Abstract
Ambient-storage-friendly, ready-to-eat (RTE) meat products are convenient in emergencies, such as earthquakes, flash floods and the current global Covid-19 lockdown. However, given the processing and long storage time of such food products, the lipid and protein components may be more susceptible to oxidation. Chicken serunding is a low-moisture, high-lipid, high-protein, RTE product that is prone to lipid oxidation and protein co-oxidation, causing product quality deterioration. The present study assessed the effects of storage temperature (25, 40, 60 °C), antioxidant (butylated hydroxyanisole, BHA), and multilayer packaging materials [metallised polyethene terephthalate (MPET) and aluminium] on the lipid oxidation and protein co-oxidation of chicken serunding during six months of storage. All lipid and protein markers elevated with increasing temperature (25 < 40 < 60 °C), indicating that storage of low-moisture meat at high temperature is not feasible. BHA was effective against lipid oxidation, as indicated by the significantly lower (p <0.05) extracted lipid content and delayed formation of malondialdehyde, a secondary lipid oxidation product. However, BHA is not effective against protein co-oxidation, as shown by the insignificant (p >0.05) effect on preventing tryptophan loss, protein carbonyl formation and Schiff base accumulation. MPET packaging with a superior light and oxygen barrier provided significant protection (p <0.05) compared to aluminium. In conclusion, low temperature (25 °C) storage of low-moisture, high-lipid, high-protein, cooked meat systems in MPET packaging is recommended for long-term storage to delay the progression of lipid oxidation and protein co-oxidation.
Ambient-storage-friendly, ready-to-eat (RTE) meat products are convenient in emergencies, such as earthquakes, flash floods and the current global Covid-19 lockdown.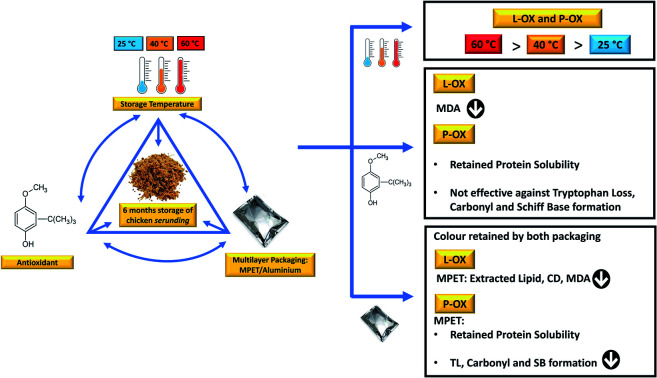
Introduction
Nutritious, ambient storable and ready meals are often required in emergencies, such as earthquakes, flash floods and the current global Covid-19 lockdown.1,2 Indeed, the various lockdown restrictions imposed globally have caused a shift in consumers' eating patterns. The closure of restaurants, coffee shops, and the potential shortage of staple foods such as rice, sugar and flour restricted daily meal preparation.3 In addition, there have been challenges for doctors and medical staff, particularly those on duty in remote or rural areas, to eat proper, satiating meals. Moreover, the closure of majority food facilities in university and college campuses has limited the food options available to students.4 With current pandemic, RTE meal becomes a convenient alternative to provide nutrition with ease. However, meat-based RTE meal is lipid and protein-rich, thus susceptible to oxidative deterioration during long-term storage. This study thus assessed the oxidation of such food system using serunding, a locally popular delicacy that is cooked under long-hour from chicken meat, known for its high-lipid (6.0–32.3%), high-protein (21.8–40.7%) and low-moisture (4.3–13.6%) character,5–7 as the representative food model.
During lipid oxidation, non-lipid substrates (majorly protein) are spontaneously attacked by highly reactive, protein-bound, lipid oxidation-derived aldehyde such as malondialdehyde (MDA).8,9 MDA, an α,β-unsaturated aldehyde, is electrophilic and attacks the nucleophilic groups on proteins, causing unfavourable alteration in the protein structure and stability,10,11 leading to the formation of Schiff base adducts (MDA-modified amino acid residues, predominantly MDA-lysine adducts)12 and a subsequent occurrence of protein co-oxidation, adversely affecting the product functionality and performance. According to Wang et al.,11 MDA could also promote protein carbonylation and loss of tryptophan fluorescence in the myofibrillar protein while Hellwig13 termed the reaction of protein with oxidized lipid product as “lipation”, where bulky modifying group on lipid is covalently attached to the nucleophilic site on amino acid. To tackle these issues, synthetic antioxidants are added commercially to various food such as baked goods, fried food, processed meat, dried cereal, processed potato, dessert mix and beverages.14,15 Despite the potential side effects of synthetic antioxidant, its application in the food industry is ongoing16 to prolong the shelf life of lipid-rich food products at a low cost.
Most studies have assessed the effects of processing on the lipid oxidation and/or protein oxidation of high-moisture raw meat and low-moisture cured meat.10,11,17 To date, no studies have investigated the effects of storage on the lipid oxidation and protein co-oxidation of a high-lipid, high-protein RTE product. The current work assessed the effectiveness of a synthetic antioxidant (butylated hydroxyanisole), storage temperature (25, 40 and 60 °C) and packaging material (MPET and aluminium containing packaging) on minimising oxidation. Physicochemical changes, lipid oxidation markers (conjugated dienes and malondialdehydes) and protein co-oxidation markers (soluble protein content, tryptophan loss, protein carbonyl, Schiff base fluorescence) were monitored over six months of storage and a principal component analysis was performed to identify the correlations between physicochemical properties (reflecting the product quality), lipid oxidation and protein co-oxidation (reflecting the extent of unfavourable oxidation activities).
Materials and methods
Materials
Fresh chicken meat was purchased from a local market in Kelantan state, Malaysia, trimmed of visible fat and stored at 4 °C before the manufacture of serunding. Two types of multilayer packaging were used, polyethene terephthalate/polyethene/metallised polyethene terephthalate/linear low-density polyethene [PET/PE/MPET/LLDPE, packaging A] and polyethene terephthalate/polyethene/aluminium/linear low-density polyethene (PET/PE/ALU/LLDPE, packaging B). The synthetic antioxidant, butylated hydroxyanisole (BHA) was obtained from Merck Millipore (Darmstadt, Germany). All other chemicals were analytical or food grade and obtained from Acros Organics (Geel, Belgium) and Sigma-Aldrich (St Louis, MO, USA). Milli-Q water was used for cooking the serunding.
Preparation of chicken serunding
The serunding was prepared as described by Wazir.7 Briefly, the chicken meat was sliced into thick pieces (5 × 5 × 10 cm), slow-cooked overnight at 70 °C in water (meat : water ratio was 1 : 1.5, w/v) until tender, drained and allowed to cool. During cooking, five slices of tamarind were added to eliminate the raw scent of meat and the residual water was collected as meat broth. The cooked meat was manually shredded to obtain fibrous meat strands. The serunding sauce containing coconut milk (25.42 g/100 g), onion (22.03 g/100 g), sugar (6.78 g/100 g), garlic (3.39 g/100 g), ginger (3.39 g/100 g), salt (1.69 g/100 g), dried chilli (1.69 g/100 g), freshly ground coriander seeds (1.69 g/100 g) and tamarind paste (0.03 g/100 g) was mixed with the meat broth, then BHA was added at 200 ppm based on the lipid content of chicken serunding. According to Malaysia's Food Act and Regulations, this is the maximum dosage allowed as an additive in a food product.18 The ingredients were concentrated and thickened in a saucepan by heating at 80–90 °C with continuous stirring to prevent charring to 50% of the initial volume, then the shredded meat was added (33.89 g/100 g) and continuously stirred until reaching the desired dryness. The entire cooking process took approximately 8 h. Fig. 1 below shows the images of chicken serunding samples and CS samples added with BHA (CS-BHA). Upon cooling, the chicken serunding was nitrogen-flushed and sealed in light-proof, gas-impermeable individual sachets of PET/PE/MPET/LLDPE (packaging A) or PET/PE/ALU/LLDPE (packaging B) in triplicate (n = 3).
Fig. 1. Images of chicken serunding (CS) samples and CS samples added with BHA (CS-BHA).
Before the production of chicken serunding samples, six preliminary experiments were conducted to obtain the optimised formulation and processing conditions to achieve a stable, consistent, and reproducible proximate product composition. The storage timeframe was selected according to the minimum shelf life of commercial serunding products (ranging between 6–12 months). During storage, samples were arranged in single layers on well-ventilated trays and incubated at 25, 40 and 60 °C. The temperature control sample was stored at −80 °C to prevent oxidation, while the packaging control sample was packed in a clear polybag and incubated at 25 °C (to represent ambient storage). The intermediate temperature of 40 °C was selected to represent the maximum temperature to maintain the primary lipid oxidation product stability, while 60 °C was selected because, at this temperature, the decomposition of hydroperoxides and initiation of secondary lipid oxidation product was further accelerated.19
Physicochemical quality assessment of chicken serunding
Proximate analysis
The proximate composition including moisture, ash, crude fat and crude protein content of chicken serunding was performed according to the AOAC official method20 in triplicate.
Fatty acid composition
Lipid was extracted per the method of Ibadullah.21 The serunding sample to solvent ratio of 1 : 5 (w/v) was added into a pre-mixed solution of chloroform : methanol (2 : 1, v/v), flushed with argon gas and allowed to stand for 30 min, then centrifuged (14 000×g, 4 °C) for 30 min. The extraction steps were repeated once and both supernatants were combined. The pooled supernatant was evaporated to obtain a dried lipid extract, which was then transesterified to produce fatty acid methyl ester (FAME) using sodium methoxide complex as the catalyst, with slight modifications of The American Oil Chemists' Society Method Ce 1-62.22 Briefly, 100 mg of oil was mixed vigorously with 5.0 mL of hexane, then sodium methoxide (250 μL) was added and the mixture was vortexed for 1 min, followed by the addition of 5.0 mL of saturated NaCl. The mixture was shaken vigorously for 15 s and allowed to stand for 10 min. The upper hexane layer was passed through anhydrous sodium sulfate granules for 20 min before injecting into a gas chromatography system (Agilent 6890N) equipped with a flame ionisation detector and an automated liquid sampler (Agilent 7683 series), fully controlled by Agilent Chemstation® Software.
To perform FAME separation, a high-polarity capillary column (DB-WAX, 30 m × 0.25 mm ID, 0.25 μm film thickness with polyethene glycol stationary phase) was used under temperature-programmed operation. To achieve optimum separation, the column was maintained at 100 °C for the first 2 min, then ramped to 230 °C at a rate of 5 °C/min−1 and maintained at 230 °C for 10 min. An injection volume of 1 μL with a split ratio of 1 : 20 was used with helium gas (flow rate = 1.0 mL min−1, pressure = 103.4 kPa) as the carrier. Peak identification was performed by comparison with the FAME standard (Supelco Park, Bellefonte, PA, USA). The fatty acid profile (%) was calculated using the area normalisation method as shown in eqn (1):
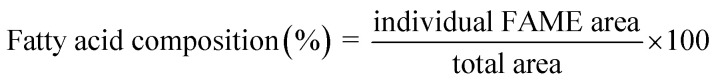 |
1 |
Colour measurement
CIE L*a*b* colour coordinates were measured using a Minolta chromameter (model CR-300, Minolta Camera Corp., Meter Division, Ramsey, NJ, USA), which consisted of a measuring head, with an 8 mm diameter measuring area, and a data processor (model DP-301). Measurements were recorded of fifteen randomly selected surface locations of the serunding sample with a primary illuminant D65 and a 0° angle observer. L*, a* and b* values were reported for lightness, redness, and yellowness, respectively. The total colour difference (ΔE0–24) between chicken serunding samples at week 0 and week 24 storage was calculated using the following equation:23,24
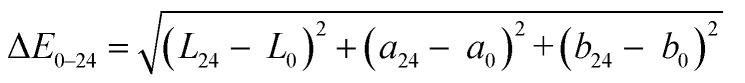 |
2 |
Scanning electron microscopy (SEM)
The surface morphology of chicken serunding was studied using a Philips XL-30 (Eindhoven, Netherlands). Samples were placed onto the sample stub, sputter coated with gold particles and observed under SEM at different magnifications of ×55 and ×330.
Lipid oxidation analysis
Total lipid content
Lipid was extracted following the procedure described in fatty acid composition section above. After two extractions, the pooled supernatant was evaporated to determine the amount of lipid, expressed as the percentage of lipid recovery (%) based on the initial sample weight. The dried lipid extract was flushed with argon, sealed, and stored at −20 °C until further analysis. The meal from the serunding sample was dried for 2–3 h at room temperature to produce a fat-free sample for use in protein analysis.
Conjugated dienes (CD)
The CD content was determined according to The American Oil Chemists' Society standard method Th 1a-64 (ref. 25) with some modifications. Briefly, 30 μL of lipid extract obtained from the lipid extraction was added to 10 mL of isooctane and the absorbance was measured at 234 nm against isooctane (blank). The CD content was calculated from Beer's Law using the molar extinction coefficient for isooctane (29 500 L mol−1 cm−1) and expressed as mmol CD/mol lipid.
Thiobarbituric acid reactive substances (TBARS)
The TBARS concentration was determined using the Food TBARS Assay Kit (Oxford Biomedical Research Inc., Rochester Hills, MI, USA). The serunding sample was mixed with distilled water at a 1 : 2 (w/v) ratio, then homogenised at 1000 rpm for 3 min to form a smooth slurry. The TBA reagent (consisting of 2.5 g of 2-thiobarbituric acid and 50 mL of proprietary acid catalyst) was added to the slurry at a 1 : 1 (v/v) ratio and vigorously agitated for 1 min using a vortex mixer. The reaction was allowed to proceed for 60 min at room temperature, then centrifugated at 15 000×g for 5 min to collect the supernatant. The TBARS concentration was determined using an MDA standard calibration curve (R2 = 0.9999, 0 to 3.0 mg L−1) and the results were expressed as mg MDA equivalent per kg sample.
Protein co-oxidation analysis
Soluble protein
The fat-free sample after lipid extraction process was mixed with sodium phosphate buffer (0.2 M, pH 7.9) containing 0.02% sodium azide and incubated overnight at 40 °C and 150 rpm constant shaking, then centrifuged at 14 000×g (30 min, 4 °C). The clear supernatant layer was retrieved and purged with a stream of argon gas at room temperature and stored at −80 °C. The soluble protein content was measured following the method previously described by Bradford26 and expressed as a percentage of the total soluble protein per sample weight (g/g, dry basis) using bovine serum albumin as the protein standard (R2 = 0.9749, 0–100 μg μL−1).
Tryptophan loss
Protein co-oxidation was monitored using a spectrofluorometric assay21 and the argon-flushed supernatant from section above. The intrinsic fluorescence from aromatic amino acids (primarily tryptophan) was calculated by recording the fluorescence emission spectra from 300 to 450 nm with optimum excitation and emission wavelengths (λex = 280 nm and λem = 330 nm), using 9 nm bandwidth, 10 nm excitation/emission slits. All spectra were recorded at room temperature. Fluorescence intensities were expressed in arbitrary units (a.u.). The tryptophan loss was calculated as follows:
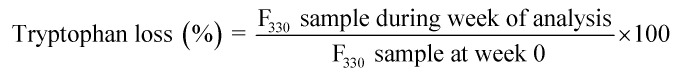 |
3 |
Protein carbonyl content
Protein carbonyl content was determined via derivatisation with 2,4-dinitrophenyl hydrazine (DNPH) as described by Soglia27 with some modifications. The serunding sample was added to phosphate buffer (20 mM, pH 6.5 containing 0.6 M NaCl) at a ratio of 1 : 10 (w/v). Four aliquots (0.2 mL each) were treated with 1.0 mL of ice-cold 10% trichloroacetic acid (TCA) to precipitate the protein. After centrifugation at 4500×g for 3 min, the supernatant was discarded. Two tubes were treated with 0.5 mL of 10 mM DNPH dissolved in 2.0 M HCl while the other two tubes were treated with 0.5 mL of 2.0 M HCl without DNPH (blank). After 1 h at room temperature, 0.5 mL of ice-cold 20% TCA was added, then the samples were centrifuged (4500×g, 3 min) and the pellet (precipitate) was collected. Excess DNPH was removed by washing the pellet three times with 1 mL of ethanol: ethyl acetate (1 : 1, v/v). The samples were re-centrifuged, the pellet was collected and dissolved in 1 mL of 6.0 M guanidine hydrochloride (pH 6.5). The absorbance was read against blank at 370 nm to estimate the carbonyl concentration (represented as protein hydrazones) and at 280 nm to estimate the protein concentration. The carbonyl content was calculated based on the molar absorptivity for protein hydrazones (22 000 M−1 cm−1) and the correction factor for hydrazone peak tail overlapping (0.43) as follows:
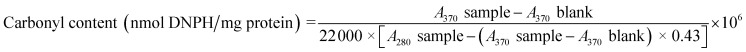 |
4 |
Schiff base content
The presumptive formation of Schiff base complexes between lipid carbonyls and protein amino groups were detected by recording λem from 400 to 650 nm with excitation at 350 nm. Emission intensity was recorded at 430 nm. All spectra were recorded using 9 nm bandwidth, 10 nm excitation/emission slits and synchronous mode, producing both excitation and emission curves displayed as an average of three spectra for each sample.
Statistical analysis
Proximate and fatty acid composition were evaluated using one-way analysis of variance (ANOVA). All lipid oxidation and protein co-oxidation markers were analysed using a general linear model. The mean differences among groups were assessed using Tukey's posthoc test at 5% significance (p <0.05). Principal component analysis (PCA) was performed to evaluate the relationship between physicochemical properties, lipid oxidation and protein co-oxidation parameters, with each replicate included as a random effect and the different treatment combinations arising from antioxidant (with and without BHA), storage temperature (25, 40 and 60 °C), storage week (0, 4, 8, 12, 16, 20, 24 weeks) and packaging material (A and B) included as fixed terms. All procedures were performed using Minitab® 16 statistical software (Minitab LLC., State College, PA, USA).
Results and discussion
Physicochemical analysis of chicken serunding
Proximate composition, scanning electron microscopy (SEM) and fatty acid composition (FAC)
The proximate composition for chicken serunding is presented in Table 1, showing that both chicken serunding (CS) and chicken serunding supplemented with BHA (CS-BHA) had a low-moisture content (<10%) and were categorised as low-moisture food.5,7,28 The addition of BHA substantially decreased the extracted fat content of CS-BHA, possibly due to less physical damage on the meat fibre during heat treatment (cooking) resulting in a lower fat content. During heating, protein–lipid radical reactions initiate protein unfolding and modification of physical protein properties, leading to an increment in hydrophobic surface area which exposes more non-polar protein side groups to the external environment.29 Zayas29 reported that the non-polar side group with a strong fat-binding ability is the primary site of the lipid–protein interaction that promotes fat absorption and increases surrounding fat uptake. The higher fat content in CS than CS-BHA could therefore be caused by the same phenomenon. The addition of BHA delays the occurrence of lipid oxidation by reducing the generation of lipid radical initiators and promoters that may otherwise react with the protein. When the protein structure is less affected, the lipophilic non-polar group is minimally exposed and the absorption of lipid-rich coconut milk into the meat fibre in CS-BHA is lowered.
Proximate composition (%) and fatty acid composition of chicken serundinga.
| Present data | 1Reported data | ||
|---|---|---|---|
| Without BHA | With BHA | ||
| Proximate composition (%) | |||
| Moisture | 7.46 ± 0.07a | 7.44 ± 0.02a | 9.61–13.56 |
| Ash | 4.97 ± 0.09b | 5.14 ± 0.03a | 3.17–5.17 |
| Fat | 32.30 ± 0.36a | 29.74 ± 0.02b | 6.04–22.22 |
| Protein | 21.77 ± 0.12a | 21.72 ± 0.03a | 25.90–30.15 |
| Carbohydrate (by difference) | 33.50 ± 0.53b | 36.14 ± 0.05a | 27.00–41.22 |
| FAC (%) | — | ||
| C6:0 | 0.73 ± 0.01b | 0.84 ± 0.01a | — |
| C8:0 | 8.43 ± 0.08a | 8.49 ± 0.41a | — |
| C10:0 | 6.50 ± 0.09a | 6.27 ± 0.32a | — |
| C12:0 | 47.85 ± 0.70a | 39.45 ± 0.80b | — |
| C14:0 | 17.29 ± 0.17a | 13.68 ± 0.55b | — |
| C14:1 | — | — | — |
| C16:0 | 8.32 ± 0.37b | 9.80 ± 0.19a | — |
| C16:1 | 0.11 ± 0.01 | — | — |
| C17:0 | — | — | — |
| C18:0 | 2.44 ± 0.29b | 3.70 ± 0.33a | — |
| C18:1, cis-9 | 5.98 ± 0.61a | 7.86 ± 0.18a | — |
| C18:2, cis-9,12 | 4.12 ± 0.05b | 8.02 ± 0.24a | — |
| Saturated | 91.65 ± 0.47a | 82.23 ± 1.50b | — |
| Monounsaturated | 6.09 ± 0.63b | 7.86 ± 0.18a | — |
| Polyunsaturated | 4.12 ± 0.05b | 8.02 ± 0.24a | — |
When the proximate composition is reported on a “per 100%” basis, a higher fat proportion in 100 g of the sample indicates a lower protein proportion.7 Both CS and CS-BHA had higher fat and lower protein content compared to the reported data, which was confirmed by SEM. The result shows that CS had a rough surface with tiny holes and cavities while CS-BHA presented a smooth surface with fewer holes and cavities. A large amount of holes and cavities in CS is responsible for the greater exposure of lipophilic protein side groups to the environment,33 thereby enhancing fat absorption relative to CS-BHA. The fat content and the SEM analysis were consistent with the fatty acid composition. From Table 1, CS-BHA had a significantly higher percentage of monounsaturated fatty acids (MUFA: 7.86%), polyunsaturated fatty acids (PUFA: 8.02%), with less saturated fatty acids (SFA: 82.23%) than CS (MUFA: 6.09%, PUFA: 4.12%, SFA: 91.65%). BHA addition (CS-BHA) reduced the SFA compared to the CS sample, indicating a substantial decrease in the absorption of coconut milk which is rich in saturated fat.
Colour
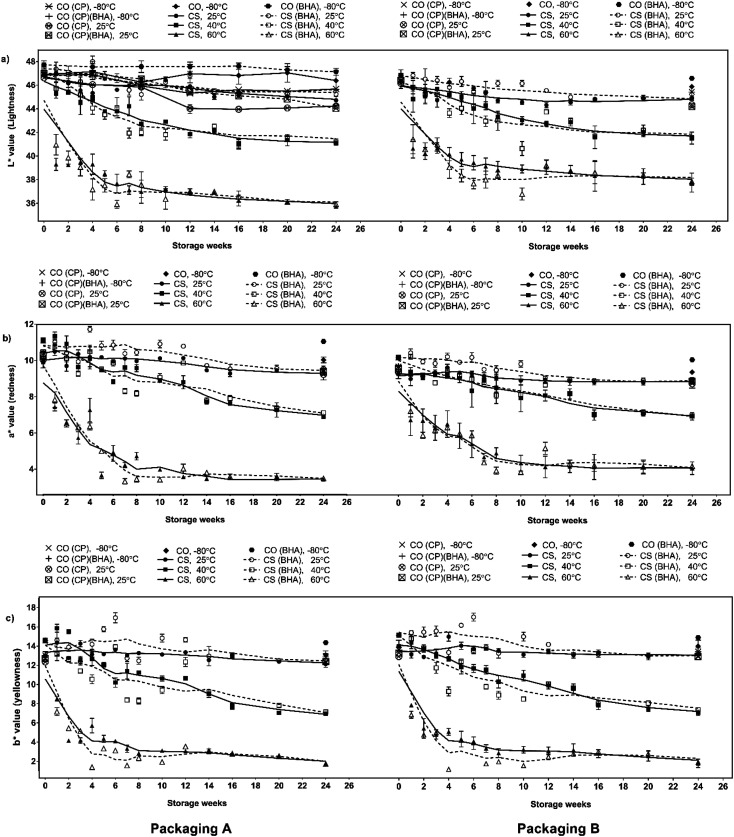
The CS and CS-BHA samples were dark brown due to (i) heat-induced denaturation of myoglobin during cooking, (ii) the Maillard reaction and (iii) aldol condensation between the lipid carbonyl and protein.7 The colour of the serunding samples is summarised in Fig. 2, and the figure legend description was tabulated in Table 2. In general, the colour changes of CS stored at 25 °C were insignificant (p >0.05), while at higher temperatures (40 and 60 °C), the colour values decreased over six months, indicating the darkening of product throughout storage.
Fig. 2. Colour measurements of (a) lightness; (b) redness and (c) yellowness on chicken serunding in packaging A and B during six-months storage.
Figure legend description for Fig. 2–4.
| CO (CP), −80 °C | Packaging control samples (clear polybag) incubated at −80 °C |
|---|---|
| CO (CP-BHA), −80 °C | Packaging control samples (clear polybag) with BHA addition incubated at −80 °C |
| CO (CP), 25 °C | Packaging control samples (clear polybag) incubated at 25 °C |
| CO (CP-BHA), 25 °C | Packaging control samples (clear polybag) with BHA addition incubated at 25 °C |
| CO, −80 °C | Temperature control samples with either packaging A or B incubated at −80 °C |
| CO (BHA), −80 °C | Temperature control samples with either packaging A or B, with BHA addition incubated at −80 °C |
| CS, 25 °C | Chicken serunding samples incubated at 25 °C |
| CS, 40 °C | Chicken serunding samples incubated at 40 °C |
| CS, 60 °C | Chicken serunding samples incubated at 60 °C |
| CS-BHA, 25 °C | Chicken serunding samples (with BHA) incubated at 25 °C |
| CS-BHA, 40 °C | Chicken serunding samples (with BHA) incubated at 40 °C |
| CS-BHA, 60 °C | Chicken serunding samples (with BHA) incubated at 60 °C |
| Packaging A | Chicken serunding samples with packaging A |
| Packaging A (BHA) | Chicken serunding samples with packaging A and BHA addition |
| Packaging B | Chicken serunding samples with packaging B |
| Packaging B (BHA) | Chicken serunding samples with packaging B and BHA addition |
This colour shift suggested that the Maillard reaction and aldol condensation were heat-dependent and aggressive at high temperatures. According to Estévez,23 the total colour difference (ΔE) higher than 2.0 is significant. Irrespective of storage temperature and packaging material, BHA addition had no significant effect on the serunding colour over six months of storage. The packaging material (packaging A: MPET vs. packaging B: aluminium) also did not affect the product colour. However, compared to the control packaging (ΔEcontrol = 4.34–5.09) both packaging A and B showed significant colour retention throughout storage at 25 °C (ΔE = 2.35–3.57), indicating that both packagings offered similar protection against light-induced degradation at 25 °C.
Lipid oxidation
Extracted lipid content
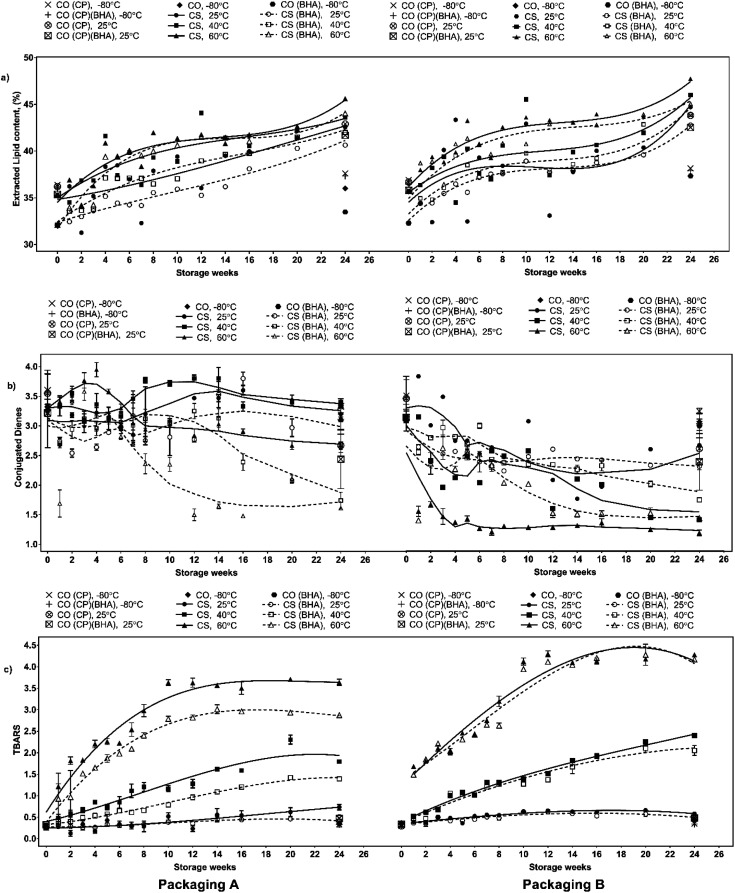
As indicated by Fig. 3a, the total percentage of extracted lipid in all serunding samples was less than 50%, with a gradual increase over six months under all storage conditions. The effect of storage temperatures on lipid oxidation in chicken serunding has been extensively reported in previous work.7
Fig. 3. Changes in lipid oxidation markers (a) extracted lipid (%), (b) conjugated dienes (CD) and (c) MDA of chicken serunding stored at different temperature in packaging A and B for 24 weeks.
In brief, the extracted lipid content in both CS and CS-BHA increased significantly (p <0.05) with increasing temperature (60 > 40 > 25 °C). The samples with BHA (CS-BHA) had a significantly lower (p <0.05) lipid content than those without antioxidant (CS) irrespective of storage temperature and packaging material. This may be attributed to the formation of phenolic radicals among BHA molecules that react with free radicals preventing further damage to the meat fibres (protein structure), thus, minimises the absorption of oil from coconut milk. The sample in packaging A (MPET) had less extracted lipid content (p <0.05) than packaging B (aluminium) during week 2 when stored at 60 °C and during week 24 when stored at 25 and 40 °C, indicating that MPET offered improved light and oxygen barrier to slow down oxidation.
Primary product: CD
CDs are generated from PUFAs as a primary lipid oxidation product in the presence of pro-oxidant/initiator catalysts such as heat, light, metal and free radicals. During CD formation, the hydrogen atom is first extracted from the methylene group on the PUFA hydrocarbon chain creating an unstable non-conjugated fatty acid, which then spontaneously shifts the double-bond position to a relatively more stable conjugated form (1–3, dienes).34 The CD analysis of the serunding samples was similar to previous studies of saturated fat-rich samples, including cooked pork meat (34–36% saturated fat, SF),35 margarine (containing 45–75% SF),36 meat sausages (38–42% SF),37 chicken breast and thigh (11–45% and 10–42% of SF, respectively).38 From Fig. 3b, CS (packaging A, stored at 25 °C and 40 °C) recorded a spiked CD content at week 10–12, much later than that stored at 60 °C, which peaked at week 4, followed by a continuous decrement until week 24, indicating that the adverse effect of high temperature rapidly speeds up the formation of primary lipid oxidation compounds, thereby deteriorating the product quality. There was no statistically significant difference observed (p >0.05) in the CD content between CS and CS-BHA at all storage temperatures and packaging material, indicating that BHA addition alone was not effective to slow down the accumulation of CD during storage.
In contrast to packaging A (MPET), the CD content in packaging B (aluminium) peaked earlier (week 2–3) regardless of antioxidant addition and storage temperature. This signified the effectiveness of packaging A to delay oxidation and decelerate the rapid formation of primary lipid oxidation products during storage. The serunding packaged in B showed an early accumulation of CD, followed by a consistent decline, indicating the early decomposition of primary lipid oxidation products into secondary products such as aldehyde and ketone, even with the addition of BHA. This suggests that aluminium caused unwanted penetration of light and oxygen that weakened the protective barrier against lipid oxidation compared to MPET, further confirming the lipid lower and higher effectiveness of packaging A against lipid oxidation.
Secondary products: MDA
MDA is the most abundant aldehyde marker generated during lipid oxidation in meat and meat products, which causes protein damage.12 Thus is frequently used in the assessment of lipid oxidation in meat products.39 Previously, MDA content has been determined by TBARS analysis of beef patties,40 pork patties,41 pork and pork meat sausages,42 chicken meatballs,43 chicken nuggets,44 and burgers.45,46Fig. 3c shows the MDA content of chicken serunding over six months of storage at three temperatures using two packaging materials. The effect of storage temperature on the MDA level has been previously described in detail by Wazir,7 highlighting that a linear increment in MDA content was observed as a function of temperature, i.e. higher storage temperature resulted in higher MDA formation. The maximum MDA content was recorded at 60 °C, followed by 40 and 25 °C irrespective of BHA addition and types of packaging. BHA addition significantly reduced the MDA level in CS-BHA (packaging A) at 40 and 60 °C up to week 16–18. However, at lower storage temperature (25 °C), CS-BHA (packaging A) showed no significant difference to CS (packaging A).
Packaging A consistently had a significantly lower (p <0.05) MDA level than packaging B, showing that packaging A was more effective at protecting high-lipid cooked meat against oxidation due to lower light and oxygen permeability (i.e., improved barrier) compared to packaging B. The combined effect of the antioxidant and packaging material on MDA level was observed at 25 °C at the end of six months' storage, whereby CS-BHA had the lowest MDA level (0.415 ± 0.01 mg kg−1) compared to CS-BHA and CS packed with either polybag (control packaging) or packaging B. These finding highlights that under favourable conditions (low storage temperature, presence of BHA, suitable packaging material), the oxidative compound (hydroperoxide) is relatively stable and the accumulation of MDA can be effectively slowed down. Previously, it has been reported that MDA formation in serunding sample with packaging A plateaued from week 16 onwards regardless of storage temperature and antioxidant addition.7 This suggests the occurrence of other reactions, such as carbonyl formation, due to the active radical transfer of carbonyl-containing moiety from MDA to other non-lipid molecules (mainly protein) via covalent attachment. Thus, the effect of the temperature, antioxidant addition and packaging materials were further assessed by tracking protein changes. The present findings confirmed that at low storage temperature (25 °C), the addition of exogenous antioxidant (BHA) and effective packaging material (packaging A, MPET) offered significant protection toward lipid stability and against lipid oxidation in low-moisture, cooked meat samples.
Protein co-oxidation
Lipid and protein oxidation can develop independently or in parallel.47 Independent, spontaneous radical-mediated oxidation imposes weaker structural modifications of protein and amino acid compared to the product from the Maillard reaction or lipation, whereby the latter involves covalent attachment of bulky modifying group to nucleophilic sites on amino acid to exert a more prominent oxidative effect.13 Since chicken serunding is a high-lipid and high-protein food system, it is most likely that radical, hydroperoxide and secondary oxidative products from lipid oxidation lead to protein oxidation, thus termed protein co-oxidation. The current study measured the extent of protein co-oxidation in chicken serunding in terms of protein solubility, depletion of tryptophan content and formation of tertiary products including protein carbonyl and Schiff base, to predict the protein quality under different combinations of storage temperature, BHA addition and packaging material.
Soluble protein content
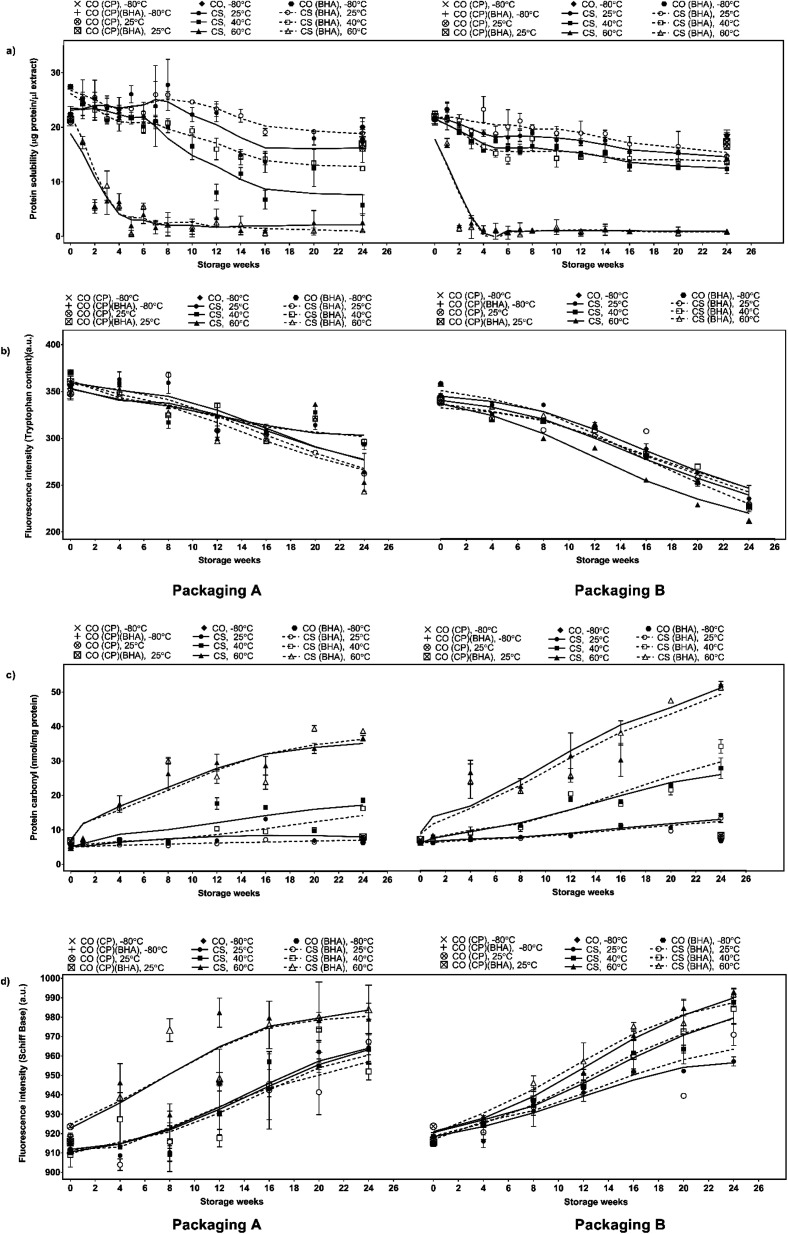
Changes in the chemical properties and protein solubility can reflect the degree of protein denaturation resulting from oxidation.48 The reaction between lipid oxidative products and protein molecules could lead to severe chemical modification on the tertiary and quaternary protein structures. The reaction causes polypeptide chains to unfold and expose hydrophobic groups to the surroundings, increasing protein–protein aggregation and disrupting protein–water interaction, resulting in the loss of solubility.29,48,49 Insolubility becomes more severe with increasing storage time and temperature as well as with elevated water activity in meat and meat products.40,50,51 As shown in Fig. 4a, a higher temperature leads to lower protein solubility irrespective of BHA addition and packaging material due to heat-induced protein denaturation, whereby at a higher temperature, the heat acts as a denaturing agent that reduces the stability of non-covalent interaction within protein molecules, prompting spontaneous structural unfolding and loss of native conformation, thus reducing the protein functionality and solubility.52 BHA significantly (p <0.05) improved protein solubility at lower temperatures (25 and 40 °C) but not at 60 °C, indicating the effect of heat as a denaturing agent surpasses the effect of BHA. At 25 and 40 °C, the higher protein solubility in CS-BHA compared to CS is in line with the lower fat content in CS-BHA. High-protein solubility is typically associated with low fat-binding capacity, as a highly soluble compound has its hydrophobic protein groups buried within the core, rendering the reaction with lipid materials that reduces the fat-binding ability.29 The samples stored in packaging A had higher protein solubility than packaging B indicating that MPET (packaging A) offered higher protein solubility retention than aluminium (packaging B) for a high-lipid, high-protein, low-moisture, cooked meat system.
Fig. 4. Changes in protein oxidation markers (a) protein solubility, (b) tryptophan content (expressed as fluorescence unit, a.u.), (c) protein carbonyls and (d) Schiff base (expressed as fluorescence unit, a.u.) of chicken serunding storage at different temperatures for 24 weeks.
Tryptophan loss
Tryptophan residue, an aromatic amino acid, is the preferential target site of lipid oxidative products, particularly MDA, to initiate protein co-oxidation. It is located primarily on the protein surface close to the lipid radical. Since it has abstractable hydrogen, it is extremely vulnerable to lipid radicals.53–55 Tryptophan loss is an indicator of changes in the protein tertiary structure that is associated with lower protein quality of cooked meat products. Previous studies have reported a loss in tryptophan content ranging from 30% to 80% during the processing of meat-based products i.e., pork burger patty and fermented sausage.56,57 However, the progression of tryptophan loss in processed meat during storage remains unclear. As depicted in Fig. 4b, there was a significantly higher loss (p <0.05) of tryptophan with increasing temperature, which was associated with the high rate of MDA formation in Fig. 3c, whereby a higher amount of MDA would actively attack tryptophan molecules and disrupt the native structure, lowering the tryptophan content. The addition of BHA did not significantly affect the tryptophan content in CS-BHA compared to CS at 25 and 60 °C, whereas packaging A significantly reduced (p <0.05) tryptophan loss compared to packaging B at all temperatures. Packaging A recorded a loss of −32.16% at 25 °C, −35.98% at 40 °C and −41.74% at 60 °C and packaging B a loss of −21.85% at 25 °C, −22.33% at 40 °C and −34.64% at 60 °C, respectively, showing that MPET (packaging A) induced less severe protein co-oxidation than aluminium (packaging B).
Tertiary product: protein carbonyl
Protein carbonylation analysis is widely applied for the evaluation of protein co-oxidation in muscle foods.58 Amino acid residues, particularly lysine, arginine, histidine and proline, are prone to oxidation, forming carbonyls that adversely affect the functionality of protein in cooked meat products.59–61 From Fig. 4c, the protein carbonyl content increases as a function of temperature, i.e. at the highest temperature of 60 °C, protein carbonyl formation is the most dominant, followed by 40 °C and 25 °C, due to the interaction between protein molecules and MDA, fragmentation of the protein backbone through the α-amidation pathway and β-scission along with direct oxidation of protein side chains containing susceptible residues such as arginine, lysine, proline and threonine,60 resulting in a net effect of carbonyl compound accumulation. The addition of BHA did not exert effective protection against the formation of protein carbonyls. The samples packaged in packaging B had a higher (p <0.05) carbonyl formation rate than packaging A at week 2 for 60 °C and week 12 for samples incubated at 25 and 40 °C. Interestingly, there was no significant difference in protein carbonyl content in samples packed with packaging B, irrespective of storage temperature and presence of BHA, indicating that the packaging material played the most critical role in promoting protein carbonyl formation during storage. These results indicate that packaging A provides superior protection against protein carbonyl formation and lowers protein co-oxidation compared to packaging B.
Tertiary product: Schiff base
Schiff base formation involves the production of stable radicals primarily from the reaction between lysine, histidine, glutamine or cysteine with reactive lipid oxidative products, which fluoresce at the conjugated structure –N CH–CH CH–.55,62,63 Specifically, it involves the reaction between an electrophilic group in the carbonyl structure of aldehyde from secondary lipid oxidative product (MDA) and a nucleophilic group on protein (electron-rich side chain of amino acid). The reaction will form a Schiff base adduct64 which then acts as an important indicator of protein co-oxidation. Schiff base fluorescence emission of chicken serunding is shown in Fig. 4d, showing that Schiff base fluorescence increased in a temperature-dependent manner, whereby higher fluorescence was observed when the storage temperature was raised (60 > 40 > 25 °C). This is due to the higher rate of lipid oxidation in samples stored at higher temperatures, which speeds up the formation of reactive lipid radicals to take part aggressively in protein co-oxidation, releasing more Schiff base compounds. BHA did not significantly reduce Schiff base formation under all circumstances, indicating the inefficiency of BHA to protect protein molecules from co-oxidative deterioration. Only samples with packaging A stored at 40 °C showed significantly lower (p <0.05) Schiff base production than packaging B at the same temperature, that is, protein co-oxidation occurred in a temperature-dependent manner, showing increasing severity as follows: 25 < 40 < 60 °C and packaging A (MPET) offered higher protection against protein deterioration than packaging B (aluminium).
Principal component analysis (PCA)
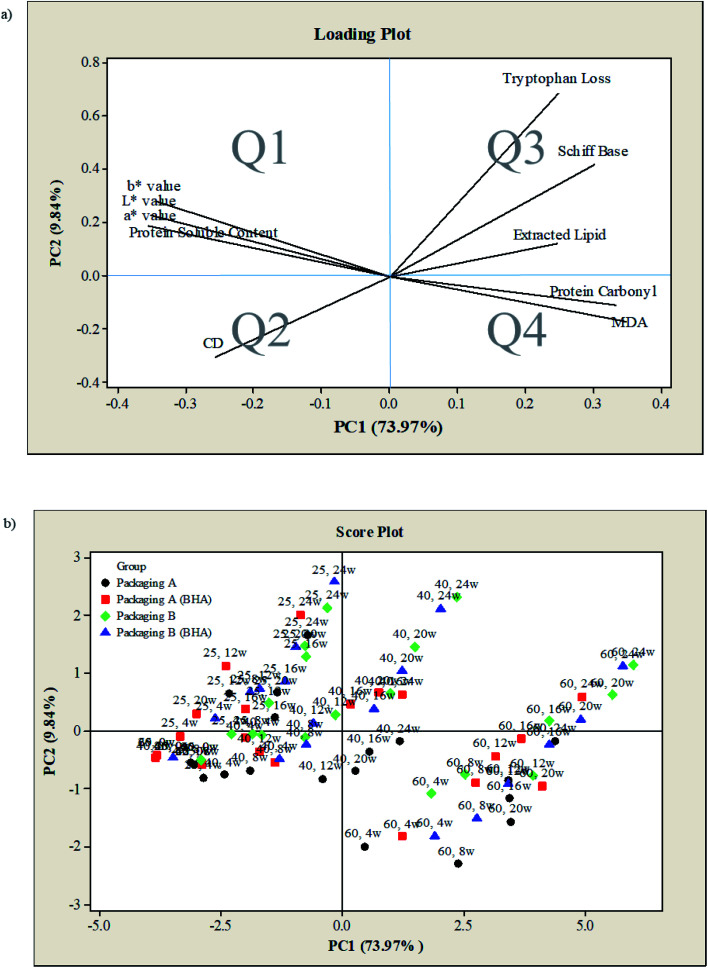
PCA was performed to evaluate the correlation between the measured parameters (colour, lipid oxidation markers, protein co-oxidation markers) under different controlled parameters (storage temperature, presence of antioxidant, packaging material) in chicken serunding. Fig. 5 shows an overlap of the first principal component (PC1) and second principal component (PC2), accounting for 83.81% of the total variance, of which PC1 and PC2 explained 73.97% and 9.84% of the variance, respectively. Both PC1 and PC2 describe the quality changes in chicken serunding in four designated quadrants (termed as Q1, Q2, Q3, and Q4). The measured parameters are depicted as a loading plot in Fig. 5a, while the controlled parameters are depicted as the score plot in Fig. 5b. From Fig. 5a, the positive-x region (Q3, Q4) of PC1 explained the variation in tryptophan loss, Schiff base, extracted lipid, protein carbonyl, and MDA, while the positive-y region (Q1, Q3) of PC2 explained the variation in L*, a*, b* colour values, soluble protein content, tryptophan loss, Schiff base and extracted lipid. This finding confirms the correlation between product colour, lipid oxidation markers and protein co-oxidation markers. From Fig. 5b, samples incubated at 60 °C were well-contained within the positive-x region (Q3, Q4), indicating that these samples suffered the most severe lipid oxidation and protein co-oxidation reaction.
Fig. 5. PCA of colour, lipid and protein markers of chicken serunding stored in different conditions (a) loading plot and (b) score plot.
Besides, samples at 60 °C were inversely correlated with colour, soluble protein content and CD (located in the negative-x region), inferring that they experienced the most severe colour degradation, had the lowest soluble protein content and underwent the most rapid CD degradation to form secondary lipid oxidation products than the other temperatures. In contrast, samples at 25 °C were well-contained within the negative-x region (Q1, Q2), indicating an inverse correlation with lipid oxidation and protein co-oxidation, thus experienced the least oxidative damage. Samples at 25 °C also positively correlated with colour, soluble protein content and CD (both in the negative-x region), inferring that these samples experienced the slightest colour change, soluble protein reduction and CD degradation that delayed the formation of secondary lipid oxidation products. The overall effect of BHA addition was insignificant, as CS and CS-BHA were not separated in the score plot. As for packaging material, samples with packaging B (blue and green) shifted toward positive-x (Q3, Q4) and positive-y (Q1, Q3) region compared to packaging A (black and red), indicating that samples with packaging B experienced a higher rate of lipid oxidation and protein co-oxidation than packaging A. PCA confirms that the changes in extracted lipid, secondary lipid oxidation product (MDA), as well as protein co-oxidation reaction (tryptophan loss, Schiff base and protein carbonyl) monitored over six months of storage, are highly affected by storage temperature and packaging material but not by antioxidant.
Conclusions
Current study elucidates the simultaneous occurrence of lipid oxidation and protein co-oxidation in a high-lipid, high-protein, low-moisture, ready-to-eat food system. Low storage temperature (25 °C) of serunding in MPET packaging offered significant protection against lipid oxidation and protein co-oxidation. In contrast, the addition of exogenous antioxidant (BHA) provided protection only against lipid oxidation and was ineffective in delaying protein co-oxidation. Comprehensive analyses encompassing multiple oxidation markers, along with evaluation of potential health risks associated with oxidised lipid and oxidised protein food products are suggested for future works.
Author contributions
H. Wazir involved in data investigation, collection and curation, as well as writing – original draft of the manuscript. S. Y. Chay helped in writing – review and editing. W. Z. W. Ibadullah conceptualized the study, provided the resources and supervised the study. The author M. Zarei validated the analyzed data. N. A. Mustapha provided the resources and supervised the study. N. Saari validated the analyzed data, acquitted the research funding, provided resources and main supervised the study.
Conflicts of interest
The authors declare no financial or commercial conflict of interest.
Supplementary Material
Acknowledgments
This research was financially supported by the Universiti Putra Malaysia, Malaysia under Putra Graduate Initiative grant (IPS-PUTRA vote no. 9438701).
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06872e
References
- Telukdarie A. Munsamy M. Mohlala P. Sustainability. 2020;12:9331. doi: 10.3390/su12229331. [DOI] [Google Scholar]
- Kumar S. Saxena S. Verma J. Gautam S. LWT–Food Sci. Technol. 2016;69:409–416. doi: 10.1016/j.lwt.2016.01.059. [DOI] [Google Scholar]
- Chaurasiya R. Pandey R. Verma P. Kek X. H. Kee D. M. H. Yeoh X. Y. Wah P. J. Rokiah R. Int. J. Bus. Sci. Appl. Manag. 2020;5:66–72. doi: 10.32535/ijabim.v5i2.859. [DOI] [Google Scholar]
- Powell P. K. Lawler S. Durham J. Cullerton K. Appetite. 2021;161:105130. doi: 10.1016/j.appet.2021.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knechtges P. L., in Food safety: Theory and practice, Jones & Bartlett Learning, Burlington, MA., 2012 [Google Scholar]
- Huda N. Fatma Y. Fazillah A. Adzitey F. Pak. J. Nutr. 2012;11:1–4. doi: 10.1186/1475-2891-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazir H. Chay S. Y. Zarei M. Hussin F. S. Mustapha N. A. Wan Ibadullah W. Z. Saari N. Antioxidants. 2019;8:486. doi: 10.3390/antiox8100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandemoortele A. and De Meulenaer B., in Encyclopedia of Food Chemistry, ed. L. Melton, F. Shahidi and P. Varelis, Academic Press, Oxford, 2019, pp. 468–477 [Google Scholar]
- Requena J. R. Fu M.-X. Ahmed M. U. Jenkins A. J. Lyons T. J. Thorpe S. R. Nephrol., Dial., Transplant. 1996;11:48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- Fuentes V. Estévez M. Ventanas J. Ventanas S. Food Chem. 2014;147:70–77. doi: 10.1016/j.foodchem.2013.09.100. [DOI] [PubMed] [Google Scholar]
- Wang Z. He Z. Emara A. Gan X. Li H. Food Chem. 2019;288:405–412. doi: 10.1016/j.foodchem.2019.02.126. [DOI] [PubMed] [Google Scholar]
- Traverso N. Menini S. Maineri E. P. Patriarca S. Odetti P. Cottalasso D. Marinari U. M. Pronzato M. A. J. Gerontol., Ser. A. 2004;59:B890–B895. doi: 10.1093/gerona/59.9.B890. [DOI] [PubMed] [Google Scholar]
- Hellwig M. J. Agric. Food Chem. 2020;68:12870–12885. doi: 10.1021/acs.jafc.0c00711. [DOI] [PubMed] [Google Scholar]
- Shahidi F., in Handbook of Antioxidants for Food Preservation, Elsevier Science, 2015 [Google Scholar]
- Movileanu I. Núñez de González M. T. Hafley B. Miller R. K. Keeton J. T. Int. J. Food Sci. 2013;2013:360732. doi: 10.1155/2013/360732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admassu S. Adv. Food Technol. Nutr. Sci. 2019;5:38–49. [Google Scholar]
- Fuentes V. Ventanas J. Morcuende D. Estévez M. Ventanas S. Meat Sci. 2010;85:506–514. doi: 10.1016/j.meatsci.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Food Act 1983, in Food Act 1983 (Act 281) and Regulations: (as at 1st March 2013), International Law Book Services, Kuala Lumpur, Malaysia, 2013 [Google Scholar]
- Schaich K. M., in Oxidative stability and shelf life of foods containing oils and fats, Elsevier, 2016, pp. 1–131 [Google Scholar]
- AOAC, Official Methods of Analysis of AOAC International, in The Association of Official Analytical Chemists, AOAC International, Maryland, North Fredick Avenue, Gaithersburg, 18th edn, 2005, https://books.google.com.my/books?id=tD8bAQAAMAAJ&q [Google Scholar]
- Ibadullah W. Z. W., Lipid protein interactions in peanut butter, Doctoral Degree, Rutgers University-Graduate School-New Brunswick, 2013, https://rucore.libraries.rutgers.edu/rutgers-lib/41943/ [Google Scholar]
- AOCS, Official Method Ce 1–62: Fatty Acid Composition by Gas Chromatography, in Official Methods and Recommended Practices of the American Oil Chemists’ Society, American Oil Chemists’ Society, Champaign, IL, USA, 2005, https://www.aocs.org/ [Google Scholar]
- Estévez M. Ventanas S. Cava R. Meat Sci. 2006;74:396–403. doi: 10.1016/j.meatsci.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Utrera M. Parra V. Estévez M. Meat Sci. 2014;96:812–820. doi: 10.1016/j.meatsci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- AOCS, Official Method Th 1a–64: Diene Value of Drying Oils, in Official Methods and Recommended Practices of the American Oil Chemists’ Society, American Oil Chemists’ Society, Urbana, IL, USA, 2011, https://www.aocs.org/ [Google Scholar]
- Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Soglia F. Petracci M. Ertbjerg P. Food Chem. 2016;197:670–675. doi: 10.1016/j.foodchem.2015.11.038. [DOI] [PubMed] [Google Scholar]
- FAO, WHOCode of Hygienic Practice for Low-Moisture Foods, Rome, Italy, 2015, https://www.fao.org/input/download/standards/13921/CXP_075e_2015.pdf [Google Scholar]
- Zayas J. F., Functionality of proteins in food, Springer science & business media, 2012 [Google Scholar]
- Maktok kita serunding ayam, https://shopee.com.my/MAKTOK-KITA-SERUNDING-AYAM-SERUNDING-DAGING-200G-i.68775626.2393134824, accessed 25 February 2021
- Serunding ayam, https://www.serundingdaging.com, accessed 25 February 2021
- Serunding nogori ayam, https://awieserunding.wordpress.com/2016/05/30/blog-post-title/, accessed 25 February 2021
- Lin Y. Wang H. Rao W. Cui Y. Dai Z. Shen Q. Food Control. 2019;98:74–81. doi: 10.1016/j.foodcont.2018.11.016. [DOI] [Google Scholar]
- Sohaib M. Anjum F. M. Sahar A. Arshad M. S. Rahman U. U. Imran A. Hussain S. Int. J. Food Prop. 2017;20:2581–2593. doi: 10.1080/10942912.2016.1246456. [DOI] [Google Scholar]
- Botsoglou E. Govaris A. Ambrosiadis I. Fletouris D. Papageorgiou G. J. Sci. Food Agric. 2014;94:227–234. doi: 10.1002/jsfa.6236. [DOI] [PubMed] [Google Scholar]
- Fruehwirth S. Egger S. Flecker T. Ressler M. Firat N. Pignitter M. Antioxidants. 2021;10:59. doi: 10.3390/antiox10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowska M. Wójciak K. M. Dolatowski Z. J. Int. J. Food Sci. Technol. 2014;49:2563–2570. doi: 10.1111/ijfs.12586. [DOI] [Google Scholar]
- Sampaio G. Saldanha T. Soares R. Torres E. Food Chem. 2012;135:1383–1390. doi: 10.1016/j.foodchem.2012.05.103. [DOI] [PubMed] [Google Scholar]
- Pereira A. L. F. and Abreu V. K. G., in Lipid peroxidation research, ed. M. A. Mansour, Intechopen, London, United Kingdon, 2020, ch. 3, pp. 29–42 [Google Scholar]
- Xia C. Wen P. Yuan Y. Yu X. Chen Y. Xu H. Cui G. Wang J. RSC Adv. 2021;11(35):21629–21641. doi: 10.1039/D1RA03151A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.-K. Kim G.-D. Jeong J.-Y. J. Food Qual. 2021;2021:6636335. [Google Scholar]
- Papastergiadis A. Mubiru E. Van Langenhove H. De Meulenaer B. J. Agric. Food Chem. 2012;60:9589–9594. doi: 10.1021/jf302451c. [DOI] [PubMed] [Google Scholar]
- Park J.-H. Lee Y.-J. Lim J.-G. Jeon J.-H. Yoon K.-S. Foods. 2021;10(7):1601. doi: 10.3390/foods10071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madane P. Das A. K. Pateiro M. Nanda P. K. Bandyopadhyay S. Jagtap P. Barba F. J. Shewalkar A. Maity B. Lorenzo J. M. Foods. 2019;8(8):307. doi: 10.3390/foods8080307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateiro M. Domínguez R. Lorenzo J. M. Foods. 2021;10:1303. doi: 10.3390/foods10061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J. M. Vargas F. C. Strozzi I. Pateiro M. Furtado M. M. Sant'Ana A. S. Rocchetti G. Barba F. J. Dominguez R. Lucini L. do Amaral Sobral P. J. Food Res. Int. 2018;114:47–54. doi: 10.1016/j.foodres.2018.07.046. [DOI] [PubMed] [Google Scholar]
- Hematyar N. Rustad T. Sampels S. Kastrup Dalsgaard T. Aquacult. Res. 2019;50:1393–1403. doi: 10.1111/are.14012. [DOI] [Google Scholar]
- Feng Y.-H. Zhang S.-S. Sun B.-Z. Xie P. Wen K.-X. Xu C.-C. Foods. 2020;9:806. doi: 10.3390/foods9060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteghlal S. Gahruie H. H. Niakousari M. Barba F. J. Bekhit A. E.-D. Mallikarjunan K. Roohinejad S. Foods. 2019;8:262. doi: 10.3390/foods8070262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taşkıran M. Olum E. Candoğan K. J. Food Process. Preserv. 2020;44(2):e14324. [Google Scholar]
- Chen X. Xu X. Liu D. Zhou G. Han M. Wang P. Food Hydrocolloids. 2018;77:524–533. doi: 10.1016/j.foodhyd.2017.10.030. [DOI] [Google Scholar]
- Damodaran S., Parkin K. L. and Fennema O. R., Fennema's food chemistry, CRC Press, 2007 [Google Scholar]
- Carvalho R. H. Ida E. I. Madruga M. S. Martínez S. L. Shimokomaki M. Estévez M. Food Chem. 2017;215:129–137. doi: 10.1016/j.foodchem.2016.07.182. [DOI] [PubMed] [Google Scholar]
- Soladoye O. Juárez M. Aalhus J. Shand P. Estévez M. Compr. Rev. Food Sci. Food Saf. 2015;14:106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Schaich K. M., in Lipid Oxidation Pathways, ed. A. Kamal-Eldin and D. Min, AOCS Press, 2008, vol. 2, ch. 8, pp. 183–274 [Google Scholar]
- Utrera M. Estévez M. J. Agric. Food Chem. 2012;60:8002–8011. doi: 10.1021/jf302111j. [DOI] [PubMed] [Google Scholar]
- Villaverde A. Ventanas J. Estévez M. Meat Sci. 2014;98:665–672. doi: 10.1016/j.meatsci.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Hu M. and Jacobsen C., in Oxidative stability and shelf life of foods containing oils and fats, Elsevier, 2016 [Google Scholar]
- Sohaib M. Anjum F. M. Arshad M. S. Imran M. Imran A. Hussain S. Lipids Health Dis. 2017;16:27. doi: 10.1186/s12944-017-0426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Xiao S. Ahn D. U. Crit. Rev. Food Sci. Nutr. 2013;53:1191–1201. doi: 10.1080/10408398.2011.577540. [DOI] [PubMed] [Google Scholar]
- Nollet L. M. and Toldrá F., in Handbook of processed meats and poultry analysis, CRC Press, 2009 [Google Scholar]
- Chio K. Tappel A. L. Biochemistry. 1969;8:2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Nadkarni D. V. Sayre L. M. Chem. Res. Toxicol. 1995;8:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- Gatellier P. Kondjoyan A. Portanguen S. Sante-Lhoutellier V. Meat Sci. 2010;85:645–650. doi: 10.1016/j.meatsci.2010.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.