Abstract
Calorie restriction (CR) is a potent modulator of longevity in multiple species. A growing body of evidence shows that sustained periods of CR without malnutrition improves risk factors involved in the pathophysiology of type 2 diabetes, cardiovascular diseases, cancer, and neurological disorders in humans. Innovative dietary strategies such as intermittent fasting and protein restriction have recently emerged as alternative approaches that improve markers of aging. Some of these newer strategies might provide benefits for healthy aging with little to no CR and therefore, compared to traditional CR, may be easier to follow. Further to providing an update of CR studies in humans, the present narrative review appraises the influence of these contemporary dietary strategies on mechanisms posited to drive CR-induced longevity in humans, including those involving energy metabolism, oxidative damage, inflammation, glucose homeostasis, and functional changes in the neuroendocrine systems. The review also discusses the utilization of these diets for populations in the current obesogenic environment, and comments on whether current research can inform an optimal diet that attenuates aging, can be easily followed, and promises to improve longevity in humans.
Keywords: Eating patterns, Intermittent fasting, Protein restriction, 5:2 diet, Healthspan, Longevity
1. Introduction
Calorie restriction (CR) without malnutrition is the most effective non-pharmacological intervention that enhances longevity and healthspan in numerous nonhuman species (Weindruch, 1996). Evidence showing the positive benefits of CR in humans has been accumulating, with many clinical trials showing weight loss and an improvement in physiological markers after short and prolonged bouts of CR (Most et al., 2017; Redman and Ravussin, 2011). However, continuous daily CR or traditional CR has a poor long-term success rate due to compromised adherence (Barte et al., 2010; Scheen, 2008), and ample availability of energy-dense foods and beverages within modern societies thwart an individual’s ability to sustain traditional CR (Swinburn and Egger, 2002). Accordingly, with the current rapidity of population aging, innovative methods are required to optimize healthspan (Dzau et al., 2019).
Novel dietary strategies have acquired prominence in recent times as viable alternatives to traditional CR (Fig. 1). Intriguingly, these approaches may stimulate positive adaptive processes without energy restriction and weight loss, challenging the dogmatic viewpoint that CR is a prerequisite of longevity-promoting diets. In view of encouraging results from animal studies, testing the efficacy of these newer dietary strategies in humans has become a contemporary research area for scientists in the aging field (Anton et al., 2018; Fontana and Partridge, 2015). To date, the most acclaimed of these approaches is intermittent fasting (IF), a dietary regimen that adjusts nutrient timings or the frequency of eating to enforce periodic bouts of fasting or extreme energy restriction, generally for 12 h or longer (Anton et al., 2018; Patterson and Sears, 2017). Approaches under this umbrella term have been theorized to increase physiological function and slow disease processes through its prolonged periods of daily energy restriction (Anton et al., 2018). Other emerging strategies manipulate macronutrient intake to stimulate improvements in health. Of these, protein restriction has received the most attention, with some arguing that a decrease in protein consumption is key to the CR-induced slowing of mammalian aging (Fontana and Partridge, 2015).
Fig. 1.
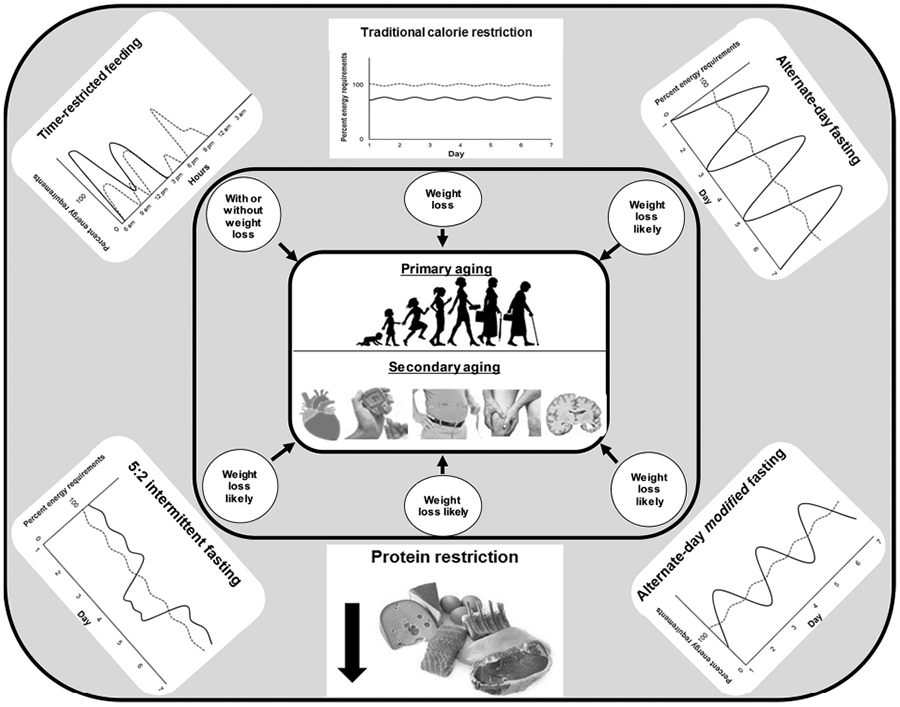
Dietary strategies that have been shown to improve markers of aging in humans. Traditional calorie restriction exerts positive aging-linked benefits largely through weight loss, but the role of weight loss on aging markers during novel dietary regimens remains equivocal. In traditional calorie restriction and intermittent fasting depictions, solid lines present typical eating patterns entailed by dietary strategy, while dashed lines represent conventional westernized eating patterns.
Research into the potential of CR-stimulated longevity has been spearheaded by Mark Mattson (Mattson, 2005; Mattson and Wan, 2005). Work expertly led by Professor Mattson has been ever-evolving and produced pioneering insights into the impact of novel dietary strategies on longevity and healthspan in animal models and humans (Anton et al., 2018; Harvie et al., 2013, 2011; Mattson et al., 2017). In this Special Issue of Ageing Research Reviews dedicated to Mark Mattson, we appraise evidence that has examined the influence of these novel dietary regimens on age-related processes in humans. Following an overview of the CR literature in humans, we will evaluate human studies that have tested the influence of IF or protein restriction on markers linked to aging and disease processes. We will also discuss issues of adherence concerning these dietary approaches, offer important directions for future research, and look to ascertain if the current evidence base pertaining to these regimens indicate novel strategies offer any superiority relative to traditional CR in humans.
2. Calorie restriction in humans
The benefits yielded by CR in humans are well established. Populations who voluntarily or involuntarily adopt CR exhibit widespread improvements in physiological functioning. This includes a population residing on a Japanese Island called Okinawa (Suzuki et al., 2001; Willcox et al., 2007; Willcox and Willcox, 2014), researchers enclosed within Biosphere 2 (Walford, 1999; Walford et al., 2002, 1992), and members of the Calorie Restriction Society International who self-impose CR, believing this will enhance their lifespan (Fontana et al., 2004; Holloszy and Fontana, 2007; Meyer et al., 2006). In a clinical context, CR interventions in humans for short periods of ≤ 10 weeks decrease resting metabolic rate (Velthuis-te Wierik et al., 1995), blood pressure (Velthuis-te Wierik et al., 1994) and glucose concentrations (Loft et al., 1995). These findings were bolstered in 2006 and 2007 when three studies were published reporting primary results from the pilot trials of the Comprehensive Assessment of Long-term Effects of Reducing Energy (CALERIE) consortium (Das et al., 2007; Heilbronn et al., 2006; Racette et al., 2006). These were the first controlled clinical trials that aimed to assess the efficacy, safety and feasibility of 6–12 month of CR without malnutrition in healthy humans without obesity. Overall, the CALERIE phase 1 trials demonstrated that modest CR of 12%–18% is feasible and does not compromise quality of life (Redman and Ravussin, 2011). Moreover, CR induced age-associated benefits, including metabolic adaptation, a reduction in markers of energy metabolism, and improvements in many risk factors for cardiometabolic disease (Das et al., 2007; Heilbronn et al., 2006; Racette et al., 2006).
The findings from CALERIE phase 1 provided promising preliminary data and informed the safety monitoring for a longer, properly powered multi-site trial. In this regard, the CALERIE phase 2 trial aimed to simulate animal studies, where CR is sustained for longer durations relative to an organisms lifespan, and establish whether CR-induced alterations become stable beyond the initial period of weight loss (Rochon et al., 2011). Briefly, 220 healthy participants who were aged 21–50 and did not have obesity were studied for two years while either consuming a 25% CR diet (CR Group, n = 145) or their usual diet ad libitum (Control Group, n = 75). Two participants in CR group dropped before the study’s initiation; therefore, 143 and 75 participants in the CR and ad libitum groups, respectively, started the study. An intensive dietary and behavioral regimen was administered to the CR group, with the aim to induce an immediate 25% CR that was maintained for the duration of the two year trial (Rochon et al., 2011; Stewart et al., 2013). The ad libitum group, on the other hand, were instructed to maintain their usual diet, eating as much or as little as they wished. Impressively, adherence to the intervention throughout the trial was determined mainly via two objective methods: the energy intake-balance method, which calculates CR via total energy expenditure and changes in body composition (Racette et al., 2012; Wong et al., 2014), and estimated weight loss trajectories derived from weight loss algorithms (Pieper et al., 2011). Calculations from the intake-balance method indicated that the CR group, over two years, averaged 11.7% CR, approximately 50% of the targeted 25% CR. There was no evidence of CR in the control group (Ravussin et al., 2015).
Remarkable resources were used in CALERIE phase 2 and the outcome measures were exhaustive (Rochon et al., 2011). Chiefly, CALERIE phase 2 showed that CR produced reductions in mass-specific resting metabolic rate and total energy expenditure, along with an accompanying decrease in triiodothyronine, leptin and oxidative stress markers (Il’yasova et al., 2018; Meydani et al., 2016; Ravussin et al., 2015; Redman et al., 2018). In tandem, these results suggest that CR in humans could improve longevity and decelerate primary aging through the rate of living and free radical theories of aging (Redman et al., 2018). That is, CR lowers energy expenditure and, in turn, decreases the production of reactive oxygen species and damage to DNA, lipids and proteins essential for normal cellular function (Redman et al., 2018). The CR group also had an attenuation of biological aging when assessed in the context of two biomarker algorithms, and this notably was observed independent of weight loss (Belsky et al., 2018). Additionally as a result of the 2-years of CR, the young and healthy participants experienced significant improvements in body composition (Das et al., 2017; Most et al., 2018), cardiometabolic risk markers (Kraus et ah, 2019), markers of inflammation (Meydani et al., 2016) and aerobic fitness (Racette et al., 2017). Collectively, these measures imply that CR improves numerous markers of disease risk and thus supports the notion that CR slows secondary aging. Importantly, CR also did not induce any negative impact on psychological and behavioral factors, such as quality of life (Martin et al., 2016), eating behaviors and appetite (Dorling et al., 2019), and memory (Leclerc et al., 2019), suggesting the CALERIE intervention was tolerable, safe and did not compromise cognition (Romashkan et al., 2016). There were, nevertheless, no CR-engendered changes in insulin-like growth factor (IGF)-l (Fontana et al., 2016b), and the CR group displayed a loss of bone mass, which could represent a drawback of prolonged CR for healthspan-enhancing purposes (Villareal et al., 2016).
On balance, CALERIE phase 2 displayed that sustained modest CR in young healthy individuals without obesity is feasible, can improve mechanistic markers linked to primary and secondary aging, and does not compromise general well-being. Such conclusions are boosted because of the control group, the increased duration of the intervention, the extensive collection of endpoints, and the greater objectivity of adherence measures relative to other human aging studies. These strengths qualify the CALERIE phase 2 trial as the current benchmark nutritional intervention to which other human studies assessing dietary strategies and age-related processes can be evaluated. With this in mind, we will hereafter appraise human studies that have investigated the effect of novel dietary strategies on age-related outcomes, providing implications and avenues for further scientific inquiry throughout.
3. Intermittent fasting
A dietary strategy that has been used as an alternative to traditional CR is IF. Various forms of IF have been proposed, but generally they involve the severe or complete restriction of energy intake for periods of 12 h or more (Anton et al., 2018; Longo and Panda, 2016). These prolonged periods of energy restriction are posited to elevate the metabolism of endogenous substrates, such as ketones, non-esterified fatty acids, and glycerol, and in turn stimulate adaptive processes that improve body composition and physiological function (Anton et al., 2018). At a cellular level, the improvements in physiological functioning seem to be mediated through a series of integrated signalling pathways that lower inflammation and oxidative stress and ultimately improve health and disease resistance. More specifically, fasting initiates a rise in the AMP:ATP ratio and AMP kinase activation, which leads to an inhibition of mammalian target of rapamycin (mTOR) and an upregulation of cell autophagy (Anton et al., 2018; de Cabo and Mattson, 2019). There is also an engagement of transcription factors such as forkhead box Os (FOXOs) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), triggering the expression of genes that are implicated in stress resistance, improved blood glucose regulation, mitochondrial biogenesis and cell survival (Anton et al., 2018; de Cabo and Mattson, 2019).
As a result of these positive molecular adaptations to fasting, IF has recently acquired significant attention, with many scientists, dietitians and media sources endorsing it as a method to enhance healthspan and longevity, particularly for those who struggle to adhere to the rigours of traditional CR. A host of IF-related strategies have been conceived, but broadly speaking, these can be categorized into four approaches: alternate-day fasting (ADF), alternate-day modified fasting (ADMF), 5:2 IF and time-restricted feeding (TRF).
3.1. Alternate-day fasting
A selection of IF-focussed studies have enforced ADF, an eating pattern which alternates days of ad libitum eating with days that virtually prohibit the consumption of calories. Halberg et al. (2005) and Soeters et al. (2009) prescribed a two-week ADF intervention to healthy males without obesity that consisted of a 20 -h fast, except for water, from 22.00 to 18.00 on alternate days. Of note, both studies were able to maintain body weight and fat mass via refeeding days, with the former instructing participants to “eat sufficient quantities of food” (Halberg et al., 2005) and the latter prescribing food intake based on energy requirements (Soeters et al., 2009). Despite finding no alterations in adiponectin, tumor necrosis factor-α and interleukin 6, Halberg and colleagues (2005) reported an increase in insulin sensitivity and adipose tissue lipolysis after ADF. Soeters and colleagues did not replicate these variations in insulin sensitivity, and also reported that adiponectin and thyroid hormone concentrations before and after the intervention were alike (Soeters et al., 2009). However, they did show that resting energy expenditure was decreased after ADF, implying a degree of metabolic adaption occurred, given their participants body weight and composition were similar after ADF (Soeters et al., 2009). Thus, despite differences in cardiometabolic outcomes, which may owe to disparities in the scientific rigour of refeeding periods, the work of Halberg et al. (2005) and Soeters et al. (2009) tentatively demonstrate that ADF could improve metabolic health and biomarkers of primary aging.
Heilbronn et al. (2005a, 2005b) applied another variation of ADF in 16 participants (8 males and 8 females) without obesity for three weeks. In this study, energy intake was restricted from midnight to the subsequent midnight on alternating days, and participants were instructed to double their food intake on ‘feast days’ to sustain energy balance. In contrast to the work of Halberg et al (2005) and Soeters et al. (2009), participants in this 3-week study lost weight, namely a mean 0.6 kg and 0.8 kg decrease in fat-free mass and fat mass, respectively (Heilbronn et al., 2005b). This implies that participants were unable to fully compensate for the fasting days on ‘feast days’, although this cannot be determined as energy intake on ‘feast days’ was not monitored. Regardless, negligible changes in resting metabolic rate, core temperature, blood pressure, blood lipids, and metabolic gene expression were seen (Heilbronn et al., 2005b, 2005a). Additionally, in anecdotal observations, Heilbronn and colleagues indicated that participants reported irritability on fasting days, questioning the sustainability of ADF, yet reported hunger was unaffected and reported fullness increased (Heilbronn et al., 2005b, 2005a). These divergent observations between indices of adherence illustrate the necessity for attrition and compliance measures in longer human ADF studies.
The effects of more sustained ADF were recently investigated by one study which prospectively compared a cohort of 60 controls who consumed a conventional western diet with a group of 30 individuals who reported completing six months or more of ADF (Stekovic et al., 2019; Tripolt et al., 2018). Analysis of food-frequency questionnaire data suggested that energy intake was ~29% lower in the group who practiced ADF (Stekovic et al., 2019), again indicating that people do not fully compensate for a day of complete energy restriction. Notwithstanding the apparently lower energy intake, analysis of body composition and blood biomarkers suggested ADF is a safe and feasible dietary regimen that can be adopted and followed by healthy participants (Stekovic et al., 2019). With regards to physiological endpoints, Stekovic et al. (2019) examined the proteome and metabolome of individuals categorized as long-term adopters of ADF. This analysis showed a significant rise in circulating lipids and a decrease in amino acids such as methionine on fasting days. These data may provide insights into the mechanisms underlying the beneficial impact of ADF in humans, since systemically low levels of methionine and other amino acids can extend lifespan in model organisms through the reduced activity of the mTOR pathway and an upregulation in cell autophagy (de Cabo and Mattson, 2019; Orentreich et al., 1993; Richie et al., 1994). Stekovic et al. (2019) also reported that the long-term ADF group displayed lower intracellular adhesion molecule-1, an improved blood lipid profile, and suppressed triiodothyronine versus the control group, despite similar age, weight and physical activity levels between groups. These results were partially corroborated by a four-week intervention where the 60 participants in the control group were randomized in a 1:1 ratio to either a control or ADF group. Here, a reduction in triiodothyronine and blood pressure were observed, but inflammatory markers were unaltered, and similar to Heilbronn and colleagues (Heilbronn et al., 2005b), ADF had no influence on resting energy expenditure and blood lipids (Stekovic et al., 2019). Furthermore, despite being reported in their methodology manuscript (Tripolt et al., 2018), data from an oral glucose tolerance test, which would have provided measures in insulin sensitivity between groups, were not delineated.
Though these studies highlight the promise of ADF, they cannot discern the influence of this strategy relative to traditional methods without an additional arm adopting traditional CR studied in parallel. Catenacci et al. (2016) were the first to test ADF relative to a CR group within the same study, to our understanding. In this study, 26 participants with obesity were randomized to either 8 weeks of CR or complete ADF. The CR group were provided foods that aimed to yield a 400 kcal/day energy deficit (~14% CR), while the ADF paradigm enforced alternate days of complete fasting and refeeding. In spite of similar responses in study attrition, leptin, fasting insulin and blood lipids, the results of Catenacci et al. (2016) revealed how humans may respond differently to CR and ADF. Only the CR group showed a decrease in mass-adjusted resting metabolic rate, whereas only ADF provoked reductions in fasting glucose concentrations, and there was a tendency for greater weight loss during ADF relative to CR (Catenacci et al., 2016). The ADF group did nonetheless show lower fat mass, resting metabolic rate and fasting insulin at baseline, which could confound between-group comparisons (Catenacci et al., 2016). In addition, although the macronutrient composition of diets was similar, the mean energy deficit was 376 kcals/day lower in the ADF group than the CR group. It is therefore plausible that the observed between-group differences are explained in whole or part by the differences in energy restriction, making it extremely difficult to pinpoint the influence of ADF independent of energy restriction.
One recent study attempted to establish the effects of ADF compared to CR in women with obesity over 8 weeks (Hutchison et al., 2019a). In this study, 88 participants were randomized in a 1:2:2:2 ratio to a control group, a traditional CR group that aimed to consume 70% of energy needs, or an ADF group at either 70% or 100% of energy requirements. In the ADF arms, on alternate days, participants consumed only a 20 kcal broth for lunch and dinner during a 24 h period that occurred after 08.00. Unsurprisingly, self-reported hunger scores were higher in the ADF groups on fasting days at six weeks of the trial, but no differences between groups were observed on feeding days, indicating that the increased energy intake on fed days could offset hunger on energy restriction days and assist compliance (Hutchison et al., 2019a). With regards to other study outcomes, 8 weeks of ADF at 100% of energy requirements reduced weight compared to the control condition, but no changes were observed with cardiometabolic endpoints. By contrast, ADF at 70% of energy needs resulted in greater improvements in weight, fasting glucose, insulin resistance, total cholesterol, low-density lipoprotein cholesterol and triglycerides compared to CR at 70% of energy needs (Hutchison et al., 2019a). However, the assessment of food records indicated that ADF elicited greater energy deficits than were prescribed (~9% during ADF at 70% energy needs and ~2% during ADF at 100% energy needs), mainly because of substandard energy consumption on feeding days (Hutchison et al., 2019a). As such, akin to the conclusions inferred from Catenacci et al. (2016), it is difficult to suggest that variations between ADF and CR occurred solely because of differences in fasting periods, as calorie intake was also affected by ADF paradigms.
3.2. Alternate-day modified fasting
Given the possible difficulties with compliance and recruitment during ADF studies, some have examined IF strategies that permit the consumption of some calories during fasting periods. Broadly, this is defined as alternate-day modified fasting (ADMF). Here, on alternating days, eating is severely restricted on one day, typically to ≤ 25% of habitual energy needs, and then a day of ad libitum eating is permitted. One of the first studies that examined ADMF on aging markers in humans instructed participants with asthma to consume 320–380 kcals/day on alternate days for 8 weeks, with ad libitum eating allowed in between (Johnson et al., 2007). Although this study only examined ten participants, nine of which adhered to the protocol, ADMF led to an 8% reduction in weight along with significant improvements in leptin, tumor necrosis factor-α and markers of oxidative stress; that is, 8-isoprostane, nitrotyrosine, protein carbonyls, and 4-hydroxynonenal adducts (Johnson et al., 2007). Johnson and colleagues also found no change in hunger on the ad libitum days, in spite of a decrease in ratings during days of low caloric intake.
Additional work has subsequently shown that ADMF is tolerable and can decrease plasma insulin of individuals who are healthy and lean (Wegman et al., 2015). Likewise, work from Varady and colleagues has assessed the effects of ADMF in humans with obesity who were otherwise healthy. Their studies have shown that 2–3 months of ADMF can lower adiposity and improve traditional cardiovascular disease markers (Bhutani et al., 2013; Varady et al., 2013, 2009), irrespective of macronutrient composition (Klempel et al., 2013) and meal timings (Hoddy et al., 2014) on energy restriction days. They also observed reductions in resting metabolic rate (Hoddy et al., 2014), leptin (Bhutani et al., 2013; Varady et al., 2013) and inflammatory markers (Bhutani et al., 2013; Varady et al., 2013). Furthermore, their work suggests participants swiftly adopt the energy intake requirements of ADMF and overall display no changes in appetite (Klempel et al., 2010), appetite-related hormones (Hoddy et al., 2016), and disordered eating behaviors (Hoddy et al., 2015).
A drawback of the present ADMF studies is that they are relatively short in comparison to traditional CR studies, in particular the two-year CALERIE phase 2 trial. Hence, the Varady lab compared the effects of ADMF, traditional CR and ad libitum eating over 12 months in 100 participants with obesity (Gabel et al., 2019; Trepanowski et al., 2018, 2017). During the first six months, a 25% energy deficit was targeted in the ADMF and CR groups, with both groups consuming equal proportions of macronutrients. The ADMF group were instructed to consume 25% of their energy requirements on “fast days” between 12.00–14.00 hours and 125% of energy on alternating “feast days”, whereas the CR group were instructed to consume 75% of their energy needs over three meals every day. Crucially, Trepanoswski and colleagues ensured that the ADMF (21%) and CR (24%) groups achieved similar energy deficits, although food records suggested participants in the ADMF group ate more on “fast days” and less on “feast days” than was prescribed (Trepanowski et al., 2018, 2017). Furthermore, at odds with CALERIE phase 2, which had an attrition rate of 9% at month 12 (Ravussin et al., 2015), attrition was relatively high (38%) in the ADMF group, with “difficulties adhering to diet” cited as the predominant reason for the high drop-out. There were largely no between-group differences in inflammatory markers, adipokines and blood pressure after six months (Trepanowski et al., 2018, 2017), yet in an analysis comprising the participants in the ADMF or CR groups who completed the first six months of the trial, insulin resistance decreased to a greater extent in the ADMF group relative to the CR group (Trepanowski et al., 2018). Similarly, another secondary analysis from this study showed that ADMF was superior to CR in decreasing insulin resistance in a subgroup of participants with homeostatic model assessment of insulin resistance values greater than 2.73 (Gabel et al., 2019). No differences between intervention groups were observed, however, in the intention-to-treat analysis of glucose and insulin resistance (Trepanowski et al., 2017). Further ambiguity surrounding these findings arises as outcome measures after baseline in the ADMF group were taken in the morning following a “feast day”, meaning it is plausible that energy intake was greater in the ADMF group compared to the CR group in hours preceding these measurements. Thus, further work is needed to establish the chronic effect of ADMF on insulin resistance relative to CR. Following the second six months of the study, where participants were instructed to maintain their dietary patterns but meet their energy needs in lieu of creating an energy deficit, similar between-group responses were seen with outcome measures (Trepanowski et al., 2017).
Although these results demonstrate that ADMF offers little or no benefit to markers of aging compared to traditional CR, a high degree of inter-individual variability existed within the ADMF group, with weight change ranging from 3.7% to −17.5% (Kroeger et al., 2018). To illuminate the potential mechanisms of this variability, Kroeger et al. (2018) investigated the changes in eating behaviors and dietary patterns during the study. This secondary analysis showed that those in the ADMF group who lost more than 5% body weight displayed no differences in dietary restraint or self-efficacy compared to those who lost less than 5% body weight (Kroeger et al., 2018). There were also no differences in appetite ratings after 12 months between those who lost more or less than 5% of their body weight. Nevertheless, individuals who lost more weight simply maintained superior adherence to the prescribed energy intake on the “fast days” than those who lost less weight (Kroeger et al., 2018), suggesting that, like traditional CR interventions, increased compliance leads to improved weight outcomes. Although the study did not report whether physiological aging markers were different between those who lost more or less than 5% body weight, it is tempting to speculate that greater adherence to the ADMF regimen would have led to improvements in markers of aging.
3.3. 5:2 intermittent fasting
Of the novel dietary approaches receiving most attention, many can be broadly categorized as 5:2 IF, which permits ad libitum eating 5 days per week and severe energy restriction or a complete fast on the other 2 days per week (Anton et al., 2018). Fasting days can be performed on either consecutive or non-consecutive days; thus, 5:2 IF enables dieters to adopt more flexible bouts of fasting, making it attractive for many individuals with inconsistent work and social commitments. Given its prominence in western dieting culture, the 5:2 IF strategy has been the subject of several studies. In one of the first, Harvie et al. (2011) randomized 107 premenopausal women with overweight or obesity to either six months of traditional CR or 5:2 IF. Both strategies aimed to restrict energy intake by 25% from baseline energy requirements, with participants in the latter group instructed to do so via two consecutive days of 75% CR followed by five days where energy intake met energy requirements. The diet strategies did not differ in terms of attrition, adherence, energy expenditure, markers of energy metabolism, inflammatory markers, and quality of life, but 5:2 IF yielded greater improvements in fasting insulin and insulin resistance compared to CR (Harvie et al., 2011). More recently, the same group reported supportive results in pre- and post-menopausal women during 5:2 IF with varying macronutrient compositions (Harvie et al., 2013). Post-trial assessments of food records did nonetheless suggest that participants in 5:2 IF groups lowered their total energy intake more compared to participants randomized to traditional CR (Harvie et al., 2013, 2011). Similarly to ADF and ADMF, 5:2 IF may be beneficial in provoking an energy deficit relative to traditional CR, though ostensible differences in energy intake make it challenging to elucidate the effect of fasting per se on endpoints.
Assessing the independent importance of the fasting period is appropriate when alterations in energy balance are equal between comparative groups. Antoni et al. (2018) attempted to control for changes in energy restriction and body weight by comparing individuals in either a CR or 5:2 IF group following equivalent weight loss. They specifically enrolled 27 individuals with overweight or obesity to either a CR group or a 5:2 IF group and assessed study endpoints at baseline and after 5% weight loss. In the 5:2 IF arm, Antoni and colleagues administered food packs that comprised 25% of energy needs and instructed participants to consume these for two consecutive days, with habitual energy intake consumed on the remaining five days. The CR group were, by contrast, informed to decrease their daily energy intake by 600 kcal. In accord with Harvie et al. (2011), there was a larger decrease in energy intake in the 5:2 IF group than the CR group, yet the time frame needed to reach 5% weight loss was similar between conditions (Antoni et al., 2018). Also in agreement with cited work (Harvie et al., 2011), 5:2 IF improved some cardiometabolic markers relative to CR, with larger reductions observed for postprandial triglycerides and C-reactive protein (Antoni et al., 2018). There was additionally a tendency for a reduction in resting energy expenditure normalized for fat-free mass in the 5:2 IF group compared to the CR group (−7.3 kJ/kg) (Antoni et al., 2018).
These promising findings notwithstanding, others have found little or no added benefit of 5:2 IF compared to daily CR. One study enrolled 112 participants with obesity to either one year of traditional CR or 5:2 IF, with the latter enforcing a 400/600 kcal (female/male) decrease in energy intake on two non-consecutive days and food intake “as usual” for the remainder of the week (Sundfør et al., 2018). In spite of higher perceptions of hunger during 5:2 IF, dietary records illustrated that both groups attained a 26–28% decrement in energy intake, had no drop-outs, and showed similar improvements in anthropometric and cardiometabolic measures at month 6 and month 12 (Sundfør et al., 2018). Analogous findings have been found in other studies that have assessed 5:2 IF in relation to traditional CR (Carter et al., 2016; Conley et al., 2018; Headland et al., 2018), with no between-group changes in quality of life (Carter et al., 2016) and attrition (Headland et al., 2018). Collectively, these studies instructed or provided participants with the option to conduct energy restriction days non-consecutively, contrasting previously cited work where fasting days were consecutive and positive changes were observed (Antoni et al., 2018; Harvie et al., 2011). These results may suggest that energy restriction periods beyond a day may be pivotal in the success of 5:2 IF relative to traditional CR. Speculatively, extension of the fasting period could elevate the reliance on free fatty acids and increase ketone bodies, ultimately upregulating transcription factors like peroxisome proliferator-activated receptor α and improving metabolic health (Anton et al., 2018). However, variations of 5:2 IF with energy restriction for either two (Pinto et al., 2019) or four (Ash et al., 2003) consecutive days has induced largely similar anthropometric and physiological changes versus traditional CR in other studies. As a result, the current body of literature questions the superiority of 5:2 IF compared to traditional CR in improving markers of aging, underscoring the need for further studies.
3.4. Time-restricted feeding
Another approach that has received recent notoriety is time-restricted feeding (TRF). This eating strategy restricts food intake to a feeding window that typically lasts less than 12 h per day during the waking phase (Anton et al., 2018). Work involving Mark Mattson was among the first to test the effect of TRF in humans (Carlson et al., 2007; Stote et al., 2007). They recruited 15 healthy participants who were neither overweight nor obese and administered two eight-week dietary regimens separated by 11 weeks in a cross-over design. One regimen necessitated that participants consumed daily energy in one meal from 17.00 to 21.00, whereas participants consumed daily energy over three meals spread throughout the day in the other. Food provision was administered to participants and was equal between treatments, and complete compliance to the protocol was gauged via random fasting glucose and triglyceride examinations. Body weight and fat mass were decreased by 2.1% and 13.0%, respectively, after TRF, yet no differences between groups were observed in body temperature, leptin or inflammatory markers (Stote et al., 2007). In fact, total cholesterol, blood pressure, liver transaminases and glucose were elevated in response to TRF (Carlson et al., 2007; Stote et al., 2007). Additionally, there was an increase in subjective appetite when the TRF intervention was compared to the regimen where intake was spread over three meals (Stote et al., 2007).
Other studies have implemented TRF during resistance training programmes in males over eight weeks (Moro et al., 2016; Tinsley et al., 2017). Both studies incorporated a control group who were instructed to consume a conventional eating pattern. However, Moro et al. (2016) allowed daily energy consumption over a seven-hour window from 13.00 to 20.00 in their TRF intervention, whilst the TRF intervention of Tinsley et al. (2017) required daily energy intake to be consumed during a four-hour period from 16.00 to 24.00. The latter study found TRF did not influence weight or fat mass change (Tinsley et al., 2017), but Moro et al. (2016) reported a larger decrease in these outcomes with their TRF intervention versus the control condition. While they found no changes in resting energy expenditure, Moro et al. (2016) also reported that TRF increased adiponectin and decreased triiodothyronine, leptin, IGF-1 and triglycerides compared to control.
Variations in protocols and study samples are clearly implicated in the mixed findings from the TRF studies reported thus far, and the implementation of TRF during resistance training may limit generalizability of some findings. However, the trial by Sutton et al. (2018) hypothesized that TRF during earlier periods of the waking phase may provoke discernible benefits via circadian rhythms. Specifically, they posited that the greater insulin sensitivity and thermic effect of food during the morning periods may provide a more suitable metabolic state for food consumption, enhancing metabolic endpoints (Sutton et al., 2018). They consequently tested this hypothesis in males with pre-diabetes who completed two, five-week eating periods in a crossover design separated by a seven-week washout. In the TRF arm, all calories were consumed between breakfast and lunch over a six-hour period, whereas in the control arm, calories were consumed over a 12 -h period. Compared to eating over 12 h, TRF for 6 h in the morning led to a 19% decrease in mean and peak insulin after an oral glucose tolerance test. Nonetheless, these results may be confounded by differences in the post-intervention measurement periods, given the extended fasting period preceding the oral glucose tolerance test in the TRF arm. With respect to secondary outcome measures, TRF improved oxidative stress and blood pressure relative to the control period, though negative changes were discovered in total cholesterol and triglycerides (Sutton et al., 2018).
The same group measured a further battery of outcome measures before and after a similar TRF eating strategy performed in healthy adults (Jamshed et al., 2019; Ravussin et al., 2019). This work enrolled 11 participants and enacted TRF for four days, preventing any long term inferences. Nevertheless, despite finding no TRF-induced changes in energy expenditure, relative to conventional eating patterns, TRF provoked a suppression of 24 -h glucose concentrations (Jamshed et al., 2019). There was also a 10% increase in expression of the gene encoding sirtuin 1, an epigenetic modifier that deacetylates FOXO and PGC-1αx, and in turn, elevates expression of genes involved in stress resistance and autophagy (de Cabo and Mattson, 2019; Rahman and Islam, 2011). Likewise, Jamshed et al. (2019) observed a 22% rise in expression of the LC3A gene, which encodes a structural aspect of autophagosomal membranes that may be linked to carcinogenesis (Bai et al., 2012; Jamshed et al., 2019). In addition, after TRF, there was a reduction in subjective appetite, which could be explained by a decrease in concentrations of the hunger hormone acylated ghrelin (Ravussin et al., 2019).
Taken together, the work led by the Peterson group indicates that early TRF may enhance many indices of cardiometabolic health, yet it is still unclear whether the time of the feeding period influences metabolic improvements as suggested by Sutton et al. (2018). One study in men with obesity recently set out to ascertain if changes in cardiometabolic markers, appetite and appetite-related hormones differed on the basis of TRF early in the day (08.00–17.00) or later in the day (12.00–21.00) (Hutchison et al., 2019b). Both early and late TRF decreased mean 24 -h glucose concentrations, and neither TRF strategy affected other outcomes, with changes in insulin, blood lipids, appetite and appetite-related hormone concentrations alike after seven days of each regimen (Hutchison et al., 2019b). These results imply that TRF can improve metabolic health irrespective of when the eating window occurs, disputing the postulations that early TRF may enhance outcomes by utilizing circadian variations in metabolism. However, evidently, longer-term studies in varying populations, including healthy individuals who are not overweight or obese, are needed to verify these findings.
4. Protein restriction
An expanding body of evidence now postulates that the restriction of protein and, in particular, essential amino acids may be the predominant dietary mediators for augmenting lifespan rather than CR (Fontana and Partridge, 2015; Grandison et al., 2009; Mirzaei et al., 2014; Pamplona and Barja, 2006). Self-reported 24 h dietary recall data from the US National Health and Nutrition Examination Survey suggests that there is a 75% elevated risk in overall mortality in individuals who consume greater than 20% of their energy from protein versus those who consume less than 10%, yet this relationship only holds when protein is derived from animal sources (Levine et al., 2014). Although causality cannot be established, this work suggests that protein, in particular from animal sources, accelerates aging in humans.
The precise mechanisms underlying improvements in healthspan during protein restriction in mammals have yet to be determined, but studies from non-human models implicate the growth hormone (GH)-IGF-1-axis in slowing age-related processes during protein restriction (Fontana et al., 2010, 2008). More specifically, protein restriction is hypothesized to inhibit the GH-IGF-1-axis, increase the activation of FOXO and downregulate mTOR activity, leading to an increase in autophagy and an inhibition of protein synthesis (de Cabo and Mattson, 2019; Fontana et al., 2010; Wang et al., 2014). In their cross-sectional analysis, Fontana et al. (2008) compared measures related to the GH-IGF-1 axis in three distinct groups of 28 individuals: a CR group who had been practicing traditional CR for at least six years, a protein restriction group that engaged in a plant-based, low protein diet for at least 2 years, and a control group who consumed a typical Western diet. Seven-day food record data indicated that the control group exhibited higher energy intake than both other diets, but there were little differences between the CR and protein restriction groups. The protein restricted group reported an average protein intake of 0.76 g/kg/day, which equated to 9.6% of total energy intake and was lower than the protein consumed by the CR and control cohorts (Fontana et al., 2008). Interestingly, despite no differences in insulin and C-reactive protein concentrations between protein restriction and CR cohorts, lower IGF-1 concentrations were evident in the protein restriction group versus the CR and control groups. To obtain causal inferences, Fontana et al. (2008) also reduced protein intake from 1.67 g/kg/day to 0.95 g/kg/day for three weeks in six individuals from the CR group and reported a 22% decrease in IGF-1 following this short intervention. As a whole, these findings led the authors to deduce that low protein intake, as opposed to low energy consumption, is the main driver in suppressing IGF-1 concentrations in humans (Fontana et al., 2008). This notion was recently corroborated in a meta-analysis that included dietary regimens that exclusively implemented CR or protein restriction (Kazemi et al., 2019).
Notwithstanding the findings of Fontana et al. (2008), evidence from larger randomized controlled trials are needed to determine the relative longevity-related benefits of protein restriction. A randomized controlled trial enrolled 38 males with overweight or obesity who were suffering from prostate cancer to either a control group who were told to maintain their usual diet, or a protein restriction group who consumed a 7%–9% protein diet that met energy needs (Eitan et al., 2017; Fontana et al., 2016a; Treviño-Villarreal et al., 2018). For an average of 43 days, the protein restriction group had their diet prepared by a metabolic kitchen, though duration of the dietary regimen was not standardized, with an average of 11 days variation between participants. Nevertheless, pre- and post-intervention assessments showed that the protein restriction group exhibited a decrease in weight, fat mass, fasting glucose, cholesterol and LDL compared to the control group (Fontana et al., 2016a; Treviño-Villarreal et al., 2018). Furthermore, though plasma leptin was unaffected, the protein restriction group displayed a rise in leptin receptor levels on extracellular vesicles compared to the control group, which may be representative of improved leptin signalling and a decrease in leptin resistance (Eitan et al., 2017). There was also an increase in the insulin sensitizing hormone fibroblast growth factor 21 (FGF21) in the protein restriction group versus the control group. Although its role is multifaceted, FGF21 appears to be central in coordinating homeostatic responses to protein restriction in mice (Hill et al., 2019, 2017), and its administration can improve insulin resistance, hyperlipidemia and body weight (Coskun et al., 2008; Kharitonenkov et al., 2005). Therefore, despite the need for further human-based studies, FGF21 could play a significant part in the improved metabolic and aging responses to protein restriction in humans.
The findings of these studies endorse the anti-aging influence of protein restriction, but additional work has sought to establish the importance of restricting specific amino acids rather than overall grams of protein. Kahleova et al. (2018) randomized 75 participants with overweight or obesity to 16 weeks of either a low protein vegan diet or control. The low protein vegan group were asked to completely restrict their intake of animal proteins concentrated with branch-chain amino acids and consume a diet rich in vegetables, grains, legumes and fruits. On the contrary, the control group were instructed to sustain their habitual dietary patterns. Compliance was established via a 3-day food record and illustrated that the low protein group decreased total protein, albeit plant- and animal-derived protein increased and decreased, respectively, compared to control (Kahleova et al., 2018). Following the intervention, which 72 participants completed, metabolic indices were unchanged in the control diet, yet the low protein vegan group had improvements in body weight (−6.5 kg), fat mass (−4.3 kg) and visceral adipose volume (−224 cm3), along with a decrease in insulin resistance. Moreover, correlational analyses indicated that a greater reduction in the consumption of animal protein and leucine, in particular, was linked to greater fat loss, whilst a decrease in histidine consumption was associated with improvements in insulin resistance after controlling for energy intake (Kahleova et al., 2018). These findings suggest that a decrease in animal protein and amino acids found highly in animal protein may be pivotal in improving metabolic health and aging, supporting observational studies associating animal-based protein and not protein derived from plants to cardiovascular disease (Bernstein et al., 2010), cancer (Alexander et al., 2011) and mortality (Levine et al., 2014). However, food record data also documented a 17.9% decrease in fat intake in the low protein group versus the control group. This is logical as many animal-based proteins are commensurate with saturated fat (Larsson and Orsini, 2014). It is plausible, therefore, that reductions in fat intake could explain, in part, the metabolic improvements seen in the low protein group, especially since fat intake has been linked to insulin resistance (Lovejoy, 2002) and was not accounted for by the authors.
Despite the elevated interest in protein restriction as a mode of attenuating the aging process, there remains no evidence on measures pertaining to primary aging. Furthermore, the studies described unfortunately did not examine changes in appetite perceptions and quality of life. These are notable missing variables, particularly since protein is the most satiating macronutrient and is often advocated during weight loss regimens as a strategy to mitigate compensatory elevations in appetite and energy intake (Batterham et al., 2006; Drummen et al., 2018). Maintaining or increasing protein consumption during energy deficits can additionally be important in attenuating reductions in fat-free mass (Drummen et al., 2018). Consequently, with the scientific interest in muscle-wasting diseases, and the link between sarcopenia and mortality (Brown et al., 2016), long-term protein restriction studies with measurements of fat-free mass, appetite and adherence are needed.
5. Areas of further study
There has been an increase in the number of studies examining novel dietary strategies, and new insights have been yielded (Table 1). However, put simply, this research is still in its infancy and equivocal findings mean a host of important aspects need to be considered before evidence-based dietary recommendations can be made. Overall, studies are needed to test the influence of these dietary strategies on human markers linked to primary aging and understand the molecular changes underlying variations in these biomarkers. This includes mass-specific energy expenditure and oxidative stress, along with markers linked to the pillars of aging such as macromolecular damage, regeneration, proteostasis and epigenetics (Kennedy et al., 2014). Most studies cited in this review have solely measured factors indicative of secondary aging, namely anthropometric and/or cardiometabolic outcomes, and very few have assessed resting energy expenditure, which is central to the rate of living theory of aging and is improved by traditional CR (Redman et al., 2018). Akin to CALERIE phase 2, studies should ideally utilize sophisticated measures, including 24 h energy expenditure during sleep and at rest, since CR-induced differences in metabolic adaptation can vary during distinct periods of the day and throughout prolonged CR bouts (Ravussin et al., 2015; Redman et al., 2018). Equally, given the posited importance of the free radical theory of aging (Harman, 1956; Redman et al., 2018), new studies should incorporate wide-ranging and stable examinations of oxidative stress, such as markers of DNA damage and reactive oxygen species (Heilbronn et al., 2006; Il’yasova et al., 2018). Studies should also be employed for substantially longer periods of time to truly gauge feasibility in community-dwelling individuals. Further, studies should ideally incorporate a broad spectrum of measures linked to biological (Belsky et al., 2018) and epigenetic (Levine et al., 2018; Lu et al., 2019) age.
Table 1.
Directionality of physiological endpoints from studies investigating new dietary strategies.
“↑” indicates increase; “↓” indicates decrease; “↔” indicates no change; “-” indicates not investigated.
More comparative paradigms that directly contrast novel dietary strategies and traditional CR are also needed. Critically, studies should seek to standardize any perturbations in energy balance to ascertain if these novel regimens foster benefits beyond traditional CR methods. Some studies within this review reported greater energy restriction in IF groups than CR controls (Catenacci et al., 2016; Harvie et al., 2013, 2011; Hutchison et al., 2019a), and while this may demonstrate the effectiveness of IF in provoking larger energy deficits, the variations in energy deficit make it challenging to determine if any differences in outcomes are the result of IF per se. Thus, if the goal is to determine any independent benefit of fasting periods, future studies should make exceptional efforts to negate any differences in the degree of energy restriction between groups. In line with this point, forthcoming studies testing the effect of macro- and micronutrient manipulations should carefully consider the design of their study to pinpoint any causal inferences, since manipulation of one nutrient will alter another when energy consumption is controlled. Researchers also need to ponder the acute effects of the observed dietary approaches on outcome measurements and differentiate the chronic impact of the strategies on metabolic markers. Indeed, in IF studies, results can be highly divergent depending on the degree of energy restriction in the hours immediately prior to measurements. For instance, prolonged fasting or energy restriction decreases fasting glucose and insulin, and decreases carbohydrate uptake and oxidation as a greater dependency is placed on endogenous fat as an energy source (Antoni et al., 2016; Clayton et al., 2018). Likewise, acute alterations in macronutrient intake causes divergent changes in glucose uptake (Lundsgaard et al., 2017). Such transient alterations in metabolism likely confound the results of postrandomization assessments, making it difficult to elucidate the chronic effects of novel eating patterns. Therefore, subsequent investigations should seek to standardize follow-up metabolic measurements to elucidate the chronic aging-related changes engendered by these novel diets. Creation of such “best practices” would serve the nutritional gerontology research community.
To facilitate the measurement of energy restriction periods, studies should seek to utilize advanced measures of energy intake where possible. Almost all studies reviewed documented compliance of their cohort using self-reported measures to determine food intake and meal timings. These tools adjust dietary behaviors through demand characteristics and provide poor validity, underestimating energy intake by as much as 800 kcal/day (Dhurandhar et al., 2015; Heitmann and Lissner, 1995). Objective assessments of dietary intake and hence adherence can be obtained with more sophisticated or costly measures such as doubly labelled water, which is considered the gold standard (Livingstone and Black, 2003; Racette et al., 2012), and weight loss trajectories (Pieper et al., 2011; Thomas et al., 2010). Doubly labelled water, however, cannot establish macronutrient intake or variations in meal timing and frequency, plus it does not provide data in near real time, so self-reported measures are also inadvertently required in nutrition studies. Accordingly, researchers in this field should contemplate novel and valid measurements to precisely determine the food intake habits integral to newer dietary strategies (Herrera and Chan, 2018). Alternatively, despite the demand for sufficient resources, and fastidious preparation required, studies may consider supplying food to participants in a controlled setting to establish more robust proof-of-principle data for dietary approaches before extending to large, long-term randomized controlled trials.
Forthcoming studies should explicitly report adherence and attrition outcomes. It is frequently assumed that, compared to traditional CR, many of the newer strategies are easier to follow (Varady et al., 2009), yet there is limited supporting data. Likewise, although some have evaluated the influence of dietary regimens over one year (Gabel et al., 2019; Sundfør et al., 2018; Trepanowski et al., 2018, 2017), the majority of studies reviewed have been short (≤ 6 months) and, to our knowledge, no studies have investigated the impact of novel dietary strategies for over 12 months. The continual adoption of a diet regimen beyond 12 months and into a weight maintenance phase is essential to gauge success, especially since weight loss maintenance is achieved by only 20%–50% of individuals in response to lifestyle interventions (Anastasiou et al., 2015; Wing and Phelan, 2005). Similar to CALERIE phase 2, which stands as a comparative trial in this field, efforts should be made to examine changes in aging and health biomarkers for time periods beyond 12 months. Further, given that attrition of newer strategies after 12 months has been both excellent (Sundfør et al., 2018) and substandard (Trepanowski et al., 2017), attrition and adherence needs to be elucidated if these novel regimens are to be universally advocated for individuals in western settings.
There is also work needed to identify factors modulating intra-individual responses in endpoints. Despite the widespread promotion of CR, some show its beneficial effects vary substantially (de Cabo and Mattson, 2019; Mattison et al., 2017). Heritable factors are likely to modulate responses to CR, since equivalent CR regimens can enhance and shorten lifespan in murine models (Liao et al., 2010; Mitchell et al., 2016). Interestingly, changes in body fat during CR could be implicated in this variation, with some showing that mice with the poorest CR-induced changes in lifespan exhibit greater reductions in adiposity (Liao et al., 2011; Mitchell et al., 2016). These results are seemingly at odds with the viewpoint that reductions in adiposity drive CR-induced benefits, although some existing evidence shows that overweight is linked to lower all-cause mortality (Flegal et al., 2013), suggesting that adipose tissue is vital in metabolic regulation during CR (de Cabo and Mattson, 2019; Mitchell et al., 2016). Work incorporating large sample sizes is needed to assess if aging-related alterations during CR and other dietary strategies are modified by intra-individual changes in adiposity. Furthermore, Moffitt and colleagues argued that aging-related studies are needed in younger individuals free from chronic disease, given pathogenic signals of diseases can assemble in the first half of the life course and confounding factors exist when studying individuals with metabolic conditions (Moffitt et al., 2017). At the other end of the spectrum, it is important to examine the impact of dietary interventions initiated in older adults. Pilot work has been done in this regard (Anton et al., 2019), but longer studies in larger samples are now needed. Last, as the efficacy of novel dietary strategies is proven, studies will need to test inventive diets that amalgamate features of the strategies covered. Such diets, which are already being studied (Brandhorst et al., 2015; Wei et al., 2017), may offer practical, more flexible plans that individuals are more likely to follow.
6. Conclusions
Though traditional CR robustly improves markers of aging in humans, manipulations in meal timing, eating frequency and macronutrient intake could engender further enhancements in the physiological endpoints involved in primary and secondary aging whilst improving adoption rates amongst the general population. Human studies have hitherto shown that energy deficits and thus weight loss may be attained with IF, as individuals fail to fully compensate for prolonged periods of severe energy restriction. Further, independent of energy balance, improvements in aging markers have been displayed with IF and protein restriction. However, inconsistency amongst findings is evident, potentially because of the diversity in protocols and the metabolic status of studied participants. There is also a scarcity of evidence that has compared novel dietary strategies with traditional CR for prolonged periods and incorporated comprehensive assessments of aging markers. Additionally, the impact of novel dietary strategies on adherence, attrition and appetite is equivocal, questioning their long-term success. It is therefore paramount that multi-arm studies in young and older individuals free from chronic disease are conducted for over 12 months before any strategy can claim superiority in retarding aging in humans situated in obesogenic settings.
Funding
This work was funded by the NIH (R01 AG029914 and U01 AG020478). It was also supported in part by P30DK072476 (Pennington/Louisiana NORC), U54 GM104940 (Louisiana Clinical and Translational Science Center), and the American Heart Association Grant #20POST35210907 (James L. Dorling).
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
References
- Alexander DD, Weed DL, Cushing CA, Lowe KA, 2011. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur. J. Cancer Prev 20, 293–307. 10.1097/CEJ.0b013e328345f985. [DOI] [PubMed] [Google Scholar]
- Anastasiou CA, Karfopoulou E, Yannakoulia M, 2015. Weight regaining: from statistics and behaviors to physiology and metabolism. Metabolism. 64, 1395–1407. 10.1016/j.metabol.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, Pahor M, 2019. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients 11, 1500. 10.3390/nu11071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, Leeuwenburgh C, Mattson MP, 2018. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity 26, 254–268. 10.1017/S0007114517003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni R, Johnston KL, Collins AL, Robertson MD, 2018. Intermittent v. Continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr 119, 507–516. 10.1017/S0007114517003890. [DOI] [PubMed] [Google Scholar]
- Antoni R, Johnston KL, Collins AL, Robertson MD, 2016. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br. J. Nutr 115, 951–959. 10.1017/S0007114515005346. [DOI] [PubMed] [Google Scholar]
- Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S, 2003. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: a randomised trial. Int. J. Obes 27, 797–802. 10.1038/sj.ijo.0802295. [DOI] [PubMed] [Google Scholar]
- Bai H, Inoue J, Kawano T, Inazawa J, 2012. A transcriptional variant of the LC3A gene is involved in autophagy and frequently inactivated in human cancers. Oncogene 31, 4397–4408. [DOI] [PubMed] [Google Scholar]
- Barte JCM, Ter Bogt NCW, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, Bemelmans WJE, 2010. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes. Rev 11, 899–906. 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ, 2006. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 4, 223–233. 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE, Anderson R, 2018. Change in the rate of biological aging in response to caloric restriction: CALERIE Biobank analysis. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 73, 4–10. 10.1093/gerona/glx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC, 2010. Major dietary protein sources and risk of coronary heart disease in women. Circulation 122, 876–883. 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Phillips SA, Norkeviciute E, Varady KA, 2013. Alternate day fasting with or without exercise : effects on endothelial function and adipokines in obese humans. ESPEN J. 8, e205–e209. [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD, 2015. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99. 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Harhay MO, Harhay MN, 2016. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia Sarcopenia Muscle 7, 290–298. 10.1002/jcsm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, Baer DJ, Egan J, Mattson MP, 2007. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 56, 1729–1734. 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Clifton PM, Keogh JB, 2016. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract 122, 106–112. 10.1016/j.diabres.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, Troy Donahoo W, 2016. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 24, 1874–1883. 10.1002/oby.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DJ, Biddle J, Maher T, Funnell MP, Sargeant JA, King JA, Hulston CJ, Stensel DJ, James LJ, 2018. 24-h severe energy restriction impairs postprandial glycaemic control in young, lean males. Br. J. Nutr 120, 1107–1116. 10.1017/S0007114518002568. [DOI] [PubMed] [Google Scholar]
- Conley M, Le Fevre L, Haywood C, Proietto J, 2018. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet 75, 65–72. 10.1111/1747-0080.12372. [DOI] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A, 2008. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027. 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Das S, Roberts S, Bhapkar M, Villareal D, Fontana L, Martin C, Racette S, Fuss P, Kraus W, Wong W, Saltzman E, Pieper C, Fielding R, Schwartz A, Ravussin E, Redman L, 2017. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am. J. Clin. Nutr 105, 913–927. 10.3945/ajcn.116.137232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, DeLany JP, Saltzman E, Roberts SB, 2007. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am. J. Clin. Nutr 85, 1023–1030. 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Mattson MP, 2019. Effects of intermittent fasting on health, gging, and disease. N. Engl. J. Med 381, 2541–2551. 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sørensen TIA, Speakman JR, Jeansonne M, Allison DB, Energy Balance Measurement Working Group, 2015. Energy balance measurement: when something is not better than nothing. Int. J. Obes 39, 1109–1113. 10.1038/ijo.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling JL, Bhapkar M, Das SK, Racette SB, Apolzan JW, Fearnbach SN, Redman LM, Myers CA, Stewart TM, Martin CK, 2019. Change in self-efficacy, eating behaviors and food cravings during two years of calorie restriction in humans without obesity. Appetite 143, 104397. 10.1016/j.appet.2019.104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummen M, Tischmann L, Gatta-Cherifi B, Adam T, Westerterp-Plantenga M, 2018. Dietary protein and energy balance in relation to obesity and co-morbidities. Front. Endocrinol. (Lausanne) 9, 443. 10.3389/fendo.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau VJ, Inouye SK, Rowe JW, Finkelman E, Yamada T, 2019. Enabling healthful aging for all — the National Academy of Medicine grand challenge in healthy longevity. N. Engl. J. Med In press. 10.1056/NEJMp1912298. [DOI] [PubMed] [Google Scholar]
- Eitan E, Tosti V, Suire CN, Cava E, Berkowitz S, Bertozzi B, Raefsky SM, Veronese N, Spangler R, Spelta F, Mustapic M, Kapogiannis D, Mattson MP, Fontana L, 2017. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell 16, 1430–1433. 10.1111/acel.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Kit BK, Orpana H, Graubard BI, 2013. Association of all-cause mortality with overweight and obesity using standard body mass index categories a systematic review and meta-analysis. JAMA - J. Am. Med. Assoc 309, 71–82. 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola SI, Alexander CM, Kimple ME, Lamming Correspondence DW, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Lamming DW, 2016a. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530. 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO, 2004. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. U. S. A 101, 6659–6663. 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD, 2010. Extending healthy life span-from yeast to humans. Science 328 (80-), 321–326. 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E, Holloszy JO, 2016b. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell 15, 22–27. 10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO, 2008. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687. 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, Varady KA, 2019. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity 27, 1443–1450. 10.1002/oby.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MDW, Partridge L, 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064. 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Henriksen M, Söderhamn N, Stallknecht B, Ploug T, Schjerling P, Dela F, 2005. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol 99, 2128–2136. 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- Harman D, 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol 11, 298–300. 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A, 2013. The effect of intermittent energy and carbohydrate restriction v. Daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr 110, 1534–1547. 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A, 2011. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes 35, 714–727. 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headland ML, Clifton PM, Keogh JB, 2018. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int. J. Obes 43, 2028–2036. 10.1038/s41366-018-0247-2. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E, 2005a. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes. Res 13, 574–581. 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, De Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, 2006. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. J. Am. Med. Assoc 295, 1539–1548. 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E, 2005b. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr 81, 69–73. 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- Heitmann BL, Lissner L, 1995. Dietary underreporting by obese individuals—is it specific or non-specific? BMJ 311, 986. 10.1136/bmj.311.7011.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera MCA, Chan CB, 2018. Narrative review of new methods for assessing food and energy intake. Nutrients 10, E1064. 10.3390/nu10081064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Albarado Di.C., McDougal DH, Berthoud HR, Münzberg H, Morrison CD, 2017. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci. Rep 15, 8209. 10.1038/s41598-017-07498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, Burke SJ, Collier JJ, Qualls-Creekmore E, Solon-Biet SM, Simpson SJ, Berthoud HR, Münzberg H, Morrison CD, 2019. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 27, 2934–2947. 10.1016/j.celrep.2019.05.022.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddy KK, Gibbons C, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Gabel K, Finlayson G, Varady KA, 2016. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin. Nutr 35, 1380–1385. 10.1016/j.clnu.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA, Meal timing during alternate day fasting: impact on body weight and cardiovascular disease risk in obese adults. Obesity 22, 2524–2531. 10.1002/oby.20909. [DOI] [PubMed] [Google Scholar]
- Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA, Safety of alternate day fasting and effect on disordered eating behaviors. Nutr. J 14, 44. 10.1186/s12937-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L, 2007. Caloric restriction in humans. Exp. Gerontol 42, 709–712. 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison AT, Liu B, Wood RE, Vincent AD, Thompson CH, O’Callaghan NJ, Wittert GA, Heilbronn LK, 2019a. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity 27, 50–58. 10.1002/oby.22345. [DOI] [PubMed] [Google Scholar]
- Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, Heilbronn LK, 2019b. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity 27, 724–732. 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Fontana L, Bhapkar M, Pieper CF, Spasojevic I, Redman LM, Das SK, Huffman KM, Kraus WE, 2018. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: the CALERIE 2 randomized clinical trial. Aging Cell 17, e12719. 10.1111/acel.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshed H, Beyl RA, Manna DLD, Yang ES, Ravussin E, Peterson CM, 2019. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11, E1234. 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP, 2007. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med 42, 665–674. 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahleova H, Fleeman R, Hlozkova A, Holubkov R, Barnard ND, 2018. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: metabolic benefits of plant protein. Nutr. Diabetes 8, 58. 10.1038/s41387-018-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi A, Speakman JR, Soltani S, Djafarian K, 2019. Effect of calorie restriction or protein intake on circulating levels of insulin like growth factor I in humans: A systematic review and meta-analysis. Clin. Nutr 10.1016/j-clnu.2019.07.030. In press. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F, 2014. Aging: a common driver of chronic diseases and a target for novel interventions. Cell 159, 709–713. 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB, 2005. FGF-21 as a novel metabolic regulator. J. Clin. Invest 115, 1627–1635. 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempel MC, Bhutani S, Fitzgibbon M, Freels S, Varady KA, 2010. Dietary and physical activity adaptations to alternate day modified fasting : implications for optimal weight loss. Nutr. J 9, 35. 10.1186/1475-2891-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Varady KA, 2013. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 62, 137–143. 10.1016/j.metabol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E, Holloszy JO, Fontana L, 2019. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7, 673–683. 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger CM, Trepanowski JF, Klempel MC, Barnosky A, Bhutani S, Gabel K, Varady KA, 2018. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: an exploratory analysis of a randomized controlled trial. Nutr. Health 24, 5–10. 10.1177/0260106017753487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Orsini N, 2014. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am. J. Epidemiol 179, 282–289. 10.1093/aje/kwt261. [DOI] [PubMed] [Google Scholar]
- Leclerc E, Trevizol AP, Grigolon RB, Subramaniapillai M, McIntyre RS, Brietzke E, Mansur RB, 2019. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 10, 1–7. 10.1017/S1092852918001566. [DOI] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner1 AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S, 2018. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany. NY) 10, 573–591. 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy K, Wei M, Cohen P, Crimmins EM, Longo VD, 2014. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417. 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF, 2010. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95. 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Gelfond JAL, Diaz V, Nelson JF, 2011. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell 10, 629–639. 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Black AE, 2003. Markers of the validity of reported energy intake. J. Nutr 133, 895S–920S. 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- Loft S, Velthuis-Te Wierik EJM, Van Den Berg H, Poulsen HE, 1995. Energy restriction and oxidative DNA damage in humans. Cancer Epidemiol. Biomarkers Prev. 4, 515–519. [PubMed] [Google Scholar]
- Longo VD, Panda S, 2016. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy JC, 2002. The influence of dietary fat on insulin resistance. Curr. Diab. Rep 2, 435–440. [DOI] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S, 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany. NY) 11, 303–327. 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundsgaard AM, Sjøberg KA, Høeg LD, Jeppesen J, Jordy AB, Serup AK, Fritzen AM, Pilegaard H, Myrmel LS, Madsen L, Wojtaszewski JFP, Richter EA, Kiens B, 2017. Opposite regulation of insulin sensitivity by dietary lipid versus carbohydrate excess. Diabetes 66, 2583–2595 https://doi.org/0.2337/db17-0046. [DOI] [PubMed] [Google Scholar]
- Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, Stewart T, Robinson L, Roberts SB, 2016. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults the CALERIE 2 randomized clinical trial. JAMA Intern. Med 176, 743–752. 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, De Cabo R, Anderson RM, 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun 8, 14063. 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 2005. Energy intake, meal frequency, and health: a neurobiological perspective. Annu. Rev. Nutr 25, 237–260. 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Longo VD, Harvie M, 2017. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev 39, 46–58. 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wan R, 2005. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem 16, 129–137. 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, Fuss PJ, Roberts SB, Holloszy JO, Fontana L, 2016. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in nonobese humans. Aging (Albany. NY) 8, 1416–1431. 10.18632/aging.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L, 2006. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol 47, 398–402. 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Suarez JA, Longo VD, 2014. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab 25, 558–566. 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Burón MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, De Cabo R, 2016. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 23, 1093–1112. 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A, 2017. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. Journals Gerontol. Ser. A. Biol. Sci. Med. Sci 72, 210–215. 10.1093/gerona/glw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A, 2016. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med 14, 1–10. 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM, 2018. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol. Metab 314, E396–E405. 10.1152/ajpendo.00261.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, Fontana L, 2017. Calorie restriction in humans: an update. Ageing Res. Rev 39, 36–45. 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA, 1993. Low methionine ingestion by rats extends life span. J. Nutr 123, 269–274. 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G, 2006. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim. Biophys. Acta - Bioenerg 1757, 496–508. 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Sears DD, 2017. Metabolic effects of intermittent fasting. Annu. Rev. Nutr 37, 371–393. 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, Martin C, Kraus W, Das S, Williamson D, Ravussin E, 2011. Development of adherence metrics for caloric restriction interventions. Clin. Trials 8, 155–164. 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AM, Bordoli C, Buckner LP, Kim C, Kaplan PC, Del Arenal IM, Jeffcock EJ, Hall WL, 2019. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: a randomized controlled trial; the Met-IER study. Clin. Nutr 10.1016/j.clnu.2019.07.014. In press. [DOI] [PubMed] [Google Scholar]
- Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, Redman LM, 2012. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. AJP Endocrinol. Metab 302, E441–E448. 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Rochon J, Uhrich ML, Villareal DT, Das SK, Fontana L, Bhapkar M, Martin CK, Redman LM, Fuss PJ, Roberts SB, Kraus WE, 2017. Effects of two years of calorie restriction on aerobic capacity and muscle strength. Med. Sci. Sports Exerc 49, 2240–2249. 10.1249/MSS.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO, 2006. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 61, 943–950. 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Islam R, 2011. Mammalian Sirt1: insights on its biological functions. Cell Commun. Signal 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM, 2019. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 27, 1244–1254. 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB, 2015. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 70, 1097–1104. 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Ravussin E, 2011. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal 14, 275–287. 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E, 2018. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 27, 805–814. 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA, 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 8, 1302–1307. 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, Hadley EC, Kraus WE, CALERIE Study Group, for the C.S, 2011. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. Journals Gerontol. Ser. A. Biol. Sci. Med. Sci 66, 97–108. 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, Bhapkar M, Kraus WE, 2016. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget 7, 19124–19133. 10.18632/oncotarget.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, 2008. The future of obesity: new drugs versus lifestyle interventions. Expert Opin. Investig. Drugs 17, 263–267. 10.1517/13543784.17.3.263. [DOI] [PubMed] [Google Scholar]
- Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans MT, Jonkers-Schuitema F, Fliers E, Sauerwein HP, Aerts JM, Serlie MJ, 2009. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am. J. Clin. Nutr 90, 1244–1251. 10.3945/ajcn.2008.27327. [DOI] [PubMed] [Google Scholar]
- Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S, Tadic J, Eisenberg T, Magnes C, Stumpe M, Zuegner E, Bordag N, Riedl R, Schmidt A, Kolesnik E, Verheyen N, Springer A, Madl T, Sinner F, de Cabo R, Kroemer G, Obermayer-Pietsch B, Dengjel J, Sourij H, Pieber TR, Madeo F, 2019. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 30, 462–476. 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- Stewart TM, Bhapkar M, Das S, Galan K, Martin CK, McAdams L, Pieper C, Redman L, Roberts S, Stein RI, Rochon J, Williamson DA, 2013. Comprehensive Assessment of Long-term Effects of reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: methods and results. Contemp. Clin. Trials 34, 10–20. 10.1016/j.cct.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP, 2007. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr 85, 981–988. 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundfør TM, Svendsen M, Tonstad S, 2018. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis 28, 698–706. 10.1016/j.numecd.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM, 2018. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221. 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Wilcox BJ, Wilcox CD, 2001. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac. J. Clin. Nutr 10, 165–171. [DOI] [PubMed] [Google Scholar]
- Swinburn B, Egger G, 2002. Preventive strategies against weight gain and obesity. Obes. Rev 3, 289–301. 10.1046/j.1467-789X.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB, 2010. A computational model to determine energy intake during weight loss. Am. J. Clin. Nutr 92, 1326–1331. 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW, 2017. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci 17, 200–207. 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, Rood J, Ravussin E, Varady KA, 2018. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin. Nutr 37, 1871–1878. 10.1016/j.clnu.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, Ravussin E, Varady KA, 2017. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med 177, 930–938. 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño-Villarreal JH, Reynolds JS, Bartelt A, Langston PK, MacArthur MR, Arduini A, Tosti V, Veronese N, Bertozzi B, Brace LE, Mejia P, Trocha K, Kajitani GS, Longchamp A, Harputlugil E, Gathungu R, Bird SS, Bullock AD, Figenshau RS, Andriole GL, Thompson A, Heeren J, Ozaki CK, Kristal BS, Fontana L, Mitchell JR, 2018. Dietary protein restriction reduces circulating VLDL triglyceride levels via CREBH-APOA5-dependent and -independent mechanisms. JCI Insight 3, e99470. 10.1172/jci.insight.99470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripolt NJ, Stekovic S, Aberer F, Url J, Pferschy PN, Schröder S, Verheyen N, Schmidt A, Kolesnik E, Narath SH, Riedl R, Obermayer-Pietsch B, Pieber TR, Madeo F, Sourij H, 2018. Intermittent fasting (alternate day fasting) in healthy, non-obese adults: protocol for a cohort trial with an embedded randomized controlled pilot trial. Adv. Ther 35, 1265–1283. 10.1007/s12325-018-0746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC, 2009. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr 90, 1138–1143. 10.3945/ajcn.2009.28380.1. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y, 2013. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr. J 12, 1–8. 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuis-te Wierik EJM, Van Den Berg H, Schaafsma G, Hendriks HEJ, Brouwer A, 1994. Energy restriction, a useful interventian to retard human ageing? Eur. J. Clin. Nutr 48, 138–148. [PubMed] [Google Scholar]
- Velthuis-te Wierik EJM, Westerterp KR, Van den Berg H, 1995. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. Int. J. Obes 19, 318–324. [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV, 2016. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Miner. Res 31, 40–51. 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford R, 1999. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: health, aging and toxicological perspectives. Toxicol. Sci 52, 61–65. 10.1093/toxsci/52.suppl_1.61. [DOI] [PubMed] [Google Scholar]
- Walford RL, Harris SB, Gunion MW, 1992. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc. Natl. Acad. Sci. U. S. A 89, 11533–11537. 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T, 2002. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 57, B211–B224. 10.1093/gerona/57.6.B211. [DOI] [PubMed] [Google Scholar]
- Wang L, Karpac J, Jasper H, 2014. Promoting longevity by maintaining metabolic and proliferative homeostasis. J. Exp. Biol 217, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman MP, Guo MH, Bennion DM, Shankar MN, Chrzanowski SM, Goldberg LA, Xu J, Williams TA, Lu X, Hsu SI, Anton SD, Leeuwenburgh C, Brantly ML, 2015. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 18, 162–172. 10.1089/rej.2014.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, Cohen P, Morgan TE, Dorff T, Hong K, Michalsen A, Laviano A, Longo VD, 2017. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med 9, eaai8700. 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, 1996. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol. Pathol 24, 742–745. 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- Willcox B, Willcox D, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb J, Suzuki M, 2007. Caloric restriction, the traditional Okinawan diet, and healthy aging. Ann. N. Y. Acad. Sci 1114, 434–455. 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, 2014. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr. Opin. Clin. Nutr. Metab. Care 10.1097/MCO.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing R, Phelan S, 2005. Long-term weight loss maintenance. Am. J. Clin. Nutr 82 (suppl), 222S–225S. 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Wong WW, Roberts SB, Racette SB, Das SK, Redman LM, Rochon J, Bhapkar MV, Clarke LL, Kraus WE, 2014. The doubly labeled water method produces highly reproducible longitudinal results in nutrition studies. J. Nutr 144, 777–783. 10.3945/jn.113.187823. [DOI] [PMC free article] [PubMed] [Google Scholar]


