Abstract
ATP synthases are macromolecular machines consisting of an ATP-hydrolysis-driven F1 motor and a proton-translocation-driven FO motor. The F1 and FO motors oppose each other’s action on a shared rotor subcomplex and are held stationary relative to each other by a peripheral stalk. Structures of resting mitochondrial ATP synthases revealed a left-handed curvature of the peripheral stalk even though rotation of the rotor, driven by either ATP hydrolysis in F1 or proton translocation through FO, would apply a right-handed bending force to the stalk. We used cryoEM to image yeast mitochondrial ATP synthase under strain during ATP-hydrolysis-driven rotary catalysis, revealing a large deformation of the peripheral stalk. The structures show how the peripheral stalk opposes the bending force and suggests that during ATP synthesis proton translocation causes accumulation of strain in the stalk, which relaxes by driving the relative rotation of the rotor through six sub-steps within F1, leading to catalysis.
Subject terms: Molecular conformation, Bioenergetics, Cryoelectron microscopy
CryoEM of mitochondrial ATP synthase frozen during rotary catalysis reveals dramatic conformational changes in the peripheral stalk subcomplex, which enable the enzyme’s efficient synthesis of ATP.
Introduction
ATP synthases use a transmembrane electrochemical proton motive force (pmf) to generate adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and inorganic phosphate (Pi). The enzyme complex consists of two molecular motors positioned to oppose each other’s action on a shared rotor subcomplex (Fig. 1a, left). The membrane-embedded FO motor is driven by proton translocation across the membrane through two offset half channels1,2 while the soluble F1 motor is powered by ATP hydrolysis. In Saccharomyces cerevisiae, the FO region contains subunits a, e, f, g, i/j, k, 8, part of subunit b, and the c10-ring of the rotor3, while the F1 region includes a trimer of catalytic subunit αβ pairs and subunits γ, δ, and ε from the rotor4. Coupling between F1 and FO requires that the two motors are held stationary relative to each other by a peripheral stalk subcomplex (Fig. 1a, green structure), which in yeast is formed from subunits b, d, h, and OSCP (the oligomycin sensitivity conferral protein).
Fig. 1. Rotation in ATP synthase.
a ATP synthase (left) consists of an F1 and an FO region with a shared rotor subcomplex (outlined in black) and a peripheral stalk (green). Rotation driven by proton translocation through the FO region, or the opposite rotation driven by ATP hydrolysis in the F1 region, are predicted to induce a right-handed bend of the peripheral stalk (right). b ATP hydrolysis or synthesis in the F1 region requires three catalytic (black, yellow, and red) and three bind dwell (light blue, blue, and green) conformations. c High-resolution structure of the yeast mitochondrial ATP synthase. d In the absence of free ATP, the peripheral stalk exhibits limited flexibility with a left-handed curvature. e During ATP hydrolysis, ATP synthase can adopt conformations that show a right-handed curvature of the peripheral stalk. f Histograms of the distribution of conformations in the absence of ATP (top, blue) and during ATP hydrolysis (bottom, purple).
During ATP synthesis, proton translocation through FO at the interface of subunit a and the c-ring causes the γδεc10 rotor (Fig. 1a, outlined in black) to turn. Rotation of subunit γ within F1 leads each αβ pairs to cycle through open, tight, and loose conformations that result in the formation of ATP. Conversely, sequential ATP hydrolysis at each of the three αβ pairs in F1 causes the γ subunit to turn in the opposite direction, rotating the proton-carrying c-ring against subunit a in FO and pumping protons across the membrane. Even with the rotor turning at hundreds of revolutions per second5,6 there is little or no ‘slip’7 and the H+:ATP ratio remains constant. In S. cerevisiae this ratio is 10:3 due to the ten proton-carrying c subunits in FO and three catalytic sites in F18. With this H+:ATP ratio, when 10× the free energy of proton translocation () is more negative than 3× the free energy of ATP hydrolysis () the FO motor overpowers the F1 motor, forcing it to synthesize ATP. When is more negative than , F1 overpowers FO and the enzyme hydrolyzes ATP to pump protons.
Synthesis or hydrolysis of each ATP molecule is associated with a ~120° rotation of the γ subunit within F1, leading to conformations of the enzyme known as rotational State 1, 2, and 3 Ref. 9. During ATP hydrolysis, which is better studied than ATP synthesis, this ~120° rotation is broken down into a ~40° sub-step as the enzyme transitions from a ‘catalytic dwell’ to a ‘binding dwell’, and an ~80° sub-step as the enzyme transitions to the next catalytic dwell5,10–12 (Fig. 1b, clockwise). ATP hydrolysis likely occurs during the ~40° sub-step while ATP binding likely occurs during the ~80° sub-step12–14. Consequently, the expected sequence of states for a 360° rotation of the rotor during ATP synthesis is State 1binding → State 1catalytic → State 2binding → State 2catalytic → State 3binding → State 3catalytic (Fig. 1b, counter-clockwise). The mismatch between these six sub-steps in F1 and the ten proton-translocation steps in FO suggests that the enzyme cycles between strained and relaxed conformations during catalysis15,16. Early cryoEM noted that the peripheral stalks of mitochondrial ATP synthases have a left-handed curvature17,18 (Fig. 1a, left). However, torque applied to the rotor following proton translocation through FO (Fig. 1a, right, blue arrows) would tend to rotate the α3β3 hexamer in the same direction as the torque, inducing a right-handed curvature of the peripheral stalk as it resists the rotation. Similarly, the opposite torque applied to the opposite end of the rotor by ATP hydrolysis in F1 (Fig. 1a, right, red arrows) would tend to rotate the membrane-embedded region of FO along with the c-ring, also inducing a right-handed curvature of the peripheral stalk as it resists the rotation17. Previously observed structures were obtained in the absence of a pmf or free ATP9,17–30, suggesting that the peripheral stalk may act as a spring that has a left-handed curvature when relaxed but a right-handed curvature under strain during catalysis17.
Results and discussion
The peripheral stalk shows pronounced bending under strain during ATP hydrolysis
We purified S. cerevisiae ATP synthase with the detergent n-Dodecyl-β-D-Maltopyranoside (DDM), which results in a monomeric preparation of the enzyme30,31, and determined its structure by cryoEM (Supplementary Figs. 1 and 2, Supplementary Tables 1 and 2). A high-resolution map of the intact complex was generated by combining multiple maps from focused refinements (Fig. 1c and Supplementary Fig. 1c). In this map, the peripheral stalk shows the left-handed curvature seen previously. Three-dimensional (3D) classification allowed particle images to be separated into six rotor positions, corresponding to the catalytic and binding dwells for each of the three main rotational states. These conformations resemble recent catalytic and binding dwell structures for an isolated bacterial F1 subcomplex imaged during ATP hydrolysis, where the absence of the peripheral stalk resulted in all catalytic dwell structures being identical and all binding dwell structures being identical32. For yeast ATP synthase imaged without ATP, the catalytic dwell structures show αβtight either in the open conformation lacking nucleotide or in a closed conformation with weak nucleotide density, and the binding dwell structures show αβtight only in an open conformation without nucleotide (Supplementary Fig. 3). The existence of αβtight in an open conformation without visible nucleotide density is likely an artifact from loss of ATP during the purification of the enzyme. Further classification of the State 1catalytic conformation resulted in classes distinguished by variability in the position of the peripheral stalk and a slight rotation of the rotor relative to subunit a. These classes were designated as State 1catalytic(a) (Fig. 1d, light blue) to State 1catalytic(d) (Fig. 1d, dark blue) in order of increasing straightening of the peripheral stalk (Supplementary Movie 1, ‘no ATP’ condition). As these structures were determined in the absence of free ATP, they likely represent energetically similar conformations that can be reached by thermal fluctuation of the enzyme structure9,26.
To test the hypothesis that the peripheral stalk of ATP synthase deforms under strain, we next added ATP to the preparation and froze cryoEM specimens. The presence of ATP in the cryoEM sample buffer introduces a concentration-dependant background noise and loss of contrast in images of DDM-solubilized ATP synthase (Supplementary Fig. 5A). Therefore, a delicate balance is required to provide sufficient ATP in solution to ensure that the enzyme is active as grids are frozen, while simultaneously maintaining sufficient contrast and image quality for image analysis. Initially, a small dataset of images was collected with a screening electron microscope where 10 mM ATP was added to the preparation at 4 °C and cryoEM specimens were frozen within 10 s. Analysis of this dataset revealed conformations of the enzyme not seen in the absence of free ATP (Supplementary Fig. 4). Therefore, a large dataset was collected for the specimen with a high-resolution microscope (Supplementary Fig. 5). Classification of the resulting dataset yielded maps showing six different F1 states, corresponding to the catalytic and binding dwell structures from each of the three main rotational states. Subclassification of these populations separated each catalytic and each binding state into conformations with increasing rotation of the rotor relative to subunit a, and increasingly strained peripheral stalks, designated as ‘a’, ‘b’, ‘c’, etc. Overall, 27 unique conformations were identified: State 1binding(a to d), State 1catalytic(a to h), State 2binding(a to b), State 2catalytic(a to e), State 3binding(a to c), and State 3catalytic(a to e). Overlaying the eight State 1catalytic(a to h) structures reveals that during ATP hydrolysis the peripheral stalk exhibits a large bending motion, transitioning from a left-handed curvature (Fig. 1e, light purple) to the predicted right-handed curvature (Fig. 1e, dark purple; Supplementary Movie 1, ‘During ATP hydrolysis’ condition). Without ATP and during ATP hydrolysis, the left-handed curvature of the peripheral stalk remains the most highly populated conformation of the enzyme (Fig. 1f).
The flexible peripheral stalk accommodates rigid rotation of the rotor during ATP hydrolysis
To facilitate comparison of the ATP synthase conformations that occur during ATP hydrolysis, backbone models of the protein structure were fit flexibly into each of the 27 maps (Fig. 2a). Remarkably, the α3β3γδεc10 models from all 18 catalytic dwell conformations could be overlaid with high-fidelity (Fig. 2b, left), as could the nine α3β3γδεc10 models from binding dwell conformations (Fig. 2b, right), with some limited flexibility at the interface between F1 and the c-ring. This observation shows that, other than being in a catalytic or binding dwell conformation, the differences between the structures are mostly due to deformation of the peripheral stalk subunits and the rotation of the c-ring relative to subunit a in FO. Comparison of the eight State 1catalytic models shows that the α3β3γδεc10 rotor can turn ~80° against subunit a in FO, or more than one-fifth of a complete revolution, before transition to the next binding dwell conformation (Fig. 2c). Bending of the peripheral stalk and not the central rotor of the complex supports suggestions that the peripheral stalk is the most compliant part of the enzyme and stores energy during rotary catalysis9,21,22,26,28,33,34.
Fig. 2. Atomic models of ATP synthase under strain during ATP hydrolysis.
a Atomic models of the enzyme were generated by flexible fitting of the atomic models of ATP synthase without ATP into maps of the strained ATP synthase conformations. b Overlay of fitted models from catalytic dwell (left) and binding dwell (right) structures show limited conformational changes in the α3β3γδεc10 subcomplex. c Overlay of models from catalytic dwell conformations show pronounced bending of the peripheral stalk while the enzyme is under strain during ATP hydrolysis.
The peripheral stalk bends by deformation of subunits d, f, and h
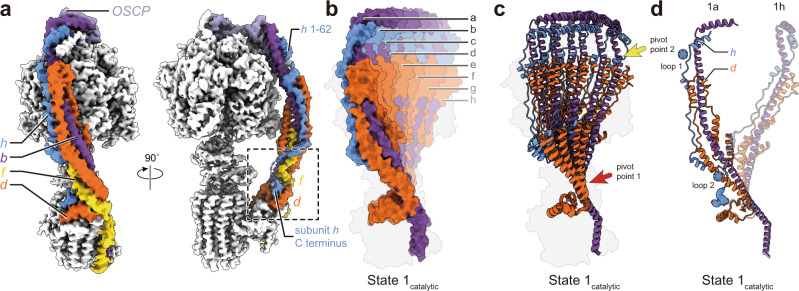
The peripheral stalk of yeast ATP synthase contains subunits b, d, h, and OSCP (Fig. 3a). Although atomic models for subunits b, d, and OSCP have been constructed from previous cryoEM of ATP synthase30, model quality for the 92-residue subunit h in earlier structures was low due to flexibility in both the peripheral stalk overall and subunit h specifically. Focused refinement of the peripheral stalk in the current structure resulted in continuous density for most of subunit h, allowing for construction of an atomic model for residues 1 to 62 based on predictions from AlphaFold35 (Fig. 3a, blue; Supplementary Fig. 6). Interestingly, despite density immediately C-terminal of His62 in subunit h appearing disordered, an additional density that interacts with subunits a, d, f, and 8 indicates that the C terminus of the protein reaches the membrane surface, as suggested previously36 (Fig. 3a, dashed box). Therefore, subunit h spans the entire distance from F1 to FO, a role usually attributed only to subunit b, and different from subunit F6, the shorter mammalian homolog of subunit h29.
Fig. 3. Deformation of the peripheral stalk of ATP synthase while under strain during ATP hydrolysis.
a Structure of the peripheral stalk shows that subunit h bridges F1 and FO. b Overlay of the eight State 1catalytic atomic models, produced by molecular dynamics flexible fitting of the atomic model of resting ATP synthase into the eight 3D maps of State 1catalytic, shows a large deformation of the peripheral stalk. c The peripheral stalk bends at two pivot points near F1 (yellow arrow) and near FO (red arrow). d Unstructured regions in subunit h (blue asterisks) allow it to withstand the bending of subunit d.
Aligning the eight structures corresponding to State 1catalytic by their FO regions reveals that the dramatic bending of the peripheral stalk is facilitated mainly by deformation of subunits b, d, and h (Fig. 3b; Supplementary Movie 1, ‘During ATP hydrolysis’ condition). In conformations that show only slight bending of the peripheral stalk, such as State 1catalytic(b to d), deviation from the relaxed State 1catalytic(a) conformation is mediated primarily by a pivot point in subunits b and d close to the membrane surface (Fig. 3c, red arrow). In the more strained conformations like State 1catalytic(h), a second pivot point in subunit b at the top of subunit d is apparent (Fig. 3c, yellow arrow). The two pivot points are located at either end of subunit d, indicating that subunit d controls where the peripheral stalk bends and likely acts to oppose the bending force, inducing the left-handed curvature of the peripheral stalk when it is not under strain. The structure of subunit d, with an α-helical hairpin that allows it to push against subunit b, is ideal for its role of applying a force that attempts to restore the relaxed conformation of the peripheral stalk during ATP hydrolysis or synthesis (Fig. 3d; Supplementary Movie 1, orange subunit). Subunit h contains two disordered regions close to the two pivot points defined by subunit d, which allows it to withstand the large conformational changes that occurs around the pivot points (Fig. 3d, blue asterisks). In contrast with the spring-like peripheral stalk seen here for the yeast ATP synthase, the unusually large peripheral stalk of algal ATP synthase from Polytomella sp., although imaged in the absence of substrate, appears mostly rigid, with the OSCP subunit that connects the catalytic domain to the rest of the peripheral stalk showing the most flexibility26.
Overall rotation cycle of yeast ATP synthase
Despite the presence of a high concentration of ATP in the buffer used for freezing specimens during ATP hydrolysis, State 1catalytic(a), the least strained of the State 1catalytic conformations, appears to have MgADP bound in its αβtight site (Fig. 4a, left). In contrast, refinement of the F1 region with particle images combined from State 1catalytic(e to h), the four most strained of the State 1catalytic conformations, resulted in a structure similar to State 1catalytic(a) but with what appears to be MgATP bound to αβtight (Fig. 4a, right). In the presence of free ATP, ATP hydrolysis occurs at the αβtight site and MgADP within the site is expected to inhibit this hydrolytic activity. Therefore, the presence of MgADP in αβtight of the non-strained conformation suggests that many of the complexes in this conformation are in the well-known MgADP inhibited state4,37. Inactive complexes have been detected previously even in the presence of free ATP38,39. In contrast, the structures that show the more strained peripheral stalks appear to be calculated from images of active enzyme particles. Density for the binding dwell conformations suggests that they contain MgADP with Pi in the αβtight site (Supplementary Fig. 7a), as was seen in the bacterial F1 region during ATP hydrolysis32.
Fig. 4. Sequence of conformations in the ATP hydrolysis and ATP synthesis cycles.
a The unstrained conformation of State 1catalytic shows density consistent with MgADP in the αβtight catalytic pair (left, yellow), suggesting an ADP-inhibited state, while the strained conformations show density consistent with MgATP (right, purple) suggesting an active state. b Measurement of the rotation of the rotor subunit γ within the F1 region shows 36° and 84° sub-steps between catalytic and binding dwell conformations. c Measurement of the rotation of the c-ring relative to subunit a in FO. Within each binding or catalytic dwell conformation the peripheral stalk becomes increasingly strained as the c-ring rotates in the ATP synthesis direction and increasingly relaxed as the c-ring rotates in the ATP hydrolysis direction. Transition between catalytic and binding dwell conformations would require back-stepping of the c-ring (shaded areas) if every conformation occurred during the rotary cycle. Colors in (b) and (c) are defined as in Fig. 1b.
To place the 27 conformations of ATP synthase observed during ATP hydrolysis into a rotational sequence, the positions of subunit γ relative to α3β3 in F1 (Fig. 4b) and of the c-ring relative to subunit a in FO (Fig. 4c) were measured and plotted on circles that represent a 360° rotation. As described above, the α3β3γδεc10 subcomplex is found in three catalytic dwell conformations and three binding dwell conformations, resulting in only six unique positions of subunit γ relative to α3β3 in F1 (Fig. 2b). Consistent with the isolated bacterial F1 region10,32, ATP hydrolysis in αβtight of the yeast catalytic dwell conformation appears to induce a slightly more open conformation of the αβ pair and a ~36° rotation of the rotor (Supplementary Fig. 7b), leaving the enzyme in a binding dwell. MgADP and Pi are then released from the αβtight site and ATP binding to the αβopen site drives an ~84° rotation of the rotor to the next catalytic dwell conformation. Repetition of this process two more times completes the 360° rotation cycle for ATP hydrolysis (Fig. 4b, clockwise arrows), while for ATP synthesis the reverse reaction is driven by rotation of the rotor in the opposite direction (Fig. 4b, counter-clockwise arrows).
In contrast to the six unique positions of subunit γ relative to α3β3 in F1, there are 27 unique positions of the c-ring relative to subunit a in FO. Plotting the angle of the c-ring relative to subunit a in FO produces a series of arcs that show the range of rotation of the ring within each catalytic or binding dwell state (Fig. 4c, black, blue, yellow, green, red, and cyan arcs). These arcs reveal that as the c-ring rotates in the ATP hydrolysis direction, each state exhibits a decreasing strain on the peripheral stalk (Fig. 4c, clockwise arrow). For example, for State 1catalytic (Fig. 4c, black arc), rotation of the c-ring in the ATP hydrolysis direction occurs during the transition from State 1catalytic(h) → State 1catalytic(a). As ATP hydrolysis in a catalytic αβtight site causes the transition from a catalytic dwell to a binding dwell, the order of states indicates that the power stroke of ATP hydrolysis forces the peripheral stalk into a more strained conformation (e.g., State 1catalytic(a) → State 1binding(d)). This strain subsequently relaxes as the c-ring continues to turn in the ATP hydrolysis direction (e.g., State 1binding(d) → State 1binding(a)). Conversely, rotation of the c-ring in the direction driven by proton translocation during ATP synthesis (Fig. 4c, counter-clockwise arrow) leads to increasing strain on the peripheral stalk (e.g., State 1binding(a) → State 1binding(d)), which relaxes as ATP is formed in the catalytic site and the enzyme transitions from a binding dwell conformation to a catalytic dwell conformation (e.g., State 1binding(d) → State 1catalytic(a)).
Notably, the most strained conformation of some of the states show less rotation of the c-ring in the ATP hydrolysis direction than less strained conformations of the preceding state (Fig. 4c, shaded areas). For example, the transition from State 1catalytic(a) to State 1binding(d) during ATP hydrolysis would involve the c-ring rotating 23° in the ATP synthesis direction. The same apparent ‘backstepping’ can be seen at the transition from State 3catalytic → State 3binding, and State 2binding → State 1catalytic. This backstepping of the c-ring would bend the peripheral stalk in the opposite direction of the applied force and is physically unlikely. Therefore, the unstrained conformations appear to show inactive complexes that are not part of the rotary sequence during substrate turnover. By extension, these data suggest that during rotary catalysis the peripheral stalk becomes strained and does not relax fully until catalysis stops. Construction of a movie showing rotation in the hydrolysis direction based on the most strained conformation of the enzyme illustrates the amount of deformation that can occur during ATP hydrolysis (Supplementary Movie 2, ‘ATP hydrolysis’ cycle). Similarly, a video can be constructed showing rotation in the ATP synthesis direction based on the most strained conformations (Supplementary Movie 2, ‘ATP synthesis’ cycle). Together, these data illustrate how in active ATP synthase the peripheral stalk can serve as a buffer for energy that deforms under strain. ATP synthase c-rings from different species can have between eight and 17 c subunits40 and the flexibility of the peripheral likely allows this variability. The storage of energy during rotation would also be expected to smooth the transmission of power between the F1 and FO motors despite symmetry mismatch, which is thought to be essential for the high turnover rate of the enzyme41,42. In the fully active enzyme, the peripheral stalk likely remains deformed as the enzyme runs, with the degree of bending dependent on the rate of turnover, and with the enzyme only becoming fully relaxed in the absence of ATP or a proton motive force. At present cryoEM appears uniquely capable of providing high-resolution insight into changes in enzyme conformation that occur during catalysis. Experiments to quantify strain within ATP synthase in solution conditions, such as single-molecule fluorescence resonance energy transfer43, could complement the findings reported here and reveal how strain changes within individual molecules during each rotation cycle.
Methods
Yeast growth and ATP synthase purification
Yeast strain USY006 containing a 6 × His tag at the N terminus of the β subunits was grown in YPGD media (1% [w/v] yeast extract, 2% [w/v] peptone, 3% [v/v] glycerol, 0.2% [w/v] glucose) with a 11 L fermenter (New Brunswick Scientific) for ~48 h at 30 °C until saturation. All purification steps were performed at 4 °C. Yeast cell walls were broken with bead beating, and cell debris was removed by centrifugation at 5000 × g for 30 min. Mitochondria were collected by centrifugation at 25,000 × g for 30 min, before being washed with phosphate buffer (50 mM sodium phosphate pH 9.0, 5 mM 6-aminocaproic acid, 5 mM benzamidine, 1 mM PMSF) for 30 min. Washed mitochondria were collected by centrifugation at 184,000 × g for 30 min, before being resuspended in buffer (50 mM Tris-HCl pH 7.4, 10% [v/v] glycerol, 1% [w/w] DDM [Anatrace], 5 mM 6-aminocaproic acid, 5 mM benzamidine, 1 mM PMSF) and solubilized with gentle shaking for one hour. Insoluble material was removed by centrifugation at 184,000 × g for 30 min, and supernatant containing solubilized protein was supplemented with 40 mM imidazole and 300 mM NaCl before being loaded onto a 5 mL HisTrap column (Cytiva) equilibrated with HisTrap buffer (50 mM Tris-HCl pH 7.4, 10% [v/v] glycerol, 0.05% [w/w], 40 mM imidazole, 300 mM NaCl, 5 mM 6-aminocaproic acid, 5 mM benzamidine, 1 mM PMSF), and washed with HisTrap buffer. ATP synthase was eluted with HisTrap buffer containing 300 mM imidazole and was loaded onto a Superose 6 Increase column (Cytiva) equilibrated with buffer (20 mM Tris-HCl pH 7.4, 10% [v/v] glycerol, 0.05% [w/w] DDM, 100 mM NaCl, 5 mM MgCl2). Fractions containing ATP synthase were pooled, and the protein was concentrated to ~15 mg/ml prior to cryoEM grid freezing or storage at −80 °C.
CryoEM specimen preparation
Glycerol in the ATP synthase preparation was removed with a Zeba Spin desalting column (Thermo Fisher Scientific [TFS]) before freezing cryoEM specimens. Holey gold films with ~2 µm holes were nanofabricated44 on 300 mesh Maxtaform copper-rhodium grids (Electron Microscopy Sciences). Specimens with 10 mM ATP were prepared by first applying 0.4 µL of 50 mM ATP in buffer (70 mM Tris-HCl pH 7.4, 0.05% [w/w] DDM, 100 mM NaCl, 55 mM MgCl2) onto a grid that had been glow-discharged in air for 2 min. Freshly prepared ATP synthase (1.6 µL) was mixed quickly with the ATP solution on the grid before blotting for 1 s in an EM GP2 grid freezing device (Leica) at 4 °C and 100% humidity and plunge frozen in liquid ethane. Specimens without ATP were prepared the same way except that the mixing step was omitted.
CryoEM data collection
Preliminary cryoEM data was collected with FEI Tecnai F20 electron microscope operated at 200 kV and equipped with a Gatan K2 Summit camera. Images with this microscope were acquired as movies with 30 fractions at 5 e/pixel/s and a calibrated pixel size of 1.45 Å/pixel. CryoEM movies for high-resolution analysis were collected with a Titan Krios G3 microscope operated at 300 kV and equipped with a Falcon 4 camera (TFS). Automated data collection was performed with EPU. For the dataset including ATP, 10,037 movies, each consisting of 30 fractions, were collected at a nominal magnification of ×59,000, corresponding to a calibrated pixel size of 1.348 Å. The exposure rate and the total exposure of the specimen were 6.1 e−/pixel/s and ~40 e−/Å2, respectively. For the ATP-free dataset, 8817 30-fraction movies were collected at a nominal magnification of ×75,000, corresponding to a calibrated pixel size of 1.046 Å. The exposure rate and the total exposure for this specimen were 4.2 e/pixel/second and ~39 e/Å2, respectively.
Image analysis
Data collection was monitored with cryoSPARC Live45 to screen and select high-quality micrographs. All other image analysis steps were performed with cryoSPARC except where mentioned. Movie fractions were aligned with patch-based motion correction and contrast transfer function (CTF) parameters were estimated with patch-based CTF estimation. After removing movies with undesirable motion or CTF fit, 7474 and 4059 movies from the dataset including ATP and the ATP-free dataset were selected for further processing, respectively. Movie fractions were aligned with MotionCor46 with a 7 × 7 grid and averaged micrographs from the aligned movies were subjected to patch-based CTF estimation. For the ATP-free dataset, particle selection was performed with Topaz47. For the dataset including ATP, templates for particle selection were generated from 2D classification of manually selected particle images. After particle selection, 2,534,488 particle images were extracted for the dataset with ATP and 442,025 particle images were extracted for the ATP-free dataset. Low-quality particle images were removed with two rounds of 2D classifications, yielding 1,109,677 and 422,765 particle images for the dataset including ATP and the ATP-free dataset, respectively. Further cleaning with ab initio 3D classification and heterogeneous refinement reduced dataset sizes to 915,825 and 379,817 particle images, respectively. The remaining particle images were classified into three classes, corresponding to the three main rotational states of the enzyme, and each class was refined with non-uniform refinement48. For the dataset including ATP, local refinement was performed with all particle images with a mask including α3β3γδε from the F1 region. CTF parameters of individual particle images were re-estimated with local CTF refinement, and masked refinement was performed again with updated CTF parameters. Image alignment parameters were then converted to Relion49 .star file format with pyem (10.5281/zenodo.3576630) and individual particle motion was corrected with Bayesian polishing50. For the ATP-free dataset, Bayesian polishing was performed with an intact ATP synthase map reconstructed with all particle images and particle images were down-sampled to a pixel size of 1.308 Å. Motion corrected images were imported back to cryoSPARC, refined, and CTF parameters re-estimated. For the dataset including ATP, particle images were initially classified into four classes. Iterative ab initio classification and heterogeneous refinement of each of the four classes yielded 27 unique rotational states of ATP synthase. The 27 structures were named State 1binding(a to d), State 1catalytic(a to h), State 2binding(a to b), State 2catalytic(a to e), State 3binding(a to c), and State 3catalytic(a to e), and had resolutions ranging from 4.4 to 7.8 Å after refinement. Masked local refinement of the F1 region of State 1catalytic(a) yielded a 3.5 Å resolution map representing the MgADP inhibited state. Local refinement of the F1 region of combined State 1catalytic(e to h) and State 1binding(a to d) yielded two 4.0 Å resolution maps. To better visualize the nucleotide density in maps, density modification51 of locally-refined maps of State 1catalytic(a), State 1catalytic(e to h), and State 1binding(a to d) was performed in Phenix52. For the ATP-free dataset, a similar 3D classification strategy yielded nine F1 states, namely State 1, 2, and 3catalytic with αβtight in a closed conformation, State 1, 2, and 3catalytic with αβtight in an open conformation, and State 1, 2, and 3binding with αβtight in an open conformation (Supplementary Fig. 1 and 3). These states included 56,739, 52,468, 24,879, 31,559, 47,065, 65,651, 19,922, 57,468, and 23,622 particle images, respectively. Local refinement of the F1 region with these images yielded maps at 3.4, 3.4, 3.6, 3.5, 3.5, 3.4, 3.7, 3.4, and 3.7 Å resolution, respectively. When the three rotational states are combined, the F1 regions of Statecatalytic with βtight closed, Statecatalytic with βtight open, and Statecatalytic with βbinding open reached 3.2, 3.2, and 3.3 Å resolution, respectively. Classification of particle images contributing to State 1catalytic from the ATP-free dataset yielded 4 classes with different c-ring positions relative to subunit a, which demonstrates the flexibility of the peripheral stalk in the absence of free ATP (Fig. 1d, Supplementary Fig. 1). Maps of these states were calculated from 41,506, 8810, 10,550, and 1984 particle images and reached 3.8, 4.4, 4.4, and 7.1 Å resolutions after refinement, respectively. A similar classification strategy was employed with the other two catalytic states, and particle images of the most relaxed State 1, 2, 3catalytic(a) (191,939 particle images) were used to calculate locally-refined maps of OSCP with its contact site on F1, the remainder of the peripheral stalk, and the FO region. These maps were combined with the map from local refinement of the F1 region of the State 1catalytic with αβtight closed using the ‘vop maximum’ function in USCF Chimera to generate a composite map of the entire complex.
Model building and refinement
To build atomic models of the F1 region, the crystal structure of yeast F1 (PDB 2HLD)53 was fitted as a rigid body into locally-refined maps of F1 in UCSF Chimera54. Models were manually adjusted in Coot55 before being imported into ISOLDE56 within ChimeraX57 to improve dihedral angles and rotamer fitting. A final round of refinement was performed with Phenix and the resulting models were evaluated with Molprobity58 and EMRinger59. To build backbone models of the full complex, a mosaic model was first assembled by rigid-body fitting of a yeast F1 crystal structure (PDB 2HLD), subunits abc10dfi from a yeast FO cryoEM structure (PDB 6B2Z)60, the peripheral stalk region without subunit h from a yeast monomer cryoEM structure (PDB 6CP3)30, and domains of a subunit h atomic model predicted with AlphaFold35 into the unsharpened maps. Molecular Dynamics Flexible Fitting61 was then performed for the 27 rotational states of the dataset including ATP to generate corresponding backbone models. Figures and movies were generated with ChimeraX and UCSF Chimera.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
H.G. was supported by an Ontario Graduate Scholarship for International Students. J.L.R. was supported by the Canada Research Chairs program. This work was supported by Canadian Institutes of Health Research grant PJT162186 (J.L.R.). CryoEM data was collected at the Toronto High-Resolution High-Throughput cryoEM facility, supported by the Canada Foundation for Innovation and Ontario Research Fund.
Author contributions
J.L.R. conceived the project and supervised the research. H.G. purified the protein, performed the cryoEM, image analysis, and atomic model building. H.G. and J.L.R. wrote the manuscript and prepared figures.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data that support this study are available from the corresponding author upon reasonable request. Cryo-EM maps generated in this study have been deposited in the Electron Microscopy Data Bank with accession numbers EMD-25930, EMD-25931, EMD-25932, EMD-25933, EMD-25934, EMD-25935, EMD-25936, EMD-25937, EMD-25938, EMD-25939, EMD-25940, EMD-25941, EMD-25942, EMD-25943, EMD-25944, EMD-25945, EMD-25946, EMD-25947, EMD-25948, EMD-25949, EMD-25950, EMD-25951, EMD-25952, EMD-25953, EMD-25954, EMD-25955, EMD-25956, EMD-25957, EMD-25958, EMD-25959, EMD-25960, EMD-25961, EMD-25962, EMD-25963, EMD-25964, EMD-25965, EMD-25966, EMD-25967, EMD-25968, EMD-25969, EMD-25970, EMD-25971, EMD-25972, EMD-25973, EMD-25974, EMD-25975, EMD-25976, EMD-25977, EMD-25978, EMD-25979, EMD-25980 and atomic models have been deposited in the Protein Data Bank with accession codes 7TJS, 7TJT, 7TJU, 7TJV, 7TJW, 7TJX, 7TJY, 7TJZ, 7TK0, 7TK1, 7TK2, 7TK3, 7TK4, 7TK5, 7TK6, 7TK7, 7TK8, 7TK9, 7TKA, 7TKB, 7TKC, 7TKD, 7TKE, 7TKF, 7TKG, 7TKH, 7TKI, 7TKJ, 7TKK, 7TKL, 7TKM, 7TKN, 7TKO, 7TKP, 7TKQ, 7TKR, 7TKS. Previously published atomic models used for atomic model building are available in the Protein Data Bank with accession codes 2HLD, 6B2Z, 6CP3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/17/2022
A Correction to this paper has been published: 10.1038/s41467-022-30544-9
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29893-2.
References
- 1.Junge W, Lill H, Engelbrecht S. ATP synthase: an electrochemical transducer with rotary mechanics. Trends Biochem. Sci. 1997;22:420–423. doi: 10.1016/s0968-0004(97)01129-8. [DOI] [PubMed] [Google Scholar]
- 2.Vik S, Antonio BJ. A mechanism of proton translocation by F1F0 ATP synthases suggested by double mutants of the a subunit. J. Biol. Chem. 1994;269:30364–9. [PubMed] [Google Scholar]
- 3.Liu S, et al. The purification and characterization of ATP synthase complexes from the mitochondria of four fungal species. Biochem. J. 2015;468:167–175. doi: 10.1042/BJ20150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 5.Bilyard T, et al. High-resolution single-molecule characterization of the enzymatic states in Escherichia coli F1-ATPase. Philos. Trans. R. Soc. B Biol. Sci. 2013;368:20120023. doi: 10.1098/rstb.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi R, Ueno H, Li C-B, Noji H. Rotary catalysis of bovine mitochondrial F1-ATPase studied by single-molecule experiments. Proc. Natl Acad. Sci. USA. 2020;117:1447–1456. doi: 10.1073/pnas.1909407117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soga N, Kimura K, Kinosita K, Yoshida M, Suzuki T. Perfect chemomechanical coupling of FoF1-ATP synthase. Proc. Natl Acad. Sci. USA. 2017;114:4960–4965. doi: 10.1073/pnas.1700801114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- 9.Zhou A, et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife. 2015;4:e10180. doi: 10.7554/eLife.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda R, Noji H, Yoshida M, Kinosita K, Itoh H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature. 2001;410:898–904. doi: 10.1038/35073513. [DOI] [PubMed] [Google Scholar]
- 11.Martin JL, Ishmukhametov R, Hornung T, Ahmad Z, Frasch WD. Anatomy of F1-ATPase powered rotation. Proc. Natl Acad. Sci. USA. 2014;111:3715–3720. doi: 10.1073/pnas.1317784111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steel BC, et al. Comparison between single-molecule and X-ray crystallography data on yeast F1-ATPase. Sci. Rep. 2015;5:8773. doi: 10.1038/srep08773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishizaka T, et al. Chemomechanical coupling in F1-ATPase revealed by simultaneous observation of nucleotide kinetics and rotation. Nat. Struct. Mol. Biol. 2004;11:142–148. doi: 10.1038/nsmb721. [DOI] [PubMed] [Google Scholar]
- 14.Adachi K, et al. Coupling of rotation and catalysis in F1-ATPase revealed by single-molecule imaging and manipulation. Cell. 2007;130:309–321. doi: 10.1016/j.cell.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Cherepanov DA, Mulkidjanian AY, Junge W. Transient accumulation of elastic energy in proton translocating ATP synthase. FEBS Lett. 1999;449:1–6. doi: 10.1016/s0014-5793(99)00386-5. [DOI] [PubMed] [Google Scholar]
- 16.Pänke O, Rumberg B. Kinetic modeling of rotary CF0F1-ATP synthase: storage of elastic energy during energy transduction. Biochim. Biophys. Acta BBA - Bioenerg. 1999;1412:118–128. doi: 10.1016/s0005-2728(99)00059-6. [DOI] [PubMed] [Google Scholar]
- 17.Lau WCY, Baker LA, Rubinstein JL. Cryo-EM structure of the yeast ATP synthase. J. Mol. Biol. 2008;382:1256–1264. doi: 10.1016/j.jmb.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein JL, Walker JE, Henderson R. Structure of the mitochondrial ATP synthase by electron cryomicroscopy. EMBO J. 2003;22:6182–6192. doi: 10.1093/emboj/cdg608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flygaard RK, Mühleip A, Tobiasson V, Amunts A. Type III ATP synthase is a symmetry-deviated dimer that induces membrane curvature through tetramerization. Nat. Commun. 2020;11:5342. doi: 10.1038/s41467-020-18993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, et al. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science. 2019;364:1068–1075. doi: 10.1126/science.aaw4852. [DOI] [PubMed] [Google Scholar]
- 21.Guo H, Suzuki T, Rubinstein JL. Structure of a bacterial ATP synthase. eLife. 2019;8:e43128. doi: 10.7554/eLife.43128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn A, Vonck J, Mills DJ, Meier T, Kühlbrandt W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science. 2018;360:eaat4318. doi: 10.1126/science.aat4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn A, et al. Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol. Cell. 2016;63:445–456. doi: 10.1016/j.molcel.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mühleip A, et al. ATP synthase hexamer assemblies shape cristae of Toxoplasma mitochondria. Nat. Commun. 2021;12:120. doi: 10.1038/s41467-020-20381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mühleip A, McComas SE, Amunts A. Structure of a mitochondrial ATP synthase with bound native cardiolipin. eLife. 2019;8:e51179. doi: 10.7554/eLife.51179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy BJ, et al. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-Fo. Science. 2019;364:eaaw9128. doi: 10.1126/science.aaw9128. [DOI] [PubMed] [Google Scholar]
- 27.Pinke G, Zhou L, Sazanov LA. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nat. Struct. Mol. Biol. 2020;27:1077–1085. doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 28.Sobti M, et al. Cryo-EM structures of the autoinhibited E. coli ATP synthase in three rotational states. eLife. 2016;5:e21598. doi: 10.7554/eLife.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spikes TE, Montgomery MG, Walker JE. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl Acad. Sci. USA. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava AP, et al. High-resolution cryo-EM analysis of the yeast ATP synthase in a lipid membrane. Science. 2018;360:eaas9699. doi: 10.1126/science.aas9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinstein JL, Walker JE. ATP synthase from Saccharomyces cerevisiae: location of the OSCP subunit in the peripheral stalk region. J. Mol. Biol. 2002;321:613–619. doi: 10.1016/s0022-2836(02)00671-x. [DOI] [PubMed] [Google Scholar]
- 32.Sobti M, Ueno H, Noji H, Stewart AG. The six steps of the complete F1-ATPase rotary catalytic cycle. Nat. Commun. 2021;12:4690. doi: 10.1038/s41467-021-25029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorgen PL, Bubb MR, Cain BD. Lengthening the second stalk of F1F0 ATP synthase in Escherichia coli. J. Biol. Chem. 1999;274:36261–36266. doi: 10.1074/jbc.274.51.36261. [DOI] [PubMed] [Google Scholar]
- 34.Sorgen PL, Caviston TL, Perry RC, Cain BD. Deletions in the second stalk of F1F0-ATP synthase in Escherichia coli. J. Biol. Chem. 1998;273:27873–27878. doi: 10.1074/jbc.273.43.27873. [DOI] [PubMed] [Google Scholar]
- 35.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein JL, Dickson VK, Runswick MJ, Walker JE. ATP synthase from Saccharomyces cerevisiae: location of subunit h in the peripheral stalk region. J. Mol. Biol. 2005;345:513–520. doi: 10.1016/j.jmb.2004.10.060. [DOI] [PubMed] [Google Scholar]
- 37.Bowler MW, Montgomery MG, Leslie AGW, Walker JE. Ground state structure of F1-ATPase from bovine heart mitochondria at 1.9 Å resolution. J. Biol. Chem. 2007;282:14238–14242. doi: 10.1074/jbc.M700203200. [DOI] [PubMed] [Google Scholar]
- 38.Hirono-Hara Y, et al. Pause and rotation of F1-ATPase during catalysis. Proc. Natl Acad. Sci. USA. 2001;98:13649–13654. doi: 10.1073/pnas.241365698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr. Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 40.Kuhlbrandt W. Structure and mechanisms of F-type ATP synthases. Annu. Rev. Biochem. 2019;88:515–549. doi: 10.1146/annurev-biochem-013118-110903. [DOI] [PubMed] [Google Scholar]
- 41.Junge W, Sielaff H, Engelbrecht S. Torque generation and elastic power transmission in the rotary FOF1-ATPase. Nature. 2009;459:364–370. doi: 10.1038/nature08145. [DOI] [PubMed] [Google Scholar]
- 42.Junge W, et al. Inter-subunit rotation and elastic power transmission in F0F1-ATPase. FEBS Lett. 2001;504:152–160. doi: 10.1016/s0014-5793(01)02745-4. [DOI] [PubMed] [Google Scholar]
- 43.Börsch M, Duncan TM. Spotlighting motors and controls of single FoF1-ATP synthase. Biochem. Soc. Trans. 2013;41:1219–1226. doi: 10.1042/BST20130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marr CR, Benlekbir S, Rubinstein JL. Fabrication of carbon films with approximately 500 nm holes for cryo-EM with a direct detector device. J. Struct. Biol. 2014;185:42–47. doi: 10.1016/j.jsb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 46.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bepler T, et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods. 2019;16:1153–1160. doi: 10.1038/s41592-019-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Punjani A, Zhang H, Fleet DJ. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods. 2020;17:1214–1221. doi: 10.1038/s41592-020-00990-8. [DOI] [PubMed] [Google Scholar]
- 49.Scheres SHW. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zivanov J, Nakane T, Scheres SHW. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ. 2019;6:5–17. doi: 10.1107/S205225251801463X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terwilliger TC, Ludtke SJ, Read RJ, Adams PD, Afonine PV. Improvement of cryo-EM maps by density modification. Nat. Methods. 2020;17:923–927. doi: 10.1038/s41592-020-0914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kabaleeswaran V, Puri N, Walker JE, Leslie AGW, Mueller DM. Novel features of the rotary catalytic mechanism revealed in the structure of yeast F1 ATPase. EMBO J. 2006;25:5433–5442. doi: 10.1038/sj.emboj.7601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 55.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 56.Croll TI. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. Sect. Struct. Biol. 2018;74:519–530. doi: 10.1107/S2059798318002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goddard TD, et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barad BA, et al. EMRinger: side chain–directed model and map validation for 3D cryo-electron microscopy. Nat. Methods. 2015;12:943–946. doi: 10.1038/nmeth.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo H, Bueler SA, Rubinstein JL. Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science. 2017;358:936–940. doi: 10.1126/science.aao4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trabuco LG, Villa E, Mitra K, Frank J, Schulten K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure. 2008;16:673–683. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support this study are available from the corresponding author upon reasonable request. Cryo-EM maps generated in this study have been deposited in the Electron Microscopy Data Bank with accession numbers EMD-25930, EMD-25931, EMD-25932, EMD-25933, EMD-25934, EMD-25935, EMD-25936, EMD-25937, EMD-25938, EMD-25939, EMD-25940, EMD-25941, EMD-25942, EMD-25943, EMD-25944, EMD-25945, EMD-25946, EMD-25947, EMD-25948, EMD-25949, EMD-25950, EMD-25951, EMD-25952, EMD-25953, EMD-25954, EMD-25955, EMD-25956, EMD-25957, EMD-25958, EMD-25959, EMD-25960, EMD-25961, EMD-25962, EMD-25963, EMD-25964, EMD-25965, EMD-25966, EMD-25967, EMD-25968, EMD-25969, EMD-25970, EMD-25971, EMD-25972, EMD-25973, EMD-25974, EMD-25975, EMD-25976, EMD-25977, EMD-25978, EMD-25979, EMD-25980 and atomic models have been deposited in the Protein Data Bank with accession codes 7TJS, 7TJT, 7TJU, 7TJV, 7TJW, 7TJX, 7TJY, 7TJZ, 7TK0, 7TK1, 7TK2, 7TK3, 7TK4, 7TK5, 7TK6, 7TK7, 7TK8, 7TK9, 7TKA, 7TKB, 7TKC, 7TKD, 7TKE, 7TKF, 7TKG, 7TKH, 7TKI, 7TKJ, 7TKK, 7TKL, 7TKM, 7TKN, 7TKO, 7TKP, 7TKQ, 7TKR, 7TKS. Previously published atomic models used for atomic model building are available in the Protein Data Bank with accession codes 2HLD, 6B2Z, 6CP3.