5.36 × 10−02 |
| Osteogenic cells | 1.94 × 10−01 |
| Skin | 5.81 × 10−02 |
| Spleen | 3.78 × 10+00 |
| Thymus | 7.52 × 10−02 |
| Thyroid | 5.86 × 10−02 |
| Urinary bladder wall | 5.61 × 10−02 |
| Uterus | 5.90 × 10−02 |
| Total body | 2.82 × 10−01 |
From Table 8, the total number of nuclear transformations that occur in liver metastases can be calculated (, which represents 72% of the total number of nuclear transformations calculated for the liver () (Table 7). Therefore, it is expected that the actual radiation dose to healthy liver cells would be less than 1 Gy per 1350 MBq administered intravenously. Although lungs and spleen doses are higher than that of the liver (Table 9), all of them are below the maximum tolerated radiation doses (MTD) recommended to the liver (MTD = 20–30 Gy), lungs (MTD = 20 Gy), and spleen (MTD = 15 Gy). Thus, for future applications, it would be necessary to develop specific dosimetric models for personalized endoradiotherapy of nanoparticles in patients.
Even though 177Lu2O3-iPSMA nanoparticles could be a viable therapeutic option for the patient evaluated in this research, it was not possible to follow up because she presented bowel obstruction due to intestinal adhesions formed as a consequence of the abdominal surgery for resection of the primary tumor. As a result, the patient had to undergo a second surgery, which complicated her condition until septic shock.
In the practice of targeted endoradiotherapy, it is necessary that the radiation absorbed dose calculations be performed as a personalized evaluation. In the case of 177Lu2O3-iPSMA application for the treatment of liver metastases, the custom dose calculation is even more critical, since the distribution of the radionanosystem can exhibit highly significant variations, depending on the patient’s liver damage. Although there is the option of administering radionanoparticles through the hepatic artery by an interventional radiology procedure to improve the distribution profile towards metastatic lesions, the intravenous route remains a less-invasive and less-expensive option, which is therefore, more feasible.
4. Discussion
As previously mentioned, 177Lu2O3-iPSMA biodistribution in the patient does not correlate with that obtained in preclinical studies after intravenous administration of the radionanosystem. However, it is expected that in most of the patients with liver neoplasms, 177Lu2O3-iPSMA nanoparticles follow the preclinical biodistribution pattern. This assertion is because the phenomenon of redistribution of radiocolloids, which involves lung uptake by induction of the pulmonary intravascular phagocytosis, occurs in patients with severe liver damage due to chronic biliary obstruction, extensive liver neoplasia, and liver infections [23,24]—three clinical conditions of the patient included in this research and not common for all patients. Therefore, the administration of 177Lu2O3-iPSMA nanoparticles to patients must be carried out under a strict personalized dosimetry procedure.
The PSMA protein is not only overexpressed in cancer cells. Although its higher expression is associated with advanced prostate cancer, PSMA is considered a multifunctional protein [12]. Among other functions, PSMA participates in the transduction of signals associated with angiogenesis and cell migration, which is why it is overexpressed in tumor neovasculature of various types of cancer, including metastatic colorectal cancer as shown in this research and in agreement with other authors [13,14]. 177Lu2O3-iPSMA nanoparticles could also be useful in the treatment of hepatocellular carcinoma, where PSMA is expressed both on the membrane of cancer cells (41% of tumors) and in the tumor neovasculature (90% of tumors) [16].
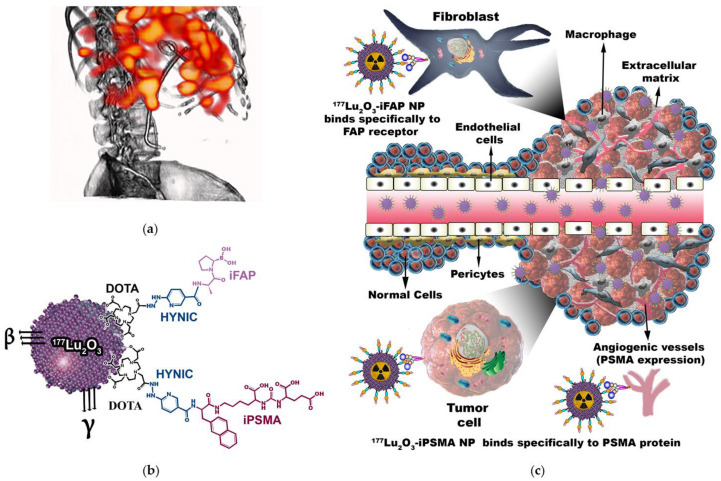
Interactions between nonmalignant and malignant cells form the tumor microenvironment. The tumor microenvironment provides essential elements involved in the processes of initiation and progression of cancer, and in the phases of metastasis and resistance to tumor therapy through intercellular communication, which is mainly regulated by dynamic networks and complex chemokines, cytokines, inflammatory enzymes, growth factors, and different components of the extracellular matrix [25]. As part of the cellular components of the tumor microenvironment, there are cancer-associated fibroblasts with high expression of FAP while PSMA expression is associated with the continuous angiogenic processes in the extracellular matrix. Therefore, the nanoplatforms containing ligands targeting FAP and PSMA, such as 177Lu2O3-iFAP, 177Lu2O3-iPSMA, or even hybrid 177Lu2O3-iFAP/iPSMA systems could improve tumor retention due to their specific binding to both fibroblasts and tumor neovasculature (Figure 7). It is expected that other polymeric and metallic nanoparticles labeled with 177Lu [26,27,28,29,30] show a behavior like that reported in this research, provided that they have the same size range and are functionalized with biomolecules directed to proteins of the tumor microenvironment.
Figure 7.
(a) Schematic “proof of concept” of a patient with colorectal cancer liver metastases with selective tumor uptake of (b) 177Lu2O3-iPSMA/177Lu2O3-iFAP nanoparticles (c) initially captured by the liver reticuloenthotelial system and by tumor metastases due to an enhanced permeability and retention (EPR) effect, but not by healthy hepatocytes of the liver parenchyma; subsequently, retained within tumors by a specific mechanism mediated both by overexpressed receptors on the surface of cancer cells (e.g., PSMA), and by receptors of the tumor microenvironment (e.g., FAP and PSMA).
It is important to mention that the 177Lu2O3-iPSMA nanoparticles developed in this research are not proposed as an option to replace prostate cancer therapies using ligands targeting PSMA, such as 177Lu-PSMA-617 or 177Lu-iPSMA [31,32]; what is proposed is the possibility of using 177Lu2O3-iPSMA for the treatment of liver metastases or tumors that express PSMA in the neovasculature, even with reduced or null expression of PSMA on the membrane of cancer cells. For example, Cuda et al. [14] reported that despite having observed PSMA expression in the neovasculature of colorectal liver metastases in ten patients when performing 68Ga-PSMA-11 imaging, none of them showed sufficient tumor avidity to be included in a 177Lu-PSMA-617 treatment protocol. It is precisely in these clinical cases that 177Lu2O3-iPSMA nanoparticles could be useful, since the first mechanism of tumor accumulation is the enhanced permeability and retention (EPR) effect, which initially takes advantage of the endothelial intercellular space size (from 400 nm up to 800 nm) of tumors, compared to 2 nm in normal endothelial cells (Figure 7). Once inside the tumor, 177Lu2O3-iPSMA nanoparticles are also retained by a specific mechanism mediated by the PSMA protein (Figure 7). The first tumor interaction of 177Lu2O3-iPSMA nanoparticles by the EPR effect, as well as their very fast uptake by the reticuloendothelial system, explains why other organs that express PSMA, such as the salivary glands, are not observed in the SPECT/CT image shown in this research (Figure A2).
Currently, 90Y-microspheres (size of 20–30 µm) are used for hepatic artery radioembolization in the treatment of liver metastases and hepatocarcinoma [33]. The radioactive microspheres clog the hepatic artery and act as a brachytherapy device [34]. The main advantage of the 177Lu2O3-iFAP and 177Lu2O3-iPSMA nanosystems is their targeted retention in the tumor microenvironment to deliver radiation doses as a localized and selective approach.
5. Conclusions
Preclinical results showed that 177Lu2O3-iPSMA and 177Lu2O3-iFAP inhibit colorectal HCT116 tumor progression due to prolonged tumor retention and a combination of 177Lu radiotherapy and iPSMA or iFAP molecular recognition. There were negligible uptake values in non-target tissues and no liver and renal toxicity evidence. The doses received by the patient’s liver metastases demonstrated the potential of 177Lu2O3-iPSMA for treating colorectal liver metastases. However, the biodistribution profile of radionanoparticles can exhibit highly significant variations, depending on the patient’s liver damage; the reason why personalized dosimetry calculations are crucial. Although, the intravenous administration of nanoparticles is less invasive and feasible, the option of administering 177Lu2O3-iPSMA and 177Lu2O3-iFAP through the hepatic artery using an interventional radiology procedure remains the next task to improve their distribution and dosimetry profile.
Acknowledgments
The authors thank the INCan/UNAM Biomedical Cancer Unit of the National Cancer Institute, Mexico, for conducting the microSPECT/CT studies. This study was conducted as a component of the activities of the “Laboratorio Nacional Investigación y Desarrollo de Radiofármacos, CONACyT” (LANIDER) and the “Consejo Mexiquense de Ciencia y Tecnología” (COMECyT) through the program for female scientists EDOMEX, grant number FICDTEM-2021-011.
Appendix A
Figure A1.
(a) PET/CT liver axial image of a patient with liver metastases of colorectal cancer (August, 2021) showing a necrotic mass without metabolic activity (approximate size of 6 cm on its major axis). (b–f) Different CT images of the patient acquired in November 2021. Observe the hepatomegaly progression, the presence of different bilomas, and dilatation of the intrahepatic bile ducts.
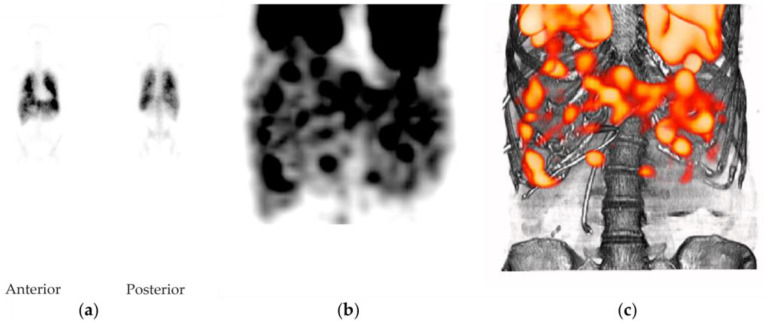
Figure A2.
(a) Anterior and posterior whole-body planar gamma images of a patient with colorectal liver metastases 1 h after intravenous administration of 177Lu2O3-iPSMA nanoparticles (185 MBq), (b) Abdominal SPECT imaging of a patient with multiple colorectal liver metastases 1 h after intravenous administration of 177Lu2O3-iPSMA nanoparticles (185 MBq), (c) Abdominal SPECT/CT imaging of a patient with multiple colorectal liver metastases 1 h after intravenous administration of 177Lu2O3-iPSMA nanoparticles (185 MBq).
Author Contributions
Conceptualization, G.F.-F., M.L.-G. and C.S.-C.; methodology, B.O.-G., N.J.-M., C.S.-C., A.A.-C., D.T.-B., T.H.-J. and M.L.-G.; formal analysis, C.S.-C., G.R.-N., G.F.-F. and R.H.-R.; writing—original draft preparation, M.L.-G. and G.F.-F.; writing—review and editing, G.F.-F.; funding acquisition, G.F.-F., R.H.-R. and C.S.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Mexican National Council of Science and Technology (CONACyT), grant CB2017-2018-A1-S-36841.
Institutional Review Board Statement
This research was approved by the Ethics Internal Committee of Use and Care of Laboratory Animals (CICUAL-ININ) of the National Institute of Nuclear Research, Approval No. 03-2018-2021. The clinical study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of “Medicina Nuclear Hospital Médica Sur” (protocol code 2021-MN01, 11 November 2021).
Informed Consent Statement
Informed consent was obtained from the patient involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cędrowska E., Pruszyński M., Gawęda W., Żuk M., Krysiński P., Bruchertseifer F., Morgenstern A., Karageorgou M.-A., Bouziotis P., Bilewicz A. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules. 2020;25:1025. doi: 10.3390/molecules25051025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferro-Flores G. Targeted nanomedicines: In the right route towards improved therapies. Curr. Cancer Ther. Rev. 2020;16:3–4. doi: 10.2174/1573394715666181224144500. [DOI] [Google Scholar]
- 3.Ramírez-Nava G., Santos-Cuevas C., Ferro-Flores G., Ocampo-García B., Chairez I., Gómez-Argumosa E., Abundiz-López L., García-Pérez F.O. Hybrid (2D/3D) Dosimetry of Radiolabeled Gold Nanoparticles for Sentinel Lymph Node Detection in Patients with Breast Cancer. Contrast Media Mol. Imaging. 2020;2020:2728134. doi: 10.1155/2020/2728134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva F., Cabral Campello M.P., Paulo A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials. 2021;14:4. doi: 10.3390/ma14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadaghiania M.S., Sheikhbahaeia S., Wernerab R.A., Pientac K.J., Pomperac M.G., Solnesa L.B., Gorinac M.A., Nae-Yuh Wang N.-Y., Roweac S.P. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177–labeled Prostate-specific Membrane Antigen–targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021;80:82–94. doi: 10.1016/j.eururo.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strosberg J., Leeuwenkamp O., Siddiqui M.K. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2021;93:102141. doi: 10.1016/j.ctrv.2020.102141. [DOI] [PubMed] [Google Scholar]
- 7.Lim K., Kim H.K., Le X.T., Nguyen N.T., Lee E.S., Oh K.T., Choi H.G., Youn Y.S. Highly Red Light-Emitting Erbium- and Lutetium-Doped Core-Shell Upconverting Nanoparticles Surface-Modified with PEG-Folic Acid/TCPP for Suppressing Cervical Cancer HeLa Cells. Pharmaceutics. 2020;12:1102. doi: 10.3390/pharmaceutics12111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller M., Espinoza S., Jüstel T., Held K.D., Anderson R.R., Purschke M. UVC-Emitting LuPO(4):Pr(3+) Nanoparticles Decrease Radiation Resistance of Hypoxic Cancer Cells. Radiat. Res. 2020;193:82–87. doi: 10.1667/RR15491.1. [DOI] [PubMed] [Google Scholar]
- 9.González-Mancebo D., Becerro A.I., Corral A., Balcerzyk M., Ocaña M. Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes. Nanomaterials. 2019;9:1153. doi: 10.3390/nano9081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Ye K., Huang X., Lin X., Ma L., Chen T. Designing lanthanide coordination nanoframeworks as X-ray responsive radiosensitizers for efficient cancer therapy. Inorg. Chem. Front. 2021;8:3433–3439. doi: 10.1039/D1QI00442E. [DOI] [Google Scholar]
- 11.Hamson E.J., Keane F.M., Tholen S., Schilling O., Gorrell M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014;8:454–463. doi: 10.1002/prca.201300095. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran A.K., Anilkumar G., Christiansen J.J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 13.Haffner M.C., Kronberger I.E., Ross J.S., Sheehan C.E., Zitt M., Mühlmann G., Ofner D., Zelger B., Ensinger C., Yang X.J., et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 2009;40:754–761. doi: 10.1016/j.humpath.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Cuda T.J., Riddell A.D., Liu C., Whitehall V.L., Borowsky J., Wyld D.K., Burge M.E., Ahern E., Griffin A., Lyons N.J.R., et al. PET Imaging Quantifying 68Ga-PSMA-11 Uptake in Metastatic Colorectal Cancer. J. Nucl. Med. 2020;61:1576–1579. doi: 10.2967/jnumed.119.233312. [DOI] [PubMed] [Google Scholar]
- 15.Arslan E., Ergül N., Karagöz Y., Gedik A.A., Çermik T.F. Recurrent Brain Metastasis of Triple Negative Breast Cancer with High Uptake in 68Ga-PSMA-11 PET/CT. Clin. Nucl. Med. 2021;46:e106–e108. doi: 10.1097/RLU.0000000000003336. [DOI] [PubMed] [Google Scholar]
- 16.Tolkach Y., Goltz D., Kremer A., Ahmadzadehfar H., Bergheim D., Essler M., Lam M., de Keizer B., Fischer H.P., Kristiansen G. Prostate-specific membrane antigen expression in hepatocellular carcinoma: Potential use for prognosis and diagnostic imaging. Oncotarget. 2019;10:4149–4160. doi: 10.18632/oncotarget.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ancira-Cortez A., Ferro-Flores G., Jiménez-Mancilla N., Morales-Avila E., Trujillo-Benítez D., Ocampo-García B., Santos-Cuevas C., Escudero-Castellanos A., Luna-Gutiérrez M. Synthesis, chemical and biochemical characterization of Lu2O3-iPSMA nanoparticles activated by neutron irradiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;117:111335. doi: 10.1016/j.msec.2020.111335. [DOI] [PubMed] [Google Scholar]
- 18.Ancira-Cortez A., Trujillo-Benítez D., Jiménez-Mancilla N., Santos-Cuevas C., Morales-Avila E., Ferro-Flores G. Synthesis and physicochemical characterization of Lu and Sm sesquioxide nanoparticles by precipitation-calcination and pulsed laser ablation in liquids. Mat. Chem. Phys. 2021;275:125229. doi: 10.1016/j.matchemphys.2021.125229. [DOI] [Google Scholar]
- 19.Trujillo-Benítez D., Luna-Gutiérrez M., Ferro-Flores G., Ocampo-García B., Santos-Cuevas C., Bravo-Villegas G., Morales-Ávila E., Cruz-Nova P., Díaz-Nieto L., García-Quiroz J., et al. Design, Synthesis and Preclinical Assessment of 99mTc-iFAP for In Vivo Fibroblast Activation Protein (FAP) Imaging. Molecules. 2022;27:264. doi: 10.3390/molecules27010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferro-Flores G., Luna-Gutiérrez M., Ocampo-García B., Santos-Cuevas C., Azorín-Vega E., Jiménez-Mancilla N., Orocio-Rodríguez E., Davanzo J., García-Pérez F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017;48:36–44. doi: 10.1016/j.nucmedbio.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Stabin M.G., Sparks R.B., Crowe E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005;46:1023–1027. doi: 10.1007/s12149-012-0593-4. [DOI] [PubMed] [Google Scholar]
- 22.Marks L.B., Yorke E.D., Jackson A., Ten Haken R.K., Constine L.S., Eisbruch A., Bentzen S.M., Nam J., Deasy J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imarisio J.J. Liver Scan Showing Intense Lung Uptake in Neoplasia and Infection. J. Nucl. Med. 1975;16:188–190. [PubMed] [Google Scholar]
- 24.Chang S.W., Ohara N. Chronic biliary obstruction induces pulmonary intravascular phagocytosis and endotoxin sensitivity in rats. J. Clin. Investig. 1994;94:2009–2019. doi: 10.1172/JCI117554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arneth B. Tumor Microenvironment. Medicina. 2020;56:15. doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilchis-Juárez A., Ferro-Flores G., Santos-Cuevas C. Molecular targeting radiotherapy with cyclo-RGDFK(C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. J. Biomed. Nanotechnol. 2014;10:393–404. doi: 10.1166/jbn.2014.1721. [DOI] [PubMed] [Google Scholar]
- 27.Viana R.D.S., Costa L.A.D.M., Harmon A.C., Gomes Filho M.A., Falcão E.H.L., Vicente M.G.H., Junior S.A., Mathis J.M. 177Lu-Labeled Eu-Doped Mesoporous SiO2 Nanoparticles as a Theranostic Radiopharmaceutical for Colorectal Cancer. ACS Appl. Nano Mater. 2020;3:8691–8701. doi: 10.1021/acsanm.0c01427. [DOI] [Google Scholar]
- 28.Mendoza-Nava H., Ferro-Flores G., Ramírez F.d.M., Ocampo-García B., Santos-Cuevas C., Aranda-Lara L., Azorín-Vega E., Morales-Avila E., Isaac-Olivé K. 177Lu-Dendrimer Conjugated to Folate and Bombesin with Gold Nanoparticles in the Dendritic Cavity: A Potential Theranostic Radiopharmaceutical. J. Nanomat. 2016;2016:1039258. doi: 10.1155/2016/1039258. [DOI] [Google Scholar]
- 29.Trujillo-Nolasco M., Cruz-Nova P., Ferro-Flores G., Gibbens-Bandala B., Morales-Avila E., Aranda-Lara L., Vargas M., Ocampo-García B. Development of 177Lu-DN(C19)-CXCR4 Ligand Nanosystem for Combinatorial Therapy in Pancreatic Cancer. J. Biomed. Nanotechnol. 2021;28:263–278. doi: 10.1166/jbn.2021.3016. [DOI] [PubMed] [Google Scholar]
- 30.Gibbens-Bandala B., Morales-Avila E., Ferro-Flores G., Santos-Cuevas C., Meléndez-Alafort L., Trujillo-Nolasco M., Ocampo-García B. 177Lu-Bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;105:110043. doi: 10.1016/j.msec.2019.110043. [DOI] [PubMed] [Google Scholar]
- 31.Sartor A.O., Morris M.J., Messman R., Krause B.J. Vision: An international, prospective, open-label, multicenter, randomized phase III study of 177Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2020;39:TPS259. doi: 10.1200/JCO.2020.38.6_suppl.TPS259. [DOI] [Google Scholar]
- 32.Santos-Cuevas C., Ferro-Flores G., García-Pérez F.O., Jiménez-Mancilla N., Ramírez-Nava G., Ocampo-García B., Luna-Gutiérrez M., Azorín-Vega E., Davanzo J., Soldevilla-Gallardo I. 177Lu-DOTA-HYNIC-Lys(Nal)-Urea-Glu: Biokinetics, Dosimetry, and Evaluation in Patients with Advanced Prostate Cancer. Contrast Media Mol. Imaging. 2018;2018:5247153. doi: 10.1155/2018/5247153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendlisz A., Van den Eynde M., Peeters M., Maleux G., Lambert B., Vannoote J., De Keukeleire K., Verslype C., Defreyne L., Van Cutsem E., et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J. Clin. Oncol. 2010;28:3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 34.Gosavi A., Puranik A.D., Shah S., Agrawal A., Purandare N.C., Shetty N., Gala K., Kulkarni S., Patkar S., Goel M., et al. Prognostic value of lung shunt fraction in hepatocellular carcinoma and unresectable liver dominant metastatic colorectal cancer undergoing transarterial radioembolisation. Nucl. Med. Commun. 2022;43:24–31. doi: 10.1097/MNM.0000000000001492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.