Abstract
Background
Spatial compartmentalization of metabolic pathways within membrane-separated organelles is key to the ability of eukaryotic cells to precisely regulate their biochemical functions. Membrane-bound organelles such as mitochondria, endoplasmic reticulum (ER) and lysosomes enable the concentration of metabolic precursors within optimized chemical environments, greatly accelerating the efficiency of both anabolic and catabolic reactions, enabling division of labor and optimal utilization of resources. However, metabolic compartmentalization also poses a challenge to cells because it creates spatial discontinuities that must be bridged for reaction cascades to be connected and completed. To do so, cells employ different methods to coordinate metabolic fluxes occurring in different organelles, such as membrane-localized transporters to facilitate regulated metabolite exchange between mitochondria and lysosomes, non-vesicular transport pathways via physical contact sites connecting the ER with both mitochondria and lysosomes, as well as localized regulatory signaling processes that coordinately regulate the activity of all these organelles.
Scope of review
This review covers how cells use membrane transporters, membrane contact sites, and localized signaling pathways to mediate inter-organelle communication and coordinate metabolism. We also describe how disruption of inter-organelle communication is an emerging driver in a multitude of diseases, from cancer to neurodegeneration.
Major conclusions
Effective communication among organelles is essential to cellular health and function. Identifying the major molecular players involved in mediating metabolic coordination between organelles will further our understanding of cellular metabolism in health and lead us to design better therapeutics against dysregulated metabolism in disease.
Keywords: Mitochondria, Metabolism, Contact sites, mTORC1, Transporters, Lysosome
1. Compartmentalization of metabolic pathways and cellular processes
Metabolic compartmentalization is found at multiple levels, from distinct organs and tissues with specialized functions to membrane-separated organelles within cells, to further micro-compartmentation through the physical association of multienzyme complexes, or metabolons [[1], [2], [3]]. Complex biological systems, such as multicellular organisms, are organized into structured compartments to achieve division of labor and optimal utilization of resources. A primary example of metabolic compartmentalization is the physical separation of metabolic pathways within membrane-separated organelles. This is beneficial for many reasons. First, subcellular compartmentalization allows for specific microenvironments, which favor optimal activity for specific enzymes. For example, acidic lysosomal pH is optimal for the degradative functions of lysosomal hydrolases [4,5], whereas peroxisomal enzymes have a basic pH optimum and a basic isoelectric point, making them better adapted for the alkaline peroxisomal pH [6]. Second, compartmentalization ensures that some futile metabolic cycles do not take place, allowing for optimized distribution of metabolites that are utilized by multiple pathways. For example, cytosolic levels of malonyl-CoA, the substrate of fatty acid synthase, regulates mitochondrial fatty acid import by inhibiting carnitine palmitoyltransferase. Because fatty acid synthesis and oxidation take place in two different cellular compartments, such regulation prevents cells to engage in futile catabolic/anabolic cycling of fatty acids, which may, instead, be stored as triglycerides in conditions of high nutrient availability [7,8]. Finally, some metabolic reactions produce toxic intermediates which, if contained and concentrated within enclosed compartments, are prevented from causing damage to the cell and can be efficiently removed. For example, ammonium, a toxic by-product of glutamine deamidation within mitochondria, is removed by mitochondrial carbamoyl-phosphate synthetase 1 (CPS1) via the urea cycle, preventing its accumulation in the cytosol under normal conditions [9].
2. Overview of organelle communication pathways
As illustrated through the examples above, compartmentalization of metabolism is important for many reasons. However, for a cell to function properly, metabolic pathways compartmentalized to different organelles must be able to crosstalk and utilize products from one pathway as intermediates for another. For example, products from mitochondrial anaplerosis, such as aspartate and citrate, are required to fuel cytosolic pathways, such as de novo purine/pyrimidine synthesis and de novo lipogenesis, respectively [10]. On the other hand, branched-chain amino acids, derived from macromolecular degradation within lysosomes, and acetyl-CoA, derived from peroxisomal fatty acid oxidation, may be used as inputs to the mitochondrial TCA cycle [10] (Figure 1). While inter-organelle exchange of small, polar metabolites occurs primarily via membrane transporters, inter-organelle transport of lipids and ions, such as Ca2+, may take place via targeted organelle-to-organelle interaction mediated by membrane contact sites (MCSs). Finally, signaling pathways that control metabolic function may be localized to specific organelle membranes, such as mechanistic target of rapamycin complex 1 (mTORC1) to the lysosome or cyclic AMP (cAMP)-dependent protein kinase (PKA) to various locations via organelle-specific A kinase-anchoring proteins (AKAPs). This allows signaling platforms to sense local metabolite concentrations and coordinate anabolism versus catabolism based on local and cellular metabolic states. In the next three sections of the review, we will describe how cells use these three modalities – membrane transporters, membrane contact sites, and localized signaling pathways – to mediate inter-organelle communication and coordinate metabolism (Figure 1).
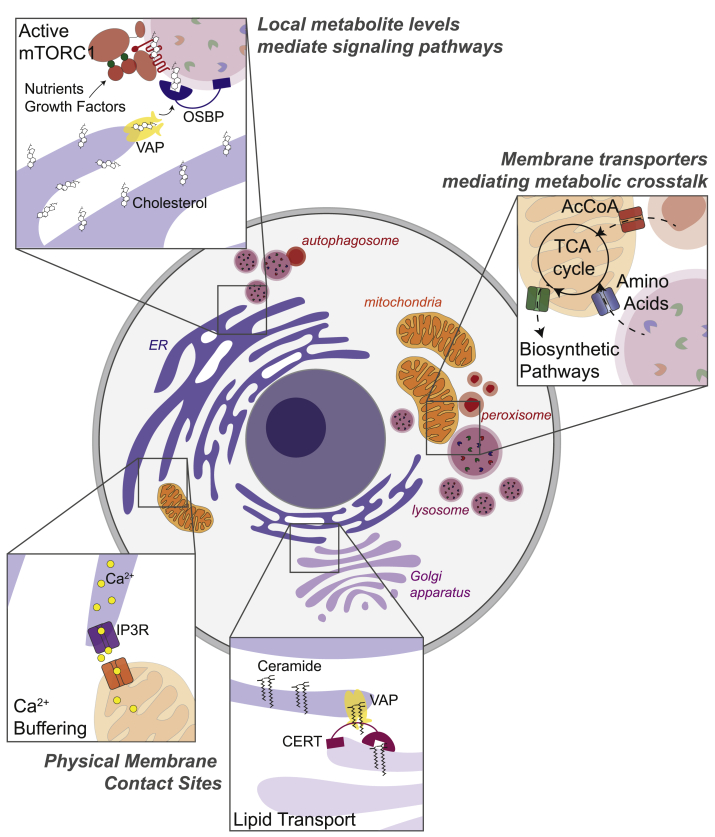
Figure 1.
Overview of inter-organelle communication pathways. Inter-organelle metabolic crosstalk is coordinated by multiple mechanisms, including i) cross-talk between solute transporters for the translocation of small, polar metabolites between organelles ii) physical membrane contact sites, notably for lipid transport and calcium signaling iii) through localized activation of signaling platforms, such as mTORC1 on the lysosomal limiting membrane, which regulates anabolism versus catabolism in response to local metabolite concentrations.
2.1. Metabolic communication via transport across organelle membranes
Regulated metabolite transport across organelle membranes is one way through which organelles facilitate complex biochemical reactions and communicate their metabolic status to one another [11]. Mitochondria, specifically, house several important biochemical pathways required to maintain cellular redox balance, to limit generation of reactive oxygen species (ROS), for calcium buffering and iron-sulfur cluster biogenesis, and to drive amino acid metabolism and fatty acid oxidation (Figure 2). Although these pathways primarily occur within the mitochondrial matrix, they require regulated exchange of substrates and products with other compartments including the cytosol, peroxisomes, the endoplasmic reticulum (ER), and lysosomes (Figure 2). Mitochondria are double membrane-bound organelles, with an inner membrane enclosing the matrix and an outer membrane separating the intermembrane space from the cytosol. The outer membrane is semi-permeable, allowing for free metabolite exchange between the intermembrane space and the cytosol. In contrast, the inner membrane is impermeable to metabolites. Therefore, a host of transporters are required to enable the import and export of the metabolites required to support the metabolic pathways enumerated above [12].
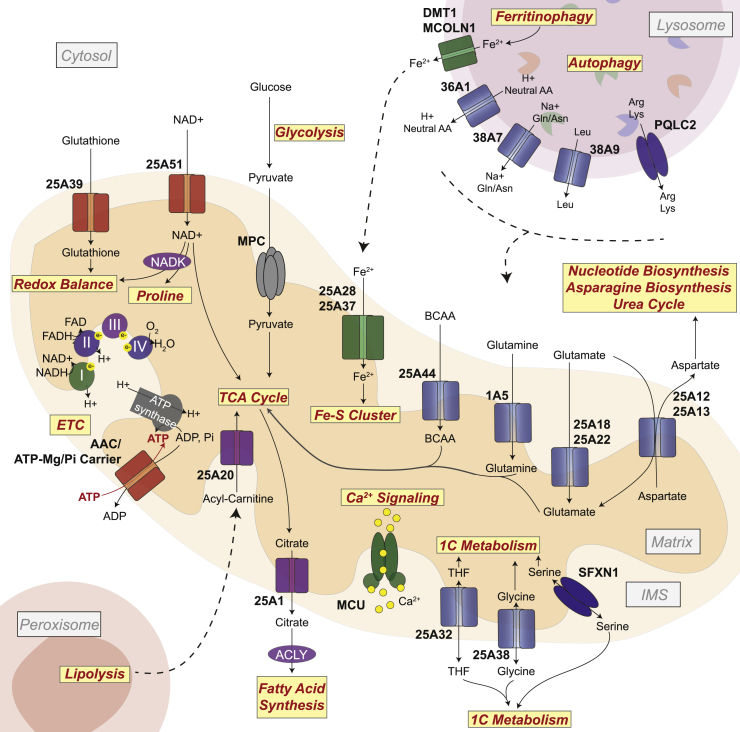
Figure 2.
Metabolic communication via transport across organelle membranes. Mitochondria are biosynthetic hubs that house several biosynthetic and catabolic pathways. Input and output metabolites of mitochondrial pathways are transported across the mitochondrial inner membrane to the intermembrane space (IMS; permeable to the cytosol) primarily via a host of SLC25-family transporters. Metabolites in the cytosol may arise from import across the plasma membrane, via cytosolic metabolic pathways, or via degradative pathways in other organelles, such as the lysosome or the peroxisome. Amino acid transporters are shown in blue, inorganic ion transporters in green, nucleotide and glutathione transporters in red, carboxylate transporters in purple and grey.
The mitochondrial carrier family (solute carrier family 25, SLC25), consisting of 53 members, is the largest solute transporter family in humans. SLC25 members transport solutes, such as amino acids, nucleotides, inorganic ions, fatty acids, and carboxylates, across the impermeable mitochondrial inner membrane [13]. Beside the SLC25 family, other uniporter complexes and the sideroflexin-family transporters also transfer various solutes across the mitochondrial inner membrane [14] (Figure 2). The molecular functions and substrate preferences of a large fraction of mitochondrial transporters have long remained mysterious. Conversely, metabolite uptake assays with purified mitochondria have suggested the existence of metabolite transporters, though their molecular identification has lagged. Recent advances in bioinformatics, proteomics and functional genomics have led to dramatic advances in our understanding of solute transport across the mitochondrial inner membrane.
2.1.1. Nucleotides
Mitochondrial adenosine diphosphate/adenosine triphosphate (ADP/ATP) carrier (AAC) imports ADP into the mitochondrial matrix, where it is converted to ATP by complex V of the electron transport chain (ETC), ATP synthase, and exports newly synthesized ATP to the cytosol to fuel energy-requiring processes [[15], [16]]. There are four AAC isoforms in humans – SLC25A4-6 and SLC25A31 – which are expressed in a tissue-dependent manner [17]. Mitochondrial adenosine may also be transported out by the ATP-Mg/Pi carriers (SLC25A23-25), which carry out the antiport of ATP-Mg (and ATP, ADP, and AMP) and Pi [18,19]. These carriers contain a calcium-regulatory domain (4 EF-hands), an amphipathic helix, and a carrier domain which transports the substrates. The EF-hands mediate calcium sensitivity, such that in the absence of Ca2+, the amphipathic helix binds to the carrier domain, inhibiting transport; conversely, in the presence of Ca2+, the EF-hands bind to the regulatory domain, allowing transport to occur [20,21].
Nicotinamide adenine dinucleotide (NAD+/NADH) is an important cofactor in mitochondria, where it provides the reducing equivalents required to connect substrate oxidation by the tricarboxylic acid (TCA) cycle to ATP production by oxidative phosphorylation (OXPHOS), mediated by the ETC. Additionally, conversion of mitochondrial NAD + to NADP + via NAD kinase 2 (NADK2) is essential for proline biosynthesis [22,23]. Mitochondrial NAD+, in mammals, was long thought to be generated by the import and conversion of nicotinamide mononucleotide (NMN) to NAD+ [24]. However, not all cell types express the mitochondrial enzymes required to convert NMN to NAD+ and stable isotope labelling experiments showed that cytosolic NAD(H) could be directly imported to mitochondria [25]. Analysis of genes coessential with ETC complex subunits and mitochondrial transporters in genome wide CRISPR/Cas9 screens uncovered SLC25A51 as a putative NAD + transporter [[26], [27], [28]]. SLC25A51-KO was associated with lower NAD + levels and overexpression of SLC25A51 in yeast mitochondria lacking endogenous nicotinamide adenine dinucleotide transporter (NDT1/NDT2) was sufficient to re-enable mitochondrial NAD + transport [26,27], a result consistent with direct transport activity.
Transport of reducing equivalents between the cytosol and mitochondria may not only occur through the transport of NAD + specifically, but also through a network of metabolic reactions, known as mitochondrial shuttles. There are two major such shuttles – the glycerol-phosphate shuttle, which invokes parts of the electron transport chain to utilize cytosolic NADH to power the conversion of flavin adenine dinucleotide (FAD) to FADH2, and the malate-aspartate shuttle, which relies on the antiport of malate and α-ketoglutarate and that of aspartate and glutamate between the cytosol and mitochondria to carry reducing equivalents from the cytosol to mitochondria, or vice versa [12]. How NAD + transport via SLC25A51 interplays with other pathways through which mitochondrial NAD + may be replenished is still an open question.
2.1.2. Amino acids
The malate-aspartate shuttle relies on SLC25A12/SLC25A13 for the import of glutamate and a proton into mitochondria and the export of aspartate. In proliferating cells, a major function of the ETC is to provide electron acceptors to boost nutrient oxidation by the TCA cycle and increase aspartate synthesis [29,30]. Aspartate synthesized in mitochondria relies on SLC25A12/SLC25A13 to be exported to the cytosol to promote various biosynthetic pathways, including nucleotide biosynthesis, redox balance, and asparagine production, required for aberrant proliferation, such as in cancer [[30], [31], [32]].
The glutamate-aspartate antiporters are also required for the urea cycle. Defects in SLC25A13 causes adult-onset type II citrullinemia – a genetic disorder associated with increased plasma ammonia, citrulline and arginine levels – due to a dysfunctional urea cycle [33]. Nitrogen released from mitochondrial amino acid de-amination reactions need to be cleared by the urea cycle. The urea cycle resides partially in mitochondria and partially in the cytosol and, to function properly, requires the one-to-one antiport of ornithine into mitochondria and, the conversion product, citrulline out of mitochondria. This transport activity is catalyzed by SLC25A15, mutations in which lead to hyperornithemia-hyperammonemia-hypercitrullinuria (HHH) syndrome [34].
Glutamine, glutamate, and branched-chain amino acids (BCAAs), valine, isoleucine, and leucine are not only nitrogen donors but also directly contribute carbons to the TCA cycle. Although the identity of mitochondrial glutamine transporters has long been a mystery, it was recently found that a variant of the plasma membrane glutamine transporter, SLC1A5 (also known as ASCT2) localizes to the mitochondrial inner membrane upon induction of expression mediated by hypoxia-inducible factor (HIF)-2α [35]. One outcome of the hypoxia program is increased glutathione (GSH) synthesis to manage ROS; upregulation of glutaminolysis by increased mitochondrial glutamine import by SLC1A5 could help support the increased requirement for GSH in solid tumors, which often display low vascularization and regions of hypoxia.
Glutamate transport from the cytosol to mitochondria relies not only on SLC25A12/SLC25A13, but also two other unidirectional glutamate transporters, SLC25A18 and SLC25A22, which are expressed in humans in a tissue-dependent manner [36]. Finally, transcriptional analysis of cold-exposed brown adipose tissue (which have upregulated BCAA uptake and catabolism to increase thermogenesis) revealed that SLC25A44 is a mitochondrial BCAA transporter [37].
One-carbon (1C) metabolism is comprised of a series of interlinking metabolic pathways that include methionine and folate cycles and altogether provide 1C (methyl) units for DNA, polyamine, amino acid, creatine, and phospholipid synthesis. Like the malate-aspartate shuttle and the urea cycle, 1C-metabolism also relies on a network of pathways residing both in the cytosol and in mitochondria. However, unlike the urea cycle, the mitochondrial and cytosolic enzymes of the 1C-metabolism pathway are seemingly redundant. Stable-isotope tracing studies suggest that mitochondria-generated formate is the main contributor to 1C units for biosynthetic pathways in cancer and that there is a specific directionality to the pathway [[38], [39], [40]]. Cytosolic serine enters mitochondria and is converted to glycine, 10-formyl-tetrahydrofolate (THF), and formate. Glycine and THF are exchanged between mitochondria and cytosol via SLC25A38 and SLC25A32 activities, respectively. A genetic screen under serine starvation in cells without cytosolic serine hydroxymethyltransferase 1 (SHMT1) revealed that sideroflexin 1 (SFXN1) is a multi-pass inner mitochondrial membrane protein that transports serine and is an integral component of 1C-metabolism [41].
Folate, a product of serine/glycine metabolism, is a vitamin cofactor required for many biochemical processes, including nucleotide, amino acid, and methyl biosynthesis; use of antifolates, such as methotrexate have thus been useful in treating cancers that rely on anabolic pathways to support proliferation [42]. Interestingly, recent studies in cancer cells grown under physiological folate concentrations revealed that there is diversity in mitochondrial vs cytosolic utilization of the 1C-pathway, largely depending on the expression and activity of SLC19A1, the plasma membrane reduced folate carrier [43].
The lysosome, as a major proteolytic compartment, in particular poses a likely source for the amino acids required for mitochondrial pathways. The limiting membrane of the lysosome contains specialized permeases that export the products of macromolecular breakdown from the lysosomal lumen to the cytosol. Examples include SLC36A1, a proton-coupled transporter for neutral amino acids [44], SLC38A7, a sodium-coupled transporter for glutamine and asparagine [45], SLC38A9, a sodium- and arginine-regulated transporter for essential amino acids such as leucine [46] and PQLC2, a uniporter for arginine and lysine [47]. This lysosome-to-mitochondria flux may be increased in KRas-driven cancers, where it provides mitochondria with several intermediates, particularly glutamine, to enable nucleotide synthesis and metabolic reprogramming [[48], [49], [50], [51]].
It is possible that close apposition between mitochondria and lysosomes (reviewed further below) may enable efficient exchange, or funneling, of metabolites between the two organelles. Such possibility has been proposed for the vacuole–mitochondria junction in yeast, the vCLAMP [52,53].
2.1.3. Inorganic ions
Mitochondria are important sites of ATP production, a process that relies on maintaining a specific membrane potential between the matrix and the intermembrane space. Both protons and inorganic ions help do this. Outside of maintaining mitochondrial membrane potential, inorganic ions are important signaling metabolites and co-factors for many mitochondrial enzymes. Since the inner membrane is impermeable to ions, specific transporters are required to import and export inorganic ions.
Phosphate, required for ATP production, is imported in by SLC25A3 in symport with a proton for ATP synthesis [54]. More recently, SLC25A3 has been implicated in transport of copper into mitochondria and cells lacking SLC25A3 show a copper-dependent defect in cyclooxygenase (COX) assembly [55,56]. Copper (Cu) has also been shown to be a modulator autophagic signaling through direct binding to the autophagy kinases, ULK1/2, and Cu-chelation therapy has been proposed to prevent autophagy-signaling in KRASG12D-driven lung tumors [57]. However, how such therapeutics would affect cellular Cu regulation and mitochondrial Cu-dependent pathways remains to be seen.
Iron, in the form of iron-sulfur clusters, is a critical cofactor for a host of TCA cycle and ETC proteins. Mitochondria import free iron via the iron transporters, SLC25A28 and SLC25A37 [58,59]. Iron is typically imported into cells via endocytosis of serum transferrin (a plasma glycoprotein that transports ferric iron) [60,61]. Once endocytosed, transferrin is proteolyzed in lysosomes (in a process known as ferritinophagy), and ferric iron (Fe3+) is converted to ferrous iron (Fe2+) by the lysosomal protein, six-transmembrane epithelial antigen of prostate 3 (STEAP3). Fe2+ is exported from lysosomes via divalent metal transporter 1 (DMT1) or mucolipin-1 (MCOLN1) and utilized by cytosolic and mitochondrial pathways. Lysosomal deacidification was associated with impaired mitochondrial respiration and TCA cycle function and, ultimately, led to non-apoptotic cell death. These effects could be rescued by iron supplementation via a non-endocytic route, suggesting a crosstalk between lysosomes and mitochondria in iron metabolism [62,63]. The importance of this iron-mobilizing pathway is further underscored by the discovery of inherited anemic syndromes resulting from mutations in DMT1 or STEAP3 [64,65].
Cells use mitochondrial calcium import to buffer cytosolic Ca2+ signals, used to regulate many different physiological processes, such as exocytosis, motility, apoptosis, and cell differentiation [66,67]. Mitochondrial Ca2+ uptake also stimulates TCA cycle dehydrogenases as well as SLC25A23-25 transporter function to catalyze increased ATP production, possibly as a mechanism to feed ATP-intensive processes that are regulated by cytosolic Ca2+ signaling [20,21,68,69]. Mitochondrial calcium buffering typically occurs at contact sites between mitochondria and the ER or plasma membrane (reviewed in more detail in a subsequent section); however, studies in isolated mitochondrial preparations suggest that mitochondria may uptake calcium even without the presence of other organellar membranes [70,71]. Comparative genomics, organelle proteomics, and cryo-EM studies revealed that the mitochondrial calcium uniporter complex is composed of four core components – the mitochondrial calcium uniporter (MCU) protein, which forms the pore [72]; the heterodimer, MICU1-MICU2, which mediates the Ca2+-dependent activation of the uniporter complex [73]; and an auxiliary subunit, essential MCU regulator (EMRE), which is essential for Ca2+ transport [74]. The mechanisms via which MICUs and EMRE mediate Ca2+-dependent gating of the MCU is the subject of intense investigation [75,76].
2.1.4. Fatty acids and carboxylates
Fatty acids and carboxylic acids are major carbon donors for mitochondrial metabolism. SLC25A20 is the carnitine/acylcarnitine carrier protein and is an important player in β-oxidation as it imports acylcarnitine into mitochondria and exports carnitine back to the cytosol [77]. SLC25A11 imports α-ketoglutarate and exports malate and is a part of the malate-aspartate shuttle [78]. Finally, the MPC1-MPC2 heterodimeric complex is an essential part of the mitochondrial pyruvate carrier, which enables pyruvate uptake into mitochondria to feed the TCA cycle [79,80].
SLC25A1 facilitates citrate export out of mitochondria, in electroneutral exchange with either another tricarboxylate, a dicarboxylate such as malate, or phosphoenolpyruvate. The citrate exported to the cytosol is primarily used by ATP-citrate lyase (ACLY), as a first step towards fatty acid synthesis [81].
The expression or activities of these transporters are often altered to meet the unique metabolic requirements of cancer cells. For instance, downregulation of MPC1 expression in multiple cancer types favors a switch to a Warburg-like metabolism characterized by lower respiration and higher glucose conversion to biomass, in turn leading to accelerated and anchorage-independent growth [82]. Furthermore, in cancer cells grown under anchorage-independent (or spheroid) conditions, SLC25A1 was shown to import citrate from the cytosol to mitochondria. This activity supported production of mitochondrial NADPH via isocitrate dehydrogenase 2 (IDH2) upon import of cytosolic citrate; NADPH production in mitochondria enabled cells growing in spheroids to mitigate mitochondrial ROS and increase growth [83].
2.1.5. Glutathione
Mitochondria are one of the most redox-active organelles and contain 10–15% of total cellular GSH pools [84,85]. The enzymes required to synthesize GSH are cytosolic, so GSH needs to be imported into mitochondria. Mitochondrial proteomics combined with pharmacological manipulation revealed that SLC25A39 (and its paralog SLC25A40) is required for mitochondrial GSH import. Interestingly, the expression of SLC25A39 only depends on GSH levels, and not oxidative stress or nuclear factor-erythroid 2-related factor 2 (NRF2) activation [86]. In another study, gene-by-gene analysis from a dual CRISPR screen showed a buffering interaction between SLC25A39 and the iron transporter, SLC25A37 [87]. Together, the two studies suggest that GSH import into mitochondria is required to maintain proper activity and stability of iron-sulfur cluster containing proteins, although the precise mechanism through which mitochondrial GSH does so is yet to be uncovered [86,87].
In summary, these recent discoveries shed light on a complex and sophisticated network of transporters, which enables communication between metabolic pathways in the mitochondrial matrix and those taking place in the cytosol, lysosome as well as other organelles (Figure 2). How the activity of this network is synchronized with that of carriers found on other organelles should be the subject of future investigation.
2.2. Metabolic communication via membrane contact sites
Whereas small, polar metabolites can readily diffuse and be exchanged between organelles, lipid exchange poses unique challenges due to their hydrophobic nature. Transfer of sterols, phospholipids and sphingolipids between organelles typically requires the establishment of physical junctions known as membrane contact sites (MCSs), and the presence at MCSs of specialized lipid-binding proteins that shield their lipid substrate molecules from the aqueous environment of the cytosol [88]. Interestingly, MCSs do not solely facilitate lipid transfer, as they also participate in the exchange of ions such as Ca2+. Ca2+ ions are polar and readily soluble, thus their transfer via MCSs may reflect the need for efficient channeling from the donor to the acceptor organelle to maintain proper concentration gradients and avoid damaging side-effects. Transfer of lipids and Ca2+ ions offer clear examples of inter-organelle metabolic communication.
2.2.1. Membrane contact sites mediated by lipid transport proteins
MCSs are regions where membranes of two organelles are held together within 10–30 nm of each other. One of the first evidence of MCSs were electron microscopy images of closely apposed ER and mitochondria [89], which were later followed by biochemical evidence of phospholipid transfer between the two compartments [90]. While MCSs can occur between any organelles, the most prevalent contact sites involve the ER. This is likely due to two major reasons: first, the ER is an extensive network that reaches virtually all parts of the cytoplasm, making it easily available to establish contacts with a variety of organelles; second, the ER is a major site of lipid synthesis, and contact sites are efficient routes of transport for lipids and their biosynthetic intermediates. The second reason is further supported by the observation that most proteins involved in mediating MCSs also contain lipid transport domains [91].
A major class of ER-organelle contacts are mediated by the vesicle-associated membrane protein (VAMP)-associated proteins (VAPs), which include VAPA/B and MOSPD1/2/3. The VAPs are inserted in the ER membrane via their C-terminal region, and they harbor an N-terminal Major Sperm Protein (MSP) globular domain, which binds to an evolutionarily conserved peptide sequence motif known as diphenylalanine [FF] in an acidic tract (FFAT; the consensus amino acid sequence being EFFDAxE) [92,93]. A multitude of lipid transport proteins (LTPs) bind to ER-localized VAPs via their respective FFAT motifs [91]. Many VAP-interacting LTPs contain pleckstrin homology (PH) domains, through which they bind to specific phosphoinositides, such as phosphatidylinositol 4-phosphate (PI4P), on the opposite organellar membrane. Thus, simultaneous binding to VAPs and PI4P enables the LTPs to span the distance between the two adjacent membranes. LTPs also contain lipid transfer domains, which, depending on the structure, may belong to one of several distinct domains known to transport specific lipid species [91,94]. With the help of these lipid transfer domains, LTPs can transport three major lipid types – phospholipids, sphingolipids, or sterols – in a directional manner.
Among VAP-binding phospholipid transfer proteins are phosphatidylcholine (PC)-transfer protein (PCTP) and Sec14 (in yeast)/phosphatidylinositol (PI)-transfer protein (PITP; in mammals). In some cases, it is possible for PITPs to bind either PI or PC. For example, Nir2, an LTP that links the Golgi apparatus with the ER, was shown to sense PC levels on the Golgi membrane and regulate the consumption of diacylglycerol via the CDP-choline pathway for PC biosynthesis [95]. Sec14, in yeast, was also shown to have a differential binding for PC and PI, suggesting distinct sensing versus transporting functions for these phospholipid transfer proteins [96].
Cholesterol transport is typically mediated by the oxysterol binding protein (OSBP)-related protein (ORP) family. ORP family proteins contain an OSBP-related ligand-binding domain (ORD), which can bind to cholesterol, oxysterols, and phospholipids. The founding member of the ORP family, OSBP1, localizes to ER-Golgi MCSs, where it transfers ER-derived cholesterol to the Golgi, while transporting PI4P in the opposite, Golgi-to-ER direction [97]. The counter-transport of cholesterol and PI4P is thought to be energetically coupled: PI4P, which is synthesized on Golgi membranes by the type-III PI4-kinase, is transported by OSBP to the ER, where it is hydrolyzed to phosphatidylinositol by the Sac1 phosphatase. The PI4P concentration gradient, in turn, energizes the delivery of cholesterol from the ER to the Golgi via alternating, coupled transport cycles [97,98].
Not all ORPs transport cholesterol. Lipidomic characterization of several yeast ORPs (known as oxysterol-binding homology, Osh, proteins) complexed with their native lipid substrates revealed that two members of this family had specificity for phosphatidylserine (PS). Phylogenetic analysis and liposome-based lipid transfer assays suggested that human ORPs, including ORP5 and ORP10, transfer PS from the ER to the plasma membrane [99].
Ceramide-transfer protein (CERT) and glycolipid-transfer protein, phosphatidylinositol-four-phosphate adaptor protein-2 (FAPP2) are two examples of sphingolipid transfer proteins [100,101]. Along with an FFAT domain and a PH domain, CERT mediates ceramide transfer at ER-Golgi MCSs via a steroidogenic acute regulatory protein (StAR)-related transfer (START) domain in its C-terminal region [100]. CERT mediates efficient transfer of C14–C20 ceramides, but not those of longer acyl chains [102]. FAPP2, on the other hand, transports glucosyl-ceramide, and both CERT and FAPP2 are essential for glycosphingolipid synthesis. As with CERT, FAPP2 is targeted to the Golgi through a PH-domain mediated interaction with PI-4P, highlighting the role of PI metabolism in sphingolipid synthesis [101].
A recurrent mutation in the MSP domain of human VAPB, P56S, is associated with an autosomal dominant form of a degenerative motor neuron disease, amyotrophic lateral sclerosis (ALS) [103]. However, it remains unclear to what degree motor neuron death is attributable to a toxic gain of function of the mutant protein, versus loss of VAPB-mediated membrane contacts and general ER homeostasis.
Mutations in LTPs may also result in a host of diseases. VPS13 are a group of glycerolipid transfer proteins that localize to various organelles, including mitochondria, late-endosome/lysosome, and Golgi, and have been shown to bind to ER-associated VAPs via FFAT domains. Loss-of-function mutations in VPS13 homologs are associated with many neurological disorders, including chorea acanthocytosis, Cohen syndrome, Parkinson's disease, and ataxia [104,105]. How the different VPS13 proteins lead to the varied neurodegenerative disorders enumerated above is still an open question. For example, mutations in VPS13C, which was shown to localize to lysosomal membranes, results in mitochondrial dysfunction associated with Parkinson's disease [105]. Whether and how this mitochondrial phenotype may result from dysfunction of Vps13C-defective endolysosomes remains to be determined.
In other situations, mutations in LTPs may lead to disruptions in overall lipid metabolism and membrane compositions. For example, mutations in CERT are associated with global developmental delay and intellectual disability [106]. Although ceramide levels were not measured in the patient studies, disruptions in sphingolipid metabolism are known to alter plasma membrane lipid composition and membrane organization [107]; such alterations may not only lead to changes in cell signaling, but also neurodegenerative diseases, such as Alzheimer's [108].
2.2.2. Mitochondria-lysosome contacts
Mitochondria and lysosomes make contacts in healthy cells distinct from both mitophagy-related and MDV-related contacts [109]. Formation of these contacts was shown to be promoted by active, GTP-bound, lysosomal RAB7. On the other hand, recruitment of TBC1D15, a RAB7 GTPase-activating protein (GAP), to mitochondria promoted GTP hydrolysis and released mitochondria-lysosome contacts. TBC1D15 recruitment depended on the mitochondrial fission-related protein, FIS1 [109]. In part due to the varied role that RAB7 plays in vesicle trafficking, it is possible that changes in endolysosomal biology may impact mitochondria-lysosome contacts via RAB7. Consistently, several lysosomal storage disorders lead to dysregulation of mitochondria-lysosome contacts, primarily due to changes in active, GTP-bound RAB7 levels [[110], [111], [112]]. Although transport of specific metabolites has yet to be uncovered at mitochondria-lysosome contacts in mammalian cells, it is tempting to speculate that physical coupling of lysosomes and mitochondria may favor the hand-off of lysosomal digestion products from lysosomal permeases to the many transporters located at the mitochondrial inner membrane.
2.2.3. Calcium transfer at membrane contact sites
The ER lumen is also the site for intracellular Ca2+ stores. Depletion of these stores drives the store-operated Ca2+ entry (SOCE) process via selective Ca2+ channels, such as the Ca2+ release-activated Ca2+ (CRAC) channel. This process occurs at small areas of the cell surface that are adjacent to junctional ER. Specifically, store depletion causes Orai1, which comprise the CRAC channel, to accumulate at these ER-plasma membrane sites; Orai1 channels are then activated by the ER membrane protein, stromal interacting molecule 1 (STIM1), which acts as a Ca2+ sensor [113,114]. Intracellular Ca2+ stores are tightly regulated, and mitochondria act as buffers for maintaining Ca2+ concentrations in the cytosol [115]. Mitochondria-associated ER membranes (MAMs) are the sites for the control of Ca2+ flux between the ER and mitochondria; at these microdomains, mitochondria are closely apposed to the ER domains enriched for the inositol 1,4,5-triphosphate (IP3)-sensitive Ca2+ channel on the ER [116].
Lipid species, including glycosphingolipids (GSLs), can regulate the architecture of Ca2+-regulating membrane systems. Deficiency in the lysosomal hydrolase, β-galactosidase (β-gal), leads to cellular buildup of the sialic acid-containing GSL, GM1-ganglioside. In turn, excess GM1-ganglioside accumulates in microdomains within MAMs, leading to increased Ca2+ flux into mitochondria, followed by Ca2+-associated opening of the mitochondrial permeability transition pore (PTP), and, finally, increased apoptosis [117]. Treatment with a cytosolic Ca2+ chelator or a drug that prevented PTP opening protected β-gal−/− MEFs or β-gal+/+ MEFs loaded with GM1 from cell death.
2.3. Localized signaling pathways coordinate metabolism
2.3.1. Mechanistic target of rapamycin complex 1 (mTORC1)
In addition to the localized nature of metabolic reactions and substrate exchange, it is increasingly appreciated that signaling pathways that control these metabolic reactions are themselves highly localized to specific organelle compartments. This compartmentalization is key to the ability of signaling factors to couple different inputs to specific metabolic outputs.
A prominent example of a compartmentalized metabolic signaling pathway is the master growth regulator, mTORC1 protein kinase [118,119]. In response to a wide variety of signals such as nutrients, growth factors, ATP, and oxygen, mTORC1 promotes anabolic processes that lead to cell mass accumulation, an obligate prerequisite for cell division, while actively suppressing catabolic processes that consume cell mass. In both yeast and metazoans, mTORC1 activation occurs at a specific cellular location – the limiting membrane of the lysosome [120,121]. Local nutrients, including amino acids, glucose, and cholesterol, trigger the physical recruitment of mTORC1 from the cytosol to the lysosomal membrane, whereas long-range signals brought by growth factors turn on the kinase activity of mTORC1. This ‘coincidence detection’ mechanism ensures that mTORC1 only triggers its downstream programs when conditions are favorable for growth [118,119].
The nutrient-dependent recruitment of mTORC1 to the lysosomal membrane is mediated by a sophisticated scaffolding complex centered on the heterodimeric Rag guanosine triphosphatases (GTPases), composed of RagA or RagB in complex with RagC or RagD [122,123]. Dedicated nutrient sensors for amino acids such as leucine, arginine, methionine, as well as cholesterol and glucose, control the nucleotide loading state of the Rag GTPase dimer [118,119]. In high nutrients, GTP-loaded RagA and GDP-loaded RagC physically bind to mTORC1 and stabilize it at the lysosomal membrane. A second GTPase, Rheb, interacts with mTORC1 at the lysosomal surface and allosterically promotes its kinase activation [124]. In turn, the mTORC1-activating, GTP-loaded form of Rheb requires the presence of growth factors and oxygen [118,119]. Thus, the coincidence detection mechanism for mTORC1 activation ultimately converges on the nucleotide loading state of the Rag and Rheb GTPases.
Triggering of mTORC1 kinase activity leads to pro-growth phosphorylation events of substrates such as S6-kinase 1 (S6K1), leading to upregulation of anabolic processes such as glycolysis, nucleotide and lipid synthesis, and 4E-binding proteins (4EBPs), triggering upregulation of protein synthesis. Moreover, catabolic processes such as autophagy initiation and lysosome biogenesis are inhibited via mTORC1-dependent phosphorylation of Unc-51 related kinases (ULK) and transcription factors EB and E3 (TFEB and TFE3) [118,119]. Phosphorylation of these as well as other substrates enable mTORC1 to affect metabolic processes occurring both at the lysosome and in other compartments, including ribosome biogenesis in the nucleus/nucleolus; the pentose phosphate pathway for nucleotide synthesis in the cytosol [125]; de novo lipid and sterol synthesis at the ER [125]; changes in mitochondrial biogenesis, dynamics and function [[126], [127], [128]]; and secretory activity at the Golgi [129].
Not only does lysosomal mTORC1 signaling regulate metabolic processes in other cellular compartments, the status of various cellular organelles also feeds back on mTORC1 activity. Low ADP/ATP ratio resulting from either glucose deprivation or mitochondrial dysfunction suppresses mTORC1 activity via the energy sensing kinase, AMPK [130,131]. In low energy states, AMPK phosphorylates the mTORC1 subunit, Raptor, as well as Tuberous Sclerosis Complex 2 (TSC2), a GTPase activating protein (GAP) for Rheb, leading to inhibition of mTORC1 kinase activity. Along with AMPK, the Heme-regulated eIF2α (HRI) kinase was recently shown to mediate mTORC1 inhibition downstream of mitochondrial stressors. HRI activates the ATF4 transcription factor, which in turn upregulates the expression of negative regulators of mTORC1 signaling, including Sestrin2 and REDD1 [132,133].
mTORC1 may also sense the status of other organelles via MCSs. The OSBP sterol carrier, which primarily resides at the ER-Golgi interface, was also shown to localize at contacts between the ER and endolysosomes via binding to PI4P generated by type II PI4-kinase on these compartments [134,135]. The OSBP-VAP complex was shown to transport, in the ER-to-lysosome direction, a pool of cholesterol that is essential for mTORC1 recruitment to the lysosome and activation of its downstream programs [134]. Because the ER is a site for the energetically expensive de novo synthesis of cholesterol, sensing of ER-derived cholesterol may provide another route to communicate cellular energy status to mTORC1.
Other metabolism-regulating kinases also display organelle-specific localization. AMPK exists in distinct cellular pools that localize to different organelles due to the unique regulatory subunit they contain [136]. A lysosome-localized AMPK pool interacts with multiple protein complexes involved in mTORC1 signaling, such as the FLCN-FNIP complex and the Ragulator-V-ATPase [[137], [138], [139]]. Lysosomal AMPK may play an especially important role in coupling cellular energy levels to lysosomal mTORC1 signaling, and it could also enable feedback regulation of AMPK signaling by lysosome-bound factors.
As reviewed above, mitochondria and lysosomes are essential for cellular metabolism and, simultaneously, contribute to signaling pathways that regulate cellular processes such as autophagy and biosynthesis. Pathological states that perturb lysosomes are often associated with mitochondrial damage and vice versa [140]. For example, lysosomal cholesterol accumulation in the neurodegenerative disease, Niemann-Pick type C (NPC), leads to aberrant cholesterol-mTORC1 signaling, impaired lysosomal proteolysis and mitophagy, and, ultimately, defective mitochondrial morphology and function [141,142]. Conversely, in a mouse model for mitochondrial myopathy (MM), caused by mutation in the mitochondrial replicative helicase, Twinkle, the resulting ETC deficiency increased mTORC1 activation and led to a multifaceted stress response, including upregulation of metabolic cytokines, remodeling of 1C metabolism and the mitochondrial unfolded protein response [143]. In both cases, pharmacological inhibition of mTORC1 corrected the disease phenotypes (mitochondrial function and lysosomal proteolysis in NPC, chronic stress response in MM, respectively), suggesting that, in these cases, aberrant mTORC1 signaling drove the disruption of organelle homeostasis [141,143].
2.3.2. Cyclic AMP (cAMP)-dependent protein kinase (PKA)
PKA, a eukaryotic Ser/Thr kinase that modulates diverse metabolic processes including lipogenesis and lipolysis, glucose homeostasis and mitochondrial respiration, is another prominent example of localized metabolic signaling [144]. PKA is composed of two regulatory subunits (RI or RII), which dimerize and bind to one catalytic subunit (C) each, blocking their kinase function. Physiological stimuli, including various hormones, trigger a raise in the intracellular concentration of cAMP, which is generated from ATP by adenylyl cyclase (AC) enzymes. In turn, cAMP binds to the PKA regulatory subunit with nanomolar affinity, triggering a conformational change that releases the C subunits from the inactive R2C2 complex, allowing them to phosphorylate nearby substrates [144,145]. Several distinct pools of PKA are bound to distinct membrane compartments via interaction of the R subunit dimer with AKAPs. 12 different AKAP proteins anchor the R2C2 complex to different membranes including the plasma membrane, mitochondria, ER, endolysosomes and lipid droplets [146]. This anchoring has important consequences on the dynamics and function of the respective organelles. For example, targeting of PKA to lipid droplets enables PKA-dependent phosphorylation of perilipin proteins, a triggering event in lipolysis [147,148]. Interestingly, several AKAPs harbor conserved FFAT-like motifs and thus bind to VAP proteins, suggesting that PKA may be activated at membrane contact sites, and that specific signals found at these structures could give rise to unique PKA-dependent signaling outputs [149].
3. Emerging paradigms for inter-organelle communication
3.1. Mitochondria-derived compartments in regulating metabolite transporters and protein quality control
Although mitochondria have long thought to be excluded from vesicular transport pathways that connect many other organelles, recent evidence suggests that mitochondria can release vesicles with unique protein compositions that enable adaptive processes. For example, in yeast, mitochondria-derived compartments or vesicles (MDCs/MDVs) sequestered SLC25A metabolite carriers away from the rest of the mitochondrial network in response to increased intracellular amino acid levels (Figure 3A). Loss of the proteins that facilitated MDV formation and vacuolar dysfunction was associated with impaired survival upon amino acid overload [150]. MDVs were also shown to be transport carriers for selective degradation of oxidized mitochondrial proteins via delivery to lysosomes, facilitating mitochondrial quality control (Figure 3A) [151,152]. Proteomic characterization of isolated MDVs shed light on some of the molecular players involved in this selective mitochondrial quality control process; however, more work is required to delineate the mechanisms through which specific mitochondrial proteins may get sequestered and removed under varying metabolic conditions [153].
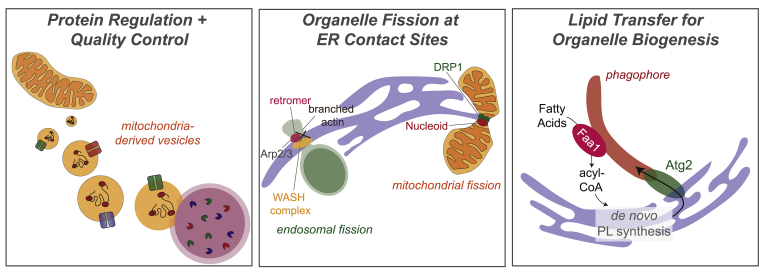
Figure 3.
Emerging paradigms for inter-organelle communication. Left: Mitochondria-derived vesicles (MDVs) may carry oxidized proteins or mitochondrial solute transporters to remove damaged proteins and regulate metabolite import to mitochondria, respectively. MDVs may fuse to lysosomes, leading to degradation of their contents. Middle: ER-mediated organelle fission. The endosomal sorting domains consist of the retromer complex, which interacts with sorting nexins, and is involved in cargo sorting. FAM21 binds to the retromer complex and recruits WASH complex components, which stimulates actin polymerization via Arp2/3 to help segregate select cargos. ER tubules are then recruited to these sorting domains and drive endosomal fission events. During mitochondrial fission, DRP1 is recruited and self-assembles at ER-mitochondria contacts. Nucleoids, for mtDNA replication, have also shown to associate with these sites. Right: Organelle biogenesis involves transfer of large amounts of lipid species. In the case of phagophore formation, phagophore-localized Faa1 may channel acyl-CoA to the ER for phospholipid (PL) synthesis. Newly synthesized PLs may mediate phagophore nucleation via Atg proteins, including Atg2.
3.2. Endosomal and mitochondrial fission at ER-organelle contact sites
ER tubules mark fission sites on early endosomes [154]. ER tubules crossed over endosomal domains marked by the Wiskott Aldrich Syndrome protein and scar homologue (WASH) complex, which is a multiprotein complex that activates actin nucleation on endosomal sorting domains, both for cargo sorting and for catalyzing fission [154]. ER-mitochondria contacts also often mark the sites for mitochondrial division. Cytosolic dynamin-related guanosine triphosphatase (GTPase)-1 (DRP1), is recruited and self-assembles at the ER-mitochondria contacts, where mitochondrial fission is observed [155].
Mitochondrial DNA (mtDNA) synthesis was spatially linked to the subset of ER-mitochondria contact sites that were coupled to mitochondrial fission [156]. Similarly, the yeast-specific ER-mitochondria encounter structure (ERMES) complex was also spatially linked to nucleoids (mtDNA-protein complexes), suggesting a conserved role for ER-mitochondria contacts in regulating mtDNA maintenance [157]. Fractionation of mitochondrial membrane preparations combined with lipidomic analysis showed that nucleoids were associated with cholesterol-enriched membranes, which are also abundant at ER-mitochondria contacts [158]. Additionally, given that the ER is a major site of phospholipid synthesis, it is possible that direct transfer of lipids from the ER to either mitochondria or endosomes at contact sites enables high membrane curvature to promote fission. Together, these observations suggest that the lipid composition at ER-organelle contacts may play an important role in organelle fission, and specifically in regulating cargo sorting within endosomes and mtDNA replication within mitochondria [154,155].
3.3. Lipid mobilization for organelle biogenesis
Making new membranes for organelle biogenesis requires transfer of large amounts of lipid, such as cholesterol and phospholipids. Key proteins mediate the transfer of these lipids from lipid sources, such as the ER, to the nucleating organelle membrane. An especially interesting paradigm is provided by autophagosome formation, which plays key roles in cellular metabolism and quality control [159]. Autophagosome formation requires the expansion of a newly synthesized isolation membrane (IM) from endomembrane-derived lipids. One of the 18 Atg proteins required for this process, Atg2, tethers the edge of the expanding IM to the ER. X-ray crystallography studies showed that Atg2 has a hydrophobic cavity that is structurally like that of Vps13, which can accommodate phospholipid acyl chains. In vitro, Atg2 mediated lipid transfer between small-sized liposomes, suggesting an important role for this protein for transferring phospholipids from the ER to the expanding IM [[160], [161], [162]].
Spatial localization of lipid synthesis enzymes is also important for membrane formation. Acyl-CoA synthetases (Faa1 and Faa4) accumulate on nucleating phagophores and channel activated fatty acids into de novo phospholipid synthesis within proximal ER. Newly synthesized phospholipids were critical for driving the assembly and elongation of phagophore membranes into autophagosomes [163].
Lipid mobilization for membrane biogenesis plays an equally important role in both health and disease. Enteroviral pathogenesis requires RNA genome replication in membrane-separated compartments, known as replication compartments (RCs). Generation of RCs requires host lipids, primarily originating from lipid droplets. Specific enteroviral proteins form membrane contact sites to facilitate mobilization of fatty acids from lipid droplets to viral RCs. This process depended on host cell lipolysis, blocking which, using inhibitors such as Atglistatin and CAY10499, led to decreased enteroviral replication [164].
4. Concluding remarks
Metabolic coordination between organelles is pivotal for cellular function. Although many advances have been made in determining the factors that mediate metabolic and physical communication between organelle pairs, much remains to be discovered. Recent advances in organelle isolation coupled with mass spectrometry is shedding light on the lipid and polar metabolite contents of organelles, and how it changes under different physiological and pathological conditions [141,[165], [166], [167]]. Proximity labeling with intact and split promiscuous biotin ligase (BirA) promise to uncover new tethering factors and metabolite transporters that selectively operate at MCSs [168,169]. Technologies such as in situ mass spectrometry imaging, isotopic labelling and fluorescent or genetically encoded RNA-based metabolite sensors will help reveal the flux of polar metabolites between different organelles [[170], [171], [172]]. Combined with functional genomics, in vitro reconstitution, and organelle-specific proteomics in different cells and tissues [173,174], these in situ labeling techniques may lead to the discovery of new pathways, or branches of known pathways, that ensure coordination of organelle function across a range of physiological states. Identifying the molecular players involved in mediating organellar metabolic coordination and the signaling repercussions of local metabolic disruptions will not only further our understanding of cellular metabolism in health, but also lead us to design better therapeutics against dysregulated metabolism in diseases, such as cancer and neurodegeneration.
Acknowledgements
This work was supported by NIH R01GM127763 and the Edward Mallinckrodt, Jr. Foundation Scholar Award to R.Z. We apologize to colleagues whose work we were not able to cite due to space limitations.
Conflict of interest
Regarding submission of review article entitled ‘Inter-Organelle Communication as a Driver of Metabolic Regulation and Cellular Homeostasis’ by Aakriti Jain as first author and Roberto Zoncu as senior author, the authors declare the following conflicts of interest: R.Z. is co-founder, stockholder and advisor for Frontier Medicines, corp.
References
- 1.Zecchin A., Stapor P.C., Goveia J., Carmeliet P. Metabolic pathway compartmentalization: an underappreciated opportunity? Current Opinion in Biotechnology. 2015;34:73–81. doi: 10.1016/j.copbio.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Diekmann Y., Pereira-Leal J.B. Evolution of intracellular compartmentalization. Biochemical Journal. 2013;449:319–331. doi: 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- 3.Ovádi J., Saks V. On the origin of intracellular compartmentation and organized metabolic systems. Molecular and Cellular Biochemistry. 2004;256–257:5–12. doi: 10.1023/b:mcbi.0000009855.14648.2c. [DOI] [PubMed] [Google Scholar]
- 4.Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett A.J. Human cathepsin B1. Purification and some properties of the enzyme. Biochemical Journal. 1973;131:809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dansen T.B., Wirtz K.W., Wanders R.J., Pap E.H. Peroxisomes in human fibroblasts have a basic pH. Nature Cell Biology. 2000;2:51–53. doi: 10.1038/71375. [DOI] [PubMed] [Google Scholar]
- 7.Kerner J., Hoppel C. Fatty acid import into mitochondria. Biochimica et Biophysica Acta. 2000;1486:1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 8.Wellen K.E., Snyder N.W. Should we consider subcellular compartmentalization of metabolites, and if so, how do we measure them? Current Opinion in Clinical Nutrition and Metabolic Care. 2019;22:347–354. doi: 10.1097/MCO.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer A.J. Channeling of ammonia from glutaminase to carbamoyl-phosphate synthetase in liver mitochondria. FEBS Letters. 1985;191:249–251. doi: 10.1016/0014-5793(85)80018-1. [DOI] [PubMed] [Google Scholar]
- 10.Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. Journal of Biological Chemistry. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 11.César-Razquin A., Snijder B., Frappier-Brinton T., Isserlin R., Gyimesi G., Bai X., et al. A call for systematic research on solute carriers. Cell. 2015;162:478–487. doi: 10.1016/j.cell.2015.07.022. (PMID: 26232220) [DOI] [PubMed] [Google Scholar]
- 12.Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology. 2018;20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruprecht J.J., Kunji E.R.S. The SLC25 mitochondrial carrier family: structure and mechanism. Trends in Biochemical Sciences. 2020;45:244–258. doi: 10.1016/j.tibs.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham C.N., Rutter J. 20,000 picometers under the OMM: diving into the vastness of mitochondrial metabolite transport. EMBO Reports. 2020;21 doi: 10.15252/embr.202050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmieri F., Klingenberg M. On the possible role of structural protein in the binding and translocation of adenine nucleotides in mitochondria. Biochimica et Biophysica Acta. 1967;131:582–585. doi: 10.1016/0005-2728(67)90018-7. [DOI] [PubMed] [Google Scholar]
- 16.Aquila H., Misra D., Eulitz M., Klingenberg M. Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 1982;363:345–349. [PubMed] [Google Scholar]
- 17.Dolce V., Scarcia P., Iacopetta D., Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Letters. 2005;579:633–637. doi: 10.1016/j.febslet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Fiermonte G., De Leonardis F., Todisco S., Palmieri L., Lasorsa F.M., Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. Journal of Biological Chemistry. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- 19.Traba J., Satrústegui J., del Arco A. Characterization of SCaMC-3-like/slc25a41, a novel calcium-independent mitochondrial ATP-Mg/Pi carrier. Biochemical Journal. 2009;418:125–133. doi: 10.1042/BJ20081262. [DOI] [PubMed] [Google Scholar]
- 20.Harborne S.P.D., King M.S., Crichton P.G., Kunji E.R.S. Calcium regulation of the human mitochondrial ATP-Mg/Pi carrier SLC25A24 uses a locking pin mechanism. Scientific Reports. 2017;7:45383. doi: 10.1038/srep45383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harborne S.P.D., Ruprecht J.J., Kunji E.R.S. Calcium-induced conformational changes in the regulatory domain of the human mitochondrial ATP-Mg/Pi carrier. Biochimica et Biophysica Acta. 2015;1847:1245–1253. doi: 10.1016/j.bbabio.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran D.H., Kesavan R., Rion H., Soflaee M.H., Solmonson A., Bezwada D., et al. Mitochondrial NADP+ is essential for proline biosynthesis during cell growth. Nature Metabolism. 2021;3:571–585. doi: 10.1038/s42255-021-00374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J., Schwörer S., Berisa M., Kyung Y.J., Ryu K.W., Yi J., et al. Mitochondrial NADP(H) generation is essential for proline biosynthesis. Science. 2021;372:968–972. doi: 10.1126/science.abd5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikiforov A., Dölle C., Niere M., Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. Journal of Biological Chemistry. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davila A., Liu L., Chellappa K., Redpath P., Nakamuru-Ogiso E., Paolella L.M., et al. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. Elife. 2018;7 doi: 10.7554/eLife.33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luongo T.S., Eller J.M., Lu M., Niere M., Raith F., Perry C., et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature. 2020;588:174–179. doi: 10.1038/s41586-020-2741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kory N., Uit de Bos J., van der Rijt S., Jankovic N., Güra M., Arp N., et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Science Advances. 2020;6:eabe5310. doi: 10.1126/sciadv.abe5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girardi E., Agrimi G., Goldmann U., Fiume G., Lindinger S., Sedlyarov V., et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat. Commun. 2020;11:6145. doi: 10.1038/s41467-020-19871-x. (PMID: 33262325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birsoy K., Wang T., Chen W.W., Freinkman E., Abu-Remaileh M., Sabatini D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162:540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan L.B., Gui D.Y., Hosios A.M., Bush L.N., Freinkman E., Vander Heiden M.G. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son J., Lyssiotis C.A., Ying H., Wang Z., Hua S., Ligorio M., et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krall A.S., Mullen P.J., Surjono F., Momcilovic M., Schmid E.W., Halbrook C.J., et al. Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metabolism. 2021;33:1013–1026.e6. doi: 10.1016/j.cmet.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K., Sinasac D.S., Iijima M., Boright A.P., Begum L., Lee J.R., et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nature Genetics. 1999;22:159–163. doi: 10.1038/9667. [DOI] [PubMed] [Google Scholar]
- 34.Camacho J.A., Mardach R., Rioseco-Camacho N., Ruiz-Pesini E., Derbeneva O., Andrade D., et al. Clinical and functional characterization of a human ORNT1 mutation (T32R) in the hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome. Pediatric Research. 2006;60:423–429. doi: 10.1203/01.pdr.0000238301.25938.f5. [DOI] [PubMed] [Google Scholar]
- 35.Yoo H.C., Park S.J., Nam M., Kang J., Kim K., Heo Y., et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metabolism. 2020;31:267–283.e12. doi: 10.1016/j.cmet.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Fiermonte G., Palmieri L., Todisco S., Agrimi G., Palmieri F., Walker J.E. Identification of the mitochondrial glutamate transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. Journal of Biological Chemistry. 2002;277:19289–19294. doi: 10.1074/jbc.M201572200. [DOI] [PubMed] [Google Scholar]
- 37.Yoneshiro T., Wang Q., Tajima K., Matsushita M., Maki H., Igarashi K., et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–619. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis C.A., Parker S.J., Fiske B.P., McCloskey D., Gui D.Y., et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Molecular Cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ducker G.S., Chen L., Morscher R.J., Ghergurovich J.M., Esposito M., Teng X., et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metabolism. 2016;23:1140–1153. doi: 10.1016/j.cmet.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y., Ling T., Lee G., Paddock M.N., Momb J., Cheng Z., et al. Mitochondrial one-carbon pathway supports cytosolic folate integrity in cancer cells. Cell. 2018;175:1546–1560.e17. doi: 10.1016/j.cell.2018.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kory N., Wyant G.A., Prakash G., Uit de Bos J., Bottanelli F., Pacold M.E., et al. SFXN1 is a mitochondrial serine transporter required for one-carbon metabolism. Science. 2018;362 doi: 10.1126/science.aat9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman I.D., Chattopadhyay S., Zhao R., Moran R. The antifolates: evolution, new agents in the clinic, and how targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Current Opinion in Investigational Drugs (London England 2000) 2010;11:1409–1423. [PubMed] [Google Scholar]
- 43.Lee W.D., Pirona A.C., Sarvin B., Stern A., Nevo-Dinur K., Besser E., et al. Tumor reliance on cytosolic versus mitochondrial one-carbon flux depends on folate availability. Cell Metabolism. 2021;33:190–198.e6. doi: 10.1016/j.cmet.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Sagné C., Agulhon C., Ravassard P., Darmon M., Hamon M., El Mestikawy S., et al. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdon Q., Boonen M., Ribes C., Jadot M., Gasnier B., Sagné C. SNAT7 is the primary lysosomal glutamine exporter required for extracellular protein-dependent growth of cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E3602–E3611. doi: 10.1073/pnas.1617066114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyant G.A., Abu-Remaileh M., Wolfson R.L., Chen W.W., Danai L.V., Vander Heiden M.G., et al. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell. 2017;171:642–654.e12. doi: 10.1016/j.cell.2017.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leray X., Conti R., Li Y., Debacker C., Castelli F., Fenaille F., et al. Arginine-selective modulation of the lysosomal transporter PQLC2 through a gate-tuning mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2021;118 doi: 10.1073/pnas.2025315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo J.Y., Teng X., Laddha S.V., Ma S., Van Nostrand S.C., Yang Y., et al. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes & Development. 2016;30:1704–1717. doi: 10.1101/gad.283416.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sousa C.M., Biancur D.E., Wang X., Halbrook C.J., Sherman M.H., et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perera R.M., Stoykova S., Nicolay B.N., Ross K.N., Fitamant J., et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361–365. doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strohecker A.M., Guo J.Y., Karsli-Uzunbas G., Price S.M., Chen G.J., Mathew R., et al. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discovery. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hönscher C., Mari M., Auffarth K., Bohnert M., Griffith J., Geerts W., et al. Cellular metabolism regulates contact sites between vacuoles and mitochondria. Developmental Cell. 2014;30:86–94. doi: 10.1016/j.devcel.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Elbaz-Alon Y., Rosenfeld-Gur E., Shinder V., Futerman A.H., Geiger T., Schuldiner M., et al. A dynamic interface between vacuoles and mitochondria in yeast. Developmental Cell. 2014;30:95–102. doi: 10.1016/j.devcel.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Fiermonte G., Dolce V., Palmieri F. Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. Journal of Biological Chemistry. 1998;273:22782–22787. doi: 10.1074/jbc.273.35.22782. [DOI] [PubMed] [Google Scholar]
- 55.Boulet A., Vest K.E., Maynard M.K., Gammon M.G., Russell A.C., Mathews A.T., et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. Journal of Biological Chemistry. 2018;293:1887–1896. doi: 10.1074/jbc.RA117.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X., Boulet A., Buckley K.M., Philips C.B., Gammon M.G., Oldfather L.E., et al. Mitochondrial copper and phosphate transporter specificity was defined early in the evolution of eukaryotes. Elife. 2021;10 doi: 10.7554/eLife.64690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsang T., Posimo J.M., Gudiel A.A., Cicchini M., Feldser D.M., Brady D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nature Cell Biology. 2020;22:412–424. doi: 10.1038/s41556-020-0481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw G.C., Cope J.J., Li L., Corson K., Hersey C., Ackermann G.E., et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 59.Chen W., Paradkar P.N., Li L., Pierce E.L., Langer N.B., Takahashi-Makise N., et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanover J.A., Willingham M.C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- 61.Cheng Y., Zak O., Aisen P., Harrison S.C., Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 62.Yambire K.F., Rostosky C., Watanabe T., Pacheu-Grau D., Torres-Odio S., Sanchez-Guerrero A., et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife. 2019;8 doi: 10.7554/eLife.51031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber R.A., Yen F.S., Nicholson S.P.V., Alwaseem H., Bayraktar E.C., Alam M., et al. Maintaining iron homeostasis is the key role of lysosomal acidity for cell proliferation. Molecular Cell. 2020;77:645–655.e7. doi: 10.1016/j.molcel.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beaumont C., Delaunay J., Hetet Gilles, Grandchamp B., de Montalembert M., Tchernia G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood. 2006;107:4168–4170. doi: 10.1182/blood-2005-10-4269. [DOI] [PubMed] [Google Scholar]
- 65.Grandchamp B., Hetet G., Kannengiesser C., Oudin C., Beaumont C., Rodrigues-Ferreira S., et al. A novel type of congenital hypochromic anemia associated with a nonsense mutation in the STEAP3/TSAP6 gene. Blood. 2011;118:6660–6666. doi: 10.1182/blood-2011-01-329011. [DOI] [PubMed] [Google Scholar]
- 66.Jouaville L.S., Ichas F., Holmuhamedov E.L., Camacho P., Lechleiter J.D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 67.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 68.Balaban R.S. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochimica et Biophysica Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hajnóczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 70.Deluca H.F., Engstrom G.W. Calcium uptake by rat kidney mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasington F.D., Murphy J.V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. Journal of Biological Chemistry. 1962;237:2670–2677. [PubMed] [Google Scholar]
- 72.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sancak Y., Markhard A.L., Kitami T., Kovács-Bogdán E., Kamer K.J., Udeshi N.D., et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan M., Zhang J., Tsai C., Orlando B.J., Rodriguez M., Xu Y., et al. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature. 2020;582:129–133. doi: 10.1038/s41586-020-2309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garg V., Suzuki J., Paranjpe I., Unsulangi T., Boyman L., Milescu L.S., et al. The mechanism of MICU-dependent gating of the mitochondrial Ca2+uniporter. Elife. 2021;10 doi: 10.7554/eLife.69312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pande S.V. A mitochondrial carnitine acylcarnitine translocase system. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:883–887. doi: 10.1073/pnas.72.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sluse F., Ranson M. Transport of 2-oxoglutarate in rat-heart mitochondria. Archives Internationales de Physiologie et de Biochimie. 1971;79:634–636. [PubMed] [Google Scholar]
- 79.Herzig S., Raemy E., Montessuit S., Veuthey J., Zamboni N., Westermann B., et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 80.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y., et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Archiv. 2004;447:689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- 82.Schell J.C., Olson K.A., Jiang L., Hawkins A.J., van Vranken J.G., Xie J., et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Molecular Cell. 2014;56:400–413. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang L., Shestov A.A., Swain P., Yang C., Parker S.J., Wang Q.A., et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deneke S.M., Fanburg B.L. Regulation of cellular glutathione. American Journal of Physiology. 1989;257:L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 85.Meredith M.J., Reed D.J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. Journal of Biological Chemistry. 1982;257:3747–3753. [PubMed] [Google Scholar]
- 86.Wang Y., Yen F.S., Zhu X.G., Timson R.C., Weber R., Xing C., et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature. 2021;599:136–140. doi: 10.1038/s41586-021-04025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi X., Reinstadler B., Shah H., To T., Bryne K., Summer L., et al. Biorxiv; 2021. Combinatorial G x G x E CRISPR screening and functional analysis highlights SLC25A39 in mitochondrial GSH transport.https://www.biorxiv.org/content/10.1101/2021.09.22.461361v1 2021.09.22.461361. [Google Scholar]
- 88.Prinz W.A. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. The Journal of Cell Biology. 2014;205:759–769. doi: 10.1083/jcb.201401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernhard W., Rouiller C. Close topographical relationship between mitochondria and ergastoplasm of liver cells in a definite phase of cellular activity. The Journal of Biophysical and Biochemical Cytology. 1956;2:73–78. doi: 10.1083/jcb.2.4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vance J.E. Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. Journal of Biological Chemistry. 1991;266:89–97. [PubMed] [Google Scholar]
- 91.Wu H., Carvalho P., Voeltz G.K. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018;361 doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loewen C.J.R., Levine T.P. A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. Journal of Biological Chemistry. 2005;280:14097–14104. doi: 10.1074/jbc.M500147200. [DOI] [PubMed] [Google Scholar]
- 93.Kaiser S.E., Brickner J.H., Reilein A.R., Fenn T.D., Walter P., Brunger A.T. Structural basis of FFAT motif-mediated ER targeting. Structure (London England 1993) 2005;13:1035–1045. doi: 10.1016/j.str.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 94.Thelen A.M., Zoncu R. Emerging roles for the lysosome in lipid metabolism. Trends in Cell Biology. 2017;27:833–850. doi: 10.1016/j.tcb.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Litvak V., Dahan N., Ramachandran S., Sabanay H., Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nature Cell Biology. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 96.Schaaf G., Ortlund E.A., Tyeryar K.R., Mousley C.J., Ile K.E., Garrett T.A., et al. Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Molecular Cell. 2008;29:191–206. doi: 10.1016/j.molcel.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mesmin B., Bigay J., Moser von Filseck J., Lacas-Gervais S., Drin G., Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 98.Jamecna D., Polidori J., Mesmin B., Dezi M., Levy D., Bigay J., et al. An intrinsically disordered region in OSBP acts as an entropic barrier to control protein dynamics and orientation at membrane contact sites. Developmental Cell. 2019;49:220–234.e8. doi: 10.1016/j.devcel.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 99.Maeda K., Anand K., Chiapparino A., Kumar A., Poletto M., Kaksonen M., et al. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- 100.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 101.D’Angelo G., Polishchuk E., Di Tullio G., Santoro M., Di Campli A., Godi A., et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 102.Kumagai K., Yasuda S., Okemoto K., Nishijima M., Kobayashi S., Hanada K. CERT mediates intermembrane transfer of various molecular species of ceramides. Journal of Biological Chemistry. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 103.Borgese N., Iacomino N., Colombo S.F., Navone F. The link between VAPB loss of function and amyotrophic lateral sclerosis. Cells. 2021;10:1865. doi: 10.3390/cells10081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guillén-Samander A., Leonzino M., Hanna M.G., Tang N., Shen H., De Camilli P. Correction: VPS13D bridges the ER to mitochondria and peroxisomes via Miro. The Journal of Cell Biology. 2021;220 doi: 10.1083/jcb.202010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar N., Leonzino M., Hancock-Cerutti W., Horenkamp F.A., Li P., Lees J.A., et al. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. The Journal of Cell Biology. 2018;217:3625–3639. doi: 10.1083/jcb.201807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 108.Díaz M., Fabelo N., Ferrer I., Marín R. ‘Lipid raft aging’ in the human frontal cortex during nonpathological aging: gender influences and potential implications in Alzheimer's disease. Neurobiology of Aging. 2018;67:42–52. doi: 10.1016/j.neurobiolaging.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 109.Wong Y.C., Ysselstein D., Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 2018;554:382–386. doi: 10.1038/nature25486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng W., Wong Y.C., Krainc D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:19266–19275. doi: 10.1073/pnas.2003236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong Y.C., Peng W., Krainc D. Lysosomal regulation of inter-mitochondrial contact fate and motility in charcot-marie-tooth type 2. Developmental Cell. 2019;50:339–354.e4. doi: 10.1016/j.devcel.2019.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim S., Wong Y.C., Gao F., Krainc D. Dysregulation of mitochondria-lysosome contacts by GBA1 dysfunction in dopaminergic neuronal models of Parkinson's disease. Nature Communications. 2021;12:1807. doi: 10.1038/s41467-021-22113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luik R.M., Wu M.M., Buchanan J., Lewis R.S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. The Journal of Cell Biology. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu M.M., Buchanan J., Luik R.M., Lewis R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. The Journal of Cell Biology. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishii K., Hirose K., Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Reports. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 117.Sano R., Annunziata I., Patterson A., Moshiach S., Gomero E., Opferman J., et al. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Molecular Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu G.Y., Sabatini D.M. Author Correction: mTOR at the nexus of nutrition, growth, ageing and disease. Nature Reviews Molecular Cell Biology. 2020;21:246. doi: 10.1038/s41580-020-0219-y. [DOI] [PubMed] [Google Scholar]
- 119.Shin H.R., Zoncu R. The lysosome at the intersection of cellular growth and destruction. Developmental Cell. 2020;54:226–238. doi: 10.1016/j.devcel.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sancak Y., Bar-Peled L., Zoncu R., Markhard A.L., Nada S., Sabatini D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Binda M., Péli-Gulli M., Bonfils G., Panchaud N., Urban J., Sturgill T.W., et al. The Vam6 GEF controls TORC1 by activating the EGO complex. Molecular Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 122.Sancak Y., Peterson T.R., Shaul Y.D., Lindquist R.A., Thoreen C.C., Bar-Peled L., et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim E., Goraksha-Hicks P., Li L., Neufeld T.P., Guan K.-L. Regulation of TORC1 by Rag GTPases in nutrient response. Nature Cell Biology. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Menon S., Dibble C.C., Talbott G., Hoxhaj G., Valvezan A.J., Takahashi H., et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Düvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morita M., Gravel S., Chénard V., Sikström K., Zheng L., Alain T., et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metabolism. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 127.Cunningham J.T., Rodgers J.T., Arlow D.H., Vazquez F., Mootha V.K., Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 128.Morita M., Prudent J., Basu K., Goyon V., Katsumura S., Hulea L., et al. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Molecular Cell. 2017;67:922–935.e5. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 129.Nüchel J., Tauber M., Nolte J.L., Mörgelin M., Türk C., Eckes B., et al. An mTORC1-GRASP55 signaling axis controls unconventional secretion to reshape the extracellular proteome upon stress. Molecular Cell. 2021;81:3275–3293.e12. doi: 10.1016/j.molcel.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Inoki K., Zhu T., Guan K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 131.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Condon K.J., Orozco J.M., Adelmann C.H., Spinelli J.B., van der Helm P.W., Roberts J.M., et al. Genome-wide CRISPR screens reveal multitiered mechanisms through which mTORC1 senses mitochondrial dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2021;118 doi: 10.1073/pnas.2022120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo X., Aviles G., Liu Y., Tian R., Unger B.A., Lin Y.T., et al. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature. 2020;579:427–432. doi: 10.1038/s41586-020-2078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lim C.-Y., Davis O.B., Shin H.R., Zhang J., Berdan C.A., Jiang X., et al. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nature Cell Biology. 2019;21:1206–1218. doi: 10.1038/s41556-019-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dong R., Saheki Y., Swarup S., Lucast L., Harper J.W., De Camilli P. Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell. 2016;166:408–423. doi: 10.1016/j.cell.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trefts E., Shaw R.J. AMPK: restoring metabolic homeostasis over space and time. Molecular Cell. 2021;81:3677–3690. doi: 10.1016/j.molcel.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Possik E., Jalali Z., Nouët Y., Yan M., Gingras M., Schmeisser K., et al. Folliculin regulates ampk-dependent autophagy and metabolic stress survival. PLoS Genetics. 2014;10 doi: 10.1371/journal.pgen.1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park H., Staehling K., Tsang M., Appleby M.W., Brunkow M.E., Margineantu D., et al. Disruption of Fnip1 reveals a metabolic checkpoint controlling B lymphocyte development. Immunity. 2012;36:769–781. doi: 10.1016/j.immuni.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang C.-S., Jiang B., Li M., Zhu M., Peng Y., Zhang Y., et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metabolism. 2014;20:526–540. doi: 10.1016/j.cmet.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 140.Deus C.M., Yambire K.F., Oliveira P.J., Raimundo N. Mitochondria-lysosome crosstalk: from physiology to neurodegeneration. Trends in Molecular Medicine. 2020;26:71–88. doi: 10.1016/j.molmed.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 141.Davis O.B., Shin H.R., Lim C.-Y., Wu E.Y., Kukurugya M., Maher C.F., et al. NPC1-mTORC1 signaling couples cholesterol sensing to organelle homeostasis and is a targetable pathway in niemann-pick type C. Developmental Cell. 2021;56:260–276.e7. doi: 10.1016/j.devcel.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yambire K.F., Fernandez-Mosquera L., Steinfeld R., Mühle C., Ikonen E., Milosevic I., et al. Mitochondrial biogenesis is transcriptionally repressed in lysosomal lipid storage diseases. Elife. 2019;8 doi: 10.7554/eLife.39598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khan N.A., Nikkanen J., Yatsuga S., Jackson C., Wang L., Pradhan S., et al. mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metabolism. 2017;26:419–428.e5. doi: 10.1016/j.cmet.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 144.Taylor S.S., Ilouz R., Zhang P., Kornev A.P. Assembly of allosteric macromolecular switches: lessons from PKA. Nature Reviews Molecular Cell Biology. 2012;13:646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim C., Cheng C.Y., Saldanha S.A., Taylor S.S. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell. 2007;130:1032–1043. doi: 10.1016/j.cell.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 146.Beene D.L., Scott J.D. A-kinase anchoring proteins take shape. Current Opinion in Cell Biology. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sanders M.A., Madoux F., Mladenovic L., Zhang H., Ye X., Angrish M., et al. Endogenous and synthetic ABHD5 ligands regulate ABHD5-perilipin interactions and lipolysis in fat and muscle. Cell Metabolism. 2015;22:851–860. doi: 10.1016/j.cmet.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pidoux G., Witczak O., Jarnæss E., Myrvold L., Urlaub H., Stokka A.J., et al. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. The EMBO Journal. 2011;30:4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mikitova V., Levine T.P. Analysis of the key elements of FFAT-like motifs identifies new proteins that potentially bind VAP on the ER, including two AKAPs and FAPP2. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schuler M.-H., English A.M., Xiao T., Campbell T.J., Shaw J.M., Hughes A.L. Mitochondrial-derived compartments facilitate cellular adaptation to amino acid stress. Molecular Cell. 2021;81:3786–3802.e13. doi: 10.1016/j.molcel.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Soubannier V., McLelland G., Zunino R., Braschi E., Rippstein P., Fon E.A., et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Current Biology. 2012;22:135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 152.Soubannier V., Rippstein P., Kaufman B.A., Shoubridge E.A., McBride H.M. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.König T., Nolte H., Altonen M.J., Tatsuta T., Krols M., Stroh T., et al. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nature Cell Biology. 2021;23:1271–1286. doi: 10.1038/s41556-021-00798-4. [DOI] [PubMed] [Google Scholar]
- 154.Rowland A.A., Chitwood P.J., Phillips M.J., Voeltz G.K. ER contact sites define the position and timing of endosome fission. Cell. 2014;159:1027–1041. doi: 10.1016/j.cell.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lewis S.C., Uchiyama L.F., Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 2016;353 doi: 10.1126/science.aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murley A., Lackner L.L., Osman C., West M., Voeltz G.K., Walter P., et al. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. Elife. 2013;2 doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gerhold J.M., Cansiz-Arda S., Löhmus M., Engberg O., Reyes A., van Rennes H., et al. Human mitochondrial DNA-protein complexes attach to a cholesterol-rich membrane structure. Scientific Reports. 2015;5:15292. doi: 10.1038/srep15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morishita H., Mizushima N. Diverse cellular roles of autophagy. Annual Review of Cell and Developmental Biology. 2019;35:453–475. doi: 10.1146/annurev-cellbio-100818-125300. [DOI] [PubMed] [Google Scholar]
- 160.Osawa T., Kotani T., Kawaoka T., Hirata E., Suzuki K., Nakatogawa H., et al. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nature Structural & Molecular Biology. 2019;26:281–288. doi: 10.1038/s41594-019-0203-4. [DOI] [PubMed] [Google Scholar]
- 161.Valverde D.P., Yu S., Boggavarapu V., Kumar N., Lees J.A., Walz T., et al. ATG2 transports lipids to promote autophagosome biogenesis. The Journal of Cell Biology. 2019;218:1787–1798. doi: 10.1083/jcb.201811139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maeda S., Otomo C., Otomo T. The autophagic membrane tether ATG2A transfers lipids between membranes. Elife. 2019;8 doi: 10.7554/eLife.45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schütter M., Giavalisco P., Brodesser S., Graef M. Local fatty acid channeling into phospholipid synthesis drives phagophore expansion during autophagy. Cell. 2020;180:135–149.e14. doi: 10.1016/j.cell.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 164.Laufman O., Perrino J., Andino, Viral Generated R. Inter-organelle contacts redirect lipid flux for genome replication. Cell. 2019;178:275–289.e16. doi: 10.1016/j.cell.2019.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen W.W., Freinkman E., Wang T., Birsoy K., Sabatini D.M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell. 2016;166:1324–1337.e11. doi: 10.1016/j.cell.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abu-Remaileh M., Wyant G.A., Kim C., Laqtom N.N., Abbasi M., Chan S.H., et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science. 2017;358:807–813. doi: 10.1126/science.aan6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tharkeshwar A.K., Trekker J., Vermeire W., Pauwels J., Sannerud R., Priestman D.A., et al. A novel approach to analyze lysosomal dysfunctions through subcellular proteomics and lipidomics: the case of NPC1 deficiency. Scientific Reports. 2017;7:41408. doi: 10.1038/srep41408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cho K.F., Branon T.C., Rajeev S., Svinkina T., Udeshi N.D., Thoudam T., et al. Split-TurboID enables contact-dependent proximity labeling in cells. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:12143–12154. doi: 10.1073/pnas.1919528117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Han Y., Branon T.C., Martell J.D., Boassa D., Shechner D., Ellisman M.H., et al. Directed evolution of split APEX2 peroxidase. ACS Chemical Biology. 2019;14:619–635. doi: 10.1021/acschembio.8b00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pareek V., Tian H., Winograd N., Benkovic S.J. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science. 2020;368:283–290. doi: 10.1126/science.aaz6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Koveal D., Díaz-García C.M., Yellen G. Fluorescent biosensors for neuronal metabolism and the challenges of quantitation. Current Opinion in Neurobiology. 2020;63:111–121. doi: 10.1016/j.conb.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ortega A.D., Takhaveev V., Vedelaar S.R., Long Y., Mestre-Farràs N., Incarnato D., et al. A synthetic RNA-based biosensor for fructose-1,6-bisphosphate that reports glycolytic flux. Cell Chemical Biology. 2021;28:1554–1568.e8. doi: 10.1016/j.chembiol.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 173.Mosen P., Sanner A., Singh J., Winter D. Targeted quantification of the lysosomal proteome in complex samples. Proteomes. 2021;9:4. doi: 10.3390/proteomes9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akter F., Ponnaiyan S., Kögler-Mohrbacher B., Bleibaum F., Damme M., Renard B.Y., et al. Biorxiv; 2020. Multi cell line analysis of lysosomal proteomes reveals unique features and novel lysosomal proteins.http://biorxiv.org/lookup/doi/10.1101/2020.12.21.423747 [DOI] [PMC free article] [PubMed] [Google Scholar]