Abstract
Calorie restriction (CR), the reduction of dietary intake below energy requirements while maintaining optimal nutrition, is the only known nutritional intervention with the potential to attenuate aging. Evidence from observational, preclinical, and clinical trials suggests the ability to increase life span by 1–5 years with an improvement in health span and quality of life. CR moderates intrinsic processes of aging through cellular and metabolic adaptations and reducing risk for the development of many cardiometabolic diseases. Yet, implementation of CR may require unique considerations for the elderly and other specific populations. The objectives of this review are to summarize the evidence for CR to modify primary and secondary aging; present caveats for implementation in special populations; describe newer, alternative approaches that have comparative effectiveness and fewer deleterious effects; and provide thoughts on the future of this important field of study.
Keywords: calorie restriction, aging, CALERIE, metabolic adaptation, intermittent fasting
INTRODUCTION
Over the past century, the average life span in higher- and lower-income countries has increased by 15 and 30 years, respectively. This is the result of lessening mortality and morbidity from food shortage, malnutrition, and infectious diseases through improved food supply and quality as well as advances in health care. In recent decades, the continuous increase in life span has slowly attenuated, and for the first time, life expectancy has reached a plateau whereby chronic diseases may balance or even outweigh modern improvements to health care (73).
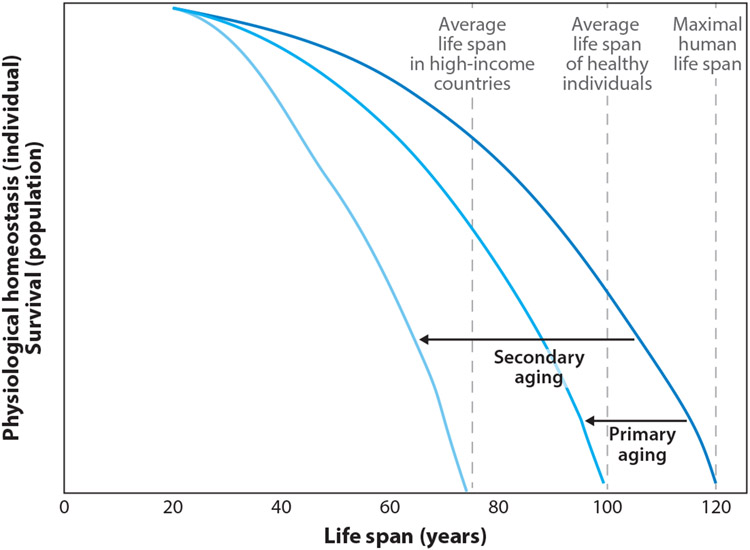
Life span is dictated by the process of aging. Aging describes the decline in physical and physiological functioning of living organisms. Aging is inevitable, but the speed or progression of aging is highly variable. The innate progression of aging is defined as primary aging (Figure 1). The acceleration of the aging process by extrinsic factors, such as excess food intake, poor fitness, or morbidity of disease, is characteristic of secondary aging. Therefore, maintenance of a normal body weight and physical functionality is generally associated with increased life span, primarily through minimization of stressors that induce secondary aging. Interestingly, sustained restriction of energy intake that is below energy requirements for weight maintenance, termed calorie restriction (CR), can further extend life span, even in normal-weight individuals.
Figure 1.
Schematic of the effects of primary and secondary aging on life span. The rate of decline in physiological functions is associated with mammalian life span. The penultimate rate of aging, whereby health span is prolonged and the majority of life is lived free of disease and with optimal physical and cognitive function, leads to above-average life span. An increase in the rate of decline of physiological function, due to the primary causes of aging and with the influence of secondary causes, leads to a shorter life.
In controlled preclinical studies using various animal models, CR has extended life span by 50–300% (43). In humans, epidemiological, observational, and randomized controlled clinical trials support the potential for CR to increase life span by 1–5 years, dependent on age at CR initiation (89). To date, however, clinical studies of CR have been limited to healthy subjects between 20 and 65 years old. While such cohorts observe clear benefits of CR, other demographic groups, such as the elderly, have seldom been studied. Yet, the penultimate study to test the effects of CR on life span in a controlled clinical setting is impractical, and may never be performed.
Unfortunately, CR carries potential risks that must be considered before it can be widely adopted as an intervention to attenuate aging and age-related diseases. First, as for many interventions, CR is vulnerable to overdose. Second, consuming fewer calories increases the risk of inadequate intake of nutrients. Third, while reducing fat mass (FM), CR may inadvertently affect muscle and bone tissue and thereby lead to unfavorable changes in physical functionality.
A practical consideration is the difficulty of adhering to CR for prolonged periods. In the most rigorous clinical studies, participants achieved only 50–75% of their prescribed energy deficit. Newer approaches for achieving CR, such as intermittent fasting or time-restricted feeding, show comparable metabolic benefits and require no reduction in daily energy intake. Allowing periods of unrestricted food intake to be interwoven with differing periods of fasting may be more widely preferred, which would lead to increased longer-term compliance.
The objectives of this review are to provide a comprehensive overview of evidence to support CR as a nutritional intervention to attenuate human aging and to present the potential risks that should be considered in specific demographic groups. We present insights into newer nutritional interventions that induce CR via alternative eating regimens with similar benefits and perhaps fewer risks, as well as future directions for this field of study and dietary practice.
MECHANISMS OF PRIMARY AND SECONDARY AGING
Advanced age is associated with increased mortality and susceptibility to disease. This increase is caused by a progressive decline in physiological function. The slope of this decline over time can be defined as aging. As depicted in Figure 1, the minimal slope allows the human life span to reach approximately 120 years. In the general population, the slope of the decline in physiological function is steeper, and the average life span is reduced to 61–83 years (128). An acceleration of this process reflects the interaction between innate aging and the extrinsic influences of the environment. The factors that explain the heterogeneity of mammalian aging are categorized into primary and secondary causes. The inevitable process of aging, which is associated with accumulation of oxidative damage and metabolic rate, is known as primary aging. Extrinsic environmental stress that accelerates primary aging and increases disease-associated mortality, such as unhealthy lifestyle and disease, is known as secondary aging.
Primary aging describes the inevitable age-associated decline in physiological and physical functions due to energy expenditure and oxidative stress. More than 100 theories exist to explain the mechanisms of the aging process, but many can be consolidated into two prevailing theories. According to the so-called rate of living theory (114), the metabolic potential, defined as energy consumed per gram of body weight per life span, is a species-specific characteristic; therefore, individuals with higher metabolic rates have shorter life spans (119). The development of this theory arose from the inverse association observed between mammalian metabolic rate (per body weight and day) and life span (49). This theory, which is still a matter of discussion, may apply only within species, not between species (121). Mechanistically, the rate of living theory is supported by the free radical theory of aging (41), which postulates that 1–3% of oxygen consumed by mitochondrial ATP production generates reactive oxygen species (ROS), that is, oxidative stress. Therefore, the more energy is expended as a function of time (i.e., age) or because of a high metabolic rate, the greater the likelihood of oxidative damage to cells and tissues will be. In turn, oxidative stress disrupts numerous molecular and cellular structures and functions (41, 90).
The acceleration of primary aging and increased mortality due to caloric excess and low physical activity occur through systemic changes in nutrient, hormonal, and immune homeostasis (45). Caloric excess leads to chronic hyperglycemia and hyperlipidemia, which in turn lead to cellular glycotoxicity and lipotoxicity, respectively, and result in systemic low-grade inflammation and oxidative stress. Low physical activity exacerbates this mechanism by reducing mitochondrial function and the antioxidative capacity of cells and tissues. Physically, caloric excess and low physical activity increase FM and cause declines in skeletal muscle mass, strength, and physical fitness. Thus, lifestyle can greatly influence susceptibility to disease (91), specifically among the elderly, who are already vulnerable due to their advanced age. Independent of the effects on primary aging, both glycotoxicity and lipotoxicity increase susceptibility to neuropathy, nephropathy, and retinopathy; insulin resistance; metabolic syndrome; cardiovascular diseases; nonalcoholic fatty liver disease; pancreatitis; and sarcopenia, among other diseases (14, 70).
SEVEN PILLARS OF AGING
The molecular, cellular, tissue-level, and organ-level changes that are affected by these stressors and cause a decline in physiological function have been described as overlapping sets of 5–10 processes (72). These have recently been termed the seven pillars of aging (58).
The seven pillars of aging include adaptation to stress, inflammation, metabolism, macromolecular damage, proteostasis, epigenetic modification, and stem cells and regeneration. Briefly, oxidative stress, or the accumulation of damaging free oxygen radicals, is a crucial signal in cells and tissues that induces pathways to restore homeostasis in a challenged system (110). Adaptations to oxidative stress include stimulation of blood flow, increased mitochondrial function, and induction of inflammation. Additionally, oxidative stress induces activation of cellular antioxidative machinery, which attempts to restore oxidative stress to physiological levels and prevent chronic accumulation of oxidative damage. With age, oxidative stress increases in response to internal and external stressors endured by an organism, and simultaneously the responsiveness of antioxidative machinery is attenuated. Combined, these changes result in molecular, cellular, and systemic damage, including tissue damage and induction of chronic inflammation.
Inflammation is the physiological response to molecular, cellular, or viral threats. With aging, systemic inflammation increases due to increased proinflammatory tissue damage, the accumulation of oxidative stress, and other intrinsic and extrinsic stressors (115). Increased chronic systemic inflammation can affect homeostasis of healthy cells and impair their function; for example, it can increase protein degradation, impair insulin signaling, and decrease production of signaling hormones by brain neurons.
Metabolism describes all processes involved in ATP production that provide energy for survival. The most prominent metabolic changes observed with aging are dysregulated nutrient sensing (25) and mitochondrial dysfunction (57). Dysregulated nutrient sensing implies decreased sensitivities to hormones such as insulin. Insulin is the master regulator of substrate production, storage, and oxidation; and impaired signaling, classically referred to as insulin resistance, is a primary contributor to glucotoxicity and lipotoxicity. Poorly functioning mitochondria increase oxidative stress, glycolytic oxidation, and lactic acid accumulation.
Macromolecular damage describes alterations to proteins, DNA, lipids, and other macromolecular components. Damage to these components impairs their function and requires increased energy for repair, breakdown, resynthesis, or elimination and increases oxidative stress, for instance, by activation of the uncoupled protein response (107).
Proteostasis describes the homeostasis of protein metabolism through coordination of protein synthesis with polypeptide folding, the conservation of protein conformation, and protein degradation. With advancing age, dysregulated proteostasis leads to misfolded and aggregated protein accumulation, which has an exaggerated impact on postmitotic cell types such as neurons, as well as on skeletal muscle proteins (44).
Epigenetic changes involve alterations in DNA methylation patterns, posttranslational modification of histones, and chromatin remodeling. Age-associated changes in these processes impair the production of enzymes and thereby inhibit critical pathways of cellular homeostasis. Epigenetic changes can be induced by environmental factors such as pollution; radiation; infection; and unhealthy lifestyle, including poor diet quality, alcohol, and smoking (34).
Stem cells are the source of cell generation for tissue growth or restoration. Over time, due to mutations and telomere shortening, stem cell pools become exhausted. Stem cell exhaustion limits production of adaptive immune cells, hematopoietic cells, and myocytes. The epigenetic changes causing stem cell exhaustion are inevitably acquired as a consequence of cell division, but they may also be accelerated by extrinsic stressors and the resultant inflammation and oxidative stress.
In human research, evaluating life span with mortality as an end point is impractical. Thus, studies of human aging must gain insight from the biological drivers of aging and hallmarks of longevity. Biomarkers of aging permit evaluation of the potential effectiveness of interventions with hypothesized benefits to the aging process. Historically, core body temperature and insulin concentrations were commonly reported biomarkers of aging that have been validated in longitudinal cohorts (113). High core body temperature is associated with increased metabolic rate and, therefore, is believed to reflect accelerated primary aging. High insulin is associated with unhealthy lifestyle, adiposity, and insulin resistance, and therefore reflects increased secondary aging. Use of modern technology has shown that methylation patterns are strongly associated with mammalian aging and alone can approximate biological age to within 1.9 years (47).
CALORIE RESTRICTION AS A NUTRITIONAL INTERVENTION TO PROMOTE HEALTHY AGING AND LONGEVITY
CR is the most prominent and well-researched intervention with the intent to attenuate mammalian aging. CR is defined as the sustained restriction of dietary energy intake as compared with the energy requirement for weight maintenance. CR requires that, while energy intake is restricted, the diet provide sufficient energy for metabolic homeostasis and be of high quality, that is, rich in micronutrients and fiber.
The optimal dose of CR will yield benefits for aging while minimizing potential harms to physical fitness and mental well-being. While preclinical studies in rodents have found beneficial effects of CR on biomarkers of aging, health span, and life span with CR ranging from 10% to 40%, the seminal study of semistarvation by Keys et al. (59), which is often discussed in the context of human CR, demonstrated that CR can likely be overprescribed. In this landmark study, CR induced by dietary restriction and increased exercise was estimated to be approximately 40%. At this degree of CR sustained over 24 weeks, body weight loss was 25%, of which approximately two-thirds was FM and one-third fat-free mass (FFM). The 40% CR led to severe adverse effects, both physical (e.g., chronic weakness, reduced aerobic capacity, and painful lower limb edema) and psychological (e.g., emotional distress, confusion, apathy, depression, hysteria, hypochondriasis, suicidal thoughts, and loss of sex drive), which emerged after 6 weeks (59). An important distinction between the diet in this semistarvation study and those of other CR studies is that not only was energy intake reduced but also diet quality (food variety, macronutrient profile, micronutrient content, etc.) was significantly curtailed. Responsible prescription of CR requires adequate intake of carbohydrate, fat, protein, and micronutrients to ensure satisfaction of the recommended daily allowance (RDA). For this reason, vitamin and mineral supplements are often included in CR diets. For nonhuman primates and humans, diets with prescriptions ranging from 10% to 30% CR have shown beneficial effects on biomarkers of aging and health span.
NONHUMAN PRIMATES AS A TRANSLATIONAL MODEL FOR CALORIE RESTRICTION
Preclinical studies in rodents provide valuable insights into potential mechanisms underpinning the beneficial effect of CR on humans. The rhesus monkey genome shares ~93% sequence identity with the human genome, and the two species have similar aging phenotypes (155). With its approximately 40-year life span, the rhesus monkey often serves as a translational model extending preclinical science to clinical applications. Two studies of independent colonies of calorie-restricted rhesus monkeys, located at the University of Wisconsin and the National Institute on Aging, are in their third decade of research. The two studies report both improvements and no changes in survival rates in the CR monkeys compared with the ad libitum–fed monkeys. However, these differences have been attributed to discrepancies in age of CR onset and animal husbandry practices (i.e., feeding protocols, treatment of age-related diseases) (81). While we await final life span data, CR monkeys from both colonies are already exhibiting signs of delayed aging compared with their ad libitum–fed counterparts, with some already surpassing longevity records in this species. The CR monkeys show delays in primary aging, as evidenced by reductions in resting metabolism (12) and metabolic cost of activity (152). There are also clear benefits to secondary markers of aging, including reductions in adiposity, particularly from the truncal regions (24), and improved glucoregulatory function and lipid levels (62, 106). Finally, compared with CR animals, ad libitum–fed animals had double the rate and more severe cases of age-associated diseases, including diabetes, cancer, cardiovascular disease, and osteoporosis (81). These nonhuman primate studies provide exciting data regarding the potential for sustained CR to keep age-associated diseases at bay while preserving physical functionality.
EPIDEMIOLOGICAL AND OBSERVATIONAL STUDIES OF CALORIE RESTRICTION AND AGING
The past century has provided several opportunities to support evidence of the benefit of CR for aging in humans. In uncontrolled observational studies of cohorts that voluntarily and involuntarily restricted caloric intake, researchers were able to study the effects of sustained CR in a free-living environment on biomarkers of aging and life span.
Okinawans
Okinawa is a small Japanese island whose residents organically practiced mild CR. Archived dietary data yield a CR estimate of 15% (147, 148), and importantly, diets composed primarily of fish and green vegetables were considered nutritionally rich (30, 146). Compared with mainland Japanese, Okinawans had a greater mean and median life span (83.8 versus 82.3 years); a higher prevalence of centenarians; and reduced mortality from age-related diseases such as cardiovascular disease, cancer, and dementia (147). Tragically, with the emergence of a Westernized diet following World War II, CR among Okinawans decreased rapidly. Today, the mean life span is 87.0 and 86.4 for girls and boys, respectively (124), which more closely reflects that of mainland Japanese and residents of many Westernized countries.
Biosphere-II
The Biosphere-II experiment provided an unexpected, involuntary opportunity to observe CR in humans. Eight volunteers were enclosed in a 3.15-acre ecosystem for 2 years for an ecological investigation (134). Diet quality was adequate, and since participants harvested 85% of their food, their diet was composed primarily of vegetables, fruits, and grains and tended to be low in protein. Due to an unexpected scarcity of food, participants’ energy intake was estimated to decline by approximately 38% for 6 months, followed by a 20% reduction once internally grown crops became more abundant (135). Individuals had a significant reduction in body mass index (BMI); systolic and diastolic blood pressure; and fasting concentrations of insulin, glucose, cholesterol, and cortisol (134, 135). Immediately after the subjects left the Biosphere enclosure, a measurement of 24-h energy expenditure in a room calorimeter revealed that they had experienced a 6% slowing of metabolism, or metabolic adaptation, in comparison to more than 800 age-, sex-, and body mass– and composition-matched controls (143). These adaptations persisted for an additional 6 months after the participants exited the ecosystem, resumed an ad libitum diet, and restored body weight (144).
CR Society International
Initiated by the late Roy Walford, a Biosphere-II participant, CR Society International is a group of free-living individuals who have voluntarily restricted their energy intake for 3 to 15 years. These individuals are reported to achieve approximately 30% CR, or energy intakes between 1,112 and 1,958 kcal/day, and 100% RDA of nutrients (37). Compared with individuals eating a standard American diet, individuals of the CR society are leaner (BMI 19.6 ± 1.9 versus 25.9 ± 2.7 kg/m2) and have a lower percentage of body fat (6.7 ± 7 versus 22 ± 8%) (37). With respect to measures of secondary aging, individuals self-practicing CR have more favorable markers of cardiometabolic health, such as lower systolic and diastolic blood pressure, fasting glucose and insulin, triglycerides, total cholesterol, and low-density lipoprotein (LDL-C), as well as higher high-density lipoprotein (HDL-C) (37) and lower levels of systemic inflammation, as characterized by C-reactive protein, tumor necrosis factor α (TNFα), interleukin-6, and cortisol, a potent anti-inflammatory mediator (35). Their skeletal muscle biopsies show that long-term CR reduces inflammatory pathways. Increased activation of protein chaperones, in particular a 1.8-fold increase in heat shock protein 70, along with increased regulation of several autophagy genes, was also evident in comparison to matched controls (153). Moreover, individuals self-practicing CR demonstrated significant improvements in the circulatory system, with a 40% lower carotid intima media thickness (37, 85) and a left ventricular elasticity that was comparable to that of individuals 16 years younger (46). Additionally, they had a heart rate variability (reflective of improved autonomic nervous system functioning) comparable to that of individuals 20 years younger (122). Notably, none of the CR society members studied to date reported any cardiovascular disease, and a review of their medical records showed less pharmacotherapy (37, 89).
While these observational studies provide insights into the possible effects of CR in community-dwelling individuals, data are sparse and limited to self-reports in many instances. Furthermore, data that would shed light on primary and secondary aging or the molecular mechanisms from the standpoint of the seven pillars of aging have not been reported. Thus, randomized controlled trials are the only means of elucidating the mechanisms of CR that may preserve biological aging and delay the onset of age-related diseases.
RANDOMIZED CONTROLLED TRIALS OF CALORIE RESTRICTION FOR HUMAN AGING
Very few randomized controlled trials have examined the effects of traditional CR on biological aging. By far the most rigorous study in humans has come from the National Institute of Aging-sponsored CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) Consortium. Over approximately 9 years, CALERIE comprised three pilot studies (CALERIE 1) followed by a large multisite randomized study (CALERIE 2). The three independent pilot studies served the overarching goal of determining the feasibility of performing different modalities of CR in humans (recruitment, compliance, and attrition), and of informing the set of measures in a large trial based on considering whether CR was induced by dietary restriction alone, increased exercise energy expenditure, or a combination of both (42, 102), or by varying diet composition (glycemic loads) (28). The findings from the 6- or 12-month pilot trials were used to develop CALERIE 2, which was a randomized controlled trial of 25% CR in more than 220 healthy, nonobese individuals for 2 years (108).
The CALERIE Calorie Restriction Studies
The CALERIE 2 intervention aimed to reduce caloric intake without enforcing a set nutrient composition and required only that the self-selected diets meet daily micronutrient requirements. To help participants understand how to self-select diets and alter the type and content of food, investigators provided participants with all meals for the first 27 days of the trial. Three 9-day diets were provided in a 3-day rotating menu. The three diets were low fat, low glycemic load, and Mediterranean. All diets varied between 20% and 35% fat, 15% and 30% protein, and 40% and 60% carbohydrate and provided a fiber ratio of 14 g/1,000 kcal. The purpose of the diet rotation was to educate participants on how the diversity of foods among the three different diets could be incorporated into their self-selected CR practice as well as to combat challenges such as hunger and cravings. Table 1 presents sample menus for each of the three diets, along with instructions for how participants could prepare the foods at home using US standard measurements. All participants were provided with a daily multivitamin and mineral supplement and additional calcium to help ensure adequate micronutrient intake.
Table 1.
Sample eating plans in the CALERIE 2 study at 1,800 kcal/daya
| Breakfast | Lunch | Dinner | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Metric | US standard |
Item | Metric | US standard |
Item | Metric | US standard |
| Low-fat eating plan | ||||||||
| Plain bagel | 90 g | 1 bagel | Wheat bread | 42 g | 2 slices | Sweet-and-sour chicken | 190 g | Recipe |
| Cream cheese | 28 g | 2 Tbsp | Ham | 80 g | 2¾ oz | Rice | 90 g | ½ cup |
| Fat-free yogurt | 120 g | ½ cup | Cheddar cheese | 15 g | ½ oz | Carrots, sliced | 110 g | ¾ cup |
| Peaches | 120 g | ½ cup | Romaine lettuce | 84 g | 1¾ cup | Pears | 130 g | ½ cup |
| Orange juice | Added to meet higher caloric needs | Tomato, chopped | 48 g | ⅓ cup | ||||
| Fat-free dressing | 36 g | 2⅓ Tbsp | ||||||
| Mustard | 5.5 g | ⅛ tsp | ||||||
| Skim milk | 242 g | 1 cup | ||||||
| Bran cookie | 64 g | 2⅓ oz | ||||||
| Low-glycemic eating plan | ||||||||
| Cereal | 30 g | ½ cup | Bean and vegetable | 205 g | Recipeb | Salsa chicken | 276 g | Recipe |
| Skim milk | 242 g | 1 cup | Pumpernickel bread | 52 g | 2 slices | Rice and beans | 145 g | Recipe |
| Grapefruit | 30 g | ⅛ cup | Tuna | 115 g | ⅔ cup | Spinach | 50 g | 1⅔ cup |
| Mayonnaise | 22 g | 1½ Tbsp | Tomato | 60 g | 1⅓ cup | |||
| Flaxseed cookie | 23.5 g | ¾ oz | Apple | 138 g | 1 apple | |||
| Skim milk | 242 g | 1 cup | Olive oil | 0 g | 2¼ tsp | |||
| Vinegar | 6 g | 1¼ tsp | ||||||
| Mediterranean eating plan | ||||||||
| Granola | 52 g | ⅔ cup | Greek wrap | Lentils with olives and feta | 185 g | Recipe | ||
| Skim milk | 242 g | 1 cup | Tortilla | 90 g | 1⅓ tortillas | Couscous | 100 g | ½ cup |
| Banana | 118 g | 1 banana | Hummus | 36 g | 2½ Tbsp | Zucchini | 150 g | ⅔ cup |
| Cucumber | 48 g | ½ cup | Strawberries | 130 g | 1 cup | |||
| Tomatoes | 48 g | ⅓ cup | ||||||
| Onions | 15 g | 1½ Tbsp | ||||||
| Olives | 10 g | 1¼ Tbsp | ||||||
| Feta cheese | 28 g | ¼ cup | ||||||
| Cheddar cheese | 45 g | ⅓ cup | ||||||
| Red grapes | 150 g | 1⅔ cup | ||||||
Diets were modified to meet caloric needs of 1,500, 1,800, and 2,400 kcal/day.
Recipe was prepared in a metabolic kitchen, and quantities were provided to participants.
CALERIE and Primary Aging
The 6-month pilot study at the Pennington Biomedical Research Center compared 25% CR achieved through (a) diet restriction alone and (b) a combination of dietary restriction (12.5%) and exercise-induced energy expenditure (12.5%) (CR+Ex) with an ad libitum control group (AL) (42). The primary hypothesis was that CR would attenuate primary aging, as represented by the presence of metabolic adaptation, which is a slowdown of energy expenditure beyond what would be expected on the basis of body size. Confirming the hypothesis was the finding that mass-adjusted sleeping energy expenditure was reduced after 6 months of CR and CR+Ex, indicating metabolic adaptation and perhaps a slowing of primary aging (Figure 2) (42). In both modalities, this metabolic adaptation was 126 + 25 kcal/day, or 6% (66), which is comparable to the metabolic adaptation observed in Biosphere-II participants. Similar findings were observed for resting metabolic rate measured in CALERIE 2, but metabolic adaptation was significantly different from the ad libitum–eating group only after 12 months (103). The lack of persistent slowing of metabolic rate in the longer-duration trial was likely confounded by adherence (~15% during the first 12 months, ~9% during the next 12 months) (103). In a subset of adherent subjects (defined as ≥5% weight loss for CR and ≤5% weight loss for AL), metabolic adaptation measured by sleeping energy expenditure was indeed confirmed after 12 and 24 months (105). Furthermore, a metabolic adaptation in total daily energy expenditure (by doubly labeled water) was observed after 6 months of CR in the pilot study (20, 42) as well as after 12 and 24 months of the multisite trial (103). The decline in total daily energy expenditure was independent of changes in spontaneous physical activity (42, 80) over 6 months but not over 12 and 24 months, suggesting that with longer-duration CR, individuals may consciously or unconsciously reduce physical activity in an effort to conserve energy (105).
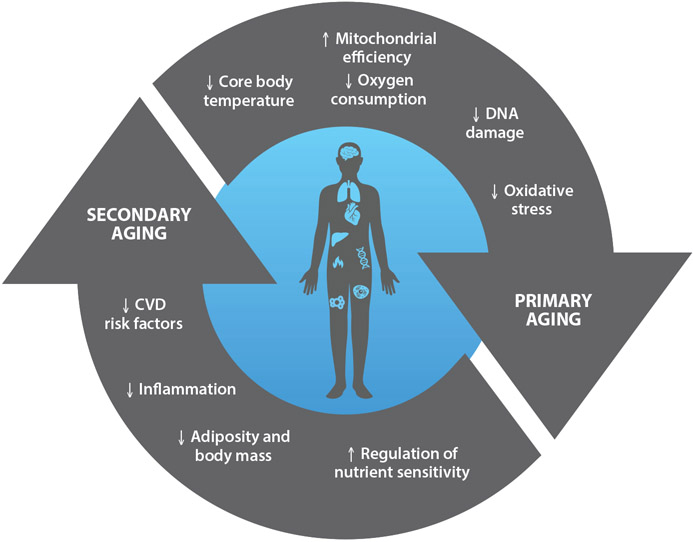
Figure 2.
Proposed effects of calorie restriction (CR) on primary and secondary aging. Primary and secondary aging act independently and synergistically to produce stressors that induce molecular, cellular, and systemic changes. CR broadly improves aging through these processes. Abbreviation: CVD, cardiovascular disease.
Slowing the rate of the energy metabolism of an organism, or reducing the rate of oxygen consumption, can reflect an increase in the overall energy efficiency of mitochondria for generating ATP or a reduction in the energy requirements of specific tissues. Improved mitochondrial energy efficiency occurs when mitochondrial respiration is closely coupled to ATP production and electron leakage is decreased. Increased energy efficiency can be achieved through increased mitochondrial capacity and reduced expression of uncoupling proteins. As hypothesized (20), CR increases mitochondrial DNA content by 35% and increases expression of genes encoding proteins involved in mitochondrial function. In CALERIE 2, individuals with higher rates of mitochondrial coupling demonstrated a greater increase in maximal ATP synthesis and ATP coupling rates compared with individuals with lower rates (120). Furthermore, CR after 12 and 24 months reduced excretion of F2-isoprostane, including isoform 2,3-dinor-iPF2α-III, a sensitive biomarker of oxidative stress (105). In support of a link between the oxidative damage theory of aging and the rate of living theory, the reduction in 2,3-dinor-iPF2αIII was associated with metabolic slowing and the degree of CR achieved (105). Unlike studies of CR in nonhuman primates (154) and short-term studies in humans (27), neither the 6- nor the 24-month CALERIE studies succeeded in reducing serum protein carbonyls (42, 105), albeit these are a less sensitive marker of oxidative stress (53). While the 6-month pilot trial showed a reduction in DNA fragmentation suggestive of less DNA damage, this reduction was not related to changes in oxygen consumption (42), indicating that other factors likely affected oxidative damage to DNA (20).
Commensurate with the observed slowdown in metabolic rate, the CALERIE studies reported decreases in core body temperature. In one of the 6-month pilot trials, declines in core body temperature occurred in both CR (−0.2 ± 0.05°C) and CR+Ex (−0.3 ± 0.08°C) participants. In the 2-year multisite trial, 24-h core temperature was also significantly reduced after 12 and 24 months, yet it was not significantly different from that of the control group (103). Interestingly, the reduction in core temperature reflected a reduction in body temperature at night only (105). Furthermore, reductions in thyroid activity are related to increases in longevity in both animals and humans (16), triiodothyronine levels declined in response to CR during the pilot trials (142), and thyroid-stimulating hormone was reduced after 1 year of CR in the multisite trial (103).
CALERIE and Secondary Aging
During the 6-month pilot at Pennington Biomedical, participants in both CR groups achieved 10% weight loss (42) with a 25% reduction in total FM and a 27% reduction in visceral fat (104), the depot that is most closely related to metabolic diseases (17). Similarly, the Washington University participants who achieved a 20% energy deficit through either exercise or CR lost comparable weight, total FM, and visceral FM (140). In the Tufts University study, all participants following 30% CR, regardless of their diet composition (high or low glycemic load), achieved significant weight loss. However, the degree of weight loss was influenced by the glycemic load of the diet and baseline insulin secretion; individuals with higher insulin secretion lost more weight on a low-glycemic diet than on a high-glycemic diet (99, 100). Since none of the pilot trials followed participants long enough to observe a weight loss plateau, the design of CALERIE 2 included a weight loss period for the first year, followed by weight maintenance in the second year. Throughout the Phase 2 trial, the change in weight was used to determine adherence. Investigators developed weight loss nomograms for each participant modeled from the Phase 1 data (98) which showed the expected weight loss trajectory if someone adhered to their 25% CR diet. Participants would weigh themselves daily, plot the weight on their personal nomogram, and receive weekly coaching to understand that more or less weight loss could be achieved by modifications to calorie intake. Since the weight loss trajectory of Phase 1 was modeled for Phase 2, it is not surprising that participants in the multisite trial also evidenced a 10% weight loss at 6, 12, and/or 24 months. Similar to the Phase 1 CR through dietary intervention alone, the majority (69%) of the weight loss consisted of FM; however, a loss of FFM (4.0 ± 0.3%) was also observed (103).
The CALERIE studies show that CR, even in already-healthy individuals with normal-range BMI, can effectively reduce both subcutaneous and visceral fat deposits, which are associated with improved cardiometabolic health (88). Moreover, benefits in ectopic fat deposition were also observed. CR reduced intrahepatic lipid content after 12 months but not after 24 months, while intramyocellular lipid remained unchanged after 12 months but was significantly decreased after 24 months (63). It is noteworthy that individuals in these trials were healthy and had very low levels of ectopic fat to begin with. Nevertheless, improvements in ectopic fat were associated with improved insulin sensitivity and cardiometabolic health (63). While the extent of FM loss was similar between males and females, sex had a significant effect on FFM loss: Males evidenced greater declines than females (29).
Cardiometabolic health.
Both the CALERIE 1 pilot studies and the 24-month CALERIE 2 trial showed significant improvements in carbohydrate metabolism with CR. For example, using an oral glucose tolerance test, 20% CR in older individuals (age 50–60) at Washington University and 30% CR in middle-aged individuals (age 24–42) at Tufts University showed improvements in insulin and glucose area under the curve (100, 140). With the use of a frequently sampled intravenous glucose tolerance test, 25% CR and CR+Ex led to a 40% and 37% increase, respectively, in insulin sensitivity after 6 months (63). These CR-mediated benefits were also observed in the 24-month trial via improvements in β-cell function (67), glucose tolerance, and fasting insulin (69, 118). Using targeted metabolomics, the pilot trials also observed an improvement in metabolic flexibility through increases in fatty acid oxidation intermediates from the fasting to the postprandial state (48).
While short-term CR resulted in no change in LDL-C or HDL-C (67), 2 years of CR induced significant and persistent declines in LDL-C and triglycerides, increases in HDL-C, and a decreased ratio of HDL-C to total cholesterol. Furthermore, declines in systolic and diastolic blood pressure and in mean arterial pressure were observed (61, 88). After 1 year, CR produced improvements in left ventricular function in healthy older adults, a benefit typically observed with exercise (109). Altogether, CR sustained for only 6 months was estimated to induce a 29% reduction in the 10-year risk for cardiovascular disease (Figure 2) (67). Importantly, the 2-year trial showed that improvements in cardiometabolic health biomarkers were robust even after controlling for weight loss (88). The reduction in risk scores for cardiovascular disease in both the short- and long-intervention trials may be attributed to declines in oxidative stress and inflammation as well as to preservation of endothelial nitric oxide function (61). It is apparent that CR offers powerful protective effects against the development of cardiometabolic diseases in individuals of young and middle age and in those already deemed healthy, that is, nonobese and not reliant on pharmacotherapy.
Inflammation.
In animal models of CR, robust declines in inflammatory markers (e.g., interleukin-1 and interleukin-8) have been observed in a variety of species, including rodents (136) and nonhuman primates (82). Additionally, systemic inflammation—measured by interleukin-6, TNFα, and C-reactive protein—appears to be a strong correlate of survival and a major determinant of longevity in centenarians (3). TNFα, which acts to impair insulin action, was significantly decreased following 24 months of CR but not after the 6-month pilot study of younger adults (42, 103). However, TNFα was reduced by 16–20% in the pilot study involving older adults and when CR was achieved through structured exercise (102). Similarly, C-reactive protein decreased slightly after 12 months but significantly at 24 months compared with the control group. Together, these findings regarding the anti-inflammatory benefits of CR are realized only after longer periods (61). However, detecting a reduction in inflammation might also be dependent on age at initiation of CR and, hence, the patients’ inflammatory state at the outset.
Biological age.
Unlike chronological aging, which advances at the same rate in everyone, biological aging refers to the gradual and progressive decline in the integrity of the body’s systems and therefore can be widely variable. In observational studies, individuals with lower rates of biological aging not only exhibit higher physical and mental functioning but also have a lower risk of chronic disease development and death (76). Considering 10 biomarkers, including serum albumin, alkaline phosphatase, C-reactive protein, total cholesterol, creatinine, glycated hemoglobin, systolic blood pressure, urea nitrogen, uric acid, and white blood cell count, Belsky et al. (10) calculated the change in biological age across the 24-month trial using two different validated equations. The Klemera–Doubal equation (60), a mortality predictor stronger than chronological age alone, showed that CR induced a slowing of biological age of 0.11 years per 12 months compared with 0.71 years in controls. Importantly, and in support of the potential health span and life span extension of CR interventions, the CR-induced preservation of biological age was independent of weight loss and was related to the degree of CR achieved (10).
CAVEATS OF CALORIE RESTRICTION INTERVENTIONS FOR AGING AND SPECIAL CONSIDERATIONS
An intervention that imposes a sustained 25% CR is safe and well tolerated. In CALERIE 2, only two participants withdrew from the study for adverse events: one for treatment-resistant anemia and one for excessive bone mass loss (≥5% compared with baseline) (112). In addition, the CALERIE studies showed no incidence of eating disorder development (150). Nevertheless, despite the numerous beneficial effects of CR, described above, sustained CR can have potential drawbacks, such as difficulty of maintaining compliance and adverse effects on mental health through weight loss–induced hunger and the loss of FFM.
Compliance
Arguably the greatest challenge for CR as a nutritional strategy to foster healthier aging is the ability to adequately sustain a reduction in calorie intake over many months and even years. As with most behavior-based interventions, compliance wavers over time. In CALERIE 1, 25% CR was prescribed and 18% CR was achieved over 6 months (104). Similarly, participants achieved 19.5 ± 0.8% CR over the first 6 months in CALERIE 2, but compliance dropped off considerably to only 9.1 ± 0.7% during the remaining 18 months (103). The high level of compliance in the short term was facilitated by food provision. Thereafter, adherence was fostered by individual- and group-based counseling by psychologists and nutritionists, weekly weight monitoring, and diet tracking (108). The decline in individual support may also have contributed to the observed decline in adherence during the intervention. After the intervention, and thus with no structured support, the level of CR was only ~5%, and consequently 54% of the initial weight loss (9.0 ± 0.6 kg) was regained (78). Furthermore, patients’ motivation may waver over time if they observe no additional obvious improvement (i.e., reduction in body weight). The compliance data from CALERIE suggest that long-term maintenance of the diet strategy could limit clinical application.
Physiologically, changes in hunger and satiety may contribute to compliance. Dietary weight loss interventions can alter eating behaviors and increase consumption of particular foods, even in the absence of hunger, which can ultimately increase weight gain (15, 145). Indeed, participants in CALERIE 2 reported increased hunger (32). Moreover, disinhibition, or lack of controlled eating, was also observed. Importantly, these changes were observed only during the first year, in which participants still lost weight, and not in the second year, when weight was maintained. Thus, sustained CR does not appear to induce hunger when body weight maintenance is achieved.
Mental and Sexual Health
In light of the Minnesota Starvation Experiment, there has been general concern about the potential detrimental effects of CR on mental health and sexual function. However, using validated quality of life, sleep, and sexual function questionnaires (79), the CALERIE studies demonstrated a positive impact on these parameters. Most measures of mood (anger, fatigue, confusion) were unaffected or even improved (less depression, less tension) in the CR group compared with the control group. Also, quality of sleep and sexual function were not affected or even improved (sexual drive and relationships). Importantly, detrimental effects can be observed with more severe CR, for instance, in CR Society International members, and may relate to low testosterone levels (36, 38).
Bone Loss
Maintaining healthy bone mineral density is a part of healthy aging. Greater bone mineral density is associated with a reduced risk of developing osteoporosis and frailty, thus offering protection from falls and related morbidities. Although loss of bone mineral density is common with weight reduction, regardless of the method, in the younger subjects studied in CALERIE 2, CR did not affect total bone mineral content (29). However, bone mass decreased at clinically important sites of osteoporotic fractures, such as the hip, femoral neck, and lumbar spine (130). These reductions were only partly proportional to changes in diet, physical activity, or body composition (10–31% in a regression analysis). This intraindividual variability in bone loss emphasizes a need for regular, close monitoring of bone health for individuals consuming a CR diet (130). Bone health monitoring is particularly important because bone mineral density peaks around age 30, followed by progressive reduction with age. Thus, accelerated reductions in bone mineral density in early to middle age may predispose individuals to developing osteopenia and fracture risk later in life, which is associated with premature mortality (74). In CALERIE 1 at Washington University, older adults experienced a reduction in bone mineral density with 11.5% CR (102, 131). With weight loss, bone loss can be expected to some extent, for instance, after bariatric surgery (116); however, whether bone loss is greater than expected on the basis of weight loss is important to assess on an individualized basis, especially among individuals whose bone mineral density may be compromised, such as the elderly (131).
Notably the reductions in bone mineral density in CALERIE persisted despite the provision of a multivitamin and mineral supplement (and additional calcium), suggesting that adaptations to bone metabolism expand beyond micronutrient availability. Individuals self-practicing CR for ~10 years or longer have low bone mineral density, but their bone quality, defined by trabecular bone microarchitecture parameters such as erosion index and surface-to-curve ratio, is not significantly different from that of age- and sex-matched controls (132). This finding suggests that bone loss may occur in the early years following initiation of CR, and that compensatory mechanisms then occur to prevent an increase in the rate of bone turnover and, in turn, may preserve bone quality (132). Longer-term clinical trials of CR are needed to properly assess whether the initially observed reductions in bone mass are attenuated over the long term, whether compensatory increases in bone quality follow, and whether there is an increased fracture risk (117), particularly in the elderly.
Loss of Fat-Free Mass
With advancing age, greater muscle mass is associated with better physical function and a lower risk of immobility. CR may induce a loss of FFM and muscle mass. In CALERIE 1, loss of FFM was observed in all CR groups. In younger individuals [25–50 years (64)], despite the reduction in muscle mass, muscle strength did not decline, but in the older cohort [50–60 years (141)], leg strength declined in proportion to the reduction in muscle mass. Loss of muscle mass and loss of strength are the primary risk factors for developing sarcopenia, a major age-related disease that is associated with increased mortality (4). However, exciting research in preclinical models suggests that CR may have a positive impact on the underlying molecular mechanisms of developing sarcopenia (23, 83). Future research is required to understand whether these findings translate to humans.
Muscle mass also affects aerobic fitness capacity and physical function. With the reduction in FFM, aerobic fitness was unaffected by CR in the younger cohort (64) but declined in the older cohort (141). This finding indicates a potentially increased risk of functional decline in older cohorts performing CR. These observations were confirmed in CALERIE 2. After 2 years of CR in 20–50-year-old subjects, reductions in FFM (29), leg lean mass (assessed by dual energy X-ray absorptiometry) (101), aerobic capacity, and leg strength (101) were observed. However, after adjustment for body mass, muscle strength and cardiorespiratory fitness were increased (versus body weight) or maintained (versus lean body mass) with CR (101). Post hoc analyses of these data revealed that greater decreases in FFM (and lean mass) occurred in subjects who achieved >12.5% CR versus those who maintained a lesser degree of CR (29). Furthermore, reductions in FFM were associated with older age, male sex, less activity-related energy expenditure, and higher baseline BMI (29) and were not influenced by protein intake (normalized to body weight) (29). Despite the more pronounced effects of CR on FFM in male subjects, aerobic capacity and leg strength were adversely affected only in women (101). It was thought that this finding is explained by the more pronounced reduction in activity-related energy expenditure in females versus males (101).
The positive aspects of CR in promoting longevity and healthy aging are entangled with concerns about long-term impacts that are difficult to measure. In young, healthy individuals, CR is safe and well tolerated and induces a host of beneficial effects on primary and secondary aging. Induced reductions in muscle and bone mass, however, require future investigation and may limit the application and clinical recommendation of CR. Importantly, lean mass reductions are associated with the degree of CR achieved; therefore, increasing CR beyond the level observed in CALERIE likely increases the risk for sarcopenia and frailty in later life, even if no clinical threshold for sarcopenia is crossed during the intervention. A post hoc analysis (102) of CALERIE data showed that increased physical activity is the most promising strategy to counteract reductions in FFM.
CALORIE RESTRICTION FOR SPECIAL POPULATIONS
Elderly
The population of elderly individuals is growing at an unprecedented rate, which has led to an acceleration in research on healthy aging (118). The profound and reproducible evidence of cardiometabolic benefits of sustained CR in healthy, nonobese young and middle-aged humans does not come without concerns for implementation in the healthy, nonobese older population (age >65 years). These concerns relate to the “healthy” degree of adiposity, muscle mass and strength, and bone mass and quality.
Large epidemiologic and population-based cohort studies have consistently demonstrated a J-shaped BMI–mortality curve, where mortality risk is lowest with a BMI of 23–24 kg/m2 (5). With advancing age (>65 years), this J-shaped BMI–mortality curve is right-shifted (lowest risk, 27–28 kg/m2) (151); thus, reducing body weight (to BMI <27 kg/m2) through CR may be associated with an increased mortality risk. Okinawan centenarians (10) and members of the CR Society International (11), who have an average BMI less than 23 kg/m2, likely benefit from long-term adaptations to CR performed over 10 or more years, as well as from their maintenance of a healthy lifestyle.
An emerging concern regarding CR in elderly individuals is sarcopenia, which is a major cause of immobility, frailty, and mortality (54, 93). The fact that CALERIE 2 observed reductions in both muscle mass and strength may be cause for concern. Older individuals may cross clinical cut points for sarcopenia diagnosis with continued CR, and having low muscle mass and low strength more than doubles the risk for all-cause mortality, even in the context of obesity (71). Further studies on CR would benefit from addressing how clinically important outcomes for sarcopenia, including gait speed, grip strength, and muscle mass, are affected by advancing age.
Despite these whole-body and tissue-level concerns regarding reductions in BMI and muscle mass and muscle function paradoxically, mechanistic studies in animals (96, 97) and humans (153) suggest that CR induces molecular adaptations that may combat sarcopenia. For example, CR induces benefits to oxidative damage (8), mitochondrial dysfunction, skeletal muscle lipid metabolite accumulation [ceramides (111), sphingolipids, and diacylglycerols (69)], dysregulated proteostasis (21), insulin resistance (31), and anabolic resistance (133)—all of which may be intrinsic to the onset and progression of sarcopenia (6). Additionally, the recent advent of top-down targeted proteomics has revealed novel molecular mechanisms underlying the sarcopenic condition (139). This more refined understanding of the molecular mechanisms underlying sarcopenia will allow for targeted approaches to investigate the potential impact of CR on these processes. These advances are particularly exciting given the recent characterization of rhesus monkey skeletal muscle proteins (55), which display delayed onset of muscle loss with CR, suggesting that CR may delay sarcopenia (23, 83).
Complementing CR with strategies to support muscle mass and function is imperative for improving overall health if CR is to benefit the elderly. Although research on this topic is in its infancy, strategies implemented alongside CR, including exercise (aerobic and/or resistance) and nutrition (high-protein diets), show promise. The LIFE (Lifestyle Intervention and Independence for Elders) randomized controlled trial suggests that, rather than nutrition, movement and physical activity are primary factors in lowering the risk of mobility impairment (33). A recent trial investigated the effect of aerobic, resistance, or combined resistance and aerobic exercise training during 6 months of CR with 1 g/kg/day protein intake in older, obese adults; it showed that the combination of aerobic and resistance training improved myocellular quality and muscle protein synthesis and preserved muscle mass (22). Information gained from additional trials in the elderly at risk for developing sarcopenia will be invaluable as parallel research to optimize strategies to support muscle mass and function of elderly individuals maintaining CR.
An additional strategy to preserve muscle mass and function alongside CR in the elderly may be increasing dietary protein intake. In this population, the RDA for protein is 0.8 g/kg/day (26); protein intake below 1 g/kg/day negatively affects muscle strength (40, 84), while intake above 1.2 g/kg/day may be optimal for skeletal muscle health with advancing age (9). Notably, the young and middle-aged adults in CALERIE 2 consumed 1.2 g/kg/day of protein, yet still observed reductions in both muscle mass and strength with weight loss. In elderly persons, the combination of exercise training and increased protein intake promotes greater improvements in muscle mass and strength than either approach alone (77). Whether this protective effect of higher protein intake translates to elderly individuals undergoing CR with or without exercise has yet to be determined.
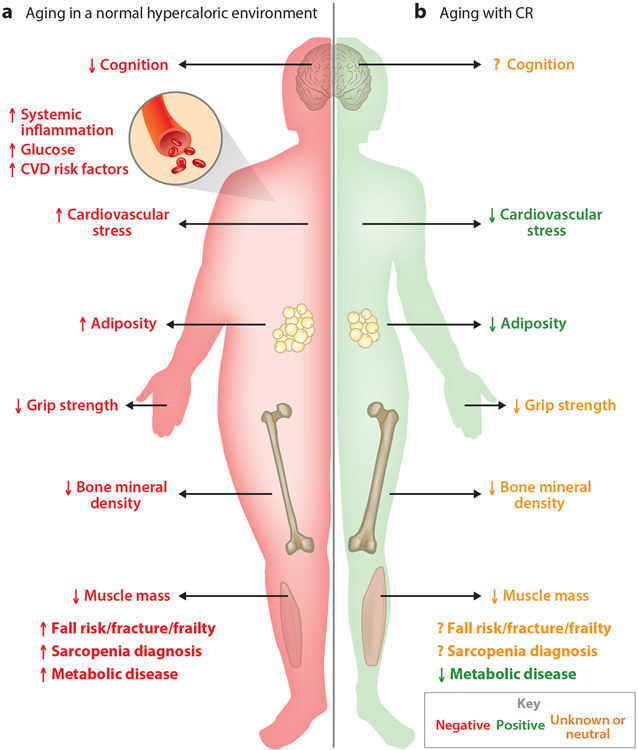
Although evidence suggests that long-term CR effectively slows cellular mechanisms of primary aging and increases health span in the third to fifth decades of life, ensuring that it does not also adversely affect the risk for sarcopenia and all-cause mortality in later aging (sixth decade of life and beyond) is imperative for assessing its overall impact on human health. Aging in the normal environment presents inherent physiological stressors on cognition, body composition, and cardiovascular and metabolic diseases (Figure 3). Loss of skeletal muscle mass and strength as well as eventual mortality is inevitable; whether long-term CR remains protective in healthy, nonobese individuals through advancing age depends on whether CR alters the intrinsic aging process sufficiently to outweigh the risks associated with reduced BMI, muscle mass, and muscle function in aging.
Figure 3.
Symmetries, differences, and unknowns in normal aging versus aging with calorie restriction (CR). (a) Aging in a hypercaloric environment presents many physiological stressors, resulting in unhealthy aging. (b) While aging with CR shares some similarities with aging in a hypercaloric environment, how these changes affect healthy aging (i.e., cognition, cardiometabolic diseases, and physical functioning) in elderly populations is unknown. Abbreviation: CVD, cardiovascular disease.
African Americans
African Americans have a lower life expectancy (13) and are at increased risk for cardiovascular diseases (94), but they have a lower prevalence of metabolic syndrome—a cluster of factors related to adiposity and diabetes—compared with whites (94). Such disparities in health and aging may be related to socioeconomic, environmental, and behavioral factors, including low physical activity and smoking, which are more prevalent in African Americans (149), but significant differences in metabolic pathways may contribute to the increased mortality of African Americans.
The most-recognized metabolic difference between African Americans and Caucasians is the difference in metabolic rate. Studies have shown that African Americans expend significantly less energy per unit of mass compared with their white counterparts (56). The metabolic origin of this lower energy expenditure has not been clearly defined. Some studies suggest that the lower metabolic rate is due to increased mitochondrial efficiency (126), while others report differences in organ size (39, 51). Similarly, measures of oxidative stress (to DNA) are lower in African Americans than in whites (52, 137). Considering the rate of living and oxidative damage theories of aging, these findings suggest lower rates of primary aging in African Americans and thus cannot explain their lower life expectancy compared with that of whites. In further support of the observation that primary aging is attenuated in African Americans, studies have shown increased telomere length (50) and increased attrition of telomeres (7). In contrast, epigenetic changes, such as gene methylation rates, are more prevalent in African Americans, suggesting advanced aging (125). More research is required to discern the genetic, environmental, lifestyle, and metabolic factors that cause the distinct differences in hallmarks of aging between African Americans and whites.
The increased prevalence of cardiovascular disease in African Americans versus whites has been partly attributed to increased subclinical inflammation (C-reactive protein), homocysteine, increased LDL oxidation, lipoprotein A, adiponectin, and plasminogen activator inhibitor 1 (94). An estimation of biological age based on information for 10 biomarkers, mostly related to secondary aging (C-reactive protein, serum creatinine, glycosylated hemoglobin, systolic blood pressure, serum albumin, total cholesterol, cytomegalovirus optical density, serum alkaline phosphatase, forced expiratory volume at 1 s, and serum urea nitrogen) found that African Americans were 3 years older than whites, after matching for chronological age (68). Moreover, the increased risk for type 2 diabetes mellitus in African Americans likely relates to a more pronounced insulin resistance, increased insulinotropic requirements due to insulin resistance and consequently faster β-cell exhaustion, and impaired hepatic insulin extraction (94).
The modulating effect of race on CR and aging has been poorly studied. In the CALERIE studies, only 10% and 15% of subjects in the CR and control groups, respectively, were African American (52, 61, 101); therefore, no effect of CR on primary aging was reported by race. Thus, it remains to be investigated whether African Americans can further reduce their metabolic rate or whether this difference is beneficial to aging.
NEW NUTRITIONAL APPROACHES TO FOSTER HEALTHY AGING
Due to the abovementioned difficulty of sustaining continuous CR (86), alternative nutritional strategies aiming to reproduce equivalent health benefits have recently emerged (Table 2). Intermittent fasting is a broad term used to describe a variety of eating patterns in which food consumption occurs during a restricted time period in order to extend the length of time spent fasting. The length of fasting is highly variable but typically ranges from 18 h to several days, and eating during the assigned time period usually occurs ad libitum (2). There are several forms of intermittent fasting, which are defined by the duration and intensity of the fast. These include time-restricted feeding (TRF; ~18-h fast), alternate-day fasting (ADF; fasting and eating every other day), and modified ADF (129).
Table 2.
Summary of physiological end points from continuous CR and intermittent fasting strategies in humans
| Continuous CR | TRF | ADF and mADF | |
|---|---|---|---|
| Primary aging | |||
| Mass-specific energy expenditure | ↓ | ↔ | ↔/↓a |
| Oxidative stress | ↓ | ↓ | ? |
| Core temperature | ↓ | ↔ | ↔ |
| Mitochondrial efficiency | ↑ | ? | ? |
| Thyroid activity | ↓ | ? | ↓ |
| Secondary aging | |||
| Body composition | |||
| BMI | ↓ | ↓ | ↓ |
| Fat mass | ↓ | ↓ | ↓ |
| Fat-free mass | ↓ | ↔ | ↓ |
| Visceral adipose tissue | ↓ | ↓ | ↓ |
| Cardiometabolic risk markers | |||
| Fasting insulin and insulin area under the curve | ↓ | ↓ | ↓ |
| Fasting glucose | ↓ | ↔ | ↓ |
| Blood pressure | ↓ | ↓ | ↓ |
| Fasting triglycerides | ↓ | ↑ | ↓ |
| Low-density lipoprotein | ↓ | ↔ | ↑/↓a |
| High-density lipoprotein | ↑ | ↔ | ↔/↓a |
| Leptin | ↓ | ↓ | ↔ |
| Inflammation | ↓ | ↔ | ↔ |
Conflicting results published.
Abbreviations: ADF, alternate-day fasting; BMI, body mass index; CR, calorie restriction; mADF, modified ADF; TRF, time-restricted feeding.
TRF is a type of intermittent fasting in which all calories are consumed within a consistent time window, usually between 6 and 12 waking hours of a day (95, 129). It is thought that eating during a restricted time interval brings metabolic processes in concert with circadian rhythms, allowing anticipatory and acute metabolic responses to synergistically maintain nutrient homeostasis (19). Chronic disruption of circadian rhythms, such as with shift work, increases factors commensurate with secondary aging and, hence, the risk of several age-associated chronic diseases (19, 75). TRF along with regulated lifestyle habits may ameliorate these risks (19). A TRF intervention in prediabetic individuals found improvements in β-cell function, insulin sensitivity, oxidative stress, and blood pressure, independent of weight loss (123). These effects are comparable to beneficial adaptations observed with continuous CR. In contrast to CR, TRF induces appetite suppression, which may contribute to superior adherence and acceptability (123). TRF may also maintain muscle mass despite fat loss (87), an area of concern with CR in aging populations. Unlike continuous CR, TRF may preserve resting energy expenditure, which negates positive impacts on primary aging yet may be superior for protection against secondary aging. It is important to note that effects from TRF may differ according to the time of day that the feeding window occurs, with early feedings superior to late feedings (123). To date, studies of TRF have been relatively short in duration (days to less than 9 weeks) and have involved very few subjects. Therefore, a proper comparison against traditional, sustained CR is not yet possible.
ADF is a form of intermittent fasting in which zero calories are consumed during fast periods (24–36 h) and ad libitum food consumption occurs on refeeding days (12–24 h). Contrary to popular belief, ADF does not result in overcompensation during refeeding days; in fact, a net 47% energy deficit was observed throughout an 8-week intervention (18). Despite a significantly greater energy deficit with ADF versus CR (−47% versus −28%), no differences were observed in weight loss, body composition, lipids, insulin sensitivity, or energy metabolism in comparison to a continuous CR diet in young to middle-aged adults (18). Therefore, in contrast to continuous CR, primary aging seems to be unaffected by ADF. With respect to secondary aging, the maintenance of energy expenditure may prevent future weight gain and protect against a decrease in health span.
Purportedly, a more tolerable approach to ADF is modified ADF, in which a small percentage of calories (i.e., ~25% of daily caloric needs) is permitted on fasting days while ad libitum eating continues on alternative days. In a trial comparing weekly energy-matched modified ADF (25% of caloric needs on fasting days; 125% of caloric needs on refeeding days) and continuous CR (75% of caloric needs daily) for 6 months, there were no differences in weight loss or changes in body composition, blood pressure, heart rate, triglycerides, fasting glucose and insulin, C-reactive protein, or homocysteine levels. Compared with CR, the modified ADF diet increased HDL levels; however, there was lower attrition and adherence with modified ADF (127). In a trial in which 25% of caloric intake was allowed on one day followed by up to 175% of caloric intake on the next, Wegman et al. (138) found declines in plasma insulin and marginal increases in expression of SIRT3, a protective diet-induced aging marker, but no other changes in markers of oxidative stress. So far, a modified ADF diet seems to produce similar benefits with regard to secondary aging; unfortunately, neither of these two hallmark studies evaluated its impact on primary aging.
Preclinical studies suggest that intermittent fasting regimes stimulate protective cellular mechanisms observed with CR, such as autophagy, mitochondrial efficiency, reduced ROS production, and declines in inflammatory cytokines (1). These molecular and cellular adaptations to intermittent fasting are initiated during the fasting phase, and in rodents they are mediated by an ~50% decline in insulin-like growth factor 1, which occurs to a lesser degree and more slowly during continuous CR (2). During prolonged periods of fasting, there is a transition from lipid synthesis and fat storage to mobilization of FFA and fatty acid–derived ketones. This transition to fatty acids and fatty acid–derived ketones, known as the metabolic switch, may be responsible for many of the underlying health adaptations to intermittent fasting.
Limited evidence suggests greater preservation of FFM with intermittent fasting due to changes in gene expression and enzymatic activity associated with mitochondrial biogenesis and autophagy with fasting (2, 87). Preservation of FFM with intermittent fasting could be valuable for elderly individuals who are at risk of sarcopenia. A few studies have proposed health benefits of intermittent fasting that exceed those evidenced by CR, yet such findings should be interpreted with caution because these studies may be underpowered to assess the small yet clinically significant changes in circulating biomarkers. A greater understanding is needed to elucidate biological aging responses from various forms of intermittent fasting, independent of weight change. At this point, research on intermittent fasting remains limited, and randomized controlled trials incorporating various strategies and populations are needed before therapeutic interventions can be prescribed.
FUTURE OF DIETARY INTERVENTIONS FOR HEALTHY AGING
To date, CR is the only nutritional intervention shown to improve life span in model organisms. The CALERIE trials demonstrated that CR can be easily adopted without the necessity to conform to a rigid eating pattern. While these trials were critical to show the safety and efficacy of prolonged CR in humans, further research on the implementation of this evidence-based approach to clinical practice is warranted. The CALERIE studies reported in the media have been critiqued for their generalized reports suggesting self-implementation of moderate to severe CR to improve life span (65). Self-implementation of CR without professional guidance from physicians, dieticians, or psychologists should be undertaken with caution, particularly in the elderly and in individuals with low BMI, depressed mood, or multiple prescriptions. Finally, it may be necessary to consider the population (e.g., young, obese, elderly) and length of prescription (long versus short term) when determining the optimal dose of CR.
Note that in nonobese elderly individuals for whom exercise interventions are not feasible, the risk of reductions in muscle mass and function may outweigh the benefits of CR. For such patients, alternate nutritional and pharmacologic strategies that mimic the benefits of CR without inducing a true energy deficit (e.g., resveratrol, metformin), and thus an inherent risk of developing sarcopenia, are intriguing areas for future research. Application of current clinical approaches like vitamin D supplementation and testosterone as adjuvant therapies to CR in elderly individuals may be warranted for those at high risk of developing sarcopenia and frailty (11).
Recently, the National Institute on Aging indicated its commitment to further research on nutrition-based approaches for healthy aging. The future research agenda of the National Institutes of Health (NIH) described the need for long-term CR interventions (i.e., 5 years) to evaluate long-term sustainability and the attenuation or amplification of biological aging (92). The NIH also attest to the need for trials to test the effectiveness, safety, and tolerability of CR interventions in older adults, as well as for comparisons of food timing and dietary interventions (i.e., intermittent fasting models, macronutrient restriction) shown to improve health, with traditional sustained CR. Although such research on the implementation science is imminent, until it is conducted, akin to the intensive lifestyle intervention of the Diabetes Prevention Program, CR will not be ready to be prescribed as a long-term nutritional intervention either for healthy individuals, particularly the elderly, or for those with multiple pharmacotherapies and preexisting health concerns. However, one might argue that individuals maintaining normal-range BMI in the modern obesogenic environment are self-imposing some degree of CR. Observational cohort studies with rigorous methods for evaluating dietary intake and biomarkers of aging will shed light on these individuals, their health span, and ultimately their life span. CR remains a vibrant area of research and a promising approach to healthy aging, yet there is still much more to learn.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Anton S, Leeuwenburgh C. 2013. Fasting or caloric restriction for healthy aging. Exp. Gerontol 48:1003–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, et al. 2018. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity 26:254–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai Y, Sasaki T, Hirose N. 2017. Demographic, phenotypic, and genetic characteristics of centenarians in Okinawa and Honshu, Japan. Part 2. Honshu, Japan. Mech. Ageing Dev 165:80–85 [DOI] [PubMed] [Google Scholar]
- 4.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, Perez-Zepeda MU, Cesari M. 2013. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 17:259–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aune D, Sen A, Prasad M, Norat T, Janszky I, et al. 2016. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aversa Z, Zhang X, Fielding RA, Lanza I, LeBrasseur NK. 2019. The clinical impact and biological mechanisms of skeletal muscle aging. Bone 127:26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, et al. 2009. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol 169:323–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaban RS, Nemoto S, Finkel T. 2005. Mitochondria, oxidants, and aging. Cell 120:483–95 [DOI] [PubMed] [Google Scholar]
- 9.Baum JI, Wolfe RR. 2015. The link between dietary protein intake, skeletal muscle function and health in older adults. Healthcare 3:529–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE. 2017. Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. J. Gerontol. A 73:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, et al. 2018. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Intern. Med 178:530–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, et al. 2003. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J. Clin. Endocrinol. Metab 88:16–23 [DOI] [PubMed] [Google Scholar]
- 13.Bond MJ, Herman AA. 2016. Lagging life expectancy for black men: a public health imperative. Am. J. Public Health 106:1167–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boren J, Taskinen MR, Olofsson SO, Levin M. 2013. Ectopic lipid storage and insulin resistance: a harmful relationship. J. Intern. Med 274:25–40 [DOI] [PubMed] [Google Scholar]
- 15.Boswell RG, Kober H. 2016. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes. Rev 17:159–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowers J, Terrien J, Clerget-Froidevaux MS, Gothie JD, Rozing MP, et al. 2013. Thyroid hormone signaling and homeostasis during aging. Endocr. Rev 34:556–89 [DOI] [PubMed] [Google Scholar]
- 17.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. 2013. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J. Am. Coll. Cardiol 62:921–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, et al. 2016. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 24:1874–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaix A, Manoogian ENC, Melkani GC, Panda S. 2019. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr 39:291–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, et al. 2007. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLOS Med. 4:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Nathan JA, Goldberg AL. 2015. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov 14:58. [DOI] [PubMed] [Google Scholar]
- 22.Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento-Villareal R, et al. 2019. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab. 30:261–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colman RJ, Beasley TM, Allison DB, Weindruch R. 2008. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J. Gerontol. A 63:556–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. 1999. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J. Gerontol. A 54:B283–90 [DOI] [PubMed] [Google Scholar]
- 25.Costantino S, Paneni F, Cosentino F. 2016. Ageing, metabolism and cardiovascular disease. J. Physiol 594:2061–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuervo M, Corbalán M, Baladía E, Cabrerizo L, Formiguera X, et al. 2009. [Comparison of dietary reference intakes (DRI) between different countries of the European Union, the United States and the World Health Organization.] Nutr. Hosp 24:384–414 (In Spanish) [PubMed] [Google Scholar]
- 27.Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, et al. 2001. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J. Clin. Endocrinol. Metab 86:355–62 [DOI] [PubMed] [Google Scholar]
- 28.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, et al. 2007. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am. J. Clin. Nutr 85:1023–30 [DOI] [PubMed] [Google Scholar]
- 29.Das SK, Roberts SB, Bhapkar MV, Villareal DT, Fontana L, et al. 2017. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am. J. Clin. Nutr 105:913–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davinelli S, Willcox DC, Scapagnini G. 2012. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun. Ageing 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFronzo RA. 1981. Glucose intolerance and aging. Diabetes Care 4:493–501 [DOI] [PubMed] [Google Scholar]
- 32.Dorling JL, Bhapkar M, Das SK, Racette SB, Apolzan JW, et al. 2019. Change in self-efficacy, eating behaviors and food cravings during two years of calorie restriction in humans without obesity. Appetite 143:104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanning J, Rejeski WJ, Chen SH, Nicklas BJ, Walkup MP, et al. 2019. A case for promoting movement medicine: preventing disability in the LIFE randomized controlled trial. J. Gerontol. A 74:1821–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feil R, Fraga MF. 2012. Epigenetics and the environment: emerging patterns and implications. Nat. Rev. Genet 13:97–109 [DOI] [PubMed] [Google Scholar]
- 35.Fontana L, Klein S, Holloszy JO. 2010. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age 32:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontana L, Klein S, Holloszy JO, Premachandra BN. 2006. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab 91:3232–35 [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Meyer TE, Klein S, Holloszy JO. 2004. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. PNAS 101:6659–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontana L, Shew JL, Holloszy JO, Villareal DT. 2005. Low bone mass in subjects on a long-term raw vegetarian diet. Arch. Intern. Med 165:684–89 [DOI] [PubMed] [Google Scholar]
- 39.Gallagher D, Albu J, He Q, Heshka S, Boxt L, et al. 2006. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am. J. Clin. Nutr 83:1062–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, et al. 2018. Low protein intake, muscle strength and physical performance in the very old: the Newcastle 85+ Study. Clin. Nutr 37:2260–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harman D 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol 11:298–300 [DOI] [PubMed] [Google Scholar]
- 42.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, et al. 2006. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295:1539–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heilbronn LK, Ravussin E. 2003. Calorie restriction and aging: review of the literature and implications for studies in humans. Am. J. Clin. Nutr 78:361–69 [DOI] [PubMed] [Google Scholar]
- 44.Hipp MS, Kasturi P, Hartl FU. 2019. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol 20:421–35 [DOI] [PubMed] [Google Scholar]
- 45.Holloszy JO. 2000. The biology of aging. Mayo Clin. Proc 75(Suppl.):S3–9 [PubMed] [Google Scholar]
- 46.Holloszy JO, Fontana L. 2007. Caloric restriction in humans. Exp. Gerontol 42:709–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horvath S 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huffman KM, Redman LM, Landerman LR, Pieper CF, Stevens RD, et al. 2012. Caloric restriction alters the metabolic response to a mixed meal: results from a randomized, controlled trial. PLOS ONE 7:e28190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. 2007. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev 87:1175–213 [DOI] [PubMed] [Google Scholar]
- 50.Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, et al. 2008. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 7:451–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter GR, Weinsier RL, Darnell BE, Zuckerman PA, Goran MI. 2000. Racial differences in energy expenditure and aerobic fitness in premenopausal women. Am. J. Clin. Nutr 71:500–6 [DOI] [PubMed] [Google Scholar]
- 52.Il’yasova D, Fontana L, Bhapkar M, Pieper CF, Spasojevic I, et al. 2018. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: the CALERIE 2 randomized clinical trial. Aging Cell 17. 10.1111/acel.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Il’yasova D, Spasojevic I, Wang F, Tolun AA, Base K, et al. 2010. Urinary biomarkers of oxidative status in a clinical model of oxidative assault. Cancer Epidemiol. Biomark. Prev 19:1506–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janssen I, Heymsfield SB, Ross R. 2002. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc 50:889–96 [DOI] [PubMed] [Google Scholar]
- 55.Jin Y, Diffee GM, Colman RJ, Anderson RM, Ge Y. 2019. Top-down mass spectrometry of sarcomeric protein post-translational modifications from non-human primate skeletal muscle. J. Am. Soc. Mass Spectrom 30:2460–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katzmarzyk PT, Most J, Redman LM, Rood J, Ravussin E. 2018. Energy expenditure and substrate oxidation in White and African American young adults without obesity. Eur. J. Clin. Nutr 72:920–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kauppila TES, Kauppila JHK, Larsson NG. 2017. Mammalian mitochondria and aging: an update. Cell Metab. 25:57–71 [DOI] [PubMed] [Google Scholar]
- 58.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, et al. 2014. Geroscience: linking aging to chronic disease. Cell 159:709–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keys AB, Brozek J, Henschel A, Mickelson O, Taylor A. 1950. The Biology of Human Starvation. Minneapolis: Univ. Minn. Press. 2 vol. [Google Scholar]
- 60.Klemera P, Doubal S. 2006. A new approach to the concept and computation of biological age. Mech. Ageing Dev 127:240–48 [DOI] [PubMed] [Google Scholar]
- 61.Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, et al. 2019. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7:673–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, et al. 1995. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am. J. Physiol. Endocrinol. Metab 268:E941–48 [DOI] [PubMed] [Google Scholar]
- 63.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, et al. 2006. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29:1337–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larson-Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E. 2010. Caloric restriction with or without exercise: the fitness versus fatness debate. Med. Sci. Sports Exerc 42:152–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Bourg E, Redman LM. 2018. Do-it-yourself calorie restriction: the risks of simplistically translating findings in animal models to humans. BioEssays 40:e1800087. [DOI] [PubMed] [Google Scholar]
- 66.Lecoultre V, Ravussin E, Redman LM. 2011. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J. Clin. Endocrinol. Metab 96:E1512–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, et al. 2009. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis 203:206–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levine ME, Crimmins EM. 2014. Evidence of accelerated aging among African Americans and its implications for mortality. Soc. Sci. Med 118:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lipina C, Hundal HS. 2017. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 8:190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu HH, Li JJ. 2015. Aging and dyslipidemia: a review of potential mechanisms. Ageing Res. Rev 19:43–52 [DOI] [PubMed] [Google Scholar]
- 71.Loenneke JP, Loprinzi PD. 2016. Obesity is associated with insulin resistance but not skeletal muscle dysfunction or all-cause mortality. Age 38:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153:1194–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ludwig DS. 2016. Lifespan weighed down by diet. JAMA 315:2269–70 [DOI] [PubMed] [Google Scholar]
- 74.Mahan LK, Escott-Stump S, Raymond JL. 2012. Krause’s Food and the Nutrition Care Process. St. Louis: Elsevier Saunders. 13th ed. [Google Scholar]
- 75.Manoogian ENC, Panda S. 2017. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev 39:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, et al. 2015. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol 44:1388–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Markofski MM, Jennings K, Timmerman KL, Dickinson JM, Fry CS, et al. 2019. Effect of aerobic exercise training and essential amino acid supplementation for 24 weeks on physical function, body composition and muscle metabolism in healthy, independent older adults: a randomized clinical trial. J. Gerontol. A 74:1598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marlatt KL, Redman LM, Burton JH, Martin CK, Ravussin E. 2017. Persistence of weight loss and acquired behaviors 2 y after stopping a 2-y calorie restriction intervention. Am. J. Clin. Nutr 105:928–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, et al. 2016. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern. Med 176:743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, et al. 2007. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 15:2964–73 [DOI] [PubMed] [Google Scholar]
- 81.Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, et al. 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun 8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mattison JA, Lane MA, Roth GS, Ingram DK. 2003. Calorie restriction in rhesus monkeys. Exp. Gerontol 38:35–46 [DOI] [PubMed] [Google Scholar]
- 83.McKiernan SH, Colman RJ, Aiken E, Evans TD, Beasley TM, et al. 2012. Cellular adaptation contributes to calorie restriction–induced preservation of skeletal muscle in aged rhesus monkeys. Exp. Gerontol 47:229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mendonça N, Granic A, Mathers JC, Hill TR, Siervo M, et al. 2018. Prevalence and determinants of low protein intake in very old adults: insights from the Newcastle 85+ Study. Eur. J. Nutr 57:2713–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. 2006. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol 47:398–402 [DOI] [PubMed] [Google Scholar]
- 86.Moreira EA, Most M, Howard J, Ravussin E. 2011. Dietary adherence to long-term controlled feeding in a calorie-restriction study in overweight men and women. Nutr. Clin. Pract 26:309–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, et al. 2016. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med 14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM. 2018. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol. Endocrinol. Metab 314:E396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Most J, Redman LM. 2017. Aging and cardiovascular disease: lessons from calorie restriction. In Nutrition and Cardiometabolic Health, ed. Bergeron N, Siri-Tarino PW, Bray GA, Krauss RM, pp. 191–208. Boca Raton, FL: CRC [Google Scholar]
- 90.Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochem. J 417:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myers J, Kokkinos P, Nyelin E. 2019. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients 11:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Natl. Inst. Aging. 2019. Planning projects for clinical trials on effects of sustained reductions in caloric intake and related dietary practices in younger and older persons. Res. Proj. U01, US Dep. Health Hum. Serv., Washington, DC [Google Scholar]
- 93.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, et al. 2006. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A 61:72–77 [DOI] [PubMed] [Google Scholar]
- 94.Osei K, Gaillard T. 2017. Disparities in cardiovascular disease and type 2 diabetes risk factors in blacks and whites: dissecting racial paradox of metabolic syndrome. Front. Endocrinol 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panda S 2016. Circadian physiology of metabolism. Science 354:1008–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV. 2016. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 30:1634–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phillips T, Leeuwenburgh C. 2005. Muscle fiber specific apoptosis and TNF-α signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 19:668–70 [DOI] [PubMed] [Google Scholar]
- 98.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, et al. 2011. Development of adherence metrics for caloric restriction interventions. Clin. Trials 8:155–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, et al. 2005. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE trial. Diabetes Care 28:2939–41 [DOI] [PubMed] [Google Scholar]
- 100.Pittas AG, Roberts SB, Das SK, Gilhooly CH, Saltzman E, et al. 2006. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity 14:2200–9 [DOI] [PubMed] [Google Scholar]
- 101.Racette SB, Rochon J, Uhrich ML, Villareal DT, Das SK, et al. 2017. Effects of two years of calorie restriction on aerobic capacity and muscle strength. Med. Sci. Sports Exerc 49:2240–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, et al. 2006. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J. Gerontol. A 61:943–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, et al. 2015. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A 70:1097–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, et al. 2007. Effect of calorie restriction with or without exercise on body composition and fat distribution. J. Clin. Endocrinol. Metab 92:865–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. 2018. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 27:805–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rezzi S, Martin FP, Shanmuganayagam D, Colman RJ, Nicholson JK, Weindruch R. 2009. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp. Gerontol 44:356–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Richardson AG, Schadt EE. 2014. The role of macromolecular damage in aging and age-related disease. J. Gerontol. A 69(Suppl. 1):S28–32 [DOI] [PubMed] [Google Scholar]
- 108.Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, et al. 2011. The CALERIE study: design and methods of an innovative 25% caloric restriction intervention. Contemp. Clin. Trials 32:874–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riordan MM, Weiss EP, Meyer TE, Ehsani AA, Racette SB, et al. 2008. The effects of caloric restriction– and exercise-induced weight loss on left ventricular diastolic function. Am. J. Physiol. Heart Circ. Physiol 294:H1174–82 [DOI] [PubMed] [Google Scholar]
- 110.Ristow M, Zarse K. 2010. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol 45:410–18 [DOI] [PubMed] [Google Scholar]
- 111.Rivas DA, Morris EP, Haran PH, Pasha EP, da Silva Morais M, et al. 2012. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J. Appl. Physiol 113:1727–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, et al. 2016. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget 7:19124–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, et al. 2002. Biomarkers of caloric restriction may predict longevity in humans. Science 297:811. [DOI] [PubMed] [Google Scholar]
- 114.Sacher GA, Duffy PH. 1979. Genetic relation of life span to metabolic rate for inbred mouse strains and their hybrids. Fed. Proc 38:184–88 [PubMed] [Google Scholar]
- 115.Salminen A, Kaarniranta K, Kauppinen A. 2012. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging 4:166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schafer AL, Kazakia GJ, Vittinghoff E, Stewart L, Rogers SJ, et al. 2018. Effects of gastric bypass surgery on bone mass and microarchitecture occur early and particularly impact postmenopausal women. J. Bone Miner Res 33:975–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seeman E, Delmas PD. 2006. Bone quality—the material and structural basis of bone strength and fragility. N. Engl. J. Med 354:2250–61 [DOI] [PubMed] [Google Scholar]
- 118.Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, et al. 2017. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr 8:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sohal RS, Allen RG. 1985. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 35:75–104 [DOI] [PubMed] [Google Scholar]
- 120.Sparks LM, Redman LM, Conley KE, Harper ME, Yi F, et al. 2017. Effects of 12 months of caloric restriction on muscle mitochondrial function in healthy individuals. J. Clin. Endocrinol. Metab 102:111–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Speakman JR. 2005. Body size, energy metabolism and lifespan. J. Exp. Biol 208:1717–30 [DOI] [PubMed] [Google Scholar]
- 122.Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. 2012. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell 11:644–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. 2018. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27:1212–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ministry of Health, Labour and Welfare. 2016. Consideration of a social model to overcome demographic aging. Annu. Health, Labour Welf. Rep., Minist. Intern. Aff. Commun., Tokyo. https://www.mhlw.go.jp/english/wp/wp-hw10/dl/summary.pdf [Google Scholar]
- 125.Tajuddin SM, Hernandez DG, Chen BH, Noren Hooten N, Mode NA, et al. 2019. Novel age-associated DNA methylation changes and epigenetic age acceleration in middle-aged African Americans and whites. Clin. Epigenet 11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toledo FGS, Dube JJ, Goodpaster BH, Stefanovic-Racic M, Coen PM, DeLany JP. 2018. Mitochondrial respiration is associated with lower energy expenditure and lower aerobic capacity in African American women. Obesity 26:903–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, et al. 2017. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med 177:930–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.UN Dep. Econ. Soc. Affairs. 2019. World Population 2019. New York: UN [Google Scholar]
- 129.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, et al. 2013. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr. J 12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Villareal DT, Fontana L, Das SK, Redman L, Smith SR, et al. 2016. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Miner. Res 31:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, et al. 2006. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch. Intern. Med 166:2502–10 [DOI] [PubMed] [Google Scholar]
- 132.Villareal DT, Kotyk JJ, Armamento-Villareal RC, Kenguva V, Seaman P, et al. 2011. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell 10:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. 2000. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab 85:4481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walford RL, Harris SB, Gunion MW. 1992. The calorically restricted low-fat nutrient-dense diet in Biosphere-2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. PNAS 89:11533–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Walford RL, Mock D, Verdery R, MacCallum T. 2002. Calorie restriction in Biosphere-2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J. Gerontol. A 57:B211–24 [DOI] [PubMed] [Google Scholar]
- 136.Wasinski F, Bacurau RF, Moraes MR, Haro AS, Moraes-Vieira PM, et al. 2013. Exercise and caloric restriction alter the immune system of mice submitted to a high-fat diet. Mediat. Inflamm 2013:395672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watters JL, Satia JA, Kupper LL, Swenberg JA, Schroeder JC, Switzer BR. 2007. Associations of antioxidant nutrients and oxidative DNA damage in healthy African-American and white adults. Cancer Epidemiol. Biomark. Prev 16:1428–36 [DOI] [PubMed] [Google Scholar]
- 138.Wegman MP, Guo MH, Bennion DM, Shankar MN, Chrzanowski SM, et al. 2015. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 18:162–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wei L, Gregorich ZR, Lin Z, Cai W,Jin Y, et al. 2018. Novel sarcopenia-related alterations in sarcomeric protein post-translational modifications (PTMs) in skeletal muscles identified by top-down proteomics. Mol. Cell Proteom 17:134–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weiss EP, Holloszy JO. 2007. Improvements in body composition, glucose tolerance, and insulin action induced by increasing energy expenditure or decreasing energy intake. J. Nutr 137:1087–90 [DOI] [PubMed] [Google Scholar]
- 141.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, et al. 2007. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J. Appl. Physiol 102:634–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weiss EP, Villareal DT, Racette SB, Steger-May K, Premachandra BN, et al. 2008. Caloric restriction but not exercise-induced reductions in fat mass decrease plasma triiodothyronine concentrations: a randomized controlled trial. Rejuvenation Res. 11:605–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. 1999. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int. J. Obes. Relat. Metab. Disord 23:715–22 [DOI] [PubMed] [Google Scholar]
- 144.Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, et al. 2000. Energy metabolism after 2 y of energy restriction: the Biosphere-2 experiment. Am. J. Clin. Nutr 72:946–53 [DOI] [PubMed] [Google Scholar]
- 145.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. 2002. Development and validation of the food-craving inventory. Obes. Res 10:107–14 [DOI] [PubMed] [Google Scholar]
- 146.Willcox BJ, Willcox DC. 2014. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr. Opin. Clin. Nutr. Metab. Care 17:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, et al. 2007. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann. N. Y. Acad. Sci 1114:434–55 [DOI] [PubMed] [Google Scholar]
- 148.Willcox BJ, Yano K, Chen R, Willcox DC, Rodriguez BL, et al. 2004. How much should we eat? The association between energy intake and mortality in a 36-year follow-up study of Japanese-American men. J. Gerontol. A 59:789–95 [DOI] [PubMed] [Google Scholar]
- 149.Williams DR, Collins C. 2001. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 116:404–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Williamson DA, Martin CK, Anton SD, York-Crowe E, Han H, et al. 2008. Is caloric restriction associated with development of eating-disorder symptoms? Results from the CALERIE trial. Health Psychol. 27:S32–42 [DOI] [PubMed] [Google Scholar]
- 151.Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. 2014. BMI and all-cause mortality in older adults: a meta-analysis. Am. J. Clin. Nutr 99:875–90 [DOI] [PubMed] [Google Scholar]
- 152.Yamada Y, Colman RJ, Kemnitz JW, Baum ST, Anderson RM, et al. 2013. Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp. Gerontol 48:1226–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang L, Licastro D, Cava E, Veronese N, Spelta F, et al. 2016. Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep. 14:422–28 [DOI] [PubMed] [Google Scholar]
- 154.Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. 2000. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 14:1825–36 [DOI] [PubMed] [Google Scholar]
- 155.Zimin AV, Cornish AS, Maudhoo MD, Gibbs RM, Zhang X, et al. 2014. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol. Direct 9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]