Abstract
Objectives
We investigated in vivo light-induced photoreceptor damage in retinitis pigmentosa (RP) using spectral-domain optical coherence tomography (SD-OCT) images.
Methods
We retrospectively reviewed patients with genetic diagnosis of EYS-associated RP. The outer nuclear layer (ONL) thickness under retinal vessels was measured on SD-OCT vertical scans. As a control, we measured adjacent ONL thickness 100 μm superior and inferior from the vessel. Same measurements were performed in healthy subjects. We calculated the ratio of ONL thickness under vessel divided by the average of adjacent ONL thickness and defined as ONL preservation ratio. In patients with RP, the length of ellipsoid zone (EZ) from the fovea was also measured with SD-OCT vertical scans.
Results
Thirty EYS-associated RP patients and 25 healthy subjects were included. In both groups, ONL thickness overshadowed by retinal vessels was not significantly different from that of adjacent area. However, ONL preservation ratio of RP was larger than that of healthy control in both superior and inferior retina (1.03 vs 0.97; p < 0.01, 1.15 vs 0.95; p < 0.01, respectively). In RP, ONL preservation ratio was significantly larger in the inferior retina than superior retina (p < 0.01). Furthermore, in RP patients, the EZ length from the fovea was always shorter in the inferior than superior retina and there was a significant difference (p < 0.01).
Conclusions
Patients with EYS-associated RP exhibited inferior-dominant photoreceptor death and the relative ONL preservation under retinal vessels. These results suggest that longitudinal environment light exposure may be correlated with the photoreceptor death.
Subject terms: Retinal diseases, Outcomes research
Introduction
Retinitis pigmentosa (RP) is characterised by degeneration of photoreceptors leading to progressive and irreversible vision loss [1]. While the progression of the disease is primarily determined by causative mutations, effect of other modifier genes [2] and environmental factors [3, 4] have been discussed. Among the environmental factors, light exposure can modify the course of RP [5, 6]. In fact, light deprivation slows retinal degeneration in more than ten strains of animal models of RP such as the Royal College of Surgeons rats and rd10 mice [7, 8]. Recently, it was reported that mice with rhodopsin mutation demonstrate a significant superior-inferior difference in photoreceptor layer thickness, and this sector pattern of photoreceptor loss is associated with light exposure [9].
In clinical settings, it is not easy to estimate the amount of light exposure and the effect on the retina. There are a few clinical reports describing the relationship between RP and light damage. For example, Berson [10] covered one eye of RP patients with an opaque scleral contact lens for 5 years, 6–8 h per day and compared the status of the retina between the both eyes. In the end, both eyes showed comparable progression as monitored by changes in visual acuity, visual fields, fundus appearance and electroretinograms. Miyake et al. [11] illustrated a patient with RP who underwent surgery for 30-year pupillary occlusion, but both fundi showed similar appearance in the distribution of bone spicule pigmentation, degree of retinal vessel attenuation, colour of the optic disc and macular status. However, these previous studies were conducted before the recent development of retinal imaging technologies and evaluated the retina only with funduscopy and functional tests.
Remarkable advance of spectral-domain optical coherence tomography (SD-OCT) images enables us to detect more subtle changes objectively [12]. In the present study, we hypothesised that the outer nuclear layer (ONL) overshadowed by retinal vessels is protected from light-induced damage and shows relatively preserved thickness compared to its adjacent light-exposed area. We included only patients with eyes shut homologue (EYS) mutant RP in order to minimise the variety of genetic factors and examined the ONL condition using SD-OCT images.
Materials and methods
This study was conducted as a part of ongoing genotype-phenotype study approved by the ethics committee at Kyoto University Graduate School of Medicine (Kyoto, Japan). All study participants gave written informed consent for the analysis. All studies were conducted adhered to the tenets of the Declaration of Helsinki.
Study subjects
We retrospectively reviewed patients with EYS-mutant RP. Genetic testing was performed as previously reported [13]. We included those who were aged over 30 and underwent SD-OCT (Spectralis2 OCT; Heidelberg Engineering, Heidelberg, Germany). Requirement of age older than 30 years was to ensure a certain amount of total light exposure. Only one eye with better image quality was studied per each patient. Patients with poor OCT image quality were excluded from this study. Healthy control individuals were selected from patients who underwent cataract or contralateral epiretinal membrane surgery between February and October in 2018 and did not have any abnormal findings in OCT imaging.
Retinal vessel and its constant and partial shadow
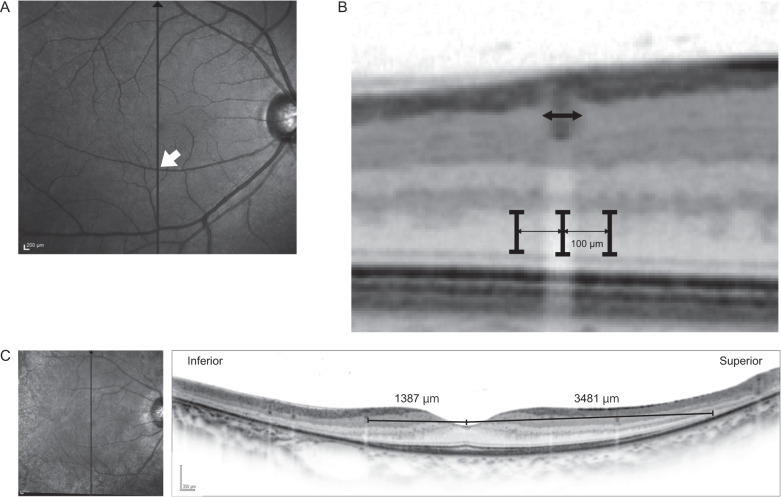
Figure 1A shows the schematic image of the eye and retinal vascular shadow. We postulated the axial length as 24 mm and the distance from pupil to retina as 20 mm based on Gullstrand–LeGrand Eye Model [14]. The area of partial and constant retinal vascular shadow under photopic environment (pupil diameter under photopic condition was assumed to be 3.5 mm) was calculated in the same way described in a previous report [15]. Namely, a 50-μm diameter retinal vessel has 80.1-μm partial shadow and 19.9-μm constant shadow, and a 100-µm diameter vessel has 125.4-µm partial and 74.6-µm constant shadow, whereas vessels whose diameter is less than 32 μm do not have constant shadow (Fig. 1B).
Fig. 1. Images of partial and constant retinal vessel shadow.
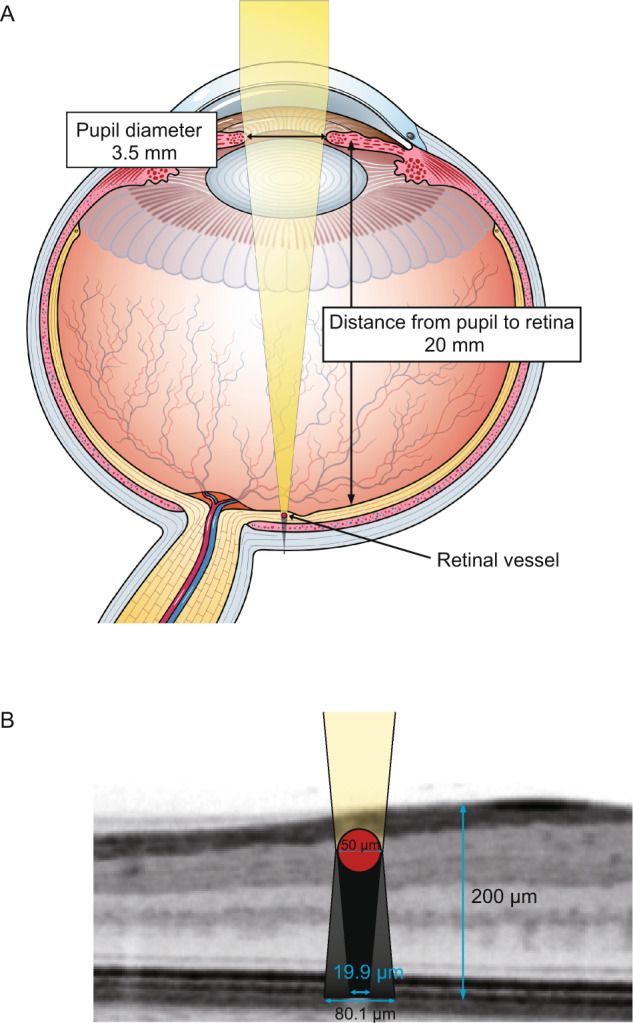
A A schematic image of the eye and retinal vessel shadow. B On the assumption that pupil diameter is 3.5 mm, axial length is 24 mm, anterior chamber depth is 4 mm and retinal thickness is 200 μm, a 50-μm diameter retinal vessel has 80.1-μm partial shadow and 19.9-μm constant shadow.
Measurement of ONL thickness and ellipsoid zone length
In SD-OCT, the vertical line scan imaging (each scan spanned 30 degrees) through the fovea was examined (Fig. 2A). The way how we performed ONL measurements was shown in Fig. 2B. First, we measured the diameter of retinal vessels in the image, and selected vessels whose diameter was 50–100 μm. We employed this range because too small vessels do not have complete shadow, and too large vessels have too wide partial shadow in the adjacent area. We manually measured ONL thickness under the vessel. Distance from the lower edge of outer plexiform layer to the upper edge of external limiting membrane (ELM) was defined as ONL thickness. As a control, we measured ONL thickness in adjacent areas: 100 μm superior and inferior from the centre of the vessel. The photoreceptor cells 100 μm away from the vessel are theoretically out of partial shadow and always exposed to light (see Fig. 1B). When there is another vessel whose shadow overlaps the measurement area, the vessels were not selected. We performed the same measurement in eyes of healthy controls. In this study, only one blood vessel was examined per each hemisphere and eyes in which both measurements could not be performed due to ELM disruption or absence of suitable vessels were excluded. All measurements were done with Heidelberg Eye Explorer. To discriminate the edge of each layer and increase the measurement accuracy, we applied unsmoothed pixels images zoomed in 800%. The measurement was done by three retinal specialists to reduce the measurement error and the average of these measurements were analysed. Intraclass correlation coefficient (ICC) was used to evaluate measurement reproducibility. We calculated the ratio of ONL thickness under the vessel divided by the average of adjacent ONL thickness and defined this as ‘ONL preservation ratio’. In patients with RP, length of ellipsoid zone (EZ) from the fovea was also manually measured in the superior and inferior retina with SD-OCT vertical scans (Fig. 2C). Patients in whom the EZ line was extended beyond the scanned area were excluded from the analysis.
Fig. 2. Measurement of ONL thickness with SD-OCT imaging.
Measurement and analysis of outer nuclear layer (ONL) thickness with the vertical line scan spectral-domain optical coherence tomography (SD-OCT) imaging. A SD-OCT 30 degrees vertical line scan imaging through the fovea was examined. B 50–100 μm retinal vessels were selected. We manually measured ONL thickness under the vessel. Note that unsmoothed images were used to determine the border of the layer precisely. As a control, we measured ONL thickness in adjacent areas: 100 μm superior and inferior from the centre of the vessel. C In patients with EYS-associated retinitis pigmentosa (RP), superior and inferior ellipsoid zone (EZ) length from the fovea was manually measured with the spectral-domain optical coherence tomography (SD-OCT) image.
Standard of care and examinations
All patients underwent a comprehensive ophthalmologic examination, including autorefractometry, best-corrected visual acuity using a Landolt chart, axial length, as well as indirect ophthalmoscopy, slit-lamp biomicroscopy, colour fundus photography, fundus autofluorescence and visual field exam using Humphrey field analyser. Electroretinogram was recorded unless the patient refused.
Data analyses
Data are expressed as mean ± standard deviation. T-tests and χ2 tests were used to compare values between groups. All analyses were performed using statistical software (SPSS Statistics 19; SPSS, Inc., Chicago, IL, USA) and statistical significance was defined as p < 0.05.
Results
Thirty patients with EYS-associated RP (10 men and 20 women) and 25 healthy individuals (11 men and 14 women) were included in this study. Mean age was 49.5 ± 13.2 years and 54.6 ± 15.0 years, respectively. Measurements with SD-OCT imaging between healthy controls and RP patients from the superior and inferior hemisphere are summarised in Table 1. Comparing the measurements between healthy controls and patients with RP, the mean retinal vessel diameter of controls tended to be larger than patients with RP. Measured vessels were located approximately 2 mm from the fovea in both groups and were slightly closer to the fovea in the RP group (p < 0.01). Not surprisingly, the mean ONL thickness was larger in controls than RP, both under vessels and in adjacent areas (p < 0.01).
Table 1.
Clinical characteristics and OCT measurements of healthy controls and patients with retinitis pigmentosa.
| Control | RP | p value (Control vs RP) | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of patients (eyes) | 25 (25) | 30 (30) | ||||||
| Age (year) | 54.6 ± 15.0 | 49.5 ± 13.2 | 0.13 | |||||
| Gender (male/female) | 11/14 | 10/20 | 0.42 | |||||
| OCT measurements in each hemisphere | Superior | Inferior | p value | Superior | Inferior | p value | Superior | Inferior |
| Vessel diameter | 74.40 ± 13.42 μm | 76.36 ± 12.90 μm | 0.60 | 68.67 ± 13.06 μm | 69.93 ± 12.13 μm | 0.70 | 0.12 | 0.06 |
| Vessel distance from the fovea | 2180 ± 489 μm | 2020 ± 479 μm | 0.25 | 2030 ± 426 μm | 1659 ± 226 μm | <0.01 | 0.23 | <0.01 |
| ONL thickness under vessels | 52.19 ± 8.55 μm | 44.36 ± 7.34 μm | <0.01 | 43.04 ± 15.33 μm | 33.31 ± 16.60 μm | 0.02 | <0.01 | <0.01 |
| Average of adjacent ONL thickness | 53.04 ± 9.76 μm | 46.57 ± 7.69 μm | <0.01 | 42.03 ± 15.28 μm | 30.36 ± 16.39 μm | <0.01 | <0.01 | <0.01 |
| ONL preservation ratio | 0.97 ± 0.04 | 0.95 ± 0.04 | 0.12 | 1.03 ± 0.06 | 1.15 ± 0.16 | <0.01 | <0.01 | <0.01 |
OCT optical coherence tomography, RP retinitis pigmentosa, ONL outer nuclear layer.
We compared ONL thickness under vessel to that in adjacent area in each group, in order to evaluate the status of overshadowed and light-exposed photoreceptor layer (Fig. 3). ICC for the ONL thickness under vessel and in adjacent areas showed good agreement among graders of 0.990 and 0.993, respectively. In healthy control, ONL thickness under the vessels tended to be thinner than the average of adjacent ONL, whereas the tendency was opposite in patients with RP. However, in both groups, ONL thickness under vessel was not significantly different from adjacent areas in both superior and inferior hemispheres (p = 0.49, 0.27 in control and p = 0.80, 0.49 in RP, respectively). Representative images of RP and healthy control are shown in Supplementary Fig. S1. In healthy control, ONL thickness under vessel was smaller than the average of adjacent areas, whereas it was opposite in a patient with RP.
Fig. 3. Comparison of ONL thickness between under vessel and average of its adjacent areas.
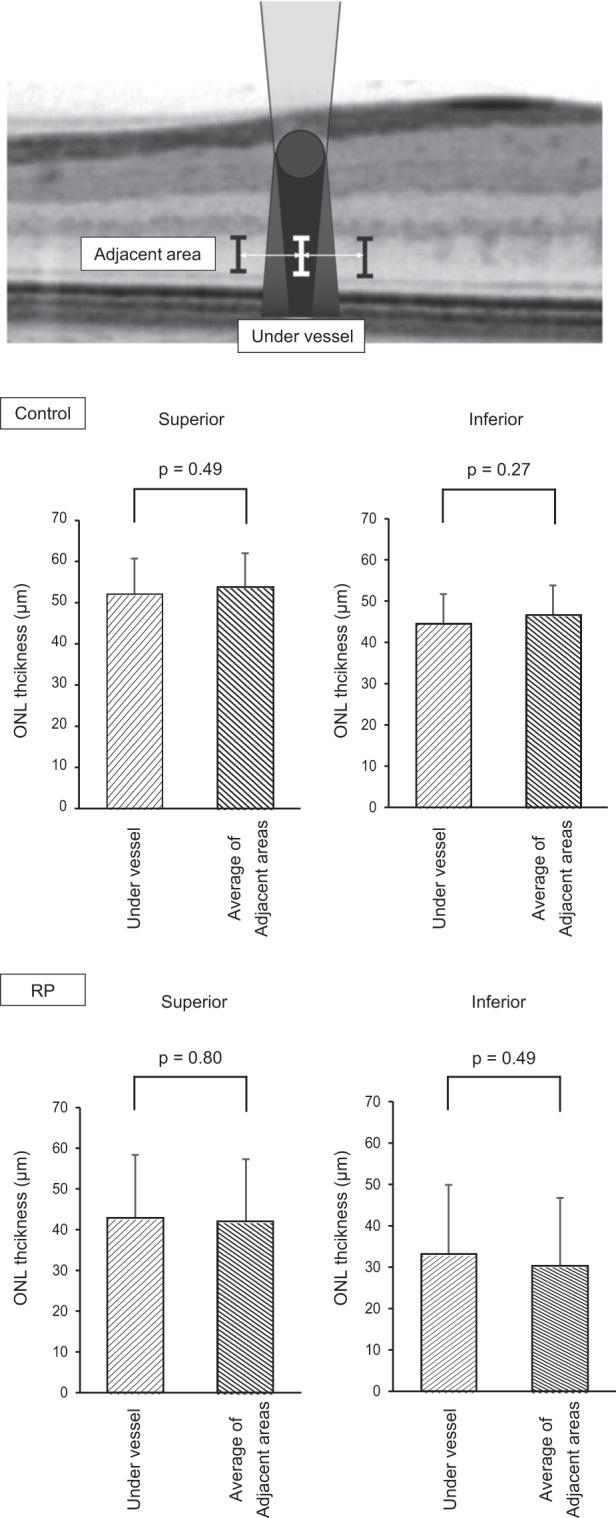
‘Under’ represents the outer nuclear layer (ONL) thickness under the vessel and ‘Adjacent 1 or 2’ shows the ONL in the adjacent light-exposed control areas. In each group, ONL thickness under vessel and in adjacent areas was compared. In both groups, ONL thickness under vessel was not significantly different from the average of control areas in both hemispheres (p = 0.27–0.80).
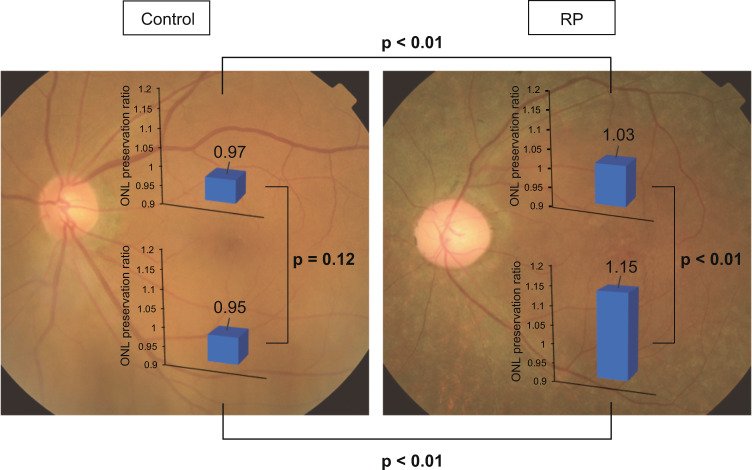
Subsequently, we compared the data on SD-OCT measurements between the superior and inferior retina in each group (Table 1). In controls, ONL thickness was always larger in the superior retina than inferior retina both under vessel and in adjacent areas (p < 0.01), despite no significant difference in vessel diameter (p = 0.60). This tendency was also observed in patients with RP: ONL was thicker in the superior retina both under vessel and in adjacent areas (p = 0.02 and <0.01, respectively), even though the vessel diameter was not different (p = 0.70), and the measured vessel was significantly closer to the fovea in the inferior retina (p < 0.01). Notably, ONL preservation ratio of RP was larger than that of healthy control in both superior and inferior retina (1.03 ± 0.06 vs 0.97 ± 0.04; p < 0.01 and 1.15 ± 0.16 vs 0.95 ± 0.04; p < 0.01, respectively). Whereas ONL preservation ratio did not show a significant difference between the superior and inferior retina in healthy control (0.97 ± 0.04 vs 0.95 ± 0.04; p = 0.12), it was significantly larger in the inferior retina than superior retina in RP group (1.03 ± 0.06 vs 1.15 ± 0.16; p < 0.01) (Fig. 4).
Fig. 4. ONL preservation ratio in superior and inferior retina in healthy controls and patients with RP.
Outer nuclear layer (ONL) preservation ratio in superior retina and inferior retina was compared in each group. The ONL preservation ratio of retinitis pigmentosa (RP) was larger than that of healthy control in both superior and inferior retina (each: p < 0.01). In addition, the ratio was larger in the inferior retina than the superior retina in RP group (p < 0.01).
Furthermore, in patients with RP, the length of EZ from the fovea was measured on SD-OCT vertical imaging. Remarkably, in all patients, EZ length was always shorter in the inferior retina than superior retina. The mean EZ length from the fovea was 3199 ± 1229 μm in the superior retina and 1917 ± 860 μm in the inferior retina (p < 0.01).
Discussion
We investigated the effect of light-induced photoreceptor damage with SD-OCT imaging by measuring ONL thickness under retinal vessels compared to adjacent control areas and the EZ length. We found that the ONL overshadowed by retinal vessels was normally thinner than adjacent area, but it was even thicker than the adjacent control area in RP. Furthermore, in RP group, ONL preservation ratio was significantly larger and the EZ length was shorter in the inferior retina where light stimulation is more intense. These findings may indicate an effect from light-induced damage in patients with RP.
In most mammals, retinal blood vessels circulate in the surface of retina and perfuse the inner retina in contrast to birds and some reptiles [16, 17]. Thus, they interfere with the light path and disturb photoreceptor cells from light reaction. This is described as the most famous flaw during vertebrate eye evolution [18] and the visual field defect due to retinal vessels is called angioscotomas [15]. Previous reports illustrated that angioscotoma is generated not only in photoreceptor layer but also by anatomical rearrangement of geniculocortical inputs [15, 19]. Light deprivation by vascular shadows induces local remodelling of geniculate afferents, in a cortical distribution that corresponds to the trajectory of blood vessels within the retina. The process is regarded as analogous phenomenon to form-deprivation amblyopia. The evidence that vascular shadows affect the visual cortex indicates that the total amount of light exposure under retinal vessels is different enough to produce physiologic changes, and the position of photoreceptor cells overshadowed by the vessels is determined in the early stage of life.
To examine the effect of light-induced damage, we defined the ONL preservation ratio. The ratio represents relative preservation of ONL overshadowed by a retinal vessel, compared to the light-exposed control area. In vertical scan, ONL thickness becomes thinner in proportion to the distance from the fovea, corresponding to the numbers and distribution of photoreceptor cells [20]. Based on this study, it is assumed that ONL thinning towards periphery can be approximated to linear curve in the area where we performed the measurements. In this case, ONL thickness under retinal vessel should be the same as the average of adjacent areas unless it is affected by any factors. Hence, we measured control ONL thickness at both sides of the vessel and averaged them. The measurement was also highly reproducible.
In the current study, normal subjects showed thinner ONL under retinal vessel than the adjacent area (Table 1 and Fig. 4). A mechanical factor such as displacement of photoreceptors due to a large retinal vessel is probably associated with the mechanism. If there is no other factor, the same tendency should have been observed also in patients with RP. However, the ONL preservation ratio was larger than 1.0 in RP group: ONL overshadowed by retinal vessels was thicker than control ONL. While retinal vessel attenuation in RP can affect the measurement, it is unlikely that the vessel attenuation led to the ONL thickening. Therefore, the even thicker ONL under vessel in RP should be the result of some other factors such as protection from light exposure.
We examined the differences between the superior and inferior retina. ONL thickness was measured at points located around 2 mm from the fovea. According to the study about the relative dose distribution of UV or visible light [21], the inferior retina (from 5° to 40°) receives the most intensive dose, and the maximum dose is situated at 20° (4 mm) below the fovea. They estimated that the light intensity relative to the maximum receive is 20% at 2 mm superior to the fovea, whereas 80% at the same position in inferior retina. Thus, 2 mm superior or inferior to the fovea looks small difference, but the amount of light exposure differs by four times. In RP group, ONL preservation ratio was significantly larger in inferior retina than superior, indicating that ONL overshadowed by vessel was especially preserved compared to light-exposed areas because light stimulation was more intense in the inferior retina. Furthermore, all patients with RP showed longer EZ in the superior retina. In fact, EZ in the superior retina was about twice as long as that in the inferior retina. This remarkable inferior-dominant photoreceptor death also supports the effect of light-induced damage. According to a recent study, some retinal genes including EYS cause sector RP, but inferior and nasal predilection is common across the different genotypes [22]. It is speculated that there is a common or down-stream mechanism related to light-induced damage.
Our study had some limitations. First, the sample size was not large. Further studies with larger sample sizes will contribute to a better understanding. Second, measurement errors could occur and influence the consequences, because we measured quite small distances. We analysed average of three independent measurements to minimise the error. The result of ICC test indicates that the measurement was at least reproducible. Third, we used the ideal eye model and did not adjust for individual eyes. However, even in an eye with 30 mm axial length, constant shadows under 50 and 100 µm vessels are calculated as 29.9 and 83.1 µm, respectively. Thus, the measurements would be appropriately done in overshadowed area and control area even in such extreme cases. Finally, the presence or degree of light-induced damage may depend on causative genes or mutations of each patient. RP is clinically and genetically heterogeneous, and the genetic diagnosis is still not easy [23]. For this reason, we included only patients with EYS-mutant RP in order to minimise the genetic factors. Nevertheless, the exact functions of EYS protein and the pathogenic mechanisms underlying EYS-associated RP remain poorly understood [24, 25]. The mechanism of action mediated by light stimulation is still unclear as well. Further studies with another genetically identified case are required for a better understanding.
In conclusion, patients with EYS-mutant RP exhibited obviously dominant photoreceptor death in the inferior retina with SD-OCT imaging. They also showed the relative ONL preservation under retinal vessels compared to the adjacent control area, particularly in the inferior retina where light exposure is more intense. These results suggest that longitudinal environment light exposure may be associated with the photoreceptor death in patients with RP, although the effect is clinically minimal.
Summary
What was known before
Light-induced photoreceptor damage was shown in animal models. However, there was no clear evidence that the phenomenon exists in human subjects.
What this study adds
Using optical coherence tomography, this study showed that outer nuclear layer thickness is relatively preserved under retinal vessels in retinitis pigmentosa.
Supplementary information
Author contributions
Design of the study: YO, AO, AU and AT; data collection: YO, AO, MM, MO, TH, SN and HOI; analysis and interpretation: YO, AO, SN and AT; manuscript preparation: YO, AO and SN; critical appraisal of the manuscript: MM, AU, MO, TH, HOI and AT; funding: AO; and administrative support: AT.
Funding
This study was supported by JSPS KAKENHI (17H06820 and 19K09929, Japan Society for the Promotion of Science, Tokyo, Japan). The authors alone are responsible for the content and writing of the paper.
Compliance with ethical standards
Conflict of interest
YO, AO, MM, AU, MO, TH, SN and HOI: none; and AT: grant from Canon (Tokyo, Japan) and JFC sales (Tokyo, Japan) and personal fee from Canon and Nidek (Gamagori, Japan).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01578-y.
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Venturini G, Rose AM, Shah AZ, Bhattacharya SS, Rivolta C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet. 2012;8:e1003040. [DOI] [PMC free article] [PubMed]
- 3.Hamel C. Retinitis pigmentosa. Orphanet. J. Rare Dis. 2006;1:40. [DOI] [PMC free article] [PubMed]
- 4.Oishi A, Noda K, Birtel J, Miyake M, Sato A, Hasegawa T, et al. Effect of smoking on macular function and retinal structure in retinitis pigmentosa. Brain Commun. 2020;2:fcaa117. [DOI] [PMC free article] [PubMed]
- 5.Paskowitz DM. Light and inherited retinal degeneration. Br J Ophthalmol. 2006;90:1060–6. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cideciyan AV, Jacobson SG, Aleman TS, Gu D, Pearce-Kelling SE, Sumaroka A, et al. In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci USA. 2005;102:5233–8. doi: 10.1073/pnas.0408892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright AF. For want of a disc, the cell was lost…. Nat Med. 2000;6:508–10. doi: 10.1038/74985. [DOI] [PubMed] [Google Scholar]
- 8.Naash ML, Peachey NS, Li ZY, Gryczan CC, Goto Y, Blanks J, et al. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Investig. Ophthalmol. Vis. Sci. 1996;37:775–82. [PubMed]
- 9.Orlans HO, Merrill J, Barnard AR, Charbel Issa P, Peirson SN, MacLaren RE. Filtration of short-wavelength light provides therapeutic benefit in retinitis pigmentosa caused by a common rhodopsin mutation. Investig Opthalmology Vis Sci. 2019;60:2733. doi: 10.1167/iovs.19-26964. [DOI] [PubMed] [Google Scholar]
- 10.Berson EL. Light deprivation and retinitis pigmentosa. Vis Res. 1980;20:1179–84. doi: 10.1016/0042-6989(80)90057-7. [DOI] [PubMed] [Google Scholar]
- 11.Miyake Y, Sugita S, Horiguch M, Yagasaki K. Light deprivation and retinitis pigmentosa. Am J Ophthalmol. 1990;110:305–6. doi: 10.1016/S0002-9394(14)76350-0. [DOI] [PubMed] [Google Scholar]
- 12.Triolo G, Pierro L, Parodi MB, De Benedetto U, Gagliardi M, Manitto MP, et al. Spectral domain optical coherence tomography findings in patients with retinitis pigmentosa. Ophthalmic Res. 2013;50:160–4. doi: 10.1159/000351681. [DOI] [PubMed] [Google Scholar]
- 13.Oishi M, Oishi A, Gotoh N, Ogino K, Higasa K, Iida K, et al. Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation sequencing. Investig Opthalmology Vis Sci. 2014;55:7369. doi: 10.1167/iovs.14-15458. [DOI] [PubMed] [Google Scholar]
- 14.Gullstrand A, Kries J. Appendices II and IV in Helmholtz’s Handbuch der Physiologischen Optik, English translation ed. JP Southall, Oxford: Butterworth-Heinemann; 1909. p. 301–358, 382–415.
- 15.Adams DL. Shadows cast by retinal blood vessels mapped in primary visual cortex. Science. 2002;298:572–6. doi: 10.1126/science.1074887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brach V. The functional significance of the avian pecten: a review. Condor. 1977;79:321. doi: 10.2307/1368009. [DOI] [Google Scholar]
- 17.Country MW. Retinal metabolism: a comparative look at energetics in the retina. Brain Res. 2017;1672:50–57. doi: 10.1016/j.brainres.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth C, Le Page M. Evolution’s greatest mistakes. N Sci. 2007;195:36–39. doi: 10.1016/S0262-4079(07)62033-8. [DOI] [Google Scholar]
- 19.Giacomantonio CE, Goodhill GJ. The effect of angioscotomas on map structure in primary visual cortex. J Neurosci. 2007;27:4935–46. doi: 10.1523/JNEUROSCI.1261-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagci AM, Shahidi M, Ansari R, Blair M, Blair NP, Zelkha R. Thickness profiles of retinal layers by optical coherence tomography image segmentation. Am J Ophthalmol. 2008;146:679–87. doi: 10.1016/j.ajo.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz L, Boëlle P, D’hermies F, Ledanois G, Virmont J. Blue light dose distribution and retinitis pigmentosa visual field defects: an hypothesis. Med Hypotheses. 2003;60:644–9. doi: 10.1016/S0306-9877(02)00391-2. [DOI] [PubMed] [Google Scholar]
- 22.Georgiou M, Grewal PS, Narayan A, Alser M, Ali N, Fujinami K, et al. Sector retinitis pigmentosa: extending the molecular genetics basis and elucidating the natural history. Am J Ophthalmol. 2021;221:299–310. doi: 10.1016/j.ajo.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias MF, Joo K, Kemp JA, Fialho SL, da Silva Cunha A, Woo SJ, et al. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog Retin Eye Res. 2018;63:107–31. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Abd El-Aziz MM, Barragan I, O’Driscoll CA, Goodstadt L, Prigmore E, Borrego S, et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008;40:1285–7. doi: 10.1038/ng.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messchaert M, Dona M, Broekman S, Peters TA, Corral-Serrano JC, Slijkerman RWN, et al. Eyes shut homolog is important for the maintenance of photoreceptor morphology and visual function in zebrafish. PLoS One. 2018;13:e0200789. doi: 10.1371/journal.pone.0200789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.