Abstract
Bacterial resistance is a naturally occurring process. However, bacterial antibiotic resistance has emerged as a major public health problem in recent years. The accumulation of antibiotics in the environment, including in wastewaters and drinking water, has contributed to the development of antibiotic resistant bacteria and the dissemination of antibiotic resistance genes (ARGs). Such can be justified by the growing consumption of antibiotics and their inadequate elimination. The conventional water treatments are ineffective in promoting the complete elimination of antibiotics and bacteria, mainly in removing ARGs. Therefore, ARGs can be horizontally transferred to other microorganisms within the aquatic environment, thus promoting the dissemination of antibiotic resistance. In this review, we discuss the efficiency of conventional water treatment processes in removing agents that can spread/stimulate the development of antibiotic resistance and the promising strategies for water remediation, mainly those based on nanotechnology and microalgae. Despite the potential of some of these approaches, the elimination of ARGs remains a challenge that requires further research. Moreover, the development of new processes must avoid the release of new contaminants for the environment, such as the chemicals resulting from nanomaterials synthesis, and consider the utilization of green and eco-friendly alternatives such as biogenic nanomaterials and microalgae-based technologies.
Keywords: antibiotic resistance, drinking water, water treatment, antibiotic resistance genes, nanotechnology, microalgae
1. Introduction
Bacterial antibiotic resistance is a major public health problem. Worldwide, about 700,000 deaths occur annually due to infections caused by resistant bacteria. In 2050, it is expected that this number will increase, reaching 10 million cases per year if no preventive measures are universally adopted [1]. In parallel, antibiotic resistance genes (ARG) and antibiotic resistant bacteria (ARB) can have an environmental distribution, which is why they have been recognized as emergent environmental pollutants [2].
The dissemination of ARB and ARG in the environment results from the inappropriate use of antibiotics in human and veterinary clinics, the incorrect elimination of expired antibiotics, the increase in discharges of pharmaceutical industrial wastewaters, and the reduced efficiency of wastewater and drinking water treatment plants [3,4]. Indeed, reducing antibiotics use per se is insufficient to control the environmental dissemination of ARBs and ARGs in drinking water [5,6,7].
The efficiency of water treatment processes plays a critical role in disseminating antibiotic resistance throughout the water distribution systems [2]. The selection of the processes used in drinking water treatment plants is based on the physicochemical characteristics of the water. Coagulation/flocculation, sedimentation, filtration, and disinfection are the most common processes used worldwide [8,9]. However, monitoring ARB and ARG prevalence after the treatments is usually neglected [7]. Moreover, recent technologies developed for water remediation, such as advanced oxidation, biological and granular activated carbon filtration, and membrane filtration, also fail to remove ARGs or even contribute to their dissemination along the water distribution systems [10,11].
Therefore, developing accessible and efficient water remediation processes to remove antibiotics, ARB, and especially ARGs remains a challenge.
Among the strategies developed in recent years, nanotechnology and microalgae-based technologies have shown great potential to address some limitations of the current water treatment processes. In addition, future studies should focus on the potential use of these technologies to control the dissemination of ARB and ARG in drinking water distribution systems. This literature review aims to discuss the limitations of conventional and advanced water treatment processes in addressing the dissemination of antibiotic resistance. On the other hand, we summarized nanotechnology and microalgae-based technologies recently reported to address antibiotic resistance in wastewaters and drinking water.
2. Antibiotic Resistance
Antibiotics are indispensable for treating infectious diseases in humans and animals, and their use in the last century allowed to improve healthcare services and increase life expectancy [12]. However, the inappropriate consumption and the incorrect disposal of antibiotics favored its accumulation in the environment, including in raw and treated drinking water [9]. The antibiotic’s presence in the environment produces a selective pressure leading to ARB and ARG dissemination. Bacterial infections are harder to treat, as commonly used antibiotics are less effective or even ineffective, which might cause uncurable infections and will undoubtedly impact medical costs and mortality numbers worldwide [13]. For this reason, antibiotic resistance has become, in recent decades, a major health concern. Several bacterial species can cause infection in humans. However, Escherichia coli and multidrug-resistant ESKAPE bacteria (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens [14,15] were, in 2017, included in the World Health Organization report, classified as critical for public health, and listed as high priority ARB [16].
2.1. Bacteria Mechanisms to Antibiotic Resistance
Bacterial species can display antibiotic resistance by intrinsic or acquired mechanisms that prevent antibiotics access to their bacterial targets or result in antibiotic inactivation [17,18,19].
2.1.1. Intrinsic Antibiotic Resistance
Intrinsic resistance is related to inherent structural or functional properties shared within a bacterial species independently of previous antibiotic exposure [19]. For example, antibiotics must cross the bacterial cell wall to reach their intracellular target. Gram-negative bacteria are intrinsically less permeable than Gram-positive due to their outer membrane, which acts as a permeability barrier [17]. Vancomycin antibiotic inhibits peptidoglycan crosslinking, and is effective in Gram-positive bacteria, but not in Gram-negative bacteria as it cannot cross the outer membrane. Despite this, antibiotics can enter the cells in Gram-negative bacteria by diffusion via porin proteins located in the outer membrane. Bacteria can express different drug efflux pumps such as ATP (adenosine triphosphate)-Binding Cassette (ABC) superfamily, Resistance Nodulation Division family, Multidrug and Toxin Extrusion superfamily, Major Facilitator Superfamily, and the Small Multidrug Resistance family, which can remove antibiotics from the cell cytoplasm to the extracellular environment [19]. For example, S. aureus expresses the efflux pump NorA that is responsible for fluoroquinolones efflux [20].
2.1.2. Acquired Antibiotic Resistance
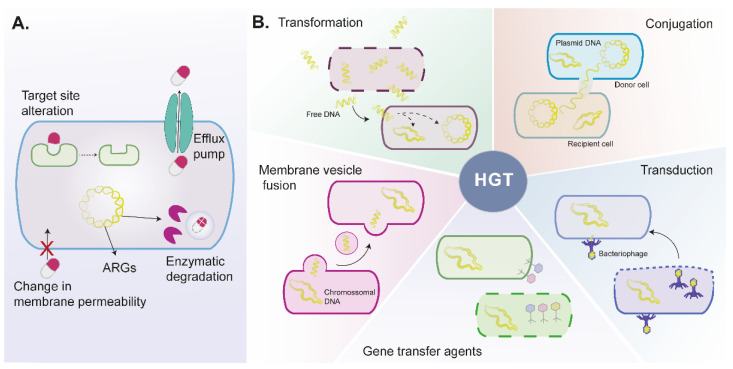
In addition to intrinsic resistance mechanisms, bacteria can also acquire antibiotic resistance mechanisms, including decreased cell permeability, increased expression of efflux pumps, modification of antibiotic targets (by genetic mutation or post-translational modification of the target), and antibiotic enzymatic inhibition or degradation (Figure 1A) [17,19,21]. To limit antibiotics’ access to cellular targets, bacteria might reduce cell permeability by altering porin proteins expression and/or function [19]. Overexpression of efflux pumps contributes to increased antibiotic extrusion, leading to low intracellular accumulation. The norA gene is found overexpressed in several strains of S. aureus, especially in methicillin-resistant strains (MRSA), and is associated with acquired resistance to fluoroquinolones such as ciprofloxacin [22,23]. Additionally, mutations codifying for changes in the antibiotic target are an important resistance mechanism against fluoroquinolones antibiotics which target the GyrA and ParC/GrlA proteins in Gram-negative and Gram-positive bacteria, respectively [24]. Bacteria can also present target changes, such as those observed in S. aureus MRSA strains, which result from acquiring the mecA gene and confers resistance against β-lactams [25]. The β-lactams action is based on its ability to inhibit penicillin binding-proteins at the bacteria cell wall. However, the cell wall of MRSA strains presents PBP2a, which is encoded by the gene mecA and is a penicillin-binding protein with low affinity to β-lactams.
Figure 1.
Schematic representation of antibiotic resistance mechanisms in bacteria. (A). Bacteria might present or acquire mechanisms to disable antibiotics, including alterations on target site, decreased permeability that impairs antibiotics cellular uptake, expression of drug efflux pumps that remove antibiotics from cells, and production of enzymes that modify or degrade antibiotics. (B). Horizontal gene transfer allows the exchange of genetic information between the same or different species by transformation, conjugation, membrane vesicle fusion, transduction, and gene transfer agents mechanisms. ARGs—antibiotic resistance genes, HGT—horizontal gene transfer.
The mechanisms mentioned can be acquired through spontaneous mutation and, more frequently, by transference of genetic material from a foreign source or horizontal gene transfer [19].
Horizontal Gene Transfer
Horizontal gene transfer of ARGs is probably the major factor contributing to the occurrence of new bacterial resistant strains [26]. The horizontal gene transfer mechanisms involve transformation, conjugation, transduction, membrane vesicle fusion, and gene transfer agents (Figure 1B) [19,27].
Transformation traduces the uptake of free DNA from a foreign competent bacteria that can be incorporated into the recipient bacteria after the death and lysis of a bacteria. In conjugation, cell–cell contact between bacteria allows the exchange of plasmids from a donor to a recipient cell. Plasmidic DNA replicates independently from the DNA chromosomic and can carry other mobile genetic elements (e.g., transposons and integrons) and several ARGs [19]. Transduction is a process mediated by bacteriophages. Bacteriophages infect bacteria, and after bacteria lysis, they can incorporate DNA fragments that will be transferred to another bacteria genome [27,28]. Bacterial membrane vesicles carry lipids, proteins, and DNA that can be released to the external environment under stressful conditions, fuse, and transform other bacteria [29]. Recently, Lee and colleagues showed that membrane vesicle fusion could transfer β-lactam resistance substances from S. aureus (MRSA strain) to E. coli, resulting in increased β-lactamase activity and conferring resistance to β-lactam antibiotics [30].
Gene transfer agents are phage-like particles that carry random DNA fragments, lacking DNA encoding machinery and self-propagating ability [31]. Gene transfer agents are produced by bacteria and released after bacteria lysis. These agents present some advantages over natural transformation and conjugation, as they can protect DNA from environmental factors and are not limited by cell-to-cell contact.
2.1.3. Biofilm Antimicrobial Resistance
Biofilm antimicrobial resistance comprises both innate and acquired mechanisms [32,33]. Biofilms are aggregates of microorganisms surrounded by a self-produced matrix of extracellular polymeric substances, attached to natural or artificial surfaces resulting from the adaptation to environmental stressors, such as antibiotics, and promote bacteria growth and survival [27,33].
Innate mechanisms of biofilms include the presence of a barrier established by the matrix of extracellular polymeric substances that limits antibiotic diffusion through the biofilm [32,33]. Additionally, hypoxic zones in deeper parts of the biofilms slow bacteria growth and enable their tolerance against antibiotics that target metabolic processes. In accordance, the efficacy of some antibiotics such as cephalothin and vancomycin is reduced in older biofilms [34]. Besides structural organization and stability conferred by the extracellular polymeric matrix, biofilms display resource capture by sorption, digestive capability, intercellular communication, and enhanced metabolic activity that promotes bacteria survival [33].
The high cell density, heterogenous bacteria population, and accumulation of mobile genetic elements enhance the horizontal gene transfer processes (transformation, conjugation, transduction, vesicle fusion, and gene transfer agents), enabling the transfer and acquisition of ARGs between the different bacteria present in the biofilm [27,33]. Moreover, the matrix of biofilms confers higher physical stability, facilitating and improving plasmid conjugation that requires cell-to-cell contact than free-living bacteria [35,36]. Different microorganisms can be associated within a biofilm allowing horizontal gene transfer and metabolic interactions, thus promoting biofilm dispersion and higher ARB dissemination in the environment [27]. Biofilms are commonly found in water distribution systems, representing a high risk for human health, and should be considered as one of the most relevant factors to be controlled in water treatment plants and water distribution systems [37].
3. Critical Factors for Antibiotic Resistance Widespread in Environment and Water Sources
In recent years, emergent evidence reinforces antibiotic resistance as a major human health problem with antibiotics, ARB, and ARG being systematically detected in diverse environmental matrices such as air, soil, groundwater, surface water, wastewater, and drinking water [2]. The main factors identified to contribute to this environmental widespread are (i) human and veterinarian inappropriate antibiotic consumption; (ii) farm and aquaculture activities; (iii) health care and pharmaceutical facilities discharge; and (iv) inefficient removal of antibiotics at water treatment plants [2,38,39,40].
3.1. Human and Veterinary Consumption
In recent decades, antibiotic consumption has risen due to human population increase, improved life quality, easier access to medicines, and overall improved health care services. Human population increase drove higher demand for animal protein and the intensification of food production [41,42].
The antibiotic consumption between 2000 and 2015 was analyzed in 76 countries, including high- and low-income countries, by Klein and colleagues [38]. Overall antibiotic consumption rate grew by 39%, with low- and middle-income countries showing the greatest increase, although antibiotic use was found to be higher in high-income countries. More recently, an analysis of antibiotic consumption by EE/EEA (European economic area) countries between 1997 and 2017 showed that β-lactams antibiotics (penicillin) remained the most consumed over the years [43]. Furthermore, the overall antibiotic consumption remained unaltered. Despite sulfonamides and trimethoprim consumption decreasing in most countries, the consumption of other antibiotics increased. The same conclusions were described in the latest report from European Centre for Disease Prevention and Control, which analyzed antibiotic consumption from 2010 and 2019 and did not notice significant differences over time [44]. These studies indicate that, despite European countries’ measures to increase awareness regarding antibiotic use and prevent antibiotic resistance dissemination, additional efforts are still required to reduce global human antibiotic consumption.
On the other hand, antibiotics are widely used in veterinary activities, farms, and aquaculture [13,40,45,46,47]. In aquaculture, antibiotics are added directly to water as a preventive measure (prophylaxis). However, aquaculture systems are rich in diverse bacteria, which favor horizontal gene transfer and, thus, the dissemination of ARG and ARB in the aquatic environment [39,48].
The antibiotics and metabolites are excreted by humans and animals through urine and feces, reaching sewage systems, as unchanged or as conjugations of glucuronic and sulfuric acid [46,49], thus contributing to water contamination. Therefore, it is necessary to be aware of the correct use of antibiotics to restrain antibiotic resistance widespread. It is expected that reducing antibiotics consumption would decrease the levels of antibiotics found in wastewater and drinking water.
3.2. Health Care Facilities and the Pharmaceutical Industry
Health care facilities are known to be a hotspot of ARB, and usually, their wastewaters are discharged without appropriate pretreatment into the sewage system [50]. Hospital wastewaters display a higher ecotoxicity risk due to the high levels of pharmaceuticals such as antibiotics, ARB, and ARGs [51,52,53]. Even though the wastewater treatment plants can remove most ARB, ARG elimination is more challenging. Consequently, high levels of ARGs and mobile genetic elements have been detected in the effluents of hospital wastewater treatment plants [51,53,54,55]. Additionally, most of the ARG detected in effluents are associated with resistance to antibiotics clinically relevant, such as β-lactams, sulfonamides, macrolides, and tetracyclines [52,54].
Similarly, effluents of the pharmaceutical industry or pharmaceutical wastewater treatment plants showed high antibiotic levels [12]. This provides high selection pressure that favors the proliferation of ARG and mobile genetic elements [56,57,58], which is responsible for their persistence in the downstream effluents [59]. So, the total elimination of antibiotics is necessary to control ARG abundance in effluents [58]. Therefore, it is urgent to establish special regulatory measures to treat industrial wastewaters to limit antibiotic resistance proliferation in the aquatic environment.
3.3. Biofilm Formation in Drinking Water Distribution Systems
Drinking water distribution systems comprise a network of extensive pipelines that deliver potable water from drinking water treatment plants to consumers. In addition, water reservoirs that allow long term water storage are important drinking water resources for the human population. However, these structures commonly harbor a great biofilm area, detaching and releasing ARBs and ARGs, causing drinking water contamination [9,10,60,61,62,63,64]. Therefore, the development of biofilms in drinking water distribution systems and inefficient water treatments are a huge risk for human health [37,63]. In accordance, previous epidemics outbreaks have been associated with contaminated drinking water [37].
To date, several reports have elucidated the risk of biofilm formation in drinking water distribution systems concerning antibiotic resistance [10,60,61,63,64,65,66]. High-throughput quantitative PCR allowed the detection of 285 ARGs and other mobile genetic elements in water samples from two drinking water treatment plants [10]. This work showed that biological activated carbon water treatment leads to ARGs increase. Additionally, the chlorination at the final water treatment step enhanced the relative abundance of ARGs. ARGs were analyzed across water distribution systems and in tap water. In tap water, ARGs’ absolute abundance, especially β-lactam resistance genes, was found to increase 6.4- to 109.2-fold compared to finished water, showing that pipeline transportation contributes to antibiotic resistance dissemination [10].
Furthermore, the detection and enrichment of mobile genetic elements, such as transposases and intI-1 genes, also suggest that they play a critical role in antibiotic resistance dissemination in drinking water. In accordance, Chan and colleagues found that 58% of the bacteria detected in the distributed water was released from the pipe biofilm [61]. More recently, sediment samples from 10 water reservoirs showed the presence of 174 ARGs, being the most prevalent the multidrug-, sulfonamide-, and vancomycin-ARGs [60]. The mobile genetic elements were identified as the main biotic factors contributing to ARGs dissemination in the analyzed sediments.
Overall, biofilm formation in drinking water distribution systems aggravates antibiotic resistance dissemination in drinking water, affecting its quality and safety. Therefore, it is necessary to develop new water remediation systems to efficiently remove ARGs at the drinking water treatment plants, inhibit biofilm formation, and eliminate the ARGs and ARBs in pipes and water reservoirs.
3.4. Inefficient Antibiotic, ARB, and ARB Removal by Water Treatment Processes
Worldwide, since the early 20th century, a combination of coagulation, sedimentation, and filtration has been applied in water treatment. Conventional water treatment plants use a combination of coagulation, flocculation, sedimentation, filtration, and disinfection units, to provide clean and safe drinking water to the public. However, these technologies present several drawbacks. Most wastewater and drinking water treatment processes require the use of hazardous chemicals, achieve low removal efficiency of contaminants, formation of by-products, and have high costs, among others [67]. While conventional water treatments effectively eliminate bacteria, the removal of ARG by processes such as coagulation, sedimentation/clarification, sand filtration, and biological activated carbon filtration might not be achieved. Indeed, these processes produced contradictory results concerning the ARG removal efficiency of different water treatment processes (Table 1).
Table 1.
ARGs removal efficiency by current water treatments.
| Antibiotic Class |
ARG | Water Treatment Process | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO + SE + SF + CL | SF | CL | OZ + CL | OZ | UV | BAC | GAC | MF | Ref | ||
| Aminoglycosides | aadA | ↓ | ↓ | ↓ | ↑ | [68] | |||||
| aadB | ND/↓ | ↑ | [69] | ||||||||
| aadE | ↓ | ↑ | |||||||||
| aphA1 | ND/↑/↓ | ||||||||||
| strA | ↑/↓ | [69,70] | |||||||||
| strB | ↑/↓ | ||||||||||
| B-lactams | blaOXA-1 | ↓ | ↑ | [70] | |||||||
| blaCTX-M | ↑ | [71] | |||||||||
| blaTEM-1 | ↓ | ↓ | ↓ | ↑ | [70,71,72] | ||||||
| Chloramphenicol | cmlA | ↓ | ↑ | ↑ | [73] | ||||||
| dfrA1 | ↑ | [71] | |||||||||
| dfrA12 | ↑ | ||||||||||
| Efflux pump | opxB | ↓ | ↓ | ↑ | [73] | ||||||
| mexF | ↑ | ↑ | ↑ | ↑ | [68] | ||||||
| mexT | ↑ | ↑ | ↑ | ↑ | |||||||
| mexW | ↑ | ↑ | ↑ | ↑ | |||||||
| Florfenicol | floR | ↓ | ↓ | ↑ | [73] | ||||||
| Lincosamides | cfr | ↓ | ↑ | ↑ | |||||||
| Macrolides | ereA | ND | [69] | ||||||||
| ermB | ND/↓ ↑ |
↓ | ↓ | ↑ | [69] [70] |
||||||
| ermC | ↑ | [70] | |||||||||
| ermF | ND | ↓ | [69,74,75] | ||||||||
| ermG | ND | ||||||||||
| ermX | ND | ||||||||||
| mefA | ND | ||||||||||
| mph(A) | ↓ | ↓ | [72] | ||||||||
| Polypeptides | bacA | ↓ | ↓ | ↓ | ↑ | [68] | |||||
| Quinolones | qepA | ↓ | ND | ↑ | [70,73] | ||||||
| qnrA | ↓ | ND | ↑ | ||||||||
| qnrB | ↑ | ND | ↑ | ↑ | |||||||
| qnrD | ND | ↑ | [73] | ||||||||
| qnrS | ND | ↑ | ↑ | ||||||||
| Sulfonamides | sul1 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑/↓ | ↑ | ↓/↑ | [68,70,71,72,73,74,75,76,77,78] | |
| sul2 | ↓ | ↓ | ↓ | ↑ | ↓/↑ | [69,70,71,73,74,75,76,78] | |||||
| sul3 | ↓ | [70] | |||||||||
| Tetracyclines | tetA | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | [69,70,71,72,73,78] | |
| tetB | ↑ | ↓ | [70,74] | ||||||||
| tetC | ↓ | ||||||||||
| tetM | ↑ | ↓ | ↑ | ↑ | ↓/↑ | [70,73,75,78] | |||||
| tetG | ND | ↑ | ↓ | [69,71,74] | |||||||
| tetL | ↓ | [79] | |||||||||
| tetO | ND/↓ | ↓ | ↓ | ↑ | ↑ | [69,73,78] | |||||
| tetQ | ND | ↓ | ↓ | ↑ | ↓ | [69,71,73,75] | |||||
| tetS | ↓ | ↑ | [73] | ||||||||
| tetW | ND/↑ | ↓ | ↑ | ↑ | ↓/↑ | [69,70,71,73,75,78] | |||||
| tetX | ND/↓ | ↓ | ↓ | ↑ | ↓/↑ | [74,78] | |||||
BAC—biological activated carbon; CL—chlorination; CO—coagulation; GAC—granular activated carbon; ND—not detected; MF—membrane filtration; OZ—ozonation; SE—sedimentation; SF—sand filtration; UV—ultraviolet; ↑—increased expression; ↓—decreased expression.
Some studies demonstrated that the conventional treatments including sand filtration and chlorination can achieve good performance in the removal of some ARGs, especially of sulfonamides resistance genes sul1, sul2, and sul3 [68,70,72,73,76], aminoglycosides ARGs aadA, aadB, and aadE [68,69], β-lactams ARGs blaOXA-1 and blaTEM [70], macrolides ARGs ereA, ermF, ermG, ermX, mefA, and mphA [69,72], quinolones ARGs qepA, qnrA, qnrD, and qnrS [70,73], and tetracyclines ARGs tetA, tetC, tetG, tetO, tetQ, tetS, and tetX [69,70,72,73] (Table 1). However, the removal efficiency of other ARGs such as aphA1, strA, strB, ermB, qnrB, and tetM showed less consistent results, with reports showing both decreases and increases in their expression [69,70,73]. Moreover, conventional water treatments were ineffective in the removal of efflux pump resistance genes mexF, mexT, and mexW, macrolide ARG ermC, and tetracycline ARG tetB [68,70].
Besides chlorination, ozonation and ultraviolet (UV) radiation are two other common disinfection technologies applied in wastewater and drinking water treatment plants and are dependent on the dose used and other physicochemical factors [80]. A process involving low-pressure UV was recently recommended as a supplementary bactericidal treatment to remove ARG in terminal water treatment [77]. The combination of UV treatment with conventional processes used in the water treatment system resulted in a higher reduction in ARB [81] and gene inactivation, and higher removal rates of blaTEM1, mphA, and tetA [72,79]. However, UV radiation increased the abundance of aadA, mexF, mexT, mexW, bacA, and sul1 resistance genes [68]. A previous study showed that UV LED was able to eliminate ARB and ARG (tetL), but resistance has reemerged naturally after disinfection [79]. Therefore, the UV/UV LED radiation process for water treatment to ARB and ARG removal is a promising strategy but requires further optimization.
In recent years, different technologies have emerged for the management of drinking water systems, such as biological activated carbon and membrane filtration technology [2,82].
Several reports have shown that biological activated carbon increases the abundance of ARGs (Table 1) [10,11,69,83,84]. This has been associated with the adhesion of bacteria to the filter surface, formation of biofilms, and consequent horizontal gene transfer [83,85]. Similar results were obtained for granular activated carbon filters [71,73]. The filtration by powder activated carbon seems to be more competent in removing the ARG [73].
Membrane filtration is another advanced water treatment process that includes ultrafiltration, nanofiltration, osmosis reverse, and forward osmosis [2,82]. The membranes act as physical barriers to limit the passage of pathogens and other water contaminants during the water treatment.
Reverse osmosis filtration proved to reduce the absolute abundance of sulfonamide (sul1 and sul2), tetracycline (tetB, tetG, and tetX), macrolides (ermF), and quinolones (qnrA, qnrB, and qnrS) resistance genes [74]. Liang and colleagues [75] showed a reduction of about 99% of the total ARGs by membrane filtration that included a step of ultrafiltration and a two-stage reverse osmosis process. Overall, 16 types of ARGs were analyzed, including tetQ, tetM, tetW, sul1, sul2, ermF, and cfrA. However, despite the significant reduction in their absolute abundance, ARGs remained at detectable levels in final water, as well as 16S rRNA that only showed a slight decrease. These results are in accordance with previous data showing that membrane technology enhances the removal of bacteria and of some ARGs in comparison to conventional water treatment processes [86]. However, ARGs are not totally removed from water samples by membrane filtration, and performance varies across different studies (Table 1) [74,75,78,86]. Le and colleagues showed that microfiltration was able to completely remove ARB and reduce some ARGs, but blaKPC, blaNDM, blaSHV, ermB, intI1, sul1, and tetO persisted after the treatment [86]. Another study noticed an increase in sulfonamides (sul1 and sul2) and tetracyclines-resistance genes (tetA, tetB, tetM, tetO, and tetX) after ultrafiltration [78].
In addition, membrane technology presents a major drawback: the risk of biofouling [82,87,88]. Microorganisms can adhere to the surface of the membranes, forming biofilms, thus supporting ARB proliferation. Diverse bacterial species, including Klebsiella sp., Staphylococcus sp., and E. coli, have been found in the biofouling of filtration membranes [87]. Additionally, chlorination pretreatment, used to prevent membrane fouling, increases the risk of ARGs in the reverse osmosis process [88]. Other approaches to reduce the risk of fouling involve membrane surface modification or membrane cleaning, which increases the associated costs.
Moreover, parameters such as molecular weight cut off and surface charge of membranes must be optimized to improve ARGs removal. A recent study demonstrated that membranes with a cut off smaller than 5000 Da and positively charged, contrarily to negatively charged DNA, enhance free DNA retention and adsorption [89].
Overall, membrane technology for water treatment and ARGs removal presented promising results but is still insufficient. Additionally, ARGs can be retained at the membranes during ultrafiltration, nanofiltration, or osmosis reverse processes but are not biodegraded. Thus, ARGs entrapped in the membranes can be released to the water in case of membranes breach or damage.
Therefore, the inefficient performance of conventional and advanced water treatment processes justifies the need for more efficient and affordable remediation systems that remove emergent pollutants, including antibiotics, ARB, and ARG, from the environment and water sources.
4. Promising Strategies to Reduce Antibiotic Resistance
The mitigation of antibiotic resistance dissemination in the environment should include strategies targeting the correct elimination of (i) antibiotics in the wastewater and drinking water treatment plants, (ii) ARBs in wastewater and drinking water treatment plants and biofilm development in drinking water distribution systems, and (iii) ARGs in both drinking water treatment plants and distribution systems. In recent years, several studies have reported new strategies for water remediation, including adsorption and degradation of pollutants based on biomaterials, nanomaterials, and microalgae. The following sections will discuss the removal efficiency of contaminants such as antibiotics, ARBs, and ARGs by these systems and the mechanisms involved.
4.1. New Approaches for Antibiotics Elimination
Despite the current processes used in wastewater and drinking water treatment plants to reduce antibiotic levels, antibiotics are still detected in drinking water [90,91]. Importantly, antibiotic accumulation imposes a high selective pressure in the environment, facilitating bacterial acquisition of resistance mechanisms [92]. A recent study showed that the presence of sulfadiazine and ciprofloxacin induced the enrichment of total bacteria and ARGs in drinking water from distribution systems compared to raw water [93]. Additionally, bacteria displayed enhanced enzymatic activities and extracellular polymeric substances production, promoting biofilm formation in the surfaces of the pipelines. Thus, antibiotics’ efficient removal in drinking water treatment plants presents a huge role in controlling antibiotic resistance dissemination. Recent strategies to overcome this issue include adsorption and degradation of antibiotics using nanotechnology and microalgae-based technologies (Table 2).
Table 2.
Recent promising strategies to efficiently eliminate antibiotics in wastewater and drinking water treatment plants.
| Strategy | Target | Removal Efficiency | Ref |
|---|---|---|---|
| Nanomaterials (nanocomposites, nanofibers, NPs) | |||
| TiO2-doped Fe3+ nano-photocatalyst | Metronidazole | 97% (ci = 80 mg/mL, pH 11, 2 h) 69.85% (pH 6, 2 h) |
[94] |
| Graphitized mesoporous carbon TiO2 nanocomposites | Ciprofloxacin | 100% (ci = 1.5 mg/L, 1.5 h) | [95] |
| V2O5-ZnO NPs coated carbon nanofibers | Ciprofloxacin Cinoxacin |
Adsorption of 87.70 mg/g (ci = 10–200 mg/L, pH 6.5, 20 min) Adsorption of 71.4 mg/g (ci = 10–200 mg/L, pH 6.5, 20 min) |
[96] |
| Ta3N5 NPs/TiO2 hollow nanosphere composite | Levofloxacin Ciprofloxacin Tetracycline hydrochloride |
93% (2 h); 89.76% after 4 cycles 93.2% (3 h) 92.2% (3 h) |
[97] |
| Silver modified ZnO nanoplates | Ofloxacin | 98% (ci = 10 mg/mL, pH 7, 2,30 h) | [98] |
| MnO2/graphene nanocomposite | Tetracycline | 99.4% | [99] |
| SnO2/Ni@N carbon nanotubes | Cephalexin | >70% (electropersulfate oxidation) | [100] |
| Boron Nitride Nanosheets | Tetracycline Ofloxacin Cephalexin |
Adsorption of 346.66 mg/g, pH 8 Adsorption of 72.50 mg/g (pH 8) Adsorption 225.0 mg/g (pH 12) |
[101] |
| Green GS-NiFe beads nanocomposite | Tetracycline (ci = 20 mg/L) |
Adsorption/degradation of 487 ± 6.85 mg/g Adsorption/degradation of 420 ± 10.21 mg/g Adsorption/degradation of 408 ± 12.35 mg/g |
[102] |
| Green bimetallic nZVI-Cu NPs (pomegranate ring extract) | Tetracycline | 72% (ci = 10 mg/L, pH 7) | [103] |
| Bentonite supported green nZVI-Cu nanocomposite | 95% (pH 7) | ||
| MnCo2O4 NPs | Ciprofloxacin | 100% (pH 3, 5 h) | [104] |
| CdS NPs | 79.50% (ci = 10 mg/mL, pH 9, 80 min) | [105] | |
| NiFe2O4 NPs loaded graphitic carbon nitride | Oxytetracycline | 100% (pH 5, 8 h) | [106] |
| ZV Cu (core) and Fe3O4 (shell) NPs | Oxytetracycline | >99% (ci = 20 mg/mL, pH3, 10 min) | [107] |
| S-doped MgO NPs | Tetracycline | 90% (pH neutral, 10 min) | [108] |
| PRB columns packed with ZVI | Tetracycline (ci = 20 mg/L, pH 6.5, 30 days) |
65% | [109] |
| PRB columns packed with MnO2 | 50% | ||
| PRB columns packed with ZVI and MnO2 | 85% (pH 6.5, 30 days) | ||
| Fe/Ni bimetallic NPs | Tetracycline | 97.4% (ci = 100 mg/mL, pH 5, 3 h) | [110] |
| Microalgae | |||
| Microcystis aeruginosa | Cefradine Amoxicillin |
37.08% 60.89% |
[111] |
| Chlorella pyrenoidosa | Cefradine Amoxicillin |
42.63% 71.25% |
|
| Haematococcus pluvialis | Sulfonamides | 42–100% (mean 93%) of sulfamerazine, sulfamethoxazole, sulfamonomethoxine | [112] |
| Selenastrum capricornutum | Macrolides Fluoroquinolones |
9–99% (mean 82%) of trimethoprim, clarithromycin azithromycin, roxithromycin 9–99% (mean 82%) of lomefloxacin, levofloxacin, flumequine |
|
| Scenedesmus quadricauda | Sulfonamides | 23–98% (mean 78%) | |
| Chlorella vulgaris | Macrolides Fluoroquinolones |
10–100% (mean 47%) 10–100% (mean 47%) of fluoroquinolones (lomefloxacin, levofloxacin, flumequine) |
|
| Chlorella vulgaris | Enrofloxacin Sulfadiazine Sulfamethazine Norfloxacin |
53–73% 11–24% 16–33% Inefficient removal |
[113] |
| Chrysosporum ovalisporum | Enrofloxacin Sulfadiazine Sulfamethazine Norfloxacin |
58–79% 10–20% 14–27% Inefficient removal |
|
| Chlorella pyrenoidosa | cefuroxime sodium | 60% (within 48 h) 92.9% (with NaHCO3 addition) |
[114] |
| Scenedesmus obliquus | Ofloxacin | 9.95–39.24% | [115] |
| Scenedesmus dimorphus | Ofloxacin | 93% | [116] |
| Spirulina sp.-derived biochar | Tetracycline | Adsorption of 61% (120 h; ↓ adsorption along with cycles) | [117] |
| Scenedesmus obliquus | Sulfamethazine Sulfamethoxazole |
31.4–62.3% (12 days) 27.7–46.8% (12 days) |
[118] |
|
Chlorella micrococcus (photo-sequencing batch reactor) |
Trimethoprim Sulfamethoxazole Sulfamethazine Sulfamerazine Norfloxacin Enrofloxacin |
91.8% 85.5% 85.5% 85.5% 98% 100% |
[119] |
| Chlorella pyrenoidosa | Sulfamethoxazole | Biodegradation of 14.9% (11 days) 99.3% (with sodium acetate addition, 5 days) |
[120] |
| Chlorella vulgaris (batch culture) | Sulfadiazine Sulfamethazine Sulfamethoxazole |
32.06% (12 days) 31.17% (12 days) 34.07% (12 days) |
[121] |
| Chlorella vulgaris biofilm membrane photobioreactor | Sulfadiazine Sulfamethazine Sulfamethoxazole |
79.2% (1 day) 76.7% (1 day) 82.1% (1 day) |
|
| M. aeruginosa | Tetracycline | 98% (2 days) | [122] |
| Chlamydomonas sp. Tai-03 | Ciprofloxacin Sulfadiazine |
100% (65.05% by biodegradation) 54.53% (35.60% by photolysis) |
[123] |
| Microalgae-bacteria consortium | Cephalexin Erythromycin |
96.54% (7 days) 92.38% (7 days) |
[124] |
| Sulfamethoxazole | 54.34% (42.86% by biodegradation) | [125] | |
NPs—nanoparticles; PRB—permeable reactive barrier; and ZVI—zero-valent iron.
4.1.1. Antibiotics Adsorption and Degradation Using Nanotechnology
Nanotechnology presents many applications, and several studies have analyzed its ability to counteract antibiotic resistance. Different nanomaterials, including nanoparticles (NPs), nanocomposites, nanotubes, and others, have been engineered to promote antibiotics removal (Table 2). Among the most promising nanomaterials, we found bimetallic and biogenic NPs as well as composites.
Bimetallic NPs have shown excellent results regarding antibiotics removal [107,110]. Ni/Fe NPs removed 97.4% of tetracycline through both adsorption and degradation mechanisms [110]. In another study, Cu (core) and Fe3O4 (shell) were combined to synthesize bimetallic NPs and showed great ability to remove oxytetracycline (>99%), improving Cu and Fe3O4 single catalytic activity [107]. Moreover, Cu@Fe3O4 presented good reusability potential, removing 97% of oxytetracycline after five cycles. However, the production of these materials is associated with the excessive use of hazardous chemicals that can produce new pollutants and present toxicity to the environment [126]. So, this process should be monitored considering the ratio of risk/benefit.
In this sense, biogenic nanomaterials emerged as a green alternative to conventional approaches to water treatment, being more eco-friendly, safer, and cost-effective. Biogenic nanomaterials, including NPs, nanorods, nanowires, and nanotubes, can be synthesized from different microorganisms (e.g., bacteria, fungi, and algae) and plants or bio-waste products instead of synthetic chemicals. These nanomaterials act as biocatalysts and adsorbents, contributing to the removal of toxic compounds such as heavy metals, hazardous dyes, pesticides, and pharmaceutical pollutants from water [126,127,128]. However, only a few studies analyzed its application and performance in the removal of antibiotics from water [102,103,129]. Biogenic platinum and palladium NPs synthesized from Desulfovibrio vulgaris bacteria allowed the remotion of ciprofloxacin and sulfamethoxazole [129]. Bio-platinum NPs showed higher catalytic activity and promoted the removal of 70% and 85% of ciprofloxacin and sulfamethoxazole, respectively. However, the reusability of Bio-platinum NPs was only ensured for three cycles. Another study that used bimetallic nZVI-Cu NPs synthesized from pomegranate rind extract showed a reduction of 72% of tetracycline [103]. Furthermore, bentonite addition enhanced NPs stability, allowing the removal of 95% of tetracycline, but, like Bio-platinum NPs, presented low reusability.
Although green synthesis of nanomaterials is a sustainable solution that should be exploited in the future for water treatment, some issues, such as biogenic NPs yield, stability, size, aggregation, reusability, and fabrication costs, are still unsolved [126,127].
Despite this, nanotechnology holds promise to remove antibiotics efficiently from water treatment plants. Moreover, nanomaterials’ application in membrane filtration technology has been shown to improve antibiotics removal [130] and inhibit biofouling [131,132]. Thin-film nanofiber membranes with UiO-66 NPs were fabricated and used in the forward osmosis process to evaluate antibiotics rejection. Membranes functionalized with the NPs increased the water flux and the rejection rate, above 99.9%, of six antibiotics (sulfamethoxazole, sulfamethazine, trimethoprim, erythromycin, chloramphenicol, and tetracycline) [130].
4.1.2. Bioadsorption, Bioaccumulation, and Biodegradation of Antibiotics by Microalgae-Based Technologies
Microalgae-based technology is an eco-friendly strategy suitable for water remediation applications. In recent years, many studies have demonstrated microalgae-based technologies’ potential and efficiency in the removal of antibiotics (Table 2). The main mechanisms used by microalgae for antibiotic removal include bioadsorption, bioaccumulation, and biodegradation [133,134,135].
Bioadsorption of antibiotics can occur in the microalgae cell membrane or into organic substances excreted by microalgae, such as exopolysaccharides. Microalgae bioadsorption capability depends on the microalgae species and their physical and chemical properties, including surface chemistry, surface area, and target antibiotic structure [133]. Chen and colleagues compared the ability of Chlorella vulgaris and Chrysosporum ovalisporum to remove sulfadiazine, sulfamethazine, enrofloxacin, and norfloxacin during 16 days [113]. Overall, C. vulgaris showed better performance, but neither microalgae could efficiently remove norfloxacin. Furthermore, microalgae capacity to remove antibiotics depends on antibiotic concentration and decreases with higher antibiotic concentration. However, desorption of antibiotics was observed on day 11 or day 16. Besides living microalgae cells, the bioadsorption of antibiotics by microalgae biomass was achieved for the removal of tetracycline [136]. So, microalgae have a great potential to remove antibiotics from water by bioadsorption, but this is not the most suitable process to efficiently remove antibiotics, as it can be reversible. Thus, antibiotics might be rereleased to the environment.
Bioaccumulation, contrarily to bioadsorption, is an active metabolic process that comprehends the uptake of antibiotics by living microalgae cells and is affected by several factors, such as temperature, pH, contact time, and antibiotic concentration [133]. Bioaccumulation can be seen as an intermediate step between bioadsorption (accumulation of antibiotics on the cell membrane) and biodegradation (intracellular degradation of antibiotics). Biodegradation is the most effective mechanism for antibiotics removal, also for being an irreversible process that can result in less toxic by-products.
As biodegradation depends on the cellular metabolism of microalgae, antibiotics removal efficiency differs among microalgae species. Chlorella pyrenoidosa and Microcystis aeruginosa abilities to remove cefradine and amoxicillin by biodegradation were analyzed [111]. C. pyrenoidosa was more efficient in the removal of the antibiotics, removing about 42% and 71% of cefradine and amoxicillin, respectively. Additionally, it showed higher tolerance to both antibiotics compared to M. aeruginosa. In another study, tetracycline removal was successfully obtained with M. aeruginosa and C. pyrenoidosa. M. aeruginosa showed a faster and more efficient removal of tetracycline than C. pyrenoidosa, about 99% within 2 days due to adsorption, bioaccumulation, and biodegradation mechanisms. On the other hand, C. pyrenoidosa contribution to tetracycline removal was achieved mainly by abiotic photolysis, hydrolysis, and cation-binding [122]. In another study, ciprofloxacin and sulfadiazine removal by Chlamydomonas sp. Tai-03 occurred mainly through biodegradation (65.05%) and photolysis (35.60%) [123].
Overall, microalgae-based processes show great potential to be applied in water remediation to remove antibiotics, which are responsible for the selective pressure on ARB and consequent ARG dissemination.
Microalgae-Bacterial Consortium
Some recent reports support the use of microalgae-bacterial consortiums for water remediation [67,124,125]. A microalgae-bacterial consortium, where Chorella sorokiniana was the predominant microalgae species, was able to remove about 54% of sulfamethoxazole from wastewater treatment plant effluents, mainly through biodegradation by bacteria. This resulted from a symbiotic action of bacteria and microalgae that act as an oxygen source for bacteria development [125]. In another study, the microalgae-bacterial consortium showed great capacity (>90%) to remove two other antibiotics, cephalexin and erythromycin, through biodegradation from wastewater treatment plant effluent [124]. In addition, a microalgae-bacterial consortium is a promising approach for the biodegradation of pharmaceutical compounds such as antibiotics compared to pure microalgae cultures [137,138]. Recently, Wang and colleagues reported enhanced chlortetracycline removal by microalgae-bacteria compared to pure microalgae, most probably due to the increasing microalgae tolerance to high concentrations of the antibiotic [67]. Moreover, the microalgae-bacterial consortium removed about 80% of chlortetracycline, at high concentrations (80 mg/L), primarily via bioadsorption, followed by biodegradation mediated by extracellular enzymatic action. Thus, these results support the microalgae-bacterial consortium as a promising strategy to eliminate antibiotics in wastewater and drinking water treatment plant facilities. However, their performance regarding ARB and ARGs occurrence must also be analyzed.
4.2. New Approaches for ARBs and ARGs Elimination
Until recently, the lack of appropriated molecular tools to evaluate the occurrence of ARB and ARG in drinking water sources constrained their risk assessment. The development of new techniques such as high-throughput quantitative PCR, metagenomics, and whole-genome sequencing [139], have contributed to analyzing the occurrence and dissemination of several ARBs and ARGs in wastewater and drinking water [60,140,141,142]. The systematic detection of ARB and ARG, even after water treatment, has raised several warnings regarding the safety of drinking water and the risks to human health. Moreover, as previously discussed, most of the water treatment processes usually applied are inefficient on ARG removal, and thus new methods must be exploited.
4.2.1. ARBs and ARGs Removal by Nanotechnology
There are already some reports describing new approaches to ARBs and ARGs elimination, based on nanomaterials with great adsorption potential, such as metallic NPs [143,144,145,146,147,148,149,150], electrocatalytic tools such as carbon nanotubes [151], and microalgae [119,124,125] (Table 3).
Table 3.
Promising strategies to eliminate ARBs and ARGs in water treatment plants and drinking water distribution systems.
| Strategy | Result | Ref |
|---|---|---|
| Nanotechnology (nanoparticles, nanocomposites, nanofibers) | ||
| GNICPs (Ginkgo biloba L. modified iron-cobalt NPs) |
↓ bacterial abundance (↓16S rRNA) ↓ ARGs: blaTEM, sul1, qnrA, acrA-02, mexB, tetM-01, ermB, mefA, ereA ↓MGEs: intI1, intI3, tnp-04, and TP614 Altered microbial community composition |
[147] |
| Cd2+ and Fe2O3 NPs | ↑ conjugative transfer frequencies ↑ cell membrane permeability ↑ antioxidant enzymes (SOD, CAT) ↑ mRNA expression of trfAp and trfBp |
[152] |
| Metallic (Cu, Zn, CuO, ZnO) NPs | ↓ bacterial growth ↓ ARGs: sul1, aadA1 and MGE: intl1 ↑ ROS production ↑ bacterial cell membrane permeability |
[143] |
| nTiO2 NPs | Adsorption of tetM-plasmid (0.06/min and 4.29 mg/g) | [144] |
| nZVI NPs | Adsorption of tetM-plasmid (0.05/min and 2.15 mg/g) ARGs fragmentation |
|
| CuO NPs (with humic acid) | ↓ absolute ARGs: macB, mexF and MGE: intl1 ↓ absolute metallic-resistance genes: copA, cusA Modulation of EPS production |
[145] |
| CNTs/AG/Ti electrode (Carbon nanotubes/agarose/titanium) |
↑ ROS production bacterial cell membrane damage ↓ ampicilin-resistant E. coli (100%, 1.8.V, 30 min) blaTEM-1 degradation (100%, 2 V (PBS), 30 min) |
[151] |
| Water-resistant cellulose foam paper coated with CuO, ZnO, or Ag2O NPs | Enhanced cellulose filter paper antibacterial activity against E. coli, P. aeruginosa, Bacillus Subtilis, and Bacillus cereus Ag2O NPs produced the highest antibacterial activity |
[148] |
| Melamine foams with Ag NPs | Antibacterial activity against E. coli | [149] |
| PVDF membrane functionalized with TiO2 NPs | 99.9% retention of tetracycline, chloramphenicol, and sulfadiazine-resistant bacteria ↓ ARGs: floR (97.8%), sul1 (99.5%), sul2 (98.8%), intI1 (93.7%), tetC (20.6%), tetW (27.2%), tetQ (2.0%) Inhibition of HGT |
[150] |
| Chitosan/biochar-nanosilver (C-Ag) composite | Sustainable antibacterial activity against E. coli (>50 days) Good reusability |
[153] |
| Carbon-based copper nanocomposites | ↓ absolute ARGs and MGEs ↓ HGT mediated by plasmids and MGEs |
[142] |
| SWNTs-PAN/TPU/PANI composite electrospun nanofiber membrane | Complete removal of S. aureus and E. coli Good durability and stability over various cycles |
[154] |
| k-carrageenan/Ag NPs film | Antimicrobial activity against Vibrio cholerae, Candida albicans, P. aeruginosa, E. coli, K. pneumoniae, and Bacillus cereus Inhibition of bacteria growth |
[146] |
| Microalgae | ||
| Chlorella micrococcus (photo-sequencing batch reactor) | ↓ 78% ARG absolute abundance ↓ Rhodocyclaceae and Burkholderiaceae bacteria families |
[119] |
| Microalgae-bacteria consortium | ↓ ARGs: blaTEM (72%) and ermB (97%) absolute abundance | [124] |
| ↑ ARG: sul1 | [125] | |
ARG—antibiotic resistance gene; CAT—catalase; EPS—extracellular polymeric substances; HGT—horizontal gene transference; NPs—nanoparticles; MGE—mobile genetic element; PRB—permeable reactive barrier; ROS—reactive oxygen species; SOD—superoxide dismutase; ZVI—zero-valent iron; ↓—decrease; and ↑—increase.
Duan and colleagues compared the efficiency of single metal nanoscale iron particles (NIPs), bimetallic (NICPs), and single and bimetallic NIPs modified with Ginkgo biloba L. (GNIPs and GNICPs, respectively) in bacteria and ARG removal [147]. Overall, bimetallic NIPs showed better performance, which was enhanced by G. biloba L. addition, due to the catalytic activity improving by cobalt and the additional active sites provided by G. biloba L. GNICPs reduced the abundance of bacteria, ARGs (blaTEM, sul1, qnrA, acrA-02, mexB, tetM-01, ermB, mefA, and ereA) and mobile genetic elements (intI-1, intI3, tnpA-04, and TP614). Notably, acrA-02, blaTEM, ermB, mefA, mexB, qnrA, and tetM-01, intI3, and TP614 were reduced to below the detection limit. Another study evaluated the nZVI and nTiO2 NPs efficiency on the removal of the ARG tetM-carrying plasmids [144]. Both NPs were able to adsorb tetM-carrying plasmids; however, only nZVI could fragment the ARG by binding to PO43− of phosphate backbones, causing its disruption during desorption.
Metallic NPs can also functionalize other materials to eliminate ARB and ARG from water. Jain and colleagues analyzed different coatings with metallic NPs (CuO, ZnO, or Ag2O) for water-resistant cellulose foam papers [148]. The coating with metallic NPs enhanced the filtration of analyzed microorganisms (E. coli, P. aeruginosa, Bacillus Subtilis, and Bacillus cereus). Moreover, foam papers coated with Ag2O NPs showed the best performance. Accordingly, melamine foams coated with Ag NPs used as filters completely removed E. coli bacteria from water, and no bacterial regrowth was registered. Synthesized Ag NPs from the pupa of green bottle fly were incorporated in a k-carrageenan film and showed great antimicrobial activity against different resistance bacteria belonging to the ESKAPE pathogens list, such as P. aeruginosa, E. coli, and K. pneumoniae [146].
Recently, another approach based on the electrocatalytic properties of carbon nanotubes coated on the titanium mesh by conductive agarose gel (CNTs/AG/Ti electrode) produced promising results concerning the inactivation of ARB and the degradation of ARGs in the aquatic environment [151]. Total inactivation of E. coli was achieved by using a potential of 1.8 V for 30 min that increased the production of reactive oxygen species and lead to bacteria cell membrane damage. In addition, the ARG blaTEM-1 was also degraded after 30 min of electrocatalytic treatment due to DNA damage.
4.2.2. ARBs and ARGs Removal by Microalgae-Based Technologies
Contrarily to antibiotics, only a few studies have addressed the removal of ARBs and ARGs by microalgae-based technologies [119,124,125] (Table 3). Rodrigues and colleagues reported that a microalgae-bacterial consortium was able to remove about 54% of sulfamethoxazole [125]. However, sulfonamide resistance gene sul1 increased during the 7 days of the experiment. An explanation could be the selective pressure caused by the remaining sulfamethoxazole. On the other hand, another microalgae-bacteria consortium was able to reduce significantly blaTEM (72%) and ermB (97%) genes. Moreover, in this study, the antibiotics analyzed, cephalexin and erythromycin, were successfully eliminated (>90%) by the consortium [124]. These observations reinforce that the occurrence of antibiotics in water, even at low levels, produce a selective pressure, resulting in ARBs and ARGs dissemination. Taking this into account, and despite the evidence showing microalgae-bacteria consortium advantages, its applicability must be further explored to understand the mechanism and efficiency on the control of ARB and ARGs in water after the treatment.
5. Conclusions and Future Perspectives
In recent years, global efforts have been developed to raise awareness about antibiotic resistance including the EU Action Plan against antibiotic resistance (2011 and 2017), the World Health Organization Global Action Plan on antimicrobial resistance (2015), and the EU Guidelines for the prudent use of antimicrobials in human health [155]. Despite this, legislation regarding antibiotics monitorization on water sources and drinking water is still missing due to the lack of knowledge about their toxicity and occurrence in the environment. In 2015, the EU Commission published a watch list of substances to monitor in the environment, including three macrolides (erythromycin, clarithromycin, and azithromycin) [156]. Since then, this list has been reviewed in 2018 [157] and in 2020 [158], to include amoxicillin and ciprofloxacin, and sulfamethoxazole and trimethoprim, respectively. Besides the relevance of monitorization of antibiotics persistence on the environment and concretely on water sources, scientific evidence of ARG dissemination in water systems highlights this as an emergent public health concern that should be considered in global surveillance programs.
In addition, the ineffectiveness of conventional and advanced treatment processes in water treatment plants in the removal of antibiotics, ARB, and mainly ARGs, reinforces the demand to develop new or complementary methods. In this way, in recent years, a few studies have highlighted the benefits of nanotechnology and microalgae-based technologies’ application in water remediation. Nanomaterials and microalgae have shown remarkable performance considering the removal of antibiotics, which is crucial to reduce the selective pressure that largely contributes to ARB and ARG dissemination. Therefore, the complementary use of nanotechnology and microalgae with water treatment processes, such as reverse and forward osmosis, hold promises to achieve efficient removal of antibiotics and ARBs. Still, nanomaterials can be engineered to optimize their biocatalytic action and adsorption properties to inhibit antibiotic resistance dissemination.
The development of novel processes to eliminate ARGs in water treatment plants and in water distribution system remains a challenge, mainly because there are no safe levels of ARGs in water. Therefore, the reduction in the total or relative abundance of ARGs does not ensure the safety and quality of water to consume. Even at low levels, ARGs can be propagated among microorganisms and contribute to antibiotic resistance dissemination.
Thus, more research is required to develop these emergent technologies and upscale to achieve a proper elimination of ARGs in water treatment plants, but also along with the water distribution systems where the formation of biofilms promotes bacterial resistance and the dissemination of ARGs by horizontal gene transfer.
Another challenge is associated with the development of processes or systems that are environmentally friendly, such as biogenic NPs and microalgae-based technology, which also contribute to avoiding the release of by-products and toxicants for the environment. Moreover, these emergent and promising strategies overcome some important limitations and disadvantages of conventional methods, namely high cost of processing, energy consumption, and instability. So, it is critical to initiate scientific actions to develop these technologies with low environmental impact, to establish efficient, stable, scalable, and cost-effective solutions to the monitoring, control, and removal of these types of emergent pollutants from various aquatic systems.
Author Contributions
Conceptualization, A.C.D. and P.C.; investigation, A.C.D. and S.R.; writing—original draft preparation, A.C.D., S.R. and P.C.; writing—review and editing, A.C.D., A.A., A.N. and P.C.; supervision, A.A., A.N. and P.C., funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Programa Operacional Regional do Centro (CENTRO-04-3559-FSE-000162), within the European Social Fund (ESF), and The Science and Technology Foundation/Ministry of Education and Science (FCT/MEC) funded the CICS-UBI projects UIDB/00709/2020 and UIDP/00709/2020 and CIMO (UIDB/00690/2020), through national funds and, where applicable, co-financed by FEDER, Portugal 2020.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Não há Tempo a Perder: Acautelar o Futuro Contra Infecções Resistentes aos Medicamentos. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 2.Zhang T., Lv K., Lu Q., Wang L., Liu X. Removal of antibiotic-resistant genes during drinking water treatment: A review. J. Environ. Sci. 2021;104:415–429. doi: 10.1016/j.jes.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Bortoloti K.D.C.S., Melloni R., Marques P.S., De Carvalho B.M.F., Andrade M.C. Qualidade microbiológica de águas naturais quanto ao perfil de resistência de bactérias heterotróficas a antimicrobianos. Eng. Sanit. Ambient. 2018;23:717–725. doi: 10.1590/s1413-41522018169903. [DOI] [Google Scholar]
- 4.Iria A. Ph.D. Thesis. Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa; Lisboa, Portugal: 2018. Efeitos da Presença de Antibióticos nas Origens de Agua. Contribuição para o Estudo da sua Remoção Através de Sistemas de Tratamento de Aguas. [Google Scholar]
- 5.Collignon P., Beggs J.J., Walsh T., Gandra S., Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health. 2018;2:e398–e405. doi: 10.1016/s2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 6.Amarasiri M., Sano D., Suzuki S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020;50:2016–2059. doi: 10.1080/10643389.2019.1692611. [DOI] [Google Scholar]
- 7.Tan Q., Li W., Zhang J., Zhou W., Chen J., Li Y., Ma J. Presence, dissemination and removal of antibiotic resistant bacteria and antibiotic resistance genes in urban drinking water system: A review. Front. Environ. Sci. Eng. 2019;13:36. doi: 10.1007/s11783-019-1120-9. [DOI] [Google Scholar]
- 8.Homem V.M.F.C. Tecnologias Alternativas de Remoção de Antibióticos de Águas Contaminadas. Faculdade de Engenharia da Universidade do Porto; Porto, Portugal: 2011. pp. 1–305. [Google Scholar]
- 9.Sanganyado E., Gwenzi W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019;669:785–797. doi: 10.1016/j.scitotenv.2019.03.162. [DOI] [PubMed] [Google Scholar]
- 10.Xu L., Ouyang W., Qian Y., Su C., Su J., Chen H. High-throughput profiling of antibiotic resistance genes in drinking water treatment plants and distribution systems. Environ. Pollut. 2016;213:119–126. doi: 10.1016/j.envpol.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J., Chen T., Chen H. Antibiotic resistome promotion in drinking water during biological activated carbon treatment: Is it influenced by quorum sensing? Sci. Total Environ. 2018;612:1–8. doi: 10.1016/j.scitotenv.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 12.Hutchings M., Truman A., Wilkinson B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Felis E., Kalka J., Sochacki A., Kowalska K., Bajkacz S., Harnisz M., Korzeniewska E. Antimicrobial pharmaceuticals in the aquatic environment—occurrence and environmental implications. Eur. J. Pharmacol. 2020;866:172813. doi: 10.1016/j.ejphar.2019.172813. [DOI] [PubMed] [Google Scholar]
- 14.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 15.Savin M., Bierbaum G., Hammerl A., Heinemann C., Parcina M., Sib E., Voigt A., Kreyenschmidt J. ESKAPE Bacteria and Extended-Spectrum-B-Lactamase- Producing Escherichia coli Isolated from Wastewater and Process Water from German Poultry Slaughterhouses. Appl. Environ. Microbiol. 2020;86:e02748-19. doi: 10.1128/AEM.02748-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrivastava S.R., Shrivastava P.S., Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018;32:76–77. doi: 10.4103/jms.jms_25_17. [DOI] [Google Scholar]
- 17.Blair J.M.A., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 18.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Beatson S.A., Paterson D.L., Walker J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:e00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadeem S.F., Gohar U.F., Tahir S.F., Mukhtar H., Pornpukdeewattana S., Nukthamna P., Moula Ali A.M., Bavisetty S.C.B., Massa S. Antimicrobial resistance: More than 70 years of war between humans and bacteria. Crit. Rev. Microbiol. 2020;46:578–599. doi: 10.1080/1040841X.2020.1813687. [DOI] [PubMed] [Google Scholar]
- 20.Costa S.S., Sobkowiak B., Parreira R., Edgeworth J.D., Viveiros M., Clark T.G., Couto I. Genetic diversity of norA, coding for a main efflux pump of Staphylococcus aureus. Front. Genet. 2019;10:1–11. doi: 10.3389/fgene.2018.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uluseker C., Kaster K.M., Thorsen K., Basiry D., Shobana S., Jain M., Kumar G., Kommedal R., Pala-Ozkok I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021;12:3003. doi: 10.3389/fmicb.2021.717809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papkou A., Hedge J., Kapel N., Young B., MacLean R.C. Efflux pump activity potentiates the evolution of antibiotic resistance across S. aureus isolates. Nat. Commun. 2020;11:3970. doi: 10.1038/s41467-020-17735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontaine F., Hequet A., Voisin-Chiret A.-S., Bouillon A., Lesnard A., Cresteil T., Jolivalt C., Rault S. First Identification of Boronic Species as Novel Potential Inhibitors of the Staphylococcus aureus NorA Efflux Pump. J. Med. Chem. 2014;57:2536–2548. doi: 10.1021/jm401808n. [DOI] [PubMed] [Google Scholar]
- 24.Malmir S., Bahreinian M., Yeganeh S.Z., Mirnejad R., Moghaddam M.M., Saberi F. Molecular Mechanisms of Resistance to Conventional Antibiotics in Bacteria. Int. J. Med. Rev. 2018;5:118–129. doi: 10.29252/ijmr-050305. [DOI] [Google Scholar]
- 25.Fishovitz J., Hermoso J., Chang M., Mobashery S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life. 2014;66:572–577. doi: 10.1002/iub.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zainab S.M., Junaidb M., Xub N., Malik R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020;187:116455. doi: 10.1016/j.watres.2020.116455. [DOI] [PubMed] [Google Scholar]
- 27.Luo A., Wang F., Sun D., Liu X., Xin B. Formation, Development, and Cross-Species Interactions in Biofilms. Front. Microbiol. 2022;12:757327. doi: 10.3389/fmicb.2021.757327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian Z., Zeng L., Xu T., Sun S., Yan S., Yang L., Huang Y., Jia J., Dou T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021;61:1049–1070. doi: 10.1002/jobm.202100201. [DOI] [PubMed] [Google Scholar]
- 29.Uddin J., Dawan J., Jeon G., Yu T., He X., Ahn J. The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery. Microorganisms. 2020;8:670. doi: 10.3390/microorganisms8050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee A.R., Park S.B., Kim S.W., Jung J.W., Chun J.H., Kim J., Kim Y.R., Lazarte J.M.S., Jang H.B., Thompson K.D., et al. Membrane vesicles (MVs) from antibiotic-resistant Staphylococcus aureus transfer antibiotic-resistance to antibiotic-susceptible Escherichia coli. J. Appl. Microbiol. 2022:1–14. doi: 10.1111/jam.15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Wintersdorff C.J.H., Penders J., Van Niekerk J.M., Mills N.D., Majumder S., Van Alphen L.B., Savelkoul P.H.M., Wolffs P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson G.G., O’Toole G.A. Innate and induced resistance mechanisms of bacterial biofilms. In: Romeo T., editor. Bacterial Biofilms. Springer; Berlin, Germany: 2008. pp. 85–105. [DOI] [PubMed] [Google Scholar]
- 33.Flemming H.C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 34.Monzón M., Oteiza C., Leiva J., Lamata M., Amorena B. Biofilm testing of Staphylococcus epidermidis clinical isolates: Low performance of vancomycin in relation to other antibiotics. Diagn. Microbiol. Infect. Dis. 2002;44:319–324. doi: 10.1016/S0732-8893(02)00464-9. [DOI] [PubMed] [Google Scholar]
- 35.Savage V.J., Chopra I., O’Neill A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013;57:1968–1970. doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Król J.E., Wojtowicz A.J., Rogers L.M., Heuer H., Smalla K., Krone S.M., Top E.M. Invasion of E. coli biofilms by antibiotic resistance plasmids. Plasmid. 2013;70:110–119. doi: 10.1016/j.plasmid.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemdan B.A., El-Taweel G.E., Goswami P., Pant D., Sevda S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: An overview of surveillance, outbreaks, and prevention. World J. Microbiol. Biotechnol. 2021;37:36. doi: 10.1007/s11274-021-03008-3. [DOI] [PubMed] [Google Scholar]
- 38.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., Goossens H., Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X., Jin G., Zhao Y., Shao X. Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Sci. Total Environ. 2020;711:134626. doi: 10.1016/j.scitotenv.2019.134626. [DOI] [PubMed] [Google Scholar]
- 40.Santos L., Ramos F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents. 2018;52:135–143. doi: 10.1016/j.ijantimicag.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Zhao R., Feng J., Liu J., Fu W., Li X., Li B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019;151:388–402. doi: 10.1016/j.watres.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruyndonckx R., Adriaenssens N., Versporten A., Hens N., Monnet D.L., Molenberghs G., Goossens H., Weist K., Coenen S. Consumption of antibiotics in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021;76:7–13. doi: 10.1093/jac/dkab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Centre for Disease Prevention and Control . Antimicrobial Consumption in the EU/EEA—Annual Epidemiological Report 2019. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2020. [Google Scholar]
- 45.Xiang Y., Yang Z., Zhang Y., Xu R., Zheng Y., Hu J., Li X., Jia M., Xiong W., Cao J. Influence of nanoscale zero-valent iron and magnetite nanoparticles on anaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction. Bioresour. Technol. 2019;294:122139. doi: 10.1016/j.biortech.2019.122139. [DOI] [PubMed] [Google Scholar]
- 46.Kovalakova P., Cizmas L., McDonald T.J., Marsalek B., Feng M., Sharma V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere. 2020;251:126351. doi: 10.1016/j.chemosphere.2020.126351. [DOI] [PubMed] [Google Scholar]
- 47.Obimakinde S., Fatoki O., Opeolu B., Olatunji O. Veterinary pharmaceuticals in aqueous systems and associated effects: An update. Environ. Sci. Pollut. Res. 2017;24:3274–3297. doi: 10.1007/s11356-016-7757-z. [DOI] [PubMed] [Google Scholar]
- 48.Watts J.E.M., Schreier H.J., Lanska L., Hale M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs. 2017;15:158. doi: 10.3390/md15060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran N.H., Reinhard M., Gin K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018;133:182–207. doi: 10.1016/j.watres.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 50.Mackull’ak T., Cverenkárová K., Stanová A., Fehér M., Tamás M., Škulcová A., Gál M., Naumowicz M., Špalkov V., Bírošová L. Hospital Wastewater—Source of Specific Micropollutants. Antibiotics. 2021;10:1070. doi: 10.3390/antibiotics10091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proia L., Adriana A., Jessica S., Carles B., Marinella F., Marta L., Luis B.J., Servais P. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere. 2018;206:70–82. doi: 10.1016/j.chemosphere.2018.04.163. [DOI] [PubMed] [Google Scholar]
- 52.Marathe N.P., Berglund F., Razavi M., Pal C., Dröge J., Samant S., Kristiansson E., Joakim Larsson D.G. Sewage effluent from an Indian hospital harbors novel carbapenemases and integron-borne antibiotic resistance genes. Microbiome. 2019;7:97. doi: 10.1186/s40168-019-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laquaz M., Dagot C., Bazin C., Bastide T., Gaschet M., Ploy M.C., Perrodin Y. Ecotoxicity and antibiotic resistance of a mixture of hospital and urban sewage in a wastewater treatment plant. Environ. Sci. Pollut. Res. 2018;25:9243–9253. doi: 10.1007/s11356-017-9957-6. [DOI] [PubMed] [Google Scholar]
- 54.Petrovich M.L., Zilberman A., Kaplan A., Eliraz G.R., Wang Y., Langenfeld K., Duhaime M., Wigginton K., Poretsky R., Avisar D., et al. Microbial and Viral Communities and Their Antibiotic Resistance Genes Throughout a Hospital Wastewater Treatment System. Front. Microbiol. 2020;11:1–13. doi: 10.3389/fmicb.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowe W.P.M., Baker-Austin C., Verner-Jeffreys D.W., Ryan J.J., Micallef C., Maskell D.J., Pearce G.P. Overexpression of antibiotic resistance genes in hospital effluents over time. J. Antimicrob. Chemother. 2017;72:1617–1623. doi: 10.1093/jac/dkx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milaković M., Vestergaard G., González-Plaza J.J., Petrić I., Kosić-Vukšić J., Senta I., Kublik S., Schloter M., Udiković-Kolić N. Effects of industrial effluents containing moderate levels of antibiotic mixtures on the abundance of antibiotic resistance genes and bacterial community composition in exposed creek sediments. Sci. Total Environ. 2020;706:136001. doi: 10.1016/j.scitotenv.2019.136001. [DOI] [PubMed] [Google Scholar]
- 57.Tong J., Lu X.T., Zhang J.Y., Sui Q., Wang R., Chen M., Wei Y. Occurrence of antibiotic resistance genes and mobile genetic elements in enterococci and genomic DNA during anaerobic digestion of pharmaceutical waste sludge with different pretreatments. Bioresour. Technol. 2017;235:316–324. doi: 10.1016/j.biortech.2017.03.104. [DOI] [PubMed] [Google Scholar]
- 58.Guo X., Yan Z., Zhang Y., Xu W., Kong D., Shan Z., Wang N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018;612:119–128. doi: 10.1016/j.scitotenv.2017.08.229. [DOI] [PubMed] [Google Scholar]
- 59.Wang K., Zhuang T., Su Z., Chi M., Wang H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental impacts. Sci. Total Environ. 2021;788:147811. doi: 10.1016/j.scitotenv.2021.147811. [DOI] [PubMed] [Google Scholar]
- 60.Zhang K., Li K., Xin R., Han Y., Guo Z., Zou W., Wei W., Cui X., Zhang Z., Zhang Y. Antibiotic resistomes in water supply reservoirs sediments of central China: Main biotic drivers and distribution pattern. Environ. Sci. Pollut. Res. 2022:1–10. doi: 10.1007/s11356-021-18095-w. [DOI] [PubMed] [Google Scholar]
- 61.Chan S., Pullerits K., Keucken A., Persson K.M., Paul C.J., Rådström P. Bacterial release from pipe biofilm in a full-scale drinking water distribution system. NPJ Biofilms Microbiomes. 2019;5:3–10. doi: 10.1038/s41522-019-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J., Li W., Tan Q., Sheng D., Li Y., Chen S., Zhou W. Effect of disinfectant exposure and starvation treatment on the detachment of simulated drinking water biofilms. Sci. Total Environ. 2022;807:150896. doi: 10.1016/j.scitotenv.2021.150896. [DOI] [PubMed] [Google Scholar]
- 63.Zhu N.J., Ghosh S., Edwards M.A., Pruden A. Interplay of Biologically Active Carbon Filtration and Chlorine-Based Disinfection in Mitigating the Dissemination of Antibiotic Resistance Genes in Water Reuse Distribution Systems. Environ. Sci. Technol. 2021;55:8329–8340. doi: 10.1021/acs.est.1c01199. [DOI] [PubMed] [Google Scholar]
- 64.Chen J., Li W., Zhang J., Qi W., Li Y., Chen S., Zhou W. Prevalence of antibiotic resistance genes in drinking water and biofilms: The correlation with the microbial community and opportunistic pathogens. Chemosphere. 2020;259:127483. doi: 10.1016/j.chemosphere.2020.127483. [DOI] [PubMed] [Google Scholar]
- 65.Liang Y., Li H., Chen Z., Yang Y., Shi D., Chen T., Yang D., Yin J., Zhou S., Cheng C., et al. Spatial behavior and source tracking of extracellular antibiotic resistance genes in a chlorinated drinking water distribution system. J. Hazard. Mater. 2022;425:127942. doi: 10.1016/j.jhazmat.2021.127942. [DOI] [PubMed] [Google Scholar]
- 66.Hu Y., Jiang L., Sun X., Wu J., Ma L., Zhou Y., Lin K., Luo Y., Cui C. Risk assessment of antibiotic resistance genes in the drinking water system. Sci. Total Environ. 2021;800:149650. doi: 10.1016/j.scitotenv.2021.149650. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., He Y., Li X., Nagarajan D., Chang J.-S. Enhanced biodegradation of chlortetracycline via a microalgae-bacteria consortium. Bioresour. Technol. 2022;343:126149. doi: 10.1016/j.biortech.2021.126149. [DOI] [PubMed] [Google Scholar]
- 68.Jia S., Bian K., Shi P., Ye L., Liu C.H. Metagenomic profiling of antibiotic resistance genes and their associations with bacterial community during multiple disinfection regimes in a full-scale drinking water treatment plant. Water Res. 2020;176:115721. doi: 10.1016/j.watres.2020.115721. [DOI] [PubMed] [Google Scholar]
- 69.Lu J., Tian Z., Yu J., Yang M., Zhang Y. Distribution and abundance of antibiotic resistance genes in sand settling reservoirs and drinking water treatment plants across the Yellow River, China. Water. 2018;10:246. doi: 10.3390/w10030246. [DOI] [Google Scholar]
- 70.Hu Y., Zhang T., Jiang L., Luo Y., Yao S., Zhang D., Lin K., Cui C. Occurrence and reduction of antibiotic resistance genes in conventional and advanced drinking water treatment processes. Sci. Total Environ. 2019;669:777–784. doi: 10.1016/j.scitotenv.2019.03.143. [DOI] [PubMed] [Google Scholar]
- 71.Xu L., Campos L.C., Canales M., Ciric L. Drinking water biofiltration: Behaviour of antibiotic resistance genes and the association with bacterial community. Water Res. 2020;182:115954. doi: 10.1016/j.watres.2020.115954. [DOI] [PubMed] [Google Scholar]
- 72.Destiani R., Templeton M.R. Chlorination and ultraviolet disinfection of antibiotic-resistant bacteria and antibiotic resistance genes in drinking water. AIMS Environ. Sci. 2019;6:222–241. doi: 10.3934/environsci.2019.3.222. [DOI] [Google Scholar]
- 73.Su H.C., Liu Y.S., Pan C.G., Chen J., He L.Y., Ying G.G. Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment system: From drinking water source to tap water. Sci. Total Environ. 2018;616:453–461. doi: 10.1016/j.scitotenv.2017.10.318. [DOI] [PubMed] [Google Scholar]
- 74.Lu J., Zhang Y., Wu J., Wang J., Cai Y. Fate of antibiotic resistance genes in reclaimed water reuse system with integrated membrane process. J. Hazard. Mater. 2020;382:121025. doi: 10.1016/j.jhazmat.2019.121025. [DOI] [PubMed] [Google Scholar]
- 75.Liang C., Wei D., Zhang S., Ren Q., Shi J., Liu L. Removal of antibiotic resistance genes from swine wastewater by membrane filtration treatment. Ecotoxicol. Environ. Saf. 2021;210:111885. doi: 10.1016/j.ecoenv.2020.111885. [DOI] [PubMed] [Google Scholar]
- 76.Hu Y., Jiang L., Zhang T., Jin L., Han Q., Zhang D., Lin K., Cui C. Occurrence and removal of sulfonamide antibiotics and antibiotic resistance genes in conventional and advanced drinking water treatment processes. J. Hazard. Mater. 2018;360:364–372. doi: 10.1016/j.jhazmat.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Zhang T., Hu Y., Jiang L., Yao S., Lin K., Zhou Y., Cui C. Removal of antibiotic resistance genes and control of horizontal transfer risk by UV, chlorination and UV/chlorination treatments of drinking water. Chem. Eng. J. 2019;358:589–597. doi: 10.1016/j.cej.2018.09.218. [DOI] [Google Scholar]
- 78.Guo X., Li J., Yang F., Yang J., Yin D. Prevalence of sulfonamide and tetracycline resistance genes in drinking water treatment plants in the Yangtze River Delta, China. Sci. Total Environ. 2014;493:626–631. doi: 10.1016/j.scitotenv.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 79.Shen L., Griffith T.M., Nyangaresi P.O., Qin Y., Pang X., Chen G., Li M., Lu Y., Zhang B. Efficacy of UVC-LED in water disinfection on Bacillus species with consideration of antibiotic resistance issue. J. Hazard. Mater. 2020;386:121968. doi: 10.1016/j.jhazmat.2019.121968. [DOI] [PubMed] [Google Scholar]
- 80.Manaia C.M., Rocha J., Scaccia N., Marano R., Radu E., Biancullo F., Cerqueira F., Fortunato G., Iakovides I.C., Zammit I., et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. Environ. Int. 2018;115:312–324. doi: 10.1016/j.envint.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 81.O’Flaherty E., Borrego C.M., Balcázar J.L., Cummins E. Human exposure assessment to antibiotic-resistant Escherichia coli through drinking water. Sci. Total Environ. 2018;616:1356–1364. doi: 10.1016/j.scitotenv.2017.10.180. [DOI] [PubMed] [Google Scholar]
- 82.Shannon M.A., Bohn P.W., Elimelech M., Georgiadis J.G., Mariñas B.J., Mayes A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. A Collect. Rev. Nat. J. 2009;452:337–346. doi: 10.1142/9789814287005_0035. [DOI] [PubMed] [Google Scholar]
- 83.Wan K., Guo L., Ye C., Zhu J., Zhang M., Yu X. Accumulation of antibiotic resistance genes in full-scale drinking water biological activated carbon (BAC) filters during backwash cycles. Water Res. 2021;190:116744. doi: 10.1016/j.watres.2020.116744. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S., Lin W., Yu X. Effects of full-scale advanced water treatment on antibiotic resistance genes in the Yangtze Delta area in China. FEMS Microbiol. Ecol. 2016;92:1–9. doi: 10.1093/femsec/fiw065. [DOI] [PubMed] [Google Scholar]
- 85.Bai X., Ma X., Xu F., Li J., Zhang H., Xiao X. The drinking water treatment process as a potential source of affecting the bacterial antibiotic resistance. Sci. Total Environ. 2015;533:24–31. doi: 10.1016/j.scitotenv.2015.06.082. [DOI] [PubMed] [Google Scholar]
- 86.Le T.H., Ng C., Tran N.H., Chen H., Gin K.Y.H. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018;145:498–508. doi: 10.1016/j.watres.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 87.Benladghem Z., Seddiki S.M.L., Mahdad Y.M. Identification of bacterial biofilms on desalination reverse osmosis membranes from the mediterranean sea. Biofouling. 2020;36:1065–1073. doi: 10.1080/08927014.2020.1851366. [DOI] [PubMed] [Google Scholar]
- 88.Wu Y.H., Wang Y.H., Xue S., Chen Z., Luo L.W., Bai Y., Tong X., Hu H.Y. Increased risks of antibiotic resistant genes (ARGs) induced by chlorine disinfection in the reverse osmosis system for potable reuse of reclaimed water. Sci. Total Environ. 2022;815:152860. doi: 10.1016/j.scitotenv.2021.152860. [DOI] [PubMed] [Google Scholar]
- 89.Slipko K., Reif D., Wögerbauer M., Hufnagl P., Krampe J., Kreuzinger N. Removal of extracellular free DNA and antibiotic resistance genes from water and wastewater by membranes ranging from microfiltration to reverse osmosis. Water Res. 2019;164:114916. doi: 10.1016/j.watres.2019.114916. [DOI] [PubMed] [Google Scholar]
- 90.Xu L., Zhang H., Xiong P., Zhu Q., Liao C., Jiang G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021;753:141975. doi: 10.1016/j.scitotenv.2020.141975. [DOI] [PubMed] [Google Scholar]
- 91.Ben Y., Hu M., Zhang X., Wu S., Wong M.H., Wang M., Andrews C.B., Zheng C. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water. Water Res. 2020;175:115699. doi: 10.1016/j.watres.2020.115699. [DOI] [PubMed] [Google Scholar]
- 92.Karkman A., Do T.T., Walsh F., Virta M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018;26:220–228. doi: 10.1016/j.tim.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Wang H., Hu C., Shen Y., Shi B., Zhao D., Xing X. Response of microorganisms in biofilm to sulfadiazine and ciprofloxacin in drinking water distribution systems. Chemosphere. 2019;218:197–204. doi: 10.1016/j.chemosphere.2018.11.106. [DOI] [PubMed] [Google Scholar]
- 94.Malakootian M., Olama N., Malakootian M., Nasiri A. Photocatalytic degradation of metronidazole from aquatic solution by TiO2-doped Fe3+ nano-photocatalyst. Int. J. Environ. Sci. Technol. 2019;16:4275–4284. doi: 10.1007/s13762-018-1836-2. [DOI] [Google Scholar]
- 95.Zheng X., Xu S., Wang Y., Sun X., Gao Y., Gao B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018;527:202–213. doi: 10.1016/j.jcis.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 96.Chaba J.M., Nomngongo P.N. Preparation of V2O5-ZnO coated carbon nanofibers: Application for removal of selected antibiotics in environmental matrices. J. Water Process. Eng. 2018;23:50–60. doi: 10.1016/j.jwpe.2018.03.003. [DOI] [Google Scholar]
- 97.Jiang Y., Jing X., Zhu K., Peng Z., Zhang J., Liu Y., Zhang W., Ni L., Liu Z. Ta3N5 nanoparticles/TiO2 hollow sphere (0D/3D) heterojunction: Facile synthesis and enhanced photocatalytic activities of levofloxacin degradation and H2 evolution. Dalt. Trans. 2018;47:13113–13125. doi: 10.1039/c8dt02343c. [DOI] [PubMed] [Google Scholar]
- 98.Kaur A., Gupta G., Ibhadon A.O., Salunke D.B., Sinha A.S.K., Kansal S.K. A Facile synthesis of silver modified ZnO nanoplates for efficient removal of ofloxacin drug in aqueous phase under solar irradiation. J. Environ. Chem. Eng. 2018;6:3621–3630. doi: 10.1016/j.jece.2017.05.032. [DOI] [Google Scholar]
- 99.Song Z., Ma Y.L., Li C.E. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total Environ. 2019;651:580–590. doi: 10.1016/j.scitotenv.2018.09.240. [DOI] [PubMed] [Google Scholar]
- 100.Duan P., Chen D., Hu X. Tin dioxide decorated on Ni-encapsulated nitrogen-doped carbon nanotubes for anodic electrolysis and persulfate activation to degrade cephalexin: Mineralization and degradation pathway. Chemosphere. 2021;269:128740. doi: 10.1016/j.chemosphere.2020.128740. [DOI] [PubMed] [Google Scholar]
- 101.Bangari R.S., Sinha N. Adsorption of tetracycline, ofloxacin and cephalexin antibiotics on boron nitride nanosheets from aqueous solution. J. Mol. Liq. 2019;293:111376. doi: 10.1016/j.molliq.2019.111376. [DOI] [Google Scholar]
- 102.Ravikumar K.V.G., Kubendiran H., Ramesh K., Rani S., Mandal T.K., Pulimi M., Natarajan C., Mukherjee A. Batch and column study on tetracycline removal using green synthesized NiFe nanoparticles immobilized alginate beads. Environ. Technol. Innov. 2020;17:100520. doi: 10.1016/j.eti.2019.100520. [DOI] [Google Scholar]
- 103.Gopal G., Sankar H., Natarajan C., Mukherjee A. Tetracycline removal using green synthesized bimetallic nZVI-Cu and bentonite supported green nZVI-Cu nanocomposite: A comparative study. J. Environ. Manag. 2020;254:109812. doi: 10.1016/j.jenvman.2019.109812. [DOI] [PubMed] [Google Scholar]
- 104.Mi X., Li Y., Ning X., Jia J., Wang H., Xia Y., Sun Y., Zhan S. Electro-Fenton degradation of ciprofloxacin with highly ordered mesoporous MnCo2O4-CF cathode: Enhanced redox capacity and accelerated electron transfer. Chem. Eng. J. 2019;358:299–309. doi: 10.1016/j.cej.2018.10.047. [DOI] [Google Scholar]
- 105.Kaur M., Mehta S.K., Kumar S. Visible light driven photocatalytic degradation of o floxacin and malachite green dye using cadmium sulphide nanoparticles. J. Environ. Chem. Eng. 2018;6:3631–3639. doi: 10.1016/j.jece.2017.04.006. [DOI] [Google Scholar]
- 106.Sudhaik A., Raizada P., Shandilya P., Singh P. Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. J. Environ. Chem. Eng. 2018;6:3874–3883. doi: 10.1016/j.jece.2018.05.039. [DOI] [Google Scholar]
- 107.Pham V.L., Kim D., Ko S. Oxidative degradation of the antibiotic oxytetracycline by Cu@Fe3O4 core-shell nanoparticles. Sci. Total Environ. 2018;631–632:608–618. doi: 10.1016/j.scitotenv.2018.03.067. [DOI] [PubMed] [Google Scholar]
- 108.Moussavi G., Mashayekh-salehi A., Kamyar Y., Anoshiravan M. The catalytic destruction of antibiotic tetracycline by sulfur-doped manganese oxide (SeMgO) nanoparticles. J. Environ. Manag. 2018;210:131–138. doi: 10.1016/j.jenvman.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Dong G., Huang L., Wu X., Wang C., Liu Y., Liu G., Wang L., Liu X., Xia H. Effect and mechanism analysis of MnO2 on permeable reactive barrier (PRB) system for the removal of tetracycline. Chemosphere. 2018;193:702–710. doi: 10.1016/j.chemosphere.2017.11.085. [DOI] [PubMed] [Google Scholar]
- 110.Dong H., Jiang Z., Zhang C., Deng J., Hou K., Cheng Y., Zhang L., Zeng G. Removal of tetracycline by Fe/Ni bimetallic nanoparticles in aqueous solution. J. Colloid Interface Sci. 2018;513:117–125. doi: 10.1016/j.jcis.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Du Y., Wang J., Li H., Mao S., Wang D., Xiang Z., Guo R., Chen J. The dual function of the algal treatment: Antibiotic elimination combined with CO2 fixation. Chemosphere. 2018;211:192–201. doi: 10.1016/j.chemosphere.2018.07.163. [DOI] [PubMed] [Google Scholar]
- 112.Kiki C., Rashid A., Wang Y., Li Y., Zeng Q., Yu C.P., Sun Q. Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways. J. Hazard. Mater. 2020;387:121985. doi: 10.1016/j.jhazmat.2019.121985. [DOI] [PubMed] [Google Scholar]
- 113.Chen S., Zhang W., Li J., Yuan M., Zhang J., Xu F., Xu H., Zheng X., Wang L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum) Environ. Pollut. 2020;263:114554. doi: 10.1016/j.envpol.2020.114554. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L., Guo R., Li H., Du Q., Lu J., Huang Y., Yan Z., Chen J. Mechanism analysis for the process-dependent driven mode of NaHCO3 in algal antibiotic removal: Efficiency, degradation pathway and metabolic response. J. Hazard. Mater. 2020;394:122531. doi: 10.1016/j.jhazmat.2020.122531. [DOI] [PubMed] [Google Scholar]
- 115.Yang L., Ren L., Tan X., Chu H., Chen J., Zhang Y., Zhou X. Removal of ofloxacin with biofuel production by oleaginous microalgae Scenedesmus obliquus. Bioresour. Technol. 2020;315:123738. doi: 10.1016/j.biortech.2020.123738. [DOI] [PubMed] [Google Scholar]
- 116.Grimes K.L., Dunphy L.J., Loudermilk E.M., Melara A.J., Kolling G.L., Papin J.A., Colosi L.M. Evaluating the efficacy of an algae-based treatment to mitigate elicitation of antibiotic resistance. Chemosphere. 2019;237:124421. doi: 10.1016/j.chemosphere.2019.124421. [DOI] [PubMed] [Google Scholar]
- 117.Choi Y.K., Choi T.R., Gurav R., Bhatia S.K., Park Y.L., Kim H.J., Kan E., Yang Y.H. Adsorption behavior of tetracycline onto Spirulina sp. (microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2020;710:136282. doi: 10.1016/j.scitotenv.2019.136282. [DOI] [PubMed] [Google Scholar]
- 118.Xiong J.Q., Govindwar S., Kurade M.B., Paeng K.J., Roh H.S., Khan M.A., Jeon B.H. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere. 2019;218:551–558. doi: 10.1016/j.chemosphere.2018.11.146. [DOI] [PubMed] [Google Scholar]
- 119.Liu H., Yao Y., Ye W., Qian R., Chen H., Liang J., Ye J. Enhanced removal of antibiotics and decreased antibiotic resistance genes in the photo-sequencing batch reactor during the aquaculture wastewater treatment. Environ. Technol. 2021:1–12. doi: 10.1080/09593330.2021.1928295. [DOI] [PubMed] [Google Scholar]
- 120.Xiong Q., Liu Y.S., Hu L.X., Shi Z.Q., Cai W.W., He L.Y., Ying G.G. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020;175:115656. doi: 10.1016/j.watres.2020.115656. [DOI] [PubMed] [Google Scholar]
- 121.Peng Y.Y., Gao F., Yang H.L., Wu H.W.J., Li C., Lu M.M., Yang Z.Y. Simultaneous removal of nutrient and sulfonamides from marine aquaculture wastewater by concentrated and attached cultivation of Chlorella vulgaris in an algal biofilm membrane photobioreactor (BF-MPBR) Sci. Total Environ. 2020;725:138524. doi: 10.1016/j.scitotenv.2020.138524. [DOI] [PubMed] [Google Scholar]
- 122.Pan M., Lyu T., Zhan L., Matamoros V., Angelidaki I., Cooper M., Pan G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021;190:116735. doi: 10.1016/j.watres.2020.116735. [DOI] [PubMed] [Google Scholar]
- 123.Xie P., Chen C., Zhang C., Su G., Ren N., Ho S.H. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 2020;172:115475. doi: 10.1016/j.watres.2020.115475. [DOI] [PubMed] [Google Scholar]
- 124.da Silva Rodrigues D.A., da Cunha C.C.R.F., do Espirito Santo D.R., de Barros A.L.C., Pereira A.R., de Queiroz Silva S., da Fonseca Santiago A., de Cássia Franco Afonso R.J. Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ. Sci. Pollut. Res. 2021;28:67822–67832. doi: 10.1007/s11356-021-15351-x. [DOI] [PubMed] [Google Scholar]
- 125.da Silva Rodrigues D.A., da Cunha C.C.R.F., Freitas M.G., de Barros A.L.C., e Castro P.B.N., Pereira A.R., de Queiroz Silva S., da Fonseca Santiago A., de Cássia Franco Afonso R.J. Biodegradation of sulfamethoxazole by microalgae-bacteria consortium in wastewater treatment plant effluents. Sci. Total Environ. 2020;749:141441. doi: 10.1016/j.scitotenv.2020.141441. [DOI] [PubMed] [Google Scholar]
- 126.Gautam P.K., Singh A., Misra K., Sahoo A.K., Samanta S.K. Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J. Environ. Manag. 2019;231:734–748. doi: 10.1016/j.jenvman.2018.10.104. [DOI] [PubMed] [Google Scholar]
- 127.Ali I., Peng C., Khan Z.M., Naz I., Sultan M., Ali M., Abbasi I.A., Islam T., Ye T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019;230:128–150. doi: 10.1016/j.jenvman.2018.09.073. [DOI] [PubMed] [Google Scholar]
- 128.Nasrollahzadeh M., Sajjadi M., Iravani S., Varma R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021;401:123401. doi: 10.1016/j.jhazmat.2020.123401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martins M., Mourato C., Sanches S., Noronha J.P., Crespo M.T.B., Pereira I.A.C. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017;108:160–168. doi: 10.1016/j.watres.2016.10.071. [DOI] [PubMed] [Google Scholar]
- 130.Mu T., Zhang Y., Shi W., Chen G., Liu Y., Huang M. A novel UiO-66/PSF-composite membrane for the rejection of multiple antibiotics: Numerical simulation and experiment verification. Chemosphere. 2021;269:128686. doi: 10.1016/j.chemosphere.2020.128686. [DOI] [PubMed] [Google Scholar]
- 131.Zhang T., Li Z., Wang W., Wang Y., Gao B., Wang Z. Enhanced antifouling and antimicrobial thin film nanocomposite membranes with incorporation of Palygorskite/titanium dioxide hybrid material. J. Colloid Interface Sci. 2019;537:1–10. doi: 10.1016/j.jcis.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 132.Gokulakrishnan S.A., Arthanareeswaran G., László Z., Veréb G., Kertész S., Kweon J. Recent development of photocatalytic nanomaterials in mixed matrix membrane for emerging pollutants and fouling control, membrane cleaning process. Chemosphere. 2021;281:130891. doi: 10.1016/j.chemosphere.2021.130891. [DOI] [PubMed] [Google Scholar]
- 133.Xiong Q., Hu L.X., Liu Y.S., Zhao J.L., He L.Y., Ying G.G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021;155:106594. doi: 10.1016/j.envint.2021.106594. [DOI] [PubMed] [Google Scholar]
- 134.Leng L., Wei L., Xiong Q., Xu S., Li W., Lv S., Lu Q., Wan L., Wen Z., Zhou W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere. 2020;238:124680. doi: 10.1016/j.chemosphere.2019.124680. [DOI] [PubMed] [Google Scholar]
- 135.Hena S., Gutierrez L., Croué J.P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021;403:124041. doi: 10.1016/j.jhazmat.2020.124041. [DOI] [PubMed] [Google Scholar]
- 136.Daneshvar E., Zarrinmehr M.J., Hashtjin A.M., Farhadian O., Bhatnagar A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018;268:523–530. doi: 10.1016/j.biortech.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 137.Norvill Z.N., Toledo-Cervantes A., Blanco S., Shilton A., Guieysse B., Muñoz R. Photodegradation and sorption govern tetracycline removal during wastewater treatment in algal ponds. Bioresour. Technol. 2017;232:35–43. doi: 10.1016/j.biortech.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 138.Tian Y., Zou J., Feng L., Zhang L., Liu Y. Chlorella vulgaris enhance the photodegradation of chlortetracycline in aqueous solution via extracellular organic matters (EOMs): Role of triplet state EOMs. Water Res. 2019;149:35–41. doi: 10.1016/j.watres.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 139.Franklin A.M., Brinkman N.E., Jahne M.A., Keely S.P. Twenty-first century molecular methods for analyzing antimicrobial resistance in surface waters to support One Health assessments. J. Microbiol. Methods. 2021;184:106174. doi: 10.1016/j.mimet.2021.106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hao H., Shi D.-Y., Yang D., Yang Z.-W., Qiu Z.-G., Liu W.-L., Shen Z.-Q., Yin J., Wang H.-R., Li J.-W., et al. Profiling of intracellular and extracellular antibiotic resistance genes in tap water. J. Hazard. Mater. 2019;365:340–345. doi: 10.1016/j.jhazmat.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 141.Han Z., Zhang Y., An W., Lu J., Hu J., Yang M. Antibiotic resistomes in drinking water sources across a large geographical scale: Multiple drivers and co-occurrence with opportunistic bacterial pathogens. Water Res. 2020;183:116088. doi: 10.1016/j.watres.2020.116088. [DOI] [PubMed] [Google Scholar]
- 142.Ji X., Tang Y., Ye J., Wu S., Hou M., Huang S., Wang R. The effect of carbon-based copper nanocomposites on Microcystis aeruginosa and the movability of antibiotic resistance genes in urban water. Chemosphere. 2022;286:131744. doi: 10.1016/j.chemosphere.2021.131744. [DOI] [PubMed] [Google Scholar]
- 143.Su Y., Wu D., Xia H., Zhang C., Shi J., Wilkinson K.J., Xie B. Metallic nanoparticles induced antibiotic resistance genes attenuation of leachate culturable microbiota: The combined roles of growth inhibition, ion dissolution and oxidative stress. Environ. Int. 2019;128:407–416. doi: 10.1016/j.envint.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 144.Lu X., Hou J., Yang K., Zhu L., Xing B., Lin D. Binding Force and Site-Determined Desorption and Fragmentation of Antibiotic Resistance Genes from Metallic Nanomaterials. Environ. Sci. Technol. 2021;55:9305–9316. doi: 10.1021/acs.est.1c02047. [DOI] [PubMed] [Google Scholar]
- 145.Li Y., Wang J., Li B., Geng M., Wang Y., Zhao J., Jin B., Li Y. Response of extracellular polymeric substances and microbial community structures on resistance genes expression in wastewater treatment containing copper oxide nanoparticles and humic acid. Bioresour. Technol. 2021;340:125741. doi: 10.1016/j.biortech.2021.125741. [DOI] [PubMed] [Google Scholar]
- 146.Abu-Saied M.A., Elnouby M., Taha T., El-Shafeey M., Alshehri A.G., Alamri S., Alghamdi H., Shati A., Alrumman S., Al-Kahtani M., et al. Potential decontamination of drinking water pathogens through k-carrageenan integrated green bottle fly bio-synthesized silver nanoparticles. Molecules. 2020;25:1936. doi: 10.3390/molecules25081936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duan W., Gao J., Wu Z., Dai H., Wang Z., Li D., Wang Y., Liu J. Enhanced removal of antibiotic resistance genes by nanoscale iron-cobalt particles modified with Ginkgo biloba L. leaf: Combining Illumina MiSeq sequencing and oligotyping analysis. Bioresour. Technol. 2021;321:124453. doi: 10.1016/j.biortech.2020.124453. [DOI] [PubMed] [Google Scholar]
- 148.Jain S., Bhanjana G., Heydarifard S., Dilbaghi N., Nazhad M.M., Kumar V., Kim K.H., Kumar S. Enhanced antibacterial profile of nanoparticle impregnated cellulose foam filter paper for drinking water filtration. Carbohydr. Polym. 2018;202:219–226. doi: 10.1016/j.carbpol.2018.08.130. [DOI] [PubMed] [Google Scholar]
- 149.Pinto J., Magrì D., Valentini P., Palazon F., Heredia-Guerrero J.A., Lauciello S., Barroso-Solares S., Ceseracciu L., Pompa P.P., Athanassiou A., et al. Antibacterial Melamine Foams Decorated with in Situ Synthesized Silver Nanoparticles. ACS Appl. Mater. Interfaces. 2018;10:16095–16104. doi: 10.1021/acsami.8b01442. [DOI] [PubMed] [Google Scholar]
- 150.Ren S., Boo C., Guo N., Wang S., Elimelech M., Wang Y. Photocatalytic Reactive Ultrafiltration Membrane for Removal of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2018;52:8666–8673. doi: 10.1021/acs.est.8b01888. [DOI] [PubMed] [Google Scholar]
- 151.Liu H., Hua X., Zhang Y.N., Zhang T., Qu J., Nolte T.M., Chen G., Dong D. Electrocatalytic inactivation of antibiotic resistant bacteria and control of antibiotic resistance dissemination risk. Environ. Pollut. 2021;291:118189. doi: 10.1016/j.envpol.2021.118189. [DOI] [PubMed] [Google Scholar]
- 152.Pu Q., Fan X.T., Sun A.Q., Pan T., Li H., Bo Lassen S., An X.L., Su J.Q. Co-effect of cadmium and iron oxide nanoparticles on plasmid-mediated conjugative transfer of antibiotic resistance genes. Environ. Int. 2021;152:106453. doi: 10.1016/j.envint.2021.106453. [DOI] [PubMed] [Google Scholar]
- 153.Hu Z., Zhang L., Zhong L., Zhou Y., Xue J., Li Y. Preparation of an antibacterial chitosan-coated biochar-nanosilver composite for drinking water purification. Carbohydr. Polym. 2019;219:290–297. doi: 10.1016/j.carbpol.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 154.Xie L., Shu Y., Hu Y., Cheng J., Chen Y. SWNTs-PAN/TPU/PANI composite electrospun nanofiber membrane for point-of-use efficient electrochemical disinfection: New strategy of CNT disinfection. Chemosphere. 2020;251:126286. doi: 10.1016/j.chemosphere.2020.126286. [DOI] [PubMed] [Google Scholar]
- 155.Peñalva G., Högberg L.D., Weist K., Vlahović-Palčevski V., Heuer O., Monnet D.L., Vandael E., Ivanov I.N., Payerl-Pal M., Skjold Selle Pedersen K., et al. Decreasing and stabilising trends of antimicrobial consumption and resistance in Escherichia coli and Klebsiella pneumoniae in segmented regression analysis, European Union/European Economic Area, 2001 to 2018. Eurosurveillance. 2019;24:1900656. doi: 10.2807/1560-7917.ES.2019.24.46.1900656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Commision E. COMMISSION IMPLEMENTING DECISION (EU) 2015/495 of 20 March 2015 Establishing a Watch List of SubStances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Volume L78/40 European Commission; Brussels, Belgium: 2015. [Google Scholar]
- 157.Commision E. COMMISSION IMPLEMENTING DECISION (EU) 2018/840 of 5 June 2018 Establishing a Watch List of SubStances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Comm. Volume 61 European Commission; Brussels, Belgium: 2018. [Google Scholar]
- 158.Cortes L.G., Marinov D., Sanseverino I., Cuenca A.N., Niegowska M., Rodriguez E.P., Lettieri T. JRC Technical Report: Selection of Substances for the 3rd Watch List under WFD. Office of the European Union; Luxembourg: 2020. [Google Scholar]