Abstract
Fertilization of an egg by multiple sperm (polyspermy) leads to lethal genome imbalance and chromosome segregation defects. In Arabidopsis thaliana, the block to polyspermy is facilitated by a mechanism that prevents polytubey (the arrival of multiple pollen tubes to one ovule). We show here that FERONIA, ANJEA, and HERCULES RECEPTOR KINASE 1 receptor-like kinases located at the septum interact with pollen tube–specific RALF6, 7, 16, 36, and 37 peptide ligands to establish this polytubey block. The same combination of RALF (rapid alkalinization factor) peptides and receptor complexes controls pollen tube reception and rupture inside the targeted ovule. Pollen tube rupture releases the polytubey block at the septum, which allows the emergence of secondary pollen tubes upon fertilization failure. Thus, orchestrated steps in the fertilization process in Arabidopsis are coordinated by the same signaling components to guarantee and optimize reproductive success.
Seed plants rely on tightly regulated fertilization mechanisms to secure fertility and reproductive success. Like in animals, the entrance of supernumerary sperm into a single egg—i.e., polyspermy—is restricted to ensure chromosomal balance and progeny health (1, 2). Fertilization in angiosperms is more complex because two sperm cells are carried by one pollen tube that grows in the maternal pistil tissues and ultimately releases its sperm cell cargo inside the ovule (3). Although hundreds of pollen tubes may grow into the transmitting tract of a pistil, usually only a single tube, in response to attractants, emerges from the septum in the vicinity of each ovule to target the ovule (Fig. 1A) (4). The block to polytubey (i.e., the emergence of multiple pollen tubes targeting an ovule) prevents the occurrence of polyspermy. This polytubey block is likely further reinforced by successful gamete fusion that triggers programmed cell death (PCD) of the persistent synergid cell, which leads to the elimination of pollen tube attractants. Thus, the first pollen tube that emerges from the septum will have the privilege of fertilizing the female gametes, providing a precondition for conspecific pollen precedence (i.e., the preferential use of pollen from the same species for fertilization) (5). If the first pollen tube fails, however, fertilization success will be ensured by fertilization recovery (6, 7), in which the polytubey block is suspended to allow the emergence of secondary pollen tubes for another chance of fertilization. Therefore, plants can (i) restrict polyspermy by enforcing the polytubey block at the septum under normal circumstances and (ii) salvage fertility by removing the polytubey block when fertilization fails. Here, we report the molecular mechanisms by which the polytubey block is implemented or suspended, when needed.
Fig. 1. FER, ANJ, and HERK1 control the polytubey block.
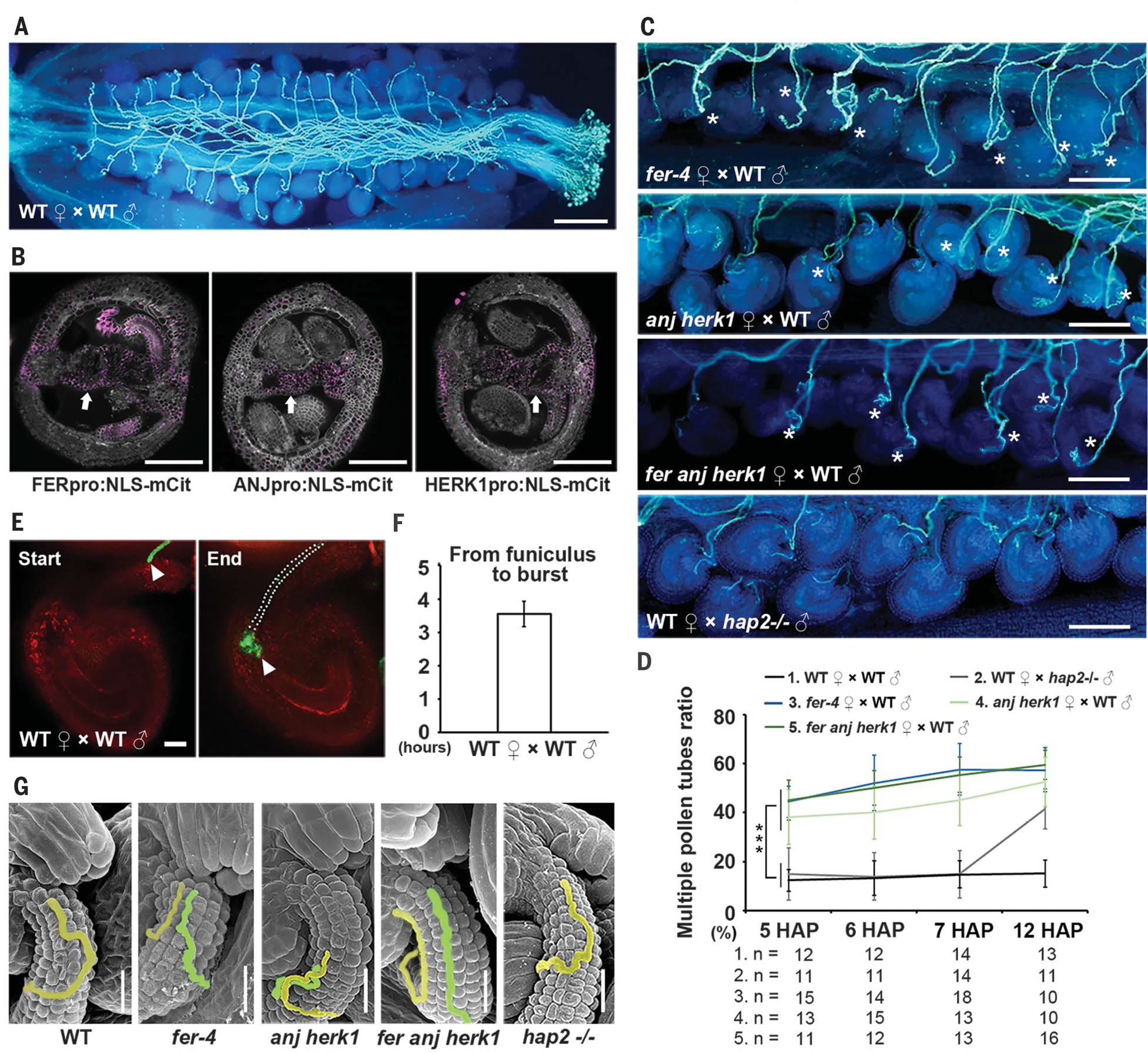
(A) A WT pistil pollinated by WT pollen showing that each ovule is targeted by a single pollen tube at 12 HAP. Scale bar, 200 μm. (B) Cross sections of pistils showing promoter:reporter expression pattern of FER, ANJ, and HERK1. Arrows indicate the septum epidermal layer. Scale bars, 100 μm. NLS-mCit, mCitrine with nuclear localization sequence. (C) Aniline blue staining showing multiple pollen tubes emerging at the septum at 5 HAP in fer-4, anj herk1, and fer anj herk1 pistils pollinated with WT pollen, but rarely in WT pistils pollinated with WT pollen and hap2−/− pollen. Asterisks indicate multiple pollen tubes. The analysis was repeated at least three times. Scale bars, 100 μm. (D) Statistical analysis of multiple pollen tube ratios, as indicated. “n” refers to the number of pistils. Data are mean values ± SDs; ***P < 0.01 (Student’s t test). (E) Semi–in vivo assay to determine the duration time of pollen tube growth along the funiculus until rupture in the embryo sac (white arrow heads indicate pollen tubes labeled with LAT52pro:GFP). Dashed dots show position of the pollen tube. Scale bar, 20 μm. GFP, green fluorescent protein. (F) Statistics of (E). Data are mean values ± SDs. (G) Scanning electron microscopy images of ovules of WT pistils pollinated with WT pollen and hap2−/− pollen and fer-4, anj herk1, and fer anj herk1 pistils pollinated with WT pollen at 5 HAP. Scale bars, 20 μm.
FERONIA, ANJEA, and HERCULES RECEPTOR KINASE 1 receptor kinases are required to establish the polytubey block
To identify factors that may establish the polytubey block, we conducted RNA sequencing (RNA-seq) analysis using transmitting tract and septum tissues and searched for candidate receptors that may perceive signals from the pollen tube. We found that seven malectin-like domain-containing receptor-like kinase (MLD-RLK) (also known as Catharanthus roseus RLK1-LIKE or CrRLK1L) genes were highly expressed [verified by real-time quantitative polymerase chain reaction (qPCR); fig. S1, A and B]. Transcriptional and translational markers showed that three of these genes—FERONIA (FER), ANJEA (ANJ), and HERCULES RECEPTOR KINASE 1 (HERK1)—were expressed in the ovule, transmitting tract, and septum epidermis (Fig. 1B and fig. S2). We further realized that a polytubey phenotype can be (i) caused by the failure of establishing the block at the septum or (ii) triggered by fertilization recovery at the later stage. To distinguish between these polytubey phenotypes, we exploited the hap2/gcs1 mutant that is defective in gamete fusion and triggers fertilization recovery (8, 9). By CRISPR-Cas9, we obtained the loss-of-function mutant hap2−/− (fig. S3) and determined that, when depositing hap2−/− pollen on wild-type (WT) pistils, the polytubey phenotype occurs at ~7 hours after pollination (HAP) (Fig. 1, C and D). To determine when the polytubey block occurs at the septum, we observed the growth behavior of WT pollen tubes in semi–in vivo assays (10). It takes a pollen tube 3.5 ± 0.4 hours (n = 3 repeats of three to five pollen tubes each) to grow along the funiculus into the ovule and burst (Fig. 1, E and F). Therefore, pollen tubes would emerge in vivo from the septum at ~4 HAP. Accordingly, a polytubey phenotype at the septum should be detectable at 4 HAP in vivo. Thus, we examined the emergence of polytubey at 5, 6, and 7 HAP, contrasting with 12 HAP, that has previously been used for characterizing fer-4 (11, 12) and/or anj herk1 mutants (13). Aniline blue staining showed that at 5 to 7 HAP, multiple pollen tubes emerged with comparable growth rates in the receptor mutants fer-4 (11), anj herk1, and fer anj herk1 (13). This was much more frequent compared with the hap2−/− mutant (Fig. 1, C and D, and figs. S4 and S5A). Scanning electron microscopy demonstrated that at 5 HAP, multiple pollen tubes grew at the funiculus of fer-4, anj herk1, or fer anj herk1 ovules. This was rarely observed in WT or hap2−/− (Fig. 1G). The comparable polytubey ratios of fer anj herk1 and fer-4 indicated that FER and/or ANJ and HERK1 receptors may form a receptor complex required for establishing the polytubey block at the septum, which occurs much earlier than the initiation of fertilization recovery in the ovule (6, 7).
Five RALF peptides from pollen tubes trigger the polytubey block
Peptides of the rapid alkalinization factor (RALF) family function upstream of MLD-RLK receptors in plant development, immunity response, pollen tube perception, and rupture (12–17). To identify candidate RALF peptides involved in establishing the polytubey block, we examined the pollen-specific MYB transcription factor mutant, myb97 myb101 myb120, which showed similar pollen tube reception defects as those observed in fer-4 and anj herk1 mutants (18, 19). We found that the emergence of multiple pollen tubes can be observed at 5 to 7 HAP in WT pistils when pollinated by myb triple mutant pollen (fig. S6), which indicates the compromised polytubey block at the septum. We thus conducted RNA-seq analysis of myb mutant pollen tubes and identified five RALF genes with expression levels that were lowered or absent in the myb triple mutant (fig. S7). They are RALF6 (At1g60625), RALF7 (At1g60815), RALF16 (At2g32835), and two noncanonical RALF36 (At2g32785) and RALF37 (At2g32788) (20), which cluster into two subclades (fig. S7). Their expression in pollen and pollen tubes was further confirmed (Fig. 2, A and B).
Fig. 2. Pollen-specific RALF6, 7, 16, 36, and 37 peptides control the polytubey block and pollen tube reception.
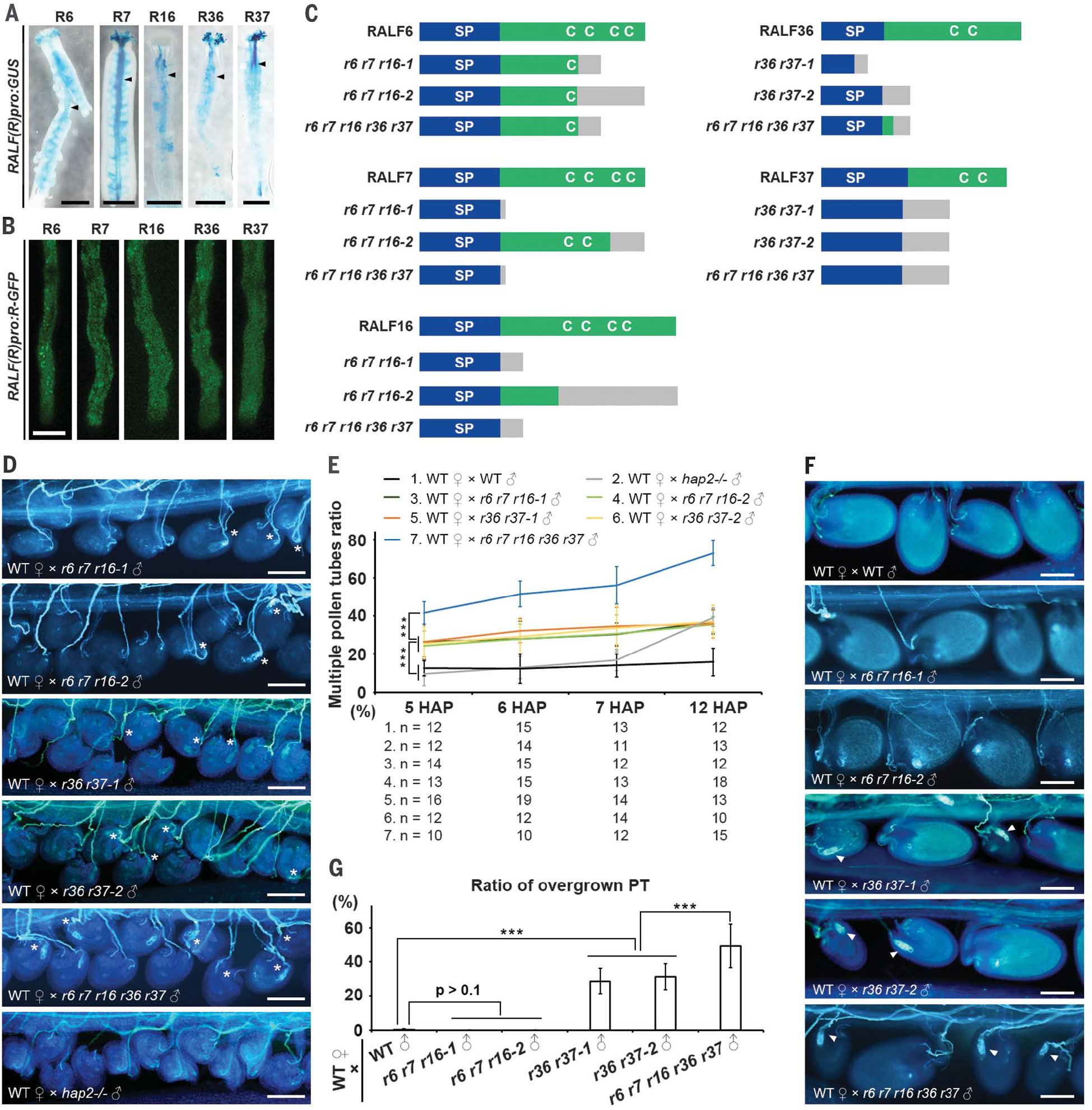
(A) Promoter:GUS plants of five RALF genes show GUS signals in the pollen tubes (black arrowheads). Scale bars, 500 μm. (B) GFP signals in pollen tubes of plants containing RALF-GFP fusion proteins expressed by their native promoter. Scale bar, 10 μm. (C) Schematic diagram of RALF6, 7, 16, 36, and 37 peptide structures showing the positions of conserved cysteine residues and their CRISPR-Cas9–edited mutant structures in ralf6 ralf7 ralf16 triple (abbreviated as r6 r7 r16), ralf36 ralf37 double (r36 r37), and ralf6 ralf7 ralf16 ralf36 ralf37 quintuple (r6 r7 r16 r36 r37) mutants. SP, signal peptide; C, conserved cysteine residue. Gray boxes indicate missense sequences resulting from frame shift mutations. (D) Aniline blue staining showing multiple pollen tubes emerging at the septum at 5 HAP in WT pistils pollinated with ralf triple, double, and quintuple mutant and hap2−/− mutant pollen. Asterisks indicate multiple pollen tubes. The analysis was repeated at least three times. Scale bars, 100 μm. (E) Statistical analysis of multiple pollen tube emergence as observed in WT and (D). “n” refers to the number of pistils. (F) Aniline blue staining showing pollen tube overgrowth at the micropyle (white arrowheads) in WT pistils pollinated with WT pollen and ralf triple, double, and quintuple mutant pollen at 48 HAP. Arrowheads indicate overgrown pollen tubes. The analysis was repeated at least three times. Scale bars, 100 μm. (G) Statistical analysis of the pollen tube (PT) overgrowth phenotype in pistils shown in (F). Data are mean values ± SDs; ***P < 0.01 (Student’s t test).
Next, we used CRISPR-Cas9 to generate ralf36 ralf37 double, ralf6 ralf7 ralf16 triple, and ralf6 ralf7 ralf16 ralf36 ralf37 quintuple mutants. They all showed normal vegetative growth behavior (Fig. 2C and figs. S8 and S9). When WT pistils were pollinated with mutant pollen, all three ralf mutants showed the polytubey phenotype at 5 to 7 HAP (Fig. 2, D and E, and fig. S5B), resembling what was observed in fer-4, anj herk1, or fer anj herk1 mutant pistils pollinated by WT pollen. The higher polytubey ratio caused by ralf quintuple than by ralf double or triple mutations suggests that the five RALF genes function collectively to establish a polytubey block. Therefore, we hypothesized that the five RALF peptides are pollen tube–produced signal molecules that are likely perceived by the FER-ANJ-HERK1 receptor complex at the septum to establish a polytubey block.
Because the FER-ANJ-HERK1 receptor complex also controls pollen tube reception at the micropyle (12–14, 21), we next investigated whether the five RALF peptides are also required for pollen tube reception. We observed that in WT pistils pollinated by ralf double and quintuple mutant pollen grains, higher ratios of pollen tube overgrowth (failures in reception) occurred at the micropyle [28.7 ± 7.4% (n = 30), 31.1 ± 7.5% (n = 30), and 49.2 ± 12.8% (n = 22) for ralf36 ralf37–1, ralf36 ralf37–2, and the ralf quintuple mutant, respectively] (Fig. 2, F and G). Consistently, fertility analysis showed that male defects led to obviously reduced fertility in the ralf double mutants (70.8 ± 7.8%, n = 30, and 68.5 ± 7.7%, n = 30, respectively) and more severely in the ralf quintuple mutant (57.1 ± 10.9%, n = 22, P < 0.01 versus ralf36 ralf37 mutants) (fig. S10). These defects were similar to those observed in fer-4, anj herk1, or fer anj herk1 mutants (fig. S11) (12–14). These findings suggest that RALF6, 7, 16, 36, and 37 are likely the long-pursued ligands of FER and ANJ-HERK1 receptors required for pollen tube reception (12, 13). As both the establishment of a polytubey block at the septum and pollen tube reception in the ovule appear to require the same signaling components, an indepth mechanistic study on their precisely controlled interaction was necessary.
FER, ANJ, and HERK1 physically interact with RALF6, 7, 16, 36, and 37 peptide ligands
In vitro pull-down assays showed that biotinylated RALF6, 7, 16, 36, and 37 bind to 6×Histagged ectodomains of FER, ANJ, and HERK1 purified from insect cells or corresponding maltose-binding protein (MBP)–tagged ectodomains purified from Escherichia coli (Fig. 3, A to F). These interactions were strengthened in a peptide dose–dependent manner (Fig. 3, G to I). Microscale thermophoresis (MST) analysis further revealed that both canonical RALF6 and noncanonical RALF36 interact with FER, ANJ, and HERK1 with low equilibrium dissociation constants (Kd) (Fig. 3J), which demonstrates that these RALFs are ligands of FER, ANJ, and HERK1. Moreover, in vitro pull-down assays showed that the addition of RALF6, 36, and 37 promoted the interactions between FER and ANJ-HERK1 receptors (Fig. 3, K and L), which suggests that the RALF6, 36, and 37 peptides may facilitate the formation of larger FER-ANJ-HERK1 heteromeric receptor complexes.
Fig. 3. FER, ANJ, and HERK1 receptors interact with RALF6, 7, 16, 36, and 37 peptide ligands.

(A to F) Pull-down assays between 6×His-tagged [(A) to (C)] and MBP-tagged [(D) to (F)] ectodomains of FER, ANJ, and HERK1 and biotinylated RALF6, 7, 16, 36, and 37 and elf24. (G to I) Pull-down assay between HA-tagged FER, ANJ, and HERK1 ectodomains and biotinylated RALF36 obtained by elevating the concentration of RALF36. (J) Binding affinity as indicated by MST analysis. CVY1 and RALF36, FER and elf24, and FER only were used as controls. (K and L) Interaction between 6×His-tagged ectodomains of FER and HA-tagged ectodomains of ANJ and HERK1 by pull-down assays in the presence or absence of RALF6, 36, and 37 peptides.
Pollen tube rupture releases the polytubey block and coordinates fertilization recovery
Because the emergence of secondary pollen tubes ensures reproductive success in cases of fertilization failure, the mechanism regulating the polytubey block has to be adjustable. We therefore investigated when and how the polytubey block is removed. After perception by the receptive synergid, the pollen tube bursts to release two sperm cells for double fertilization within ~20 min (4, 22–26). FER-mediated pollen tube reception and production of reactive oxygen species (ROS) in the filiform apparatus region of the ovule have been shown to be required for pollen tube rupture (21). We hypothesized that the presence of RALF peptides is required not only for establishing but also for maintaining the polytubey block at the septum. Thus, pollen tube rupture that naturally terminates the production of RALF peptides would weaken or remove the polytubey block. To test this hypothesis, by using antibodies against RALF36 and 37, we detected immunofluorescence signals in the cell wall of the whole shank region in intact WT pollen tubes but not in the ralf36 ralf37 mutant (Fig. 4, A, D, and E, and fig. S12). This indicates that the polytubey block can be maintained as long as the pollen tube grows inside the ovule. However, the intensity of RALF36 and 37 signals in the cell wall declined rapidly 2 min after pollen tube rupture (Fig. 4, B, D, and E) and was no longer detectable after 15 min (Fig. 4, C, D, and E), whereas released pollen tube content showed strong immunofluorescence (Fig. 4, B and C). Thus, pollen tube rupture results in the loss of RALF peptides that are required to maintain the polytubey block at the septum and allows secondary pollen tubes to exit the septum.
Fig. 4. Pollen tube rupture removes the polytubey block and coordinates fertilization recovery.
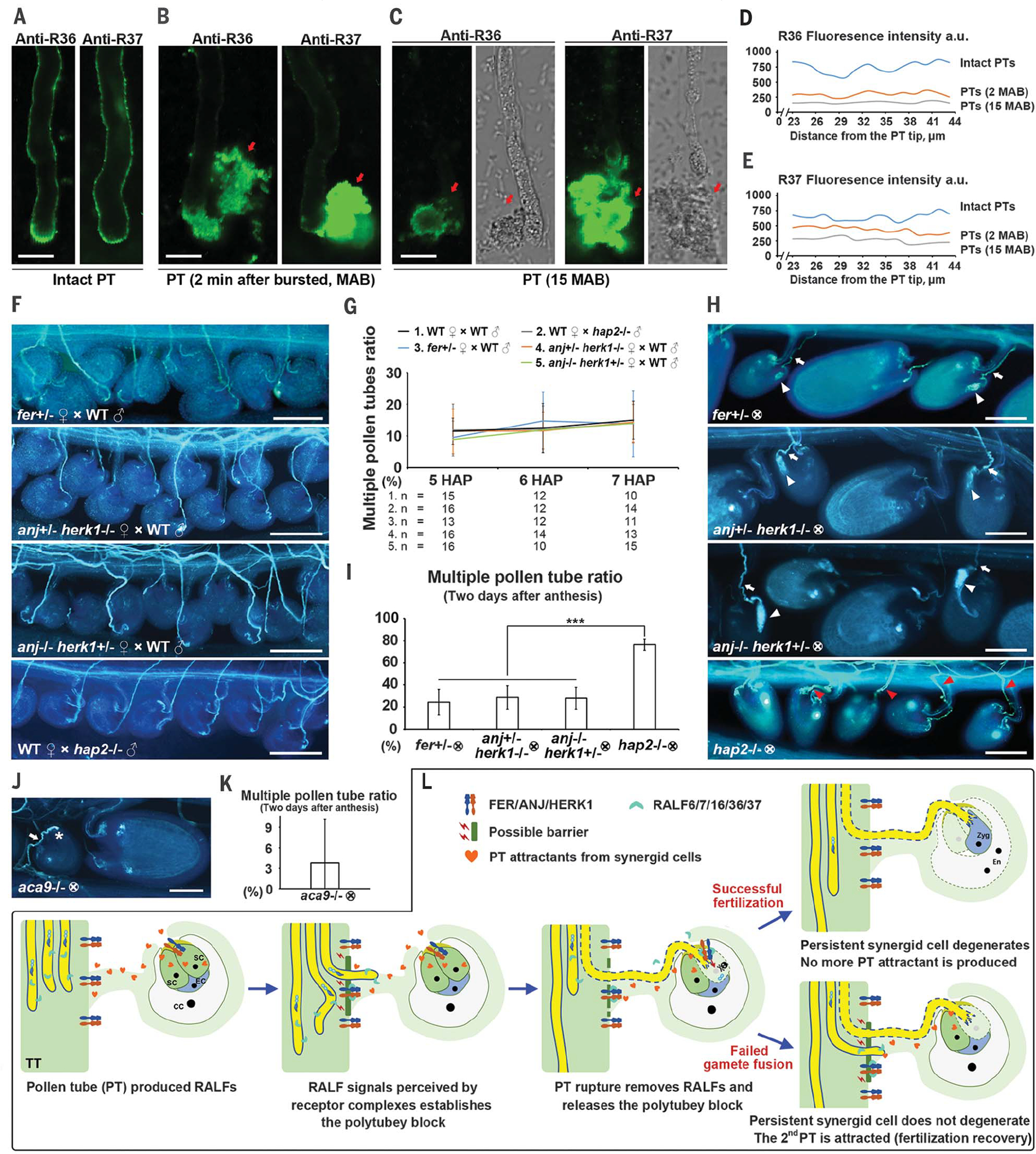
(A) Immunofluorescence imaging of RALF36 and 37 on the surface of intact pollen tubes. (B and C) Shortly after pollen tube burst (2 min), RALF36- and RALF37-derived fluorescence signal on the pollen tube surface decreased substantially (B) and could scarcely be detected at 15 min (C). Cytosolic RALF36 and 37 (red arrows) were released after pollen tube burst at the tip. The analysis was repeated at least three times. Scale bars, 10 μm. (D and E) Quantification of immunofluorescence intensity along the shank of the pollen tube surface at stages shown in (A) to (C). Intact pollen tubes and pollen tubes 2 and 15 min after burst (MAB) were measured (n = 17, 18, and 23 for anti-R36, and n = 22, 20, and 18 for anti-R37). a.u., arbitrary units. (F) Aniline blue staining of pollen tube emerging status in fer+/−, anj+/−herk1−/−, and anj−/−herk1+/− pollinated with WT pollen and WT pistil pollinated with hap2−/− pollen at 5 HAP. The analysis was repeated at least three times. Scale bars, 100 μm. (G) Statistical analysis of the multiple pollen tube ratio at stages indicated in pistils shown in WT and (F). “n” refers to the number of pistils. (H) Aniline blue staining of self-crossed fer+/− and hap2−/− mutants 2 days after anthesis. White arrowheads indicate overgrown pollen tubes, white arrows mark single pollen tubes in the self-crossed fer+/− mutant, and red arrowheads point toward multiple pollen tubes in the self-crossed hap2−/− mutant. The analysis was repeated at least three times. Scale bars, 100 μm. (I) Statistical analysis of multiple pollen tubes ratio in pistils shown in (H). (J) Aniline blue staining of self-crossed aca9−/− mutant 2 days after anthesis. White arrow indicates the single pollen tube, and white asterisk indicates blocked pollen tube. Scale bar, 100 μm. (K) Statistical analysis of multiple pollen tubes ratio in pistils shown in (J). (L) Model of how pollen-specific RALFs act on female tissue located at FER, ANJ, and HERK1 receptor complexes to jointly coordinate establishment, maintenance, and removal of the polytubey block during fertilization progression. TT, transmitting tract; SC, synergid cell; EC, egg cell; CC, central cell; Zyg, zygote; En, endosperm. Data are mean values ± SDs; ***P < 0.01 (Student’s t test).
To genetically test this hypothesis, we examined the receptor mutants fer+/− (11), anj−/− herk1+/−, and anj+/− herk1−/− (13). Like WT, these heterozygous mutants should be able to establish and maintain the polytubey block at the septum. Compared with the hap2−/− control, none of the three heterozygous mutant pistils exhibited significant emergence of multiple WT pollen tubes at 5 to 7 HAP (Fig. 4, F and G, and fig. S5C). Absence of FER or ANJ-HERK1 in synergids was previously reported to result in failure of pollen tube rupture and impaired fertility, and pollen tube overgrowth can be easily visualized in the mutant ovule (12, 13, 21). We therefore investigated whether selfed heterozygous receptor mutants would show a polytubey phenotype that is triggered by fertilization recovery. Two days after anthesis, in those ovules with failed events of pollen tube rupture (i.e., pollen tube overgrowth), only low levels of polytubey were observed [fer+/−, 24.3 ± 11.8% (n = 28); anj−/− herk1+/−, 28.6 ± 10.5% (n = 16); and anj+/− herk1−/−, 27.9 ± 9.8% (n = 21)], which were much lower than that of the hap2−/− mutant (76.4 ± 5.2%, n = 12) (Fig. 4, H and I), indicating that the removal of the polytubey block required for fertilization recovery was compromised. We next investigated another mutant, aca9 [autoinhibited calcium adenosine triphosphatase (ATPase) 9], which showed defects in pollen tube rupture in the ovule (27) (Fig. 4J). Like the receptor mutants, aca9 did not produce an increased level of polytubey 2 days after anthesis [3.8 ± 6.4% (n = 20)] (Fig. 4, J and K). This further confirms that the removal of the polytubey block at the septum depends on pollen tube rupture in the embryo sac. Taken together, the FER, ANJ, and HERK1 receptor complexes not only mediate pollen tube–synergid recognition and subsequent pollen tube rupture in the embryo sac (12, 13), but also function to trigger the removal of the polytubey block at the septum for fertilization recovery.
Discussion
Here, we have elucidated a molecular mechanism of how FER, ANJ, and HERK1 receptor complexes located at the septum interact with pollen tube–produced RALF6, 7, 16, 36, and 37 peptide ligands to coordinately establish, maintain, and terminate the polytubey block and thus regulate the emergence of pollen tubes to ultimately prevent polyspermy and to ensure reproductive success. On the basis of this and previous studies, we suggest the following model (Fig. 4L): (i) Pollen tube attractants secreted from the synergid cells trigger the nearest pollen tube to exit the transmitting tract. (ii) RALFs secreted from this pollen tube activate FER, ANJ, and HERK1 signaling in septum epidermal cells to establish the polytubey block that prevents the emergence of additional pollen tubes. This male-female jointly established polytubey block remains activated during pollen tube growth into the ovule as a result of the continuous production of RALF peptides by the first-emerged pollen tube. (iii) After successful recognition by the same receptor complex (FER-ANJ-HERK1) in synergid cells, the pollen tube ruptures to release two sperm cells, fertilization will be completed within 20 min (21–26), and the polytubey block is then removed as RALFs quickly disappear from the ruptured pollen tube. (iv) Once fertilization is successful, the persistent synergid cell undergoes fertilization-dependent PCD and fuses with the fertilized central cell. Pollen tube attractants are dispersed, modified, and degraded (11, 28–30), which reduces the attraction of further pollen tubes from the septum despite the release of the polytubey block. Polyspermy is thus prevented. (v) When gamete fusion fails, the persistent synergid cell remains alive and continues to produce pollen tube attractants. Because the polytubey block is removed shortly after pollen tube rupture, secondary pollen tubes are able to emerge from the septum to salvage fertilization. The secondary pollen tube reestablishes the polytubey block, which also explains the low rate of tertiary pollen tubes (6, 23, 30).
This study demonstrates how Arabidopsis regulates the emergence of a single pollen tube at the septum to target each ovule and how fertilization success and recovery are interconnected with the activity of the same receptor complexes. It will now be essential to elucidate the downstream mechanism or mechanisms that block the emergence of secondary pollen tubes at the septum. Further components of this polytubey block may include nitric oxide (11); secretion of cell wall components; and ROS that mediate FER-controlled pollen tube rupture (21), pollen hydration (31), and self-incompatibility (32).
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Ye for providing fer-4 and myb97 myb101 myb120 mutant seeds; L. Smith for sharing anj, herk1, anj herk1, and fer anj herk1 mutant seeds; J. F. Harper for providing aca9 mutant seeds; and C. Li and Q. Duan for sharing fer+/− mutant seeds.
Funding:
L.-J.Q. was funded by the National Natural Science Foundation of China (grant nos. 31991202, 31830004, 31620103903, and 31621001), S.Z. was supported by the Young Elite Scientists Sponsorship Program by the China Association of Science and Technology (2019QNRC001), Z.G. was supported by a NSFC Young Scientists Fund (31900161), A.Y.C. was funded by the US Natural Science Foundation (IOS-1645854, MCB-1715764, and MCB-0955910), J.D. was funded by the National Institute of Health (R01GM109080), and T.D. was supported by the German Research Foundation DFG (SFB924).
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability:
All data are available in the main text or the supplementary materials.
REFERENCES AND NOTES
- 1.Wolf DP, Dev. Biol. 64, 1–10 (1978). [DOI] [PubMed] [Google Scholar]
- 2.Evans JP, Mol. Reprod. Dev. 87, 341–349 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Zhang J et al. , Nat. Plants 3, 17079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong S, Qu L-J, Curr. Opin. Plant Biol. 51, 7–14 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Zhong S et al. , Science 364, eaau9564 (2019).31147494 [Google Scholar]
- 6.Beale KM, Leydon AR, Johnson MA, Curr. Biol. 22, 1090–1094 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasahara RD et al. , Curr. Biol. 22, 1084–1089 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T, Nat. Cell Biol. 8, 64–71 (2006). [DOI] [PubMed] [Google Scholar]
- 9.von Besser K, Frank AC, Johnson MA, Preuss D, Development 133, 4761–4769 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Palanivelu R, Preuss D, BMC Plant Biol. 6, 7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan Q et al. , Nature 579, 561–566 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Escobar-Restrepo JM et al. , Science 317, 656–660 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Galindo-Trigo S et al. , EMBO Rep. 21, e48466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge Z, Dresselhaus T, Qu L-J, Trends Plant Sci. 24, 978–981 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR, Science 343, 408–411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C et al. , eLife 4, e06587 (2015). [Google Scholar]
- 17.Stegmann M et al. , Science 355, 287–289 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Leydon AR et al. , Curr. Biol. 23, 1209–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y et al. , PLOS Genet. 9, e1003933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abarca A, Franck CM, Zipfel C, Plant Physiol. 187, 996–1010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan Q et al. , Nat. Commun. 5, 3129 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Hamamura Y et al. , Curr. Biol. 21, 497–502 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Sprunck S et al. , Science 338, 1093–1097 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Denninger P et al. , Nat. Commun. 5, 4645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamamura Y et al. , Nat. Commun. 5, 4722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dresselhaus T, Sprunck S, Wessel GM, Curr. Biol. 26, R125–R139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiøtt M et al. , Proc. Natl. Acad. Sci. U.S.A. 101, 9502–9507 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Völz R, Heydlauff J, Ripper D, von Lyncker L, Groβ-Hardt R, Dev. Cell 25, 310–316 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Maruyama D et al. , Cell 161, 907–918 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Yu X et al. , Nature 592, 433–437 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Liu C et al. , Science 372, 171–175 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Zhang L et al. , Curr. Biol. 31, 3004–3016.e4 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.


