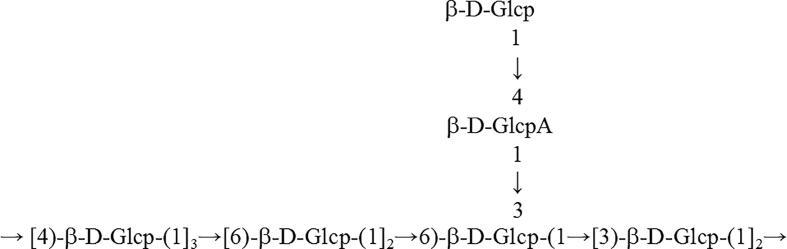Highlights
-
•
Ganoderma lucidum crude polysaccharide (GLP) exhibited protective effect on liver damage in mice caused by restraint stress through improving oxidative status.
-
•
Two polysaccharides, including a neutral β-glucan and an acidic β-glucan containing glucuronic acid were purified from GLP by anion-exchange chromatography (AEC) and gel filtration.
-
•
Acidic polysaccharide demonstrated stronger hepatoprotective effect in vitro compared to neutral polysaccharide.
-
•
Anion-exchange chromatography (AEC) is an effective technique for separate β-glucan into neutral and ionic fractions by different ionic strength buffer.
Keywords: Ganoderma lucidum polysaccharides, β-glucans, Anion-exchange chromatography (AEC), Restraint stress, Hepatoprotective
Abstract
In this study, Ganoderma lucidum crude polysaccharide (GLP) was found to have protective effect on liver damage in mice caused by restraint stress through improving oxidative status. Two polysaccharides, including a neutral β-glucan (GLPB2) and an acidic β-glucan (GLPC2) were purified from GLP through anion-exchange chromatography (AEC) combined with gel permeation. GLPC2, with an average molecular weight of 20.56 kDa, exhibited stronger hepatoprotective effect against H2O2-induced liver injury in HepG2 cells compared to GLPB2. Glycosidic residues and NMR analysis comprehensively revealed that GLPC2 contained d-Glcp-(1→, →3)-d-Glcp-(1→, →4)-d-Glcp-(1→, →6)-d-Glcp-(1→, →3, 6)-d-Glcp-(1 → and → 4)-d-GlcpA-(1 → . AEC can be an effective technique for separating β-glucans into neutral and acidic fractions by different ionic strength buffer. The findings provided a theoretical basis for the potential application of G. lucidum polysaccharides as a hepatoprotective in food and pharmaceutical industry.
1. Introduction
Mushroom polysaccharides act generally as an obligatory part of cell walls in fruiting bodies, mycelia and other parts. More than that, they display effective bioactivities including immunomodulatory, anti-tumor, and anti-inflammatory etc. (He et al., 2020, Kothari et al., 2018, Ruthes et al., 2016, Synytsya and Novák, 2013; Y. Wang et al., 2017). Besides their benefit to human health, it was worthy to mentioned that most mushroom polysaccharides were found to be comparatively nontoxic against liver and kidneys and then even no significant side effects (Zhao et al., 2014), which make them a research hot spot in natural medicine and functional food industries, especially for liver protection (Y. Wang et al., 2017).
As a vital organ, the liver acts an important role in metabolism, taking biological transformation and detoxification (J. Xiao et al., 2019). Stress, excessive diet, alcohol and drugs abuse can cause liver injure, which may further develop into chronic fatigue, coagulopathy, liver failure and even cancers (Luo et al., 2021). Liver diseases which develop from liver injury have brought enormous burdens for society and economics. Thus, it is highly demanded to search for effective and safe hepatoprotective agents, for which mushrooms are a vital source.
Ganoderma lucidum (Polyporaceae) is a well-known edible and medicinal mushroom, which has been used widely in medicinal and functional food industry (Lu, He, Sun, Zhang, Linhardt, & Zhang, 2020). The polysaccharides in G. lucidum are believed as one of the most important contributors to its multiple medicinal and health benefits, such as anti-cancer, anti-diabetes, and immune-modulation (Lu, He, Sun, Zhang, Linhardt, & Zhang, 2020). Over 200 polysaccharides with different molecular weights and different glycosidic linkages had been published from Ganoderma spp. (Y. Wang et al., 2017), and the number continually increased recently. However, most of them were neutral. Furthermore, hetero-polysaccharide occupied Ganoderma polysaccharides, their detailed characterization especially in terms of specific glycosidic linkages is still unclear, which made the structure–activity relationship research very difficult.
In this study, we reported the protective effects of G. lucidum crude polysaccharides (GLP) against acute liver injury induced by restraint stress in vivo. Two main polysaccharides, including a neutral β-glucan (GLPB2) and an acidic β-glucan (GLPC2) were purified from GLP. Moreover, the chemical properties and hepatoprotective effect in vitro of GLPB2 and GLPC2 were investigated. Knowing the relationship between structural features and activities of G. lucidum polysaccharides gave novel insights into the application of G. lucidum polysaccharides as a hepatoprotective and antioxidant agent or adjuvant in medicine or food industry.
2. Experimental
2.1. Materials and reagents
The fruiting bodies of G. lucidum were obtained from Yuewei Edible Fungi Technology Co. Ltd., Guangzhou, China (S. Chen, Yong, Zhang, Su, Jiao, & Xie, 2017). DEAE Sepharose™ FF and Sephacryl S-200 HR were purchased from Cytiva (GE healthcare). Monosaccharide standards, series dextrans kit, trifluoroacetic acid (TFA), and 1-phenyl-3-methyl-5-pyrazolone (PMP) were purchased from Sigma-Aldrich LLC. Dialysis bags (MWCO = 3500 Da), dimethyl sulfoxide (DMSO), sodium borohydride (NaBH4), iodomethane (CH3I), and other chemical reagents were obtained from Guangzhou Mingwang Bio-Technology Co., Ltd.. Deuterium oxide (99.8% D) was obtained from CIL Technology. Vitamin C (VC) powder was purchased from Shanghai Yuanye Biotech Co.. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), catalase (CAT) and superoxide dismutase (SOD) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute.
2.2. Extraction and isolation
Before extraction, the fruiting bodies of G. lucidum (2.5 kg) was dried overnight at 60 °C, and then powdered and defatted by soaking with 95% ethanol for two times. Then the defatted residue was air-dried and extracted with deionized water for two times (80 °C, 30 L and 3 h for each). The extracting solution was filtrated, vacuum concentrated and then dialyzed with deionized water for 36 h. The retentate was precipitated by adding four times the initial volume of 95% ethanol. After storage at 4 °C for 48 h, the precipitate was obtained after siphon treatment, and the precipitate was re-dissolved in deionized water. G. lucidum crude polysaccharides (GLP) were acquired after deproteination by Savage method and freeze-drying. The total carbohydrates content was measured by phenol–sulfuric method.
GLP was fully dissolved in distilled water (20 mg/mL) and centrifugated (8000 rpm × 10 min). The supernatant was then subjected on a DEAE Sepharose™ FF column (50 cm × 3.6 cm) and fractioned with 0.05, 0.1, 0.3 and 0.5 M NaCl solution stepwise, and the collected eluants were assayed by phenol–sulfuric method. Two major fractions (GLPB and GLPC) collected from 0.3 M and 0.5 M NaCl solution eluants were further subjected on a Sephacryl S-200 HR column (60 cm × 2.6 cm) to afford two main polysaccharides GLPB2 and GLPC2, respectively. The procedures were repeated to obtain adequate GLPB2 and GLPC2.
2.3. Molecular weights (Mw) of GLPB2 and GLPC2
The Mw of GLPB2 and GLPC2 were evaluated by high performance gel permeation chromatography (HPGPC) equipped with two connected columns of ACQUITY APC AQ450 and AQ200 (Waters, Germany) at 35 °C. The mobile phase was 25 mmol/L NaH2PO4-Na2HPO4 (pH = 6.7) with a flow rate of 0.6 mL/min. Dextrans (Mw from 5 kDa to 670 kDa) was used to draw the standard curve. Mw was calculated by Waters EmpowerTM GPC software.
2.4. Monosaccharide composition
Monosaccharide composition analysis of GLP, GLPB2 and GLPC2 was performed using PMP pre-column derivation and HPLC analysis (Dai et al., 2010; S. Zhang, He, Chen, & Ding, 2019). Samples (each 3 mg) were fully hydrolyzed with TFA respectively. After removing TFA, the hydrolysate were reacted with NaOH and PMP, and stopped by adding HCl. The PMP derivatives were analyzed by HPLC with a YMC-Pack ODS-AM column (250 mm × 4.6 mm, 5 μm) eluted with 15% acetonitrile containing KH2PO4-K2HPO4 (0.1 M, pH = 6.7) at a flow rate of 1.0 mL/min. The monosaccharide standards were processed and analyzed as above.
2.5. Linkage patterns
GLPC2R was firstly obtained from carboxyl reduction reaction of GLPC2 (Kim & Carpita, 1992). GLPC2 and GLPC2R were methylated using Haworth’s method (Haworth, 1915) and Ciucanu’s method (Ciucanu & Kerek, 1984). The methylated polysaccharide was fully hydrolyzed with TFA, the hydrolysate was prepared for partially methylated alditol acetates (PMAA), and then detected on a Thermo Fisher GC-MS apparatus equipped with a TR-5 column (30 m × 0.25 mm × 0.25 μm). The temperature programming was 120–250 °C at 3 °C/min, followed by 250 °C held for 5 min.
2.6. FT-IR and NMR spectra
Dried GLPC2 (1 mg) and KBr powder were mixed and fully ground to make a sheet. FT-IR spectrum was acquired on a Jasco FT-IR 480 plus spectrophotometer with frequency range of 4000–500 cm−1.
GLPC2 (35 mg) was exchanged with D2O and centrifuged. The supernatant was lyophilized and then re-dissolved with 0.5 mL of D2O. NMR spectroscopic data were measured on a Bruker AVANCE III 600 spectrometer at 25 °C.
2.7. Scanning electron microscopy (SEM) and atomic force microscope (AFM) analysis
In SEM analysis, GLPC2 was mounted on aluminum stubs and coated with a gold layer. The surface morphologies and section-cross microstructure were observed by employing a Hitachi S-3000 N scanning electron microscope (JEOL Ltd., Tokyo, Japan).
In AFM analysis, GLPC2 solution (10 μg/mL) was prepared and then the solution (5 μL) was dropped onto the surface of a clean mica sheet and dried. The surface topology was scanned using a Bruker MultiMode8 atomic force microscope.
2.8. In vivo hepatoprotective effect of G. lucidum crude polysaccharides GLP
The animal experimental protocols were approved by Institute of Microbiology, Guangdong Academy of Sciences (IMGAS) Ethics Committee (GT-IACUC201807032). Male BALB/c mice (7 weeks) were purchased from Guangdong Medical Laboratory Animal Center, Guangzhou. The mice were housed in cages under controlled humidity (50 ± 5%), temperature (23 ± 2 °C) and 12 h day/night cycle. The mice were free to get food and water during the experiments. After one week of adjustable feeding, the mice were randomly divided into six groups (n = 8): normal control (N), model control (M), positive control (Vc), GLP-100, GLP-200 and GLP-400 groups. Mice in Vc group were administrated with Vc at a dose of 200 mg/kg/day. Mice in GLP-100, GLP-200 and GLP-400 groups were given GLP at a dose of 100, 200 and 400 mg/kg/day, respectively. Mice in N and M groups were given equal volume of water, respectively. After 7 days, each mouse except those in N group was confined to a 50 mL centrifuge tube drilled at the bottom for 18 h after the last administration. Then, the mice were sacrificed. Blood was collected from eyeballs and liver was harvested. Serum was obtained after centrifugation for 10 min at 4000 r/min. The content of ALT, AST, GSH-Px, MDA, CAT and SOD were determined in accordance with the kits’ instructions.
2.9. In vitro hepatoprotective activities
2.9.1. Cell viability
HepG2 cells (1 × 104 per well) were incubated in 96-well plates for 24 h, and then treated with different concentrations of GLPB2 or GLPC2 (0.1, 0.2, and 0.4 mg/mL). After 24 h treatment, the medium was replaced with 200 μL complete DMEM containing 10 μL of CCK-8. The plate was incubated at 37 °C for 4 h and its OD was measured at 450 nm on a microplate reader.
2.9.2. Determination of ALT and AST levels
As described above, HepG2 cells were cultured in 96-well plates for 24 h and then added with H2O2 (100 μg/mL) and further incubated for 2 h. Then the cells were treated with different concentrations of GLPB2 and GLPC2 (0.1, 0.2, and 0.4 mg/mL) and incubated for 24 h. ALT and AST were measured using commercially kits according to the manufacturer's instructions.
3. Results and discussion
3.1. Extraction of G. ludicum crude polysaccharides (GLP) and purification of polysaccharides GLPB2 and GLPC2
G. ludicum crude polysaccharides (GLP) were prepared through water extraction and different impurity removal methods (Fig. S1). The total carbohydrate content of GLP was 52.4 ± 4.1%, and it was composed of mannose (10.1%), glucose (74.7%), glucuronic acid (6.5%), galactose (3.9%), xylose (3.4%) and fucose (1.4%) by PMP pre-column derivation and HPLC analysis (Fig. S3). Then, GLP was further purified by different chromatographic columns. As shown in Fig. S2, two polysaccharides GLPB2 and GLPC2 were obtained from GLP on a DEAE Sepharose™ FF column by different concentrations of sodium chloride aqueous solutions, followed by a Sephacryl S-200 column. According to the HPGPC analysis, GLPB2 and GLPC2 showed single symmetric peaks, respectively. The Mw of GLPB2 was calculated as 22.51 kD and GLPC2 was 20.56 kD.
3.2. Monosaccharide composition
Monosaccharide composition of GLPB2 and GLPC2 were analyzed by PMP-HPLC method. As shown in Fig. S3, GLPB2 was mainly composed of mannose (7.5%) and glucose (85.3%), with trace glucuronic acid (0.9%), galactose (1.6%), xylose (1.9%) and fucose (1.8%). GLPC2 consisted of mannose (5.9%), glucuronic acid (9.0%) and glucose (80.4%), with small amount of galactose (1.8%), xylose (1.8%) and fucose (0.9%). The results of monosaccharide composition analysis implied that both GLPB2 and GLPC2 may be glucans.
3.3. Protective effect of GLP on liver damage in restraint stress-induced mice
Dysfunctional mitochondria or reduced antioxidant defense may increase the production of free radicals, which further lead to the development of oxidative stress (Sies, 2015). Long-term exposure to stress may trigger problems in mental and physical health and cause life-style diseases (Moore & Cunningham, 2012). It has been reported that stress can cause glucose and lipid metabolism disorder, immunocompromise and acute liver damage (M. Pan et al., 2020, Yan et al., 2020). Nowadays, diseases caused by stress-related have received widespread attention. Relieving stress was very important in keeping health (López-Otín & Kroemer, 2021). Previous studies have reported that G. lucidum triterpenoids and G. lucidum crude polysaccharides presented hepatoprotective effect against liver injury caused by chemicals (Hu et al., 2020, Wu et al., 2016). However, the protective effect of G. lucidum polysaccharides on oxidative stress induced liver damage was rarely reported. Hence, we focused on the effect of GLP on liver damage caused by oxidative stress.
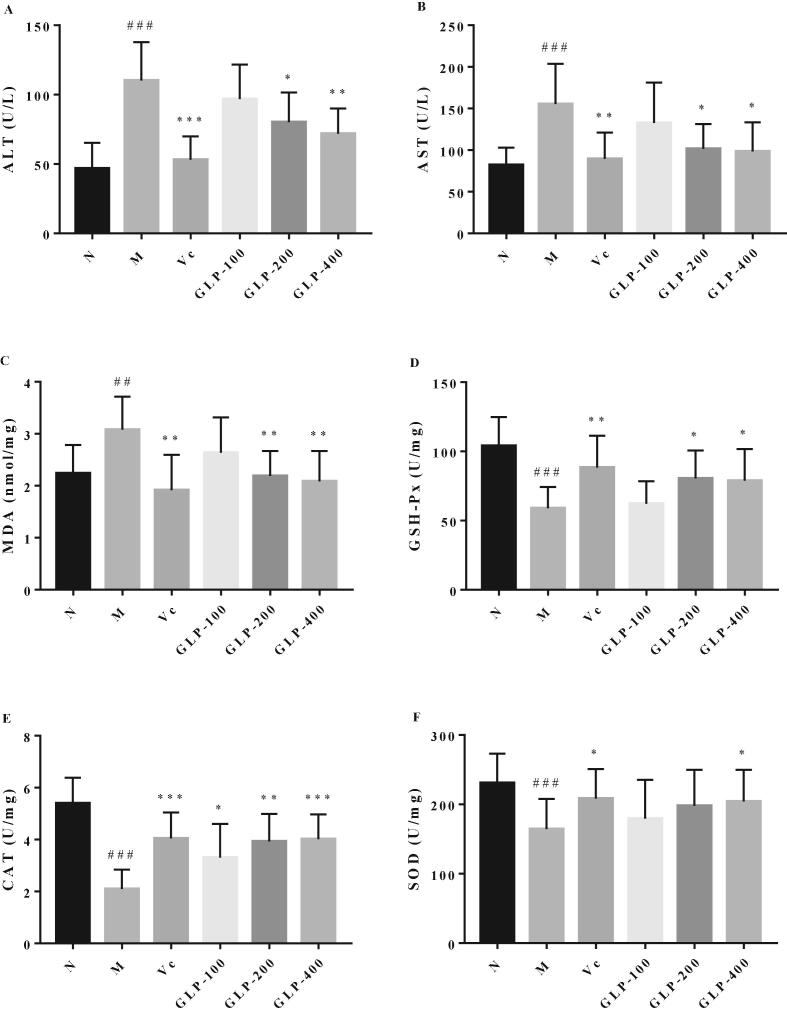
ALT and AST are considered as the major markers of liver damage. In this in vivo study, plasma ALT and AST in M group apparently increased to 110.18 ± 9.19 and 155.29 ± 16.11 U/L respectively, compared to those in N group (46.79 ± 6.19 and 82.19 ± 6.95 U/L), which indicated the liver was damaged after being stressed for 18 h. It was worthy to note that GLP decreased plasma ALT and AST levels in a dose-dependent manner (Fig. 1), depicting that GLP could attenuate acute liver damage induced by restraint stress.
Fig. 1.
Effect of GLP on ALT (A), AST (B), MDA (C), GSH-Px (D), CAT (E) and SOD (F) levels in mice with restraint stress. Data were presented as mean ± SD. ##P < 0.01, ###P < 0.001, compared to N group; * P < 0.05, **P < 0.01, ***P < 0.001, compared to M group.
Free radical reactions induced by stress can further cause harmful change for cell membranes, proteins and DNA, increase lipid peroxidation, and reduce antioxidant substances endogenously (Zhai et al., 2012). The related mechanism of GLP for attenuating stress was further investigated by observing the improvement effect of GLP on oxidative damage. The MDA, GSH-Px, CAT and SOD level in liver were determined, and the results were also shown in Fig. 1. Compared to N group (2.24 ± 0.18 nmol/mg), the hepatic MDA content of M group (3.08 ± 0.21 nmol/mg) was significantly higher (P < 0.01). However, the MDA content was significantly decreased by administration of GLP (200 and 400 mg/kg) as well as VC (200 mg/kg). The hepatic GSH-Px level (103.92 ± 6.95 U/mg) significantly was decreased to 58.93 ± 5.13 U/mg by being stressed (P < 0.001). Obviously, GLP (200 and 400 mg/kg) increased the hepatic GSH-Px level to 80.46 ± 6.75 and 78.76 ± 7.62 U/mg, respectively. As the positive control, VC also significantly increase the hepatic GSH-Px level (88.31 ± 7.66 U/mg) compared with the stressed mice (P < 0.01). Similarly, stress decreased the hepatic CAT level, and GLP (100, 200 and 400 mg/kg) and VC increased CAT activity. Hepatic SOD activity significantly was decreased after being stressed (164.5 ± 12.6 vs. 230.9 ± 12.2 U/mg), and 400 mg/kg of GLP significantly increased the hepatic SOD level to 204.1 ± 13.2 U/mg (P < 0.05). Above all, GLP may relieve acute liver damage by enhancing the activity of antioxidant enzymes and inhibiting the production of lipid peroxide.
3.4. Protective effect of GLPB2 and GLPC2 against H2O2-induced HepG2 cells in vitro
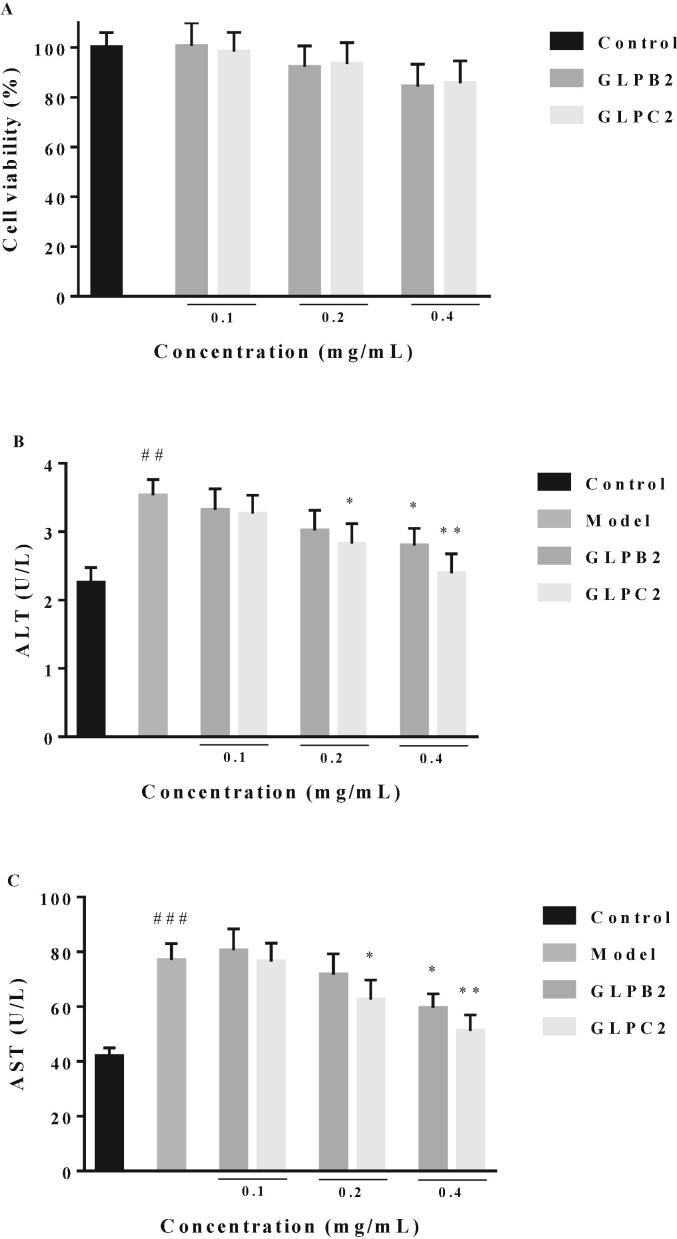
Cellular oxidative damage is a well-established general mechanism for cell and tissue injury and primarily caused by ROS. GLP had protective effects on restraint stress-induced acute liver damage by improving the oxidative status. Thus, the protective effects of GLPB2 and GLPC2 against H2O2-induced HepG2 cells were tested. Hydrogen peroxide is a toxic substance for the liver cells and can be converted into hydroxyl radicals and oxygen radicals. Previous studies had confirmed the hepatoprotective effect of polysaccharides based on their effect on the viability, ALT and AST activity of H2O2-induced HepG2 cells (Qu et al., 2020). ALT and AST activities were considered as indicators to measure the degree of hepatocyte injury. Preliminarily, the cytotoxicity of GLPB2 and GLPC2 were determined based on the viability of HepG2 cells. As shown in Fig. 2A, both GLPB2 and GLPC2 exerted no obvious toxicity against HepG2 cells at the concentration from 0.1 to 0.4 mg/mL. Then, Both GLPB2 and GLPC2 were further examined for their protective effects against H2O2-induced HepG2 cells damage. As shown in Fig. 2B and C, ALT and AST activities in HepG2 cells were increased after being induced by H2O2. Pretreatment with GLPB2 and GLPC2 prominently inhibited ALT and AST activities in a concentration-dependent manner. Moreover, GLPC2 showed stronger inhibitory activity than GLPB2. According to the results of the monosaccharide analysis and the hepatoprotective assay, it is concluded that the stronger hepatoprotective activity of GLPC2 may be partly attributed to the glucuronic acid content.
Fig. 2.
Hepatoprotective activities of GLPB2 and GLPC2. Cell viability (A) and ALT (B) and AST (C) activities in H2O2-induced HepG2 cells treated with various concentrations of GLPB2 and GLPC2. ##P < 0.01, ###P < 0.001, compared to N group; * P < 0.05, **P < 0.01, compared to M group.
Continuous research has reported that polysaccharides exerted hepatoprotective effect through various mechanisms including the pathological process of inflammation, apoptosis and oxidative stress. Polysaccharides could regulate NF-κB, JAK/STAT, TGF-β, PI3K/AKT, MAPK, caspase cascade, p53 and Nrf2-Keap1 pathways, lipid metabolism as well as cytochrome P450 enzymes (Qu et al., 2020). Therefore, further research is needed to verify the hepatoprotective effect of GLPC2 in vivo and to study the underlying mechanism.
3.5. Linkage patterns analysis
Carboxyl reduction and methylation analysis were performed to determine the linkage types of GLPC2 and the GC-MS date were listed in Table S1. GLPC2 mainly contained T-Glcp, 1, 3-Glcp, 1, 4-Glcp, 1, 6-Glcp, and 1, 3, 6-Glcp, with a molar ratio of about 1:2:3:2:1. Besides, GC-MS analysis also revealed small trace of T-Manp and 1, 6-Galp. GLPC2R, the reduction products of GLPC2, included T-Glcp, 1, 3-Glcp, 1, 4-Glcp, 1, 6-Glcp, and 1, 3, 6-Glcp, with a molar ratio of 1:2:4:2:1. The results implied that GlcA in GLPC2 existed as 1, 4-linkages.
3.6. FT-IR and NMR spectra
The typical absorption signals of GLPC2 can be observed from its FT-IR spectrum (Fig. S4). The peak at 3415.0 cm−1 was attributed to the characteristic O—H stretching absorption. Bands at 2923.5, 1375.3 and 1262.8 cm−1 were assigned to the C—H stretching absorption, C—H bending vibration and H—C—H twisting vibration, respectively (Venkatesan, Qian, Ryu, Kumar, & Kim, 2011; S. Zhang, He, Chen, & Ding, 2019). The band at 895 cm−1 indicated the β-glucopyranosyl linkages of GLPC2 (Fu et al., 2019, Shi et al., 2020).
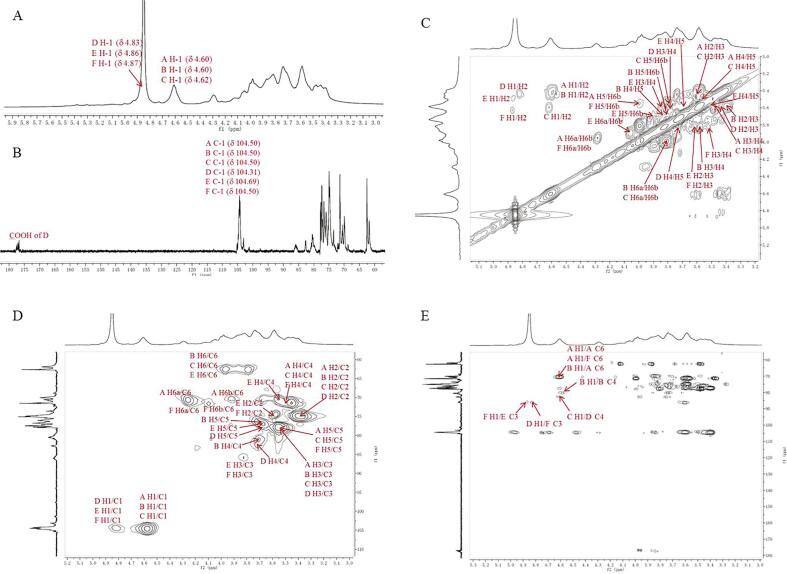
The 1H and 13C NMR spectra of GLPC2 were presented in Fig. 3A and B. In 1H NMR spectrum, the main peaks of δH 4.60–4.62 and δH 4.82–4.87 ppm indicated that all the glycosidic residues were β-configurated. Correspondingly, the signals of the anomeric carbons were observed at about 104.50 ppm in 13C NMR spectrum. The downfield signals at about δC 86 and 80 were assigned to the substituted C-3 and C-4 of glycosidic residues, respectively. The downfield peak at about δC 70 ppm was attributed to the substituted C-6 of glycosidic residues. The strong peak at δC 62.69 was from the unsubstituted C-6 of glycosidic residues. Based on NMR assignments reported in literatures (Y. Chen et al., 2017, Dong et al., 2012, Fu et al., 2019; D. Huang, Hou, Zhang, Zhang, & Yan, 2020; Y. Wang et al., 2017; H. Zhang, Nie, Cui, Xu, Ding, & Xie, 2017), the HSQC correlation observed at 4.60/104.53 and 4.59/104.53 (Fig. 3C) were assigned as H-1/C-1 of 1, 6-β-d-Glcp (marked as residue A) and 1, 4-β-d-Glcp (B), respectively. Another correlation of 4.60/104.53 was ascribed to H-1/C-1 of T-β-d-Glcp (C). 4.82/104.31 was from H-1/C-1 of 1, 4-β-d-GlcpA (D). Signals at 4.86/104.69 was assigned as H-1/C-1 of 1, 3-β-d-Glcp (E) and 4.87/104.63 was assigned as H-1/C-1 of 1, 3, 6-β-d-Glcp (F). The cross peaks of 3.84/85.87 and 3.85/86.02 were from H-3/C-3 of 1, 3-β-d-Glcp (E) and 1, 3, 6-β-d-Glcp (F), respectively. 4.27, 4.02/70.59, and 4.28, 3.93/70.62 were assigned as H2-6/C-6 of 1, 6-β-d-Glcp (A) and 1, 3, 6-β-d-Glcp (F) respectively. In the same way, the other proton and carbon signals for these residues were assigned by analysis of HSQC combined with 1H–1H COSY spectra (Fig. 3D). The NMR assignments of glycosidic residues A − E were listed in Table 1.
Fig. 3.
1H (A), 13C (B), 1H–1H COSY (C), HSQC (D) and HMBC (E) spectra of GLPC2.
Table 1.
1H and 13C NMR assignment of GLPC2 (in D2O).
| H-1 C-1 |
H-2 C-2 |
H-3 C-3 |
H-4 C-4 |
H-5 C-5 |
H-6 C-6 |
|
|---|---|---|---|---|---|---|
| →6)-β-d-Glcp-(1→ (A) |
4.60 104.53 |
3.45 74.97 |
3.58 77.52 |
3.49 71.55 |
3.55 77.86 |
4.28, 3.93 70.59 |
| →4)-β-d-Glcp-(1→ (B) |
4.59 104.53 |
3.39 75.24 |
3.58 77.52 |
3.84 80.49 |
3.68 75.70 |
3.99, 3.81 62.69 |
|
β-d-Glcp-(1→ (C) |
4.60 104.53 |
3.45 74.97 |
3.58 77.52 |
3.49 71.55 |
3.55 77.86 |
3.99, 3.81 62.53 |
| →4)-β-d-GlcpA-(1→ (D) |
4.82 104.31 |
3.42 74.92 |
3.59 76.85 |
3.79 82.89 |
3.71 77.95 |
176.94 |
| →3)-β-d-Glcp-(1→ (E) |
4.86 104.69 |
3.63 75.15 |
3.85 86.02 |
3.58 69.94 |
3.69 76.70 |
4.05, 3.89 61.97 |
| →3,6)-β-d-Glcp-(1→ (F) |
4.87 104.63 |
3.61 74.93 |
3.84 85.72 |
3.47 71.38 |
3.55 77.44 |
4.28, 3.93 70.62 |
The observed HMBC correlation (Fig. 3E) of δ 4.60/70.59 (AH1/AC6), 4.60/70.62 (AH1/FC6), and 4.60/70.59 (BH1/AC6) suggested that C-6 of residue A was linked to O-1 of residue A, O-1 of residue B, as well as O-1 of residue F. Cross signal at 4.60/80.49 (BH1/BC4) indicated that C-4 of residue B was connected to O-1 of residue B. The correlation of 4.87/86.02 (FH1/EC3) indicated C-3 of residue E was linked to O-1 of residue F. These results suggested that GLPC2 had the backbone of → 3)-β-d-Glcp-(1→, →4)-β-d-Glcp-(1→, →6)-β-d-Glcp-(1→, and → 3, 6)-β-d-Glcp-(1→, with a branching point at C-3 of → 3, 6)-β-d-Glcp-(1 → . The branch was consisted of → 4)-β-d-Glcp-(1→ (D) and T-β-d-Glcp (C) from the observed correlations of 4.82/85.57 (DH1/FC3) and 4.62/82.78 (CH1/DC4), respectively. Finally, Combined with the results of monosaccharide composition, and GC–MS analysis, a putative structure for GLPC2 was proposed as shown in Fig. 4.
Fig. 4.
Possible repeated unit of GLPC2.
Previous studies have revealed that both glucans and heteroglycans were found in G. lucidum (Y. Liu et al., 2014, Lu et al., 2020; J. Wang, Ma, Zhang, Fang, Jiang, & Phillips, 2011). Glucans were composed of 1, 3-, 1, 4-, 1, 6-, 1, 3, 6-, and 1, 4, 6-β-d-Glcp residues with different Mw (Bao et al., 2002, Kao et al., 2012, Li et al., 2020; W. Liu, Wang, Pang, Yao, & Gao, 2010). However, G. lucidum heteroglycans possessed different monosaccharide combination including mannose, rhammose, glucose, galactose, xylose, fucose and arabinose with different linkage patterns (Bao, Wang, Dong, Fang, & Li, 2002; S. Huang et al., 2011, Li et al., 2020; D. Pan, Wang, Chen, Hu, & Zhou, 2015; C. Xiao, Wu, Zhang, Xie, Cai, & Tan, 2017). Different strains, cultivated patterns, growing periods may cause the complexity and diversity of Ganoderma polysaccharides. Besides, most of G. lucidum polysaccharides are neutral polysaccharides and the acidic polysaccharides had yet received little attention. Our results revealed that acid glucans were also contained in G. lucidum, which further enriched the understanding of chemical diversity of G. lucidum polysaccharides.
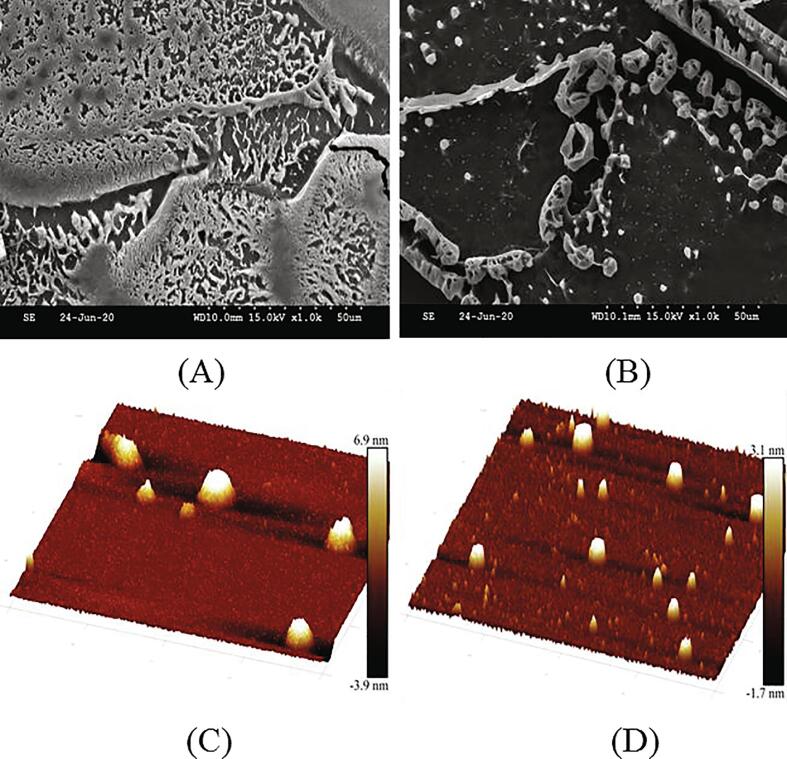
3.7. Morphological analysis of GLPC2
SEM and AFM are considered as powerful tools to observe the microstructure and morphological characteristics of macromolecular. As shown in Fig. 5A and B, GLPC2 had spherical or columnar crannied conglomerations on the smooth surface. It can be seen from Fig. 5C and D visually that GLPC2 comprised rigid short-rod conformation. The AFM images were in accordance with the results of SEM. It is demonstrated that β-d-glucans polysaccharide possessed a high Mw (>2.5 × 104) and presented a triple helix tertiary structure (Synytsya & Novák, 2013). The low Mw would destruct the hydrogen bond, which lead to the failure of the triple helix structure formation (Li et al., 2020).
Fig. 5.
SEM (A, B) and AFM (C, D) analysis of GLPC2.
4. Conclusion
G. lucidum crude polysaccharide GLP was found to relieve oxidative stress of acute liver injury in restraint mice. Two glucans, named GLPB2 and GLPC2, were purified from GLP. GLPB2 and GLPC2 possessed similar Mw but different monosaccharide compositions. In addition, GLPC2 showed stronger hepatoprotective activity compared to GLPB2 in vitro, indicating that the content of uronic acid may influence the hepatoprotective bioactivities of polysaccharides. Glycosidic residues and NMR analysis comprehensively revealed that GLPC2 contained d-Glcp-(1→, →3)-d-Glcp-(1→, →4)-d-Glcp-(1→, →6)-d-Glcp-(1→, →3, 6)-d-Glcp-(1 → and → 4)-d-GlcpA-(1 → . Besides, AEC could be an effective technique for separating glucans into neutral and ionic fractions by utilizing different ionic strength buffer. Our current study may contribute to the improved utilization of G. lucidum polysaccharides for liver protection. Future work on structure–activity relationship of GLPs and the hepatoprotective mechanism is in progress.
CRediT authorship contribution statement
Shaodan Chen: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Funding acquisition. Xiaoying Guan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Tianqiao Yong: Writing – original draft. Xiong Gao: Writing – review & editing. Chun Xiao: Writing – review & editing. Yizhen Xie: Writing – review & editing. Diling Chen: Writing – review & editing. Huiping Hu: Writing – review & editing. Qingping Wu: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81803393, 31901696), Guangdong Basic and Applied Basic Research Foundation (2020A1515011337), Guangdong Province Science and Technology Project (2019A050520003), Science and Technology Program of Guangzhou (202002030225), Guangdong Province Agricultural Science and Technology Innovation System (2020KJ103) and GDAS' Project of Science and Technology Development (2020GDASYL-20200104016).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100204.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bao X.F., Wang X.S., Dong Q., Fang J.N., Li X.Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry. 2002;59(2):175–181. doi: 10.1016/s0031-9422(01)00450-2. [DOI] [PubMed] [Google Scholar]
- Chen S., Yong T., Zhang Y., Su J., Jiao C., Xie Y. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Frontiers in Chemistry. 2017;5:85. doi: 10.3389/fchem.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li X.H., Zhou L.Y., Li W., Liu L., Wang D.D.…Lu Y.M. Structural elucidation of three antioxidative polysaccharides from Tricholoma lobayense. Carbohydrate Polymers. 2017;157:484–492. doi: 10.1016/j.carbpol.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Ciucanu I., Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydrate Research. 1984;131(2):209–217. [Google Scholar]
- Dai J., Wu Y., Chen S.W., Zhu S., Yin H.P., Wang M., Tang J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydrate Polymers. 2010;82(3):629–635. [Google Scholar]
- Dong Q., Wang Y., Shi L., Yao J., Li J., Ma F., Ding K. A novel water-soluble beta-D-glucan isolated from the spores of Ganoderma lucidum. Carbohydrate Research. 2012;353:100–105. doi: 10.1016/j.carres.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Fu Y., Shi L., Ding K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. International Journal of Biological Macromolecules. 2019;141:693–699. doi: 10.1016/j.ijbiomac.2019.09.046. [DOI] [PubMed] [Google Scholar]
- Haworth W.N. A new method of preparing alkylated sugars. Journal of the Chemical Society. 1915;107:8–16. [Google Scholar]
- He X., Fang J., Guo Q., Wang M., Li Y., Meng Y., Huang L. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydrate Polymers. 2020;229:115548. doi: 10.1016/j.carbpol.2019.115548. [DOI] [PubMed] [Google Scholar]
- Hu Z., Du R., Xiu L., Bian Z., Ma C., Sato N.…Wang X. Protective effect of triterpenes of Ganoderma lucidum on lipopolysaccharide-induced inflammatory responses and acute liver injury. Cytokine. 2020;127:154917. doi: 10.1016/j.cyto.2019.154917. [DOI] [PubMed] [Google Scholar]
- Huang D., Hou X., Zhang D., Zhang Q., Yan C. Two novel polysaccharides from rhizomes of Cibotium barometz promote bone formation via activating the BMP2/SMAD1 signaling pathway in MC3T3-E1 cells. Carbohydrate Polymers. 2020;231:115732. doi: 10.1016/j.carbpol.2019.115732. [DOI] [PubMed] [Google Scholar]
- Huang S.Q., Li J.W., Li Y.Q., Wang Z. Purification and structural characterization of a new water-soluble neutral polysaccharide GLP-F1-1 from Ganoderma lucidum. International Journal of Biological Macromolecules. 2011;48(1):165–169. doi: 10.1016/j.ijbiomac.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Kao P.F., Wang S.H., Hung W.T., Liao Y.H., Lin C.M., Yang W.B. Structural characterization and antioxidative activity of low-molecular-weights beta-1,3-glucan from the residue of extracted Ganoderma lucidum fruiting bodies. Journal of Biomedicine and Biotechnology. 2012;2012:673764. doi: 10.1155/2012/673764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-B., Carpita N.C. Changes in esterification of the uronic-acid groups of cell-wall polysaccharides during elongation of maize coleoptiles. Plant Physiology. 1992;98(2):646–653. doi: 10.1104/pp.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari D., Patel S., Kim S.-K. Anticancer and other therapeutic relevance of mushroom polysaccharides: A holistic appraisal. Biomedicine & Pharmacotherapy. 2018;105:377–394. doi: 10.1016/j.biopha.2018.05.138. [DOI] [PubMed] [Google Scholar]
- Li J., Gu F., Cai C., Hu M., Fan L., Hao J., Yu G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. International Journal of Biological Macromolecules. 2020;143:806–813. doi: 10.1016/j.ijbiomac.2019.09.141. [DOI] [PubMed] [Google Scholar]
- Liu W., Wang H., Pang X., Yao W., Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. International Journal of Biological Macromolecules. 2010;46(4):451–457. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang J., Tang Q., Yang Y., Guo Q., Wang Q.…Cui S.W. Physicochemical characterization of a high molecular weight bioactive beta-D-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydrate Polymers. 2014;101:968–974. doi: 10.1016/j.carbpol.2013.10.024. [DOI] [PubMed] [Google Scholar]
- López-Otín C., Kroemer G. Hallmarks of Health. Cell. 2021;184(1):33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- Lu J., He R., Sun P., Zhang F., Linhardt R.J., Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. International Journal of Biological Macromolecules. 2020;150:765–774. doi: 10.1016/j.ijbiomac.2020.02.035. [DOI] [PubMed] [Google Scholar]
- Luo M., Xu H., Dong Y., Shen K., Lu J., Yin Z.…Sun Y. The Mechanism of Dehydrating Bimodules in trans-Acyltransferase Polyketide Biosynthesis: A Showcase Study on Hepatoprotective Hangtaimycin. Angewandte Chemie-International Edition. 2021;60(35):19139–19143. doi: 10.1002/anie.202106250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.J., Cunningham S.A. Social position, psychological stress, and obesity: A systematic review. Journal of the Academy of Nutrition and Dietetics. 2012;112(4):518–526. doi: 10.1016/j.jand.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Pan D., Wang L., Chen C., Hu B., Zhou P. Isolation and characterization of a hyperbranched proteoglycan from Ganoderma lucidum for anti-diabetes. Carbohydrate Polymers. 2015;117:106–114. doi: 10.1016/j.carbpol.2014.09.051. [DOI] [PubMed] [Google Scholar]
- Pan M.H., Zhu S.R., Duan W.J., Ma X.H., Luo X., Liu B.…He R.R. “Shanghuo” increases disease susceptibility: Modern significance of an old TCM theory. Journal of Ethnopharmacology. 2020;250:112491. doi: 10.1016/j.jep.2019.112491. [DOI] [PubMed] [Google Scholar]
- Qu J., Huang P., Zhang L., Qiu Y., Qi H., Leng A., Shang D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. International Journal of Biological Macromolecules. 2020;161:24–34. doi: 10.1016/j.ijbiomac.2020.05.196. [DOI] [PubMed] [Google Scholar]
- Ruthes A.C., Smiderle F.R., Iacomini M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydrate Polymers. 2016;136:358–375. doi: 10.1016/j.carbpol.2015.08.061. [DOI] [PubMed] [Google Scholar]
- Shi W., Zhong J., Zhang Q., Yan C. Structural characterization and antineuroinflammatory activity of a novel heteropolysaccharide obtained from the fruits of Alpinia oxyphylla. Carbohydrate Polymers. 2020;229:115405. doi: 10.1016/j.carbpol.2019.115405. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synytsya A., Novák M. Structural diversity of fungal glucans. Carbohydrate Polymers. 2013;92(1):792–809. doi: 10.1016/j.carbpol.2012.09.077. [DOI] [PubMed] [Google Scholar]
- Venkatesan J., Qian Z.J., Ryu B., Kumar N.A., Kim S.K. Preparation and characterization of carbon nanotube-grafted-chitosan-Natural hydroxyapatite composite for bone tissue engineering. Carbohydrate Polymers. 2011;83(2):569–577. [Google Scholar]
- Wang J., Ma Z., Zhang L., Fang Y., Jiang F., Phillips G.O. Structure and chain conformation of water-soluble heteropolysaccharides from Ganoderma lucidum. Carbohydrate Polymers. 2011;86(2):844–851. [Google Scholar]
- Wang Y., Liu Y., Yu H., Zhou S., Zhang Z., Wu D.…Zhang J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble beta-glucan from the spores of Ganoderma lucidum. Carbohydrate Polymers. 2017;167:337–344. doi: 10.1016/j.carbpol.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Wu J.G., Kan Y.J., Wu Y.B., Yi J., Chen T.Q., Wu J.Z. Hepatoprotective effect of ganoderma triterpenoids against oxidative damage induced by tert-butyl hydroperoxide in human hepatic HepG2 cells. Pharmaceutical Biology. 2016;54(5):919–929. doi: 10.3109/13880209.2015.1091481. [DOI] [PubMed] [Google Scholar]
- Xiao C., Wu Q., Zhang J., Xie Y., Cai W., Tan J. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 down-regulated hepatic glucose regulatory enzymes in diabetic mice. Journal of Ethnopharmacology. 2017;196:47–57. doi: 10.1016/j.jep.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Xiao J., Wang F., Wong N.K., He J., Zhang R., Sun R.…Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. Journal of Hepatology. 2019;71(1):212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Yan C., Luo Z., Li W., Li X., Dallmann R., Kurihara H.…He R.-R. Disturbed Yin-Yang balance: Stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1. Acta Pharmaceutica Sinica B. 2020;10(3):383–398. doi: 10.1016/j.apsb.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y.J., He R.R., Tsoi B., Li Y.F., Li X.D., Tsuruoka N.…Kurihara H. Protective effect of extract of chicken meat on restraint stress-induced liver damage in mice. Food & Function. 2012;3(6):662. doi: 10.1039/c2fo10275g. [DOI] [PubMed] [Google Scholar]
- Zhang H., Nie S., Cui S.W., Xu M., Ding H., Xie M. Characterization of a bioactive polysaccharide from Ganoderma atrum: Re-elucidation of the fine structure. Carbohydrate Polymers. 2017;158:58–67. doi: 10.1016/j.carbpol.2016.11.088. [DOI] [PubMed] [Google Scholar]
- Zhang S., He F., Chen X., Ding K. Isolation and structural characterization of a pectin from Lycium ruthenicum Murr and its anti-pancreatic ductal adenocarcinoma cell activity. Carbohydrate Polymers. 2019;223:115104. doi: 10.1016/j.carbpol.2019.115104. [DOI] [PubMed] [Google Scholar]
- Zhao T., Mao G., Feng W., Mao R., Gu X., Li T.…Wu X. Isolation, characterization and antioxidant activity of polysaccharide from Schisandra sphenanthera. Carbohydrate Polymers. 2014;105:26–33. doi: 10.1016/j.carbpol.2014.01.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.