Summary
Lipids are a complex and diverse group of molecules with crucial roles in many physiological processes, as well as in the onset, progression, and maintenance of cancers. Fatty acids and cholesterol are the building blocks of lipids, orchestrating these crucial metabolic processes. In the liver, lipid alterations are prevalent as a cause and consequence of chronic hepatitis B and C virus infections, alcoholic hepatitis, and non-alcoholic fatty liver disease and steatohepatitis. Recent developments in lipidomics have also revealed that dynamic changes in triacylglycerols, phospholipids, sphingolipids, ceramides, fatty acids, and cholesterol are involved in the development and progression of primary liver cancer. Accordingly, the transcriptional landscape of lipid metabolism suggests a carcinogenic role of increasing fatty acids and sterol synthesis. However, limited mechanistic insights into the complex nature of the hepatic lipidome have so far hindered the development of effective therapies.
Keywords: Non-alcoholic fatty liver disease, hepatocellular carcinoma, cholangiocarcinoma, metabolomics, lipidomics
Abbreviations: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; ALD, alcohol-related liver disease; BAs, bile acids; CCA, cholangiocarcinoma; Cer, ceramide(s); CPT, carnitine palmitoyltransferase; DNL, de novo lipogenesis; ELOV1-6, elongation of very-long-chain fatty acids; FA, fatty acid; FABP, fatty acid-binding protein; FADS2, fatty acid desaturase 2; FAO, fatty acid oxidation; FASN, fatty acid synthase; FXR, farnesoid X receptor; HCC, hepatocellular carcinoma; HMGCR, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; HSCs, hepatic stellate cells; LA, linoleic acid; LPC, lysophosphatidylcholine; LXR, liver X receptor; MUFA, monounsaturated fatty acid; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PC, phosphatidylcholine; PPARs, peroxisome proliferator-activated receptors; PSC, primary sclerosing cholangitis; PUFA, polyunsaturated fatty acid; S1P, sphingosine-1-phosphate; SCD, stearoyl-CoA desaturase; SE, sterol esters; SFA, saturated fatty acid; SM, sphingomyelin; SREBP, sterol regulatory element-binding protein; TERT, telomerase reverse transcriptase; TG, triglycerides; TLR, Toll-like receptor
Key points.
-
•
Lipidomic alterations are a common feature of primary liver cancers (hepatocellular carcinoma and cholangiocarcinoma) and their risk factors.
-
•
Unique changes in the lipid landscape of hepatocellular carcinoma and cholangiocarcinoma allow for differential diagnosis of these malignancies.
-
•
Hepatocellular carcinoma and cholangiocarcinoma show differential dependency on de novo lipogenesis.
-
•
Transcriptional deregulation of lipid metabolism differs between hepatocellular carcinoma and cholangiocarcinoma.
Introduction
Liver cancer is the fourth leading cause of cancer-related deaths worldwide,1 and incidence and mortality rates are steadily increasing.2 It is estimated that, by 2025, more than 1 million people will be affected by primary liver cancer annually,3 posing a severe health challenge and societal burden. The most frequent types of primary liver cancer are hepatocellular carcinoma (HCC), accounting for up to 90%4 of all cases, and cholangiocarcinoma (CCA), accounting for 10-15%.5,6 The complex heterogeneity of these malignancies makes their early diagnosis and the development of therapies difficult. The common risk factors for liver cancer development are chronic HBV4 and HCV infections (whose frequency has decreased considerably due to successful vaccination programmes and antiviral drugs),7 alcohol abuse,1 and metabolic diseases including non-alcoholic fatty liver disease (NAFLD),8 ranging from simple steatosis to non-alcoholic steatohepatitis (NASH),8 obesity,1 and diabetes mellitus.1 Additional risk factors include aflatoxin exposure in HCC,1 and inflammation of the biliary tract in CCA,9 with underlying causes including primary sclerosing cholangitis (PSC), cholestasis, bile stones and liver fluke infestation.
Metabolic alterations are a well-established hallmark of cancer.10 The liver is the central organ for metabolism in the body[11], [12], [13], [14], [15]; thus, metabolic processes are often highly altered in liver cancer (reviewed in16). Distinct metabolic alterations have been uncovered in glucose, nucleotide, amino acid, and lipid metabolism in liver cancer.16 Dysregulation of lipids plays important roles in both the development17 and the progression18 of liver cancer, which is a consequence of lipids being a vast and multifarious group of complex structured biomolecules. Lipids are involved in diverse biological processes in the body from energy storage19 and metabolism,20 to epigenetic regulation,21 signal transduction,22 immunoregulation,23 inflammation,24 and cell-cell recognition.25
The study of the lipidome and its dynamic nature used to pose a significant technical challenge. However, advances in mass spectrometry and chromatography techniques in the past decade have provided deeper insights into the metabolic heterogeneity and biological function(s) of the lipidome in both normal homeostasis and disease.[26], [27], [28], [29] In this review, we will highlight the major lipidomic rearrangements that occur in the development and progression of liver cancer, focusing on lipids structural function and roles in energy storage and signal transduction.
The origin and role(s) of hepatic lipids
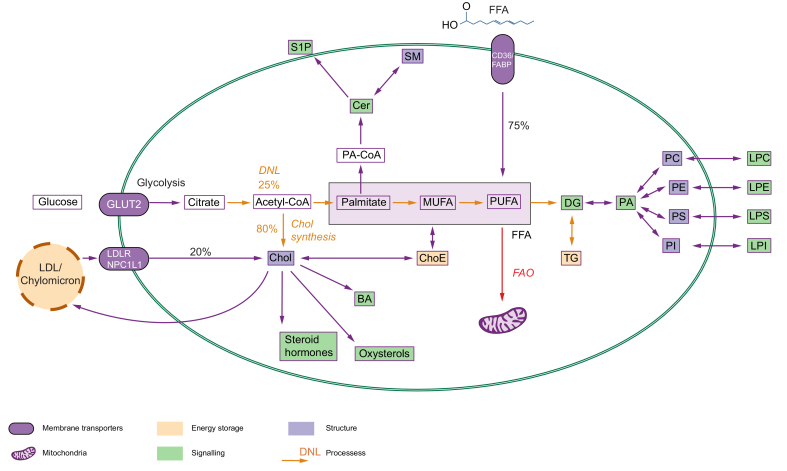
Fatty acids (FAs), including carboxylic acids with a chain from 2 to 36 carbon atoms,28 and cholesterol, consisting of 4 linked hydrocarbon rings,30 are the fundamental building blocks of all lipids. The hepatic FA pool is mainly dependent on the FA uptake of serum non-esterified FAs from dietary sources31 (in the fed state) or adipose tissue lipolysis31,32 (in the fasting state) (Fig. 1). However, 15-25% of all FAs originate from a process termed de novo lipogenesis (DNL).32,33 This process allows for FA synthesis up to the Δ9 position, while other FAs need to be taken up from dietary sources.34,35 Contrary to FAs, the majority (80%) of cholesterol is synthesised internally, and almost 50% of cholesterol synthesis is controlled by the liver.36 With a body mass of 70 kg, a human contains around 100 grams of cholesterol with a synthesis rate of 1.2 grams per day.37 Whereas cholesterol can be sufficiently synthesised, the dietary intake can range from 300-500 mg per day.37 FA and cholesterol are the backbone of a very diverse group of biomolecules that can be classified based on their structure, chemical properties (such as hydrophobicity or hydrophilicity), and biological function(s)30 (Fig. 1).
Fig. 1.
Simplified representation of the major metabolic pathways responsible for the uptake, transport, synthesis, and utilisation of lipids in the liver.
FA and cholesterol are the building blocks of most complex lipids. They can either be synthesised (orange arrows) via DNL (up to 25% of FA pool) and cholesterol synthesis (up to 80% of cholesterol pool) or they can be taken directly from the circulation. FA are subjected to FAO (red arrow) via a series of catabolic reactions, which are carried out in the mitochondria to generate ATP or used to form complex lipids. Lipids play structural (blue), signalling (green) or energy storage (yellow) functions. BA, bile acids; Cer, ceramides; Chol, cholesterol; ChoE, cholesterol esters; DG, diglyceride; DNL, de novo lipogenesis; FAO, fatty acid oxidation; FFA, free fatty acids; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphoinositide; LPS, lysophosphatidylserine; MUFA, monounsaturated FA; PA, phosphatidate; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphoinositide; PS, phosphatidylserine; PUFA, polyunsaturated FA; S1P, sphingosine-1-phosphate; SM, sphingomyelin; TG, triglyceride.
Energy storage
The human body stores energy as fat and carbohydrates. The neutral storage of FAs in the healthy liver is in the form of triglycerides (TGs), which are 3 FAs attached to a glycerol moiety, and sterol esters (SEs), in which FA is esterified to sterol.30 Neutral lipids (SEs and TGs) are stored in lipid droplets, and in a healthy liver, these lipids should not exceed 5%.38 FAs stored in TGs and SEs can be utilised at any time during liver homeostasis to generate energy (ATP) via fatty acid oxidation (FAO) or be transported to other organs in very-low-density lipoprotein.
Structural lipids
Glycerophospholipids, sphingolipids, and cholesterol are major building blocks of the cellular membrane (Fig. 1). Glycerophospholipids include phosphatidylcholine (PC), phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, and phosphatidic acid. PC accounts for more than 50% of the phospholipids in most eukaryotic membranes.39 The second most abundant lipid in the mammalian membrane is cholesterol, which accounts for 30% of lipids, and increases the lipid-packing density to maintain a high membrane fluidity.40 Lastly, sphingomyelin (SM) is the most abundant sphingolipid in mammalian cells and this lipid plays a crucial role in the formation of sterol-enriched ordered membrane domains and in cell-cell signalling.39
Signalling molecules
Lipids act as first (extracellular) and second (intracellular) messengers in signal transduction and molecular recognition processes (reviewed in39,41). As such, membrane glycerolipids and sphingolipids transduce signals through hydrolysis to generate bioactive molecules: ceramides and sphingosine-1-phosphate (S1P),42 while steroids (oxysterols, bile acids [BAs], steroid hormones) and FAs interact directly with receptors,[43], [44], [45], [46], [47] such as CD36 (FA translocase) (Fig. 1).
Lipid alterations in liver diseases associated with the development of liver cancer
Prominent steatosis is caused by FA uptake and DNL exceeding FAO and secretion,[48], [49], [50] and is a shared feature underlying several risk factors of liver cancer. Accordingly, increased intrahepatic lipid accumulation is observed in viral hepatitis,51,52 alcoholic hepatitis,53 and among individuals suffering from metabolic diseases (obesity,54 diabetes,55 and NAFLD56,57), all of which pose a risk for liver cancer development. Conversely, steatosis is rarely observed alongside PSC or primary biliary cholangitis,58 which are conditions associated with BA deregulation.59,60
Viral hepatitis
HBV and HCV infections are important risk factors for liver cancer development. HBV is the main aetiology for HCC in most regions of Asia, Africa, and South America. HCV is the predominant cause in Western Europe, North America, and Japan.1
Hepatic steatosis is often associated with both HBV and HCV infections,51,52,61 as well as being observed in HBx (HBx protein is crucial in HBV tumorigenesis) transgenic62,63 and HCV transgenic mouse models.64 Hence, viral hepatitis leads to prominent changes in both the serum61 and hepatic (tumour and tumour-adjacent compartments)65 lipidomes (Table 1). Indeed, the blood FA composition is significantly altered in HBV- and HCV-infected patients. As such, serum levels of saturated FAs (SFAs) and monounsaturated FAs (MUFAs) are significantly increased[66], [67], [68] in HBV-positive patients, and increase in parallel with disease severity.67,69 Concurrently, the class of polyunsaturated FA (PUFAs) is depleted.[66], [67], [68] This change in FA composition is also observed in mouse HBV-positive liver tumours.70 Moreover, PUFAs are necessary for HCV particle replication71 as knockdown of fatty acid desaturase 2 (FADS2) - the first step in PUFA synthesis – impairs HCV virus particle production. Similarly, FAs are involved in stabilisation of the HBx protein.72 HBx can also increase the cholesterol levels in HCC cells, both in vitro73 and in vivo.74 Conversely, in both patients and HCV transgenic mice, cholesterol,66,67 TG,66 and lysophosphatidylcholine (LPC)75,76 of longer FA chains have been shown to be significantly depleted.77
Table 1.
Deregulation of blood (and tissue) lipids as risk factors for liver cancer, HCC and CCA.
| Serum/plasma |
Tissue |
||||||
|---|---|---|---|---|---|---|---|
| Lipid | Viral hepatitis | Alcoholic hepatitis | NAFLD | PSC59 | CCA59 | HCC | HCC T vs. SL |
| SFA | ↑[66], [67], [68] | ↑82 | ↑86,87,91,92 | = | = | ↑117,118,121 | ↑118 |
| MUFA | ↑[66], [67], [68] | ↑82 | ↑86,87,91,92 | = | = | ↑117,118,121 | ↑118 |
| PUFA | ↓[66], [67], [68] | ↑82 | ↑86,87,91,92 | = | = | ↓90,118,122 | ↓ |
| TG | ↓66 | ↑82 | ↑86,87,91,92,99,100 | ↑ | = | ↑90 | ↓216 |
| Cholesterol | ↓66,67 | ↑ | ↑ | ↑122,134,140,141↓146 | |||
| BA | ↑86,87,91,92 | ↑ | ↑ | ↑59,90 | ↓311 | ||
| Cholesterol ester | ↑ | ↓ | ↑90 | ↑216,249 | |||
| LPC | ↓75,76 | ↓82 | ↓100,103 | ↑ | ↓ | ↓135 | = |
| PC | ↓100,103 | ↑134 | |||||
| Ceramide | ↑86,87,91,92,107,312 | = | = | ↑123 | ↑124,132 ↓132 |
||
| S1P | ↑ | ↑ | ↑123 | ↑[126], [127], [128] | |||
| Sphingomyelin | ↓ | ↑135 | ↑134 | ||||
Data for PSC and CCA are based on single reference (Banales et al.59)
↑Upregulated metabolites; ↓ downregulated metabolites.
BA, bile acids; CCA, cholangiocarcinoma; HCC, hepatocellular carcinoma; LPC, lysophosphatidylcholine; MUFA, monounsaturated fatty acid; NAFLD, non-alcoholic fatty liver disease; PC, phosphatidylcholine; PSC, primary sclerosing cholangitis; PUFA, polyunsaturated fatty acid; S1P, sphingosine-1-phosphate; SFA, saturated fatty acid; SL, surrounding liver; SM, sphingomyelin; T, tumour; TG, triglyceride.
Alcohol-related liver disease
Excessive alcohol consumption is the main aetiological factor for liver cancer development in Central and Eastern Europe.1 In patients with alcohol-related liver disease (ALD), FAs that accumulate in the liver are predominantly released from the adipose tissue.78 Ethanol increases the uptake of FAs by the liver in vivo79 and in vitro80 leading to intrahepatic accumulation of TGs.81 Abstinence has been shown to reduce serum FA and LPC levels, while TGs stay elevated82 in patients with ALD (Table 1). Moreover, in several models, FA synthesis pathways are significantly upregulated in mice fed alcohol ad libitum in their drinking water.[83], [84], [85]
Non-alcoholic fatty liver disease
NAFLD, ranging from steatosis to its progressive form NASH, is the most common liver disease in the developed world,1 and is an important risk factor for liver cancer development. NAFLD is associated with prominent changes in both the hepatic and serum lipidomes at the onset of steatosis, but as the disease progresses to NASH, only certain TGs[86], [87], [88] and steroids89 change progressively. However, NAFLD-HCC is reflected by a complete rearrangement of the serum lipidome90 (Table 1). Patients with NAFLD have significantly increased levels of FAs, TGs, ceramides (Cer), and BAs, while phospholipids in the blood are depleted.86,87,91,92 In NAFLD, the most important metabolic dysregulation is a result of high lipolysis and non-esterified FAs released into the bloodstream.93 Hepatic FA profiles in patients with NAFLD are severely deregulated.86,92 Specifically, SFAs and PUFAs are significantly increased in NAFLD compared to normal livers.92 Conversely, in murine models, a higher consumption of n-6 FAs leads to the onset of NASH by inducing mitochondrial dysfunction and altered apoptosis.94 Furthermore, palmitic acid and linoleic acid (LA) have been found to modulate the immune response in murine models of NASH.95 Palmitic acid and LA stimulate neutrophils95 as well as macrophages24 to express and secrete inflammatory proteins (for example, interleukin-6, interleukin-10, chemokine (C-C motif) ligand 2, interferon-γ, and tumour necrosis factor). LA also upregulates carnitine palmitoyltransferase (CPT) leading to increased apoptosis of CD4+ T cells.96 This LA-mediated loss of intrahepatic CD4+ T cells, but not CD8+ T lymphocytes, results in HCC progression.97 On a background of NASH, CD8+ T cells promote the incidence of murine HCC because of impaired tumour surveillance and increased tissue damage by lymphocytes.98
NAFLD is characterised by a significant increase of TGs in the circulation86,99,100 and liver.86,92 These are TGs with longer carbon chains and fewer double bonds.92,101,102 Among the lipids increased in both the blood and livers of NAFLD patients are Cer,86,103,104 with an increase in dihydroceramides105,106 that are basic markers of de novo Cer synthesis.107 As such, murine models have shown a decrease in hepatic steatosis when levels of liver Cer are lowered by an increase in acid ceramidase activity108 or deletion of dihydroceramide desaturase 1109. Moreover, several studies have shown that BA levels are increased in the liver,110,111 plasma91,110,112 and faeces112 of patients with NASH. Elevated plasma levels of glycocholate, taurocholate, and taurochenodeoxycholate91,110 are associated with progressive liver deterioration and dysfunction. Furthermore, increased levels of cholic, chenodeoxycholic, and deoxycholic acids are present in liver tissue,111 leading to altered expression and activity of genes involved in BA, lipid and carbohydrate metabolism, energy expenditure, and inflammation.113 Meanwhile, PCs and LPCs (particularly classes that contain PUFAs) are depleted in livers86 and blood obtained from patients with NAFLD100 and NASH.103 Interestingly, sphingolipids, phospholipids, and TGs are putative biomarkers of NAFLD progression.87,103 Furthermore, cholesterol promotes NAFLD development.114
Primary sclerosing cholangitis
Previously, studies with relatively limited sample sizes (n <30) have investigated lipidomic changes in patients with PSC compared to healthy individuals.59,115,116 The most comprehensive lipidomic study59 has shown prominent changes in patients with PSC compared to healthy individuals, reporting over 150 altered metabolites. Overall, serum obtained from patients with PSC comprised augmented levels of BAs, phosphatidylethanolamines, PCs and LPCs, and lysophosphatidylinositols, as well as decreased levels of some FA, SM and TG species (Table 1). Previously, FA deregulation has been observed in PSC.115 The liver plays a major role in cholesterol clearance via BA secretion; thus, it is not surprising that BAs are among the most deregulated lipids.59,60,115,116 Particularly, taurine and glycine conjugates of primary BAs have been found elevated in patients with PSC compared to non-cholestatic individuals.59,60,115,116
Deregulation of lipid metabolism in liver cancer
Deregulated lipid metabolism has been strongly associated with the onset and progression of HCC in several epidemiological studies90,[117], [118], [119] as well as in in vitro and in vivo modelling (Table 1). In comparison, CCA lipidomic studies are currently limited to biomarker discovery16,59,120; thus, a comprehensive investigation of the biliary tract and CCA lipidome landscapes are still lacking.
Lipidomic landscape is deregulated in liver cancer
Several lipidomic studies have investigated the blood lipidome to understand the progressive nature of CCA,59 HCC61,67,75,76,117,121 of viral origin, and more recently NAFLD-HCC.90 As such, the FA composition in the circulation dynamically changes as the liver deteriorates and progresses towards HCC59 (this is not seen in CCA). Several SFAs and MUFAs are increased during disease progression: chronic hepatitis -> cirrhosis -> HCC.117,121 Particularly, the MUFAs (16:1) and (18:1) progressively increase during development of viral-associated HCC117,118; however, these observations have not been corroborated in NAFLD-HCC.90 Conversely, serum levels of PUFAs are decreased in the blood of patients with HCC.90,118,122
Sphingolipids are an important lipid class that is upregulated in HCC90,123,124 and CCA.125 As such, S1P, a biologically active sphingolipid, has been shown to promote cell proliferation, migration, invasion, and epithelial-to-mesenchymal transition (EMT) in HCC[126], [127], [128] as well as lymph node metastasis in CCA.125 Accordingly, sphingosine-1-phosphate receptor (S1PR) could be a potential therapeutic target in HCC, as it is known to promote HCC invasion[129], [130], [131] and progression131 (Fig. 2). Similarly, Cer as a class accumulate in the serum of patients with HCC,123 but the function of specific Cer remains unknown and contradictory.132,133 Furthermore, an increase of SM (40:1) in mice134 and SM (18:2/24:1) in patients with HCC,135 as well as the utility of SM as a biomarker in distinguishing HCC and CCA59 suggest that sphingolipid metabolism may present a therapeutic target in liver cancer.136,137
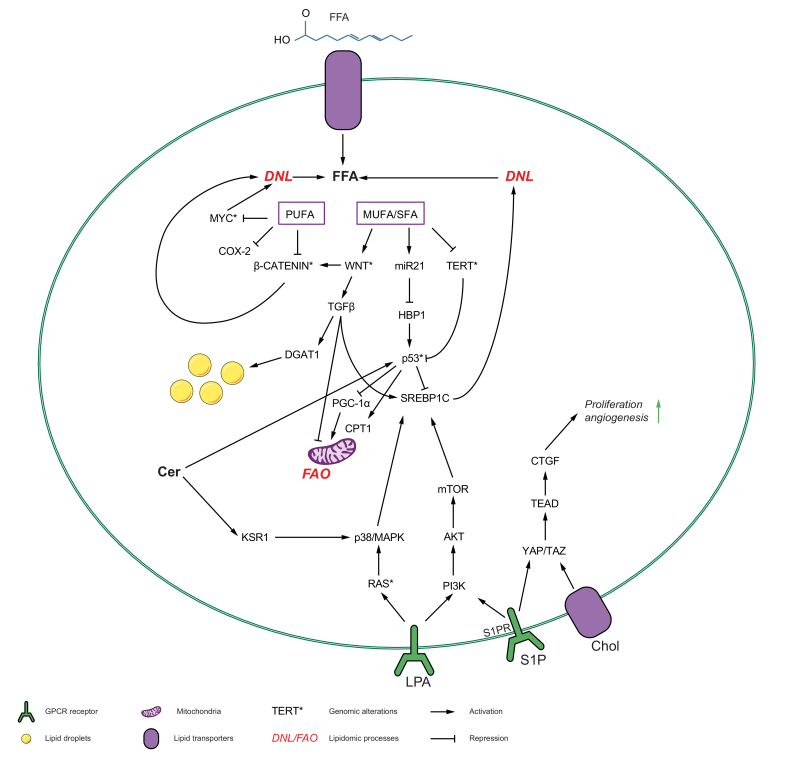
Fig. 2.
The interplay between lipid metabolism and oncogene pathways that leads to tumorigenesis in the liver.
Various lipids influence and are influenced by the recurrently deregulated oncogenic pathways, p53, RAS/MAPK, PI3K/AKT/mTOR signalling axis, Wnt/β-catenin signalling axis, TGF-β signalling axis and myc and TAZ/YAP pathways to cause hepatocarcinogenesis. The inhibited genes are marked with (⊥) and activated with (↓). The recurrent molecular alterations in oncogenes are marked with (∗). CL, cardiolipin; CTGF, connective tissue growth factor; DNL, de novo lipogenesis; FA, fatty acid; GlcCer, glucosylceramide; mTOR, mammalian target of rapamycin; NPC1, NPC intracellular cholesterol transporter 1; PI3K, phosphoinositide 3-kinase; PDK1, phosphoinositide-dependent protein kinase 1; S1P, sphingosine-1-phosphate; SL, sphingolipid; TG, triglycerides; TGF-β, transforming growth factor-β; YAP, Yes-associated protein.
Phospholipids are also significantly implicated in hepatocarcinogenesis.133,138 MUFA-PCs accumulate in HCC tumours,134 while PUFA-PCs and SFA-PCs are depleted. Moreover, MUFA-PCs are associated with a switch in the proliferative capacity of hepatocytes and with the onset of HCC.134 Additionally, LPCs, a highly anti-inflammatory class of molecules, are progressively decreased during chronic hepatitis and HCC onset.75,139 LPCs are highly upregulated in PSC, but significantly depleted in CCA,59 which follows the opposite trajectory compared to HCC. We can only speculate that LPCs are increased in PSC as a response to bile duct inflammation.
Several studies have demonstrated that a high-fat, high-cholesterol diet can trigger HCC in mice,122,134,140,141 via a neoplastic transformation of hepatocytes caused by broad transcriptional deregulation of genes involved in metabolic pathways (`metabolism in cancer´ hallmark) and calcium signalling.140 It has been shown that statins, which block hepatic cholesterol synthesis, protect against HCC142 and CCA143 development, as well as resulting in decreased risk of mortality.144 On the other hand, a higher serum cholesterol level was shown to be reflective of conserved liver function and decreased mortality.145 A recent population study showed that low serum cholesterol in patients (not using statins) was significantly associated with an increased risk of developing HCC.119 Interestingly, in patients with HCV-associated HCC, serum cholesterol levels and genes involved in the cholesterol synthesis pathway are significantly reduced.61 High serum levels of cholesterol suppress HCC tumorigenesis through the activation of natural killer cells146; however, further studies are necessary to understand this disagreement. This may not be the situation in CCA, where high levels of cholesterol have been observed in the sera of patients.147
The nature and regulation of BAs is less controversial than that of cholesterol itself. Conjugated primary BAs are significantly elevated at the stage of cirrhosis and continue to increase with the progression of HCC.148,149 The role of BAs in the development of HCC is well-established and was extensively reviewed elsewhere.150 Fundamentally, BAs activate farnesoid X receptor (FXR) and G-protein coupled BA receptor 1 (GPBAR1) that both control numerous oncogenic processes, including inflammation, oxidative stress, and the regulation of many cancer-related genes.150 BAs act through FXR in different cell types and organs, including the gut,151 hepatocytes,152 hepatic stellate cells (HSCs)152 and immune cells.153 Conversely, a stepwise increase in plasma-conjugated BAs has been observed in the trajectory from healthy control to benign biliary disease, and further to CCA.154 Indeed, conjugated BAs promote the growth of CCA through control and activation of the NF-kB pathway and decreased expression of FXR.155,156
Lastly, steroid hormones play an important role in hepatocarcinogenesis.157,158 Oestrogen shows a significantly protective effect against HCC,159 while elevated serum oestrogen and high expression levels of oestrogen-related proteins are associated with CCA160,161 and poor clinical outcomes.161 Thus, tamoxifen may potentially aid CCA therapy.162 As such, the use of oral contraception is associated with an increased risk of CCA,158 but not HCC development.163
Lipids reshape oncogenic processes
While many studies have investigated how alterations of lipids alter transcriptomic processes, there are still gaps in our knowledge of how specific lipids alter the metabolic landscape in liver diseases. Herein, we will highlight how specific lipids, acting as signalling molecules, may promote tumorigenesis (Fig. 2, Table 2). We will focus on alterations in cancer cells, as lipid reprogramming of immune cells in the tumour microenvironment has been reviewed elsewhere.164
Table 2.
Ability of various lipids to promote or inhibit tumour growth in HCC and CCA.
| Lipid | Impact | Mechanism | Ref |
|---|---|---|---|
| Fatty acids | |||
| SFA | Telomere shortening in obesity | Increase DNA methylation in TERT promoter | 123,165 |
| Oleic acid | Promote HCC | deregulate p53 signalling via miR21-HBP1 axis | 132,168 |
| Palmitoleic acid (16:1) | Promote HCC | Rising lipolysis and increased oxidative stress via mitochondrial beta oxidation, as well as increases insulin sensitivity, Wnt and TGFβ activation | 117,118 |
| Linoleic acid (18:2) | Promote NAFLD-HCC | Linoleic acid upregulates CPT1 to induce CD4(+) T cell apoptosis, hence mitochondrial function disruption along with increased FADS2 expression in tumours | 96,97,118 |
| Vaccenic and erucic acid | Promote NAFLD-HCC | Upregulation of SCD and ELOVL6 causes upregulation of these fatty acids | 117 |
| n-3 PUFA | Inhibit CCA | n-3 PUFAs suppressed c-Myc and inhibits CCA tumour growth | 171 |
| EPA, DHA |
Inhibit HCC |
Simultaneous inhibition of COX-2 and beta-catenin |
313 |
| Glycerophospho-lipids | |||
| Cardiolipin |
Promote HCC |
mTORC2 promotes cardiolipin synthesis, leading to accumulation in liver and then tumorigenesis, most probably via enhanced oxidative phosphorylation |
124 |
| Phospholipids | |||
| LPA (16:0) | Promote HCC | LPCs are metabolized by phospholipase D to produce LPAs that are potent mitogens, mediating their tumorigenic effect by the PI3K/AKT/mTOR signalling pathway or p38 MAPK signalling pathway | [175], [176], [177],314 |
| LPC (20:4) | Inhibit HCC | Low LPC indicate inflammatory and oxidative stress, through apoptosis induction through the death ligands (Fas and/or TNF-alpha) pathway | 135,178 |
| MUFA-PC |
Promote HCC |
Increased lipogenesis, fatty acyl desaturation, de novo synthesis of PC, and PC remodeling and decreased β-oxidation |
134 |
| Sphingolipids | |||
| S1P | Promote HCC and CCA | S1P activates YAP, PI3K/AKT and TFG-β1 production in HCC cells | [125], [126], [127], [128] |
| Glucosylceramide | Promote HCC | mTORC2 promotes glucosylceramide accumulation which increases tumorigenesis | 124 |
| (SM) 18:2/24:1 | Promote HCC | Enrichment might be due to a specific diet; more studies are needed | 123,135 |
| C16 | Promote HCC | Long-chain ceramides may have proliferative effects for HCC, RAS activation, regulatory ligand of p53 | 123,135,172 |
| C12:0, 16:0, 18:1 and 24:1 ceramides |
Promote HCC |
Associated with cannabinoid receptor activation and SCD downregulation |
132 |
| Sterols | |||
| Cholesteryl ester | Promote NAFLD-HCC | PTEN/PI3K/AKT/mTOR signalling pathway is responsible for cholesteryl ester accumulation which then leads to tumorigenesis | 249 |
| Cholesterol | Promote HCC | Upregulation of TAZ to promote fibrotic NASH | 114,249 |
CCA, cholangiocarcinoma; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HCC, hepatocellular carcinoma; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; MUFA, monounsaturated fatty acid; NASH, non-alcoholic steatohepatitis; PC, phosphatidylcholine; PUFA, polyunsaturated fatty acid; S1P, sphingosine-1-phosphate; SM, sphingomyelin.
FAs play a crucial role in the regulation of several oncogenic processes (Fig. 2, Table 2). For instance, telomere length and telomerase reverse transcriptase (TERT) activity are affected by FAs.165 In obese children, the TERT promoter was shown to be hypermethylated, reducing TERT activity compared to in control individuals. Telomere length in leukocytes obtained from obese children showed a positive association with total SFAs and docosahexaenoic acid, as well as a negative association with the arachidonic acid to docosahexaenoic acid ratio.165 Conversely, TERT deficiency in murine models promotes hepatic injury, increasing steatosis upon high-fat diet feeding, and inhibits liver regeneration via the activation of the tumour suppressor p53-peroxisome proliferator-activated receptor gamma coactivator 1 alpha (p53-PGC1α) axis.166 However, TERT expression and its activity are restored with cancer initiation as 80% of HCCs exhibit TERT promoter mutations or gene amplifications.167 Moreover, oleic acid upregulates miR21, which promotes steatosis, G1/S transition and proliferation through the miR21-HMG-box transcription factor 1 (HBP1)-p53 axis.168 In ALD, hepatic FAs suppress the LAMP-2 (lysosome-associated membrane protein 2) autophagy flux pathway through ER stress signalling and increase hepatic injury169 (Table 2). Furthermore, palmitic acid treatment leads to transcriptional upregulation of Wnt and transforming growth factor-β (TGF-β) signalling, activating EMT.170 In CCA, it has been demonstrated in vitro that n-3 PUFAs may inhibit c-myc expression.171
Among sphingolipids, Cer16 has been shown to be an important activator of the p53 pathway.172 Cer16 binds the DNA-binding domain of p53 and disrupts its complex with MDM2 (mouse double minute 2), leading to p53 accumulation and transcriptional activation, contributing to the apoptotic process and hepatic liver injury.123 Indeed, Cer16 is significantly upregulated in serum123 and tumour tissues obtained from patients with HCC.173 Moreover, Cer activates KSR1 (kinase suppressor of Ras 1), acting as a positive regulator of the RAS-RAF-MAPK pathway,174 which is frequently deregulated in liver cancers (Table 2).
Phospholipids can act as signal transductors as well as directly bind to G-protein-coupled receptors, with both oncogenic and tumour suppressive roles. Lysophosphatidic acid (16:0) is a potent regulator of the PI3K/Akt, mTOR (mammalian target of rapamycin), and p38 MAPK signalling pathways, increasing HCC cell migration, invasion, and adhesion.[175], [176], [177] Conversely, LPC (20:4) was shown to induce Fas and tumour necrosis factor-α pathways, resulting in apoptosis and thus playing a tumour suppressive role178 (Table 2).
Sterol lipids, particularly cholesterol, may play important roles in liver cancer. Increased cholesterol in hepatocytes upregulates the transcriptional regulator TAZ (WWTR1), and thus promotes NASH.114 Elevated TAZ activity leads to the synthesis and secretion of IHH (Indian hedgehog)179 and activation of HSCs.179 Currently, no association has been shown between TAZ and CCA (Table 2).
Collectively, these studies suggest that prominent lipidomic rearrangements occur before carcinogenesis and continue to play a role during liver cancer progression. Several types of lipids have been shown to interact with oncogenes, altering the signalling activity of many pathways, and likely contributing to tumour formation through these interactions. This emphasises possible opportunities for future preventative treatments.
Molecular alterations in liver cancer promote lipid remodelling
Metabolic stress contributes to increased reactive oxygen species levels that may result in mutational processes, with the accumulation of somatic mutations in chronic liver disease eventually leading to cancer. A recent study in patients with ALD and NAFLD showed that 3 master regulators of lipid processing and storage – FOXO1 (forkhead box protein O1), CIDEB (cell death inducing DFFA like effector B) and GPAM (glycerol-3-phosphate acyltransferase, mitochondrial) – are frequent targets of convergent somatic mutations.167 Recurrent mutations in RAS, TP53, MYC, and CTNNB1 have also been shown to alter the lipidome of liver tumours (Fig. 2).
Mutations in the RAS-RAF pathway are frequent in HCC167 and CCA,180 and lead to transcriptional activation of fatty acid synthase (FASN), which promotes lipogenesis.181 Furthermore, increased Wnt and Myc activities intensify FA desaturation and elevate unsaturated fatty acyl groups in phospholipids in a RAS-dependent manner. Through this metabolic reprogramming, stearoyl-CoA desaturase (SCD) was identified as a putative therapeutic target.182
Mutated TP53 is a dominant driver-gene in many cancers, including HCC167 and CCA,180 regulating cellular metabolism (reviewed in183). On the one hand, wild-type p53 is a potent repressor of sterol regulatory element-binding proteins (SREBP-1/2) that regulate DNL and cholesterol synthesis, respectively.184,185 On the other hand, wild-type p53 promotes FAO through expression of lipin 1, sirtuin 1, and CPT, maintaining lipid homeostasis in the liver.186,187
The role of the Wnt pathway in reprograming cancer metabolism has been extensively studied and reviewed elsewhere.188 Many of the recurrent mutations in this pathway are not druggable (though it has received significant attention in the area of small molecule development). However, exploiting the lipidomic changes inflicted by the mutations in Wnt could present an indirect therapeutic strategy. Overall, there is a convincing rationale to target the Wnt/β-catenin pathway in liver cancers189; however, the effect on lipid metabolism has received less attention. The activation of the Wnt pathway leads to the release of β-catenin, its translocation to the nucleus and consequently to increased expression of peroxisome proliferator-activated receptor-α (Pparα)190 resulting in increased FAO in this subset of HCC. Moreover, inhibition of Wnt/β-catenin may lead to downregulation of DNL and FA desaturation, which is frequently upregulated in liver cancer. Thus, further studies to investigate the effects of lipid-targeted therapies are warranted.
Transcriptionally deregulated lipid metabolism pathways
PPARs, SREBPs, and liver X receptors (LXRs) are key hepatic transcriptional regulators of enzymes involved in lipid metabolism (reviewed in191). As cofactors, lipids can bind directly to transcription factors, modulating the expression of lipid metabolism in a feedback loop. As such, PUFAs and 4-phenyl butyric acid have been shown to directly bind to PPARs,22,192 which can contribute to the development of HCC. It has been implied that the PPARα-SCD1 axis is important to maintain the stemness of HCC cells by promoting the nuclear accumulation of β-catenin.193 Furthermore, LXRs are activated by oxysterols and their activation can trigger lipotoxicity in liver cancer,194 while their inactivation leads to NAFLD-HCC development.195 As such, the transcriptomic landscape of lipid metabolism is significantly deregulated in liver cancer[196], [197], [198] (Fig. 3).
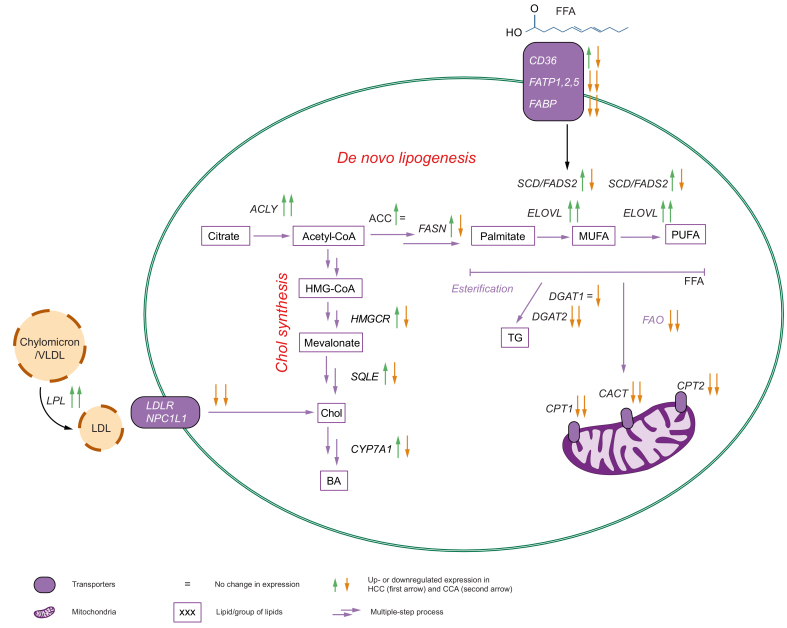
Fig. 3.
Transcriptionally deregulated lipidomic processes are different in HCC compared to CCA.
The alteration in gene expression profiles of tumour vs. surrounding tissue in HCC (first arrow) and CCA (second arrow). The upregulated genes are marked with red ↑, and downregulated with ↓. ACLY, acetyl-CoA by ATP citrate lyase; ACC, acetyl-CoA carboxylase; BA, bile acids; CCA, cholangiocarcinoma; Chol, cholesterol; DGAT1/2, diglyceride acyltransferase 1 and 2; DNL, de novo lipogenesis; ELOVLs, elongation of very-long-chain fatty acids; FADS2, fatty acid desaturase 2; FAO, fatty acid oxidation; FASN, fatty acid synthase; FATPs, fatty acid transport proteins; FFA, free fatty acids; HCC, hepatocellular carcinoma; HMGCR, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase; LPL, lipoprotein lipase; MUFA, monounsaturated FA; PUFA, polyunsaturated FA; SCD, stearoyl-CoA desaturase; SQLE, squalene epoxidase; TG, triglyceride.
FA uptake and transport
FAs are required for tumour cell proliferation to provide new phospholipids for plasma membranes. Their availability in serum is dependent on the dietary supply and TG lipolysis by lipoprotein lipase.199,200 FAs are intracellularly translocated through CD36170,201 and/or FA transporters of the SLC27 family.202 SLC27A1 and lipoprotein lipase are upregulated in CCA tumour tissue compared to the adjacent normal liver parenchyma, which suggests an increased demand and dependency on exogenous FAs in tumour tissue.203 In HCC, high CD36 expression is associated with diminished overall survival (TCGA KMplotter, p = 0.047). Furthermore, CD36-mediated uptake and trafficking via fatty acid-binding proteins (FABP1 and FABP4) correlate with increased EMT and activation of the Wnt/β-catenin and TGF-β signalling pathways170 (Fig. 3).
De novo lipogenesis and triglyceride synthesis
DNL is significantly upregulated in the proliferative class of HCC204,205 and it is predictive of patient prognosis.206 Therefore, rate-limiting genes of DNL, such as ATP citrate lyase (ACLY),207 acetyl-CoA carboxylase (ACC),[208], [209], [210] and FASN,17,[210], [211], [212], [213] are frequently upregulated in HCCs compared to the normal adjacent liver tissue. Interestingly, in CCA, tumour cells show lower dependency on DNL and instead a higher addiction to exogenous FAs.203 Thus, DNL as a process, and rate-limiting genes in particular, may present an attractive therapeutic option for obesity, NAFLD and HCC, but not CCA (Table 2). Furthermore, it has been suggested that, in HCC, DNL is glucose-derived209 and thus, restricting HCC cells access to glucose results in diminished DNL activity.214 However, a recent study implicated fructose and sucrose, rather than glucose, as substrates in DNL215 in the healthy liver. Thus, in HCC, the substrates driving DNL require comprehensive studies. Indeed, several HCC studies have focused on DNL inhibition as a therapeutic option, but such work remains in its infancy.206,208,216
ACLY is the first step in DNL and has been shown to promote HCC transcriptionally by interacting with NONO (non-POU domain-containing octamer binding protein).217 Overall, ACLY was shown to regulate stemness, migration, and invasion of HCC cells via the Wnt/β-catenin signalling pathway.207 In CCA, the expression of ACLY is higher in tumour tissues compared to the surrounding tissue,218 however, its role remains unknown.
Parts of acetyl-CoA are carboxylated to malonyl-CoA by ACC, which is the primary rate-limiting enzyme in this process. Either inhibition of ACC itself or deletion of AMPK-targeted ACC phosphorylation sites lead to a significant decreased tumour burden and attenuated DNL in diethylnitrosamine-induced HCC.208 Also, in vitro, these modifications result in decreased proliferation and viability of HCC cells.17 Accordingly, several inhibitors designed to block ACC activity and reduce lipogenesis have led to significant reductions in the accumulation of hepatic TG and activation of HSCs.219,220 Still, liver-specific ACC knockout in mice with diethylnitrosamine-induced HCC led to a significant increase in the tumour burden and altered redox regulation.206 Additionally, complete deletion of ACC1 and ACC2 abrogates acetogenic lipogenesis but fails to protect murine livers from increased lipid accumulation, likely due to inhibition of FAO as the compensatory mechanism.221 Taken together, these data suggest that ACC inhibition, but not deletion, could be a beneficial treatment option for patients with HCC.
The next step in DNL is palmitate synthesis, which is catalysed from malonyl-CoA by FASN. The role of FASN in HCC is dependent on the model. First, overexpression of FASN alone or in combination with either N-Ras, c-Met, or SCD1 is not sufficient to promote HCC.222 However, FASN expression is essential in the development of HCC in both the AKT222 and AKT/Ras203 models and FASN inhibition delays Pten/c-Met-driven HCC.216 As such, stabilisation of FASN by glyceronephosphate O-acyltransferase promotes DNL and formation of liver tumours in mice.212 Interestingly, FASN expression is dispensable for CCA formation in AKT/Notch intracellular domain 1- and AKT/Ras-driven models,203 and KDM5C-mediated repression of FASN was shown to correlate with reduced CCA cell proliferation and invasion.223
Finally, palmitate, which is the end-product of DNL, can undergo a series of desaturation and elongation reactions that are catalysed by SCD, FADS2, and elongation of very-long-chain fatty acids (ELOVL1-6), respectively. In HCC, upregulation of SCD has been comprehensively described in mice, rats, tumour cells, and patients.134,198,224,225 SCD has been shown to be crucial for proliferation of HCC cells,134,224 for development of HCC in mice,198,225 and is present at higher levels in more aggressive HCCs in patients.198 Additionally, a subset of HCC cell lines is metabolically flexible since they upregulate FADS2 and utilise this as an alternative desaturation pathway when SCD is inhibited,226 suggesting that targeting both desaturation pathways would be necessary to impair HCC growth. Furthermore, suppression of ELOVL6 in HCC cells led to reduced proliferation, tighter cell-cell junctions, and increased lipid accumulation,227 as well as reduced HCC tumour growth in vivo and increased survival.227
The role of TG synthesis in liver cancer development and progression remains elusive. It is implied that TG synthesis is downregulated in HCC.228 The formation of TG from acetyl-CoA and diacylglycerol is catalysed by evolutionarily unrelated enzymes (diglyceride acyltransferase [DGAT]1 and DGAT2) that are downregulated in HCC compared with matched normal tissues.228 Higher expression of DGAT2 results in longer overall survival.228 These data were corroborated in vitro and in vivo, demonstrating that overexpression of DGAT2 curbs cell proliferation and diminishes tumour growth.228 While comprehensive studies linking TG synthesis to HCC development and progression are lacking, overexpression of DGAT1 and DGAT2 in hepatocytes229,230 leads to steatosis and lipid accumulation,231 which is one of the key long-term causes of hepatocarcinogenesis. However, DGAT1 is important for the maintenance of HCC in vitro, silencing it reverts HCC cells to a dedifferentiated and stem cell-like phenotype.232 Interestingly, in vitro, if DGAT1 is silenced in HCC cells, the cells compensate by upregulating DGAT2.233 Hence, inhibition of DGAT2 in vivo234 ameliorated liver steatosis. Another less studied enzyme in the TG synthesis pathway is monoacylglycerol O-acyltransferase, which catalyses the synthesis of TGs, may contribute to hepatic steatosis in vivo235,236 and could be an important therapeutic target for the treatment of NAFLD.237
Fatty acid oxidation
CPT1 and CPT2 deliver long-chain FAs to the mitochondria for oxidation and thereby generate ATP and NADPH.238 In both HCC and CCA, the expression levels of CPT1 and CPT2 are downregulated (TCGA,239,240 www.firebrowse.org). In fact, downregulation of CPT2 was shown to protect against lipotoxicity241 in an E2F2-dependant manner in HCC.242 Furthermore, downregulation of acylcarnitine translocase (SLC25A20) in the mitochondrial matrix is observed in both HCC and CCA (TCGA, www.firebrowse.org) and was shown to suppress FAO and promote HCC proliferation as well as metastasis.243 Furthermore, enzymes in the FAO process, such as medium-chain acyl-CoA dehydrogenase244 and long-chain acyl-CoA dehydrogenase,245 have potential tumour suppressor roles in HCC.244,245 Therefore, FA utilisation for structural and messenger molecules, rather than storage or energy sources, supports HCC development and progression.
Cholesterol and BA synthesis
In addition to FA synthesis, other lipogenesis pathways have also been demonstrated to be deregulated during HCC development. The rate-limiting enzyme of cholesterol synthesis (and target of statins) 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) is upregulated in HCC246 and CCA247 and thus, the use of statins is associated with reduced risk of liver cancer development.143,248 Furthermore, increased expression of squalene epoxidase249 has been implicated in the development of NAFLD-HCC. Interestingly, a decreased ability to utilise circulating cholesterol was associated with increased HCC proliferation and metastasis.250 Furthermore, cholesterol synthesis was shown to support HCC growth in the absence of FASN,216 which indicates crosstalk between DNL and cholesterol synthesis. Similarly, increased cholesterol and BA synthesis, through PPARα activation, cause cholestasis, liver damage, and finally CCA development.251
Diagnostic potential of circulating lipids
Most lipids detected in human serum or plasma remain stable and are well correlated with the liver lipidome.86 Therefore, the lipidome in circulation is an attractive source of biomarkers.16,107
Several studies exploited serum FA as a diagnostic tool.88,252 Recently, it has been shown that a combination of several FAs and PC (18:2) can robustly distinguish patients with NAFLD-HCC from those with HCC of other aetiologies and non-cancerous controls,88 providing a potential tool for non-invasive surveillance.
The increased serum/plasma levels of TGs are a well-known biomarker of liver dysfunction in cholestasis, ALD, NAFLD, and HBV-,87,88,103,[253], [254], [255] but not HCV-associated hepatitis.256 Similarly, increased TG levels have been identified as risk factors for CCA.147,257 Furthermore, diminished levels of circulating TG in patients with HCC are associated with worse overall survival.258 However, due to the unspecific nature of these changes, they remain a generic biomarker of liver dysfunction. On the other hand, the changes in specific TG species may be more useful. The major differences observed with the development of NAFLD and its progression to NASH are increasing serum levels of saturated and monounsaturated TGs,87,103 many of which progressively increase with the onset of HCC,88,258 particularly in the absence of cirrhosis. Conversely, the depletion of cholesterol esters, another energy storage class of lipids, has diagnostic potential in HCC18,59 and CCA.59
Differences in the abundance of structural lipids have also been exploited, with specific sphingolipids having biomarker potential. As an example, following increased levels of C16-ceramides, S1P has been shown to distinguish patients with HCC from those with cirrhosis.18,259 The depletion of serum SMs in HCC could distinguish patients with HCC from healthy controls, while SMs were significantly altered between HCC and CCA and could thus distinguish between these malignancies.59 Furthermore, several LPCs have shown diagnostic value in CCA260 and HCC.18,75,259
The increase in lipidomic studies in liver cancer has led to an abundance of novel biomarkers, many of which show significantly higher diagnostic potential than alpha-fetoprotein or carbohydrate antigen 19-9. However, most of these studies lack external validation cohorts. Furthermore, most of these studies lack absolute quantification of metabolites, their reference ranges, and the cut-off values that could be considered as diagnostic. As a result, they have limited clinical value and require further development before clinical use.
Therapeutic opportunities
For decades, lipid metabolism has been an attractive target for the development of new therapies that could alleviate the burden of chronic liver diseases and hence reduce the risk of cancer. Furthermore, similar genes orchestrating lipid metabolism in chronic liver diseases have recently been investigated as therapeutic targets in cancer treatment (Table S1).
Treatment of underlying diseases to prevent liver cancer
Several inhibitors of DNL, FAO, or cholesterol synthesis have reached clinical trials and use (Table S1). In DNL, drugs that target ACC have progressed significantly. The ACC inhibitor firsocostat is the most prominent, having shown promising results in mice,261 as well as significantly reducing steatosis in patients with NAFLD in phase II clinical trials.[262], [263], [264] The FASN inhibitor TVB-2640 has also shown promise in humans211,265 as has the inhibitor cerulenin in mice.213 A PPAR inhibitor targeting the intracellular transport of FAs (lobeglitazone) has reached phase VI clinical trials in NAFLD,266 where it reduced intrahepatic fat content and improved glycaemic and lipid profiles in 38 patients with NAFLD and type 2 diabetes. Other PPAR inhibitors such as pioglitazone,267 IVA337 (lanifibranor)268 and elafibranor269 have also shown promise, but are at the earlier phases of clinical trials. Perhaps the most noteworthy inhibitors in NAFLD and PSC treatment are LXR and FXR agonists such as oltipraz,270 obeticholic acid,271,272 Px-104,273 and MET409.274 Interestingly, the FXR agonist obeticholic acid has shown promise as a therapeutic target in both NAFLD271 and PSC.272 Another noteworthy pathway is the cholesterol biosynthesis pathway; statins, which suppress HMGCR, have been studied in both NAFLD and PSC (Table S1) and successfully lowered LDL cholesterol in NASH.275 As far as sphingolipid metabolism is concerned, there have been no clinical studies yet, but the SK2 inhibitor K145276 and the S1P1R inhibitor fingolimid277 have ameliorated NAFLD in mouse models.
Targeting lipid metabolism in liver cancer treatment
Since lipids partake in liver cancer development and progression through various pathways and there is some progress targeting these in the pre-malignant liver, it is not surprising that there is considerable interest in exploiting lipid metabolism pathways in liver cancer treatment. Many pre-clinical studies have focused on DNL: the ACC inhibitor AICAR showed an anti-cancer growth effect in vitro,278 and ND-654 improved survival of HCC tumour-bearing rats.208 The FASN inhibitor orlistat has also displayed antitumor activity in vitro279,280 and in murine models, along with the inhibitors C75,281 triclosan21,282 and EGCG283 showing promise in vitro. The SCD inhibitor CAY10566 has also been successful in ameliorating HCC in vitro224,284 and in vivo285 and the DGAT inhibitor tussilagone reduced TG synthesis in vitro.286 Interestingly, sorafenib, which is an inhibitor of tyrosine kinases in HCC,287 targets liver cancer cells by acting on the SCD1 pathway in vitro288 and in human liver tumours289 and, in return, SCD inhibition sensitises the tumour to sorafenib treatment.289 Targeting FAO by CPT1 inhibition with etomoxir is another pathway that has successfully reduced HCC occurrence in vivo,190,[290], [291], [292] however these studies are limited to the pre-clinical setting (Table S1). Furthermore, the SREBP inhibitor betulin,293 the FXR agonist INT-767294 and simvastatin295 (a HMGCR and PPAR inhibitor) significantly ameliorate HCC in a pre-clinical setting. Several statins are under investigation in combination therapy for HCC (Table S1). Atorvastatin is in phase IV clinical trials for HCC but it is still at the recruiting phase (NCT03024684), while pravastatin in combination with sorafenib failed to improve patient outcomes.296
In CCA treatment, targeting sphingolipid metabolism or more specifically the enzyme sphingosine kinase 2 (encoded by the gene SPHK2) with the inhibitor ABC294640 has shown promise in vitro,297,298 in vivo299 and in a clinical trial setting.136 In addition, the ASBT inhibitor Bamet-UD2300 has shown significant anticancer effects in a pre-clinical setting, while the role of statins in CCA has not been investigated in a controlled clinical setting. Furthermore, obeticholic acid, which has shown promise in ameliorating NAFLD and PSC, managed to decrease proliferation of CCA cells in vitro.301
Dietary intervention as preventative and therapeutic strategy
In addition to drugs, dietary combination strategies may also be helpful.302 n-3 PUFAs have been implicated as a way to reduce hepatic steatosis and inflammation in ALD,109 NAFLD,303,304 and NASH.305 The supplementation of n-3 PUFAs has been shown in a meta-analysis to reduce HCC risk by up to 51%,306 results that have been mimicked in vitro307 and in mice.308 In fact, reducing the ratio (n-6:n-3) FAs in the diet has been shown to impair liver steatosis in vivo.309,310 Therefore, we can speculate that patients with HCC, particularly patients with underlying NAFLD or NASH aetiologies, may benefit from a dietary supplement in combination with their pharmacological therapy.
Conclusion and future perspectives
Despite the increasing interest in the investigation of lipidomic rearrangements in liver cancer, targeting lipid metabolism in a therapeutic setting has not been successful. The dynamic nature of the lipidome and lack of mechanistic insights into the role(s) of individual lipids in the development of liver cancer significantly hinder the development of new therapies. Our current knowledge allows us to exploit the human lipidome as a non-invasive diagnostic and prognostic tool; however, dissecting the mechanism of lipid metabolism and homeostasis in liver cancer development and progression is necessary. This review highlights the fact that while we are making great strides to unravel the role of lipids in the development and progression of liver cancer, directed mechanistic studies to understand lipids are necessary (Fig. 4).
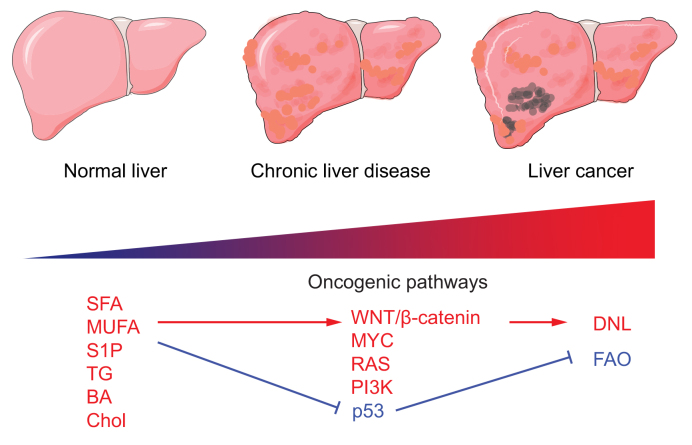
Fig. 4.
Outline of the lipidomic alterations in liver cancer progression.
Progression from the normal liver through chronic liver disease (hepatic steatosis to NASH) and into primary liver cancers. Oncogenic pathways activated by a change in different metabolic classes (SFA; MUFA; S1P; TG; BA; Chol) during neoplastic onset. Activation of DNL and p53-mediated inactivation of FAO. BA, bile acid; Chol, cholesterol; DNL, de novo lipogenesis; FAO, fatty acid oxidation; MUFA, monounsaturated fatty acid; NASH, non-alcoholic steatohepatitis; S1P, sphingosine-1-phosphate; SFA, saturated fatty acid; TG, triglyceride.
Financial support
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No 801481, Dansk Kræftforskningsfond (FID2014816). The laboratory of JBA is supported by competitive funding from the Novo Nordisk Foundation (14040, 0058419), Danish Cancer Society (R167-A10784, R278-A16638), NEYE foundation and the Danish Medical Research Council (1030-00070B).
Authors’ contributions
B.P. and M.L. researched data for the article; B.P., M.L, and J.B.A wrote and edited the manuscript before submission.
Conflict of Interest
Authors declare no conflicts of interest
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100479.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 4.Akinyemiju T., Abera S., Ahmed M., Alam N., Alemayohu M.A., Allen C., et al. Global Burden of Disease Liver Cancer C The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeOliveira M.L., Cunningham S.C., Cameron J.L., Kamangar F., Winter J.M., Lillemoe K.D., et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakeeb A., Pitt H.A., Sohn T.A., Coleman J., Abrams R.A., Piantadosi S., et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. discussion 473-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanwal F., Kramer J., Asch S.M., Chayanupatkul M., Cao Y., El-Serag H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005 e1001. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnusson I., Schumann W.C., Bartsch G.E., Chandramouli V., Kumaran K., Wahren J., et al. Noninvasive tracing of Krebs cycle metabolism in liver. J Biol Chem. 1991;266:6975–6984. [PubMed] [Google Scholar]
- 12.Diraison F., Large V., Brunengraber H., Beylot M. Non-invasive tracing of liver intermediary metabolism in normal subjects and in moderately hyperglycaemic NIDDM subjects. Evidence against increased gluconeogenesis and hepatic fatty acid oxidation in NIDDM. Diabetologia. 1998;41:212–220. doi: 10.1007/s001250050892. [DOI] [PubMed] [Google Scholar]
- 13.Large V., Brunengraber H., Odeon M., Beylot M. Use of labeling pattern of liver glutamate to calculate rates of citric acid cycle and gluconeogenesis. Am J Physiol. 1997;272:E51–E58. doi: 10.1152/ajpendo.1997.272.1.E51. [DOI] [PubMed] [Google Scholar]
- 14.Jones J.G., Solomon M.A., Cole S.M., Sherry A.D., Malloy C.R. An integrated (2)H and (13)C NMR study of gluconeogenesis and TCA cycle flux in humans. Am J Physiol Endocrinol Metab. 2001;281:E848–E856. doi: 10.1152/ajpendo.2001.281.4.E848. [DOI] [PubMed] [Google Scholar]
- 15.Jones J.G., Solomon M.A., Sherry A.D., Jeffrey F.M., Malloy C.R. 13C NMR measurements of human gluconeogenic fluxes after ingestion of [U-13C]propionate, phenylacetate, and acetaminophen. Am J Physiol. 1998;275:E843–E852. doi: 10.1152/ajpendo.1998.275.5.E843. [DOI] [PubMed] [Google Scholar]
- 16.Satriano L., Lewinska M., Rodrigues P.M., Banales J.M., Andersen J.B. Metabolic rearrangements in primary liver cancers: cause and consequences. Nat Rev Gastroenterol Hepatol. 2019;16:748–766. doi: 10.1038/s41575-019-0217-8. [DOI] [PubMed] [Google Scholar]
- 17.Calvisi D.F., Wang C., Ho C., Ladu S., Lee S.A., Mattu S., et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Chen J., Huang C., Li N., Zou L., Chia S.E., et al. Comparison of hepatic and serum lipid signatures in hepatocellular carcinoma patients leads to the discovery of diagnostic and prognostic biomarkers. Oncotarget. 2018;9:5032–5043. doi: 10.18632/oncotarget.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuerschner L., Moessinger C., Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9:338–352. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 20.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D., Zhao T., Long K., Wu M., Zhang Z. Triclosan down-regulates fatty acid synthase through microRNAs in HepG2 cells. Eur J Pharmacol. 2021;907:174261. doi: 10.1016/j.ejphar.2021.174261. [DOI] [PubMed] [Google Scholar]
- 22.Chen S.Z., Ling Y., Yu L.X., Song Y.T., Chen X.F., Cao Q.Q., et al. 4-phenylbutyric acid promotes hepatocellular carcinoma via initiating cancer stem cells through activation of PPAR-alpha. Clin Transl Med. 2021;11:e379. doi: 10.1002/ctm2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek A.E., Yu Y.A., He S., Wardell S.E., Chang C.Y., Kwon S., et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864. doi: 10.1038/s41467-017-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu H., Tang B., Lang J., Du Y., Cao B., Jin L., et al. High-fat diet promotes macrophage-mediated hepatic inflammation and aggravates diethylnitrosamine-induced hepatocarcinogenesis in mice. Front Nutr. 2020;7:585306. doi: 10.3389/fnut.2020.585306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima N., Hakomori S. Synergistic effect of two cell recognition systems: glycosphingolipid-glycosphingolipid interaction and integrin receptor interaction with pericellular matrix protein. Glycobiology. 1991;1:623–630. doi: 10.1093/glycob/1.6.623. [DOI] [PubMed] [Google Scholar]
- 26.Han X., Gross R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Zech T., Ejsing C.S., Gaus K., de Wet B., Shevchenko A., Simons K., et al. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–476. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmelzer K., Fahy E., Subramaniam S., Dennis E.A. The lipid maps initiative in lipidomics. Methods Enzymol. 2007;432:171–183. doi: 10.1016/S0076-6879(07)32007-7. [DOI] [PubMed] [Google Scholar]
- 29.Graessler J., Schwudke D., Schwarz P.E., Herzog R., Shevchenko A., Bornstein S.R. Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients. PLoS One. 2009;4:e6261. doi: 10.1371/journal.pone.0006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahy E., Subramaniam S., Brown H.A., Glass C.K., Merrill A.H., Jr., Murphy R.C., et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Mendenhall C.L. Origin of hepatic triglyceride fatty acids: quantitative estimation of the relative contributions of linoleic acid by diet and adipose tissue in normal and ethanol-fed rats. J Lipid Res. 1972;13:177–183. [PubMed] [Google Scholar]
- 32.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diraison F., Moulin P., Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 34.Burr G.O., Burr M.M. Nutrition classics from The Journal of Biological Chemistry 82:345-367, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev. 1973;31:248–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 35.Rieckenhoff I.G., Holman R.T., Burr G.O. Polyethenoid fatty acid metabolism; effect of dietary fat on polyethenoid fatty acids of rat tissues. Arch Biochem. 1949;20:331–340. [PubMed] [Google Scholar]
- 36.Turley S.D., Andersen J.M., Dietschy J.M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981;22:551–569. [PubMed] [Google Scholar]
- 37.Repa J.J., Mangelsdorf D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 38.European Association for the Study of the L. European Association for the Study of D. European Association for the Study of O EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 39.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X., Barraza K.M., Beauchamp J.L. Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air-water interface. Proc Natl Acad Sci U S A. 2018;115:3255–3260. doi: 10.1073/pnas.1722323115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wymann M.P., Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 42.Overkleeft H.S., Renkema G.H., Neele J., Vianello P., Hung I.O., Strijland A., et al. Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. J Biol Chem. 1998;273:26522–26527. doi: 10.1074/jbc.273.41.26522. [DOI] [PubMed] [Google Scholar]
- 43.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson N.E., Kotarsky K., Owman C., Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 45.Soroosh P., Wu J., Xue X., Song J., Sutton S.W., Sablad M., et al. Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc Natl Acad Sci U S A. 2014;111:12163–12168. doi: 10.1073/pnas.1322807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DuSell C.D., Umetani M., Shaul P.W., Mangelsdorf D.J., McDonnell D.P. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umetani M., Domoto H., Gormley A.K., Yuhanna I.S., Cummins C.L., Javitt N.B., et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 48.Falcon A., Doege H., Fluitt A., Tsang B., Watson N., Kay M.A., et al. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2010;299:E384–E393. doi: 10.1152/ajpendo.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doege H., Baillie R.A., Ortegon A.M., Tsang B., Wu Q., Punreddy S., et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Doege H., Grimm D., Falcon A., Tsang B., Storm T.A., Xu H., et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283:22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasui K., Harano Y., Mitsuyoshi H., Tsuji K., Endo M., Nakajima T., et al. Steatosis and hepatic expression of genes regulating lipid metabolism in Japanese patients infected with hepatitis C virus. J Gastroenterol. 2010;45:95–104. doi: 10.1007/s00535-009-0133-8. [DOI] [PubMed] [Google Scholar]
- 52.Machado M.V., Oliveira A.G., Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361–1367. doi: 10.1111/j.1440-1746.2011.06801.x. [DOI] [PubMed] [Google Scholar]
- 53.Connor C.L. Fatty infiltration of the liver and the development of cirrhosis in diabetes and chronic alcoholism. Am J Pathol. 1938;14:347–364 349. [PMC free article] [PubMed] [Google Scholar]
- 54.Seppala-Lindroos A., Vehkavaara S., Hakkinen A.M., Goto T., Westerbacka J., Sovijarvi A., et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 55.Scherer T., Lindtner C., O'Hare J., Hackl M., Zielinski E., Freudenthaler A., et al. Insulin regulates hepatic triglyceride secretion and lipid content via signaling in the brain. Diabetes. 2016;65:1511–1520. doi: 10.2337/db15-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holt H.B., Wild S.H., Wood P.J., Zhang J., Darekar A.A., Dewbury K., et al. Non-esterified fatty acid concentrations are independently associated with hepatic steatosis in obese subjects. Diabetologia. 2006;49:141–148. doi: 10.1007/s00125-005-0070-x. [DOI] [PubMed] [Google Scholar]
- 57.Teng F., Jiang J., Zhang J., Yuan Y., Li K., Zhou B., et al. The S100 calcium-binding protein A11 promotes hepatic steatosis through RAGE-mediated AKT-mTOR signaling. Metabolism. 2021;117:154725. doi: 10.1016/j.metabol.2021.154725. [DOI] [PubMed] [Google Scholar]
- 58.Bosch D.E., Yeh M.M. Primary sclerosing cholangitis is protective against nonalcoholic fatty liver disease in inflammatory bowel disease. Hum Pathol. 2017;69:55–62. doi: 10.1016/j.humpath.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Banales J.M., Inarrairaegui M., Arbelaiz A., Milkiewicz P., Muntane J., Munoz-Bellvis L., et al. Serum metabolites as diagnostic biomarkers for cholangiocarcinoma, hepatocellular carcinoma, and primary sclerosing cholangitis. Hepatology. 2019;70:547–562. doi: 10.1002/hep.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sang C., Wang X., Zhou K., Sun T., Bian H., Gao X., et al. Bile acid profiles are distinct among patients with different etiologies of chronic liver disease. J Proteome Res. 2021;20:2340–2351. doi: 10.1021/acs.jproteome.0c00852. [DOI] [PubMed] [Google Scholar]
- 61.Wu J.M., Skill N.J., Maluccio M.A. Evidence of aberrant lipid metabolism in hepatitis C and hepatocellular carcinoma. HPB (Oxford) 2010;12:625–636. doi: 10.1111/j.1477-2574.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin H.C., Chen Y.F., Hsu W.H., Yang C.W., Kao C.H., Tsai T.F. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev Res (Phila) 2012;5:952–962. doi: 10.1158/1940-6207.CAPR-12-0001. [DOI] [PubMed] [Google Scholar]
- 63.Wu Y.F., Fu S.L., Kao C.H., Yang C.W., Lin C.H., Hsu M.T., et al. Chemopreventive effect of silymarin on liver pathology in HBV X protein transgenic mice. Cancer Res. 2008;68:2033–2042. doi: 10.1158/0008-5472.CAN-07-2450. [DOI] [PubMed] [Google Scholar]
- 64.Moriya K., Fujie H., Shintani Y., Yotsuyanagi H., Tsutsumi T., Ishibashi K., et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 65.Haberl E.M., Weiss T.S., Peschel G., Weigand K., Kohler N., Pauling J.K., et al. Liver lipids of patients with hepatitis B and C and associated hepatocellular carcinoma. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22105297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arain S.Q., Talpur F.N., Channa N.A., Ali M.S., Afridi H.I. Serum lipid profile as a marker of liver impairment in hepatitis B Cirrhosis patients. Lipids Health Dis. 2017;16:51. doi: 10.1186/s12944-017-0437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao R., Cheng J., Fan C., Shi X., Cao Y., Sun B., et al. Serum metabolomics to identify the liver disease-specific biomarkers for the progression of hepatitis to hepatocellular carcinoma. Sci Rep. 2015;5:18175. doi: 10.1038/srep18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arain S.Q., Talpur F.N., Channa N.A., Ali M.S., Afridi H.I. Serum lipids as an indicator for the alteration of liver function in patients with hepatitis B. Lipids Health Dis. 2018;17:36. doi: 10.1186/s12944-018-0683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng H., Chen M., Lu S., Zhao L., Ji J., Gao H. Metabolic characterization of hepatitis B virus-related liver cirrhosis using NMR-based serum metabolomics. Metabolomics. 2017;13:121. [Google Scholar]
- 70.Teng C.F., Hsieh W.C., Yang C.W., Su H.M., Tsai T.F., Sung W.C., et al. A biphasic response pattern of lipid metabolomics in the stage progression of hepatitis B virus X tumorigenesis. Mol Carcinog. 2016;55:105–114. doi: 10.1002/mc.22266. [DOI] [PubMed] [Google Scholar]
- 71.Hofmann S., Krajewski M., Scherer C., Scholz V., Mordhorst V., Truschow P., et al. Complex lipid metabolic remodeling is required for efficient hepatitis C virus replication. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1041–1056. doi: 10.1016/j.bbalip.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Cho H.K., Kim S.Y., Yoo S.K., Choi Y.H., Cheong J. Fatty acids increase hepatitis B virus X protein stabilization and HBx-induced inflammatory gene expression. FEBS J. 2014;281:2228–2239. doi: 10.1111/febs.12776. [DOI] [PubMed] [Google Scholar]
- 73.Cui M., Xiao Z., Sun B., Wang Y., Zheng M., Ye L., et al. Involvement of cholesterol in hepatitis B virus X protein-induced abnormal lipid metabolism of hepatoma cells via up-regulating miR-205-targeted ACSL4. Biochem Biophys Res Commun. 2014;445:651–655. doi: 10.1016/j.bbrc.2014.02.068. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Wu T., Hu D., Weng X., Wang X., Chen P.J., et al. Intracellular hepatitis B virus increases hepatic cholesterol deposition in alcoholic fatty liver via hepatitis B core protein. J Lipid Res. 2018;59:58–68. doi: 10.1194/jlr.M079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu T., Zheng X., Yang M., Zhao A., Li M., Chen T., et al. Serum lipid alterations identified in chronic hepatitis B, hepatitis B virus-associated cirrhosis and carcinoma patients. Sci Rep. 2017;7:42710. doi: 10.1038/srep42710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun J., Zhao Y., Qin L., Li K., Zhao Y., Sun H., et al. Metabolomic profiles for HBV related hepatocellular carcinoma including alpha-fetoproteins positive and negative subtypes. Front Oncol. 2019;9:1069. doi: 10.3389/fonc.2019.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lerat H., Kammoun H.L., Hainault I., Merour E., Higgs M.R., Callens C., et al. Hepatitis C virus proteins induce lipogenesis and defective triglyceride secretion in transgenic mice. J Biol Chem. 2009;284:33466–33474. doi: 10.1074/jbc.M109.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang M., Zhang X.J., Feng K., He C., Li P., Hu Y.J., et al. Dietary alpha-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci Rep. 2016;6:26826. doi: 10.1038/srep26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berk P.D., Zhou S., Bradbury M.W. Increased hepatocellular uptake of long chain fatty acids occurs by different mechanisms in fatty livers due to obesity or excess ethanol use, contributing to development of steatohepatitis in both settings. Trans Am Clin Climatol Assoc. 2005;116:335–344. discussion 345. [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou S.L., Gordon R.E., Bradbury M., Stump D., Kiang C.L., Berk P.D. Ethanol up-regulates fatty acid uptake and plasma membrane expression and export of mitochondrial aspartate aminotransferase in HepG2 cells. Hepatology. 1998;27:1064–1074. doi: 10.1002/hep.510270423. [DOI] [PubMed] [Google Scholar]
- 81.Zhong W., Zhao Y., Tang Y., Wei X., Shi X., Sun W., et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Israelsen M., Kim M., Suvitaival T., Madsen B.S., Hansen C.D., Torp N., et al. Comprehensive lipidomics reveals phenotypic differences in hepatic lipid turnover in ALD and NAFLD during alcohol intoxication. JHEP Rep. 2021;3:100325. doi: 10.1016/j.jhepr.2021.100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji C., Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 84.Ji C., Shinohara M., Vance D., Than T.A., Ookhtens M., Chan C., et al. Effect of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver. Alcohol Clin Exp Res. 2008;32:1049–1058. doi: 10.1111/j.1530-0277.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.You M., Fischer M., Deeg M.A., Crabb D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 86.Ooi G.J., Meikle P.J., Huynh K., Earnest A., Roberts S.K., Kemp W., et al. Hepatic lipidomic remodeling in severe obesity manifests with steatosis and does not evolve with non-alcoholic steatohepatitis. J Hepatol. 2021;75:524–535. doi: 10.1016/j.jhep.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 87.Mayo R., Crespo J., Martinez-Arranz I., Banales J.M., Arias M., Minchole I., et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun. 2018;2:807–820. doi: 10.1002/hep4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewinska M., Santos-Laso A., Arretxe E., Alonso C., Zhuravleva E., Jimenez-Aguero R., et al. The altered serum lipidome and its diagnostic potential for Non-Alcoholic Fatty Liver (NAFL)-associated hepatocellular carcinoma. EBioMedicine. 2021;73:103661. doi: 10.1016/j.ebiom.2021.103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caussy C., Ajmera V.H., Puri P., Hsu C.L., Bassirian S., Mgdsyan M., et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non-alcoholic fatty liver disease. Gut. 2019;68:1884–1892. doi: 10.1136/gutjnl-2018-317584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewinska M.S.-L.,A., Arretxe E., Alonso C., Zhuravleva E., Jimenez-Aguero R., Eizaguirre E., et al. 2021. The altered serum lipidome and its diagnostic potential for Non-Alcoholic Fatty Liver (NAFL)-associated hepatocellular carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puri P., Daita K., Joyce A., Mirshahi F., Santhekadur P.K., Cazanave S., et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O., et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 93.Bugianesi E., Gastaldelli A., Vanni E., Gambino R., Cassader M., Baldi S., et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 94.Schuster S., Johnson C.D., Hennebelle M., Holtmann T., Taha A.Y., Kirpich I.A., et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. J Lipid Res. 2018;59:1597–1609. doi: 10.1194/jlr.M083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Windt D.J., Sud V., Zhang H., Varley P.R., Goswami J., Yazdani H.O., et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347–1360. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown Z.J., Fu Q., Ma C., Kruhlak M., Zhang H., Luo J., et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9:620. doi: 10.1038/s41419-018-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma C., Kesarwala A.H., Eggert T., Medina-Echeverz J., Kleiner D.E., Jin P., et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pfister D., Nunez N.G., Pinyol R., Govaere O., Pinter M., Szydlowska M., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kotronen A., Velagapudi V.R., Yetukuri L., Westerbacka J., Bergholm R., Ekroos K., et al. Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia. 2009;52:684–690. doi: 10.1007/s00125-009-1282-2. [DOI] [PubMed] [Google Scholar]
- 100.Oresic M., Hyotylainen T., Kotronen A., Gopalacharyulu P., Nygren H., Arola J., et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia. 2013;56:2266–2274. doi: 10.1007/s00125-013-2981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Westerbacka J., Kotronen A., Fielding B.A., Wahren J., Hodson L., Perttila J., et al. Splanchnic balance of free fatty acids, endocannabinoids, and lipids in subjects with nonalcoholic fatty liver disease. Gastroenterology. 2010;139:1961–1971 e1961. doi: 10.1053/j.gastro.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 102.Kotronen A., Seppanen-Laakso T., Westerbacka J., Kiviluoto T., Arola J., Ruskeepaa A.L., et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58:203–208. doi: 10.2337/db08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gorden D.L., Myers D.S., Ivanova P.T., Fahy E., Maurya M.R., Gupta S., et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Promrat K., Longato L., Wands J.R., de la Monte S.M. Weight loss amelioration of non-alcoholic steatohepatitis linked to shifts in hepatic ceramide expression and serum ceramide levels. Hepatol Res. 2011;41:754–762. doi: 10.1111/j.1872-034X.2011.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luukkonen P.K., Zhou Y., Sadevirta S., Leivonen M., Arola J., Oresic M., et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Apostolopoulou M., Gordillo R., Koliaki C., Gancheva S., Jelenik T., De Filippo E., et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care. 2018;41:1235–1243. doi: 10.2337/dc17-1318. [DOI] [PubMed] [Google Scholar]
- 107.Masoodi M., Gastaldelli A., Hyotylainen T., Arretxe E., Alonso C., Gaggini M., et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021 doi: 10.1038/s41575-021-00502-9. [DOI] [PubMed] [Google Scholar]
- 108.Xia J.Y., Holland W.L., Kusminski C.M., Sun K., Sharma A.X., Pearson M.J., et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaurasia B., Tippetts T.S., Mayoral Monibas R., Liu J., Li Y., Wang L., et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365:386–392. doi: 10.1126/science.aav3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dasarathy S., Yang Y., McCullough A.J., Marczewski S., Bennett C., Kalhan S.C. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2011;23:382–388. doi: 10.1097/MEG.0b013e328345c8c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aranha M.M., Cortez-Pinto H., Costa A., da Silva I.B., Camilo M.E., de Moura M.C., et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur J Gastroenterol Hepatol. 2008;20:519–525. doi: 10.1097/MEG.0b013e3282f4710a. [DOI] [PubMed] [Google Scholar]
- 112.Mouzaki M., Wang A.Y., Bandsma R., Comelli E.M., Arendt B.M., Zhang L., et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chavez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, Type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694 e1673. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 114.Wang X., Cai B., Yang X., Sonubi O.O., Zheng Z., Ramakrishnan R., et al. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31:969–986 e967. doi: 10.1016/j.cmet.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bell L.N., Wulff J., Comerford M., Vuppalanchi R., Chalasani N. Serum metabolic signatures of primary biliary cirrhosis and primary sclerosing cholangitis. Liver Int. 2015;35:263–274. doi: 10.1111/liv.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trottier J., Bialek A., Caron P., Straka R.J., Heathcote J., Milkiewicz P., et al. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: a pilot study. Dig Liver Dis. 2012;44:303–310. doi: 10.1016/j.dld.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 117.Zhou L., Wang Q., Yin P., Xing W., Wu Z., Chen S., et al. Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Anal Bioanal Chem. 2012;403:203–213. doi: 10.1007/s00216-012-5782-4. [DOI] [PubMed] [Google Scholar]
- 118.Muir K., Hazim A., He Y., Peyressatre M., Kim D.Y., Song X., et al. Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma. Cancer Res. 2013;73:4722–4731. doi: 10.1158/0008-5472.CAN-12-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cho Y., Cho E.J., Yoo J.J., Chang Y., Chung G.E., Jeong S.M., et al. Association between lipid profiles and the incidence of hepatocellular carcinoma: a nationwide population-based study. Cancers (Basel) 2021;13 doi: 10.3390/cancers13071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Padthaisong S., Phetcharaburanin J., Klanrit P., Li J.V., Namwat N., Khuntikeo N., et al. Integration of global metabolomics and lipidomics approaches reveals the molecular mechanisms and the potential biomarkers for postoperative recurrence in early-stage cholangiocarcinoma. Cancer Metab. 2021;9:30. doi: 10.1186/s40170-021-00266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ismail I.T., Elfert A., Helal M., Salama I., El-Said H., Fiehn O. Remodeling lipids in the transition from chronic liver disease to hepatocellular carcinoma. Cancers (Basel) 2020;13 doi: 10.3390/cancers13010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vlock E.M., Karanjit S., Talmon G., Farazi P.A. Reduction of polyunsaturated fatty acids with tumor progression in a lean non-alcoholic steatohepatitis-associated hepatocellular carcinoma mouse model. J Cancer. 2020;11:5536–5546. doi: 10.7150/jca.48495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grammatikos G., Schoell N., Ferreiros N., Bon D., Herrmann E., Farnik H., et al. Serum sphingolipidomic analyses reveal an upregulation of C16-ceramide and sphingosine-1-phosphate in hepatocellular carcinoma. Oncotarget. 2016;7:18095–18105. doi: 10.18632/oncotarget.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guri Y., Colombi M., Dazert E., Hindupur S.K., Roszik J., Moes S., et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32:807–823 e812. doi: 10.1016/j.ccell.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 125.Hirose Y., Nagahashi M., Katsuta E., Yuza K., Miura K., Sakata J., et al. Generation of sphingosine-1-phosphate is enhanced in biliary tract cancer patients and is associated with lymphatic metastasis. Sci Rep. 2018;8:10814. doi: 10.1038/s41598-018-29144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng J.C., Wang E.Y., Yi Y., Thakur A., Tsai S.H., Hoodless P.A. S1P stimulates proliferation by upregulating CTGF expression through S1PR2-mediated YAP activation. Mol Cancer Res. 2018;16:1543–1555. doi: 10.1158/1541-7786.MCR-17-0681. [DOI] [PubMed] [Google Scholar]
- 127.Bao M., Chen Z., Xu Y., Zhao Y., Zha R., Huang S., et al. Sphingosine kinase 1 promotes tumour cell migration and invasion via the S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 2012;32:331–338. doi: 10.1111/j.1478-3231.2011.02666.x. [DOI] [PubMed] [Google Scholar]
- 128.Zeng Y., Yao X., Chen L., Yan Z., Liu J., Zhang Y., et al. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/ syndecan-1/TGF-beta autocrine loop. Oncotarget. 2016;7:63324–63337. doi: 10.18632/oncotarget.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yokota T., Nojima H., Kuboki S., Yoshitomi H., Furukawa K., Takayashiki T., et al. Sphingosine-1-phosphate Receptor-1 promotes vascular invasion and EMT in hepatocellular carcinoma. J Surg Res. 2021;259:200–210. doi: 10.1016/j.jss.2020.11.044. [DOI] [PubMed] [Google Scholar]
- 130.Zhang S.L., Liu L. microRNA-148a inhibits hepatocellular carcinoma cell invasion by targeting sphingosine-1-phosphate receptor 1. Exp Ther Med. 2015;9:579–584. doi: 10.3892/etm.2014.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoshida T., Tsuchiya A., Kumagai M., Takeuchi S., Nojiri S., Watanabe T., et al. Blocking sphingosine 1-phosphate receptor 2 accelerates hepatocellular carcinoma progression in a mouse model of NASH. Biochem Biophys Res Commun. 2020;530:665–672. doi: 10.1016/j.bbrc.2020.07.099. [DOI] [PubMed] [Google Scholar]
- 132.Yang J., Tian Y., Zheng R., Li L., Qiu F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol Lett. 2019;18:1530–1538. doi: 10.3892/ol.2019.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krautbauer S., Meier E.M., Rein-Fischboeck L., Pohl R., Weiss T.S., Sigruener A., et al. Ceramide and polyunsaturated phospholipids are strongly reduced in human hepatocellular carcinoma. Biochim Biophys Acta. 2016;1861:1767–1774. doi: 10.1016/j.bbalip.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 134.Hall Z., Chiarugi D., Charidemou E., Leslie J., Scott E., Pellegrinet L., et al. Lipid remodeling in hepatocyte proliferation and hepatocellular carcinoma. Hepatology. 2021;73:1028–1044. doi: 10.1002/hep.31391. [DOI] [PubMed] [Google Scholar]
- 135.Cotte A.K., Cottet V., Aires V., Mouillot T., Rizk M., Vinault S., et al. Phospholipid profiles and hepatocellular carcinoma risk and prognosis in cirrhotic patients. Oncotarget. 2019;10:2161–2172. doi: 10.18632/oncotarget.26738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Britten C.D., Garrett-Mayer E., Chin S.H., Shirai K., Ogretmen B., Bentz T.A., et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:4642–4650. doi: 10.1158/1078-0432.CCR-16-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi W., Zhang S., Ma D., Yan D., Zhang G., Cao Y., et al. Targeting SphK2 reverses acquired resistance of regorafenib in hepatocellular carcinoma. Front Oncol. 2020;10:694. doi: 10.3389/fonc.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Z., Zhang Z., Mei H., Mao J., Zhou X. Distribution and clinical relevance of phospholipids in hepatocellular carcinoma. Hepatol Int. 2020;14:544–555. doi: 10.1007/s12072-020-10056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou L., Ding L., Yin P., Lu X., Wang X., Niu J., et al. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. J Proteome Res. 2012;11:5433–5442. doi: 10.1021/pr300683a. [DOI] [PubMed] [Google Scholar]
- 140.Liang J.Q., Teoh N., Xu L., Pok S., Li X., Chu E.S.H., et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat Commun. 2018;9:4490. doi: 10.1038/s41467-018-06931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsuchida T., Lee Y.A., Fujiwara N., Ybanez M., Allen B., Martins S., et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 2018;69:385–395. doi: 10.1016/j.jhep.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wong Y.J., Qiu T.Y., Ng G.K., Zheng Q., Teo E.K. Efficacy and safety of statin for hepatocellular carcinoma prevention among chronic liver disease patients: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55:615–623. doi: 10.1097/MCG.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 143.Lavu S., Therneau T.M., Harmsen W.S., Mara K.C., Wongjarupong N., Hassan M., et al. Effect of statins on the risk of extrahepatic cholangiocarcinoma. Hepatology. 2020;72:1298–1309. doi: 10.1002/hep.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thrift A.P., Natarajan Y., Liu Y., El-Serag H.B. Statin use after diagnosis of hepatocellular carcinoma is associated with decreased mortality. Clin Gastroenterol Hepatol. 2019;17:2117–2125 e2113. doi: 10.1016/j.cgh.2018.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaplan D.E., Serper M.A., Mehta R., Fox R., John B., Aytaman A., et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology. 2019;156:1693–1706 e1612. doi: 10.1053/j.gastro.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 146.Qin W.H., Yang Z.S., Li M., Chen Y., Zhao X.F., Qin Y.Y., et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158:1713–1727. doi: 10.1053/j.gastro.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 147.Andreotti G., Chen J., Gao Y.T., Rashid A., Chang S.C., Shen M.C., et al. Serum lipid levels and the risk of biliary tract cancers and biliary stones: a population-based study in China. Int J Cancer. 2008;122:2322–2329. doi: 10.1002/ijc.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thomas C.E., Luu H.N., Wang R., Xie G., Adams-Haduch J., Jin A., et al. Association between pre-diagnostic serum bile acids and hepatocellular carcinoma: the Singapore Chinese health study. Cancers (Basel) 2021;13 doi: 10.3390/cancers13112648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sydor S., Best J., Messerschmidt I., Manka P., Vilchez-Vargas R., Brodesser S., et al. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC. Clin Transl Gastroenterol. 2020;11:e00131. doi: 10.14309/ctg.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu L., Feng J., Li J., Yu Q., Ji J., Wu J., et al. The gut microbiome-bile acid axis in hepatocarcinogenesis. Biomed Pharmacother. 2021;133:111036. doi: 10.1016/j.biopha.2020.111036. [DOI] [PubMed] [Google Scholar]
- 151.Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W., et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garrido A., Kim E., Teijeiro A., Sanchez Sanchez P., Gallo R., Nair A., et al. Histone acetylation of bile acid transporter genes plays a critical role in cirrhosis. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mencarelli A., Renga B., Migliorati M., Cipriani S., Distrutti E., Santucci L., et al. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol. 2009;183:6657–6666. doi: 10.4049/jimmunol.0901347. [DOI] [PubMed] [Google Scholar]
- 154.Zhang X., Yang Z., Shi Z., Zhu Z., Li C., Du Z., et al. Analysis of bile acid profile in plasma to differentiate cholangiocarcinoma from benign biliary diseases and healthy controls. J Steroid Biochem Mol Biol. 2021;205:105775. doi: 10.1016/j.jsbmb.2020.105775. [DOI] [PubMed] [Google Scholar]
- 155.Dai J., Wang H., Shi Y., Dong Y., Zhang Y., Wang J. Impact of bile acids on the growth of human cholangiocarcinoma via FXR. J Hematol Oncol. 2011;4:41. doi: 10.1186/1756-8722-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dai J., Wang H., Dong Y., Zhang Y., Wang J. Bile acids affect the growth of human cholangiocarcinoma via NF-kB pathway. Cancer Invest. 2013;31:111–120. doi: 10.3109/07357907.2012.762781. [DOI] [PubMed] [Google Scholar]
- 157.Kemp C.J., Leary C.N., Drinkwater N.R. Promotion of murine hepatocarcinogenesis by testosterone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci U S A. 1989;86:7505–7509. doi: 10.1073/pnas.86.19.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Petrick J.L., McMenamin U.C., Zhang X., Zeleniuch-Jacquotte A., Wactawski-Wende J., Simon T.G., et al. Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank. Br J Cancer. 2020;123:316–324. doi: 10.1038/s41416-020-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei Q., Guo P., Mu K., Zhang Y., Zhao W., Huai W., et al. Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95:804–816. doi: 10.1038/labinvest.2015.63. [DOI] [PubMed] [Google Scholar]
- 160.Kaewlert W., Sakonsinsiri C., Namwat N., Sawanyawisuth K., Ungarreevittaya P., Khuntikeo N., et al. The importance of CYP19A1 in estrogen receptor-positive cholangiocarcinoma. Horm Cancer. 2018;9:408–419. doi: 10.1007/s12672-018-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hunsawong T., Singsuksawat E., In-chon N., Chawengrattanachot W., Thuwajit C., Sripa B., et al. Estrogen is increased in male cholangiocarcinoma patients' serum and stimulates invasion in cholangiocarcinoma cell lines in vitro. J Cancer Res Clin Oncol. 2012;138:1311–1320. doi: 10.1007/s00432-012-1207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pawar P., Ma L., Byon C.H., Liu H., Ahn E.Y., Jhala N., et al. Molecular mechanisms of tamoxifen therapy for cholangiocarcinoma: role of calmodulin. Clin Cancer Res. 2009;15:1288–1296. doi: 10.1158/1078-0432.CCR-08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McGlynn K.A., Sahasrabuddhe V.V., Campbell P.T., Graubard B.I., Chen J., Schwartz L.M., et al. Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project. Br J Cancer. 2015;112:1266–1272. doi: 10.1038/bjc.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X., Ramadori P., Pfister D., Seehawer M., Zender L., Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021 doi: 10.1038/s41568-021-00383-9. [DOI] [PubMed] [Google Scholar]
- 165.Liu X., Liu X., Shi Q., Fan X., Qi K. Association of telomere length and telomerase methylation with n-3 fatty acids in preschool children with obesity. BMC Pediatr. 2021;21:24. doi: 10.1186/s12887-020-02487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alves-Paiva R.M., Kajigaya S., Feng X., Chen J., Desierto M., Wong S., et al. Telomerase enzyme deficiency promotes metabolic dysfunction in murine hepatocytes upon dietary stress. Liver Int. 2018;38:144–154. doi: 10.1111/liv.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 168.Wu H., Ng R., Chen X., Steer C.J., Song G. MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut. 2016;65:1850–1860. doi: 10.1136/gutjnl-2014-308430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guo W., Zhong W., Hao L., Dong H., Sun X., Yue R., et al. Fatty acids inhibit LAMP2-mediated autophagy flux via activating ER stress pathway in alcohol-related liver disease. Cell Mol Gastroenterol Hepatol. 2021;12:1599–1615. doi: 10.1016/j.jcmgh.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nath A., Li I., Roberts L.R., Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. doi: 10.1038/srep14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yao L., Han C., Song K., Zhang J., Lim K., Wu T. Omega-3 polyunsaturated fatty acids upregulate 15-PGDH expression in cholangiocarcinoma cells by inhibiting miR-26a/b expression. Cancer Res. 2015;75:1388–1398. doi: 10.1158/0008-5472.CAN-14-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fekry B., Jeffries K.A., Esmaeilniakooshkghazi A., Szulc Z.M., Knagge K.J., Kirchner D.R., et al. C16-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat Commun. 2018;9:4149. doi: 10.1038/s41467-018-06650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uranbileg B., Ikeda H., Kurano M., Enooku K., Sato M., Saigusa D., et al. Increased mRNA levels of sphingosine kinases and S1P lyase and reduced levels of S1P were observed in hepatocellular carcinoma in association with poorer differentiation and earlier recurrence. PLoS One. 2016;11:e0149462. doi: 10.1371/journal.pone.0149462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yin X., Zafrullah M., Lee H., Haimovitz-Friedman A., Fuks Z., Kolesnick R. A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation. Cell Physiol Biochem. 2009;24:219–230. doi: 10.1159/000233248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Skill N.J., Scott R.E., Wu J., Maluccio M.A. Hepatocellular carcinoma associated lipid metabolism reprogramming. J Surg Res. 2011;169:51–56. doi: 10.1016/j.jss.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 176.Xu M., Liu Z., Wang C., Yao B., Zheng X. EDG2 enhanced the progression of hepatocellular carcinoma by LPA/PI3K/AKT/ mTOR signaling. Oncotarget. 2017;8:66154–66168. doi: 10.18632/oncotarget.19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhu B., Shi S., Ma Y.G., Fan F., Yao Z.Z. Lysophosphatidic acid enhances human hepatocellular carcinoma cell migration, invasion and adhesion through P38 MAPK pathway. Hepatogastroenterology. 2012;59:785–789. doi: 10.5754/hge11427. [DOI] [PubMed] [Google Scholar]
- 178.Sakakima Y., Hayakawa A., Nakao A. Phosphatidylcholine induces growth inhibition of hepatic cancer by apoptosis via death ligands. Hepatogastroenterology. 2009;56:481–484. [PubMed] [Google Scholar]
- 179.Wang X., Zheng Z., Caviglia J.M., Corey K.E., Herfel T.M., Cai B., et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brindley P.J., Bachini M., Ilyas S.I., Khan S.A., Loukas A., Sirica A.E., et al. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gouw A.M., Eberlin L.S., Margulis K., Sullivan D.K., Toal G.G., Tong L., et al. Oncogene KRAS activates fatty acid synthase, resulting in specific ERK and lipid signatures associated with lung adenocarcinoma. Proc Natl Acad Sci U S A. 2017;114:4300–4305. doi: 10.1073/pnas.1617709114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yao Y., Sun S., Wang J., Fei F., Dong Z., Ke A.W., et al. Canonical Wnt signaling remodels lipid metabolism in zebrafish hepatocytes following ras oncogenic insult. Cancer Res. 2018;78:5548–5560. doi: 10.1158/0008-5472.CAN-17-3964. [DOI] [PubMed] [Google Scholar]
- 183.Chen L.L., Wang W.J. p53 regulates lipid metabolism in cancer. Int J Biol Macromol. 2021;192:45–54. doi: 10.1016/j.ijbiomac.2021.09.188. [DOI] [PubMed] [Google Scholar]
- 184.Yahagi N., Shimano H., Matsuzaka T., Najima Y., Sekiya M., Nakagawa Y., et al. p53 Activation in adipocytes of obese mice. J Biol Chem. 2003;278:25395–25400. doi: 10.1074/jbc.M302364200. [DOI] [PubMed] [Google Scholar]
- 185.Moon S.H., Huang C.H., Houlihan S.L., Regunath K., Freed-Pastor W.A., Morris JPt, et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2019;176:564–580 e519. doi: 10.1016/j.cell.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Assaily W., Rubinger D.A., Wheaton K., Lin Y., Ma W., Xuan W., et al. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol Cell. 2011;44:491–501. doi: 10.1016/j.molcel.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 187.Derdak Z., Villegas K.A., Harb R., Wu A.M., Sousa A., Wands J.R. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol. 2013;58:785–791. doi: 10.1016/j.jhep.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mo Y., Wang Y., Zhang L., Yang L., Zhou M., Li X., et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J Cancer. 2019;10:3789–3797. doi: 10.7150/jca.31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.He S., Tang S. WNT/beta-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. doi: 10.1016/j.biopha.2020.110851. [DOI] [PubMed] [Google Scholar]
- 190.Senni N., Savall M., Cabrerizo Granados D., Alves-Guerra M.C., Sartor C., Lagoutte I., et al. beta-catenin-activated hepatocellular carcinomas are addicted to fatty acids. Gut. 2019;68:322–334. doi: 10.1136/gutjnl-2017-315448. [DOI] [PubMed] [Google Scholar]
- 191.Wang Y., Viscarra J., Kim S.J., Sul H.S. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Forman B.M., Chen J., Evans R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ma X.L., Sun Y.F., Wang B.L., Shen M.N., Zhou Y., Chen J.W., et al. Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer. 2019;19:760. doi: 10.1186/s12885-019-5963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rudalska R., Harbig J., Snaebjornsson M.T., Klotz S., Zwirner S., Taranets L., et al. LXRα activation and Raf inhibition trigger lethal lipotoxicity in liver cancer. Nat Cancer. 2021;2:201–217. doi: 10.1038/s43018-020-00168-3. [DOI] [PubMed] [Google Scholar]
- 195.Shimizu Y., Tamura T., Kemmochi A., Owada Y., Ozawa Y., Hisakura K., et al. Oxidative stress and Liver X Receptor agonist induce hepatocellular carcinoma in Non-alcoholic steatohepatitis model. J Gastroenterol Hepatol. 2021;36:800–810. doi: 10.1111/jgh.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Peng X., Chen Z., Farshidfar F., Xu X., Lorenzi P.L., Wang Y., et al. Molecular characterization and clinical relevance of metabolic expression subtypes in human cancers. Cell Rep. 2018;23:255–269.e254. doi: 10.1016/j.celrep.2018.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ally A., Balasundaram M., Carlsen R., Chuah E., Clarke A., Dhalla N., et al. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341.e1323. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Budhu A., Roessler S., Zhao X., Yu Z., Forgues M., Ji J., et al. Integrated metabolite and gene expression profiles identify lipid biomarkers associated with progression of hepatocellular carcinoma and patient outcomes. Gastroenterology. 2013;144:1066–1075 e1061. doi: 10.1053/j.gastro.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim J.K., Fillmore J.J., Chen Y., Yu C., Moore I.K., Pypaert M., et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Merkel M., Weinstock P.H., Chajek-Shaul T., Radner H., Yin B., Breslow J.L., et al. Lipoprotein lipase expression exclusively in liver. A mouse model for metabolism in the neonatal period and during cachexia. J Clin Invest. 1998;102:893–901. doi: 10.1172/JCI2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Luo X., Zheng E., Wei L., Zeng H., Qin H., Zhang X., et al. The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis. 2021;12:328. doi: 10.1038/s41419-021-03596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Anderson C.M., Stahl A. SLC27 fatty acid transport proteins. Mol Aspects Med. 2013;34:516–528. doi: 10.1016/j.mam.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li L., Che L., Tharp K.M., Park H.M., Pilo M.G., Cao D., et al. Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology. 2016;63:1900–1913. doi: 10.1002/hep.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rebouissou S., Nault J.C. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72:215–229. doi: 10.1016/j.jhep.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 205.Zhou Y., Tao J., Calvisi D.F., Chen X. Role of lipogenesis rewiring in hepatocellular carcinoma. Semin Liver Dis. 2021 doi: 10.1055/s-0041-1731709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nelson M.E., Lahiri S., Chow J.D., Byrne F.L., Hargett S.R., Breen D.S., et al. Inhibition of hepatic lipogenesis enhances liver tumorigenesis by increasing antioxidant defence and promoting cell survival. Nat Commun. 2017;8:14689. doi: 10.1038/ncomms14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Han Q., Chen C.A., Yang W., Liang D., Lv H.W., Lv G.S., et al. ATP-citrate lyase regulates stemness and metastasis in hepatocellular carcinoma via the Wnt/beta-catenin signaling pathway. Hepatobiliary Pancreat Dis Int. 2021;20:251–261. doi: 10.1016/j.hbpd.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 208.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29:174–182 e175. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang M.D., Wu H., Fu G.B., Zhang H.L., Zhou X., Tang L., et al. Acetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology. 2016;63:1272–1286. doi: 10.1002/hep.28415. [DOI] [PubMed] [Google Scholar]
- 210.Yahagi N., Shimano H., Hasegawa K., Ohashi K., Matsuzaka T., Najima Y., et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41:1316–1322. doi: 10.1016/j.ejca.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 211.Syed-Abdul M.M., Parks E.J., Gaballah A.H., Bingham K., Hammoud G.M., Kemble G., et al. Fatty acid synthase inhibitor TVB-2640 reduces hepatic de Novo lipogenesis in males with metabolic abnormalities. Hepatology. 2020;72:103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 212.Gu L., Zhu Y., Lin X., Tan X., Lu B., Li Y. Stabilization of FASN by ACAT1-mediated GNPAT acetylation promotes lipid metabolism and hepatocarcinogenesis. Oncogene. 2020;39:2437–2449. doi: 10.1038/s41388-020-1156-0. [DOI] [PubMed] [Google Scholar]
- 213.Cheng G., Palanisamy A.P., Evans Z.P., Sutter A.G., Jin L., Singh I., et al. Cerulenin blockade of fatty acid synthase reverses hepatic steatosis in ob/ob mice. PLoS One. 2013;8:e75980. doi: 10.1371/journal.pone.0075980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cassim S., Raymond V.A., Dehbidi-Assadzadeh L., Lapierre P., Bilodeau M. Metabolic reprogramming enables hepatocarcinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle. 2018;17:903–916. doi: 10.1080/15384101.2018.1460023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Geidl-Flueck B., Hochuli M., Nemeth A., Eberl A., Derron N., Kofeler H.C., et al. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J Hepatol. 2021;75:46–54. doi: 10.1016/j.jhep.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 216.Che L., Chi W., Qiao Y., Zhang J., Song X., Liu Y., et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2020;69:177–186. doi: 10.1136/gutjnl-2018-317581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ding H., Liu J., Wang C., Su Y. NONO promotes hepatocellular carcinoma progression by enhancing fatty acids biosynthesis through interacting with ACLY mRNA. Cancer Cell Int. 2020;20:425. doi: 10.1186/s12935-020-01520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hao Q., Li T., Zhang X., Gao P., Qiao P., Li S., et al. Expression and roles of fatty acid synthase in hepatocellular carcinoma. Oncol Rep. 2014;32:2471–2476. doi: 10.3892/or.2014.3484. [DOI] [PubMed] [Google Scholar]
- 219.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:394–406 e396. doi: 10.1016/j.cmet.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bates J., Vijayakumar A., Ghoshal S., Marchand B., Yi S., Kornyeyev D., et al. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J Hepatol. 2020;73:896–905. doi: 10.1016/j.jhep.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 221.Chow J.D., Lawrence R.T., Healy M.E., Dominy J.E., Liao J.A., Breen D.S., et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab. 2014;3:419–431. doi: 10.1016/j.molmet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li L., Pilo G.M., Li X., Cigliano A., Latte G., Che L., et al. Inactivation of fatty acid synthase impairs hepatocarcinogenesis driven by AKT in mice and humans. J Hepatol. 2016;64:333–341. doi: 10.1016/j.jhep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang B., Zhou B.H., Xiao M., Li H., Guo L., Wang M.X., et al. KDM5C represses FASN-mediated lipid metabolism to exert tumor suppressor activity in intrahepatic cholangiocarcinoma. Front Oncol. 2020;10:1025. doi: 10.3389/fonc.2020.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang G.M., Jiang Q.H., Cai C., Qu M., Shen W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett. 2015;358:180–190. doi: 10.1016/j.canlet.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 225.Falvella F.S., Pascale R.M., Gariboldi M., Manenti G., De Miglio M.R., Simile M.M., et al. Stearoyl-CoA desaturase 1 (Scd1) gene overexpression is associated with genetic predisposition to hepatocarcinogenesis in mice and rats. Carcinogenesis. 2002;23:1933–1936. doi: 10.1093/carcin/23.11.1933. [DOI] [PubMed] [Google Scholar]
- 226.Vriens K., Christen S., Parik S., Broekaert D., Yoshinaga K., Talebi A., et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. 2019;566:403–406. doi: 10.1038/s41586-019-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Su Y.C., Feng Y.H., Wu H.T., Huang Y.S., Tung C.L., Wu P., et al. Elovl6 is a negative clinical predictor for liver cancer and knockdown of Elovl6 reduces murine liver cancer progression. Sci Rep. 2018;8:6586. doi: 10.1038/s41598-018-24633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y., Li T., Jin Y., Shen J. Dgat2 reduces hepatocellular carcinoma malignancy via downregulation of cell cycle-related gene expression. Biomed Pharmacother. 2019;115:108950. doi: 10.1016/j.biopha.2019.108950. [DOI] [PubMed] [Google Scholar]
- 229.de la Rosa Rodriguez M.A., Deng L., Gemmink A., van Weeghel M., Aoun M.L., Warnecke C., et al. Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes. Mol Metab. 2021;47:101168. doi: 10.1016/j.molmet.2021.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kim J.H., Nagappan A., Jung D.Y., Suh N., Jung M.H. Histone demethylase KDM7A contributes to the development of hepatic steatosis by targeting diacylglycerol acyltransferase 2. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shen X., Liang X., Ji X., You J., Zhuang X., Song Y., et al. CD36 and DGAT2 facilitate the lipid-lowering effect of chitooligosaccharides via fatty acid intake and triglyceride synthesis signaling. Food Funct. 2021;12:8681–8693. doi: 10.1039/d1fo01472b. [DOI] [PubMed] [Google Scholar]
- 232.Chang S., Sung P.S., Lee J., Park J., Shin E.C., Choi C. Prolonged silencing of diacylglycerol acyltransferase-1 induces a dedifferentiated phenotype in human liver cells. J Cell Mol Med. 2016;20:38–47. doi: 10.1111/jcmm.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McFie P.J., Chumala P., Katselis G.S., Stone S.J. DGAT2 stability is increased in response to DGAT1 inhibition in gene edited HepG2 cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158991. doi: 10.1016/j.bbalip.2021.158991. [DOI] [PubMed] [Google Scholar]
- 234.Yenilmez B., Wetoska N., Kelly M., Echeverria D., Min K., Lifshitz L., et al. An RNAi therapeutic targeting hepatic DGAT2 in a genetically obese mouse model of nonalcoholic steatohepatitis. Mol Ther. 2021 doi: 10.1016/j.ymthe.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ramon-Krauel M., Pentinat T., Bloks V.W., Cebria J., Ribo S., Perez-Wienese R., et al. Epigenetic programming at the Mogat1 locus may link neonatal overnutrition with long-term hepatic steatosis and insulin resistance. FASEB J. 2018 doi: 10.1096/fj.201700717RR. [DOI] [PubMed] [Google Scholar]
- 236.Wolf Greenstein A., Majumdar N., Yang P., Subbaiah P.V., Kineman R.D., Cordoba-Chacon J. Hepatocyte-specific, PPARgamma-regulated mechanisms to promote steatosis in adult mice. J Endocrinol. 2017;232:107–121. doi: 10.1530/JOE-16-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hall A.M., Kou K., Chen Z., Pietka T.A., Kumar M., Korenblat K.M., et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J Lipid Res. 2012;53:990–999. doi: 10.1194/jlr.P025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ma Y., Temkin S.M., Hawkridge A.M., Guo C., Wang W., Wang X.Y., et al. Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett. 2018;435:92–100. doi: 10.1016/j.canlet.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Farshidfar F., Zheng S., Gingras M.C., Newton Y., Shih J., Robertson A.G., et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cancer Genome Atlas Research Network. Electronic address wbe. Cancer Genome Atlas Research N Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341 e1323. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fujiwara N., Nakagawa H., Enooku K., Kudo Y., Hayata Y., Nakatsuka T., et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493–1504. doi: 10.1136/gutjnl-2017-315193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gonzalez-Romero F., Mestre D., Aurrekoetxea I., O'Rourke C.J., Andersen J.B., Woodhoo A., et al. E2F1 and E2F2-mediated repression of CPT2 establishes a lipid-rich tumor-promoting environment. Cancer Res. 2021;81:2874–2887. doi: 10.1158/0008-5472.CAN-20-2052. [DOI] [PubMed] [Google Scholar]
- 243.Yuan P., Mu J., Wang Z., Ma S., Da X., Song J., et al. Down-regulation of SLC25A20 promotes hepatocellular carcinoma growth and metastasis through suppression of fatty-acid oxidation. Cell Death Dis. 2021;12:361. doi: 10.1038/s41419-021-03648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ma A.P.Y., Yeung C.L.S., Tey S.K., Mao X., Wong S.W.K., Ng T.H., et al. Suppression of ACADM-mediated fatty acid oxidation promotes hepatocellular carcinoma via aberrant Cav1/SREBP-1 signaling. Cancer Res. 2021 doi: 10.1158/0008-5472.CAN-20-3944. [DOI] [PubMed] [Google Scholar]
- 245.Zhao X., Qin W., Jiang Y., Yang Z., Yuan B., Dai R., et al. ACADL plays a tumor-suppressor role by targeting Hippo/YAP signaling in hepatocellular carcinoma. NPJ Precis Oncol. 2020;4:7. doi: 10.1038/s41698-020-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kawata S., Takaishi K., Nagase T., Ito N., Matsuda Y., Tamura S., et al. Increase in the active form of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human hepatocellular carcinoma: possible mechanism for alteration of cholesterol biosynthesis. Cancer Res. 1990;50:3270–3273. [PubMed] [Google Scholar]
- 247.Tomacha J., Dokduang H., Padthaisong S., Namwat N., Klanrit P., Phetcharaburanin J., et al. Targeting fatty acid synthase modulates metabolic pathways and inhibits cholangiocarcinoma cell progression. Front Pharmacol. 2021;12:696961. doi: 10.3389/fphar.2021.696961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Singh S., Singh P.P., Singh A.G., Murad M.H., Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 249.Liu D., Wong C.C., Fu L., Chen H., Zhao L., Li C., et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aap9840. [DOI] [PubMed] [Google Scholar]
- 250.Chen Z., Chen L., Sun B., Liu D., He Y., Qi L., et al. LDLR inhibition promotes hepatocellular carcinoma proliferation and metastasis by elevating intracellular cholesterol synthesis through the MEK/ERK signaling pathway. Mol Metab. 2021;51:101230. doi: 10.1016/j.molmet.2021.101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Manieri E., Folgueira C., Rodriguez M.E., Leiva-Vega L., Esteban-Lafuente L., Chen C., et al. JNK-mediated disruption of bile acid homeostasis promotes intrahepatic cholangiocarcinoma. Proc Natl Acad Sci U S A. 2020;117:16492–16499. doi: 10.1073/pnas.2002672117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gao R., Cheng J., Fan C., Shi X., Cao Y., Sun B., et al. Serum metabolomics to identify the liver disease-specific biomarkers for the progression of hepatitis to hepatocellular carcinoma. Scientific Rep. 2015;5 doi: 10.1038/srep18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lieber C.S., Jones D.P., Decarli L.M. Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest. 1965;44:1009–1021. doi: 10.1172/JCI105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fu K., Wang C., Gao Y., Fan S., Zhang H., Sun J., et al. Metabolomics and lipidomics reveal the effect of hepatic Vps33b deficiency on bile acids and lipids metabolism. Front Pharmacol. 2019;10:276. doi: 10.3389/fphar.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yan L.B., Liao J., Han N., Zhou L.Y., Wang X.E., Wang Y.J., et al. Association between hepatitis B virus infection and metabolic syndrome in Southwest China: a cross-sectional study. Sci Rep. 2020;10:6738. doi: 10.1038/s41598-020-62609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Butt A.A., Yan P., Simon T.G., Chung R.T., Abou-Samra A.B., Team Es Changes in circulating lipids level over time after acquiring HCV infection: results from ERCHIVES. BMC Infect Dis. 2015;15:510. doi: 10.1186/s12879-015-1268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu Q., He X.D., Yu L., Liu W., Tao L.Y. The metabolic syndrome and risk factors for biliary tract cancer: a case-control study in China. Asian Pac J Cancer Prev. 2012;13:1963–1969. doi: 10.7314/apjcp.2012.13.5.1963. [DOI] [PubMed] [Google Scholar]
- 258.Liu X., Li M., Wang X., Dang Z., Jiang Y., Wang X., et al. Effect of serum triglyceride level on the prognosis of patients with hepatocellular carcinoma in the absence of cirrhosis. Lipids Health Dis. 2018;17:248. doi: 10.1186/s12944-018-0898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ressom H.W., Xiao J.F., Tuli L., Varghese R.S., Zhou B., Tsai T.H., et al. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90–100. doi: 10.1016/j.aca.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liang Q., Liu H., Zhang T., Jiang Y., Xing H., Zhang H. Serum metabolomics uncovering specific metabolite signatures of intra- and extrahepatic cholangiocarcinoma. Mol Biosyst. 2016;12:334–340. doi: 10.1039/c5mb00572h. [DOI] [PubMed] [Google Scholar]
- 261.Matsumoto M., Yashiro H., Ogino H., Aoyama K., Nambu T., Nakamura S., et al. Acetyl-CoA carboxylase 1 and 2 inhibition ameliorates steatosis and hepatic fibrosis in a MC4R knockout murine model of nonalcoholic steatohepatitis. PLoS One. 2020;15:e0228212. doi: 10.1371/journal.pone.0228212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Alkhouri N., Lawitz E., Noureddin M., DeFronzo R., Shulman G.I. GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH) Expert Opin Investig Drugs. 2020;29:135–141. doi: 10.1080/13543784.2020.1668374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lawitz E.J., Coste A., Poordad F., Alkhouri N., Loo N., McColgan B.J., et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2018;16:1983–1991 e1983. doi: 10.1016/j.cgh.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 264.Stiede K., Miao W., Blanchette H.S., Beysen C., Harriman G., Harwood H.J., Jr., et al. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: a randomized, double-blind, crossover study. Hepatology. 2017;66:324–334. doi: 10.1002/hep.29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Loomba R., Mohseni R., Lucas K.J., Gutierrez J.A., Perry R.G., Trotter J.F., et al. TVB-2640 (FASN inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterology. 2021;161:1475–1486. doi: 10.1053/j.gastro.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 266.Lee Y.H., Kim J.H., Kim S.R., Jin H.Y., Rhee E.J., Cho Y.M., et al. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. J Korean Med Sci. 2017;32:60–69. doi: 10.3346/jkms.2017.32.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yoneda M., Endo H., Nozaki Y., Tomimoto A., Fujisawa T., Fujita K., et al. Life style-related diseases of the digestive system: gene expression in nonalcoholic steatohepatitis patients and treatment strategies. J Pharmacol Sci. 2007;105:151–156. doi: 10.1254/jphs.fm0070063. [DOI] [PubMed] [Google Scholar]
- 268.Francque S.M., Bedossa P., Ratziu V., Anstee Q.M., Bugianesi E., Sanyal A.J., et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 269.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159 e1145. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 270.Kim W., Kim B.G., Lee J.S., Lee C.K., Yeon J.E., Chang M.S., et al. Randomised clinical trial: the efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:1073–1083. doi: 10.1111/apt.13981. [DOI] [PubMed] [Google Scholar]
- 271.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kowdley K.V., Vuppalanchi R., Levy C., Floreani A., Andreone P., LaRusso N.F., et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Traussnigg S., Halilbasic E., Hofer H., Munda P., Stojakovic T., Fauler G., et al. Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien Klin Wochenschr. 2021;133:441–451. doi: 10.1007/s00508-020-01735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Harrison S.A., Bashir M.R., Lee K.J., Shim-Lopez J., Lee J., Wagner B., et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75:25–33. doi: 10.1016/j.jhep.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 275.Pockros P.J., Fuchs M., Freilich B., Schiff E., Kohli A., Lawitz E.J., et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019;39:2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 276.Shi Y., Wei Q., Liu Y., Yuan J. The alleviating effect of sphingosine kinases 2 inhibitor K145 on nonalcoholic fatty liver. Biochem Biophys Res Commun. 2021;580:1–6. doi: 10.1016/j.bbrc.2021.09.060. [DOI] [PubMed] [Google Scholar]
- 277.Rohrbach T.D., Asgharpour A., Maczis M.A., Montefusco D., Cowart L.A., Bedossa P., et al. FTY720/fingolimod decreases hepatic steatosis and expression of fatty acid synthase in diet-induced nonalcoholic fatty liver disease in mice. J Lipid Res. 2019;60:1311–1322. doi: 10.1194/jlr.M093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jose C., Hebert-Chatelain E., Bellance N., Larendra A., Su M., Nouette-Gaulain K., et al. AICAR inhibits cancer cell growth and triggers cell-type distinct effects on OXPHOS biogenesis, oxidative stress and Akt activation. Biochim Biophys Acta. 2011;1807:707–718. doi: 10.1016/j.bbabio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 279.Zhang C., Sheng L., Yuan M., Hu J., Meng Y., Wu Y., et al. Orlistat delays hepatocarcinogenesis in mice with hepatic co-activation of AKT and c-Met. Toxicol Appl Pharmacol. 2020;392:114918. doi: 10.1016/j.taap.2020.114918. [DOI] [PubMed] [Google Scholar]
- 280.You B.J., Chen L.Y., Hsu P.H., Sung P.H., Hung Y.C., Lee H.Z. Orlistat displays antitumor activity and enhances the efficacy of paclitaxel in human hepatoma Hep3B cells. Chem Res Toxicol. 2019;32:255–264. doi: 10.1021/acs.chemrestox.8b00269. [DOI] [PubMed] [Google Scholar]
- 281.Gao Y., Lin L.P., Zhu C.H., Chen Y., Hou Y.T., Ding J. Growth arrest induced by C75, A fatty acid synthase inhibitor, was partially modulated by p38 MAPK but not by p53 in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:978–985. doi: 10.4161/cbt.5.8.2883. [DOI] [PubMed] [Google Scholar]
- 282.Miranda J.F., Scarinci L.D., Ramos L.F., Silva C.M., Goncalves L.R., de Morais P.F., et al. The modulatory effect of triclosan on the reversion of the activated phenotype of LX-2 hepatic stellate cells. J Biochem Mol Toxicol. 2020;34:e22413. doi: 10.1002/jbt.22413. [DOI] [PubMed] [Google Scholar]
- 283.Huang C.H., Tsai S.J., Wang Y.J., Pan M.H., Kao J.Y., Way T.D. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 284.de Lima Luna A.C., Forti F.L. Modulation of SCD1 activity in hepatocyte cell lines: evaluation of genomic stability and proliferation. Mol Cell Biochem. 2021 doi: 10.1007/s11010-021-04167-5. [DOI] [PubMed] [Google Scholar]
- 285.Zhou Y., Zhong L., Yu S., Shen W., Cai C., Yu H. Inhibition of stearoyl-coenzyme A desaturase 1 ameliorates hepatic steatosis by inducing AMPK-mediated lipophagy. Aging (Albany NY) 2020;12:7350–7362. doi: 10.18632/aging.103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Park H.R., Yoo M.Y., Seo J.H., Kim I.S., Kim N.Y., Kang J.Y., et al. Sesquiterpenoids isolated from the flower buds of Tussilago farfara L. inhibit diacylglycerol acyltransferase. J Agric Food Chem. 2008;56:10493–10497. doi: 10.1021/jf801978r. [DOI] [PubMed] [Google Scholar]
- 287.Lencioni R., Marrero J., Venook A., Ye S.L., Kudo M. Design and rationale for the non-interventional global investigation of therapeutic DEcisions in hepatocellular carcinoma and of its treatment with sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–1041. doi: 10.1111/j.1742-1241.2010.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Liu G., Kuang S., Cao R., Wang J., Peng Q., Sun C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019;33:10089–10103. doi: 10.1096/fj.201802619RR. [DOI] [PubMed] [Google Scholar]
- 289.Ma M.K.F., Lau E.Y.T., Leung D.H.W., Lo J., Ho N.P.Y., Cheng L.K.W., et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J Hepatol. 2017;67:979–990. doi: 10.1016/j.jhep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 290.Xu A., Wang B., Fu J., Qin W., Yu T., Yang Z., et al. Diet-induced hepatic steatosis activates Ras to promote hepatocarcinogenesis via CPT1alpha. Cancer Lett. 2019;442:40–52. doi: 10.1016/j.canlet.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 291.Merrill C.L., Ni H., Yoon L.W., Tirmenstein M.A., Narayanan P., Benavides G.R., et al. Etomoxir-induced oxidative stress in HepG2 cells detected by differential gene expression is confirmed biochemically. Toxicol Sci. 2002;68:93–101. doi: 10.1093/toxsci/68.1.93. [DOI] [PubMed] [Google Scholar]
- 292.Ren M., Xu H., Xia H., Tang Q., Bi F. Simultaneously targeting SOAT1 and CPT1A ameliorates hepatocellular carcinoma by disrupting lipid homeostasis. Cell Death Discov. 2021;7:125. doi: 10.1038/s41420-021-00504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yin F., Feng F., Wang L., Wang X., Li Z., Cao Y. SREBP-1 inhibitor Betulin enhances the antitumor effect of Sorafenib on hepatocellular carcinoma via restricting cellular glycolytic activity. Cell Death Dis. 2019;10:672. doi: 10.1038/s41419-019-1884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cariello M., Peres C., Zerlotin R., Porru E., Sabba C., Roda A., et al. Long-term administration of nuclear bile acid receptor FXR agonist prevents spontaneous hepatocarcinogenesis in Abcb4(-/-) mice. Sci Rep. 2017;7:11203. doi: 10.1038/s41598-017-11549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Feng J., Dai W., Mao Y., Wu L., Li J., Chen K., et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1alpha/PPAR-gamma/PKM2-mediated glycolysis. J Exp Clin Cancer Res. 2020;39:24. doi: 10.1186/s13046-020-1528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jouve J.L., Lecomte T., Bouche O., Barbier E., Khemissa Akouz F., Riachi G., et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:516–522. doi: 10.1016/j.jhep.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 297.Ding X., Chaiteerakij R., Moser C.D., Shaleh H., Boakye J., Chen G., et al. Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget. 2016;7:20080–20092. doi: 10.18632/oncotarget.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ding X., Zhang Y., Huang T., Xu G., Peng C., Chen G., et al. Targeting sphingosine kinase 2 suppresses cell growth and synergizes with BCL2/BCL-XL inhibitors through NOXA-mediated MCL1 degradation in cholangiocarcinoma. Am J Cancer Res. 2019;9:546–561. [PMC free article] [PubMed] [Google Scholar]
- 299.Beljanski V., Lewis C.S., Smith C.D. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther. 2011;11:524–534. doi: 10.4161/cbt.11.5.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lozano E., Monte M.J., Briz O., Hernandez-Hernandez A., Banales J.M., Marin J.J., et al. Enhanced antitumour drug delivery to cholangiocarcinoma through the apical sodium-dependent bile acid transporter (ASBT) J Control Release. 2015;216:93–102. doi: 10.1016/j.jconrel.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 301.Di Matteo S., Nevi L., Costantini D., Overi D., Carpino G., Safarikia S., et al. The FXR agonist obeticholic acid inhibits the cancerogenic potential of human cholangiocarcinoma. PLoS One. 2019;14:e0210077. doi: 10.1371/journal.pone.0210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tran Q., Lee H., Kim C., Kong G., Gong N., Kwon S.H., et al. Revisiting the Warburg effect: diet-based strategies for cancer prevention. Biomed Res Int. 2020;2020:8105735. doi: 10.1155/2020/8105735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Bae J.S., Park J.M., Lee J., Oh B.C., Jang S.H., Lee Y.B., et al. Amelioration of non-alcoholic fatty liver disease with NPC1L1-targeted IgY or n-3 polyunsaturated fatty acids in mice. Metabolism. 2017;66:32–44. doi: 10.1016/j.metabol.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 304.Nemoto N., Suzuki S., Kikuchi H., Okabe H., Sassa S., Sakamoto S. Ethyl-eicosapentaenoic acid reduces liver lipids and lowers plasma levels of lipids in mice fed a high-fat diet. In Vivo. 2009;23:685–689. [PubMed] [Google Scholar]
- 305.Konuma K., Itoh M., Suganami T., Kanai S., Nakagawa N., Sakai T., et al. Eicosapentaenoic acid ameliorates non-alcoholic steatohepatitis in a novel mouse model using melanocortin 4 receptor-deficient mice. PLoS One. 2015;10:e0121528. doi: 10.1371/journal.pone.0121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Gao M., Sun K., Guo M., Gao H., Liu K., Yang C., et al. Fish consumption and n-3 polyunsaturated fatty acids, and risk of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Causes Control. 2015;26:367–376. doi: 10.1007/s10552-014-0512-1. [DOI] [PubMed] [Google Scholar]
- 307.Kang S., Huang J., Lee B.K., Jung Y.S., Im E., Koh J.M., et al. Omega-3 polyunsaturated fatty acids protect human hepatoma cells from developing steatosis through FFA4 (GPR120) Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:105–116. doi: 10.1016/j.bbalip.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 308.Weylandt K.H., Krause L.F., Gomolka B., Chiu C.Y., Bilal S., Nadolny A., et al. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-alpha. Carcinogenesis. 2011;32:897–903. doi: 10.1093/carcin/bgr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Jeyapal S., Kona S.R., Mullapudi S.V., Putcha U.K., Gurumurthy P., Ibrahim A. Substitution of linoleic acid with alpha-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis. Sci Rep. 2018;8:10953. doi: 10.1038/s41598-018-29222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Valenzuela R., Espinosa A., Gonzalez-Manan D., D'Espessailles A., Fernandez V., Videla L.A., et al. N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS One. 2012;7:e46400. doi: 10.1371/journal.pone.0046400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Huang Q., Tan Y., Yin P., Ye G., Gao P., Lu X., et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 2013;73:4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 312.Simon J., Ouro A., Ala-Ibanibo L., Presa N., Delgado T.C., Martinez-Chantar M.L. Sphingolipids in non-alcoholic fatty liver disease and hepatocellular carcinoma: ceramide turnover. Int J Mol Sci. 2019;21 doi: 10.3390/ijms21010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lim K., Han C., Dai Y., Shen M., Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Patterson A.D., Maurhofer O., Beyoglu D., Lanz C., Krausz K.W., Pabst T., et al. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011;71:6590–6600. doi: 10.1158/0008-5472.CAN-11-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.