Summary
Background
α-Klotho is a geroprotective protein that can attenuate or alleviate deleterious changes with ageing and disease. Declines in α-Klotho play a role in the pathophysiology of multiple diseases and age-related phenotypes. Pre-clinical evidence suggests that boosting α-Klotho holds therapeutic potential. However, readily clinically-translatable, practical strategies for increasing α-Klotho are not at hand. Here, we report that orally-active, clinically-translatable senolytics can increase α-Klotho in mice and humans.
Methods
We examined α-Klotho expression in three different human primary cell types co-cultured with conditioned medium (CM) from senescent or non-senescent cells with or without neutralizing antibodies. We assessed α-Klotho expression in aged, obese, and senescent cell-transplanted mice treated with vehicle or senolytics. We assayed urinary α-Klotho in patients with idiopathic pulmonary fibrosis (IPF) who were treated with the senolytic drug combination, Dasatinib plus Quercetin (D+Q).
Findings
We found exposure to the senescent cell secretome reduces α-Klotho in multiple nonsenescent human cell types. This was partially prevented by neutralizing antibodies against the senescence-associated secretory phenotype (SASP) factors, activin A and Interleukin 1α (IL-1α). Consistent with senescent cells’ being a cause of decreased α-Klotho, transplanting senescent cells into younger mice reduced brain and urine α-Klotho. Selectively removing senescent cells genetically or pharmacologically increased α-Klotho in urine, kidney, and brain of mice with increased senescent cell burden, including naturally-aged, diet-induced obese (DIO), or senescent cell-transplanted mice. D+Q increased α-Klotho in urine of patients with IPF, a disease linked to cellular senescence.
Interpretation
Senescent cells cause reduced α-Klotho, partially due to their production of activin A and IL-1α. Targeting senescent cells boosts α-Klotho in mice and humans. Thus, clearing senescent cells restores α-Klotho, potentially opening a novel, translationally-feasible avenue for developing orally-active small molecule, α-Klotho-enhancing clinical interventions. Furthermore, urinary α-Klotho may prove to be a useful test for following treatments in senolytic clinical trials.
Funding
This work was supported by National Institute of Health grants AG013925 (J.L.K.), AG062413 (J.L.K., S.K.), AG044271 (N.M.), AG013319 (N.M.), and the Translational Geroscience Network (AG061456: J.L.K., T.T., N.M., S.B.K., S.K.), Robert and Arlene Kogod (J.L.K.), the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.), and the Noaber Foundation (J.L.K.). The previous IPF clinical trial was supported by the Claude D. Pepper Older Americans Independence Centers at WFSM (AG021332: J.N.J., S.B.K.), UTHSCA (AG044271: A.M.N.), and the Translational Geroscience Network.
Keywords: Cellular senescence, α-Klotho, Senolytics
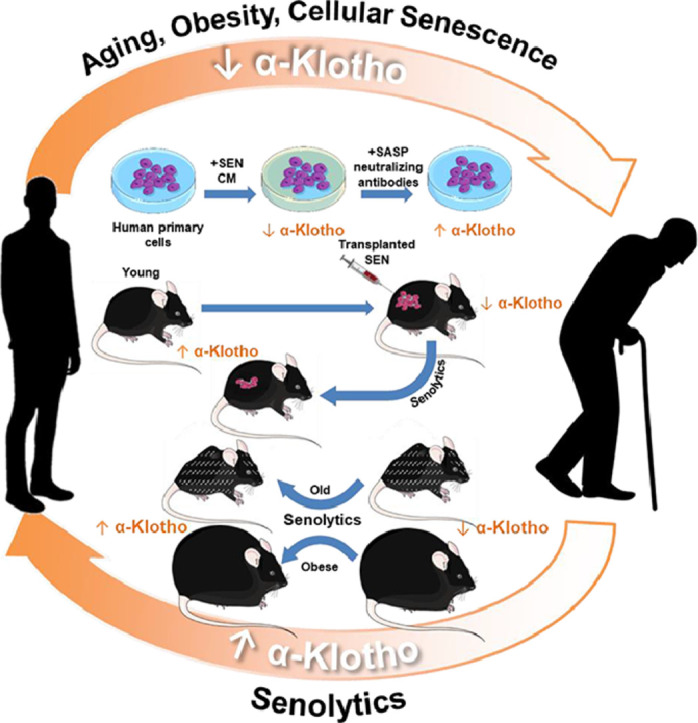
Graphical abstract
Senescent cells, the burden of which is increased with ageing and in obesity, appear to cause decreases in α-Klotho. Senolytics, which reduce the increased senescent cell burden resulting from ageing or obesity, can partially restore α-Klotho, potentially adding to benefits of senolytics.
Research in context.
Evidence before this study
α-Klotho levels decline and senescent cells accumulate with ageing in mice and humans. α-Klotho- deficient mice exhibit accelerated ageing-like phenotypes. α-Klotho overexpression increases lifespan and delays or prevents onset of many age-related diseases. Similarly, accumulation of senescent cells is associated with tissue dysfunction in multiple organs and multiple age-related diseases, recapitulating effects of α-Klotho deficiency. Clearance of senescent cells through genetic targeting or senescent cell-targeting (senolytic) drugs alleviates or prevents many age-related diseases, as does overexpressing α-Klotho. Hence, we speculate α-Klotho and cellular senescence are inversely and potentially mechanistically interrelated.
Added value of this study
We demonstrated senescent cell CM reduces α-Klotho in cultured non-senescent human umbilical vein endothelial cells (HUVECs), human primary kidney glomerular endothelial cells (HKEs), and human brain astrocytes (HBAs). Effects of senescent cell CM were partially attenuated by neutralizing antibodies against the SASP factors, activin A and IL-1α. Transplanting senescent cells into younger mice decreased kidney and brain α-Klotho. Genetically reducing highly p16Ink4a-expressing cells in old INK-ATTAC mice or administering the senolytics, Dasatinib plus Quercetin (D+Q) or Fisetin, to young mice transplanted with senescent cells, young DIO mice, or naturally-aged mice increased urine, kidney, and/or brain α-Klotho. Treating patients with IPF, a cellular senescence-related disease, with D+Q increased urinary α-Klotho.
Implications of all the available evidence
α-Klotho and cellular senescence are inversely related and causally linked to the genesis and progression of age-related diseases. Targeting senescent cells increases the geroprotective factor α-Klotho, potentially amplifying beneficial effects of senolytic drugs. Our study also opens a novel, translationally-feasible avenue for developing orally-active small molecules to increase α-Klotho, which may also be a useful biomarker for senescent cell burden or senolytic drug activity in clinical trials.
Alt-text: Unlabelled box
Introduction
α-Klotho is a geroprotective factor that exerts anti-physiological stress effects1 and protects against oxidative damage, hypoxia, and cytotoxic drugs.2,3 Soluble α-Klotho is an endocrine protein that regulates multiple biological processes, including phosphate homeostasis, mineral metabolism,4 and signaling by insulin-like growth factor 1 (IGF-1),5,6 mammalian target of rapamycin (mTOR),7,8 cyclic adenosine monophosphate (cAMP),9,10 p53/ p21CIP1,11,12 and Wnt proteins.2,13,14 α-Klotho is synthesized and secreted by the distal renal tubule and so is present in urine.15, 16, 17 It is also highly expressed in the choroid plexus and other regions of the brain and to a lesser extent in adipose tissue. Several preclinical studies have implicated α-Klotho as a molecule that impacts lifespan, health-span, and renal and cognitive function. α-Klotho declines with ageing in mice and humans.2,18 Mice deficient in α-Klotho develop premature ageing-like phenotypes, including shortened lifespan, atherosclerosis-like vascular dysfunction, cognitive impairment, sarcopenia, physical dysfunction, cardiac hypertrophy and fibrosis, and osteopenia.2,19, 20, 21, 22, 23, 24, 25, 26 Conversely, high α-Klotho levels attenuate age-related functional declines. Mice overexpressing α-Klotho have increased lifespan, enhanced cognition, delayed age-related vascular dysfunction, decreased diabetes-related inflammation, and improved skeletal muscle regeneration.27, 28, 29 As α-Klotho holds therapeutic potential, various strategies to increase α-Klotho have been proposed. However, despite extensive effort there are few approaches for increasing or restoring α-Klotho that are either feasible to advance into clinical trials or that have shown efficacy in trials. This led us to seek a novel approach based on the inter-relatedness of fundamental ageing mechanisms.
Along with reductions in α-Klotho, senescent cells accumulate with ageing. Cellular senescence is a cell fate involving essentially permanent replicative arrest, extensive changes in metabolic state, and resistance to apoptosis. Senescent cell abundance is increased in multiple age-related diseases and disorders in experimental animals and humans.30, 31, 32 Cellular senescence can be associated with a SASP33, 34, 35 that entails release of pro-inflammatory and tissue-damaging cytokines, chemokines, extracellular matrix-destroying proteases, bioactive lipids, metabolites, nucleotides, exosomes, pro-apoptotic factors, and factors that impede stem cell, progenitor, and tissue function and can spread senescence to previously normal cells, both locally and systemically.33, 34, 35, 36, 37 Reducing senescent cell burden with senolytics, a class of drugs that selectively clears senescent cells, extends health-span in mice36,38 and has effects similar to those of overexpressing or administering recombinant α-Klotho.11,39, 40, 41, 42, 43 Conversely, transplanting senescent cells into young mice shortens health-span and causes frailty, early onset of age-related phenotypes and diseases, and premature death from the same causes of death as naturally-aged mice.36 Collectively, these findings suggest a feed-forward cycle, with deficiency of α-Klotho contributing to increased senescent cell burden11,44 and hence increased SASP factors that, in turn, might further exacerbate α-Klotho deficiency. Therefore, we speculated that α-Klotho and cellular senescence are inversely and potentially causally interrelated. If so, decreasing senescent cell abundance using orally-active senolytics could be a clinically effective and practical way to increase α-Klotho.
To determine if senolytics increase α-Klotho and explore translational relevance, we performed post hoc specimen analyses of urinary α-Klotho in three different mouse models and the first human study of the senolytic drug combination, D+Q, in persons with IPF. IPF is a progressive, fatal age-related disease linked to both cellular senescence and reduced α-Klotho. Bleomycin inhalation induces lung senescent cell accumulation together with an IPF-like syndrome in mice that is alleviated by decreasing senescent cell burden genetically or with senolytics.45 Also, lung α-Klotho is lowered by Bleomycin administration. Transgenic mice overexpressing α-Klotho are protected from Bleomycin-induced pulmonary fibrosis.46,47 Patients with IPF have decreased plasma α-Klotho48 as well as an increased burden of senescent cells.45 α-Klotho is lower in lung fibroblasts cultured from IPF patients than control subjects without IPF.47 We previously reported that the senolytic drug combination, D+Q, may alleviate physical dysfunction in IPF patients49 (a single-arm phase I pilot trial, ClinicalTrials.gov identifier: NCT02874989). Here, we tested the hypothesis that α-Klotho and cellular senescence are causally linked in mice and humans by determining effects of senolytics on urinary α-Klotho levels in naturally aged, diet-induced obese (DIO), or senescent cell-transplanted mice. We then assayed urinary α-Klotho in specimens from the senolytic drug study in patients with IPF to determine if effects of senolytics on α-Klotho in mice are also apparent in humans.
Methods
Study design overview
We conducted a translational study of α-Klotho by first establishing α-Klotho signalling mechanisms in cultured human primary senescent cells, then we explored the casual links between α-Klotho and senescent cells in vivo following genetic clearance of senescent cells and senolytic drug administration in naturally aged, DIO, and senescent cell-transplanted mice administered senolytic drugs vs. vehicle. Finally, we measured urinary α-Klotho before and after senolytic drug treatment in a post hoc analysis of a previous single-arm, open-label trial49 in older adults with IPF.
Ethics statement
Mice were humanely euthanized at defined end points and all experimental procedures had prior approval from the Institutional Animal Care and Use Committee at Mayo Clinic. All human study procedures complied with the Declaration of Helsinki and the informed consent and study documents were approved by the Institutional Review Boards at Wake Forest School of Medicine (WFSM) and the University of Texas Health Science Center San Antonio (UTHSCSA) and were reviewed by Data and Safety Monitoring Boards at both clinical sites conducting the previous clinical trial of senolytics for IPF.49 The nature, benefits, and risks of that study had been explained to the patient volunteers and their written informed consent was obtained prior to participation in that previous study (NCT02874989). Deidentified urine samples from that study collected from the patients with IPF were used here for exploratory analyses of α-Klotho.
Reagents
Human primary cells were obtained from commercial sources, passed quality control procedures, and were certified by the commercial sources. All reagents were validated by the manufacturer and/or has been previously cited in the literature. Detailed information about these reagents is provided in Supplementary Table 1.
Animals
Wild-type C57BL/6 mice (young = 8-month-old, male) were purchased from Charles River and maintained in the Mayo Clinic animal facility. INK-ATTAC mice were developed by J.L.K, T.T., J. van Deursen, and D.J. Baker.50 In INK-ATTAC mice, the ATTAC gene devised by Trujillo et al.51 is driven by a senescence-related p16Ink4a promoter fragment devised by Wang et al.52 The fusion protein product of the ATTAC “suicide” gene contains a mutated FKBP moiety that can be cross-linked by AP20187, thereby activating the ATTAC protein caspase-8 moieties, causing apoptosis. INK-ATTAC mice were bred, genotyped, and aged at Mayo Clinic's animal facility, which is pathogen-free and maintained at 23–24 °C under a 12 h. light, 12 h. dark regimen. Mice had free access to water and a 20% protein by weight, 5% fat (13.2% fat by calories), and 6% fiber diet (Lab Diet). The INK-ATTAC mouse strain is patented by Mayo Clinic can be requested under a material transfer agreement. DIO mice were fed a diet in which 60% of calories were from fat (Research Diets) for 7–8 months before experiments.
Human IPF trial
This is a post hoc analysis of a previously published observational study.49 That study was conducted at the Clinical Research Units of WFSM (n = 2) and UTHSCSA (n = 18). Intermittent D (100 mg/day) plus Q (1250 mg/day) were orally administered over three consecutive days for three weeks (i.e. 9 total participant administered dosing days) at the two outpatient clinical research centers using a single-arm, open-label design, as previously described.49
Cell transplantation
Wild-type C57BL/6 mice were randomly assigned to be transplanted with senescent (induced by 10Gy x-ray) or non-senescent adipocyte progenitors or vehicle (phosphate buffered saline [PBS]) and matched for body weight across groups. Mouse preadipocytes were isolated and cultured as previously described.36 Mice were anesthetized using Isoflurane and 2 million cells were transplanted intraperitoneally (i.p.) in 150 µl PBS through a 22G needle, as previously described.
Drug treatments
INK-ATTAC mice were randomly assigned to AP20187 or vehicle groups. AP20187 was purchased from Clontech. Vehicle (10% ethanol, 30% polyethylene glycol, 60% Phosal) or AP20187 in vehicle was injected i.p. (10 mg/kg) for 3 consecutive days every 2 weeks for 4 weeks. Wild-type C57BL/6 mice were randomly assigned to D+Q, Fisetin (F), or vehicle treatments. Treatments were started at age 26–27 months for old animals, and for DIO mice after 7-8 months of high fat-feeding beginning at 4 months of age. Mice were treated every 20 days with D+Q, F, or vehicle by oral gavage for 3 consecutive days in each of 3 cycles over 2 months (9 doses in total).
Cell culture
HKEs (ScienCell Research Laboratories, #4000), HUVECs (Lonza, # C2519A), and HBAs (ScienCell Research Laboratories, #1800) were cultured following the suppliers’ directions. All cells purchased from commercial sources were validated by the manufacturers (Supplementary Table 1). Briefly, CM from senescent or non-senescent HKEs, HUVECs, or HBAs was filtered (0.2 µm) before being added to target non-senescent HKEs, HUVECs, or HBAs, respectively, with or without neutralizing antibodies against Interleukin-1β (IL-1β; Biolegend, #508202, RRID:AB_315514), Interleukin-18 (IL-18; Thermo Fisher Scientific, #PA5-47803, RRID:AB_2606212), CXCL1 (R&D Systems, #MAB275, RRID:AB_2292460), Interleukin-6 (IL-6; R&D Systems, #MAB2061, RRID:AB_354281), activin A (R&D Systems, #MAB3381), Tumour Necrosis Factor α (TNF-α) (Cell Signaling Technology, #7321S), or Plasminogen Activator Inhibitor-1 (PAI-1; R&D Systems, #MAB1786) for 48 h. Cells were then collected for assay by qPCR.
Non-senescent HKEs, HUVECs, and HBAs were co-cultured with recombinant human IL-1α protein (R&D Systems, #00-LA-010) or recombinant human activin A (R&D Systems, #338-AC) for 48 h, when cells were assayed by qPCR.
qPCR
Each cDNA sample was generated by reverse transcription using 1 µg RNA following the manufacturer's protocol (Thermo Fisher Scientific). Reverse transcription involved incubation for 10 min at 25 °C, 120 min at 37 °C, 5 min at 85 °C, and holding at 4 °C using Taqman Fast Advanced Master Mix (Thermo Fisher Scientific). TBP was used as a control. Data were analysed by the ∆∆Ct method.
Human and mouse urinary α-Klotho
α-Klotho was assayed by ELISA in 1:100 diluted mouse urine (IBL America, #27601). Human urine α-Klotho was assayed by ELISA (IBL America, #27998, RRID:AB_2750859). Urine α-Klotho measurements are expressed as a function of urine volume, creatinine (R&D Systems, #KGE005), and/or cystatin C (R&D Systems, #MSCTC0).
Senescent cell quantification by mass cytometry (CyTOF)
CyTOF experiments were performed as described.53
Immunofluorescent staining
Mouse brains were processed for paraffin embedding, cut into 4 µm-thick sagittal sections, deparaffinized in Histoclear (National Diagnostics), and rehydrated in decreasing percentages of ethanol diluted in water. Prior to staining, antigen retrieval was performed by incubating sections in Tris EDTA pH9 buffer in a steamer for 20 min and cooling to room temperature. Sections were washed with PBS and blocked with 5% Normal Goat Serum and 0.3% Tween20 in PBS-BSA 0.1% for 30 min. Rabbit anti-mouse α-Klotho antibody (Invitrogen, #MA5-32784) was diluted 1:50 in blocking solution, added to sections, and incubated overnight at 4 °C. Slides were washed with PBS and incubated with secondary goat anti-rabbit 647 antibody (Invitrogen, A-21244, RRID: AB_2535812) for 1 h at room temperature in the dark. Slides were washed and mounted in ProLong Gold Antifade mountant with DAPI (Invitrogen, P36935).
Imaging and analysis
Stained α-Klotho mouse whole brain sections were scanned using an Axio Scanner Z1 (Zeiss) at 20x magnification. Photoshop CC 19.1 (Adobe, Inc.) was used to virtually dissect the choroid plexus, whole cerebellum, and background samples, avoiding major blood vessels with erythrocytes or tissue folding that could interfere with fluorescence intensity calculations. Using ImageJ FIJI,54 the region of interest (ROI) was selected for analysis to exclude blood vessels and folding of tissue that can interfere with intensity measurements. ROI areas were quantified for raw intensity and then corrected with background on the same section. Results are shown as the corrected mean intensity, calculated as corrected intensity divided by area.
Western blotting
Tissue extracts were homogenized using a Bead Mill 24 homogenizer (Waltham) and lysed in NETN buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40), 5 mM NaF, a protease inhibitor cocktail (Millipore Sigma), and 5 mM nicotinamide. After incubating 30 min at 4 °C, samples were centrifuged at 15,000 rpm for 10 min at 4 °C. Protein concentrations of the supernatants were determined by the Bradford protein assay (BioRad). Lysates were separated by SDS-PAGE and transferred by electrophoresis to PVDF membranes (Millipore Sigma). Membranes were immunoblotted with primary mouse α-Klotho antibody (R&D Systems, AF1819) at 1:1000 dilution and GAPDH antibody (Cell Signaling Technology, 97166) at 1:1000 dilution. Enhanced chemiluminescence detection was performed using SuperSignal West Pico or Femto Chemiluminescence Substrate (Thermo Scientific). All blots were imaged with a GelDoc Go Imaging System (BioRad).
Statistical analyses
At least four independent replicates were studied in all cell culture experiments. Randomisation: Mice were randomized using a random number generator in all experiments. Blinding: Experiments were conducted with the investigators being blinded as to treatment, group allocation for immunofluorescence (IF) staining, ELISA assays, quantitative PCR (qPCR), and quantification of protein expression. Sample size determination: Sample sizes were chosen based on the means and variation of preliminary data to achieve at least 80% power and allow for a 5% type I error. Inclusion/exclusion: The animals were matched for age, gender, and body weight. No animals or experimental data were excluded. Inclusion and exclusion criteria for the clinical trial from which de-identified urine was acquired for post hoc analysis have been published in.49 All data were plotted and analyzed for statistical significance using Prism 9.0 (GraphPad) for cell culture and animal experiments. We reported R2 (same as η2), calculated in Prism, as the effect size. Comparisons between a single treated group and vehicle were made using non-paired T-tests. When comparisons were made between multiple treatment levels, a mixed effects repeated measures model was used, accounting for the repeated measures within each subject. For IPF clinical trial data, the following statistical methods were used and due to the wide range of the values, a log transformation was performed before analysis. Zeros (n = 3) were replaced with half of the minimum non-zero value before log transformation. Imputation using half of the minimum was used to impute zeros due to under-detection.55 This imputation method56 is available in MetaboAnalyst 2.0, a web server for data analysis and interpretation. MetaboAnalyst 2.0 allows selected analyses to avoid divide-by-zero problems to process missing values, which can be replaced by the half of the minimum value found in the dataset by default. Wilcoxon signed rank tests (two-sided) were used to test differences before and after treatment in human trial data since the data were not normally distributed. Both Spearman's rho and Kendall's tau are non-parametric tests for association and test the same null hypothesis and both control for type I error at the nominal level. In our study, we chose to use Spearman's rank correlation to test the association between urinary α-Klotho and SASP factors in urine.
Role of funder
The funders had no role in the study design, data collection, analysis, interpretation, decision to publish, or writing the manuscript.
Results
Senescent cells decrease α-Klotho through paracrine mechanisms
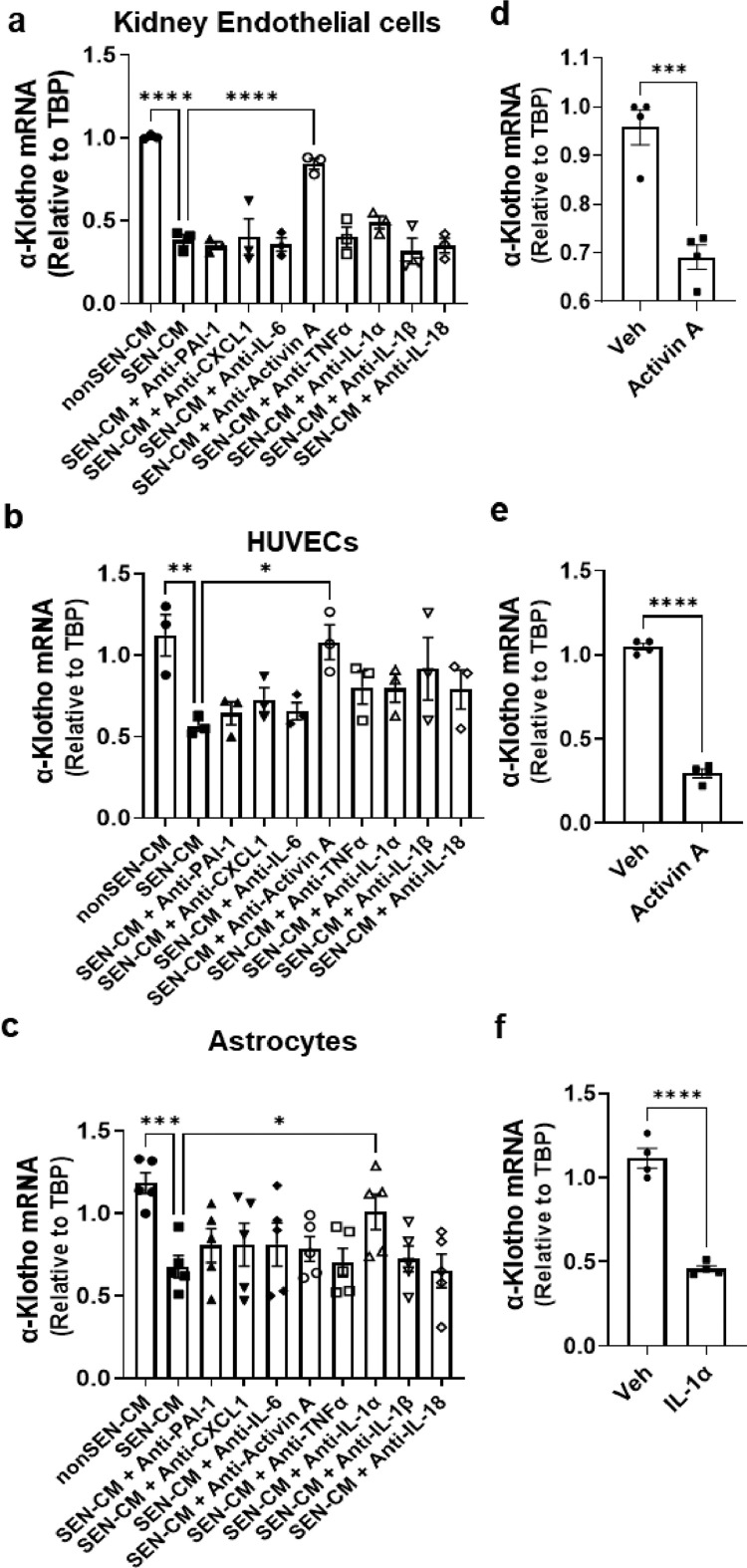
HKEs, HUVECs, and HBAs were radiated (20Gy X-ray). By 25 days after radiation, the cells had become senescent (Supplementary Fig. 1). To determine if senescent cells can directly impact α-Klotho expression, HKEs, HUVECs, and HBA were exposed to CM from cultured senescent or non-senescent human HKEs, HUVECs, or HBAs, respectively. Senescent cell CM exposure resulted in decreased α-Klotho expression in the non-senescent cells (Figure 1A–C, Supplementary Fig. 2). These reductions were attenuated in non-senescent HKEs or HUVECs by neutralizing antibodies against activin A, and in non-senescent HBAs by anti-IL-1α (Figure 1A–C). Conversely, treating human non-senescent HKEs, HUVECs, or HBAs with recombinant activin A or IL-1α caused decreased expression of α-Klotho (Figure 1D). Thus, components of the SASP can decrease α-Klotho in non-senescent cells. Our results are consistent with findings by others that activin A, a transforming growth factor-β (TGF-β) superfamily member, binds to its receptor, ActRllA, to activate TGF-β signaling. Activated TGF-β/Smad signaling reduces both α-Klotho mRNA and protein by altering α-Klotho gene promoter methylation.57, 58, 59 However, since targeting the individual SASP factors, activin A or IL-1α, was not sufficient to fully restore α-Klotho across all cell types, multiple factors might need to be targeted to prevent SASP-induced reductions in α-Klotho in vivo. Alternatively, eliminating senescent cells with senolytics, thereby reducing all SASP factors with a single intervention, might be a more encompassing approach.
Figure 1.
Senescent cell conditioned medium decreases α-Klotho; blocking particular SASP factors with neutralizing antibodies partially restores α-Klotho. (a-c) Conditioned media (CM) collected from senescent or non-senescent (a) human primary kidney endothelial cells (HKEs; one-way ANOVA: F = 19.10, p < 0.0001, R2 = 0.89; n = 3; ****p < 0.0001); (b) human umbilical vein endothelial cells (HUVECs; one-way ANOVA: F = 3.02, p = 0.02, R2 = 0.58, n = 3; *p = 0.02, **p = 0.01); or (b) human brain astrocytes (HBAs; one-way ANOVA: F = 7.93, p = 0.02, R2 = 0.66; n = 5; ***p < 0.001, *p = 0.03) were applied to non-senescent HKEs, HUVECs, or HBAs, respectively, with or without antibodies against PAI-1, CXCL1, IL-6, activin A, TNF-α, IL-1α, IL-1β, or IL-18 for 48 h. α-Klotho mRNA was assayed by qPCR. Means ± SEM. (d) Primary HKEs (n = 4), (e) HUVECs (n = 4), or (f) HBAs (n = 4) were treated with recombinant activin A or IL-1α. α-Klotho was assayed by qPCR 24 h after treatments. Means ± SEM; unpaired T-tests; ***p < 0.001, ****p < 0.0001.
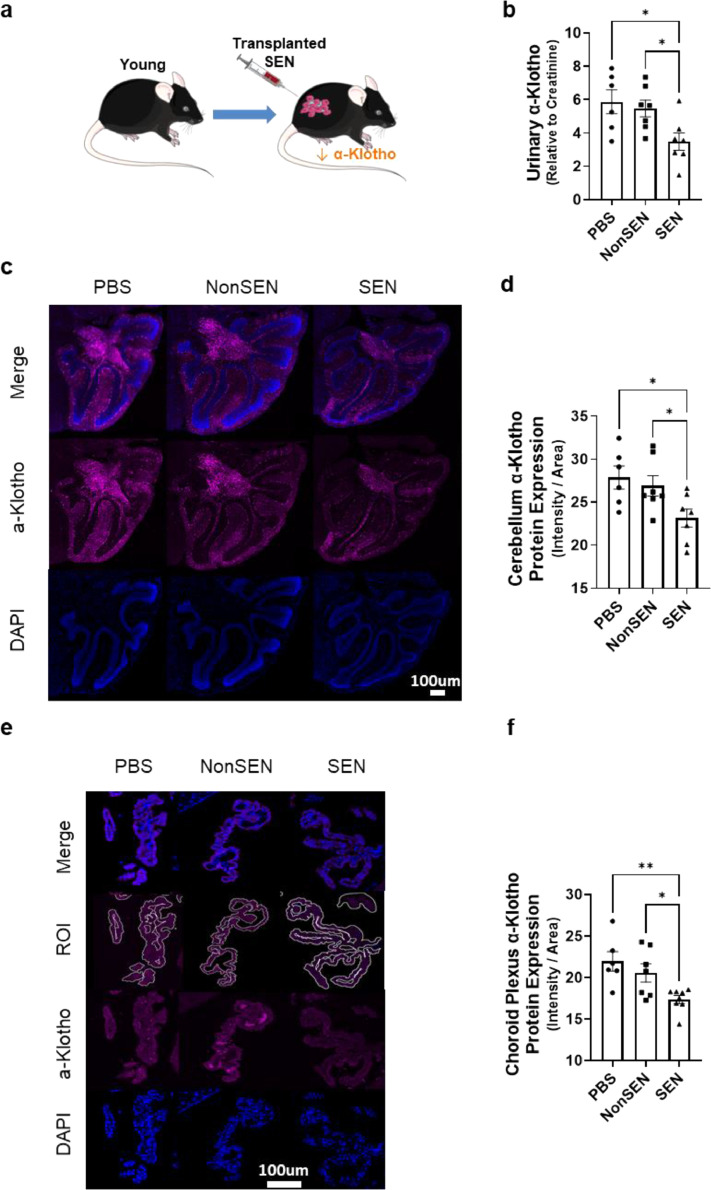
To test causality further, we ascertained if senescent cells can decrease α-Klotho in vivo by transplanting small numbers of senescent or non-senescent adipocyte progenitors vs. vehicle (PBS) i.p. into young mice (8-month-old) (Figure 2A). Transplanting these mice with senescent cells decreased urinary, cerebellar, and choroid plexus α-Klotho protein (Figure 2B–F) compared to control non-senescent cell transplanted- or PBS-treated mice.
Figure 2.
Transplanting senescent cells decreases urinary and brain α-Klotho. (a) 8-month-old male mice were transplanted i.p. with 2 million senescent or non-senescent mouse preadipocytes in 150 µl PBS through a 22-G needle. (b) Urinary α-Klotho was assayed by ELISA and expressed as a function of creatinine (one-way ANOVA: F = 6.45, p = 0.01, R2 = 0.42; n = 7; **p < 0.01, *p = 0.02). Representative images of immunofluorescent (IF) α-Klotho stains of cerebellum (c) and choroid plexus (e) are shown. ROI (Region of Interest, outlined in white) were quantified by ImageJ (d,f) from mice that had been transplanted with senescent or non-senescent cells. Means ± SEM; one-way ANOVA and post hoc Tukey's tests: F = 4.94, p = 0.02, R2 = 0.38. *p = 0.02, *p = 0.03 in (d), F = 7.84; p <0.01, R2 = 0.38, **p < 0.01, *p = 0.01 in (f).
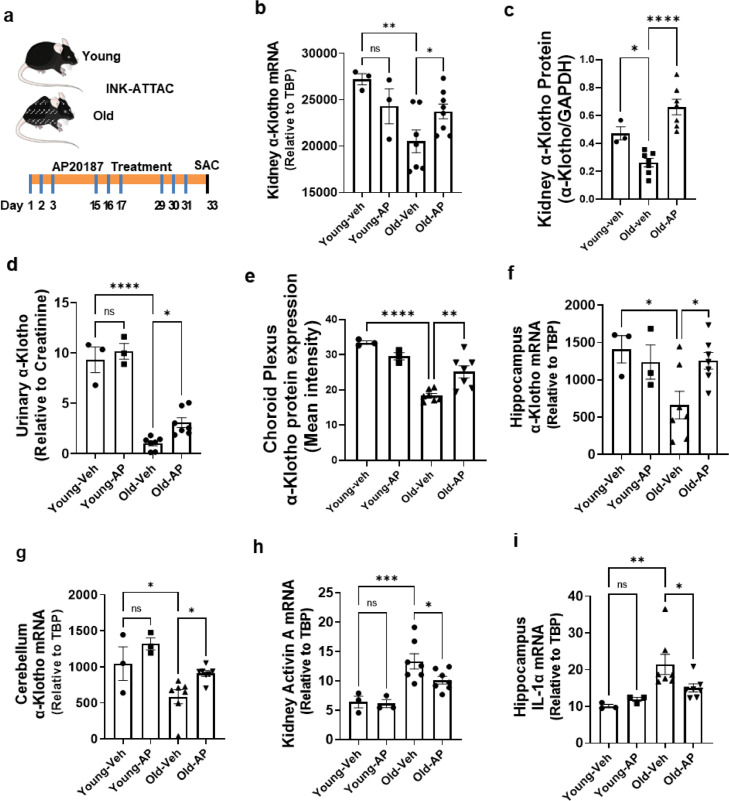
Genetic clearance of p16Ink4a-expressing senescent cells increases α-Klotho
Next, we determined if removal of p16Ink4a-expressing cells, many of which are senescent, increases α-Klotho in old mice. To accomplish this, we used transgenic INK-ATTAC mice, in which the drug, AP20187, dimerizes the ATTAC “suicide” caspase-8 moiety-containing fusion protein,51 causing death of cells that highly express p16Ink4a. Conditions alleviated by decreasing p16Ink4a+ cells in INK-ATTAC mice parallel those alleviated by increasing α-Klotho, including age- or high fat diet-induced adipose tissue and metabolic dysfunction, age-related osteoporosis, and bleomycin-induced lung fibrosis.45,60, 61, 62 Eight- and 26–27-month-old INK-ATTAC mice were administrated 10 mg/kg AP20187 in cycles of 3 consecutive days every 15 days and euthanized 5 days after the third 3-day course (9 doses in total) of AP20187 (Figure 3A). Kidney, urinary, and brain α-Klotho, which was lower in old than younger INK-ATTAC mice, was increased by AP20187 in the old mice (Figure 3B–D,E–G, Supplementary Fig. 4). Consistent with results in our in vitro experiment (Figure 1A–F), activin A and IL-1α were high in kidneys and brains of old INK-ATTAC mice, respectively, but decreased after AP20187 treatment (Figure 3H,I), supporting the hypothesis that senescent cells contribute to the age-related decline in α-Klotho in part through the SASP factors, activin A and IL-1α.
Figure 3.
Genetic clearance of highly p16Ink4a-expressing cells increases α-Klotho. Young (8-month-old) or old (27-29-month-old) INK-ATTAC male mice were treated with vehicle or AP20187 (n = 3 young+ vehicle; n = 3 young+ AP20187; n = 7 old + vehicle; n = 7 old + AP20187) to dimerize the FKBP-caspase-8 fusion protein expressed in highly p16Ink4a-expressing cells to selectively eliminate p16Ink4a+ cells. AP20187 (10 mg/kg) or vehicle was administered i.p. every 2 weeks for 6 weeks. (a) Schematic view of treatments. (b) Kidney α-Klotho mRNA was assayed by qPCR. One-way ANOVA, F = 4.81, p = 0.01, R2 = 0.46; *p = 0.03, **p < 0.01. (c) α-Klotho protein was assayed relative to GAPDH (Western blots in Supplementary Fig. 7). One-way ANOVA: F = 4.81, p = 0.01, R2 = 0.46; *p < 0.05, ****p < 0.0001. (d) Mouse urine was collected 2 days after the last dose of AP20187. Urinary α-Klotho was assayed by ELISA and expressed as a function of creatinine. One-way ANOVA, F = 61.53, p < 0.00001, R2 = 0.90; *p = 0.04, ****p < 0.0001. Choroid Plexus (e) α-Klotho protein was assayed by immunofluorescence. One-way ANOVA, F = 20.98, R2 = 0.80, **p < 0.01, ****p < 0.0001. Hippocampal (f) and cerebellar (g) α-Klotho mRNA was assayed by qPCR. Activin A mRNA in kidney (h) and IL-1α mRNA in hippocampus (i) were assayed by qPCR. Means ± SEM; one-way ANOVA and post hoc Tukey's tests; F = 3.84, p = 0.038, R2 = 0.40; *p = 0.04 in (f); *p = 0.04, F = 7.87, p < 0.01, R2 = 0.60; *p = 0.03 in (g); F = 10.85; p < 0.001, R2 = 0.64; *p = 0.03, ***p < p < 0.0001 in (h); F = 5.49; p = 0.01, R2 = 0.49; *p < 0.05, **p = 0.01 in (i).
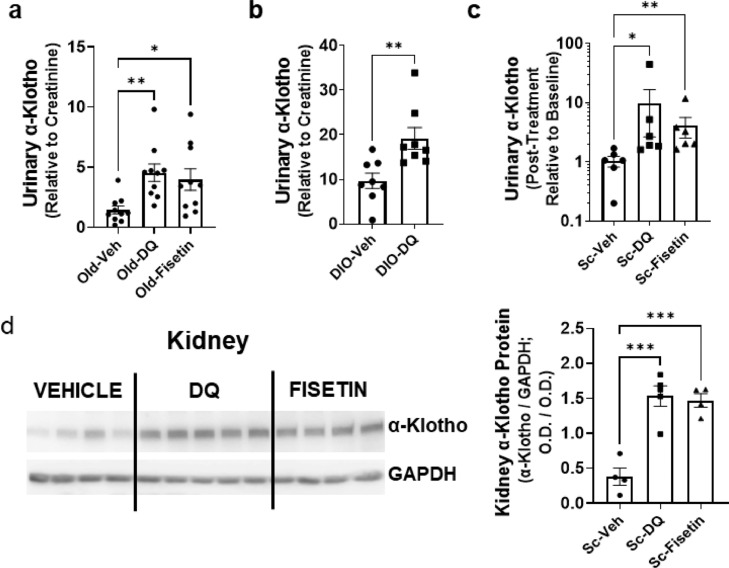
Senolytics increase α-Klotho in vivo
Senolytics are agents that reduce senescent cell burden.63 Senolytics, which were discovered independently from developing INK-ATTAC mice and do not depend on p16Ink4a expression to target senescent cells. Indeed, not all senescent cells have high p16Ink4a and not all cells that highly express p16Ink4a, such as activated macrophages, are senescent.64 Rather, senolytic agents act by transiently disabling the Senescent Cell anti-Apoptotic Pathways (SCAPs) that protect those senescent cells with a pro-apoptotic SASP from their own cytotoxic microenvironment.31,38,65 Treating mice orally with the senolytics, D+Q or F,38,65,66 increased urine α-Klotho in aged mice (Figure 4A). In young wild-type DIO mice, which have an increased burden of senescent cells compared to lean mice53,67, 68, 69 and a decline in urinary α-Klotho (Supplementary Fig. 3), D+Q was effective in increasing urinary α-Klotho (Figure 4B, Supplementary Fig. 4). Both D+Q and F increased urinary α-Klotho in young mice that had been transplanted with senescent cells (Figure 4C). Kidney α-Klotho, which was lower in old than young mice (Supplementary Fig. 3), was increased by D+Q in the old mice (Figure 4D).
Figure 4.
Senolytics increase urinary and kidney α-Klotho in old or obese mice. (a) Naturally-aged male mice (28-29-month-old, n = 10 in each group) were treated with vehicle, Dasatinib plus Quercetin (D+Q), or Fisetin. Urinary α-Klotho was measured by ELISA and expressed as a function of creatinine. One-way ANOVA: F = 6.39; p < 0.05, R2 = 0.30; *p = 0.02, **p < 0.01. (b) DIO mice (male, 10-month-old, n = 8) were treated with vehicle or D+Q and urinary α-Klotho was assayed by ELISA and expressed as a function of creatinine. Unpaired T test; **p < 0.01. (c) Six-month-old male mice (n = 6 in each group) were transplanted i.p. with senescent preadipocytes. After 3 months, they were treated with vehicle, D+Q, or Fisetin. Urinary α-Klotho was assayed by ELISA and expressed as a function of creatinine. One-way ANOVA and post hoc Tukey's tests: Kruskal-Wallis statistic=10.19; *p = 0.02, **p < 0.01. (d) Kidney α-Klotho protein was also assayed in the same transplanted mice by Western blotting. One-way ANOVA: F = 24.24; p < 0.001, R2 = 0.83; ***p < 0.001.
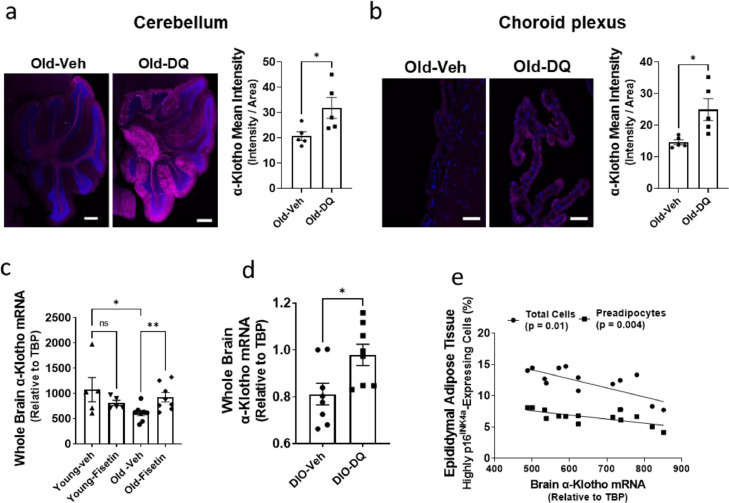
The brain is another primary site of α-Klotho production.70, 71, 72 We found senolytics increase α-Klotho protein in the cerebellum and choroid plexus (Figure 5A,B) as well as α-Klotho mRNA in whole brains of old mice in which α-Klotho decreases with ageing (Figure 5C). In young obese mice, senolytics increased brain α-Klotho (Figure 5D). Furthermore, α-Klotho was inversely related to adipose tissue senescent cell burden in untreated obese mice (Figure 5E).
Figure 5.
Senolytics increase brain α-Klotho in old mice. Naturally-aged mice (female, 28-month-old) were treated with vehicle (n = 5) or D+Q (n = 5). Brain α-Klotho was analysed by IF. (a) Representative IF images of α-Klotho expression (red) in the cerebellum are shown. Mean intensities of red florescence in the cerebellum were quantified by ImageJ. Unpaired T-tests; *p = 0.03. (b) Brain choroid plexus α-Klotho (purple), representative IF images. Mean intensities of florescence in the choroid plexus were quantified by ImageJ. Unpaired T-tests; *p = 0.02. (c) Young (female, 6-month-old, n = 5 in vehicle and treated groups) and naturally-aged mice (female, 22-month-old, n = 8 in vehicle and treated groups) were treated with Fisetin or vehicle. α-Klotho in whole brain was assayed by qPCR. One-way ANOVA and post hoc Tukey's tests: Kruskal-Wallis statistic=9.49; *p = 0.02, **p < 0.01. (d) DIO mice (male, 8-9-month-old) were treated with D+Q (n = 8) or vehicle (n = 8). Brain α-Klotho was assayed by qPCR. Mann-Whitney test; *p = 0.03. (e) Correlation between brain α-Klotho mRNA and peripheral p16Ink4a-expressing adipose tissue progenitor cells was quantified by CyTOF. Spearman correlation analysis.
To determine if senolytics increase α-Klotho through direct, off-target effects in α-Klotho-expressing non-senescent cells, we exposed human cultured non-senescent preadipocytes or astrocytes to D+Q or F (Supplementary Fig. 5). Treatment with senolytics did not increase α-Klotho in these cultured non-senescent cells. Treating young INK-ATTAC mice with AP20187 or D+Q also did not increase kidney α-Klotho (Supplementary Fig. 6A). Furthermore, senolytics did not increase α-Klotho in urine of young mice (Figure 6B). Thus, senescent cell targeting strategies do not appear to increase α-Klotho when senescent cell burden is low, consistent with increases in α-Klotho being due to removal of senescent cells, rather than other mechanisms.
Figure 6.
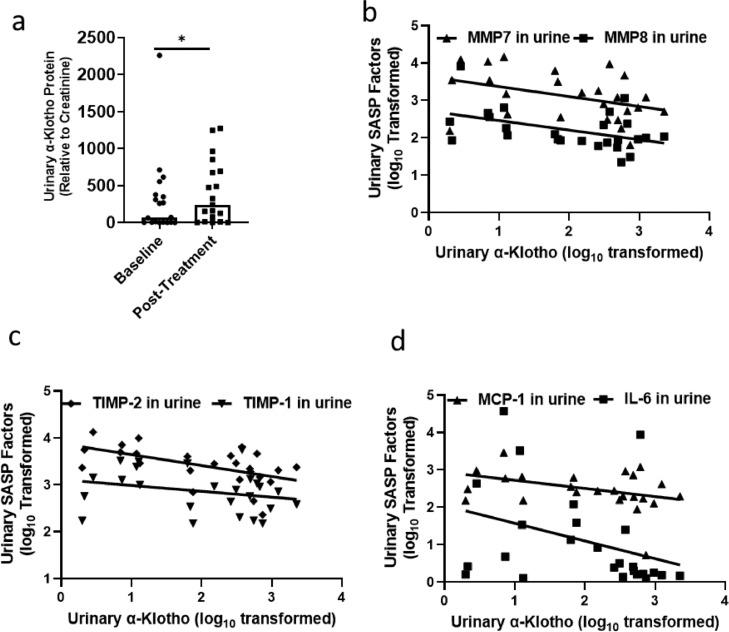
Senolytics increase α-Klotho in human urine; urinary SASP factors are inversely related to urinary α-Klotho. Subjects (n = 20) with idiopathic pulmonary fibrosis (IPF) were administered 3 courses of D+Q for 3 sequential days each (total 9 doses over 3 weeks). (a) Urinary α-Klotho and SASP factors were assayed at baseline and 5 days after the last D+Q treatment. Urinary α-Klotho in subjects with IPF was increased (Baseline: 293.49±115.48, Post-treatment: 392.79±95.24, normalized to urinary creatinine) by D+Q. Wilcoxon signed rank tests (two-sided) were used to test differences before and after treatment. *p = 0.04. (b–d) Spearman correlations of urinary α-Klotho and SASP factors are shown. FDR-corrected R2 and p values are in Supplementary Table 2.
Senolytics increase α-Klotho in humans
We recently reported that treating patients who have IPF with 9 doses of D+Q over 3 weeks alleviated physical dysfunction, with increased gait distance and speed, ability to rise out of a chair, and improved short physical performance battery scores 5 days after the last dose of senolytics.49 In these subjects, urinary α-Klotho was increased after (392.79 ± 95.24, normalized to urinary creatinine), compared to before (293.49 ± 115.48, normalized to urinary creatinine), D+Q administration (Figure 6A). We also found that urinary SASP factors were inversely correlated with urinary α-Klotho (Figure 6B–D, Supplementary Table 2).
Discussion
Senolytics appear to be an effective small molecule, orally-active interventional strategy for increasing α-Klotho. Senolytics eliminate mouse and human senescent cells in vitro and reduce senescent cell burden across a range of animal models as well as in humans.31,36,38,66 The senolytic combination, D+Q, improves cardiovascular function in old mice, the first demonstration that reducing senescent cell abundance improves health-span in naturally-aged mice,38 and is effective in alleviating multiple ageing phenotypes and chronic diseases across a number of mouse models.36,38,45,49,53,62,73, 74, 75, 76, 77, 78, 79 D+Q may also alleviate physical dysfunction in humans with IPF.49
Besides senolytics, other small molecules that may increase α-Klotho include PPARγ agonists,80 losartan,81 statin HMG-CoA reductase inhibitors,82 and vitamin D derivatives.83 Whether the latter agents affect α-Klotho through effects on senescent cells or other mechanisms remains to be determined. At least in the case of cultured HUVECs, angiotensin II exposure can promote cells to become senescent.84 This angiotensin II-driven senescence was prevented by α-Klotho as well as an angiotensin II-inhibiting peptide that also increased α-Klotho, suggesting links between α-Klotho and senescence in HUVECs. This also implies that α-Klotho could potentially have either senomorphic (suppression of senescence) or senolytic effects, which needs further investigation.
A potential advantage of oral senolytics over other approaches is that senolytics are effective when administered intermittently in a “hit-and-run” fashion. Elimination half-lives of some senolytics are short (<4 h for D, 11 h for Q, and 4 h for F) and senescent cells take weeks to over a month to re-develop, at least in cell culture,31,36,62,85 enabling a “hit-and-run” administration strategy. Additionally, senolytics may be more practical for increasing α-Klotho than recombinant α-Klotho protein or related peptides,40,41 since the latter may need to be administered repeatedly and by injection, rather than intermittently and orally. Furthermore, α-Klotho protein or related peptides may not readily act across the blood brain barrier. However, D+Q reduces brain senescent cell burden in mice that are models of Alzheimer's disease78 as well as in obese mice.75
Our results indicate that assaying urinary α-Klotho could be a useful approach for monitoring effects of senolytics during clinical trials or, eventually, if senolytics are translated into clinical practice, in the course of treating patients. First morning urine assays have the advantage of providing an integrated representation of α-Klotho production over several hours, as opposed to the instantaneous view from blood tests. In addition, urine collections are less invasive than venipuncture. Furthermore, urine tests are probably less confounded by the more extensive presence of cells of various types in blood than urine and possible effects of other proteins in blood that might bind to α-Klotho. α-Klotho is a complex molecule. Changing its conformation by binding to other proteins might affect availability of α-Klotho epitopes for antibody binding, and hence interfere with reliable detection by immunoassay.
Our study has caveats regarding the precise mechanisms through which senolytics increase α-Klotho: is this by reducing bystander effects of senescent cells or removing α-Klotho deficient senescent cells? We will dissect such potential mechanisms in future studies. Additional future studies are needed to: (1) ascertain if CSF α-Klotho increases after administering senolytics to humans with Alzheimer's disease (currently being studied in the clinical trials, NCT04785300 and NCT04685590), (2) compare blood indices of senescent cell burden to urinary α-Klotho in patients across the age spectrum in other current and future clinical studies of senolytics, and (3) ascertain why in pre-eclampsia, a condition that we found is associated with increased senescent cells86 and that has been linked to decreased urinary α-Klotho,87 plasma α-Klotho is increased.88 This discrepancy between α-Klotho in urine and plasma in pre-eclampsia is consistent with the previous finding that in chronic kidney disease (CKD), plasma α-Klotho is not related to kidney function and does not predict adverse outcomes.89 These findings in pre-eclampsia suggest that, while cellular senescence appears to be a major regulator of α-Klotho production, it is unlikely to be the only one.
The results here showing interactions between α-Klotho and cellular senescence add to evidence supporting our “Unitary Theory of Targeting Fundamental Ageing Mechanisms”90,91: that targeting any one fundamental ageing mechanism may positively impact most or all of the others. Cellular senescence can contribute to other “pilars of ageing”, including inflammation, fibrosis, DNA damage, mitochondrial dysfunction, ROS generation, NAD+ depletion, protein aggregation, failed autophagy, lipotoxicity, and stem/progenitor cell dysfunction. Conversely, other fundamental ageing processes can lead to cellular senescence. This may partly explain why targeting even a single fundamental ageing process, such as senescence, appears to delay, prevent, or alleviate multiple age-related diseases and disorders as a group.30,90, 91, 92 We speculate that senolytics might impact many, or perhaps even all of the pillars of ageing,93 with increased α-Klotho resulting from administering senolytics being an example. Examples of the impact of senolytics on other pillars of ageing include: (1) preventing or reversing age-related decreases in the ecto-enzyme CD38, which metabolizes nicotinamide adenine dinucleotide (NAD+), leading to restoration of NAD+ levels,94 (2) attenuating age-related inflammation and fibrosis,37,45,75,95, 96, 97, 98 (3) reducing the extent of age-related declines in mitochondrial function and blunting ROS generation,99 (4) decreasing DNA damage,100 (5) restoring insulin sensitivity,53,60 and (6) blunting age-related increases in mTOR (manuscript in preparation). Additionally, targeting senescent cells can alleviate age- and chronic disease-related progenitor cell dysfunction: senolytics enhance adipose progenitor,36,53 osteoblast progenitor,62 and cardiac progenitor cell function76 and restore neurogenesis75,77 in ageing animals or animals with cellular senescence-related conditions. If our Unitary Theory holds true, it will be critical to test whether combining individual interventions targeting interlinked or independent fundamental ageing mechanisms has less than additive, additive, or synergistic effects.
Declaration of interests
Y.Z., T.T., N.G., T.P., A.K.P., and J.L.K. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. No conflicts of interest, financial or otherwise, are declared by the other authors.
Acknowledgments
Contributors
Y.Z., L.G.P.L.P., J.M.E.N., N.G., T.P., A.X., U.T., E.O.W.G., and T.C. performed experiments and verified the data in this study. K.O.J. and C.L.I. bred, genotyped, and pre-treated mice. L.G.P.L.P. and K.S. conducted immunofluorescence staining and quantification. J.C. performed statistical analyses. J.N.J., A.M.N., N.M., S.B.K., S.K., T.T., and J.L.K. designed or conducted the previous human clinical trial. J.L.K., Y.Z., and T.T. designed the current study and wrote the manuscript. A.K.P., D.J., and M.J.S. reviewed and edited the manuscript. All authors read, edited, and approved the final version of the manuscript.
Acknowledgements
We thank Victoria S. Shea and Jacqueline L. Armstrong for administrative help during manuscript submission. We thank Dr. Darren J. Baker for providing feedback and comments on this work. This work was supported by National Institute of Health grants AG013925 (J.L.K.), AG062413 (J.L.K., S.K.), AG044271 (N.M.), AG013319 (N.M.), and the Translational Geroscience Network (AG061456: J.L.K., T.T., N.M., S.B.K., S.K.), Robert and Arlene Kogod (J.L.K.), the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.), and the Ted Nash Long Life (J.L.K.) and Noaber Foundations (J.L.K.). The previous IPF clinical trial was supported by the Claude D. Pepper Older Americans Independence Centers at WFSM (AG021332: J.N.J., S.B.K.) and UTHSCA (AG044271: A.M.N.), and the Translational Geroscience Network.
Data sharing statement
All data associated with this study are in the article or the Supplementary Materials. The INK-ATTAC mouse strain is patented by Mayo Clinic and should be requested under a material transfer agreement.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2022.103912.
Contributor Information
Yi Zhu, Email: Zhu.Yi@Mayo.edu.
James L. Kirkland, Email: Kirkland.James@Mayo.edu.
Appendix. Supplementary materials
References
- 1.Maique J., Flores B., Shi M., et al. High phosphate induces and klotho attenuates kidney epithelial senescence and fibrosis. Front Pharmacol. 2020;11:1273. doi: 10.3389/fphar.2020.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuro OM. The klotho proteins in health and disease. Nat Rev Nephrol. 2019;15(1):27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 3.Kuro OM. Klotho and endocrine fibroblast growth factors: Markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transpl. 2019;34(1):15–21. doi: 10.1093/ndt/gfy126. [DOI] [PubMed] [Google Scholar]
- 4.Hu M.C., Shiizaki K., Kuro-o M., Moe OW. Fibroblast growth factor 23 and klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delcroix V., Mauduit O., Tessier N., et al. The role of the anti-aging protein klotho in igf-1 signaling and reticular calcium leak: impact on the chemosensitivity of dedifferentiated liposarcomas. Cancers. 2018;10(11):439. doi: 10.3390/cancers10110439. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaconstantinou J. Insulin/igf-1 and ros signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol. 2009;299(1):89–100. doi: 10.1016/j.mce.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daneshgar N., Dai DF. Ros, klotho and mtor in cardiorenal aging. Aging. 2020;12(20):19830–19831. doi: 10.18632/aging.104209. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C., Ma X., Zhou Y., Liu Y., Shao Y., Wang Q. Klotho restraining egr1/tlr4/mtor axis to reducing the expression of fibrosis and inflammatory cytokines in high glucose cultured rat mesangial cells. Exp Clin Endocrinol Diabetes. 2019;127(9):630–640. doi: 10.1055/s-0044-101601. [DOI] [PubMed] [Google Scholar]
- 9.Rakugi H., Matsukawa N., Ishikawa K., et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31(1):82–87. doi: 10.1007/s12020-007-0016-9. PMID: 17709902. [DOI] [PubMed] [Google Scholar]
- 10.Yang J., Matsukawa N., Rakugi H., et al. Upregulation of camp is a new functional signal pathway of klotho in endothelial cells. Biochem Biophys Res Commun. 2003;301(2):424–429. doi: 10.1016/s0006-291x(02)03056-5. [DOI] [PubMed] [Google Scholar]
- 11.Ikushima M., Rakugi H., Ishikawa K., et al. Anti-apoptotic and anti-senescence effects of klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339(3):827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 12.Xie L., Wang Y., Li Q., et al. The hif-1alpha/p53/mirna-34a/klotho axis in retinal pigment epithelial cells promotes subretinal fibrosis and exacerbates choroidal neovascularization. J Cell Mol Med. 2021;25(3):1700–1711. doi: 10.1111/jcmm.16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurosu H., Ogawa Y., Miyoshi M., et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fon Tacer K., Bookout A.L., Ding X., et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24(10):2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinek T., Wolf I., Modan-Moses D. The longevity hormone klotho is a new player in the interacion of the growth hormone/insulin-like growth factor 1 axis. Pediatr Endocrinol Rev. 2016;14(1):9–18. doi: 10.17458/PER.2016.RWM.LongevityHormoneKlotho. [DOI] [PubMed] [Google Scholar]
- 16.Typiak M., Piwkowska A. Antiinflammatory actions of klotho: Implications for therapy of diabetic nephropathy. Int J Mol Sci. 2021;22(2):956. doi: 10.3390/ijms22020956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuro-o M. Overview of the fgf23-klotho axis. Pediatr Nephrol. 2010;25(4):583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 18.Cheikhi A., Barchowsky A., Sahu A., et al. Klotho: an elephant in aging research. J Gerontol A Biol Sci Med Sci. 2019;74(7):1031–1042. doi: 10.1093/gerona/glz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul C., Amaral A.P., Oskouei B., et al. Fgf23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuro-o M., Matsumura Y., Aizawa H., et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi H., Manabe N., Miyaura C., Chikuda H., Nakamura K. Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104(3):229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suga T., Kurabayashi M., Sando Y., et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol. 2000;22(1):26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- 23.Kamemori M., Ohyama Y., Kurabayashi M., Takahashi K., Nagai R., Furuya N. Expression of klotho protein in the inner ear. Hear Res. 2002;171(1-2):103–110. doi: 10.1016/s0378-5955(02)00483-5. [DOI] [PubMed] [Google Scholar]
- 24.Nagai T., Yamada K., Kim H.C., et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17(1):50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- 25.Shimada T., Takeshita Y., Murohara T., et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110(9):1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 26.Fan J., Sun Z. The antiaging gene klotho regulates proliferation and differentiation of adipose-derived stem cells. Stem Cells. 2016;34(6):1615–1625. doi: 10.1002/stem.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W., Xiao T., Han W., et al. Klotho inhibits pkcalpha/p66shc-mediated podocyte injury in diabetic nephropathy. Mol Cell Endocrinol. 2019;494:110490. doi: 10.1016/j.mce.2019.110490. [DOI] [PubMed] [Google Scholar]
- 28.He T., Xiong J., Huang Y., et al. Klotho restrain rig-1/nf-kappab signaling activation and monocyte inflammatory factor release under uremic condition. Life Sci. 2019;231 doi: 10.1016/j.lfs.2019.116570. [DOI] [PubMed] [Google Scholar]
- 29.Sahu A., Clemens Z.J., Shinde S.N., et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nature Aging. 2021;1:1148–1161. doi: 10.1038/s43587-021-00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchkonia T., Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA. 2018;320(13):1319–1320. doi: 10.1001/jama.2018.12440. [DOI] [PubMed] [Google Scholar]
- 31.Kirkland J.L., Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justice J.N., Gregory H., Tchkonia T., et al. Cellular senescence biomarker p16ink4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2018;73(7):939–945. doi: 10.1093/gerona/glx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppe J.P., Patil C.K., Rodier F., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic ras and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuilman T., Peeper DS. Senescence-messaging secretome: Sms-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 35.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M., Pirtskhalava T., Farr J.N., et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandhaya-Pillai R., Miro-Mur F., Alijotas-Reig J., Tchkonia T., Kirkland J.L., Schwartz S. Tnfalpha-senescence initiates a stat-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging. 2017;9(11):2411–2435. doi: 10.18632/aging.101328. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y., Tchkonia T., Pirtskhalava T., et al. The achilles' heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P., et al. Suppression of aging in mice by the hormone klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y., Zeng C.Y., Li X.H., Yang T.T., Kuang X., Du JR. Klotho overexpression improves amyloid-beta clearance and cognition in the app/ps1 mouse model of alzheimer's disease. Aging Cell. 2020;19(10):e13239. doi: 10.1111/acel.13239. Epub ahead of print. PMID: 32964663; PMCID: PMC7576297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hum J.M., O'Bryan L.M., Tatiparthi A.K., et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. J Am Soc Nephrol. 2017;28(4):1162–1174. doi: 10.1681/ASN.2015111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao D., Wang S., Lin Y., Sun Z. In vivo aav delivery of glutathione reductase gene attenuates anti-aging gene klotho deficiency-induced kidney damage. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welc S.S., Wehling-Henricks M., Kuro O.M., Thomas K.A., Tidball JG. Modulation of klotho expression in injured muscle perturbs wnt signalling and influences the rate of muscle growth. Exp Physiol. 2020;105(1):132–147. doi: 10.1113/EP088142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Oliveira RM. Klotho rnai induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580(24):5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 45.Schafer M.J., White T.A., Iijima K., et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Q., Chen Y., Shen S., et al. Klotho antagonizes pulmonary fibrosis through suppressing pulmonary fibroblasts activation, migration, and extracellular matrix production: a therapeutic implication for idiopathic pulmonary fibrosis. Aging. 2020;12(7):5812–5831. doi: 10.18632/aging.102978. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes J.W., Duncan D., Helton S., et al. Role of fibroblast growth factor 23 and klotho cross talk in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019;317(1):L141–LL54. doi: 10.1152/ajplung.00246.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buendia-Roldan I., Machuca N., Mejia M., Maldonado M., Pardo A., Selman M. Lower levels of alpha-klotho in serum are associated with decreased lung function in individuals with interstitial lung abnormalities. Sci Rep. 2019;9(1):10801. doi: 10.1038/s41598-019-47199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Justice J.N., Nambiar A.M., Tchkonia T., et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker D.J., Wijshake T., Tchkonia T., et al. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trujillo M.E., Pajvani U.B., Scherer P.E., et al. Apoptosis through targeted activation of caspase 8 (“attac-mice”): novel mouse models of inducible and reversible tissue ablation. Cell Cycle. 2005;4(9):1141–1145. doi: 10.4161/cc.4.9.2030. [DOI] [PubMed] [Google Scholar]
- 52.Wang W., Wu J., Zhang Z., Tong T. Characterization of regulatory elements on the promoter region of p16(ink4a) that contribute to overexpression of p16 in senescent fibroblasts. J Biol Chem. 2001;276(52):48655–48661. doi: 10.1074/jbc.M108278200. [DOI] [PubMed] [Google Scholar]
- 53.Palmer A.K., Xu M., Zhu Y., et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3):e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schindelin J., Arganda-Carreras I., Frise E. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia J., Mandal R., Sinelnikov I.V., Broadhurst D., Wishart DS. Metaboanalyst 2.0–a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40:W127–W133. doi: 10.1093/nar/gks374. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia J., Psychogios N., Young N., Wishart DS. Metaboanalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. doi: 10.1093/nar/gkp356. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nordholm A., Egstrand S., Gravesen E., et al. Circadian rhythm of activin a and related parameters of mineral metabolism in normal and uremic rats. Pflug Arch. 2019;471(8):1079–1094. doi: 10.1007/s00424-019-02291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugiura H., Yoshida T., Shiohira S., et al. Reduced klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol. 2012;302(10):F1252–F1264. doi: 10.1152/ajprenal.00294.2011. [DOI] [PubMed] [Google Scholar]
- 59.Yang X.H., Zhang B.L., Zhang X.M., et al. Egcg attenuates renal damage via reversing klotho hypermethylation in diabetic db/db mice and hk-2 cells. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/6092715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu M., Palmer A.K., Ding H., et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer A.K., Tchkonia T., LeBrasseur N.K., Chini E.N., Xu M., Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farr J.N., Xu M., Weivoda M.M., et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirkland J.L., Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015;68:19–25. doi: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall B.M., Balan V., Gleiberman A.S., et al. P16(ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging. 2017;9(8):1867–1884. doi: 10.18632/aging.101268. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yousefzadeh M.J., Zhu Y., McGowan S.J., et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y., Doornebal E.J., Pirtskhalava T., et al. New agents that target senescent cells: the flavone, fisetin, and the bcl-xl inhibitors, a1331852 and a1155463. Aging. 2017;9(3):955–963. doi: 10.18632/aging.101202. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minamino T., Orimo M., Shimizu I., et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 68.Tchkonia T., Morbeck D.E., Von Zglinicki T., et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmer A.K., Gustafson B., Kirkland J.L., Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62(10):1835–1841. doi: 10.1007/s00125-019-4934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vo H.T., Laszczyk A.M., King GD. Klotho, the key to healthy brain aging? Brain Plast. 2018;3(2):183–194. doi: 10.3233/BPL-170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clinton S.M., Glover M.E., Maltare A., et al. Expression of klotho mrna and protein in rat brain parenchyma from early postnatal development into adulthood. Brain Res. 2013;1527:1–14. doi: 10.1016/j.brainres.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li S.A., Watanabe M., Yamada H., Nagai A., Kinuta M., Takei K. Immunohistochemical localization of klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29(4):91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 73.Roos C.M., Zhang B., Palmer A.K., et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogrodnik M., Miwa S., Tchkonia T., et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogrodnik M., Zhu Y., Langhi L.G.P., et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29(5):1061-77.e8. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis-McDougall F.C., Ruchaya P.J., Domenjo-Vila E., et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. 2019;18(3):e12931. doi: 10.1111/acel.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musi N., Valentine J.M., Sickora K.R., et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang P., Kishimoto Y., Grammatikakis I., et al. Senolytic therapy alleviates abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an alzheimer's disease model. Nat Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nath K.A., O'Brien D.R., Croatt A.J., et al. The murine dialysis fistula model exhibits a senescence phenotype: pathobiological mechanisms and therapeutic potential. Am J Physiol Renal Physiol. 2018;315(5):F1493–F14F9. doi: 10.1152/ajprenal.00308.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H., Li Y., Fan Y., et al. Klotho is a target gene of ppar-gamma. Kidney Int. 2008;74(6):732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 81.Lim S.C., Liu J.J., Subramaniam T., Sum CF. Elevated circulating alpha-klotho by angiotensin ii receptor blocker losartan is associated with reduction of albuminuria in type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst. 2014;15(4):487–490. doi: 10.1177/1470320313475905. [DOI] [PubMed] [Google Scholar]
- 82.Kuwahara N., Sasaki S., Kobara M., et al. Hmg-coa reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol. 2008;123(2):84–90. doi: 10.1016/j.ijcard.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 83.Hajialilo M., Noorabadi P., Tahsini Tekantapeh S., Malek Mahdavi A. Endothelin-1, alpha-klotho, 25(oh) vit d levels and severity of disease in scleroderma patients. Rheumatol Int. 2017;37(10):1651–1657. doi: 10.1007/s00296-017-3797-z. [DOI] [PubMed] [Google Scholar]
- 84.Romero A., San Hipolito-Luengo A., Villalobos L.A., et al. The angiotensin-(1-7)/mas receptor axis protects from endothelial cell senescence via klotho and nrf2 activation. Aging Cell. 2019;18(3):e12913. doi: 10.1111/acel.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkland J.L., Tchkonia T., Zhu Y., Niedernhofer L.J., Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65(10):2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suvakov S., Cubro H., White W.M., et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ. 2019;10(1):49. doi: 10.1186/s13293-019-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suvakov S., Ghamrawi R., Cubro H., et al. Epigenetic and senescence markers indicate an accelerated ageing-like state in women with preeclamptic pregnancies. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loichinger M.H., Towner D., Thompson K.S., Ahn H.J., Bryant-Greenwood GD. Systemic and placental alpha-klotho: effects of preeclampsia in the last trimester of gestation. Placenta. 2016;41:53–61. doi: 10.1016/j.placenta.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seiler S., Wen M., Roth H.J., et al. Plasma klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int. 2013;83(1):121–128. doi: 10.1038/ki.2012.288. [DOI] [PubMed] [Google Scholar]
- 90.Tchkonia T., Palmer A.K., Kirkland JL. New horizons: Novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J Clin Endocrinol Metab. 2021;106(3):e1481–e14e7. doi: 10.1210/clinem/dgaa728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirkland J.L., Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wissler Gerdes E.O., Zhu Y., Weigand B.M., et al. Cellular senescence in aging and age-related diseases: implications for neurodegenerative diseases. Int Rev Neurobiol. 2020;155:203–234. doi: 10.1016/bs.irn.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy B.K., Berger S.L., Brunet A., et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chini C., Hogan K.A., Warner G.M., et al. The nadase cd38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular nad(+) decline. Biochem Biophys Res Commun. 2019;513(2):486–493. doi: 10.1016/j.bbrc.2019.03.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogrodnik M., Evans S.A., Fielder E., et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20(2):e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saccon T.D., Nagpal R., Yadav H., et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J Gerontol A Biol Sci Med Sci. 2021 doi: 10.1093/gerona/glab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iske J., Seyda M., Heinbokel T., et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. 2020;11(1):4289. doi: 10.1038/s41467-020-18039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moncsek A., Al-Suraih M.S., Trussoni C.E., et al. Targeting senescent cholangiocytes and activated fibroblasts with b-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (mdr2(-/-)) mice. Hepatology. 2018;67(1):247–259. doi: 10.1002/hep.29464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vizioli M.G., Liu T., Miller K.N., et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020;34(5-6):428–445. doi: 10.1101/gad.331272.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yousefzadeh M., Henpita C., Vyas R., Soto-Palma C., Robbins P., Niedernhofer L. DNA damage-how and why we age? Elife. 2021;10 doi: 10.7554/eLife.62852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.