Peripherally administered capsaicin increases the depolymerization of axonal microtubules leading to axonal ablation and long-lasting analgesia for chronic pain from trigeminal neuropathy in mice.
Keywords: Capsaicin, Analgesia, TRPV1, Microtubule, Paclitaxel, Neuropathic pain
Abstract
Capsaicin is a specific agonist of transient receptor potential vanilloid 1 (TRPV1), which is enriched in nociceptors. Capsaicin not only produces acute pain but also leads to long-lasting analgesia in patients with chronic pain. Although capsaicin-induced TRPV1 and Ca2+/calpain-dependent ablation of axonal terminals is necessary for long-lasting analgesia, the mechanisms underlying capsaicin-induced ablation of axonal terminals and its association with analgesia are not fully understood. Microtubules are composed of tubulin polymers and serve as a main axonal cytoskeleton maintaining axonal integrity. In this study, we hypothesized that capsaicin would increase the depolymerization of microtubules and lead to axonal ablation and analgesia for trigeminal neuropathic pain. Paclitaxel, a microtubule stabilizer, decreased capsaicin-induced ablation of axonal terminals in time-lapsed imaging in vitro. Capsaicin increases free tubulin in dissociated sensory neurons, which was inhibited by paclitaxel. Consistently, subcutaneous injection of paclitaxel prevented capsaicin-induced axonal ablation in the hind paw skin. Capsaicin administration to the facial skin produced analgesia for mechanical hyperalgesia in mice with chronic constriction injury of the infraorbital nerve, which was prevented by the coadministration of paclitaxel and capsaicin. Whole-mount staining of facial skin showed that paclitaxel reduced capsaicin-induced ablation of peptidergic afferent terminals. Despite the suggested involvement of TRPV1 Ser801 phosphorylation on microtubule integrity, capsaicin-induced analgesia was not affected in TRPV1 S801A knock-in mice. In conclusion, capsaicin-induced depolymerization of axonal microtubules determined capsaicin-induced ablation of nociceptive terminals and the extent of analgesia. Further understanding of TRPV1/Ca2+-dependent mechanisms of capsaicin-induced ablation and analgesia may help to improve the management of chronic pain.
1. Introduction
Posttraumatic trigeminal neuropathic pain often occurs because of facial trauma or iatrogenic reasons such as a third molar extraction.14 Because posttraumatic trigeminal neuropathic pain is more resistant to first-line therapy compared with other neuropathic pain conditions,23,30 developing an effective strategy to treat these conditions with fewer adverse side effects is necessary.
Capsaicin is an ingredient of chili peppers and a specific agonist of transient receptor potential vanilloid 1 (TRPV1), a Ca2+-permeable ion channel enriched in nociceptors.13 When capsaicin is applied to the skin it induces a burning pain in animals and humans. Interestingly, such nociception is often followed by prolonged analgesia that attenuates preexisting, persistent pain. Currently, the topical application of capsaicin (an 8% patch) is approved for treating postherpetic neuralgia, HIV-associated neuropathy, and diabetic neuropathy.1,3 Topical capsaicin attenuates not only self-reported pain but also mechanical allodynia with greater efficacy than pregabalin.15 Injectable capsaicin is also under development for the treatment of chronic pain from knee osteoarthritis.6 Although the peripheral administration of capsaicin provides nonaddictive, nonopioidergic treatment for chronic pain, mechanisms underlying capsaicin-induced analgesia are poorly understood, and further improvement of the therapy is hindered.
Although multiple hypotheses have been proposed to explain the analgesic effects of topical capsaicin, structural ablation of TRPV1-expressing afferent terminals localized to the application site is postulated to be a major contributor to long-lasting analgesia.1,3,11 Recent preclinical studies have established the causal contribution of capsaicin-induced ablation of afferent terminals to prolonged analgesia for trigeminal neuropathic pain.42,43 In mice with chronic constriction injury of the infraorbital nerve (ION-CCI), a single subcutaneous injection of capsaicin to the face produces 2-week long analgesia for mechanical allodynia and ongoing pain.43 Ca2+-dependent protease calpain mediates capsaicin-induced ablation of TRPV1-expressing cutaneous afferents, and pharmacological inhibition of calpain prevents capsaicin-induced analgesia for neuropathic pain.42,43 Therefore, capsaicin-induced ablation of afferent terminals is an initial step necessary for producing analgesia.
Capsaicin-induced axonal ablation is primarily mediated by a Ca2+ influx through the TRPV1 pore,42 suggesting that Ca2+-dependent events occur at the immediate vicinity of TRPV1. Interestingly, TRPV1 is known to be directly bound to microtubules, which stabilizes them.19 The phosphorylation of TRPV1 Ser801 within the carboxy-terminal domain interferes with the binding of microtubules with TRPV1 and destabilizes the microtubule.22 Furthermore, mechanisms of microtubule disruption also involve Ca2+-dependent processes. Microtubules are matured by polymerization of multiple tubulin dimers, but high concentrations of Ca2+ enhance depolymerization, presumably by increasing the rate of guanosine triphosphate hydrolysis.32 Axonal microtubules are the stiffest structures that maintain structural integrity and molecular traffic,17 and their disruption leads to axonal degeneration.28 However, it is not known whether microtubule depolymerization mediates capsaicin-induced axonal ablation and affects capsaicin-induced analgesia for neuropathic pain.
In this study, we hypothesized that capsaicin increases depolymerization of microtubules, which induce axonal ablation and, hence, mediates analgesia for trigeminal neuropathic pain. To test this hypothesis, we investigated the effects of microtubule stabilization on capsaicin-induced axonal ablation and analgesia for trigeminal neuropathic pain in mice.
2. Methods
2.1. Animals
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were performed under the University of Maryland–approved Institutional Animal Care and Use Committee protocol. We used male and female mice. The number of male and female mice used for the in vivo assays is indicated in the figure legend. For in vitro assays using dissociated dorsal root ganglia (DRG), the neurons were obtained from both male and female mice, and dissociated neurons that came from multiple mice were pooled for the assays regardless of sex. C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME; stock no. 000664). To visualize TRPV1-lineage axons, we used a genetically engineered mouse model that selectively expresses a membrane-bound fluorophore in TRPV1-expressing neurons. We crossed 2 lines of genetically engineered mice. The first was TRPV1Cre (TRPV1tm1(cre)Bbm/J; The Jackson Laboratory, Stock No. 017769) in which Cre recombinase is expressed under the control of the TRPV1 promoter.9 TRPV1Cre mice10 express Cre recombinase under the control of the TRPV1 promoter and, when crossed with a Cre-dependent reporter line, label afferents that have expressed TRPV1 at any stage of development (TRPV1-lineage). Of these TRPV1-LN neurons, approximately half express TRPV1 in adult sensory ganglia.10 The second line was ROSAmT/mG (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; The Jackson Laboratory, Stock No. 007676), which constitutively expresses tdTomato in every cell without Cre recombination. The offspring express membrane-tethered enhanced green fluorescence protein (GFP) in neurons expressing Cre recombinase, which permits for the identification of TRPV1-lineage neurons by GFP signals (TRPV1GFP mice). Homozygote CalcaCre mice (Jackson laboratory; stock No. 033168), in which Cre recombinase is knocked into the Calca locus, were used as Calca knockout mice for the validation of antibodies.8 The generation of TRPV1 S801A mice has been described previously.25 Littermate, wild-type (WT) mice were used as controls. All experiments were performed on adult mice (22-30 g) unless otherwise indicated. Animals were group-housed under standard conditions with ad libitum access to water and food.
2.2. Culture of dorsal root ganglion neurons in microfluidic chambers
We cultured DRG neurons in microfluidic devices (Xona Microfluidics, Inc, Research Triangle Park, NC) following the manufacturer's instructions as described previously.42 In this system, soma and axons of DRG neurons are segregated and independently manipulated. Autoclaved rectangular glass coverslips were coated with poly-d-ornithine and laminin. Microfluidic devices (SND150 with 150 μm microgrooves or TCND500 with 500 μm microgrooves) were autoclaved, attached to the glass coverslip, and kept in 100-mm culture plates. Primary sensory neurons were dissociated from adult mice as described previously.42 Mice (4-9 weeks old) were anesthetized using a cocktail of ketamine (80 mg/kg) and xylazine (10 mg/kg). Dorsal root ganglia were dissected out and collected in cold Puck saline (171 mM NaCl, 6.7 mM KCl, 1.4 mM Na2HPO4, 0.5 mM KH2PO4, and 6.0 mM glucose, pH 7.3). The ganglia were incubated in 5 mL of Dulbecco's modified Eagle medium (DMEM)/F12 medium containing collagenase type IV (1 mg/mL) at 37°C for 30 minutes. The ganglia were incubated for an additional 15 minutes after the addition of trypsin (0.25%) and ethylenediaminetetraacetic acid EDTA) (0.025%). The tissues were triturated with flame-polished Pasteur pipettes. After spinning down, the neurons were resuspended in DMEM/F12 medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/fungizone and plated onto the chamber. Dorsal root ganglion neurons dissociated from 1 mouse were plated onto 5 chambers. The neurons were maintained in DMEM/F12 containing 10% FBS and 1% penicillin/streptomycin/fungizone at 37°C in a 5% CO2 incubator. Culture medium was replaced every 3 days.
2.3. Ca2+ imaging assay
To monitor changes in cytosolic Ca2+, we performed ratiometric Ca2+ imaging using Fura-2AM as previously described.44 One day after dissociation, DRG neurons were loaded with Fura-2AM for 40 minutes at 37°C in calcium imaging buffer (130 mM NaCl, 3 mM KCl, 0.5 mM MgCl2, 0.9 mM CaCl2, 10 mM HEPES, 10 mM sucrose, and 1.2 mM NaHCO3 (pH 7.45, 320 mOsm adjusted with mannitol)). Ratiometric Ca2+ imaging was performed using an inverted fluorescence microscope (Eclipse Ti; Nikon Instrument, Melville, NY), an optical filter changer (Lambda 10-B; Sutter Instrument, Novato, CA), and a cooled CCD camera (Nikon Instruments, Inc, Tokyo, Japan). After a 15-minute wash period for de-esterification, dual images (excitation at 340 nm and 380 nm; emission at 510 nm) were collected every 2 seconds using NIS-Elements (Nikon Instruments). The Fura response was defined as the ratio of background-subtracted emissions measured during excitation at 340 and 380 nm. Data in each experimental group were obtained from at least 4 independent experimental repeats.
2.4. Free tubulin assay and western blot analysis
The amount of free tubulin was evaluated through assays as previously described34,46 using a commercially available kit (Cytoskeleton, Inc, Denver, CO; Cat # BK038). DRG neurons were dissociated and plated onto a 12-well plate. The dissociated neurons were harvested using lysis and microtubule-stabilizing buffer containing 100 µM guanosine triphosphate, 1 mM adenosine triphosphate, and a protease inhibitor cocktail at 37°C. The lysate was centrifuged for 5 minutes at 2000×g at 37°C. Supernatants were centrifuged at 100,000×g for 30 minutes at 37°C. The supernatants were then immediately moved onto ice. After measuring protein concentrations, 5x sodium dodecyl sulfate loading buffer was added, which was heated to 95°C for 2 minutes, and loaded onto a 4% to 12% Bis-Tris NuPAGE gel. After electroporation, the gel was transferred to a polyvinylidene difluoride membrane (at 500 mA for 1.5 hours on ice). The membrane was probed using anti-β-tubulin antibody (Cat #: ATN01) diluted to 1:2000. As a control, the membrane was also probed with an antiglyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody diluted to 1:20000. After incubating with secondary antibodies, bands were visualized using enhanced chemiluminescence reagents.
2.5. Immunohistochemical analysis of transient receptor potential vanilloid 1–lineage afferent terminals
To assess capsaicin-induced ablation of TRPV1-lineage nerve terminals in vivo, we performed an immunohistochemical analysis in the skin after the injection of capsaicin. In adult TRPV1GFP mice, hind paws were injected with either vehicle (20 µL; 25% PEG300 and 75% H2O) or capsaicin (10 µg in 20 µL of the vehicle). For consistency, injections were performed under anesthesia (80 mg/kg ketamine and 10 mg/kg xylazine). Animals were euthanized and perfused transcardially using 3.7% formaldehyde in phosphate-buffered saline. The skin was cryoprotected in sucrose and cryosectioned (30 µm). Immunohistochemistry was performed using a specific antibody against GFP (1:1000; rabbit polyclonal; A11122, Invitrogen, Thermo Fisher Scientific, Waltham, MA).12
2.6. Whole-mount immunohistochemistry of facial skin
The protocol for whole-mount immunohistochemistry was performed as previously described27 with modifications. Connective tissues and hairs present in the facial skin were removed using a pair of blunt forceps, and the skin was fixed in 3.7% formalin in 0.1 M phosphate buffer (PBS) overnight at 4°C. The next day, the skin was washed with PBS with 1% Triton X-100 (PBST; pH 7.4) for 30 minutes ten times at room temperature. The primary antibody against calcitonin gene–related peptide (CGRP) (1:1000; rabbit polyclonal; MilliporeSigma, Burlington, MA) was diluted in a blocking buffer (75% PBST, 20% dimethyl sulfoxide; 5% normal goat serum). The skin tissues were incubated for 4 days on a shaker at room temperature and washed with PBST for 30 minutes ten times. The secondary antibody (1:1000; goat anti-rabbit Cy3) was prepared in the blocking buffer, and the tissues were incubated for 4 days at room temperature. The tissues were washed with PBST (for 30 minutes ten times at room temperature) and subsequently treated with 50%, 80%, and 100% methanol for 5 minutes, 5 minutes, and 20 minutes, respectively (2 times each). The skin was then treated with BABB (benzyl alcohol and benzyl benzoate; 1:2 mixture) for 20 minutes at room temperature. Subsequently, the skin was transferred onto microscopic slides, covered with coverslips using BABB as the mounting medium, and stored at 4°C for confocal microscopy.
2.7. Image acquisition and quantification of afferent terminals in the skin
For quantifying epidermal fibers from the GFP-stained hind paw skin sections, Z-stack images were captured using an inverted epifluorescence microscope (Axiovert 200M microscope; ZEISS, Jena, Germany) with Apotome. Three images per tissue section, 5 sections per hind paw (a total of 15 images from 1 hind paw), were examined. The number of epidermal fibers passing through the epidermis–dermis junction (under the nuclei of basal epithelial cells identified by DAPI staining) was counted in each image, and values from 15 images were averaged.
The whole-mount stained skin was imaged using a Nikon W1 Spinning Disk confocal microscope at X10 objective. Consecutive Z-stack images were captured at 0.5-μm depth intervals from the selected skin spots until the fluorescence signal became undetectable. The Z projection was applied to a stack of all imaging sections, and single merged imaging of the skin spot was created. The raw images were processed to remove nonspecific fluorescence in the skin. All of the images were quantified and analyzed with ImageJ software. For all images, the background grayscale was eliminated, and binary images were generated. CGRP+ area and the entire region of interest area were measured, and the ratio of the CGRP+ area/region of interest area was calculated.
2.8. Trigeminal neuropathic pain model
To produce the trigeminal neuropathic pain model, we performed ION-CCI.43 Loose ligatures of the unilateral infraorbital nerve were performed using an intraoral approach to keep the orofacial skin intact for the measurement of mechanical sensitivity. Mice were anesthetized with an intraperitoneal injection of mixed ketamine (100 mg/kg) and xylazine (10 mg/kg). The head of the mouse was positioned in lateral recumbency, and 4 limbs were fixed on the table with adhesive tape. A 5 to 7-mm long incision was made along the gingivobuccal margin in the buccal mucosa, beginning immediately next to the first molar. The ION was freed from the surrounding connective tissues by a glass rod and visualized using a surgical microscope. At 3 to 4 mm from the nerve where its branches emerge from the infraorbital fissure, the ION was loosely tied with 2 chromic gut (4.0) ligatures, 2 mm apart. The incision was closed using veterinary tissue glue. The sham-operated mice received only unilateral nerve exposure without ligatures.
2.9. Behavioral assay for mechanical sensitivity
All behavioral tests were conducted under blind conditions. To reduce any effects of restraint, the mouse was habituated for 20 minutes/day for 3 days to stand on or lean against a regular leatherwork hand glove worn by the experimenter. The von Frey assay was performed using up–down methods as previously described.43 A series of calibrated von Frey filaments with bending forces ranging from 0.008 to 4 g were applied to the orofacial skin. For assessing primary or secondary hyperalgesia, these filaments were applied to the skin just behind the vibrissa pad within the infraorbital territory (V2). A brisk or active withdrawal of the head from the probing filament was defined as a nocifensive response. Each von Frey filament was applied 5 times at intervals of a few seconds. The response frequencies [(the number of responses/number of stimuli) × 100%] to a range of von Frey filament forces were determined, and S-R curves were plotted. Mechanical thresholds were measured as the lowest intensity at which animals escaped from the stimuli over 50% of chance. In the mouse ION-CCI model, mechanical hyperalgesia and allodynia develop in the maxillary V2 skin within 2 weeks and are maintained for many months.43 To determine the effects of capsaicin injection on mechanical hyperalgesia, capsaicin was injected on day 14 after ION-CCI. A single bolus of either vehicle (20 µL, 25% PEG300 and 75% H2O) or capsaicin (10 µg in 20 µL of the vehicle) was subcutaneously injected into the skin just behind the vibrissa pad in which von Frey testing was performed.
2.10. Drugs
Paclitaxel was obtained from Tocris Bioscience (Bristol, United Kingdom). All other chemicals and drugs were purchased from Sigma-Aldrich (St. Louis, MO). For testing the effects of pharmacological manipulations on capsaicin-induced ablation of afferent terminals in vivo, we injected inhibitors into one hind paw and vehicle into the other hind paw before capsaicin injection. The animals were then euthanized after 6 to 7 days for immunohistochemical assessment of intraepidermal fiber density in the hind paw glabrous skin. To determine the effects of paclitaxel, we injected 10 µL of 2 mg/mL paclitaxel. To determine the effects of capsaicin injection on mechanical hyperalgesia in mice with ION-CCI, capsaicin was injected on day 14 after ION-CCI. A single bolus of either vehicle (20 µL; 25% PEG300 and 75% H2O) or capsaicin (10 µg in 20 µL of the vehicle) was subcutaneously injected into the skin just behind the vibrissa pad in which von Frey testing was performed.
2.11. Data analysis
Statistical analyses were performed using Prism (Graphpad Software Inc, San Diego, CA). Mean values between the 2 groups were compared using the Student t-test. Comparisons among the 4 groups with 2 variables were performed using a 2-way analysis of variance followed by the Sidak post hoc test. Proportions of unaffected fibers (survival curves) in different groups were compared using the log-rank test. The data are expressed as mean ± SEM, and n numbers in experimental groups were included in the graphs either inside bars or in the parentheses next to group names.
3. Results
3.1. Paclitaxel decreased capsaicin-induced ablation of axonal terminals in vitro
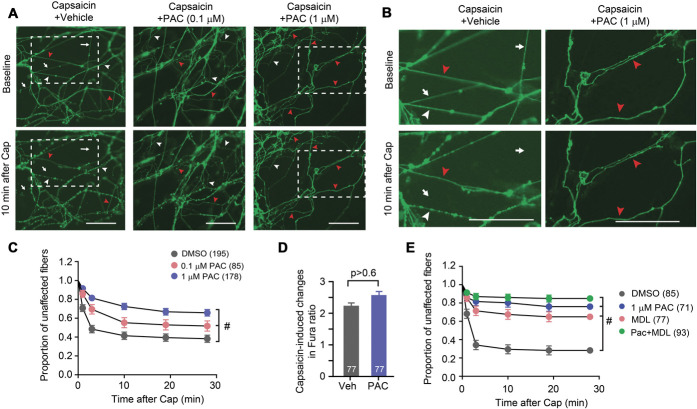
We established an in vitro model for the real-time monitoring of axonal ablation using a multicompartmental microfluidic chamber and dissociated sensory neurons from TRPV1GFP mice. Time-lapse imaging of GFP was performed in an axonal compartment before and after the application of capsaicin (100 µM). GFP-positive axons began to exhibit blebbing or beading (white arrowheads in Fig. 1A), disruption of continuity, and fragmentation (arrows in Fig. 1A) as early as 1 minute after capsaicin application. Approximately 60% of GFP-positive axons were affected after 10 minutes (Figs. 1A and B). To determine whether stabilization of microtubules altered capsaicin-induced axonal ablation, we tested the effects of paclitaxel. Paclitaxel is a widely used chemotherapeutic agent, which exerts its effects by binding to microtubules to stabilize them against depolymerization.18,24 In our study, we determined whether capsaicin-induced ablation was influenced by increased stability from pretreatment with paclitaxel. The pretreatment of axons with paclitaxel for 1 hour significantly attenuated capsaicin-induced ablation of GFP-positive axons (Figs. 1A and B). Pretreatment with paclitaxel slowed down the rate and reduced the extent of degeneration in a concentration-dependent manner, and only 30% of GFP-positive axons were affected by capsaicin on coapplication with 1 µM paclitaxel. The inhibitory effect of paclitaxel was not due to interference with TRPV1 functioning, as evidenced by a Ca2+ imaging assay showing that pretreatment with 1 µM paclitaxel did not attenuate the capsaicin-evoked Ca2+ increase in sensory neurons (Fig. 1C). It has been previously shown that MDL28170, an inhibitor of calpains, prevents capsaicin-induced axonal ablation.42 Pretreatment with paclitaxel or MDL alone was sufficient to prevent capsaicin-induced, degenerative changes to a comparable degree, suggesting the 2 compounds possibly affect common mechanisms (Fig. 1E).
Figure 1.
Paclitaxel inhibits capsaicin-induced axonal ablation. (A) Representative time-lapsed imaging of axonal terminals from microfluidic chambers (MFCs). Fluorescence signals from GFP were monitored before and 10 minutes after the axonal compartment was exposed to capsaicin (100 µM) plus vehicle or paclitaxel (PAC), 0.1 µM or 1 µM as indicated. Scale bar, 50 µm; arrows, examples of ablated fibers; white arrow heads, beaded fibers; red arrow heads, fibers not affected. Dotted boxes, the areas shown in panel B. (B) Enlarged images of the areas indicated in panel A. Scale bar, 50 µm. (C) Proportion of unaffected fibers after the application of 100 µM capsaicin (Cap) and paclitaxel. The survival proportion is plotted as mean ± SEM. Numbers in parentheses represent the number of individual GFP-expressing fibers examined in each group. Results based on the log-rank test, #P < 0.0001. (D) Capsaicin-induced change of the Fura ratio in the soma of dissociated sensory neurons under the indicated conditions. Numbers within bars represent the number of neurons quantified in each group. Results base on the Student t-test. (E) Proportion of unaffected fibers after the application of 100 µM capsaicin with DMSO, 1 µM paclitaxel, 10 µM MDL28170, and paclitaxel plus MDL28170. The survival proportion is plotted as mean ± SEM. Numbers in parentheses represent the number of individual GFP-expressing fibers examined in each group. Results are based on the log-rank test, #P < 0.0001. GFP, green fluorescence protein.
3.2. Capsaicin decreased microtubule stability in sensory neurons
To further determine whether capsaicin-induced activation of TRPV1 leads to microtubule destabilization, we analyzed the amount of unpolymerized free tubulin in dissociated sensory neurons (Fig. 2). One hour pretreatment of dissociated DRG with paclitaxel reduced the amount of free tubulin, which confirmed the stabilizing effects of paclitaxel on tubulin. Exposure of dissociated neurons to capsaicin (10 µM) increased the amount of free tubulin, suggesting that capsaicin enhanced the depolymerization of tubulin. This effect was suppressed by pretreatment with paclitaxel (1 µM), which is consistent with its inhibitory effects on capsaicin-induced ablation of the nerve fiber.
Figure 2.
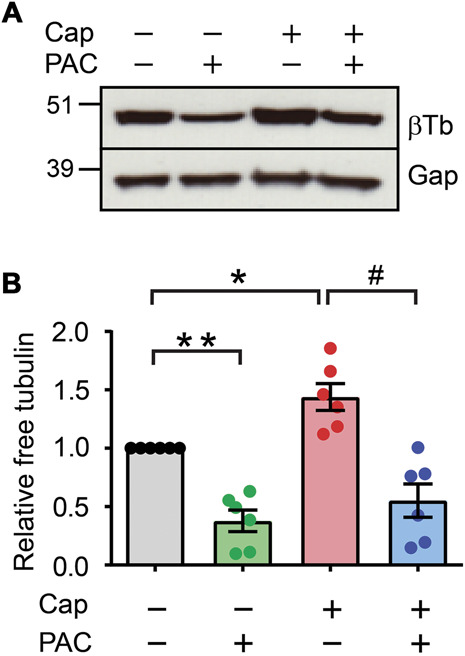
Paclitaxel decreases capsaicin-induced depolymerization of microtubules in sensory neurons. (A) The amount of free tubulin in dissociated sensory neurons from C57BL/6 mice. The neurons were exposed to capsaicin (Cap; 100 µM; 30 minutes), paclitaxel (PAC; 1 µM; 30 minutes before and during capsaicin), or vehicle controls. Unpolymerized tubulin was isolated from polymerized tubulin in a microtubule stabilization buffer at 37°C using a microtubule assay kit. Western blot was performed using antibodies against β-tubulin (βTb) followed by GAPDH (Gap). (B) Quantification of relative free tubulin to Veh/Veh. *P < 0.05; **0.005; ****P < 0.0001 in the Bonferroni posttest after 1-way ANOVA. Numbers within bars represent the n of each group. ANOVA, analysis of variance.
3.3. Paclitaxel decreased capsaicin-induced ablation of epidermal transient receptor potential vanilloid 1–lineage axons in vivo
We determined the effects of microtubule stabilization on capsaicin-induced ablation of axonal terminals in vivo. In the hind paws of TRPV1GFP mice, we injected capsaicin + paclitaxel or capsaicin + vehicle subcutaneously into the right and left hind paws, respectively. After 7 days, the mice were euthanized, and sections of plantar skin were immunostained using a GFP antibody (Figs. 3A and B). The number of intraepidermal fibers in the skin injected with capsaicin plus paclitaxel was significantly greater than the number in the skin injected with capsaicin plus vehicle. These results are consistent with the inhibitory effects of paclitaxel on capsaicin-induced axonal ablation in vitro.
Figure 3.
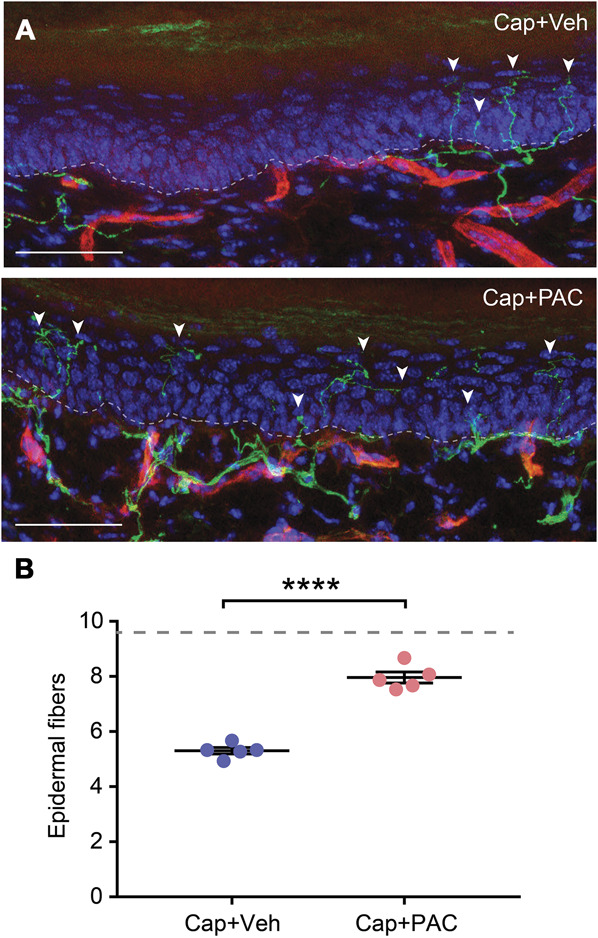
Paclitaxel decreases capsaicin-induced ablation of epidermal nerve fibers in the glabrous skin of the hind paw. (A) Examples of naive hind paw skin or hind paw skin at 7 days after the intraplantar injection of capsaicin (CAP) (10 μg in 20 μL of the vehicle) plus vehicle or capsaicin plus paclitaxel (PAC) (20 ug/10 uL) in adult transient receptor potential vanilloid 1 (TRPV1)GFP mice. Green signals represent GFP labeled by the specific antibody. Red signals represent ubiquitously expressed tdTomato. Blue signals represent DAPI. The dotted line demarcates the epidermis–dermis junction under basal epithelial cells. Arrowheads indicate counted epidermal nerve fibers. Scale bar, 50 μm. (B) Intraepidermal GFP-expressing nerve terminals were quantified. N = 5 in each group (3 males and 2 females/group). ****P < 0.0001 in the Student t-test. The dotted line represents the average number of epidermal fibers in the naive hind paw (9.6) in our previous study. GFP, green fluorescence protein.
3.4. Paclitaxel decreased capsaicin-induced analgesia for mechanical hyperalgesia in trigeminal neuropathy
In a previous study, we showed that capsaicin-induced ablation in the facial skin is necessary for capsaicin-induced analgesia for mechanical hyperalgesia in mice with ION-CCI.43 Because we found that stabilization of microtubules using paclitaxel decreased capsaicin-induced ablation of axonal terminals, we tested whether paclitaxel can prevent capsaicin-induced analgesia. Unilateral ION-CCI, but not a sham, induced robust mechanical hyperalgesia in the maxillary V2 region in the ipsilateral, but not in the contralateral, side. On a single injection of paclitaxel or vehicle into the facial skin, mechanical sensitivity was not significantly different in either the sham or ION-CCI group in the ipsilateral and contralateral sides for 2 weeks after injection (Figs. 4A and B). In a separate group of mice, 2 weeks after ION-CCI, we injected capsaicin (10 µg) into the maxillary V2 skin by subcutaneous injection under anesthesia. Mechanical sensitivity was tested after 3 and 7 days. A single injection of capsaicin in mice with ION-CCI produced a significant increase in the mechanical threshold of V2 skin after 3 days and 7 days (Fig. 4). Capsaicin did not affect the mechanical threshold in the sham group or on the contralateral side. This is consistent with the analgesic effects of capsaicin on mechanical hyperalgesia in mice with ION-CCI.43 By contrast, coadministration of capsaicin with paclitaxel did not increase the mechanical threshold and effectively prevented capsaicin-induced analgesia. These results suggest that a single, local administration of paclitaxel alone to the facial skin does not produce or exacerbate mechanical hyperalgesia under our conditions, but the combined administration of paclitaxel and capsaicin prevents the analgesic effects of capsaicin.
Figure 4.
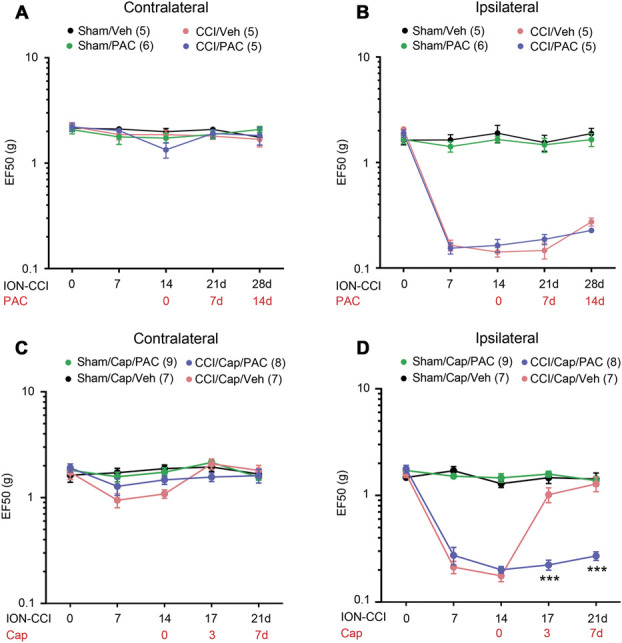
Paclitaxel decreases capsaicin-induced analgesia for mechanical hyperalgesia in mice with chronic constriction injury of the infraorbital nerve (ION-CCI). Changes in mechanical sensitivity in the contralateral (A and C) or ipsilateral (B and D) side of skin behind the vibrissa pad within the infraorbital territory (V2) after a sham or ION-CCI procedure. Paclitaxel (PAC; 40 µg in 20 µL) or vehicle (Veh) alone (A and B) or a combination of capsaicin (Cap; 10 µg) and PAC or Veh (C and D) were injected 2 weeks after surgery. (A–B) N = 6 in sham/PAC (3 males and 3 females) and N = 5 in the other groups (3 males and 2 females). (C–D) N = 6 in each group (3 males and 3 females/group). Two-way repeated measures ANOVA followed by the Sidak posttest; ***P < 0.001 (vs. CCI/Cap/Veh). ANOVA, analysis of variance.
3.5. Paclitaxel decreased capsaicin-induced ablation of nerve terminals in the facial skin
Because quantifying epidermal nerve terminals in hairy skin sections was challenging, we performed whole-mount staining of facial skin to assess the extent of capsaicin-induced ablation of afferent terminals in the facial skin. We evaluated the density of CGRP-containing nerves to assess capsaicin-induced ablation of cutaneous afferents because TRPV1 and CGRP are highly colocalized. The specificity of the primary antibody against CGRP was validated by comparing the immunolabeling in trigeminal ganglia from WT and CalcaCre/Cre mice, in which the Calca gene was disrupted (Fig. 5A). Eight facial skin samples obtained from 2 groups of mice (ION-CCI injected with capsaicin plus paclitaxel, CCI/Cap/Pac and ION-CCI injected with capsaicin plus vehicle, CCI/Cap/Veh) were processed for whole-mount staining at the same time as the quantification of immunolabeling. CGRP+ afferent terminals were significantly greater in the CCI/Cap/Pac group compared with the CCI/Cap/Veh group (Figs. 5B and C). These results are consistent with in vitro and hind paw data and support the idea that the depolymerization of microtubules leads to capsaicin-induced ablation, which is necessary for capsaicin-induced analgesia for trigeminal neuropathic pain.
Figure 5.
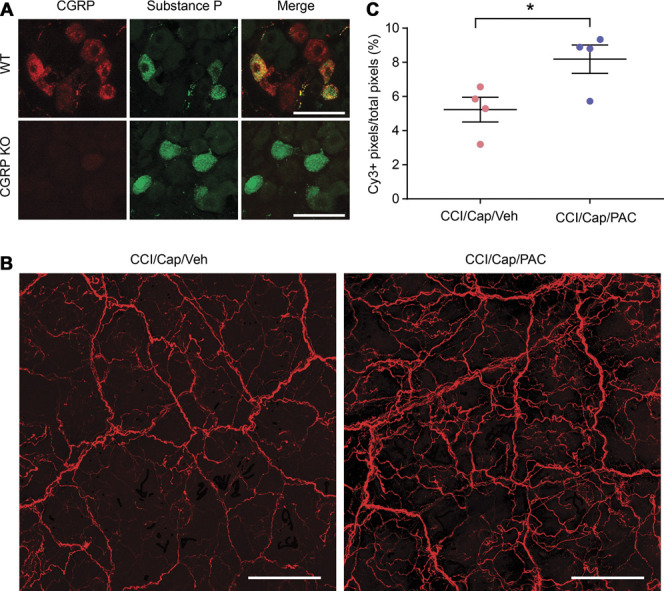
Whole-mount staining of calcitonin gene–related peptide (CGRP). (A) Validation of primary antibody against CGRP. Scale bar, 50 µm. (B) Representative projected images from whole-mount staining of facial skin behind the vibrissa pad within the infraorbital territory from mice with ION-CCI injected with capsaicin plus vehicle (CCI/cap/Veh) or with capsaicin plus paclitaxel (CCI/Cap/PAC) as indicated. Scale bar, 200 µm. (C) Quantification of CGRP+ afferent terminals. *P < 0.05 in the Student t-test. ION-CCI, chronic constriction injury of the infraorbital nerve.
3.6. Transient receptor potential vanilloid 1 S801 phosphorylation did not influence capsaicin-induced analgesia
The carboxy-terminal domain of TRPV1 directly binds to and stabilizes microtubules.22 Interestingly, the phosphorylation of TRPV1 S801 residue in the carboxy-terminal domain interferes with TRPV1–microtubule binding, suggesting that TRPV1 S801 phosphorylation may affect the efficacy of capsaicin-induced analgesia. To test this, we used a mouse model in which the S801 residue was mutated to alanine, preventing phosphorylation at the given site.25 TRPV1S801A/S801A mutant mice showed identical development of mechanical hyperalgesia as WT after ION-CCI (Fig. 6). Capsaicin injection to the facial skin produced the same extent of analgesia in WT and TRPV1 S801A mice, suggesting that TRPV1 S801 phosphorylation does not influence capsaicin-induced analgesia.
Figure 6.
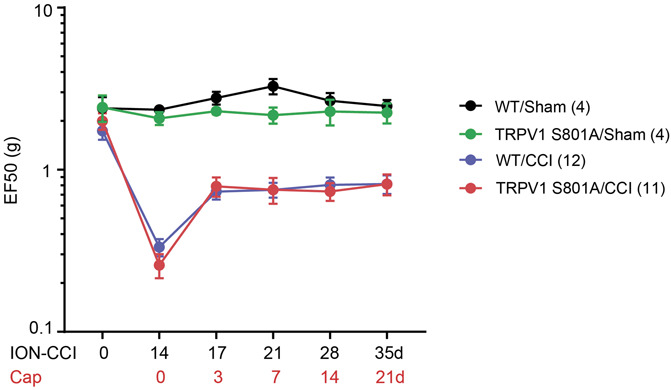
Capsaicin-induced analgesia is not affected by the prevention of phosphorylation in Ser800 of transient receptor potential vanilloid 1 (TRPV1). Changes in mechanical sensitivity in the ipsilateral side of V2 skin after chronic constriction injury of the infraorbital nerve (ION-CCI) in wild-type (WT) or TRPV1 S801A mice. Mechanical hyperalgesia was attenuated by the injection of capsaicin (10 µg) to the facial skin. Two-way repeated measures ANOVA followed by the Sidak posttest showed no significant difference. N = 12 in WT (9 males and 3 females) and 11 in TRPV1 S801A (7 males and 4 females). ANOVA, analysis of variance; TRVP1, transient receptor potential vanilloid.
4. Discussion
We determined the effects of microtubule stabilization by paclitaxel on capsaicin-induced axonal ablation and analgesia for trigeminal neuropathic pain in mice. Capsaicin administration can cause desensitization of TRPV1,26 inhibition of nociceptor firing,29 or a decrease in mechanotransduction.5 These effects of capsaicin on primary afferents could potentially contribute to analgesic effects immediately after capsaicin injection. However, we do not believe these early events contribute to long-lasting analgesia. Instead, capsaicin-induced long-term analgesia is the likely outcome of the ablation of TRPV1-expressing afferent terminals. High concentrations of capsaicin lead to long-term defunctionalization associated with the ablation of TRPV1-expressing afferent terminals in humans.1,35,40 Recent studies have shown that capsaicin-induced TRPV1/Ca2+/calpain-mediated ablation of axonal terminals is necessary for producing long-lasting analgesia in a mouse model of neuropathic pain. Focal injection of capsaicin into the facial skin attenuated mechanical hyperalgesia lasting for more than 2 weeks in an ION-CCI model, which was abolished by the inhibition of capsaicin-induced ablation of afferent terminals in the skin.42,43 These data indicate that capsaicin-induced ablation of TRPV1+ afferent terminals is necessary to induce long-lasting analgesia for mechanical hyperalgesia. The results from the current study further support this notion. This hypothesis is based on the presumption that TRPV1+ nerve terminals remaining in the skin after ION-CCI contribute to the maintenance of mechanical hyperalgesia. Although peripheral neuropathy decreases epidermal peptidergic afferent terminals,33 our whole-mount staining showed ample projections of CGRP+ afferent terminals in the facial skin after ION-CCI. Reduced capsaicin-induced ablation by paclitaxel and its association with reduced analgesia suggests that remaining peptidergic afferent terminals contribute to the maintenance of mechanical hyperalgesia, and their ablation is associated with analgesia. The remaining nociceptors may be abnormally hyperactive “irritable nociceptors”16 that mediate heat and mechanical hyperalgesia in a subpopulation of patients with peripheral neuropathy.4 Microneurographic recordings in patients with neuropathic pain have shown spontaneous firing of c nociceptors, which are associated with ongoing pain and hyperalgesia.7,37,38 Capsaicin administration can improve pain management in this group of patients for whom pain is maintained by hyperactive nociceptors.
Because intraperitoneal or intravenous administration of paclitaxel alone induces chemotherapy-induced peripheral neuropathy and persistent hyperalgesia,41 there is a possibility that the lack of analgesia by capsaicin on coadministration of paclitaxel is due to the superimposition of paclitaxel-induced neuropathic pain with capsaicin-induced analgesia. However, we do not believe our data can be explained by this alternative hypothesis. A single subcutaneous injection of 20 µg of paclitaxel into the facial skin of mice in the sham or ION-CCI group did not result in any signs of mechanical hyperalgesia in the sham group or increased mechanical hyperalgesia in the ION-CCI group during the 2-week observation period. Indeed, development of trigeminal peripheral neuropathy and facial pain is rare.41 Furthermore, paclitaxel-induced neuropathic pain is bilateral.41 After facial paclitaxel injection, no sign of hyperalgesia was shown from the contralateral side in mice with ION-CCI or sham surgery. Moreover, paclitaxel-induced neuropathy depends on the cumulative dose of administered paclitaxel, and a single injection of a higher dose does not show axonal or myelin damage of the peripheral nerves.31 Consistently, the density of peptidergic afferent terminals at the injected skin site were greater in the group with paclitaxel plus capsaicin injection than the group with capsaicin alone. These results argue against the possibility that reduced capsaicin-induced analgesia by the coadministration of paclitaxel is due to paclitaxel-induced neuropathy.
Transient receptor potential vanilloid 1 activation has been suggested to contribute to cytoskeleton remodeling and thus influence nociception.19,20 The activation of TRPV1 results in the rapid disassembly of microtubules, and TRPV1 activation mainly affects dynamic microtubules that contain tyrosinated tubulins, whereas stable microtubules are apparently unaffected. However, it is not known whether the TRPV1-induced regulation of microtubules mediates capsaicin-induced ablation of afferent terminals and, more importantly, analgesia for neuropathic pain. Paclitaxel enhances the polymerization of tubulin to stabilize microtubules against depolymerization by Ca2+, which readily depolymerize normal microtubules.24 Stabilization of microtubules by paclitaxel decreased capsaicin-induced increases of free tubulin in DRG neurons. Furthermore, capsaicin-induced ablation of afferent terminals in vitro and in vivo (both in the hind paw and facial skin) was prevented by paclitaxel. Importantly, coadministration of paclitaxel and capsaicin prevented capsaicin-induced analgesia for mechanical hyperalgesia. These data strongly suggest that capsaicin-induced depolymerization of microtubules leads to axonal ablation and analgesia.
We previously showed that a Ca2+ influx through a TRPV1 pore is necessary for capsaicin-induced degeneration of afferent terminals.42 However, an increase in intra-axonal Ca2+ alone is not sufficient to mimic capsaicin-induced degeneration, suggesting that a TRPV1-mediated Ca2+ influx may be preferentially linked with downstream Ca2+-dependent effectors, which would lead to degeneration. Our previous studies42,43 and current study suggest that capsaicin-induced TRPV1-mediated axonal Ca2+ influx leads to the ablation of axonal terminals and analgesia through 2 critical factors: calpain activation and microtubule depolymerization. Microtubules directly bind to the carboxy-terminal domain of TRPV1, which determines the stability of microtubules.21 Because microtubules bind to the region near the ion conducting pore, microtubules bound to TRPV1 can be particularly susceptible to a Ca2+ influx through the TRPV1 pore. Despite clear involvement of calpain activation and microtubule depolymerization in capsaicin-induced degeneration of afferent terminals, we do not know whether these 2 mechanisms occur independently or in series. Both the activation of calpain and the depolymerization of microtubules are induced by the increase in intracellular Ca2+32 after TRPV1 activation by capsaicin. Thus, it is possible that the activation of calpain and the depolymerization of microtubules occur independently. However, the Ca2+-dependent depolymerization of microtubules alone is unlikely to be sufficient to explain the extent of capsaicin-induced ablation of nerve terminals and long-lasting analgesia because the inhibition of calpains produced the robust prevention of ablation of afferent terminals and analgesia to the same degree as microtubule stabilization. Individual inhibition of calpains and microtubule depolymerization robustly prevented capsaicin-induced ablation, and simultaneous inhibition did not show substantial additivity, suggesting microtubule depolymerization may be downstream of calpain activation. Although it is not known whether the activation of calpain leads to the degradation of microtubules or free tubulins, calpains can hydrolyze microtubule-associated proteins, which, in turn, destabilizes microtubules.45 Therefore, the activation of calpain can indirectly destabilize microtubules by hydrolyzing microtubule-associated protein. The destabilized microtubules by calpain activation can be further depolymerized by the increased local Ca2+. Therefore, a series of events involving TRPV1/Ca2+/calpains/microtubule depolymerization leads to the execution of capsaicin-induced ablation. An alternative pathway of TRPV1 regulation of microtubules that does not involve ionic conduction has also been suggested and, instead, involves the phosphorylation of the TRPV1 S801 residue.22 However, our assay using TRPV1 S801A mice did not show a difference in capsaicin-induced analgesia, suggesting that the regulation of microtubules through TRPV1 S801 phosphorylation is not a significant factor influencing the therapeutic effects of capsaicin. Further studies on the soluble or structural contributors of capsaicin/TRPV1/microtubule regulation of axonal ablation are warranted.
Given well-established capsaicin-induced ablation of cutaneous nerve terminals in humans,3,11,35,40 TRPV1/Ca2+/calpain/microtubule mechanisms should primarily explain capsaicin-induced analgesia in humans. It is unclear, however, whether capsaicin produces analgesia through TRPV1-independent mechanisms in humans. In mice, capsaicin does not ablate afferent terminals in DRG neurons from TRPV1 knockout mice or in TRPV1-negative neurons.42 In humans, the administration of capsaicin fully denervates PGP9.5-expressing intraepidermal fibers,40 suggesting a possible TRPV1-independent ablation by capsaicin based on the assumption that a substantial proportion of intraepidermal fibers in humans does not express TRPV1. However, a microneurography study has shown that 90% of c nociceptors from human leg skin are capsaicin sensitive.36 A recent in situ hybridization study with human DRG has also suggested that TRPV1 is expressed in 75% of all sensory afferents—including most nociceptive neurons—whereas TRPV1 expression is limited in 35% of sensory afferents that are mainly peptidergic in mice.39 Therefore, the extent of capsaicin-induced ablation of nociceptive afferents from the skin can be greater in humans than mice. Although we cannot exclude a possible contribution of TRPV1-independent mechanisms, we presume that the TRPV1/Ca2+/calpain/microtubule mechanism primarily mediates capsaicin-induced analgesia in humans as well. Our results also suggest that capsaicin-induced analgesia may not be effective in patients under ongoing chemotherapy using paclitaxel or other taxane stabilizing microtubules. Because neuropathy induced by microtubule-targeting drugs demonstrates individual variations that are likely underpinned by genetic variations,2 the efficacy of capsaicin may be also affected by genetic variations in genes involved in microtubule stabilization. It is also possible that drugs enhancing microtubule depolymerization might enhance the ablative effects of capsaicin, even at lower doses, which could lead to decreased procedural pain.
In conclusion, capsaicin-induced depolymerization of axonal microtubules determines capsaicin-induced ablation of nociceptive terminals and the extent of analgesia. Further understanding of TRPV1/Ca2+-dependent mechanisms of capsaicin-induced ablation and analgesia may help to improve the efficacy of this important treatment.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank Dr. Michael Caterina for providing the protocol for whole-mount staining. This study was supported by National Institutes of Health grant R01 DE027735 and R35 DE030045 (M.K.C.). All data will be made available to other investigators upon request to the corresponding author.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Vipin Arora, Email: varora@umaryland.edu.
Tingting Li, Email: Tli3@umaryland.edu.
Sinu Kumari, Email: skumari@umaryland.edu.
Sheng Wang, Email: swang2@umaryland.edu.
Jamila Asgar, Email: jasga@umaryland.edu.
References
- [1].Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 2011;107:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Argyriou AA, Bruna J, Genazzani AA, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat Rev Neurol 2017;13:492–504. [DOI] [PubMed] [Google Scholar]
- [3].Arora V, Campbell JN, Chung MK. Fight fire with fire: neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol Ther 2021;220:107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral Neuropathic Pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal 2015;8:ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Campbell JN, Stevens R, Hanson P, Connolly J, Meske DS, Chung MK, Lascelles BDX. Injectable capsaicin for the management of pain due to osteoarthritis. Molecules 2021;26:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campero M, Bostock H, Baumann TK, Ochoa JL. A search for activation of C nociceptors by sympathetic fibers in complex regional pain syndrome. Clin Neurophysiol 2010;121:1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 2013;503:111–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 2011;31:10119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 2011;31:5067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chung MK, Campbell JN. Use of capsaicin to treat pain: mechanistic and therapeutic considerations. Pharmaceuticals (Basel) 2016;9:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chung MK, Jue SS, Dong X. Projection of non-peptidergic afferents to mouse tooth pulp. J Dent Res 2012;91:777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol 2011;704:615–36. [DOI] [PubMed] [Google Scholar]
- [14].Chung MK, Wang S, Oh SL, Kim YS. Acute and chronic pain from facial skin and oral mucosa: unique neurobiology and challenging treatment. Int J Mol Sci 2021;22:5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cruccu G, Nurmikko TJ, Ernault E, Riaz FK, McBride WT, Haanpaa M. Superiority of capsaicin 8% patch versus oral pregabalin on dynamic mechanical allodynia in patients with peripheral neuropathic pain. Eur J Pain 2018;22:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209–27. [DOI] [PubMed] [Google Scholar]
- [17].Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 2010;463:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gornstein E, Schwarz TL. The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology 2014;76:175–83. [DOI] [PubMed] [Google Scholar]
- [19].Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+ -sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem 2004;91:1092–103. [DOI] [PubMed] [Google Scholar]
- [20].Goswami C, Dreger M, Otto H, Schwappach B, Hucho F. Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J Neurochem 2006;96:254–66. [DOI] [PubMed] [Google Scholar]
- [21].Goswami C, Hucho TB, Hucho F. Identification and characterisation of novel tubulin-binding motifs located within the C-terminus of TRPV1. J Neurochem 2007;101:250–62. [DOI] [PubMed] [Google Scholar]
- [22].Goswami C, Kuhn J, Dina OA, Fernandez-Ballester G, Levine JD, Ferrer-Montiel A, Hucho T. Estrogen destabilizes microtubules through an ion-conductivity-independent TRPV1 pathway. J Neurochem 2011;117:995–1008. [DOI] [PubMed] [Google Scholar]
- [23].Haviv Y, Zadik Y, Sharav Y, Benoliel R. Painful traumatic trigeminal neuropathy: an open study on the pharmacotherapeutic response to stepped treatment. J Oral Facial Pain Headache 2014;28:52–60. [DOI] [PubMed] [Google Scholar]
- [24].Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol 1994;5(Suppl 6):S3–6. [PubMed] [Google Scholar]
- [25].Joseph J, Qu L, Wang S, Kim M, Bennett D, Ro J, Caterina M, Chung MK. Phosphorylation of TRPV1 S801 contributes to modality-specific hyperalgesia in mice. J Neurosci 2019;39:9954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Joseph J, Wang S, Lee J, Ro JY, Chung MK. Carboxyl-terminal domain of transient receptor potential vanilloid 1 contains distinct segments differentially involved in capsaicin- and heat-induced desensitization. J Biol Chem 2013;288:35690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011;147:1615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma M. Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol Dis 2013;60:61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ma XL, Zhang FX, Dong F, Bao L, Zhang X. Experimental evidence for alleviating nociceptive hypersensitivity by single application of capsaicin. Mol Pain 2015;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McQuay HJ, Tramer M, Nye BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. PAIN 1996;68:217–27. [DOI] [PubMed] [Google Scholar]
- [31].Mimura Y, Kato H, Eguchi K, Ogawa T. Schedule dependency of paclitaxel-induced neuropathy in mice: a morphological study. Neurotoxicology 2000;21:513–20. [PubMed] [Google Scholar]
- [32].O'Brien ET, Salmon ED, Erickson HP. How calcium causes microtubule depolymerization. Cell Motil Cytoskeleton 1997;36:125–35. [DOI] [PubMed] [Google Scholar]
- [33].Peleshok JC, Ribeiro-da-Silva A. Delayed reinnervation by nonpeptidergic nociceptive afferents of the glabrous skin of the rat hindpaw in a neuropathic pain model. J Comp Neurol 2011;519:49–63. [DOI] [PubMed] [Google Scholar]
- [34].Pipeleers DG, Pipeleers-Marichal MA, Sherline P, Kipnis DM. A sensitive method for measuring polymerized and depolymerized forms of tubulin in tissues. J Cell Biol 1977;74:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004;127:1606–15. [DOI] [PubMed] [Google Scholar]
- [36].Schmelz M, Schmid R, Handwerker HO, Torebjork HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000;123:560–71. [DOI] [PubMed] [Google Scholar]
- [37].Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. PAIN 2012;153:42–55. [DOI] [PubMed] [Google Scholar]
- [38].Serra J, Duan WR, Locke C, Sola R, Liu W, Nothaft W. Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in C-nociceptors in patients with painful diabetic neuropathy: a randomized controlled trial. PAIN 2015;156:2175–83. [DOI] [PubMed] [Google Scholar]
- [39].Shiers S, Klein RM, Price TJ. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. PAIN 2020;161:2410–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci 1998;18:8947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol 2020;324:113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang S, Asgar J, Joseph J, Ro JY, Wei F, Campbell JN, Chung MK. Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J Biol Chem 2017;292:8291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang S, Bian C, Yang J, Arora V, Gao Y, Wei F, Chung MK. Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. eNeuro 2020;7:ENEURO.0118-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. PAIN 2015;156:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yoshimura N, Tsukahara I, Murachi T. Calpain and calpastatin in porcine retina. Identification and action on microtubule-associated proteins. Biochem J 1984;223:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci 2005;25:5488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]