Abstract
Background
There is a need for the development of comprehensive, global, evidence‐based guidance for stakeholder engagement in guideline development. Stakeholders are any individual or group who is responsible for or affected by health‐ and healthcare‐related decisions. This includes patients, the public, providers of health care and policymakers for example. As part of the guidance development process, Multi‐Stakeholder Engagement (MuSE) Consortium set out to conduct four concurrent systematic reviews to summarise the evidence on: (1) existing guidance for stakeholder engagement in guideline development, (2) barriers and facilitators to stakeholder engagement in guideline development, (3) managing conflicts of interest in stakeholder engagement in guideline development and (4) measuring the impact of stakeholder engagement in guideline development. This protocol addresses the second systematic review in the series.
Objectives
The objective of this review is to identify and synthesise the existing evidence on barriers and facilitators to stakeholder engagement in health guideline development. We will address this objective through two research questions: (1) What are the barriers to multi‐stakeholder engagement in health guideline development across any of the 18 steps of the GIN‐McMaster checklist? (2) What are the facilitators to multi‐stakeholder engagement in health guideline development across any of the 18 steps of the GIN‐McMaster checklist?
Search Methods
A comprehensive search strategy will be developed and peer‐reviewed in consultation with a medical librarian. We will search the following databases: MEDLINE, Cumulative Index to Nursing & Allied Health Literature (CINAHL), EMBASE, PsycInfo, Scopus, and Sociological Abstracts. To identify grey literature, we will search the websites of agencies who actively engage stakeholder groups such as the AHRQ, Canadian Institutes of Health Research (CIHR) Strategy for Patient‐Oriented Research (SPOR), INVOLVE, the National Institute for Health and Care Excellence (NICE) and the PCORI. We will also search the websites of guideline‐producing agencies, such as the American Academy of Pediatrics, Australia's National Health Medical Research Council (NHMRC) and the WHO. We will invite members of the team to suggest grey literature sources and we plan to broaden the search by soliciting suggestions via social media, such as Twitter.
Selection Criteria
We will include empirical qualitative and mixed‐method primary research studies which qualitatively report on the barriers or facilitators to stakeholder engagement in health guideline development. The population of interest is stakeholders in health guideline development. Building on previous work, we have identified 13 types of stakeholders whose input can enhance the relevance and uptake of guidelines: Patients, caregivers and patient advocates; Public; Providers of health care; Payers of health services; Payers of research; Policy makers; Program managers; Product makers; Purchasers; Principal investigators and their research teams; and Peer‐review editors/publishers. Eligible studies must describe stakeholder engagement at any of the following steps of the GIN‐McMaster Checklist for Guideline Development.
Data Collection and Analysis
All identified citations from electronic databases will be imported into Covidence software for screening and selection. Documents identified through our grey literature search will be managed and screened using an Excel spreadsheet. A two‐part study selection process will be used for all identified citations: (1) a title and abstract review and (2) full‐text review. At each stage, teams of two review authors will independently assess all potential studies in duplicate using a priori inclusion and exclusion criteria. Data will be extracted by two review authors independently and in duplicate according to a standardised data extraction form.
Main Results
The results of this review will be used to inform the development of guidance for multi‐stakeholder engagement in guideline development and implementation. This guidance will be official GRADE (Grading of Recommendations Assessment, Development and Evaluation) Working Group guidance. The GRADE system is internationally recognised as a standard for guideline development. The findings of this review will assist organisations who develop healthcare, public health and health policy guidelines, such as the World Health Organization, to involve multiple stakeholders in the guideline development process to ensure the development of relevant, high quality and transparent guidelines.
1. BACKGROUND
1.1. The problem, condition or issue
Health guidelines are ‘systematically developed evidence‐based statements which assist providers, recipients and other stakeholders in making informed decisions about appropriate health interventions’ (WHO, 2003). Guidelines play a crucial role in the delivery of evidence‐based medicine. The systematic examination of evidence promoted by evidence‐based medicine is done through a comprehensive search of the literature, critical appraisal of the quality of the evidence and interpretation of the findings in light of patients' preferences and societal values (Guyatt, 2008). These guidelines assist health practitioners, patients, caregivers, policymakers and other stakeholders to make informed decisions about health practice, public health and health policy. It is increasingly recognised that the engagement of multiple stakeholders in guideline development improves recommendation relevancy, uptake, implementation and sustainability (Esmail, 2015; Gagliardi, 2012; Moulding, 1999).
Stakeholders are ‘any individual or group who is responsible for or affected by health‐ and healthcare‐related decisions that can be informed by research evidence’ (Concannon, 2012). This broad definition includes, but is not limited to, patients, caregivers, the public, patient advocates, providers (e.g., nurses), policymakers (e.g., health ministries), payers of health services (e.g., insurers, public payers) and health research (e.g., national research institutes), purchasers (e.g., employers), researchers, product makers (e.g., drug makers) and peer review editors. Several entities, including the World Health Organization (WHO), the Canadian Institute for Health Research (CIHR), the National Institute for Health and Care Excellence (NICE) and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group recommend the inclusion of stakeholder groups in the guideline development and implementation process (Akl, 2017; CIHR, 2014; NICE, 2014; WHO, 2003). The Guidelines International Network (GIN) McMaster Checklist for Guideline Development, the global standard for guideline development, identifies ‘consumer and stakeholder involvement’ as Step 6 in the guideline development process (Schünemann, 2014).
There is a growing body of research into the methods for engaging stakeholders in guideline development (Armstrong, 2017; Cluzeau, 2012), as well as approaches to measuring the impact of stakeholder engagement in guideline development (Cottrell, 2014; Ray, 2017). For example, a 2017 review of the incorporation of patients' values and preferences in guidelines included guidance documents from 56 different organisations (Selva et al., 2017). However, there remains a need for the development of comprehensive, global, evidence‐based guidance for stakeholder engagement in health guideline development that brings together the vast literature amassed to date. The Multi‐Stakeholder Engagement Consortium (MuSE Consortium) was established in 2015 to advance methods and approaches used in stakeholder engaged health research. The consortium addresses research of all kinds—including reviews and guidelines – and includes more than 80 researchers, trainees and stakeholders from Australia, Brazil, Canada, Germany, Italy, Lebanon, the Netherlands, the Philippines, Switzerland, the UK and the USA. The team membership of MuSE includes researchers, policymakers, guideline developers, research funders, clinicians, patients, patient representatives and policymakers from various organisations including the Agency for Healthcare Research and Quality (AHRQ), the Campbell Collaboration, Cochrane, GRADE Working Group, Public Health Agency of Canada, Patient‐Centered Outcomes Research Institute (PCORI), Research and Development (RAND) Corporation, the World Health Organization (WHO), Joanna Briggs Institute (JBI) and multiple universities (Petkovic, 2020). As part of the consortium's work, a working group was formed to develop global guidance on stakeholder engagement in guideline development according to the 18‐step GIN‐McMaster Checklist. The working group set out to conduct four concurrent systematic reviews to summarise the evidence on: (1) existing guidance for stakeholder engagement in guideline development, (2) barriers and facilitators to stakeholder engagement in guideline development, (3) managing conflicts of interest in stakeholder engagement in guideline development and (4) measuring the impact of stakeholder engagement in guideline development. This protocol addresses the second systematic review in the series.
1.2. Definitions
For the purpose of this review, we define:
Guidelinesas ‘systematically developed evidence‐based statements which assist providers, recipients and other stakeholders in making informed decisions about appropriate health interventions’ (WHO, 2003).
Stakeholdersas ‘any individual or group who is responsible for or affected by health‐ and healthcare‐related decisions that may be informed by research evidence’ (Concannon et al., 2012).
Engagementas an approach to ensure the contribution of stakeholders towards the development of the guideline, completion of any of the stages of the guideline, or dissemination of the guideline and its recommendations (Frank, 2020; Pollock, 2018).
Levels of engagementas the intensity of involvement in the guideline development process (Oliver, 2008; Pollock, 2019). We operationalise two levels for the purpose of this review: (1) advisory/feedback or (2) participation in decision‐making or knowledge translation.
1.3. Description of the phenomena of interest
Stakeholder engagement in guideline development is the approach to gather input or contribution from stakeholders ‘towards the development of a guideline, completion of any stages of a guideline, or dissemination, uptake or evaluation of a guideline and its recommendations’ (Pollock, 2018). The term ‘stakeholders’ is inclusive of multiple individuals within a single stakeholder group (such as several patients) and multiple stakeholders across several stakeholder groups (such as patients, policymakers and providers of care). Engagement refers to a meaningful and active partnership among stakeholder groups (CIHR, 2019; Staniszewska, 2017) to develop, complete or disseminate a guideline. Other terms for engagement include ‘public involvement’, ‘consumer engagement’, ‘Patient and Public Involvement (PPI)’, ‘coproduction’, ‘co‐creation’, ‘activation’ and ‘involvement’.
Stakeholder engagement in research can occur at different levels of intensity, ranging from low‐intensity communication (i.e., stakeholders are informed of research processes) up to high‐intensity coproduction (i.e., stakeholders are equal members of the research team and participate in shared decision‐making) (Concannon, 2012). From previous work (Crowe, 2017; Oliver, 2008; Pollock, 2019), we identify two levels of engagement in guideline development, namely (1) advisory/feedback, and (2) participation in decision‐making or knowledge translation. These levels were selected for our operational use, but we acknowledge that within these, there may be different levels.
There are many activities required for effective stakeholder engagement in guideline development (see Figure 1). The engagement of stakeholders from multiple disciplines or specialities is typically recommended to take account of a wide range of opinions. Indeed, the greater diversity in stakeholders' background increases the knowledge and skills available to make decisions (Oliver, 2018). However, the ability to gather and apply input from multiple stakeholders in a way that meaningfully reflects and addresses stakeholders' interests is not easily achieved, as increased diversity may also reduce group cohesiveness (Oliver, 2018). In their review of theoretical literature, Brodbeck and colleagues (Brodbeck, 2007) proposed that groups can out perform individual decision makers under conditions which promote sharing of knowledge and mutual learning. Oliver and colleagues (Oliver, 2018) built on this model, and suggested that these conditions include diverse group membership, skilled facilitation, time for knowledge sharing, following formal consensus processes, and integrating divergent perspectives. Stakeholder engagement is a complex process, and achieving these conditions requires both individual and organisational adoption. Organisational cultures and relationships act as a backdrop to these stakeholder engagement activities (Oliver, 2018). There are a multitude of individual‐ and organisational‐level factors that can affect a stakeholder's ability to engage in the guideline development process, or a guideline developer's ability to effectively engage them. Barriers to stakeholder engagement are defined as any variable, condition or factor that may impede stakeholder engagement in guideline development, while facilitators are any variable, condition or factor that may promote stakeholder engagement in guideline development. Such determinants may include knowledge, skills, time and resources, for example, and may occur across one or more steps of guideline development.
Figure 1.
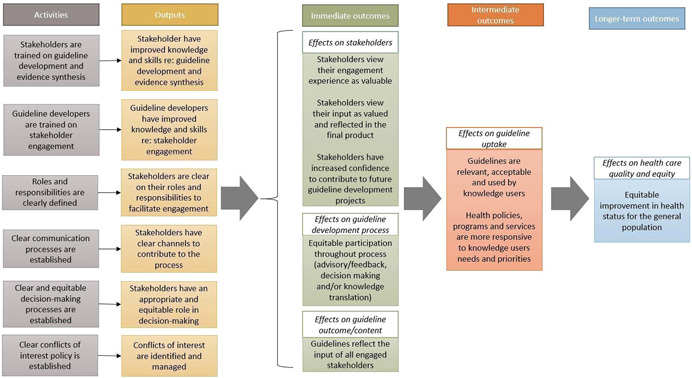
Logic model of the effects of stakeholder engagement in guideline development
1.4. Why it is important to do this review
With increased recognition of the value of stakeholder engagement in guideline development, there is a need for expert opinion and evidence‐based guidance on when and how to engage stakeholders. This review of barriers and facilitators to stakeholder engagement in guideline development will help fill this gap. It will build on existing reviews of barriers and facilitators, synthesise the current evidence‐base, and examine a broad range of stakeholder groups.
To date, several systematic reviews have been conducted on the topic of stakeholder engagement in guideline development, but few are explicitly focused on barriers and facilitators to engagement. Oxman and colleagues (Oxman et al., 2006) conducted a series of reviews to advise WHO to ensure that health care recommendations are informed by the best available research evidence. Relevant reviews in this series included group composition (Fretheim, 2006a), group processes (Fretheim, 2006b) and consumer involvement (Schünemann, 2006). However, these reviews were not systematic nor exhaustive, and prioritised existing syntheses over primary empirical studies. Relatedly, Oliver and colleagues (Oliver, 2018) synthesised theoretical literature to learn about the structure, processes and environment of committees generally, which has applications for health guideline development.
Much of the existing barrier and facilitator syntheses focus specifically on patients. Légaré and colleagues (Légaré, 2011) reviewed published and unpublished literature regarding the effectiveness of patient and public engagement programs in clinical practice guidelines and described the barriers and facilitators to these programs. Barriers included recruiting challenges, lack of representation of certain patient and public groups, and participants' lack of familiarity with medical and scientific terminology. Facilitators included the provision of training and support. Similarly, Grant and colleagues (Grant, 2018) conducted a rapid review and qualitative evidence synthesis to make inferences about how feasible online methods for patient engagement would be during clinical practice guideline development. The authors highlighted important considerations for patient and caregiver participation related to skills, time and resources. Notably, both of these reviews only considered patient and public involvement and did not consider the engagement of other stakeholder groups such as healthcare providers or policymakers, for example. Recently, Selva and colleagues (Selva, 2017) reviewed guidance documents for developing clinical guidelines to assess how these documents address the incorporation of patients' views. They identified the need for additional guidance in this area.
Cluzeau and colleagues (Cluzeau, 2012; Kelson, 2012; Kunz, 2012) conducted a literature review and workshop to answer six questions related to stakeholder engagement in guideline development, including identification of the potential barriers and facilitators to integrating stakeholder involvement. They defined stakeholders as ‘all those who have a legitimate interest in a guideline. They include healthcare professionals, patients and caregivers, public and private funding bodies, managers, employers and manufacturers’. They noted that integrating different, sometimes competing, stakeholder perspectives can be challenging. Other barriers included issues with potential bias among stakeholder views, and the costly nature of the process. In contrast, they found that educating stakeholders and ensuring effective communication enabled effective stakeholder engagement.
In sum, there is a lack of systematic reviews which have synthesised barriers and facilitators to multi‐stakeholder engagement in health guideline development. The results of this review will be used to inform the development of guidance for multi‐stakeholder engagement in guideline development and implementation. This guidance will be official GRADE (Grading of Recommendations Assessment, Development and Evaluation) Working Group guidance. The GRADE system is internationally recognised as a standard for guideline development (Guyatt, 2008). The findings of this review will assist organisations who develop healthcare, public health and health policy guidelines, such as the World Health Organization, to involve multiple stakeholders in the guideline development process to ensure the development of relevant, high quality and transparent guidelines.
2. OBJECTIVES
The objective of this review is to identify and synthesise the existing evidence on barriers and facilitators to stakeholder engagement in health guideline development. We will address this objective through two research questions:
-
1.
What are the barriers to multi‐stakeholder engagement in health guideline development across any of the 18 steps of the GIN‐McMaster checklist?
-
2.
What are the facilitators to multi‐stakeholder engagement in health guideline development across any of the 18 steps of the GIN‐McMaster checklist?
3. METHODS
The methods for this systematic review will follow the Cochrane Handbook for Systematic Reviews of Interventions and the Handbook for Synthesizing Qualitative Research, as appropriate (Higgins, 2019; Sandelowski, 2007). These methods were developed by a multi‐stakeholder research team that engaged investigators, trainees, providers of care, policymakers and patient representatives.
3.1. Criteria for including and excluding studies
3.1.1. Types of study designs
We will include empirical qualitative and mixed‐method primary research studies which qualitatively report on the barriers or facilitators to stakeholder engagement in health guideline development. This includes qualitative research studies using common methods such as interviews, focus groups, or surveys to collect participant experiences; case studies of existing programmes; and process evaluation studies. We will also include published guidelines which report lessons learned only if these statements are derived from empirical qualitative methods and the details of these methods are available in a published report. Mixed‐method and intervention studies (randomised or non‐randomised designs) will be eligible only if they apply qualitative methods and qualitatively report barriers and facilitators to engagement, independent of their quantitative findings.
Conference abstracts, commentaries, editorials, protocols, systematic reviews, meta‐ethnographies and other literature reviews will be excluded.
3.1.2. Population of interest
The population of interest is stakeholders in health guideline development. We define stakeholders as ‘any individual or group who is responsible for or affected by health‐ and healthcare‐related decisions’ (Concannon, 2012). Building on previous work, we have identified 13 types of stakeholders whose input can enhance the relevance and uptake of guidelines (Concannon, 2012, 2019; Tugwell, 2006). We will include studies which report on the barriers or facilitators to engagement of one or several of these stakeholder groups at any level of intensity at any step of the guideline development process. For comparisons with previously published stakeholder frameworks, please see Supporting Information Appendix 7. For the purposes of this review, have grouped them as follows:
Patients, caregivers and patient advocates
Public
Providers of health care
Payers of health services
Payers of research
Policy makers
Program managers
Product makers
Purchasers
Principal investigators and their research teams, and
Peer‐review editors/publishers
We recognise that within each of these categories, there is diversity and heterogeneity in types of stakeholders. For example, we recognise that patients and caregivers have differing characteristics and experiences, and their engagement brings diverse value to guideline development. The categories outlined above are for operational purposes and are based on prior research (Concannon, 2012, 2019; Tugwell, 2006). Studies which consider a single group of stakeholders or multiple groups of stakeholders will be eligible for inclusion. If a study includes multiple stakeholder groups, findings will be coded for each stakeholder group separately.
3.1.3. Phenomena of interest
Eligible studies must describe stakeholder engagement at any of the following steps of the GIN‐McMaster Checklist for Guideline Development (Supporting Information Appendix 9) (Schünemann, 2014):
-
1.
Organization, budget, planning and training
-
2.
Priority‐setting
-
3.
Guideline group membership
-
4.
Establishing guideline group processes
-
5.
Identifying target audience and topic selection
-
6.
Consumer and stakeholder involvement
-
7.
Conflict of interest considerations
-
8.
Question generation
-
9.
Considering importance of outcomes and interventions, values, preferences and utilities
-
10.
Deciding what evidence to include and searching for evidence
-
11.
Summarising evidence and considering additional information
-
12.
Judging quality, strength or certainty of body of evidence
-
13.
Developing recommendations and determining their strength
-
14.
Wording of recommendations and of considerations about implementation, feasibility and equity
-
15.
Reporting and peer review
-
16.
Dissemination and implementation
-
17.
Evaluation and use
-
18.
Updating
This checklist was selected because it represents the global standard for guideline development. Eligible studies must report primary data in their results on barriers and facilitators to stakeholder engagement at at least one step of the checklist. Studies which report on barriers and facilitators at one step, multiple steps, or across all steps will be considered for inclusion.
3.1.4. Outcomes of interest
The primary outcomes of interest are any barriers and facilitators to stakeholder engagement in health guideline development.
Barriers are defined as any variable or condition that impedes stakeholder engagement in guideline development or implementation.
Facilitators are defined as any variable or condition that promotes stakeholder engagement in guideline development or implementation.
For qualitative evidence, we will analyse first‐ and second‐order constructs related to stakeholders' perceptions and experiences of barriers and facilitators to their engagement in guideline development and implementation. These include participant quotations from interviews or focus groups, excerpts or quotations from documentary analysis, narrative descriptive summaries, author hypotheses, explanations and recommendations, themes and sub‐themes. First order‐constructs are defined as participant quotes and participant observations, while recognising that in secondary analysis these represent the participants' views as selected by the study authors in evidencing their second‐order constructs. Second‐order constructs are study authors' themes/concepts and interpretations.
3.1.5. Setting
We will place no restrictions on geographic setting. Studies focused on health guideline development from any country in the world are eligible for inclusion.
3.1.6. Language
No language restrictions will be applied.
3.1.7. Publication date
No publication date restrictions will be applied.
3.2. Search methods for identification of studies
This review is part of a series of four reviews conducted by the MuSE working group on stakeholder engagement in guideline development. As such, one comprehensive search strategy was developed and peer‐reviewed in consultation with a medical librarian in accordance with PRESS guidelines (McGowan, 2016). A second medical librarian reviewed the search strategy.
3.2.1. Electronic databases
We will search the following databases: MEDLINE (OVID) (Supporting Information Appendix 1), Cumulative Index to Nursing & Allied Health Literature (CINAHL; EBSCO) (Supporting Information Appendix 2), EMBASE (OVID) (Supporting Information Appendix 3), PsycInfo (OVID) (Supporting Information Appendix 4), Scopus (Supporting Information Appendix 5) and Sociological Abstracts (Supporting Information Appendix 6). Limits will not be placed on date, study design or language.
3.2.2. Searching other resources
To identify grey literature, we will search the websites of agencies who actively engage stakeholder groups such as the AHRQ, Canadian Institutes of Health Research (CIHR) Strategy for Patient‐Oriented Research (SPOR), INVOLVE, the National Institute for Health and Care Excellence (NICE) and the PCORI. We will also search the websites of guideline‐producing agencies, such as the American Academy of Pediatrics, Australia's National Health Medical Research Council (NHMRC) and the WHO. We will invite members of the team to suggest grey literature sources and we plan to broaden the search by soliciting suggestions via social media, such as Twitter.
3.2.3. Citations and reference lists
Backward and forward citation tracking will be performed on included articles to identify further eligible studies. We will review reference lists of relevant reviews to identify eligible primary studies for inclusion.
3.2.4. Contacting experts
We will contact authors of included studies to ask for suggested studies. We will also ask our MuSE Consortium members for potentially eligible primary studies that were not identified through the database search.
3.2.5. Screening of studies
All identified citations from electronic databases will be imported into Covidence software for screening and selection. Documents identified through our grey literature search will be managed and screened using an Excel spreadsheet. A two‐part study selection process will be used for all identified citations: (1) a title and abstract review and (2) full‐text review. At each stage, teams of two review authors will independently assess all potential studies in duplicate using a priori inclusion and exclusion criteria. We will resolve any disagreements through discussion or, if required, we will consult a third review author. We will produce a PRISMA flow diagram which reports the number of studies included and excluded at each stage. Reasons for exclusion will be provided for all studies assessed at full‐text.
3.3. Description of methods used in primary research
Relevant studies will have employed a qualitative or mixed‐method design. For example:
Brouwers et al. (2017) (mixed‐methods; Brouwers, 2017). Cancer patients, survivors, family members and caregivers were recruited to explore optimal approaches to patient and caregiver engagement in the development of cancer practice guidelines. Participants attended a workshop, completed a survey, or participated in a telephone interview. Quantitative data were analysed using Microsoft Excel, and qualitative data were analysed using thematic analysis. Authors report on barriers and facilitators to engaging in guideline development.
Atkins et al. (2013) (qualitative; Atkins, 2013). Members of three National Institute of Health and Clinical Excellence (NICE) advisory groups on acute physical, mental and public health were interviewed to investigate how they conceptualise evidence and experience the process. Participants were interviewed at the beginning and end of the life of the group. Interview transcripts were analysed using thematic and content analysis to identify main themes.
3.4. Criteria for determination of independent findings
3.4.1. Multiple reports of the same study
If a guideline development process is evaluated by more than one publication and is published in multiple reports, we will only include the most recent report or any additional report with unique outcome assessments (e.g., secondary analysis reports). If several reports of the same guideline process are identified and all publications report on different outcomes, each individual report must meet eligibility criteria for inclusion. Eligible reports of the same study will be analysed as a single study. Information on study sample sizes, guideline details, grant numbers and so on will be used to identify multiple reports from single studies and multiple studies in single reports. The authors of the reports will be contacted if it is unclear whether reports and studies provide independent findings.
3.4.2. Multiple studies in single reports
If more than one study is described in a single report, each study within the report will be coded separately.
3.4.3. Multiple studies involving the same guideline development team
Reports published by the same guideline development team are likely to follow similar stakeholder engagement practices, and so multiple reports published by a single guideline team will be aggregated.
3.5. Details of study coding categories
Data will be extracted by two review authors independently and in duplicate according to a standardised data extraction form (see Supporting Information Appendix 8). Disagreements will be discussed and resolved by a third reviewer. The data extraction form will be pilot tested by two review authors and coding categories will be clarified iteratively, as needed. Data will be extracted on basic study characteristics and methods including:
Study objective
Study setting
Type of guideline
Population and participant characteristics, including categorisation according to stakeholder group and equity‐relevant characteristics according to PROGRESS‐Plus (O'Neill, 2014).
Recruitment methods
Data collection methods
Analysis methods
In addition, we will extract information on study:
Limitations
Recommendations
Conclusions
Funding
Conflict of Interest
For qualitative outcomes, we will extract data using codes adopted from the Theoretical Domains Framework (Atkins, 2017). We will extract stakeholders' perceptions and experiences regarding the barriers and facilitators to their engagement in health guideline development from the results and discussion sections, including participant quotations from interviews or focus groups, excerpts or quotations from documentary analysis, narrative descriptive summaries, author hypotheses, explanations and recommendations, themes and sub‐themes.
Discrepancies in the data extraction process will be resolved by a third reviewer.
3.5.1. Dealing with missing data
Missing data is an inherent component of the qualitative research process. Since meanings and interpretations are the outputs of qualitative studies, data are always missing because there are some questions that respondents do not want to answer, others they circumvent, and aspects of their experiences which may not be reported (Singh, 2013). In such situations, the silence on these issues in itself becomes powerful data. Our findings will be mapped against the GIN‐McMaster Checklist and we will report which steps of the checklist do have supporting qualitative evidence and those which do not, thereby highlighting gaps in evidence and opportunities for new qualitative inquiry.
3.6. Critical appraisal
Two review authors will assess the methodological rigour of all included studies independently and in duplicate. We will assess the quality of qualitative studies and the qualitative components of mixed method studies using the Critical Appraisal Skills Programme (CASP) qualitative appraisal research tool (Singh, 2013). Discrepancies in the critical appraisal process will be resolved by a third reviewer.
3.7. Treatment of qualitative research
3.7.1. Identification of key findings
Our included studies will employ a qualitative approach to inquiry. We will use the ‘best fit’ framework method as a systematic and flexible approach to analysing the qualitative data (Booth, 2015; Carroll, 2013; Gale, 2013). Framework‐based synthesis, using the ‘best fit’ method, is a highly pragmatic and useful strategy for analysing a range of questions (Dixon‐Woods, 2011) and is supported by guideline development literature (Flemming, 2019). Framework analysis is a five‐stage process that includes familiarisation with the data, identifying a thematic framework, indexing (applying the framework), charting and mapping and interpretation (Spencer & Ritchie, 1994).
Implementing new or changing existing stakeholder engagement practices, in organisations who develop guidelines, requires a change in individual and collective behaviour. Changing behaviour requires an understanding of the influences on behaviour in the context in which they occur. We selected the Theoretical Domains Framework (TDF) for extracting and analysing our barriers and facilitator data (Atkins, 2017). Developed through a multidisciplinary consensus approach and subsequent validation, TDF consolidates overlapping behavioural theories into 14 domains encompassing 84 theoretical constructs, and provides a theoretical lens through which to view the cognitive, affective, social and environmental influences on behaviour and implementation.
Data will be analysed deductively, using the TDF domains to generate a framework of preliminary themes. Whilst the domains are purposively designed to be broad groupings of the possible factors to influence behaviour, the intent is to explore the important domains in further detail. Data will be coded independently by two reviewers. Disagreements will be resolved by discussion. The entire review team will be involved in developing key findings.
3.7.2. Mapping of key findings
All identified key findings will be mapped to the 18 topics (146 steps) of the guideline development process outlined by the GIN‐McMaster checklist (Shunemann 2014). We will present this information as a matrix indicating the barriers and facilitators that exists for each stakeholder group and for each step of guideline development. The findings of this mapping exercise will be combined with the results of the other reviews in this series (Khabsa, 2022; Petkovic, 2022) to develop international guidance on stakeholder engagement in guideline development.
3.8. Certainty of evidence
We will use the Confidence in the Evidence from Reviews of Qualitative research (CERQual) tool to assess the confidence of our findings. This tool is a method for assessing the strength of qualitative review evidence, similar to how the GRADE approach assesses the strength of quantitative evidence (Lewin et al., 2018). CERQual bases the evaluation on four criteria: (a) methodological limitations of included studies supporting a review finding, (b) the relevance of included studies to the review question, (c) the coherence of the review finding and (d) the adequacy of the data contributing to a review finding. Final CERQual assessments will be presented as a CERQual Evidence Profile.
CONTRIBUTIONS OF AUTHORS
Conceiving the review: PT, VW, TC, JP
Designing the review: JP, AR, VW, PT, TC
Coordinating the review: AR, OM, JP
Writing the protocol: AR, OM, JP, PA, JK, LL
Providing general advice on the review: EAA, MS, RM, VW, LD, NS, PC, SG
Securing funding for the review: PT, VW, JP
DECLARATIONS OF INTEREST
None.
PUBLISHED NOTES
None.
SOURCES OF SUPPORT
Internal sources
-
•
No sources of support provided
External sources
-
•
Canadian Institutes for Health Research, Canada
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
The MuSE project has been funded by the Canadian Institutes of Health Research. The authors acknowledge the valuable contributions and advice provided by the entire MuSE Consortium membership throughout the many stages of this project. We thank Ms. Layal Hneiny of the American University of Beirut who helped refine and translate the search strategies.
Magwood, O. , Riddle, A. , Petkovic, J. , Lytvyn, L. , Khabsa, J. , Atwere, P. , Akl, E. A. , Campbell, P. , Welch, V. , Smith, M. , Mustafa, R. A. , Limburg, H. , Dans, L. F. , Skoetz, N. , Grant, S. , Concannon, T. , & Tugwell, P. (2022). PROTOCOL: Barriers and facilitators to stakeholder engagement in health guideline development: A qualitative evidence synthesis. Campbell Systematic Reviews, 18, e1237. 10.1002/cl2.1237
REFERENCES
- ADDITIONAL REFERENCES
- Akl, E. A. , Welch, V. , Pottie, K. , Eslava‐Schmalbach, J. , Darzi, A. , Sola, I. , Katikireddi, S. V. , Singh, J. , Murad, M. H. , Meerpohl, J. , Stanev, R. , Lang, E. , Matovinovic, E. , Shea, B. , Agoritsas, T. , Alexander, P. E. , Snellman, A. , Brignardello‐Petersen, R. , Gloss, D. , … Tugwell, P. (2017). GRADE equity guidelines 2: Considering health equity in GRADE guideline development: Equity extension of the guideline development checklist. Journal of Clinical Epidemiology, 90, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, M. J. , Rueda, J. D. , Gronseth, G. S. , & Mullins, C. D. (2017). Framework for enhancing clinical practice guidelines through continuous patient engagement. Health Expectations, 20(1), 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins, L. , Francis, J. , Islam, R. , O'Connor, D. , Patey, A. , Ivers, N. , Foy, R. , Duncan, E. M. , Colquhoun, H. , Grimshaw, J. M. , & Lawton, R. (2017). A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implementation Science, 12(1), 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins, L. , Smith, J. A. , Kelly, M. P. , & Michie, S. (2013). The process of developing evidence‐based guidance in medicine and public health: A qualitative study of views from the inside. Implementation Science, 8(1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, A. , & Carroll, C. (2015). How to build up the actionable knowledge base: the role of “best fit” framework synthesis for studies of improvement in healthcare. BMJ Quality & Safety, 24(11), 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck, F. C. , Kerschreiter, R. , Mojzisch, A. , & Schulz‐Hardt, S. (2007). Group decision making under conditions of distributed knowledge: The information asymmetries model. The Academy of Management Review, 32(2), 459–479. [Google Scholar]
- Brouwers, M. C. , Vukmirovic, M. , Spithoff, K. , & Makarski, J. (2017). Understanding optimal approaches to patient and caregiver engagement in the development of cancer practice guidelines: A mixed methods study. BMC Health Services Research, 17(1), 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institutes for Health Research . (2014). Strategy for Patient‐Oriented Research—Patient Engagement Framework. https://cihr-irsc.gc.ca/e/48413.html
- Canadian Institutes for Health Research (CIHR) . (2019). Strategy for Patient‐Oriented Research—Patient Engagement Framework. https://cihr-irsc.gc.ca/e/48413.html
- Candy, B. , King, M. , Jones, L. , & Oliver, S. (2011). Using qualitative synthesis to explore hetergeneity of complex interventions. BMC Medical Research Methodology, 11, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, C. , Booth, A. , Leaviss, J. , & Rick, J. (2013). “Best fit” framework synthesis: refining the method. BMC Medical Research Methodology, 13(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluzeau, F. , Wedzicha, J. A. , Kelson, M. , Corn, J. , Kunz, R. , Walsh, J. , & Schünemann, H. J. (2012). Takeholder involvement: How to do it right: article 9 in integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proceedings of the American Thoracic Society, 9(5), 269–273. [DOI] [PubMed] [Google Scholar]
- Concannon, T. W. , Grant, S. , Welch, V. , Petkovic, J. , Selby, J. , Crowe, S. , Synnot, A. , Greer‐Smith, R. , Mayo‐Wilson, E. , Tambor, E. , Tugwell, P. , & Multi Stakeholder Engagement (MuSE), C. (2019). Practical guidance for involving stakeholders in health research. Journal of General Internal Medicine, 34(3), 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon, T. W. , Meissner, P. , Grunbaum, J. A. , McElwee, N. , Guise, J.‐M. , Santa, J. , Conway, P. H. , Daudelin, D. , Morrato, E. H. , & Leslie, L. K. (2012). A new taxonomy for stakeholder engagement in patient‐centered outcomes research. Journal of General Internal Medicine, 27(8), 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell, E. , Whitlock, E. , Kato, E. , Uhl, S. , Belinson, S. , Chang, C. , Hoomans, T. , Meltzer, D. , Noorani, H. , Robinson, K. , Schoelles, K. , Motu'apuaka, M. , Anderson, J. , Paynter, R. , & Guise, J.‐M. (2014). Defining the benefits of stakeholder engagement in systematic reviews. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK196184 [PubMed]
- Crowe, S. (2017). Who inspired my thinking?—Sherry Arnstein. Research for All, 1, 143–146. [Google Scholar]
- Dixon‐Woods, M. (2011). Using framework‐based synthesis for conducting reviews of qualitative studies. BMC Medicine, 9(1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmail, L. , Moore, E. , & Rein, A. (2015). Evaluating patient and stakeholder engagement in research: Moving from theory to practice. Journal of Comparative Effectiveness Research, 4(2), 133–145. [DOI] [PubMed] [Google Scholar]
- Flemming, K. , Booth, A. , Garside, R. , Tunçalp, Ã. ƒ– , & Noyes, J. (2019). Qualitative evidence synthesis for complex interventions and guideline development: Clarification of the purpose, designs and relevant methods. BMJ Glob Heal, 4(Suppl. 1), e000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, L. , Morton, S. C. , Guise, J. M. , Jull, J. , Concannon, T. , Tugwell, P. , Frank, L. , Morton, S. C. , Guise, J. M. , Jull, J. , Concannon, T. , & Tugwell, P. (2020). The essential enterprise: The critical role of accreditation in the 21st century. Journal of General Internal Medicine, 35(1), 304–314. [Google Scholar]
- Fretheim, A. , Schünemann, H. J. , & Oxman, A. D. (2006a). Improving the use of research evidence in guideline development: 3. Group composition and consultation process. Health Research Policy and Systems/BioMed Central, 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretheim, A. , Schünemann, H. J. , & Oxman, A. D. (2006b). Improving the use of research evidence in guideline development: 5. Group processes. Health research policy and systems/BioMed Central, 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi, A. R. , & Brouwers, M. C. (2012). Integrating guideline development and implementation: Analysis of guideline development manual instructions for generating implementation advice. Implementation Science: IS, 7, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, N. K. , Heath, G. , Cameron, E. , Rashid, S. , & Redwood, S. (2013). Using the framework method for the analysis of qualitative data in multi‐disciplinary health research. BMC Medical Research Methodology, 13(1), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, S. , Hazlewood, G. S. , Peay, H. L. , Lucas, A. , Coulter, I. , Fink, A. , & Khodyakov, D. (2018). Practical considerations for using online methods to engage patients in guideline development. The patient, 11(2), 155–166. [DOI] [PubMed] [Google Scholar]
- Guyatt, G. , Cook, D. , & Haynes, B. (2004). Evidence based medicine has come a long way. BMJ, 329, 990–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt, G. H. , Oxman, A. D. , Vist, G. E. , Kunz, R. , Falck‐Ytter, Y. , Alonso‐Coello, P. , & Schunemann, H. J. (2008). GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ, 336, 336–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Altman, D. G. , Gøtzsche, P. C. , Jüni, P. , Moher, D. , Oxman, A. D. , Savović, J. , Schulz, K. F. , Weeks, L. , & Sterne, J. A. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. J. & Welch, V. A. (Eds.). (2019). Cochrane handbook for systematic reviews of interventions (2nd ed). Version 6.0. Cochrane. [Google Scholar]
- Hong, Q. N. , Pluye, P. , Fabregues, S. , Bartlett, G. , Boardman, F. , Cargo, M. , Dagenais, P. , Gagnon, M.‐P. , Griffiths, F. , Nicolau, B. , O'Cathain, A. , Rousseau, M.‐C. , & Vedel, I. (2018). Mixed methods appraisal tool (MMAT). McGill University Version 2018. http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT__criteria-manual_2018-08-01_ENG.pdf [DOI] [PubMed]
- Kelson, M. , Akl, E. A. , Bastian, H. , Cluzeau, F. , Curtis, J. R. , Guyatt, G. , Montori, V. M. , Oliver, S. , Schünemann, H. J. , & ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline, D. (2012). Integrating values and consumer involvement in guidelines with the patient at the center: Article 8 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proceedings of the American Thoracic Society, 9(5), 229–233. [DOI] [PubMed] [Google Scholar]
- Khabsa, J. , Petkovic, J. , Riddle, A. , Lytvyn, L. , Magwood, O. , Atwere, P. , Campbell, P. , Katikireddi, S. V. , Merner, B. , Nasser, M. , Chang, S. , Jaramillo Garcia, A. P., Limburg, H. , Guise, J. , Tugwell, P. , & Akl, E. A. (2022). Conflict of interest issues when engaging stakeholders in health and healthcare guideline development: a systematic review. Cochrane Database of Systematic Reviews, CA000251. [DOI] [PMC free article] [PubMed]
- Kunz, R. , Fretheim, A. , Cluzeau, F. , Wilt, T. J. , Qaseem, A. , Lelgemann, M. , Kelson, M. , Guyatt, G. , Schünemann, H. J. , & ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline, D. (2012). Guideline group composition and group processes: Article 3 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proceedings of the American Thoracic Society, 9(5), 229–233. [DOI] [PubMed] [Google Scholar]
- Légaré, F. , Boivin, A. , van der Weijden, T. , Pakenham, C. , Burgers, J. , Légaré, J. , St‐Jacques, S. , & Gagnon, S. (2011). Patient and public involvement in clinical practice guidelines: A knowledge synthesis of existing programs. Medical Decision Making, 31(6), E45–E74. [DOI] [PubMed] [Google Scholar]
- Lewin, S. , Booth, A. , Glenton, C. , Munthe‐Kaas, H. , Rashidian, A. , Wainwright, M. , Bohren, M. A. , Tunçalp, Ö. , Colvin, C. J. , Garside, R. , Carlsen, B. , Langlois, E. V. , & Noyes, J. (2018). Applying GRADE‐CERQual to qualitative evidence synthesis findings: Introduction to the series. Implementation Science, 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, J. , Sampson, M. , Salzwedel, D. M. , Cogo, E. , Foerster, V. , & Lefebvre, C. (2016). PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology, 75, 40–46. [DOI] [PubMed] [Google Scholar]
- Moulding, N. T. , Silagy, C. A. , & Weller, D. P. (1999). A framework for effective management of change in clinical practice: Dissemination and implementation of clinical practice guidelines. BMJ Quality & Safety, 8(3), 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . (2014). Developing NICE guidelines: The manual. https://www.nice.org.uk/process/pmg20/chapter/introduction-and-overview [PubMed]
- Noyes, J. , Booth, A. , Cargo, M. , Flemming, K. , Harden, A. , Harris, J. , Garside, R. , Hannes, K. , Pantoja, T. , & Thomas, J. (2016). Chapter 21: Qualitative evidence. In Higgins J. P. T., thomas J., Chandler J., Cumpston M., Li T., Page M. J., & Welch V. A. (Eds.), Cochrane Handbooks for Systematic Reviews of Interventions (2nd ed., pp. 525–546). John Wiley & Sons. [Google Scholar]
- O'Neill, J. , Tabish, H. , Welch, V. , Petticrew, M. , Pottie, K. , Clarke, M. , Evans, T. , Pardo Pardo, J. , Waters, E. , White, H. , & Tugwell, P. (2014). Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology, 67(1), 56–64. [DOI] [PubMed] [Google Scholar]
- Oliver, S. , Hollingworth, K. , Briner, R. , Swann, C. , & Roche, C. (2018). Effective and efficient committee work: A systematic overview of multidisciplinary literatures. Evidence Base: A journal of evidence reviews in key policy areas, (2), 1–21. [Google Scholar]
- Oliver, S. R. , Rees, R. W. , Clarke‐Jones, L. , Milne, R. , Oakley, A. R. , Gabbay, J. , Stein, K. , Buchanan, P. , & Gyte, G. (2008). A multidimensional conceptual framework for analysing public involvement in health services research. Health Expectations, 11(1), 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman, A. D. , Fretheim, A. , Schünemann, H. J. , & SURE . (2006). Improving the use of research evidence in guideline development: Introduction. Health Research Policy and Systems, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic, J. , Riddle, A. , Akl, E. A. , Khabsa, J. , Lytvyn, L. , Atwere, P. , Campbell, P. , Chalkidou, K. , Chang, S. M. , Crowe, S. , Dans, L. , Jardali, F. E. , Ghersi, D. , Graham, I. D. , Grant, S. , Greer‐Smith, R. , Guise, J. M. , Hazlewood, G. , Jull, J. , … Tugwell, P. (2020). Protocol for the development of guidance for stakeholder engagement in health and healthcare guideline development and implementation. Systematic Reviews, 9(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic, J. , Riddle, A. , Lytvyn, L. , Khabsa, J. , Akl, E. A. , Welch, V. , Magwood, O. , Atwere, P. , Graham, I. D. , Grant, S. , John, D. , Katikireddi, S. V. , Langlois, E. , Mustafa, R. A. , Todhunter-Brown, A. , Schünemann, H. , Smith, M. , Stein, A. T. , Concannon, T. , & Tugwell, P. (2022). Guidance for stakeholder engagement in health guideline development: A scoping review. Cochrane Database of Systematic Reviews, CA000252. [Google Scholar]
- Pollock, A. , Campbell, P. , Struthers, C. , Synnot, A. , Nunn, J. , Hill, S. , Goodare, H. , Morris, J. , Watts, C. , & Morley, R. (2018). Stakeholder involvement in systematic reviews: A scoping review. Systematic Reviews, 7(1), 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, A. , Campbell, P. , Struthers, C. , Synnot, A. , Nunn, J. , Hill, S. , Goodare, H. , Morris, J. , Watts, C. , & Morley, R. (2019). Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. Journal of Health Services Research & Policy, 24(4), 245–255. [DOI] [PubMed] [Google Scholar]
- Ray, K. N. , & Miller, E. (2017). Strengthening stakeholder‐engaged research and research on stakeholder engagement. Journal of Comparative Effectiveness Research, 6(4), 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, J. , & Spencer, L. (1994). Qualitative data analysis for applied policy research. In Bryman A., & Burgess R. (Eds.), Analyzing qualitative data. (pp. 173–194). Routledge. 10.4324/9780203413081_chapter_9 [DOI] [Google Scholar]
- Sandelowski, M. , & Barroso, J. (2007). Handbook for synthesizing qualitative research. Springer Publishing Company. [Google Scholar]
- Schünemann, H. J. , Wiercioch, W. , Etxeandia, I. , Falavigna, M. , Santesso, N. , Mustafa, R. , Ventresca, M. , Brignardello‐Petersen, R. , Laisaar, K. T. , Kowalski, S. , Baldeh, T. , Zhang, Y. , Raid, U. , Neumann, I. , Norris, S. L. , Thornton, J. , Harbour, R. , Treweek, S. , Guyatt, G. , … Akl, E. A. (2014). Guidelines 2.0: Systematic development of a comprehensive checklist for a successful guideline enterprise. Canadian Medical Association Journal, 186(3), E123–E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann, H. J. , Fretheim, A. , & Oxman, A. D. (2006). Improving the use of research evidence in guideline development: 10. Integrating values and consumer involvement. Health Research Policy and Systems/BioMed Central, 4, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva, A. , Sanabria, A. J. , Pequeño, S. , Zhang, Y. , Solà, I. , Pardo‐Hernandez, H. , Selva, C. , Schünemann, H. , & Alonso‐Coello, P. (2017). Incorporating patients' views in guideline development: A systematic review of guidance documents. Journal of Clinical Epidemiology, 88, 102–112. [DOI] [PubMed] [Google Scholar]
- Singh, J. (2013). Critical appraisal skills programme. Journal of Pharmacology and Pharmacotherapeutics, 4(1), 76. [Google Scholar]
- Singh, S. , & Richards, L. (2003). Finding “central” themes in qualitative research. Qualitative Research Journal, 33(11), 5–17. [Google Scholar]
- Staniszewska, S. , Brett, J. , Simera, I. , Seers, K. , Mockford, C. , Goodlad, S. , Altman, D. G. , Moher, D. , Barber, R. , Denegri, S. , Entwistle, A. , Littlejohns, P. , Morris, C. , Suleman, R. , Thomas, V. , & Tysall, C. (2017). GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. BMJ, 358, j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. , Hernan, M. A. , Reeves, B. C. , Savovic, J. , Berkman, N. D. , Viswanathan, M. , Henry, D. , Altman, D. G. , Ansari, M. T. , Bourton, I. , Carpenter, J. R. , Phelan, A. , Churchill, R. , Deeks, J. J. , Hrobjartsson, A. , Kirkham, J. , Juni, P. , Loke, Y. K. , Pigott, T. D. , … Higgins, J. P. T. (2016). ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ, 355, i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwell, P. , Robinson, V. , Grimshaw, J. , & Santesso, N. (2006). Systematic reviews and knowledge translation. Bulletin of the World Health Organization, 84(8), 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grootel, L. , van Wesel, F. , O'Mara‐Eves, A. , Thomas, J. , Hox, J. , & Boeije, H. (2017). Using the realist perspective to link theory from qualitative evidence synthesis to quantitative studies: Broadening the matrix approach. Research synthesis methods, 8(3), 303–311. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2003). WHO handbook for guideline development (2nd ed.). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


