Introduction
The potential role of “leaky gut” or reduced barrier function with increased intestinal permeability has been considered an important factor in intestinal and extra-intestinal diseases. Reversing the impairment of barrier function in diseases associated with mucosal damage may be necessary but may not be sufficient to reverse the disease pathogenesis as in inflammatory bowel disease or coeliac disease. In these diseases, the underlying immune dysfunction is likely to be a significant factor in perpetuating the disease. Nevertheless, gastrointestinal and extra-intestinal diseases that are not associated with predominant inflammation in the small intestine or colon have drawn attention to the potential role of reduced barrier function in disease pathogenesis. These illnesses range from eosinophilic esophagitis to non-alcoholic fatty liver disease and to diverse neuropsychiatric diseases, as summarized elsewhere [1]. There are also diverse systemic consequences associated with gut barrier dysfunction including increased inflammation or oxidative stress and decreased insulin sensitivity affecting tissues or organs such as the liver, fat, skeletal, muscle, and brain [2].
This article, designed to be based almost exclusively on data acquired in humans in vivo, focuses on four objectives: first, to review recently validated and optimized measurements of intestinal and colonic barrier function; second, to summarize the evidence for the avoidance of foods that are deleterious to barrier function; and third, to summarize the evidence for substances in foods that strengthen the intestinal barrier and how these can be supplemented in the diet. It is relevant to acknowledge that there is still a dearth of evidence that these dietary components improve barrier function, and therefore, a fourth objective is to introduce potential pharmacological approaches in development for enhancing intestinal barrier function.
Components of the Intestinal Barrier
The intestinal barrier components and cellular elements are described in detail elsewhere [2–5]. The barrier is a dynamic entity interacting with and responding to various stimuli. In the lumen, there is degradation of bacteria and antigens by gastric acid, bile acids, and pancreatic juice. Bile has direct antimicrobial effects on gut microbes [6]; the deleterious effects of reduced bile, as in cirrhosis, are demonstrated by the reduction in normal gram-positive microbiota such as Blautia and Ruminococcaceae, and by an increase in the pro-inflammatory taxa, Enterobacteriaceae [7]. In addition, commensal bacteria in the lumen inhibit colonization by pathogens through the effects of antimicrobial substances. In addition, a microclimate composed of the unstirred water layer, glycocalyx, and mucus prevents bacterial adhesion and presents defense mechanisms such as secreted immunoglobulin A (IgA) as well as the physical barrier provided by the glycocalyx and mucus.
The mucus layer is an important physical barrier [8] that consists of an inner firmly adherent layer where bacteria are sparse and where secreted peptides with antibacterial functions (e.g., defensins and lysozyme) are present, and a thicker and loosely adherent outer layer where bacteria and bacterial products are abundant. The composition and distribution of the mucus layer vary along the GI tract. The mucus is thicker in the colon than in the small bowel. In addition, the firm inner mucus layer of colon is dense with a high concentration of Muc2, which is a gel-forming densely packed mucin [9]; in contrast, in the small intestine, the same Muc2 mucin is not attached and directly forms a soluble mucus gel [10]. Mucus prevents antigens, toxins, and bacteria [11] from directly contacting the epithelial cells. The structure of mucus glycoproteins consists of a central protein core (abundant in serine, threonine, and proline) and O-glycosylated hexoses and hexosamines that are oriented almost perpendicular to the protein core like a bottle brush, forming a gel-like sieve overlying the intestinal epithelium.
There is regional variation in the pore size of the barrier along the gut: 4-5 Å at the villus tip to over 20 Å at the base of the crypt in the small bowel, consistent with a decreasing gradient of paracellular permeability from crypt to villus adjacent intestinal epithelial cells. The colonic epithelium is less permeable than the small intestinal epithelium. Epithelial cells, connected by apical junctional complexes, transport luminal content, and they react to noxious stimuli by secretion of chloride and antimicrobial peptides. From the apical to the basal domains of enterocytes, there are three sets of intercellular junctions: tight junction [zonula occludens (ZO)], adherens junction (zonula adherens), and desmosome; together they comprise the apical junctional complex and support the dense microvillus brush border [12]. TJ are involved in regulating the epithelial barrier function and intercellular transport, the other cell-to-cell adhesion structures attach adjacent enterocytes or colonocytes [12].
There are two distinct paracellular epithelial permeability pathways [4,12,13]: the ‘leak’ and ‘pore’ pathways which are regulated by tight junctions and define intestinal permeability. The paracellular pathway accepts molecules with molecular mass <600 Da [14,15]. In contrast, an ‘unrestricted’ pathway is associated with pathological states, is independent of tight junctions, and provides access of luminal antigens to the lamina propria. In the presence of erosions or ulcers, bacteria gain access to the mucosa.
Goblet cells produce and release mucins which constitute the glycoprotein to maintain the mucosal barrier. The Paneth cells in the epithelial layer are most numerous in the crypts and produce defensins and several antibiotic peptides and proteins when exposed to bacteria or bacterial products such as lipopolysaccharide.
Three other types of cells interspersed among the enterocytes are: first, enteroendocrine cells that secrete peptides or hormones such as GLP-2 (which is involved in epithelial regeneration and repair, and induction of synthesis of tight junction proteins); second, tuft cells which produce IL-25 (involved in innate immunity) and IL-13 (which controls IgE responses and stimulates goblet cell hyperplasia and mucus production); and third, M cells which are found exclusively in the small intestine. These M cells are involved in antigen uptake and thereby initiate antigen-specific mucosal immune responses such as the induction of secretory immunoglobulin A (sIgA).
The lamina propria contains elements of innate and acquired immunity including cells such as plasma cells, B and T lymphocytes, and mast cells, secreting IgA, cytokines, chemokines, and mast cell proteases. The final elements of the barrier involve endocrine and secretomotor mechanisms mediated by the enteric nervous system which result in water secretion and intestinal motility, thereby transporting potentially injurious elements downstream and ultimately excretion.
Although transcellular permeability is not a major focus of this article, acute inflammation and ischemia increase transmucosal transport of bacteria and bacterial products via transcellular uptake such as endocytosis (reviewed in ref. [13]). Transcellular transport [14,15] constitutes the major pathway for molecules that are beyond the molecular weight accepted by the paracellular pathways (>600 Da); these include substances such as food antigens and peptides that are produced by processing of proteins and subsequently undergo further degradation in acidic and lysosomal compartments during passage through the cell [15]. The transcellular permeability process therefore involves endocytosis at the apical membrane of epithelial cells and transcytosis toward the lamina propria with release as amino acids or partially degraded at the basolateral pole of enterocytes. Early endosomes containing partially degraded food antigens in the form of peptides of ~1,500 Da molecular weight meet the major histocompatibility complex (MHC) class II compartment, leading to the formation of exosomes. These are small membrane vesicles (40 – 90 nm) that can diffuse in the basement membrane and interact with local dendritic cells and thereby lead to peptide presentation to T cells.
It is clear that in vivo assessment of intestinal permeability using small inert molecules can only assess the paracellular permeability and does not necessarily correlate with the uptake of larger dietary antigens via the transcellular pathway.
Diagnosis of “Leaky Gut”
If consideration of “leaky gut” is to enter the clinical practice of mainstream gastroenterology and nutrition, it is essential to develop and use accurate measurements of “leaky gut”. In addition, in order to maximally impact the field and perform studies in large numbers of patients or healthy participants which is essential to document relatively small effect sizes with perturbations or treatments in uninflamed intestine, it is essential to develop and validate methods that do not require mucosal biopsies from the small bowel or colon. Thus, structure and function claims [16] submitted to regulatory agencies regarding nutrients and supplements require primary evidence from human studies demonstrating that a dietary component is causally associated with maintaining or restoring normal gut barrier structure (e.g., mucus layer thickness) or function of human intestinal barrier (e.g., “normal” permeability or epithelial cell immune function).
Considerations in Selection of Oral Probes for Measuring Intestinal Permeability in Humans In Vivo
The factors considered to be needed to embark on barrier function measurements in otherwise healthy humans are: first, demonstration of the absence of confounding by exogenous intake which would reduce accuracy of the measurements; second, ease of performance or availability of the assay, such as, availability of a chemical or “kit-based” assay may be preferable to liquid chromatography/mass spectrometry, but the “kit” assay should match the sensitivity and accuracy of the latter; third, affordable cost of the probe molecules and the test (e.g,, a stable isotope-labeled sugar is more expensive than other natural sugars); and fourth, test reproducibility, as demonstrated by small variance within individuals on replicate studies, and well-defined interindividual coefficients of variation to plan sample size for future studies.
Recent Advances in Measurements of Human “Leaky Gut” In Vivo
Elsewhere [1,16], the diverse methods to assess intestinal permeability were reviewed: First, orally ingested probe molecules (a direct functional assessment of the epithelial barrier, specifically the paracellular pathway); second, circulating endotoxins [lipopolysaccharide (LPS) and LPS-binding protein], serum zonulin (which is an intestinal tight junction modulator) or intestinal fatty acid-binding protein levels (I-FABP); these measurements reflect damage to the epithelium and constitute indirect assessments of the barrier. Zonulins are 47 kDa paracrine proteins released by several cell lines in the body including epithelial cells lining the small intestine. The intestinal zonulin is particularly relevant since it is a physiological modulator of the intercellular tight junctions, important in antigen trafficking, and therefore it appears to be a key player in regulation of the mucosal immune response as in celiac disease and type 1 diabetes mellitus [17]. These alterations of tight junctions and their associations with these diseases illustrate the potential importance of the paracellular pathway to the immunobiology of disease states. I-FABP is an enterocyte-derived protein that serves as an intestinal biomarker of enterocyte damage and ischemia; its appearance in the circulation indicates damage to mature enterocytes and may be accompanied by infiltration of bacterial endotoxins within the blood circulation, as has been described in spondyloarthritis associated with inflammatory bowel disease [18].
A third category consists of endoscopic measurements using confocal microscopy and mucosal impedance. The range of molecular weights of the probe substances were also reviewed elsewhere. Serum lipopolysaccharide, zonulin, and I-FABP may reflect the increased permeability associated with mucosal diseases. Recent conflicting information on the sensitivity of serum zonulin in the irritable bowel syndrome (IBS) or non-celiac gluten sensitivity, as well as controversy regarding sensitivity of commercial assays for zonulin [19–21] (which measure additional compounds in addition to the critically important biomarker, prehaptoglobulin-2) may question the applicability of such serum markers based on potentially flawed ELISA assays for in vivo measurements of permeability or for assessing effects of elements in the diet on intestinal permeability in non-ulcerating or non-inflammatory diseases. Therefore, careful review of the method used is necessary when the investigation of intestinal permeability used serum zonulin by ELISA.
However, a report by Seethaler et al. involved a cross-sectional study of 51 non-obese and 27 obese individuals without any documented intestinal diseases [22]. They used the lactulose/mannitol ratio (LMR), based on a 6-hour urine collection after oral ingestion of the sugars, as an established marker of intestinal permeability (though the timed urine collection probably reflected both small bowel and colonic barriers), and assessed the performance of 6 potential surrogate biomarkers: albumin, calprotectin, and zonulin measured in feces, as well as intestinal fatty acid binding protein (I-FABP), lipopolysaccharide-binding protein (LBP), and zonulin, measured in plasma. Correlation analyses and multiple linear regression models were conducted to assess possible associations between LMR and the proposed biomarkers, and they evaluated potential effects of age, body mass index (BMI), and sex. The best correlations with LMR were observed with plasma LBP and fecal zonulin, and the results were independent of age, sex, and BMI, except for fecal zonulin where the correlation with LMR was associated with BMI. The authors concluded that plasma LBP is a promising biomarker for intestinal permeability in adults, and fecal zonulin is a potential biomarker in overweight and obese individuals [22].
In a recent validation study [23], urinary secretion of orally administered sugar probes was measured in 60 healthy male and female adults, aged 18 to 70 years. All participated in 3 randomized studies with otherwise standardized diets (2 studies with 16.25g fiber and 1 study with 32.5g fiber in the diet). At each test, the following sugars were ingested: 200mg 12C-mannitol, 100mg 13C-mannitol, 200mg rhamnose (three monosaccharides), 1g sucralose, and 1g lactulose (two disaccharides). The usual dosing recommendation of the lactulose to mannitol ratio of 5:1 was followed. Standardized meals were administered from 24 hours before and during 24 hours post-administration of the sugars with 3 urine collections: 0-2, 2-8, and 8-24 hours. These collection periods had previously been validated [24] as the best for evaluation of the small bowel (0-2h), combined small bowel and colon (2-8h), and exclusively colonic permeability (8-24h). Sugars were measured by HPLC-MS [25,26]. Measurements of the same sugars were also performed at baseline, that is prior to the administration of the oral probe molecules, to assess the potential for environmental contamination. Sugar levels >3-fold above lower limits of quantitation were identified in the 3 studies performed in the 60 participants: 12C-mannitol in all participants (reflecting presence of this sugar in diverse foods and even skin preparations); sucralose (reflecting its use as a sweetener in several foods and beverages) in up to 8 participants; and rhamnose in up to 3 participants. The median contamination with 12C-mannitol was about 12mg which would be 6% of the orally administered dose (200mg) and 20% of the total 12C-mannitol excretion over 24h. In addition, the 75th percentile of baseline mannitol content in baseline urine was 25mg and the highest values exceeded 50mg (Figure 1).
Figure 1. 12C-mannitol in urine samples collected at baseline during three test days in 60 healthy participants.
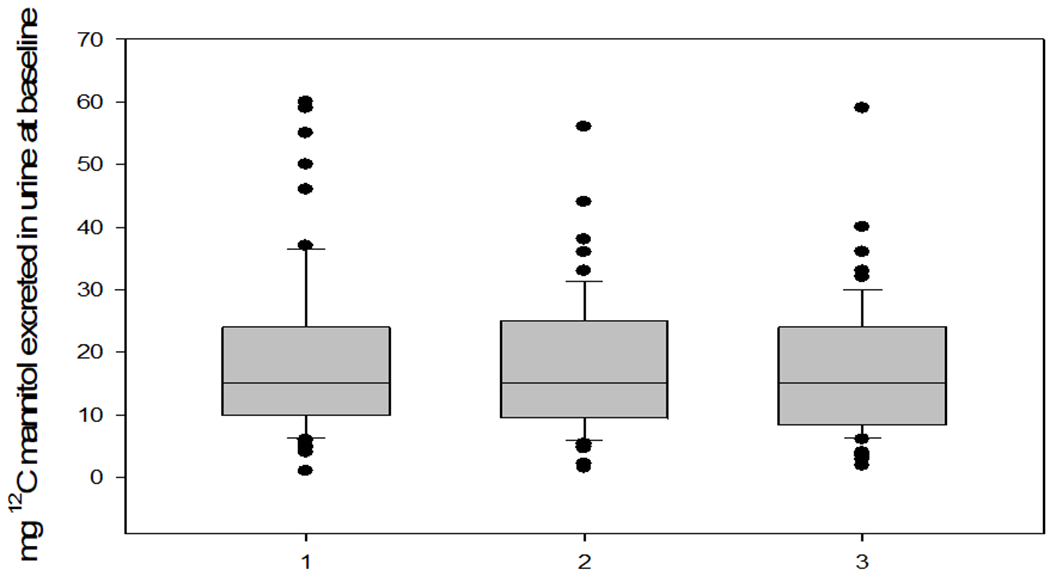
Reproduced from ref. 23, Khoshbin et al. Gastroenterology 2021.
These data suggest that the time-honoured use of mannitol as a probe for measuring intestinal permeability in humans is unacceptable because of environmental contamination unless the dose of administered mannitol is increased to minimize the impact by the contaminant. However, the effect of using a larger amount of mannitol (e.g. 2 or 5g) is that it automatically results in the need for, typically, a 5-fold higher lactulose dose with potential interference by the lactulose of gut fluid fluxes by the osmotic load.
The recent study documented normal value data for all individual sugars and the commonly used disaccharide top monosaccharide excretion ratios in adults [23] (Table 1). The median COVinter was 76.5% (10-90%ile: 34.6-111.0) for the different sugars measured in urine collections. There were no significant effects of sex, age, or BMI on permeability measurements in healthy participants. Importantly, intraindividual saccharide excretions were consistent, with minimal differences in excretion of sugars when the same diet containing 16.25g fiber was ingested, and minor differences observed with diets containing 16.25g compared to 32.5g fiber diets.
Table 1. Median (interquartile range, IQR) mg sugar excretions with fiber 16.25g/day (median of 2 studies) vs. fiber 32.5g/day; note sequence of studies was randomized (double-blind).
Reproduced with permission from ref. 23, Khoshbin et al Gastroenterology 2021
| Saccharide (dose used) | Timed urine collection | Median (IQR) mg or ratio avg. of 2 studies with 16.25g fiber/day | Median (IQR) mg or ratio with 32.5g fiber/day |
|---|---|---|---|
| 13C-mannitol 100mg | 0–2 h | 15.25 (12.0, 20.5) | 13.0 (10.0,18.0) |
| 2–8 h | 13.22 (9.62, 17.25) | 15.0 (8.7, 20.5) | |
| 8–24 h | 1.8 (1.15, 2.82) | 2.35 (1.5, 3.6) | |
| 0–24 h | 32.05 (25.55, 37.57) | 31.25 (25.75, 39.9) | |
| Rhamnose 200mg | 0–2 h | 11.48 (9.40, 14.85) | 10.55 (7.54, 14.05) |
| 2–8 h | 10.85 (8.06, 15.15) | 12.55 (7.275, 16.9) | |
| 8–24 h | 3.12 (1.75, 4.56) | 2.19 (1.50, 3.64) | |
| 0–24 h | 26.04 (21.43, 33.15) | 26.14 (20.05, 34.33) | |
| Lactulose 1000mg | 0–2 h | 1.2 (0.9, 1.95) | 1.10 (0.7, 1.65) |
| 2–8 h | 1.575 (1.17, 2.40) | 1.75 (1.3, 2.8) | |
| 8–24 h | 0.29 (0.29, 0.30) | 0.29 (0.29, 1.0) | |
| 0–24 h | 3.42 (2.49, 4.69) | 4.04 (3.09, 5.345) | |
| Sucralose 1000mg | 0–2 h | 6.895 (4.91, 10.38) | 6.655 (4.59, 9.765) |
| 2–8 h | 9.92 (7.52, 12.77) | 10.85 (8.25, 15.15) | |
| 8–24 h | 4.75 (3.28, 6.72) | 5.445 (3.69, 7.88) | |
| 0–24 h | 22.26 (17.96, 29.98) | 24.31 (20.17, 30.51) | |
| %L/RR | 0–2 h | 0.021 (0.016,0.028) | 0.021 (0.016, 0.028) |
| %L/RR | 8–24 h | 0.027 (0.018,0.035) | 0.044 (0.031,0.064) |
| %L/13CMR | 0–2 h | 0.008 (0.006,0.010) | 0.008 (0.006,0.011) |
| %L/13CMR | 8–24 h | 0.02 (0.015,0.033) | 0.023 (0.014,0.034) |
| %S/RR | 0–2 h | 0.122 (0.103, 0.147) | 0.123 (0.101, 0.142) |
| %S/RR | 8–24 h | 0.352 (0.245,0.445) | 0.404 (0.318, 0.619) |
| %S/13CMR | 0–2 h | 0.047 (0.040,0.056) | 0.047 (0.037, 0.052) |
| %S/13CMR | 8–24 h | 0.295 (0.199, 0.388) | 0.212 (0.144, 0.342) |
L=lactulose; 13CM=13C-mannitol; R=rhamnose; S=sucralose; L/RR=lactulose to rhamnose ratio; L/13CMR=lactulose to 13C-mannitol ratio; S/RR=sucralose to rhamnose ratio; S/13CMR=sucralose to 13C-mannitol ratio
The median excretions/24h expressed as percent of administered dose for 13C-mannitol, rhamnose, lactulose, and sucralose were respectively ~30%, ~15%, 0.32%, and 2.3%. 13C-mannitol and rhamnose excretion profiles reflected mainly small intestinal permeability. The data demonstrate that, in the uninflamed and non-ulcerated intestine (in contrast to colitis with ~4% lactulose excretion during 8-24h) [27], there is a very small percent of excretion of lactulose, reflecting the minimal absorption in such human participants, as well as the bacterial metabolism of lactulose in the colon [28]. The proportion of disaccharide absorbed in the small bowel (0-2 h excretion) or the combination of the small bowel and colon (2-8h excretion) was much greater for sucralose than lactulose, possibly reflecting lack of colonic bacterial metabolism and favouring sucralose as the disaccharide probe. This is countered by the potential contamination by dietary intake. 13C-mannitol measurements were also shown to be feasible in patients with diarrhea-predominant IBS (IBS-D) [23].
With a robust, relatively simple, valid measurement of intestinal permeability available, the diagnosis of “leaky gut” could be entertained for patients with non-inflammatory intestinal diseases. However, there are no approved medications proven to specifically target and restore normal barrier function, although the zonulin inhibitor, larazotide, has been tested in several diseases: celiac disease, type 1 diabetes, inflammatory bowel disease, Kawasaki disease, and respiratory (infective and non-infective) diseases [29]. However, a dose response study in celiac disease showed efficacy for a 0.5mg dose with regard to symptoms, but not for permeability measurements, whereas 1mg and 2mg doses were no different than placebo for any endpoint [30]. At present, therefore, we can consider two management approaches to improve intestinal barrier function: avoidance of foods that damage the barrier or ingestion of foods that strengthen the barrier. The next sections address the evidence in support of these two approaches.
Avoidance of Foods that Are Deleterious to Barrier Function in Health
The effects of components in the diet on intestinal permeability in animal models, cell or membrane based studies in vitro, and in human studies in vivo or in mucosal biopsies in vitro have been extensively reviewed elsewhere [27,31]. This section focuses on the current best evidence of negative effects of high-fat diet and emulsifiers (Figure 2) particularly in studies in humans in vivo, as well as the proposed mechanisms resulting in those effects.
Figure 2.
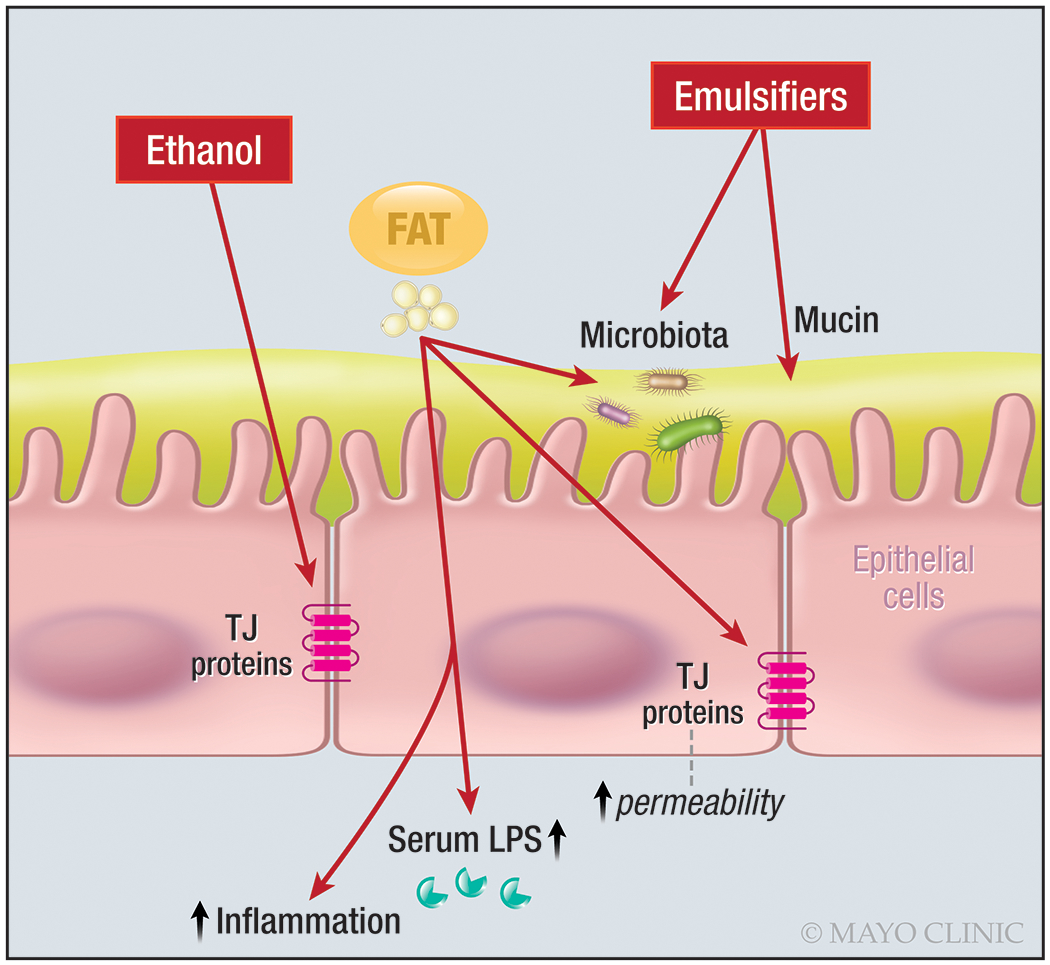
Summary of injurious effects on intestinal barrier of fat, emulsifiers, and alcohol
A. Negative effects of a high-fat diet on intestinal permeability
Mechanisms:
Consumption of excess dietary fats can increase intestinal permeability, modulating the expression and distribution of tight junctions through different mechanisms [32]:
Stimulation of a shift to hydrophobic bile acids such as deoxycholic acid which can disrupt the barrier
Transient reduction in microbes that enhance the gut barrier such microbes, including Lactobacillus spp., Bifidobacterium spp., Bacteriodetes spp., Clostridiales spp., and Akkermansia muciniphilia
Augmentation of microbes involved in decreasing barrier integrity such as Oscillibacter spp., and Desulfovibrio spp. (e.g. Bilophila wadsworthia) which deconjugate taurocholic acid producing hydrogen sulphide gas, thereby inducing oxidative stress and apoptosis of intestinal epithelial cells
Stimulation of pro-inflammatory signaling cascades
Increasing barrier-disrupting cytokines [TNFα, IL 1β, IL6, and interferon γ (IFNγ)] and decreasing barrier-forming cytokines (IL10, IL17, and IL22).
Documented effects of high fat diet on markers of intestinal permeability in humans:
A review has documented the effects of a high-fat diet on microbiota in animals and humans, and associations with alterations in microbiota [33]. Table 2 shows examples of damaging effects on intestinal barrier function of food intake and particularly effects of high-fat diet [34–37]: However, the effects are not consistent in the literature; for example, the effect of fat on barrier function is only shown by measuring serum LPS or LBP [34,36], and, to date, there is no documentation of increased permeability based on direct permeability measurement [37].
Table 2.
Examples of effects of food intake and particularly effects of high-fat diet on intestinal barrier function
| Author/Ref. # | Sample | Treatment/Duration | Main Results | Comment |
|---|---|---|---|---|
| Amar et al., ref. 34; Cani et al., ref. 35 | 1,015 healthy people randomly recruited in France | 3-day food record; plasma LPS tested in 201 male participants | Association between food intake (positively correlated with fat intake) and plasma LPS. | Experimental data in mice suggest that fat was more efficient in transporting bacterial LPS from the intestinal lumen into the bloodstream. |
| Lyte et al., ref. 36 | 20 healthy adults (mean age 25±3.2 years) | Control diet (olive oil - 20%) HFD with omega-3 (fish oil, 35%) HFD with omega-6 (grape seed oil, 35%) Diet rich in saturated fat (coconut oil, 35%) |
Serum endotoxin concentration increased during postprandial period after consumption of a high-saturated fat meal, but decreased after the meal with n-3 | The n-6 meal did not affect postprandial endotoxin concentration in relation to the control meal. There was no postprandial effect on inflammatory biomarkers after meals. |
| Bowser et al., ref. 37 | 13 normal weight and sedentary adult males (mean age 22.2±1.6 years); all weight stable | 2-week control diet [55% CHO, 30% fat (9% saturated), and 15% protein]; HFD for 5 days [55% fat (25% saturated fat), 30% CHO, and 15% protein]; HFM challenge [820 kcal: 25% CHO, 12% protein, 63% fat (~26% saturated fat)] before and following the 5-day HFD |
No significant changes in gastroduodenal, small intestinal (p=0.084 LM ratio), or colonic permeability following HFD. |
Fasting endotoxin concentrations increased (1.2±0.1 vs. 2.3±0.4 Eu/mL, p=0.04) following HFD, but postprandial serum endotoxin concentrations area under the curve did not change following HFD. |
CHO=carbohydrate; HFD=high fat diet; HFM=high fat meal; LM ratio=lactulose to mannitol ratio; LPS=lipopolysaccharides
B. Effects of emulsifiers on mucosal barrier and inflammation
Emulsifiers are complex molecules that contain hydrophilic and lipophilic sections (often in a head to tail configuration) that maintain a dispersion of either fat molecules in liquid suspension or water-soluble components in a hydrophobic environment, extending the stability of such mixtures and slowing the rate of phase separation [38]. These properties maintain the texture, hydration, plasticity, fluidity, consistency, viscosity, volume, structural integrity, color, heat resistance, mold resistance, mouth feel and taste in multi-phase processed foods and beverages.
Mechanisms:
Bancil et al. [39] reviewed the mechanisms resulting in emulsifier-induced damage to the intestinal barrier, decreased microbial diversity and mucosal inflammation. These mechanisms include increased bacterial expression of flagellin and LPS, enhancing bacterial translocation through to the epithelial layer as the mucus is altered, effectively decreasing the gut barrier. Different emulsifiers have dissimilar effects on mucus [40]. For example, carboxymethylcellulose (CMC) decreased mucus pore size which resulted in significantly slower E. coli speed and particle diffusion rates through mucus. Conversely, polysorbate 80 (Tween) exposure minimally impacted mucus microstructure and particle diffusion, but increased E. coli speed in mucus. Both emulsifiers cause thinning of the mucus layer, leading to bacteria encroaching epithelial cells, altering membrane-associated proteins (e.g. ZO-1), and higher levels of bacterial translocation.
Emulsifiers also activate inflammatory pathways through Bcl-10 and TLR-4, which in turn activates the NF-kB cascade, leading to the secretion of pro-inflammatory cytokines such as TNF-α and IL-6 and subsequent development of mucosal inflammation. The effects of CMC and P80 have also been demonstrated through experiments involving transplantation to germ-free recipient mice of a suspension prepared in a mucosal simulator of the human intestinal microbial ecosystem [41].
Documented effects of emulsifiers on inflammatory markers in humans:
The effects of emulsifiers have been shown in two types of human studies: trials restricting emulsifiers, and trials of diets restricting emulsifiers as part of other diets, as summarized in Table 3 [42–46]. These data need to be interpreted with caution, since the diets involved other dietary interventions, and the effects on inflammation cannot be directly attributed to altered intestinal permeability which was not measured in any of the trials.
Table 3.
Effects of emulsifiers in human studies based on trials restricting emulsifiers or trials of diets restricting emulsifiers as part of other diets.
| Study | Design | Participants | Intervention | Outcome | |||
|---|---|---|---|---|---|---|---|
| Tolerance | Disease Activity | Microbiome | Inflammatory Markers | ||||
| Diets Free of Emulsifiers | |||||||
| Bhattacharyya et al. ref. 42 | Randomized, double-blind, placebo-controlled trial | 12 CUC | Carrageenan-free diet for 12 months vs carrageenan-containing diet (resupplementation) | 3 patients declined further participation due to reluctance to comply with the diet | At the 1-year endpoint, 3/5 patients in the carrageenan-containing diet group relapsed; only 1/7 relapsed in the no-carrageenan diet (p=0.046) | Not measured | At end of study (compared to baseline), there was increase in IL-6 and fecal calprotectin in the carrageenan-containing diet group, but not in the carrageenan-free diet |
| Sandall et al. ref. 43 | Uncontrolled feasibility study | 20 Crohn’s in remission | Low emulsifier diet for 2 weeks: ≥75% reduction in frequency of emulsifier intake between baseline and intervention was achieved in 19/20 | FR-QOL improved significantly on the low emulsifier diet | Crohn’s disease-related symptoms (measured using the PRO-2 Questionnaire) as well as perceived disease control (assessed by the IBD Control-8 Questionnaire) improved significantly between baseline and the low emulsifier diet. | Not measured | Not measured |
| Diets Restricting Emulsifiers as Part of Other Diet | |||||||
| Sigall-Boneh et al. ref. 44 | Uncontrolled retrospective study | 47 children and young adults with active Crohn’s disease (PCDAI >7.5 or HBI ≥4) | CDED plus 50% PEN, or CDED alone for 6 weeks; then an additional 6-week stepdown diet | 5 patients were not compliant (2 did not comply at all; 3 complied most of the time, with 2/3 achieving full remission) | Remission occurred in 70% of children and 69% of adults. Seven patients used the diet without PEN, with 6/7 entering remission. | Not measured | Normalization of previously elevated CRP in 70% of patients in remission |
| Levine et al. ref. 45 | Randomized controlled trial | 78 children with mild to moderate CD (PCDAI ≥10 and ≤40) |
Group 1 (n=40): CDED plus 50% of energy from PEN for 6 wk (stage 1), followed by CDED with 25% PEN from weeks 7 to 12 (stage 2) Group 2 (n=38): EEN for 6 wk, followed by a free diet with 25% PEN from weeks 7 to 12 |
Combination of CDED and PEN was tolerated by 39 children (97.5%), whereas EEN was tolerated by 28 children (73.6%) (P=0.002) | At week 12, 28 of 37 (75.6%) children given CDED plus PEN were in corticosteroid-free remission compared with 14 of 31 (45.1%) given EEN and then PEN (P=0.01). | CDED plus PEN was associated with reduction of fecal Proteo-bacteria | In children given CDED plus PEN, corticosteroid-free remission was associated with sustained reductions in CRP and fecal calprotectin |
| Svolos et al. ref. 46 | Randomized open-label pilot study | 5 children with active Crohn’s disease (PCDAI ≥ 12.5) and 28 healthy adults received CD-TREAT diet for 8 weeks | CD-TREAT restricts components such as gluten, lactose, emulsifiers, fiber, CHO, and alcohol for 8 weeks | In healthy adults, CD-TREAT was easier to comply with than EEN. 4 children completed the 8-wk trial; 1 child withdrew due to symptom exacerbation | 4 children (80%) experienced a clinical response and 3 (60%) entered remission | Total fecal bacteria decreased with both diets plus similar changes to β-diversity and fecal bacterial metabolites | Children receiving CD-TREAT had significant decrease in fecal calprotectin (mean change −918±555 mg/kg; P=0.002) |
CDED=Crohn’s disease exclusion diet; CD-TREAT=Crohn’s disease TReatment with EATing; CHO=carbohydrates; CRP=C-reactive protein; EEN=exclusive enteral nutrition; FR-QOL=food-related quality of life; HBI=Harvey-Bradshaw Index; IL=interleukin; PCDAI=Pediatric Crohn’s Disease Activity Index; PEN=partial enteral nutrition
C. Negative effects of alcohol on intestinal permeability
Both acute and chronic alcohol use in humans alters the small intestinal barrier [47]. In mice [48], chronic alcohol administration led to altered microbiota composition, intestinal bacterial overgrowth, and bacterial translocation, compared with the control group.
Mechanism:
Ethanol disrupts intestinal permeability by directly damaging the cells and altering tight junction structures (Figure 2). In healthy participants, single oral intake of alcohol (1g/kg) induced altered histology in the duodenum [49], and excessive alcohol intake in 11 healthy participants altered rectal mucosal cells with goblet cell depletion and induced inflammation with mononuclear cell infiltration [50]. Intra-duodenal administration of 20g alcohol resulted in increased permeability based on ratios of excretion of the disaccharides, lactulose (0-5h, consistent with increased small intestinal permeability) and sucralose (5-24h tracking colonic permeability) [51]. In the same study, duodenal mucosal biopsies showed reduced expression of ZO-1 and occludin, as well as increased phosphorylation of MAP kinase isoforms with alcohol compared to placebo treatment [51]. These human data are supported by results in Caco-2 cell studies that showed alcohol induced a change in expression of the tight junction-associated proteins, ZO-1 and claudin-1, which are two major sites of alcohol action, thus increasing intestinal epithelial barrier permeability [52]. The reduced ZO-1 expression has been associated with increased miR-212 expression in colonic mucosal biopsies from patients with alcoholic liver disease [53]. miR-212 inhibits ZO-1 protein synthesis and disrupts ZO-1’s organizational network.
There is also evidence of Candida as the most abundant genus in the fecal mycobiota in alcohol use disorder, whereas genus Penicillium dominated the mycobiome of nonalcoholic controls, and there was also a lower fungal diversity in alcohol users [54]. However, there was no associated increase in intestinal permeability as measured using plasma zonulin or lipopolysaccharide binding protein levels in association with the altered fecal mycobiota in alcohol use disorder.
Documented effects of alcohol on markers of intestinal permeability in humans:
In men with chronic alcohol abuse and alcoholic liver disease compared with nonalcoholic controls [55], there was increased permeability to larger molecules such as PEG 10,000 (estimated diameter 46 Å) which was used to mimic the molecular weight of endotoxins (5000-8000 Da). Thus, PEG 10,000 was identified in urine, and plasma endotoxin (LPS) was increased five-fold in the alcoholics in comparison to controls. In addition, there was a significant correlation between permeability measured by PEG 4000 and plasma endotoxins in the plasma in the alcoholic group. It is, nevertheless relevant to note that, although these PEG molecules have similar molecular weight to LPS, the latter’s chemistry includes both a hydrophilic region (sugar chain) and a hydrophobic lipid region that leads to the formation of micelles with far greater apparent molecular weight (perhaps reaching 106 Da) in aqueous environments; therefore, despite the observed correlation, it is unclear whether PEG and LPS reflected the same permeability pathway(s). In a separate study, only alcoholics with chronic liver disease showed increased urinary L/M ratio, in contrast to alcoholics without liver disease or patients with non-alcohol-related liver disease [56], suggesting an effect of alcohol rather than a nonspecific effect of liver disease. In another study [57], chronic alcohol abuse was associated with increased intestinal permeability, measured using 51Cr-labeled EDTA, which persisted even after 2 weeks of cessation of alcohol intake.
Alcohol use is also a significant factor in the alteration in short chain fatty acids (SCFAs) and permeability observed in association with circadian variation [58], which is discussed below.
Evidence for Food Substances that Strengthen the Intestinal Barrier and their Supplementation in Diet
Several potential treatment strategies [5] have been proposed against gut barrier dysfunction, including direct immune treatments, inhibition of signaling factors, implantation of selected microbes, fecal microbiota transplantation, microbial metabolites, probiotics, inhibition of regulatory factors induced by other diseases, hormone treatment, cure of an underlying disease or dietary supplementation, healthy diet and good health. This article focuses on dietary components and how they can be supplemented in the diet.
A. Dietary fiber
Dietary fiber (reviewed elsewhere [27]) provides carbohydrate polymers with 10 or more monomeric units that are not hydrolyzed by endogenous enzymes in human small intestine. Fiber may be soluble or insoluble, but both types undergo fermentation by microbiota, with insoluble fiber increasing stool bulk and soluble fiber increasing fermentation, leading to production of metabolites such as SCFAs. The outer mucus layer interacts with gut microbiota and is degraded to glycans that are foraged by microbiota. In vivo studies in mice revealed that glycan-consuming microbiota use the glycans of the mucus layer as part of their diet in the absence of enough amounts of dietary fiber. Thus, availability of dietary fiber can modify the mucus layer by changing the mucus consumption through microbiota-accessible carbohydrates (non-digestible by host enzymes) found within dietary fiber. Dietary fiber leading to the production of SCFAs can also help regulate the intestinal mucosal immune barrier.
Inulin is an oligosaccharide or polysaccharide (based on its chain length) that is present in plant sources such as wheat, barley, and garlic. In a study performed in healthy male volunteers [59], participants who took inulin for 8 weeks had significantly lower lactulose/mannitol (L/M) ratio and serum zonulin and higher levels of mucosal GLP-2. However, it is important to note that the methods used were suboptimal, including use of 10g lactulose and 5g mannitol as sugar probes, urine collection from 0-5h (which, as noted in the methodology section, does not define the region of gut assessed), and the use of serum levels that do not necessarily reflect intestinal permeability.
B. Short chain fatty acids (SCFAs)
The SCFAs, acetate, propionate, and butyrate, enter colonocytes via simple diffusion or via facilitated diffusion by solute transporters, SLC16a1 or SLC5a8. SCFAs support host cellular metabolism and gut immune system colonocytes, neutrophils, and T cells, which are components of intestinal barrier function.
Mechanisms:
Based on evidence predominantly from cell lines or preclinical models, SCFAs enhance barrier function through different mechanisms:
Butyrate provides energy for the colonocytes and regulates hypoxia-inducible factor-1 (HIF-1), which modulates the efficiency of epithelial TJ CLDN1 (gene coding for claudin-1 [60,61])
SCFAs modulate the size and function of the T cell network in the gut through G protein-coupled receptor 43 (GPR43)-dependent mechanisms [62] and through histone deacetylase inhibition [63].
Documented effects on intestinal permeability:
While the literature is replete with studies in experimental animals, three examples document the information regarding the relevance of SCFAs to human colonic barrier function. First, decrease in gut-derived plasma SCFAs correlated with increased colonic permeability associated with circadian oscillation as documented in shift workers [64]. Second, in vitro studies of T84 and Caco-2 cells showed that physiological concentrations of SCFAs immediately enhanced barrier function of the colonic epithelium through cholesterol-rich microdomain in the plasma membrane [65]. Third, organoid studies based on human colonic mucosal biopsies showed that fermentation of 2’-O-fucosyllactose (2’FL) which led to an increase of Bifidobacteria, accompanied by an increase of SCFAs, in particular butyrate, resulted in claudin-5 being significantly upregulated, thereby enhancing barrier function [66].
C. Polyphenols, anthocyanins, and ellagitannins
Polyphenols, including flavonoids and stilbenes, represent a ubiquitous group of secondary metabolites in fruits and vegetables and are part of the average human diet. The polyphenol, resveratrol, has been shown to enhance gut barrier function and to reverse the dysbiosis associated with high-fat diet in mice. The improvement in barrier function with resveratrol compared to the untreated high-fat diet mice [67] was demonstrated with both serum lipopolysaccharide and FITC-dextran in in vivo measurements. In addition, heat inactivation treatment of the flavonols [68], galangin and kaempferol, inhibited their benefits to improve barrier function in rat intestinal epithelial (IEC-6) cells. Quercetin inhibited the redistribution of ZO-1 protein induced in the tight junction by indomethacin and prevented the decrease of ZO-1 and occludin expression induced by indomethacin [69]. Similarly, quercetin enhanced intestinal barrier function through the assembly of ZO-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells [70]. However, there are no formal trials of the effects of polyphenols on intestinal permeability in vivo in humans.
Consumption of polyphenol-rich food products can be achieved through diverse foods [71]. Anthocyanin pigments are a subfamily of flavonoids that are responsible for colors in fruits; they play a major role in the antioxidant function of fruits such as berries and grapes. In a mouse model of obesity, treatment with anthocyanin enhanced barrier function [72]. No human studies are available that directly assess intestinal barrier, but beneficial effects of New Zealand black currant anthocyanin-rich extract on oxidative stress associated with exercise (which also impacts intestinal barrier function) have been documented in human studies [73].
Ellagitannins are a diverse class of hydrolysable polymers that are formed when ellagic acid and a hydroxyl group, like glucose, bind together. They are considered a type of antioxidant with free radical scavenging activity, as well as antimicrobial, anti-cancer, anti-nutritional, and cardio-protective properties [74].
In vivo, in rats fed a high-fat diet, pomegranate peel polyphenols reduced chronic low-grade inflammatory responses by modulating gut microbiota, and this was also associated with reduced circulating endotoxin and evidence of reduced inflammation and colonic tissue damage [75]. In the same study, punicalagin and urolithin A (the main microbiota metabolites of pomegranate ellagitannins) increased tight junction protein expression levels which were decreased by LPS, and they reversed the LPS-induced inflammatory response in Caco-2 cells [75]. There is additional evidence [76] that ellagic acid (a metabolite of punicalagin) and urolithin A affect intestinal barrier function in distinct ways. Ellagic acid strengthens the barrier per se, while urolithin A protects against inflammation-induced barrier dysfunction. There are no studies evaluating effects of ellagitannins on intestinal permeability in humans in vivo.
Sources of anthocyanins (ANCs) and ellagitannins (ETs):
Berries, red grapes and red wine are important sources of anthocyanins. The main ANCs present in our diet are: cyanidin-3-glucoside which is the most frequent ANC found in raspberries, blackberries, elderberries, purple corn and black carrots; malvidin-3-glucoside is the major ANC found in red grapes and wines; pelargonidin-3-glucoside found in strawberries; and blueberries contain a mixture of delphinidin, malvidin and cyanidin derivatives. The highest ANC content is found in chokeberries (400 to 1500 mg/100g fresh weight), black currants (100 to 500 mg/100g fresh weight), blackberries (50 to 350 mg/100g fresh weight), blueberries (60 to 300 mg/100g fresh weight), and purple corn (1500 mg/100g fresh weight).
Pomegranates and nuts contain important quantities of ellagitannins (150–500 mg/100 mL pomegranate juice; ~1600 mg/100g walnuts), although they can also be found in berries at lower concentrations (50–350 mg/100g fresh weight raspberries; 25–80 mg/100g fresh weight strawberries). Ellagitannins most commonly ingested by humans are punicalagin, peduncalagin and sanguiin.
D. Glutamine
Glutamine is an L-alpha-amino acid. It is the most abundant free amino acid in human blood. Glutamine is needed for several functions in the body including for the synthesis of proteins as well as an energy source. Glutamine can be synthesized by the body and can also be obtained from the diet. Proposed actions of glutamine in intestinal cells are protection against apoptosis and cellular stresses, anti-inflammation, and maintaining intestinal tissue integrity [77], as well as enhancing tight junction protein expression [78] and correcting for the reduced glutamine synthase associated with increased miR-29a expression in gut mucosa of patients with diarrhea- predominant irritable bowel syndrome (IBS) [79].
Glutamine normalized intestinal hyperpermeability (where hyperpermeability was defined as an elevated urinary L/M ratio >0.07) in patients with IBS [80]. In addition, human studies have shown that glutamine ameliorates mucositis associated with radiation and chemotherapy, an effect that is attributed to its function as a nitrogen donor in intracellular metabolism [81] as well as its function of enhancing recovery of normal tissues. Several other studies have shown that enteral glutamine reduces intestinal permeability or evidence of intestinal mucosal damage in patients with severe burns [82,83] or in patients with Crohn’s disease [84]. The evidence regarding reducing intestinal mucosal damage was measured using plasma diamine oxidase activity, and permeability was measured in all the studies using the lactulose to mannitol ratio. In contrast, in patients with nutritional depletion and increased intestinal permeability, intravenous glutamine did not have a significant effect on the abnormal barrier function [85].
There are multiple foods and supplements that are high in glutamine content including the following foods: meats, fish, eggs, nuts, beans, and milk. Supplements that are high in glutamine include: high-protein milks which contain protein powder, water with protein powder, protein drinks, nasogastric or gastric tube formulas, and preparations in which gluten is added either to nutrient powder or multiple dextran or sucrose and trehalose. These are shown in Table 4 from a recent review [81].
Table 4. Available supplements containing glutamine.
Reproduced with permission from ref. 81, Anderson & Lalla. Nutrients 2020;12:1675
| Nutrients with High Glutamine | Supplement or Product (Source) |
|---|---|
| Dietary: foods high in glutamine | High protein foods (meats, fish, eggs, nuts, beans, milk) |
| High-protein milk + protein powder | FairLife milk + carnation breakfast (Nestle) |
| Water + protein powder | Beneprotein (Nestle), Whey powder, or plant protein powder |
| Protein drinks | Boost, Ensure, Core Power, Pediasure, Peptamen |
| NG- and G-tube formulas | Nutren (Nestle), Vital (Abbott), Jevity (Carewell) |
| Glutamine added to nutrient powder | Juven (Abbott) |
| Glutamine powder + maltodextrin | Glutasolve (Nestle) |
| Glutamine + sucrose + trehalose | Healios (Enlivity) |
G-tube=gastrostomy tube; NG=nasogastric tube
E. Vitamin D
Patients with IBS who had a low vitamin D level at baseline had increased permeability, which improved with long-term low-FODMAP diet [86]. In a clinical trial conducted in patients with Crohn’s disease, 2,000 IU/day of vitamin D or placebo were administered for 3 months, with lactulose and mannitol used as markers of small intestine permeability, sucrose for gastroduodenal permeability, and sucralose for combined small and large bowel permeability. The trial showed that vitamin D may improve gastroduodenal permeability (sucrose excretion) relative to the deterioration observed on placebo; however, there were no significant improvements in small intestinal or colonic permeability with vitamin D [87].
F. Zinc
Zinc carnosine, a health food supplement, stabilizes small bowel integrity and stimulates gut repair processes post-indomethacin treatment. This was demonstrated in a placebo-controlled trial in which intestinal permeability was measured using the lactulose to rhamnose ratio [88]. The mechanism of benefit appears to be related to a proliferative response to zinc carnosine, which was demonstrated in a cell assay using the HT29 cell line.
G. Probiotics
Effects of prebiotics, symbiotics, and probiotics have been extensively reviewed elsewhere [31], and the in vivo human studies [89–92] that have included studies of intestinal barrier function in diseases/models associated with increased permeability are summarized in Table 5. Other effects attributed to prebiotics include decreases in pathogenic bacteria; increases in Bifidobacteria, and Lactobacilli, and short chain fatty acid production; and improvement in host immunity.
Table 5.
Human in vivo studies that have included studies of intestinal barrier function in diseases/models associated with increased permeability
| Human Studies with Probiotics Including Intestinal Permeability/Barrier | ||||
|---|---|---|---|---|
| Probiotic (ref.) | Study Design | # Participants, Treatment Duration | Effect on Permeability | Other Effects |
| Viable vs. sonicated probiotics (89) | Randomized, double-blind, placebo-control trial | 28 critically ill patients; 7 days | No significant difference in intestinal permeability measured by lactulose: mannitol ratio | |
| Bifidobacterium adolescentis IVS-1 vs. B. lactis BB12, + GOS prebiotic, 6 treatment arms (90) | Aspirin challenge; randomized, double-blind, lactose control trial | 94 obese (BMI 30-40) patients; 3 weeks | Bifidobacterium IVS-1 but not B. lactis BB-12 reduced permeability (sucralose:lactulose ratio); GOS prebiotic also effective alone | No synergistic effect between prebiotic and probiotic |
| L. plantarum WCFS1, CIP104448, TIFN101 or placebo (91) | Indomethacin stressor; Double-blind, placebo-control, 4-way crossover trial | 10 healthy; 7-day oral treatments with 4-week washouts between each | Indomethacin increased lactulose:rhamnose ratio; no difference between baseline and on treatment lactulose:rhamnose ratio for any treatment vs. placebo | Integrin pathway and Actinin α4 gene upregulated by L.plantarum TIFN 101; Claudin 5 gene downregulated by L.plantarum WCFS1; Claudin 19 gene downregulated by L.plantarum CIP48 |
| 3-wk kefir supplementation compared with 3-wk milk supplementation (92) | Crossover intervention study | 28 overweight asymptomatic adults | Greater improvement on serum zonulin levels with kefir | Similar improvement in lipid profile and serum glucose with both supplementations. CRP, adiponectin, and appetite unaffected. |
CRP=C-reactive protein; GOS=galacto-oligosaccharides
Dietary Components to Strengthen Intestinal Barrier
Table 6 provides examples of key dietary components associated with potential to have positive effects on overall gut integrity; the table specifies the compound or bacterial strains as well as the common food sources that contain the specified chemical compound. Further studies are required to prove the effects on intestinal permeability and clinical effects in disease.
Table 6.
Key dietary components associated with potential to have positive effects on overall gut integrity
| Component* | Common Food Sources |
|---|---|
| PREBIOTIC FIBER | |
| Beta Glucan | barley, mushroom, oat |
|
Fructans: Fructo-oligosaccharide Inulin Oligofructose |
asparagus, banana, barley, chicory root, garlic, honey, Jerusalem artichoke, leek, nectarine, onion, scallion, rye, wheat |
| Galacto-oligosaccharide | cashew, legume (chickpea, red kidney bean, soybean, split pea), milk, pistachio, squash (butternut, pumpkin) |
| Pectin | apple, banana, broccoli, carrot, dried pea, grapefruit, lemon, orange, potato, tomato |
| Resistant starch | banana, legume (black bean, dried pea, fava bean, lentil, pinto bean, soybean), whole grain (barley, oat) (Cooling of cooked starchy food (e.g. legume, pasta, potato, rice) increases resistant starch content) |
| Xylo-oligosaccharide | bamboo shoots and other vegetables, fruit, honey, milk |
| POLYPHENOL Subclass | |
| Flavonoid: Anthocyanin | black bean, blackberry, black currant, black raspberry, blueberry, cherry, cranberry, eggplant, pecan, purple sweet potato, red cabbage, red grape, red (or blood) orange, red radish, red raspberry |
| Tannin: Ellagitannin | almond, blackberry, blueberry, cranberry, pecan, pomegranate, raspberry, strawberry, walnut |
| PROBIOTIC Bacteria/Yeast | |
|
Bifidobacterium
Escherichia coli Lactobacilli Saccharomyces |
Fermented dairy and nondairy sources: kefir, kimchi, kombucha, miso, sauerkraut, tempeh, yogurt |
| AMINO ACID | |
| Glutamine | • Animal-based source: dairy (cheese, milk, yogurt), egg, meat, poultry, seafood • Plant-based source: almond, cashew, kale, legume (chickpea, kidney bean, lentil, peanut, soybean), mushroom (shiitake), pistachio, seed (pumpkin, sunflower), red cabbage, spinach, tomato, whole grain (oat, quinoa, wheat) (USDA food composition data resources accessed for glutamic acid) L-glutamine used as a food additive and nutritional supplement |
| MINERAL | |
| Zinc | •Animal-based source: dairy (cheese, milk, yogurt), egg, meat (red), poultry (dark), shellfish (crab, lobster, oyster) •Plant-based source: almond, legume (bean, lentil, pea), potato, seed (chia, pumpkin, sunflower), walnut, whole grain (oat, quinoa, wheat) (Phytates lower the bioavailability of zinc from plant-derived foods) Zinc-L-carnosine used as a nutritional supplement |
| MACRONUTRIENT | |
| Fat | Modify a typical Western-style diet high in fat (e.g. saturated fat). A Mediterranean diet eating pattern with focus on healthy unsaturated fats (olive oil, nuts), plant- based choices (fruits, vegetables, whole grains) and lean protein sources (fish, legumes) may favorably impact intestinal gut function. |
Commercially produced components (e.g., inulin, oligosaccharides, pectin, resistant starch, bacteria/yeast strains, L-glutamine, zinc-L-carnosine) are used as food additives/nutritional supplements.
The U.S. Department of Agriculture (USDA) food composition data resource at https://fdc.nal.usda.gov/about-us.html was accessed to obtain information on food sources.
Potential Pharmacological Approaches to Restoring Normal Barrier Function
Several approaches are being developed based on greater mechanistic understanding of the control of the “leak pathway” which typically allows passage of molecules >8Å in diameter.
Given the pivotal role of TNF-α and myosin-light chain kinase (MLCK) as the main regulators of the leak paracellular pathway [93], there is evidence for:
adalimumab and infliximab as regulators of barrier permeability, that contribute to their overall anti-inflammatory effects in inflammatory bowel diseases (IBD). In active IBD, claudin 2 expression and MLCK activity are both increased, suggesting that both pore and leak pathways are involved in disease-associated impairment of barrier function (reviewed in reference #12).
a small molecule, divertin, that prevents MLCK1 recruitment by pro-inflammatory cytokines (including TNF-α, interleukin-1β, and several related molecules) to the peri-junctional actomyosin ring. Such recruitment of MLCK1 leads to molecular reorganization of tight junction structure and composition, including occludin endocytosis. Thus, it has been demonstrated that divertin restores barrier function after TNF-induced barrier loss and prevents disease progression in experimental chronic inflammatory bowel disease [94].
Other drugs and targets may regulate intestinal permeability and are currently undergoing research as potential therapies. These include monoclonal antibodies to IL-13 and interferon γ, and drugs targeting receptors (e.g., α2- adrenergic receptors with dexmedetomidine, glucagon-like peptide 2, glucocorticoids) or ion channels (e.g. chloride channel with lubiprostone) or mast cell stabilizers.
Conclusions
Recent advances provide an opportunity to identify “leaky gut” and more accurately explore the pathophysiological contributions to diverse disease states. These rigorous studies of intestinal barrier function are essential to more clearly understand the role played by abnormal intestinal barrier function in the etiopathogenesis of disease. Equally important, there are also several dietary components that have been associated with damage to the intestinal barrier including fat, emulsifiers, and alcohol. Conversely several chemical entities and novel pharmacological approaches appear to have beneficial effects on the intestinal barrier and, with the establishment of accurate measurements of the barrier, one can anticipate that more rigorous studies will validate the utility of these food components to restore intestinal barrier function.
Key Messages.
Several pathophysiological states have been associated with “leaky gut”.
Advances in measurement of altered intestinal permeability using serum biomarkers or urinary excretion of orally administered sugar probes should facilitate objective measurements of impaired intestinal permeability.
Some dietary factors that damage the intestinal barrier include high fat diet, emulsifiers, and alcohol.
Several dietary components have been reported to restore intestinal barrier function resulting from perturbations, and these components include prebiotics, probiotics, amino acids, minerals, and modifying dietary intake of components that damage the barrier. Pharmacological approaches in development, such as divertin, target MAP kinase, which is an important factor in the leak pathway of permeability
These advances should facilitate claims submitted to regulatory agencies that nutrients and supplements require primary evidence from human studies demonstrating that a dietary component is causally associated with maintaining or restoring normal gut barrier structure.
Acknowledgements:
The authors thank Margaret Gall, R.D.N., L.D., Mayo Clinic Division of Endocrinology, Diabetes and Metabolism, for developing the data for Table 6 based on literature search on the chemical constituents of dietary components; and Cindy Stanislav for secretarial assistance.
Support:
Dr. Camilleri receives funding for studies on intestinal permeability from National Institutes of Health (grant #R01-DK115950) and from the Institute for the Advancement of Food and Nutrition Sciences (IAFNS) (through an ILSI North America Carbohydrate Committee grant). IAFNS is a nonprofit science organization that pools funding from industry and advances science through the in-kind and financial contributions from private and public sector members.
Footnotes
Disclosures: Dr. Camilleri has submitted a patent application on “Methods and materials for assessing intestinal permeability; Publication number: 20190145953” with colleagues at Mayo Clinic. Dr. A. Vella has no conflicts of interest.
REFERENCES
- 1.Camilleri M Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019;68:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Régnier M, Van Hul M, Knauf C, et al. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J Endocrinol 2021;248:R67–R82. [DOI] [PubMed] [Google Scholar]
- 3.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 2010;22:718–33. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, Whitley CS, Haribabu B, et al. Regulation of intestinal barrier function by microbial metabolites Cell Mol Gastroenterol Hepatol 2021;11:1463–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 2005;29:625–51. [DOI] [PubMed] [Google Scholar]
- 7.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelaseyed T, Bergström JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 2014;260:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008;105:15064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atuma C, Strugula V, Allen A, et al. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol 2001;280:G922–9. [DOI] [PubMed] [Google Scholar]
- 11.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 2020;69:2232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 2017;14:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 14.Hollander D, Kaunitz JD. The “Leaky Gut”: tight junctions but loose associations? Dig Dis Sci 2020;65:1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 2010;3:247–59. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Lyle BJ, Madsen KL, et al. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol 2019;317:G17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood Heickman LK, DeBoer MD, Fasano A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes Metab Res Rev 2020;36:e3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luchetti MM, Ciccia F, Avellini C, et al. Gut epithelial impairment, microbial translocation and immune system activation in inflammatory bowel disease-associated spondyloarthritis. Rheumatology 2021;60:92–102. [DOI] [PubMed] [Google Scholar]
- 19.Talley NJ, Holtmann GJ, Jones M, et al. Zonulin in serum as a biomarker fails to identify the IBS, functional dyspepsia and non-coeliac wheat sensitivity. Gut 2020;69:1–3. [DOI] [PubMed] [Google Scholar]
- 20.Barbaro MR, Cremon C, Morselli-Labate AM, et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity Gut 2020;69:1966–74. [DOI] [PubMed] [Google Scholar]
- 21.Fasano A Zonulin measurement conundrum: add confusion to confusion does not lead to clarity. Gut 2020. Nov 11:gutjnl-2020–323367. doi: 10.1136/gutjnl-2020-323367. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Seethaler B, Basrai M, Neyrinck AM, et al. Biomarkers for assessment of intestinal permeability in clinical practice. Am J Physiol – Gastrointest Liver Physiol 2021;321:G11–7. [DOI] [PubMed] [Google Scholar]
- 23.Khoshbin K, Khanna L, Maselli D, et al. Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults. Gastroenterology 2021;161:463–475.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol 2011;301:G919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 2010;22:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grover M, Camilleri M, Hines J, et al. 13C-mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 2016;28:1114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol 2020;319:G589–G608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meddings J, Gibbons I. Discrimination of site‐specific alterations in gastrointestinal permeability in the rat. Gastroenterology 1998;114:83–92. [DOI] [PubMed] [Google Scholar]
- 29.Troisi J, Venutolo G, Terracciano C, et al. The therapeutic use of the zonulin inhibitor AT-1001 (Larazotide) for a variety of acute and chronic inflammatory diseases. Curr Med Chem 2021. Jan 3. doi: 10.2174/0929867328666210104110053. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Leffler DA, Kelly CP, Green PH, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 2015;148:1311–9.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M Human intestinal barrier: effects of stressors, diet, prebiotics, and probiotics. Clin Transl Gastroenterol 2021;12:e00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, et al. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr 2020;11:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netto Candido TL, Bressan J, de Cássia Gonçalves Alfenas R. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr Hosp 2018;35:1432–40. [DOI] [PubMed] [Google Scholar]
- 34.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008;87:1219–23. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–72. [DOI] [PubMed] [Google Scholar]
- 36.Lyte JM, Gabler NK, Hollis JH. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis 2016:15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowser SM, McMillan RP, Boutagy NE, et al. Serum endotoxin, gut permeability and skeletal muscle metabolic adaptations following a short term high fat diet in humans. Metabolism 2020;103:154041. [DOI] [PubMed] [Google Scholar]
- 38.Glade MJ, Meguid MM. Nutrition and food: A glance at … dietary emulsifiers, the human intestinal mucus and microbiome, and dietary fiber. Nutrition 2016;32:609–14. [DOI] [PubMed] [Google Scholar]
- 39.Bancil AS, Sandall AM, Rossi M, et al. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: mechanistic insights in inflammatory bowel disease. J Crohn’s Colitis 2021;15:1068–79. [DOI] [PubMed] [Google Scholar]
- 40.Lock JY, Carlson TL, Wang CM, et al. Acute exposure to commonly ingested emulsifiers alters intestinal mucus structure and transport properties. Sci Rep 2018;8:10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017;66:1414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya S, Shumard T, Xie H, et al. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr Healthy Aging 2017;4:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandall AM, Cox SR, Lindsay JO, et al. Emulsifiers impact colonic length in mice and emulsifier restriction is feasible in people with Crohn’s disease. Nutrients 2020;12:2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis 2014;20:1353–60. [DOI] [PubMed] [Google Scholar]
- 45.Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019;157:440–50.e8. [DOI] [PubMed] [Google Scholar]
- 46.Svolos V, Hansen R, Nichols B, et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 2019;156:1354–67.e6. [DOI] [PubMed] [Google Scholar]
- 47.Stӓrkel P, Leclercq S, de Timary P, et al. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci (Lond) 2018;132:199–212. [DOI] [PubMed] [Google Scholar]
- 48.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011;53:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottfried EB, Korsten MA, Lieber CS. Alcohol-induced gastric and duodenal lesions in man. Am J Gastroenterol 1978;70:587–92. [PubMed] [Google Scholar]
- 50.Brozinsky S, Fani K, Grosberg SJ, et al. Alcohol ingestion induced changes in the human rectal mucosa: light and electron microscopic studies. Dis Colon Rectum 1978;21:329–35. [DOI] [PubMed] [Google Scholar]
- 51.Elamin E, Masclee A, Troost F, et al. Ethanol impairs intestinal barrier function in humans through mitogen activated protein kinase signaling: a combined in vivo and in vitro approach. PLoS One 2014;9:e107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Tong J, Chang B, et al. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep 2014;9:2352–6. [DOI] [PubMed] [Google Scholar]
- 53.Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res 2008;32:355–64. [DOI] [PubMed] [Google Scholar]
- 54.Lang S, Duan Y, Liu J, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology 2020;71:522–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parlesak A, Schӓfer C, Schutz T, et al. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 2000;32:742–7. [DOI] [PubMed] [Google Scholar]
- 56.Keshavarzian A, Holmes EW, Patel M, et al. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 1999;94:200–7. [DOI] [PubMed] [Google Scholar]
- 57.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1984;1:179–82. [DOI] [PubMed] [Google Scholar]
- 58.Swanson GR, Siskin J, Gorenz A, et al. Disrupted diurnal oscillation of gut-derived Short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Transl Res 2020;221:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo F, Linsalata M, Clemente C, et al. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr Res 2012;32:940–6. [DOI] [PubMed] [Google Scholar]
- 60.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015;17:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saeedi BJ, Kao DJ, Kitzenberg DA, et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell 2015;26:2252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MH, Kang SG, Park JH,et al. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013;145:396–406.e1. [DOI] [PubMed] [Google Scholar]
- 63.Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015;8:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swanson GR, Siskin J, Gorenz A, et al. Disrupted diurnal oscillation of gut-derived short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Transl Res 2020;221:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 2008;100:297–305. [DOI] [PubMed] [Google Scholar]
- 66.Šuligoj T, Vigsnæs LK, Van den Abbeele P, et al. Effects of human milk oligosaccharides on the adult gut microbiota and barrier function. Nutrients 2020;12:2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang P, Wang J, Li D, et al. Targeting the gut microbiota with resveratrol: a demonstration of novel evidence for the management of hepatic steatosis. J Nutr Biochem 2020;81:108363. [DOI] [PubMed] [Google Scholar]
- 68.Fan J, Zhao X-H, Li T-J. Heat treatment of galangin and kaempferol inhibits their benefits to improve barrier function in rat intestinal epithelial cells. J Nutr Biochem 2021;87:108517. [DOI] [PubMed] [Google Scholar]
- 69.Carrasco-Pozo C, Morales P, Gotteland M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression. J Agric Food Chem 2013;61:5291–7. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr 2009;139:965–74. [DOI] [PubMed] [Google Scholar]
- 71.Guglielmetti S, Bernardi S, Del Bo C, et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: study protocol of the MaPLE randomised controlled trial. BMC Geriatr 2020;20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cremonini E, Daveri E, Mastaloudis A, et al. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol 2019;26:101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyall KA, Hurst SM, Cooney J, et al. Short-term blackcurrant extract consumption modulates exercise induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am J Physiol Regul Integr Comp Physiol 2009;297:R70–81. [DOI] [PubMed] [Google Scholar]
- 74.Smeriglio A, Barreca D, Bellocco E, et al. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol 2017;174:1244–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao R, Long X, Yang J, et al. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct 2019;10:8273–85. [DOI] [PubMed] [Google Scholar]
- 76.Hering NA, Luettig J, Jebautzke B, et al. The punicalagin metabolites ellagic acid and urolithin A exert different strengthening and anti-inflammatory effects on tight junction-mediated intestinal barrier function in vitro. Front Pharmacol 2021;12:610164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim MH, Kim H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int J Mol Sci 2017;18:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Zhang C, Wu G, et al. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J Nutr 2015;145:25–31. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Q, Souba WW, Croce CM, et al. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010;59:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou QQ, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019;68:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson PM, Lalla RV. Glutamine for amelioration of radiation and chemotherapy- associated mucositis during cancer therapy. Nutrients 2020;12:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng X, Yan H, You Z, et al. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 2004;30:135–9. [DOI] [PubMed] [Google Scholar]
- 83.Zhou YP, Jiang ZM, Sun YH, et al. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. J Parenter Enteral Nutr 2003;27:241–5. [DOI] [PubMed] [Google Scholar]
- 84.Benjamin J, Makharia G, Ahuja V, et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig Dis Sci 2012;57:1000–12. [DOI] [PubMed] [Google Scholar]
- 85.Hulsewé KW, van Acker BA, Hameeteman W, et al. Does glutamine-enriched parenteral nutrition really affect intestinal morphology and gut permeability? Clin Nutr 2004;23:1217–25. [DOI] [PubMed] [Google Scholar]
- 86.Linsalata M, Riezzo G, Orlando A, et al. The relationship between low serum vitamin D levels and altered intestinal barrier function in patients with IBS diarrhoea undergoing a long-term low-FODMAP diet: novel observations from a clinical trial. Nutrients 2021;13:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomized double-blind placebo-controlled study. United European Gastroenterol J 2015;3:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahmood A, FitzGerald AJ, Marchbank T, et al. Zinc carnosine, a health food supplement that stabilizes small bowel integrity and stimulates gut repair processes. Gut 2007;56:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alberda C, Gramlich L, Meddings J, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2007;85:816–23. [DOI] [PubMed] [Google Scholar]
- 90.Krumbeck JA, Rasmussen HE, Hutkins RW, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018;6:121, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mujagic Z, de Vos P, Boekschoten MV, et al. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep 2017;7:40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jenko Pražnikar Z, Kenig S, Vardjan T, et al. Effects of kefir or milk supplementation on zonulin in overweight subjects. J Dairy Sci 2020;103:3961–70. [DOI] [PubMed] [Google Scholar]
- 93.Zuo L, Kuo W-T, Turner JR. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell Mol Gastroenterol Hepatol 2020;10:327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He W-Q, Wang J, Sheng J-Y, et al. Contributions of myosin light chain kinase to regulation of epithelial paracellular permeability and mucosal homeostasis. Int J Mol Sci 2020;21:993. [DOI] [PMC free article] [PubMed] [Google Scholar]


