During the worldwide spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), age and comorbidities were identified as the key risk factors associated with infection severity and death.1,2 Even higher fatality rates were observed in kidney transplant recipients, probably because of their impaired immune response because of the long-term use of immunosuppressive drugs, in addition to the high prevalence of comorbidities such as diabetes and cardiovascular diseases.3,4
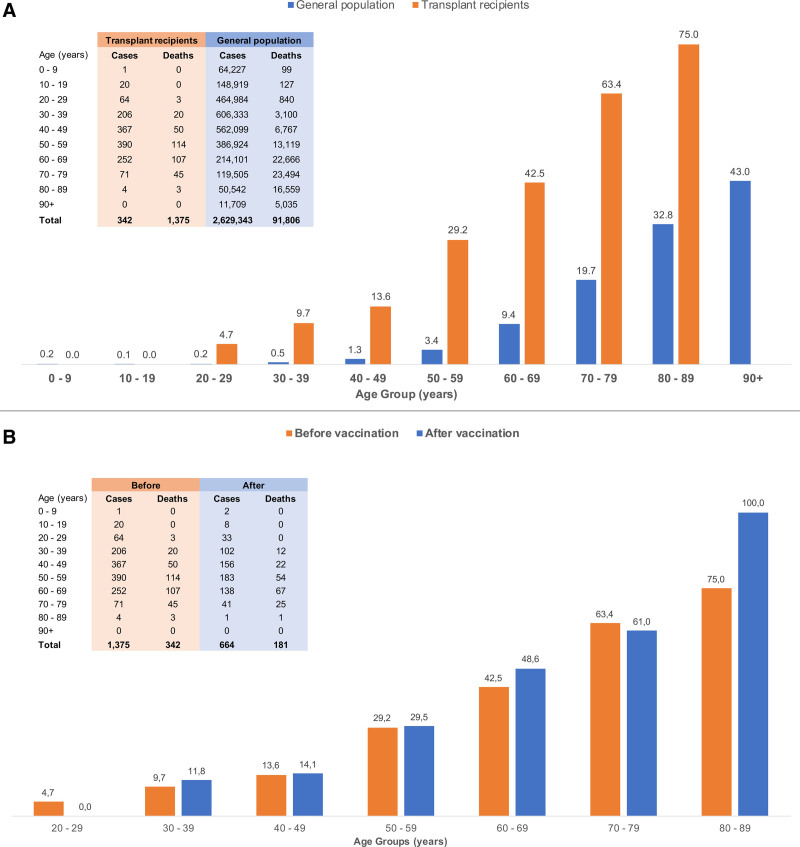
To evaluate the magnitude of this problem, we compared the coronavirus disease 2019 (COVID-19)–associated age-adjusted case fatality rates among kidney transplant recipients with the overall population in the state of São Paulo, Brazil, between March 2020 and March 2021 (Figure 1A). The incidence of COVID-19 was 11.7% among 11 715 kidney transplant recipients followed up in our transplant center (n = 1375), compared with 5.8% in the 45 million persons living in that state (n = 2.63 million).5 The 2-fold higher incidence of COVID-19 observed in kidney transplant recipients is probably influenced by increased awareness, closer follow-up, and immediate testing provided by the multidisciplinary healthcare team dedicated to this group of patients. In contrast, the overall crude fatality rate was >7-fold higher among the kidney transplant recipients compared with the state population (24.9% versus 3.5%), respectively, with higher fatality rates observed across all age strata. Importantly, the fatality rates were 23-fold higher (8.5% versus 0.37%) among young kidney transplant recipients aged 20 to 39 y (Figure 1A), who are known to have a lower prevalence of comorbidities, underscoring the negative impact of the chronic use of immunosuppressive drugs in this population.
FIGURE 1.
The COVID-19–associated age-adjusted case fatality rates among kidney transplant recipients and the general population in the state of São Paulo, Brazil. A, Comparison of COVID-19–associated case fatality rates, stratified by age, between kidney transplant recipients and the general population of the state of São Paulo, Brazil, before the COVID-19 immunization. Among 1375 kidney transplant recipients diagnosed with COVID-19 between March 2020 and March 2021, 342 died, reaching a case fatality rate of 24.9%. In the same period; 2.63 million people were diagnosed with COVID-19 in the state of São Paulo; and 91 806 died, yielding a case fatality rate of 3.5%. The data of the general population were extracted from the public and anonymized official registry provided by the government of the State of São Paulo.5 B, Comparison of COVID-19–associated case fatality rates, stratified by age, before and after the immunization of kidney transplant recipients. Between March 2020 and March 2021, before vaccination, there were 342 deaths among 1375 kidney transplant recipients diagnosed with COVID-19, reaching an overall case fatality rate of 24.9%. The immunization of the kidney transplant recipients at the transplant center started on March 20, 2021, as part of the prospective phase IV study with CoronaVac. Between May 10, 2021, 28 d after the second dose of vaccine, and January 4, 2022, there were 181 deaths among 664 patients diagnosed with COVID-19, yielding a case fatality rate of 27.3%. COVID-19, coronavirus disease 2019.
This scenario underscored the need to develop effective therapeutic and preventive strategies specific to this population. We started by reinforcing awareness about the disease and nonpharmacological preventive strategies while waiting for effective and safe pharmacological treatments and vaccines.6 Subsequently, we investigated the use of convalescent plasma in kidney transplant recipients with up to 10 d of SARS-CoV-2 mild infection (real-time polymerase chain reaction–confirmed). Because no benefit in disease progression or death was observed among the first 58 treated patients, compared with a propensity-scored matched control group, the study was prematurely terminated.7 Similar findings were observed in an international multicenter trial in nontransplanted patients, which was also terminated because of the lack of efficacy.8 We had no access to other agents providing passive immune protection, such as the monoclonal antibodies directed against SARS-CoV-2, although to date, there are no data regarding the efficacy of these agents in transplanted patients.9
After the emergency approval of vaccines, the Brazilian national vaccination program started in January 2021, giving priority to older people, considering the high risk of mortality, and healthcare workers, based on increased risk of infection. The comparative analysis of the COVID-19 fatality rates between solid organ transplant recipients and the general population, shown in Figure 1A, was decisive for the Brazilian government to grant priority access to vaccination for this group of patients in May 2021.
In parallel, we performed a large clinical trial in 3371 kidney transplant recipients to evaluate the effectiveness, reactogenicity, and immunogenicity of an inactivated virus vaccine (NCT04801667). We observed a low seroconversion rate of 15% after the first dose10 and 43% 4 wk after the second dose.11 Importantly, seroconversion after 2 doses of the BNT162b2 vaccine in kidney transplant recipients varied from 22% to 37.5%,12 and similar figures were observed with the mRNA-1273 SARS-CoV-2 vaccine,13 confirming the impaired immune response, regardless of the vaccine platform, associated with the chronic use of transplant-related immunosuppressive drugs. These data stimulated interest in the use of additional “booster” doses of vaccines. Although preliminary data suggest an increase in seroconversion rates and neutralizing antibody titers after the third homologous dose of mRNA14 or inactivated virus,15 the conversion rates are still low compared with the general population. These results prompted us to investigate the additive effects of a third dose of a heterologous BNT162b2 vaccine or a fourth homologous dose of CoronaVac for patients who were still seronegative after 2 or 3 doses of CoronaVac, respectively. The clinical effectiveness of these strategies remains to be determined. Using an interrupted time-series analysis, we observed a trend reduction in the incidence of cases 15 d after the second dose of CoronaVac from 64 of 1000 persons at risk (before vaccination) to 42 of 1000 persons at risk (after vaccination), but we did not observe impact in the case fatality rate.11 As shown in Figure 1B, the case fatality rate was not reduced after vaccination, regardless of the age group. These single-center data have been confirmed in a recent analysis of a large data registry from the United Kingdom, showing that real-life use of BNT162b2 and ChAdOx1-S vaccines did not protect solid organ transplant recipients from laboratory-confirmed SARS-CoV-2 infection, and only the ChAdOx1-S provided a modest reduction in short-term risk of death.16
More recently, we started a phase I/II randomized clinical trial to evaluate the safety, pharmacokinetics, dosing, and preliminary efficacy of high titer equine heterologous serum anti–SARS-CoV-2, in collaboration with the Butantan Institute (NTC04834089). Although this strategy addresses one of the issues associated with the lack of efficacy of the convalescent plasma, namely, the inconsistent and low anti–SARS-CoV-2 antibody titer, with the possibility of still being effective against emerging new variants, the use of monoclonal or polyclonal antibodies shares the same practical implementation challenges, given the need to start the therapy within a very few days after symptom onset.9
We had no access to remdesivir, tocilizumab, and tofacitinib, although these agents have shown inconsistent efficacy in the overall population and have not been studied in solid organ transplant recipients.17,18 The use of 2 new oral antiviral drugs, Molnupiravir (NCT04405739) and Paxlovid (NCT04960202), has been associated with reduced incidence of hospitalization and deaths.19 Although these drugs have not been tested in solid organ transplant recipients, their use as treatment and possibly as postexposure prophylaxis strategy may be an attractive strategy in this population, although the known drug interactions between immunosuppressive and antiretroviral drugs may limit the use with Paxlovid.
The data provided in Figure 1A highlighted the disproportional higher COVID-19 fatality rates in the kidney transplanted population compared with the general population. Furthermore, data shown in Figure 1B and in the UK registry analysis underscore that vaccination is not enough for the transplant recipient, as recently highlighted by Chapman and Wigmore.20 Clinical effectiveness of pharmacological interventions, including vaccines, remains dismal, urging further investigation for effective therapies. The known poor vaccine response among patients receiving immunosuppressive agents indicates that they should be included in early trials20 exploring the efficacy and safety of ascending concentrations of inactivated whole-virion, adenoviral particles, and mRNA concentration, and different schedules, including the number of doses and time intervals, alternatives that are now under investigation. Therefore, monoclonal and polyclonal antibodies and the new antiviral oral drugs might be considered as rescue therapies for patients with breakthrough infections among transplanted patients. Yet, until such strategies are widely available, we must strongly advocate the maintenance of the use of the nonpharmacological measures, wearing masks and social distancing, which consistently showed effectiveness during the worst periods of the pandemic, even when new variants emerged, as delta and now omicron.
Footnotes
The authors declare no funding or conflicts of interest.
J.M.-P., R.D.F., and L.R.R.-M. participated in data analysis and in the writing of the article. M.P.C. and H.T.-S. participated in the writing of the article.
REFERENCES
- 1.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Requião-Moura LR, de Sandes-Freitas TV, Viana LA, et al. High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: results from the Brazilian multicenter cohort study. PLoS One. 2021;16:e0254822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modelli de Andrade LG, de Sandes-Freitas TV, Requião-Moura LR, et al. Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients. Am J Transplant. 2022;22:610–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plano São Paulo. Retomada Consciente. Available at https://www.saopaulo.sp.gov.br/planosp/simi/dados-abertos. Accessed January 5, 2022.
- 6.Cristelli MP, Viana LA, Fernandes RA, et al. Kidney transplantation in the time of COVID-19: dilemmas, experiences, and perspectives. Transpl Infect Dis. 2021;23:e13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristelli MP, Junior DML, Viana LA, et al. Efficacy of convalescent plasma to treat mild to moderate COVID-19 in kidney transplant patients: a propensity score matching analysis. Transplantation. 2022;106:e92–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bégin P, Callum J, Jamula E, et al. ; CONCOR-1 Study Group. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27:2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina-Pestana J, Cristelli MP, Viana LA, et al. Clinical impact, reactogenicity, and immunogenicity after the first CoronaVac dose in kidney transplant recipients. Transplantation. 2022;106:e95–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina-Pestana J, Covas DT, Viana LA, et al. Inactivated whole-virus vaccine triggers low response against SARS-CoV-2 infection among renal transplant patients: prospective phase 4 study results. Transplantation. [Epub ahead of print. December 7, 2021]. doi:10.1097/TP.0000000000004036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021;100:1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina-Pestana J, Covas DT, Viana LA, et al. Homologous third dose of inactivated whole-virion vaccine fails to elicit a robust immune response among kidney seronegative transplant recipients. Transplantation. [Epub ahead of print. December 28, 2021]. doi:10.1097/TP.0000000000004029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. [Epub ahead of print. January 4, 2022]. doi:10.1097/TP.0000000000004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guimarães PO, Quirk D, Furtado RH, et al. ; STOP-COVID Trial Investigators. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MS, An MH, Kim WJ, et al. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. Plos Med. 2020;17:e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer W, Eron JJ, Holman W, et al. Molnupiravir, an oral antiviral treatment for COVID-19. medRxiv. Preprint posted online June 17, 2021. doi:10.1101/2021.06.17.21258639 [Google Scholar]
- 20.Chapman JR, Wigmore SJ. Simple vaccination is not enough for the transplant recipient. Transplantation. [Epub ahead of print. January 4, 2022]. doi:10.1097/TP.0000000000004064 [DOI] [PMC free article] [PubMed] [Google Scholar]