Abstract
Introduction
The remarkable efficacy and effectiveness of COVID-19 vaccines have been described in healthy individuals, but kidney transplant recipients have been excluded from these studies. Therefore, real-world evidence of these vaccines can guide clinicians in predicting complications in kidney transplant recipients and how many doses of vaccines are protective. In this study, we aimed to investigate the impact of the COVID-19 vaccines on kidney transplant recipients with SARS-CoV-2 infection.
Material and method
This matched case-control study included vaccinated kidney transplant recipients with COVID-19 from two centers between 1 May and 1 October 2021. All patients in the vaccinated group received a minimum of two doses of the vaccine and were diagnosed with COVID-19 at least one month after the last dose. Each vaccinated patient was matched with an unvaccinated kidney transplant recipient diagnosed with COVID. The endpoints were all-cause mortality, hospitalization, intensive care unit admission, acute kidney injury, cytokine storm, and acute respiratory distress syndrome.
Results
The median age of vaccinated seventy-two participants was 45 years, and 41 of the participants were men in the vaccinated group. Four patients in the vaccinated group and nine patients in the control group died during follow-up (p = 0.247). Seventeen patients in the vaccinated group, thirty-four participants in the control group were hospitalized (p = 0.004); five vaccinated patients and ten unvaccinated patients were followed-up in the ICU during follow-up (p = 0.168). Thirteen of the vaccinated and twelve unvaccinated patients developed acute kidney injury (p = 0.16). The occurrence of cytokine storm (n = 4 vs. n = 11; p = 0.061) and acute respiratory distress syndrome (n = 5 vs. n = 10; p = 0.168) was higher in the patient group compared to the control group.
Conclusion
COVID-19 remains a fatal disease despite advancing treatment modalities and preventive strategies. COVID-19 vaccines can't prevent death in all kidney transplant recipients, but they decrease hospitalization rate and duration in most patients.
Keywords: COVID-19, SARS-CoV-2, Kidney transplantation, CoronaVac, BNT162b2, Vaccine effectiveness
1. Introduction
Kidney transplant recipients face high mortality rates, allograft damage, acute respiratory syndrome, and cytokine storm during Coronavirus disease 2019 (COVID-19) [1], [2], [3]. Although there are treatment options for COVID-19, there is no cure yet. The remarkable efficacy and effectiveness of COVID-19 vaccines have been described in healthy individuals, but kidney transplant recipients have been excluded from these studies [4]. Therefore, most recommendations for kidney transplant recipients are based on measurements of immune responses to vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On the other hand, the correlation between the immune response and the clinic effect has been demonstrated in healthy individuals, but this effect wasn't confirmed in immunocompromised patients yet [5], [6], [7].
There is an ongoing debate about the number of doses and timing of the COVID-19 vaccine schedule in kidney transplant recipients. Two doses were recommended early in the pandemic, and guidelines were later revised to three doses of the vaccine (two doses plus a booster dose). Due to efficacy and effectiveness concerns, four (additional plus booster) doses were recommended recently [7], [8]. Despite all the improvements in treatment modalities and vaccination for COVID-19, deaths and complications occur. In addition, there are growing concerns about new variants in the virus and vaccine ineffectiveness. Also, data on how many vaccine doses are still necessary and sufficient in immunocompromised patients are limited. In addition, real-world evidence of these vaccines can guide clinicians in predicting complications in kidney transplant recipients. In this study, we aimed to investigate the impact of the COVID-19 vaccines on kidney transplant recipients with SARS-CoV-2 infection.
2. Material and method
2.1. Study population
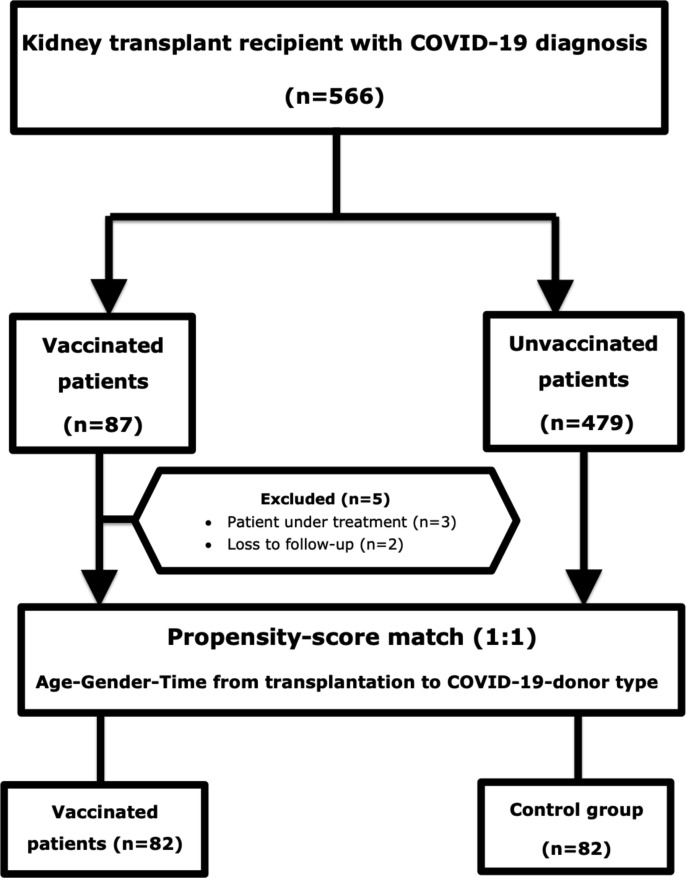
This matched case-control study included vaccinated kidney transplant recipients with COVID-19 from two centers between 1 May and 1 October 2021. All patients in the vaccinated group received a minimum of two doses of the vaccine. All the vaccinated patients were diagnosed with COVID-19 at least one month after the last dose. Exclusion criteria were patients currently under treatment for COVID-19 (n = 3) and loss of follow-up after diagnosis (n = 2). Eighty-two participants were followed for at least fourteen days or until death. Each vaccinated patient was matched with an unvaccinated kidney transplant recipient diagnosed with COVID between 1 April 2020 and 1 October 2021. Age, gender, type of donor, and time from transplantation to COVID-19 diagnosis were matched using propensity score-match between groups (Fig. 1 ). No patient had a history of previous COVID-19 in both groups. The Istanbul Medical Faculty Medical Ethics Committee approved this study.
Fig. 1.
Flow chart diagram of the patient and control group.
2.2. Data collection
Demographic data (age, sex, comorbidities, etiology of primary kidney disease, duration of post-transplant follow-up, type of donor, induction therapy, maintenance immunosuppression, and medication), clinical characteristics (post-infection follow-up, duration of hospitalization, presenting symptoms, contact history, symptoms, examination findings, and laboratory results), and medication during COVID-19 (antiviral, antibiotic, cytokine‐targeted, anticoagulation treatment, and oxygen therapy) were extracted from electronic medical records.
2.3. Vaccination schedule
All the participants in the vaccinated group were administered at least two doses of either CoronaVac (Sinovac Life Sciences) or BNT162b2 (Pfizer-BioNTech). The first dose was applied on 17 January for CoronaVac and 18 March 2021 for BNT162b2, and the second dose was administered one month later. Booster doses were administered three to six months after the second. Also, some of the patients who received two doses of CoronaVac received boosters of BNT162b2. While twenty-one patients were vaccinated with two doses and fourteen patients with three doses of CoronaVac. Also, two doses of BNT162b2 were administered to twenty kidney transplant recipients, and two CoronaVac and one BNT162b2 to twenty-seven participants.
2.4. Patient management
All patients underwent blood testing at admission. Laboratory investigations included a complete blood count and serum C-reactive protein (CRP), interleukin-6, markers of myocardial damage (creatine kinase, troponin I, lactate dehydrogenase), tests of secondary hemostasis profile (prothrombin time, activated partial thromboplastin time), serum biochemical tests (including renal and liver function, and electrolytes), procalcitonin, fibrinogen, d-dimer. Laboratory tests were repeated daily for inpatients and weekly for outpatients for two weeks. In addition, the modification of diet in the renal disease study equation (MDRD) was used to calculate the estimated glomerular filtration rate (eGFR).
Patients were divided into four groups according to the severity of the disease. Asymptomatic patients and patients with mild disease (oxygen saturation above 93%, no lung involvement on chest computed tomography) were followed up in the outpatient clinic weekly. Indications for hospitalization were moderate (oxygen saturation above 90%, respiratory rate under 30 breaths/min) and severe disease (oxygen saturation under 90%, respiratory rate above 30 breaths/min), cytokine storm (persistent fever, high or increasing CRP, ferritin, and D-dimer, liver function test abnormalities, hypofibrinogenemia with cytopenia in the forms of lymphopenia and thrombocytopenia). Criteria for admission to the intensive care unit (ICU) were the partial pressure of arterial oxygen, the inspiratory oxygen fraction (PaO2 / FiO2) ratio<300, oxygen saturation<90%, and PaO2 below 70 mm Hg despite 5 L/minute oxygen therapy, and persistent hypotension (systolic blood pressure < 90 mm Hg or mean arterial pressure < 65 mm Hg). The national COVID-19 guidelines were used for the diagnosis and treatment of the patients [9]. The Kidney Disease Improving Global Outcomes guidelines graded acute kidney injury [10].
2.5. Treatment regimens
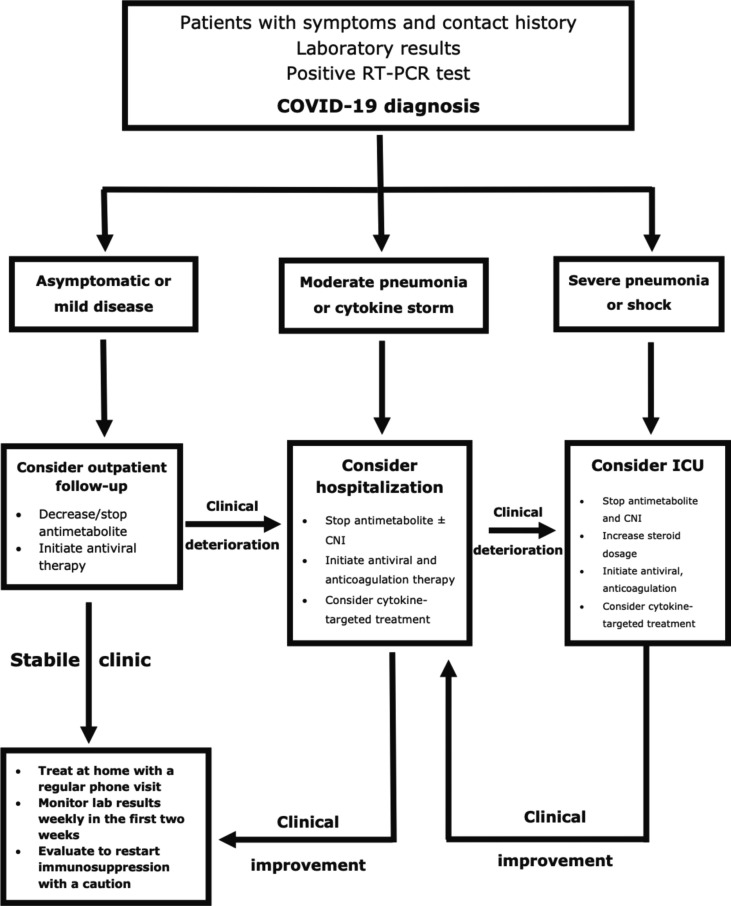
A standard protocol was used to manage immunosuppression, antiviral, and cytokine‐targeted therapy (Fig. 2 ). The immunosuppressive regimen was not changed if the patient was asymptomatic. Antimetabolites (mycophenolate derivatives and azathioprine) were discontinued in the remaining cases. Trough levels were maintained between 4 and 6 ng/dL for tacrolimus, 25–75 ng/dL for cyclosporine, and 3–7 ng/dL for everolimus for a patient with stable clinic course and were stopped in hypoxemic patients. The calcineurin inhibitor and mammalian target of rapamycin inhibitor levels were monitored twice a week in hospitalized patients, once a week in outpatients.
Fig. 2.
Treatment scheme for COVID‐19 in kidney transplant recipients.
The patients were treated with hydroxychloroquine (400 mg twice a day for the first, and then 200 mg twice a day for four days via oral administration) in the first wave. Also, favipiravir (1600 mg twice a day for the first, and then 600 mg twice a day for the following four days via oral administration) was used in the later period of the pandemic. Tocilizumab (400–800 mg once a day for the two days via intravenous infusion) or anakinra (100–600 mg once a day for seven to fourteen days or until hospital discharge via subcutaneous injection) are used to treat cytokine storm. The administration of antibiotics was based on the infection specialist's decision in the presence of confirmed or suspected invasive bacterial infection. In addition, the patients were monitored for adverse drug reactions during the hospital stay.
2.6. Supportive care
Prophylactic-dose low molecular weight heparin was used for inpatients unless there were contraindications. Patients with deep vein thrombosis or pulmonary embolism were treated with therapeutic-dose anticoagulation. Doses were adjusted according to the risk of bleeding in the patient. Oxygen treatment was provided to patients whose oxygen saturation was below 92% via a nasal cannula and with a non-rebreather mask if the former was insufficient. If respiratory failure persisted, invasive mechanical ventilation was applied following non-invasive ventilation.
2.7. Outcomes
The primary endpoint was all-cause mortality. The secondary endpoints were hospitalization, intensive care unit admission, acute kidney injury, cytokine storm, and acute respiratory distress syndrome.
2.8. Statistical analysis
All parameters (patient demographics, clinical presentations, laboratory values at admission, treatment regimens, and outcomes) were classified according to the vaccinated and unvaccinated patients. Categorical variables were summarized with numbers and percentages. Quantitative variables are summarized with means and standard deviations or medians and interquartile range where appropriate. While chi-square and Fisher's exact test were performed for qualitative variables, the Mann-Whitney U test was used for quantitative variables with the nonparametric distribution. A p-value of<0.05 was considered significant.
3. Results
3.1. Patient characteristics
The median age of vaccinated eighty-two participants was 45 years (interquartile range [IQR], 38–63), and 41 (50%) of the participants were men in the vaccinated group. The median time from transplantation to COVID-19 diagnosis was 113 months (IQR, 36–174) in the vaccinated group.
The prevalence of heart disease (n = 13 [15.9%] vs. n = 10 [12.2%]; p = 0.460), pre-existing lung disease (n = 1 [1.2%] vs. n = 5 [6.1%]; p = 0.096), and post-transplant diabetes mellitus (n = 16 [19.5%] vs. n = 14 [17.1%]; p = 0.714) were similar between the groups. However, chronic hypertension (n = 43 [52.4%] vs. n = 60 [73.2%]; p = 0.010) was more common in the control group compared to the patient group.
Sixty-three (76.8%) of the patient population underwent kidney transplantation from a living donor. Also, there was no significant difference between the groups in terms of anti-T-lymphocyte globulin use (n = 45 [54.9%] vs. n = 48 [58.5%]; p = 0.636), previous rejection episodes (n = 7 [8.5%] vs. n = 11 [13.4%]; p = 0.304), and BK virus nephropathy (n = 2 [2.4%] vs. n = 2 [2.4%]; p = 1). The most common maintenance immunosuppressive agents in both groups were tacrolimus, mycophenolate derivatives, and corticosteroids. The demographic characteristics and immunosuppression regimen of patients are shown in Table 1 .
Table 1.
Patients’ demographic characteristics and immunosuppression regimen.
| Vaccinated patients (n = 82) |
Control group (n = 82) |
p-value | |
|---|---|---|---|
| Age (year) | 45 (38–63) | 47 (39–60) | 0.588 |
| Sex | |||
| Male | 41 (50) | 41 (50) | 1 |
| Female | 41 (50) | 41 (50) | |
| Duration from tx to COVID-19 (months) | 113 (36–174) | 106 (36–171) | 0.844 |
| Time from vaccination to COVID-19 (months) | 114 (78–158) | ||
| Etiology of CKD | 0.200 | ||
| Hypertensive nephropathy | 8 (9.8) | 5 (6.1) | |
| Diabetic nephropathy | 8 (9.8) | 12 (14.6) | |
| Chronic glomerulonephritis | 11 (13.4) | 12 (14.6) | |
| CAKUT | 13 (15.9) | 20 (24.4) | |
| Other | 14 (17.1) | 5 (6.1) | |
| Unknown | 28 (34.1) | 28 (34.1) | |
| Comorbid diseases | |||
| Pre-existing lung disease | 1 (1.2) | 5 (6.1) | 0.096 |
| Previous heart disease | 13 (15.9) | 10 (12.2) | 0.460 |
| Chronic hypertension | 43 (52.4) | 60 (73.2) | 0.010 |
| Post-transplant diabetes mellitus | 16 (19.5) | 14 (17.1) | 0.714 |
| Type of donor | |||
| Living | 63 (76.8) | 63 (76.8) | 1 |
| Cadaver | 19 (23.2) | 19 (23.2) | |
|
Induction therapy ATLG |
45 (54.9) |
48 (58.5) |
0.636 |
| Posttransplant complications | 7 (8.5) | 11 (13.4) | 0.304 |
| Allograft rejection | 2 (2.4) | 2 (2.4) | 1 |
| BK virus nephropathy CMV viremia |
0 | 0 | |
| Maintenance immunosuppression at admission | |||
| Tacrolimus | 63 (76.8) | 68 (82.9) | 0.330 |
| Cyclosporine A | 11 (13.4) | 8 (9.8) | 0.464 |
| Everolimus | 4 (4.9) | 5 (6.1) | 0.731 |
| Sirolimus | 1 (1.2) | 2 (2.4) | 0.271 |
| Mycophenolate derivatives | 70 (85.4) | 73 (89) | 0.483 |
| Azathioprine | 8 (9.8) | 3 (3.7) | 0.129 |
| Steroids | 77 (93.9) | 81 (98.8) | 0.210 |
Abbreviations: ATLG: anti-T-lymphocyte globulin; CAKUT: congenital abnormalities of the urinary tract; CKD: chronic kidney disease; CMV: cytomegalovirus; tx: transplantation.
P-values compared vaccinated patients and control group, obtained from the Chi-Square test, Fisher’s exact test, or Mann–Whitney U test. Data were presented as n (%) or median [Interquartile range 25–75] unless otherwise noted. Bold indicates statistically significant associations (p < 0.05).
3.2. Clinical presentations
Cough (n = 52 [65.9%] vs. n = 49 [59.8%]; p = 0.426) was the most frequent symptom at admission in both groups, shown in Table 2 . Diarrhea (n = 28 [34.1%] vs. n = 16 [19.5%]; p = 0.034) was more common in the vaccinated group than in the control group. Lymphocyte count was lower in the control compared to the vaccinated patients (900 [IQR, 560–1370] vs. 1060 [IQR, 760–1600]; p = 0.173). Also, a significant difference was found in the serum albumin levels (4.1 [IQR, 3.6–4.4] vs. 3.7 [IQR, 3.4–4.2]; p = 0.024) (Table 2). The median serum creatinine levels at admission were similar between the groups (1.2 [IQR, 0.9–1.6] vs. 1.3 [IQR, 1–1.7]; p = 0.465).
Table 2.
Patients’ clinical characteristics and laboratory results at admission.
| Vaccinated patients (n = 82) |
Control group (n = 82) |
p-value | |
|---|---|---|---|
| Presentation symptoms | |||
| Fever | 36 (43.9) | 42 (51.2) | 0.348 |
| Cough | 52 (65.9) | 49 (59.8) | 0.426 |
| Dyspnea | 20 (25.3) | 18 (22) | 0.615 |
| Diarrhea | 28 (34.1) | 16 (19.5) | 0.034 |
| Initial examination findings | |||
| Pulse rate (/min) | 60 (60–64) | 78 (60–90) | <0.001 |
| SpO2 value (%) | 98 (95–99) | 98 (95–99) | 0.687 |
| Respiratory rate (/min) | 18 (16–18) | 18 (18–20) | 0.004 |
| Blood pressure (mmHg) | |||
| Systolic | 110 (110–120) | 120 (110–125) |
0.003 |
| Diastolic | 80 (70–80) | 80 (78–80) | 0.022 |
| Laboratory results at admission | |||
| Serum creatinine (mg/dL) | 1.2 (0.9–1.6) | 1.3 (1–1.7) | 0.465 |
| Leucocyte count (/mm3) | 6000 (4500–8100) | 6650 (4690–8340) | 0.540 |
| Lymphocyte count (/mm3) | 1060 (760–1600) | 900 (560–1370) | 0.173 |
| Hemoglobin (g/dL) | 13 (11.3–14) | 12.8 (11.3–14) | 0.530 |
| Platelet count (/mm3) | 196 (159–258) | 205 (152–271) | 0.521 |
| Serum CRP levels (mg/L) | 16 (6–35) | 18 (3–46) | 0.972 |
| Serum ALT levels (IU/L) | 21 (15–30) | 18 (14–24) | 0.274 |
| Serum AST levels (IU/L) | 22 (17–32) | 20 (17–27) | 0.138 |
| Serum LDH levels (IU/L) | 215 (184–261) | 238 (186–307) | 0.243 |
| Serum D-dimer (ng/mL) | 338 (179–658) | 420 (305–772) | 0.057 |
| Serum Ferritin (ng/mL) | 232 (111–573) | 326 (99–646) | 0.556 |
| Serum Albumin (g/dL) | 4.1 (3.6–4.4) | 3.7 (3.4–4.2) | 0.024 |
| Serum IL-6 (pg/mL) | 1.5 (1.1–11) | 1.6 (0.8–4.5) | 0.637 |
| Procalcitonin (ng/mL) | 0.08 (0.04–0.21) | 0.08 (0.04–0.53) | 0.953 |
Abbreviations: SpO2: blood oxygen saturation levels; CRP: C-reactive protein; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; IL-6: Interleukin 6. P-values compared vaccinated patients and control group, obtained from the Chi-Square test, Fisher’s exact test, or Mann–Whitney U test. Data were presented as n (%) or median [Interquartile range 25–75] unless otherwise noted. Bold indicates statistically significant associations (p < 0.05).
3.3. Treatment regimens
Antimetabolites were discontinued in 66 (80.5%) vaccinated and 69 (84.1%) unvaccinated patients (p = 0.539). Also, calcineurin inhibitors and mycophenolate derivative were stopped in 9 (11%) patients in the vaccinated group and 10 (12.2%) patients in the control group (p = 0.807). In addition, hydroxychloroquine was used only in 17 (20.7%) unvaccinated patients. Usage of favipiravir (60 [73.1%] vs. 64 [78%]; p = 0.467), anakinra (3 [3.7] vs. 4 [4.9]; p = 0.699) and tocilizumab (1 [1.2%] vs. 2 [2.4%]; p = 0.560) were similar between the groups. Usage of antibiotics (18 [22.5%] vs. 38 [46.3%]; p < 0.001) and anticoagulation (17 [20.7%] vs. 34 [41.5%]; p = 0.004) were significantly higher in the control group compared to the vaccinated group. Duration of hospitalization was longer in the control group compared to the patient group (8 [IQR, 5–12] vs. 11 [IQR, 7–21]; p < 0.001).
3.4. Outcomes
Four patients in the vaccinated group (4.9%) and nine patients (11%) in the control group died during follow-up (p = 0.247). Seventeen patients (20.7%) in the vaccinated group, thirty-four participants (41.5%) in the control group were hospitalized (p = 0.004). Also, five vaccinated patients (6.1%) and ten unvaccinated patients (12.2%) were followed up in the ICU during follow-up (p = 0.168). Five vaccinated (6.1%) and ten unvaccinated patients (12.2%) received mechanical ventilation (p = 0.168), shown in Table 3 . In addition, one vaccinated patient died from multiorgan failure secondary to cytokine storm, and three were lost due to respiratory failure (Table 4 ). Two of the deceased patients received two doses of CoronaVac, while one patient received two doses of BNT162b2, and one patient was vaccinated with two doses of CoronaVac and one dose of BNT162b2. Any adverse events did not occur, and no drug interactions were detected during follow-up.
Table 3.
Patients’ vaccine, treatment regimens and outcomes.
| Vaccinated patients (n = 82) |
Control group (n = 82) |
p-value | |
|---|---|---|---|
| Duration of hospitalization (days) | 8 (5–12) | 11 (7–21) | <0.001 |
| Follow-up after COVID-19 | 57 (32–81) | 134 (104–203) | 0.812 |
| Vaccine Regimens | |||
| Two doses of CoronoVac | 21 (25.6) | ||
| Two doses of BNT162b2 | 20 (24.4) | ||
| Three doses of CoronoVac | 14 (17.1) | ||
| Two doses of CoronoVac and one of BNT162b2 | 27 (32.9) | ||
| Withdrawal of IS agent | |||
| Calcineurin inhibitors | 9 (11) | 10 (12.2) | 0.807 |
| Antimetabolites | 66 (80.5) | 69 (84.1) | 0.539 |
| Treatment of infection | |||
| Hydroxychloroquine | 0 | 17 (20.7) | <0.001 |
| Favipiravir | 60 (73.1) | 64 (78) | 0.467 |
| Cytokine‐targeted therapy | 4 (4.9) | 6 (7.3) | 0.514 |
| Anakinra | 3 (3.7) | 4 (4.9) | 0.699 |
| Tocilizumab | 1 (1.2) | 2 (2.4) | 0.560 |
| Anticoagulation | 17 (20.7) | 34 (41.5) | 0.004 |
| Antibiotics | 18 (22.5) | 38 (46.3) | <0.001 |
| Ventilation devices | |||
| Nasal cannula | 9 (11) | 20 (24.3) | 0.244 |
| Non-invasive ventilation | 4 (4.9) | 8 (9.8) | 0.230 |
| Mechanical ventilation | 5 (6.1) | 10 (12.2) | 0.168 |
| Acute kidney injury | 13 (15.9) | 20 (24.4) | 0.160 |
| Stage 1 | 9 (11) | 7 (8.8) | 0.599 |
| Stage 2 | 3 (3.7) | 6 (7.3) | 0.495 |
| Stage 3 | 1 (1.2) | 7 (8.5) | 0.029 |
| Endpoints | |||
| Cytokine storm | 4 (4.9) | 11 (13.4) | 0.061 |
| ARDS | 5 (6.1) | 10 (12.2) | 0.168 |
| Hospitalized patient | 17 (20.7) | 34 (41.5) | 0.004 |
| Follow-up in ICU | 5 (6.1) | 10 (12.2) | 0.168 |
| Death | 4 (4.9) | 9 (11) | 0.247 |
Abbreviations; IS: immunosuppression; IVIG: intravenous immunoglobulin; ARDS: acute respiratory distress syndrome; ICU: intensive care unit.
P-values compared vaccinated patients and control group obtained from the Chi-Square test, Fisher’s exact test, or Mann–Whitney U test. Data were presented as n (%) or median [Interquartile range 25–75] unless otherwise noted. Bold indicates statistically significant associations (p < 0.05).
Table 4.
Demographics, immunosuppressives and treatment regimens of vaccinated death patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Sex | Female | Female | Female | Female |
| Age (years) | 52 | 49 | 60 | 46 |
| Etiology of CKD | Unknown | Diabetic nephropathy | Polycystic kidney disease | Polycystic kidney disease |
| Type of donor | Cadaver | Living | Living | Living |
| Induction regimen | ATLG | ATLG | ATLG | ATLG |
| Maintenance IS | Tac + MMF + Steroid | Tac + MMF + Steroid | Tac + MMF + Steroid | Tac + MMF + Steroid |
| Time from Tx to COVID-19 (months) | 49 | 143 | 182 | 74 |
| Doses of vaccination | Two doses | Two doses | Three doses | Two doses |
| Type of vaccines | CoronaVac | CoronaVac | Two CoronaVac and one BNT162b2 |
BNT162b2 |
| Time from the last vaccine to hospitalization (days) | 92 | 33 | 45 | 57 |
| Length of hospitalization (days) | 15 | 21 | 22 | 11 |
| Severity of COVID-19 | Critically severe | Critically severe | Critically severe | Critically severe |
| Cytokine storm | Yes | Yes | Yes | Yes |
|
Changes of IS during COVID-19 |
Withdrawal of TAC + MMF | Withdrawal of TAC + MMF | Withdrawal of TAC + MMF | Withdrawal of TAC + MMF |
| Antiviral agents | Favipiravir | Favipiravir | Favipiravir | Favipiravir |
| Anticoagulation | Enoxaparin | Enoxaparin | Enoxaparin | Enoxaparin |
Abbreviations; TAC: Tacrolimus; MMF: Mycophenolate mofetil.
Thirteen of the vaccinated (15.9%) and twenty unvaccinated patients (24.4%) developed acute kidney injury (p = 0.16). However, stage three acute kidney injury (n = 1 [1.2%] vs. n = 7 [8.5%]; p = 0.029) was more common in controls patients than in the vaccinated. The occurrence of cytokine storm (n = 4 [4.9%] vs. n = 11 [13.4%]; p = 0.061) and acute respiratory distress syndrome (n = 5 [6.1%] vs. n = 10 [12.2%]; p = 0.168) was higher in the patient group compared to the control group, but these did not reach statistical significance.
4. Discussion
COVID-19 remains a significant cause of complications and death in kidney transplant recipients despite advancing treatment protocols and vaccines. This study examined COVID-19 outcomes in vaccinated and unvaccinated kidney transplant recipients with similar risk factors. The hospitalization rate was lower in the vaccinated patients than in the control group (n = 17 vs. n = 34, p = 0.004). Also, the median hospital stay was shorter in the vaccinated group than in the control group (8 vs. 11; p < 0.001). The mortality rate was 4.9% in the vaccinated group and 11% in the unvaccinated group (p = 0.247).
While BNT162b2 was reported to have 100% efficacy and 86% effectiveness, the CoronaVac has 100% efficacy and 70% to 90% effectiveness in preventing death in healthy individuals with COVID-19 [11], [12]. Although the ratios vary by a variant of the virus and study population, vaccines are the best method of preventing death in a healthy population with COVID-19 [7]. However, data regarding the effectiveness of vaccines in preventing death in kidney transplant recipients is insufficient and controversial. A few controversial studies investigate whether two doses of vaccine reduce mortality in kidney transplant recipients. Although this has been shown in UK registry analysis, another study did not confirm this effect. [13], [14]. In our study, although the mortality rate in the vaccinated patients was lower than in the unvaccinated group, this did not reach statistical significance. This condition may be explained by the unknown effectiveness of the inactivated virus vaccine in kidney transplant recipients, the low total mortality rate, and the low number of patients. On the other hand, this study demonstrated that some kidney transplant recipients still suffer from various complications during COVID-19 despite three doses of vaccines.
After excluding kidney transplant recipients in phase III trials, measuring B- and T-cell response to the COVID-19 vaccine has become a research model [15], [16]. BNT162b2 and CoronaVac are well tolerated and protective against COVID-19 in healthy adults. However, in kidney transplant recipients, mRNA-based BNT162b2 has been shown to induce a higher nucleocapsid antibody response and T cell response than the inactivated vaccine, CoronoVac [17]. Also, the antibody response persists longer in BNT162b2 and is more effective against the omicron variant than the CoronoVac in a healthy population [18]. On the other hand, in another study, no antibody response was detected in 51.4% of the patients, despite four doses of the BNT162b2 vaccine in kidney transplant recipients [19]. Also, no antibody response was determined in 82% of the kidney transplant recipients who received two doses of CoronaVac [16]. However, there has been no standardized assay and threshold limit in kidney transplant recipients yet. According to our research, vaccines have reduced the severity of the disease and the length of hospital stay. On the other hand, it was impossible to conclude the required vaccine doses and more effective vaccine type due to the limited number of patients, different protocols, and lack of immunity measurement.
The effectiveness of BNT162b2 and CoronaVac in preventing hospitalizations with COVID-19 in healthy individuals was previously shown to be 80–90% and 80%, respectively [12], [20]. The COVID-19 vaccines decreased the hospitalization rate and hospitalization duration in our study. Also, the higher antibiotic usage may be explained by the elevation of nosocomial infections due to the longer hospitalization duration in the control group.
Our study has several limitations; the number of patients was small, the follow-up period was short, PCR was collected only from symptomatic patients, and strain identification and immune response measurements were not available. These restrictions did not allow us to make definitive decisions, so a prospective study is needed to confirm our results. The strengths of this study consisted of the presence of matching control groups and standard treatment regimens of both centers.
In conclusion, COVID-19 remains a fatal disease despite advancing treatment modalities and preventive strategies. Vaccines decrease hospitalization rate and duration in most patients, but complications can occur if kidney transplant recipients have only received two doses of the COVID-19 vaccine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors of this manuscript have no conflicts of interest.
Data availability statement
This research obeyed human studies guidelines and was ethically conducted by the World Medical Association Declaration of Helsinki. Istanbul Medical Faculty Medical Ethics Committee approved this study.
Author Contributions
ED and AT designed the study, ED, AT, HD and ASA participated in the writing of the paper. ED and AT analyzed the data; ED made the table; ED, HD, SS, ASA, SS, and AT drafted and revised the paper. All authors approved the final version of the manuscript.
Disclosure
The authors declare no conflicts of interest.
Funding
The authors declare no sources of funding for this study.
References
- 1.Hilbrands L.B., Duivenvoorden R., Vart P., Franssen C.F.M., Hemmelder M.H., Jager K.J., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duivenvoorden R, Vart P, Noordzij M, et al. Clinical, Functional, and Mental Health Outcomes in Kidney Transplant Recipients 3 Mo After a Diagnosis of COVID-19. Transplantation. Article in Press. https://doi.org/10.1097/TP.0000000000004075 [DOI] [PMC free article] [PubMed]
- 3.Pérez‐Sáez M.J., Blasco M., Redondo‐Pachón D., Ventura‐Aguiar P., Bada‐Bosch T., Pérez‐Flores I., et al. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020;20(11):3182–3190. doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. [accessed 8 December 2021]. [PubMed]
- 6.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.US Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available at https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised [accessed 1 April 2022].
- 9.Coronavirus Scientific Advisory Board. Coronavirus Disease 2019 guidelines of patient management and treatment. Turkish Ministry of Health. Available at https://covid19.saglik.gov.tr/TR-66301/covid-19-rehberi.html [accessed 8 December 2021].
- 10.Kellum J.A., Lameire N., Aspelin P., et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 11.Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks-Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep 2021;70:396–401. https://doi.org/10.15585/mmwr.mm7011e3external icon. [DOI] [PMC free article] [PubMed]
- 12.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravanan R., Mumford L., Ushiro-Lumb I., Callaghan C., Pettigrew G., Thorburn D., et al. Two Doses of SARS-CoV-2 Vaccines Reduce Risk of Death Due to COVID-19 in Solid Organ Transplant Recipients: preliminary Outcomes From a UK Registry Linkage Analysis. Transplantation. 2021;105(11):e263–e264. doi: 10.1097/TP.0000000000003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEvoy CM, Lee A, Misra PS, et al. Real-world Effectiveness of 2-dose SARS-CoV-2 Vaccination in Kidney Transplant Recipients. medRxiv 2021.09.21.21263457 https://doi.org/10.1101/2021.09.21.21263457
- 15.Ou M.T., Boyarsky B.J., Motter J.D., Greenberg R.S., Teles A.T., Ruddy J.A., et al. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation. 2021;105(10):2170–2174. doi: 10.1097/TP.0000000000003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eren Sadioğlu R., Demir E., Evren E., Aktar M., Şafak S., Artan A.S., et al. Antibody response to two doses of inactivated SARS-CoV-2 vaccine (CoronaVac) in kidney transplant recipients. Transpl Infect Dis. 2021;23(6) doi: 10.1111/tid.v23.610.1111/tid.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seija M., Rammauro F., Santiago J., Orihuela N., Zulberti C., Machado D., et al. Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant. Clin Kidney J. 2022;15(3):527–533. doi: 10.1093/ckj/sfab291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Q., Zhou R., Wang Y., Zhao M., Liu N.a., Li S., et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. eBioMedicine. 2022;77:103904. doi: 10.1016/j.ebiom.2022.103904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamar N., Abravanel F., Marion O., Romieu-Mourez R., Couat C., Del Bello A., et al. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA–Based Vaccine in Recipients of a Solid Organ Transplant. JAMANetw Open. 2021;4(11):e2136030. doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study BMJ 2021;373:n1088 https://doi.org/10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This research obeyed human studies guidelines and was ethically conducted by the World Medical Association Declaration of Helsinki. Istanbul Medical Faculty Medical Ethics Committee approved this study.