Abstract
Background:
Experimental evidence indicates that exposure to certain pollutants is associated with liver damage. Per- and polyfluoroalkyl substances (PFAS) are persistent synthetic chemicals widely used in industry and consumer products and bioaccumulate in food webs and human tissues, such as the liver.
Objective:
The objective of this study was to conduct a systematic review of the literature and meta-analysis evaluating PFAS exposure and evidence of liver injury from rodent and epidemiological studies.
Methods:
PubMed and Embase were searched for all studies from earliest available indexing year through 1 December 2021 using keywords corresponding to PFAS exposure and liver injury. For data synthesis, results were limited to studies in humans and rodents assessing the following indicators of liver injury: serum alanine aminotransferase (ALT), nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, or steatosis. For human studies, at least three observational studies per PFAS were used to conduct a weighted -score meta-analysis to determine the direction and significance of associations. For rodent studies, data were synthesized to qualitatively summarize the direction and significance of effect.
Results:
Our search yielded 85 rodent studies and 24 epidemiological studies, primarily of people from the United States. Studies focused primarily on legacy PFAS: perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulfonic acid. Meta-analyses of human studies revealed that higher ALT levels were associated with exposure to PFOA ( 6.20, ), PFOS ( 3.55, ), and PFNA ( 2.27, ). PFOA exposure was also associated with higher aspartate aminotransferase and gamma-glutamyl transferase levels in humans. In rodents, PFAS exposures consistently resulted in higher ALT levels and steatosis.
Conclusion:
There is consistent evidence for PFAS hepatotoxicity from rodent studies, supported by associations of PFAS and markers of liver function in observational human studies. This review identifies a need for additional research evaluating next-generation PFAS, mixtures, and early life exposures. https://doi.org/10.1289/EHP10092
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a public health epidemic.1 In parallel with the growing obesity epidemic, prevalence of NAFLD has significantly increased in recent years and become one of the most common causes of chronic liver disease globally.2,3 The prevalence of NAFLD is estimated to be about 25% worldwide, whereas cases in the United States are expected to number , or about one-third of all adults, by 2030.4 Untreated, NAFLD may progress to more serious liver injury such as nonalcoholic steatohepatitis (NASH), cirrhosis, and end-stage liver disease.5
Exposure to environmental chemicals has emerged as a significant contributor to liver disease, including NAFLD. Experimental evidence indicates that exposure to per- and polyfluorinated substances (PFAS), a class of endocrine-disrupting chemicals, has the ability to promote metabolic changes that can result in fatty liver.6 PFAS are synthetic chemicals widely used in industry and consumer products such as stain-resistant fabric and fire retardants.7,8 The stable chemical properties that make PFAS ideal for industrial use also allow them to persist and accumulate in the environment,9 which is of concern because of the potential for long-term human health effects. Recent biomonitoring studies have emphasized the ubiquitous nature of PFAS exposure and have indicated that four congeners of PFAS account for most known human exposure: perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexanesulfonic acid (PFHxS), and perfluorononanoic acid (PFNA).10,11 Significant sources of exposure include drinking water,12,13 food,14,15 indoor and outdoor air,16,17 and early life placental or breast milk exposure.18–20 PFAS are detected in the serum of nearly all U.S. adults21,22 and accumulate in body tissues, such as in the liver.23–25 This bioaccumulation, coupled with the long half-lives of many PFAS,26,27 leads to concern about the potential for PFAS to disrupt liver homeostasis should they continue to accumulate in human tissue even if industrial use is abated.
Research evaluating hepatotoxic effects of PFAS has greatly increased in the peer‐reviewed literature; however, conclusions remain inconsistent. In animal studies, PFAS have consistently induced steatosis and lipid accumulation in mice,28 rats,29 zebrafish,30 chickens,31 frogs,32 and primates.33 Despite this, it is difficult to extrapolate directly from animal results to human health effects in part due to species differences in PFAS elimination and half-lives.34
Evaluations of occupationally exposed workers have not consistently reported associations between PFAS exposure and liver enzymes or liver disease,35–38 although recent analyses of other populations have reported positive associations between PFAS and liver enzymes indicative of liver injury.39–42 Epidemiological studies have also reported associations between PFAS exposure and cholesterol,43–47 triglycerides,38,45,47 bilirubin,40 and uric acid,40 further supporting a relationship between PFAS exposure and liver injury given that these are additional biomarkers of metabolic disruption, NAFLD, and advanced liver disease.48–50
Indeed, the association between PFAS exposure and NAFLD in humans remains challenging to evaluate given the difficulty in obtaining biopsy-confirmed NAFLD histological data, and thus liver injury is typically assessed using serum biomarkers of hepatotoxicity or imaging assessments of hepatic steatosis.51 Alanine aminotransferase (ALT) in particular is considered a specific biomarker of liver injury and is widely used in epidemiological studies.51–53 A recent review summarized the state of the literature regarding toxic effects of PFAS on many adverse health effects, including liver disease, lipid dysregulation, and other metabolic outcomes.54 Fenton et al.54 provided an overview of the evidence for hepatoxicity across human and animal studies, as well as a discussion of possible mechanisms underlying this relationship. In contrast, the purpose of the present review is to specifically evaluate the effects of PFAS exposure on NAFLD and markers of NAFLD, with a focus on the liver enzymes commonly used in human epidemiological research. To our knowledge, this is the first systematic review and meta-analysis integrating both the epidemiological (human) and experimental (rodent) evidence for an effect of PFAS exposure on liver enzymes and related markers of liver injury.
Materials and Methods
This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The review protocol was registered in PROSPERO (CRD42020158911).55
Search Strategy
We systematically searched two databases, PubMed and Embase, for human and rodent studies evaluating the association between exposure to PFAS and markers of liver injury from earliest available online indexing through 1 December 2021. For PubMed, the search strategy was as follows: (NAFLD OR “nonalcoholic fatty liver disease” OR “nonalcoholic fatty liver disease” OR NASH OR “nonalcoholic steatohepatitis” OR “nonalcoholic steatohepatitis” OR “nonalcoholic fatty liver” OR “fatty liver” OR steatosis OR ALT OR “alanine aminotransferase” OR AST OR “aspartate aminotransferase” OR GGT OR “gamma-glutamyl transferase” OR “gamma glutamyl transferase” OR CK18 OR “cytokeratin 18” OR ALP OR “alkaline phosphatase” OR “liver enzymes” OR “liver damage” OR “liver injury” OR “liver fibrosis” OR “liver weight”) AND (Perfluoroalkyl OR Polyfluoroalkyl OR Perfluorinated OR polyfluorinated OR perfluoro* OR polyfluoro* OR PFAS* [tiab] OR PFOS [tiab] OR ((perfluorooctanesulfonic OR perfluorooctane sulfonic) AND acid) OR “perfluorooctane sulfonate” OR PFOA [tiab] OR “perfluorooctanoic” acid OR perfluorooctanoate OR PFHxS [tiab] OR ((perfluorohexane sulfonic OR perfluorohexanesulfonic) AND acid) OR “perfluorohexane sulfonate” OR perfluorohexanesulfonate OR PFNA [tiab] OR “perfluorononanoic acid” OR perfluorononanoate OR GenX [tiab] OR “hexafluoropropylene oxide dimer acid” OR PFOSA [tiab] OR “perfluorooctane sulfonamide” OR PFUnDA [tiab] OR “perfluorodecanoic acid” OR perfluoroundecanoate PFDA OR “perfluorodecanoic acid” OR perfluorodecanoate OR PFBS OR “perfluorobutane sulfonic acid” OR “perfluorobutane sulfonate”.
For Embase, the search terms were (NAFLD OR “nonalcoholic fatty liver disease” OR “nonalcoholic fatty liver disease” OR NASH OR “nonalcoholic steatohepatitis” OR “nonalcoholic steatohepatitis” OR “nonalcoholic fatty liver” OR “fatty liver” OR steatosis OR ALT OR “alanine aminotransferase” OR AST OR “aspartate aminotransferase” OR GGT OR “gamma-glutamyl transferase” OR “gamma glutamyl transferase” OR CK18 OR “cytokeratin 18” OR ALP OR “alkaline phosphatase” OR “liver enzymes” OR “liver damage” OR “liver injury” OR “liver fibrosis” OR “liver weight”) AND (Perfluoroalkyl OR Polyfluoroalkyl OR Perfluorinated OR polyfluorinated OR perfluoro* OR polyfluoro* OR PFAS*:ab,ti OR PFOS:ab,ti OR ((perfluorooctanesulfonic OR perfluorooctane sulfonic) AND acid) OR “perfluorooctane sulfonate” OR PFOA:ab,ti OR “perfluorooctanoic acid” OR “perfluorooctanoate” OR PFHxS:ab,ti OR ((perfluorohexane sulfonic OR perfluorohexanesulfonic) AND acid) OR “perfluorohexane sulfonate” OR perfluorohexanesulfonate OR PFNA:ab,ti OR “perfluorononanoic acid” OR perfluorononanoate OR GenX:ab,ti OR “hexafluoropropylene oxide dimer acid” OR PFOSA:ab,ti OR “perfluorooctane sulfonamide” OR PFUnDA:ab,ti OR “perfluorodecanoic acid” OR “perfluoroundecanoate PFDA” OR “perfluorodecanoic acid” OR perfluorodecanoate OR PFBS OR “perfluorobutane sulfonic acid” OR “perfluorobutane sulfonate”. We also screened the references of recent reviews for eligible studies.
Study Selection
Studies were eligible for inclusion if they met the following criteria: (a) were original experimental or observational research published in English (i.e., not a review, meta-analysis, abstract, editorial, letter, or commentary); (b) conducted in humans, mice, or rats; (c) assessed one or more PFAS; and (d) reported data on serum ALT, NAFLD, NASH, or steatosis. ALT was chosen as the biomarker of interest because of its relative specificity to liver disease and use in previous literature on PFAS exposure and NAFLD. Other markers of liver disease—such as bilirubin, alkaline phosphatase, albumin, and uric acid—were not included because alterations in these biomarkers may suggest damage to other organ systems or liver diseases with alternate causes (e.g., cancer, alcoholic fatty liver).51,56,57 Secondary outcomes were extracted, if available, and included serum aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT), cytokeratin-18 (CK-18), liver histopathology, and relative liver weight (animals only). For the purpose of this review, increases in liver weight were presumed to be adverse, given our focus on additional measures of liver injury (e.g., enzymes, histopathology). However, increases in liver weight alone may be an adaptive response in rodents and do not always indicate that an injury has occurred.58 Two reviewers (S.R. and E.C.) independently performed an initial screening of titles and abstracts and then evaluated potentially relevant studies based on full-text reviews. Any discrepancies were resolved by discussion with a third reviewer (N.S.).
Data Extraction
In human studies, the following information was extracted from each article: first author, publication year, country, year and method of exposure assessment and outcome assessment, study design, population characteristics, sample size, confounders, and results [adjusted coefficients and odds ratios with standard errors (SEs) or 95% confidence intervals]. In rodent studies, the following information was extracted: first author, year, study design, species/strain, sex, sample size, age, exposure, frequency and duration of exposure, administration route, dose, diet, outcome results, and SE. Data were independently extracted by two reviewers (S.R. and E.C.) and compared for accuracy. Any discrepancies were resolved through discussion with a third reviewer (N.S.).
Quality Assessment
Human and rodent study quality was independently evaluated by two reviewers (S.R. and E.C.) using the Office of Health Assessment and Translation (OHAT) Risk of Bias tool,59,60 with discrepancies resolved through discussion. The OHAT Risk of Bias tool was used to evaluate threats to internal validity and assess the risk of bias. The OHAT tool was chosen for its ability to evaluate cross-sectional studies, which are not considered in other quality rating systems, and applicability to both human and rodent studies.61,62
Six of the 10 domains in the OHAT tool were relevant to observational human studies; those pertaining to randomization and blinding were not applicable. Eight domains were relevant to experimental rodent studies; domains that addressed participant selection and confounding were not relevant. For each domain, a study was evaluated for definitely low risk of bias (), probably low risk of bias (), probably high risk of bias (–), and definitely high risk of bias (– –). In domains where the study did not provide enough information to evaluate bias, an assignment of “probably high” risk was given with the notation “NR” for “not reported.” Specific criteria for each domain are described in the section “Description of domains in Office of Health Assessment and Translation (OHAT) Risk of Bias tool” in the Supplemental Material.
Data Synthesis and Meta-Analysis
In human studies, we conducted meta-analyses between exposure to each of the four selected PFAS and serum concentrations of each of three liver enzymes (ALT, AST, and GGT), which were reported in at least three studies of similar design (e.g., cross-sectional, longitudinal). Because of the heterogeneous methodologies (e.g., log-transformation or natural log-transformation of the exposure, the outcome, or both) and noncomparable effect estimates, it was not possible to directly pool effect estimates across studies. For example, the effect estimate from a study that both exposure and outcome cannot be pooled with a study that natural log-transformed only the exposure, and pooling only studies that had similar transformation methodologies may introduce selection bias. Thus, we used a weighted -scores method to summarize results. -Scores were calculated using adjusted coefficients from linear regression analyses of PFAS and their SE.63 Although the magnitude of the effect cannot be determined using this method, a weighted -score allows for determination of the statistical significance and direction of the relationship. For each PFAS-liver enzyme relationship, a weighted-average -score was calculated where weights were the square root of the sample size. Studies in populations of age (presumed to be either in early stages of puberty or prepubertal based on normal range of puberty in girls and boys)64,65 were excluded from this calculation to account for developmental effects and included in sensitivity analyses. For different studies with overlapping populations, only the study with the largest population was included. In studies that reported multiple models, we used the effect estimate from the most highly adjusted model. Although the inclusion criteria did not exclude studies with categorical measures, none of the studies in the present review used exclusively categorical measures. The -score was calculated using the overall , not those stratified by sex, weight, or other factors, unless an overall was not available. However, when studies reported stratified analyses by sex in addition to overall population results, we included the stratified results to see whether sex-specific differences might exist when multiple studies are compared. Where possible, additional sensitivity analyses were performed and -scores were calculated a) separately by sex, b) after excluding the largest study, c) for studies using National Health and Nutrition Examination Survey (NHANES) data, and d) including populations of age. The purpose of these analyses was to determine whether a) the relationships differed by sex, b) they were driven primarily by a single large study, c) the relationship differed between the general population of the United States and populations from other countries or those occupationally exposed, and d) including children changed the direction or statistical significance of the relationship.
In rodent studies, substantial differences in study design (e.g., length of exposure, exposure vehicle, dose) meant that meta-analyses were not feasible. Data were synthesized and displayed graphically. We used strip plots adapted from Thayer et al.66 to summarize the direction of the effect of PFAS dose (in milligrams per kilogram of body weight or parts per million) on ALT across all eligible studies. Additional plots were used to summarize the effects of PFAS exposure on additional liver enzymes and relative liver weight in those studies that reported secondary outcomes. Some studies provided data on groups treated with PFAS combined with nonstandard diets or supplements; for these, we selected as control the group on standard diet with no PFAS or supplement exposure. PFAS plus experimental diet or supplement were included as exposure groups. All analyses were conducted in R (version 4.0.2; R Development Core Team).
Results
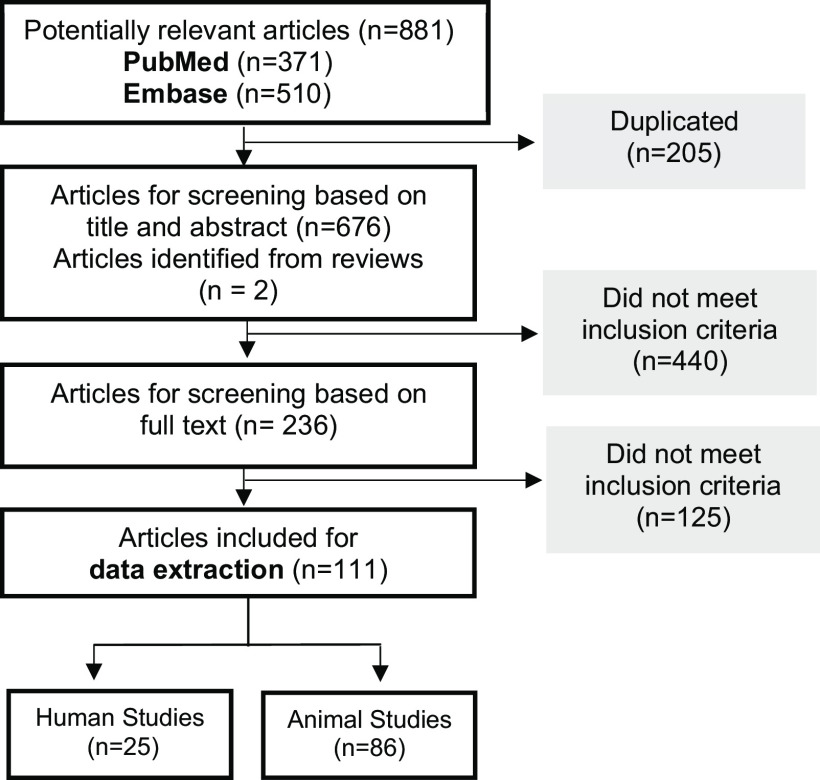
Our search produced 881 articles from PubMed () and Embase (), 205 of which were duplicates (Figure 1). After title and abstract screening and full-text review, 109 studies met the eligibility criteria. Two additional studies were identified from review articles (see the section “Review articles screened for additional eligible articles” in the Supplemental Material). Of the 111 total studies, 25 were observational human studies and 86 were experimental rodent studies. Extracted data used in -score calculations for human studies and in visual data synthesis for animal studies are available in Excel Tables S1 and S2, respectively.
Figure 1.
Flow chart of the study selection.
The characteristics of human studies included in this review are shown in Table 1. Eighteen studies included populations from the United States,35–40,43,44,67–76 7 included populations from Europe,36–38,42,77–79 and 2 from Asia.41,80 Years of PFAS exposure assessment ranged from 195139 to 2016.41,67,74 Sixteen studies were cross-sectional and 6 had a longitudinal design. Two studies, Darrow et al.39 and Olsen et al.,37 included both cross-sectional and longitudinal data.
Table 1.
Human studies on per- and polyfluorinated chemicals and biomarkers or outcomes of liver injury included for systematic review.
| Reference | Populationa | Year of exposure assessment | Exposure assessmentb | Year of outcome assessment | Outcomec | Confounding | Results |
|---|---|---|---|---|---|---|---|
| Attanasio67,169 | NHANES adolescents (USA) 353 (M), 305 (F) |
2013–2016 | Geometric mean (SE) PFOAd (M), (F); PFOSd (M), (F); PFNAd (M), (F); PFHxSd (M), (F) |
Same as exposure | ALT (U/L),d GGT (U/L),d AST (U/L)d | Adjusted for age, race/ethnicity, weight category, poverty–income ratio, tobacco exposure, and education. | Males: PFOA and PFNA were associated with lower ALT. PFNA was associated with lower AST. There was no association between any PFAS and GGT. Females: PFOA and PFNA were associated with higher ALT. PFOA, PFOS, and PFNA were associated with higher AST. PFOA and PFOS were associated with higher GGT. |
| Bassler et al.68 | C8 Health Study adults (USA) 200 |
2006 | Mean (SE) PFOAd ; PFOSd ; PFNAd ; PFHxSd |
Same as exposure | CK18 (U/mL)d | Adjusted for e-GFR, alcohol consumption category, BMI, age, and sex. | CK18-M30 and CK18-M65 were positively associated with PFOA, PFNA, and PFHxS, and there was a positive trend with PFOS. |
| Darrow et al.39 | C8 Health Study adults (USA) 28,047 |
1951–2006 (cumulative); 2005–2006 (cross-sectional) |
PFOA (modeled cumulative exposure) Median PFOAd |
2005–2006 (enzymes); 2008–2011 (liver disease) |
Liver disease (enlarged liver, fatty liver, or cirrhosis), ALT (U/L),d GGT (U/L)d | Adjusted for age, sex, BMI, alcohol consumption, regular exercise, smoking status, education, insulin resistance, fasting status, history of working at DuPont plant, and race. | Cross-sectional PFOA and longitudinal (estimated) PFOA were positively associated with ALT. There was no relationship between PFOA and liver disease. |
| Emmett et al.69 | Residents (adults and children) of Little Hocking (USA) 371 |
Not Specified | Median (IQR) PFOA |
Same as exposure | Liver disease, ALT (U/L), GGT (U/L), AST (U/L) | No adjustment for covariates. | No linear association between PFOA and ALT, GGT, or AST. Having abnormal AST levels was associated with lower PFOA. There was no relationship between liver disease and PFOA. |
| Gilliland et al.165 | Male employees of PFOA plant Adults (USA) 115 |
1985–1989 | Mean (range) Total fluorine 3.3 (0– ) (surrogate for PFOA) |
Same as exposure | ALT (IU/dL), AST (IU/dL), GGT (IU/dL) | Age, cigarette use, alcohol use, and BMI | Total serum fluorine was not associated with ALT, AST, or GGT. ALT, AST, and GGT levels did not differ by level of fluorine exposure. There was a significant interaction between serum fluorine and BMI: There was a positive association between serum fluorine and both ALT and AST in people with obesity. |
| Gallo et al.70 | C8 Health Study adults (USA) 46,452 |
2005–2006 | Median (IQR) PFOAd ; PFOSd |
Same as exposure | ALT (U/L),d GGT (U/L)d | Adjusted for alcohol consumption, socioeconomic status, fasting status, race, month of blood sample collection, age, sex, smoking, BMI, physical activity, and insulin resistance. | PFOA and PFOS were positively associated with ALT. |
| Gleason et al.40 | NHANES adults and adolescents (USA) 4,333 |
2007–2010 | Median (IQR) PFOAd ; PFOSd ; PFNAd ; PFHxSd |
Same as exposure | ALT (U/L),d GGT (U/L),d AST (U/L)d | Adjusted for age, sex, race/ethnicity, BMI, poverty, smoking, and alcohol consumption. | PFHxS, PFOA, and PFNA were positively associated with ALT. PFOA and PFNA were positively associated with GGT. PFHxS was positively associated with AST. |
| Jain71 | NHANES adults (USA) 9,523 |
2003–2014 | PFOA (ng/mL)e; PFOS (ng/mL)e |
Same as exposure | ALT (U/L),e GGT (U/L),e AST (U/L)e | Adjusted for sex, race/ethnicity, smoking status, age, BMI, diabetes status, hypertension status, fasting time, poverty–income ratio, survey year, and alcohol consumption. | PFOA and PFOS were inconsistently associated with ALT, GGT, and AST when stratified by glomerular function stage and obesity status. |
| Jain and Ducatman72 | NHANES adults (USA) 2,883 |
2011–2014 | Geometric mean (95% CI) PFOAe (non-obese); (obese); PFOSe (non-obese); (obese); PFNAe (non-obese); (obese); PFHxSe (non-obese); (obese) |
Same as exposure | ALT (U/L),e GGT (U/L),e AST (U/L)e | Adjusted for sex, race/ethnicity, age, age-squared, poverty–income ratio, physical activity, BMI, and serum cotinine. | Positive associations between PFOA, PFHxS, and PFNA and ALT were observed in participants with obesity. In those with obesity, PFOA and PFNA were also positively associated with GGT. Additional PFAS: PFDA was not found to be associated with liver enzymes. |
| Jin et al.73 | Children with NAFLD (USA) 74 |
2007–2015 | Median (IQR) PFOA ; PFOS ; PFHxS |
Same as exposure | Histological severity of NAFLD | — | Higher PFOS, PFOA, and PFHxS concentrations were associated with more severe NAFLD (NASH, fibrosis, lobular/portal inflammation, NAFLD activity score). |
| Khalil et al.74 | Dayton Obese Cohort children (USA) 48 |
2016 | Median (IQR) PFOA ; PFOS ; PFNA ; PFHxS |
Same as exposure | ALT (U/L), AST (U/L) | Adjusted for age, sex, race, and multiple testing. | There were no significant relationships between PFAS and ALT or AST. |
| Lin et al.75 | NHANES adults (USA) 2,216 |
1999–2003 | Mean (SE) PFOAe ; PFOSe ; PFNAe ; PFHxSe |
Same as exposure | ALT (U/L), GGT (U/L)e | Adjusted for age, sex, race/ethnicity, smoking, alcohol consumption, education level, BMI, HOMR-IR, metabolic syndrome, iron saturation status. | PFOA was positively associated with ALT and GGT, with a stronger effect in those with obesity. |
| Mora et al.76 | Project Viva children (USA) 508 (longitudinal); 630 (cross-sectional) |
1999–2002 (longitudinal); 2007–2010 (cross-sectional) | Median (IQR) Longitudinal: PFOA (maternal) ; PFOS (maternal) ; PFNA (maternal) ; PFHxS (maternal) ; Cross-sectional: PFOA (child) ; PFOS (child) ; PFNA (child) ; PFHxS (child) |
2007–2010 (longitudinal, cross-sectional) | ALT (U/L) | Longitudinal: Adjusted for maternal education, prenatal smoking, gestational age at blood draw, sex, race/ethnicity, and age at ALT measurements. Cross-Sectional: Adjusted for maternal education, prenatal smoking, sex, race/ethnicity, and age. |
There was an inverse but not statistically significant inverse relationship between maternal PFOS, PFOA, and PFHxS exposure and ALT in girls. Higher childhood PFOA and PFOS concentrations were associated with lower ALT. Additional PFAS: Maternal EtFOSAA and MeFOSAA were not associated with liver enzymes. |
| Mundt et al.35 | Employees at a chemical manufacturer (USA) 592 |
1976–2003 | High, low, no exposure PFNA |
1989–2003 | ALT (U/L), GGT (U/L), AST (U/L) | Adjusted for age and BMI. | PFNA exposure was not associated with mean ALT, GGT, or AST. |
| Nian et al.41 | Adult residents of Shenyang, China 1,605 |
2015–2016 | Median (IQR) PFOA ; PFOS ; PFNA ; PFHxS |
Same as exposure | ALT (U/L),d GGT (U/L),d AST (U/L)d | Adjusted for age, sex, career, income, education, alcohol consumption, smoking, giblet/seafood consumption, physical activity, and BMI. | PFOA, PFOS, and PFNA were positively associated with ALT. There were also positive associations between PFOA and AST and GGT. Additional PFAS: PFDA was positively associated with ALT. |
| Olsen et al.36 | Male employees at two fluorochemical manufacturers (Antwerp, Belgium, and Decatur, Alabama) 178 (1995); 149 (1997) |
1995, 1997 | Mean PFOS (Antwerp, 1995); (Decatur, 1995); (Antwerp, 1997); (Decatur, 1997) |
Same as exposure | ALT (U/L), GGT (U/L), AST (U/L) | Adjusted for age, BMI, alcohol use, and smoking. | PFOS exposure was not associated with ALT, GGT, or AST. |
| Olsen et al.37 | Employees at two fluorochemical manufacturers (Antwerp, Belgium, and Decatur, Alabama) 263 (Decatur), 255 (Antwerp), 174 (longitudinal) |
1994–2000 (longitudinal); 2000 (cross-sectional) | Geometric mean (95% CI): PFOA (Antwerp); (Decatur); PFOS (Antwerp); (Decatur) |
2000 | ALT (U/L), GGT (U/L), AST (U/L) | Adjusted for age, BMI, alcohol use, smoking, and location. | Those in the highest quartile of PFOS exposure had higher mean ALT. PFOS was not associated with increased odds of elevated ALT or GGT. There were no associations between PFOS or PFOA and liver enzymes in the longitudinal analysis. |
| Olsen and Zobel38 | Male employees at three fluorochemical manufacturers (Antwerp, Belgium; Decatur, Alabama; Cottage Grove, Minnesota) 196 (Antwerp), 188 (Decatur), 122 (Cottage Grove) |
2000 | Mean (SD) PFOAd (Antwerp); (Decatur); (Cottage Grove) |
Same as exposure | ALT (U/L),d GGT (U/L),d AST (U/L)d | Adjusted for age, BMI, and alcohol use. | There were no significant linear associations between PFOA and ALT, GGT, or AST, or between PFOA and elevated liver enzymes. |
| Rantakokko et al.77 | Kupio Obesity Surgery Study adult participants (Finland) 161 |
2005–2010 | Median (5th, 95th percentile) PFOAe ; PFOSe ; PFNAe ; PFHxSe |
Same as exposure 12 months post (ALT) |
ALT (U/L),e steatosis, NASH, lobular inflammation, liver cell ballooning | Adjusted for age, fasting insulin, and weight change. | There were no significant associations between PFOA, PFOS, PFNA, or PFHxS and ALT at either baseline or 12 months later. PFOA, PFNA, and PFHxS were inversely associated with lobular inflammation at baseline. Additional PFAS: PFHxA was associated with ALT at 12 months. PFDA and sum of PFCA were associated with lobular inflammation at baseline. |
| Sakr et al.44 | Employees at the Washington Works polymer manufacturing site (USA) 205 |
1979–2007 | Mean (SD) PFOA |
1980–2007 | ALT (U/L), GGT (U/L), AST (U/L) | Adjusted for age, sex, BMI, and decade of hire. | There was a positive association between PFOA and AST. |
| Sakr et al.43 | Employees at Washington Works polymer manufacturing site (USA) 1,018 |
2004 | Mean (SD) PFOA |
Same as exposure | ALT (U/L),d GGT (U/L),d AST (U/L)d | Adjusted for age, sex, BMI, alcohol consumption, family history of heart attack, and use of lipid-lowering medications. | There was a positive association between PFOA and GGT. |
| Salihovic et al.42 | Older adults (Sweden) 1,002 |
2001–2014 | Median (IQR) PFOAd ; PFOSd ; PFNAd ; PFHxSd |
2006–2014 | ALT (ukat/L), GGT (ukat/L) | Adjusted for sex, LDL and HDL cholesterol, serum triglycerides, BMI, fasting glucose levels, statin use, and smoking. | There were positive associations between PFOA, PFOS, PFNA, and PFHxS and ALT. There was also a positive association between PFOA and GGT. Additional PFAS: PFHpA was positively associated with ALT, and PFUnDA was positively associated with GGT. |
| Sen et al.79 | Adults undergoing laparoscopic bariatric surgery without other risk factors for NAFLD (Sweden) 105 |
Not Specified | Median (min–max) PFOA ; Br-PFOS ; L-PFOS ; PFNA ; PFHxS |
Same as exposure | NAFLD), NASH), macrosteatosis), necroinflammatory activity), fibrosis | None | Positive associations were observed between PFAS (PFOA, PFOS, PFNA, and PFHxS) and macrosteatosis. PFOA and PFOS were positively associated with necroinflammation and NASH. PFNA was negatively associated with NASH. PFOS was positively associated with fibrosis. |
| Stratakis et al.78 | Children in the HELIX cohort (UK, France, Spain, Lithuania, Norway, Greece) 1,105 |
2005–2009 (prenatal) | PFAS mixture Median (IQR) PFOA ; PFOS ; PFNA ; PFHxS |
2014–2015 | Liver injury risk (ALT, AST, or GGT percentile) | Adjusted for cohort, maternal age, maternal education, maternal prepregnancy BMI, child ethnicity, child age, and child sex. | Higher prenatal PFAS exposure was associated with increased ALT, AST, and GGT, and with being at increased risk of liver injury. Additional PFAS: PFUnDA was included in the mixture analysis. |
| Yamaguchi et al.80 | Japanese residents with no occupational PFAS exposure 608 |
2008–2010 | Median (IQR) PFOAe ; PFOSe |
Same as exposure | ALT (IU/L), AST (IU/L), GGT (IU/L) | Adjusted for age, sex, BMI, regional block, and smoking, and alcohol intake. | PFOA and PFOS were significantly positively correlated with ALT and AST. There was also a significant positive correlation with GGT, but not after adjustment for alcohol intake. |
Note: —, not available; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CI, confidence interval; CK18, cytokeratin 18; eGFR, estimated glomerular filtration rate; EtFOSAA, -ethyl perfluorooctane sulfonamidoacetic acid; F, female; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; HELIX, Human Early Life Exposome; HOMR-IR, Homeostatic Model Assessment of Insulin Resistance; IQR, interquartile range; LDL, low-density lipoprotein; M, male; max, maximum; MeFOSAA, -methylperfluorooctane sulfonamidoacetic acid; min, minimum; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NHANES, National Health and Nutrition Examination Survey; PFAS, per- and polyfluorinated substances; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFUnDA, perfluoroundecanoic acid; SD, standard deviation; SE, standard error.
aSample sizes given here represent the maximum number of subjects available for at least one of the analyses of interest. Specific analyses may have slightly different sample sizes.
bBlood concentration of PFOA, PFOS, PFNA, PFHxS only. Exposure concentrations are reported where available.
cOutcomes listed here are limited to liver enzymes (ALT, AST, GGT), NAFLD, NASH, and liver histopathology. Studies may have reported additional outcomes.
dNatural log (ln) transformed.
e transformed.
Of the 86 eligible rodent studies, experiments investigating PFOA and PFOS were the most common. Other PFAS included PFNA, PFHxS, perfluorobutyrate (PFBA), perfluorobutanesulfonic acid (PFBS), perfluorodecanoic acid (PFDA), perfluorododecanoic acid (PFDoA), perfluoroundecanoic acid (PFUA), perfluorohexanoic acid (PFHxA), and hexafluoropropylene oxide dimer acid (GenX). Experimental animal study designs varied widely in choice of dosing scheme, duration of exposure, and exposure route (Table 2). Doses ranged from 0.02 to body weight and lasted for as little as 1 d to as long as 2 y. The most common route of exposure was oral gavage, although additional studies exposed animals to PFAS through drinking water, diet, inhalation, intraperitoneal injection, or dermal contact. Some study conditions were intended to mimic occupational or environmental human exposure levels (e.g., Blake et al.81), whereas others, such as Crebelli et al.82 or Lieder et al.,83 chose dose levels based on the no or lowest observed adverse effect level (NOAEL or LOAEL).
Table 2.
Animal studies on per- and polyfluorinated chemicals and biomarkers or outcomes of liver injury included for systematic review.
| Reference | Exposure | Dose | Species/strain/sex | Exposure route | Duration of exposure | Outcomes | Main findingsa |
|---|---|---|---|---|---|---|---|
| Bagley et al.29 | , | and | Rats; Sprague Dawley; male and female | Diet | 3 wk | Steatosis, ALT, AST, GGT, relative liver weight, liver histopathology | PFOS induces steatosis in male but not female rats, and it was not attenuated by choline supplementation. |
| Bijland et al.123 | , , | PFOS: ; PFHxS: ; PFBS: |
Mice; APOE*3-Leiden.CETP; male | Diet (Western diet) | 4–6 wk | Steatosis | PFOS and PFHxS, but not PFBS-induced steatosis. |
| Blake et al.81 | , GenX | PFOA: 1 and ; GenX: 2 and |
Mice; CD-1; female (dams) | Gavage | E1.5–11.5, E1.5–17.5 | ALT, AST, relative liver weight, liver histopathology | PFOA and GenX exposure resulted in increased liver weights and altered liver histopathology. AST was elevated in the highest PFOA and GenX exposure groups at E17.5. |
| Botelho et al.101 | PFOA | 0.002%, 0.005%, 0.01%, and 0.02% wt/wt | Mice; C57BL/6; male | Diet | 10 d | ALT, relative liver weight, liver histopathology | PFOA exposure increased liver weight in all dose groups. ALT was significantly elevated in the highest dose group. Histopathological alterations were observed after PFOA exposure. |
| Butenhoff et al.147 | 0.3, 1, 3, and | Rats; Sprague Dawley; male and female ( parents, pups) | Gavage (); () | 44 d ( males), 14 d prior to mating–PND 22 ( females); prenatal – PND22 () | ALT ( only); AST ( only), relative liver weight, liver histopathology | Relative liver weight was increased in males at the 3- and dose levels only. There was no observed effect of PFHxS on liver histopathology or enzymes. | |
| Butenhoff et al.93 | , | PFOA: ; PFBA, 28 d: 6, 30, and ; PFBA, 90 d: 1.2, 6, and |
Rats; Sprague Dawley; male and female | Gavage | 28 d (PFOA, PFBA); 90 d (PFBA) | ALT, AST, relative liver weight, liver histopathology | 28-d study: In males only, liver weight was increased in 30- and PFBA dose groups and after PFOA exposure. ALT was elevated in both sexes after PFOA exposure and returned to normal in males after 21 d of recovery. No change in ALT or AST was observed after PFBS exposure. Histopathological changes were observed in male rats in the PFBA and PFOA groups. 90-d study: In males only, liver weight was increased after PFBA exposure. There was no change in ALT or AST in either sex. Histological changes were observed in male rats in the dose group. |
| Butenhoff et al.95 | 0.5, 2, 5, and | Rats; Sprague Dawley; male and female | Diet | 2 y | ALT, AST, relative liver weight, liver histopathology | ALT was increased in the highest dose group at wk 14 and 53, in males only. No changes were observed in AST. PFOA induced histopathological changes and increased liver weight. | |
| Butenhoff et al.94 | 30 and | Rats; Sprague Dawley; male and female | Diet | 2 y | ALT, AST, relative liver weight, liver histopathology | ALT, AST, and liver weight were elevated in males exposed to PFOA. PFOA also induced histopathological changes, which were more severe in males than in females. | |
| Butenhoff et al.122 | POSF | 30, 100, and vol/vol | Rats; Sprague Dawley; male and female | Inhalation | 13 wk (6 h/d, 5 d/wk) | ALT, relative liver weight, liver histopathology | Liver weight increased following exposure. ALT was elevated in male rats but returned to normal after a 13-wk recovery period. |
| Chang et al.146 | 0.3, 1, and | Mice; CD-1; male and female ( parents, pups) | Gavage (); () | 42 d ( males); 14 d prior to mating–LD22 ( females); () | ALT, AST, GGT, relative liver weight, liver histopathology | There was no observed effect of PFHxS on liver histopathology or enzymes in either the or generations. Liver weight was increased in males and females at the 1- and dose levels, and in males and females at PND21 and -36 at the dose level. | |
| Chappell et al.158 | GenX | 0.1, 0.5, and | Mice; CD-1; male and female | Gavage | 90 d | Steatosis, liver histopathology | Histopathological changes, but no steatosis, were observed in the highest dose group. |
| Chengelis et al.155 | PFHxA | 10, 50, and | Rats; Sprague Dawley; male and female | Gavage | 90 d | ALT, AST, liver histopathology | ALT and liver weight were elevated in males at the dose level. Histopathological changes were also only observed in males at the highest dose. |
| Crebelli et al.82 | PFOA, PFBA | PFOA: 0.1, 1, and ; PFBA: |
Mice; C57BL/6; female | Drinking water | 5 wk | ALT, AST, liver histopathology | PFOA exposure at increased ALT and AST and resulted in histopathological changes. Mild histopathological changes were observed after PFBA exposure. |
| Cui et al.170 | PFOA | Mice; and C57BL/6J (WT); male | Gavage | 28 d | ALT, AST, relative liver weight, liver histopathology | PFOA exposure increased ALT, AST, and liver weights in both strains. | |
| Curran et al.113 | 2, 20, 50, and | Rats; Sprague Dawley; male | Diet | 28 d | ALT, AST, relative liver weight | ALT was increased in male rats at the highest dose level and AST in female rats at the highest dose level. Liver weights were increased following PFOS exposure in both sexes. | |
| Das et al.28 | , PFNA, | Mice; Sv/129 (WT) and ; male |
Gavage | 7 d | Steatosis, relative liver weight, liver histopathology | Steatosis was induced after exposure to any PFAS in WT mice and after exposure to PFNA and PFHxS in mice, as well as in control mice. Liver weight increased after all exposures in both strains. |
|
| Deng et al.124 | and | Mice; C57BL/6; male | Gavage | 1 d | Steatosis, ALT, AST, liver histopathology | Coexposure to PCB126 increased lipid droplets and inflammation in the liver. ALT and AST were also elevated in the coexposed group. | |
| Ding et al.151 | PFDoA | 0.02, 0.05, 0.2, and | Rats; Sprague Dawley; male | Gavage | 110 d | Steatosis, ALT, AST, relative liver weight | PFDoA induced steatosis and histopathological changes at doses . There were no changes to ALT or AST following exposure. Liver weight was increased at all dose levels. |
| Elcombe et al.114 | 20 and | Rats; Sprague Dawley; male | Diet | 1, 7, and 28 d | ALT, AST, relative liver weight, liver histopathology | Liver weight was increased in the highest dose group after 7 and 28 d. No changes were observed in ALT or AST. Histopathological alterations increased with duration of treatment. | |
| Elcombe et al.115 | 20 and | Rats; Sprague Dawley; male | Diet | 7 d | ALT, AST, relative liver weight, liver histopathology | Increases in relative liver weight reduced after 28 d of recovery. ALT and AST were not elevated. Alterations to liver histopathology did not completely resolve after 28, 56, or 84 d of recovery. | |
| Fang et al.145 | PFNA | 0.2, 1, and | Rats; Sprague Dawley; male | Gavage | 14 d | ALT, AST | PFNA exposure increased ALT and AST in the dose group. |
| Fang et al.144 | PFNA | 0.2, 1, and | Rats; Sprague Dawley; male (diabetic) | Gavage | 7 d | ALT, AST | PFNA exposure increased ALT levels in the 1- and dose groups. |
| Foreman et al.149 | PFBA | 35, 175, and | Mice; Sv/129 (WT), , and ; male | Gavage | 28 d | ALT, relative liver weight, liver histopathology | PFBA induced hepatocellular hypertrophy in WT and mice, and focal necrosis in WT. ALT was not elevated in any dose group or strain. |
| Guo et al.84 | 0.4, 2, and | Mice; BALB/c; male | Gavage | 28 d | ALT, AST, relative liver weight, liver histopathology | ALT and AST increased dose dependently. PFOA exposure increased liver weight and induced histopathological changes. | |
| Guo et al.90,171 | PFOA, | 0.4, 2, and | Mice; BALB/c; male | Gavage | 28 d | Steatosis, ALT, AST, relative liver weight, liver histopathology | GenX induced mild steatosis in the highest dose group, and PFOA induced steatosis in the 2- and dose groups. ALT and AST were elevated in the highest PFOA exposure group. Liver weight increased at all exposure levels. |
| Hamilton et al.125 | PFOS | , , , and | Mice; Cyp2b-null and hCYP2B6; male and female | Gavage | 21 d | Steatosis, ALT | ALT was increased after of PFOS exposure, but less so with coexposure to HFD. Coexposure to HFD exacerbated PFOS-induced steatosis, more so in hCYP2B6 mice. |
| Han et al.116 | 1 and | Rats; Sprague Dawley; male | Gavage | 28 d | ALT, AST, liver histopathology | ALT and AST levels increased following PFOS exposure. Changes in liver histopathology were observed. | |
| Han et al.117 | 1 and | Rats; Sprague Dawley; male | Gavage | 28 d | ALT, AST, relative liver weight, liver histopathology | ALT and AST levels increased following PFOS exposure. PFOS exposure induced histopathological changes and increases in liver weight. | |
| Huang et al.126 | PFOS | and | Mice; Kunming; male | Gavage | 21 d | Steatosis, ALT, AST, relative liver weight, liver histopathology | PFOS induced steatosis, increased ALT and AST levels, and increased liver weight. GSPE supplementation attenuated steatosis, enzyme changes, and liver weight increases in PFOS-exposed mice. |
| Huck et al.127 | PFOS |
and |
Mice; C57BL/6J; male | Diet | 6 wk | Steatosis, relative liver weight, liver histopathology | PFOS induced steatosis in mice fed standard diet. Steatosis did not develop in mice. A similar pattern was observed for liver weight |
| Hui et al.85 | PFOA | 1 and | Mice; BALB/c; male | Gavage | 7 d | ALT, liver histopathology | PFOA exposure resulted in increased ALT and altered liver histopathology. |
| Kato et al.152 | PFDoA | 0.1, 0.5, and | Rats; Sprague Dawley; male and female (dams and nonpregnant females) | Gavage | 42 d and 14 d prior to mating–LD5 (dams) | ALT, AST, GGT, relative liver weight, liver histopathology | No changes in ALT or GGT were observed. AST was significantly elevated in nonpregnant females 14 d after exposure ended. Liver weight increased following PFDoA exposure. Histopathological changes were observed in both sexes. |
| Kim et al.148 | PFDA | Rats; Sprague Dawley; female | Intraperitoneal injection | — | ALT, AST, GGT, relative liver weight | No changes in ALT, AST, or GGT were observed at either Wk 2 or Wk 8. Relative liver weight was increased at both 2 and 8 wk postexposure. | |
| Kim et al.118 | 1.25, 5, and | Rats; Sprague Dawley; male and female | Gavage | 28 d | ALT, AST, GGT, relative liver weight, liver histopathology | AST increased in the highest dose group in males only. Altered liver histopathology was also observed in males. Liver weight increased in the highest dose group for both sexes. | |
| Lai et al.128 | PFOS | Mice; CD-1; male and female | postnatally | E1–E18.5 | ALT, AST | Elevated ALT and AST was observed in PFOS-exposed offspring after a DEN challenge. | |
| Li et al.86 | PFOA | 1, 2.5, 5, and | Mice; Kunming; female | Prenatal | GD1–GD17 | ALT, AST, relative liver weight, liver histopathology | ALT, AST, and liver weight were increased on PND21 following prenatal PFOS exposure. Histopathological alterations were observed. |
| Li et al.102 | and | Mice; C57BL/6; male | Gavage | 2, 8, and 16 wk | Steatosis, ALT, liver histopathology | No change in ALT was observed for PFOA alone, and reversed ALT increases and steatosis induced by HFD. PFOA alone and increased liver weight. |
|
| Liang et al.141 | PFOS | 0.5 and | Mice; Kunming; female (dams) | Gavage | E0.5–E20.5 | Steatosis, liver histopathology | PFOS-induced histopathological changes and steatosis in dams at the highest dose level. |
| Lieder et al.83 | 60, 200, and | Rats; Sprague Dawley; male and female | Gavage | 90 d | ALT, AST, relative liver weight, liver histopathology | No changes in ALT, AST, relative liver weight, or liver histopathology were observed after PFBS exposure. | |
| Liu et al.103 | PFOA | and | Mice; Kunming; male | Gavage | 14 d | ALT, AST, liver histopathology | PFOA increased ALT and AST levels and altered liver histopathology, but this was attenuated with coexposure to GSPE. |
| Luo et al.153 | PFDA | Mice; and 129/Sv (WT) | Intraperitoneal injection | One injection | ALT, AST, relative liver weight, liver histopathology | In WT mice, ALT and AST were both elevated 5 d after PFDA exposure. ALT returned to baseline levels 10 d after exposure. There were no changes in ALT or AST in mice, and no changes to liver histopathology in either strain after 5 d. Liver weight increased after PFDA exposure in both strains. | |
| Lv et al.119 | PFOS | 0.5 and | Rats; Wistar; male and female | Prenatal and lactational | GD0-PND21 | Steatosis, liver histopathology | Histopathological changes and steatosis were observed in pups from the highest dose group 19 wk after weaning. |
| Lv et al.129 | PFOS | and | Mice; strain not reported; male | Gavage | 3 wk | ALT, AST, relative liver weight, liver histopathology | Nar coexposure attenuated changes in ALT, AST, liver weight, and histopathology induced by PFOS. |
| Marques et al.139 | 0.0003% wt/wt, , and | Mice; C57BL/6N; male | Diet | 10 wk | Steatosis, relative liver weight, liver histopathology | PFOS exposure induced steatosis in HFD and H-SD groups. PFOS also increased liver weight in all diet groups. | |
| Marques et al.130 | PFOA, , , PFAS mixture | and | Mice; CD-1; female (dams) and male and female (pups) | Gavage (dams); (pups) | Gestation (GD1–birth) and lactation (birth–PND21) | ALT, relative liver weight | ALT was elevated only in dams fed a standard diet and PFOS. PFOA and PFAS mixture exposure increased liver weights in both diet groups for dams. PFAS exposure generally increased liver weight in pups. |
| Martin et al.99 | , | PFOA: ; PFOS: |
Rats; Sprague Dawley; male | Gavage | 1, 3, and 5 d | Steatosis, ALT, liver histopathology | Steatosis and increased liver weight were observed in both treatment groups after 3 and 5 d. Additional histopathological alterations were observed, more frequently after longer exposures. No changes in ALT were observed. |
| Minata et al.96 | 12.5, 25, and | Mice; 129S4/SvlmJ (WT) and ; male | Gavage | 4 wk | Steatosis, ALT, AST, liver histopathology | Dose-dependent increases in ALT and AST were observed following PFOA exposure. Steatosis was present to a greater extent in all mice than in WT mice. Liver weights increased in all exposed mice. Histopathological evaluation suggests that the mode of toxicity is different in and WT mice. | |
| Nakagawa et al.97 | 1.0 and | Mice; Sv/129 (WT), , and ; male | Gavage | 6 wk | Steatosis, ALT, relative liver weight, liver histopathology | Histopathological alterations differed across the three strains. Steatosis was observed in and mice. ALT was elevated in all mice at the highest dose. Liver weight was increased in all exposed mice. | |
| Owumi et al.112 | PFOA | , (), and () | Rats; Wistar; male | Gavage | 28 d | ALT, AST, GGT, relative liver weight, liver histopathology | PFOA exposure increased ALT, AST, and GGT, but not when coexposed to NAC. NAC coexposure mitigated histopathological alterations induced by PFOA. There were no changes in relative liver weight. |
| Pfohl et al.131 | PFOS, PFNA | and | Mice; C57BL/6J; male | Diet | 12 wk | Steatosis, relative liver weight | Steatosis was present in all treatment groups, but coexposure to HFD mitigated its development. Liver weight was increased in all treatment groups. |
| Pouwer et al.87 | 10, 300, and | Mice; APOE*3-Leiden.CETP; male | Diet | 4 and 6 wk | Steatosis, ALT, liver histopathology | ALT and liver weight w ere increased in the highest dose group. Some steatosis was observed in the 10- and dose groups. | |
| Qazi et al.104 | PFOA, | PFOA: 0.002% wt/wt; PFOS: 0.005% wt/wt |
Mice; C57BL/6; male | Diet | 10 d | ALT, AST, liver histopathology | No changes in ALT or AST were observed for either exposure. Both PFAS-induced histopathological changes. |
| Qazi et al.132 | 10 d: 0.004% wt/wt and ; 28 d: 0.0001% wt/wt and |
Mice; C57BL/6; male | Diet | 10 and 28 d | ALT, AST, relative liver weight, liver histopathology | Coexposure of PFOS and Con A increased ALT and AST levels. Histopathological alterations were observed and liver weight increased with PFOS exposure in all study conditions. | |
| Qazi et al.105 | PFOA | 10 d: ; 28 d: 0.00005% wt/wt and |
Mice; C57BL/6; male | Diet | 10 and 28 d | ALT, AST, relative liver weight, liver histopathology | Coexposure of PFOS and Con A increased ALT and AST levels in the 10-d study. Substantial histopathological alterations were only observed with PFOS exposure in the 10-d study. Liver weight increased in both exposure groups in the 10-d study, and only in the PFOA group in the 28-d study. |
| Qin et al.133 | PFOS | and | Mice; C57BL/6J; male | Gavage | 4 wk | Steatosis, ALT, AST, relative liver weight | PFOS exposure exacerbated steatosis in HFD-fed mice. ALT, AST, and liver weights were increased in both PFOA-exposed groups. |
| Quist et al.106 | Prenatal: 0.01, 0.1, 0.3, and ; Postnatal: , , , and |
Mice; CD-1; female | Prenatal | GD1–GD17 | ALT, AST, relative liver weight, liver histopathology | PFOA did not alter ALT or AST. Histopathological alterations were observed were observed on PND21 and became more severe by PND91 in a dose-dependent fashion. Liver weights were increased at PND21 but not at PND91. | |
| Rigden et al.92 | PFOA | 10, 33, and | Rats; Sprague Dawley; male | Gavage | 3 d | ALT, AST | Elevated ALT was observed in the dose group only 4 d after the end of treatment, and no changes in AST were observed. |
| Roth et al.134 | PFAS mixture (PFOS, PFOA, PFNA, PFHxS, GenX) | Mice; C57BL/6J; male and female | Drinking water | 12 wk | ALT, relative liver weight, liver histopathology | ALT and liver weight increased following PFAS exposure in both males and females. PFAS exposure also resulted in alterations to liver histopathology, with more inflammation observed in females. | |
| Schlezinger et al.98 | PFOA | Mice; WT, , and ; male and female | Drinking water | 6 wk | Steatosis, relative liver weight, liver histopathology | Steatosis was present after treatment with PFOA in mice, mice, and male WT mice. Liver weights increased in all genotypes. | |
| Seacat et al.120 | 0.5, 2.0, 5.0 and | Rats; Sprague Dawley; male and female | Diet | 4 and 14 wk | ALT, AST, GGT, relative liver weight, liver histopathology | ALT was increased in females at 4 wk and males at 14 wk in the highest dose group. Liver weight was increased in both sexes at 14 wk. Histopathological alterations were observed in 5- and exposed males and exposed females. | |
| Shao et al.172 | PFOA | Mice; CD-1; male (pups) | Prenatal | GD13-delivery | ALT, AST, liver histopathology | ALT and AST were elevated in mice exposed prenatally to PFOA. PFOA induced hepatic inflammation and histopathological alterations. | |
| Shi et al.173 | PFOA | and groups | Mice; C57BL/6J; male | Gavage | 1D | ALT, AST, and GGT | ALT, AST, and GGT were increased after PFOA exposure. These increases were mitigated with LAB exposure. PFOA also increased liver weight, which was not reduced with LAB exposure. |
| Son et al.107 | 2, 10, 50, and | Mice; CD-1; male | Drinking water | 21 d | ALT, AST, relative liver weight, liver histopathology | ALT, AST, and liver weight increased dose dependently. Altered liver histopathology was present after PFOA exposure. | |
| Su et al.135 | PFOS | , VC, and VC | Mice; CD-1; male | Gavage | 21 d | Steatosis, ALT, AST, liver histopathology | VC supplementation ameliorated elevations in ALT, AST, and steatosis induced by PFOS. VC supplementation also improved histopathological alterations following PFOS exposure. |
| Takahashi et al.156 | PFUA | 0.1, .03, and | Rats; Sprague Dawley; male and female (dams) | Gavage | 42 d and 14 d prior to mating–LD4 (dams) | ALT, AST, GGT, relative liver weight, liver histopathology | ALT was increased in males at the dose level. Liver weights were elevated in males at dose 0.3 and and in females at . PFUA induced histopathological changes at doses in both sexes. |
| Tan et al.108 | PFOA | and | Mice; C57BL/6N; male | Diet | 3 wk | ALT, AST, relative liver weight, liver histopathology | PFOA exposure increased ALT and liver weight. Coexposure to HFD exacerbated this and induced more severe histopathological changes. |
| Van Esterik et al.100 | 3, 10, 30, 100, 300, 1,000, and | Mice; C57BL/6JxFVB; male | 14 d prior to mating–LD21 | Steatosis, relative liver weight, liver histopathology | PFOA-exposed offspring fed a HFD after weaning had increased liver weight, and more severe histopathological alterations. Steatosis was observed in the highest dose group. | ||
| Wan et al.136 | PFOS | 1, 5 and | Mice; CD-1; male | Gavage | 3, 7, 14, and 21 d | Steatosis, liver histopathology | PFOS-induced steatosis in a dose- and time-dependent fashion. |
| Wan et al.136 | PFOS | 1 and | Rats; Sprague Dawley; male | Gavage | 28 d | ALT, AST, liver histopathology | PFOS exposure increased ALT and AST levels and caused histopathological alterations. |
| Wang et al.142 | PFNA | 0.2, 1, and | Mice; BALB/c; male | Gavage | 14 d | ALT, AST, relative liver weight | ALT and AST were elevated in the group. Liver weight increased in all dose groups. |
| Wang et al.157 | GenX | Mice; CD-1; male | Gavage | 28 d | ALT, AST, relative liver weight, liver histopathology | GenX exposure resulted in increased liver weight, mild steatosis, and histopathological alterations. | |
| Wang et al.137 | PFOS | 0.3, 3, and | Mice; C57BL/6J; male | Gavage | 16 d | ALT, AST, GGT, relative liver weight, liver histopathology | PFOS exposure increased ALT levels at all doses and GGT at the highest dose. Histopathology was altered and liver weights increased in all exposure groups. |
| Wang et al.154 | PFDA | , , and | Mice; CD-1; male | Drinking water | 12 d | Steatosis, ALT, AST, liver histopathology | PFDA induced steatosis. GTPs and EGCG were protective against increases in ALT and AST and against histopathological alterations. |
| Wang et al.109 | PFOA | 14 d: 3 and ; 30 d: 2.5, 5, and |
Mice; C57BL/6J; male | Gavage | 14 and 30 d | ALT, AST, GGT, relative liver weight, liver histopathology | PFOA exposure increased ALT levels, altered liver histopathology and increased liver weight. |
| Weatherly et al.150 | PFBA | 3.75%, 7.5%, and 15% vol/vol | Mice; ; male and female | Dermal | 28 d | ALT, relative liver weight, liver histopathology | There were no observed increases in ALT. Relative liver weight increased after exposure to PFBA. |
| Wu et al.174 | PFOA | Mice; Kunming; male | Gavage | 1 d | ALT and AST | ALT and AST levels were not significantly increased following exposure. | |
| Wu et al.91 | PFOA | 1 and | Mice; Kunming; female | Gavage | 21 d | ALT, AST, relative liver weight, liver histopathology | PFOA exposure increased ALT, AST, and relative liver weight in the highest dose group only. Liver histopathology was altered in both dose groups. |
| Xing et al.138 | PFOS | 14 d: 30, 40, 50, 60, and ; 30 d : 2.5, 5, and |
Mice; C57BL/6J; male | Gavage | 14 and 30 d |
ALT, AST GGT, liver histopathology |
PFOS exposure resulted in histopathological alteration and increased ALT and AST in a dose-dependent fashion. |
| Yahia et al.88 | PFOA | 1, 5, and | Mice; CD-1; female (dams) | Gavage | GD0–GD17/18 | ALT, AST, GGT, relative liver weight, liver histopathology | Histopathological alterations and elevated ALT, AST, and GGT were observed in the highest dose group. PFOA exposure increased liver weight in a dose-dependent fashion. |
| Yan et al.89 | PFOA, PFOS | PFOA: 0.08, 0.31, 1.25, 5, and ; PFOS: 1.25 and |
Mice; BALB/c; male | Gavage | 28 d | ALT, AST, relative liver weight | ALT and AST were increased at the highest PFOA and PFOS exposure group. Liver weight increased in all but the lowest dose of PFOA. |
| Yan et al.175 | PFOA | 4-PBA and 4-PBA | Mice; BALB/c; male | Gavage | 28 d | ALT, AST, relative liver weight | ALT and liver weight increased in all PFOA-exposed groups. AST increased in the PFOA-only treatment group. |
| Yang et al.110 | PFOA | 2.5, 5, and | Mice; Kunming; male | Gavage | 14 d | ALT, AST, relative liver weight, liver histopathology | ALT levels increased in a dose-dependent manner. AST was increased at the two highest dose levels. Histopathological alterations and liver weight increases were seen in all dose groups, and were more severe at the highest dose. |
| Zhang et al.140 | 0.003% wt/wt, 0.006% wt/wt , 0.012% wt/wt , and |
Mice; C57BL/6; male | Diet | 21 d (mMCD) and 6 wk (CS) | Steatosis, ALT, relative liver weight, liver histopathology | PFOS increased ALT and liver weight, and induced histopathological changes and steatosis. Toxicity was exacerbated in the group and attenuated with CS coexposure. | |
| Zhang et al.143 | PFNA | Mice; C57BL/6 (WT), , and CAR-null; male | Intraperitoneal injection | One injection | ALT, relative liver weight, liver histopathology | PFNA increased liver weight in all three strains after 14 d. After 1 wk, ALT was elevated in the WT and CAR-null mice. Alterations in histopathology were observed after 14 and 90 d. | |
| Zou et al.111 | PFOA | and | Mice; Kunming; male | Gavage | 14 d | ALT, AST, liver histopathology | Coexposure to Que decreased PFOA induced ALT and AST levels and ameliorated histopathological changes. |
Notes: 4-PBA, 4-phenylbutyrate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAR, constitutive antigen receptor; Con A, Concanavalin A; CS, choline supplementation; DEN, diethylnitrosamine; E, embryonic day; EGCG, epi-gallocatechin-3-gallate; GD, gestation day; GenX, hexafluoropropylene dimer acid; GGT, gamma-glutamyl transferase; GSPE, grape seed proanthocyanidin extract; GTP, green tea polyphenol; HFD, high-fat diet; hPPAR, humanized peroxisome proliferator-activated receptor; H-SD, high-fat diet to standard diet; , potassium ion; LAB, lactic acid bacteria; LD, lactation day; LFD, low-fat diet; mMCD, marginal methionine/choline-deficient diet; NAC, -acetylcysteine; Nar, naringin; , ammonium ion; PCB, polychlorinated biphenyl; PFAS, per- and polyfluorinated substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonate; PFDA perfluorodecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUA, perfluoroundecanoic acid; PHDoA, perfluorododecanoic acid; PND, postnatal day; POSF, perfluorooctanesulfonyl fluoride; PPAR, peroxisome proliferator-activated receptor; Que, quercetin; SD, standard diet; VC, vitamin C; WT, wild type.
Findings presented here are limited to those related to the markers of liver injury investigated in this review.
Results on OHAT risk of bias ratings are provided in Tables S1 and S2. No studies were excluded based on risk of bias. For human studies, risk of bias was often “definitely low” or “probably low” for all domains, but some were determined to have higher risk of bias because they did not adequately account for confounders related to NAFLD or NASH (e.g., alcohol use, body mass index, smoking). Most animal studies were determined to have “probably high” risk of bias for domains relating to blinding of researchers or concealment of experimental assignments, because most studies were either not blinded or did not report it. Animal studies generally received positive ratings on all other domains.
Exposure to PFOA
Human studies.
Eight cross-sectional studies assessing the relationship between PFOA and ALT in adults and adolescents ( of age) were included in the weighted -score calculation.38,40,41,43,67,70,72,75 A weighted -score of 6.20 () indicated a positive relationship between PFOA and ALT (Table 3). This positive relationship remained across sensitivity analyses (Table S3). A weighted -score for PFOA and ALT was also calculated for the three available longitudinal studies and was statistically significant ( 5.12; ; Table 3).39,42,44 Only two studies examined the effect of PFOA exposure on ALT levels in children of age, reporting no statistically significant associations.74,76 In adults, there was a positive relationship between PFOA exposure and GGT ( 4.13, )38,40,41,43,67,69,70,72,75 (Table S4), and this remained statistically significant after removing the largest study and after restricting the calculation to only NHANES participants (Table S3). There was no statistically significant relationship between PFOA and AST ( 1.95, ) in adults (Table S4).38,40,41,43,67,69,72 Two longitudinal analyses did not find any associations between PFOA and other liver enzymes.37,39 One, Salihovic et al.,42 did find a positive association between PFOA and GGT.
Table 3.
Strip plots for the -scores of the analyses of PFAS on ALT.
| Reference | Population | Age (y) | Sex | Weight | Exposure | PFAS Blood Conc. | -Score (-value) | |
|---|---|---|---|---|---|---|---|---|
| PFOA (cross-sectional studies) | ||||||||
| Sakr et al.43,a | GHS | Overall | All | 1,024 | PFOA | b | 1.53 (0.13) | |
| Olsen and Zobel38,a | Plant employees | 21–67 | Male | All | 506 | PFOA | b | (0.56) |
| Emmett et al.69 | Little Hocking, Ohio | 2–90 | Overall | All | 371 | PFOA | c | 0.45 (0.67) |
| Gallo et al.70,a | C8HP | Overall | All | 46,452 | PFOA | c | 12.32 () | |
| Darrow et al.39 | C8HP | Overall | All | 28,047 | PFOA | NS | 6.72 () | |
| Darrow et al.39 | C8HP | Male | All | 12,364 | PFOA | c | 4.63 () | |
| Darrow et al.39 | C8HP | Female | All | 15,683 | PFOA | c | 3.92 () | |
| Nian et al.41,a | I C8HP | 22–95 | Overall | All | 1,605 | PFOA | c | 4.23 () |
| Lin et al.75,a | NHANES 1999–2003 | Overall | All | 2,197 | PFOA | b | 2.99 (0.003) | |
| Lin et al.75 | NHANES 1999–2003 | Male | All | 1,063 | PFOA | b | 1.85 (0.064) | |
| Lin et al.75 | NHANES 1999–2003 | Female | All | 1,134 | PFOA | b | 1.65 (0.098) | |
| Gleason et al.40,a | NHANES 2007–2010 | Overall | All | 4,333 | PFOA | d | 3.10 (0.002) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Non-obese | 1,082 | PFOA | d | 0.22 (0.84) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Obese | 1,801 | PFOA | d | 3.17 (0.002) | |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Male | All | 354 | PFOA | d | (0.022) |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Female | All | 305 | PFOA | d | 2.35 (0.019) |
| Mora et al.76 | Project Viva | 6–11 | Overall | All | 630 | PFOA | c | (0.74) |
| Mora et al.76 | Project Viva | 6–11 | Male | All | 332 | PFOA | c | (0.24) |
| Mora et al.76 | Project Viva | 6–11 | Female | All | 298 | PFOA | c | (0.050) |
| Khalil et al.74 | DCH | 8–12 | Overall | Obese | 48 | PFOA | c | 1.62 (0.11) |
| Weighted | 6.20 () | |||||||
| PFOA (longitudinal studies) | ||||||||
| Sakr et al.44,a | GHS | Overall | All | 205 | PFOA | b | 1.06 (0.29) | |
| Darrow et al.39,a | C8HP | Overall | All | 28,047 | PFOA | NS | 5.88 () | |
| Darrow et al.39 | C8HP | Male | All | 12,364 | PFOA | c | 4.57 () | |
| Darrow et al.39 | C8HP | Female | All | 15,683 | PFOA | c | 3.92 () | |
| Salihovic et al.42,a | Swedish | 70 | Overall | All | 1,002 | PFOA | c | 5.20 () |
| Mora et al.76 | Project Viva | 6–11 | Overall | All | 508 | PFOA | c | (0.19) |
| Mora et al.76 | Project Viva | 6–11 | Male | All | 273 | PFOA | c | (0.38) |
| Mora et al.76 | Project Viva | 6–11 | Female | All | 235 | PFOA | c | (0.19) |
| Weighted | 5.12 () | |||||||
| PFOS (cross-sectional studies) | ||||||||
| Gallo et al.70,a | C8HP | Overall | All | 46,452 | PFOS | c | 6.53 () | |
| Nian et al.41,a | I C8HP | 22–95 | Overall | All | 1,605 | PFOS | c | 2.31 (0.021) |
| Lin et al.75,a | NHANES 1999–2003 | Overall | All | 2,216 | PFOS | b | 1.90 (0.057) | |
| Gleason et al.40,a | NHANES 2007–2010 | Overall | All | 4,333 | PFOS | c | 1.19 (0.24) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Non-obese | 1,082 | PFOS | d | (0.31) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Obese | 1,801 | PFOS | d | 1.26 (0.21) | |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Male | All | 354 | PFOS | d | 0.21 (0.85) |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Female | All | 305 | PFOS | d | 1.86 (0.063) |
| Mora et al.76 | Project Viva | 6–11 | Overall | All | 630 | PFOS | c | (0.29) |
| Mora et al.76 | Project Viva | 6–11 | Male | All | 332 | PFOS | c | (0.50) |
| Mora et al.76 | Project Viva | 6–11 | Female | All | 298 | PFOS | c | (0.23) |
| Khalil et al.74 | DCH | 8–12 | Overall | Obese | 48 | PFOS | c | 0.16 (0.88) |
| Weighted | 3.55 () | |||||||
| PFNA (cross-sectional studies) | ||||||||
| Nian et al.41,a | I C8HP | 22–95 | Overall | All | 1,605 | PFNA | c | 3.86 () |
| Lin et al.75,a | NHANES 1999–2003 | Overall | All | 2,216 | PFNA | b | 1.55 (0.12) | |
| Gleason et al.40,a | NHANES 2007–2010 | Overall | All | 4,333 | PFNA | d | 3.51 () | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Non-obese | 1,082 | PFNA | d | 0.47 (0.65) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Obese | 1,801 | PFNA | d | 3.53 () | |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Male | All | 354 | PFNA | d | (0.013) |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Female | All | 305 | PFNA | d | 3.02 (0.003) |
| Mora et al.76 | Project Viva | 6–11 | Overall | All | 630 | PFNA | c | (0.003) |
| Mora et al.76 | Project Viva | 6–11 | Male | All | 332 | PFNA | c | () |
| Mora et al.76 | Project Viva | 6–11 | Female | All | 298 | PFNA | c | (0.19) |
| Khalil et al.74 | DCH | 8–12 | Overall | Obese | 48 | PFNA | c | (0.86) |
| Weighted | 2.27 (0.023) | |||||||
| PFHxS (cross-sectional studies) | ||||||||
| Nian et al.41,a | I C8HP | 22–95 | Overall | All | 1,605 | PFHxS | c | 0.39 (0.71) |
| Lin et al.75,a | NHANES 1999–2003 | Overall | All | 2,216 | PFHxS | b | 0.40 (0.71) | |
| Gleason et al.40,a | NHANES 2007–2010 | Overall | All | 4,333 | PFHxS | d | 2.61 (0.009) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Non-obese | 1,082 | PFHxS | d | 0.26 (0.81) | |
| Jain and Ducatman72,a | NHANES 2011–2014 | Overall | Obese | 1,801 | PFHxS | d | 3.33 () | |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Male | All | 354 | PFHxS | d | 0.49 (0.64) |
| Attanasio67,a | NHANES 2013–2016 | 12–19 | Female | All | 305 | PFHxS | d | 2.35 (0.019) |
| Mora et al.76 | Project Viva | 6–11 | Overall | All | 630 | PFHxS | c | 0.00 (1.0) |
| Mora et al.76 | Project Viva | 6–11 | Male | All | 332 | PFHxS | c | (0.52) |
| Mora et al.76 | Project Viva | 6–11 | Female | All | 298 | PFHxS | c | 0.78 (0.44) |
| Khalil et al.74 | DCH | 8–12 | Overall | Obese | 48 | PFHxS | c | 0.08 (0.94) |
| Weighted | 1.42 (0.15) | |||||||
Notes: Both overall and sex-specific results are presented where available. ALT, alanine aminotransferase; C8HP, C8 Health Project; DCH, Dayton Children’s Hospital; GHS, General Health Survey; I C8HP, Isomers of C8 Health Project; NHANES, National Health and Nutrition Examination Survey; NS, not specified; PFAS, per- and polyfluorinated substances; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid.
aThe weighted -score calculation was performed for those of age, using the larger of overlapping cohorts.
bMean.
cMedian.
dGeometric mean.
Rodent studies.
Thirty-two studies assessed exposure to PFOA in mice and 5 studies assessed exposure to PFOA in rats (Table 2). Overall, exposure to PFOA in rodents was associated with elevated mean serum ALT (Figure 2). Twenty-one mouse studies observed a statistically significant difference in mean serum ALT in treatment groups relative to unexposed controls. Of these, 10 studies observed a statistically significant positive association at higher doses and no effect at lower doses, suggesting a dose-dependent relationship.81,82,84–91 However, these results did not reveal an obvious threshold for lowest dose of observed effect. Of the 4 studies in Sprague Dawley rats, 3 found a statistically significant relationship between PFOA exposure and ALT.92–94 Most studies included only males, and the few studies including both males and females observed no consistent differences by sex in effects on ALT levels.94,95 Studies also reported elevated AST or liver weight in PFOA-exposed rodents (Figures S1 and S2). PFOA exposure in adult mice and rats frequently induced steatosis.28,84,87,90,96–99 Only 1 study investigated prenatal PFOA exposure and development of steatosis in adulthood and no association was found.100 Other reported histopathological alterations included hepatocellular hypertrophy and necrosis in both mice28,81,82,84,86–88,96,97,101–111 and rats.93,94,99,112
Figure 2.
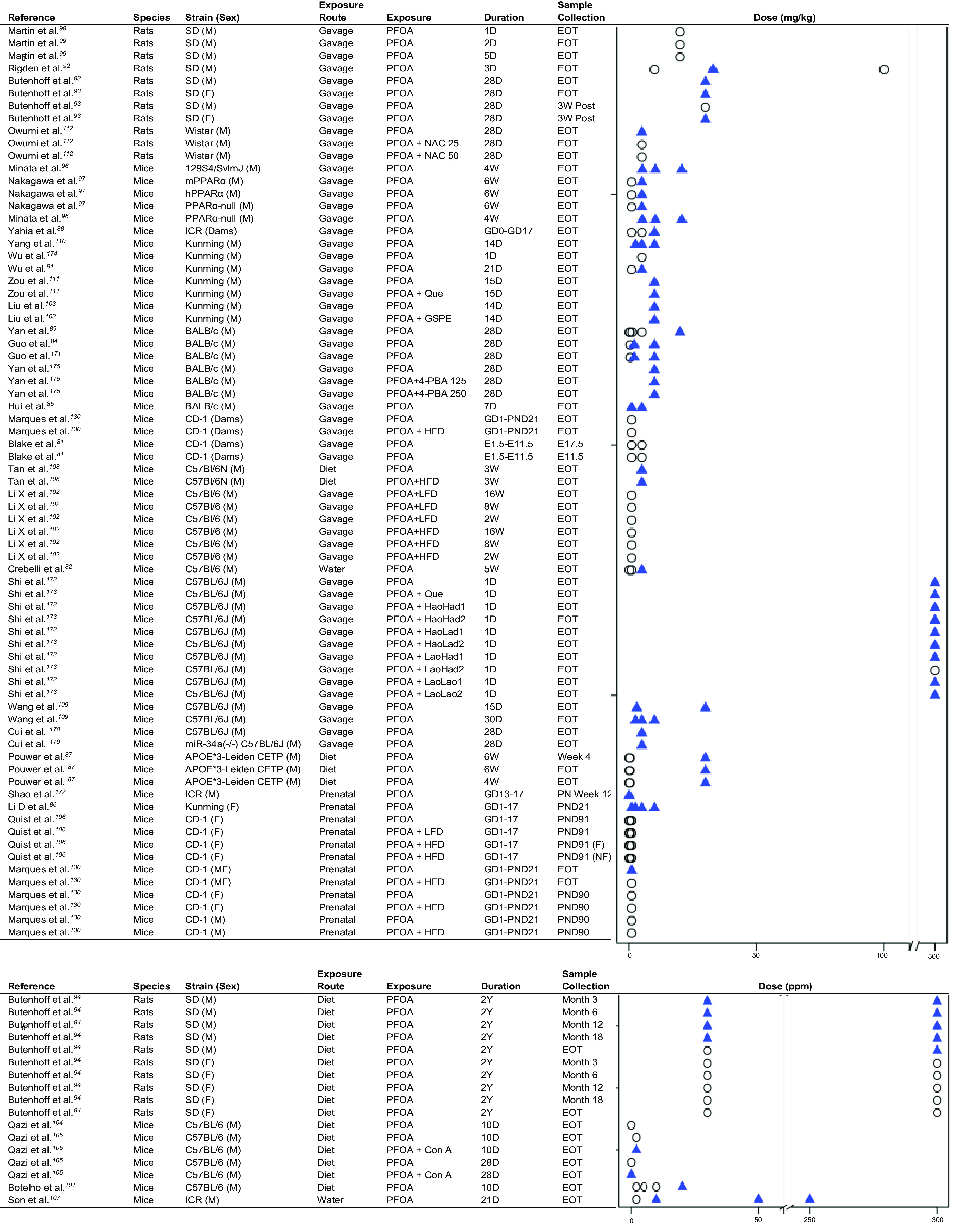
Strip plots for PFOA and ALT in animal studies. Triangles indicate a significant increase in ALT relative to control. Circles indicate no significant change in ALT relative to control. Additional exposures in Shi et al.173 refer to lactic acid bacterial strains. An accessible version of this figure is available in Table S5. Note: 4-PBA, 4-phenylbutyric acid; ALT, alanine aminotransferase; Con A, concanavalin A; D, day; E, embryonic day; EOT, end of treatment; F, female; GD, gestational day; GSPE, grape seed proanthocyanidin extract; HFD, high-fat diet; hPPAR, humanized peroxisome proliferator-activated receptor; LFD, low-fat diet; M, male; mPPAR, mouse peroxisome proliferator-activated receptor; NAC, -acetylcysteine; PFOA, perfluorooctanoic acid; PND, postnatal day; Que, quecertin; SD, Sprague Dawley; W, week; Y, year.
Exposure to PFOS
Human studies.
Six cross-sectional studies assessing the relationship between PFOS and ALT in adults and adolescents ( of age) were included in the weighted -score calculation.40,41,67,70,72,75 A weighted -score of 3.55 () suggested a positive association between PFOS and ALT (Table 3). After including two studies in children ( of age),74,76 the association remained statistically significant ( 3.27, ); however, the association was no longer statistically significant in sensitivity analyses that removed the largest study70 ( 1.11, ) or that restricted the analysis to only those studies using NHANES data40,67,72,75 ( 0.90, ) (Table S3). No statistically significant associations between PFOS and ALT were reported in children in either cross-sectional74,76 or longitudinal76 analyses. Weighted -scores did not suggest a relationship between PFOS and GGT when including all eligible studies ( 1.13, )40,41,67,70,72,75 or in sensitivity analyses (Table S3) or between PFOS and AST ( 0.37, ) in adults (Table S4).40,41,67,72 One longitudinal analysis reported a positive association with ALT,42 but none found any relationship between PFOS and other liver enzymes.37,42
Rodent studies.
Among rodent studies, 13 studies assessed exposure to PFOS in rats29,95,99,113–122 and 19 assessed PFOS exposure in mice28,89,104,123–138 (Table 2). PFOS exposure consistently increased serum ALT in mice (Figure 3). This effect was also observed in rats, although several studies did not report any effect of PFOS on ALT levels.99,114,118 Many mouse studies also observed increases in AST after PFOS exposure (Figure S3), and both mouse and rat studies reported increases in liver weight following PFOS exposure (Figure S4). PFOS exposure was also shown to induce steatosis in mice and rats.99,118,123,125–127,131,133,139–141 Prenatal exposure also resulted in steatosis in Wistar rats.119 Hepatocellular hypertrophy and necrosis were also consistently observed after PFOS exposure in both mice104,129,132,135,137,138 and rats.29,95,99,113–118,120–122
Figure 3.
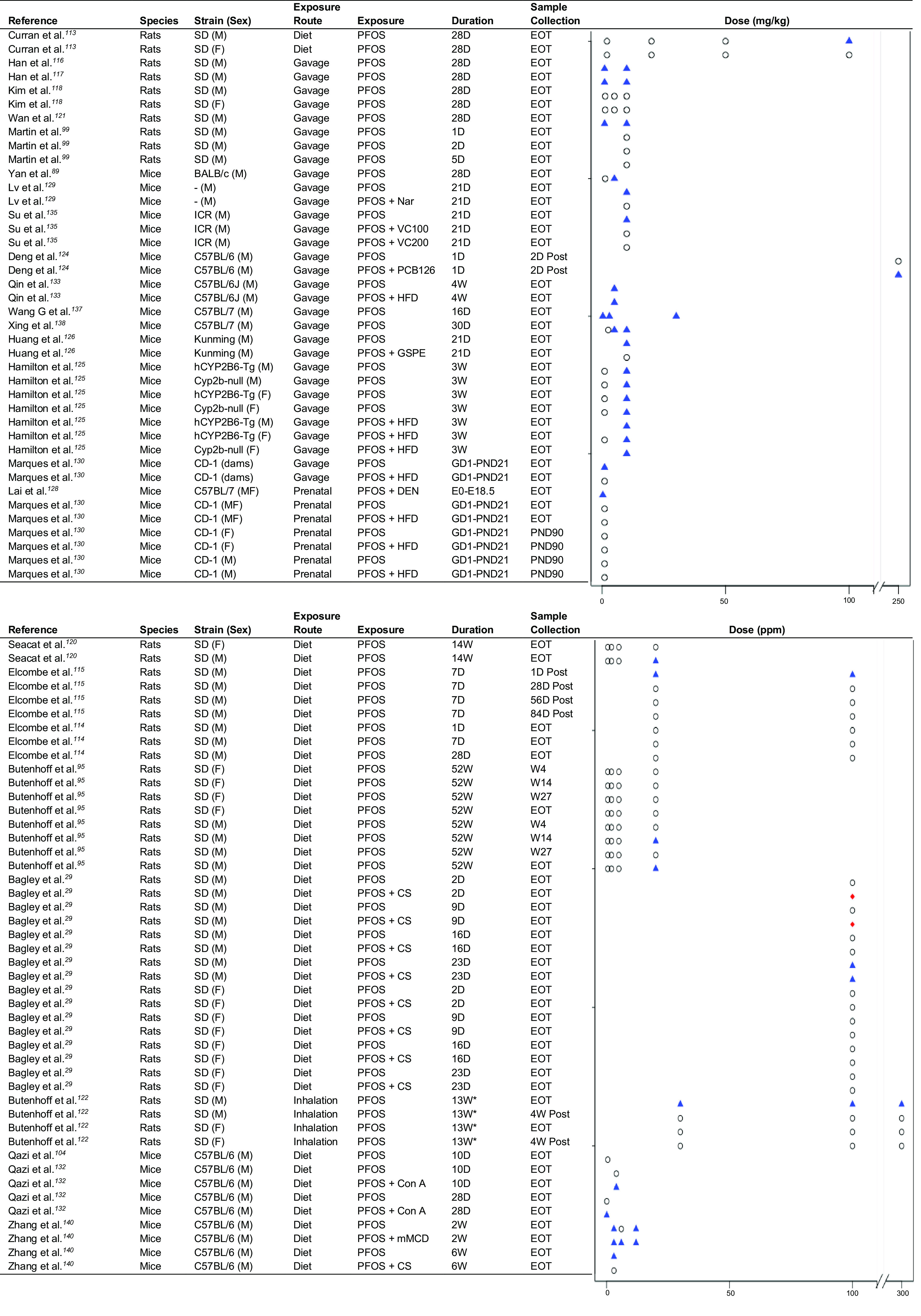
Strip plots for PFOS and ALT in rodent studies. Triangles indicate a significant increase in ALT relative to control. Diamonds indicate a significant decrease in ALT relative to control. Circles indicate no significant change in ALT relative to control. Plots are ordered by species and strain. In the study by Butenhoff et al.122, atmospheric exposure occurred for 5 h/d, 5 d/wk. An accessible version of this figure is available in Table S6. Note: ALT, alanine aminotransferase; CS, choline supplementation; Con A, concanavalin A; D, day; DEN, diethylnitrosamine; EOT, end of treatment; F, female; GD, gestational day; GSPE, grape seed proanthocyanidin extract; HFD, high-fat diet; M, male; mMCD, marginal methionine/choline-deficient diet; Nar, naringin; PCB, polychlorinated biphenyl; PFOS, perfluorooctanesulfonic acid; PND, postnatal day; SD, Sprague Dawley; VC, vitamin C; W, week.
Exposure to PFNA
Human studies.
Five cross-sectional studies assessing the relationship between PFNA and ALT in adults and adolescents were included in the weighted -score calculation.40,41,67,72,75 A weighted -score of 2.27 () suggested a positive relationship between PFNA and ALT (Table 3). Owing to the limited number of available studies, no sensitivity analyses were performed for this weighted -score. Mora et al.76 reported a statistically significant negative association in cross-sectional analyses of PFNA and ALT in boys only, although no statistically significant associations were found for children overall in either cross-sectional or longitudinal analyses by either Mora et al.76 or Khalil et al.74 There was no relationship between PFNA and GGT ( 1.45, )40,41,67,72,75 or AST ( 0.95, ) in adults (Table S4).40,41,67,72 Mundt et al.35 found no difference in mean ALT, GGT, or AST between production workers with low, high, or no occupational exposure to PFNA. Salihovic et al. reported a positive association between PFNA and ALT, but no relationship was found between PFNA and GGT in a longitudinal analysis.42
Rodent studies.
Six studies evaluated exposure to PFNA and markers of liver injury in mice or rats. Results consistently demonstrated elevated ALT, steatosis, and hepatocellular hypertrophy in treatment groups compared with controls in both mice28,131,142,143 and rats.144,145
Exposure to PFHxS
Human studies.
Five cross-sectional studies assessing the relationship between PFHxS and ALT in adults and adolescents were included in the weighted -score calculation.40,41,67,72,75 A weighted -score of 1.42 () did not suggest any relationship between PFHxS and ALT (Table 3). No sensitivity analyses were performed for this weighted -score because of the limited number of available studies. One longitudinal study reported a positive association between PFHxS and ALT.42 Studies in children reported no relationship between PFHxS and ALT.74,76 Likewise, weighted -scores did not indicate a relationship between PFHxS and GGT ( 0.66, )40,41,67,72,75 or between PFHxS and AST ( 1.50, ) in adults (Table S4).40,41,67,72
Rodent studies.
Five studies examined the effects of PFHxS on liver outcomes. Two studies in mice130,146 and one in rats147 investigated the effects of PFHxS exposure on liver enzymes. No alterations in ALT or AST were observed in adult male rats or rat dams, or in mouse dams or pups.130,146,147 However, PFHxS-induced steatosis and hepatocellular hypertrophy at doses of in the one rat and two mouse studies that reported histopathological results.28,123,147
Exposure to Other PFAS
Findings among studies assessing exposure to other PFAS (PFDA, PFHxA, PFHpA, PFBS, PFBA, PFDoA, PFHxA, PFDoA, and GenX) were not consistent (Table 1). For instance, Nian et al. observed a positive relationship between PFDA and ALT in humans,41 whereas several other human studies found no relationship.42,72,77 Positive associations of human exposure to PFHxA77 and PFHpA42 with ALT were observed. Our search identified only one study that evaluated the effects of PFAS as a mixture in humans and found that higher prenatal PFAS exposure was associated with increased risk for livery injury in childhood, based on ALT, AST, and GGT percentiles.78 This finding suggests that, even if certain individual PFAS exert minor or no effects on the liver, the overall effect of multiple exposures may be detrimental.
No changes in ALT were reported after exposure to PFDA in rats,148 PFBS in rats,83 PFBA in mice or rats,82,93,149,150 or PFDoA in rats.151,152 Elevated ALT was reported following exposure to PFDA in mice,153,154 and PFHxA155 and PFUA156 in male but not female rats . PFDA154 and PFDoA151 exposure was also shown to result in steatosis in mice and rats, respectively, whereas PFBS exposure in mice did not.123
Few rodent studies evaluated the effects of GenX exposure on liver injury, and no eligible human studies evaluated this relationship. In mice, three studies reported that exposure to GenX resulted in steatosis90,157 or histopathological changes,81 although there were no changes in liver enzyme levels . A fourth study in mice did not find any significant histopathological changes or steatosis following GenX administration.158
Two studies in mice evaluated the effects of PFAS mixtures. In one, a mixture of PFOA, PFOS, and PFHxS was not found to alter ALT levels in pregnant dams fed either standard or high-fat diet or in their offspring.130 In the other, a mixture of PFOS, PFOA, PFNA, PFHxS, and GenX was found to increase ALT levels and alter liver histopathology in adult males and females.134
Discussion
This systematic review summarizes the body of evidence linking markers of liver injury with exposure to PFOA, PFOS, PFHxS, and PFNA, the most commonly studied PFAS. Meta-analysis in human studies provided convincing evidence that exposure to PFOA, PFOS, and PFNA are associated with higher serum ALT. Rodent studies have consistently demonstrated a positive relationship between exposure to PFOA and PFOS and serum ALT as well as relative liver weight, which may indicate accumulation of excess liver fat. We also found evidence to suggest a positive association between PFNA and ALT. Findings in rodents were largely consistent across studies that differed in exposure routes and duration. Many rodent studies exposed animals to doses far above expected human exposures; this is due to differences in PFAS elimination and half-lives in mice and rats relative to humans34 and does not preclude comparison with human research. The findings of the present review indicate consistency of results across human and rodent studies, adding support to the idea that associations found in observational human studies may be causal.
Per- and polyfluorinated compounds were first detected in the blood of occupationally exposed workers in the 1970s and in the general population in the 1990s, which brought awareness of their potential health risks.7 The hydrophobic and oleophobic properties of the carbon–fluorine bond make PFAS ideal for industrial use in flame retardants and surfactants yet also allow them to persist in the environment, with concerning implications for long-term health effects. Although manufacturers started to phase out the production of PFOS and other long-chain PFAS in the early 2000s, the Centers for Disease Control and Prevention still reports widespread PFAS exposure in U.S. adults, demonstrating their persistence in biological systems and the continued public health relevance of the present review.21,22 Of additional concern, newer PFAS that have replaced the legacy PFAS for industrial use, such as GenX, have similar chemical structure and properties. The limited studies of these replacement PFAS suggest that they may have toxic effects similar to the legacy chemicals.157,159
The exact mechanism of PFAS hepatotoxicity remains unresolved. PFAS are thought to promote liver inflammation and triglyceride accumulation through activation of both human and mouse peroxisome proliferator-activated receptor alpha () and other receptors given their structural similarities with fatty acids.28,96–98,143,149,153,158,160 Consequently, altered lipid metabolism has been associated with PFAS exposure in both human46,54,73,78 and animal studies.28,32,85,129,136 Although much of the mechanistic research has been done using mouse models, cell-culture studies evaluating comparability of this mechanism in both mouse and human receptors have demonstrated that PFAS similarly activate human 161–163 However, PFAS-induced liver injury and steatosis may not depend on alone.164 Alternate or complementary mechanisms may involve activation of constitutive androstane receptor (CAR),98,143 down-regulation of nuclear factor erythroid 2-related factor 2 (NRF2),121,129 and up-regulation of nuclear factor kappa-light-chain-enhancer of activated B cells nuclear factor-kappa B (NF-ĸB).145 An additional possibility suggests that PFAS may reduce the bioavailability of choline, leading to steatosis as a result of choline deficiency.29,140
Several studies in mice examined the effects of PFAS exposure with coexposure to either a dietary supplement or high-fat diet. Supplementation with antioxidants was consistently found to ameliorate PFAS-induced liver injury.103,111,126,129,135,137 The effects of PFAS exposure in populations consuming high-fat diets were mixed; studies have found that PFAS exposure in rodents exacerbates the effect of high-fat diets on liver injury,108,125,139 although others have reported potentially protective effects.102,127,131 It is possible that the mechanisms by which PFAS induce liver injury are altered when liver homeostasis is already disrupted. These findings have not been replicated or studied extensively in humans, although there is some evidence that the relationship between PFAS and ALT may be mediated by metabolic disease or obesity.72,165
The parallel findings in experimental rodent studies identified in the present review address the limitations of observational findings and provide comprehensive evidence to suggest hepatotoxic effects of PFAS exposure. Many human studies, because of limited access to histopathological and imaging data for asymptomatic participants, limit analyses to liver enzymes and other biomarkers than can be easily measured in blood samples. Although levels of ALT and other enzymes are relatively specific indicators of liver injury, the exact nature or severity of the injury cannot be determined without more invasive procedures.51 However, it is well understood that populations that have higher levels of ALT also experience higher mortality and morbidity related to liver disease, and mild elevations of ALT in individuals may suggest the presence of NAFLD.57 Animal studies report similar increases in liver enzymes and pathological alterations to the structure and function of the liver. Indeed, changes to serum biomarkers of liver function following PFAS exposure are often accompanied by histopathological changes or steatosis in rodents,126,135,140 suggesting that associations between PFAS and ALT, AST, and GGT may be indicative of liver disease. However, only one study in humans reported both histological and liver enzyme data.77 Some rodent studies reported histological alterations without associated changes in liver enzymes,29,81,99,151 demonstrating the limitations of liver enzymes as markers of liver health. Recently, metabolomics79,131 and mixtures78,130,134 methods have emerged as more focused approaches to uncovering the relationship and mechanism between PFAS and liver injury and account for realistic exposure conditions, which may address this limitation. Most human studies identified by this review were cross-sectional, which precludes causal conclusions, and were conducted using different methods of data transformation and control of potential confounders. Far more studies have been conducted in rodents, and these findings, in conjunction with the limited number of longitudinal human studies, support a direct effect of PFAS on liver injury.
Still, there are a number of understudied factors in both epidemiolocal and experimental studies that require evaluation to elucidate the relationship between PFAS and liver injury. In this review, we have identified few studies in humans or rodents that evaluated sex-specific histological effects of PFAS exposure. Attanasio67 reported positive associations between PFAS and ALT in female adolescents and negative associations in male adolescents, whereas studies in adults did not observe any sex-specific differences.39,75 Some evidence for sex-specific differences was also reported in rats, with elevated ALT observed more frequently in male rats following PFAS exposure,29,94,95,120,122 and sex-specific differences in the elimination half-lives of PFOA and PFOS have also been reported for rodents.34 Many rodent studies were limited to males alone, which narrows the scope of findings and potential for mechanistic understanding. PFAS have been found to exert differential health effects by sex among other disease outcomes,166,167 and thus, the sex specificity of PFAS toxicity merits further investigation. Early life exposure to PFAS is another potentially significant factor that requires additional investigation. In humans, Stratakis et al. 78 reported that prenatal PFAS exposure was significantly associated with elevated liver enzymes in childhood; however, Mora et al. 76 observed modest inverse associations between maternal PFAS concentration and child ALT levels. In rodents, in utero and perinatal PFAS exposure was associated with elevated liver enzymes and liver weight, steatosis, and other histopathological alterations.86,100,106,119,128 These significant findings in rodent studies warrant the need for further consideration in humans. In both human and rodent studies, we found that most studies focused on the relationship between a single PFAS exposure and liver injury. As NHANES and other surveillance programs have indicated, multiple PFAS can regularly be detected in individuals and these exposures are highly correlated.21,40,67,72,75 Research suggests that effects of PFAS mixtures, as well as the interaction between PFAS and other environmental exposures (e.g., diet, polychlorinated biphenyls) may exert synergistic or antagonistic effects.168 Only two animal studies appear to have investigated this possibility to date, and the study designs differ in vehicle and duration of exposure, as well as in life stage and PFAS mixture composition, making it difficult to draw conclusions or extrapolate to humans.130,134 Only one study in humans investigated the liver effects of PFAS mixtures rather than single exposures and found convincing evidence for synergistic effects.78 Rapidly evolving methods for assessing exposure–mixture effects in population studies have potential to unravel the complex relationships between environmental exposures and liver injury.
To our knowledge, this is the first systematic review of the literature on PFAS exposure and liver injury and one of few reviews to consider both observational human and experimental rodent evidence for the effects of environmental exposures on health. We focused on ALT as a specific indicator of liver injury in occupationally exposed and general human populations and have followed PRISMA guidelines to limit the risk of bias in data synthesis and reporting of results. We found significant heterogeneity in the analyses, which limited our ability to perform a traditional meta-analysis and obtain a pooled effect estimate for human studies. However, evidence from experimental rodent studies consistently supported the results from human studies and indicates that PFAS exposure may contribute to markers of liver injury such as elevated liver enzymes, steatosis, and histopathological alterations.
Conclusion
Data from human studies consistently demonstrate an association between PFOA, PFOS, and PFNA and markers of liver injury: ALT, AST, and GGT. Complementary evidence from experimental rodent studies provides biological plausibility that this association may be causal. Insufficient evidence in both human and rodent studies exists to conclude that PFHxS and other PFAS have hepatoxic effects, possibly due to the low number of available studies. That there are positive associations between PFAS and ALT levels in humans suggests that PFAS exposure may contribute to the growing NAFLD epidemic. Future research should evaluate the full spectrum of NAFLD (including inflammation, hepatocellular injury, steatosis, and fibrosis) through histopathology or imaging, as well as consider additional investigation on lesser studied PFAS and PFAS mixtures to elucidate potential synergistic effects.
Supplementary Material
Acknowledgments
This work was conceptualized and supervised by L.C. and N.S. The methodology was developed by L.C., N.S., D.V.C., and S.P.E. All analyses and data curation was performed and the original manuscript draft was written by S.R. and E.C. Critical review and editing of the manuscript was provided by L.C., N.S., S.P.E., D.I.W., D.V., D.C., T.J., S.A.X., R.K., S.S., V.V., M.A.L., H.R., D.V.C., and R.M. The financial support for this project was acquired by L.C. Sadly, Dr. Hugo Rosen passed away during revisions and did not review the final version of the paper. The authors agreed that Dr. Rosen should remain a co-author on the published article. All other authors provided final approval of this manuscript.
We gratefully acknowledge R. Jain for providing standard errors for the analyses extracted from Jain 71 and Jain and Ducatman 72.
The results reported herein correspond to specific aims of grant R01ES030691 (L.C., R.M., D.V.C., S.P.E., D.I.W., D.V.) from the National Institutes of Health (NIH) National Institute of Environmental Health Science (NIEHS). Additional funding from the NIEHS included R21ES029681 (L.C., R.M., D.V.C., D.V.), R01ES029944 (L.C., D.V.C., D.I.W., D.V., S.R.), R01ES030364 (L.C., R.M., D.V.C., D.I.W., D.V., M.A.L., S.P.E., N.S., E.C.) R01ES032712 (V.V.), R21ES028903 (R.M., D.V., D.I.W., L.C.), P30ES007048 (R.M., L.C., D.V.C., S.P.E., S.R.), R21ES029328 (D.V.), P30ES023515 (D.V.), U2C ES030859 (D.I.W.), and T32ES013678 (E.C.). Additional funding from the NIH included P01CA196569, R01CA140561, R01ES016813, P30DK048522 (D.V.C.). Funding from the U.S. Department of Agriculture (USDA) National Institute of Food and Agriculture included the Hatch project [1002182 (M.A.L.)] and funding from USDA Agricultural Research Service agreement #58-3092-0-001 (S.S.).
References
- 1.Mitra S, De A, Chowdhury A. 2020. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol 5:16, PMID: , 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. 2013. From NAFLD to NASH to cirrhosis—new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 10(11):627–636, PMID: , 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Zhang C, Zhan YT. 2018. Nonalcoholic fatty liver disease cirrhosis: a review of its epidemiology, risk factors, clinical presentation, diagnosis, management, and prognosis. Can J Gastroenterol Hepatol 2018:2784537, PMID: , 10.1155/2018/2784537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. 2018. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67(1):123–133, PMID: , 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt EM. 2004. Nonalcoholic steatohepatitis. Semin Liver Dis 24(1):3–20, PMID: , 10.1055/s-2004-823098. [DOI] [PubMed] [Google Scholar]
- 6.Foulds CE, Trevino LS, York B, Walker CL. 2017. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol 13(8):445–457, PMID: , 10.1038/nrendo.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: , 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K. 2008. Estimating consumer exposure to PFOS and PFOA. Risk Anal 28(2):251–269, PMID: , 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 9.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: , 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 38(17):4489–4495, PMID: , 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 11.Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3:344–350, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada K, Saito N, Sasaki K, Inoue K, Koizumi A. 2003. Perfluorooctane sulfonate contamination of drinking water in the Tama River, Japan: estimated effects on resident serum levels. Bull Environ Contam Toxicol 71(1):31–36, PMID: , 10.1007/s00128-003-0126-x. [DOI] [PubMed] [Google Scholar]
- 13.Skutlarek D, Exner M, Färber H. 2006. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res Int 13(5):299–307, PMID: , 10.1065/espr2006.07.326. [DOI] [PubMed] [Google Scholar]
- 14.EFSA CONTAM Panel (EFSA Panel on Contaminants in the Foot Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, et al. 2020. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18(9):e06223, PMID: , 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domingo JL, Nadal M. 2017. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem 65(3):533–543, PMID: , 10.1021/acs.jafc.6b04683. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K, Harada K, Saito N, Tsutsui T, Nakanishi S, Tsuzuki H, et al. 2003. Impact of airborne perfluorooctane sulfonate on the human body burden and the ecological system. Bull Environ Contam Toxicol 71(2):408–413, PMID: , 10.1007/s00128-003-0179-x. [DOI] [PubMed] [Google Scholar]
- 17.Shoeib M, Harner T, Ikonomou M, Kannan K. 2004. Indoor and outdoor air concentrations and phase partitioning of perfluoroalkyl sulfonamides and polybrominated diphenyl ethers. Environ Sci Technol 38(5):1313–1320, PMID: , 10.1021/es0305555. [DOI] [PubMed] [Google Scholar]
- 18.So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, et al. 2006. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol 40(9):2924–2929, PMID: , 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- 19.Aimuzi R, Luo K, Chen Q, Wang H, Feng L, Ouyang F, et al. 2019. Perfluoroalkyl and polyfluoroalkyl substances and fetal thyroid hormone levels in umbilical cord blood among newborns by prelabor caesarean delivery. Environ Int 130:104929, PMID: , 10.1016/j.envint.2019.104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, et al. 2019. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int 130:104874, PMID: , 10.1016/j.envint.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(CDC) Centers for Disease Control and Prevention. 2021. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA: CDC. [Google Scholar]
- 22.U.S. EPA (U.S. Environmental Protection Agency). 2021. Fact Sheet: 2010/2015 PFOA Stewardship Program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program [accessed 22 April 2021].
- 23.Kärrman A, Domingo JL, Llebaria X, Nadal M, Bigas E, van Bavel B, et al. 2010. Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples. Environ Sci Pollut Res Int 17(3):750–758, PMID: , 10.1007/s11356-009-0178-5. [DOI] [PubMed] [Google Scholar]
- 24.Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, et al. 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59:354–362, PMID: , 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, et al. 2019. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124:482–492, PMID: , 10.1016/j.envint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, et al. 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 106:135–143, PMID: , 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: , 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, et al. 2017. Perfluoroalkyl acids—induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 378:37–52, PMID: , 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagley BD, Chang SC, Ehresman DJ, Eveland A, Zitzow JD, Parker GA, et al. 2017. Perfluorooctane sulfonate-induced hepatic steatosis in male Sprague Dawley rats is not attenuated by dietary choline supplementation. Toxicol Sci 160(2):284–298, PMID: , 10.1093/toxsci/kfx185. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Lv S, Nie S, Liu J, Tong S, Kang N, et al. 2016. Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish. Aquat Toxicol 176:45–52, PMID: , 10.1016/j.aquatox.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Ni H, Guo Y, Lin Y, Ji J, Jin C, et al. 2021. Hexafluoropropylene oxide dimer acid (HFPO-DA) induced developmental cardiotoxicity and hepatotoxicity in hatchling chickens: roles of peroxisome proliferator activated receptor alpha. Environ Pollut 290:118112, PMID: , 10.1016/j.envpol.2021.118112. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, He J, Li N, Gao N, Du Q, Chen B, et al. 2019. Lipid accumulation responses in the liver of Rana nigromaculata induced by perfluorooctanoic acid (PFOA). Ecotoxicol Environ Saf 167:29–35, PMID: , 10.1016/j.ecoenv.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 33.Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, et al. 2002. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol Sci 69(1):244–257, PMID: , 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- 34.Pizzurro DM, Seeley M, Kerper LE, Beck BD. 2019. Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul Toxicol Pharmacol 106:239–250, PMID: , 10.1016/j.yrtph.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Mundt DJ, Mundt KA, Luippold RS, Schmidt MD, Farr CH. 2007. Clinical epidemiological study of employees exposed to surfactant blend containing perfluorononanoic acid. Occup Environ Med 64(9):589–594, PMID: , 10.1136/oem.2007.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen GW, Burris JM, Mandel JH, Zobel LR. 1999. Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees. J Occup Environ Med 41(9):799–806, PMID: , 10.1097/00043764-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Olsen GW, Burris JM, Burlew MM, Mandel JH. 2003. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med 45(3):260–270, PMID: , 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 38.Olsen GW, Zobel LR. 2007. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health 81(2):231–246, PMID: , 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- 39.Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. 2016. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a Mid-Ohio Valley community. Environ Health Perspect 124(8):1227–1233, PMID: , 10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleason JA, Post GB, Fagliano JA. 2015. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res 136:8–14, PMID: , 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Nian M, Li QQ, Bloom M, Qian ZM, Syberg KM, Vaughn MG, et al. 2019. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: isomers of C8 Health Project in China. Environ Res 172:81–88, PMID: , 10.1016/j.envres.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Salihovic S, Stubleski J, Kärrman A, Larsson A, Fall T, Lind L, et al. 2018. Changes in markers of liver function in relation to changes in perfluoroalkyl substances—a longitudinal study. Environ Int 117:196–203, PMID: , 10.1016/j.envint.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. 2007. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med 49(10):1086–1096, PMID: , 10.1097/JOM.0b013e318156eca3. [DOI] [PubMed] [Google Scholar]
- 44.Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. 2007. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med 49(8):872–879, PMID: , 10.1097/JOM.0b013e318124a93f. [DOI] [PubMed] [Google Scholar]
- 45.Canova C, Barbieri G, Zare Jeddi M, Gion M, Fabricio A, Daprà F, et al. 2020. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environ Int 145:106117, PMID: , 10.1016/j.envint.2020.106117. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Barregard L, Xu Y, Scott K, Pineda D, Lindh CH, et al. 2020. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environ Health 19(1):33, PMID: , 10.1186/s12940-020-00588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 170(10):1268–1278, PMID: , 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra P, Gill RK, Saksena S, Alrefai WA. 2020. Disturbances in cholesterol homeostasis and non-alcoholic fatty liver diseases. Front Med (Lausanne) 7:467, PMID: , 10.3389/fmed.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J, Zhong R, Liu C, Tang Y, Gong J, Chang J, et al. 2016. Association between bilirubin and risk of non-alcoholic fatty liver disease based on a prospective cohort study. Sci Rep 6:31006, PMID: , 10.1038/srep31006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Q, Yu J, Zhang X, Liu S, Ge Y. 2016. Association of the serum uric acid level with liver histology in biopsy-proven non-alcoholic fatty liver disease. Biomed Rep 5(2):188–192, PMID: , 10.3892/br.2016.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannini EG, Testa R, Savarino V. 2005. Liver enzyme alteration: a guide for clinicians. CMAJ 172(3):367–379, PMID: , 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark JM, Brancati FL, Diehl AM. 2003. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98(5):960–967, PMID: , 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 53.Ioannou GN, Weiss NS, Boyko EJ, Kahn SE, Lee SP. 2005. Contribution of metabolic factors to alanine aminotransferase activity in persons with other causes of liver disease. Gastroenterology 128(3):627–635, PMID: , 10.1053/j.gastro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. 2021. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem 40(3):606–630, PMID: , 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costello E, Stratakis N, Chatzi L. 2020. The emerging role of perfluoroalkyl substances in liver injury and nonalcoholic fatty liver disease. PROSPERO 2020:CRD42020158911. [accessed 4 December 2019]. [Google Scholar]
- 56.Muiesan ML, Agabiti-Rosei C, Paini A, Salvetti M. 2016. Uric acid and cardiovascular disease: an update. Eur Cardiol 11(1):54–59, PMID: , 10.15420/ecr.2016:4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwo PY, Cohen SM, Lim JK. 2017. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 112(1):18–35, PMID: , 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 58.Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, et al. 2012. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes—conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol 40(7):971–994, PMID: , 10.1177/0192623312448935. [DOI] [PubMed] [Google Scholar]
- 59.NTP (National Toxicology Program). 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Washington DC: National Institute of Environmental Health Sciences, Division of the National Toxicology Program, OHAT. [Google Scholar]
- 60.NTP. 2015. OHAT Risk of Bias Rating Tool for Human and Animal Studies. Washington DC: National Institute of Environmental Health Sciences, Division of the National Toxicology Program, OHAT. [Google Scholar]
- 61.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. 2021. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Ontario, Canada: Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 22 March 2021]. [Google Scholar]
- 62.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928, PMID: , 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willer CJ, Li Y, Abecasis GR. 2010. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26(17):2190–2191, PMID: , 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buttke DE, Sircar K, Martin C. 2012. Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES (2003–2008). Environ Health Perspect 120(11):1613–1618, PMID: , 10.1289/ehp.1104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDowell MA, Brody DJ, Hughes JP. 2007. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Adolesc Health 40(3):227–231, PMID: , 10.1016/j.jadohealth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120(6):779–789, PMID: , 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Attanasio R. 2019. Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: analyses of NHANES 2013–2016. Environ Pollut 254(pt B):113061, PMID: , 10.1016/j.envpol.2019.113061. [DOI] [PubMed] [Google Scholar]
- 68.Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, et al. 2019. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut 247:1055–1063, PMID: , 10.1016/j.envpol.2019.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, et al. 2006. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J Occup Environ Med 48(8):771–779, PMID: , 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, et al. 2012. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 120(5):655–660, PMID: , 10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain RB. 2019. Concentration of selected liver enzymes across the stages of glomerular function: the associations with PFOA and PFOS. Heliyon 5(7):e02168, PMID: , 10.1016/j.heliyon.2019.e02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain RB, Ducatman A. 2019. Selective associations of recent low concentrations of perfluoroalkyl substances with liver function biomarkers: NHANES 2011 to 2014 data on US adults aged ≥20 years. J Occup Environ Med 61(4):293–302, PMID: , 10.1097/JOM.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 73.Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, et al. 2020. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: an untargeted metabolomics approach. Environ Int 134:105220, PMID: , 10.1016/j.envint.2019.105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, et al. 2018. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: a pilot study. Environ Res 160:314–321, PMID: , 10.1016/j.envres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 75.Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY, et al. 2010. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol 105(6):1354–1363, PMID: , 10.1038/ajg.2009.707. [DOI] [PubMed] [Google Scholar]
- 76.Mora AM, Fleisch AF, Rifas-Shiman SL, Woo Baidal JA, Pardo L, Webster TF, et al. 2018. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int 111:1–13, PMID: , 10.1016/j.envint.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rantakokko P, Männistö V, Airaksinen R, Koponen J, Viluksela M, Kiviranta H, et al. 2015. Persistent organic pollutants and non-alcoholic fatty liver disease in morbidly obese patients: a cohort study. Environ Health 14(1):79, PMID: , 10.1186/s12940-015-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stratakis N, Conti DV, Jin R, Margetaki K, Valvi D, Siskos AP, et al. 2020. Prenatal exposure to perfluoroalkyl substances associated with increased susceptibility to liver injury in children. Hepatology 72(5):1758–1770, PMID: , 10.1002/hep.31483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sen P, Qadri S, Luukkonen PK, Ragnarsdottir O, McGlinchey A, Jäntti S, et al. 2022. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J Hepatol 76(2):283–293, PMID: , 10.1016/j.jhep.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 80.Yamaguchi M, Arisawa K, Uemura H, Katsuura-Kamano S, Takami H, Sawachika F, et al. 2013. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J Occup Health 55(3):184–194, PMID: , 10.1539/joh.12-0264-oa. [DOI] [PubMed] [Google Scholar]
- 81.Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, et al. 2020. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ Health Perspect 128(2):27006, PMID: , 10.1289/EHP6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crebelli R, Caiola S, Conti L, Cordelli E, De Luca G, Dellatte E, et al. 2019. Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul Toxicol Pharmacol 106:169–177, PMID: , 10.1016/j.yrtph.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Lieder PH, Chang SC, York RG, Butenhoff JL. 2009. Toxicological evaluation of potassium perfluorobutanesulfonate in a 90-day oral gavage study with Sprague–Dawley rats. Toxicology 255(1–2):45–52, PMID: , 10.1016/j.tox.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Guo H, Wang J, Yao J, Sun S, Sheng N, Zhang X, et al. 2019. Comparative hepatotoxicity of novel PFOA alternatives (perfluoropolyether carboxylic acids) on male mice. Environ Sci Technol 53(7):3929–3937, PMID: , 10.1021/acs.est.9b00148. [DOI] [PubMed] [Google Scholar]
- 85.Hui Z, Li R, Chen L. 2017. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene 622:67–71, PMID: , 10.1016/j.gene.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 86.Li D, Zhang L, Zhang Y, Guan S, Gong X, Wang X. 2019. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ Sci Pollut Res Int 26(18):18866–18875, PMID: , 10.1007/s11356-019-05258-z. [DOI] [PubMed] [Google Scholar]
- 87.Pouwer MG, Pieterman EJ, Chang SC, Olsen GW, Caspers MPM, Verschuren L, et al. 2019. Dose effects of ammonium perfluorooctanoate on lipoprotein metabolism in APOE∗3-Leiden.CETP mice. Toxicol Sci 168(2):519–534, PMID: , 10.1093/toxsci/kfz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yahia D, El-Nasser MA, Abedel-Latif M, Tsukuba C, Yoshida M, Sato I, et al. 2010. Effects of perfluorooctanoic acid (PFOA) exposure to pregnant mice on reproduction. J Toxicol Sci 35(4):527–533, PMID: , 10.2131/jts.35.527. [DOI] [PubMed] [Google Scholar]
- 89.Yan S, Wang J, Zhang W, Dai J. 2014. Circulating microRNA profiles altered in mice after 28 d exposure to perfluorooctanoic acid. Toxicol Lett 224(1):24–31, PMID: , 10.1016/j.toxlet.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Guo H, Sheng N, Guo Y, Wu C, Xie W, Dai J. 2021. Exposure to GenX and its novel analogs disrupts fatty acid metabolism in male mice. Environ Pollut 291:118202, PMID: , 10.1016/j.envpol.2021.118202. [DOI] [PubMed] [Google Scholar]
- 91.Wu X, Xie G, Xu X, Wu W, Yang B. 2018. Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environ Sci Pollut Res Int 25(5):4787–4793, PMID: , 10.1007/s11356-017-0872-7. [DOI] [PubMed] [Google Scholar]
- 92.Rigden M, Pelletier G, Poon R, Zhu J, Auray-Blais C, Gagnon R, et al. 2015. Assessment of urinary metabolite excretion after rat acute exposure to perfluorooctanoic acid and other peroxisomal proliferators. Arch Environ Contam Toxicol 68(1):148–158, PMID: , 10.1007/s00244-014-0058-y. [DOI] [PubMed] [Google Scholar]
- 93.Butenhoff JL, Bjork JA, Chang SC, Ehresman DJ, Parker GA, Das K, et al. 2012. Toxicological evaluation of ammonium perfluorobutyrate in rats: twenty-eight-day and ninety-day oral gavage studies. Reprod Toxicol 33(4):513–530, PMID: , 10.1016/j.reprotox.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Butenhoff JL, Kennedy GL Jr, Chang SC, Olsen GW. 2012. Chronic dietary toxicity and carcinogenicity study with ammonium perfluorooctanoate in Sprague–Dawley rats. Toxicology 298(1–3):1–13, PMID: , 10.1016/j.tox.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Butenhoff JL, Chang SC, Olsen GW, Thomford PJ. 2012. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology 293(1–3):1–15, PMID: , 10.1016/j.tox.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Minata M, Harada KH, Kärrman A, Hitomi T, Hirosawa M, Murata M, et al. 2010. Role of peroxisome proliferator-activated receptor-alpha in hepatobiliary injury induced by ammonium perfluorooctanoate in mouse liver. Ind Health 48(1):96–107, PMID: , 10.2486/indhealth.48.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakagawa T, Ramdhan DH, Tanaka N, Naito H, Tamada H, Ito Y, et al. 2012. Modulation of ammonium perfluorooctanoate-induced hepatic damage by genetically different PPARα in mice. Arch Toxicol 86(1):63–74, PMID: , 10.1007/s00204-011-0704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF. 2020. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol 405:115204, PMID: , 10.1016/j.taap.2020.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin MT, Brennan RJ, Hu W, Ayanoglu E, Lau C, Ren H, et al. 2007. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci 97(2):595–613, PMID: , 10.1093/toxsci/kfm065. [DOI] [PubMed] [Google Scholar]
- 100.van Esterik JCJ, Sales LB, Dollé MET, Håkansson H, Herlin M, Legler J, et al. 2016. Programming of metabolic effects in C57BL/6JxFVB mice by in utero and lactational exposure to perfluorooctanoic acid. Arch Toxicol 90(3):701–715, PMID: , 10.1007/s00204-015-1488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Botelho SC, Saghafian M, Pavlova S, Hassan M, DePierre JW, Abedi-Valugerdi M. 2015. Complement activation is involved in the hepatic injury caused by high-dose exposure of mice to perfluorooctanoic acid. Chemosphere 129:225–231, PMID: , 10.1016/j.chemosphere.2014.06.093. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Wang Z, Klaunig JE. 2019. The effects of perfluorooctanoate on high fat diet induced non-alcoholic fatty liver disease in mice. Toxicology 416:1–14, PMID: , 10.1016/j.tox.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 103.Liu W, Xu C, Sun X, Kuang H, Kuang X, Zou W, et al. 2016. Grape seed proanthocyanidin extract protects against perfluorooctanoic acid-induced hepatotoxicity by attenuating inflammatory response, oxidative stress and apoptosis in mice. Toxicol Res (Camb) 5(1):224–234, PMID: , 10.1039/c5tx00260e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qazi MR, Abedi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M. 2010. Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol 10(11):1420–1427, PMID: , 10.1016/j.intimp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 105.Qazi MR, Hassan M, Nelson BD, Depierre JW, Abedi-Valugerdi M. 2013. Sub-acute, moderate-dose, but not short-term, low-dose dietary pre-exposure of mice to perfluorooctanoate aggravates concanavalin A-induced hepatitis. Toxicol Lett 219(1):1–7, PMID: , 10.1016/j.toxlet.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 106.Quist EM, Filgo AJ, Cummings CA, Kissling GE, Hoenerhoff MJ, Fenton SE. 2015. Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol Pathol 43(4):546–557, PMID: , 10.1177/0192623314551841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Son HY, Kim SH, Shin HI, Bae HI, Yang JH. 2008. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch Toxicol 82(4):239–246, PMID: , 10.1007/s00204-007-0246-x. [DOI] [PubMed] [Google Scholar]
- 108.Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, et al. 2013. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 8(4):e61409, PMID: , 10.1371/journal.pone.0061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang G, Pan R, Liang X, Wu X, Wu Y, Zhang H, et al. 2021. Perfluorooctanoic acid-induced liver injury is potentially associated with gut microbiota dysbiosis. Chemosphere 266:129004, PMID: , 10.1016/j.chemosphere.2020.129004. [DOI] [PubMed] [Google Scholar]
- 110.Yang B, Zou W, Hu Z, Liu F, Zhou L, Yang S, et al. 2014. Involvement of oxidative stress and inflammation in liver injury caused by perfluorooctanoic acid exposure in mice. Biomed Res Int 2014:409837, PMID: , 10.1155/2014/409837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zou W, Liu W, Yang B, Wu L, Yang J, Zou T, et al. 2015. Quercetin protects against perfluorooctanoic acid-induced liver injury by attenuating oxidative stress and inflammatory response in mice. Int Immunopharmacol 28(1):129–135, PMID: , 10.1016/j.intimp.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 112.Owumi S, Bello T, Oyelere AK. 2021. N-acetyl cysteine abates hepatorenal toxicities induced by perfluorooctanoic acid exposure in male rats. Environ Toxicol Pharmacol 86:103667, PMID: , 10.1016/j.etap.2021.103667. [DOI] [PubMed] [Google Scholar]
- 113.Curran I, Hierlihy SL, Liston V, Pantazopoulos P, Nunnikhoven A, Tittlemier S, et al. 2008. Altered fatty acid homeostasis and related toxicologic sequelae in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS). J Toxicol Environ Health A 71(23):1526–1541, PMID: , 10.1080/15287390802361763. [DOI] [PubMed] [Google Scholar]
- 114.Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Butenhoff JL. 2012. Hepatocellular hypertrophy and cell proliferation in Sprague–Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARα and CAR/PXR. Toxicology 293(1–3):16–29, PMID: , 10.1016/j.tox.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 115.Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Noker PE, et al. 2012. Evaluation of hepatic and thyroid responses in male Sprague Dawley rats for up to eighty-four days following seven days of dietary exposure to potassium perfluorooctanesulfonate. Toxicology 293(1–3):30–40, PMID: , 10.1016/j.tox.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 116.Han R, Hu M, Zhong Q, Wan C, Liu L, Li F, et al. 2018. Perfluorooctane sulphonate induces oxidative hepatic damage via mitochondria-dependent and NF-κB/TNF-α-mediated pathway. Chemosphere 191:1056–1064, PMID: , 10.1016/j.chemosphere.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 117.Han R, Zhang F, Wan C, Liu L, Zhong Q, Ding W. 2018. Effect of perfluorooctane sulphonate-induced Kupffer cell activation on hepatocyte proliferation through the NF-κB/TNF-α/IL-6-dependent pathway. Chemosphere 200:283–294, PMID: , 10.1016/j.chemosphere.2018.02.137. [DOI] [PubMed] [Google Scholar]
- 118.Kim HS, Jun Kwack S, Sik Han E, Seok Kang T, Hee Kim S, Young Han S. 2011. Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate. J Toxicol Sci 36(2):201–210, PMID: , 10.2131/jts.36.201. [DOI] [PubMed] [Google Scholar]
- 119.Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, et al. 2013. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol 28(9):532–542, PMID: , 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- 120.Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, et al. 2003. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183(1–3):117–131, PMID: , 10.1016/S0300-483X(02)00511-5. [DOI] [PubMed] [Google Scholar]
- 121.Wan C, Han R, Liu L, Zhang F, Li F, Xiang M, et al. 2016. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicol Appl Pharmacol 295:85–93, PMID: , 10.1016/j.taap.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 122.Butenhoff JL, Olsen GW, Chang S. 2017. Toxicological response of Sprague Dawley rats from inhalation exposure to perfluorooctane sulfonyl fluoride (POSF). Toxicol Lett 271:38–49, PMID: , 10.1016/j.toxlet.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 123.Bijland S, Rensen PCN, Pieterman EJ, Maas ACE, van der Hoorn JW, van Erk MJ, et al. 2011. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol Sci 123(1):290–303, PMID: , 10.1093/toxsci/kfr142. [DOI] [PubMed] [Google Scholar]
- 124.Deng P, Wang C, Wahlang B, Sexton T, Morris AJ, Hennig B. 2020. Co-exposure to PCB126 and PFOS increases biomarkers associated with cardiovascular disease risk and liver injury in mice. Toxicol Appl Pharmacol 409:115301, PMID: , 10.1016/j.taap.2020.115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hamilton MC, Heintz MM, Pfohl M, Marques E, Ford L, Slitt AL, et al. 2021. Increased toxicity and retention of perflourooctane sulfonate (PFOS) in humanized CYP2B6-Transgenic mice compared to Cyp2b-null mice is relieved by a high-fat diet (HFD). Food Chem Toxicol 152:112175, PMID: , 10.1016/j.fct.2021.112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang T, Zhang Y, Zhang W, Lin T, Chen L, Yang B, et al. 2020. Attenuation of perfluorooctane sulfonate-induced steatohepatitis by grape seed proanthocyanidin extract in mice. Biomed Res Int 2020:8818160, PMID: , 10.1155/2020/8818160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huck I, Beggs K, Apte U. 2018. Paradoxical protective effect of perfluorooctanesulfonic acid against high-fat diet-induced hepatic steatosis in mice. Int J Toxicol 37(5):383–392, PMID: , 10.1177/1091581818790934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lai KP, Li JW, Cheung A, Li R, Billah MB, Chan TF, et al. 2017. Transcriptome sequencing reveals prenatal PFOS exposure on liver disorders. Environ Pollut 223:416–425, PMID: , 10.1016/j.envpol.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 129.Lv Z, Wu W, Ge S, Jia R, Lin T, Yuan Y, et al. 2018. Naringin protects against perfluorooctane sulfonate-induced liver injury by modulating NRF2 and NF-κB in mice. Int Immunopharmacol 65:140–147, PMID: , 10.1016/j.intimp.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 130.Marques ES, Agudelo J, Kaye EM, Modaresi SMS, Pfohl M, Bečanová J, et al. 2021. The role of maternal high fat diet on mouse pup metabolic endpoints following perinatal PFAS and PFAS mixture exposure. Toxicology 462:152921, PMID: , 10.1016/j.tox.2021.152921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pfohl M, Marques E, Auclair A, Barlock B, Jamwal R, Goedken M, et al. 2021. An ’omics approach to unraveling the paradoxical effect of diet on perfluorooctanesulfonic acid (PFOS) and perfluorononanoic acid (PFNA)-induced hepatic steatosis. Toxicol Sci 180(2):277–294, PMID: , 10.1093/toxsci/kfaa172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qazi MR, Hassan M, Nelson BD, DePierre JW, Abedi-Valugerdi M. 2013. Both sub-acute, moderate-dose and short-term, low-dose dietary exposure of mice to perfluorooctane sulfonate exacerbates concanavalin A-induced hepatitis. Toxicol Lett 217(1):67–74, PMID: , 10.1016/j.toxlet.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 133.Qin Y, Gu T, Ling J, Luo J, Zhao J, Hu B, et al. 2021. PFOS facilitates liver inflammation and steatosis: an involvement of NLRP3 inflammasome-mediated hepatocyte pyroptosis. Preprint posted online October 23, 2021. J Appl Toxicol, PMID: , 10.1002/jat.4258. [DOI] [PubMed] [Google Scholar]
- 134.Roth K, Yang Z, Agarwal M, Liu W, Peng Z, Long Z, et al. 2021. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ Int 157:106843, PMID: , 10.1016/j.envint.2021.106843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Su M, Liang X, Xu X, Wu X, Yang B. 2019. Hepatoprotective benefits of vitamin C against perfluorooctane sulfonate-induced liver damage in mice through suppressing inflammatory reaction and ER stress. Environ Toxicol Pharmacol 65:60–65, PMID: , 10.1016/j.etap.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 136.Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CKC. 2012. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim Biophys Acta 1820(7):1092–1101, PMID: , 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 137.Wang G, Sun S, Wu X, Yang S, Wu Y, Zhao J, et al. 2020. Intestinal environmental disorders associate with the tissue damages induced by perfluorooctane sulfonate exposure. Ecotoxicol Environ Saf 197:110590, PMID: , 10.1016/j.ecoenv.2020.110590. [DOI] [PubMed] [Google Scholar]
- 138.Xing J, Wang G, Zhao J, Wang E, Yin B, Fang D, et al. 2016. Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J mouse models. Environ Pollut 210:388–396, PMID: , 10.1016/j.envpol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 139.Marques E, Pfohl M, Auclair A, Jamwal R, Barlock BJ, Sammoura FM, et al. 2020. Perfluorooctanesulfonic acid (PFOS) administration shifts the hepatic proteome and augments dietary outcomes related to hepatic steatosis in mice. Toxicol Appl Pharmacol 408:115250, PMID: , 10.1016/j.taap.2020.115250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang L, Krishnan P, Ehresman DJ, Smith PB, Dutta M, Bagley BD, et al. 2016. Perfluorooctane sulfonate–choline ion pair formation: a potential mechanism modulating hepatic steatosis and oxidative stress in mice. Toxicol Sci 153(1):186–197, PMID: , 10.1093/toxsci/kfw120. [DOI] [PubMed] [Google Scholar]
- 141.Liang X, Xie G, Wu X, Su M, Yang B. 2019. Effect of prenatal PFOS exposure on liver cell function in neonatal mice. Environ Sci Pollut Res Int 26(18):18240–18246, PMID: , 10.1007/s11356-019-05245-4. [DOI] [PubMed] [Google Scholar]
- 142.Wang J, Yan S, Zhang W, Zhang H, Dai J. 2015. Integrated proteomic and miRNA transcriptional analysis reveals the hepatotoxicity mechanism of PFNA exposure in mice. J Proteome Res 14(1):330–341, PMID: , 10.1021/pr500641b. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y, Zhang Y, Klaassen CD, Cheng X. 2018. Alteration of bile acid and cholesterol biosynthesis and transport by perfluorononanoic acid (PFNA) in mice. Toxicol Sci 162(1):225–233, PMID: , 10.1093/toxsci/kfx237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang X, Gao G, Zhang X, Wang H. 2015. Perfluorononanoic acid disturbed the metabolism of lipid in the liver of streptozotocin-induced diabetic rats. Toxicol Mech Methods 25(8):622–627, PMID: , 10.3109/15376516.2015.1053649. [DOI] [PubMed] [Google Scholar]
- 145.Fang X, Zou S, Zhao Y, Cui R, Zhang W, Hu J, et al. 2012. Kupffer cells suppress perfluorononanoic acid-induced hepatic peroxisome proliferator-activated receptor α expression by releasing cytokines. Arch Toxicol 86(10):1515–1525, PMID: , 10.1007/s00204-012-0877-4. [DOI] [PubMed] [Google Scholar]
- 146.Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, et al. 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD-1 mice. Reprod Toxicol 78:150–168, PMID: , 10.1016/j.reprotox.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 147.Butenhoff JL, Chang SC, Ehresman DJ, York RG. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod Toxicol 27(3–4):331–341, PMID: , 10.1016/j.reprotox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 148.Kim SC, Hong JT, Jang SJ, Kang WS, Yoo HS, Yun YP. 1998. Formation of 8-oxodeoxyguanosine in liver DNA and hepatic injury by peroxisome proliferator clofibrate and perfluorodecanoic acid in rats. J Toxicol Sci 23(2):113–119, PMID: , 10.2131/jts.23.2_113. [DOI] [PubMed] [Google Scholar]
- 149.Foreman JE, Chang SC, Ehresman DJ, Butenhoff JL, Anderson CR, Palkar PS, et al. 2009. Differential hepatic effects of perfluorobutyrate mediated by mouse and human PPAR-α. Toxicol Sci 110(1):204–211, PMID: , 10.1093/toxsci/kfp077. [DOI] [PubMed] [Google Scholar]
- 150.Weatherly LM, Shane HL, Lukomska E, Baur R, Anderson SE. 2021. Systemic toxicity induced by topical application of heptafluorobutyric acid (PFBA) in a murine model. Food Chem Toxicol 156:112528, PMID: , 10.1016/j.fct.2021.112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ding L, Hao F, Shi Z, Wang Y, Zhang H, Tang H, et al. 2009. Systems biological responses to chronic perfluorododecanoic acid exposure by integrated metabonomic and transcriptomic studies. J Proteome Res 8(6):2882–2891, PMID: , 10.1021/pr9000256. [DOI] [PubMed] [Google Scholar]
- 152.Kato H, Fujii S, Takahashi M, Matsumoto M, Hirata-Koizumi M, Ono A, et al. 2015. Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environ Toxicol 30(11):1244–1263, PMID: , 10.1002/tox.21996. [DOI] [PubMed] [Google Scholar]
- 153.Luo M, Tan Z, Dai M, Song D, Lin J, Xie M, et al. 2017. Dual action of peroxisome proliferator-activated receptor alpha in perfluorodecanoic acid-induced hepatotoxicity. Arch Toxicol 91(2):897–907, PMID: , 10.1007/s00204-016-1779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang D, Gao Q, Wang T, Kan Z, Li X, Hu L, et al. 2020. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res Int 127:108628, PMID: , 10.1016/j.foodres.2019.108628. [DOI] [PubMed] [Google Scholar]
- 155.Chengelis CP, Kirkpatrick JB, Radovsky A, Shinohara M. 2009. A 90-day repeated dose oral (gavage) toxicity study of perfluorohexanoic acid (PFHxA) in rats (with functional observational battery and motor activity determinations). Reprod Toxicol 27(3–4):342–351, PMID: , 10.1016/j.reprotox.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 156.Takahashi M, Ishida S, Hirata-Koizumi M, Ono A, Hirose A. 2014. Repeated dose and reproductive/developmental toxicity of perfluoroundecanoic acid in rats. J Toxicol Sci 39(1):97–108, PMID: , 10.2131/jts.39.97. [DOI] [PubMed] [Google Scholar]
- 157.Wang J, Wang X, Sheng N, Zhou X, Cui R, Zhang H, et al. 2017. RNA-sequencing analysis reveals the hepatotoxic mechanism of perfluoroalkyl alternatives, HFPO2 and HFPO4, following exposure in mice. J Appl Toxicol 37(4):436–444, PMID: , 10.1002/jat.3376. [DOI] [PubMed] [Google Scholar]
- 158.Chappell GA, Thompson CM, Wolf JC, Cullen JM, Klaunig JE, Haws LC. 2020. Assessment of the mode of action underlying the effects of GenX in mouse liver and implications for assessing human health risks. Toxicol Pathol 48(3):494–508, PMID: , 10.1177/0192623320905803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Caverly Rae JM, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL. 2015. Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague–Dawley rats. Toxicol Rep 2:939–949, PMID: , 10.1016/j.toxrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, et al. 2019. Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep 6(3):80–94, PMID: , 10.1007/s40572-019-00232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. 2008. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol Sci 106(1):162–171, PMID: , 10.1093/toxsci/kfn166. [DOI] [PubMed] [Google Scholar]
- 162.Behr AC, Plinsch C, Braeuning A, Buhrke T. 2020. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol In Vitro 62:104700, PMID: , 10.1016/j.tiv.2019.104700. [DOI] [PubMed] [Google Scholar]
- 163.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci 92(2):476–489, PMID: , 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 164.Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE, Fenton SE. 2015. Perfluorooctanoic acid (PFOA)-induced liver lesions in two strains of mice following developmental exposures: PPARα is not required. Toxicol Pathol 43(4):558–568, PMID: , 10.1177/0192623314558463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gilliland FD, Mandel JS. 1996. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men. Am J Ind Med 29(5):560–568, PMID: , . [DOI] [PubMed] [Google Scholar]
- 166.Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. 2018. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ Pollut 242(pt A):894–904, PMID: , 10.1016/j.envpol.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. 2010. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect 118(5):686–692, PMID: , 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect 124(1):A6–A9, PMID: , 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Attanasio R. 2019. Association between perfluoroalkyl acids and liver function: data on sex differences in adolescents. Data Brief 27:104618, PMID: , 10.1016/j.dib.2019.104618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cui R, Li C, Wang J, Dai J. 2019. Induction of hepatic miR-34a by perfluorooctanoic acid regulates metabolism-related genes in mice. Environ Pollut 244:270–278, PMID: , 10.1016/j.envpol.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 171.Guo H, Chen J, Zhang H, Yao J, Sheng N, Li Q, et al. 2021. Exposure to GenX and its novel analogs disrupts hepatic bile acid metabolism in male mice. Environ Sci Technol Preprint posted online August 24, 2021, PMID: , 10.1021/acs.est.1c02471. [DOI] [PubMed] [Google Scholar]
- 172.Shao W, Xu J, Xu C, Weng Z, Liu Q, Zhang X, et al. 2021. Early-life perfluorooctanoic acid exposure induces obesity in male offspring and the intervention role of chlorogenic acid. Environ Pollut 272:115974, PMID: , 10.1016/j.envpol.2020.115974. [DOI] [PubMed] [Google Scholar]
- 173.Shi L, Pan R, Lin G, Liang X, Zhao J, Zhang H, et al. 2021. Lactic acid bacteria alleviate liver damage caused by perfluorooctanoic acid exposure via antioxidant capacity, biosorption capacity and gut microbiota regulation. Ecotoxicol Environ Saf 222:112515, PMID: , 10.1016/j.ecoenv.2021.112515. [DOI] [PubMed] [Google Scholar]
- 174.Wu X, Liang M, Yang Z, Su M, Yang B. 2017. Effect of acute exposure to PFOA on mouse liver cells in vivo and in vitro. Environ Sci Pollut Res Int 24(31):24201–24206, PMID: , 10.1007/s11356-017-0072-5. [DOI] [PubMed] [Google Scholar]
- 175.Yan S, Zhang H, Wang J, Zheng F, Dai J. 2015. Perfluorooctanoic acid exposure induces endoplasmic reticulum stress in the liver and its effects are ameliorated by 4-phenylbutyrate. Free Radic Biol Med 87:300–311, PMID: , 10.1016/j.freeradbiomed.2015.06.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.