Abstract
Background
This study aimed to assess the longitudinal health-related quality of life (HRQOL) using the Expanded Prostate Cancer Index Composite (EPIC) and HRQOL change between the nerve-sparing technique in Japanese men treated with robot-assisted radical prostatectomy (RARP).
Methods
A total of 573 patients who received RARP were included in this study. EPIC questionnaire was administered before treatment and up to 36 months after RARP. Clinical recovery was defined as half of the standard deviation of the baseline score for each domain. We divided all patients into recovery group or nonrecovery group. The time from survey to each domain recovery was calculated using the Kaplan–Meier method. We compared the sexual and urinary score change between groups using analysis of variance to confirm the effect of nerve-sparing technique.
Results
The median age was 67 years (interquartile range, 62–71 years). The mean score of all urinary domains worsened noticeably after 1 month. All postoperative urinary summary, function, and incontinence scores were significantly lower than preoperative scores up to 3 years post-RARP. Postoperative sexual summary and functional scores were significantly lower than preoperative score at all follow-up times throughout the 36 months. The recovery rate for the urinary incontinence domain was the lowest (44.5%), whereas the recovery rate for the urinary irritative–obstructive domain was the highest (73.7%). In the sexual domain, the bother domain had a higher recovery rate (73.0%) than the functional domain (29.7%). Although the recovery of sexual domains was slower compared with other domains, by 36 months after RARP, almost all values had recovered. Compared with other technique groups, bilateral intrafascial nerve-sparing group showed significantly decreased change in subscale scores before and after RARP in several sexual and urinary domain.
Conclusion
The time course and extent of functional and bother domain recovery documented in this study may prove useful for RARP patient selection in Japan.
Keywords: Nerve-sparing surgery, Prostate cancer, Quality of life, Robot-assisted radical prostatectomy
1. Introduction
In Japan, the prevalence of prostate cancer (CaP) is ranked third after gastric cancer and lung cancer in 2017, whereas the number of CaP patients has dramatically increased over the last five decades.1 As the median life expectancy after treatment for CaP is 13.8 years2 and the relative survival rate for CaP is almost 100%, the most important clinical outcomes are long-term functional outcomes of treatment, complications, and satisfaction.
At present, the gold standard for localized CaP treatment is robot-assisted radical prostatectomy (RARP). RARP is offered at many centers in Japan because it is a minimally invasive procedure and the less burdensome treatment option for patients. For example, there are urinary incontinence and erectile dysfunction as complications of RARP. These complications consequently decrease the quality of life (QOL).3, 4, 5 Therefore, urologists should place importance on not only oncological cure control but also maintenance of urinary and sexual function in patients after CaP treatment.
Several existing CaP patient-reported-outcome instruments such as the UCLA-Prostate Cancer Index6 and the Expanded Prostate Cancer Index Composite (EPIC)7 can accurately evaluate CaP health-related QOL (HRQOL) changes over time.8 The EPIC is one of the most commonly used questionnaires in urological research9, which measures the side effects and associated HRQOL of CaP therapies.10 In EPIC, the questionnaire assesses four domains, namely, urinary, bowel, sexual, and hormonal. Each domain comprises two subscales: symptom severity (function subscale) and the annoyance associated with these side effects (bother subscale). The urinary domain is also separated into two other subscales, combining both function and bother parameters, namely, a urinary incontinence subscale and a urinary irritation/obstruction subscale. A summary score is also generated for each domain, corresponding to the mean of the function and bother subscales.
Unfortunately, there are very few reports of QOL comparing oncological, perioperative, functional outcomes before and after RARP in Japanese patients.11 The primary aim of this study is to assess recovery objectively in urinary and sexual function after RARP using the EPIC questionnaire (which has been validated in Japan) with respect to baseline preoperative function and to explore the time course of postoperative HRQOL convalescence in a series of patients who underwent RARP in a single Asian center.
2. Materials and methods
2.1. Study populations
We retrospectively analyzed patients who underwent RARP for CaP between April 2012 and August 2018 at Juntendo University Hospital, Tokyo, Japan. Eligible men had clinically localized (clinical TNM classification T1, T2, or limited T3), biopsy-proven adenocarcinoma of the prostate. Clinicopathological characteristics of patients, such as age, preoperative prostate-specific antigen (PSA), the number of positive biopsy cores, the rate of positive biopsy cores, prostate volume, Gleason score, clinical T stage, neoadjuvant androgen deprivation therapy (ADT) or not, nerve-sparing status, pathological T stage, and D'Amico risk classification12, were obtained from medical records. Patients with previous prostate surgery or the post-RARP ADT, chemotherapy, and radiation therapy were excluded from this analysis. The decision to perform cavernosal nerve-sparing was made by the patient and surgeon. The patients were divided into five groups according to their respective nerve-sparing technique: group 0, non-nerve-sparing; group 1, unilateral intrafascial nerve-sparing (ITR-NS); group 2, unilateral interfascial nerve-sparing (ITE-NS); group 3, bilateral ITR-NS; and group 4, bilateral ITE-NS.
2.2. Instruments for HRQOL assessments
We used the Japanese-validated version of the EPIC questionnaire.13 EPIC was administered before RARP and at 1, 3, 6, 9, 12, 15, 18, 24, 30, and 36 months post-RARP. The calculated summary scores and each subscale score post-RARP were compared with the baseline (preoperative) scores. Any case with a score higher than the value obtained by subtracting half of the standard deviation (SD) of the baseline score from the baseline score after RARP was categorized into the recovery group in each subscale.14
2.3. Statistical methods
We used paired t-tests to assess differences in subscale scores at each time point after RARP compared with preoperative scores. We analyzed clinical data differences between recovery group and nonrecovery group by Pearson's chi-square. Time from survey to each domain recovery was calculated using the Kaplan–Meier method. We compared the Δ score (prescore—nadir score after RARP) in each subscale according to the nerve-sparing technique groups using analysis of variance (ANOVA). All statistical analysis was conducted using statistical software package: R version 2.14.0. Two-tailed P ˂ 0.05 were considered as a statistically significant difference.
3. Results
3.1. Patient's characteristics
Table 1 shows the clinicopathological characteristics of the patients in this cohort (n = 573). The median age was 67 years (interquartile range [IQR], 62–71), and the median preoperative PSA level was 7.3 ng/mL (IQR, 5.5–10.2). In this study, 177 patients (30.9%) received neoadjuvant ADT.
Table 1.
Baseline demographic and medical characteristics of study participants
| Patients (n) | 573 |
| Median age (yr), (IQR) | 67 (62–71) |
| Median preoperative PSA (ng/mL), (IQR) | 7.3 (5.5–10.2) |
| Neoadjuvant ADT (%) | 177 (30.9) |
| Median prostate volume (mL), (IQR) | 27 (20.3–35.0) |
| Clinical T stage (%) | |
| cT1 | 176 (30.7) |
| cT2 | 358 (62.5) |
| cT3 | 3 (6.1) |
| Unknown | 4 (0.7) |
| Median number of positive cores/PBx cores, (IQR) | 3 (2–5)/14 (12–16) |
| Median rate of positive biopsy cores (%), (IQR) | 25.0 (12.5–41.7) |
| Gleason score (%) | |
| ≤6 | 210 (36.7) |
| 7 | 258 (45.0) |
| 8 | 95 (16.6) |
| ≥9 | 10 (1.7) |
| Nerve-sparing status (%) | |
| None | 255 (44.5) |
| Unilateral | 161 (28.1) |
| Bilateral | 154 (26.9) |
| Unknown | 3 (0.5) |
| Pathological T stage (%) | |
| ≤pT2 | 458 (79.9) |
| pT3 | 115 (20.1) |
| Positive surgical margin (%) | 140 (24.4) |
ADT, androgen deprivation therapy; IQR, interquartile range; PBx, prostate biopsy; PSA, prostate-specific antigen.
We performed bilateral nerve-sparing RARP in 154 patients (26.9%) and unilateral nerve-sparing RARP in 161 patients (28.1%).
The most frequent pathological T stage was pT2c. Positive surgical margin was observed in 24.4% of patients.
3.2. The changes in each subscale scores pre- and post-RARP
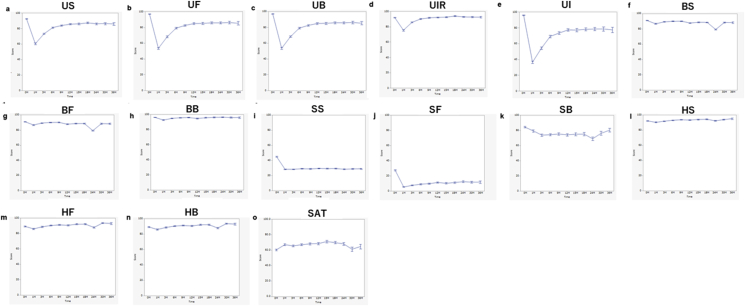
We showed the changes over time in each subscale scores up to 3 years after RARP (Fig. 1). These figures show QOL mean ± standard error scores of each subscale over the follow-up period.
Fig. 1.
Longitudinal changes in urinary, bowel, sexual, hormonal, and satisfactory domain mean scores over time. Bars represent mean ± SE. (A) Urinary summary scores (US). (B) Urinary function scores (UF). (C) Urinary bother scores (UB). (D) Urinary irritative–obstructive scores (UIR). (E) Urinary incontinence scores (UI). (F) Bowel summary scores (BS). (G) Bowel function scores (BF). (H) Bowel bother scores (BB). (I) Sexual summary scores (SS). (J) Sexual function scores (SF). (K) Sexual bother scores (SB). (L) Hormonal summary scores (HS). (M) Hormonal function scores (HF). (N) Hormonal bother scores (HB). (O) Satisfactory scores (SAT).
At baseline (before RARP), most men reported good urinary function and incontinence (median 100) and minimal urinary bother and urinary irritative obstruction (median 92.9). Urinary scores exhibited a significant decline 1 month after treatment. These scores improved and stabilized in the subsequent months but remained significantly lower than the baseline score at most follow-up times. Almost all postoperative urinary scores, except the urinary irritative–obstructive domain, were significantly lower than baseline score according to t-test at each time point (Fig. 1). All postoperative urinary summary (Fig. 1A), function (Fig. 1B), and incontinence scores (Fig. 1E) over the entire follow-up period (3 years) were significantly lower than preoperative scores. Although the postoperative urinary bother scores (Fig. 1C) at certain time points were not statistically different, most follow-up months showed significantly lower scores compared with baseline. Postoperative 1-, 3-, 6-, and 18-month urinary irritative–obstructive scores (Fig. 1D) were significantly lower than preoperative scores by using t-test.
Almost all bowel scores and the effect of bowel habits on QOL were unchanged in this study (Fig. 1F–H). Although almost all postoperative bowel summary scores (Fig. 1F), except 9 months post-RARP, were significantly lower than preoperative scores, the difference was slight. Unlike the bowel function scores (Fig. 1G), which remained significantly below baseline at all follow-up times after RARP, the postoperative bowel bother score (Fig. 1H) was significantly lower than preoperative that score only at 1, 3, and 12 months after RARP.
At baseline, most men reported low sexual summary (Fig. 1I; median 42.3) and function (Fig. 1J; median 25.9) scores but good sexual bother scores (Fig. 1K; median 100). At all times after RARP up to 36 months, postoperative sexual summary (Fig. 1I) and sexual function (Fig. 1J) scores were significantly lower than preoperative scores. The mean sexual functional and bother scores reached its nadir at 1-month post-RARP. Although postoperative sexual bother scores, up to 30 months, were significantly lower than preoperative scores, there was no significant difference after 3 years.
Postoperative satisfactory scores (Fig. 1O), up to 2 years, were significantly higher than preoperative scores according to the t-test.
3.3. Recovery group vs. nonrecovery group
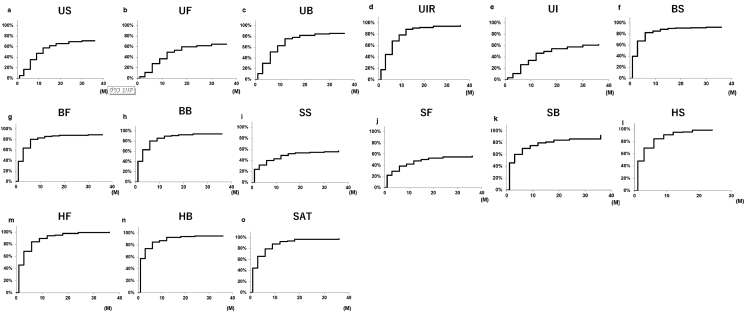
There were no significant differences in neoadjuvant ADT, nerve-sparing status, and D'Amico risk classification between the recovery group and the nonrecovery group. To expand the information represented by mean score changes, we showed the distribution of patients in recovery group and nonrecovery group post-PARP (Supplementary Fig. 1).
Fig. 2 shows Kaplan–Meier curves representing the achievement of recovery in each subscale. Although there were differences in the recovery time in each urinary domain, more than 60% of patients had recovered in each urinary subscale domain at 36-months post-PARP (Fig. 2A, summary: 62.8%; Fig. 2B, function: 64.4%; Fig. 2C, bother: 76.5%; Fig. 2D, irritative obstructive: 87.2%; Fig. 2E, incontinence: 62.0%). Urinary irritative–obstructive scores (Fig. 2D) indicated that 77.8% of patients recovered at 1-year post-PARP, compared with 47.1% in urinary continence scores (Fig. 2E). With regard to bowel domain, patients exhibited a high rate of recovery in both function (Fig. 2G; 81.3%) and bother domains (Fig. 2H; 94.0%) at 3 years after RARP. Although all sexual domains were low before RARP and the recovery for this domain was comparatively slower, by 36 months post-RARP (Fig. 2J, function: 98.6%; Fig. 2K, bother: 87.7%). The slowest recovery was the sexual summary domain, for which the 6-, 12-, 24-, and 36-month potency recovery rates were 23.6%, 30.4%, 34.9%, and 39.7%, respectively. Just like the bowel domain, many cases recovered early, and almost all cases recovered after 3 years in the hormonal functional (Fig. 2M; 96.7%) and bother domain (Fig. 2H; 96.9%).
Fig. 2.
Kaplan–Meier analysis of the proportion of subjects returning to recovery urinary, bowel, sexual, hormonal, and satisfactory scorea) over time. (A) Urinary summary scores (US). (B) Urinary function scores (UF). (C) Urinary bother scores (UB). (D) Urinary irritative–obstructive scores (UIR). (E) Urinary incontinence scores (UI). (F) Bowel summary scores (BS). (G) Bowel function scores (BF). (H) Bowel bother scores (BB). (I) Sexual summary scores (SS). (J) Sexual function scores (SF). (K) Sexual bother scores (SB). (L) Hormonal summary scores (HS). (M) Hormonal function scores (HF). (N) Hormonal bother scores (HB). (O) Satisfactory scores (SAT).
a)Recovery group defined as change score that was greater than (preoperative value − ½ SD preoperative value) for respective subscales.
3.4. The effect of different nerve-sparing techniques on urinary and sexual scores
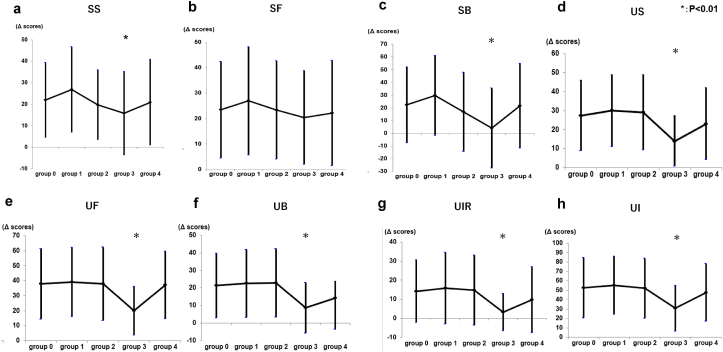
We compared the Δ score (prescore—nadir score after RARP) in each subscale score between groups using ANOVA analysis to confirm the effect of nerve-sparing technique. Δ score in sexual summary subscale (P = 0.006) and sexual bother scores (P < 0.001) were significantly different, and it tended to be lower in group 3 (bilateral ITR-NS; Fig. 3A and C). For all subscales in the urinary domain, Δ score were significantly different, and it tended to be lower in group 3 (bilateral ITR-NS; Fig. 3D–H).
Fig. 3.
The Δ score (prescore – nadir score after RARP) for each nerve-sparing technique groups in sexual and urinary scores. Group 0, non-nerve-sparing; group 1, unilateral ITR-NS; group 2, unilateral ITE-NS; group 3, bilateral ITR-NS; 4, bilateral ITE-NS. (A) Sexual summary scores (SS; mean ± SD). Group 0, 21.9 ± 17.5; group 1, 26.8 ± 19.9; group 2, 19.7 ± 16.2; group 3, 15.8 ± 19.4; group 4, 20.9 ± 19.9. ANOVA demonstrated significant differences between groups (P = 0.006). (B) Sexual function scores (SF; mean ± SD). Group 0, 23.5 ± 19.0; group 1, 26.9 ± 21.4; group 2, 23.4 ± 19.4; group 3, 20.4 ± 18.4; group 4, 22.2 ± 20.7. There were no significant differences between groups (P = 0.345). (C) Sexual bother scores (SB; mean ± SD). Group 0, 22.4 ± 29.9; group 1, 29.7 ± 31.4; group 2, 16.8 ± 31.2; group 3, 4.1 ± 31.4; group 4, 21.7 ± 33.2. ANOVA demonstrated significant differences between groups (P < 0.001). (D) Urinary summary scores (US; mean ± SD). Group 0, 27.4 ± 18.6; group 1, 29.9 ± 18.9; group 2, 29.1 ± 19.7; group 3, 13.9 ± 13.3; group 4, 23.0 ± 18.9. There are significant differences between groups (P < 0.001). (E) Urinary function scores (UF; mean ± SD). Group 0, 37.8 ± 23.4; group 1, 39.0 ± 23.2; group 2, 37.9 ± 24.5; group 3, 19.9 ± 16.4; group 4, 37.0 ± 22.6. There are significant differences between groups (P < 0.001). (F) Urinary bother scores (UB; mean ± SD). Group 0, 21.4 ± 18.4; group 1, 22.5 ± 19.3; group 2, 22.8 ± 19.5; group 3, 8.7 ± 14.3, group 4, 14.2 ± 17.9. There are significant differences between groups (P < 0.001). (G) Urinary irritative–obstructive scores (UIR; mean ± SD). Group 0, 14.2 ± 16.4; group 1, 15.8 ± 18.7; group 2, 14.8 ± 18.4; group 3, 3.25 ± 9.8; group 4, 9.8 ± 17.3. There are significant differences between groups (P < 0.001). (H) Urinary incontinence scores (UI; mean ± SD). Group 0, 52.8 ± 32.2; group 1, 55.2 ± 30.9; group 2, 52.1 ± 31.8; group 3, 30.7 ± 24.2; group 4, 47.7 ± 30.6. There are significant differences between groups (P < 0.001).
4. Discussion
Radical prostatectomy was previously associated with worse urinary control in the immediate postoperative period. Regarding urinary treatment–related effects, the prostatectomy group showed worse adverse effects for incontinence but better results on urinary irritative–obstructive symptoms compared with brachytherapy 3 years after treatment.15 These findings were consistent with the results reported in the present study. Continual improvement of urinary control beyond 2 years after open radical prostatectomy (ORP) has also been reported.16 Yaxley and colleagues previously showed that surgical technique was not associated with either urinary or sexual functional outcomes.17 In our study, among patients with urinary obstructive-irritative symptoms after RARP, improvement was observed in 73.7% of patients up to three years post-RARP. Our results confirm previously reported early improvement in urinary obstruction-irritation in patients treated with radical prostatectomy (RP).10,11,18 However, Litwin reported that in the group without CaP (n = 598, median age, 73 years), 31% reported incontinence.19 Therefore, it is very difficult to evaluate the effect of RP on urinary incontinence.
In general, RP was not associated with a change in bowel function. Bowel dysfunction was more common after radiation therapy than RP.20 The patients in the RARP group had better QOL with regard to bowel function compared with brachytherapy group.21 In our study, 80% of RP subjects returned to baseline bowel function within six months of surgery. Our data corroborate outcomes from Wei et al6, and from CaPSURE22, all of which showed a minimal effect on bowel symptoms after RP.
Donovan et al20 reported that there was a notable decline in the sexual scores after RP, with subsequent partial recovery. Erectile function remained worse in the prostatectomy group at all time points, and although there was limited recovery to 21% with erections firm enough for intercourse at 36 months. However, this rate declined again to 17% at 6 years post-RP.20 Although the functional domain clearly had a lower recovery rate (29.7%) than the bother domain (73.0%) in this study, it was of a similar degree to previous reports. Previous studies showed that a greater return to baseline function for patients receiving RARP compared with laparoscopic radical prostatectomy (LRP)23 and sexual function scores did not differ significantly between RARP and ORP group at 6 and 12 weeks after operation.17 In our study, a higher proportion of cases recovered preoperative value in the sexual burden domain (73.0%) than the functional domain (29.7%). Parker et al reported that mean sexual bother did not return to the baseline mean; only 44% of patients achieved their individual baseline by 36 months,18 which is markedly lower than that reported in our study. This may be attributed to characteristics specific to Japanese patients. However, further evaluation would be required to clarify any long-term changes.
After Walsh's anatomic nerve-sparing technique in 1982,24 several anatomical studies have provided deeper insight into the neuroanatomy of the prostate and adjacent tissue, which formed the basis for ensuring good oncologic and functional outcomes after radical prostatectomy. The nerve-sparing technique remains the target anatomical approach to achieve better functional outcomes related to potency and continence. Depending on the dissection plane chosen during the procedure, ITR-NR and ITE-NS technical variations have been identified.25 Interfascial dissection of neurovascular bundles (NVBs) involves a dissection lateral to the prostatic fascia at the anterolateral and posterolateral aspects of the prostate.26 The NVB may be more prone to partial resection with interfascial dissection because this dissection does not allow for the preservation of more fascial layers at the anterolateral surface of the prostate, presumably resulting in an oncologically safer approach.27 In a systematic review, Weng et al reported that although there were no differences in perioperative parameters between both nerve-sparing radical prostatectomy, ITR-NS had advantages in terms of both continence and potency recovery compared with ITE-NS.28 Our study outlines the important finding that men undergoing bilateral ITR-NS exhibited significantly lower in each subscale scores of sexual and urinary domains after RARP compared with non-nerve-sparing and ITE-NS. It is unclear about the rationale why ITR-NS technique has a better effect on the urinary irritative–obstructive scores than the bilateral ITE-NS.
Some limitations of this study should be taken into account. First, not all changes in HRQOL scores had clinical significance. However, the subscale score thresholds that should be considered clinically relevant have not been defined. So, there exists a longstanding challenge in its interpretation. In some reports on HRQOL, authors recommend that a clinically meaningful change in function is defined as a change of greater than half the SD in HRQOL score.29,30 Previous studies have found that half the SD or one-third the SD are appropriate choices for a distribution-based minimally important difference (MID) cut point, with half the SD representing a medium-sized effect and one-third the SD representing a small effect.31 In contrast, Skolarus et al found the variety of domains, such as the bowel and vitality/hormonal domains, to have the lowest MID range (4- to 6-point change should be considered clinically relevant), whereas the sexual domain had the greatest MID values (10–12).32 Willis et al defined the recovery of each case using the preoperative value in each domain.23 Unfortunately, the Japanese-validated EPIC questionnaire has not defined the range of MID. Therefore, we defined the recovery in each case as obtaining a value greater than the value obtained by subtracting half the SD from the preoperative value in each subscale. Second, the primary limitation of our study is the relatively short follow-up of 3 years. Follow-up for an additional 5–10 years 5,33 is required to fully inform decisions involving the tradeoff between the shorter-term effects of the management strategies shown here and the longer course of progression and treatment of CaP in the context of the onset of other life-threatening conditions. Third, one important limitation in this study was the small number of patients that were included.
Observational patient registry research including HRQOL for CaP has begun at multicenter in Asia, and it is expected to accumulate the large and long-term QOL data.34
5. Conclusion
Despite these limitations, the present study offers a comparative longitudinal analysis of HRQOL outcomes after RARP for Japanese CaP patients. Compared with urinary incontinence domain, urinary irritative and obstructive domain had less postoperative decline, and recovery was quick. The recovery of sexual bother occurred faster than sexual function. In step with the aging population, the total number of patients with CaP in Japan is expected to increase dramatically. Therefore, we should consider not only the effectiveness of treatments but also the impact on patients’ QOL.
Conflicts of interest
None of the contributing authors have any conflicts of interest, including specific financial interests, relationships, and affiliations relevant to the subject matter or materials discussed in the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2021.08.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Percentage of recovery group and nonrecovery group in each subscale.
References
- 1.Wakao F., Higashi T., Katanoda K. Foundation for Promotion of Cancer Research; Tokyo: 2018. Cancer statistics in Japan 2017.http://ganjoho.jp/data/reg_stat/statistics/brochure/2017/cancer_statistics_2017.pdfhttp://ganjoho.jp/data/reg_stat/statistics/brochure/2017/cancer_statistics_2017.pdf [Internet] [cited 2018 Dec 22]. Available from: [Google Scholar]
- 2.Walz J., Gallina A., Saad F., Montorsi F., Perrotte P., Shariat S.F., et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol. 2007;25:3576–3581. doi: 10.1200/JCO.2006.10.3820. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra V., Novara G., Ahlering T.E., Costello A., Eastham J.A., Graefen M., et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–430. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Wei J.T., Dunn R.L., Sandler H.M., McLaughlin P.W., Montie J.E., Litwin M.S., et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 5.Johansson E., Steineck G., Holmberg L., Johansson J.E., Nyberg T., Ruutu M., et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–899. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 6.Litwin M.S., Hays R.D., Fink A., Ganz P.A., Leake B., Brook R.H. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Wei J.T., Dunn R.L., Litwin M.S., Sandler H.M., Sanda M.G. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 8.Rnic K., Linden W., Tudor I., Pullmer R., Vodermaier A. Measuring symptoms in localized prostate cancer: a systematic review of assessment instruments. Prostate Cancer Prostatic Dis. 2013;16:111–122. doi: 10.1038/pcan.2013.1. [DOI] [PubMed] [Google Scholar]
- 9.Dowrick A.S., Wootten A.C., Murphy D.G., Costello A.J. “We used a validated questionnaire”: what does this mean and is it an accurate statement in urologic research? Urology. 2015;85:1304–1310. doi: 10.1016/j.urology.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 10.Sanda M.G., Dunn R.L., Michalski J., Sandler H.M., Northouse L., Hembroff L., et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 11.Miyake H., Miyazaki A., Furukawa J., Hinata N., Fujisawa M. Prospective assessment of time-dependent changes in quality of life of Japanese patients with prostate cancer following robot-assisted radical prostatectomy. J Robot Surg. 2016;10 doi: 10.1007/s11701-016-0565-0. 2017-7. [DOI] [PubMed] [Google Scholar]
- 12.D’ Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A., et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 13.Kakehi Y., Takegami M., Suzukamo Y., Namiki S., Arai Y., Kamoto T., et al. Health related quality of life in Japanese men with localized prostate cancer treated with current multiple modalities assessed by a newly developed Japanese version of the Expanded Prostate Cancer Index Composite. J Urol. 2007;177:1856–1861. doi: 10.1016/j.juro.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 14.Levinson A.W., Lavery H.J., Ward N.T., Su L.M., Pavlovich C.P. Is a return to baseline sexual function possible? An analysis of sexual function outcomes following laparoscopic radical prostatectomy. World J Urol. 2011;29:29–34. doi: 10.1007/s00345-010-0616-5. [DOI] [PubMed] [Google Scholar]
- 15.Chien G.W., Slezak J.M., Harrison T.N., Jung H., Gelfond J.S., Zheng C., et al. Health-related quality of life outcomes from a contemporary prostate cancer registry in a large diverse population. BJU Int. 2017;120:520–529. doi: 10.1111/bju.13843. [DOI] [PubMed] [Google Scholar]
- 16.Pardo Y., Guedea F., Aguiló F., Fernández P., Macías V., Mariño A., et al. Quality-of-Life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 17.Yaxley J.W., Coughlin G.D., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 18.Parker W.R., Wang R., He C., Wood D.P., Jr. Five year expanded prostate cancer index composite-based quality of life outcomes after prostatectomy for localized prostate cancer. BJU Int. 2011;107:585–590. doi: 10.1111/j.1464-410X.2010.09579.x. [DOI] [PubMed] [Google Scholar]
- 19.Litwin M.S. Health related quality of life in older men without prostate cancer. J Urol. 1999;161:1180–1184. [PubMed] [Google Scholar]
- 20.Donovan J.L., Hamdy F.C., Lane J.A., Mason M., Metcalfe C., Walsh E., et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard P., Davis J.W., Frank S.J., Kim J., Pettaway C.A., Pugh T.J., et al. Quality of life after brachytherapy or bilateral nerve-sparing robot-assisted radical prostatectomy for prostate cancer: a prospective cohort. BJU Int. 2018;121:540–548. doi: 10.1111/bju.14021. [DOI] [PubMed] [Google Scholar]
- 22.Litwin M.S., Sadetsky N., Pasta D.J., Lubeck D.P. Bowel function and bother after treatment for early stage prostate cancer: a longitudinal quality of life analysis from CaPSURE. J Urol. 2004;172:515–519. doi: 10.1097/01.ju.0000129236.56712.e7. [DOI] [PubMed] [Google Scholar]
- 23.Willis D.L., Gonzalgo M.L., Brotzman M., Feng Z., Trock B., Su L.M. Comparison of outcomes between pure laparoscopic vs robot-assisted laparoscopic radical prostatectomy: a study of comparative effectiveness based upon validated quality of life outcomes. BJU Int. 2012;109:898–905. doi: 10.1111/j.1464-410X.2011.10551.x. [DOI] [PubMed] [Google Scholar]
- 24.Walsh P.C., Donker P.J. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 25.Walz J., Burnett A.L., Costello A.J., Eastham J.A., Graefen M., Guillonneau B., et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–192. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Zorn K.C., Gofrit O.N., Orvieto M.A., Mikhail A.A., Zagaja G.P., Shalhav A.L. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755–762. doi: 10.1016/j.eururo.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Menon M., Kaul S., Bhandari A., Shrivastava A., Tewari A., Hemal A. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174:2291–2296. doi: 10.1097/01.ju.0000181825.54480.eb. [DOI] [PubMed] [Google Scholar]
- 28.Weng H., Zeng X.T., Li S., Meng X.Y., Shi M.J., He D.L., et al. Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: a systematic review and meta-analysis. Sci Rep. 2017;7:11454. doi: 10.1038/s41598-017-11878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman G.R., Sloan J.A., Wyrwich K.W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 30.Gay H., Michalski J., Hamstra D.A., Wei J.T., Dunn R.L., Klein E.A., et al. Neoadjuvant androgen deprivation therapy (NADT) Leads to Immediate Impairment of Vitality/Hormonal and Sexual Quality of Life: Results of a Multi-Center, Prospective Study. Urology. 2013;82:1363–1368. doi: 10.1016/j.urology.2013.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revicki D., Hays R.D., Cella D., Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Skolarus T.A., Dunn R.L., Sanda M.G., Chang P., Greenfield T.K., Litwin M.S., et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015;85:101–105. doi: 10.1016/j.urology.2014.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerleau C., Guizard A.V., Daubisse-Marliac L., Heutte N., Mercier M., Grosclaude P., et al. the French Network of Cancer Registries (FRANCIM) Long-term quality of life among localised prostate cancer survivors: QALIPRO population-based study. Eur J Cancer. 2016;63:143–153. doi: 10.1016/j.ejca.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Uemura H., Ye D., Lee J.Y., Chiong E., Pu Y.S., et al. Prostate cancer in Asia: design of a patient registry to inform real-world treatments, outcomes, and quality of life. Prostate Int. 2019;7:108–113. doi: 10.1016/j.prnil.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentage of recovery group and nonrecovery group in each subscale.