Abstract
Objective:
To evaluate the relationship between hypertensive diseases in pregnancy and kidney function later in life.
Methods:
We evaluated measured GFR using iothalamate urinary clearance in 725 women of the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Women were classified by self-report as nulliparous (n=62), a history of normotensive pregnancies (n=544), a history of hypertensive pregnancies (n=102), or a history of preeclampsia (n= 17). We compared adjusted associations among these 4 groups with mGFR using generalized estimating equations to account for familial clustering. Chronic Kidney Disease (CKD) was defined as mGFR of less than 60 ml/min/1.73 m2 or Urinary Albumin-Creatinine Ratio (UACR) ≥ 30 mg/g.
Results:
Among women with kidney function measurements (mean age 59 ± 9 years, 53% African American), those with a history of hypertensive pregnancy had lower mGFR (–4.66 ml/min/1.73 m2, 95% CI −9.12, −0.20) compared to women with a history of normotensive pregnancies. Compared to women with a history of normotensive pregnancies, women with a history of hypertensive pregnancy also had higher odds of mGFR < 60 ml/min/1.73 m2 (OR 2.09, 95% CI 1.21, 3.60). Additionally, women with a history of hypertensive pregnancy had greater odds for CKD (OR 4.89, 95% CI 1.55, 15.44), after adjusting for age, race, education, smoking history, hypertension, body mass index, and diabetes.
Conclusions:
A history of hypertension in pregnancy is an important prognostic risk factor for kidney disease. To our knowledge, this is the first and largest investigation demonstrating the association between HDP and subsequent kidney disease using measured GFR in a large biracial cohort.
Keywords: Pregnancy, Hypertension, Kidney function, Chronic Kidney Disease, Genetic Epidemiology Network of Arteriopathy
INTRODUCTION
Kidney disease is the ninth leading cause of death in the United States.1 Among 15% of United States adults affected by kidney disease, many are women and unaware that they have kidney disease.2 Traditional risk factors such as diabetes mellitus and hypertension are well established in the evolution of kidney disease. However, the long-term effects of hypertensive diseases in pregnancy (HDP) on kidney function have not been systematically examined, particularly in well characterized multiracial cohorts.
HDP constitute the spectrum of chronic hypertension, gestational hypertension, preeclampsia-eclampsia, and chronic hypertension with superimposed preeclampsia. Abnormal placentation has been implicated as the cause of the hemodynamic changes resulting in cardiac and kidney damage in HDP.3 However, traditional wisdom has questioned whether these changes resolve completely after delivery or if a history of HDP may lead to kidney dysfunction, particularly, later in life.
Women with a history of HDP have greater risk for stroke and cardiovascular diseases later in life. 3–12 Despite this evidence, few studies have examined the long-term effects of HDP specifically on kidney function. Some studies have shown that a history of preeclampsia is associated with an increased risk of albuminuria13,14 and a prior study also demonstrated an increased risk of albuminuria later in life in women with a history of hypertension in pregnancy.15 These studies have been limited in that most of them have examined this association without using estimated or measured glomerular filtration rate (GFR) to assess kidney function.
Therefore, we assessed the long-term effects of HDP on kidney function, independent of traditional risk factors, in a well characterized biracial cohort of women using measured GFR using iothalamate urinary clearance (mGFR). Additionally, we assessed the long-term effects of HDP on kidney function by Cystatin C estimations of GFR because of its clinical accessibility. We hypothesized that women with a history of HDP would be more likely to have reduced kidney function compared to women with a history of normotensive pregnancies.
METHODS
Study population
The Genetic Epidemiology Network of Arteriopathy (GENOA) study is one of the four research networks of the National Heart, Lung, and Blood Institute (NHLBI) Family Blood pressure program (FBPP) established in 1995 to investigate the genetic determinants of hypertension among multiple racial groups. GENOA recruited sibships in which at least two siblings had essential hypertension diagnosed before the age of 60 years (Phase I, 1995–2000).16 All other siblings were invited to participate, irrespective of their hypertension status.17,18 These included African Americans from Jackson, Mississippi (N = 1854) and whites from Rochester, Minnesota (N = 1583). The GENOA study was approved by the Institutional Review Boards of each field center. All participants provided written informed consent.
Inclusion and Exclusion Criteria
We analyzed data from 1,578 participants that underwent GFR measurements during the third study examination. We excluded 534 men, 257 women without iothalamate clearance measurements, 58 women missing pregnancy history, and 4 missing data on gender at the third examination, leading to an analytic cohort of 725 participants in our study.
Pregnancy History
A validated standard questionnaire administered by a trained interviewer was used to collect information on HDP history. 19 Women who answered “Yes” to the question, “Have you had at least one pregnancy lasting more than 6 months?” were queried on the number of pregnancies and the development of hypertension in each pregnancy. Those who self-reported a previous history of hypertension in any pregnancy lasting more than 6 months were also asked if they had protein in their urine during the pregnancy and if they received a diagnosis of preeclampsia during any of the pregnancies. Pregnancy history categories were defined as nulliparous, normotensive pregnancies, hypertensive pregnancy, or preeclampsia.
Kidney Function Measurements
Blood and urine samples from each participant were collected and processed by standardized protocols after an overnight fast at the third examination. GFR was measured from the urinary clearance of iothalamate. A 300 mg subcutaneous dose of non-radiolabeled iothalamate was given to each participant in the upper outer arm, followed by a 45 min wait (equilibrium phase) to allow blood levels to become stable. A voided urine (UE) and a blood plasma sample (P1) were collected, during the equilibrium phase. After 45–75 minutes, when the participant had the urge to void, a second urine (U1) and a blood (P2) sample were collected, and urine volume was recorded. Incomplete bladder emptying (i.e. residual <10 mL or <10% of voided volume; and a minimum of 100 mL urine voided) was excluded by assessing bladder volume with an ultrasonic bladder scanner. Iothalamate was assayed with capillary electrophoresis using the Beckman Pace 2100 Analyzer and Beckman System Gold software (Fullerton CA) at the centralized laboratory in Mayo Clinic, Rochester, Minnesota. mGFR was calculated using the formula: 1) Volume of urine collected per minute or flow (mL/min) = volume U1 /collection time in mins; and 2) GFR (in mL/min) = (U1 x Flow)/(P1+P2)/2. mGFR was indexed to ideal BSA was expressed in ml/minute per 1.73 m2. 20,21,22
Details for estimating GFR using Cystatin C in the GENOA study have been described in previous studies.21 Briefly, serum cystatin C was measured by an automated particle-enhanced nephelometric immunoassay (Dade Behring BN II Nephelometer; Dade Behring, Deerfield IL) at the Department of Laboratory Medicine and Pathology in Mayo Clinic, Rochester, Minnesota. Cystatin C-estimated GFR was calculated with the CKD-EPI cystatin C equation.23
Urinary albumin was measured by immunoturbidimetry utilizing an antibody to human albumin in an automated immunoprecipitin analysis system (Diasorin Inc., Stillwater, MN) and urinary creatinine was measured using a Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis IN) through an automated kinetic, fixed-time spectrophotometric method at the centralized laboratory in Mayo Clinic, Rochester, Minnesota. Concurrently, quality control samples with different urinary albumin and creatinine concentrations were run each day during the measurements. All samples were measured in duplicate, and the mean of the duplicates were recorded. Urinary Albumin-Creatinine Ratio (UACR) was calculated by dividing albumin concentration in milligrams by urine creatinine concentration in grams. 24,25
Clinical Covariates
We defined CKD as GFR of less than 60 ml/min/1.73 m2 or UACR ≥ 30 mg/g. 26,27 “We defined hypertension as an average systolic blood pressure≥140 mmHg or diastolic blood pressure ≥90 mmHg from two measurements, previous diagnosis of hypertension or use of blood pressure–lowering medication, and diabetes as fasting glucose ≥126 mg/dL or use of diabetes medications. Body mass index (BMI) was calculated as weight (kg)/height (m)2. We defined “ever smoker” as participants who reported ever smoking more than 100 cigarettes. Dyslipidemia was defined as use of lipid-lowering medication, fasting serum triglyceride ≥1.69 mmol/L, fasting serum total cholesterol ≥5.18 mmol/L, or fasting serum high-density lipoprotein (HDL) concentration <1.04 mmol/L. Coronary heart disease (CHD) was defined by self-report of coronary angioplasty, coronary bypass surgery, balloon dilatation, stent placement, or history of myocardial infarction. Self-report of cerebral hemorrhage and/or stroke defined cerebrovascular disease. Age, education level, medication use, personal and family histories, history of menopause and hormone replacement were obtained using standardized questionnaires”.28
Statistical Analysis
We compared the baseline characteristics of participants across pregnancy history categories with a “single” reference group (normotensive pregnancy) using the Kruskal-Wallis tests, Chi-square test and Fisher’s exact tests, as appropriate based on the underlying distribution. Continuous variables were presented as means with standard deviations. Categorical variables in frequencies and percentages.
Adjusted associations for kidney function measurements were compared among these groups with generalized linear models utilizing generalized estimating equations with Huber-White sandwich robust estimators to account for the correlation among siblings.. We sequentially adjusted the models as follows: Model 1: age, and race; and Model 2: additionally, adjusting for education, smoking, hypertension, BMI, and diabetes. Appropriate families and link functions were used in our models for continuous and categorical outcomes. We assessed effect modification by race/ethnicity by adding an interaction term between HDP and race-ethnicity. Plots of adjusted average kidney function measurements with 95% confidence bands across pregnancy history categories were constructed to visually depict results from Model 2. All statistical analyses were performed with STATA version 15 (STATA Corp, College Station, TX). A 2-sided P value <0.05 was considered statistically significant.
RESULTS
Among 725 women (mean age 60 ± 9 years at the time of kidney function measurements), 550 (53%) were African American, 62 (9%) were self-identified as nulliparous, 544 (75%) reported normotensive pregnancies, 102 (14%) reported at least one hypertensive pregnancy, and 17 (2%) reported at least one pregnancy with preeclampsia (Table 1). Women with a history of preeclampsia were more likely to be ever smokers and to have cerebrovascular disease compared to other groups. Women with a history of a hypertensive pregnancy were more likely to be hypertensive at the time of the exam (p<0.001). Nulliparous women were more likely to have completed higher levels of education compared to other groups.
TABLE 1:
Baseline characteristics of participants
| VARIABLE | Normotensive Pregnancy (N= 544) | Nulliparous (N= 62) | Hypertensive Pregnancy (N= 102) | Preeclamptic Pregnancy (N= 17) | P-value |
|---|---|---|---|---|---|
| Age (years) | 60 (8) | 54 (10) | 59 (9) | 58 (9) | < .001 |
| Race, n (%) | |||||
| African American | 306 (56.2) | 18 (29.0) | 55 (53.9) | 5 (29.4) | |
| White | 238 (43.8) | 44 (71.0) | 47 (46.1) | 12 (70.6) | |
| Education, n (%) | .03 | ||||
| Less than 8 years | 15 (2.8) | 2 (3.2) | 7 (6.9) | 1 (5.9) | |
| 9–11 years | 55 (10.1) | 2 (3.2) | 12 (11.8) | 2 (11.7) | |
| 12 years/GED | 202 (37.1) | 14 (22.6) | 32 (31.3) | 3 (17.7) | |
| > 12 years | 272 (50.0) | 44 (71.0) | 51 (50.0) | 11 (64.7) | |
| History of smoking, n (%) | 189 (34.7) | 22 (35.5) | 22 (21.6) | 7 (41.2) | .05 |
| Body Mass Index, n (%) | .02 | ||||
| Underweight (BMI <18.5) | 3 (0.6) | 0 | 0 | 0 | |
| Normal Weight (BMI =18.5–24.9) | 84 (15.4) | 13 (21.0) | 9 (8.8) | 3 (17.7) | |
| Overweight (BMI = 25.0 − <30) | 185 (34.0) | 19 (30.7) | 20 (19.6) | 3 (17.7) | |
| Obese (BMI ≥ 30) | 272 (50.0) | 30 (48.3) | 73 (71.6) | 11 (64.6) | |
| Hypertension, n (%) | 356 (65.4) | 42 (67.7) | 94 (92.2) | 14 (82.4) | < 0.001 |
| Dyslipidemia, n (%) | 420 (77.2) | 48 (77.4) | 65 (63.7) | 11 (64.7) | 0.03 |
| Diabetes, n (%) | 81 (14.9) | 7 (11.29) | 19 (18.6) | 5 (29.4) | 0.38 |
| Aspirin Use, n (%) | 159 (29.2) | 19 (30.7) | 35 (34.3) | 6 (35.3) | 0.88 |
| Hormone Replacement Therapy, n (%) | 191 (35.1) | 27 (43.6) | 34 (33.3) | 7 (41.2) | 0.53 |
| Family History of Hypertension, n (%) | 523 (96.1) | 59 (95.2) | 101 (99.0) | 17 (100) | 0.41 |
| Family History of CHD, n (%) | 280 (51.5) | 31 (50.0) | 56 (54.9) | 10 (58.8) | 0.86 |
| Family History of Diabetes, n (%) | 328 (60.3) | 28 (45.2) | 55 (53.9) | 11 (64.7) | 0.10 |
| History of CHD, n (%) | 17 (3.1) | 3 (4.8) | 4 (3.9) | 0 (0) | 0.76 |
| History of CVD, n (%) | 9 (1.7) | 2 (3.2) | 1 (1.0) | 3 (17.7) | < 0.01 |
n=725; The P-values of Continuous variables were calculated with Kruskal-Wallis test and the differences between categorical variables were tested with the Chi-square test and Fisher’s exact tests. Results are reported as means with standard deviations, frequencies, and percentages for categorical variables.
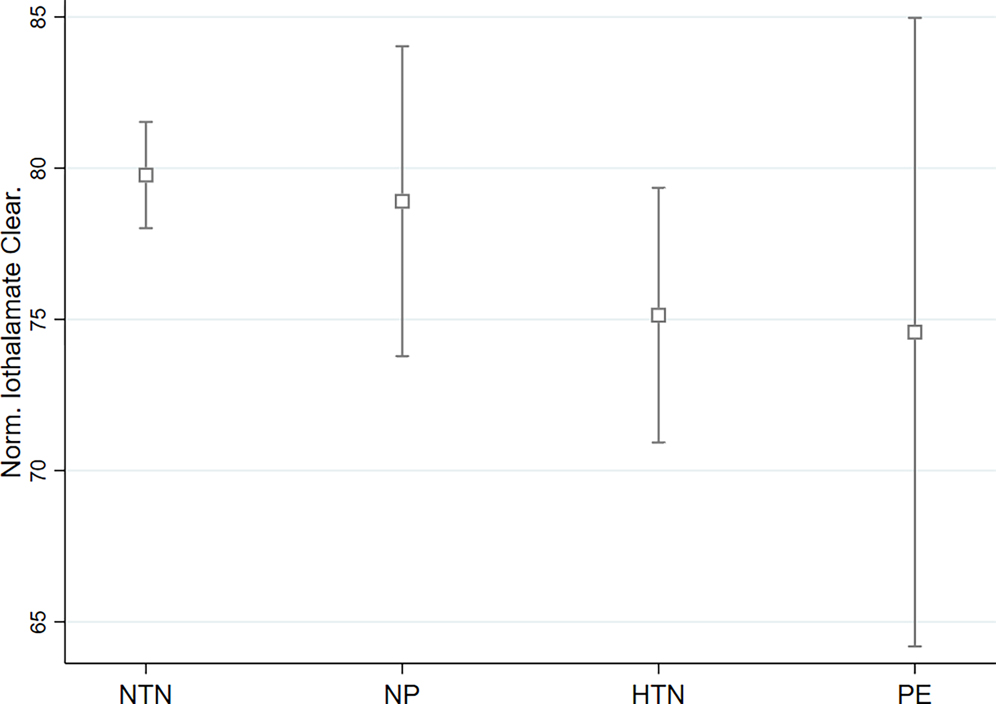
After adjusting for age, and race (Model 1), women with a history of at least one hypertensive pregnancy had lower mGFR (p = 0.02) compared to women with a history of normotensive pregnancies (Table 2). With further adjustment for age, race, education, smoking history, hypertension, BMI, and diabetes (model 2), women with a history of at least one hypertensive pregnancy had lower mGFR (p = 0.04) compared to women with a history of normotensive pregnancies (Table 2). Plots for adjusted average measures of kidney function with 95% confidence bands across pregnancy history categories from Model 2 (adjusting for age, race, education, smoking history, hypertension, BMI, and diabetes) are shown in Figure 1 and Supplemental Figure 1.
TABLE 2:
Differences in mGFR <60 ml/1.73m2 Later in Life Across Pregnancy History categories Compared to Women with Normotensive pregnancies *
| Model | Normotensive pregnancy | Nulliparous | Hypertensive pregnancy | Preeclamptic Pregnancy |
|---|---|---|---|---|
| Unadjusted | reference | 3.06 (−2.63, 8.75) P=.29 | −5.25 (−9.80, −0.69) P=.02 | −4.13 (−14.68, 6.42) P= .44 |
| Model 1 | reference | −1.46 (−6.96,4.03) P=.60 | −5.36 (−9.68, −1.03) P=.02 | −6.07 (−16.02, 3.87) P=.23 |
| Model 2 | reference | −1.14 (−6.64,4.35) P=.68 | −4.66 (−9.12, −0.20) P=.04 | −5.45 (−15.41, 4.50) P=.28 |
All values for mGFR are expressed as adjusted β coefficients, with 95% confidence intervals and P−values; P−values in bold are significant; Model 1: adjusted for age and race; Model 2: plus level of education, smoking history, hypertension, Body Mass Index (BMI), and diabetes
mean age of the participants at the time of kidney function measurements was 59 ± 9 years
Figure 1.
Plots for adjusted average measures of kidney function with 95% confidence bands across pregnancy history categories (from Model 2)
NP- Nulliparous; NTN-Normotensive Pregnancy; HTN-Hypertensive pregnancy; PE-Preeclamptic Pregnancy
After adjusting for age and race (Model 1), women with a history of at least one hypertensive pregnancy had higher odds of mGFR < 60 ml/min/1.73 m2 (p<0.01), and compared to women with a history of normotensive pregnancies (Table 3). With further adjustment for age, race, education, smoking history, hypertension, BMI, and diabetes (Model 2), women with a history of at least one hypertensive pregnancy also had higher odds of mGFR < 60 ml/min/1.73 m2 (p= 0.01) compared to women with a history of normotensive pregnancies. We observed a similar trend with participants with a history of preeclampsia. However, our results did not achieve statistical significance due to the modest number of participants in this category with mGFR assessments for our study.
TABLE 3:
Odds of mGFR <60 ml/1.73m2 Later in Life Across Pregnancy Groups with Normotensive pregnancies as the reference group*
| Model | Normotensive pregnancy | Nulliparous | Hypertensive pregnancy | Preeclamptic Pregnancy |
|---|---|---|---|---|
| Unadjusted | 1 (reference) | 0.95 (0.47, 1.93) P=.91 | 1.91 (1.16, 3.14) P=.01 | 1.65 (0.51, 5.24) P=.40 |
| Model 1 | 1 (reference) | 1.38 (0.67, 2.86) P=.37 | 2.00 (1.20, 3.34) P<.01 | 2.06 (0.62, 6.89) P=.24 |
| Model 2 | 1 (reference) | 1.49 (0.73, 3.06) P=.27 | 2.09 (1.21, 3.60) P=.01 | 2.12 (0.69, 6.52) P=.19 |
All values for mGFR are expressed as adjusted OR, Odds ratios, with 95% confidence intervals and P-values; P-values in bold are significant; Model 1: adjusted for age and race; Model 2: plus level of education, smoking history, hypertension, Body Mass Index (BMI), and diabetes
mean age of the participants at the time of kidney function measurements was 59 ± 9 years
Our results for participants that had Cystatin C estimations of GFR are presented Supplemental Table 1 and 2.
In a subset of our analytic cohort (participants with available data on both mGFR and UACR; n= 380), women with a history of at least one hypertensive pregnancy had significantly greater odds of CKD (p<0.01) compared to women with a history of normotensive pregnancies, after adjusting for age, race, education, smoking history, hypertension, BMI, and diabetes (Model 2) (Table 4). Similar results were observed in the age and race adjusted model (Model 1).
TABLE 4:
Odds of chronic kidney disease (CKD)* later in life related to history of hypertensive disease in pregnancy.
| Model | Normotensive pregnancy | Nulliparous | Hypertensive pregnancy |
|---|---|---|---|
| Unadjusted | 1 (reference) | 2.32 (0.26, 20.43) P=.45 | 5.14 (1.65, 16.00) P<.01 |
| Model 1 | 1 (reference) | 2.44 (0.27, 21.54) P=.42 | 5.03 (1.61, 15.74) P<.01 |
| Model 2 | 1 (reference) | 2.26 (0.27, 18.88) P=.45 | 4.89 (1.55, 15.44) P<.01 |
n=380; All values for mGFR are expressed as adjusted OR, Odds ratios, with 95% confidence intervals and P-values; P-values in bold are significant; Model 1:adjusted for age and race; Model 2: plus level of education, smoking history, hypertension, Body Mass Index (BMI), and diabetes
CKD defined as mGFR <60 ml/min/1.73 m2 or UACR ≥30 mg/g
mean age of the participants at the time of kidney function measurements was 59 ± 9 years
A summary of the number of participants with abnormal kidney function measurements by pregnancy history category are also presented Supplemental Table 3 and our results when we combined women with a history of hypertensive pregnancies and those with a history of preeclampsia, collectively as “HDP” category and compared them with women with a history of normotensive pregnancies (our reference group) are also presented in Supplemental Table 4, 5, and 6.
Testing for Interaction
We did not observe any statistically significant interaction between race and pregnancy history category in our analysis for any measure of kidney function (all p >0.11).
DISCUSSION
Our findings from this well characterized biracial cohort support our hypothesis that a history of hypertension in pregnancy is associated with lower kidney function later in life and a higher odds of CKD. To our knowledge, this is first and largest investigation demonstrating the association between HDP and subsequent kidney disease using measured GFR in a large biracial cohort. Additionally, our study is the first to investigate the relationship between HDP and kidney disease later in life through the combination of mGFR and UACR measurements at a clinically relevant threshold. In a retrospective study of Taiwanese women with insurance claims data followed for about 6.3 years, women with a history of HDP had over double the risk for end-stage kidney disease compared with women with a history normotensive pregnancies.29 We found hypertensive pregnancy was associated with lower GFR compared with normotensive pregnancies, and about 5 fold higher odds of CKD. A major strength of our study was the use of mGFR.
A cross-sectional analysis of women in the Family Blood Pressure Program (FBPP) between 2000 and 2004, found a greater risk of albuminuria (urine albumin-creatinine ratio greater than 25 mg/g) in women with at least one hypertensive pregnancy compared to women with normotensive pregnancies. 15 In our study, we also found that a history of hypertension in pregnancy was associated with lower mGFR compared with women with normotensive pregnancies. However, we extended these findings using a direct measurement of GFR by iothalamate urinary clearance, in addition to eGFR. Furthermore, we examined HDP subtypes (preeclampsia and hypertensive pregnancy) to demonstrate possible distinct changes in kidney function among these groups.
Barrett and colleagues showed in a Swedish registry-based cohort study that women with a history of hypertensive pregnancy and preeclampsia had higher risk of developing CKD, with adjusted hazard ratios of 1.49 and 1.92, respectively, compared with women with normotensive pregnancies. 30 Our results (OR of 4.89 in history of hypertensive pregnancy versus normotensive pregnancies) suggest that a history of hypertensive pregnancy may have even greater impact on kidney function later in life, although these conclusions should be interpreted cautiously given the different study designs (retrospective cohort in theirs vs cross sectional in ours) and different statistical modeling. Further, our study was performed in a multi-racial cohort including 56% African American women who have higher rates of CKD. Additionally, we relied on kidney function measurements for the ascertainment of CKD among pregnancy history categories in our study.
Investigators from the Record linkage study evaluated the long term effects of HDP on kidney function. They reported that the odds of having an eGFR less than 60 ml/min/1.73 m2 was about 1.4 times in women with a history of hypertensive pregnancy and about 1.9 times in women with a history of preeclampsia compared to women who had normotensive pregnancies, in adjusted models. 31 We found that a history hypertensive pregnancy was associated with twice the odds of having an mGFR less than 60 ml/min/1.73 m2 later in life. Women with a history of preeclampsia also had twice the odds of having an mGFR of less than 60 ml/min/1.73 m2 in our study. However, this result did not achieve statistically significance, likely due to the small sample size of women in this category (about 2% of our study sample). Insomuch as the Record linkage study had a larger number of participants compared to ours, we adopted a different approach toward assessing kidney function in our study by defining CKD as “GFR of less than 60 ml/min/1.73 m2 or UACR ≥ 30 mg/g”, and we demonstrated that a history hypertensive pregnancy was associated with more risk for CKD (about 5 times increased odds compared to women with normotensive pregnancies) later in life using measured GFR. Additionally, the mean age of the women in their study was about 25 years vs 59 years, in ours (we had a older analytical cohort, giving room for better ascertainment of the relationship between a history of HDP and kidney function several decades later despite adjusting for age in our statistical models).
Our study extends the findings from previous observational studies about the relationship between HDP and kidney function by showing associations of hypertensive pregnancy with kidney function decline later in life before end stage kidney disease (ESKD) develops. Evaluation of kidney disease requires accurate and precise GFR determination. Thus, our use of measured GFR provides a superior approach of evaluating kidney function because of its better precision, accuracy, and its higher propensity for the detection of early kidney function decline compared with estimated GFR. 32–35
Further, our results support the American Heart Association’s recommendation for close monitoring of women with a history of HDP 36 and paves the way for future longitudinal studies to investigate the association between HDP and kidney function decline.
Our study has some limitations. Due to the cross-sectional design, we cannot infer causality and we cannot rule out residual confounding including pregnancy related factors and parity. The information concerning the age(s) when participants that conceived had their pregnancies were not captured in the GENOA study. Thus, we could not directly calculate the interval between each participant’s pregnancy and the time when GFR measurements were performed. Additionally, given that the GFR measurements in our study were assessed at a single time, we could not compare the rate of GFR decline over time among participants by pregnancy history category. The FBPP recruited participants through sibships from families with histories of hypertension; hence our findings may not be generalizable to populations with lower rates of hypertension. However, we tried to correct this by carefully adjusting for hypertension in our models. We had a small number of participants who had a history of preeclampsia. Thus, the study may have been underpowered to detect meaningful associations of preeclampsia with HDP subtypes and kidney function. Finally, as pregnancy history was ascertained by questionnaires, there is a possibility of misclassification due to recall bias. However, the validity of the questionnaires used in our study, has been shown to be consistently high from previous population-based studies. 15,19
CONCLUSION
In a well characterized biracial cohort, a history of HDP was associated with poorer kidney function later in life, compared to women with a history of normotensive pregnancies. However, this finding should be interpreted cautiously and requires further study.
Supplementary Material
Acknowledgments
We would like to thank the families that participated in the GENOA study.
Sources of Funding
This work was supported in part by grants U01-HL054463 (THM) from the National Heart Lung and Blood Institute of the National Institutes of Health. MEH has been supported by 1K08DK099415 from the National Institute of Diabetes and Digestive and Kidney Diseases and P20GM10357 and 5U54GM115428 from ttheNational Institutes of General Medical Sciences. STL is partially supported by the Mississippi Center for Clinical and Translational Research and Mississippi Center of Excellence in Perinatal Research COBRE funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers 5U54GM115428 and P20GM121334. VDG was supported by the grant R01HL136348 from the National Institutes of Health. STT was supported by the grant R01DK073537 from the National Institutes of Health R01DK073537
ABBREVIATIONS LIST
- AHA
American Heart Association
- BMI
Body mass index
- CKD
Chronic Kidney Disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- GFR
Glomerular Filtration Rate
- HDP
Hypertensive diseases in pregnancy
- HR
Hazard Ratio
- HX
History
- mGFR
GFR measured with iothalamate urinary clearance
- NP
Nulliparous
- NTN
Normotensive Pregnancy
- HTN
Hypertensive pregnancy
- PE
Preeclamptic Pregnancy
- UACR
Urinary Albumin-Creatinine Ratio
Footnotes
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures
None
REFERENCES
- 1.Centers for Disease Control and Prevention. Chronic Kidney Disease Initiative. Chronic Kidney Disease Basics. Available from: https://www.cdc.gov/kidneydisease/basics.html. Accessed January 20,2020 [Google Scholar]
- 2.Centers for Disease Control and Prevention. Chronic Kidney Disease Initiative. Chronic Kidney Disease in the United States, 2019. Available from: https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf. Accessed January 20,2020 [Google Scholar]
- 3.Furuya M, Ishida J, Aoki I, Fukamizu A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vascular health and risk management. 2008;4(6):1301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG. 2005. Mar;112(3):286–92 [DOI] [PubMed] [Google Scholar]
- 5.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008. Nov;156(5):918–30. [DOI] [PubMed] [Google Scholar]
- 6.Garovic VD, Bailey KR, Boerwinkle E, Hunt SC, Weder AB, Curb D, Mosley TH Jr, Wiste HJ, Turner ST. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens. 2010. Apr;28(4):826–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Therapeutic advances in cardiovascular disease. 2008. Aug;2(4):249–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007. Nov 10;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001. Jun 23;357(9273):2002–6 [DOI] [PubMed] [Google Scholar]
- 10.Jónsdóttir LS, Arngrímsson R, Geirsson RT, Sigvaldason H, Sigfússon N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995. Nov;74(10):772–6 [DOI] [PubMed] [Google Scholar]
- 11.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003. Apr 19;326(7394):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Männistö T, Mendola P, Vääräsmäki M, Järvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013. Feb 12;127(6):681–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nisell H, Lintu H, Lunell NO, Möllerström G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol. 1995. Nov;102(11):876–81 [DOI] [PubMed] [Google Scholar]
- 14.Bar J, Kaplan B, Wittenberg C, Erman A, Boner G, Ben-Rafael Z, et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant. 1999. May;14(5):1129–32 [DOI] [PubMed] [Google Scholar]
- 15.Kattah AG, Asad R, Scantlebury DC, Bailey KR, Wiste HJ, Hunt SC, et al. Hypertension in pregnancy is a risk factor for microalbuminuria later in life. J Clin Hypertens (Greenwich). 2013. Sep;15(9):617–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Investigators FBPP. Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP). Hypertension. 2002. Jan;39(1):3–9 [DOI] [PubMed] [Google Scholar]
- 17.Kamimura D, Suzuki T, Wang W, deShazo M, Hall JE, Winniford MD, Kullo IJ, et al. Higher plasma leptin levels are associated with reduced left ventricular mass and left ventricular diastolic stiffness in black women: insights from the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Hypertens Res. 2018. Aug;41(8):629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamimura D, Suzuki T, Furniss AL, Griswold ME, Kullo IJ, Lindsey ML, et al. Elevated serum osteoprotegerin is associated with increased left ventricular mass index and myocardial stiffness. J Cardiovasc Med (Hagerstown). 2017. Dec;18(12):954–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diehl CL, Brost BC, Hogan MC, Elesber AA, Offord KP, Turner ST, et al. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008. May;198(5): e11–3 [DOI] [PubMed] [Google Scholar]
- 20.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med 1916. 17:863–71. [Google Scholar]
- 21.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease [published correction appears in Kidney Int. 2013 Aug;84(2):419]. Kidney Int. 2013;83(6):1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson DM, Bergert JH, Larson TS, Liedtke RR. GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis.1997. Nov;30(5):646–52 [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. NEngl J Med. 2012. Jul 5;367(1):20–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genetic Epidemiology Network of Arteriopathy (GENOA). Study Description. Available from: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/GetPdf.cgi?id=phd003593.1. Accessed July 26,2020
- 25.Freedman BI, Beck SR, Rich SS, et al. A genome-wide scan for urinary albumin excretion in hypertensive families. Hypertension. 2003;42(3):291–296. [DOI] [PubMed] [Google Scholar]
- 26.National Kidney Foundation. Quick Reference Guide on Kidney Disease Screening. Available from: https://www.kidney.org/kidneydisease/siemens_hcp_quickreference. Accessed July 26,2020
- 27.National Institute of Diabetes and Digestive and Kidney Diseases. Explaining Your Kidney Test Results: A Tear-off Pad for Clinical Use. Available from: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-education-outreach/explain-kidney-test-results. Accessed January 4, 2020 [Google Scholar]
- 28.Oshunbade AA, Hamid A, Lirette ST, et al. Hypertensive diseases in pregnancy, cardiac structure and function later in life: Insights from the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Pregnancy Hypertens. 2020;21:184–190. [DOI] [PubMed] [Google Scholar]
- 29.Wang IK, Muo CH, Chang YC, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;185(3):207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett PM, McCarthy FP, Evans M, Kublickas M, Perry IJ, Stenvinkel P, Khashan AS, Kublickiene K. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: A Swedish registry-based cohort study. PLoS Med. 2020. Aug 14;17(8):e1003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayansina D, Black C, Hall SJ, Marks A, Millar C, Prescott GJ, Wilde K, Bhattacharya S. Long term effects of gestational hypertension and pre-eclampsia on kidney function: Record linkage study. Pregnancy Hypertens. 2016. Oct;6(4):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Strengths and limitations of estimated and measured GFR. Nat Rev Nephrol. 2019. Dec;15(12):784. [DOI] [PubMed] [Google Scholar]
- 33.MacPhee IAM. Measured Versus Estimated Glomerular Filtration Rate in the Assessment of Living Kidney Donors: eGFR Has Its Limitations. Transplantation. 2018. Oct;102(10):1595–1596. [DOI] [PubMed] [Google Scholar]
- 34.Bjornstad P, Karger AB, Maahs DM. Measured GFR in Routine Clinical Practice-The Promise of Dried Blood Spots. Adv Chronic Kidney Dis. 2018. Jan;25(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020. Jan;16(1):51–64. [DOI] [PubMed] [Google Scholar]
- 36.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women−-2011 Update: A Guideline From the American Heart Association. Circulation. 2011. Mar 22;123(11):1243–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.