Abstract
In order to overcome the multidrug resistance mediated by P-glycoprotein-like transporters in Leishmania spp., we have studied the effects produced by derivatives of the flavanolignan silybin and related compounds lacking the monolignol unit on (i) the affinity of binding to a recombinant C-terminal nucleotide-binding domain of the L. tropica P-glycoprotein-like transporter and (ii) the sensitization to daunomycin on promastigote forms of a multidrug-resistant L. tropica line overexpressing the transporter. Oxidation of the flavanonol silybin to the corresponding flavonol dehydrosilybin, the presence of the monolignol unit, and the addition of a hydrophobic substituent such as dimethylallyl, especially at position 8 of ring A, considerably increased the binding affinity. The in vitro binding affinity of these compounds for the recombinant cytosolic domain correlated with their modulation of drug resistance phenotype. In particular, 8-(3,3-dimethylallyl)-dehydrosilybin effectively sensitized multidrug-resistant Leishmania spp. to daunomycin. The cytosolic domains are therefore attractive targets for the rational design of inhibitors against P-glycoprotein-like transporters.
Drug resistance has become a major impediment to the treatment of diseases caused by protozoan parasites, which threaten the life of nearly one-quarter of the human population. Among parasitic infections, World Health Organization statistics show that the incidence of leishmaniasis has increased 42-fold between 1985 and 1998 and has become the second leading cause of death (18) worldwide. Chemotherapy remains the only effective way to control infections. The conventional clinical drugs, pentavalent antimonials in the form of Glucantime and Pentostam (reviewed in reference 27), are not very efficient due to their toxicity and the increased appearance of drug resistance (14). ATP-binding cassette (ABC) transporters have been found to be involved in Leishmania species in vitro selected for metal resistance (reviewed in reference 30). The multidrug resistance (MDR) phenotype due to P-glycoprotein (Pgp)-like transporters has been extensively characterized in cancer cells (1, 13) and protozoan parasites (41), including Plasmodium (45) and Leishmania (4, 5, 17) spp. Pgp is an ATP-dependent pump that exports a wide range of drugs from the cell, decreasing their intracellular concentration and preventing their cytotoxic activity. Pgp belongs to the ABC superfamily of transporters. It consists of two homologous halves, each comprising a transmembrane domain involved in drug efflux, and a cytosolic nucleotide-binding domain (NBD) responsible for ATP binding and hydrolysis. Pgp can be inhibited in vitro by agents such as verapamil and cyclosporine, which compete with drug binding to the transmembrane domains (16). However, most of these inhibitors are also pumped substrates and therefore require high concentrations for effective inhibition. These concentrations produce undesirable side effects. In addition, these classical modulators of drug efflux in cancer cells only poorly sensitize the MDR phenotype in Leishmania parasites (4, 17, 33). Thus, new classes of more specific, nontransported inhibitors of Pgp-like transporters with lower host toxicity need to be developed. Recently, it has been described that NBDs can be the target for inhibitors of Pgp-like transporters (7, 11, 33). Flavonoids, which constitute a well-known class of natural inhibitors of different ATP-binding proteins (28), with contradictory modulation effects on different MDR cells (8, 15, 33, 37–39), bind to transporter NBDs. They interact with both the ATP-binding site and a vicinal hydrophobic region (7, 9, 33), inhibiting drug efflux and reversing the resistance phenotype of an L. tropica MDR line (33). Their efficient modulation of drug efflux has been correlated with their affinity binding to the transporter cytosolic domain (33).
Silymarin is a mixture of flavanolignans isolated from the medicinal plant Silybum marianum, with silybin (or silybinin) (Fig. 1A) as the main component (31). These natural compounds are well-established hepatoprotectants and are used in Europe and Asia for the clinical treatment of liver diseases with different aetiologies (reviewed in references 25 and 32). Silymarin is well tolerated as a therapeutic agent and is largely devoid of adverse effects (25, 32). It has been recently marketed in the United States and in Europe as a nutritional supplement. Silymarin may directly affect cholesterol metabolism and is therefore considered as a potential hypocholesterolemic (41). In vitro studies indicate that silymarin and silybin may help to prevent and treat breast, prostate, skin and ovarian cancers (32, 36, 46). Silybin also appeared to be synergistic with doxorubicin in a doxorubicin-resistant cell line, probably by inhibiting Pgp function (36). Thus, silybin, either alone or in combination with other cytotoxic drugs, is currently being tested in patients with advanced ovarian cancer (36).
FIG. 1.
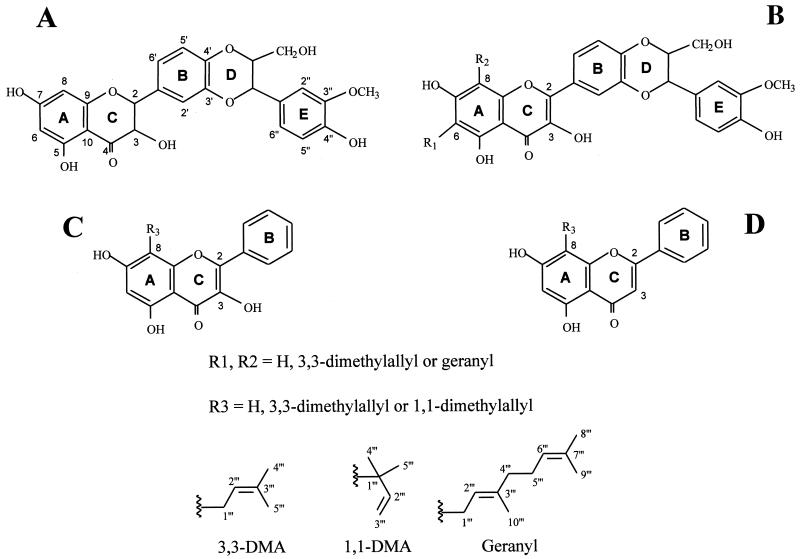
Chemical structures of silybin and derivatives. (A) The flavanol silybin with a reduced 2,3-bond, a hydroxyl group at position 3 and the monolignol unit (rings D and E) adjacent to ring B. (B) The flavonol DHS with oxidized 2,3-bond and derivatives substituted by 3,3-DMA or geranyl groups at either position 6 (R1) or 8 (R2) of ring A. (C) The flavonol galangin, lacking the monolignol unit, and derivatives substituted at position 8 (R3). (D) The flavone chrysin, lacking both the monolignol unit and the hydroxyl group at position 3, and 8-substituted derivatives (R3).
The binding of flavonoids to Pgp (7, 33) and their modulation of drug efflux in an L. tropica MDR line are class dependent (33). Flavones and flavonols display better binding affinities than the corresponding isoflavones and flavanones. The present study has tested the ability of the flavanonol silybin, plus its oxidized and hydrophobically substituted derivatives, to bind to purified recombinant C-terminal NBD (NBD2) of a Leishmania Pgp-like transporter and to sensitize the MDR phenotype. The oxidation of silybin to its corresponding flavonol and the prenylation of the latter, especially at position 8, dramatically increased the binding affinity for NBD2 and the sensitization of an L. tropica MDR line overexpressing the transporter, thereby inhibiting growth in the presence of the daunomycin.
MATERIALS AND METHODS
Chemical compounds.
HECAMEG [6-O-[(N-heptylcarbamoyl)methyl]-α-d-glucopyranoside] was purchased from Calbiochem, daunomycin was obtained from Pharmacia & Upjohn (Barcelona, Spain), and imidazole (catalog reference I 0250) was from Sigma. Commercial flavonoids were obtained from either Aldrich (galangin) or Sigma (chrysin and silybin). 1,1-DMA-chrysin (3), 1,1-DMA-galangin (2) and the derivatives of silybin (26) were synthesized as described. 3,3-DMA-chrysin was from the Natural Products Laboratory collections of compounds.
Synthesis of 8-(3,3-DMA)-galangin.
To a stirred solution of 1.2 g of galangin (4.4 mmol) and 1.6 g of tetraethylammonium iodide (6.2 mmol) in 70 ml of 10% aqueous tetramethylammonium hydroxide was added 1 ml of prenyl bromide (8.7 mmol) dropwise at room temperature. After a 90-min reaction, the medium was acidified to pH 1 (with HCl, 1 N) and extracted with ethyl acetate. Isolation of 8-(3,3-DMA)-galangin (0.19 g, 0.6 mmol, 14%) from the ethyl acetate extract was carried out by medium pressure liquid chromatography on a C18 reversed-phase column using a gradient of methanol in water as solvent. The 1H nuclear magnetic resonance (acetone-d6, 300 MHz) variables were as follows: δ 12.02 (1H, s, 5-OH), δ 8.30 (2H, m, H-2′+6′), δ 7.55 (3H, m, H-3′+4′+5′), δ 6.40 (1H, s, H-6), δ 5.30 (1H, brt, J = 6.5 Hz, H-2), δ 3.59 (2H, d, J = 6.5 Hz, H-1 ), δ 1.83 (3H, s, H-5 ), and δ 1.67 (3H, s, H-4 ). For electron impact mass spectrometry (EIMS) (70 eV), the m/z (rel. int.) values were 338 [M]+ (94), 323 (100), 283 (72), and 270 (42). For high-resolution EIMS, the m/z value was 338.1155 (as calculated for C20H18O5 = 338.1154).
Parasite culture and in vitro experiments.
The wild-type L. tropica LRC strain was a clone obtained by agar plating (19). An L. tropica line highly resistant to daunomycin (DNM-R150) was maintained in the presence of 150 μM daunomycin and used as previously described (4). This resistant line had an MDR phenotype similar to that of tumor cells, with cross-resistance to several drugs and an overexpressed drug efflux Pgp-like transporter (4). Promastigote forms were grown at 28°C in RPMI 1640-modified medium (Gibco) (20) and supplemented with 20% heat-inactivated fetal bovine serum (Gibco). The growth sensitivity of wild-type and drug-resistant parasites to modulators of drug efflux was ascertained as described earlier (33, 34).
Overexpression, protein purification, and interaction of Leishmania NBD2 with silybin and derivatives.
The recombinant NBD2 from Leishmania Pgp-like transporter was overexpressed in E. coli M15 (pREP4) cells and purified by affinity chromatography (33). Fluorescence experiments were performed at 25.0 ± 0.1°C using an SLM-Aminco 8000C spectrofluorimeter with spectral bandwidths of 2 and 4 nm for excitation and emission, respectively. The measurements were corrected for wavelength dependence on the excitation light intensity by using rhodamine B in the reference channel. All spectra were corrected for buffer Raman effect and for dilution. The intrinsic fluorescence of NBD2 (0.2 to 0.5 μM final concentration) was measured in 1 ml of diluting buffer (50 mM potassium phosphate, pH 8.0; 1 M NaCl; 20% [wt/vol] glycerol, 0.05% [wt/vol] HECAMEG; 1 mM β-mercaptoethanol; 10 mM imidazole), with increasing concentrations of silybin or derivatives dissolved in dimethyl sulfoxide. The emission spectrum was measured in the range of 300 to 350 nm upon excitation at 288 nm (a wavelength which minimized imidazole interference [33, 34]). Ligand binding was monitored by the quenching of emission fluorescence produced upon addition of increasing ligand concentrations. Corrections for the inner-filter effect and dimethyl sulfoxide addition (up to a 2% final concentration) were determined under the same conditions, by using a mixture of N-acetyltryptophanamide and N-acetyltyrosinamide in the same ratio (3:7) as the tryptophan and tyrosine residues present in NBD2. Curve fitting of ligand binding-related fluorescence quenching was analyzed with the Grafit program (Erithacus Software) (12). This allowed the determination of the apparent dissociation constant (Kd) and maximal quenching of fluorescence.
RESULTS
Interaction of NBD2 with silybin and derivatives.
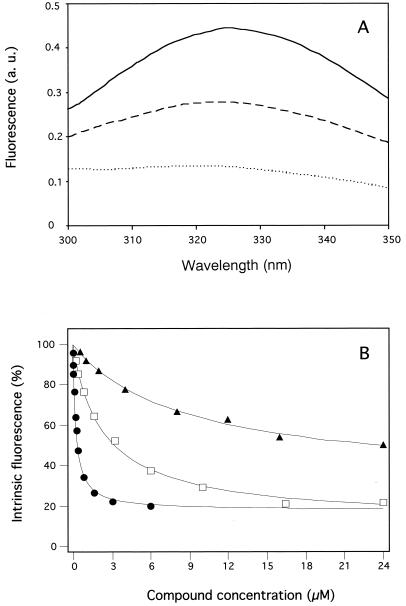
Incubation of recombinant NBD2 with the naturally occurring flavanolignan silybin (Fig. 1A) produced the concentration-dependent quenching of protein intrinsic fluorescence illustrated in Fig. 2A. Detailed analysis of binding (Fig. 2B) gave an apparent dissociation constant (Kd) of 9.2 ± 1.0 μM. Oxidation of the 2,3-bond of ring C in silybin to the corresponding flavonol dehydrosilybin (DHS) (Fig. 1B), gave a fourfold-higher binding affinity for the domain (Fig. 2B, with a Kd of 2.3 ± 0.2 μM). A further 3.5- to 21-fold increase in binding affinity was produced by addition of hydrophobic substituents (dimethylallyl [DMA] or geranyl) at either position 6 or position 8 of ring A, with Kd values in the nanomolar range (Table 1). The effect was dependent on both the nature and the position of the hydrophobic substituent. Thus, a 3,3-DMA group at position 6 or 8 of ring A increased the binding affinity a further 2.5- to 3-fold more, respectively, than a geranyl group at the same positions (Table 1). In addition, the hydrophobic substitution at position 8 of ring A by either prenyl or geranyl substituent gave twofold-greater affinity than substitution at position 6 (Table 1). Thus, the 8-(3,3-DMA)-DHS derivative gave the highest binding affinity, with a Kd of 0.11 ± 0.02 μM. This value was 85-fold less than that obtained with unmodified silybin.
FIG. 2.
Interaction of recombinant Leishmania NBD2 with silybin and derivatives monitored by the quenching of protein intrinsic fluorescence. (A) Spectral modification upon interaction with silybin. The fluorescence spectrum of 0.5 μM NBD2 was recorded after excitation at 288 nm, in the absence (continuous line) or presence of either 4 μM (broken line) or 16 μM (dotted line) silybin; the traces were obtained by buffer subtraction before correction for inner-filter effect. (B) The concentration-dependent binding of silybin and derivatives was analyzed by quenching of NBD2 intrinsic fluorescence, determined by spectral integration from 300 to 350 nm, and corrected for the inner-filter effect in the presence of increasing concentrations of either silybin (▴), DHS (□), or 8-(3,3-DMA)-DHS (●) added as a dimethyl sulfoxide solution.
TABLE 1.
Effects of DHS prenylation on the affinity of binding to Leishmania NBD2a
| DHS derivative | Kd (μM ± SD) | % Maximal quenching ± SD |
|---|---|---|
| 6-Geranyl-DHS | 0.67 ± 0.06 | 56.7 ± 1.4 |
| 8-Geranyl-DHS | 0.31 ± 0.05 | 72.0 ± 2.2 |
| 6-(3,3-Dimethylallyl)-DHS | 0.27 ± 0.05 | 76.1 ± 2.4 |
| 8-(3,3-Dimethylallyl)-DHS | 0.11 ± 0.02 | 76.1 ± 1.7 |
The NBD2 domain was incubated, under the conditions of Fig. 2B, with the indicated hydrophobic derivatives of DHS. The dissociation constant and maximal quenching values were determined by fitting with the Graft program using the Erithacus software (see Materials and Methods).
The effect of prenylation at position 8 was further studied within the compounds galangin and chrysin (Fig. 1C and D), lacking the monolignol unit. When compared to DHS, the binding affinity for NBD2 was reduced fourfold for galangin and eightfold for chrysin that also lacks an hydroxyl group at position 3 of ring C (Table 2). In both cases, prenylation by 3,3-DMA at position 8 also markedly increased (6- to 15-fold) the binding affinity. A double increase (12- to 28-fold) was even obtained with the 1,1-DMA substituent.
TABLE 2.
Binding affinities of different prenylated compounds lacking the monolignol unita-
| Compound | Kd (μM ± SD) | % Maximal quenching ± SD | Source or reference |
|---|---|---|---|
| Galangin | 9.2 ± 1.0 | 75.9 ± 3.9 | 33 |
| 8-(3,3-Dimethylallyl)-galangin | 0.62 ± 0.24 | 80.0 ± 6.4 | This study |
| 8-(1,1-Dimethylallyl)-galangin | 0.34 ± 0.09 | 85.0 ± 4.0 | This study |
| Chrysin | 17.6 ± 5.9 | 81.3 ± 8.6 | 33 |
| 8-(3,3-Dimethylallyl)-chrysin | 2.9 ± 0.3 | 84.3 ± 2.0 | This study |
| 8-(1,1-Dimethylallyl)-chrysin | 1.4 ± 0.2 | 75.4 ± 2.7 | 33 |
Sensitization of promastigote forms by silybin and its derivatives.
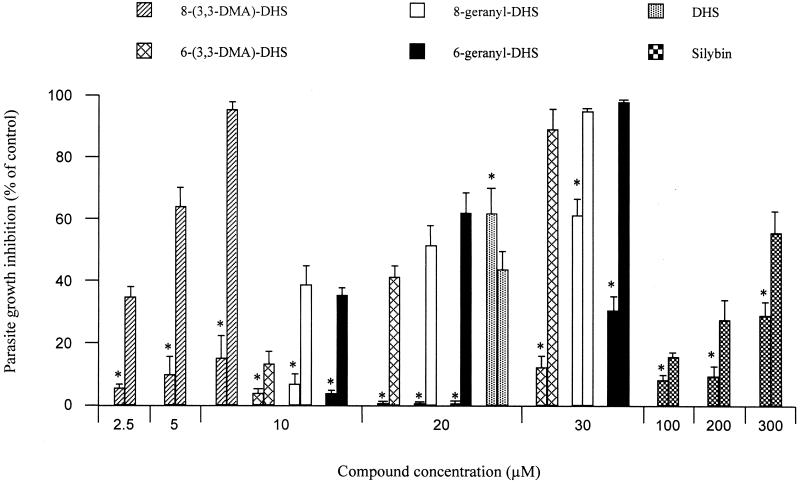
The resistance to daunomycin in the MDR L. tropica line is mainly due to the overexpression of a Pgp-like transporter involved in drug efflux (4) which limits drug accumulation. We tested whether the binding of silybin derivatives to the cytosolic domain of the Pgp-like transporter could inhibit drug pumping and overcome drug resistance. Potential modulators were assessed for their ability to inhibit the growth of resistant parasites in the presence of daunomycin, in comparison with wild-type parasites in absence of drug. Figure 3 shows that a 72-h incubation of resistant parasites with 150 μM daunomycin in the presence of different silybin derivatives gave differential dose-dependent growth inhibition (GI). These studies demonstrate the importance of silybin oxidation to DHS and the prenylation of the latter. Consistent with the binding analysis, 8-(3,3-DMA)-DHS was the most efficient sensitizer. It gave more than 95% GI at 10 μM and showed only a minor toxic effect in the wild-type line. The other hydrophobically substituted derivatives also showed considerable sensitization of the cells (89 to 98% GI in the resistant line) at a threefold-higher concentration (30 μM). However, this concentration of geranyl derivatives gave significant cytotoxicity in the wild-type parasites (31 to 62% GI). In contrast, unmodified silybin only gave modest sensitization, even at much higher concentrations (100 to 300 μM). Silymarin also did not reverse the resistant phenotype at high concentrations such as 250 μg/ml (data not shown). Finally, DHS showed considerable cytotoxicity on wild-type parasite (ca. 62% GI at 20 μM). This hampered studies of its sensitization of the MDR phenotype.
FIG. 3.
Sensitization by silybin derivatives in a daunomycin-resistant L. tropica line. Cell growth of either wild-type or resistant parasites was determined after incubation at 28°C for 72 h. Wild-type parasites (∗) were incubated in the presence of different concentrations of silybin derivatives. Resistant parasites were incubated with the same concentrations of silybin derivatives in the presence of 150 μM daunomycin. The results are expressed as the percentage of growth inhibition observed in each cell line compared to the absence of modulator (control cells). The data are means with standard deviations for three experiments performed in duplicate.
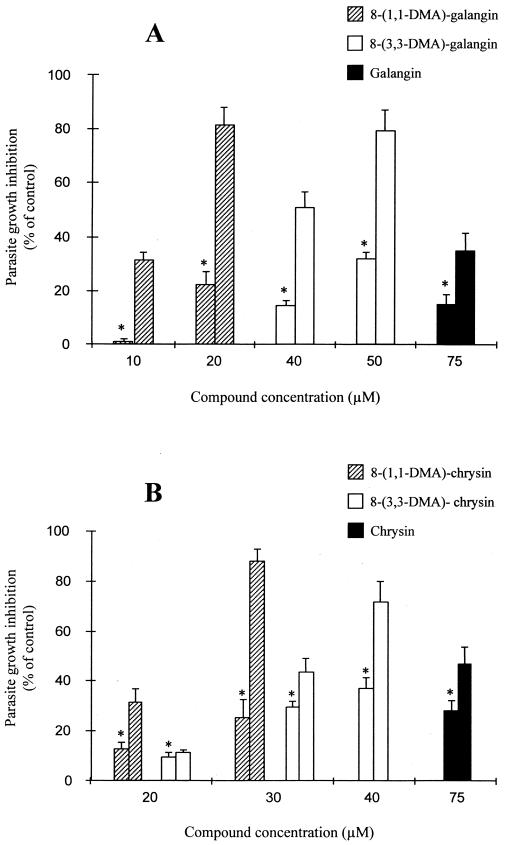
The reversal of parasite resistance to daunomycin was also tested with prenylated derivatives of galangin and chrysin. Figure 4A shows that higher concentrations of galangin derivatives were required to produce ca. 80% GI in the resistant line [20 μM 8-(1,1-DMA)-galangin or 50 μM 8-(3,3-DMA)-galangin] compared with 8-(3,3-DMA)-DHS (5 to 10 μM), while nonprenylated galangin produced a slight sensitization at 75 μM. The chrysin derivatives were even less efficient (Fig. 4B): 30 μM 8-(1,1-DMA)-chrysin produced a 88% GI in the resistant line, and 40 μM 8-(3,3-DMA)-chrysin gave 71% GI, but this concentration was relatively cytotoxic for the wild-type line. The nonsubstituted chrysin produced a small effect at 75 μM. Interestingly, in both galangin and chrysin derivatives, the 1,1-DMA substitution gave better sensitivity to daunomycin that the 3,3-DMA substitution.
FIG. 4.
Sensitization of growth caused by prenylated derivatives lacking the monolignol unit. Wild-type (∗) and resistant parasites were incubated under the conditions of Fig. 3 with different prenylated derivatives of galangin (A) and chrysin (B). The data are means with standard deviations for three experiments performed in duplicate.
DISCUSSION
We show here that oxidized and prenylated derivatives of the therapeutic agent silybin exhibit high binding affinity to the recombinant cytosolic domain of Leishmania Pgp-like transporter and revert the MDR of an L. tropica line that overexpresses the transporter, drawing attention to the importance of the monolignol unit within the above compounds.
Silybin is a natural flavanolignan with many positive therapeutic properties and few adverse effects in animals and humans. Although its cellular target is unknown, it increased the cytotoxicity of doxorubicin in a doxorubicin-resistant cell line (36). In order to circumvent MDR phenotype in Leishmania, we have studied silybin binding to recombinant NBD2 of the L. tropica multidrug transporter, the effects of its oxidation to DHS, and the effects of different hydrophobic substitutions on ring A, previously described as critical for the increase the binding of flavones to the cytosolic domain of Leishmania Pgp-like transporter (33). These results, together with comparative data for derivatives lacking the monolignol unit, provide important structure-activity information. (i) First of all, these results demonstrate the significance of the oxidation of 2,3-bond within the silybin ring C to its corresponding flavonol DHS, which may reinforce the mimicry of the adenine moiety of ATP, as previously suggested from the higher binding affinity of the flavone apigenin compared to its reduced analogue naringenin (7, 33). Conversely, the reduction of the 2,3-double bond of flavones to give flavanones resulted in a decrease of the competitive inhibition of H+,K+-ATPase with respect to ATP (29). (ii) They demonstrate the importance of the addition of a prenyl or geranyl hydrophobic substituent on ring A that could increase the interaction with the hydrophobic region vicinal to the ATP site (33). (iii) The monolignol unit (rings D and E in Fig. 1A and B) within flavanolignans produces significant effects with a five- to sixfold-higher affinity for 8-(3,3-DMA)-DHS with respect to 8-(3,3-DMA)-galangin and a fourfold higher affinity for DHS with respect to galangin. Additional studies are needed to determine its specific role in the interaction with the domain. (iv) These results further demonstrate the preference for hydrophobic substitution at position 8 over position 6 of ring A, suggesting some differences in binding orientation of the differently substituted compounds. (v) These results show the more efficient effect of prenylation compared to geranylation despite a lower hydrophobicity. (vi) Finally, these studies show the systematically more efficient effect of the 1,1-DMA compared to 3,3-DMA.
Similar in vitro results with some of these silybin derivatives have been obtained in parallel studies with the cytosolic domain of mammalian Pgp (26), except that the geranyl substitution was more efficient than the prenyl one. These differential results may indicate some differences between the cytosolic domains of Leishmania and mammalian transporters, possibly at the level of the hydrophobic interacting region.
The same sequence in efficiency has also been obtained from the in vitro sensitization studies in an MDR Leishmania line, so that compounds that display higher binding affinity for the recombinant NBD2 most efficiently sensitize the MDR phenotype. Thus, although the reversing effects of the compounds could in some cases be partially covered by their intrinsic cytotoxicity, as was observed for geranyl-DHS, the importance of the monolignol unit is evident from the higher reversion of resistance obtained with 8-(3,3-DMA)-DHS with respect to 8-(3,3-DMA)-galangin, as well as the role of prenylation, especially 1,1-DMA, at position 8 of ring A. Thus, 8-(3,3-DMA)-DHS is the most active MDR-sensitizing agent ever described for Leishmania. Work is in progress to synthesize the 8-(1,1-DMA)-DHS derivative that would probably bind with even higher affinity to the domain and sensitize MDR at even lower concentrations. The single exception to the correlation between binding to NBD2 and MDR sensitization was the absence of any reversion by DHS. This appears to be due to the high cytotoxicity caused by this compound (ca. 62% GI in wild-type parasites at 20 μM) compared to silybin (29% at 300 μM). Similarly, a fourfold-higher GI for DHS compared to that for silybin has been described in a human ovarian carcinoma cell line (36). This effect could be due to higher mimicry with ATP after oxidation of the silybin 2,3-bond, thereby favoring additional binding to other ATP-binding proteins. Indeed, flavonols such as DHS, with a hydroxyl at position 3 and a 2,3-double bond in addition to the hydroxyl at position 5 and the ketone at position 4, contain all the requirements to bind to ATP-binding site, as previously shown not only for L. tropica (33) and mammalian (7, 11) multidrug transporters but also for crystallized CDK2 (10) and HcK (40).
We propose that prenylation of the ring A within these compounds might generate more specific inhibitors of Pgp-like transporters by strengthening the interaction with its cytosolic domains, possibly with the hydrophobic region vicinal to the ATP site. This would, on the one hand, increase the reversal efficiency on the MDR parasites and, on the other hand, lower the affinity for other cellular ATP-binding proteins, as deduced from the significant decrease of the cytotoxicity on wild-type parasites after prenylation (ca. 10% GI for 40 μM 6-prenyl-DHS compared to nearly 100% GI for DHS at the same concentration; data not shown)
A number of observations have indicated flavonoid antagonism toward ATP: (i) the binding of kaempferide or derivatives to recombinant NBD2 from either mammalian (7) or Leishmania (33) multidrug transporter was partly prevented or displaced by ATP; (ii) flavonoid inhibition of several ATP-utilizing enzymes involves competitive interaction at the ATP-binding site (28), as studied in detail for H+,K+-ATPase (29) and as clearly demonstrated by crystallization experiments with the protein kinases CDK2 (10) and Hck (40); and (iii) the flavonol quercetin, on the one hand, competitively inhibited the ATPase activity of a recombinant NBD2 from the cystic fibrosis transmembrane conductance regulator (another ABC protein) (35) and, on the other hand, prevented Hoechst 33342 transport by mammalian Pgp, partly by inhibiting its ATPase activity (38). We show here that the sensitization caused by silybin derivatives in an MDR L. tropica line correlates with their affinity of binding to the cytosolic NBD2, which is consistent with a previous correlation between the binding affinity of other flavonoids for NBD2 and the increase of intracellular daunomycin accumulation monitored by flow cytometry (33). All of these results suggest that the reversion of drug resistance caused by silybin derivatives was produced by direct interaction with the nucleotide-binding domain of the Pgp-like transporter. However, other factors such as interaction with membrane phospholipids, altering the lipid packing density and the diffusion rate of the drug (6), or modulation of the multidrug transporter expression (22) may contribute to the observed sensitization of MDR. In addition, it is possible that prenylated derivatives might mainly interact at the hydrophobic region characterized within the NBDs or bind to the drug-binding sites within the transmembrane domains of the Pgp-like multidrug transporter. Quercetin has indeed been proposed to interact at the mammalian Pgp site where the transported drugs Hoechst 33342 and colchicine bind, which stimulated the transport of anthracyclines (38). Additional work is therefore required to further characterize the flavonoid-binding site(s).
In India, recent clinical studies showed that approximately 50% of patients with leishmaniasis fail to achieve parasite clearance after a standard dose of pentavalent antimonials (42). The in vivo data are supported by in vitro monitoring of drug sensitivity of fresh clinical isolates obtained at the same study site (24). The high rate of therapeutic failure to these usual drugs in cases of leishmaniasis call for new rational approaches to develop alternative drugs. Some pharmaceutical compounds, such as azoles and rifampin (27), and other drugs considered as potential leishmanicidal agents, such as doxorubicin (23), taxol (21) and alkyl lysophospholipids (44), are known substrates of Pgps or other ABC transporters and thus could induce an MDR phenotype. The development of inhibitors which block the MDR mechanism is a promising potential way to circumvent resistance to drugs. Our studies also provide useful models for understanding how similar defense mechanisms can be overcome in other protozoan parasites such as Plasmodium, Entamoeba, and Trichomonas spp., where ABC transporters have been associated with drug resistance.
ACKNOWLEDGMENTS
This work was supported by the Spanish Grants PM97-0139 (F.G.), PM98-0115 (S.C.), and CICYT-FEDER IFD97-0747-C04-03 (S.C.) and the Convenio CSIC-CNRS between F.G. and A.D.P. (1997–2000). J.M.P.-V. received a fellowship from the Junta de Andalucía (Spain), F.J.P.-V. received a fellowship from the Ministerio de Educación y Cultura (Spain), G.C. received fellowship from the Ligue Nationale contre le Cancer (Haute-Savoie, France), and M.M. received a fellowship from the French Ministry of National Education and Technological Research. Financial support from the Association pour la Recherche sur le Cancer (ARC 9147) and the Ligue Nationale contre le Cancer (Rhône 1999) is also acknowledged.
We thank Pilar Navarro for the help in parasite culture. We also acknowledge Pharmacia & Upjohn (Barcelona, Spain) for providing the daunomycin used in this study and Brian Monk for improving the English of the manuscript.
REFERENCES
- 1.Ambudkar S V, Dey S, Hrycyna C A, Ramachandra M, Pastan I, Gottesman M. Biochemical, cellular and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 2.Barron D, El Aidi C, Mariotte A M. 13C nuclear magnetic resonance analysis of two prenyl flavonols from Platanus acerifolia buds. Phytochem Anal. 1994;5:309–314. [Google Scholar]
- 3.Barron D, Mariotte A M. Syntheses of 8-C-(1,1-dimethylallyl) flavones and 3-methyl flavonols. Nat Prod Lett. 1994;4:21–28. [Google Scholar]
- 4.Chiquero M J, Pérez-Victoria J M, O'Valle F, Gonzalez-Ros J M, del Moral R, Ferragut J A, Castanys S, Gamarro F. Altered drug membrane permeability in a multidrug-resistant Leishmania tropica line. Biochem Pharmacol. 1998;55:131–139. doi: 10.1016/s0006-2952(97)00385-7. [DOI] [PubMed] [Google Scholar]
- 5.Chow L M C, Wong A K C, Ullman B, Wirth D F. Cloning and functional analysis of an extrachromosomally amplified multidrug resistance-like gene in Leishmania enrietti. Mol Biochem Pharmacol. 1993;60:195–208. doi: 10.1016/0166-6851(93)90131-g. [DOI] [PubMed] [Google Scholar]
- 6.Clarke R, Van den Berg H W, Murphy R F. Reduction of the membrane fluidity of human breast cancer cells by tamoxifen and 17 beta-estradiol. J Natl Cancer Inst. 1990;82:1702–1705. doi: 10.1093/jnci/82.21.1702. [DOI] [PubMed] [Google Scholar]
- 7.Conseil G, Baubichon-Cortay H, Dayan G, Jault J M, Barron D, Di Pietro A. Flavonoids: a new class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA. 1998;95:9831–9836. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Critchfield J W, Welsh C J, Phang J M, Yeh G C. Modulation of adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells: activation of P-glycoprotein as a possible mechanism. Biochem Pharmacol. 1994;48:1437–1445. doi: 10.1016/0006-2952(94)90568-1. [DOI] [PubMed] [Google Scholar]
- 9.Dayan G, Jault J M, Baubichon-Cortay H, Baggetto L G, Renoir J M, Baulieu E E, Gros P, Di Pietro A. Binding of steroid modulators to recombinant cytosolic domain from mouse P-glycoprotein in close proximity to the ATP site. Biochemistry. 1997;36:15208–15215. doi: 10.1021/bi9718696. [DOI] [PubMed] [Google Scholar]
- 10.De Azevedo W F, Jr, Mueller-Dieckmann H J, Schulze-Gahmen U, Worland P J, Sausville E, Kim S H. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc Natl Acad Sci USA. 1996;93:2735–2740. doi: 10.1073/pnas.93.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Pietro A, Dayan G, Conseil G, Steinfels E, Krell T, Trompier D, Baubichon-Cortay H, Jault J M. P-glycoprotein-mediated resistance to chemoterapy in cancer cells: using recombinant cytosolic domains to establish structure-function relationship. Braz J Med Biol Res. 1999;32:925–939. doi: 10.1590/s0100-879x1999000800001. [DOI] [PubMed] [Google Scholar]
- 12.Divita G, Goody R S, Gautheron D C, Di Pietro A. Structural mapping of catalytic site with respect to alpha-subunit and noncatalytic site in yeast mitochondrial F1-ATPase using fluorescence resonance energy transfer. J Biol Chem. 1993;268:13178–13186. [PubMed] [Google Scholar]
- 13.Endicott J A, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 14.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugere B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferté J, Kühnel J M, Chapuis G, Rolland Y, Lewin G, Schwaller M A. Flavonoid-related modulators of multidrug resistance: synthesis, pharmacological activity, and structure-activity relationships. J Med Chem. 1999;42:478–489. doi: 10.1021/jm981064b. [DOI] [PubMed] [Google Scholar]
- 16.Ford J M, Hait W N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42:155–199. [PubMed] [Google Scholar]
- 17.Henderson D M, Sifri C D, Rodgers M, Wirth D F, Hendrickson N, Ullman B. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol. 1992;12:2855–2865. doi: 10.1128/mcb.12.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirst S I, Stapley L A. Parasitology: the dawn of a new millennium. Parasitol Today. 2000;16:1–3. doi: 10.1016/s0169-4758(99)01589-6. [DOI] [PubMed] [Google Scholar]
- 19.Iovannisci D M, Ullman B. High efficiency plating method for Leishmania promastigotes in semi-defined or completely-defined medium. J Parasitol. 1983;69:633–636. [PubMed] [Google Scholar]
- 20.Jackson P R, Lawrie J M, Stiteler J M, Hawkins D W, Wohlhieter J A, Rowtin E D. Detection and characterization of Leishmania species and strains from mammals and vectors by hybridization and restriction endonuclease digestion of kinetoplast DNA. Vet Parasitol. 1986;20:195–215. doi: 10.1016/0304-4017(86)90100-7. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor P, Sachdeva M, Madhubala R. Effect of the microtubule stabilising agent taxol on leishmanial protozoan parasites in vitro. FEMS Microbiol Lett. 1999;176:429–435. doi: 10.1111/j.1574-6968.1999.tb13693.x. [DOI] [PubMed] [Google Scholar]
- 22.Kioka N, Hosokawa N, Komano T, Hirayoshi K, Nagata K, Ueda K. Quercetin, a bioflavonoid, inhibits the increase of human multidrug resistance gene (MDR1) expression caused by arsenite. FEBS Lett. 1992;301:307–309. doi: 10.1016/0014-5793(92)80263-g. [DOI] [PubMed] [Google Scholar]
- 23.Kole L, Das L, Das P K. Synergistic effect of interferon-gamma and mannosylated liposome-incorporated doxorubicin in the therapy of experimented visceral leishmaniasis. J Infect Dis. 1999;180:811–820. doi: 10.1086/314929. [DOI] [PubMed] [Google Scholar]
- 24.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 25.Luper S. A review of plants used in the treatment of liver diseases: part 1. Altern Med Rev. 1998;3:410–421. [PubMed] [Google Scholar]
- 26.Maitrejean M, Comte G, Barron D, El Kirat K, Conseil G, Di Pietro A. The flavanolignan silybin and its hemisynthetic derivatives, a novel series of potential modulators of P-glycoprotein. Bioorg Med Chem Lett. 2000;10:157–160. doi: 10.1016/s0960-894x(99)00636-8. [DOI] [PubMed] [Google Scholar]
- 27.Marsella R, Ruiz de Gopegui R. Leishmaniasis: a re-emerging zoonosis. Int J Dermatol. 1998;37:801–814. doi: 10.1046/j.1365-4362.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- 28.Middleton E, Kandaswami C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In: Harborne J B, editor. The flavonoids: advances in research since 1986. London, England: Chapman Hall; 1993. pp. 619–652. [Google Scholar]
- 29.Murakami S, Muramatsu M, Tomisawak K. Inhibition of gastric H+,K+-ATPase by flavonoids: a structure-activity study. J Enzyme Inhib. 1999;14:151–166. doi: 10.3109/14756369909036551. [DOI] [PubMed] [Google Scholar]
- 30.Ouellette M, Légaré D, Haimeur A, Grondin K, Roy G, Brochu C, Papadopoulou B. ABC transporters in Leishmania and their role in drug resistance. Drug Res Updates. 1998;I:43–48. doi: 10.1016/s1368-7646(98)80213-6. [DOI] [PubMed] [Google Scholar]
- 31.Pelter A, Hansel R. The structure of silybin (Silybum substance E6), the first flavanolignan. Tetrahedron Lett. 1968;1968:2911–2916. [Google Scholar]
- 32.Pepping J. Milk tistle: Silibum marianum. Am J Health-Syst Pharm. 1999;56:1195–1197. doi: 10.1093/ajhp/56.12.1195. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Victoria J M, Chiquero M J, Conseil G, Dayan G, Di Pietro A, Barron D, Castanys S, Gamarro F. Correlation between the affinity of flavonoids binding to cytosolic site of Leishmania tropica multidrug transporter and their efficiency to revert parasite resistance to daunomycin. Biochemistry. 1999;38:1736–1743. doi: 10.1021/bi982455v. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Victoria J M, Tincusi B M, Jiménez I A, Bazzocchi I L, Gupta M P, Castanys S, Gamarro F, Ravelo A G. New natural sesquiterpenes as modulators of daunomicyn resistance in a multidrug-resistant Leishmania tropica line. J Med Chem. 1999;42:4388–4393. doi: 10.1021/jm991066b. [DOI] [PubMed] [Google Scholar]
- 35.Randack C, Auerswald E A, Assfalg-Machleidt Y, Reenstra W W, Machleidt W. Inhibition of ATPase, GTPase and adenylate kinase activities of the second nucleotide-binding fold of the cystic fibrosis transmembrane conductance regulator by genistein. Biochem J. 1999;340:227–235. [PMC free article] [PubMed] [Google Scholar]
- 36.Scambia G, De Vincenzo R, Ranelletti F O, Panici P B, Ferrandina G, D'Agostino G, Fattorossi A, Bombardelli E, Mancuso S. Antiproliferative effect of silybin on gynaecological malignacies: synergism with cisplatin and doxorubicin. Eur J Cancer. 1996;32:877–882. doi: 10.1016/0959-8049(96)00011-1. [DOI] [PubMed] [Google Scholar]
- 37.Scambia G, Ranelletti F O, Benedetti Panici P, De Vincenzo R, Bonanno G, Ferrandina G, Piantelli M, Bussa S, Rumi C, Cianfriglia M, Mancuso S. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol. 1994;34:459–464. doi: 10.1007/BF00685655. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro A B, Ling V. Effect of quercetin on Hoechst 33342 transport by purified and reconstituted P-glycoprotein. Biochem Pharmacol. 1997;53:587–596. doi: 10.1016/s0006-2952(96)00826-x. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro A B, Ling V. Positivily cooperative sites for drug transport by P-glycoprotein with disting drug specificities. Eur J Biochem. 1997;250:130–137. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 40.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 41.Skottova N, and Krecman V. Silymarin as a potential hypocholesteroalemic drug. Physiol Res. 1998;47:1–7. [PubMed] [Google Scholar]
- 42.Sundar S, Singh V P, Murray H W. Current epidemic of visceral leishmaniasis in India. Acta Parasitol Turc. 1997;21:128. [Google Scholar]
- 43.Ullman B. Multidrug resistance and P-glycoproteines in parasitic protozoa. J Bioenerg Biomembr. 1995;27:77–84. doi: 10.1007/BF02110334. [DOI] [PubMed] [Google Scholar]
- 44.Urbina J A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;114:91–99. [PubMed] [Google Scholar]
- 45.Wilson C M, Serrano A E, Wasley A, Bogenschutz M P, Shankar A H, Wirth D F. Amplification of a gene related to the mammalian mdr genes in drug resistance Plasmodium falciparum. Science. 1989;244:1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- 46.Zi X, Agarwal R. Silybinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci USA. 1999;96:7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]