Abstract
Chronic liver disease is reaching epidemic proportions with the increasing prevalence of obesity, nonalcoholic liver disease, and alcohol overuse worldwide. Most patients are not candidates for liver transplantation even if they have end-stage liver disease. There is growing evidence of a gut microbial basis for many liver diseases, therefore, better diagnostic, prognostic, and therapeutic approaches based on knowledge of gut microbiota are needed. We review the questions that need to be answered to successfully translate our knowledge of the intestinal microbiome and the changes associated with liver disease into practice.
Keywords: Cirrhosis, Hepatic Encephalopathy, Fecal Microbial Transplant, Diet
Liver diseases form a major worldwide burden with increasing morbidity and mortality.1,2 The ultimate impact of chronic liver disease (CLD) is affected by disease etiology interacting with host genetics,3,4 comorbid conditions,5 socioeconomic status,6 and dietary differences. If we look at the most common etiologies of CLD, alcohol use–associated liver disease (ALD), hepatitis C virus infection (HCV), and nonalcoholic fatty liver disease (NAFLD) are more common in North America and Europe,7,8 and the predominant etiologies in Asia and Africa are hepatitis B virus infection, HCV, NAFLD, and ALD.9 Etiologies prevalent in the West, such as NAFLD and ALD, have major lifestyle components. With the obesity epidemic and increasing longevity of the population, the population impact of liver diseases is projected to increase.10 The common end stage of liver disease progression regardless of etiology is cirrhosis, which can result in decompensation and development of hepatocellular cancer.2,11 Changes in the gut microbiota composition and function have a critical relationship with liver health from precirrhotic stages to cirrhosis, decompensation, and requirement for liver transplantation.12 Alterations in the gut–liver axis have far-reaching consequences pertaining to the occurrence, progression, prognostication, and treatment of the major liver diseases.13 This review will focus on major human experiences and current and future opportunities for targeting the gut bacteria in liver disease. This is not meant to be an exhaustive review, but rather a synthesis of several studies that determines the overview of our current knowledge, barriers, and future directions. These are also summarized in Figure 1.
Figure 1.
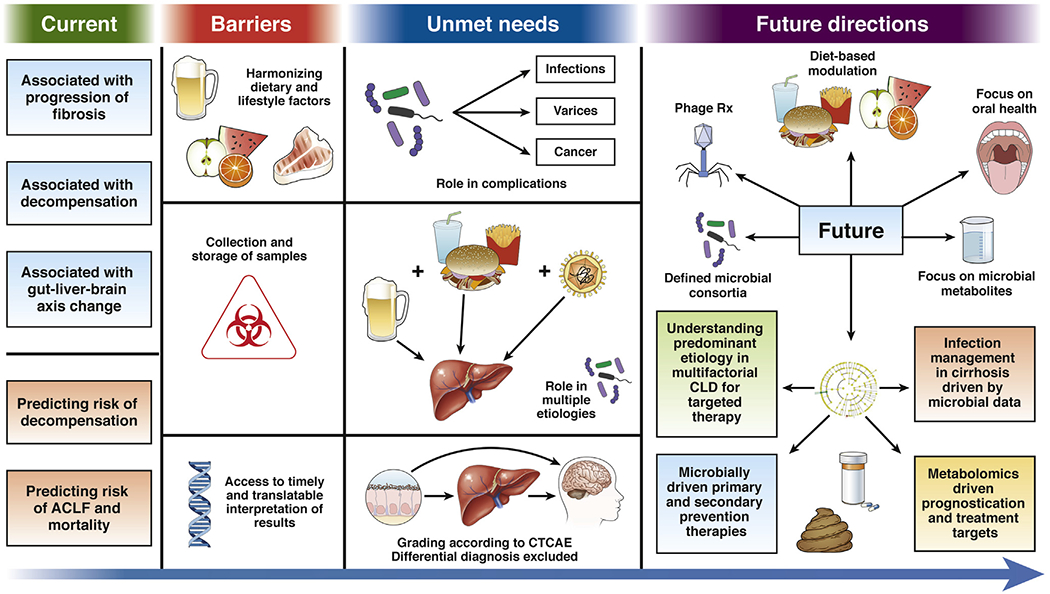
Current state of microbiome-based diagnostic and therapeutic strategies in CLD: barriers to implementation, deficiencies, and predicted future approaches.
Pre-Cirrhotic Changes in Microbiota and Relationship to Etiology
There is usually a prolong pre-cirrhotic period during which liver inflammation and fibrosis progress with alteration in the gut–liver axis with continued exposure to the etiology. In general, progression in pre-cirrhosis stages involves a reduction in overall diversity, reduction in phyla with predominantly beneficial bacteria (Firmicutes), and increase in phyla Bacteroidetes and Proteobacteria that tend to contain pathobionts.12,14
In viral hepatitis (hepatitis B and hepatitis C), the stool microbiome shows a loss of diversity and increase in potential pathobionts, such as Enterobacteriaceae and others, such as Bacteroides, well before cirrhosis.15–19 There is a potential homeostatic role of interferons in the gut, by which they eliminate pathogenic bacteria and promote growth of beneficial bacteria.20 However, the studies that examined the microbiome in patients with cirrhosis pre- and post-treatment for HCV using interferon-based therapies failed to show reversal of dysbiosis.21 The impact of direct-acting antivirals, however, has shown improvement in the microbiome profile in patients with HCV-associated cirrhosis.22 The evidence for pre-cirrhosis microbial involvement in NAFLD and alcohol-related liver disease is relatively more robust. In NAFLD, we have seen an increase in the relative abundance of pathobionts, such as Enterobacteriaceae and Escherichia coli in the stool, blood, and in liver biopsies.23–25 This intrahepatic dysbiosis is probably a feature of all CLDs, but needs to be studied in other etiologies. As fibrosis in NAFLD progresses, dysbiosis worsens, with increased abundance of pathogenic bacteria and reduced abundance of Firmicutes.26,27 Due to the overlap of diabetes mellitus (DM), obesity, and other comorbidities, understanding the contribution of these individual pathologic states to the dysbiosis in NAFLD in understandably difficult. A study looking into the impact of DM on microbiota in cirrhosis found that insulin-dependent DM was associated with dysbiosis, with increased stool Bacteroidaceae and lower Ruminococceae indicating a differential impact of DM based on the severity of DM (insulin-dependent vs non–insulin-dependent).28 Similarly, as the exact contribution of obesity to the dysbiotic milieu in NASH is unclear, a study looking into the impact of obesity found that lean NASH patients have a 3-fold lower abundance of Firmicutes (Faecalibacterium and Ruminococcus), along with a deficiency of Lactobacillus compared with obese NASH patients. This study indicated that the microbial signature of lean NASH is different, or that obesity has its own impact in dysbiosis in CLD.29
In alcoholic hepatitis (AH), that is, before CLD onset, use of alcohol results in significant dysbiosis with increased intestinal permeability that is variably reversed after successful alcohol cessation.30 With continued alcohol misuse, there is further reduction in diversity and an increase in relative abundances of pathogenic bacteria, such as Enterobacteriaceae and Enterococcaceae.31–33 The impact of dysbiosis in ALD is potentially relatively greater than other etiologies, likely due to the direct toxicity of alcohol to the intestinal barrier and microbiome before CLD onset. There is limited evidence about the role of the microbiome in autoimmune hepatitis–related CLD, with one study reporting an increase in Veillonella, which is a potential pathobiont.34 Finally, in cholestatic liver diseases, studies have also consistently shown an overall lower diversity in microbiota. Primary biliary cholangitis showed a distinct pattern with 8 genera (Haemophilus, Veillonella, Clostridium, Lactobacillus, Streptococcus, Pseudomonas, Klebsiella, and an unknown genus in the family of Enterobacteriaceae) being significantly increased in primary biliary cholangitis,35 while primary sclerosing cholangitis patients tended to have increased pathobionts (Veillonella).36–38 There are several studies in NAFLD and nonalcoholic steatohepatitis (NASH) that determined that the microbiota structure and functional aspects worsen as fibrosis progresses. The study by Boursier et al26 found that advanced fibrosis and NASH were associated with changes in carbohydrate and lipid metabolism as they noted enrichment in functional categories. Similarly, Loomba et al27 also found that in NAFLD/NASH with advanced fibrosis, there were changes in abundance of pathways for carbon metabolism and detoxification in the microbiota. This is important to note because it tells us that despite dysbiosis, the microbiome adapts its function to maintain nutrition. In cirrhosis, fecal microbiota–predicted functionality showed functions related to vitamin and oxidative stress metabolism were more likely, and the salivary microbiome–predicted functionality was more for endotoxin production.39
Ultimately, despite differing etiologies associated with pre-cirrhotic liver disease, the overall microbiome changes follow a relatively predictable pattern in human studies currently published. However, several areas need further research. For example, we need more data in hepatitis B virus–related liver disease, especially the effects of antiviral treatment on the microbiome. An unresolved issue that needs teasing out is the individual imprint of each component of the metabolic syndrome and of DM in NASH patients, which has proven to be technically challenging. Due to the long natural history of the disease progression, this is difficult to elucidate in the current cross-sectional or even longitudinal human studies, particularly in determining whether the microbiome changes precede the liver disease development, is complicit in progression, or is a result of the liver disease. In an ideal world, once we find that patients have evidence of pre-cirrhotic CLD, favorable alterations in gut microbiota could potentially prevent disease progression. This would have the greatest impact on liver disease progression and potentially public health. However, this is difficult to translate into practice because, at an early stage in the disease, most patients are either not aware of their disease or the natural history of disease spans several decades. There are multiple logistical and potential patient-related factors that would hinder a preventive approach to dysbiosis in these early stages.
Role of Microbiota in Causation and Progression of Liver Diseases
The role of liver disease etiology vs microbiota in causation and progression of disease is important to consider. The preponderance of evidence points towards a noncausal but contributory role of the microbiome and the current accepted hypothesis is that the human intestinal microbiome, when stressed by various disease processes, undergoes dysbiosis, which accelerates liver fibrosis/cirrhosis development through up-regulation of inflammation and subsequently advanced fibrosis/cirrhosis that contributes to its complications, such as hepatic encephalopathy (HE).40 The data to support this narrative are relatively robust in ALD and NAFLD. For example, alcohol consumption results in direct toxicity to the liver via dysbiosis mediated through small intestinal bacterial overgrowth/large intestinal bacterial overgrowth or direct microbial toxicity, and by direct local injury to the intestinal barrier with resultant increased bacterial translocation and increased inflammation.30,41–43
As liver fibrosis progresses, there are simultaneous changes in the metabolic function of the liver that influence the microbiome. With progression of liver fibrosis, there is a reduction in bile acid (BA) production, with lower BAs noted in the intestine, which link directly to bacterial dysbiosis.44,45 This is because secondary BAs (transformation of which can only be done by selected colonic microbes, such as Clostridium cluster XVIa via 7α dehydroxylation) are the most potent stimulators of the FXR in the ileum.46–48 We know that FXR directly regulated BA production from the liver and, therefore, with dysbiosis there is lesser secondary BA-mediated FXR up-regulation, along with a resultant reduction in the BA pool, which further promotes dysbiosis.47 In addition to BAs, several other metabolites, such as aromatic amino acid moieties, phospholipids, and trimethylamine oxide, can mediate the interface between microbiota and the hosts.49,50 In addition, products from specific microbes, such as cytolysin from Enterococcus faecalis and candidalysin from Candida can also impact host metabolism and outcome.51,52 Similarly, in NAFLD, we know of the direct toxicity of adipose tissue to hepatocytes as seen by balloon degeneration on pathology, but via microbial dysbiosis there is associated upregulation in inflammation, changes to Bas, and progression of disease.12,40 Therefore, it is likely that the liver disease etiology can trigger dysbiosis, which then propagates disease in the early stages and causes functional changes pertaining to BA metabolism. In addition to BA, several other gut microbial products, such as trimethylamines, ammonia, tryptophan, and short-chain fatty acids (SCFAs), can also influence disease progression.53–56 The interaction of the microbiota and their products from a functional perspective might be more relevant than their composition and needs to be investigated further.
To better understand the role of the microbiome in liver disease, the use of germ-free (GF) animal models can be helpful. Llopis et al,31 for example, found that fecal transfer of gut microbiota from patients with severe AH into GF mice resulted in a more severe hepatitis in mice (GF and conventional) than fecal microbiota transplantation (FMT) from an alcoholic patient with no AH, indicating that the microbiome had bacteria or other compounds that had a significant role in worsening disease severity. Another recent important study found that mice exposed to cytolysin-positive Enterococcus faecalis developed alcohol-related liver injury at greater rates than those that were not exposed to these bacteria, showing that pathogenic microbiota determine the severity of liver injury.52 The impact of microbiota on cirrhosis and alcohol has also been studied in the context of cirrhosis. Stool transplanted from human donors were able to replicate BA profiles, hepatic enzyme expression, and intestinal and systemic inflammation, without the active liver injury etiologic agent in GF mice.57 In another study, when GF mice with cirrhosis developed hyperammonemia but without neuroinflammation, as opposed to cirrhotic conventional mice that exhibited hyperammonemia and neuroinflammation with concomitant intestinal dysbiosis.58 This indicated that the microbiome could have a pivotal role in complications, such as HE and BA physiology in human cirrhosis. However, transplanting the microbiota alone was not able to replicate the complete morphology and phenotype of the liver disease, but made the recipient animal more prone to the disease etiology once they were exposed to it. Therefore, the current evidence suggests that microbiota are complicit but not causative in CLD.
In addition to bacteria, there is emerging evidence of the role of fungi and viruses in the pathogenesis and advancing of CLDs. In alcohol-related CLD and in cirrhosis, there are alterations in gut fungal composition that can predict outcomes.59,60 Virome changes in patients with NAFLD, AH, and cirrhosis have also been described that can potentially modulate the impact of CLD directly or indirectly by affecting gut bacteria.61–63
There are immense clinical implications for defining the microbial basis of liver disease in the development of microbially based interventions that reduce disease severity and slow down progression toward cirrhosis, and prevent complications such as HE; infections; and death from cirrhosis.
Diet, Gut Microbiota, and Liver Diseases
The bulk of data for liver disease and microbiota come from North America and Europe. Complicating the epidemiology of CLD is the obesity pandemic, with Asian populations showing higher visceral adiposity and insulin resistance, despite lower body mass indices.64 Obesity and accompanying dysbiosis can complicate all etiologies of CLD. Regardless, in North America and Europe, there appears to be an growing association between disease progression and then again with decompensation with cirrhosis with increases in pathogenic bacteria from phyla such as Bacteroidetes and Proteobacteria.65,66 In Asia, where the most common etiology is hepatitis B virus, a few studies in patients with cirrhosis have found increased Proteobacteria and Fusobacteria.67,68 Data from the African subcontinent are lacking, although a small study from Egypt noted higher Prevotella and Faecalibacterium in HCV patients with Childs B cirrhosis.69 Given the role of diet in influencing microbiota, and given the vastly distinct dietary practices between the East and West, direct comparisons of gut microbial data from these continents might not be entirely accurate, but, despite all of these differences, we still see similar trends in gut dysbiosis.
Diet has a major role in shaping the gut microbiome. Several studies have determined the roles played by diet and ethnicity across the lifespan. A landmark study compared the stool microbiome from children in rural west Africa with that of children from the European Union and noted that African children who consumed more dietary fibers and no processed food had a significantly higher enrichment in Bacteroidetes and depletion in Firmicutes, significant under-representation of Enterobacteriaceae (Shigella and Escherichia), and they also had higher fecal SCFA.70 In adults, David et al71 found that specific diets can modulate the microbiome, but the effects are short-lived and reverse after 2 days. The study found that an animal-based diet increased bile-tolerant microorganism (such as Alistipes, Bilophila, and Bacteroides) and decreased the levels of Firmicutes (such as Roseburia, Eubacterium rectale, and Ruminococcus bromii). This tells us that the human microbiome starts evolving at an early age and continues to evolve with us as our diets change. Therefore, this needs to be accounted for in CLD-related studies.
Dietary studies in patients with CLD need to be performed with a microbiome focus. In a randomized controlled trial of a modified alternate-day calorie restriction vs standard diet in patients with NAFLD, the modified alternate-day calorie restriction group had reduced liver steatosis and fibrosis scores on shear wave elastography at the end of 8 weeks.72 No studies on specific diets and the microbiome have been done in ALD or viral hepatitis. Recently, additional data have emerged on the benefits of a Mediterranean diet in a non-CLD elderly population. The multicenter European trial found that this specific diet, given during a period of 1 year, attenuated loss of gut microbial diversity, improved certain taxa that were associated with improved cognition, and reduced risk of frailty and reduced inflame-aging (eg, Faecalibacterium prausnitzii).73 Special studies of diet and disease pose their own challenges, for those interested, a recent review sums up the existential road blocks in setting up a diet-based microbiome interventional study and how to overcome them.74
To understand how CLD and its complications are influenced by different diets, recent studies have evaluated 3 unique populations of patients with cirrhosis from the United States, Mexico, and Turkey.75 On looking at a North American population compared with a Turkish population (with predominant dietary intake of foods rich in bacterial cultures, such as fermented milk), the Turkish population had a higher α-diversity and lower risk of 90-day hospitalization and admissions. In contrast, more from renal injury was seen in the Turkish population as opposed to the US cohort, which had admissions from infections. Another recent study comparing saliva, stool, and gut–brain axis in patients with cirrhosis from the United States and Mexico noted that Mexican patients had lower consumption of dietary protein and higher animal fat and the cohort had lower bacterial diversity and higher hospitalizations. Prevotellaceae was found to be associated with minimal hepatic encephalopathy (MHE) and hospitalizations in the Mexican cohort.76 As more pathogenic taxa appear to predominate with a more animal-based processed food diet, we might be able to draw conclusions on what type of diet a person is consuming. In the same vein, we can tailor a subject’s diet to try and encourage the growth of beneficial bacteria by studying their fecal microbiota. For example, a fiber-rich diet (indigestible fibers particularly) that promotes SCFA producers will enhance colonic health and improve overall microbial diversity.77
Ultimately, the impact of diet on microbiota in CLD needs to be controlled for74 before comparing across populations. In addition, dietary interventions as primary or secondary mediators of improvement in liver disease via the microbiota need to be explored.
Interaction of Gut–Brain Axis in Liver Diseases
We know that even in the absence of liver disease, the microbiome plays a role in modulating mood disorders78 and potentially cognition.79 Altered brain function in CLD is a consequence of the gut–liver–brain axis, which can influence the course even before cirrhosis. Alcohol-related disease is a prime example where one can see the full spectrum of influence of the gut–liver–brain axis. The brain is already impacted and rendered susceptible by the effects of alcohol, leading to cognitive impairment, mood disorders, post-traumatic stress disorder (PTSD) and other psychiatric comorbidities that are worsened by nutritional deficiencies.80,81 An important study of gut–liver–brain axis in alcohol use disorder (AUD) found that depression, anxiety, and alcohol craving correlated with increased intestinal permeability and that patients with high intestinal permeability continued to have depression, anxiety, and alcohol craving, even after alcohol withdrawal.30 A more recent publication on manipulation of this axis in AUD found that these cravings, consumption, and long-term AUD-related hospitalizations can potentially be reduced after FMT, but not in placebo in patients with AUD.82
The widely prevalent form of cognitive dysfunction in cirrhosis is an anamnestic type—referred to as minimal or covert hepatic encephalopathy.83 This can progress to overt HE, which can manifest as lethargy, disorientation, asterixis, and coma. HE, which is the classic microbiome-related complication of the gut–liver–axis, is a result of systemic endotoxemia and inflammation that ultimately aggravates neuroinflammation, which is an important factor that underpins the pathogenesis of HE. For many years, ammonia was noted to be central to the pathogenesis of HE, but it was then discovered that systemic inflammation was necessary for ammonia to exert its neurotoxic effects.84 The role of specific microbiota in the progression of HE and systemic inflammation, including the influence of sex, is being described increasingly.85,86 In mice, it was found that the gut microbiome is necessary for systemic inflammation and neuroinflammation that are characteristic of HE.87 Clinically, MHE requires specialized cognitive tests, such as the Psychometric Hepatic Encephalopathy Score, inhibitory control test and EncephalApp Stroop for diagnosis.83 These tests are, however, best administered in a specialized setting and require expertise. This can be circumvented by use of gut microbial data. Examination of salivary and stool microbiota in patients who were tested for different cognitive tests (Psychometric Hepatic Encephalopathy Score, inhibitory control test, and EncephalApp Stroop) showed that specific stool and salivary microbial signatures could be used to exclude MHE if easy access to MHE is not available.88
There is also a synergistic interaction between MHE and PTSD via the gut microbiota, leading to greater dysbiosis in patients with PTSD compared with others.89 These are relevant issues because PTSD, alcohol misuse, and cirrhosis often co-exist.90,91 Microbial changes might be helpful in differentiating MHE from PTSD and could help us define newer therapies helping subjects with and without military-related trauma.90,91
A major challenge in leveraging the gut–brain axis is the aging CLD population. Elderly patients tend to have microbial compositions that are more pathogenic,92 which worsens with comorbidities, hospitalizations, and antibiotic exposure towards greater gram-negative pathobionts.93 Older (older than 65 years) patients with cirrhosis tend to have an altered gut–liver–brain axis, with increased inflammation and impaired cognitive performance focused on memory compared with similar aged noncirrhotic controls.94 More importantly, in the study on correlation network analysis, similar patterns were noted in the elderly cirrhotic and noncirrhotic groups, which found that elderly patients already have an underlying altered gut–brain axis.95 This has major implications for liver transplantation because age-related changes in the gut–brain axis can persist despite a liver transplantation. This aging population tends to have multiple other comorbidities and psychiatric illness requiring extra care to ensure optimal post-transplantation outcomes. Table 1 lists some important gut–brain axis studies showing how the gut microbiome influences brain function.
Table 1.
Microbiome-Based Axes in Chronic Liver Disease
| Focus | Important studies | Important Findings | Barriers |
|---|---|---|---|
| Gut-liver axis | |||
| Alcohol-related CLD | Tuomisto et al, 2014133 | Active alcohol intake results in dysbiosis in the entire intestine, liver, ascitic fluid, and serum and worsens the dysbiosis. The pattern of dysbiosis in AUD shows increased pathogenic taxa like all other etiologies of cirrhosis. | Cost, collection, interpretation expertise |
| Mutlu et al, 201241 | |||
| Santiago et al, 2016134 | |||
| Bajaj et al, 2017135 | |||
| Non-alcoholic CLD | Caussy et al, 2019111 | Increased Enterobacteriaceae and Streptococcus with reduction in Akkermansia in cirrhosis in NASH. Universal dysbiosis from oral cavity to colon occurs in cirrhosis with a distal migration of oral origin species in cirrhosis. Overall similar dysbiotic patterns of increased pathogenic taxa noted that worsens in the sickest patients that require hospitalization. | |
| Ponziani et al, 201822 | |||
| Qin et al, 201468 | |||
| Chen et al, 2016136 | |||
| Bajaj et al, 201465 | |||
| Bajaj et al, 201528 | |||
|
| |||
| Gut–liver–brain axis | |||
| Cirrhosis-MHE studies | Zhang et al, 2013137 | MHE is also associated with distal migration of oral species. Stool and salivary microbial signatures can be specific to MHE. Certain autonomic taxa are independently associated with good cognition. | |
| Bajaj et al, 201988 | |||
| Cirrhosis-OHE studies | Bajaj et al, 201465 | Increased pathogenic bacteria with reduction of autochthonous taxa in stool and salivary microbiome in OHE, irrespective of etiology. Salivary and stool microbiota predicted readmissions and outcomes. | |
| Bajaj et al, 201528 | |||
| Bajaj et al, 201539 | |||
| Aging population | Bajaj et al, 201695 | Elderly patients regardless of liver disease or cognitive dysfunction have and altered gut–brain axis. Those with MHE had lower relative abundance of autochthonous and oral origin families, but higher Bacteroides abundance mirroring dysbiotic patterns in younger ages. | |
|
| |||
| Gut–liver–brain axis: brain function | |||
| Magnetic resonance imaging (MRI)/magnetic resonance spectroscopy (MRS) | Bajaj et al, 201695 | Cirrhotic patients demonstrated significant changes on MRS. Autochthonous taxa correlated negatively with MRS. Pathogenic taxa correlated positively in controls and cirrhotic patients. MRI/MRS of the brain in cirrhosis reflects microbial dysbiosis. | Cost of MRS is prohibitive for day-to-day practice |
| Ahluwalia et al, 2016138 | |||
|
| |||
| Gut–liver–brain axis in coexistent psychiatric states | |||
| PTSD | Bajaj et al, 201989 | Lower diversity and autochthonous taxa with higher pathobionts in PTSD patients compared with those without PTSD regardless of HE status. The gut–brain axis is a significant contributor in psychiatric states and pathogenic taxa seem to be involved irrespective of liver disease. | Lower numbers, diagnosis of PTSD may be challenging outside the VA system |
OHE, overt hepatic encephalopathy; VA, Veterans Affairs.
The current evidence shows that gut microbial function and composition have major roles in HE, but data on patients without cirrhosis, especially related to behaviors that drive CLD, are emerging.
Microbiota in Critical Care and Peri-Liver Transplantation for Patients With Cirrhosis
The microbiome is an excellent biosensor and is truly reflective of the susceptibility of the patient toward further decompensation. Stool microbiota have been analyzed in the critical care setting of acute and chronic liver failure (ACLF). In a large multicenter study, stool microbiota samples taken on admission have shown predictive potential for hospitalization course and risk for ACLF, that is, those who went on to develop ACLF and its high 30-day morbidity and mortality. Those patients had a relatively higher abundance of Enterococcaceae and bacteria of the phylum Proteobacteria with relatively lower Lachnospiraceae.96 Another study looking at ACLF noted a higher abundance of Pasteurellaceae in the ACLF groups (compared with healthy controls), which was an independent predictor of mortality97 and another study of ACLF in hepatitis B noted Prevotellaceae to be an independent predictor of short-term mortality.98 Another recent metagenomics single-center study confirmed these changes at a deeper level in patients who developed ACLF and mortality.99 In a multicenter study of 602 inpatients with cirrhosis, serum collected on admission showed several microbially generated metabolites that were able to predict the development of ACLF and mortality independent of clinical factors.49 Another multicenter study of 650 patients with decompensated cirrhosis and 181 ACLF patients with serum metabolite examination found significant differences in people who had already developed ACLF. The study noted overwhelming systemic inflammation of ACLF, which was the primary reason for the altered metabolic pathways and that many of the metabolites were of microbial origin.100 AH is another major etiology of ACLF and has significant mortality if not treated.101,102 Alcoholic liver disease and AH result in significant microbial dysbiosis,103–105 as noted previously in this review. Recently, a large multicenter trial suggested that metabolomics, in particular microbial metabolic pathways, could be leveraged to predict 30-day mortality with good accuracy.106 These data suggest that gut microbial composition and products could be leveraged to predict outcomes.
This advanced sick population usually gets urgently listed for liver transplantation if infections are controlled. After transplantation, in addition to compositional change (gut microbiota diversity restoration, increase in autochthonous with decreases in potentially pathogenic taxa), there is resolution of microbial functionality evidenced by reduced endotoxemia, higher secondary, oxo, and iso-BAs.107 This sick population is also susceptible to infections and, if infected by a multidrug-resistant organism, the microbiome post liver transplantation can have persistent dysbiosis and lower diversity.108 More studies to document long-term microbiota changes post liver transplantation are currently needed.
Given the multiple potential confounders inherent to the critical care and post-transplantation settings, the data are consolidating around microbial composition and function being important modulators of outcomes in these settings.
Gut Microbiota As Biosensors in Liver Disease and Potential Translation Into Practice
The strong correlations noted between advancing liver diseases and the gut microbiome and the ease of generating large amounts of data emerging from these studies can revolutionize our approach to diagnostics and prognostics in CLD. Diagnosis of CLD using the microbiota is still in its infancy, but through a combination of metagenomics, clinical metadata, and liver functions tests, headway is being made to differentiate cirrhosis from earlier fibrosis stages.109 Advanced fibrosis, which is an important predictor of mortality in NAFLD, can be inferred from the fecal microbiota to help identify higher-risk groups.27,110 From the perspective of outpatients, gut microbiota can prove to be a useful tool to predict admission, MHE, and who has advanced fibrosis. As one example, the salivary dysbiosis ratio (Lachnospiraceae + Ruminococcaceae + Veillonellaceae/Streptococcaceae in salivary microbiome), a simple practical clinical tool was found to predict admissions at 90 days in decompensated cirrhosis patients with and without HE.39 A study in NAFLD cirrhosis found that 27 bacterial features on stool microbial analysis, along with age, sex, and body mass index, were predictive of NAFLD cirrhosis.111
Given the predicted pattern of dysbiosis noted in decompensated cirrhosis, the cirrhosis dysbiosis ratio (ie, Lachnospiraceae + Ruminococcaceae + Veillonellaceae/Enterobacteriaceae + Bacteroidaceae) was conceived as a tool to predict those at risk for decompensation (for all common CLD etiologies admitted to hospital) where a lower score predicted a worse outcome and had strong predictive values.65 Stool microbiota can also be used to predict who can have readmissions at 90 days,28 and recurrence of acute HE as the fecal microbial profile for those with acute HE is different from those with other decompensating events. Notably, in one study, abundance of Veillonella parvula increased the most during HE compared with those without HE. Other operational taxonomical units were also found to be relatively abundant during the HE episode and associated with HE recurrence and mortality.112 Often overlooked but commonly prescribed are proton pump inhibitors (PPIs), which worsen dysbiosis due to changes to the gastric pH in health and in CLD.113,114 In a study looking at readmissions in patients with cirrhosis on and off PPIs, PPI users had lower autochthonous taxa and higher readmissions at 30 and 90 days. In a sub-cohort of the study, initiation of PPI resulted in increased oral-origin microbial taxa in the fecal microbiome that reduced with PPI withdrawal.115 Table 2 enumerates a few studies that used microbial data to predict hospitalizations and diagnosis cirrhosis in the inpatient and outpatient setting.
Table 2.
Outcomes and Microbiota/ Microbiota Products
| Focus | Important studies | Findings | Barriers |
|---|---|---|---|
| Diagnosis of cirrhosis | Oh et al, 2020109 | AUC of 0.91 for diagnosis of cirrhosis based on microbiota and other variables | Cost, availability and mixing compensated and decompensated cirrhosis |
|
| |||
| Outcomes in outpatients | |||
| Hospitalizations | Bajaj et al, 201465 | Microbial patterns in stool and saliva predicted admissions | Cost, availability |
| Bajaj et al, 201528 | |||
| Bajaj et al, 201539 | |||
| Bajaj et al, 2020154 | |||
| Hepatic encephalopathy | Bajaj et al, 201465, | Microbial patterns in stool predicted readmissions for HE | Cost, availability |
| Bajaj et al, 201528 | |||
| Sung et al, 2019112 | |||
| Impact of medications | Loomba et al, 2020127 | Aldafermin (FGF19 analogue) use was associated with increase in Veillonella, chronic opioid use resulted in dysbiosis and associated endotoxemia. PPI use in cirrhosis resulted in oral-origin microbiota in the stool and was associated with higher readmissions. | |
| Acharya et al, 2017139 | |||
| Bajaj et al, 2018115 | |||
|
| |||
| Outcomes in inpatients | |||
| ACLF and organ failure | Bajaj et al, 201465 | Microbial patterns of dysbiosis and microbial metabolites, predicted mortality and development of ACLF | Cost, availability, knowledge of interpretation |
| Chen et al, 201597 | |||
| Bajaj et al, 201996 | |||
| Moreau et al, 2019100 Bajaj et al, 202049 | |||
| Sole et al, 202099 | |||
| Readmissions and death | Bajaj et al, 201465 | Dysbiosis was associated with mortality, predicted decompensation, and mortality in all etiologies of CLD. Cytolysin a fungal and bacterial cytotoxin was associated with severity of ALD but not with NASH. | Cost, availability |
| Chen et al, 201597 | |||
| Bajaj et al, 2018140 | |||
| Sung et al, 2019112 | |||
| Bajaj et al, 202049 | |||
| Sole et al, 202099 | |||
| Duan et al, 201952 | |||
| Lang et al, 2020141 | |||
AUC, area under the curve; FGF, fibroblast growth factor.
Hepatocellular carcinoma (HCC), which is a consequence of CLD of all etiologies, also bears a significant microbial imprint. A small study noted that irrespective of CLD etiology, HCC was associated with intestinal E coli overgrowth that predicts the presence of HCC with an receiver operating characteristic of 0.742.116 Another large study from China noted that HCC was associated with a reduction in butyrate-producing genera (eg, Alistipes), and pathogenic lipopolysaccharide-producing genera (eg, Klebsiella) were increased. Interestingly, the study noted an increased diversity in the microbiome in the patients with HCC.117 In NAFLD-related HCC, a study from Italy noted increased Bacteroides and Ruminococcaceae with reduced Bifidobacterium.118 There are very limited data available on the association of the microbiome with cholangiocarcinoma, with only 1 study showing enrichment of Stenotrophomonas in nonparasitic cholangiocarcinoma tumor tissue.119 These human studies show patterns of association, but have an admixture of patients with and without cirrhosis and focused on specific etiologies with modest differentiation within the cirrhosis with and without HCC. Therefore, the data are encouraging but not mature enough to replace current methods for screening for HCC.
Currently, due to lack of awareness and availability, use of microbiota as a diagnostic and prognostic test has not gained mainstream acceptance. A foreseeable logistical issue could be collection of a stool sample in the decompensated patient with cirrhosis. However, this can easily be bypassed by providing a salivary sample. A large multicenter study proved the potential benefits of using on-admission serum for analysis of microbially generated metabolites in predicting the development of ACLF and mortality, which was independent of all clinical factors.49 These findings need to be validated in other cohorts, but show that gut microbiota and their products could be a potential prognostic tool even in this extreme situation. Collection and storage of samples to send to outside institutions, along with potentially prolonged turnaround times, can be fraught with logistic challenges. The perception that microbiota testing is expensive, a barrier to clinical utility, has been addressed in a Markov model analysis that found significant cost savings by analyzing stool microbiota and recognizing higher-risk patients, compared with the costs of readmission, death, and liver transplantation.120
Microbe-Based Therapies
Microbiota manipulation in the management of CLD is evolving rapidly (Table 3). The relative contribution of these approaches will become clearer as more data from current trials become available. Probiotics, which are being explored in several studies in NAFLD to improve steatosis and fibrosis, have had mixed results.121 The studies were smallscale but randomized studies that used multiple different probiotic and synbiotic preparations and were summed up in a recent review.122 Notably, most of the preparations contain Lactobacillus species, which produce SCFAs and are beneficial to colonic health. A small pilot study of short-term (5 days) oral supplementation with Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 was associated with restoration of the bowel flora and greater improvement in alcohol-induced liver injury than standard therapy alone.123 Probiotics have shown potential in prevention of HE and even CLDs, as shown in Table 3.
Table 3.
Microbiome-Based Targeted Therapies
| Focus | Important Studies | Findings | Barriers |
|---|---|---|---|
| Direct microbiome therapies | |||
| Probiotics | Agrawal et al, 2012142 | Probiotics pre cirrhosis can improve NAFLD histology, in cirrhosis can reduce dysbiosis, and are effective for reversing secondary prevention of OHE, reducing hospitalizations for decompensating events, but may or may not improve cognition (MHE) | Duration of administration, standardization of formulations |
| Bajaj et al, 2014143 | |||
| Dhiman et al, 2014124 | |||
| Duseja et al, 2019121 | |||
| FMT to prevent disease | Philips et al, 2017144 | FMT was safe, reduced Proteobacteria and increased Actinobacteria post FMT, improved liver disease severity, reduced RR for death at 3 mo and improved survival at 1 y in severe AH. In chronic hepatitis B, FMT group showed microbial changes and reduced HBeAg. FMT increased diversity post FMT in all PSC patients with 3 of 10 experiencing a >50% reduction in ALP, reduction in shortterm alcohol craving and consumption, and AUD-related hospitalizations. | More data needed, expertise, cost, safety, and long-term consequences |
| Philips et al, 2018145 | |||
| Ren et al, 2017146 | |||
| Allegretti et al, 2019147 | |||
| Bajaj et al, 202082 | |||
| FMT to prevent decompensation | Bajaj et al, 2017148 | FMT increased diversity and beneficial taxa along with cognition on short-term follow-up. Brain function, hospitalizations, and cirrhosis-related decompensation, including OHE events remained low on long-term follow-up with persistent engraftment. | |
| Bajaj et al, 2017149 | Can be associated with infections if donors are not screened adequately. | ||
| Bajaj et al, 2019155 | |||
| Bajaj et al, 2019129 | |||
| DeFilipp et al, 2020150 | |||
|
| |||
| Microbiome-augmenting strategies | |||
| FXR/FGF19 analogue | Harrison et al, 2020151 | Aldafermin (FGF19 analogue) use for 24 wk resulted in improvement in NASH and fibrosis. | Still in trial phase |
| Dietary | Bajaj et al, 201875 | In the decompensated stage, Turkish patients had higher beneficial taxa, such as Ruminococcaceae and other Clostridiales, and a lower Enterococcaceae vs US-based patient. Vegetable use and Prevotellaceae, which increase with vegetable use were associated with protection from hospitalization in United States, Mexican and Turkish cohorts. | Standardization of diet, cultural differences, harmonization of diet in microbiota studies |
| Cox et al, 2020152 | High protein, high fermented milk products, and greater vegetables were associated with better outcomes | ||
| Bajaj et al, 202076 | |||
| Periodontal therapy/cleaning | Bajaj et al, 2018153 | In patients with HE there was reduction in Enterobacteriaceae and Streptococcaceae, increase in Ruminococcaceae in stool microbiome and reduction in cirrhosis salivary Pasteurellaceae and Streptococcaceae post periodontal cleaning. | Larger-scale data needed; dental coverage is limited or not emphasized. |
ALP, alkaline phosphatase; FGF, fibroblast growth factor; HBeAg, hepatitis B e antigen; OHE, overt hepatic encephalopathy; PSC, primary sclerosing cholangitis.
A well-known commercial probiotic preparation, previously known as VSL#3 (112.5 billion bacteria/capsule), which is now marketed in the United States as Visbiome.124,125 The study of the use of the formulation that is now marketed as Visbiome, to prevent complication of cirrhosis, focusing on HE showed a significant benefit in disease severity and a trend towards prevention of HE. Similarly, another commercial preparation available in most pharmacies is Lactobacillus rhamnosus GG. As all of the available trials used different preparations or different durations of study (short term for 1 week and some for longer between 8 weeks to 6 months) and none of these products have been scrutinized by the US Food and Drug Administration, we cannot advocate for a single preparation, and probiotics have not gained much favor in clinical practice. BA and microbial modulation using the FXR–FGF-19 axis is being studied extensively in NASH.126 There are several trials targeting the FXR–FGF-19 axis currently progressing in NASH and a recent study has demonstrated that Veillonella might be a marker of potential responders to an FGF19 analog Aldafermin.127 This is an exciting approach to potentially personalize treatments and select those enriched in biomarkers that predict study response in similar NASH studies.
FMT, which involves the transfer of donor stool into recipients, has been studied in small-scale studies for recurrent HE management, primary sclerosing cholangitis management, and in AH. The strongest current evidence comes from the management of HE, where oral capsules and rectal enemas were given in addition to standard of care (lactulose + rifaximin) in patients with recurrent HE. In the trial with FMT enemas, patients were given broad-spectrum antibiotics before FMT. Although the study showed improvement in cognition and improvement in bacterial diversity, a follow-up study noted that immediately post antibiotics, there was a collapse of microbial diversity.128 Hence, this might not be the best approach, and on a subsequent trial, the investigators did not follow this pathway. Long-term follow-up of the FMT patients given broad-spectrum antibiotics with rectal enemas found that on principal component analysis, there was a clustering of the long-term microbiota, with microbiota obtained at days 7 and 15 after FMT compared with pre-FMT microbiota, indicating that there was engraftment of the FMT long-term.129 Important FMT studies are documented in Table 3. Phase 1 studies in primary sclerosing cholangitis and AH have also confirmed the relative safety of this approach, even in more advanced patients. Despite the use of FMT for Clostridioides difficile clinically, its use in HE and cirrhosis requires additional precautions. A recent small study confirmed its efficacy in patients with CLD,130 and another study noted that patients with cirrhosis required more FMT treatments than those without cirrhosis.131 Once FMT is administered, patients need to be monitored closely short- and longer-term. Serious complications, such as infections from multidrug-resistant organisms, have been reported,132 prompting the US Food and Drug Administration to issue guidelines for monitoring patients and selecting donors. FMT should be used with caution in CLD and under appropriate regulatory supervision with careful donor selection.
Future Directions
There is growing evidence that the human microbiota has not only a potential strong imprint in causation, but also in progression and worsening of CLD. There remain several uncertainties (Table 4). Further advocacy towards microbiome-based testing and targeted therapies is needed. We need to work with industry to make this more accessible and cost-effective. Microbial testing needs to be adapted more widely, given its relative ease of collection and vast information that can be gathered with a single sample. These can help in diagnosing, prognosticating, and potentially personalizing therapeutic options. Multiple groups are currently enrolling patients in clinical FMT trials for prevention and treatment of CLD and as therapeutic options for preventing mortality from decompensation. The results of all these trials are eagerly awaited. Further microbial therapies should focus on oral–gut axis, targeted dietary interventions, exploring phage therapies beyond drug-resistant infections, and finessing treatments with defined microbial consortia. The approach of the future towards microbiome manipulation therefore needs to be a more proactive multidisciplinary and multifaceted one if we are to leverage the microbiome in our favor.
Table 4.
Uncertainties in Microbial Changes in Human Studies
| Uncertainty |
|---|
| Variations between studies with respect to depth of coverage, diet, ethnicity, sex, and etiology |
| Determination of whether microbiota cause or are a consequence of liver disease |
| Microbial function and metabolites need to be elucidated |
| Nonbacterial constituents: viruses, fungi, protists |
| Cost–benefit analysis and clinical translation |
Funding
Supported in part by Veterans Affairs Merit Review I0CX00176, National Center for Advancing Translational Sciences R21TR002024 and R21TR003095 to Jasmohan S. Bajaj.
Abbreviations used in this paper:
- ACLF
acute and chronic liver failure
- AH
alcoholic hepatitis
- ALD
alcohol use–associated liver disease
- AUD
alcohol use disorder
- BA
bile acid
- CLD
chronic liver disease
- DM
diabetes mellitus
- FMT
fecal microbiota transplantation
- GF
germ-free
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HE
hepatic encephalopathy
- MHE
minimal hepatic encephalopathy
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PPI
proton pump inhibitor
- PTSD
post-traumatic stress disorder
- SCFA
short-chain fatty acid
Biographies


Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70:151–171. [DOI] [PubMed] [Google Scholar]
- 2.Yoon Y, Chen CM. Surveillance Report No. 105. Liver Cirrhosis Mortality in the United States: National, State, and Regional Trends, 2000–2013. Washington, DC: National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research, Alcohol Epidemiologic Data System, 2016. [Google Scholar]
- 3.Trepo E, Romeo S, Zucman-Rossi J, et al. PNPLA3 gene in liver diseases. J Hepatol 2016;65:399–412. [DOI] [PubMed] [Google Scholar]
- 4.Dong XC. PNPLA3-A potential therapeutic target for personalized treatment of chronic liver disease. Front Med (Lausanne) 2019;6:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology 2014;146:147–156; quiz e15–e16. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen P, Vilstrup H, Andersen PK, et al. Socioeconomic status and survival of cirrhosis patients: a Danish nationwide cohort study. BMC Gastroenterol 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 8.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–735. [DOI] [PubMed] [Google Scholar]
- 9.Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia–Pacific region. Nat Rev Gastroenterol Hepatol 2019;16:57–73. [DOI] [PubMed] [Google Scholar]
- 10.Asrani SK, Kouznetsova M, Ogola G, et al. Increasing health care burden of chronic liver disease compared with other chronic diseases, 2004-2013. Gastroenterology 2018;155:719–729.e4. [DOI] [PubMed] [Google Scholar]
- 11.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol 2020;72:1003–1027. [DOI] [PubMed] [Google Scholar]
- 13.Acharya C, Bajaj JS. The microbiome in cirrhosis and its complications. Clin Gastroenterol Hepatol 2019;17:307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann P, Chu H, Duan Y, et al. Gut microbiota in liver disease: too much is harmful, nothing at all is not helpful either. Am J Physiol Gastrointest Liver Physiol 2019;316:G563–G573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue T, Nakayama J, Moriya K, et al. Gut dysbiosis associated with hepatitis C virus infection. Clin Infect Dis 2018;67:869–877. [DOI] [PubMed] [Google Scholar]
- 16.Preveden T, Scarpellini E, Milic N, et al. Gut microbiota changes and chronic hepatitis C virus infection. Expert Rev Gastroenterol Hepatol 2017;11:813–819. [DOI] [PubMed] [Google Scholar]
- 17.Heidrich B, Vital M, Plumeier I, et al. Intestinal microbiota in patients with chronic hepatitis C with and without cirrhosis compared with healthy controls. Liver Int 2018;38:50–58. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Wu Z, Xu W, et al. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol 2011;61:693–703. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Y, Chen S, Fu Y, et al. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat 2020;27:143–155. [DOI] [PubMed] [Google Scholar]
- 20.Kotredes KP, Thomas B, Gamero AM. The protective role of type I interferons in the gastrointestinal tract. Front Immunol 2017;8:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj JS, Sterling RK, Betrapally NS, et al. HCV eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Aliment Pharmacol Ther 2016;44:638–643. [DOI] [PubMed] [Google Scholar]
- 22.Ponziani FR, Putignani L, Paroni Sterbini F, et al. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther 2018;48:1301–1311. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601–609. [DOI] [PubMed] [Google Scholar]
- 24.Lelouvier B, Servant F, Paisse S, et al. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: a pilot analysis. Hepatology 2016;64:2015–2027. [DOI] [PubMed] [Google Scholar]
- 25.Sookoian S, Salatino A, Castano GO, et al. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut 2020;69:1483–1491. [DOI] [PubMed] [Google Scholar]
- 26.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054–1062.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj JS, Betrapally NS, Hylemon PB, et al. Gut microbiota alterations can predict hospitalizations in cirrhosis independent of diabetes mellitus. Sci Rep 2015;5:18559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte SMB, Stefano JT, Miele L, et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: a prospective pilot study. Nutr Metab Cardiovasc Dis 2018;28:369–384. [DOI] [PubMed] [Google Scholar]
- 30.Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 2014;111:E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830–839. [DOI] [PubMed] [Google Scholar]
- 32.Llorente C, Jepsen P, Inamine T, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun 2017;8:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinzone MR, Celesia BM, Di Rosa M, et al. Microbial translocation in chronic liver diseases. Int J Microbiol 2012;2012:694629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Li Y, Yan L, et al. Alterations of gut microbiome in autoimmune hepatitis. Gut 2020;69:569–577. [DOI] [PubMed] [Google Scholar]
- 35.Tang R, Wei Y, Li Y, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 2018;67:534–541. [DOI] [PubMed] [Google Scholar]
- 36.Kummen M, Holm K, Anmarkrud JA, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017;66:611–619. [DOI] [PubMed] [Google Scholar]
- 37.Ruhlemann MC, Heinsen FA, Zenouzi R, et al. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut 2017;66:753–754. [DOI] [PubMed] [Google Scholar]
- 38.Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016;65:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajaj JS, Betrapally NS, Hylemon PB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 2015;62:1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol 2020;72:558–577. [DOI] [PubMed] [Google Scholar]
- 41.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 2012;302:G966–G978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 1987;4:8–14. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res 2015;39:763–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kakiyama G, Hylemon PB, Zhou H, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol 2014;306:G929–G937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids 2014;86:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridlon JM, Kang DJ, Hylemon PB. Bile salt bio-transformations by human intestinal bacteria. J Lipid Res 2006;47:241–259. [DOI] [PubMed] [Google Scholar]
- 48.Ridlon JM, Kang DJ, Hylemon PB. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe 2010;16:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bajaj JS, Reddy KR, O’Leary JG, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute on chronic liver failure and death in patients with cirrhosis. Gastroenterology 2020;159:1715–1730.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McPhail MJW, Shawcross DL, Lewis MR, et al. Multivariate metabotyping of plasma predicts survival in patients with decompensated cirrhosis. J Hepatol 2016;64:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu H, Duan Y, Lang S, et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol 2020;72:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen YM, Liu Y, Zhou RF, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 55.De Chiara F, Thomsen KL, Habtesion A, et al. Ammonia scavenging prevents progression of fibrosis in experimental nonalcoholic fatty liver disease. Hepatology 2020;71:874–892. [DOI] [PubMed] [Google Scholar]
- 56.Hendrikx T, Schnabl B. Indoles: metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J Intern Med 2019;286:32–40. [DOI] [PubMed] [Google Scholar]
- 57.Kang DJ, Hylemon PB, Gillevet PM, et al. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol Commun 2017;1:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang DJ, Betrapally NS, Ghosh SA, et al. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology 2016;64:1232–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang AM, Inamine T, Hochrath K, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest 2017;127:2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bajaj JS, Liu EJ, Kheradman R, et al. Fungal dysbiosis in cirrhosis. Gut 2018;67:1146–1154. [DOI] [PubMed] [Google Scholar]
- 61.Lang S, Demir M, Martin A, et al. Intestinal Virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology 2020;159:1839–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang L, Lang S, Duan Y, et al. Intestinal virome in patients with alcoholic hepatitis [published online ahead of print July 12, 2020]. Hepatology doi: 10.1002/hep.31459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajaj JS, Sikaroodi M, Shamsaddini A, et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy [published online ahead of print September 30, 2020]. Gut doi: 10.1136/gutjnl-2020-322470. [DOI] [PubMed] [Google Scholar]
- 64.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bajaj JS, Hylemon PB, Ridlon JM, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 2012;303:G675–G685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011;54:562–572. [DOI] [PubMed] [Google Scholar]
- 68.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- 69.Aly AM, Adel A, El-Gendy AO, et al. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog 2016;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johari MI, Yusoff K, Haron J, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep 2019;9:11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020;69:1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson AJ, Zheng JJ, Kang JW, et al. A guide to diet-microbiome study design. Front Nutr 2020;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bajaj JS, Idilman R, Mabudian L, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 2018;68:234–247. [DOI] [PubMed] [Google Scholar]
- 76.Bajaj JS, Torre A, Rojas ML, et al. Cognition and hospitalizations are linked with salivary and faecal microbiota in cirrhosis cohorts from the USA and Mexico. Liver Int 2020;40:1395–1407. [DOI] [PubMed] [Google Scholar]
- 77.Makki K, Deehan EC, Walter J, et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018;23:705–715. [DOI] [PubMed] [Google Scholar]
- 78.Bear TLK, Dalziel JE, Coad J, et al. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv Nutr 2020;11:890–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novotny M, Klimova B, Valis M. Microbiome and cognitive impairment: can any diets influence learning processes in a positive way? Front Aging Neurosci 2019;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davis BC, Bajaj JS. Effects of alcohol on the brain in cirrhosis: beyond hepatic encephalopathy. Alcohol Clin Exp Res 2018;42:660–667. [DOI] [PubMed] [Google Scholar]
- 81.Butterworth RF. Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab Brain Dis 2016;31:1211–1215. [DOI] [PubMed] [Google Scholar]
- 82.Bajaj JS, Gavis E, Fagan A, et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder [published online ahead of print August 4, 2020]. Hepatology, doi: 10.1002/hep.31496. [DOI] [PubMed] [Google Scholar]
- 83.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 84.Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys 2013;536:189–196. [DOI] [PubMed] [Google Scholar]
- 85.Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385–1396. [DOI] [PubMed] [Google Scholar]
- 86.Saboo K, Shamsaddini A, Iyer MV, et al. Sex is associated with differences in gut microbial composition and function in hepatic encephalopathy [published online ahead of print July 15, 2020]. J Hepatol doi: 10.1016/j.jhep.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu R, Kang JD, Sartor RB, et al. Neuroinflammation in murine cirrhosis is dependent on the gut microbiome and is attenuated by fecal transplant. Hepatology 2020;71:611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bajaj JS, Fagan A, White MB, et al. Specific gut and salivary microbiota patterns are linked with different cognitive testing strategies in minimal hepatic encephalopathy. Am J Gastroenterol 2019;114:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bajaj JS, Sikaroodi M, Fagan A, et al. Post-traumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am J Physiol Gastrointest Liver Physiol 2019;317:G661–G669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bajaj JS, Sharma A, Dudeja PK. Targeting gut microbiome interactions in service-related gastrointestinal and liver diseases of veterans: meeting summary. Gastroenterology 2019;157:1180–1183.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hemmings SMJ, Malan-Muller S, van den Heuvel LL, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 2017;79:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011;108(Suppl 1):4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartosch S, Fite A, Macfarlane GT, et al. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 2004;70:3575–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bajaj JS, Duarte-Rojo A, Xie JJ, et al. Minimal hepatic encephalopathy and mild cognitive impairment worsen quality of life in elderly patients with cirrhosis. Clin Gastroenterol Hepatol 2020;18:3008–3016.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bajaj JS, Ahluwalia V, Steinberg JL, et al. Elderly patients have an altered gut-brain axis regardless of the presence of cirrhosis. Sci Rep 2016;6:38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajaj JS, Vargas HE, Reddy KR, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17:756–765.e3. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y, Guo J, Qian G, et al. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol 2015;30:1429–1437. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Zhao R, Shi D, et al. Characterization of the circulating microbiome in acute-on-chronic liver failure associated with hepatitis B. Liver Int 2019;39:1207–1216. [DOI] [PubMed] [Google Scholar]
- 99.Sole C, Guilly S, Da Silva K, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics. relationship with acute-on-chronic liver failure and prognosis [published online ahead of print September 14, 2020]. Gastroenterology doi: 10.1053/j.gastro.2020.08.054. [DOI] [PubMed] [Google Scholar]
- 100.Moreau R, Claria J, Aguilar F, et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol 2020;72:688–701. [DOI] [PubMed] [Google Scholar]
- 101.Maddrey WC, Boitnott JK, Bedine MS, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–199. [PubMed] [Google Scholar]
- 102.Thursz MR, Forrest EH, Ryder S, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;373:282–283. [DOI] [PubMed] [Google Scholar]
- 103.Smirnova E, Puri P, Muthiah MD, et al. Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology 2020;72:271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lang S, Fairfied B, Gao B, et al. Changes in the fecal bacterial microbiota associated with disease severity in alcoholic hepatitis patients. Gut Microbes 2020;12:1785251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Puri P, Liangpunsakul S, Christensen JE, et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology 2018;67:1284–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao B, Duan Y, Lang S, et al. Functional microbiomics reveals alterations of the gut microbiome and host co-metabolism in patients with alcoholic hepatitis. Hepatol Commun 2020;4:1168–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bajaj JS, Kakiyama G, Cox IJ, et al. Alterations in gut microbial function following liver transplant. Liver Transpl 2018;24:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Annavajhala MK, Gomez-Simmonds A, Macesic N, et al. Colonizing multidrug-resistant bacteria and the longitudinal evolution of the intestinal microbiome after liver transplantation. Nat Commun 2019;10:4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oh TG, Kim SM, Caussy C, et al. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab 2020;32:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lang S, Farowski F, Martin A, et al. Prediction of advanced fibrosis in non-alcoholic fatty liver disease using gut microbiota-based approaches compared with simple non-invasive tools. Sci Rep 2020;10:9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caussy C, Tripathi A, Humphrey G, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun 2019;10:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sung CM, Lin YF, Chen KF, et al. Predicting clinical outcomes of cirrhosis patients with hepatic encephalopathy from the fecal microbiome. Cell Mol Gastroenterol Hepatol 2019;8:301–318.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bajaj JS, Cox IJ, Betrapally NS, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol 2014;307:G951–G957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bajaj JS, Acharya C, Fagan A, et al. Proton pump inhibitor initiation and withdrawal affects gut microbiota and readmission risk in cirrhosis. Am J Gastroenterol 2018;113:1177–1186. [DOI] [PubMed] [Google Scholar]
- 116.Grat M, Wronka KM, Krasnodebski M, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant Proc 2016;48:1687–1691. [DOI] [PubMed] [Google Scholar]
- 117.Ren Z, Li A, Jiang J, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019;68:1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019;69:107–120. [DOI] [PubMed] [Google Scholar]
- 119.Chng KR, Chan SH, Ng AHQ, et al. Tissue Microbiome profiling identifies an enrichment of specific enteric bacteria in Opisthorchis viverrini associated cholangiocarcinoma. EBioMedicine 2016;8:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bajaj JS, Acharya C, Sikaroodi M, et al. Cost-effectiveness of integrating gut microbiota analysis into hospitalisation prediction in cirrhosis. GastroHep 2020;2:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duseja A, Acharya SK, Mehta M, et al. High potency multistrain probiotic improves liver histology in nonalcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol 2019;6:e000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie C, Halegoua-DeMarzio D. Role of probiotics in non-alcoholic fatty liver disease: does gut microbiota matter? Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kirpich IA, Solovieva NV, Leikhter SN, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 2008;42:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147:1327–1337.e3. [DOI] [PubMed] [Google Scholar]
- 125.Tursi A, Brandimarte G, Papa A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 2010;105:2218–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wahlstrom A, Sayin SI, Marschall HU, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 2016;24:41–50. [DOI] [PubMed] [Google Scholar]
- 127.Loomba R, Ling L, Dinh DM, et al. The commensal microbe Veillonella as a marker for response to an FGF19 analog in nonalcoholic steatohepatitis [published online ahead of print August 13, 2020]. Hepatology doi: 10.1002/hep.31523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bajaj JS, Kakiyama G, Savidge T, et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 2018;68:1549–1558. [DOI] [PubMed] [Google Scholar]
- 129.Bajaj JS, Fagan A, Gavis EA, et al. Long-term outcomes of fecal microbiota transplantation in patients with cirrhosis. Gastroenterology 2019;156:1921–1923.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meighani A, Alimirah M, Ramesh M, et al. Fecal microbiota transplantation for clostridioides difficile infection in patients with chronic liver disease. Int J Hepatol 2020;2020:1874570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pringle PL, Soto MT, Chung RT, et al. Patients with cirrhosis require more fecal microbiota capsules to cure refractory and recurrent Clostridium difficile infections. Clin Gastroenterol Hepatol 2019;17:791–793. [DOI] [PubMed] [Google Scholar]
- 132.DeFilipp Z, Bloom PP, Hohmann EL, et al. Drug-resistant bacteremia after fecal microbiota transplant. Reply. N Engl J Med 2020;382:1961–1962. [DOI] [PubMed] [Google Scholar]
- 133.Tuomisto S, Pessi T, Collin P, et al. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Santiago A, Pozuelo M, Poca M, et al. Alteration of the serum microbiome composition in cirrhotic patients with ascites. Sci Rep 2016;6:25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bajaj JS, Kakiyama G, Zhao D, et al. Continued alcohol misuse in human cirrhosis is associated with an impaired gut-liver axis. Alcohol Clin Exp Res 2017;41:1857–1865. [DOI] [PubMed] [Google Scholar]
- 136.Chen Y, Ji F, Guo J, et al. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep 2016;6:34055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Z, Zhai H, Geng J, et al. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol 2013;108:1601–1611. [DOI] [PubMed] [Google Scholar]
- 138.Ahluwalia V, Betrapally NS, Hylemon PB, et al. Impaired gut-liver-brain axis in patients with cirrhosis. Sci Rep 2016;6:26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Acharya C, Betrapally NS, Gillevet PM, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:319–331. [DOI] [PubMed] [Google Scholar]
- 140.Bajaj JS, Thacker LR, Fagan A, et al. Gut microbial RNA and DNA analysis predicts hospitalizations in cirrhosis. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lang S, Demir M, Duan Y, et al. Cytolysin-positive Enterococcus faecalis is not increased in patients with non-alcoholic steatohepatitis. Liver Int 2020;40:860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Agrawal A, Sharma BC, Sharma P, et al. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol 2012;107:1043–1050. [DOI] [PubMed] [Google Scholar]
- 143.Bajaj JS, Heuman DM, Hylemon PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 2014;39:1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Philips CA, Pande A, Shasthry SM, et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017;15:600–602. [DOI] [PubMed] [Google Scholar]
- 145.Philips CA, Phadke N, Ganesan K, et al. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol 2018;37:215–225. [DOI] [PubMed] [Google Scholar]
- 146.Ren YD, Ye ZS, Yang LZ, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 2017;65:1765–1768. [DOI] [PubMed] [Google Scholar]
- 147.Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am J Gastroenterol 2019;114:1071–1079. [DOI] [PubMed] [Google Scholar]
- 148.Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bajaj JS, Fagan A, Sikaroodi M, et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl 2017;23:907–914. [DOI] [PubMed] [Google Scholar]
- 150.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 2019;381:2043–2050. [DOI] [PubMed] [Google Scholar]
- 151.Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of aldafermin, an engineered fgf19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis [published online ahead of print August 8, 2020]. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 152.Cox IJ, Idilman R, Fagan A, et al. Metabolomics and microbial composition increase insight into the impact of dietary differences in cirrhosis. Liver Int 2020;40:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bajaj JS, Matin P, White MB, et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol 2018;315:G824–G837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bajaj JS, Torre A, Rojas ML, et al. Cognition and hospitalizations are linked with salivary and faecal microbiota in cirrhosis cohorts from the USA and Mexico. Liver Int 2020;40:1395–1407. [DOI] [PubMed] [Google Scholar]
- 155.Bajaj JS, Salzman NH, Acharya C, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology 2019;70:1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]


