Abstract
Probiotics are microorganisms (including bacteria, yeasts and moulds) that confer various health benefits to the host, when consumed in sufficient amounts. Food products containing probiotics, called functional foods, have several health-promoting and therapeutic benefits. The significant role of yeasts in producing functional foods with promoted health benefits is well documented. Hence, there is considerable interest in isolating new yeasts as potential probiotics. Survival in the gastrointestinal tract (GIT), salt tolerance and adherence to epithelial cells are preconditions to classify such microorganisms as probiotics. Clear understanding of how yeasts can overcome GIT and salt stresses and the conditions that support yeasts to grow under such conditions is paramount for identifying, characterising and selecting probiotic yeast strains. This study elaborated the adaptations and mechanisms underlying the survival of probiotic yeasts under GIT and salt stresses. This study also discussed the capability of yeasts to adhere to epithelial cells (hydrophobicity and autoaggregation) and shed light on in vitro methods used to assess the probiotic characteristics of newly isolated yeasts.
Keywords: autoaggregation, coaggregation, gastric, intestine, probiotics
1. Introduction
The term ‘probiotics’ refers to ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ [1]. Food products containing probiotics are called functional foods and they provide health benefits, such as antihypertensive, hypoglycaemic, antioxidant [2,3,4] and immunomodulatory effects [5,6,7,8,9]. Numerous clinical studies have proven the beneficial effects of probiotics. These benefits could be conveyed either via the consumption of probiotics, as a dietary supplement, or via food products, as a probiotic vehicle [10,11,12]. Increasing awareness of the health attributes of functional foods amongst consumers has corresponded with an increased demand for the characterisation of new isolates that could be identified as novel probiotic microorganisms. As a result, there have been many medical and industrial food interests in isolating new probiotic species/strains with health-promoting benefits [13].
The International Scientific Association for Probiotics and Prebiotics published a consensus paper, regarding the characteristics of probiotics [1]. The potential probiotic microorganism must meet several criteria to be considered probiotic. The essential criteria are tolerance to the gastrointestinal tract (GIT) conditions [14] (e.g., acidic/alkaline pH, digestive enzymes and bile acids/salts), attachment to mucus and epithelial cells and sensitivity to antibiotics [15]. More importantly, the probiotic should be tested in clinical trials to be officially named a probiotic. Hill et al. [1] stated that probiotic properties are related to strain specificity, not to a particular species or genus of microorganisms.
Yeast cells are eukaryotic microorganisms ~10 times larger in size than bacteria, enabling them to act as a steric hindrance against pathogenic bacteria, thus, enhancing their prospects to be a probiotic candidate [16]. Several studies have reported the ability of new yeast isolates to resist GIT conditions, tolerate salt stress, adhere to epithelial cells and possess antimicrobial activity against various pathogens [17,18,19]. This characterisation led to research progress to employ potential probiotic yeast strains in functional foods [20,21,22,23]. However, amongst all yeast genera, only a few Saccharomyces cerevisiae strains have been recognised and are available commercially as probiotics for human consumption [24]. The in vitro characterisation of potential probiotic yeasts is an essential preliminary step before clinical trials. The fulfilled probiotic criteria of yeasts pave the way for conducting investigation and validation for animal models and human trials and for using yeast probiotics commercially in functional foods and for therapeutic purposes [25].
The use of probiotics in foods or dietary components provides superior health benefits to conventional food products [26]. Ogunremi et al. [27] reported using the probiotic strain of Pichia kudriavzevii OG23 to produce fermented cereal-based food, with higher antioxidant activity and various flavour compounds [27]. The combination of S. cerevisiae var. boulardii and inulin developed symbiotic yogurt. Sarwar et al. [28] reported improved product texture and an increased amount of desirable volatile compounds. In addition, the probiotic strain S. cerevisiae var. boulardii is used to produce alcohol-free beer [29].
Probiotic yeasts have also gained importance in promoting animal nutrition and health. In the past, yeast as a probiotic was employed as a feed additive, because of its rich source of fibre, protein, minerals, organic acids and B vitamins [30]. Adding viable and nonviable yeast cells to animal feed promotes health and growth [31]. Probiotic yeast (S. cerevisiae) has positively impacted poultry health by increasing egg production, improving feed intake and reducing plasma cholesterol [32,33]. In addition, S. cerevisiae has been used in ruminant feed to reduce lactate accumulation [34]. In aquaculture, stimulating the enzymatic antioxidative response of farmed fish has been reported using Debaryomyces hansenii as a dietary supplement [35].
Despite the importance of yeasts in food industries, they could contribute to the spoilage of different foods and might be pathogenic for the host. The traditional identification methods of microorganisms, based on physiological, biochemical and morphological characteristics, are insufficient and inaccurate to classify the yeast as nonpathogenic. Conventional methods are time consuming and require significant human skill. Therefore, the characterisation of yeasts at the genomic level might be decisive in distinguishing between nonpathogenic and pathogenic yeast strains. Recently, molecular methods, such as polymerase chain reaction-based techniques, mitochondrial DNA restriction analysis and chromosome electrophoretic analysis, have been effectively used in yeast strain identification [36,37]. Furthermore, rapid and reliable fragment analysis tools have been established to identify foodborne yeasts, such as random amplified polymorphic DNA, pulsed-field gel electrophoresis and restriction fragment length polymorphism [38,39,40,41,42].
The applications of yeast probiotics have been addressed in several reviews [43,44,45]. However, the assessment methods of potential probiotic characteristics of newly isolated yeasts have not been addressed. Therefore, there is a need for a comprehensive review of the assessment methods of new isolated yeasts as potential probiotics and the appropriateness of these in vitro assessment methods used. Thus, this review identified the newly isolated yeasts characterised as potential probiotics, assessed the characterisation methods and highlighted the mechanistic effects of the assessment methods on yeasts.
2. The Gastric Environment
Digestion is the process that degrades food substances into nutrients (e.g., phytochemicals, micronutrients and macronutrients) to release them into the bloodstream [46]. This process comprises mechanical and chemical digestion. Mechanical digestion breaks down food substances into small particles, as a prelude to effective chemical digestion [47], whereas chemical digestion is responsible for catabolising other food molecules via various digestive enzymes, to absorb them into the bloodstream [48]. Peristaltic contractions of the smooth muscle display mechanical digestion in the stomach, consisting of propulsion, churning and grinding [48]. Only tiny particles (<2 mm) can enter the duodenum [47]. The bigger particles are churned back towards the stomach for further mechanical and chemical digestion [49]. The stomach provides an environment for a fundamental part of chemical digestion. The gastric mucosa contains two glands, the so-called oxyntic and pyloric glands, associated with chemical digestion [50].
Oxyntic glands are found in the stomach. Their parietal cells produce hydrochloric acid (HCl; 160 mmol/L, pH 0.8) [51]. HCl is important to exterminate pathogenic microorganisms ingested together with foods or drinks [52] and to activate pepsin from the zymogen pepsinogen, which is secreted by the chief cells of oxyntic glands [50]. Another intrinsic function of HCl is protein denaturation, to facilitate enzymatic digestion by pepsin. Pepsin breaks down the peptide bonds of proteins at the optimal pH of 2.0–3.0 into individual amino acids, which are released into the bloodstream [47,50]. The pyloric glands are located in the stomach antrum [53]. They form the hormone gastrin secreted by their enteroendocrine G-cells, which act to induce the creation of HCl [51,53]. In addition, mucus secreted by mucous cells of the pyloric glands plays a significant role in protecting the gastric surface from the acidic medium in the stomach [54].
2.1. Tolerance to Gastric Conditions
Several studies have defined the growth inhibition of microorganisms subjected to GIT conditions [55,56,57]. Acids can diffuse passively through the cell membrane and, thus, access the cytoplasm, dissociating into charged derivatives and protons [58]. Proton accumulation in the intracellular cytoplasm may decrease the intracellular pH and, subsequently, affect the transmembrane pH gradient [59], which contributes to the proton-motive force and minimises the amount of energy obtainable for cellular growth [60,61]. Furthermore, internal acidification reduces the inhibition of the action of acid-sensitive microorganisms, but extremely low internal pH induces damage to DNA and enzymes and denatures proteins, resulting in cell death [62,63]. Besides accumulating dissociated organic acids in the cytoplasm, this condition has a destructive effect on cellular physiology [64]. Gastric stress experiments with different potential probiotic yeasts are summarised in Table S1 (pH 2.0, 0.0133 g/L pepsin, 2.5 h and 37 °C). The survival rate of Metschnikowia pulcherrima isolated from fermented table olives reached 96.4% [65]. This survival percentage was relatively lower in Candida adriatica collected from olive oil (23.4%) [66].
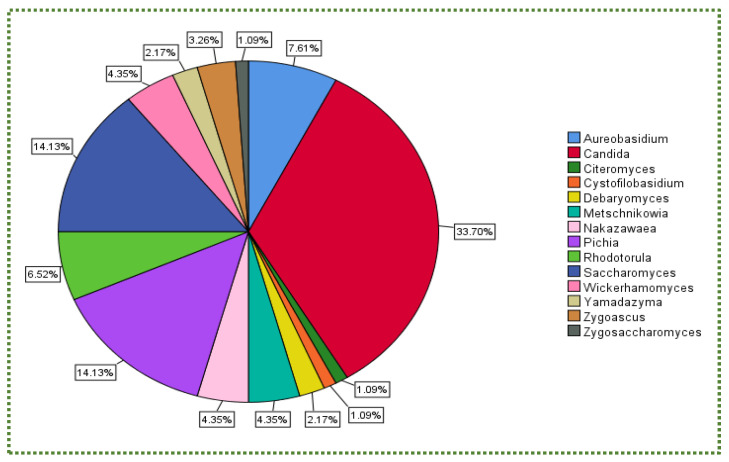
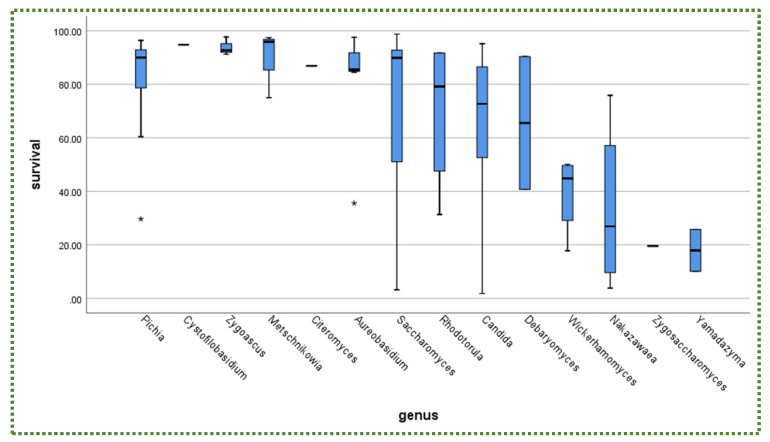
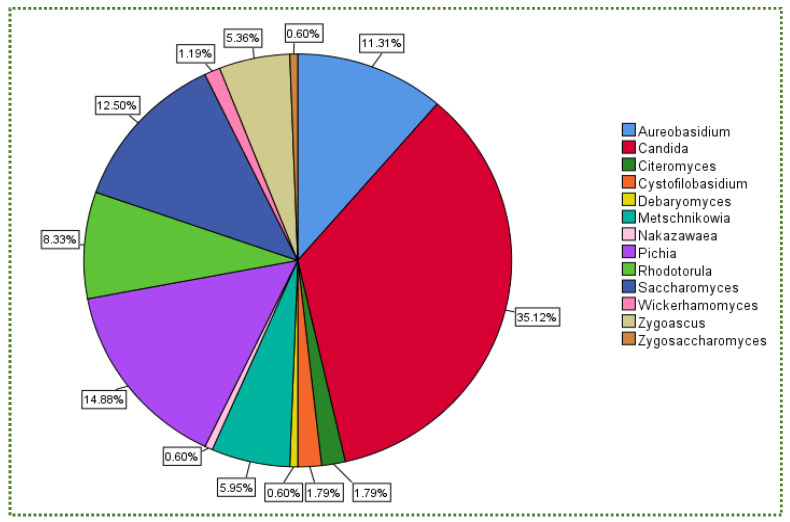
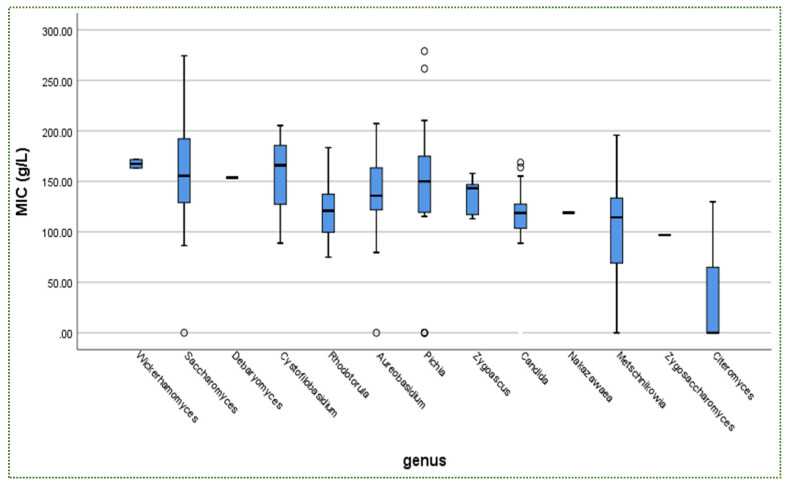
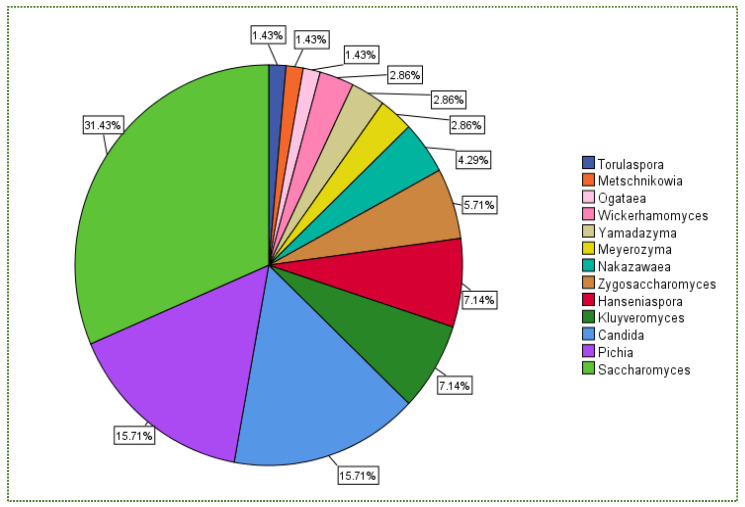
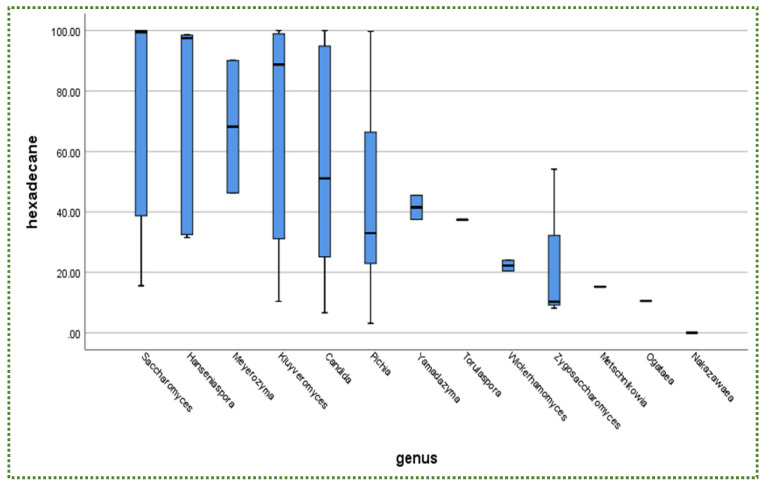
Figure 1 illustrates the percentage of the total number of isolated yeast genera subjected to gastric and intestinal conditions. The boxplot chart in Figure 2 displays the percent survival rate under gastric conditions. Although Candida did not exhibit the highest survival rate (Figure 2), it was the most frequently isolated genus at 34%, followed by Pichia and Saccharomyces (14% for each) and Aureobasidium (7.6%; Figure 1).
Figure 1.
Total number (%) of isolated yeast genera under gastrointestinal conditions.
Figure 2.
Survival rate (%) of yeasts under gastric conditions in descending order according to genera. * is outliners.
2.2. Assessment Methods of Gastric Tolerance
Three factors must be considered when adopting such a method for screening GIT stress tolerance. These factors are derived from the digestion process in vivo: (1) diffusion of gastrointestinal fluids into the food matrix, (2) synchronisation of mechanical digestion with chemical digestion and (3) sequence of enzymatic degradation. Therefore, time is a decisive parameter.
The significant challenges in the stomach are the extreme acidity, pH from 2.0 to 3.0, and the presence of digestive enzymes, such as pepsin [67]. This condition inhibits most microorganisms, including yeasts [68]. To qualify as probiotics, yeast strains have to survive the gastric conditions and reach the gut alive, where they will exert their function [69]. Some microorganisms, including yeasts, possess the capability to survive and grow in an acidic medium. The survival of selected yeast strains against digestion conditions is usually evaluated in vitro, in a gastric-like environment, where simulated gastric juice is prepared in a buffer solution at a low pH level (preferably pH 2.0) in the presence of pepsin, for given time intervals at 37 °C [70]. This method aims to quantify strain viability after being subjected to gastric juice. However, many researchers [18,71,72] used only acidic pH without adding pepsin to evaluate pH tolerance. They overlooked assessing gastric tolerance as a precondition to consider such microorganisms as probiotics, leading to inaccurate outcomes.
For all studies in this review (Table S1), a buffer solution consisting of NaCl, KH2PO4, CaCl2 and KCl was used. Cells of each yeast strain were resuspended in gastric fluid and incubated for 2.5 h at 37 °C [65,66,70,73,74]. Given the importance of mechanical movement, no studies (Table S1) mentioned the application of mechanical movement while assessing the gastric tolerance of potential yeast probiotics as a part of their assays, which could result in an imprecise assessment.
2.3. Mechanisms of Gastric Tolerance
The underlying mechanism for surviving yeast under low pH is modifying the yeast cell wall [75,76]. Strong inorganic acids, such as HCl, in the stomach and yeast cells adjusted to low pH comprise a mechanism that activates the cell wall integrity pathway [77,78]. It depends on the transmission of the signals of stressed walls to Rho1 GTPase, leading to the formation of various carbohydrate polymers used for remodelling the cell wall [79]. Another mechanism for resisting the strong inorganic acid is a general stress response pathway, an essential response against any conditional alteration, where the conductance of the protein kinase C pathway is fundamental [76].
In addition, under low pH, the temporal inefficiency of the glucose-sensing pathway in yeast and growth inhibition are the main causatives of the activity reduction in protein kinase A, thereby liberating general stress responses and driving to remodel cell gene expression, to adjust to the low-pH condition [76,78,80]. Furthermore, calcium metabolism could affect yeast responses to low external pH [81], in which the deletion of either Mid1p or Cch1pm, as calcium channels, is vital to yeast cells when subjected to inorganic acid stress [82,83].
Adjusting the membrane lipid composition in Candida glabrata by mediator subunits led to increases in acid tolerance, up to pH 2.0, indicating the significant role of lipid homoeostasis [84]. Fletcher et al. [85] proved that acid tolerance could be acquired by altering sterol composition and reducing iron uptake, such as in S. cerevisiae, as its acidic tolerance developed to pH 2.8. In line with previous studies, the use of pepsin and low pH (2.0) is recommended to mimic human gastric conditions and, thus, obtain an accurate assessment when examining new isolates in gastric juice.
3. Intestinal Conditions
The small intestine is associated with pancreatic enzymes and bile released from the liver to continue digestion. The secretions of the pancreas and duodenum mix with digesta and chyme [47]. As peristalsis occurs, mechanical digestion goes on, slightly. The small intestine environment is neutral to slight alkalinity because of the bicarbonate produced by the pancreas. It allows the digestive enzymes secreted by the duodenum and pancreas (e.g., pancreatic amylase, pancreatic lipase and trypsinogen) to act optimally at pH 6–7 [86,87]. Pancreatic amylase is responsible for hydrolysing starch into maltose and maltotriose [88]. At the same time, pancreatic lipase is associated with a coenzyme, named colipase, for hydrolysing triglycerides to produce diacylglycerols and monoacylglycerols [88,89]. Trypsinogen is a zymogen of trypsin. It is converted to its active form (trypsin) by enterokinase, and trypsin converts other pancreatic zymogens to their active forms [90].
Pancreatic zymogens transfer to the common bile duct, creating the ampulla of Vater, and empty its contents into the duodenum, where pancreatic zymogen activation occurs. Liver cells form bile, which is stored in the gallbladder and carried by the common bile duct [91]. Bile contains a mixture of bile salts, fatty acids, cholesterol, electrolytes and bilirubin that make the total solution basic with an average pH of 8.2 [92,93]. Bile salts and acids pass to the small intestine, where they function as detergents for waste products from the blood and are critical for breaking down fat into fatty acids [94].
3.1. Tolerance to Intestinal Conditions
The major obstacles to yeast survival are high bile salt and organic acid concentrations. Moreover, Pais et al. [95] highlighted that pancreatic and hydrolytic enzymes, secondary metabolic products of the gut microbiome and epithelial brush border in the small intestine, might destroy microorganisms, including yeasts. Bile salts are generally created from cholesterol in the liver and secreted into the intestine to contribute to the digestive process [96]. Previously, Urdaneta and Casadesús [97] stated that bile salts have detergent properties, and as a result, they can be toxic to the GIT microbiota. However, some microorganisms resist bile salts and hydrolytic enzymes [98].
Alkaline stress is another challenge that inevitably exposes yeast strains in the intestinal tract. For example, S. cerevisiae grows in acidic pH better than in alkaline conditions [77]. These strains do not proliferate when pH exceeds 8.0–8.2 [99]. Indeed, Schizosaccharomyces pombe growth was inhibited, even at neutral pH [100]. Nevertheless, some yeast species, such as Yarrowia lipolytica, are resistant to alkaline conditions up to pH 10–11 [101,102]. Previous studies confirmed that pH changes lead to upregulated and downregulated gene expression in S. cerevisiae [103,104,105].
The active transporters of the plasma membrane are activated by the permanent proton gradient between extracellular and intracellular media, which is protected by respective fungal orthologs, for instance, in S. cerevisiae by Pma1p H+-ATPase [106]. The importance of this gradient comes from its functional uptake of several vital compounds [61]. Acidic stress affects it slightly, but alkaline stress damages it [106]. When cells cannot neutralise a circumambient alkaline medium, microorganisms suffer from starvation for nutrients, such as glucose and phosphate [107,108]. In addition, extreme external pH reduces the ionisation of primary transition metals, leading to starvation for these metals (e.g., iron and copper) [109].
Table S2 summarises various yeast strains undergoing in vitro intestinal conditions (pH 8, 0.1 g/L pancreatin, 3.0 g/L bile salts, 3.5 h and 37 °C). Bonatsou et al. [74] tested the response of Rhodotorula diobovatum isolated from Greek black olives, which displayed only a 3.83% survival rate. Other species isolated from the same source (Rhodotorula mucilaginosa) reached 53.40%. The differences in the survival rate were species- and strain-dependent.
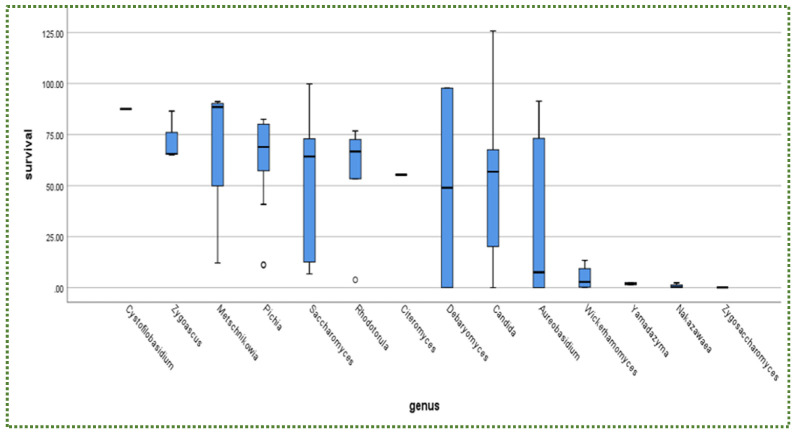
The percentage of the total number of isolated yeast genera undergoing intestinal conditions is presented in Figure 1 (pH 8, 0.1 g/L pancreatin, 3.0 g/L bile salts, 3.5 h and 37 °C). Figure 3 shows that Cystofilobasidium achieved the highest survival rate, whereas Zygoascus occupied the second rank, followed by Metschnikowia and Pichai, which, relatively, retracted compared to their position in gastric tolerance.
Figure 3.
Survival rate (%) of yeasts under intestinal conditions. o = outliners.
3.2. Assessment Methods of Intestinal Tolerance
Intestinal tolerance is a prerequisite to consider yeast as a probiotic, in addition to its survival under notable pH and temperature variations, through their passage from the stomach to the small intestine [110]. The assessment of intestinal tolerance of the potential yeast probiotic is mainly performed based on a similar concept to the in vitro gastric condition test. The optimum concentration of the bile in the human gut environment and the actual human temperature range are 0.3–0.6% and 36.5–37.5 °C, respectively [111]. Thus, isolated yeasts are commonly incubated at 37 °C for ~3.5 h after resuspension in an intestinal-like fluid, containing pancreatin and bile salts at pH 8.0. Both sodium phosphate dibasic heptahydrate and sodium chloride were used to make a buffer solution for experiments of intestinal tolerance in recently reviewed studies.
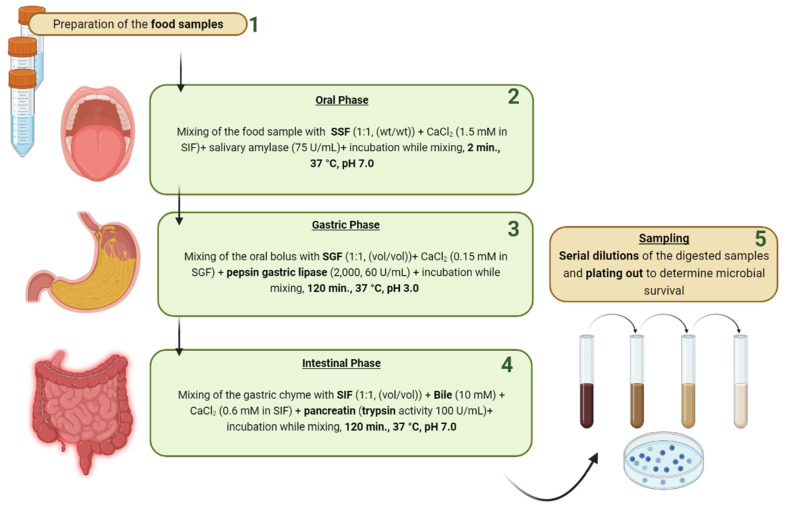
The INFOGEST in vitro digestion model (Figure 4) is widely applied in food research. This method includes three successive stages that simulate the digestion process in the upper GIT in vivo. The modality to implement INFOGEST is based on pH values, enzyme activity and the ionic strength of electrolyte solutions used in oral, gastric and intestinal phases. Furthermore, the digestion durations are 2.0, 120 and 120 min, respectively, under agitation status [112]. Thus far, according to the three factors mentioned in Section 2.2, the INFOGEST static model is considered a more reliable method to evaluate the GIT stress tolerance for microorganisms because it is almost the most simulated in vivo GIT. However, to the best of the authors’ knowledge, up to now, there is no application of the INFOGEST assay on the assessment of the GIT stress tolerance of yeast probiotics. By contrast, screening for this essential probiotic criterion of a potential probiotic bacterium by the INFOGEST model has been applied by many researchers [113,114,115].
Figure 4.
Schematic in vitro digestion method. SSF, simulated salivary fluid; SGF, simulated gastric fluid; SIF, simulated intestinal fluid.
3.3. Mechanisms of Intestinal Tolerance
According to FAO/WHO [116], resistance to bile acids/salts is a precondition for probiotic yeast to survive in the small intestine through the passage in the GIT. Bile salts have strong toxic effects on the cellular membrane of microorganisms [117], in terms of their fluidity, charge and hydrophobicity, perturbing cellular homoeostasis and motive oxidative stress [118]. However, several microorganisms can overcome the toxic effects of bile salts by employing bile salt hydrolases (BSH) of the intestinal microbiome, minimising the toxic influence of conjugated bile [119]. The mechanism of bile resistance in yeast is not entirely understood [120]. Despite that, bile salt resistance in probiotic yeasts has been shown by several studies [18,121]. Recently, a BSH, an enzyme responsible for hydrolysing bile salts, was investigated in vitro and detected in Saccharomyces boulardii [122], which might contribute to understanding yeast bile resistance.
Numerous mechanisms have been suggested to explain yeast resistance regarding yeast alkaline tolerance. One of them occurs in S. cerevisiae, where yeast plasma membrane Ena1 P-type ATPase acts on the cell’s efflux K+ and Na+ cations, counteracting the internal alkaline medium [106,123]. Another mechanism in S. cerevisiae, the protein kinase A pathway, is inhibited under alkaline stress, leading to the remodelling of Msn2 and Msn4 gene expression of stress-responsive transcriptional activators, to offset environmental alkalisation [124]. Moreover, Casamayor et al. [107] documented that S. cerevisiae resorts to glycogen mobilisation to respond to alkaline pH stress, to compromise glucose uptake. The vacuolar H+-ATPase enzyme is also essential to regulate intracellular pH in yeast cells [125,126]. Another strategy is adopted by Y. lipolytica to cope with alkaline stress, using polyphosphate storage molecules. The elevated pH of the cytosol drives polyphosphate hydrolysis, which compensates for the phosphate storage and restores the proper pH of the intracellular medium [101,127]. Simulating the intestinal fluid in the presence of pancreatin bile salts, preparing them at a similar concertation to the natural digestive system and setting a pH range from 7.8 to 8.0 are keys to an accurate appraisal of new isolates against intestinal juice.
4. Salt Conditions
In several food processes, probiotic cells are constantly subjected to environmental stresses, including excessive salinity, which forms hyperosmotic stress (salt stress). Probiotic yeasts could be exposed to hyperosmotic stress during the food production process, such as in the production of fermented food and certain cheese varieties [111,128].
In recent years, there have been a variety of food matrices targeted as probiotic vehicles containing significant amounts of NaCl [129]. For instance, a high-brine solution has been used as a flavour improver and preservative agent [130]. Fermentation of black olives occurs in 80–100 g/L brine [131], whereas the salt concentration in soy sauce reaches up to 18% [132] or higher. During the fermentation process, salt can repress the growth of moulds and some yeasts, the main causative agents of food spoilage, and can suppress the growth of specific foodborne pathogens, such as Listeria monocytogenes and Staphylococcus aureus [133,134]. Further, salt preservative properties are associated with shelf-life extension, flavour improvement and fermented food products [130,135].
4.1. Salt Stress
Salt tolerance can be a critical feature for selected yeast strains, thus, partially qualifying them as probiotics in traditional food fermentation or processing. However, an elevated saline environment can damage enzyme structure, suppress metabolic enzyme activity and retard fermentation [136]. Moreover, Heinisch and Rodicio [137] documented that high salinity may drive cell plasmolysis, and intracellular water molecules could diffuse out of the cells. Hence, owing to the lack of timely and efficient responses, high-salt conditions could cause growth inhibition or yeast death [138]. However, some yeast strains can survive and grow under such an environment, relying on strain-specific abilities to detect and respond to salt stress [139]. Table S3 presents the assessed non-inhibitory concentration (NIC) and minimum inhibitory concentration (MIC) values (g/L) measured under NaCl conditions for some yeast strains. S. cerevisiae isolated from Kalamata table olive fermentation and subjected to NaCl tolerance test at pH 3.5, 5.0 and 6.5 showed MIC values of 146.73, 174.8 and 143.8 g/L, whereas the same source was used to collect Zygoascus hellenicus and demonstrated MIC values of 125, 147 and 129 g/L.
From the same data, Candida was the most isolated genus (35%) amongst the 13 genera (Figure 5). Although it did not achieve the highest mean MIC value (Figure 6), which was recorded for Wickerhamomyces, it was not halophilic.
Figure 5.
Total number (%) of isolated yeast genera after NaCl stress.
Figure 6.
MIC (g/L) of yeasts under NaCl conditions in descending order according to genera. o = outliners.
4.2. Assessment Methods of Salt Tolerance
Lately, salinity tolerance test was carried out in vitro by adding yeast suspensions to appropriate broth (e.g., yeast malt broth), supplemented with different NaCl concentrations (e.g., 0–250 g/L) at various pH values (e.g., 3.5, 5.5 and 6.5). The growth of the strains was measured as optical density (OD) at 420 nm, in an automatic spectrophotometer for a specific period (e.g., 12 h for a fixed interval, such as 7 days). The NIC and MIC at different NaCl/pH values for each period were calculated by comparing the area of the curves under OD/time for the control, where salt was absent to the area of the curves where salt at different pH values was used [65,70]. Because salt is used in many processed foods, the inaccuracy in assessing its tolerance may suppress the growth of putative probiotic strains in the food product. Accordingly, yeast strains subjected to salt stress for short periods (2 h) may not give correct information, regarding the ability of the strains to tolerate salt (Bonatsou et al.) [74].
4.3. Mechanisms of Salt Tolerance
Hyperosmotic stress induces water efflux, resulting in cell shrinkage, excessive cellular solute concentrations and abolished cell turgor pressure [138,140]. Microorganisms counteract such influences by coping with various energetic mechanisms that modify cellular energy homoeostasis and reduce microbial growth [134]. Cells adjust their intracellular osmotic pressure by producing osmolytes, which are small organic solutes (such as glycerol and trehalose) that help in retaining intracellular water [141,142].
Hyperosmotic stress tolerance in yeasts is adopted via the high osmolarity glycerol (HOG) pathway, where mitogen-induced protein kinase is activated by leveraging two cell-membrane-bound receptors [140,143]. More specifically, sho1p and sln1p reveal osmotic alterations, leading to the stimulation of HOG pathway genes [106,144] and resulting in downstream triggered genes related to salt stress resistance [145]. When conducting a salt tolerance test to evaluate novel probiotic yeasts, it is recommended to apply different NaCl concentrations at various pH values to estimate each environment in fermented food matrices and targeted food processing protocols. The results could indicate the strains that may survive and dominate during fermentation.
5. Autoaggregation
Autoaggregation is associated with promoting colonisation in the human intestine, immunomodulation of the colonic mucosa and prevention of pathogenic infections [146,147]. Intestinal epithelial cells are covered by the mucosal glycocalyx layer that mainly contains electrolytes, immunoglobulins, glycolipids and glycosylated proteins (mucins) with sugar residues [148]. Adhesion property is gained by particular cell surface proteins, named ‘flocculins’ or ‘adhesions’, on the yeast cell surface that bind sugar residues, as on mucins in epithelial cells or certain amino acids on the surface of the other cells [149].
The autoaggregation property is represented as the ability of yeast strains to self-aggregate and produce flocs as a survival response, which extends a competitive advantage over other microorganisms, including enteric bacterial pathogens in severe conditions, such as human GIT [25]. This ability is an essential property of selected probiotics. Fernandez-Pacheco Rodríguez et al. [150] noted that probiotics should adequately adhere to epithelial cells to colonise the intestinal mucosa and exert their functional effects. In addition, the creation of cellular aggregates expands cell protection in an adverse environment [151]. Because yeast cells are larger and heavier than bacteria, they sediment faster and in a higher amount [152].
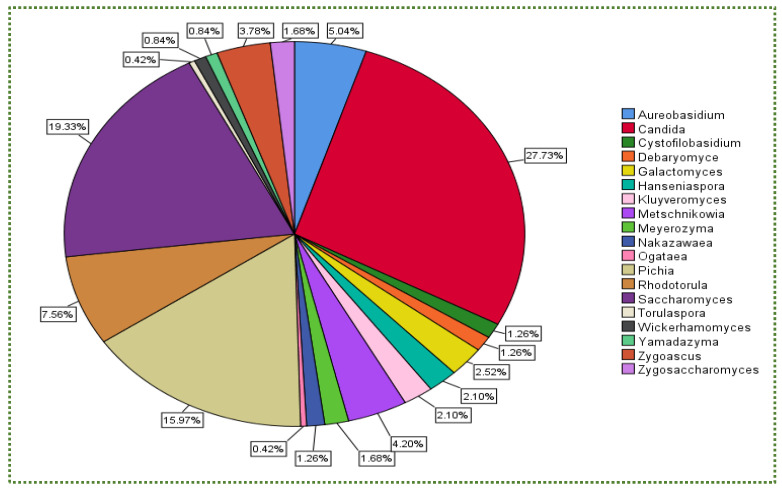
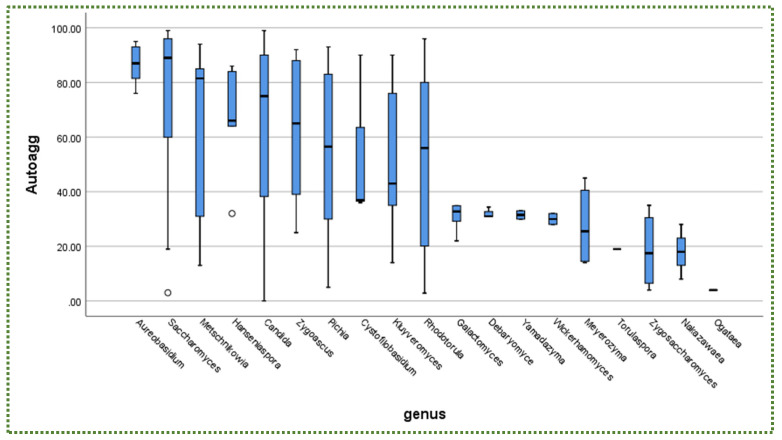
The outlines of the autoaggregation capacity of the selected yeast strains, measured in different periods, are shown in Table S4. Candida molendinolei isolated from Kalamata table olive fermentation exhibited 42%, 48% and 93% autoaggregation percentages after 2, 4 and 24 h of incubation. After 4–24 h of incubation, the yeast strains continuously increased their capability to make cellular aggregates. Candida tropicalis is another Candida species, collected from fermented Portuguese table olives, demonstrating ~86% autoaggregation. Nakazawaea wickerhamii isolated from olive oil displayed only 18%. This variance showed that the autoaggregation capability is substantially strain-dependent [153]. In another perspective, the most frequently isolated genus amongst yeast genera was Candida with 27.7%, followed by Saccharomyces and Pichia with 19.3% and 16%, respectively (Figure 7), whereas the highest autoaggregation percentage was exhibited by Aureobasidium and Saccharomyces (Figure 8).
Figure 7.
Total number (%) of isolated yeast genera that underwent the autoaggregation test.
Figure 8.
Autoaggregation (%) in descending order according to yeast genera. o = outliners.
5.1. Assessment Methods of Autoaggregation
Generally, the autoaggregation percentage of selected yeast strains proceeds in vitro via the resuspension of the suspended strains in phosphate-buffered saline (PBS) or NaCl solution (mostly 0.9%, w/v), and the test is carried out in a disposable plastic cuvette. The absorbance is measured at 600 nm, using an automatic spectrophotometer at different time intervals, without shaking the cell suspensions [65,73]. Yeast strains isolated by Gut et al. [25] and Fernandez-Pacheco Rodríguez et al. [150] had undergone autoaggregation evaluation for only 2 h or 30 min, respectively. Quantifying the autoaggregate capacity of yeast strains under a short time could not reflect their actual capacity. In vitro, the median transit time through the gut is 28.7 h [154,155], and the mean short intestinal transit time is 4.2 h [156], where the autoaggregation phenomenon is expected to activate its function.
5.2. Mode of Action of Autoaggregation
Autoaggregation (or, to be exact, ‘flocculation’) in yeasts is a complex phenomenon that takes place predominantly upon sugar depletion throughout the plated exponential or stationary phase [157]. In this circumstance, autoaggregation is influenced by the differences in cell wall composition, the presence and morphological type of cell appendages and the protruding macromolecules from the cell wall [158].
Di Gianvito et al. [159] demonstrated that flocculation occurs in two main phases: equal cell–cell adhesion is formed on glycan–glycan interaction and the glycan–lectin binding. First, glycan–glycan interaction is fixed by specific proteins, known as flocculins, zymolectins, adhesins or lectins (lectin-like theory) [160]. Then, residual mannose in the cell wall is bound by any proteins that protrude from the cell surface [161]. Ca2+ ions in the environment are significant because of their contribution to sugar binding and maintaining the correct flocculin conformation [160,162]. To gain the correct autoaggregation result, it is advisable to perform the test at gradual time intervals and suspend the strains in a saline buffer solution at the simulated NaCl concentration in the human GIT.
6. Hydrophobicity
Another property of potential probiotic strains is cell surface hydrophobicity. As in autoaggregation, hydrophobicity is crucial in reflecting the ability of probiotic adhesion and colonisation in the epithelial cells of the human GIT, where they may extend prophylactic and therapeutic impacts [163], resulting in the prevention of pathogen colonisation by their interactions [164]. The tendency of microorganisms to adhesion can increase according to the surface type [165]. Thus, cells with high hydrophobic properties attach more strongly to hydrophobic surfaces [166]. Surface hydrophobicity in mammals, including humans, is very high on the top of the gastric mucosa and the colon [167]. This hydrophobic property is attributed to the surface-active phospholipid layer that lines the mucus top that coats the epithelium [168]. Van Tassell and Miller [169] stated that, initially, the reversible and weak physical binding between probiotics and mucosa has occurred through nonspecific contact by hydrophobicity and spatial recognition. Subsequently, stable binding has been established between probiotic adhesins, usually surface-anchored proteins and complementary receptors in the mucus or intestinal epithelial cells, effectively colonising the GIT [169].
The correlation between the microbial ability to colonise the GIT and hydrophobicity has been studied in vivo and has been debated widely [1,170,171]. Nevertheless, this is not a compulsory characteristic, as yeasts are commonly excreted in the faeces because of competition with the GIT microbiota [172]. Therefore, probiotic yeasts should be regularly consumed to conserve appropriate balance in the host track, considering that the probiotic impact is dose-dependent [15].
The hydrophobicity of the studied yeast strains towards n-hexadecane is presented in Table S5. Interestingly, all strains isolated from fermented foods by Menezes et al. [173] resulted in >90% hydrophobicity, confirming that fermented foods could serve as carriers for probiotic microorganisms [174], including yeast. From another viewpoint, Saccharomyces is the most often isolated genus (Figure 9), followed by Pichia and Candida, regarding the ratio of the isolated yeast genus. However, these latter two genera hold the fifth (55.47%) and sixth (45.35%) positions, respectively, in terms of their ability towards hydrophobicity (Figure 10).
Figure 9.
Total number (%) of isolated yeast genera measured as the hydrophobicity ability towards n-hexadecane.
Figure 10.
Hydrophobicity (%) towards n-hexadecane in descending order according to yeast genera.
6.1. Assessment Methods of Hydrophobicity
The technique often used in vitro to assess hydrophobicity is measuring microbial interaction with hydrocarbons, such as n-hexadecane. This method is based on the microorganism’s affinity to a nonpolar solvent (e.g., n-hexadecane) [175]. Initially, strains are resuspended in buffer (e.g., 0.1 M KNO3 or 0.1 M PBS, pH 7). After measuring OD600 nm as the initial absorbance, the addition of n-hexadecane, xylene and octane to independent samples is conducted. After incubation at 37 °C for 60 min, the absorbance of the aqueous phase is measured [22,66,150].
Gut et al. [25], Zullo and Ciafardini [66] and Menezes et al. [173] used only n-hexadecane to examine the capacity of putative yeast probiotics, which may give unsatisfactory results. By contrast, microcell adhesion to the intestinal epithelium is linked to several factors, such as lectins, passive forces, lipoteichoic acid and hydrophobic forces [176,177]. Therefore, the ability to be hydrophobic is not determined by the presence of a particular hydrocarbon solvent. For example, Linder [178] stated that the self-assembly of filamentous fungi refers to hydrophobins and surface-active proteins, whereas Hazen and Hazen [179] found that the main factor of the hydrophobic feature in Candida albicans is the surface fibrils. Thus, the greater the variety of nonpolar solvents used to evaluate hydrophobicity, the more accurate the results.
6.2. Mechanisms of Hydrophobicity
Hydrophobicity is a physicochemical characteristic of the microbial cell surface, where a cell surface protein and lipoteichoic acids mediate a nonspecific interaction between microbial and host cells [180,181]. Lara-Hidalgo et al. [182] reported that strains with >40% hydrophobicity are hydrophobic. In this respect, hydrocarbons of the host cell will be bound by those strains and will be shifted from the aqueous to the organic phase of the environment [183]. Hydrophobicity has been attributed to complex interactions between hydrophobic and hydrophilic (negatively and positively charged) components on the microbial cell surface. To evaluate the cell surface hydrophobicity of newly isolated yeasts, different hydrocarbons, such as n-hexadecane, xylene and octane, should be used to mimic the adhesion efficiency of the intestinal epithelium.
7. Insights and Future Recommendations
Studying gastrointestinal and salt tolerance in vitro has revealed the possibility of yeast cells surviving under harsh conditions. Thorough knowledge of the mode of action that supports the adaptation of such gastrointestinal and salt stresses can promote yeasts as probiotics. Simulating the required conditions in the food industry and mimicking the biological processes to which yeasts in the human body are exposed are the decisive keys to accurately assess the probiotic properties of potential yeast isolates.
In addition, there is no relationship between yeast availability (Figure 1 and Figure 4) and its survivability (Figure 2, Figure 3 and Figure 5) under stresses and activities (autoaggregation and hydrophobicity), as reviewed by this study. This review also showed that Pichia and Cystofilobasidium achieved the highest survival rate under GIT stresses, indicating that it is valuable to focus on their molecular and genetic mechanisms to advance to in vivo trials, to exploit them in the production of functional foods and probiotic dietary supplements. This review included mainly the more robust probiotic properties (GIT and salt tolerance) presented by fermented food compared to unfermented food. In addition, the higher capability of cell surface properties, autoaggregation and hydrophobicity were demonstrated by isolates that achieved higher survivability under GIT stress (positive correlation).
The differences between bacteria and yeasts dictate the necessity to determine the mechanisms adopted by yeast probiotics to overcome extreme conditions. In this context, yeast resistance to bile acids/salts requires further studies. Further investigation is recommended to evaluate potential yeast probiotics in the food industry, such as survivability under heat stress and the ability to produce exopolysaccharides and safety aspects, including antibiotic sensitivity and the absence of virulence genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8040365/s1, Table S1: The survivability of selected yeast strains undergo in-vitro gastric conditions. Table S2: The survivability of selected yeast strains undergoes in-vitro intestinal conditions. Table S3: Evaluated of NIC (non-inhibitory concentration) and MIC (minimum inhibitory concentration) values (g/L) measured under salt (NaCl) for the yeast strains. Table S4: The percentage auto-aggregation capacity of the selected yeast strains, incubated at 37C and measured at different time intervals. Table S5: The percentage of hydrophobicity capacity of the selected yeast strains, incubated at 37C and measured at different time intervals.
Author Contributions
N.S.A., writing—original draft, formal analysis; T.M.O., A.N.O., A.A.A.-N., S.-Q.L., N.P.S. and V.A., writing—review and editing; writing—review and editing; M.M.A., conceptualization, writing—original draft, funding, supervising, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC were funded by the United Arab Emirates University (UAEU), Al-Ain, UAE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Ashaolu T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020;130:110625. doi: 10.1016/j.biopha.2020.110625. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S., Al-Shabib N.A. Functional Food Products and Sustainable Health. Springer; Berlin/Heidelberg, Germany: 2020. [Google Scholar]
- 4.Wang X., Han M., Zhang M., Wang Y., Ren Y., Yue T., Gao Z. In vitro evaluation of the hypoglycemic properties of lactic acid bacteria and its fermentation adaptability in apple juice. LWT. 2021;136:110363. doi: 10.1016/j.lwt.2020.110363. [DOI] [Google Scholar]
- 5.Dargahi N., Johnson J., Donkor O., Vasiljevic T., Apostolopoulos V. Immunomodulatory effects of Streptococcus thermophilus on U937 monocyte cell cultures. J. Funct. Foods. 2018;49:241–249. doi: 10.1016/j.jff.2018.08.038. [DOI] [Google Scholar]
- 6.Dargahi N., Johnson J., Apostolopoulos V. Streptococcus thermophilus alters the expression of genes associated with innate and adaptive immunity in human peripheral blood mononuclear cells. PLoS ONE. 2020;15:e0228531. doi: 10.1371/journal.pone.0228531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dargahi N., Matsoukas J., Apostolopoulos V. Streptococcus thermophilus ST285 alters pro-inflammatory to anti-inflammatory cytokine secretion against multiple sclerosis peptide in mice. Brain Sci. 2020;10:126. doi: 10.3390/brainsci10020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dargahi N., Johnson J.C., Apostolopoulos V. Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics. 2021;1:396–415. doi: 10.3390/biologics1030023. [DOI] [Google Scholar]
- 9.Dargahi N., Johnson J., Donkor O., Vasiljevic T., Apostolopoulos V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas. 2019;119:25–38. doi: 10.1016/j.maturitas.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Lee S.J., Bose S., Seo J.-G., Chung W.-S., Lim C.-Y., Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: A randomized double-blind controlled clinical trial. Clin. Nutr. 2014;33:973–981. doi: 10.1016/j.clnu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Wong R.K., Yang C., Song G.-H., Wong J., Ho K.-Y. Melatonin regulation as a possible mechanism for probiotic (VSL# 3) in irritable bowel syndrome: A randomized double-blinded placebo study. Dig. Dis. 2015;60:186–194. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 12.Gu J., Ahn-Jarvis J.H., Riedl K.M., Schwartz S.J., Clinton S.K., Vodovotz Y. Characterization of black raspberry functional food products for cancer prevention human clinical trials. J. Agric. Food Chem. 2014;62:3997–4006. doi: 10.1021/jf404566p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daba G.M., Elnahas M.O., Elkhateeb W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. 2021;173:79–89. doi: 10.1016/j.ijbiomac.2021.01.110. [DOI] [PubMed] [Google Scholar]
- 14.Vera-Pingitore E., Jimenez M.E., Dallagnol A., Belfiore C., Fontana C., Fontana P., von Wright A., Vignolo G., Plumed-Ferrer C. Screening and characterization of potential probiotic and starter bacteria for plant fermentations. LWT-Food Sci. Technol. 2016;71:288–294. doi: 10.1016/j.lwt.2016.03.046. [DOI] [Google Scholar]
- 15.FAO. WHO . Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. FAO; Rome, Lazio, Italy: WHO; Geneva, Switzerland: 2002. [Google Scholar]
- 16.Czerucka D., Piche T., Rampal P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 17.Silva T., Reto M., Sol M., Peito A., Peres C., Peres C., Malcata F.X. Characterization of yeasts from Portuguese brined olives, with a focus on their potentially probiotic behavior. LWT-Food Sci. Technol. 2011;44:1349–1354. doi: 10.1016/j.lwt.2011.01.029. [DOI] [Google Scholar]
- 18.Zahoor F., Sooklim C., Songdech P., Duangpakdee O., Soontorngun N. Selection of potential yeast probiotics and a cell factory for xylitol or acid production from honeybee samples. Metabolites. 2021;11:312. doi: 10.3390/metabo11050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perricone M., Bevilacqua A., Corbo M.R., Sinigaglia M. Technological characterization and probiotic traits of yeasts isolated from Altamura sourdough to select promising microorganisms as functional starter cultures for cereal-based products. Food Microbiol. 2014;38:26–35. doi: 10.1016/j.fm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Greppi A., Saubade F., Botta C., Humblot C., Guyot J.-P., Cocolin L. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 2017;62:169–177. doi: 10.1016/j.fm.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Rai A.K., Appaiah K.A. Application of native yeast from Garcinia (Garcinia xanthochumus) for the preparation of fermented beverage: Changes in biochemical and antioxidant properties. Food Biosci. 2014;5:101–107. doi: 10.1016/j.fbio.2013.11.008. [DOI] [Google Scholar]
- 22.Amorim J.C., Piccoli R.H., Duarte W.F. Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages. Food Res. Int. 2018;107:518–527. doi: 10.1016/j.foodres.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Di Cagno R., Filannino P., Cantatore V., Polo A., Celano G., Martinovic A., Cavoski I., Gobbetti M. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. Int. J. Food Microbiol. 2020;323:108591. doi: 10.1016/j.ijfoodmicro.2020.108591. [DOI] [PubMed] [Google Scholar]
- 24.Arévalo-Villena M., Fernandez-Pacheco P., Castillo N., Bevilacqua A., Pérez A.B. Probiotic capability in yeasts: Set-up of a screening method. LWT. 2018;89:657–665. doi: 10.1016/j.lwt.2017.11.047. [DOI] [Google Scholar]
- 25.Gut A.M., Vasiljevic T., Yeager T., Donkor O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods. 2019;58:56–66. doi: 10.1016/j.jff.2019.04.046. [DOI] [Google Scholar]
- 26.Ayyash M., Liu S.-Q., Al Mheiri A., Aldhaheri M., Raeisi B., Al-Nabulsi A., Osaili T., Olaimat A. In vitro investigation of health-promoting benefits of fermented camel sausage by novel probiotic Lactobacillus plantarum: A comparative study with beef sausages. LWT. 2019;99:346–354. doi: 10.1016/j.lwt.2018.09.084. [DOI] [Google Scholar]
- 27.Ogunremi O.R., Agrawal R., Sanni A.I. Development of cereal-based functional food using cereal-mix substrate fermented with probiotic strain—Pichia kudriavzevii OG32. Food Sci. Nutr. 2015;3:486–494. doi: 10.1002/fsn3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarwar A., Aziz T., Al-Dalali S., Zhao X., Zhang J., Ud Din J., Chen C., Cao Y., Yang Z. Physicochemical and microbiological properties of synbiotic yogurt made with probiotic yeast Saccharomyces boulardii in combination with inulin. Foods. 2019;8:468. doi: 10.3390/foods8100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senkarcinova B., Graça Dias I.A., Nespor J., Branyik T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT. 2019;100:362–367. doi: 10.1016/j.lwt.2018.10.082. [DOI] [Google Scholar]
- 30.Rosa C., Peter G. Biodiversity and Ecophysiology of Yeasts. Springer; Berlin/Heidelberg, Germany: 2006. [Google Scholar]
- 31.Roto S.M., Rubinelli P.M., Ricke S.C. An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front. Vet. Sci. 2015;2:28. doi: 10.3389/fvets.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassanein S.M., Soliman N.K. Effect of Probiotic (Saccharomyces cerevisiae) Adding to Diets on Intestinal Microflora and Performance of Hy-Line Layers Hens. J. Am. Sci. 2010;6:159–169. [Google Scholar]
- 33.Vohra A., Syal P., Madan A. Probiotic yeasts in livestock sector. Anim. Feed Sci. Technol. 2016;219:31–47. doi: 10.1016/j.anifeedsci.2016.05.019. [DOI] [Google Scholar]
- 34.Marden J.P., Julien C., Monteils V., Auclair E., Moncoulon R., Bayourthe C. How does live yeast differ from sodium bicarbonate to stabilize ruminal pH in high-yielding dairy cows? J. Dairy Sci. 2008;91:3528–3535. doi: 10.3168/jds.2007-0889. [DOI] [PubMed] [Google Scholar]
- 35.Reyes-Becerril M., Tovar-Ramírez D., Ascencio-Valle F., Civera-Cerecedo R., Gracia-López V., Barbosa-Solomieu V. Effects of dietary live yeast Debaryomyces hansenii on the immune and antioxidant system in juvenile leopard grouper Mycteroperca rosacea exposed to stress. Aquaculture. 2008;280:39–44. doi: 10.1016/j.aquaculture.2008.03.056. [DOI] [Google Scholar]
- 36.Andrighetto C., Psomas E., Tzanetakis N., Suzzi G., Lombardi A. Randomly amplified polymorphic DNA (RAPD) PCR for the identification of yeasts isolated from dairy products. Lett. Appl. Microbiol. 2000;30:5–9. doi: 10.1046/j.1472-765x.2000.00589.x. [DOI] [PubMed] [Google Scholar]
- 37.Querol A., Ramon D. The application of molecular techniques in wine microbiology. Trends Food Sci. Technol. 1996;7:73–78. doi: 10.1016/0924-2244(96)81300-8. [DOI] [Google Scholar]
- 38.Liu T., Li Y., Sadiq F.A., Yang H., Gu J., Yuan L., Lee Y.K., He G. Predominant yeasts in Chinese traditional sourdough and their influence on aroma formation in Chinese steamed bread. Food Chem. 2018;242:404–411. doi: 10.1016/j.foodchem.2017.09.081. [DOI] [PubMed] [Google Scholar]
- 39.Soll D.R. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 2000;13:332–370. doi: 10.1128/CMR.13.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K.W., Chen Y.C., Lin Y.H., Chou H.H., Li S.Y. The molecular epidemiology of serial Candida tropicalis isolates from ICU patients as revealed by multilocus sequence typing and pulsed-field gel electrophoresis. Infect. Genet. Evol. 2009;9:912–920. doi: 10.1016/j.meegid.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Arastehfar A., Boekhout T., Butler G., Buda De Cesare G., Dolk E., Gabaldón T., Hafez A., Hube B., Hagen F., Hovhannisyan H., et al. Recent trends in molecular diagnostics of yeast infections: From PCR to NGS. FEMS Microbiol. Rev. 2019;43:517–547. doi: 10.1093/femsre/fuz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prillinger H., Molnár O., Eliskases-Lechner F., Lopandic K. Phenotypic and genotypic identification of yeasts from cheese. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1999;75:267–283. doi: 10.1023/A:1001889917533. [DOI] [PubMed] [Google Scholar]
- 43.Kunyeit L., Anu-Appaiah K.A., Rao R.P. Application of probiotic yeasts on candida species associated infection. J. Fungi. 2020;6:189. doi: 10.3390/jof6040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saber A., Alipour B., Faghfoori Z., Yari Khosroushahi A. Cellular and molecular effects of yeast probiotics on cancer. Crit. Rev. Microbiol. 2017;43:96–115. doi: 10.1080/1040841X.2016.1179622. [DOI] [PubMed] [Google Scholar]
- 45.Shurson G.C. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018;235:60–76. doi: 10.1016/j.anifeedsci.2017.11.010. [DOI] [Google Scholar]
- 46.Liu D., Dhital S., Wu P., Chen X.D., Gidley M.J. In Vitro Digestion of Apple Tissue Using a Dynamic Stomach Model: Grinding and Crushing Effects on Polyphenol Bioaccessibility. J. Agric. Food Chem. 2020;68:574–583. doi: 10.1021/acs.jafc.9b05649. [DOI] [PubMed] [Google Scholar]
- 47.Camilleri M. Integrated Upper Gastrointestinal Response to Food Intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Welcome M.O. Gastrointestinal Physiology. Springer; Berlin/Heidelberg, Germany: 2018. Chemical Digestion, Absorption, and Transport; pp. 871–972. [Google Scholar]
- 49.Meyer J.H., Ohashi H., Jehn D., Thomson J.B. Size of liver particles emptied from the human stomach. Gastroenterology. 1981;80:1489–1496. doi: 10.1016/0016-5085(81)90262-6. [DOI] [PubMed] [Google Scholar]
- 50.Carlson B.M. Chapter 12—The Digestive System. In: Carlson B.M., editor. The Human Body. Academic Press; Cambridge, MA, USA: 2019. pp. 321–355. [Google Scholar]
- 51.Herdt T.H. 29—Secretions of the Gastrointestinal Tract. In: Klein B.G., editor. Cunningham’s Textbook of Veterinary Physiology. 6th ed. W.B. Saunders; St. Louis, MO, USA: 2020. pp. 307–315. [Google Scholar]
- 52.Schubert M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2003;19:519–525. doi: 10.1097/00001574-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Schubert M.L. Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr. Opin. Gastroenterol. 2017;33:430–438. doi: 10.1097/MOG.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 54.Berin M.C., Furuta G.T., Aceves S.S. 67—Gastrointestinal Mucosal Immunology. In: Adkinson N.F., Bochner B.S., Burks A.W., Busse W.W., Holgate S.T., Lemanske R.F., O’Hehir R.E., editors. Middleton’s Allergy. 8th ed. W.B. Saunders; London, UK: 2014. pp. 1084–1094. [Google Scholar]
- 55.da Silva Guedes J., Pimentel T.C., Diniz-Silva H.T., Tayse da Cruz Almeida E., Tavares J.F., Leite de Souza E., Garcia E.F., Magnani M. Protective effects of β-glucan extracted from spent brewer yeast during freeze-drying, storage and exposure to simulated gastrointestinal conditions of probiotic lactobacilli. LWT. 2019;116:108496. doi: 10.1016/j.lwt.2019.108496. [DOI] [Google Scholar]
- 56.Rui Y., Wan P., Chen G., Xie M., Sun Y., Zeng X., Liu Z. Simulated digestion and fermentation in vitro by human gut microbiota of intra- and extra-cellular polysaccharides from Aspergillus cristatus. LWT. 2019;116:108508. doi: 10.1016/j.lwt.2019.108508. [DOI] [Google Scholar]
- 57.Sekova V.Y., Dergacheva D.I., Tereshina V.M., Isakova E.P., Deryabina Y.I. Carbohydrate Spectrum of Extremophilic Yeasts Yarrowia lipolytica under pH Stress. Microbiology. 2018;87:173–182. doi: 10.1134/S0026261718020133. [DOI] [Google Scholar]
- 58.Guerzoni M.E., Serrazanetti D.I., Vernocchi P., Gianotti A. Handbook on Sourdough Biotechnology. Springer; Boston, MA, USA: 2013. Physiology and biochemistry of sourdough yeasts; pp. 155–181. [Google Scholar]
- 59.Orij R., Brul S., Smits G.J. Intracellular pH is a tightly controlled signal in yeast. Biochim. Biophys. Acta Gen. Subj. 2011;1810:933–944. doi: 10.1016/j.bbagen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Horák J. Yeast nutrient transporters. Biochim. Biophys. Acta Int. J. Biochem. Biophys. 1997;1331:41–79. doi: 10.1016/S0304-4157(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 61.Guan N., Liu L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020;104:51–65. doi: 10.1007/s00253-019-10226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaichumjai P., Valyasevi R., Assavanig A., Kurdi P. Isolation and characterization of acid-sensitive Lactobacillus plantarum with application as starter culture for Nham production. Food Microbiol. 2010;27:741–748. doi: 10.1016/j.fm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Estruch F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 2000;24:469–486. doi: 10.1111/j.1574-6976.2000.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 64.Viljoen B.C. Yeast Ecological Interactions. Yeast’Yeast, Yeast’Bacteria, Yeast’Fungi Interactions and Yeasts as Biocontrol Agents. In: Querol A., Fleet G., editors. Yeasts in Food and Beverages. Springer; Berlin/Heidelberg, Germany: 2006. pp. 83–110. [Google Scholar]
- 65.Bonatsou S., Karamouza M., Zoumpopoulou G., Mavrogonatou E., Kletsas D., Papadimitriou K., Tsakalidou E., Nychas G.J.E., Panagou E.Ζ. Evaluating the probiotic potential and technological characteristics of yeasts implicated in cv. Kalamata natural black olive fermentation. Int. J. Food Microbiol. 2018;271:48–59. doi: 10.1016/j.ijfoodmicro.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 66.Zullo B.A., Ciafardini G. Evaluation of physiological properties of yeast strains isolated from olive oil and their in vitro probiotic trait. Food Microbiol. 2019;78:179–187. doi: 10.1016/j.fm.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Palla M., Agnolucci M., Calzone A., Giovannetti M., Di Cagno R., Gobbetti M., Rizzello C.G., Pontonio E. Exploitation of autochthonous Tuscan sourdough yeasts as potential starters. Int. J. Food Microbiol. 2019;302:59–68. doi: 10.1016/j.ijfoodmicro.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Puppala K.R., Ravi Kumar V., Khire J., Dharne M. Dephytinizing and Probiotic Potentials of Saccharomyces cerevisiae (NCIM 3662) Strain for Amelioration of Nutritional Quality of Functional Foods. Probiotics Antimicrob. Proteins. 2019;11:604–617. doi: 10.1007/s12602-018-9394-y. [DOI] [PubMed] [Google Scholar]
- 69.Ragavan M.L., Das N. In Vitro Studies on Therapeutic Potential of Probiotic Yeasts Isolated from Various Sources. Curr. Microbiol. 2020;77:2821–2830. doi: 10.1007/s00284-020-02100-5. [DOI] [PubMed] [Google Scholar]
- 70.Porru C., Rodríguez-Gómez F., Benítez-Cabello A., Jiménez-Díaz R., Zara G., Budroni M., Mannazzu I., Arroyo-López F.N. Genotyping, identification and multifunctional features of yeasts associated to Bosana naturally black table olive fermentations. Food Microbiol. 2018;69:33–42. doi: 10.1016/j.fm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Du Toit M., Franz C.M.A.P., Dicks L.M.T., Schillinger U., Haberer P., Warlies B., Ahrens F., Holzapfel W.H. Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholesterol levels, faeces pH and faeces moisture content. Int. J. Food Microbiol. 1998;40:93–104. doi: 10.1016/S0168-1605(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 72.Kenfack C.H.M., Kaktcham P.M., Ngoufack F.Z., Wang Y.R., Yin L., Zhu T. Screening and Characterization of Putative Probiotic Lactobacillus Strains from Honey Bee Gut (Apis mellifera) J. Adv. Microbiol. 2018;10:1–18. doi: 10.9734/JAMB/2018/40780. [DOI] [Google Scholar]
- 73.Oliveira T., Ramalhosa E., Nunes L., Pereira J.A., Colla E., Pereira E.L. Probiotic potential of indigenous yeasts isolated during the fermentation of table olives from Northeast of Portugal. Innov. Food Sci. Emerg. Technol. 2017;44:167–172. doi: 10.1016/j.ifset.2017.06.003. [DOI] [Google Scholar]
- 74.Bonatsou S., Benitez A., Rodriguez-Gomez F., Panagou E.Z., Arroyo-Lopez F.N. Selection of yeasts with multifunctional features for application as starters in natural black table olive processing. Food Microbiol. 2015;46:66–73. doi: 10.1016/j.fm.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Kapteyn J., Ter Riet B., Vink E., Blad S., De Nobel H., Van Den Ende H., Klis F.M. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2001;39:469–480. doi: 10.1046/j.1365-2958.2001.02242.x. [DOI] [PubMed] [Google Scholar]
- 76.Lucena R.M., Dolz-Edo L., Brul S., de Morais M.A., Smits G. Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes. 2020;11:656. doi: 10.3390/genes11060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen A.K.-L., Gelling C., Rogers P.L., Dawes I.W., Rosche B. Response of Saccharomyces cerevisiae to stress-free acidification. J. Microbiol. 2009;47:1–8. doi: 10.1007/s12275-008-0167-2. [DOI] [PubMed] [Google Scholar]
- 78.de Lucena R.M., Elsztein C., de Barros Pita W., de Souza R.B., Júnior S.d.S.L.P., de Morais Junior M.A. Transcriptomic response of Saccharomyces cerevisiae for its adaptation to sulphuric acid-induced stress. Antonie Leeuwenhoek. 2015;108:1147–1160. doi: 10.1007/s10482-015-0568-2. [DOI] [PubMed] [Google Scholar]
- 79.Levin D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Melo H., Bonini B., Thevelein J., Simões D., Morais M.A., Jr. Physiological and molecular analysis of the stress response of Saccharomyces cerevisiae imposed by strong inorganic acid with implication to industrial fermentations. J. Appl. Microbiol. 2010;109:116–127. doi: 10.1111/j.1365-2672.2009.04633.x. [DOI] [PubMed] [Google Scholar]
- 81.Brandão R.L., Rosa J.C.C., Nicoli J.R., Almeida M.V.S., do Carmo A.P., Queiros H.T., Castro I.M. Investigating acid stress response in different Saccharomyces strains. J. Mycol. 2014;2014:178274. [Google Scholar]
- 82.Claret S., Gatti X., Doignon F., Thoraval D., Crouzet M. The Rgd1p Rho GTPase-activating protein and the Mid2p cell wall sensor are required at low pH for protein kinase C pathway activation and cell survival in Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:1375–1386. doi: 10.1128/EC.4.8.1375-1386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Lucena R., Elsztein C., Simoes D., de Morais M.A., Jr. Participation of CWI, HOG and Calcineurin pathways in the tolerance of Saccharomyces cerevisiae to low pH by inorganic acid. J. Appl. Microbiol. 2012;113:629–640. doi: 10.1111/j.1365-2672.2012.05362.x. [DOI] [PubMed] [Google Scholar]
- 84.Lin X., Qi Y., Yan D., Liu H., Chen X., Liu L. CgMED3 changes membrane sterol composition to help Candida glabrata tolerate low-pH stress. Appl. Environ. Microbiol. 2017;83:e00972-17. doi: 10.1128/AEM.00972-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fletcher E., Feizi A., Bisschops M.M.M., Hallström B.M., Khoomrung S., Siewers V., Nielsen J. Evolutionary engineering reveals divergent paths when yeast is adapted to different acidic environments. Metab. Eng. 2017;39:19–28. doi: 10.1016/j.ymben.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 86.Abbott A.M., Armstrong L., Jensen E.H. Chapter 68—Small Intestine. In: Yeo C.J., editor. Shackelford’s Surgery of the Alimentary Tract. 7th ed. W.B. Saunders; Philadelphia, PA, USA: 2013. pp. 839–863. [Google Scholar]
- 87.Kiela P.R., Ghishan F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016;30:145–159. doi: 10.1016/j.bpg.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silk D.B. Digestion and absorption of carbohydrate protein and fat. Contemp. Issues Clin. Biochem. 1986;4:7–40. [PubMed] [Google Scholar]
- 89.Alexander S.P.H. Diacylglycerol Lipase (DAG Lipase) In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2009. pp. 1–6. [Google Scholar]
- 90.Chen J.-M., Radisky E.S., Férec C. Chapter 576—Human Trypsins. In: Rawlings N.D., Salvesen G., editors. Handbook of Proteolytic Enzymes. 3rd ed. Academic Press; Cambridge, MA, USA: 2013. pp. 2600–2609. [Google Scholar]
- 91.Semrin M.G., Russo M.A. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Elsevier; Amsterdam, The Netherlands: 2010. Anatomy, histology, embryology, and developmental anomalies of the stomach and duodenum; pp. 773–788.e2. [Google Scholar]
- 92.Hofmann A.F. Bile acid secretion, bile flow and biliary lipid secretion in humans. Hepatology. 1990;12:17S–25S. [PubMed] [Google Scholar]
- 93.Drury D.R., McMaster P.D., Rous P. Observations on some causes of gall stone formation. III. The relation of the reaction of the bile to experimental cholelitiasis. J. Exp. Med. 1924;39:403–424. doi: 10.1084/jem.39.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maldonado-Valderrama J., Wilde P., MacIerzanka A., MacKie A. The role of bile salts in digestion. Adv. Colloid Interface Sci. 2011;165:36–46. doi: 10.1016/j.cis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Pais P., Almeida V., Yılmaz M., Teixeira M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi. 2020;6:78. doi: 10.3390/jof6020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aguilar-Ballester M., Herrero-Cervera A., Vinué Á., Martínez-Hervás S., González-Navarro H. Impact of cholesterol metabolism in immune cell function and atherosclerosis. Nutrients. 2020;12:2021. doi: 10.3390/nu12072021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Urdaneta V., Casadesús J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. 2017;4:163. doi: 10.3389/fmed.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Merchán A.V., Benito M.J., Galván A.I., Ruiz-Moyano Seco de Herrera S. Identification and selection of yeast with functional properties for future application in soft paste cheese. LWT. 2020;124:109173. doi: 10.1016/j.lwt.2020.109173. [DOI] [Google Scholar]
- 99.Canadell D., García-Martínez J., Alepuz P., Pérez-Ortín J.E., Ariño J. Impact of high pH stress on yeast gene expression: A comprehensive analysis of mRNA turnover during stress responses. Biochim. Biophys. Acta Gene Regul. Mech. 2015;1849:653–664. doi: 10.1016/j.bbagrm.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Tominaga A., Higuchi Y., Mori H., Akai M., Suyama A., Yamada N., Takegawa K. Catechol O-methyltransferase homologs in Schizosaccharomyces pombe are response factors to alkaline and salt stress. Appl. Microbiol. Biotechnol. 2019;103:4881–4887. doi: 10.1007/s00253-019-09858-0. [DOI] [PubMed] [Google Scholar]
- 101.Zvyagilskaya R., Parchomenko O., Abramova N., Allard P., Panaretakis T., Pattison-Granberg J., Persson B.L. Proton-and sodium-coupled phosphate transport systems and energy status of Yarrowia lipolytica cells grown in acidic and alkaline conditions. J. Membr. Biol. 2001;183:39–50. doi: 10.1007/s00232-001-0054-9. [DOI] [PubMed] [Google Scholar]
- 102.Peñalva M.A., Arst H.N., Jr. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 2004;58:425–451. doi: 10.1146/annurev.micro.58.030603.123715. [DOI] [PubMed] [Google Scholar]
- 103.Causton H.C., Ren B., Sang Seok K., Harbison C.T., Kanin E., Jennings E.G., Tong Ihn L., True H.L., Lander E.S., Young R.A. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell. 2001;12:323–337. doi: 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Serrano R., Ruiz A., Bernal D., Chambers J.R., Ariño J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: Evidence for calcium-mediated signalling. Mol. Microbiol. 2002;46:1319–1333. doi: 10.1046/j.1365-2958.2002.03246.x. [DOI] [PubMed] [Google Scholar]
- 105.Serrano R., Martín H., Casamayor A., Ariño J. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J. Biol. Chem. 2006;281:39785–39795. doi: 10.1074/jbc.M604497200. [DOI] [PubMed] [Google Scholar]
- 106.Skoneczny M., Skoneczna A. Stress Response Mechanisms in Fungi: Theoretical and Practical Aspects. Springer; Cham, Switzerland: 2018. Response mechanisms to chemical and physical stresses in yeast and filamentous fungi; pp. 35–85. [Google Scholar]
- 107.Casamayor A., Serrano R., Platara M., Casado C., Ruiz A., Ariño J. The role of the Snf1 kinase in the adaptive response of Saccharomyces cerevisiae to alkaline pH stress. Biochem. J. 2012;444:39–49. doi: 10.1042/BJ20112099. [DOI] [PubMed] [Google Scholar]
- 108.Serra-Cardona A., Petrezsélyová S., Canadell D., Ramos J., Ariño J. Coregulated expression of the Na+/phosphate pho89 transporter and ena1 Na+-ATPase allows their functional coupling under high-pH stress. Mol. Cell. Biol. 2014;34:4420–4435. doi: 10.1128/MCB.01089-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Higuchi Y., Mori H., Kubota T., Takegawa K. Analysis of ambient pH stress response mediated by iron and copper intake in Schizosaccharomyces pombe. J. Biosci. Bioeng. 2018;125:92–96. doi: 10.1016/j.jbiosc.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Sen S., Mansell T.J. Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genet. Biol. 2020;137:103333. doi: 10.1016/j.fgb.2020.103333. [DOI] [PubMed] [Google Scholar]
- 111.Helmy E., Soliman S., Abdel-Ghany T.M., Ganash M. Evaluation of potentially probiotic attributes of certain dairy yeast isolated from buffalo sweetened Karish cheese. Heliyon. 2019;5:e01649. doi: 10.1016/j.heliyon.2019.e01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carrière F., et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14:991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 113.Ayyash M., Abdalla A., Alhammadi A., Senaka Ranadheera C., Affan Baig M., Al-Ramadi B., Chen G., Kamal-Eldin A., Huppertz T. Probiotic survival, biological functionality and untargeted metabolomics of the bioaccessible compounds in fermented camel and bovine milk after in vitro digestion. Food Chem. 2021;363:130243. doi: 10.1016/j.foodchem.2021.130243. [DOI] [PubMed] [Google Scholar]
- 114.Ta L.P., Bujna E., Antal O., Ladányi M., Juhász R., Szécsi A., Kun S., Sudheer S., Gupta V.K., Nguyen Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021;183:1136–1144. doi: 10.1016/j.ijbiomac.2021.04.170. [DOI] [PubMed] [Google Scholar]
- 115.Gomez-Mascaraque L.G., Morfin R.C., Pérez-Masiá R., Sanchez G., Lopez-Rubio A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT-Food Sci. Technol. 2016;69:438–446. doi: 10.1016/j.lwt.2016.01.071. [DOI] [Google Scholar]
- 116.FAO. WHO . Report of a Joint FAO/WHO Expert Consultation on Guidelines for the Evaluation of Probiotics in Food. FAO; Rome, Lazio, Italy: WHO; Geneva, Switzerland: 2002. [Google Scholar]
- 117.Begley M., Hill C., Gahan C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Begley M., Gahan C.G.M., Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Elkins C.A., Moser S.A., Savage D.C. Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species. Microbiology. 2001;147:3403–3412. doi: 10.1099/00221287-147-12-3403. [DOI] [PubMed] [Google Scholar]
- 120.Martins F.S., Miranda I.C., Rosa C.A., Nicoli J.R., Neves M.J. Effect of the trehalose levels on the screening of yeast as probiotic by in vivo and in vitro assays. Braz. J. Microbiol. 2008;39:50–55. doi: 10.1590/S1517-83822008000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hossain M.N., Afrin S., Humayun S., Ahmed M.M., Saha B.K. Identification and Growth Characterization of a Novel Strain of Saccharomyces boulardii Isolated from Soya Paste. Front. Nutr. 2020;7:27. doi: 10.3389/fnut.2020.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hernández-Gómez J.G., López-Bonilla A., Trejo-Tapia G., Ávila-Reyes S.V., Jiménez-Aparicio A.R., Hernández-Sánchez H. In vitro bile salt hydrolase (BSH) Activity screening of different probiotic microorganisms. Foods. 2021;10:674. doi: 10.3390/foods10030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakayama H., Yoshida K., Shinmyo A. Yeast plasma membrane Ena1p ATPase alters alkali-cation homeostasis and confers increased salt tolerance in tobacco cultured cells. Biotechnol. Bioeng. 2004;85:776–789. doi: 10.1002/bit.20021. [DOI] [PubMed] [Google Scholar]
- 124.Casado C., González A., Platara M., Ruiz A., Ariño J. The role of the protein kinase A pathway in the response to alkaline pH stress in yeast. Biochem. J. 2011;438:523–533. doi: 10.1042/BJ20110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilms T., Swinnen E., Eskes E., Dolz-Edo L., Uwineza A., Van Essche R., Rosseels J., Zabrocki P., Cameroni E., Franssens V., et al. The yeast protein kinase Sch9 adjusts V-ATPase assembly/disassembly to control pH homeostasis and longevity in response to glucose availability. PLoS Genet. 2017;13:e1006835. doi: 10.1371/journal.pgen.1006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diab H.I., Kane P.M. Loss of vacuolar H+-ATPase (V-ATPase) activity in yeast generates an iron deprivation signal that is moderated by induction of the peroxiredoxin TSA2. J. Biol. Chem. 2013;288:11366–11377. doi: 10.1074/jbc.M112.419259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang S.Y., Huang T.K., Kuo H.F., Chiou T.J. Role of vacuoles in phosphorus storage and remobilization. J. Exp. Bot. 2017;68:3045–3055. doi: 10.1093/jxb/erw481. [DOI] [PubMed] [Google Scholar]
- 128.Capusoni C., Arioli S., Donzella S., Guidi B., Serra I., Compagno C. Hyper-Osmotic Stress Elicits Membrane Depolarization and Decreased Permeability in Halotolerant Marine Debaryomyces hansenii Strains and in Saccharomyces cerevisiae. Front. Microbiol. 2019;10:64. doi: 10.3389/fmicb.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deparis Q., Claes A., Foulquié-Moreno M.R., Thevelein J.M. Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res. 2017;17:fox036. doi: 10.1093/femsyr/fox036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gandhi A., Shah N.P. Effect of salt stress on morphology and membrane composition of Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum, and their adhesion to human intestinal epithelial-like Caco-2 cells. J. Dairy Sci. 2016;99:2594–2605. doi: 10.3168/jds.2015-10718. [DOI] [PubMed] [Google Scholar]
- 131.Romero-Gil V., Bautista-Gallego J., Rodríguez-Gómez F., García-García P., Jiménez-Díaz R., Garrido-Fernández A., Arroyo-López F.N. Evaluating the individual effects of temperature and salt on table olive related microorganisms. Food Microbiol. 2013;33:178–184. doi: 10.1016/j.fm.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 132.Hou L., Wang M., Wang C., Wang C., Wang H. Analysis of salt-tolerance genes in Zygosaccharomyces rouxii. Appl. Biochem. Biotechnol. 2013;170:1417–1425. doi: 10.1007/s12010-013-0283-2. [DOI] [PubMed] [Google Scholar]
- 133.Song Y.R., Jeong D.Y., Baik S.H. Effects of indigenous yeasts on physicochemical and microbial properties of Korean soy sauce prepared by low-salt fermentation. Food Microbiol. 2015;51:171–178. doi: 10.1016/j.fm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 134.Yao S.J., Zhou R.Q., Jin Y., Zhang L.Q., Huang J., Wu C.D. Co-culture with Tetragenococcus halophilus changed the response of Zygosaccharomyces rouxii to salt stress. Process Biochem. 2020;95:279–287. doi: 10.1016/j.procbio.2020.02.021. [DOI] [Google Scholar]
- 135.Fleet G.H. Yeast spoilage of foods and beverages. In: Kurtzman C.P., Fell J.W., Boekhout T., editors. The Yeasts: A Taxonomic Study. Volume 1. Elsevier Science; San Diego, CA, USA: 2011. pp. 53–63. [Google Scholar]
- 136.Hong J., Li W., Lin B., Zhan M., Liu C., Chen B.Y. Deciphering the effect of salinity on the performance of submerged membrane bioreactor for aquaculture of bacterial community. Desalination. 2013;316:23–30. doi: 10.1016/j.desal.2013.01.015. [DOI] [Google Scholar]
- 137.Heinisch J.J., Rodicio R. Biology of Microorganisms on Grapes, in Must and in Wine. Springer; Berlin/Heidelberg, Germany: 2017. Stress responses in wine yeast; pp. 377–395. [Google Scholar]
- 138.Hohmann S. An integrated view on a eukaryotic osmoregulation system. Curr. Genet. 2015;61:373–382. doi: 10.1007/s00294-015-0475-0. [DOI] [PubMed] [Google Scholar]
- 139.Sharma A., Sharma S.C. Physiological Basis for the Tolerance of Yeast Zygosaccharomyces bisporus to Salt Stress. HAYATI J. Biosci. 2017;24:176–181. doi: 10.1016/j.hjb.2017.11.001. [DOI] [Google Scholar]
- 140.Pascual-Ahuir A., Manzanares-Estreder S., Timón-Gómez A., Proft M. Ask yeast how to burn your fats: Lessons learned from the metabolic adaptation to salt stress. Curr. Genet. 2018;64:63–69. doi: 10.1007/s00294-017-0724-5. [DOI] [PubMed] [Google Scholar]
- 141.Dakal T.C., Solieri L., Giudici P. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014;185:140–157. doi: 10.1016/j.ijfoodmicro.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 142.Hounsa C.G., Brandt E.V., Thevelein J., Hohmann S., Prior B.A. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology. 1998;144:671–680. doi: 10.1099/00221287-144-3-671. [DOI] [PubMed] [Google Scholar]
- 143.Brewster J.L., Gustin M.C. Hog1: 20 years of discovery and impact. Sci. Signal. 2014;7:re7. doi: 10.1126/scisignal.2005458. [DOI] [PubMed] [Google Scholar]
- 144.Rodrigues-Pousada C.A., Nevitt T., Menezes R., Azevedo D., Pereira J., Amaral C. Yeast activator proteins and stress response: An overview. FEBS Lett. 2004;567:80–85. doi: 10.1016/j.febslet.2004.03.119. [DOI] [PubMed] [Google Scholar]
- 145.Dhar R., Sägesser R., Weikert C., Yuan J., Wagner A. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J. Evol. Biol. 2011;24:1135–1153. doi: 10.1111/j.1420-9101.2011.02249.x. [DOI] [PubMed] [Google Scholar]
- 146.Isenring J., Geirnaert A., Lacroix C., Stevens M.J.A. Bistable auto-aggregation phenotype in Lactiplantibacillus plantarum emerges after cultivation in in vitro colonic microbiota. BMC Microbiol. 2021;21:268. doi: 10.1186/s12866-021-02331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galdiero F., Carratelli C.R., Nuzzo I., Bentivoglio C., Galdiero M. Phagocytosis of bacterial aggregates by granulocytes. Eur. J. Epidemiol. 1988;4:456–460. doi: 10.1007/BF00146398. [DOI] [PubMed] [Google Scholar]
- 148.Monteagudo-Mera A., Rastall R.A., Gibson G.R., Charalampopoulos D., Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019;103:6463–6472. doi: 10.1007/s00253-019-09978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Verstrepen K.J., Klis F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006;60:5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- 150.Fernandez-Pacheco Rodríguez P., Arévalo-Villena M., Zaparoli Rosa I., Briones Pérez A. Selection of potential non-Sacharomyces probiotic yeasts from food origin by a step-by-step approach. Food Res. Int. 2018;112:143–151. doi: 10.1016/j.foodres.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 151.Suvarna S., Dsouza J., Ragavan M.L., Das N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018;27:745–753. doi: 10.1007/s10068-018-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lima M.D.S.F.D., Souza K.M.S.D., Albuquerque W.W.C., Teixeira J.A.C., Cavalcanti M.T.H., Porto A.L.F. Saccharomyces cerevisiae from Brazilian kefir-fermented milk: An in vitro evaluation of probiotic properties. Microb. Pathog. 2017;110:670–677. doi: 10.1016/j.micpath.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 153.García-Cayuela T., Korany A.M., Bustos I., de Cadiñanos L.P.G., Requena T., Peláez C., Martínez-Cuesta M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014;57:44–50. doi: 10.1016/j.foodres.2014.01.010. [DOI] [Google Scholar]
- 154.Nandhra G.K., Mark E.B., Di Tanna G.L., Haase A.M., Poulsen J., Christodoulides S., Kung V., Klinge M.W., Knudsen K., Borghammer P., et al. Normative values for region-specific colonic and gastrointestinal transit times in 111 healthy volunteers using the 3D-Transit electromagnet tracking system: Influence of age, gender, and body mass index. Neurogastroenterol. Motil. 2020;32:e13734. doi: 10.1111/nmo.13734. [DOI] [PubMed] [Google Scholar]
- 155.Asnicar F., Leeming E.R., Dimidi E., Mazidi M., Franks P.W., Al Khatib H., Valdes A.M., Davies R., Bakker E., Francis L., et al. Blue poo: Impact of gut transit time on the gut microbiome using a novel marker. Gut. 2021;70:1665–1674. doi: 10.1136/gutjnl-2020-323877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roland B.C., Ciarleglio M.M., Clarke J.O., Semler J.R., Tomakin E., Mullin G.E., Pasricha P.J. Small Intestinal Transit Time Is Delayed in Small Intestinal Bacterial Overgrowth. J. Clin. Gastroenterol. 2015;49:571–576. doi: 10.1097/MCG.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 157.Gil-Rodríguez A.M., Carrascosa A.V., Requena T. Yeasts in foods and beverages: In vitro characterisation of probiotic traits. LWT-Food Sci. Technol. 2015;64:1156–1162. doi: 10.1016/j.lwt.2015.07.042. [DOI] [Google Scholar]
- 158.Stewart G.G. Yeast flocculation—sedimentation and flotation. Fermentation. 2018;4:28. doi: 10.3390/fermentation4020028. [DOI] [Google Scholar]
- 159.Di Gianvito P., Tesnière C., Suzzi G., Blondin B., Tofalo R. FLO5 gene controls flocculation phenotype and adhesive properties in a Saccharomyces cerevisiae sparkling wine strain. Sci. Rep. 2017;7:10786. doi: 10.1038/s41598-017-09990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miki B.L.A., Poon N.H., James A.P., Seligy V.L. Possible mechanism for flocculation interactions governed by gene FLO1 in Saccharomyces cerevisiae. J. Bacteriol. 1982;150:878–889. doi: 10.1128/jb.150.2.878-889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Touhami A., Hoffmann B., Vasella A., Denis F.A., Dufrêne Y.F. Aggregation of yeast cells: Direct measurement of discrete lectin-carbohydrate interactions. Microbiology. 2003;149:2873–2878. doi: 10.1099/mic.0.26431-0. [DOI] [PubMed] [Google Scholar]
- 162.Goossens K.V.Y., Ielasi F.S., Nookaew I., Stals I., Alonso-Sarduy L., Daenen L., Van Mulders S.E., Stassen C., Van Eijsden R.G.E., Siewers V., et al. Molecular mechanism of flocculation self-recognition in yeast and its role in mating and survival. mBio. 2015;6:e00427-15. doi: 10.1128/mBio.00427-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu Q., Yu Z., Tian F., Zhao J., Zhang H., Zhai Q., Chen W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020;19:23. doi: 10.1186/s12934-020-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Somashekaraiah R., Shruthi B., Deepthi B.V., Sreenivasa M.Y. Probiotic properties of lactic acid bacteria isolated from neera: A naturally fermenting coconut palm nectar. Front. Microbiol. 2019;10:1382. doi: 10.3389/fmicb.2019.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krasowska A., Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014;4:112. doi: 10.3389/fcimb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kochkodan V., Tsarenko S., Potapchenko N., Kosinova V., Goncharuk V. Adhesion of microorganisms to polymer membranes: A photobactericidal effect of surface treatment with TiO2. Desalination. 2008;220:380–385. doi: 10.1016/j.desal.2007.01.042. [DOI] [Google Scholar]
- 167.Lugea A., Salas A., Casalot J., Guarner F., Malagelada J.R. Surface hydrophobicity of the rat colonic mucosa is a defensive barrier against macromolecules and toxins. Gut. 2000;46:515–521. doi: 10.1136/gut.46.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farhadi A., Banan A., Fields J., Keshavarzian A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 169.Van Tassell M.L., Miller M.J. Lactobacillus adhesion to mucus. Nutrients. 2011;3:613–636. doi: 10.3390/nu3050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McGinnis M.R. Pathogenesis of indoor fungal diseases. Med. Mycol. 2004;42:107–117. doi: 10.1080/13693780410001661473. [DOI] [PubMed] [Google Scholar]
- 171.Ehrmann M.A., Kurzak P., Bauer J., Vogel R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002;92:966–975. doi: 10.1046/j.1365-2672.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- 172.França R.C., Conceição F.R., Mendonça M., Haubert L., Sabadin G., de Oliveira P.D., Amaral M.G., Silva W.P., Moreira Â.N. Pichia pastoris X-33 has probiotic properties with remarkable antibacterial activity against Salmonella Typhimurium. Appl. Microbiol. Biotechnol. 2015;99:7953–7961. doi: 10.1007/s00253-015-6696-9. [DOI] [PubMed] [Google Scholar]
- 173.Menezes A.G.T., Ramos C.L., Cenzi G., Melo D.S., Dias D.R., Schwan R.F. Probiotic Potential, Antioxidant Activity, and Phytase Production of Indigenous Yeasts Isolated from Indigenous Fermented Foods. Probiotics Antimicrob Proteins. 2020;12:280–288. doi: 10.1007/s12602-019-9518-z. [DOI] [PubMed] [Google Scholar]
- 174.Rezac S., Kok C.R., Heermann M., Hutkins R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018;9:1785. doi: 10.3389/fmicb.2018.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Goktas H., Dikmen H., Demirbas F., Sagdic O., Dertli E. Characterisation of probiotic properties of yeast strains isolated from kefir samples. Int. J. Dairy Technol. 2021;74:715–722. doi: 10.1111/1471-0307.12802. [DOI] [Google Scholar]
- 176.Romero-Luna H.E., Hernández-Sánchez H., Ribas-Aparicio R.M., Cauich-Sánchez P.I., Dávila-Ortiz G. Evaluation of the probiotic potential of Saccharomyces cerevisiae Strain (C41) isolated from Tibicos by in vitro studies. Probiotics Antimicrob. Proteins. 2019;11:794–800. doi: 10.1007/s12602-018-9471-2. [DOI] [PubMed] [Google Scholar]
- 177.Servin A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 178.Linder M.B. Hydrophobins: Proteins that self assemble at interfaces. Curr. Opin. Colloid Interface Sci. 2009;14:356–363. doi: 10.1016/j.cocis.2009.04.001. [DOI] [Google Scholar]
- 179.Hazen K.C., Hazen B.W. Surface hydrophobic and hydrophilic protein alterations in Candida albicans. FEMS Microbiol. Lett. 1993;107:83–87. doi: 10.1111/j.1574-6968.1993.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 180.Willaert R.G. Adhesins of yeasts: Protein structure and interactions. J. Fungi. 2018;4:119. doi: 10.3390/jof4040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hirayama S., Furukawa S., Ogihara H., Morinaga Y. Yeast mannan structure necessary for co-aggregation with Lactobacillus plantarum ML11-11. Biochem. Biophys. Res. Commun. 2012;419:652–655. doi: 10.1016/j.bbrc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 182.Lara-Hidalgo C., Dorantes-Álvarez L., Hernández-Sánchez H., Santoyo-Tepole F., Martínez-Torres A., Villa-Tanaca L., Hernández-Rodríguez C. Isolation of yeasts from guajillo pepper (Capsicum annuum L.) fermentation and study of some probiotic characteristics. Probiotics Antimicrob. Proteins. 2019;11:748–764. doi: 10.1007/s12602-018-9415-x. [DOI] [PubMed] [Google Scholar]
- 183.Kaczorek E., Chrzanowski Ł., Pijanowska A., Olszanowski A. Yeast and bacteria cell hydrophobicity and hydrocarbon biodegradation in the presence of natural surfactants: Rhamnolipides and saponins. Bioresour. Technol. 2008;99:4285–4291. doi: 10.1016/j.biortech.2007.08.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.