Abstract
TIMELESS (TIM) was first identified as a molecular cog in the Drosophila circadian clock. Almost three decades of investigations have resulted in an insightful model describing the critical role of Drosophila TIM (dTIM) in circadian timekeeping in insects, including its function in mediating light entrainment and temperature compensation of the molecular clock. Furthermore, exciting discoveries on its sequence polymorphism and thermosensitive alternative RNA splicing have also established its role in regulating seasonal biology. Although mammalian TIM (mTIM), its mammalian paralog, was first identified as a potential circadian clock component in 1990s due to sequence similarity to dTIM, its role in clock regulation has been more controversial. Mammalian TIM has now been characterized as a DNA replication fork component and has been shown to promote fork progression and participate in cell cycle checkpoint signaling in response to DNA damage. Despite defective circadian rhythms displayed by mtim mutants, it remains controversial whether the regulation of circadian clocks by mTIM is direct, especially given the interconnection between the cell cycle and circadian clocks. In this review, we provide a historical perspective on the identification of animal tim genes, summarize the roles of TIM proteins in biological timing and genomic stability, and draw parallels between dTIM and mTIM despite apparent functional divergence.
Keywords: cell cycle, circadian clock, DNA replication, Drosophila timeless, mammalian timeless , seasonal biology, timeout
Drosophila TIMELESS (dTIM) is a key component of the fly circadian clock, which is essential for regulating daily rhythms in physiology and behavior. However, whether its mammalian paralog (mTIM) is important for the functionality of the clock has been controversial. This review summarizes the roles of TIM proteins in biological timing and genomic stability and draws parallels between dTIM and mTIM despite apparent functional divergence.

Abbreviations
- CLK
CLOCK
- CRY
CRYPTOCHROME
- CYC
CYCLE
- dTIM
Drosophila TIM
- mTIM
mammalian TIM
- PER
PERIOD
- PTM
posttranslational modification
- TIM
TIMELESS
- TTFL
transcription–translation feedback loop
Introduction
Circadian rhythms are common features in all domains of life and are driven by molecular clockworks [1, 2, 3, 4, 5, 6]. Molecular clocks incorporate a range of environmental time cues, such as light–dark and temperature signals, and metabolic signals to orchestrate daily rhythms in physiology and behavior [4, 6, 7]. This allows organisms to synchronize their biology to their external environment, thereby promoting organismal health and fitness [8, 9, 10, 11]. The animal circadian clock is powered by cell‐autonomous interlocked transcription–translation feedback loops (TTFLs) [6]. In the primary TTFL in Drosophila, which relies heavily on Drosophila TIM (dTIM) function, transcription factors CLOCK (CLK) (ortholog of mammalian CLOCK) and CYCLE (CYC) (ortholog of mammalian BMAL1) are positive elements that heterodimerize and activate the expression of negative elements, PERIOD (PER) (ortholog of mammalian PER1, PER2, and PER3) and dTIM (functionally replaced by CRYPTOCHROMEs (CRYs) in mammalian clockworks). In addition to core clock components, CLK‐CYC also activates the transcription of other clock‐controlled output genes [12, 13, 14], often in tissue‐specific manner [15, 16, 17]. To complete the TTFL, PER, and dTIM form a repressor complex that enters the nucleus in a time‐of‐day‐dependent manner [18, 19, 20, 21, 22] to repress CLK‐CYC transcription activity [23, 24, 25]. This repression is relieved when both PER and dTIM are degraded in a proteasome‐dependent manner [26, 27, 28, 29, 30]. In addition to its role within the molecular clock, thermosensitive alternative splicing of dtim RNA [31, 32, 33, 34] and light sensitivity [35, 36, 37, 38] of TIM protein are key features that allow dTIM to function at the interface between circadian and seasonal timing.
In the mammalian clock, CRYs replace TIM to partner with PERs to maintain circadian rhythms [39, 40, 41, 42, 43, 44]. Whether mammalian TIM (mTIM) is a key component of the mammalian clock has been heavily debated since it was first characterized [45, 46, 47]. On the other hand, evidence supporting the role of mTIM in DNA replication and DNA damage response is strong. We will discuss the controversial role of mTIM in timekeeping below.
This review summarizes the various roles played by dTIM in Drosophila circadian clocks, in the regulation of seasonal biology, and other non‐circadian processes. We will then discuss the circadian and non‐circadian functions of mTIM, highlighting data that either support its role in circadian timekeeping or are in conflict with the notion. Finally, we conclude the review by summarizing recent findings on the potential functional parallel between dTIM and mTIM.
Drosophila TIM plays critical roles in circadian timekeeping
Drosophila TIM in the molecular clock
Circadian timekeeping relies on cycling genes and proteins that maintain a free‐running period of approximately 24 h. Investigations to elucidate the inner workings of the molecular clockwork started around 50 years ago, when Konopka and Benzer [48] isolated the first three clock mutants in Drosophila melanogaster via genetic screening. The mutations were all located in the same loci, which were later confirmed as the key clock gene, period (per) [49, 50, 51, 52, 53]. Hardin et al. [54] suggested that PER may feedback to repress its own mRNA expression to establish molecular oscillations that manifest into behavioral and physiology rhythms. In the next few years, taking advantage of high throughput genetic screening in Drosophila, Sehgal et al. [55] identified dtim as the second clock gene. This gene encodes a protein with novel structure at the time and the only recognizable sequence feature the authors highlighted was a stretch of acidic residues [56]. The arrhythmic PER nuclear localization as well as locomotor activity in dtim null mutants has led to the model illustrating how the coordination of per and dtim may generate 24‐h free‐running period via negative feedback: (a) transcriptional activation of per and dtim in midday due to the absence of nuclear PER; (b) PER and dTIM heterodimerize and enter the nucleus at dusk; (c) increasing amount of nuclear PER blocks per and dtim mRNA transcription and accumulation at night; (d) nuclear PER and dTIM decline because of inhibited mRNA production and subsequent protein turnover in late night to early morning (Fig. 1) [57]. This model was eventually expanded to incorporate CLK [58, 59] and CYC [60] after their characterization, thereby establishing the TTFL model of the Drosophila clock.
Fig. 1.
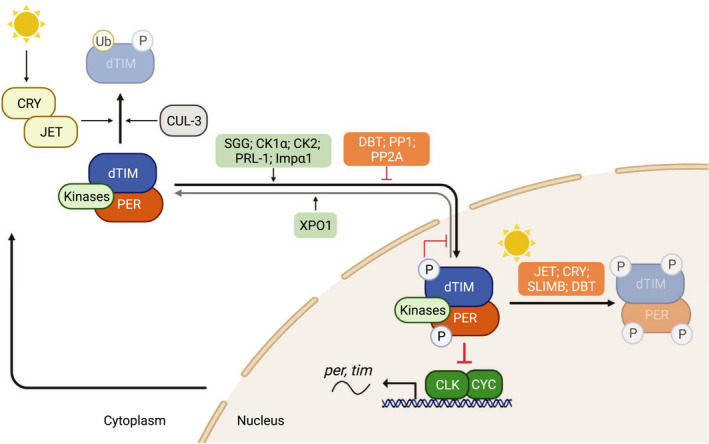
Drosophila TIM (dTIM) is a core component of the molecular oscillator. During the day, CLK‐CYC heterodimers activate the transcription of rhythmic genes, including per and tim in the nucleus [6]. In the cytoplasm, dTIM undergoes proteasomal degradation mediated by CRYPTOCHROME (CRY) [35, 36, 37, 38] and JETLAG (JET) [28, 88] upon light exposure. CULLIN‐3 (CUL‐3) has also been observed to mediate dTIM degradation in a light‐independent manner [27]. Early in the night, SHAGGY (SGG) [68], casein kinase 1α (CK1α) [187], casein kinase 2 (CK2) [69, 70], Importin‐α1 (Impα1) [64] and phosphatase of regenerating liver‐1 (PRL‐1) [75] promote nuclear accumulation of PER‐dTIM complex. This is antagonized by DOUBLETIME (DBT) [25], protein phosphatase 1 (PP1) [74] and protein phosphatase 2A (PP2A) [73]. Once PER‐dTIM complex is in the nucleus, CK2‐dependent phosphorylation of dTIM (S1404) inhibits PER‐dTIM nuclear export by exportin 1 (XPO1) complex, retaining PER‐dTIM complex in the nucleus [67]. At midnight, nuclear PER–dTIM complex interacts with CLK‐CYC and represses their transcriptional activity [23, 25]. From late night to early morning, CRY and JET mediate light‐dependent TIM degradation [28, 88], whereas DBT and SUPERNUMERARY LIMBS (SLIMB) mediate PER degradation [26, 29]. There have also been reports suggesting the involvement of SLIMB in TIM degradation [27].
As a negative component in the molecular clockwork, dTIM does not have intrinsic repression activity. Instead, it is essential in maintaining rhythmic PER expression and activity (Fig. 1). This is strongly supported by observations that PER rhythmic expression and behavioral rhythmicity are abolished in dtim null mutant [18] and mutants that are defective in TIM nuclear entry [61, 62]. Early studies suggest that dTIM binds to and blocks the cytoplasmic localization domain (CLD) of PER and thus reduces PER cytoplasmic retention [63]. Another study described a mechanism by which dTIM antagonizes the activity of DOUBLETIME (DBT, homolog of mammalian casein kinase 1 delta/epsilon) in inhibiting PER nuclear entry [22]. dTIM also acts as the major cargo recognized by the Importin‐α1 (IMPα1) nuclear entry machinery, thus transporting PER into the nucleus [64]. Saez et al. [61] identified a functional nuclear localization signal (NLS) that is potentially recognized by IMPα1 (Fig. 2). Once in the nucleus, dTIM appears to be bound to PER constitutively and facilitates PER repression [25, 65]. Sun et al. [66] suggested that dTIM may act as a scaffold to promote PER‐CLK interaction. Alternatively, dTIM may facilitate yet‐to‐be‐characterized CLK kinase(s) [23, 24, 67] in the PER‐dTIM repressor complex to phosphorylate CLK and inactivate transcriptional activity.
Fig. 2.
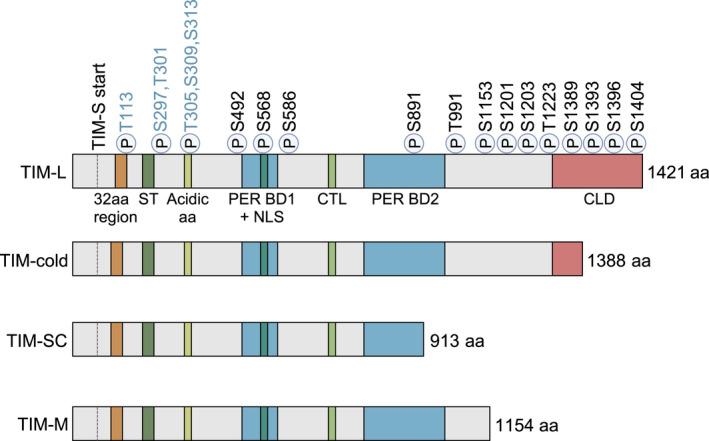
Schematic illustrating domain structure of TIM isoforms generated from alternative splicing. All amino acid numbering is based on the TIM‐L1421 isoform. ‘TIM‐S start’ denotes alternative translation start site for TIM‐S. Previously described domains of TIM: 32 amino acid region (amino acid [aa] 260–291) [188], also known as serine‐rich domain (SRD) (aa 260–292) [71]; serine/threonine (ST)‐rich region (aa 293–312) [72]; a stretch of acidic amino acid residues (acidic aa) (aa 383–412) [56]; PER binding domain 1 (PER BD1) (aa 536–610) [61]; nuclear localization sequence (NLS) (aa 558–593) [61]; C‐terminal tail‐like sequence (CTL) (aa 640–649) [87]; PER binding domain 2 (PER BD2) (aa 747–946) [61]; and cytoplasmic localization domain (CLD) (aa 1261–1421) [61]. P = phosphorylation sites [62, 67, 72, 75]. Phosphorylation sites in black = identified via mass spectrometry; blue = identified via in vivo functional analysis but have not been validated by mass spectrometry or phospho‐specific antibodies. TIM‐cold, TIM‐SC, TIM‐M isoforms are based on Shakhmantsir et al., Foley et al., Martin Anduaga et al. [31, 32, 33].
dTIM function is extensively regulated by posttranslational modifications (PTMs). Notably, phosphorylation is the best‐studied protein modification to achieve dTIM time‐of‐day specific functions. Casein kinase 2 (CK2) and SHAGGY [SGG, homolog of mammalian glycogen synthase kinase‐3β (GSK3β)] have been shown to phosphorylate both PER and dTIM and promote nuclear entry [68, 69, 70, 71, 72] (Fig. 1). Interestingly, once in the nucleus, PER‐dTIM complexes are subjected to phosphorylation‐dependent nuclear export, providing an additional means to control nuclear accumulation [21, 67]. Protein phosphatases also participate in regulating PER‐dTIM nuclear accumulation [73, 74, 75]. Over the past 10 years, site‐specific functions of dTIM phosphorylation have been characterized in a few studies (Fig. 2). In vivo functional analysis leveraging mutagenesis of dTIM protein revealed that T113 is critical for rhythmic dTIM expression [62]. Mutating T113 to non‐phosphorylatable alanine (A) abolishes dTIM nuclear entry, whereas mutations at a nearby proline (P115) produce similar defects. Combining genetic and biochemical studies, Top et al. [72] showed that SGG and CK2 phosphorylate five residues at ST region (S297, T301, T305, S309, and S313) to promote dTIM nuclear accumulation. Interestingly, SGG and CK2 appear to regulate PER‐dTIM only in a subset of clock neurons, which may contribute to the divergent functions of specific neuronal groups within the circadian neuronal circuitry. This could potentially explain how alteration in TIM phosphorylation in flies carrying timblind allele (A1128V, L1131M) results in lengthened locomotor activity rhythms but normal eclosion rhythms [76]. Activity and eclosion rhythms are two well characterized output of the Drosophila clock and are normally altered to the same extent in most fly mutants, including the three per mutants Konopka and Benzer identified in 1971 [48]. The mechanisms by which kinases phosphorylate PER‐dTIM in specific neurons remain unclear. Since alternative pre‐mRNA splicing patterns were observed in different clock neurons including for sgg mRNAs [77], we speculate that this may result in cell‐type‐specific posttranslational modification programs for key clock proteins, including dTIM.
Recently, two studies harnessed mass spectrometry proteomics to identify dTIM phosphorylation sites [67, 75] (Fig. 2). Kula‐Eversole et al. [75] identified five dTIM phosphorylation sites in Drosophila S2R+ cells coexpressing dTIM and relevant kinases (SGG and CK2). S586 and T991 are shown to be dephosphorylated by Phosphatase of Regenerating Liver‐1 (PRL‐1), which in turn promotes dTIM nuclear accumulation. In Cai et al. [67], we identified 12 phosphorylation sites in PER‐bound dTIM from Drosophila tissues. In particular, we showed that S1404 phosphorylation inhibits the interaction between dTIM and the nuclear export complex, thereby promoting dTIM nuclear accumulation. S1404 phosphorylation status in fly tissues was confirmed using phosphospecific antibody.
In addition to nuclear accumulation, phosphorylation also regulates dTIM protein turnover. CULLIN‐3 (CUL‐3) and SKP1‐CUL1‐F‐box‐protein/SUPERNUMERARY LIMB complex (SCF/SLIMB) differentially facilitates dTIM degradation depending on its phosphorylation status [27, 78], thus fine‐tuning dTIM phase‐specific functions (Fig. 1). Besides phosphorylation, O‐GlcNAcylation at multiple residues on dTIM was also identified [67]. Since O‐GlcNAcylation modifies serine/threonine residues and regulates the function of many proteins including PER and CLK [79, 80, 81, 82], it will be interesting to determine how the two types of PTMs coordinate to regulate dTIM phase‐specific functions. Given O‐GlcNAcylation is nutrient‐sensitive, this could be a mechanism by which metabolic signals can integrate with time‐of‐day environmental signals to promote robust circadian rhythms.
Finally, besides PTMs, dtim expression is regulated by posttranscriptional mechanisms. Carbon catabolite repression‐negative on TATA‐less deadenylation complex (CCR4‐NOT) has been shown to regulate dtim mRNA stability to support phase‐specific dTIM function [83]. Drosophila tim also exhibits alternative splicing pattern in response to environmental conditions, which will be described later.
Drosophila TIM and light entrainment of circadian rhythms
To confer fitness, a circadian clock must be synchronized to local time. Environmental time cues such as daily light–dark or temperature cycles entrain the circadian clock [84]. Identification of clock genes paved the way to investigations on molecular components that mediate clock entrainment. Two years after the identification of dtim in 1994, four exciting papers showed that dTIM displays light sensitivity, thus coupling the molecular clockwork to photic input from the environment [35, 36, 37, 38] (Fig. 1). CRY is the major photoreceptor that mediates TIM light‐dependent degradation [85, 86, 87]. Light induces CRY conformational change, thus enabling CRY to bind to dTIM. Thereafter, E3 ubiquitin ligase JETLAG (JET) along with CRY promotes rapid TIM proteasomal degradation [28, 87, 88] upon yet uncharacterized TIM tyrosine phosphorylation [89]. QUASIMODO (QSM), a light‐responsive protein expressing predominantly in CRY‐negative clock neurons, also trigger dTIM degradation upon light exposure [90]. dTIM degradation promotes PER turnover, thus resetting the circadian clock [37].
Drosophila TIM and temperature compensation of the circadian clock
Whereas rates of chemical reactions are often temperature‐dependent on a molecular level, a clock is only meaningful if its period length stays constant over a wide range of temperatures. The circadian clock has the property of temperature compensation; its pace is stable over a wide range of temperatures [84]. PER was first identified to participate in this process. A repetitive threonine‐glycine (Thr‐Gly) tract in PER exhibits more flexible conformation in higher temperature [91], which correlates with the observation that flies expressing PER with a deletion in the Thr–Gly tract display impaired temperature compensation of the circadian clock [92]. In wild D. melanogaster populations, the Thr–Gly tract is polymorphic in length; this is adaptive and enables flies to maintain the pace of the clock in environments with different range of temperatures [93].
dtim has also been demonstrated to contribute to temperature compensation of the clock. At the posttranscriptional level, manipulating dtim thermosensitive splicing results in defective temperature compensation [32, 33]. Elucidating the function of each dtim isoform under different temperatures could help understand how they regulate temperature compensation in future studies. At the posttranslational level, mutant lines bearing a number of amino acid substitutions, timrit (P1116A) and timblind , exhibit impaired temperature compensation [94, 95]. The mechanism by which dTIM regulates temperature compensation remains unclear. One possibility is that temperature directly modulates PER‐dTIM interaction. Another possibility is that temperature may indirectly modulate site‐specific phosphorylation to regulate phase‐specific functions of PER‐dTIM and achieve temperature compensation. In mammalian systems, temperature has been shown to determine the priority of competing phosphorylation sites to regulate PER2 turnover rate [96, 97]. Therefore, mass spectrometry‐based phosphorylation site mapping in combination with molecular genetics may further expand our understanding of how dTIM phosphorylation confers temperature compensation in flies.
Sequence polymorphism and alternative splicing of Drosophila tim regulates seasonal biology
To prepare for seasonal changes, plants and animals rely on internal photoperiodic timers, allowing them to undergo physiological and behavioral changes to survive unfavorable times [98, 99, 100]. Genetic analysis of wild D. melanogaster populations as well as molecular studies revealed that polymorphism at the dtim locus facilitates seasonal adaptation (Fig. 3A). ls‐tim is a derived dtim allele that evolved 300–3000 years ago in Europe [101] and has a G nucleotide insertion upstream of the original ATG translational start site [102, 103]. This generates an extra ATG 23 amino acids upstream of the TIM‐S start codon. ls‐tim allele thus generates two protein isoforms: TIM‐S and a 23‐aa longer TIM‐L (Fig. 2) (TIM‐S and TIM‐L were originally named S‐TIM and L‐TIM but we are renaming them to follow the convention used in more recent publications describing other TIM protein isoforms resulting from alternative pre‐mRNA splicing). TIM‐L displays reduced light sensitivity, largely due to its reduced binding affinity to CRY [88]. Since light‐dependent degradation of dTIM is critical to the resetting of the clock, reduced light sensitivity is thought to keep the molecular clockwork rhythmic in long summer days [104]. Furthermore, in anticipation of the onset of winter, flies carrying ls‐tim alleles enter reproductive dormancy earlier in autumn as compared with flies carrying only s‐tim alleles [103]. This is expected to be adaptive for flies inhabiting higher latitudes where harsh conditions are common in winter. For this reason, it was surprising that Tauber et al. [103] initially found the highest ls‐tim allele frequency in southeastern Italy and decrease of ls‐tim as the sampling distance increases both northward and southward. Subsequent analysis now suggests that this derived allele is only 300–3000 years old; it is still under selection and has not yet achieved fixation [101]. In fact, more extensive sampling in Spain [101] and in North America [105] reported a strong latitudinal cline where ls‐tim allele increases in frequency as latitude increases.
Fig. 3.
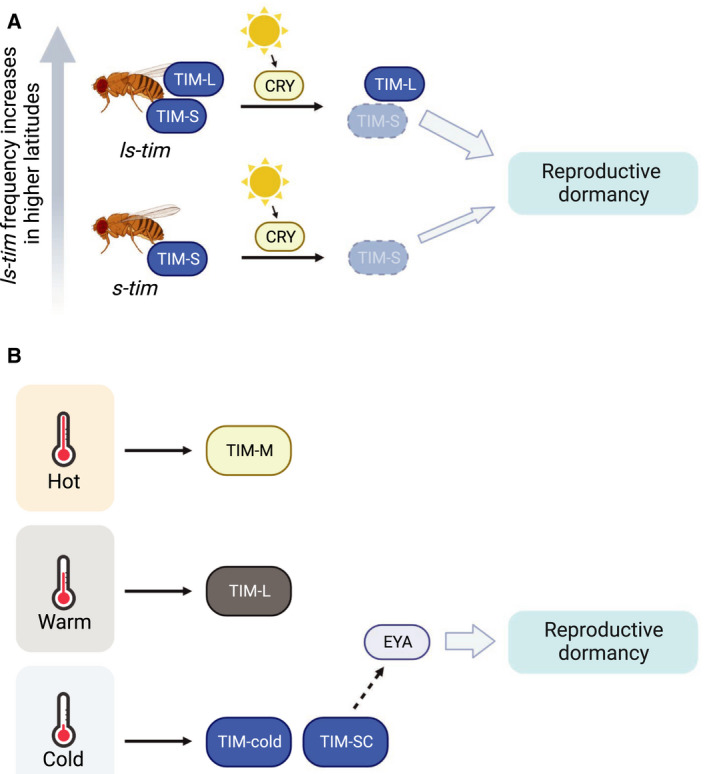
Role of Drosophila TIM in regulating seasonal biology. (A) Flies carrying s‐tim allele express TIM‐S, whereas flies carrying ls‐tim allele express both TIM‐L and TIM‐S. Sampling of flies in North America [105] and on the eastern side of the Iberian Peninsula [101] showed that ls‐tim allele frequency exhibits a latitudinal cline and increases with latitude. Since TIM‐L is less susceptible to light‐activated CRY‐dependent degradation, flies carrying ls‐tim allele interpret light signal differently and have higher inducibility of reproductive dormancy at the onset of winter to survive harsh conditions [103]. (B) High temperature promotes accumulation of TIM‐M isoform [31]. TIM‐L is the major isoform at warm temperature [32]. Cold temperature promotes the accumulation of TIM‐SC and TIM‐cold isoforms [32, 33]. TIM‐SC can potentially stabilize EYES ABSENT (EYA) to promote reproductive dormancy [34].
In addition to sequence polymorphism at the dtim locus, dtim displays thermosensitive alternative splicing. This has been proposed to be a temperature sensing mechanism to regulate D. melanogaster seasonal biology. In response to temperature changes, dtim produces four splice variants: tim‐cold, tim‐short and cold (tim‐sc), tim‐M (also called tim‐tiny), and tim‐L (full‐length isoform) (Fig. 2). At moderate temperature (25 °C), constitutively spliced tim‐L is the major isoform and produces full‐length TIM [32] (Fig. 3B). tim‐cold and tim‐sc are major isoforms in colder temperatures (10–18 °C) [32, 33, 34, 106], whereas tim‐tiny intron is retained in higher temperatures, resulting in high levels of tim‐M isoform (29–35 °C) [31, 32, 107]. Thermosensitive alternative splicing is also observed in three other Drosophila species, indicating this could be a conserved mechanism across the genus [32]. Less is known regarding the functional divergence of each dtim splice variant and how the pattern of splicing modulates the circadian clock in different seasonal conditions. Since some splicing events generate truncated TIM proteins, they could differentially affect TIM function in the circadian clock. For example, the TIM‐SC protein lacks the C‐terminal CLD and part of PER‐binding domain, which may compromise nuclear accumulation of the PER‐dTIM complex. Further functional studies on TIM isoforms are required to test this hypothesis.
There has been a substantial amount of evidence to support the role of dtim in regulating seasonal biology in addition to the studies mentioned above. They include the observed correlation between tim alleles and photoperiodic diapause in D. triauraria [108], changes in tim expression levels in response to photoperiod in several insect species [109, 110], and differential photosensitive alternative splicing of tim observed in cold‐adapted D. montana populations collected in a wide latitudinal range [111]. We recently provided evidence supporting the role of dTIM in seasonal physiology in D. melanogaster [34] (Fig. 3B). We showed that dtim null mutants fail to enter reproductive dormancy in simulated winter condition, while flies overexpressing dtim exhibit higher incidence of reproductive dormancy. We report evidence indicating that the cold‐induced and light‐insensitive isoform TIM‐SC facilitates the accumulation of EYES ABSENT (EYA) protein in winter condition, an event that is sufficient to promote reproductive dormancy. It remains unclear why TIM‐SC is not subjected to light‐dependent degradation and how it interacts with EYA. One possibility is that the truncated protein reduces the binding affinity to CRY and/or JET, and somehow stabilizes EYA via yet unknown mechanisms. A temperature‐dependent alternative splicing event is also observed in frequency (frq), a key repressor in the Neurospora clockwork [112, 113, 114]. It is possible that this temperature‐regulated event also contributes to Neurospora seasonal adaptation.
What is the mechanism by which temperature regulates dtim alternative splicing? So far, splicing regulator P‐element somatic inhibitor (PSI) [33] and triple small nuclear ribonuclearprotein (tri‐snRNP) spliceosome [31] have been shown to regulate dtim splicing. Temperature is known to modulate alternative splicing at multiple levels, including the expression of splicing‐related genes [115, 116], PTMs [117], spliceosome assembly [118], and spliceosome localization [119, 120].
Non‐circadian roles of Drosophila TIM
The fact that dTIM is expressed and differentially regulated in non‐clock cells has led to the investigation of non‐circadian roles of dTIM. A few studies revealed unexpected results regarding dTIM circadian expression pattern and light sensitivity in non‐clock cells. dTIM and its binding partner PER remain constitutively cytoplasmic in the fly ovary, which is known to lack intracellular molecular clocks [121, 122, 123]. This is unlike the subcellular shuttling of PER‐dTIM observed consistently in clock neurons. Furthermore, dTIM in the follicle cells is not susceptible to light‐induced degradation [123, 124]. It is noteworthy that egg‐laying rhythms persist under constant light, in contrast to the arrhythmic eclosion and locomotor activity rhythms in the same condition [125]. Whether the peculiar PER‐dTIM behavior in ovaries relates to rhythmic egg laying under constant light remains unclear. Although dtim null mutants display reduced fitness in terms of female fertility and fecundity [123], it has been proposed that this is likely due to the overall loss of the circadian clock [11]. To examine non‐circadian roles of dtim, it is necessary to manipulate dtim specifically in target cells/tissues. One possibility is that dtim expressed in non‐clock cells has a residual role in maintaining chromosome integrity inferred from its ancestral paralog dTIMEOUT, the homolog of mTIM [126] (Fig. 4A). The non‐circadian function of mTIM will be discussed below.
Fig. 4.
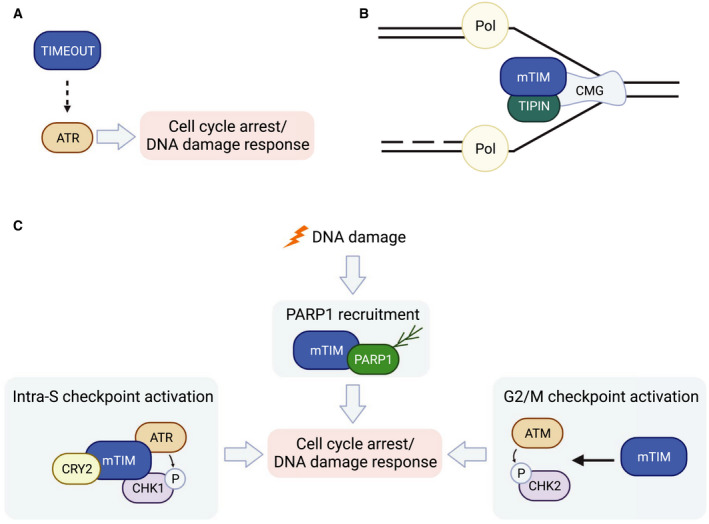
Drosophila TIMEOUT and mammalian TIMELESS in genome maintenance. (A) Drosophila TIMEOUT interacts with Ataxia telangiectasia and Rad3‐related (ATR) (genetically) to maintain genomic stability [126]. (B) mTIM and Tim‐interacting protein (TIPIN) couple replicative DNA helicase CMG (CDC45, MCM2‐7, GINS) and DNA polymerase (Pol) [145, 146] in progressing replication fork. (C) In response to DNA damage, mTIM physically interacts with and recruits poly [ADP‐ribose] polymerase 1 (PARP1) to damaged sites [153, 154]. ATR and ataxia telangiectasia mutated (ATM) can both sense DNA damage and phosphorylate checkpoint kinase 1/2 (CHK1/2) [189]. This is dependent on a number of partner proteins including mTIM [140, 152, 153].
Debate on mammalian TIM function in circadian timekeeping
Evidence supporting the role of mammalian TIM in the circadian clock
Whether mTIM is a core component in the mammalian clock has been controversial. Due to their sequence similarity, mTIM was first identified as the homolog of dTIM in late 1990s [127, 128, 129, 130]. Because of its rhythmic mRNA expression in the mammalian brain [127, 131] and physical interaction to core clock proteins mPER1/2/3 [130, 132] and CRY1/2 [133, 134, 135, 136], mTIM was implicated as a clock protein. In addition, short‐term mTIM knockdown causes phase resetting, whereas long‐term knockdown of mTIM disrupts circadian neuronal activity rhythms [132]. Recently, Kurien et al. [137] reported a mutation in human TIM (hTIM) that causes familial advanced sleep phase syndrome (FASPS), reviving the discussion of the potential role of mTIM in mammalian clockworks. This mutation inhibits TIM nuclear accumulation and destabilizes PER/CRY2 repressor complex at the molecular level.
Evidence contradicting a direct role of mammalian TIM in regulating circadian rhythms
Multiple lines of evidence argue against a direct role of mTIM in the molecular clock. Homozygous mTim mutant mice are lethal in embryonic stage, whereas other homozygous clock mutants remain viable, suggesting a critical non‐circadian role of mTIM [45]. The binding of mTIM to CRY1/2 does not necessarily support a circadian role of mTIM given that CRY1/2 also participates in non‐circadian processes. CRY1 and CRY2 are known to modulate DNA damage response [138] and cell proliferation [139], and the interaction of mTIM‐CRY1 and mTIM‐CRY2 are critical for checkpoint activation [140, 141]. Furthermore, phylogenetic analysis revealed that mTIM is an ortholog of dTIMEOUT [142]. Drosophila TIMEOUT is the widely conserved ancestral paralog of dTIM among eukaryotes that originated from gene duplication at the time of Cambrian Explosion [45, 46, 143]. Unlike dTIM, dTIMEOUT is an essential gene in Drosophila development and maintenance of chromosome integrity [126].
Non‐circadian roles of mammalian TIM
There have been extensive investigations focusing on non‐circadian roles of mTIM (Fig. 4B,C). Similar to its yeast homolog topoisomerase 1‐associated factor 1 (tof1) [144], mTIM and its evolutionally conserved partner Tim‐interacting protein (TIPIN) maintain replisome stability [145, 146] and promote fork progression through hard‐to‐replicate regions [147, 148, 149, 150, 151]. In response to DNA damage, mTIM collaborates with cardinal signaling kinases ataxia telangiectasia‐mutated checkpoint kinase 1 (ATR‐CHK1) [140, 152], ataxia telangiectasia and Rad3‐related checkpoint kinase 2 (ATM‐CHK2) [153], and poly [ADP‐ribose] polymerase 1 (PARP1) [154, 155] to facilitate proper checkpoint control and DNA repair [156, 157, 158]. Because of its role in genome maintenance, it is not surprising that mTIM dysregulation is commonly found in many cancer types [153, 159, 160]. Specifically, mTIM promotes cancer development by protecting cancer cells from replication stress and cell cycle arrest [153, 161, 162]. Thus, mTIM appears to be a promising target for anticancer treatment. However, given its ability to influence the circadian clock, the side effect of clock disruption needs to be considered, as clock disruption has been linked to increased risks of many diseases including metabolic disorders and cancers [163, 164].
Considering the role of mTIM discussed in this section, it is noteworthy that the period shortening phenotype on the molecular clock resulting from the mTIM(R1081X) mutation is limited to proliferative cells [137]. Since the circadian clock ticks regardless of cell proliferation status, why was the period shortening phenotype only observed in proliferating cells? We speculate that mTIM modulates the circadian clock through its role in other cellular processes occurring only in proliferating cells. Specifically, its elevated expression in proliferative tissues such as spleen and thymus are consistent with its cell cycle‐related function [137, 165]. DNA damage has been shown to induce a circadian phase shift [166, 167, 168], with mTIM downregulation attenuating this effect [165]. Interestingly, the FASPS mutation found in hTIM lacks the C‐terminal domain critical for mTIM‐mediated DNA repair and checkpoint activation through replication stress response regulator SDE2 and PARP1 binding, respectively [153, 154, 162]. Taken together, it is plausible that the period shortening effect in proliferating cells can be attributed to a non‐circadian role of mTIM.
Despite functional divergence of mTIM and dTIM, there are still some parallels. Drosophila TIMEOUT is expressed in the optic lobe of adult Drosophila and contributes to light entrainment, analogous to light sensitivity of dTIM [126]. Decreased dTIM and mTIM nuclear accumulation in Drosophila and mammals respectively both lead to similar outcome in circadian rhythms at the molecular and behavioral levels [67, 137] (Fig. 5). This highlights an unexpected functional parallel between mTIM and dTIM in circadian regulation.
Fig. 5.
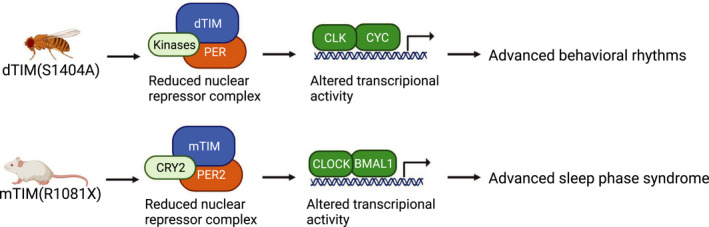
Functional parallel between Drosophila and mammalian TIM. Drosophila TIM(S1404A) elevates PER‐dTIM nuclear export [67]. The reduced abundance of nuclear PER‐dTIM repressor complex leads to altered phosphorylation status of CLK and transcriptional activity of CLK‐CYC, resulting in advanced behavioral rhythms. Mammalian TIM (R1081X) results in reduced nuclear mTIM [137], similar to the phenotype observed in dTIM(S1404A). This promotes destabilization of PER2‐CRY2 repressor complex, thus altering transcriptional activity of CLOCK‐BMAL1 and resulting in advanced sleep phase syndrome. Phosphorylation status of CLOCK or BMAL1 was not examined in [137].
Conclusion and perspectives
The very name of the timeless gene hints at its critical function in biological timing. Since its discovery, almost three decades ago in D. melanogaster, a large body of work have uncovered the role of dTIM as a cardinal clock protein necessary to maintain circadian timekeeping, mediate light entrainment, and modulate temperature compensation. Thermosensitive splicing of tim mRNA in combination with the light sensitivity of dTIM protein enables its role in regulating seasonal physiology. Its ancestral paralog timeout (mTIM in mammals) surprisingly plays a distinct role in the maintenance of genomic stability. An important unanswered question regarding the role of dTIM in biological rhythms is how splice variants affect dTIM protein function in response to thermal and photic cues. The answer would clarify how the circadian clock interplays with seasonal timing. Another area of interest is to elucidate how mTIM regulates the molecular clockwork and potentially sits at the intersection between circadian clocks and cell cycle regulation. This would further shed light on the functional similarity and divergence of the two TIM paralogs. More importantly, this would extend our understanding of the interconnection between the circadian clock and the cell cycle. Circadian regulation of the cell cycle has been found in all domains of life [169, 170, 171, 172, 173, 174, 175, 176, 177, 178], and the cell cycle also influences the phase and amplitude of circadian rhythms [166, 179, 180]. Given the accumulating evidence on circadian regulation of the cell cycle in the context of cancer and tissue regeneration upon injury [181, 182, 183, 184, 185, 186], understanding the interaction of the circadian clock and the cell cycle could pave the way for innovative therapeutics for cancer and improved recovery of patients who suffered injuries.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
YDC wrote the initial draft of the manuscript with input from JCC. JCC edited the manuscript for submission.
Acknowledgements
We thank Gary K. Chow for critical reading of the manuscript. This work was funded by NIH R01 DK124068 to JCC. We apologize to any colleagues whose works were not included in this review owing to space limitations.
References
- 1. Johnson CH, Zhao C, Xu Y & Mori T (2017) Timing the day: what makes bacteria clocks tick? Nat Rev Microbiol 15, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swan JA, Golden SS, LiWang A & Partch CL (2018) Structure, function, and mechanism of the core circadian clock in cyanobacteria. J Biol Chem 293, 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cox KH & Takahashi JS (2019) Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol 63, R93–R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creux N & Harmer S (2019) Circadian rhythms in plants. Cold Spring Harb Perspect Biol 11, a034611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunlap JC & Loros JJ (2017) Making time: conservation of biological clocks from fungi to animals. Microbiol Spectr 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patke A, Young MW & Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21, 67–84. [DOI] [PubMed] [Google Scholar]
- 7. Steed G, Ramirez DC, Hannah MA & Webb AAR (2021) Chronoculture, harnessing the circadian clock to improve crop yield and sustainability. Science 372, eabc9141. [DOI] [PubMed] [Google Scholar]
- 8. Ouyang Y, Andersson CR, Kondo T, Golden SS & Johnson CH (1998) Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA 95, 8660–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woelfle MA, Ouyang Y, Phanvijhitsiri K & Johnson CH (2004) The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14, 1481–1486. [DOI] [PubMed] [Google Scholar]
- 10. Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ & Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival and competitive advantage. Science 309, 630–633. [DOI] [PubMed] [Google Scholar]
- 11. Horn M, Mitesser O, Hovestadt T, Yoshi T, Rieger D & Helfrich‐Föster C (2019) The circadian clock improves fitness in the fruit fly, Drosophila melanogaster . Front Physiol 10, 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Claridge‐Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N & Young MW (2001) Circadian regulation of gene expression systems in the Drosophila head. Neuron 32, 657–671. [DOI] [PubMed] [Google Scholar]
- 13. McDonald MJ & Rosbash M (2001) Microarray analysis and organization of circadian gene expression in Drosophila . Cell 107, 567–578. [DOI] [PubMed] [Google Scholar]
- 14. Abruzzi KC, Rodriguez J, Mente JS, Desrochers J, Zadina A, Luo W, Tkachev S & Rosbash M (2011) Drosophila CLOCK target gene characterization: implications for circadian tissue‐specific gene expression. Genes Dev 25, 2374–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111, 16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi‐Benyahya O, Cooper HM et al. (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beytebiere JR, Trott AJ, Greenwell BJ, Osborne CA, Vitet H, Spence J, Yoo SH, Chen Z, Takahashi JS, Ghaffari N et al. (2019) Tissue‐specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer‐enhancer interactions. Genes Dev 33, 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vosshall LB, Price JL, Sehgal A, Saez L & Young MW (1994) Block in nuclear localization of period protein by a second clock mutation, timeless . Science 263, 1606–1609. [DOI] [PubMed] [Google Scholar]
- 19. Price JL, Dembinska ME, Young MW & Rosbash M (1995) Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless . EMBO J 14, 4044–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B & Young MW (1998) double‐time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94, 83–95. [DOI] [PubMed] [Google Scholar]
- 21. Ashmore L, Sathyanarayanan S, Silvestre DW, Emerson MM, Schotland P & Sehgal A (2003) Novel insights into the regulation of the timeless protein. J Neurosci 23, 7810–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cyran SA, Yiannoulos G, Buchsbaum AM, Saez L & Young MW (2005) The double‐time protein kinase regulates the subcellular localization of the Drosophila clock protein period . J Neurosci 25, 5430–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu W, Zheng H, Houl JH, Dauwalder B & Hardin PE (2006) PER‐dependent rhythms in CLK phosphorylation and E‐box binding regulate circadian transcription. Genes Dev 20, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu W, Zheng H, Price JL & Hardin PE (2009) DOUBLETIME plays a noncatalytic role to mediate CLOCK phosphorylation and repress CLOCK‐dependent transcription within the Drosophila circadian clock. Mol Cell Biol 29, 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menet JS, Abruzzi KC, Desrochers J, Rodriguez J & Rosbash M (2010) Dynamic PER repression mechanisms in the Drosophila circadian clock: from on‐DNA to off‐DNA. Genes Dev 24, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grima B, Lamouroux A, Chélot E, Papin C, Limbourg‐Bouchon B & Rouyer F (2002) The F‐box protein Slimb controls the levels of clock proteins Period and Timeless. Nature 420, 178–182. [DOI] [PubMed] [Google Scholar]
- 27. Grima B, Dognon A, Lamouroux A, Chélot E & Rouyer F (2012) CULLIN‐3 controls TIMELESS oscillations in the Drosophila circadian clock. PLoS Biol 10, e1001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koh K, Zheng X & Sehgal A (2006) JETLAG resets the Drosophila circadian clock by promoting light‐induced degradation of TIMELESS. Science 312, 1809–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiu JC, Vanselow JT, Kramer A & Edery I (2008) The phospho‐occupancy of an atypical SLIMB‐binding site on PERIOD that is phosphorylated by DOUBLETIME controls the pace of the clock. Genes Dev 22, 1758–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiu JC, Ko KW & Edery I (2011) NEMO/NLK phosphorylates PERIOD to initiate a time‐delay phosphorylation circuit that sets circadian clock speed. Cell 145, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shakhmantsir I, Nayak S, Grant GR & Sehgal A (2018) Spliceosome factors target timeless (tim) mRNA to control clock protein accumulation and circadian behavior in Drosophila . eLife 7, e39821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin Anduaga A, Evantal N, Patop IL, Bartok O, Weiss R & Kadener S (2019) Thermosensitive alternative splicing senses and mediates temperature adaptation in Drosophila . eLife 8, e44642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foley LE, Ling J, Joshi R, Evantal N, Kadener S & Emery P (2019) Drosophila PSI controls circadian period and the phase of circadian behavior under temperature cycle via tim splicing. eLife 8, e50063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abrieux A, Xue Y, Cai Y, Lewald KM, Nguyen HN, Zhang Y & Chiu JC (2020) EYES ABSENT and TIMELESS integrate photoperiodic and temperature cues to regulate seasonal physiology in Drosophila . Proc Natl Acad Sci USA 117, 15293–15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee C, Parikh V, Itsukaichi T, Bae K & Edery I (1996) Resetting the Drosophila clock by photic regulation of PER and a PER‐TIM complex. Science 271, 1740–1744. [DOI] [PubMed] [Google Scholar]
- 36. Hunter‐Ensor M, Ousley A & Sehgal A (1996) Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell 84, 677–685. [DOI] [PubMed] [Google Scholar]
- 37. Zeng H, Qian Z, Myers MP & Rosbash M (1996) A light‐entrainment mechanism for the Drosophila circadian clock. Nature 380, 129–135. [DOI] [PubMed] [Google Scholar]
- 38. Myers MP, Wager‐Smith K, Rothenfluh‐Hilfiker A & Young MW (1996) Light‐induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science 271, 1736–1740. [DOI] [PubMed] [Google Scholar]
- 39. Miyazaki K, Mesaki M & Ishida N (2001) Nuclear entry mechanism of rat PER2 (rPER2): role of rPER2 in nuclear localization of CRY protein. Mol Cell Biol 21, 6651–6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yagita K, Tamanini F, Yasuda M, Hoeijmakers JHJ, van der Horst GTJ & Okamura H (2002) Nuclearcytoplasmic shuttling and mCRY‐dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J 21, 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SIH, Draetta GF & Pagano M (2007) SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of Cryptochrome proteins. Science 316, 900–904. [DOI] [PubMed] [Google Scholar]
- 42. Godinho SIH, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR et al. (2007) The after‐hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316, 897–900. [DOI] [PubMed] [Google Scholar]
- 43. Siepka SM, Yoo S‐H, Park J, Song W, Kumar V, Hu Y, Lee C & Takahashi JS (2007) Circadian mutant Overtime reveals F‐box protein FBXL3 regulation of Cryptochrome and Period gene expression. Cell 129, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brenna A, Olejniczak I, Chavan R, Ripperger JA, Langmesser S, Cameroni E, Hu Z, Virgilio CD, Dengele J & Albrecht U (2019) Cyclin‐dependent kinase 5 (CDK5) regulates the circadian clock. eLife 8, e50925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gotter AL, Manganaro T, Weaver DR, Kolakowski LF, Possidente B, Sriram S, MacLaughlin DT & Reppert SM (2000) A time‐less function for mouse timeless . Nat Neurosci 3, 755–756. [DOI] [PubMed] [Google Scholar]
- 46. Gotter AL (2006) A Timeless debate: resolving TIM's noncircadian roles with possible clock function. NeuroReport 17, 1229–1233. [DOI] [PubMed] [Google Scholar]
- 47. Mazzoccoli G, Laukkanen MO, Vinciguerra M, Colangelo T & Colantuoni V (2016) A Timeless link between circadian patterns and disease. Trends Mol Med 22, 68–81. [DOI] [PubMed] [Google Scholar]
- 48. Konopka RJ & Benzer S (1971) Clock mutants of Drosophila melanogaster . Proc Natl Acad Sci USA 68, 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bargiello TA & Young MW (1984) Molecular genetics of a biological clock in Drosophila . Proc Natl Acad Sci USA 81, 2142–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamblen M, Zehring WA, Kyriacou CP, Reddy P, Yu Q, Wheeler DA, Zwiebel LJ, Konopka RJ, Rosbash M & Hall JC (1986) Germ‐line transformation involving DNA from the period locus in Drosophila melanogaster: overlapping genomic fragments that restore circadian and ultradian rhythmicity to per 0 and per ‐ mutants. J Neurogenet 3, 249–291. [DOI] [PubMed] [Google Scholar]
- 51. Baylies MK, Bargiello TA, Jackson FR & Young MW (1987) Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. Nature 326, 390–392. [DOI] [PubMed] [Google Scholar]
- 52. Yu Q, Colot HV, Kyriacou CP, Hall JC & Rosbash M (1987) Behaviour modification by in vitro mutagenesis of a variable region within the period gene of Drosophila. Nature 326, 765–769. [DOI] [PubMed] [Google Scholar]
- 53. Lorenz LJ, Hall JC & Rosbash M (1989) Expression of a Drosophila mRNA is under circadian control during pupation. Development 107, 869–880. [DOI] [PubMed] [Google Scholar]
- 54. Hardin PE, Hall JC & Rosbash M (1990) Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540. [DOI] [PubMed] [Google Scholar]
- 55. Sehgal A, Price JL, Man B & Young MW (1994) Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless . Science 263, 1603–1606. [DOI] [PubMed] [Google Scholar]
- 56. Myers MP, Wager‐Smith K, Wesley CS, Young MW & Sehgal A (1995) Positional cloning and sequence analysis of the Drosophila clock gene, timeless . Science 270, 805–808. [DOI] [PubMed] [Google Scholar]
- 57. Sehgal A, Rothenfluh‐Hilfiker A, Hunter‐Ensor M, Chen Y, Myers MP & Young MW (1995) Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science 270, 808–810. [DOI] [PubMed] [Google Scholar]
- 58. Allada R, White NE, So WV, Hall JC & Rosbash M (1998) A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless . Cell 93, 791–804. [DOI] [PubMed] [Google Scholar]
- 59. Darlington TK, Wager‐Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TD, Weitz CJ, Takahashi JS & Kay SA (1998) Closing the circadian loop: CLOCK‐induced transcription of its own inhibitors per and tim . Science 280, 1599–1603. [DOI] [PubMed] [Google Scholar]
- 60. Rutila JE, Suri V, Le M, So WV, Rosbash M & Hall JC (1998) CYCLE is a second bHLH‐PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless . Cell 93, 805–814. [DOI] [PubMed] [Google Scholar]
- 61. Saez L, Derasmo M, Meyer P, Stieglitz J & Young MW (2011) A key temporal delay in the circadian cycle of Drosophila is mediated by a nuclear localization signal in the timeless protein. Genetics 188, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hara T, Koh K, Combs DJ & Sehgal A (2011) Post‐translational regulation and nuclear entry of TIMELESS and PERIOD are affected in new timeless mutant. J Neurosci 31, 9982–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saez L & Young MW (1996) Regulation of nuclear entry of the Drosophila clock proteins period and timeless . Neuron 17, 911–920. [DOI] [PubMed] [Google Scholar]
- 64. Jang AR, Moravcevic K, Saez L, Young MW & Sehgal A (2015) Drosophila TIM binds importin α1, and acts as an adapter to transport PER to the nucleus. PLOS Genet 11, e1004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meyer P, Saez L & Young MW (2006) PER‐TIM interactions in living Drosophila cells: an interval timer for the circadian clock. Science 311, 226–229. [DOI] [PubMed] [Google Scholar]
- 66. Sun WC, Jeong EH, Jeong HJ, Ko HW, Edery I & Kim EY (2010) Two distinct modes of PERIOD recruitment onto dCLOCK reveal a novel role for TIMELESS in circadian transcription. J Neurosci 30, 14458–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cai YD, Xue Y, Truong CC, Carmen‐Li JD, Ochoa C, Vanselow JT, Murphy KA, Li YH, Liu X, Kunimoto BL et al. (2021) CK2 inhibits TIMELESS nuclear export and modulates CLOCK transcriptional activity to regulate circadian rhythms. Curr Biol 31, 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martinek S, Inonog S, Manoukian AS & Young MW (2001) A role for the segment polarity gene shaggy/GSK‐3 in the Drosophila circadian clock. Cell 105, 769–779. [DOI] [PubMed] [Google Scholar]
- 69. Lin JM, Kilman VL, Keegan K, Paddock B, Emery‐Le M, Rosbash M & Allada R (2002) A role for casein kinase 2alpha in the Drosophila circadian clock. Nature 420, 816–820. [DOI] [PubMed] [Google Scholar]
- 70. Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T & Jackson FR (2003) A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci 6, 251–257. [DOI] [PubMed] [Google Scholar]
- 71. Meissner RA, Kilman VL, Lin JM & Allada R (2008) TIMELESS is an important mediator of CK2 effects on circadian clock function in vivo . J Neurosci 28, 9732–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Top D, Harms E, Syed Y, Adams EL & Saez L (2016) GSK‐3 and CK2 kinases converge on Timeless to regulate the master clock. Cell Rep 16, 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sathyanarayanan S, Zheng X, Xiao R & Sehgal A (2004) Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell 116, 603–615. [DOI] [PubMed] [Google Scholar]
- 74. Fang Y, Sathyanarayanan S & Sehgal A (2007) Post‐translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1). Genes Dev 21, 1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kula‐Eversole E, Lee DH, Samba I, Yildirim E, Levine DC, Hong HK, Lear BC, Bass J, Rosbash M & Allada R (2021) Phosphatase of Regenerating Liver‐1 selectively times circadian behavior in darkness via function in PDF neurons and dephosphorylation of TIMELESS. Curr Biol 31, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wülbeck C, Szabo G, Shafer OT, Helfrich‐Förster C & Stanewsky R (2005) The novel Drosophila tim (blind) mutation affects behavioral rhythms but not periodic eclosion. Genetics 169, 751–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Q, Abruzzi KC, Rosbash M & Rio DC (2018) Striking circadian neuron diversity and cycling of Drosophila alternative splicing. eLife 7, e35618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Szabó Á, Papin C, Cornu D, Chélot E, Lipinszki Z, Udvardy A, Redeker V, Mayor U & Rouyer F (2018) Ubiquitylation dynamics of the clock cell proteome and TIMELESS during a circadian cycle. Cell Rep 23, 2273–2282. [DOI] [PubMed] [Google Scholar]
- 79. Kim EY, Jeong EH, Park S, Jeong H‐J, Edery I & Cho JW (2012) A role for O‐GlcNAcylation in setting circadian clock speed. Genes Dev 26, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kaasik K, Kivimäe S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptáček LJ et al. (2013) Glucose sensor O‐GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab 17, 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li YH, Liu XL, Vanselow JT, Zheng H, Schlosser A & Chiu JC (2019) O‐GlcNAcylation of PERIOD regulates its interaction with CLOCK and timing of circadian transcriptional repression. PLoS Genet 15, e1007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu X, Blaženović I, Contreras AJ, Phan TM, Tabuloc CA, Li YH, Ji J, Fiehn O & Chiu JC (2021) Hexosamine biosynthetic pathway and O‐GlcNAc‐processing enzymes regulate daily rhythms in protein O‐GlcNAcylation. Nat Commun 12, 4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Grima B, Papin C, Martin B, Chélot E, Ponien P, Jacquet E & Rouyer F (2019) PERIOD‐controlled deadenylation of the timeless transcript in the Drosophila circadian clock. Proc Natl Acad Sci USA 116, 5721–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pittendrigh CS (1974) Circadian oscillations in cells and the circadian organization of multicellular systems. In The Neurosciences Third Study Program (Schmitt FO & Worden FG, eds), pp. 437–458. MIT Press, Cambridge, MA. [Google Scholar]
- 85. Ceriani MF, Darlington TK, Más DS, Petti AA, Weitz J & Kay SA (1999) Light‐dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285, 553–556. [DOI] [PubMed] [Google Scholar]
- 86. Ozturk N, Selby CP, Annayev Y, Zhong D & Sancar A (2011) Reaction mechanism of Drosophila cryptochrome . Proc Natl Acad Sci USA 108, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vaidya AT, Top D, Manahan CC, Tokuda JM, Zhang S, Pollack L, Young MW & Crane BR (2013) Flavin reduction activates Drosophila cryptochrome . Proc Natl Acad Sci USA 110, 20455–20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Peschel N, Chen KF, Szabo G & Stanewsky R (2009) Light‐dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless . Curr Biol 19, 241–247. [DOI] [PubMed] [Google Scholar]
- 89. Naidoo N, Song W, Hunter‐Ensor M & Sehgal A (1999) A role for the proteasome in the light response of the timeless clock protein. Science 285, 1737–1741. [DOI] [PubMed] [Google Scholar]
- 90. Chen KF, Peschel N, Zavodska R, Sehadova H & Stanewsky R (2011) QUASIMODO, a novel GPI‐anchored zone pellucida protein involved in light input to the Drosophila circadian clock. Curr Biol 21, 719–729. [DOI] [PubMed] [Google Scholar]
- 91. Castiglione‐Morelli MA, Guantieri V, Villani V, Kyriacou CP, Costa R & Tamburro AM (1995) Conformational study of the Thr‐Gly repeat in the Drosophila clock protein, PERIOD. Proc Natl Acad Sci USA 260, 155–163. [DOI] [PubMed] [Google Scholar]
- 92. Ewer J, Hamblen‐Coyle M, Rosbash M & Hall JC (1990) Requirement for period gene expression in the adult and not during development for locomotor activity rhythms of imaginal Drosophila melanogaster . J Neurogenet 7, 31–73. [DOI] [PubMed] [Google Scholar]
- 93. Sawyer LA, Hennessy JM, Peixoto AA, Rosato E, Parkinson H, Costa R & Kyriacou CP (1997) Natural variation in a Drosophila clock gene and temperature compensation. Science 278, 2117–2120. [DOI] [PubMed] [Google Scholar]
- 94. Matsumoto A, Tomioka K, Chiba Y & Tanimura T (1999) tim rit lengthens circadian period in a temperature‐dependent manner through suppression of PERIOD protein cycling and nuclear localization. Mol Cell Biol 19, 4343–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Singh S, Giesecke A, Damulewicz M, Fexova S, Mazzotta GM, Stanewsky R & Dolezel D (2019) New Drosophila circadian clock mutants affecting temperature compensation induced by targeted mutagenesis of Timeless . Front Physiol 10, 1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kidd PB, Young MW & Sigma ED (2015) Temperature compensation and temperature sensation in the circadian clock. Proc Natl Acad Sci USA 112, E6284–E6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhou M, Kim JK, Eng GWL, Forger DB & Virshup DM (2015) A Period2 phosphoswitch regulates and temperature compensates circadian period. Mol Cell 60, 77–88. [DOI] [PubMed] [Google Scholar]
- 98. Yanovsky MJ & Kay SA (2003) Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Biol 4, 265–275. [DOI] [PubMed] [Google Scholar]
- 99. Merlin C, Iiams SE & Lugena AB (2020) Monarch butterfly migration moving into the genetic era. Trends Genet 36, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Reiter RJ & Sharma R (2021) Central and peripheral actions of melatonin on reproduction in seasonal and continuous breeding mammals. Gen Comp Endocrinol 300, 1136020. [DOI] [PubMed] [Google Scholar]
- 101. Zonato V, Vaniò S, Costa R, Tauber E & Kyriacou CP (2018) Inverse European latitudinal cline at the timeless locus of Drosophila melanogaster reveals selection on a clock gene: population genetics of ls‐tim . J Biol Rhythms 33, 15–23. [DOI] [PubMed] [Google Scholar]
- 102. Sandrelli F, Tauber E, Pegoraro M, Mazzotta G, Risotto P, Landskron J, Stanewsky R, Piccin A, Rosato E, Zordan M et al. (2007) A molecular basis for natural selection at the timeless locus in Drosophila melanogaster . Science 316, 1898–1900. [DOI] [PubMed] [Google Scholar]
- 103. Tauber E, Zordan M, Sandrelli F, Pegoraro M, Osterwalder N, Breda C, Daga A, Selmin A, Monger K, Benna C et al. (2007) Natural selection favors a newly derived timeless allele in Drosophila melanogaster . Science 316, 1895–1898. [DOI] [PubMed] [Google Scholar]
- 104. Beer K & Helfrich‐Föster C (2020) Model and nom‐model insects in chronobiology. Front Behav Neurosci 14, 601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pegoraro M, Zonato V, Tyler ER, Fedele G, Kyriacou CP & Tauber E (2017) Geographical analysis of diapause inducibility in European Drosophila melanogaster populations. J Insect Physiol 98, 238–244. [DOI] [PubMed] [Google Scholar]
- 106. Boothroyd C, Wijnen H, Naef F, Saez L & Young MW (2007) Integration of light and temperature in the regulation of circadian gene expression in Drosophila . PLoS Genet 3, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Montelli S, Mazzotta G, Vanin S, Caccin L, Corrà S, De Pittà C, Boothroyd C, Greem EW, Kyriacou CP & Costa R (2015) period and timeless mRNA splicing profiles under natural conditions in Drosophila melanogaster . J Biol Rhythms 30, 217–227. [DOI] [PubMed] [Google Scholar]
- 108. Yamada H & Yamamoto MT (2011) Association between circadian clock genes and diapause incidence in Drosophila triauraria . PLoS One 6, e27493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stehlík J, Závodská R, Shimada K, Sauman I & Kostál V (2008) Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata . J Biol Rhythms 23, 129–139. [DOI] [PubMed] [Google Scholar]
- 110. Huang X, Poelchau MF & Armbruster PA (2015) Global transcriptional dynamics of diapause induction in non‐blood‐fed and blood‐fed Aedes albopictus . PLoS Negl Trop Dis 9, e0003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tapanainen R, Parker DJ & Kankare M (2018) Photosensitive alternative splicing of the circadian clock gene timeless is population specific in a cold‐adapted fly, Drosophila montana . G3: Genes ‐ Genomes ‐ Genetics 8, 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu Y, Garceau NY, Loros JJ & Dunlap JC (1997) Thermally regulated translational control of FRQ mediates aspects of temperature responses in the Neurospora circadian clock. Cell 89, 477–486. [DOI] [PubMed] [Google Scholar]
- 113. Colot HV, Loros JJ & Dunlap JC (2005) Temperature‐modulated alternative splicing and promoter use in the Circadian clock gene frequency . Mol Biol Cell 16, 5563–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Diernfellner A, Colot HV, Dintsis O, Loros JJ, Dunlap JC & Brunner M (2007) Long and short isoforms of Neurospora clock protein FRQ support temperature‐compensated circadian rhythms. FEBS Lett 581, 5759–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee BH, Kapoor A, Zhu J & Zhu JK (2006) STABILIZED1, a stress‐upregulated nuclear protein, is required for pre‐mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis . Plant Cell 18, 1736–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kim GD, Cho YH, Lee BH & Yoo SD (2017) STABILIZED1 modulates pre‐mRNA splicing for thermotolerance. Plant Physiol 173, 2370–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu GT, Jiang JF, Liu XN, Jiang JZ, Sun L, Duan W, Li RM, Wang Y, Lecourieux D, Liu CH et al. (2019) New insights into the heat responses of grape leaves via combined phosphoproteomic and acetylproteomic analyses. Hortic Res 6, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schlaen RG, Mancini E, Sanchez SE, Perez‐Santángelo S, Rugnone ML, Simpson CG, Brown JWS, Zhang X, Chernomoretz A & Yanovsky MJ (2015) The spliceosome assembly factor GEMIN2 attenuates the effects of temperature on alternative splicing and circadian rhythms. Proc Natl Acad Sci USA 112, 9382–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Weber C, Nover L & Fauth M (2008) Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J 56, 517–530. [DOI] [PubMed] [Google Scholar]
- 120. Reddy ASN, Day IS, Göhring J & Barta A (2012) Localization and dynamics of nuclear speckles in plants. Plant Physiol 158, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Saez L & Young MW (1988) In situ localization of the per clock protein during development of Drosophila melanogaster . Mol Cell Biol 8, 5378–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hardin PE ( 1994) Analysis of period mRNA cycling in Drosophila head and body tissues indicates that body oscillators behave differently from head oscillators. Mol Cell Biol 14, 7211–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Beaver LM, Rush BL, Gvakharia BO & Giebultowicz JM (2003) Noncircadian regulation and function of clock genes period and timeless in oogenesis of Drosophila melanogaster . J Biol Rhythms 18, 463–472. [DOI] [PubMed] [Google Scholar]
- 124. Rush BL, Murad A, Emery P & Giebultowicz JM (2006) Ectopic CRYPTOCHROME renders TIM light sensitive in the Drosophila ovary. J Biol Rhythms 21, 272–278. [DOI] [PubMed] [Google Scholar]
- 125. Howlader G & Sharma VK (2006) Circadian regulation of egg‐laying behavior in fruit flies Drosophila melanogaster . J Insect Physiol 52, 779–785. [DOI] [PubMed] [Google Scholar]
- 126. Benna C, Bonaccorsi S, Wülbeck C, Helfrich‐Förster C, Gatti M, Kyriacou CP, Costa R & Sandrelli F (2010) Drosophila timeless2 is required for chromosome stability and circadian photoreception. Curr Biol 20, 346–352. [DOI] [PubMed] [Google Scholar]
- 127. Koike N, Hida A, Numano R, Hirose M, Sakaki Y & Tei H (1998) Identification of the mammalian homologues of the Drosophila timeless gene, Timeless1 . FEBS Lett 441, 427–431. [DOI] [PubMed] [Google Scholar]
- 128. Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP et al. (1998) Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK‐BMAL1‐induced transcription. Neuron 21, 1101–1113. [DOI] [PubMed] [Google Scholar]
- 129. Zylka MJ, Shearman LP, Levine JD, Jin X, Weaver DR & Reppert SM (1998) Molecular analysis of mammalian timeless. Neuron 21, 1115–1122. [DOI] [PubMed] [Google Scholar]
- 130. Takumi T, Nagamine Y, Miyake S, Matsubara C, Taguchi K, Takekida S, Sakakida Y, Nishikawa K, Kishimoto T, Niwa S et al. (1999) A mammalian ortholog of Drosophila timeless, highly expressed in SCN and retina, forms a complex with mPER1. Genes Cells 4, 67–75. [DOI] [PubMed] [Google Scholar]
- 131. Tischkau SA, Barnes JA, Lin FJ, Myers EM, Barnes JW, Meyer‐Bernstein EL, Hurst WJ, Burgoon PW, Chen D, Sehgal A et al. (1999) Oscillation and light induction of timeless mRNA in the mammalian circadian clock. J Neurosci 19, RC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Barnes JW, Tischkau SA, Barnes JA, Mitchell JW, Burgoon PW, Hickok JR & Gillette MU (2003) Requirement of mammalian Timeless for circadian rhythmicity. Science 302, 439–442. [DOI] [PubMed] [Google Scholar]
- 133. Griffin EA Jr, Staknis D & Weitz CJ (1999) Light‐independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286, 768–771. [DOI] [PubMed] [Google Scholar]
- 134. Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH & Reppert SM (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205. [DOI] [PubMed] [Google Scholar]
- 135. Field MD, Maywood ES, O’Brien JA, Weaver DR, Reppert SM & Hastings MH (2000) Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 25, 437–447. [DOI] [PubMed] [Google Scholar]
- 136. Gotter AL ( 2003) Tipin, a novel timeless‐interacting protein, is developmentally co‐expressed with timeless and disrupts its self‐association. J Mol Biol 331, 167–176. [DOI] [PubMed] [Google Scholar]
- 137. Kurien P, Hsu PK, Leon J, Wu D, McMahon T, Shi G, Xu Y, Lipzen A, Pennacchio LA, Jones CR et al. (2019) TIMELESS mutation alters phase responsiveness and causes advanced sleep phase. Proc Natl Acad Sci USA 116, 12045–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shafi AA, McNair CM, McCann JJ, Alshalalfa M, Shostak A, Severson TM, Zhu Y, Bergman A, Gordon N, Mandigo AC et al. (2021) The circadian cryptochrome, CRY1, is a pro‐tumorigenic factor that rhythmically modulates DNA repair. Nat Commun 12, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Huber AL, Papp SJ, Chan AB, Henriksson E, Jordan SD, Kriebs A, Nguyen M, Wallace M, Li Z, Metallo CM & Lamia KA (2016) CRY2 and FBXL3 cooperatively degrade c‐MYC. Mol Cell 64, 774–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ünsal‐Kaçmaz K, Mullen TE, Kaufmann WK & Sancar A (2005) Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol 25, 3109–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kang TH & Leem SH (2014) Modulation of ATR‐mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res 42, 4427–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Benna C, Scannapieco P, Piccin A, Sandrelli F, Zordan M, Rosato E, Kyriacou CP, Valle G & Costa R (2000) A second timeless gene in Drosophila shares greater sequence similarity with mammalian tim . Curr Biol 10, R512–R513. [DOI] [PubMed] [Google Scholar]
- 143. Rubin EB, Shemesh Y, Cohen M, Elgavish S, Elgavish S, Robertson HM & Bloch G (2006) Molecular and phylogenetic analyses reveal mammalian‐like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res 16, 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Foss EJ ( 2001) Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae . Genetics 157, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gotter AL, Suppa C & Emanuel BS (2006) Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork‐associated factors. J Mol Biol 366, 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cho W‐H, Kang Y‐H, An Y‐Y, Tappin I, Hurwitz J & Lee J‐K (2013) Human Tim‐Tipin complex affects the biochemical properties of the replicative DNA helicase and DNA polymerases. Proc Natl Acad Sci USA 110, 2523–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Urtishak KA, Smith KD, Chanoux RA, Greenberg RA, Johnson FB & Brown EJ (2009) Timeless maintains genomic stability and suppresses sister chromatid exchange during unperturbed DNA replication. J Biol Chem 284, 8777–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Smith KD, Fu MA & Brown EJ (2009) Tim‐Tipin dysfunction creates an indispensable reliance on the ATR‐Chk1 pathway for continued DNA synthesis. J Cell Biol 187, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Calì F, Bharti SK, Di Perna R, Brosh RM, Pisani FM (2016) Tim/Timeless, a member of the replication fork protection complex, operates with the Warsaw breakage syndrome DNA helicase DDX11 in the same fork recovery pathway. Nucleic Acids Res 44, 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Shyian M, Albert B, Zupan AM, Ivanitsa V, Charbonnet G, Dilg D & Shore D (2020) Fork pausing complex engages topoisomerases at the replisome. Genes Dev 34, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Westhorpe R, Keszthelyi A, Minchell NE, Jones D & Baxter J (2020) Separable functions of Tof1/Timeless in intra‐S‐checkpoint signalling, replisome stability and DNA topological stress. Nucleic Acids Res 48, 12169–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kemp MG, Akan Z, Yilmaz S, Grillo M, Smith‐Roe SL, Kang TH, Cordeiro‐Stone M, Kaufmann WK, Abraham RT, Sancar A et al. (2010) Tipin‐replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress. J Biol Chem 285, 16562–16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yang X, Wood PA & Hrushesky WJM (2010) Mammalian TIMELESS is required for ATM‐dependent CHK2 activation and G2/M checkpoint control. J Biol Chem 285, 3030–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Xie S, Mortusewicz O, Ma HT, Herr P, Poon RYC, Helleday T & Qian (2015) Timeless interacts with PARP‐1 to promote homologous recombination repair. Mol Cell 60, 163–176. [DOI] [PubMed] [Google Scholar]
- 155. Young LM, Marzio A, Perez‐Duran P, Reid DA, Meredith DN, Roberti D, Star A, Rothenberg E, Ueberheide B & Pagano M (2015) TIMELESS forms a complex with PARP1 distinct from its complex with TIPIN and plays a role in the DNA damage response. Cell Rep 13, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chou DM & Elledge SJ (2006) Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function. Proc Natl Acad Sci USA 103, 18143–18147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ünsal‐Kacmaz K, Chastain PD, Qu PP, Minoo P, Cordeiro‐Stone M, Sancar A & Kaufmann WK (2007) The human Tim/Tipin complex coordinates an Intra‐S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol 27, 3131–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gotter AL, Suppa C & Emanuel BS (2007) Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork‐associated factors. J Mol Biol 366, 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mao Y, Fu A, Leaderer D, Zheng T, Chen K & Zhu Y (2013) Potential cancer‐related role of circadian gene TIMELESS suggested by expression profiling and in vitro analyses. BMC Cancer 13, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yoshida K, Sato M, Hase T, Elshazley M, Yamashita R, Usami N, Taniguchi T, Yokoi K, Nakamura S, Kondo M, Girard L, Minna JD & Hasegawa Y (2013) TIMELESS is over expressed in lung cancer and its expression correlates with poor patient survival. Cancer Sci 104, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bianco JN, Bergoglio V, Lin YL, Pillaire MJ, Schmitz AL, Gilhodes J, Lusque A, Mazières J, Lacroix‐Triki M, Roumeliotis TI et al. (2019) Overexpression of Claspin and Timeless protects cancer cells from replication stress in a checkpoint‐independent manner. Nat Commun 10, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Rageul J, Park JJ, Zeng PP, Lee EA, Yang J, Hwang S, Lo N, Weinheimer AS, Schärer OD, Yeo JE et al. (2020) SDE2 integrates into the TIMELESS‐TIPIN complex to protect stalled replication forks. Nat Commun 11, 5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pan A, Schernhammer ES, Sun Q & Hu FB (2011) Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Medicine 8, e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kinouchi K & Sassone‐Corsi P (2020) Metabolic rivalry: circadian homeostasis and tumorigenesis. Nat Rev Cancer 20, 645–661. [DOI] [PubMed] [Google Scholar]
- 165. Engelen E, Janssens RC, Yagita K, Smits VA, van der Horst GTJ & Tamanini F (2013) Mammalian TIMELESS is involved in period determination and DNA damage‐dependent phase advancing of the circadian clock. PLoS One 8, e56623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pregueiro AM, Liu Q, Baker CL, Dunlap JC & Loros JJ (2006) The Neurospora checkpoint kinase 2: a regulatory link between the circadian and cell cycles. Science 313, 644–649. [DOI] [PubMed] [Google Scholar]
- 167. Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R & van der Horst GT (2008) Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol 18, 286–291. [DOI] [PubMed] [Google Scholar]
- 168. Papp SJ, Huber AL, Jordan SD, Kriebs A, Nguyen M, Moresco JJ, Yates JR & Lamia KA (2015) DNA damage shifts circadian clock time via Hausp‐dependent Cry1 stabilization. eLife 4, e04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F & Okamura H (2003) Control mechanism of the circadian clock for the timing of cell division in vivo. Science 302, 255–259. [DOI] [PubMed] [Google Scholar]
- 170. Yang Q, Pando BF, Dong G, Golden SS & van Oudenaarden A (2010) Circadian gating of the cell cycle revealed in single cyanobacteria cells. Science 327, 1522–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A et al. (2012) Brain and muscle Arnt‐like protein‐1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB‐induced DNA damage in the epidermis. Proc Natl Acad Sci USA 109, 11758–11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bouchard‐Cannon P, Mendoza‐Viveros L, Yuen A, Kærn M & Cheng HY (2013) The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell‐cycle entry and exit. Cell Rep 5, 961–973. [DOI] [PubMed] [Google Scholar]
- 173. Karpowicz P, Zhang Y, Hogenesch JB, Emery P & Perrimon N (2013) The circadian clock gates the intestinal stem cell regenerative state. Cell Rep 3, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hong CI, Zámborszky J, Baek M, Labiscsak L, Ju K, Lee H, Larrondo LF, Gotik A, Chong HS, Belden WJ et al. (2014) Circadian rhythms synchronize mitosis in Neurospora crassa . Proc Natl Acad Sci USA 111, 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Miyagishima SY, Fuji T, Sumiya N, Hirooka S, Nakano A, Kabeya Y & Nakamura M (2014) Translation‐independent circadian control of the cell cycle in a unicellular photosynthetic eukaryote. Nat Commun 5, 3807. [DOI] [PubMed] [Google Scholar]
- 176. Matsu‐ura T, Dovzhenok A, Aihara E, Rood J, Le H, Ren Y, Rosselot AE, Zhang T, Lee C, Obrietan K et al. (2016) Intercellular coupling of the cell cycle and circadian clock in adult stem cell culture. Mol Cell 64, 900–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Fung‐Uceda J, Lee K, Seo PJ, Polen S, De Veylder L & Mas P (2018) The circadian clock sets the time of DNA replication licensing to regulate growth in Arabidopsis . Dev Cell 45, 101–113.e4. [DOI] [PubMed] [Google Scholar]
- 178. Liao Y & Rust MJ (2021) The circadian clock ensures successful DNA replication in cyanobacteria. Proc Natl Acad Sci USA 118, e2022516118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gamsby JJ, Loros JJ & Dunlap JC (2009) A phylogenetically conserved DNA damage response resets the circadian clock. J Biol Rhythms 24, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Liu X, Dang Y, Matsu‐Ura T, He Y, He Q, Hong CI & Liu Y (2017) DNA replication is required for circadian clock function by regulating rhythmic nucleosome composition. Mol Cell 67, 203–213.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dakup P & Gaddameedhi S (2017) Impact of the circadian clock on UV‐induced DNA damage response and photocarcinogenesis. Photochem Photobiol 93, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Shostak A (2017) Circadian clock, cell division, and cancer: from molecules to organism. Int J Mol Sci 18, 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gaucher J, Montellier E & Sassone‐Corsi P (2018) Molecular cogs: interplay between circadian clock and cell cycle. Trends Cell Biol 28, 368–379. [DOI] [PubMed] [Google Scholar]
- 184. Ruby CL, Major RJ & Hinrichsen RD (2021) Regulation of tissue regeneration by the circadian clock. Eur J Neurosci 53, 3576–3597. [DOI] [PubMed] [Google Scholar]
- 185. Lubov JE, Cvammen W & Kemp MG (2021) The impact of the circadian clock on skin physiology and cancer development. Int J Mol Sci 22, 6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sancar A & Van Gelder RN (2021) Clocks, cancer, and chronochemotherapy. Science 371. 10.1126/science.abb0738 [DOI] [PubMed] [Google Scholar]
- 187. Lam VL, Li YH, Liu X, Murphy KA, Diehl JS, Kwok RS & Chiu JC (2018) CK1α collaborates with DOUBLETIME to regulate PERIOD function in the Drosophila circadian clock. J Neurosci 38, 10631–10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ousley A, Zafarullah K, Chen Y, Emerson M, Hickman L & Sehgal A (1998) Conserved regions of the timeless (tim) clock gene in Drosophila analyzed through phylogenetic and functional studies. Genetics 148, 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Blackford AN & Jackson SP (2017) ATM, ATR, and DNA‐PK, the trinity at the heart of the DNA damage response. Mol Cell 66, 801–817. [DOI] [PubMed] [Google Scholar]


