Abstract
Anti-cytokine autoantibodies (ACAAs) can cause immunodeficiency or dysregulate immune responses. They may phenocopy genetically defined primary immunodeficiencies. We review current anti-Type 1 and 2 interferon, anti-interleukin-12/23, anti-interleukin-17, anti-granulocyte-macrophage-colony-stimulating-factor autoantibodies, HLA associations, disease associations, and mechanistically based treatment options. Suspecting and identifying patients at the onset of symptoms should ameliorate disease and improve outcomes.
Keywords: anti-cytokine autoantibodies, anti-interferon-gamma, anti-interferon-alpha, anti-interleukin-23, anti-interleukin-12, anti-interleukin-17, anti-granulocyte-macrophage colony stimulating factor, opportunistic infections, adult-onset immunodeficiency, protein alveolar proteinosis, chronic mucocutaneous candidiasis
INTRODUCTION
High titer neutralizing anti-cytokine autoantibodies (ACAAs), typically polyclonal IgG, are increasingly recognized in diverse infectious and/or immunological conditions (1–3). Anti-interferon-gamma (IFN-γ) autoantibodies are seen in disseminated mycobacterial disease; anti-interleukin-17 (IL-17) in chronic mucocutaneous candidiasis (CMC); anti-granulocyte-macrophage-colony-stimulating-factor (GM-CSF) in cryptococcosis, nocardiosis and pulmonary alveolar proteinosis; anti-IL-6 in staphylococcal sepsis; and anti-Type I interferons in viral infections and severe COVID-19 (4–12).
ACAAs are common, even in healthy individuals (12, 13). They may affect cytokine biology by diminishing or augmenting signaling or by altering their half-life in the circulation (13–19). In contrast to pathogenic ACAAs associated with immunodeficiency, regulatory ACAAs are not as inhibitory, and are typically detected at lower binding titers (20, 21). With age, the frequency of ACAAs increases, and their respective titers and functionality may change in the setting of endogenous ligands, such as in ARDS and sepsis, or anti-Type 1 interferons during the onset of acute COVID19 (22, 23). The probability that ACAAs are deterministic (have an immunoregulatory function) rather than stochastic (appear independent of physiological effects) increases as their neutralizing activity increases.
Anti-interferon-γ autoantibodies
Interferon-γ is the key macrophage-activating factor secreted by αβ+ (including natural killer T (NKT), mucosal associated invariant T (MAIT) cells, conventional CD4+ and CD8+ T cells), γδ+ T cells, B cells, NK cells, and some innate lymphoid cells (ILCs) (24). Patients with neutralizing anti-IFN-γ autoantibodies (or any of the known inborn errors of IFN-γ due to defects in 16 distinct genes) are prone to mycobacterial disease and related infections by intra-macrophagic microbes (25, 26). Over 600 cases have been reported since the first fatal cases were described in 2004 (27–29). There is a preponderance of HIV-uninfected individuals of East Asian descent (including Filipino, Thai, Taiwanese, Laotian, Japanese, Chinese but not Koreans) with HLA-DRB1*15:02/16:02 and HLA-DQB1*05:01/05:02 (30, 31). However, recent reports in Caucasians, two children, a Surinamese individual of African descent, a Sri-Lankan man, and an HIV-positive individual with opportunistic infections years after immune reconstitution, suggest that this autoimmune phenomenon may be more widespread (32–36).
Lymph nodes, bones/joints, and lungs are most affected, with occasional soft tissue and skin involvement (in the form of reactive neutrophilic dermatosis, erythema nodosum, or exanthematous pustulosis) (37, 38). Lymph node histopathology may be mistaken for T-cell lymphoma (50% show monoclonality, 33% may be indistinguishable from angioimmunoblastic T-cell lymphoma by criteria), IgG4-related disease, or multicentric Castleman disease. These episodes of mimicry highlight the need to culture and stain for mycobacteria in the evaluation of lymphadenopathy or to at least consider anti-IFN-γ autoantibodies before chemotherapy or corticosteroids (39–41). The microbial spectrum includes Salmonella, Burkholderia, Bacillus species, Cryptococcus, Talaromyces, Coccidioides, Histoplasma, Toxoplasma and varicella-zoster, herpes simples and cytomegalovirus (4, 42–45).
Anti-IFN-γ autoantibodies block IFN-γ binding to its receptor and downstream phosphorylation of Signal Transducer and Activator of Transcription 1 (STAT1). Blockade of IFN-γ induced STAT1 phosphorylation by patient plasma or serum in vitro is the simplest assay for anti-IFN-γ autoantibodies (46, 47). However, one of the most common and clinically accessible tests is the QuantiFERON Gold In-Tube, which relies on detection of IFN-γ elaborated in response to antigen or mitogen; it is blocked by anti-IFN-γ autoantibodies. Therefore, either a positive or a negative QuantiFERON Gold result indicate that IFN-γ produced in response to mitogen was detected and argue strongly against the presence of anti-IFN-γ autoantibodies; in contrast, an indeterminate result is consistent with anti-IFN-γ autoantibodies (48, 49).
Exogenous IFN-γ does not ameliorate disease since these autoantibodies are typically quite high titer (4, 50). In contrast, immunomodulation with cyclophosphamide, rituximab, bortezomib, abatacept and daratumumab have all been partially successful in severe or refractory cases (37, 51–55). These reports suggest that reducing autoantibody-producing B-cells or plasma cells may help control infections (56). However, not all cases require immune directed therapy and in many patients the levels of anti-IFN-γ autoantibodies declines over time in conjunction with treatment of the mycobacterial disease (38). The direction of the causality here is unresolved.
Anti-IL-12 and IL-23 autoantibodies
IL-12 and IL-23 share the p40 subunit and both use the IL12Rβ1 receptor and are essential for optimal production of IFN-γ (57, 58). Therefore, it is unsurprising that autoantibodies against them are also associated with opportunistic infections. Anti-IL12 and anti-IL23 autoantibodies are found in 33–45% of patients with thymoma overall, but in patients with recurrent sinopulmonary or disseminated infections, anti-IL12 and anti-IL23 frequencies increase to >95% (16, 59–61) and unpublished data).
Anti-GM-CSF autoantibodies
Cryptococcosis (particularly C. gattii) and nocardiosis may disseminate to the brain in seemingly immunocompetent individuals and have been associated with neutralizing anti-GM-CSF autoantibodies, which also cause autoimmune pulmonary alveolar proteinosis (aPAP)(62–66). Anti-GM-CSF-mediated disruption of STAT5 signaling and PU.1 nuclear translocation results in alveolar macrophage dysfunction, intra-alveolar accumulation of surfactant lipoproteinaceous material, and the insidious onset of interstitial lung disease (67, 68). Anti-GM-CSF autoantibodies are associated with HLA-DRB1*08:03 in Japanese patients with aPAP (69). Treatments for aPAP have included whole lung lavage, inhaled recombinant human GM-CSF, and rituximab (70–72). Anti-GM-CSF autoantibodies concentration are lower in bronchoalveolar lavage than in the blood, hence exogenous inhaled GM-CSF, unlike exogenous IFN-γ, may overcome some of the neutralizing activity at the site of tissue pathology (73). Rituximab is less effective against anti-GM-CSF autoantibodies than anti-IFN-γ autoantibodies, suggesting that anti-GM-CSF autoantibodies may be produced by long-lived plasma cells and memory B-cells which no longer express CD20 (74).
Anti-IL-17 autoantibodies
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) patients with biallelic mutations in AIRE and to a lesser degree, patients with thymic epithelial tumors, have been described to have neutralizing autoantibodies to Th17 cytokines including IL17A/F, IL22, and Type 1 interferons (5, 6). Chronic mucocutaneous candidiasis (CMC), characterized by chronic, non-invasive Candida spp. infections of the skin, nails, and mucus membranes is the most notable infectious manifestation in APECED patients, whereas in thymoma patients, CMC may be accompanied by recurrent sinopulmonary infections exacerbating bronchiectasis or overshadowed by disseminated infections associated with anti-IL12/23 autoantibodies or Good syndrome (61, 75). While these anti-IL17 autoantibodies phenocopy some of the primary immune defects (IL17F, IL17RA, IL17RF, ACT1), recent data have also shown excessive IFN-γ production by mucosal T cells to cause candidiasis in APECED (76). Therefore, the exact role of anti-IL-17 autoantibodies in APECED is still under investigation.
Anti-Type I interferon autoantibodies
Neutralizing autoantibodies against Type I IFNs have been associated with >10% of life-threatening COVID-19 cases in multiple cohorts, particularly in men over sixty (11, 77–79). These neutralizing anti-IFN-α and anti-IFN-ω autoantibodies are found in 4% of the population > 70 years, in 5–6% of patients with systemic lupus erythematosus, in 59–64 % of thymoma patients with myasthenia gravis, and in 100% of APECED patients (15, 22, 61, 80, 81). Clinical penetrance for severe COVID-19 is incomplete across these diverse populations with pre-existing anti-Type I interferon autoantibodies (82). Some APECED patients with high titer neutralizing anti-IFN-α and anti-IFN-ω autoantibodies who contracted SARS-CoV-2 have had only mild COVID-19, whereas in the general population, no individuals with mild COVID-19 had detectable anti-IFN-α and anti-IFN-ω autoantibodies (78, 83, 84). Anti-IFN-α and anti-IFN-ω autoantibodies in APECED patients are unchanged by COVID-19. In contrast, serial sampling during and after severe COVID-19 in otherwise normal people identified highly dynamic and declining levels of anti-IFN-α, sometimes to undetectable levels in convalescence (Elana Shaw, submitted). Neutralization of IFN-β-induced STAT1 phosphorylation in vitro was seen in only 2% of those with anti-IFN-α and anti-IFN-ω autoantibodies (11, 22, 84). Preemptive use of IFN-β in an individual with with incontinentia pigmenti and autoantibodies against IFN-α and IFN-ω was successful despite a high initial viral load of SARS-CoV-2 (85). Individualized approaches will be needed in those with chronic autoimmunity and multiple ACAAs, since some may have compensatory alterations in interacting cytokine pathways (85, 86). Outside of COVID-19, neutralizing Type 1 IFNs autoantibodies have been found when looked for in unusually severe viral illnesses (12, 87).
CONCLUSIONS
High throughput autoantibody screening will certainly identify new targets and new mechanisms of infectious and organ-specific diseases. We recommend screening for anti-interferons, anti-IL17, anti-GM-CSF and anti-IL23 in appropriate patients with otherwise unknown etiologies for opportunistic infections and/or in those with known autoimmunity, but more importantly, correlating binding specificities with appropriate functional assays. The clinical penetrance of ACAAs is incomplete, suggesting that these syndromes result from a combination of factors including environmental and genetic ones. It is imperative to consider ACAAs in the setting of unexplained or severe infections, as directed therapies may ameliorate their effects. These often silent and underappreciated modulators of severe infections are increasing in recognition and clinical importance.
Figure 1:
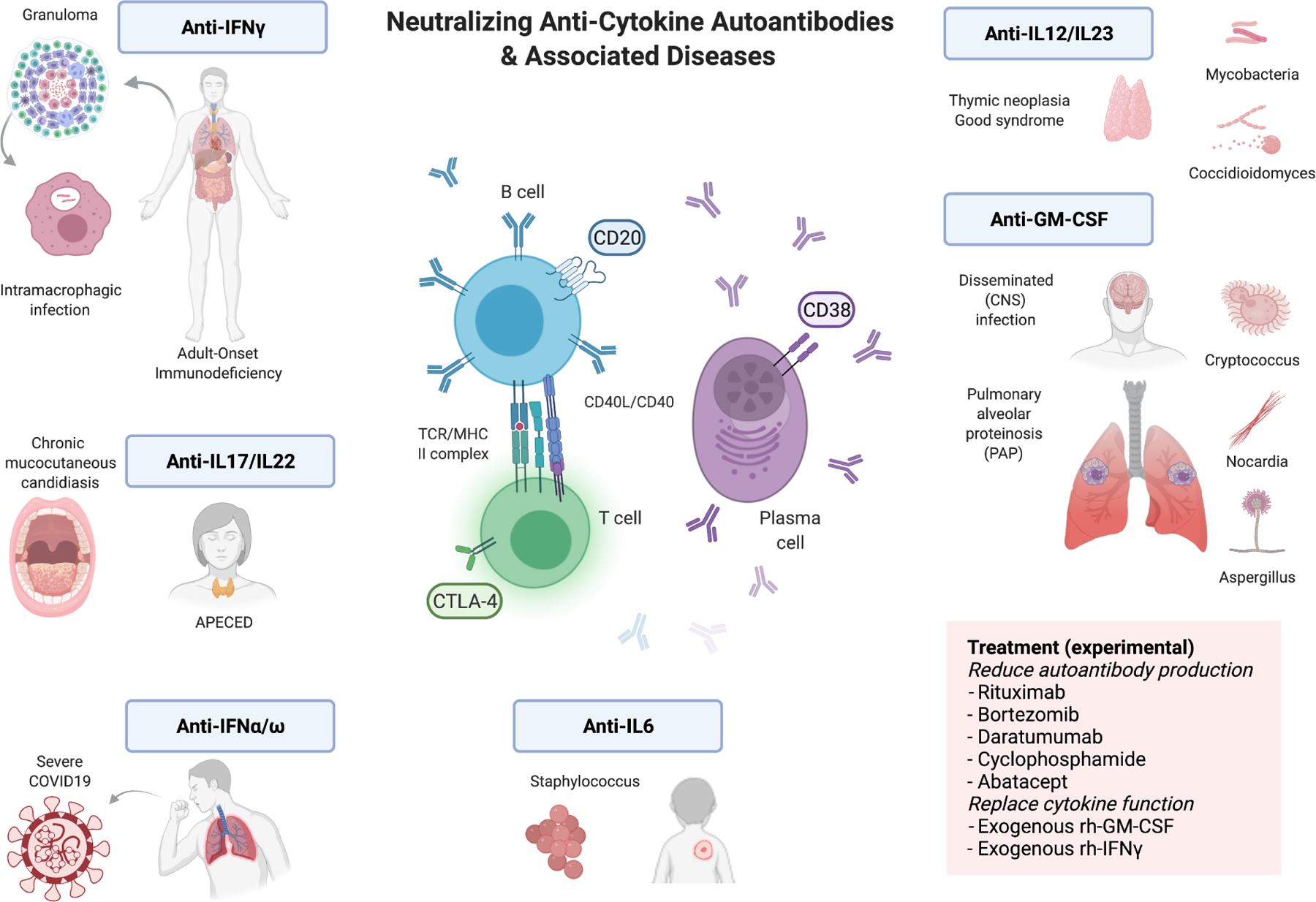
Pictorial abstract of the disease associations and mechanistic-based therapy of anti-cytokine autoantibodies.
Funding
This work was supported with funds from the NIAID Division of Intramural Research.
LIST OF ABBREVIATIONS (alphabetical)
- ACAAs
anti-cytokine autoantibodies
- aPAP
autoimmune protein alveolar proteinosis
- APECED
autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy
- ARDS
adult respiratory distress syndrome
- CMC
chronic mucocutaneous candidiasis
- COVID19
coronavirus virus disease 2019
- IFN-α
interferon-alpha
- IFN-β
interferon-beta
- IFN-γ
interferon-gamma
- IFN-ω
interferon-omega
- IL12
interleukin-12
- IL17
interleukin-17
- IL-22
interleukin-22
- IL23
interleukin-23
- GM-CSF
granulocyte-macrophage colony stimulating factor
- STAT1
Signal Transducer and Activator of Transcription
- STAT5
Signal Transducer and Activator of Transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Neither authors have potential conflicts of interest to declare.
References
- 1.Browne SK. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol 2014;32:635–57. [DOI] [PubMed] [Google Scholar]
- 2.Barcenas-Morales G, Cortes-Acevedo P, Doffinger R. Anticytokine autoantibodies leading to infection: early recognition, diagnosis and treatment options. Curr Opin Infect Dis 2019;32(4):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ku CL, Chi CY, von Bernuth H, Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum Genet 2020;139(6–7):783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012;367(8):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puel A, Döffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010;207(2):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010;207(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 2013;190(8):3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen LB, Rocha Pereira N, Figueiredo C, Fiske LC, Ressner RA, Hong JC, et al. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis 2015;60(7):1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloomfield M, Parackova Z, Cabelova T, Pospisilova I, Kabicek P, Houstkova H, et al. Anti-IL6 Autoantibodies in an Infant With CRP-Less Septic Shock. Front Immunol 2019;10:2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanki T, Onoue I, Nagasaka K, Takayasu A, Ebisawa M, Hosoya T, et al. Suppression of elevations in serum C reactive protein levels by anti-IL-6 autoantibodies in two patients with severe bacterial infections. Ann Rheum Dis 2013;72(6):1100–2. [DOI] [PubMed] [Google Scholar]
- 11.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370(6515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari R, Rosen LB, Lisco A, Gilden D, Holland SM, Zerbe CS, et al. Primary and Acquired Immunodeficiencies Associated With Severe Varicella-Zoster Virus Infections. Clin Infect Dis 2021;73(9):e2705–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda Y, Toda G, Hashimoto N, Umeda N, Miyake K, Yamanaka M, et al. Naturally occurring anti-interferon-alpha 2a antibodies in patients with acute viral hepatitis. Clin Exp Immunol 1991;85(1):80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunder-Plassmann G, Sedlacek PL, Sunder-Plassmann R, Derfler K, Swoboda K, Fabrizii V, et al. Anti-interleukin-1 alpha autoantibodies in hemodialysis patients. Kidney Int 1991;40(4):787–91. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Tatouli IP, Rosen LB, Hasni S, Alevizos I, Manna ZG, et al. Distinct Functions of Autoantibodies Against Interferon in Systemic Lupus Erythematosus: A Comprehensive Analysis of Anticytokine Autoantibodies in Common Rheumatic Diseases. Arthritis Rheumatol 2016;68(7):1677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kärner J, Pihlap M, Ranki A, Krohn K, Trebusak Podkrajsek K, Bratanic N, et al. IL-6-specific autoantibodies among APECED and thymoma patients. Immun Inflamm Dis 2016;4(2):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappellano G, Orilieri E, Woldetsadik AD, Boggio E, Soluri MF, Comi C, et al. Anti-cytokine autoantibodies in autoimmune diseases. Am J Clin Exp Immunol 2012;1(2):136–46. [PMC free article] [PubMed] [Google Scholar]
- 18.Caruso A, Foresti I, Gribaudo G, Bonfanti C, Pollara P, Dolei A, et al. Anti-interferon-gamma antibodies in sera from HIV infected patients. J Biol Regul Homeost Agents 1989;3(1):8–12. [PubMed] [Google Scholar]
- 19.Takasaki J, Ogawa Y. Anti-interleukin-8 autoantibody in the tracheobronchial aspirate of infants with chronic lung disease. Pediatr Int 2001;43(1):48–52. [DOI] [PubMed] [Google Scholar]
- 20.Bayat A, Burbelo PD, Browne SK, Quinlivan M, Martinez B, Holland SM, et al. Anti-cytokine autoantibodies in postherpetic neuralgia. J Transl Med 2015;13:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen MB, Svenson M, Bendtzen K. Human anti-interleukin 1 alpha antibodies. Immunol Lett 1991;30(1):133–9. [DOI] [PubMed] [Google Scholar]
- 22.Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in. Sci Immunol 2021;6(62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burbelo PD, Seam N, Groot S, Ching KH, Han BL, Meduri GU, et al. Rapid induction of autoantibodies during ARDS and septic shock. J Transl Med 2010;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ijzermans JN, Marquet RL. Interferon-gamma: a review. Immunobiology 1989;179(4–5):456–73. [DOI] [PubMed] [Google Scholar]
- 25.Shih HP, Ding JY, Yeh CF, Chi CY, Ku CL. Anti-interferon-γ autoantibody-associated immunodeficiency. Curr Opin Immunol 2021;72:206–14. [DOI] [PubMed] [Google Scholar]
- 26.Notarangelo LD, Bacchetta R, Casanova JL, Su HC. Human inborn errors of immunity: An expanding universe. Sci Immunol 2020;5(49). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höflich C, Sabat R, Rosseau S, Temmesfeld B, Slevogt H, Döcke WD, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 2004;103(2):673–5. [DOI] [PubMed] [Google Scholar]
- 28.Döffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis 2004;38(1):e10–4. [DOI] [PubMed] [Google Scholar]
- 29.Chawansuntati K, Rattanathammethee K, Wipasa J. Minireview: Insights into anti-interferon-γ autoantibodies. Exp Biol Med (Maywood) 2021;246(7):790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pithukpakorn M, Roothumnong E, Angkasekwinai N, Suktitipat B, Assawamakin A, Luangwedchakarn V, et al. HLA-DRB1 and HLA-DQB1 Are Associated with Adult-Onset Immunodeficiency with Acquired Anti-Interferon-Gamma Autoantibodies. PLoS One 2015;10(5):e0128481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku CL, Lin CH, Chang SW, Chu CC, Chan JF, Kong XF, et al. Anti-IFN-γ autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol 2016;137(3):945–8.e8. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell E, Rosen LB, LaRue RW, Fabre V, Melia MT, Auwaerter PG, et al. The first US domestic report of disseminated Mycobacterium avium complex and anti-interferon-γ autoantibodies. J Clin Immunol 2014;34(8):928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew WK, Thoon KC, Chong CY, Tan NWH, Cheng DT, Chan BSW, et al. Juvenile-Onset Immunodeficiency Secondary to Anti-Interferon-Gamma Autoantibodies. J Clin Immunol 2019;39(5):512–8. [DOI] [PubMed] [Google Scholar]
- 34.van de Vosse E, van Wengen A, van der Meide WF, Visser LG, van Dissel JT. A 38-year-old woman with necrotising cervical lymphadenitis due to Histoplasma capsulatum. Infection 2017;45(6):917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keragala BSDP, Gunasekera CN, Yesudian PD, Guruge C, Dissanayaka BS, Liyanagama DP, et al. Disseminated Mycobacterium simiae infection in a patient with adult-onset immunodeficiency due to anti-interferon-gamma antibodies - a case report. BMC Infect Dis 2020;20(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu UI, Hung CC, Chang SY, Jhong YT, Sun HY, Wang JT, et al. Neutralizing antiinterferon-γ autoantibodies causing disseminated Mycobacterium avium complex infection in an HIV-infected patient on successful combination antiretroviral therapy. AIDS 2017;31(18):2557–9. [DOI] [PubMed] [Google Scholar]
- 37.Hong GH, Ortega-Villa AM, Hunsberger S, Chetchotisakd P, Anunnatsiri S, Mootsikapun P, et al. Natural History and Evolution of Anti-Interferon-γ Autoantibody-Associated Immunodeficiency Syndrome in Thailand and the United States. Clin Infect Dis 2020;71(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jutivorakool K, Sittiwattanawong P, Kantikosum K, Hurst CP, Kumtornrut C, Asawanonda P, et al. Skin Manifestations in Patients with Adult-onset Immunodeficiency due to Anti-interferon-gamma Autoantibody: A Relationship with Systemic Infections. Acta Derm Venereol 2018;98(8):742–7. [DOI] [PubMed] [Google Scholar]
- 39.Yuan CT, Wang JT, Sheng WH, Cheng PY, Kao CJ, Wang JY, et al. Lymphadenopathy Associated With Neutralizing Anti-interferon-gamma Autoantibodies Could Have Monoclonal T-cell Proliferation Indistinguishable From Malignant Lymphoma and Treatable by Antibiotics: A Clinicopathologic Study. Am J Surg Pathol 2021;45(8):1138–50. [DOI] [PubMed] [Google Scholar]
- 40.Thingujam B, Syue LS, Wang RC, Chen CJ, Yu SC, Chen CC, et al. Morphologic Spectrum of Lymphadenopathy in Adult-onset Immunodeficiency (Anti-interferon-γ Autoantibodies). Am J Surg Pathol 2021. [DOI] [PubMed] [Google Scholar]
- 41.Wu UI, Wang JT, Sheng WH, Sun HY, Cheng A, Hsu LY, et al. Incorrect diagnoses in patients with neutralizing anti-interferon-gamma-autoantibodies. Clin Microbiol Infect 2020;26(12):1684.e1–.e6. [DOI] [PubMed] [Google Scholar]
- 42.Chi CY, Lin CH, Ho MW, Ding JY, Huang WC, Shih HP, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) 2016;95(25):e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wipasa J, Chaiwarith R, Chawansuntati K, Praparattanapan J, Rattanathammethee K, Supparatpinyo K. Characterization of anti-interferon-γ antibodies in HIV-negative immunodeficient patients infected with unusual intracellular microorganisms. Exp Biol Med (Maywood) 2018;243(7):621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chetchotisakd P, Anunnatsiri S, Nithichanon A, Lertmemongkolchai G. Cryptococcosis in Anti-Interferon-Gamma Autoantibody-Positive Patients: a Different Clinical Manifestation from HIV-Infected Patients. Jpn J Infect Dis 2017;70(1):69–74. [DOI] [PubMed] [Google Scholar]
- 45.Qiu Y, Zhang J, Li B, Shu H. Bacillus cereus isolated from a positive bone tissue culture in a patient with osteolysis and high-titer anti-interferon-γ autoantibodies: A case report. Medicine (Baltimore) 2019;98(43):e17609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shima K, Sakagami T, Tanabe Y, Aoki N, Moro H, Koya T, et al. Novel assay to detect increased level of neutralizing anti-interferon gamma autoantibodies in non-tuberculous mycobacterial patients. J Infect Chemother 2014;20(1):52–6. [DOI] [PubMed] [Google Scholar]
- 47.Merkel PA, Lebo T, Knight V. Functional Analysis of Anti-cytokine Autoantibodies Using Flow Cytometry. Front Immunol 2019;10:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suárez I, Lehmann C, Gruell H, Graeb J, Kochanek M, Fätkenheuer G, et al. Repurposing QuantiFERON for Detection of Neutralizing Interferon-γ Autoantibodies in Patients With Nontuberculous Mycobacterial Infections. Clin Infect Dis 2017;65(3):518–21. [DOI] [PubMed] [Google Scholar]
- 49.Wu UI, Chuang YC, Sheng WH, Sun HY, Jhong YT, Wang JY, et al. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon-γ autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect 2018;24(2):159–65. [DOI] [PubMed] [Google Scholar]
- 50.Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012;119(17):3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chetchotisakd P, Anunnatsiri S, Nanagara R, Nithichanon A, Lertmemongkolchai G. Intravenous Cyclophosphamide Therapy for Anti-IFN-Gamma Autoantibody-Associated. J Immunol Res 2018;2018:6473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laisuan W, Pisitkun P, Ngamjanyaporn P, Suangtamai T, Rotjanapan P. Prospective Pilot Study of Cyclophosphamide as an Adjunct Treatment in Patients With Adult-Onset Immunodeficiency Associated With Anti-interferon-γ Autoantibodies. Open Forum Infect Dis 2020;7(2):ofaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koizumi Y, Sakagami T, Nishiyama N, Hirai J, Hayashi Y, Asai N, et al. Rituximab Restores IFN-γ-STAT1 Function and Ameliorates Disseminated Mycobacterium avium Infection in a Patient with Anti-Interferon-γ Autoantibody. J Clin Immunol 2017;37(7):644–9. [DOI] [PubMed] [Google Scholar]
- 54.Czaja CA, Merkel PA, Chan ED, Lenz LL, Wolf ML, Alam R, et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-γ autoantibody. Clin Infect Dis 2014;58(6):e115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochoa S, Ding L, Kreuzburg S, Treat J, Holland SM, Zerbe CS. Daratumumab (Anti-CD38) for Treatment of Disseminated Nontuberculous Mycobacteria in a Patient With Anti-Interferon-γ Autoantibodies. Clin Infect Dis 2021;72(12):2206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koizumi Y, Mikamo H. Anti-Interferon Gamma Autoantibody and Disseminated Nontuberculous Mycobacteria Infection: What Should Be Done to Improve Its Clinical Outcome? Clin Infect Dis 2021;72(12):2209–11. [DOI] [PubMed] [Google Scholar]
- 57.Martínez-Barricarte R, Markle JG, Ma CS, Deenick EK, Ramírez-Alejo N, Mele F, et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci Immunol 2018;3(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 2015;21(7):719–29. [DOI] [PubMed] [Google Scholar]
- 59.Meager A, Vincent A, Newsom-Davis J, Willcox N. Spontaneous neutralising antibodies to interferon--alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet 1997;350(9091):1596–7. [DOI] [PubMed] [Google Scholar]
- 60.Meager A, Wadhwa M, Dilger P, Bird C, Thorpe R, Newsom-Davis J, et al. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol 2003;132(1):128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, Kristosturyan E, et al. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood 2010;116(23):4848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuo CY, Wang SY, Shih HP, Tu KH, Huang WC, Ding JY, et al. Disseminated Cryptococcosis Due to Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Absence of Pulmonary Alveolar Proteinosis. J Clin Immunol 2017;37(2):143–52. [DOI] [PubMed] [Google Scholar]
- 63.Viola GM, Malek AE, Rosen LB, DiNardo AR, Nishiguchi T, Okhuysen PC, et al. Disseminated cryptococcosis and anti-granulocyte-macrophage colony-stimulating factor autoantibodies: An underappreciated association. Mycoses 2021;64(6):576–82. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka N, Watanabe J, Kitamura T, Yamada Y, Kanegasaki S, Nakata K. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte-macrophage colony stimulating factor. FEBS Lett 1999;442(2–3):246–50. [DOI] [PubMed] [Google Scholar]
- 65.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 1999;190(6):875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 2014;5(2):e00912–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004;103(3):1089–98. [DOI] [PubMed] [Google Scholar]
- 68.Papiris SA, Tsirigotis P, Kolilekas L, Papadaki G, Papaioannou AI, Triantafillidou C, et al. Pulmonary alveolar proteinosis: time to shift? Expert Rev Respir Med 2015;9(3):337–49. [DOI] [PubMed] [Google Scholar]
- 69.Sakaue S, Yamaguchi E, Inoue Y, Takahashi M, Hirata J, Suzuki K, et al. Genetic determinants of risk in autoimmune pulmonary alveolar proteinosis. Nat Commun 2021;12(1):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kavuru MS, Malur A, Marshall I, Barna BP, Meziane M, Huizar I, et al. An open-label trial of rituximab therapy in pulmonary alveolar proteinosis. Eur Respir J 2011;38(6):1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trapnell BC, Inoue Y, Bonella F, Morgan C, Jouneau S, Bendstrup E, et al. Inhaled Molgramostim Therapy in Autoimmune Pulmonary Alveolar Proteinosis. N Engl J Med 2020;383(17):1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leth S, Bendstrup E, Vestergaard H, Hilberg O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology 2013;18(1):82–91. [DOI] [PubMed] [Google Scholar]
- 73.Malur A, Kavuru MS, Marshall I, Barna BP, Huizar I, Karnekar R, et al. Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasis. Respir Res 2012;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soyez B, Borie R, Menard C, Cadranel J, Chavez L, Cottin V, et al. Rituximab for autoimmune alveolar proteinosis, a real life cohort study. Respir Res 2018;19(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez B, Browne SK. Good syndrome, bad problem. Front Oncol 2014;4:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Break TJ, Oikonomou V, Dutzan N, Desai JV, Swidergall M, Freiwald T, et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 2021;371(6526). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Troya J, Bastard P, Planas-Serra L, Ryan P, Ruiz M, de Carranza M, et al. Neutralizing Autoantibodies to Type I IFNs in >10% of Patients with Severe COVID-19 Pneumonia Hospitalized in Madrid, Spain. J Clin Immunol 2021;41(5):914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goncalves D, Mezidi M, Bastard P, Perret M, Saker K, Fabien N, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunology 2021;10(8):e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Wijst MGP, Vazquez SE, Hartoularos GC, Bastard P, Grant T, Bueno R, et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med 2021;13(612):eabh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meloni A, Furcas M, Cetani F, Marcocci C, Falorni A, Perniola R, et al. Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab 2008;93(11):4389–97. [DOI] [PubMed] [Google Scholar]
- 81.Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Kärner J, et al. AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell 2016;166(3):582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koning R, Bastard P, Casanova JL, Brouwer MC, van de Beek D, Investigators wtAUMCC- B. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med 2021;47(6):704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meisel C, Akbil B, Meyer T, Lankes E, Corman VM, Staudacher O, et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J Clin Invest 2021;131(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bastard P, Orlova E, Sozaeva L, Lévy R, James A, Schmitt MM, et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med 2021;218(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bastard P, Lévy R, Henriquez S, Bodemer C, Szwebel TA, Casanova JL. Interferon-β Therapy in a Patient with Incontinentia Pigmenti and Autoantibodies against Type I IFNs Infected with SARS-CoV-2. J Clin Immunol 2021;41(5):931–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Prost N, Bastard P, Arrestier R, Fourati S, Mahévas M, Burrel S, et al. Plasma Exchange to Rescue Patients with Autoantibodies Against Type I Interferons and Life-Threatening COVID-19 Pneumonia. J Clin Immunol 2021;41(3):536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walter JE, Rosen LB, Csomos K, Rosenberg JM, Mathew D, Keszei M, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest 2015;125(11):4135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


