Abstract
Relatively unstable cyclic imines, generated in situ from their corresponding alicyclic amines via oxidation of their lithium amides with simple ketone oxidants, engage aryl lithium compounds containing a leaving group on an ortho-methylene functionality to provide polycyclic isoindolines in a single operation. The scope of this transformation includes pyrrolidine, piperidine, azepane, azocane, and piperazines.
Graphical Abstract

Given the prevalence of saturated azacycles as core structures of numerous bioactive materials,1 the development of methods for the synthesis of more complex amines via the C–H bond functionalization of their parent heterocycles continues to inspire the development of diverse synthetic strategies.2,3 Despite the tremendous progress that has been achieved in this area, there is a lack of available methods that enable direct amine annulation via concurrent N–H and α-C–H bond functionalization as an attractive entry to polycyclic amines (Scheme 1). While redox-annulations (Scheme 1a) are highly appealing in this regard, these transformations remain limited to aldehydes containing activated pronucleophiles.4 For instance, direct access to polycyclic isoindolines via redox-annulation of alicyclic amines and aryl aldehydes would be highly desirable (Scheme 1b), given the privileged nature of such compounds in drug discovery and the need for multiple-step sequences when accessing these materials via traditional approaches.5,6 However, due to other, more facile reaction pathways, this transformation has remained elusive.7 Here we report a new approach to the synthesis of polycyclic isoindolines, proceeding in a single operation from commercial materials.
Scheme 1.
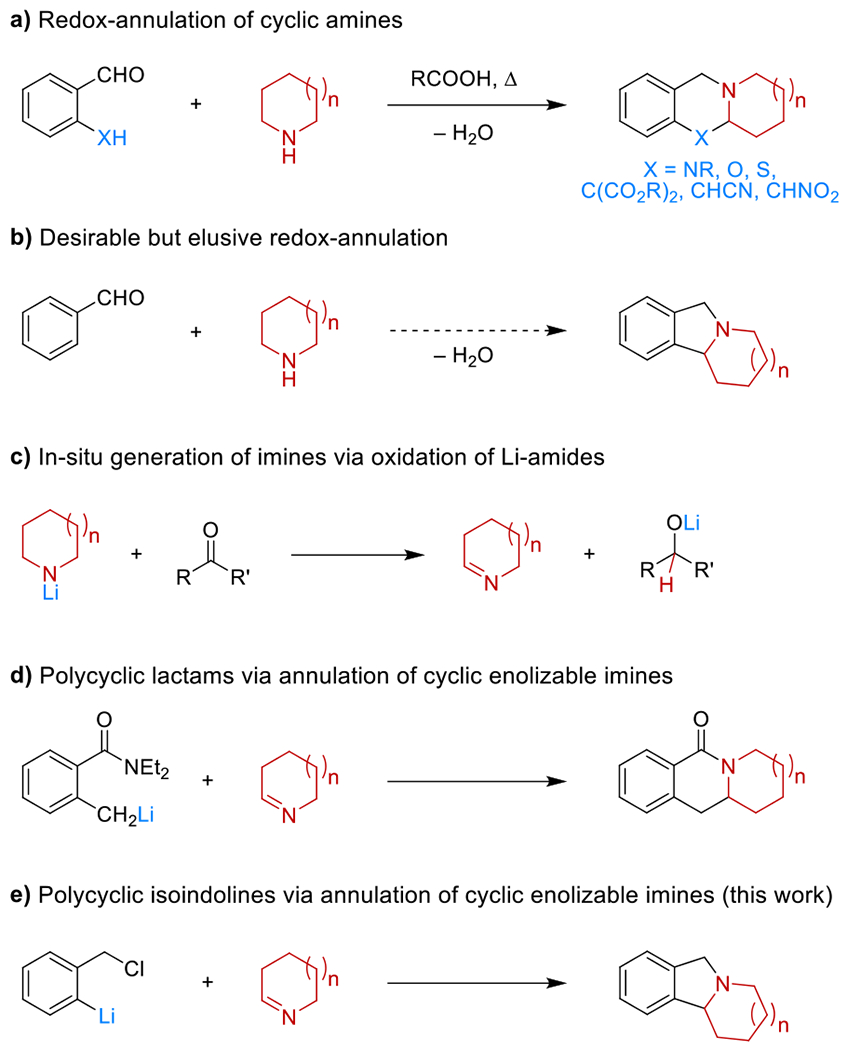
Overview of methods for amine α-C–H/N–H annulation
Capitalizing on the well-documented ability of lithium amides to serve as reductants,8 we recently developed a strategy to access relatively unstable enolizable cyclic imines in situ from lithium amides and simple ketone oxidants (Scheme 1c).9 This approach enabled the synthesis of various α-functionalized amines.9 In addition, we could show that transient imines undergo annulation with lithiated o-toluamides to form polycyclic lactams (Scheme 1d).9f We reasoned that isoindolines may be accessible via the process outlined in Scheme 1e, utilizing an aryl lithium nucleophile containing a leaving group on an o-methylene functionality. This nucleophile was envisioned to be accessible via lithium–halogen exchange. This idea was evaluated with 1-bromo-2-(chloromethyl)benzene (1a)10 and 1-ethylpiperazine. Selected experiments are summarized in Table 1. Following significant optimization, the use of a mixed solvent system (ether/THF) in the lithiation of 1a was found to be critical. Under the optimized conditions, product 2e was obtained in 63% yield. Interestingly, performing the entire reaction sequence in just ether or THF as the only solvent led to dramatically reduced yields.
Table 1.
Reaction development.a

| ||
|---|---|---|
| entry | deviation from optimized conditions | yield (%) |
|
| ||
| 1 | none | 63 |
| 2b | reaction performed at rt for 3 h | 41 |
| 3c | reaction performed at −78 °C for 3 h | 61 |
| 4d | amine as the limiting reagent | 33 |
| 5e | 1 equiv of LiCl used as additive | 14 |
| 6 | entire reaction performed in ether | 23 |
| 7 | entire reaction performed in THF | 16 |
Reactions were performed with 0.5 mmol of 1a. Yields correspond to isolated yields of chromatographically purified product. A mixture of ether (1 mL) and THF (1 mL) was used for the lithiation of 1a. Cyclic imine was prepared in situ by adding n-BuLi (2 equiv) to a solution of 1-ethylpiperazine (2 equiv) in ether (1 mL) at −78 °C, followed by the addition of trifluoroacetophenone (2.05 equiv).
Reaction immediately warmed up to rt after the addition of the in-situ generated imine.
Reaction was carried out at −78 °C throughout without warming to rt.
1.5 equiv of the 2-bromobenzyl chloride used with respect to the in-situ generated imine.
Lithium chloride was added as a 0.5 M THF solution before the Li-halogen exchange reaction.
To evaluate the scope of this transformation, a series of readily available o-chloromethyl-arylbromides were subjected to Li–Br exchange with n-BuLi, followed by addition of cyclic imines generated in situ as described previously (Scheme 2). A range of polycyclic isoindolines 2 were obtained in acceptable to good yields. Substituents on the aryl ring were tolerated in any position. Amines with different ring sizes were readily accommodated. 4-Substituted piperidines and bicyclic amines underwent annulation to furnish products with high levels of diastereoselectivity (products 2h–j). Precedent for this type of reaction is extremely limited and all prior examples involve the use of stable, non-enolizable acyclic imines.11
Scheme 2. Scope of the reaction.
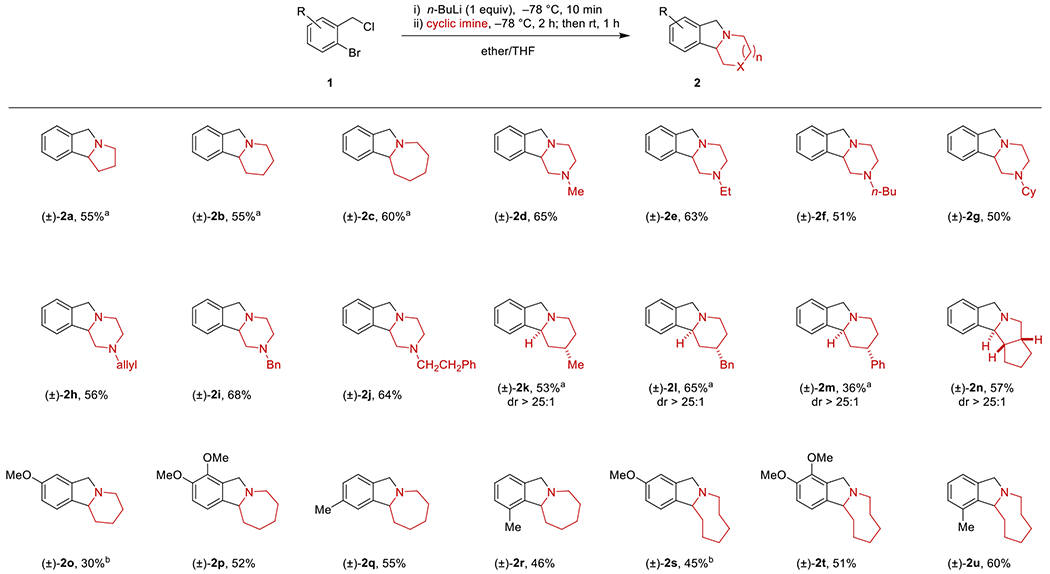
Reactions were performed with 0.5 mmol of 1. Yields correspond to isolated yields of chromatographically purified product. A mixture of ether (1 mL) and THF (1 mL) was used for the lithiation of 1. Cyclic imines were prepared in situ by adding n-BuLi (2 equiv) to a solution of the corresponding cyclic amine (2 equiv) in ether (1 mL) at −78 °C, followed by the addition of trifluoroacetophenone (2.05 equiv). a A mixture of ether (1.8 mL) and THF (0.2 mL) was used for the lithiation of 1. b THF (1 mL) was used for the lithiation and cyclic imine was prepared in situ by adding n-BuLi (2 equiv) to a solution of cyclic amine (2 equiv) in ether (1.5 mL) at −78 °C, followed by the addition of trifluoroacetophenone (2.05 equiv) in 1 mL of ether.
In conclusion, we have achieved annulation reactions of o-chloromethyl-aryllithiums with enolizable cyclic imines, species that were prepared in situ via the oxidation of the corresponding lithiated amines with a ketone oxidant. This methodology allows for the facile construction of various polycyclic isoindolines in a single operation, dramatically simplifying access to these materials.
Supplementary Material
ACKNOWLEDGMENT
Financial support from the NIH–NIGMS (grant no. R01GM101389) is gratefully acknowledged. Mass spectrometry instrumentation was supported by a grant from the NIH (S10 OD021758-01A1).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details, characterization data, X-ray data, and copies of NMR spectra (PDF).
REFERENCES
- (1).(a) Taylor RD; MacCoss M; Lawson ADG, Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859 [DOI] [PubMed] [Google Scholar]; (b) Vitaku E; Smith DT; Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- (2).For a general overview of amine C–H bond functionalization, see; (a) Dutta S; Li B; Rickertsen DRL; Valles DA; Seidel D, C–H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods. SynOpen 2021, 05, 173–228. [DOI] [PMC free article] [PubMed] [Google Scholar]; Other selected reviews; (b) Campos KR, Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev 2007, 36, 1069–1084 [DOI] [PubMed] [Google Scholar]; (c) Jazzar R; Hitce J; Renaudat A; Sofack-Kreutzer J; Baudoin O, Functionalization of Organic Molecules by Transition-Metal-Catalyzed C(sp3)-H Activation. Chem. Eur. J 2010, 16, 2654–2672 [DOI] [PubMed] [Google Scholar]; (d) Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW, Direct alpha-Functionalization of Saturated Cyclic Amines. Chem. Eur. J 2012, 18, 10092–10142 [DOI] [PubMed] [Google Scholar]; (e) Peng B; Maulide N, The Redox-Neutral Approach to C-H Functionalization. Chem. Eur. J 2013, 19, 13274–13287 [DOI] [PubMed] [Google Scholar]; (f) Girard SA; Knauber T; Li C-J, The Cross-Dehydrogenative Coupling of C sp3-H Bonds: A Versatile Strategy for C-C Bond Formations. Angew. Chem. Int. Ed 2014, 53, 74–100 [DOI] [PubMed] [Google Scholar]; (g) Haibach MC; Seidel D, C-H Bond Functionalization through Intramolecular Hydride Transfer. Angew. Chem. Int. Ed 2014, 53, 5010–5036 [DOI] [PubMed] [Google Scholar]; (h) Beatty JW; Stephenson CRJ, Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res 2015, 48, 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Cheng M-X; Yang S-D, Recent Advances in the Enantioselective Oxidative α-C–H Functionalization of Amines. Synlett 2017, 28, 159–174 [Google Scholar]; (j) Chu JCK; Rovis T, Complementary Strategies for Directed C(sp3)−H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed 2018, 57, 62–101 [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Stateman LM; Nakafuku KM; Nagib DA, Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Edwards PM; Schafer LL, Early transition metal-catalyzed C–H alkylation: hydroaminoalkylation for Csp3–Csp3 bond formation in the synthesis of selectively substituted amines. Chem. Commun 2018, 54, 12543–12560 [DOI] [PubMed] [Google Scholar]; (m) Gonnard L; Guérinot A; Cossy J, Transition metal-catalyzed α-alkylation of amines by C(sp3)‒H bond activation. Tetrahedron 2019, 75, 145–163 [Google Scholar]; (n) Liu S; Zhao Z; Wang Y, Construction of N-Heterocycles through Cyclization of Tertiary Amines. Chem. Eur. J 2019, 25, 2423–2441 [DOI] [PubMed] [Google Scholar]; (o) Antermite D; Bull JA, Transition Metal-Catalyzed Directed C(sp3)–H Functionalization of Saturated Heterocycles. Synthesis 2019, 51, 3171–3204 [Google Scholar]; (p) Zhang T; Wu Y-H; Wang N-X; Xing Y, Advances in C(sp3)–H Bond Functionalization via Radical Processes. Synthesis 2019, 51, 4531–4548 [Google Scholar]; (q) Trowbridge A; Walton SM; Gaunt MJ, New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev 2020, 120, 2613–2692 [DOI] [PubMed] [Google Scholar]; (r) Kapoor M; Singh A; Sharma K; Hua Hsu M, Site-Selective C(sp3)−H and C(sp2)−H Functionalization of Amines Using a Directing-Group-Guided Strategy. Adv. Synth. Catal 2020, 362, 4513–4542 [Google Scholar]; (s) An X-D; Xiao J, Recent advances in hydride transfer-involved C(sp3)–H activation reactions. Org. Chem. Front 2021, 8, 1364–1383 [Google Scholar]; (t) Basak S; Winfrey L; Kustiana BA; Melen RL; Morrill LC; Pulis AP, Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes. Chem. Soc. Rev 2021, 50, 3720–3737 [DOI] [PubMed] [Google Scholar]; (u) Caplin MJ; Foley DJ, Emergent synthetic methods for the modular advancement of sp3-rich fragments. Chem. Sci 2021, 12, 4646–4660; [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Ohno S; Miyoshi M; Murai K; Arisawa M, Non-Directed β- or γ-C(sp3)–H Functionalization of Saturated Nitrogen-Containing Heterocycles. Synthesis 2021, 53, 2947–2960 [Google Scholar]; (w) He Y; Zheng Z; Yang J; Zhang X; Fan X, Recent advances in the functionalization of saturated cyclic amines. Org. Chem. Front 2021, 8, 4582–4606. [Google Scholar]
- (3).Selected recent examples of mechanistically diverse methods for amine C–H bond functionalization; (a) Ohmatsu K; Suzuki R; Furukawa Y; Sato M; Ooi T, Zwitterionic 1,2,3-Triazolium Amidate as a Catalyst for Photoinduced Hydrogen-Atom Transfer Radical Alkylation. ACS Catal 2020, 10, 2627–2632 [Google Scholar]; (b) Roque JB; Kuroda Y; Jurczyk J; Xu L-P; Ham JS; Göttemann LT; Roberts CA; Adpressa D; Saurí J; Joyce LA; Musaev DG; Yeung CS; Sarpong R, C–C Cleavage Approach to C–H Functionalization of Saturated Aza-Cycles. ACS Catal. 2020, 10, 2929–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rand AW; Yin H; Xu L; Giacoboni J; Martin-Montero R; Romano C; Montgomery J; Martin R, Dual Catalytic Platform for Enabling sp3 α C–H Arylation and Alkylation of Benzamides. ACS Catal. 2020, 10, 4671–4676 [Google Scholar]; (d) Liu W; Babl T; Röther A; Reiser O; Davies HML, Functionalization of Piperidine Derivatives for the Site-Selective and Stereoselective Synthesis of Positional Analogues of Methylphenidate. Chem. Eur. J 2020, 26, 4236–4241 [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Verma P; Richter JM; Chekshin N; Qiao JX; Yu J-Q, Iridium(I)-Catalyzed α-C(sp3)–H Alkylation of Saturated Azacycles. J. Am. Chem. Soc 2020, 142, 5117–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Walker MM; Koronkiewicz B; Chen S; Houk KN; Mayer JM; Ellman JA, Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C–H Arylation and Epimerization. J. Am. Chem. Soc 2020, 142, 8194–8202 [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen L; Yang Y; Liu L; Gao Q; Xu S, Iridium-Catalyzed Enantioselective α-C(sp3)–H Borylation of Azacycles. J. Am. Chem. Soc 2020, 142, 12062–12068 [DOI] [PubMed] [Google Scholar]; (h) Xu L-P; Roque JB; Sarpong R; Musaev DG, Reactivity and Selectivity Controlling Factors in the Pd/Dialkylbiarylphosphine-Catalyzed C–C Cleavage/Cross-Coupling of an N-Fused Bicyclo α-Hydroxy-β-Lactam. J. Am. Chem. Soc 2020, 142, 21140–21152 [DOI] [PubMed] [Google Scholar]; (i) Feng K; Quevedo RE; Kohrt JT; Oderinde MS; Reilly U; White MC, Late-stage oxidative C(sp3)–H methylation. Nature 2020, 580, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Sarver PJ; Bacauanu V; Schultz DM; DiRocco DA; Lam Y.-h.; Sherer EC; MacMillan DWC, The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem 2020, 12, 459–467 [DOI] [PubMed] [Google Scholar]; (k) McManus JB; Onuska NPR; Jeffreys MS; Goodwin NC; Nicewicz DA, Site-Selective C–H Alkylation of Piperazine Substrates via Organic Photoredox Catalysis. Org. Lett 2020, 22, 679–683 [DOI] [PubMed] [Google Scholar]; (l) Cao L; Zhao H; Tan Z; Guan R; Jiang H; Zhang M, Ruthenium-Catalyzed Hydrogen Evolution o-Aminoalkylation of Phenols with Cyclic Amines. Org. Lett 2020, 22, 4781–4785 [DOI] [PubMed] [Google Scholar]; (m) Chen Y; Wan H-L; Huang Y; Liu S; Wang F; Lu C; Nie J; Chen Z; Yang G; Ma C, B(C6F5)3-Catalyzed β-Functionalization of Pyrrolidines Using Isatins via Borrowing Hydrogen: Divergent Access to Substituted Pyrrolidines and Pyrroles. Org. Lett 2020, 22, 7797–7803 [DOI] [PubMed] [Google Scholar]; (n) Oeschger R; Su B; Yu I; Ehinger C; Romero E; He S; Hartwig J, Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 2020, 368, 736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Short MA; Blackburn JM; Roizen JL, Modifying Positional Selectivity in C–H Functionalization Reactions with Nitrogen-Centered Radicals: Generalizable Approaches to 1,6-Hydrogen-Atom Transfer Processes. Synlett 2020, 31, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Kim Y; Heo J; Kim D; Chang S; Seo S, Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer. Nat. Commun 2020, 11, 4761. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Trindade AF; Faulkner EL; Leach AG; Nelson A; Marsden SP, Fragment-oriented synthesis: β-elaboration of cyclic amine fragments using enecarbamates as platform intermediates. Chem. Commun 2020, 56, 8802–8805 [DOI] [PubMed] [Google Scholar]; (r) Holmberg-Douglas N; Choi Y; Aquila B; Huynh H; Nicewicz DA, β-Functionalization of Saturated Aza-Heterocycles Enabled by Organic Photoredox Catalysis. ACS Catal. 2021, 11, 3153–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Yi M-J; Zhang H-X; Xiao T-F; Zhang J-H; Feng Z-T; Wei L-P; Xu G-Q; Xu P-F, Photoinduced Metal-Free α-C(sp3)–H Carbamoylation of Saturated Aza-Heterocycles via Rationally Designed Organic Photocatalyst. ACS Catal. 2021, 11, 3466–3472 [Google Scholar]; (t) Aguilera EY; Sanford MS, Palladium-Mediated Cγ−H Functionalization of Alicyclic Amines. Angew. Chem. Int. Ed 2021, 60, 11227–11230 [DOI] [PMC free article] [PubMed] [Google Scholar]; (u) Chang Y; Cao M; Chan JZ; Zhao C; Wang Y; Yang R; Wasa M, Enantioselective Synthesis of N-Alkylamines through β-Amino C–H Functionalization Promoted by Cooperative Actions of B(C6F5)3 and a Chiral Lewis Acid Co-Catalyst. J. Am. Chem. Soc 2021, 143, 2441–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Yue W-J; Day CS; Martin R, Site-Selective Defluorinative sp3 C–H Alkylation of Secondary Amides. J. Am. Chem. Soc 2021, 143, 6395–6400 [DOI] [PubMed] [Google Scholar]; (w) Koperniku A; Schafer LL, Zirconium Catalyzed Hydroaminoalkylation for the Synthesis of α-Arylated Amines and N-Heterocycles. Chem. Eur. J 2021, 27, 6334–6339. [DOI] [PubMed] [Google Scholar]
- (4).For a comprehensive review, see; (a) Chen W; Seidel D, Condensation-Based Methods for the C–H Bond Functionalization of Amines. Synthesis 2021, 53, 3869–3908. Selected examples of redox-annulations [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang C; De CK; Mal R; Seidel D, alpha-Amination of Nitrogen Heterocycles: Ring-Fused Aminals. J. Am. Chem. Soc 2008, 130, 416–417 [DOI] [PubMed] [Google Scholar]; (c) Zheng L; Yang F; Dang Q; Bai X, A Cascade Reaction with Iminium Ion Isomerization as the Key Step Leading to Tetrahydropyrimido[4,5-d]pyrimidines. Org. Lett 2008, 10, 889–892 [DOI] [PubMed] [Google Scholar]; (d) Zhang C; Das D; Seidel D, Azomethine ylide annulations: facile access to polycyclic ring systems. Chem. Sci 2011, 2, 233–236 [Google Scholar]; (e) Dieckmann A; Richers MT; Platonova AY; Zhang C; Seidel D; Houk KN, Metal-Free α-Amination of Secondary Amines: Computational and Experimental Evidence for Azaquinone Methide and Azomethine Ylide Intermediates. J. Org. Chem 2013, 78, 4132–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Richers MT; Breugst M; Platonova AY; Ullrich A; Dieckmann A; Houk KN; Seidel D, Redox-Neutral α-Oxygenation of Amines: Reaction Development and Elucidation of the Mechanism. J. Am. Chem. Soc 2014, 136, 6123–6135 [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Kang Y; Chen W; Breugst M; Seidel D, Asymmetric Redox-Annulation of Cyclic Amines. J. Org. Chem 2015, 80, 9628–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Chen W; Seidel D, Redox-Annulation of Cyclic Amines and β-Ketoaldehydes. Org. Lett 2016, 18, 1024–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Li J; Fu Y; Qin C; Yu Y; Li H; Wang W, Asymmetric synthesis of isoquinolinonaphthyridines catalyzed by a chiral Bronsted acid. Org. Biomol. Chem 2017, 15, 6474–6477 [DOI] [PubMed] [Google Scholar]; (j) Liu Y; Wu J; Jin Z; Jiang H, Synthesis of 1,2-Fused Bicyclic Imidazolidin-4-ones by Redox-Neutral Cyclization Reaction of Cyclic Amines and α-Ketoamides. Synlett 2018, 29, 1061–1064 [Google Scholar]; (k) Paul A; Chandak HS; Ma L; Seidel D, Redox-Annulations of Cyclic Amines with ortho-Cyanomethylbenzaldehydes. Org. Lett 2020, 22, 976–980 [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Rickertsen DRL; Ma L; Paul A; Abboud KA; Seidel D, Traceless Redox-Annulations of Alicyclic Amines. SynOpen 2020, 04, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Speck K; Magauer T, The chemistry of isoindole natural products. Beilstein J. Org. Chem 2013, 9, 2048–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Selected examples for the synthesis of polycyclic isoindolines; (a) Meyers AI; Santiago B, C2 symmetric amines. III. An asymmetric synthesis of (S,S)-1,3-dialkyl lsoindolines by sequential formamidine alkylation. Tetrahedron Lett. 1995, 36, 5877–5880 [Google Scholar]; (b) Alsarabi AE; Canet J-L; Troin Y, Short diastereoselective synthesis of cis- and trans-hexahydropyrido[2,1-a]isoindole derivatives. Tetrahedron Lett. 2004, 45, 9003–9006 [Google Scholar]; (c) Ben-Othman R; Othman M; Ciamala K; Knorr M; Strohmann C; Decroix B, Synthesis of diversely functionalized pyrrolizidines and indolizidines using olefin ring-closing metathesis. Tetrahedron 2009, 65, 4846–4854 [Google Scholar]; (d) Zang Q; Javed S; Porubsky P; Ullah F; Neuenswander B; Lushington GH; Basha FZ; Organ MG; Hanson PR, Synthesis of a Unique Isoindoline/Tetrahydroisoquinoline-based Tricyclic Sultam Library Utilizing a Heck-aza-Michael Strategy. ACS Comb. Sci 2012, 14, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ali IAI, Synthesis of 3-substituted N-allylisoindolinone derivatives by the acetate method. Monatsh. Chem 2014, 145, 803–810 [Google Scholar]; (f) Xie Y-Y; Wang Y-C; He Y; Hu D-C; Wang H-S; Pan Y-M, Catalyst-free synthesis of fused 1,2,3-triazole and isoindoline derivatives via an intramolecular azide–alkene cascade reaction. Green Chem. 2017, 19, 656–659. [Google Scholar]
- (7).For a detailed discussion, see:; Ma L; Paul A; Breugst M; Seidel D, Redox-Neutral Aromatization of Cyclic Amines: Mechanistic Insights and Harnessing of Reactive Intermediates for Amine α- and β-C−H Functionalization. Chem. Eur. J 2016, 22, 18179–18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For an early review on the ability of lithium amides to serve as reductants, see; (a) Reduction with lithium dialkylamides. Majewski M; Gleave DM J. Organomet. Chem 1994, 470, 1–16. Selected key contributions [Google Scholar]; (b) Über Lithium-diäthylamid als Hydrid-Donator. Wittig G; Schmidt HJ; Renner H Chem. Ber 1962, 95, 2377–2383 [Google Scholar]; (c) Zur Reaktionsweise N-metallierter acyclischer und cyclischer sekundärer Amine. Wittig G; Hesse A Liebigs Ann. Chem 1971, 746, 149–173 [Google Scholar]; (d) Hydrid-Übertragung von Lithium-pyrrolidid auf Azomethine. Wittig G; Hesse A Liebigs Ann. Chem 1971, 746, 174–184 [Google Scholar]; (e) Über die Reaktivität von metallierten Aminen als Hydrid-Donatoren. Wittig G; Häusler G Liebigs Ann. Chem 1971, 746, 185–199. [Google Scholar]
- (9).(a) Chen W; Ma L; Paul A; Seidel D, Direct α-C–H bond functionalization of unprotected cyclic amines. Nat. Chem 2018, 10, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Paul A; Seidel D, α-Functionalization of Cyclic Secondary Amines: Lewis Acid Promoted Addition of Organometallics to Transient Imines. J. Am. Chem. Soc 2019, 141, 8778–8782 [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen W; Paul A; Abboud KA; Seidel D, Rapid functionalization of multiple C–H bonds in unprotected alicyclic amines. Nat. Chem 2020, 12, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Paul A; Kim JH; Daniel SD; Seidel D, Diversification of Unprotected Alicyclic Amines by C−H Bond Functionalization: Decarboxylative Alkylation of Transient Imines. Angew. Chem. Int. Ed 2021, 60, 1625–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kim JH; Paul A; Ghiviriga I; Seidel D, α-C–H Bond Functionalization of Unprotected Alicyclic Amines: Lewis-Acid-Promoted Addition of Enolates to Transient Imines. Org. Lett 2021, 23, 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen W; Seidel D, α-C–H/N–H Annulation of Alicyclic Amines via Transient Imines: Preparation of Polycyclic Lactams. Org. Lett 2021, 23, 3729–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Valles DA; Dutta S; Paul A; Abboud KA; Ghiviriga I; Seidel D, α,α′-C–H Bond Difunctionalization of Unprotected Alicyclic Amines. Org. Lett 2021, 23, 6367–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Parham WE; Jones LD; Sayed YA, Selective halogen-lithium exchange in bromophenylalkyl halides. J. Org. Chem 1976, 41, 1184–1186. [Google Scholar]
- (11).Bradsher CK; Hunt DA, Schiff bases as external and internal electrophiles in reactions of functionalized organolithium reagents. A new route to isoindoline derivatives and 1,2,3,4-tetrahydroisoquinolines. J. Org. Chem 1981, 46, 327–330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


