Abstract
Post-COVID-19 telogen effluvium has been largely reported as a sequela in the post-acute phase of COVID-19, causing major emotional distress among the affected patients. The affected individuals are further exposed to a vast amount of misinformation from the internet and social media and it is important for physicians to be familiar with the phenomenon and provide appropriate counselling to their patients regarding this condition. This article aims to review the evidence-based complementary strategies that can help enhance hair regrowth after post-COVID-19 hair loss, from psychological support and patient education to the importance of optimal nutrition and potential indications and benefits of oral nutritional supplementation, as well as the role of both topical and injectable hair growth stimulators.
Keywords: COVID-19, SARS-CoV-2, hair loss, telogen effluvium, hair growth
Introduction
Since the ensue of this worldwide pandemic there has been a vast amount of scientific articles aiming to describe the individuals’ lasting health consequences after the acute phase of COVID-19, with hair loss being one of the most common effects. The phenomenon is considered as telogen effluvium (TE), a diffuse hair loss due to dysregulation of the hair growth cycles.1
TE is by far the most common form of hair loss observed during the ongoing pandemic.2 However, alopecia areata (AA), another nonscarring hair loss, has also been reported after SARS-CoV-2 infection in small sample size studies.3 The disorder is believed to be immune-mediated, occurring as a response to the inflammatory process that affects anagen hair follicles. Contrary to TE, the hair loss is usually patchy, nondiffuse. The causal link between COVID-19 and AA onset is not clarified and the disease will not be discussed in this review since TE and AA are distinct entities, with different management options.
Clinical trials have also addressed the possible connection between alopecia androgenetica (AGA), another form of chronic hair loss, and the severity of COVID‐19.4 In this respect, we want to clarify that AGA is the only hair loss disorder that has been investigated as a determinative factor for COVID-19 severity, and not as a COVID-19 sequela.5 It was stipulated that androgen receptor activity stimulate TMPRSS transcription, an important part of the viral entry of COVID-19 via cleavage of ACE2 and priming of spike protein. ACE2 and androgen receptors can be found on chromosome X giving us insight into why males are predominantly affected. On the same note, androgen sensitivity may point to the reason why children may not be as severely affected as their elders when met with COVID-19.6 We did not find any research that further investigated the severity of post-COVID-19 hair loss in patients previously diagnosed with AGA. Our paper focuses on acute hair loss as a consequence of COVID-19, not on the relationship between previous chronic hair loss disorders and COVID-19 severity, hence this review will not include further discussions on this topic.
Approximately 25% of COVID-19 patients suffer from acute classic telogen effluvium in the first two to three months after the infection, with women being at greater risk than men.7 Yet, an early onset telogen effluvium after COVID-19 has been observed within less than 2 months after COVID-19 infection, especially in patients affected by more severe forms of COVID-19.8 This early-onset telogen phase may be related to the direct effect of high levels of pro-inflammatory cytokines on hair follicle cells, since severe cases of SARS-CoV-2 are typically characterized by higher levels of pro-inflammatory cytokines and reduced host antiviral responses.1 Furthermore, pro-inflammatory states caused by COVID-19 could cause the formation of microthrombi in the hair follicles, which could lead to occluding hair follicles’ blood supply.9
Further histopathological and/or immunohistochemical studies are warranted to elucidate the precise etiopathogenesis of this phenomenon,1,10 but, at this moment, it is believed the condition can be triggered by both the systemic viral infection itself as well as the stressors related to the COVID-19 pandemic.
Although spontaneous improvement is expected for most patients with post-COVID-19 TE, patients regularly seek effective ways to enhance the process of hair regeneration. A massive increase in searches using the keywords “hair loss” has been noticed in Google Trends since the ensue of the COVID-19 pandemic.11 Due to the proliferation of misinformation and unverified information about post-COVID-19 hair loss, these highly vulnerable patients may be easily misled into trusting non-evidence-based practices that promise to treat this condition.
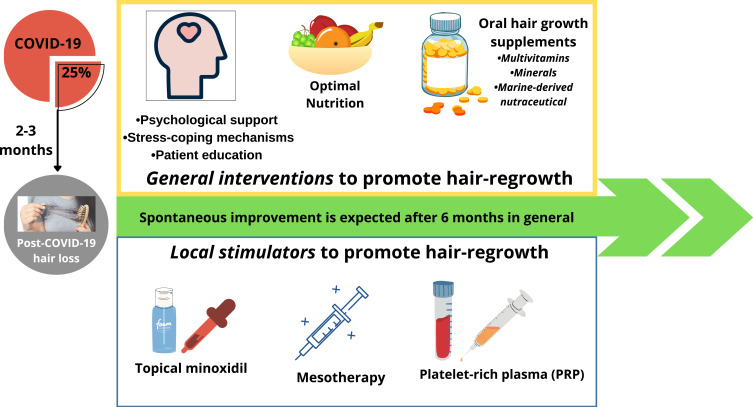
This article proposes a holistic approach that reviews only the evidence-based complementary strategies (both general measures as well as local treatments) that can help reduce post-COVID-19 hair loss and promote hair re-growth: (i) General measures: patient education and stress-coping mechanism, dietary interventions and oral hair growth supplements; (ii) local treatments: from topical therapy with minoxidil to minimally invasive scalp injections (mesotherapy and platelet-rich plasma therapy) (Figure 1). The clinical decision of initiating any of these treatment modalities should be made on an individual case-by-case basis, after careful evaluation of all psychosocial and physiological factors, and integration of patient preferences.
Figure 1.
Schematic representation of the complementary strategies that can help reduce post-COVID-19 hair loss and promote hair re-growth.
Patient Psychological Support and Education
New onset diffuse hair loss can markedly affect a person’s psychosocial well-being,12 even more in patients previously infected with SARS-CoV-2 (Figure 2). And emotional distress is well known to be an inciting trigger for the induction of hair shedding,13 thus, these patients often find themselves trapped in a vicious cycle.
Figure 2.
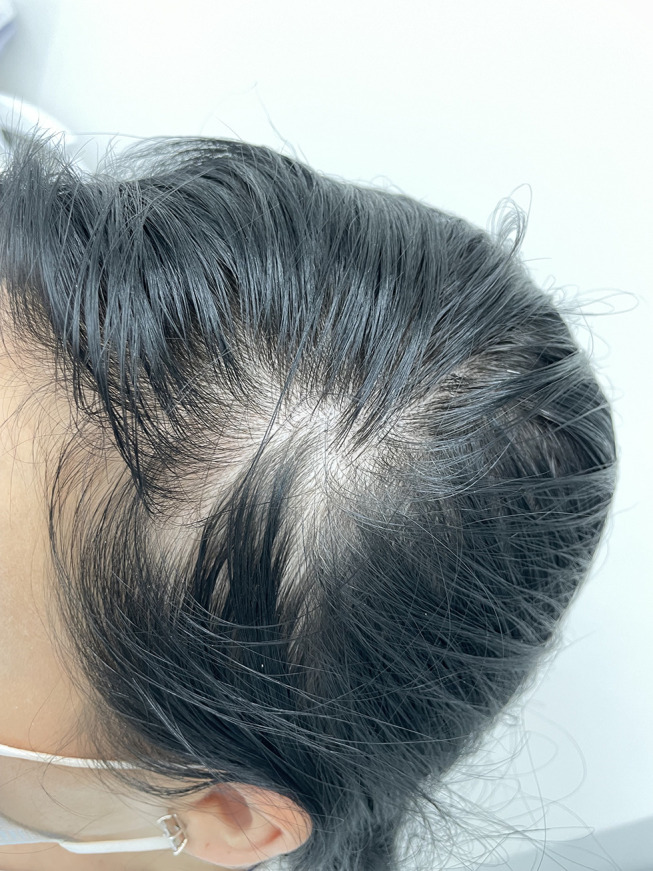
Acute reduction in total hair density in a 37-year-old female who is recovering from moderate SARS-CoV-2 infection. The diffuse hair loss is most noticeable in the frontotemporal region and first developed within two months after COVID-19. The patient also reported high levels of anxiety caused by the acute hair loss.
So, expressing empathy and support for the patient’s anxiety and psychological stress is an important component of patient management. Since the ensue of this worldwide pandemic, in addition to the illness itself, many other COVID-19 related stressors can further enhance hair loss, such as social isolation, work-related changes, or financial stress, among others. A holistic approach, with integration of stress-coping strategies into the management of these patients, could be particularly useful.14 Regular physical activity, yoga, meditation, adequate amounts of sleep are all powerful but often overlooked stress management tools.15
Regarding patient education, is essential to instruct the patients about the nature of the hair loss, namely that infectious illnesses and fever can affect the hair growth lifecycle, by forcing more hair follicles than normal to prematurely enter the shedding (telogen) phase. As a consequence, some patients may experience noticeable hair shedding two to three months after COVID-19.16 Additionally, patients should be instructed that an earlier than classic TE has been reported, and they may experience hair loss even in the first month after the infection.8
Most patients are anxious that they will gradually lose all the hair on the scalp, and is important to clarify that this is not expected since the shedding is known to affect up to 30% of scalp hair.17 It is also necessary to reassure the patient that fortunately, the disorder is reversible. The hair loss is usually self-limiting and spontaneous improvement is expected after 6 to 12 months from the onset of the condition to noticeable improvement.18
Nutrition
Optimal nutrition is a crucial strategy that can mitigate the hair loss consequences associated with COVID-19, through nutritional modulation of the immune system. It is also important to take into account that anosmia/ageusia are symptoms frequently reported in COVID-19 and can lead to decreased appetite and nutritional deficiencies that can either induce or aggravate hair fall.19
Thus, consumption of healthy foods should be a top priority to maintain adequate nutritional status and a well-functioning immune system, reducing susceptibility to long-term sequels from COVID-19 in general.20 It is known that improved nutrition containing micronutrients such as vitamins (A, B complex, C, D, and E) and minerals (iron, zinc) have a potential role to augment the immune system.
Micronutrients that help maintain a well-functioning immune system can be particularly found in fresh foods (citrus fruits and vegetables), whole grain foods (brown rice, oats), lean meat, fish, low-fat dairy, and healthy fats (nuts, seeds, olive oil and fish oil).21 On the other hand, an unhealthy diet consisting of sugars, saturated fats (fatty acids), and refined carbohydrates (pastries, white bread) leads to chronic inflammation and lowers the body’s ability to defend against the effects of the virus.
Oral Hair Growth Supplements
Dietary supplements are a large and vibrant industry, with globally estimated sales that exceed $100 billion.22 While some ingredients and formulations have shown evidence supporting their use in commercial supplements for hair regrowth,23 many others have gained huge popularity mainly with the aid of extensively disseminated marketing messages on social media and on the internet in general.
Patients suffering from post-COVID-19 hair loss can be easily deceived into believing the promises of stimulating hair growth, even for products that have not been properly assessed in clinical trials. Given this context, is essential for physicians to educate and counsel their patients about the ingredients and formulations that have demonstrated benefit in high-quality randomized clinical trials, their correct use and indications.
Oral micronutrient supplementation is a viable approach to alleviate the severity of post-COVID-19 hair loss and support hair regrowth and should be administered to individuals in whom deficiencies are detected. The utility of oral micronutrient supplementation in improving the course of TE in the absence of detected deficiencies is unconfirmed and larger studies are needed to confirm their impact on the course of this condition. In this section, we are referring only to ingredients and formulations with published evidence supporting their validity and utility in this condition.
Multivitamins
Vitamin D
Research data has shown a correlation between TE and low serum vitamin D levels24–26 and therefore when evaluating patients with post-COVID-19 hair loss, testing for 25-hydroxyvitamin D levels (to assess for vitamin D deficiency) is indicated. Vitamin D receptors are expressed in hair follicle cells and possess the ability to modulate keratinocyte proliferation and hair growth cycling.27
Meanwhile, in COVID-19 vitamin D is a supplement that gained a lot of popularity due to its ability to boost the immune system.28
Vitamin E
Vitamin E is a well-known powerful antioxidant. Tocopherols and tocotrienols are the vitamin E derivatives commonly used in hair growth oral supplements. Beoy et al demonstrated their ability to increase hair growth, due to the ability to inhibit lipid peroxidation and reduce hair follicle oxidative stress.29
Biotin
Biotin, also known as vitamin B7, is one of the most popular nutrients in many over-the-counter hair growth supplements, mainly due to its function in keratin production. However, despite its substantial advertising in this field, in patients suffering from hair loss, it is still controversial if oral biotin supplements do offer benefits, irrespective of serum biotin levels. In 2017 Patel el al conducted a systematic review of the use of biotin for hair loss conditions30 and concluded that only patients suffering from biotin deficiency do achieve clinical improvement after daily biotin supplementation, whereas no benefit is seen in individuals with normal baseline biotin levels.
Minerals
Zinc
Zinc is a trace element that is a potent promoter of hair follicle recovery31 and dysregulations in zinc metabolism were proved to play an essential role in many forms of hair loss, especially TE.32 There is also evidence supporting the role of zinc supplementation for significant increase in hair thickness in females suffering from hair loss.33
Based on the available data, as well as on the popularity that zinc has gained in the COVID era as an immune booster, it is recommended that zinc be supplied in patients with post-COVID-19 hair loss, if the baseline concentration of zinc is low.
Iron
Iron deficiency is the most frequently detected nutritional deficiency worldwide, affecting approximately 20–25% of the general population.34,35
It is well known that iron deficiency anaemia is linked to hair loss, and the affected patients can benefit from oral iron supplementation.36–38 Whether iron supplementation is also beneficial for patients with TE in the absence of iron deficiency anaemia is debatable, but data suggest benefit.38 In patients with post-COVID-19 hair loss, serum ferritin levels should be evaluated to assessed iron storage and oral iron supplementation could enhance hair re-growth whenever serum levels of ferritin are beneath the reference threshold.39 The reference intervals for serum ferritin may vary across laboratories, but generally, the normal ranges are between 30 to 200 ng/mL for women and 30 to 300 ng/mL for men.40
Marine-Derived Nutraceutical
Several studies have shown that oral administration of bioactive substances derived from the marine environment can have a significant impact on hair regrowth in various hair loss conditions.41
Complex formulations containing hydrolyzed marine collagen type I and type III,42 shark and mollusk powder43 and other marine proteins can function as a highly efficient complementary treatment to decrease hair shedding and also enhance hair regrowth. The exact mechanism of action of these compounds is still not elucidated but the available evidence supports their use.
Topical Minoxidil
Minoxidil is an antihypertensive agent that uses its vasodilation properties to favour and increase blood circulation in the hair follicle. It also has the ability to activate prostaglandin synthase-1, an enzyme that promotes hair growth.44 It is primarily indicated for the treatment of androgenetic alopecia, and some physicians also prescribe it for chronic TE, whereas the efficacy of topical minoxidil on acute TE has not been properly evaluated in high-quality clinical studies.
It is well known that minoxidil has the ability to stimulate the transition of hair follicles from the shedding (telogen) phase of the hair cycle to the growing (anagen) phase and further prolong this phase.45 As previously stated, post-COVID-19 hair loss is characterized by a gradual remission and that is the point when initiation of treatment with topical minoxidil might be useful as a promoter for hair regrowth and maintenance.
In a limited amount of cases, patients may experience increased hair shedding during the first eight weeks of treatment with topical minoxidil. This simply indicates that minoxidil stimulates the release of telogen hair - thus boosting the end of the telogen phase, to subsequently stimulate anagen transition of hair follicles. It is important to inform the patients that this paradoxical phenomenon may occur, it is only transitory, and therapy should not be discontinued.46
Administration instructions include application of topical 5% or 2% minoxidil in a foam or solution, once or twice daily, to the entire scalp.45 Continuous therapy for 4 months may be necessary for beneficial effects to become visible.
For patients preferring plant-derived hair growth stimulators, rosemary oil can constitute a feasible option. In a randomized comparative study by Panahi et al, it was shown that twice daily application of rosemary oil can be as effective as 2% minoxidil, by improving microcirculation surrounding the hair follicle.47
Mesotherapy
The term mesotherapy refers to “treatment of the mesoderm, the middle layer of skin”.48 The technique consists of multiple intradermal microinjections of low doses of active substances, such as multivitamins, minerals, plant extracts, conventional medications and other bioactive substances directly into the dermal layer of the skin.
The direct inoculation of substances into the dermis (intradermotherapy) is an effective way to improve the nutrition of hair follicles located at this level, thus overcoming the epidermal barrier that is extensively lowering penetration of topically administered drugs. Furthermore, the trauma induced by multiple injections stimulates the activation of the dermal papillae, and local production of cytokines and growth factors at the affected sites, consequently supporting anagen hair regrowth.49
Intradermal and intramuscular scalp injections of botulinum toxin (BTX) have also been investigated as adjunctive therapeutic strategies for the treatment of various non-scarring hair loss disorders, but the effectiveness and safety were not clearly demonstrated.50 BTX is an injectable neurotoxin originally prescribed for medical disorders characterized by muscular hyperactivity,51 and it has been afterwards widely employed in cosmetic dermatology, for the treatment of dynamic facial rhytides.52 Due to its unique versatility, BTX is also being used in many other dermatologic conditions, in off label regimen.53 In a recent pilot animal study, is has been used experimentally to investigate its effect on hair follicle cell regeneration under continuous stress conditions. The authors concluded that BTX may be a positive indicator for hair loss treatment but future studies are warranted.54
The efficacy of mesotherapy on hair loss conditions still needs to be defined in high-quality, peer-reviewed clinical trials. Available data shows it can be used as a valuable adjuvant treatment modality for non-scarring alopecia in general, and the decision of initiating mesotherapy as a complementary therapy to promote hair regrowth after post-COVID-19 hair loss should be made on an individual case-by-case basis.
Platelet-Rich Plasma (PRP)
PRP is an innovative treatment in the field of regenerative medicine, with the highest level of evidence in hair re-growth, mainly alopecia areata and androgenetic alopecia. In the latter, a recent study showed that PRP therapy and treatment with autologous human follicle mesenchymal stem cells (HF-MSCs) have comparable results.55
PRP contains high concentrations of autologous growth factors and other signaling molecules derived from blood platelets. These are injected into the target tissue, thus making it possible to deliver larger amounts of growth factors than usual blood circulation can supply in the affected areas.56 At this level, PRP activates follicular stem cells and promotes regeneration of hair follicles, prolongs the anagen phase of the hair cycle and improves the function of the hair follicle.
To obtain clinical benefits, is necessary that the PRP preparation and composition is properly analysed, by its concentration of platelets, the filtration of white cells, and level of red blood cells contamination.57
Only few peer reviewed studies investigated the efficacy of PRP application in the treatment of TE and the results are controversial. A recent case series, which included 9 patients suffering from accelerated hair loss associated with COVID-19, found PRP injections to provide a satisfactory solution after 4 sessions of treatment.58
Considering the treatment is minimally invasive, with low costs, lacks adverse effects and has gained massive popularity for the treatment of hair loss, it could represent a useful complementary treatment to enhance hair regrowth in post-COVID-19 hair loss. The decision should be made on an individual case-by-case basis.
Conclusion
Telogen effluvium after COVID-19 is one of the postinfectious manifestations which is expected to be reported among recovering patients worldwide. The diffuse loss of “handfuls” of hair has a high-visual impact that is likely to attract dissemination of this condition on the internet and lay media along with misinformation regarding therapeutic options with “miraculous effectiveness” in treating post-COVID-19 hair loss. To enhance the process of hair regrowth, no single intervention is sufficient. Instead, a holistic approach integrating the general and local measures discussed in this article is the most appropriate strategy.
General measures include: patients’ education in understanding the disorder is reversible, integration of stress-coping strategies, optimal nutrition containing micronutrients such as vitamins (A, B complex, C, D, and E) and minerals (iron, zinc), and oral hair growth supplements for individuals in whom micronutrient deficiencies are detected. Local treatments with published evidence supporting their validity and utility in this condition include the topical vasodilator minoxidil, as well as techniques based on minimally invasive scalp injections in which active substances and high concentrations of autologous growth factors are directly delivered into the dermal layer of the scalp (mesotherapy and platelet-rich plasma therapy).
At this moment, scientific evidence does not point to an objective “best” option for patients suffering from post-COVID-19 hair loss. Considerations in simply waiting for spontaneous resolution of the disorder or choosing the appropriate complementary strategies that can help enhance hair regrowth after post-COVID-19 hair loss should be made on an individual case-by-case basis, after careful evaluation of all physiological and psychosocial factors, and integration of patient preferences.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Olds H, Liu J, Luk K, Lim HW, Ozog D, Rambhatla PV. Telogen effluvium associated with COVID-19 infection. Dermatol Ther. 2021;34(2):e14761. doi: 10.1111/dth.14761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharquie KE, Jabbar RI. COVID-19 infection is a major cause of acute telogen effluvium. Ir J Med Sci. 2021. doi: 10.1007/s11845-021-02754-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57–61. doi: 10.1016/j.jdin.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wambier CG, Vano-Galvan S, McCoy J, et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the “Gabrin sign”. J Am Acad Dermatol. 2020;83(2):680–682. doi: 10.1016/j.jaad.2020.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goren A, McCoy J, Wambier CG, et al. What does androgenetic alopecia have to do with COVID-19? An insight into a potential new therapy. Dermatol Ther. 2020;33(4):e13365. doi: 10.1111/dth.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wambier CG, Goren A, Vano-Galvan S, et al. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev Res. 2020;81(7):771–776. doi: 10.1002/ddr.21688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11–18. doi: 10.1016/j.jdin.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Arrones OM, Lobato-Berezo A, Gomez-Zubiaur A, et al. SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35(3):e181–e183. doi: 10.1111/jdv.17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutlu O, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33(6):e14096. doi: 10.1111/dth.14096 [DOI] [PubMed] [Google Scholar]
- 12.Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2012;66(3):e97–102. doi: 10.1016/j.jaad.2010.11.042 [DOI] [PubMed] [Google Scholar]
- 13.Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen effluvium: a review of the literature. Cureus. 2020;12(5):e8320. doi: 10.7759/cureus.8320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivetti N, Barruscotti S. Management of telogen effluvium during the COVID-19 emergency: psychological implications. Dermatol Ther. 2020;33(4):e13648. doi: 10.1111/dth.13648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violant-Holz V, Gallego-Jimenez MG, Gonzalez-Gonzalez CS, et al. Psychological health and physical activity levels during the COVID-19 pandemic: a systematic review. Int J Environ Res Public Health. 2020;17(24):9419. doi: 10.3390/ijerph17249419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrantes TF, Artounian KA, Falsey R, et al. Time of onset and duration of post-COVID-19 acute telogen effluvium. J Am Acad Dermatol. 2021;85(4):975–976. doi: 10.1016/j.jaad.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trueb RM. Systematic approach to hair loss in women. J Dtsch Dermatol Ges. 2010;8(4):284–297, 284–298. doi: 10.1111/j.1610-0387.2010.07261.x [DOI] [PubMed] [Google Scholar]
- 18.Mieczkowska K, Deutsch A, Borok J, et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021;60(1):122–124. doi: 10.1111/ijd.15313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoier A, Chaaban N, Andersen BV. Possibilities for maintaining appetite in recovering COVID-19 patients. Foods. 2021;10(2):464. doi: 10.3390/foods10020464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020;3(1):74–92. doi: 10.1136/bmjnph-2020-000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamulka J, Jeruszka-Bielak M, Gornicka M, Drywien ME, Zielinska-Pukos MA. Dietary supplements during COVID-19 outbreak. Results of google trends analysis supported by PLifeCOVID-19 online studies. Nutrients. 2020;13(1):54. doi: 10.3390/nu13010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adelman MJ, Bedford LM, Potts GA. Clinical efficacy of popular oral hair growth supplement ingredients. Int J Dermatol. 2021;60(10):1199–1210. doi: 10.1111/ijd.15344 [DOI] [PubMed] [Google Scholar]
- 24.Cheung EJ, Sink JR, English JC III. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15(10):1235–1237. [PubMed] [Google Scholar]
- 25.Gerkowicz A, Chyl-Surdacka K, Krasowska D, Chodorowska G. The role of vitamin D in non-scarring alopecia. Int J Mol Sci. 2017;18(12):2653. doi: 10.3390/ijms18122653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayak K, Garg A, Mithra P, Manjrekar P. Serum vitamin D3 levels and diffuse hair fall among the student population in south India: a case-control study. Int J Trichology. 2016;8(4):160–164. doi: 10.4103/ijt.ijt_57_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demay MB. The hair cycle and vitamin D receptor. Arch Biochem Biophys. 2012;523(1):19–21. doi: 10.1016/j.abb.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Vimaleswaran KS, Forouhi NG, Khunti K. Vitamin D and covid-19. BMJ. 2021;372:n544. doi: 10.1136/bmj.n544 [DOI] [PubMed] [Google Scholar]
- 29.Beoy LA, Woei WJ, Hay YK. Effects of tocotrienol supplementation on hair growth in human volunteers. Trop Life Sci Res. 2010;21(2):91–99. [PMC free article] [PubMed] [Google Scholar]
- 30.Patel DP, Swink SM, Castelo-Soccio L. A review of the use of biotin for hair loss. Skin Appendage Disord. 2017;3(3):166–169. doi: 10.1159/000462981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plonka PM, Handjiski B, Popik M, Michalczyk D, Paus R. Zinc as an ambivalent but potent modulator of murine hair growth in vivo- preliminary observations. Exp Dermatol. 2005;14(11):844–853. doi: 10.1111/j.1600-0625.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 32.Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol. 2013;25(4):405–409. doi: 10.5021/ad.2013.25.4.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siavash M, Tavakoli F, Mokhtari F. Comparing the effects of zinc sulfate, calcium pantothenate, their combination and minoxidil solution regimens on controlling hair loss in women: a randomized controlled trial. J Res Pharm Pract. 2017;6(2):89–93. doi: 10.4103/jrpp.JRPP_17_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almohanna HM, Ahmed AA, Tsatalis JP, Tosti A. The role of vitamins and minerals in hair loss: a review. Dermatol Ther (Heidelb). 2019;9(1):51–70. doi: 10.1007/s13555-018-0278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coad J, Conlon C. Iron deficiency in women: assessment, causes and consequences. Curr Opin Clin Nutr Metab Care. 2011;14(6):625–634. doi: 10.1097/MCO.0b013e32834be6fd [DOI] [PubMed] [Google Scholar]
- 36.Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin d in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26(2):101–107. doi: 10.1159/000346698 [DOI] [PubMed] [Google Scholar]
- 37.Poonia K, Thami GP, Bhalla M, Jaiswal S, Sandhu J. NonScarring diffuse hair loss in women: a clinico-etiological study from tertiary care center in North-West India. J Cosmet Dermatol. 2019;18(1):401–407. doi: 10.1111/jocd.12559 [DOI] [PubMed] [Google Scholar]
- 38.Trost LB, Bergfeld WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol. 2006;54(5):824–844. doi: 10.1016/j.jaad.2005.11.1104 [DOI] [PubMed] [Google Scholar]
- 39.Rushton DH. Nutritional factors and hair loss. Clin Exp Dermatol. 2002;27(5):396–404. doi: 10.1046/j.1365-2230.2002.01076.x [DOI] [PubMed] [Google Scholar]
- 40.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med. 2004;351(15):1548–1563. doi: 10.1056/NEJMcpc049016 [DOI] [PubMed] [Google Scholar]
- 41.Suleria HA, Osborne S, Masci P, Gobe G. Marine-based nutraceuticals: an innovative trend in the food and supplement industries. Mar Drugs. 2015;13(10):6336–6351. doi: 10.3390/md13106336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17(5):558–565. [PubMed] [Google Scholar]
- 43.Rizer RL, Stephens TJ, Herndon JH, Sperber BR, Murphy J, Ablon GR. A marine protein-based dietary supplement for subclinical hair thinning/loss: results of a multisite, double-blind, placebo-controlled clinical trial. Int J Trichology. 2015;7(4):156–166. doi: 10.4103/0974-7753.171573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777–2786. doi: 10.2147/DDDT.S214907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of Telogen Effluvium in India. Int J Trichology. 2019;11(3):107–112. doi: 10.4103/ijt.ijt_23_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abeles AM, Pillinger MH, Solitar BM, Abeles M. Narrative review: the pathophysiology of fibromyalgia. Ann Intern Med. 2007;146(10):726–734. doi: 10.7326/0003-4819-146-10-200705150-00006 [DOI] [PubMed] [Google Scholar]
- 47.Panahi Y, Taghizadeh M, Marzony ET, Sahebkar A. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13(1):15–21. [PubMed] [Google Scholar]
- 48.Konda D, Thappa DM. Mesotherapy: what is new? Indian J Dermatol Venereol Leprol. 2013;79(1):127–134. doi: 10.4103/0378-6323.104689 [DOI] [PubMed] [Google Scholar]
- 49.Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32(4):564–569. doi: 10.1111/jdv.14722 [DOI] [PubMed] [Google Scholar]
- 50.Carloni R, Pechevy L, Postel F, Zielinski M, Gandolfi S. Is there a therapeutic effect of botulinum toxin on scalp alopecia? Physiopathology and reported cases: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2020;73(12):2210–2216. doi: 10.1016/j.bjps.2020.05.035 [DOI] [PubMed] [Google Scholar]
- 51.Popescu MN, Petca RC, Beiu C, et al. Efficiency of different preparations of botulinum toxin type A, Xeomin and Dysport, in the management of spastic upper limb after stroke. Rev Chim. 2019;70(10):3490–3494. doi: 10.37358/RC.19.10.7582 [DOI] [Google Scholar]
- 52.Carruthers A. Botulinum toxin type A: history and current cosmetic use in the upper face. Dis Mon. 2002;48(5):299–322. doi: 10.1053/mda.2001.25138 [DOI] [PubMed] [Google Scholar]
- 53.Martina E, Diotallevi F, Radi G, Campanati A, Offidani A. Therapeutic use of botulinum neurotoxins in dermatology: systematic review. Toxins (Basel). 2021;13(2):120. doi: 10.3390/toxins13020120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung BH, Song SH, Yoon SJ, Koo JH, Yoo KY. The effect of botulinum toxin on hair follicle cell regeneration under continuous stress conditions: a pilot animal study. Neurotox Res. 2022;40(1):103–110. doi: 10.1007/s12640-021-00453-8 [DOI] [PubMed] [Google Scholar]
- 55.Gentile P, Scioli MG, Bielli A, et al. Platelet-rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re-growth in androgenetic alopecia. biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7(2):27. doi: 10.3390/biomedicines7020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roohaninasab M, Goodarzi A, Ghassemi M, Sadeghzadeh-Bazargan A, Behrangi E, Najar Nobari N. Systematic review of platelet-rich plasma in treating alopecia: focusing on efficacy, safety, and therapeutic durability. Dermatol Ther. 2021;34(2):e14768. doi: 10.1111/dth.14768 [DOI] [PubMed] [Google Scholar]
- 57.Popescu MN, Iliescu MG, Beiu C, et al. Autologous platelet-rich plasma efficacy in the field of regenerative medicine: product and quality control. Biomed Res Int. 2021;2021:4672959. doi: 10.1155/2021/4672959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Islek A, Karaaslan E, Simsek S, Merve Cetin F. Platelet-rich plasma treatment for accelerated androgenetic alopecia pattern hair loss after COVID-19 infection: a case series. J Cosmet Dermatol. 2022;21(2):590–594. doi: 10.1111/jocd.14721 [DOI] [PubMed] [Google Scholar]