Summary
Background
To examine global patterns of gastric cancer in 2020 and the projected burden in 2040.
Methods
Data on primary gastric cancer were extracted from the GLOBOCAN database for the year 2020. Age-standardized incidence and mortality rates were calculated by sex, country, world region and level of human development index (HDI) for 185 countries. The predicted burden of incidence and mortality in 2040 was calculated based on demographic projections.
Findings
In total, ∼1.1 million new cases and 770,000 deaths of gastric cancer were estimated in 2020. Incidence rates were on average 2-fold higher in males than females (15.8 and 7.0 per 100,000, respectively) with variation across countries. Highest incidence rates were observed in Eastern Asia for both males and females (32.5 and 13.2, respectively); males residing in Japan (48.1), Mongolia (47.2) and Korea (39.7) had the highest rates in the world. Incidence was lowest in Africa with incidence rates < 5 per 100,000. Highest mortality rates were observed in Eastern Asia for both males (21.1) and females (8.8). A lower share of deaths was observed in very high HDI countries compared to medium and low HDI countries. The annual burden of gastric cancer is predicted to increase to ∼1.8 million new cases and ∼1.3 million deaths by 2040.
Interpretation
These estimates of the global burden of gastric cancer pinpoint countries and regions of high incidence and mortality in need of cancer control initiatives. Primary prevention through eradication of H. pylori and behavioural changes such as reducing salt intake, smoking, obesity, and alcohol, remains key in stomach cancer control.
Funding
No direct funding was received. All authors had access to the included study data and all authors agreed with the final decision to submit for publication.
Keywords: Gastric cancer, Incidence, Mortality, Epidemiology
Research in context.
Evidence before this study
While gastric cancer has decreased in many countries globally, 1.1 million people were diagnosed with the disease and 770,000 subsequent deaths occurred in 2020. We searched PubMed with the terms “gastric cancer”, “stomach cancer”, “incidence”, “mortality”, “global” and “international” for previous studies published between 1/1/1994 and 22/3/2022 about determinants and trends of incidence and mortality of gastric cancer globally. Previous studies have shown reductions in gastric cancer incidence across world regions possibly linked to improvements in food preservation practices as well as improvements in economic development leading to reduction in the prevalence of Helicobacter pylori infection with reports of increases in younger adults also observed suggesting the role of lifestyle factors such as obesity.
Added value of this study
We provide comprehensive estimates of the most up-to-date and future global burden of gastric cancer in 185 countries worldwide and show that it remains a high incidence and mortality cancer in some global regions, including Eastern Asia. Countries with the highest incidence globally include Mongolia, Japan and the Republic of Korea and similar patterns were observed by age group with highest incidence in Eastern Asia countries in both <60 and 60+ year olds. If current rates remain stable, future projections indicate that 1.8 million cases and 1.3 million deaths are expected to occur in 2040 which is 66% and 71% higher than that estimated in 2020.
Implications of all the available evidence
If no further action was taken, gastric cancer is expected to contribute to a substantial number of cases in many countries and an important cause of cancer mortality. It is important that surveillance of trends in younger adults are monitored and risk factors further investigated to understand reported increases in this group. We provide information that will allow governments and researchers to prioritize funding and research in cancer control according to current regional and national estimates on the burden of gastric cancer.
Alt-text: Unlabelled box
Introduction
Gastric cancer is the 5th most common cancer and the 4th leading cause of cancer death worldwide in 2020.1 Despite some improvements, international benchmarking studies have shown that gastric cancer survival remains low, even in high income countries, confirming that a focus on primary and secondary prevention is much needed.2,3
Gastric cancer incidence and mortality have been declining for several decades in most parts of the world, much of which has been attributed to economic development and better food preservation practices linked to refrigeration during the transport and storage of food. Contrary to previous reports of continuously declining incidence trends in all ages combined,2,4,5 recent reports have documented increases in incidence rates in younger adults (< 50 years),6,7 particularly for non-cardia tumours in wealthier populations.8,9
Gastric cancers can be separated into two main topographical subsites; while most gastric cancers occur in distal regions of the stomach (non-cardia), globally about 18% of all gastric cancers arise in the cardia, the part of the stomach adjoining the oesophageal-gastric junction.10 Both anatomical subsites have overlapping risk factors; smoking, heavy alcohol consumption, foods preserved by salting but also distinct aetiologies. Cardia gastric cancer has been associated with gastroesophageal reflux and obesity, while 90% of non-cardia cancers were attributable to Helicobacter pylori (H. pylori) infection.11,12
Considering the strong association of gastric cancer with modifiable risk factors, therein a largely preventable disease, it is important to investigate and report up-to-date estimates of the current burden for cancer control planning. This paper describes the current epidemiology of gastric cancer worldwide using data from IARC's GLOBOCAN 2020 database.
Methods
Data sources and methods
The GLOBOCAN 2020 database was used to derive the number of new cases of, and deaths from, gastric cancers (International Classification of Diseases, 10th revision: C16) for 185 countries or territories, by sex and 18 age groups (0–4, 5–9, …, 80–84, 85 and over).13, 14, 15 Population data for 2020 were extracted from the United Nations (UN) website to estimate rates.16 The method to describe data sources and estimation hierarchy have been described in detail elsewhere.13 In brief, the national cancer incidence and mortality were estimated using the best available sources of cancer incidence and mortality data within a given country based on recorded data prior to the COVID-19 pandemic. The same methods were used to derive the 2020 estimates to those used previously17, 18, 19; wherever possible giving priority to short-term predictions of observed high quality data.
We present tables and figures of new cases and deaths of gastric cancers including the age-standardized rate (ASR) per 100,000 using the world standard population20,21 to allow comparisons between populations adjusted for differences in age structures. ASRs and their corresponding 95% confidence intervals are also presented and can be found in the supplementary materials (supplementary Tables 1 and 2) of this study.
The results are presented by sex, age group (< 60 and 60 + years), country, and aggregated, across 20 UN-defined world regions,16 and according to the UN's four-tier Human Development Index (HDI) in 2020,22 the latter a means to assess the cancer burden at varying levels of development (low, medium, high and very high HDI). Throughout we use the terms transitioning, emerging and lower HDI countries/economies as synonyms for nations classified as low or medium HDI, and transitioned or higher HDI countries/economies for those classified as high or very high HDI. In addition, we presented mortality and incidence ratio (M:I ratio) which was calculated as the ratio of mortality to incidence rates. We also provide a prediction of the future number of gastric cancer cases and deaths worldwide for the year 2040, based on demographic projections and scenarios of annually increasing (+1, +2, +3%), stable or decreasing (−1, −2, −3%) rates from the baseline year of 2020. Incidence and mortality predictions were calculated by applying the 2020 rates (and each of the increasing or decreasing scenarios described) to the predicted population data as estimated by the United Nations Development Programme (UNDP).
The data including corresponding uncertainty intervals are available at the Global Cancer Observatory (GCO, https://gco.iarc.fr) which includes facilities for the tabulation and graphical visualization of the database, including explorations of the current15 and future23 burden for gastric cancer and also other cancer types.
Ethics
Ethics approval was not required for this study as the study used existing non-identifiable data that were aggregated and population-level.
Role of the funding source
No direct funding was received. All authors had access to the included study data and all authors agreed with the final decision to submit for publication.
Results
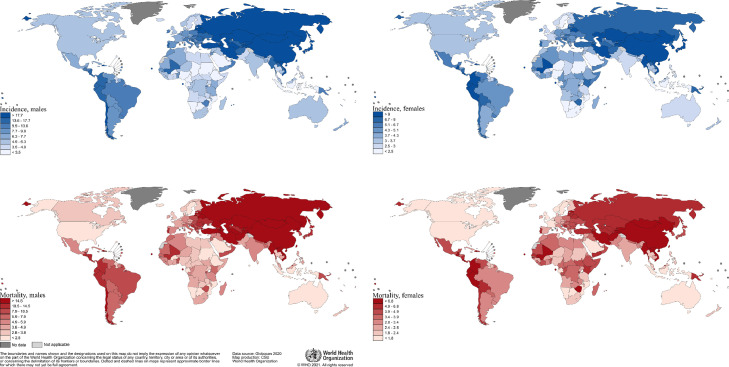
In 2020, an estimated 1.1 million cases (720,000 males and 370,000 females) of gastric cancer were diagnosed worldwide, ranking among the top 3 most common cancers in 19 countries, in 4 of which (Bhutan, Kyrgyzstan, Cape Verde and Tajikistan) it is the most common cancer. Of all gastric cancer cases, 60% were observed in Eastern Asia, 43.9% in China alone. Gastric cancer was more commonly diagnosed in males, with 66% of all cases occurring in men. Incidence rates in males ranged from less than 5 per 100,000 person-years in several African countries to over 30 per 100,000 person-years in Eastern Asian countries (Table 1, Figure 1).
Table 1.
New cases and deaths, age standardised incidence and mortality rates of gastric cancer (ICD10, C16) by sex, world region and Human Development Index (HDI), GLOBOCAN 2020.
| Incidence |
Mortality |
M:I ratio |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
Males | Females | Total | |||||
| World region | Number of cases | ASR | Number of cases | ASR | Number of deaths | ASR | Number of deaths | ASR | |||
| Africa | |||||||||||
| Eastern Africa | 4,919 | 4.9 | 5,042 | 4.2 | 4,261 | 4.3 | 4,454 | 3.7 | 0.9 | 0.9 | 0.9 |
| Middle Africa | 1,731 | 4.6 | 1,614 | 3.8 | 1,535 | 4.1 | 1,437 | 3.4 | 0.9 | 0.9 | 0.9 |
| Northern Africa | 5,414 | 5.4 | 3,983 | 3.5 | 4,489 | 4.5 | 3,238 | 2.9 | 0.8 | 0.8 | 0.8 |
| Southern Africa | 1,096 | 4.7 | 754 | 2.4 | 954 | 4.2 | 621 | 2.0 | 0.9 | 0.8 | 0.9 |
| Western Africa | 4,340 | 4.8 | 3,509 | 3.5 | 3,860 | 4.3 | 3,096 | 3.1 | 0.9 | 0.9 | 0.9 |
| America | |||||||||||
| Caribbean | 2,549 | 9.0 | 1,696 | 5.0 | 2,091 | 7.3 | 1,303 | 3.7 | 0.8 | 0.8 | 0.8 |
| Central America | 7,470 | 8.7 | 6,355 | 6.1 | 6,019 | 7.0 | 4,814 | 4.6 | 0.8 | 0.8 | 0.8 |
| South America | 30,520 | 12.1 | 19,027 | 6.0 | 24,449 | 9.6 | 14,716 | 4.6 | 0.8 | 0.8 | 0.8 |
| Northern America | 18,175 | 5.4 | 11,597 | 3.1 | 8,090 | 2.3 | 5,301 | 1.3 | 0.4 | 0.5 | 0.4 |
| Asia | |||||||||||
| Eastern Asia | 452,324 | 32.5 | 204,025 | 13.2 | 295,349 | 21.1 | 139,862 | 8.8 | 0.7 | 0.7 | 0.7 |
| All but China | 120,695 | 42.6 | 57,146 | 16.1 | 38,837 | 12.2 | 22,585 | 5.0 | 0.3 | 0.4 | 0.3 |
| China | 331,629 | 29.5 | 146,879 | 12.3 | 256,512 | 22.8 | 117,277 | 9.6 | 0.8 | 0.8 | 0.8 |
| South-Eastern Asia | 24,142 | 7.3 | 15,621 | 4.0 | 20,106 | 6.2 | 12,708 | 3.2 | 0.8 | 0.8 | 0.8 |
| South Central Asia | 67,701 | 7.4 | 34,975 | 3.7 | 59,832 | 6.6 | 29,763 | 3.1 | 0.9 | 0.9 | 0.9 |
| All but India | 27,015 | 10.8 | 15,439 | 5.8 | 23,402 | 9.4 | 12,940 | 4.9 | 0.9 | 0.8 | 0.9 |
| India | 40,686 | 6.1 | 19,536 | 2.9 | 36,430 | 5.5 | 16,823 | 2.5 | 0.9 | 0.9 | 0.9 |
| Western Asia | 13,235 | 11.4 | 7,921 | 6.1 | 11,157 | 9.8 | 6,429 | 4.9 | 0.8 | 0.8 | 0.8 |
| Europe | |||||||||||
| Central-Eastern Europe | 39,925 | 17.4 | 25,591 | 7.1 | 30,443 | 13.1 | 19,575 | 5.1 | 0.8 | 0.8 | 0.8 |
| Northern Europe | 7,263 | 6.2 | 4,214 | 3.1 | 4,830 | 3.9 | 2,938 | 2.0 | 0.7 | 0.7 | 0.7 |
| Southern Europe | 18,628 | 10.2 | 11,927 | 5.0 | 13,143 | 6.7 | 8,313 | 3.2 | 0.7 | 0.7 | 0.7 |
| Western Europe | 17,900 | 8.2 | 10,590 | 3.8 | 11,039 | 4.6 | 6,716 | 2.1 | 0.6 | 0.6 | 0.6 |
| Oceania | |||||||||||
| Australia/New Zealand | 1,799 | 6.4 | 877 | 2.8 | 839 | 2.7 | 516 | 1.5 | 0.5 | 0.6 | 0.5 |
| Melanesia | 333 | 9.9 | 226 | 6.2 | 260 | 8.0 | 175 | 4.8 | 0.8 | 0.8 | 0.8 |
| Micronesia/Polynesia | 59 | 9.7 | 36 | 5.5 | 42 | 7.1 | 30 | 4.5 | 0.7 | 0.8 | 0.8 |
| HDI | |||||||||||
| Low HDI | 12,195 | 5.4 | 10,034 | 3.8 | 10,784 | 4.8 | 8,856 | 3.4 | 0.9 | 0.9 | 0.9 |
| Medium HDI | 77,748 | 7.7 | 44,267 | 4.1 | 67,794 | 6.7 | 37,558 | 3.5 | 0.9 | 0.8 | 0.9 |
| High HDI | 397,829 | 21.2 | 189,074 | 9.0 | 310,756 | 16.6 | 150,478 | 7.0 | 0.8 | 0.8 | 0.8 |
| Very high HDI | 231,586 | 15.3 | 126,102 | 6.6 | 113,335 | 7.2 | 69,040 | 3.3 | 0.5 | 0.5 | 0.5 |
| World | 719,523 | 15.8 | 369,580 | 7.0 | 502,788 | 11.0 | 266,005 | 4.9 | 0.7 | 0.7 | 0.7 |
M:I, mortality: incidence; ASR, age-standardised rate per 100,000 population; HDI, human development index.
Figure 1.
Worldwide gastric cancer incidence and mortality rates (age-adjusted according to the world standard population, per 100 000) in males and females in 2020.
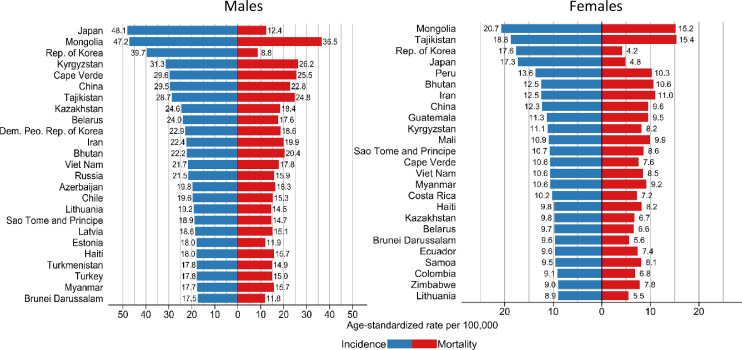
Incidence rates were highest in males in Japan (48.1), Mongolia (47.2) and the Republic of Korea (39.7) (Figure 2). Incidence rates were consistently lower in females compared to their male counterparts, with highest incidence in females observed in Mongolia (20.7), Tajikistan (18.8) and Republic of Korea (17.6). Similar patterns in incidence were noted by age group, with highest incidence rates observed in Eastern Asia for both < 60 and 60 + years (Table 2). A higher proportion of patients diagnosed <60 years were observed in African and South Central Asian regions compared with their older counterparts due to the younger age structure.
Figure 2.
Age-standardised incidence and mortality rates per 100,000 of the 25 highest incidence countries of gastric cancer, GLOBOCAN 2020.
Table 2.
New cases and deaths, age standardised incidence and mortality rates of gastric cancer (ICD-10, C16) by age group, world region, GLOBOCAN 2020
ASR, age-standardized rate per 100,000 population.
| Incidence |
Mortality |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <60 years |
≥ 60 years |
< 60 years |
≥ 60 years |
|||||||||
| Population | Number of cases | % of all cases | ASR | Number of cases | % of all cases | ASR | Number of cases | % of all cases | ASR | Number of cases | % of all cases | ASR |
| Africa | ||||||||||||
| Eastern Africa | 4,647 | 46.7 | 1.8 | 5,314 | 53.3 | 26.1 | 3,824 | 43.9 | 1.5 | 4,891 | 56.1 | 24.0 |
| Middle Africa | 1,288 | 38.5 | 1.4 | 2,057 | 61.5 | 26.6 | 1,073 | 36.1 | 1.1 | 1,899 | 63.9 | 24.6 |
| Northern Africa | 3,904 | 41.5 | 1.9 | 5,493 | 58.5 | 24.7 | 2,972 | 38.5 | 1.4 | 4,755 | 61.5 | 21.3 |
| Southern Africa | 750 | 40.5 | 1.3 | 1,100 | 59.5 | 19.7 | 601 | 38.2 | 1.1 | 974 | 61.8 | 17.4 |
| Western Africa | 3,519 | 44.8 | 1.5 | 4,330 | 55.2 | 24.6 | 2,948 | 42.4 | 1.3 | 4,008 | 57.6 | 23.0 |
| America | ||||||||||||
| Caribbean | 1,141 | 26.9 | 2.5 | 3,104 | 73.1 | 42.5 | 829 | 24.4 | 1.8 | 2,565 | 75.6 | 34.4 |
| Central America | 4,690 | 33.9 | 2.8 | 9,135 | 66.1 | 43.5 | 3,162 | 29.2 | 1.9 | 7,671 | 70.8 | 35.9 |
| South America | 13,728 | 27.7 | 3.1 | 35,819 | 72.3 | 54.7 | 9,745 | 24.9 | 2.2 | 29,420 | 75.1 | 44.1 |
| Northern America | 7304 | 24.5 | 1.8 | 22,468 | 75.5 | 23.7 | 2,938 | 21.9 | 0.7 | 10,453 | 78.1 | 10.3 |
| Asia | ||||||||||||
| Eastern Asia | 170,428 | 26.0 | 7.3 | 485,921 | 74.0 | 143.9 | 95,223 | 21.9 | 4.1 | 339,988 | 78.1 | 99.3 |
| All but China | 23,036 | 13.0 | 7.6 | 154,805 | 87.0 | 195.5 | 5763 | 9.4 | 2.0 | 55,659 | 90.6 | 58.7 |
| China | 147,392 | 30.8 | 7.3 | 331,116 | 69.2 | 127.9 | 89,460 | 23.9 | 4.4 | 284,329 | 76.1 | 108.5 |
| South-Eastern Asia | 15,671 | 39.4 | 2.2 | 24,092 | 60.6 | 31.8 | 11,191 | 34.1 | 1.6 | 21,623 | 65.9 | 28.3 |
| South Central Asia | 41,173 | 40.1 | 2.2 | 61,503 | 59.9 | 31.9 | 34,835 | 38.9 | 1.9 | 54,760 | 61.1 | 28.4 |
| All but India | 15,751 | 37.1 | 2.9 | 26,703 | 62.9 | 51.0 | 13,093 | 36.0 | 2.5 | 23,249 | 64.0 | 44.4 |
| India | 25,422 | 42.2 | 2.0 | 34,800 | 57.8 | 24.8 | 21,742 | 40.8 | 1.7 | 31,511 | 59.2 | 22.4 |
| Western Asia | 7282 | 34.4 | 2.9 | 13,874 | 65.6 | 54.3 | 5335 | 30.3 | 2.1 | 12,251 | 69.7 | 47.6 |
| Europe | ||||||||||||
| Central-Eastern Europe | 14,425 | 22.0 | 4.0 | 51,091 | 78.0 | 70.1 | 9,948 | 19.9 | 2.7 | 40,070 | 80.1 | 53.5 |
| Northern Europe | 1802 | 15.7 | 1.4 | 9675 | 84.3 | 29.8 | 1,019 | 13.1 | 0.8 | 6749 | 86.9 | 19.4 |
| Southern Europe | 4924 | 16.1 | 2.4 | 25,631 | 83.9 | 47.9 | 2,815 | 13.1 | 1.4 | 18,641 | 86.9 | 32.6 |
| Western Europe | 5617 | 19.7 | 2.3 | 22,873 | 80.3 | 34.9 | 2,679 | 15.1 | 1.1 | 15,076 | 84.9 | 21.1 |
| Oceania | ||||||||||||
| Australia/New Zealand | 570 | 21.3 | 1.7 | 2106 | 78.7 | 27.4 | 228 | 16.8 | 0.7 | 1127 | 83.2 | 13.4 |
| Melanesia | 197 | 35.2 | 2.3 | 362 | 64.8 | 53.7 | 132 | 30.3 | 1.6 | 303 | 69.7 | 44.9 |
| Micronesia/Polynesia | 30 | 31.6 | 2.5 | 65 | 68.4 | 46.8 | 18 | 25.0 | 1.5 | 54 | 75.0 | 38.6 |
| HDI | ||||||||||||
| Low HDI | 9808 | 44.1 | 1.8 | 12,421 | 55.9 | 27.1 | 8,245 | 42.0 | 1.5 | 11,395 | 58.0 | 24.9 |
| Medium HDI | 51,801 | 42.5 | 2.5 | 70,214 | 57.5 | 32.7 | 42,267 | 40.1 | 2.0 | 63,085 | 59.9 | 29.4 |
| High HDI | 180,241 | 30.7 | 5.2 | 406,662 | 69.3 | 92.2 | 113,471 | 24.6 | 3.2 | 347,763 | 75.4 | 78.0 |
| Very high HDI | 61,171 | 17.1 | 3.2 | 296,517 | 82.9 | 69.6 | 27,489 | 15.1 | 1.5 | 154,886 | 84.9 | 33.8 |
| World | 303,090 | 27.8 | 3.8 | 786,013 | 72.2 | 70.1 | 191,515 | 24.9 | 2.4 | 577,278 | 75.1 | 50.8 |
ASR, age-standardised rate per 100,000 population; HDI, human development index; HDI, human development index.
In terms of mortality due to gastric cancer, 770,000 deaths (65% were males) were observed worldwide in 2020. In 42 countries, gastric cancer ranks among the three most common causes of cancer death; in 13 countries it ranks as first. 56.6% of all global deaths occurred in Eastern Asia, with 48.6% of all global deaths occurring in China alone. Mortality rates in males ranged from less than 5 per 100,000 in Northern America, Northern Europe, Australia/ New Zealand regions to more than 20 per 100,000 in Eastern Asia regions. Mortality rates were highest in Mongolia (36.5 per 100,000) and Tajikistan (15.4) for males and females, respectively (Figures 1, 2).
Incidence was higher in transitioned (high and very high HDI) countries, which however had a lower share of deaths compared to medium and low HDI countries, where gastric cancer ranks among the top causes of cancer-related death. Most countries showed a direct correlation between incidence and mortality, although this was less marked in higher HDI categories (Table 1). M:I ratios ranged from 0.9 in low HDI countries to 0.7 in very high HDI countries. Despite being two of the highest incidence countries, Japan and the Republic of Korea had conversely lower mortality rates (M:I ratios of 0.3 [Japan] and 0.2 [Republic of Korea], in both males and females (Figure 2).
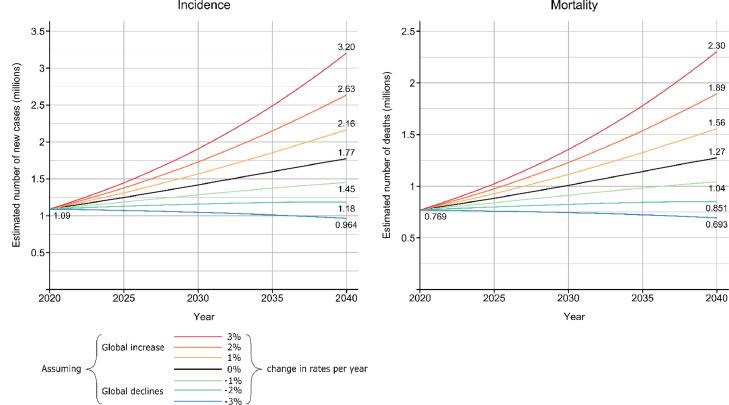
Assuming current rates remain the same, the annual number of new gastric cancer cases is predicted to increase by 62% from 1.09 million cases to 1.77 million cases by 2040 (Figure 3). If rates decreased by 2% per year, new cases would reach 1.18 million. If mortality rates remain the same, there would be 1.27 million deaths expected by 2040 (Figure 3), whereas if rates decrease by 2% per year, the expected number of deaths would reach 0.85 million.
Figure 3.
Projected number of cases of gastric cancer worldwide from 2020 to 2040.
Each line represents the estimated cases and deaths based on different scenarios of national rates of increase (or decrease) each year. For example, the black line, represents a 0% increase (or decrease) in incidence / mortality rates each year, and therefore indicates what the predicted number of cases or deaths will be if rates remained the same as in 2020.
Discussion
More than 1 million cases of gastric cancer were estimated to be diagnosed in 2020 with considerable geographic variation. Eastern Asian and Eastern European regions showed highest incidence rates in both males and females and globally about two thirds of all cases occur in men. Incidence and mortality are highly correlated reflecting poor survival and highlighting the importance of preventing cases or earlier diagnosis. By 2040 we expected a 62% rise in the number of cases to 1.77 million if the rates were to continue.
Some countries in Eastern Asia and Eastern Europe have the highest incidence in the world and are known to also have high prevalence of the established risk factors of gastric cancer. People of Japanese and Korean ancestry have higher incidence of gastric cancer in the US, which suggests that in addition to the different prevalence of East-Asian-type cytotoxin-associated gene A (cagA) strains and Western-type cagA strains of H. Pylori, cultural/dietary factors may be important.24,25 The main risk factor for gastric cancer is H. pylori infection with 90% of non-cardia gastric cases associated with the bacterium.11 A decline in H. pylori prevalence and an improvement in food storage and hygiene in recent decades have resulted in decreasing trends in gastric cancer incidence.26 Additionally, previous studies have found an interactive effect between H. pylori sero-positivity and current smoking and gastric cancer risk.27 Based on historical trends, globally an estimated 2% and 0.7% annual decrease in men and women in medium HDI countries has been reported that varied only slightly by HDI groups.28 Yet, despite these changes, and future predictions that it already has or will become a rare disease in a number of countries, incidence remains high in some regions, accompanied by persistent high mortality rates.6 High prevalence of H. pylori in countries in South America and Africa have been reported, although lower incidence of gastric cancer in Africa (known as the “African enigma”) has been observed and needs further investigation.29,30 One explanation is the geographical variation of H. pylori strains with populations where Eastern Asian type CagA is predominant have higher incidence than populations where Western type CagA is found.31,32 Moreover, consumption of salted and smoked foods are also known to increase the risk of gastric cancer and likely play an interactive role in the risk of this cancer. It is believed that glandular mucus contains glycans that inhibit the biosynthesis of glycolipids in the cell wall of H. pylori, and that a high-salt diet increases superficial mucus cell mucus and decreases glandular mucus cell mucus.33,34 There has been a large-scale reduction of salt intake in recent decades due to programmes driven to reduce hypertension which could have impacted incidence of gastric cancer.35 Such programmes should continue and be expanded to all countries not only for prevention of gastric cancer but cardiovascular disease, a major contributor to the burden of non-communicable disease.36
Despite reports on major declines in the incidence of gastric cancer, recent studies have observed increases in younger age groups (<50 years), particularly in low-incidence settings.6,8,9 The changes in incidence observed in younger generations over time could be a result of drastic decreases in prevalence of H. Pylori,37 and of changes in (the prevalence of) risk factors, such as dietary habits and obesity. Moreover, recent increases in autoimmune gastritis and dysbiosis of the gastric microbiome may be behind the observed shift towards more tumours occurring close to the esophago-gastric junction. It has been reported that early-onset gastric cancer (< 60 years) is genetically and clinically distinct from traditional gastric cancer.38 Further surveillance of this age group is warranted to confirm risk factors, particularly as such changes in incidence could be masked by overall decreases.
Prognosis of gastric cancer remains poor in most world regions, shown by the close correlation between incidence and mortality. The vast majority of gastric cancer cases present at late disease stage i.e., regional and distant disease in high-income countries.39 While the balance between benefits, harms and costs of routine screening in low-risk populations is still unclear,40 the implementation of population-based screening in high-risk regions has shown some promise.41 Studies have also shown that screening in high-risk sub-populations, e.g. certain race or ethnic groups in low-risk countries could be cost-effective.42,43 Two of the countries with the highest incidence rates globally, yet considerably lower mortality are Japan and the Republic of Korea. In 1953, gastric cancer screening using an indirect X-ray truck was started in Japan, and the number of people receiving screening increased rapidly with introduction of the Health Care Law for the Elderly in 1982. The introduction of national screening programmes in 2016 in Japan and 2002 in the Republic of Korea using either upper gastrointestinal series or endoscopy for men and women ≥ 40 years have demonstrated a shift in stage at diagnosis and relatively high survival proportions of over 60%.44,45 It is yet to be confirmed whether this will translate into true mortality reductions and how much of these survival increases are attributable to lead time and detection bias.46,47 Furthermore, recent improvements in diagnostic techniques through endoscopic imaging such as the Gastrointestinal Artificial Intelligence Diagnosis System (GRAIDS) show promise for improving the effectiveness of diagnosing gastric cancer, even in less experienced and low volume hospitals.48 In high-risk populations, eradication of H. pylori, is considered in combination with pepsinogen screening for gastric cancer.49 The cost-effectiveness of this strategy is currently being explored in several ongoing randomized controlled trials. Finally, other non-invasive markers, such as microRNA, for detection of precancerous lesions are being investigated with further validation studies needed.50,51 Despite some high-incidence countries like Japan showing low mortality rates, most high-incidence countries (such as Mongolia) continue to have high gastric cancer mortality, highlighting the need for more focus on preventive cancer control measures. Further to prevention strategies, socioeconomic disparities in the risk of gastric cancer has been reported suggesting higher incidence among low socioeconomic populations possibly due to dietary habits, obesity and smoking in this group.52 Disparities have also been reported elsewhere, for example Alaskan Native people had a higher incidence rate, poorer survival and younger age at diagnosis compared to Non-Hispanic people.53 This suggests that if the gap between socioeconomic status (SES) were to be reduced then the prevalence of SES-related risk factors would also be reduced which could ultimately impact national incidence of gastric cancer.54
Given that these data were based on estimates, where in some regions, particularly in low- and middle- income countries, complete coverage of cancer data was not available (or of limited quality), interpretations should be made with care.13 Validity of estimates is dependent on the degree of representativeness and quality of the source information.13 For example, the incidence in Africa is low, despite H. pylori studies indicating a high prevalence, and it is unclear whether this is due to data quality issues; competing risks; or other differences. Further research to investigate the true incidence and determinants of gastric cancer incidence in Africa may be useful in this regard. The Global Initiative for Cancer Registry Development (https://gicr.iarc.fr/) is an ongoing initiative that seeks to improve cancer registration in low- and middle-income countries in Africa, South and Central America and Asia for example, where surveillance systems are often limited or do not exist.55 Cancers of the esophageal-gastric junction could be classified as either stomach or esophagus and are considered a separate disease entity. Future research by subsite would be beneficial to further understand the etiology and clinical management of junctional cancers, i.e. cardia gastric cancers are known to be associated with obesity and gastroesophageal reflux disease. Information on anatomic subsite (cardia and non-cardia) were not available for this current study which seeks to provide a snapshot of the current burden of gastric cancer worldwide as well as future predictions of this burden based on current data. The collection and availability of data by subsite, particularly in low incidence countries would be useful to further understand the burden of this cancer, and changes in the risk factor landscape that likely drive shifting patterns in incidence by subsite, as previous studies have shown.10 Lastly, our figures are based on projections of cancer estimates before the COVID-19 pandemic. Recent studies have indicated that endoscopy services have been largely impacted by the COVID pandemic, which may result in reduced cancer detection.56,57 Further research is warranted to understand the full impact of the pandemic on the cancer burden with reported delays in surgeries and if the inequality gap has widened for some sites.
In conclusion, despite declining trends, gastric cancer remains an important cause of cancer mortality contributing to 8% of all cancer-related deaths in 2020. Whilst screening strategies are important for early detection in high-risk populations, the reduction in prevalence of H. pylori infection as well as the reduction in other known risk factors is key to prevention of gastric cancer. Surveillance of trends in younger adults is needed to monitor if increases in incidence are set to continue for this group. It is important that research and government initiatives focus their efforts on preventive measures that could modify the prevalence of risk factors for long-term public health benefits.
Funding
No direct funding was received.
Contributors
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Study concept and design: IS. Data collection and interpretation of data: EM, MA, IS, JF. Data analysis: JV, ML, JF. Drafting the manuscript: EM, MA, IS, MCC, AG, ATK, TM, FM, RHAV. Critical revision of the manuscript for important intellectual content: all authors.
Data sharing statement
The data including corresponding uncertainty intervals are available at the Global Cancer Observatory (GCO, https://gco.iarc.fr).
Declaration of interests
R.H.A.V. received a grant from Bristol Myers Squibb and another grant (more than 3 years ago) from Roche. All the other authors report no conflicts.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101404.
Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021:caac.21660. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Rutherford M.J., Bardot A., et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo G., Zhang Y., Guo P., Wang L., Huang Y., Li K. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int J Cancer. 2017;141:1333–1344. doi: 10.1002/ijc.30835. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M., Karim-Kos H.E., Coebergh J.W., et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European cancer observatory. Eur J Cancer. 2015;51:1164–1187. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Arnold M., Park J.Y., Camargo M.C., Lunet N., Forman D., Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69:823–829. doi: 10.1136/gutjnl-2019-320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heer E.V., Harper A.S., Sung H., Jemal A., Fidler-Benaoudia M.M. Emerging cancer incidence trends in Canada: the growing burden of young adult cancers. Cancer. 2020;126:4553–4562. doi: 10.1002/cncr.33050. [DOI] [PubMed] [Google Scholar]
- 8.Anderson W.F., Rabkin C.S., Turner N., Fraumeni J.F., Rosenberg P.S., Camargo M.C. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018;110:608–615. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camargo M.C., Anderson W.F., King J.B., et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–1649. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold M., Ferlay J., Van Berge Henegouwen M.I., Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 11.Mukaisho K.I., Nakayama T., Hagiwara T., Hattori T., Sugihara H. Two distinct etiologies of gastric cardia adenocarcinoma: interactions among pH, Helicobacter pylori, and bile acids. Front Microbiol. 2015;6:11. doi: 10.3389/fmicb.2015.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 13.Ferlay J., Colombet M., Soerjomataram I., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021 doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 14.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021:caac.21660. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J., Laversanne M., Ervik M., et al. International Agency for Research on Cancer; Lyon, France: 2020. Global Cancer Observatory: Cancer Today.https://gco.iarc.fr/today/home [cited 2021 Apr 30]. Available from. [Google Scholar]
- 16.United Nations Statistics Division. Standard country or area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49/overview/. Accessed 28 April 2021.
- 17.Ferlay J., Colombet M., Soerjomataram I., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 19.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 20.Segi M. Cancer mortality for selected sites in 24 countries (1950-1957). 2nd ed. Sendai: Tohoku University of Medicine; 1960
- 21.Doll R., Payne P., Waterhouse J. 1st ed. Springer; 1966. Cancer Incidence in Five Continents - A Technical Report. [Google Scholar]
- 22.United Nations Development Programme . United Nations; New York: 2020. Human Development Report 2020. [Google Scholar]
- 23.Ferlay J., Laversanne M., Ervik M., et al. International Agency for Research on Cancer; Lyon, France: 2020. Global Cancer Observatory: Cancer Tomorrow.https://gco.iarc.fr/tomorrow/en [cited 2021 Apr 30]. Available from. [Google Scholar]
- 24.Shah S.C., McKinley M., Gupta S., Peek R.M., Martinez M.E., Gomez S.L. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology. 2020;159:1705–1714. doi: 10.1053/j.gastro.2020.07.049. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunari O., Shiota S., Suzuki R., et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012;50:876–883. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuccio L. Gastric cancer, Helicobacter pylori infection and other risk factors. World J Gastrointest Oncol. 2010;2:342. doi: 10.4251/wjgo.v2.i9.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butt J., Varga M.G., Wang T., et al. Smoking, Helicobacter pylori serology, and gastric cancer risk in prospective studies from China, Japan, and Korea. Cancer Prev Res. 2019;12:667–674. doi: 10.1158/1940-6207.CAPR-19-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soerjomataram I., Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol. 2021;2021:1–10. doi: 10.1038/s41571-021-00514-z. [DOI] [PubMed] [Google Scholar]
- 29.Smith S., Fowora M., Pellicano R. Infections with Helicobacter pylori and challenges encountered in Africa. World J Gastroenterol. 2019;25:3183. doi: 10.3748/wjg.v25.i25.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curado M.P., de Oliveira M.M., de Araújo Fagundes M. Prevalence of Helicobacter pylori infection in Latin America and the Caribbean populations: a systematic review and meta-analysis. Cancer Epidemiol. 2019;60:141–148. doi: 10.1016/j.canep.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Yamaoka Y., Kato M., Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J.Y., Forman D., Waskito L.A., Yamaoka Y., Crabtree J.E. Epidemiology of Helicobacter pylori and CagA-positive infections and global variations in gastric cancer. Toxins. 2018;10:163. doi: 10.3390/toxins10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato S., Tsukamoto T., Mizoshita T., et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558–1566. doi: 10.1002/ijc.21810. [DOI] [PubMed] [Google Scholar]
- 34.Kawakubo M., Ito Y., Okimura Y., et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 35.He F.J., MacGregor G.A. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52:363–382. doi: 10.1016/j.pcad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 36.He F.J., MacGregor G.A. Role of salt intake in prevention of cardiovascular disease: controversies and challenges. Nat Rev Cardiol. 2018;15:371–377. doi: 10.1038/s41569-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 37.Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017;20:3–7. doi: 10.1007/s10120-016-0658-5. [DOI] [PubMed] [Google Scholar]
- 38.Bergquist J.R., Leiting J.L., Habermann E.B., et al. Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery. 2019;166:547–555. doi: 10.1016/j.surg.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Arnold M., Morgan E., Bardot A., et al. International variation in oesophageal and gastric cancer survival 2012-2014: differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study) Gut. 2021;0:1–12. doi: 10.1136/gutjnl-2021-325266. gutjnl-2021-325266. [DOI] [PubMed] [Google Scholar]
- 40.Lansdorp-Vogelaar I., Meester R.G.S., Laszkowska M., Escudero F.A., Ward Z.J., Yeh J.M. Cost-effectiveness of prevention and early detection of gastric cancer in Western countries. Best Pract Res Clin Gastroenterol. 2021;50–51 doi: 10.1016/j.bpg.2021.101735. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X., Li M., Chen S., et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology. 2018;155:347–354. doi: 10.1053/j.gastro.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Saumoy M., Schneider Y., Shen N., Kahaleh M., Sharaiha R.Z., Shah S.C. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology. 2018;155:648–660. doi: 10.1053/j.gastro.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Shah S.C., Canakis A., Peek R.M., Saumoy M. Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Clin Gastroenterol Hepatol. 2020;18:3026–3039. doi: 10.1016/j.cgh.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung K.W., Won Y.J., Kong H.J., et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–430. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda T., Saika K. Cancer burden in Japan based on the latest cancer statistics: need for evidence-based cancer control programs. Ann Cancer Epidemiol. 2018;2 [Google Scholar]
- 46.Hamashima C., Kato K., Miyashiro I., et al. Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2018;48:673–683. doi: 10.1093/jjco/hyy077. [DOI] [PubMed] [Google Scholar]
- 47.Jun J.K., Choi K.S., Lee H.Y., et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328. doi: 10.1053/j.gastro.2017.01.029. e7. [DOI] [PubMed] [Google Scholar]
- 48.Luo H., Xu G., Li C., et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645–1654. doi: 10.1016/S1470-2045(19)30637-0. [DOI] [PubMed] [Google Scholar]
- 49.Leja M., Park J.Y., Murillo R., et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ Open. 2017;7:e016999. doi: 10.1136/bmjopen-2017-016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei H., Pu K., Liu X.G., et al. The diagnostic value of circulating microRNAs as a biomarker for gastric cancer: a meta-analysis. Oncol Rep. 2019;41:87–102. doi: 10.3892/or.2018.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuoka T., Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J Gastroenterol. 2018;24:2818–2832. doi: 10.3748/wjg.v24.i26.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uthman O, Jadidi E, Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health. 2013;67:854–860. doi: 10.1136/jech-2012-201108. [DOI] [PubMed] [Google Scholar]
- 53.Martinson H.A., Shelby N.J., Alberts S.R., Olnes M.J. Gastric cancer in Alaska native people: a cancer health disparity. World J Gastroenterol. 2018;24:2722–2732. doi: 10.3748/wjg.v24.i25.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willis K., Hajizadeh M. Socioeconomic inequalities in gastric cancer incidence in Canada: 1992–2010. Acta Oncol. 2020;59:1333–1337. doi: 10.1080/0284186X.2020.1764098. [DOI] [PubMed] [Google Scholar]
- 55.Ferlay J., Colombet M., Soerjomataram I., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 56.Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a national endoscopy database analysis. Gut. 2021;70:537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 57.Lantinga M.A., Theunissen F., Ter Borg P.C.J., Bruno M.J., Ouwendijk R.J.T., Siersema P.D. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53:166–170. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.