Abstract
Environmental factors, including pollutants and lifestyle, constitute a significant role in severe, chronic pathologies with an essential societal, economic burden. The measurement of all environmental exposures and assessing their correlation with effects on individual health is defined as the exposome, which interacts with our unique characteristics such as genetics, physiology, and epigenetics. Epigenetics investigates modifications in the expression of genes that do not depend on the underlying DNA sequence. Some studies have confirmed that environmental factors may promote disease in individuals or subsequent progeny through epigenetic alterations. Variations in the epigenetic machinery cause a spectrum of different disorders since these mechanisms are more sensitive to the environment than the genome, due to the inherent reversible nature of the epigenetic landscape. Several epigenetic mechanisms, including modifications in DNA (e.g., methylation), histones, and noncoding RNAs can change genome expression under the exogenous influence. Notably, the role of long noncoding RNAs in epigenetic processes has not been well explored in the context of exposome-induced tumorigenesis. In the present review, our scope is to provide relevant evidence indicating that epigenetic alterations mediate those detrimental effects caused by exposure to environmental toxicants, focusing mainly on a multi-step regulation by diverse noncoding RNAs subtypes.
Keywords: epigenetic reprogramming, environment-related toxicants, tumorigenesis, biomarkers, noncoding RNAs, exposome
1. Introduction
The central dogma in genetics is that information in our cells flows only in one direction, from DNA to RNA, then to proteins. It was an absolute dogma that has now been essentially debunked due to the role of the environment in the modulation of gene expression [1]. Environmental factors, including pollutants and lifestyle, constitute a significant role in severe, chronic pathologies with social and economic consequences [2].
The measurement of all environmental exposures and assessing their correlation with effects on individual health is defined as the exposome [3]. An individual’s exposome begins before birth and includes insults from environmental and occupational sources. In fact, the exposome interacts with our unique characteristics such as genetics, physiology, and epigenetics. The “environmental exposure” is a complex construct that encompasses exposures either physical, chemical, biological, or societal [4]. Historically, Christopher Paul Wild defined the exposome in 2005 as the totality of an individual’s exposure experience from conception until death and its impact on chronic diseases [5]. As a concept, it was put forward to stress the necessity of appropriate tools development for exposure assessment when applied to the study of human disease’s etiology [6].
The adaptation of cells to environmental factors causing stress relies on a wide range of tightly controlled regulatory mechanisms. The epigenetic landscape represents the platform where multiple environmental factors interact with the complex genetic milieu, resulting in alterations in the expression gene that shape many aspects of health and disease [7]. Changes in the organization and the structure of chromatin structure are associated with the transcriptional response to stress caused by the environment, and in some cases, can impart the memory of stress exposure to subsequent generations through mechanisms of epigenetic inheritance [8].
Epigenetics investigates modifications in the expression of genes that do not depend on the underlying DNA sequence. Some studies have confirmed that environmental factors, such as toxicants, may promote a phenotype or a disease in an individual or even in the subsequent progeny through epigenetic alterations [9,10]. Several epigenetic mechanisms, including modifications in DNA (e.g., methylation), histones, and non-protein coding RNAs (ncRNAs) can change genome expression under the exogenous influence [11]. Notably, the role of long noncoding RNAs (lncRNAs) in epigenetic processes has recently been highlighted in more detail [12]. The latent interest in epigenetics has resulted in breakthroughs in seminal concepts in diseases ranging from autoimmune conditions to cancer, congenital diseases, mental retardation, endocrine diseases, pediatric diseases, neuropsychiatric disorders, and many others [1,13].
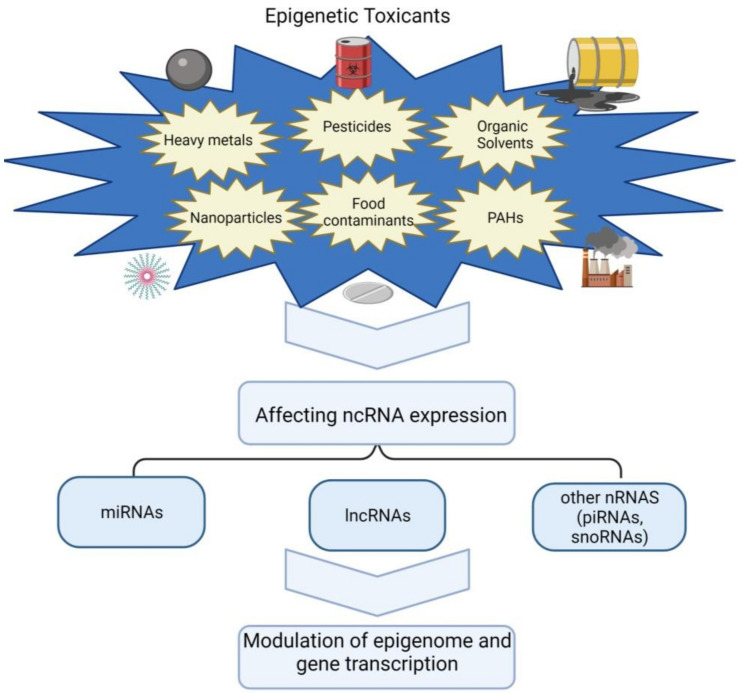
Discrepancies in the homeostatic functions of the epigenetic machinery cause a range of different disorders since these mechanisms (“epigenome”) are more sensitive to the environmental status (lack of nutrient consumption, physicochemical exposures, and psychological stress) than the genome, especially during early development due to the inherently dynamic nature of the epigenetic landscape [14]. Besides the factors above, exposure to pollutants such as arsenic, nickel, cadmium, mercury, benzene, dioxin, bisphenol A, and diethylstilbestrol can alter the epigenetic regulation of the genome. These changes can cause abnormal gene expression, leading to various cancer types and other diseases [15]. Here, we review current evidence indicating that epigenetic alterations mediate those detrimental effects caused by exposure to environmental toxicants, focusing mainly on a direct regulation by a diversity of ncRNAs subtypes (Figure 1).
Figure 1.
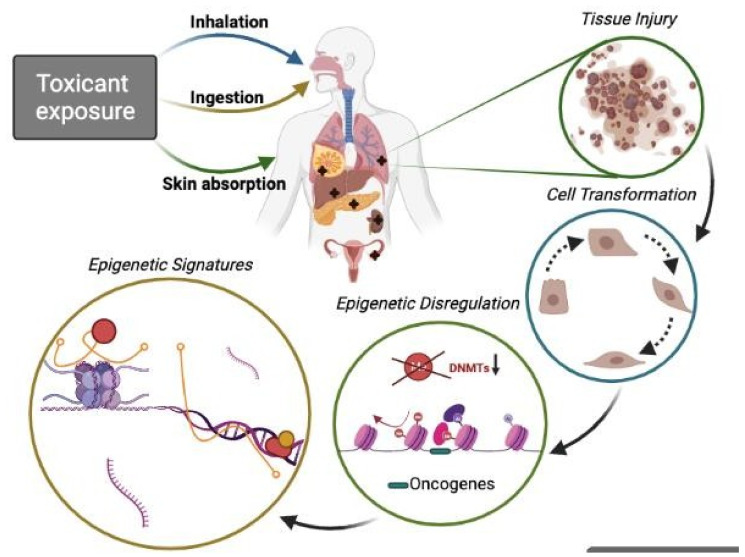
Epigenetic toxicants lead to the phenotypic transformation of normal cells. People exposed (by inhalation, food or water ingestion, and skin contact) to certain pollutants suffer potential tissue injury in the lung, mammary gland, liver, pancreas, skin, colon, ovary, and hematological tissue among other target organs. It is well known that acute or chronic exposures to toxicants are associated with malignant cell transformation and, thus, pollution-related diseases, including cancer. Aberrant genetic modifications (DNA methylation, histone modifications, and ncRNAs can transform cells and disturb the expression of genes involved in homeostasis maintenance. Recently much attention has been given to ncRNAs with their role in pathophysiological conditions and signaling pathways such as oncogenesis, cell survival, altered apoptosis, and cell adhesion. Thus, ncRNAs (miRNA, lncRNA) are vital mediator molecules. Moreover, other important regulators, such as ncRNA-associated proteins forming multi-component complexes on specific loci at specific time points that conform complex epigenetic signatures, are also crucial during the cell transformation process. Studying those epigenetic signatures may improve the understanding of the biology of different pollution-related cancer types.
2. ncRNA Subtypes, Biogenesis, and Turnover
2.1. miRNAs
MicroRNAs (miRNAs) are the smallest ncRNAs, 20–25 nucleotides long, and do not encode proteins [16]. They bind in a complementary manner in the 3′ untranslated region (3′ UTR) of messenger RNAs (mRNAs) and mostly target them for degradation and blocking of their translation [17]. A miRNA can bind numerous mRNAs to inhibit their translation. Therefore, miRNAs are considered crucial post-transcriptional regulators of gene expression [16]. However, under certain cellular contexts (for instance, cell cycle arrest) they are recruited with AGO2 and FXR1 to specific loci with AU-rich elements (AREs) to ultimately activate translation [18,19,20]. Interestingly, recent evidence has called into question that the function of miRNAs is exclusively in the cytoplasm as a post-transcriptional mechanism targeting only mRNAs. In contrast, nuclear miRNAs potentially act during transcriptional silencing of bi-directionally expressed genes involving the formation of miRNA-ncRNA duplexes and nucleolus organization [21,22].
Genes coding for miRNAs are transcribed by RNA polymerase II (POLII) in the nucleus, where the primary miRNAs (pri-miRNAs) are capped, spliced, and polyadenylated. One miRNA, or clusters of two or more miRNAs, are produced from a primary transcript [16]. The double-stranded RNAse III DROSHA, inside a nuclear protein complex called Microprocessor, exerts the important role of cleaving the immature long pri-miRNAs. One essential cofactor that supports the activity of this protein complex is the double-stranded RNA (dsRNA)-binding protein DiGeorge syndrome critical region 8, DGCR8. Two RNase III domains inside DROSHA are necessary for the cleavage of one strand of the dsRNA to release a hairpin-shaped precursor miRNAs (pre-miRNAs) of approximately 60–70-nucleotides long [17]. Next, the export of the pre-miRNAs to the cytoplasm is mediated by exportin 5 (XPO5), and these are then cleaved by DICER1, an RNase III enzyme, originating from dsRNA to produce the mature miRNA duplex with 2-nucleotide 3′ overhangs of 22 nucleotides. DICER1 associates with transactivation-responsive RNA-binding protein (TRBP), which attaches dsRNA. TRBP physically bridges DICER1 with the Argonaute proteins (AGO1, AGO2, AGO3, or AGO4) and with members of the GW182 protein family during the assembly of the miRNA-induced silencing complex (miRISC). The silencing domain within GW proteins interacts with the deadenylase complex, which shortens the miRNA poly(A) tail. The mRNA-decapping enzyme 1 (DCP1)–DCP2 complex removes the 7-methylguanylate (m7G) cap. Consequently, the unprotected 5′ end is degraded by 5′–3′ exoribonuclease 1 (XRN1). In this manner, a specific mRNA is still stable at early stages, but its translation is inhibited, whereas, at later stages, mRNAs with short poly(A) tails are degraded [17]. Recent evidence showed that the increased production of miRNAs is proportional to the concentration of target mRNAs. Increased processivity of AGO2-associated DICER in the presence of target mRNA also contributes to the higher biogenesis of mature miRNAs [23].
Several classes of non-canonical, Drosha-independent or Dicer-independent miRNAs have been identified. One example of miRNAs that are Microprocessor-independent is related to “mirtrons”, which originate from spliced introns that function as pre-miRNAs and can be immediately exported to the cytoplasm for further processing by DICER [16]. Potential miRNAs can also originate from small nucleolar RNAs (snoRNAs) and transfer RNA (tRNA) fragments, and some of them can even be loaded into the RISC complex. Another Microprocessor-independent miRNA type is originated from the 5′-end of POLII-transcribed genes. Early transcription termination liberates the hairpins and serves as a DICER substrate. These miRNA precursors have a 5′7-methylguanylate (m7G) cap, which is not removed by the Microprocessor and facilitates its nuclear export by the cap-binding complex–exportin 1 (EXP1) pathway [16]. In particular, the existence of DICER-independent miRNAs arose from the detailed analysis of AGO2 activity, an RNase H-like endonuclease in AGO2-knockout mice [24] and zebrafish [25]. In other studies, focused on the precursor of the erythrocyte-specific miR-451, it was demonstrated that sequences that are too short to be processed by DICER produce abundant miRNA loads. Thus, miR-451 is bound by AGO2 and cleaved within its stem for mature and functional miRNA formation. This crucial process is conserved during erythrocyte maturation [24,25].
2.2. lncRNAs
Long noncoding RNAs (lncRNAs) lack protein-coding properties and extend more than 200 nucleotides. Although the proportion of functional lncRNAs is still undetermined and investigation is required to support evidence of functionality of the majority of lncRNAs, it is well documented that a growing number of lncRNAs have determinant roles in different mechanisms of gene regulation [26]. These large molecules of RNA can be spliced and polyadenylated, although some of them bind to ribosomes [27]. LncRNAs were originally described to have equivalent chromatin features to protein-coding genes [28]. However, recent work has highlighted the differences in the abundance of precise histone marks and splicing efficiency between lncRNAs and coding RNAs [29], as well as additional specifications among lncRNA subsets that differ in their chromatin landscapes [30].
LncRNAs are fundamental to different mechanisms such as gene imprinting, cells, and tissue differentiation, antiviral response, and the development of cancers. Among the broad mechanisms of action of transcriptional regulation by lncRNAs, those considered most relevant and universal is the interaction with chromatin-modifying complexes, obstruction of the transcriptional machinery, maintenance of the structure of nuclear speckles, regulation of splicing, regulation of mRNA degradation, modulation of protein translation and stability, and acting as molecular sponges for miRNAs [31]. However, while lncRNAs can be dysregulated in various human diseases known to include environmental factors as etiology, it is unclear how specific the interactions between lncRNA and mediators of the cellular response to environmental exposures could be. Most of the available data are derived from cell studies, and no data generated from population-based studies have been published [32]. In addition, as highly specific as lncRNAs are, depending on the target tissue, they represent potential diagnostic markers and, in consequence, therapeutic targets in cancer biology. Developing RNA-targeting therapeutics means a tremendous opportunity for modulating lncRNAs endogenous activity. Several preclinical approaches have been developed in this context, and others are currently under development by several pharmaceutical companies [33].
2.3. Other ncRNA Biotypes (siRNA, piRNAs, snoRNAs)
Although miRNAs are by far the most studied class of small-ncRNA biotypes in many fields, including cancer, environmental toxicology, and risk assessment, research of other RNA biotypes such as small nucleolar RNAs (snoRNAs), short interfering RNAs (siRNAs), and PIWI-interacting RNAs (piRNAs) may lead to similar breakthroughs based on their distinct cellular functions. First, siRNAs are 21- and 22-nucleotides RNAs generated by ribonuclease III (RNase III) and cleaved from longer double-stranded RNAs (dsRNAs) involved in post-transcriptional gene silencing in mammals and plants. Silencing is initiated by dsRNA that is homologous in sequence to the silenced gene [12]. Similar to miRNAs, siRNAs depend on DICER enzymes to excise them from their precursors [34] and AGO proteins to support their silencing-effector functions [35]. Generally, siRNA assembles into functional siRISC; one strand is separated and degraded. Then, siRNA-containing siRISC binds to AGO2 protein. This complex finally recognizes its targets by base pairing, and silencing occurs through one of several molecular mechanisms [36]. PIWI-interacting RNAs (piRNAs) are 26–32 nucleotides in length and bind to the PIWI protein family; they are abundant in spermatogenic cells, responsible for stem cell self-renewal, and involved in transposon silencing. piRNAs have been found to act in somatic cells and are crucial in guiding epigenetic regulation [37]. The biogenesis of piRNAs, initially described in Drosophila spp. and in C. elegans, begins with the transcription of a long, single-stranded precursor derived from a coding- gene transcript, transposons, tRNA, ribosomal RNA (rRNA), or intergenic loci by RNA POLII. Furthermore, a DICER/DROSHA-independent manner makes their processing. The precursor is exported from the nucleus into yeast bodies and further processed into smaller segments in yeast. piRNAs then form a complex with PIWI proteins assisted by shutdown (SHU) and heat shock protein 90 (HSP90). Their mature form is completed when the enzyme HENMT1 adds a methyl group to the 2′ carbon of the ribose on the 3′ end of the transcript. It can reenter the nucleus to induce transposon silencing and epigenetic regulation [38]. The abnormal expression of piRNA is associated with various cancers such as gastric, breast, renal, colorectal, and lung cancer [39].
Small nucleolar RNAs (snoRNAs) are short (60–300 nucleotides long) crucial biomolecules that participate importantly in ribosome biogenesis. They also have a role in the chemical modifications of some RNA biotypes, such as rRNAs, tRNAs, and small nuclear RNAs. There are two classes according to their conserved sequence elements: the C/D box snoRNAs (hairpins containing a sizeable internal loop, bounded by the C/C′ box and the Box D/D’ motifs), and the H/ACA box snoRNAs (two stem-loop structures separated by a conserved single-stranded motif with a consensus sequence of ANANNA) that are predicted to direct site-specific pseudo-uridylation and 2′-O-methylation of rRNA, to guide pre-rRNA processing and to act as molecular chaperones [40]. snoRNAs are derived from either mono- or polycistronic transcription units. In contrast, most of them are encoded within introns of pre-mRNAs and are transcribed mainly by RNA POLII. However, RNA POLIII and their final maturation require exonucleolytic trimming via two pathways: a splicing-dependent and splicing-independent pathway [41]. Splicing of the pre-mRNA results in a debranched precursor further processed by exonucleases to develop a mature snoRNA [42]. Because of their essential roles in biological processes, dysregulations in their expression can actively contribute to the promotion of carcinogenesis in the lung [43] and breast tissue [44].
3. Epigenetic Alterations Induced by Toxicants
Environmental toxicants are widespread worldwide and include various chemicals, from combustion products to contaminating trace metals and residual organic compounds used in daily life [45]. Those considered pollutants or chemical agents are chemical elements or compounds whose state and physicochemical characteristics allow them to encounter individuals. Their main routes of entry into the body are respiratory, dermal, and digestive [46]. Exposure to varying levels and types of these contaminants is linked to a substantial harmful impact on human health. For this reason, the environmental health and toxicology fields have recently focused on improving the understanding of how these contaminants impact biological processes so they can be prevented [45]. The following sections introduce a list of the main toxins that act at the epigenetic level, according to the four primary sources of exposure to chemical agents (Figure 2 and Supplementary Table S1).
Figure 2.
Classification of common and emerging epigenetic toxicants. The most common epigenetic pollutants are classified based on their chemical structure and associated epigenetic effects on different biological targets. Notably, the role of long noncoding RNAs (lncRNAs) in epigenetic processes has been recently highlighted as the primary mediator of the cellular response to environmental pollution.
3.1. Toxics in the Air
According to the United States National Ambient Air Quality Standard (NAAQS), there are six principal air pollutants: nitrogen dioxide (NO2), carbonic oxide (also known as carbon monoxide, CO), ozone (O3), sulfurous acid anhydride (also known as sulfur dioxide, SO2), lead (Pb), and particulate matter classified as a particulate matter of ≤10 μm (PM10) and ≤2.5 μm (PM2.5) [47,48]. Air pollution has numerous harmful effects and contributes to cardiovascular and lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), and metabolic disorders. Air pollution can induce changes in DNA methylation [49], histone modifications [50], as well as regulation by ncRNAs [51].
3.1.1. Ozone
O3 is an environmental toxicant caused by photochemical reactions in automobile exhaust gases [49,50,51,52,53,54]. Respiratory exposure to ozone causes inflammation and injury to the respiratory tract. A recent study investigated the impact of atmospheric pollutants on the murine lung. The results show that the ozone-induced damage increases the markers of inflammation and the number of EV-sized particles in the BALF in murine lungs. Moreover, high-throughput small RNA sequencing identified the altered expression of several microRNAs in pulmonary extracellular vesicles, including miR-22-3p [55].
3.1.2. Carbon Monoxide
Carbon monoxide (CO) competes with oxygen and alters the hemoglobin dissociation curve. Once it enters into the circulation, it binds to the Heme group of hemoglobin, displacing the oxygen and forming a hematic complex called carboxyhemoglobin, which hinders oxygen transport to cells and tissues, causing generalized cellular hypoxia. Hypoxia is known to affect cells with higher oxygen demand, particularly myocytes and neurons, which might have been linked to a higher risk of cardiovascular and neurodegenerative diseases [56,57]. Recently, novel epigenetic signatures were identified to be associated with the uptake of carbon monoxide (DLCO) per alveolar volume (VA) (DLCO/VA), using the single-breath technique in 2674 individuals. Two CpG sites (cg05575921 and cg05951221) significantly associated with CO uptake were identified. Furthermore, they found a positive association between hypomethylation of the AHRR gene (cg05575921) and the expression of EXOC3 in whole blood, suggesting that EXOC3 is an excellent candidate through which smoking-induced AHRR hypomethylation could affect the exchange of pulmonary gases [58].
3.1.3. Lead
Lead (Pb) is a xenobiotic metal and toxic pollutant. Lead-contamination primary sources are associated with the inhalation of contaminated air and ingestion of contaminated food [59]. Pb affects the nervous system and is associated with cancer and neurodegenerative disorders [59,60]. A group of Caucasian male employees with higher lead concentration in their blood exhibited complete CpG methylation, including the CDKN2A promoter, whereas the group with lower lead concentration in their blood showed partial methylation. Therefore, results suggested that DNA methylation could be involved in the mechanism by which it induces neurotoxicity [61]. However, additional studies are required to determine the specific effect in the DNA methylation profile in neuronal cells using in vivo and in vitro experiments.
3.1.4. Sulfur Dioxide
Sulfur dioxide (SO2) is a colorless gas that condenses at −10 °C and solidifies at −72 °C. It is soluble in water and organic solvents. During its oxidation process in the atmosphere, it forms sulfates that can be transported in PM10 and, in the presence of humidity, form acids that are components of PM2.5 [61,62]. One study reported that the co-exposure of PM2.5 and SO2 at low doses leads to neuronal apoptosis, reduction in the postsynaptic density, and synergistic neurodegeneration by the activity of miR-337-5p [63].
3.1.5. Nitrogen Dioxide
Nitrogen dioxide (NO2) is a gas that generates inflammation bronchial hyperresponsiveness and causes an imbalance in the Th1/Th2 immune response. Furthermore, prenatal exposure to NO2 is also associated with respiratory infections and asthma development during childhood [64,65]. A longitudinal panel study was conducted among 40 university students with four repeated measurements in Shanghai, finding that short-term exposure to NO2 was associated with hypomethylation of NOS2A, hypermethylation of ARG2, respiratory inflammation, as well as impaired lung function [66].
3.1.6. Particulate Matter
Considering all atmospheric pollutants, particulate matter (PM) is considered a profound health problem because they are made up of a complex combination of elemental and organic carbon, nitrates, volatile organic compounds, sulfates, polycyclic aromatic hydrocarbons, metals, and biological components [67]. PM is classified based on its diameter, including PM10 and PM2.5 [68]. Fine particulate matter enters the lower respiratory tract due to its low diameter, while 10 µm-particles remain in the upper respiratory tract. Thus, most epidemiological reports indicate that PM2.5 is the most harmful fraction for public health [67,69]. In general, PM comprises heavy metals and polycyclic aromatic hydrocarbons, which can induce modifications in DNA methylation, histone modifications, and, importantly, ncRNA expression. Specifically, several studies have revealed the effects of PM2.5 on the deregulation of miRNAs such as miR-4516 and miR-32, whose upregulation is observed in lung cancer cells. Moreover, the downregulation of RPL37, a target of miR-4516, enhanced the expression of LC3B, a critical hallmark of autophagy [70]. Meanwhile, miR-32 might function as a tumor suppressor to repress EMT [71]. Conversely, PM2.5 affects the expression of miR-194-3p and miR-16 in human bronchial epithelial cells (CSE-HBEpiCs) treated with cigarette smoke extracts, as well as in human hepatocellular carcinoma (HCC) cells. miR-194-3p inhibition, in turn, enhances apoptosis [72] while miR-16 deregulation could enhance metastasis and EMT features [73]. More recently, studies demonstrated that PM2.5 also transform normal cells by lncRNA alteration. For example, MEG3 (maternally expressed gene 3) over-expression promotes autophagy and apoptosis by increasing TP53 (a tumor suppressor) in HBE cells after treatment with arterial traffic ambient PM2.5 (TAPM2.5) and wood smoke PM2.5 (WSPM2.5) [74]. MEG3 is also upregulated in CSE-treated 16HBE cells and COPD tissues, leading to cell proliferation impairment by downregulation of miR-218 [75]. Another upregulated lncRNA by PM2.5 is LOC101927514, which binds to STAT3 to raise an inflammatory state in 16HBE cells [76].
Organic and Elemental Carbon
Organic carbon, or secondary organic aerosol, is the main component of PM2.5. It represents between 20–80% of the total mass of these particles and has various chemical forms. Carbonaceous aerosols can be released from multiple sources, including mobile devices, biomass burning, meat cooking, and fossil fuel burning, and are also believed to be generated through the gas phase oxidation of precursors in the atmosphere [77,78]. Elemental carbon in the atmosphere is a dark-colored, low-volatile material that does not evolve below 700 °C. Elemental carbon is also an essential component of PM2.5 and is emitted into the atmosphere by various combustion processes, such as fossil fuels and biomass burning. This type of carbon influences the regional and global climate by directly absorbing solar radiation, changing cloud precipitation, acting as ice nuclei or cloud condensation through coating with soluble species, improving the fusion of the snow and ice cap when deposited on these surfaces [79,80]. During phagocytosis and metabolism, elemental carbon and organic carbon have produced highly reactive oxygen species (ROS) concentrations. ROS causes acute and chronic inflammatory diseases, including cardiovascular disease, asthma, and cancer. Furthermore, many lines of evidence demonstrate that ROS can induce epigenetic changes such as DNA methylation or microRNA differential expression [81]. In addition, ROS induced by PM2.5 exposure may promote the expression of some lncRNAs such as loc146880 [82], which expression was positively correlated with RCC2 [83] that encodes the protein RCC2/TD-60, required for cell cycle progression [84]. Furthermore, it was shown that RCC2 can bind and stimulate the effect of the lncRNA LCPAT1 (lung cancer progression-association transcript 1) on cell autophagy and EMT after exposure to PM2.5 and CSE (cigarette smoke extracts) in lung cancer cells [85]. Therefore, high loc146880 and LCPAT1 levels might be associated with lung cancer development [82,83,85].
Sulfates
Sulfates (SO4) are mainly secondary components that originate from the oxidation of SO2, although they may be present as a primary component derived from sea salt or mineral matter, such as gypsum. Sulfate is closely related to the release of CO2 into the atmosphere [86]. In the Normative Study of Aging, 1406 blood samples from 706 elderly participants were analyzed for DNA methylation changes in sulfate-associated repetitive elements, a prolonged exposure to SO4 particles showed significant association with hypomethylation of the long-interspersed nucleotide element-1 (LINE-1) retrotransposon and the short interspersed nuclear element (SINE) Alu [87].
Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAH) are mainly present in the organic component (Po) of PM2.5 [88] as by-products of combustion and enter the human body from various sources, including inhaling gasoline, diesel-fueled engines, coal, coke, oil burners, eating grilled and smoked meats, and cigarette smoking [63]. Reports have shown PAHs as etiological agents of several types of cancer including colon, pancreas, breast, lung, and prostate. However, the biological pathways by which contaminants cause adverse health effects are largely unknown [89]. Moreover, exposure to PAHs has been investigated to alter glutathione, DNA, and RNA (hydroxy) methylation levels, the formation of DNA PAH-adducts [89], as well as the expression of ncRNAs. For example, some breast tumor-related genes such as EPHB2 (Ephrin type-B receptor 2) and LONP1 (Lon Peptidase 1) present altered methylation under PAH and NO2 exposures [68]. On the other hand, chronic Po exposure in lung adenocarcinoma cells enhanced their cancer stem cell properties through a long noncoding loc107985872-notch1 signaling pathway which could be recapitulated in vivo [90,91]. Similarly, MALAT1 could interact with miR-204 which results in increased ZEB1 (an EMT-related transcription factor) function which in turn enhances the EMT and malignant transformation in lung bronchial epithelial cells after Po exposure [92].
3.1.7. Indoor Air Pollutants
There are five types of sources of pollution in an ordinary home. The first source to be recognized was the combustion of fuels in heating and cooking food. The most used gases as fuel are natural gas (methane) and liquefied gas (propane-butane), which mainly produce NO2 and CO (described previously in Section 3.1.5 and Section 3.1.2, respectively). If wood is used for heating in chimneys or cooking (this is the case in many countries in the world), then PM 2.5 and PM 10 are added and a series of PAHs (described in Section 3.1 and Section 6 and Polyciclic Aromatic Hydrocarbons, respectively).
The second source of internal contamination is the result of natural and synthetic materials used in carpets, foam insulation, interior decoration papers, and furniture. The glues used in chipboard, for example, produce formaldehyde. Latex rugs are a source of phenyl cyclohexane emissions. Asbestos used in building materials for its heat resistance properties can cause the emission of asbestos fibers indoors if they are not adequately sealed. In offices, some types of photocopiers and computer printers are a source of toxic organic substances such as toluene. Indeed, the air in many modern buildings is particularly polluted due to the combination of office equipment, synthetic carpets, and poor ventilation [93,94].
The third possible source of internal contamination is the leakage of toxic gases through the ground of the house or from the sewage services due to potential contamination into the pipes. In the United States and China, the largest source of gas emissions though the ground is the radioactive gas radon [95,96]. Many of the commercial products used domestically, such as furniture cleaners, glues, cleaning agents, cosmetic deodorizers, pesticides, and solvents used at home, contribute to the toxicity of the indoor ambient air. These products are the fourth source of production of internal contamination. The fifth source of pollution is cigarette smoke that emits PM. Not only is a pollutant toxic by itself, but it also increases the risk of diseases from other toxic compounds present in indoor environments [97,98].
Formaldehyde
Using proteomic analysis, previous groups have identified that formaldehyde can cause epigenetic changes in human cells that lead to the phosphorylation of histone H3. In addition, cells from the pulmonary tract exposed to this aldehyde in the gaseous state displayed 89 miRNAs significantly downregulated. Those alterations were potentially implicated in signal transduction mechanisms associated with the regulation of the endocrine system, inflammatory response, and cancer, indicating that formaldehyde exposure can change the expression profile of miRNAs, making them potential pathogenic drivers of formaldehyde-induced diseases. The authors suggested that this dysregulation could cause the onset and progression of heart and cardiovascular diseases by modulating the expression levels of ncRNAs [99], as it may occur in congenital heart disease (CHD), where several circRNAs may be dysregulated as shown in fetal heart rat samples exposed to formaldehyde compared to the control group [100].
Asbestos
Asbestos is a natural mineral consisting of extremely fine fibers that can become trapped in the lung upon inhalation. Genomic DNA mutations and gene rearrangements that cause lung cancer are extensively reported, with biomarkers and targeted therapies already in clinical use [101]. However, our current understanding of how asbestos fibers trapped in the lungs cause epigenetic changes and lung cancer is incomplete. Redox reactions on the surface of fibers have been shown to generate ROS that in turn damage DNA, causing genetic and epigenetic alterations that reduce the activity of tumor suppressor genes. Asbestos-associated lung cancer exhibits less methylation variability than lung cancers in general, and to a substantial extent, the variability is restricted to promoter regions [102]. Additionally, asbestos exposure is highly related to malignant pleural mesothelioma (MPM) development [103], in which various markers of DNA methylation, ncRNAs, and histone modifications have been proposed as diagnostic markers involved in the progression of MPM [104]. In this sense, lncRNA-RP1 and miR-2053 were part of a four-RNA signature in serum, in combination with the DNA damage regulated autophagy modulator 1 (DRAM1) and arylsulfatase A (ARSA) exclusively in MPM patients [105]. Furthermore, miR-16 (inhibitor of cell proliferation and migration), miR-17 (potential tumor suppressor), miR-126 (inhibitor of VEGF activity), and miR-486 (antifibrotic) were downregulated in MPM samples. Together, these miRNAs can represent the basis of the mechanism involved in MPM progression [106]. Since GAS5 (Growth arrest-specific transcript 5) might act as an oncogene released by the tumors and upregulated in serum of MPM subjects, it is suggested as a potential circulating marker [107].
Toluene
It has been reported that toluene increases CYP2E1 mRNA and modifies its protein activity on leukocytes. A venous blood study was performed on workers exposed to toluene in the air and twenty-four administrative workers in Guanajuato, Mexico. This study indicated that the methylation of CYP2E1 and IL6 promoters significantly increased in the group exposed to toluene. In addition, the levels of methylation in the promoter region of CYP2E1 were higher in smokers exposed to toluene in comparison with non-smokers. A significant correlation was also found between the methylation of CYP2E1 and the methylation at the promoter region of GSTP1 and SOD1 [108]. A recent study proposed the long noncoding NEAT1 and a panel of four miRNAs (miR-301a-3p, miR-16-5p, miR-15b-5p, and miR-15a-5p) as an efficient epigenomic signature related with toluene-induced neurodegeneration [109]. Conversely, another study showed that the effects of chronic combined exposure to volatile organic compounds (VOCs, toluene, ethylbenzene, and xylene) are more unfavorable than those resulting from a single compound exposure [84]. Specifically, MALAT1 and eight coding genes (CACNG8, CLIP2, CNTNAP3, FMR1, GLS2, SULT4A1, TP73, and WNT7B) expressions were significantly reduced by DNA hypermethylation in VOC-exposed subjects [110].
Radon
Recently, ncRNAs have been highlighted as early biomarkers of toxicity to radon exposure. In a cohort study performed with 144 miners, potential biomarkers were analyzed. Hematological parameters were statistically different in the underground group, while the levels of certain cyclins and cyclin-dependent kinases (e.g., CDK2, CDK4, CDK6) were higher. A miRNA microarray screening showed that five miRNAs (among them miR-19a, miR-335, and miR-451a) were downregulated (>2 fold) in the underground group, suggesting that specific miRNAs can be used as potential indicators of radon-mediated injury [111].
Mercury
Mercury (Hg) is a metal widely used in the industry [85]. Inhalation of mercury vapor can harm the nervous, renal, gastrointestinal, respiratory, and immune systems [112]. A recent study revealed an increased expression of histone deacetylase 4 (HDAC4), transcription factors specificity protein 1 (SP1) and 4 (SP4) in neuroblastoma (SH-SY5Y) Hg-treated cells. The results confirmed that the siRNA-mediated knockdown of MAPK14 (P38), SP1, SP4, HDAC4, or BDNF blocked Hg-induced cell death significantly. All these results suggested that MAPK14/SP1-SP4/HDAC4/BDNF may represent a new effector pathway involved in Hg-mediated neurotoxicity [113].
Cigarette Smoking
The vulnerability to cigarette smoke (CS) is one of the significant threats to human health worldwide. Numerous chemical substances present in tobacco contribute to its toxicity: PAHs, N-nitrosamines, heavy metals (e.g., cadmium, arsenic), aromatic mines, and alkaloids (e.g., nicotine and its metabolite cotinine) [114]. For decades, it has been known that smoking considerably increases the risk of cardiovascular diseases, different cancer types, as well as respiratory diseases [115]. CS is a complex chemical mixture containing thousands of compounds, several known carcinogens, co-carcinogens, and mutagens [12]. CS induces a significant increase in the expression of several chromatin modification enzymes, including DNA methyltransferases, histone acetyltransferases, histone methyltransferases, SET-domain proteins, kinases, and ubiquitinases [115]. Moreover, several epigenome-wide association studies (EWAS) identified a correlation between modifications in DNA methylation in smokers’ blood and their smoking status [116]. With regard to ncRNA regulation, several studies report that cigarette smoke extracts increase the HOX transcript antisense RNA (HOTAIR) levels in human bronchial epithelial (HBE) cells. HOTAIR mediates epithelial-to-mesenchymal transition (EMT), a process involved in the malignant transformation of cells [12,117,118]. In addition, HOTAIR induces epigenetic silencing of CDKN1A via enhancer of zeste homolog 2 (EZH2)-mediated tri-methylation of Lys 27 of histone H3, which contributes to cell cycle changes [116]. Similar studies indicate that the lncRNA MALAT1 is also involved in cigarette smoke-induced EMT and malignant transformation in HBE cells [119].
3.2. Toxics in Water
The quality of water sources widely varies depending on local geology, agricultural activities, and industrial or municipal sources of pollution. As a rule, groundwater contains more mineral pollutants than surface water due to its passage along rocked beads on the earth’s surface, which are mineral rich. On the other hand, surface waters tend to contain more biological pollutants and more organic pollution than groundwater. Water sources can include both organic and inorganic industrial wastes [120,121]. Contaminants getting into drinking water can do it in a variety of ways. One is through pipeline corrosion which releases metals such as lead (described in Section 3.1.3), copper, cadmium, iron, zinc, nickel, and others into the water supply. In addition, there are problems caused by the by-products of chlorine when used as a disinfectant, being the most important the organic molecules called Trihalomethanes (THMs), of which the best known is chloroform. THMs are produced by naturally reacting chlorine with organic matter such as rotten leaves or soil and with industrial pollutants of organic origin [122,123].
3.2.1. Arsenic
Arsenic (As) is a natural environmental toxicant found in drinking water, soil, and food [124]. Chronic exposure to As is involved with various tumorigenic events, including those from the respiratory tract and the bladder [125]. In human cells from the embryonic kidney (HEK239T) and cervix-carcinoma (HELA), As reduced global acetylation of lysine 16 of histone H4 (H4K16) in a dose-dependent manner [125]. As a possible mechanism, arsenic decreased histone acetyltransferase (HAT) by direct binding to cysteine into the Cys2HisCys (C2HC) zinc finger domain [125]. In addition, the analysis of CD4+ cells from women exposed to arsenic showed that individuals with high urinary arsenic concentrations had significantly lower levels of global trimethylated Lys-9 of histone H3 (H3K9me3) compared to women with lower urinary arsenic concentrations. The authors suggested that decreased histone methylation caused by As could lead to the expression of genes involved in tumorigenesis [126].
3.2.2. Cadmium
Cadmium (Cd) is a human carcinogen [127] derived from industrial activities. The primary source of Cd exposure is food intake [128]. In human bronchial epithelial cells (16HBE), Cd inactivates tumor suppressors and genes associated with DNA repair through the hypermethylation of DNA, as a result of the elevated expression of DNA methyltransferases (DNMT1 and DNMT3A) [129]. Furthermore, human lymphocyte cells treated with Cd increased their proliferation, expression levels of the DNA methyltransferases DNMT1 and DNMT3B, and global methylation. In contrast, transcription levels of cyclin-dependent kinase inhibitor 2A (CDKN2A) were reduced compared to control cells. Still, its expression levels were rescued using a DNA methyltransferase inhibitor (5-aza-dC) with a concomitant reduction in proliferation even with Cd treatment. These data suggested that the aberrant increase in the Cd-exposed lymphocytes is induced by hypermethylation of the CDKN2A promoter [127].
3.2.3. Chromium
Chromium (Cr) is a toxic and carcinogenic contaminant that directly reacts with DNA, forming adducts and inducing mutations. Cr compounds cause lung, gastrointestinal, and skin cancer [130]. New research studies show that human bronchial epithelial cells (BEAS2B) treated with Cr increased the levels of NUPR1 mRNA and a reduction in acetylated Lys-16 of histone 4 (H4K16ac). Significantly, overexpression of NUPR1 induces anchorage-independent cell growth, while its knockdown prevents Cr-induced cell transformation. These findings suggest a deleterious epigenetic mechanism of Cr through the induction of NUPR1 expression and the decrease in H4K16 acetylation [130]. The promoter of MLH1, an essential DNA mismatch-repair protein, showed slightly increased methylation after Cr exposure in normal lung tissue. In tumor tissues exposed to Cr, the methylation of the MLH1 promoter was remarkably higher [131]. These findings suggest that Cr-mediated carcinogenesis drives epigenetic changes in MLH1 in normal airway epithelial cells. The methyltransferase SUV39H1 might be involved in the hypermethylation process in promoters followed by the accumulation of mutations in DSB repair genes such as RAD50, which could lead to lung cancer progression [131].
3.2.4. Nickel
Nickel (Ni) is a toxic and carcinogenic metal used in industry [132]. The primary route of human Ni exposure is inhalation; thus, it causes multiple respiratory conditions such as irritation, inflammation, edema, and cancer [132]. A recent study showed that both epigenome and transcriptome undergo robust changes after the termination of the nickel exposure in BEAS-2B cells [133]. Upregulated transcripts correlated with a significant increase in the levels of H3K4me3 and the loss of repressive H3K27me3 after Ni exposure. These H3K4me3-gain and H3K4me3-loss regions correlate as well with several transcriptional activators and repressors, suggesting that these loci are important regions during dynamic chromatin changes [133].
3.2.5. Copper
Copper (Cu) is a vital trace element within the human body, but it is also toxic [134]. The main sources of Cu from food include vegetables, potatoes, beef, and nuts. A group showed an association between DNA methylation at specific loci and Cu exposure by correlating Cu-related CpG sites, lower high-density lipoprotein cholesterol (HDL-C), and higher C reactive protein (CRP) levels, which are cardiovascular risk factors. This evidence suggested that the DNA methylation profile after Cu exposure modifies lipid metabolism and inflammation [134]. However, experimental evidence is necessary to support these observations and verify the specific mechanism by which Cu increases DNA methylation.
3.2.6. Trihalomethanes
Trihalomethanes (THM) are undesired disinfection by-products (DBPs) formed during water treatment. Cytotoxicity of these compounds has been associated with chloroform and trichloroacetic acid exposure. Studies have reported changes in the epigenetic landscape due to global DNA methylation and proto-oncogenes hypomethylation after exposure to THM, as described in rodents. However, there is limited human evidence of DNA methylation changes in retrotransposons or other regions of the genome [135,136].
3.3. Toxicants in Food
Nutrition is one of the most studied and better understood environmental epigenetic factors [137]. It is proposed that both dietary nutrition and exposure to environmental toxicants are two factors that are intrinsically intertwined in health outcomes [138]. Dietary compounds are thought to effectively prevent epigenetically dysregulated conditions [139]. Nutrients can directly inhibit the activity of epigenetic enzymes such as DNMT, HDAC, or HAT or can alter the substrate availability necessary for their enzymatic activity. Consequently, it modifies the expression of critical genes and impacts overall health and longevity [137].
Food is a significant source of environmental toxicants, including bisphenol analogs, phthalates, PFASs, polychlorinated biphenyl (PCBs), particulate matter, parabens, heavy metals, as well as other emerging contaminants. Due to their hydrophobicity, many of these agents accumulate in fats during food processing and consequently in the fatty tissues of animal bodies [138]. Thus, variations in food consumption and the resultant exposure to additional levels of toxicants may vary, particularly in “Western diets”, which contain high protein and fat amounts [138]. In addition, food additives such as nitrates are related to an increased cancer risk from digestive organs. However, intake of nitrates from diet and other sources such as drinking water was not associated with these types of cancer [140].
In recent decades, it has been suggested that microbiota influence gene expression. However, we are still in the first stage of the discovery of biological processes in bacteria that regulate the expression of eukaryotic genes within the host in the context of metabolic diseases [141]. Furthermore, the relationship between the microbiota and the induced epigenetic programming has been recently explored. For example, volatile short-chain fatty acids have been proposed as mediators in the host [142]. However, most of the molecular processes that favor epigenetic changes remain to be deeply studied. Despite water quality being affected by increasing microbial pollution, how microbial agents would affect our epigenetic reprogramming is unclear.
3.3.1. Glyphosate
N-(phosphonomethyl) glycine (glyphosate) is one of the ingredients in herbicides formulation, whereas the primary source of exposure is through its residues in food. Some studies have associated glyphosate-based herbicides with non-Hodgkin lymphoma (NHL), acute myeloid leukemia (AML), and endocrine-disrupting activities [143]. In this context, human peripheral blood mononuclear cells (PBMCs) incubated with glyphosate modified its global methylation profile, showing low expression of CDKN2A and TP53, and high expression of the cell cycle drivers CDKN1A and BCL2 compared to untreated cells [143]. Similarly, PBMCs treated with glyphosate and its metabolite AMPA showed higher expression of genes involved in the DNA methylation process and deacetylation of histone proteins [144]. Therefore, glyphosate and AMPA exposure was related to expression changes in chromatin remodelers in these cells. In parallel, it is essential to evaluate the mechanism that induces these changes at the transcriptional level and its implications at the cellular level. On the other hand, in human embryonic kidney 293 (HEK293) cells treated with glyphosate, the transcription of cell cycle progression (EGR1, AP1, FOS, and MRTL) and regulation genes (CCNB1, CCND1, and CKN1A) was activated. According to this evidence, glyphosate enhances cell proliferation [143].
3.3.2. Dioxin
Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) is a persistent and environmental toxicant that accumulates in the fat tissue due to its lipophilic nature [145]. In mice treated with dioxin, cardiomyocyte-like Nkx2-5+ cells were hypomethylated during cardiogenesis affecting cell signaling and cardiovascular development functions. On the other hand, embryonic stem cells did not show any changes in their methylation status in response to dioxin. The authors of this work suggested the impaired activity of TET proteins because of dioxin exposure [146]. The authors found 4821 genes differentially expressed in the same position, only 240 genes differentially methylated, and 111 genes that showed a significant correlation between DNA methylation and gene expression [146].
3.3.3. Bisphenol A
Bisphenol A (BPA) is an organic synthetic compound widely used in plastic manufacturing, packaging of food and drinks, medical devices, thermal paper, and dental materials. BPA represents a contamination source in air and soil, as well as in beverages and food. It accumulates in several human tissues and is potentially harmful to human health [145,147]. BPA is also a xenoestrogen that acts as an endocrine disruptor by binding multiple targets inside and outside the nucleus. Furthermore, it is a weak carcinogen that may have long-term effects on the reproductive system by altering the epigenome [145]. In neonatal rats, BPA promoted phosphatidylinositol 3 kinase (PI3K)/AKT signaling activation by inducing histone methyltransferase EZH2 inactivation and the consequent reduction in the levels of trimethylated Lys-27 of histone 3 (H3K27me3) [148].
3.3.4. Phthalate
Di(2-Ethylhexyl) phthalate (DEHP) is a plasticizer in polyvinyl chloride (PVC)-based materials. Exposure to DEHP often occurs via oral ingestion, inhalation, and dermal absorption, associated with reproductive alterations [149,150]. Upregulation of essential adipogenesis-related genes (Pparγ, C/Ebpα, and Fabp4) and acetylation of Lys-9 of histone 3 (H3K9ac) has been studied to be dose-dependent in mouse mesenchymal stem cells treated with benzyl butyl phthalate (BBP) [151]. In addition, these authors validated the downregulation of the histone methyltransferases Setdb1 and G9a (responsible for the di-methylation of H3K9) and the upregulation of the histone acetyltransferases Gcn5 and Ep300, along with the histone deacetylases Hdac3 and Hdac10. Notably, Pparγ knockdown reverted these epigenetic modifications in mesenchymal cells. Hence, it seems that BBP enhances the adipogenesis of MSCs by altering the balance between histone modifications through Pparγ [151]. Therefore, it will be essential to determine a specific interaction mechanism between BBP and Pparγ that promotes the observed changes in epigenetic regulators.
3.4. Toxics in Consumer Products
Increasing amounts and varieties of chemicals are used in consumer products, while our understanding of their exposure pathways and associated human health risks still lags [152]. The following sections will summarize the epigenetic alterations related to some of the most used toxic agents.
3.4.1. Nanotechnology
In the last decade, the nanotechnology field has become increasingly relevant in different aspects of human life [153]. Nanomaterials (NMs) are natural or engineered materials with one or more external dimensions in the size range of 1–100 nm [10,154]. Nanoparticles (NPs) and NMs are used in many daily life products including medicines, household items, food, toys, among others [153,155]. The increasing usage of NMs increases the probability of human exposure, favoring the considerable risk of nanotoxicity. “Toxico-omics” research has been performed on these materials. However, the epigenetic modulations in humans originating from exposure to these toxicants have not been widely studied [156]. Because of their ability to enter the human body via inhalation and skin penetration and subsequently interact with intracellular structures, several concerns over their potential toxicity to workers and end-users have been raised [10]. In addition, their effects on epigenetic markers remain a novel area in the field of nanotoxicology, with limited and inconclusive information, as well as several knowledge gaps [157,158]. The epigenetic aspects of the effect of nanomaterials on cells help to provide a better understanding of when and how nanomaterials may impact genome integrity and guide application strategies for nanomaterials in biomedicine. However, there is limited information about the effects of NPs (e.g., SiO2, AuNP, AgNP), quantum dots, and copper oxide (CuO) on the epigenetic landscape [159].
It is important to highlight that besides ncRNAs, histone post-translational modifications (PTMs) including methylation and acetylation are covalent modifications that have an essential role in gene regulatory function by modulating chromatin structure [8,45]. Generally, the repressive state of chromatin is enriched in H3K9 and H3K27 trimethylation (H3K9me3 and H3K27me3), while H3K4me3 is enriched at promoter regions and associated with more accessible chromatin [160]. Besides methylation and acetylation, other modifications such as phosphorylation of histones are indicative of toxicity-induced damage or environmental stress [161]. In the context of nanotechnology products, NMs and NPs are reported to induce disruption of the normal patterns of histone modifications and changes in DNA methylation. One of the most common alterations generated by prolonged exposure to a range of NMs and NPs is increased phosphorylation of histone H2AX at serine-139 (γH2AX) [155]. γH2AX is induced in response to various types of DNA damage and it is one of the earliest biomarkers of DNA damage in different pathologies [162,163]. In most of these reports, the induction of oxidative stress was carried out in parallel with the increase in the formation of γH2AX. Nevertheless, the oxidative stress independent of the phosphorylation of histone H2AX was also shown [155]. Recently, the effect of ncRNAs in association with different engineered NMs in an exposure model in mouse airways has been reported [164].
Titanium Dioxide
Nanoscale titanium dioxide (TiO2) is manufactured on a large scale, with various applications in consumer products. Different human cell lines (Caco-2, colorectal; HepG2, liver; NL20, lung; and A-431, skin) were exposed to TiO2 nanoparticles to assess the effects on global methylation. The reduction in global levels of DNA methylation and the expression of the DNA methyltransferases MBD2 and UHRF1 were demonstrated in A431, Caco-2, and HepG2 cell lines exposed to TiO2 nanoparticles. Eight genes (BNIP3, CDKN1A, DNAJC15, GADD45A, SCARA3, GDF15, INSIG1, and TP53) were proven to be methylated in promoter regions. The altered expression of these genes is directly associated with the etiology of different diseases. The results also revealed aberrant expression of epigenetic regulators implicated in DNA methylation (MBD2, DNMT1, DNMT3a, DNMT3b, and UHRF1) in cells exposed to TiO2 [165].
Gold Nanoparticles
Gold nanoparticles (AuNP) have great potential for various biomedical applications, but their extensive application is dependent on possible toxicity in the liver, which is their leading accumulation site. A study conducted to determine the cytotoxic effects induced by AuNP of different sizes (15 nm to 60 nm), shapes (nanospheres or nanostars), and coating (citrate or 11-mercaptoundecanoic acid MUA) in human HepaRG cells and rat hepatocytes, proved that AuNPs have low toxicity in liver cells. Among all AuNPs tested, the most miniature 15 nm spheres showed the highest toxicity [166]. Early observations indicated that the exposure of human fetal fibroblasts to AuNP affected the expression of 19 genes, for example, miR-155 and PROS1, and induced global chromatin condensation [167]. Moreover, a most recent study which was performed in liver cancer cells demonstrated the altered expression of miRNAs (mainly miR-34a) in response to the application of AuNP [168]. Interestingly, both works concluded that AuNPs do not affect DNA methylation levels. Nevertheless, current studies aim to synthesize novel AuNPs for anti-cancer therapeutics focused on ncRNA inhibition [169].
Silica
Silicon-based materials are used in many applications, including drug delivery, dietary supplements, implants, and dental fillers. Silica nanoparticles (SiNPs) interact with immunocompetent cells and induce toxicity [170]. Recent studies proposed that these particles could alter gene expression by modulating epigenetic marks. The possible relationship between exposure to particles and these mechanisms could represent a critical factor in carcinogenicity. An investigation compared the effects of two transforming particles, NM-203 pyrogenic amorphous silica nanoparticle and Min-U-Sil® (U.S. Silica Co., Frederick, MD, USA) 5 crystalline silica particle on the Bhas42 cell line. Short-term use of Min-U-Sil® 5 decreased global DNA methylation and increased the expression of DNMT3a and DNMT3b, responsible for de novo DNA methylation. Treatment with NM-203 did not affect the expression of these enzymes nor DNA methylation levels. Furthermore, differences in global histone H4 acetylation status and HDAC protein levels were only observed in cells treated with Min-U-Sil® 5. Finally, both types of particles induced a strong expression of c-MYC at the early stages of cell transformation, which correlated with the enrichment of RNA polymerase II and active histone marks in its promoter region [171].
The Balance between the Beneficial and Detrimental Effects of Nanomaterials and Nanoparticles
The main goal of nanotechnology is to develop smart nanomaterials that improve life quality without inducing adverse effects [172]. This fact explains why their wide usage and the global market are increasing in an exponential manner [173]. There are many pros and cons for their adoption; one of the most promising applications of NMs is in the field of medicine and pharmacology. For example, nanoparticles and nanomaterials have been designed as drug delivery systems and therapeutics. Besides their potential application as antitumor drugs [174], they can be used as carriers to deliver proteins, vaccine antigens, and drugs to a specific site of action [175]. A particular application in the context of ncRNA-based therapy (discussed in Section 5.10) is that nanoparticles have been developed for the safe transference of nuclei acids and increasing their stability to reach their targets [176,177]. Moreover, the application of NMs for the improvement of the environment is promising for the reduction in contaminants. NMs such as photocatalyst, nanosized adsorbents, nanomembranes, have been developed as emerging technologies for water purification [178].
On the other hand, NMs may have a double-sword effect. Their widely used application should be taken with caution as the exponential growth of nanotechnology usage imposes concerns over its impact on human and environmental health and safety (EHS) [173]. It is important to recognize that information about their environmental fate, transformation, transport, and accumulation in other environmental compartments (e.g., air, air, soil, and water) are still under study. Attention has been focused on their accumulation by aquatic environments [179]. Furthermore, there is evidence indicating that many NPs possess DNA-damaging activity; however, the conclusions are still controversial [173] As epigenetic mechanisms have an important role in the onset and progression of diseases, some attention should be given to obtain more inside correlation between epigenetic alterations and diseases, specifically for understanding the pathogenesis associated with nanomaterials. There are still some questions regarding the nano-size effect of materials (optical, thermal, magnetic, and the induction of reactive species, in the regulation of the epigenetic landscape, remain open. Taking both pros and cons of nanoparticles and nanomaterials, we consider that more research should be performed in the field of nanotoxicology to provide indications regarding their widespread usage in the future. As epigenetics represents a novel endpoint in the field of nanotoxicology [172], it should be important to increase the evidence for epigenetic toxicity in human-based biological models to distinguish between adverse health effects of NP exposure, in contrast to adaptative mechanisms to these nanoparticles. Possibly in the future, novel NMs could be developed to counterattack the effects of other NMs.
3.4.2. Pharmaceutically Active Compounds
Pharmaceutically active compounds (PhACs) are a group of compounds that include hormones, antibiotics, and painkillers. They have adverse effects on numerous life forms because of their toxicity and can be found profoundly in wastewater (for instance, from hospitals) and surface waters [180]. Recently, the epigenetic effects caused by PhACs in aquatic animals have been investigated [181,182], but their consequences on human health remain unknown. In addition, very few studies have evaluated the effect of different epigenetic toxicant combinations. Multiple studies which assessed the impact of mixed industrial chemicals (e.g., p,a-DDE, Li, Fe, Ni, Sb, Pb, Bi, V, As, S) [183], mixtures of endocrine disruptors derived from plastics (BPA, DEHP, DBP) [184], and total xenoestrogens [185] on aquatic animals showed that PhACs influence DNA methylation. However, their impact on ncRNAs signature remains to be studied.
Diethylstilbestrol
Diethylstilbestrol (DES) is a synthetic nonsteroidal estrogen with high estrogenic activity [186]. DES induces a reduction in the expression of P450scc in the early stages of steroidogenesis. Recently, it was reported that the mouse cell line treated with DES, not only shows a reduction in the expression of P450scc but also induces histone modifications in its gene. This effect was further validated by ChIP assay, which confirmed the high occupancy of histone deacetylases on the P450scc promoter region upon treatment of TTE1 cell lines with DES. Such findings opened a novel panorama regarding epigenetic reprogramming caused by toxic compounds in a biological context associated with reproduction [186].
3.4.3. Parabens
Parabens are preservatives widely used in consumer products, including cosmetics and food. In recent years, the effects of low doses of parabens on human health have been a matter of controversy [187]. The impact of prenatal paraben exposure on infant overweight was investigated by combining epidemiological data from a mother–child cohort with experimental approaches. Mothers who reported using cosmetic products containing parabens had elevated concentrations of them in their urine. Consistently, maternal exposure of mice to parabens increased food intake and weight in female pups. The effect was accompanied by an epigenetic modification in the neuronal enhancer of Pro-opiomelanocortin (POMC1), leading to reduced hypothalamic POMC expression [188].
3.4.4. Aluminum Hydrochloride
Aluminum hydrochloride is a potential neurotoxic agent in cosmetic antiperspirants [189] which is associated with the risk of cognitive disorders such as neurodegenerative diseases. Moreover, aluminum exposure in rats was shown to cause spatial learning damage and impairs memory functions, mediated by increased expression of histone deacetylase 6 (HDAC6), reduced H3K9, and H4K12 acetylation at the promoter of the brain-derived neurotrophic factor (BDNF), which will eventually inhibit BDNF expression [190].
4. Effects of Epigenetic Toxicants in Phenotypic Transformation
Epimutations in Pollution-Associated Diseases
Every single toxicant described above displays harmful effects that differ among cell types. Consequently, various epigenetic changes trigger the activation, deactivation, or modulation of biological processes, which leads to the development of pollution-related diseases, including cancer. The following sections will describe the epigenetic implications focusing on ncRNAs as mediators of cellular responses upon exposure to toxicants in the human body. Furthermore, we will highlight seminal studies elucidating their relationship with pathological processes.
Glyphosate is extensively used in agriculture, forestry, urban areas, and aquaculture [191]. It is not approved for applications in the aquatic environment. Nevertheless, it can be easily detected in surface and groundwater [192]. Therefore, studies have indicated the presence of glyphosate in the human body, mainly in blood and urine [193,194], with cytotoxic effects in liver, lung, and nervous system [195]. In addition, this herbicide has been associated with alterations in methylation in the breast [196], blood [144], kidney [197], and lung fibroblasts [198]. In addition, it increases the expression of genes coding for enzymes that regulate DNA methylation processes, i.e., DNMT1 (maintenance methyltransferase) and DMNT3A (de novo methyltransferase) in human PBMCs [144]. Increased DNMT1 and HDAC3 activities contribute to the condensation of chromatin, preventing transcriptional factors (TF) binding, resulting in the inhibition of transcription [144] related to neoplastic transformation. DNMT1 mediates the hypermethylation of RASAL1, which encodes an inhibitor of the RAS oncoprotein in primary human fibroblasts, causing their activation. Moreover, pathological hypermethylation by DNMT1, and therefore transcriptional dysregulation, can be induced by long-term exposure to the pro-fibrotic transforming growth factor TGF-β1, while increased DNMT3a expression induces methylome modifications in organ fibrosis [197]. However, it is currently unclear if glyphosate might promote the expression of TGF-β1 in humans, although one report showed that glyphosate exposure induced inflammatory response in other species through TGF-β1 upregulation [199].
Exposure to nickel (Ni), mainly insoluble Ni compounds, is related to the development of different types of cancer such as lung cancer [200] through disruption of epigenetic processes. Some studies demonstrated an alteration in methylation states in A549 cells due to an increase in di- and tri-methylation on H3K4 upon exposure to Ni [201]. Moreover, it is known that histone H4 has a high-affinity binding site for Ni in its N-terminal tail; thus, the exposure to Ni could lead to changes in the chromatin structure of target genes [202]. In addition, some ncRNAs may play a role in nickel-induced cell transformation and carcinogenesis; for example, miR-21 is upregulated in mouse lungs by Ni nanocompounds exposure in a dose-dependent manner. miR-21 enhances TGF-β/SMAD signaling events and finally promotes lung fibrosis [203]. Furthermore, in both in vitro and in vivo studies, mouse primary peripheral blood monocytes were exposed to Ni, which led to the upregulation of miR-21 and further downregulation of RECK. MMP-2 and MMP-9 expression increased, and their activity resulted in excessive degradation of the ECM, causing reduced structural integrity [204]. Moreover, Ni and hypoxia inhibit the demethylase activity of JMJD1A and cause SPRY2 repression in BEAS-2b cells [205]. Indeed, SPRY2 downregulation contributes to cellular transformation in various human cancers, as in non-small cell lung cancer [206]. Chronic exposure to Ni also induces the upregulation of ZEB1, a gene associated with EMT that causes persistent downregulation of its repressors (e.g., miR-205, miR-200a, and miR-200b) [207]. Something similar occurs in rat lung tissues, where Ni oxide nanoparticles (NiO NPs) cause pulmonary fibrosis via activating TGF-β1 and a decrease in MEG3 expression, a lncRNA that inhibits the levels of TGF-β1 and therefore the EMT occurrence [208].
Cadmium (Cd) can be ingested, inhaled, and absorbed by the skin because of its ubiquitous presence in the environment. Chronic exposure even in low doses has been related to kidney, liver, and cardiovascular damage, having adverse effects during reproduction and pregnancy [209]. Cd also has teratogenic solid and mutagenic effects due to aberrant alterations of multiple ncRNAs involved in pathophysiological conditions and signaling pathways that lead to the development of lung, breast, prostate, pancreas, urinary bladder, and kidney cancer, among others [210]. In vitro and in vivo studies have been performed to characterize some ncRNA signatures related to Cd exposure. For example, chronic Cd leads to a malignant phenotype in human prostate epithelial cells, where miR-96 and miR- 9 were upregulated; miR-181d, miR-205, miR-125a-5p, miR-373, miR-146b-5p, miR-138, miR-155, miR-222, let-7b, and let-7c were downregulated. These miRNAs have previously been related to pathways such as oncogenesis, cell survival, altered apoptosis, and cell adhesion [211].
Cd also affects miRNAs expression in the kidneys, specifically the renal cortex, leading to acute kidney injury, as seen in male Sprague Dawley rats treated with CdCl2, where expression of a nine-miRNA signature (miR-21-5p, miR-34a-5p, miR-146b-5p, miR-149-3p, miR-224-5p, miR-451-5p, miR-1949, miR-3084a-3p, and miR-3084c-3p) showed significantly increased levels. In contrast, the expression of miR-193b-3p, miR-455-3p, and miR-342-3p significantly decreased compared to control conditions [212]. Furthermore, patients with acute renal damage have higher miR-21 levels in their urine than healthy individuals [212]. On the other side, Cd also transformed 16HBE cells. Among the miRNAs, miR-27b-3p appeared to be upregulated, while miR-944 was downregulated in Cd-treated cells. Consequently, CCM2 upregulation was induced. Additionally, it encodes a scaffold protein that promotes RHOA breakdown by interaction with SMAD. In endothelial cells, RHOA is necessary for the appropriate cytoskeletal organization, cell–cell interactions, and lumen formation [213].
In addition, Cd is highly present in cigarettes and upregulates the expression of miR-101 and miR-144 that target the CFTR 3′UTR to suppress its expression in 16HBE cells. This was also demonstrated in C57BL/6 female mice exposed to cigarettes, where an upregulation of miR-101 resulted in the suppression of CFTR in the lungs [176]. Moreover, miR-101 is more expressed in lung samples from patients with severe COPD than control patients [214]. In the same Cd-transformed 16HBE cells, a differential lncRNA expression has been reported, a 369-lncRNA signature was upregulated, whereas a ninety-lncRNA signature was downregulated. The lncRNA ENST00000414355 was highly expressed, and its silencing reduced the expression of DNA damage-related genes (ATM, ATR, and ATRIP), while DNA repair-related genes increased [215]. In Sprague Dawley rats exposed to Cd, the expression of ENST00000414355 corresponded with the expression of its target genes [215]. The same animal model showed a positive correlation between MALAT1 and BAX, TGF-β1, and STAT3 expression. Thus, MALAT1 knockdown in Cd-transformed 16HBE cells inhibited cell proliferation, apoptosis, migration, and invasion [216]. Since the liver is the principal site for Cd deposition, several investigations have focused on liver harm by Cd exposure. A report demonstrated that at a high concentration of Cd in HepG2 cells, many miRNAs belonging to the let-7 family (tumor suppressor miRNAs) were dysregulated [217]. Furthermore, in HepG2 cells, the lncRNA MT1DP is induced by Cd exposure, thus promoting cell death by activating RHOC-CCN1/2-AKT signaling and the consequent acceleration of Ca2+ influx, resulting in accelerated and elevated Cd toxicity [130]. MT1DP further enhances the repressive function of miR-365 that decreases NRF2 levels, a protein that responds to Cd-induced oxidative stress and participates in removing intracellular ROS in the HepG2 cell line, thus aggravating oxidative stress in hepatocytes [218].
Exposure to chromium (Cr) occurs mainly via inhalation or dermal absorption, although nowadays hexavalent chromium [Cr(VI)] is highly present as a toxicant in drinking water [219]. It has become a significant public health concern with toxic effects in the skin, oral, digestive, hepatic, respiratory, and renal systems [219], capable of inducing carcinogenesis through both genetic and epigenetic mechanisms, as evaluated in mice [220] and human cells [221]. People highly exposed to Cr may have high concentrations in the urine associated with the expression of miR-21 and miR-155, promoting chronic kidney diseases [222]. In addition to urine, Cr has been detected in blood where it induces miR-142-3p, miR-19a-3p, and miR-19b-3p downregulation, and in contrast with upregulation of miR-941. Among them, miR-19a-3p is highly expressed in ovarian and gastric cancer, although the mechanism inducing Cr-mediated cellular transformation is unknown [223]. Decreased miR-3940-5p [224] and miR-143 levels in plasma are also linked to high Cr concentration in blood. In BEAS cells exposed to Cr, miR-143 was reduced, whereas IL6 (a cytokine that regulates cell differentiation and proliferation) levels were significantly higher than control cells. Therefore, the overexpression of miR-143 decreased the expression of IL6, inhibiting at the same time HIF-1α and NF-κB P65 expression, thus affecting tumor growth [225].
In 16HBE-Cr cells, the relative expression of lncRNAs increased or decreased gradually in a dose-dependent manner. For instance, RP11-817O13.6 increased first (0.63 μmol/L) and then decreased at the higher dose (10 μmol/L), suggesting that the ncRNA profiling may change according to the amount, the time of exposure, and even the cell type, as seen in BEAS-2B-Cr (1 µM) cells where miR-143 expression levels were much higher than in the lung cancer cells A549 and H2195, that further lead to Cr (VI)-induced cell malignant transformation via upregulation of insulin-like growth factor-1 receptor (IGF-IR) and insulin receptor substrate-1 (IRS1) expression [226]. Moreover, the interleukin-8 is strongly induced by Cr (VI) through the activation of the IGF-IR/IRS1 axis, followed by the activation of the downstream ERK/hypoxia-induced factor HIF-1α/NF-κB signaling pathway, resulting in enhanced angiogenesis [227]. Other authors have suggested different Cr(VI) mechanisms such as the miR-21-PDCD4 signaling axis [226] and by inducing cancer stem cell (CSC)-like properties with increasing c-MYC (target of miR-494) levels through the downregulation of miR-494 at a chronic low dose (0.25 µM) [228].
All these mentioned works attempted to describe some action mechanisms of epigenetic toxicants mainly by in vitro experiments. However, in most of them, only a single ncRNA molecule is highlighted and represented as a unique mediator of crucial biological pathways that lead to the phenotypic transformation of the cell. This approach sets aside other essential factors such as associated proteins, methylation changes, participation of multi-component structures, and loci at specific times of the ncRNAs of interest. Therefore, the importance of conducting robust, unbiased, and integrative studies emerges to assess potential multi-omics technologies and the implementation of biochemical and molecular experiments able to describe epigenetic processes with high precision.
5. ncRNA Dysregulation in Pollution-Related Cancer
An increasing number of studies suggest that mutations or abnormal expression of ncRNAs are closely related to various diseases, including cancer [229]. Particularly, miRNAs have an oncogenic or tumor-suppressive function; even miRNA expression is globally suppressed in tumor cells compared with normal tissue, suggesting that miRNA biogenesis might be impaired in cancer [230] (Figure 3). For example, mercury chloride treatment in the human embryonic kidney (HEK-293) and human umbilical vein endothelial (HUVEC) cells induced the overexpression of miR-92a and miR-486. This work suggested that miR-92a and miR-486 moderate the NF-κB pathway activation during mercury toxicity [114]. However, it is not yet clear how miR-92a and miR-486 are upregulated in workers and cells after mercury exposure.
Figure 3.
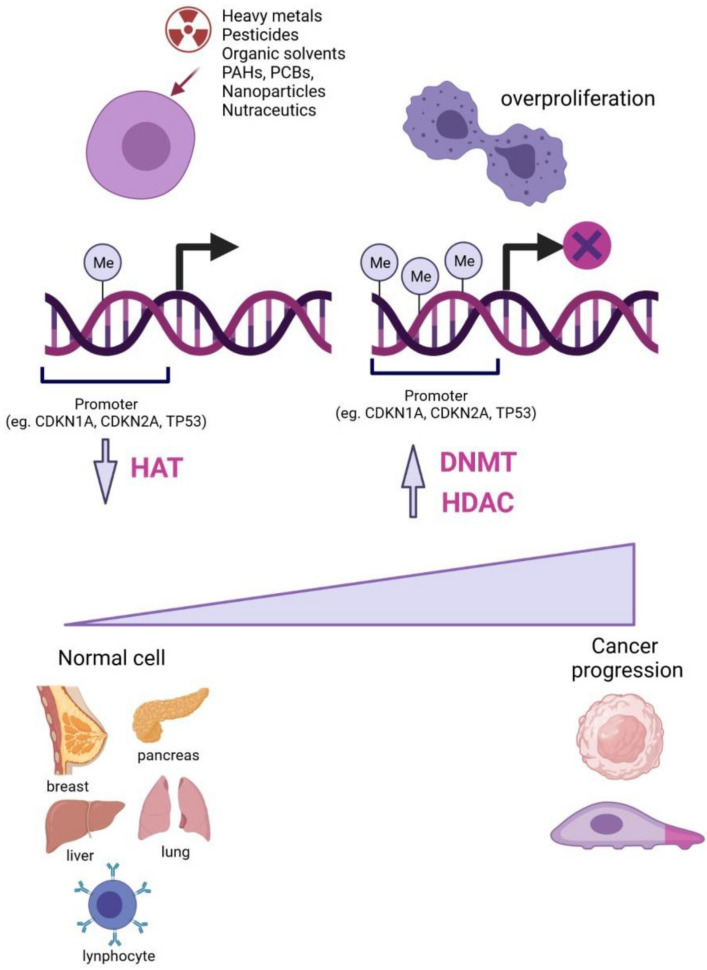
Epigenetic toxicants in humans induce chromatin structure changes. Exposure to toxicants is associated with hypermethylation in cell cycle regulators genes, causing hyperproliferation and cell transformation. PAHS: Polycyclic aromatic hydrocarbons; PCBs: polychlorinated biphenyl; HATs: histone acetyltransferases; DNMT: DNA methyltransferases; HDAC: histone deacetyltransferases.
5.1. ncRNA Dysregulation in Liver Cancer
In HepG2 cells treated with Cd, the lncRNA MT1DP/RhoC interaction activates the CCN1/2-AKT pathway. MT1H competes with miR-214 to avoid MT1DP suppression, triggering cell death. MT1DP decreases NRF2 activity, an antioxidant gene transcription factor, to evoke oxidative stress through the elevation of miR-365, which directly represses NRF2 transcription [208]. These studies reveal a new mechanism underlying the MT1DP-conducted signaling promoting Cd toxicity in hepatocytes.
The compound hexahydro-1,3,5-trinitro-1,3,5-triazine (also known as cyclomethylenetrinitramine, RDX) is an environmental toxicant resulting from human activities, such as military actions. Additionally, the effect on the global expression profile of miRNAs in B6C3F1 mice exposed to RDX was reported. Interestingly, the alteration in miRNAs profiles showed that the brain is the most affected organ upon RDX exposure, compared to the liver [231]. Moreover, RDX exposure increases the expression of miR-206, reducing BDNF and tankyrase 2, poly(ADP-ribose) polymerase tankyrase 2 (TNKS2) cluster gene expression potential targets in the brain, which may influence the neurotoxic effect by RDX. Moreover, many cancer-related miRNAs were significantly affected by RDX exposure [231].
5.2. ncRNA Dysregulation in Ovarian Cancer
A ncRNA signature may predict the origin of different histologic types of cancer even with a different tumor grade. In the case of ovarian cancer (OC), many authors have demonstrated specific miRNA and lncRNA expression patterns from various approaches. High-grade ovarian serous carcinoma (HGOSC) is the most common ovarian epithelial malignancy, and it shares similar histologic features with serous uterine carcinoma (USC) that make the diagnosis difficult [232]. A panel of 11-miRNAs, identified with a random forest model, could discriminate between OSC and USC, among them, miR-196b, miR-532, miR-886-3b, and miR-99b exhibited lower levels in HGOSC compared with USC, whereas miR-29c, miR-155, miR-454, miR-146b5p, miR-486-5p, miR-27a, miR-192, and miR-141 exhibited higher expression in HGOSC. Moreover, a nested cross-validated 6-miRNA predictive model was able to classify the tumors with 91.5% accuracy [232]. Although other studies show different signatures with significantly up-modulated miR-200 family and miR-141 in a well-differentiated OC subtype, suggesting their role in developing a specific epithelial OC subtype [233]. Likewise, lncRNA signatures have been related to critical clinical aspects in patients with OC, for example, LINC00861, LEMD1-AS1, and LOC101927151 may act as prognostic factors [234], and the expression of lncSERTAD2-3, lncSOX4-1, lncHRCT1-1, and lncMYC-2 (also PVT1) are independent prognostic markers of relapse and survival for stage I OC [235]. Additionally, patients with high or low risk have distinctive lncRNA signatures [236]. In addition, the predictive value may be independent of other clinic-pathological factors, as in the case of the eleven-lncRNA signature validated for the prediction of both early and late survival [237]. Interestingly, other studies have established lncRNAs- and multi-RNAs-based models that could predict the response to treatment [238] and the recurrence risks [239], respectively.
5.3. ncRNA Dysregulation in Breast Cancer
Concerning breast cancer (BC), several investigations aimed to establish a ncRNA signature associated with specific characteristics of the different BC subtypes. For example, triple-negative breast cancer (TNBC), compared to other breast cancer subtypes, has the highest incidence and poorest prognosis. A logistic regression model identified an eight-miRNA signature associated with tumor recurrence and a decreased survival in high-risk patients [240]. Detection of BC at an early stage can prevent the development of metastasis and death. Therefore, another approach consists of characterizing molecular signatures based on tissue profiles and validating them with serum profiles [241]. In this way, four miRNAs have been validated for patients at the early stage, although in this case, most of the used samples were from Hispanic patients, while serum samples were from Chinese patients [241]. Putatively the lack of a uniform cohort may reduce the classification accuracy of the signature.
Using a support vector machine (SVM)-based classifier model, a thirty-four-miRNA signature could categorize patients into early and advanced BC stages with more than 80% accuracy, including four miRNAs associated with poor prognosis [242]. Clinical risk factors also contribute to prognosis prediction. A six-miRNA signature and risk factors as age, tumor-node-metastasis (TNM) stage, and some molecular characteristics (receptor for estrogens and progesterone, as well as human epithelial growth factor receptor 2-HER2 status) can predict 5-year overall survival (OS), with a predictive accuracy (0.75) higher than TNM stage (0.65) which is generally used for prognostic assessment and determine specific treatments [243]. Meanwhile, with the least absolute shrinkage and selection operator (LASSO) regression method, 17-miRNA-based OS, we set to predict a 13-miRNA-based recurrence-free survival (RFS) classifier signature [244].
With the same method, and highlighting the importance of the tumor immunology, eight immune-related lncRNAs correlated with OS [245] and a six-lncRNA signature could separate two immune risk subgroups, where cohorts of high risk showed reduced levels of CD8-T cells and the immunity-related signature compared to cohorts of low risk, suggesting a role of CD8-T cells as a prognostic feature [246]. In addition, another ten-lncRNA signature for RFS [247] and an eight-lncRNA signature that can divide a cohort into two risk groups with different survival times [248] have been further validated as independent predictors. A lncRNA signature may help predict the sensitivity and outcome response to chemical treatment, a tamoxifen efficacy-related lncRNA signature (TLS) was obtained to predict the response to tamoxifen of ER+ BC patients, demonstrating that patients with increased levels of TLS were more resistant to tamoxifen therapy as these lncRNAs interfered with cell cycle arrest induced by the drug [249]. Even a circRNAs signature is associated with docetaxel resistance in BC-cell lines where circABCB1 was upregulated, whereas circEPHA3.1 and circEPHA3.2 are downregulated [250].
5.4. ncRNA Dysregulation in Pancreatic Cancer
Pancreatic cancer (PC) has the worst prognosis of all cancers. More than 90% of PC patients are diagnosed at late stages, suggesting that RNA signatures should have a potential role as biomarkers. Recently, a thirteen-miRNA signature was developed which helps to better evaluate and predict patients with a high OS [251]. Some miRNAs (miR-103-2, miR-126, miR-340, miR-374b, and miR-627) were associated in addition with other characteristics, such as alcohol consumption [251], suggesting the importance of integrating them into other clinical characteristics of the patients to obtain more specific signatures. The pancreatic ductal adenocarcinoma (PAAD) is the most frequent subtype where a five-miRNAs panel (miR-125a, miR-328, miR-376b, and miR-376c, miR-1301) could increase the capacity of prognostic prediction for patient survival [252]. Meanwhile, by a prospective study for longer follow-up times (which may represent tumors in earlier stages of tumorigenesis), positive associations between the over-expression of four-miRNAs (miR-10a, miR-10b, miR-21-5p, and miR-30c) and PAAD development may be set [253]. Although other two-miRNAs (miR-33a-3p and miR-320a), in addition to the serum carbohydrate antigen 19.9 (CA19.9), used for monitoring response to treatment, increased the identification of patients with PC or premalignant intraductal papillary mucinous neoplasm (IPMNs) from healthy people with an accuracy of 0.95 [254]. In another study, the application of a three-miRNA signature (miR-125a-3p, miR-5100, and miR-642b-3p) for PC diagnosis yielded the same accuracy [255]. However, through a LASSO regression model, a five-lncRNA signature was identified which was shown to be correlated with better survival [256]. In another study, a four-lncRNA signature (ABHD11-AS1, HOPPIT, LINC00460, and miR-205HG) demonstrated to have a negative correlation with OS, whereas a two-miRNA signature (C9orf139 and miR-600HG) correlated to OS in a positive manner [257].
5.5. ncRNA Dysregulation in Colorectal Cancer
Several lines of evidence indicated that genes associated with the immune response together with lncRNAs serve as predictor markers for OS in colon cancer patients. Additionally, Lin et al. identified a lncRNA signature associated with the immune response for prognosis in patients. A highly expressed lncRNA signature (AC008760.1, AC009237.14, AC083809.1, AL391422.4, AL445645.1, LINC01063, and LINC01234) was associated with poor prognosis; on the contrary, the expression of AC016027.1 indicated an association with good prognosis [258]. Pichler et al. analyzed genome-wide miRNA sequencing data of 228 colorectal cancer patients from The Cancer Genome Atlas dataset to identify miRNAs significantly associated with survival. A six-miRNA signature (miR-92b-3p, miR-188-3p, miR-221-5p, miR-331-3p, miR-425-3p, and miR-497-5p) was identified strongly predicting patient survival in the screening cohort. High miR-188-3p expression proved to be an independent prognostic factor since its exogenous expression increased migration of cancer cells in vitro and the formation of metastases in vivo. Mechanistically, the pro-migratory role of miR-188-3p is mediated by direct interaction with MLLT4, a novel biomarker associated with cell migration in colorectal cancer [259].
5.6. ncRNA Dysregulation in Lung Cancer
MALAT1 is one of the most assessed lncRNAs involved in important biological processes. In fact, numerous molecules, and their corresponding signaling pathways, have been validated as targets of MALAT1 in lung cancer. MALAT1 activates the epithelial-mesenchymal (EMT) process and increases brain metastasis-initiating lung cancer cells [260]. In addition, MALAT1/miR-101-3p/MCL1 axis mediates cisplatin sensitivity in cisplatin-resistant A549/DDP cells [261]. Another vastly assessed oncogenic lncRNA in lung cancer is XIST (X-inactive specific transcript), which was reported to be increased in NSCLS cell lines compared to normal human bronchial epithelial cell lines. Concordantly, XIST silencing suppressed proliferation, invasion, migration, and finally programmed cell death [262]. Furthermore, the association between expression levels of lncRNAs panels and patient prognosis has been evaluated in several investigations. For example, a high-throughput study has shown the potential of ADAMTS9-AS2, AC011483.1, TTTY16, AC006238.1, LINC00462, and CACNA2D3-AS1 in the prediction of OS of patients with lung cancer [263]. Gong et al. have recently compared RNA-seq data with the TCGA repository to find lncRNAs with predictive potential in lung cancer. This analysis identified 84 dysregulated lncRNAs in lung cancer tissues, among which ten lncRNAs were mainly associated with the survival of patients. In addition, LINC01537 has been recognized as the most significant lncRNA whose downregulation was verified in the subsequent analysis [264].
Up to now, numerous investigations exist analyzing lncRNA expression data in lung cancer, and robust validations in non-dependent datasets. However, the lncRNA landscape in NSCLC is far from comprehensive, mainly because of the minor independent validation inconsistency in targets and sample sets [259]. Recently, Acha-Sagredo et al. validated a twelve-lncRNA signature (upregulated FEZF1-AS1, LINC00673, LINC01214, LINC01929, NUTM2A-AS1, and PCAT6; and downregulated ADAMTS9-AS2, FENDRR, LANCL1-AS1, LINC00968, PCAT19, and SVIL-AS1) which is aberrantly expressed in NSCLC cancer. In addition, abnormal DNA methylation was observed in the promoters of genes such as FENDRR, FEZF1-AS1, and SVIL-AS1. FEZF1-AS1 and LINC01929 improved patient survival, according to the Cancer Genome Atlas (TCGA) set [265]. In a different approach focused on miRNAs, human bronchial epithelial cell line (BEAS-2B), as well as lung cancer tissues from patients exposed to arsenic showed an apparent, sustained upregulation of miR-301a by IL6/STAT3/SMAD4 signaling pathway, which contributed to carcinogenesis after chronic exposure of arsenic [126].
5.7. ncRNA Dysregulation in Kidney Cancer
Renal cell carcinoma (RCC) is the most common malignant tumor of adult kidneys (3.7% of all adult cancers worldwide) [266]. Although lncRNAs are seen as promising biomarkers for cancer initiation and progression, their prognostic significance in RCC is not fully understood. Liu et al. determined the ability of lncRNAs as predictors of poor prognosis in clear cell RCC (ccRCC). A total of 525 patients were analyzed based on the cohort of kidney renal clear cell carcinoma (KIRC) on the TCGA repository. Hierarchical clustering of the KIRC cohort allowed the identification of 26 differentially expressed lncRNAs (11 downregulated and 15 upregulated lncRNAs). Further survival analysis identified a significant thirty-lncRNA signature for the prediction of ccRCC prognosis, with four differentially expressed lncRNAs (HAR1B, miR155HG, PVT1, and TCL6) that correlated with OS [266].
In addition, the function of miRNAs in oncogenesis has been studied in RCC cells. Notably, it was revealed that tumor suppressor genes such as APC, MEG3, and PTEN are targets of oncogenic miRNAs such as miR-7, miR-301a, miR-22, miR-193a-3p, and miR-671-5p, suggesting a role of miRNAs in the pathogenesis of RCC. In contrast, tumor suppressor miRNAs (downregulated in RCC cells) have functional roles in the induction of apoptosis and cell cycle arrest. Genes associated with epithelial-to-mesenchymal transition (EMT) such as HIF-1a, HOTAIR, SLUG, and ZEB1 are targets of this tumor suppressor miRNAs. Therefore, their downregulation, in consequence, promotes the enhancement of EMT [267]. Furthermore, signatures based on miRNAs can be used for the classification of subtypes in RCC. For example, Youssef et al. classified different subtypes of RCC (e.g., clear cell, papillary, oncocytoma, and chromophobe RCC) with sensitivity values [268].
5.8. ncRNA Dysregulation in Acute Leukemia
Acute lymphoblastic leukemia (ALL) is known as the most common form of pediatric leukemia. One of its main characteristics is the rapid growth of abnormally developing lymphoid cells within the bone marrow and the reduced production of normal blood cells [269]. Based on RNA sequencing approaches, different studies demonstrated that lncRNAs and miRNAs are crucial in the pathogenesis of progenitor B-cell acute lymphoblastic leukemia (B-ALL). Even though the number of studies is limited, they indicate that the expression of lncRNAs is modified in this type of leukemia [231]. For example, Dinesh Rao and colleagues found “B-ALL-associated long RNAs” or BALR, differentially expressed in B-ALL (BALR-1, BALR-6, BALR-2) as well as LINC00958. Particularly, the lncRNA BALR-2 was correlated with poor OS and poor response to the pharmacological treatment (prednisone) [269,270].
In addition, miRNAs have vital biological importance during the development of B-cells and may contribute to associated malignancies. For example, the miRNA database from ALL and LeukmiR predicted 861 miRNAs associated with the disease [271]. Furthermore, the expression of miRNAs can discriminate between the different types and subtypes of leukemia. For example, a twenty-seven-miRNA signature was identified in 72 patients, which was differentially expressed among ALL and acute myeloid leukemia (AML) samples [272]. In another study, a four-miRNA signature (let-7b, miR-128a, miR-128b, and miR-223) discriminated against two leukemic types. A subsequent study also confirmed that miR-128a and miR128b (in addition to miR-34a, miR-213, miR-210, miR-130b, and miR-146a) were differentially expressed and played a significant role in ALL [273]. In addition, miRNA signatures were useful to predict prognosis and relapse. Upon miRNA profiling using paired samples, Han et al. identified a three-miRNA signature (miR-27a, miR-223, and miR-708) in children with ALL-associated relapse [274]. Similarly, other miRNAs (miR151-5p, miR-451, and miR-1290) have been identified as a putative signature for relapse occurrence when the miRNAs profile correlated with patient diagnosis and clinical outcomes [275]. Beyond miRNAs and lncRNAs, other ncRNAs have implications in leukemia, such as circRNAs [276] and snoRNAs, although they have not been extensively studied in B-ALL. Nevertheless, they have been proposed as biomarkers in hematological diseases [269].
5.9. ncRNA Dysregulation in Testis Cancer
Testis cancer is the most prevalent solid tumor in young men (15 to 40 years old), with a constant increase in the frequency of the cases [277]. LncRNAs that alter testicular tumor pathogenesis such as XIST, H19, SPRY4-IT, NLC1-1C, and HOTTIP, but most of them have not been evaluated in clinical studies, except for XIST. Previous reports have suggested the expression of the lncRNA XIST, and epigenetic marks related to the inactivation of the X chromosome as specific biomarkers [277]. In particular, the demethylation of XIST 5′ is augmented in seminomas compared to non-seminomas or normal tissue. In consequence, this biomarker has a potential value for the assessment of the quality of spermatogenesis in individuals [278].
Besides lncRNAs, the miR-371-373 cluster has been suggested as putative markers in patients with testis cancer. Some groups evidenced that miR-302/367 and miR-371a-3p and expression significantly differs between testicular tumors and healthy testicular tissue. Several clinical studies verified that these miRNAs greatly improved the sensitivity and specificity of conventional markers [279]. However, all these studies have certain limitations that need to be addressed. Although advanced bioinformatic methods were implemented, most reviewed papers did not describe the quality of the RNA-seq dataset used; in some works, available microarray was downloaded. Additionally, other researchers assessed miRNAs expression by a single- or multiplex qRT- PCR methodology. Moreover, only a few explained the population characteristics from which the sequencing data were obtained, mainly ethnicity. On the other hand, these works mention that data were obtained mainly from Asian or European populations. Then, this implies that the proposed signature may not be strictly valid for a global population, only for specific ethnic groups or none. Furthermore, most authors mention that their validation method should be improved by increasing the sample size, using data from prospective studies, and integrating into vitro or in vivo experimental data to confirm the results. The experimental validation is crucial to determine a more precise signature. As expected, this needs to be unbiased and integrative in the sense of making use of other kinds of omics-derived, high-quality sequencing data. In addition, the simultaneous consideration of multiple clinical factors and already known biomarker tests proved in biofluids (plasma, urine, saliva, etc.) would enhance the precision of the potential signatures to achieve a high enough specificity [280] and finally be considered clinically useful.
5.10. Potential Role of ncRNAs as Therapeutic Agents in Exposome-Associated Diseases
Since ncRNAs have a functional role in modulating the expression of multiple downstream gene targets and associated pathways in cancer and other diseases, there is a rationale that suggests that ncRNAs-based therapies are promising as therapeutic options [176]. Different ncRNA-based strategies have been proposed for therapeutical purposes: small interfering RNAs (siRNAs), antisense oligonucleotides (ASOs), short hairpin RNAs (shRNAs), anti-microRNAs (anti-miRNAs), miRNA mimics, miRNA sponges, therapeutic circular RNAs (cirRNAs), and CRISPR-Cas9-based gene editing [281]. Several clinical trials have confirmed that ncRNAs are successful for the treatment of complex diseases including cancer [176,282]. According to a recent review by Winkler et al., 11 therapeutics based on ncRNAs have been approved by the FDA and/or the European Medicines Agency (EMA), and many other ncRNA approaches are under phase II or III clinical testing. The majority of the approved therapeutics correspond to siRNAs or ASOs that promote downregulation of genes, or that target pre-mRNA splicing. It is important to remark that there are no ncRNA-based strategies that have entered clinical testing so far [215]. However, recent investigations highlight their potential as ncRNA-based therapeutics. For example, Battistelli et al. studied a mutant form of the ncRNA HOTAIR to reduce the epithelial-to-mesenchymal transition (EMT). Their results showed that the HOTAIR mutant form was able to reduce the binding of HOTAIR to Snail, impairing EMT [283].
Although ncRNAs represent a novel therapeutical option, there are still some limitations to their adoption in clinical practice [184]. Trials have reported ambivalent results with some studies reporting positive effects whereas others showed limited success or inducing toxicity [185]. Issues related to specificity (undesired on-target effects), delivery, and tolerability hamper their translation into the clinical scenario [184,185]. However, novel approaches to overcome these limitations are under investigation such as chemical modification of the backbone of the nucleic acid sequence [284], and the use of nanoparticles for improving their delivery and stability as they offer protection against degradation by nucleases [146,285].
Several technological advances have increased the capacity to design and manufacture most ncRNA mimics. However, a clear limitation for their use is the current lack of deep understanding of their functional roles in oncogenesis and their downstream genetic targets [286]. For example, the therapeutic application of transfer RNAs, circular RNAs, small nucleolar RNAs, and piwi-interacting RNAs is still at its infancy [286].
6. Transgenerational Inheritance after Epi-Toxicants Exposure
Recently, the concept that parental experiences can shape the offspring’s behavior and physiology has stimulated new perspectives in the epigenetics field, with an emphasis on disease risk and resilience. In addition, epigenetic transgenerational inheritance has revealed that parents’ exposure to deleterious factors such as stress, toxicants, or abnormal dietary intakes can lead to germline-mediated inheritance of epigenetic modifications even without a constant impact on the environment [287].
The fundamentals of cellular reprogramming rely on epigenetic mechanisms beyond and above intrinsic DNA sequence; meaning that without epigenetic reprogramming, those epigenetic marks which are acquired during development or those externally induced by the environment, cannot be erased. If epigenetic reprogramming fails, such epigenetic marks can be transmitted from one generation to the next one. In other words, parental (F0 generation) exposure to toxic environments can influence germlines (female ova and male sperm) as well. This implies that the next generation (F1 generation) is still considered exposed, and the F2 generation will be the first transgenerational “unexposed” generation [287]. In mammals, these mechanisms took longer to be proved convincingly, compared to simpler organisms such as nematodes (C. elegans), where evolution seems to ensure across generations the deletion of potential harmful experiences during parental lifetime.
Currently used chemotherapeutic treatments have been proven to cause effects in the F1 generation, but not in the F2 generation since the main off-target effect of these drugs is exacerbated DNA damage [288]. A transgenerational phenotype, also known as a genetic trait, requires permanent germline reprogramming, mainly due to the methylation state. For instance, primordial germ cells (PGCs) acquire genome-wide de novo methylation within one day of development and subsequent migration into the genital ridge. However, after their entry, there is a rapid removal of DNA methylation of regions within imprinted and non-imprinted loci in males and female embryos [289]. Afterwards, germ cells in the gonad undergo a process in which they are methylated during gonadal sex determination [290].
The fact that an environmental factor (for example, an endocrine disruptor such as BPA) can reprogram the germline and promote a transgenerational disease state has significant implications for evolutionary biology and disease etiology. Although epigenetic inheritance is commonly associated with plant inbreeding [291], some pioneering studies describing this phenomenon in animals date back to the 1950s. Indeed, by the seminal studies of Conrad Waddington, who discovered in Drosophila melanogaster that up to seven generations display the exact wing structure change induced by heat shock in F0 [292]. Slowly, the definition suggested by Waddington shifted toward the concept of heritability. However, some reports of rodents appeared almost 50 years later. In 2005, Anway et al. demonstrated that the exposure of gestating rats to vinclozolin (an antiandrogenic fungicide) or methoxychlor (an estrogenic pesticide) induced an adult phenotype from F1 until F4 generations, including decreased spermatogenic capacity and increased incidence of male infertility. Interestingly, this work correlated DNA methylation patterns to the altered reproductive phenotype [293]. Other reports demonstrated that transgenes defined mice’s specific active or inactive transgenerational inheritance. For instance, the obese, diabetic, tumor susceptible, and yellow fur phenotype in mice is not only linked to the ectopic expression of the agouti protein. Still, it is directly induced by a transposable element located 100 kb upstream of the agouti A gene [294]. Even if this process is not likely conserved in humans, several epialleles for cancer predisposition (for instance, the MLH1 locus for colorectal cancer or the DAPK loci for lymphocytic leukemia) depend on specific polymorphisms on the DNA sequence and are maintained in every generation. Moreover, these individuals display young-onset cancer by non-Mendelian inheritance through their reversal in the germline [295,296].
At this point, it is essential to set a clear difference between intergenerational, such as in utero exposure to nutrients, hormones, toxins at embryonic stages, and actual transgenerational mechanisms [297,298]. In addition, in subsequent generations, there may be an increased susceptibility to disease since they would respond in an aberrant manner to the same environmental agents, which therefore develops into generational toxicology, processes that usually involve transposable elements and are generally termed as “germline epimutations” [299].
Epigenetic marks in germ cells, which are vulnerable to environmental stimuli and capable of directing cellular fates during development, may mediate the effects of parental environmental exposures on the offspring’s behavior and physiology. Events of post-translational histone modification, DNA demethylation, and ncRNA expression panels in sperm have been implicated as transmitters of parental experiences (stress, nutrition, drug abuse). Rodent models examining paternal transmission have identified epigenetic signatures in mature sperm as possible substrates of transgenerational programming, namely patterns of (1) histone modifications (mostly at active marks such as H3K4me3, or inactive marks such as H3K9me2/3 and H3K27me3), (2) DNA methylation on CG, CH and CHH motifs, and (3) populations of noncoding RNAs (miRNAs, siRNAs, piRNAs, tRNAs, and lncRNAs). Therefore, different RNA subtypes are of primary interest, as they may be altered through intercellular communication via epididymosomes even in transcriptionally inert mature sperm, where DNA condensation impedes other epigenetic changes [300]. However, the identity of specific ncRNA signatures in sperm responsive to environmental clues is not yet clear.
Consistent evidence in rodents has suggested the impact of transgenerational inheritance in complex diseases as hepatic steatosis induced by tributyltin (TBT, a fungicide with obesogenic properties), which increased white adipose tissue and adipocyte size and number [301]. Furthermore, recent works suggest a chromatin-remodeling effect of TBT beyond DNA methylation, which is conserved in mice and humans and can ultimately be reconstructed [302]. Noteworthy is the potential implications for human health derived from recent studies suggesting that humans might be susceptible to environmental disruptions of chromatin organization.
Besides the prominent effects of DNA methylation and transposable elements for reprogramming and transgenerational epigenetic inheritance, their impact on mammals suggests having noncoding RNAs (ncRNAs) as key mediator molecules. As a clear example, the locus Rasgrf1 requires Mili and Miwi2 (PIWI-interacting RNAs, piRNAs) for de novo promoter methylation in differentially methylated regions (DMR) in the male germline [303]. Therefore, the role of ncRNAs is becoming more evident as a mechanistic link between paternal environmental exposure and their offspring phenotype. In F0 mice, an early maternal separation causes the downregulation of the piRNA cluster 110 [304], while a high-fat and high-sugar diet induces the differential expression of 190 piRNAs distributed in 63 clusters [305], indicating that acquired stress and food-induced trait inheritance, at least, is enhanced by ncRNA-dependent regulation, and observation later verified as well in rats in a miRNA let7c-dependent manner [306].
From the different ncRNA biotypes, the regulation of transgenerational inheritance has been studied more extensively for miRNAs and tRNAs. It is now accepted that small ncRNAs can mediate non-Mendelian inheritance of traits acquired during life, and they are, in fact, abundant in mammals’ sperm [307]. The epigenetic modification of the mouse Kit gene regulation is a clear example of miRNA-mediated inheritance. The wild-type progeny of Kit heterozygous parents shows an altered Kit phenotype (whitetail) for consecutive generations by miR-221- and miR-222-direct targeting [308]. The exposure to microRNAs of the early embryonic genome seemed at the time convincing and promising to induce a permanent and heritable epigenetic change in gene expression.
Rodgers et al. demonstrated in 2015, through zygote microinjection, a nine-miRNA (miR-29c, miR-30a, miR-30c, miR-32, miR-193-5p, miR-204, miR-375, miR-532-3p, and miR-698) signature in sperm, related to paternal stress, which induces a reduction in the levels of mRNAs from the mother in zygotes at early stages, ultimately reprogramming gene expression in the offspring’s hypothalamic–pituitary–adrenal (HPA) stress axis, and recapitulating the phenotype of offspring stress dysregulation [309]. Of note, single miRNA injections did not affect the responsivity to the activated HPA axis in terms of corticosterone levels, which highlights the necessity of an orchestrated miRNA panel response. Of relevance for stress response and metabolic regulation, miR-375 targets catenin B1 (Ctnnb1) in the mouse hippocampus. Notably, the reported miRNAs signature altered in mice induced rather systemic effects, and their expression was affected in serum and brain cortical regions [304].
Another example of small noncoding RNAs that are involved in transgenerational stress comes from 5′ halves of tRNAs, as found in the F3 generation sperm of rats that were pregnant during vinclozolin exposure [310]. Interestingly, another research group suggested that comparable behavioral, metabolic, and molecular effects were induced by either direct exposure to unexpected-maternal stress (MSUS) during early postnatal life or by injection of sperm small ncRNAs from MSUS males. In summary, cumulative evidence suggests that the environment itself can induce epigenetic transgenerational inheritance and, consequently, pollution-associated diseases, which indicates the relevance of further studies to evaluate its role as critical determinants influencing the etiologic, toxicologic, and progression factors of disease.
7. Future Perspective: Biomonitoring and Novel 3D Epigenomic Technologies
Although new drugs for several human cancer subtypes, presumably induced by epigenetic pollutants, are not yet approved at a clinical stage, the confluence of recent factors related to the development of sequencing technologies to analyze the human epigenome may change this scenario. Moreover, other factors such as an increased interest in research associated with environmental epigenetics, an improved understanding of the etiology and markers of cancer subtypes and their epigenetic landscape, and the promising results from immunotherapy might as well open new scopes.
The number of vulnerable individuals waiting for curative therapies is growing, in parallel to the growth in global industrial development. There are 1.9 million individuals living with pollution-associated diseases within the US alone, 3.9 million in Europe, and an estimated 19.3 million people worldwide, most of these cases due to cancer (2022 American Cancer Society) [311]. As it has been recently estimated, air pollution causes up to 3.1 million premature deaths worldwide every year, corresponding to 3.2% of the global disease burden [312]. Regionally, countries with low and middle incomes in the regions of Southeast Asia and Western Pacific had the most significant burden related to air pollution (2.2 and 2.8 million deaths, respectively). More than 287,000 deaths occurred in Europe, 131 000 in America, 600,000 in Africa, and 394,000 in the Eastern Mediterranean region [312]. On top of it, a currently unknown number of individuals harbor pre-symptomatic pathology, mainly children living around highly contaminated regions, which then represents an under-estimated population of individuals who may benefit from future treatments. A critical component for the approval of new therapies is selecting, validating, and deploying tools for disease screening and treatment monitoring. Here, we provide an overview of the ncRNA biomarkers acting as the driving force for pathogenesis. The development of sequencing technologies such as RNA-seq, small RNA-seq, single-cell RNA-seq (scRNA-seq), and nascent RNA-seq (GRO-seq, PRO-seq) significantly improves the integrative approaches for ncRNA research [313]. Recently developed techniques can be used to screen functional ncRNAs, such as global RNA interactions with DNA by deep sequencing (GRID-seq) to determine all potential chromatin-interacting RNAs [314]. Another important tool comes from public databases since they provide major references based on theoretical analysis, sequencing data, and even experimental verification, which guide the identification and the functional investigation of ncRNAs involved in human diseases [313].
Tremendous efforts are desired to discover and utilize ncRNAs as biomarkers in clinical diagnosis, calling for technological advancement in the analysis of circulating ncRNAs in biospecimens. Point-of-care testing based on microfluidic technology allows faster detection and diagnosis of diseases near the patient site than conventional lab-based testing [315]. It focuses on developing miniature laboratory-based procedures into user-friendly platforms that are portable, simple, and disposable [316]. This technology has been proposed for the integration for the extraction and purification of ncRNA from body fluids and in situ analysis. However, it is an ongoing challenge to integrate all these beneficial elements in a singular, convenient, and proof platform [316].
As technologies evolve, global interactions between ncRNA influencing chromatin modifications are still not covered. For example, lncRNAs exert their functionality by tuning chromatin architecture, resulting in the alteration in transcription readouts [317]. As Mishra and Kanduri referred, there are no unifying principles to understand the chromatin associations with lncRNAs [317]. Therefore, in the context of environmental exposures, the integrative mechanism that mediates the effect of ncRNA on chromatin remodeling processes is something that could be explored in the future to understand the effects of the exposome in a global manner.
Even though extensive research directed to characterize reprogrammable epigenetic signatures in pollution-associated diseases is still pending. Preparing the healthcare system for the advent of disease-modifying therapies against the effects of industrialization is imperative. It will be necessary to identify the most suitable early biomarkers to facilitate appropriate upcoming treatment, primarily for affected populations. We aimed to summarize recent developments in environmental biomonitoring and the validation of ncRNA signatures for pollution-associated pathologies. We discussed potential approaches that could be adopted to screen for and clarify the underlying pathology in people seeking medical advice because of late-stage symptoms. Future clinical trials should incorporate both CT scanning and radio-imaging and biomarker approaches by next-generation sequencing to assess the nuclear changes and the biological response at the same time. Given the potential barriers which may impede access to proper therapy in isolated communities located in high-exposure areas, the need to expand treatment options beyond specialized centers towards fluid biomarkers by non-invasive approaches is evident. While the proposed recommendations need empirical data for support, they represent a testable scenario regarding how upcoming clinical trials could be designed and treatments could be delivered in the clinic with the help of biomarkers. We briefly review recent data regarding ncRNA biomarkers for pollution-associated cancer in humans, highlighting the underlying epigenetic dysregulation in these patients. There is an evident need for further research into comorbidities induced by common environmental pollutants to suggest how different biomarkers could be used (most likely in combination as “disease epigenetic signatures”) to facilitate the development and clinical implementation of novel drug candidates against pollution-associated diseases.
Acknowledgments
We thank all the members of the EPIGEN laboratory for their resourceful comments on the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom12040513/s1, Table S1: Clinical and molecular relationship between toxicants and ncRNAs [318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396,397,398,399,400,401,402,403,404,405,406,407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431].
Author Contributions
M.Á.O.-S., S.L., P.S., G.B. and K.R. contributed to the conception and design of the study; M.Á.O.-S., I.R.-D., A.P.-G., A.M.-H., M.Á.C.-G., S.L., P.S. and K.R. wrote the manuscript; S.L., P.S. and G.B. helped with constructive discussions. All authors have read and agreed to the published version of the manuscript.
Funding
Guillermo Barreto is funded by the “Centre National de la Recherche Scientifique” (CNRS, France), “Délégation Centre-Est” (CNRS-DR6), the “Lorraine Université” (LU, France) through the initiative “Lorraine Université d’Excellence” (LUE). Karla Rubio was funded by the “Consejo de Ciencia y Tecnología del Estado de Puebla” (CONCYTEP, Puebla, Mexico) through the initiative International Laboratory EPIGEN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang L., Lu Q., Chang C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020;1253:3–55. doi: 10.1007/978-981-15-3449-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Vrijheid M. The exposome: A new paradigm to study the impact of environment on health. Thorax. 2014;69:876–878. doi: 10.1136/thoraxjnl-2013-204949. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen R., Schymanski E.L., Barabási A.-L., Miller G.W. The exposome and health: Where chemistry meets biology. Science. 2020;367:392–396. doi: 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boardman J.D., Daw J., Freese J. Defining the Environment in Gene–Environment Research: Lessons From Social Epidemiology. Am. J. Public Health. 2013;103:S64–S72. doi: 10.2105/AJPH.2013.301355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 6.Debord D.G., Carreón T., Lentz T.J., Middendorf P.J., Hoover M.D., Schulte P.A. Use of the “Exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2016;184:302–314. doi: 10.1093/aje/kwv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhubi A., Cook E.H., Guidotti A., Grayson D.R. Epigenetic Mechanisms in Autism Spectrum Disorder. Int. Rev. Neurobiol. 2014;115:203–244. doi: 10.1016/b978-0-12-801311-3.00006-8. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizio P., Garvis S., Palladino F. Histone Methylation and Memory of Environmental Stress. Cells. 2019;8:339. doi: 10.3390/cells8040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skinner M.K., Manikkam M., Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J., Loh X.J., Luo Y., Ge S., Fan X., Ruan J. Insights into the epigenetic effects of nanomaterials on cells. Biomater. Sci. 2019;8:763–775. doi: 10.1039/C9BM01526D. [DOI] [PubMed] [Google Scholar]
- 11.Bollati V., Baccarelli A. Environmental epigenetics. Heredity. 2010;105:105–112. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson O., Baccarelli A.A. Environmental Health and Long Non-coding RNAs. Curr. Environ. Health Rep. 2016;3:178–187. doi: 10.1007/s40572-016-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javierre B.M., Fernandez A.F., Richter J., Al-Shahrour F., Martin-Subero J.I., Rodriguez-Ubreva J., Berdasco M., Fraga M.F., O’Hanlon T.P., Rider L.G., et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20:170–179. doi: 10.1101/gr.100289.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota T. Epigenetic alterations induced by environmental stress associated with metabolic and neurodevelopmental disorders. Environ. Epigenetics. 2016;2:dvw017. doi: 10.1093/eep/dvw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fragou D., Fragou A., Kouidou S., Njau S., Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicol. Mech. Methods. 2011;21:343–352. doi: 10.3109/15376516.2011.557878. [DOI] [PubMed] [Google Scholar]
- 16.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019;20:5–20. doi: 10.1038/s41580-018-0059-1. Erratum in Nat. Rev. Mol. Cell Biol. 2019, 20, 321. [DOI] [PubMed] [Google Scholar]
- 17.Miguel V., Lamas S., Espinosa-Diez C. Role of non-coding-RNAs in response to environmental stressors and consequences on human health. Redox Biol. 2020;37:101580. doi: 10.1016/j.redox.2020.101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truesdell S.S., Mortensen R.D., Seo M., Schroeder J.C., Lee J.H., LeTonqueze O., Vasudevan S. MicroRNA-mediated mRNA Translation Activation in Quiescent Cells and Oocytes Involves Recruitment of a Nuclear microRNP. Sci. Rep. 2012;2:srep00842. doi: 10.1038/srep00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasudevan S., Steitz J.A. AU-Rich-Element-Mediated Upregulation of Translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh I., Contreras A., Cordero J., Rubio K., Dobersch S., Günther S., Jeratsch S., Mehta A., Krüger M., Graumann J., et al. MiCEE is a ncRNA-protein complex that mediates epigenetic silencing and nucleolar organization. Nat. Genet. 2018;50:990–1001. doi: 10.1038/s41588-018-0139-3. [DOI] [PubMed] [Google Scholar]
- 22.Rubio K., Singh I., Dobersch S., Sarvari P., Günther S., Cordero J., Mehta A., Wujak L., Cabrera-Fuentes H., Chao C.-M., et al. Inactivation of nuclear histone deacetylases by EP300 disrupts the MiCEE complex in idiopathic pulmonary fibrosis. Nat. Commun. 2019;10:1–16. doi: 10.1038/s41467-019-10066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose M., Bhattacharyya S.N. Target-dependent biogenesis of cognate microRNAs in human cells. Nat. Commun. 2016;7:12200. doi: 10.1038/ncomms12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheloufi S., Dos Santos C.O., Chong M.M.W., Hannon G.J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cifuentes D., Xue H., Taylor D.W., Patnode H., Mishima Y., Cheloufi S., Ma E., Mane S., Hannon G.J., Lawson N.D., et al. A Novel miRNA Processing Pathway Independent of Dicer Requires Argonaute2 Catalytic Activity. Science. 2010;328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statello L., Guo C.-J., Chen L.-L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. Correction in 2021, 22, 159, doi:10.1038/s41580-021-00330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Orera J., Messeguer X., Subirana J., Alba M.M. Long non-coding RNAs as a source of new peptides. eLife. 2014;3:e03523. doi: 10.7554/eLife.03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlackow M., Nojima T., Gomes T., Dhir A., Carmo-Fonseca M., Proudfoot N.J. Distinctive Patterns of Transcription and RNA Processing for Human lincRNAs. Mol. Cell. 2016;65:25–38. doi: 10.1016/j.molcel.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marques A.A., Hughes J., Graham B., Kowalczyk M.S., Higgs D.R., Ponting C.P. Chromatin signatures at transcriptional start sites separate two equally populated yet distinct classes of intergenic long noncoding RNAs. Genome Biol. 2013;14:R131. doi: 10.1186/gb-2013-14-11-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchese F.P., Raimondi I., Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C.H., Chen Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int. J. Biochem. Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 34.Ketting R.F., Fischer S.E., Bernstein E., Sijen T., Hannon G.J., Plasterk R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamura K., Ishizuka A., Siomi H., Siomi M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar A., Maji R.K., Saha S., Ghosh Z. piRNAQuest: Searching the piRNAome for silencers. BMC Genom. 2014;15:555. doi: 10.1186/1471-2164-15-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rayford K., Cooley A., Rumph J., Arun A., Rachakonda G., Villalta F., Lima M., Pratap S., Misra S., Nde P. piRNAs as Modulators of Disease Pathogenesis. Int. J. Mol. Sci. 2021;22:2373. doi: 10.3390/ijms22052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo B., Li D., Du L., Zhu X. piRNAs: Biogenesis and their potential roles in cancer. Cancer Metastasis Rev. 2020;39:567–575. doi: 10.1007/s10555-020-09863-0. [DOI] [PubMed] [Google Scholar]
- 40.Ojha S., Malla S., Lyons S.M. snoRNPs: Functions in Ribosome Biogenesis. Biomolecules. 2020;10:783. doi: 10.3390/biom10050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirose T., Shu M.-D., Steitz J.A. Splicing-Dependent and -Independent Modes of Assembly for Intron-Encoded Box C/D snoRNPs in Mammalian Cells. Mol. Cell. 2003;12:113–123. doi: 10.1016/S1097-2765(03)00267-3. [DOI] [PubMed] [Google Scholar]
- 42.Ooi S.L., Samarsky D.A., Fournier M.J., Boeke J.D. Intronic snoRNA biosynthesis in Saccharomyces cerevisiae depends on the lariat-debranching enzyme: Intron length effects and activity of a precursor snoRNA. RNA. 1998;4:1096–1110. doi: 10.1017/S1355838298980785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mei Y.-P., Liao J.-P., Shen J., Yu L., Liu B.L., Liu L., Li R.-Y., Ji L., Dorsey S.G., Jiang Z.-R., et al. Small nucleolar RNA 42 acts as an oncogene in lung tumorigenesis. Oncogene. 2012;31:2794–2804. doi: 10.1038/onc.2011.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson D., Roberts J.T., King V.M., Houserova D., Barnhill E.C., Crucello A., Polska C.J., Brantley L.W., Kaufman G.C., Nguyen M., et al. Human snoRNA-93 is processed into a microRNA-like RNA that promotes breast cancer cell invasion. NPJ Breast Cancer. 2017;3:1–12. doi: 10.1038/s41523-017-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsit C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015;218:71–79. doi: 10.1242/jeb.106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tohyama C. Towards comprehensive health risk assessments of chemicals for occupational and environmental health. Ind. Health. 2017;55:199–200. doi: 10.2486/indhealth.55-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farhadi Z., Gorgi H.A., Shabaninejad H., Delavar M.A., Torani S. Association between PM2.5 and risk of hospitalization for myocardial infarction: A systematic review and a meta-analysis. BMC Public Health. 2020;20:1–12. doi: 10.1186/s12889-020-8262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng F., Tsuji G., Zhang J.-Z., Chen Z., Furue M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019;132:105063. doi: 10.1016/j.envint.2019.105063. [DOI] [PubMed] [Google Scholar]
- 49.Rider C.F., Carlsten C. Air pollution and DNA methylation: Effects of exposure in humans. Clin. Epigenetics. 2019;11:1–15. doi: 10.1186/s13148-019-0713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C., Xu J., Chen Y., Guo X., Zheng Y., Wang Q., Chen Y., Ni Y., Zhu Y., Joyce B.T., et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ. Health. 2015;14:65. doi: 10.1186/s12940-015-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Ma J., Li X., Chan M.T.V., Wu W.K.K., Wu Z., Shen J. Aberrantly expressed long non-coding RNAs in air pollution-induced congenital defects. J. Cell. Mol. Med. 2019;23:7717–7725. doi: 10.1111/jcmm.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasahara D.I., Shore S.A. IL-33, diet-induced obesity, and pulmonary responses to ozone. Respir. Res. 2020;21:1–15. doi: 10.1186/s12931-020-01361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tashiro H., Cho Y., Kasahara D.I., Brand J.D., Bry L., Yeliseyev V., Abu-Ali G., Huttenhower C., Shore S.A. Microbiota Contribute to Obesity-related Increases in the Pulmonary Response to Ozone. Am. J. Respir. Cell Mol. Biol. 2019;61:702–712. doi: 10.1165/rcmb.2019-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shore S.A. Mechanistic Basis for Obesity-related Increases in Ozone-induced Airway Hyperresponsiveness in Mice. Ann. Am. Thorac. Soc. 2017;14:S357–S362. doi: 10.1513/AnnalsATS.201702-140AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith G.J., Tovar A., Kanke M., Wang Y., Deshane J.S., Sethupathy P., Kelada S.N.P. Ozone-induced changes in the murine lung extracellular vesicle small RNA landscape. Physiol. Rep. 2021;9:e15054. doi: 10.14814/phy2.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romito B.T., McBroom M.M., Bryant D., Gamez J., Merchant A., E Hill S. The effect of SANGUINATE® (PEGylated carboxyhemoglobin bovine) on cardiopulmonary bypass functionality using a bovine whole blood model of normovolemic hemodilution. Perfusion. 2019;35:19–25. doi: 10.1177/0267659119850681. [DOI] [PubMed] [Google Scholar]
- 57.Ozkan S., Salt O., Durukan P., Sen A., Bulbul E., Duman A., Kavalci C. The relationship among plasma copeptin, carboxyhemoglobin, and lactate levels in carbon monoxide poisoning. Hum. Exp. Toxicol. 2019;39:311–318. doi: 10.1177/0960327119886063. [DOI] [PubMed] [Google Scholar]
- 58.Terzikhan N., Xu H., Edris A., Bracke K.R., Verhamme F.M., Stricker B.H.C., Dupuis J., Lahousse L., O’Connor G.T., Brusselle G.G. Epigenome-wide association study on diffusing capacity of the lung. ERJ Open Res. 2020;7 doi: 10.1183/23120541.00567-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T., Zhang J., Xu Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int. J. Environ. Res. Public Health. 2020;17:4878. doi: 10.3390/ijerph17134878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovatsi L., Georgiou E., Ioannou A., Haitoglou C., Tzimagiorgis G., Tsoukali H., Kouidou S. p16promoter methylation in Pb2+-exposed individuals. Clin. Toxicol. 2010;48:124–128. doi: 10.3109/15563650903567091. [DOI] [PubMed] [Google Scholar]
- 61.Wu M.-F., Chen Y.-H., Chen H.-C., Huang W.-C. Interactions among Obstructive Sleep Apnea Syndrome Severity, Sex, and Obesity on Circulatory Inflammatory Biomarkers in Patients with Suspected Obstructive Sleep Apnea Syndrome: A Retrospective, Cross-Sectional Study. Int. J. Environ. Res. Public Health. 2020;17:4701. doi: 10.3390/ijerph17134701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu W., Li J., Zhang W., Wang Z., Wu J., Ge X., Wu J., Cao Y., Xie Y., Ying D., et al. Emission of sulfur dioxide from polyurethane foam and respiratory health effects. Environ. Pollut. 2018;242:90–97. doi: 10.1016/j.envpol.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 63.Ku T., Chen M., Li B., Yun Y., Li G., Sang N. Synergistic effects of particulate matter (PM2.5) and sulfur dioxide (SO2) on neurodegeneration via the microRNA-mediated regulation of tau phosphorylation. Toxicol. Res. 2017;6:7–16. doi: 10.1039/C6TX00314A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fenech S., Aquilina N.J. Trends in ambient ozone, nitrogen dioxide, and particulate matter concentrations over the Maltese Islands and the corresponding health impacts. Sci. Total Environ. 2019;700:134527. doi: 10.1016/j.scitotenv.2019.134527. [DOI] [PubMed] [Google Scholar]
- 65.Ritz B., Hoffmann B., Peters A. The Effects of Fine Dust, Ozone, and Nitrogen Dioxide on Health. Dtsch Arztebl Int. 2019;51–52:881–886. doi: 10.3238/arztebl.2019.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Y., Niu Y., Xia Y., Liu C., Lin Z., Wang W., Ge Y., Lei X., Wang C., Cai J., et al. Effects of personal nitrogen dioxide exposure on airway inflammation and lung function. Environ. Res. 2019;177:108620. doi: 10.1016/j.envres.2019.108620. [DOI] [PubMed] [Google Scholar]
- 67.Seo M.Y., Kim S.-H., Park M.J. Air pollution and childhood obesity. Clin. Exp. Pediatr. 2020;63:382–388. doi: 10.3345/cep.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahay D., Terry M.B., Miller R. Is breast cancer a result of epigenetic responses to traffic-related air pollution? A review of the latest evidence. Epigenomics. 2019;11:701–714. doi: 10.2217/epi-2018-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown J.S., Gordon T., Price O., Asgharian B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013;10:12. doi: 10.1186/1743-8977-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X., Lv Y., Hao J., Sun H., Gao N., Zhang C., Lu R., Wang S., Yin L., Pu Y., et al. Role of microRNA-4516 involved autophagy associated with exposure to fine particulate matter. Oncotarget. 2016;7:45385–45397. doi: 10.18632/oncotarget.9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang D., Ma M., Zhou W., Yang B., Xiao C. Inhibition of miR-32 activity promoted EMT induced by PM2.5 exposure through the modulation of the Smad1-mediated signaling pathways in lung cancer cells. Chemosphere. 2017;184:289–298. doi: 10.1016/j.chemosphere.2017.05.152. [DOI] [PubMed] [Google Scholar]
- 72.Zhou T., Zhong Y., Hu Y., Sun C., Wang Y., Wang G. PM2.5 downregulates miR-194-3p and accelerates apoptosis in cigarette-inflamed bronchial epithelium by targeting death-associated protein kinase 1. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:2339–2349. doi: 10.2147/COPD.S168629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H., Li Z. microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma. Open Med. 2019;14:673–682. doi: 10.1515/med-2019-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., Zheng M., Pu J., Zhou Y., Hong W., Fu X., Peng Y., Zhou W., Pan H., Li B., et al. Identification of abnormally expressed lncRNAs induced by PM2.5 in human bronchial epithelial cells. Biosci. Rep. 2018;38:BSR20171577. doi: 10.1042/BSR20171577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song B., Ye L., Wu S., Jing Z. Long non-coding RNA MEG3 regulates CSE-induced apoptosis and inflammation via regulating miR-218 in 16HBE cells. Biochem. Biophys. Res. Commun. 2019;521:368–374. doi: 10.1016/j.bbrc.2019.10.135. [DOI] [PubMed] [Google Scholar]
- 76.Tan Y., Wang Y., Zou Y., Zhou C., Yi Y., Ling Y., Liao F., Jiang Y., Peng X. LncRNA LOC101927514 regulates PM2.5-driven inflammation in human bronchial epithelial cells through binding p-STAT3 protein. Toxicol. Lett. 2019;319:119–128. doi: 10.1016/j.toxlet.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Khuzestani R.B., Schauer J.J., Shang J., Cai T., Fang D., Wei Y., Zhang L., Zhang Y. Source apportionments of PM2.5 organic carbon during the elevated pollution episodes in the Ordos region, Inner Mongolia, China. Environ. Sci. Pollut. Res. 2018;25:13159–13172. doi: 10.1007/s11356-018-1514-4. [DOI] [PubMed] [Google Scholar]
- 78.Peng X.-L., Hao Q.-J., Wen T.-X., Ji D.-S., Liu Z.-R., Wang Y.-S., Chen J.-B., Jiang C.-S. [Pollution Characteristics of Organic Carbon and Elemental Carbon in Atmospheric Aerosols in Beibei District, Chongqing] Huan jing ke xue Huanjing kexue. 2018;39:3502–3510. doi: 10.13227/j.hjkx.201711205. [DOI] [PubMed] [Google Scholar]
- 79.Xing L., Li G., Pongpiachan S., Wang Q., Han Y., Cao J., Tipmanee D., Palakun J., Aukkaravittayapun S., Surapipith V., et al. Quantifying the contributions of local emissions and regional transport to elemental carbon in Thailand. Environ. Pollut. 2020;262:114272. doi: 10.1016/j.envpol.2020.114272. [DOI] [PubMed] [Google Scholar]
- 80.Plato N., Lewné M., Gustavsson P. A historical job-exposure matrix for occupational exposure to diesel exhaust using elemental carbon as an indicator of exposure. Arch. Environ. Occup. Health. 2019;75:321–332. doi: 10.1080/19338244.2019.1644277. [DOI] [PubMed] [Google Scholar]
- 81.Sagai M. Toxic Components of PM2.5 and Their Toxicity Mechanisms—On the Toxicity of Sulfate and Carbon Components. Nippon Eiseigaku Zasshi. 2019;74:19004. doi: 10.1265/jjh.19004. [DOI] [PubMed] [Google Scholar]
- 82.Deng X., Feng N., Zheng M., Ye X., Lin H., Yu X., Gan Z., Fang Z., Zhang H., Gao M., et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2016;1861:112–125. doi: 10.1016/j.bbagen.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Feng N., Ching T., Wang Y., Liu B., Lin H., Shi O., Zhang X., Zheng M., Zheng X., Gao M., et al. Analysis of Microarray Data on Gene Expression and Methylation to Identify Long Non-coding RNAs in Non-small Cell Lung Cancer. Sci. Rep. 2016;6:37233. doi: 10.1038/srep37233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yenjerla M., Panopoulos A., Reynaud C., Fotedar R., Margolis R.L. TD-60 is required for interphase cell cycle progression. Cell Cycle. 2013;12:837–841. doi: 10.4161/cc.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin H., Zhang X., Feng N., Wang R., Zhang W., Deng X., Wang Y., Yu X., Ye X., Li L., et al. LncRNA LCPAT1 Mediates Smoking/Particulate Matter 2.5-Induced Cell Autophagy and Epithelial-Mesenchymal Transition in Lung Cancer Cells via RCC2. Cell. Physiol. Biochem. 2018;47:1244–1258. doi: 10.1159/000490220. [DOI] [PubMed] [Google Scholar]
- 86.Zheng L., Chen X., Dong X., Wei X., Jiang C., Tang Q. Using δ34S–SO4 and δ18O–SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicol. Environ. Saf. 2019;181:231–240. doi: 10.1016/j.ecoenv.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Madrigano J., Baccarelli A., Mittleman M., Wright R., Sparrow D., Vokonas P.S., Tarantini L., Schwartz J. Prolonged Exposure to Particulate Pollution, Genes Associated with Glutathione Pathways, and DNA Methylation in a Cohort of Older Men. Environ. Health Perspect. 2011;119:977–982. doi: 10.1289/ehp.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Q., Liu M., Yu Y., Li Y. Characterization and source apportionment of PM2.5-bound polycyclic aromatic hydrocarbons from Shanghai city, China. Environ. Pollut. 2016;218:118–128. doi: 10.1016/j.envpol.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 89.Duca R.-C., Grova N., Ghosh M., Do J.-M., Hoet P.H.M., Vanoirbeek J.A.J., Appenzeller B.M.R., Godderis L. Exposure to Polycyclic Aromatic Hydrocarbons Leads to Non-monotonic Modulation of DNA and RNA (hydroxy)methylation in a Rat Model. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-28911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo H., Feng Y., Yu H., Xie Y., Luo F., Wang Y. A novel lncRNA, loc107985872, promotes lung adenocarcinoma progression via the notch1 signaling pathway with exposure to traffic-originated PM2.5 organic extract. Environ. Pollut. 2020;266:115307. doi: 10.1016/j.envpol.2020.115307. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y., Zhong Y., Hou T., Liao J., Zhang C., Sun C., Wang G. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol. Environ. Saf. 2019;178:159–167. doi: 10.1016/j.ecoenv.2019.03.086. [DOI] [PubMed] [Google Scholar]
- 92.Luo F., Wei H., Guo H., Li Y., Feng Y., Bian Q., Wang Y. LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM2.5 in lung bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2019;317:L87–L98. doi: 10.1152/ajplung.00073.2019. [DOI] [PubMed] [Google Scholar]
- 93.Zhao G., Zou J., Zhang T., Li C., Zhou S., Jiao F. Recent progress on removal of indoor air pollutants by catalytic oxidation. Rev. Environ. Health. 2020;35:311–321. doi: 10.1515/reveh-2019-0102. [DOI] [PubMed] [Google Scholar]
- 94.Bhargava B., Malhotra S., Chandel A., Rakwal A., Kashwap R.R., Kumar S. Mitigation of indoor air pollutants using Areca palm potted plants in real-life settings. Environ. Sci. Pollut. Res. 2020;28:8898–8906. doi: 10.1007/s11356-020-11177-1. [DOI] [PubMed] [Google Scholar]
- 95.Ye W., Zhang X., Gao J., Cao G., Zhou X., Su X. Indoor air pollutants, ventilation rate determinants and potential control strategies in Chinese dwellings: A literature review. Sci. Total Environ. 2017;586:696–729. doi: 10.1016/j.scitotenv.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 96.Siza C., Morrison M., Harris S., Hatch T., Tyler M. Assessment of Community Awareness and Practices Concerning Indoor Air Pollutants—Madison County, Alabama, June 2017. MMWR. Morb. Mortal. Wkly. Rep. 2018;67:447–450. doi: 10.15585/mmwr.mm6715a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vincent M., Chemarin C. Impact sanitaire de la pollution particulaire minérale à l’intérieur des locaux. Rev. des Mal. Respir. 2011;28:496–502. doi: 10.1016/j.rmr.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 98.Yadav I.C., Devi N.L., Li J., Zhang G. Polychlorinated biphenyls and organochlorines pesticides in indoor dust: An exploration of sources and health exposure risk in a rural area (Kopawa) of Nepal. Ecotoxicol. Environ. Saf. 2020;195:110376. doi: 10.1016/j.ecoenv.2020.110376. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Yang Y., He X., Yang P., Zong T., Sun P., Sun R., Yu T., Jiang Z. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J. Cell. Mol. Med. 2021;25:5358–5371. doi: 10.1111/jcmm.16602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Yang Y., Ju H., He X., Sun P., Tian Y., Yang P., Song X.-X., Yu T., Jiang Z. Comprehensive profile of circRNAs in formaldehyde induced heart development. Food Chem. Toxicol. 2022;162:112899. doi: 10.1016/j.fct.2022.112899. [DOI] [PubMed] [Google Scholar]
- 101.Singh N., Baby D., Rajguru J.P., Patil P.B., Thakkannavar S.S., Pujari V.B. Inflammation and cancer. Ann. Afr. Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng Y.Y., Rath E.M., Linton A., Yuen M.L., Takahashi K., Lee K. The Current Understanding Of Asbestos-Induced Epigenetic Changes Associated With Lung Cancer. Lung Cancer Targets Ther. 2020;11:1–11. doi: 10.2147/LCTT.S186843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilk E., Krówczyńska M. Malignant mesothelioma and asbestos exposure in Europe: Evidence of spatial clustering. Geospat. Health. 2021;16:1. doi: 10.4081/gh.2021.951. [DOI] [PubMed] [Google Scholar]
- 104.Rozitis E., Johnson B., Cheng Y.Y., Lee K. The Use of Immunohistochemistry, Fluorescence in situ Hybridization, and Emerging Epigenetic Markers in the Diagnosis of Malignant Pleural Mesothelioma (MPM): A Review. Front. Oncol. 2020;10:1742. doi: 10.3389/fonc.2020.01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matboli M., Shafei A.E., Ali M.A., Gaber A.I., Galal A., Tarek O., Marei M., Khairy E., El-Khazragy N., Anber N., et al. Clinical significance of serum DRAM1 mRNA, ARSA mRNA, hsa-miR-2053 and lncRNA-RP1-86D1.3 axis expression in malignant pleural mesothelioma. J. Cell. Biochem. 2018;120:3203–3211. doi: 10.1002/jcb.27586. [DOI] [PubMed] [Google Scholar]
- 106.Mozzoni P., Ampollini L., Goldoni M., Alinovi R., Tiseo M., Gnetti L., Carbognani P., Rusca M., Mutti A., Percesepe A., et al. MicroRNA Expression in Malignant Pleural Mesothelioma and Asbestosis: A Pilot Study. Dis. Markers. 2017;2017:1–10. doi: 10.1155/2017/9645940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weber D.G., Casjens S., Brik A., Raiko I., Lehnert M., Taeger D., Gleichenhagen J., Kollmeier J., Bauer T.T., Brüning T., et al. Circulating long non-coding RNA GAS5 (growth arrest-specific transcript 5) as a complement marker for the detection of malignant mesothelioma using liquid biopsies. Biomark. Res. 2020;8:1–11. doi: 10.1186/s40364-020-00194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiménez-Garza O., Baccarelli A., Byun H.-M., Márquez-Gamiño S., Barrón-Vivanco B.S., Albores A. CYP2E1 epigenetic regulation in chronic, low-level toluene exposure: Relationship with oxidative stress and smoking habit. Toxicol. Appl. Pharmacol. 2015;286:207–215. doi: 10.1016/j.taap.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 109.Yu S.Y., Koh E.J., Kim S.H., Song B., Lee J.S., Son S.W., Seo H., Hwang S.Y. Analysis of multi-omics data on the relationship between epigenetic changes and nervous system disorders caused by exposure to environmentally harmful substances. Environ. Toxicol. 2021;37:802–813. doi: 10.1002/tox.23444. [DOI] [PubMed] [Google Scholar]
- 110.Yu S.Y., Koh E.J., Kim S.H., Lee S.Y., Lee J.S., Son S.W., Hwang S.Y. Integrated analysis of multi-omics data on epigenetic changes caused by combined exposure to environmental hazards. Environ. Toxicol. 2021;36:1001–1010. doi: 10.1002/tox.23099. [DOI] [PubMed] [Google Scholar]
- 111.Sun L., Pan Y., Wang X., Gao G., Wu L., Piao C., Ruan J., Liu J. Screening for Potential Biomarkers in Peripheral Blood From Miners Exposed to Radon Radiation. Dose-Response. 2020;18:1559325820904600. doi: 10.1177/1559325820904600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding E., Guo J., Bai Y., Zhang H., Liu X., Cai W., Zhong L., Zhu B. MiR-92a and miR-486 are potential diagnostic biomarkers for mercury poisoning and jointly sustain NF-κB activity in mercury toxicity. Sci. Rep. 2017;7:15980. doi: 10.1038/s41598-017-13230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guida N., Laudati G., Mascolo L., Valsecchi V., Sirabella R., Selleri C., Di Renzo G., Canzoniero L.M.T., Formisano L. p38/Sp1/Sp4/HDAC4/BDNF Axis Is a Novel Molecular Pathway of the Neurotoxic Effect of the Methylmercury. Front. Neurosci. 2017;11:8. doi: 10.3389/fnins.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zong D., Liu X., Li J., Ouyang R., Chen P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin. 2019;12:1–25. doi: 10.1186/s13072-019-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sundar I.K., Rahman I. Gene expression profiling of epigenetic chromatin modification enzymes and histone marks by cigarette smoke: Implications for COPD and lung cancer. Am. J. Physiol. Cell. Mol. Physiol. 2016;311:L1245–L1258. doi: 10.1152/ajplung.00253.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaur G., Begum R., Thota S., Batra S. A systematic review of smoking-related epigenetic alterations. Arch. Toxicol. 2019;93:2715–2740. doi: 10.1007/s00204-019-02562-y. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y., Luo F., Xu Y., Wang B., Zhao Y., Xu W., Shi L., Lu X., Liu Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015;282:9–19. doi: 10.1016/j.taap.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 118.Rubio K., Castillo-Negrete R., Barreto G. Non-coding RNAs and nuclear architecture during epithelial-mesenchymal transition in lung cancer and idiopathic pulmonary fibrosis. Cell. Signal. 2020;70:109593. doi: 10.1016/j.cellsig.2020.109593. [DOI] [PubMed] [Google Scholar]
- 119.Lu L., Luo F., Liu Y., Liu X., Shi L., Lu X., Liu Q. Posttranscriptional silencing of the lncRNA MALAT1 by miR-217 inhibits the epithelial–mesenchymal transition via enhancer of zeste homolog 2 in the malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015;289:276–285. doi: 10.1016/j.taap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 120.Hasan K., Shahriar A., Jim K.U. Water pollution in Bangladesh and its impact on public health. Heliyon. 2019;5:e02145. doi: 10.1016/j.heliyon.2019.e02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kurwadkar S. Occurrence and distribution of organic and inorganic pollutants in groundwater. Water Environ. Res. 2019;91:1001–1008. doi: 10.1002/wer.1166. [DOI] [PubMed] [Google Scholar]
- 122.Tracy J.W., Guo A., Liang K., Bartram J., Fisher M. Sources of and Solutions to Toxic Metal and Metalloid Contamination in Small Rural Drinking Water Systems: A Rapid Review. Int. J. Environ. Res. Public Health. 2020;17:7076. doi: 10.3390/ijerph17197076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fisher M.B., Guo A.Z., Tracy J.W., Prasad S.K., Cronk R.D., Browning E.G., Liang K.R., Kelly E.R., Bartram J.K. Occurrence of Lead and Other Toxic Metals Derived from Drinking-Water Systems in Three West African Countries. Environ. Health Perspect. 2021;129:47012. doi: 10.1289/EHP7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhong M., Huang Z., Wang L., Lin Z., Cao Z., Li X., Zhang F., Wang H., Li Y., Ma X. Malignant Transformation of Human Bronchial Epithelial Cells Induced by Arsenic through STAT3/miR-301a/SMAD4 Loop. Sci. Rep. 2018;8:13291. doi: 10.1038/s41598-018-31516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu D., Wu D., Zhao L., Yang Y., Ding J., Dong L., Hu L., Wang F., Zhao X., Cai Y., et al. Arsenic Trioxide Reduces Global Histone H4 Acetylation at Lysine 16 through Direct Binding to Histone Acetyltransferase hMOF in Human Cells. PLoS ONE. 2015;10:e0141014. doi: 10.1371/journal.pone.0141014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pournara A., Kippler M., Holmlund T., Ceder R., Grafström R., Vahter M., Broberg K., Wallberg A.E. Arsenic alters global histone modifications in lymphocytes in vitro and in vivo. Cell Biol. Toxicol. 2016;32:275–284. doi: 10.1007/s10565-016-9334-0. [DOI] [PubMed] [Google Scholar]
- 127.Yuan D., Ye S., Pan Y., Bao Y., Chen H., Shao C. Long-term cadmium exposure leads to the enhancement of lymphocyte proliferation via down-regulating p16 by DNA hypermethylation. Mutat. Res. Toxicol. Environ. Mutagen. 2013;757:125–131. doi: 10.1016/j.mrgentox.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 128.Gao M., Chen M., Li C., Xu M., Liu Y., Cong M., Sang N., Liu S. Long non-coding RNA MT1DP shunts the cellular defense to cytotoxicity through crosstalk with MT1H and RhoC in cadmium stress. Cell Discov. 2018;4:1–19. doi: 10.1038/s41421-017-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou Z.-H., Lei Y.-X., Wang C.-X. Analysis of Aberrant Methylation in DNA Repair Genes During Malignant Transformation of Human Bronchial Epithelial Cells Induced by Cadmium. Toxicol. Sci. 2011;125:412–417. doi: 10.1093/toxsci/kfr320. [DOI] [PubMed] [Google Scholar]
- 130.Chen D., Kluz T., Fang L., Zhang X., Sun H., Jin C., Costa M. Hexavalent Chromium (Cr(VI)) Down-Regulates Acetylation of Histone H4 at Lysine 16 through Induction of Stressor Protein Nupr1. PLoS ONE. 2016;11:e0157317. doi: 10.1371/journal.pone.0157317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsuboi M., Kondo K., Soejima S., Kajiura K., Kawakita N., Toba H., Kawakami Y., Yoshida M., Takizawa H., Tangoku A. Chromate exposure induces DNA hypermethylation of the mismatch repair gene MLH1 in lung cancer. Mol. Carcinog. 2019;59:24–31. doi: 10.1002/mc.23125. [DOI] [PubMed] [Google Scholar]
- 132.Martinez-Zamudio R., Ha H.C. Environmental epigenetics in metal exposure. Epigenetics. 2011;6:820–827. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jose C.C., Wang Z., Tanwar V.S., Zhang X., Zang C., Cuddapah S. Nickel-induced transcriptional changes persist post exposure through epigenetic reprogramming. Epigenetics Chromatin. 2019;12:1–15. doi: 10.1186/s13072-019-0324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Long P., Wang Q., Zhang Y., Zhu X., Yu K., Jiang H., Liu X., Zhou M., Yuan Y., Liu K., et al. Profile of copper-associated DNA methylation and its association with incident acute coronary syndrome. Clin. Epigenetics. 2021;13:1–12. doi: 10.1186/s13148-021-01004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.A Salas L., Bustamante M., Gonzalez J.R., Gracia-Lavedan E., Moreno V., Kogevinas M., Villanueva C.M. DNA methylation levels and long-term trihalomethane exposure in drinking water: An epigenome-wide association study. Epigenetics. 2015;10:650–661. doi: 10.1080/15592294.2015.1057672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Villanueva C.M., Cordier S., Font-Ribera L., Salas L.A., Levallois P. Overview of Disinfection By-products and Associated Health Effects. Curr. Environ. Health Rep. 2015;2:107–115. doi: 10.1007/s40572-014-0032-x. [DOI] [PubMed] [Google Scholar]
- 137.Tiffon C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018;19:3425. doi: 10.3390/ijms19113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang Y., Fang M. Nutritional and Environmental Contaminant Exposure: A Tale of Two Co-Existing Factors for Disease Risks. Environ. Sci. Technol. 2020;54:14793–14796. doi: 10.1021/acs.est.0c05658. [DOI] [PubMed] [Google Scholar]
- 139.Hodjat M., Rahmani S., Khan F., Niaz K., Navaei-Nigjeh M., Nejad S.M., Abdollahi M. Environmental toxicants, incidence of degenerative diseases, and therapies from the epigenetic point of view. Arch. Toxicol. 2017;91:2577–2597. doi: 10.1007/s00204-017-1979-9. [DOI] [PubMed] [Google Scholar]
- 140.Saigenji K. [Tests required for the diagnosis of gastric and duodenal ulcer and their significance] Nursing Tech. 1988;34:858–859. [PubMed] [Google Scholar]
- 141.Devaux C.A., Raoult D. The Microbiological Memory, an Epigenetic Regulator Governing the Balance Between Good Health and Metabolic Disorders. Front. Microbiol. 2018;9:1379. doi: 10.3389/fmicb.2018.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woo V., Alenghat T. Host–microbiota interactions: Epigenomic regulation. Curr. Opin. Immunol. 2017;44:52–60. doi: 10.1016/j.coi.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Woźniak E., Reszka E., Jablonska E., Balcerczyk A., Broncel M., Bukowska B. Glyphosate affects methylation in the promoter regions of selected tumor suppressors as well as expression of major cell cycle and apoptosis drivers in PBMCs (in vitro study) Toxicol. Vitr. 2019;63:104736. doi: 10.1016/j.tiv.2019.104736. [DOI] [PubMed] [Google Scholar]
- 144.Woźniak E., Reszka E., Jabłońska E., Michałowicz J., Huras B., Bukowska B. Glyphosate and AMPA Induce Alterations in Expression of Genes Involved in Chromatin Architecture in Human Peripheral Blood Mononuclear Cells (In Vitro) Int. J. Mol. Sci. 2021;22:2966. doi: 10.3390/ijms22062966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharavanan V.J., Sivaramakrishnan M., Sivarajasekar N., Senthilrani N., Kothandan R., Dhakal N., Sivamani S., Show P.L., Awual R., Naushad M. Pollutants inducing epigenetic changes and diseases. Environ. Chem. Lett. 2019;18:325–343. doi: 10.1007/s10311-019-00944-3. [DOI] [Google Scholar]
- 146.De Gannes M., Ko C.-I., Zhang X., Biesiada J., Niu L., E Koch S., Medvedovic M., Rubinstein J., Puga A. Dioxin Disrupts Dynamic DNA Methylation Patterns in Genes That Govern Cardiomyocyte Maturation. Toxicol. Sci. 2020;178:325–337. doi: 10.1093/toxsci/kfaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cimmino I., Fiory F., Perruolo G., Miele C., Beguinot F., Formisano P., Oriente F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020;21:5761. doi: 10.3390/ijms21165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Greathouse K.L., Bredfeldt T., I Everitt J., Lin K., Berry T., Kannan K., Mittelstadt M.L., Ho S.-M., Walker C.L. Environmental Estrogens Differentially Engage the Histone Methyltransferase EZH2 to Increase Risk of Uterine Tumorigenesis. Mol. Cancer Res. 2012;10:546–557. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chou C., Huang H., Yang C., Dahms H., Liang S., Wang T., Kuo P., Hsi E., Tsai E., Chiu C. Reduced camptothecin sensitivity of estrogen receptor-positive human breast cancer cells following exposure to di(2-ethylhexyl)phthalate (DEHP) is associated with DNA methylation changes. Environ. Toxicol. 2019;34:401–414. doi: 10.1002/tox.22694. [DOI] [PubMed] [Google Scholar]
- 150.Shahidehnia M. Epigenetic Effects of Endocrine Disrupting Chemicals. J. Environ. Anal. Toxicol. 2016;6:4. doi: 10.4172/2161-0525.1000381. [DOI] [Google Scholar]
- 151.Sonkar R., Powell C.A., Choudhury M. Benzyl butyl phthalate induces epigenetic stress to enhance adipogenesis in mesenchymal stem cells. Mol. Cell. Endocrinol. 2016;431:109–122. doi: 10.1016/j.mce.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 152.Li D., Suh S. Health risks of chemicals in consumer products: A review. Environ. Int. 2019;123:580–587. doi: 10.1016/j.envint.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 153.Gupta R., Xie H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018;37:209–230. doi: 10.1615/JEnvironPatholToxicolOncol.2018026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bleeker E.A., de Jong W.H., Geertsma R., Groenewold M., Heugens E.H., Koers-Jacquemijns M., van de Meent D., Popma J.R., Rietveld A.G., Wijnhoven S.W., et al. Considerations on the EU definition of a nanomaterial: Science to support policy making. Regul. Toxicol. Pharmacol. 2013;65:119–125. doi: 10.1016/j.yrtph.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 155.Pogribna M., Hammons G. Epigenetic Effects of Nanomaterials and Nanoparticles. J. Nanobiotechnol. 2021;19:1–18. doi: 10.1186/s12951-020-00740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gedda M.R., Babele P.K., Zahra K., Madhukar P. Epigenetic Aspects of Engineered Nanomaterials: Is the Collateral Damage Inevitable? Front. Bioeng. Biotechnol. 2019;7:228. doi: 10.3389/fbioe.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shyamasundar S., Ng C.T., Yung L.Y.L., Dheen S.T., Bay B.H. Epigenetic mechanisms in nanomaterial-induced toxicity. Epigenomics. 2015;7:395–411. doi: 10.2217/epi.15.3. [DOI] [PubMed] [Google Scholar]
- 158.Sierra M.I., Valdés A., Fernández A., Torrecillas R., Fraga M.F. The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome. Int. J. Nanomed. 2016;11:6297–6306. doi: 10.2147/IJN.S120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patil N.A., Gade W.N., Deobagkar D.D. Epigenetic modulation upon exposure of lung fibroblasts to TiO2 and ZnO nanoparticles: Alterations in DNA methylation. Int. J. Nanomed. 2016;11:4509–4519. doi: 10.2147/ijn.s110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Black J.C., Van Rechem C., Whetstine J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Valdiglesias V., Giunta S., Fenech M., Neri M., Bonassi S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat. Res. Mutat. Res. 2013;753:24–40. doi: 10.1016/j.mrrev.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 162.Kopp B., Dario M., Zalko D., Audebert M. Assessment of a panel of cellular biomarkers and the kinetics of their induction in comparing genotoxic modes of action in HepG2 cells. Environ. Mol. Mutagen. 2018;59:516–528. doi: 10.1002/em.22197. [DOI] [PubMed] [Google Scholar]
- 163.Dobersch S., Rubio K., Singh I., Günther S., Graumann J., Cordero J., Castillo-Negrete R., Huynh M.B., Mehta A., Braubach P., et al. Positioning of nucleosomes containing γ-H2AX precedes active DNA demethylation and transcription initiation. Nat. Commun. 2021;12:1–20. doi: 10.1038/s41467-021-21227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ndika J., Karisola P., Kinaret P., Ilves M., Alenius H. Profiling Non-Coding RNA Changes Associated with 16 Different Engineered Nanomaterials in a Mouse Airway Exposure Model. Cells. 2021;10:1085. doi: 10.3390/cells10051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pogribna M., Koonce N.A., Mathew A., Word B., Patri A.K., Lyn-Cook B., Hammons G. Effect of titanium dioxide nanoparticles on DNA methylation in multiple human cell lines. Nanotoxicology. 2020;14:534–553. doi: 10.1080/17435390.2020.1723730. [DOI] [PubMed] [Google Scholar]
- 166.Enea M., Pereira E., Costa J., Soares M.E., da Silva D.D., Bastos M.D.L., Carmo H.F. Cellular uptake and toxicity of gold nanoparticles on two distinct hepatic cell models. Toxicol. Vitr. 2020;70:105046. doi: 10.1016/j.tiv.2020.105046. [DOI] [PubMed] [Google Scholar]
- 167.Ng C.-T., Dheen S.T., Yip W.-C.G., Ong C.N., Bay B.-H., Yung L.-Y.L. The induction of epigenetic regulation of PROS1 gene in lung fibroblasts by gold nanoparticles and implications for potential lung injury. Biomaterials. 2011;32:7609–7615. doi: 10.1016/j.biomaterials.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 168.Brzóska K., Grądzka I., Kruszewski M. Silver, Gold, and Iron Oxide Nanoparticles Alter miRNA Expression but Do Not Affect DNA Methylation in HepG2 Cells. Materials. 2019;12:1038. doi: 10.3390/ma12071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abdullah A.S., Sayed I.E.T.E., El-Torgoman A.M.A., Alghamdi N.A., Ullah S., Wageh S., Kamel M.A. Preparation and Characterization of Silymarin-Conjugated Gold Nanoparticles with Enhanced Anti-Fibrotic Therapeutic Effects against Hepatic Fibrosis in Rats: Role of MicroRNAs as Molecular Targets. Biomedicines. 2021;9:1767. doi: 10.3390/biomedicines9121767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen L., Liu J., Zhang Y., Zhang G., Kang Y., Chen A., Feng X., Shao L. The toxicity of silica nanoparticles to the immune system. Nanomedicine. 2018;13:1939–1962. doi: 10.2217/nnm-2018-0076. [DOI] [PubMed] [Google Scholar]
- 171.Seidel C., Kirsch A., Fontana C., Visvikis A., Remy A., Gaté L., Darne C., Guichard Y. Epigenetic changes in the early stage of silica-induced cell transformation. Nanotoxicology. 2017;11:923–935. doi: 10.1080/17435390.2017.1382599. [DOI] [PubMed] [Google Scholar]
- 172.Dusinska M., Tulinska J., El Yamani N., Kuricova M., Liskova A., Rollerova E., Rundén-Pran E., Smolkova B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017;109:797–811. doi: 10.1016/j.fct.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 173.Koedrith P., Rahman M., Jang Y.J., Shin D.Y., Seo Y.R. Nanoparticles: Weighing the Pros and Cons from an Eco-genotoxicological Perspective. J. Cancer Prev. 2021;26:83–97. doi: 10.15430/JCP.2021.26.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huang Y., Fan C.-Q., Dong H., Wang S.-M., Yang X.-C., Yang S.-M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017;12:1815–1825. doi: 10.2147/IJN.S127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Han J., Zhao D., Li D., Wang X., Jin Z., Zhao K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers. 2018;10:31. doi: 10.3390/polym10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hueso M., Mallén A., Suñé-Pou M., Aran J.M., Suñé-Negre J.M., Navarro E. ncRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery. Int. J. Mol. Sci. 2021;22:11596. doi: 10.3390/ijms222111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics — challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ghadimi M., Zangenehtabar S., Homaeigohar S. An Overview of the Water Remediation Potential of Nanomaterials and Their Ecotoxicological Impacts. Water. 2020;12:1150. doi: 10.3390/w12041150. [DOI] [Google Scholar]
- 179.Sun T.Y., Bornhöft N.A., Hungerbuehler K., Nowack B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016;50:4701–4711. doi: 10.1021/acs.est.5b05828. [DOI] [PubMed] [Google Scholar]
- 180.Majumder A., Gupta B., Gupta A.K., Majumder A., Gupta B., Gupta A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019;176:108542. doi: 10.1016/j.envres.2019.108542. [DOI] [PubMed] [Google Scholar]
- 181.Song X., Wang X., Bhandari R.K. Developmental abnormalities and epigenetic alterations in medaka (Oryzias latipes) embryos induced by triclosan exposure. Chemosphere. 2020;261:127613. doi: 10.1016/j.chemosphere.2020.127613. [DOI] [PubMed] [Google Scholar]
- 182.Guyon A., Smith K.F., Charry M.P., Champeau O., Tremblay L.A. Effects of Chronic Exposure to Benzophenone and Diclofenac on DNA Methylation Levels and Reproductive Success in a Marine Copepod. J. Xenobiotics. 2018;8:1–3. doi: 10.4081/xeno.2018.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guillette L.J., Parrott B.B., Nilsson E., Haque M., Skinner M.K. Epigenetic programming alterations in alligators from environmentally contaminated lakes. Gen. Comp. Endocrinol. 2016;238:4–12. doi: 10.1016/j.ygcen.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vilahur N., Bustamante M., Byun H.-M., Fernandez M.F., Marina L.S., Basterrechea M., Ballester F., Murcia M., Tardón A., Fernández-Somoano A., et al. Prenatal exposure to mixtures of xenoestrogens and repetitive element DNA methylation changes in human placenta. Environ. Int. 2014;71:81–87. doi: 10.1016/j.envint.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Warita K., Mitsuhashi T., Sugawara T., Tabuchi Y., Tanida T., Wang Z.-Y., Matsumoto Y., Yokoyama T., Kitagawa H., Miki T., et al. Direct effects of diethylstilbestrol on the gene expression of the cholesterol side-chain cleavage enzyme (P450scc) in testicular Leydig cells. Life Sci. 2010;87:281–285. doi: 10.1016/j.lfs.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 187.Meng F., Jiao X., Chen F., Zhang X., Duan Z., Ding Z., Wu D., Wang Y., Zhang S., Miao Y., et al. Isobutylparaben Negatively Affects Porcine Oocyte Maturation Through Increasing Oxidative Stress and Cytoskeletal Abnormalities. Environ. Mol. Mutagen. 2020;61:433–444. doi: 10.1002/em.22356. [DOI] [PubMed] [Google Scholar]
- 188.Leppert B., Strunz S., Seiwert B., Schlittenbauer L., Schlichting R., Pfeiffer C., Röder S., Bauer M., Borte M., Stangl G.I., et al. Maternal paraben exposure triggers childhood overweight development. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-019-14202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silvestre A.L.P., Milani M.I., Rossini E.L., Pezza L., Pezza H.R. A paper platform for colorimetric determination of aluminum hydrochloride in antiperspirant samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018;204:432–435. doi: 10.1016/j.saa.2018.06.049. [DOI] [PubMed] [Google Scholar]
- 190.Li J., Zhang D.-D., Wang C.-Q., Shi M., Wang L.-L. Protective effects of low-intensity pulsed ultrasound on aluminum overload-induced cerebral damage through epigenetic regulation of brain-derived neurotrophic factor expression. Biosci. Rep. 2019;39:BSR20181185. doi: 10.1042/BSR20181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Matozzo V., Fabrello J., Masiero L., Ferraccioli F., Finos L., Pastore P., Di Gangi I.M., Bogialli S. Ecotoxicological risk assessment for the herbicide glyphosate to non-target aquatic species: A case study with the mussel Mytilus galloprovincialis. Environ. Pollut. 2018;233:623–632. doi: 10.1016/j.envpol.2017.10.100. [DOI] [PubMed] [Google Scholar]
- 192.Espinoza-Montero P.J., Vega-Verduga C., Alulema-Pullupaxi P., Fernández L., Paz J.L. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules. 2020;25:5550. doi: 10.3390/molecules25235550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zouaoui K., Dulaurent S., Gaulier J.-M., Moesch C., Lachâtre G. Determination of glyphosate and AMPA in blood and urine from humans: About 13 cases of acute intoxication. Forensic Sci. Int. 2013;226:e20–e25. doi: 10.1016/j.forsciint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 194.Soukup S.T., Merz B., Bub A., Hoffmann I., Watzl B., Steinberg P., Kulling S.E. Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: Results of the cross-sectional KarMeN study in Germany. Arch. Toxicol. 2020;94:1575–1584. doi: 10.1007/s00204-020-02704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hao Y., Zhang Y., Ni H., Gao J., Yang Y., Xu W., Tao L. Evaluation of the cytotoxic effects of glyphosate herbicides in human liver, lung, and nerve. J. Environ. Sci. Health Part B. 2019;54:737–744. doi: 10.1080/03601234.2019.1633215. [DOI] [PubMed] [Google Scholar]
- 196.Duforestel M., Nadaradjane A., Bougras-Cartron G., Briand J., Olivier C., Frenel J.-S., Vallette F.M., Lelièvre S.A., Cartron P.-F. Glyphosate Primes Mammary Cells for Tumorigenesis by Reprogramming the Epigenome in a TET3-Dependent Manner. Front. Genet. 2019;10:885. doi: 10.3389/fgene.2019.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bechtel W., McGoohan S., Zeisberg E.M., Müller G.A., Kalbacher H., Salant D.J., Müller C.A., Kalluri R., Zeisberg M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Negreros M., Hagood J.S., Espinoza C.R., Balderas-Martinez Y.I., Selman M., Pardo A. Transforming growth factor beta 1 induces methylation changes in lung fibroblasts. PLoS ONE. 2019;14:e0223512. doi: 10.1371/journal.pone.0223512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ma J., Zhu J., Wang W., Ruan P., Rajeshkumar S., Li X. Biochemical and molecular impacts of glyphosate-based herbicide on the gills of common carp. Environ. Pollut. 2019;252:1288–1300. doi: 10.1016/j.envpol.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 200.Zhu Y., Costa M. Metals and molecular carcinogenesis. Carcinogenesis. 2020;41:1161–1172. doi: 10.1093/carcin/bgaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou X., Li Q., Arita A., Sun H., Costa M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol. Appl. Pharmacol. 2009;236:78–84. doi: 10.1016/j.taap.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zoroddu M.A., Peana M., Medici S., Casella L., Monzani E., Costa M. Nickel binding to histone H4. Dalton Trans. 2009;39:787–793. doi: 10.1039/B916019C. [DOI] [PubMed] [Google Scholar]
- 203.Mo Y., Zhang Y., Wan R., Jiang M., Xu Y., Zhang Q. miR-21 mediates nickel nanoparticle-induced pulmonary injury and fibrosis. Nanotoxicology. 2020;14:1175–1197. doi: 10.1080/17435390.2020.1808727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mo Y., Zhang Y., Mo L., Wan R., Jiang M., Zhang Q. The role of miR-21 in nickel nanoparticle-induced MMP-2 and MMP-9 production in mouse primary monocytes: In vitro and in vivo studies. Environ. Pollut. 2020;267:115597. doi: 10.1016/j.envpol.2020.115597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen H., Kluz T., Zhang R., Costa M. Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis. 2010;31:2136–2144. doi: 10.1093/carcin/bgq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sutterlüty H., Mayer C.-E., Setinek U., Attems J., Ovtcharov S., Mikula M., Mikulits W., Micksche M., Berger W. Down-Regulation of Sprouty2 in Non–Small Cell Lung Cancer Contributes to Tumor Malignancy via Extracellular Signal-Regulated Kinase Pathway-Dependent and -Independent Mechanisms. Mol. Cancer Res. 2007;5:509–520. doi: 10.1158/1541-7786.MCR-06-0273. [DOI] [PubMed] [Google Scholar]
- 207.Jose C.C., Jagannathan L., Tanwar V.S., Zhang X., Zang C., Cuddapah S. Nickel exposure induces persistent mesenchymal phenotype in human lung epithelial cells through epigenetic activation of ZEB1. Mol. Carcinog. 2018;57:794–806. doi: 10.1002/mc.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhan H., Chang X., Wang X., Yang M., Gao Q., Liu H., Li C., Li S., Sun Y. LncRNA MEG3 mediates nickel oxide nanoparticles-induced pulmonary fibrosis via suppressing TGF-β1 expression and epithelial-mesenchymal transition process. Environ. Toxicol. 2021;36:1099–1110. doi: 10.1002/tox.23109. [DOI] [PubMed] [Google Scholar]
- 209.Genchi G., Sinicropi M.S., Lauria G., Carocci A., Catalano A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health. 2020;17:3782. doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gu S., Dai J., Qu T., He Z. Emerging Roles of MicroRNAs and Long Noncoding RNAs in Cadmium Toxicity. Biol. Trace Element Res. 2019;195:481–490. doi: 10.1007/s12011-019-01859-4. [DOI] [PubMed] [Google Scholar]
- 211.Ngalame N.N.O., Waalkes M.P., Tokar E.J. Silencing KRAS Overexpression in Cadmium-Transformed Prostate Epithelial Cells Mitigates Malignant Phenotype. Chem. Res. Toxicol. 2016;29:1458–1467. doi: 10.1021/acs.chemrestox.6b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fay M.J., Alt L.A.C., Ryba D., Salamah R., Peach R., Papaeliou A., Zawadzka S., Weiss A., Patel N., Rahman A., et al. Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex. Toxics. 2018;6:16. doi: 10.3390/toxics6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu Q., Zheng C., Shen H., Zhou Z., Lei Y. MicroRNAs-mRNAs Expression Profile and Their Potential Role in Malignant Transformation of Human Bronchial Epithelial Cells Induced by Cadmium. BioMed Res. Int. 2015;2015:1–13. doi: 10.1155/2015/902025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hassan F., Nuovo G.J., Crawford M., Boyaka P., Kirkby S., Nana-Sinkam S.P., Cormet-Boyaka E. MiR-101 and miR-144 Regulate the Expression of the CFTR Chloride Channel in the Lung. PLoS ONE. 2012;7:e50837. doi: 10.1371/journal.pone.0050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhou Z., Liu H., Wang C., Lu Q., Huang Q., Zheng C., Lei Y. Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology. Sci. Rep. 2015;5:srep15293. doi: 10.1038/srep15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huang Q., Lu Q., Chen B., Shen H., Liu Q., Zhou Z., Lei Y. LncRNA-MALAT1 as a novel biomarker of cadmium toxicity regulates cell proliferation and apoptosis. Toxicol. Res. 2017;6:361–371. doi: 10.1039/C6TX00433D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fabbri M. Whole genome analysis and microRNAs regulation in HepG2 cells exposed to cadmium. ALTEX. 2012;29:173–182. doi: 10.14573/altex.2012.2.173. [DOI] [PubMed] [Google Scholar]
- 218.Gao M., Li C., Xu M., Liu Y., Cong M., Liu S. LncRNA MT1DP Aggravates Cadmium-Induced Oxidative Stress by Repressing the Function of Nrf2 and is Dependent on Interaction with miR-365. Adv. Sci. 2018;5:1800087. doi: 10.1002/advs.201800087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sun H., Brocato J., Costa M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015;2:295–303. doi: 10.1007/s40572-015-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stout M.D., Herbert R.A., Kissling G.E., Collins B.J., Travlos G.S., Witt K.L., Melnick R.L., Abdo K.M., Malarkey D.E., Hooth M.J. Hexavalent Chromium Is Carcinogenic to F344/N Rats and B6C3F1 Mice after Chronic Oral Exposure. Environ. Health Perspect. 2009;117:716–722. doi: 10.1289/ehp.0800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen Q.Y., Murphy A., Sun H., Costa M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 2019;377:114636. doi: 10.1016/j.taap.2019.114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Amrani I., Haddam N., Garat A., Allorge D., Zerimech F., Schraen S., Taleb A., Merzouk H., Edme J.-L., Lo-Guidice J.-M. Exposure to metal fumes and circulating miRNAs in Algerian welders. Int. Arch. Occup. Environ. Health. 2019;93:553–561. doi: 10.1007/s00420-019-01509-1. [DOI] [PubMed] [Google Scholar]
- 223.Jia J., Li T., Yao C., Chen J., Feng L., Jiang Z., Shi L., Liu J., Chen J., Lou J. Circulating differential miRNAs profiling and expression in hexavalent chromium exposed electroplating workers. Chemosphere. 2020;260:127546. doi: 10.1016/j.chemosphere.2020.127546. [DOI] [PubMed] [Google Scholar]
- 224.Li Y., Li P., Yu S., Zhang J., Wang T., Jia G. miR-3940-5p associated with genetic damage in workers exposed to hexavalent chromium. Toxicol. Lett. 2014;229:319–326. doi: 10.1016/j.toxlet.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 225.Wang L., Qiu J.-G., He J., Liu W.-J., Ge X., Zhou F.-M., Huang Y.-X., Jiang B.-H., Liu L.-Z. Suppression of miR-143 contributes to overexpression of IL-6, HIF-1α and NF-κB p65 in Cr(VI)-induced human exposure and tumor growth. Toxicol. Appl. Pharmacol. 2019;378:114603. doi: 10.1016/j.taap.2019.114603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pratheeshkumar P., Son Y.-O., Divya S.P., Turcios L., Roy R.V., Hitron J.A., Wang L., Kim D., Dai J., Asha P., et al. Hexavalent chromium induces malignant transformation of human lung bronchial epithelial cells via ROS-dependent activation of miR-21-PDCD4 signaling. Oncotarget. 2016;7:51193–51210. doi: 10.18632/oncotarget.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.He J., Qian X., Carpenter R., Xu Q., Wang L., Qi Y., Wang Z.-X., Liu L.-Z., Jiang B.-H. Repression of miR-143 Mediates Cr (VI)–Induced Tumor Angiogenesis via IGF-IR/IRS1/ERK/IL-8 Pathway. Toxicol. Sci. 2013;134:26–38. doi: 10.1093/toxsci/kft101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Z., Lin H.-P., Li Y., Tao H., Yang P., Xie J., Maddy D., Kondo K., Yang C. Chronic Hexavalent Chromium Exposure Induces Cancer Stem Cell-Like Property and Tumorigenesis by Increasing c-Myc Expression. Toxicol. Sci. 2019;172:252–264. doi: 10.1093/toxsci/kfz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang J., Song Y.-X., Ma B., Wang J.-J., Sun J.-X., Chen X.-W., Zhao J.-H., Yang Y.-C., Wang Z.-N. Regulatory Roles of Non-Coding RNAs in Colorectal Cancer. Int. J. Mol. Sci. 2015;16:19886–19919. doi: 10.3390/ijms160819886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lin S., Gregory R.I. MicroRNA biogenesis pathways in cancer. Nat. Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang B., Pan X. RDX Induces Aberrant Expression of MicroRNAs in Mouse Brain and Liver. Environ. Health Perspect. 2009;117:231–240. doi: 10.1289/ehp.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hui P., Gysler S.M., Uduman M., Togun T.A., Prado D.E., Brambs C.E., Nallur S., Schwartz P.E., Rutherford T.J., Santin A.D., et al. MicroRNA signatures discriminate between uterine and ovarian serous carcinomas. Hum. Pathol. 2018;76:133–140. doi: 10.1016/j.humpath.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 233.Iorio M.V., Visone R., Di Leva G., Donati V., Petrocca F., Casalini P., Taccioli C., Volinia S., Liu C.-G., Alder H., et al. MicroRNA Signatures in Human Ovarian Cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 234.Zheng M., Hu Y., Gou R., Nie X., Li X., Liu J., Lin B. Identification three LncRNA prognostic signature of ovarian cancer based on genome-wide copy number variation. Biomed. Pharmacother. 2020;124:109810. doi: 10.1016/j.biopha.2019.109810. [DOI] [PubMed] [Google Scholar]
- 235.Martini P., Paracchini L., Caratti G., Mello-Grand M., Fruscio R., Beltrame L., Calura E., Sales G., Ravaggi A., Bignotti E., et al. lncRNAs as Novel Indicators of Patients’ Prognosis in Stage I Epithelial Ovarian Cancer: A Retrospective and Multicentric Study. Clin. Cancer Res. 2016;23:2356–2366. doi: 10.1158/1078-0432.CCR-16-1402. [DOI] [PubMed] [Google Scholar]
- 236.Meng C., Zhou J.-Q., Liao Y.-S. Autophagy-related long non-coding RNA signature for ovarian cancer. J. Int. Med Res. 2020;48:300060520970761. doi: 10.1177/0300060520970761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang Y., Zhou H., Zhang M., Xing L., Yang C., Xia B., Lou G. Integrated analysis of a competing endogenous RNA network reveals an 11-lncRNA prognostic signature in ovarian cancer. Aging. 2020;12:25153–25171. doi: 10.18632/aging.104116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Xu L., Wu Y., Che X., Zhao J., Wang F., Wang P., Qu X., Liu Y., Li Z. Cox-LASSO Analysis Reveals a Ten-lncRNA Signature to Predict Outcomes in Patients with High-Grade Serous Ovarian Cancer. DNA Cell Biol. 2019;38:1519–1528. doi: 10.1089/dna.2019.4826. [DOI] [PubMed] [Google Scholar]
- 239.Zhang Y., Ye Q., He J., Chen P., Wan J., Li J., Yang Y., Li X. Recurrence-Associated Multi-RNA Signature to Predict Disease-Free Survival for Ovarian Cancer Patients. BioMed Res. Int. 2020;2020:1618527-19. doi: 10.1155/2020/1618527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hong H.-C., Chuang C.-H., Huang W.-C., Weng S.-L., Chen C.-H., Chang K.-H., Liao K.-W., Huang H.-D. A panel of eight microRNAs is a good predictive parameter for triple-negative breast cancer relapse. Theranostics. 2020;10:8771–8789. doi: 10.7150/thno.46142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yu X., Liang J., Xu J., Li X., Xing S., Li H., Liu W., Liu D., Xu J., Huang L., et al. Identification and Validation of Circulating MicroRNA Signatures for Breast Cancer Early Detection Based on Large Scale Tissue-Derived Data. J. Breast Cancer. 2018;21:363–370. doi: 10.4048/jbc.2018.21.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sathipati S.Y., Ho S.-Y. Identifying a miRNA signature for predicting the stage of breast cancer. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-34604-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lai J., Wang H., Pan Z., Su F. A novel six-microRNA-based model to improve prognosis prediction of breast cancer. Aging. 2019;11:649–662. doi: 10.18632/aging.101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tang J., Ma W., Zeng Q., Tan J., Cao K., Luo L. Identification of miRNA-Based Signature as a Novel Potential Prognostic Biomarker in Patients with Breast Cancer. Dis. Markers. 2019;2019:3815952-17. doi: 10.1155/2019/3815952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ma W., Zhao F., Yu X., Guan S., Suo H., Tao Z., Qiu Y., Wu Y., Cao Y., Jin F. Immune-related lncRNAs as predictors of survival in breast cancer: A prognostic signature. J. Transl. Med. 2020;18:1–13. doi: 10.1186/s12967-020-02522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li Z., Li Y., Wang X., Yang Q. Identification of a Six-Immune-Related Long Non-coding RNA Signature for Predicting Survival and Immune Infiltrating Status in Breast Cancer. Front. Genet. 2020;11:680. doi: 10.3389/fgene.2020.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang Y., Wang Y., Tian G., Jiang T. Long non-coding RNA-based signatures to improve prognostic prediction of breast cancer. Medicine. 2020;99:e22203. doi: 10.1097/MD.0000000000022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu Z., Li M., Hua Q., Li Y., Wang G. Identification of an eight-lncRNA prognostic model for breast cancer using WGCNA network analysis and a Cox-proportional hazards model based on L1-penalized estimation. Int. J. Mol. Med. 2019;44:1333–1343. doi: 10.3892/ijmm.2019.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang G., Chen X., Liang Y., Wang W., Fang Y., Shen K. Long Noncoding RNA Signature and Disease Outcome in Estrogen Receptor-Positive Breast Cancer Patients Treated with Tamoxifen. J. Breast Cancer. 2018;21:277–287. doi: 10.4048/jbc.2018.21.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Huang P., Li F., Mo Z., Geng C., Wen F., Zhang C., Guo J., Wu S., Li L., Brünner N., et al. A Comprehensive RNA Study to Identify circRNA and miRNA Biomarkers for Docetaxel Resistance in Breast Cancer. Front. Oncol. 2021;11:669270. doi: 10.3389/fonc.2021.669270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhou X., Huang Z., Xu L., Zhu M., Zhang L., Zhang H., Wang X., Li H., Zhu W., Shu Y., et al. A panel of 13-miRNA signature as a potential biomarker for predicting survival in pancreatic cancer. Oncotarget. 2016;7:69616–69624. doi: 10.18632/oncotarget.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liang L., Wei D., Li J., Luo D., Chen G., Dang Y., Cai X. Prognostic microRNAs and their potential molecular mechanism in pancreatic cancer: A study based on The Cancer Genome Atlas and bioinformatics investigation. Mol. Med. Rep. 2017;17:939–951. doi: 10.3892/mmr.2017.7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Duell E.J., Lujan-Barroso L., Sala N., McElyea S.D., Overvad K., Tjonneland A., Olsen A., Weiderpass E., Busund L.-T., Moi L., et al. Plasma microRNAs as biomarkers of pancreatic cancer risk in a prospective cohort study. Int. J. Cancer. 2017;141:905–915. doi: 10.1002/ijc.30790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vila-Navarro E., Duran-Sanchon S., Vila-Casadesús M., Moreira L., Ginès À., Cuatrecasas M., Lozano J.J., Bujanda L., Castells A., Gironella M. Novel Circulating miRNA Signatures for Early Detection of Pancreatic Neoplasia. Clin. Transl. Gastroenterol. 2019;10:e00029. doi: 10.14309/ctg.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shams R., Saberi S., Zali M., Sadeghi A., Ghafouri-Fard S., Aghdaei H.A. Identification of potential microRNA panels for pancreatic cancer diagnosis using microarray datasets and bioinformatics methods. Sci. Rep. 2020;10:1–15. doi: 10.1038/s41598-020-64569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhou C., Wang S., Zhou Q., Zhao J., Xia X., Chen W., Zheng Y., Xue M., Yang F., Fu D., et al. A Long Non-coding RNA Signature to Improve Prognostic Prediction of Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2019;9:1160. doi: 10.3389/fonc.2019.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shi X., Zhao Y., He R., Zhou M., Pan S., Yu S., Xie Y., Li X., Wang M., Guo X., et al. Three-lncRNA signature is a potential prognostic biomarker for pancreatic adenocarcinoma. Oncotarget. 2018;9:24248–24259. doi: 10.18632/oncotarget.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lin Y., Pan X., Chen Z., Lin S., Chen S. Identification of an Immune-Related Nine-lncRNA Signature Predictive of Overall Survival in Colon Cancer. Front. Genet. 2020;11:318. doi: 10.3389/fgene.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pichler M., Stiegelbauer V., Vychytilova-Faltejskova P., Ivan C., Ling H., Winter E., Zhang X., Goblirsch M., Wulf-Goldenberg A., Ohtsuka M., et al. Genome-Wide miRNA Analysis Identifies miR-188-3p as a Novel Prognostic Marker and Molecular Factor Involved in Colorectal Carcinogenesis. Clin. Cancer Res. 2016;23:1323–1333. doi: 10.1158/1078-0432.CCR-16-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shen L., Chen L., Wang Y., Jiang X., Xia H., Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J. Neuro-Oncol. 2014;121:101–108. doi: 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 261.Wang H., Wang L., Zhang G., Lu C., Chu H., Yang R., Zhao G. MALAT1/miR-101-3p/MCL1 axis mediates cisplatin resistance in lung cancer. Oncotarget. 2017;9:7501–7512. doi: 10.18632/oncotarget.23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ghafouri-Fard S., Shoorei H., Anamag F.T., Taheri M. The Role of Non-Coding RNAs in Controlling Cell Cycle Related Proteins in Cancer Cells. Front. Oncol. 2020;10:608975. doi: 10.3389/fonc.2020.608975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Li R., Yang Y.-E., Jin J., Zhang M.-Y., Liu X., Yin Y.-H., Qu Y.-Q. Identification of lncRNA biomarkers in lung squamous cell carcinoma using comprehensive analysis of lncRNA mediated ceRNA network. Artif. Cells Nanomed. Biotechnol. 2019;47:3246–3258. doi: 10.1080/21691401.2019.1647225. [DOI] [PubMed] [Google Scholar]
- 264.Gong W., Yang L., Wang Y., Xian J., Qiu F., Liu L., Lin M., Feng Y., Zhou Y., Lu J. Analysis of Survival-Related lncRNA Landscape Identifies A Role for LINC01537 in Energy Metabolism and Lung Cancer Progression. Int. J. Mol. Sci. 2019;20:3713. doi: 10.3390/ijms20153713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Acha-Sagredo A., Uko B., Pantazi P., Bediaga N.G., Moschandrea C., Rainbow L., Marcus M.W., Davies M.P.A., Field J.K., Liloglou T. Long non-coding RNA dysregulation is a frequent event in non-small cell lung carcinoma pathogenesis. Br. J. Cancer. 2020;122:1050–1058. doi: 10.1038/s41416-020-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu H., Ye T., Yang X., Lv P., Wu X., Zhou H., Zeng J., Tang K., Ye Z. A Panel of Four-lncRNA Signature as a Potential Biomarker for Predicting Survival in Clear Cell Renal Cell Carcinoma. J. Cancer. 2020;11:4274–4283. doi: 10.7150/jca.40421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ghafouri-Fard S., Shirvani-Farsani Z., Branicki W., Taheri M. MicroRNA Signature in Renal Cell Carcinoma. Front. Oncol. 2020;10:596359. doi: 10.3389/fonc.2020.596359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Youssef Y.M., White N.M., Grigull J., Krizova A., Samy C., Mejia-Guerrero S., Evans A., Yousef G.M. Accurate Molecular Classification of Kidney Cancer Subtypes Using MicroRNA Signature. Eur. Urol. 2011;59:721–730. doi: 10.1016/j.eururo.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 269.Rodriguez P., Paculova H., Kogut S., Heath J., Schjerven H., Frietze S. Non-Coding RNA Signatures of B-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021;22:2683. doi: 10.3390/ijms22052683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Fernando T.R., Rodriguez-Malave N.I., Waters E.V., Yan W., Casero D., Basso G., Pigazzi M., Rao D.S. LncRNA Expression Discriminates Karyotype and Predicts Survival in B-Lymphoblastic Leukemia. Mol. Cancer Res. 2015;13:839–851. doi: 10.1158/1541-7786.MCR-15-0006-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rawoof A., Swaminathan G., Tiwari S., A Nair R., Kumar L.D. LeukmiR: A database for miRNAs and their targets in acute lymphoblastic leukemia. Database. 2020;2020:baz151. doi: 10.1093/database/baz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mi S., Lu J., Sun M., Li Z., Zhang H., Neilly M.B., Wang Y., Qian Z., Jin J., Zhang Y., et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2007;104:19971–19976. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhang H., Luo X.-Q., Zhang P., Huang L.-B., Zheng Y.-S., Wu J., Zhou H., Qu L.-H., Xu L., Chen Y.-Q. MicroRNA Patterns Associated with Clinical Prognostic Parameters and CNS Relapse Prediction in Pediatric Acute Leukemia. PLoS ONE. 2009;4:e7826. doi: 10.1371/journal.pone.0007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Han B.-W., Feng D.-D., Li Z.-G., Luo X.-Q., Zhang H., Li X.-J., Zhang X.-J., Zheng L.-L., Zeng C.-W., Lin K.-Y., et al. A set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Hum. Mol. Genet. 2011;20:4903–4915. doi: 10.1093/hmg/ddr428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Avigad S., Verly I.R., Lebel A., Kordi O., Shichrur K., Ohali A., Hameiri-Grossman M., Kaspers G.J., Cloos J., Fronkova E., et al. miR expression profiling at diagnosis predicts relapse in pediatric precursor B-cell acute lymphoblastic leukemia. Genes Chromosom. Cancer. 2015;55:328–339. doi: 10.1002/gcc.22334. [DOI] [PubMed] [Google Scholar]
- 276.Gaffo E., Boldrin E., Molin A.D., Bresolin S., Bonizzato A., Trentin L., Frasson C., Debatin K.-M., Meyer L.H., Kronnie G.T., et al. Circular RNA differential expression in blood cell populations and exploration of circRNA deregulation in pediatric acute lymphoblastic leukemia. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-50864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bresesti C., Vezzoli V., Cangiano B., Bonomi M. Long Non-Coding RNAs: Role in Testicular Cancers. Front. Oncol. 2021;11:605606. doi: 10.3389/fonc.2021.605606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lobo J., Nunes S.P., Gillis A.J.M., Barros-Silva D., Miranda-Gonçalves V., Berg A.V.D., Cantante M., Guimarães R., Henrique R., Jerónimo C., et al. XIST-Promoter Demethylation as Tissue Biomarker for Testicular Germ Cell Tumors and Spermatogenesis Quality. Cancers. 2019;11:1385. doi: 10.3390/cancers11091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Regouc M., Belge G., Lorch A., Dieckmann K.-P., Pichler M. Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers. 2020;12:749. doi: 10.3390/cancers12030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wentzensen N., Wacholder S. From Differences in Means between Cases and Controls to Risk Stratification: A Business Plan for Biomarker Development. Cancer Discov. 2013;3:148–157. doi: 10.1158/2159-8290.CD-12-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nava V.E., Perera P.-Y., Kumar N., Jain M. Noncoding-RNA-Based Therapeutics with an Emphasis on Prostatic Carcinoma—Progress and Challenges. Vaccines. 2022;10:276. doi: 10.3390/vaccines10020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Juliano R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44:6518–6548. doi: 10.1093/nar/gkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Battistelli C., Garbo S., Riccioni V., Montaldo C., Santangelo L., Vandelli A., Strippoli R., Tartaglia G.G., Tripodi M., Cicchini C. Design and functional validation of a mutant variant of the lncRNA HOTAIR to counteract Snail function in Epithelial-to-Mesenchymal Transition. Cancer Res. 2020;81:103–113. doi: 10.1158/0008-5472.CAN-20-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Egli M., Manoharan M. Re-Engineering RNA Molecules into Therapeutic Agents. Accounts Chem. Res. 2019;52:1036–1047. doi: 10.1021/acs.accounts.8b00650. [DOI] [PubMed] [Google Scholar]
- 285.Das S.K., Menezes M.E., Bhatia S., Wang X.-Y., Emdad L., Sarkar D., Fisher P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2014;230:259–271. doi: 10.1002/jcp.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Toden S., Zumwalt T.J., Goel A. Non-coding RNAs and potential therapeutic targeting in cancer. Biochim. et Biophys. Acta. 2020;1875:188491. doi: 10.1016/j.bbcan.2020.188491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Skinner M.K. What is an epigenetic transgenerational phenotype?: F3 or F2. Reprod. Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Morris I.D. Sperm DNA damage and cancer treatment1. Int. J. Androl. 2002;25:255–261. doi: 10.1046/j.1365-2605.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 289.Hajkova P., Erhardt S., Lane N., Haaf T., El-Maarri O., Reik W., Walter J., Surani M. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 2002;117:15–23. doi: 10.1016/S0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 290.Reik W., Walter J. Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 291.Ptashne M. Epigenetics: Core misconcept. Proc. Natl. Acad. Sci. USA. 2013;110:7101–7103. doi: 10.1073/pnas.1305399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Waddington C.H. Canalization of Development and Genetic Assimilation of Acquired Characters. Nature. 1959;183:1654–1655. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 293.Anway M.D., Cupp A.S., Uzumcu M., Skinner M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Morgan H.D., Sutherland H., Martin D.I., Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 295.Raval A., Tanner S.M., Byrd J.C., Angerman E.B., Perko J.D., Chen S.-S., Hackanson B., Grever M.R., Lucas D.M., Matkovic J.J., et al. Downregulation of Death-Associated Protein Kinase 1 (DAPK1) in Chronic Lymphocytic Leukemia. Cell. 2007;129:879–890. doi: 10.1016/j.cell.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hitchins M.P., Rapkins R.W., Kwok C.-T., Srivastava S., Wong J., Khachigian L., Polly P., Goldblatt J., Ward R. Dominantly Inherited Constitutional Epigenetic Silencing of MLH1 in a Cancer-Affected Family Is Linked to a Single Nucleotide Variant within the 5′UTR. Cancer Cell. 2011;20:200–213. doi: 10.1016/j.ccr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 297.Daxinger L., Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 298.Lim J.P., Brunet A. Bridging the transgenerational gap with epigenetic memory. Trends Genet. 2013;29:176–186. doi: 10.1016/j.tig.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nilsson E.E., Sadler-Riggleman I., Skinner M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics. 2018;4:dvy016. doi: 10.1093/eep/dvy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Belleannee C., Calvo É., Caballero J., Sullivan R. Epididymosomes Convey Different Repertoires of MicroRNAs throughout the Bovine Epididymis1. Biol. Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- 301.Chamorro-García R., Sahu M., Abbey R.J., Laude J., Pham N., Blumberg B. Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Exposure to the Obesogen Tributyltin in Mice. Environ. Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Diaz-Castillo C., Chamorro-Garcia R., Shioda T., Blumberg B. Transgenerational Self-Reconstruction of Disrupted Chromatin Organization After Exposure To An Environmental Stressor in Mice. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-49440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Watanabe T., Tomizawa S.-I., Mitsuya K., Totoki Y., Yamamoto Y., Kuramochi-Miyagawa S., Iida N., Hoki Y., Murphy P.J., Toyoda A., et al. Role for piRNAs and Noncoding RNA in de Novo DNA Methylation of the Imprinted Mouse Rasgrf1 Locus. Science. 2011;332:848–852. doi: 10.1126/science.1203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Gapp K., Jawaid A., Sarkies P., Bohacek J., Pelczar P., Prados J., Farinelli L., Miska E., Mansuy I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Grandjean V., Fourré S., De Abreu D.A.F., Derieppe M.-A., Remy J.-J., Rassoulzadegan M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015;5:18193. doi: 10.1038/srep18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Barbosa T.D.C., Ingerslev L.R., Alm P.S., Versteyhe S., Massart J., Rasmussen M., Donkin I., Sjögren R., Mudry J.M., Vetterli L., et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2016;5:184–197. doi: 10.1016/j.molmet.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kawano M., Kawaji H., Grandjean V., Kiani J., Rassoulzadegan M. Novel Small Noncoding RNAs in Mouse Spermatozoa, Zygotes and Early Embryos. PLoS ONE. 2012;7:e44542. doi: 10.1371/journal.pone.0044542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rassoulzadegan M., Grandjean V., Gounon P., Vincent S., Gillot I., Cuzin F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 309.Rodgers A.B., Morgan C.P., Leu N.A., Bale T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Schuster A., Skinner M.K., Yan W. Ancestral vinclozolin exposure alters the epigenetic transgenerational inheritance of sperm small noncoding RNAs. Environ. Epigenetics. 2016;2:dvw001. doi: 10.1093/eep/dvw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 312.Babatola S.S. Global burden of diseases attributable to air pollution. J. Public Health Afr. 2018;9:813. doi: 10.4081/jphia.2018.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sun Y.-M., Chen Y.-Q. Principles and innovative technologies for decrypting noncoding RNAs: From discovery and functional prediction to clinical application. J. Hematol. Oncol. 2020;13:1–27. doi: 10.1186/s13045-020-00945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li X., Zhou B., Chen L., Gou L.-T., Li H., Fu X.-D. GRID-seq reveals the global RNA–chromatin interactome. Nat. Biotechnol. 2017;35:940–950. doi: 10.1038/nbt.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sachdeva S., Davis R.W., Saha A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021;8:602659. doi: 10.3389/fbioe.2020.602659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang Y.-M., Trinh M.P., Zheng Y., Guo K., Jimenez L.A., Zhong W. Analysis of circulating non-coding RNAs in a non-invasive and cost-effective manner. TrAC Trends Anal. Chem. 2019;117:242–262. doi: 10.1016/j.trac.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mishra K., Kanduri C. Understanding Long Noncoding RNA and Chromatin Interactions: What We Know So Far. Non-Coding RNA. 2019;5:54. doi: 10.3390/ncrna5040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Agency for Toxic Substances and Disease Registry ToxFAQsTM for DDT, DDE, and DDD. [(accessed on 10 February 2022)];2020 Available online: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=80&toxid=20. [PubMed]
- 319.Agency for Toxic Substances and Disease Registry Polychlorinated Biphenyls (PCBs) Toxicity. [(accessed on 10 February 2022)];2014 Available online: https://www.atsdr.cdc.gov/csem/polychlorinated-biphenyls/adverse_health.html.
- 320.American Cancer Society Perfluorooctanoic Acid (PFOA), Teflon, and Related Chemicals. 2022. [(accessed on 10 February 2022)]. Available online: https://www.cancer.org/cancer/cancer-causes/teflon-and-perfluorooctanoic-acid-pfoa.html.
- 321.Alli L.A. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip. Toxicol. 2015;8:146–150. doi: 10.1515/intox-2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Alvarado A., Blanco R., Mora E. El cromo como elemento esencial en los humanos. Rev. Costarric. De Cienc. Médicas. 2002;23:55–68. [Google Scholar]
- 323.Anyanwu B.O., Orisakwe O.E. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. J. Environ. Sci. Health Part C. 2020;38:204–244. doi: 10.1080/26896583.2020.1782116. [DOI] [PubMed] [Google Scholar]
- 324.Avissar-Whiting M., Veiga K.R., Uhl K.M., Maccani M.A., Gagne L.A., Moen E.L., Marsit C.J. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010;29:401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Awadalla A., Mortada W.I., Abol-Enein H., Shokeir A.A. Correlation between blood levels of cadmium and lead and the expression of microRNA-21 in Egyptian bladder cancer patients. Heliyon. 2020;6:e05642. doi: 10.1016/j.heliyon.2020.e05642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Bae H.-S., Ryu D.-Y., Choi B.-S., Park J.-D. Urinary Arsenic Concentrations and their Associated Factors in Korean Adults. Toxicol. Res. 2013;29:137–142. doi: 10.5487/TR.2013.29.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Baker B.A., Cassano V.A., Murray C. Arsenic Exposure, Assessment, Toxicity, Diagnosis, and Management. J. Occup. Environ. Med. 2018;60:e634–e639. doi: 10.1097/JOM.0000000000001485. [DOI] [PubMed] [Google Scholar]
- 328.Beck R., Bommarito P., Douillet C., Kanke M., Del Razo L.M., García-Vargas G., Fry R.C., Sethupathy P., Stýblo M. Circulating miRNAs Associated with Arsenic Exposure. Environ. Sci. Technol. 2018;52:14487–14495. doi: 10.1021/acs.est.8b06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Blockhuys S., Wittung-Stafshede P. Roles of Copper-Binding Proteins in Breast Cancer. Int. J. Mol. Sci. 2017;18:871. doi: 10.3390/ijms18040871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Boffetta P., Jourenkova N., Gustavsson P. Riesgo de cáncer por exposición ocupacional y ambiental a hidrocarbu-ros aromáticos policíclicos. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/A:1018465507029. [DOI] [PubMed] [Google Scholar]
- 331.Born S.C. Chapter 62. dioxins. In: Olson K.R., editor. Poisoning & Drug Overdose. 6th ed. McGraw Hill; New York, NY, USA: 2012. [(accessed on 10 February 2022)]. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=391§ionid=42069876. [Google Scholar]
- 332.Bouwman H., Becker P.J., Schutte C.H. Malaria control and longitudinal changes in levels of DDT and its me-tabolites in human serum from KwaZulu. Bull. World Health Organ. 1994;72:921–930. [PMC free article] [PubMed] [Google Scholar]
- 333.Brucker N., Charão M.F., Moro A.M., Ferrari P., Bubols G., Sauer E., Fracasso R., Durgante J., Thiesen F., Duarte M.M., et al. Atherosclerotic process in taxi drivers occupationally exposed to air pollution and co-morbidities. Environ. Res. 2014;131:31–38. doi: 10.1016/j.envres.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 334.Burstyn I., Kromhout H., Partanen T., Svane O., Langård S., Ahrens W., Kauppinen T., Stücker I., Shaham J., Heederik D., et al. Polycyclic Aromatic Hydrocarbons and Fatal Ischemic Heart Disease. Epidemiology. 2005;16:744–750. doi: 10.1097/01.ede.0000181310.65043.2f. [DOI] [PubMed] [Google Scholar]
- 335.CDC Polycyclic Aromatic Hydrocarbons (PAHs). Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2009 Available online: https://www.epa.gov/sites/default/files/2014-03/documents/pahs_factsheet_cdc_2013.pdf.
- 336.CDC Dichlorodiphenyltrichloroethane (DDT). Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2009 Available online: https://www.cdc.gov/biomonitoring/pdf/ddt_factsheet.pdf.
- 337.CDC Lead Compounds (as Pb). Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2014 Available online: https://www.cdc.gov/niosh/idlh/7439921.html.
- 338.CDC Smoking and Cardiovascular Disease. Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2014 Available online: https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/pdfs/fs_smoking_CVD_508.pdf.
- 339.CDC ToxFAQs™—DDT, DDE y DDD. Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2016 Available online: https://www.atsdr.cdc.gov/es/toxfaqs/es_tfacts35.html.
- 340.CDC Bisphenol A (BPA) Factsheet. Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2017 Available online: https://www.cdc.gov/biomonitoring/BisphenolA_FactSheet.html.
- 341.CDC Sources of Lead Exposure. Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2020 Available online: https://www.cdc.gov/nceh/lead/prevention/sources.htm.
- 342.CDC Health Effects of Cigarette Smoking. Centers for Disease Control and Prevention. [(accessed on 10 February 2022)];2021 Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm.
- 343.Centers for Disease Control and Prevention. [(accessed on 25 June 2020)]; Available online: https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html.
- 344.Chuang S.-C., Chen H.-C., Sun C.-W., Chen Y.-A., Wang Y.-H., Chiang C.-J., Chen C.-C., Wang S.-L., Chen C.-J., Hsiung C.A. Phthalate exposure and prostate cancer in a population-based nested case-control study. Environ. Res. 2019;181:108902. doi: 10.1016/j.envres.2019.108902. [DOI] [PubMed] [Google Scholar]
- 345.Cobellis L., Colacurci N., Trabucco E., Carpentiero C., Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009;23:1186–1190. doi: 10.1002/bmc.1241. [DOI] [PubMed] [Google Scholar]
- 346.Cohn B.A., Wolff M.S., Cirillo P.M., Sholtz R.I. DDT and Breast Cancer in Young Women: New Data on the Significance of Age at Exposure. Environ. Health Perspect. 2007;115:1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Collaborative on Health and the Environment Arsenic. 2019. [(accessed on 10 February 2022)]. Available online: https://www.healthandenvironment.org/environmental-health/environmental-risks/chemical-environment-overview/arsenic.
- 348.Craig S. Chapter 78. Glyphosate. In: Olson K.R., editor. Poisoning & Drug Overdose. 6th ed. McGraw Hill; New York, NY, USA: 2012. [(accessed on 10 February 2022)]. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=391§ionid=42069892. [Google Scholar]
- 349.Dann A.B., Hontela A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2010;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 350.Deng Q., Huang S., Zhang X., Zhang W., Feng J., Wang T., Hu D., Guan L., Li J., Dai X., et al. Plasma microRNA Expression and Micronuclei Frequency in Workers Exposed to Polycyclic Aromatic Hydrocarbons. Environ. Health Perspect. 2014;122:719–725. doi: 10.1289/ehp.1307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Dinwiddie M.T., Terry P.D., Chen J. Recent Evidence Regarding Triclosan and Cancer Risk. Int. J. Environ. Res. Public Health. 2014;11:2209–2217. doi: 10.3390/ijerph110202209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Dioni L., Sucato S., Motta V., Iodice S., Angelici L., Favero C., Cavalleri T., Vigna L., Albetti B., Fustinoni S., et al. Urinary chromium is associated with changes in leukocyte miRNA expression in obese subjects. Eur. J. Clin. Nutr. 2016;71:142–148. doi: 10.1038/ejcn.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Du Z., Chai X., Li X., Ren G., Yang X., Yang Z. Nano-CuO causes cell damage through activation of dose-dependent autophagy and mitochondrial lncCyt b-AS/ND5-AS/ND6-AS in SH-SY5Y cells. Toxicol. Mech. Methods. 2021;32:37–48. doi: 10.1080/15376516.2021.1964665. [DOI] [PubMed] [Google Scholar]
- 354.Falagan-Lotsch P., Murphy C.J. Network-based analysis implies critical roles of microRNAs in the long-term cellular responses to gold nanoparticles. Nanoscale. 2020;12:21172–21187. doi: 10.1039/D0NR04701E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Gao H., Yang B.-J., Li N., Feng L.-M., Shi X.-Y., Zhao W.-H., Liu S.-J. Bisphenol A and Hormone-Associated Cancers: Current progress and perspectives. Medicine. 2015;94:e211. doi: 10.1097/MD.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Garshick E., Laden F., Hart J.E., Davis M.E., Eisen E.A., Smith T.J. Lung Cancer and Elemental Carbon Exposure in Trucking Industry Workers. Environ. Health Perspect. 2012;120:1301–1306. doi: 10.1289/ehp.1204989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Gaum P.M., Gube M., Schettgen T., Putschögl F.M., Kraus T., Fimm B., Lang J. Polychlorinated biphenyls and depression: Cross-sectional and longitudinal investigation of a dopamine-related Neurochemical path in the German HELPcB surveillance program. Environ. Health. 2017;16:106. doi: 10.1186/s12940-017-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gerona R.R., Pan J., Zota A.R., Schwartz J.M., Friesen M., Taylor J.A., Hunt P.A., Woodruff T.J. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health. 2016;15:50. doi: 10.1186/s12940-016-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Gonskikh Y., Gerstl M., Kos M., Borth N., Schosserer M., Grillari J., Polacek N. Modulation of mammalian translation by a ribosome-associated tRNA half. RNA Biol. 2020;17:1125–1136. doi: 10.1080/15476286.2020.1744296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Government of Spain DDT. 2020. [(accessed on 10 February 2022)]. Available online: https://prtr-es.es/DDT,15620,11,2007.html.
- 361.Harari F., Barregard L., Östling G., Sallsten G., Hedblad B., Forsgard N., Borné Y., Fagerberg B., Engström G. Blood Lead Levels and Risk of Atherosclerosis in the Carotid Artery: Results from a Swedish Cohort. Environ. Health Perspect. 2019;127:127002. doi: 10.1289/EHP5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Hollensteiner J., Schneider D., Poehlein A., Daniel R. Complete Genome of Roseobacter ponti DSM 106830T. Genome Biol. Evol. 2020;12:1013–1018. doi: 10.1093/gbe/evaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hong Y.-S., Song K.-H., Chung J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health. 2014;47:245–252. doi: 10.3961/jpmph.14.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Iida T., Todaka T. Measurement of Dioxins in Human Blood: Improvement of Analytical Method. Ind. Health. 2003;41:197–204. doi: 10.2486/indhealth.41.197. [DOI] [PubMed] [Google Scholar]
- 365.Kalinina T.S., Kononchuk V.V., Ovchinnikov V.Y., Chanyshev M.D., Gulyaeva L.F. Expression of the miR-190 family is increased under DDT exposure in vivo and in vitro. Mol. Biol. Rep. 2018;45:1937–1945. doi: 10.1007/s11033-018-4343-0. [DOI] [PubMed] [Google Scholar]
- 366.Kim J.H., Cho Y.H., Hong Y.-C. MicroRNA expression in response to bisphenol A is associated with high blood pressure. Environ. Int. 2020;141:105791. doi: 10.1016/j.envint.2020.105791. [DOI] [PubMed] [Google Scholar]
- 367.Konieczna A., Rutkowska A., Rachoń D. Health risk of exposure to Bisphenol A (BPA) Rocz. Panstw. Zakl. Hig. 2015;66:5–11. [PubMed] [Google Scholar]
- 368.Lee H.-W., Jose C.C., Cuddapah S. Epithelial-mesenchymal transition: Insights into nickel-induced lung diseases. Semin. Cancer Biol. 2021;76:99–109. doi: 10.1016/j.semcancer.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lee T.-W., Kim D.H., Ryu J.Y. Association between urinary polycyclic aromatic hydrocarbons and hypertension in the Korean population: Data from the Second Korean National Environmental Health Survey (2012–2014) Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-74353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lim J.-H., Song M.-K., Cho Y., Kim W., Han S.O., Ryu J.-C. Comparative analysis of microRNA and mRNA expression profiles in cells and exosomes under toluene exposure. Toxicol. Vitr. 2017;41:92–101. doi: 10.1016/j.tiv.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 371.López-Carrillo L., Hernández-Ramírez R.U., Calafat A.M., Torres-Sánchez L., Galván-Portillo M., Needham L.L., Ruiz-Ramos R., Cebrián M.E. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ. Health Perspect. 2010;118:539–544. doi: 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Luz A., DeLeo P., Pechacek N., Freemantle M. Human health hazard assessment of quaternary ammonium compounds: Didecyl dimethyl ammonium chloride and alkyl (C12–C16) dimethyl benzyl ammonium chloride. Regul. Toxicol. Pharmacol. 2020;116:104717. doi: 10.1016/j.yrtph.2020.104717. [DOI] [PubMed] [Google Scholar]
- 373.Messerlian C., Wylie B.J., Mínguez-Alarcón L., Williams P.L., Ford J.B., Souter I.C., Calafat A.M., Hauser R. Urinary Concentrations of Phthalate Metabolites and Pregnancy Loss Among Women Conceiving with Medically Assisted Reproduction. Epidemiology. 2016;27:879–888. doi: 10.1097/EDE.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Mohamed F., Gawarammana I., Robertson T., Roberts M., Palangasinghe C., Zawahir S., Jayamanne S., Kandasamy J., Eddleston M., Buckley N.A., et al. Acute Human Self-Poisoning with Imidacloprid Compound: A Neonicotinoid Insecticide. PLoS ONE. 2009;4:e5127. doi: 10.1371/journal.pone.0005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Mundhe S.A., Birajdar S.V., Chavan S.S., Pawar N.R. Imidacloprid poisoning: An emerging cause of potentially fatal poisoning. Indian J. Crit. Care Med. 2017;21:786–788. doi: 10.4103/ijccm.IJCCM_152_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Muzembo B.A., Iwai-Shimada M., Isobe T., Arisawa K., Shima M., Fukushima T., Nakayama S.F. Dioxins levels in human blood after implementation of measures against dioxin exposure in Japan. Environ. Health Prev. Med. 2019;24:6. doi: 10.1186/s12199-018-0755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Nassan F.L., Mínguez-Alarcón L., Williams P.L., Dadd R., Petrozza J.C., Ford J.B., Calafat A.M., Hauser R. Urinary triclosan concentrations and semen quality among men from a fertility clinic. Environ. Res. 2019;177:108633. doi: 10.1016/j.envres.2019.108633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.National Academy of Sciences 2 Phthalate Exposure Assessment in Humans. In Phthalates and Cumulative Risk Assessment: The Tasks Ahead; 2008. [(accessed on 10 February 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK215044/
- 379.National Institute of Environmental Health Science Bisphenol A (BPA) [(accessed on 10 February 2022)];2021 Available online: https://www.niehs.nih.gov/health/topics/agents/sya-bpa/index.cfm.
- 380.National Research Council (US) Committee on Measuring Lead in Critical Populations . Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations. National Academies Press; Washington, DC, USA: 1993. [(accessed on 10 February 2022)]. 2. Adverse Health Ef-fects of Exposure to Lead. Available online: https://www.ncbi.nlm.nih.gov/books/NBK236465. [PubMed] [Google Scholar]
- 381.New Jersey Departament of Health Hazardous Substance Fact Sheet. [(accessed on 10 February 2022)];2010 Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0709.pdf.
- 382.Oregon State University Imidacloprid. 2010. [(accessed on 10 February 2022)]. Available online: http://npic.orst.edu/factsheets/imidagen.html#exposed.
- 383.Papoutsopoulou S., Satsangi J., Campbell B.J., Probert C.S. Review article: Impact of cigarette smoking on intestinal inflammation-direct and indirect mechanisms. Aliment. Pharmacol. Ther. 2020;51:1268–1285. doi: 10.1111/apt.15774. [DOI] [PubMed] [Google Scholar]
- 384.Pan X., Hu J., Xia W., Zhang B., Liu W., Zhang C., Yang J., Hu C., Zhou A., Chen Z., et al. Prenatal chromium exposure and risk of preterm birth: A cohort study in Hubei, China. Sci. Rep. 2017;7:3048. doi: 10.1038/s41598-017-03106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Pan American Health Organization (w.d.). Arsenic. [(accessed on 10 February 2022)]. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=9201:2013-arsenic&Itemid=40132&lang=es.
- 386.Parvez S., Gerona R.R., Proctor C., Friesen M., Ashby J.L., Reiter J.L., Lui Z., Winchester P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health. 2018;17:1–12. doi: 10.1186/s12940-018-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Perkins J.T., Petriello M.C., Newsome B.J., Hennig B. Polychlorinated biphenyls and links to cardiovascular disease. Environ. Sci. Pollut. Res. 2015;23:2160–2172. doi: 10.1007/s11356-015-4479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Persson E.C., Graubard B.I., Evans A.A., London W.T., Weber J.-P., Leblanc A., Chen G., Lin W., McGlynn K.A. Dichlorodiphenyltrichloroethane and risk of hepatocellular carcinoma. Int. J. Cancer. 2012;131:2078–2084. doi: 10.1002/ijc.27459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Pesatori A.C., Consonni D., Rubagotti M., Grillo P., Bertazzi P.A. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: Twenty years of follow-up. Environ. Health. 2009;8:39. doi: 10.1186/1476-069X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Poothong S., Papadopoulou E., Padilla-Sánchez J.A., Thomsen C., Haug L.S. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020;134:105244. doi: 10.1016/j.envint.2019.105244. [DOI] [PubMed] [Google Scholar]
- 391.Pozuelos G.L., Kagda M.S., Schick S., Girke T., Volz D.C., Talbot P. Experimental Acute Exposure to Thirdhand Smoke and Changes in the Human Nasal Epithelial Transcriptome: A Randomized Clinical Trial. JAMA Netw. Open. 2019;2:e196362. doi: 10.1001/jamanetworkopen.2019.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Rahimzadeh M.R., Rahimzadeh M.R., Kazemi S., Moghadamnia A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017;8:135–145. doi: 10.22088/cjim.8.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Rezg R., El-Fazaa S., Gharbi N., Mornagui B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014;64:83–90. doi: 10.1016/j.envint.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 394.Rivers A.R., Burns A.S., Chan L.-K., Moran M.A. Experimental Identification of Small Non-Coding RNAs in the Model Marine Bacterium Ruegeria pomeroyi DSS-3. Front. Microbiol. 2016;7:380. doi: 10.3389/fmicb.2016.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Schecter A., Stanley J., Boggess K., Masuda Y., Mes J., Wolff M., Fürst P., Furst C., Wilson-Yang K., Chisholm B. Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environ. Health Perspect. 1994;102:149–158. doi: 10.1289/ehp.94102s1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Shankar A., Xiao J., Ducatman A. Perfluorooctanoic Acid and Cardiovascular Disease in US Adults. Arch. Intern. Med. 2012;172:1397–1403. doi: 10.1001/archinternmed.2012.3393. [DOI] [PubMed] [Google Scholar]
- 397.Shanker A., Venkateswarlu B. Chromium: Environmental Pollution, Health Effects and Mode of Action. Encycl. Environ. Health. 2011:650–659. doi: 10.1016/b978-0-444-52272-6.00390-1. [DOI] [Google Scholar]
- 398.Sharma M.K., Kumar M. Sulphate contamination in groundwater and its remediation: An overview. Environ. Monit. Assess. 2020;192:1–10. doi: 10.1007/s10661-019-8051-6. [DOI] [PubMed] [Google Scholar]
- 399.Shelnutt S., Kind J., Allaben W. Bisphenol A: Update on newly developed data and how they address NTP’s 2008 finding of “Some Concern”. Food Chem. Toxicol. 2013;57:284–295. doi: 10.1016/j.fct.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 400.Shvedova A.A., Yanamala N., Kisin E.R., Khailullin T.O., Birch M.E., Fatkhutdinova L. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLoS ONE. 2016;11:e0150628. doi: 10.1371/journal.pone.0150628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Skarha J., Mínguez-Alarcón L., Williams P.L., Korevaar T.I., De Poortere R.A., Broeren M.A., Ford J.B., Eliot M., Hauser R., Braun J.M. Cross-sectional associations between urinary triclosan and serum thyroid function biomarker concentrations in women. Environ. Int. 2018;122:256–262. doi: 10.1016/j.envint.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Steenland K., Boffetta P. Lead and cancer in humans: Where are we now? Am. J. Ind. Med. 2000;38:295–299. doi: 10.1002/1097-0274(200009)38:3<295::AID-AJIM8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 403.Steenland K., Fletcher T., Stein C.R., Bartell S.M., Darrow L., Lopez-Espinosa M.-J., Ryan P.B., Savitz D.A. Review: Evolution of evidence on PFOA and health following the assessments of the C8 Science Panel. Environ. Int. 2020;145:106125. doi: 10.1016/j.envint.2020.106125. [DOI] [PubMed] [Google Scholar]
- 404.Sun D., Zhao T., Long K., Wu M., Zhang Z. Triclosan down-regulates fatty acid synthase through microRNAs in HepG2 cells. Eur. J. Pharmacol. 2021;907:174261. doi: 10.1016/j.ejphar.2021.174261. [DOI] [PubMed] [Google Scholar]
- 405.Sun L., Xu A., Li M., Xia X., Li P., Han R., Fei G., Zhou S., Wang R. Effect of Methylation Status of lncRNA-MALAT1 and MicroRNA-146a on Pulmonary Function and Expression Level of COX2 in Patients With Chronic Obstructive Pulmonary Disease. Front. Cell Dev. Biol. 2021;9:667624. doi: 10.3389/fcell.2021.667624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Tang F., Wang H., Chen E., Bian E., Xu Y., Ji X., Yang Z., Hua X., Zhang Y., Zhao B. LncRNA-ATB promotes TGF-β-induced glioma cells invasion through NF-κB and P38/MAPK pathway. J. Cell. Physiol. 2019;234:23302–23314. doi: 10.1002/jcp.28898. [DOI] [PubMed] [Google Scholar]
- 407.Tani H., Numajiri A., Aoki M., Umemura T., Nakazato T. Short-lived long noncoding RNAs as surrogate indicators for chemical stress in HepG2 cells and their degradation by nuclear RNases. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-56869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.University of Tennessee Toxicity Profiles. Formal Toxicity Summary for Cadmium. [(accessed on 10 February 2022)];2020 Available online: https://rais.ornl.gov/tox/profiles/cadmium.html.
- 409.University of Tennessee Toxicity Profiles. Formal Toxicity Summary for Chromium. [(accessed on 10 February 2022)];2020 Available online: https://rais.ornl.gov/tox/profiles/chromium.html.
- 410.United States Environmental Protection Agency (EPA) Polychlorinated Biphenyls (PCBs) [(accessed on 10 February 2022)];2021 Available online: https://www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls-pcbs.
- 411.United States Environmental Protection Agency (EPA) PFOA, PFOS and Other PFAS. [(accessed on 10 February 2022)];2021 Available online: https://www.epa.gov/pfas/pfas-explained.
- 412.US Department of Health and Human Services Toxicological Profile for Glyphosate. Agency for toxic Subtances and Disease Registry. [(accessed on 10 February 2022)];2020 Available online: https://www.atsdr.cdc.gov/toxprofiles/tp214.pdf. [PubMed]
- 413.Wahlang B., Petriello M.C., Perkins J.T., Shen S., Hennig B. Polychlorinated biphenyl exposure alters the expression profile of microRNAs associated with vascular diseases. Toxicol. Vitr. 2016;35:180–187. doi: 10.1016/j.tiv.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wang W., Shim Y.K., Michalek J.E., Barber E., Saleh L.M., Choi B.Y., Wang C.-P., Ketchum N., Costello R., Marti G.E., et al. Serum microRNA profiles among dioxin exposed veterans with monoclonal gammopathy of undetermined significance. J. Toxicol. Environ. Health Part A. 2020;83:269–278. doi: 10.1080/15287394.2020.1749919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wani A.L., Ara A., Usmani J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Weatherly L.M., Gosse J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health. Part B Crit. Rev. 2017;20:447–469. doi: 10.1080/10937404.2017.1399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Woeller C.F., Thatcher T.H., Thakar J., Cornwell A., Smith M.R., Jones D.P., Hopke P.K., Sime P.J., Krahl P., Mallon T.M., et al. Exposure to Heptachlorodibenzo-p-dioxin (HpCDD) Regulates microRNA Expression in Human Lung Fibroblasts. J. Occup. Environ. Med. 2019;61:S82–S89. doi: 10.1097/JOM.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.World Health Organization Regional Office for Europe, Copenhagen, Denmark. Chapter 6.4 Chromium. 2000. [(accessed on 10 February 2022)]. Available online: https://www.euro.who.int/__data/assets/pdf_file/0017/123074/AQG2ndEd_6_4Chromium.PDF.
- 419.World Health Organization Preventing Disease through Healthy Environments Exposure to Dioxins and Dioxin-like Substances: A Major Public Health Concern. 2010. [(accessed on 10 February 2022)]. Available online: https://www.who.int/ipcs/features/dioxins.pdf.
- 420.World Health Organization Preventing Disease through Healthy Environments. Exposure to Cadmium: A Major Public Health Concern. 2019. [(accessed on 10 February 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/329480/WHO-CED-PHE-EPE-19.4.3-eng.pdf?ua=1.
- 421.World Health Organization IARC Monograph on Glyphosate. 2022. [(accessed on 10 February 2022)]. Available online: https://www.iarc.who.int/featured-news/media-centre-iarc-news-glyphosate/
- 422.World Health Organization, International Agency for Research on Cancer Triclosan (TCS) (Compound) 2022. [(accessed on 10 February 2022)]. Available online: http://exposome-explorer.iarc.fr/compounds/1420.
- 423.World Health Organization Regional Office for Europe, Copenhagen, Denmark. Chapter 5.10 Polychlorinated bi-phenyls (PCBs) 2000. [(accessed on 10 February 2022)]. Available online: https://www.euro.who.int/__data/assets/pdf_file/0016/123064/AQG2ndEd_5_10PCBs.PDF.
- 424.World Health Organization Regional Office for Europe, Copenhagen, Denmark. Chapter 8.1 Environmental Tobacco Smoke. 2000. [(accessed on 10 February 2022)]. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/123087/AQG2ndEd_8_1ETS.PDF.
- 425.Xu M., Yu Z., Hu F., Zhang H., Zhong L., Han L., An Y., Zhu B., Zhang H. Identification of differential plasma miRNA profiles in Chinese workers with occupational lead exposure. Biosci. Rep. 2017;37:BSR20171111. doi: 10.1042/BSR20171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Xu Y., Jurkovic-Mlakar S., Li Y., Wahlberg K., Scott K., Pineda D., Lindh C.H., Jakobsson K., Engström K. Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water. Environ. Int. 2020;136:105446. doi: 10.1016/j.envint.2019.105446. [DOI] [PubMed] [Google Scholar]
- 427.Yan W., Yue H., Ji X., Li G., Sang N. Prenatal NO2 exposure and neurodevelopmental disorders in offspring mice: Transcriptomics reveals sex-dependent changes in cerebral gene expression. Environ. Int. 2020;138:105659. doi: 10.1016/j.envint.2020.105659. [DOI] [PubMed] [Google Scholar]
- 428.Zefferino R., Piccoli C., Ricciardi N., Scrima R., Capitanio N. Possible Mechanisms of Mercury Toxicity and Cancer Promotion: Involvement of Gap Junction Intercellular Communications and Inflammatory Cytokines. Oxidative Med. Cell. Longev. 2017;2017:1–6. doi: 10.1155/2017/7028583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Zhang B.-Z., Hu G.-L., Lu L.-Y., Hu S.-F., Li Y.-S., Su X., Dong W.-Y., Zhen C.-A., Liu R.-Q., Kong F.-B., et al. Identification of differentially expressed microRNAs under imidacloprid exposure in Sitobion miscanthi. Pestic. Biochem. Physiol. 2021;177:104885. doi: 10.1016/j.pestbp.2021.104885. [DOI] [PubMed] [Google Scholar]
- 430.Zhou Y., He L., Liu X.-D., Guan H., Li Y., Huang R.-X., Zhou P.-K. Integrated Analysis of lncRNA and mRNA Transcriptomes Reveals New Regulators of Ubiquitination and the Immune Response in Silica-Induced Pulmonary Fibrosis. BioMed Res. Int. 2019;2019:1–11. doi: 10.1155/2019/6305065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Zota A.R., Geller R.J., Vannoy B.N., Marfori C.Q., Tabbara S., Hu L.Y., A Baccarelli A., Moawad G.N. Phthalate Exposures and MicroRNA Expression in Uterine Fibroids: The FORGE Study. Epigenetics Insights. 2020;13:2516865720904057. doi: 10.1177/2516865720904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.