Abstract
Objectives
The oral cavity is one of the main entry sites for SARS-CoV-2. Gingival keratinocytes express transmembrane serine protease 2 (TMPRSS2), responsible for priming the SARS-CoV-2 spike protein. We investigated whether periodontitis increased the expression of TMPRSS2.
Methods
To investigate gene expression in periodontitis, we analyzed the expression of specific genes from (1) the Gene Expression Omnibus (GEO) dataset of 247 human gingival tissues and (2) an experimentally-induced periodontitis mouse model. Human gingival tissues with or without periodontitis were immunohistochemically stained using an anti-TMPRSS2 antibody. Analysis of the TMPRSS2 promoter was performed using a ChIP-Atlas dataset. TMPRSS2 expression was detected in cultured human keratinocytes using quantitative reverse transcription (qRT)-PCR and Western blot analysis.
Results
GEO dataset analysis and an experimentally-induced periodontitis model revealed increased expression of TMPRSS2 in periodontitis gingiva. The keratinocyte cell membrane in periodontitis gingiva was strongly immunohistochemically stained for TMPRSS2. Using ChIP-Atlas and GEO datasets, we screened for transcription factors that bind to the TMPRSS2 promoter region. We found one candidate, estrogen receptor 1 (ESR1), highly expressed in periodontitis gingiva. Analysis of the GEO dataset revealed a correlation between ESR1 and TMPRSS2 expression in gingival tissues. An ESR1 ligand induced TMPRSS2 expression in cultured keratinocytes.
Conclusions
Periodontitis increases TMPRSS2 expression in the cell membrane of gingival keratinocytes.
Keywords: COVID-19, SARS-CoV-2, Periodontitis, Keratinocytes, Gingiva
Abbreviations: COVID-19, coronavirus infection 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane serine protease 2; NHEKs, normal human epithelial keratinocytes; GEO, Gene Expression Omnibus; ACE2, angiotensin-converting enzyme 2; E2, 17β-estradiol; DHT, 5α-dihydrotestosterone; ES, estrogen receptor
1. Introduction
The coronavirus infection 2019 (COVID-19) pandemic has spread to more than 150 million people worldwide since the first outbreak of SARS-CoV-2 infection in Wuhan, China, in December 2019 [1]. COVID-19 causes fever, malaise, dry cough, sore throat, dyspnea, and respiratory complications that often deteriorate into severe acute respiratory syndrome and death [2]. Generally, the nasal and oral cavities are critical entry points for microbial pathogens. Since SARS-CoV-2 is present in saliva and nasopharyngeal secretions, it is thought to be transmitted through the oral and nasal mucosa.
The SARS-CoV-2 spike protein is primed by host membrane serine proteases including transmembrane protease serine 2 (TMPRSS2) and FURIN. This priming of spike is a preliminary step for ACE2 binding, which is required for SARS-CoV-2 to gain entry into host cells [3,4]. High expression of TMPRSS2 enhances SARS-CoV-2 invasion and inhibition of TMPRSS2 activity decreased the invasion of SARS-CoV-2 into lung epithelial cells [3]. In vivo experiments with TMPRSS2-deficient mice showed a reduced inflammatory response in the lungs following infection with SARS-CoV-2, which suggested a reduction in COVID-19 severity [5]. Indeed, the epithelium of the nasal cavity and bronchi, which co-express ACE2 and TMPRSS2, are possible SARS-CoV-2 entry sites [6].
SARS-CoV-2 was detected by RT-PCR in gingival tissue and gingival crevicular fluid, suggesting that SARS-CoV-2 infects gingival keratinocytes [7,8]. Immunohistochemistry has also shown that SARS-CoV-2 invasion-related molecules such as ACE2, FURIN, and TMPRSS2 are expressed in healthy gingival keratinocytes [9]. Clinical data from patients with periodontitis suggest that periodontitis is associated with COVID-19 severity [10]. However, it is not well understood how periodontitis affects the expression of genes required for activation of SARS-CoV-2 invasion in gingival tissue. In this study, we investigated the effects of periodontitis on the expression of TMPRSS2, a priming protease for the SARS-CoV-2 spike protein.
2. Subjects and methods
2.1. Materials
Human interleukin (IL)-1β and human IL-6 were obtained from Otsuka Pharmaceutical Co. (Osaka, Japan). Human recombinant hepatocyte growth factor (HGF) was generously provided by Mitsubishi Pharma Co. Ltd (Tokyo, Japan). 17β-estradiol (E2) was obtained from Fujifilm Wako Pure Chemical Co. (Tokyo, Japan) and 5α-dihydrotestosterone (DHT) was purchased from Tokyo Chemical Industry (Tokyo, Japan).
2.2. Acquisition and analysis of gingival tissue GeneChip data from the GEO database
GeneChip expression data were downloaded from the GSE10334 dataset [11] in the NCBI GEO database. Total gingival tissues (247) were collected from 90 subjects, who had alveolar bone defects, no systemic diseases such as diabetes mellitus, and were not current tobacco users. The subjects were classified into two groups: (1) periodontitis or (2) healthy tissue. Total RNA from the gingival tissue was reverse transcribed, and biotin-labeled cRNA was synthesized. After hybridization with a human genome array, the expression data were normalized and summarized using the log scale robust multi-array analysis [11]. Violin plots were generated with Seaborn in Python and were used to show the distribution of gene expression between healthy and periodontitis samples.
2.3. Mouse model of experimental periodontitis
Male C57BL/6 mice were obtained from CLEA Japan, Inc. (Tokyo, Japan) and maintained in accordance with protocols approved by the Animal Care and Use Committee at Kagoshima University. To induce experimental periodontitis, the right maxillary first molar was ligated with a 0.2 mm wire for 7 days [12]. Gingivae surrounding the ligature (right) and non-ligature (left) maxillary first molars were detached from the mouse under a stereomicroscope [12]. Total RNA obtained from both gingivae was reverse-transcribed into cDNA as described previously [12].
2.4. Immunohistochemical analyses of gingival tissue
The immunohistochemical analyses were conducted on samples derived from 12 patients with periodontitis who did not have systemic diseases such as diabetes mellitus and were not current tobacco users. Gingival tissues from 14 specimens were collected from the 12 subjects and classified into two groups: (1) a periodontal diseased site which was BoP positive and had a PPD value ≧4 mm and (2) a healthy site which was BoP negative and had a PPD value ≦3 mm. After the specimens were fixed in 4% paraformaldehyde and embedded into paraffin, the paraffin sections were stained with hematoxylin-eosin (HE) or immunohistochemically stained with anti-TMPRSS2 antibody (ab92323; Abcam, Cambridge, UK) followed by counterstaining with hematoxylin.
2.5. Cell culture
Normal human epithelial keratinocytes (NHEKs) were obtained from juvenile foreskin, which was purchased from Takara Bio Inc. (Otsu, Japan). The cells were cultured to subconfluency in serum-free medium containing supplements according to the manufacturer's instructions [13]. Twenty-four hours later, the medium was changed to calcium-free minimum essential medium MEM (Thermo Fisher, Waltham, USA) containing 1.3 mM calcium plus 10% chelated fetal calf serum (FCS). Subsequently, the cells were stimulated with cytokines and sex hormones.
2.6. qRT-PCR and western blot analyses
Total RNA was isolated from NHEKs or mouse gingivae using Isogen II (Nippon Gene, Tokyo, Japan) and was used as a template for cDNA synthesis with RivaTra Ace (Toyobo, Tokyo, Japan). qRT-PCR was performed using the following primer sets: human TMPRSS2 forward, 5′- GAACTCAGGGTCACCACCAG-3’; human TMPRSS2 reverse 5′-CTGTGCGGGATAGGGGTTTT-3’; human keratin 10 (KRT10) forward, 5′-TTGAAACAATCCCTGGAAGC-3’; human KRT10 reverse, 5′-TGCACACAGTAGCGACCTTC-3’; human keratin 14 (KRT14) forward, 5′-GGCCTGCTGAGATCAAAGAC-3’; human KRT14 reverse, 5′-GTCCACTGTGGCTGTGAGAA-3’; human Rpl13a forward, 5′-AAGTACCAGGCAGTGACAGC-3’; human Rpl13a reverse, 5′-TTCTCCACGTTCTTCTCGGC-3’; mouse IL-1β forward, 5′-TGTGAAATGCCACCTTTTGA-3’; mouse IL-1β reverse, 5′-GTAGCTGCCACAGCTTCTCC-3’; mouse TMPRSS2 forward, 5′-CAAGGCACTCAGACCTGGAG-3’; mouse TMPRSS2 reverse, 5′-GGCCCTTGGTTTTCTTGCAG-3’; mouse Rpl13a forward, 5′-GCTTACCTGGGGCGTCTG-3’; and mouse Rpl13a reverse, 5′-ACATTCTTTTCTGCCTGTTTCC. The qRT-PCRs were conducted with SYBR Green I (Cambrex, Rockland, ME, USA) and 0.5 U Excel Taq DNA polymerase (SMOBIO Technology, Inc., Hsinchu, Taiwan) under the following conditions: 95 °C for 5 min, followed by 40 PCR cycles at 95 °C for 30 s, 60 °C for 20 s, and 72 °C for 40 s. The relative quantity was obtained using the comparative threshold method, and the results were normalized against a human or mouse Rpl13a control.
RIPA buffer cell lysates were subjected to Western blot analyses with anti-TMPRSS2 or anti-β-actin antibodies (Santa Cruz Biotechnology, Santa Cruz, USA) as described previously [13].
2.7. Bioinformatics analyses for gene expression screening
Analyses of the GEO dataset (GSE10334), which contains the transcriptome of healthy and diseased gingival tissues was quantitated as the base-2 logarithmic expression value of healthy tissue subtracted by that of diseased tissue (logFc). The adjusted p-values show differences in gene expression between the two groups. We selected 1000 genes whose expression was significantly increased in periodontitis tissue with an adjusted p-value of 3.4 × 10−8 or less and a logFC of 0.1 or more. We utilized the ChIP-Atlas (http://chip-atlas.org/) database to select the top 50 transcription factors ranked according to their binding affinity to the human TMPRSS2 promoter region. Subsequently, we examined whether the 23 factors from the GEO dataset were included in the list of 50 transcription factors from the ChIP-Atlas analyses.
2.8. Statistical analyses
We utilized the Bonferroni criterion and q-value to analyze the expression values of each gene in gingival tissues from healthy sites and periodontitis lesions obtained from the GEO database and mouse gingivae on ligature and non-ligature sides [14]. The experimental data from cultured cells was statistically analyzed by one-way ANOVA with the multiple comparison technique. In order to analyze the correlation between the expression levels of two genes, the Pearson correlation coefficient was calculated.
3. Results
3.1. TMPRSS2 expression in gingival tissues containing periodontitis lesions
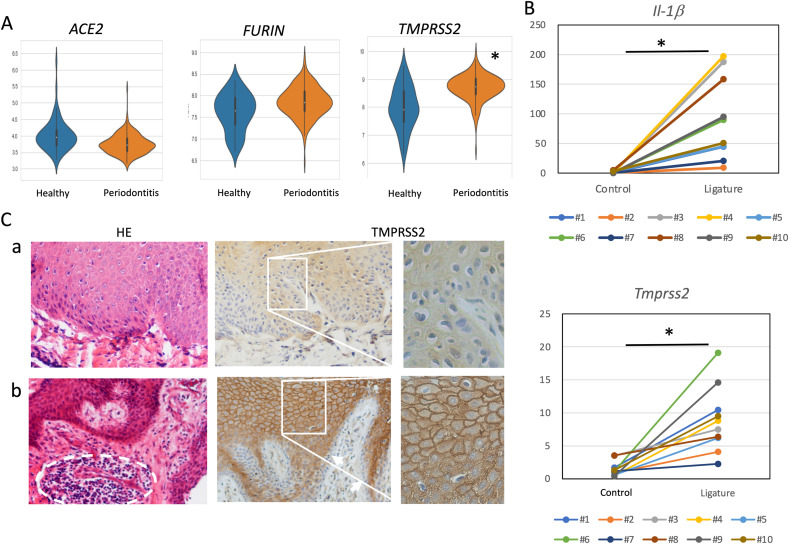
We used the GEO dataset (GSE10334) [11], which comprises a transcriptome analysis of 247 gingival tissues, to analyze the expression of the SARS-CoV-2 invasion-related genes, ACE2, FURIN, and TMPRSS2 from healthy and diseased individuals. The expression levels of these genes were compared between 183 periodontitis gingiva and 64 healthy gingiva. TMPRSS2 was expressed at a statistically significant higher level in gingiva with periodontitis than in healthy gingiva (Fig. 1 A, Table 1 ). However, the expression levels of ACE2 and FURIN in the gingiva of periodontitis lesions were similar to the levels found in healthy gingiva (Fig. 1A, Table 1). In order to confirm that periodontitis increases the expression of TMPRSS2 in gingival tissue, experimental periodontitis was induced by ligating wire to the maxillary right first molar of mice. In all mice, compared to the control side, the gingiva of the ligated first molar showed an increase in expression of IL-1β, a proinflammatory cytokine, as well as an increase in the expression of TMPRSS2 (Fig. 1B). The differences in the expression of IL-1β and TMPRSS2 in gingiva with and without induced experimental periodontitis were statistically significant (Fig. 1B).
Fig. 1.
TMPRSS2 expression in gingival lesions with periodontitis. (A) Violin plots of ACE2, TMPRSS2, and FURIN expression in gingival tissues from the GEO dataset, depicted in orange (healthy) and violet (periodontitis). ∗p < 0.05 vs. healthy samples. (B) IL-1β and TMPRSS2 expression in experimental periodontitis-induced gingivae. Ten mice (#1–#10) were subjected to experimental periodontitis induced by ligation of the maxillary right first molar. Total RNA was collected from the gingivae on the ligature (Ligature) and non-ligature (Control) sides and the levels of IL-1β and TMPRSS2 mRNAs were quantitated. The connected lines are individual lines. ∗p < 0.05. (C) Hematoxylin-eosin staining (HE; left panels) of a representative healthy gingiva (a) and periodontitis gingiva (b) as well as immunohistochemical staining (center and right panels) with anti-TMPRSS2 antibody using DAB. The dotted circles indicate the areas of inflammatory cell infiltration. The arrows indicate the basal layer of the gingival epithelium. Magnification: ×100 (left and center panels) and ×200 (right panels).
Table 1.
Gingival gene expression in healthy and periodontitis tissues.
| Gene symbol | Expression value average |
Ratio: Periodontitis/Healthyb | Adjusted p-valuec | |
|---|---|---|---|---|
| Healthy | Periodontitis | |||
| ACE2 | 4 | 3.7 | 0.8 | 3.5E−03 |
| TMPRSS2a | 8 | 8.7 | 1.7 | 1.5E−12 |
| FURIN | 7.6 | 7.9 | 1.2 | 6.9E−01 |
| IL-1βa | 8.3 | 9.3 | 2.1 | 1.6E−11 |
| IL-6a | 6.4 | 7.2 | 1.7 | 3.1E−03 |
| TNF-α | 5.3 | 5.4 | 1.1 | 1.0E−01 |
| HGFa | 4.2 | 4.4 | 1.2 | 5.3E−04 |
| SPP1 | 5.9 | 6.1 | 1.1 | 2.1E−01 |
| IFN-γ | 4.7 | 4.7 | 1.0 | 4.8E−02 |
| ESR1a | 4.9 | 5.4 | 1.5 | 1.6E−06 |
| ESR2 | 4.2 | 4.4 | 1.2 | 5.3E−02 |
| AR | 5.8 | 5.5 | 0.8 | 1.2E−01 |
Genes whose expression was statistically significantly upregulated in periodontitis tissues.
Since each gene expression value is expressed as a logarithm with a base of 2, the difference in the average expression values was used to calculate the ratio of gene expression between periodontitis and healthy samples.
The expression of each gene in gingival tissue samples was divided into two groups, periodontitis patients and healthy subjects. The Bonferroni test was utilized to obtain the p-values.
In order to investigate the localization of TMPRSS2 in gingiva, healthy and periodontitis gingival tissues were immunohistochemically stained with anti-TMPRSS2 antibody. Infiltration of inflammatory cells was observed by HE staining in seven samples of periodontitis gingiva. Moreover, in all of the samples, the cell membrane of keratinocytes in the spinous layer was strongly stained with anti-TMPRSS2 antibody (Fig. 1C–b, Fig. S1B). These immunohistochemical data confirm the results of the GEO dataset analysis described above, which suggested that periodontitis increases the expression of TMPRSS2 in gingival tissue. In addition, the keratinocytes in the spinous layer of the gingival tissue were stained with anti-TMPRSS2 antibody, while keratinocytes in the basal layer of the gingival epithelium were hardly stained (Fig. 1C, Fig. S1). Therefore, our data suggested that differentiation of keratinocytes increased the expression of TMPRSS2.
3.2. The effects of inflammatory cytokines on TMPRSS2 expression in human keratinocytes
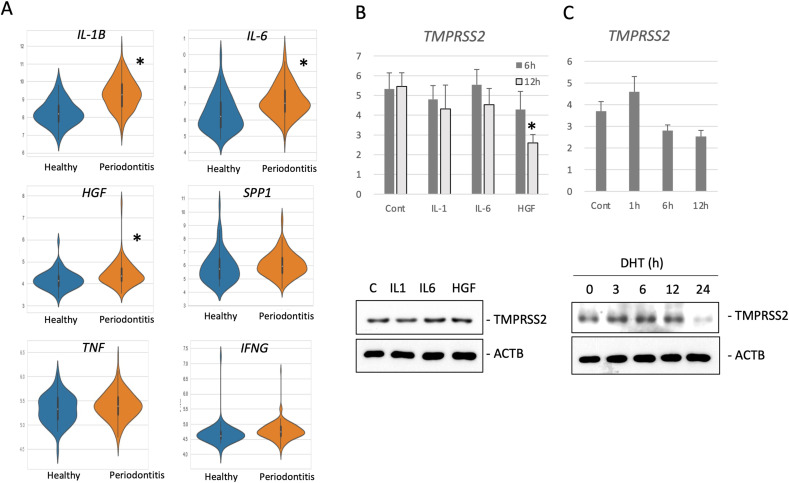
Periodontal tissue exhibiting inflammation has increased production of cytokines such as TNF-α, IL-1, IL-6, osteopontin (SPP1), HGF, and IFN-γ. Moreover, the levels of some of these cytokines are reported to be elevated in gingival crevicular fluid (GCF) [[15], [16], [17], [18], [19], [20]]. Transcriptome analysis of the GEO dataset (GSE10334) showed that IL-1β gene expression was predominantly upregulated in gingival tissues from periodontitis lesions. Furthermore, the expression of IL-6 and HGF was also increased in gingival tissues from periodontitis lesions, albeit to a lesser extent than IL-1β. However, the marginally increased expression of SPP1 and TNF-α in gingival tissues from periodontitis lesions were not statistically significant (Fig. 2 A, Table 1).
Fig. 2.
Analysis of gene expression in gingival tissue with periodontitis. (A) Violin plots of IL-1β, IL-6, HGF, SPP1, TNF-α, and IFN-γ expression in gingival tissues from the GEO dataset depicted in orange (healthy) and violet (periodontitis). ∗p < 0.05 vs. healthy samples. (B) NHEKs were stimulated with 10 ng/ml IL-1β, 10 ng/ml IL-6, 20 ng/ml HGF, 10 ng/ml TNF-α, or 10 ng/ml IFN- γ for 6 or 12 h. Total RNA was collected from the cells to detect TMPRSS2 mRNA expression by qRT-PCR. The mRNA levels were normalized to the Rp113a mRNA levels. Means ± SE, n = 3. p < 0.05 vs. control. The cell lysates were subjected to Western blot analysis with anti-TMPRSS2 (TMPRSS2) or anti-β-actin (ACTB) antibodies. (C) NHEKs were stimulated with 100 nM DHT for 0, 1, 3, 6, 12, or 24 h. The levels of TMPRSS2 mRNA were quantified as described above and Western blots were performed as described above.
In order to examine whether these cytokines induce TMPRSS2 expression in keratinocytes, we cultured and stimulated NHEKs with IL-1β, IL-6 or HGF. However, none of these cytokines enhanced TMPRSS2 expression at either the protein or mRNA level (Fig. 2B). In addition, androgen is reported to increase TMPRSS2 gene expression in cancer cells [21]. Hence, NHEKs were stimulated with DHT, which is an active androgen form, followed by analysis of the expression levels of TMPRSS2. However, DHT did not enhance TMPRSS2 expression (Fig. 2C).
3.3. Screening for transcription factors involved in the upregulation of TMPRSS2 expression in periodontitis disease
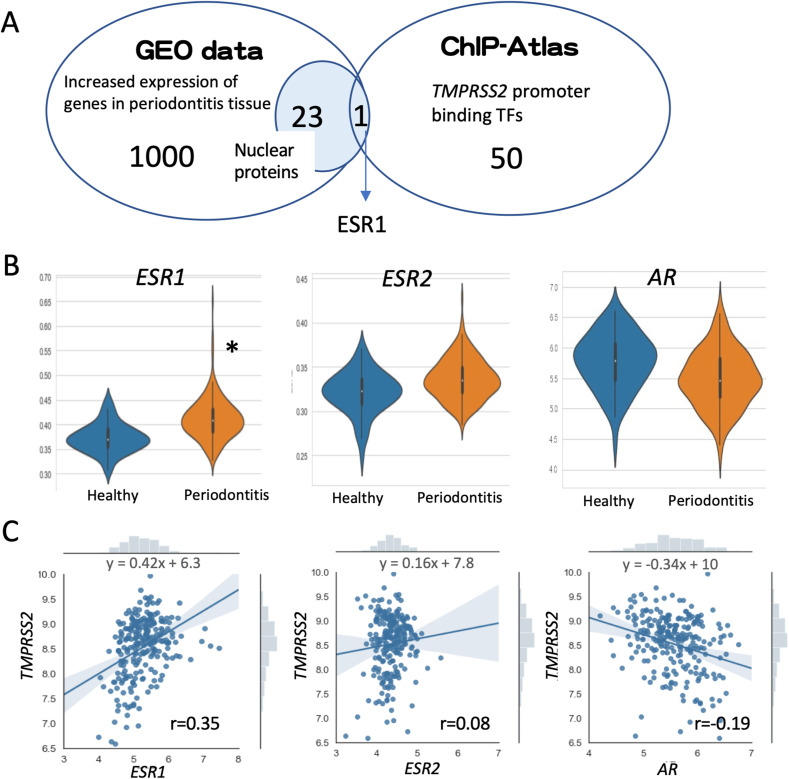
In order to uncover the transcription factors involved in the upregulation of TMPRSS2 expression in periodontitis, we performed the following screening. First, we selected 1000 genes whose expression was significantly increased in tissues exhibiting periodontitis from published GEO datasets (GSE10334) [11], and we selected 23 genes encoding nuclear-localized proteins. Next, we selected 50 transcription factors that bind to the promoter region of the human TMPRSS2 gene from ChIP-Atlas, a ChIP-seq database, and checked if any of the genes matched the selected 23 genes (Fig. 3 A). The only gene that showed a match was the ESR1 gene. Furthermore, the transcriptome analysis of gingival tissues revealed that not only was ESR1 expression significantly increased in periodontitis gingival tissues, but also there was a weak correlation between ESR1 and TMPRSS2 expression (r = 0.35). In contrast, there was no correlation between TMPRSS2 and ESR2 (r = 0.08) or androgen receptor (AR) expression (r = −0.19) (Fig. 3B and C).
Fig. 3.
Screening for transcription factors with increased expression in tissues exhibiting periodontitis and that bind to the TMPRSS2 promoter. (A) Twenty-three nuclear-localized proteins with increased expression in tissues exhibiting periodontitis were selected using the GEO dataset and 50 proteins that bind to the TMPRSS2 promoter were selected using ChIP-Atlas. ESR1 was identified as the only transcription factor common to both datasets. (B) Violin plots of ESR1, ESR2, and AR expression in gingival tissues from the GEO dataset depicted in orange (healthy) and violet (periodontitis). (C) Correlation between the expression levels of TMPRSS2 and ESR1, ESR2, or AR. The regression line formula for TMPRSS2 and each gene is shown. The correlation coefficient (r) is also shown.
3.4. Effects of E2 on TMPRSS2 expression in human keratinocytes
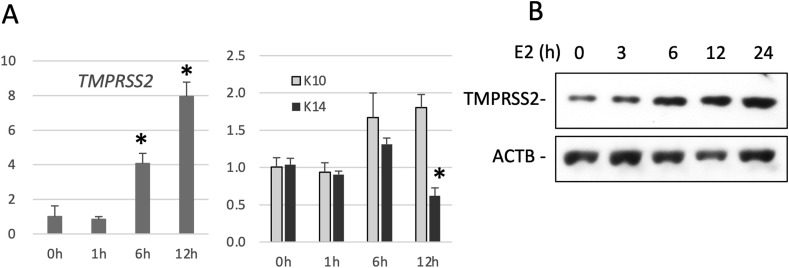
Lastly, we examined if ESR signaling upregulates TMPRSS2 expression in NHEKs. We stimulated NHEKs with E2 and analyzed the expression of TMPRSS2. Consequently, E2 increased TMPRSS2 expression at the mRNA level and protein level (Fig. 4 A and B). Furthermore, the increase in TMPRSS2 expression was synchronized with the expression of KRT10, which is expressed in differentiated keratinocytes. In contrast, E2 treatment did not induce the expression of KRT14, which is expressed in undifferentiated keratinocytes (Fig. 4A) [22]. This phenomenon agrees with our immunohistochemical observations, in which TMPRSS2 was expressed in differentiated keratinocytes.
Fig. 4.
Treatment with E2 increases TMPRSS2 expression in human keratinocytes. (A) NHEKs were stimulated with 5 nM E2 for 0, 1, 6, or 12 h. Total RNA was collected from the cells in order to detect TMPRSS2, KRT10 (K10), or KRT14 (K14) mRNA expression by qRT-PCR. The mRNA expression levels were normalized to the Rpl13a mRNA levels. Means ± SE, n = 3. ∗p < 0.05. (B) NHEKs were stimulated with 5 nM E2 for 0, 3, 6, 12, or 24 h. The cell lysates were then subjected to Western blot analysis with anti-TMPRSS2 (TMPRSS2) or anti-β-actin (ACTB) antibodies.
4. Discussion
In this study, we found that TMPRSS2 expression was increased in gingival tissues from periodontitis lesions and that this phenomenon was associated with increased ESR1 signaling. TMPRSS2 is expressed on the plasma membrane of keratinocytes from periodontitis gingiva. TMPRSS2 proteolytically cleaves the SARS-CoV-2 spike protein [3,4]. SARS-CoV-2 has also been detected in the gingival tissue of deceased COVID-19 patients [7]. Taken together, these data lead to the hypothesis that periodontitis is a risk factor that may contribute to the proteolytic priming of the SARS-CoV-2 spike protein, which activates the binding of SARS-CoV-2 to its receptor. However, a similar hypothesis has been previously proposed [23]. Based on the observations that chronic periodontitis increased osteopontin levels in GCF and that osteopontin increased FURIN expression [18,24], it was hypothesized that increased FURIN expression due to periodontitis could increase the sensitivity to COVID-19. In our study, we focused on TMPRSS2 instead of FURIN for the following three reasons: (1) TMPRSS2 was highly expressed in gingival periodontal lesions compared to healthy gingival tissues, but periodontitis did not affect the expression of FURIN and SPP1, the latter of which has been reported to increase the expression of FURIN (Table 1). (2) TMPRSS2 expression was observed in the plasma membrane of differentiated keratinocytes on and near the gingival surface (Fig. 1B). In contrast, FURIN is expressed mostly in the basal layer of keratinocytes [9]. (3) Before the entry of SARS-CoV-2 into host cells, TMPRSS2, which has serine protease activity, cleaves both the viral spike protein and the viral receptor, ACE2, while FURIN only cleaves the spike protein of SARS-CoV-2 [3,25,26]. In addition, it has been reported that the cleavage of SARS-CoV-2 spike protein by FURIN is not essential for infection with SARS-CoV-2 [27].
It has been reported that the SARS-CoV-2 receptor, ACE2, is highly expressed in the mucosa of the oral cavity, including the tongue [28]. Oral symptoms in patients with COVID-19 include a taste disorder and oral mucosal lesions such as irregular ulcers, small blisters, petechiae, and desquamative gingivitis [[29], [30], [31]]. However, these observations do not indicate that SARS-CoV-2 infection causes necrosis of oral epithelial cells. In general, severe systemic disorders with dysregulated immunity are more likely to be susceptible to oral opportunistic infections that may result in oral mucosal lesions such as ulcers, erosions, and abscesses [32]. It has been previously reported that in COVID-19 patients, oral lesions are presumed to be caused by oral opportunistic infections such as Candida, herpes simplex, and cytomegalovirus [9,29,31,32]. However, these reports do not rule out infection of the oral epithelium, and in fact, SARS-CoV-2 has been found to infect gingival tissue keratinocytes [7]. Further studies are required to determine whether infection of the oral mucosa with SARS-CoV-2 is responsible for the oral lesions.
According to international statistics, the morbidity and mortality of COVID-19 is higher in males than in females [33]. The ACE2 gene is located on the X chromosome and is thought to be more highly expressed in females. However, there is no evidence that ACE2 is involved in the gender differences of COVID-19 [34]. Instead, a large Italian cohort study has shown that TMPRSS2 is more highly expressed in male bronchial epithelial cells than in female bronchial epithelial cells [35]. It has been suggested that this difference in TMPRSS2 expression between males and females is responsible for the difference in susceptibility and severity of COVID-19. In addition, it has been reported that TMPRSS2 expression in androgen-sensitive adenocarcinomas is regulated by androgens [36]. In contrast, the present study suggested that TMPRSS2 expression in human keratinocytes was not regulated by androgens. Since these data suggest that the regulation of TMPRSS2 expression may differ among cell types, further studies are required to determine whether the gender difference in TMPRSS2 expression in lung epithelium is due to androgen. On the other hand, a meta-analysis of clinical studies showed that periodontitis is more common in the female population [37], which indicates that local susceptibility to infection is not necessarily a factor in systemic infection. Coronavirus infections such as SARS-CoV and MERS-CoV as well as general viral infections also show a male bias in severity and mortality due to systemic infection [34]. Based on these observations, the gender differences may be explained by the fact that females are more likely to have increased antiviral activity because the gene TLR7 (which plays an important role in viral immunity) is located on the X chromosome. Thus, differences in the local expression of TMPRSS2 in the lung epithelium may have no effect on the gender biases in COVID-19 morbidity and mortality [38].
Common risk factors for COVID-19 severity and periodontitis include diabetes, aging, smoking habits, and cardiovascular disease. Therefore, clinical studies have shown a relationship between periodontitis and COVID-19 severity after adjusting for these potential confounders [10]. One of the hypothetical mechanisms for the relationship between periodontitis and COVID-19 severity is that periodontal pockets act as viral reservoirs [10,39]. The present study showed that periodontitis promotes TMPRSS2 expression in gingival keratinocytes and induced the transfer of TMPRSS2 to the cell membrane of keratinocytes. These data prompted us to hypothesize that periodontitis increases the levels of primed SARS-CoV-2 in periodontal pockets. The presence of SARS-CoV-2 in the gingival epithelium of COVID-19 patients, combined with our data, supports this hypothesis [7]. Moreover, the finding that the oral viral load of SARS-CoV-2 is associated with the severity of COVID-19 also supports this hypothesis. Future clinical studies aiming to detect SARS-CoV-2 in the GCF of severe COVID-19 patients will provide a better understanding of the mechanism of gingival infection [40].
5. Conclusion
The expression of TMPRSS2, a priming protease for SARS-CoV-2, was increased and localized to the plasma membrane of keratinocytes in gingival tissues with periodontitis. Its increased expression was also associated with more robust ESR1 signaling.
Funding
This work was supported by KAKENHI from the Japan Society for the Promotion of Science (Grant 20K09909).
Ethical statement
The Ethics Committee of Kagoshima University approved this study (Approval Number: 200159epi) and all patients provided their written informed consent to participate in all procedures associated with this study. Animal experiments were approved by the Experimental Animal Research Committee of Kagoshima University (Permission number: D21031) and carried out according to the Kagoshima University Animal Experimentation Regulations.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Tomokazu Ohnishi: Conceptualization, design, data acquisition, data analysis, data interpretation, Writing – original draft, critically revised the manuscript, final approval of the manuscript and agreed to be accountable for all aspects of the work. Toshiaki Nakamura: design, data acquisition, data analysis, critically revised the manuscript, final approval of the manuscript and agreed to be accountable for all aspects of the work. Kaori Shima: design, data acquisition, data analysis, critically revised the manuscript, final approval of the manuscript and agreed to be accountable for all aspects of the work. Kazuyuki Noguchi: design, data acquisition, data analysis, critically revised the manuscript, final approval of the manuscript and agreed to be accountable for all aspects of the work. Norika Chiba: design, data analysis, critically revised the manuscript, final approval of the manuscript and agreed to be accountable for all aspects of the work. Tetsuya Matsuguchi: design, data analysis, data interpretation, Writing – original draft, critically revised the manuscript, final approval of the manuscript and agreed to be accountable for all aspects of the work.
Acknowledgement
We would like to thank the Department of Periodontology at Kagoshima University Hospital for collecting the gingival tissues.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.job.2022.04.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93 doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes Matuck B., Dolhnikoff M., Maia G.V.A., Isaac Sendyk D., Zarpellon A., Costa Gomes S., et al. Periodontal tissues are targets for Sars-Cov-2: a post-mortem study. J Oral Microbiol. 2020;13:1848135. doi: 10.1080/20002297.2020.1848135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S., Mohindra R., Chauhan P.K., Singla V., Goyal K., Sahni V., et al. SARS-CoV-2 detection in gingival crevicular fluid. J Dent Res. 2021;100:187–193. doi: 10.1177/0022034520970536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi W., Kubota N., Shimizu T., Saruta J., Fuchida S., Kawata A., et al. Existence of SARS-CoV-2 entry molecules in the oral cavity. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marouf N., Cai W., Said K.N., Daas H., Diab H., Chinta V.R., et al. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol. 2021;48:483–491. doi: 10.1111/jcpe.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demmer R.T., Behle J.H., Wolf D.L., Handfield M., Kebschull M., Celenti R., et al. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 2008;79:2112–2124. doi: 10.1902/jop.2008.080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnishi T., Okamoto A., Kakimoto K., Bandow K., Chiba N., Matsuguchi T. Involvement of Cot/Tp12 in bone loss during periodontitis. J Dent Res. 2010;89:192–197. doi: 10.1177/0022034509353405. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi T., Hisadome M., Kusuyama J., Chiba N., Amir M.S., Kanekura T., et al. Ultraviolet B irradiation decreases CXCL10 expression in keratinocytes through endoplasmic reticulum stress. J Cell Biochem. 2021;122:1141–1156. doi: 10.1002/jcb.29936. [DOI] [PubMed] [Google Scholar]
- 14.Storey J.D., Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maulani C., Masulili S.L.C., Priyadharsini S., Susmiarsih T.P., Auerkari E.I. Positive correlation between the level of interferon-gamma and the severity of periodontitis. Pesqui. Bras. Odontopediatria Clín. Integr. 2019;19 [Google Scholar]
- 16.Offenbacher S., Barros S.P., Singer R.E., Moss K., Williams R.C., Beck J.D. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78:1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi T., Daikuhara Y. Hepatocyte growth factor/scatter factor in development, inflammation and carcinogenesis: its expression and role in oral tissues. Arch Oral Biol. 2003;48:797–804. doi: 10.1016/s0003-9969(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 18.Sharma C.G., Pradeep A.R. Plasma and crevicular fluid osteopontin levels in periodontal health and disease. J Periodontal Res. 2007;42:450–455. doi: 10.1111/j.1600-0765.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 19.Rakmanee T., Calciolari E., Olsen I., Darbar U., Griffiths G.S., Petrie A., et al. Expression of growth mediators in the gingival crevicular fluid of patients with aggressive periodontitis undergoing periodontal surgery. Clin Oral Invest. 2019;23:3307–3318. doi: 10.1007/s00784-018-2752-z. [DOI] [PubMed] [Google Scholar]
- 20.Duarte P.M., de Lorenzo Abreu L., Vilela A., Feres M., Giro G., Miranda T.S. Protein and mRNA detection of classic cytokines in corresponding samples of serum, gingival tissue and gingival crevicular fluid from subjects with periodontitis. J Periodontal Res. 2019;54:174–179. doi: 10.1111/jre.12617. [DOI] [PubMed] [Google Scholar]
- 21.Ko C.J., Huang C.C., Lin H.Y., Juan C.P., Lan S.W., Shyu H.Y., et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 2015;75:2949–2960. doi: 10.1158/0008-5472.CAN-14-3297. [DOI] [PubMed] [Google Scholar]
- 22.Strudwick X.L., Lang D.L., Smith L.E., Cowin A.J. Combination of low calcium with Y-27632 rock inhibitor increases the proliferative capacity, expansion potential and lifespan of primary human keratinocytes while retaining their capacity to differentiate into stratified epidermis in a 3D skin model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madapusi Balaji T., Varadarajan S., Rao U.S.V., Raj A.T., Patil S., Arakeri G., et al. Oral cancer and periodontal disease increase the risk of COVID 19? A mechanism mediated through furin and cathepsin overexpression. Med Hypotheses. 2020;144:109936. doi: 10.1016/j.mehy.2020.109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V., Behera R., Lohite K., Karnik S., Kundu G.C. p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res. 2010;70:10381–10391. doi: 10.1158/0008-5472.CAN-10-1470. [DOI] [PubMed] [Google Scholar]
- 25.Fuentes-Prior P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J Biol Chem. 2021;296:100135. doi: 10.1074/jbc.REV120.015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papa G., Mallery D.L., Albecka A., Welch L.G., Cattin-Ortola J., Luptak J., et al. Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell-cell fusion. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amorim Dos Santos J., Normando A.G.C., Carvalho da Silva R.L., Acevedo A.C., De Luca Canto G., Sugaya N., et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2021;100:141–154. doi: 10.1177/0022034520957289. [DOI] [PubMed] [Google Scholar]
- 30.Brandão T.B., Gueiros L.A., Melo T.S., Prado-Ribeiro A.C., Nesrallah A., Prado G.V.B., et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131:e45–e51. doi: 10.1016/j.oooo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hockova B., Riad A., Valky J., Sulajova Z., Stebel A., Slavik R., et al. Oral complications of ICU patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med. 2021;10 doi: 10.3390/jcm10040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sedghizadeh P.P., Mahabady S., Allen C.M. Opportunistic oral infections. Dent Clin. 2017;61:389–400. doi: 10.1016/j.cden.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mjaess G., Karam A., Aoun F., Albisinni S., Roumeguere T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30:484–487. doi: 10.1016/j.purol.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alwani M., Yassin A., Al-Zoubi R.M., Aboumarzouk O.M., Nettleship J., Kelly D., et al. Sex-based differences in severity and mortality in COVID-19. Rev Med Virol. 2021;31:e2223. doi: 10.1002/rmv.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asselta R., Paraboschi E.M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020;12:10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Janne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Helal O., Gostemeyer G., Krois J., Fawzy El Sayed K., Graetz C., Schwendicke F. Predictors for tooth loss in periodontitis patients: systematic review and meta-analysis. J Clin Periodontol. 2019;46:699–712. doi: 10.1111/jcpe.13118. [DOI] [PubMed] [Google Scholar]
- 38.Carrel L., Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 39.Badran Z., Gaudin A., Struillou X., Amador G., Soueidan A. Periodontal pockets: a potential reservoir for SARS-CoV-2? Med Hypotheses. 2020;143:109907. doi: 10.1016/j.mehy.2020.109907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrera D., Serrano J., Roldan S., Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Invest. 2020;24:2925–2930. doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.