Highlights
-
•
Terpenes and terpenoids are the main bioactive compounds of essential oils (EOs).
-
•
EOs and their major constituents confer several biological activities.
-
•
EOs are potential as natural food preservatives.
Keywords: Essential oil, Anticancer, Antimicrobial, Anti-inflammatory, Food preservatives, Terpenes
Chemical compounds studied in this article: Carvacrol (PubChem CID: 10364), Linalool (PubChem CID: 6549), Hinokitiol (PubChem CID: 3611), Bakuchiol (PubChem CID: 5468522), Limonene (PubChem CID: 22311), Eugenol (PubChem CID: 3314), Myrcene (PubChem CID: 31253), α-Terpineol (PubChem CID: 17100), Atractylone (PubChem CID: 3080635), Carvone (PubChem CID: 7439)
Abstract
Essential oils (EOs) are volatile and concentrated liquids extracted from different parts of plants. Bioactive compounds found in EOs, especially terpenes and terpenoids possess a wide range of biological activities including anticancer, antimicrobial, anti-inflammatory, antioxidant, and antiallergic. Available literature confirms that EOs exhibit antimicrobial and food preservative properties that are considered as a real potential application in food industry. Hence, the purpose of this review is to present an overview of current knowledge of EOs for application in pharmaceutical and medical industries as well as their potential as food preservatives in food industry.
Introduction
The notion to promote the use of natural products in daily life have spread worldwide in the last several decades. Of all, essential oils (EOs) have been one of the most utilized natural products (Carpena, Nuñez-Estevez, Soria-Lopez, Garcia-Oliveira, & Prieto, 2021). Essential oils are highly concentrated hydrophobic liquid derived from a variety of plants and defined based on their chemical and physical properties. The pharmacological effect of EOs have been extensively examined: ranging from antimicrobial (Burt, 2004, Falleh et al., 2020), antihelminthic (Inouye, Takizawa, & Yamaguchi, 2001), antiviral (Silva, Figueiredo, Byler, & Setzer, 2020), antiulcer (Dordević et al., 2007), antioxidant (Falleh et al., 2020, Mimica-Dukić et al., 2003), anti-inflammatory (Silva et al., 2015), insecticide (Isman & Machial, 2006), larvacidal (Jantan, Ping, Visuvalingam, & Ahmad, 2003), immunomodulatory (Mediratta, Sharma, & Singh, 2002), and antinociceptive properties (Abdollahi, Karimpour, & Monsef-Esfehani, 2003).
Essential oils have long been used as flavorings in the food industry (Pandey, Kumar, Singh, Tripathi, & Bajpai, 2017). Of thousands different EOs known at present, around 300 are commercially marketed in the flavor and fragrances products (Hyldgaard, Mygind, & Meyer, 2012). In addition to their aromatic qualities, the antimicrobial properties of EOs against a wide range of microorganisms have also provided convincing evidence that EOs are suitable candidates to be used as natural food preservatives (Falleh et al., 2020). Among all chemical components of EOs, terpenes and terpenoids have been comprehensively studied and reported to play key roles in humans health (Perveen, 2018).
Terpenes (pinene, myrcene, limonene, terpinene, p-cymene) are characterized as compounds with simple hydrocarbons structures while terpenoids (oxygen-containing hydrocarbons) are defined as modified class of terpenes with different functional groups and oxidized methyl groups moved or removed at various positions (Perveen, 2018). Terpenes have been reported to exert antimicrobial activities against both the antibiotic-susceptible and antibiotic-resistant bacteria, mainly via their abilities to promote cell rupture and inhibition of protein and DNA synthesis (Álvarez-Martínez, Barrajón-Catalán, Herranz-López, & Micol, 2021). Carvacrol, carvone, eugenol, geraniol, and thymol were among the terpenes that demonstrated antibacterial action against Staphylococcus aureus (Gallucci et al., 2009). In addition, terpenoids have been shown as one of secondary metabolites produced by aromatic and medicinal plants that played a key role in disease resistance. For example, monoterpenoids are antibacterial in nature, causing disruption in microbe multiplication and development, as well as interfering with their physiological and metabolic activities (Burt, 2004). Some botanical compounds, such as azadirachtin, carvone, menthol, ascaridol, methyl eugenol, toosendanin, and volkensin, have been shown to yield antimicrobial and antifungal properties, as well as insect pest repellent properties (Isman and Machial, 2006, Pandey et al., 2016, Pandey et al., 2017).
In addition to their various pharmacological effects in humans, the antimicrobial capabilities of EOs’ terpenes and terpenoids against foodborne microbes and their beneficial use in food as flavoring additives shall serve as excellent alternatives to the standard bactericides and fungicides currently used in the food industry (Perricone, Arace, Corbo, Sinigaglia, & Bevilacqua, 2015). This review will thus provide an overview to the current knowledge about the potential role of terpenes and terpenoids, as main bioactive compound of EOs, in human health and their industrial potential as natural food preservatives (Fig. 1). This, in turn, shall provide prompt opportunities to cultivate more and better ideas and research avenues that can promote extensive pharmaceutical applications of EOs’ constituents in the daily life.
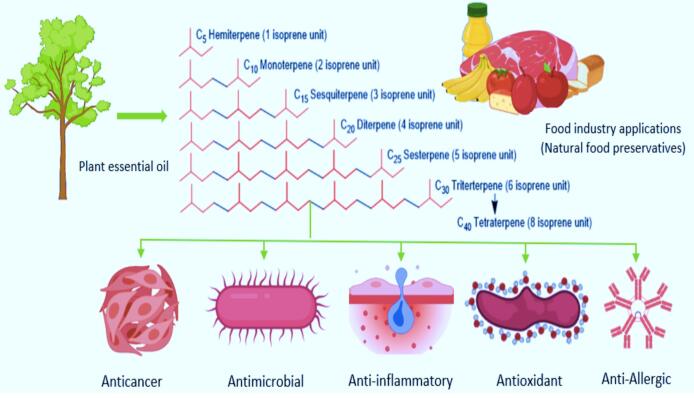
Fig. 1.
The potential benefits of terpenes and terpenoids found in EOs.
Bioactive compounds of essential oils
Essential oils are concentrated liquids of complex mixtures of aromatic hydrophobic oily volatile compounds and can be extracted from different parts of plants such as bark, buds, flowers, fruits, leaves, peels, roots, seeds, twigs, or whole plant from a single botanical source (Falleh et al., 2020, Stephane and Jules, 2020). International Organization for Standardization (ISO) defines that EOs are products obtained from raw material of vegetables by pressing or distillation processes (Stevanovic, Sieniawska, Glowniak, Obradovic, & Pajic-Lijakovic, 2020).
Essential oils possess a strong odor and usually colorless especially when fresh, but some exceptions are known such as pale yellow (yellow mandarin), blue (chamomile), orange (sweet orange), and green (bergamot). EOs may be easily oxidizable with age by air, heat, or light exposure which proceed to the dark color. Hence, EOs need to be stored in a cool and dry place (Stephane & Jules, 2020). Structurally, the chemical constituents of EOs can be classified into four groups: terpenes, terpenoids, phenylpropanoids, and other constituents (Hyldgaard et al., 2012, Pandey et al., 2017).
Terpenes
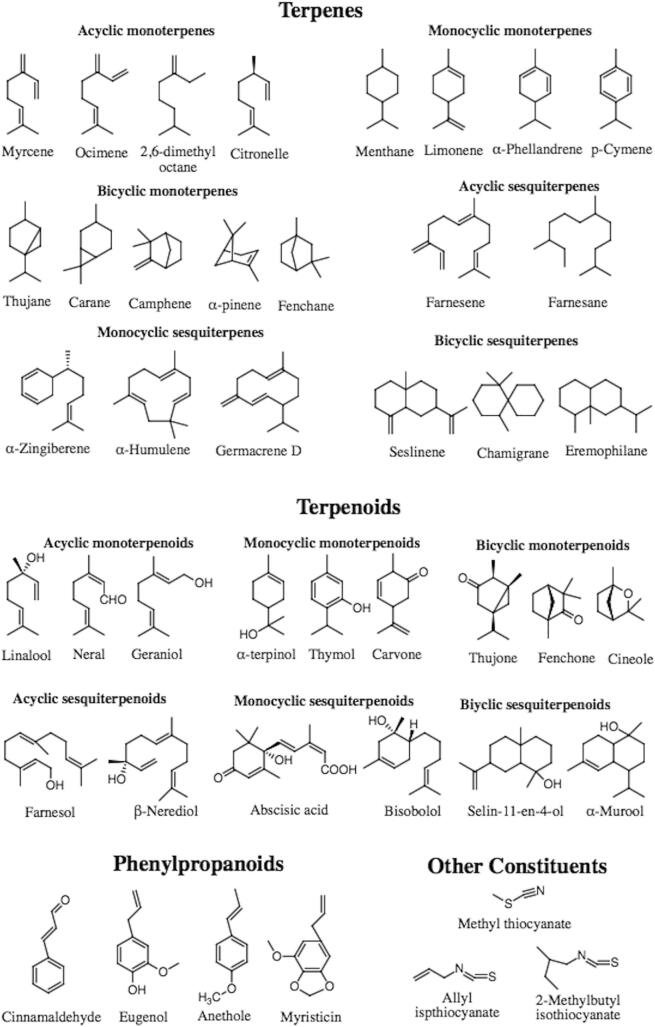
Terpenes or isoprenoids are the major constituents found in EOs with molecular structures containing carbon backbones of 2-methylbuta-1,3-diene (isoprene units) which can be rearranged into cyclic structures (Hyldgaard et al., 2012). The number of isoprene units are primarily responsible for structural diversity of terpenes. Hemiterpenes are formed by one isoprene unit (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetraterpenes (C40) (Bhavaniramya, Vishnupriya, Al-Aboody, Vijayakumar, & Baskaran, 2019). Hemiterpenes are a minor part of terpenes found in EOs. The most outstanding HT, isoprene which is emitted from the herbs and leaves of many trees such as conifers, oaks, poplars, and willows. Examples of hemiterpenes include angelic, tiglic, isovaleric, and senecioic acids. Monoterpenes are the predominant components of EOs (90%), followed by sesquiterpenes (Falleh et al., 2020). Diterpenes, triterpenes, and tetraterpenes with their oxygenated derivatives are also detected in small amounts (Stephane & Jules, 2020). Examples of bioactive compounds of EO are presented in Fig. 2.
Fig. 2.
The chemical structures of bioactive compounds of EOs.
Terpenoids
Terpenoids are another type of terpenes containing oxygen molecules that are constructed via biochemical modifications (removal or addition of methyl groups) (Pandey et al., 2017). Terpenoids can be divided into alcohols, aldehydes, esters, ether, epoxides, ketones, and phenols. Examples of terpenoids are: carvacrol, citronellal, geraniol, linalool, linalyl acetate, piperitone, menthol, and thymol (Hyldgaard et al., 2012). These bioactive compounds confer several biological activities such as anticancer (Potočnjak, Gobin, & Domitrović, 2018), anti-allergic (Kobayashi et al., 2016), antibacterial (Guimarães et al., 2019), and antioxidant (Wang, Chen, & Hou, 2019).
Terpenes and terpenoids are synthesized by the mevalonic acid (MVA) pathway in the cytosol and the 2C-methyl-d-erythritol-4-phosphate (MEP) pathway in the plastid for the formation of precursors: isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Oldfield and Lin, 2012, Stephane and Jules, 2020).
Phenylpropanoids
Phenylpropanoids are synthesized by the shikimic acid pathway and their basic structure from the six-carbon aromatic phenol group linked usually to the three-carbon propene tail of cinnamic acid, oxygenated in the third/fourth/fifth position frequently possess a carbon–carbon double bond (Stevanovic et al., 2020). Examples of phenylpropanoids such as anethole, cinnamaldehyde, eugenol, isoeugenol, myristicin, safrole, and vanillin. In a recent study, anethole has been reported to yield anticancer activity (Contant, Rouabhia, Loubaki, Chandad, & Semlali, 2021). Moreover, myristicin has been described to exert antiproliferative and anti-inflammatory properties (Seneme, Dos Santos, Silva, Franco, & Longato, 2021) and safrole has been reported to promote diverse biological activities, such as antidiabetic, antimicrobial, analgesic, and antifungal activities (Eid & Hawash, 2021).
Other constituents
EOs contain several derivatives of amino acids such as alanine, isoleucine, leucine, valine, and methionine. Polyketides, lipids, and sulfur derivatives are rarely found in EOs such as jasmonic acid, methyl jasmonate, cis-jasmone, (Z)-3-hexenal, allicin (Pandey et al., 2017, Stevanovic et al., 2020).
Green extraction and purification methods of terpenes and terpenoids
Extraction is the most essential stage in extracting and purifying active compounds from natural sources. Natural chemicals can be extracted via maceration, infusion, soxhletation, percolation, or digesting methods (Giacometti et al., 2018). Due to growing energy prices, CO2 emissions, and other associated environmental issues, extraction techniques for the chemical, food, and pharmaceutical sectors have received much interest in recent years. One of the most popular topics in this approach is the development of methods and procedures to achieve maximal extraction at a low cost and in an environmentally friendly. In general, the methods involve the following steps (Fig. S2, Supplementary material): (a) breaking plant cells to release their chemical constituents; (b) extracting the sample using a suitable solvent—or through distillation or compound trapping; (c) separating the desired terpene from undesired contents of extracts that confound analysis and quantification; and (d) analyzing the product using an appropriate method (Asl & Khajenoori, 2021).
In this chapter, the processes in isolation of terpene and terpenoid chemicals in plants and the technique of determining structure elucidation were presented (Fig. S3, Supplementary material). The pre-treatment stage is the most time-consuming. This stage is determined by the physical condition of the sample, which might be solid or liquid. Several techniques are employed to decrease the size of the solid sample, including steaming, dry distillation, and heating, which is subsequently milled. For liquid samples, another option is to employ solvent treatment or chemical treatment. This method is critical in sample preparation to improve extraction efficiency. Traditional terpene and terpenoids extraction methods include maceration, soxhlet extraction, solvent extraction, pressurized liquid extraction, and hydrodistillation (Isidore, Karim, & Ioannou, 2021). Solvent extraction used such as petroleum ether, ether, and hexane for isolating mono and sesquiterpenoids. Less polar sesquiterpene lactones, diterpenes, sterols, and triterpenoids may also be extracted using ether and chloroform. Oxygenated diterpenoids, sterols, and triterpenoids are found in the ethyl acetate and acetone extracts. Highly oxygenated, polar triterpenes, as well as triterpenoid, are extracted using ethanol, methanol, and water. Total extraction using polar solvents including acetone, aqueous methanol (80%), and ethanol, followed by re-extraction with hexane, chloroform, and ethyl acetate, resulted in sequential extraction of terpenoids. Whereas, modern methods include supercritical carbon dioxide extraction, static headspace (HS) extraction, microwave-assisted hydrodistillation (MWHD), supercritical fluid extraction (SFE) and ultrasound-assisted extraction (UAE). Supercritical fluid extraction (SFE) with carbon dioxide as the solvent is considered a “green extraction” method in comparison to traditional extraction methods because it uses a solvent that is essentially non-toxic, has a low potential for artifact formation, and CO2 can be obtained in high purity suitable for the production of food grade extracts. The inclusion of polarity modifiers such as EtOH, as well as the development of SFE apparatus capable of producing pressures in excess of 600 bar, enabled the extraction of several intermediate polarity compounds (Essien, Young, & Baroutian, 2020). UAE was useful for the green production of solvent-free terpenoid-rich peppermint extracts. MWHD is better than traditional techniques since it requires fewer extraction times and less solvent. It operates at atmospheric pressure, utilizing microwaves and gravity without solvents (Pavlić et al., 2021). Furthermore, recent report suggested that HS was the most efficient extraction method to recover terpenoids from various food matrices, compared to hydrodistillation and pressurized liquid extraction (Triaux, Petitjean, Marchioni, Steyer, & Marcic, 2021). Compound extraction generates a complicated mixture of compounds. Specific compounds are now isolated using purification methods. Purification procedures varies due to variations in terpene and terpenoids structure, and they are depending on the chemical characteristics of the target molecule, the physical quality and amount of initial plant material, and the availability of equipment and reagents. The properties of a particular terpene will define the optimum extraction method (Yang et al., 2020, Zhang and Hong, 2020). This is followed by compound identification, characterization, and authentication namely Thin Layer CT (Chromatostrip), High-Performance Counter-Current Chromatography (HPCCC), Liquid-Liquid Extraction (LLE), High Performance Liquid Chromatography UV (HPLC-UV), Gas Chromatography Mass Spectrometry (GC–MS), Liquid Chromatograph (LC), High Performance Thin Layer Chromatography (HPTLC), Fast-GC Analysis and Ultra-High-Performance Liquid Chromatography (UHPLC) (Rodríguez-Llorente et al., 2020, Uwineza and Waśkiewicz, 2020).
Role of terpenes and terpenoids on human health
In recent decades, numerous studies have reported that terpenes and terpenoids are essential in supporting human health. These bioactive compounds consisting of several isoprene units is the largest class of organic compounds produced in the EOs of various plants. It has a significant role to treat various types of diseases, in many studies in vitro and in vivo using as anticancer agents, antimicrobial, anti-inflammatory, antioxidants, antiallergic, neuroprotective, anti-aggregator, anti-coagulation, sedative and analgesic through the activity of monoterpenes, sesquiterpenes, diterpenes, triterpenes, and tetraterpenes and glycoside compounds (Zhao, Jiang, Li, Yan, & Zhang, 2016). The content of this compound can be found in several nutritional and health products of humans because it is a source of vitamins A, E, K, and coenzyme Q10. Even carotenoid and tocopherol compounds in this group are essential sources of vitamins, mostly in animals, including humans (Rodriguez-Concepcion et al., 2018). The application of terpenes compounds in everyday human life and health is widely used in pharmaceutical, nutraceutical, food and beverage products, cosmetics, perfumes, synthetic chemicals, aroma and flavor additives, rubber products, and the biofuel industry (Tetali, 2019).
Anticancer
Cancer is a regenerative disease that affects anybody part's abnormal and uncontrolled cell growth. It can attack or spread to various other parts of the body and interfere with the normal function of homeostasis of its host cells (Sung et al., 2021). Today worldwide, it is one of the leading causes of morbidity and mortality in the contemporary world, number second after cardiovascular disease and being one in six human deaths and constitutes 30% of all premature deaths occurring in adults (30–69 years). In addition, the number of cancer deaths is predicted to continue to grow and multiply. This cancer is caused by the influence of internal factors, inability, and external factors in lifestyle patterns carried out every day. A food diet can protect from risk factors for carcinogenesis in the human body (El-Sherif, El-Sherif, Taylor, & Ayakannu, 2021).
Terpenes and terpenoids are the largest class of organic compounds produced in the EOs of various plants and have a significant role in the prevention of malignancies in cancer (Silva, Nascimento, Silva, Silva, & Aguiar, 2021). The anticancer potencies of terpenes and terpenoids have been reported extensively in recent years (Table 1). Sheikh, Sarker, Kamarudin, and Mohan (2017) studied the effect of citral on the proliferation of human colorectal cancer HCT116 and HT29 cells. They found that citral decreased the expression of Bc-2 and Bcl-xL while inducing p53 protein phosphorylation and Bax expression. Citral also elevated mitochondrial-mediated apoptosis via augmentation of intracellular ROS and attenuated the total GSH levels (Sheikh et al., 2017).
Table 1.
Examples of terpene compounds in essential oils with potential anticancer effects.
| Classes | Compounds | Plant | Target cancers | Cancer effects | Type of assay | Reference |
|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | Myrcene | PC | Human lung adenocarcinoma (A549) | Increased apoptosis via caspase induction (IC50 0.5 μg/ml) | MTT | (Bai & Tang, 2020) |
| Cyclic monoterpene | Limonene | Navel orange (Essential oil) | Lung adenocarcinoma (A549) | Reduced cell proliferation (IC50 22.10 µg/ml) | MTT | (Yang et al., 2017) |
| Bicyclic monoterpene | α-Pinene | PC | Human liver cancer (HepG2) | Reduced cell growth (IR 39.3%) | MTT | (Xu et al., 2018) |
| Acyclic monoterpene | Linalool | PC | Human ovarian cancer (SKOV3ip1, A2780, HeyA8) | Increased apoptotis and cytotoxicity | MTT | (Han, Cho et al., 2016) |
| Oxygenated monoterpene | α-Thujone | Thuja occidentalis L. | Human glioblastoma multiforme (T98G), and human glioblastoma (U-87 MG) | Induction of cell death, reduced proliferation and invasive | TB exclusion | (Pudełek et al., 2019) |
| Terpineols Monoterpene | α-Terpineol | Navel orange (Essential oil) | Lung adenocarcinoma (A549) | Increased caspase-dependent cell death and reduced proliferation (IC50 51.37 µg/ml for 24 h) | MTT | (Yang et al., 2017) |
| Oxygenated monoterpene | 1,8-cineole | PC | Head and neck squamous cell carcinoma (HNSCC) | Reduced proliferation, Wnt/β-catenin activity | MTT | (Roettger et al., 2017) |
| Cyclic monoterpene | α-Phellandrene | PC | Melanoma(B-16/F-10), and Murine (S-180) | Antinociceptive and tumor-reducing effect (CI50 436.0 and 217.9 μg/ml) | MTT | (Pinheiro-Neto et al., 2021) |
| Bicyclic Monoterpene | 3-carene | Gannan Navel (Essential oil) | Human lung cancer (A549) | Reduced proliferation (IC50 70.80 µg/ml) | MTT | (Yang et al., 2017) |
| Hydroxylated monoterpene | Perillyl alcohol | PC | Hepatoma cell | Suppress cell invasion and migration | Transwell | (Ma et al., 2016) |
| Terpenophenol | Bakuchiol | PC | Human gastric cancer (NUGC3) | Increased cell death and reduced cancer cell viability (IC50 120 µg/ml for 24 h) | MTT | (Lv & Liu, 2017) |
| Monoterpenoid phenol | Carvacrol | PC | Human Caucasian gastric adenocarcinoma (AGS) | Increased cell apoptosis, inhibited proliferation, GSH-reducing effects on cell (IC50 82.57 μmol/l) | CellTiter-Glo | (Günes-Bayir et al., 2017) |
| Citral isomers | Geranial, Neral, and Citral | PC | Colorectal cancer (HCT116 and HT29) | Increased mitochondrial-mediated apoptosis, inhibited cell growth (IC50 52.63 μM, and 91.5 μM for 72 h) | MTT | (Sheikh et al., 2017) |
| Tropolone monoterpene | Hinokitiol | Purity ≥ 90% | Human adenocarcinoma (A549) | Reduced cell migration and chemoprevention | MTT | (Jayakumar et al., 2018) |
| Bicyclic Monoterpene | Myrtenal | PC | Melanoma (B16F0, B16F10 and SkMel-5) | Decreased tumor cells migration and invasion | MTT | (Martins et al., 2019) |
| Oxygenated monoterpen | Carvone | PC | Breast ductal carcinoma (MCF-7) | Protective effect against tumor (IC50 14.22 μM) | MTT | (Abbas, Kandil, & Abbas, 2020) |
Note: PC = Pure Compound.
Similarly, Jayakumar et al. (2018) evaluated the effect of hinokitiol on the migration of A549 lung cancer cells. They observed that hinokitiol inhibited the migration of A549 cells via several mechanisms, including suppression of MMPs, induction of antioxidant enzymes (catalase and superoxide dismutase), and activation of caspases-9 and -3. It also significantly induced the cytochrome c expression (Jayakumar et al., 2018).
α-Thujone is another compound that has anticancer activity. In a study carried out by Pudełek et al. (2019), the effect of α-thujone on the malignancy of glioblastoma multiforme (GBM) cells was investigated. They found that α-thujone exerts the attenuating effect on the proliferation and viability of GBM cells. They also indicated that α-thujone exhibits anti-invasive and pro-apoptotic effects on GBM cells (Pudełek et al., 2019).
Furthermore, it has been reported by Potočnjak et al. (2018) that carvacrol exhibits anticancer activity against HeLa cells, a human cervical cancer cell line. Carvacrol induced cytotoxicity on HeLa cells and enhanced cisplatin (CP)-induced expression of light chain 3 beta in a manner dependent on the inhibition of mitogen-activated protein kinase (MEK). This compound, interestingly, also increased HeLa cells resistance on CP by suppressing apoptosis in an extracellular signal-regulated kinase (ERK1)/ERK2-independent manner and promoting the modulation of autophagy in an ERK1/ERK2-dependent manner (Potočnjak et al., 2018). In addition, carvacrol is a potential source of anticancer agent against the human gastric adenocarcinoma (AGS) cells. Carvacrol inhibited AGS cell proliferation and demonstrated genotoxic, ROS generating, and glutathione-reducing effects (Günes-Bayir et al., 2017).
Han et al. (2016) studied the ability of linalool-incorporated nanoparticles (LIN-NPs) as novel anticancer agents. They reported that LIN-NP had significant apoptotic and cytotoxicity activity against epithelial ovarian cancer cells (Han, Cho et al., 2016). Moreover, linalool has also been reported to exert anticancer activity against hepatocellular carcinoma HepG2 cells through modulation of Ras/MAPK and Akt/mTOR pathways (Rodenak-Kladniew et al., 2018).
In a study by Ma et al. (2016), the anticancer activity of perillyl alcohol was demonstrated. They found that perillyl alcohol has an inhibitory effect on protein synthesis of hypoxia-inducible factor 1α (HIF-1α) which causes a decrease in HCT116 cell growth in a xenograft colon tumor model. In addition, perillyl alcohol was also able to increase the expression of p53 and p21, decrease the expression of cyclin D1, c-Myc, and S-phase kinase-associated protein 2 (Skp2) and induce cell cycle catch in phase G1 in cancer cells (Ma et al., 2016).
The monoterpene myrtenal has also been reported to exhibit anticancer properties. Myrtenal plays an important role in controlling tumor malignancy and metastasis through inhibition of V-ATPase enzymes, disruption of H + flow to V-ATPase-dependent extracellular matrix, induction of autophagy, and alteration of ion gradients (Martins, Arruda, Costa, Jerdy, De Souza, Santos, De Freitas, Kanashiro, De Carvalho, Sant'anna, Antunes, Martinez-Zaguilan, Souad, Okorokova-Façanha, & Façanha, 2019). Lastly, bakuchiol, an example of meroterpene, has been reported to exert anticancer activity in the human stomach cancer cells. Preliminary investigation demonstrated that bakuchiol yield its anticancer activity via the induction of mitochondrial-dependent cell death and modulation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) pathways of Nagoya University-Gastric Cancer-3 (NUGC3) cells (Lv & Liu, 2017).
Antimicrobial
The antimicrobial activity of EOs has been reported in many studies, but most of these studies attribute such action to the most common compounds without analyzing it independently (Guimarães et al., 2019). Infections caused by sensitive and drug-resistant bacteria or other microorganisms seriously harm human health and global public health. Therefore, finding and developing new antimicrobial agents to combat multidrug-resistant organisms is essential (Liu et al., 2020).
The use of terpene and terpenoid compounds as antimicrobial agents is very promising. It has been used in several industrial fields, including cosmetics, foods, and some health care products, such as hair tonics, toothpaste, eyelid cleansers, hair restorers, skin soaps, and body lotions (El Hachlafi et al., 2021). Terpene and terpenoid compounds have been reported to yield bactericidal effects (Guimarães et al., 2019). For example, limonene have synergistic modulation effects with gentamicin antibiotics in the inhibition of Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli as and several resistant bacteria (Costa et al., 2019). In addition, a combination of limonene and ε-Polylysine demonstrates an additive and useful synergistic effect against E. coli, S. aureus, Bacillus subtilis and Saccharomyces cerevisiae (Zahi, El Hattab, Liang, & Yuan, 2017),
Monoterpene terpineol’s isomers (α-terpineol, terpinen-4-ol, and δ-terpineol) have a good inhibitory effect on several Gram-negative bacteria, especially Shigella flexneri with bacterial membrane permeability mechanisms resulting in the release of nucleic acids and proteins along with a decrease in membrane potential. In addition to that, the release of AKP indicates that the bacterial cell wall is destroyed, thus the potential use of terpineol in food as a natural antibacterial agent that is capable to destroy cell membranes and walls, resulting in bacterial cell death (Huang et al., 2021). Borneol and citral also have synergistic effects as bacteriostatic and antibiofilm agents in the packaging materials and common surface disinfectants. The synergistic effects of borneol and citral demonstrates a good elimination effect on the formation of biofilms produced by Listeria monocytogenes and Pseudomonas aeruginosa, most likely attributed to the ability of those two components to increase porosity and lytic effect on bacterial cell membranes as particularly suitable for use as promising food additives (Wang et al., 2021).
Hinokitiol is effective against Candida strain panels with several azole-resistant mechanisms and inhibits the growth of Candida albicans (Jin et al., 2021). The mechanism of action of hinokitiol is attributed to the chelated effect of intracellular iron fungus and inhibits the respiration of fungal cells but has little effect on the mammalian cells. It has been reported that hinokitiol also inhibits the activity of mitochondrial respiratory chains complex I and II and reduces the potential of mitochondrial membranes, thereby reducing intracellular ATP synthesis and increasing the intracellular reproductive stress. Thus, hinokitiol shows low potential to induce resistance in some Candida species and greatly improves the survival of Candida-infected Galleria mellonella (Jin et al., 2021). Furthermore, hinokitiol has also been demonstrated to yield prospective antibacterial effect with low or no negative effect on the human host or environment (Suzuki et al., 2019).
Other examples of EO with antimicrobial effect are eugenol (Devi, Nisha, Sakthivel, & Pandian, 2010). Eugenol showed rapid bactericidal action against Salmonella enterica serovar Typhimurium. Eugenol also demonstrated excellent bactericidal activity against strains of S. aureus. The compounds carveol, citronellol, and geraniol have a rapid bactericidal effect against E. coli. Furthermore, carvacrol, l-carveol, eugenol, trans-geraniol, and thymol, showed higher activity when compared to sulfanilamide. The inhibition of microbial growth and the death of bacterial cells is based on the loss of integrity in the function of cellular membranes (Guimarães et al., 2019). Terpenes and other terpenoid compounds, such as bakuchiol, α-pinene, linalool, champene, geraniol, 1,8-cineole, α-phellandrene, 3-carene, p-cymene, perillyl alcohol, bornyl acetate, and citral isomers, also have been reported to yield inhibitory effects on the growth of microorganisms (Table 2). These convincing data further indicate the potential use of these compounds as efficient antimicrobial agents in the food industry (Zahi et al., 2017), thus has a valuable contribution in supporting human health.
Table 2.
Examples of terpene compounds in essential oils with the potential antimicrobial effects.
| Classes | Compounds | Plant | Microorganism strain | Antimicrobial effects | Type of assay | Reference |
|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | Myrcene | PC | Enterococcus faecalis (ATCC 29212), Streptococcus mutans (ATCC 25175), S. mutans (ATCC 35668), S. mutans (ATCC UA159), and Lactobacillus rhamnosus (ATCC 53103) | Bacterial growth (MIC > 8 × 102 CFU/ml) | Agar dilution | (Chaves-Quirós et al., 2020) |
| Terpineols Monoterpene | α-Terpineol | PC | Escherichia coli (CICC 21530), Shigella flexneri (CICC 21534), and Salmonella enterica (CICC 21513) | Good inhibitory effects against several gram-negative bacteria (MIC 1.531, 0.766, and 1.531 mg/ml) | Two-fold dilutions | (Huang et al., 2021) |
| Tropolone monoterpene | Hinokitiol | PC | Bacillus subtilis, S. aureus, E. coli, and Pseudomonas aeruginosa | (MIC 80, 160, 80, and 320 mg/ml) | Agar dilution | (Suzuki et al., 2019) |
| Bicyclic monoterpene | Borneol | Purity ≥ 98% | E. coli (ATCC 43894), Listeria monocytogenes (ATCC 19118), S. aureus (ATCC 23235), P. aeruginosa (ATCC 27853) | Bacteriostatic activity (MIC 1.25, 5, 5, 10 mg/ml) | Double microdilution | (Wang et al., 2021) |
| Terpenophenol | Bakuchiol | PC | S. aureus (ATCC29213), MRSA N315, MRSA NCTC10442, E. coli (ATCC25922), P. aeruginosa (ATCC9027), Acinetobacter baumannii (ATCC17978), A. baumannii (R2889), Klebsiella pneumoniae (ATCC10031), K. pneumoniae (ATCC14581) | Antibacterial activity (MIC 6.25, 3.125, 3.125, > 100, >50, >50, >50, 12.5, and > 50 μl/ ml) | Broth dilution method | (Li et al., 2021) |
| Cyclic monoterpene | d-Limonene | PC | E. coli (ATCC 8739), S. aureus (ATCC 6538), B. subtilis (ATCC 6633), Saccharomyces cerevisiae (ATCC 9763) | Exhibit strong synergistic (MIC 1, 1, 1, and 0.5 µg/ml) | Serial Dilution | (Zahi et al., 2017) |
| Acyclic monoterpene Alcohol | Geraniol | PC | B. cereus (MTCC 430), E. coli(MTCC 443) | Antimicrobial effect (MIC 1200 µl/ml all tested) | BHI broth | (Syed & Sarkar, 2018) |
| Oxygenated monoterpene | 1,8-cineole | Purity 99% | S. aureus (ATCC 25923), MRSA clinical isolate, P. aeruginosa (ATCC 27853), E. coli (ATCC 25922), K. pneumoniae (ATCC 700603), Enterococcus faecalis (ATCC 51299) Candida albicans (ATCC 90028) | Effect against microorganisms (MIC 128, 128, 256, 32, 64, 128, and 32 g/l) | Serial double dilutions | (Şimşek & Duman, 2017) |
| Cyclic monoterpene | α-Phellandrene | Purity > 99% | Penicillium cyclopium | Inhibit the mycelia growth (MIC 1.7 ml/L) | Agar dilution | (Zhang, Sun, Chen, Zeng, & Wang, 2017) |
| Bicyclic Monoterpene | (+)-3-carene | Purity ≥ 90.0% | Brochothrix thermosphacta (ACCC 03,870), and Pseudomonas fluorescens (ATCC 13,525) | Had strong antibacterial activity (MIC 20 ml/L) | Agar dilution | (Shu et al., 2020) |
| Alkylbenzene Monoterpene | p-Cymene | Salvia officinalis (Essential oil) | E. coli (ATCC 8739), S. aureus (ATCC 6538), C. albicans (ATCC 10231) | Antibacterial activity (MIC 7.5, >15, and 3.75 μl/ml) | Broth microdilution | (Cutillas, Carrasco, Martinez-Gutierrez, Tomas, & Tudela, 2017) |
| Oxygenated monoterpene | Bornyl acetate | S. officinalis (Essential oil) | E. coli (ATCC 8739), S. aureus (ATCC 6538), C. albicans(ATCC 10231) | Antibacterial activity (MIC > 15, 15, and > 15 μl/ml) | Broth microdilution | (Cutillas et al., 2017) |
| Monoterpenoid phenol | Carvacrol | PC | B. cereus (MTCC 430), E. coli (MTCC 443) | Antimicrobial effect (MIC 400 µl/ml all tested) | BHI broth | (Syed & Sarkar, 2018) |
| Acyclic monoterpene Alcohol | Linalool | PC | P. aeruginosa (ATCC9027) | Antibacterial activity (MIC 431 µg/ml) | Medium dilution | (Liu et al., 2020) |
| Citral isomer | Citral | PC | C. albicans (SC5314), C. tropicalis (ATCC1369), S. aureus (ATCC25923) | Antimicrobial action (MIC 0.0313, 0.0156, and 0.0313 v/v%) | Serial microdilutions | (Gao et al., 2020) |
Note: PC = Pure Compound.
Anti-inflammatory
Recent decades have shown that terpenes and terpenoids are physiologically important to alleviate various symptoms caused by inflammation, most likely by inhibiting multiple pathological steps in the inflammatory process (Kim, Song, Cho, & Lee, 2020). Inflammation is a protective response of the host to non-self objects that are usually generated by microbial infection and/or tissue damage. Dysregulation of inflammatory responses can lead to acute and chronic inflammatory diseases that cause excessive or long-lasting tissue damage (Chen et al., 2017).
Macrophages, one of key immune cells, play a central role in many different immune pathological phenomena during inflammation, including overproduction of pro-inflammatory cytokines and inflammatory mediators, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α), and nitric oxide (NO) synthesized by non-reduced NO synthase (iNOS), and prostaglandin E2 (PGE-2) synthesized by cyclooxygenase-2 (COX-2). Lastly, a central transcription factor, nuclear-κB (NF-κB) factor, holds a central role in the expression of pro-inflammatory genes during inflammation (Liu, Zhang, Joo, & Sun, 2017).
Some cellular processes, including oxidative stress and autophagy, also play an essential role in inflammation. Reactive oxygen species (ROS), which results from several sources, including mitochondria, mediate increased leukocyte migration and junctional permeability through various signaling mechanisms. In addition, a recent study showed that ROS regulates the release of IL-1β by directly interfering with NF-κB signals (Warnatsch et al., 2017).
The application of terpenes and terpenoids to alleviate inflammation has been shown successful in the mitigation of respiratory inflammation, atopic dermatitis, arthritis, and neuroinflammation (Kim, Song et al., 2020). Table 3 shows that terpene compounds and terpenoids are beneficial as an anti-inflammatory in some disease conditions.
Table 3.
Examples of terpene compounds in EO with the potential anti-inflammatory effects.
| Classes | Compounds | Plant | Type of inflammation | Anti-inflammatory effects | Assay/method | Reference |
|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | Myrcene | PC | Renal tissues of rats | Inhibited the activities of inflammatory cytokine, pro-inflammatory signalling | ELISA | (Yang & Liao, 2021) |
| Cyclic monoterpene | R-(+)-limonene | Purity > 99%(Sigma Aldrich, St. Louis, MO, USA) | Gastric ulcer in rats | Decreased the levels of TNF-a, IL-6, and IL-1β and increased the level of IL-10 | ELISA kits | (De Souza et al., 2019) |
| Bicyclic monoterpene | Borneol | PC | Acute pancreatitis mice model | Inhibited TNF-α, IL-1β, IL-6, and modulated Nrf2/NF-κB pathway | ELISA | (Bansod et al., 2021) |
| Tropolone monoterpene | Hinokitiol | PC | Inflammation in primary human keratinocytes | Inhibited LPS-mediated up-regulation of pro-inflammatory factors including tumor necrosis factor alpha, IL-6, and prostaglandin E2 (PGE2), and Sirt1 activity | Quantitative Real-Time PCR | (Lee et al., 2017) |
| Terpenophenol | Bakuchiol | Psoralea corylifolia (Seeds essential oil) | Cerebral ischemic injury in mouse BV-2 microglia | Exhibit its anti-inflammatory property via activating Nrf2 signaling | Immuno-fluorescence staining | (Xu, Gao, Wang, Yang, & Xie, 2021) |
| Bicyclic monoterpene | α-Pinene | PC | Focal cerebral ischemia–reperfusion in rats | Neuroprotective effect during ischemic stroke through attenuating neuroinflammation. | ELISA | (Khoshnazar, Parvardeh, & Bigdeli, 2020) |
| Acyclic monoterpene Alcohol | Linalool | Boswellia carterii | Ear edema model and a formalin-inflamed hind paw model | More potent pharmacological effects on hind paw inflammation and COX-2 overexpression | Immuno-histochemistry | (Li, Yang, Li, Zhang, & Tang, 2016) |
| Acyclic monoterpene Alcohol | Geraniol | PC | Lungs in mice model | Improved the inflammatory changes | ELISA | (Lin et al., 2021) |
| Cyclic monoterpene | α-Phellandrene | PC | Wound healing | Suppressed the overproduction of pro-inflammatory cytokines of IL-6 and TNF-α. | ELISA | (De Christo Scherer et al., 2019) |
| Hydroxylated monoterpene | Perillyl alcohol | Purity 96% | Lung tissue in rats | Inhibited cellular inflammation | ELISA | (Beik et al., 2021) |
| Citral isomer | Citral | Purity ≥ 95% | Hyperalgesia and pleurisy in mice | Anti-inflammatory activities | ELISA | (Campos, Lima, Trindade, Souza, Mota, Heimfarth, & Thangaraj, 2019) |
| Monoterpenoid phenol | Carvacrol | PC | Asthma in rats | Reduced of AEC, IgE, IL-4, IL-5, IL-13, TNF-α, IFN-γ, iNOS and MDA | Colorimetric and Quantitative Real-Time PCR | (Ezz-Eldin, Aboseif, & Khalaf, 2020) |
| Oxygenated monoterpene | 1,8-cineole | PC | Upper Ileum Tissues | Prevented low-grade inflammation | Quantitative Real-Time PCR | (Jiang et al., 2021) |
Note: PC = Pure Compound.
The prospective anti-inflammatory effect of myrcene in the treatment of renal inflammation was tested in the adrenalectomized rat models (Islam et al., 2020). The mechanisms were suggested to be associated with the downregulation of pro-inflammatory cytokine (IL-1β, IL-6, and TNF-α) and anti-inflammatory markers (IL-4 and IL-10) as well as the upregulation of endogenous antioxidants such as catalase (CAT), superoxide dismutases (SODs) and glutathione (GSH) (Islam et al., 2020).
Limonene, a cyclic monoterpene, has been reported to exert gastroprotective effect in rats (De Souza et al., 2019). The precise mechanism of its gastroprotective action remains unclear. However, current experimental evidence suggested that this effect may be due to its ability in the modulation of the oxidative stress (via upregulation of endogenous antioxidant glutathione peroxidase) and the alleviation of inflammatory responses (via inhibition of NF-κB-mediated gene expression) (De Souza et al., 2019).
Several terpenes and terpenoids such as (+)-α-terpineol, (−)-β-pinene, and (+)-α-pinene reported to reduce the expression of genes associated with inflammation (IL-4 and IL-13) and secretion of β-hexosaminidase in RBL-2H3 cells stimulated by LPS. The application of these compounds in the treatment of inflammatory conditions and the basis for the development of new anti-inflammatory drugs (Yang, Choi, Kim, Eom, & Park, 2021). Borneol, for example, has been reported to significantly increase the activation of nuclear factors E2-related factor 2 (Nrf2) and the expression of superoxide dismutase (SOD) 1 but at the same time downregulate the expression of NF-κB and p65. Treatment with borneol significantly inhibited the pro-inflammatory expression of cytokines and has the potential to alleviate cerulein-induced acute pancreatitis by reducing oxidative damage and inflammation of the pancreas in a manner dependent on the modulating of the Nrf2/NF-κB pathway (Bansod et al., 2021).
Another example is bakuchiol, a meroterpene in the class of terpenophenol, and hinokitiol, a natural monoterpenoid. Bakuchiol has been demonstrated to be effective in the suppression of pro-inflammatory cytokine expression and mitigation of delayed hypersensitivity responses compared to the control group (Kumar et al., 2021). Hinokitiol inhibits LPS-mediated regulation, including tumor necrosis factor-alpha, IL-6, and prostaglandin E2 (PGE2). Hinokitiol is suggested to act through the inhibition of LPS-mediated proinflammatory signals via the activation of histone sirt1 deacetylase in primary human keratinocytes. Hence, hinokitiol has been suggested as a potential therapeutic agent in the treatment of inflammatory skin diseases such as psoriasis (Lee, Moon, Lee, & Park, 2017).
Antioxidant
Some EOs have an important role in reducing oxidative stress and often used to prevent several chronic diseases. Chamazulene, a bicyclic sesquiterpene derivative from EOs of Matricaria chamomilla, was able to balance ROS level on bovine aortic endothelial cells-1 (BAECs), which increase due to high glucose and H2O2 treatment (Querio et al., 2018). Ursolic acid which isolated from Entada abyssinica is a pentacyclic triterpenoid carboxylic acid. Ursolic acid has antioxidant activity with IC50 1.43 ± 0.080, 2.87 ± 1.19, and 7.04 ± 1.29 µg/ml by using FRAP, DPPH, and ABTS method, respectively (Dzoyem et al., 2017). FRAP, DPPH, and ABTS are radical compound which can assist in the measurement of antioxidant activity through color change. DPPH will change into a non-radical compound (diphenylpicrylhydrazine), characterized by a change in the color of the solution from purple to pale yellow, if its free electrons bind to the hydrogen atom of an antioxidant compound. In the ABTS method, the solution will change from blue or green to colorless due to the acceptance of proton donors from antioxidant compounds. Meanwhile, in the FRAP method, the solution will change color from yellow to blue when ferri-tripyridyl-triazine (Fe(III)TPTZ) becomes ferro-tripyridyl-triazine (Fe(II)TPTZ) due to electron transfer from antioxidant compounds (Moon & Shibamoto, 2009).
Some terpeneoids (α-pinene, limonene, nerol, terpinol, geraniol, linalool, and myrcene) are responsible for specific aroma in wine. α-Pinene showed the strongest radical scavenging effect with IC50 12.57 ± 0.18 mg/ml, followed by limonene and nerol with IC50 13.35 ± 0.26 and 26.08 ± 2.28 mg/ml, respectively. This is in line with the result of reducing power assay, where α-pinene has the greatest reducing power among others (213.7 ± 5.27 mg/ml), followed by limonene (133.48 ± 6.22 mg/ml), nerol (79.15 ± 3.75 mg/ml), terpinol (73.92 ± 3.34 mg/ml), geraniol (73.78 ± 3.94 mg/ml), linalool (43.97 ± 1.09), and the lowest was myrcene (21.59 ± 1.14 mg/ml) (Wang et al., 2019).
Magnolia biondii, one of traditional Chinese herb, contain many active components in its essential oil. Of all, α-terpineol showed the highest % rate of scavenging (84.1%) followed by c-cadinene (83.9%), geraniol (75.5%), linalool (65.5%), and citronellol (58.1%) by DPPH assays. Similar results were also obtained in the ABTS assays, where these five components had the highest scavenging activity compared to the others, ranging from 89.1% to 83.0% (Nie et al., 2020). Essential oil of Cinnamon contains cinnamaldehyde which has high scavenging rate of 93.0% by using DPPH assay (Farag, Alagawany, & Tufarelli, 2017). Eugenol and isoeugenol which found in a variety of plants including cinnamon, basil, clove, spices, and nutmeg have quite promising antioxidant activity, with EC50 of 22.1 ± 3.5 and 17.6 ± 4.1 µg/ml for DPPH assay, 146.5 ± 5.6 and 87.9 ± 4.7 µg/ml for ABTS assay, and 11.2 ± 1.5 and 18.4 ± 1.2 µg/ml for FRAP assay, respectively. These results indicate that isoeugenol has slightly higher scavenging activity than eugenol (Zhang, Zhang, Xu, & Hu, 2017).
Anti-allergic
Allergic diseases are characterized by inflammation with infiltration of T cells and granulocytes (eosinophils, neutrophils, and mast cells). Mast cells play a major role in almost all allergic diseases, generally in the end reaction of allergic disease (Modena, Dazy, & White, 2016). Mast cells begin to synthesize prostanoids and proinflammatory leukotrienes, and subsequently produce inflammatory cytokines, such as IL-4, IL-5, IL-13, IL-1α/β, thereby stimulating the activation of other cells such as neutrophils, monocytes, basophils, eosinophils and lymphocytes. Anti-allergic compounds derived from plants, animals, and microbes, with various mechanisms of action such as binding to the epitope present in allergens, affecting gut microbiota and intestinal epithelial cells, changing antigen presentation and T cell differentiation, and inhibiting effector cell degranulation (Yang, Li, Xue, Huang, & Wang, 2021).
Some terpenes and terpenoids have been studied for anti-allergic activity. Atractylone (Atr), one of sesquiterpene components from Atractylodes japonica, can inhibit rat peritoneal mast cells (RPMC) degranulation, tryptase and histamine release, and intracellular calcium levels. Also, reduced tryptase and histamine release from PMA plus A23187-stimulated HMC-1 cells, led to decreased actvity and expression of histidine decarboxylase in activated HMC-1 cells. Atr decreased levels of histamine, IgE, IL-4, IL-5, IL-6, vascular endothelial growth factor, and IL-13 in the serum of PCA-induced mice. Atr has activity as a treatment for allergic reactions mediated by mast cells (Han, Moon et al., 2016). Carvone (S- and R-) at concentrations 10 mg/kg via oral route has been reported to reduce the total eosinophil and leukocytes counts in the murine model of airway allergic inflammation induced by sensitization and challenge with ovalbumin (OVA). S-carvone significantly increased the concentrations of IFN- and neutrophil counts in the BAL of allergic mice. R-carvone could stimulate IL-10 synthesis and inhibit IgE secretion (Ribeiro-Filho et al., 2020). β-Amyrin, 2α,3α,23- trihydroxyursa-12,20(30)-dien-28-oic acid, and euscaphic acid at 10 µM, inhibited histamine release with percentage of inhibitions of 46.7, 57.9, and 54.2%, respectively. In addition, β-amyrin and 2α,3α,23- trihydroxyursa-12,20(30)-dien-28-oic acid showed strong inhibition of TNF- α and IL-6 in the test for pro-inflammatory cytokines (Choi, Kim, Kim, & Kim, 2016).
Citronellol, one of major component from geranium EOs, at concentrations 0.5 mM can inhibit degranulation of mast cells by 69.4% and significantly inhibit IgE- induced tumor necrosis factor -α (TNF-α) production (Kobayashi et al., 2016). Vernodalin isolated from Vernonia amygdalina increase filaggrin (FLG) mRNA expression levels and reduce IL-33mRNA expression in mice (vs. control group) also significantly reduce the dermatitis score (vs. control) in regard to ear skin lesions at 100 µg/ml via topical administration (Hirota & Ngatu, 2018). Mojabanchromanol, a constituent isolated from Sargassum horneri, has anti-allergy effect on bone marrow-derived cultured mast cells. Mojabanchromanol shows inhibitory effect on the β-hexosaminidase release and inhibited the mRNA expression levels of allergic cytokines in bone marrow cultured mast cells (BMCMCs) (Kim, Han et al., 2020).
Bioaccessibility and bioavailability of terpenes and terpenoids
Bioavailability is a term that refers to a measure of the rate and fraction of the initial dose of a drug that successfully reaches the site of action or body fluids of the target drug to produce the desired biologic effect (Currie, 2018). Bioavailability includes two interrelated parts, namely: bioaccessibility and bioactivity. Bioaccessibility is defined as the quantity or fraction released from the food matrix in the digestive tract that is available for absorption (Thakur et al., 2020).
Studies on the bioavailability and bioaccessibility of a compound are generally carried out by in vitro or in vivo methods. Simulation of the condition of the gastrointestinal system by adjusting the pH, digestive enzymes and some chemical reactions that occur, is the most widely used in vitro digestion method (Jones, Caballero, & Davidov-Pardo, 2019). In vitro methods can also be carried out using a Caco-2 cell model wherein the absorbed target compound is collected on the basolateral side of the monolayer model cell (Jones et al., 2019). In vivo methods were carried out using animal models prior to clinical studies.
The matrix of medicinal plants is related to the bioavailability of the terpenes they contain because they must pass through digestion in the mouth and stomach before accessing the small intestine. Furthermore, these compounds will undergo mechanical and enzymatic actions, changes in pH conditions, as well as water-soluble transformations, which occur mainly in the liver and other tissues such as the lungs, gastrointestinal, kidney, blood and brain. Terpenes describe high lipophilic behavior that influences their solubility in the aqueous phase of the gut lumen, thus incorporation with the lipid phase becomes very important in the bioavailability of terpenes, either during digestion or during food processing (Mouhid et al., 2017). Even though intravenous administration is assumed has maximal (100%) bioavailability of EOs, skin application, oral intake, and inhalation are the primary intake routes of EOs (Stevanovic et al., 2020). Some studies have been conducted in order to understand bioavailability and bioaccessibilty of terpenes and terpenoids (Table S1).
Terpenoids easily enter the body by penetration through the skin due to their lipophilic properties although the amount depends on the area of skin applied, skin characteristics, concentration of compounds and exposure time. Following oral administration of terpenoids, the upper gastrointestinal tract did not have a significant role in the absorption. However, using the route of inhalation, terpenoids may be absorbed by the lungs so that they are available systemically. This absorption depends on the type of compound and the respiratory mechanics of the subject. Enterohepatic circulation, pulmonary and renal excretion play a role in the elimination of terpenoids in the form of feces, expired air, and urine. A minor part is eliminated along with the feces, and the rest through the urine as terpene conjugates and in the expired air with CO2. Drug biotransformation occurs in the liver by phase I and phase II reactions, but in many terpenoids it takes only one phase to eliminate these volatile compounds (Jäger and Höferl, 2020, Kohlert et al., 2000). Camphor undergoes phase II metabolism to 5-exo-hydroxycamphor. Menthol and peppermint oil are excreted through the kidneys as menthol glucuronide. Terpenoids have high clearance and a short elimination half-life making accumulation of their metabolites are impossible.
Some EOs were used as penetration enhancer of drug via transdermal administration. Galangal EO was added to flurbiprofen microemulsion gel to increase bioavailability of flurbiprofen as anti-inflammatory drugs (Dong et al., 2020). Menthol and menthone can enhance penetration of ligustrazine hydrochloride, an anti-platelet aggregation, on transdermal absorption (Wang et al., 2017). In the oral administration, the main absorption site, the intestine, the absorption rate of EOs depends on lipophilicity, polarity, solubility, and molecular weight (Stevanovic et al., 2020). Due to different physiological and chemical condition of absorption site in GI tract, different rates of absorption can be happened in different terpenes. For example, sesquiterpene lactones and diterpene lactones, which have similar structures, have often been reported to have changeable pharmacokinetics, particularly unstable absorption and extensive metabolism. Although this compound is quite permeable in the intestinal epithelium, its absorption can be unstable due to the influence of gastrointestinal pH and efflux transporters (P-glycoprotein). P-gp expression increases from the proximal to the distal end of the small intestine causing a different absorption area of the P-gp substrate. Sesquiterpene lactone and diterpene lactone tend to be absorbed most effectively in the duodenum, followed by the jejunum, ileum and colon (Liu et al., 2019). In addition, differences in pH in the GI tract also affect terpene absorption, where the pH of the stomach, duodenum and colon is 1.2, 5.5 and 7.0, respectively (Wang et al., 2020).
Potential and limitation application of EO as natural food preservatives
One of the crucial matters in the food industry is to provide safe and healthy food. Thereby, to improve safety and extend the shelf life of food products, many synthetic preservatives have been permitted to be used in the food industry to prevent contamination of foodborne pathogens and/or to control spoilage (Yousefi, Khorshidian, & Hosseini, 2020). Nevertheless, following the consumers' preference to consume food products with natural substances, the food industry has also been exploiting the use of natural preservatives in their products. In this sense, EOs exhibit various activities including antibacterial, antioxidant and antifungal properties that are considered as alternative eco-friendly food preservatives (Pandey et al., 2017).
Several EOs and their constituents were approved by the United States Food and Drug Administration (FDA) as Generally Recognized as Safe (GRAS) status to be used as flavorings and food preservatives (Ben Jemaa et al., 2017). The registered EOs have GRAS status including basil, cinnamon, clove, coriander, ginger, lavandin, menthol, nutmeg, oregano, rose, sage, and thyme EOs. Likewise, the reported EO constituent comprise carvacrol, carvone, citral, cinnamaldehyde, eugenol, limonene, linalool, thymol, and vanillin. The main constituents of EOs such as terpenes, play an important role in food safety without affecting the quality (Falleh et al., 2020).
EOs have the potential to be used as a food preservative for various food products (Table 4) such as meat, breads, grains, fruits, vegetables, milk, and dairy products (Pandey et al., 2017). Various pathogens including E. coli, Clostridium spp., Salmonella spp., Campylobacter jejuni, Aeromonas hydrophila, S. cerevisiae, Penicillium expansum, and Listeria monocytogenes involved in the spoilage of food products. Among them, L. monocytogenes has been reported as the main causative agent of serious diseases in humans and animals (Yousefi et al., 2020).
Table 4.
Examples of conducted studies on preservation of food products by essential oils.
| Food product | Investigated essential oil | Main bioactive compounds | Key findings | Reference |
|---|---|---|---|---|
| Bread | Cymbopogon citratus (lemongrass) | (z)-Citral (62.58%), cis-verbenol (6.29%), geranyl acetate (5.36%), isoeugenol (4.52%), caryophyllene (3.91%) | The vapor of lemongrass EO (750 µl of EO/Lair) could inhibit Penicillium expansum inoculated on bread for 21 days at 20 °C. | (Mani López, Valle Vargas, Palou, & López Malo, 2018) |
| Cake | Thymus vulgaris | Thymol (53.57%), p-cymene (15.51%), limonene (7.14%), carvacrol (6.93%), trans-caryophyllene (3.26%), α-pinene (2.80%) | Addition of encapsulated thyme essential oil (0.60 mg/ml) in the cake formulation enhanced the shelf life of the product for 30 days of storage. | (Gonçalves et al., 2017) |
| Dry fruits | Mentha cardiaca L. | Carvone (59.6%), limonene (23.3%), β-myrcene (2.5%), 1,8-cineole (2.1%), β-bourbonene (1.5%), cis-dihydrocarvone (1.5%) | M. cardiaca EO showed strong antifungal activity (MIC 1.25 µl/ml) against biodeterioration fungi of dry fruits and potentially to reduce aflatoxin secretion. | (Dwivedy, Prakash, Chanotiya, Bisht, & Dubey, 2017) |
| Green gram seeds | Lippia alba | Geranial (36.94%), neral (29.32%), myrcene (18.65%), α-caryophyllene (2.07%), eugenol (1.82%), α-phelandrine (1.02%) | Utilization of a dose of 80 μl/0.25 L of Lippia alba oil in green gram seeds significantly inhibited the proliferation of fungal and production of aflatoxin B1 without affecting the seed germination rate during storage. | (Pandey, Sonker, & Singh, 2016) |
| Orangina fruit juice | Eucalyptus globulus essential oil | 1,8-cineole (94.03%), α-pinene (2.93%), γ-terpinene (1.93%), α-phellandrene (0.59%), β-pinene (0.20%), myrcene (0.19%) | EGEO (0.8 to 4 μl/ml) was effective and potent to reduce S. cerevisiae growth in the fruit juice of Orangina. | (Boukhatem et al., 2020) |
| Pineapple juice | Cymbopogon citratus D.C. Stapf. essential oil (CCEO) | Geraniol (46.16%), neral (31.74%), geranyl-acetate (4.34%), caryophyllene (2.02%), 6-methyl-5-hepten-2-ona (1.77%), dipentyl-ketone (1.06%), linalool (1.03%) | The incorporation of CCEO in pineapple juice at all tested concentrations (5, 2.5, and 1.25 μl/ml) caused a decrease in viable counts of E. coli, L. monocytogenes, and Salmonella enterica. | (Leite et al., 2016) |
| Chicken breast fillets | Zingiber officinale(Ginger) | α-Zingiberene (24.96%), β-sesquiphellandrene (12.74%), sesquisabinene hydrate (6.19%), camphene (5.90%), zingiberenol (4.26%), (E)-citral (3.93%), sabinene (3.75%), (E)-farnesene (3.73%), and italicene (3.21%) | Ginger essential oils nanoemulsion (6%) significantly reduced L. monocytogenes growth in refrigerated chicken filets during 12 days of storage. | (Noori, Zeynali, & Almasi, 2018) |
| Chicken meatballs | Ziziphora clinopodioides | Carvacrol (65.22%), thymol (19.51%), p-cymene (4.86%),and γ-terpinene (4.63%) | Z. clinopodioides EO (0.3%) efficiently inhibited the growth of L. monocytogenes in chicken meatball during 12 days storage 4 °C without any unfavorable sensory properties. | (Shahbazi, Karami, & Shavisi, 2018) |
| Dry Fermented Sausages | Juniperus communis L. | β-myrcene (14.12%), sabinene (9.51%), d,l-limonene (8.36%), 4-terpineol (6.88%), α-amorphene (5.43%), β-pinene (5.39%), caryophyllene (3.94%), p-cymene (3.92%), germacrene D (3.81%), | Juniperus communis EO can be utilized to control foodborne pathogens (L. monocytogenes, Salmonella spp., E. coli, and sulfite-reducing clostridia) in dry fermented sausages during storage period (225 days). The sample with 0.10 µl/g of Juniperus communis EO had untypical flavor. | (Tomović et al., 2020) |
| Ground beef | Melaleuca alternifolia (tea tree) | Terpinen-4-ol (43.1%), γ-terpinene (22.8%), α-terpinene (9.3%), α-terpineol (5.2%), terpinolene (3.5%), and α-pinene (3.0%) | The incorporation of 1.5% v/w Melaleuca alternifolia EO in ground beef was effective against L. monocytogenes with MIC and MBC values of 0.10 μl/g and 0.15 μl/ml, respectively. Melaleuca alternifolia EO was not significantly effective in the sample with the suspension at 1.5 × 108 CFU/ml. | (Silva, Figueiredo, Stamford, & Silva, 2019) |
| Minced beef meat | Citrus limon (lemon) | β-Pinene (25.44%), limonene (39.74%), linalool (2.16%), α-terpineol (7.30%), linalyl acetate (3.01%), acetate geranyl (3.03%), nerolidol (6.91%), acetate neryl (1.74%), and farnesol (4.28%). | The application of Citrus limon EO (0.06 and 0.312 mg/g) can be considered as a natural substance in controlling growth of L. monocytogenes during storage at 4 °C of minced beef meat. | (Ben Hsouna, Ben Halima, Smaoui, & Hamdi, 2017) |
| Sausages | Thyme essential oil | Thymol (38.2%), p-cymene (25.4%) and terpineol with γ-terpinene (16.2%), α-pinene (2.2%) | Thyme EO inhibited development of L. monocytogenes in sausages. The main constituents of thyme EO related to thymol that disrupts membrane cells and reduces the activity of ATPase of L. monocytogenes. | (Blanco-Lizarazo, Betancourt-Cortés, Lombana, Carrillo-Castro, & Sotelo-Díaz, 2017) |
| Turkey meat | Zataria multiflora Boiss and Bunium persicum Boiss | Zataria multiflora Boiss: carvacrol (51.55%), thymol (25.49%), p-cymene (5.23%), and γ-terpinene (4.44%). Bunium persicum Boiss: cumic aldehyde (38.39%), p-cymene (18.36%), and 2-caren-10-al (13.26%) | Nanoemulsion of BEO and ZEO could extend the shelf life of turkey meat to 9 days. The chitosan-loaded nanoemulsion containing ZEO 1% provided the best antimicrobial activity. Nanoemulsion containing BEO and ZEO significantly decreased the population of Salmonella Enteritidis and L. monocytogenes about 3 log CFU/g and 2 log CFU/g, respectively. | (Keykhosravy, Khanzadi, Hashemi, & Azizzadeh, 2020) |
| UHT milk | Syzygium aromaticum(clove), Cinnamomum zeylanicum (cinnamon), Myrtus communis (myrtle), and Lavandula stoechas (lavender) | Syzygium aromaticum (clove): eugenol (74.5%), caryophyllene (20.4%), aceteugenol (2.6%), β‐selinene (2%). Cinnamomum zeylanicum (cinnamon): Cinnamaldehyde (89%), camphene (3.5%), α‐terpineol (1.6%), 1,8‐cineole (1.6%). Myrtus communis (myrtle): Butanoic acid, 2-methyl, 2-methylbutyl ester (74.6%), 1,8‐cineole (11.5%), d‐limonene (6.5%), linalool (1.9%). Lavandula stoechas (lavender): camphor (35.4%), α‐fenchone (32.5%), 1,8‐cineole (7.4%), camphene (4.3%), α‐pinene (2.8%). | The combination of 2.4% S. aromaticum, 38.2% L. stoechas, and 59.4% C. zeylanicum significantly restricted the viability of E. coli to 1 × 106 CFU.ml−1. These findings are to be taken into consideration for a successful application of these essential oils as food preservatives in milk and dairy industries. | (Falleh et al., 2019) |
In a study by Khaleque et al. (2016), the effect of clove and cinnamon EO against L. monocytogenes in ground beef were determined (Khaleque et al., 2016). They found that 10% of crude and commercial clove EOs could be effective to decrease contamination and growth of L. monocytogenes. Furthermore, recent findings indicated that Eucalyptus globulus EO could reduce S. cerevisiae growth in the Orangina fruit juice (Boukhatem, Boumaiza, Nada, Rajabi, & Mousa, 2020).
Regardless, the main limitations for using EOs as food preservatives are their organoleptic effects (Falleh et al., 2020). In fact, EOs intense aroma sometimes adversely affects the organoleptic characteristics of food matrices. Several strategies have been considered to overcome this hurdle. Carpena et al. (2021) suggested that encapsulation is considered as a promising technique to minimize the EOs organoleptic impact. EOs may be used in active packaging as encapsulated molecules into microemulsions or nanoemulsions (Carpena et al., 2021). Active packaging is an innovative packaging technology with an extra function to extend product shelf life, ensure food quality and safety, and improve the appearance of the packaged food (Fang, Zhao, Warner, & Johnson, 2017). In addition, it also has been reported that Thymus capitatus (thyme EO) and its nanoemulsion were able to entirely inhibit Gram positive bacterial (S. aureus, Bacillus licheniformis, and Enterococcus hirae) in contaminated milk (Ben Jemaa et al., 2017).
Concluding remarks and future perspectives
The information compiled in this review demonstrates that EOs and their main active constituent(s) are crucial in pharmaceutical and medical industries with several potential activities such as anticancer, antimicrobial, anti-inflammatory, antioxidant, and anti-allergic. Nevertheless, more studies are necessarily required to understand the mechanism behind the biological properties of EOs. Major bioactive compounds of EOs need to be clearly elucidated to improve their potential efficiencies in disease management. In addition to their function in health, EOs show high potential to be used as natural food preservatives in the food industry. However, organoleptic impact and probable contamination of EOs in food products have been suggested to limit their use. Indeed, careful and thorough investigations are urgently needed to reduce the disadvantages of EOs to meet the needs for food industry applications. Accordingly, scientific efforts need to be further initiated and developed to evaluate the effects of incorporating EOs into packaging systems and to ensure their safety for the consumers and the environment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Funding for open access charge: Universidade de Vigo/CISUG.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100217.
Contributor Information
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd.
Firzan Nainu, Email: firzannainu@unhas.ac.id.
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abbas M.M., Kandil Y.İ., Abbas M.A. R-(-)-carvone attenuated doxorubicin induced cardiotoxicity in vivo and potentiated its anticancer toxicity in vitro. Balkan Medical Journal. 2020;37:98–103. doi: 10.4274/balkanmedj.galenos.2019.2019.7.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahi M., Karimpour H., Monsef-Esfehani H.R. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacological Research. 2003;48:31–35. [PubMed] [Google Scholar]
- Álvarez-Martínez F.J., Barrajón-Catalán E., Herranz-López M., Micol V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine. 2021;90 doi: 10.1016/j.phymed.2021.153626. [DOI] [PubMed] [Google Scholar]
- Asl A.H., Khajenoori M. CRC Press; Boca Raton: 2021. Green extraction in separation technology. [Google Scholar]
- Bai X., Tang J. Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Natural Product Communications. 2020;15 1934578X20961189. [Google Scholar]
- Bansod S., Chilvery S., Saifi M.A., Das T.J., Tag H., Godugu C. Borneol protects against cerulein-induced oxidative stress and inflammation in acute pancreatitis mice model. Environmental Toxicology. 2021;36:530–539. doi: 10.1002/tox.23058. [DOI] [PubMed] [Google Scholar]
- Beik A., Najafipour H., Joukar S., Rajabi S., Iranpour M., Kordestani Z. Perillyl alcohol suppresses monocrotaline-induced pulmonary arterial hypertension in rats via anti-remodeling, anti-oxidant, and anti-inflammatory effects. Clinical and Experimental Hypertension. 2021;43:270–280. doi: 10.1080/10641963.2020.1860080. [DOI] [PubMed] [Google Scholar]
- Ben Hsouna A., Ben Halima N., Smaoui S., Hamdi N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids in Health and Disease. 2017;16 doi: 10.1186/s12944-017-0487-5. 146-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Jemaa M., Falleh H., Neves M.A., Isoda H., Nakajima M., Ksouri R. Quality preservation of deliberately contaminated milk using thyme free and nanoemulsified essential oils. Food Chemistry. 2017;217:726–734. doi: 10.1016/j.foodchem.2016.09.030. [DOI] [PubMed] [Google Scholar]
- Bhavaniramya S., Vishnupriya S., Al-Aboody M.S., Vijayakumar R., Baskaran D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain & Oil Science and Technology. 2019;2:49–55. [Google Scholar]
- Blanco-Lizarazo C.M., Betancourt-Cortés R., Lombana A., Carrillo-Castro K., Sotelo-Díaz I. Listeria monocytogenes behaviour and quality attributes during sausage storage affected by sodium nitrite, sodium lactate and thyme essential oil. Food Science and Technology International. 2017;23:277–288. doi: 10.1177/1082013216686464. [DOI] [PubMed] [Google Scholar]
- Boukhatem M.N., Boumaiza A., Nada H.G., Rajabi M., Mousa S.A. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (Orangina Fruit Juice) Applied Sciences. 2020;10:5581. [Google Scholar]
- Burt S. Essential oils: Their antibacterial properties and potential applications in foods–A review. International Journal of Food Microbiology. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Campos, C. A., Lima, B. S., Trindade, G. G. G., Souza, E. P. B. S. S., Mota, D. S. A., Heimfarth, L., … Thangaraj, P. 2019. Anti-hyperalgesic and anti-inflammatory effects of citral with β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes in animal models. Life Sciences, 229, 139-148. [DOI] [PubMed]
- Carpena M., Nuñez-Estevez B., Soria-Lopez A., Garcia-Oliveira P., Prieto M.A. Essential oils and their application on active packaging systems: A review. Resources. 2021;10:7. [Google Scholar]
- Chaves-Quirós C., Usuga-Usuga J.-S., Morales-Uchima S.-M., Tofiño-Rivera A.-P., Tobón-Arroyave S.-I., Martínez-Pabón M.-C. Assessment of cytotoxic and antimicrobial activities of two components of Cymbopogon citratus essential oil. Journal of Clinical and Experimental Dentistry. 2020;12:e749–e754. doi: 10.4317/jced.56863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J.…Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.G., Kim T.H., Kim S.-H., Kim J.A. Anti-allergic Inflammatory Triterpenoids Isolated from the Spikes of Prunella vulgaris. Natural Product Communications. 2016;11 1934578X1601100111. [PubMed] [Google Scholar]
- Contant C., Rouabhia M., Loubaki L., Chandad F., Semlali A. Anethole induces anti-oral cancer activity by triggering apoptosis, autophagy and oxidative stress and by modulation of multiple signaling pathways. Scientific Reports. 2021;11:13087. doi: 10.1038/s41598-021-92456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M.D.S., Rocha J.E., Campina F.F., Silva A.R.P., Da Cruz R.P., Pereira R.L.S.…Coutinho H.D.M. Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. European Journal of Pharmaceutical Sciences. 2019;128:158–161. doi: 10.1016/j.ejps.2018.11.036. [DOI] [PubMed] [Google Scholar]
- Currie G.M. Pharmacology, Part 2: Introduction to Pharmacokinetics. Journal of Nuclear Medicine Technology. 2018;46:221–230. doi: 10.2967/jnmt.117.199638. [DOI] [PubMed] [Google Scholar]
- Cutillas A.-B., Carrasco A., Martinez-Gutierrez R., Tomas V., Tudela J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Antienzymatic, and Antimicrobial Bioactivities. Chemistry & Biodiversity. 2017;14 doi: 10.1002/cbdv.201700102. [DOI] [PubMed] [Google Scholar]
- De Christo Scherer M.M., Marques F.M., Figueira M.M., Peisino M.C.O., Schmitt E.F.P., Kondratyuk T.P.…Fronza M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. Journal of Tissue Viability. 2019;28:94–99. doi: 10.1016/j.jtv.2019.02.003. [DOI] [PubMed] [Google Scholar]
- De Souza M.C., Vieira A.J., Beserra F.P., Pellizzon C.H., Nóbrega R.H., Rozza A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine. 2019;53:37–42. doi: 10.1016/j.phymed.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Devi K.P., Nisha S.A., Sakthivel R., Pandian S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. Journal of Ethnopharmacology. 2010;130:107. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Dong J., Zhu X.-M., Wu F.-Y., Yang B.-Q., Feng H., Dong Y.-F.…Chen J. Development of galangal essential oil-based microemulsion gel for transdermal delivery of flurbiprofen: Simultaneous permeability evaluation of flurbiprofen and 1,8-cineole. Drug Development and Industrial Pharmacy. 2020;46:91–100. doi: 10.1080/03639045.2019.1706548. [DOI] [PubMed] [Google Scholar]
- Dordević S., Petrović S., Dobrić S., Milenković M., Vucićević D., Zizić S., Kukić J. Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. Journal of Ethnopharmacology. 2007;109:458–463. doi: 10.1016/j.jep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Dwivedy A.K., Prakash B., Chanotiya C.S., Bisht D., Dubey N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food and Chemical Toxicology. 2017;106:175–184. doi: 10.1016/j.fct.2017.05.043. [DOI] [PubMed] [Google Scholar]
- Dzoyem J.P., Melong R., Tsamo A.T., Tchinda A.T., Kapche D.G.W.F., Ngadjui B.T.…Eloff J.N. Cytotoxicity, antimicrobial and antioxidant activity of eight compounds isolated from Entada abyssinica (Fabaceae) BMC Research Notes. 2017;10:118. doi: 10.1186/s13104-017-2441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid A.M., Hawash M. Biological evaluation of Safrole oil and Safrole oil Nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complementary Medicine and Therapies. 2021;21:159. doi: 10.1186/s12906-021-03324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hachlafi N., Lakhdar F., Khouchlaa A., Bakrim S., El Omari N., Balahbib A.…Bouyahya A. Health benefits and pharmacological properties of hinokitiol. Processes. 2021;9:1680. [Google Scholar]
- El-Sherif A., El-Sherif S., Taylor A.H., Ayakannu T. Ovarian Cancer: Lifestyle, Diet and Nutrition. Nutrition and Cancer. 2021;73:1092–1107. doi: 10.1080/01635581.2020.1792948. [DOI] [PubMed] [Google Scholar]
- Essien S.O., Young B., Baroutian S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends in Food Science & Technology. 2020;97:156–169. [Google Scholar]
- Ezz-Eldin Y.M., Aboseif A.A., Khalaf M.M. Potential anti-inflammatory and immunomodulatory effects of carvacrol against ovalbumin-induced asthma in rats. Life Sciences. 2020;242 doi: 10.1016/j.lfs.2019.117222. [DOI] [PubMed] [Google Scholar]
- Falleh H., Ben Jemaa M., Djebali K., Abid S., Saada M., Ksouri R. Application of the mixture design for optimum antimicrobial activity: Combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. Journal of Food Processing and Preservation. 2019;43 [Google Scholar]
- Falleh H., Ben Jemaa M., Saada M., Ksouri R. Essential oils: A promising eco-friendly food preservative. Food Chemistry. 2020;330 doi: 10.1016/j.foodchem.2020.127268. [DOI] [PubMed] [Google Scholar]
- Fang Z., Zhao Y., Warner R.D., Johnson S.K. Active and intelligent packaging in meat industry. Trends in Food Science & Technology. 2017;61:60–71. [Google Scholar]
- Farag M.R., Alagawany M., Tufarelli V. In vitro antioxidant activities of resveratrol, cinnamaldehyde and their synergistic effect against cyadox-induced cytotoxicity in rabbit erythrocytes. Drug and Chemical Toxicology. 2017;40:196–205. doi: 10.1080/01480545.2016.1193866. [DOI] [PubMed] [Google Scholar]
- Gallucci M.N., Oliva M., Casero C., Dambolena J., Luna A., Zygadlo J., Demo M. Antimicrobial combined action of terpenes against the food-borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour and Fragrance Journal. 2009;24:348–354. [Google Scholar]
- Gao S., Liu G., Li J., Chen J., Li L., Li Z.…Zhang S. Antimicrobial Activity of Lemongrass Essential Oil (Cymbopogon flexuosus) and Its Active Component Citral Against Dual-Species Biofilms of Staphylococcus aureus and Candida Species. Frontiers in Cellular and Infection Microbiology. 2020;10 doi: 10.3389/fcimb.2020.603858. 603858-603858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti J., Bursać Kovačević D., Putnik P., Gabrić D., Bilušić T., Krešić G.…Režek Jambrak A. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Research International. 2018;113:245–262. doi: 10.1016/j.foodres.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Gonçalves N.D., Pena F.D.L., Sartoratto A., Derlamelina C., Duarte M.C.T., Antunes A.E.C., Prata A.S. Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Research International. 2017;96:154–160. doi: 10.1016/j.foodres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Guimarães A.C., Meireles L.M., Lemos M.F., Guimarães M.C.C., Endringer D.C., Fronza M., Scherer R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24:2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günes-Bayir A., Kiziltan H.S., Kocyigit A., Güler E.M., Karatas E., Toprak A. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anti-Cancer Drugs. 2017;28 doi: 10.1097/CAD.0000000000000491. [DOI] [PubMed] [Google Scholar]
- Han H.D., Cho Y.-J., Cho S.K., Byeon Y., Jeon H.N., Kim H.-S.…Lee J.-W. Linalool-incorporated nanoparticles as a novel anticancer agent for epithelial ovarian carcinoma. Molecular Cancer Therapeutics. 2016;15:618. doi: 10.1158/1535-7163.MCT-15-0733-T. [DOI] [PubMed] [Google Scholar]
- Han N.-R., Moon P.-D., Nam S.-Y., Ryu K.-J., Yoou M.-S., Choi J.-H.…Jeong H.-J. Inhibitory effects of atractylone on mast cell-mediated allergic reactions. Chemico-Biological Interactions. 2016;258:59–68. doi: 10.1016/j.cbi.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Hirota R., Ngatu N.R. In: Occupational and Environmental Skin Disorders: Epidemiology, Current Knowledge and Perspectives for Novel Therapies. Ngatu N.R., Ikeda M., editors. Springer Singapore; Singapore: 2018. Experimental Anti-Allergic and Immunomodulatory Effects of Vernonia amygdalina-Derived Biomaterials, Vernodalin and Its Leaf Extracts. [Google Scholar]
- Huang J., Yang L., Zou Y., Luo S., Wang X., Liang Y.…Wei Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiologica. 2021;66:59–67. doi: 10.1007/s12223-020-00818-0. [DOI] [PubMed] [Google Scholar]
- Hyldgaard M., Mygind T., Meyer R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Takizawa T., Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. Journal of Antimicrobial Chemotherapy. 2001;47:565–573. doi: 10.1093/jac/47.5.565. [DOI] [PubMed] [Google Scholar]
- Isidore E., Karim H., Ioannou I. Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants. 2021;10:942. doi: 10.3390/antiox10060942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A.U.S., Hellman B., Nyberg F., Amir N., Jayaraj R.L., Petroianu G., Adem A. Myrcene attenuates renal inflammation and oxidative stress in the adrenalectomized rat model. Molecules. 2020;25:4492. doi: 10.3390/molecules25194492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isman M.B., Machial C.M. In: Advances in Phytomedicine. Rai M., Carpinella M.C., editors. Elsevier; 2006. Chapter 2 Pesticides based on plant essential oils: from traditional practice to commercialization. [Google Scholar]
- Jäger W., Höferl M. In: Handbook of Essential Oils. 3rd ed. Başer K.H.C., Buchbauer G., editors. CRC Press; Boca Raton: 2020. Metabolism of Terpenoids in Animal Models and Humans. [Google Scholar]
- Jantan I., Ping W.O., Visuvalingam S.D., Ahmad N.W. Larvicidal activity of the essential oils and methanol extracts of Malaysian plants on Aedes aegypti. Pharmaceutical Biology. 2003;41:234–236. [Google Scholar]
- Jayakumar T., Liu C.-H., Wu G.-Y., Lee T.-Y., Manubolu M., Hsieh C.-Y.…Sheu J.-R. Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis. International Journal of Molecular Sciences. 2018;19:939. doi: 10.3390/ijms19040939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Luo M., Ma W., Ma S., Wang Y., Zhang K. Protective Effects of 1,8-Cineole Microcapsules against inflammation and gut microbiota imbalance associated weight loss induced by heat stress in broiler chicken. Frontiers in Pharmacology. 2021;11 doi: 10.3389/fphar.2020.585945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Zhang M., Lu J., Duan X., Chen J., Liu Y.…Lou H. Hinokitiol chelates intracellular iron to retard fungal growth by disturbing mitochondrial respiration. Journal of Advanced Research. 2021 doi: 10.1016/j.jare.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Caballero S., Davidov-Pardo G. In: Advances in Food and Nutrition Research. Lim L.-T., Rogers M., editors. Academic Press; 2019. Chapter Six - Bioavailability of nanotechnology-based bioactives and nutraceuticals. [DOI] [PubMed] [Google Scholar]
- Keykhosravy K., Khanzadi S., Hashemi M., Azizzadeh M. Chitosan-loaded nanoemulsion containing Zataria Multiflora Boiss and Bunium persicum Boiss essential oils as edible coatings: Its impact on microbial quality of turkey meat and fate of inoculated pathogens. International Journal of Biological Macromolecules. 2020;150:904–913. doi: 10.1016/j.ijbiomac.2020.02.092. [DOI] [PubMed] [Google Scholar]
- Khaleque M.A., Keya C.A., Hasan K.N., Hoque M.M., Inatsu Y., Bari M.L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT. 2016;74:219–223. [Google Scholar]
- Khoshnazar M., Parvardeh S., Bigdeli M.R. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. Journal of Stroke and Cerebrovascular Diseases. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104977. [DOI] [PubMed] [Google Scholar]
- Kim H.-S., Han E.J., Fernando I.P.S., Sanjeewa K.K.A., Jayawardena T.U., Kim H.-J.…Ahn G. Anti-allergy effect of mojabanchromanol isolated from Sargassum horneri in bone marrow-derived cultured mast cells. Algal Research. 2020;48 [Google Scholar]
- Kim T., Song B., Cho K.S., Lee I.-S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. International Journal of Molecular Sciences. 2020;21:2187. doi: 10.3390/ijms21062187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Sato H., Yorita M., Nakayama H., Miyazato H., Sugimoto K., Jippo T. Inhibitory effects of geranium essential oil and its major component, citronellol, on degranulation and cytokine production by mast cells. Bioscience, Biotechnology, and Biochemistry. 2016;80:1172–1178. doi: 10.1080/09168451.2016.1148573. [DOI] [PubMed] [Google Scholar]
- Kohlert C., Van Rensen I., März R., Schindler G., Graefe E.U., Veit M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Medica. 2000;66:495–505. doi: 10.1055/s-2000-8616. [DOI] [PubMed] [Google Scholar]
- Kumar A., Sawhney G., Kumar Nagar R., Chauhan N., Gupta N., Kaul A.…Yadav G. Evaluation of the immunomodulatory and anti-inflammatory activity of Bakuchiol using RAW 264.7 macrophage cell lines and in animal models stimulated by lipopolysaccharide (LPS) International Immunopharmacology. 2021;91 doi: 10.1016/j.intimp.2020.107264. [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Moon J.-H., Lee Y.-J., Park S.-Y. SIRT1, a Class III Histone Deacetylase, Regulates LPS-Induced Inflammation in Human Keratinocytes and Mediates the Anti-Inflammatory Effects of Hinokitiol. Journal of Investigative Dermatology. 2017;137:1257–1266. doi: 10.1016/j.jid.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Leite C.J.B., De Sousa J.P., Da Costa Medeiros J.A., Da Conceição M.L., Dos Santos Falcão-Silva V., De Souza E.L. Inactivation of Escherichia coli, Listeria monocytogenes, and Salmonella enteritidis by Cymbopogon citratus D.C. Stapf. Essential Oil in Pineapple Juice. Journal of Food Protection. 2016;79:213–219. doi: 10.4315/0362-028X.JFP-15-245. [DOI] [PubMed] [Google Scholar]
- Li H., Liu J., Liu C.-F., Li H., Luo J., Fang S.…Lin S. Design, synthesis, and biological evaluation of membrane-active bakuchiol derivatives as effective broad-spectrum antibacterial agents. Journal of Medicinal Chemistry. 2021;64:5603–5619. doi: 10.1021/acs.jmedchem.0c02059. [DOI] [PubMed] [Google Scholar]
- Li X.-J., Yang Y.-J., Li Y.-S., Zhang W.K., Tang H.-B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. Journal of Ethnopharmacology. 2016;179:22–26. doi: 10.1016/j.jep.2015.12.039. [DOI] [PubMed] [Google Scholar]
- Lin L., Long N., Qiu M., Liu Y., Sun F., Dai M. The inhibitory efficiencies of geraniol as an anti-inflammatory, antioxidant, and antibacterial, natural agent against methicillin-resistant Staphylococcus aureus infection in vivo. Infection and drug resistance. 2021;14:2991–3000. doi: 10.2147/IDR.S318989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Cai J., Chen H., Zhong Q., Hou Y., Chen W., Chen W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microbial Pathogenesis. 2020;141 doi: 10.1016/j.micpath.2020.103980. [DOI] [PubMed] [Google Scholar]
- Liu J.Y., Geng T., Duan K., Gao X., Huang C.J., Wang J.J.…Xiao W. Cellular pharmacokinetics and pharmacodynamics mechanisms of ginkgo diterpene lactone and its modulation of P-glycoprotein expression in human SH-SY5Y cells. Biomedical Chromatography. 2019;33 doi: 10.1002/bmc.4692. [DOI] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Liu B. Anti-tumor effects of bakuchiol on human gastric carcinoma cell lines are mediated through PI3K/AKT and MAPK signaling pathways. Molecular Medicine Reports. 2017;16:8977–8982. doi: 10.3892/mmr.2017.7696. [DOI] [PubMed] [Google Scholar]
- Ma J., Li J., Wang K.S., Mi C., Piao L.X., Xu G.H.…Jin X. Perillyl alcohol efficiently scavenges activity of cellular ROS and inhibits the translational expression of hypoxia-inducible factor-1α via mTOR/4E-BP1 signaling pathways. International Immunopharmacology. 2016;39:1–9. doi: 10.1016/j.intimp.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Mani López E., Valle Vargas G.P., Palou E., López Malo A. Penicillium expansum inhibition on bread by lemongrass essential oil in vapor phase. Journal of Food Protection. 2018;81:467–471. doi: 10.4315/0362-028X.JFP-17-315. [DOI] [PubMed] [Google Scholar]
- Martins, B. X., Arruda, R. F., Costa, G. A., Jerdy, H., De Souza, S. B., Santos, J. M., De Freitas, W. R., Kanashiro, M. M., De Carvalho, E. C. Q., Sant'anna, N. F., Antunes, F., Martinez-Zaguilan, R., Souad, S., Okorokova-Façanha, A. L. & Façanha, A. R. 2019. Myrtenal-induced V-ATPase inhibition - A toxicity mechanism behind tumor cell death and suppressed migration and invasion in melanoma. Biochimica et Biophysica Acta (BBA) - General Subjects, 1863, 1-12. [DOI] [PubMed]
- Mediratta P.K., Sharma K.K., Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. Journal of Ethnopharmacology. 2002;80:15–20. doi: 10.1016/s0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Mimica-Dukić N., Bozin B., Soković M., Mihajlović B., Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Medica. 2003;69:413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- Modena B.D., Dazy K., White A.A. Emerging concepts: Mast cell involvement in allergic diseases. Translational Research. 2016;174:98–121. doi: 10.1016/j.trsl.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Moon J.-K., Shibamoto T. Antioxidant assays for plant and food components. Journal of Agricultural and Food Chemistry. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- Mouhid L., Corzo-Martínez M., Torres C., Vázquez L., Reglero G., Fornari T., Ramírez De Molina A. Improving in vivo efficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems. Journal of Oncology. 2017;2017:7351976. doi: 10.1155/2017/7351976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J.-Y., Li R., Jiang Z.-T., Wang Y., Tan J., Tang S.-H., Zhang Y. Screening and evaluation of radical scavenging active compounds in the essential oil from Magnolia biondii Pamp by electronic nose coupled with chemical methodology. Industrial Crops and Products. 2020;144 [Google Scholar]
- Noori S., Zeynali F., Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. [Google Scholar]
- Oldfield E., Lin F.Y. Terpene biosynthesis: Modularity rules. Angewandte Chemie (International ed. in English) 2012;51:1124–1137. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A.K., Kumar P., Singh P., Tripathi N.N., Bajpai V.K. Essential oils: Sources of antimicrobials and food preservatives. Frontiers in Microbiology. 2017;7 doi: 10.3389/fmicb.2016.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A.K., Sonker N., Singh P. Efficacy of some essential oils against Aspergillus flavus with special reference to lippia alba oil an inhibitor of fungal proliferation and aflatoxin B1 production in green gram seeds during storage. Journal of Food Science. 2016;81:M928–M934. doi: 10.1111/1750-3841.13254. [DOI] [PubMed] [Google Scholar]
- Pavlić B., Teslić N., Zengin G., Đurović S., Rakić D., Cvetanović A.…Zeković Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.127724. [DOI] [PubMed] [Google Scholar]
- Perricone M., Arace E., Corbo M.R., Sinigaglia M., Bevilacqua A. Bioactivity of essential oils: A review on their interaction with food components. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.00076. 76-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perveen S. In: Terpenes and Terpenoids. Perveen S., Al-Taweel A., editors. IntechOpen; 2018. Introductory Chapter: Terpenes and Terpenoids. [Google Scholar]
- Pinheiro-Neto F.R., Lopes E.M., Acha B.T., Gomes L.D.S., Dias W.A., Reis Filho A.C.D.…Almeida F.R.D.C. α-Phellandrene exhibits antinociceptive and tumor-reducing effects in a mouse model of oncologic pain. Toxicology and Applied Pharmacology. 2021;418 doi: 10.1016/j.taap.2021.115497. [DOI] [PubMed] [Google Scholar]
- Potočnjak I., Gobin I., Domitrović R. Carvacrol induces cytotoxicity in human cervical cancer cells but causes cisplatin resistance: Involvement of MEK-ERK activation. Phytotherapy Research. 2018;32:1090–1097. doi: 10.1002/ptr.6048. [DOI] [PubMed] [Google Scholar]
- Pudełek M., Catapano J., Kochanowski P., Mrowiec K., Janik-Olchawa N., Czyż J., Ryszawy D. Therapeutic potential of monoterpene α-thujone, the main compound of Thuja occidentalis L. essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia. 2019;134:172–181. doi: 10.1016/j.fitote.2019.02.020. [DOI] [PubMed] [Google Scholar]
- Querio G., Antoniotti S., Foglietta F., Bertea C.M., Canaparo R., Gallo M.P., Levi R. Chamazulene Attenuates ROS Levels in Bovine Aortic Endothelial Cells Exposed to High Glucose Concentrations and Hydrogen Peroxide. Frontiers in Physiology. 2018;9 doi: 10.3389/fphys.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Filho J., Da Silva Brandi J., Ferreira Costa H., De Paula C., Medeiros K., Alves Leite J.…Regina Piuvezam M. Carvone Enantiomers Differentially Modulate IgE-Mediated Airway Inflammation in Mice. International Journal of Molecular Sciences. 2020;21:9209. doi: 10.3390/ijms21239209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenak-Kladniew B., Castro A., Stärkel P., De Saeger C., García De Bravo M., Crespo R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sciences. 2018;199:48–59. doi: 10.1016/j.lfs.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D.…Zhu C. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Llorente D., Cañada-Barcala A., Álvarez-Torrellas S., Águeda V.I., García J., Larriba M. A review of the use of eutectic solvents, terpenes and terpenoids in liquid–liquid extraction processes. Processes. 2020;8:1220. [Google Scholar]
- Roettger A., Bruchhage K.-L., Drenckhan M., Ploetze-Martin K., Pries R., Wollenberg B. Inhibitory Effect of 1,8-Cineol on β-Catenin Regulation, WNT11 Expression, and Cellular Progression in HNSCC. Frontiers in oncology. 2017;7 doi: 10.3389/fonc.2017.00092. 92-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneme E.F., Dos Santos D.C., Silva E.M.R., Franco Y.E.M., Longato G.B. Pharmacological and therapeutic potential of myristicin: A literature review. Molecules. 2021;26:5914. doi: 10.3390/molecules26195914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi Y., Karami N., Shavisi N. Effect of Ziziphora clinopodioides essential oil on shelf life and fate of Listeria monocytogenes and Staphylococcus aureus in refrigerated chicken meatballs. Journal of Food Safety. 2018;38 [Google Scholar]
- Sheikh B.Y., Sarker M.M.R., Kamarudin M.N.A., Mohan G. Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomedicine & Pharmacotherapy. 2017;96:834–846. doi: 10.1016/j.biopha.2017.10.038. [DOI] [PubMed] [Google Scholar]
- Shu H., Zhang W., Yun Y., Chen W., Zhong Q., Hu Y.…Chen W. Metabolomics study on revealing the inhibition and metabolic dysregulation in Pseudomonas fluorescens induced by 3-carene. Food Chemistry. 2020;329 doi: 10.1016/j.foodchem.2020.127220. [DOI] [PubMed] [Google Scholar]
- Silva J.K.R.D., Figueiredo P.L.B., Byler K.G., Setzer W.N. Essential Oils as Antiviral Agents. Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In-Silico Investigation. International Journal of Molecular Sciences. 2020;21:3426. doi: 10.3390/ijms21103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C.D.S., Figueiredo H.M.D., Stamford T.L.M., Silva L.H.M.D. Inhibition of Listeria monocytogenes by Melaleuca alternifolia (tea tree) essential oil in ground beef. International Journal of Food Microbiology. 2019;293:79–86. doi: 10.1016/j.ijfoodmicro.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Silva G.L., Luft C., Lunardelli A., Amaral R.H., Melo D.A., Donadio M.V.…De Oliveira J.R. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. Anais da Academia Brasileira de Cienc. 2015;87:1397–1408. doi: 10.1590/0001-3765201520150056. [DOI] [PubMed] [Google Scholar]
- Silva B.I.M., Nascimento E.A., Silva C.J., Silva T.G., Aguiar J.S. Anticancer activity of monoterpenes: A systematic review. Molecular Biology Reports. 2021;48:5775–5785. doi: 10.1007/s11033-021-06578-5. [DOI] [PubMed] [Google Scholar]
- Şimşek M., Duman R. Investigation of Effect of 1,8-cineole on Antimicrobial Activity of Chlorhexidine Gluconate. Pharmacognosy Research. 2017;9:234–237. doi: 10.4103/0974-8490.210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephane F.F.Y., Jules B.K.J. In: Essential Oils - Bioactive Compounds, New Perspectives and Applications. Oliveira M.S.D., Costa W.A.D., Silva S.G., editors. IntechOpen; 2020. Terpenoids as Important Bioactive Constituents of Essential Oils. [Google Scholar]
- Stevanovic Z.D., Sieniawska E., Glowniak K., Obradovic N., Pajic-Lijakovic I. Natural Macromolecules as Carriers for Essential Oils: From Extraction to Biomedical Application. Frontiers in Bioengineering and Biotechnology. 2020:8. doi: 10.3389/fbioe.2020.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Suzuki R., Inoue Y., Murata I., Nomura H., Isshiki Y., Hashimoto M.…Kanamoto I. Preparation, characterization, and study of the antimicrobial activity of a Hinokitiol-copper(II)/γ-cyclodextrin ternary complex. Journal of Molecular Structure. 2019;1194:19–27. [Google Scholar]
- Syed I., Sarkar P. Ultrasonication-assisted formation and characterization of geraniol and carvacrol-loaded emulsions for enhanced antimicrobial activity against food-borne pathogens. Chemical Papers. 2018;72:2659–2672. [Google Scholar]
- Tetali S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta. 2019;249:1–8. doi: 10.1007/s00425-018-3056-x. [DOI] [PubMed] [Google Scholar]
- Thakur N., Raigond P., Singh Y., Mishra T., Singh B., Lal M.K., Dutt S. Recent updates on bioaccessibility of phytonutrients. Trends in Food Science & Technology. 2020;97:366–380. [Google Scholar]
- Tomović V., Šojić B., Savanović J., Kocić-Tanackov S., Pavlić B., Jokanović M.…Vujadinović D. New Formulation towards Healthier Meat Products: Juniperus communis L. Essential Oil as Alternative for Sodium Nitrite in Dry Fermented Sausages. Foods. 2020;9:1066. doi: 10.3390/foods9081066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triaux Z., Petitjean H., Marchioni E., Steyer D., Marcic C. Comparison of headspace, hydrodistillation and pressurized liquid extraction of terpenes and terpenoids from food matrices—Qualitative and quantitative analysis. Journal of Analytical Chemistry. 2021;76:284–295. [Google Scholar]
- Uwineza P.A., Waśkiewicz A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules. 2020;25:3847. doi: 10.3390/molecules25173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-Y., Chen Y.-W., Hou C.-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. International Journal of Food Properties. 2019;22:230–238. [Google Scholar]
- Wang J., Dong C., Song Z., Zhang W., He X., Zhang R.…Yuan C. Monocyclic monoterpenes as penetration enhancers of ligustrazine hydrochloride for dermal delivery. Pharmaceutical Development and Technology. 2017;22:571–577. doi: 10.1080/10837450.2016.1189936. [DOI] [PubMed] [Google Scholar]
- Wang W., Ren Z., Wang L., Cai Y., Ma H., Fang L., Su J. Nanoparticle-stabilized encapsulation of borneol and citral: Physicochemical characteristics, storage stability, and enhanced antibacterial activities. Journal of Food Science. 2021;86:4554–4565. doi: 10.1111/1750-3841.15910. [DOI] [PubMed] [Google Scholar]
- Wang W., Yan X., Li Q., Chen Z., Wang Z., Hu H. Adapted nano-carriers for gastrointestinal defense components: Surface strategies and challenges. Nanomedicine: Nanotechnology, Biology and Medicine. 2020;29 doi: 10.1016/j.nano.2020.102277. [DOI] [PubMed] [Google Scholar]
- Warnatsch A., Tsourouktsoglou T.-D., Branzk N., Wang Q., Reincke S., Herbst S.…Papayannopoulos V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity. 2017;46:421–432. doi: 10.1016/j.immuni.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Gao X., Wang L., Yang M., Xie R. Bakuchiol ameliorates cerebral ischemia-reperfusion injury by modulating NLRP3 inflammasome and Nrf2 signaling. Respiratory Physiology & Neurobiology. 2021;292 doi: 10.1016/j.resp.2021.103707. [DOI] [PubMed] [Google Scholar]
- Xu Q., Li M., Yang M., Yang J., Xie J., Lu X.…Chen W. α-pinene regulates miR-221 and induces G(2)/M phase cell cycle arrest in human hepatocellular carcinoma cells. Bioscience Reports. 2018;38 doi: 10.1042/BSR20180980. BSR20180980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Chen H., Chen H., Zhong B., Luo X., Chun J. Antioxidant and anticancer activities of essential oil from gannan navel orange peel. Molecules (Basel, Switzerland) 2017;22:1391. doi: 10.3390/molecules22081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Chen X., Li Y., Guo S., Wang Z., Yu X. Advances in pharmacological activities of terpenoids. Natural Product Communications. 2020;15 1934578X20903555. [Google Scholar]
- Yang J., Choi W.-S., Kim K.-J., Eom C.-D., Park M.-J. Investigation of active anti-inflammatory constituents of essential oil from Pinus koraiensis (Sieb. et Zucc.) Wood in LPS-Stimulated RBL-2H3 Cells. Biomolecules. 2021;11:817. doi: 10.3390/biom11060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Li C., Xue W., Huang L., Wang Z. Natural immunomodulating substances used for alleviating food allergy. Critical Reviews in Food Science and Nutrition. 2021:1–19. doi: 10.1080/10408398.2021.1975257. [DOI] [PubMed] [Google Scholar]
- Yang L., Liao M. Influence of myrcene on inflammation, matrix accumulation in the kidney tissues of streptozotocin-induced diabetic rat. Saudi Journal of Biological Sciences. 2021;28:5555–5560. doi: 10.1016/j.sjbs.2020.11.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi M., Khorshidian N., Hosseini H. Potential Application of Essential Oils for Mitigation of Listeria monocytogenes in Meat and Poultry Products. Frontiers Nutrition. 2020;7 doi: 10.3389/fnut.2020.577287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahi M.R., El Hattab M., Liang H., Yuan Q. Enhancing the antimicrobial activity of d-limonene nanoemulsion with the inclusion of ε-polylysine. Food Chemistry. 2017;221:18–23. doi: 10.1016/j.foodchem.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Zhang C., Hong K. Production of Terpenoids by Synthetic Biology Approaches. Frontiers in Bioengineering and Biotechnology. 2020;8 doi: 10.3389/fbioe.2020.00347. 347-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-H., Sun H.-L., Chen S.-Y., Zeng L., Wang T.-T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Botanical Studies. 2017;58:13. doi: 10.1186/s40529-017-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.-L., Zhang L.-F., Xu J.-G., Hu Q.-P. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food & Nutrition Research. 2017;61:1353356. doi: 10.1080/16546628.2017.1353356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.-D., Jiang L.-L., Li H.-Y., Yan P.-F., Zhang Y.-L. Chemical components and pharmacological activities of terpene natural products from the genus paeonia. Molecules. 2016;21:1362. doi: 10.3390/molecules21101362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.