Abstract
β-cells are insulin-producing cells in the pancreas that maintain euglycemic conditions. Pancreatic β-cell maturity and function are regulated by a variety of transcription factors that enable the adequate expression of the cellular machinery involved in nutrient sensing and commensurate insulin secretion. One of the key factors in this regulation is MAF bZIP transcription factor A (MafA). MafA expression is decreased in type 2 diabetes, contributing to β-cell dysfunction and disease progression. The molecular biology underlying MafA is complex, with numerous transcriptional and post-translational regulatory nodes. Understanding these complexities may uncover potential therapeutic targets to ameliorate β-cell dysfunction. This article will summarize the role of MafA in normal β-cell function and disease, with a special focus on known transcriptional and post-translational regulators of MafA expression
Keywords: MafA, pancreatic beta cells, beta cell maturity, transcription factors, diabetes
1. Introduction
Pancreatic β-cells are critical in maintaining euglycemia by responding to blood glucose oscillations. β-cell dysfunction is a feature of diabetes mellitus which is characterized by abnormally high blood glucose levels. β-cells are responsible for the synthesis, storage and secretion of insulin, a hormone that is paramount for metabolic homeostasis. One hundred years after the successful isolation of insulin by Banting and collaborators, studies on insulin and β-cells have determined many of the factors needed for normal β-cell function, which is best defined by the appropriate expression of canonical β-cell factors involved in glucose-stimulated insulin secretion. One of these factors is the MAF bZIP transcription factor A (MafA). MafA, a large basic leucine zipper (bZIP) transcription factor (TF), derives its name from the viral oncogene v-maf, which was isolated from musculoaponeurotic fibrosarcoma in chicken [1]. The seven members of the MAF family were subsequently identified by homology of their bZIP domain with v-maf and are divided into two subgroups according to molecular size: large MAFs (Maf, MafA, MafB and Nlr) and small MAFs (MafF, MafG and MafK), which lack a transactivation domain. These factors have roles in development and terminal differentiation across multiple tissues [2,3,4], but have also been identified in disease pathogenesis [5]. For instance, MafA was first identified as the trans-acting factor binding the RIPE3b/C1 element of the insulin promoter [6,7,8] and has since been characterized as an important TF for β-cell function that is dysregulated in diabetes progression [9,10]. These studies and others emphasized the critical role of MafA for β-cell function and suggested that elucidating mechanisms of MafA regulation would deepen our understanding of β-cell pathophysiology and potentially uncover therapeutic targets for the amelioration of β-cell dysfunction and diabetes progression.
Here, we review the transcriptional and post-transcriptional regulation of MafA, with a focus on post-translational modifications (PTMs) and their effects on MafA stability and transcriptional activity. In addition, this review will discuss β-cell transcriptional targets of MafA. Other MAF factors and the role of MafA in non-β-cells are reviewed elsewhere [5]. Because of the major role MafA plays in β-cells, our goal is to summarize mouse and human studies and to provide a cohesive picture of how MafA is regulated in β-cells.
2. MafA Regulates β-Cell Function
The importance of MafA for glucose homeostasis was shown in MafA-deficient mice, which displayed glucose intolerance after weaning due to impaired glucose-stimulated insulin secretion (GSIS) and age-dependent diabetes progression [11,12]. MafA-deficient mice had decreased mRNA levels of Ins1, Ins2, Pdx1, Neurod1 and Slc2a2, suggesting that MafA is essential for the transcriptional identity of β-cells. MafA works synergistically with Neurod1 and Pdx1 to transactivate the insulin promoter, which is an effect that could not be fully replicated with MafB nor Maf [13]. Along these lines, MafA promotes insulin transcription when overexpressed in rat islets [14] and when ectopically expressed in chick embryonic endoderm [15] or in other non-β-cell lines of endodermal origin [16,17].
Several studies suggest that MafA is dispensable for β-cell development but plays an essential role in β-cell maturation and glucose responsiveness of adult β-cells. In rodents, Mafa expression is low at birth, increasing during the postnatal period [18,19]. Interestingly, premature expression of MafA in pancreatic endocrine progenitors prevents differentiation into hormone-expressing cells [20,21], suggesting that the induction of MafA expression in β-cells is time sensitive. Consistently, in mice with whole pancreas MafA knockout (Mafa∆panc), the effects of MafA loss are not apparent until three weeks of age, when the KO mice showed lower β-cell mass, decreased insulin expression, and impaired glucose tolerance compared to controls [22]. Similarly, at twelve weeks of age in whole-body MafA knockout mice, there is a reduced β-cell:α-cell ratio, decreased islet insulin content and β-cell dedifferentiation into MafB-expressing, progenitor-like cells [12]. These results may recapitulate some aspects of normal islet development. MafB is actually the predominant large MAF protein expressed during pancreatic development in both α- and β-cells [4]. MafB can upregulate Ins1 and Ins2 transcription [8], but in adult mice, MafB is mainly expressed in α-cells and upregulates Gcg gene expression [4,23]. The decrease in β-cell MafB expression after birth is at least partly due to methylation, specifically by DNA methyltransferase 3a (Dnmt3a) which binds and represses at a region −1032 to −838 upstream of the Mafb transcriptional start site [24]. Mafb∆panc mice have higher blood glucose levels at P1, but two weeks after birth, blood glucose levels normalize. Likewise, at E15.5, Mafb∆panc mice have a lower number of insulin+ and glucagon+ cells compared to controls, but two weeks after birth, cell numbers became roughly the same [25]. MafB does seem to have a role in enhancing postnatal β-cell function under metabolic stress (pregnancy, high-fat diet) [24,26], but MafA remains critical for proper β-cell function and glucose homeostasis in mature β-cells, with forced MafB expression unable to compensate for MafA loss in adult mice [24].
The MAFA expression pattern in humans is similar to that observed in rodents. In human β-cells, MAFA mRNA expression increases postnatally in an age-dependent manner [27]. In contrast, MAFB expression in humans differs from that observed in mice. In juvenile islets (<9 years old), MAFB is expressed in a larger portion of β-cells compared to MAFA [24], and MAFB expression in human β-cells peaks at this stage, but it still can be detected though life [25]. However, similar to mice, human MAFB is required for the derivation of insulin producing β-like cells from pluripotent stem cells, suggesting a conserved role in endocrine differentiation in humans [28].
Reduced MafA expression is associated with diabetes progression in mice and human patients. In islets isolated from diabetic db/db mice, MafA is decreased due to hyperglycemia-associated oxidative stress and c-Jun activity [9]. Similarly, islets from subjects with type 2 diabetes (T2D) display a marked decrease in MAFA mRNA levels and protein expression [10,29,30]. Several studies have shown possible mechanisms for this phenomenon. In β-cells from subjects with T2D [31] and in in vitro studies mimicking oxidative stress [10], MafA was found primarily in the cytoplasm rather than properly localized in the nucleus. However, when MafA levels were “rescued” in db/db mice, GSIS and β-cell mass improved, suggesting that preserving MafA expression in β-cells can still mitigate diabetes progression [32]. scRNA-seq analysis of human β-cells supports this concept as well, wherein metabolically inflexible β-cells show lower MAFA activity as compared to healthy β-cells [33]. This finding is thought to align with previous patterns of T2D-induced oxidative stress, in which case MafA activity is high under healthy islet conditions but can also increase as a protective mechanism against acute oxidative stress [34]. In a different scRNA-seq analysis of human islets, β-cells co-expressing MAFA/MAFB had increased expression of genes related to β-cell identity, glucose metabolism and exocytosis compared to β-cells that only expressed one or neither TF [35]. Since MAFA and MAFB are capable of forming heterodimers [8,36], it is possible that the co-expression of both can enhance the β-cell maturity transcriptional program. These and other studies have documented the functional and transcriptional heterogeneity of β-cells [33,35,37]. In this regard, intra-islet MAFA expression is highly variable, which has been reported to contribute to optimal β-cell function, since homogeneous overexpression of MAFA and PDX1 through the islet led to defects in Ca2+ fluxes and impaired insulin secretion [38].
3. MafA Target Genes
MafA transcriptional activity is necessary for proper postnatal β-cell function. MafA was first identified as a TF of insulin that binds to the C1 element of the insulin gene promoter. MAF proteins, including MafA, bind to the MAF-recognition elements (MAREs), through a DNA consensus sequence TGCTGAC(G)TCAGCA [39]. However, in β-cells, the MARE sequence within the C1 element deviates slightly from the consensus sequence. The MARE sequence in C1 element of the rat and mouse Ins2 promoter is TGCAGCTTCAGCC whereas the equivalent in the human INS promoter is TGCAGCCTCAGCC (the underlined nucleotides indicate deviations from the MARE consensus sequence) [7,16,40]. Interestingly, in addition to the C1 element, three other MARE sites (MARE1, MARE2 and MARE3) were also identified in the rat Ins2 and human INS promoter. MafA can bind to MARE2 and MARE3 in the rat Ins2 promoter while MafA can bind to MARE1 in the human INS promoter [16].
Although MafA can activate the insulin promoter alone, the addition of Neurod1 and Pdx1, which bind the E box and A box of the insulin promoter, respectively, synergistically promote insulin transcription [13]. Consistently, mice with homozygous CRISPR-Cas9-introduced mutations in the C1 box of the Ins1 and Ins2 gene promoters are glucose intolerant. These C1 box mutations also prevented MafA from activating the promoter in luciferase assays, further supporting the importance of MafA in upregulating the promoter of its target genes [41]. Human mutations characterized by a deletion of the C1 and E1 elements of the INS promoter have been shown to cause permanent neonatal diabetes, which may be attributed to the disruption of MAFA and NEUROD1 binding sites [42,43]. Other factors are also involved in this regulation. For example, Gli-similar 3 (Glis3), a Krüppel-like zinc finger transcription factor, activates insulin transcription by recruiting histone acetyltransferase Creb-binding protein (CBP) and by acting as a scaffold for the MafA, NeuroD1 and Pdx1 complex [44].
Beyond insulin, MafA increases the expression of a wide range of genes involved in β-cell function, including genes implicated in glucose sensing, insulin processing, Ca2+ influx and oxidative phosphorylation. Many studies have identified a plethora of MafA target genes, all having important roles in proper β-cell function, further supporting MafA’s critical role in β-cells. While some studies have found direct MafA target genes (i.e., MafA directly binds to the promoter of the target genes), other studies describe genes that are differentially expressed when MafA protein is overexpressed or silenced, without showing direct MafA binding to the target gene promoters (i.e., MafA knockout or overexpression causes a decrease or increase in the target gene expression, respectively). Some of these MafA-target genes are summarized in Table 1. The lack of promoter binding evidence does not necessarily mean MafA cannot bind to the promoter of these genes, but rather that MafA may regulate these genes through intermediaries rather than direct promoter binding.
Table 1.
MafA target genes. (*) denotes evidence that MafA directly binds target gene promoter.
| Gene Symbol | Gene Name | Gene Function | Reference |
|---|---|---|---|
| Cacng4 * | Voltage-dependent calcium channel gamma-4 subunit | Enhances L-type Ca2+-mediated Ca2+ entry into β-cell | [45] |
| Chrnb4 * | Cholinergic receptor nicotinic beta 4 | Subunit of the nicotinic acetylcholine receptor | [46] |
| Cox6a2 * | Cytochrome C oxidase subunit 6A2 | One out of 13 subunits of cytochrome C oxidase complex (Complex IV), the last enzyme in the electron transport chain | [47] |
| G6pc2 * | Glucose-6-phosphate catalytic subunit related protein | Islet-specific enzyme that hydrolyzes glucose-6-phosphate, limits basal insulin secretion | [48] |
| Gck | Glucokinase | Phosphorylates glucose to glucose-6-phosphate in pancreatic islets and hepatocytes. Considered the β-cell glucose sensor | [49] |
| Glp1r | Glucagon-like peptide 1 receptor | Receptor for Glucagon-like peptide 1 (Glp1), a stimulator of insulin secretion | [49] |
| Ins1 * | Insulin I | One of two insulin genes in mouse, on chromosome 19 | [11] |
| Ins2 * | Insulin II | One of two insulin genes in mouse, on chromosome 7 | [11] |
| Maob * | Monoamine oxidase B | Metabolizes monoamine neurotransmitters, specifically benzylamine, dopamine and phenylethylamine | [50] |
| Neurod1 | Neurogenic differentiation 1 | Transactivator of genes important for β-cell maturation and function, including insulin | [49] |
| Nkx6.1 | NK6 homeobox1 | TF involved in β-cell development and regulation of genes involved in mature β-cell function | [49,51] |
| Pcsk1 | Proprotein convertase subtilisin/kexin type 1 | Proprotein convertase, which processes proinsulin in β-cells | [49] |
| Pcx | Pyruvate carboxylase | Catalyzes the conversion of pyruvate to oxaloacetate | [49] |
| Pdx1 * | Pancreatic and duodenal homeobox 1 | TF important pancreas development and for mature β-cell function | [49,52] |
| PPP1R1A | Protein phosphatase 1, regulatory inhibitor subunit 1A | Regulates cAMP/PKA signaling pathway Promotes Glp1-induced GSIS |
[53] |
| Prlr | Prolactin Receptor | Involved in increasing β-cell mass during pregnancy | [54] |
| Slc2a2 * | Solute Carrier Family 2 Member 2 | Glucose transporter 2, transmembrane glucose transporter with a high Km for glucose | [49,55] |
| Slc80a8 | Solute carrier family 30 member 8 | Zinc transporter on insulin granules in β-cells | [19,46] |
4. Regulation of MafA Transcription
The first level of MafA regulation is through Mafa gene expression. The Mafa promoter consists of six regions (R1-R6), which contain highly conserved sequences 25 kb upstream the 5′ MafA untranslated region (UTR). R1 spans from −9389 to −9194, R2 spans from −8420 to −8293, R3 spans from −8118 to −7750, R4 spans from −6622 to −6441, R5 spans from −6217 to −6031 and R6 spans from −250 to +56. Of these, R3 has been shown to be most necessary, but not sufficient, for transcriptional initiation mediated by direct TF binding [56,57]. Many transcription factors bind R3 on the Mafa promoter, including Pax6, Nkx6.1, Nkx2.2, Pdx1, Hnf1a, Foxa2 and Isl1. Changes in these transcription factors directly correlate with Mafa expression. However, there are some exceptions. Neurod1, another important insulin transcription factor, can bind to R3 of the Mafa promoter, but Neurod1−/− mice do not have detectable changes in MafA staining [58]. However, in a more recent study, Neurod1∆endo mice had lower Mafa mRNA levels, suggesting that Neurod1 may regulate Mafa transcription [59]. Additionally, Pax4 is a negative regulator of Mafa. Pax4 overexpression limits R3-mediated promoter activity, but there is limited evidence of direct Pax4 binding to R3 via ChIP, possibly due to Pax4 regulating Mafa in an indirect manner [60].
Other TFs bind elsewhere in the MafA promoter or regulate Mafa transcription through other mechanisms, as summarized in Table 2. For instance, Onecut1, a TF involved in pancreatic endocrine development that is upregulated in adult db/db mice, acts as a negative regulator of Mafa transcription by preventing Foxa2 from interacting with the Mafa promoter, highlighting the importance of the transcriptional regulation of Mafa in metabolic disease states [61].
Table 2.
Known transcriptional regulators of Mafa gene expression.
| Protein Symbol | Protein Name | Mafa Transcription Mechanism | Reference |
|---|---|---|---|
| Pax6 | Paired box protein Pax-6 | Binds R1, R3 and R6 of the Mafa promoter | [58] |
| Nkx6.1 | NK6 homeobox 1 | Binds R3 of the Mafa promoter | [58] |
| Nkx2.2 | NK2 homeobox 2 | Binds R3 of the Mafa promoter | [57] |
| Pdx1 | Pancreatic and duodenal homeobox 1 | Binds R3 and R6 of the Mafa promoter | [57,58] |
| Hnf1a | Hepatocyte nuclear factor 1-alpha | Binds R3 of the Mafa promoter | [56] |
| Foxa2 | Forkhead box A2 | Binds R3 of the Mafa promoter | [57] |
| Isl1 | Insulin gene enhancer protein ISL-1 | Binds R3 of the Mafa promoter | [62] |
| Neurod1 | Neurogenic differentiation 1 | Binds R3 of the Mafa promoter | [57] |
| Pax4 | Paired box protein Pax-4 | Negative regulator of Mafa, potentially by interfering other factors from binding R3 of the Mafa promoter | [60] |
| Mafb | Transcription factor MafB | Binds R3 of the Mafa promoter | [58,63] |
| Onecut1 | One cut domain, family member 1 | Prevents Foxa2 from binding to the Mafa promoter | [61] |
| Foxo1 | Forkhead box O1 | Binds to the forkhead element of the Mafa promoter | [34] |
| Thra | Thyroid hormone receptor alpha | Binds to Thyroid hormone response element (TRE), which are located from −1927 to −1946 and from +647 to +659 (named Site 2 and Site 3, respectively) | [64] |
| CREB | cAMP responsive element binding protein | Constitutively binds to the cAMP response element (CRE), spanning from −1342 to −1346, of the Mafa promoter | [65] |
Foxo1, a TF involved in a number of metabolic pathways, is inactive in resting β-cells, but after acute high glucose or H2O2 exposure, translocates to the nucleus where it enhances Mafa expression by binding the forkhead element in the Mafa promoter [34,66]. Overexpression of Foxo1 in mouse β-cells increased Mafa mRNA and decreased age-dependent β-cell mass decline [67]. Conversely, the loss of Foxo1 is associated with reduced expression of MafA and other important β-cell TFs, eventually leading to a loss of cell identity in conditions of stress [68]. Other mechanisms are also at play. For example, active thyroid hormone (T3), bound to the thyroid hormone receptor, directly transactivates the Mafa promoter [64]. Similarly, transcriptional elements downstream of GLP1R, such as CREB-transcription factors and CREB-regulated transcription coactivator 2 (Crtc2), also regulate Mafa transcription. CREB constitutively binds the cAMP response element of the Mafa promoter, while Crtc2 binds the Mafa promoter following forskolin treatment, enhancing Mafa transcription. This MafA expression-potentiating mechanism is impaired by chronic high glucose levels [65].
The transcription of Mafa in β-cells is also sensitive to acute changes in glucose levels. In low glucose conditions, Mafa mRNA levels are low, but after acute high glucose exposure, Mafa transcription increases. This glucose-dependent transcriptional regulation is mediated by the hexosamine biosynthesis pathway and is thought to be independent of glycolysis [69]. However, under metabolically stressful conditions (i.e., hyperglycemia), Mafa transcription paradoxically decreases [10,70]. Oxidative stress causes lower binding of certain TFs (Pdx1 and Nkx6.1) to the MafA promoter, which at least partly contributes to lower Mafa mRNA [10]. Palmitate, a fatty acid that inhibits insulin gene expression, also decreased glucose-stimulated Mafa transcription, possibly by decreasing Pdx1 nuclear localization and binding to the Mafa promoter [71]. Additionally, palmitate may increase c-Jun N-terminal kinase (JNK) activity, which previously was found to correlate negatively with Mafa transcription under low glucose conditions, thereby causing a decrease in Mafa transcription [72,73]. Likewise, aldosterone, a key player in the renin–angiotensin–aldosterone system (RAAS), can mediate β-cell dysfunction by enhancing JNK activity, causing a decrease in Mafa at the transcriptional level [74].
Mafa gene expression is also affected by non-coding RNAs (ncRNA). A number of studies have identified microRNAs (miRNA)—short ncRNAs that base-pair with their target mRNA at the 3′-untranslated region (UTR) to promote mRNA instability and repression in protein translation [75]—that regulate Mafa gene expression [76,77,78,79]. One study found that thioredoxin-interacting protein (Txnip) decreases insulin gene expression by upregulating miR-204, which targets the 3′-UTR of Mafa mRNA, resulting in mRNA degradation and decreased protein translation [76]. However, this effect could not be replicated in human islets [80], likely due to a mismatch between miR-204 and its target 3′-UTR of human MAFA that is not present in the 3′-UTR of rat Mafa [80]. Other miRNAs that affect Mafa gene expression include miR-149, which negatively regulates Mafa after arsenite exposure [77]; miR-30d, which positively regulates Mafa by inhibiting Mitogen-activated protein 4 kinase 4 (Map4k4) [78]; and miR-24, which negatively regulates Mafa [79]. Most of these miRNAs have been studied in mouse islets or cell lines; despite sequence homology [78], effects in human β-cells still require experimental validation.
Mafa is also affected by long non-coding RNAs (lncRNA). LncRNAs are transcripts of >200 bp that that lack protein-coding potential, but have biological roles through chromatin remodeling, imprinting induction, regulation of splicing and mRNA translation [81]. For example, the lncRNA Meg3 binds Ezh2, a methyltransferase, which causes suppression of gene expression of three repressive transcriptional regulators (Rad21, Smc3, and Sin3a) via promoter methylation, culminating in an increase in Mafa expression [82]. In humans, the MEG3 promoter is hypermethylated in islets from human subjects with T2D, suggesting that MEG3 expression is downregulated in T2D, which may contribute to decreased MAFA expression [83,84].
5. MafA Post-Translational Modifications
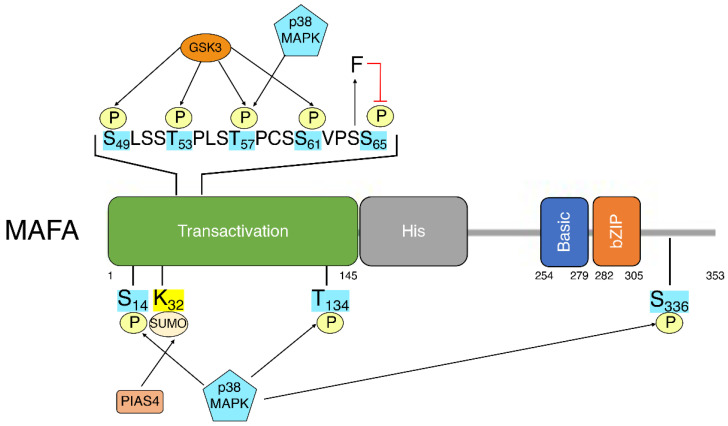
The previously discussed studies indicate the importance and potential mechanisms of Mafa transcriptional regulation. Post-translational modifications (PTMs) add a second layer of regulation, allowing for dynamic changes in MafA activity or stability in response to changes in β-cell environment. While most documented PTMs are found in the N-terminal transactivation domain of MafA [85], other regions of MafA, such as the extended homology region, the basic domain and the leucine-zipper, affect DNA recognition and binding and are also likely affected by the addition of PTMs [5] (Figure 1).
Figure 1.
Linear schematic of MAFA depicting MAFA domains and known post-translationally modified residues. Transactivation: transactivation domain. His: histamine-rich region. Basic: basic domain. bZIP: Leucine zipper.
5.1. Phosphorylation
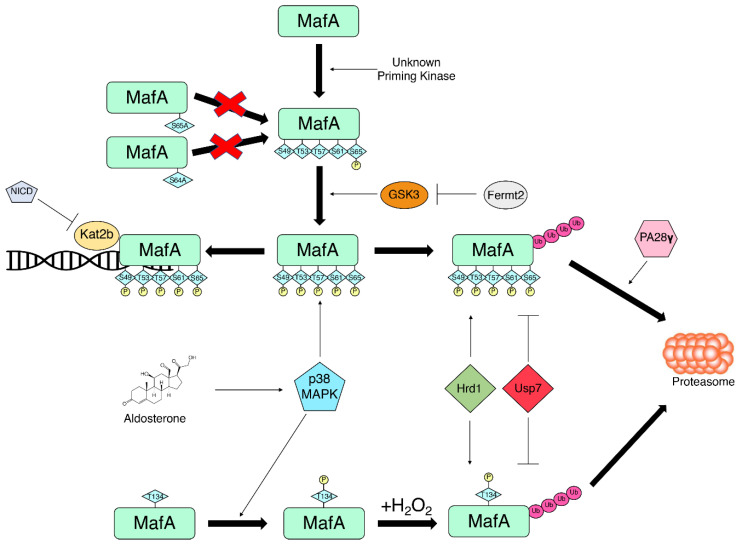
The most studied PTM of MafA is phosphorylation. In fact, even before MafA was identified as the TF binding to the C1 box of the insulin promoter, phosphorylation was deemed necessary for DNA binding [86]. MafA is highly phosphorylated under basal conditions, which is reduced by H2O2-induced oxidative stress and likely other stress. Phosphorylation of MafA increases MafA transcriptional activity and enhances its interactions with binding partners such as Neurod1 [87], which allows for MafA dimerization [85], and increases DNA binding [88]. A number of MafA phosphorylation sites have been identified through mass spectrometry [85], and subsequently characterized [89]. Of these, phosphorylation of Ser65, discussed more extensively later in this review, has been the most studied as it increased MafA transactivation but paradoxically decreased protein stability [90]. However, numerous other Ser/Thr residues are also phosphorylated, including Thr134 by p38MAPK [91], which is increased with oxidative stress and leads to MafA degradation [92,93]. Interestingly, in addition to decreasing Mafa mRNA levels, aldosterone can also mediate β-cell dysfunction by enhancing p38MAPK-induced MafA phosphorylation, in turn causing MafA degradation [74].
The most studied phosphorylated residues are those in the MafA transactivation domain associated with glycogen synthase kinase 3 (GSK3) [94,95]. Most GSK3 target proteins contain a “priming” residue that must be phosphorylated by another kinase before GSK3 can phosphorylate its target residues. GSK3 often phosphorylates its targets in a sequential manner, on every fourth residue that is serine or threonine after the priming residue is phosphorylated [96]. The mechanism for GSK3-mediated MafA phosphorylation is no different. GSK3 cannot phosphorylate its target residues until Ser65 of MafA is phosphorylated by an unknown “priming” kinase. After Ser65 is phosphorylated, GSK3 sequentially phosphorylate MafA on Ser61, Thr57, Thr53 and Ser49. GSK3-mediated phosphorylation not only increases MafA activity but also increases MafA degradation. The S65A mutation, which prevents the unknown priming kinase from phosphorylating MafA, or mutating all the residues targeted by GSK3, results in a very stable MafA protein as compared to WT MafA [95]. Consistent with the importance of this regulation, a recent study showed that MafA stability is also affected by the GSK3-regulator Fermt2 (also known as Kindlin-2), an integrin activator [97]. Fermt2 increases Gsk3b Ser9 phosphorylation, which decreases Gsk3b activity. Thus, β-cell-specific Fermt2-knockout mice show reduced islet MafA and impaired GSIS, leading to glucose intolerance. The study did not investigate whether Fermt2 decreased Gsk3b-mediated MafA phosphorylation, but the results suggest that MafA regulation by Fermt2 is important in the intricate regulation underlying MafA stability and activity.
Interestingly, another study identified MAFA S64F missense mutations in two families, which led to hypophosphorylation of MafA [98]. Phenotypically, males with this mutation were more likely to develop T2D, while the females were more likely to develop hyperinsulinemia-induced hypoglycemia. These phenotypes were replicated in mice as well [99]. Molecularly, S64F mutation increased MafA stability under both low and high glucose conditions and decreased phosphorylation, suggesting the hypothesis that S64F may prevent the priming kinase from phosphorylating Ser65, possibly through steric inhibition. More studies would be needed to further support the hypothesis, but this work highlights the importance of MafA phosphorylation in maintaining proper β-cell function in humans.
5.2. SUMOylation
Small ubiquitin-like modifier (SUMO) has been shown on MafA lysine residues. Despite its name, SUMOylation does not target proteins for proteasomal degradation but rather affects protein function, stability and transport. SUMOylation occurs on lysine residues at a consensus Ψ-K-X-E motif, where Ψ represents a hydrophobic amino acid. SUMOylation of Lys32 occurs at this motif (VK32KE) [100,101], and similarly in MafB and Maf at Lys32 and Lys33, respectively [100]. In immortalized β-cell lines (MIN6 and INS-1), low-glucose conditions and oxidative stress caused increased endogenous MafA SUMOylation, suggesting a physiological role of this PTM [101]. SUMOylation of MafA resulted in a decrease in MafA transcriptional activity. Conversely, preventing MafA SUMOylation with a K32R mutation resulted in increased transactivation of the insulin promoter [100,101]. Intriguingly, MafA SUMOylation did not significantly impact protein stability [100]. These results suggest that in β-cells, SUMOylation may act as a mechanism to decrease MafA-mediated transactivation, but not stability, in low-glucose and oxidative conditions. Consistently, the E3 SUMO protein ligase protein inhibitor of activated STAT4 (PIAS4) enhanced MafA SUMOylation and negatively regulated the insulin promoter, though PIAS4 interaction with the bZIP domain of MafA is sufficient for transcriptional repression, independently from SUMOylation [102].
5.3. Ubiquitination
Ubiquitin acts in pleiotropic ways, depending on the pattern of ubiquitination. Polyubiquitination usually marks a protein for proteasomal degradation, but monoubiquitination can regulate DNA repair and gene expression [103,104]. MafA has been shown to be polyubiquitinated in the lysine-rich C-terminal domain of MafA, causing MafA proteasomal degradation [90]. Currently, no ubiquitinated lysine on MafA has been identified thus far, and no study, to our knowledge, has investigated the role MafA monoubiquitination.
MafA ubiquitination is largely contingent on MafA phosphorylation. A number of studies have shown that blocking MafA phosphorylation, in particular at S65, significantly decreases ubiquitination and increases MafA half-life [90,94,95]. Commensurately, GSK3 inhibitors also increase MafA half-life [94,95]. Furthermore, proteasomal activation with PA28γ induces degradation of WT MafA but does not induce degradation of non-phosphorylatable MafA mutant nor dephosphorylated MafA due to GSK3 inhibition, further suggesting that phosphorylation is a necessary step to MafA proteasome-dependent degradation [105].
Although MafA ubiquitin status is dependent on its phosphorylation status, there are mechanisms that protect MafA phosphorylation from degradation. K(lysine) acetyltransferase 2B (Kat2b), also known as p300/CBP-associated factor (PCAF), is believed to enhance the transactivation potential of GSK3-phosphorylated MafA by protecting it from degradation [94]. Kat2b decreases MafA ubiquitination, thereby increasing the transcriptional activity of MafA through stabilization. In the context of metabolic disease, disruption of MafA–Kat2b interaction results in the loss of β-cell maturity. Our studies investigating Notch signaling in β-cells found that forced Notch signaling in β-cells caused MafA degradation, leading to impaired GSIS and thereby glucose intolerance. We found that active Notch expression reduces Kat2b–MafA complex and that Kat2b silencing alone is able to cause a loss of MafA and impaired GSIS [106]. These findings emphasize the importance of Kat2b in MafA stability through preventing ubiquitination.
However, MafA ubiquitination may be even more complex, as two novel regulator proteins for MafA have also been identified: ubiquitin-specific protease 7 (USP7) and HMG-CoA reductase degradation protein 1 (HRD1), also known as synoviolin. USP7 is a ubiquitin protease that cleaves ubiquitin off its substrate while HRD1 is an endoplasmic reticulum-resident E3 ubiquitin ligase [107,108]. Re-CLIP/MS analysis of endogenous MafA-binding partners in a β-cell line (βTC-3 cells) identified Usp7 [109]. Co-expression of MafA with USP7 decreased MafA ubiquitination and stabilized MafA [107]. By contrast, HRD1 increased the ubiquitin status of MafA while HRD1 knockdown decreased MafA ubiquitination and increased MafA stability [108]. The identification of USP7 and HRD1 as MafA ubiquitin protease and E3 ligase, respectively, provides more novel therapeutic targets that could potentially stabilize MafA to increase MafA target gene expression (summarized in Figure 2).
Figure 2.
Regulators of MafA phosphorylation and ubiquitination fine-tune the balance between MafA transcriptional activity and MafA degradation.
5.4. Other PTMs
MafA may be regulated by other PTMs. MafG can be acetylated at Lys53, Lys60, Lys71 and Lys76, which increases its transcriptional activity [110]. Indeed, as mentioned before, Kat2b, an acetyltransferase, regulates MafA, but specific deacetylases that regulate MafA have not yet been identified. In addition, MafA acetylation has not yet been described. Similarly, although UDP-N-acetylglucosaminyl transferase (OGT), which causes O-glycosylation on its targets, is involved in glucose-dependent Mafa gene expression [69], no evidence has yet been shown for O-glycosylation of MafA [95]. Studies investigating MafA PTMs deepened our understanding of MafA regulation. Future studies on novel MafA PTMs or novel findings on known MafA PTMs can uncover potential therapeutic targets to ameliorate β-cell dysfunction.
6. Conclusions
In the last two decades, many studies have investigated MafA in the context of β-cell function. Since its discovery as a regulator of insulin transcription, MafA has also been identified as a TF for numerous other genes involved in the acquisition of β-cell functional maturity status, including genes involved in glucose sensing and insulin secretion. Consistently, the loss of β-cell MafA in mice leads to glucose intolerance and diabetes progression. In humans, decreased β-cell MAFA correlates with T2D, further supporting the importance of MAFA in maintaining β-cell function. Many studies have identified regulators of Mafa transcription, but MafA also shows important post-translational regulation through phosphorylation, SUMOylation and ubiquitination. Further elucidation of the molecular underpinnings of MafA regulation may provide novel therapeutic targets for the treatment and prevention of diabetes.
Acknowledgments
We thank members of the Pajvani laboratory for insightful discussion.
Author Contributions
Conceptualization, J.L. and A.B.; writing—original draft preparation, J.L. and M.C.; writing—review and editing, J.L., M.C., U.B.P. and A.B.; supervision, U.B.P. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH T32DK00732 (to M.C.), R01DK132661 (to U.P.) and the “Programa de Atracción de Talento” (2020-T1/BMD-20162, Comunidad Autónoma de Madrid, Spain) to A.B.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nishizawa M., Kataoka K., Goto N., Fujiwara K.T., Kawai S. V-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc. Natl. Acad. Sci. USA. 1989;86:7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reza H.M., Yasuda K. Roles of Maf family proteins in lens development. Dev. Dyn. 2004;229:440–448. doi: 10.1002/dvdy.10467. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C., Guo Z.M. Multiple functions of Maf in the regulation of cellular development and differentiation. Diabetes Metab. Res. Rev. 2015;31:773–778. doi: 10.1002/dmrr.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artner I., Le Lay J., Hang Y., Elghazi L., Schisler J.C., Henderson E., Sosa-Pineda B., Stein R. MafB: An activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 5.Eychene A., Rocques N., Pouponnot C. A new MAFia in cancer. Nat. Rev. Cancer. 2008;8:683–693. doi: 10.1038/nrc2460. [DOI] [PubMed] [Google Scholar]
- 6.Olbrot M., Rud J., Moss L.G., Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc. Natl. Acad. Sci. USA. 2002;99:6737–6742. doi: 10.1073/pnas.102168499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka K., Han S.I., Shioda S., Hirai M., Nishizawa M., Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J. Biol. Chem. 2002;277:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka T.A., Zhao L., Artner I., Jarrett H.W., Friedman D., Means A., Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka T.A., Kaneto H., Miyatsuka T., Yamamoto T., Yamamoto K., Kato K., Shimomura I., Stein R., Matsuhisa M. Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes. 2010;59:1709–1720. doi: 10.2337/db08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo S., Dai C., Guo M., Taylor B., Harmon J.S., Sander M., Robertson R.P., Powers A.C., Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Moriguchi T., Kajihara M., Esaki R., Harada A., Shimohata H., Oishi H., Hamada M., Morito N., Hasegawa K., et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura W., Takahashi S., Yasuda K. MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia. 2015;58:566–574. doi: 10.1007/s00125-014-3464-9. [DOI] [PubMed] [Google Scholar]
- 13.Aramata S., Han S.I., Yasuda K., Kataoka K. Synergistic activation of the insulin gene promoter by the beta-cell enriched transcription factors MafA, Beta2, and Pdx1. Biochim. Biophys. Acta. 2005;1730:41–46. doi: 10.1016/j.bbaexp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L., Guo M., Matsuoka T.A., Hagman D.K., Parazzoli S.D., Poitout V., Stein R. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J. Biol. Chem. 2005;280:11887–11894. doi: 10.1074/jbc.M409475200. [DOI] [PubMed] [Google Scholar]
- 15.Artner I., Hang Y., Guo M., Gu G., Stein R. MafA is a dedicated activator of the insulin gene in vivo. J. Endocrinol. 2008;198:271–279. doi: 10.1677/JOE-08-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka T.A., Artner I., Henderson E., Means A., Sander M., Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc. Natl. Acad. Sci. USA. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuoka T.A., Kaneto H., Stein R., Miyatsuka T., Kawamori D., Henderson E., Kojima I., Matsuhisa M., Hori M., Yamasaki Y. MafA regulates expression of genes important to islet beta-cell function. Mol. Endocrinol. 2007;21:2764–2774. doi: 10.1210/me.2007-0028. [DOI] [PubMed] [Google Scholar]
- 18.Aguayo-Mazzucato C., Koh A., El Khattabi I., Li W.C., Toschi E., Jermendy A., Juhl K., Mao K., Weir G.C., Sharma A., et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia. 2011;54:583–593. doi: 10.1007/s00125-010-2026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artner I., Hang Y., Mazur M., Yamamoto T., Guo M., Lindner J., Magnuson M.A., Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He K.H., Juhl K., Karadimos M., El Khattabi I., Fitzpatrick C., Bonner-Weir S., Sharma A. Differentiation of pancreatic endocrine progenitors reversibly blocked by premature induction of MafA. Dev. Biol. 2014;385:2–12. doi: 10.1016/j.ydbio.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura W., Bonner-Weir S., Sharma A. Expression of MafA in pancreatic progenitors is detrimental for pancreatic development. Dev. Biol. 2009;333:108–120. doi: 10.1016/j.ydbio.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hang Y., Yamamoto T., Benninger R.K., Brissova M., Guo M., Bush W., Piston D.W., Powers A.C., Magnuson M., Thurmond D.C., et al. The MafA transcription factor becomes essential to islet beta-cells soon after birth. Diabetes. 2014;63:1994–2005. doi: 10.2337/db13-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh M.C., Jung Y., Ugboma C.M., Shimbo M., Kuno A., Basha W.A., Kudo T., Oishi H., Takahashi S. MafB Is Critical for Glucagon Production and Secretion in Mouse Pancreatic alpha Cells In Vivo. Mol. Cell. Biol. 2018;38:e00504-17. doi: 10.1128/MCB.00504-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cyphert H.A., Walker E.M., Hang Y., Dhawan S., Haliyur R., Bonatakis L., Avrahami D., Brissova M., Kaestner K.H., Bhushan A., et al. Examining How the MAFB Transcription Factor Affects Islet beta-Cell Function Postnatally. Diabetes. 2019;68:337–348. doi: 10.2337/db18-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad E., Dai C., Spaeth J., Guo M., Cyphert H.A., Scoville D., Carroll J., Yu W.M., Goodrich L.V., Harlan D.M., et al. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. Am. J. Physiol. Endocrinol. Metab. 2016;310:E91–E102. doi: 10.1152/ajpendo.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiafukaiti G., Maimaiti S., Ogata K., Kuno A., Kudo T., Shawki H.H., Oishi H., Takahashi S. MafB Is Important for Pancreatic beta-Cell Maintenance under a MafA-Deficient Condition. Mol. Cell. Biol. 2019;39:e00080-19. doi: 10.1128/MCB.00080-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arda H.E., Li L., Tsai J., Torre E.A., Rosli Y., Peiris H., Spitale R.C., Dai C., Gu X., Qu K., et al. Age-Dependent Pancreatic Gene Regulation Reveals Mechanisms Governing Human beta Cell Function. Cell Metab. 2016;23:909–920. doi: 10.1016/j.cmet.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell R., Carnese P.P., Hennings T.G., Walker E.M., Russ H.A., Liu J.S., Giacometti S., Stein R., Hebrok M. Loss of the transcription factor MAFB limits beta-cell derivation from human PSCs. Nat. Commun. 2020;11:2742. doi: 10.1038/s41467-020-16550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnavion R., Jaafar R., Kerr-Conte J., Assade F., van Stralen E., Leteurtre E., Pouponnot C., Gargani S., Pattou F., Bertolino P., et al. Both PAX4 and MAFA are expressed in a substantial proportion of normal human pancreatic alpha cells and deregulated in patients with type 2 diabetes. PLoS ONE. 2013;8:e72194. doi: 10.1371/journal.pone.0072194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler A.E., Robertson R.P., Hernandez R., Matveyenko A.V., Gurlo T., Butler P.C. Beta cell nuclear musculoaponeurotic fibrosarcoma oncogene family A (MafA) is deficient in type 2 diabetes. Diabetologia. 2012;55:2985–2988. doi: 10.1007/s00125-012-2666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinti F., Bouchi R., Kim-Muller J.Y., Ohmura Y., Sandoval P.R., Masini M., Marselli L., Suleiman M., Ratner L.E., Marchetti P., et al. Evidence of beta-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuoka T.A., Kaneto H., Kawashima S., Miyatsuka T., Tochino Y., Yoshikawa A., Imagawa A., Miyazaki J., Gannon M., Stein R., et al. Preserving Mafa expression in diabetic islet beta-cells improves glycemic control in vivo. J. Biol. Chem. 2015;290:7647–7657. doi: 10.1074/jbc.M114.595579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son J., Ding H., Farb T.B., Efanov A.M., Sun J., Gore J.L., Syed S.K., Lei Z., Wang Q., Accili D., et al. BACH2 inhibition reverses beta cell failure in type 2 diabetes models. J. Clin. Investig. 2021;131:e153876. doi: 10.1172/JCI153876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitamura Y.I., Kitamura T., Kruse J.P., Raum J.C., Stein R., Gu W., Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Shrestha S., Saunders D.C., Walker J.T., Camunas-Soler J., Dai X.Q., Haliyur R., Aramandla R., Poffenberger G., Prasad N., Bottino R., et al. Combinatorial transcription factor profiles predict mature and functional human islet alpha and beta cells. JCI Insight. 2021;6:e151621. doi: 10.1172/jci.insight.151621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benkhelifa S., Provot S., Lecoq O., Pouponnot C., Calothy G., Felder-Schmittbuhl M.P. mafA, a novel member of the maf proto-oncogene family, displays developmental regulation and mitogenic capacity in avian neuroretina cells. Oncogene. 1998;17:247–254. doi: 10.1038/sj.onc.1201898. [DOI] [PubMed] [Google Scholar]
- 37.Camunas-Soler J., Dai X.Q., Hang Y., Bautista A., Lyon J., Suzuki K., Kim S.K., Quake S.R., MacDonald P.E. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Cell Metab. 2020;31:1017–1031.e1014. doi: 10.1016/j.cmet.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasteska D., Fine N.H.F., Ashford F.B., Cuozzo F., Viloria K., Smith G., Dahir A., Dawson P.W.J., Lai Y.C., Bastidas-Ponce A., et al. PDX1(LOW) MAFA(LOW) beta-cells contribute to islet function and insulin release. Nat. Commun. 2021;12:674. doi: 10.1038/s41467-020-20632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu X., Guanga G.P., Wan C., Rose R.B. A novel DNA binding mechanism for maf basic region-leucine zipper factors inferred from a MafA-DNA complex structure and binding specificities. Biochemistry. 2012;51:9706–9717. doi: 10.1021/bi301248j. [DOI] [PubMed] [Google Scholar]
- 40.Kataoka K., Noda M., Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol. Cell. Biol. 1994;14:700–712. doi: 10.1128/mcb.14.1.700-712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguchi H., Miyagi-Shiohira C., Nakashima Y., Kinjo T., Saitoh I., Watanabe M. Mutations in the C1 element of the insulin promoter lead to diabetic phenotypes in homozygous mice. Commun. Biol. 2020;3:309. doi: 10.1038/s42003-020-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garin I., Edghill E.L., Akerman I., Rubio-Cabezas O., Rica I., Locke J.M., Maestro M.A., Alshaikh A., Bundak R., del Castillo G., et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc. Natl. Acad. Sci. USA. 2010;107:3105–3110. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akerman I., Maestro M.A., De Franco E., Grau V., Flanagan S., Garcia-Hurtado J., Mittler G., Ravassard P., Piemonti L., Ellard S., et al. Neonatal diabetes mutations disrupt a chromatin pioneering function that activates the human insulin gene. Cell Rep. 2021;35:108981. doi: 10.1016/j.celrep.2021.108981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ZeRuth G.T., Takeda Y., Jetten A.M. The Kruppel-like protein Gli-similar 3 (Glis3) functions as a key regulator of insulin transcription. Mol. Endocrinol. 2013;27:1692–1705. doi: 10.1210/me.2013-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luan C., Ye Y., Singh T., Barghouth M., Eliasson L., Artner I., Zhang E., Renstrom E. The calcium channel subunit gamma-4 is regulated by MafA and necessary for pancreatic beta-cell specification. Commun. Biol. 2019;2:106. doi: 10.1038/s42003-019-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganic E., Singh T., Luan C., Fadista J., Johansson J.K., Cyphert H.A., Bennet H., Storm P., Prost G., Ahlenius H., et al. MafA-Controlled Nicotinic Receptor Expression Is Essential for Insulin Secretion and Is Impaired in Patients with Type 2 Diabetes. Cell Rep. 2016;14:1991–2002. doi: 10.1016/j.celrep.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagai Y., Matsuoka T.A., Shimo N., Miyatsuka T., Miyazaki S., Tashiro F., Miyazaki J.I., Katakami N., Shimomura I. Glucotoxicity-induced suppression of Cox6a2 expression provokes beta-cell dysfunction via augmented ROS production. Biochem. Biophys. Res. Commun. 2021;556:134–141. doi: 10.1016/j.bbrc.2021.03.148. [DOI] [PubMed] [Google Scholar]
- 48.Martin C.C., Flemming B.P., Wang Y., Oeser J.K., O’Brien R.M. Foxa2 and MafA regulate islet-specific glucose-6-phosphatase catalytic subunit-related protein gene expression. J. Mol. Endocrinol. 2008;41:315–328. doi: 10.1677/JME-08-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Brun T., Kataoka K., Sharma A.J., Wollheim C.B. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia. 2007;50:348–358. doi: 10.1007/s00125-006-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganic E., Johansson J.K., Bennet H., Fex M., Artner I. Islet-specific monoamine oxidase A and B expression depends on MafA transcriptional activity and is compromised in type 2 diabetes. Biochem. Biophys. Res. Commun. 2015;468:629–635. doi: 10.1016/j.bbrc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Aigha I.I., Abdelalim E.M. NKX6.1 transcription factor: A crucial regulator of pancreatic beta cell development, identity, and proliferation. Stem Cell Res. Ther. 2020;11:459. doi: 10.1186/s13287-020-01977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanhoose A.M., Samaras S., Artner I., Henderson E., Hang Y., Stein R. MafA and MafB regulate Pdx1 transcription through the Area II control region in pancreatic beta cells. J. Biol. Chem. 2008;283:22612–22619. doi: 10.1074/jbc.M802902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cataldo L.R., Vishnu N., Singh T., Bertonnier-Brouty L., Bsharat S., Luan C., Renstrom E., Prasad R.B., Fex M., Mulder H., et al. The MafA-target gene PPP1R1A regulates GLP1R-mediated amplification of glucose-stimulated insulin secretion in beta-cells. Metabolism. 2021;118:154734. doi: 10.1016/j.metabol.2021.154734. [DOI] [PubMed] [Google Scholar]
- 54.Eto K., Nishimura W., Oishi H., Udagawa H., Kawaguchi M., Hiramoto M., Fujiwara T., Takahashi S., Yasuda K. MafA is required for postnatal proliferation of pancreatic beta-cells. PLoS ONE. 2014;9:e104184. doi: 10.1371/journal.pone.0104184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ono Y., Kataoka K. MafA, NeuroD1, and HNF1beta synergistically activate the Slc2a2 (Glut2) gene in beta-cells. J. Mol. Endocrinol. 2021;67:71–82. doi: 10.1530/JME-20-0339. [DOI] [PubMed] [Google Scholar]
- 56.Hunter C.S., Maestro M.A., Raum J.C., Guo M., Thompson F.H., 3rd, Ferrer J., Stein R. Hnf1alpha (MODY3) regulates beta-cell-enriched MafA transcription factor expression. Mol. Endocrinol. 2011;25:339–347. doi: 10.1210/me.2010-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raum J.C., Gerrish K., Artner I., Henderson E., Guo M., Sussel L., Schisler J.C., Newgard C.B., Stein R. FoxA2, Nkx2.2, and PDX-1 regulate islet beta-cell-specific mafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol. Cell. Biol. 2006;26:5735–5743. doi: 10.1128/MCB.00249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raum J.C., Hunter C.S., Artner I., Henderson E., Guo M., Elghazi L., Sosa-Pineda B., Ogihara T., Mirmira R.G., Sussel L., et al. Islet beta-cell-specific MafA transcription requires the 5′-flanking conserved region 3 control domain. Mol. Cell. Biol. 2010;30:4234–4244. doi: 10.1128/MCB.01396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romer A.I., Singer R.A., Sui L., Egli D., Sussel L. Murine Perinatal beta-Cell Proliferation and the Differentiation of Human Stem Cell-Derived Insulin-Expressing Cells Require NEUROD1. Diabetes. 2019;68:2259–2271. doi: 10.2337/db19-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu He K.H., Lorenzo P.I., Brun T., Jimenez Moreno C.M., Aeberhard D., Vallejo Ortega J., Cornu M., Thorel F., Gjinovci A., Thorens B., et al. In vivo conditional Pax4 overexpression in mature islet beta-cells prevents stress-induced hyperglycemia in mice. Diabetes. 2011;60:1705–1715. doi: 10.2337/db10-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto K., Matsuoka T.A., Kawashima S., Takebe S., Kubo F., Miyatsuka T., Kaneto H., Shimomura I. A novel function of Onecut1 protein as a negative regulator of MafA gene expression. J. Biol. Chem. 2013;288:21648–21658. doi: 10.1074/jbc.M113.481424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Du A., Hunter C.S., Murray J., Noble D., Cai C.L., Evans S.M., Stein R., May C.L. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Artner I., Blanchi B., Raum J.C., Guo M., Kaneko T., Cordes S., Sieweke M., Stein R. MafB is required for islet beta cell maturation. Proc. Natl. Acad. Sci. USA. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aguayo-Mazzucato C., Zavacki A.M., Marinelarena A., Hollister-Lock J., El Khattabi I., Marsili A., Weir G.C., Sharma A., Larsen P.R., Bonner-Weir S. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62:1569–1580. doi: 10.2337/db12-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanchet E., Van de Velde S., Matsumura S., Hao E., LeLay J., Kaestner K., Montminy M. Feedback inhibition of CREB signaling promotes beta cell dysfunction in insulin resistance. Cell Rep. 2015;10:1149–1157. doi: 10.1016/j.celrep.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Accili D., Talchai S.C., Kim-Muller J.Y., Cinti F., Ishida E., Ordelheide A.M., Kuo T., Fan J., Son J. When beta-cells fail: Lessons from dedifferentiation. Diabetes Obes. Metab. 2016;18((Suppl. 1)):117–122. doi: 10.1111/dom.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao X., Chen C., Guo P., Zhang T., Fischbach S., Fusco J., Shiota C., Prasadan K., Dong H., Gittes G.K. Forkhead Box Protein 1 (FoxO1) Inhibits Accelerated beta Cell Aging in Pancreas-specific SMAD7 Mutant Mice. J. Biol. Chem. 2017;292:3456–3465. doi: 10.1074/jbc.M116.770032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanderford N.L., Andrali S.S., Ozcan S. Glucose induces MafA expression in pancreatic beta cell lines via the hexosamine biosynthetic pathway. J. Biol. Chem. 2007;282:1577–1584. doi: 10.1074/jbc.M605064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leenders F., Groen N., de Graaf N., Engelse M.A., Rabelink T.J., de Koning E.J.P., Carlotti F. Oxidative Stress Leads to beta-Cell Dysfunction Through Loss of beta-Cell Identity. Front. Immunol. 2021;12:690379. doi: 10.3389/fimmu.2021.690379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hagman D.K., Hays L.B., Parazzoli S.D., Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J. Biol. Chem. 2005;280:32413–32418. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao M., Long Y., Tong Y., Wan J., Tong N. Activation of PPARdelta up-regulates the expression of insulin gene transcription factor MafA and ameliorates glucose-induced insulin secretion impaired by palmitate. Mol. Cell. Biochem. 2012;366:183–189. doi: 10.1007/s11010-012-1296-9. [DOI] [PubMed] [Google Scholar]
- 73.Vanderford N.L., Cantrell J.E., Popa G.J., Ozcan S. Multiple kinases regulate mafA expression in the pancreatic beta cell line MIN6. Arch Biochem. Biophys. 2008;480:138–142. doi: 10.1016/j.abb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen F., Liu J., Wang Y., Wu T., Shan W., Zhu Y., Han X. Aldosterone induces clonal beta-cell failure through glucocorticoid receptor. Sci. Rep. 2015;5:13215. doi: 10.1038/srep13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu G., Chen J., Jing G., Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 2013;19:1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Q., Yang Q., Xu H., Xue J., Chen C., Yang X., Gao X., Liu Q. miR-149 Negative Regulation of mafA Is Involved in the Arsenite-Induced Dysfunction of Insulin Synthesis and Secretion in Pancreatic Beta Cells. Toxicol. Sci. 2019;167:116–125. doi: 10.1093/toxsci/kfy150. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X., Mohan R., Ozcan S., Tang X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic beta-cells. J. Biol. Chem. 2012;287:31155–31164. doi: 10.1074/jbc.M112.362632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Y., Sun Y., Zhou Y., Zhang Y., Zhang T., Li Y., You W., Chang X., Yuan L., Han X. MicroRNA-24 promotes pancreatic beta cells toward dedifferentiation to avoid endoplasmic reticulum stress-induced apoptosis. J. Mol. Cell Biol. 2019;11:747–760. doi: 10.1093/jmcb/mjz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marzinotto I., Pellegrini S., Brigatti C., Nano R., Melzi R., Mercalli A., Liberati D., Sordi V., Ferrari M., Falconi M., et al. miR-204 is associated with an endocrine phenotype in human pancreatic islets but does not regulate the insulin mRNA through MAFA. Sci. Rep. 2017;7:14051. doi: 10.1038/s41598-017-13622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arnes L., Sussel L. Epigenetic modifications and long noncoding RNAs influence pancreas development and function. Trends Genet. 2015;31:290–299. doi: 10.1016/j.tig.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang N., Zhu Y., Xie M., Wang L., Jin F., Li Y., Yuan Q., De W. Long Noncoding RNA Meg3 Regulates Mafa Expression in Mouse Beta Cells by Inactivating Rad21, Smc3 or Sin3alpha. Cell. Physiol. Biochem. 2018;45:2031–2043. doi: 10.1159/000487983. [DOI] [PubMed] [Google Scholar]
- 83.Kameswaran V., Golson M.L., Ramos-Rodriguez M., Ou K., Wang Y.J., Zhang J., Pasquali L., Kaestner K.H. The Dysregulation of the DLK1-MEG3 Locus in Islets From Patients with Type 2 Diabetes Is Mimicked by Targeted Epimutation of Its Promoter With TALE-DNMT Constructs. Diabetes. 2018;67:1807–1815. doi: 10.2337/db17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kameswaran V., Bramswig N.C., McKenna L.B., Penn M., Schug J., Hand N.J., Chen Y., Choi I., Vourekas A., Won K.J., et al. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab. 2014;19:135–145. doi: 10.1016/j.cmet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo S., Vanderford N.L., Stein R. Phosphorylation within the MafA N terminus regulates C-terminal dimerization and DNA binding. J. Biol. Chem. 2010;285:12655–12661. doi: 10.1074/jbc.M110.105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao L., Cissell M.A., Henderson E., Colbran R., Stein R. The RIPE3b1 activator of the insulin gene is composed of a protein(s) of approximately 43 kDa, whose DNA binding activity is inhibited by protein phosphatase treatment. J. Biol. Chem. 2000;275:10532–10537. doi: 10.1074/jbc.275.14.10532. [DOI] [PubMed] [Google Scholar]
- 87.Han S.I., Tsunekage Y., Kataoka K. Phosphorylation of MafA enhances interaction with Beta2/NeuroD1. Acta Diabetol. 2016;53:651–660. doi: 10.1007/s00592-016-0853-1. [DOI] [PubMed] [Google Scholar]
- 88.Matsuoka T., Zhao L., Stein R. The DNA binding activity of the RIPE3b1 transcription factor of insulin appears to be influenced by tyrosine phosphorylation. J. Biol. Chem. 2001;276:22071–22076. doi: 10.1074/jbc.M010321200. [DOI] [PubMed] [Google Scholar]
- 89.Benkhelifa S., Provot S., Nabais E., Eychene A., Calothy G., Felder-Schmittbuhl M.P. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol. Cell. Biol. 2001;21:4441–4452. doi: 10.1128/MCB.21.14.4441-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo S., Burnette R., Zhao L., Vanderford N.L., Poitout V., Hagman D.K., Henderson E., Ozcan S., Wadzinski B.E., Stein R. The stability and transactivation potential of the mammalian MafA transcription factor are regulated by serine 65 phosphorylation. J. Biol. Chem. 2009;284:759–765. doi: 10.1074/jbc.M806314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sii-Felice K., Pouponnot C., Gillet S., Lecoin L., Girault J.A., Eychene A., Felder-Schmittbuhl M.P. MafA transcription factor is phosphorylated by p38 MAP kinase. FEBS Lett. 2005;579:3547–3554. doi: 10.1016/j.febslet.2005.04.086. [DOI] [PubMed] [Google Scholar]
- 92.Kondo T., El Khattabi I., Nishimura W., Laybutt D.R., Geraldes P., Shah S., King G., Bonner-Weir S., Weir G., Sharma A. p38 MAPK is a major regulator of MafA protein stability under oxidative stress. Mol. Endocrinol. 2009;23:1281–1290. doi: 10.1210/me.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El Khattabi I., Sharma A. Preventing p38 MAPK-mediated MafA degradation ameliorates beta-cell dysfunction under oxidative stress. Mol. Endocrinol. 2013;27:1078–1090. doi: 10.1210/me.2012-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rocques N., Abou Zeid N., Sii-Felice K., Lecoin L., Felder-Schmittbuhl M.P., Eychene A., Pouponnot C. GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol. Cell. 2007;28:584–597. doi: 10.1016/j.molcel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Han S.I., Aramata S., Yasuda K., Kataoka K. MafA stability in pancreatic beta cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol. Cell. Biol. 2007;27:6593–6605. doi: 10.1128/MCB.01573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu K., Lai Y., Cao H., Bai X., Liu C., Yan Q., Ma L., Chen D., Kanaporis G., Wang J., et al. Kindlin-2 modulates MafA and beta-catenin expression to regulate beta-cell function and mass in mice. Nat. Commun. 2020;11:484. doi: 10.1038/s41467-019-14186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iacovazzo D., Flanagan S.E., Walker E., Quezado R., de Sousa Barros F.A., Caswell R., Johnson M.B., Wakeling M., Brandle M., Guo M., et al. MAFA missense mutation causes familial insulinomatosis and diabetes mellitus. Proc. Natl. Acad. Sci USA. 2018;115:1027–1032. doi: 10.1073/pnas.1712262115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walker E.M., Cha J., Tong X., Guo M., Liu J.H., Yu S., Iacovazzo D., Mauvais-Jarvis F., Flanagan S.E., Korbonits M., et al. Sex-biased islet beta cell dysfunction is caused by the MODY MAFA S64F variant by inducing premature aging and senescence in males. Cell Rep. 2021;37:109813. doi: 10.1016/j.celrep.2021.109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanai K., Reza H.M., Kamitani A., Hamazaki Y., Han S.I., Yasuda K., Kataoka K. SUMOylation negatively regulates transcriptional and oncogenic activities of MafA. Genes Cells. 2010;15:971–982. doi: 10.1111/j.1365-2443.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 101.Shao C., Cobb M.H. Sumoylation regulates the transcriptional activity of MafA in pancreatic beta cells. J. Biol. Chem. 2009;284:3117–3124. doi: 10.1074/jbc.M806286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Onishi S., Kataoka K. PIASy is a SUMOylation-independent negative regulator of the insulin transactivator MafA. J. Mol. Endocrinol. 2019;63:297–308. doi: 10.1530/JME-19-0172. [DOI] [PubMed] [Google Scholar]
- 103.Sadowski M., Sarcevic B. Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residues proximal to the lysine. Cell Div. 2010;5:19. doi: 10.1186/1747-1028-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komander D., Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 105.Kanai K., Aramata S., Katakami S., Yasuda K., Kataoka K. Proteasome activator PA28{gamma} stimulates degradation of GSK3-phosphorylated insulin transcription activator MAFA. J. Mol. Endocrinol. 2011;47:119–127. doi: 10.1530/JME-11-0044. [DOI] [PubMed] [Google Scholar]
- 106.Bartolome A., Zhu C., Sussel L., Pajvani U.B. Notch signaling dynamically regulates adult beta cell proliferation and maturity. J. Clin. Investig. 2019;129:268–280. doi: 10.1172/JCI98098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He Y., Wang S., Tong J., Jiang S., Yang Y., Zhang Z., Xu Y., Zeng Y., Cao B., Moran M.F., et al. The deubiquitinase USP7 stabilizes Maf proteins to promote myeloma cell survival. J. Biol. Chem. 2020;295:2084–2096. doi: 10.1074/jbc.RA119.010724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu T., Zhang S., Xu J., Zhang Y., Sun T., Shao Y., Wang J., Tang W., Chen F., Han X. HRD1, an Important Player in Pancreatic beta-Cell Failure and Therapeutic Target for Type 2 Diabetic Mice. Diabetes. 2020;69:940–953. doi: 10.2337/db19-1060. [DOI] [PubMed] [Google Scholar]
- 109.Scoville D.W., Cyphert H.A., Liao L., Xu J., Reynolds A., Guo S., Stein R. MLL3 and MLL4 Methyltransferases Bind to the MAFA and MAFB Transcription Factors to Regulate Islet beta-Cell Function. Diabetes. 2015;64:3772–3783. doi: 10.2337/db15-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hung H.L., Kim A.Y., Hong W., Rakowski C., Blobel G.A. Stimulation of NF-E2 DNA binding by CREB-binding protein (CBP)-mediated acetylation. J. Biol. Chem. 2001;276:10715–10721. doi: 10.1074/jbc.M007846200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.