Abstract
While the immune system is essential for survival, an excessive or prolonged inflammatory response, such as that resulting from sustained heavy alcohol use, can damage the host and contribute to psychiatric disorders. A growing body of literature indicates that the immune system plays a critical role in the development and maintenance of alcohol use disorder (AUD). As such, there is enthusiasm for treatments that can restore healthy levels of inflammation as a mechanism to reduce drinking and promote recovery. In this qualitative literature review, we provide a conceptual rationale for immune therapies and discuss progress in medications development for AUD focused on the immune system as a treatment target. This review is organized into sections based on primary signaling pathways targeted by the candidate therapies, namely: (a) toll-like receptors, (b) phosphodiesterase inhibitors, (c) peroxisome proliferator-activated receptors, (d) microglia and astrocytes, (e) other immune pharmacotherapies, and (f) behavioral therapies. As relevant within each section, we examine the basic biological mechanisms of each class of therapy and evaluate preclinical research testing the role of the therapy on mitigating alcohol-related behaviors in animal models. To the extent available, translational findings are reviewed with discussion of completed and ongoing randomized clinical trials and their findings to date. An applied and clinically focused approach is taken to identify the potential clinical applications of the various treatments reviewed. We conclude by delineating the most promising candidate treatments and discussing future directions by considering opportunities for immune treatment development and personalized medicine for AUD.
Keywords: Alcohol use disorder, Inflammation, Medications development, Immune system, Neuroinflammation, Heavy alcohol use, Immune therapy, Neuroimmune modulator, Addiction
1. Introduction
A growing body of literature indicates that the immune system plays a critical role in the development and maintenance of alcohol use disorder (AUD) (Mayfield and Harris, 2017). Hence, there is increasing interest in the development of medications and therapies that target the immune system in an effort to treat AUD. This review addresses the conceptual rationale for immune treatments and highlights the potential for treatment approaches modulating the immune system to mitigate mechanisms contributing to AUD. The link between the immune system and AUD is supported by both basic and clinical findings.
Briefly, the immune system, which is comprised of both innate and adaptive immune mechanisms, serves as the body’s primary defense against pathogens and is critical for human well-being and health (Slavich and Irwin, 2014). Although the brain is protected by the blood–-brain barrier (BBB), it has resident immune defenses, including innate immune cells, to help protect against threats (Coleman and Crews, 2018). Microglia are considered resident macrophages of the brain and, along with astrocytes and neurons, contain receptors capable of immune signaling (Coleman and Crews, 2018). Innate immune signaling in the periphery can cross the BBB through several mechanisms, including immune-mediated active transport and disruptions in the BBB (Banks, 2015; Erickson et al., 2018; Quan and Banks, 2007). The innate immune branch responds rapidly and includes immune cells like monocytes and dendritic cells that circulate throughout the body. It is the first line of defense against bacterial infection or tissue injury and can initiate inflammatory cascades and activate adaptive immune processes (Medzhitov, 2008). Adaptive immunity takes over when the innate immune response is insufficient; it is slower but more specific (Bonilla and Oettgen, 2010). Adaptive immune mechanisms like T and B lymphocytes target antigens through an immunological memory of the pathogen (Slavich and Irwin, 2014).
During initial innate immune activation, inflammatory responses are triggered by detection of conserved features of microbes, termed pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) (Bonilla and Oettgen, 2010). LPS is an endotoxin component of the outer membrane of Gram-negative bacteria (Raetz and Whitfield, 2002). LPS levels are shown to be elevated in individuals with AUD (Qin et al., 2008); however, these levels normalize after 3 weeks of abstinence (Leclercq et al., 2012). Toll-like receptors (TLRs) are a common family of receptors found on immune cells and are known to recognize PAMPs and subsequently activate transcription factors, including nuclear factor-κB (NF-κB), interferon (IFN) regulatory factors, and cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) (Medzhitov, 2008; Aurelian et al., 2016; Balan et al., 2018). These activated factors then drive the expression of proinflammatory immune protein molecules, termed cytokines, which are released from immune cells, coordinate inflammatory cell functions, and have wide-ranging effects on physiological and behavioral responses (Dinarello, 2000). Types of cytokines have specific mechanisms and proinflammatory cytokine types include interleukin (IL)-1, IL-2, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) (Erickson et al., 2019).
Alcohol is thought to alter immune signaling and increase neuroinflammation via two primary mechanisms: (a) indirectly by initiating systemic production of proinflammatory cytokines; and (b) directly through actions in the brain, whereby alcohol and potentially alcohol-induced neural damage (de la Monte and Kril, 2014) stimulate the release of inflammatory molecules (Crews and Vetreno, 2016). Individuals with AUD display peripheral immune dysregulation and are vulnerable to viral or bacterial infections (Keshavarzian et al., 2009). Systemic inflammation appears to be induced by alcohol when it acts on peripheral immune receptors in the gut (Erickson et al., 2019) and also by breaking down lymphatic duct lining and endothelial cell junctions, allowing inflammatory molecules to leak into the bloodstream, termed “leaky gut” (Gorky and Schwaber, 2016). The resultant proinflammatory molecules in the periphery then provoke neuroinflammation, i.e., an inflammatory response within the central nervous system (CNS) as opposed to in the periphery. This provocation occurs through several mechanisms, such as inflammatory molecules crossing the BBB via immune-mediated active transport or by entering the brain through disruptions in the BBB (Banks, 2015; Quan and Banks, 2007). Additionally, receptor binding of inflammatory cytokines at vagal afferent sites (e.g., in stomach and liver) rapidly results in the transduction of inflammatory signaling in the CNS (Quan and Banks, 2007). Proinflammatory molecules in the brain impact neural circuit functioning and neuronal plasticity (Erickson et al., 2019). Notably, neuroinflammation can be both adaptive, such as in response to brain injury to promote repair, or maladaptive, such as in response chronic social stressors (DiSabato et al., 2016). Thus, while the immune system is essential for survival, an excessive or prolonged inflammatory response, such as that resulting from sustained heavy alcohol use, can damage the host and contribute to psychiatric and physical disorders (Slavich and Irwin, 2014).
Initial support for the relationship between alcohol use and neuroinflammation came from gene expression studies of post-mortem brain tissue (McBride et al., 2014; Osterndorff-Kahanek et al., 2015). These studies demonstrated consistent upregulation in the expression of genes involved in inflammatory responses in the brains of individuals with AUD (Liu et al., 2006; Liu et al., 2004; Robinson et al., 2014). Similar findings were obtained in a reverse-translation (i.e., leveraging insights from human studies to inform mechanistic and preclinical work) to rodents exposed to chronic ethanol. Voluntary ethanol consumption increased cytokines and chemokines in the CNS and periphery for mice (Pascual et al., 2015) and monkeys (Beattie et al., 2018). In rats, 24–48 h of ethanol withdrawal, a critical window of reinstatement, resulted in the upregulation of mRNA proinflammatory expression of innate immune markers (e.g., TNF-α, IL-1β) in cortical tissue (Freeman et al., 2012; Whitman et al., 2013). These findings indicate immune signaling upregulated during alcohol withdrawal may contribute to the maintenance of AUD. Importantly, immune factors mediate not only neuroinflammation but a broad set of neural functions, including neurotransmitter systems and synaptic function, neurogenesis and neurodevelopment, and endocrine function (Cui et al., 2014). For instance, research suggests that alcohol disrupts the ability of astrocytes to properly regulate glutamate homeostasis, which contributes to the development of sustained drinking (Bachtell et al., 2017). Emerging work also supports the involvement of neuroimmune signaling in adolescent binge drinking and subsequent changes in brain physiology (Crews et al., 2019; Crews et al., 2017; Montesinos et al., 2016; Pascual et al., 2018). Further, preclinical work suggests that neuroinflammation and modulation of immune signaling induced by chronic alcohol use heighten motivation for intake, enhances alcohol-related reward, and contributes to substance-related cognitive impairments and depression-like behavior (Alfonso-Loeches et al., 2010; Blednov et al., 2018; Breese et al., 2008; Briones and Woods, 2013; Frank et al., 2011).
Evidence for heightened CNS activation of inflammatory signaling in human samples with AUD is very limited and further research is necessary to establish the neuroimmune hypothesis of AUD. Studies evaluating this hypothesis have largely used positron emission tomography (PET) to image the translator protein (TSPO), a mitochondrial protein that is upregulated during neuroinflammation. Three studies have reported reduced binding of PET TSPO ligands in the brains of individuals with AUD relative to controls (Feldman et al., 2020); however, these findings are discrepant with in vitro animal studies and complicated by genotype specific responding to TSPO ligands (Kreisl et al., 2013). Elevations in TSPO mRNA in postmortem brains from individuals with an AUD provide initial evidence for the neuroinflammation hypothesis (De Carvalho et al., 2021). Several studies demonstrate elevated peripheral inflammation in clinical AUD samples. Whereas this work has been largely correlational, it generally supports the hypothesized link between inflammation and AUD. In treatment-seeking individuals, elevated levels of circulating LPS were found at treatment onset but decreased after 3-weeks of detoxification, reaching levels comparable to controls (Leclercq et al., 2012). Proinflammatory proteins, including TNF-α, IL-6, and C-reactive protein (CRP), were positively correlated with craving at treatment entry among individuals with AUD (Leclercq et al., 2012). However, not all studies have found elevations in LPS proinflammatory protein levels, indicating that this peripheral inflammatory response may be present in only a subset of individuals with AUD (Adams et al., 2020). To what extent alcohol induction of peripheral inflammation increases neuroimmune signaling in clinical samples is not known, although this link is established in basic studies. Translational work is beginning to guide these efforts. For example, when fecal microbiota were transplanted from patients with AUD to germ-free mice (Leclercq et al., 2020), CNS alterations in myelination, neurotransmission, and inflammation occurred, with evidence of an increased expression of proinflammatory cytokines and chemokines and elevated markers of microglial activation. To that end, novel treatment targets, such as peripheral and neural immune pathways, represent an important direction in the development of novel and more effective treatment options for AUD (Litten et al., 2016; Ray et al., 2014) and psychiatric diseases more broadly. While the current review focuses on the application of immune interventions for AUD, literature in this area is broad and recent reviews have addressed other topics relevant to immunity and AUD in detail (Coleman and Crews, 2018; Erickson et al., 2019; Cui et al., 2014; Crews et al., 2017; Gao et al., 2019; Jimenez-Gonzalez et al., 2021).
In this qualitative literature review, we discuss recent advances in medications development for AUD focusing on the immune system as a treatment target. Based on the implication of immune mechanisms to the phenomenology of AUD, briefly reviewed above, there is enthusiasm for treatments that can restore healthy levels of inflammation and immune signaling as a mechanism to reduce drinking and promote recovery (see Fig. 1). This review is organized into sections based on the primary signaling pathway targeted by the candidate therapies, namely: (a) TLRs, (b) phosphodiesterase (PDE) inhibitors, (c) peroxisome proliferator-activated receptors (PPARs), (d) microglia and astrocytes, (e) other immune pharmacotherapies, and (f) behavioral therapies. We use these categories for organizational purposes, as we recognize that these distinctions are inherently arbitrary and that there is complex interplay across signaling pathways and molecules. After reviewing the relevant literature, we consider future directions and opportunities for treatment development and personalized medicine for AUD. As the field continues to evolve and more clinical studies are added to the robust preclinical literature on alcohol and inflammation, a refined understanding of immune targets for AUD will continue to emerge. Consistent with the ongoing challenge of translational science in AUD (Ray et al., 2021), we emphasize avenues for applying these findings to clinical populations. To that end, we take an applied and clinically focused approach to identifying potential clinical applications of the various treatments reviewed herein. We contend that biological and clinical plausibility are both necessary in order to optimize treatment development.
Fig. 1.
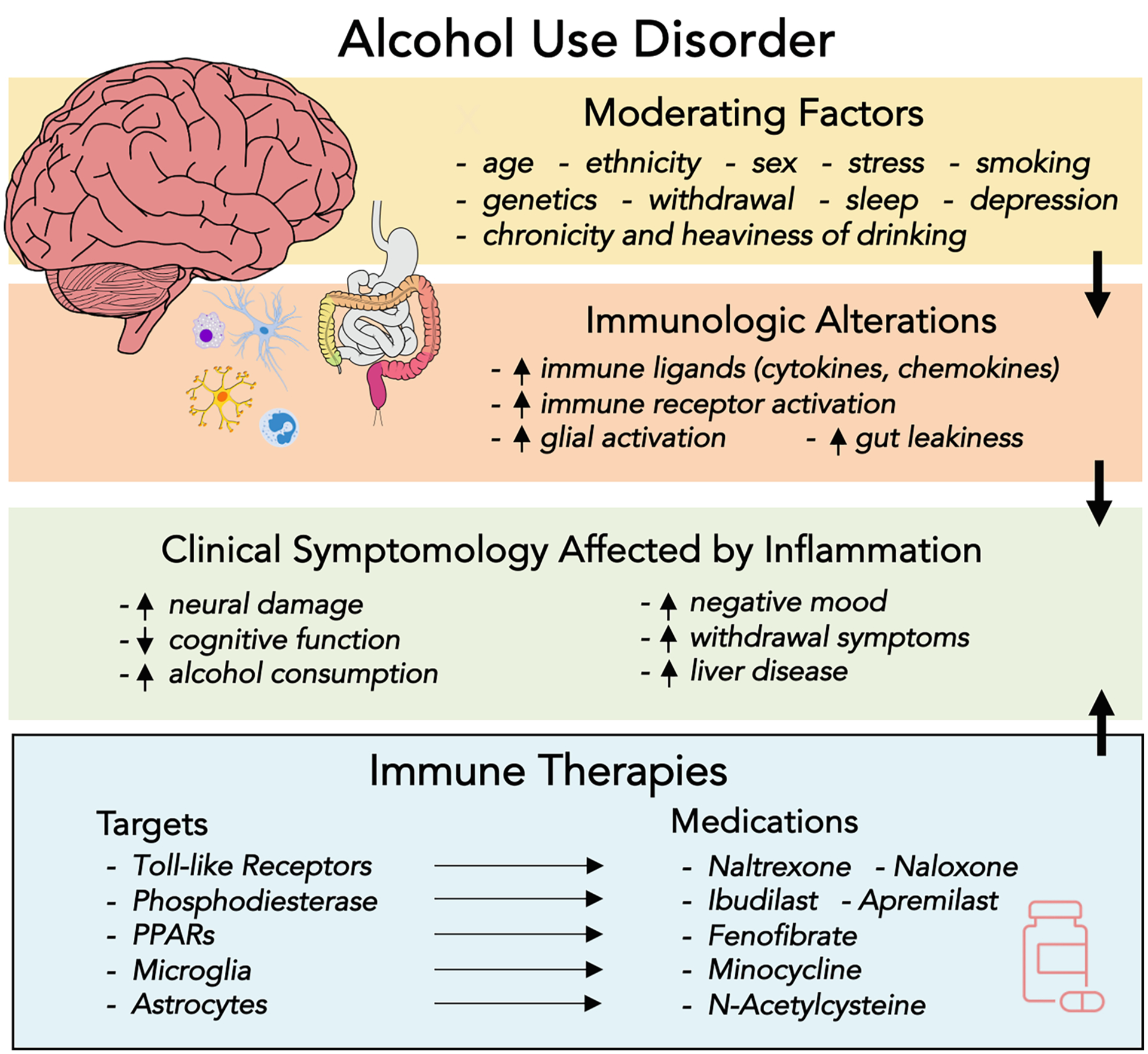
Brain-Immune Interactions in Alcohol Use Disorder. Potential moderators of the relationship between alcohol use disorder (AUD) and the immune system include factors such as age, sex, stress, sleep, and smoking. Multiple aspects of the immune system are altered by chronic alcohol consumption, including increased concentrations of proinflammatory immune ligands, increased immune receptor and glial activation, and breakdown of down lymphatic duct lining and endothelial cell junctions (i.e., gut leakiness). In return, inflammation and immune imbalance are thought to affect clinical symptoms of AUD, ranging from negative mood and cognitive dysfunction to withdrawal symptoms and liver disease. The pharmacological immune therapies discussed in the current review act on specific immune targets to potentially mitigate the effects of immunologic alterations and associated clinical symptomatology in AUD; PPARs = peroxisome proliferator-activated receptors.
1.1. Targets for candidate immune therapies for AUD
The following sections describes various candidate immune therapies for AUD. Within each section, we examine the basic biological mechanisms of each class of therapy and evaluate the basic and preclinical research testing the role of the therapy in mitigating alcohol behaviors in animal models. To the extent available, translations findings are reviewed with discussion of completed and ongoing interventional trials and findings to date.
1.1.1. Toll-Like receptors
TLRs are members of the IL-1 receptor/TLR superfamily. As reviewed above, TLRs, along with proinflammatory cytokines and their associated receptors, share signaling pathways that converge on NF-κB, an innate immune transcription factor that regulates inflammatory cytokine expression (Crews et al., 2017). These pathways are widely implicated in alcohol-induced neuroinflammation (Crews et al., 2017; Bajo et al., 2016; Montesinos et al., 2017), with human brain tissue from individuals with AUD showing an upregulation of several TLRs.
Ten TLRs have been identified in humans and the most widely studied subtype within this family is TLR4, which is thought to contribute significantly to alcohol-related neuroimmune activation. TLR4 activation plays an important role in regulating neuroimmune signals that influence alcohol intake. The TLR4 signal is innately activated in neurons from alcohol-preferring rats and TLR4-MyD88 proinflammatory cytokines are inhibited after acute exposure (Muralidharan et al., 2018). The TLR4 signal is activated through the non-canonical TLR4 binding of the GABAAR α2 subunit (Balan et al., 2018; Liu et al., 2011). During alcohol self-administration, this signal is sustained through increased expression of the stress hormone corticotropin-releasing factor (CRF) and its feedback regulation of TLR4 signaling (Balan et al., 2018; June et al., 2015). The balance of the resulting pro-and anti-inflammatory chemokines likely contributes to the transition to alcohol dependence. Further, in neurons from the central nucleus of the amygdala (CeA) and ventral tegmental area (VTA), TLR4 signals through the chemokine CCL2 (Aurelian and Balan, 2019; Zhou et al., 2011) localizing in dopaminergic neurons, and inducing the expression of tyrosine hydroxylase through CREB signal (Aurelian et al., 2016; Banisadr et al., 2005). In CeA neurons, CCL2 is localized to the synapse and transported via axons to downstream brain regions, such as the bed nucleus of the stria terminalis, and is thought to affect behaviors such as anxiety. Neurons are thus an important target for chemokine function independent of inflammation but related to relevant behavior, including impulsivity and alcohol intake (Harper et al., 2020).
Results from studies of TLR-affecting medications in the context of AUD implicate TLRs as promising targets for the development of AUD therapeutics (see Table 1). TLR4 blockade by opioid antagonists, including naltrexone and naloxone, has been extensively tested in animal models. Both the (+) and (−) isomers of naltrexone and naloxone are considered TLR4 antagonists (Skolnick et al., 2014; Wang et al., 2016). The fact that the opioid-inactive (+) isomer acts similarly on TLR4 as the opioid-active (−) isomer, denotes an immune mechanism likely independent of the opioid effects of these pharmacotherapies (Wang et al., 2016; Hutchinson et al., 2008). In regards to alcohol effects, TLR4 blockade by (+)-naltrexone reduces binge drinking in adolescent mice (Jacobsen et al., 2018) and decreases ethanol preference (Jacobsen et al., 2018). In healthy mice using opioid-inactive naloxone to block TLR4 reduced acute alcohol-induced motor impairment and sedation (Wu et al., 2012). In contrast, another study found that (+)-naloxone produced very modest inhibition of intake among rodents and only at the highest dose (Harris et al., 2017). Moreover, nalmefene, another opioid receptor antagonist that inhibits TLR4 signaling, reduced ethanol-induced inflammation and binge-like drinking behaviors in adolescent female mice by preventing TLR4 activation (Montesinos et al., 2017).
Table 1.
Toll-Like Receptors.
| Immunotherapy | Potential Immune Target | Animal Study Findings | Human Study Findings | References |
|---|---|---|---|---|
| Toll-Like Receptors (TLRs): | ||||
| Naltrexone | TLR4 | ↓ binge drinking in adulthood ↓ alcohol preference ↓ immune-related gene mRNA expression |
↓ return to drinking ↓ heavy drinking days |
(Jonas et al., 2014) (Jacobsen et al., 2018) (Jacobsen et al., 2018) |
| Naloxone | TLR4 | ↓ alcohol-induced sedation ↓ alcohol-induced motor impairment |
– | (Wu et al., 2012) (Harris et al., 2017) |
| Nalmefene | TLR4 | ↓ alcohol-induced neuroinflammation ↓ binge drinking |
↓ heavy drinking days ↓ drinks per drinking day |
(Karhuvaara et al., 2007) (Montesinos et al., 2017) |
| Sulfasalazine | IKKβ inhibition | ↓ ethanol intake and preference | – | (Truitt et al., 2016) |
| TPCA-1 | IKKβ inhibition | ↓ ethanol intake and preference | – | (Truitt et al., 2016) |
| Amlexanox | TLR3/TRIF inhibition | ↓ ethanol consumption | – | (McCarthy et al., 2018) |
| T5342126 | TLR4 | ↓ ethanol consumption ↓ microglial activation marker |
– | (Bajo et al., 2016) |
While TLR4 is the most studied member of the TLR family in the context of AUD, other TLR pathways modulating NF-κB signaling, including TLR2 and TLR3 (Erickson et al., 2019) are similarly implicated in the neuroimmune effects of alcohol in several preclinical studies (Pascual et al., 2015; Blednov et al., 2017; Fernandez-Lizarbe et al., 2013; McCarthy et al., 2018). Given that NF-κB is a target of multiple TLRs, it is likely that multiple receptors work in concert and convergently act on NF-κB. Therefore, exploring pharmacological antagonists of TLR subclasses beyond TLR4 may be worthwhile. Neuroimmune therapies that bypass TLR binding to act directly on NF-κB have also shown promise in preclinical work for the treatment of AUD. Immuno-therapies such as sulfasalazine and TPCA-1 act on NF-κB through IKKβ, an inhibitor of the NF-κB kinase subunit beta. Both of these pharmacological inhibitors of IKKβ have been shown to decrease ethanol consumption and preference in mice (Truitt et al., 2016). Amlexanox, another NF-κB inhibitor and anti-inflammatory, anti-allergic pharmacological immunomodulator involved in several TLR pathways (Reilly et al., 2013) reduced ethanol consumption and preference in mice completing a two-bottle-choice paradigm (McCarthy et al., 2018).
While the majority of work exploring neuroimmune modulators of TLR and NF-κB are preclinical in nature, numerous human studies have evaluated established opioid antagonists, such as naltrexone and nalmefene. Naltrexone is FDA-approved for treatment of AUD and nalmefene is approved in Europe for harm-reduction (Swift and Aston, 2015). Naltrexone was associated with drinking reductions, including longer latency to return to any drinking and reduction in heavy drinking days (Jonas et al., 2014). Nalmefene was also associated with moderate reductions in heavy drinking days and drinks per drinking day (Karhuvaara et al., 2007). Importantly, existing human studies have not specifically examined their neuroimmune mechanisms and instead focus on opioid receptor mechanisms. Therefore, it remains uncertain whether drinking outcomes relate to neuroimmune properties.
In sum, it is likely that several subclasses of TLRs work together along with other neuroimmune factors to influence drinking-related behaviors. While naltrexone and nalmefene are well-established pharmacotherapies for addiction, questions remain about the biological mechanisms (e.g., opioid and/or immune system) through which they affect AUD-related behavioral outcomes in humans. Experimental trials seeking to test their specific anti-inflammatory actions would help address this knowledge gap, particularly by comparing the opioid-inactive vs. -active isomers. NF-κB, IKKε, and direct TLR inhibitors, such as sulfasalazine and amlexanox, have not yet progressed to use in human clinical trials for AUD, although preclinical results demonstrate beneficial effects on ethanol consumption and preference, which support their potential for further medications development. Several of these compounds demonstrate safety and tolerability in other clinical samples for the treatment of inflammatory medical conditions like rheumatoid arthritis and ulcerative colitis (Liu et al., 2006; Plosker and Croom, 2005). However, while sulfasalazine shows a relatively safe side effect profile, serious adverse events like low white blood cell count (i.e., leukopenia) are known to occur in rare cases (Plosker and Croom, 2005). Future work that tests novel TLR inhibitors for their safety, particularly medication × alcohol interactions, and early efficacy markers would be critical in facilitating the progression of medications development for this class of drugs from preclinical to clinical studies of AUD.
1.1.2. Phosphodiesterase inhibitors
Cyclic nucleotide PDEs are a family of phosphohydrolases. PDEs are the only known enzymes to regulate the intracellular levels of cAMP and cyclic guanosine monophosphate (cGMP) (Wen et al., 2018). Thus, PDEs play a critical role in regulating the intracellular levels of cAMP and cGMP as well as their downstream signal transductions. There are 11 PDE subtypes widely distributed in the CNS (Menniti et al., 2006), which can be divided into three categories based on their substrate specificity: (1) cAMP specific; (2) cGMP specific; and (3) dual-substrate PDEs. These subtypes are differentially distributed in the brain and have unique roles regulating neuronal function, indicating that targeted inhibition of specific isoforms may provide the best therapeutic benefits.
cAMP and cGMP signaling pathways play a key role in neural functions and synaptic transmission in the CNS as well as the downregulation of NF-κB and proinflammatory cytokine release (Wen et al., 2018; Parry and Mackman, 1997). PDEs play a crucial role in maintaining cyclic nucleotide levels, and therefore, regulate intracellular signaling cascades that use cAMP and cGMP as second messengers. Of particular importance, PDEs modulate the cAMP protein kinase (PKA) pathway, which has been implicated in the regulation of response to acute and chronic alcohol exposure (Logrip, 2015). Acute alcohol exposure leads to activation of cAMP signal transduction; conversely, chronic alcohol exposure attenuates this signaling pathway in a brain-region specific manner (Wen et al., 2018). Alcohol withdrawal, thought to be an important driver of severe AUD, also decreases cAMP signal transduction in the cortex and amygdala in the rat (Pandey et al., 2003). cGMP signaling may also be involved in alcohol-drinking behavior; however, it has been less studied than cAMP. Rats exposed to chronic ethanol have increased cGMP levels in various brain regions including the striatum, hippocampus, and cortex; abstinence from ethanol lowers the cGMP levels back to normal (Uzbay et al., 2004). Given the critical role of these signaling pathways in alcohol drinking behaviors, normalization of their signaling is of interest for treating AUD. Specifically, PDE sub-family inhibitors have been proposed as promising therapeutics for AUD (see Table 2).
Table 2.
Phosphodiesterase Inhibitors.
| Immunotherapy | Potential Immune Target | Animal Study Findings | Human Study Findings | References |
|---|---|---|---|---|
| Phosphodiesterase (PDE) Inhibitors: | ||||
| Ibudilast | PDE3 − 4–10 & − 11 | ↓ ethanol consumption | ↓ alcohol craving ↑ mood outcomes ↓ heavy drinking days clinical trial underway: NCT03594435 completed clinical trials: NCT03489850 NCT02025998 |
(Bell et al., 2015) (Ray et al., 2017) (Grodin et al., 2021) |
| Rolipram | PDE4 | ↓ ethanol intake and preference | – | (Hu et al., 2011) (Wen et al., 2012) (Blednov et al., 2014) (Franklin et al., 2015) (Gong et al., 2017) (Ozburn et al., 2020) |
| Mesopram | PDE4 | ↓ ethanol intake and preference | – | (Blednov et al., 2014) |
| Piclamilast | PDE4 | ↓ ethanol intake and preference | – | (Blednov et al., 2014) |
| CDP840 | PDE4 | ↓ ethanol intake and preference | – | (Blednov et al., 2014) |
| Apremilast | PDE4 | ↑ ethanol-inducedsedation and intoxication no effect on ethanol CPP or withdrawal ↓ ethanol intake and preference |
clinical trial completed: NCT03175549 |
(Blednov et al., 2018) (Blednov et al., 2018) |
| Rofumilast | PDE4 | ↓ ethanol intake and preference | – | (Liu et al., 2017) |
| TP-10 | PDE10 | ↓ relapse-like alcohol self-administration | – | (Logrip et al., 2014) |
Note. CPP = conditioned place preference
PDE inhibitors have been widely studied using preclinical animal models of AUD with particular focus on PDE4 inhibition, as PDE4 is expressed in several brain regions that underly the reinforcing effects of alcohol (e.g., nucleus accumbens, amygdala, and VTA) (Perez-Torres et al., 2000). Rolipram, a selective PDE4 inhibitor, has shown promising preclinical efficacy. Rolipram reduced alcohol intake and preference in several strains of mice (Hu et al., 2011; Blednov et al., 2014; Ozburn et al., 2020), decreased alcohol seeking in alcohol-preferring drinking rats (Wen et al., 2012; Franklin et al., 2015), and attenuated abstinence-like anxious and depressive behavior in mice (Gong et al., 2017). Despite these promising findings, rolipram does not have a desirable side effect profile in humans as it commonly induces significant nausea and emesis thought to be caused by its high affinity for the PDE4 subtype D. Other selective PDE4 inhibitors have been evaluated in preclinical mouse models, including mesopram, piclamilast, and CDP840 (Blednov et al., 2014). In a 24-hour two-bottle choice test, all three compounds showed efficacy at reducing ethanol intake and preference but only mesopram produced long-lasting reductions. Yet, these compounds are not without their own side effect concerns, as there is a correlation between high affinity binding of these PDE4 inhibitors and emetic activity (Gong et al., 2017). Roflumilast is a second generation PDE4 inhibitor with FDA approval for chronic obstructive pulmonary disease. In mice, roflumilast decreased ethanol intake and preference in two drinking paradigms and did not impact sucrose or quinine drinking (Liu et al., 2017). However, it was much less potent for reducing drinking than rolipram, which may be attributable to its poor ability to penetrate the BBB. Finally, apremilast, a partial competitive PDE4 inhibitor, is FDA-approved for the treatment of psoriasis. Apremilast has a better side effect profile than the PDE4 inhibitors reviewed above, possibly because it does not demonstrate PDE4 subfamily (A to D) selectivity (Schafer et al., 2010). Favorably, apremilast reduced ethanol intake and preference in mice but did not modify sucrose preference, indicating its effects may be alcohol-specific (Blednov et al., 2018). This compound may impact ethanol consumption and preference by increasing the aversive properties of ethanol, including decreasing functional tolerance and increasing sedative effects (Blednov et al., 2018).
Other preclinical work has investigated the inhibition of other PDE-subtypes. PDE10 inhibition has been evaluated in preclinical rat models through TP-10, a specific PDE10A inhibitor (Logrip et al., 2014). TP-10 reduced alcohol self-administration in alcohol-preferring and dependent- and non-dependent rats. However, TP-10 also reduced saccharin self-administration, indicating that it may have a broader effect on reinforcing substances, which could limit translation to humans. Of note, inhibitors of PDE1 (vinpocetine), PDE3 (olprinone, milrinone), PDE5 (zaprinast), and a non-selective PDE inhibitor (propentofylline) have all been tested in animal models with null results (Blednov et al., 2014). Finally, ibudilast, which is a selective PDE inhibitor, with preferential inhibition of PDE3A, PDE4, PDE10A, and PDE11A, has been tested in preclinical research (Gibson et al., 2006). Ibudilast reduced drinking and relapse in multiple animal models of AUD, and critically, has been shown to preferentially reduce drinking in dependent, compared to non-dependent mice (Bell et al., 2015). Specifically, ibudilast reduced drinking by~50% in alcohol-preferring and high-alcohol drinking rats, during both maintenance and relapse tests. It is suspected that ibudilast’s effects on alcohol drinking are primarily driven by the inhibition of PDE4 and PDE10A.
At present two completed randomized controlled trials investigating the effect of PDE inhibition in humans with AUD have been published. A human laboratory trial of ibudilast with a crossover design was conducted in a non-treatment seeking sample with AUD. Ibudilast decreased tonic craving for alcohol and improved mood following alcohol cue and stress exposure (Ray et al., 2017). A two-week experimental medicine trial of ibudilast conducted by the same laboratory similarly enrolled a non-treatment seeking sample with AUD and results demonstrated that ibudilast reduced rates of heavy drinking and neural alcohol cue-reactivity compared with placebo (Grodin et al., 2021).
Taken together, PDE inhibitors represent promising novel compounds to treat AUD and may be particularly effective at reducing alcohol preference, relapse, and negative mood associated with withdrawal. Ibudilast and apremilast have the best translational potential, particularly due to their tolerability, and are under investigation in large scale clinical trials. Specifically, ibudilast (50 mg, bis in die (b.i.d. or twice a day) is being evaluated in a 12-week randomized clinical trial in treatment-seeking individuals with AUD (NCT03594435) with a primary outcome of percent heavy drinking days and an additional aim to examine peripheral markers of inflammation, and depressive symptomology. Apremilast (50 mg, b.i.d.) is being investigated in a two-week clinical trial in non-treatment-seeking individuals with AUD (NCT03175549) to assess alcohol cue-induced craving and drinking. At this time, first generation PDE4 inhibitors do not show translational potential due to their unfavorable side effect profile. However, next-generation PDE4 inhibitors, particularly those targeting the PDE4B subtype, hold promise as they are designed with human translation at the forefront. While PDE10A inhibitors may have translational potential, more preclinical work must be done to evaluate if PDE10A inhibition causes unfavorable, wide-ranging reductions in reward seeking behaviors. Future research should validate the immunomodulatory actions of PDE inhibitors by measuring medication-induced changes in markers of inflammation in samples of AUD and further connect these changes to meaningful clinical outcomes.
1.1.3. Peroxisome proliferator-activated receptors
PPARs are transcription factors and members of the nuclear hormone receptor superfamily that have been tested for their potential role in addiction processes (Ray et al., 2014; Cippitelli et al., 2017). PPARs form a ligand-activated heterodimer partnership with retinoid X receptors; this dimer binds to a particular DNA sequence element, referred to as the peroxisome proliferator response element. PPAR actions can attenuate proinflammatory innate immune signaling (Michalik et al., 2006) and regulate other cellular and physiological processes, such as glucose metabolism, cellular differentiation and proliferation, and lipid-homeostasis. PPARs are thought to modulate pathways involved in NF-κB and nitric oxide (NO) production and inhibit expression of TNF-α (Berger and Moller, 2002; Scirpo et al., 2015). The three known isoforms, PPARα, PPARβ/δ, and PPARγ, are each transcribed from different genes (Berger and Moller, 2002) and are located in peripheral tissues and neural regions implicated in AUD (Moreno et al., 2004). These isoforms are activated by eicosanoids and fatty acids and display broad albeit tissue-specific expression patterns (Michalik et al., 2006). PPARα is highly expressed in organs carrying out catabolism of fatty acids, PPARβ/δ shows the broadest expression patterns, and PPARγ is expressed in adipose tissue and more widely in the brain, gut, and immune cells. Generally, PPARs are distributed throughout the brain in neuronal and glial cell types and are suggested to be involved in neuromodulation through the regulation of genes encoding for neurotransmitter receptors, metabolism, and release (Moreno et al., 2004). The PPARα and PPARγ isoforms are of particular interest to the addictions field, as their receptors may be involved in modulation of dopamine and GABA transmission in mesocorticolimbic circuitries as well as providing neuroprotection against oxidative damage (Ray et al., 2014; Mascia et al., 2011; Melis et al., 2008).
PPAR agonists are anti-inflammatory compounds used to treat insulin resistance in diabetes and hyperlipidemia (Chigurupati et al., 2015) and show promise as immune therapies for AUD (see Table 3) and CNS diseases more broadly (Erickson et al., 2019; Le Foll et al., 2013). Early preclinical work on these targets evidenced the role of PPARs in regulating ethanol intake, stress-induced ethanol seeking, and withdrawal (Le Foll et al., 2013). The PPARγ isoform is suspected to be expressed in dopaminergic cells, as it colocalizes with tyrosine hydroxylase in the VTA (Le Foll et al., 2013; Stopponi et al., 2013). In alcohol-preferring male mice, PPAR agonists modulated treatment-response genes in the amygdala, prefrontal cortex, and liver, suggesting these AUD-relevant gene targets may mediate reductions in ethanol intake (Ferguson et al., 2014). The PPARγ agonist pioglitazone has been tested extensively in animal models of AUD. Pioglitazone affected several measures of alcohol-related behaviors in rats, including reductions in voluntary drinking, lever pressing, and reinstatement of alcohol-seeking behavior, but not prevention of cue-induced relapse (Stopponi et al., 2011). Behavioral modifications were not due to changes in alcohol metabolism or blood glucose levels, suggesting that alcohol-related changes were not due to metabolic effects (Stopponi et al., 2011). A later investigation in rats combined pioglitazone with an FDA-approved medication for AUD, naltrexone, and revealed larger reductions for alcohol drinking with this combined administration (Stopponi et al., 2013). These findings illustrate the potential added benefit of combining neuroimmune therapies with existing, approved medications to treat AUD. Intriguingly, pioglitazone may also have anxiolytic properties involving areas of the VTA and amygdala, as it modulated yohimbine stress-induced reinstatement of alcohol seeking (Fotio et al., 2020), along with neuroprotective properties that prevent alcohol-induced neuronal and cognitive damage (Cippitelli et al., 2017).
Table 3.
Peroxisome Proliferator-Activated Receptors.
| Immunotherapy | Potential Immune Target | Animal Studies Findings | Human Studies Findings | References |
|---|---|---|---|---|
| Peroxisome Proliferator-Activated Receptors (PPARs): | ||||
| Gemfibrozil | PPARα activation | ↓ ethanol intake | – | (Barson et al., 2009) |
| Fenofibrate | PPARα activation | ↓ ethanol consumption and preference ↓ self-administration ↑ ethanol intake |
clinical trial completed: NCT02158273 | (Ferguson et al., 2014) (Karahanian et al., 2014) (Blednov et al., 2015) (Blednov et al., 2016) (Blednov et al., 2016) (Haile and Kosten, 2017) (Ozburn et al., 2020) |
| Pioglitazone | PPARγ activation | ↓ ethanol intake and preference ↑ protection against alcohol-induced impairment ↓ ethanol self-administration ↓ ethanol cue-induced reinstatement ↓ ethanol-induced IL-6 & IL-1β expression |
poor safety and tolerability clinical trial underway: NCT03864146 clinical trials halted: NCT03860753 NCT01631630 |
(Blednov et al., 2015) (Stopponi et al., 2011) (Stopponi et al., 2013) (Cippitelli et al., 2017) (Fotio et al., 2020) (Schwandt et al., 2020) |
| Tesaglitazar | PPARα/PPARγ activation | ↓ ethanol consumption and preference no effect on ethanol intake |
– | (Ferguson et al., 2014) (Blednov et al., 2015) (Blednov et al., 2016) (Blednov et al., 2016) (Ozburn et al., 2020) |
| Bezafibrate | PPARα/PPARγ/PPARδ activation | ↓ ethanol intake and preference no effect on ethanol intake |
– | (Ferguson et al., 2014) (Blednov et al., 2015) |
| N-acylethanolamines (OEA) | PPARα in intestinal cells | ↓ ethanol cue-induced reinstatement ↓ behavioral symptoms of withdrawal ↓ ethanol-induced IL-1β, COX-2, TNF-α, iNOS, MCP-1 ↓ ethanol-induced expression of HMGB1, TLR4, NF-κB |
decreases in inhibition correlate with reduced alcohol intake | (Bilbao et al., 2016) (van Kooten et al., 2016) (Antón et al., 2017) |
| Aspirin | PPAR | ↓ ethanol intake ↓ relapse-like binge drinking |
– | (Israel et al., 2021) |
Note. IL = interleukin; COX = cyclooxygenase; iNOS = inducible nitric oxide synthase; TNF-α = tumor necrosis factor-α; MCP-1 = monocyte chemotactic protein-1; HMGB1 = high mobility group box protein 1; NF-κB = nuclear factor-κB; TLR4 = toll-like receptor 4
Several other PPAR agonists have been tested in animal models. In mice, fenofibrate (PPARα), tesaglitazar (dual agonist: PPARα/γ), and bezafibrate (pan agonist: PPARα/γ/δ) were independently tested (Ferguson et al., 2014; Blednov et al., 2015). While fenofibrate and tesaglitazar produced long-lasting reductions in alcohol intake, bezafibrate produced mostly null results. Fenofibrate and tesaglitazar also reduced novelty response and increased acute withdrawal severity (Blednov et al., 2016), but did not modify conditioned place preference for alcohol (Blednov et al., 2016). In a rat model, fenofibrate treatment had dose-dependent effects on self-administration and reduced both the reinforcing and motivational effects of alcohol (Haile and Kosten, 2017). The mechanisms by which fenofibrate reduces alcohol consumption may be partially due to its effects on genes involved in energy metabolism, as its administration resulted in increased levels of blood acetaldehyde, which is aversive and similar to the effects of disulfiram, an FDA-approved medication for AUD. The effects of PPAR agonists may not be uniform, with mice’ responsiveness depending on drinking paradigm, sex, and genotype (Ozburn et al., 2020; Blednov et al., 2016; Blednov et al., 2016). For example, Blednov and colleagues (Blednov et al., 2016; Blednov et al., 2016) determined that males exhibited larger changes in alcohol consumption than females during fenofibrate and tesaglitazar administration.
Importantly, PPAR activation may exert effects on alcohol behaviors through both central and peripheral immune modulation (Erickson et al., 2019) and this corresponds to an increased interest in the function of peripheral inflammation in AUD. Several PPARα agonists with actions in the periphery, such as the intestinal tract, have been tested in animal models. Oleoylethanolamide (OEA) is endocannabinoid-like compound with anti-inflammatory properties mediated by PPARα activation that may reduce the permeability of intestinal cells (i.e., “leaky gut”) (Antón et al., 2017; Karwad et al., 2017). In animal models, OEA, a known satiety factor, blocked cue-induced reinstatement of alcohol-seeking and reduced withdrawal severity (Antón et al., 2017; Bilbao et al., 2016). Further, OEA reduced levels of neural and peripheral proinflammatory markers, such as IL-1β and COX-2 during alcohol consumption (Antón et al., 2017). The over-the-counter medication, aspirin, has anti-inflammatory properties, which may be mediated by PPARγ activation (Yiqin et al., 2009). In rats, the co-administration of aspirin and n-acetylcysteine (NAC) inhibited chronic alcohol intake by 70% with aspirin administration alone inhibiting chronic intake by 50% (Israel et al., 2021).
Testing of several PPAR agonists has moved to human samples of heavy drinking but no randomized trial data for samples of AUD have been published. A clinical trial was conducted for a dietary supplement containing the precursor of OEA in young adult heavy drinkers (van Kooten et al., 2016). This supplement significantly improved performance on a Go/No-Go task of inhibition, which was correlated with reductions in drinking (van Kooten et al., 2016); yet measures of alcohol use or inflammatory markers were not collected. Importantly, an experimental medicine study (NCT01631630) of pioglitazone resulted in premature termination due to concern over myopathy risk (i.e., a neuromuscular disorder) in the active treatment group (Schwandt et al., 2020). While several PPAR agonists are FDA-approved medications for medical conditions such as diabetes and dyslipidemia, they have shown unfavorable side-effect profiles and as a result, regulatory agencies have issued caution for future clinical trials (Wright et al., 2014). Moreover, PPARγ and dual agonists have shown concerning long-term effects on weight gain, fluid accumulation, cardiac safety, and tumor development (Wright et al., 2014; Amato, 2012). Despite these concerns, future work aims to optimize subtype interaction profiles to develop safer and more effective treatment options (Wright et al., 2014; Amato, 2012). At present, one human clinical trial is underway to test the effects of pioglitazone (45 mg/day) on alcohol use and biomarkers (NCT03864146); another trial was terminated due to the COVID-19 pandemic (NCT03860753). Researchers completed a clinical trial of fenofibrate for AUD and while trial results have yet to be published, reporting indicates that no serious adverse events occurred (NCT02158273).
Overall, evidence on PPAR agonists to date demonstrate their promising potential to reduce alcohol consumption and mitigate alcohol-related consequences in AUD. The majority of this work has been completed in animal models and shows that PPAR agonists may reduce the motivational and reinforcing features of alcohol, potentially by modulating dopaminergic signaling in the VTA and amygdala (Fotio et al., 2020). Findings across compounds have been mixed as to whether PPAR agonists’ known metabolic actions, along with their anti-inflammatory properties, contribute to their effects on alcohol intake, with research suggesting that these agonists target neurons and modulate synaptic transmission more prominently than neuroimmune regulation (Ferguson et al., 2014; Stopponi et al., 2011; Haile and Kosten, 2017). This investigation is limited by the lack of studies validating PPAR agonists’ effects on markers of inflammation. Other initial findings suggest that these agonists may attenuate stress-induced alcohol consumption (Fotio et al., 2020) and exert neuroprotective benefits (Cippitelli et al., 2017). Medications mitigating alcohol-induced neural damage are highly sought after in CNS therapeutics. PPAR agonists with actions in the periphery, such as aspirin and OEA, show initial promise for reducing alcohol use and proinflammatory signaling and warrant safety and efficacy testing in humans. Human clinical trials for two of the most promising compounds, fenofibrate and pioglitazone, are emerging. However, long-term side effect profiles of certain PPAR agonists are of concern and should be tracked closely (Wright et al., 2014; Amato, 2012).
1.1.4. Microglia and astrocytes
Microglia and astrocytes act as immune mediators in the brain, releasing and responding to immune signals (Nimmerjahn et al., 2005), and are implicated in alcohol-induced neuroimmune responses (Erickson et al., 2019; Erickson et al., 2019). Microglia have been shown to regulate escalation of drinking and alcohol dependence-induced changes in neuronal function (Warden et al., 2020). Activated M1 microglia are thought to secrete TNF-α, IL-6, and IL-1β, while anti-inflammatory microglia, M2, release TGF-β and IL-10 (Tang and Le, 2016). Astrocytes have the critical function of regulating synaptic glutamate levels through glutamate transporters (i.e., Glutamate Transporter 1 (GLT-1)) (Verkhratsky et al., 2015). The expression and function of astrocytic glutamate transporters are modulated by proinflammatory cytokines (Tilleux and Hermans, 2007) as well as alcohol, whereby chronic alcohol downregulates the expression of GLT-1 (Sari, 2013). Astrocyte-specific calcium signaling can regulate ethanol intake as well as the acute stimulatory and sedative-hypnotic effects of ethanol in mice (Erickson et al., 2021). Glial cells may also play an important role in the modulation of dopamine activity relevant to addiction through the release of cytokines over dopaminergic neurons (Jimenez-Gonzalez et al., 2021). Furthermore, a recent study identifying transcriptomic patterns associated with alcohol dependence found that the largest number of cell-type specific genes with altered expression in individuals with alcohol dependence were detected in astrocytes and microglia (Brenner et al., 2020). Therefore, these glial cells represent potential new targets for medications focusing on the neuroimmune aspects of AUD (see Table 4).
Table 4.
Microglia and Astrocytes.
| Immunotherapy | Potential Immune Target | Animal Studies Findings | Human Studies Findings | References |
|---|---|---|---|---|
| Microglia: | ||||
| Minocycline | Microglial activation inhibition | ↓ ethanol intake in adult mice no effect of ethanol intake in adolescent mice ↓ relapse-like ethanol consumption ↓ withdrawal-induced anxiety ↑ ethanol-induced motor impairment ↓ ethanol-induced TNF-α |
no effect on subjective response to alcohol no effect on cytokine levels clinical trial completed: NCT02187211 clinical trial underway: NCT04210713 |
(Wu et al., 2011) (Agrawal et al., 2011) (Agrawal et al., 2014) (Lainiola and Linden, 2017) (Gajbhiye et al., 2018) (Petrakis et al., 2019) |
| Astrocytes: | ||||
| N-acetylcysteine | Glutamate transporter 1 (GLT-1) | ↓ ethanol intake no effect on ethanol cue-induced reinstatement ↓ relapse-like binge drinking ↓ ethanol-seeking behavior ↓ ethanol-induced proinflammatory cytokines prevent ethanol-induced decreases in anti-inflammatory cytokines |
↓ alcohol consumption clinical trials completed: NCT03216954 NCT02791945 NCT01214083 NCT02911285 clinical trials underway: NCT03707951 NCT03238300 NCT03879759 NCT02966873 NCT03120468 |
(Weiland et al., 2015) (Schneider et al., 2017) (Lebourgeois et al., 2018) (Squeglia et al., 2018) (Israel et al., 2021) |
| Ceftriaxone | GLT-1 | ↓ ethanol cue-induced reinstatement ↓ ethanol intake ↓ relapse-like ethanol intake ↓ withdrawal symptoms |
– | (Sari et al., 2011) (Lee et al., 2013) (Qrunfleh et al., 2013) (Alhaddad et al., 2014) (Abulseoud et al., 2014) (Das et al., 2015) (Weiland et al., 2015) (Sari et al., 2016) |
| Clavulanic acid | GLT-1 | ↓ ethanol intake | – | (Hakami and Sari, 2017) |
Note. TNF-α = tumor necrosis factor-α
To date, only one medication targeting microglia has been explored in preclinical models. Minocycline, a broad-spectrum antibiotic that crosses the BBB, is a microglial attenuator (Romero-Sandoval et al., 2000) shown to alter immune and cytokine expression in the brain and periphery (Garrido-Mesa and Zarzuelo, 2013). Results from minocycline studies for AUD are inconclusive. In male and female mice, minocycline modestly reduced alcohol intake in a free-choice voluntary drinking model (Agrawal et al., 2011). The effects of minocycline may be non-specific, as it reduced both alcohol and water intake in mouse models (Lainiola and Linden, 2017). Moreover, minocycline’s beneficial effects on alcohol reductions were limited to adult vs. adolescent mice (Agrawal et al., 2014). However, other results suggest that minocycline modulates a host of AUD-related behaviors including reductions in alcohol-induced sedation, withdrawal-related anxiety, and alcohol reinstatement (Gajbhiye et al., 2018; Wu et al., 2011).
Medications targeting astrocytic GLT-1, which aids in the regulation of extracellular glutamate, include n-acetylcysteine (NAC), ceftriaxone, and clavulanic acid. Astrocytic compounds have been more extensively studied in animal models and are relevant to AUD as glutamate expression is known to be dysregulated in AUD and contribute to alcohol withdrawal. NAC is an over-the-counter dietary supplement and anti-oxidant precursor to glutathione used to treat acetaminophen poisoning and cystic fibrosis (Ooi et al., 2011). In rat models, NAC reduced ethanol-seeking and self-administration (Lebourgeois et al., 2018) but did not prevent cue-primed ethanol reinstatement (Weiland et al., 2015). NAC may protect against chronic alcohol-induced neuroinflammation in the frontal cortex and hippocampus, as it prevented both increases in proinflammatory cytokines and decreases in anti-inflammatory cytokines in rat models (Schneider et al., 2017). Moreover, the co-administration of NAC and aspirin reduced ethanol intake and relapse binge drinking in ethanol-preferring rats (Israel et al., 2021). Ceftriaxone, a beta-lactam antibiotic, showed promising preclinical results for AUD-related behaviors as well. Ceftriaxone attenuated cue-primed reinstatement of alcohol-seeking (Weiland et al., 2015), reduced alcohol consumption (Lee et al., 2013), and attenuated relapse-like consumption across rodent models (Alhaddad et al., 2014; Qrunfleh et al., 2013). Alcohol withdrawal syndrome was alleviated in a rat model of ethanol withdrawal by ceftriaxone treatment (Abulseoud et al., 2014). Clavulanic Acid, another beta-lactam antibiotic, increased the expression of GLT-1 and attenuated ethanol consumption and preference (Hakami and Sari, 2017). Importantly, clavulanic acid attenuated alcohol consumption at a 20–40-fold lower dose than ceftriaxone and therefore shows higher potential for clinical translation, as large dose-to-body-weight ratios are unfeasible to use in human samples (Shen et al., 2019).
Among these glial targeting compounds, only minocycline and NAC have been translated into human clinical samples of addiction. A completed clinical study found no beneficial effect of a short-term minocycline treatment on inflammation or subjective response to alcohol among heavy drinkers (Petrakis et al., 2019). Currently underway is a clinical trial of minocycline testing alcohol use, craving, and neurocognitive impairment in AUD (NCT04210713). Additionally, in a secondary analysis of a clinical trial for cannabis use disorder (CUD), NAC treatment reduced alcohol consumption by 30% (Squeglia et al., 2018). Several other clinical trials will examine the potential effectiveness of NAC in both adolescent and adult samples of AUD (e.g., NCT03216954, NCT03707951). These trials will include combination pharmacotherapy, samples with comorbid psychopathology, and neuroimaging methods that will test NAC’s ability to modulate cortical levels of relevant metabolites and neural reactivity to alcohol cues.
In sum, microglia and astrocytes present promising targets for medications development for AUD. Compounds targeting astrocytes may be particularly useful in normalizing glutamate expression and treating withdrawal symptoms. The vast majority of existing studies have involved animal models, but several compounds demonstrate translational potential to clinical development. While ceftriaxone appears unlikely to translate due to its required dose size, clavulanic acid’s efficacy at a much lower dose is promising for translation. Clavulanic acid has shown safety and tolerability in human clinical samples, as it is FDA-approved for clinical use in combination with an amoxicillin antibiotic. Minocycline and NAC are also FDA-approved treatments for other medical conditions and ongoing clinical trials aim to test their effects on AUD-related outcomes. While minocycline is generally well-tolerated in humans, it is less commonly prescribed than similar antibiotics because it increases risk for irreversible pigmentation, hepatotoxicity, and lupus-erythematosus-like syndrome (Garrido-Mesa and Zarzuelo, 2013; Smith and Leyden, 2005). Overall, NAC appears to be the most promising glia-targeting AUD treatment with multiple ongoing clinical trials. Orally administered NAC is well-tolerated with long-term use being associated with only mildly adverse effects (e.g., nausea, diarrhea) (LaRowe et al., 2006). NAC is being tested as an AUD treatment specifically for adolescents, which represents a novel prospect, as no pharmacotherapies are currently approved for adolescents with AUD (Hammond, 2016; Winslow et al., 2016). Future research in this area can benefit from assessing biobehavioral and psychosocial factors to elucidate the mechanisms (e.g., withdrawal alleviation, neuroprotection) through which NAC and other glia-targeting neuroimmune therapies might reduce drinking and promote recovery.
1.1.5. Other immune pharmacotherapies
Compounds with specific targets differing from those covered have been explored as potential immune treatments for AUD. Indomethacin, a selective cyclooxygenase-2 (COX-2) inhibitor, has been investigated for its protective effects against alcohol-induced neuronal and cognitive damage (Pascual et al., 2015; Vetreno et al., 2018; Vetreno et al., 2018). Indomethacin is a potent nonsteroidal anti-inflammatory drug (NSAID) targeting COX isozymes involved in peripheral and neural inflammatory responses (Remmel et al., 2004). An initial investigation in rats reported dose-dependent reductions in alcohol self-administration (George, 1989). More recent work has focused on adolescence, a developmental period when the brain is especially sensitive to alcohol’s neurotoxic effects. In adolescent rodents, indomethacin alone (Pascual et al., 2007) and in combination with exercise (Vetreno et al., 2018; Vetreno et al., 2018) blocked ethanol-induced neuronal cell death and behavioral deficits.
Using transcriptome-based drug discovery methods, researchers identified several novel compounds with potential for reducing excessive alcohol use (Ferguson et al., 2018). Gene expression profiles of heavy drinking mice were compared with gene expression signatures of thousands of compounds and the most promising targets were selected via computational modeling. A sizeable proportion of the compounds identified are thought to have anti-inflammatory properties, including terreic acid and pergolide, which were then validated in mice models (Ferguson et al., 2018). Terreic acid is a Bruton’s tyrosine kinase (BTK) inhibitor (Kawakami et al., 1999), which is an important component in signaling pathways of B-cell receptors and malignancies, TLRs, and chemokine receptors (Kim, 2019). Pergolide is a dopamine and serotonin receptor agonist thought to have anti-inflammatory properties, yet this mechanism is poorly understood (Bendele et al., 1991). Findings showed that pergolide and terreic acid significantly reduced alcohol intake in HDID-1 mice (Ferguson et al., 2018). However, terreic acid appeared to have more selective effects on alcohol intake with pergolide decreasing water and saccharin intake as well (Ferguson et al., 2018).
Endogenous neuroactive steroids, termed “neurosteroids”, are implicated in neuroimmune signaling in AUD. These steroids are synthesized in the brain and have a range of genomic and non-genomic actions, including modulation of GABAAR-mediated neurotransmission, TLR-dependent signaling (i.e., blocking TLR-MyD88 binding) (Balan et al., 2021), and CRF signaling, with the potential to target complex symptomatology of AUD (Gatta et al., 2021; Morrow et al., 2020; Reddy, 2010). Neurosteroids that are positive modulators of GABAARs, such as allopregnanolone and pregnenolone, demonstrate anticonvulsant, sedative, and anxiolytic effects. Research shows that chronic alcohol exposure depletes neurosteroids in human serum and brains of rodents and monkeys; this depletion contributes to psychological and behavioral adaptations, which are further exacerbated by withdrawal and binge drinking (Morrow et al., 2020; Finn and Jimenez, 2018). Neurosteroids are being investigated as potential treatments given their ability to restore homeostasis in these functions (Morrow et al., 2020) and reduce alcohol intake (see relevant reviews (Morrow et al., 2020; Finn and Jimenez, 2018; Tomaselli and Valĺee, 2019). In several preclinical studies, allopregnanolone or the precursor pregnenolone reduced ethanol intake, preference, or reinforcement in male alcohol-preferring rodents at high doses, demonstrating initial efficacy (Ford et al., 2005; Janak et al., 1998; Rezvani and Levin, 2014). However, neurosteroids may actually increase ethanol consumption and reinstatement at low doses or in non-dependent breeds (Morrow et al., 2020; Ramaker et al., 2014).
Cannabidiol (CBD), a non-psychoactive component of the cannabis plant, has received considerable attention as a possible therapeutic for illnesses including AUD (Turna et al., 2019). CBD exhibits diverse biological effects, such as on learning and memory, immune system, appetitive behaviors, and neuroprotection by interacting with the body’s endocannabinoid system and possibly other receptors like serotonin and opioid (Turna et al., 2019). Research supports CBD’s anti-inflammatory effects with immune signaling actions in the periphery and CNS; anti-inflammatory targets of CBD include CB1, CB2, TRPV1, GPR55, and 5-HT1 serotonin receptors with downstream actions on PPARγ, COX-2 enzymes, NF-κB, etc. (Burstein, 2015; Pellati et al., 2018). Several studies have tested whether CBD administration can reduce alcohol intake and related harms in preclinical models (see systematic review (Turna et al., 2019), including alcohol’s neurotoxic effects, motivation and intake, and hepatoxicity). Findings consistently support CBD as a candidate pharmacotherapy for AUD. In rodent models, CBD treatment reduced voluntary alcohol consumption (Viudez-Martínez et al., 2018) and prevented cue- and stress-elicited alcohol reinstatement (Gonzalez-Cuevas et al., 2018). While, the majority of this work has yet to examine CBD’s impact on immune markers, one study testing hepatoxicity found that CBD attenuated alcohol-induced increases in liver enzymes, mRNA expression of cytokines TNF-α and IL-1β, and several chemokines (Wang et al., 2017). These results suggest that CBD’s ability to prevent liver damage is partially attributable to immune processes. Other evidence from in vitro models demonstrates cannabinoids’ potential to reduce intestinal permeability, which might have therapeutic implications (Alhamoruni et al., 2010).
Research on compounds reviewed in this section remains in early stages and is largely restricted to preclinical models not yet translated to human samples of AUD (see Table 5). However, one human laboratory trial of the neuroactive steroid, dutasteride, was completed and enrolled males reporting light and heavy drinking patterns. Participants were randomized in a crossover design to both placebo and 4 mg dutasteride pretreatment before alcohol administration (Covault et al., 2014). Results were encouraging, such that males with heavy drinking patterns reported fewer heavy drinking days in the two weeks following pretreatment for dutasteride vs. placebo and further, the compound was well tolerated. Clinical trials of neuroactive steroids for other psychiatric conditions similarly demonstrate safety and tolerability with no serious adverse effects reported, yet mild sedative effects may occur (Morrow et al., 2020). Continuing, animal models show that indomethacin may be a particularly promising compound for preventing alcohol-induced neurocognitive deficits. Indomethacin administration, however, can cause gastrointestinal toxicity due to its action as a partial COX-1 inhibitor and this may be particularly concerning when alcohol is concurrently consumed. Yet, analogues of indomethacin with less severe side effect profiles may become available (Blobaum et al., 2013) and may warrant safety and efficacy testing in humans. Development of medications that attenuate alcohol-related neurocognitive impairments in adults and adolescents are merited as these deficits (e.g., inhibitory control, working memory) contribute to continued alcohol use by interfering with goal-directed decision making, self-regulation, and treatment (Bates et al., 2006). Using the bioinformatic approach described above (Ferguson et al., 2018), terreic acid proved to be most selective for reducing heavy drinking in mice. While several second-generation BTK inhibitors show clinical promise, the safety profile of terreic acid (Kawakami et al., 1999) and its translatability to humans remains unclear (Kim, 2019).
Table 5.
Other Immune Pharmacotherapies and Behavioral Therapies.
| Immunotherapy | Potential Immune Target | Animal Study Findings | Human Study Findings | References |
|---|---|---|---|---|
| Other Potential Immune Pharmacotherapies: | ||||
| Indomethacin | COX-2 enzyme inhibitor | ↑ protection against ethanol-induced brain damage ↓ ethanol-induced NF-κB phosphorylation ↓ ethanol-induced COX-2 & iNOS expression |
– | (George, 1989) (Pascual et al., 2007) (Vetreno et al., 2018) (Vetreno et al., 2018) |
| Pergolide | Dopamine/serotonin receptor agonist | ↓ ethanol intake | – | (Ferguson et al., 2018) |
| Terreic acid | Bruton’s tyrosine kinase (BTK) inhibitor | ↓ ethanol intake | – | (Ferguson et al., 2018) |
| Cannabidiol | Diverse actions (e.g., COX-2 enzyme inhibition, PPARγ activation) | ↓ ethanol intake ↓ cue- and stress-induced ethanol seeking ↑ protection against ethanol-induced brain damage ↓ ethanol-induced liver damaged ↓ impulsive choice ↓ ethanol-induced liver inflammation |
clinical trials underway: NCT03252756 NCT04205682 NCT03248167 |
(Turna et al., 2019) (Wang et al., 2017) |
| Neuroactive steroids | Toll-like receptor (TLR) 4, TLR7 | ↓ ethanol intake, preference, and operant responding in select rodents at high doses ↑ ethanol intake and operant responding in low doses |
↓ alcohol use in males with heavy drinking patterns clinical trials underway: NCT03872128 NCT02582905 NCT04098302 NCT04015869 |
(Morrow et al., 2020) (Rezvani and Levin, 2014) (Covault et al., 2014) |
| Behavioral Therapies: | ||||
| Mindfulness-Based Relapse Prevention | Downstream stress pathways | – | ↑ mindfulness practice predicted ↓ IL-6 and ↓ drinking clinical trial completed: NCT01056484 clinical trial underway: NCT02994043 |
(McClintock et al., 2019) (Zgierska et al., 2019) |
Note. IL = interleukin; COX-2 = cyclooxygenase-2; iNOS = inducible nitric oxide synthase; TNF-α = tumor necrosis factor-α; NF-κB = nuclear factor-κB; PPARγ = peroxisome proliferator-activated receptor γ.
As such, the next step would be replication of these promising results in additional animal models followed by research on the safety of this compound or other BTK inhibitors in humans. A major benefit of using this bioinformatics approach to select promising compounds for AUD is many of these compounds are FDA-approved for other medical conditions, thus shortening development time and reducing research costs. CBD, through its diverse biological actions, appears to advantageously target several AUD domains including liver damage, intestinal permeability, and motivation but the degree to which these effects are attributable to immune mechanisms is undetermined and warrants further research (Turna et al., 2019). CBD proves to be safe and tolerable in a range of clinical samples (Larsen and Shahinas, 2020) but translational challenges exist, including the low bioavailability of oral CBD in humans and potential contraindication with liver impairment (Turna et al., 2019). Randomized clinical trials of CBD are ongoing and will serve to translate these exciting preclinical findings to human AUD samples (NCT03252756; NCT04205682). One pilot trial will examine CBD dosing and its effects on withdrawal symptoms among inpatients. An 8-week trial of CBD will also assess changes in self-reported and biomarkers of alcohol use among treatment-seeking individuals with AUD. Moreover, randomized clinical trials of several neuroactive steroids for the treatment of AUD are also underway (NCT03872128; NCT02582905; NCT04098302; NCT04015869). These trials include crucial investigation into sex differences and the effect of neurosteroids on alcohol intake, withdrawal, stress reactivity, and mood symptoms. In sum, the complexity of the body’s immunological pathways and the phenotypic heterogeneity seen in AUD will result in the continued identification of novel immune targets.
1.1.6. Behavioral interventions
In addition to the pharmacotherapies reviewed above, behavioral interventions may also mitigate heavy drinking and elevations in proinflammatory levels observed in AUD (see Table 5). While the anti-inflammatory effects of mind–body therapies have been explored in the context of chronic disease, depression, and aging (Bower and Irwin, 2016; Morgan et al., 2014), this area of research has only recently emerged in the context of AUD (McClintock et al., 2019). Mind-body therapies promote self-regulation and positive affect while decreasing stress reactivity and negative affectivity. Relevantly, heavy alcohol use is known to alter the body’s natural biological stress system (Sinha, 2009) and stress increases alcohol craving and use. These therapies are hypothesized to interact with the neuroimmune system through downstream stress reactivity pathways, and thereby reverse activation of inflammatory mechanisms (Bower and Irwin, 2016). Existing research illustrates that mind–body therapies reduce proinflammatory gene expression profiles in healthy adults and those with medical or psychiatric conditions (Bower and Irwin, 2016). Mindfulness-Based Relapse Prevention (MBRP) is a mind–body therapy specifically designed for individuals with addiction (Grant et al., 2017). MBRP is typically delivered in 2-hour group sessions aimed to cultivate increased awareness of present-moment cognitive, emotional, and physical states, especially as they relate to cravings and withdrawal (Grant et al., 2017).
Few randomized trials of MBRP have been conducted in AUD populations and findings on its effectiveness have been mixed (Bowen et al., 2009; Zgierska et al., 2019). MBRP may be most effective for individuals with severe AUD or comorbid mood symptomatology (Roos et al., 2017), which is supported by literature linking depression and inflammation (Miller and Raison, 2016). One trial connecting biological markers to behavior examined the impact of MBRP on peripheral proinflammatory levels in adults with alcohol dependence (McClintock et al., 2019). While significant decreases in IL-6 following MBRP were not detected, greater time spent practicing mindfulness predicted lower levels of circulating IL-6, suggesting regular mindfulness practice might reduce peripheral proinflammatory levels (McClintock et al., 2019).
One clinical trial underway will extend this research by exploring immunological, epigenetic, and neurobiological changes associated with MBRP in AUD (NCT02994043). Additional trials seek to further test MBRP efficacy, identify predictors of positive outcomes, and mechanisms of behavior change (NCT03842670; NCT0214783). Availability of behavioral interventions that serve to treat AUD maintenance factors (e. g., stress reactivity) differing from those typically targeted in existing evidence-based therapies, is a needed contribution to the field. Further, medications for AUD are largely under prescribed due to provider and patient factors and thus a group therapy option, with potentially novel anti-inflammatory actions, is critical.
1.2. Conclusions and future directions
A host of treatments targeting the immune system show promise for treating AUD. The guiding principle in this review is a translational focus on the biological and clinical plausibility of the immune therapies tested. We contend that, in order to push medications development forward, treatments’ clinical applications and utility is equally as important as an understanding of their biological mechanisms. Considerations in the translation from preclinical to clinical medications development include dosage and target engagement. While most of the discussed medications will be administered to humans orally, chronically, and at doses selected to prevent toxicity, most rodent studies use acute intraperitoneal injection administration with doses that produce blood levels much greater than would be achieved in humans. Relatedly, the exact peripheral and/or central mechanisms of action through which many of the discussed medications act to reduce alcohol intake remain unclear. For instance, certain compounds do not readily cross the BBB, indicative of low engagement at brain targets. Brain effects can be more easily achieved by increasing dosage in rodents, yet this is often unfeasible in humans. Along with compound availability, adverse event profile, and commercialization potential, these translational and clinical applications must be considered in order to feasibly reach, and safely and effectively treat individuals suffering from AUD (Litten et al., 2020).
Given these considerations, numerous treatments show significant promise even when held to the highest standards of clinical plausibility. For instance, two PDE4 inhibitors are in advanced stages of testing for AUD, apremilast (Blednov et al., 2018) and ibudilast (Ray et al., 2017). Pioglitazone (Blednov et al., 2015) and fenofibrate (Haile and Kosten, 2017), both PPAR agonists, have been extensively tested for in animals models (Stopponi et al., 2013), and have moved into clinical trials for AUD. Moreover, ongoing clinical trials for NAC are wide-ranging and will test this treatment’s efficacy in combination with more established AUD pharmacotherapy in adolescent samples and in adult samples with comorbid psychopathology. However, careful attention to side effect profiles and tolerability is necessary as immune research progresses into human samples with heavy alcohol use. For example, a trial of pioglitazone for AUD was halted over myopathy risk concerns (Schwandt et al., 2020). More research testing the neuroimmune hypothesis of AUD in human samples is also needed. In addition to pharmacotherapies, mind–body therapies, particularly MBRP, show potential to restore healthy levels of inflammation through downstream stress-reactivity pathways (McClintock et al., 2019). In brief, while in its early stages, the future of immune therapies in AUD appears bright, consistent with its application to other psychiatric disease states.
Based on the premise that immune therapies deserve careful attention for the indication of AUD, the next obstacle is establishing an effective compound screening model (Ray et al., 2021). Recognition that novel compounds and mechanisms may call for novel screening methods, is key in facilitating progression from preclinical to clinical settings. The endpoint of reduced alcohol consumption remains a gold-standard for AUD trials, yet initial efficacy testing in non-treatment-seeking samples may require a broad set of endpoints, including safety and tolerability. Other important outcomes may include treatment effects on mood, neurocognition, biomarkers of peripheral and neural immune signaling, and withdrawal symptoms. Future research may also benefit from examining medication effects in the context of experimental laboratory paradigms, such as alcohol self-administration or stress- and cue-reactivity, as these methods afford efficient early efficacy testing, that is faster and less costly than full-scale clinical trials (Bujarski and Ray, 2016). Moving forward, screening should consider all aspects of medications and therapy development to advance understanding of how treatments interface with immune processes and their clinically relevant effects on brain and behavior.
After initial human testing, clinical trials should consider a broad range of factors involved in AUD recovery (Witkiewitz et al., 2020). As recently redefined, recovery from AUD is a “process by which individuals substantially reduce or eliminate AUD symptoms while enhancing one’s social support and psychosocial functioning in order to build resilience to relapse” (PA 18-619). To that end, the combination of pharmacotherapy with synergistic and evidence-based behavioral therapy may be critical to reaching recovery endpoints beyond reductions in alcohol use. Identification of optimal combinations has resulted in success across areas of medicine. Likewise, identifying subgroups of treatment responders through precision medicine approaches can boost medication effect sizes dramatically (Litten et al., 2020). Would individuals showing a particular set of vulnerabilities in their AUD presentation, such as “leaky gut”, elevated peripheral proinflammatory markers, or depressive symptomatology, be best suited for therapies targeting the immune system? Further, would specific individual inflammatory profiles show better responses to these treatments targeting inflammation and disruption of immune signaling? These are the type of questions we envision having high translational value as indexed by a high potential to inform clinical care and to improve treatment efficacy. Another approach with the potential to inform medications development for AUD, including immune therapies, is the use of pharmacoepidemiology. As datasets from closed health systems become more detailed and informative, questions about the efficacy of immune therapies for heavy alcohol use may become accessible. Such approaches have already proven helpful when characterizing opioid use, a high priority area (Hudson et al., 2017). While pharmacoepidemiology offers an emerging tool in this area, one of the limitations is the fact that a full clinical picture may not emerge until treatment-seeking individuals attempting to change their drinking are considered in efficacy trials.
In closing, this qualitative review of immune therapies for AUD demonstrates optimism with regard to the biological and clinical plausibility of treatments that can restore healthy immune function as a means of promoting AUD recovery. As the field progresses with clinical testing, literature calls for adjustments in the way AUD medications are developed with a particular focus on how novel treatment mechanisms can be effectively captured in clinical samples. To that end, efficacy screening models (i.e., human laboratory trials, neuroimaging) sensitive to the unique effect of immune modulation on psychology, behavior, and biomarkers are critical. Overreliance on models used to capture medication effects on standard phenotypes (e.g., craving or subjective response to alcohol) may result in ‘missing the signal’ from immune treatments on other key components of addiction (e.g., affect and neurocognition). Moving towards clinical testing and randomized controlled trials, a broad definition of recovery along with identification of predictors of treatment response are central to establishing the utility of novel immune treatments. A nuanced understanding of treatment effects in turn can advance the much-anticipated precision medicine approach to AUD.
Funding
This work was supported by the National Institute on Drug Abuse grant to UCLA [No. 5T32DA024635], the National Institute on Alcohol Abuse and Alcoholism grants [Nos. F32AA027699, K24AA025704, F31AA028976], and UCLA.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mayfield J, Harris RA. The Neuroimmune Basis of Excessive Alcohol Consumption. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(1):376-. doi: 10.1038/npp.2016.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 140 (3), 774–815. 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Crews FT, 2018. Innate Immune Signaling and Alcohol Use Disorders. Handb Exp Pharmacol. 248, 369–396. 10.1007/164_2018_92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, 2015. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun. 44, 1–8. 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Banks WA, Dantzer R, 2018. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol Rev. 70 (2), 278–314. 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA, 2007. Brain-immune communication pathways. Brain Behav Immun. 21 (6), 727–735. 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature. 454 (7203), 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Bonilla FA, Oettgen HC, 2010. Adaptive immunity. J Allergy Clin Immunol. 125 (2 Suppl 2), S33–S40. 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Raetz CRH, Whitfield C, 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem. 71 (1), 635–700. 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong J-S, Crews FT, 2008. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 5 (1), 10. 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Stärkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P, 2012. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 26 (6), 911–918. 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Aurelian L, Warnock KT, Balan I, Puche A, June H. TLR4 signaling in VTA dopaminergic neurons regulates impulsivity through tyrosine hydroxylase modulation. Transl Psychiatry. 2016;6:e815. doi: 10.1038/tp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan I, Warnock KT, Puche A, Gondre-Lewis MC, Aurelian L, 2018. Innately activated TLR4 signal in the nucleus accumbens is sustained by CRF amplification loop and regulates impulsivity. Brain Behav Immun. 69, 139–153. 10.1016/j.bbi.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, 2000. Proinflammatory cytokines. Chest. 118 (2), 503–508. 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Erickson EK, Grantham EK, Warden AS, Harris RA, 2019. Neuroimmune signaling in alcohol use disorder. Pharmacol Biochem Behav. 177, 34–60. 10.1016/j.pbb.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ, 2014. Human alcohol-related neuropathology. Acta Neuropathol. 127 (1), 71–90. 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, 2016. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl). 233 (9), 1543–1557. 10.1007/s00213-015-3906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, 2009. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 50 (3), 538–547. 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorky J, Schwaber J, 2016. The role of the gut-brain axis in alcohol use disorders. Prog Neuropsychopharmacol Biol Psychiatry. 65, 234–241. 10.1016/j.pnpbp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSabato DJ, Quan N, Godbout JP, 2016. Neuroinflammation: the devil is in the details. J Neurochem. 139 (Suppl 2), 136–153. 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding Z-M, Edenberg HJ, Liang T, Rodd ZA, Bell RL, 2014. Changes in gene expression within the extended amygdala following binge-like alcohol drinking by adolescent alcohol-preferring (P) rats. Pharmacol Biochem Behav. 117, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterndorff-Kahanek EA, Becker HC, Lopez MF, Farris SP, Tiwari GR, Nunez YO, Harris RA, Mayfield RD, Bardoni B, 2015. Chronic ethanol exposure produces time-and brain region-dependent changes in gene coexpression networks. PloS one. 10 (3), e0121522. 10.1371/journal.pone.0121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD, 2006. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 31 (7), 1574–1582. 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Dodd PR, Randall PK, Harris RA, Mayfield RD, 2004. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J Neurochem. 90 (5), 1050–1058. 10.1111/j.1471-4159.2004.02570.x. [DOI] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA, 2014. Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans. Int Rev Neurobiol. 118, 13–39. 10.1016/B978-0-12-801284-0.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Aragón CMG, Guerri C, 2015. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology. 89, 352–359. 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Beattie MC, Reguyal CS, Porcu P, Daunais JB, Grant KA, Morrow AL. Neuroactive Steroid (3α, 5α) 3-hydroxypregnan-20-one (3α, 5α-THP) and Pro-inflammatory Cytokine MCP-1 Levels in Hippocampus CA 1 are Correlated with Voluntary Ethanol Consumption in Cynomolgus Monkey. Alcoholism: Clinical and Experimental Research. 2018;42(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K, Brureau A, Vadigepalli R, Staehle MM, Brureau MM, Gonye GE, Hoek JB, Hooper DC, Schwaber JS, 2012. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. Journal of Neuroinflammation. 9 (1) 10.1186/1742-2094-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR, 2013. The Cytokine m RNA Increase Induced by Withdrawal from Chronic Ethanol in the Sterile Environment of Brain is Mediated by CRF and HMGB 1 Release. Alcoholism: Clinical and Experimental Research. 37 (12), 2086–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Shurtleff D, Harris RA, 2014. Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol. 118, 1–12. 10.1016/B978-0-12-801284-0.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, Comer SD, 2017. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend. 180, 156–170. 10.1016/j.drugalcdep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS, Vetreno RP, 2019. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol Clin Exp Res. 43 (9), 1806–1822. 10.1111/acer.v43.910.1111/acer.14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Walter TJ, Coleman LG Jr., Vetreno RP, 2017. Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl). 234 (9–10), 1483–1498. 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J, Guerri C, 2016. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 53, 159–171. 10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Guerri C, 2018. Role of the innate immune system in the neuropathological consequences induced by adolescent binge drinking. J Neurosci Res. 96 (5), 765–780. 10.1002/jnr.v96.510.1002/jnr.24203. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C, 2010. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 30 (24), 8285–8295. 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Da Costa AJ, Harris RA, Messing RO, 2018. Apremilast Alters Behavioral Responses to Ethanol in Mice: II. Increased Sedation, Intoxication, and Reduced Acute Functional Tolerance. Alcohol Clin Exp Res. 42 (5), 939–951. 10.1111/acer.2018.42.issue-510.1111/acer.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA, 2008. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 33 (4), 867–876. 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Woods J, 2013. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 254, 324–334. 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF, 2011. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 25 (Suppl 1), S21–S28. 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, McPherson KL, Biesecker CL, Wiers CE, Manza P, Volkow ND, Wang G-J, 2020. Neuroimaging of inflammation in alcohol use disorder: A review. Science China. Information Sciences 63 (7) 10.1007/s11432-019-2857-5. [DOI] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB, 2013. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 33 (1), 53–58. 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho LM, Wiers CE, Sun H, Wang GJ, Volkow ND, 2021. Increased transcription of TSPO, HDAC2, and HDAC6 in the amygdala of males with alcohol use disorder. Brain Behav. 11 (2), e01961 10.1002/brb3.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C, Conigrave JH, Lewohl J, Haber P, Morley KC, 2020. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav Immun. 89, 501–512. 10.1016/j.bbi.2020.08.002. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Le Roy T, Furgiuele S, Coste V, Bindels LB, Leyrolle Q, et al. , 2020. Gut Microbiota-Induced Changes in beta-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep. 33 (2), 108238 10.1016/j.celrep.2020.108238. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB, 2016. Discovery, Development, and Adoption of Medications to Treat Alcohol Use Disorder: Goals for the Phases of Medications Development. Alcoholism, Clinical and Experimental Research. 40 (7), 1368–1379. 10.1111/acer.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Roche DJO, Heinzerling K, Shoptaw S, 2014. Opportunities for the development of neuroimmune therapies in addiction. International Review of Neurobiology. 118, 381–401. 10.1016/B978-0-12-801284-0.00012-9. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr., 2017. The role of neuroimmune signaling in alcoholism. Neuropharmacology. 122, 56–73. 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Ahmad MF, Nagy LE, Tsukamoto H, 2019. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 70 (2), 249–259. 10.1016/j.jhep.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gonzalez A, Gomez-Acevedo C, Ochoa-Aguilar A, Chavarria A, 2021. The Role of Glia in Addiction: Dopamine as a Modulator of Glial Responses in Addiction. Cell Mol Neurobiol. 10.1007/s10571-021-01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Grodin EN, Leggio L, Bechtholt AJ, Becker H, Feldstein Ewing SW, Jentsch JD, King AC, Mason BJ, O’Malley S, MacKillop J, Heilig M, Koob GF, 2021. The future of translational research on alcohol use disorder. Addict Biol. 26 (2) 10.1111/adb.v26.210.1111/adb.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Montgomery SE, Cates LN, Nadav T, Delucchi AM, Cheng K, Yin H, Crawford EF, Roberts AJ, Roberto M, 2016. Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol and Alcoholism (Oxford, Oxfordshire). 51 (5), 541–548. 10.1093/alcalc/agw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Gil A, Guerri C, 2017. Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcoholism, Clinical and Experimental Research. 41 (7), 1257–1270. 10.1111/acer.2017.41.issue-710.1111/acer.13416. [DOI] [PubMed] [Google Scholar]
- Muralidharan S, Lim A, Catalano D, Mandrekar P, 2018. Human Binge Alcohol Intake Inhibits TLR4-MyD88 and TLR4-TRIF Responses but Not the TLR3-TRIF Pathway: HspA1A and PP1 Play Selective Regulatory Roles. J Immunol. 200 (7), 2291–2303. 10.4049/jimmunol.1600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan I, Warnock KT, Puche A, Gondre-Lewis MC, June H, Aurelian L, 2018. The GABAA Receptor alpha2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse. Brain Sci. 8 (4) 10.3390/brainsci8040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL Jr, et al. , 2011. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 108 (11), 4465–4470. 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L, 2015. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 40 (6), 1549–1559. 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelian L, Balan I, 2019. GABAAR alpha2-activated neuroimmune signal controls binge drinking and impulsivity through regulation of the CCL2/CX3CL1 balance. Psychopharmacology (Berl). 236 (10), 3023–3043. 10.1007/s00213-019-05220-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H, 2011. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem. 116 (3), 406–414. 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Gosselin R-D, Mechighel P, Kitabgi P, Rostène W, Parsadaniantz SM, 2005. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. J Comp Neurol. 489 (3), 275–292. 10.1002/(ISSN)1096-986110.1002/cne.v489:310.1002/cne.20598. [DOI] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Todd CA, Balan I, Aurelian L, Criswell HE, Breese GR, 2020. Phenotyping CCL2 Containing Central Amygdala Neurons Controlling Alcohol Withdrawal-Induced Anxiety. Front Cell Neurosci. 14 10.3389/fncel.2020.58058310.3389/fncel.2020.580583.s00110.3389/fncel.2020.580583.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Davis H, Arnelle D, Deaver D, 2014. Translational potential of naloxone and naltrexone as TLR4 antagonists. Trends Pharmacol Sci. 35 (9), 431–432. 10.1016/j.tips.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR, 2016. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol. 173 (5), 856–869. 10.1111/bph.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, et al. , 2008. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone. The European journal of neuroscience. 28 (1), 20–29. 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JHW, Buisman-Pijlman FTA, Mustafa S, Rice KC, Hutchinson MR, 2018. The efficacy of (+)-Naltrexone on alcohol preference and seeking behaviour is dependent on light-cycle. Brain, Behavior, and Immunity. 67, 181–193. 10.1016/j.bbi.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JHW, Buisman-Pijlman FT, Mustafa S, Rice KC, Hutchinson MR, 2018. Antagonising TLR4-TRIF signalling before or after a low-dose alcohol binge during adolescence prevents alcohol drinking but not seeking behaviour in adulthood. Neuropharmacology. 128, 460–473. 10.1016/j.neuropharm.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, et al. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. British Journal of Pharmacology. 2012;165(5):1319–29. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM, de Guglielmo G, Lasek AW, Logrip ML, Vendruscolo LF, Roberts AJ, Roberts E, George O, Mayfield J, Billiar TR, Hackam DJ, Mayfield RD, Koob GF, Roberto M, Homanics GE, 2017. Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 37 (5), 1139–1155. 10.1523/JNEUROSCI.2002-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Chernis J, Costa AD, Mayfield J, Harris RA, 2017. Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcoholism: Clinical and Experimental Research. 41 (3), 516–530. 10.1111/acer.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, Guerri C, 2013. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. Journal of Neurochemistry. 126 (2), 261–273. 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- McCarthy GM, Warden AS, Bridges CR, Blednov YA, Harris RA, 2018. Chronic ethanol consumption: role of TLR3/TRIF-dependent signaling. Addiction biology. 23 (3), 889–903. 10.1111/adb.2018.23.issue-310.1111/adb.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt JM, Blednov YA, Benavidez JM, Black M, Ponomareva O, Law J, et al. Inhibition of IKKβ Reduces Ethanol Consumption in C57BL/6J Mice. eNeuro. 2016;3(5). doi: 10.1523/ENEURO.0256-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Chiang S-H, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR, 2013. An inhibitor of the protein kinases TBK1 and IKK-ε improves obesity-related metabolic dysfunctions in mice. Nature Medicine. 19 (3), 313–321. 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift RM, Aston ER, 2015. Pharmacotherapy for alcohol use disorder: current and emerging therapies. Harv Rev Psychiatry. 23 (2), 122–133. 10.1097/HRP.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC, 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 311 (18), 1889. 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Karhuvaara S, Simojoki K, Virta A, Rosberg M, Loyttyniemi E, Nurminen T, Kallio A, Mäkelä R, 2007. Targeted nalmefene with simple medical management in the treatment of heavy drinkers: a randomized double-blind placebo-controlled multicenter study. Alcoholism, Clinical and Experimental Research. 31 (7), 1179–1187. 10.1111/acer.2007.31.issue-710.1111/j.1530-0277.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Zeng X, Chen Q, Cai Y, Chen F, Wang Y, Zhou H, Lin M, Shi J, Wang Z, Zhang Y, 2006. An evaluation on the efficacy and safety of amlexanox oral adhesive tablets in the treatment of recurrent minor aphthous ulceration in a Chinese cohort: a randomized, double-blind, vehicle-controlled, unparallel multicenter clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 102 (4), 475–481. 10.1016/j.tripleo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Plosker GL, Croom KF, 2005. Sulfasalazine: a review of its use in the management of rheumatoid arthritis. Drugs. 65 (13), 1825–1849. 10.2165/00003495-200565130-00008. [DOI] [PubMed] [Google Scholar]
- Wen R-T, Zhang F-F, Zhang H-T, 2018. Cyclic nucleotide phosphodiesterases: potential therapeutic targets for alcohol use disorder. Psychopharmacology. 235 (6), 1793–1805. 10.1007/s00213-018-4895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Faraci WS, Schmidt CJ, 2006. Phosphodiesterases in the CNS: targets for drug development. Nature Reviews Drug Discovery. 5 (8), 660–670. [DOI] [PubMed] [Google Scholar]
- Parry GC, Mackman N, 1997. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 159 (11), 5450–5456. [PubMed] [Google Scholar]
- Logrip ML, 2015. Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol. 49 (8), 795–802. 10.1016/j.alcohol.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, 2003. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 27 (3), 396–409. 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Uzbay IT, Celik T, Aydin A, Kayir H, Tokgoz S, Bilgi C, 2004. Effects of chronic ethanol administration and ethanol withdrawal on cyclic guanosine 3’,5’-monophosphate (cGMP) levels in the rat brain. Drug Alcohol Depend. 74 (1), 55–59. 10.1016/j.drugalcdep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G, 2000. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 20 (3–4), 349–374. 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang H-T, 2011. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology. 218 (2), 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Harris RA, 2014. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci. 8, 129. 10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Metten P, Potretzke S, Townsley KG, Blednov YA, Crabbe JC, 2020. Effects of Pharmacologically Targeting Neuroimmune Pathways on Alcohol Drinking in Mice Selectively Bred to Drink to Intoxication. Alcoholism: Clinical and Experimental Research. 44 (2), 553–566. 10.1111/acer.v44.210.1111/acer.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R-T, Zhang M, Qin W-J, Liu Q, Wang W-P, Lawrence AJ, Zhang H-T, Liang J-H, 2012. The Phosphodiesterase-4 (PDE4) Inhibitor Rolipram Decreases Ethanol Seeking and Consumption in Alcohol-Preferring Fawn-Hooded Rats. Alcoholism: Clinical and Experimental Research. 36 (12), 2157–2167. 10.1111/acer.2012.36.issue-1210.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding Z-M, McBride WJ, Bell RL, 2015. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4). Psychopharmacology. 232 (13), 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M-F, Wen R-T, Xu Y, Pan J-C, Fei N, Zhou Y-M, Xu J-P, Liang J-H, Zhang H-T, 2017. Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology (Berl). 234 (20), 3143–3151. 10.1007/s00213-017-4697-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Hao P-D, Yang M-F, Sun J-Y, Mao L-L, Fan C-D, Zhang Z-Y, Li D-W, Yang X-Y, Sun B-L, Zhang H-T, 2017. The phosphodiesterase-4 inhibitor roflumilast decreases ethanol consumption in C57BL/6J mice. Psychopharmacology. 234 (16), 2409–2419. 10.1007/s00213-017-4631-8. [DOI] [PubMed] [Google Scholar]
- Schafer P, Parton A, Gandhi A, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. British journal of pharmacology. 2010;159(4):842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP, 2014. Phosphodiesterase 10A Regulates Alcohol and Saccharin Self-Administration in Rats. Neuropsychopharmacology. 39 (7), 1722–1731. 10.1038/npp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LCD, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, MacKenzie FL, Nagasawa M, Stevens PA, MacKenzie SJ, 2006. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. European journal of pharmacology. 538 (1–3), 39–42. [DOI] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC, 2015. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 20 (1), 38–42. 10.1111/adb.2015.20. issue-110.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K, 2017. Development of the Neuroimmune Modulator Ibudilast for the Treatment of Alcoholism: A Randomized, Placebo-Controlled. Human Laboratory Trial. Neuropsychopharmacology. 42 (9), 1776–1788. 10.1038/npp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Bujarski S, Towns B, Burnette E, Nieto S, Lim A, Lin J, Miotto K, Gillis A, Irwin MR, Evans C, Ray LA, 2021. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial. Transl Psychiatry. 11 (1) 10.1038/s41398-021-01478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Da Costa AJ, Tarbox T, Ponomareva O, Messing RO, Harris RA, 2018. Apremilast Alters Behavioral Responses to Ethanol in Mice: I. Reduced Consumption and Preference. Alcohol Clin Exp Res. 42 (5), 926–938. 10.1111/acer.2018.42.issue-510.1111/acer.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Domi E, Ubaldi M, Douglas JC, Li HW, Demopulos G, et al. , 2017. Protection against alcohol-induced neuronal and cognitive damage by the PPARgamma receptor agonist pioglitazone. Brain Behav Immun. 64, 320–329. 10.1016/j.bbi.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O’Rahilly S, Palmer CNA, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W, 2006. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 58 (4), 726–741. 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE, 2002. The mechanisms of action of PPARs. Annu Rev Med. 53 (1), 409–435. 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M, 2015. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 62 (5), 1551–1562. 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP, 2004. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 123 (1), 131–145. 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR, 2011. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 69 (7), 633–641. 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, Pistis M, 2008. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 28 (51), 13985–13994. 10.1523/JNEUROSCI.3221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigurupati S, Dhanaraj SA, Balakumar P, 2015. A step ahead of PPARgamma full agonists to PPARgamma partial agonists: therapeutic perspectives in the management of diabetic insulin resistance. Eur J Pharmacol. 755, 50–57. 10.1016/j.ejphar.2015.02.043. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R, 2013. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr Drug Targets. 14 (7), 768–776. 10.2174/1389450111314070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, et al. , 2013. Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res. 37 (8), 1351–1360. 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Ferguson LB, Most D, Blednov YA, Harris RA, 2014. PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology. 86, 397–407. 10.1016/j.neuropharm.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. , 2011. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 69 (7), 642–649. 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Fotio Y, Borruto AM, Benvenuti F, Demopulos G, Gaitanaris G, Roberto M, et al. , 2020. Activation of peroxisome proliferator-activated receptor gamma reduces alcohol drinking and seeking by modulating multiple mesocorticolimbic regions in rats. Neuropsychopharmacology. 10.1038/s41386-020-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, et al. , 2015. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res. 39 (1), 136–145. 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA. PPAR Agonists: I. Role of Receptor Subunits in Alcohol Consumption in Male and Female Mice. Alcohol Clin Exp Res. 2016;40(3):553–62. doi: 10.1111/acer.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA, 2016. PPAR Agonists: II. Fenofibrate and Tesaglitazar Alter Behaviors Related to Voluntary Alcohol Consumption. Alcohol Clin Exp Res. 40 (3), 563–571. 10.1111/acer.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Kosten TA, 2017. The peroxisome proliferator-activated receptor alpha agonist fenofibrate attenuates alcohol self-administration in rats. Neuropharmacology. 116, 364–370. 10.1016/j.neuropharm.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón María, Alén Francisco, Gómez de Heras Raquel, Serrano Antonia, Pavón Francisco Javier, Leza Juan Carlos, García-Bueno Borja, Rodríguez de Fonseca Fernando, Orio Laura, 2017. Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-kB danger signaling in rat frontal cortex and depressive-like behavior induced by ethanol binge administration. Addict Biol. 22 (3), 724–741. 10.1111/adb.2017.22.issue-310.1111/adb.12365. [DOI] [PubMed] [Google Scholar]
- Karwad MA, Macpherson T, Wang B, Theophilidou E, Sarmad S, Barrett DA, et al. , 2017. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARalpha. FASEB J. 31 (2), 469–481. 10.1096/fj.201500132. [DOI] [PubMed] [Google Scholar]
- Bilbao Ainhoa, Serrano Antonia, Cippitelli Andrea, Pavón Francisco J., Giuffrida Andrea, Suárez Juan, García-Marchena Nuria, Baixeras Elena, Gómez de Heras Raquel, Orio Laura, Alén Francisco, Ciccocioppo Roberto,Cravatt Benjamin F., Parsons Loren H., Piomelli Daniele, Rodríguez de Fonseca Fernando, 2016. Role of the satiety factor oleoylethanolamide in alcoholism. Addict Biol. 21 (4), 859–872. 10.1111/adb.2016.21.issue-410.1111/adb.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiqin Y, Meilin X, Jie X, Keping Z, 2009. Aspirin inhibits MMP-2 and MMP-9 expression and activity through PPARalpha/gamma and TIMP-1-mediated mechanisms in cultured mouse celiac macrophages. Inflammation. 32 (4), 233–241. 10.1007/s10753-009-9125-3. [DOI] [PubMed] [Google Scholar]
- Israel Yedy, Quintanilla María Elena, Ezquer Fernando, Morales Paola, Santapau Daniela, Berríos-Cárcamo Pablo, Ezquer Marcelo, Olivares Belen, Herrera-Marschitz Mario, 2021. Aspirin and N-acetylcysteine co-administration markedly inhibit chronic ethanol intake and block relapse binge drinking: Role of neuroinflammation-oxidative stress self-perpetuation. Addict Biol. 26 (1) 10.1111/adb.v26.110.1111/adb.12853. [DOI] [PubMed] [Google Scholar]
- van Kooten MJ, Veldhuizen MG, de Araujo IE, O’Malley SS, Small DM, 2016. Fatty acid amide supplementation decreases impulsivity in young adult heavy drinkers. Physiol Behav. 155, 131–140. 10.1016/j.physbeh.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Diazgranados N, Umhau JC, Kwako LE, George DT, Heilig M, 2020. PPARγ activation by pioglitazone does not suppress cravings for alcohol, and is associated with a risk of myopathy in treatment seeking alcohol dependent patients: a randomized controlled proof of principle study. Psychopharmacology (Berl). 237 (8), 2367–2380. 10.1007/s00213-020-05540-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MB, Bortolini M, Tadayyon M, Bopst M, 2014. Minireview: Challenges and opportunities in development of PPAR agonists. Mol Endocrinol. 28 (11), 1756–1768. 10.1210/me.2013-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato AA, de Assis Rocha Neves F. Idealized PPARgamma-Based Therapies: Lessons from Bench and Bedside. PPAR Res. 2012;2012:978687. doi: 10.1155/2012/978687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson Jessica R., Karatayev Olga, Chang Guo-Qing, Johnson Deanne F., Bocarsly Miriam E., Hoebel Bartley G., Leibowitz Sarah F., 2009. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol. 43 (6), 433–441. 10.1016/j.alcohol.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Fernandez K, Israel Y, 2014. Fenofibrate–a lipid-lowering drug–reduces voluntary alcohol drinking in rats. Alcohol. 48 (7), 665–670. 10.1016/j.alcohol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F, 2005. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science. 308 (5726), 1314–1318. 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Erickson EK, Blednov YA, Harris RA, Mayfield RD, 2019. Glial gene networks associated with alcohol dependence. Sci Rep. 9 (1), 10949. 10.1038/s41598-019-47454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden Anna S., Wolfe Sarah A., Khom Sophia, Varodayan Florence P., Patel Reesha R., Steinman Michael Q., Bajo Michal, Montgomery Sarah E., Vlkolinsky Roman, Nadav Tali, Polis Ilham, Roberts Amanda J., Dayne Mayfield, R., Adron Harris, R., Roberto Marisa, 2020. Microglia Control Escalation of Drinking in Alcohol-Dependent Mice: Genomic and Synaptic Drivers. Biol Psychiatry. 88 (12), 910–921. 10.1016/j.biopsych.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W, 2016. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Molecular Neurobiology. 53 (2), 1181–1194. 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M, Hertz L, 2015. Why are astrocytes important? Neurochemical Research. 40 (2), 389–401. 10.1007/s11064-014-1403-2. [DOI] [PubMed] [Google Scholar]
- Tilleux Sébastien, Hermans Emmanuel, 2007. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. Journal of Neuroscience Research. 85 (10), 2059–2070. 10.1002/(ISSN)1097-454710.1002/jnr.v85: 1010.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Sari Y, 2013. Potential therapeutic role of glutamate transporter 1 for the treatment of alcohol dependence. OA alcohol. 1 (1), 6. 10.13172/2053-0285-1-1-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EK, DaCosta AJ, Mason SC, Blednov YA, Mayfield RD, Harris RA, 2021. Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacology. 46 (3), 500–508. 10.1038/s41386-020-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner E, Tiwari GR, Kapoor M, Liu Y, Brock A, Mayfield RD, 2020. Single cell transcriptome profiling of the human alcohol-dependent brain. Hum Mol Genet. 29 (7), 1144–1153. 10.1093/hmg/ddaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: Focus on glial-modulating targets. Current opinion in investigational drugs (London, England : 2000). 2008;9(7):726–34. [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A Fau - Gálvez J, Gálvez J. Minocycline: far beyond an antibiotic. British Journal of Pharmacology. 2013;169(2):337–52. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE, 2011. Minocycline Reduces Ethanol Drinking. Brain, behavior, and immunity. 25 (Suppl 1), S165–S169. 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainiola M, Linden A-M, 2017. Alcohol intake in two different mouse drinking models after recovery from the lipopolysaccharide-induced sickness reaction. Alcohol (Fayetteville, N.Y.) 65, 1–10. 10.1016/j.alcohol.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Agrawal Rajiv G., Owen Julie A., Levin Patricia S., Hewetson Aveline, Berman Ari E., Franklin Scott R., Hogue Ryan J., Chen Yukun, Walz Chris, Colvard Benjamin D., Nguyen Jonathan, Velasquez Oscar, Al-Hasan Yazan, Blednov Yuri A., Fowler Anna-Kate, Syapin Peter J., Bergeson Susan E., 2014. Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcoholism, Clinical and Experimental Research. 38 (2), 428–437. 10.1111/acer.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajbhiye SV, Tripathi RK, Petare A, Potey AV, Shankar A, 2018. Minocycline in Alcohol Withdrawal Induced Anxiety and Alcohol Relapse in Rats. Current Clinical Pharmacology. 13 (1), 65–72. 10.2174/1574884713666180228110310. [DOI] [PubMed] [Google Scholar]
- Wu Yue, Lousberg Erin L., Moldenhauer Lachlan M., Hayball John D., Robertson Sarah A., Coller Janet K., Watkins Linda R., Somogyi Andrew A., Hutchinson Mark R., 2011. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain, Behavior, and Immunity. 25, S155–S164. 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ooi SA-O, Green R, Pak SA-O, 2011. N-Acetylcysteine for the Treatment of Psychiatric Disorders: A Review of Current Evidence. J Psychiatry Neurosci. 32 (2), 78–86. 10.1503/jpn.100057. [DOI] [Google Scholar]
- Lebourgeois Sophie, González-Marín María Carmen, Jeanblanc Jerome, Naassila Mickael, Vilpoux Catherine, 2018. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addiction Biology. 23 (2), 643–652. 10.1111/adb.2018.23.issue-210.1111/adb.12521. [DOI] [PubMed] [Google Scholar]
- Weiland A, Garcia S, Knackstedt LA, 2015. Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Frontiers in Pharmacology. 6 10.3389/fphar.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Ricardo, Bandiera Solange, Souza Débora Guerini, Bellaver Bruna, Caletti Greice, Quincozes-Santos André, Elisabetsky Elaine, Gomez Rosane, 2017. N-acetylcysteine Prevents Alcohol Related Neuroinflammation in Rats. Neurochemical Research. 42 (8), 2135–2141. 10.1007/s11064-017-2218-8. [DOI] [PubMed] [Google Scholar]
- Lee MR, Ruby CL, Hinton DJ, Choi S, Adams CA, Young Kang N, et al. , 2013. Striatal adenosine signaling regulates EAAT2 and astrocytic AQP4 expression and alcohol drinking in mice 38, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y, 2014. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 231 (20), 4049–4057. 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y, 2013. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. Journal of Psychopharmacology (Oxford, England). 27 (6), 541–549. 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi D-S, 2014. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2 39, 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Sari Y, 2017. β-Lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neuroscience Letters. 657, 140–145. 10.1016/j.neulet.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Jie, Swift Brandon, Mamelok Richard, Pine Samuel, Sinclair John, Attar Mayssa, 2019. Design and Conduct Considerations for First-in-Human Trials. Clin Trans Sci. 12 (1), 6–19. 10.1111/cts.v12.110.1111/cts.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis Ismene L., Ralevski Elizabeth, Gueorguieva Ralitza, Sloan Matthew E., Devine Lesley, Yoon Gihyun, Arias Albert J., Sofuoglu Mehmet, 2019. Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology. 236 (10), 3013–3021. 10.1007/s00213-019-05205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tomko RL, Baker NL, McClure EA, Book GA, Gray KM, 2018. The effect of N-acetylcysteine on alcohol use during a cannabis cessation trial. Drug and Alcohol Dependence. 185, 17–22. 10.1016/j.drugalcdep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Leyden JJ, 2005. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 27 (9), 1329–1342. 10.1016/j.clinthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- LaRowe Steven D., Mardikian Pascale, Malcolm Robert, Myrick Hugh, Kalivas Peter, McFarland Krista, Saladin Michael, McRae Aimee, Brady Kathleen, 2006. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 15 (1), 105–110. 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CJ, 2016. The Role of Pharmacotherapy in the Treatment of Adolescent Substance Use Disorders. Child Adolesc Psychiatr Clin N Am. 25 (4), 685–711. 10.1016/j.chc.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow BT, Onysko M, Hebert M, 2016. Medications for Alcohol Use Disorder. Am Fam Physician. 93 (6), 457–465. [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL, 2011. Ceftriaxone, a Beta-Lactam Antibiotic, Reduces Ethanol Consumption in Alcohol-Preferring Rats. Alcohol and Alcoholism (Oxford, Oxfordshire). 46 (3), 239–246. 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y, 2015. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 97, 67–74. 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Toalston JE, Rao PSS, Bell RL, 2016. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience. 326, 117–125. 10.1016/j.neuroscience.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno Ryan P., Crews Fulton T., Arendt Thomas, 2018. Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin. PLoS One. 13 (10), e0204500. 10.1371/journal.pone.0204500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Lawrimore CJ, Rowsey PJ, Crews FT, 2018. Persistent Adult Neuroimmune Activation and Loss of Hippocampal Neurogenesis Following Adolescent Ethanol Exposure: Blockade by Exercise and the Anti-inflammatory Drug Indomethacin. Front Neurosci. 12, 200. 10.3389/fnins.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmel RP, Crews BC, Kozak KR, Kalgutkar AS, Marnett LJ, 2004. Studies on the metabolism of the novel, selective cyclooxygenase-2 inhibitor indomethacin phenethylamide in rat, mouse, and human liver microsomes: identification of active metabolites. Drug Metab Dispos. 32 (1), 113–122. 10.1124/dmd.32.1.113. [DOI] [PubMed] [Google Scholar]
- George FR, 1989. The role of arachidonic acid metabolites in mediating ethanol self-administration and intoxication. Ann N Y Acad Sci. 559, 382–391. 10.1111/j.1749-6632.1989.tb22624.x. [DOI] [PubMed] [Google Scholar]
- Pascual Maria, Blanco Ana M., Cauli Omar, Miñarro Jose, Guerri Consuelo, 2007. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 25 (2), 541–550. 10.1111/ejn.2007.25.issue-210.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Ferguson Laura B, Ozburn Angela R, Ponomarev Igor, Metten Pamela, Reilly Matthew, Crabbe John C, Harris R Adron, Mayfield R Dayne, 2018. Genome-Wide Expression Profiles Drive Discovery of Novel Compounds that Reduce Binge Drinking in Mice. Neuropsychopharmacology. 43 (6), 1257–1266. 10.1038/npp.2017.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Hartman SE, Kinoshita E, Suzuki H, Kitaura J, Yao L, Inagaki N, Franco A, Hata D, Maeda-Yamamoto M, Fukamachi H, Nagai H, Kawakami T, 1999. Terreic acid, a quinone epoxide inhibitor of Bruton’s tyrosine kinase. Proc Natl Acad Sci U S A. 96 (5), 2227–2232. 10.1073/pnas.96.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, 2019. Development of BTK inhibitors for the treatment of B-cell malignancies. Arch Pharm Res. 42 (2), 171–181. 10.1007/s12272-019-01124-1. [DOI] [PubMed] [Google Scholar]
- Bendele AM, Spaethe SM, Benslay DN, Bryant HU, 1991. Anti-inflammatory activity of pergolide, a dopamine receptor agonist. J Pharmacol Exp Ther. 259 (1), 169–175. [PubMed] [Google Scholar]
- Balan I, Aurelian L, Schleicher R, Boero G, O’Buckley T, Morrow AL, 2021. Neurosteroid allopregnanolone (3alpha,5alpha-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl Psychiatry. 11 (1), 145. 10.1038/s41398-021-01266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Guidotti A, Saudagar V, Grayson DR, Aspesi D, Pandey SC, et al. Epigenetic Regulation of GABAergic Neurotransmission and Neurosteroid Biosynthesis in Alcohol Use Disorder. Int J Neuropsychopharmacol. 2021;24(2):130–41. doi: 10.1093/ijnp/pyaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow A. Leslie, Boero Giorgia, Porcu Patrizia, 2020. A Rationale for Allopregnanolone Treatment of Alcohol Use Disorders: Basic and Clinical Studies. Alcohol Clin Exp Res. 44 (2), 320–339. 10.1111/acer.v44.210.1111/acer.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, 2010. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 186, 113–137. 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Jimenez VA, 2018. Dynamic Adaptation in Neurosteroid Networks in Response to Alcohol. Handb Exp Pharmacol. 248, 55–78. 10.1007/164_2017_82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli Giovanni, Vallée Monique, 2019. Stress and drug abuse-related disorders: The promising therapeutic value of neurosteroids focus on pregnenolone-progesterone-allopregnanolone pathway. Frontiers in Neuroendocrinology. 55, 100789. 10.1016/j.yfrne.2019.100789. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA, 2005. Neurosteroid modulators of GABA(A) receptors differentially modulate Ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 29 (9), 1630–1640. 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak Patricia H., Redfern Jane E.M., Samson Herman H., 1998. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 22 (5), 1106–1112. [PubMed] [Google Scholar]
- Rezvani AH, Levin ED, 2014. Assessment of pregnenolone effects on alcohol intake and preference in male alcohol preferring (P) rats. Eur J Pharmacol. 740, 53–57. 10.1016/j.ejphar.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Phillips TJ, Finn DA, 2014. Differences in the reinstatement of ethanol seeking with ganaxolone and gaboxadol. Neuroscience. 272, 180–187. 10.1016/j.neuroscience.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turna Jasmine, Syan Sabrina K., Frey Benicio N., Rush Brian, Costello Mary Jean, Weiss Mark, MacKillop James, 2019. Cannabidiol as a Novel Candidate Alcohol Use Disorder Pharmacotherapy: A Systematic Review. Alcohol Clin Exp Res. 43 (4), 550–563. 10.1111/acer.2019.43.issue-410.1111/acer.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S, 2015. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 23 (7), 1377–1385. 10.1016/j.bmc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- Pellati Federica, Borgonetti Vittoria, Brighenti Virginia, Biagi Marco, Benvenuti Stefania, Corsi Lorenzo, 2018. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress. Inflammation, and Cancer. Biomed Res Int 2018, 1–15. 10.1155/2018/1691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez-Martínez Adrián, García-Gutiérrez María S., Navarrón Carmen María, Morales-Calero María Isabel, Navarrete Francisco, Torres-Suárez Ana Isabel, Manzanares Jorge, 2018. Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict Biol. 23 (1), 154–164. 10.1111/adb.12495. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cuevas Gustavo, Martin-Fardon Remi, Kerr Tony M., Stouffer David G., Parsons Loren H., Hammell Dana C., Banks Stan L., Stinchcomb Audra L., Weiss Friedbert, 2018. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology. 43 (10), 2036–2045. 10.1038/s41386-018-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yuping, Mukhopadhyay Partha, Cao Zongxian, Wang Hua, Feng Dechun, Haskó György, Mechoulam Raphael, Gao Bin, Pacher Pal, 2017. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci Rep. 7 (1) 10.1038/s41598-017-10924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan SE, 2010. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 335 (1), 92–102. 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR, 2014. Dutasteride reduces alcohol’s sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl). 231 (17), 3609–3618. 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobaum AL, Uddin MJ, Felts AS, Crews BC, Rouzer CA, Marnett LJ, 2013. The 2’-Trifluoromethyl Analogue of Indomethacin Is a Potent and Selective COX-2 Inhibitor. ACS Med Chem Lett. 4 (5), 486–490. 10.1021/ml400066a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF, 2006. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychol Addict Behav. 20 (3), 241–253. 10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C, Shahinas J, 2020. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J Clin Med Res. 12 (3), 129–141. 10.14740/jocmr4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Irwin MR, 2016. Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun. 51, 1–11. 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One. 2014;9(7):e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock AS, Goldberg SB, Coe CL, Zgierska AE, 2019. Mindfulness practice predicts interleukin-6 responses to a mindfulness-based alcohol relapse prevention intervention. J Subst Abuse Treat. 105, 57–63. 10.1016/j.jsat.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2009. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 14 (1), 84–98. 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Colaiaco B, Motala A, Shanman R, Booth M, Sorbero M, et al. , 2017. Mindfulness-based Relapse Prevention for Substance Use Disorders: A Systematic Review and Meta-analysis. J Addict Med. 11 (5), 386–396. 10.1097/ADM.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen Sarah, Chawla Neharika, Collins Susan E., Witkiewitz Katie, Hsu Sharon, Grow Joel, Clifasefi Seema, Garner Michelle, Douglass Anne, Larimer Mary E., Marlatt Alan, 2009. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 30 (4), 295–305. 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska Aleksandra E., Burzinski Cindy A., Mundt Marlon P., McClintock Andrew S., Cox Jennifer, Coe Christopher L., Miller Michael M., Fleming Michael F., 2019. Mindfulness-based relapse prevention for alcohol dependence: Findings from a randomized controlled trial. J Subst Abuse Treat. 100, 8–17. 10.1016/j.jsat.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Bowen S, Witkiewitz K, 2017. Baseline patterns of substance use disorder severity and depression and anxiety symptoms moderate the efficacy of mindfulness-based relapse prevention. J Consult Clin Psychol. 85 (11), 1041–1051. 10.1037/ccp0000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 16 (1), 22–34. 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten Raye Z., Falk Daniel E., Ryan Megan L., Fertig Joanne, Leggio Lorenzo, 2020. Five Priority Areas for Improving Medications Development for Alcohol Use Disorder and Promoting Their Routine Use in Clinical Practice. Alcohol Clin Exp Res. 44 (1), 23–35. 10.1111/acer.v44.110.1111/acer.14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA, 2016. Experimental psychopathology paradigms for alcohol use disorders: Applications for translational research. Behav Res Ther. 86, 11–22. 10.1016/j.brat.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Montes KS, Schwebel FJ, Tucker JA, 2020. What Is Recovery? Alcohol Res. 40 (3), 01. 10.35946/arcr.v40.3.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson Teresa J., Painter Jacob T., Martin Bradley C., Austen Mark A., Williams James S., Fortney John C., Sullivan Mark D., Edlund Mark J., 2017. Pharmacoepidemiologic analyses of opioid use among OEF/OIF/OND veterans. Pain. 158 (6), 1039–1045. 10.1097/j.pain.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]


