Abstract
Soybeans have traditionally been a staple part of the human diet being highly rich in protein and lipid content. In an addition to the high nutritional components, soybeans have several functional components, like isoflavones, saponins, lecithin, and oligosaccharides. Soybeans emerge as a healthy functional food option. Isoflavones are most notable functional component of soybeans, exhibiting antioxidant activity while preventing plant-related diseases (e.g., antimicrobial and antiherbivore activities) and having positive effects on the life quality of plants. Isoflavones are thus sometimes referred to as phytochemicals. The latest research trends evince substantial interest in the biological efficacy of isoflavones in the human body as well as in plants and their related mechanisms. However, there is little information on the relationship between isoflavones and plants than beneficial human effects. This review discusses what is known about the physiological communication (transport and secretion) between isoflavones and plants, especially in soybeans.
Keywords: Soybean-derived isoflavone, Plant, Rhizobium, Symbiotic interaction, Environmental adaptation
Introduction
Soybeans [Glycine max (L.) Merr.] are widely recognized as a food source with high nutritional value. Recent studies on their effects on maintaining health and preventing or ameliorating diseases have also led soybeans to be recognized as a food source rich in functionality (Chatterjee et al., 2018; Messina, 2016). Soybean protein, as a nutrient, has been shown to lower blood pressure and cholesterol levels (Chatterjee et al., 2018). Furthermore, although soybean components, such as lectin and trypsin inhibitors, have been originally seen as harmful, new beneficial physiological functions for these components have now been identified, including diabetes prevention and improvement, and antitumor activity (Anderson and Wolf, 1995). Phospholipids are lipids abundant in soybeans; they reduce lipids in plasma where they are the major components and lower total cholesterol (Ramdath et al., 2017). Soybean oligosaccharides are carbohydrates that promote the proliferation of probiotic bacteria, such as Bifidus and Lactobacillus species (Kushida et al., 2018). Small molecule compounds in soybeans have also been shown to display various functions. Phytic acid, an inositol attached to six phosphates, saponins, and isoflavones, which are mainly responsible for the bitter taste of soybeans, exhibits antitumor and antioxidant effects (Kang et al., 2010).
Isoflavones are a group of flavonoid-based compounds abundant in soybeans, and especially in the hypocotyl (Chung et al., 2017). These have demonstrated similarities in structure and biological functions to estrogen which is the female hormone, and hence termed as phytoestrogens. The use of phytoestrogens is a potential alternative strategy of nutrient uptake and environmental adaptation in rhizobium-dependent conditions in plants (Krizova et al., 2019). Currently, isoflavones have emerged as a vital component of many food-based medicinal and commercialized products which includes beverages, cosmetic products and also, health supplements (Zaheer and Humayoun Akhtar, 2017). This is attributed to the interest in medicinal and functional effects of soybeans as food products. The functional application of soybean isoflavones on plant growth is also gaining attention. This review focuses on the functionality of soy isoflavones, especially physiological communication (transport and secretion), which have recently received much attention in plants.
Composition, content, structure, and metabolism of isoflavones
Isoflavone content in soybean
Soybean is the only bean that contains prominent isoflavone levels (He and Chen, 2013; souces: Isoflavone Content of Selected Foods (Release 2.1) USDA; http://www.ars.usda.gov/nutrientdata). The isoflavone content in soybeansis slightly different in different countries. For example, 100 g soybean contains 118.28 mg isoflavones in China, 130.65 mg in Japan, 159.98 mg in the United States, and 178.81 mg/100 g (the highest) in Korea. The global average is 154.53 mg/100 g (He and Chen, 2013). These findings demonstrate that the cultivating and growth conditions modulate the isoflavone content in soybean. Overall, soybeans have about 0.2–0.3% isoflavone content as per the dry weight. The recommended daily intake of isoflavones is 40–50 mg/day; to achieve this, 25 g boiled soybean or 100 g common tofu should be consumed daily (Krizova et al., 2019).
Isoflavone biosynthesis
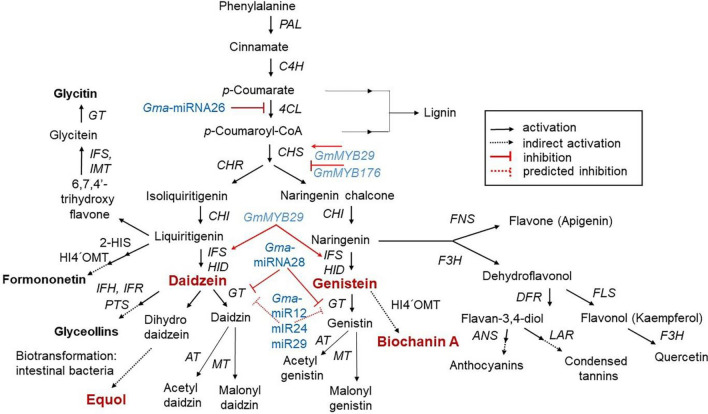
The biosynthetic pathway for isoflavones is a well-regulated flux channeling pathway compromising of several multiple pathways, with common substrates. Also, a group of secondary plant metabolites of the phenylpropanoid pathway also form a part of the multiple pathway of isoflavones (Garcia-Calderon et al., 2020). The main precursor for the isoflavone biosynthesis is amino acid l-phenylalanine. This metabolism in the first step of the pathway is stripped of its amine group to give cinnamic acid through nonoxidative deamination via the enzyme phenylalanine ammonia lyase. Next, the cinnamic acid is converted into p-coumaroyl CoA by enzymes cinnamate 4-hydroxylase and 4-coumarate CoA ligase (4CL). The crucial rate limiting enzymes here belong to the multigenic chalcone synthase (CHS) family, however, this is not found at detectable levels in all copies/seeds. Amongst the homologous copies, CHS7 and CHS8 are the ones with the highest expression levels in seeds and there is variability between seeds. Both these catalyze the conversion of p–coumaroyl CoA to naringenin chalcone. The other vital enzymes for isoflavone synthesis are the chalcone isomerase that converts chalcones to flavanones, and chalcone reductase enzyme which is required for daidzein and glycitein formation (Fig. 1; Gupta et al., 2017; Yu et al., 2000).
Fig. 1.
Schematic representation of the phenylpropanoid pathway depicting the key intermediates and enzymes for isoflavone biosynthesis, as well as partial regulation pathway. Isoflavones are biosynthesized via a phenylalanine-dependent pathway. GmMYB29 activates CHS expression, and GmMYB176 inhibits CHS expression. In addition, Gma-miRNA26, Gma-miRNA269, and Gma-miRNA28 suppress the gene expression or enzymatic activity of 4CL, IFS, and glucosyltransferase (GT), respectively. The dotted lines indicate the unclear role of miRNA in the pathway (Gupta et al., 2017; Yu et al., 2000). This isoflavone biosynthetic pathway is adapted from the USDA Database for the Isoflavone Content of Selected Foods Release 2.1 (http://www.ars.usda.gov/nutrientdata). ANS anthocyanidin synthase, AT acetyltransferase, 4CL 4-coumarate:CoA ligase, C4H cinnamic acid 4-hydroxylae, CHI chalcone isomerase, CHR chalcone reductase, CHS chalcone synthase, DFR dihydroflavonol reductase, F3H flavanone 3-hydroxylase, FNS flavone synthase, FLS flavonol synthase, GT glycosyltransferase, 12´H isoflavone hydroxylase, HI4´OMT 2,7,4´-trihydroxyisoflavanone 4´-O-methyltransferase, HID 2-hydroxyisoflavone dehydratase, 2-HIS 2-hydroxyisoflavone synthase, IFH isoflavone hydroxylase, IFS isoflavone synthase, IFR isoflavone reductase, IMT isoflavone malonyl transferase, LAR flavan-3,4-diol reductase, MT malonyltransferase, PAL phenylalanine ammonia lyase, PTS pterocarpan synthase, PTS pterocarpan synthase
The enzyme isoflavone synthase (IFS; cytochrome P450 monooxygenase) identifies isoflavone-producing plants (especially soybean) and segregates them from those without isoflavone (Yu et al., 2000). There are two IFS genes (IFS1 and IFS2) with a difference of 14 amino acids among them. The first reaction of isoflavone biosynthesis is catalyzed by both isoforms of IFS to produce 2-hydroxyisoflavanone, which is further dehydrated to give daidzein and genistein via 2-hydroxyisoflavanone dehydratase in the cytosol of the soybean seed (Akashi et al., 2005). Both metabolites are next glucosylated to daidzin and genistin via enzyme UDP-glucose:isoflavone 7-O-glucosyltransferase followed by being malonylated to malonyldaidzin/genistin via enzyme malonyl-CoA:isoflavone 7-O-glucoside 6″-O-malonyltransferase. All these conversions take place in the vacuoles (Ahmad et al., 2017). Studies indicated indicate that daidzein and genistein are released from the roots into the rhizosphere via two mechanisms, one is directly via membrane transport using an ATP-binding cassette (ABC)-type transporter and the other is an indirect mechanism via isoflavone glucosides being hydrolyzed to daidzein and genistein in the apoplast by enzyme isoflavone-conjugated hydrolyzing β-glucosidase (ICHG) (Chatterjee et al., 2018; Gupta et al., 2017; Sugiyama et al., 2017). The phenylpropanoid pathway also contributes to the synthesis of lignins, stilbene, phlobaphenes, proanthocyanidins, and anthocyanins via multiple pathways, facilitating the isoflavone pathway (Dong and Lin, 2021; Falcone Ferreyra et al., 2012). The differences in isoflavone accumulation in different soybean cultivars result from genetic and environmental interactions, the regulation of which is still unclear.
All steps in isoflavone biosynthesis are regulated by multiple genes that regulate the production of the different intermediate metabolites (Gupta et al., 2017). Recently, Gupta et al. (2017) demonstrated that there were five microRNAs including the Gma-miRNA12, Gma-miRNA24, Gma-miRNA26, Gma-miRNA28, and Gma-miRNA29 which might have a plausible role in isoflavone biosynthesis. The expression of Gma-miRNA26 and Gma-miRNA28 and their corresponding target genes (Glyma.10G197900 and Glyma.09G127200) is directly correlated with the total isoflavone content (Gupta et al., 2017). Additionally, some myeloblastosis (MYB) transcription factors (TFs) responsible for the regulation of the isoflavone biosynthesis pathway have been detected in soybeans (Sarkar et al., 2019). For example, the R1-type MYB TF GmMYB176 regulates the expression of the CHS8 gene, and the R2R3-type MYB TFs GmMYB39 and GmMYB100 suppressing the expression of structural biosynthesis genes are involved in isoflavone biosynthesis (Chu et al., 2017; Sarkar et al., 2019). The R2R3-type MYB TF GmMYB29 (Glyma.20G209700) has been reported recently to activate IFS2 and CHS8 gene promoters (Chu et al., 2017; Sarkar et al., 2019). GmMYB29 upregulation or downregulation in hairy roots caused improved or reduced isoflavone levels, respectively (Chu et al., 2017). Thus, these results suggested the need for future research to better understand the molecular mechanism involved in isoflavone biosynthesis. This would facilitate the alteration of isoflavone biosynthesis, which would ultimately be applied to the production of cultivars that would meet the requirements.
Structure and type of isoflavones
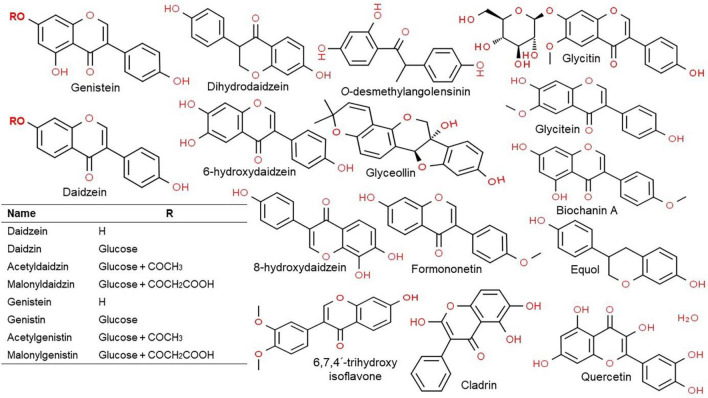
Isoflavones have a 3-phenylchrome based structure. The most commonly known isoflavones have been genistein, daidzein, formononetin, biochanin A, and the coumestans. The soy isoflavones have been divided into two types, based on their chemical structure, the glycosides and aglycones. About 12 isoflavones have so far been identified in soybean, which includes three aglycones (genistein, daidzein, and glycitein), and 3 glycosides (daidzin, genistin, and glycitin) and also malonyl glycoside and acetyl glycoside (Jung et al., 2020a, 2020b). In soybean, the isoflavone glycosides are found in the form of malonyl glycoside (Fig. 2; Krizova et al., 2019). For absorption in the intestines, glycosides having a polysaccharide-attached with either 6″-O-acetylglucosides, or 6″-O-malonylglucosides is converted further into aglycones. This is mediated by enzyme β-glucosidase secreted by the intestinal microflora. These aglycones are free isoflavones without the sugar (Izumi et al., 2000; Szeja et al., 2017), and are mainly distributed in the epicotyl and hypocotyl of the plant, with variability in their contents depending on cultivars and growth conditions (Andres et al., 2015; Miadokova, 2009).
Fig. 2.
Chemical structures for the major classes of isoflavones and their metabolites. Structures have been drawn using ChemSpider
Variation in isoflavone content
Isoflavone content in soybean seeds varies during maturation; the amount increases approximately 35 days after flowering. In particular, genistin and malonyl-genistin show an increase in accumulation in the late maturation phase, whereas daidzin and malonyl-daidzin show an increased concentration at a steady rate of increase throughout maturation. Concurrently, glycitin and malonyl-glycitin contents stayed the same or slightly decreased (Gupta et al., 2017; Kudou et al., 2014).
In one study, soybean isoflavone content was isolated from the leaf, stem, root, flower, shoot, and seed; in each part, it was influenced more by environmental factors than genetic factors. In the seeds harvested from soybeans cultivated at high temperatures, isoflavone content decreased (Morgan et al., 2014; Mureşan et al., 2020). In each part of the soybean seed, isoflavone content in the cotyledon decreased in higher temperatures; however, isoflavone content in the hypocotyl was consistent. Isoflavone content in the whole soybean and hypocotyl ranged from 9.4 to 194.9 mg/100 g and 135.6 to 1503.7 mg/100 g, respectively (Eum et al., 2020; Zhu et al., 2005). Isoflavone content was influenced by the temperature during maturation and decreased significantly with increased temperatures (Zhang et al., 2014). Thus, increasing the temperature during maturation resulted in larger decreases in isoflavone content. This variation in isoflavone content due to maturation temperature was slightly larger than that due to the choice of soybean cultivar. Glycitin and its malonyl glycoside were found only in soybean hypocotyl (Yuan et al., 2009).
The amount of isoflavones produced by soybeans depends mainly on growing conditions and the cultivar (Krizova et al., 2019; Miladinovic et al., 2019). Isoflavone concentrations increase sharply during stressful conditions (e.g., lowered humidity, pathogen attack, or plant diseases) and are subjective to environmental conditions like soil fertility harvest period and climatic conditions, such as temperature and precipitation. The overall concentration of isoflavone is further predisposed to postharvest processing (Krizova et al., 2019; Messina et al., 2021).
Secretion, transport, and the role of isoflavone in soybean plants
Accumulation during soybean growth stages
Extensive research has been conducted on the effects of genetic and environmental changes on the isoflavone content of soybean seeds due to the agricultural importance of soybean crops. However, little is known about isoflavone content changes at different soybean growth stages (Sugiyama, 2019). In a recent study on leaf flavonoids at the growth and developmental stages, daidzein and genistein content increased in leaves from R5 to R6 (Sugiyama et al., 2016). In another recent study investigating isoflavone content in tissues and root secretions during growth and development under nutritional stress, daidzein was most abundant at V3, whereas malonyl genistein was abundant in leaves from V7 to R6. Daidzein and genistein contents were very low at V3 and V7, and aglycones were not detected at R4 and R6 (Sugiyama et al., 2016). Two isoflavones, daidzein and genistein, accumulated in the form of malonyl glucosides or glucosides in leaves. The ratio of genistein to daidzein derivatives was 3.5:1 at V3 and approximately 1:2 from V7 to R6 (Sugiyama et al., 2016). Malonyl daidzein was most abundant from V3 to R6 in roots; however, the amounts of malonyl daidzein and daidzein were almost identical at VE. The levels of daidzein and genistein were higher in the roots than in the leaves. The ratio of daidzein to genistein derivatives in the roots was 2.5:1 at VE and increased from 4:1 to 5:1 from V3 to R6, respectively. In particular, daidzein was a predominant isoflavone in soybean root exudates during the vegetative stages (VE to V7). Only trace amounts of malonyl glucosides and glucosides were present at VE, which increased slightly during development. At the reproductive stage, malonyl glucoside and glucoside levels were similar aglycone levels (Sugiyama et al., 2016).
The changes in isoflavone content in leaves and roots during growth and development were relatively low, suggesting that isoflavone content remained constant under normal growth conditions. The ratio of daidzein and genistein derivatives was high in the roots and low in the leaves, except at V3, wherein the levels were reversed in the roots and leaves (Sugiyama et al., 2016). This difference in isoflavone content between tissues suggested a tissue-specific expression of CHS and chalcone reductase, the major enzymes involved in 5-deoxyflavonoid synthesis (Dao et al., 2011; Sugiyama et al., 2016). In contrast to isoflavone content in tissues, isoflavone secretion was dependent on growth stage. In other words, isoflavone secretion into the rhizosphere reached a maximum and gradually decreased at V3 (Sugiyama et al., 2016), suggesting that isoflavone-mediated biological interactions are important at the vegetative stage. Daidzein content at VE was 225 ± 99 pmol/g fresh weight (FW). This suggested that daidzein was the predominant isoflavone secreted into the rhizosphere, followed by isoflavones secreted during the growth stages (V3, 37 ± 18 pmol/g FW; V7, 9.9 ± 5.6 pmol/g FW; R4, 2.4 ± 2.4 pmol/g FW; R6, 3.5 ± 1.7 pmol/g FW; Sugiyama et al., 2016, 2017). A large amount of daidzein secreted into the rhizosphere at the seedling stage presumably contributes to symbiosis with rhizobia at the vegetative stage.
The content and distribution of other isoflavones in the leaves and roots did not change dynamically during the developmental stages. However, isoflavones in root exudates were different during the developmental stages, particularly reproductive stages, and under nitrogen-deficient conditions in plants (Moran et al., 2014; Sugiyama et al., 2016). In the roots, there are two feasible pathways for isoflavone production. ICHG is a major regulator of the secretion of malonyl glucosides and glucosides (Sugiyama et al., 2016; Suzuki et al., 2006). ICHG expression was increased at vegetative stages, particularly V3 and V7, and decreased at reproductive stages during soybean development (Sugiyama et al., 2016, 2017), consistent with the result that showed a higher daidzein content in the root exudates of soybeans at the vegetative stages (Fujimatsu et al., 2020; Sugiyama et al., 2016). Although daidzein and genistein secretions significantly increased in nitrogen-deficient growth conditions, the expression of ICHG was not effected by any kind of nitrogen or phosphate deficiency (Sugiyama et al., 2016).
ICHG expression was reduced during reproductive stages. In line with this, malonyl daidzein and other glucosides were present in root exudates (Matsuda et al., 2020). Daidzein and genistein from soybean seeds and root exudates induced nod gene expression (Kape et al., 1992). When soybeans were grown under nitrogen-deficient conditions, isoflavone content in the roots increased; thus, isoflavone secretion into the rhizosphere also increased (Subramanian et al., 2006; Sugiyama et al., 2016). These secreted isoflavones were analyzed in young seedlings during active growth under nitrogen-deficient conditions but not in mature plants (Fujimatsu et al., 2020; Sugiyama et al., 2016). Isoflavone content in leaves or roots did not increase for 1 week due to nitrogen deficiency, but the secretion of isoflavones, particularly daidzein and genistein, increased by about 8-fold and 15-fold, respectively (Sugiyama et al., 2016). Because ICHG expression was not induced in roots, this suggested that ATP-dependent transporters contribute to isoflavone secretion during nitrogen deficiency along with ICHG-mediated secretion (Sugiyama et al., 2007, 2016). Root exudation of amino acids and organic acids in soybeans is induced by phosphate deficiency. In contrast, although isoflavone secretion was slightly decreased, isoflavone content in tissues did not significantly change (Canarini et al., 2019; Sugiyama et al., 2016).
Transport and secretion
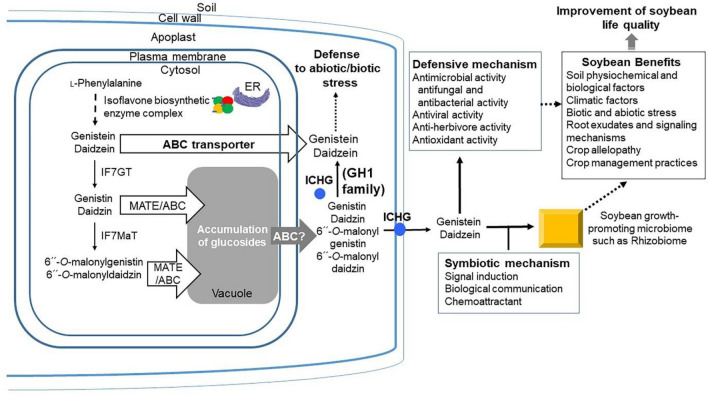
Plants have effective mechanisms for the safe intracellular storage of synthesized molecules or possible extracellular exudation. Based on previous studies, there are three main pathways for the transport of flavonoids and similar metabolites (Falcone Ferreyra et al., 2012; Petrussa et al., 2013; Zhao, 2015). The first pathway involves transport through ABC proteins that obtain energy by ATP hydrolysis in the subdomains. This broad family of proteins is classified into several subfamilies. There are specific subfamilies of multidrug resistance-associated protein (MRP) transporters that transport flavonoids in some plants. The second pathway involves transport by multidrug and toxin extrusion (MATE) proteins involved in MATE, which are dependent on the proton/membrane potential. Energy for transport is indirectly obtained when several pumps, such as plasma membrane H+-ATPases or vacuolar H+-pyrophosphatase, maintain sufficient gradients. The third pathway involves transport inside vesicles produced by organelles, which is another possible way to transport flavonoids and other secondary metabolites in plant cells (Fig. 3; Ku et al., 2020; Sugiyama, 2019; Taneja et al., 2016). The flavonoid transport mechanism is also related to glutathione-S-transferase activity, which plays a role in the sequestration of these substances into vacuoles (Nianiou-Obeidat et al., 2017).
Fig. 3.
Isoflavone transport mechanism dependent on vesicular or multidrug and toxin extrusion (MATE)/ATP-binding cassette (ABC) proteins and the effect of inhibitors. Genistein and daidzein synthesized from l-phenylalanine in the presence of endoplasmic reticulum (ER)-coupled isoflavone biosynthetic enzyme complex in the cytosol are released to apoplasts by ABC. Furthermore, metabolites processed via the isoflavone-7-O-glucosyltransferase (IF7GT) and isoflavone-7-O-glucoside-6′′-O-malonyltransferase (IF7MaT) are transported to vacuoles by MATE proteins. Accumulated metabolites in vacuoles, such as genistin, daidzin, 6′′-O-malonylgenistin, and 6′′-O-malonyldaidzin, are released to apoplasts by ABC transporters and converted to genistein and daidzein in the presence of isoflavone conjugate-hydrolyzing β-glucosidase (ICHG) expressed in the apoplast and cell wall. The biosynthesized genistein and daidzein are involved in defense mechanisms against abiotic and biotic stress in soybean plant cells and exhibit a wide range of biological effects, including normal cell growth development and defense against abiotic and biotic stress, through symbiotic mechanisms within the rhizosphere in the soil. They eventually lead to improved soybean life quality (Ku et al., 2020; Sugiyama, 2019; Taneja et al., 2016)
Daidzein and genistein metabolites are secreted either directly from the roots to the rhizosphere mediated by the ABC-type membrane transporters or also indirectly based on hydrolysis of isoflavone glucosides and secretion into the apoplast. Furthermore, ICHG, which exists in the apoplast, hydrolyzes isoflavone glucosides to daidzein and genistein (Sugiyama et al., 2017). Many secondary metabolites in plants, including flavonoids, are stored in vacuoles of cells in the form of glycosides with acyl substituents. One of the biochemical roles of acylation is to confer biochemical stability to products stored in vacuoles of plant cells. The indiscriminate degradation of stored glycosides by microbial glycosidases can be prevented by acylation (Falcone Ferreyra et al., 2012; Petrussa et al., 2013; Zhao, 2015). Therefore, cells require enzymes to convert glycoside-conjugated isoflavones into free isoflavones (Krizova et al., 2019). G. max ICHG (GmICHG), which can directly hydrolyze isoflavone conjugates in malonylated forms, enables the host-controlled production of isoflavone aglycones (Ahmad et al., 2017; Suzuki et al., 2006). GmICHG, a cationic glycoprotein localized in the cell walls and intercellular space (called apoplast) of soybean roots, plays a major role in the extracellular secretion pathway through endoplasmic reticulum-Golgi trafficking (Suzuki et al., 2006). However, the selective pathway of the GmICHG precursor has not yet been elucidated. GmICHG binds to pectic polysaccharides in plant cell walls and apoplasts in plants. It is intrinsically a homodimer with a 58-kDa subunit (Suzuki et al., 2006).
Recently, the CtICHG protein of guar (Cyamopsis tetragonoloba) was purified, and its biochemical properties were revealed. It exists as a trimer with a molecular weight of approximately 150 kDa and is active against isoflavone-conjugated glycosides (daidzein and genistein) but inactive against other flavonoid conjugates. In addition, CtICHG protein showed β-glucosidase activity along with isoflavone conjugate-hydrolyzing properties (Asati and Sharma, 2019). Furthermore, the ICHG of Medicago sativa is a homodimer and has a 40.3-kDa subunit (Zubieta et al., 2001). In addition to CtICHG, GmICHG can cleave malonylated and acylated conjugates (Suzuki et al., 2006). CtICHG protein is relatively stable at high temperatures, and its enzyme activity is completely inhibited by cobalt (Co2+) and mercury (Hg2+) ions (Asati and Sharma, 2019). Most ICHG enzyme inhibition mechanisms are achieved by inducing conformational changes in proteins with the formation of sulfhydryl linkages by interacting with cysteine residues in the presence of Hg2+ (Ismail and Hayes, 2005; Jung et al., 2020a, 2020b; Suzuki et al., 2006). The inactivation of CtICHG in the presence of Co2+ ions results in the complete denaturation of the protein by irreversible binding of amino acids that exist at the ICHG active site with Co2+ ions. Other ions, such as Mg2+, Mn2+, Ca2+, Na+, Cu2+, and Fe3+, also inhibit enzyme activity at all concentrations. These ions also form metal complexes with proteins, altering protein structures and eventually inactivating them (Suzuki et al., 2006; Yoo et al., 2013). In summary, soybean roots release isoflavones, such as daidzein and genistein, into the rhizosphere and a small area around the root. These compounds act as signal enhancers by signaling symbiotic rhizomes to form nodules in the roots.
Plants of the Fabaceae family (i.e., Medicago truncatula) are used as models to study isoflavonoid transport (Garcia-Calderon et al., 2020; Kubes et al., 2019). Nevertheless, similar transport processes in soybean, an important crop and a major source of metabolites with therapeutic potential, have not been extensively studied. Recently, the isoflavone secretion mechanism in a Trifolium pratense suspension culture was identified (Zaklos-Szyda and Budryn, 2020). Increased isoflavone content after two vanadium compound (1 µM NH4VO3 and 1 µM VOSO4) treatments for 24 h was significantly reduced by transporter inhibitors, such as MRP subfamily ABC inhibitors and MATE suppressors. The isoflavone concentration in the cell suspension solution was lower than the basal level in the presence of these inhibitors (Zaklos-Szyda and Budryn, 2020). Transporter inhibitors include brefeldin A (BA), ammonium chloride (NH4Cl), sodium orthovanadate (Na3VO4), gramicidin, verapamil, probenecid, and glibenclamide (Kubes et al., 2019). In particular, brefeldin A blocks the vesicular transport in the nutrient medium and causes decrease in the isoflavone concentration (Fig. 4; Kubes et al., 2019).
Fig. 4.
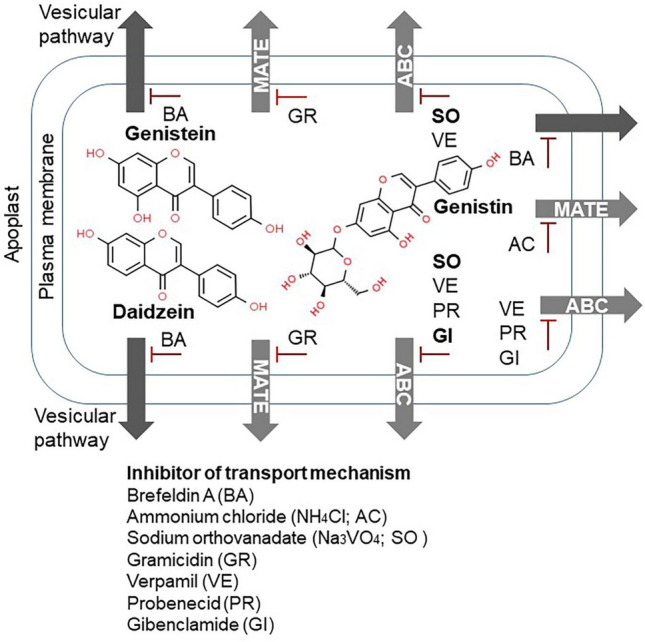
Inhibitors affecting plasma-membrane transport mechanism (vesicular or multidrug and toxin extrusion [MATE]/ATP-binding cassette [ABC] pathways) of genistein, daidzein, and genistin in soybean plant cells. Inhibitors indicated in bold significantly affect isoflavone contents in both the nutrient medium and the dry mass of seedling plants (Kubes et al., 2019). AC ammonium chloride (NH4Cl), GR gramicidin, BA brefeldin A, SO sodium orthovanadate (Na3VO4), VE verapamil, PR probenecid, GI glibenclamide
Potential of rhizobacteria
Plants secrete a significant proportion of photosynthetic substances from their roots into the rhizosphere (Olanrewaju et al., 2019). However, the mechanisms and genes involved in root exudation have not yet been fully elucidated. Plant growth stages and nutrient deficiency, such as nitrogen, phosphate, and iron, significantly influence metabolite secretion into the rhizosphere (Igiehon and Babalola, 2018). For example, the amount of root exudate in rice is lowest at the seedling stage, gradually increasing until flowering and decreasing again at plant maturity (Sugiyama et al., 2016). Metabolites secreted by Arabidopsis roots dynamically change during development, which is closely related to the transcriptome of microbial communities in the rhizosphere (Korenblum et al., 2020). However, little is known about the developmental and nutritional regulation of soybean root exudation. Two potential pathways involved in isoflavone secretion have been proposed: (1) ATP-dependent transport of isoflavone aglycones (especially genistein) and (2) secretion of daidzein and genistein into the apoplasts by ICHG-induced hydrolysis of isoflavone glucosides (Sugiyama, 2019; Sugiyama et al., 2016).
Rhizobacteria are also considered biofertilizers because they can enhance plant growth either directly or indirectly through various activities. The various roles of rhizobacteria include nitrogen fixation, nutrient solubilization, biosynthesis of phytohormones (e.g., indole-3-acetic acid, ethylene, and gibberellin), antibiotic properties, antifungal metabolite production, hydrolytic enzymes (i.e., lytic enzymes), siderophore formation, and acquisition of inducible systemic resistance to pathogens in plants (Ahemad and Kibret, 2014; Olanrewaju et al., 2017). Rhizobacteria are known as biocontrol agents or biopesticides because they inhibit of plant pathogens through antagonism and competition. They are also called bioremediators, rhizoremediators, or phytoremediators, because they contribute to the decomposition of organic pollutants and the reduction of metal toxicity in contaminated soil (Backer et al., 2018). Similarly, rhizobacteria that produce plant hormones are collectively referred to as phytostimulators (Aloo et al., 2019). Therefore, studies based on plant growth-promoting rhizobacteria (PGPR) have shown that they can improve various properties related to plant growth (Alberton et al., 2020; Bukhat et al., 2020; Wang et al., 2021), such as sprout growth, root length, biomass, seed germination, and leaf size (Backer et al., 2018).
The diverse microbial communities thriving in the rhizosphere have significant and different positive effects on the growth and health parameters of the plants which includes nutrition, disease control, and tolerance to abiotic and biotic stresses (Ahkami et al., 2017). In addition to climate, soil type, plant species, plant genotype, and growth stage regulate the diversity and composition of microbial communities in the rhizosphere (Dastogeer et al., 2020). Studies on microbial communities in the soybean rhizosphere showed more symbiotic rhizobia than bulk soil (Han et al., 2020; Sugiyama, 2019). During soybean growth in the field, microbial communities changed in the rhizosphere but not in bulk soil (Sugiyama et al., 2014). These findings suggested that plant growth is affected more by changes in microbial communities in the rhizosphere than environmental factors. Bradyrhizobium sp. and other potential PGPRs, such as Bacillus sp., are more abundant in the rhizosphere than in bulk soil. For example, Paenibacillus polymyxa BFKC01 improved not only the nutrient availability of soybean plants but also the plant’s ability to produce phytohormones and withstand biotic and abiotic stresses (Dalio et al., 2018; Jahan et al., 2019; Jayaraman et al., 2012; Kubes et al., 2019; Olanrewaju et al., 2019; Veitch, 2013). Therefore, rhizospheres containing isoflavones (daidzein and genistein) can improve the ability to adapt to external environmental changes and supply nutrients required by plants through various microbial communities, including rhizobacteria. This suggested that isoflavones play a very important role in this process.
Functionality of isoflavones in plants
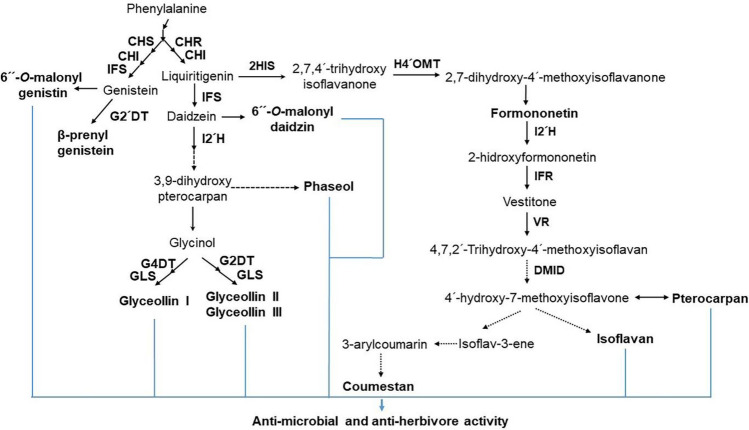
Isoflavones take part in various plant-microbe interactions, such as defense and symbiosis. In defense, the antioxidant activity of isoflavones is utilized for counteracting stress-activated reactive oxygen species (ROS). They act as precursors for the production of phytoalexins, the plant metabolites which have been found to have potent antibacterial, antiviral effects, and/or antiherbivore effects (Fig. 5; Chu et al., 2017; Dixon, 2001; Jayaraman et al., 2012; Olanrewaju et al., 2019; Singh et al., 2021). To protect leguminous plant soybean from pathogens, such as Phytophthora sojae and Macrophomina phaseolina, daidzein-derived phytoalexins, such as glyceollins and prenylated pterocarpans, are secreted (Lygin et al., 2013; Sukumaran et al., 2018). In symbiosis, in leguminous plants containing beans, soybeans, peas, chickpeas, peanuts, lentils, alfalfa, and clover, isoflavones also act as signal molecules in the production of nitrogen-fixing root nodules (Abdel-Lateif et al., 2012). Furthermore, the soybean roots also release the isoflavones, daidzein and genistein, into the rhizosphere, and these compounds further then stimulate the rhizobia to form nodules in the roots (Sugiyama, 2019; Sugiyama et al., 2017).
Fig. 5.
Relationship between isoflavone products and antimicrobial activity. Phenylalanine-derived biosynthetic isoflavone products, including 6″-O-malonylgenistein, glyceollins (I–III), coumestan, isoflavan, and pterocarpan, have antimicrobial and antiherbivore activities (Dixon, 2001; Olanrewaju et al., 2019; Singh et al., 2021). CHI chalcone isomerase, CHR chalcone reductase, CHS chalcone synthase, DMID 7,2'-dihydroxy-4'-methoxy-isoflavanol dehydratase, G2´DT glycinol 2-dimethylallyltransferase, G4´DT glycinol 4-dimethylallyltransferase, GLS glyceollin synthase, 2-HIS 2-hydroxyisoflavanone synthase, H4´OMT 2,7,4′-trihydroxyisoflavanone 4′-O-methyltransferase, I2´H isoflavone 2-hydroxylase, IFR isoflavone reductase, IFS isoflavone synthase, VR vestitone reductase
In humans, the phytoestrogenic and antioxidant properties of isoflavones have been implicated in the prevention and improvement of certain diseases, such as cancers, cardiovascular diseases, neurological diseases, climacteric syndrome, obesity, inflammation, and aging, in an isoflavone-dependent manner (Miadokova, 2009; Yu et al., 2016). Lately, isoflavones have also been incorporated in soy-based infant formulas (Testa et al., 2018).
The environmental conditions, such as those mediated by heavy metals, causes changes in various organismal structures and processes and this has been seen to be mediated either directly or indirectly for isoflavones biosynthesis via the ROS production (Krizova et al., 2019). Heavy metals, such as vanadium, inhibit plant growth and development when present in high concentrations (Dutta et al., 2018). Vanadium compounds can bind to certain enzymes instead of phosphate, including protein kinases, ribonucleases, or ATPases (Kubes et al., 2019; Pessoa et al., 2015; Skalicky et al., 2018). Despite these toxic effects, vanadium compounds have also been examined as favorable plant elicitors with different effects on the production and exudation of secondary metabolites from in vitro cell cultures, and these compounds have also been tested as potential drugs for the treatment of various diseases (Skalicky et al., 2018). Moreover, vanadium compounds upregulate gene expression and increase enzyme activity related to the phenylpropanoid pathway, thus resulting in flavonoid synthesis and release (Kubes et al., 2019).
Plants survive in complex, dynamic, or sometimes unfavorable environmental conditions through their natural immune systems, such as systematic acquired resistance and induced systemic resistance (Conrath, 2006; Romera et al., 2019). In plants, active defense compounds called phytoalexins are low-molecular-weight molecules induced during stress and pathogen attacks. These phytoestrogens exhibit antifungal, antibacterial, antiviral, and antioxidant properties (Krizova et al., 2019).
Conclusion and perspectives
The amount of isoflavones secreted by soybeans into the rhizosphere is higher during their vegetative growth stages, when ICHG expression increases, than during their reproductive stages. This suggests that increased secretion of isoflavone malonylglucosides may accumulate in vacuoles. Under nitrogen-deficient conditions, the metabolite genistein along with daidzein, is secreted in excess which indicates a plausible mechanism that an ATP-dependent transporter facilates the secretion of genistein. In soybean roots, the two pathways of isoflavone production have distinct physiological roles (ICHG-mediated secretion during vegetative growth and ATP-dependent transport during nitrogen deficiency), though many processes and the mechanism still remain unclear. The metabolites which are secreted from the roots, collectively called root exudates act as the primary factors regulating the rhizosphere microbial communities, which further modulates the downstream pathway and parameters of plant growth and health. In the soybean rhizosphere, the overall structure of the microbial communities, especially the bacteria communities, also undergo changes during growth of the soya beans. This therefore indicates that overall growth of the plant especially that of the root exudates, further in turn regulates the rhizosphere bacterial communities.
This review is focused on the bioactivity of the isoflavone identified so far, but it doesn’t involve any previous studies covering the enhancement of life quality of soybean-derived isoflavones or studies which examine the possibility of their potential use in functional products. Most studies up till now, have demonstrated the benefits of high isoflavone doses does on human health especially the doses that exceed the average human consumption by several tens or hundreds of times. Notwithstanding the practical constraints as mentioned above, it is an established fact that soybean-derived isoflavones have the potential to enhance the quality of life in plants; nevertheless, further additional studies to delineate the underlying mechanisms of isoflavone activity are required.
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (NRF-2019R1I1A1A01058109).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Lateif K, Bogusz D, Hocher V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signaling and Behavior. 2012;7:636–641. doi: 10.4161/psb.20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. Journal of King Saud University-Science. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- Ahkami AH, Allen White R, Handakumbura PP, Jansson C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere. 2017;3:233–243. doi: 10.1016/j.rhisph.2017.04.012. [DOI] [Google Scholar]
- Ahmad MZ, Li P, Wang J, Rehman NU, Zhao J. Isoflavone malonyltransferases GmIMaT1 and GmIMaT3 differently modify isoflavone glucosides in soybean (Glycine max) under various stresses. Frontiers in Plant Science. 2017;8:735. doi: 10.3389/fpls.2017.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi T, Aoki T, Ayabe S. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiology. 2005;137:882–891. doi: 10.1104/pp.104.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberton D, Valdameri G, Rotuno Moure VR, Monteiro RA, de Oliveira Pedrosa F, Marcelo Müller-Santos M, de Souza EM. What did we learn from plant growth-promoting rhizobacteria (PGPR)-grass associations studies through proteomic and metabolomic approaches? Frontiers in Sustainable Food Systems. 2020;4:607343. doi: 10.3389/fsufs.2020.607343. [DOI] [Google Scholar]
- Aloo BN, Makumba BA, Mbega ER. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiological Research. 2019;219:26–39. doi: 10.1016/j.micres.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Anderson RL, Wolf WJ. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. Journal of Nutrotion. 1995;125:581S–588S. doi: 10.1093/jn/125.3_Suppl.581S. [DOI] [PubMed] [Google Scholar]
- Andres S, Hansen U, Niemann B, Palavinskas R, Lampen A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food and Function. 2015;6:2017–2025. doi: 10.1039/C5FO00308C. [DOI] [PubMed] [Google Scholar]
- Asati V, Sharma PK. Purification and characterization of an isoflavones conjugate hydrolyzing beta-glucosidase (ICHG) from Cyamopsis tetragonoloba (guar) Biochemistry and Biophysics Reports. 2019;20:100669. doi: 10.1016/j.bbrep.2019.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in Plant Science. 2018;9:1473. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhat S, Imran A, Javaid S, Shahid M, Majeed A, Naqqash T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiological Research. 2020;238:126486. doi: 10.1016/j.micres.2020.126486. [DOI] [PubMed] [Google Scholar]
- Canarini A, Kaiser C, Merchant A, Richter A, Wanek W. Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Frontiers in Plant Science. 2019;10:157. doi: 10.3389/fpls.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee C, Gleddie S, Xiao CW. Soybean bioactive peptides and their functional properties. Nutrients. 2018;10:1211. doi: 10.3390/nu10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Wang J, Zhu Y, Liu S, Zhou X, Zhang H, Wang CE, Yang W, Tian Z, Cheng H, Yu D. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genetetics. 2017;13:e1006770. doi: 10.1371/journal.pgen.1006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IM, Oh JY, Kim SH. Comparative study of phenolic compounds, vitamin E, and fatty acids compositional profiles in black seed-coated soybeans (Glycine max (L.) Merrill) depending on pickling period in brewed vinegar. Chemistry Central Journal. 2017;11:64. doi: 10.1186/s13065-017-0298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U. Systemic acquired resistance. Plant Signaling Behavior. 2006;1:179–184. doi: 10.4161/psb.1.4.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalio RJD, Herlihy J, Oliveira TS, McDowell JM, Machado M. Effector biology in focus: A primer for computational prediction and functional characterization. Molecular Plant-Microbe Interactions. 2018;31:22–33. doi: 10.1094/MPMI-07-17-0174-FI. [DOI] [PubMed] [Google Scholar]
- Dao TT, Linthorst HJ, Verpoorte R. Chalcone synthase and its functions in plant resistance. Phytochemistry Reviews. 2011;10:397–412. doi: 10.1007/s11101-011-9211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastogeer KMG, Tumpa FH, Sultana A, Akter MA, Chakraborty A. Plant microbiome–an account of the factors that shape community composition and diversity. Current Plant Biology. 2020;23:100161. doi: 10.1016/j.cpb.2020.100161. [DOI] [Google Scholar]
- Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Dong NQ, Lin HX. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. Journal of Integrative Plant Biology. 2021;63:180–209. doi: 10.1111/jipb.13054. [DOI] [PubMed] [Google Scholar]
- Dutta S, Mitra M, Agarwal P, Mahapatra K, De S, Sett U, Roy S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signaling Behavior. 2018;13:e1460048. doi: 10.1080/15592324.2018.1460048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum HL, Park Y, Yi TG, Lee JW, Ha KS, Choi IY, Park NI. Effect of germination environment on the biochemical compounds and anti-inflammatory properties of soybean cultivars. PLoS One. 2020;15:e0232159. doi: 10.1371/journal.pone.0232159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Frontiers in Plant Science. 2012;3:222. doi: 10.3389/fpls.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimatsu T, Endo K, Yazaki K, Sugiyama A. Secretion dynamics of soyasaponins in soybean roots and effects to modify the bacterial composition. Plant Direct. 2020;4:e00259. doi: 10.1002/pld3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calderon M, Perez-Delgado CM, Palove-Balang P, Betti M, Marquez AJ. Flavonoids and isoflavonoids biosynthesis in the model legume Lotus japonicus; Connections to nitrogen metabolism and photorespiration. Plants (basel) 2020;9:774. doi: 10.3390/plants9060774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta OP, Nigam D, Dahuja A, Kumar S, Vinutha T, Sachdev A, Praveen S. Regulation of isoflavone biosynthesis by miRNAs in two contrasting soybean genotypes at different seed developmental stages. Frontiers in Plant Science. 2017;8:567. doi: 10.3389/fpls.2017.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Ma Q, Chen Y, Tian B, Xu L, Bai Y, Chen W, Li X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME Journal. 2020;14:1915–1928. doi: 10.1038/s41396-020-0648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Chen JQ. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Science and Human Wellness. 2013;2:146–161. doi: 10.1016/j.fshw.2013.08.002. [DOI] [Google Scholar]
- Igiehon NO, Babalola OO. Rhizosphere microbiome modulators: Contributions of nitrogen fixing bacteria towards sustainable agriculture. International Journal of Environmental Research and Public Health. 2018;15:574. doi: 10.3390/ijerph15040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail B, Hayes K. Beta-glycosidase activity toward different glycosidic forms of isoflavones. Journal of Agricultural and Food Chemistry. 2005;53:4918–4924. doi: 10.1021/jf0404694. [DOI] [PubMed] [Google Scholar]
- Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. Journal of Nutrition. 2000;130:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- Jahan MA, Harris B, Lowery M, Coburn K, Infante AM, Percifield RJ, Ammer AG, Kovinich N. The NAC family transcription factor GmNAC42-1 regulates biosynthesis of the anticancer and neuroprotective glyceollins in soybean. BMC Genomics. 2019;20:149. doi: 10.1186/s12864-019-5524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman D, Forshey KL, Grimsrud PA, Ane JM. Leveraging proteomics to understand plant-microbe interactions. Frontiers in Plant Science. 2012;3:44. doi: 10.3389/fpls.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Kim YJ, Kim AT, Jang D, Kim MS, Seo DH, Nam TG, Rha CS, Park CS, Kim DO. Enrichment of polyglucosylated isoflavones from soybean isoflavone aglycones using optimized amylosucrase transglycosylation. Molecules. 2020;25:181. doi: 10.3390/molecules25010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Rha CS, Baik MY, Baek NI, Kim DO. A brief history and spectroscopic analysis of soy isoflavones. Food Science and Biotechnology. 2020;29:1605–1617. doi: 10.1007/s10068-020-00815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Badger TM, Ronis MJ, Wu X. Non-isoflavone phytochemicals in soy and their health effects. Journal of Agricultural and Food Chemistry. 2010;58:8119–8133. doi: 10.1021/jf100901b. [DOI] [PubMed] [Google Scholar]
- Kape R, Parniske M, Brandt S, Werner D. Isoliquiritigenin, a strong nod gene- and glyceollin resistance-inducing flavonoid from soybean root exudate. Applied Environmental Microbiology. 1992;58:1705–1710. doi: 10.1128/aem.58.5.1705-1710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenblum E, Dong Y, Szymanski J, Panda S, Jozwiak A, Massalha H, Meir S, Rogachev I, Aharoni A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:3874–3883. doi: 10.1073/pnas.1912130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizova L, Dadakova K, Kasparovska J, Kasparovsky T. Isoflavones. Molecules. 2019;24:1076. doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku YS, Ng MS, Cheng SS, Lo AW, Xiao Z, Shin TS, Chung G, Lam HM. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients. 2020;12:1717. doi: 10.3390/nu12061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes J, Skalicky M, Tumova L, Martin J, Hejnak V, Martinkova J. Vanadium elicitation of Trifolium pratense L. cell culture and possible pathways of produced isoflavones transport across the plasma membrane. Plant Cell Reports. 2019;38:657–671. doi: 10.1007/s00299-019-02397-y. [DOI] [PubMed] [Google Scholar]
- Kudou S, Fleury Y, Welti D, Magnolato D, Uchida T, Kitamura K, Okubo K. Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill) Agricultural and Biological Chemistry. 2014;55:2227–2233. [Google Scholar]
- Kushida M, Okouchi R, Iwagaki Y, Asano M, Du MX, Yamamoto K, Tsuduki T. Fermented soybean suppresses visceral fat accumulation in mice. Molecular Nutrition and Food Research. 2018;62:e1701054. doi: 10.1002/mnfr.201701054. [DOI] [PubMed] [Google Scholar]
- Lygin AV, Zernova OV, Hill CB, Kholina NA, Widholm JM, Hartman GL, Lozovaya VV. Glyceollin is an important component of soybean plant defense against Phytophthora sojae and Macrophomina phaseolina. Phytopathology. 2013;103:984–994. doi: 10.1094/PHYTO-12-12-0328-R. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Nakayasu M, Aoki Y, Yamazaki S, Nagano AJ, Yazaki K, Sugiyama A. Diurnal metabolic regulation of isoflavones and soyasaponins in soybean roots. Plant Direct. 2020;4:e00286. doi: 10.1002/pld3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8:754. doi: 10.3390/nu8120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M, Mejia SB, Cassidy A, Duncan A, Kurzer M, Nagato C, Ronis M, Rowland I, Sievenpiper J, Barnes S. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: a technical review of the observational and clinical data. Critical Review in Food Science and Nutrition. 2021 doi: 10.1080/10408398.2021.1895054. [DOI] [PubMed] [Google Scholar]
- Miadokova E. Isoflavonoids - an overview of their biological activities and potential health benefits. Interdisciplinary Toxicology. 2009;2:211–218. doi: 10.2478/v10102-009-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miladinovic J, Dordevic V, Balesevic-Tubic S, Petrovic K, Ceran M, Cvejic J, Bursac M, Miladinovic D. Increase of isoflavones in the aglycone form in soybeans by targeted crossings of cultivated breeding material. Scientific Reports. 2019;9:10341. doi: 10.1038/s41598-019-46817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Garrido P, Cabello E, Alonso A, Gonzalez C. Effects of estradiol and genistein on the insulin signaling pathway in the cerebral cortex of aged female rats. Experimental Gerontology. 2014;58:104–112. doi: 10.1016/j.exger.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Morgan HE, Dillaway D, Edwards TM. Estrogenicity of soybeans (Glycine max) varies by plant organ and developmental stage. Endocrine Disruptors. 2014;2:e28490. doi: 10.4161/endo.28490. [DOI] [Google Scholar]
- Mureşan L, Clapa D, Borsai O, Rusu T, WangTTY Park JB. Potential impacts of soil tillage system on isoflavone concentration of soybean as functional food ingredients. Land. 2020;9:386. doi: 10.3390/land9100386. [DOI] [Google Scholar]
- Nianiou-Obeidat I, Madesis P, Kissoudis C, Voulgari G, Chronopoulou E, Tsaftaris A, Labrou NE. Plant glutathione transferase-mediated stress tolerance: functions and biotechnological applications. Plant Cell Reports. 2017;36:791–805. doi: 10.1007/s00299-017-2139-7. [DOI] [PubMed] [Google Scholar]
- Olanrewaju OS, Glick BR, Babalola OO. Mechanisms of action of plant growth promoting bacteria. World Journal of Microbiology and Biotechnology. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO. Plant health: feedback effect of root exudates-rhizobiome interactions. Applied Microbiology and Biotechnology. 2019;103:1155–1166. doi: 10.1007/s00253-018-9556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa JC, Etcheverry S, Gambino D. Vanadium compounds in medicine. Coordination Chemistry Reviews. 2015;301:24–48. doi: 10.1016/j.ccr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A. Plant flavonoids–biosynthesis, transport and involvement in stress responses. International Journal of Molecular Science. 2013;14:14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: A review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9:324. doi: 10.3390/nu9040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Garcia MJ, Lucena C, Martinez-Medina A, Aparicio MA, Ramos J, Alcantara E, Angulo M, Perez-Vicente R. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Frontiers in Plant Science. 2019;10:287. doi: 10.3389/fpls.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar MAR, Watanabe S, Suzuki A, Hashimoto F, Anai T. Identification of novel MYB transcription factors involved in the isoflavone biosynthetic pathway by using the combination screening system with agroinfiltration and hairy root transformation. Plant Biotechnology (tokyo) 2019;36:241–251. doi: 10.5511/plantbiotechnology.19.1025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Kaur I, Kariyat R. The multifunctional roles of polyphenols in plant-herbivore interactions. International Journal of Molecular Science. 2021;22:1442. doi: 10.3390/ijms22031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalicky M, Kubes J, Hejnak V, Tumova L, Martinkova J, Martin J, Hnilickova H. Isoflavones production and possible mechanism of their exudation in Genista tinctoria L. suspension culture after treatment with vanadium compounds. Molecules. 2018;23:1619. doi: 10.3390/molecules23071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant Journal. 2006;48:261–273. doi: 10.1111/j.1365-313X.2006.02874.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama A. The soybean rhizosphere: Metabolites, microbes, and beyond-A review. Journal of Advanced Research. 2019;19:67–73. doi: 10.1016/j.jare.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Shitan N, Yazaki K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiology. 2007;144:2000–2008. doi: 10.1104/pp.107.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Ueda Y, Zushi T, Takase H, Yazaki K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLoS One. 2014;9:e100709. doi: 10.1371/journal.pone.0100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Yamazaki Y, Yamashita K, Takahashi S, Nakayama T, Yazaki K. Developmental and nutritional regulation of isoflavone secretion from soybean roots. Bioscience, Biotechnology and Biochemistry. 2016;80:89–94. doi: 10.1080/09168451.2015.1062714. [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Yamazaki Y, Hamamoto S, Takase H, Yazaki K. Synthesis and secretion of isoflavones by field-grown soybean. Plant and Cell Physiology. 2017;58:1594–1600. doi: 10.1093/pcp/pcx084. [DOI] [PubMed] [Google Scholar]
- Sukumaran A, McDowell T, Chen L, Renaud J, Dhaubhadel S. <>prenyltransferase. The Plant Journal. 2018;96(5):966–981. doi: 10.1111/tpj.14083. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takahashi S, Watanabe R, Fukushima Y, Fujita N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayam T. An isoflavone conjugate-hydrolyzing beta-glucosidase from the roots of soybean (Glycine max) seedlings: purification, gene cloning, phylogenetics, and cellular localization. Journal of Biological Chemistry. 2006;281:30251–30259. doi: 10.1074/jbc.M605726200. [DOI] [PubMed] [Google Scholar]
- Szeja W, Grynkiewicz G, Rusin A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Current Organic Chemistry. 2017;21:218–235. doi: 10.2174/1385272820666160928120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja I, Raju KS, Wahajuddin M. Dietary isoflavones as modulators of drug metabolizing enzymes and transporters: Effect on prescription medicines. Critical Reviews in Food Science and Nutrition. 2016;56:S95–S109. doi: 10.1080/10408398.2015.1045968. [DOI] [PubMed] [Google Scholar]
- Testa I, Salvatori C, Di Cara G, Latini A, Frati F, Troiani S, Principi N, Esposito S. Soy-based infant formula: Are phyto-oestrogens still in doubt? Frontiers in Nutrition. 2018;5:110. doi: 10.3389/fnut.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch NC. Isoflavonoids of the leguminosae. Natural Product Reports. 2013;30:988–1027. doi: 10.1039/c3np70024k. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang H, Li Y, Li Q, Yan W, Zhang Y, Wu Z, Zhou Q. Plant growth-promoting rhizobacteria isolation from rhizosphere of submerged macrophytes and their growth-promoting effect on Vallisneria natans under high sediment organic matter load. Microbial Biotechnology. 2021;14:726–736. doi: 10.1111/1751-7915.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D, Hara T, Fujita N, Waki T, Noguchi A, Takahashi S, Nakayama T. Transcription analyses of GmICHG, a gene coding for a beta-glucosidase that catalyzes the specific hydrolysis of isoflavone conjugates in Glycine max (L.) Merr. Plant Science. 2013;208:10–19. doi: 10.1016/j.plantsci.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Yu O, Jung W, Shi J, Croes RA, Fader GM, McGonigle B, Odell JT. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiology. 2000;124:781–794. doi: 10.1104/pp.124.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Bi X, Yu B, Chen D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients. 2016;8:361. doi: 10.3390/nu8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Liu YB, Peng J, Wang JH, Liu X. Changes of isoflavone profile in the hypocotyls and cotyledons of soybeans during dry heating and germination. Journal of Agricultural and Food Chemistry. 2009;57:9002–9010. doi: 10.1021/jf902248b. [DOI] [PubMed] [Google Scholar]
- Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Critical Reviews in Food Science and Nutrition. 2017;57:1280–1293. doi: 10.1080/10408398.2014.989958. [DOI] [PubMed] [Google Scholar]
- Zaklos-Szyda M, Budryn G. The effects of Trifolium pratense L. sprouts' phenolic compounds on cell growth and migration of MDA-MB-231, MCF-7 and HUVEC cells. Nutrients. 2020;12:257. doi: 10.3390/nu12010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ge Y, Han F, Li B, Yan S, Sun J, Wang L. Isoflavone content of soybean cultivars from maturity group 0 to VI grown in Northern and Southern China. Journal of American Oil Chemists' Society. 2014;91:1019–1028. doi: 10.1007/s11746-014-2440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Flavonoid transport mechanisms: how to go, and with whom. Trends in Plant Science. 2015;20:576–585. doi: 10.1016/j.tplants.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Zhu D, Hettiarachchy NS, Horax R, Chen P. Isoflavone contents in germinated soybean seeds. Plant Foods for Human Nutrition. 2005;60:147–151. doi: 10.1007/s11130-005-6931-0. [DOI] [PubMed] [Google Scholar]
- Zubieta C, He XZ, Dixon RA, Noel JP. Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nature Structural Biology. 2001;8:271–279. doi: 10.1038/85029. [DOI] [PubMed] [Google Scholar]