Abstract
Federal statutes authorize several agencies to protect human populations from chemical emergencies and provide guidance to evacuate, clean, and re-occupy affected areas. Each of the authorized federal agencies have developed programs to provide managers, public health officials, and regulators, with a rapid assessment of potential hazards and risks associated with chemical emergencies. Emergency responses vary based on exposure scenarios, routes, temporal considerations, and the substance(s) present. Traditional chemical assessments and derivation of toxicity values are time-intensive, typically requiring large amounts of human epidemiological and experimental animal data. When a rapid assessment of health effects is needed, an integrated computational approach of augmenting extant toxicity data with in vitro (new alternative toxicity testing methods) data can provide a quick, evidence-based solution. In so doing, multiple streams of data can be used, including literature searches, hazard, dose-response, physicochemical, and environmental fate and transport property data, in vitro cell bioactivity testing and toxicogenomics. The field of toxicology is moving towards increased use of this approach as it transforms from observational to predictive science. The challenge is to objectively and transparently derive toxicity values using this approach to protect human health and the environment. Presented here are examples and efforts toward rapid risk assessment that demonstrate unified, parallel, and complementary work to provide timely protection in times of chemical emergency.
Keywords: emergency response, risk assessment, federal agencies, alternative toxicity testing, NAMs, computational tools
Chemical risk assessment is central to the evaluation and interpretation of the effects of chemical exposures on the natural environment and human health. The general goal is to assure the public that no harm is expected or inform them what to expect from the exposure dose or concentration. The scope of assessment changes based on its purpose, be it emergency response, clean-up of contaminated sites, selection of less-toxic alternatives, or national-level regulatory actions. Regardless of scope, a common challenge in assessing risk is a lack of hazard and dose-response data to determine potential human health risk(s) associated with chemical exposures. As such, chemical risk assessment is transitioning to a more predictive science through increased incorporation of New Approach Methods (NAMs) based data (EPA, 2020). In emergencies, the primary objective of chemical evaluation is to provide timely information that guides decision-making, leading to protection from chemical exposures and helping people return to and reoccupy their communities, workplaces, or living facilities. In practical applications of risk assessment in emergency response, assumptions and uncertainties are integrated by necessity and pragmatically into the precautionary principle; a complete or comprehensive understanding of the toxicity and its associated biological mechanisms for the chemical of concern often is unknown or unavailable (Goldstein, 2001).
The need to characterize risk from chemical exposures is an intrinsic part of the mission of the Agency for Toxic Substances and Disease Registry (ATSDR), the U.S. Environmental Protection Agency (EPA); and the U.S. Department of Defense (DoD), each having a slightly different purpose or scope. Here we describe the similarities and differences associated with collecting toxicology data and developing tools to address specific functional chemical evaluation requirements for each agency in characterizing human health risks associated with environmental exposures.
ATSDR: Responding to Community Needs
ATSDR protects communities from harmful health effects related to exposure to natural and manufactured hazardous substances (ATSDR, 2021). ATSDR investigates environmental exposures to hazardous substances and recommends action(s) to reduce harmful exposures and their health consequences. ATSDR responds to environmental health emergencies, investigates emerging environmental health threats, conducts research on the health effects of hazardous waste sites, and provides emergency response assistance, in collaboration with federal, state, and local agencies.
The Office of Emergency Management (OEM) coordinates ATSDR’s response to requests for rapidly needed information concerning the health implications of chemical spills. ATSDR staff members include trained emergency response coordinators with knowledge and experience to identify the human health issues arising from acute releases of hazardous chemicals. An emergency response coordinator is available 24/7 and can be reached through the Centers for Disease Control and Prevention’s Emergency Operations Center. The coordinator has access to toxicologists, epidemiologists, public health assessors, chemists, physicians, modelers, risk communicators, logisticians, and budget personnel. In the event of an acute release of hazardous materials, OEM works with other federal agencies and state and local authorities to develop the appropriate public health response under an incident management system, compliant with the National Incident Management System. Many acute exposure scenarios occur during and after hurricanes, tornados, highway accidents, train derailments, warehouse fires, and other unforeseen events. These events further endanger communities through potential exposures during and after the release of hazardous materials into air or water systems. OEM can assess environmental monitoring data and help develop site safety plans, sampling plans, fact sheets, and other communication tools for the event.
ATSDR routinely provides assessments of environmental data to the on-scene coordinator or incident commander managing a response and specific recommendations for community actions, such as evacuation or shelter in place. When OEM receives a request regarding a chemical incident, the time limitations and requirements of the incident are recorded. In the initial problem formulation stage, the emergency response coordinator documents the identified chemical(s), chemical structures, routes of exposure, and health effects of interest. Ideally, ATSDR’s response includes input from its subject matter experts. The coordinator asks responders about potential human exposures at the scene and might make assumptions based on the types of buildings near the incident (e.g., homes, industrial complexes) if information is not available.
A search of chemical databases and scientific literature for pre-established health guidance values for the event or query chemical could yield a variety of measures, including minimal risk levels (MRLs), reference doses (RfDs), reference concentrations (RfCs), acute exposure guideline levels (AEGLs), emergency response planning guidelines (ERPGs), and provisional peer-reviewed toxicity values (PPRTVs), with their pros and cons (EPA, 2021 a, b, c, d; Woodall, 2005). Three options become available for derivation of needed guidance values: use of existent values, de novo values, or computer (in silico) modeling (Figure 1).
Figure 1.
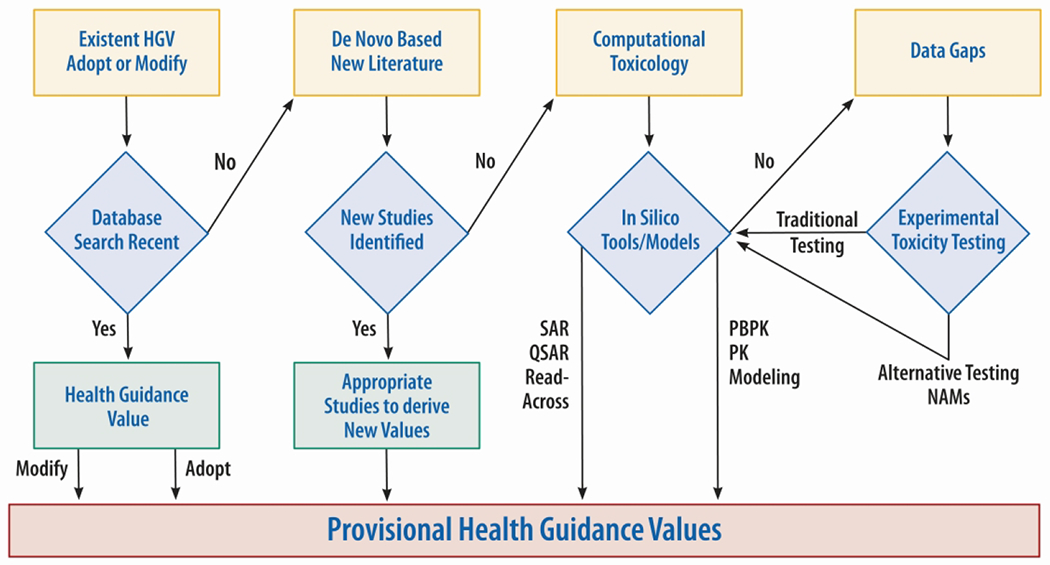
The Agency for Toxic Substances and Disease Registry’s process to derive a health guidance value (HGV) for emergency response.
For the query chemical, if guidance values are found, they may be adopted or modified appropriately under the incident-specific situation. Health guidance values based on a different route or duration of exposure, or a different form of the chemical, might not be appropriate.
If no health guidance values are identified for the query chemical, a literature search of the scientific databases is conducted to identify recent research and toxicity studies on it. If a new, appropriate study is identified that meets most of the requirements of an ideal evidence-based study, the data are used to derive de novo provisional toxicity guidance values akin to MRLs by applying the MRL development protocol (ATSDR, 1996). Such values are derived based on known and assumed conditions pertaining to that specific emergency event. When developing de novo levels in a crisis, sufficient time often might not be available to establish certainty; therefore, maximum uncertainty values are used for each extrapolation.
When definitive studies on certain chemicals are not available, the emergency response coordinator consults with subject matter experts in toxicology to compute appropriate values using in silico tools such as structure activity relationships (SAR), quantitative structure activity relationships (QSAR), read-across, pharmacokinetic (PK) or physiologically based pharmacokinetic (PBPK) modeling. Within a short period, an analysis of toxicity, potential toxicities, the model selection process, confidence in the estimates, and a comparison of results of the model with any known information are provided. The guidance values thus derived are not and should not be applied to other situations without a separate follow-up evaluation of their suitability by the professionals involved, before use in those latter crises.
If minimal data are not available or if in silico model predictions are not adequate to evaluate query chemical toxicity, data gaps are identified and experimental toxicity testing and data generation are recommended. Such testing is traditionally conducted by the National Institute of Environmental Health Sciences (NIEHS) and its National Toxicology Program. However, with the recent proliferation of rapid toxicity testing methods such as new approach methods (NAMs), NIEHS and EPA’s Center for Computational Toxicology and Exposure have invested significant resources in in vitro toxicity testing systems, which resulted in the Tox21™/ToxCast™ high throughput screening testing programs. These programs are gaining prominence among risk assessors because a tremendous volume of cell bioactivity data is generated across a broad chemical landscape, however the applicability of such data in risk-based decision-making is still at evaluation stage (EPA, 2015). Increasing the benefits from these data requires further understanding of the underlying mechanisms of toxicity and the limitations of the cell systems and assays used in these programs.
Often at ATSDR, MRLs are adopted or modified and used in emergency responses, much like health assessors routinely use them to derive environmental media evaluation guides for site-specific assessments (ATSDR, 2005b). The guides are estimated contaminant concentrations not expected to result in adverse noncancer health effects. They use default values for body weight, drinking water consumption, and soil intake for adults and for children. Environmental media evaluation guides, sometimes referred to as comparison values, allow for direct comparison of environmental concentrations to concentrations that would not be expected to cause harm, given conservative exposure assumptions. In emergencies, other parameters for values, such as body weights and ingestion rates, may be used to better tailor the comparison values to the populations potentially affected by the event. Thus, issues such as susceptible populations, exposure scenarios, and other pertinent issues can be considered while deriving specific environmental concentrations of possible concern.
Incident managers can use these types of risk analysis to inform and justify response decisions on-site. The available information on the substance, data gaps, and associated assumptions to address those gaps, and the conclusions and recommendations need to be understood and documented. In many ways, documentation of assumptions and decision-making is the key to safe and effective management of responses to hazardous material incidents. Such incidents can be challenging, especially because many communities rarely have chemical spills. Therefore, individual medical personnel have limited experience in dealing with these incidents. Volume III of ATSDR’s Managing Hazardous Materials Incidents series provides emergency medical services personnel with chemical-specific guidance on how to appropriately decontaminate, treat, and provide follow-up care to exposed persons, and measures to protect themselves (ATSDR, 2001).
EPA: Evaluating Environmental Chemicals
EPA program and regional offices make decisions affecting human health in diverse contexts, including emergency responses to chemical spills and releases, screening and prioritization of contaminated sites, and human health assessment of chemicals found in environmental media. Assessing and addressing the risks requires that data on potential exposure, hazard, and dose-response are quickly assembled and communicated.
EPA evaluates a wide range of chemicals, including legacy compounds (e.g., polyaromatic hydrocarbons, polychlorinated biphenyls) and emerging contaminants (e.g., per- and polyfluoroalkyl substances, nanomaterials), often in the context of emergency response. In practice, emergency response to a chemical spill typically is not a single type of event, but rather entails gradations of response with varied exposure scenarios and routes, temporal considerations, and priorities of hazards. The basic problem to consider is whether chemicals in the contaminated environmental media represent an immediate threat to life or health. If they do, then prompt evacuation and extensive risk remediation measures become the priority. Making that decision typically does not warrant significant or extensive characterization of human health effects data.
The response to other environmental emergencies entails identification of chemicals, characterization of potential exposure(s) and hazard(s), and selection of optimal mitigation approach(es) within a matter of hours up to 1–2 days. After a matter of days, an environmental chemical threat effectively transitions into site- or media-specific prioritization, screening, and assessment, as appropriate. The common factor among these gradations of emergency response is the need to rapidly compile human health, ecological, physicochemical property, environmental fate and transport, and exposure information for chemical evaluation.
As such in an emergency, the chemical toxicity values used by EPA program, regional, and state purviews can be highly variable. This can be particularly true for most novel and emerging contaminants and some legacy compounds that have not been formally evaluated. Risk assessment and remediation practitioners end up using a variety of approaches and methods to establish some baseline for exposure and hazard potential.
To ensure federal and state mission success, particularly for emergency response scenarios, the integration of existing data (albeit often limited) with NAMs data will be critically useful in advancing chemical evaluation and assessment. NAMs is a broadly descriptive term for any non-animal technology, methodology, or approach, or combination thereof, that can be used to provide information on chemical hazard and risk assessment (NIEHS, 2018). NAMs data represent a wealth of available information spanning in silico and cheminformatic (e.g., SAR and read-across; predicted physicochemical properties), in vitro cell bioactivity, toxicogenomics, empirical or predicted exposure(s), high-throughput toxicokinetics, and environmental fate and transport predictions (Kavlock et al., 2018; EPA, 2020).
Systematic collection, assemblage, and review of traditional human epidemiological and experimental animal bioassay data, when available, and NAMs data is the basic construct of an integrated approach to testing and assessment (OECD, 2021). The approach draws hazard conclusions when supported by integrating data from different levels of biological organization, filling data-gaps where possible, and scoping weight-of-evidence for fit-for-purpose application(s). Optimally, the approach results in qualitative and quantitative metrics that aid risk evaluation (e.g., screening, prioritization, assessment). In practice, widely varying datasets will be available for chemicals of potential concern. In some cases, sufficient hazard data is available to drive a decision, relegating NAMs data to more of a data-gap filling role. For data-poor chemicals, NAMs data might be the primary driver for hazard and dose-response interpretations.
Although collection, curation, and coherent staging of such a diverse array of available traditional bioassay and NAMs information seemingly might be intractable, EPA has committed significant resources to doing this over the past several years. The CompTox Chemicals Dashboard (referred to as the “Dashboard”) is a publicly available interactive database that provides empirical and predicted data for approximately 883,000 chemicals (EPA, 2021). Dashboard users can obtain information for one specific chemical if desired, or thousands of chemicals via batch search. Users may select discrete data elements (e.g., existing human health values only) or can obtain a full complement of traditional and NAMs data and modeled outputs across several information domains (e.g., mammalian toxicology, in vitro cell bioactivity, physicochemical properties, environmental fate and transport, exposure, product use). In an emergency response, only a relatively narrow scope of exposure and hazard and toxicology information (e.g., acute or short-term exposure durations) is typically applicable. As such, quick assembly and delivery of key values and data is the primary objective, versus comprehensive collection and evaluation of potentially voluminous, diverse, and complex data and modeled outputs.
EPA’s Office of Research and Development provides readily accessible, properly scoped information to emergency responders and others tasked with mitigating emerging contaminant threats. The Center for Computational Toxicology and Exposure in that office is developing decision-based workflows, collectively referred to as RapidTox, with EPA programmatic and regional end-user input, to fill this need (EPA, 2019). RapidTox workflows, for decision contexts such as emergency response, are designed to expedite the assembly and delivery of information specifically relevant to the end-user’s defined application. For example, for an emergency response, EPA partners and state and public agencies are tasked with mitigating chemicals in an acute exposure scenario. EPA and partners compile basic information on chemistry (e.g., structure, physicochemical properties, environmental fate, transport), acute human health (e.g., acute MRLs), or ecological toxicity values, acute duration hazard, and dose response (e.g., points-of-departure), on an aggressively short timeline. Personnel who evaluate emerging contaminants (e.g., chemists, toxicologists, risk assessors, ecologists, hydrologists, project managers) have specific information needs for their respective role(s) in risk-based decision-making. Some of these needs overlap, many more are specific to application, skillset, or expertise. As such, the RapidTox workflows and associated CompTox Chemicals Dashboard (EPA, 2019, 2021) are informed by and evolving with an increased understanding between the platform builders, risk decision translators, and end-user practitioners, be it for emergency response or other chemical evaluation decision needs.
Readily accessible fit-for-purpose information via the modules within RapidTox workflows might revolutionize the efficiency of making critical decisions that affect human health and ecological viability, be it under emergency response or longer-term risk remediation decision contexts.
DoD: Protecting Military Personnel
Providing for our national defense is often, by definition, a risky endeavor. It requires the use of unique substances under special conditions required for research, testing, training, and warfighting. Protecting the health of military personnel and their families within the military while ensuring the sustainable use of testing and training ranges are critical goals in maintaining a ready force. We must be able to predict the effects from exposures that could degrade the mission, often with greater accuracy and less uncertainty.
In developing military-specific substances, such as those used as medications for countermeasures, insect repellants, fire extinguishing agents, warfighter gear, propellants, smokes, and specialized coatings, all must be shown to be safe for warfighter use and sustainable for use on ranges. Therefore, the toxicology requirements of the military — to ensure public health of its personnel, fight against unpredictable mission requirements, and provide new tools for winning conflicts in unconventional scenarios — are diverse and problem-specific.
Additional scenarios include those where military assistance is required for national emergency response or peacekeeping missions, including hurricanes, floods, wildfires, and disease outbreaks. These could result in exposures to substances in unexpected combinations and exposure routes. Often, toxicity data to provide field commanders with accurate estimates of risk from exposure are lacking. Also lacking are fast, feasible, and accurate means for characterizing exposure. As with first responders, military personnel often must use indicators of adverse effect to help determine go/no go decisions (Ciottone, 2018).
Given the variety of potential missions and interests, sound problem formulation is essential. Various tools are needed to characterize exposure, toxicity, and health-related risks relative to those of the mission (Table 1).
Table 1.
U.S. Department of Defense general considerations for addressing risk to military personnel during mission operations of variable risk.
| Conditions | Exposure Information | Toxicity Information | Relative Level of Uncertainty | Decision | Comments |
|---|---|---|---|---|---|
| Warfighting Operations | None-qualitative | Qualitative | High | Go/No go | Use real-time symptomology to mitigate risks relative to mission. |
| Peace-keeping Operations | Ad hoc analytical data | Toxicity benchmarks available for substances being analyzed. | Medium | Mitigate exposures, medical intervention, if required. | Exposure data variable depending on time point, weather, analyte list and troop location. |
| Training Operations | Specific analytical data collected. | Benchmarks available. | Low | Mitigate exposures | Use engineering controls. |
| System Design, Research, Development, Testing, and Design | Analytical chemistry data available. | Toxicology benchmarks available. | Low | Evaluate chemical alternatives; personal protective equipment, general engineering controls. | Use to assist in weapon system design. |
Constraints in toxicity data are evaluated as to the feasibility and reliability of getting accurate exposure information, potential health effects, and the mission. Military populations are relatively healthy, and considerations typically used to adjust exposure benchmarks for the general population might not be useful or relevant.
Optimizing the collection of toxicology data and providing that information in an understandable format to those developing new systems provides the most efficient means of accomplishing public health and mission goals. Toxicologists work alongside system researchers and developers to provide data and recommendations early in the process, when changes or alternatives can be easily made (Eck et al. 2013). Phased approaches to the collection of toxicity data best occur early — parallel to designing molecules using computer models — and have been conducted with investigators and others.
Chemists begin with in silico models that provide specific predictions important to performance. Toxicologists can use other models (e.g., quantitative structural activity and property relationships) and read-across techniques to provide relative indications of toxicity in prospective uses, even before the substance is synthesized (Figure 3). When small quantities are synthesized, focused in vitro work can confirm quantitative structural activity and property relationship predictions (ASTM, 2016). Data from NAMs can augment computational approaches to help refine further testing and rank alternatives. When scale-up can be demonstrated (i.e., synthesis of kilogram amounts), focused in vivo work can be conducted to provide data for safety data sheets and personal protective equipment recommendations. After confirming constituents within formulation and meeting functional military specifications, in vivo studies may be expanded, yet focused, based on suspected target of toxicity and expected exposure regimes. Testing protocols can be developed for expected occupational exposure scenarios, and other testing can be prescribed (e.g., acute and sub-chronic aquatic testing) for expected manufacturing requirements, such as wastewater disposal and potential for environmental release. Finally, other data gaps are filled, depending on expected exposure potential, release, and outcome of other tests (USAPHC, 2021). This approach has been used successfully in several DoD programs.
Figure 3.
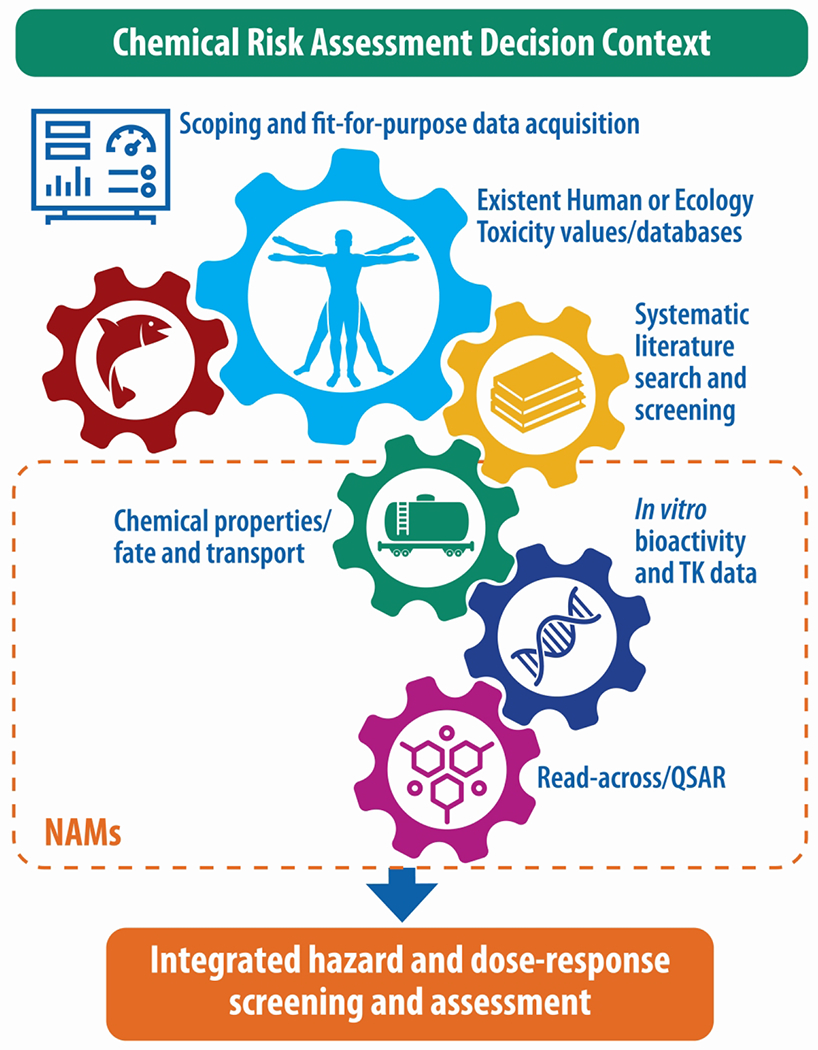
Integration of multiple data streams to inform integrated hazard assessment The U.S. Environmental Protection Agency is tasked with evaluating chemicals under multiple risk-based decision contexts, including emergency response. Scoping and problem formulation is a key first step in determining types and volume of data needed to address a given decision context. The data streams may include various gradations (based on availability) of existent human health and/or ecological toxicity values, and hazard and dose-response from traditional human or experimental animal bioassays, coupled with new approach methods (NAMs) (e.g., empirical or predicted physical chemistry, environmental fate, and transport; in vitro cell-based bioactivity and toxicokinetics [TK]; and structure-activity/read-across).
Once collected, information must be interpreted. Data are used in relative ways to ascertain which substances are optimal in relation to public health and the environment. Toxicity data must be evaluated relative to information on bioaccumulation, environmental persistence, and environmental fate. Toxicity data can be binned into toxicity categories such as those of the Globally Harmonized System (GHS, 2021). These data and interpretations are provided to researchers, developers, manufacturers, and users through tools such as toxicity assessments that also provide an evaluation and hazard assessment of other available data.
In all, 14 entities within DoD perform toxicology evaluation, research, testing, or all three. Seven of those entities have laboratories that collect toxicology data, each having different missions and areas of specialties. To enable the military to respond quickly to an unknowable, complex future, the Tri-Service Toxicology Consortium was established to help coordinate toxicology services, where appropriate. The consortium meets three times annually and has more than 30 coordinated projects. The consortium serves as a technical organization to support DoD, and participation is voluntary.
DISCUSSION
Rapid identification of data that inform decisions about exposure and toxicity of chemicals, individually or in mixtures, is critical for protection of human health and the environment. Some decision contexts involve exposure scenarios (e.g., acute, immediate) that necessitate delivery of relevant chemical information in a matter of hours to days. Other decision contexts (e.g., long-term, sub-chronic, chronic) have the advantage of time, where greater breadth and depth of analysis over weeks or months is typically acceptable. A common theme across risk decision contexts is the challenge of evaluating chemicals that have little to no available toxicity data. The advent of NAMs and development of platforms to deliver a broader array of data streams provides tremendous future opportunity for converging principles and practices in chemical evaluation, regardless of application. Validated high throughput assays and computational models have been used in EPA’s Endocrine Disruptor Screening Program (EDSP) to screen 1,800 chemicals, pesticides and substances for their ability to cause adverse effects (EPA, 2015). In some instances, even short-term rapid tests such as the murine local lymph node assay (LLNA) are being used to develop more efficient QSAR models (Alves et al., 2016). In another study, multiple non-animal testing strategies incorporating in vitro, in chemico, and in silico inputs demonstrated equivalent or superior performance to the LLNA when compared with animal and human data for skin sensitization (Kleinstreuer, et al., 2018).
As shown, ATSDR, EPA, and DoD have multiple areas of mutual endeavor pertaining to chemical evaluation and assessment, regardless of the decision context. Emergency responses often represent the most complex and taxing problem for federal and state partners because of the inherent rapidity with which decisions need to be made for public health.
In the 21st century, toxicology testing, NAMs, and risk assessment afford opportunities to coordinate and collaborate in ways that are resource efficient, animal sparing, and complementary for chemical evaluation decisions (Figure 3). After the Deepwater Horizon accident, a battery of in vitro high throughput assays were used to predict real-time toxicity for eight commercial dispersants (Judson et al., 2010). The study focused on the estrogen and androgen receptors, but also included assays probing other biological pathways. No activity was seen in any androgen receptor assay. A few dispersants showed a weak estrogen receptor signal in one assay and others did not show any activity. Cytotoxicity values for six of the dispersants were statistically indistinguishable with median LC50 values (~100 ppm) and two showed significantly less cytotoxic than the others (>1,000 ppm). This demonstrated that in certain emergency scenarios, a similar rapid screening effort could be used to make time-sensitive decisions based on potential hazard and risk (Carmicheal et al., 2012). Transcriptomic analyses and innovative methods and technology were used to understand mechanisms of complex toxicity resulting from environmental pollutants (Xu et al., 2017; Reyero et al., 2013). NAMs might help identify candidate points of departure, generated from various data sources, including in vitro, in vivo, and in silico, for potential use in the development of human health risk assessment values.
Identifying and understanding toxic effects from exposures to chemicals often involves the use of incomplete data, leading to extrapolations, assumptions, and associated uncertainties. Such incomplete data can be augmented with in vitro toxicity tests to draw rapid conclusions in an emergency scenario. After the accidental chemical spill of 4-methyl-1-cyclohexanemethanol (4-MCHM) in Elk River, West Virginia, in 2014, a quantitative toxicogenomics approach was used that included proteomics analysis in yeast cells and transcriptional analysis in human cells (Lan et al., 2015). Although 4-MCHM is considered moderately toxic, previous limited acute toxicity evaluation indicated that its metabolites were likely more toxic in yeast and human cells (Lan et al., 2015). Crude MCHM and its constituents cause slight to moderate skin and eye irritation in rodents. They are neither mutagenic nor predicted to be carcinogenic. Multiple prediction models were used to study the metabolite formation and their potential toxicity. (Paustenbauch et al, 2015). Even though several constituents were thought to be possible developmental toxicants, 1,4-cyclohexanedimethanol, 1,4-cyclohexanedicarboxylic acid, and dimethyl 1,4-cyclohexanedicarboxylate did not cause developmental toxicity in rats. Collectively, the findings and predictions indicated that crude MCHM did not pose an apparent toxicological risk to humans at 1 ppm, the health advisory level set by ATSDR and the Centers for Disease Control and Prevention for household water.
Although NAM tools, platforms, and data are increasingly used to fill data gaps for chemical evaluation, use of such information as the primary basis for hazard and dose-response assessment is still in its infancy. An added challenge in emergency responses is the need for data accuracy and defensibility — the health and well-being of affected communities or populations might literally hang in the balance. As such, there is an inherent reliance upon traditional human or experimental animal assay data (e.g., duration-relevant toxicity values, exposure metrics) for decision-making during an emergency. Currently, NAM data are rarely considered by emergency response personnel because they need to be validated thoroughly before use. This includes, but is not limited to, lack of clarity of how non-traditional assay or modeling data informs hazard and dose-response in acute or short-term exposures, challenges with risk communication based on atypical data streams, and assumptions by some that NAM data are spurious or too uncertain to support emergency response decision-making. The challenge for gaining acceptance of NAM data in emergency response and many other chemical evaluation contexts might be best addressed through bi-directional collaboration. NAM data generators and translators should engage with the intended end-user community (e.g., field risk assessors and emergency response personnel) to develop consensus and better understand the issues and data needs in a rapidly evolving situation. Likewise, end-users should engage with NAM data developers to better understand the diversity of data streams and qualitative and quantitative information that can be obtained across the platform. They also can help inform areas where NAM data could be readily integrated and those that need more work to address assumptions and uncertainties that might preclude application. Informed public health decisions can be made as we continue to develop, validate, and implement different methods and tools, using insights gained from in vitro and in silico approaches, high throughput screening, and toxicity testing (Kavlock et al., 2018).
The National Toxicology Program’s work to rapidly assess hazards for classes of chemicals with little to no toxicological data are at the nexus between toxicological chemical evaluation and end-user application. This approach, called Rapid Evaluation and Assessment of Chemical Toxicity, or REACT, is designed to provide regulators and responders with hazard information on chemicals and mixtures to address environmental and public health challenges in a timely manner (NIEHS, 2017). The program focuses on literature-based rapid evidence mapping, computational methods including quantitative structural activity relationship and in vitro-to-in vivo extrapolation models, in vitro high-throughput toxicity screening, and short-term in vivo toxicogenomic studies. These tools can be used in combination or individually, depending on the data needs, to support a given decision or response (NIEHS, 2017).
Another program that can assist in deriving provisional toxicity values is the Integrated Chemical Environment (ICE) (NIEHS, 2021). This web-based resource provides access to high-quality curated data that can be efficiently linked to sets of assay responses and model predictions mapped to a health outcome or toxicity endpoint. ICE currently includes in vivo experimental animal test data, reference chemical information, in vitro assay data (including Tox21™/ToxCast™ high-throughput screening data), and in silico model predictions. ICE also includes reference chemical lists, supporting data sets, and computational predictions for properties such as physicochemical parameters and clearance rates, which are often needed in model development. It serves as an alternative to searching and pooling data from a variety of disparate sources. The ICE data integrator function allows users to query high-quality in vivo and in vitro test results by chemical and by endpoint (e.g., acute systemic toxicity, endocrine disruption, skin sensitization). The ICE user environment is an excellent example of leveraging information across different levels of biological organization and data streams to inform chemical evaluation across decision-making foci.
CONCLUSION
ATSDR, DoD, EPA, and other federal and state agencies have established approaches, protocols, and data sources for informing responses to chemical emergencies or crises to fit their respective mandates and public health missions. Each of the federal agencies have developed programs to meet their legislative mandates that can provide managers, public health officials, and regulators with rapid assessment of the potential hazard and risk associated with chemical emergencies. The activities coordinated among these federal agencies demonstrate unified, parallel, and complementary work that provides timely protection of the public in times of chemical emergency.
The field of predictive toxicology has been growing and developing rapidly in the last two decades, particularly in the development of ever more sophisticated in vitro assays that effectively reflect the properties of human organ systems in vivo. As new methods and approaches come into increased use, the scientific community must continue to orient to and foster the transition of toxicological testing from a resource-intensive and time-consuming observational endeavor to an animal-sparing and higher-throughput predictive science, while continuing to provide scientifically defensible and situationally plausible conclusions and recommendations.
One way to promote this transition is to integrate limited traditional toxicity data from epidemiological and experimental animal bioassay with in vitro and high throughput testing data. That will allow rapid conclusions to be drawn using multi-stream data from diverse levels of biological organization, while building confidence in such new data use. This approach will help advance the chemical evaluation and risk assessment process. Experience gained by repeated use of this approach will bring clarity and understanding of the toxicological mechanism and, in due course, can be expected to minimize uncertainty in the chemical evaluation process.
Figure 2.
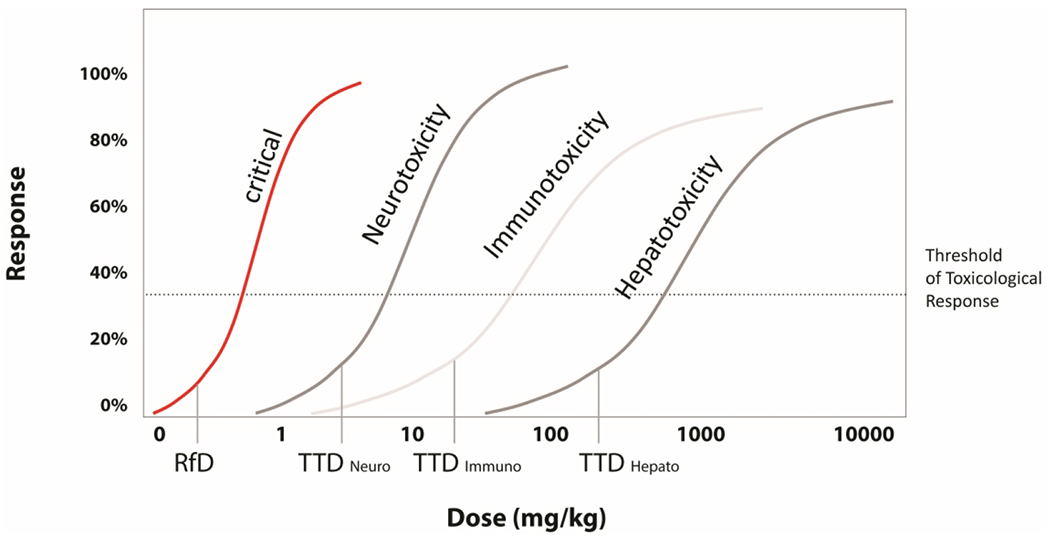
Relationship between the minimal risk level (MRL) and target organ toxicity doses (TTD). An MRL is derived for the most protective critical effect and is an estimate of the daily human exposure to a hazardous substance that is likely to be without appreciable risk of adverse noncancer health effect. TTDs are derived for secondary effects other than critical effects (ATSDR, 1996; 2004).
Figure 4.

U.S. Department of Defense conceptual framework for the development of toxicity data needed to evaluate relative hazard. Green and red in confidence and cost bars reflect general cost and confidence of hazard; green denotes relative low cost and high confidence; red is relative high cost and low certainty. (DEM-Val = demonstration/validation stage in weapons system development).
ACKNOWLEDGMENTS
The authors appreciate Donald Meadows for editorial/technical support; Pat Brady and Angela Glaude-Hosch for Graphics Services.
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Publisher's Disclaimer: Disclaimer: The views and opinions expressed in this paper/article are those of the individual authors, and do not necessarily reflect the official policies and positions of the U.S. Government, the U.S. Department of Health and Human Services (Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry), the U.S. Department of Defense (U.S. Army), or the U.S. Environmental Protection Agency.
Partially presented at the Society of Toxicology annual meetings in Baltimore, MD March 10 -14, 2019.
REFERENCES
- Alves VM, Capuzzi SJ, Muratov E, Braga RC, Thornton T, Fourches D, Strickland J, Kleinstreuer N, Andrade CH, and Tropsha A (2016). QSAR models of human data can enrich or replace LLNA testing for human skin sensitization. Green Chem. 18, 6501–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTM (American Society for Testing and Materials). (2016). Standard Guide for Assessing the Environmental and Human Health Impacts of New Compounds for Military Use. E 2552-16, Volume 11.06, Section 11, Water and Environmental Technology, Biological Effects and Environmental Fate, Biotechnology. ASTM International, Conshohocken, Pennsylvania. [Google Scholar]
- ATSDR. (1996). Minimal Risk Levels for Priority Substances and Guidance for Derivation. Federal Register 61, 101: 25873–25882. Available at: https://www.govinfo.gov/content/pkg/FR-1996-05-23/pdf/96-12991.pdf. Accessed September 28, 2021. [Google Scholar]
- ATSDR. (2001). Managing Hazardous Materials Incidents (MHMIs). Available at: https://www.atsdr.cdc.gov/MHMI/index.html. Accessed April 20, 2021.
- ATSDR. (2004). Interaction Profiles for Benzene, Toluene, Ethylbenzene, and Xylenes. Available at: https://www.atsdr.cdc.gov/interactionprofiles/index.html. Accessed February 28, 2021.
- ATSDR. (2005a). Public Health assessment of Conrail Rail Yard, Elkhart, Elkhart County, Indiana. EPA Facility ID: IND000715490. August 11, 2005. Available at: https://www.atsdr.cdc.gov/HAC/pha/ConrailRailYd/ConrailRailYardPHA081105.pdf. Accessed September 8, 2021.
- ATSDR. (2005b). Public Health Assessment Guidance Manual (Update). Available at: https://www.atsdr.cdc.gov/hac/phamanual/pdfs/phagm_final1-27-05.pdf. Accessed September 28, 2021.
- ATSDR. (2006). Health Consultation Endicott Area Investigation Broome County, New York. Available at: https://www.atsdr.cdc.gov/HAC/pha/EndicottAreaInvestigation113006/EndicottAreaInvestigationHC113006.pdf. Accessed September 9, 2021.
- ATSDR. (2014). Availability of Draft Toxicological Profiles. Federal Register. 79, 240: 74093–74094. Available at: https://www.govinfo.gov/content/pkg/FR-2014-12-15/pdf/2014-29258.pdf. Accessed September 28, 2021. [Google Scholar]
- ATSDR. (2018). Draft Guidance for the Preparation of Toxicological Profiles. Available at: https://www.atsdr.cdc.gov/toxprofiles/guidance/profile_development_guidance.pdf. Accessed September 9, 2021.
- ATSDR. (2020). Health Consultation: Per- and Polyfluoroalkyl Substances (PFAS) in the Pease Tradeport Public Water System. EPA PWS ID: 1951020; Pease Air Force Base; Portsmouth, Newington, and Greenland, New Hampshire. EPA Facility ID: NH7570024847. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service, Atlanta, Georgia. [Google Scholar]
- ATSDR. (2021). Agency for Toxic Substances and Disease Registry. Available at: https://www.atsdr.cdc.gov/. Accessed February 28, 2021.
- Carmichael RH, Jones AL, Patterson HK, Walton WC, Pérez-Huerta A, Overton EB, Dailey M, and Willett KL (2012). Assimilation of oil-derived elements by oysters due to the Deepwater Horizon oil spill. Environ. Sci. Technol. 46, 12787–12795. [DOI] [PubMed] [Google Scholar]
- Ciottone GR (2018). Toxidrome recognition in chemical weapon attacks. N. Engl. J. Med. 378, 1611–1620. [DOI] [PubMed] [Google Scholar]
- Eck WS, Watts K, Lieb NJ, and Johnson MS (2013). Toward environmentally-sustainable acquisition: a phased approach. Army A L&T, April-June: 51–56. https://asc.army.mil/web/wp-content/uploads/2013/04/April-June2013_army_al.pdf
- GHS. (2021). Globally Harmonized System of Classification and Labelling of Chemicals. Available at: https://www.chemsafetypro.com/Topics/GHS/GHS_Classification_Criteria.html.
- EPA. (2015). Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment. Available at: https://www.federalregister.gov/documents/2015/06/19/2015-15182/use-of-high-throughput-assays-and-computational-tools-endocrine-disruptor-screening-program-notice. Accessed September 9, 2021.
- EPA. (2019). Development of a RapidTox Dashboard to Inform Risk Assessment. Available at: https://www.epa.gov/sites/default/files/2019-05/documents/comptox_cop_lambert_v1.pdf. Accessed September 9, 2021.
- EPA. (2020). New Approach Methods Work Plan. EPA 615B20001/June 2020. Available at: https://www.epa.gov/chemical-research/epa-new-approach-methods-work-plan-reducing-use-animals-chemical-testing. Accessed September 9, 2021.
- EPA. (2021). CompTox Chemicals Dashboard 883 Thousand Chemicals. Available at: https://comptox.epa.gov/dashboard/. Accessed April 20, 2021.
- EPA. (2021a). Acute Exposure Guideline Levels (AEGLs). Available at: https://www.epa.gov/aegl . Accessed September 9, 2021.
- EPA. (2021b). Integrated Risk Information System. Available at: https://www.epa.gov/iris. Accessed September 9, 2021.
- EPA. (2021c). Professional Reviewed Toxicity Values (PPRTVs). Available at: https://www.epa.gov/pprtv. Accessed September 9, 2021.
- EPA. (2021d). Acute Exposure Guideline Levels (AEGLs). Available at: https://www.epa.gov/aegl. Accessed September 9, 2021.
- Goldstein B (2001). The precautionary principle also applies to public health actions. Am. J. Public Health. 91, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MS (2018). Toward development of occupational exposure levels for insensitive munition components. Toxicol. Ind. Health. 34, 1. [DOI] [PubMed] [Google Scholar]
- Judson RS, Martin MT, Reif DM, Houck KA, Knudsen TB, Rotroff DM, Xia M, Sakamuru S, Huang R, Shinn P, Austin CP, Kavlock RJ and Dix DJ (2010). Analysis of eight oil spill dispersants using rapid, in vitro tests for endocrine and other biological activity. Environ Sci Technol. 44, 5979–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock RJ, Bahadori T, Barton-Maclaren TS, Gwinn MR, Rasenberg M, and Thomas RS (2018). Accelerating the pace of chemical risk assessment. Chem. Res. Toxicol 31, 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstreuer NC, Hoffmann S, Alépée N, Allen D, Ashikaga T, Casey W, Clouet E, Cluzel M, Desprez B, Gellatly N, Göbel C, Kern PS, Klaric M, Kühnl J, Martinozzi-Teissier S, Mewes K, Miyazawa M, Strickland J, van Vliet E, Zang Q, and Petersohn D (2018). Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit. Rev. Toxicol 48, 359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Hu M, Gao C, Alshawabkeh A, and Gu A (2015). Toxicity assessment of 4-methyl-1-cyclohexanemethanol and its metabolites in response to a recent chemical spill in West Virginia, USA. Environ. Sci. Technol 49, 6284–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz MM, Poirier KA, and Colman JT (1997). Risk assessment of chemical mixtures: fine-tuning the hazard index approach. J. Clean Technol. Environ. Toxicol Occnp. Med 6, 189–204. [Google Scholar]
- NIEHS. (2018). A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States. Available at: https://ntp.niehs.nih.gov/iccvam/docs/roadmap/iccvam_strategicroadmap_january2018_document_508.pdf.
- NIEHS. (2017). Rapid Evaluation and Assessment of Chemical Toxicity (REACT) of Per and Polyalkyl Substances (PFAS). Available at: https://ntp.niehs.nih.gov/ntp/about_ntp/bsc/2017/december/presentations/07devito_508.pdf. Accessed September 28, 2021.
- NIEHS. (2021). ICE: Integrated Chemical Environment. Available at: https://ntp.niehs.nih.gov/whatwestudv/niceatm/comptox/ct-ice/ice.html. Accessed April 20, 2021.
- OECD. (2021). Integrated Approaches to Testing and Assessment (IATA). Available at: https://www.oecd.org/chemicalsafetv/risk-assessment/iata-integrated-approaches-to-testing-and-assessment.htm
- Paustenbach DJ, Winans B, Novick RM, and Green SM (2015). The toxicity of crude 4-methylcyclohexanemethanol (MCHM): review of experimental data and results of predictive models for its constituents and a putative metabolite. Crit. Rev. Toxicol 45(Suppl 2), 1–55. [DOI] [PubMed] [Google Scholar]
- Pohl HR, Roney N, Wilbur S, Hansen H, and De Rosa CT (2003). Six interaction profiles for simple mixtures. Chemosphere. 53, 183–197. [DOI] [PubMed] [Google Scholar]
- Pohl HR, and Abadin HG (2008). Chemical mixtures: evaluation of risk for child-specific exposures in a multi-stressor environment. Toxicol. Appl. Pharmacol 233, 116–125. [DOI] [PubMed] [Google Scholar]
- Reyero NG, Raghavachari N, Showmaker K, Liu P, Jafari N, Barker N, Willette KL, Corrales J, Patterson HK, Carmichael RH, and Baldwin D (2013). Genomics Research Group (GRG): elucidating the effects of the Deepwater Horizon oil spill on the Atlantic oyster using global transcriptome analysis. J. Biomol. Tech 24(Suppl), S68–S69. [Google Scholar]
- USAPHC. (2021). Guide to Performing a Developmental Environment, Safety, and Occupational Health Evaluation (DESHE). Technical Guide 389. U.S. Army Public Health Center, Toxicology Directorate. Aberdeen Proving Ground, Maryland. [Google Scholar]
- Woodall GM (2005). Acute health reference values: overview, perspective, and current forecast of needs. Jour. Tox. Environ. Health, Part A 68, 901–926. [DOI] [PubMed] [Google Scholar]
- Xu EG, Mager EM, Grosell M, Hazard SE, Hardiman G, and Schlenk D (2017). Novel transcriptome assembly and comparative toxicity pathway analysis in mahi-mahi (Coryphaena hippurus) embryos and larvae exposed to Deepwater Horizon oil. Sci. Rep 7, 44546. [DOI] [PMC free article] [PubMed] [Google Scholar]


