Abstract
Fungal infections impact the lives of at least 12 million people every year, killing over 1.5 million. Wide-spread use of fungicides and prophylactic antifungal therapy have driven resistance in many serious fungal pathogens, and there is an urgent need to expand the current antifungal arsenal. Recent research has focused on improving azoles, our most successful class of antifungals, by looking for synergistic interactions with secondary compounds. Synergists can co-operate with azoles by targeting steps in related pathways, or they may act on mechanisms related to resistance such as active efflux or on totally disparate pathways or processes. A variety of sources of potential synergists have been explored, including pre-existing antimicrobials, pharmaceuticals approved for other uses, bioactive natural compounds and phytochemicals, and novel synthetic compounds. Synergy can successfully widen the antifungal spectrum, decrease inhibitory dosages, reduce toxicity, and prevent the development of resistance. This review highlights the diversity of mechanisms that have been exploited for the purposes of azole synergy and demonstrates that synergy remains a promising approach for meeting the urgent need for novel antifungal strategies.
Keywords: antifungal, azole, synergy, mycosis, resistance, Candida, dermatophytes, natural products
1. Introduction
1.1. The Burden of Fungal Disease
Fungal pathogens present an ever-increasing threat to global health. An estimated 1.5 million people are killed by fungal infections every year, and the incidence of several serious mycoses is growing [1,2]. It is likely that the global fungal burden is under-estimated, as several invasive fungal infections are under-reported in the developed world due to their association with other predisposing illnesses [3,4,5]. Australian clinics have recently seen a near-doubling of systemic candidaemia caused by drug-resistant Candida glabrata, with increasing rates of invasive candidaemia seen in Europe and the USA [6,7,8]. Candida auris, an emerging yeast pathogen infamous for its high tolerance to most important antifungals, has been the cause of several recent outbreaks, both before and during the COVID-19 pandemic [9,10,11,12]. Drug-resistant biofilms of various species of Candida have become increasingly responsible for fatal nosocomial infections [13]. Lethal infections with Aspergillus fumigatus and Cryptococcus sp. also remain a pressing concern, together causing an estimated 400,000 deaths per year, with chronic pulmonary aspergillosis severely affecting close to 3 million people [2]. Morbidity of non-life-threatening topical fungal infections is also increasing at an alarming rate. Cutaneous dermatophytosis affects 12 to 13 million people a year, and nail infections are extraordinarily difficult to treat, with less than 13% of cases fully resolved [14,15]. Decreasing susceptibility to topical antifungals has been observed in Exophiala dermatitidis and Malassezia sp. [15,16,17,18].
The increased incidence of emerging systemic, superficial, and cutaneous fungal pathogens has increased the demand for novel antifungal medications. However, despite the advances over the past four decades in bringing azoles and echinocandins to market, the currently available antifungals still operate via a limited number of mechanisms (Figure 1) and unfortunately all have significant problems, including toxicity, difficulty of administration, limited bioavailability and efficacy, and an often high cost [19,20,21].
Figure 1.
Mechanisms of action of commercially available antifungals. (a) Polyenes bind to ergosterol in the plasma membrane, forming pores that permit the efflux of vital small solutes like potassium ions and simple sugars [45]. (b) Echinocandins operate by inhibiting 1,3-β-D-glucan synthase in the fungal membrane, depriving the cell wall of glucans and therefore its structural integrity [46]. (c) Azoles inhibit Erg11 (lanosterol 14α-demethylase) preventing the biosynthesis of ergosterol and resulting in a build-up of toxic methyl-sterols that incorporate into the plasma membrane. The result is a loss of membrane structure and inhibition of growth [47]. (d) Allylamines inhibit ergosterol biosynthesis by antagonising squalene epoxidase Erg1, which converts squalene to squalene epoxide. As well as preventing the biosynthesis of ergosterol, this results in the build-up of squalene, which is deposited into lipid vesicles that disrupt the plasma membrane [48]. (e) Griseofulvin binds to tubulin in the fungal cell, preventing the formation of microtubules and arresting mitosis [49]. (f) 5-flucytosine is a pyrimidine analogue that is converted to 5-fluorouracil inside the cell. This fluoridated nucleotide is incorporated into mRNA, halting ribosomal processing and inhibiting protein translation. 5-fluorouracil also antagonises Cdc21, or thymidylate synthase, preventing the biosynthesis of thymidine nucleotides and inhibiting DNA synthesis [50].
Current therapies are also being increasingly compromised by antifungal resistance. Widespread over-use of agricultural fungicides appears to be driving cross-resistance in a range of pathogenic moulds and yeasts, and the use of prophylactic and over-the-counter azoles for superficial infections can promote the acquisition of resistance [22,23,24,25]. While recent innovations in anti-retroviral therapy have dramatically reduced the rates of fungal infection associated with HIV-AIDS, the case fatality rates of invasive fungal infections have remained constant [2,26,27]. Due to its propensity to up-regulate active efflux pumps, C. glabrata is an emerging cause of recurrent candidiasis, and multiple-drug resistance has been observed in an alarming proportion of clinical isolates of C. auris [28,29,30]. Clearly, there is an urgent and unmet need for new approaches to treat fungal infections [31].
1.2. Azole Antifungals
Arguably the most successful class of drug in the antifungal toolbox is the azoles. Azoles operate by binding to and inhibiting lanosterol 14α-demethylase, a fungal cytochrome P450 enzyme essential to the biosynthesis of ergosterol (Figure 1) [32]. First-generation triazoles, such as itraconazole and fluconazole, have low host toxicity, and the more important azoles are administered orally and have excellent pharmacodynamic properties [33]. Itraconazole and the second-generation triazole voriconazole have high respiratory bioavailability and fluconazole has superb neural pharmacokinetics, making it well suited as a maintenance therapy for cryptococcal meningitis. A promising new azole prodrug, isavuconazonium, was approved by the FDA in 2015, while a new generation of tetrazoles is currently in clinical and agrochemical development [34,35,36,37,38,39]. Older azoles like fluconazole are cheap, off-patent and widely available, including in developing countries where they are needed the most. The currently available prescription and over-the-counter azoles, and the mycoses for which they are commonly indicated, are detailed in Table 1.
Table 1.
Currently available azole antifungals and associated mycoses.
| Class | Application | Azole | Brand | Mycosis | Notes | Ref. |
|---|---|---|---|---|---|---|
| Imidazole | Topical | butoconazole | Gynazole-1, Mycelex-3 | uncomplicated and recurrent vaginal candidiasis | [65] | |
| climbazole | Squaphane, Pitiren | dandruff and seborrhoeic dermatitis caused by Malassezia sp. | [66] | |||
| clotrimazole † | Lotrimin | oral and vaginal candidiasis, and tinea versicolor, cruris and pedis | WHO Essential Medicine | [66,67] | ||
| eberconazole | Ebernet | cutaneous candidiasis and dermatophytosis | Approved in EU in 2015 | [68] | ||
| econazole | Spectrazole, Ecostatin | tinea pedis and cruris, vaginal candidiasis | Also repels clothes moths | [69] | ||
| flutrimazole | Flusporan, Topiderm | cutaneous dermatophytosis including tinea pedis | [70] | |||
| isoconazole | Icaden, Travogen | tinea pedis and vaginal candidiasis | Effective against Gram-positive bacteria | [71] | ||
| ketoconazole † | Nizoral | seborrhoeic dermatitis, dandruff, tinea and cutaneous candidiasis | Also systemic | [72] | ||
| luliconazole | Luzu | tinea pedis and cruris and other dermatophytoses | FDA-approved in 2013 | [73] | ||
| miconazole † | Monistat, Desenex | dermatophytosis and cutaneous, oral and vaginal candidiasis | WHO Essential Medicine | [74] | ||
| oxiconazole | Oxistat, Oxizole | dermatophytoses and cutaneous candidiasis | [75] | |||
| sertaconazole | Ertaczo, Dermofix | tinea pedis and vaginal candidiasis | Also anti-inflammatory and anti-pruritic | [76,77] | ||
| sulconazole | Exelderm | dermatophytoses | Also anti-carpet beetle | [78,79] | ||
| tioconazole | Vagistat-1 | onychomycosis, dermatophytoses and vaginal candidiasis | Also called thioconazole | [80] | ||
| Systemic | ketoconazole | Nizoral (oral) | mycoses caused by Candida, Histoplasma and Coccidioides | Systemic use for extreme cases only | [81] | |
| Triazole | Topical | efinaconazole | Jublia, Clenafin | onychomycosis | Low cure rate, but higher than other drugs | [82] |
| fluconazole † | Diflucan | dermatophytoses and cutaneous candidiasis | WHO Essential Medicine, more commonly systemic | [21] | ||
| terconazole | Terazol | acute and chronic vaginal candidiasis | [83] | |||
| Systemic | fluconazole † | Diflucan | candidiasis, cryptococcosis, histoplasmosis, blastomycosis | WHO Essential Medicine, oral or intravenous | [21] | |
| fosfluconazole | Prodif | prophylaxis in the immunocompromised | Fluconazole prodrug | [84,85] | ||
| fosravuconazole | Nailin | onychomycosis | Ravuconazole prodrug | [86] | ||
| isavuconazonium | Cresemba | mucormycosis and invasive aspergillosis | Isavuconazole prodrug | [35,87] | ||
| itraconazole † | Sporanox, Orungal | aspergillosis, histoplasmosis, coccidioidomycosis and blastomycosis | WHO Essential Medicine | [48,88] | ||
| posaconazole | Noxafil, Posanol | invasive candidiasis, aspergilosis, mucormycosis and scedosporiosis | FDA-approved in 2006 | [89] | ||
| voriconazole † | Vfend | aspergillosis, candidiasis, penicilliosis, histoplasmosis and fusariosis | WHO Essential Medicine | [90] |
† Most commonly prescribed azole antifungals. Other azole antifungals no longer on the market include ravuconazole, a triazole similar to voriconazole which was discontinued after Phase-III clinical trials and the thiazole abafungin, which is no longer available [91].
Azoles are a vital tool in the fight against fungal infection. It has been projected that the antifungal market will be USD 13 billion by 2026, with roughly 2.4 billion of that allocated to azoles: USD 1.4 billion in oral and intravenous drugs, and USD 1 billion in topical and over-the-counter solutions [40,41]; however, azoles have limitations. Many filamentous fungal pathogens are not sensitive to azoles, particularly fluconazole [33]. Second-generation triazoles like voriconazole can be toxic to the host [42]. Azoles are often used prophylactically in neutropenic and transplant patients, but this can encourage the development of resistance [23,43]. Azole-based fungicides are used in agriculture on a massive scale, and cross-resistance between these and azole antifungals is seen with alarming frequency [22]. Candida auris particularly represents an escalating threat to the usefulness of azoles, as it is frequently highly azole-tolerant and often multi-drug resistant [44].
There are various mechanisms of resistance to azole antifungals. Mutations in ERG11 (also known as CYP51), which encodes the target enzyme lanosterol 14α-demethylase, can prevent enzyme binding, or ERG11 expression can be up-regulated via changes to its promoter or regulating transcription factors or via gene duplication and aneuploidy [51]. Azoles can be actively excluded from the cell via membrane bound ABC and MFS efflux pumps [52,53,54], which can be increased in expression via aneuploidy or by alterations to transcription factors leading to constitutive expression. Mutations in ERG3 have also been found to increase resistance, thought to be via prevention of the build-up of toxic intermediates [55,56,57,58,59,60,61]. Frequently, these resistance mechanisms lead to cross-resistance across azole drug types, and although the research community is working to derive new azole antifungals by modelling lanosterol 14α-demethylase crystal structures [62,63,64], the increased resistance seen in clinical strains can undermine their use as a monotherapy.
1.3. Antimicrobial Synergy
Recent expiries in patent protection of early-generation azoles have led to generic alternatives for voriconazole, fluconazole, posaconazole and efinaconazole entering the market and have made space for innovations. There is a particular increasing interest in enhancing the antifungal activity of current azoles using drug synergy.
Synergy occurs when two compounds produce an increased inhibitory effect beyond what would be expected by adding the effects of the compounds individually [92]. Significant synergy is determined by the Fractional Inhibitory Concentration Index (FICI), which is calculated as the sum of the ratios of the Minimum Inhibitory Concentration (MIC) of the drugs when used alone and together, according to the following equation:
When the FICI of two drugs is ≤0.5 their interaction is considered synergistic, and when this is >4 it is considered antagonistic. An FICI between 0.5 and 4 is considered indifferent [92].
Antimicrobial synergy can overcome resistance and lower inhibitory dosages to within clinically achievable levels [93,94]. Synergy can expand the spectra of activity of the individual drugs, making azoles a viable option against pathogens that may be otherwise resistant. Antimicrobial synergy can also make otherwise fungistatic drugs fungicidal, including many azoles [95,96].
Antimicrobial synergy has been exploited in the clinic for years for the treatment of HIV and malaria [97,98], and the most successful induction therapy for cryptococcal meningitis is a synergistic combination of amphotericin B and 5-flucytosine. Synergy between two drugs can be potentiated by their co-operation on multiple enzymes belonging to the same pathway; for example, sulfamethoxazole and trimethoprim are antibacterial agents that target the folic acid biosynthesis pathway at two different sites to synergistically improve both the spectrum and potency of bacterial inhibition [99]. Synergy can also be produced between drugs that inhibit different processes: for example, amphotericin B damages the fungal plasma membrane and 5-flucytosine interrupts translation and replication [100,101].
1.4. Aims and Scope of This Review
The mechanism of action of azoles is well understood (Figure 1) and various synergists have been described that operate on related (Figure 2) or quite disparate (Figure 3) pathways or mechanisms. In this review, we describe recent studies that have reported azole synergists. There are several excellent earlier reviews that have considered aspects of azole synergy [52,102,103]; here we provide an update with a particular focus on developments made over the past six years. Table 2 provides an overview of these findings presented as a heatmap, with the extent of synergy demonstrated between commonly used azoles and secondary agents in blue, and the spectrum of fungi found to be affected in yellow. Below we explore each of the different classes of synergists and their potential as combination therapies.
Figure 2.
Proposed mechanism of synergy between azoles and inhibitors that operate on the ergosterol biosynthesis and mevalonate pathways. In fungi, the synthesis of ergosterol occurs primarily in the endoplasmic reticulum, with the final product packaged into vesicles to be incorporated into the membrane [268]. Azole drugs inhibit Erg11, preventing lanosterol from being converted into dimethyltrienol and leading to the build-up of toxic methyl sterols. These are incorporated into the membrane instead of mature ergosterol, causing a loss of membrane structure and an inability to divide and resulting in the fungistatic arrest of growth [47]. Synergistic inhibitors co-operate at points up- and down-stream of Erg11, increasing the generation of toxic ergosterol precursors and other terpene-derived metabolites. The mevalonate pathway, upstream from the ergosterol biosynthesis pathway, is responsible for the biosynthesis of squalene, a precursor to all fungal membrane sterols. Statins like atorvastatin inhibit the HMG-CoA reductases Hmg1 and Hmg2, which are responsible for the production of mevalonate from HMG-CoA [269]. Further downstream, bisphosphonates like zoledronate inhibit farnesyl pyrophosphate synthase, or Erg20, which catalyses the production of farnesyl pyrophosphate from dimethylallyl pyrophosphate [270]. In the ergosterol biosynthesis pathway, squalene is converted into squalene epoxide by Erg1, a squalene epoxidase, which can be inhibited by allylamines like terbinafine [271]. Downstream from Erg11, dimethyltrienol is converted again into dimethylzymosterol by Erg24, a sterol reductase that is inhibited by morpholine antifungals such as amorolfine [47,272]. The resulting destabilisation of the cell membrane means synergy can often produce a fungicidal effect in the pathogen.
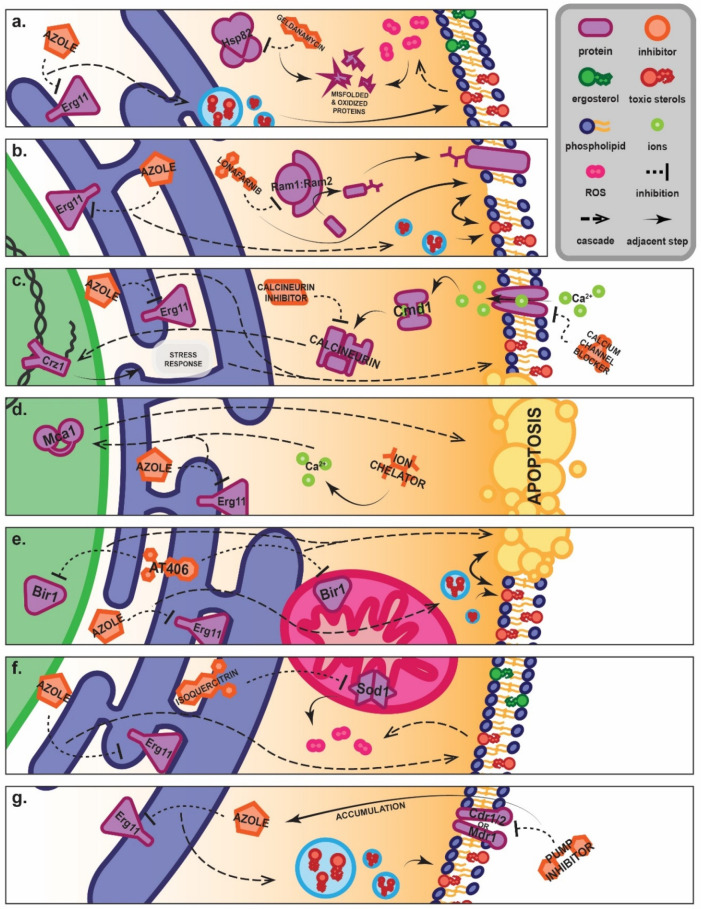
Figure 3.
Proposed novel mechanisms of synergy between azoles and inhibitors that operate on entirely separate pathways. (a) HSP82 inhibitors like geldanamycin prevent the association of Hsp82 with proteins, inhibiting proper folding of nascent proteins and degradation of senescent proteins. Accumulation of toxic oxygen radicals results in oxidation of proteins, which would ordinarily be degraded. HSP82 inhibitor–azole synergy therefore appears to rise from the accumulation of oxidatively damaged, toxic proteins [195]. (b) Inhibition of protein farnesylation by farnesyltransferase (Ram1:Ram2) inhibitors such as lonafarnib results in reduced translocation of membrane-bound proteins. This decline in the population of membrane proteins combines synergistically with the azole-induced build-up of toxic sterols, resulting in increased membrane instability [199]. (c) Calcium channel blockers and calcineurin inhibitors prevent the activation of the calcineurin complex by calmodulin (Cmd1). This results in an inability of calcineurin to dephosphorylate Crz1, which would ordinarily mobilise it to the nucleus. Crz1 is a transcription factor responsible for the regulation of several stress-related genes. Calcium channel blockers and calcineurin inhibitors therefore impair the cellular stress response, sensitising the cell to the antifungal effect of azoles [182,280,296]. (d) Ion chelators like DIBI and D-penicillamine bind to ions and disrupt cellular ion homeostasis. Evidence suggests that it is the disturbance of calcium homeostasis that results in the promotion of metacaspase (Mca1)-dependent apoptosis when paired with azoles, synergistically enhancing the fungicidal effect [202,253]. (e) AT406 is an antagonist of the inhibition of apoptosis proteins (IAPs) such as Bir1, which is present in both the mitochondrion and the nucleus. There is evidence that membrane weakness due to toxic sterol build-up improves the pro-apoptotic effects of AT406 [256]. (f) Some novel synergists, such as isoquercitrin, have demonstrated the ability to inhibit mitochondrial superoxide dismutase, Sod1. Sod1 becomes unable to neutralise harmful reactive oxygen species that accumulate during azole treatment, resulting in rapid accumulation of radicals and potentiating a toxic oxidative effect [200]. (g) Direct and indirect inhibitors of both ABC transporters such as Cdr1 and MFS transporters such as Mdr1 prevent the active efflux of toxic compounds such as fluconazole out of the cell, resulting in an accumulation of the drug and extending its antifungal effect. In turn, the destabilised membrane may reduce or prevent incorporation of transmembrane proteins including pumps, further reducing the efflux capabilities of the cell [173,183,240].
Table 2.
Interactions between azoles and synergists and their spectrum of activity described in published studies.
| Azole Synergy | Synergy in % Strains Tested | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Synergist | Fluconazole | Itraconazole | Voriconazole | Isavuconazole | Posaconazole | Ketoconazole | Miconazole | C. albicans | AR Candida | A. fumigatus | AR Aspergillus | Dermatophytes | Other | Notes 1 | Ref. |
| Antimicrobials | ||||||||||||||||
| Antifungals | terbinafine | Incl. Scedosporium sp. and Pythium sp. | [104,105,106,107,108,109,110,111,112] | |||||||||||||
| caspofungin | Dep. on mechanism of resistance | [113,114,115,116] | ||||||||||||||
| anidulafungin | [114,117,118] | |||||||||||||||
| micafungin | Incl. C. auris | [115,116,118] | ||||||||||||||
| natamycin | Incl. Fusarium sp. | [119] | ||||||||||||||
| ciclopirox | [120] | |||||||||||||||
| flucytosine | Incl. C. auris | [121] | ||||||||||||||
| voriconazole | Limited synergy in the Mucorales | [122] | ||||||||||||||
| amorolfine | Onychomycosis clinical trials | [123,124,125,126] | ||||||||||||||
| K20 | Also clotrimazole, incl. C. neoformans | [127] | ||||||||||||||
| oxadiazole pept. | [128] | |||||||||||||||
| Antibacterials | sulfamethoxazole | Incl. C. auris | [129,130] | |||||||||||||
| sulfa- antibiotics | Some effective vs. biofilms and in vivo | [130] | ||||||||||||||
| doxycycline | Effective against biofilms and in vivo | [131,132,133] | ||||||||||||||
| tigecycolline | Incl. Fusarium sp., limited anti-biofilm | [132,134,135] | ||||||||||||||
| minocycline | Incl. C. neoformans, Scedosporium sp. | [133,136,137,138,139] | ||||||||||||||
| gentamicin | Effective against biofilms | [140] | ||||||||||||||
| linezolid | Little synergy, but reduced dosage | [141] | ||||||||||||||
| polymyxin B | Incl. Rhodotorula and Lichtheimia sp. | [142] | ||||||||||||||
| colistin | Incl. C. auris | [96,143,144] | ||||||||||||||
| Antiparasitics | pyrvinium pam. | [145,146] | ||||||||||||||
| chloroquine | [147] | |||||||||||||||
| artemisinins | Effective against biofilms | [146] | ||||||||||||||
| INK128 | Incl. Fusarium and Exophiala sp. | [148] | ||||||||||||||
| mefloquine | Incl. C. neoformans | [149] | ||||||||||||||
| Antivirals | saquinavir | Incl. Histoplasma capsulatum | [150] | |||||||||||||
| ritonavir | Incl. Histoplasma capsulatum | [150] | ||||||||||||||
| adamantanamine | Switch from fungistatic to fungicidal | [151] | ||||||||||||||
| ribavirin | Effective against biofilms and in vivo | [152] | ||||||||||||||
| lopinavir | Incl. C. auris | [153] | ||||||||||||||
| Efflux Inhibitors | ||||||||||||||||
| Calcium Inhibitors | tetrandrine | Effective in vivo | [154,155,156,157] | |||||||||||||
| verapamil | Dep. on mechanism of resistance | [158,159] | ||||||||||||||
| Other | eucalyptal D | Natural product | [160] | |||||||||||||
| dodenoic acid | [161] | |||||||||||||||
| azoffluxin | Incl. C. auris | [162] | ||||||||||||||
| ospemifeme | Incl. C. neoformans and C. auris | [163] | ||||||||||||||
| phialocephalarin | [164] | |||||||||||||||
| palmarumycin P3 | [164] | |||||||||||||||
| geraniol | Effective in vivo, natural product | [165] | ||||||||||||||
| Repurposed Drugs | ||||||||||||||||
| Statins | lovastatin | Incl. Rhizopus sp. | [166,167,168] | |||||||||||||
| atorvastatin | Incl. Rhizopus sp., Cryptococcus sp. | [166,167,169] | ||||||||||||||
| fluvastatin | Incl. Rhizopus sp. | [166,167] | ||||||||||||||
| simvastatin | Incl. Rhizopus sp., Cryptococcus sp. | [166,167,170] | ||||||||||||||
| pitavastatin | [171] | |||||||||||||||
| Bisphosphonates | risedronate | Incl. Cryptococcus sp. | [172] | |||||||||||||
| alendronate | Incl. Cryptococcus sp. | [172] | ||||||||||||||
| zoledronate | Incl. Cryptococcus sp. | [172] | ||||||||||||||
| Immunomodulators | promethazine | [173,174] | ||||||||||||||
| terfenadine | Effective against biofilms | [175] | ||||||||||||||
| ebastine | Effective against biofilms | [175] | ||||||||||||||
| dexamethasone | Effective against biofilms | [176] | ||||||||||||||
| budesonide | Effective in vivo | [177] | ||||||||||||||
| methotrexate | [178] | |||||||||||||||
| Psychoactives | bromperidol | [179] | ||||||||||||||
| fluoxetine | Effective in vivo, C. albicans only | [180] | ||||||||||||||
| haloperidol | [173] | |||||||||||||||
| sertraline | Incl. Trichosporon asahii | [181] | ||||||||||||||
| Calcineurin Inhibitors | cyclosporine | Incl. S. cerevisiae | [182,183,184,185,186,187] | |||||||||||||
| tacrolimus | Incl. S. cerevisiae | [183,188,189,190,191,192,193] | ||||||||||||||
| Other | PPIs | Proton pump inhibitors | [194] | |||||||||||||
| geldanamycin | [195] | |||||||||||||||
| ponatinib | Incl. C. neoformans | [196] | ||||||||||||||
| HSP990 | Incl. C. neoformans | [197] | ||||||||||||||
| givinostat | [198] | |||||||||||||||
| lonafarnib | Incl. E. dermatitidis | [199] | ||||||||||||||
| isoquercitrin | [200] | |||||||||||||||
| EDTA | Incl. C. deuterogattii | [201] | ||||||||||||||
| D-penicillamine | Copper ion chelator | [202] | ||||||||||||||
| licofelone | Effective against biofilms | [203] | ||||||||||||||
| phenylbutyrate | [204] | |||||||||||||||
| 17-AAG | Incl. E. dermatitidis | [205] | ||||||||||||||
| ketamine | [206] | |||||||||||||||
| ibuprofen | [207] | |||||||||||||||
| chlorhexidine | Incl. C. auris | [208] | ||||||||||||||
| ganetespib | [209] | |||||||||||||||
| HMA | Incl. Cryptococcus sp. | [210] | ||||||||||||||
| Natural Products | ||||||||||||||||
| Ess. Oil Extracts | thymol | [211] | ||||||||||||||
| carvacrol | Effective against biofilms of C. auris | [211,212] | ||||||||||||||
| acetophenone | [213] | |||||||||||||||
| osthole | [214] | |||||||||||||||
| houttuyfonate | [215] | |||||||||||||||
| menthol | [216] | |||||||||||||||
| tyrosol | Effective against biofilms | [217] | ||||||||||||||
| allyl isothiocyan. | Effective against biofilms | [218] | ||||||||||||||
| butylphthalide | Effective against biofilms | [219] | ||||||||||||||
| glabridin | [220] | |||||||||||||||
| chito-oligosacch. | [221] | |||||||||||||||
| oridonin | [222] | |||||||||||||||
| Crude Ess. Oils | sea-buckthorn | [223] | ||||||||||||||
| guava leaf | [224] | |||||||||||||||
| frankincense | [225] | |||||||||||||||
| TTO | [226] | |||||||||||||||
| Alkaloids | berberine | Incl. S. cerevisiae and T. marneffei | [227,228,229,230,231,232] | |||||||||||||
| palmatine | Effective against biofilms | [233,234] | ||||||||||||||
| harmine | [235] | |||||||||||||||
| Other Terpenoids | guttiferone | [236] | ||||||||||||||
| farnesol | Effective against C. auris biofilms | [237] | ||||||||||||||
| asiatic acid | Effective in vivo | [238] | ||||||||||||||
| Other Phenols | magnolol | [239] | ||||||||||||||
| diorcinol | Extreme decrease in required dosage | [240] | ||||||||||||||
| proanthocyan. | [241] | |||||||||||||||
| epigallocatechin | Effective against biofilms | [242] | ||||||||||||||
| asarone | Also clotrimazole | [243] | ||||||||||||||
| pyrogallol | [244] | |||||||||||||||
| Peptides | lactoferrin | Other incl. Cryptococcus sp. | [201,245] | |||||||||||||
| beauvericin | [246] | |||||||||||||||
| Novel Compounds | ||||||||||||||||
| ATTAF-1 and -2 | Novel azole derivatives | [247] | ||||||||||||||
| 31 and 42 | Novel azole derivatives | [248] | ||||||||||||||
| 15 and 24 | Isoquinolone and phthalazinone deriv. | [249] | ||||||||||||||
| B-7b | Novel berberine derivative | [250] | ||||||||||||||
| LQFM-79-81 | Novel guttiferone-A derivatives | [236] | ||||||||||||||
| phenylpentanol | Novel phenylpentanol derivatives | [171] | ||||||||||||||
| AR-12 | Novel celecoxib derivative | [251] | ||||||||||||||
| SCY-078 | Glucan synthase inhibitor | [252] | ||||||||||||||
| DIBI | Ion chelator | [253] | ||||||||||||||
| 1 – 34c | Novel caffeic acid derivative | [254] | ||||||||||||||
| chalcones | [255] | |||||||||||||||
| AT406 | IAP Inhibitor, incl. E. dermatitidis | [256] | ||||||||||||||
| B2 | Piperidone derivative, incl. C. neoformans | [257] | ||||||||||||||
| H1-J10 | Novel HSP90/HDAC inhibitors | [258] | ||||||||||||||
| L1-C2 | Novel lipopeptides | [259] | ||||||||||||||
| AZD8055 | Novel TOR inhibitor | [260] | ||||||||||||||
| KEY: | extremely strong synergy | synergistic in all strains tested | ||||||||||||||
| strong synergy | synergistic in >20% strains tested | |||||||||||||||
| weak synergy | synergistic in <20% strains tested | |||||||||||||||
| borderline synergy | not synergistic in any strains tested | |||||||||||||||
1. “Incl.” refers to species included under “Other”.
2. Synergy between Azoles and Currently Available Antimicrobials
Existing antimicrobial pharmaceuticals that have been proven safe or tolerable for humans and have approval from regulatory bodies such as the FDA make an attractive starting point for antifungal synergy. Theoretically, combining antimicrobials might broaden their activity spectrum to include pathogens that are not susceptible to either drug as a monotherapy [261]. Although combining azoles with other classes of antifungals could be expected to often give synergy, certain antibacterial, antiparasitic and antiviral drugs have also been shown to interact synergistically.
2.1. Azole-Antifungal Synergy
Numerous antifungals, including azoles, allylamines and morpholines, target different points of the ergosterol biosynthesis pathway, as summarised in Figure 2. Given that the mechanisms of action of these drugs are often closely related, they are prime targets for investigation as potential azole synergists. Terbinafine is an allylamine that has been investigated across a broad array of azoles and various fungal and oomycete pathogens and was found to synergise with some systemic azoles (Table 2), particularly against azole-resistant C. albicans isolates. A combined treatment strategy using terbinafine and fluconazole was also shown to resolve persistent oropharyngeal thrush in a clinical setting [104,105,106]. For other species of Candida, however, terbinafine-azole synergy is weaker and only seen in azole-sensitive isolates, although the combination shifts inhibition from fungistatic to fungicidal [107]. Terbinafine and azoles have proven effective against clinical isolates of Scedosporium prolificans, a hard-to-treat pathogen of the lungs, sinuses and brain, at clinically achievable concentrations [108,109]. For other pathogens, terbinafine–azole synergy is narrow in spectrum, with only a very few isolates of Aspergillus, azole-resistant non-albicans Candida species, azole-resistant dermatophytes and Pythium insidiosum, (a fungus-like oomycete that is the cause of often-fatal pythiosis) affected [106,107,110,111,262,263].
Antifungals from the echinocandin class, which act by weakening the cell wall, were considered potentially attractive as azole synergists when they first became available; however, for most combinations, the pairs either synergise strongly but in a select subset of isolates, or work across a global spectrum of isolates but with only weakly synergistic interactions (Table 2) [113,114,115,116,117]. One particularly promising pair is micafungin and voriconazole, which strongly inhibits azole- and echinocandin-resistant Candida auris, bringing the required dosages of both to well within clinically achievable levels [115]. In a recent survey, anidulafungin, caspofungin and micafungin all displayed strong synergy with isavuconazole in C. auris [118]. Given the extreme level of resistance to azoles demonstrated by many C. auris isolates and the threat of emerging resistance to echinocandins, which are currently the front-line antifungal for C. auris infection, these combinations may warrant immediate clinical application [264,265,266].
Broadly speaking, most other market antifungals have demonstrated weak or no synergy with azoles. The topical polyene natamycin weakly synergises with voriconazole against a narrow range of azole-susceptible pathogens, while ciclopirox synergises strongly with itraconazole but only in a small number of dermatophytes [119,120]. Flucytosine and even voriconazole have been tested with other azoles but were indifferent or only weakly synergistic for various fungal species, including C. auris and pathogens in the order Mucorales [121,122]. Amorolfine, however, which acts on the ergosterol biosynthesis pathway subsequent to azoles (Figure 2) displayed more promise, synergising with systemic and topical azoles against dermatophytes in vitro, and demonstrating efficacy in an open randomised clinical trial of notoriously refractive onychomycosis [123,124,125]. While promising, this combined treatment strategy for topical fungal infections is yet to make it to market.
A few recently developed novel antifungals that are not yet available commercially appear promising as azole synergists. K20, a novel fungus-specific amphiphilic aminoglycoside that inhibits Fusarium spp. and a variety of yeast pathogens, displayed strong synergy with a wide variety of systemic azoles for almost all Candida isolates tested [127,267]. Oxadiazole-tagged macrocyclic peptides, which are capable of inhibiting azole-resistant C. glabrata and C. tropicalis strains also interacted synergistically with fluconazole, but with less synergy and a narrower spectrum of activity than K20 (Table 2) [128].
2.2. Azole–Antibacterial Synergy
Among the antibacterials, the sulfa-based drugs have shown the greatest potential to date for azole synergy (Table 2). These inhibit folic acid biosynthesis and several, including the widely available antibacterial sulfamethoxazole, have displayed strong synergy with azoles in most azole-resistant Candida isolates, including isolates of C. auris [129,130]. Tetracycline-based antibacterial agents have demonstrated synergy with azoles specifically against azole-resistant pathogens (Table 2). Doxycycline, tigecycline and minocycline could all potentially be used to improve therapy with azoles for persistent cases of candidiasis, aspergillosis and fusariosis [131,132,133,134,135,136,137]. Minocycline in particular has recently demonstrated potential as an itraconazole synergist in C. neoformans and Scedosporium sp. [138,139]. This synergy has been consistently reproduced in in vivo infection models and against sessile forms of Candida, with some demonstrating efficacy against biofilms. Colistin is a last-resort antibiotic that has recently displayed promising synergy in vitro with isavuconazole in Aspergillus spp. and Candida auris, and in vivo in Candida albicans [93,96,143,144,252,253].
Other antibacterial compounds have failed to display any real potential as combined antifungal therapies. Gentamicin was synergistic with fluconazole in biofilms produced by some resistant Candida species. Linezolid failed to produce true synergy with any azoles tested but it did reduce the required dosage of both drugs to a clinically achievable level for a limited spectrum of fungi (Table 2) [140,141]. While reducing the dose is one of the main goals when developing novel combination treatments, in the wake of more viable therapeutic leads it seems unlikely that drugs like linezolid will see further development. Furthermore, the in vivo use of voriconazole and clarithromycin, another antibacterial found to be synergistic in vitro, was found to cause acute kidney damage, illustrating the potential for undesirable consequences when exploiting antimicrobial synergy [273].
2.3. Azole–Antiparasitic Synergy
Only a limited number of antiparasitic compounds have been explored for azole synergy in recent years, but some of them show promise. Chloroquine and artemisinin interacted synergistically with azoles against azole-resistant Candida strains, while pyrvinium pamoate–azole synergy was observed in a broad suite of dermatophytes [145,146,147,148]. Mefloquine and related compounds displayed limited synergy in C. neoformans, but did potentiate a strong fungicidal effect when combined with fungistatic fluconazole [149].
2.4. Azole–Antiviral Synergy
Antivirals have recently been investigated as potential antifungal synergists, with the anti-retrovirals showing the most promise. Saquinavir and ritonavir were effective at synergistically cooperating with azoles against Histoplasma, a systemic fungal pathogen [150]. Other antivirals like ribavirin and 2-adamantanine have shown promise at treating biofilms of azole-resistant C. albicans and potentiating the antifungal activity of azoles from fungistatic to fungicidal, respectively [151,152]. Lopinavir is an antiviral that shows extremely strong synergy with voriconazole in a majority of azole-resistant C. auris strains, and certainly warrants further investigation [153].
3. Active Efflux Modulators
The active, ATP-dependent transport of antimicrobial compounds out of the fungal cell is one of the most concerning mechanisms of antifungal resistance emerging today. It is the principal mechanism of azole tolerance in C. glabrata and in a significant portion of C. auris and azole-resistant C. albicans isolates [44,274,275]. There is also evidence that active efflux is responsible for azole resistance in some Aspergillus species and dermatophytes [276,277]. Due to the importance of pumps for resistance in bacterial pathogens and malarial parasites, inhibition of active efflux is a popular target in antimicrobial discovery and the development of combined antifungal treatments [278,279].
Most efflux pump inhibitors that have been investigated as azole synergists interact directly with the membrane-bound pump. Several drugs that affect the intracellular homeostasis of calcium by blocking calcium channels have been found to have some affinity for membrane-bound transporters, particularly tetrandrine and verapamil. Traditionally used as immunomodulators and vasodilators, these have produced antifungal synergy with a variety of azoles. Tetrandrine combined with posaconazole has been proposed as an effective solution for persistent and temporary candidiasis [154,155,156,157,158,159,280]. Eucalyptal D and dodecenoic acid are essential oil extracts that have been shown to directly antagonise ABC transporters [160,161]. These exhibited strong synergy with fluconazole and itraconazole, respectively, in azole-resistant Candida. Azoffluxin, an oxindole Cdr1 inhibitor, has been shown to synergise strongly with fluconazole in all non-clade III C. auris and azole-resistant C. albicans strains, both in vitro and in vivo [162]. Other receptor antagonists may be cross-reactive to membrane-bound transporters; for example, ospemifeme is a promising therapeutic lead that has a broad spectrum of activity and synergises very potently with fluconazole (Figure 3g) [159,163].
Several efflux inhibitors that show promising synergy with azoles do not directly bind pump proteins but interfere with efflux through other mechanisms. Palmarumycin P3 and phialocephalarin B are naturally occurring quinone derivatives that synergise with fluconazole in pump-dependent, azole-resistant C. albicans. It appears that the mechanism of synergy is due to the ability of these derivatives to directly inhibit nuclear transcription factors, modulating the expression of the principal pump-coding gene MDR1 [164]. Geraniol is a unique synergist that causes the localisation of pumps to become dysregulated, preventing them from being incorporated into the membrane and resulting in weak synergy [165]. The controversial anti-cancer drug ponatinib is able to inhibit active efflux in multiple yeast pathogens by interfering with the proton motive force, producing strong synergy with fluconazole in all strains tested [196]. Other potential co-drugs like cationic triphenylphosphonium have been shown to improve the activity of efflux pump inhibitors, suggesting the potential development of a triple-drug antifungal treatment strategy [281].
Many other compounds currently being investigated for their ability to synergise with azoles promote the downstream inhibition of efflux, despite no direct action on the pumps themselves or their expression. Haloperidol and promethazine are repurposed drugs that have displayed the ability to modulate active efflux. Both show great promise as azole adjuvants for the treatment of cutaneous mycoses and dandruff [18,173,174]. Thymol and carvacrol are terpenoids extracted from thyme leaves that interact synergistically with azoles and inhibit efflux in azole-resistant Candida, including C. auris [211,212,282]. Numerous other natural products with synergistic potential have displayed similar indirect anti-efflux properties [203,211,223,227,233].
It should be noted that fungal drug efflux pumps have an extremely broad spectrum of substrates that they transport [283]. Given this, many of the compounds discussed in this review may be substrates of efflux pumps that compete with azoles, preventing the transport of the azole and prolonging its effect. This competition may contribute to the synergy observed between some drug pairs where the underlying mechanism is currently unknown.
4. Repurposing Other Pharmaceuticals
Repurposing existing drugs can short-cut the process of drug development and regulatory approval. In recent years, a popular approach in drug discovery has been to screen libraries of existing compounds for novel repurposed uses, including antifungal activity. The resulting “hits” may have completely different mechanisms of action to any other currently available antifungal, opening new avenues for drug design. Many of these repurposed antimicrobials are also tested for their capacity as antifungal adjuvants [284,285].
4.1. Statins
Statins are common anti-cholesterol medications and until recently were considered one of the most promising routes for antifungal discovery. Statins operate on HMG-CoA reductase in the mevalonate pathway, upstream from azole-targeted demethylases (Figure 2) [269]. Unfortunately, however, as demonstrated in Table 2, synergy between azoles and statins is often minor or bordering on indifferent. In addition, while it might appear that some statin–azole pairings have a decent spectrum of activity, most studies have tested only 1–3 strains per species. Their applicability to a wide variety of mycoses is therefore difficult to gauge [166,167,168,169,170]. Where synergy has been observed, the mechanism was shown to be primarily driven by the co-operation of the drug pairs on the same pathway (Figure 2) [286]. Newer statins such as pitavastatin have been found to exhibit extremely strong antifungal synergy with fluconazole in azole-resistant Candida, which may reignite future interest [171]. Statins can unfortunately have adverse off-target effects and drug interactions that range from unpleasant to lethal, especially for already vulnerable mycosis patients. These include diabetes, liver cirrhosis, irreparable damage to skeletal muscle and sexual dysfunction [287,288].
4.2. Bisphosphonates
Bisphosphonates are anti-osteoporosis drugs and show promising synergy with fluconazole. Like statins, bisphosphonates operate on the mevalonate pathway where they target farnesyl pyrophosphate synthase (or Erg11; Figure 2) [289]. Of the bisphosphonates tested, zoledronate resulted in strong synergy across numerous strains of Cryptococcus tested in vitro and in an in vivo model and significantly limited the development of antifungal resistance [172]. Due to their propensity to bind to bone mineral and their implication in osteonecrosis, market bisphosphonates have limited applicability for the treatment of invasive mycoses [289,290]; however, their antifungal synergy and potent immunostimulatory properties make them attractive lead compounds for further development [291].
4.3. Repurposing Miscellaneous Pharmaceuticals
Although most fail to produce strong, broad-spectrum synergy and may have undesirable anti-inflammatory or immunosuppressive effects, certain immunomodulators could be useful as lead compounds for development as synergists to treat Candida biofilms [175,176,177,178,292]. The antihistamine promethazine appears potentially attractive as a novel topical anti-dandruff and anti-tinea treatment, as it synergised strongly with azoles in all strains of dermatophytes tested [18,173,174].
Some psychoactive drugs have displayed limited synergy with azoles along a narrow spectrum of activity. Bromperidol and fluoxetine show limited potential as azole adjuvants for the treatment of candidiasis, while the commonly prescribed antidepressant sertraline synergised weakly with azoles in the opportunistic yeast Trichosporon [179,180,181]. Haloperidol is an antipsychotic that may be more promising as a topical combined treatment due to its strong synergy with fluconazole and itraconazole in many strains of dermatophytes [18,173].
Two of the most attractive groups of compounds for developing new synergists are calcineurin inhibitors and calcium channel blockers. These drugs modulate calcium ion homeostasis, which is vital for cellular signalling, and have been considered promising antifungal leads for a variety of diverse pathogens for more than a decade [184,185,186,293]. Calcium channel blockers have also been shown to further enhance the synergy between fluconazole and doxycycline in a series of three-way checkerboards [131]. The calcium channel blockers tetrandrine and verapamil are discussed above in the context of their role in efflux, but their ability to disturb calcium homeostasis is likely also part of their antifungal effect. Other inhibitors like cyclosporine and tacrolimus target calcineurin and calmodulin, a calcium-activated complex responsible for the up-regulation of genes related to growth and the fungal stress response (Figure 3c) [18,182,183,184,185,187,294]. Tacrolimus specifically produces significant synergy for the majority of dermatophyte species, while cyclosporine reliably and potently interacts with fluconazole in C. albicans [183,188,189]. It should also be noted that calcineurin inhibitors like tacrolimus directly affect the ATPase activity of efflux pumps in addition to acting on calcineurin, thereby both directly and indirectly inhibiting active efflux [295].
The growing drug repurposing initiative has yielded synergists with novel mechanisms of action with significant therapeutic potential, as illustrated in Figure 3. Fungal membrane proton pumps are vital enzymes, generating the membrane potential required for membrane-bound transporter function and the uptake of nutrients required for ATP synthesis, thereby enabling ATP-dependent drug efflux [52]. A wide variety of proton pump inhibitors have been shown to produce strong synergy with fluconazole in azole-resistant Candida isolates [194]. HSP90 (HSP82 in yeast) inhibitors like geldanamycin and ganetespib repress the fungal response to stress, reducing the survival of yeasts during azole-induced inhibition (Figure 3a) [195,197,209]. Histone deacetylase inhibitors have been popular in the development of antifungal therapies but surprisingly few have been tested for synergy with azoles. One exception is givinostat, a potential anti-aspergillosis treatment when paired with posaconazole [198]. Lonafarnib inhibits farnesyltransferase, preventing vital post-translational modifications of fungal proteins and producing moderate azole synergy in Aspergillus (Figure 3b) [199]. Other recently discovered azole synergists with more limited utility inhibit superoxide dismutase, chemosensitising C. albicans to oxidative stressors induced by azole treatment (Figure 3f) [200]. Others such as DIBI, lactoferrin, D-penicillamine and EDTA chelate ions vital for enzymatic function, resulting in dysregulated apoptosis in the cell, but these result in only a weak or narrow-spectrum antifungal synergy (Figure 3d) [201,202,245,253].
Some repurposed pharmaceuticals interact synergistically with azoles through a mechanism that has yet to be fully elucidated. Licofelone failed to make it through clinical trials as an anti-arthritic but was shown to abrogate azole resistance in biofilms of C. albicans [203]. Phenylbutyrate is an aromatic fatty acid used to treat hyperammonaemia and has been shown to weakly synergise with various systemic azoles against resistant Candida species [204]. Other repurposed inhibitors including sedatives, antiseptics, diuretics and analgesics have displayed some promising synergy with azoles against a narrow spectrum of strains that may warrant further exploration [205,206,207,208,210].
5. Azole Synergy with Natural Products
Nature has long been the source of novel compounds, including important anti-cancer and antimicrobial chemotherapies [297]. Table 3 lists naturally produced synergists that have been included in this review alongside their original biological source.
Table 3.
Bioactive natural products named in this review and their original biological source(s).
| Source | ||||
|---|---|---|---|---|
| Type | Synergist | Common Name | Latin Name | Ref. |
| Essential Oil Extracts | thymol | thyme, ajwain, wild bergamot | Thymus vulgaris, Trachyspermum ammi, Monarda fistulosa | [211] |
| carvacrol | oregano, thyme, marjoram | Origanum vulgare, Thymus vulgaris, Origanum majorana | [211] | |
| acetophenone | apple, apricot, beef, cheese, croton | Malus domestica, Prunus armeniaca, Bos taurus, Croton sp. | [213] | |
| osthole | snowparsley, wild celery, shishiudo | Cnidium monnieri, Angelica archangelica, Angelica pubescens | [214] | |
| houttuyfonate | fish mint | Houttuynia cordata | [215] | |
| menthol | wild mint, peppermint | Mentha arvensis, Mentha piperita | [216] | |
| tyrosol | olive, argan | Olea europaea, Argania spinosa | [217] | |
| allyl isothiocyanate | mustard, radish, horseradish, wasabi | Sinapis alba, Raphanus raphanistrum, Armoracia rusticana | [218] | |
| butylphthalide | celery | Apium graveolens | [219] | |
| glabridin | liquorice | Glycyrrhiza glabra | [220] | |
| oridonin | blushred | Rabdosia rubescens | [222] | |
| Crude Essential Oils | sea-buckthorn | sea-buckthorn | Hippophae rhamnoides | [223] |
| guava leaf | guava, pineapple guava | Psidium guajava, Acca sellowiana | [224] | |
| frankincense | Indian frankincense | Boswellia serrata | [225] | |
| TTO | tea tree | Melaleuca alternifolia | [226] | |
| Alkaloids | berberine | barberry, tree turmeric, prickly poppy | Berberis vulgaris, Berberis aristate, Argemone mexicana | [228,229,230,231,232] |
| palmatine | Amur cork tree, yanhusuo | Phellodendron amurense, Corydalis yanhusuo | [233,234] | |
| harmine | wild rue, ayahuasca | Peganum harmala, Banisteriopsis caapi | [235] | |
|
Other
terpenoids |
guttiferone | boarwood root | Symphonia globulifera | [236] |
| farnesol | plants, animals and fungi | [237] | ||
| Other phenols | magnolol | Chinese magnolia, southern magnolia | Magnolia officinalis, Magnolia grandiflora | [239] |
| diorcinol | fungal symbiont | Epichloe bromicola | [240] | |
| proanthocyanidin | pine bark, cranberries, grape seeds | Pinus sp., Vaccinium oxycoccus, Vitis labrusca | [241] | |
| epigallocatechin | black tea, white tea, green tea | Camellia sinensis | [242] | |
| asarone | sweet flag, wild ginger | Acorus calamus, Asarum sp. | [243] | |
| Peptides | lactoferrin | bovine and human (milk, mucous) | Bos taurus, Homo sapiens | [201,245] |
| beauvericin | white muscardine, Fusarium | Beauveria bassiana, Fusarium sp. | [246] | |
Some essential oils and essential oil extracts have shown strong potential as lead compounds, but most are only effective at concentrations that are not clinically achievable or are active only in particular isolates. Thymol and carvacrol are well-characterised terpenoids from thyme and oregano essential oils (Table 3) and have been found to synergise with fluconazole in wild-type C. albicans and have displayed some activity in C. auris biofilms [211,212,282]. Acetophenone is a small ketone present in many foods that showed promise as a topical antifungal when paired with ketoconazole [213]. Osthole from tonka bean oil and houttuyfonate from fish mint oil both displayed excellent synergy with azoles in azole-resistant species of Candida [214,215]. Oridonin is a staple of traditional Chinese medicine that has displayed strong synergy with common azoles in resistant isolates of Candida [222]. Menthol extracted from mint synergised well with itraconazole, but only for a fraction of the Candida strains tested [216]. Glabridin from liquorice root synergised with voriconazole in all A. fumigatus strains tested [220]. Several oil extracts displayed excellent anti-biofilm activity when combined with fluconazole, including chito-oligosaccharides, tyrosol from olive oil, allyl isothiocyanate from mustard oil and butylphthalide from celery oil [217,218,219,221]. Some crude essential oils have also been investigated for potential synergy, including oils from Indian frankincense, tea tree, sea buckthorn and guava leaf. While some promising anti-Candida and anti-dermatophyte synergy was observed, these crude oils are too complex to be called therapeutic leads, and more refined bioactive fractions need to be identified [223,224,225,226].
Alkaloid and terpenoid metabolites make up many of the most promising naturally derived synergists. The alkaloid phytochemicals berberine and palmatine are particularly attractive, displaying synergy with fluconazole in both planktonic and biofilm forms of nearly all strains tested in the Candida genus, Saccharomyces cerevisiae and Talaromyces marneffei [227,228,229,230,231,232,233,234]. Harmine is an alkaloid that interacts extremely synergistically with multiple systemic azoles, but only in a fraction of the strains tested, while guttiferone, a terpenoid, synergises less acutely but with more total strain coverage, particularly in non-albicans species of Candida [235,236]. Berberine and guttiferone are two of the very few phytochemicals actually taken past the point of a therapeutic lead, undergoing chemical modifications and development into more synergistic novel derivatives in further studies [236,250,298]. Farnesol is an isoprenoid that displays limited or no synergy in planktonic forms of C. auris but is highly synergistic against its biofilms. This contrasts with asiatic acid, a terpenoid that synergises with fluconazole only in planktonic Candida or in vivo, but not in biofilms [238]. Farnesol may, thus, be a promising tool for fighting highly problematic biofilms of C. auris [237].
Phenol derivatives are an important class of organic phytochemicals, often vital for mediating the plant response to stress [299]. Many phenolics have been shown to have antimicrobial activity, and several may be potential synergistic azole co-drugs [300]. Both the lignan magnolol and the diphenol diorcinol have proven effective against every Candida strain tested, with the former synergising well with fluconazole and the latter not technically synergising but sharply decreasing the required inhibitory dosage, bringing it to well within a clinically achievable concentration [239,240]. In contrast, proanthocyanidin, a plant polyphenol, synergised with fluconazole very weakly and in only a small number of the azole-resistant non-albicans Candida isolates tested [241]. Other phenolics show greater promise, particularly for the treatment of cutaneous or oropharyngeal candidiasis. Epigallocatechin gallate and asarones both synergise with topical azoles, with the former particularly effective against Candida biofilms [242,243]. Pyrogallol is a phenol that synergises with various market antifungals by inhibiting active efflux in both azole-susceptible and azole-resistant Candida [244]. Catechol, while unable to inhibit Candida itself, potentiates the antifungal activity of azoles and polyenes, and prevents up-regulation of virulence-associated genes. Curiously, catechol did not reduce the viability of Candida biofilms, but did reduce their hydrophobicity [301].
Antimicrobial peptides are found in cells from all taxonomic Kingdoms and are vital for the defence against infection, potentially synergising with other antimicrobials [302]. Synergy has been seen with the milk protein lactoferrin with azoles in resistant isolates of Candida, but not Cryptococcus [201,245]. Beauvericin is an antibiotic and insecticidal peptide derivative called a depsipeptide that synergises well with fluconazole. With only a limited group of azole-susceptible strains of C. albicans tested, however, it remains unclear whether it can be called a truly promising therapeutic lead [246].
6. Azole Synergy with Novel Compounds
Numerous new compounds that have been shown to have good antifungal activity as a monotherapy have also been found to synergize with azoles. Synergy is often inconsistent for azole-susceptible and azole-resistant strains, however. Consistent with the cross-resistance observed between azoles, novel azole derivatives are generally effective only in sensitive yeasts and not in resistant ones [247,248]. Novel azoles conjugated with triphenylphosphonium cations have displayed improved mitochondrial targeting and have shown an improved fungicidal effect when combined with Hsp90 inhibitors [303].
Several of the more promising novel compounds currently under investigation are chemically modified derivatives of promising therapeutic leads. Derivatives of the aforementioned isoquinolone and phthalazine, natural metabolites berberine, piperidol, caffeic acid and guttiferone, the anti-inflammatory celecoxib, and phenylpentanol all demonstrated strong synergy with fluconazole in a significant portion of Candida strains tested, and in particular in azole-resistant strains [171,236,249,250,251,254,257]. Other promising novel compounds like beta-glucan synthase inhibitor SCY-078, ion chelator DIBI, TOR inhibitor AZD8055, efflux modulators, and a group of novel antifungal chalcones have all proven highly synergistic against azole-resistant C. albicans, C. glabrata and other Candida species with reduced sensitivity to azoles [173,252,253,255,260]. A group of novel ultra-short cationic lipopeptides were not able to fully synergise with fluconazole, but did interact additively [259].
Two promising classes of compounds that have been developed in the past year are “dual-inhibitors”, which are single compounds that are designed to attack more than one druggable target at once. One group of these that was strongly antifungal contains a piperazine moiety, allowing it to inhibit 14α-demethylase, and a zinc binding group to inhibit HDAC [304]. Another used fluconazole conjugated with COX inhibitors and was able to consistently inhibit pathogenic Candida [305]. As these dual inhibitors are single compounds, they cannot be considered truly synergistic; however, a class of novel Hsp90/HDAC dual inhibitors has displayed strong synergy with fluconazole in azole-resistant Candida [258].
An interesting novel antifungal mechanism of action is the promotion of dysregulated apoptosis in the fungal cell. Control of apoptosis in yeasts is partially governed by the regulation of Inhibitors of Apoptosis Proteins (IAPs), which prevent progression to cell death [306]. A new IAP antagonist, AT406, promotes apoptosis in C. albicans and the outbreak pathogen Exophiala dermatitidis, sensitising the cell to the oxidative stress produced by the azoles (Figure 3e) [256]. This is a truly novel mode of fungicidal action that may be a source of an entirely new class of azole synergists.
7. Conclusions
With the rise of opportunistic and emerging fungal pathogens and increasing rates of antifungal resistance, there is an urgent need for new therapies in our antifungal toolbox. As this review has shown, a reliable path to success is to improve commonly used azole antifungals with synergists, and there is substantial diversity in the compounds and approaches that have yielded synergy. Although high-throughput screening has become a popular method for discovering new therapeutic agents, a rational, target-based approach to drug discovery may yield more reliable and effective therapeutic leads. Compounds co-operating with azoles on the ergosterol and mevalonate biosynthesis pathways have displayed consistent synergy in various fungal pathogens. Rational drug design, building on a known mechanism of action or starting with already approved drugs with known pharmacokinetic data, may take newly developed drugs into market more rapidly. On the other hand, hypothesis-free drug screening initiatives may yield novel synergies that would otherwise be undiscovered, opening new avenues for drug design.
There are several gaps in current studies that await further research. From Table 2, there is a clear focus on developing combined treatment strategies to combat Candida, particularly azole-resistant clinical isolates, and with a few notable exceptions there is a paucity of data exploring azole synergy in Cryptococcus and filamentous fungi. There is also a focus on improving the systemic azoles, particularly fluconazole, while for topical pathogens where oral bioavailability is not required, more could be gained by exploring other azoles. Finally, any translation of synergy into clinical use must deal with the issue of co-administration of two (or more) compounds. New systems that package drugs into nanoparticle delivery systems or co-crystallise compounds into a single formulation, may enable the development of single-dose synergistic treatments [307,308]. There are currently no azole-based antifungal combinations used to treat mycoses, but the need for new treatments and the threats to azoles from intrinsic and acquired resistance make drug synergy an increasingly attractive avenue for antifungal development.
Acknowledgments
The authors would like to extend their gratitude to Kenya Fernandes for her assistance proof-reading the final draft of this review.
Author Contributions
Conceptualization, A.K. and D.A.C.; investigation, A.K.; resources, D.A.C.; writing—original draft preparation, A.K.; writing—review and editing, D.A.C.; visualization, A.K.; supervision, D.A.C.; project administration, D.A.C.; funding acquisition, D.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enoch D.A., Yang H., Aliyu S.H., Micallef C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017;1508:17–65. doi: 10.1007/978-1-4939-6515-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Bongomin F., Gago S., Oladele R., Denning D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schelenz S., Barnes R.A., Kibbler C.C., Jones B.L., Denning D.W. Standards of Care for Patients with Invasive Fungal Infections within the United Kingdom: A National Audit. J. Infect. 2009;58:145–153. doi: 10.1016/j.jinf.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Bitar D., Lortholary O., le Strat Y., Nicolau J., Coignard B., Tattevin P., Che D., Dromer F. Population-Based Analysis of Invasive Fungal Infections, France, 2001-2010. Emerg. Infect. Dis. 2014;20:1149–1155. doi: 10.3201/eid2007.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees J.R., Pinner R.W., Hajjeh R.A., Brandt M.E., Reingold A.L. The Epidemiological Features of Invasive Mycotic Infections in the San Francisco Bay Area, 1992-1993: Results of Population-Based Laboratory Active Surveillance. Clin. Infect. Dis. 1998;27:1138–1150. doi: 10.1093/clinids/27.5.1138. [DOI] [PubMed] [Google Scholar]
- 6.Chapman B., Slavin M., Marriott D., Halliday C., Kidd S.E., Arthur I., Bak N., Heath C., Kennedy K., Morrissey C.O., et al. Changing Epidemiology of Candidaemia in Australia. J. Antimicrob. Chemother. 2017;72:1103–1108. doi: 10.1093/jac/dkx047. [DOI] [PubMed] [Google Scholar]
- 7.Tsay S.V., Mu Y., Williams S., Epson E., Nadle J., Bamberg W.M., Barter D.M., Johnston H.L., Farley M.M., Harb S., et al. Burden of Candidemia in the United States, 2017. Clin. Infect. Dis. 2020;71:E449–E453. doi: 10.1093/cid/ciaa193. [DOI] [PubMed] [Google Scholar]
- 8.Astvad K.M.T., Johansen H.K., Røder B.L., Rosenvinge F.S., Knudsen J.D., Lemming L., Schønheyder H.C., Hare R.K., Kristensen L., Nielsen L., et al. Update from a 12-Year Nationwide Fungemia Surveillance: Increasing Intrinsic and Acquired Resistance Causes Concern. J. Clin. Microbiol. 2018;56:e01564-17. doi: 10.1128/JCM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre D.W., Sheppard A.E., Madder H., Moir I., Moroney R., Quan T.P., Griffiths D., George S., Butcher L., Morgan M., et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018;379:1322–1331. doi: 10.1056/NEJMoa1714373. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Gaitán A., Moret A.M., Tasias-Pitarch M., Aleixandre-López A.I., Martínez-Morel H., Calabuig E., Salavert-Lletí M., Ramírez P., López-Hontangas J.L., Hagen F., et al. An Outbreak Due to Candida auris with Prolonged Colonisation and Candidaemia in a Tertiary Care European Hospital. Mycoses. 2018;61:498–505. doi: 10.1111/myc.12781. [DOI] [PubMed] [Google Scholar]
- 11.Schelenz S., Hagen F., Rhodes J.L., Abdolrasouli A., Chowdhary A., Hall A., Ryan L., Shackleton J., Trimlett R., Meis J.F., et al. First Hospital Outbreak of the Globally Emerging Candida auris in a European Hospital. Antimicrob. Resist. Infect. Control. 2016;5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prestel C., Anderson E., Forsberg K., Lyman M., de Perio M.A., Kuhar D., Edwards K., Rivera M., Shugart A., Walters M., et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:56–57. doi: 10.15585/mmwr.mm7002e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas L.J. Candida Biofilms and Their Role in Infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 14.Seebacher C., Bouchara J.-P., Mignon B. Updates on the Epidemiology of Dermatophyte Infections. Mycopathologia. 2008;166:335–352. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A.K., Paquet M., Simpson F.C. Therapies for the Treatment of Onychomycosis. Clin. Dermatol. 2013;31:544–554. doi: 10.1016/j.clindermatol.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhoff L., Olsowski M., Rath P.-M., Steinmann J. Exophiala dermatitidis: Key Issues of an Opportunistic Fungal Pathogen. Virulence. 2019;10:984–998. doi: 10.1080/21505594.2019.1596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cafarchia C., Iatta R., Immediato D., Puttilli M.R., Otranto D. Azole Susceptibility of Malassezia pachydermatis and Malassezia furfur and Tentative Epidemiological Cut-off Values. Med. Mycol. 2015;53:743–748. doi: 10.1093/mmy/myv049. [DOI] [PubMed] [Google Scholar]
- 18.Iatta R., Puttilli M.R., Immediato D., Otranto D., Cafarchia C. The Role of Drug Efflux Pumps in Malassezia pachydermatis and Malassezia furfur Defence against Azoles. Mycoses. 2017;60:178–182. doi: 10.1111/myc.12577. [DOI] [PubMed] [Google Scholar]
- 19.Moen M.D., Lyseng-Williamson K.A., Scott L.J. Liposomal Amphotericin B. Drugs. 2009;69:361–392. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 20.Kneale M., Bartholomew J.S., Davies E., Denning D.W. Global Access to Antifungal Therapy and Its Variable Cost. J. Antimicrob. Chemother. 2016;71:3599–3606. doi: 10.1093/jac/dkw325. [DOI] [PubMed] [Google Scholar]
- 21.Brammer K.W., Farrow P.R., Faulkner J.K. Pharmacokinetics and Tissue Penetration of Fluconazole in Humans. Clin. Infect. Dis. 1990;12:S318–S326. doi: 10.1093/clinids/12.Supplement_3.S318. [DOI] [PubMed] [Google Scholar]
- 22.Verweij P.E., Snelders E., Kema G.H., Mellado E., Melchers W.J. Azole Resistance in Aspergillus fumigatus: A Side-Effect of Environmental Fungicide Use? Lancet Infect. Dis. 2009;9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 23.Goldman M., Cloud G.A., Smedema M., Lemonte A., Connolly P., Mckinsey D.S., Kauffman C.A., Moskovitz B., Wheat L.J., Flanigan C., et al. Does Long-Term Itraconazole Prophylaxis Result in In Vitro Azole Resistance in Mucosal Candida albicans Isolates from Persons with Advanced Human Immunodeficiency Virus Infection? Antimicrob. Agents Chemother. 2000;44:1585–1587. doi: 10.1128/AAC.44.6.1585-1587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggero M.A., Topal J.E. Development of Echinocandin-Resistant Candida albicans Candidemia Following Brief Prophylactic Exposure to Micafungin Therapy. Transpl. Infect. Dis. 2014;16:469–472. doi: 10.1111/tid.12230. [DOI] [PubMed] [Google Scholar]
- 25.Bastos R.W., Carneiro H.C.S., Oliveira L.V.N., Rocha K.M., Freitas G.J.C., Costa M.C., Magalhães T.F.F., Carvalho V.S.D., Rocha C.E., Ferreira G.F., et al. Environmental Triazole Induces Cross-Resistance to Clinical Drugs and Affects Morphophysiology and Virulence of Cryptococcus gattii and C. neoformans. Antimicrob. Agents Chemother. 2018;62:e01179-17. doi: 10.1128/AAC.01179-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamba S., Lortholary O., Sawadogo A., Millogo A., Guiguemdé R.T., Bretagne S. Decreasing Incidence of Cryptococcal Meningitis in West Africa in the Era of Highly Active Antiretroviral Therapy. AIDS. 2012;26:1039–1041. doi: 10.1097/QAD.0b013e328352d1d8. [DOI] [PubMed] [Google Scholar]
- 27.D’Arminio Monforte A., Sabin C.A., Phillips A., Sterne J., May M., Justice A., Dabis F., Grabar S., Ledergerber B., Gill J., et al. The Changing Incidence of AIDS Events in Patients Receiving Highly Active Antiretroviral Therapy. Arch. Intern. Med. 2005;165:416–423. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- 28.Bennett J.E., Izumikawa K., Marr K.A. Mechanism of Increased Fluconazole Resistance in Candida glabrata during Prophylaxis. Antimicrob. Agents Chemother. 2004;48:1773–1777. doi: 10.1128/AAC.48.5.1773-1777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigues C.F., Silva S., Henriques M. Candida glabrata: A Review of Its Features and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:673–688. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhary A., Sharma C., Meis J.F. Candida auris: A Rapidly Emerging Cause of Hospital-Acquired Multidrug-Resistant Fungal Infections Globally. PLOS Pathog. 2017;13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denning D.W., Bromley M. How to Bolster the Antifungal Pipeline. Science (1979) 2015;347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 32.Odds F.C., Brown A.J.P., Gow N.A.R. Antifungal Agents: Mechanisms of Action. Trends Microbiol. 2003;11:272–279. doi: 10.1016/S0966-842X(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 33.Allen D., Wilson D., Drew R., Perfect J. Azole Antifungals: 35 Years of Invasive Fungal Infection Management. Expert Rev. Anti-Infect. Ther. 2015;13:787–798. doi: 10.1586/14787210.2015.1032939. [DOI] [PubMed] [Google Scholar]
- 34.Andes D., Kovanda L., Desai A., Kitt T., Zhao M., Walsh T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018;62:e00585-18. doi: 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miceli M.H., Kauffman C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015;61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 36.Brand S.R., Degenhardt T.P., Person K., Sobel J.D., Nyirjesy P., Schotzinger R.J., Tavakkol A. A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Efficacy and Safety of Orally Administered VT-1161 in the Treatment of Recurrent Vulvovaginal Candidiasis. Am. J. Obstet. Gynecol. 2018;218:624.e1–624.e9. doi: 10.1016/j.ajog.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Wiederhold N.P., Xu X., Wang A., Najvar L.K., Garvey E.P., Ottinger E.A., Alimardanov A., Cradock J., Behnke M., Hoekstra W.J., et al. In Vivo Efficacy of VT-1129 against Experimental Cryptococcal Meningitis with the Use of a Loading Dose-Maintenance Dose Administration Strategy. Antimicrob. Agents Chemother. 2018;62:e01315-18. doi: 10.1128/AAC.01315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monk B.C., Keniya M.V. Roles for Structural Biology in the Discovery of Drugs and Agrochemicals Targeting Sterol 14α-Demethylases. J. Fungi. 2021;7:67. doi: 10.3390/jof7020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hargrove T.Y., Garvey E.P., Hoekstra W.J., Yates C.M., Wawrzak Z., Rachakonda G., Villalta F., Lepesheva G.I. Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity. Antimicrob. Agents Chemother. 2017;61:e00570-17. doi: 10.1128/AAC.00570-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antifungal Drugs Market Size 2019|Demand|Industry Forecast. [(accessed on 8 March 2021)]. Available online: https://www.reportsanddata.com/report-detail/antifungal-drugs-market.
- 41.Global Antifungal Drugs Market Report 2020|Orbis Research. [(accessed on 8 March 2021)]. Available online: https://www.orbisresearch.com/reports/index/global-antifungal-drugs-market-report-2020.
- 42.Zonios D., Yamazaki H., Murayama N., Natarajan V., Palmore T., Childs R., Skinner J., Bennett J.E. Voriconazole Metabolism, Toxicity, and the Effect of Cytochrome P450 2C19 Genotype. J. Infect. Dis. 2014;209:1941–1948. doi: 10.1093/infdis/jiu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann P.A., McNicholas P.M., Chau A.S., Patel R., Mendrick C., Ullmann A.J., Cornely O.A., Patino H., Black T.A. Impact of Antifungal Prophylaxis on Colonization and Azole Susceptibility of Candida Species. Antimicrob. Agents Chemother. 2009;53:5026–5034. doi: 10.1128/AAC.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaabane F., Graf A., Jequier L., Coste A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019;10:2788. doi: 10.3389/fmicb.2019.02788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brajtburg J., Powderly W.G., Kobayashi G.S., Medoff G. Amphotericin B: Current Understanding of Mechanisms of Action. Antimicrob. Agents Chemother. 1990;34:183–188. doi: 10.1128/AAC.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlin D.S. Current Perspectives on Echinocandin Class Drugs. Future Microbiol. 2011;6:441–457. doi: 10.2217/fmb.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgopapadakou N.H. Antifungals: Mechanism of Action and Resistance, Established and Novel Drugs. Curr. Opin. Microbiol. 1998;1:547–557. doi: 10.1016/S1369-5274(98)80087-8. [DOI] [PubMed] [Google Scholar]
- 48.Leyden J. Pharmacokinetics and Pharmacology of Terbinafine and Itraconazole. J. Am. Acad. Dermatol. 1998;38:S42–S47. doi: 10.1016/S0190-9622(98)70483-9. [DOI] [PubMed] [Google Scholar]
- 49.de Carli L., Larizza L. Griseofulvin. Mutat. Res./Rev. Genet. Toxicol. 1988;195:91–126. doi: 10.1016/0165-1110(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 50.Vermes A. Flucytosine: A Review of Its Pharmacology, Clinical Indications, Pharmacokinetics, Toxicity and Drug Interactions. J. Antimicrob. Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 51.Heilmann C.J., Schneider S., Barker K.S., Rogers P.D., Morschhäuser J. An A643T Mutation in the Transcription Factor Upc2p Causes Constitutive ERG11 Upregulation and Increased Fluconazole Resistance in Candida albicans. Antimicrob. Agents Chemother. 2010;54:353–359. doi: 10.1128/AAC.01102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes A.R., Cardno T.S., Strouse J.J., Ivnitski-Steele I., Keniya M.V., Lackovic K., Monk B.C., Sklar L.A., Cannon R.D. Targeting Efflux Pumps to Overcome Antifungal Drug Resistance. Future Med. Chem. 2016;8:1485–1501. doi: 10.4155/fmc-2016-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon R.D., Lamping E., Holmes A.R., Niimi K., Baret P.V., Keniya M.V., Tanabe K., Niimi M., Goffeau A., Monk B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamping E., Monk B.C., Niimi K., Holmes A.R., Tsao S., Tanabe K., Niimi M., Uehara Y., Cannon R.D. Characterization of Three Classes of Membrane Proteins Involved in Fungal Azole Resistance by Functional Hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:1150–1165. doi: 10.1128/EC.00091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selmecki A., Forche A., Berman J. Aneuploidy and Isochromosome Formation in Drug-Resistant Candida albicans. Science (1979) 2006;313:367–370. doi: 10.1126/science.1128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selmecki A.M., Dulmage K., Cowen L.E., Anderson J.B., Berman J. Acquisition of Aneuploidy Provides Increased Fitness during the Evolution of Antifungal Drug Resistance. PLoS Genet. 2009;5:e1000705. doi: 10.1371/journal.pgen.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lockhart S.R., Frade J.P., Etienne K.A., Pfaller M.A., Diekema D.J., Balajee S.A. Azole Resistance in Aspergillus fumigatus Isolates from the ARTEMIS Global Surveillance Study Is Primarily Due to the TR/L98H Mutation in the Cyp51A Gene. Antimicrob. Agents Chemother. 2011;55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez-Jimenez I., Lucio J., Amich J., Cuesta I., Arroyo R.S., Alcazar-Fuoli L., Mellado E. A CYP51b Mutation Contributes to Azole Resistance in Aspergillus fumigatus. J. Fungi. 2020;6:315. doi: 10.3390/jof6040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leonardelli F., Macedo D., Dudiuk C., Cabeza M.S., Gamarra S., Garcia-Effron G. Aspergillus fumigatus Intrinsic Fluconazole Resistance Is Due to the Naturally Occurring T301I Substitution in Cyp51Ap. Antimicrob. Agents Chemother. 2016;60:5420–5426. doi: 10.1128/AAC.00905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vermitsky J.P., Edlind T.D. Azole Resistance in Candida glabrata: Coordinate Upregulation of Multidrug Transporters and Evidence for a Pdr1-like Transcription Factor. Antimicrob. Agents Chemother. 2004;48:3773–3781. doi: 10.1128/AAC.48.10.3773-3781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hagiwara D., Miura D., Shimizu K., Paul S., Ohba A., Gonoi T., Watanabe A., Kamei K., Shintani T., Moye-Rowley W.S., et al. A Novel Zn2-Cys6 Transcription Factor AtrR Plays a Key Role in an Azole Resistance Mechanism of Aspergillus fumigatus by Co-Regulating Cyp51A and Cdr1B Expressions. PLoS Pathog. 2017;13:e1006096. doi: 10.1371/journal.ppat.1006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keniya M.V., Sabherwal M., Wilson R.K., Woods M.A., Sagatova A.A., Tyndall J.D.A., Monk B.C. Crystal Structures of Full-Length Lanosterol 14α-Demethylases of Prominent Fungal Pathogens Candida albicans and Candida glabrata Provide Tools for Antifungal Discovery. Antimicrob. Agents Chemother. 2018;62:e01134-18. doi: 10.1128/AAC.01134-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheng C., Miao Z., Ji H., Yao J., Wang W., Che X., Dong G., Lü J., Guo W., Zhang W. Three-Dimensional Model of Lanosterol 14α-Demethylase from Cryptococcus neoformans: Active-Site Characterization and Insights into Azole Binding. Antimicrob. Agents Chemother. 2009;53:3487–3495. doi: 10.1128/AAC.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis R.E., Kontoyiannis D.P. Rationale for Combination Antifungal Therapy. Pharmacotherapy. 2001;21:149S–164S. doi: 10.1592/phco.21.12.149S.34505. [DOI] [PubMed] [Google Scholar]
- 65.Droegemueller W., Adamson D.G., Brown D., Cibley L., Fleury F., Lepage M.E., Henzl M.R. Three-Day Treatment with Butoconazole Nitrate for Vulvovaginal Candidiasis. Obstet. Gynecol. 1984;64:530–534. [PubMed] [Google Scholar]
- 66.Schmidt A. In Vitro Activity of Climbazole, Clotrimazole and Silver-Sulphadiazine against Isolates of Malassezia pachydermatis. J. Vet. Med. Ser. B. 1997;44:193–197. doi: 10.1111/j.1439-0450.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 67.Mendling W., Plempel M. Vaginal Secretion Levels after 6 Days, 3 Days and 1 Day of Treatment with 100, 200 and 500 Mg Vaginal Tablets of Clotrimazole and Their Therapeutic Efficacy. Chemotherapy. 1982;28:43–47. doi: 10.1159/000238151. [DOI] [PubMed] [Google Scholar]
- 68.Fernández-Torres B., Inza I., Guarro J. In Vitro Activities of the New Antifungal Drug Eberconazole and Three Other Topical Agents against 200 Strains of Dermatophytes. J. Clin. Microbiol. 2003;41:5209–5211. doi: 10.1128/JCM.41.11.5209-5211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thienpont D., van Cutsem J., van Nueten J.M. Biological and Toxicological Properties of Econazole, a Broad Spectrum Antimycotic. Arzneim.-Forsch./Drug Res. 1975;25:224–230. [PubMed] [Google Scholar]
- 70.Gerven F., van Odds F.C. The Anti-Malassezia furfur Activity In Vitro and in Experimental Dermatitis of Six Imidazole Antifungal Agents: Bifonazole, Clotrimazole, Flutrimazole, Ketoconazole, Miconazole and Sertaconazole. Mycoses. 1995;38:389–393. doi: 10.1111/j.1439-0507.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 71.Veraldi S. Isoconazole Nitrate: A Unique Broad-Spectrum Antimicrobial Azole Effective in the Treatment of Dermatomycoses, Both as Monotherapy and in Combination with Corticosteroids. Mycoses. 2013;56:3–15. doi: 10.1111/myc.12054. [DOI] [PubMed] [Google Scholar]
- 72.Green C.A., Farr P.M., Shuster S. Treatment of Seborrhoeic Dermatitis with Ketoconazole: II. Response of Seborrhoeic Dermatitis of the Face, Scalp and Trunk to Topical Ketoconazole. Br. J. Dermatol. 1987;116:217–221. doi: 10.1111/j.1365-2133.1987.tb05815.x. [DOI] [PubMed] [Google Scholar]
- 73.Khanna D., Bharti S. Luliconazole for the Treatment of Fungal Infections: An Evidence-Based Review. Core Evid. 2014;9:113. doi: 10.2147/CE.S49629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Cutsem J.M., Thienpont D. Miconazole, a Broad-Spectrum Antimycotic Agent with Antibacterial Activity. Chemotherapy. 1972;17:392–404. doi: 10.1159/000220875. [DOI] [PubMed] [Google Scholar]
- 75.Polak A. Oxiconazole, a New Imidazole Derivative. Evaluation of Antifungal Activity In Vitro and In Vivo. Arzneim.-Forsch./Drug Res. 1982;32:17–24. [PubMed] [Google Scholar]
- 76.Liebel F., Lyte P., Garay M., Babad J., Southall M.D. Anti-Inflammatory and Anti-Itch Activity of Sertaconazole Nitrate. Arch. Dermatol. Res. 2006;298:191–199. doi: 10.1007/s00403-006-0679-8. [DOI] [PubMed] [Google Scholar]
- 77.Carrillo-Muñoz A.J., Giusiano G., Ezkurra P.A., Quindós G. Sertaconazole: Updated Review of a Topical Antifungal Agent. Expert Rev. Anti-Infect. Ther. 2005;3:333–342. doi: 10.1586/14787210.3.3.333. [DOI] [PubMed] [Google Scholar]
- 78.Benfield P., Stephen P. Sulconazole: A Review of Its Antimicrobial Activity and Therapeutic Use in Superficial Dermatomycoses. Drugs. 1988;35:143–153. doi: 10.2165/00003495-198835020-00004. [DOI] [PubMed] [Google Scholar]
- 79.Sunderland M.R., Cruickshank R.H., Leighs S.J. The Efficacy of Antifungal Azole and Antiprotozoal Compounds in Protection of Wool from Keratin-Digesting Insect Larvae. Text. Res. J. 2014;84:924–931. doi: 10.1177/0040517513515312. [DOI] [Google Scholar]
- 80.Jevons S., Gymer G.E., Brammer K.W., Cox D.A., Leeming M.R. Antifungal Activity of Tioconazole (UK-20,349), a New Imidazole Derivative. Antimicrob. Agents Chemother. 1979;15:597–602. doi: 10.1128/AAC.15.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dismukes W.E., Stamm A.M., Graybill J.R., Craven P.C., Stevens D.A., Stiller R.L., Sarosi G.A., Medoff G., Gregg C.R., Gallis H.A., et al. Treatment of Systemic Mycoses with Ketoconazole: Emphasis on Toxicity and Clinical Response in 52 Patients. National Institute of Allergy and Infectious Diseases Collaborative Antifungal Study. Ann. Intern. Med. 1983;98:13–20. doi: 10.7326/0003-4819-98-1-13. [DOI] [PubMed] [Google Scholar]
- 82.Elewski B.E., Rich P., Pollak R., Pariser D.M., Watanabe S., Senda H., Ieda C., Smith K., Pillai R., Ramakrishna T., et al. Efinaconazole 10% Solution in the Treatment of Toenail Onychomycosis: Two Phase III Multicenter, Randomized, Double-Blind Studies. J. Am. Acad. Dermatol. 2013;68:600–608. doi: 10.1016/j.jaad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 83.van Cutsem J., van Gerven F., Zaman R., Janssen P.A.J. Terconazole—A New Broad-Spectrum Antifungal. Chemotherapy. 1983;29:322–331. doi: 10.1159/000238215. [DOI] [PubMed] [Google Scholar]
- 84.Sobue S., Tan K., Layton G., Eve M., Sanderson J.B. Pharmacokinetics of Fosfluconazole and Fluconazole Following Multiple Intravenous Administration of Fosfluconazole in Healthy Male Volunteers. Br. J. Clin. Pharmacol. 2004;58:20–25. doi: 10.1111/j.1365-2125.2004.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi D., Nakamura T., Shigematsu R., Matsui M., Araki S., Kubo K., Sato H., Shirahata A. Fosfluconazole for Antifungal Prophylaxis in Very Low Birth Weight Infants. Int. J. Pediatr. 2009;2009:274768. doi: 10.1155/2009/274768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watanabe S., Tsubouchi I., Okubo A. Efficacy and Safety of Fosravuconazole L-Lysine Ethanolate, a Novel Oral Triazole Antifungal Agent, for the Treatment of Onychomycosis: A Multicenter, Double-Blind, Randomized Phase III Study. J. Dermatol. 2018;45:1151–1159. doi: 10.1111/1346-8138.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., Mullane K.M., Perfect J.R., Thompson G.R., Alangaden G.J., Brown J.M., Fredricks D.N., Heinz W.J., et al. Isavuconazole Treatment for Mucormycosis: A Single-Arm Open-Label Trial and Case-Control Analysis. Lancet Infect. Dis. 2016;16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 88.Odds F.C. Itraconazole—A New Oral Antifungal Agent with a Very Broad Spectrum of Activity in Superficial and Systemic Mycoses. J. Dermatol. Sci. 1993;5:65–72. doi: 10.1016/0923-1811(93)90072-W. [DOI] [PubMed] [Google Scholar]
- 89.Torres H.A., Hachem R.Y., Chemaly R.F., Kontoyiannis D.P., Raad I.I. Posaconazole: A Broad-Spectrum Triazole Antifungal. Lancet Infect. Dis. 2005;5:775–785. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- 90.Hoffman H.L., Rathbun R.C. Review of the Safety and Efficacy of Voriconazole. Expert Opin. Investig. Drugs. 2002;11:409–429. doi: 10.1517/13543784.11.3.409. [DOI] [PubMed] [Google Scholar]
- 91.BioCentury—Abasol Abafungin Regulatory Update. [(accessed on 16 March 2021)]. Available online: https://www.biocentury.com/article/126706/abasol-abafungin-regulatory-update.
- 92.Odds F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 93.Xu X., Xu L., Yuan G., Wang Y., Qu Y., Zhou M. Synergistic Combination of Two Antimicrobial Agents Closing Each Other’s Mutant Selection Windows to Prevent Antimicrobial Resistance. Sci. Rep. 2018;8:7237. doi: 10.1038/s41598-018-25714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coates A.R.M., Hu Y., Holt J., Yeh P. Antibiotic Combination Therapy against Resistant Bacterial Infections: Synergy, Rejuvenation and Resistance Reduction. Expert Rev. Anti-Infect. Ther. 2020;18:5–15. doi: 10.1080/14787210.2020.1705155. [DOI] [PubMed] [Google Scholar]
- 95.Zubko E.I., Zubko M.K. Co-Operative Inhibitory Effects of Hydrogen Peroxide and Iodine against Bacterial and Yeast Species. BMC Res. Notes. 2013;6:272. doi: 10.1186/1756-0500-6-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bibi M., Murphy S., Benhamou R.I., Rosenberg A., Ulman A., Bicanic T., Fridman M., Berman J. Combining Colistin and Fluconazole Synergistically Increases Fungal Membrane Permeability and Antifungal Cidality. ACS Infect. Dis. 2021;7:377–389. doi: 10.1021/acsinfecdis.0c00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trickey A., May M.T., Vehreschild J.J., Obel N., Gill M.J., Crane H.M., Boesecke C., Patterson S., Grabar S., Cazanave C., et al. Survival of HIV-Positive Patients Starting Antiretroviral Therapy between 1996 and 2013: A Collaborative Analysis of Cohort Studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bell A. Antimalarial Drug Synergism and Antagonism: Mechanistic and Clinical Significance. FEMS Microbiol. Lett. 2005;253:171–184. doi: 10.1016/j.femsle.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 99.Hitchings G.H. Mechanism of Action of Trimethoprim-Sulfamethoxazole. J. Infect. Dis. 1973;128:S433–S436. doi: 10.1093/infdis/128.Supplement_3.S433. [DOI] [PubMed] [Google Scholar]
- 100.Bennett J.E., Dismukes W.E., Duma R.J., Medoff G., Sande M.A., Gallis H., Leonard J., Fields B.T., Bradshaw M., Haywood H., et al. A Comparison of Amphotericin B Alone and Combined with Flucytosine in the Treatment of Cryptoccal Meningitis. N. Engl. J. Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 101.Stamm A.M., Diasio R.B., Dismukes W.E., Shadomy S., Cloud G.A., Bowles C.A., Karam G.H., Espinel-Ingroff A. Toxicity of Amphotericin B plus Flucytosine in 194 Patients with Cryptococcal Meningitis. Am. J. Med. 1987;83:236–242. doi: 10.1016/0002-9343(87)90691-7. [DOI] [PubMed] [Google Scholar]
- 102.Campitelli M., Zeineddine N., Samaha G., Maslak S. Combination Antifungal Therapy: A Review of Current Data. J. Clin. Med. Res. 2017;9:451–456. doi: 10.14740/jocmr2992w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson M.D., MacDougall C., Ostrosky-Zeichner L., Perfect J.R., Rex J.H. Combination Antifungal Therapy. Antimicrob. Agents Chemother. 2004;48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weig M., Müller F.M.C. Synergism of Voriconazole and Terbinafine against Candida albicans Isolates from Human Immunodeficiency Virus-Infected Patients with Oropharyngeal Candidiasis. Antimicrob. Agents Chemother. 2001;45:966–968. doi: 10.1128/AAC.45.3.966-968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghannoum M.A., Elewski B. Successful Treatment of Fluconazole-Resistant Oropharyngeal Candidiasis by a Combination of Fluconazole and Terbinafine. Clin. Diagn. Lab. Immunol. 1999;6:921–923. doi: 10.1128/CDLI.6.6.921-923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perea S., Gonzalez G., Fothergill A.W., Sutton D.A., Rinaldi M.G. In Vitro Activities of Terbinafine in Combination with Fluconazole, Itraconazole, Voriconazole, and Posaconazole against Clinical Isolates of Candida glabrata with Decreased Susceptibility to Azoles. J. Clin. Microbiol. 2002;40:1831–1833. doi: 10.1128/JCM.40.5.1831-1833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cantón E., Pemân J., Gobernado M., Viudes A., Espinel-Ingroff A. Synergistic Activities of Fluconazole and Voriconazole with Terbinafine against Four Candida Species Determined by Checkerboard, Time-Kill, and Etest Methods. Antimicrob. Agents Chemother. 2005;49:1593–1596. doi: 10.1128/AAC.49.4.1593-1596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meletiadis J., Mouton J.W., Rodriguez-Tudela J.L., Meis J.F.G.M., Verweij P.E. In Vitro Interaction of Terbinafine with Itraconazole against Clinical Isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 2000;44:470–472. doi: 10.1128/AAC.44.2.470-472.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meletiadis J., Mouton J.W., Meis J.F.G.M., Verweij P.E. In Vitro Drug Interaction Modeling of Combinations of Azoles with Terbinafine against Clinical Scedosporium prolificans Isolates. Antimicrob. Agents Chemother. 2003;47:106–117. doi: 10.1128/AAC.47.1.106-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryder N.S., Leitner I. Synergistic Interaction of Terbinafine with Triazoles or Amphotericin B against Aspergillus Species. Med. Mycol. 2001;39:91–95. doi: 10.1080/mmy.39.1.91.95. [DOI] [PubMed] [Google Scholar]
- 111.Bidaud A.L., Schwarz P., Chowdhary A., Dannaoui E. In Vitro Antifungal Combination of Terbinafine with Itraconazole against Isolates of Trichophyton Species. Antimicrob. Agents Chemother. 2021;66:e0144921. doi: 10.1128/AAC.01449-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barchiesi F., Di Francesco L.F., Scalise G. In Vitro Activities of Terbinafine in Combination with Fluconazole and Itraconazole against Isolates of Candida albicans with Reduced Susceptibility to Azoles. Antimicrob. Agents Chemother. 1997;41:1812–1814. doi: 10.1128/AAC.41.8.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mavridou E., Meletiadis J., Rijs A., Mouton J.W., Verweij P.E. The Strength of Synergistic Interaction between Posaconazole and Caspofungin Depends on the Underlying Azole Resistance Mechanism of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015;59:1738–1744. doi: 10.1128/AAC.04469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buil J.B., Brüggemann R.J.M., Denardi L.B., Melchers W.J.G., Verweij P.E. In Vitro Interaction of Isavuconazole and Anidulafungin against Azole-Susceptible and Azole-Resistant Aspergillus fumigatus Isolates. J. Antimicrob. Chemother. 2020;75:2582–2586. doi: 10.1093/jac/dkaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fakhim H., Chowdhary A., Prakash A., Vaezi A., Dannaoui E., Meis J.F., Badali H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017;61:e01056-17. doi: 10.1128/AAC.01056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fakhim H., Vaezi A., Dannaoui E., Sharma C., Mousavi B., Chowdhary A., Meis J.F., Badali H. In Vitro Combination of Voriconazole with Micafungin against Azole-Resistant Clinical Isolates of Aspergillus fumigatus from Different Geographical Regions. Diagn. Microbiol. Infect. Dis. 2018;91:266–268. doi: 10.1016/j.diagmicrobio.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 117.Pfaller M.A., Messer S.A., Deshpande L.M., Rhomberg P.R., Utt E.A., Castanheira M. Evaluation of Synergistic Activity of Isavuconazole or Voriconazole plus Anidulafungin and the Occurrence and Genetic Characterization of Candida auris Detected in a Surveillance Program. Antimicrob. Agents Chemother. 2021;65:e02031-20. doi: 10.1128/AAC.02031-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caballero U., Kim S., Eraso E., Quindós G., Vozmediano V., Schmidt S., Jauregizar N. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics. 2021;10:355. doi: 10.3390/antibiotics10040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sradhanjali S., Yein B., Sharma S., Das S. In Vitro Synergy of Natamycin and Voriconazole against Clinical Isolates of Fusarium, Candida, Aspergillus and Curvularia spp. Br. J. Ophthalmol. 2018;102:142–145. doi: 10.1136/bjophthalmol-2017-310683. [DOI] [PubMed] [Google Scholar]
- 120.Gupta A.K., Kohli Y. In Vitro Susceptibility Testing of Ciclopirox, Terbinafine, Ketoconazole and Itraconazole against Dermatophytes and Nondermatophytes, and In Vitro Evaluation of Combination Antifungal Activity. Br. J. Dermatol. 2003;149:296–305. doi: 10.1046/j.1365-2133.2003.05418.x. [DOI] [PubMed] [Google Scholar]
- 121.Bidaud A.L., Botterel F., Chowdhary A., Dannaouia E. In Vitro Antifungal Combination of Flucytosine with Amphotericin B, Voriconazole, or Micafungin against Candida auris Shows No Antagonism. Antimicrob. Agents Chemother. 2019;63:e01393-19. doi: 10.1128/AAC.01393-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macedo D., Leonardelli F., Dudiuk C., Vitale R.G., del Valle E., Giusiano G., Gamarra S., Garcia-Effron G. In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis. J. Fungi. 2019;5:5. doi: 10.3390/jof5010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polak A. Combination of Amorolfine with Various Antifungal Drugs in Dermatophytosis. Mycoses. 2009;36:43–49. doi: 10.1111/j.1439-0507.1993.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 124.Tamura T., Asahara M., Yamamoto M., Yamaura M., Matsumura M., Goto K., Rezaei-Matehkolaei A., Mirhendi H., Makimura M., Makimura K. In Vitro Susceptibility of Dermatomycoses Agents to Six Antifungal Drugs and Evaluation by Fractional Inhibitory Concentration Index of Combined Effects of Amorolfine and Itraconazole in Dermatophytes. Microbiol. Immunol. 2014;58:1–8. doi: 10.1111/1348-0421.12109. [DOI] [PubMed] [Google Scholar]
- 125.Lecha M. Amorolfine and Itraconazole Combination for Severe Toenail Onychomycosis; Results of an Open Randomized Trial in Spain. Br. J. Dermatol. 2008;145:21–26. doi: 10.1111/j.1365-2133.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 126.Laurent A., Monod M. Production of Trichophyton rubrum Microspores in Large Quantities and Its Application to Evaluate Amorolfine/Azole Compound Interactions In Vitro. Mycoses. 2017;60:581–586. doi: 10.1111/myc.12632. [DOI] [PubMed] [Google Scholar]
- 127.Shrestha S.K., Grilley M., Anderson T., Dhiman C., Oblad J., Chang C.-W.T., Sorensen K.N., Takemoto J.Y. In Vitro Antifungal Synergy between Amphiphilic Aminoglycoside K20 and Azoles against Candida Species and Cryptococcus neoformans. Med. Mycol. 2015;53:837–844. doi: 10.1093/mmy/myv063. [DOI] [PubMed] [Google Scholar]
- 128.Revie N.M., Robbins N., Whitesell L., Frost J.R., Appavoo S.D., Yudin A.K., Cowen L.E. Oxadiazole-Containing Macrocyclic Peptides Potentiate Azole Activity against Pathogenic Candida Species. mSphere. 2020;5:e00256-20. doi: 10.1128/mSphere.00256-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eldesouky H.E., Li X., Abutaleb N.S., Mohammad H., Seleem M.N. Synergistic Interactions of Sulfamethoxazole and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Int. J. Antimicrob. Agents. 2018;52:754–761. doi: 10.1016/j.ijantimicag.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 130.Eldesouky H.E., Mayhoub A., Hazbun T.R., Seleema M.N. Reversal of Azole Resistance in Candida albicans by Sulfa Antibacterial Drugs. Antimicrob. Agents Chemother. 2018;62:e00701-17. doi: 10.1128/AAC.00701-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao Y., Zhang C., Lu C., Liu P., Li Y., Li H., Sun S. Synergistic Effect of Doxycycline and Fluconazole against Candida albicans Biofilms and the Impact of Calcium Channel Blockers. FEMS Yeast Res. 2013;13:453–462. doi: 10.1111/1567-1364.12048. [DOI] [PubMed] [Google Scholar]
- 132.Hooper R.W., Ashcraft D.S., Pankey G.A. In Vitro Synergy with Fluconazole plus Doxycycline or Tigecycline against Clinical Candida glabrata Isolates. Med. Mycol. 2019;57:122–126. doi: 10.1093/mmy/myy008. [DOI] [PubMed] [Google Scholar]
- 133.Gu W., Yu Q., Yu C., Sun S. In Vivo Activity of Fluconazole/Tetracycline Combinations in Galleria mellonella with Resistant Candida albicans Infection. J. Glob. Antimicrob. Resist. 2018;13:74–80. doi: 10.1016/j.jgar.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 134.Venturini T.P., Rossato L., Chassot F., Keller J.T., Piasentin F.B., Santurio J.M., Alves S.H. In Vitro Synergistic Combinations of Pentamidine, Polymyxin B, Tigecycline and Tobramycin with Antifungal Agents against Fusarium spp. J. Med. Microbiol. 2016;65:770–774. doi: 10.1099/jmm.0.000301. [DOI] [PubMed] [Google Scholar]
- 135.Hacioglu M., Tan A.S.B., Dosler S., Inan N., Otuk G. In Vitro Activities of Antifungals Alone and in Combination with Tigecycline against Candida albicans Biofilms. PeerJ. 2018;2018:e5263. doi: 10.7717/peerj.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao L., Sun Y., Yuan M., Li M., Zeng T. In Vitro and In Vivo Study on the Synergistic Effect of Minocycline and Azoles against Pathogenic Fungi. Antimicrob. Agents Chemother. 2020;64:e00290-20. doi: 10.1128/AAC.00290-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tan J., Jiang S., Tan L., Shi H., Yang L., Sun Y., Wang X. Antifungal Activity of Minocycline and Azoles Against Fluconazole-Resistant Candida Species. Front. Microbiol. 2021;12:1185. doi: 10.3389/fmicb.2021.649026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan L., Shi H., Chen M., Wang Z., Yao Z., Sun Y. In Vitro Synergistic Effect of Minocycline Combined with Antifungals against Cryptococcus neoformans. J. Med. Mycol. 2022;32:101227. doi: 10.1016/j.mycmed.2021.101227. [DOI] [PubMed] [Google Scholar]
- 139.Yang F., Sun Y., Lu Q. The Synergistic Effect of Minocycline and Azole Antifungal Drugs against Scedosporium and Lomentospora Species. BMC Microbiol. 2022;22:21. doi: 10.1186/s12866-021-02433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lu M., Yu C., Cui X., Shi J., Yuan L., Sun S. Gentamicin Synergises with Azoles against Drug-Resistant Candida albicans. Int. J. Antimicrob. Agents. 2018;51:107–114. doi: 10.1016/j.ijantimicag.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 141.Lu M., Yang X., Yu C., Gong Y., Yuan L., Hao L., Sun S. Linezolid in Combination With Azoles Induced Synergistic Effects Against Candida albicans and Protected Galleria mellonella Against Experimental Candidiasis. Front. Microbiol. 2019;9:3142. doi: 10.3389/fmicb.2018.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yousfi H., Ranque S., Rolain J.M., Bittar F. In Vitro Polymyxin Activity against Clinical Multidrug-Resistant Fungi. Antimicrob. Resist. Infect. Control. 2019;8:66. doi: 10.1186/s13756-019-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwarz P., Djenontin E., Dannaoui E. Colistin and Isavuconazole Interact Synergistically In Vitro against Aspergillus nidulans and Aspergillus niger. Microorganisms. 2020;8:1447. doi: 10.3390/microorganisms8091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwarz P., Bidaud A.-L., Dannaoui E. In Vitro Synergy of Isavuconazole in Combination with Colistin against Candida auris. Sci. Rep. 2020;10:21448. doi: 10.1038/s41598-020-78588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gao L., Sun Y., He C., Zeng T., Li M. Synergy between Pyrvinium Pamoate and Azoles against Exophiala dermatitidis. Antimicrob. Agents Chemother. 2018;62:e02361-17. doi: 10.1128/AAC.02361-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Cremer K., Lanckacker E., Cools T.L., Bax M., de Brucker K., Cos P., Cammue B.P.A., Thevissen K. Artemisinins, New Miconazole Potentiators Resulting in Increased Activity against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2015;59:421–426. doi: 10.1128/AAC.04229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Y., Wan Z., Liu W., Li R. Synergistic Activity of Chloroquine with Fluconazole against Fluconazole-Resistant Isolates of Candida Species. Antimicrob. Agents Chemother. 2015;59:1365–1369. doi: 10.1128/AAC.04417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao L., Sun Y., He C., Li M., Zeng T., Lu Q. INK128 Exhibits Synergy with Azoles against Exophiala spp. and Fusarium spp. Front. Microbiol. 2016;7:1658. doi: 10.3389/fmicb.2016.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Montoya M.C., Beattie S., Alden K.M., Krysan D.J. Derivatives of the Antimalarial Drug Mefloquine Are Broad-Spectrum Antifungal Molecules with Activity against Drug-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 2020;64:e02331-19. doi: 10.1128/AAC.02331-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brilhante R.S.N., Caetano É.P., Riello G.B., de M. Guedes G.M., de Souza Collares Maia Castelo-Branco D., Fechine M.A.B., de Oliveira J.S., de Camargo Z.P., de Mesquita J.R.L., Monteiro A.J., et al. Antiretroviral Drugs Saquinavir and Ritonavir Reduce Inhibitory Concentration Values of Itraconazole against Histoplasma capsulatum Strains In Vitro. Braz. J. Infect. Dis. 2016;20:155–159. doi: 10.1016/j.bjid.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.LaFleur M.D., Sun L., Lister I., Keating J., Nantel A., Long L., Ghannoum M., North J., Lee R.E., Coleman K., et al. Potentiation of Azole Antifungals by 2-Adamantanamine. Antimicrob. Agents Chemother. 2013;57:3585–3592. doi: 10.1128/AAC.00294-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang M., Yan H., Lu M., Wang D., Sun S. Antifungal Activity of Ribavirin Used Alone or in Combination with Fluconazole against Candida albicans Is Mediated by Reduced Virulence. Int. J. Antimicrob. Agents. 2020;55:105804. doi: 10.1016/j.ijantimicag.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 153.Eldesouky H.E., Salama E.A., Lanman N.A., Hazbun T.R., Seleem M.N. Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2020;65:e00684-20. doi: 10.1128/AAC.00684-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao Y.J., Liu W.D., Shen Y.N., Li D.M., Zhu K.J., Zhang H. The Efflux Pump Inhibitor Tetrandrine Exhibits Synergism with Fluconazole or Voriconazole against Candida parapsilosis. Mol. Biol. Rep. 2019;46:5867–5874. doi: 10.1007/s11033-019-05020-1. [DOI] [PubMed] [Google Scholar]
- 155.Li S.-X., Song Y.-J., Jiang L., Zhao Y.-J., Guo H., Li D.-M., Zhu K.-J., Zhang H. Synergistic Effects of Tetrandrine with Posaconazole Against Aspergillus fumigatus. Microb. Drug Resist. 2017;23:674–681. doi: 10.1089/mdr.2016.0217. [DOI] [PubMed] [Google Scholar]
- 156.Shi J., Li S., Gao A., Zhu K., Zhang H. Tetrandrine Enhances the Antifungal Activity of Fluconazole in a Murine Model of Disseminated Candidiasis. Phytomedicine. 2018;46:21–31. doi: 10.1016/j.phymed.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 157.Li S.X., Song Y.J., Zhang L.L., Shi J.P., Ma Z.L., Guo H., Dong H.Y., Li Y.M., Zhang H. An In Vitro and In Vivo Study on the Synergistic Effect and Mechanism of Itraconazole or Voriconazole Alone and in Combination with Tetrandrine against Aspergillus fumigatus. J. Med. Microbiol. 2015;64:1008–1020. doi: 10.1099/jmm.0.000120. [DOI] [PubMed] [Google Scholar]
- 158.Zeng Q., Zhang Z., Chen P., Long N., Lu L., Sang H. In Vitro and In Vivo Efficacy of a Synergistic Combination of Itraconazole and Verapamil Against Aspergillus fumigatus. Front. Microbiol. 2019;10:1266. doi: 10.3389/fmicb.2019.01266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Afeltra J., Vitale R.G., Mouton J.W., Verweij P.E. Potent Synergistic In Vitro Interaction between Nonantimicrobial Membrane-Active Compounds and Itraconazole against Clinical Isolates of Aspergillus fumigatus Resistant to Itraconazole. Antimicrob. Agents Chemother. 2004;48:1335–1343. doi: 10.1128/AAC.48.4.1335-1343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu J., Liu R., Sun F., An L., Shang Z., Kong L., Yang M. Eucalyptal D Enhances the Antifungal Effect of Fluconazole on Fluconazole-Resistant Candida albicans by Competitively Inhibiting Efflux Pump. Front. Cell. Infect. Microbiol. 2019;9:211. doi: 10.3389/fcimb.2019.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang D.L., Hu Y.L., Yin Z.X., Zeng G.S., Li D., Zhang Y.Q., Xu Z.H., Guan X.M., Weng L.X., Wang L.H. Cis-2-Dodecenoic Acid Mediates Its Synergistic Effect with Triazoles by Interfering with Efflux Pumps in Fluconazole-Resistant Candida albicans. Biomed. Environ. Sci. 2019;32:199–209. doi: 10.3967/bes2019.027. [DOI] [PubMed] [Google Scholar]
- 162.Iyer K.R., Camara K., Daniel-Ivad M., Trilles R., Pimentel-Elardo S.M., Fossen J.L., Marchillo K., Liu Z., Singh S., Muñoz J.F., et al. An Oxindole Efflux Inhibitor Potentiates Azoles and Impairs Virulence in the Fungal Pathogen Candida auris. Nat. Commun. 2020;11:6429. doi: 10.1038/s41467-020-20183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eldesouky H.E., Salama E.A., Hazbun T.R., Mayhoub A.S., Seleem M.N. Ospemifene Displays Broad-Spectrum Synergistic Interactions with Itraconazole through Potent Interference with Fungal Efflux Activities. Sci. Rep. 2020;10:6089. doi: 10.1038/s41598-020-62976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xie F., Chang W., Zhang M., Li Y., Li W., Shi H., Zheng S., Lou H. Quinone Derivatives Isolated from the Endolichenic Fungus Phialocephala fortinii Are Mdr1 Modulators That Combat Azole Resistance in Candida albicans. Sci. Rep. 2016;6:33687. doi: 10.1038/srep33687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh S., Fatima Z., Ahmad K., Hameed S. Fungicidal Action of Geraniol against Candida albicans Is Potentiated by Abrogated CaCdr1p Drug Efflux and Fluconazole Synergism. PLoS ONE. 2018;13:e0203079. doi: 10.1371/journal.pone.0203079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nyilasi I., Kocsubé S., Krizsán K., Galgóczy L., Pesti M., Papp T., Vágvölgyi C. In Vitro Synergistic Interactions of the Effects of Various Statins and Azoles against Some Clinically Important Fungi. FEMS Microbiol. Lett. 2010;307:175–184. doi: 10.1111/j.1574-6968.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- 167.Nyilasi I., Kocsubé S., Krizsán K., Galgóczy L., Papp T., Pesti M., Nagy K., Vágvölgyi C. Susceptibility of Clinically Important Dermatophytes against Statins and Different Statin-Antifungal Combinations. Med. Mycol. 2013;52:140–148. doi: 10.3109/13693786.2013.828160. [DOI] [PubMed] [Google Scholar]
- 168.Zhou Y., Yang H., Zhou X., Luo H., Tang F., Yang J., Alterovitz G., Cheng L., Ren B. Lovastatin Synergizes with Itraconazole against Planktonic Cells and Biofilms of Candida albicans through the Regulation on Ergosterol Biosynthesis Pathway. Appl. Microbiol. Biotechnol. 2018;102:5255–5264. doi: 10.1007/s00253-018-8959-8. [DOI] [PubMed] [Google Scholar]
- 169.de Q. Ribeiro N., Costa M.C., Magalhães T.F.F., Carneiro H.C.S., Oliveira L.V., Fontes A.C.L., Santos J.R.A., Ferreira G.F., de S. Araujo G.R., Alves V., et al. Atorvastatin as a Promising Anticryptococcal Agent. Int. J. Antimicrob. Agents. 2017;49:695–702. doi: 10.1016/j.ijantimicag.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 170.Brilhante R.S., Caetano E.P., Oliveira J.S., Castelo-Branco D., Souza E.R., Alencar L.P., Cordeiro R., Bandeira T., Sidrim J.J., Rocha M.F. Simvastatin Inhibits Planktonic Cells and Biofilms of Candida and Cryptococcus Species. Braz. J. Infect. Dis. 2015;19:459–465. doi: 10.1016/j.bjid.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Eldesouky H.E., Salama E.A., Li X., Hazbun T.R., Mayhoub A.S., Seleem M.N. Repurposing Approach Identifies Pitavastatin as a Potent Azole Chemosensitizing Agent Effective against Azole-Resistant Candida Species. Sci. Rep. 2020;10:7525. doi: 10.1038/s41598-020-64571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kane A., Campbell L., Ky D., Hibbs D., Carter D. The Antifungal and Synergistic Effect of Bisphosphonates in Cryptococcus. Antimicrob. Agents Chemother. 2021;65:e01753-20. doi: 10.1128/AAC.01753-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aneke C.I., Rhimi W., Otranto D., Cafarchia C. Synergistic Effects of Efflux Pump Modulators on the Azole Antifungal Susceptibility of Microsporum canis. Mycopathologia. 2020;185:279–288. doi: 10.1007/s11046-019-00419-7. [DOI] [PubMed] [Google Scholar]
- 174.Brilhante R.S.N., de Oliveira J.S., de Jesus Evangelista A.J., Pereira V.S., Alencar L.P., de Souza Collares Maia Castelo-Branco D., Câmara L.M.C., de Lima-Neto R.G., de A. Cordeiro R., Sidrim J.J.C., et al. In Vitro Effects of Promethazine on Cell Morphology and Structure and Mitochondrial Activity of Azole-Resistant Candida tropicalis. Med. Mycol. 2017;56:1012–1022. doi: 10.1093/mmy/myx088. [DOI] [PubMed] [Google Scholar]
- 175.Dennis E.K., Garneau-Tsodikova S. Synergistic Combinations of Azoles and Antihistamines against Candida Species In Vitro. Med. Mycol. 2019;57:874–884. doi: 10.1093/mmy/myy088. [DOI] [PubMed] [Google Scholar]
- 176.Sun W., Wang D., Yu C., Huang X., Li X., Sun S. Strong Synergism of Dexamethasone in Combination with Fluconazole against Resistant Candida albicans Mediated by Inhibiting Drug Efflux and Reducing Virulence. Int. J. Antimicrob. Agents. 2017;50:399–405. doi: 10.1016/j.ijantimicag.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 177.Li X., Yu C., Huang X., Sun S. Synergistic Effects and Mechanisms of Budesonide in Combination with Fluconazole against Resistant Candida albicans. PLoS ONE. 2016;11:e0168936. doi: 10.1371/journal.pone.0168936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang J., Gao L., Yu P., Kosgey J.C., Jia L., Fang Y., Xiong J., Zhang F. In Vitro Synergy of Azole Antifungals and Methotrexate against Candida albicans. Life Sci. 2019;235:116827. doi: 10.1016/j.lfs.2019.116827. [DOI] [PubMed] [Google Scholar]
- 179.Holbrook S.Y.L., Garzan A., Dennis E.K., Shrestha S.K., Garneau-Tsodikova S. Repurposing Antipsychotic Drugs into Antifungal Agents: Synergistic Combinations of Azoles and Bromperidol Derivatives in the Treatment of Various Fungal Infections. Eur. J. Med. Chem. 2017;139:12–21. doi: 10.1016/j.ejmech.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 180.Gu W., Guo D., Zhang L., Xu D., Sun S. The Synergistic Effect of Azoles and Fluoxetine against Resistant Candida albicans Strains Is Attributed to Attenuating Fungal Virulence. Antimicrob. Agents Chemother. 2016;60:6179–6188. doi: 10.1128/AAC.03046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cong L., Liao Y., Yang S., Yang R. In Vitro Antifungal Activity of Sertraline and Synergistic Effects in Combination with Antifungal Drugs against Planktonic Forms and Biofilms of Clinical Trichosporon asahii Isolates. PLoS ONE. 2016;11:e0167903. doi: 10.1371/journal.pone.0167903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jia W., Zhang H., Li C., Li G., Liu X., Wei J. The Calcineruin Inhibitor Cyclosporine a Synergistically Enhances the Susceptibility of Candida albicans Biofilms to Fluconazole by Multiple Mechanisms. BMC Microbiol. 2016;16:113. doi: 10.1186/s12866-016-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tome M., Zupan J., Tomičić Z., Matos T., Raspor P. Synergistic and Antagonistic Effects of Immunomodulatory Drugs on the Action of Antifungals against Candida glabrata and Saccharomyces cerevisiae. PeerJ. 2018;2018:e4999. doi: 10.7717/peerj.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Onyewu C., Blankenship J.R., Del Poeta M., Heitman J. Ergosterol Biosynthesis Inhibitors Become Fungicidal When Combined with Calcineurin Inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003;47:956–964. doi: 10.1128/AAC.47.3.956-964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uppuluri P., Nett J., Heitman J., Andes D. Synergistic Effect of Calcineurin Inhibitors and Fluconazole against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2008;52:1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Steinbach W.J., Schell W.A., Blankenship J.R., Onyewu C., Heitman J., Perfect J.R. In Vitro Interactions between Antifungals and Immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2004;48:1664–1669. doi: 10.1128/AAC.48.5.1664-1669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Marchetti O., Moreillon P., Glauser M.P., Bille J., Sanglard D. Potent Synergism of the Combination of Fluconazole and Cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 2000;44:2373–2381. doi: 10.1128/AAC.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao L., Sun Y., He C., Zeng T., Li M. Synergistic Effects of Tacrolimus and Azoles against Exophiala dermatitidis. Antimicrob. Agents Chemother. 2017;61:e00948-17. doi: 10.1128/AAC.00948-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gao L., Sun Y. In Vitro Interactions of Antifungal Agents and Tacrolimus against Aspergillus Biofilms. Antimicrob. Agents Chemother. 2015;59:7097–7099. doi: 10.1128/AAC.01510-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Denardi L.B., Mario D.A.N., Loreto É.S., Santurio J.M., Alves S.H. Synergistic Effects of Tacrolimus and Azole Antifungal Compounds in Fluconazole-Susceptible and Fluconazole-Resistant Candida glabrata Isolates. Braz. J. Microbiol. 2015;46:125–129. doi: 10.1590/S1517-838246120120442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kubiça T.F., Denardi L.B., Azevedo M.I., Oliveira V., Severo L.C., Santurio J.M., Alves S.H. Antifungal Activities of Tacrolimus in Combination with Antifungal Agents against Fluconazole-Susceptible and Fluconazole-Resistant Trichosporon asahii Isolates. Braz. J. Infect. Dis. 2016;20:539–545. doi: 10.1016/j.bjid.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Borba-Santos L.P., Reis de Sá L.F., Ramos J.A., Rodrigues A.M., de Camargo Z.P., Rozental S., Ferreira-Pereira A. Tacrolimus Increases the Effectiveness of Itraconazole and Fluconazole against Sporothrix spp. Front. Microbiol. 2017;8:1759. doi: 10.3389/fmicb.2017.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang J., Tan J., Yang L., He Y. Tacrolimus, Not Triamcinolone Acetonide, Interacts Synergistically with Itraconazole, Terbinafine, Bifonazole, and Amorolfine against Clinical Dermatophyte Isolates. J. De Mycol. Med. 2018;28:612–616. doi: 10.1016/j.mycmed.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 194.Lu M., Yan H., Yu C., Yuan L., Sun S. Proton Pump Inhibitors Act Synergistically with Fluconazole against Resistant Candida albicans. Sci. Rep. 2020;10:498. doi: 10.1038/s41598-019-57174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jia C., Zhang J., Zhuge Y., Xu K., Liu J., Wang J., Li L., Chu M. Synergistic Effects of Geldanamycin with Fluconazole Are Associated with Reactive Oxygen Species in Candida tropicalis Resistant to Azoles and Amphotericin B. Free Radic. Res. 2019;53:618–628. doi: 10.1080/10715762.2019.1610563. [DOI] [PubMed] [Google Scholar]
- 196.Liu L., Jiang T., Zhou J., Mei Y., Li J., Tan J., Wei L., Li J., Peng Y., Chen C., et al. Repurposing the FDA-Approved Anticancer Agent Ponatinib as a Fluconazole Potentiator by Suppression of Multidrug Efflux and Pma1 Expression in a Broad Spectrum of Yeast Species. Microb. Biotechnol. 2022;15:482–498. doi: 10.1111/1751-7915.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li L.P., An M.M., Shen H., Huang X., Yao X., Liu J., Zhu F., Zhang S.Q., Chen S.M., He L.J., et al. The Non-Geldanamycin Hsp90 Inhibitors Enhanced the Antifungal Activity of Fluconazole. Am. J. Transl. Res. 2015;7:2589–2602. [PMC free article] [PubMed] [Google Scholar]
- 198.Sun Y., Gao L., He C., Wu Q., Li M., Zeng T. Givinostat Exhibits In Vitro Synergy with Posaconazole against Aspergillus spp. Med. Mycol. 2016;55:myw131. doi: 10.1093/mmy/myw131. [DOI] [PubMed] [Google Scholar]
- 199.Qiao J., Sun Y., Gao L., He C., Zheng W. Lonafarnib Synergizes with Azoles against Aspergillus spp. and Exophiala spp. Med. Mycol. 2018;56:452–457. doi: 10.1093/mmy/myx072. [DOI] [PubMed] [Google Scholar]
- 200.Kim S., Woo E.-R., Lee D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida albicans. IUBMB Life. 2019;71:283–292. doi: 10.1002/iub.1973. [DOI] [PubMed] [Google Scholar]
- 201.Lai Y.-W., Campbell L.T., Wilkins M.R., Pang C.N.I., Chen S., Carter D.A. Synergy and Antagonism between Iron Chelators and Antifungal Drugs in Cryptococcus. Int. J. Antimicrob. Agents. 2016;48:388–394. doi: 10.1016/j.ijantimicag.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 202.Li Y., Jiao P., Li Y., Gong Y., Chen X., Sun S. The Synergistic Antifungal Effect and Potential Mechanism of D-Penicillamine Combined With Fluconazole Against Candida albicans. Front. Microbiol. 2019;10:2853. doi: 10.3389/fmicb.2019.02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu X., Li T., Wang D., Yang Y., Sun W., Liu J., Sun S. Synergistic Antifungal Effect of Fluconazole Combined with Licofelone against Resistant Candida albicans. Front. Microbiol. 2017;8:2101. doi: 10.3389/fmicb.2017.02101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun W., Zhang L., Lu X., Feng L., Sun S. The Synergistic Antifungal Effects of Sodium Phenylbutyrate Combined with Azoles against Candida albicans via the Regulation of the Ras–CAMP–PKA Signalling Pathway and Virulence. Can. J. Microbiol. 2019;65:105–115. doi: 10.1139/cjm-2018-0337. [DOI] [PubMed] [Google Scholar]
- 205.Gao L., Sun Y., He C., Li M., Zeng T. In Vitro Interactions between 17-AAG and Azoles against Exophiala dermatitidis. Mycoses. 2018;61:853–856. doi: 10.1111/myc.12824. [DOI] [PubMed] [Google Scholar]
- 206.de Andrade Neto J.B., da Silva C.R., Barroso F.D., do Amaral Valente Sá L.G., de Sousa Campos R., S Aires do Nascimento F.B., Sampaio L.S., da Silva A.R., da Silva L.J., de Sá Carneiro I., et al. Synergistic Effects of Ketamine and Azole Derivatives on Candida Spp. Resistance to Fluconazole. Future Microbiol. 2020;15:177–188. doi: 10.2217/fmb-2019-0082. [DOI] [PubMed] [Google Scholar]
- 207.Ahangarkani F., Khodavaisy S., Mahmoudi S., Shokohi T., Rezai M.S., Fakhim H., Dannaoui E., Faraji S., Chowdhary A., Meis J.F., et al. Indifferent Effect of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Combined with Fluconazole against Multidrug-Resistant Candida auris. Curr. Med. Mycol. 2019;5:26–30. doi: 10.18502/cmm.5.3.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hao W., Wang Y., Xi Y., Yang Z., Zhang H., Ge X. Activity of Chlorhexidine Acetate in Combination with Fluconazole against Suspensions and Biofilms of Candida auris. J. Infect. Chemother. 2022;28:29–34. doi: 10.1016/j.jiac.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 209.Yuan R., Tu J., Sheng C., Chen X., Liu N. Effects of Hsp90 Inhibitor Ganetespib on Inhibition of Azole-Resistant Candida albicans. Front. Microbiol. 2021;12:1280. doi: 10.3389/fmicb.2021.680382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vu K., Blumwald E., Gelli A. The Antifungal Activity of HMA, an Amiloride Analog and Inhibitor of Na+/H+ Exchangers. Front. Microbiol. 2021;12:1055. doi: 10.3389/fmicb.2021.673035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ahmad A., Khan A., Manzoor N. Reversal of Efflux Mediated Antifungal Resistance Underlies Synergistic Activity of Two Monoterpenes with Fluconazole. Eur. J. Pharm. Sci. 2013;48:80–86. doi: 10.1016/j.ejps.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 212.Shaban S., Patel M., Ahmad A. Improved Efficacy of Antifungal Drugs in Combination with Monoterpene Phenols against Candida auris. Sci. Rep. 2020;10:1162. doi: 10.1038/s41598-020-58203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.de Aguiar F.L.L., de Morais S.M., dos Santos H.S., Albuquerque M.R.J.R., Bandeira P.N., de Brito E.H.S., Rocha M.F.G., dos Santos Fontenelle R.O. Antifungal Activity and Synergistic Effect of Acetophenones Isolated from Species Croton against Dermatophytes and Yeasts. J. Med. Plants Res. 2016;10:216–222. doi: 10.5897/jmpr2016.6048. [DOI] [Google Scholar]
- 214.Li D.D., Chai D., Huang X.W., Guan S.X., Du J., Zhang H.Y., Sun Y., Jiang Y.Y. Potent In Vitro Synergism of Fluconazole and Osthole against Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2017;61:e00436-17. doi: 10.1128/AAC.00436-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shao J., Cui Y., Zhang M., Wang T., Wu D., Wang C. Synergistic In Vitro Activity of Sodium Houttuyfonate with Fluconazole against Clinical Candida albicans Strains under Planktonic Growing Conditions. Pharm. Biol. 2017;55:355–359. doi: 10.1080/13880209.2016.1237977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sharifzadeh A., Khosravi A.R., Shokri H., Tari P.S. Synergistic Anticandidal Activity of Menthol in Combination with Itraconazole and Nystatin against Clinical Candida glabrata and Candida krusei Isolates. Microb. Pathog. 2017;107:390–396. doi: 10.1016/j.micpath.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 217.de A. Cordeiro R., Teixeira C.E.C., Brilhante R.S.N., Castelo-Branco D.S.C.M., Alencar L.P., de Oliveira J.S., Monteiro A.J., Bandeira T.J.P.G., Sidrim J.J.C., Moreira J.L.B., et al. Exogenous Tyrosol Inhibits Planktonic Cells and Biofilms of Candida Species and Enhances Their Susceptibility to Antifungals. FEMS Yeast Res. 2015;15:12. doi: 10.1093/femsyr/fov012. [DOI] [PubMed] [Google Scholar]
- 218.Raut J.S., Bansode B.S., Jadhav A.K., Karuppayil S.M. Activity of Allyl Isothiocyanate and Its Synergy with Fluconazole against Candida albicans Biofilms. J. Microbiol. Biotechnol. 2017;27:685–693. doi: 10.4014/jmb.1607.07072. [DOI] [PubMed] [Google Scholar]
- 219.Gong Y., Liu W., Huang X., Hao L., Li Y., Sun S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole against Candida albicans. Front. Microbiol. 2019;10:1461. doi: 10.3389/fmicb.2019.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nabili M., Aslani N., Shokohi T., Hedayati M.T., Hassanmoghadam F., Moazeni M. In Vitro Interaction between Glabridin and Voriconazole against Aspergillus fumigatus Isolates. Rev. Iberoam. De Micol. 2021;38:145–147. doi: 10.1016/j.riam.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 221.Ganan M., Lorentzen S.B., Gaustad P., Sørlie M. Synergistic Antifungal Activity of Chito-Oligosaccharides and Commercial Antifungals on Biofilms of Clinical Candida Isolates. J. Fungi. 2021;7:718. doi: 10.3390/jof7090718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen H., Li H., Duan C., Song C., Peng Z., Shi W. Reversal of Azole Resistance in Candida albicans by Oridonin. J. Glob. Antimicrob. Resist. 2021;24:296–302. doi: 10.1016/j.jgar.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 223.Sadowska B., Budzyńska A., Stochmal A., Żuchowski J., Różalska B. Novel Properties of Hippophae Rhamnoides L. Twig and Leaf Extracts—Anti-Virulence Action and Synergy with Antifungals Studied In Vitro on Candida Spp. Model. Microb. Pathog. 2017;107:372–379. doi: 10.1016/j.micpath.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 224.da Gabriella G., Pippi B., Dalla Lana D.F., Amaral A.P.S., Teixeira M.L., de Souza K.C.B., Fuentefria A.M. Reversal of Fluconazole Resistance Induced by a Synergistic Effect with Acca sellowiana in Candida glabrata Strains. Pharm. Biol. 2016;54:2410–2419. doi: 10.3109/13880209.2016.1158286. [DOI] [PubMed] [Google Scholar]
- 225.Sadhasivam S., Palanivel S., Ghosh S. Synergistic Antimicrobial Activity of Boswellia serrata Roxb. Ex Colebr. (Burseraceae) Essential Oil with Various Azoles against Pathogens Associated with Skin, Scalp and Nail Infections. Lett. Appl. Microbiol. 2016;63:495–501. doi: 10.1111/lam.12683. [DOI] [PubMed] [Google Scholar]
- 226.Roana J., Mandras N., Scalas D., Campagna P., Tullio V. Antifungal Activity of Melaleuca alternifolia Essential Oil (TTO) and Its Synergy with Itraconazole or Ketoconazole against Trichophyton rubrum. Molecules. 2021;26:461. doi: 10.3390/molecules26020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yang Z., Wang Q., Ma K., Shi P., Liu W., Huang Z. Fluconazole Inhibits Cellular Ergosterol Synthesis to Confer Synergism with Berberine against Yeast Cells. J. Glob. Antimicrob. Resist. 2018;13:125–130. doi: 10.1016/j.jgar.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 228.Li D.D., Xu Y., Zhang D.Z., Quan H., Mylonakis E., Hu D.D., Li M.B., Zhao L.X., Zhu L.H., Wang Y., et al. Fluconazole Assists Berberine to Kill Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2013;57:6016–6027. doi: 10.1128/AAC.00499-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wei G.X., Xu X., Wu C.D. In Vitro Synergism between Berberine and Miconazole against Planktonic and Biofilm Candida Cultures. Arch. Oral Biol. 2011;56:565–572. doi: 10.1016/j.archoralbio.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 230.Iwazaki R.S., Endo E.H., Ueda-Nakamura T., Nakamura C.V., Garcia L.B., Filho B.P.D. In Vitro Antifungal Activity of the Berberine and Its Synergism with Fluconazole. Int. J. Gen. Mol. Microbiol. 2010;97:201–205. doi: 10.1007/s10482-009-9394-8. [DOI] [PubMed] [Google Scholar]
- 231.Quan H., Cao Y.Y., Xu Z., Zhao J.X., Gao P.H., Qin X.F., Jiang Y.Y. Potent In Vitro Synergism of Fluconazole and Berberine Chloride against Clinical Isolates of Candida albicans Resistant to Fluconazole. Antimicrob. Agents Chemother. 2006;50:1096–1099. doi: 10.1128/AAC.50.3.1096-1099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Luo H., Pan K.-S., Luo X.-L., Zheng D.-Y., Andrianopoulos A., Wen L.-M., Zheng Y.-Q., Guo J., Huang C.-Y., Li X.-Y., et al. In Vitro Susceptibility of Berberine Combined with Antifungal Agents Against the Yeast Form of Talaromyces marneffei. Mycopathologia. 2019;184:295–301. doi: 10.1007/s11046-019-00325-y. [DOI] [PubMed] [Google Scholar]
- 233.Wang T., Shao J., Da W., Li Q., Shi G., Wu D., Wang C. Strong Synergism of Palmatine and Fluconazole/Itraconazole Against Planktonic and Biofilm Cells of Candida Species and Efflux-Associated Antifungal Mechanism. Front. Microbiol. 2018;9:2892. doi: 10.3389/fmicb.2018.02892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Campos R.D., da Silva C.R., de Andrade Neto J.B., Sampaio L.S., Aires do Nascimento F.B.S., de Moraes M.O., Cavalcanti B.C., Magalhães H.I.F., Gomes A.O., Lobo M.D.P., et al. Antifungal Activity of Palmatine against Strains of Candida Spp. Resistant to Azoles in Planktonic Cells and Biofilm. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:3657–3669. doi: 10.20546/ijcmas.2018.702.435. [DOI] [Google Scholar]
- 235.Li X., Wu X., Gao Y., Hao L. Synergistic Effects and Mechanisms of Combined Treatment With Harmine Hydrochloride and Azoles for Resistant Candida albicans. Front. Microbiol. 2019;10:2295. doi: 10.3389/fmicb.2019.02295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ribeiro de Carvalho R., Chaves Silva N., Cusinato M., Tranches Dias K.S., dos Santos M.H., Viegas Junior C., Silva G., Tranches Dias A.L. Promising Synergistic Activity of Fluconazole with Bioactive Guttiferone-A and Derivatives against Non-albicans Candida Species. J. De Mycol. Med. 2018;28:645–650. doi: 10.1016/j.mycmed.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 237.Nagy F., Vitális E., Jakab Á., Borman A.M., Forgács L., Tóth Z., Majoros L., Kovács R. In Vitro and In Vivo Effect of Exogenous Farnesol Exposure Against Candida auris. Front. Microbiol. 2020;11:957. doi: 10.3389/fmicb.2020.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang Y., Lu C., Zhao X., Wang D., Liu Y., Sun S. Antifungal Activity and Potential Mechanism of Asiatic Acid Alone and in Combination with Fluconazole against Candida albicans. Biomed. Pharmacother. 2021;139:111568. doi: 10.1016/j.biopha.2021.111568. [DOI] [PubMed] [Google Scholar]
- 239.Sun L.-M., Liao K., Liang S., Yu P.-H., Wang D.-Y. Synergistic Activity of Magnolol with Azoles and Its Possible Antifungal Mechanism against Candida albicans. J. Appl. Microbiol. 2015;118:826–838. doi: 10.1111/jam.12737. [DOI] [PubMed] [Google Scholar]
- 240.Li Y., Chang W., Zhang M., Li X., Jiao Y., Lou H. Synergistic and Drug-Resistant Reversing Effects of Diorcinol D Combined with Fluconazole against Candida albicans. FEMS Yeast Res. 2015;15:1. doi: 10.1093/femsyr/fov001. [DOI] [PubMed] [Google Scholar]
- 241.Moraes R.C., Carvalho A.R., Lana A.J.D., Kaiser S., Pippi B., Fuentefria A.M., Ortega G.G. In Vitro Synergism of a Water Insoluble Fraction of Uncaria tomentosa Combined with Fluconazole and Terbinafine against Resistant Non-Candida albicans Isolates. Pharm. Biol. 2017;55:406–415. doi: 10.1080/13880209.2016.1242631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Behbehani J.M., Irshad M., Shreaz S., Karched M. Synergistic Effects of Tea Polyphenol Epigallocatechin 3-O-Gallate and Azole Drugs against Oral Candida Isolates. J. De Mycol. Med. 2019;29:158–167. doi: 10.1016/j.mycmed.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 243.Kumar S.N., Aravind S.R., Sreelekha T.T., Jacob J., Kumar B.S.D. Asarones from Acorus calamus in Combination with Azoles and Amphotericin B: A Novel Synergistic Combination to Compete Against Human Pathogenic Candida Species In Vitro. Appl. Biochem. Biotechnol. 2015;175:3683–3695. doi: 10.1007/s12010-015-1537-y. [DOI] [PubMed] [Google Scholar]
- 244.Yao D., Zhang G., Chen W., Chen J., Li Z., Zheng X., Yin H., Hu X. Pyrogallol and Fluconazole Interact Synergistically In Vitro against Candida glabrata through an Efflux-Associated Mechanism. Antimicrob. Agents Chemother. 2021;65:e0010021. doi: 10.1128/AAC.00100-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kobayashi T., Kakeya H., Miyazaki T., Izumikawa K., Yanagihara K., Ohno H., Yamamoto Y., Tashiro T., Kohno S. Synergistic Antifungal Effect of Lactoferrin with Azole Antifungals against Candida albicans and a Proposal for a New Treatment Method for Invasive Candidiasis. Jpn. J. Infect. Dis. 2011;64:292–296. doi: 10.7883/yoken.64.292. [DOI] [PubMed] [Google Scholar]
- 246.Shekhar-Guturja T., Tebung W.A., Mount H., Liu N., Köhler J.R., Whiteway M., Cowen L.E. Beauvericin Potentiates Azole Activity via Inhibition of Multidrug Efflux, Blocks Candida albicans Morphogenesis, and Is Effluxed via Yor1 and Circuitry Controlled by Zcf29. Antimicrob. Agents Chemother. 2016;60:7468–7480. doi: 10.1128/AAC.01959-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fakhim H., Emami S., Vaezi A., Hashemi S.M., Faeli L., Diba K., Dannaoui E., Badali H. In Vitro Activities of Novel Azole Compounds ATTAF-1 and ATTAF-2 against Fluconazole-Susceptible and-Resistant Isolates of Candida Species. Antimicrob. Agents Chemother. 2017;61:e01106-16. doi: 10.1128/AAC.01106-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shi C., Liu C., Liu J., Wang Y., Li J., Xiang M. Anti-Candida Activity of New Azole Derivatives Alone and in Combination with Fluconazole. Mycopathologia. 2015;180:203–207. doi: 10.1007/s11046-015-9899-9. [DOI] [PubMed] [Google Scholar]
- 249.Mood A.D., Premachandra I.D.U.A., Hiew S., Wang F., Scott K.A., Oldenhuis N.J., Liu H., Van Vranken D.L. Potent Antifungal Synergy of Phthalazinone and Isoquinolones with Azoles Against Candida albicans. ACS Med. Chem. Lett. 2017;8:168–173. doi: 10.1021/acsmedchemlett.6b00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li L.P., Liu W., Liu H., Zhu F., Zhang D.Z., Shen H., Xu Z., Qi Y.P., Zhang S.Q., Chen S.M., et al. Synergistic Antifungal Activity of Berberine Derivative B-7b and Fluconazole. PLoS ONE. 2015;10:e0126393. doi: 10.1371/journal.pone.0126393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Koselny K., Green J., DiDone L., Halterman J.P., Fothergill A.W., Wiederhold N.P., Patterson T.F., Cushion M.T., Rappelye C., Wellington M., et al. The Celecoxib Derivative AR-12 Has Broad-Spectrum Antifungal Activity In Vitro and Improves the Activity of Fluconazole in a Murine Model of Cryptococcosis. Antimicrob. Agents Chemother. 2016;60:7115–7127. doi: 10.1128/AAC.01061-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ghannoum M., Long L., Larkin E.L., Isham N., Sherif R., Borroto-Esoda K., Barat S., Angulo D. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob. Agents Chemother. 2018;62:e00244-18. doi: 10.1128/AAC.00244-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Savage K.A., del Carmen Parquet M., Allan D.S., Davidson R.J., Holbein B.E., Lilly E.A., Fidel P.L. Iron Restriction to Clinical Isolates of Candida albicans by the Novel Chelator Dibi Inhibits Growth and Increases Sensitivity to Azoles In Vitro and In Vivo in a Murine Model of Experimental Vaginitis. Antimicrob. Agents Chemother. 2018;62:e02576-17. doi: 10.1128/AAC.02576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dai L., Zang C., Tian S., Liu W., Tan S., Cai Z., Ni T., An M., Li R., Gao Y., et al. Design, Synthesis, and Evaluation of Caffeic Acid Amides as Synergists to Sensitize Fluconazole-Resistant Candida albicans to Fluconazole. Bioorganic Med. Chem. Lett. 2015;25:34–37. doi: 10.1016/j.bmcl.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 255.Wang Y.H., Dong H.H., Zhao F., Wang J., Yan F., Jiang Y.Y., Jin Y.S. The Synthesis and Synergistic Antifungal Effects of Chalcones against Drug Resistant Candida albicans. Bioorganic Med. Chem. Lett. 2016;26:3098–3102. doi: 10.1016/j.bmcl.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 256.Sun Y., Gao L., He C., Li M., Zeng T. In Vitro Interactions between IAP Antagonist AT406 and Azoles against Planktonic Cells and Biofilms of Pathogenic Fungi Candida albicans and Exophiala dermatitidis. Med. Mycol. 2018;56:1045–1049. doi: 10.1093/mmy/myx150. [DOI] [PubMed] [Google Scholar]
- 257.Yang W., Tu J., Ji C., Li Z., Han G., Liu N., Li J., Sheng C. Discovery of Piperidol Derivatives for Combinational Treatment of Azole-Resistant Candidiasis. ACS Infect. Dis. 2021;7:650–660. doi: 10.1021/acsinfecdis.0c00849. [DOI] [PubMed] [Google Scholar]
- 258.Li C., Tu J., Han G., Liu N., Sheng C. Heat Shock Protein 90 (Hsp90)/Histone Deacetylase (HDAC) Dual Inhibitors for the Treatment of Azoles-Resistant Candida albicans. Eur. J. Med. Chem. 2022;227:113961. doi: 10.1016/j.ejmech.2021.113961. [DOI] [PubMed] [Google Scholar]
- 259.Czechowicz P., Neubauer D., Nowicka J., Kamysz W., Gościniak G. Antifungal Activity of Linear and Disulfide-Cyclized Ultrashort Cationic Lipopeptides Alone and in Combination with Fluconazole against Vulvovaginal Candida spp. Pharmaceutics. 2021;13:1589. doi: 10.3390/pharmaceutics13101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sun Y., Tan L., Yao Z., Gao L., Yang J., Zeng T. In Vitro and In Vivo Interactions of TOR Inhibitor AZD8055 and Azoles against Pathogenic Fungi. Microbiol. Spectr. 2022;10:e0200721. doi: 10.1128/spectrum.02007-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chang Y.-L., Yu S.-J., Heitman J., Wellington M., Chen Y.-L. New Facets of Antifungal Therapy. Virulence. 2017;8:222–236. doi: 10.1080/21505594.2016.1257457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cavalheiro A.S., Maboni G., de Azevedo M.I., Argenta J.S., Pereira D.I.B., Spader T.B., Alves S.H., Santurio J.M. In Vitro Activity of Terbinafine Combined with Caspofungin and Azoles against Pythium insidiosum. Antimicrob. Agents Chemother. 2009;53:2136–2138. doi: 10.1128/AAC.01506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Argenta J.S., Santurio J.M., Alves S.H., Pereira D.I.B., Cavalheiro A.S., Spanamberg A., Ferreiro L. In Vitro Activities of Voriconazole, Itraconazole, and Terbinafine Alone or in Combination against Pythium insidiosum Isolates from Brazil. Antimicrob. Agents Chemother. 2008;52:767–769. doi: 10.1128/AAC.01075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kordalewska M., Lee A., Park S., Berrio I., Chowdhary A., Zhao Y., Perlin D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018;62:e00238-18. doi: 10.1128/AAC.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pristov K.E., Ghannoum M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019;25:792–798. doi: 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 267.Shrestha S.K., Chang C.-W.T., Meissner N., Oblad J., Shrestha J.P., Sorensen K.N., Grilley M.M., Takemoto J.Y. Antifungal Amphiphilic Aminoglycoside K20: Bioactivities and Mechanism of Action. Front. Microbiol. 2014;5:671. doi: 10.3389/fmicb.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pichler H., Gaigg B., Hrastnik C., Achleitner G., Kohlwein S.D., Zellnig G., Perktold A., Daum G. A Subfraction of the Yeast Endoplasmic Reticulum Associates with the Plasma Membrane and Has a High Capacity to Synthesize Lipids. Eur. J. Biochem. 2001;268:2351–2361. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- 269.Istvan E.S., Deisenhofer J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science (1979) 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 270.van Beek E., Pieterman E., Cohen L., Löwik C., Papapoulos S. Farnesyl Pyrophosphate Synthase Is the Molecular Target of Nitrogen-Containing Bisphosphonates. Biochem. Biophys. Res. Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 271.Balfour J.A., Faulds D. Terbinafine: A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Potential in Superficial Mycoses. Drugs. 1992;43:259–284. doi: 10.2165/00003495-199243020-00010. [DOI] [PubMed] [Google Scholar]
- 272.Polak A. Preclinical Data and Mode of Action of Amorolfine. Dermatology. 1992;184:3–7. doi: 10.1159/000247588. [DOI] [PubMed] [Google Scholar]
- 273.Mishima E., Maruyama K., Nakazawa T., Abe T., Ito S. Acute Kidney Injury from Excessive Potentiation of Calcium-Channel Blocker via Synergistic CYP3A4 Inhibition by Clarithromycin Plus Voriconazole. Intern. Med. 2017;56:1687–1690. doi: 10.2169/internalmedicine.56.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Miyazaki H., Miyazaki Y., Geber A., Parkinson T., Hitchcock C., Falconer D.J., Ward D.J., Marsden K., Bennett J.E. Fluconazole Resistance Associated with Drug Efflux and Increased Transcription of a Drug Transporter Gene, PDH1, in Candida glabrata. Antimicrob. Agents Chemother. 1998;42:1695–1701. doi: 10.1128/AAC.42.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Albertson G.D., Niimi M., Cannon R.D., Jenkinson H.F. Multiple Efflux Mechanisms Are Involved in Candida albicans Fluconazole Resistance. Antimicrob. Agents Chemother. 1996;40:2835–2841. doi: 10.1128/AAC.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fraczek M.G., Bromley M., Buied A., Moore C.B., Rajendran R., Rautemaa R., Ramage G., Denning D.W., Bowyer P. The Cdr1B Efflux Transporter Is Associated with Non-Cyp51a-Mediated Itraconazole Resistance in Aspergillus Fumigatus. J. Antimicrob. Chemother. 2013;68:1486–1496. doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- 277.Martinez-Rossi N.M., Peres N.T.A., Rossi A. Antifungal Resistance Mechanisms in Dermatophytes. Mycopathologia. 2008;166:369–383. doi: 10.1007/s11046-008-9110-7. [DOI] [PubMed] [Google Scholar]
- 278.Webber M.A. The Importance of Efflux Pumps in Bacterial Antibiotic Resistance. J. Antimicrob. Chemother. 2003;51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 279.Sanchez C.P., McLean J.E., Rohrbach P., Fidock D.A., Stein W.D., Lanzer M. Evidence for a Pfcrt-Associated Chloroquine Efflux System in the Human Malarial Parasite Plasmodium falciparum. Biochemistry. 2005;44:9862–9870. doi: 10.1021/bi050061f. [DOI] [PubMed] [Google Scholar]
- 280.Liu S., Yue L., Gu W., Li X., Zhang L., Sun S. Synergistic Effect of Fluconazole and Calcium Channel Blockers against Resistant Candida albicans. PLoS ONE. 2016;11:e0150859. doi: 10.1371/journal.pone.0150859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Chang W., Liu J., Zhang M., Shi H., Zheng S., Jin X., Gao Y., Wang S., Ji A., Lou H. Efflux Pump-Mediated Resistance to Antifungal Compounds Can Be Prevented by Conjugation with Triphenylphosphonium Cation. Nat. Commun. 2018;9:5102. doi: 10.1038/s41467-018-07633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Miranda-Cadena K., Marcos-Arias C., Mateo E., Aguirre-Urizar J.M., Quindós G., Eraso E. In Vitro Activities of Carvacrol, Cinnamaldehyde and Thymol against Candida Biofilms. Biomed. Pharmacother. 2021;143:112218. doi: 10.1016/j.biopha.2021.112218. [DOI] [PubMed] [Google Scholar]
- 283.Prasad R., Rawal M.K. Efflux Pump Proteins in Antifungal Resistance. Front. Pharmacol. 2014;5:202. doi: 10.3389/fphar.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Perfect J.R. The Antifungal Pipeline: A Reality Check. Nat. Rev. Drug Discov. 2017;16:603–616. doi: 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 286.Cabral M.E., Figueroa L.I.C., Fariña J.I. Synergistic Antifungal Activity of Statin-Azole Associations as Witnessed by Saccharomyces cerevisiae- and Candida utilis-Bioassays and Ergosterol Quantification. Rev. Iberoam. De Micol. 2013;30:31–38. doi: 10.1016/j.riam.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 287.Thompson P.D., Panza G., Zaleski A., Taylor B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016;67:2395–2410. doi: 10.1016/j.jacc.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 288.Shaukat A., Benekli M., Vladutiu G.D., Slack J.L., Wetzler M., Baer M.R. Simvastatin–Fluconazole Causing Rhabdomyolysis. Ann. Pharmacother. 2003;37:1032–1035. doi: 10.1345/aph.1C467. [DOI] [PubMed] [Google Scholar]
- 289.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ruggiero S.L., Mehrotra B., Rosenberg T.J., Engroff S.L. Osteonecrosis of the Jaws Associated with the Use of Bisphosphonates: A Review of 63 Cases. J. Oral Maxillofac. Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 291.Cabillic F., Toutirais O., Lavoué V., de La Pintière C.T., Daniel P., Rioux-Leclerc N., Turlin B., Mönkkönen H., Mönkkönen J., Boudjema K., et al. Aminobisphosphonate-Pretreated Dendritic Cells Trigger Successful Vγ9Vδ2 T Cell Amplification for Immunotherapy in Advanced Cancer Patients. Cancer Immunol. Immunother. 2010;59:1611–1619. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Singh N. Antifungal Prophylaxis for Solid Organ Transplant Recipients: Seeking Clarity amidst Controversy. Clin. Infect. Dis. 2000;31:545–553. doi: 10.1086/313943. [DOI] [PubMed] [Google Scholar]
- 293.Shirazi F., Kontoyiannis D.P. The Calcineurin Pathway Inhibitor Tacrolimus Enhances the In Vitro Activity of Azoles against Mucorales via Apoptosis. Eukaryot. Cell. 2013;12:1225–1234. doi: 10.1128/EC.00138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cyert M.S. Calcineurin Signaling in Saccharomyces cerevisiae: How Yeast Go Crazy in Response to Stress. Biochem. Biophys. Res. Commun. 2003;311:1143–1150. doi: 10.1016/S0006-291X(03)01552-3. [DOI] [PubMed] [Google Scholar]
- 295.Tegos G.P., Haynes M., Jacob Strouse J., Md. T. Khan M., Bologa C.G., Oprea T.I., Sklar L.A. Microbial Efflux Pump Inhibition: Tactics and Strategies. Curr. Pharm. Des. 2012;17:1291–1302. doi: 10.2174/138161211795703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Stathopoulos-Gerontides A., Guo J.J., Cyert M.S. Yeast Calcineurin Regulates Nuclear Localization of the Crz1p Transcription Factor through Dephosphorylation. Genes Dev. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Harvey A.L., Edrada-Ebel R., Quinn R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 298.Xu Y., Wang Y., Yan L., Liang R.M., di Dai B., Tang R.J., Gao P.H., Jiang Y.Y. Proteomic Analysis Reveals a Synergistic Mechanism of Fluconazole and Berberine against Fluconazole-Resistant Candida albicans: Endogenous ROS Augmentation. J. Proteome Res. 2009;8:5296–5304. doi: 10.1021/pr9005074. [DOI] [PubMed] [Google Scholar]
- 299.Dangles O. Antioxidant Activity of Plant Phenols: Chemical Mechanisms and Biological Significance. Curr. Org. Chem. 2012;16:692–714. doi: 10.2174/138527212799957995. [DOI] [Google Scholar]
- 300.Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jothi R., Sangavi R., Kumar P., Pandian S.K., Gowrishankar S. Catechol Thwarts Virulent Dimorphism in Candida albicans and Potentiates the Antifungal Efficacy of Azoles and Polyenes. Sci. Rep. 2021;11:21049. doi: 10.1038/s41598-021-00485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bahar A., Ren D. Antimicrobial Peptides. Pharmaceuticals. 2013;6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wang X., Liu J., Chen J., Zhang M., Tian C., Peng X., Li G., Chang W., Lou H. Azole-Triphenylphosphonium Conjugates Combat Antifungal Resistance and Alleviate the Development of Drug-Resistance. Bioorganic Chem. 2021;110:104771. doi: 10.1016/j.bioorg.2021.104771. [DOI] [PubMed] [Google Scholar]
- 304.Zhu T., Chen X., Li C., Tu J., Liu N., Xu D., Sheng C. Lanosterol 14α-Demethylase (CYP51)/Histone Deacetylase (HDAC) Dual Inhibitors for Treatment of Candida tropicalis and Cryptococcus neoformans Infections. Eur. J. Med. Chem. 2021;221:113524. doi: 10.1016/j.ejmech.2021.113524. [DOI] [PubMed] [Google Scholar]
- 305.Elias R., Basu P., Fridman M. Fluconazole-COX Inhibitor Hybrids: A Dual-Acting Class of Antifungal Azoles. J. Med. Chem. 2022;65:2361–2373. doi: 10.1021/acs.jmedchem.1c01807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Uren A.G., Beilharz T., O’Connell M.J., Bugg S.J., van Driel R., Vaux D.L., Lithgow T. Role for Yeast Inhibitor of Apoptosis (IAP)-like Proteins in Cell Division. Proc. Natl. Acad. Sci. USA. 1999;96:10170–10175. doi: 10.1073/pnas.96.18.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Scorzoni L., de Paula e Silva A.C.A., Marcos C.M., Assato P.A., de Melo W.C.M.A., de Oliveira H.C., Costa-Orlandi C.B., Mendes-Giannini M.J.S., Fusco-Almeida A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017;8:36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Weng J., Wong S.N., Xu X., Xuan B., Wang C., Chen R., Sun C.C., Lakerveld R., Kwok P.C.L., Chow S.F. Cocrystal Engineering of Itraconazole with Suberic Acid via Rotary Evaporation and Spray Drying. Cryst. Growth Des. 2019;19:2736–2745. doi: 10.1021/acs.cgd.8b01873. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.