Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines are capable of inducing combined humoral and cellular immunity. Which effect is more relevant for their potent protective effects is unclear, but isolated T cell responses without seroconversion in healthy household members of individuals with Coronavirus disease 19 (COVID-19) suggest that T cell responses effectively protect against clinical infection. Oncologic patients have an outsize risk of unfavorable outcomes after SARS-CoV-2 infection and therefore were prioritized when vaccines first became available, although the quality of their immune response to vaccination was expected to be suboptimal, as has been confirmed in subsequent studies. Inherently, patients with anti-CD19 chimeric antigen receptor (CAR) T cell therapy-mediated B cell aplasia would be incapable of generating humoral responses, so that assessment of the vaccine-induced cellular immunity is all the more important to gauge whether the vaccine can induce meaningful protection. A salient difference between T cell and humoral responses is the former's relative impassiveness to mutations of the antigen, which is more relevant than ever since the advent of the omicron variant. The objective of this study was to assess the immune cell composition and spike protein-specific T cell responses before and after the first and second doses of SARS-CoV-2 mRNA vaccine in a cohort of juvenile CD19 CAR T cell therapy recipients with enduring B cell aplasia. The prospective study included all patients age >12 years diagnosed with multiply relapsed B cell precursor acute lymphoblastic leukemia and treated with anti-CD19 CAR T cell (CAR-T19) therapy in our center. The primary endpoint was the detection of cell-mediated and humoral responses to vaccine (flow cytometry and anti-S immunoglobulin G, respectively). Secondary endpoints included the incidence of vaccine-related grade 3 or 4 adverse events, exacerbation of graft-versus-host disease (GVHD), relapse, and the influence of the vaccine on CAR T cells and lymphocyte subsets. Even though one-half of the patients exhibited subnormal lymphocyte counts and marginal CD4/CD8 ratios, after 2 vaccinations all showed brisk T-cell responsiveness to spike protein, predominantly in the CD4 compartment, which quantitatively was well within the range of healthy controls. No severe vaccine-related grade 3 or 4 adverse events, GVHD exacerbation, or relapse was observed in our cohort. We posit that SARS-CoV-2 mRNA vaccines induce meaningful cellular immunity in patients with isolated B cell deficiency due to CAR-T19 therapy.
Key Words: Anti-CD19 CAR T cell-induced B cell aplasia, CAR T cell therapy, SARS-CoV-2 vaccination, Immune response
INTRODUCTION
Coronavirus 19 disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); its course can be severe, with overall mortality approaching 1% among unvaccinated adults [1]. Patients with malignant diseases and an impaired immune system, especially recipients of hematopoietic stem cell transplantation (HSCT) and immune cell therapy, have an increased risk of an aggravated course of the disease or death 2, 3, 4 and thus were granted prioritized access despite the expectation of less-than-optimal vaccine responsiveness.
In December 2020/January 2021, first the US Food and Drug Administration (FDA) and then the European Medicines Agency (EMA) approved the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech; Pfizer, New York, NY) for the prevention of COVID-19, based on a Phase III study that showed 94.6% clinical efficacy in the general population [5]. Humoral responses after 1 dose of this vaccine are low, whereas the second dose strongly enhances the serologic response. It is thus recommended that hematology-oncology patients and HSCT recipients should receive their vaccinations 3 to 4 weeks apart [6]. More recently, in May 2021, the EMA also approved BNT162b2 for children age 12 to 15 years; it is important to note that prior to that date, adolescent hematology-oncology patients did not have access to SARS-CoV-2 vaccines. A reduced-dose SARS-CoV-2 vaccine for children age 5 to 11 years was approved and released in December 2021.
The effectiveness of SARS-CoV-2 vaccination in patients with hematologic malignancies appears to be influenced by the therapy and the underlying disease. Several studies have shown blunted immune responses to mRNA vaccines in solid organ transplant recipients [7] as well as in patients with multiple myeloma, chronic lymphoblastic leukemia, lymphoma, and myeloproliferative malignancies and in HSCT recipients compared with healthy individuals 8, 9, 10, 11, 12. Unsurprisingly, especially poor serologic responses were seen in B lineage non-Hodgkin lymphoma treated with depleting anti-CD20 antibodies within 12 months before vaccination [11].
Although the FDA, European Society for Blood and Marrow Transplantation (EBMT), American Society of Transplantation and Cellular Therapy, American Society of Hematology, and National Marrow Donor Program recommend SARS-CoV-2 vaccination for immunosuppressed patients 13, 14, 15, limited data are available on its efficacy and safety in patients undergoing chimeric antigen receptor (CAR) immune effector cell therapy.
CD19-targeting CAR T cell therapy (CAR-T19) is a relevant treatment option for patients with refractory and recurrent lymphoid malignancies, especially acute lymphoblastic leukemia and diffuse large B cell lymphoma. Owing to absolute B cell aplasia after successful CAR-T19 therapy, a humoral immune response is not expected after vaccination. There are limited data on the cellular response rate of CAR-T19 recipients after SARS-CoV-2 vaccination. Two small series of adult patients reported response rates of 0% and 36% [16,17]; similar data are not available for children and adolescents after CAT-T19 therapy.
All approved vaccines induce a combined humoral and cellular immune response in the majority of healthy recipients, whereas in cancer patients typically both responses are blunted, at least at the cohort level. Thus, the benefits of one response over the other are impossible to dissect. However, cocktails of recombinant neutralizing antibodies appear to provide clinically meaningful protection from progression to severe COVID-19. On the other hand, studies of household members of COVID-19-positive individuals who neither got sick nor developed a humoral response showed robust SARS-CoV-2-specific T cell responses [18]. What emanates, therefore, is that both branches of the adaptive immune system by themselves provide some protection against COVID-19 disease. The question whether B cell-deficient patients are able to mount specific T cell immunity in response to SARS-CoV-2 vaccination is thus more than just academic.
In this study, we aimed to evaluate the safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine in young patients who had previously received CAR-T19 therapy and remained B cell aplastic.
METHODS
Study Design and Participants
The prospective study included 8 patients age >12 years (and hence eligible for SARS-CoV-2 vaccination) diagnosed with multiply relapsed B cell precursor acute lymphoblastic leukemia and treated with CAR-T19 therapy between 2016 and 2021. Patients were eligible for this study if they met the EBMT criteria for COVID-19 vaccination (version 6.0; May 27, 2021), including age >12 years and at least a 3-month interval between cell infusion and vaccination. Seven of 8 patients had undergone HSCT prior to relapse and CAR-T19 therapy and thus were at risk of GVHD, but neither showed signs of nor received treatment for acute or chronic GVHD at the time of vaccination. None of the patients had contracted SARS-CoV-2 infection prior to vaccination.
For benchmarking of our observations in the CAR-T19 cohort, we similarly tested 10 random healthy controls (6 females and 4 males) who were either double-vaccinated with an mRNA vaccine (Comirnaty/BNT162b2; BioNTech, Mainz, Germany; n = 5) or Spikevax/mRNA1273; Moderna, Cambridge, MA; n = 1) or a combination of vector-based (Vaxzevria; AstraZeneca, Gothenburg, Sweden) and mRNA (Comirnaty) vaccination (n = 4), depending on availability and prioritization. Mean age of the control group was 34.3 years (range, 24 to 46 years).
All patients and healthy vaccine recipients signed informed consent to provide blood samples and clinical information. The study was approved by Goethe University Medical Center's Ethics Committee (case 2021-180).
Patients were vaccinated through the national German vaccination program that started in January 2021 for patients age >18 years and in June 2021 for patients age >12 years. To ascertain whether they were SARS-CoV-2-naïve, all patients had a baseline serology test for anti-nucleocapsid antibodies, a baseline IgG level, and quantification of peripheral CAR T cells and corona-specific T cells, as well as determination of lymphocyte subsets. These tests were repeated 4 weeks after the first and 4 weeks after the second vaccination. Assessment of acute or chronic GVHD was done during the visits 4 weeks after the first and second vaccinations. In personal or telephone contact, we asked the patients about vaccine-related side effects (eg, pain at the injection site, headache, fever, allergy, arthralgia, weakness) or GVHD exacerbation. We graded acute and chronic GVHD according to the EBMT-NIH-CIBMTR Task Force position statement on standardized terminology and guidance for GVHD [19].
The study's primary endpoint was the detection of cell-mediated and humoral responses to vaccine (flow cytometry and anti-S immunoglobulin G, respectively).
Secondary endpoints included the incidence of vaccine-related grade 3 or 4 adverse events, GVHD exacerbation, relapse, and the influence of the vaccine on CAR T cells and lymphocyte subsets.
Determination of Lymphocyte Subsets
We examined the frequency and concentration of lymphocytes, CD3+ T cells, CD3+CD4+ T4 and CD3+CD8+ T8 T helper cells, CD56+CD3-natural killer (NK) cells, and CD19+ B cells. The differentiation of the T8 and T4 compartments into naïve (CD45RA+CD62L+; Tnaïve), central memory (CD45R0+CD62L+; TCM), effector memory (CD45R0+CD62L-; TEM) and effector memory RA (CD45RA+CD62L-; TEMRA) T cells, as well as regulatory T cells (CD4+CD25+CD127dim/neg; Tregs), were queried. EDTA-anticoagulated peripheral blood samples were collected, kept at room temperature, and analyzed within 24 hours. Flow cytometry was performed as a dual-platform analysis (FC500 flow cytometer; Beckman Coulter, Brea, CA). Monoclonal antibodies against CD45, CD56, CD19, CD3, CD4, CD25, CD45RA, CD62L, CD45R0, CD127, and CD8 (all purchased from Beckman Coulter) were conjugated with fluorescein isothiocyanate, phycoerythrin (PE), phycoerythrin-Texas Red tandem, phycoerythrin-cyanine-5, and phycoerythrin-cyanine7 for staining. In brief, whole blood was mixed with the respective antibody and incubated at room temperature for 15 minutes in the dark. An automated lyse/no wash procedure with a fixation step was performed using the TQ-Prep Workstation (Beckman Coulter). The reference range of healthy donors was taken from the literature [20,21].
Detection of CAR T Cells
First, a NH4Cl-based erythrocyte lysing solution (Beckman Coulter) was added to 200 µL of PBS-EDTA, incubated for 10 minutes at room temperature, and then washed with PBS buffer containing .5% human serum albumin. Following 15 minutes of incubation with CD19 CAR Detection Reagent, a biotinylated recombinant extracellular domain of CD19 (Miltenyi Biotec, Bergisch Gladbach, Germany), cells were washed twice and then incubated for 15 minutes with anti-biotin-PE (Miltenyi Biotec) and 7-AAD, CD3- allophycocyanin (APC), and CD45-KrO (Beckman Coulter). After a final washing step, cells were acquired on a Navios flow cytometer (Beckman Coulter). CAR T cells were defined as CD45+/7-AAD-/lymphocytes/CD3+/anti-CD19 CAR+.
SARS-CoV-2-Specific T-Cell Responses
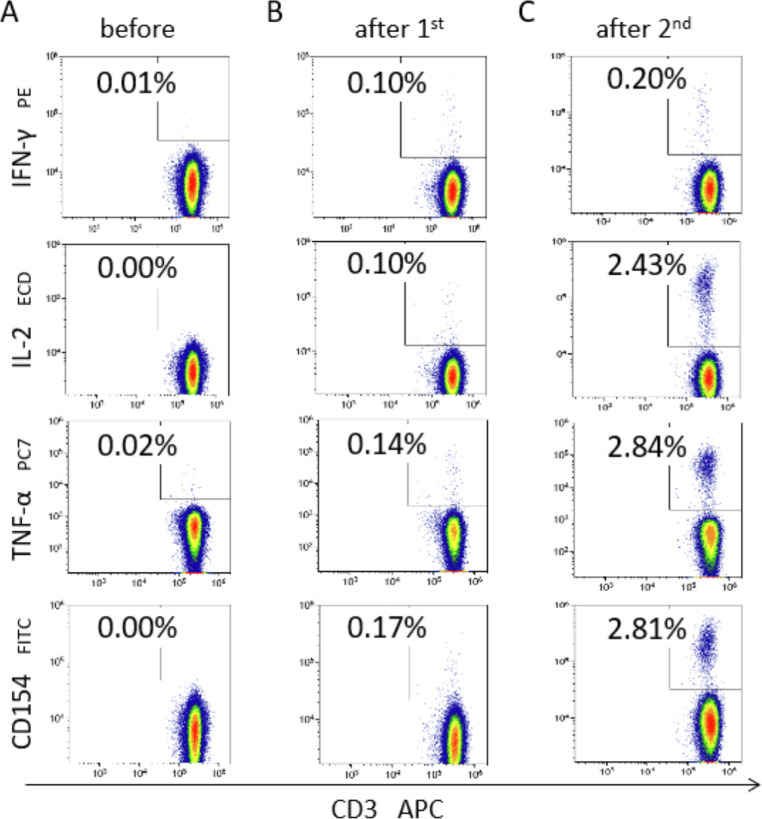
T cell response was evaluated by stimulating fresh heparinized peripheral blood in vitro with PepTivator SARS-CoV-2 Prot_S Complete (Miltenyi Biotec), a pool of lyophilized overlapping peptides covering almost the full sequence of SARS-CoV-2 spike protein (amino acids 5 to 1273) dissolved in sterile water/10% DMSO solution. Positive controls using CytoStim and negative controls were included in each experiment, performed using the SARS-CoV-2 T Cell Analysis Kit (Whole Blood), Human (Miltenyi Biotec) according to the manufacturer's instructions. Lymphocyte subsets were then prepared for flow cytometry quantification by mixing with antibodies against CD45, CD56, CD14, CD20, CD3, CD4, CD8, IFN-γ, IL-2, TNF-α, and CD154 (CD40L) conjugated with KrO, Brilliant Violet 786, Pacific Blue, VioBlue, APC, APC-Vio 770, APC-Alexa Fluor 700, PE, PE-Vio 615, PE-Vio 770, and Vio Bright B515. Flow cytometry was performed using a 13-color/3-laser flow cytometer (DxFLEX; Beckman Coulter). Detection of 150,000 CD3+ events was targeted for each sample. Kaluza software (Beckman Coulter) was then used for precise gating. A positive reaction to antigen stimulation was defined as the difference between negative controls and stimulation samples in all IFN-γ+, IL-2+, TNF-α+, and CD154+/CD40+ levels for CD4+ and CD8+ T cells, as well as for CD56+CD3- NK cells. Double-reactive (IL-2+CD154+/CD40L+ or IFN-γ+TNF-α+) T cells were calculated as well. Exemplary flow cytometry plots with the frequency of SARS-CoV-2-specific cells in one patient are shown in Figure 1 .
Figure 1.
Exemplary FACS plots. The figure shows exemplary flow cytometry density plots of SARS-CoV-2 specific T-cells in a patient before and after 1st and 2nd vaccinations with an mRNA vaccine. Plots, gated on T helper cells, show CD3 (X-axis) over the cytokines IFN-γ, IL-2, TNF-α and CD154, respectively (top to bottom, Y-axis).
Qualitative and Quantitative SARS-CoV-2 IgG/IgM Measurement
Qualitative detection of SARS-CoV-2 nucleocapsid protein-specific IgG (SARS-CoV-2-IgG chemiluminescent microparticle immunoassay [CMIA]; Abbott, Abbott Park, IL) and SARS-CoV-2 spike protein-specific IgM (SARS-CoV2-IgM CMIA; Abbott) antibodies, and quantitative detection of SARS-CoV-2 Spike IgG (SARS-CoV-2 IgG II Quant CMIA; Abbott) were performed using the automated Abbott Alinity i [22] platform according to the manufacturer's recommendations.
Statistical Methods and Software
Continuous variables are described as the median and range of values. Descriptive statistics were calculated in Excel (Microsoft, Redmond, WA), and graphs were created using GraphPad Prism (GraphPad Software, San Diego, CA). The precise gating was performed with CXP and Kaluza software (Beckman Coulter). Comparisons among 3 groups (before, after first vaccination, and after second vaccination) and between 2 groups (before and after second vaccination) were assessed as paired, not normally distributed samples using the Friedmann test and Wilcoxon rank-sum test, respectively. Comparisons after the second vaccination between patients and healthy controls were done using the Mann-Whitney Utest for unpaired non-normally distributed samples. P-values <.05 were considered significant.
RESULTS
All patients were in complete remission (CR) at the time of vaccination, with persistent CAR T cells and B cell aplasia. Two patients were treated with B cell-depleting monoclonal antibodies; both had received rituximab within 6 months before and 3 months after vaccination. Another patient received inotuzumab and was treated with donor lymphocyte infusion. All patients were in complete remission (CR) at the time of vaccination, with persistent CAR-T19 cells and B-cell aplasia. Two patients were treated with B-cell-depleting monoclonal antibodies. Both had received rituximab within six months before and within three months after vaccination and both patients had persistence of CAR-T cells and B-cell aplasia through this procedure. One additional patient received inotuzumab and was treated with donor lymphocyte infusions. Seven of 8 patients were under regular IgG substitution, with a median IgG trough level of 722 mg/dL (range, 171 to 1112 mg/dL). None of the patients showed signs of acute or chronic GVHD, and none of the patients had had a SARS-CoV-2 infection prior to vaccination. The median patient age was 18.7 years (specifically, patients were 12, 13, 15, 17, 19, 19, 23, and 28 years old). Patient characteristics are summarized in Table 1 ).
Table 1.
Patient Characteristics (N = 8)
| Characteristic | Value |
|---|---|
| Age, yr, median (range) | 18.7 (12-28) |
| Sex, male/female, n | 4/4 |
| Diagnosis, n | |
| cALL | 4 |
| Pre-B-ALL | 4 |
| Time from CAR-T19 therapy to vaccination, mo, median (range) | 48 (39-148) |
| Vaccine type: Pfizer-BioNTech, n (%) | 8 (100) |
| Disease state at vaccination: CR, n (%) | 8 (100) |
| Prior HSCT before CAR-T19 therapy, n (%) | 7 (87.5) |
| Patients with complete B cell aplasia, n (%) | 7 (87.5) |
| IgG level, per mg/dL, median (range) | 722 (171-1114) |
| Patients under regular IgG substitution, n (%) | 7 (87.5) |
| Exposure to anti-CD20/22 antibodies 6 mo before vaccination, n (%) | 2 (29) |
| Total lymphocyte count before first vaccination, /µL, median (range) | 977 (350-2240) |
| Prior exposure to SARS-CoV-2, n (%) | 0 (0) |
cALL indicates common acute lymphoblastic leukemia; B-ALL, B cell acute lymphoblastic leukemia.
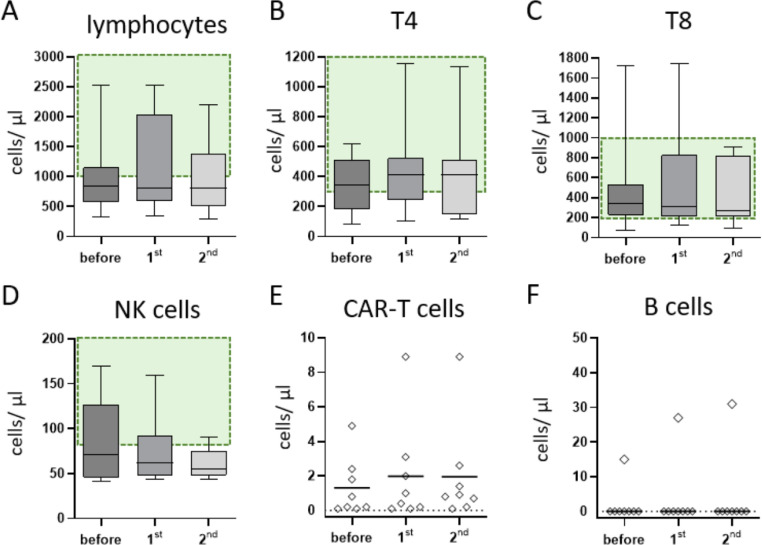
Lymphocyte counts were numerically normal in 3 of the 8 patients and decreased in the other 5 (Figure 2 A). The CD8+ T cell count was age-matched normal in 7 of the 8 patients, but absolute CD4+ T helper cell lymphopenia was predominant in 4. In the 4 patients classified as normal, the value was in the lower normal range. The CD4:CD8 ratios were skewed slightly in favor of CD8+ cells, with a median of 1.05 (range, .3 to 1.7; normal, .9 to 3.0), but mostly not outright pathologic. No significant changes in lymphocyte subpopulations were observed by vaccination status (Figure 2B-D). After vaccination, no change in CAR T cell persistence was observed in any of the patients (Figure 2E), and all patients maintained a deep remission of their underlying malignancy. Seven of the 8 patients had no B cells detected, as expected (Figure 2F).
Figure 2.
Lymphocyte subpopulations and persistence of CAR-T cells. Shown are selected lymphocyte subsets before and after the 1st and 2nd vaccination dose. The reference ranges for healthy controls are shown in green. In the median, the patients show absolute lymphocyte values slightly below the reference range (A), whereas the median absolute cell counts for T-helper and cytotoxic T-cells were in the lower reference range (B+C). Median NK-cells were slightly decreased (D). CAR T-cell concentrations were stable over the vaccination period (E). All but one patient showed complete B-cell aplasia (F).
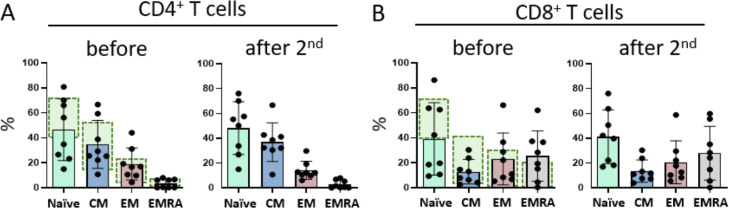
Differentiation of the CD4+ and CD8+ T cells into Tnaïve, TCM, TEM, and TEMRAcells was stable over time (Figure 3 A,B). There is no significant difference in the subgroups of T4 and T8 cells in the Tnaïve, TCM, TEM, and TEMRAsubgroups before versus after the second vaccination. Analyzing the patients’ individual courses showed a very slight tendency toward an increase in TCM T4 cells.
Figure 3.
T-cell differentiation. Flow cytometric measurements of naïve, central, effector memory, and EMRA T-cells for T-helper (A) and cytotoxic T-cells (B) illustrate the paucity of naïve T-cells in both compartments. Normal values for the respective T-cell subsets of healthy adolescents are highlighted in green. Vaccination did not significantly alter the composition of T cells.
Tolerability and Safety
None of the patients experienced severe (grade 3 or 4) vaccine-associated adverse events. Four of 7 patients reported pain at the injection site, and 1 patient developed a fever. No patient presented with acute or chronic GVHD at the time of vaccination or developed signs of GVHD thereafter (7 at risk). No patient developed post-vaccination cytopenia.
Vaccine Efficacy
Humoral response
Humoral response to SARS-CoV-2 vaccination was routinely monitored in all patients. The patient with minimal CD19+ cell concentrations between 15 and 31/µL despite CAR T cell persistence was the only patient to show a borderline antibody level (7.4 binding antibody units/mL). As expected, the other, fully B cell aplastic patients showed no humoral immune response.
Cellular response
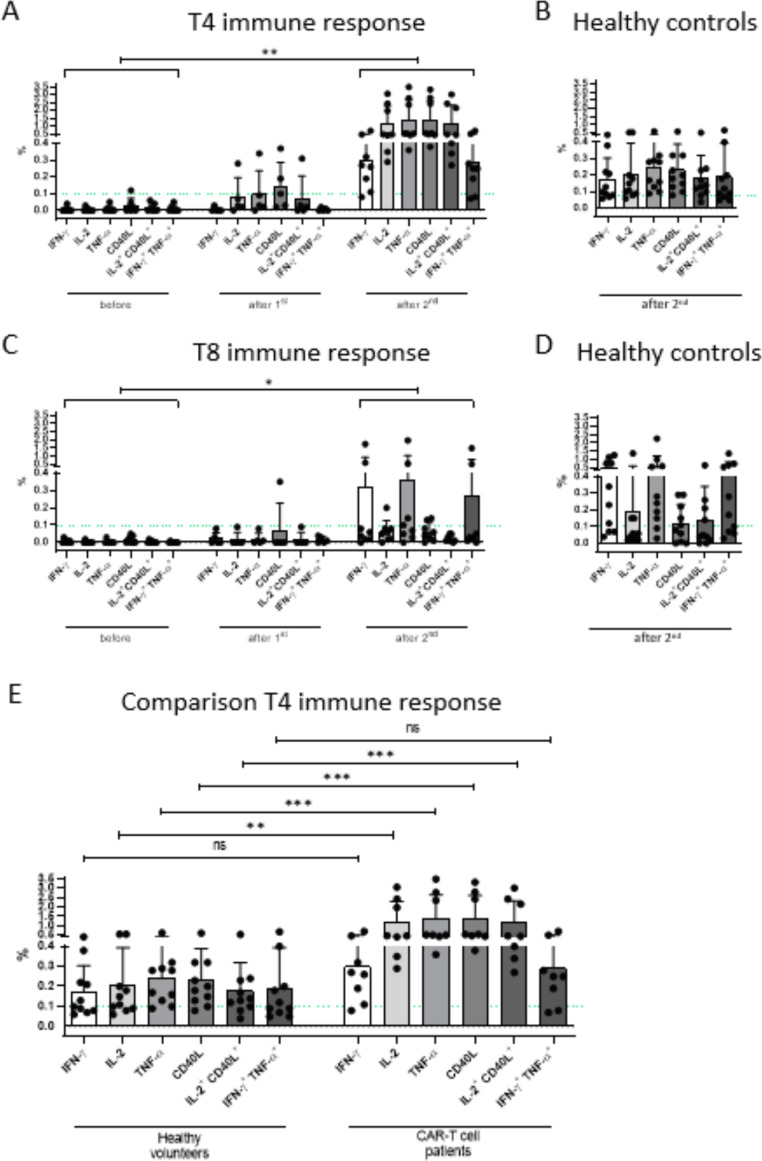
SARS-CoV-2-specific CD4+ T helper cell reactivity was detectable in all 8 patients treated with CAR-T19 therapy (100%). This specific response related to the cytokines IFN-γ, IL-2, TNF-α, and CD40L showed a significant increase after the second vaccination compared with the readings before the first vaccination (P = .0078; Figure 4 A). Comparing the immune response before and after vaccination, patients showed significantly higher frequencies of SARS-CoV-2-reactive T helper cells for IL-2 (P< .013), TNF-α (P< .0017), CD40L (P< .0019), and IL-2+CD40L+ (P< .002). Nonsignificantly higher readings were obtained for IFN-y+CD4+ T cells. SARS-CoV-2-specific T helper cell concentrations ranged between .5 and 12 /µL. A vaccine response for T8 cells was also seen in all patients (Figure 4C), albeit a relatively weaker response than in the T4 compartment. Compared with healthy similarly vaccinated controls, the frequency of S protein-reactive T4 cells was even higher (Figure 4E). For TNF-α CD40L- and IL-2+CD40L-producing T-helper cytokines, the comparison between CAR-T patients and healthy volunteers was highly significant at P< .001. For IL-2 alone, there was P< .01. For IFN-γ alone and INF-γ + TNF-α co-producing T4, there was no statistical significance (Figure 4E).
Figure 4.
T-cell response to SARS-CoV-2 mRNA vaccination. SARS-CoV-2-specific T-cell responses of helper (A) and cytotoxic cells (C) is shown for CAR-T patients and healthy controls (B, D). Patients after CAR-T cell therapy showed a significant increase in specific T-helper cells after the 2nd vaccination (A). Compared with healthy controls (E), significantly higher levels of SARS-CoV-2 specific CD4+ T-cells were obtained for IL-2, TNFα, CD40L, and IL-2+CD40L. Similar outcomes, albeit less pronounced than for helper T-cells, were observed for cytotoxic T-cells (C). Antigen-specific cytotoxic T-cells from healthy vaccine recipients and CAR-T19 patients were similar in frequency (D).
The immune response appears to be a multicytokine-producing population (Supplementary Figure S1). These polyfunctional T cells form the major portion and produce primarily CD40L, IL-2, and CD154 (Supplementary Figure S1B). The IFN-γ- producing population is a much smaller and independent population.
Patient Follow-Up
All 8 patients maintained regular social contacts, including attending work or school. Three patients contracted SARS-CoV-2; although the viruses were not sequenced, the timing of the infection suggests 1 delta infection and 2 omicron infections. Two patients had mild symptoms (rhinitis and mild fever); 1 patient's infection was caught during routine screening during an outpatient department visit. Out of an abundance of caution, the latter patient received sotrovimab 1 day after the positive PCR result, and he remained asymptomatic throughout.
DISCUSSION
Our data show that despite B cell aplasia and often subnormal T cell concentrations in CAR-T19-treated patients, SARS-CoV-2 vaccines elicit potent cellular vaccination responses dominated by T helper reactivity, quantitively at least on par with the responses seen in normal volunteers. The fact that a strong cellular vaccine response is regularly seen retrospectively validates the decision to prioritize CAR-T19 recipients’ access to SARS-CoV-2 vaccination.
Even for at-risk groups such as our present cohort, SARS-CoV-2 vaccination was not available in children age <12 years until December 2021, that is, for 80% of our CAR-T19 patients. Thus, experience with the vaccine in this age group in general, and in a pediatric-adolescent B cell aplastic cohort in particular, is limited to nonexistent. The uniformity of our data supports definitive conclusions despite the study's modest size. These conclusions are that the BNT162b2 mRNA COVID-19 vaccine Comirnaty is safe in patients with B cell aplasia after CAR-T19 therapy, does not cause cytopenia, and does not exacerbate GVHD. Predictably, in the absence of B cells, patients do not mount a humoral response. In contrast, the cellular response was at least as strong as in healthy vaccination controls, even though one-half of the patients were T lymphocytopenic. Vaccinations did not deplete CAR-T19 cells, trigger CAR-T19 cell-mediated autoimmune phenomena, or interfere with leukemia control—in short, they were quite safe and tolerable in our cohort.
Despite the observed robust cellular immunity to spike protein, the patients were advised to continue to be cautious. Therefore, we can only speculate about the protective value of isolated T cell responses. According to an earlier report, some household members of COVID-19 patients who remained SARS-CoV-2 PCR-negative as well as anti-SARS-CoV-2 antibody-negative during quarantine were later found to have mounted isolated SARS-CoV-2-specific T cell responses [18]. The authors proposed that these were at least somewhat protective, preventing the development of clinical COVID-19. We therefore posit that likely vaccinated CAR-T19 patients enjoy meaningful immunity, even if possibly not as potent as if both branches of the adaptive immune system could contribute, and indeed our limited clinical data seem to support this. Despite their predicted very high risk of severe COVID, none of the 3 patients eventually diagnosed with SARS-CoV-2 infection developed significant symptoms. The risks for severe COVID and death from SARS-CoV2 for the omicron variant in nonimmunized individuals seem to be only about one-half as high as those for the delta variant [23]. Extrapolated to our cohort, this would still imply very high morbidity and mortality if vaccination did not induce meaningful cellular immunity. CAR-T19 clinical guidelines thus far had recommended vaccination in analogy to other vaccines but remained moot on the (clinical) value of immunization in this patient cohort [15,24]. Our response rate of 100% is much higher than that in the only other report on vaccine-induced T cell immunity in CAR-T19 patients [17]. Even though slightly different technology was used for SARS-CoV-2-specific T cell detection, the response rate was only 50% (6 of 12) in that adult cohort. We propose that the 2-fold higher response rate in our patient cohort is related to their young age [25,26].
In summary, we have demonstrated robust and potentially clinically meaningful T cell responses to SARS-CoV-2 vaccines in patients with CAR-T19-mediated humoral immunodeficiency. Like the SARS-CoV-2 vaccine, most other vaccines (with the exception of polysaccharide vaccines) also induce adaptive T cell responses. On the basis of the evidence provided here, we therefore propose reconsidering the attitude toward vaccinating CAR-T19 patients with nonlive vaccines [27]. It will be important to supplement these with investigation of antigen-specific T cell responses [15].
ACKNOWLEDGMENTS
Financial disclosure: There was no outside funding for this project.
Conflict of interest statement: There are no conflicts of interest to report.
Footnotes
Financial disclosure: See Acknowledgments on page XXX.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.04.017.
Appendix. Supplementary materials
REFERENCES
- 1.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piñana JL, Martino R, García-García I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez-MacGregor M, Lei X, Zhao H, Scheet P, Giordano SH. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol. 2022;8:69–78. doi: 10.1001/jamaoncol.2021.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enssle JC, Campe J, Schwenger A, et al. Severe impairment of T-cell responses to BNT162b2 immunization in patients with multiple myeloma patients. Blood. 2022;139:137–142. doi: 10.1182/blood.2021013429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roeker LE, Knorr DA, Pessin MS, et al. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34:3047–3049. doi: 10.1038/s41375-020-01030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5:3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarisch A, Wiercinska E, Daqiq-Mirdad S, et al. SARS-CoV-2 specific T-cells are generated in less than half of allogeneic HSCT recipients failing to seroconvert after COVID-19 vaccination [e-pub ahead of print]. Eur J Immunol. doi: 10.1002/eji.202149771, https://www.ebmt.org/sites/default/files/2022-01/COVID%20vaccines%20version%208.3%20-%202022-01-03.pdf. [DOI] [PMC free article] [PubMed]
- 13.American Society of Hematology. ASH-ASTCT COVID-19 vaccination for HCT and CAR T cell recipients: frequently asked questions. 2022. Available at: https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients.
- 14.Jarisch A, Wiercinska E, Daqiq-Mirdad S, et al. SARS-CoV-2-specific T cells are generated in less than half of allogeneic HSCT recipients failing to seroconvert after COVID-19 vaccination. Eur J Immunol. 2022 Apr 7:10.1002/eji.202149771. 10.1002/eji.202149771. Epub ahead of print. PMID: 35389515; PMCID: PMC9087431. [DOI] [PMC free article] [PubMed]
- 15.Hayden PJ, Roddie C, Bader P, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA) Ann Oncol. 2022;33:259–275. doi: 10.1016/j.annonc.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138:1278–1281. doi: 10.1182/blood.2021012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-a single-center prospective cohort study. Transplant Cell Ther. 2021;27:788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallais F, Velay A, Nazon C, et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27:113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53:1401–1415. doi: 10.1038/s41409-018-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 21.Huenecke S, Fryns E, Wittekindt B, et al. Percentiles of lymphocyte subsets in preterm infants according to gestational age compared to children and adolescents. Scand J Immunol. 2016;84:291–298. doi: 10.1111/sji.12474. [DOI] [PubMed] [Google Scholar]
- 22.Seo JD, Song DY, Nam Y, et al. Evaluation of analytical performance of Alinity i system on 31 measurands. Pract Lab Med. 2020;22:e00185. doi: 10.1016/j.plabm.2020.e00185. PMID: 33204790; PMCID: PMC7652776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy. Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) Haematologica. 2020;105:297–316. doi: 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 26.Huenecke S, Behl M, Fadler C, et al. Age-matched lymphocyte subpopulation reference values in childhood and adolescence: application of exponential regression analysis. Eur J Haematol. 2008;80:532–539. doi: 10.1111/j.1600-0609.2008.01052.x. [DOI] [PubMed] [Google Scholar]
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.