Abstract
Research with animals and humans has demonstrated that chronic stress exposure can impact key biological aging pathways such as inflammation and DNA damage, suggesting a mechanism through which stress may increase risk for age-related disease. However, it is less clear whether these effects extend to other hallmarks of the aging process, such as cellular senescence. Male SCID mice were exposed to 14 days of restraint stress, with (n = 6) or without (n = 10) propranolol administration, or a non-stress control condition (n = 10). Normal femoral bone marrow leukocytes were isolated from engrafted leukemia cells that had been injected prior to the stressor, as the mice were also under a cancer challenge. We performed whole genome transcriptional profiling to assess indicators of biological aging: cell stress, DNA damage repair, cellular senescence markers p16INK4a and p21, and the pro-inflammatory senescence-associated secretory phenotype (SASP). ANCOVAs that adjusted for tumor load and Fisher's pairwise comparisons revealed that stressed mice had enhanced p16INK4a (p = .02) and p21 (p = .004), lower DNA damage repair (p < .001), and higher SASP (p = .03) gene expression than control mice. Stressed mice also showed up-regulated beta-adrenergic (CREB) and inflammatory (NF-кB, AP-1) and down-regulated cell stress (Nrf2) transcription factor activity relative to control mice (ps < .01). Propranolol reversed CREB and Nrf2 activity (ps < .03). Findings suggest that chronic stress exposure can impact several key biological aging pathways within bone marrow leukocytes and these effects may be partially mediated by sympathetic beta-adrenergic receptor activation.
Keywords: Chronic stress, Biological aging, Cellular senescence, Gene expression
1. Introduction
There is growing evidence that exposure to chronic psychosocial stress can impact key biological aging pathways in animals and humans, suggesting a potential mechanism through which chronic stress may increase risk for age-related diseases such as cancer, cardiovascular disease, type 2 diabetes, dementia, and early mortality (Epel, 2009; Shalev et al., 2013). Chronic stress—through prolonged or repeated activation of the sympathoadrenal system—is associated with an increased production of catecholamines (e.g., noradrenaline) that bind to adrenergic receptors on the surface of cells. This initiates a stress-signaling cascade characterized by increased inflammatory signaling and cellular energy production, which can result in cell stress—a state of cellular imbalance in which the production of oxidants exceeds the cell's antioxidant capacity (Aschbacher et al., 2013; Bergamini et al., 2005; Marón et al., 2019). Cells under stress activate compensatory responses to promote detoxification, build antioxidant reserve, and activate repair processes in response to cellular injury. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key regulator of the cellular response to oxidative stress (Bouvier et al., 2017; Baird and Dinkova-Kostova, 2011; Singh et al., 2010), and heat shock factors (HSFs) also play a role in protecting cells from damage under stressful conditions (Kim et al., 2012; Tower, 2009; Oda et al., 2018). Cell stress that is prolonged or insufficiently repaired can lead to excess DNA damage that initiates a state of permanent cell growth arrest termed cellular senescence (Campisi and D’ Adda Di Fagagna, 2007; Coppé et al., 2010).
Intracellular proteins p16INK4a and p21 have been identified as robust markers of cellular senescence that are correlated with chronological age in mice and humans (Campisi and D’Adda Di Fagagna, 2007). Expression of these cell cycle inhibitors in response to DNA damage is thought to have evolved as a protective mechanism to prevent the replication of damaged cells that could develop into cancer or other malignancies. However, although senescent cells are unable to proliferate, they remain metabolically active and are associated with a heightened release of pro-inflammatory cytokines, chemokines, and growth factors referred to as the senescence-associated secretory phenotype (SASP) (Copp é et al., 2010). Therefore, the accumulation of senescent cells can itself become damaging and accelerate aging through release of the SASP, which is thought to be a driver of age-related disease and a source of chronic inflammation (“inflammaging”) observed with older age (Campisi and D’Adda Di Fagagna, 2007; Coppé et al., 2010; Kirkland and Tchkonia, 2017; Franceschi and Campisi, 2014; Collado et al., 2007; Effros et al., 2005; Özcan et al., 2016; Rodier and Campisi, 2011). Importantly, research with mice has demonstrated that removal of p16INK4a-positive cells can prevent or slow the deterioration of several tissues and organs associated with aging (Baker et al., 2011; Demaria et al., 2017).
A well-established literature has linked chronic psychosocial stress with increased inflammatory signaling (e.g., NF-кB and AP-1 transcription factor activity) and circulating markers (e.g., IL-6) in animals and humans (Irwin and Cole, 2011; Powell et al., 2013). Experimental animal studies have demonstrated the role of sympathetically-mediated adrenergic receptor activity in this inflammatory response (Bierhaus et al., 2003; DeRijk et al., 1994). Research with animals and humans suggests that glucocorticoid receptor (GR) activity also plays a role in stress-related inflammatory signaling. Although acute increases in glucocorticoids inhibit inflammatory signaling, chronic elevations in response to stress are thought to lead to insensitivity or down-regulation of GRs that further contribute to a heightened pro-inflammatory response (Miller et al., 2002; Cohen et al., 2012). Indeed, chronic stress exposure has been associated with reduced GR and enhanced CREB and NF-кB transcription factor activation as well as elevated pro-inflammatory gene expression (Powell et al., 2013; Cole et al., 2007; Cole, 2014; Rohleder et al., 2009; Miller et al., 2008, 2014).
The impact of chronic stress on other hallmarks of aging, including cell stress, DNA damage and repair, and cellular senescence pathways, has been less well characterized. For instance, research with mice and rats has linked stress exposure to elevated markers of cell stress, including lower Nrf2 translocation to the nucleus and increased heat shock proteins (Bouvier et al., 2017; Fleshner et al., 2004; Johnson et al., 2005). Other research has demonstrated that mice exposed to chronic stress and humans exposed to an acute laboratory stressor showed increased DNA damage and decreased DNA repair processes (Aschbacher et al., 2013; Nishio et al., 2007; Consiglio et al., 2010; Hara et al., 2013; Forsberg et al., 2015; Flint et al., 2005; Knickelbein et al., 2008). To our knowledge, only two studies have examined links between chronic stress and cellular senescence. In the first study, mice exposed to subordination stress showed increased p53, which stimulates the p21-encoding gene Cdkn1a, and a trend toward increased p16INK4a expression in the liver and spleen (Razzoli et al., 2018). In the second study, mice that were administered catecholamines showed increased p53 and p21 in bone marrow cells and endothelial cells in cardiac tissue (Katsuumi et al., 2018). These findings are supported by an observational study in humans, in which chronic stress exposure was associated with enhanced p16INK4a gene expression in leukocytes (Rentscher et al., 2019).
The present study extends this literature by adopting an experimental restraint stress model to investigate the effects of chronic psychosocial stress on biological aging. Mice were exposed to 14 days of restraint stress with or without concurrent propranolol administration (a beta-adrenergic antagonist), or a non-stress control condition, and were under a cancer challenge as they had been injected with pre-B acute lymphoblastic leukemia (ALL) cells prior to the onset of the stressor (Lamkin et al., 2012). Given the innervation of the bone marrow by sympathetic nervous system fibers (Nance and Sanders, 2007; Felten et al., 1991; Sloan et al., 2008), we aimed to assess the effects of stress on several key indicators of biological aging in normal femoral bone marrow leukocytes: cell stress, DNA damage repair, cellular senescence, and the pro-inflammatory senescence-associated secretory phenotype (SASP). As cellular senescence is a state of permanent cell cycle arrest that is associated with a heightened pro-inflammatory phenotype, senescent cells within the bone marrow microenvironment may have a particularly negative impact on tissue homeostasis and immune function, and contribute to the development or progression of age-related disease (Katsuumi et al., 2018; Abdul-Aziz et al., 2019; Heidt et al., 2014; Yousefzadeh et al., 2021). Based on previous research, we hypothesized that mice exposed to chronic stress would show greater expression of cellular senescence markers p16INK4a and p21 and the SASP, as well as decreased DNA damage repair, compared to control mice. Consistent with these patterns of gene expression, we also expected that mice exposed to chronic stress would show altered a priori-specified transcription factor activity relative to control mice: beta-adrenergic (up-regulated CREB), inflammatory (up-regulated NF-κB and AP-1, down-regulated glucocorticoid receptor [GR]), and cell stress (down-regulated Nrf2, up-regulated HSFs). Finally, based on previous research on the role of beta-adrenergic receptor activation in the stress-signaling cascade, we hypothesized that treatment with propranolol would reduce the effects of chronic stress exposure on these transcriptomic indicators.
2. Materials and methods
2.1. Animals
Severe combined immunodeficient mice (CB17/Icr-Prkdcscid; Charles River Laboratories) were 6- to 8-week-old males. Mice were housed under BSL2 barrier conditions on an individually ventilated cage (IVC) rack in dual filter disposable cages (Innovive, Inc.) in groups of 4–5 mice per cage, with corn cob bedding and ad libitum access to food and water on a 12:12 light:dark cycle at 22 °C (Lamkin et al., 2012). Bone marrow samples from a total of 26 mice were available for whole transcriptome examination in the present study (n = 10 for control condition; n = 10 for chronic restraint stress; n = 6 chronic restraint stress + propranolol). All experimental procedures with live animals described below were approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles (#2007-155).
2.2. Procedures
2.2.1. Chronic stress procedure and β-adrenergic receptor blockade
Mice were randomly assigned to home cage control conditions or 2 hours per day of physical restraint for 14 consecutive days. Mice were restrained in a confined space that prevented them from moving freely but did not press on them (Thaker et al., 2006). This paradigm has been shown to induce chronic stress as evidenced by neuroendocrine activation (Thaker et al., 2006; Manni et al., 2008), weight loss (Smagin et al., 1999), and anxiety-like behaviors (Hermann et al., 1994). To determine whether beta-adrenergic receptor activation mediated the effects of chronic stress on biological aging, propranolol hydrochloride (Sigma, #P8688) in phosphate buffered saline (PBS) vehicle was administered via Alzet osmotic mini-pumps (DURECT Corporation) implanted subcutaneously on the lateral dorsal side of the mouse near the scapula to deliver a dose of 2 mg/kg/d beginning 8 days before initiation of the restraint stress and continuing throughout the experimental period.
2.2.2. Bone marrow collection
Femoral bone marrow was collected with cold PBS, supplemented with 1% fetal bovine serum (FBS), and subjected to red blood cell lysis buffer (BD Biosciences, #555899). Given that the mice had been injected with human pre-B acute lymphoblastic leukemia (ALL) cells at one day prior to initiation of the chronic restraint stress period, fluorescent activated cell sorting was used to separate ALL cells from normal mouse leukocytes at the conclusion of the experiment. To mark ALL cells, bone marrow cell samples were incubated with PE-conjugated antibodies against human CD10 (BD Biosciences, #555375), washed, and sorted on a FACSAria II High-Speed Cell Sorter with FACSDiva software (BD Biosciences). Sorted human-CD10− mouse leukocytes were stored at −80 °C until processing for total RNA extraction. To control for potential effects of leukemia burden on biological aging in normal mouse leukocytes, the amount of ALL tumor load, as measured in Lamkin et al. (2012), was used as a covariate in the present analyses.
2.2.3. Whole transcriptome analysis
Total RNA from normal mouse leukocyte samples was extracted (Qiagen RNeasy Mini Kit, #74104) and cleared of contaminating DNA with on-column DNase digestion (Qiagen RNase-Free DNase Set, #79254). Total RNA samples (∼1 μg) were assayed in a single batch using microarray-based whole genome transcriptional profiling with Illumina MouseRef-8 v2.0 Expression Beadchips in the University of California, Los Angeles Neuroscience Genomics Core (UNGC). Quantile normalization (Gallón et al., 2013) was applied to values of the 18,138 assayed transcripts, and normalized data were then log2 transformed.
2.3. Measures
2.3.1. Cellular senescence markers
To determine the effect of chronic stress on cellular senescence, we first analyzed transcript abundance for the conventional biomarkers p16INK4a (Cdkn2a) and p21 (Cdkn1a).
2.3.2. Transcriptome representation analysis for DNA damage repair and the senescence-associated secretory phenotype (SASP)
To determine the effect of chronic stress on DNA damage repair and the SASP, we used a transcriptome representation analysis (TRA) (Powell et al., 2013) to analyze the expression of gene sets that have empirically shown substantial differential expression for the indicated cellular property in previous reference studies. The reference DNA damage repair transcriptome included the validated 12-member gene set from Collin et al. (2018) (Supplementary Table 2), which was down-regulated in senescent cells and associated with increased DNA damage (e.g., arising from collapsed replication forks or oxidative stress). The reference SASP transcriptome included the validated 57-member gene set from Coppé et al. (2010) (Supplementary Table 3).
2.3.3. Transcription control pathways
To assess the effect of chronic stress on transcription factor activity consistent with these gene expression patterns, we tested for a significant difference in the prevalence of transcription factor binding motifs (TFBMs) in the promoters of up-vs. down-regulated genes for beta-adrenergic cAMP response element-binding protein (CREB), pro-inflammatory nuclear factor–kappa B (NF-κB) and activator protein-1 (AP-1), anti-inflammatory glucocorticoid receptor (GR), and cell stress-associated nuclear factor erythroid 2–related factor 2 (Nrf2) and heat shock factors (HSF-1 and HSF-2) by using 2-group differential expression analysis with the Transcription Element Listening System (TELiS) (Cole et al., 2005). Binding motif definitions for these transcription factors were retrieved from the TRANSFAC database (V$CREB_01, V$NFKAPPAB_01, V$AP1_C, V$GR_06, V$NRF2_01, V$HSF1_01, and V$HSF2_01, respectively), and differential gene expression was defined as a ≥ 25% difference (i.e., 1.25-fold) in the mean log2-transformed values between (a) the stress group and the control group, and (b) the stress + propranolol group and the control group, adjusting for ALL tumor load. For each motif, the analysis averaged results derived from nine parametric variations of promoter length (300 bp relative to RefSeq transcription start site, 600 bp, and 1000 bp to +200) and target TFBM match stringency (MatSim = 0.80, 0.90, 0.95).
2.4. Data analysis
Analyses were conducted in the R statistical environment, version 3.6.1 (R Core Team, 2019). All distributions were examined for outliers and winsorization was applied to normalize distributions for p16INK4a and the composite DDR and SASP transcriptome scores (Wilcox, 2017); scores were winsorized at the 90/10th percentile, resulting in two winsorized data points per group in each analysis.
To test the hypotheses that chronic stress promotes cellular senescence and does so through beta-adrenergic signaling, we first conducted univariate analyses of covariance on the log2-transformed gene counts for cellular senescence markers p16INK4a and p21, where the independent variable was stress group (Control vs. Stress vs. Stress + Propranolol) and the covariate was ALL tumor load. Femoral ALL tumor load was measured through bioluminescent imaging of luciferase-tagged ALL cells as photons per second emanating from a region of interest (ROI) that encircled the femur, with ROI size and exposure time kept constant across all subjects (see Lamkin et al., 2012 for full details). Fisher's pairwise comparisons tested differences in gene counts between the groups (i.e., Stress vs. Control; Stress + Propranolol vs. Control). TRA results were determined by conducting a univariate analysis of covariance on composite transcriptome scores, where the composite transcriptome score for each subject was computed by taking the mean of the z-score transformed gene expression values for each gene in the given reference set for that subject.
For each TFBM examined, the mean value of all possible prevalence ratios for each two-group comparison (i.e., Stress vs. Control; Stress + Propranolol vs. Control) was tested for significant deviation from a null population mean ratio of 1 with a single sample t-test. Significance of the reversal of the mean ratio by propranolol for a given TFBM was determined by conducting a paired samples t-test on the 9 difference scores between log2 ratios from the 2 comparisons (i.e., the Stress/Control ratio minus the Stress + Propranolol/Control ratio), matched on the 9 parametric variations of promoter length and target TFBM match stringency.
3. Results
3.1. Gene expression patterns
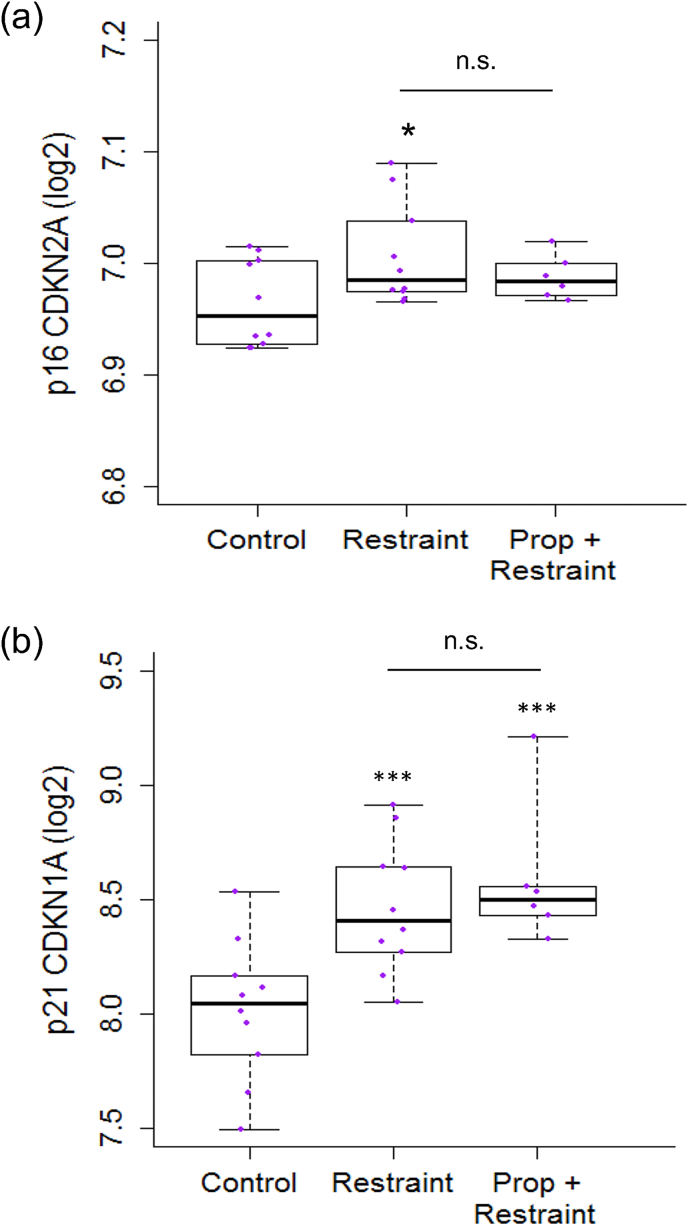
Consistent with hypotheses, there was a marginal overall effect of stress condition on gene expression of cellular senescence marker p16INK4a (p = .06; Supplementary Table 1). Fisher's pairwise comparisons revealed that mice exposed to chronic stress had higher expression of the p16INK4a-encoding gene (Cdkn2a) than control mice (t = −2.52, p = .02; Fig. 1). Mice exposed to chronic stress with propranolol administration did not differ from control mice (p = .29) or stressed mice without propranolol (p = .29). ALL tumor load was not associated with p16INK4a gene expression (p = .25). Also as hypothesized, there was a significant effect of stress condition on expression of cellular senescence marker p21 (p = .002; Supplementary Table 1). Fisher's pairwise comparisons revealed that mice exposed to chronic stress had higher expression of the p21-encoding gene (Cdkn1a) than control mice (t = −3.26, p = .004; Fig. 1). However, mice exposed to chronic stress with propranolol administration also had higher expression of p21 than control mice (t = −3.67, p = .001), and did not differ from stressed mice without propranolol (p = .41). ALL tumor load was not associated with p21 gene expression (p = .50).
Fig. 1.
Effects of restraint stress on expression of cellular senescence markers p16INK4a and p21 in mouse bone marrow cells.
(a) Five-number summary box plot of expression of cellular senescence marker p16INK4a (Cdkn2a; winsorized at 90/10th percentile) in bone marrow cells of mice randomized to Control (n = 10), Restraint (n = 10), or Propranolol (Prop) + Restraint (n = 6) conditions on a log2 scale centered at zero. (b) Box plot as in (a) of expression of cellular senescence marker p21 (Cdkn1a). ∗p < .05, ∗∗p < .01, ∗∗∗p < .001 vs. Control.
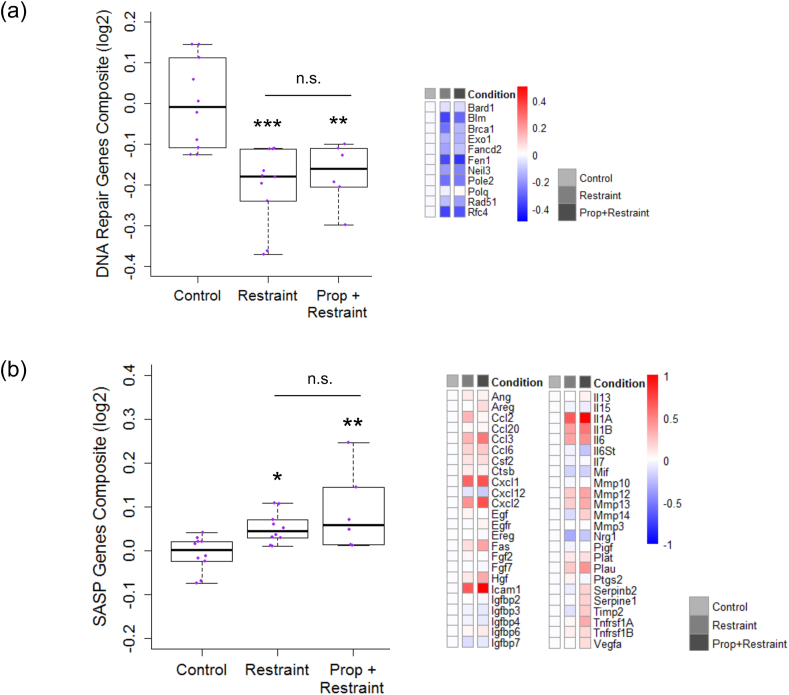
Also as hypothesized, there was a significant effect of stress condition on expression of DNA damage repair genes (p < .001; Supplementary Table 1). Fisher's pairwise comparisons revealed that mice exposed to chronic stress had higher expression of DNA damage repair genes than control mice (t = 4.56, p < .001; Fig. 2). However, mice exposed to chronic stress with propranolol administration also had higher expression of DNA damage repair genes than control mice (t = 3.47, p = .002) and did not differ from stressed mice without propranolol (p = .64). ALL tumor load was not associated with DNA damage repair gene expression (p = .30).
Fig. 2.
Effects of restraint stress on the DNA repair transcriptome and SASP transcriptome in mouse bone marrow cells.
(a) Five-number summary box plot of composite scores of the DNA repair transcriptome (winsorized at 90/10th percentile) in bone marrow cells of mice randomized to Control (n = 10), Restraint (n = 10), or Propranolol (Prop) + Restraint (n = 6) conditions on a log2 scale centered at zero. Reference set for DNA repair genes was bioinformatically identified and empirically validated by Collin et al. (2018). Heatmap of mean fold-changes for distinct DNA repair genes in Restraint and Prop + Restraint vs. Control group on log2 scale. (b) Box plot as in (a) of composite scores of senescence-associated secretory phenotype (SASP) transcriptome (winsorized at 90/10th percentile) and heatmap of mean fold-changes for distinct SASP genes. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001 vs. Control.
As expected, there was a significant effect of stress condition on expression of the senescence-associated secretory phenotype (SASP; p = .006; Supplementary Table 1). Fisher's pairwise comparisons revealed that mice exposed to chronic stress had higher expression of SASP genes than control mice (t = −2.36, p = .03; Fig. 2). However, mice exposed to chronic stress with propranolol administration also had higher expression of SASP genes than control mice (t = −3.46, p = .002), and did not differ from stressed mice without propranolol (p = .17). ALL tumor load was not associated with SASP gene expression (p = .46).
3.2. Transcription control pathways
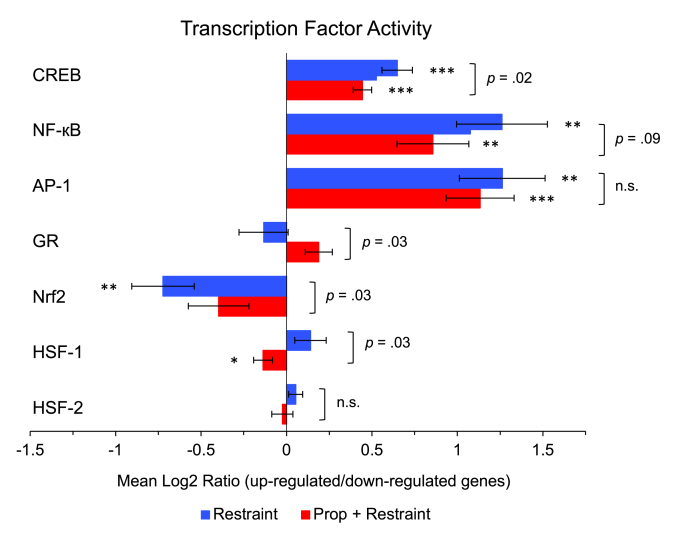
Secondary promoter-based bioinformatics analyses assessed the inferred activity of a priori-selected transcription factors involved in beta-adrenergic (CREB), inflammatory (NF-κB, AP-1, GR), and cell stress (Nrf2, HSF-1, HSF-2) responses. Analyses identified 434 differentially expressed genes (201 up-regulated, 231 down-regulated) with ≥ 25% difference (i.e., 1.25-fold) in mean log2-transformed values between mice exposed to chronic stress and control mice, adjusting for ALL tumor load. Analyses also identified 919 differentially expressed genes (396 up-regulated, 523 down-regulated) with ≥ 25% difference (i.e., 1.25-fold) in mean log2-transformed values between mice exposed to chronic stress with propranolol treatment and control mice, adjusting for ALL tumor load. The two lists of differentially expressed genes were submitted to the Transcription Element Listening System (TELiS) (Cole et al., 2005), which contains data on the prevalence of the transcription factor binding motifs (TBFMs) for a priori-selected transcription factors, to assess the likelihood of whether the TBFMs were over- or under-represented in the promoter regions of the genes.
As hypothesized, relative to control mice, mice exposed to chronic stress showed up-regulated beta-adrenergic CREB (Mean Log2 Ratio [MLR] = 1.57, SE = 1.06, p < .001) and pro-inflammatory NF-κB (MLR = 2.40, SE = 1.20, p = .001) and AP-1 (MLR = 2.40, SE = 1.19, p = .001) transcription factor activity (Fig. 3). Also as expected, mice exposed to chronic stress showed down-regulated Nrf2 (MLR = 0.61, SE = 1.14, p = .004) transcription factor activity relative to control mice. There were no differences between stressed and control mice for GR (p = .38), HSF-1 (p = .17), or HSF-2 (p = .23) transcription factors.
Fig. 3.
Effects of restraint stress on beta-adrenergic, inflammatory, and cell stress transcription factor activity.
Beta-adrenergic (CREB), inflammatory (NF-кB, AP-1, GR), and cell stress (Nrf2, HSF-1, HSF-2) transcription factor activity in bone marrow cells of mice randomized to Restraint (n = 10) or Propranolol (Prop) + Restraint (n = 6) vs. Control (n = 10) conditions, expressed as a Mean Log2 Ratio of transcription factor binding motif (TFBM) prevalence in the promoter regions of up-regulated versus down-regulated genes, averaged across nine parametric variations of promoter length and target TFBM match stringency. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001 vs. null difference of zero. Brackets represent mean difference tests between Restraint and Prop + Restraint conditions.
Relative to control mice, mice exposed to chronic stress with propranolol treatment also showed up-regulated beta-adrenergic CREB (MLR = 1.36, SE = 1.04, p < .001) and pro-inflammatory NF-κB (MLR = 1.81, SE = 1.16, p = .004) and AP-1 (MLR = 2.19, SE = 1.15, p < .001) transcription factor activity (Fig. 3). Mice exposed to chronic stress with propranolol treatment also showed marginally up-regulated anti-inflammatory GR (MLR = 1.14, SE = 1.06, p = .06), marginally down-regulated Nrf2 (MLR = 0.76, SE = 1.13, p = .06), and down-regulated HSF-1 (MLR = 0.91, SE = 1.04, p = .04) transcription factor activity relative to control mice. There were no differences between stressed mice with propranolol treatment and control mice for HSF-2 (p = .70) transcription factor.
Consistent with expectations, propranolol treatment significantly reversed the mean log2 ratios (stressed vs. control mice) for several transcription factors. On average, propranolol decreased CREB activation by 13% (mean difference = −0.28, p = .02), marginally decreased NF-κB by 24% (mean difference = −0.40, p = .09), and increased anti-inflammatory GR by 19% (mean difference = 0.25, p = .03). Propranolol treatment also increased Nrf2 activation by 25% (mean difference = 0.33, p = .03) and decreased HSF-1 activation by 17% (mean difference = −0.28, p = .03). Propranolol did not significantly change the mean log2 ratios for AP-1 (p = .19) or HSF-2 (p = .29).
4. Discussion
This study adopted an experimental restraint stress model to investigate the effects of chronic stress on transcriptomic indicators of biological aging in mouse bone marrow leukocytes, including cell stress, DNA damage repair, cellular senescence, and the pro-inflammatory senescence-associated secretory phenotype (SASP). As expected, mice exposed to chronic restraint stress showed enhanced expression of cellular senescence markers p16INK4a and p21 and the SASP and reduced expression of DNA damage repair genes compared to control mice. Consistent with these patterns of gene expression, mice exposed to chronic stress also showed increased beta-adrenergic (CREB) and pro-inflammatory (NF-κB, AP-1) transcription factor activity, and decreased Nrf2 transcription factor activity relative to control mice. It is also important to note that, although the mice were under a cancer challenge, the effects of stress on biological aging were independent from femoral ALL tumor load, and tumor load was not associated with indicators of biological aging.
Findings from this study are consistent with previous research that has linked different forms of chronic stress to increased markers of biological aging in mice. For instance, although research has shown that Nrf2 depletion exacerbates age-related increases in cellular senescence markers p16INK4a and p21 (Fulop et al., 2018), only one study has examined the effects of stress on Nrf2, finding that social defeat stress prevented translocation of Nrf2 to the nucleus in rats (Bouvier et al., 2017). In addition, mice exposed to social isolation, forced swimming, and restraint stress showed increased DNA damage and decreased repair in the brain and peripheral blood cells (Nishio et al., 2007; Consiglio et al., 2010; Hara et al., 2013; Forsberg et al., 2015; Flint et al., 2005). Emerging evidence also suggests that subordination stress is associated with increased p53, which stimulates the p21-encoding gene Cdkn1a, and possibly p16INK4a in the liver and spleen (Razzoli et al., 2018). Studies with humans are consistent with these findings, such that chronic stressors, including informal caregiving, bereavement, and work-related stress, have been associated with greater oxidative stress, DNA damage and repair processes, and cellular senescence marker p16INK4a (Rentscher et al., 2019; Gidron et al., 2006; Robles and Carroll, 2011). It is important to note that although there is a well-established literature linking chronic stress and inflammation in mice and humans, this is the first study to specifically link chronic stress exposure to increased expression of pro-inflammatory SASP factors.
Contrary to expectations, treatment with propranolol (a beta-adrenergic antagonist) did not reduce the effects of chronic stress on expression of cellular senescence, DNA damage repair, or the pro-inflammatory SASP. However, propranolol did reverse beta-adrenergic (CREB) and cell stress (Nrf2) transcription factor activity, suggesting that these effects may be partially mediated by beta-adrenergic receptor activation. Although previous research has demonstrated that beta-adrenergic antagonists can reduce the effects of stress on inflammatory signaling, cell stress, and DNA damage (Bierhaus et al., 2003; Johnson et al., 2005; Hara et al., 2013), it is possible that the observed effects also occur though other pathways, including activation of alpha-adrenergic receptors (Bierhaus et al., 2003; Johnson et al., 2005). Although this study was not designed to test this hypothesis, in vitro studies have shown that inflammatory (e.g., NF-κB) transcription factor activity was reduced by both alpha- and beta-adrenergic antagonists (Bierhaus et al., 2003). Somewhat unexpectedly, chronic stress did not have a significant effect on glucocorticoid receptor (GR) transcription factor activity. This finding is surprising in light of previous research demonstrating that animals and humans exposed to chronic stress showed increased GR resistance in splenocytes (Reber et al., 2007; Stark et al., 2001; Avitsur et al., 2001; Niraula et al., 2018; Quan et al., 2003) and peripheral leukocytes (Powell et al., 2013; Miller et al., 2002, 2008, 2014; Cole et al., 2007; Rohleder et al., 2009; Schmidt et al., 2010; Walsh et al., 2018; Cole, 2008). The role of GR transcription factor activity during the response to chronic stress in mouse bone marrow leukocytes remains a question for future investigation.
Findings from this study should be considered in light of its limitations, which suggest directions for future research. First, this study included mice with severe combined immunodeficiency (SCID) because they are ideal models for the engraftment of human cells. However, because SCID mice lack adaptive immune cells (i.e., B and T lymphocytes), the assessment of biological aging markers in this study was derived primarily from the innate immune cell compartment (e.g., monocytes, natural killer cells). In addition, SCID mice have a deficiency in non-homologous end-joining, which is one pathway for DNA double-strand break repair; however, previous research found that SCID mice showed a slightly lower but still significant increase in p21 (Cdkn1a) expression following radiation exposure compared to wild type mice, suggesting that other DNA damage and repair pathways may be activated (Rudqvist et al., 2018). In this study, the experimental design included all SCID mice so that the only difference between the groups was exposure to chronic stress, and an effect of stress on expression of DNA repair and other biological aging processes was observed despite this deficiency. Future research may benefit from using a humanized murine model to study the effects of chronic stress on biological aging in mice facing a cancer challenge without severe immunodeficiency. Second, this study included male mice only, which precluded the investigation of sex differences. Given that previous research also tested the effects of chronic stress and catecholamine exposure on cellular senescence in male mice (Razzoli et al., 2018; Katsuumi et al., 2018), the present study contributes to our understanding of these effects in males; however, it will be important for future research to test these effects in females as well as potential sex differences in stress-induced biological aging. Third, although previous research has linked p16INK4a and DNA repair mRNA levels to protein levels (Mekki et al., 1999; Collin et al., 2018; Hara et al., 1996; Li et al., 1994), some SASP factors such as damage-associated molecular patterns (DAMPs) are not initiated by the transcriptome (Basisty et al., 2020). Future research that combines transcriptomic and proteomic analysis will provide a more complete picture of the chronic stress-induced senescence response in bone marrow and other cell types. Finally, although the overall pattern of findings from this study suggests several molecular pathways through which psychosocial stress impacts biological aging, the study did not employ a knock-out model or pharmacological blockade of these molecular mechanisms to directly test for mediation, and this is an important direction for future investigation.
Despite these limitations, this is the first study to demonstrate that chronic psychosocial stress activates several key biological aging processes in mouse bone marrow leukocytes, including cell stress, DNA damage repair, cellular senescence, and the pro-inflammatory SASP, supporting the hypothesis that chronic psychosocial stress may accelerate biological aging and be a key mechanism driving increased risk for age-related disease, such as dementia, cardiovascular disease, and early mortality. Findings from this study also suggest that these effects may be partially mediated by beta-adrenergic receptor signaling, which initiates the cell stress-signaling cascade characterized by increased inflammation and cellular energy production. Excess cell stress for prolonged periods leads to DNA damage and can initiate cellular senescence. Importantly, given that cellular senescence is a state of permanent cell cycle arrest that is associated with a heightened pro-inflammatory phenotype, senescent immune cells in the bone marrow microenvironment may have particularly detrimental consequences for tissue homeostasis, immune function, and the development and progression of age-related diseases such as cancer (Katsuumi et al., 2018; Abdul-Aziz et al., 2019; Heidt et al., 2014; Yousefzadeh et al., 2021). Our results support a role of chronic psychosocial stress in driving biological aging pathways within bone marrow leukocytes, and offer a mechanism through which stress may accelerate aging and risk for age-related disease. Given the innervation of the bone marrow by the sympathetic nervous system (Nance and Sanders, 2007; Felten et al., 1991; Sloan et al., 2008), future research that examines the impact of chronic psychosocial stress on biological aging within progenitor cells in the bone marrow microenvironment is warranted.
Ethics statement
This research was conducted in accordance with ethical standards and the Institutional Animal Care and Use Committee of the University of California, Los Angeles.
Funding
This work was supported by the National Institutes of Health [grant numbers K01AG065485, K07CA188237], American Cancer Society [grant number 128660-RSG-15-187-01-PCS], and UCLA Cousins Center for Psychoneuroimmunology.
Author contributions
All authors made substantial contributions to and approved the final version of this manuscript. The original study was conceived and designed, and the data were acquired and analyzed by D.M.L. Hypotheses regarding biological aging pathways were generated by K.E.R. and J.E.C. The manuscript was drafted by K.E.R. and D.M.L. and revised critically for intellectual content by K.E.R., J.E.C, L.R.P., and D.M.L.
Data availability statement
The gene expression data that support the findings of this study are openly available in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/, reference number [GSE157133].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100461.
Contributor Information
Kelly E. Rentscher, Email: krentscher@mcw.edu.
Judith E. Carroll, Email: JCarroll@mednet.ucla.edu.
Lilian R. Polsky, Email: LPolsky@mednet.ucla.edu.
Donald M. Lamkin, Email: dlamkin@ucla.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdul-Aziz A.M., Sun Y., Hellmich C., et al. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood. 2019;133(5):446–456. doi: 10.1182/blood-2018-04-845420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K., O'Donovan A., Wolkowitz O.M., Dhabhar F.S., Su Y., Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38(9):1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R., Stark J.L., Sheridan J.F. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 2001;39(4):247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Baker D.J., Wijshake T., Tchkonia T., et al. Clearance of p16 Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N., Kale A., Jeon O.H., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1) doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini C., Gambetti S., Dondi A., Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharmaceut. Des. 2005;10(14):1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Wolf J., Andrassy M., et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier E., Brouillard F., Molet J., et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatr. 2017;22(12):1701–1713. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- Campisi J., D'Adda Di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Doyle W.J., et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. U. S. A. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav. Immun. 2008;22(7):1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W. Human social Genomics. PLoS Genet. 2014;10(8) doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W., Yan W., Galic Z., Arevalo J., Zack J.A. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21(6):803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- Cole S.W., Hawkley L.C., Arevalo J.M., Sung C.Y., Rose R.M., Cacioppo J.T. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):1–13. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M., Blasco M.A., Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Collin G., Huna A., Warnier M., Flaman J.M., Bernard D. Transcriptional repression of DNA repair genes is a hallmark and a cause of cellular senescence article. Cell Death Dis. 2018;9(3):1–14. doi: 10.1038/s41419-018-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio A.R., Ramos A.L.L.P., Henriques J.A.P., Picada J.N. DNA brain damage after stress in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(4):652–656. doi: 10.1016/j.pnpbp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5(1):99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M., O'Leary M.N., Chang J., et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRijk R.H., Boelen A., Tilders F.J.H., Berkenbosch F. Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology. 1994;19(2):155–163. doi: 10.1016/0306-4530(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Effros R.B., Dagarag M., Spaulding C., Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 2005;205(27):147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Epel E.S. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Basel) 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Felten S.Y., Felten D.L. In: Psychoneuroimmunology. Ader R., Felten D.L., Cohen N., editors. Academic Press; 1991. Innervation of lymphoid tissue; pp. 27–69. [DOI] [Google Scholar]
- Fleshner M., Campisi J., Amiri L., Diamond D.M. Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29(9):1142–1152. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Flint M.S., Carroll J.E., Jenkins F.J., Chambers W.H., Han M.L., Baum A. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. J. Neuroimmunol. 2005;167(1–2):34–44. doi: 10.1016/j.jneuroim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Forsberg K., Aalling N., Wörtwein G., et al. Dynamic regulation of cerebral DNA repair genes by psychological stress. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015;778:37–43. doi: 10.1016/j.mrgentox.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Campisi J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. Journals Gerontol - Ser A Biol Sci Med Sci. 2014;69(S1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Fulop G.A., Kiss T., Tarantini S., et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. GeroScience. 2018;40(5–6):513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallón S., Loubes J.M., Maza E. Statistical properties of the quantile normalization method for density curve alignment. Math. Biosci. 2013;242(2):129–142. doi: 10.1016/j.mbs.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Gidron Y., Russ K., Tissarchondou H., Warner J. The relation between psychological factors and DNA-damage: a critical review. Biol. Psychol. 2006;72(3):291–304. doi: 10.1016/J.BIOPSYCHO.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Hara E., Smith R., Parry D., Tahara H., Stone S., Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell Biol. 1996;16(3):859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M.R., Sachs B.D., Caron M.G., Lefkowitz R.J. Pharmacological blockade of a β2AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle. 2013;12(2):219–224. doi: 10.4161/cc.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidt T., Sager H.B., Courties G., et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann G., Beck F.M., Tovar C.A., Malarkey W.B., Allen C., Sheridan J.F. Stress-induced changes attributable to the sympathetic nervous system during experimental influenza viral infection in DBA/2 inbred mouse strain. J. Neuroimmunol. 1994;53(2):173–180. doi: 10.1016/0165-5728(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Cole S.W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.D., Campisi J., Sharkey C.M., Kennedy S.L., Nickerson M., Fleshner M. Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J. Appl. Physiol. 2005;99(5):1789–1795. doi: 10.1152/japplphysiol.00390.2005. [DOI] [PubMed] [Google Scholar]
- Katsuumi G., Shimizu I., Yoshida Y., et al. Catecholamine-induced senescence of endothelial cells and bone marrow cells promotes cardiac dysfunction in mice. Int. Heart J. 2018;59(4):837–844. doi: 10.1536/ihj.17-313. [DOI] [PubMed] [Google Scholar]
- Kim G., Meriin A.B., Gabai V.L., et al. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11(4):617–627. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J.L., Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein K.Z., Flint M., Jenkins F., Baum A. Psychological stress and oxidative damage in lymphocytes of aerobically fit and unfit individuals. J. Appl. Biobehav. Res. 2008;13(1):1–19. doi: 10.1111/j.1751-9861.2008.00025.x. [DOI] [Google Scholar]
- Lamkin D.M., Sloan E.K., Patel A.J., et al. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav. Immun. 2012;26(4):635–641. doi: 10.1016/j.bbi.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Nichols M.A., Shay J.W., Xiong Y. Cancer Research; 1994. Transcriptional Repression of the D-type Cyclin-dependent Kinase Inhibitor Pl6 by the Retinoblastoma Susceptibility Gene Product pRb.https://aacrjournals.org/cancerres/article/54/23/6078/500663/Transcriptional-Repression-of-the-D-Type-Cyclin . Published. [PubMed] [Google Scholar]
- Manni L., Fausto V., Fiore M., Aloe L. Repeated restraint and nerve growth factor Administration in male and female mice: effect on sympathetic and cardiovascular mediators of the stress response. Curr. Neurovascular Res. 2008;5(1):1–12. doi: 10.2174/156720208783565654. [DOI] [PubMed] [Google Scholar]
- Marón F.J.M., Ferder L., Saraví F.D., Manucha W. Hypertension linked to allostatic load: from psychosocial stress to inflammation and mitochondrial dysfunction. Stress. 2019;22(2):169–181. doi: 10.1080/10253890.2018.1542683. [DOI] [PubMed] [Google Scholar]
- Mekki Y., Catallo R., Bertrand Y., et al. Enhanced expression of p16(ink4a) is associated with a poor prognosis in childhood acute lymphoblastic leukemia. Leukemia. 1999;13(2):181–189. doi: 10.1038/sj.leu.2401303. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Cohen S., Ritchey A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Sze J., et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatr. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Murphy M.L.M., Cashman R., et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav. Immun. 2014;41(1):191–199. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance D.M., Sanders V.M. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav. Immun. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula A., Wang Y., Godbout J.P., Sheridan J.F. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J. Neurosci. 2018;38(9):2328–2340. doi: 10.1523/JNEUROSCI.2568-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y., Nakano Y., Deguchi Y., et al. vol. 29. 2007. (Social Stress Induces Oxidative DNA Damage in Mouse Peripheral Blood Cells). [DOI] [Google Scholar]
- Özcan S., Alessio N., Acar M.B., et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 2016;8(7):1316–1329. doi: 10.18632/aging.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Sekimoto T., Kurashima K., Fujimoto M., Nakai A., Yamashita T. Acute HSF1 depletion induces cellular senescence through the MDM2-p53-p21 pathway in human diploid fibroblasts. J. Cell Sci. 2018;131(9) doi: 10.1242/jcs.210724. [DOI] [PubMed] [Google Scholar]
- Powell N.D., Sloan E.K., Bailey M.T., et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via -adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N., Avitsur R., Stark J.L., et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J. Neuroimmunol. 2003;137(1–2):51–58. doi: 10.1016/S0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Nyuyki-Dufe K., Gurney A., et al. Social stress shortens lifespan in mice. Aging Cell. 2018;17(4) doi: 10.1111/acel.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber S.O., Birkeneder L., Veenema A.H., et al. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148(2):670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- Rentscher K.E., Carroll J.E., Repetti R.L., Cole S.W., Reynolds B.M., Robles T.F. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16 INK4a. Psychoneuroendocrinology. 2019;102 doi: 10.1016/j.psyneuen.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles T.F., Carroll J.E. Restorative biological processes and health. Soc Personal Psychol Compass. 2011;5(8):518–537. doi: 10.1111/j.1751-9004.2011.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F., Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N., Marin T.J., Ma R., Miller G.E. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J. Clin. Oncol. 2009;27(18):2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- Rudqvist N., Laiakis E.C., Ghandhi S.A., et al. Global gene expression response in mouse models of DNA repair deficiency after gamma irradiation. Radiat. Res. 2018;189(4):337–344. doi: 10.1667/RR14862.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D., Reber S.O., Botteron C., et al. Chronic psychosocial stress promotes systemic immune activation and the development of inflammatory Th cell responses. Brain Behav. Immun. 2010;24(7):1097–1104. doi: 10.1016/j.bbi.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Shalev I., Entringer S., Wadhwa P.D., et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Vrishni S., Singh B.K., Rahman I., Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic. Res. 2010;44(11):1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- Sloan E.K., Capitanio J.P., Cole S.W. Stress-induced remodeling of lymphoid innervation. Brain Behav. Immun. 2008;22(1):15–21. doi: 10.1016/j.bbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin G.N., Howell L.A., Redmann S., Ryan D.H., Harris R.B.S. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;276(5 45–5):1461–1468. doi: 10.1152/ajpregu.1999.276.5.r1461. [DOI] [PubMed] [Google Scholar]
- Stark J.L., Avitsur R., Padgett D.A., Campbell K.A., Beck F.M., Sheridan J.F. Social stress induces glucocorticoid resistance in macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(6 49–6) doi: 10.1152/ajpregu.2001.280.6.r1799. [DOI] [PubMed] [Google Scholar]
- Thaker P.H., Han L.Y., Kamat A.A., et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Tower J. Hsps and aging. Trends Endocrinol. Metabol. 2009;20(5):216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.P., Ewing L.J., Cleary J.L., et al. Development of glucocorticoid resistance over one year among mothers of children newly diagnosed with cancer. Brain Behav. Immun. 2018;69:364–373. doi: 10.1016/j.bbi.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R. Modern statistics for the social and behavioral sciences. Mod Stat Soc Behav Sci. 2017 doi: 10.1201/9781315154480. Published online August 15. [DOI] [Google Scholar]
- Yousefzadeh M.J., Flores R.R., Zhu Y., et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021 doi: 10.1038/s41586-021-03547-7. Published online May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The gene expression data that support the findings of this study are openly available in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/, reference number [GSE157133].