Summary
The ε4 allele of the apolipoprotein E gene (APOE4) is a strong genetic risk factor for Alzheimer’s disease (AD) and several other neurodegenerative conditions including Lewy body dementia (LBD). The three APOE alleles encode protein isoforms which differ from one another only at amino acid positions 112 and 158; apoE2 (C112, C158), apoE3 (C112, R158), and apoE4 (R112, R158). Despite progress, it remains unclear how these small amino acid differences in apoE sequence among the three isoforms lead to profound effects on aging and disease-related pathways. Here, we propose a novel “ApoE Cascade Hypothesis” in AD and age-related cognitive decline that the biochemical and biophysical properties of apoE impact a cascade of events at the cellular and systems levels ultimately impacting aging-related pathogenic conditions including AD. As such, apoE-targeted therapeutic interventions are predicted to be more effective by addressing the biochemical phase of the cascade.
In brief:
In this review, Martens et al. propose a novel “ApoE Cascade Hypothesis” that the biochemical and biophysical properties of apoE impact a cascade of events at the cellular and systems levels ultimately leading to Alzheimer’s disease and age-related cognitive decline.
Introduction
The ε4 allele of the apolipoprotein E gene (APOE4) vastly increases the risk for Alzheimer’s disease (AD) compared to the more common APOE3 allele, while APOE2 is protective (Corder et al., 1993; Corder et al., 1994; Farrer et al., 1997; Saunders et al., 1993). APOE4 not only increases the risk but also lowers the age at onset of AD in a dose-dependent manner (Corder et al., 1993; Sando et al., 2008). In addition to AD, APOE4 is also associated with the risk for age-related cognitive decline in non-demented individuals as well as other neurodegenerative conditions such as Lewy body dementia (LBD) and TDP-43 pathology in AD (Bras et al., 2014; Dhana et al., 2021; Guerreiro et al., 2018; Tsuang et al., 2013; Wennberg et al., 2018; Yang et al., 2018). Despite these strong genetic associations, the molecular pathobiology underlying the differential effects of the three apoE isoforms remains puzzling.
AD is a progressive neurodegenerative disease neuropathologically characterized by the deposition of amyloid-β (Aβ) cleaved from amyloid precursor protein (APP) as senile plaques and hyperphosphorylated tau as neurofibrillary tangles in the brain (Alzheimer's Association, 2021). Given that Aβ accumulation appears to precede the onset of other AD phenotypes such as neocortical tauopathy and cognitive impairment; Aβ may contribute to diverse pathways related to the disease onset and progression. As such, the “amyloid cascade hypothesis” has long been considered central to the pathogenesis of AD (Hardy and Higgins, 1992). Indeed, this hypothesis is well supported by genetic evidence from autosomal dominant AD (ADAD) cases in which mutations in APP, PSEN1, or PSEN2 are causatively involved in AD development by increasing APP amyloidogenic processing and Aβ production or its seeding propensity. However, with >99% of AD cases being sporadic (Bekris et al., 2010) where a variety of mixed pathologies are present in AD brains, the linearity and broader relevance of the “amyloid cascade hypothesis” is at times challenged. Further, the therapeutic efficacy of various Aβ-targeting approaches on cognitive decline during the symptomatic phase of AD are limited despite effectiveness in reducing brain Aβ deposition (Knopman et al., 2021). Other hypotheses such as the “cellular phase of AD” or a consideration of the other elements both downstream but also independent of Aβ (Musiek and Holtzman, 2015) have been proposed to link broader pathways impacted in AD, integrating both the effect of pathological tau as well as other brain cell types in particular astrocytes, microglia, oligodendrocytes, and vascular cells.
How apoE pathobiology fits into these existing hypotheses represents an opportunity for exploring therapeutic avenues targeting apoE in AD and related dementias. Interestingly, a recent case report showed that carrying two copies of the APOE3 p.R136S referred to as APOE3 Christchurch mutation is linked with preserved cognition at a much later age than expected despite high brain amyloid levels due to FAD-linked PSEN1 p.E280A mutation (Arboleda-Velasquez et al., 2019). This APOE polymorphism is located in the receptor binding region of apoE and has been suggested to be protective by reducing apoE binding to the heparan sulfate proteoglycan (HSPG) (Arboleda-Velasquez et al., 2019). We have also recently reported that a rare apoE3 variant, APOE3 p.V236E referred to as APOE3 Jacksonville variant, reduces amyloid plaques and neuronal damage by preventing apoE self-oligomerization and promoting lipid metabolism (Liu et al., 2021). These studies support the notion that changes in the biochemical properties of apoE such as receptor binding, oligomerization, and lipid metabolism have differential impacts on cellular functions which manifest as phenotypic changes leading to eventual effects on disease onset. Mounting evidence has demonstrated the importance of apoE in the pathogenesis of AD and age-related cognitive decline (Frisoni et al., 2022); however, there has not been a central hypothesis that links the apoE isoform-related molecular events to the cellular changes and eventually to the disease manifestation of AD. Herein, based on accumulating evidence from biochemical, cellular, animal, and human studies, we propose a novel “ApoE Cascade Hypothesis” in AD and age-related cognitive decline that the biochemical and biophysical properties of apoE initiate a cascade of events at the cellular and systems levels ultimately impacting aging-related pathogenic conditions including AD (Figure 1), thus preventative or therapeutic interventions are likely to be more effective by targeting the apoE biochemical phase of the cascade.
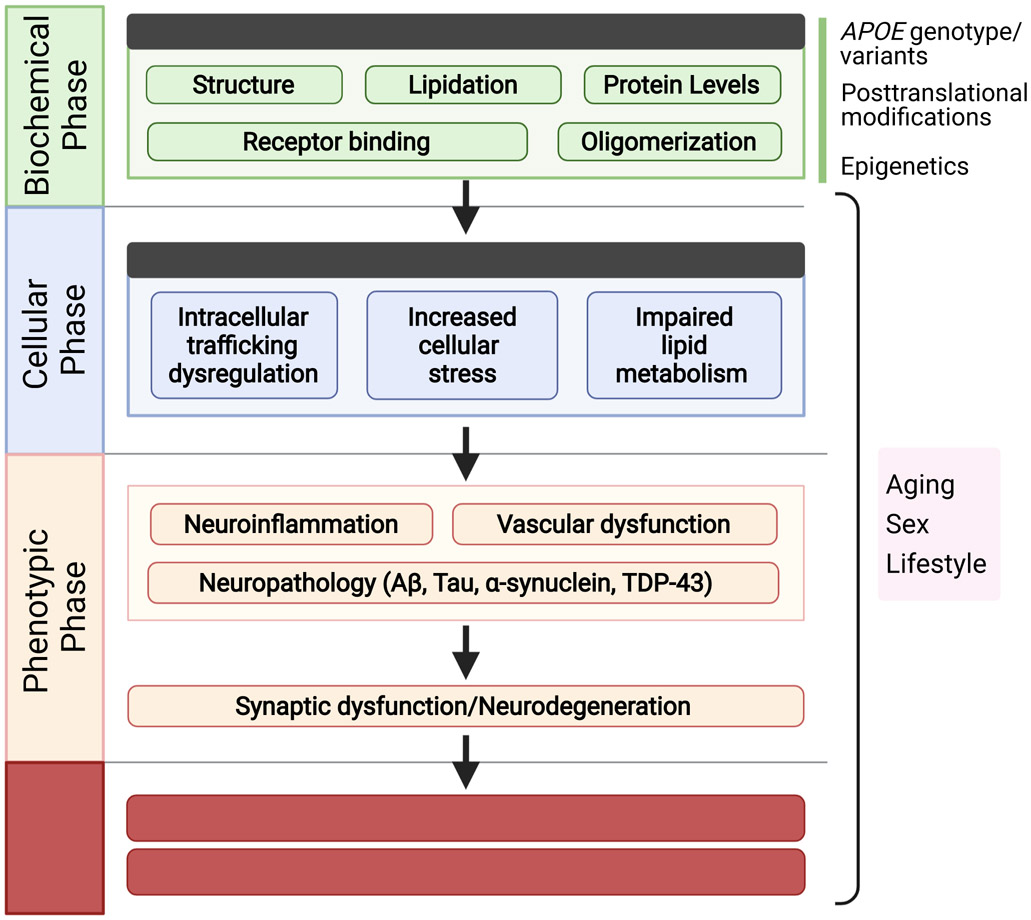
Figure 1. ApoE cascade hypothesis.
The cascade starts from the different biochemical and biophysical properties including apoE structure, lipidation, protein levels, receptor binding, and oligomerization. These biochemical/biophysical differences are then propagated to functional effects on cellular homeostasis including cellular stress, endosomal-lysosomal trafficking, as well as lipid dysregulation. Not depicted here, some of these cellular effects can be either cell autonomous in cells expressing abundant apoE (astrocytes and microglia in the brain, hepatocytes and macrophages in the periphery, and vascular mural cells interfacing the periphery with the brain), or non-cell autonomous (e.g., secreted apoE from one cell type binding to apoE receptors on another including neurons). These cellular effects are further relayed to trackable phenotypes at the systems level highlighted by neuroinflammation, vascular dysfunction, and neuropathologies, leading to synaptic dysfunction/loss, neurodegeneration, and eventual age-related cognitive decline and AD.
Overview of ApoE Cascade Hypothesis
This cascade starts from the biochemical and biophysical properties of apoE including apoE structure, lipidation, oligomerization, protein levels, and receptor binding (Figure 1). APOE genotype or rare variants, epigenetics, posttranslational modifications, aging, sex, and lifestyle can all affect this phase. These biochemical/biophysical differences are then propagated to functional effects on cellular homeostasis including events known to be differentially impacted by apoE isoforms such as cellular stress (autophagy, mitochondria stress, ER stress), endosomal-lysosomal trafficking, and lipid metabolism. Some of these cellular effects can be either cell autonomous in cells expressing abundant apoE (astrocytes and reactive microglia in the brain, hepatocytes and macrophages in the periphery, and vascular mural cells interfacing the periphery with the brain), or non-cell autonomous (e.g., by binding to apoE receptors on neurons which themselves express little apoE). These cellular effects lead to systems level phenotypes highlighted by neuroinflammation, vascular dysfunction, neuropathology, synaptic loss, and neurodegeneration, leading to age-related cognitive decline and other aging-related pathological conditions such as AD. In simpler terms, the qualitative and/or quantitative changes of apoE depending on its isoforms and other modifications during aging trigger the cascade of pathogenic events leading to cognitive impairment and dementia. Biological events associated with aging such as oxidative stress, cellular senescence, chronic inflammation, glial activation, and lifestyle including sleep pattern, diet, and activity level can contribute to the cascade both in an apoE-dependent, through the biochemical phase, and in an apoE-independent manner by directly impacting cellular and phenotypic phases.
In this review, we will focus on discussing the roles of apoE in the development of age-related cognitive decline and AD as they relate to this ApoE Cascade Hypothesis.
Biochemical phase in the ApoE Cascade Hypothesis
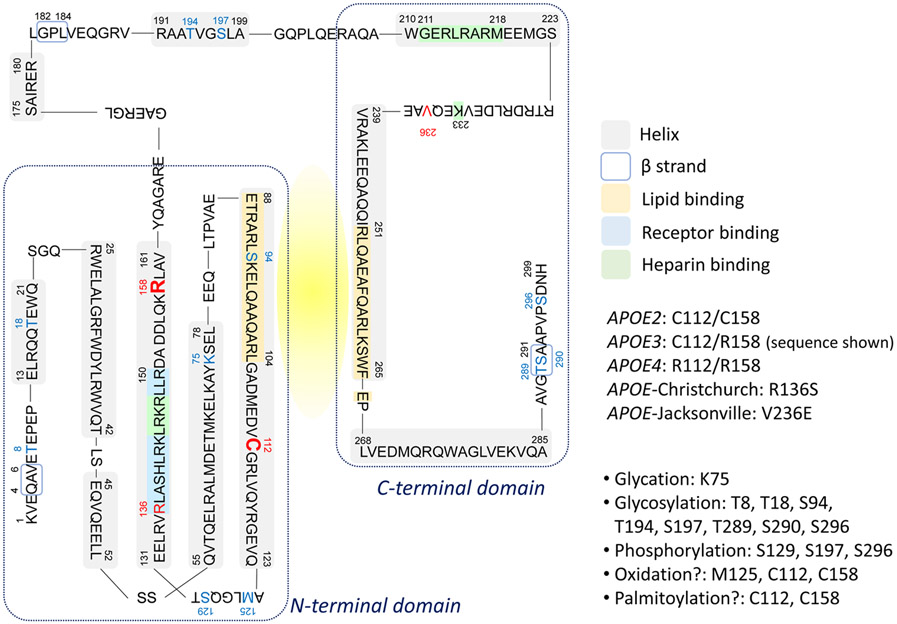
ApoE is a 299-amino acid glycoprotein composed of the N-terminal domain (residues 1–167), a hinge region (residues 168–205), and the C-terminal domain (residues 206–299) (Chen et al., 2021) (Figure 2: apoE3 amino acid sequence shown). While the receptor-binding region (residues 136–150) is within the N-terminal four helix bundle, the lipid-binding region (residues 244-272) is in the C-terminal domain. By interacting with cellular membranes through ABC transporters, apoE incorporates membrane lipids and forms lipoprotein particles. Subsequently, the lipidated apoE particles transport and distribute lipids from cell to cell through binding to cell-surface apoE receptors including the low-density lipoprotein receptor (LDLR), the LDLR-related protein 1 (LRP1), and HSPGs (Herz and Bock, 2002; Herz and Chen, 2006; Wahrle et al., 2004). The isoform-dependent biochemical and biophysical properties of apoE to interact with lipids and receptors are intimately linked to its functions in health and disease (Huang and Mahley, 2014; Yamazaki et al., 2019).
Figure 2. ApoE amino acid sequence and potential post translational modification sites.
Amino acid sequence of apoE3 is depicted. Key functional regions and residues that differ among apoE isoforms and variants, as well as known or potential posttranslational modification sites are marked.
ApoE structure, lipidation, and receptor binding.
NMR structural studies indicate that the structured helix regions of apoE3 are bound by several unstructured intrinsically disordered regions (IDRs) and smaller flexible regions (Chen et al., 2011; Frieden et al., 2017). The relatively unstable structural feature could allow apoE to be incorporated in different sizes of lipid particles with diverse compositions. While the pocket between the part of Helix 3 in the N-terminal helical bundle (residues 88-104) and the C-terminal domain (residues 251-266), brought together by several salt bridges, is possibly the initial lipid binding site (Frieden et al., 2017), the structural differences in apoE isoforms may differently influence the lipid recognition and the curvature of lipoprotein particles. Helix 4 in the N-terminal helical bundle contains a receptor-binding region enriched in positively charged Lys and Arg residues, providing the binding site to negatively charged moieties in apoE receptors (Chen et al., 2021). Although several models regarding the conformational changes of the N-terminal domain are hypothesized in the lipid-bound form of apoE (Chen et al., 2021; Hatters et al., 2006a), the receptor-binding region is likely undocked upon lipid-binding, increasing the accessibility to apoE receptors and enabling efficient cellular lipid delivery.
The three major apoE isoforms encoded by each corresponding APOE allele differ in two amino acid residues at positions 112 and 158 (apoE2: Cys112/Cys158; apoE3: Cys112/Arg158; apoE4: Arg112/Arg158) (Mahley and Rall, 2000). In vitro lipid efflux assays for cholesterol or phospholipids showed the superior role of apoE2 to apoE3 and apoE4 as a lipid acceptor (Michikawa et al., 2000). In cerebrospinal fluid (CSF), the size of apoE/lipoprotein particles is apoE isoform-dependent following the order of apoE2>apoE3>apoE4 (Heinsinger et al., 2016; Lanfranco et al., 2020). The same apoE isoform-dependent apoE/lipoprotein particle size in mouse brains has also been reported supporting the notion that apoE2 as a better lipid transporter (Hu et al., 2015). Since residue 112 is connected to the lipid-binding site of Helix 3 and residue 158 is located behind the lipid-binding domain (Chen et al., 2011; Frieden et al., 2017), these amino acid differences could substantially impact the apoE properties in forming lipoprotein particles and receptor binding. In the periphery, apoE2 and apoE3 bind preferentially to HDL while apoE4 binds to VLDL, and this is thought to be due to the presence of the Arg residue at amino acid position 112 leading to altered domain interaction between the N- and C-terminal domains (Weisgraber, 1990). In addition, Cys158 in apoE2 alters the conformation of the positively charged receptor binding domain, thus reducing its affinity for the LDLR (Mahley et al., 2009). This reduced affinity of apoE2 to LDLR results in decreased clearance of triglyceride-rich lipoprotein particles and increases the risk of developing Type III hyperlipoproteinemia in small group of apoE2 homozygous individuals (Mahley et al., 1999).
ApoE protein levels.
In plasma, apoE concentrations are isoform-dependent where apoE2 is higher and apoE4 is lower compared to apoE3 (Rasmussen et al., 2015). For the apoE levels in the CNS, the result is mixed depending on the quantification methods used. ELISA measurements revealed that apoE2-TR mice display highest levels of apoE in brain parenchyma, CSF, and ISF followed by apoE3-TR mice, then apoE4-TR mice (Riddell et al., 2008; Shinohara et al., 2016; Ulrich et al., 2013). However, using stable isotope amino acid labeling and mass spectrometry, Wildsmith et al found no isoform-dependent differences in apoE levels and its turnover rate between apoE3 and apoE4 in CSF of young cognitively normal individuals as well as apoE-TR mice (Wildsmith et al., 2012). The same study also reported that the level of apoE2 appears to be higher. The lack of isoform-dependent difference in apoE levels in CSF was later confirmed in another study in non-AD and AD subjects (Martinez-Morillo et al., 2014). More recently, a study using induced pluripotent stem cell (iPSC)-derived astrocytes and cerebral organoids showed apoE4 being associated with higher apoE levels compared to apoE3 (Lin et al., 2018) while another study did not find such a difference in iPSC-derived cerebral organoids (Zhao et al., 2020a). The reason for these discrepancies is not entirely clear, but one possibility is that the structural differences among the apoE isoforms affect epitope presentation leading to different apoE concentrations by ELISA. Moreover, the isoform-dependent apoE levels may be due to differences in receptor binding ability, structural stability, and oligomerization propensity.
ApoE oligomerization.
As lipid-free forms of apolipoproteins are not conformationally stable in general, they possess misfolding and self-oligomerization propensities (Hatters and Howlett, 2002). While the apoE C-terminal domain is predominantly assembled as coiled-coil dimeric or tetrameric species in vitro (Choy et al., 2003), the full-length apoE is prone to form soluble protofilament-like amyloid fibrils with a high α-helical conformation in an isoform-dependent manner (apoE4 > apoE3 > apoE2) (Hatters et al., 2006b). The greater apoE4 aggregation propensity is also seen in human brains (Liu et al., 2021). Additionally, the isoform-dependent propensity of apoE to form amorphous aggregates is hindered by lipidation in vitro (Hubin et al., 2019).
While strong evidence from human clinical and animal model studies suggests that a major mechanism by which APOE4 increases the risk of AD is by driving earlier and more abundant amyloid pathology in the brain (Christensen et al., 2010; Koffie, 2012; Kok, 2009; Liu et al., 2017a; Morris, 2010; Polvikoski, 1995; Reiman et al., 2009; Schmechel, 1993; Tiraboschi, 2004), in the absence of an APOE4 allele, any change that affects the biochemical and biophysical properties of apoE will have a greater cascading impact to the subsequent phases.
ApoE posttranslational modifications.
Posttranslational modification of proteins is a well-known phenomenon that affects protein structure and dynamics (Mann and Jensen, 2003). The differential posttranslational modifications of apoE isoforms also play an important role in modulating its function (Figure 2). A number of posttranslational modifications of apoE have been reported including glycation (Shuvaev et al., 1999), glycosylation (Flowers et al., 2020; Ke et al., 2020; Lee et al., 2010), phosphorylation (Jaros et al., 2012; Raftery et al., 2005), and oxidation (Jolivalt et al., 1996; Miyata and Smith, 1996; Strittmatter et al., 1993). In clinical studies, plasma levels of posttranslationally modified apoE (glycosylation, methylation, demethylation, and dihydroxylation) have been reported to increase in breast cancer patients (Uen et al., 2015), and the increased apoE citrullination is observed in the synovial fluid of rheumatoid arthritis patients (van Beers et al., 2013). Aging also impacts protein biochemical properties and functions through posttranslational modifications including oxidation and glycation (Santos and Lindner, 2017). Further studies should refine how aging-, apoE isoform-, and disease status-dependent changes in posttranslational modifications impact the structural and biochemical features of apoE under physiological and pathological conditions.
ApoE epigenetic modifications.
Epigenetic modification is another example of modifiers that can alter the biochemical phase of the ApoE Cascade Hypothesis. Tulloch et al. reported increased DNA methylation of APOE 3’ DNA in postmortem AD brains compared to control brains in a tissue- and APOE genotype-specific manner (Tulloch et al., 2018). The increased APOE DNA methylation has been shown to negatively correlate with total APOE mRNA levels (Lee et al., 2020), which may result in reduced apoE protein levels. Other epigenetic modifications of APOE such as chromatin remodeling and noncoding RNA have been reported (Yu and Foraker, 2015), but further investigation is needed to examine their impact on apoE protein levels.
Cellular phase of ApoE Cascade Hypothesis
The disruption of biochemical and biophysical properties of apoE such as misfolding and self-assembly of apoE (“structure” and “oligomerization”), decreased binding to lipids (“lipidation”), and decreased production or increased degradation of apoE (“protein levels”) can negatively impact apoE-dependent cellular functions in a cell-type specific manner leading to the second phase of the ApoE Cascade Hypothesis called the “cellular phase”.
ApoE is mainly produced by astrocytes, reactive microglia, vascular mural cells, and choroid plexus cells in the brain (Kang et al., 2018; Xu et al., 2006). Under stress conditions, neurons display an enhanced lipid metabolism accompanied by apoE production, perhaps to repair damaged membrane (Najm et al., 2019). Since excess intracellular lipid accumulation can cause cellular stress, apoE may also play a predominant role in transporting lipids from intracellular to extracellular space. However, lipids are also important for cellular homeostasis. Subcellular organelle membranes consist primarily of lipids, dividing/proliferating or damaged cells may require sufficient lipid supplies for membrane remodeling or repair. The binding of apoE/lipoprotein particles with cell surface apoE receptors and subsequent endocytosis are essential mechanisms for cell-to-cell lipid distribution in the brain (Figure 3). Therefore, the “ApoE Cascade Hypothesis” predicts that disruption of apoE-mediated cellular lipid homeostasis initiates a pathogenic cascade that contributes to AD-related cellular dysfunction. In addition to apoE isoforms, biochemical properties, and concentrations, brain cell type-specific apoE metabolism and functions through cell-autonomous and non-cell-autonomous mechanisms may also significantly modulate the cellular phase of AD and age-related cognitive decline (De Strooper and Karran, 2016). As such, there is a strong need to define how apoE properties and quantity affect each brain cell type, and how they are involved in the disease phenotypes at the cellular level.
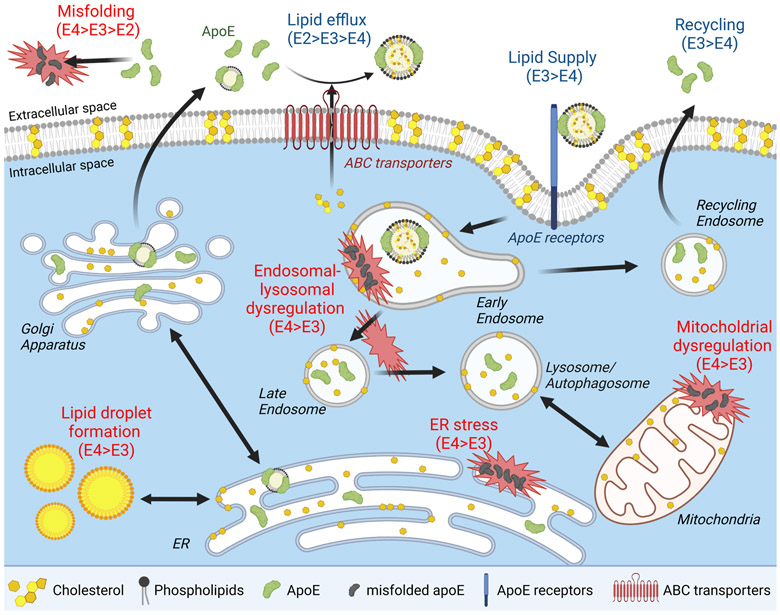
Figure 3. ApoE and cellular homeostasis.
ApoE traffics through the secretory pathway as a non-lipidated or lipidated protein into the extracellular space. ABC transporters load membrane and traffic intracellular lipids onto apoE to produce nascent apoE/lipoprotein particles. ApoE-containing lipid particles can undergo further lipid modifications and are taken up by various cells through receptor-mediated endocytosis by binding to apoE receptors. This process supplies cells with diverse lipids including phospholipids and cholesterols necessary to maintain cellular homeostasis and support synaptic integrity and plasticity. The endocytosed particles and their components are transported to lysosome/autophagosome through late endosomes or recycled back to the extracellular space through recycling endosomes. ApoE isoforms impact cellular homeostasis by differentially modulating membrane trafficking, ER stress, and mitochondria function due to their individual effects on protein homeostasis, aggregation, and lipid metabolism.
ApoE and intracellular trafficking dysregulation.
As the enlargement of endosomes is often detected as a cytopathological hallmark in early stages of AD, endosomal-lysosomal dysregulation is one of the central pathways in the cellular phase of AD pathogenesis (Nixon, 2005; Small and Petsko, 2020; Van Acker et al., 2019). Intriguingly, this phenotype appears to be exacerbated by APOE4 both in the brains of AD patients (Cataldo et al., 2000) and aged APOE-targeted replacement (TR) mice (Nuriel et al., 2017), independently of the amyloid pathology. A transcriptomics study has revealed that genes involved in endosomal-lysosomal pathways are enriched in the brains of apoE4-TR mice compared to apoE3-TR mice (Nuriel et al., 2017). Chen and colleagues previously reported that apoE4 reduces cell surface levels of apoER2, a neuronal signaling receptor for Reelin and apoE, as well as glutamate receptors by sequestering them in the endocytic compartments, thereby reducing synaptic activity (Chen et al., 2010; Lane-Donovan et al., 2014). The low pH environment of endosomes induces structurally labile apoE4 to form a molten globule which leads to reduced cell surface apoER2 expression due to dysregulation of endosomal intracellular trafficking (Xian et al., 2018). Our group found that apoE4 also suppresses cell surface insulin receptor (IR) and impairs IR trafficking by aggregating and retaining IR in the early endosomes (Zhao et al., 2017). As a result, the downstream signaling and the effects of insulin-induced glycolysis and mitochondrial respiration are significantly suppressed by apoE4. Altogether, these studies suggest that apoE4 may suppress various signaling cascades by impairing the trafficking of cell surface receptors.
While phosphoinositides contribute to vesicular transport by regulating vesicular budding, membrane fusion and cytoskeleton dynamics (De Craene et al., 2017), brain levels of phosphoinositol biphosphate (PIP2) are also decreased in APOE4 carriers regardless of AD stage (Zhu et al., 2015). Overexpression of phosphatidylinositol binding clathrin assembly protein (PICALM) restores endocytic defects caused by APOE4 in iPSC-derived astrocytes (Narayan et al., 2020). Thus, defining the link between apoE and phosphoinositide metabolism may provide important clues to uncover the pathogenic mechanism contributing to impaired vesicle trafficking in AD. In addition, altered apoE solubility and membrane association under acidic conditions in lysosomes may directly destabilize the vesicle membranes and cause lysosomal leakage (Van Acker et al., 2019). Since phospholipid asymmetries in the endomembrane system likely trigger exocytic/endocytic vesicle budding (Huijbregts et al., 2000), it is also possible that apoE-mediated membrane lipid modifications indirectly influence the cellular trafficking.
ApoE and cellular stress.
Whereas mitochondria and ER are essential organelles in maintaining cellular homeostasis, the dysregulation of the ER-mitochondria axis has been implicated in the pathogenesis of several age-related neurodegenerative diseases including AD (Filadi et al., 2017; Swerdlow et al., 2014; Wang et al., 2020). Of note, apoE is also involved in ER stress and mitochondrial dysfunction in AD pathogenesis (Dose et al., 2016). In apoE-TR mice, apoE4 increases eukaryotic initiation factor-2α (eIF2α) phosphorylation which indicates aggravated ER stress responses in the brain (Machlovi et al., 2022; Segev et al., 2013). The mitochondrial dynamics such as fusion and fission is also altered in the presence of apoE4, which is accompanied by impaired mitophagy in mouse brains (Simonovitch et al., 2019). While the misfolding of non-lipidated apoE may lead to those cellular stress responses, the domain-domain interaction in apoE4 and fragmented apoE are also possibly involved in this mechanism (Dose et al., 2016). In addition, apoE4 facilitates the physical interaction between mitochondria and ER through mitochondria-associated ER membranes (MAMs) (Tambini et al., 2016). Indeed, the critical roles of MAMs have been increasingly recognized in regulating proper cellular functions including calcium signaling and energy homeostasis (Eysert et al., 2020; Veeresh et al., 2019), and apoE may differently regulate ER-mitochondria functions through MAM formation depending on apoE isoforms or biochemical properties. Furthermore, ER stress and mitochondrial dysregulation are also associated with the formation of lipid droplets, which have been shown to accumulate in different brain cell types including neurons, astrocytes and microglia during aging and AD (Ralhan et al., 2021). Supporting the biochemical to cellular cascade, reduced receptor binding of apoE4 to LRP1 (biochemical phase) has been shown to upregulate cyclophilin A expression leading to increased cellular stress (cellular phase) through NF-κB pathway activation in pericytes (Bell et al., 2012). This can result in neuroinflammation and vascular dysfunction (phenotypic phase), which precede age-related cognitive impairment and AD.
ApoE and lipid dysregulation.
Lipid droplets contain non-polar lipids such as triglycerides and cholesterol esters, and are often considered as organelles budded from the ER and associated with other organelles. They regulate cellular metabolism and buffer lipotoxicity (Olzmann and Carvalho, 2019) but can also be pathogenic when dysregulated. Interestingly, apoE4 is associated with greater lipid droplet formation in astrocytes compared to apoE3 (Sienski et al., 2021); however, it is suppressed by apoE4 in neurons (Qi et al., 2021). The study by Qi and colleagues (Qi et al., 2021) highlights an effect of apoE4 impacted by its structure and protein levels (biochemical phase) in decreasing sequestration of fatty acid into lipid droplets in neurons, and in reducing its transport to astrocytes. As such, apoE4 is associated with decreased fatty acid degradation and lipid accumulation, leading to lipid dysregulation and accumulation of lipid droplets in astrocytes and increased mitochondrial stress (cellular phase). A similar finding of impaired lipid transport from neurons to astrocytes by apoE4 has also been reported using a drosophila model (Liu et al., 2017b). The impaired lipid metabolism can lead to synaptic dysfunction and neurodegeneration in neuropathology-dependent and independent manner (Chew et al., 2020) (phenotypic phase).
Interestingly, glial lipid metabolism appears to be most affected by apoE. Significant alterations were found in cholesterol esters and other lipids predominantly in microglia and to some extent in astrocytes with little change in whole brain in Apoe-KO mice (Nugent et al., 2020). This suggests a focus on apoE effects in glial lipid metabolism will likely provide important insights into apoE-related pathways in the normal brain, aging, and in AD. Thus, cell autonomous or non-autonomous apoE functions in lipid metabolism and cellular stress responses might differ depending on brain cell types during the cellular phase but converge to impact phenotypic outcomes.
Phenotypic phase of ApoE Cascade Hypothesis
While neurodegeneration is fundamental in the phenotypic phase of AD and age-related cognitive decline (De Strooper and Karran, 2016), apoE-mediated lipid metabolism and cellular dysregulation undoubtedly participate in the pathogenic process through both neuropathology-dependent and independent pathways as already described. In AD brains, apoE and Aβ frequently co-deposit in amyloid plaques (Cho et al., 2001). ApoE deficiency in mice vastly reduces brain Aβ deposition as fibril plaques and cerebral amyloid angiopathy (CAA) (Bales et al., 1997; Holtzman et al., 2000; Kim et al., 2011), suggesting that apoE promotes aggregation and fibrillization of Aβ in AD and CAA. When apoE is hyperlipidated upon ABCA1 overexpression, Aβ deposition is significantly reduced (Wahrle et al., 2008). ApoE2 is reported to be hyperlipidated compared to apoE3 and apoE4 in human CSF (Heinsinger et al., 2016) and in culture medium of immortalized astrocytes derived from apoE-TR mice (Morikawa et al., 2005) supporting the notion that increased lipidation of apoE protects against AD by reducing Aβ deposition.
Of note, APOE genotype has also been associated with the occurrence and severities of diverse neuropathologies including tau, α-synuclein, and TDP-43 in addition to Aβ (Belloy et al., 2019). Histological studies found colocalization of apoE with neurofibrillary tangles in AD brains (Benzing and Mufson, 1995; Richey et al., 1995), whereas apoE3 likely has a greater binding affinity than apoE4 to non-phosphorylated tau and prevents its phosphorylation in vitro (Hoe et al., 2006; Strittmatter et al., 1994). ApoE fragments are also detected within Lewy bodies in the brains of Parkinson’s disease patients (Rohn and Mack, 2018). Supporting this, deletion of apoE has been shown to increase α-synuclein solubility in SynA30P transgenic mice (Gallardo et al., 2008). Together, these lines of evidence indicate that apoE is involved in the development of various neuropathologies by impacting protein aggregation and deposition in an isoform-dependent manner. Further studies should define how apoE biochemical properties contribute to protein aggregation during aging and in aging-related pathological conditions. In addition, emerging evidence indicates that apoE produced by microglia or produced by other cells that act on microglia impacts the immune response in the brain during aging and AD (Guerreiro, 2018; Shi et al., 2019; Shi et al., 2017). In fact, APOE is ranked as one of the highest disease-associated microglia (DAM) genes, which are associated with aging, amyloid, and tau (Deczkowska, 2018; Krasemann, 2017; Rangaraju, 2018; Song and Colonna, 2018; Ulrich, 2018). ApoE along with phospholipids have been demonstrated as ligands for the triggering receptor expressed on myeloid cells 2 (TREM2), which is also a strong AD risk gene expressed in microglia (Atagi, 2015; Bailey et al., 2015; Wang et al., 2015; Yeh et al., 2016). Thus, apoE is a critical factor regulating AD-related neuroinflammation, although how microglia-expressed apoE influences the pathologies compared to astrocytic apoE still needs further investigation. Other common phenotypes in AD are the disturbances of cerebrovascular integrity and function. APOE4 is also a strong genetic risk factor for multiple vascular conditions including hypercholesterolemia, atherosclerosis, vascular cognitive impairment, and cerebral amyloid angiopathy (Davidson, 2006; Rannikmae, 2014; Shinohara, 2016; Sun, 2015). The presence of APOE4 is associated with increased severity of white matter hyperintensities, accelerated pericyte degeneration, and compromised blood-brain barrier integrity (Halliday, 2013; 2016; Schilling, 2013; Sudre, 2017). Thus, a better understanding of the biology and pathobiology regarding how apoE isoforms produced by different brain cell types and their biochemical properties impact cerebrovascular functions will provide new insights in AD-related phenotypes.
Targeting apoE-initiated cascade events in the disease process.
The ApoE Cascade Hypothesis proposed here should guide the design of novel therapeutic strategies against age-related cognitive decline and AD. The pharmacological, genetic, or lifestyle interventions that alter the biochemical and biophysical properties of apoE (biochemical phase) will lead to changes in subsequent phases of this cascade. A study by Xian et al. reported that the low pH environment of endosome induces structurally labile apoE4 to form a molten globule (biochemical Phase) leading to reduced cell surface apoER2 level (cellular Phase) (Xian et al., 2018). Pharmacological reduction of the pH in endosomes by inhibiting NHE6 reverses the apoE4-mediated endosomal dysfunction and restores synaptic function. In a follow up study by the same group, deletion of NHE6 reduces apoE-mediated amyloid plaque buildup in an animal model of AD (phenotypic phase) (Pohlkamp et al., 2021).
Interventions to increase lipidation, reduce oligomerization, increase or decrease the protein levels or receptor binding of apoE depending on apoE isoforms, as well as the use of structural correctors are all strategies that are being or should be investigated for treating AD and age-related cognitive decline (Chen et al., 2012; Liao et al., 2018; Tai et al., 2014; Zhao et al., 2014). Some of the strategies show promising results against amyloid pathology (Xiong et al., 2021), but others need future optimization to reduce potential toxic side effects (Tai et al., 2014). Due to its complex biology and pathobiology of apoE, future interventions may benefit from the use of bi-functional molecules or those that enable cell type-specific delivery of drugs. Target engagement of therapeutic interventions to alter the biochemical and biophysical properties of apoE, followed by the investigation of their impact on the cellular phase of apoE cascade should be validated using in vitro or in vivo assays (Hughes et al., 2011). The validation of their effects on the phenotypic phase will require more complex model systems such as animal models and human iPSC-derived cerebral organoids (Park et al., 2021; Singh and Seed, 2021).
ApoE Cascade Hypothesis in other age-related disorders.
The biochemical properties of apoE can also cascade down to cause age-related disorders. APOE4 has been found to be a genetic risk factor for Lewy body dementia (LBD). Using animal models and human iPSC models, our group have demonstrated a pathogenic role of APOE4 in exacerbating α-synuclein pathology independent of amyloid (Davis et al., 2020; Zhao et al., 2020a; Zhao et al., 2020b). Our group has also reported an association between the APOE2 genotype and risk of tauopathies such as progressive supranuclear palsy and corticobasal degeneration (Zhao et al., 2018). These findings suggest that APOE2 status may influence the risk and progression of primary tauopathy. ApoE2 and apoE4 increases the risk of cardiovascular diseases through different mechanisms. The low affinity of apoE2 for LDLR leads to reduced clearance of triglyceride-rich VLDL which is prone to form atherosclerotic plaques while the preference of apoE4 for VLDL is associated with higher plasma LDL cholesterol which results in increased coronary heart disease risk (Mahley, 2016). While apoE2 is protective against AD and AD-related neuropathologies, it is a risk factor for age-related macular degeneration (AMD) (Thakkinstian et al., 2006). The main pathological features of AMD is the formation of lipid-rich drusen, yellow deposits under the retina (Wang et al., 2010). A study by Levy et al. reveals that subretinal mononuclear phagocytes from apoE2-TR mice exhibit increased subretinal inflammation, promoting choroidal neovascularization in subretinal space (Levy et al., 2015). Although the exact mechanism of how apoE2 increases the risk of AMD remains unknown, disruption of lipid metabolism in retina cells has been suggested in an animal model (Saadane et al., 2018). Altogether, this evidence supports the generalizability of the ApoE Cascade Hypothesis to decipher pathogenic mechanisms of other apoE-related conditions.
Concluding remarks
How apoE isoforms, differing only by a single amino acid from one another, have such profound effects on the risk of AD and related dementias, has been puzzling the apoE field for almost three decades (Yamazaki et al., 2019). To this end, we propose a potential “butterfly effect” of apoE on AD and age-related cognitive decline referred to as “ApoE Cascade Hypothesis”; a collection of differences in structural and biochemical properties depending on apoE isoforms, posttranslational modifications, and/or altered apoE expression initiate a cascade of events at the cellular and systems levels during aging, thus driving AD-related pathogenic conditions (Figure 1). Whereas most proteins encoded by AD risk genes have been shown to impact lipid metabolism, immune response, or membrane trafficking (Kanekiyo and Bu, 2014), apoE is involved in all three pathways. Furthermore, apoE has been shown to contribute to the development of amyloid pathology (A), tauopathy (T), and neurodegeneration (N), collectively known as ATN classification, as well as neuroinflammation and cerebrovascular dysfunction at the presymptomatic stage of AD. Therefore, it is reasonable to hypothesize that apoE triggers multifaceted pathways in AD. Although APOE4 is the strongest genetic risk factor for AD impacting 50-70% of all cases, it is not a causative gene (Corder et al., 1993; Corder et al., 1994; Farrer et al., 1997; Saunders et al., 1993). Moreover, it is still not entirely clear how cell type, disease status, and apoE isoform collectively or individually modulate the biochemical phase of apoE. Interestingly, recent work from our group revealed that the apoE lipoprotein particle sizes are affected by both cell type (astrocytes vs. microglia) and apoE isoform (Huynh et al., 2019). There is a dire need to further address these critical gaps in knowledge to better design mechanism-based therapeutic strategies. Thus, secondary modifiers such as age, sex, and other genetic/epigenetic or environmental factors may accelerate or decelerate the apoE cascade in AD and age-related cognitive decline.
Although the field has learned so much about the ways apoE contributes to AD, much work is still needed to further support or strengthen the “ApoE Cascade Hypothesis”. First, the limited structural information for lipid-bound apoE produced by different brain cell types and the differential effects of apoE isoforms call for focused efforts in addressing apoE structural properties related to lipid association and protein oligomerization. Second, much of the information on apoE is derived from the detrimental effects of apoE4, whereas the field can gain greater insights by understanding the protective mechanisms of apoE2, as well as the rare apoE3-Christchurch (Arboleda-Velasquez et al., 2019) and apoE3-Jacksonville (Liu et al., 2021) variants. Third, there is increasing evidence suggesting a contributing role of peripheral apoE, thus understanding how apoE isoforms expressed by the liver and macrophages represents an opportunity for greater appreciation on how peripheral system impacts the brain and AD. Despite the need of more knowledge, we believe that the “ApoE Cascade Hypothesis” can guide the design of therapeutic strategies for AD and related dementias by targeting early events such as apoE structure, apoE concentration, posttranslational modifications, oligomerization, receptor binding, and/or lipidation.
Acknowledgement
This work is supported by NIH grant U19AG069701 to all listed authors with G.B., D.M.H., and A.M.G. as Co-Principal Investigators. Authors also receive other NIH grants that relate to studies on apoE. G.B. and D.M.H. are further supported by grants from the Cure Alzheimer’s Fund. AMG is supported by grants from the JPB Foundation and the Neurodegeneration Consortium. The authors would like to thank Dr. Ana-Caroline Raulin and Ms. Shelby Ross for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
GB consults for SciNeuro and Lexeo, has consulted for Vida Ventures, AbbVie, E-Scape, and Eisai, is on the scientific advisory board of Kisbee Therapeutics, and serves as a Co-Editor-in-Chief for Molecular Neurodegeneration. DMH is as an inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies. DMH co-founded and is on the scientific advisory board of C2N Diagnostics. C2N Diagnostics has licensed certain anti-tau antibodies to AbbVie for therapeutic development. DMH is on the scientific advisory board of Denali, Genentech, and Cajal Neurosciences and consults for Eli Lilly. DMH receives sponsored research agreements to Washington University from NextCure, C2N Diagnostics, Yumanity, Eli Lilly, and Novartis. AMG is on the scientific advisory board of Genentech and has consulted for Cognition Therapeutics and AbbVie. Other authors declare no competing interests.
References
- Alzheimer's Association (2021). 2021 Alzheimer's disease facts and figures. Alzheimers Dement 17, 327–406. 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Lopera F, O'Hare M, Delgado-Tirado S, Marino C, Chmielewska N, Saez-Torres KL, Amarnani D, Schultz AP, Sperling RA, et al. (2019). Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med 25, 1680–1683. 10.1038/s41591-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atagi Y (2015). Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem 290. 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CC, DeVaux LB, and Farzan M (2015). The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J. Biol. Chem 290. 10.1074/jbc.M115.677286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. (1997). Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet 17, 263–264. 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Yu CE, Bird TD, and Tsuang DW (2010). Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 25, 213–227. 10.1177/0891988710383571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al. (2012). Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516. 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloy ME, Napolioni V, and Greicius MD (2019). A Quarter Century of APOE and Alzheimer's Disease: Progress to Date and the Path Forward. Neuron 101, 820–838. 10.1016/j.neuron.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing WC, and Mufson EJ (1995). Apolipoprotein E immunoreactivity within neurofibrillary tangles: relationship to Tau and PHF in Alzheimer's disease. Exp Neurol 132, 162–171. 10.1016/0014-4886(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, Hernandez DG, Nalls MA, Clark LN, Honig LS, et al. (2014). Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet 23, 6139–6146. 10.1093/hmg/ddu334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, and Nixon RA (2000). Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 157, 277–286. 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HK, Liu Z, Meyer-Franke A, Brodbeck J, Miranda RD, McGuire JG, Pleiss MA, Ji ZS, Balestra ME, Walker DW, et al. (2012). Small molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons. J Biol Chem 287, 5253–5266. 10.1074/jbc.M111.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Q, and Wang J (2011). Topology of human apolipoprotein E3 uniquely regulates its diverse biological functions. Proc. Natl Acad. Sci. USA 108. 10.1073/pnas.1106420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, and Herz J (2010). ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A 107, 12011–12016. 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Strickland MR, Soranno A, and Holtzman DM (2021). Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 109, 205–221. 10.1016/j.neuron.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew H, Solomon VA, and Fonteh AN (2020). Involvement of Lipids in Alzheimer's Disease Pathology and Potential Therapies. Front Physiol 11, 598. 10.3389/fphys.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Hyman BT, Greenberg SM, and Rebeck GW (2001). Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Abeta aggregation. J Neuropathol Exp Neurol 60, 342–349. 10.1093/jnen/60.4.342. [DOI] [PubMed] [Google Scholar]
- Choy N, Raussens V, and Narayanaswami V (2003). Inter-molecular coiled-coil formation in human apolipoprotein E C-terminal domain. J Mol Biol 334, 527–539. 10.1016/j.jmb.2003.09.059. [DOI] [PubMed] [Google Scholar]
- Christensen DZ, Schneider-Axmann T, Lucassen PJ, Bayer TA, and Wirths O (2010). Accumulation of intraneuronal Abeta correlates with ApoE4 genotype. Acta Neuropathol. 119. 10.1007/s00401-010-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses A, Haines J, and Pericak-Vance MA (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr., Rimmler JB, Locke PA, Conneally PM, Schmader KE, and et al. (1994). Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7, 180–184. 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Davidson Y (2006). Apolipoprotein E epsilon4 allele frequency in vascular dementia. Dement. Geriatr. Cogn. Disord 22. 10.1159/000092960. [DOI] [PubMed] [Google Scholar]
- Davis AA, Inman CE, Wargel ZM, Dube U, Freeberg BM, Galluppi A, Haines JN, Dhavale DD, Miller R, Choudhury FA, et al. (2020). APOE genotype regulates pathology and disease progression in synucleinopathy. Sci Transl Med 12. 10.1126/scitranslmed.aay3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene JO, Bertazzi DL, Bar S, and Friant S (2017). Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways. Int J Mol Sci 18. 10.3390/ijms18030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, and Karran E (2016). The Cellular Phase of Alzheimer's Disease. Cell 164, 603–615. 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Deczkowska A (2018). Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173. 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Dhana K, Aggarwal NT, Rajan KB, Barnes LL, Evans DA, and Morris MC (2021). Impact of the Apolipoprotein E4 allele on the Relationship Between Healthy Lifestyle and Cognitive Decline: A Population-based Study. Am J Epidemiol. 10.1093/aje/kwab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dose J, Huebbe P, Nebel A, and Rimbach G (2016). APOE genotype and stress response - a mini review. Lipids Health Dis 15, 121. 10.1186/s12944-016-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysert F, Kinoshita PF, Mary A, Vaillant-Beuchot L, Checler F, and Chami M (2020). Molecular Dysfunctions of Mitochondria-Associated Membranes (MAMs) in Alzheimer's Disease. Int J Mol Sci 21. 10.3390/ijms21249521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, and van Duijn CM (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama 278, 1349–1356. [PubMed] [Google Scholar]
- Filadi R, Theurey P, and Pizzo P (2017). The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 62, 1–15. 10.1016/j.ceca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Flowers SA, Grant OC, Woods RJ, and Rebeck GW (2020). O-glycosylation on cerebrospinal fluid and plasma apolipoprotein E differs in the lipid-binding domain. Glycobiology 30, 74–85. 10.1093/glycob/cwz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C, Wang H, and Ho CMW (2017). A mechanism for lipid binding to apoE and the role of intrinsically disordered regions coupled to domain-domain interactions. Proc. Natl Acad. Sci. USA 114. 10.1073/pnas.1705080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Altomare D, Thal DR, Ribaldi F, van der Kant R, Ossenkoppele R, Blennow K, Cummings J, van Duijn C, Nilsson PM, et al. (2022). The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci 23, 53–66. 10.1038/s41583-021-00533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo G, Schluter OM, and Sudhof TC (2008). A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci 11, 301–308. 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- Guerreiro R (2018). Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol. 17. 10.1016/S1474-4422(17)30400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, Shepherd CE, Parkkinen L, Darwent L, Heckman MG, et al. (2018). Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol 17, 64–74. 10.1016/S1474-4422(17)30400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR (2013). Relationship between cyclophilin A levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein E4 carriers and blood-brain barrier breakdown. JAMA Neurol. 70. 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab 36. 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, and Higgins GA (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185. 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hatters DM, and Howlett GJ (2002). The structural basis for amyloid formation by plasma apolipoproteins: a review. Eur Biophys J 31, 2–8. 10.1007/s002490100172. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, and Weisgraber KH (2006a). Apolipoprotein E structure: insights into function. Trends Biochem Sci 31, 445–454. 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Zhong N, Rutenber E, and Weisgraber KH (2006b). Amino-terminal domain stability mediates apolipoprotein E aggregation into neurotoxic fibrils. J Mol Biol 361, 932–944. 10.1016/j.jmb.2006.06.080. [DOI] [PubMed] [Google Scholar]
- Heinsinger NM, Gachechiladze MA, and Rebeck GW (2016). Apolipoprotein E genotype affects size of ApoE complexes in cerebrospinal fluid. J. Neuropathol. Exp. Neurol 75. 10.1093/jnen/nlw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, and Bock HH (2002). Lipoprotein receptors in the nervous system. Annu Rev Biochem 71, 405–434. 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Herz J, and Chen Y (2006). Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci 7, 850–859. 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Freeman J, and Rebeck GW (2006). Apolipoprotein E decreases tau kinases and phospho-tau levels in primary neurons. Mol Neurodegener 1, 18. 10.1186/1750-1326-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, and Hyman BT (2000). Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer's disease model. Ann Neurol 47, 739–747. [PubMed] [Google Scholar]
- Hu J, Liu CC, Chen XF, Zhang YW, Xu H, and Bu G (2015). Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Abeta metabolism in apoE4-targeted replacement mice. Mol Neurodegener 10, 6. 10.1186/s13024-015-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, and Mahley RW (2014). Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis 72 Pt A, 3–12. 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubin E, Verghese PB, van Nuland N, and Broersen K (2019). Apolipoprotein E associated with reconstituted high-density lipoprotein-like particles is protected from aggregation. FEBS Lett 593, 1144–1153. 10.1002/1873-3468.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, and Philpott KL (2011). Principles of early drug discovery. Br J Pharmacol 162, 1239–1249. 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts RP, Topalof L, and Bankaitis VA (2000). Lipid metabolism and regulation of membrane trafficking. Traffic 1, 195–202. [DOI] [PubMed] [Google Scholar]
- Huynh TV, Wang C, Tran AC, Tabor GT, Mahan TE, Francis CM, Finn MB, Spellman R, Manis M, Tanzi RE, et al. (2019). Lack of hepatic apoE does not influence early Abeta deposition: observations from a new APOE knock-in model. Mol Neurodegener 14, 37. 10.1186/s13024-019-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaros JA, Martins-de-Souza D, Rahmoune H, Rothermundt M, Leweke FM, Guest PC, and Bahn S (2012). Protein phosphorylation patterns in serum from schizophrenia patients and healthy controls. J Proteomics 76 Spec No., 43–55. 10.1016/j.jprot.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Jolivalt C, Leininger-Muller B, Drozdz R, Naskalski JW, and Siest G (1996). Apolipoprotein E is highly susceptible to oxidation by myeloperoxidase, an enzyme present in the brain. Neurosci Lett 210, 61–64. 10.1016/0304-3940(96)12661-6. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, and Bu G (2014). The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer's disease. Front Aging Neurosci 6, 93. 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Ebbert MTW, Baker KE, Cook C, Wang X, Sens JP, Kocher JP, Petrucelli L, and Fryer JD (2018). Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J Exp Med 275, 2235–2245. 10.1084/jem.20180653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke LY, Chan HC, Chen CC, Chang CF, Lu PL, Chu CS, Lai WT, Shin SJ, Liu FT, and Chen CH (2020). Increased APOE glycosylation plays a key role in the atherogenicity of L5 low-density lipoprotein. FASEB J 34, 9802–9813. 10.1096/fj.202000659R. [DOI] [PubMed] [Google Scholar]
- Kim J, Jiang H, Park S, Eltorai AE, Stewart FR, Yoon H, Basak JM, Finn MB, and Holtzman DM (2011). Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci 31, 18007–18012. 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM (2012). Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 135. 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok E (2009). Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann. Neurol 65. 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Krasemann S (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47. 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane-Donovan C, Philips GT, and Herz J (2014). More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron 83, 771–787. 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco MF, Ng CA, and Rebeck GW (2020). ApoE Lipidation as a Therapeutic Target in Alzheimer's Disease. Int J Mol Sci 21. 10.3390/ijms21176336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EG, Tulloch J, Chen S, Leong L, Saxton AD, Kraemer B, Darvas M, Keene CD, Shutes-David A, Todd K, et al. (2020). Redefining transcriptional regulation of the APOE gene and its association with Alzheimer's disease. PLoS One 15, e0227667. 10.1371/journal.pone.0227667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kockx M, Raftery MJ, Jessup W, Griffith R, and Kritharides L (2010). Glycosylation and sialylation of macrophage-derived human apolipoprotein E analyzed by SDS-PAGE and mass spectrometry: evidence for a novel site of glycosylation on Ser290. Mol Cell Proteomics 9, 1968–1981. 10.1074/mcp.M900430-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O, Lavalette S, Hu SJ, Housset M, Raoul W, Eandi C, Sahel JA, Sullivan PM, Guillonneau X, and Sennlaub F (2015). APOE Isoforms Control Pathogenic Subretinal Inflammation in Age-Related Macular Degeneration. J Neurosci 35, 13568–13576. 10.1523/JNEUROSCI.2468-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Li A, Xiong M, Bien-Ly N, Jiang H, Zhang Y, Finn MB, Hoyle R, Keyser J, Lefton KB, et al. (2018). Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation. J Clin Invest 128, 2144–2155. 10.1172/JCI96429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, et al. (2018). APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 98, 1294. 10.1016/j.neuron.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Murray ME, Li X, Zhao N, Wang N, Heckman MG, Shue F, Martens Y, Li Y, Raulin AC, et al. (2021). APOE3-Jacksonville (V236E) variant reduces self-aggregation and risk of dementia. Sci Transl Med 13, eabc9375. 10.1126/scitranslmed.abc9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Zhao N, Fu Y, Wang N, Linares C, Tsai CW, and Bu G (2017a). ApoE4 Accelerates Early Seeding of Amyloid Pathology. Neuron 96, 1024–1032 e1023. 10.1016/j.neuron.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletic-Savatic M, and Bellen HJ (2017b). The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab 26, 719–737 e716. 10.1016/j.cmet.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlovi SI, Neuner SM, Hemmer BM, Khan R, Liu Y, Huang M, Zhu JD, Castellano JM, Cai D, Marcora E, and Goate AM (2022). APOE4 confers transcriptomic and functional alterations to primary mouse microglia. Neurobiol Dis 164, 105615. 10.1016/j.nbd.2022.105615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW (2016). Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl) 94, 739–746. 10.1007/s00109-016-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, and Rall SC Jr. (1999). Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res 40, 1933–1949. [PubMed] [Google Scholar]
- Mahley RW, and Rall SC Jr. (2000). Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1, 507–537. 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, and Huang Y (2009). Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res 50 Suppl, S183–188. 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M, and Jensen ON (2003). Proteomic analysis of post-translational modifications. Nat Biotechnol 21, 255–261. 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- Martinez-Morillo E, Hansson O, Atagi Y, Bu G, Minthon L, Diamandis EP, and Nielsen HM (2014). Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer's disease patients and controls. Acta Neuropathol 127, 633–643. 10.1007/s00401-014-1266-2. [DOI] [PubMed] [Google Scholar]
- Michikawa M, Fan QW, Isobe I, and Yanagisawa K (2000). Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem 74, 1008–1016. 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- Miyata M, and Smith JD (1996). Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet 14, 55–61. 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Fryer JD, Sullivan PM, Christopher EA, Wahrle SE, DeMattos RB, O'Dell MA, Fagan AM, Lashuel HA, Walz T, et al. (2005). Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-beta. Neurobiol Dis 19, 66–76. 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Morris JC (2010). APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67. 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, and Holtzman DM (2015). Three dimensions of the amyloid hypothesis: time, space and 'wingmen'. Nat Neurosci 18, 800–806. 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm R, Jones EA, and Huang Y (2019). Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer's disease. Mol Neurodegener 14, 24. 10.1186/s13024-019-0324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Sienski G, Bonner JM, Lin YT, Seo J, Baru V, Haque A, Milo B, Akay LA, Graziosi A, et al. (2020). PICALM Rescues Endocytic Defects Caused by the Alzheimer's Disease Risk Factor APOE4. Cell Rep 33, 108224. 10.1016/j.celrep.2020.108224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA (2005). Endosome function and dysfunction in Alzheimer's disease and other neurodegenerative diseases. Neurobiol Aging 26, 373–382. 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, et al. (2020). TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron 105, 837–854 e839. 10.1016/j.neuron.2019.12.007. [DOI] [PubMed] [Google Scholar]
- Nuriel T, Peng KY, Ashok A, Dillman AA, Figueroa HY, Apuzzo J, Ambat J, Levy E, Cookson MR, Mathews PM, and Duff KE (2017). The Endosomal-Lysosomal Pathway Is Dysregulated by APOE4 Expression in Vivo. Front Neurosci 11, 702. 10.3389/fnins.2017.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann JA, and Carvalho P (2019). Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20, 137–155. 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC, Jang SY, Lee D, Lee J, Kang U, Chang H, Kim HJ, Han SH, Seo J, Choi M, et al. (2021). A logical network-based drug-screening platform for Alzheimer's disease representing pathological features of human brain organoids. Nat Commun 12, 280. 10.1038/s41467-020-20440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlkamp T, Xian X, Wong CH, Durakoglugil MS, Werthmann GC, Saido TC, Evers BM, White CL 3rd, Connor J, Hammer RE, and Herz J (2021). NHE6 depletion corrects ApoE4-mediated synaptic impairments and reduces amyloid plaque load. Elife 10. 10.7554/eLife.72034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvikoski T (1995). Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N. Engl. J. Med 333. 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- Qi G, Mi Y, Shi X, Gu H, Brinton RD, and Yin F (2021). ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep 34, 108572. 10.1016/j.celrep.2020.108572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M, Campbell R, Glaros EN, Rye KA, Halliday GM, Jessup W, and Garner B (2005). Phosphorylation of apolipoprotein-E at an atypical protein kinase CK2 PSD/E site in vitro. Biochemistry 44, 7346–7353. 10.1021/bi0504052. [DOI] [PubMed] [Google Scholar]
- Ralhan I, Chang CL, Lippincott-Schwartz J, and Ioannou MS (2021). Lipid droplets in the nervous system. J Cell Biol 220. 10.1083/jcb.202102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju S (2018). Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease. Mol. Neurodegener 13. 10.1186/s13024-018-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannikmae K (2014). APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J. Neurol. Neurosurg. Psychiatry 85. 10.1136/jnnp-2013-306485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, and Frikke-Schmidt R (2015). Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol 77, 301–311. 10.1002/ana.24326. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, et al. (2009). Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A 106, 6820–6825. 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey PL, Siedlak SL, Smith MA, and Perry G (1995). Apolipoprotein E interaction with the neurofibrillary tangles and senile plaques in Alzheimer disease: implications for disease pathogenesis. Biochem Biophys Res Commun 208, 657–663. 10.1006/bbrc.1995.1389. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, et al. (2008). Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci 28, 11445–11453. 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, and Mack JM. (2018). Apolipoprotein E Fragmentation within Lewy Bodies of the Human Parkinson's Disease Brain. Int J Neurodegener Dis 1. 10.23937/IJND-2017/1710002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadane A, Petrov A, Mast N, El-Darzi N, Dao T, Alnemri A, Song Y, Dunaief JL, and Pikuleva IA (2018). Mechanisms that minimize retinal impact of apolipoprotein E absence. J Lipid Res 59, 2368–2382. 10.1194/jlr.M090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando SB, Melquist S, Cannon A, Hutton ML, Sletvold O, Saltvedt I, White LR, Lydersen S, and Aasly JO (2008). APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from central Norway. BMC Neurol 8, 9. 10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AL, and Lindner AB (2017). Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid Med Cell Longev 2017, 5716409. 10.1155/2017/5716409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, and et al. (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43, 1467–1472. 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schilling S (2013). APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology 81. 10.1212/WNL.0b013e31829bfda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE (1993). Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl Acad. Sci. USA 90. 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev Y, Michaelson DM, and Rosenblum K (2013). ApoE epsilon4 is associated with eIF2alpha phosphorylation and impaired learning in young mice. Neurobiol Aging 34, 863–872. 10.1016/j.neurobiolaging.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Shi Y, Manis M, Long J, Wang K, Sullivan PM, Remolina Serrano J, Hoyle R, and Holtzman DM (2019). Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med 216, 2546–2561. 10.1084/jem.20190980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, et al. (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527. 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M (2016). Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. 132. 10.1007/s00401-016-1580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Kanekiyo T, Yang L, Linthicum D, Shinohara M, Fu Y, Price L, Frisch-Daiello JL, Han X, Fryer JD, and Bu G (2016). APOE2 eases cognitive decline during Aging: Clinical and preclinical evaluations. Ann Neurol 79, 758–774. 10.1002/ana.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Fujii J, Kawasaki Y, Itoh H, Hamaoka R, Barbier A, Ziegler O, Siest G, and Taniguchi N (1999). Glycation of apolipoprotein E impairs its binding to heparin: identification of the major glycation site. Biochim Biophys Acta 1454, 296–308. 10.1016/s0925-4439(99)00047-2. [DOI] [PubMed] [Google Scholar]
- Sienski G, Narayan P, Bonner JM, Kory N, Boland S, Arczewska AA, Ralvenius WT, Akay L, Lockshin E, He L, et al. (2021). APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med 13. 10.1126/scitranslmed.aaz4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovitch S, Schmukler E, Masliah E, Pinkas-Kramarski R, and Michaelson DM (2019). The Effects of APOE4 on Mitochondrial Dynamics and Proteins in vivo. J Alzheimers Dis 70, 861–875. 10.3233/JAD-190074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, and Seed TM (2021). How necessary are animal models for modern drug discovery? Expert Opin Drug Discov 16, 1391–1397. 10.1080/17460441.2021.1972255. [DOI] [PubMed] [Google Scholar]
- Small SA, and Petsko GA (2020). Endosomal recycling reconciles the Alzheimer's disease paradox. Sci Transl Med 12. 10.1126/scitranslmed.abb1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WM, and Colonna M (2018). The identity and function of microglia in neurodegeneration. Nat. Immunol 19. 10.1038/s41590-018-0212-1. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, and Roses AD (1994). Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci U S A 91, 11183–11186. 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, and Roses AD (1993). Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A 90, 8098–8102. 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH (2017). APOE epsilon4 status is associated with white matter hyperintensities volume accumulation rate independent of AD diagnosis. Neurobiol. Aging 53. 10.1016/j.neurobiolaging.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Sun JH (2015). Genetics of vascular dementia: systematic review and meta-analysis. J. Alzheimers Dis 46. 10.3233/JAD-143102. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, and Khan SM (2014). The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta 1842, 1219–1231. 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Koster KP, Luo J, Lee SH, Wang YT, Collins NC, Ben Aissa M, Thatcher GRJ, and LaDu MJ (2014). Amyloid-beta pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo. J Biol Chem 289, 30538–30555. 10.1074/jbc.M114.600833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini MD, Pera M, Kanter E, Yang H, Guardia-Laguarta C, Holtzman D, Sulzer D, Area-Gomez E, and Schon EA (2016). ApoE4 upregulates the activity of mitochondria-associated ER membranes. EMBO Rep 17, 27–36. 10.15252/embr.201540614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkinstian A, Han P, McEvoy M, Smith W, Hoh J, Magnusson K, Zhang K, and Attia J (2006). Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet 15, 2784–2790. 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P (2004). Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 62. 10.1212/01.WNL.0000128091.92139.0F. [DOI] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, et al. (2013). APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 70, 223–228. 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch J, Leong L, Thomson Z, Chen S, Lee EG, Keene CD, Millard SP, and Yu CE (2018). Glia-specific APOE epigenetic changes in the Alzheimer's disease brain. Brain Res 1698, 179–186. 10.1016/j.brainres.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uen YH, Liao CC, Lin JC, Pan YH, Liu YC, Chen YC, Chen WJ, Tai CC, Lee KW, Liu YR, et al. (2015). Analysis of differentially expressed novel post-translational modifications of plasma apolipoprotein E in Taiwanese females with breast cancer. J Proteomics 126, 252–262. 10.1016/j.jprot.2015.05.038. [DOI] [PubMed] [Google Scholar]
- Ulrich JD (2018). ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med 215. 10.1084/jem.20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM, Jiang H, Cirrito JR, and Holtzman DM (2013). In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener 8, 13. 10.1186/1750-1326-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker ZP, Bretou M, and Annaert W (2019). Endo-lysosomal dysregulations and late-onset Alzheimer's disease: impact of genetic risk factors. Mol Neurodegener 14, 20. 10.1186/s13024-019-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, Oosterink E, Bozic B, and Pruijn GJ (2013). The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum 65, 69–80. 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- Veeresh P, Kaur H, Sarmah D, Mounica L, Verma G, Kotian V, Kesharwani R, Kalia K, Borah A, Wang X, et al. (2019). Endoplasmic reticulum-mitochondria crosstalk: from junction to function across neurological disorders. Ann N Y Acad Sci 1457, 41–60. 10.1111/nyas.14212. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, et al. (2008). Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest 118, 671–682. 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, and Holtzman DM (2004). ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 279, 40987–40993. 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, and Curcio CA (2010). Abundant lipid and protein components of drusen. PLoS One 5, e10329. 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao F, Ma X, Perry G, and Zhu X (2020). Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Mol Neurodegener 15, 30. 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. (2015). TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell 160, 1061–1071. 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber KH (1990). Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res 31, 1503–1511. [PubMed] [Google Scholar]
- Wennberg AM, Tosakulwong N, Lesnick TG, Murray ME, Whitwell JL, Liesinger AM, Petrucelli L, Boeve BF, Parisi JE, Knopman DS, et al. (2018). Association of Apolipoprotein E epsilon4 With Transactive Response DNA-Binding Protein 43. JAMA Neurol 75, 1347–1354. 10.1001/jamaneurol.2018.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsmith KR, Basak JM, Patterson BW, Pyatkivskyy Y, Kim J, Yarasheski KE, Wang JX, Mawuenyega KG, Jiang H, Parsadanian M, et al. (2012). In vivo human apolipoprotein E isoform fractional turnover rates in the CNS. PLoS One 7, e38013. 10.1371/journal.pone.0038013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X, Pohlkamp T, Durakoglugil MS, Wong CH, Beck JK, Lane-Donovan C, Plattner F, and Herz J (2018). Reversal of ApoE4-induced recycling block as a novel prevention approach for Alzheimer's disease. Elife 7. 10.7554/eLife.40048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Jiang H, Serrano JR, Gonzales ER, Wang C, Gratuze M, Hoyle R, Bien-Ly N, Silverman AP, Sullivan PM, et al. (2021). APOE immunotherapy reduces cerebral amyloid angiopathy and amyloid plaques while improving cerebrovascular function. Sci Transl Med 13. 10.1126/scitranslmed.abd7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, and Huang Y (2006). Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26, 4985–4994. 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Zhao N, Caulfield TR, Liu CC, and Bu G (2019). Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol 15, 501–518. 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA, Bennett DA, Schneider JA, and De Jager PL (2018). Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE epsilon4 haplotype status: a community-based cohort study. Lancet Neurol 17, 773–781. 10.1016/S1474-4422(18)30251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FL, Wang Y, Tom I, Gonzalez LC, and Sheng M (2016). TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91. 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Yu CE, and Foraker J (2015). Epigenetic considerations of the APOE gene. [corrected]. Biomol Concepts 6, 77–84. 10.1515/bmc-2014-0039. [DOI] [PubMed] [Google Scholar]
- Zhao J, Fu Y, Liu CC, Shinohara M, Nielsen HM, Dong Q, Kanekiyo T, and Bu G (2014). Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway. J Biol Chem 289, 11282–11292. 10.1074/jbc.M113.526095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Fu Y, Yamazaki Y, Ren Y, Davis MD, Liu CC, Lu W, Wang X, Chen K, Cherukuri Y, et al. (2020a). APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer's disease patient iPSC-derived cerebral organoids. Nat Commun 11, 5540. 10.1038/s41467-020-19264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Attrebi ON, Ren Y, Qiao W, Sonustun B, Martens YA, Meneses AD, Li F, Shue F, Zheng J, et al. (2020b). APOE4 exacerbates alpha-synuclein pathology and related toxicity independent of amyloid. Sci Transl Med 12. 10.1126/scitranslmed.aay1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Liu CC, Van Ingelgom AJ, Linares C, Kurti A, Knight JA, Heckman MG, Diehl NN, Shinohara M, Martens YA, et al. (2018). APOE epsilon2 is associated with increased tau pathology in primary tauopathy. Nat Commun 9, 4388. 10.1038/s41467-018-06783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Liu CC, Van Ingelgom AJ, Martens YA, Linares C, Knight JA, Painter MM, Sullivan PM, and Bu G (2017). Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron 96, 115–129 e115. 10.1016/j.neuron.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhong M, Elder GA, Sano M, Holtzman DM, Gandy S, Cardozo C, Haroutunian V, Robakis NK, and Cai D (2015). Phospholipid dysregulation contributes to ApoE4-associated cognitive deficits in Alzheimer's disease pathogenesis. Proc Natl Acad Sci U S A 112, 11965–11970. 10.1073/pnas.1510011112. [DOI] [PMC free article] [PubMed] [Google Scholar]