Abstract
Background
Open angle glaucoma (OAG) is an important cause of blindness worldwide. Laser trabeculoplasty, a treatment modality, still does not have a clear position in the treatment sequence.
Objectives
The objective of this review was to study the effects of laser trabeculoplasty for OAG.
Search methods
We identified trials from CENTRAL in The Cochrane Library, MEDLINE, EMBASE, LILACS and manual searching. We also contacted researchers in the field.
Selection criteria
We included randomised controlled trials comparing laser trabeculoplasty with no intervention, with medical treatment, or with surgery. We also included trials comparing different technical modalities of laser trabeculoplasty.
Data collection and analysis
Two authors independently assessed trial quality and extracted the data. We contacted trial investigators for missing information.
Main results
This review included 19 trials involving 2137 participants. Only five trials fulfilled the criteria of good methodological quality. One trial compared laser trabeculoplasty with topical beta‐blocker to no intervention in early glaucoma. The risk of glaucoma progression was higher in the control group at six years of follow up (risk ratio (RR) 0.71 95% confidence interval (CI) 0.53 to 0.95). No difference in health‐related quality of life was observed between the two groups. Three trials compared laser trabeculoplasty to medication (regimens used before the 1990s) in people with newly diagnosed OAG. The risk of uncontrolled intraocular pressure (IOP) was higher in the medication group compared to the trabeculoplasty group at six months and two years of follow up. Three trials compared laser trabeculoplasty with trabeculectomy. The risk of uncontrolled IOP was significantly higher in the trabeculoplasty group at six months but significant heterogeneity was observed at two years. Diode and selective laser are compared to argon laser trabeculoplasty in three trials and there is some evidence showing a comparable effect in controlling IOP at six months and one year of follow up.
Authors' conclusions
Evidence suggests that, in people with newly diagnosed OAG, the risk of uncontrolled IOP is higher in people treated with medication used before the 1990s when compared to laser trabeculoplasty at two years follow up. Trabeculoplasty is less effective than trabeculectomy in controlling IOP at six months and two years follow up. Different laser technology and protocol modalities were compared to the traditional laser trabeculoplasty and more evidence is necessary to determine if they are equivalent or not. There is no evidence to determine the effectiveness of laser trabeculoplasty compared to contemporary medication (prostaglandin analogues, topical anhydrase inhibitors and alpha2‐agonists) and also with contemporary surgical techniques. Also there should be further investigation in to the effectiveness of laser trabeculoplasty in specific racial groups, specific diagnostic groups, such as pseudoexfoliation and pigmentary glaucoma and different stages of OAG. More research is also required determining cost‐effectiveness of laser trabeculoplasty in the management of glaucoma.
Plain language summary
Laser trabeculoplasty for open angle glaucoma
Glaucoma is a chronic progressive disease of the optic nerve which, if not treated, leads to visual field decay and blindness at end stages. Intraocular pressure (IOP) decreasing is shown to diminish the progression of the disease, and could be achieved by the chronic use of hypotensive eyedrops, incisional surgery or laser trabeculoplasty. There is still great controversy about where in the treatment scale laser trabeculoplasty is positioned, although this technique has already been described three decades ago. This treatment consists of application of laser spots in the trabecular meshwork, the structure responsible for the aqueous humor drainage, leading to an increase in the outflow facility through it and in consequence, decreasing IOP. It is an interesting form of treatment since it does not depend on chronic instillation of eyedrops, as does medical treatment, and also does not have too many complications, as does incisional surgery. This review included 19 trials (2137 participants). One trial compared laser trabeculoplasty associated with a hypotensive eyedrop with no intervention, and at six years of follow up the risk of visual field decay was greater in non treated participants. Three trials compared hypotensive eyedrops with trabeculoplasty, and the risk of uncontrolled IOP was greater at two years in the laser group. It is necessary to mention that the eyedrops used in these trials differ significantly from the ones used currently, since these trials were developed a decade ago. Three other trials compared trabeculoplasty with trabeculectomy and the risk of uncontrolled IOP was higher in the laser group at six months of follow up. There is some evidence showing that diode laser and selective trabeculoplasty have similar effect in controlling IOP when compared to argon laser trabeculoplasty. Comparisons of different lasers and different techniques of application were done in the remaining trials, but there is still not enough evidence to determine which is the best treatment protocol. Further research is necessary to compare trabeculoplasty with new hypotensive eyedrops and also the results of laser therapy in people of different ethnicities, since some studies suggest that they have a different response to this kind of laser therapy. More research is required to analyse cost‐effectiveness of these interventions.
Background
Epidemiology
In 1990 there were an estimated five million people worldwide blind because of glaucoma (Thylefors 1994) with a projected 8.4 million blind by 2010 (Quigley 2006). In white people the prevalence of primary open angle glaucoma (POAG) ranges from 0.8% to 3.0% (Bonomi 1998; Cedrone 1997; Dielemans 1994; Leske 1983; Tielsch 1991; Wensor 1998; Wolfs 2000). Several studies suggest that people of African origin have four to five times higher rates of POAG compared with people of european or asian origin (Buhrmann 2000; Leske 1994; Mason 1989; Tielsch 1991). In one recent epidemiological publication (Hyman 2001) these ethnic differences in POAG prevalence were confirmed. The prevalence of blindness and visual impairment was significantly higher in the African‐origin group and the leading cause was POAG (28%), as much as age‐related lens opacities.
Presentation and diagnosis
One of the problems of glaucoma diagnosis is the lack of agreement on the definition of the disease (Bathija 1998). Glaucoma is nowadays described as a group of disorders with a common denominator; a characteristic optic neuropathy. Various risk factors are associated with glaucoma but increased intraocular pressure (IOP) is consistently one of the most important (Shields 2005). Primary open angle glaucoma has these characteristics, but also an open, normal‐appearing anterior chamber angle with no ocular or systemic abnormality that might account for the elevated IOP, that is consistently above 21 mmHg, at least in one eye (Shields 2005). Normal tension glaucoma has similar characteristics but IOP is not the most influential causative factor (Shields 2005). However, it is now widely accepted that dichotomising the disease into normal tension and POAG around an IOP of 21 mmHg or any other statistically derived figure is arbitrary and that there is a continuity or gradation of risk with increasing IOP which reflects the extent to which the disease is determined by elevated pressure. At lower levels of IOP, other mechanisms are believed to influence progression of the disease.
Glaucoma secondary to pigment dispersion syndrome (pigmentary glaucoma) is a form of glaucoma where although the anterior chamber angle is open, there is an unusually heavy dispersion of pigment, which may be significantly involved in the pathogenesis of elevated IOP. Pseudoexfoliation glaucoma or capsular glaucoma is another form of glaucoma with open anterior chamber angle, but it is associated with a deposition of a proteinaceous material in the anterior segment of the eye. All these four entities are included in a group called open angle glaucoma (OAG). Glaucomatous neuropathy leads to visual field loss, initially in an arcuate or paracentral pattern extending to the periphery and ultimately to loss of central vision.
Treatment options
Reduction of IOP is still the goal of OAG treatment and it has been shown in a systematic review to be important in preventing visual field deterioration (Maier 2005; Vass 2007). Medical therapy is usually the first line therapy and there are currently many combinations of hypotensive topical medicines that can lead to a satisfactory IOP reduction (Realini 2002). Surgical approaches are usually reserved for cases in which good IOP control is not achieved with medication (Shields 2005) because of the risks of surgical complications. Laser trabeculoplasty is a non‐invasive technique that has been employed as first line or adjunctive therapy, or in order to avoid or delay surgical procedures.
The treatment of the trabecular meshwork with laser in human eyes was first described by Krasnov 1973. He believed the ruby laser caused a 'puncture' of the meshwork and thus an improvement in aqueous filtration. Later, Worthen 1974 described a series of uncontrolled OAG patients treated with argon laser, calling the procedure a laser trabeculotomy. Wise 1979 followed for 18 months a series of 56 cases submitted to trabecular argon laser treatment and considered this to be an effective alternative to filtration surgery. With a longer follow up, Schwartz 1985 observed a decreasing success rate over time and poorer IOP control in black people. Some authors observed an improvement in visual fields, probably related to laser trabeculoplasty induced IOP reduction (Traverso 1986), but this finding was not reproduced in other studies (AGIS; GLT). Peripheral anterior synechiae (PAS) and IOP spikes were frequently described complications of laser trabeculoplasty (GLT). Recently, other technologies have been used for trabeculoplasty. A Q‐switched 532‐nm Nd:YAG laser has been used with an apparently satisfactory IOP reduction (Chung 1998; Kim 2000; Lai 2004; Lanzetta 1999; Latina 1998; Nagar 2005).
It has been suggested that in early cases of OAG, laser trabeculoplasty treatment may be as effective as medical treatment or could delay the need for medical or surgical treatment. Similar results have been described in advanced cases, when laser trabeculoplasty treatment is compared with surgical treatment. In these advanced cases, laser trabeculoplasty seemed to be more effective in promoting visual field stabilisation in black people, than a surgical approach (AGIS; GLT).
Rationale for a systematic review
Although these data suggest a positive effect of laser trabeculoplasty for controlling OAG, there is no consensus on the role of laser trabeculoplasty in the treatment pathway for OAG and its use varies widely in practice in different parts of the world. This reflects underlying uncertainty of its effectiveness and hence there is a need for a systematic summary of all the best evidence of the effectiveness of this intervention.
Objectives
The objective of this review was to investigate the effects of laser trabeculoplasty for treating OAG when compared to medication, incisional glaucoma surgery or no intervention. We also wished to investigate the effectiveness of different laser trabeculoplasty technologies for treating OAG.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
Participants in the trials were people with any diagnosis of OAG. This included primary, secondary pigment dispersion, corticosteroid‐induced glaucoma and exfoliation or pseudoexfoliation syndromes. Gender, age and nationality were not used as exclusion criteria.
Types of interventions
We included trials where any laser trabeculoplasty technique was compared with one or more of the following:
medical ocular hypotensive therapy;
laser trabeculoplasty combined with medical ocular hypotensive therapy;
glaucoma drainage surgery;
an alternative laser trabeculoplasty technique;
no intervention (untreated control groups).
Types of outcome measures
Primary outcomes
(1) failure to control IOP according to each study definition; (2) failure to stabilise visual field progression (the progression is determined by criteria defined in each study protocol); (3) failure to stabilise optic neuropathy (optic disc deterioration is determined by criteria defined in each study protocol).
Secondary outcomes
(1) necessity of adding or changing the medical therapeutic regimen in consequence of uncontrolled IOP and visual field or optic disc damage progression; (2) adverse effects (severe, minor) including: IOP spikes; uveitis; cyclitis; hyphema; PAS formation; corneal edema; persistent IOP elevation; loss of vision (central island); bronchialspasm; (3) quality of life measures as available in the trial reports; (4) economic data as available in the trial reports.
A minimum six months follow up was required. We also collected outcomes at 12 and 24 months when possible.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) in The Cochrane Library, MEDLINE, EMBASE and LILACS (Latin American and Caribbean Literature on Health Sciences). There were no language or date restrictions in the searches for trials. The databases were last searched on 15 June 2007.
See: Appendices for details of search strategies for each database.
Searching other resources
The authors of the included studies and experts in the field were contacted to find out if they knew of any published or unpublished RCTs of laser trabeculoplasty for OAG which had not been identified. We used the Science Citation Index to search for reports that cited the studies included in this review. We also searched the reference lists of included study reports to check for details of further relevant trials.
Data collection and analysis
Assessment of search results
Two authors screened the abstracts of all publications that were found by the searches. Full text reports of all potentially relevant studies were obtained and were assessed against the inclusion criteria.
Assessment of methodological quality
In order to ensure that variation was not caused by systematic errors in the design of the study, two authors independently assessed methodological quality of the selected trials using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006):
allocation concealment: was the assignment of the group concealed before randomisation?
losses to follow up: were there equal drop out rates between the groups?
intention‐to‐treat analysis: were all participants analysed in the group to which they were randomised?
masking was not included as a quality criterion since it is very difficult to mask either providers or receivers of treatment to their allocation. However, masking of those making measurements of primary outcomes was considered as an additional quality criterion.
Trial quality was graded as follows: A ‐ low risk of bias (adequate); B ‐ moderate risk of bias (unclear); C ‐ high risk of bias (inadequate).
Authors of the trials graded B were contacted for further information. The trials graded C were excluded from this review.
Data collection
Tables were used to display characteristics of eligible trials and the reasons for exclusion of trials. Two authors independently extracted data. Any disagreement was discussed and the decisions documented. Where necessary the authors of the studies were contacted to help resolve the issue.
Data synthesis and analysis
One review author entered the data into RevMan 4.2 to perform statistical analysis. Wherever possible an intention‐to‐treat analysis was performed.
Dichotomous outcomes were analysed by calculating the risk ratio for each trial with 95% confidence intervals. The risk ratios from the individual trials were combined though meta‐analysis. When overall results were significant, the number needed to treat to produce one outcome was calculated by combining the overall risk ratio with an estimate of the prevalence of the event in the control group of the trials. Continuous outcomes were analysed according to their difference in mean treatment effects (weighted mean) and standard deviation.
We used a fixed‐effect model for the majority of comparisons unless there was significant heterogeneity between studies when we used a random‐effects model. In some cases we present both analyses.
Heterogeneity in the results of the trials was assessed both by inspection of graphical presentations and by performing a chi‐square test and an I2 test. Where heterogeneity existed we explored this by separate analysis of the following prespecified subgroups: (1) different technologies used in the intervention (argon laser, Nd:YAG laser, etc); (2) where laser treatment was provided in conjunction with ocular hypotensive drugs; (3) ethnic characteristics of people participating in the trials.
Results
Description of studies
Results of the search
The electronic searches identified a total of 706 reports. From these, 59 potentially relevant studies were identified and full copies were obtained. From the 36 RCTs that we identified, only 19 met the inclusion criteria (AGIS; Bergea 1992; Blyth 1999; Brancato 1991; Chung 1998; Damji 1999; Elsas 1989; EMGT; Gandolfi 2005; GLT; Grayson 1993; Grayson 1994; Hugkulstone 1990; Moorfields PTT; Moriarty 1988; Rouhiainen 1988; Sherwood 1987; Smith 1984; Watson 1984). Two trials were excluded because they included participants with OHT and no optic nerve damage (Lai 2004; Nagar 2005). Sixteen trials were excluded because the reported follow up was less than six months (Douglas 1987; Englert 1997; Huk 1991; Moriarty 1993; Popiela 2000; Shin 1996; Traverso 1984; Weinreb 1983; Weinreb 1983a), because of pseudo randomisation (Agarwal 2006; Heijl 1984; Hollo 1996; Tuulonen 1989), incompatible interventions (Demailly 1989a) or not included outcomes (Brancato 1988; Frenkel 1997). Sixteen other studies were not RCTs. The authors are awaiting further information about the methodology of three other studies (Gandolfi 2004a; Krasnov 1982; Schrems 1988). Contact with the authors of the trials and experts in the area did not identify any additional trials.
Included studies
See Characteristics of included studies for further details.
Types of participants
The 19 trials randomised 2746 eyes of 2137 participants. All the participants had OAG. The majority had POAG and some had pseudoexfoliation syndrome and pigment dispersion syndrome. There were two major groups of participants: those who were newly diagnosed as glaucomatous and needed initial therapy and those who already had glaucoma diagnosed but showed signs of progression even with the use of maximal antihypertensive medical therapy. The majority of the trials included both white and black participants, although in some of the trials (Bergea 1992; Brancato 1991; EMGT) the majority of participants were white. Moriarty 1988 evaluated the effectiveness of laser trabeculoplasty in black people. In AGIS the authors conducted a subgroup analysis according to ethnicity (white or black). Some studies used one eye of each participant. Other trials included each eye separately in the analysis; only one considering lack of independence between eyes (Damji 1999). Other trials used both eyes as either treated or control units for analysis (GLT; Hugkulstone 1990).
Types of interventions
Laser trabeculoplasty was mainly performed with argon laser, with a 50 micron spot size, 50 to 100 burns, with 0.8 to 2.0 Watts, 0.1 second exposure. Argon laser trabeculoplasty was compared with conventional trabeculectomy (AGIS; Moorfields PTT), with medication (Bergea 1992; Gandolfi 2005; GLT; Moorfields PTT; Moriarty 1988; Sherwood 1987) and with trabeculoplasty performed with other types of lasers such as diode laser (Brancato 1991; Blyth 1999; Chung 1998) and ND:Yag laser (Damji 1999). In one study (EMGT), participants were randomised to receive laser trabeculoplasty and ocular hypotensive medication (a selective beta‐blocker) or no treatment. Studies were included that compared laser trabeculoplasty with different exposure time (Hugkulstone 1990), different power levels (Rouhiainen 1988), monochromatic versus bichromatic wavelengths (Smith 1984) and the extent of the trabecular meshwork that was treated (Elsas 1989; Grayson 1993; Grayson 1994).
Types of outcomes
All included trials reported success rates with a minimum six month follow up. The exact definition of success varied across trials, but IOP at or below 21 mmHg with or without medication is an inclusive definition. Others used a 20% decrease from initial IOP as primary outcome (Damji 1999). Some studies considered the need for filtering surgery as failure criteria (Elsas 1989; Grayson 1993; Watson 1984). In addition, glaucoma progression defined as progression of visual field parameters, optic disk deterioration and visual acuity decay were considered as failure criteria for some trials (EMGT; GLT). Peripheral anterior synechiae formation was a complication that defined failure of the procedure in one of the trials (Rouhiainen 1988). Some trials (Blyth 1999; Damji 1999; Hugkulstone 1990; Watson 1984) reported the final mean and standard deviation of the IOP and others (Damji; GLT) reported the mean change and the standard deviation of the IOP from entry. The higher the change from entry the better the result was. Intraocular pressure spikes in the initial hours after laser trabeculoplasty (Damji 1999; Elsas 1989; Hugkulstone 1990), uveitis after laser treatment (Damji 1999), systemic adverse events and ocular adverse events (EMGT; Gandolfi 2005), such as need for cataract surgery, trabeculectomy or self reported eye conditions (reduction of visual acuity, floaters and conjunctivitis) were adverse events reported in some studies.
Excluded studies
See Characteristics of excluded studies for further details.
Risk of bias in included studies
Two authors independently assessed the methodological quality of included studies according to the guidelines developed by the Cochrane Eyes and Vision Group. The general quality of the studies was low. Five trials were graded at relatively low risk of bias (AGIS; Bergea 1992; EMGT; GLT; Sherwood 1987) and fulfilled all the criteria as referenced in the Cochrane Eyes and Vision Group guidelines (seeTable 1).
1. Quality assessment of included studies.
| TRIAL | Selection bias | Performance bias | Detection bias | Attrition bias |
| AGIS | A | D | A | A |
| Bergea 1992 | A | D | A | A |
| Blyth 1999 | B | D | B | A |
| Brancato 1991 | B | D | A | A |
| Chung 1998 | A | D | B | A |
| Damji 1999 | A | D | B | A |
| Elsas 1989 | B | D | B | B |
| EMGT | A | D | A | A |
| Gandolfi 2005 | B | D | B | A |
| GLT | A | D | A | A |
| Grayson 1993 | B | D | C | C |
| Grayson 1994 | B | D | C | C |
| Hugkulstone 1990 | B | D | A | C |
| Moorfields PTT | A | D | A | B |
| Moriarty 1988 | B | D | C | A |
| Rouhiainen 1988 | A | D | C | B |
| Sherwood 1987 | A | D | A | A |
| Smith 1984 | B | D | B | B |
| Watson 1984 | A | D | C | C |
Selection bias
Randomisation in 10 trials was adequately concealed (AGIS; Bergea 1992; Chung 1998; Damji 1999; EMGT; GLT; Moorfields PTT; Rouhiainen 1988; Sherwood 1987; Watson 1984). The methods of concealment were reported in 12 trials. For Rouhiainen 1988 further information was obtained after personal contact with the author.
Performance bias
Both care providers and recipients were aware of the assigned therapy they would receive as it is not possible to mask laser treatment when compared to another treatment modality. For this reason trials in this review were classified as D (not used).
Detection bias
The assessment of the outcomes was not masked for the majority of trials apart from AGIS; Bergea 1992; Brancato 1991; Gandolfi 2004a; GLT; Hugkulstone 1990 and Sherwood 1987. In these trials, although the participants and physicians knew the treatment they received since laser trabeculoplasty is a surgical intervention, visual field and optic disc readings were performed in a reading centre and IOP was obtained by a masked examiner.
Attrition bias
The majority of the included RCTs had similar rates of follow up in the comparison groups and intention‐to‐treat analysis was performed for almost all of them (AGIS; Bergea 1992; Blyth 1999; Brancato 1991; Chung 1998; Damji 1999; EMGT; Gandolfi 2005; GLT; Moriarty 1988; Sherwood 1987). Elsas 1989; Grayson 1993; Grayson 1994; Smith 1984; Watson 1984 did not describe the final sample or the losses to follow up. In Hugkulstone 1990, Moorfields PTT and Rouhiainen 1988 the analyses were not done on an intention‐to‐treat basis.
Effects of interventions
1. Laser trabeculoplasty and topical beta‐blocker versus no treatment
The EMGT enrolled 255 eyes of participants with newly diagnosed OAG. The eyes were assigned to receive a selective beta‐blocker (betaxolol) and one week later laser trabeculoplasty or no treatment. Since this was the only study comparing the intervention with no treatment it was not possible to perform a meta‐analysis. The goal of the study was to analyse natural history of glaucoma, although it included all OAG, which could have different physiopathology (normal tension glaucoma and pseudoexfoliative glaucoma). The main outcomes were glaucoma progression (visual field and optic disc lesion progression, both confirmed in reading centres).
Failure to control IOP EMGT did not report this outcome in the published paper or after personal contact with the authors.
Glaucoma progression At a minimum period of follow up of 48 months, 39/129 participants showed glaucoma progression in the laser trabeculoplasty group (30%) while 62/126 in the control group showed progression (49%).
Ocular and systemic adverse effects Laser trabeculoplasty associated with topical beta‐blocker seems to increase the risk of ocular and systemic adverse effects when compared with no treatment, but this was not statistically significant. Ocular adverse effects were in general mild and included redness, dryness, blurred vision and transient discomfort. In participants treated with laser trabeculoplasty and beta‐blocker, 28/129 developed ocular adverse events compared to 18/126 in the control group (RR 1.52, 95% CI 0.89 to 2.60). For systemic adverse effects 5/129 participants in the treatment group and 1/126 in the control group were reported (RR 4.88, 95% CI 0.58 to 41.22). Decreased visual acuity was reported as an adverse effect and there was no difference between the groups after four years of follow up.
Health‐related quality of life (HRQOL) The EMGT used a Swedish translation of the 25‐item National Eye Institute Visual Function Questionnaire self‐administered at three and six years after randomisation. Two hundred and thirty three out of 255 (91.4%) participants answered the first questionnaire (treated group n = 116, control group n = 117). At six years 167/255 participants (treated group n = 84, control group n = 83) completed the questionnaire (65.5%). There was a group of participants who were lost to follow up during the trial (10), others died (32) and 46 participants answered the first but not the second questionnaire at six years of follow up. At three years the lowest scores were related to general health and the highest ones related to lack of dependency of others. There was no significant difference between the treated and the control group. Lower scores were correlated to poorer visual acuity.
After three years, from the second questionnaire, it was possible to observe a decrease in the composite score (‐2.6 mean, +‐8.4 SD, P < 0.001) and compared with participants randomised to treatment, control participants seemed to have a larger decrease but this difference was small and was not statistically significant. These data were not presented in the publication. Larger decreases in the composite scores through the years were correlated to decrease in visual acuity, female gender and older age at first administration.
2. Argon laser trabeculoplasty versus medication in newly diagnosed participants
Four trials analysed this comparison (Bergea 1992; Gandolfi 2005; GLT; Moorfields PTT). Gandolfi 2005 only reported incidence of bronchospasm as a primary outcome.
Failure to control IOP (Analysis 1.1) When compared with medication, laser trabeculoplasty reduced the chance of uncontrolled IOP at six months and 24 months in newly diagnosed participants. The follow up in the Moorfields PTT study extended for five years and at this time point, the risk of uncontrolled IOP was greater in the laser trabeculoplasty group, although this was not statistically significant (RR 1.83, 95% CI 0.93 to 3.61). The definition of uncontrolled IOP varied between the trials. Bergea 1992 defined it as a peak over 26 mmHg in a daily curve that was reproduced one week later. In GLT the definition was not a specific value but was defined as the need for an additional medication. However, the protocol specified the need for an additional medicine if the IOP was 22 mmHg or more on two consecutive visits 14 days apart or 80% of the reference IOP. In Moorfields PTT when the IOP exceeded 22 mmHg it was considered a failure. Some heterogeneity could be observed between the results at six and 24 months suggesting a reduction in effectiveness over time.
1.1. Analysis.
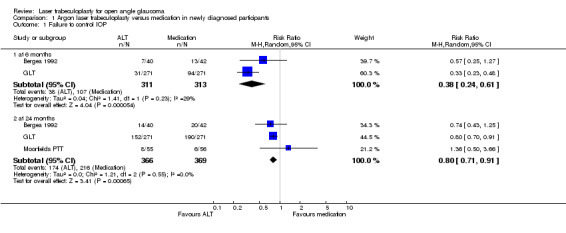
Comparison 1 Argon laser trabeculoplasty versus medication in newly diagnosed participants, Outcome 1 Failure to control IOP.
Visual field progression (Analysis 1.2) With regard to visual field progression, laser trabeculoplasty seemed to reduce the risk of deterioration when compared with medication but this was not statistically significant at one year (RR 0.77, 95% CI 0.46 to 1.28) or at two years of follow up (RR 0.70, 95% CI 0.42 to 1.16) as described in two trials (Bergea 1992; GLT) for newly diagnosed participants. In Moorfields PTT there was not a precisely reported dichotomous outcome indicating how many participants developed visual field deterioration in each group; but there is a statement that in the medicine treated group there was a significant deterioration in the visual field when compared with participants in the laser‐treated group. These data are not presented graphically. With regard to the visual field measurements, Bergea 1992 used Goldman manual perimetry and the field plots were evaluated in a masked fashion by two independent observers. In Moorfields PTT, the visual field was tested with a Friedman apparatus initially but was replaced after two years with an automated Humphrey field analyser. In GLT, an Octopus automated perimeter, models 201 or 2000 were used.
1.2. Analysis.
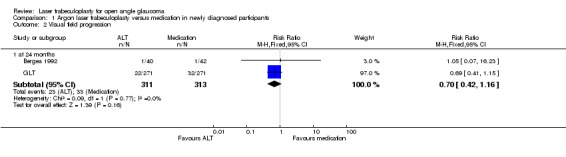
Comparison 1 Argon laser trabeculoplasty versus medication in newly diagnosed participants, Outcome 2 Visual field progression.
Optic neuropathy progression (Analysis 1.3) There were no differences in the risk of progression of optic neuropathy in terms of the appearance of the optic nerve at 24 months between the groups treated with laser trabeculoplasty or medication (Bergea 1992; GLT) (RR 0.73, 95% CI 0.44 to 1.20).
1.3. Analysis.
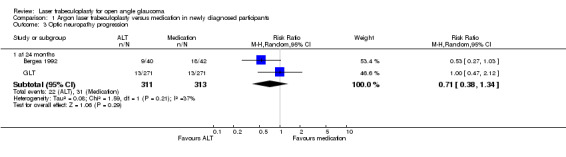
Comparison 1 Argon laser trabeculoplasty versus medication in newly diagnosed participants, Outcome 3 Optic neuropathy progression.
Adverse effects: peripheral anterior synechiae (PAS) formation (Analysis 1.4) Bergea 1992 and GLT described this outcome. The risk of PAS formation was greater in the laser trabeculoplasty group (RR 11.15, 95% CI 5.63 to 22.09). Systemic adverse effects: decrease of 20% or more in the forced expiratory volume after a metacholine test Gandolfi 2005 presented the results of a trial in which a subclinical bronchial reactivity after treatment of glaucomatous participants with laser trabeculoplasty or timolol eye drops was analysed. At both periods, three and four years, there was a tendency of a reduced risk ratio in bronchial reactivity in the trabeculoplasty group, but it was not statistically significant.
1.4. Analysis.
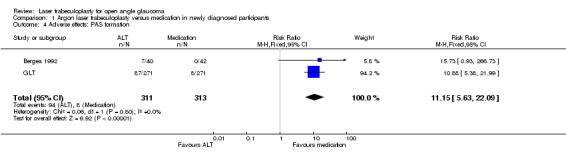
Comparison 1 Argon laser trabeculoplasty versus medication in newly diagnosed participants, Outcome 4 Adverse effects: PAS formation.
3. Argon laser trabeculoplasty versus medication in participants on maximal medical therapy
Failure to control IOP Moriarty 1988 and Sherwood 1987 evaluated the effectiveness of laser trabeculoplasty in a group of participants using maximal tolerated antiglaucomatous medical therapy. At 12 months of follow up, in Sherwood 1987, two participants had uncontrolled IOP in 24 people randomised to laser trabeculoplasty, while all participants in the medication group had failed (RR 0.08 95% IC 0.02 to 0.31). In Moriarty 1988, also at 12 months of follow up, 8/25 failed in the laser trabeculoplasty group while 17/22 in the medication group (RR 0.41 95% IC 0.22 to 0.77). We observed a considerably heterogeneity when combining these studies (P = 0.001) and because of that we did not present data in a meta‐analysis. The failure criteria (IOP > or = 22 mmHg) in these two trials seems to be very similar. The major difference was that in Moriarty 1988 the sample comprised participants of African origin while in Sherwood 1987 the majority were probably white. These findings are detailed in the discussion section.
4. Argon laser trabeculoplasty versus trabeculectomy
Three trials compared effectiveness of laser trabeculoplasty with trabeculectomy: AGIS, Moorfields PTT; Watson 1984.
Failure to control IOP (Analysis 2.1) Failure to control IOP was more frequently observed in the laser trabeculoplasty group when compared with trabeculectomy at six months (RR 3.4, 95% CI 1.60 to 6.18) and two years (RR 2.03, 95% CI 1.38 to 2.98). We included the first interventions of AGIS and considered failure when the participant met the criteria for reintervention, secondary to uncontrolled IOP. This information was received after personal contact with the study co‐ordinator. At two years of follow up some heterogeneity was observed in the comparison (I2 = 50.8%). Watson 1984 did not describe this dichotomous outcome.
2.1. Analysis.
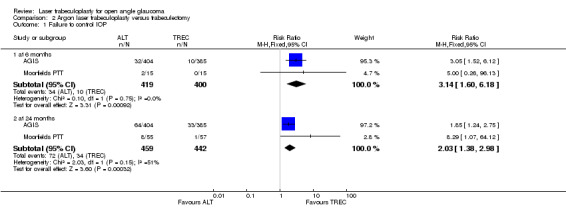
Comparison 2 Argon laser trabeculoplasty versus trabeculectomy, Outcome 1 Failure to control IOP.
Optic neuropathy progression
Although optic disc was photographed yearly in Moorfields PTT and after six months in Watson 1984, optic disc progression was not reported and was not considered a failure criterion in these two studies.
5. Diode laser trabeculoplasty (DLT) versus argon laser trabeculoplasty
Three trials analysed this comparison (Blyth 1999; Brancato 1991; Chung 1998).
Failure to control IOP This outcome was described in Blyth 1999 and Brancato 1991. At six months of follow up there was no significant difference between groups in the risk of uncontrolled IOP (RR 1.0, 95% CI 0.26 to 3.81). At one year of follow up the risk of uncontrolled IOP seemed to be greater in the diode trabeculoplasty group, but that was not statistically significant (RR 3.0, 95% CI 0.37 to 24.17). At two years the results suggested the opposite, but the risk difference was not statistically significant (RR 0.50, 95% CI 0.10 to 2.43). Unfortunately these data could not be combined in a meta‐analysis as the outcomes were described at different timepoints.
Adverse effects: early IOP spikes (Analysis 3.1) Chung 1998 observed an increase in IOP from baseline of 2 to 6 mmHg in the first hour postoperatively in two eyes of the diode group and five eyes in the argon laser group. After 24 hours from the laser procedures in both groups no eye experienced elevated IOP greater than 3 mmHg from baseline. Brancato 1991 evaluated IOP after trabeculoplasty within two hours postoperatively and did not observe any IOP elevation greater than 5 mmHg in either group. In Blyth 1999 two eyes in each group experienced an increase of more than 5 mmHg after two hours postoperatively.
3.1. Analysis.
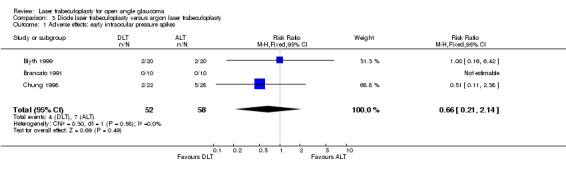
Comparison 3 Diode laser trabeculoplasty versus argon laser trabeculoplasty, Outcome 1 Adverse effects: early intraocular pressure spikes.
Adverse effects: PAS formation In Brancato 1991 no goniosynechiae formation was observed, while in Chung 1998 some eyes presented at three months with synechiae and in Blyth 1999, four eyes of the argon group developed PAS. There was no statistically significant difference in the risk ratio between the groups (RR 0.54; 95% CI 0.17 to 1.76).
6. Nd:Yag laser trabeculoplasty versus argon laser trabeculoplasty
One trial compared the effects of treatment with Nd:Yag laser (also called selective laser trabeculoplasty (SLT)) with argon laser trabeculoplasty (Damji 1999). The major outcomes described in this trial were 20% or more IOP decrease from initial values at six and 12 months and adverse effects (uveitis, early IOP spikes and PAS formation). Values for IOP at six months, mean change in IOP at six months and change in medications were also outcomes described, but they were not included as outcomes in this review.
Failure to control IOP Considering IOP lowering of less than 20% from initial values a primary outcome in the most recent publication, authors reported 35 eyes had uncontrolled IOP from 89 eyes randomised to SLT treatment at one year of follow up and 27 eyes from 87 randomised to laser trabeculoplasty. The risk of uncontrolled IOP was not different between these two groups at this follow up (RR 1.27 95% IC 0.84 to 1.90). These data were not presented in a meta‐analysis graph since it is the only trial comparing these treatments.
Adverse effects: uveitis one hour post laser ; early IOP spikes and PAS formation Flare and cells were checked clinically one hour post laser and graded on a scale from 0 (no reaction) to 4 (very marked reaction). There was no difference observed in mean score of flare in anterior chamber between the eyes treated with SLT (1.00 SD 0.6) and eyes treated with laser trabeculoplasty (0.8 SD 0.6).
7. Monochromatic wavelength trabeculoplasty versus bichromatic wavelength trabeculoplasty
Smith 1984 analysed the effect of trabeculoplasty conducted with a continuous‐wave laser (green) compared to the standard technology performed with the blue‐green continuous‐wave laser.
Failure to control IOP Failure was considered when the IOP achieved levels at which it was necessary to provide further glaucoma intervention in order to prevent glaucoma progression. There was no statistically significant difference in the risk ratio for uncontrolled IOP between the eyes that received trabeculoplasty with monochromatic or bichromatic wavelength (RR 0.57, 95% CI 0.18 to 1.83).
Adverse effects: PAS formation There was no statistically significant difference in the risk ratio of PAS formation between the two groups (RR 1.30, 95% CI 0.63 to 2.68).
8. Two stage trabeculoplasty versus one stage trabeculoplasty
Elsas 1989 analysed the effectiveness of laser trabeculoplasty performed in one session (one stage), treating 360 degrees of the angle and in two sessions four weeks apart, treating 180 degrees of the angle in each session and reported risk of field progression and IOP spikes as outcomes. Grayson 1993 stratified eyes in three groups: group one was randomised to receive 100 burns over 360 degrees over the trabecular meshwork, group two received 50 burns over 180 degrees in two sessions and group three received 50 burns over 360 also in two sessions. The outcome measure reported was the need for further intervention which was not an included outcome in this review. Only at seven years follow up did there appear to be a significant benefit of a two stage procedure.
Visual field progression In Elsas 1989 there was no statistically significant difference in risk ratio of visual field progression between the two groups (RR 3.3, 95% CI 0.14 to 76.46).
Adverse effects: IOP spikes We defined spikes as IOP elevations above 22 mmHg. In Elsas 1989 six eyes in the two stages group developed IOP elevation, while 11 eyes in the one stage group showed this increase. There was not a statistically significant difference in risk ratio in these two groups (RR 0.60, 95% CI 0.28 to 1.31).
9. Superior trabeculoplasty versus inferior trabeculoplasty
In Grayson 1994, the primary outcome was the need for further glaucoma surgery, which was not included as an outcome in this review. There was no difference at two years of follow up in the need for further surgery between the group in which the meshwork was treated superiorly (18/40) and the group treated inferiorly (23/53).
10. Argon laser trabeculoplasty applied at different power levels
Rouhiainen 1988 is the only trial that compared different power levels for laser trabeculoplasty. Four groups were randomised to receive laser at 500 mW, 600 mW, 700 mW and 800 mW. We used 500 mW as standard technique and compared the other three power levels with the first. The authors described an adverse effect as outcome (PAS formation). Effectiveness in controlling IOP, visual field decay and optic nerve progression were unpublished data and no additional information was obtained from the authors.
Adverse effects: PAS formation with 500 mW laser trabeculoplasty versus 600 mW laser trabeculoplasty versus 700 mW laser trabeculoplasty versus 800 mW laser trabeculoplasty Two eyes in 29 randomised to 500 mW developed PAS formation at six months of follow up. There was no statistically significant difference in risk ratio of PAS formation when compared to the group of eyes treated with 600 mW, in which 7/30 developed PAS (RR 3.38; 95% CI 0.77 to 14.96), and when compared to eyes treated with 700 mW in which 5/30 developed PAS (RR 2.42, 95% CI 0.51 to 11.48). When eyes treated with 500 mW were compared to those treated with 800 mW, there was an increased risk of synechiae formation (12/30 developed PAS) (RR 5.80, 95% CI 1.42 to 23.69).
11. Argon laser trabeculoplasty using 0.1 seconds versus 0.2 seconds
Time of exposure is a parameter used during laser trabeculoplasty analysed in Hugkulstone 1990. Group one was eyes treated with 0.1 seconds of time exposure, and group two the fellow eye of each participant treated with a 0.2 seconds time exposure laser. Outcomes described were mean IOP at six, 12 and 18 months (which are not outcomes included in this review), and the presence of IOP spikes at one hour. Intraocular pressures were marginally but not significantly lower in the patients with longer treatment duration.
Adverse effects: Intraocular pressure spikes at one hour At one hour of follow up 7/33 eyes treated with energy delivered at 0.1 seconds and 10/33 eyes treated with 0.2 seconds developed an increase of 10 mmHg or more. There was no statistically significant difference in risk ratio (RR 0.70, 95% CI 0.30 to 1.62).
Discussion
Laser trabeculoplasty and its role in OAG management is still controversial and there is much variation in practice. There is doubt especially about the duration of the effect and concern that pressure control may abruptly fail at some unspecified time after treatment. Trial evidence does not adequately address this issue though there is a suggestion that compared to medical treatment, the relative effectiveness is less at two years than at six months.
This review included evidence of the effects of laser trabeculoplasty as a first intervention and in participants already using maximal medical tolerated therapy. It included trials published in the last 22 years during which period there have been numerous developments in glaucoma management. Topical carbonic anhydrase inhibitors, alpha‐agonists and prostaglandin analogues were introduced after 1990. The trials comparing medication and argon laser trabeculoplasty were usually designed before this decade (Bergea 1992; GLT; Moorfields PTT; Moriarty 1988; Sherwood 1987). Incisional surgery has also changed in the past few decades. The use of antimetabolites to augment trabeculectomy is increasingly common and an effective means of decreasing the risk of failure in controlling IOP (Wilkins 2005). Trials comparing trabeculoplasty and trabeculectomy were designed before the 1990s and antimetabolites were not used in these studies (Moorfields PTT; Watson 1984). In AGIS, 205 eyes of 378 treated initially with trabeculectomy were operated on after 1990 and probably augmented with 5‐Fluorouracil or mitomycin C.
The main outcomes in this review were uncontrolled IOP and glaucoma progression, detected by progressive visual field damage and progressive optic neuropathy. Unfortunately few trials had documented these last two outcomes, and some of them had such a short follow‐up (six months) that it would probably not be possible to detect progression. These trials with shorter follow up and without detection of glaucomatous neuropathy progression were included, not only because they met the inclusion criteria but also because maintaining IOP control even in the short term may result in less discomfort, inconvenience and costs for patients. The concept of target IOP, which defines failure in IOP control, has also evolved over this period. AGIS was one of the trials that concluded that a group of glaucomatous participants had an increased risk of progression when their IOP was consistently over 18 mmHg. In the majority of trials, uncontrolled IOP was considered to be consistently greater than 22 mmHg (Blyth 1999; Elsas 1989; GLT; Moorfields PTT; Moriarty 1988; Sherwood 1987). Even in AGIS, failure was determined when IOP was higher than 22 mmHg for certain groups. Bergea 1992 considered failure eyes in which daily curves of IOP had measurements over 26 mmHg. Other trials defined failure as the need for further surgical intervention (which was usually linked to uncontrolled IOP) (Grayson 1993; Smith 1984; Watson 1984)) or reducing IOP to less than 20% of initial values (Brancato 1991; Damji 1999).
Visual field testing has also changed in the past few decades. Because of this and inherent long term fluctuation in test performance, it was difficult to standardise visual field progression as an outcome. Health‐related quality of life is also an important outcome, but EMGT was the only trial in this review which attempted to estimate it.
Secondary outcomes were related to adverse effects and were: PAS formation, early IOP spikes, especially when different laser trabeculoplasty techniques were compared (Blyth 1999; Brancato 1991; Chung 1998; Damji 1999; Elsas 1989; Hugkulstone 1990; Rouhiainen 1988; Smith 1984), uveitis Damji 1999, ocular and general side effects (EMGT; Gandolfi 2004a).
With regard to methodological quality, only five trials were considered of good quality in all criteria measured (AGIS, Bergea 1992, EMGT, GLT, Sherwood 1987). If we included evidence from these trials alone, there is even less information to inform practice.
EMGT was a carefully designed RCT, planned in the early 1990s. It showed that treating early glaucoma with laser trabeculoplasty and a selective beta‐blocker reduced the risk of glaucoma progression. It was interesting to observe that the risk of ocular or systemic adverse effects was not greater in the treatment group even though the study was unmasked. Also there was not a statistically significant difference in health‐related quality of life between the treated and untreated participants.
The most important outcomes in this review, in our opinion, were those obtained from the comparison of laser trabeculoplasty, medication and trabeculectomy. Bergea 1992, GLT and Moorfields PTT were trials that described the effect of laser trabeculoplasty compared with medication in newly diagnosed participants. The results of these three trials suggested that at six and 24 months argon laser decreased the risk of uncontrolled IOP compared to medicine though the size of the effect was less at 24 months compared to six months suggesting a reduction in effect over time. The heterogeneity observed at six months in this comparison, although not statistically significant by the Chi square test (P = 0.23), is greater than 20% when analysed by I2 test (29%). Maybe this heterogeneity could be explained by differences in the samples, outcomes and also because in GLT both eyes were included in the analysis, without taking into account the lack of independence between them (they were actually used as independent treated and control units). But both the concept of IOP control and the medical treatments available have changed and these trials were designed before these changes occurred. There is no evidence that with these modern developments laser trabeculoplasty would decrease the risk of uncontrolled IOP. Further research is necessary to address this question.
Visual field was also a primary outcome, and there was no difference in the risk of decay in this function comparing trabeculoplasty or medication. It is interesting that in GLT there was an improvement in the results of visual field after randomisation, in both groups, suggesting a learning effect, common in this kind of psychophysical analysis, but which could also lead to erroneous conclusions. Another consideration is that there is much heterogeneity in the instruments for visual field analysis. In Bergea 1992 Goldman manual perimetry was used and the plotted charts were evaluated in a masked fashion by two independent observers. In Moorfields PTT visual field was tested with a Friedman Mark III analyser initially and after two years, a Humphrey field analyser was used. In GLT the Octopus automated perimeter models 201 or 2000, were used.
Optic disc evaluation also showed no difference in progression between participants treated with laser trabeculoplasty or medication between two and seven years of follow up, and we could observe some heterogeneity in this comparison (i2 = 37.2%). This finding could reflect heterogeneity in the methods of optic disc evaluation between trials.
In terms of complications, not surprisingly argon laser trabeculoplasty causes a higher risk of PAS formation when compared to medication. Considering systemic side effects, the risk of topical beta‐blocker induced bronchospasm after a metacholine test when compared to laser trabeculoplasty in asymptomatic participants was not statistically significant (Gandolfi 2005). Although this study was considered of good methodological quality, it is the only trial to examine this outcome and was small and probably lacked power to detect an effect.
Moriarty 1988 and Sherwood 1987 evaluated outcomes in participants already on maximal tolerated medical antiglaucoma therapy. Argon laser trabeculoplasty decreased the risk of uncontrolled IOP but there was considerable heterogeneity between the two trials. Racial differences and severity of glaucoma could explain the observed heterogeneity.
AGIS, Moorfields PTT and Watson 1984 all compared laser trabeculoplasty with trabeculectomy. At six months, trabeculectomy (only in a few cases mitomycin‐augmented) was associated with a reduced risk of uncontrolled IOP compared to laser trabeculoplasty. If this result is extrapolated to current glaucoma management, this difference in IOP reduction would be probably more pronounced, since antimetabolites are used more often and achieve a greater reduction in IOP. We could not detect a statistically significant difference between groups at 24 months because in combining the studies for this comparison, heterogeneity was too high; using both the random and fixed‐effect models for analysis.
Considering different techniques for argon laser trabeculoplasty, treating 360 degrees in one session or 180 degrees in two sessions did not affect the risk of progression of visual field loss after six months and did not increase the risk of postoperative IOP spikes (Elsas 1989). The sample in this trial was small and further studies would help to confirm these findings. The only factor that significantly increased the risk of PAS formation was using higher power settings, such as 800 mW (Rouhiainen 1988).
Other lasers were analysed in this review. Nd:Yag laser trabeculoplasty, also called SLT was evaluated in one trial which did not demonstrate any additional benefit from the new technology with possibly an increased risk of inflammation (Damji 1999). Selective laser trabeculoplasty can only be used for this purpose while diode laser which is cheap has multiple applications and is also portable. Brancato 1991 and Blyth 1999 showed that the chance of uncontrolled IOP was similar to participants treated with argon laser trabeculoplasty or diode at six months and one year and two years. The risk of early IOP spikes and PAS formation were also similar in both groups. Finally a monochromatic wave laser, manufactured by MIRA, was compared with the traditional bichromatic wavelength. There was no difference in the risk of uncontrolled IOP or of PAS formation. This laser was not approved by the FDA and consequently not used in USA.
Authors' conclusions
Implications for practice.
In newly diagnosed OAG participants, the risk of uncontrolled IOP at six months and two years of follow up is higher when medical treatment is instituted compared to laser trabeculoplasty but this evidence does not include any of the more recently introduced medications.
On the other hand in participants already on maximal antiglaucoma medical therapy, the risk of uncontrolled IOP at six months of follow up is higher when laser trabeculoplasty is used compared to trabeculectomy though laser trabeculoplasty can still achieve a reducion in IOP in these circumstances.
Diode laser and selective trabeculoplasty had similar side effects when compared to laser trabeculoplasty. Selective laser trabeculoplasty has similar effectiveness in controlling IOP at six and 12 months to laser trabeculoplasty, but this evidence needs repetition in other randomised cllinical trials and SLT lacks the flexibility of other laser modes which can be used for other indications. The findings suggest that laser trabeculoplasty might be a useful initial treatment in people with early glaucoma when the availability of medicines or their cost or both might reduce the likelihood of compliance in the real world. This might be of particular relevance in poorer countries but concern will remain about the duration of effect. Certainly, rigorous follow up is needed after laser treatment so that timely additional treatments can be offered when the initial effect wears off.
Different protocols of laser trabeculoplasty have similar risks of uncontrolled IOP, but higher power levels such as 800 mW seemed to increase the risk of PAS formation. There is some evidence to suggest that there is differential effectiveness of laser trabeculoplasty in different ethnic minority groups particularly black African‐Americans (AGIS) and African‐Caribbean (Moriarty 1988) people and perhaps people from Africa itself alhough there are no trials reporting the effectiveness of laser trabeculoplasty in Africa. There is no doubt that laser trabeculoplasty can have an effect on IOP control though in some practices, it has fallen out of fashion. A treatment delivered at a single sitting which is not invasive and has minimal side effects and is relatively cheap is probably worth looking at again very closely.
Implications for research.
Further RCTs of current medical treatment compared to argon, diode laser and selective trabeculoplasty in different stages of OAG are required, with a more recent concept of target pressure as an outcome or at least achieving remission where field and or disc progression is prevented regardless of the IOP. Other pragmatic outcome measures should be considered such as delaying the need for surgery in people with early glaucoma or high risk OHT where effective medicines are expensive or hard to obtain a reliable and sustainable supply or both.
Secondly, further RCTs are necessary in different populations particularly in poorer countries where the availability of medicine is less certain and relatively more costly. This is of particular relevance to communities of black African origin throughout the world. Results of laser trabeculoplasty should be compared to medication and trabeculectomy, to determine if there is actually a racial difference in response to laser. Pseudoexfoliation syndrome and pigmentary glaucoma are subgroups in which it is thought there is a better response to laser treatment but this needs substantiation in properly conducted studies. In addition, a cost‐effectiveness analysis related to new prospective studies would be very important to determine possible economic advantages in performing laser trabeculoplasty.
Much can be done to improve the quality of RCTs addressing this important question. Trialists have assumed that masking of participants is not possible but since such a large placebo effect was demonstrated in the European Glaucoma Prevention Study, it has become all the more important to provide single if not double masking. Double masking might be achievable with the use of a specially devised pair of gonioscopy lenses, one of which absorbs laser energy. In such a circumstance a standard laser power would have to be used and some degree of unmasking may occur if the operator notes blanching of trabecular pigment or bubbling but this is still probably better than no masking. In any event, masking of intervention status by those determining outcome should always be employed.
The other vitally important question is whether we can gain a better understanding of the determinants of responsiveness to laser trabeculoplasty; who responds and who does not and why? Related to this is discovering what determines duration of effect and whether there is anyway this can be predicted?
What's new
| Date | Event | Description |
|---|---|---|
| 5 November 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Eyes and Vision Group editorial team developed and executed the electronic search strategies for this review. We are grateful to Stefano Miglior and Mark Wilkins for peer review comments on the protocol for this review and to Bernardo Soares, Catey Bunce and Jenny Burr for peer reviewing and/or advising on this review. The principal investigators of the majority of the included trials were contacted and we are very grateful for additional information provided.
Appendices
Appendix 1. CENTRAL search strategy used for Issue 2, 2007
#1 MeSH descriptor Glaucoma, Open‐Angle #2 open near angle near glaucoma* #3 poag #4 primary near glaucoma* #5 chronic near glaucoma* #6 secondary near glaucoma* #7 low near tension near glaucoma* #8 low near pressure near glaucoma* #9 normal near tension near glaucoma* #10 normal near pressure near glaucoma* #11 pigment near glaucoma* #12 MeSH descriptor Exfoliation Syndrome #13 exfoliat* near syndrome* #14 exfoliat* near glaucoma* #15 pseudoexfoliat* near syndrome* #16 pseudoexfoliat* near glaucoma* #17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 MeSH descriptor Trabeculectomy #19 trabeculectom* or trabeculotom* #20 argon laser* or ALT #21 trabeculoplast* #22 (#18 OR #19 OR #20 OR #21) #23 (#17 AND #22)
Appendix 2. MEDLINE search strategy used on OVID up to June 2007
1. exp clinical trial/ [publication type] 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp glaucoma open angle/ 14. (simple$ adj3 glaucoma$).tw. 15. (open adj2 angle adj2 glaucoma$).tw. 16. POAG.tw. 17. (primary adj2 glaucoma$).tw. 18. (chronic adj2 glaucoma$).tw. 19. (secondary adj2 glaucoma$).tw. 20. (low adj2 tension adj2 glaucoma$).tw. 21. (low adj2 pressure adj2 glaucoma$).tw. 22. (normal adj2 tension adj2 glaucoma$).tw. 23. (normal adj2 pressure adj2 glaucoma$).tw. 24. (pigment$ adj2 glaucoma$).tw. 25. exp exfoliation syndrome/ 26. (exfoliat$ adj2 syndrome$).tw. 27. (exfoliat$ adj2 glaucoma$).tw. 28. (pseudoexfoliat$ adj2 syndrome$).tw. 29. (pseudoexfoliat$ adj2 glaucoma$).tw. 30. or/13‐29 31. exp trabeculectomy/ 32. (trabeculectom$ or trabeculotom$).tw. 33. (argon laser$ or ALT).tw. 34. trabeculoplast$.tw. 35. or/31‐34 36. 30 and 35 37. 12 and 36
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE search strategy used on OVID up to June 2007
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp open angle glaucoma/ 34. (open adj2 angle adj2 glaucoma$).tw. 35. POAG.tw. 36. (primary adj2 glaucoma$).tw. 37. (chronic adj2 glaucoma$).tw. 38. (secondary adj2 glaucoma$).tw. 39. (low adj2 tension adj2 glaucoma$).tw. 40. (low adj2 pressure adj2 glaucoma$).tw. 41. (normal adj2 tension adj2 glaucoma$).tw. 42. (normal adj2 pressure adj2 glaucoma$).tw. 43. (pigment$ adj2 glaucoma$).tw. 44. exp exfoliation syndrome/ 45. (exfoliat$ adj2 syndrome$).tw. 46. (exfoliat$ adj2 glaucoma$).tw. 47. (pseudoexfoliat$ adj2 syndrome$).tw. 48. (pseudoexfoliat$ adj2 glaucoma$).tw. 49. or/33‐48 50. exp trabeculectomy/ 51. (trabeculectom$ or trabeculotom$).tw. 52. (argon laser$ or ALT).tw. 53. trabeculoplast$.tw. 54. or/50‐53 55. 49 and 54 56. 32 and 55
Appendix 4. LILACS search terms used on 15 June 2007
glaucoma and trabecul$
Data and analyses
Comparison 1. Argon laser trabeculoplasty versus medication in newly diagnosed participants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to control IOP | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 2 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.24, 0.61] |
| 1.2 at 24 months | 3 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.71, 0.91] |
| 2 Visual field progression | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 at 24 months | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.16] |
| 3 Optic neuropathy progression | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 at 24 months | 2 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.34] |
| 4 Adverse effects: PAS formation | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.15 [5.63, 22.09] |
Comparison 2. Argon laser trabeculoplasty versus trabeculectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to control IOP | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 2 | 819 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.60, 6.18] |
| 1.2 at 24 months | 2 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.38, 2.98] |
| 2 Failure to control IOP | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 2 | 819 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [1.59, 6.16] |
| 2.2 at 24 months | 2 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.74, 10.43] |
2.2. Analysis.
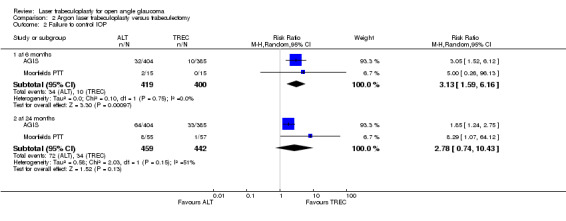
Comparison 2 Argon laser trabeculoplasty versus trabeculectomy, Outcome 2 Failure to control IOP.
Comparison 3. Diode laser trabeculoplasty versus argon laser trabeculoplasty.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects: early intraocular pressure spikes | 3 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.21, 2.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AGIS.
| Methods | Allocation: randomised, centralised, stratified list generated by a formal procedure, assigned the eye to one of the two surgical sequences. Both eyes could be enrolled if they both met inclusion criteria at the same time and they were randomised separately Masking: evaluator (whenever possible). If the evaluator gained knowledge of the treatment assigned, they noted this on the examination form Follow up: 5 years Centres: 11 | |
| Participants | N = 591 participants (789 eyes) Diagnosis: 1. POAG in a phakic eye 2. OAG in a phakic eye four weeks or more after laser iridotomy, provided the eye is not inflamed, steroid medication has not been used for a week, and less of one twelfth of the trabecular meshwork circumference is blocked by PAS 3. study eye on maximal medical therapy tolerated 4. at least 1 visual field test before the eligibility test 5. study eye meets at least 1 of the 9 combinations of criteria for consistent elevated IOP, glaucomatous visual field defect and optic disk rim deterioration specified in the study 6. visual acuity of 20/80 or better (Snellen) 7. visual field score at least 1 and not more than 16 8. study eye treatable with either with trabeculoplasty or trabeculectomy 9. patients able to cooperate 10. patients sign consent form Age: 35 to 80 years old (median 67 year old) Gender: 46% male; 54% female Race: 42% white, 56% black, 2% other History: hereditary 38% (first degree), hypertension 50%, vascular disorder 20%, diabetes 20% | |
| Interventions | 1. ALT + trabeculectomy + trabeculectomy (n = 404) 2. Trabeculectomy + ALT + trabeculectomy (n = 385) | |
| Outcomes | Early failure (6 weeks): IOP > initial levels, SVFD Late failure (more than 6 weeks): when met again the eligibility criteria with maximal medical therapy (eligibility criteria are explicited in the AGIS protocol and combines visual field severity with IOP levels) | |
| Notes | Considering this study has three interventions in each group, we will consider the results just of the first intervention, which were obtained by personal contact | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bergea 1992.
| Methods | Allocation: randomised Masking: not mentioned Follow up: 24 months Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 82 participants (82 eyes) Diagnosis: 1. newly diagnosed simple or capsular glaucoma 2. untreated mean daytime IOP between 25 and 50 mmHg 3. trabecular meshwork visible at least 3/4 at gonioscopy 4. reproducible visual field defect at automated perimetry (Competer 350, threshold program 30 degrees) or Goldmann perimetry. Eye included: the worse, but fellow eye received the same therapy if necessary Exclusion criteria: participants with other causes of visual field loss, participants that could not cooperate with a reliable visual field examination, gonioscopy or fundus examination, severe visual field in one eye (PV less than 100), visual acuity less than 0.3 at Snellen's fraction, refractive errors greater than + ‐ 5.00, aphakia or pseudophakia, ocular inflammation, corneal disease, age below 50 years Age: at least 50 years old (70.8) Sex: not mentioned Race: all Caucasian History: all phakic, 29 participants had bilateral glaucomatous damage, no description of concomitant pathologies | |
| Interventions | 1. ALT (n = 40). 2 sessions 1 month apart (randomly assigned superiorly or inferiorly). 50 spots, 50 micra, 0.1 seconds. No postoperative steroids were used 2. Ocular hypotensive medication (pilocarpine 4%, 3 times daily) (n = 42). Extra medications were not mentioned in the first publication. Stepped medication described in 1992: 1. beta‐blocker; 2. oral acetazolamide; 3. ALT; 4. surgery | |
| Outcomes | Failure: 1. 2 daily IOP curve > 26 mmHg 1 week apart 2. IOP reduction < 4 mmHg on 2 following IOP curves (1 week apart) 3. clinically evident visual field decay 4. adverse reaction that necessitates change in medication Success: no indication for additional therapy Continuous data of pressure for superior or inferior trabeculoplasty Optic nerve progression (documented with stereophotographs analysed by a 2 masked examiners, projected simultaneously) | |
| Notes | 1 participant deceased after 10 months of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Blyth 1999.
| Methods | Allocation: randomised (table of random numbers) Masking: not mentioned Follow up: 14 months ITT analysis: not mentioned | |
| Participants | N = 40 participants (40 eyes) Diagnosis: 1. POAG for which maximum medical therapy had failed to control IOP at less than 22 mmHg. Maximum therapy for this study was topical timolol 0.25% twice daily and pilocarpine 2% 4 times a day 2. no evidence of pseudoexfoliation or pigment dispersion syndrome 3. the absence of corneal opacities which might preclude view of the trabecular meshwork 4. no previous surgery or trabecular photocoagulation 5. the participant should be willing and capable of giving informed consent to the treatment and able to complete follow‐up visits Age: mean 65.8 years in DLT group and 67.8 years in ALT group Sex: not mentioned History: not mentioned | |
| Interventions | 1. DLT (n = 20) 2. ALT (n = 20) | |
| Outcomes | Failure: 1. IOP > or equal 22 mmHg 2. PAS formation 3. Continuous IOP data | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brancato 1991.
| Methods | Allocation: randomised Masking: evaluator masked for gonioscopy and tonometry. Follow up: 12 months (7 to 20) ITT analysis: not mentioned (describe one loss of follow up). We used all randomised to analyse failure | |
| Participants | N = 20 participants Diagnosis: OAG (excluded eyes with closed angles, aphakic and pseudophakic, juvenile glaucoma and miopia over 3 diopters) Age: not mentioned Race: not mentioned Sex: not mentioned | |
| Interventions | 1. DLT (n =10) 2. ALT (n =10) | |
| Outcomes | Failure: less of 20% IOP reduction or need of changing in medication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chung 1998.
| Methods | Allocation: randomised, by a third person, random numbers table. If the patient had both eyes to be treated they were randomised separately. Masking: until patient signed informed consent Follow up: 5 years Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 46 participants (50 eyes) Diagnosis: OAG with maximally tolerated medical therapy. Included POAG, pseudoexfoliation syndrome, pigmentary glaucoma, patients with mixed mechanisms and NTG Age: 1. 76 years old +‐ 2.8; 2. 71 years old +‐ 2.5 Race: not mentioned History: 6 eyes in group 1 received intraocular surgeries before DLT and 5 eyes in group 2 (ALT) received previously intraocular surgeries | |
| Interventions | 1. DLT (n = 22) 2. ALT (n = 28) | |
| Outcomes | Failure: need of trabeculectomy Side effects: discomfort, PAS formation, inflammation | |
| Notes | No difference of IOP between groups at any time. 4 participants had both eyes randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Damji 1999.
| Methods | Allocation: randomised by a blocked randomisation schedule (computered generated), third party Masking: no Follow up: 12 months Centres: 1 ITT analysis: used | |
| Participants | N = 152 participants (176 eyes). Both eyes included but correlation between eyes was accounted. Diagnosis: OAG, primary, pigmentary or pseudoexfoliation) on maximal medical therapy or failed previous ALT (180/360, more than 6 months previously), had 2 sighted eyes. Exclusion criteria: advanced visual field defect, previous glaucoma surgery done, corneal disease, use of systemic steroids during the study Age: 1. 69.7 years (10.52); 2. 69.5 years (11.54) Race: not mentioned Sex: 72 male, 104 female | |
| Interventions | 1. SLT (n = 89) 2. ALT (n = 87) | |
| Outcomes | Primary outcome: less of 20% IOP reduction from initial values at 6 months and one year. Secondary: anterior chamber reaction, IOP spikes at one hour post laser and PAS formation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Elsas 1989.
| Methods | Allocation: randomised, methods not specified. When both eye were eligible, one eye was randomised to each group Masking: not specified Follow up: 6 months Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 34 participants (40 eyes) Diagnosis: IOP equal or greater than 25 mmHg in a pre laser curve in a patient with POAG or capsular glaucoma; glaucomatous cupping of the optic disc and glaucomatous visual field defect; no earlier glaucoma treatment Age: 1. 72.5 years (54 to 89); 2. 70.2 years (62 to 89) Race: not specified History: not specified | |
| Interventions | 1. ALT in 2 stages. Treatment of 180 degrees of trabecular meshwork in each stage (n = 19) 2. ALT in 1 stage. Treatment of 360 degrees of trabecular meshwork (n = 21) Treatment protocol: 50 micra spot size, 0.1 seconds, 0.8 to 2.0 W | |
| Outcomes | Failure: IOP > 22 mmHg with hypotensive medication Visual field deterioration (confirmed with manual perimetry) Optic disc deterioration (detected by biomicroscopy) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
EMGT.
| Methods | Allocation: randomised (set of sequentially numbered, opaque, sealed envelopes provided by a Data centre) Masking: computerised visual field and fundus photographs read by masked graders (Disc Photography Reading Center). IOP evaluator was also masked (and the status of collecting data was recorded at each study visit) Follow‐up: at least 4 years Centres: 2 clinical, 1 reading and 1 co‐ordinating ITT analysis: used Randomisation: January 1993 to April 1997 | |
| Participants | N = 255 participants (if possible both eyes with correction of correlation between fellow eyes) Diagnosis: Men and women with newly diagnosed, previously untreated COAG (primary, normal tension, exfoliative glaucoma). The diagnosis required repeatable visual fields defects in at least one eye, detected by static computerised perimetry (Humphrey 24‐2 Full‐Threshold program). Exclusion criteria included: advanced visual field defects (MD ‐16 dB or threat to fixation), visual acuity less than 0.5, mean IOP greater than 30 mmHg, lens opacities exceeding N1, C1 or P1 in the Lens Opacities Classification System II. Participants with glaucomatous visual field defect in both eyes eligible only if MD ‐10dB or better in one eye and ‐16dB in the other eye Age: mean 68.1 years (4.9) Race: not mentioned, but probably 100% white History: 20% had family history of glaucoma, 38% had systemic hypertension, 6% had myocardial infarction, 4% had diabetes, 9% had vasospasm, 10% had migraine 24% had both eyes eligible | |
| Interventions | 1. No treatment (n = 126) 2. Betaxolol and ALT, performed one week after inclusion (n = 129) Technique: full 360 degree ALT was administered If the eligible eye achieved 25 mmHg in 2 consecutive visits or the other eye 35 mmHg in 1 visit, latanoprost 50 micrograms/ml was administered once daily | |
| Outcomes | Primary: glaucoma progression (visual field changes or optic disk changes). Visual field progression was defined as worsening of 3 consecutive points in the Glaucoma Change Probability map, confirmed in 3 consecutive visual fields. Optic disc progression should be detected by a masking reader in a flicker chronoscopy and side by side comparison in 2 consecutive visits Secondary: explore natural history, explore the factors that may influence progression, change in IOP over time, vision‐related quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gandolfi 2005.
| Methods | Allocation: randomised, methods not specified Masking: not mentioned Follow up: 4 years (3 years and 1 year after cross over) ITT analysis: | |
| Participants | N = 32 participants randomised. Diagnosis: participants with POAG: IOP greater than 22 mmHg in both eyes (mean of the 2 highest readings of the daily phasing); glaucomatous visual field defects in at least 1 eye as assessed by a computer assisted static perimetry (Octopus G1). In case of a unilateral field defect, the fellow eye had to show optic disc cupping consistent with glaucomatous optic neuropathy Exclusion criteria: previous antiglaucoma treatment, smoking, history of allergic and respiratory disease, including asthma and atopy assessed by skin prick testing. Participants who experienced IOP > or = 22 mmHg during follow up were excluded Age: 44 to 67 years Gender: 17 women and 15 men History: not mentioned | |
| Interventions | 1. ALT (n = 16) 2.0.5% timolol twice daily (n = 16) No other treatment was administered to these participants during the follow up. If the IOP reached 22 mmHg or more the participant was excluded | |
| Outcomes | Primary: change in the PC20, the provocative concentration that reduced at least 20% of the forced expirated volume, presented in a logarithmic transformed value | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
GLT.
| Methods | Allocation: randomised. Blocked on patient: 1 eye 1 intervention, fellow eye the other Masking: IOP evaluator, visual field and optic disc reading centres Follow up: the study was controlled for 2 years, but there was a follow up until 9 years on an observational basis (no managed treatment) Centres: 8 clinical, 1 co‐ordinating, 1 photography reading centre, 1 visual field reading centre ITT analysis: | |
| Participants | N = 271 participants (542 eyes). At follow up (after 2 years) 203 participants were followed. Diagnosis: age > or = 35 years, IOP in both eyes > or = 22 mmHg on 2 consecutive visits, IOP ratio between 0.67 and 1.5 inclusive, glaucomatous field defect in at least 1 eye or disc abnormality in the presence of extremely elevated IOP, best corrected visual acuity 20/70 or better in both eyes, informed consent Exclusion criteria: history of glaucoma other than POAG, usage of topical or systemic antihypertensive medication within the last 6 months, severe paracentral or central field defect, contraindication for use of trial medication, previous eye surgery, goniosynechiae more than 10 degree, evidence of diabetic retinopathy, current use of corticosteroids, epinephrine or clonidine Age: median 61 years Race: 45% white, 43% black History: 15% diabetes; 12% coronary disease, 10% peripheral vascular disease, 37% hypertension, 7% anemia, 4% using alfa blocker, 6% using beta blocker | |
| Interventions | 1. ALT first followed by topical medication if needed (n = 271) 2. Medication first, applied according a stepped regimen (n = 271) | |
| Outcomes | Failure criteria: 1. Primary: prescription of more than first line medication Reduction of IOP was considered inadequate if the measurements were of 22 mmHg or more at 2 consecutive visits 2 weeks apart or above 80% of the reference IOP. The reference IOP could change if there was a visual field decay 2. Secondary: deterioration of visual field. Visual fields were examined using Program 32 on the Octopus 201 or 2000 automated perimeter. The analysis was numerically and clinically 3. Deterioration of optic disc (subjective assessment) 4. Change in IOP 5. Deterioration of visual acuity 6. Need of further glaucoma intervention 7. Intraocular spikes observed at 4 hours of the first application (data used) 8. PAS formation at 3 months of follow up | |
| Notes | At 2 years of follow up uncontrolled IOP was defined as need of one medication to the LF group and add a second medication to MF group. Early IOP spikes were considered after the first laser application | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Grayson 1993.
| Methods | Allocation: randomised (not mentioned allocation criteria, but were stratified by race before randomisation) Masking: not mentioned Follow up: 90 months Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 36 participants (45 eyes) Diagnosis: POAG and exfoliation syndrome, no previous ocular surgery, age 50 years or older, current treatment with maximally tolerated ocular hypotensive medication Age: 57 to 92 years (mean 75 +‐ 1.4) Race: 27 white and 9 black History: not mentioned | |
| Interventions | 1. ALT (100 burns, applied 360 degrees over the meshwork) (n = 15) 2. ALT (50 burns, applied over 180 degrees of the meshwork) (n = 15) 3. ALT (50 burns, applied over 360 degrees of the meshwork) (n = 15) | |
| Outcomes | Failure criteria: further intervention required (after completion of 100 burns of ALT) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Grayson 1994.
| Methods | Allocation: randomised Masking: not mentioned Follow up: 24 months Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 80 participants (102 eyes) Diagnosis: POAG and exfoliative glaucoma requiring initial ALT Age: not specified Race: not specified History: not specified | |
| Interventions | 1. ALT receiving 50 burns to the superior 180 degrees of the trabecular meshwork (n = 49 eyes) 2. ALT receiving 50 burns to the inferior 180 degrees of the trabecular meshwork (n = 53 eyes) | |
| Outcomes | Failure criteria: further intervention required (either completion of ALT or filtration surgery) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hugkulstone 1990.
| Methods | Allocation: randomised (one eye allocated to receive treatment with 0.2s and the fellow eye received 0.1s) Masking: evaluator (notes in chart) Follow up: 6 months and posteriorly a new analysis with 24 months of follow up Centres: 1 ITT: not mentioned | |
| Participants | N= 33 participants randomised (64 eyes) N=26 participants followed for 24 months Diagnosis: POAG on topical hypotensive medication who had IOP of 21 mmHg or more and/or who showed deteriorating visual fields, using a suprathreshold static visual field analyser (Friedmann). Pseudoesfooliation, pigment dispersion syndrome and other secondary glaucomas were excluded) Age: 73.4 (8.3) years Race: not speciefied History: not specified | |
| Interventions | 1. ALT with duration of 0.1 sec in OD and with 0.3 seconds each spot in OS (n=17) 2. ALT with duration of 0.2 sec in OD and with 0.1 seconds each spot in OS (n=16). All eyes received a 50 micra spot size, applied to all 360 degrees of the trabecular meshwork in one or two sessions. The power setting was started at 0.9 W | |
| Outcomes | Failure criteria: Adverse effects: IOP spikes after one hour of laser application | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moorfields PTT.
| Methods | Allocation: randomised (computer selection) Masking: not mentioned Follow up: 6 months to 8 years Centres: 1 ITT analysis: not mentioned (77 randomised; 44 analysed in the first publication in 1984) The worse eye analysis was performed in cases of bilateral glaucoma or random allocation | |
| Participants | N = 77 participants randomised (44 participants with a minimum of six months of follow up were analysed) Diagnosis: OAG newly diagnosed Inclusion criteria: IOP equal or greater 24 mmHg on 2 different occasions, cup to disc ratio equal or greater than 0.6 and/or notching, and/or pallor of the neuroretinal rim, glaucomatous visual field loss using the Friedmann Field Analyser Posterior publication = 168 participants Age: varied between 60.8 and 67.1 years History: not mentioned Race: not mentioned | |
| Interventions | 1. Medical treatment (pilocarpine and/or sympatomimetics and/or timolol and/or carbonic anhydrase inhibitor (n = 15). Medical regimen could have been changed to control IOP. n2 (post publication) = 56 2. Surgical treatment (trabeculectomy) (n = 15); n (post publication) = 57 3. ALT (plus pilocarpine if IOP not controlled). It was performed in 2 sessions 2 weeks apart, 50 burns over 180 degrees each session, 50 micra spot size, 0.1 seconds, 0.5‐1.0 W. 0.3% prednisolone was used 4 times a day for 4 days and pilocarpine if necessary. Pilocarpine was used 2% four times a day 1 week prior the laser treatment. Participants in this group could receive pilocarpine 2% to control their pressure during the follow up. n = 15; post publication n = 55 | |
| Outcomes | Failure criteria: IOP equal or greater than 22 mmHg after 3 months of treatment or visual field loss greater than 2% per annum | |
| Notes | If failure occurred the second line treatment was undertaken and again randomly allocated Success: 6 months: surgery = 100%; med = 87%; laser = 86%; 3 years: surgery = 99.9%; med = 89.2%; laser = 85% The visual field deterioration is determined by a mean score, but is shown in Table 4 (1994). There is not an absolute number. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Moriarty 1988.
| Methods | Allocation: randomised Masking: not mentioned Follow up: 12 months Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 30 participants (48 eyes) Diagnosis: Phakic POAG with IOP of 22 mmHg or greater despite maximal medical therapy. These participants had glaucomatous optic disc cupping and glaucomatous visual field changes on Goldmann perimetry. In bilateral cases right eyes were randomised and left eye received the opposite. Exclusion criteria: Developmental, aphakic, inflammatory, haemolitic and pseudo‐exfoliative types of glaucoma were not eligible. Age: 62 years (27 to 77 years) Race: black History: not mentioned | |
| Interventions | 1. ALT (n = 25 eyes). Technique: 50 micra in the anterior portion of the trabecular meshwork, 0.1 sec of duration, power was adjusted to 800 to 1000mW. 50 burns placed over nasal 180 degrees at first application. If after 3 months the IOP remained high the procedure was repeated at the 180 degrees temporally. Eyes received prednisolone acetate 1% if there was uveitis 2. Full tolerable antiglaucoma medical treatment (n = 23 eyes) ‐ pilocarpine 4% and acetazolamide orally. 4 participants also used timolol | |
| Outcomes | Success criteria: successful control: IOP < 22 mmHg Improvement: reduction in IOP of 5 mmHg but keeping levels of more than 22 mmHg No improvement: reduction in IOP of 4 mmHg or less but keeping levels of 22 mmHg or greater Deterioration: rise in IOP of 5 mmHg or more | |
| Notes | The authors describe the results of each success group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rouhiainen 1988.
| Methods | Allocation: randomised (each eye). Methods of allocation: picking from a hat Masking: not mentioned Centres: 1 ITT analysis: not applied | |
| Participants | N = 120 participants Diagnosis: POAG (60 participants) and capsular glaucoma (60 participants). All eyes receiving maximal tolerated antiglaucoma medication insufficient to control the glaucoma Age: 71 years (51 to 87) Race: not mentioned History: not mentioned | |
| Interventions | 1. ALT (n = 29), 50 micra, 0.1 sec, power level 500 mW 2. ALT, 50 micra, 0.1 sec, power level 600 mW (n = 30) 3. ALT, 50 micra, 0.1 sec, power level 600 mW (n = 30) 4. ALT, 50 micra, 0.1 sec, power level 800 mW (n = 30) . Medication was kept unchanged. No anti‐inflammatory drugs were used | |
| Outcomes | Failure criteria: 1. PAS formation 2. IOP > or = 21 mmHg or no decrease of IOP < 3 mmHg | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sherwood 1987.
| Methods | Allocation: randomised (one eye selected by computer, the other eye selected as control) Masking: evaluator and second visual field evaluator Follow up: 35 months (30 to 40) Centres: 1 ITT analysis: not mentioned, but losses were described | |
| Participants | N = 25 participants (50 eyes) Diagnosis: bilateral POAG as judged by elevation of IOP (above 21 mmHg), glaucomatous disc change and visual field loss. No previous eye surgery or evidence of other eye disease. Patients taking in both eyes the maximum antiglaucoma medication that could be tolerated. Despite treatment IOP was consistently exceeding 21 mmHg and considered to be inadequately controlled. Age: 72, 54 years (50 to 90) Race: not specified History: not mentioned | |
| Interventions | 1. ALT (n = 25) (360 degrees, 100 burns, 0.1 seconds, 150 to 350 micra size spots) continued with maximal medical therapy. The participants received topical prednisolone 1% 4 times a day for one week after the treatment 2. MMT (n = 25) | |
| Outcomes | Success criteria: IOP decrease of 20% or more from the baseline examination; no IOP readings above 21 mmHg; stable visual fields by Goldmann perimetry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Smith 1984.
| Methods | Allocation: randomised Masking: not mentioned Follow up: 2 to 14 months (9.5 months) Centres: 1 ITT analysis: not mentioned | |
| Participants | N = 100 participants (100 eyes) Diagnosis: COAG, phakic eyes, with visual field and/or optic nerve damage (not specified by which methods) and judged to be uncontrolled with maximum tolerated medical therapy Age: 1. 64.0 years (mean); 2. 69.4 years (mean) Race: 89% white participants, 11% black participants History: not mentioned | |
| Interventions | 1. Bichromatic wavelength (blue‐green) ALT, performed with 80 burns over 360 degrees of the anterior portion of the trabecular meshwork (n = 50 eyes) 2. Monochromatic wavelength (green) ALT applied with the same technique (n = 50 eyes) In both groups eyes were kept with MMT tolerated | |
| Outcomes | Failure: need for filtering surgery | |
| Notes | Green manufactured by MIRA; Blue‐green manufactured by Coherent Radiation Model 900 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Watson 1984.
| Methods | Allocation: randomised (blocked, by a third party not involved). If a participant required a second eye to be treated this was subjected to the same randomisation procedure as the first Masking: not mentioned Follow up: 6 months Centres: 2 ITT analysis: not mentioned | |
| Participants | N = 61 participants (95 eyes) Diagnosis: OAG classified as severe or with evidence of progression of disease or not responding to medical treatment. 7 participants had NTG, 1 was aphakic and 1 had pseudoexfoliation (2 eyes), but all had open angles Age: 70 years (range 38 to 86 years) Race: not mentioned History: not mentioned Race: not mentioned | |
| Interventions | 1. ALT (n = 46). Blue/green laser source, applied 180 degrees of the trabecular meshwork, 50 burns, 50 micra spots 2. Trabeculectomy (n = 48). Standard technique with a fornix based flap | |
| Outcomes | Continuous data; need for medication; need for filtering surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ALT: argon laser trabeculoplasty COAG: chronic open angle glaucoma DLT: diode laser trabeculoplasty IOP: intraocular pressure ITT: intention‐to‐treat MMT: maximal medical therapy NTG: normal tension glaucoma OAG: open angle glaucoma PAS: peripheral anterior synechiae POAG: primary open angle glaucoma SVFD: sustained visual field defect
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2006 | Allocation: peudo randomisation |
| Brancato 1988 | Outcome: continuous intraocular pressure |
| Demailly 1989a | Interventions: Argon laser trabeculoplasty versus vascular medication |
| Douglas 1987 | Follow up: 6 weeks |
| Englert 1997 | Follow up: 3 months |
| Frenkel 1997 | Outcome: continuous intraocular pressure |
| Heijl 1984 | Allocation: pseudo randomisation |
| Hollo 1996 | Allocation: (pseudorandomisation) right eye of each participant received argon laser trabeculoplasty and left eye received Q‐switched Nd:Yag laser trabeculoplasty (non‐randomised) |
| Huk 1991 | Follow up: 24 hours |
| Lai 2004 | Participants: people with primary open angle glaucoma and ocular hypertension |
| Moriarty 1993 | Follow up: 8 weeks |
| Nagar 2005 | Participants: people with primary open angle glaucoma and ocular hypertension |
| Popiela 2000 | Follow up: 3 months |
| Shin 1996 | Follow up: 35 days |
| Traverso 1984 | Follow up: 16 weeks |
| Tuulonen 1989 | Allocation: pseudorandomisation (participants born in even years received medication and participants born in odd years received argon laser trabeculoplasty) |
| Weinreb 1983 | Follow up: 24 hours |
| Weinreb 1983a | Follow up: 2 months |
Contributions of authors
Coordinating the review: CRM Data collection for the review: CRM Screening search results: CRM, AP Screening retrieved papers against inclusion criteria: CRM, AP Appraising quality of papers: CRM, AP Extracting data from papers: CRM, AP Writing to authors of papers for additional information: CRM Data management for the review: CRM Entering data into RevMan: CRM Writing the review: CRM, RW
Sources of support
Internal sources
Universidade Federal de Sao Paulo, Ophthalmology Department, Brazil.
External sources
Brazilian Cochrane Center, Brazil.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
AGIS {published and unpublished data}
- AGIS (Advanced Glaucoma Intervention Study) Investigators. The advanced glaucoma intervention study, 8: Risk of cataract formation after trabeculectomy. Archives of Ophthalmology 2001;119(12):1771‐9. [DOI] [PubMed] [Google Scholar]
- AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 9. Comparison of glaucoma outcomes in black and white patients within treatment groups. American Journal of Ophthalmology 2001;132(3):311‐20. [DOI] [PubMed] [Google Scholar]
- Anonymous. The Advanced Glaucoma Intervention Study (AGIS): 3. Baseline characteristics of black and white patients. Ophthalmology 1998;105(7):1137‐45. [DOI] [PubMed] [Google Scholar]
- Anonymous. The Advanced Glaucoma Intervention Study (AGIS): 4. Comparison of treatment outcomes within race. Seven‐year results. Ophthalmology 1998;105(7):1146‐64. [DOI] [PubMed] [Google Scholar]
- Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race. Ten‐year results. Ophthalmology 2004;111(4):651‐64. [DOI] [PubMed] [Google Scholar]
- Gaasterland DE, Blackwell B, Dally LG, Caprioli, J, Katz LJ, Ederer F, et al. The Advanced Glaucoma Intervention Study (AGIS): 10. Variability among academic glaucoma subspecialists in assessing optic disc notching. Transactions of the American Ophthalmological Society 2001;99:177‐85. [PMC free article] [PubMed] [Google Scholar]
- Schwartz AL, VanVeldhuisen PC, Gaasterland DE, Ederer F, Sullivan EK, Cyrlin MN, et al. The Advanced Glaucoma Intervention Study (AGIS): 5. Encapsulated bleb after initial trabeculectomy. American Journal of Ophthalmology 1999;127(1):8‐19. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. Advanced Glaucoma Intervention Study (AGIS): 2. Visual field: method of scoring and reliability. Ophthalmology 1994;101(8):1445‐55. [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods, and baseline characteristics of study patients. Controlled Clinical Trials 1994;15(4):299‐325. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 11. Risk factors for failure of trabeculectomy and argon laser trabeculoplasty. American Journal of Ophthalmology 2002;134(4):481‐93. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. American Journal of Ophthalmology 2002;134(4):499‐512. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. American Journal of Ophthalmology 2000;130(4):429‐40. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study, 6: effect of cataract on visual field and visual acuity. Archives of Ophthalmology 2000;118(12):1639‐52. [DOI] [PubMed] [Google Scholar]
Bergea 1992 {published data only}
- Bergea B, Bodin L, Svedbergh B. Primary argon laser trabeculoplasty vs pilocarpine IV. Long‐term effects on optic nerve head. Acta Ophthalmologica Scandinavica 1995;73(3):216‐21. [DOI] [PubMed] [Google Scholar]
- Bergea B, Bodin L, Svedbergh B. Primary argon laser trabeculoplasty vs pilocarpine. II: Long‐term effects on intraocular pressure and facility of outflow. Study design and additional therapy. Acta Ophthalmologica 1994;72(2):145‐54. [DOI] [PubMed] [Google Scholar]
- Bergea B, Bodin L, Svedbergh B. Primary argon laser trabeculoplasty vs pilocarpine. III. Long‐term effects on visual fields. Acta Ophthalmologica Scandinavica 1995;73(3):207‐215. [DOI] [PubMed] [Google Scholar]
- Bergea B, Svedbergh B. Primary argon laser trabeculoplasty vs. pilocarpine. Short‐term effects. Acta Ophthalmologica 1992;70(4):454‐60. [DOI] [PubMed] [Google Scholar]
- Svedbergh B, Bodin L, Bergea B. Primary argon laser trabeculoplasty versus pilocarpine. Investigative Ophthalmology and Visual Science 1994;35:Abstract 3762. [Google Scholar]
Blyth 1999 {published data only}
- Blyth CPJ, Moriarty AP, McHugh JDA. Diode laser trabeculoplasty versus argon laser trabeculoplasty in the control of primary open angle glaucoma. Lasers in Medical Science 1999;14(2):105‐8. [DOI] [PubMed] [Google Scholar]
- Moriarty AP, McHugh JD, Ffytche TJ, Marshall JM, Spalton DJ, Moriarty BJ. Diode laser trabeculoplasty (DLT) versus argon laser trabeculoplasty (ALT) in primary open angle glaucoma. American Academy of Ophthalmology 1994:107. [Google Scholar]
Brancato 1991 {published data only}
- Brancato R, Carassa R, Trabucchi G. Diode laser compared with argon laser for trabeculoplasty. American Journal of Ophthalmology 1991;112(1):50‐5. [DOI] [PubMed] [Google Scholar]
Chung 1998 {published data only}
- Chung PY, Schuman JS, Netland PA, Lloyd‐Muhammad RA, Jacobs DS. Five‐year results of a randomized, prospective, clinical trial of diode vs argon laser trabeculoplasty for open‐angle glaucoma. American Journal of Ophthalmology 1998;126(2):185‐90. [DOI] [PubMed] [Google Scholar]
Damji 1999 {published data only}
- Damji KF, Bovell AM, Hodge WG, Rock W, Shah K, Buhrman R, Pan I. Selective laser trabeculoplasty versus argon laser trabeculoplasty: results from a 1‐year randomised clinical trial trabeculoplasty: results from a 1‐year randomised clinical trial. British Journal of Ophthalmology 2006;90(12):1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damji KF, Shah KC, Rock WJ, Bains HS, Hodge WG. Selective laser trabeculoplasty v argon laser trabeculoplasty: a prospective randomised clinical trial. British Journal of Ophthalmology 1999;83(6):718‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge WG, Damji KF, Rock W, Buhrmann R, Bovell AM, Pan Y. Baseline IOP predicts selective laser trabeculoplasty success at 1 year post‐treatment: results from a randomised clinical trial. British Journal of Ophthalmology 2005;89(9):1157‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsas 1989 {published data only}
- Elsas T, Johnsen H, Brevik TA. The immediate pressure response to primary laser trabeculoplasty ‐ a comparison of one‐ and two‐stage treatment. Acta Ophthalmologica 1989;67(6):664‐8. [DOI] [PubMed] [Google Scholar]
EMGT {published data only}
- Heijl A, Leske MC, Bengtsson B, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmologica Scandinavia 2003;81(3):286‐93. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression ‐ Results from Early Manifest Glaucoma Trial. Archives of Ophthalmology 2002;120(10):1268‐79. [DOI] [PubMed] [Google Scholar]
- Hyman L, Komaroff E, Heijl A, Bengtsson B, Leske MC, Early Manifest Glaucoma Trial Group. Treatment and vision‐related quality of life in Early Manifest Glaucoma Trial. Ophthalmology 2005;112(9):1505‐13. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment ‐ The Early Manifest Glaucoma Trial. Archives of Ophthalmology 2003;121(1):48‐56. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 1999;106(11):2144‐53. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Komaroff E. Factors for progression and glaucoma treatment: The Early Manifest Glaucoma Trial. Current Opinion in Ophthalmology 2004;15(2):102‐6. [DOI] [PubMed] [Google Scholar]
Gandolfi 2005 {published data only}
- Gandolfi S, Chetta A, Cimino K, Mora P, Sangermani C, Tardini G. Bronchial reactivity in healthy individuals undergoing long‐term treatment with beta‐blockers. Archives of Ophthalmology 2005;123(1):35‐8. [DOI] [PubMed] [Google Scholar]
GLT {published data only}
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow‐up study: 7. Results. American Journal of Ophthalmology 1995;120(6):718‐31. [DOI] [PubMed] [Google Scholar]
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT). 2. Results of argon laser trabeculoplasty versus topical medicines. Ophthalmology 1990;97(11):1403‐13. [PubMed] [Google Scholar]
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT): 3. Design and methods. Controlled Clinical Trials 1991;12(4):504‐24. [DOI] [PubMed] [Google Scholar]
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT): 5. Subgroup differences at enrollment. Ophthalmic Surgery 1993;24(4):232‐40. [PubMed] [Google Scholar]
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT): 6. Treatment group differences in visual field changes. American Journal of Ophthalmology 1995;120(1):10‐22. [DOI] [PubMed] [Google Scholar]
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial: 4. Contralateral effects of timolol on the intraocular pressure of eyes treated with ALT. Ophthalmic Surgery 1991;22(6):324‐9. [PubMed] [Google Scholar]
- Kiri A, Spaeth G, Beckman H, Sternberg A, GLT Research Group. Optic disc characteristics of blacks and whites in the glaucoma laser trial (GLT). Investigative Ophthalmology and Visual Science 1994;35:Abstract 1187. [Google Scholar]
- Sternberg A, Kiri A, Beckman H, Spaeth G, GLT Research Group. Quantitative optic disk characteristics in hypertensive POAG, and diabetic POAG patients in the glaucoma laser trial. Investigative Ophthalmology and Visual Science 1994;35:Abstract 394. [Google Scholar]
- Wilensky JT, Beckman H, Meiner CL, Ritch R, Sternberg AL, Glaucoma Laser Trabeculoplasty Study Group. Contribution of each treatment session to IOP reduction resulting from split therapy ALT in the Glaucoma Laser Trial (GLT). Investigative Ophthalmology and Visual Science 1994;35:Abstract 3761. [Google Scholar]
Grayson 1993 {published data only}
- Grayson D, Ritch R, Camras C, Lustgarten J, Podos S. Influence of treatment protocol on the long‐term efficacy of argon laser trabeculoplasty. Journal of Glaucoma 1993;2(7):7‐12. [PubMed] [Google Scholar]
- Lustgarten J, Podos S M, Ritch R, Fischer R, Stetz D, Zborowski L, Boas R. Laser trabeculoplasty. A prospective study of treatment variables. Archives of Ophthalmology 1984;102(4):517‐9. [DOI] [PubMed] [Google Scholar]
Grayson 1994 {published data only}
- Grayson D, Chi T, Liebmann J, Ritch R. Initial argon laser trabeculoplasty to the inferior vs superior half of trabecular meshwork. Archives of Ophthalmology 1994;112(4):446‐7. [DOI] [PubMed] [Google Scholar]
Hugkulstone 1990 {published data only}
- Hugkulstone CE. Argon laser trabeculoplasty with standard and long duration. Acta Ophthalmologica 1990;68(5):579‐81. [DOI] [PubMed] [Google Scholar]
- Hugkulstone CE. Two‐year follow‐up of intra‐ocular pressure control with long duration argon laser trabeculoplasty. Ophthalmologica 1993;71(3):327‐31. [DOI] [PubMed] [Google Scholar]
Moorfields PTT {published data only}
- Hitchings R, Migdal C, Fitzke F. Intraocular pressure: does it protect the Visual Fields?. Glaucoma Update IV Berlin Heidelberg. Springer‐Verlag, 1991. [Google Scholar]
- Hitchings R, Migdal C, Gregory W, Poinooswamy D, Fitzke F. The use of Humphrey Perimeter to identify visual loss. Annual Congress of Royal College of Ophthalmologists. May, 1993.
- Hitchings R, Migdal C, Wormald R, Poinooswamy D, Fitzke F. The primary treatment trial: Changes in the visual field analysis by computerised‐assisted perimetry. Eye 1994;8(Pt 1):117‐20. [DOI] [PubMed] [Google Scholar]
- McHam M, Migdal C, Netland P. Early trabeculectomy in the management of primary open angle glaucoma. International Ophthalmology Clinics 1994;34(3):163‐72. [DOI] [PubMed] [Google Scholar]
- Migdal C. What is the appropriate treatment for patients with primary open angle glaucoma: medicine, laser or primary surgery. Ophthalmic Surgery 1995;26(2):108‐9. [PubMed] [Google Scholar]
- Migdal C, Clark P, Hitchings C. Glaucomatous field changes related to the method and the degree of intraocular pressure control. Documenta Ophthalmologica 1987;49:371‐6. [Google Scholar]
- Migdal C, Gregory W, Hitchings R. Long‐term functional outcome after early surgery compared with laser and medicine in open‐angle glaucoma. Ophthalmology 1994;101(10):1651‐6. [DOI] [PubMed] [Google Scholar]
- Migdal C, Hitchings R. Control of chronic simple glaucoma with primary medical, surgical and laser treatment. Transactions of the Ophthalmological Societies of the United Kingdom 1986;105(Pt 6):653‐6. [PubMed] [Google Scholar]
- Migdal C, Hitchings R. Primary therapy for chronic simple glaucoma the role of argon laser trabeculoplasty. Transactions of the Ophthalmological Societies of the United Kingdom 1984;104(Pt 1):62‐6. [PubMed] [Google Scholar]
- Migdal C, Hitchings R. The role of early surgery for open angle glaucoma. Ophthalmology Clinics of North America 1991;4(4):853‐9. [Google Scholar]
- Migdal C, Hitchings R, Gregory M. Effect of mean IOP levels on visual field preservation in treated glaucoma patients. Annual Congress of Royal College of Ophthalmologists. May 1993.
Moriarty 1988 {published data only}
- Moriarty BJ, Char JN, Acheson RW, Dunn DT. Argon laser trabeculoplasty in primary open‐angle glaucoma ‐ results in black Jamaican population. International Ophthalmology 1988;12(4):217‐21. [DOI] [PubMed] [Google Scholar]
Rouhiainen 1988 {published data only}
- Rouhiainen HJ, Terasvirta ME, Tuovinen EJ. Peripheral anterior synechiae formation after trabeculoplasty. Archives of Ophthalmology 1988;106(2):189‐91. [DOI] [PubMed] [Google Scholar]
- Rouhiainen HJ, Terasvirta ME, Tuovinen EJ. The effect of some treatment variables on the results of trabeculoplasty. Archives of Ophthalmology 1988;106(5):611‐3. [DOI] [PubMed] [Google Scholar]
Sherwood 1987 {published data only}
- Sherwood MB, Lattimer J, Hitchings RA. Laser trabeculoplasty as supplementary treatment for primary open angle glaucoma. British Journal of Ophthalmology 1987;71(3):188‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1984 {published data only}
- Smith J. Argon laser trabeculoplasty: comparison of bichromatic and monochromatic wavelengths. Ophthalmology 1984;91(4):355‐60. [PubMed] [Google Scholar]
Watson 1984 {published data only}
- Watson PG, Allen ED, Graham CM, Porter GP, Pickering MS. Argon laser trabeculoplasty or trabeculectomy a prospective randomised block study. Transactions of the Ophthalmological Societies of the United Kingdom 1984;104(Pt 1):55‐61. [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2006 {published data only}
- Agarwal HC, Poovali S, Sihota R, Dada T. Comparative evaluation of diode laser trabeculoplasty vs frequency doubled Nd :YAG laser trabeculoplasty in primary open angle glaucoma. Eye 2006;20:1352–6. [DOI] [PubMed] [Google Scholar]
Brancato 1988 {published data only}
- Brancato R, Menchini U, Pece A, Bandello F, Serini P, Fantaguzzi S. Laser trabeculoplasty: argon laser or krypton laser? [Trabeculoplastie au laser: laser a l'argon ou laser au krypton?]. Ophtalmologie 1988;2(3):221‐2. [PubMed] [Google Scholar]
Demailly 1989a {published data only}
- Demailly P, Lehrer M, Kretz G. Argon laser trabeculoretraction in normal pressure glaucoma. A prospective study on the tonometric and perimetric effect. Journal Francais d' Ophtalmologie 1989;12(3):183‐9. [PubMed] [Google Scholar]
Douglas 1987 {published data only}
- Douglas GR, Wijsman K. Effects of laser trabeculoplasty on intraocular pressure in the medically untreated eye. Canadian Journal of Ophthalmology 1987;22(3):157‐60. [PubMed] [Google Scholar]
Englert 1997 {published data only}
- Englert JA, Cox TA, Allingham RR, Shields MB. Argon vs diode laser trabeculoplasty. American Journal of Ophthalmology 1997;124(5):627‐31. [DOI] [PubMed] [Google Scholar]
Frenkel 1997 {published data only}
- Frenkel RE, Shin DH, Epstein DL, Hertzmark E, Bohn JL, Hong YJ. Laser trabeculoplasty: how little is enough. Ophthalmic Surgery & Lasers 1997;28(11):900‐4. [PubMed] [Google Scholar]
Heijl 1984 {published data only}
- Heijl A. One‐ and two‐session laser trabeculoplasty. A randomized, prospective study. Acta Ophthalmologica 1984;62(5):715‐24. [DOI] [PubMed] [Google Scholar]
Hollo 1996 {published data only}
- Hollo G. Argon and low energy, pulsed Nd:YAG laser trabeculoplasty. A prospective, comparative clinical and morphological study. Acta Ophthalmologica Scandinavica 1996;74(2):126‐31. [DOI] [PubMed] [Google Scholar]
Huk 1991 {published data only}
- Huk B, Garus HJ, Bleckmann H. Anti‐inflammatory treatment after argon laser trabeculoplasty. Ophthalmologica 1991;203(1):24‐9. [DOI] [PubMed] [Google Scholar]
Lai 2004 {published data only}
- Lai JSM, Chua JKH, Tham CCY, Lam DSC. Five‐year follow up of selective laser trabeculoplasty in Chinese eyes. Clinical Experimental Ophthalmology 2004;32(4):368‐72. [DOI] [PubMed] [Google Scholar]
Moriarty 1993 {published data only}
- Moriarty AP, McHugh JD, Spalton DJ, Ffytche TJ, Shah SM, Marshall J. Comparison of the anterior chamber inflammatory response to diode and argon laser trabeculoplasty using a laser flare meter. Ophthalmology 1993;100(8):1263‐7. [DOI] [PubMed] [Google Scholar]
Nagar 2005 {published data only}
- Nagar M, Ogunyomade A, O'Brart DP, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. British Journal of Ophthalmology 2005;89(11):1413‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Popiela 2000 {published data only}
- Popiela G, Muzyka M, Szelepin L, Cwirko M, Nizankowska MH. Use of YAG‐Selecta laser and argon laser in the treatment of open angle glaucoma [Zastosowanie lasera YAG‐Selecta i lasera argonowego w leczeniu jaskry otwartego kata]. Klinica Oczna 2000;102(2):129‐33. [PubMed] [Google Scholar]
Shin 1996 {published data only}
- Shin DH, Frenkel RE, David R, Cheetham JK. Effect of topical anti‐inflammatory treatment on the outcome of laser trabeculoplasty. The Fluorometholone‐Laser Trabeculoplasty Study Group. American Journal of Ophthalmology 1996;122(3):349‐54. [DOI] [PubMed] [Google Scholar]
Traverso 1984 {published data only}
- Traverso CE, Greenidge KC, Spaeth GL. Formation of peripheral anterior synechiae following argon laser trabeculoplasty. A prospective study to determine relationship to position of laser burns. Archives of Ophthalmology 1984;102(6):861‐3. [DOI] [PubMed] [Google Scholar]
Tuulonen 1989 {published data only}
- Tuulonen A, Koponen J, Alanko HI, Airaksinen PJ. Laser trabeculoplasty versus medication treatment as primary therapy for glaucoma. Acta Ophthalmologica 1989;67(3):275‐80. [DOI] [PubMed] [Google Scholar]
Weinreb 1983 {published data only}
- Weinreb RN, Ruderman J, Juster R, Zweig K. Immediate intraocular pressure response to argon laser trabeculoplasty. American Journal of Ophthalmology 1983;95(3):279‐86. [DOI] [PubMed] [Google Scholar]
Weinreb 1983a {published data only}
- Weinreb RN, Ruderman J, Juster R, Wilensky JT. Influence of the number of laser burns administered on the early results of argon laser trabeculoplasty. American Journal of Ophthalmology 1983;95(3):287‐92. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gandolfi 2004a {published data only}
- Gandolfi SA, Sangermani C, Cimino L, Ungaro N, Tardini, Viswanathan A, et al. Is there a non IOP related effect of brimonidine on visual field progression in human glaucoma?. Investigative Ophthalmology and Visual Science 2004;45:E abstract 2298. [Google Scholar]
Krasnov 1982 {published data only}
- Krasnov MM, Akopian VS, Il'ina TS, Kazakova EL. Laser treatment of primary open‐angle glaucoma. Randomized comparative studies. Cyclotrabeculospasis and trabeculoplasty. Vestnik Oftalmologii 1982;5:18‐22. [PubMed] [Google Scholar]
Schrems 1988 {published data only}
- Schrems W, Hofmann G, Krieglstein GK. Therapy of open‐angle glaucoma with the argon and neodymium laser. Fortschritte der Ophthalmologie 1988;85(1):119‐23. [PubMed] [Google Scholar]
Additional references
Bathija 1998
- Bathija R, Gupta N, Zangwill L, Weinreb RN. Changing definition of glaucoma. Journal of Glaucoma 1998;7(3):165‐9. [PubMed] [Google Scholar]
Bonomi 1998
- Bonomi L, Marchini G, Marraffa M, Bernardi P, Franco I, Perfetti S, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna‐Neumarkt Study. Ophthalmology 1998;105(2):209‐15. [DOI] [PubMed] [Google Scholar]
Buhrmann 2000
- Buhrmann RR, Quigley HA, Barron Y, West SK, Oliva MS, Mmbaga BB. Prevalence of glaucoma in a rural East African population. Investigative Ophthalmology and Visual Science 2000;41(1):40‐8. [PubMed] [Google Scholar]
Cedrone 1997
- Cedrone C, Culasso F, Cesareo M, Zapelloni A, Cedrone P, Cerulli L. Prevalence of glaucoma in Ponza, Italy: a comparison with other studies. Ophthalmic Epidemiology 1997;4(2):59‐72. [DOI] [PubMed] [Google Scholar]
Dielemans 1994
- Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, Jong PT. The prevalence of primary open‐angle glaucoma in a population‐based study in The Netherlands. The Rotterdam Study. Ophthalmology 1994;101(11):1851‐5. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] Section 6. In: The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & Sons, Ltd.
Hyman 2001
- Hyman L, Wu SY, Connell AM, Schachat A, Nemesure B, Hennis A, et al. Prevalence and causes of visual impairment in The Barbados Eye Study. Ophthalmology 2001;108(10):1751‐6. [DOI] [PubMed] [Google Scholar]
Kim 2000
- Kim YJ, Moon CS. One‐year follow‐up of laser trabeculoplasty using Q‐switched frequency‐doubled Nd:YAG laser of 523 nm wavelength. Ophthalmic Surgery and Lasers 2000;31(5):394‐9. [PubMed] [Google Scholar]
Krasnov 1973
- Krasnov MM. Laseropuncture of anterior chamber angle in glaucoma. American Journal of Ophthalmology 1973;75(4):674‐8. [DOI] [PubMed] [Google Scholar]
Lanzetta 1999
- Lanzetta P, Menchini U, Virgili G. Immediate intraocular pressure response to selective laser trabeculoplasty. British Journal of Ophthalmology 1999;83(1):29‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latina 1998
- Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G. Q‐switched 532‐nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology 1998;105(11):2082‐8; discussion 2089‐90. [DOI] [PubMed] [Google Scholar]
Leske 1983
- Leske MC. The epidemiology of open‐angle glaucoma: a review. American Journal of Epidemiology 1983;118(2):166‐91. [DOI] [PubMed] [Google Scholar]
Leske 1994
- Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Archives of Ophthalmology 1994;112(6):821‐9. [DOI] [PubMed] [Google Scholar]
Maier 2005
- Maier PC, Funk J, Schwarzer G, Antes G, Fal k‐Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta‐analysis of randomised controlled trials. BMJ 2005;331(7509):134‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 1989
- Mason RP, Kosoko O, Wilson MR, Martone JF, Cowan CLJ, Gear JC, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies. Part I. Prevalence findings. Ophthalmology 1989;96(9):1363‐8. [DOI] [PubMed] [Google Scholar]
Quigley 2006
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 2006;90(3):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Realini 2002
- Realini T, Fechtner RD. 56,000 ways to treat glaucoma. Ophthalmology 2002;109(11):1955‐6. [DOI] [PubMed] [Google Scholar]
Schwartz 1985
- Schwartz AL, Love DC, Schwartz MA. Long‐term follow‐up of argon laser trabeculoplasty for uncontrolled open‐angle glaucoma. Archives of Ophthalmology 1985;103(10):1482‐4. [DOI] [PubMed] [Google Scholar]
Shields 2005
- Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G, Shields MB. Shields' Textbook of Glaucoma. 5th Edition. Philadelphia: Lippinicott Williams & Wilkins, 2005. [Google Scholar]
Thylefors 1994
- Thylefors B, Negrel AD. The global impact of glaucoma. Bulletin of the World Health Organization 1994;72(3):323‐6. [PMC free article] [PubMed] [Google Scholar]
Tielsch 1991
- Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open‐angle glaucoma. The Baltimore Eye Survey. JAMA 1991;266(3):369‐74. [PubMed] [Google Scholar]
Traverso 1986
- Traverso CE, Spaeth GL, Starita RJ, Fellman RL, Greenidge KC, Poryzees E. Factors affecting the results of argon laser trabeculoplasty in open‐angle glaucoma. Ophthalmic Surgery 1986;17(9):554‐9. [PubMed] [Google Scholar]
Vass 2007
- Vass C, Hirn C, Sycha T, Findl O, Bauer P, Schmetterer L. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database of Systematic Reviews 2007, Issue 4. [Art. No.: CD003167. DOI: 10.1002/14651858.CD003167.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wensor 1998
- Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology 1998;105(4):733‐9. [DOI] [PubMed] [Google Scholar]
Wilkins 2005
- Wilkins M, Indar A, Wormald R. Intra‐operative mitomycin C for glaucoma surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [Art. No.: CD002897. DOI: 10.1002/14651858.CD002897.pub2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wise 1979
- Wise JB, Witter SL. Argon laser therapy for open‐angle glaucoma. A pilot study. Archives of Ophthalmology 1979;97(2):319‐22. [DOI] [PubMed] [Google Scholar]
Wolfs 2000
- Wolfs RC, Borger PH, Ramrattan RS, Klaver CC, Hulsman CA, Hofman A, et al. Changing views on open‐angle glaucoma: definitions and prevalences ‐ The Rotterdam Study. Investigative Ophthalmology and Visual Science 2000;41(11):3309‐21. [PubMed] [Google Scholar]
Worthen 1974
- Worthen DM, Wickham MG. Argon laser trabeculotomy. Transactions of the American Academy of Ophthalmology 1974;78:371‐5. [PubMed] [Google Scholar]


