Abstract
As the number of households that raise dogs and cats is increasing, there is growing interest in animal health. The gut plays an important role in animal health. In particular, the microbiome in the gut is known to affect both the absorption and metabolism of nutrients and the protective functions of the host. Using probiotics on pets has beneficial effects, such as modulating the immune system, helping to reduce stress, protecting against pathogenic bacteria and developing growth performance. The goals of this review are to summarize the relationship between probiotics/the gut microbiome and animal health, to feature technology used for identifying the diversity of microbiota composition of canine and feline microbiota, and to discuss recent reports on probiotics in canines and felines and the safety issues associated with probiotics and the gut microbiome in companion animals.
Keywords: Probiotics, Gut microbiome, Companion animal, Canine, Feline
INTRODUCTION
Terms such as ‘companion animals’ apply to households with pets, companion dogs and companion cats that are frequently encountered in the surroundings. The word ‘companion’, with which we are already familiar, refers to an animal that lives with humans and was first proposed by zoologist and Nobel Prize winner Konrad Lorenz at an international symposium held in Vienna, Austria in 1983 [1]. Households that raise these pets accounted for 29.7% of the total households in Korea, with 6.04 million households at the end of 2020 [2]. As the number of people raising companion animals is increasing, the relationship between humans and companion animals is further developing.
Most pet owners currently treat their pets as family, colleagues, and friends [3]. Pet humanization, a phenomenon that recognizes companion animals as family members and treats them as individuals with emotions, has been established as a global trend [4]. In Korea, the trend of pet humanization is also spreading, with 88.9% of companion households and 64.3% of general households agreeing to the phrase ‘pets are part of the family’ [2]. A typical example is that the pets do the same things as humans do, such as having birthday parties for dogs and cats, sleeping with the owner in the bed, and others. Companion animals have become an increasingly important part of human life, and therefore, the health and well-being of pets have increasingly attracted interest in recent decades [5].
Dogs and cats have evolved into carnivores with high-protein diets and have relatively simple gastrointestinal tracts (GITs) [5,6]. Cats are carnivores that rely on high-protein animal tissues to meet their unique nutritional requirements in the wild and consume protein-containing feed to meet their nutrients in the case of household felines. They are metabolically adapted to low glucose utilization and high protein metabolism [5,7]. Although dogs share many anatomical and metabolic characteristics with cats, they are metabolically omnivorous and can digest, absorb and metabolize significant amounts of carbohydrates [8].
The gut plays an important role in animal health, and the GIT contains a complex microbial community. A healthy gut is known to affect host physiology and well-being. This microbial ecosystem acts in several ways, affecting both the absorption and metabolism of nutrients and the protective functions of the host. Probiotics are defined as ‘living microorganisms that provide health benefits to the host when administered in appropriate amounts’ [9]. Recently, gut-related probiotic products aimed at pets, particularly dogs and cats, have also gained in popularity among owners [10]. The benefits of using probiotics for pets include their modulation of the immune system, help in reducing stress, protection against infections caused by intestinal pathogens and growth performance development [11]. As dogs and cats become family members, the number of studies about dogs and cats has been increasing. Among their topics, knowledge about the gut microbiome and probiotics in dogs and cats is still expanding. However, published papers on the application of probiotics in companion animals are significantly limited compared to those in humans. The purpose of this review is to describe the current knowledge about the gut microbial communities in dogs and cats in relation to probiotics.
PROBIOTICS FOR COMPANION ANIMALS
Probiotics that are living and beneficial microbiota have been used for companion animal’s health [9]. As people’s desire to have their pets for a long time has increased, interest in probiotics has also attracted more attention [10]. Probiotics provide beneficial health effects to the host animal by altering the gastrointestinal (GI) flora. The GI benefits for dogs and cats include maintaining a balanced and healthy gut microbiome, preventing diarrhea, and managing small intestinal bacterial overgrowth and inflammatory bowel disorders [12]. Since dogs and cats have different dietary needs and digestive systems, their needs for and effects from probiotics differ.
Probiotics for canines
Canines are considered animal models for the study of the human microbiome because of the high structural and functional similarity between the canine and human microbiomes [13]. The study of the dog microbiome can be predictive of the human microbiome. Thus, the study of dogs offers two advantages not only directly for dogs but also for its potentially benefits for humans [14]. Although the beneficial effects of probiotics have been extensively studied in humans and animals, the exact mechanisms of probiotic-based immune modulation are not entirely clear, and the efficacy of probiotic applications varies depending on many different factors [15]. Recent reports of using probiotics in canines are shown in Tables 1 and 2.
Table 1. List of canine and feline origin bacterial strains used as probiotic properties in canines and felines.
| Bacterial strains | Amount | Source | Group | Tested for | Result | Reference |
|---|---|---|---|---|---|---|
| Bifidobacterium animalis AHC7 | 2 × 1010 CFU/day | Canine | Young adult dogs with acute diarrhea | Assessment for managing acute diarrhea | Reduced diarrhea compared to placebo group | [22] |
|
Lactobacillus
rhamnosus MP01 Lactobacillus plantarum MP02 |
109 CFU/day | Canine | 1 month old puppies | Assessments for preventing gastrointestinal infection in puppies | Significantly increased
Lactobacillus and
Faecalibacterium in
fecal Significantly increased SCFAs concentration in feces Prevented gastrointestinal infection |
[23] |
| Lactobacillus murinus LbP2 | 5 × 109 CFU/day | Canine | Dogs with canine distemper virus (CDV)-associated diarrhea | Assessment of fecal and mental status | Fecal consistency, mental status and appetite were significantly improved | [24] |
| Lactobacillus johnsonii CPN23 | 2.3 × 108 CFU/day | Canine | Adult female Labrador dogs | Assessment of nutrient digestibility and fecal fermentative metabolites | Increased crude fiber
digestibility Increased concentrations of SCFAs in feces Reduction in fecal ammonia concentration |
[25] |
| Lactobacillus fermentum CCM 7421 | 107–109 CFU/day | Canine | Dogs suffering from gastrointestinal disorder | Assessment of blood samples and composition of the fecal microbiome | Improved total protein, cholesterol
and ALT in blood samples Increased lactic acid bacteria population and decreased clostridia population along with some of the gram-negative bacterial genera Modulate liquid feces to normal consistency (dogs with diarrhea) |
[26] |
| Lactobacillus fermentum AD1 | 3 mL of 109 CFU/mL | Canine | Healthy dogs | Assessment of blood sample and composition of fecal microbiome | Significantly increased total lipid
and total protein in the blood Significantly decreased glucose concentration in the bloodstream Significantly increased the number of lactobacilli and enterococci in the feces |
[27] |
| Bifidobacterium animalis B/12 | 1 mL of 1.04 × 109 CFU/mL | Canine | Healthy dogs | Assessment of blood samples and composition of fecal microbiome | Significantly decreased concentration
of triglycerides and albumin in blood
serum Increased ALT and ALP Increased acetic, acetoacetic and valeric acids in feces |
[28] |
| Lactobacillus johnsonii CPN23 | 108 CFU/mL (0.1 mL/kg BW) | Canine | Adult female dogs | Assessment of blood sample profile | Decreased plasma glucose and
cholesterol level Increased HDL/LDL ratio |
[29] |
| Enterococcus faecium DSM 32820 | 109 CFU/day | Canine | Healthy dog | Assessment of blood sample profile | Decreased serum glucose concentration | [30] |
| Lactobacillus acidophilus DSM13241 | 2 × 108 CFU/day | Feline | Healthy adult cats | Assessment for improving intestinal health in cats | Increased numbers of beneficial
Lactobacillus and L.
acidophilus groups in feces and decreased numbers
of Clostridium spp. and Enterococcus
faecalis Decreased fecal pH and plasma endotoxin concentrations resulting in systemic and immunomodulatory changes in treated cats |
[41] |
| Enterococcus hirae | 2.85–4.28 × 108 CFU/day | Feline | Kittens | Assessment for preventing atypical Enteropathogenic E. coli (EPEC) in kittens | Highly effective at promoting
intestinal colonization and fecal shedding of live E.
hirae during
administration. Ameliorated the effects of atypical EPEC experimental infection on intestinal function and water loss |
[42] |
| Enterococcus faecium SF68 | 5 × 109 CFU/day | Feline | Kittens | Effects of Enterococcus faecium strain SF68 supplementation on immune function | The percentage of CD4+ lymphocytes was significantly higher in the treatment group | [43] |
SCFAs, short-chain fatty acids; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BW, body weight; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2. List of bacterial strains of non-canine and non-feline origin when used for their probiotic properties in canines and felines.
| Bacterial strains | Amount | Source | Group | Tested for | Result | Reference |
|---|---|---|---|---|---|---|
|
Lactobacillus casei
Zhang Lactobacillus plantarum P-8 Bifidobacterium animalis subsp. Lactis V9 |
2 × 109 CFU/g (2 g for young, 4 g for training, 10 g for elderly dogs) |
Lactobacillus casei
Zhang (koumiss) Lactobacillus plantarum P-8 (Fermented dairy products in China) Bifidobacterium animalis subsp. Lactis V9 (Feces of a healthy Mongolian child) |
Young, training and elderly dogs | Assessment of nutrition, immunity and composition of fecal microbiome | Promoted the average daily feed intake
of elderly dogs Improved average daily weight gain om all dogs Enhanced the level of serum IgG, IFN-α, and fecal secretory IgA (sIgA), while reducing the TNF-α. Increased beneficial bacteria and decreased potentially harmful bacteria |
[14] |
| Lactobacillus acidophilus D2/CSL | 5.0 × 109 CFU/kg of diet | Gastrointestinal (GI) tract of a healthy adult chicken | Healthy dogs | Assessment of nutritional and fecal status | Higher body condition score than
control group Positive effect on fecal consistency |
[31] |
| Enterococcus faecium SF68 | 5 × 108 CFU/day | Feces of a healthy breast-fed baby | Dogs with diarrhea | Assessment of the effect of administering metronidazole with Enterococcus faecium SF68 to treat diarrhea | Dual therapy that administrates
metronidazole with Enterococcus faecium SF68
improved diarrhea more than administering metronidazole
alone Giardia cysts were eliminated from the dual treatment group |
[32] |
| Enterococcus faecium SF68 | 5 × 108 CFU/day | Feces of a healthy breast-fed baby | Healthy dogs | Assessment of blood sample profile | Mean cholesterol concentration
significantly decreased Mean triglyceride concentration significantly increased |
[33] |
| Lactobacillus acidophilus D2/CSL (CECT 4529) | 5 × 109 CFU/kg of food | Conventional foods such as milk, yogurt and dietary supplements | Healthy adult cats | Assessment of the effects on nutritional condition and fecal quality | Improved fecal quality
parameters Increased Lactobacillus count and decreased total coliform bacteria counts |
[44] |
| Enterococcus faecium SF68 | 2.1 × 109 CFU/day | A healthy breast-fed newborn baby | Cats | Effects on Enterococcus faecium SF68 in diarrhea | The percentage of cats with diarrhea was significantly lower in the probiotic group when compared with the placebo group | [45] |
| Enterococcus faecium SF68 | 1/4 can of canned food mixed with Enterococcus faecium | A healthy breast-fed newborn baby | Young adult cats | Description of the GI abnormalities associated with the administration of amoxicillin-clavulanate to cats and an assessment of whether feeding with Enterococcus faecium SF68 could ameliorate those abnormalities | The total diarrhea scores for days
1–11 were significantly lower in the cats fed
Enterococcus faecium SF68 compared to the
cats fed the placebo Feeding Enterococcus faecium SF68 can lessen some associated clinical abnormalities |
[46] |
| Enterococcus faecium SF68 | 5 × 108 CFU/day | A healthy breast-fed newborn baby | Cats with chronic Feline Herpes virus-1 (FHV-1) infection | Assessment of the effect of feeding Enterococcus faecium SF68 in clinical signs of FHV-1 infection | Fecal microbial diversity was
maintained throughout the study in cats supplemented with
Enterococcus faecium SF68, indicating a
more stable microbiome in cats receiving Enterococcus
faecium SF68 Lessened morbidity associated with chronic FHV-1 infection in some cats |
[47] |
| Proviable®-DC (7 bacterial species) | 5 × 109 CFU of a mixture of seven bacterial species per day | Multistrain probiotic product | Adult cat | Improvement in stool character | Improved diarrhea symptoms after 21-day feeding | [48] |
| Lactobacillus plantarum | 1 × 108 CFU/mL | Mare’s milk | Cats with chronic gingivostomatitis | Assessment of preventive and therapeutic oral pathology | The administration of the probiotic to
the two immunosuppressed cats affected by gingivostomatitis led
to an improvement in the time of recurrence The symptoms of chronic feline gingivostomatitis disappeared after two weeks of administration The ulceration, inflammation and pain of the oral cavity decreased, thalism and halitosis disappeared |
[49] |
| Probiotic combination | Lactobacillus casei 4 × 108 CFU, Lactobacillus rhamnosus 3 × 108 CFU, Lactobacillus acidophilus 5 × 107 CFU, Lactobacillus bulgaricus 1 × 107 CFU, Bifidobacterium infantis 4 × 107 CFU, Bifidobacterium breve 5 × 107 CFU, Streptococcus thermophilus 1 × 108 CFU | Multistrain probiotic product | 8-month-old male cats | Management of feline idiopathic cystitis (FIC) using probiotics | Probiotic combination treatment effectively managed this disease due the effect of bactericidal, anti-inflammatory, and immunomodulatory actions | [50] |
| Proviable®-DC (7 bacterial species) | 5 × 109 CFU of a mixture of seven bacterial species per day | Multistrain probiotic product | Healthy cats | Assessment of a multispecies on the fecal microbiome of healthy cats | Increased abundance of probiotic bacteria in the feces of healthy cats | [85] |
IgG, Immunoglobulin G; IFN, Interferon; TNF, tumor necrosis factor.
GI disorders are one of the most common health problems in dogs [16,17]. Regardless of the cause, most GI disorders present with acute or chronic diarrhea, or, in some cases, vomiting or anorexia [5,18,19]. Many previous studies have shown positive results regarding the treatment of dogs with different types of probiotics [20,21]. Dogs on diets supplemented with 2 × 1010 CFU/day canine-derived probiotic Bifidobacterium animalis AHC7 had a significantly more rapid resolution of acute diarrhea than dogs that received placebo [22]. The administration of Lactobacillus rhamnosus MP01 and L. plantarum MP02, two strains isolated from canine milk, decreased the Faecalibacterium in feces [23]. Supplementing 5 × 109 CFU/day of L. murinus LbP2 in dogs improved their stool output, fecal consistency, mental status, and appetite compared to the control [24]. A total of 15 adult female dogs who were given 2.3 × 108 CFU/day canine-origin probiotic L. johnsonii CPN23 exhibited increased fiber digestibility and concentrations of short-chain fatty acids in their feces and reduced fecal ammonia concentrations compared to the control [25]. Dogs that consumed 107–109 CFU/day canine-derived probiotic L. fermentum CCM 7421 displayed an increased lactic acid bacteria population, reduced Clostridia population and some gram-negative bacterial genera. Additionally, dogs that consumed probiotics showed improved total protein, cholesterol and alanine transaminase in blood samples [26]. Three milliliters of 109 CFU/mL of the new potential probiotic L. fermentum AD1 significantly increased total lipids and total protein and significantly decreased the glucose concentration in the bloodstream [27]. Dogs fed 1.04 × 109 CFU/mL B. animalis B/12 showed a significantly decreased concentration of triglycerides and albumin and increased acetic, acetoacetic, and valeric acid in feces [28]. Supplementing 108 CFU/mL canine-origin probiotic L. johnsonii CPN23 in adult female dogs decreased their plasma glucose and cholesterol levels and increased the high-density lipoprotein and low-density lipoprotein ratio [29]. Dogs receiving Enterococcus faecium DSM 32820 had optimal fecal consistency throughout the experiment, significantly stimulated phagocytic activity and a metabolic burst activity of leukocytes and lower serum glucose concentrations [30]. Heathy dogs receiving 5 × 109 CFU/kg L. acidophilus D2/CSL showed higher body condition scores than the control dogs and there was a positive effect on their fecal consistency [31]. The probiotic feed additive contained three different bacterial strains, namely, L. casei Zhang, L. plantarum P-8, and B. animalis subsp. lactis V9 promoted the average daily feed intake, improved average daily weight gain, increased beneficial bacteria and decreased potentially harmful bacteria [14]. The probiotic E. faecium SF68 improved diarrhea symptoms compared to the control, and Giardia cysts were eliminated [32]. Adding 5 × 108 CFU/day E. faecium SF68 significantly increased the triglyceride concentration and decreased the cholesterol concentration [33].
The gut microbiome greatly affects the health and disease of the host so maintaining it in good condition is important for the health of the host [34]. Many factors influence the composition of the gut microbiome and aging is one of the greatest impacts [35]. After all, this aging which is defined as the gradual changes that occur after maturation in various organs, resulting in decreased functional capacity in the gut microbiome is thought to be somehow related to the health of the host [36]. Masuoka et al. [34] conducted the experiment with dogs of 5 different age groups (pre-weanling, weanling, young, aged and senile) and analyzed the composition of their intestinal microbiota of dogs in different age groups. As a result, the composition of the dog’s intestinal microbiota changed with age. Lactobacillus and Bifidobacterium were found to decrease as the dog aged. This experiment showed that the gut microbiome of dogs can be changed regarding the age at the level of bacterial groups and species. Further studies are needed to be done to identify whether different probiotics are needed for different phases of life.
Probiotics for felines
Cats have trillions of live bacteria in their bodies, which are mostly in their intestines [21]. Each cat’s bacterial population is different for individuals and can be changed based on diet, health status, and lifestyle choices [37,38]. During times of stress and infection, the microbiome balance can increase the number of bad bacteria, disrupting the system’s balance and potentially causing digestive problems such as decreased appetite, vomiting, diarrhea or stool changes [39,40]. Supplementing probiotics for felines can be one of the best ways to add good bacteria to the cat body [21]. Recent reports of using probiotics in felines are listed in Tables 1 and 2.
Although many studies have investigated the use of probiotics in dogs, studies in cats are relatively scarce. Few studies on probiotic usage in cats have been reported to date, and because of differences in host physiologic characteristics and the diet, the probiotic efficacy in cats cannot be extrapolated from studies in dogs [41]. The purpose of this review paper is to discuss various results about treating cats with different types of probiotics. Kittens receiving 2.85–4.28 × 108 CFU/day E. hirae showed high intestinal colonization and fecal shedding of live E. hirae during administration [42]. Supplementing 2 × 108 CFU/day L. acidophilus DSM13241 as a probiotic in healthy adult cats increased the numbers of beneficial L. and L. acidophilus groups in feces and decreased the numbers of Clostridium spp. and E. faecalis. It also decreased the fecal pH and plasma endotoxin concentrations and resulted in systemic and immunomodulatory changes in treated cats [41]. Kittens fed 5 × 109 CFU/day E. faecium SF68 showed a significantly higher percentage of CD4+ lymphocytes than controls [43]. Healthy adult cats fed 5 × 109 CFU/kg L. acidophilus D2/CSL had better results in terms of their fecal quality parameters and had increased Lactobacillus counts and decreased total coliform bacteria counts [44]. The percentage of cats with diarrhea was significantly lower in the 2.1 × 109 CFU/day E. faecium SF68 group than in the control group [45]. Young adult cats receiving E. faecium SF68 had significantly lower total diarrhea scores for days 1–11 compared to the control. Additionally, feeding E. faecium SF68 could lessen some associated clinical abnormalities [46]. Feeding Enterococcus faecium SF68 in cats with chronic feline herpesvirus-1 (FHV-1) infection showed fecal microbial diversity throughout the study which indicates a more stable microbiome. It also lessened the morbidity associated with chronic FHV-1 infections [47]. Healthy cats with 5 × 109 CFU from a mixture of seven bacterial species per day (Proviable®-DC) showed an increased abundance of probiotic bacteria in the feces. Probiotics also improved diarrhea after 21 days of feeding [48]. Cats with chronic gingivostomatitis that were fed 1 × 108 CFU/mL L. plantarum showed many positive results in gingivostomatitis symptoms. There was an improvement in the time of recurrence, and the symptoms of chronic feline gingivostomatitis disappeared after two weeks of administration. Additionally, ulceration, inflammation and oral cavity pain decreased, and thalism and halitosis disappeared [49]. Giving multistrain probiotic products to 8-month-old male cats with feline idiopathic cystitis effectively managed this disease due to the effects of bactericidal, anti-inflammatory, and immunomodulatory actions [50].
The health and disease of the host are affected by gut microbiota, maintaining the gut microbiota is getting more important as cats get aged. Masuoka et al. [51] conducted the experiment with cats of 5 different age groups (pre-weanling, weanling, young, aged and senile) and analyzed the composition of their intestinal microbiota of cats in different age groups. The results suggested that the composition of the cat’s gut microbiome changed with age, whereas the change was different from that of dogs. Bifidobacterium which predominated in the gut of dogs did not appear to be important in the gut of cats. Instead, enterococci appeared to be the main lactic acid-producing bacteria in cats. Ultimately, the results of this study indicated that the compositions of the gut microbiome between dogs and cats are different and those compositions are changing with aging. Not only are different probiotics might need for dogs and cats but also for regarding aging. Further studies are needed to use different probiotics for different phases of life.
GUT MICROBIOME FOR COMPANION ANIMALS
Gut microbiome and nutrient metabolism
Microorganisms affect the absorption of nutrients in the host and provide beneficial metabolites in return for using host nutrients [52]. Each intestine harbors a different unique microbial ecosystem due to anatomical and physiological differences [53]. Additionally, each animal harbors a different and unique microbial profile. For example, at the species and strain levels, only a few overlap between individual animals. However, the bacterial phyla, order and genera are shared by most mammals [54].
The most predominant bacterial gene category in the canine gut is carbohydrate metabolism, such as that related to mannose, oligosaccharide and raffinose metabolism. The fermentation of carbohydrates by colonic organisms such as Bacteroides, Roseburia, Ruminococcus and Lachnospiraceae results in the synthesis of short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate which are sources of energy for the host [20]. SCFAs have beneficial effects on host health, including immunomodulatory effects, anti-diarrheic effects, and a regulatory effect on GI motility. In the case of felines, which are obligate carnivores, consuming raw meat increased Clostridium and Eubacterium, which are known to produce SCFAs [55].
The synthesis of vitamin K and several components of vitamin B are important functions of the intestinal microbiota [56,57]. Vitamin K, which is included in fat-soluble vitamins, plays an important role in prothrombin coagulation factor activity. Therefore, there is a risk of intestinal bleeding in cases of vitamin K deficiency [58]. Vitamin B12 (also known as cobalamin) is important for many aspects of a dog’s health [59]. It is crucial for a healthy nervous system and brain function as well as for the formation and growth of blood cells [60]. Additionally, it is needed to maintain healthy digestion [61]. As a result of a metagenome analysis using dog feces, genes affecting lipoprotein lipase activity in adipocytes were identified in intestinal microbial genes, confirming that microorganisms are also related to lipid metabolism [62].
Gut microbiome and the immune system
The microbiome plays an important role in the immune system of the intestinal tract. In particular, early microbial exposure significantly affects gut microbiome formation and immune modulation, which affects susceptibility to intestinal diseases [63]. When comparing animals born through vaginal delivery with germ-free animals through cesarean section, the germ-free animals have fewer and smaller peyer’s patches, mesenteric lymph nodes and CD4+ T cells in the lamina propria of the gut wall [64]. In germ-free animals, a reduction in B cells, macrophages and neutrophils was confirmed [65]. Additionally, in germ-free animals, immunoglobulin was found at a level of 2%, which was significantly lower than that in normal healthy animals [66]. The microbiome also plays a role as a signal indicating health [65]. This characteristic is expected because animals evolved in coexistence with symbiotic microorganisms for a very long time [67]. Microorganisms that coexist with animals communicate directly and effectively with their host’s immune system through metabolites and nutrients [64,65].
Identifying diversity in the canine and feline microbiomes
The intestine is a major part of the body that influences host health. Numerous microbes form the complex microbial community in the GIT. Disrupting the gut microbiome may cause dysbiosis and lead to several diseases and disorders, such as diarrhea, allergies and obesity [21]. GI disease caused by the dysbiosis of the gut microbial community is also generally observed in dogs and cats [68,69]. Knowing the diversity and taxonomic bacterial distribution of the gut microbiota of healthy dogs and cats is important as a baseline in future studies evaluating GI diseases in dogs and cats [70,71].
Previous studies have focused on the cultivation of intestinal content to characterize and identify the microbiota [72-74]. Most of the bacterial groups cultivated from the canine intestine belonged to Enterobacteriaceae, Bacteroides, Clostridium, Lactobacillus, and Bifidobacterium spp. [75,76]. However, culturing the bacteria to evaluate the complex diversity of the microbiota had limitations. Bacterial species that can be cultivated by using bacterial culture techniques are only a small portion of microbiota composition [77,78], anaerobic bacteria can be easily damaged during sample handling [79,80], the cost used for culture techniques is expensive [80], a great amount of time is used for isolation and cultivation [80,81]. A novel molecular method that uses the 16S ribosomal RNA (rRNA)-enabled the evaluation of the diversity and abundance of bacteria in the sample without culturing [82,83].
The development of next-generation sequencing technologies has helped to characterize bacterial communities and to understand interactions between hosts and bacteria. Using next-generation sequencing, dog and cat organ microbiotas have been described. These microbiota include those of the GIT [70, 84-86], skin [87], oral cavity [88,89], nasal cavity [90], and vagina [91].
Composition of canine and feline microbiome
All animals, including dogs and cats, harbor numerous microorganisms in the GIT [8]. Dogs and cats have different microbiota compositions and they also differ in the same species [21]. There are lots of factors that can affect microbiota compositions such as age [92-94], breed [8,95,96], diet composition [39,92,94], disease [92,93], environment [92,96,97], food type [93,98] and sex [99,100].
The gut microbiome which is highly related to a healthy life could be affected by dogs’ breed. There was a relationship between GI conditions and dog breeds [101,102]. According to You and Kim’s experiment [103], there was a difference in microbial composition in Poodle and Maltese groups. Also, phylum Fusobacterium was differed by breeds (Maltese, Poodle, and Miniature Schnauzer). From these data, they suggested that there might be differences in the gut microbiome composition depending on the dog breeds. According to Lehtimäki et al.’s experiment [104], living conditions have a significant impact on the skin microbiome in humans and dogs, but not the gut microbiome. Dogs living in rural and urban environments participated in the study. The skin microbiome was more diverse among individuals in rural areas compared to urban areas. This study showed that the living environment had a much greater effect on the skin microbiome than the guts of dogs. Experiments on changes in the gut microbiome of cats according to breeds and living environments have been limited. Further research is needed as with dogs. According to Older et al.’s [96] experiment, breed and living environment played an important role in shaping the cat skin microbiome. In particular, it seems that the hair coat and grooming according to the cat breeds have a great influence on the microbiome of the cat’s skin microbiome.
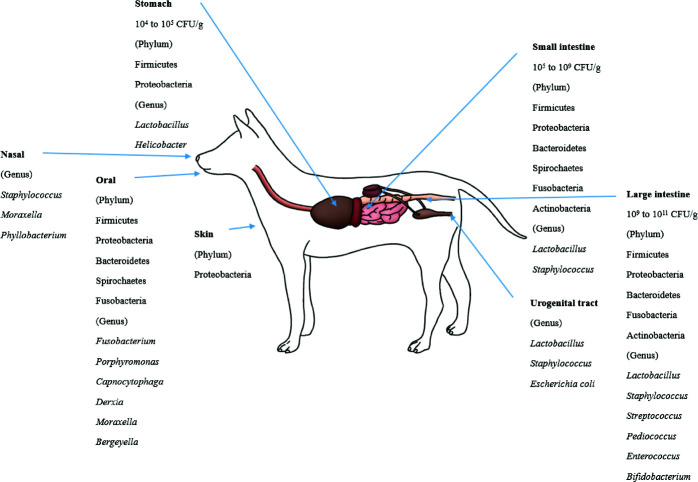
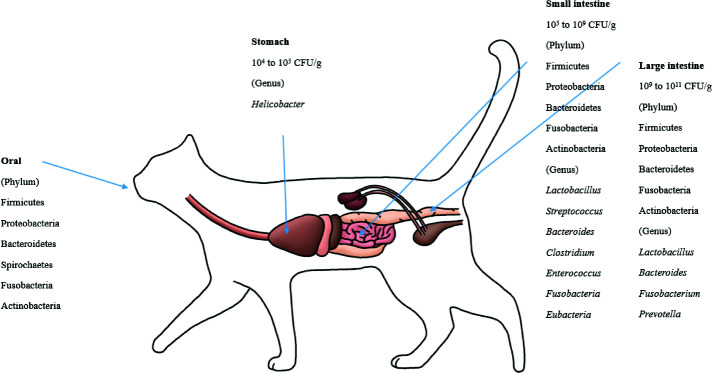
The bacterial count in the stomach is between 104 and 105 CFU/g [105]. In the duodenum and jejunum, the bacterial counts are generally low (105 CFU/g) but can reach 109 CFU/mL in some dogs and cats [106]. The ileum contains an increasing number of diverse microbiota, mostly at 107 CFU/mL. The bacterial counts in the colon are between 109 and 1011 CFU/g [38,73].
The healthy canine stomach has a comparably low number of total bacteria. Most belonged to Proteobacteria (99.6%), and few belonged to Firmicutes (0.3%). The dominant species are Helicobacter and Lactobacillus spp. [85]. Using 16S rRNA sequences, four phyla (Firmicutes, Fusobacteria, Bacteroidetes and Proteobacteria) predominated in the small intestine [70]. The duodenum of healthy canines consisted of six phyla. Firmicutes predominated followed by Proteobacteria, Bacteroidetes, Spirochaetes, Fusobacteria and Actinobacteria [37,107]. Healthy dog microbiota in the jejunum were evaluated, and the most predominant phylum was Proteobacteria (46%), followed by Firmicutes (15%), Actinobacteria (11.2%), Spirochaetes (14.2%), Bacteroidetes (6.2%) and Fusobacteria (5.4%) [84]. The ileum microbiota of healthy dogs predominantly consists of Fusobacteria, Firmicutes and Bacteroidetes [70].
Lactobacillales was present in all parts of the intestines (22% in the duodenum and 10% in the jejunum). Enterobacterales were more frequently detected in the small intestine than in the colon. Clostridiales were highly abundant in the duodenum (40%), jejunum (39%), ileum (25%) and colon (26%) [70]. Facultative anaerobic Lactobacillus strains predominated in the jejunal microbiota, and L. acidophilus was the most abundant among them [108]. In the jejunal samples, facultative anaerobic and anaerobic bacteria were similarly detected, while anaerobic bacteria predominated in the fecal samples. The number of bacteria in the jejunum was 102 to 106 CFU/g, while the number in the feces was 108 to 1011 CFU/g. Despite the lower number in the small intestine, some microbial groups were more prevalent in the small intestine than in feces: staphylococci, 64% versus 36%; non fermentative gram-negative rods, 27% versus 9%; and yeasts, 27% versus 5% [73].
Firmicutes, Bacteroidetes, Fusobacteria, Proteobacteria and Actinobacteria were the most abundant phyla in the fecal microbiota of healthy dogs [70, 86, 109]. However, Fusobacteria (39.17%) were dominant, followed by Bacteroidetes (33.36%) and Firmicutes (15.81%) in healthy adult Miniature Schnauzer dogs [86], while the abundances of Fusobacteria, Bacteroidetes and Firmicutes were similar (approximately 30% each) in six Hound dogs [109].
Clostridia was the most predominant bacterial class in the dog fecal microbiota [109]. At the genus level, Lactobacillus was the most predominant, followed by Bifidobacterium, Enterococcus, Streptococcus and Pediococcus, in the dog fecal microbiota [110]. Many Lactobacillus spp. including L. casei, L. salivarius, L. rhamnosus, L. mucosae, L. fermentum, L. reuteri, L. animalis, L. acidophilus and L. johnsonii were the most frequently isolated ones from the feces. L. reuteri, L. animalis, and L. johnsonii were the most predominant species in dogs [110-112]. Weissella confuse, Pediococcus acidilactici, Enterococcus spp. and B. animalis ssp. lactis were also frequently isolated from dog feces [111-113]. At the fungal kingdom-phylum level, Ascomycota, Basidiomycota, Glomeromycota, and Zygomycota were detected in dog feces [109].
In the skin microbiota, the most predominant phyla and families were Proteobacteria and Oxalobacteriaceae [87]. At the oral microbiota phylum level, Bacteroidetes (60%) was the most predominant, followed by Proteobacteria (20.8%), Firmicutes (11.4%), Fusobacteria (4.7%) and Spirochaetes (1.7%). At the genus level, the oral microbiota consisted of Porphyromonas (39.2%), Fusobacterium (4.5%), Capnocytophaga (3.8%), Derxia (3.7%), Moraxella (3.3%) and Bergeyella (2.7%) [88]. In the nasal microbiota of healthy dogs, Moraxella spp. was the most abundant species, followed by Phyllobacterium spp., Staphylococcus spp., and Cardiobacteriaceae [90]. The most frequently isolated bacteria from the dog’s vaginal tract were Lactobacillus, Escherichia coli and Staphylococcus pseudointermedius [21,91].
The feline GIT has different bacterial species than other animals. Helicobacter is known to reside in the stomachs of cats [114]. For the microbiota composition of the GI (stomach, duodenum, jejunum, ileum, and colon) contents, which were collected from 5 healthy felines, Firmicutes (68%) predominated, followed by Proteobacteria (14%), Bacteroidetes (10%), Fusobacteria (5%) and Actinobacteria (4%). At the order level, Clostridiales (54%) prevailed, followed by Lactobacillales, Bacteroidales, Campylobacterales, and Fusobacteriales [37]. Based on several studies, it is known that Bacteroides spp., Clostridium spp., Enterococcus spp., Streptococcus spp., Fusobacteria spp., and Eubacteria spp. are present in the small intestines of felines [69,115]. Representative lactic acid bacteria present in the GIT of felines include L. acidophilus, L. salivarius, L. johnsonii, L. reuteri and L. sakei, which are typical intestinal lactic acid bacteria found in animals, including humans, although the amount varies by individual [21,37]. The major phyla in the feline fecal microbiota were Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria [69,109,116]. These four phyla make up more than 99% of the fecal microbiota [69]. Handl et al. [109] reported that Firmicutes was the most prevalent phylum in the fecal microbiota, followed by Bacteroidetes and Actinobacteria; however, Tun et al. [116] reported that Bacteroidetes was the most predominant phylum, followed by Firmicutes and Proteobacteria. Bacteroides, Fusobacterium, and Prevotella were the most predominant genera in the feline fecal microbiota, which indicated that these genera play a major role in the feline intestine [117]. Fungi, archaea and viruses compose a minor part of intestinal microbial communities. Ascomycota was the only phylum of fungi detected in cats [109,116].
Malassezia spp. were the most prevalent fungi in feline skin mycobiota. M. restricta and M. globosa were the most predominant fungal species in all cat breeds [96]. M. pachydermatis is known as a yeast that is present in the skin microbiome, yet it can also act as a pathogen that can cause dermatitis [118]. The phylum level of the oral microbiota is generally conserved between cats. These phyla are predominated by Proteobacteria (75.2%), followed by Bacteroidetes (9.3%), Firmicutes (6.7%), SR1 (2.7%), Spirochaetes (1.8%), Fusobacteria (1.3%), and Actinobacteria (0.6%) [89]. The composition of the canine and feline microbiota is shown in Figs. 1 and 2.
Fig. 1. The dynamic community of nasal, oral and gut microbiota in canines ([21], [37], [38], [70], [73], [86]–[88], [90], [91], [107]–[113]).
Fig. 2. The microbial community of feline nasal, oral and gut environments ([37],[69],[89], [114],[115],[117]).
SAFETY ISSUES OF PROBIOTICS AND THE GUT MICROBIOME IN COMPANION ANIMALS
The Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO) defined probiotics as “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host” [9]. Tremendous scientific evidence for the efficacy of probiotic candidates has been available for decades, but insufficient information about their safety is available. While known to be safe in general, a few adverse effects associated with probiotics use have been documented in patients [119,120]. Moreover, there is a lack of information on the inherent characteristics of each probiotic strain that may be associated with health risks [121].
The European Food Safety Authority (EFSA) recommended the qualified presumption of safety (QPS) status for microorganisms used in feed and food production in 2003. Based on the QPS guidelines, microbes that produce toxins or possess virulence factors that may contribute to their pathogenicity cannot be used as probiotics. In addition, it must be ensured that there are no acquired genes encoding antimicrobial resistance (AMR). The existence of knowledge, including a history of use, ecology, industrial application, clinical reports, and a public database, is considered important evidence for evaluating the safety of microbial species [122]. QPS list includes several taxonomic units for bacteria, yeasts, and viruses [123], of which Lactobacillus and Bifidobacterium are representative because their reasonable certainty of no harm has been supported by an extensive record of safe use [124].
With the recent focus on the beneficial effects of probiotics in companion animals and their relationship to gut microflora and health, probiotic products are being increasingly marketed in the form of feed additives, dietary supplements, and probiotic-containing foods [125]. Although there have been no reports of adverse events when probiotics are administered to small animals, safety concerns remain to be addressed [126]. The microorganisms used in feed additives require safety verification for target animals, manufacturers, and owners/consumers. In particular, Enterococcus, known as canine and feline intestinal commensal bacteria, have been used as probiotics for small animals, but their use is restricted in some countries because of the risk of host infection by AMR gene transfer [125,127]. Rinkinen [128] demonstrated that some Enterococcus faecium strains promoted the adherence of the zoonotic pathogen Campylobacter jejuni in the intestines of canines. Notably, the administration of E. faecium strain SF68 (deposited as strain NCIMB 10415), which originated from infant feces in 1968, reduced the occurrence of diarrhea in dogs and cats housed in animal shelters [45], and it has been verified that the strain may not cause any safety concerns for companion animals and their owners [129-131]. Due to these conflicting outcomes, stringent safety evaluations are required in a strain-specific manner with regard to probiotic use.
Host specificity is considered an important criterion for selecting probiotic candidates, primarily due to differences in physiological structure, immune systems, and microbial composition [132,133]. However, most commercial probiotics used as feed additives originate from humans and are verified by human-based methods and criteria [134]. The clinical results from Weese and Anderson [135] showed that Lactobacillus rhamnosus GG, a commercial probiotic strain isolated from a healthy human GIT, may not be suitable for use in canines because of its short persistence. In addition, in an in vitro test, probiotic strains of canine origin inhibited the adhesion of enterotoxigenic Clostridium perfringens to canine jejunal chyme more efficiently than non-canine strains [128]. Recent studies have focused on strains isolated from the intestines of healthy dogs and cats to demonstrate their impact on pathogen inhibition, attenuation of inflammatory status, and modulation of the gut microbiome [136,137]. Host specificity has been discussed with respect to probiotic efficacy in most publications but must also be addressed from a safety perspective. Furthermore, clinical outcomes for the safe use of probiotics in target animal species should be documented. Given the host specificity and broad diversity of potential probiotic candidates for small animals, the availability of novel species with no record of use requires attention [133]. For a complete characterization, documentation of genetic and biochemical properties and long-term clinical trials are needed. Additional efforts are required to standardize safety assessment methods for novel species to be considered QPS or as having a generally recognized as safe status based on the opinions of regulatory bodies and expert panels [138].
Quality control issues associated with probiotics relate to products intended for animals as well as humans [21]. The global market for probiotics for companion animals is growing, but insufficient quality control regulations create serious problems for the safety of consumers and target animals. Some investigators have disclosed that many commercial animal probiotics or pet foods that claim to contain probiotics did not contain microbial species listed on the label or even contain other species. Moreover, bacterial viability, a key concept to stipulate probiotics, was inconsistent with labeled values expressed in colony-forming units [139-141]. The current low level of quality control may lead to exposure to unknown health risks not only for the animals but also for the owners and the environment. Recent advances in meta-omics technologies (metagenomics, metatranscriptomics, metaproteomics, and metabolomics) have promoted a correct evaluation of the quality of probiotic products. For the multistrain product VSL#3, Mora and colleagues [142] successfully identified microbial taxa with metagenomics and viability by flow cytometry and confirmed reproducibility using metaproteomics. Metagenomic approaches have also enabled the analysis of genes related to safety concerns in a culture-independent manner. Stringent oversight by regulatory bodies, high manufacturer awareness, and the development of rigorous evaluation methods by researchers are needed for the safe selection and production of probiotic candidates intended for both companion animal and human use.
CONCLUSION
Although not enough research has been conducted on the probiotics and gut microbiome thus far, because the number of companion animal people and the companion animal market are growing, research related to the companion animal microbiome is also growing. Recently, in-depth research has been performed to identify the functionality of probiotics and the gut microbiome of companion animals and to make them with various materials, ranging from feed, snacks, supplies, and treatments for the diseases of companion animals. The current evidence suggests that specific probiotic strains and/or their defined combinations may be useful in canine and feline nutrition, therapy and care. However, probiotics and the gut microbiota used in the present study are of human origin; thus, the companion animal-specific health benefits are not unclear. Therefore, the most important step is to secure pet-originated microorganisms for their health claims. Moreover, detailed in vivo designs and trials using companion animals are needed to identify and characterize newly isolated pet-originated microbiomes with an impact on health maintenance in both dogs and cats. Corroborations of these health-promoting effects and microbiological safety issues should be assessed regarding potential probiotics and the gut microbiome for animal health and welfare.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (NRF-2021R1A2C3011051).
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Lee D, Goh TW, Kang MG, Choi HJ, Yeo SY, Yang J, Huh CS, Kim YY, Kim Y.
Data curation: Lee D, Goh TW, Kim Y. Formal analysis: Lee D, Goh TW, Kim Y.
Writing - original draft: Lee D, Goh TW, Kang MG, Choi HJ, Yeo SY, Yang J, Huh CS, Kim YY, Kim Y.
Writing - review & editing: Lee D, Goh TW, Kang MG, Choi HJ, Yeo SY, Yang J, Huh CS, Kim YY, Kim Y.
Ethics approval and consent to participate
This manuscript does not require IRB/IACUC approval because there are no human and animal participants.
REFERENCES
- 1.Krebs JR, Sjolander S. Konrad zacharias lorenz, 7 November 1903 - 27 February 1989. Biogr Mem Fell R Soc. 1992;38:209–28. doi: 10.1098/rsbm.1992.0011. [DOI] [PubMed] [Google Scholar]
- 2.Hwang EK, Sohn KP. Companion animal in Korea report. Seoul: KB financial group; 2021. [Google Scholar]
- 3.Mosteller J. Animal-companion extremes and underlying consumer themes. J Bus Res. 2008;61:512–21. doi: 10.1016/j.jbusres.2007.07.004. [DOI] [Google Scholar]
- 4.Do S, Phungviwatnikul T, de Godoy MRC, Swanson KS. Nutrient digestibility and fecal characteristics, microbiota, and metabolites in dogs fed human-grade foods. J Anim Sci. 2021;99:skab028. doi: 10.1093/jas/skab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redfern A, Suchodolski J, Jergens A. Role of the gastrointestinal microbiota in small animal health and disease. Vet Rec. 2017;181:370. doi: 10.1136/vr.103826. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald ML, Rogers QR, Morris JG. Nutrition of the domestic cat, a mammalian carnivore. Annu Rev Nutr. 1984;4:521–62. doi: 10.1146/annurev.nu.04.070184.002513. [DOI] [PubMed] [Google Scholar]
- 7.Clauss M, Kleffner H, Kienzle E. Carnivorous mammals: nutrient digestibility and energy evaluation. Zoo Biol. 2010;29:687–704. doi: 10.1002/zoo.20302. [DOI] [PubMed] [Google Scholar]
- 8.Deng P, Swanson KS. Gut microbiota of humans, dogs and cats: current knowledge and future opportunities and challenges. Br J Nutr. 2015;113:S6–17. doi: 10.1017/S0007114514002943. [DOI] [PubMed] [Google Scholar]
- 9.Morelli L, Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol. 2012;46:S1–2. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 11.Sarowska J, Choroszy-Król I, Regulska-Ilow B, Frej-Mądrzak M, Jama-Kmiecik A. The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med. 2013;22:759–66. [PubMed] [Google Scholar]
- 12.Case LP, Daristotle L, Hayek MG, Raasch MF. Canine and feline nutrition: a resource for companion animal professionals. 3rd ed. London: Elsevier Health Sciences; 2010. [DOI] [Google Scholar]
- 13.Coelho LP, Kultima JR, Costea PI, Fournier C, Pan Y, Czarnecki-Maulden G, et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome. 2018;6:72. doi: 10.1186/s40168-018-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Huang W, Hou Q, Kwok LY, Laga W, Wang Y, et al. Oral administration of compound probiotics improved canine feed intake, weight gain, immunity and intestinal microbiota. Front Immunol. 2019;10:666. doi: 10.3389/fimmu.2019.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culligan EP, Hill C, Sleator RD. Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut Pathog. 2009;1:19. doi: 10.1186/1757-4749-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchodolski JS. Companion animals symposium: microbes and gastrointestinal health of dogs and cats. J Anim Sci. 2011;89:1520–30. doi: 10.2527/jas.2010-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondo E, Marliani G, Accorsi PA, Cocchi M, Di Leone A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet J. 2019;9:253–8. doi: 10.4314/ovj.v9i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unterer S, Busch K. Acute hemorrhagic diarrhea syndrome in dogs. Vet Clin Small Anim Pract. 2021;51:79–92. doi: 10.1016/j.cvsm.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci (Elite Ed). 2012;4:1404–19. doi: 10.2741/e470. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Pan Z, Yang R, Bi Y, Xiong X. The canine gastrointestinal microbiota: early studies and research frontiers. Gut Microbes. 2020;11:635–54. doi: 10.1080/19490976.2019.1704142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grześkowiak Ł, Endo A, Beasley S, Salminen S. Microbiota and probiotics in canine and feline welfare. Anaerobe. 2015;34:14–23. doi: 10.1016/j.anaerobe.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley RL, Minikhiem D, Kiely B, O’Mahony L, O’Sullivan D, Boileau T, et al. Clinical benefits of probiotic canine-derived Bifidobacterium animalis strain AHC7 in dogs with acute idiopathic diarrhea. Vet Ther. 2009;10:121–30. [PubMed] [Google Scholar]
- 23.Fernández L, Martínez R, Pérez M, Arroyo R, Rodríguez JM. Characterization of Lactobacillus rhamnosus MP01 and Lactobacillus plantarum MP02 and assessment of their potential for the prevention of gastrointestinal infections in an experimental canine model. Front Microbiol. 2019;10:1117. doi: 10.3389/fmicb.2019.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delucchi L, Fraga M, Zunino P. Effect of the probiotic Lactobacillus murinus LbP2 on clinical parameters of dogs with distemper-associated diarrhea. Can J Vet Res. 2017;81:118–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Pattanaik AK, Sharma S, Gupta R, Jadhav SE, Dutta N. Comparative assessment of canine-origin Lactobacillus johnsonii CPN23 and dairy-origin Lactobacillus acidophillus NCDC 15 for nutrient digestibility, faecal fermentative metabolites and selected gut health indices in dogs. J Nutr Sci. 2017;6:e38. doi: 10.1017/jns.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strompfová V, Kubašová I, Lauková A. Health benefits observed after probiotic Lactobacillus fermentum CCM 7421 application in dogs. Appl Microbiol Biotechnol. 2017;101:6309–19. doi: 10.1007/s00253-017-8425-z. [DOI] [PubMed] [Google Scholar]
- 27.Strompfová V, Marciňáková M, Simonová M, Bogovič-Matijašić B, Lauková A. Application of potential probiotic Lactobacillus fermentum AD1 strain in healthy dogs. Anaerobe. 2006;12:75–9. doi: 10.1016/j.anaerobe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Strompfová V, Pogány Simonová M, Gancarčíková S, Mudroňová D, Farbáková J, Mad’ari A, et al. Effect of Bifidobacterium animalis B/12 administration in healthy dogs. Anaerobe. 2014;28:37–43. doi: 10.1016/j.anaerobe.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Pattanaik AK, Sharma S, Jadhav SE. Species-specific probiotic Lactobacillus johnsonii CPN23 supplementation modulates blood biochemical profile and erythrocytic antioxidant indices in Labrador dogs. Indian J Anim Sci. 2016;86:918–24. [Google Scholar]
- 30.Strompfová V, Kubašová I, Ščerbová J, Maďari A, Gancarčíková S, Mudroňová D, et al. Oral administration of bacteriocin-producing and non-producing strains of Enterococcus faecium in dogs. Appl Microbiol Biotechnol. 2019;103:4953–65. doi: 10.1007/s00253-019-09847-3. [DOI] [PubMed] [Google Scholar]
- 31.Marelli SP, Fusi E, Giardini A, Martino PA, Polli M, Bruni N, et al. Effects of probiotic Lactobacillus acidophilus D2/CSL (CECT 4529) on the nutritional and health status of boxer dogs. Vet Rec. 2020;187:e28. doi: 10.1136/vr.105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenimore A, Martin L, Lappin MR. Evaluation of metronidazole with and without Enterococcus faecium SF68 in shelter dogs with diarrhea. Top Companion Anim Med. 2017;32:100–3. doi: 10.1053/j.tcam.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Lucena R, Novales M, Blanco B, Hernández E, Ginel PJ. Effect of probiotic Enterococcus faecium SF68 on liver function in healthy dogs. J Vet Intern Med. 2019;33:2628–34. doi: 10.1111/jvim.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuoka H, Shimada K, Kiyosue-Yasuda T, Kiyosue M, Oishi Y, Kimura S, et al. Transition of the intestinal microbiota of dogs with age. Biosci Microbiota Food Health. 2017;36:27–31. doi: 10.12938/bmfh.BMFH-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsuoka T. Establishment of intestinal bacteriology. Biosci Microbiota Food Health. 2014;33:99–116. doi: 10.12938/bmfh.33.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong PJ. Changes in body composition and energy balance with aging. Vet Clin Nutr. 1996;3:83–7. [Google Scholar]
- 37.Ritchie LE, Steiner JM, Suchodolski JS. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol Ecol. 2008;66:590–8. doi: 10.1111/j.1574-6941.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- 38.Suchodolski JS. Intestinal microbiota of dogs and cats: a bigger world than we thought. Vet Clin North Am Small Anim Pract. 2011;41:261–72. doi: 10.1016/j.cvsm.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J Gastroenterol. 2014;20:16489–97. doi: 10.3748/wjg.v20.i44.16489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willard MD. Feline inflammatory bowel disease: a review. J Feline Med Surg. 1999;1:155–64. doi: 10.1016/S1098-612X(99)90204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall-Jones ZV, Baillon MLA, Croft JM, Butterwick RF. Effects of Lactobacillus acidophilus DSM13241 as a probiotic in healthy adult cats. Am J Vet Res. 2006;67:1005–12. doi: 10.2460/ajvr.67.6.1005. [DOI] [PubMed] [Google Scholar]
- 42.Watson VE, Jacob ME, Bruno-Bárcena JM, Amirsultan S, Stauffer SH, Píqueras VO, et al. Influence of the intestinal microbiota on disease susceptibility in kittens with experimentally-induced carriage of atypical enteropathogenic Escherichia coli. Vet Microbiol. 2019;231:197–206. doi: 10.1016/j.vetmic.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veir JK, Knorr R, Cavadini C, Sherrill SJ, Benyacoub J, Satyaraj E, et al. Effect of supplementation with Enterococcus faecium (SF68) on immune functions in cats. Vet Ther Res Appl Vet Med. 2007;8:229–38. [PubMed] [Google Scholar]
- 44.Fusi E, Rizzi R, Polli M, Cannas S, Giardini A, Bruni N, et al. Effects of Lactobacillus acidophilus D2/CSL (CECT 4529) supplementation on healthy cat performance. Vet Rec Open. 2019;6:e000368. doi: 10.1136/vetreco-2019-000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bybee SN, Scorza AV, Lappin MR. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J Vet Intern Med. 2011;25:856–60. doi: 10.1111/j.1939-1676.2011.0738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres-Henderson C, Summers S, Suchodolski J, Lappin MR. Effect of Enterococcus faecium strain SF68 on gastrointestinal signs and fecal microbiome in cats administered amoxicillin-clavulanate. Top Companion Anim Med. 2017;32:104–8. doi: 10.1053/j.tcam.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Lappin MR, Veir JK, Satyaraj E, Czarnecki-Maulden G. Pilot study to evaluate the effect of oral supplementation of Enterococcus faecium SF68 on cats with latent feline herpesvirus 1. J Feline Med Surg. 2009;11:650–4. doi: 10.1016/j.jfms.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart ML, Suchodolski JS, Steiner JM, Webb CB. Open-label trial of a multi-strain synbiotic in cats with chronic diarrhea. J Feline Med Surg. 2012;14:240–5. doi: 10.1177/1098612X11434386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segovia BM, Torras MDLÁC. Communication of the results of the treatment with probiotics in two cats with chronic gingivostomatitis. Open J Vet Med. 2018;8:9–14. doi: 10.4236/ojvm.2018.82002. [DOI] [Google Scholar]
- 50.Sofyan MS, Rosman N, Krisnu B, Kamaludeen JB, Dadi TB, Pertiwi H. Management of feline idiopathic cystitis (FIC) using probiotic combination treatment. Indian Vet J. 2019;96:20–2. [Google Scholar]
- 51.Masuoka H, Shimada K, Kiyosue-Yasuda T, Kiyosue M, Oishi Y, Kimura S, et al. Transition of the intestinal microbiota of cats with age. PLOS ONE. 2017;12:e0181739. doi: 10.1371/journal.pone.0181739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suchodolski JS, Ruaux CG, Steiner JM, Fetz K, Williams DA. Application of molecular fingerprinting for qualitative assessment of small-intestinal bacterial diversity in dogs. J Clin Microbiol. 2004;42:4702–8. doi: 10.1128/JCM.42.10.4702-4708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suchodolski JS, Ruaux CG, Steiner JM, Fetz K, Williams DA. Assessment of the qualitative variation in bacterial microflora among compartments of the intestinal tract of dogs by use of a molecular fingerprinting technique. Am J Vet Res. 2005;66:1556–62. doi: 10.2460/ajvr.2005.66.1556. [DOI] [PubMed] [Google Scholar]
- 54.Ritchie LE, Burke KF, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Characterization of fecal microbiota in cats using universal 16S rRNA gene and group-specific primers for Lactobacillus and Bifidobacterium spp. Vet Microbiol. 2010;144:140–6. doi: 10.1016/j.vetmic.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 55.Pilla R, Suchodolski JS. The gut microbiome of dogs and cats, and the influence of diet. Vet Clin North Am Small Anim Pract. 2021;51:605–21. doi: 10.1016/j.cvsm.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Altveş S, Yildiz HK, Vural HC. Interaction of the microbiota with the human body in health and diseases. Biosci Microbiota Food Health. 2020;39:23–32. doi: 10.12938/bmfh.19-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterson CT, Rodionov DA, Osterman AL, Peterson SN. B vitamins and their role in immune regulation and cancer. Nutrients. 2020;12:3380. doi: 10.3390/nu12113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vermeer CV. Vitamin K: the effect on health beyond coagulation – an overview. Food Nutr Res. 2012;56:5329. doi: 10.3402/fnr.v56i0.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kather S, Grützner N, Kook PH, Dengler F, Heilmann RM. Review of cobalamin status and disorders of cobalamin metabolism in dogs. J Vet Intern Med. 2020;34:13–28. doi: 10.1111/jvim.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss DJ, Wardrop KJ. Schalm’s veterinary hematology. New York, NY: John Wiley & Sons; 2011. [Google Scholar]
- 61.Xu H, Zhao F, Hou Q, Huang W, Liu Y, Zhang H, et al. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019;10:2618–29. doi: 10.1039/C9FO00087A. [DOI] [PubMed] [Google Scholar]
- 62.Swanson KS, Dowd SE, Suchodolski JS, Middelbos IS, Vester BM, Barry KA, et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011;5:639–49. doi: 10.1038/ismej.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nash MJ, Frank DN, Friedman JE. Early microbes modify immune system development and metabolic homeostasis-the “restaurant” hypothesis revisited. Front Endocrinol. 2017;8:349. doi: 10.3389/fendo.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamada N, Núñez G. Role of the gut microbiota in the development and function of lymphoid cells. J Immunol. 2013;190:1389–95. doi: 10.4049/jimmunol.1203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomkovich S, Jobin C. Microbiota and host immune responses: a love–hate relationship. Immunology. 2016;147:1–10. doi: 10.1111/imm.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tizard IR, Jones SW. The microbiota regulates immunity and immunologic diseases in dogs and cats. Vet Clin North Am Small Anim Pract. 2018;48:307–22. doi: 10.1016/j.cvsm.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–35. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 68.Qin X. What is human inflammatory bowel disease (IBD) more like: Johne’s disease in cattle or IBD in dogs and cats? Inflamm Bowel Dis. 2008;14:138. doi: 10.1002/ibd.20240. [DOI] [PubMed] [Google Scholar]
- 69.Minamoto Y, Hooda S, Swanson KS, Suchodolski JS. Feline gastrointestinal microbiota. Anim Health Res Rev. 2012;13:64–77. doi: 10.1017/S1466252312000060. [DOI] [PubMed] [Google Scholar]
- 70.Suchodolski JS, Camacho J, Steiner JM. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol Ecol. 2008;66:567–78. doi: 10.1111/j.1574-6941.2008.00521.x. [DOI] [PubMed] [Google Scholar]
- 71.Inness VL, McCartney AL, Khoo C, Gross KL, Gibson GR. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridisation with special reference to Desulfovibrio spp. J Anim Physiol Anim Nutr. 2007;91:48–53. doi: 10.1111/j.1439-0396.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 72.Werner M, Suchodolski JS, Lidbury JA, Steiner JM, Hartmann K, Unterer S. Diagnostic value of fecal cultures in dogs with chronic diarrhea. J Vet Intern Med. 2021;35:199–208. doi: 10.1111/jvim.15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mentula S, Harmoinen J, Heikkilä M, Westermarck E, Rautio M, Huovinen P, et al. Comparison between cultured small-intestinal and fecal microbiotas in beagle dogs. Appl Environ Microbiol. 2005;71:4169–75. doi: 10.1128/AEM.71.8.4169-4175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.German AJ, Day MJ, Ruaux CG, Steiner JM, Williams DA, Hall EJ. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med. 2003;17:33–43. doi: 10.1111/j.1939-1676.2003.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 75.Benno Y, Nakao H, Uchida K, Mitsuoka T. Impact of the advances in age on the gastrointestinal microflora of beagle dogs. J Vet Med Sci. 1992;54:703–6. doi: 10.1292/jvms.54.703. [DOI] [PubMed] [Google Scholar]
- 76.Buddington RK. Postnatal changes in bacterial populations in the gastrointestinal tract of dogs. Am J Vet Res. 2003;64:646–51. doi: 10.2460/ajvr.2003.64.646. [DOI] [PubMed] [Google Scholar]
- 77.Greetham HL, Giffard C, Hutson RA, Collins MD, Gibson GR. Bacteriology of the Labrador dog gut: a cultural and genotypic approach. J Appl Microbiol. 2002;93:640–6. doi: 10.1046/j.1365-2672.2002.01724.x. [DOI] [PubMed] [Google Scholar]
- 78.Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Møller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol. 2002;68:673–90. doi: 10.1128/AEM.68.2.673-690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holland KT. Anaerobic bacteria. Cham: Springer Science & Business Media; 2013. [Google Scholar]
- 80.Lagier JC, Dubourg G, Million M, Cadoret F, Bilen M, Fenollar F, et al. Culturing the human microbiota and culturomics. Nat Rev Microbiol. 2018;16:540–50. doi: 10.1038/s41579-018-0041-0. [DOI] [PubMed] [Google Scholar]
- 81.Pereira AC, Cunha MV. An effective culturomics approach to study the gut microbiota of mammals. Res Microbiol. 2020;171:290–300. doi: 10.1016/j.resmic.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol Immunol. 2002;46:535–48. doi: 10.1111/j.1348-0421.2002.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 83.Lan PTN, Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of cecal microbiota in chicken by the use of 16S rDNA clone libraries. Microbiol Immunol. 2002;46:371–82. doi: 10.1111/j.1348-0421.2002.tb02709.x. [DOI] [PubMed] [Google Scholar]
- 84.Suchodolski JS, Dowd SE, Westermarck E, Steiner JM, Wolcott RD, Spillmann T, et al. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009;9:210. doi: 10.1186/1471-2180-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Mazcorro JF, Lanerie DJ, Dowd SE, Paddock CG, Grützner N, Steiner JM, et al. Effect of a multi-species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol Ecol. 2011;78:542–54. doi: 10.1111/j.1574-6941.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 86.Hand D, Wallis C, Colyer A, Penn CW. Pyrosequencing the canine faecal microbiota: breadth and depth of biodiversity. PLOS ONE. 2013;8:e53115. doi: 10.1371/journal.pone.0053115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodrigues Hoffmann A, Patterson AP, Diesel A, Lawhon SD, Ly HJ, Elkins Stephenson C, et al. The skin microbiome in healthy and allergic dogs. PLOS ONE. 2014;9:e83197. doi: 10.1371/journal.pone.0083197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sturgeon A, Stull JW, Costa MC, Weese JS. Metagenomic analysis of the canine oral cavity as revealed by high-throughput pyrosequencing of the 16S rRNA gene. Vet Microbiol. 2013;162:891–8. doi: 10.1016/j.vetmic.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 89.Sturgeon A, Pinder SL, Costa MC, Weese JS. Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet J. 2014;201:223–9. doi: 10.1016/j.tvjl.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 90.Dorn ES, Tress B, Suchodolski JS, Nisar T, Ravindran P, Weber K, et al. Bacterial microbiome in the nose of healthy cats and in cats with nasal disease. PLOS ONE. 2017;12:e0180299. doi: 10.1371/journal.pone.0180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hutchins RG, Vaden SL, Jacob ME, Harris TL, Bowles KD, Wood MW, et al. Vaginal microbiota of spayed dogs with or without recurrent urinary tract infections. J Vet Intern Med. 2014;28:300–4. doi: 10.1111/jvim.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pilla R, Suchodolski JS. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front Vet Sci. 2020;6:498. doi: 10.3389/fvets.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bermingham EN, Young W, Butowski CF, Moon CD, Maclean PH, Rosendale D, et al. The fecal microbiota in the domestic cat (Felis catus) is influenced by interactions between age and diet; a five year longitudinal study. Front Microbiol. 2018;9:1231. doi: 10.3389/fmicb.2018.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reddy KE, Kim HR, Jeong JY, So KM, Lee S, Ji SY, et al. Impact of breed on the fecal microbiome of dogs under the same dietary condition. J Microbiol Biotechnol. 2019;29:1947–56. doi: 10.4014/jmb.1906.06048. [DOI] [PubMed] [Google Scholar]
- 96.Older CE, Diesel AB, Lawhon SD, Queiroz CRR, Henker LC, Rodrigues Hoffmann A. The feline cutaneous and oral microbiota are influenced by breed and environment. PLOS ONE. 2019;14:e0220463. doi: 10.1371/journal.pone.0220463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Du G, Huang H, Zhu Q, Ying L. Effects of cat ownership on the gut microbiota of owners. PLOS ONE. 2021;16:e0253133. doi: 10.1371/journal.pone.0253133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bermingham EN, Young W, Kittelmann S, Kerr KR, Swanson KS, Roy NC, et al. Dietary format alters fecal bacterial populations in the domestic cat (Felis catus) MicrobiologyOpen. 2013;2:173–81. doi: 10.1002/mbo3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scarsella E, Stefanon B, Cintio M, Licastro D, Sgorlon S, Dal Monego S, et al. Learning machine approach reveals microbial signatures of diet and sex in dog. PLOS ONE. 2020;15:e0237874. doi: 10.1371/journal.pone.0237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J Men’s Health. 2020;38:48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serpell JA, Duffy DL. Dog breeds and their behavior. In: Horowitz A, editor. Domestic dog cognition and behavior: the scientific study of Canis familiaris. Berlin: Springer; 2014. pp. 31–57. editor. p. [DOI] [Google Scholar]
- 102.Kathrani A, Werling D, Allenspach K. Canine breeds at high risk of developing inflammatory bowel disease in the south-eastern UK. Vet Rec. 2011;169:635. doi: 10.1136/vr.d5380. [DOI] [PubMed] [Google Scholar]
- 103.You I, Kim MJ. Comparison of gut microbiota of 96 healthy dogs by individual traits: breed, age, and body condition score. Animals. 2021;11:2432. doi: 10.3390/ani11082432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lehtimäki J, Sinkko H, Hielm-Björkman A, Laatikainen T, Ruokolainen L, Lohi H. Simultaneous allergic traits in dogs and their owners are associated with living environment, lifestyle and microbial exposures. Sci Rep. 2020;10:21954. doi: 10.1038/s41598-020-79055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kil DY, Swanson KS. Companion animals symposium: role of microbes in canine and feline health. J Anim Sci. 2011;89:1498–505. doi: 10.2527/jas.2010-3498. [DOI] [PubMed] [Google Scholar]
- 106.Karen LJ. Small intestinal bacterial overgrowth. Vet Clin North Am Small Anim Pract. 1999;29:523–50. doi: 10.1016/S0195-5616(99)50033-8. [DOI] [PubMed] [Google Scholar]
- 107.Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579–89. doi: 10.1111/j.1574-6941.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 108.Tang Y, Manninen TJ, Saris PE. Dominance of Lactobacillus acidophilus in the facultative jejunal Lactobacillus microbiota of fistulated beagles. Appl Environ Microbiol. 2012;78:7156–9. doi: 10.1128/AEM.01975-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol. 2011;76:301–10. doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 110.Kim SY, Adachi Y. Biological and genetic classification of canine intestinal lactic acid bacteria and bifidobacteria. Microbiol Immunol. 2007;51:919–28. doi: 10.1111/j.1348-0421.2007.tb03983.x. [DOI] [PubMed] [Google Scholar]
- 111.Beasley SS, Manninen TJK, Saris PEJ. Lactic acid bacteria isolated from canine faeces. J Appl Microbiol. 2006;101:131–8. doi: 10.1111/j.1365-2672.2006.02884.x. [DOI] [PubMed] [Google Scholar]
- 112.Silva BC, Jung LRC, Sandes SHC, Alvim LB, Bomfim MRQ, Nicoli JR, et al. In vitro assessment of functional properties of lactic acid bacteria isolated from faecal microbiota of healthy dogs for potential use as probiotics. Benef Microbes. 2013;4:267–75. doi: 10.3920/BM2012.0048. [DOI] [PubMed] [Google Scholar]
- 113.Bunešová V, Vlková E, Rada V, Ročková Š, Svobodová I, Jebavý L, et al. Bifidobacterium animalis subsp. lactis strains isolated from dog faeces. Vet Microbiol. 2012;160:501–5. doi: 10.1016/j.vetmic.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 114.Araujo IC, Mota SB, de Aquino MHC, Ferreira AMR. Helicobacter species detection and histopathological changes in stray cats from Niterói, Brazil. J Feline Med Surg. 2010;12:509–11. doi: 10.1016/j.jfms.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston KL, Swift NC, Forster-van Hijfte M, Rutgers HC, Lamport A, Ballàvre O, et al. Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease. J Am Vet Med Assoc. 2001;218:48–51. doi: 10.2460/javma.2001.218.48. [DOI] [PubMed] [Google Scholar]
- 116.Tun HM, Brar MS, Khin N, Jun L, Hui RKH, Dowd SE, et al. Gene-centric metagenomics analysis of feline intestinal microbiome using 454 junior pyrosequencing. J Microbiol Methods. 2012;88:369–76. doi: 10.1016/j.mimet.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alessandri G, Milani C, Mancabelli L, Longhi G, Anzalone R, Lugli GA, et al. Deciphering the bifidobacterial populations within the canine and feline gut microbiota. Appl Environ Microbiol. 2020;86:e02875–19. doi: 10.1128/AEM.02875-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buommino E, Nocera FP, Parisi A, Rizzo A, Donnarumma G, Mallardo K, et al. Correlation between genetic variability and virulence factors in clinical strains of Malassezia pachydermatis of animal origin. New Microbiol. 2016;39:216–23. [PubMed] [Google Scholar]
- 119.Jacobi CA, Schulz C, Malfertheiner P. Treating critically ill patients with probiotics: beneficial or dangerous? Gut Pathog. 2011;3:2. doi: 10.1186/1757-4749-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kochan P, Chmielarczyk A, Szymaniak L, Brykczynski M, Galant K, Zych A, et al. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient—is the time right to revise probiotic safety guidelines? Clin Microbiol Infect. 2011;17:1589–92. doi: 10.1111/j.1469-0691.2011.03614.x. [DOI] [PubMed] [Google Scholar]
- 121.Sanders ME, Akkermans LMA, Haller D, Hammerman C, Heimbach JT, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–85. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.EFSA [European Food Safety Authority] Opinion of the Scientific Committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J. 2005;226:1–12. doi: 10.2903/j.efsa.2005.226. [DOI] [Google Scholar]
- 123.European Commission On a generic approach to the safety assessment of microorganisms used in feed/food and feed/food production. A working paper open for comment [Internet] 2003. https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_out178.pdf [cited 2021 Nov 4]. Available at.
- 124.Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess. 2011 Apr;:1–645. [PMC free article] [PubMed] [Google Scholar]
- 125.Baffoni L. Probiotics and prebiotics for the health of companion animals. In: Di Gioia D, Biavati B, editors. Probiotics and prebiotics in animal health and food safety. Cham: Springer; 2018. pp. 175–95. p. [DOI] [Google Scholar]
- 126.Schmitz SS. Value of probiotics in canine and feline gastroenterology. Vet Clin North Am Small Anim Pract. 2021;51:171–217. doi: 10.1016/j.cvsm.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 127.FEEDAP [EFSA Panel on Additives, Products or Substances used in Animal Feed] Guidance on the safety assessment of Enterococcus faecium in animal nutrition. EFSA J. 2012;10:2682. doi: 10.2903/j.efsa.2012.2682. [DOI] [Google Scholar]
- 128.Rinkinen M, Jalava K, Westermarck E, Salminen S, Ouwehand AC. Interaction between probiotic lactic acid bacteria and canine enteric pathogens: a risk factor for intestinal Enterococcus faecium colonization? Vet Microbiol. 2003;92:111–9. doi: 10.1016/S0378-1135(02)00356-5. [DOI] [PubMed] [Google Scholar]
- 129.EFSA [European Food Safety Authority Opinion of the scientific panel on additives and products or substances used in animal feed (FEEDAP) on the efficacy and safety of the coccidiostat Koffogran. EFSA J. 2004;2:16. doi: 10.2903/j.efsa.2004.16. [DOI] [Google Scholar]
- 130.FEEDAP [EPSA Panel on Additives], Products or Substances used in Animal Feed] Scientific opinion on the safety and efficacy of Cylactin® (Enterococcus faecium) as a feed additive for cats and dogs. EFSA J. 2013;11:3098. doi: 10.2903/j.efsa.2013.3098. [DOI] [Google Scholar]
- 131.Holzapfel W, Arini A, Aeschbacher M, Coppolecchia R, Pot B. Enterococcus faecium SF68 as a model for efficacy and safety evaluation of pharmaceutical probiotics. Benef Microbes. 2018;9:375–88. doi: 10.3920/BM2017.0148. [DOI] [PubMed] [Google Scholar]
- 132.Dogi CA, Perdigón G. Importance of the host specificity in the selection of probiotic bacteria. J Dairy Res. 2006;73:357–66. doi: 10.1017/S0022029906001993. [DOI] [PubMed] [Google Scholar]
- 133.Park H, Yeo S, Arellano K, Kim HR, Holzapfel W. Role of the gut microbiota in health and disease. In: Di Gioia D, Biavati B, editors. Probiotics and prebiotics in animal health and food safety. Cham: Springer; 2018. pp. 35–62. p. [DOI] [Google Scholar]
- 134.Yeo S, Lee S, Park H, Shin H, Holzapfel W, Huh CS. Development of putative probiotics as feed additives: validation in a porcine-specific gastrointestinal tract model. Appl Microbiol Biotechnol. 2016;100:10043–54. doi: 10.1007/s00253-016-7812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weese JS, Anderson MEC. Preliminary evaluation of Lactobacillus rhamnosus strain GG, a potential probiotic in dogs. Can Vet J. 2002;43:771–4. [PMC free article] [PubMed] [Google Scholar]
- 136.Strompfová V, Lauková A, Ouwehand AC. Lactobacilli and enterococci — potential probiotics for dogs. Folia Microbiol. 2004;49:203–7. doi: 10.1007/BF02931403. [DOI] [PubMed] [Google Scholar]
- 137.Kainulainen V, Tang Y, Spillmann T, Kilpinen S, Reunanen J, Saris PEJ, et al. The canine isolate Lactobacillus acidophilus LAB20 adheres to intestinal epithelium and attenuates LPS-induced IL-8 secretion of enterocytes in vitro. BMC Microbiol. 2015;15:4. doi: 10.1186/s12866-014-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cunningham M, Azcarate-Peril MA, Barnard A, Benoit V, Grimaldi R, Guyonnet D, et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021;29:667–85. doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Weese JS, Arroyo L. Bacteriological evaluation of dog and cat diets that claim to contain probiotics. Can Vet J. 2003;44:212–6. [PMC free article] [PubMed] [Google Scholar]
- 140.Weese JS, Martin H. Assessment of commercial probiotic bacterial contents and label accuracy. Can Vet J. 2011;52:43–6. [PMC free article] [PubMed] [Google Scholar]
- 141.Metras BN, Holle MJ, Parker VJ, Miller MJ, Swanson KS. Assessment of commercial companion animal kefir products for label accuracy of microbial composition and quantity. J Anim Sci. 2020;98:skaa301. doi: 10.1093/jas/skaa301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mora D, Filardi R, Arioli S, Boeren S, Aalvink S, de Vos WM. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: the case of VSL#3. Microb Biotechnol. 2019;12:1371–86. doi: 10.1111/1751-7915.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]