Abstract
Background
At a global level, the COVID-19 disease outbreak has had a major impact on health services and has induced disruption in routine care of health institutions, exposing cancer patients to severe risks. To provide uninterrupted tumor treatment throughout a pandemic lockdown is a major obstacle. Coronavirus disease (COVID-19) and its causative virus, SARS-CoV-2, stance considerable challenges for the management of oncology patients. COVID-19 presents particularly severe respiratory and systemic infection in aging and immunosuppressed individuals, including patients with cancer.
Objective
In the present review, we focused on emergent evidence from cancer sufferers that have been contaminated with COVID-19 and cancer patients who were at higher risk of severe COVID-19, and indicates that anticancer treatment may either rise COVID-19 susceptibility or have a duple therapeutic impact on cancer as well as COVID-19; moreover, how SARS-CoV-2 infection impacts cancer cells. Also, to assess the global effect of the COVID-19 disease outbreak on cancer and its treatment.
Methods
A literature survey was conducted using PubMed, Web of Science (WOS), Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), and VIral Protein domain DataBase (VIP DB) between Dec 1, 2019 and Sep 23, 2021, for studies on anticancer treatments in patients with COVID-19. The characteristics of the patients, treatment types, mortality, and other additional outcomes were extracted and pooled for synthesis.
Results
This disease has a huge effect on sufferers who have cancer(s). Sufferers of COVID-19 have a greater percentage of tumor diagnoses than the rest of the population. Likewise, cancer and highest proportion is lung cancer sufferers are more susceptible to COVID-19 constriction than the rest of the population.
Conclusion
Sufferers who have both COVID-19 and tumor have a considerably elevated death risk than single COVID-19 positive patients overall. During the COVID-19 pandemic, there was a reduction in the screening of cancer and detection, and also deferral of routine therapies, which may contribute to an increase in cancer mortality there in future.
Keywords: SARS-CoV-2, Cancer, COVID-19, Treatment, Chemotherapy
Graphical abstract
1. Introduction
Coronavirus illness (COVID-19) seems to be an infection induced by a novel virus that has never been seen in humankind. Cough, diarrhea, and in the most acute cases, inflammation are signs of this infection, which induces lung disease. According to the event descriptions and testing procedures used in the affected countries, 2.5 million reports of infection were recorded in 193 countries, resulting in 165,000 deaths, with two-thirds of those deaths occurring in Europe. The whole pandemic stunned the entire planet including its contagiousness, rapid spread through all subgroups, and ferocity in terms of fatalities. Infection of SARS-CoV-2 can cause asymptomatic infection to extreme lower respiratory tracts disease with lung invades, which can lead to acute respiratory distress syndrome (ARDS) and death (Chuang et al., 2020). The unique coronavirus (CoV) infection pandemic is posing a serious threat to global health systems (Guan et al., 2020).
Any victims with COVID-19, such as tumor patients are known to be in greater danger than others. Cancer progresses in an immunosuppressed setting, adding to the proof which oncology victims are more susceptible to infections, which is exacerbated by such oncologic therapies (e.g., chemotherapy, radiotherapy). Medical oncologists have reorganized their regular clinical practice in light of the ongoing crisis, putting in place preventive mechanisms (Indinia et al., 2020). Due to the scarcity of COVID-19 information in oncology patients, no proof guidelines have been made to date. COVID-19 diffusion in tumor victims is just not as widespread as predicted, according to results from minor case collection (Liang et al., 2020).
Various complications (such as coronary disease, diabetes, and chronic obstructive pulmonary disease [COPD]) are linked to an increased threat of infections and serious events. The correlation between CoV and tumor victims may not be clear due to the virus's unique pathogenesis in humans and the pathways of new oncologic therapies. CoVs, unlike certain modern viruses, have not been found to induce a worsening of infection in immunosuppressed people (Wan et al., 2020a). COVID-19 relies on the owner's immune reaction in addition to overt viral pathogenicity. CoV inflammation causes an unregulated aberrant immune reaction to external stimuli in some people, resulting in pulmonary tract harm (Prompetchara et al., 2020).
Many oncology patients also modified the characteristics as immuno-compromised subjects after the advent of antitumor immunotherapy (e.g., immune checkpoint inhibitors [ICIs]). Rather, the tumor therapy they undergo boosts their immune response in a way. This may indicate that these individuals are more susceptible to CoV illnesses. The interaction of CoV and ICIs has the potential to complicate COVID-19′s clinical trajectory, which could exacerbate ICI-related adverse impacts (Ester et al., 2020).
The latest CoV pandemic poses a major challenge to tumor victims who are immunosuppressed. There is also a scarcity of information mostly on new CoV breathing disease (COVID-19) in cancer victims. CoVs, unlike certain viruses, haven't been shown to induce further deadly illness in immunosuppressed people. In addition to specific virus pathogenicity, COVID-19 infection causes an unregulated aberrant inflammatory reaction in some people, resulting in lung tissue harm. COVID-19 may thus pose a potential danger to cancer survivors who are receiving immunotherapy (e.g., ICIs). Patients, particularly those are receiving intensive oncologic treatment, may be among the most susceptible members of the infectious population. Just certain case studies or observational trials with scant medical data and restricted sample sizes have been conducted so far. There is a scarcity of specific evidence on tumor background, anti-tumor therapy, respiratory course, laboratory testing and death rates (Jing et al., 2020). Including the fact that patients undergoing successful anti-cancer therapy are underreported in the articles listed, people with cancer may have an increased chance of disease and serious incidents (Xiaonan et al., 2020).
The influenced countries' oncology divisions had to change rapidly and form new organizations with a clear sense of targets. As a result, various guidelines and treatment strategies have been formulated to improve departmental structure and operation. With the purpose of offering and continuing to offer adequate treatment to every cancer sufferer. Many organizations including science associations also provided practical guidelines under these unusual situations. The first guidelines in Europe focused mostly on how to secure tumor sufferers (You et al., 2020).
The objective of this review is to assess the global impact of COVID-19 on cancer, with an emphasis on the identification and care of COVID-19 victims, and to address strategies for treating cancer sufferers in this pandemic and to discuss how SARS-CoV-2 infection impacts on cancer.
1.1. Significant summary
-
➢
According to combined studies, 1%–3.9% of worldwide COVID-19 patients had tumors. Among subgroups of COVID-19 cases that became seriously sick or dead, the percentage of people with an underlying malignancy was found to be significantly higher (7.3%–20.3%).
-
➢
SARS-CoV-2 contamination is more common in cancer patients (0.79%–8.3%).
-
➢
Cancer patients who inherit COVID-19 have far poorer results than those cancer-negative people (mortality range from 11.4%–35.5%).
-
➢
The effect of current systemic antitumor treatment upon result of SARS-CoV-2 contamination is a source of controversy.
-
➢
The redirection of biomedical services to COVID-19 frontline administration has caused a reduction in tumor screening which has had an effect on cancer treatment. This might lead to an increase in cancer-related deaths in the near future.
2. Materials and Methods
A comprehensive online publication was conducted by searching for research papers through Google search and PubMed database to study the COVID-19 in cancer patients. Boolean generators were utilized for the corresponding key-words. SARS-CoV-2, and novel corona viruses in association with cancer, oncology, tumor, anti-tumor therapy and malignancy, Radiotherapy and COVID-19, COVID-19 and cancer guidance and advice. We compiled almost all English-language summaries and papers. Some Chinese publications with their abstracts written in English were also included.
3. Results
3.1. COVID-19 and cancer biology
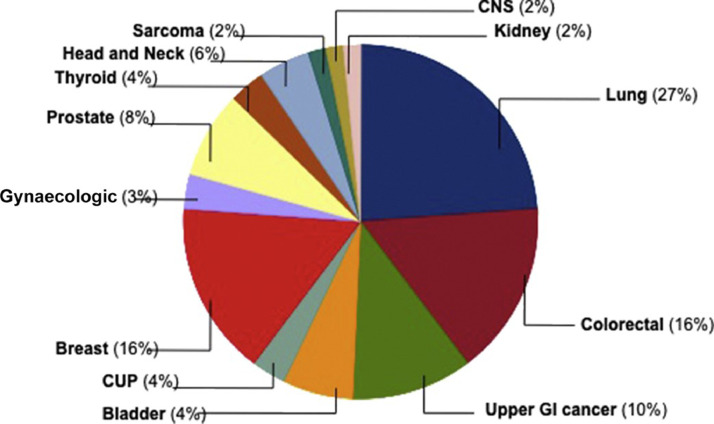
COVID-19 may be particularly harmful to tumor patients, which can be mostly attributed to the compromised immune process from the diseases people got and treatment they received. Such compromised immune process makes patients particularly vulnerable to fend off contamination through the new coronavirus. So many researches have looked at whether systemic cancer treatments including chemotherapy and selective therapies make patients more susceptible to COVID-19. Concerning tumor characteristics and active cancer treatments, utmost common primary tumor sites were lung cancer (27%), followed by colorectal (16%) and breast cancer (16%) and others as presented in the pie chart (Fig. 1 ) (Yarza et al., 2020).
Fig. 1.
Cancer patients infected with SARS-CoV-2 were distributed according to primary tumor location. Cancer of Unknown Primary (CUP) stands for cancer with no specific cause; CNS stands for central nervous system; and GI stands for gastrointestinal. Reproduced with permission from (Yarza et al., 2020).
An overactive immune reaction defined as "cytokine storm" that can harm lungs as well as other tissues, is one of COVID-19′s most severe side effects. Cancer patients who receive immune-activating therapies including blockers of checkpoints, T-cell therapies of chimeric antigen receptors (CARs), and bi-specific T-cell engagers (BiTEs) are also at threat of health problems if the immune reaction elicited by these strategies attacks natural, safe tissue. Patients who receive CAR T-cell therapies or BiTEs can experience cytokine release disorder, which is close to the cytokine storm shown in COVID-19 victims. COVID-19, according to the new study, although not completely conclusive, may intensify cytokine discharge syndrome in people receiving such immunotherapies. While patients may be worried about the elevated risk of COVID-19 as a consequence of treating cancer, this does not prevent them from receiving therapy. In certain cases, cancer drugs will extend life and even cure the cancer, it's essential to have in mind the purposes of treatment and to address the costs and benefits of treatment with the psychiatrist in your specific situation (Ziad et al., 2020).
3.2. Role in cancer
These viruses are believed to specifically attack the pulmonary part of humans. COVID-19 is the seventh coronavirus to infect people. COVID-19 is a biologically unique virus in and of itself, and few function of the new corona-virus in tumor has been well revealed so far. Comorbidities including asthma and cancer predispose positive COVID-19 suffers to worse health conditions, comparable to other serious acute respiratory incidents (SARS-CoV-2, MERS-CoV-1) (Yotsana and Michael, 2020).
The level of fatalities among COVID-19 cancer victims as a comorbid diagnosis was 7.6%, compared to 3.8% for overall COVID-19 community, as per World Health Organization (WHO) (World Health Organization WHO, 2020). As a result, cancer plays a role in COVID-19 pathogenesis. In the United States, upwards of half a million patients are doing treatment, and more than 1.5 million citizens will be in treatment of tumor. Sufferers who are undergoing in action chemotherapy or radiation therapy, or have previously undergone transplantation, are in even greater danger. Even though tumor victims are at a higher threat of serious effects from infection with the SARS-CoV-2 virus, research on COVID-19 in cancer people is still scarce. Immune dysfunction and prolonged inflammation can play a role in COVID-19 positive tumor people's poor results. As a result, it is critical to investigate the root pathways that bind COVID-19 to cancer. A greater awareness of the mechanistic relationship between COVID-19 and cancer would aid in the prevention of infection's harmful consequences as well as the development of new treatments. It's also vital to consider the balance of choosing chemotherapy and antiviral drug therapies, as well as the timing for such treatment. Chemotherapy must be delayed for some people until antiviral course is completed, whereas some can be exposed to viral disease therapies while receiving antitumor medication (Timothy et al., 2020).
3.3. Role of angiotensin converting enzyme 2 (ACE2)
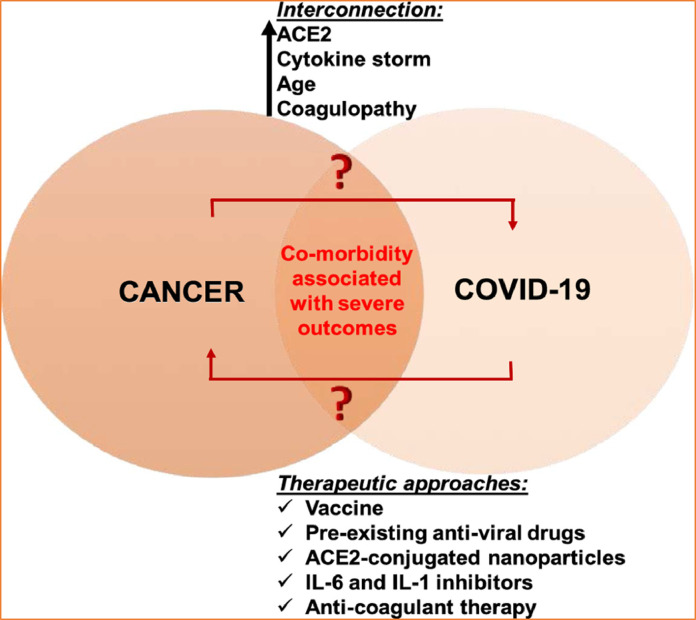
With regards to the biological interconnection between COVID-19 and cancer, ACE-2, cytokine storm, age, and coagulopathy are a few strong factors that connect COVID-19 and cancer (Borchardt and Harrys, 2014; Vickers et al., 2002). A deeper understanding of these connecting links may guide us in finding novel anti-viral and anti-cancer therapeutic options (Fig. 2 ). ACE2 is a carboxypeptidase that converts angiotensin-I to angiotensin 1–9, and angiotensin-II to angiotensin 1–7. It has a significant role in cardiac regulation and also has a protective impact in severe lung injury. Similarly, SARS-CoV and SARS-CoV-2 also enter into the human cells via ACE2 (Wani et al., 2020; Xu and Zhu, 2019; Wan et al., 2020b). The spike protein of the SARS-CoV-2 virus is able to replicate and develop by binding to ACE2, and being transported together into the cell (Lu et al., 2020).
Fig. 2.
The presentation of interrelated connection between COVID-19 and cancer. Reproduced with permission from (Feng et al., 2011).
Recent literature suggests that ACE2 protects mice in contradiction of acute lung injury and avian influenza. Some of the H5N1 infected patients who had higher ACE2 levels in their blood serum presented well outcomes to avian influenza infection and treating mice with human ACE2 prevented lung injury (Feng et al., 2011). Inappropriately, inadequate research work has been done to confirm the mechanistic link between ACE2 expression and SARS-CoV-2 infection in cancer. Therefore, it would be valuable to test whether levels of ACE2 increases/decreases in several tissues of cancer patients and COVID-19 infected patients and how this affects COVID-19 infection in these patients.
3.4. Common risk factors for severe COVID-19 and cancer
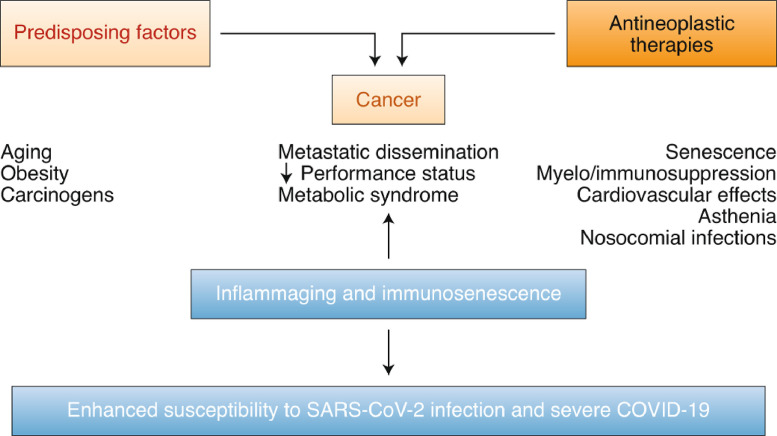
In light of these intriguing data sets, it is significant to clarify the relationship between severe COVID-19 as well as preexisting pro-inflammatory and immunosuppressing circumstances associated with cancer (Fig. 3 ) and its treatments (Table 1 and 2). Here, we discuss the common risk factors between severe COVID-19 and cancer.
Fig. 3.
Tangled relation between cancer as well as the comorbidity or treatment in relation to COVID-19 vulnerability. Reproduced with permission from (Derosa et al., 2020).
Table 1.
Mortality data from selected studies on patients with cancer and COVID-19.
| Countries | Total number of patients with cancer and COVID-19 | Number of deaths | Death rate | Time period of study (2020) | Refs. |
|---|---|---|---|---|---|
| USA, Canada, Spain | 928 | 121 | 13% | 17 March−16 April | (Kuderer et al., 2020) |
| Italy | 909 | 150 | 16.5% | Up–30 March | (Trapani et al.,2020) |
| UK Coronavirus CancerMonitoring Project | 800 | 226 | 28% | 18 March–26 April | (Lee et al., 2020) |
| New York, USA | 423 | 51 | 12% | 10 March–7 April | (Robilotti et al., 2020b)) |
| TERAVOLT Registry | 400 | 141 | 35.5% | 26 March−12 April | (Horn et al., 2020) |
| New York, USA | 218 | 61 | 28% | 18 March−8 April | (Mehta et al., 2020) |
| Europe chronic lymphatic leukaemia | 190 | 55 | 28 March−22 May | (Scarfo et al., 2020) | |
| Brazilian National Cancer Institute | 181 | 60 | 33.1% | 30 April−26 May | (deMelo et al., 2020) |
| Gustave Roussy Cancer Campus,Villejuif, France | 137 | 20 | 14.6% | 14 March−15 April | (Fabrice et al., 2020) |
| China | 105 | 12 | 11.4% | 1 January−24 February | (Mengyuan et al., 2020) |
| New York, USA | 102 | 25 | 25% | 12 March−6 May | (Luo et al., 2020a)) |
Table 2.
Drugs with anti-cancer effects and redeployment to become anti-virals.
| Drugs | Mode of action | Cancer indication | Antiviral indication | Type of study | Ongoing clinical trials |
| Interferon-based therapies | |||||
| IFNα2b or IFNβ1b, alone or combined with antivirals | Stimulation of innateimmunity | RCC, melanoma,HCC, CML, hairy-cell Leukemia (Galimberti et al., 2020),AIDS-relatedKaposi sarcomaFollicular lymphomaCondylomata acuminata | Hepatitis B and C | Clinical trial againstCOVID19 (Sallard et al., 2020; Lu, H., 2020; Sheahan et al., 2020) | NCT04344600, NCT04350671, NCT04343768, NCT04343976, NCT04254874, NCT04320238, ChiCTR2000029387, NCT04315948, NCT04276688 |
| IFNγ | Activation of lungmacrophages | Cancer (Garcia et al., 2018) cancercomorbidities: e.g., COPD, idiopathicpulmonary fibrosis | Synergy with IFNβ to block SARS-CoV-2 replication | Clinical trial withinhaled IFNγ (Smaldone, 2018) | |
| TLR3 agonistsHiltonol(poly-ICLC) | Induction of type IIFNs and protectionagainst respiratoryvirus–inducedimmunopathology (Kumaki et al., 2017) | Adjuvant for cancer vaccines Therapeutic in addition to immunogenic chemotherapy | Preclinical studyin BALB/c miceagainst SARS-CoV: prophylactic and therapeutic effects of poly-ICLC by intranasal route (Kumaki et al., 2017) | No trials | |
| Immune checkpoint inhibitors | |||||
| Anti-PD-1 AbsPembrolizumabNivolumab | Reactivation of exhausted antiviral and antitumor CTLs (Hirsch et al., 2017) | Multiple stage IIIc IV cancer indications (various cancers) (Hirsch et al., 2017) | Prevention of EBV-induced HLH (Liu et al., 2020a) Non-inferiority in case of concomitant NSCLC and COVID-19 (Luo et al., 2020b) | Retrospective analysis on (Weiss et al., 2013) patients with lung cancer adjusted for gender and smoking status Randomized trials with anti-PD-1 | NCT04335305: pembrolizumabNCT04333914: nivolumab |
| IL-6–JAK–STAT3 | |||||
| Anti-IL-6R AbsTocilizumab | Cytokine storm andHLH, prevention ofimmunothrombosis,lung and systemicinflammation,reduction ofneutrophilia (Moots et al., 2017) | FDA approved foriatrogenic responsesto immunostimulation (e.g., CART cell therapies) andmyeloproliferativeneoplasms.In assessment formultiple myeloma,many solid andhematologicalmalignancies andacute GVHD (Ocana et al., 2017) | FDA approved in China for severe COVID-19 | Pilot studies orRandomized clinical trials Observationalstudy in 21 Chinesepatients withCOVID-19TranslationalResearch studies (Guo et al., 2020; Liao et al., 2020) | NCT04332094, NCT04359667, NCT04317092, NCT0433291, NCT04335071, NCT04346355, NCT04306705, NCT04331795, NCT04377659, NCT04377750, NCT04363853, NCT04320615, NCT04315480, NCT04331808, NCT04310228, NCT0433391, NCT04339712, NCT04331808 |
| Androgen-deprivation therapy | |||||
| Androgenreceptordeprivationtherapies (ADT) | ADTs decreaseTMPRSS2 in lungand prostatetissues (Mikkonen et al., 2010) | Prostate cancer(expressing TMPRSS2) (Lucas et al., 2014) | TMPRSS2 inducesspike protein priming;its inhibition bycamostat mesylatehas antiviral effect | Retrospective Italian study, n = 4532 patients; ADT decreased COVID-19 incidence(OR: 4.05) (Lucas et al., 2014) | NCT04397718 |
| Other small molecules | |||||
| Anti-CD26/DDP4Begelomab | Alternate receptorfor SARS-CoV-2 (inaddition to ACE2) (Vankadari and Wilce 2020) Preservation of Cxcl10 biologicalactivity and anticancer synergisticeffects betweenCD26 blockade andanti-PD-1 Ab (Nabavi et al., 2020) | Steroid refractory acute GVHD(Nabavi et al., 2020) | MERS Diabetes (Iacobellis, 2020) | No trials | |
| ImatinibMesylate or saracatinib | Abl kinase activityinvolved incoronavirus fusionwith endosomalmembrane as well ascell–cell fusion latein infection (Inoue et al., 2007) | FDA approved for CML and GIST | Infectious bronchitisvirus (IBV)SARS-CoV1MERS (Nabavi et al., 2020) | Randomized clinical trial | NCT04357613, NCT04356495,NCT04346147 |
| Ibrutinib | TKI Bruton kinaseand IL-2-inducibleT cell kinaseinhibitor that bluntsT cell activation andreduces cytokinerelease syndrome (Treon et al., 2020) | Steroid-refractorychronic GVHDCLL Waldenström disease (Galimberti et al., 2020) | Effects on host immunecells; no direct antiviralactivity | Retrospective observation in Waldenstrommacroglobulinemia: reduced COVID-19incidence (Treon et al., 2020) | No trials |
| Zotatifin;Plitidepsin | eIF4A inhibitor;eEF1A inhibitor | Preclinical activity against multiple forms of KRAS mutant andreceptor tyrosine kinase mutant cancers currentlybeing evaluated inmultiple myeloma | eIF4H, an Nsp9interactor, is a partner ofeIF4A; eIF4A inhibitorzotatifin shows strongantiviral effect eIF4Ainhibitor ternatin-4 hasantiviral effects | Phase 1/2 clinicaltrial in patients witha targeted set ofsolid tumors (Gordon et al., 2020) | NCT04092673NCT04382066 |
| Anakinra | Interleukin (IL)−1receptor antagonist | Currently beingevaluated to prevent or treat severe side effectsin patients receiving CAR-T cell therapy (NCT04148430) | Clinical trials incytokine stormsyndrome secondaryto COVID-19 (Cavalli et al., 2020) | NCT04443881 | |
| Other strategies | |||||
| BCG | Trained immunityEpigeneticreprogrammingof myeloid cells (Mitroulis et al., 2018) | FDA approved innon-muscle invasivebladder urothelialcancers | No dataNegative epidemiologicaldata (Hamiel et al., 2014) | Phase 3 randomized controlled clinicaltrials | NCT04328441, NCT04327206,NCT04379336, NCT04327206,NCT04348370 |
| Chlorpromazine | High concentrationsin lung and salivaAnti-inflammatory(more IL10, less TNFα, IFNα)Antiproliferativevia suppressionof AKT–mTOR orsirtuin 1 inhibition | Phenothiazinederivative used to treat psychotic disordersControl of nauseaand vomiting incisplatin-treated cancer patientsWeak indication in drug repurposing:antineoplastic properties in colon cancer and glioblastoma in vitro | Inhibition of clathrin-dependent endocytosis(Gadina et al., 1991) | Clinical trials | NCT04366739, NCT04354805 |
| Low-doseRadiotherapy | ImmunomodulationReprogrammingof iNOS+/ M1 phenotype of pulmonary macrophages(Meziani et al., 2021) | Low-grade lymphomaLung cancer | Pneumonia | Preclinical models oflung inflammationinduced by TLR3 orTLR4L (Meziani et al., 2021) | NCT04377477, NCT04390412,NCT04366791, NCT04380818,NCT04394182 |
| Vitamin D3(cholecalciferol) | Extra skeletalbioactivity inprevention ofinfections, T1D andT2D, cardiovasculardisease, obesity,asthma, inflammatorybowel disease andcancers (colon,breast, prostate andovarian) | Induction of apoptosis,stimulation of celldifferentiation andanti-inflammatoryand antiproliferativeeffects and inhibition ofangiogenesis, invasionand metastasisReduction of death bycancer but no benefitin preventing cancer incidence. | Protective effect ofvitamin D in settings ofpneumonia, cytokinehyperproduction andARDS (Huang et al., 2020)Vitamin D repurposedfor influenza A H5N1virus–induced lunginjury | Observationalstudies: Dailysupplementation with 2000–5000 IU/day vitamin D3 in older adults with Parkinson disease may offer protection against COVID-19 (Hribar et al., 2020; Manson et al., 2019)Genetic studies:Vitamin D receptorgene (VDR)alleles associatedwith increased susceptibility to respiratory or viral infections (Jolliffe et al., 2018) | NCT04399746,NCT04344041,NCT04372017, NCT04386850 |
Ab, antibody; ADT, androgen deprivation therapy; ARDS, acute respiratory distress syndrome; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; EBV, Epstein–Barr virus; EMA, European Medical Agency; GVHD, graft-versus-host disease; HCC, hepatocarcinoma; HLH, hemophagocytic lymphohistiocytosis; iNOS, inducible nitric acid synthase; NSCLC, non-small-cell lung cancer; poly-ICLC, polyinosinic–polycytidylic acid; RCC, renal-cell carcinoma.
The predisposing factors for cancer are aging, obesity, metabolic syndrome, and exposure to carcinogens. Also, aging, obesity, and metabolic syndrome are represented comorbidities that influence vulnerability to and sternness of SARS-CoV-2 infection. In cancer-infected patients, metastatic spreading and weak Eastern Cooperative Oncology Group (ECOG) performance status also favor COVID sternness (Derosa et al., 2020).
3.4.1. Aging, immunosenescence and inflammaging
Aging is one of the utmost common causes, increasing the occurrences of both cancer and SARS-CoV-2 infection, with significantly potential commonalities connecting to immunosenescence, inflammaging (Fig. 3), and their treatments are presented in Table 2. Immunosenescence outlines a status of lessening the function of the body immune system-related responses to vaccination, infection, and cancer, also an augmented occurrence of devastating autoimmune diseases in the aging populace (Pawelec, G., 2018) For example, C-reactive protein levels are related to aging CD8+ T cells, plasma blasts as well as granulocytes in aging persons (Stevenson et al., 2018) Particularly, in patients with COVID-19, lesser T cell counts are related to clinical indicators of inflammation, for example, D-dimers, ferritin, and C-reactive protein, while high quantities of plasma blasts are related to severe disease (Mathew et al., 2020). Interleukin 6 (IL6), which has been referred to as the ‘gerontologist's cytokine’ (Ershler and Keller, 2000), is normally present at low levels in the blood but is increased with aging (Nelke et al., 2019) and correlates with death (Puzianowska-Kuźnicka et al., 2016). IL6 is involved in the pathogenesis of numerous chronic ailments, including cancer (Weiss et al., 2013). The IL6–JAK–STAT3 pathway is hyperactivated in various types of cancer, driving the propagation, survival, and intrusiveness of cancer cells and suppressing the anti-tumor immune response (Dulos et al., 2012).
Therefore, tactics directing this pathway have already received US Food and Drug Administration (FDA) approval to treat inflammatory conditions or myeloproliferative neoplasms, and to manage certain adverse effects of chimeric antigen receptor-expressing T cells (CAR T cells) (Johnson et al., 2018).
3.4.2. Metabolic syndrome, cancer and COVID-19
Numerous meta-analyses have reported an association between type 2 diabetes (T2D) and cancer, with the strongest relationship found for liver and pancreatic cancer, followed by endometrial cancer (Oberaigner et al., 2014; Dong et al., 2021). Likewise, severely obese persons with T2D are more probable to become infected by SARS-CoV-2, and are at a higher risk of problems and demise from COVID-19. Interestingly, persons with T2D were also at augmented risk for SARS as well as MERS (Kulcsar et al., 2019). Related to this, insulin is a vital hormonal garnish of tumor metabolism and development in obesity-related to insulin resistance (Perry and Shulman, 2020) and treatment of T2D during COVID-19 is being instigated to mitigate disease severity (Longo et al., 2020) which may compromise the integrity of the intestinal barrier that located in SARS-CoV-2 replication (Thaiss et al., 2018).
3.4.3. Immunosuppression, lymphopenia, neutrophilia and interferon deficiency
Through their involvement in immunosurveillance, lymphocytes control the occurrence, development, and therapeutic response of cancers (Galluzzi et al., 2017). CD4+ and CD8+ T lymphocytes recognize tumor cells expressing immunodominant determinants presented by chief histocompatibility complex class II and class I, respectively. CD4+ lymphopenia, a hallmark of immunosuppressive viral infection, occurs in about 20% of patients with advanced pancreatic cancer, melanoma, non-Hodgkin's lymphoma and breast cancer (Bedimo et al., 2009).
Lymphopenia regularly escorts cancer diagnosis, treatment, or progression and is a side effect of chemotherapy and steroids. Radiotherapy also decreases lymphocyte counts (Meye, 1970). An augmented number of blood neutrophils is often combined with reduced lymphocyte counts, resulting from the elevation of the neutrophil-to-lymphocyte ratio. High neutrophil-to-lymphocyte ratio is a poor prognostic indicator and forecasts short cancer-specific progression-free survival after blockade of programmed cell death protein 1 (PD-1), as well as severe COVID-19 (Ocana et al., 2017)
3.4.4. Psychological effect of COVID-19 pandemic on cancer patients
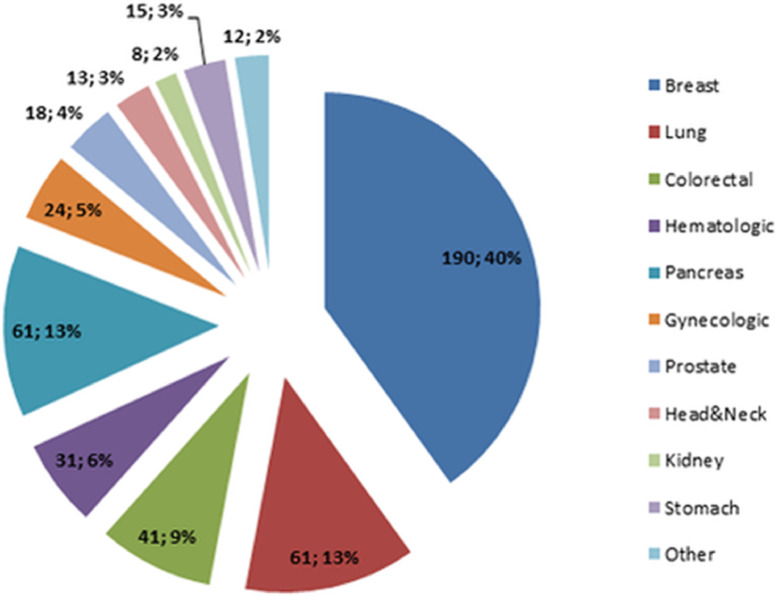
Investigation have found among defendants, the most common cancer diagnoses were breast cancer (190 patients; 40%), lung cancer (61 patients; 13%), pancreatic cancer (61 patients; 13%), colorectal cancer (41 patients; 8%), hematologic malignancies (31 patients; 6%), gynecologic cancer (24 patients; 5%), prostate cancer (18 patients; 4%), stomach cancer (15 patients; 3%), head and neck cancer (13 patients; 3%), kidney cancer (8 patients; 2%) and other such melanoma, sarcoma and testicular cancer (11 patients; 3%). Overall, in a population characterized by a high level of emotional vulnerability the CoV-19 pandemic had a marginal effect, and only a small percentage of patients stated a rise in their emotional vulnerability (Fig. 4 ) (Eva et al., 2021).
Fig. 4.
Changes to cancer treatment caused by the COVID-19 pandemic. Reproduced with permission from (Eva et al., 2021).
3.5. Impact of COVID-19 in various countries
3.5.1. Impact of COVID-19 in India
The nationwide lockdown to combat the COVID-19 disease outbreak had a detrimental effect on chemotherapy care, with a substantial decrease in the percentage of people opting for cancer-specific treatment. To minimize the number of hospital visits, the trend of chemotherapy prescriptions shifted to a longer interval and a longer path. Even though COVID-19 new cases were observed in the population, the removal of travel limits increased in numbers of patients seeking advice (Pandey et al., 2020; Wani et al., 2021).
3.5.2. Impact of COVID-19 in China
Anti-viral treatment and opposing his RT-PCR on 2 occasions, a COVID-19 positive people with early phase colon cancer was effectively diagnosed with colectomy without complications (Guilherme et al., 2020). Chinese doctors changed not only the treatment methods for cancer patients but also the medical protocols (Eva et al., 2021). When it comes to lung cancer, maintaining a high index of suspicion for COVID-19 contamination and safeguarding sensitive patients are top priorities, cancer of the lungs is the much vulnerable type of tumor to COVID-19 contamination, according to three Chinese cohorts. China said that, after the COVID-19 pandemic, China saw a shift in approach in the treatment of oncology people (Xu et al., 2020).
3.5.3. Impact of COVID in Italy
Italy was among the first countries to see a dramatic rise in the prevalence and mortality level of COVID-19 cases, by the end of March 2020, there have been over 100,000 incidents and up to 11,600 deaths. Italy quickly protested for a lack of staff, and several oncologists were called in to help with the COVID-19 war. As a result, some areas were converted to only accept COVID-19 patients. Focused on their existing experience, an Italian group of young oncologists proposed several steps to respond to the situation. Several elective operations in colorectal surgery, for example, were restricted in many facilities around the world, but colorectal cancer operations were not included in this policy and continued alongside emergent procedures (Di Saverio et al., 2020). In the absence of guidelines, few rectal cancer specialists choose to use oral capecitabine instead of 5-FU wherever necessary and to use short-course radiation treatment in the neoadjuvant environment when postponing surgery (De Felice and Petrucciani, 2020). A northern Italian association of radiation oncologists suggested an equation that focused on attempting to handle cancer patients with hypo fractionated protocols wherever possible, while withholding or delaying radiation treatment for benign illness, just handle reported or extremely suspect COVID-19 events in the adjuvant environment (Filippi et al., 2020).
3.5.4. Impact of COVID-19 in France
Oncology practice in France was similar to China and Italy, but several French organizations currently published standardized recommendations for the treatment of particular cancers. All of these proposals were in line with suggestions made by a panel of French experts commissioned by the leader on March 14th, 2021 the Public Health Council held a meeting to discuss the SARS-CoV-2 virus and solid cancers (You et al., 2020).
3.6. The pandemic's effect on cancer care
In response to the disease outbreak, cancer clinics around the world implemented procedures like a divided process, prioritizing some subsets of people with cancer for urgent care while delaying treatment for others (Beddok et al., 2020; Wani et al., 2022). Mauri et al. summarised core recommendations from 63 standards in specialized associations across the planet. Health services are selectively mobilized to the treatment of clinicians with COVID-19 in hospitals servicing communities with a heavy caseload of SARS-CoV-2 disease, including New Delhi, Mumbai, Milan, Madrid, and New York, possibly jeopardizing normal operations like cancer testing and treatment (Mauri et al., 2020).
Since the pandemic, another problem that doctors caring for is deciding which systemic care is best for the metastatic tumor people have had from the various options available, particularly in light of new evidence that hospital entry or repeated hospital visits could be possible causes for cancer sufferers contracting the SARS-CoV-2 virus. People with this disease in low-income and middle-income countries have faced increasingly daunting difficulties (Trehan and Vijay, 2021). During this pandemic, guidelines support the utilization of regimens with reduced levels of cytopenia in the area of genitourinary cancers, where various therapeutic methods such as chemotherapy, selective treatments, hormonal therapies, immunotherapeutic, or radionuclides may be provided to patients (Gillessen and Thomas, 2020).
A recent study demonstrates that some patients with cancer face a higher risk of death if affected by COVID-19, which is predominantly driven by older age, smoking, the presence of active disease and associated risk factors. Patients who are otherwise healthy and have curable malignancies present COVID-19-related mortality rates similar to the general population, which should not have delayed access to cancer treatment. The oncology community is trying to thoughtfully balance the fear of COVID-19 against the dire consequences of not treating cancer in an effective or timely manner (Cannistra et al., 2020).Our data endorse the recommendations to minimize the risk of SARS-CoV-2 infection in patients with cancer with active preventive measures, especially in subgroups of patients with recognized poor prognostic factors, and to perform close monitoring in the case of exposure to the virus or COVID-19 related symptoms. The mortality data from selected studies on patients with cancer and COVID-19 are presented in Table 1.
3.7. Treatment of COVID-19 in cancer patients
3.7.1. Oxygen therapy for COVID-19 patients
The most important symptomatic treatment for COVID-19 patients is oxygen therapy (Yang et al., 2020). For cancer patients with COVID-19, there was a higher percentage of patients who received oxygen therapy (Zhang et al., 2020a). The higher proportion of COVID-19 patients with cancer requiring oxygen therapy and mechanical ventilation may be related to more severe disease and an immunosuppressive state in cancer patients, who are more susceptible to secondary lung infection with other pathogens.
3.7.2. Antiviral treatment for COVID-19 patients
Currently, there is no antiviral drug that is specifically effective against SARS-CoV-2. Several clinical studies have indicated that remdsivir, arbidol, and chloroquine may have moderate benefits for treating COVID-19 (Wang et al., 2020a; Grein et al., 2020). Larger clinical studies need to confirm these results. For cancer patients with COVID-19, the use of antiviral drugs did not yield any different outcomes compared with general COVID-19 patients. About 71.4% of cancer patients with COVID-19 received at least one antiviral agent, including arbidol, lopinavir/ritonavir, ganciclovir, and ribavirin, while 32.1% received two or more antiviral agents (Zhang et al., 2020b; Dai et al., 2020).
3.7.3. Immune enhancement therapy for COVID-19 patients
Given that COVID-19 cancer patients may have systemic immunosuppression, intravenous immunoglobulin may be a promising treatment of COVID-19. One study showed that 12 out of 28 cancer patients with COVID-19 received intravenous immunoglobulin treatment. However, the study could not provide adequate information about efficacy due to the limited sample size and lack of a randomized control group (Zhang et al., 2020a).
3.7.4. Anti-inflammatory therapy for COVID-19 patients
The rationale for anti-inflammatory therapy is based on the premise that COVID-19 induces a cytokine storm with deleterious effects on tissues (Tobaiqy et al., 2020). In a controlled, open-label trial, the use of dexamethasone in hospitalized patients with COVID-19 resulted in lower 28-day mortality among those receiving either invasive mechanical ventilation or oxygen alone (Horby et al., 2020). For cancer patients with COVID-19, the use of systemic corticosteroids remains controversial. Given that cancer patients are already at a higher risk of opportunistic infections, the use of corticosteroids may not be effective in mitigating COVID-19 symptoms. Indeed, one study showed that corticosteroids did not reduce the incidence of severe events in cancer patients with COVID-19 (Zhang et al., 2020b). Blood purification therapy is an alternative treatment to reduce cytokine storms and benefit critically ill COVID-19 patients. One report showed that the therapy was effective in managing cytokine storms and pathogenic antibodies in three critically ill COVID-19 patients with profound inflammations (Ma et al., 2020). However, larger randomized data were lacking. Furthermore, multi-disciplinary efforts are needed to achieve increased availability of blood purification therapy for COVID-19 cancer patients.
3.7.5. Convalescent plasma therapy for COVID-19 patients
Convalescent plasma therapy has also been explored to alleviate COVID-19 symptoms (Tobaiqy et al., 2020). Of note, there are some potential risks and ethical issues associated with its usage, including thrombotic risk and the selection of donors. Given that cancer patients with COVID-19 may have a more rapid disease progression, convalescent plasma therapy may be particularly beneficial in this population. To date, there is no report about the effectiveness of convalescent plasma therapy in this patient population.
3.7.6. Therapies for COVID-19 associated with anti-tumor therapies
It is known that immune tolerance is a key part of tumorigenesis and anti-tumor therapy resistance (Dong, 2020). Similar to cancer therapy, one method of vaccine development may be a T cell epitope vaccine to enhance the T cell recognition of virus-infected cells. The regimen used to prevent or reduce the cytokine storm in cancer patients during CAR-T cell therapy may also be used to reduce the risk of cytokine storm in COVID-19 patients. It is known that IL-6 is a critical cytokine involved in cancer and inflammation. High levels of IL6 predict poor prognoses of patients with COVID-19 (Zhao, 2020). Among 129 patients hospitalized for COVID-19, those who received tocilizumab in addition to standard treatment were significantly less likely to need ventilation or die within 2 weeks, when compared with those who received standard treatment alone. Therefore, antibodies targeting the IL6 receptor (tocilizumab and sarilumab), I-6 (siltuximab),and other receptor antagonists (α1-adrenergic receptor antagonist, prazosin) for mitigating cytokine storm are promising therapeutic strategies for the treatment of cancer patients with COVID-19 (Konig et al., 2020). Besides IL-6, other cytokines such as type-I interferon, IL1β, IL7, IL17, and TNFα are central to the pathophysiology of COVID-19 (Jamilloux et al., 2020). In particular, IL17 is a critical cytokine associated with immune responses in both cancer and COVID-19 patients (Cafarotti, 2020). Given that anti-IL17 antibodies have demonstrated a therapeutic role in the treatment of cancer and lung infection by H1N1 and AIDS41, this approach might be useful to control COVID-19 in cancer patients.
4. Conclusions and future directions
Despite all of the attempts, identifying the best solution for people with cancer in the face of the COVID-19 challenge is proving difficult. The treatment of people with cancer must be complex and tailored to each patient's diagnosis, the services available at each hospital, and the expertise of each physician. The existing COVID-19 predicament has a lesser effect on healthy, appropriate broods and adolescents, though demanding its lethal toll among all other sections of the populace: the sick, the unfit, and the elderly, including patients with cancer. Cancer predisposes to severe COVID-19 for several reasons i.e. (a) cancer patients fall into general at-risk categories because of their average progressive age, predisposing factors i.e. obesity as well as smoking, then comorbidities i.e. type-2 diabetes including high blood pressure; (b) cancer fundamentally has undesirable effects on patients’ general health status; (c) anticancer treatments i.e. radiotherapy, chemotherapy, and surgery may enervate the body-immune system which leads to immunosenescence as well as inflammaging. However, whether cancer as such is a sovereign risk factor for severe COVID-19 remains to be clarified. It is important that throughout the COVID-19 eruption, morbidity and mortality of cancer patients may have been significantly affected by the viral disease itself, and by the enormous burden wielded by COVID-19 in the healthcare system, which led to the delay of the antineoplastic therapies.
Throughout the existing COVID-19 pandemic, oncologic divisions are often provoked with the challenge of treating patients with both cancers as well as COVID-19, increasing a strong squabble for exploring treatment methods that might concurrently progress both ailments. Numerous medications which have direct repressing impacts on SARS-CoV-2 reproduction in vitro studies (Rocca et al., 2013; Chittezhath et al., 2014) (which are still need further clinical trials for the establishment Table 3 ) are also known for their potential anti-cancer impacts, such drugs including imatinib mesylate and inhibitors of cap-dependent translation, might have a dual therapeutic activity in contradiction of cancer and COVID-19. Assumed the doubts about the benefits of PD-1 and/or PD-L1/IL6R antagonist (Robilotti et al., 2020a), other options are being examined i.e. passive transfer of neutralizing anti-SARS-CoV-2 antibodies for weak patients at a mild to the serious phase of COVID- 19 (Hansen et al., 2020). Lastly, active vaccination will be a greater choice for patients to get rid of the high risk of evolving severe COVID-19 nonetheless still capable of rising defensive anti-viral T-cell responses (Weiss et al., 2013).
Table 3.
Antiviral drugs proposed against SARS-CoV-2 infection displaying antitumor effects.
| Antiviral drugs | FDA indication | Anti-CoV-2effectin vitro | Antitumor effectin vitro | Antitumor effectin vivo | Anticancer mechanism | Ongoing clinical trialsfor viral or cancerindications |
| Azithromycin | Acute bacterial sinusitisAcute bacterialinfections in COPDCommunity-acquired pneumoniaPharyngitis/tonsillitis Skin infectionsUrethritis and cervicitis Genital ulcer disease | Yes (Andreani et al., 2020) | BC cellsPancreatic cancerCells (Mukai et al., 2016) HCC cells (Abdel-Hamid et al., 2017) Colon cancer, GCCells (Qiao et al., 2018) Myeloma cells (Moriya et al., 2013) | In miceLung cancer (Moriya et al., 2013) | Inhibition of angiogenesis (suppression of VEGF receptor 2 signaling) Apoptosis via both intrinsic and extrinsic pathway (Abdel-Hamid et al., 2017) | NCT04341207: (+hydroxychloroquine) SARS-CoV-2 incancer patients;NCT04369365:COVID-19 prophylaxisin cancer patients;NCT04392128:(+hydroxychloroquine) COVID-19 treatmentin patients withhematologicalmalignancies |
| Camostat mesylate | No | Yes (Hoffmann et al., 2020) | No | No | No | No |
| Favipiravir | No | Yes (Wang et al., 2020b) | No | No | No | No |
| Hydroxychloroquine | COVID-19 (Chinawith cautions)MalariaAutoimmuneDiseases | Yes (Liu et al., 2020b) | Glioblastoma cells (Kim et al., 2010) Stomach cancer Cells (Kim et al., 2019) Pancreatic cancerCells (Yang et al., 2014) Kidney cancercells (Park et al., 2016) Endometrial cancer cells (Fukuda, T. et al. 2015) MyelomaCells (Jarauta et al., 2016) LymphoblasticCells (Hounjet et al., 2019) Lymphoma cells (Masud et al., 2016)Melanoma cells (Lakhter et al., 2013) | In mice:Neuroblastoma(Cournoyer et al., 2019) Gastric cancer (Wang et al., 2019) Prostate cancer (Saleem et al., 2012)Melanoma (Hall et al., 2018)In humans:Phase 1 trialin melanoma,glioblastoma andmyeloma (Saleem et al., 2012) | Autophagy inhibition (Levy et al., 2017) Akt signaling pathway inhibition G2/M cell cycle arrest (Jiang et al., 2008) Increase in CTLResponse (Xu et al., 2014) | Viral indications:NCT04341207: SARS-CoV-2 in cancer patients; NCT04381988:COVID-19 prevention in patients receivingRT; NCT04392128:(+ azithromycin)COVID-19 andhematologicalmalignancyCancer indications:NCT01266057,NCT01023737,NCT01480154: advancedcancer; NCT03774472,NCT03774472,NCT03032406:BC; NCT03377179:cholangiocarcinoma;NCT04214418,NCT04145297:gastrointestinaladenocarcinoma;NCT04201457:glioma; NCT03037437:HCC; NCT02722369,NCT00977470: lungcancer; NCT04163107:myeloma; NCT03754179,NCT02257424:melanoma;NCT03081702: ovariancancer; NCT03598595:osteosarcoma;NCT04132505,NCT03825289,NCT01506973,NCT01494155:pancreatic cancer;NCT03513211,NCT04011410,NCT04011410: prostatecancer; NCT01023737,NCT03015324,NCT04316169: solidtumors |
| Lopinavir | HIV | Yes, at very high dose (Choy et al., 2020) | Primary effusionlymphoma cells (Kariya et al., 2014)Melanoma cells (Paskas et al., 2019)Lymphoblastic and myeloid leukemia Cells (Maksimovic et al., 2015) | In mice:Prostate cancerColon cancer (Selvakumaran et al., 2013) | Caspase-dependentApoptosis (Kariya et al., 2014) Suppression of NF-κB activity by inhibition of KK phosphorylation in PEL cells (Kariya et al., 2014) Inhibition of proliferation,Transient activation of Akt and inhibition of P70 S6 kinase (Paskas et al., 2019) ER stressCell signaling induction, including Akt and mTOR (Meier et al., 2017) | No |
| Nitazoxanide | Giardia lambliadiarrhea; Cryptosporidiumparvum diarrhea | Yes (Anastasiou et al., 2020) | Glioblastoma cells (Wang et al., 2018) | In mice:CRC (Senkowski et al., 2015) | G1-phase cell cyclearrest, inhibition ofprotein translation viathe mTOR–c-Myc–p27 pathway (Ripani et al., 2020) | No |
| Nafamostat | No | Yes (De Felice et al., 2020) | GC cells CRC cellsGallbladder cancer cellsPancreaticcancer cellsHCC cells (Haruki et al., 2013) | In mice:HCC (Haruki et al., 2013) GallbladderCancer (Iwase et al., 2013)PancreaticCancer (Gocho et al., 2013) | Induce mitochondria dependent apoptosisCanonical NF-κBsignaling blockadeTNFR1-stimulatedcleavage of caspasefamily members (Iwase et al., 2013) | No |
| Oseltamivir | Influenza virusinfection | Limited(Srinivas et al., 2020) | BC cells (Thulasiraman et al., 2019) | In canines and mice: Increasemammary tumoraggressiveness(deOliveira et al., 2015) | Increase of cleavedcaspase 3 expression (Thulasiraman et al., 2019) | No |
| Penciclovir | Recurrent herpes labialis | Yes (Hongyan et al., 2020) | Oral hairyLeukoplakia (Moura and Haddad, 2010) | No | Accumulation ofcells in S phase andapoptotic death (Shaw et al., 2001) | No |
| Ribavirin | HCVHCC co-infectedwith HIV | Yes (Wang et al., 2020c) (high dose) | Glioblastoma cells (Volpin et al., 2017)HNSCC cells (Dunn et al., 2018) Cervical and colon cancer cells (Xi et al., 2018) | In mice:Glioma, Glioblastoma (Volpin et al., 2017)RCC291 | GTP depletion in HeLa cervical cancer cells (Volpin et al., 2017) Growth inhibition, STAT1 depletion and IFNγ (Dominguez et al., 2019) | NCT02109744:AML; NCT01268579,NCT02308241:HPV-related malignancies;NCT03585725: indolent follicular lymphoma and mantle cell lymphoma;NCT02940496: livercancer; NCT01268579:tonsil and/or base oftongue SCC |
| Ritonavir | HIV | No | ThymomasEL4-T cells (Moawad 2014)Cervical cancerCells (Bandiera et al., 2016) Myeloma cells (Dalva et al., 2015) Lymphoblastoid B cells (Dewan et al., 2009) | In mice:Thymoma (Moawad 2014)Prostate cancer (Ikezoe et al., 2004) In humans:Glioma—noEfficacy (Laurent et al., 2004) | Cell signaling induction including Akt and mTOR (Dalva et al., 2015) Suppression ofpro-survival BCL-2family member andMCL-1 expression (Dalva et al., 2015) Suppression ofNF-κB transcriptionalActivation (Dewan et al., 2009) | NCT02948283: multiplemyeloma, CLL |
| Umifenovir | No | Yes (Wang et al., 2020) | No | No | No | No |
BC, breast cancer; BCL-2, B cell lymphoma 2; CLL, chronic lymphocytic leukemia; COPD, chronic obstructive pulmonary disease; CTL, cytotoxic T lymphocytes; CRC, colorectal cancer; eIF4E, eukaryotic initiation factor 4; ERK, extracellular signal-regulated kinase; EZH2, enhancer of zeste homolog 2; GC, gastric cancer; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous-cell carcinoma; HPV, human papillomavirus; IL, interleukin; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NSCLC, non-small-cell lung cancer; RCC, renal-cell carcinoma; SCC, squamous-cell carcinoma; STAT1, signal transducers and activators of transcription protein; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
However, to evaluate their efficacy and safety coronavirus vaccines will have to undergo worldwide large-scale phase-3 clinical trials. All these possibilities need urgent investigation to permit clinical oncologists to steer between cancer and COVID-19 in complete obedience with the Hippocratic Oath: primum non nocere—first, not harm. Beyondthe clinical assumptions, oncologists must keep in mind that if the COVID-19 epidemic spread, the possibility of raised cancer therapy being unavailable is larger than the risk of a SARS-CoV-2 ailment in a cancer victim.
Ethical Approval
Not applicable.
Data Availability
All data of this paper will be available on request.
Funding
Nil.
Declaration of Competing Interest
All authors of this manuscript declared that no conflict of interest exists.
CRediT authorship contribution statement
Mohammad Ali: Conceptualization, Writing – original draft, Data curation. Shahid Ud Din Wani: Methodology, Software, Supervision. Mubashir Hussain Masoodi: Project administration, Supervision. Nisar Ahmad Khan: Formal analysis, Investigation. H.G. Shivakumar: Formal analysis, Investigation. Riyaz M. Ali Osmani: Formal analysis. Khalid Ahmed Khan: Data curation, Supervision.
Acknowledgments
Acknowledgement
We thank the Al-Ameen College of Pharmacy, Bengaluru, India and Dean, School of Applied Science and Technology, University of Kashmir, Srinagar, India, for their incessant support.
ORCID
Shahid Ud Din Wani, https://orcid.org/0000–0001–9860–0124.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ccmp.2022.100041.
Appendix. Supplementary materials
References
- Andreani J., Marion, Le B., Isabelle D., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:1–5. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hamid N.I., El-Azab M.F., Moustafa Y.M. Macrolide antibiotics differentially influence human HepG2 cytotoxicity and modulate intrinsic/extrinsic apoptotic pathways in rat hepatocellular carcinoma model. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390:379–395. doi: 10.1007/s00210-016-1337-0. [DOI] [PubMed] [Google Scholar]
- Anastasiou I.A., Eleftheriadou I., Tentolouris A., Tsilingiris D., Tentolouris N. In vitro data of current therapies for SARS-CoV-2. Curr. Med. Chem. 2020;27:4542–4548. doi: 10.2174/0929867327666200513075430. [DOI] [PubMed] [Google Scholar]
- Borchardt R.A., Harrys A.T. Challenges in managing hepatitis C virus infection in cancer patients. World J. Gastroenterol. 2014;20(11):2771–2772. doi: 10.3748/wjg.v20.i11.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedimo R.J., McGinnis K.A., Dunlap M., et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J. Acquir. Immune Defic. Syndr. 2009;52:203–208. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddok A., Valentin C., Mathieu M., et al. Post-lockdown management of oncological priorities and postponed radiation therapy following the COVID-19 pandemic: experience of the institute curie. Radiother. Oncol. 2020;150:12–14. doi: 10.1016/j.radonc.2020.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C., Wang Y., Li X., Lili R., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistra S.A., Hafft y B.G., Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J. Clin. Oncol. 2020;38:2206–2207. doi: 10.1200/JCO.20.00858. [DOI] [PubMed] [Google Scholar]
- Cavalli G., Giacomo D.L., Corrado C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournoyer S., Anissa A., Assila B., et al. GX15-070 (Obatoclax), a Bcl-2 family proteins inhibitor engenders apoptosis and pro-survival autophagy and increases chemosensitivity in neuroblastoma. BMC Cancer. 2019;19:1–14. doi: 10.1186/s12885-019-6195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y., Kaewpreedee P., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178:1–6. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittezhath M., Manprit K.D., Jyue Y.L., et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41:815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Cafarotti S. Severe acute respiratory syndrome-coronavirus-2 infection and patients with lung cancer: the potential role of interleukin-17 target therapy. J. Thorac. Oncol. 2020;15:e101–e103. doi: 10.1016/j.jtho.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa L., Melenotte C., Griscelli F., et al. The immuno-oncological challenge of COVID-19. Nat. Cancer. 2020;1:946–964. doi: 10.1038/s43018-020-00122-3. [DOI] [PubMed] [Google Scholar]
- Dulos J., Carven G.J., van Boxtel S.J., et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 2012;35(2):169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- Dong H., Jimi C., Jun G.G. Metabolic syndrome and the risk of COVID-19 infection: a nationwide population-based case-control study. Nutr. Metab. Cardiovasc. Dis. 2021;31(9):2596–2604. doi: 10.1016/j.numecd.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Saverio S., Pata F., Gallo G., Carrano F., Scorza A., Sileri P., et al. Coronavirus pandemic and colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020;22(6):625–634. doi: 10.1111/codi.15056. [DOI] [PubMed] [Google Scholar]
- De Felice F., Petrucciani N. Treatment approach in locally advanced rectal cancer during coronavirus (COVID-19) pandemic: long course or short course? Colorectal Dis. 2020;22:641–649. doi: 10.1111/codi.15058. The Association of Coloproctology of Great Britain and Ireland. [DOI] [PubMed] [Google Scholar]
- deMelo A.C., Luiz C.S.T., Jesse L.S., Lucas Z.A., Ana C.P., Luciana O.R.R., et al. Cancer in patients with COVID-19: a report from the Brazilian National Cancer Institute. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0241261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deOliveira J.T., Ana L.S., Catarina G., et al. Anti-influenza neuraminidase inhibitor oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS One. 2015;10(4):1–22. doi: 10.1371/journal.pone.0121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L.A., Matthew G.F., Eric J.S., et al. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck. 2018;40:233–241. doi: 10.1002/hed.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez G., Dominique C.P., Alma C.B., et al. Growth inhibition and transcriptional effects of ribavirin in lymphoma. Oncol. Rep. 2019;42:1248–1256. doi: 10.3892/or.2019.7240. [DOI] [PubMed] [Google Scholar]
- Dalva A., Richa B., Maylyn M., et al. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clin. Cancer Res. 2015;21:1161–1171. doi: 10.1158/1078-0432.CCR-14-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M.Z., Mariko T., Harutaka K., et al. An HIV protease inhibitor, ritonavir, targets the nuclear factor-κB and inhibits the tumor growth and infiltration of EBV-positive lymphoblastoid B cells. Int. J. Cancer. 2009;124:622–629. doi: 10.1002/ijc.23993. [DOI] [PubMed] [Google Scholar]
- Dong H. Seeking and destroying the evils from the inside‑translating cancer immunity to fight COVID‑19. Cancer Immunol. Immunother. 2020;69:911–912. doi: 10.1007/s00262-020-02580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester di, G., Giuseppe, B., Gianluca, P., Stefano, S., Fabrizia, C., Giovanni de, G., Massimo, C., 2020. Management of older people during the COVID-19 outbreak: recommendations from an Italian experience. Int. j. of geriatric psyc., 35(7), 803–805. 10.1002/gps.5318 [DOI] [PubMed]
- Ershler W.B., Keller E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Eva P., Daniela T., Lucia C., Jessica I., Enrico T., et al. Psychological impact of COVID-19 pandemic on oncological patients: a survey in Northern Italy Michela Rimondini Valeria Donisi. PLoS One. 2021;16:1–13. doi: 10.1371/journal.pone.0248714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Lei N., Huanying W., Liang F., Xiaochun F., et al. Overexpression of ACE2 produces antitumor effects via inhibition of angiogenesis and tumor cell invasion in vivo and in vitro. Oncol. Rep. 2011;26(5):1157–1164. doi: 10.3892/or.2011.1394. [DOI] [PubMed] [Google Scholar]
- Filippi A.R., Elvio R., Stefano M.M., Renzo C. Letter from Italy: first practical indications for radiation therapy departments during COVID-19 outbreak. Int. J. Radiat. Oncol. 2020;107(3):598–599. doi: 10.1016/j.ijrobp.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrice B., Adrien D., Didier D., et al. Effectiveness and safety of nivolumab in the treatment of lung cancer patients in France: preliminary results from the real-world EVIDENS study. Oncoimmunology. 2020;9(1):1–8. doi: 10.1080/2162402X.2020.1744898. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T., et al. The anti-malarial chloroquine suppresses proliferation and overcomes cisplatin resistance of endometrial cancer cells via autophagy inhibition. Gynecol. Oncol. 2015;137:538–545. doi: 10.1016/j.ygyno.2015.03.053. [DOI] [PubMed] [Google Scholar]
- Guan W., Ni Z., Yu H., Liang W., Ou C., He J., Liu L., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- Gillessen S., Thomas P. Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic. Eur. Urol. 2020;77(6):667–668. doi: 10.1016/j.eururo.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe COVID-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti S., Chiara B., Claudia B., et al. The CoV-2 outbreak: how hematologists could help to fight COVID-19. Pharmacol. Res. 2020;157:1–11. doi: 10.1016/j.phrs.2020.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Melody S., Aitziber B., et al. Trial Watch: immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology. 2018;7:1–16. doi: 10.1080/2162402X.2018.1433982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocho T., Tadashi U., Kenei F., et al. Combination chemotherapy of serine protease inhibitor nafamostat mesilate with oxaliplatin targeting NF-κB activation for pancreatic cancer. Cancer Lett. 2013;333:89–95. doi: 10.1016/j.canlet.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Guo C., Bin L., Huan M. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-17834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Gwendolyn M.J., Mehdi B., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadina M., Bertini R., Mengozzi M., et al. Protective effect of chlorpromazine on endotoxin toxicity and TNF production in glucocorticoid-sensitive and glucocorticoid-resistant models of endotoxic shock. J. Exp. Med. 1991;173:1305–1310. doi: 10.1084/jem.173.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme H., Fernanda F., Gongora A.B.L., et al. SARS-CoV-2 testing for asymptomatic adult cancer patients before initiating systemic treatments: a systematic review. Ecancermedicalscience. 2020;14:1100. doi: 10.3332/ecancer.2020.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L., Jennifer G.W., Valter T., Li-Ching H., Annalisa T., Luis G.P.A., et al. Thoracic cancers international COVID-19 collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. J. Clin. Oncol. 2020;38(18):1–6. [Google Scholar]
- Hirsch T., Rothoeft T., Teig N., Bauer J.W., Pellegrini G., De Rosa L., Scaglione D., Reichelt J., Klausegger A., Kneisz D., Romano O., Secone Seconetti A., Contin R., Enzo E., Jurman I., Carulli S., Jacobsen F., Luecke T., Lehnhardt M., Fischer M., De Luca M. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiel PO, Hamiel U, Boyko V, Graph-Barel C, Reichman B, Lerner-Geva L. Trajectories of HbA1c Levels in Children and Youth with Type 1 Diabetes. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Cong Z., Qiang L., et al. Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. PLoS Pathog. 2020;16(3):1–16. doi: 10.1371/journal.ppat.1008341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar C.A., Cobbold P.H., Church F.C. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson's disease. Brain Sci. 2020;10:1–8. doi: 10.3390/brainsci10050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Hannah K.W., Simon S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounjet J., Roger H., Marco B.S., et al. The anti-malarial drug chloroquine sensitizes oncogenic NOTCH1 driven human T-ALL to γ-secretase inhibition. Oncogene. 2019;38:5457–5468. doi: 10.1038/s41388-019-0802-x. [DOI] [PubMed] [Google Scholar]
- Hall E.A., Jon E.R., Zhihua P., et al. Novel organometallic chloroquine derivative inhibits tumor growth. J. Cell. Biochem. 2018;119:5921–5933. doi: 10.1002/jcb.26787. [DOI] [PubMed] [Google Scholar]
- Haruki K., Hiroaki S., Yuki F., et al. Inhibition of nuclear factor-κB enhances the antitumor effect of tumor necrosis factor-α gene therapy for hepatocellular carcinoma in mice. Surgery. 2013;154:468–478. doi: 10.1016/j.surg.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Hongyan Z., Conghua X., Yihua H. Treatment and outcome of a patient with lung cancer infected with severe acute respiratory syndrome coronavirus-2. Journal of thoracic oncology : official publication of the International Association for the Study of Lung. Cancer. 2020;15(5):e63–e64. doi: 10.1016/j.jtho.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Alina B., Kristen E.P., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with COVID-19 – preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indinia A., Erika R., Michele G., Claudia B., et al. Coronavirus infection and immune system: an insight of COVID-19 in cancer patients. Crit. Rev. Oncol. Hematol. 2020;153:1–8. doi: 10.1016/j.critrevonc.2020.103059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Nobuyuki T., Yoshinori T., et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase R., Koichiro H., Yuki F., et al. Combination chemotherapy of nafamostat mesylate with gemcitabine for gallbladder cancer targeting nuclear factor-κB activation. J. Surg. Res. 2013;184:605–612. doi: 10.1016/j.jss.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Ikezoe T., Yasuko H., Tamotsu T., et al. HIV-1 protease inhibitor, ritonavir: a potent inhibitor of CYP3A4, enhanced the anticancer effects of docetaxel in androgen-independent prostate cancer cells in vitro and in vivo. Cancer Res. 2004;64:7426–7431. doi: 10.1158/0008-5472.CAN-03-2677. [DOI] [PubMed] [Google Scholar]
- Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020;162:1–3. doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Wen O., Melvin L.K.C., Conghua X. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe D.A., Claire L.G., Charles A.M., et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 2018;120:891–900. doi: 10.1017/S000711451800209X. [DOI] [PubMed] [Google Scholar]
- Jarauta V., Paula J., Oscar G., et al. Inhibition of autophagy with chloroquine potentiates carfilzomib-induced apoptosis in myeloma cells in vitro and in vivo. Cancer Lett. 2016;382:1–10. doi: 10.1016/j.canlet.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Jiang P.D., Zhao Y.L., Yang S.Y., et al. Effects of chloroquine diphosphate on proliferation and apoptosis of human leukemic K562 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16(4):768–771. In this issue. [PubMed] [Google Scholar]
- Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Toni K.C., Dimpy P.S., Yu S., Samuel M.R., Donna R.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y., Salazar A.M., Wandersee M.K., Barnard D.L. Prophylactic and therapeutic intranasal administration with an immunomodulator, Hiltonol® (Poly IC:LC), in a lethal SARS-CoV-infected BALB/c mouse model. Antivir. Res. 2017;139:1–12. doi: 10.1016/j.antiviral.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.L., Robin W., Anne R., et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro Oncol. 2010;12:389–400. doi: 10.1093/neuonc/nop046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.Y., Annie J.K., Jeonget J.Y., et al. Combination therapy with a PI3K/mTOR dual inhibitor and chloroquine enhances synergistic apoptotic cell death in Epstein-Barr virus-infected gastric cancer cells. Mol. Cells. 2019;42:448–459. doi: 10.14348/molcells.2019.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya R., Manabu T., Shinya S., et al. HIV protease inhibitor Lopinavir induces apoptosis of primary effusion lymphoma cells via suppression of NF-κB pathway. Cancer Lett. 2014;342:52–59. doi: 10.1016/j.canlet.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20):1–18. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M.F., Powell M., Staedtke V., Bai R.Y., Thomas D.L., Fischer N., et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J Clin. Invest. 2020;130:3345–3347. doi: 10.1172/JCI139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Weijie G., Ruchong C., Wei W., Jianfu L., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Xiang Z., Juan L., Peihua N., Yang B., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M., Paola C., Maria I.M., et al. Treating type 2 diabetes in COVID-19 patients: the potential benefits of injective therapies. Cardiovasc. Diabetol. 2020;19:1–5. doi: 10.1186/s12933-020-01090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y., Jean B.C., Vasileios A., Roland A., Vartika B., Naomi A.C., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, J., Rizvi, H., Preeshagul, I.R., Egger, J.V., Hoyos, D., Bandlamudi, C., et al., 2020. COVID-19 in patients with lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology, 31(10), 1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Liu P., Xiangyu P., Chong C., et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood. 2020;135:826–833. doi: 10.1182/blood.2019003886. [DOI] [PubMed] [Google Scholar]
- Luo J., Hira R., Jacklynn V.E., et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Yang L., Jing Y., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Lucas J.M., Cynthia H., Tom K., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ruiyuan C., Mingyue X., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhter A.J., Ravi P.S., Yang S., et al. Chloroquine promotes apoptosis in melanoma cells by inhibiting BH3 domain-mediated PUMA degradation. J. Invest. Dermatol. 2013;133:2247–2254. doi: 10.1038/jid.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent N., Sophie de B., Guillamo J.S., et al. Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in vivo. Mol. Cancer Ther. 2004;3:129–136. [PubMed] [Google Scholar]
- Mathew D., Josephine R.G., Amy E.B., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;4(369):1–18. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K.K. Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch. Surg. 1970;101:114–121. doi: 10.1001/archsurg.1970.01340260018003. [DOI] [PubMed] [Google Scholar]
- Mauri T., Elena S., Eleonora S., et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit. Care Med. 2020;48(8):1129–1134. doi: 10.1097/CCM.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Sanjay G., Rafi K., Daniel C., Mendel G., Ana A.V., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengyuan D., Dianbo L., Miao L., Fuxiang Z., Guiling L., Zhen C., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moots R.J., Anthony S., William R., et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology. 2017;56:541–549. doi: 10.1093/rheumatology/kew370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziani L., Robert C., Classe M., Da Costa B., Mondini M., Clémenson C., Alfaro A., Mordant P., Ammari S., Le Goffic R., Deutsch E. Low Doses of Radiation Increase the Immunosuppressive Profile of Lung Macrophages During Viral Infection and Pneumonia. International journal of radiation oncology, biology, physics. 2021;110(5):1283–1294. doi: 10.1016/j.ijrobp.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Janne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Manson J.E., Nancy R.C., Min L., et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai S., Shota Mo., Masaki H., et al. Macrolides sensitize EGFR-TKI-induced non-apoptotic cell death via blocking autophagy flux in pancreatic cancer cell lines. Int. J. Oncol. 2016;48:45–54. doi: 10.3892/ijo.2015.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya S., Xiao-Fang C., Seiichiro K., et al. Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int. J. Oncol. 2013;42:1541–1550. doi: 10.3892/ijo.2013.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masud A.M., Ryusho K., Azusa K., et al. Inhibition of autophagy by chloroquine induces apoptosis in primary effusion lymphoma in vitro and in vivo through induction of endoplasmic reticulum stress. Apoptosis. 2016;21:1191–1201. doi: 10.1007/s10495-016-1277-7. [DOI] [PubMed] [Google Scholar]
- Maksimovic I., Marija M., Mirna B., et al. The NO-modified HIV protease inhibitor as a valuable drug for hematological malignancies: role of p70S6K. Leuk. Res. 2015;39:1088–1095. doi: 10.1016/j.leukres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Meier S., Riemer J., Narendran A. The HIV protease inhibitor, nelfinavir, as a novel therapeutic approach for the treatment of refractory pediatric leukemia. Onco Targets Ther. 2017;10:2581–2593. doi: 10.2147/OTT.S136484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura M.D.G., Haddad J.P.A., et al. A new topical treatment protocol for oral hairy leukoplakia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;110(5):611–617. doi: 10.1016/j.tripleo.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Mitroulis I., Ruppova K., Wang B., Chen L.S., Grzybek M., Grinenko T., Eugster A., Troullinaki M., Palladini A., Kourtzelis I., Chatzigeorgiou A., Schlitzer A., Beyer M., Joosten L., Isermann B., Lesche M., Petzold A., Simons K., Henry I., Dahl A., Chavakis T., et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172(1–2):147–161.e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad E.Y. Identifying the optimal dose of ritonavir in the treatment of malignancies. Metab. Brain Dis. 2014;29:533–540. doi: 10.1007/s11011-013-9448-5. [DOI] [PubMed] [Google Scholar]
- Ma J., Xia P., Zhou Y., Liu Z., Zhou X., Wang J., et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelke C., Dziewas R., Minnerup J., Meuth S.G., Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S.F., Solomon H., Emilio C., et al. Lessons learned from SARS-CoV and MERS-CoV: fDA-approved Abelson tyrosine-protein kinase 2 inhibitors may help us combat SARS-CoV-2. Arch. Med. Sci. 2020;16:519–5521. doi: 10.5114/aoms.2020.94504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberaigner W., Christoph E., Karin O., et al. Increased cancer incidence risk in type 2 diabetes mellitus: results from a cohort study in Tyrol/Austria. BMC Public Health. 2014;14:1–9. doi: 10.1186/1471-2458-14-1058. http://www.biomedcentral.com/1471-2458/14/1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana A., Nieto-Jiménez C., Pandiella A., Templeton A.J. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol. Cancer. 2017;16(1):137. doi: 10.1186/s12943-017-0707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Chutitorn K., Tanapat P. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Age and immunity: what is ‘immunosenescence’? Exp. Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuźnicka M., et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing. 2016;13(12):1–12. doi: 10.1186/s12979-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J., Shulman G.I. Mechanistic links between obesity, insulin, and cancer. Trends Cancer. 2020;6:75–78. doi: 10.1016/j.trecan.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Mala R., Neelam C., Mridula P., Ravindra S., et al. Impact of the coronavirus disease 2019 pandemic on cancer care delivery: a single-center retrospective study. Cancer Res. Stat. Treat. 2020;3(4):683–691. [Google Scholar]
- Park E.J., Kyoung-jin M., Kyeong S.C., et al. Chloroquine enhances TRAIL-mediated apoptosis through up-regulation of DR5 by stabilization of mRNA and protein in cancer cells. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep22921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskas S., Emanuela M., Maria S.B., et al. Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Invest. New Drugs. 2019;37:1014–1028. doi: 10.1007/s10637-019-00733-3. [DOI] [PubMed] [Google Scholar]
- Qiao X., Wang X., Shang Y., Li Y., Chen S.Z. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells in-vitro and in-vivo. Cancer Commun. 2018;38:1–13. doi: 10.1186/s40880-018-0309-9. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robilotti E.V., Esther B.N., Peter A.M., Thierry R., Rocio P.J., Marilia B., et al. Determinants of severity in cancer patients with COVID-19 illness. Nat. Med. 2020;26(8):1–5. doi: 10.1101/2020.05.04.20086322. [DOI] [PubMed] [Google Scholar]
- Ripani P., Delp J., Bode K., et al. Thiazolides promote G1 cell cycle arrest in colorectal cancer cells by targeting the mitochondrial respiratory chain. Oncogene. 2020;39:2345–2357. doi: 10.1038/s41388-019-1142-6. [DOI] [PubMed] [Google Scholar]
- Rocca Y.S., María P.R., Juan M.A., et al. Altered phenotype in peripheral blood and tumorassociated NK cells from colorectal cancer patients. Innate Immun. 2013;19:76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- Robilotti E.V., Esther N.B., Mini K., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson A.J., Daniel L.M., Sarah E.H., Adele M.T., Paul R., et al. Trajectories of inflammatory biomarkers over the eighth decade and their associations with immune cell profiles and epigenetic ageing. Clin. Epigenetics. 2018;10(1):1–10. doi: 10.1186/s13148-018-0585-x. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarfo L., Thomas C., Gian M.R., Giulia Q., Marina M., Candida V., et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL campus. Leukemia. 2020;34:2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Lescure F.X., Yazdanpanah Y., et al. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020;178:1–5. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Amy C.S., Sarah R.L., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon β against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem A., Dmitri D., Urmila S., et al. Effect of dual inhibition of apoptosis and autophagy in prostate cancer. Prostate. 2012;72:1374–1381. doi: 10.1002/pros.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumaran M., Amaravadi R.K., Vasilevskaya I.A., O'Dwyer P.J. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin. Cancer Res. 2013;19:2995–3007. doi: 10.1158/1078-0432.CCR-12-1542. [DOI] [PubMed] [Google Scholar]
- Senkowski W., Xiaonan Z., Maria H.O., et al. Three-dimensional cell culture-based screening identifies the anthelmintic drug nitazoxanide as a candidate for treatment of colorectal cancer. Mol. Cancer Ther. 2015;14(6):1504–1516. doi: 10.1158/1535-7163. [DOI] [PubMed] [Google Scholar]
- Srinivas P., Sacha G., Koval C. Antivirals for COVID-19. Clevel. Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc030. [DOI] [PubMed] [Google Scholar]
- Smaldone G.C. Repurposing of gamma interferon via inhalation delivery. Adv. Drug Deliv. Rev. 2018;133:87–92. doi: 10.1016/j.addr.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Shaw M.M., Gürr W.K., Watts P.A., Littler E., Field H.J. Ganciclovir and penciclovir, but not acyclovir, induce apoptosis in herpes simplex virus thymidine kinase-transformed baby hamster kidney cells. Antivir. Chem. Chemother. 2001;12(3):175–186. doi: 10.1177/095632020101200305. [DOI] [PubMed] [Google Scholar]
- Timothy P.H., Gerald A.E., Christopher M.B. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat. Rev. Clin. Oncol. 2020;17(5):268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehan H.S., Vijay S.K. Slit-lamp infection protector cover for COVID-19. Cornea. 2021;40(3):1–2. doi: 10.1097/ICO.0000000000002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani D., Antonio M., Giuseppe C. The experience on corona virus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region. Eur. J. Cancer. 2020;132:199–206. doi: 10.1016/j.ejca.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon S.P., Jorge J.C., Alan P.S., et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulasiraman P., Kelbie K., Kathleen M., et al. Neuraminidase 1 regulates proliferation, apoptosis and the expression of Cadherins in mammary carcinoma cells. Mol. Cell. Biochem. 2019;462:207–215. doi: 10.1007/s11010-019-03623-7. [DOI] [PubMed] [Google Scholar]
- Thaiss C.A., Maayan L., Inna G., et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A., et al. Therapeutic management of patients with COVID-19: a systematic review. Infect. Prev. Pract. 2020;2 doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C., Paul H., Virendar K., Larry D., James G. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Vankadari N., Wilce J.A. Emerging Wuhan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpin F., Casaos J., Sesen J., et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene. 2017;36:3037–3047. doi: 10.1038/onc.2016.457. [DOI] [PubMed] [Google Scholar]
- Wan W., Yanli X., Ruqin G., Roujian L., Kai H., Guizhen Wu, Wenjie T. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Jian S., Rachel G., Ralph S.B., Fang L. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7):e00127. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T.W., Arnesen H., Seljeflot I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013;62:1008–1013. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Wang M., Ruiyuan C., Leike Z., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Linqing L., Yucheng Z., et al. Hydroxychloroquine enhances the antitumor effects of BC001 in gastric cancer. Int. J. Oncol. 2019;55:405–414. doi: 10.3892/ijo.2019.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen S., Zhendong L., et al. Nitazoxanide, an antiprotozoal drug, inhibits late-stage autophagy and promotes ING1-induced cell cycle arrest in glioblastoma. Cell Death Dis. 2018;9:1–15. doi: 10.1038/s41419-018-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ruiyuan C., Huanyu Z., et al. The anti-influenza virus drug arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:1–5. doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S.U.D., Gautam S, P; Qadrie Z.L. An Overview on COVID-19: clinical features, treatment and prevention. Coronaviruses. 2020;2(3):291–295. doi: 10.2174/2666796701999200818145609. [DOI] [Google Scholar]
- Wani S.U.D., Khan N.A., Thakur G., et al. Utilization of artificial intelligence in disease prevention: diagnosis, treatment, and implications for the healthcare workforce. Healthcare. 2022;10(4):608. doi: 10.3390/healthcare10040608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S.U.D., Gautam S.P., Ali M. Impact of SARS-CoV-2 mutations on global travel and the increasing number of Re-infections: a risk-assessment perspective. The Open COVID Journal. 2021;1:196–204. doi: 10.2174/2666958702101010196. [DOI] [Google Scholar]
- Xu R., Zhu J. Comment on: pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: metaanalysis of 153 cohorts with 15,713 patients: meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer. 2019;127:167. doi: 10.1016/j.lungcan.2018.09.018. [DOI] [PubMed] [Google Scholar]
- Xu X., Mingfeng H., Tiantian L., Wei S., Dongsheng W., Binqing F., et al. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Koichi A., Shuzhao L., et al. Autophagy is essential for effector CD8+ T cell survival and memory formation. Nat. Immunol. 2014;15(12):1152–1161. doi: 10.1038/ni.3025. 15, 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi C., Ling W., Jie Y., Hui Y., et al. Inhibition of eukaryotic translation initiation factor 4E is effective against chemo-resistance in colon and cervical cancer. Biochem. Biophys. Res. Commun. 2018;503:2286–2292. doi: 10.1016/j.bbrc.2018.06.150. [DOI] [PubMed] [Google Scholar]
- Xiaonan Z., Yun T., Yun L., Gang L., Feng L., Zhigang Y. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- You B., Ravaud A., Canivet A., Ganem G., Giraud P., Guimbaud R., et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21(5):619–621. doi: 10.1016/S1470-2045(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarza R., Mateo B., Diana P., Flora L.L., Diego J.C., Alicia C.L. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsana N., Michael R.K. The impact of COVID-19 on cancer risk and treatment. Cell Mol. Bioeng. 2020;13(4):285–291. doi: 10.1007/s12195-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Rajeshkumar N.V., Xiaoxu W., et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–913. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziad A.M., Stanley P., Maria D., Van K., Alimuddin Z. Middle East respiratory syndrome. Lancet. 2020;395(10229):1063–1077. doi: 10.1016/S0140-6736(19)33221-0. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO)., 2020. Estimating mortality from COVID-19. Available from: https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19.WHO/2019-nCoV/Sci_Brief/Mortality/2020.1 [Last accessed on October 21, 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data of this paper will be available on request.